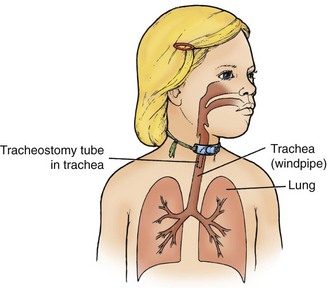
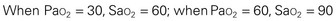The Child with Disturbance of Oxygen and Carbon Dioxide Exchange
http://evolve.elsevier.com/wong/ncic
The Child with Respiratory Dysfunction, Ch. 32
Discharge Planning and Home Care, Ch. 26
Family-Centered Home Care, Ch. 25
High Risk Related to Disturbed Respiratory Function, Ch. 10
Physical Examination: Chest, Lungs, Ch. 6
Preparation for Diagnostic and Therapeutic Procedures, Ch. 27
Shock, Ch. 29
Respiratory Tract Structure and Function
Disorders of the respiratory tract occur frequently in infancy and childhood. Anatomically, several factors influence the manner in which children, particularly infants, respond to respiratory disturbances.
The respiratory tract consists of many complex structures. The primary function of these structures is to distribute air and exchange gases so that cells are supplied with oxygen (O2) for body metabolism while carbon dioxide (CO2), the volatile product of metabolism, is removed. The nose, pharynx, larynx, trachea, bronchi, and lungs are structures of the respiratory system through which gases enter the body. The circulatory system distributes gases to and from the millions of cells throughout the body. All the structures of the respiratory system, except the minute air sacs (alveoli) of the lung tissue, function in air distribution. It is within the alveoli that gas exchange takes place.
Structure
The thoracic cavity, located in the bony framework provided by the ribs, vertebrae, and sternum, consists of three major sections: the three-lobed lung on the right; the two-lobed lung on the left; and the mediastinum, or the space between the lungs. The mediastinum contains the esophagus, trachea, large blood vessels, and heart. Smooth parietal pleura line the entire thoracic cavity and adhere to the ribs and superior surface of the diaphragm. Each lung is encased in a separate visceral pleural sac that, when inflated, lies against the parietal pleura. Normally the two pleural membranes are separated by only enough fluid to lubricate the surface for painless movement during filling and emptying of the lungs. In disease states this space may contain air (pneumothorax), fluid (pleural effusion), serum (hydrothorax), blood (hemothorax), or pus (pyothorax, also known as empyema). Inflammation of the pleura causes the painful friction of pleurisy during respiratory movements.
Chest
The chest has a relatively round configuration at birth but changes gradually to one that is more or less flattened in the anteroposterior (front-to-back) diameter in adulthood. In some lung diseases, chronic overinflation causes changes in these measurements. For example, in severe obstructive lung disease (e.g., asthma, cystic fibrosis) the anteroposterior measurement approaches the transverse (side-to-side) measurement to produce the so-called barrel chest. Periodic measurements provide clues to the course of the lung disease or the efficacy of therapy. Increased size indicates progressive obstructive lung disease.
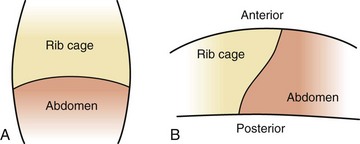
The elliptic shape of the ribs and the angle at which they are attached to the spine allow the thorax to change size during respiration. Contraction of the intercostal muscles lifts the ribs from a downward angle to a more horizontal angle, which increases both the anteroposterior and the lateral dimensions of the chest (Fig. 31-1, A). This also changes the diameter of the bronchi; the diameter increases during inspiration and decreases during expiration, an important factor when the bronchi are narrowed as a result of obstruction or inflammation. Contraction and relaxation of the diaphragm cause the chest cavity to lengthen and shorten, which also increases the volume of the chest cavity during inspiration. Normal expiration is passive, although contraction of the internal intercostal muscles pulls the rib cage downward, and contraction of the abdominal muscles forces the diaphragm upward to actively decrease the chest size. (See Fig. 6-30.)
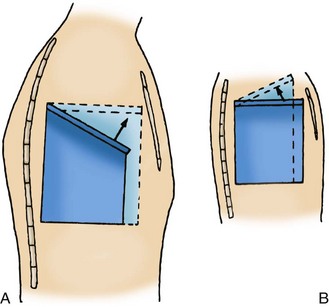
Fig. 31-1 Mechanisms of respiratory excursion. A, Downward and lateral position of rib in adult and expansion of lung capacity on thoracic inspiration. B, More horizontal position of rib in infant and decreased expansion of lung capacity on thoracic inspiration.
An adult’s ribs articulate with the vertebrae and sternum from a downward and lateral angle. During inspiration the respiratory muscles contract and the thorax enlarges. In the newborn infant, however, the ribs articulate with the spine at a horizontal rather than a downward slope; consequently, during inspiration the diameter of the chest decreases (Fig. 31-1, B). The infant relies almost entirely on diaphragmatic-abdominal breathing. During inspiration the diaphragm is forced downward, increasing the available space for lung expansion; the intercostal muscles serve primarily as stabilizing forces. Respiration is facilitated by the processes of (1) compliance, the elastic property of lung tissue that allows it to expand and recoil; and (2) resistance, which affects the amount of flow through the airways (see p. 1185).
Variations occur in lung volume relative to posture. In the upright position the evenly distributed weight of the abdominal contents contributes to uniform application of negative intrathoracic pressure. However, in the supine position the abdominal contents apply weight caudally to create a nonuniform distribution of positive pressure to the diaphragm. Consequently, lung volume is increased in the upright position and decreased in the supine position. In addition, the mechanical attachment of the diaphragm to the rib cage is such that contraction elevates the rib cage in the upright position but in the supine position tends to pull in the rib cage (Fig. 31-2).
In the newborn the diaphragm is attached higher in front. Therefore this already stretched diaphragm is unable to contract as far or as forcefully as that of the older infant or child. Young infants are also less able to withstand diaphragmatic fatigue because of fewer energy-producing components. Abdominal distention from gas or fluid can impede diaphragmatic excursion significantly.
Airways
The rigid nasal structures, which are lined with ciliated mucous membranes, serve as passageways for air, warming and moistening air, filtering its impurities, and destroying microorganisms that come in contact with immune defenses in the mucosa. In infancy the nasal passages are narrow, and infants are primarily nose breathers. Any factor that decreases the size of the nasal passages and increases airway resistance, such as nasal mucosal swelling and mucus accumulation, hampers breathing and feeding.
The upper airway (oronasopharynx, pharynx, larynx, and upper part of the trachea) is shared by both the respiratory and the alimentary tracts, and many of the muscles in this area participate in several complex acts. However, the sequence of airway muscle activation is different in breathing and swallowing. The upper airway dilates during inspiration and constricts during exhalation. During some activities these dimensions are modified. For example, inspiration is short during crying, coughing, and sneezing, but with crying the larynx and pharynx dilate. The net result of swallowing is closure of the upper airway with interruption of airflow. Consequently, the timing and magnitude of muscle activation have important implications for airway size and patency.
The pharynx is a passageway for the entry and exit of air, and it plays a role in phonation by helping to produce vowel sounds. The pharynx contains the palatine and lingual tonsils, which are involved in infection control.
The larynx, situated at the upper end of the trachea, is made of a rigid circular framework of cartilage and contains the epiglottis and glottis (vocal cords). These structures prevent solids or liquids from entering the airway during swallowing, and the vibrations of the vocal cords produce voice sounds. In infancy the glottis is located more cephalad (toward the head) than in later childhood, and the laryngeal reflexes are active. The epiglottis is longer and projects farther posteriorly in infants. The narrowest portion of the larynx is at the level of the cricoid cartilage. In the infant and young child the ciliated columnar epithelium below the vocal cords is loosely bound with areolar connective tissue and is therefore more susceptible to edema formation. Swelling of the glottis and epiglottis produces hoarseness and often life-threatening obstruction of this portion of the airway.
The lower airway is made up of the lower trachea, mainstem bronchi, segmental bronchi, subsegmental bronchioles, terminal bronchioles, and alveoli. The trachea, which is composed of smooth muscle supported by C-shaped rings of cartilage, ensures an open airway to the bronchi and lungs. The trachea divides at the carina into two primary bronchi. The right one is situated slightly more vertical than the left, which causes aspirated objects to lodge more frequently in the right bronchus. Each bronchus enters the lung on its respective side, where it divides into secondary bronchi that continue to branch and divide into progressively smaller bronchioles. The entire bronchial tree is lined with mucous membrane and is composed of spiral smooth muscle supported by rings of cartilage. As the bronchioles become smaller, the cartilaginous rings become increasingly irregular and then disappear completely in the smallest bronchioles, the walls of which consist of only a single layer of cells (Fig. 31-3). There is a range of 23 to 26 levels of branches divided into two categories: the conducting airways and the terminal respiratory units. These branch levels are called generations.
Pathophysiology Review
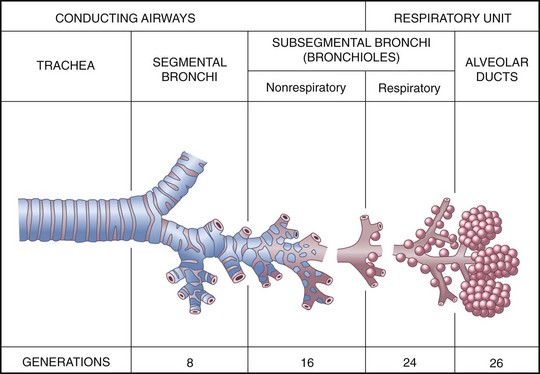
Fig. 31-3 Structures of the lower airway. (Redrawn from Thompson JM, McFarland GK, Hirsch JE, et al: Mosby’s clinical nursing, ed 5, St Louis, 2002, Mosby.)
All the structures are subject to obstruction from edema or foreign objects, but the degree of obstruction from constriction of smooth muscle differs. The diameter of the relatively rigid upper airway is less subject to constriction than the lower airway structures, which contain little cartilaginous support. The highly reactive bronchiolar smooth muscle of the lower airway structures can cause life-threatening obstruction during bronchospasm. The airway cartilage in young infants is soft and compressible; therefore the intrathoracic airways are highly reactive to stimuli, such as vagal nerve stimulation.
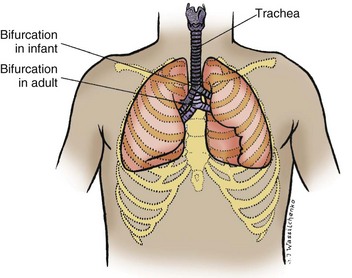
The airways of the newborn have little smooth muscle, but in children 4 to 5 months of age they contain sufficient muscle to cause narrowing in response to irritating stimuli. By 1 year of age, smooth muscle development and reactivity are comparable to those in the adult. Growth of the respiratory system follows the general growth curve during the early weeks of life, but the airways grow faster than the thoracic and cervical portions of the vertebral column. Consequently, the larynx and trachea descend in relation to the upper spine. For example, the bifurcation of the trachea that lies opposite the third thoracic vertebra in the infant descends to a position opposite the fourth in adulthood (Fig. 31-4). Likewise, the cricoid cartilage descends from a position opposite the fourth cervical vertebra in the infant to opposite the sixth cervical vertebra in the adult. These anatomic changes produce differences in the angle of access to the trachea at various ages, and the nurse must consider this when the infant or child is positioned for resuscitation and airway clearance.
The function of the tracheobronchial tree is to distribute air to the alveoli of the lung. A variety of diseases and conditions, such as mucosal swelling, muscular contraction, and mechanical obstruction by mucus or a foreign body (FB), can cause localized or generalized airway occlusion.
Respiratory Units
![]() The two cone-shaped lungs consist of the bronchi; bronchioles; and innumerable small air sacs, or alveoli. Through these thin-walled sacs, gas exchange occurs by simple diffusion between the inspired air and the bloodstream. The amount of gas exchanged depends on many factors, including the amount and composition of air inhaled, thickness of the alveolar wall, adequacy of circulation to the alveoli, and substances within the alveoli that either prevent their inflation (e.g., surface-active surfactant) or prevent gas exchange (e.g., fluids).
The two cone-shaped lungs consist of the bronchi; bronchioles; and innumerable small air sacs, or alveoli. Through these thin-walled sacs, gas exchange occurs by simple diffusion between the inspired air and the bloodstream. The amount of gas exchanged depends on many factors, including the amount and composition of air inhaled, thickness of the alveolar wall, adequacy of circulation to the alveoli, and substances within the alveoli that either prevent their inflation (e.g., surface-active surfactant) or prevent gas exchange (e.g., fluids).
![]() Animation—Bronchi and Bronchioles
Animation—Bronchi and Bronchioles
With age, changes take place in the air passages that increase the respiratory surface area. The major changes are in the number and size of alveoli and in the increased branching of terminal bronchioles. Although the number of conducting airways is complete early in fetal life, the air sacs are shallow with wide necks and have few shared walls, or septa, at birth. This promotes patency but limits surface area for gas exchange. The alveoli are large with thick septa that have little elastic recoil (not unlike the emphysemic lung). During the first year, bronchioles continue to branch, and the globular alveoli formed earlier in the terminal units rapidly increase in number with each generation. These alveoli partition and divide existing alveoli to form smaller lobular units separated by thinner septa, thus enlarging the area available for gas exchange.
Alveoli increase steadily in number, but it is unclear when septal division ceases and an increase in size begins. It appears to occur sometime during middle childhood, although evidence indicates that an increase in the number of alveoli for each terminal airway takes place at puberty. Approximately nine times more alveoli are present at age 12 years than at birth. In later stages of growth the structures lengthen and enlarge. In addition, collateral pathways of ventilation develop, including pores through alveolar walls and possibly pathways between bronchioles.
All these factors have significant implications for respiratory disorders in children. Infants and young children have less alveolar surface area for gas exchange, the narrowly branching peripheral airways become easily obstructed, and lack of collateral pathways inhibits ventilation beyond obstructed units. Consequently, young children are subject to obstruction and atelectasis, especially as a result of repeated infection.
A variety of pathologic conditions affect lung growth. A postural defect such as kyphoscoliosis reduces the number of alveoli. Infections of the respiratory tract (e.g., coxsackievirus) can permanently alter lung development, resulting in decreased numbers of small airways. Replication of alveoli is inhibited, so the remaining alveoli are large but decreased in number. Changes in hormone levels influence lung growth. Glucocorticosteroids, thyroxine, and prolactin enhance lung development, but lack of thyroid hormone results in immature lungs. Biochemical substances that enhance lung growth are theophylline, estrogen, isoxsuprine, epidermal growth factor, and heroin injected during pregnancy. Some medications such as phenobarbital or excess insulin inhibit lung growth.
Function
![]() Respiratory movements are first evident at approximately 20 weeks of gestation, and throughout fetal life amniotic fluid is exchanged in the alveoli. In the neonate the respiratory rate is rapid to meet the needs of a high metabolism. During growth the respiratory rate steadily decreases until it levels off at maturity. (See inside back cover.) The volume of air inhaled increases with the growth of the lungs and is closely related to body size. In addition, a qualitative difference exists in expired air at different ages. During growth the amount of oxygen in the expired air gradually decreases and the amount of carbon dioxide increases.
Respiratory movements are first evident at approximately 20 weeks of gestation, and throughout fetal life amniotic fluid is exchanged in the alveoli. In the neonate the respiratory rate is rapid to meet the needs of a high metabolism. During growth the respiratory rate steadily decreases until it levels off at maturity. (See inside back cover.) The volume of air inhaled increases with the growth of the lungs and is closely related to body size. In addition, a qualitative difference exists in expired air at different ages. During growth the amount of oxygen in the expired air gradually decreases and the amount of carbon dioxide increases.
Ventilation, the exchange of gases in the lung, results from changes in pressure gradients created by changes in the size of the thoracic cavity. Contraction of the diaphragm and external intercostal muscles increases the size of the thorax and decreases the intrathoracic pressure. As a result, air moves from the atmosphere, which has a higher pressure, into the lungs, which have a lower pressure. The principles of artificial or mechanical ventilation are based on this concept. Mechanical (artificial) respiratory devices increase the pressure entering the air passages (positive pressure breathing devices) or lower the pressure around the body (negative pressure ventilator).
The two primary forces that affect the mechanics of breathing are compliance and resistance; conditions that either increase or decrease these two forces are listed in Box 31-1. Compliance is a measure of chest wall and lung distensibility. It represents the relative ease with which the chest and lungs expand with increasing volume and then collapse away from the pleural wall with decreasing volume (elastic recoil). The two major factors determining compliance are (1) alveolar surface tension, which is lowered by surfactant, a lipoprotein at the air-fluid interface that allows alveolar expansion and prevents alveolar collapse; and (2) elastic recoil, the tendency of the lungs to return to the resting state after inspiration (a passive process that requires no muscular effort). Other factors influencing compliance include the degree of tissue hydration, lung blood volume, surface forces at the air-fluid interface, and chest or lung tissue pathologic state (e.g., fibers of elastin or collagen). Factors that interfere with compliance and recoil increase the work of breathing.
Compliance is normally high in the newborn and infant because of a more pliant (flexible) rib cage. This greater compliance causes the rib cage to be easily distorted with increased negative pressure in the pleural cavity or when factors inhibit the stabilizing action of the intercostal muscles. As the child grows, chest wall compliance decreases and elastic recoil increases; therefore ventilation becomes progressively more efficient. In pathologic states an increase in compliance indicates that the lungs or chest wall is abnormally easy to inflate and has lost some elastic recoil, such as in asthma. A decrease in compliance indicates that the lungs or chest wall is abnormally stiff or difficult to inflate, such as in respiratory distress syndrome (McCance and Huether, 2010).
Any condition that decreases or increases compliance or increases airway resistance results in increased work of breathing (increased respiratory rate, retractions, nasal flaring). When respiratory muscle fatigue develops, respiratory failure will occur.
Resistance is determined primarily by airway size. The body must overcome three sources of resistance during breathing: tissue resistance in the chest wall (about 20% resistance); tissue resistance in the lungs (about 15% resistance); and, most important, flow resistance in the airways (which often increases with respiratory disease). The four factors determining resistance are flow rate velocity, gas viscosity, length of airway, and airway diameter. If any of the first three variables increases, resistance to airflow also increases. If airway diameter decreases, resistance increases exponentially.
The small diameter of children’s airways increases the potential risk of any condition that reduces airway size. Fig. 31-5 illustrates the difference that airway size plays in older children’s and infants’ responses to airway compromise.
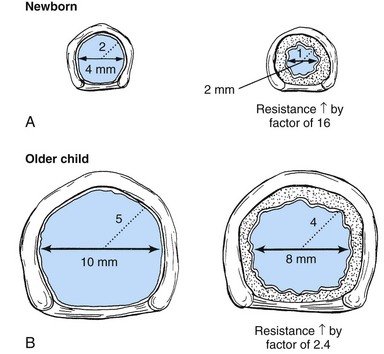
Fig. 31-5 Effects of 1 mm of circumferential edema in small neonate and older child. A, Neonate possesses a larynx approximately 4 mm in diameter and 2 mm in radius. If 1 mm of circumferential edema develops, it will halve the airway radius and increase resistance to air flow by a factor of 16. B, Older child possesses a larynx approximately 10 mm in diameter and 5 mm in radius. The 1 mm of circumferential edema will reduce the radius by 20% (from 5 mm to 4 mm) and increase resistance to air flow by a factor of 2.4. (From Hazinski MF, editor: Nursing care of the critically ill child, ed 2, St Louis, 1992, Mosby.)
The diameter of the airways and thus the airflow are determined by the balance of forces that tend to widen or narrow the airways. One of these is neural regulation of bronchial smooth muscles mediated through autonomic nerves. Sympathetic impulses relax the airways; parasympathetic impulses constrict them. Reflex constriction occurs in response to irritating inhalants such as dust, smoke, or sulfur dioxide; arterial hypoxemia and hypercapnia; cold air; and some drugs, such as acetylcholine and histamine. Other factors that alter airway size are peribronchial pressure, which tends to narrow the airways, and intraluminal pressure, which tends to keep the airways open. For example, forced expiration causes increased peribronchial pressure and hence narrowing of the airways; a positive pressure breathing apparatus increases intraluminal pressure, keeping the airways open.
Gas Exchange
Gases in the blood are measured by the partial pressures (tensions) of the individual gases and are expressed in millimeters of mercury. With oxygen therapy it is important to understand the relationship between the concentration of the inspired gas and the partial pressure of that gas in the arteries (Pao2). Inspired oxygen is expressed as the fraction of inspired oxygen (Fio2), with 1.0 indicating 100% oxygen, 0.5 indicating 50% oxygen, and so on. Patients breathing room air have an Fio2 of 0.21 because ambient air contains 21% oxygen.
Ambient air is composed of 21% oxygen, trace amounts of carbon dioxide, and 79% nitrogen (N). Water vapor (H2O) also exerts pressure. The water vapor does not change with the barometric pressure (Pb) but exerts a constant pressure of 47 mm Hg when the gas is fully saturated at body temperature. Each gas contributes to the total barometric pressure as follows:
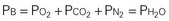
At sea level, the total pressure of gases in the atmosphere and the blood (Pb) is always equal to 760 mm Hg.
The significance of inspired gases lies in the Fio2 and the pressure it exerts (Pio2). At sea level this can be calculated as follows:
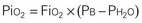
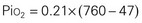
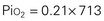
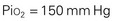
When the Fio2 increases (e.g., to 50%), the pressure exerted also increases:
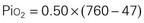
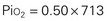
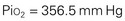
As the inspired gas travels down the airway and reaches the alveoli, the pressure drops as carbon dioxide is added to the mixture. Ambient air contains only traces of carbon dioxide. As the gas diffuses from the capillary blood to the alveoli, however, the amount and pressure of carbon dioxide in the alveoli increase to the carbon dioxide levels in the venous blood (approximately 40 mm Hg). By subtracting the Pco2 from the Pio2, one can determine the alveolar oxygen pressure (PAo2). The PAco2 is first divided by 0.8. This correlation factor, or respiratory quotient (RQ), is used to calculate the ratio of oxygen absorbed to carbon dioxide eliminated. The alveolar pressure can then be expressed as:
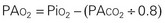
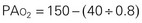
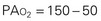
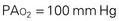
Because normal venous Po2 is approximately 40 mm Hg, a gradient is created when the PAo2 is 100 mm Hg and diffusion occurs between the alveoli and capillary blood. When the patient’s PAo2 decreases, the Fio2 can be raised to increase the PAo2, thereby increasing the gradient for diffusion. Because carbon dioxide is more soluble than oxygen, it diffuses 21 times faster; therefore diffusion of carbon dioxide from the blood to the alveoli is not impaired. The amount of oxygen that diffuses into the blood and the amount of carbon dioxide removed by the lungs depend on several factors (Box 31-2).
Oxygen and Carbon Dioxide Transport: Once oxygen has diffused from the alveolus to the pulmonary capillary, it is transported throughout the body in two ways. A small amount (Pao2) is transported as a solute dissolved in the plasma and the water of red blood cells. A larger portion (40 to 70 times as much) is carried by hemoglobin as oxyhemoglobin. Because each gram of hemoglobin can combine with 1.34 ml of oxygen, the transport capacity is largely determined by the amount of hemoglobin present. Thus children with severe anemia tend to be fatigued and breathe more rapidly. In addition, increasing the amount of oxygen delivered to the alveoli can increase the amount carried by the blood only in relation to the amount of hemoglobin present. For example, at a Pao2 of 100 mm Hg, hemoglobin is 97.5% saturated. Hemoglobin saturation is commonly termed arterial oxygen saturation (Sao2) or oxyhemoglobin saturation. The nonlinear relationship between the Pao2 and the Sao2 is described by the oxyhemoglobin dissociation curve (see Fig. 31-10).
Carbon dioxide is carried in the blood in a number of ways. A small amount (Paco2) is transported dissolved in the plasma and the water of red blood cells. A large amount (more than half) hydrates to form carbonic acid, which dissociates and is carried as bicarbonate and hydrogen ions. The remaining carbon dioxide combines with certain plasma proteins and hemoglobin. The association of carbon dioxide with hemoglobin is accelerated by an increasing Paco2 and a decreasing Pao2 and is decreased by the opposite conditions. The diffusion of carbon dioxide into the alveoli is very rapid. Thus the equilibrium between the Paco2 of the pulmonary capillaries and the alveoli is achieved promptly. Transport between blood and tissue cells is accomplished down a diffusion gradient, just as it is between the blood and the alveoli.
Regulation of Respiration: The mechanisms that control respiration are divided into two categories: (1) a neural system that maintains a coordinated, rhythmic respiratory cycle and regulates the depth of respiration; and (2) a chemical system that regulates alveolar ventilation and maintains normal blood gas pressures.
Neural control in the respiratory center is located in three areas: a pneumotaxic center, which modulates the respiratory frequency and depth; an apneustic center, which produces an inspiratory spasm and is modulated by the pneumotaxic and medullary centers and by vagal afferent impulses; and the medullary respiratory centers, both inspiratory and expiratory, which regulate the rhythmicity of respirations. Impulses from other areas also affect the respiratory centers. Proprioceptive vagal impulses in the lung parenchyma are sensitive to stretching. When lungs become stretched, impulses are transmitted by the vagus nerve to the respiratory center, which inhibits further inflation and prevents overdistention (the Hering-Breuer reflex). The cerebral cortex also helps control respirations by voluntary inhibition or acceleration of the rate and depth of respirations. Reflex apnea can result from sudden painful stimulation, sudden cold stimulation, and stimulation to the larynx or pharynx (the choking reflex, which serves to prevent aspiration).
Chemical control is mediated by specialized structures—central chemoreceptors, located in the medulla, and peripheral chemoreceptors, located in the great vessels—that respond to changes in pH, Pco2, and Po2. Peripheral chemoreceptors of greatest physiologic importance are the carotid bodies, located at the division of the common carotid artery into its external and internal branches, and the aortic bodies that lie between the ascending aorta and the pulmonary artery. Carbon dioxide and hydrogen ions control respiration by acting directly on the respiratory center; the peripheral chemoreceptors respond to changes in Po2. Thus an increase in ventilation can result from either (1) stimulation of the respiratory center by an increased Paco2 or pH; or (2) a decreased Pao2, which stimulates the carotid and aortic bodies. These bodies then transmit signals to the brain to excite the respiratory center.
The lungs also have an important role in acid-base balance. Less rapid than the chemical buffers, the respiratory mechanism begins to act within 1 to 3 minutes to make adjustments in pH by eliminating or retaining carbon dioxide. When the levels of carbon dioxide are altered sufficiently, the respiratory centers in the brain respond by either increasing or decreasing the rate and depth of respiration. For example, when the pH of the blood drops, as from increased exercise, a compensatory increase in respirations rids the body of the carbon dioxide derived from carbonic acid, which is formed from buffered acid metabolites. Carbon dioxide buildup from breath holding produces the same response, again increasing the carbonic acid and reducing the serum pH. Therefore the lungs serve as compensatory organs in metabolic disturbances and respond quite rapidly.
Defenses of the Respiratory Tract
The respiratory tract has several anatomic and biochemical characteristics that provide natural defenses against the many biologic and inanimate agents that can damage respiratory tissues. Intact defenses help repel and resist the impact of injurious agents; factors that reduce the integrity of these mechanisms increase the vulnerability of these tissues to invasion and disease. Respiratory tract defenses include:
Lymphoid tissues—Faucial, lingual, and pharyngeal tonsils (adenoids) and other pharyngeal lymphoid tissues form a protective circle around the entrance to the respiratory tract. These help localize and contain invading organisms so they can be destroyed by the body’s humoral defense mechanisms.
Mucous blanket—The epithelium of the respiratory tract secretes sticky mucus to which airborne organisms adhere.
Ciliary action—The mucus secreted by the columnar epithelium of the respiratory tract is kept flowing, carrying microorganisms and other foreign agents away from the lungs to be coughed or swallowed.
Epiglottis—The epiglottis and the epiglottis reflex protect the respiratory tract from invading material, including infectious exudate from the upper tract, and prevent such material from being aspirated into the lower tract.
Cough—The expulsive force of the cough reflex propels foreign material out of the lower tract.
Position changes—Changes in body position encourage drainage of tracheobronchial passages.
Lymphatics—Lymphatics draining the terminal bronchi and bronchioles remove invading organisms, which are filtered and destroyed in the regional lymph nodes.
Humoral defenses—Organisms and other foreign material are removed or destroyed by phagocytes, enzymes, and immunoglobulins, especially immunoglobulin A, secreted by the bronchial epithelium.
Some children have conditions (e.g., chronic asthma, cystic fibrosis, and the various immunodeficiency disorders) that predispose them to infection as a result of interference with the efficiency of these mechanisms. Frequent, intense exposure to organisms that accompany conditions of crowding or continual exposure to irritating substances in the air results in breakdown of healthy defenses. Concurrent illness, malnutrition, or fatigue reduces the efficiency of natural defenses. Drying of the mucous membranes also inhibits the activity of humoral defenses, such as immunoglobulins.
Assessment of Respiratory Function
Information about the child’s respiratory status is obtained from observations of physical signs and behavior. However, to make a useful assessment, the nurse must know what to look for and how to interpret findings. (See Physical Examination: Chest, Chapter 6.) Auscultation of the lung fields is helpful in identifying specific pathologic conditions and in assessing the child’s responses to treatment. Auscultation is essential when determining airway patency. Palpation and percussion provide information regarding areas of pain and tissue density. Chapter 6 describes breath sounds and their terminology.
Respiration
Assess the configuration of the chest and the pattern of respiratory movement, including rate, regularity, symmetry of movements, depth, effort expended in respiration, and use of accessory muscles of respiration. To determine deviations, the nurse must know the normal type and rate of respiration in relation to the child’s size and age. (See inside back cover.) Respirations (ventilations) are best determined when the child is sleeping or quietly awake.
Tachypnea (rapid respirations) often occurs with anxiety, elevated temperature, severe anemia, and metabolic acidosis. It may also be associated with respiratory alkalosis caused by psychoneurosis and with central nervous system (CNS) disturbances. By observing changes in respiratory rate, the nurse can follow and evaluate the progress of disorders that contribute to low compliance, such as the pneumonias, pulmonary edema, and pleural effusion.
Alterations in the depth of respirations—too deep (hyperpnea) or too shallow (hypopnea)—are recognized as abnormal only in the extremes. Hyperpnea is noted with fever, severe anemia, respiratory alkalosis associated with psychosis, CNS disturbances, and respiratory acidosis that accompanies disorders such as diabetic ketoacidosis or diarrhea. Hypoventilation may occur with metabolic alkalosis in conditions such as hypertrophic pyloric stenosis and respiratory acidosis that accompanies diaphragmatic paralysis or CNS depression. Hypoventilation in preterm infants may occur as a result of pulmonary immaturity, absence of adequate substrate to support respiratory muscle activity, neurologic insult, and neurologic immaturity. Children with neuromuscular diseases such as spinal muscular atrophy may also exhibit hypoventilation. Congenital central hypoventilation syndrome, or Ondine curse, is a rare CNS defect in which respiratory failure occurs as a result of the respiratory system’s failure to respond to increasing carbon dioxide levels; this condition has been known to occur in children with Hirschsprung disease and is often manifested on the first day of life (Haddad, 2007).
Associated Observations
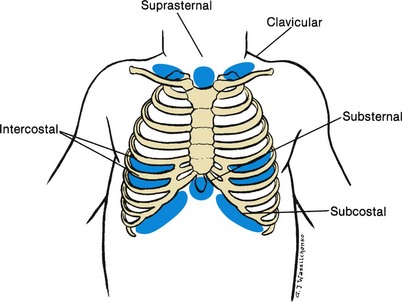
![]() Retractions, or a sinking in of soft tissues relative to the cartilaginous and bony thorax, may occur in some pulmonary disorders. In disease states (particularly in severe airway obstruction), retractions become extreme. Subcostal retraction, observed anteriorly at the lower costal margins, indicates a flattened diaphragm because it not only lowers the floor of the thorax, but also pulls on the rib cage in response to a greater than normal decrease in intrathoracic pressure. In severe airway obstruction, retractions extend to the supraclavicular areas and the suprasternal notch (Fig. 31-6).
Retractions, or a sinking in of soft tissues relative to the cartilaginous and bony thorax, may occur in some pulmonary disorders. In disease states (particularly in severe airway obstruction), retractions become extreme. Subcostal retraction, observed anteriorly at the lower costal margins, indicates a flattened diaphragm because it not only lowers the floor of the thorax, but also pulls on the rib cage in response to a greater than normal decrease in intrathoracic pressure. In severe airway obstruction, retractions extend to the supraclavicular areas and the suprasternal notch (Fig. 31-6).
![]() Anatomy Review—Location of Retractions
Anatomy Review—Location of Retractions
Nasal flaring is a sign of respiratory distress and a significant finding in an infant. The enlargement of the nostrils helps reduce nasal resistance and maintains airway patency. Nasal flaring may be intermittent or continuous and should be described as minimum or marked.
Head bobbing in a sleeping or exhausted infant is a sign of dyspnea. The head, supported on the caregiver’s arm only at the suboccipital area, bobs forward with each inspiration. This is caused by neck flexion resulting from contraction of the scalene and sternocleidomastoid muscles. Noisy breathing such as “snoring” is frequently associated with hypertrophied adenoidal tissue, choanal obstruction, polyps, or an FB in the nasal passages.
Stridor, which is a high-pitched, noisy respiration, is usually an indication of narrowing of the upper airway, either as a result of edema and inflammation, or in association with an upper airway obstruction, often from mucus secretions or possibly from a foreign object. Stridor may be inspiratory or expiratory. Common causes in children include croup, epiglottitis, FB, or tracheitis (Boat and Green, 2007).
Grunting is frequently a sign of pain in older children, suggesting acute pneumonia or pleural involvement. It is also observed in pulmonary edema and is a characteristic of respiratory distress in newborns and infants. It is the body’s attempt at more efficient respirations. Grunting serves to increase end-respiratory pressure and thus prolong the period of oxygen and carbon dioxide exchange across the alveolocapillary membrane.
Wheezing is a continuous musical sound originating from vibrations in narrowed airways (Watts and Goodman, 2007). Wheezing is primarily heard on expiration and may be either polyphonic (with widespread narrowing of the airways [e.g., asthma]), or monophonic (single-pitched sound produced in the larger airways [e.g., tracheomalacia]). Infants may have wheezing as a result of increased airway resistance and a compliant chest wall; there is evidence that inflammatory mediators such as histamines, leukotrienes, and interleukins may also contribute to wheezing in infants (Watts and Goodman, 2007). Older children often have wheezing with a lower respiratory tract infection as a result of inflammation, bronchospasm, and accumulated secretions, all of which serve to narrow the airways and produce the characteristic wheezing sound.
Color changes of the skin, especially mottling, pallor, and cyanosis, are important. Except for the peripheral bluish discoloration (acrocyanosis) resulting from circulatory stasis in the newborn or the mottling or peripheral cyanosis resulting from a cool environment, mottling and cyanosis are significant and usually indicate cardiopulmonary disease.
Chest pain may be a complaint of older children and may have a variety of causes, both pulmonary and nonpulmonary. It may be caused by disease of any of the chest structures—esophagus, pericardium, diaphragm, pleura, or chest wall. Parietal pleural pain is usually localized over the affected area and is aggravated by respiratory movements. The pain of diaphragmatic pleural irritation may be referred to the base of the neck posteriorly and anteriorly or to the abdomen. Most pleural pain is related to respiration; therefore respiratory movements are shallow and rapid and may be accompanied by grunting, especially in the younger patient.
Clubbing, or proliferation of tissue about the terminal phalanges, accompanies a variety of conditions, frequently those associated with chronic hypoxia, primarily cardiac defects, and chronic pulmonary disease (e.g., cystic fibrosis). Although clubbing often worsens with lung disease, it does not accurately reflect disease progression. The degree of clubbing depends on the extent to which the nail base is lifted on the dorsal surface of the phalanx by the tissue proliferation. The greater the angle formed above the finger or toe at the skin-nail junction, the more pronounced the clubbing, especially when there is a decided curvature to the nail (Fig. 31-7).
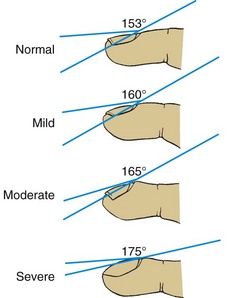
Fig. 31-7 Stages of clubbing. Degree of angle formed above finger at skin-nail junction indicates extent of clubbing. Angle greater than 160 degrees and decided curvature of nail are good criteria for presence of clubbing. (Modified from Waring WW: The history and physical examination. In Chernick V, editor: Kendig’s disorders of the respiratory tract in children, ed 6, Philadelphia, 1998, Saunders.)
Cough is often associated with respiratory disease, although it may suggest other disorders (Box 31-3). It serves as a protective mechanism and an indicator of irritation. A cough can be initiated voluntarily but is usually a result of a complex reflex consisting of three components: afferent nerve fibers, the cough center, and efferent nerve fibers. The respiratory epithelium contains afferent receptors that are sensitive to mechanical or chemical stimuli. These receptors are concentrated in the areas of the larynx, the carina, and the bifurcations of the large and medium-sized bronchi. When a stimulus is applied to these areas, impulses are transmitted via the vagus nerve to the cough center in the brainstem. Efferent impulses travel via the vagus, phrenic, and spinal motor nerves to the larynx, intercostal muscles, diaphragm, abdominal muscles, and pelvic floor. An inspiratory gasp and closure of the glottis are followed by contraction of muscles in the chest wall, diaphragm, abdomen, and pelvic floor. The resulting compression and increase in pleural, alveolar, and subglottic pressure cause a sudden opening of the glottis and immediate release of trapped air at extremely rapid expiratory flow rates, which forces undesirable material from the respiratory tract.
Inflammation or infection in the upper or lower respiratory tract may produce coughing. Some types of cough are characteristic of specific diseases. For example, a severe cough is associated with measles and cystic fibrosis, and the paroxysmal cough accompanied by an inspiratory “whoop” is typical of pertussis in small children. A brassy, nonproductive cough is part of the symptomatology of croup and FB aspiration. Because there are no cough receptors in the alveoli, a cough may be absent in a child with pneumonia in the early stages of the disease but is a common feature during active pneumonia and recovery. The nurse assesses a cough according to the features listed in Box 31-4.
Diagnostic Procedures
Several procedures are available for assessing respiratory function and diagnosing respiratory disease. All these procedures require preparation and support of the child and the family to ensure cooperation and accurate results (see Family-Centered Care box). These procedures not only are useful in diagnosis, but also provide information that guides nursing interventions, such as positioning, use of supplemental oxygen, and monitoring oxygenation and respiratory status.
Pulmonary Function Tests
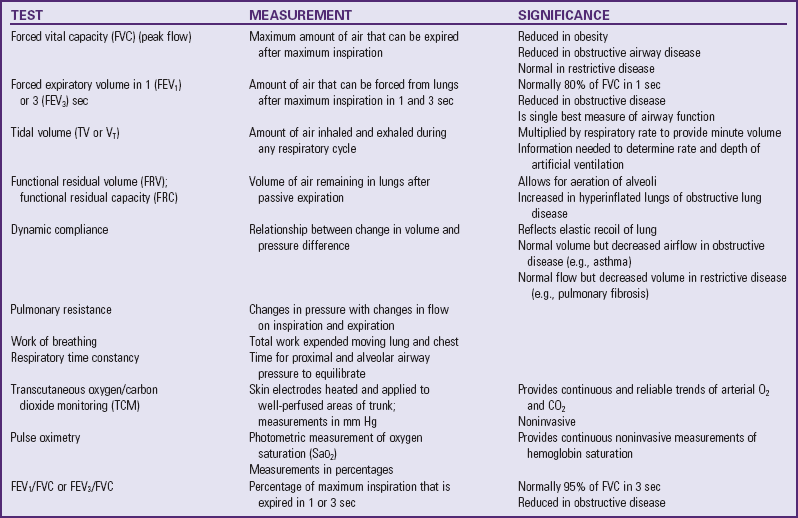
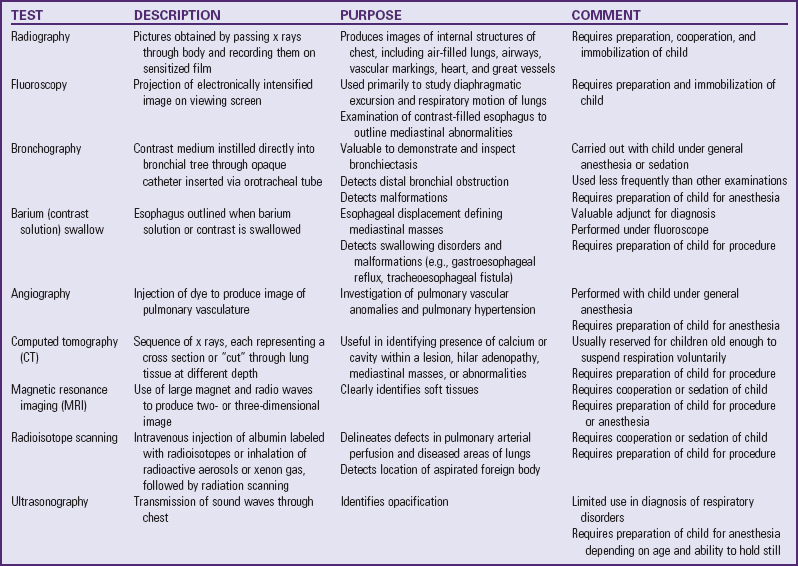
Noninvasive pulmonary mechanics are often measured at the bedside of infants and children with the use of pneumotachography or spirometry. However, information obtained limits diagnosis because the same functional abnormality may occur in different diseases. These tests are useful to evaluate the severity and course of a disease and to study the effects of treatment (Table 31-1 and Fig. 31-8).
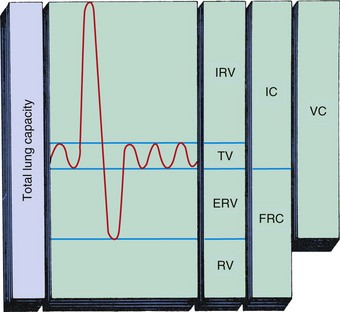
Fig. 31-8 Divisions of total lung capacity. Total lung capacity (TLC) is maximum amount of air contained in lungs. TLC is divided into four primary volumes: IRV, inspiratory reserve volume; TV, tidal volume; ERV, expiratory reserve volume; and RV, residual volume. Capacities are combinations of two or more lung volumes. These include inspiratory capacity (IC), functional residual capacity (FRC), and vital capacity (VC). (From Shapiro BA, Harrison RA, Walton R: Clinical application of blood gases, ed 3, St Louis, 1982, Mosby.)
Radiology and Other Diagnostic Procedures
Radiography is used frequently in diagnostic evaluation of children (Table 31-2). Although no definitive information exists on the effects of low-dose radiation, providers take action to protect vulnerable areas from possible damage. When possible, technicians and others try to prevent unnecessary exposure of the child (and nursing personnel), and they protect the more radiosensitive areas. Careful protection of the patient’s immature gonads with lead shields is essential. Other sensitive areas are the thyroid gland, ocular lens, and bone marrow.
Although nurses have limited control over the length, frequency, and correct application of the x-ray beam, they can make certain that the infant or child receives proper protection from possible hazards. Lead shields, correctly placed and consistently applied to areas not needed for diagnostic purposes, are essential. Play and modification of methodology effectively reduce the trauma sometimes associated with the procedure and gain the child’s cooperation. Nurses, regardless of age and pregnancy status, should use protective equipment to guard against unnecessary radiation exposure during diagnostic examinations.
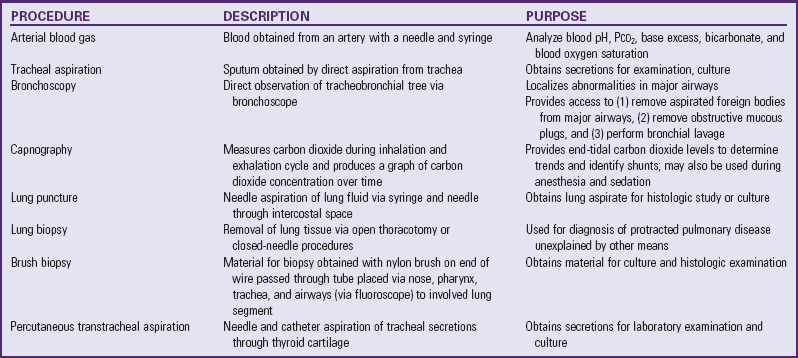
Several other procedures are used to diagnose lung disorders (Table 31-3). Most require specialized equipment and skills. All require preparation of the child.
Blood Gas Determination
Blood gas measurements are sensitive indicators of change in respiratory status in acutely ill patients (Table 31-4). They provide valuable information regarding lung function, lung adequacy, and tissue perfusion and are essential for monitoring conditions involving hypoxemia, carbon dioxide retention, and pH. For the acutely ill patient, this information also guides decisions regarding therapeutic interventions, such as adjusting mechanical ventilator settings, modifying chest physiotherapy (CPT), administering oxygen, or positioning the child for maximum ventilation. Both invasive and noninvasive methods are available (see Atraumatic Care box).
TABLE 31-4
note: The Sao2 printed with blood gas reports cannot be used as a standard to confirm oximetry readings. Blood gas analyzers provide only approximate blood oxygen saturations based on calculations using measured blood gases, pH, and Pao2.
*Values for child are for those ages 7-19 yr.
Modified from Custer JW, Rau RE, editors, Johns Hopkins Hospital Department of Pediatrics: The Harriet Lane handbook, ed 18, St Louis, 2009, Mosby.
Pulse oximetry provides a continuous or intermittent noninvasive method of determining oxygen saturation (Sao2). A sensor composed of a light-emitting diode (LED) and a photodetector is placed in opposition around a foot, hand, finger, toe, or earlobe, with the LED placed on top of the nail when digits are used (Fig. 31-9). The diode emits red and infrared lights that pass through the skin to the photodetector. The photodetector measures the amount of each type of light absorbed by functional hemoglobins (those capable of carrying oxygen). Hemoglobin saturated with oxygen (oxyhemoglobin) absorbs more infrared light than does hemoglobin not saturated with oxygen (deoxyhemoglobin). A microprocessor determines the difference between absorption of the red and infrared light. The percentage of the total normal hemoglobin that is oxygenated is displayed on a monitor.
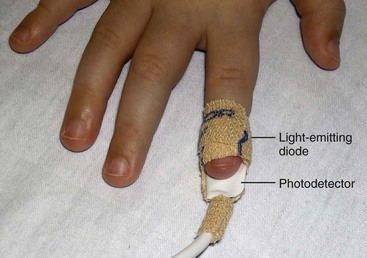
Fig. 31-9 Oximeter sensor on right second finger. Note that sensor is positioned with light-emitting diode opposite photodetector.
Pulsatile blood flow is the primary physiologic factor that influences accuracy of the pulse oximeter. In most infants with continuous pulse oximetry monitoring, the nurse must change the electrode site at least every 3 to 4 hours to prevent pressure necrosis (in infants with poor perfusion or disrupted skin integrity). In infants with poor perfusion and temperature problems such as hypothermia or sensitive skin, the probe may need more frequent changing. In an active or crying infant motion artifact may make the reading more difficult to obtain.
Another noninvasive method is transcutaneous monitoring (TCM), which provides continuous monitoring of transcutaneous partial pressure of oxygen in arterial blood (tcPao2) and, with some devices, of carbon dioxide in arterial blood (tcPaco2). An electrode is attached to the warmed skin to facilitate arterialization of cutaneous capillaries. The site of the electrode must be changed every 3 to 4 hours (or more frequently according to skin status) to avoid burning the skin, and the machine must be calibrated with every site change. This monitoring is used frequently in neonatal intensive care units, but it may not reflect an accurate Pao2 in infants with impaired local circulation.
The Pao2 can be correlated with the Sao2 by means of the oxyhemoglobin dissociation curve (Fig. 31-10), although changes in Pao2 do not cause identical (linear) changes in Sao2. The curve represents the relationship between Pao2 (measured in the blood) and Sao2 (measured by the pulse oximeter). As seen on the graph, when the Pao2 is 60 mm Hg, the Sao2 is 90%. Increasing the Pao2 above this point does not significantly increase Sao2 or greatly improve oxygen delivery to the tissues. At this point, further increases in the Pao2 will only increase the dissolved oxygen in the blood and will not, under normal conditions, contribute significantly to the arterial oxygen content. On the lower part of the curve, however, even small changes in the Pao2 produce large changes in saturation. This is an advantage at the tissue level, especially in low oxygen states (hypoxia) because a small decrease in Pao2 will cause a relatively large unloading of oxygen to the tissues.
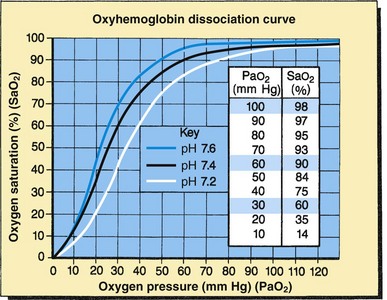
Fig. 31-10 Oxyhemoglobin dissociation curve. Changes in affinity of hemoglobin for oxygen shift the position of oxyhemoglobin dissociation curve. Standard curve (black): Assumes normal pH (7.4), temperature, and Pco2 levels. Shift to left (blue): Increased oxygen affinity of hemoglobin: increased pH, decreased temperature and Pco2. Shift to right (white): Decreased oxygen affinity of hemoglobin: decreased pH, increased temperature and Pco2.
Oximetry is insensitive to hyperoxia because hemoglobin approaches 100% saturation for all Pao2 readings above approximately 100 mm Hg, which is a potentially dangerous situation for the preterm infant at risk for developing oxidative stress. Oxidative stress may lead to complications such as bronchopulmonary dysplasia and retinopathy of prematurity. (See Chapter 10.) Therefore the preterm infant being monitored with oximetry should have a range of upper limits identified, such as 89% to 93%, and a protocol should be established for decreasing oxygen when saturations are high.
Several factors affect the degree to which oxygen combines with hemoglobin. A shift of the curve to the left causes an increased affinity of hemoglobin for oxygen, but the oxygen is not easily released to the tissues. This represents an increase in the Sao2 if it is measured against the same Pao2 of the normal oxyhemoglobin dissociation curve. This left shift can be caused by an increase in blood pH or a decrease in Paco2 or body temperature.
A shift of the curve to the right causes a decreased affinity of hemoglobin for oxygen but improved oxygen release to the tissues. This represents a lower Sao2 if measured against the same Pao2 of the normal oxyhemoglobin dissociation curve. This rightward shift can be caused by a decrease in blood pH or an increase in Paco2 or body temperature.
Oximetry offers several advantages over TCM. Oximetry (1) does not require heating the skin, thus reducing the risk of burns; (2) eliminates a delay period for transducer equilibration; and (3) maintains an accurate measurement regardless of the patient’s age or skin characteristics or the presence of lung disease.
Applying the sensor correctly is essential for accurate Sao2 measurements. Because the sensor must identify every pulse beat to calculate the Sao2, movement can interfere with sensing. Some devices synchronize the oxygen saturation reading with the heartbeat, thereby reducing the interference caused by motion. Sensors are not placed on extremities used for blood pressure monitoring or with indwelling arterial catheters, since pulsatile blood flow can be affected. It is recommended that the probe site be changed according to manufacturer guidelines.
It is important to make certain that sensory connectors and oximeters are compatible. Wiring that is incompatible can generate considerable heat at the tip of the sensor, causing second- and third-degree burns under the sensors. Pressure necrosis can also occur from sensors attached too tightly. Therefore inspect the skin under the sensor frequently.
Ambient light from ceiling lights and phototherapy or high-intensity heat and light from radiant warmers can interfere with readings. Therefore cover the sensor to block these light sources. Intravenous dyes; green, purple, or black nail polish; nonopaque synthetic nails; and possibly ink used for footprinting can also cause inaccurate Sao2 measurements. The nurse should remove the dyes or, in the case of porcelain nails, use a different area used for the sensor. Skin color, thickness, and edema do not affect the readings. Elevated levels of carboxyhemoglobin, methemoglobin, and fetal hemoglobin affect the accuracy of the device because it can only distinguish between oxyhemoglobin and deoxyhemoglobin; therefore the child with carbon monoxide poisoning may have a normal Sao2 reading but an abnormal (low) Pao2.
Arterial blood gas (ABG) sampling helps to evaluate gas exchange and oxygenation and may be performed on blood from an artery or a capillary. Historically, some controversy surrounds the collection of “arterialized” capillary blood for blood gas measurements. However, many believe it to be a safe, convenient, and relatively accurate method, and that capillary blood gas (CBG) can accurately reflect the arterial pH and Pco2 in most pediatric disease states (Yildizdas, Yapicioglu, Yilmaz, et al, 2004; Bilan, Behbahan, and Khosroshahi, 2008). The blood samples are obtained by a heel stick after dilation of the vascular bed by warming. The first drop of blood is discarded, and subsequent blood is collected directly into heparinized capillary tubes held in a horizontal position.
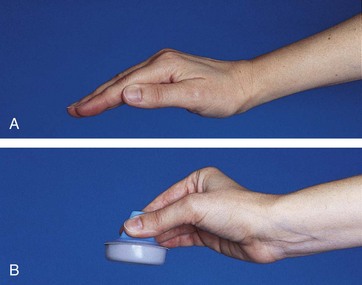
ABG samples may also be obtained through an indwelling arterial catheter or by arterial puncture. The sites most commonly used for arterial puncture include the radial, dorsalis pedis, posterior tibial, and femoral arteries. A catheter may also be placed in the neonate’s umbilical artery for ABG sampling. The femoral artery is the last choice because hemorrhage and hematomas are difficult to control in this area and the risk for limb ischemia is high if the femoral artery is damaged (Curley and Moloney-Harmon, 2001). Risks of arterial puncture include pain, artery damage, decreased perfusion to the extremity distal to the puncture site, thrombosis, and hemorrhage (see Atraumatic Care box). Before a radial artery puncture, perform the Allen test to assess adequacy of the collateral circulation. To perform the test, elevate the extremity distal to the puncture site and blanch it by squeezing gently (such as making a fist). The two arteries supplying blood flow to the extremity (such as the radial and ulnar arteries in the wrist) are then occluded. Lower the extremity, and remove pressure from one artery (such as the ulnar). If color returns to the blanched extremity in less than 5 seconds, this indicates collateral circulation.
An accurate ABG or CBG requires unclotted whole or capillary blood; therefore use a heparinized syringe or capillary tube to draw blood samples. Do not allow air bubbles to enter the sample, since air alters the blood gas concentration. Many institutions use prepackaged ABG sampling kits, which allow air-free samples to be drawn without the need for heparin dilutions. The amount collected depends on the child’s size. Depending on the laboratory facilities, as little as 0.1 ml may be sufficient in small infants. After obtaining the blood sample, pack it in ice to reduce blood cell metabolism and have it taken to the laboratory immediately for analysis. Table 31-4 lists normal ABG and pH measurements on room air at sea level for adults and children 7 to 19 years of age.
Although ABG values are similar for children and adults, newborns can have slightly lower values and still be considered normal. For example, normal pH values for a newborn range from 7.26 to 7.29, the average Pao2 is 70 mm Hg, the average Paco2 is 33 mm Hg, and the average bicarbonate is 20 mEq/L.
ABG values also depend on the concentration of oxygen the child is breathing. The arterial Po2 should rise in proportion to the oxygen concentration being inhaled. Therefore, when evaluating ABG values, consider the following: the percentage of oxygen administered (if any), the child’s body temperature (as little as 1° F can alter the blood gas values 5% to 8%), and the presence of anxiety (if children hyperventilate, carbon dioxide is exhaled) or crying (can cause breath holding, resulting in decreased Pao2).
The significance of ABG determination is related primarily to the relationships among the following three parameters: pH, Po2, and Pco2. (See Acid-Base Imbalance, Chapter 28.) Any change in a blood gas value must be compared with the other values and with previous readings, as well as with the child’s clinical appearance and behavior, medical history, and associated physiologic factors.
Clinical indications for blood gas analysis include changes in pulse oximetry, color (e.g., mottling, pallor, cyanosis, or duskiness), depth or rate of respirations (e.g., shallow and rapid), behavior or sensorium, and vital signs. Blood gas analysis is also used to determine adequacy of treatment and optimal ventilator settings in infants and children on supplemental oxygen and noninvasive and invasive mechanical ventilation. The nurse may or may not obtain the blood sample by arterial puncture, depending on the institution’s policies. In any event, nurses must understand the results of blood gas analyses because these results provide essential information to guide nursing interventions (e.g., changing the position, performing suction, administering prescribed drugs, or notifying the practitioner).
One approach to determine a simple acid-base disturbance:
• Evaluate the pH to determine whether acidosis or alkalosis is present.
• Evaluate the Pco2 to determine whether the imbalance is respiratory.
• Evaluate the bicarbonate levels to determine whether the imbalance is metabolic.
In a patient with a mixed acid-base disorder, compensatory factors (renal, pulmonary, or both) have been set in motion to equilibrate the blood pH. (See Acid-Base Imbalance, Chapter 28.)
Respiratory Therapy
The indication for administration of oxygen is hypoxemia (reduced blood oxygenation). Oxygen is delivered by mask, nasal cannula, tent, hood, face tent, or ventilator (Table 31-5). The mode of delivery is selected on the basis of the concentration needed and the child’s ability to cooperate in its use. The concentration of oxygen delivered should be regulated according to the individual child’s needs. There are hazards related to its use; therefore continue oxygen only as long as needed and at the prescribed amount. Because medical-grade oxygen from piped systems or tanks is anhydrous, humidification of the gas before administration to the patient is essential.
TABLE 31-5
ADVANTAGES AND DISADVANTAGES OF VARIOUS OXYGEN-DELIVERY SYSTEMS
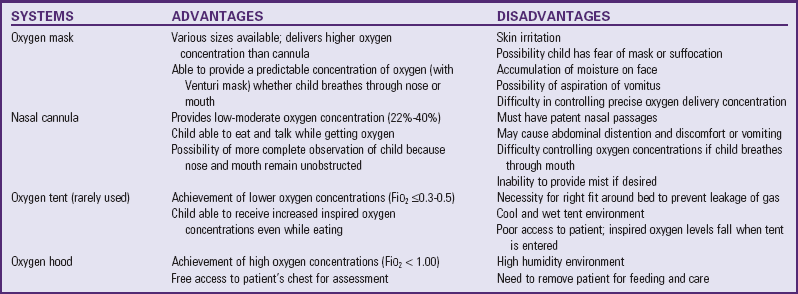
Modified from Hazinski MF, editor: Nursing care of the critically ill child, ed 2, St Louis, 1992, Mosby.
Oxygen therapy is frequently administered in the hospital, although increasing numbers of children are receiving oxygen in the home. It is the responsibility of the nurse or respiratory care practitioner to ensure uninterrupted delivery of the appropriate oxygen concentration and to monitor the child’s response to the therapy.
Oxygen Administration
Oxygen delivered to infants is well tolerated by using a plastic hood (Fig. 31-11). Low and high concentrations of oxygen can be easily maintained in this head hood, and most nursing procedures can continue without interrupting the oxygen delivery. At least 4 to 5 L/min of flow is necessary to maintain oxygen concentrations and remove the exhaled carbon dioxide.
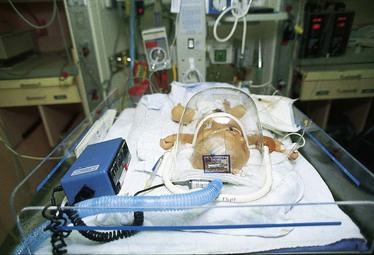
Fig. 31-11 Oxygen administered to infant by means of plastic hood. Note oxygen analyzer (blue machine).
The humidified oxygen should not be blown directly into the face of an infant in a hood. Cold fluid or air applied to the face stimulates receptors that trigger the diving reflex, which causes bradycardia and shunting of blood from peripheral to central circulation. The oxygen hood should not rub against the infant’s neck, chin, or shoulder.
Older infants and children can use a nasal cannula or prongs. Nasal cannula may also be used for lower concentrations of oxygen for infants and children who do not require high oxygen concentrations; the cannula has two soft prongs, which are inserted into the nares, providing flow into the nasopharynx. Skin care of the nasal alae is important to prevent breakdown.
Oxygen masks are available in pediatric sizes. The simple oxygen mask fits over the patient’s nose and oxygen is delivered as the child breathes. The simple oxygen mask is used for short-term oxygen therapy, and it should be used at oxygen flow rates greater than 5 to 6 L/min to minimize carbon dioxide rebreathing. The simple mask has side holes to permit room air to enter into the mask to be mixed with oxygen. Signs of carbon dioxide rebreathing include somnolence, dizziness, headache, tingling, and eventually unconsciousness. A partial rebreathing mask is similar to the simple mask in that the plastic reservoir fits over the child’s face; the partial rebreather has a reservoir at the base of the mask that receives expired air and fresh gas so lower oxygen flow rates (<4 L/min) can be used. The nonrebreathing mask is similar to the partial rebreathing mask, but the former has a one-way valve that limits rebreathing carbon dioxide. Another valve on the nonrebreathing mask’s reservoir ensures that room air is not mixed with oxygen, thus providing higher oxygen concentrations (provided the mask fits correctly).
At times the child or infant may become quite agitated with the use of a mask, and a blow-by method may be used to provide low oxygen concentrations (<30%), humidification, or aerosolized medication. The blow by can be made with any oxygen tubing, corrugated tubing, or a mask held approximately 2 to 3 inches from the child’s nose and mouth. It is recognized that this method is not the optimal choice of oxygen delivery.
Face masks may not be well tolerated by children, since the fit must be snug to the face to ensure adequate oxygen delivery. A face tent or bucket is often better tolerated, since this soft piece of plastic sits beneath the child’s chin and allows for the direction of oxygen up to the area of the mouth and nose without having the mouth and nose enclosed by plastic (Curley and Moloney-Harmon, 2001).
Historically the oxygen tent (often referred to as a croup tent) was used as a satisfactory means for oxygen administration in older infants and some small children; however, these are rarely used in developed countries. A tent does not require any device to come into direct contact with the face, but the concentration of oxygen within the tent is difficult to control and to maintain above 30% to 50%. A major difficulty with the use of the tent is keeping the tent closed so that oxygen concentration is maintained; the humidification also presents a problem as the linens and child’s clothing becomes saturated quite easily and must be changed often. Closely monitor the child’s temperature in an oxygen tent.
Oxygen Toxicity
Oxygen is essential to life and a valuable therapeutic aid. Prolonged exposure to high oxygen tensions, however, can damage lung tissue. Although the exact pathogenesis of the pulmonary changes is unclear, evidence indicates damage to lung capillaries, which causes diffuse microhemorrhagic changes, diminished mucus flow, inactivation of surfactant, and altered ciliary function. The result of these changes is a gradual impairment of alveolar ventilation.
Atelectasis may occur as a result of the “washing out” of nitrogen from the alveoli by the high concentrations of oxygen. This is more likely to occur in persons with low tidal volume and retention of mucus or other secretions.
Oxygen-induced carbon dioxide narcosis is a physiologic hazard of oxygen therapy that may occur in persons with chronic pulmonary disease. It is rare in children, except those with cystic fibrosis. These children have chronic alveolar hypoventilation with a concomitant chronic carbon dioxide retention and hypoxemia. The respiratory center has adapted to the continuously higher Paco2 levels, and therefore hypoxia becomes the more powerful stimulus to respiration. When the Pao2 is elevated during oxygen administration, the hypoxic drive is removed, causing progressive hypoventilation and increased Paco2 levels, and the child rapidly becomes unconscious. Carbon dioxide narcosis can also be induced by the administration of sedation in these patients.
Other suspected toxic effects of oxygen include changes in the renal tubules, sympathoadrenal medullary stimulation precipitating neurogenic seizures, and an increased rate of destruction of red blood cells. In extremely preterm infants the risk of retinopathy of prematurity is a major concern in oxygen administration, although the exact correlation between the two is unclear. (See Chapter 10.)
Aerosol Therapy
Aerosol therapy can be effective in depositing medication directly into the airway. However, the value of aerosolized water, or “mist therapy,” is controversial. Continuous administration of mist, or aerosolized water, often viewed as a traditional and helpful remedy, is not a treatment of choice for most inflammatory conditions of the airways. The exception is the child with mild viral croup, who might benefit from cool-mist therapy, including a walk outside in the cool, humid night air. The effectiveness of this practice, however, has also been questioned. Mist therapy may not help the child with reactive airway disease and croup because humidity may worsen the bronchospasm.
This route of administration can be useful in avoiding the systemic side effects of certain drugs and in reducing the amount of drug necessary to achieve the desired effect. Bronchodilators, steroids, and antibiotics, suspended in particulate form, can be inhaled so that the medication reaches the small airways. Aerosol therapy is particularly challenging in children who are too young to cooperate with controlling the rate and depth of breathing. Administration of this therapy requires skill, patience, and creativity.
Medications can be aerosolized or nebulized with air or with oxygen-enriched gas. Hand-held nebulizers are common. The medicated mist is discharged into a small plastic mask, which the child holds over the nose; for older children the mouthpiece may be used instead of the mask. (See Fig. 32-12.) To avoid particle deposition in the nose and pharynx, instruct the child to take slow, deep breaths through an open mouth during treatment. For home or school, use an air compressor–driven nebulizer to force air through the liquid medication to form the aerosol. Compact, portable units can be obtained or rented from health equipment companies.
The metered dose inhaler (MDI) is a self-contained, hand-held device that allows for intermittent delivery of a specified amount of medication. (See Fig. 32-11.) Many bronchodilators are available in this form and are used successfully by children with asthma or cystic fibrosis. (See Chapter 32.) For children less than 5 or 6 years of age or children who have difficulty learning to use an MDI, a spacer device or holding chamber attached to the MDI can help coordinate breathing and aerosol delivery. The spacer allows the aerosolized particles to remain in suspension for a longer time. The MDI should be attached to a spacer when an inhaled corticosteroid is administered to prevent yeast infections in the mouth if the child is too young to rinse the mouth after the treatment. Dry powder inhalers such as the Rotahaler and Turbuhaler are also commonly used for inhaled medications.
A major nursing responsibility during aerosol therapy is to assess the effectiveness of the treatment, the patient’s tolerance of the procedure, and the patient’s ability to perform the procedure and use equipment correctly. Assess breath sounds, work of breathing, and pulse oximetry readings before and after treatments. Young children who become upset with a mask held close to the face may become fatigued from fighting the procedure and may appear worse during and immediately after the therapy. It may be necessary to spend a few minutes calming the child after the therapy and allowing vital signs to return to baseline levels to accurately assess changes in breath sounds and work of breathing. Alternatively, if the child’s condition permits, the end of a 1-inch wide tubing (using a 6-inch pigtail) may be used to administer aerosol therapy (similar to blow-by oxygen administration).
Bronchial (Postural) Drainage
Bronchial drainage is indicated whenever excessive fluid or mucus in the bronchi is not removed by normal ciliary activity and cough. Positioning the child to take maximum advantage of gravity facilitates removal of secretions. Postural drainage can be effective in children with chronic pulmonary illness characterized by thick mucus secretions, such as cystic fibrosis. Postural drainage may be used in combination with percussion, which serves to facilitate the loosening of secretions in the lower airways.
Postural drainage is carried out three or four times daily (or as necessary) and is more effective when it follows other respiratory therapy, such as bronchodilator or nebulized medication. Bronchial drainage is generally performed before meals (or 1 to  hours after meals) to minimize the chance of vomiting and is repeated at bedtime. The duration of treatment depends on the child’s condition and tolerance—usually 20 to 30 minutes. Several positions facilitate drainage from all major lung segments (Fig. 31-12); all positions are not used at each session. Children usually cooperate for four to six positions, but more than six tends to exceed their limits of tolerance. Older children can tolerate longer periods.
hours after meals) to minimize the chance of vomiting and is repeated at bedtime. The duration of treatment depends on the child’s condition and tolerance—usually 20 to 30 minutes. Several positions facilitate drainage from all major lung segments (Fig. 31-12); all positions are not used at each session. Children usually cooperate for four to six positions, but more than six tends to exceed their limits of tolerance. Older children can tolerate longer periods.
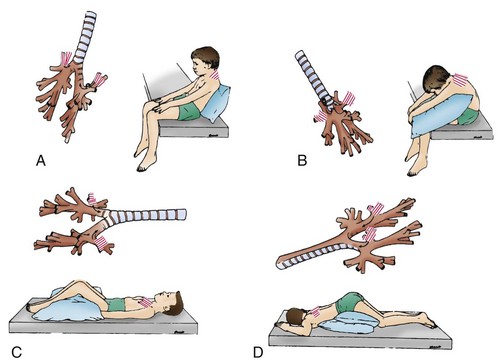
Fig. 31-12 Bronchial drainage positions for all major lung segments of child. For each position, model of tracheobronchial tree is projected beside child to show segmental bronchus (striped) being drained and pathway of secretions out of bronchus. Drainage platform is horizontal. Striped area on child’s chest or back indicates area to be cupped or vibrated by therapist. A, Apical segment of right upper lobe and apical subsegment of apical-posterior segment of left upper lobe. B, Posterior segment of right upper lobe and posterior subsegment of apical-posterior segment of left upper lobe. C, Anterior segments of both upper lobes. Child should be rotated slightly away from side being drained. D, Superior segments of both lower lobes. (Modified from Chernick V, editor: Kendig’s disorders of the respiratory tract of children, ed 6, Philadelphia, 1998, Saunders.)
In the hospital an older child can be positioned over an elevated knee rest. Small children and infants can be positioned with pillows or on the parent’s or therapist’s lap and legs (Fig. 31-13). Infants should not be placed in the Trendelenburg (head-down) position, since they do not have an autonomic regulation of blood flow to the head. Special modifications of the techniques are required in children whose conditions contraindicate head-down positioning, such as those with head injuries, some types of surgical incisions or burns, and casts or traction. At home small children can be positioned on a padded slant board, parent’s lap, bed, or couch. Children who require postural drainage over a period of months or years may benefit from specially constructed tables padded and adjusted to their individual needs. Individualize the position used and the frequency and duration of treatment.
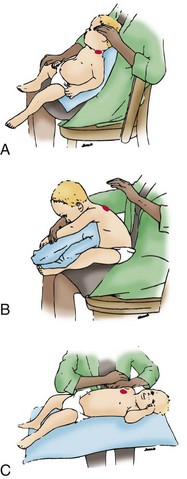
Fig. 31-13 Bronchial drainage positions for all major lung segments of infant. Procedure is most easily carried out in therapist’s lap. Therapist’s hand indicates area (solid red) to be cupped or vibrated. A, Apical segment of left upper lobe. B, Anterior segment of left upper lobe. C, Posterior basal segment of right lower lobe. (Modified from Cystic Fibrosis Foundation: Infant segmental bronchial drainage, Rockville, Md, n.d., The Foundation.)
Chest Physiotherapy
CPT usually refers to the use of postural drainage in combination with adjunctive techniques that are thought to enhance the clearance of mucus from the airway. These are often referred to as airway clearance techniques. These techniques include manual or mechanical percussion, vibration, and squeezing of the chest; cough; forceful expiration (exhalation) or huffing; and breathing exercises. Special mechanical devices (e.g., vest-type percussors) are also currently used to perform CPT in children with chronic pulmonary illness such as cystic fibrosis (see p. 1280). Additional methods include the flutter device (see p. 1284), Acapella, and intrapulmonary percussive ventilation (Marks, 2008). Postural drainage in combination with forced expiration has been beneficial.
Common techniques used in association with postural drainage include manual percussion of the chest wall and percussion with mechanical devices such as a high-frequency hand-held chest compression device. Nurses may be responsible for this procedure, and they should become skilled in the technique. The patient is dressed in a light shirt and placed in a postural drainage position. The practitioner then gently but firmly strikes the chest wall with a cupped hand (Fig. 31-14, A). For infants and small children, special devices are available for percussing small areas (Fig. 31-14, B). A “popping,” hollow sound, not a slapping sound, should be the result. The procedure should be done only over the rib cage and should be painless. Percussion can be performed with a soft, circular mask (adapted to maintain air trapping) or a percussion cup marketed to aid in loosening secretions (see Fig. 31-14, B).
Vibration can be used to help move secretions cephalad during exhalations. Larger children may benefit from a more powerful vibrator such as a high-frequency chest compression device. This therapy is subject to patient tolerance, and pulse oximetry is an excellent monitoring tool for therapy tolerance.
CPT is contraindicated when patients have pulmonary hemorrhage, pulmonary embolism, end-stage renal disease, increased intracranial pressure, osteogenesis imperfecta, or minimum cardiac reserves. Avoid the head-down positions in children with gastroesophageal reflux (Marks, 2008). McIlwaine (2007) notes that the head-down position is detrimental and should not be used; Naylor, McLean, Chow, and colleagues (2006) also recommend that the head-down position be used infrequently because of an increase in cardiovascular adverse effects. Guidelines for performing CPT are given in the Nursing Care Guidelines box.
Squeezing is sometimes useful while the child is in the drainage position. Direct the child to take a deep breath and then exhale through the mouth rapidly and as completely as possible. The depth of the expiratory effort is increased by brief, firm pressure from the practitioner’s hands compressing the sides of the chest. This decreases the volume of the tracheobronchial tree and facilitates the expression of secretions. Inspiration after the activity often stimulates a deep, productive cough. Another technique to force exhalation is to use abdominal thrusts in conjunction with a MAC device (see discussion below).
Encourage deep breathing when the child is relaxed and in the desired position for drainage. Direct the child to take several deep breaths using diaphragmatic breathing. The use of deep breathing enlarges the tracheobronchial tree, enabling air to circulate around and through secretions that are not affected by usual tidal volumes. Exhalations after these deep breaths often carry secretions and may stimulate a cough. Other methods that can be employed to stimulate deep breathing are the use of incentive spirometers and incorporation of play that extends the expiratory time and increases expiratory pressure. For example, play may include blowing pinwheel toys, moving small items by blowing through a straw, blowing cotton balls or a Ping-Pong ball on a table, preventing a tissue from falling by blowing it against a wall, blowing up balloons (under supervision), singing loudly (especially songs with a lot of words between breaths), or blowing soap bubbles.
With or without stimulation, encourage children to cough, not to suppress a cough, and not to waste strength and energy with repeated weak and ineffective coughs. One or two hard coughs after a deep breath are more efficient. Because many children have difficulty coughing when in a dependent position, have them sit up while they cough. Having the child hug a stuffed toy or a small pillow offers comfort, as well as physical support, during coughing. As an alternative, reinforce the child’s efforts by encircling the chest with your hands and compressing the sides of the lower chest in synchrony with the cough. This is less fatiguing and increases the effectiveness of the cough efforts.
Mechanical-assisted cough (MAC) devices are available for children with acute or chronic pulmonary disease and neuromuscular disease to assist in clearing the airway when the cough reflex is ineffective or diminished. These devices may be used with CPT to enhance the removal of mucus from the airways. The mechanical cough insufflator-exsufflator (MIE) has been evaluated and found to be safe and effective in the daily management of respiratory function (Fauroux, Guillemot, Aubertin, et al, 2008; Homnick, 2007). The MIE delivers positive inspiratory pressures at a set rate, followed by negative pressure exsufflation coordinated with the patient’s own breathing rhythm. The exsufflation is designed to mimic a cough reflex so mucus can be effectively cleared. Airway suctioning after exsufflation is accomplished as necessary to clear the airways. In children the MIE may be connected directly to a tracheostomy or used with a mouthpiece or face mask.
Breathing and postural exercises are useful techniques with motivated children and children with kyphoscoliosis, cystic fibrosis, asthma, and bronchiectasis. Breathing exercises are part of a total therapy program and are more convenient when performed in association with bronchial drainage.
The goals of breathing exercises are to (1) develop more effective diaphragmatic and lower intercostal breathing; (2) relax all muscles, especially those of the upper chest, shoulder girdle, and neck; and (3) attain correct posture. The number and type of exercises depend on the child’s age, motivation, and strength and the type and extent of the physiologic disturbance. Select breathing exercises to meet the specific child’s needs. The most important exercises are diaphragmatic breathing and side bending, with emphasis on abdominal expansion and lateral expansion.
Mechanical Ventilation
![]() If a child’s respiratory status is deteriorating and the respiratory effort is excessive or inadequate, mechanically assisted ventilation may become necessary (Box 31-5).
If a child’s respiratory status is deteriorating and the respiratory effort is excessive or inadequate, mechanically assisted ventilation may become necessary (Box 31-5).
A variety of methods are available for controlling or assisting ventilation. Temporary assistance can be provided by a hand-operated self-inflating ventilation bag with a mask and a nonreturnable valve to prevent rebreathing, commonly referred to as a bag and valve mask (BVM). With the mask placed on the nose and mouth, the bag is rhythmically compressed, forcing gas from the bag into the patient’s airways. The self-inflating bag is equipped with a reservoir to deliver a high percentage of oxygen. To avoid barotrauma, self-inflating bags should have a preset (or adjustable) pop-off valve that allows maximum peak inspiratory pressure of 30 to 35 cm H2O (Curley and Moloney-Harmon, 2001).
Another type of bag used for manual ventilation is the flow-inflating Mapleson bag. This anesthesia bag inflates only if a continuous flow of oxygen is available. The bag is commonly used in the operating room or intensive care unit and requires trained personnel to use it. Regardless of the type of manual ventilation bag used, establish an open airway by correct positioning with the patient’s chin directed forward and the neck extended to the “sniffing” position. It is important not to hyperextend an infant’s neck, since this can occlude the airway.
![]() Several types of noninvasive positive pressure ventilation (NPPV) devices can support pediatric patients who have respiratory difficulties in acute, chronic, and home settings. NPPV does not require endotracheal (ET) intubation or a tracheostomy, but the ventilation is instead delivered via a nasal or oral mask attached to a ventilation system. Three types of NPPV can be delivered: (1) continuous positive airway pressure (CPAP), (2) intermittent positive pressure breathing (IPPB), and (3) bilevel positive airway pressure (BiPAP). CPAP provides a constant flow of positive pressure to prevent collapse of the alveoli. IPPB is a type of CPAP used intermittently to deliver aerosol medications. BiPAP provides constant positive pressure at two different pressure settings—one for inspiration and one for expiration. BiPAP has been used for pediatric patients with obstructive sleep apnea, tracheomalacia, diaphragm paralysis, progressive neuromuscular disorders, cystic fibrosis, and asthma (Curley and Moloney-Harmon, 2001). Noninvasive ventilation modes such as BiPAP are used frequently in children with neuromuscular disease to maintain adequate ventilation, especially during sleep, and are said to have prolonged life expectancy in many children (Kennedy and Martin, 2009).
Several types of noninvasive positive pressure ventilation (NPPV) devices can support pediatric patients who have respiratory difficulties in acute, chronic, and home settings. NPPV does not require endotracheal (ET) intubation or a tracheostomy, but the ventilation is instead delivered via a nasal or oral mask attached to a ventilation system. Three types of NPPV can be delivered: (1) continuous positive airway pressure (CPAP), (2) intermittent positive pressure breathing (IPPB), and (3) bilevel positive airway pressure (BiPAP). CPAP provides a constant flow of positive pressure to prevent collapse of the alveoli. IPPB is a type of CPAP used intermittently to deliver aerosol medications. BiPAP provides constant positive pressure at two different pressure settings—one for inspiration and one for expiration. BiPAP has been used for pediatric patients with obstructive sleep apnea, tracheomalacia, diaphragm paralysis, progressive neuromuscular disorders, cystic fibrosis, and asthma (Curley and Moloney-Harmon, 2001). Noninvasive ventilation modes such as BiPAP are used frequently in children with neuromuscular disease to maintain adequate ventilation, especially during sleep, and are said to have prolonged life expectancy in many children (Kennedy and Martin, 2009).
![]() Animation—Positive Pressure Ventilation
Animation—Positive Pressure Ventilation
Negative pressure ventilators create a subatmospheric pressure around the chest wall and create negative abdominal pressure, which allows the diaphragm to distend and pull air into the chest. Negative pressure ventilators come in many forms: the cuirass, the iron lung, and raincoats or ventilator suits. This form of ventilation can be used continuously or intermittently (at night or during naptime) to allow rest for patients with neuromuscular disease.
For acute respiratory support, mechanical ventilation replaces the function of the diaphragm and thoracic chest wall muscles. For the delivery of positive pressure ventilation by a ventilator, the patient will have an ET tube or a tracheostomy. Positive pressure ventilation inflates the lungs by creating pressure at the airway opening that is greater than the intraalveolar pressure. This results in improved gas distribution within the lung because of re-recruitment or reopening of partially collapsed lung segments. The overall effect is improvement of gas exchange.
Ventilators are usually classified according to the factors that regulate cycling. The method by which inspiration is terminated can be categorized as pressure cycled, volume cycled, or time cycled (Box 31-6). High-frequency ventilation uses a rapid cycling rate and delivers small tidal volumes with each cycle. Different high-frequency ventilation techniques include high-frequency oscillation ventilation, high-frequency positive pressure ventilation, high-frequency flow interruption, and high-frequency jet ventilation. With each high-frequency ventilation strategy, lung volume is held relatively constant, the cycle of inflation and deflation associated with conventional ventilation is reduced, and gas exchange is maintained with a lower incidence of lung injury (Curley and Moloney-Harmon, 2001).
Research is ongoing to support the benefits of numerous ventilation strategies in the pediatric population. The PALISI Network (Pediatric Acute Lung Injury and Sepsis Investigators; http://pedsccm.org/PALISI_network.php) is a collaborative group of investigators who study ways to optimize ventilation strategies in pediatric patients.
Critically ill children on mechanical ventilation are at risk for acquisition of ventilator-associated pneumonia (VAP). Evidence-based guidelines for the prevention of VAP have been published elsewhere (Centers for Disease Control and Prevention, 2004). Recommendations for nurses working with mechanically ventilated patients include appropriate hand hygiene measures; wearing gloves to handle respiratory secretions or contaminated objects; elevating the head of the bed 30 to 45 degrees; and routine oral hygiene, which includes oropharyngeal suctioning of secretions (Centers for Disease Control and Prevention, 2004).
An invasive technique to provide respiratory support is extracorporeal membrane oxygenation (ECMO). ECMO is a form of cardiopulmonary bypass that provides both pulmonary and cardiac support using an external oxygenation device and a pump. (See Chapter 10.)
Increasing attention is being paid to lung protective strategies, which are aimed at decreasing the effects of high distending pressures, lung barotrauma, and associated problems such as acute lung injury and bronchopulmonary dysplasia. Lung protective strategies include the use of permissive hypercapnia and ventilation with low inspiratory tidal volume pressure (Rogovik and Goldman, 2008).
Nursing Care Management
The regulation and maintenance of mechanical ventilators are often the responsibility of respiratory care practitioners. However, the nurse should understand the function of the ventilator and how to detect signs of malfunction and deviations from the desired settings. The nursing process in the care of the pediatric patient on mechanical ventilation involves baseline and continuous respiratory assessment and provision of optimum ventilation through interventions such as preventing accidental or unplanned extubation; positioning for optimum ventilation and comfort; suctioning the airway only as necessary; monitoring for potential side effects of mechanical ventilation (e.g., pneumothorax); preventing infection; ensuring that adequate humidification is provided; and providing support, comfort, and reassurance to the child and the family. It is important to help the family understand why the intubated child cannot speak when an ET tube is in place. The nurse can enhance the family’s role in providing emotional support to the child by allowing family involvement in the child’s care. With mechanical ventilation, nutrition and hydration needs must be met with either gastrostomy feedings or parenteral nutrition. (See Chapter 10 for assisted and controlled ventilation in the neonate.)
Nursing assessment of the child requiring mechanical ventilation focuses on physical examination, vital signs, response to treatment, pulmonary status, oxygenation, comfort, airway patency (e.g., obstruction or unplanned extubation and dislodgment), laboratory analysis (ABGs), and pulse oximetry. All infants and children who are intubated and on mechanical ventilation should be placed on a cardiorespiratory monitor.
![]() When an infant or child is intubated, medications are usually administered for achieving a successful, atraumatic intubation and decreasing the child’s anxiety. Because pain and agitation are often difficult to distinguish in children, it is important that nurses assess and treat agitation in the ventilated infant and child to decrease the potential for self-harm, maximize the effects of the therapy (oxygenation of tissues), and prevent self-extubation (Brinker, 2004). Rapid sequence intubation (RSI) is commonly performed in pediatric (and some neonatal) patients to induce an unconscious, neuromuscular blocked condition to avoid the use of positive pressure ventilation and the risk of possible aspiration (Bottor, 2009). Atropine, fentanyl, and vecuronium or rocuronium are drugs commonly used during RSI. In neonates endotracheal intubation is often a stressful event, and hypoxia and pain are commonly associated with routine intubation; RSI in neonates may serve to prevent such adverse events (Bottor, 2009).
When an infant or child is intubated, medications are usually administered for achieving a successful, atraumatic intubation and decreasing the child’s anxiety. Because pain and agitation are often difficult to distinguish in children, it is important that nurses assess and treat agitation in the ventilated infant and child to decrease the potential for self-harm, maximize the effects of the therapy (oxygenation of tissues), and prevent self-extubation (Brinker, 2004). Rapid sequence intubation (RSI) is commonly performed in pediatric (and some neonatal) patients to induce an unconscious, neuromuscular blocked condition to avoid the use of positive pressure ventilation and the risk of possible aspiration (Bottor, 2009). Atropine, fentanyl, and vecuronium or rocuronium are drugs commonly used during RSI. In neonates endotracheal intubation is often a stressful event, and hypoxia and pain are commonly associated with routine intubation; RSI in neonates may serve to prevent such adverse events (Bottor, 2009).
![]() Animation—Intubation in Infant
Animation—Intubation in Infant
Objective assessment tools to measure anxiety in intubated children should guide medication administration; the Comfort scale has been used in critically ill ventilated patients (Brinker, 2004). Medications used for sedation in children on mechanical ventilation include opioids (fentanyl and morphine), benzodiazepines (midazolam [Versed] and lorazepam [Ativan]), and neuromuscular blockade agents (pancuronium, vecuronium, and rocuronium). Propofol may be used short term in intubated children. Prolonged use of sedating agents may cause withdrawal, physical dependence, and tolerance, and plans should be implemented to counteract untoward effects, especially during the weaning process (Brinker, 2004).
Other important criteria to assess include nutritional status, intake and output (urinary output should be at least 2 ml/kg/hr for the infant and younger child and 1 ml/kg/hr for the older child), and skin integrity (especially around the face and lips for the child with an ET tube, and around the neck and stoma for the child with a tracheostomy). In some cases there is an increase in the amount of oral or nasal secretions, which requires appropriate perioral skin care. The child’s lips and mouth may be dry and uncomfortable; therefore provision of oral care and moisture is important to decrease discomfort.
Weaning the patient from a ventilator involves gradual physical and psychologic withdrawal from dependence on the mechanical device. Criteria for beginning the weaning process vary with the primary disease.
If the intubated child is receiving nasogastric feedings and is at risk for aspiration, feedings are usually stopped a few hours before extubation. Steroids may be administered before the extubation to control laryngeal edema. The child should remain on a cardiorespiratory monitor, and resuscitation and reintubation equipment must be available at the bedside.
CPT and suctioning are ordinarily performed just before tube removal, and cool mist or a noninvasive form of oxygen delivery (nasal cannula, face mask) is initiated immediately after extubation. The nurse monitors the child for respiratory distress and observes adequacy of oxygenation through ABG measurements or pulse oximetry. The most common complications after extubation are airway edema and pain, fatigue, and atelectasis. Stridor results from airway edema and often responds to nebulized racemic epinephrine. This can be given several times to reduce airway swelling and avoid reintubation.
Endotracheal Airways
![]() An artificial airway is commonly used in association with artificial ventilation and in children with upper airway obstruction (Box 31-7). ET intubation can be accomplished by the nasal (nasotracheal), oral (orotracheal), or direct tracheal (tracheostomy) routes. Oral intubation is usually the method of choice for emergency situations, but for prolonged intubation a nasotracheal tube is often used. Nasotracheal intubation facilitates oral hygiene and provides more stable fixation, which reduces the complication of tracheal erosion and the danger of accidental extubation. Nasotracheal intubation also allows for age-appropriate oral development such as learning to suck on a pacifier. This normal development decreases the possibility that the patient will develop oral aversions and perhaps even later feeding difficulties as a result of being intubated. One potential disadvantage of nasal intubation is erosion of the nasal septum; therefore meticulous skin care and monitoring for skin breakdown are essential.
An artificial airway is commonly used in association with artificial ventilation and in children with upper airway obstruction (Box 31-7). ET intubation can be accomplished by the nasal (nasotracheal), oral (orotracheal), or direct tracheal (tracheostomy) routes. Oral intubation is usually the method of choice for emergency situations, but for prolonged intubation a nasotracheal tube is often used. Nasotracheal intubation facilitates oral hygiene and provides more stable fixation, which reduces the complication of tracheal erosion and the danger of accidental extubation. Nasotracheal intubation also allows for age-appropriate oral development such as learning to suck on a pacifier. This normal development decreases the possibility that the patient will develop oral aversions and perhaps even later feeding difficulties as a result of being intubated. One potential disadvantage of nasal intubation is erosion of the nasal septum; therefore meticulous skin care and monitoring for skin breakdown are essential.
![]() Animation—Intubation, Incorrect Placement
Animation—Intubation, Incorrect Placement
Use only uncuffed ET tubes in children less than 8 years of age (Curley and Moloney-Harmon, 2001). Although infants have been successfully maintained on ET tubes for longer periods, tracheostomy is usually considered in some infants and older children who require intubation for an extended period. However, infants may experience complications as a result of prolonged intubation, including tracheomalacia. Therefore it is not uncommon to attempt early weaning. Prolonged mechanical ventilation in infants has also been associated with the development of bronchopulmonary dysplasia, or chronic lung disease.
The decision to change from an ET tube to a tracheostomy is made on an individual basis. The tracheostomy allows the child to speak (by temporarily occluding the opening with a special speaking device) and eat and also facilitates clearing of secretions. Suctioning an ET tube is carried out with the same care as suctioning a tracheostomy.
The practice of instilling sterile saline in the ET tube or tracheostomy before suctioning is still common in many intensive care units. However, the routine use of saline is not recommend because of increased incidence of oxygen desaturation, increased intracranial pressure, increased arterial blood pressure, and nosocomial infection (Ridling, Martin, Bratton, et al, 2003; Celik and Kanan, 2006; Morrow and Argent, 2008; Gardner and Shirland, 2009). The conclusions drawn from neonatal and adult studies are that routinely instilling saline is not supported by research. A review of 14 studies contends that normal saline (NS) does not liquefy or thin out thick secretions in the endotracheal tube; the researchers pointed out that sputum recovery with NS instillation was minimal and did not outweigh the adverse effects of routine NS instillation (Halm and Krisko-Hagel, 2008). Policies about instillation of sterile saline while suctioning vary among institutions (see Evidence-Based Practice box). Evidence-based guidelines for ET suctioning in neonates and infants have been published elsewhere (Gardner and Shirland, 2009).
Complications: ![]() The most severe complication related to immediate intubation is hypoxia with accompanying bradycardia. Closely monitor patients during intubation attempts, and if hypoxia occurs, discontinue the procedure until vital signs are stable. Ventilation with BVM and oxygen is reinstituted. Other complications include aspiration; trauma to the mouth, teeth, and trachea; epistaxis; creation of air leaks; and vagal-mediated changes in vital signs. The most common sequela of intubation is a sore throat, which disappears within 48 to 72 hours without therapy, although a humidified atmosphere is beneficial. Other complications include traumatic laryngitis, infection, glottic edema, and mucosal lesions of the larynx secondary to pressure exerted by the rigid ET tube. The most severe sequela of intubation is subglottic stenosis secondary to fibrosis.
The most severe complication related to immediate intubation is hypoxia with accompanying bradycardia. Closely monitor patients during intubation attempts, and if hypoxia occurs, discontinue the procedure until vital signs are stable. Ventilation with BVM and oxygen is reinstituted. Other complications include aspiration; trauma to the mouth, teeth, and trachea; epistaxis; creation of air leaks; and vagal-mediated changes in vital signs. The most common sequela of intubation is a sore throat, which disappears within 48 to 72 hours without therapy, although a humidified atmosphere is beneficial. Other complications include traumatic laryngitis, infection, glottic edema, and mucosal lesions of the larynx secondary to pressure exerted by the rigid ET tube. The most severe sequela of intubation is subglottic stenosis secondary to fibrosis.
Tracheostomy
![]() Tracheotomy is a surgical opening in the trachea between the second and fourth tracheal rings (Fig. 31-15). The tracheostomy tube is inserted through the exterior tracheal stoma into the trachea to provide an airway. Congenital or acquired structural defects, such as subglottic stenosis, tracheomalacia, and vocal cord paralysis, account for many long-term tracheostomies. A tracheotomy may be necessary in an emergency situation for epiglottitis, croup, or FB aspiration. These tracheostomies usually remain in place for a short time. An infant or child requiring long-term ventilatory support may also have a tracheostomy.
Tracheotomy is a surgical opening in the trachea between the second and fourth tracheal rings (Fig. 31-15). The tracheostomy tube is inserted through the exterior tracheal stoma into the trachea to provide an airway. Congenital or acquired structural defects, such as subglottic stenosis, tracheomalacia, and vocal cord paralysis, account for many long-term tracheostomies. A tracheotomy may be necessary in an emergency situation for epiglottitis, croup, or FB aspiration. These tracheostomies usually remain in place for a short time. An infant or child requiring long-term ventilatory support may also have a tracheostomy.
Pediatric tracheostomy tubes are usually made of plastic or Silastic (Fig. 31-16). The most common types are the Hollinger, Jackson, Aberdeen, and Shiley tubes. These tubes are constructed with a more acute angle than adult tubes, and they soften at body temperature, conforming to the contours of the trachea. Because these materials resist the formation of crusted respiratory secretions, they are made without an inner cannula.
Tracheostomy Care
Before the tracheotomy is performed, it is important to prepare the child and family. Teaching before the procedure should include the child (if age appropriate), family, and other primary caregivers. It should address the child’s appearance with a tracheostomy, the communication method to be used after the procedure, and postoperative procedures. If time permits, medical play or hands-on experience with tracheostomy supplies and a tour of the pediatric intensive care unit are helpful to decrease anxiety.
The child returns from the operating room with the tracheostomy tube in place and long sutures (stay sutures) taped to the chest. These sutures are attached to the tracheal rings and can be used to hold the stoma open in the event of accidental decannulation. In approximately 5 days a tract is formed in the trachea, subcutaneous tissue, and skin, at which time the stay sutures are no longer required. The nurse should tell the child that removal of the stay sutures is not painful.
Children who have undergone a tracheotomy must be closely monitored for complications such as hemorrhage, edema, aspiration, accidental decannulation, tube obstruction, and the entrance of free air into the pleural cavity. Nursing care focuses on maintaining a patent airway, facilitating the removal of secretions, providing humidified air or oxygen, offering comfort care, cleansing the stoma, monitoring the child’s ability to swallow, and teaching while simultaneously preventing complications.
Because the child may be unable to signal for help, direct observation of the child and the use of respiratory and cardiac monitors are essential. Perform respiratory assessments (including breath sounds and work of breathing, vital signs, pulse oximetry, tightness of the tracheostomy ties, and the type and amount of secretions) every 15 minutes until the patient is stable and then every 1 to 2 hours for the first 24 hours. Perform assessments thereafter every 2 to 4 hours or more frequently if needed.
Position the child with the head of the bed raised, or in the position most comfortable to the child. Suction catheters, the suction source, gloves, sterile saline, sterile gauze for wiping away secretions, scissors, an extra tracheostomy tube of the same size with ties already attached, another tracheostomy tube one size smaller, and the obturator are kept at the bedside. Provide a source of humidification, since the normal humidification and filtering functions of the airway have been bypassed. Intravenous fluids ensure adequate hydration until the child is able to swallow sufficient amounts of fluids.
Suctioning: The airway must remain patent and may require frequent suctioning during the first few hours after a tracheotomy to remove mucous plugs and excessive secretions. However, although it is necessary to prevent obstruction of the airway, suctioning is not without inherent risks, including atelectasis, hypoxemia, trauma, infection, bronchospasm, and increased mucus production (Ireton, 2007). Ireton (2007) emphasizes the importance of evidence-based practice for effective and safe tracheostomy suctioning in children. However, suctioning is often based on tradition rather than evidence and further research is needed to clarify best practice.
Proper vacuum pressure and suction catheter size are important to prevent atelectasis and decrease hypoxia from the suctioning procedure. Vacuum pressure should range from 60 to 100 mm Hg for infants and children and from 40 to 60 mm Hg for preterm infants. Unless secretions are thick and tenacious, the lower range of negative pressure is recommended. Tracheal suction catheters are available in a variety of sizes. The catheter selected should have a diameter one half the diameter of the tracheostomy tube. If the catheter is too large, it can block the airway and cause atelectasis and hypoxemia. The catheter is constructed with a side port so that the catheter is introduced without suction and removed while simultaneous intermittent suction is applied by covering the port with the thumb (Fig. 31-17). Catheters with three holes (versus a single hole) are recommended to decrease inflammatory reactions (Ireton, 2007). The catheter is inserted just slightly beyond the end (0.5 cm) of the tracheostomy tube to prevent tracheal edema and inflammation; the use of a premeasured suction catheter is recommended to avoid deep suctioning. Routine instillation of NS for suctioning is not recommended (Ireton, 2007; Morrow and Argent, 2008).
Fig. 31-17 Tracheostomy suctioning. A, Insertion, port open. B, Withdrawal, port occluded. Note that catheter is inserted just slightly beyond end of tracheostomy tube. C, Closed tracheal suctioning of child’s tracheostomy.
Counting one–one thousand, two–one thousand, three–one thousand, and so on while suctioning is a simple means for monitoring the time. For infants it is recommended that the suction time be limited to less than 5 seconds, whereas for children suctioning for less than 10 seconds is recommended (Ireton, 2007). Without a safeguard, the airway may be obstructed for too long. The child may be hyperoxygenated and hyperventilated with 100% oxygen before and after suctioning (using a BVM or increasing the Fio2 ventilator setting) to prevent hypoxia. However, these practices vary somewhat throughout the nation (Paul-Allen and Ostrow, 2000; Sole, Byers, Ludy, et al, 2003), and some recommend hyperoxygenation only if the child is on 40% oxygen or has not tolerated suctioning for brief periods without significant desaturations (Ireton, 2007).
Closed tracheal suctioning systems that allow for uninterrupted oxygen delivery may also be used. In a closed suctioning system a suction catheter is directly attached to the ventilator tubing. This system has several advantages. First, there is no need to disconnect the patient from the ventilator, which allows for better oxygenation. Second, the suction catheter is enclosed in a plastic sheath, which reduces the risk of the caregiver’s exposure to the patient’s secretions. Morrow and Argent (2008) indicate there is currently no strong evidence to support closed- or open-system suctioning.
The child is allowed to rest for 30 to 60 seconds after each aspiration to allow oxygen saturation to return to normal. Then, the process is repeated until the trachea is clear. Suctioning should be limited to about three aspirations in one period. Oximetry is an effective feedback tool to monitor suctioning and prevent hypoxia.
In the acute care setting, aseptic technique is used during care of the tracheostomy. Secondary infection is a major concern because the air entering the lower airway bypasses the natural defenses of the upper airway. Standard Precautions are recommended, and the nurse should wear gloves during suctioning procedure, although a sterile glove is needed only on the hand touching the catheter. It is recommended that the nurse follows the institution protocols for the use of nonsterile and sterile gloves during suctioning. Use a new sterile suction catheter and sterile gloves each time in the acute care setting. In the home care setting nonsterile gloves may be worn, and the suction catheter may be rinsed with water internally and cleansed with alcohol on the external surface (Sherman, Davis, Albamonte-Petrick, et al, 2000).
Nursing Care Management: The tracheostomy stoma requires daily care. Assessments of the stoma area include observations for signs of infection and breakdown of the skin. Keep the skin clean and dry, and gently remove secretions around the stoma with warm soap and water or saline. Do not use hydrogen peroxide with sterling silver tracheostomy tubes because it tends to pit and stain the silver surface. Peroxide is no longer recommended for stoma care but may be used to clean secretions or crusts that are adhered to the trach tube flanges. The nurse should be aware of wet tracheostomy dressings, which can predispose the peristomal area to skin breakdown. Several products are available to prevent or treat excoriation. The Allevyn tracheostomy dressing is a hydrophilic sponge with a polyurethane back that is highly absorptive. Other possible barriers to help maintain skin integrity include the use of hydrocolloid wafers (e.g., DuoDERM CGF, Hollister Restore, Mepilex Lite) under the tracheostomy flanges and extrathin hydrocolloid wafers under the chin (see Research Focus box).
Myers (2008) states there are abundant data indicating that agents such as povidone-iodine, Dakin solution, and hydrogen peroxide are cytotoxic to living cells, even when diluted. Therefore, until there are sufficient data to indicate otherwise, the practice of using hydrogen peroxide on tracheostomy stoma is no longer recommended.
Tracheostomy ties made of a durable, nonfraying material hold the tracheostomy tube in place. The ties are changed daily and when soiled. Ties fastened with self-adhering Velcro closures are available and are commonly used. These devices are made of a soft, cushioning, slightly stretchy, and comfortable material. They are increasingly popular because of their ease of use and ability to maintain better skin integrity. If Velcro ties are not available, cotton ties can be looped through the tracheostomy flanges and tied snugly in a triple knot at the side of the neck before the soiled ties are cut and removed. The ties should be tight enough to allow just a fingertip to be inserted between the ties and the neck (Fig. 31-18). It is easier to ensure a snug fit if the child’s head is flexed rather than extended while the ties are being secured. Routine tracheostomy tube changes are usually carried out weekly after a tract has been formed to minimize formation of granulation tissue. The first change is usually performed by the surgeon. Subsequent changes are performed by the nurse and, if the child is discharged home with the tracheostomy, by either a parent or a visiting nurse. Ideally, two caregivers participate in the procedure to assist with positioning the child.
The tracheostomy tube is changed using strict aseptic technique. A gown and eye protection should be worn to change the tracheostomy. Sterile gloves may be worn for insertion of the sterile trach tube, but clean gloves may be used for trach tubes that are cleansed and reused. Tube changes should occur before meals or 2 hours after the last meal. Continuous feedings should be turned off at least an hour before a tube change. Prepare the new tube by inserting the obturator and attaching new ties. Suction the child as necessary before the procedure to minimize secretions, then restrain and position the child with the neck slightly extended. One caregiver removes the old ties and removes the tube from the stoma. Insert the new tube gently into the stoma (using a downward and forward motion that follows the curve of the trachea), remove the obturator, and secure the ties. Assess the adequacy of ventilation after a tube change because the tube can be inserted into the soft tissue surrounding the trachea. Therefore carefully monitor breath sounds and respiratory effort.
Supplemental oxygen is always delivered with a humidification system to prevent drying of the respiratory mucosa. Humidification of room air for an established tracheostomy can be intermittent if secretions remain thin enough to be coughed or suctioned from the tracheostomy. Direct humidification via tracheostomy collar can be provided during naps and at night so that the child is able to be up and around unencumbered during much of the day. Room humidifiers are also used successfully.
The inner cannula, if used, should be removed with each suctioning, cleaned with sterile saline and pipe cleaners to remove crusted material, dried thoroughly, and reinserted.
Emergency Care: Tube Occlusion and Accidental Decannulation: Occlusion of the tracheostomy tube is life threatening. Infants and children are at greater risk than adults because of the smaller diameter of the tube. Patency of the tube is maintained with suctioning and routine tube changes to prevent formation of crusts that can occlude the tube.
Accidental decannulation also requires immediate tube replacement. Some children have a fairly rigid trachea, so that the airway remains partially open when the tube is removed. However, others have malformed or flexible tracheal cartilage, which causes the airway to collapse when the tube is removed or dislodged. Because many infants and children with upper airway problems have little airway reserve, if replacement of the dislodged tube is impossible, insert a smaller tube. Provide ventilation with a BVM to the stoma if the child is apneic. Bag-valve-stoma ventilation can be challenging, since it is often difficult to attain a good seal between the mask and the stoma. If these ventilations are ineffective (as evidenced by minimum chest movement with breaths or other signs of ineffective breathing such as cyanosis or decreased pulse oximetry readings), the stoma can be occluded with the gloved finger of one provider while another provider does BVM ventilation to the patient’s mouth and nose. If the stoma cannot be cannulated with another tracheostomy tube, oral intubation should be performed.
Decannulation
The tracheostomy tube is removed as soon as it is no longer needed. Airway problems of short duration (e.g., FB obstruction) usually allow early removal, but some conditions (e.g., tracheomalacia, tracheostenosis, vocal cord paralysis) may require that the tracheostomy tube remain in place indefinitely.
Opinions differ regarding the best means for removing the tube, especially after lengthy intubation. The usual procedure may take up to several months to wean or “downsize” the child to the smallest possible tracheostomy tube. Once this has been accomplished and the child’s respiratory status is unimpaired for 24 hours, the tube is occluded, and then removed within the next 24 hours. A small bandage is usually placed over the open stoma, which will close within a short time. The procedure is carried out in a clinical setting where continuous observation is available and emergency reintubation can be accomplished without delay, if necessary. After successful decannulation, the child remains under close observation for an additional period.
Home Care of the Child with a Tracheostomy
The early return of the infant or child with a tracheostomy to a home setting can reduce developmental delays or social handicaps related to prolonged hospitalization. Placement in the home also allows for reestablishment of routines and a regular schedule of normal activities. Physical, occupational, and speech therapy continues in the home setting. Nursing care may also be continued in the home through private-duty care or by routine, frequent nursing visits.
Preparing the family to care for the child with a tracheostomy at home is multifaceted (see Family-Centered Care box). Teaching sessions should be short, and written material must accompany instructions to reinforce what is taught. The family must be able to demonstrate tracheostomy care before the child is discharged from the hospital. Home-based care of a tracheostomy includes suctioning the tracheostomy; cleaning the stoma; changing the tracheostomy ties; changing the tracheostomy tube; adapting the home environment; and recognizing warning signs of obstruction, infection, or a worsening condition.
To prepare for any emergency, the family must learn infant or child cardiopulmonary resuscitation (CPR). The local utility company and local emergency medical service (EMS) should be notified of the child’s condition and the equipment used in the home. Prior notification allows for a quick response if help is needed.
The home should have all the necessary equipment before the child arrives. Supplies include sterile saline; a portable suction machine (and a DeLee suction trap); connecting tubing for suction; suction catheters; tracheostomy dressings; twill tape or self-adhering tracheostomy ties; pipe cleaners or a tracheostomy brush; an extra tracheostomy tube; a BVM; and a cool mist humidifier. Many children receive oxygen at home, so this too must be in place. Finally, an apnea monitor or pulse oximeter may be needed in some situations.
Encourage the family to take the child out of the home for routine outings. Two people should always be present when traveling because the child may need attention while riding in the car or at the destination. In addition to routine child care supplies, the family should always bring the portable suction machine, suction catheters, gloves, and an extra tracheostomy tube.
Management of the Tracheostomy: Clean technique and thorough, strict hand washing are taught for suctioning, cleaning the tracheostomy site, and changing the tracheostomy tube. After initial use, the catheter is rinsed with sterile water and then stored between uses in a clean cup or jar once it has dried sufficiently. The catheter may be used and cleansed appropriately as long as it remains intact and secretions can be visualized in the catheter.
Skin at the tracheostomy site is assessed for areas of breakdown or drainage. The area can be cleansed with an antibacterial soap and water.
Encourage the family to avoid frequently suctioning the child because this increases mucus production and irritates the mucosal lining. The care provider should be alert to changes in the child’s secretions regarding the amount, color, or viscosity. Awareness of these changes can prompt early medical interventions if necessary.
The family must be able to remove a plugged, clogged, or obstructed tracheostomy tube and replace it with a clean one. This situation can result in life-threatening circumstances. Older children and adolescents should be taught to care for their tracheostomies. The child should be encouraged to assume as much of his or her care as is developmentally appropriate. Independence is enhanced as the child takes responsibility for tracheostomy care.
Home Environment: Changes in the home environment may be necessary before bringing the child home. Toys, blankets, clothing, and pets that shed fine hair or lint, as well as aerosols, powders, dust, and smoke, should be avoided. Fine particles from any of these items can accumulate in the tracheostomy tube and obstruct the airway. Toys that have small removable parts (that could be placed in the tracheostomy tube) should also be eliminated.
Clothing should have a loose-fitting collar that does not cover the tracheostomy tube opening (Fig. 31-19). When the child is outside, the artificial nose or a thin cloth such as a bandanna or face mask can be placed loosely over the tracheostomy tube to prevent cold air, dust, dirt, or sand from entering the tube. The child can be bathed in a tub filled with shallow water, although it is important to ensure that no water or soap enters the tracheostomy tube. If this does occur, the tracheostomy should be suctioned immediately. Older children may shower if they are able to tolerate plugging the tube while under the shower spray.
Vocalization: The life of a child with a tracheostomy should be normalized. After the child returns home, the family should establish routines that allow the child to renew skills and enhance childhood development. Verbalization and speaking are major tasks that are often overlooked. Vocalization for the child with a tracheostomy has recently become a reality. Several tracheostomy speaking valves have been created to aid in the development of uninterrupted speech without the necessity of finger occlusion. When the speaking valve is used, air enters through the tracheostomy but is expelled over the vocal cords and through the mouth and nose. This creates a more normal passage of air through the upper airway.
A speaking valve offers many benefits. The child develops an improved self-image, since the tracheostomy can be disguised and finger occlusion for speech is not needed. The ability to swallow improves, because pressure can now accumulate as a result of the decreased amount of air released from the tracheostomy. This also allows for the creation of back pressure into the lungs. The lungs then remain open for improved gas exchange. Other advantages of this redirection of air by a speaking valve into the upper airway include improved senses of smell and taste. Secretion production is decreased because of normal evaporation, and secretions can now be coughed into the mouth, decreasing the amount of suctioning required.
Several speaking valves are available. The Passy-Muir valve is a one-way valve that attaches to the hub of all types and sizes of tracheostomies. The Passy-Muir valve will not function properly without an air leak around the tracheostomy tube and is contraindicated if there is no air leak around the tube or there is an upper airway obstruction (Kaut, Turcott, and Lavery, 1996). Therefore a cuffed tracheostomy tube must be fully deflated when using the Passy-Muir valve. If the cuff is not deflated and the Passy-Muir valve is attached, the patient will lose his or her airway. This valve can be used in infants and in children who are ventilator assisted (Engleman and Turnage-Carrier, 1997).
Two types of speaking valves are available for adolescents and adults. The first, the Kistner valve—a part of all Kistner tracheostomy tubes—is made of thin, soft plastic and does not protrude into the trachea. (Jackson metal tracheostomy tubes can also be used with a Kistner valve.) The second type is the Tucker valve, which is built into the inner cannula as a one-way leaflet. The leaflet opens on inspiration to allow air in and closes on expiration to force air into the upper airway. The Tucker valve inner cannula can be used with Tucker tracheostomy tubes sizes 4 to 9 and with Jackson tracheostomy tubes sizes 4 to 8. Tucker valves can only be used with sterling silver tracheostomy tubes.
Tracheostomy speaking valves are inappropriate for use in children who require an inflated cuff tracheostomy; who have a laryngectomy, severe tracheostenosis, or copious or excessive secretions; or who are unconscious or seriously ill.
Socialization: School-age children can be in a regular classroom environment and participate in school activities as their physical abilities allow. They should be encouraged to interact with their peers to facilitate development of socialization skills. Participation in ability-appropriate extracurricular activities is also advocated.
Many children with tracheostomies benefit from attending summer camps for children with tracheostomies who may or may not be ventilator dependent. Camping environments provide the child with independent living and a normal camping experience. Some camping sites allow the family to vacation together yet provide special care and assistance for the child with a tracheostomy.
Respiratory Emergency
![]() An inadequate supply of oxygen results in blood hypoxemia and tissue hypoxia; inadequate carbon dioxide removal causes hypercapnia. Often both gases may be insufficiently exchanged. In general, the term respiratory insufficiency is applied to two conditions: (1) when there is increased work of breathing but gas exchange function remains near normal, and (2) when normal blood gas tensions cannot be maintained and hypoxemia and acidosis develop secondary to carbon dioxide retention.
An inadequate supply of oxygen results in blood hypoxemia and tissue hypoxia; inadequate carbon dioxide removal causes hypercapnia. Often both gases may be insufficiently exchanged. In general, the term respiratory insufficiency is applied to two conditions: (1) when there is increased work of breathing but gas exchange function remains near normal, and (2) when normal blood gas tensions cannot be maintained and hypoxemia and acidosis develop secondary to carbon dioxide retention.
![]() Animation—Respiratory Failure, Infant
Animation—Respiratory Failure, Infant
Respiratory failure is the inability of the respiratory apparatus to maintain adequate gas exchange. This process involves pulmonary dysfunction that generally results in impaired alveolar-capillary gas exchange, which can lead to hypoxemia or hypercapnia. Respiratory arrest is the cessation of respiration. Respiratory failure is the most common cause of cardiopulmonary arrest in children (Rotta and Wiryawan, 2003).
Apnea is generally defined as cessation of breathing for more than 20 seconds or for a shorter period when associated with hypoxemia or bradycardia (Dudell and Stoll, 2007). Apnea can be (1) central, in which both airflow and chest wall movement are absent; (2) obstructive, in which airflow is absent but chest wall motion is present; and (3) mixed, in which both central and obstructive components are present.
Effective pulmonary gas exchange requires clear airways, normal lungs and chest wall, and adequate pulmonary circulation. Anything that affects these functions or their relationships can compromise respiration.
Respiratory dysfunction may have an abrupt or an insidious onset. Respiratory failure can occur as an emergency situation or may be preceded by gradual and progressive deterioration of respiratory function. Most clinical manifestations are nonspecific and are affected by variations among individual patients and differences in the severity and duration of inadequate gas exchange.
The diagnosis of respiratory failure is determined by the combined application of three sources of information:
1. Presence or history of a condition that might predispose to respiratory failure
Conditions That Predispose to Respiratory Failure
Respiratory disorders are classified according to three dominant functional abnormalities, although all three types may be present in the disease. In obstructive lung disease there is increased resistance to airflow in either the upper or the lower respiratory tract. Obstruction can result from anomalies (tracheomalacia, choanal atresia, vocal paralysis), aspiration (meconium, mucus, vomitus, FB), infection (epiglottitis, pneumonia, pertussis, severe tonsillitis), tumors (hemangioma, cystic hygroma), anaphylaxis, and laryngospasm from local irritation (intubation, drowning, aspiration).
In restrictive lung disease, impaired lung expansion results from loss of lung volume, decreased distensibility, or chest wall disturbance. Causes of pulmonary restriction include respiratory distress syndrome, pneumonia, cystic fibrosis, pneumothorax, pulmonary edema, pleural effusion, near drowning, congenital diaphragmatic hernia, abdominal distention, muscular dystrophy, paralytic conditions (poliomyelitis, botulism), and severe structural obstructions such as severe scoliosis.
In primary inefficient gas transfer there is insufficient alveolar ventilation for carbon dioxide removal or impaired oxygenation of pulmonary capillary blood as a result of dysfunction of the respiratory control mechanism or a diffusion defect. Causes of respiratory center depression include cerebral trauma (birth injuries); intracranial tumors; CNS infection (meningitis, encephalitis, sepsis); overdose with barbiturates, opioids, or benzodiazepines (diazepam [Valium] or midazolam); severe asphyxia (hypercapnia, hypoxemia); and tetanus. Pulmonary diffusion defects include pulmonary edema, fibrosis, embolism, or hypertension; collagen disorders; Pneumocystis carinii pneumonia; anemia; and hemorrhage.
Recognition of Respiratory Failure
Respiratory failure that occurs as a result of acute obstruction of a major airway or cardiac arrest is sudden and readily apparent. Gradual and more covert development of signs and symptoms is less easily recognized. Insufficient alveolar ventilation from any cause ultimately leads to hypoxemia and hypercapnia. However, in some situations severe respiratory distress may be present without significant carbon dioxide retention, and hypoxemia may occur without clinically detectable cyanosis. Therefore evaluation of respiratory adequacy is based on both clinical assessment and laboratory studies. Nursing observation and judgment are vital to successful management of respiratory failure. Nurses must be able to assess a situation and initiate appropriate action within moments.
Unless respiratory arrest occurs suddenly, signs of hypoxemia and hypercapnia are usually subtle in their development, becoming more obvious as respiratory failure progresses. The unknowing observer may attribute early signs such as mood changes and restlessness to other causes, and some signs can be altered by other factors. Clinical manifestations of respiratory failure are listed in Box 31-8.
In clinical situations in which impaired ventilation can be anticipated or clinical manifestations indicate impending hypoxemia, serial measurements of blood gases should be obtained and monitored to detect impending respiratory failure, and therapy should be implemented before respiratory acidosis becomes extreme.
Nursing Care Management
![]() The interventions used in the management of respiratory failure are often dramatic, requiring special skills, and are frequently emergency procedures. If respiratory arrest occurs, the primary objectives are to recognize the situation and immediately initiate resuscitative measures, such as opening the airway and positioning, administering supplemental oxygen and positive pressure ventilation, suctioning, performing CPR, or intubating if the child’s status continues to deteriorate. When the situation is not an arrest, the suspicion of respiratory failure is confirmed by assessment, and the severity is defined by capillary or arterial blood gas analysis. Interventions such as administering supplemental oxygen, opening the airway, positioning, stimulation, suctioning, and early intubation may avert an arrest. When severity is established, an attempt is made to determine the underlying cause by thorough evaluation.
The interventions used in the management of respiratory failure are often dramatic, requiring special skills, and are frequently emergency procedures. If respiratory arrest occurs, the primary objectives are to recognize the situation and immediately initiate resuscitative measures, such as opening the airway and positioning, administering supplemental oxygen and positive pressure ventilation, suctioning, performing CPR, or intubating if the child’s status continues to deteriorate. When the situation is not an arrest, the suspicion of respiratory failure is confirmed by assessment, and the severity is defined by capillary or arterial blood gas analysis. Interventions such as administering supplemental oxygen, opening the airway, positioning, stimulation, suctioning, and early intubation may avert an arrest. When severity is established, an attempt is made to determine the underlying cause by thorough evaluation.
![]() Nursing Care Plan—The Child with Respiratory Failure
Nursing Care Plan—The Child with Respiratory Failure
Treatment of respiratory dysfunction involves both specific and nonspecific therapy. Specific therapies are directed toward reversal of the causative factors. However, nonspecific measures are necessary to maintain oxygenation and enhance carbon dioxide removal until specific methods take effect. The major reasons for implementing nonspecific treatments are (1) unknown etiology, (2) lack of specific treatment for a known cause, (3) lack of time for a specific treatment to take effect, and (4) need for specialized personnel or equipment for specific treatment.
The principles of management are to (1) maintain ventilation and maximize oxygen delivery, (2) correct hypoxemia and hypercapnia, (3) treat the underlying cause, (4) minimize extrapulmonary organ failure, (5) apply specific and nonspecific therapy to control oxygen demands, and (6) anticipate complications. Monitoring the patient’s condition is critical.
Observation and Monitoring
![]() The nurse monitors the child to anticipate respiratory failure, determine a course of action, and assess the patient’s response to treatment. If close, continuous monitoring is required, the child is transferred to a pediatric intensive care unit. The child is kept as comfortable as possible, and observation is geared toward general appearance, responsiveness, pulse oximetry, and vital signs. The child is positioned to allow maximum lung expansion and comfort, such as sitting upright or leaning forward (depending on respiratory status).
The nurse monitors the child to anticipate respiratory failure, determine a course of action, and assess the patient’s response to treatment. If close, continuous monitoring is required, the child is transferred to a pediatric intensive care unit. The child is kept as comfortable as possible, and observation is geared toward general appearance, responsiveness, pulse oximetry, and vital signs. The child is positioned to allow maximum lung expansion and comfort, such as sitting upright or leaning forward (depending on respiratory status).
![]() Animation—Inefficient Bag Ventilation
Animation—Inefficient Bag Ventilation
Prone positioning improves oxygenation and lung mechanics in adult patients with acute lung injury and acute respiratory distress syndrome. A multicenter pediatric trial for prone positioning showed it to be ineffective in improving oxygenation in acute lung injury or acute respiratory distress syndrome (Curley, Hibberd, Fineman, et al, 2005). At this point, the only indications for its use are in the case of bronchial compression by the heart or the need to augment secretion clearance. The nurse monitors the child’s cardiac and respiratory status by observation and by electronic means. However, no monitoring equipment can replace conscientious nursing observations (Box 31-9), which should focus primarily on the child’s airway, oxygenation, ventilation, and tissue perfusion.
Because one goal of therapy is to control the body’s oxygen demands, assessments of fever and pain should be frequent. Both conditions (as well as cold stress) can dramatically increase oxygen requirements, especially in younger children, and therefore increase respiratory effort. Measure oxygenation by the use of pulse oximetry or blood gas monitoring.
Family Support
Children who are in respiratory distress often relax after an airway is established and their respiratory effort is assisted. However, they are anxious and frightened when they are unable to communicate; therefore it is important to effectively manage the child’s anxiety. This may be accomplished initially with mild sedation until the child’s ventilatory status has improved.
It is often frightening for young children to discover they are unable to make vocal sounds, including crying. It is also stressful to parents to watch their child’s inability to vocalize and helplessness. It is important to talk to children and reassure them that their voices will return when the breathing tube (ET tube or tracheostomy) is removed.
Parents have many concerns relative to ET tubes and ventilators. Before intubation, the nurse should discuss with them the reasons for the decision to implement the therapy, the expected results, and the approximate length of time it will remain in place. Parents are often concerned about the (often) life-threatening implications generated by the need for the procedure and the possible long-term effects on the child, both physiologic and psychologic. They are also concerned about the long-term residual effects on the brain and on the child’s psychologic status. Parents who must face the possibility of caring for the child with a tracheostomy or a home ventilator have additional worries regarding their ability to assume this responsibility (see p. 1206).
For families whose child has had a respiratory arrest, support focuses on keeping the family informed of the child’s status and helping them cope with a near-death experience or an actual death. (See Chapter 23.) Knowing that their child requires CPR is a frightening and often overwhelming experience for parents. Uncertainty regarding outcome—both mortality and morbidity—is a primary concern. Traditionally family members have not been allowed to be present during resuscitation efforts (see Evidence-Based Practice box).
Nurses can serve as the family’s advocate by either being present with them or making certain a support person, such as a clergy member, is present. After the child’s recovery or death, the family needs continued support and thorough medical information regarding lifesaving measures, the prognosis if the child survives, and the cause of death if the child dies.
Cardiopulmonary Resuscitation
Cardiac arrest in the pediatric population is less often of cardiac origin than a result of prolonged hypoxemia secondary to inadequate oxygenation, ventilation, and circulation (shock). Some causes include injuries, suffocation (e.g., FB aspiration), smoke inhalation, anaphylaxis, apparent life-threatening event, and infection. In small infants the small size of the airway may easily be compromised by improper positioning with the chin resting on the chest. This is easy to remedy by positioning the infant with the chin elevated (but not hyperextended) so the airway is open. This is common in newborns or infants who are not positioned properly in an infant seat or car restraint seat.
Respiratory arrest is associated with a better survival rate than cardiac arrest. Recent studies indicate that infants survived out-of-hospital cardiac arrest (including survival to hospital discharge) at a rate similar to that of adults—3.3% for infants and 4.5 % for adults. However, older children and adolescents survived out-of-hospital cardiac arrest at higher rates—9.1% and 8.9%, respectively (Atkins, Everson-Stewart, Sears, et al, 2009). Ventricular fibrillation, previously believed to be rare in children, occurred in 25% of patients with in-hospital pediatric cardiac arrest and 7% of those with out-of-hospital pediatric cardiac arrest (Topjian, Nadkarni, and Berg, 2009). Studies demonstrated that more males required resuscitation in prehospital settings, and the overall survival rate was approximately 9% (Young, Gausche-Hill, McClung, et al, 2004).
Complete apnea signals the need for rapid and vigorous action to prevent cardiac arrest. In such situations nurses must be prepared to initiate action immediately. In the hospital, emergency equipment should be readily available in areas in which respiratory arrest might take place, and the status of this resuscitation equipment should be checked at least once daily. Regardless of the cause of the arrest, basic procedures are carried out and modified somewhat according to the child’s size.
Outside the hospital the first action in an emergency is to quickly assess the extent of any injury and determine whether the child is unconscious. A child who is struggling to breathe but conscious should be transported immediately to an advanced life support (ALS) facility, allowing the child to maintain whatever position affords the most comfort. Attempting to transport a child by personal vehicle wastes valuable time in obtaining help. Transport by EMS is recommended. Services in larger communities can institute ALS immediately or en route to a medical facility.
An unconscious child is managed with care to prevent additional trauma if a head or spinal cord injury has been sustained. The circumstances in which the child is found offer clues to a possible injury. For example, a child who has been thrown from a bicycle or has fallen from a tree is more likely to have sustained trauma than a child who is discovered in bed. The child should not be moved unless there is a life-threatening condition such as a fire; if moved, the victim must be appropriately immobilized with spinal and cervical immobilization devices.
Resuscitation Procedure
![]() The American Heart Association (2005) implemented several changes in CPR guidelines that incorporate the use of the automatic external defibrillator (AED) as part of the treatment of cardiorespiratory arrest in children 1 year of age and older. The 2005 guidelines state that AEDs can be safely and effectively used in children ages 1 to 8 years; however, there are insufficient data to support or refute the use of AEDs in children younger than 1 year old. Appropriate-sized pediatric pads must be used for small children. Health care providers are advised to give children 1 year and older a defibrillatory shock after providing approximately five cycles of CPR (approximately 2 minutes of cycles of 30 compressions and two ventilations by the lone rescuer), provided the AED is sensitive to pediatric rhythms, the device is capable of delivering a pediatric dosage of 2 joules/kg, and a shockable rhythm (usually ventricular fibrillation) is present. In a hospital situation, where weight-based defibrillation dosing is possible, manual defibrillation is the mode of choice instead of AED. When using an AED, health care providers are advised to give adults and children older than 8 years a defibrillatory shock within 5 minutes of collapse outside the hospital and within 3 minutes in the hospital.
The American Heart Association (2005) implemented several changes in CPR guidelines that incorporate the use of the automatic external defibrillator (AED) as part of the treatment of cardiorespiratory arrest in children 1 year of age and older. The 2005 guidelines state that AEDs can be safely and effectively used in children ages 1 to 8 years; however, there are insufficient data to support or refute the use of AEDs in children younger than 1 year old. Appropriate-sized pediatric pads must be used for small children. Health care providers are advised to give children 1 year and older a defibrillatory shock after providing approximately five cycles of CPR (approximately 2 minutes of cycles of 30 compressions and two ventilations by the lone rescuer), provided the AED is sensitive to pediatric rhythms, the device is capable of delivering a pediatric dosage of 2 joules/kg, and a shockable rhythm (usually ventricular fibrillation) is present. In a hospital situation, where weight-based defibrillation dosing is possible, manual defibrillation is the mode of choice instead of AED. When using an AED, health care providers are advised to give adults and children older than 8 years a defibrillatory shock within 5 minutes of collapse outside the hospital and within 3 minutes in the hospital.
Changes in resuscitation procedure for the lay rescuer are discussed in the following section. The sequence of CPR steps for the health care provider is discussed in both the text and Fig. 31-20.
Fig. 31-20 Summary of basic life support maneuvers for infants, children, and adults (newborn-neonatal information not included). CPR, Cardiopulmonary resuscitation; HCP, maneuvers used only by health care provider; AED, automated external defibrillator. (From American Heart Association: 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, Circulation 112[24 Suppl 1]:IV-166, 2005.)
If two rescuers are present, one rescuer should begin CPR while the second rescuer activates the EMS system by calling 911 and obtaining an AED. Pediatric rescuers provide five cycles of basic life support (approximately 2 minutes) before activating EMS; each cycle consists of 30 chest compressions and two ventilations. Because pediatric arrests are most commonly caused by respiratory arrest, maintaining ventilation is primary.
Open the Airway: For effective CPR, place the victim on the back on a firm, flat surface, using appropriate precautions. With loss of consciousness the tongue, which is attached to the lower jaw, relaxes and falls back, obstructing the airway. To open the airway, the lay rescuer positions the head with a head tilt–chin lift maneuver. Health professionals should open the airway using either a head tilt–chin lift or jaw thrust maneuver. A head tilt is accomplished by placing one hand on the victim’s forehead and applying firm, backward pressure with the palm to tilt the head back. Place the fingers of the free hand under the bony portion of the lower jaw near the chin to lift and bring the chin forward (chin lift). This supports the jaw and helps tilt the head back (Fig. 31-21).
The jaw thrust is accomplished by grasping the angles of the victim’s lower jaw and lifting with both hands, one on each side, displacing the mandible upward and outward. The jaw thrust is recommended for use only by health care workers. In suspected neck injuries, use the jaw thrust method while the cervical spine is completely immobilized. After a patent airway is restored by removal of foreign material and secretions (if indicated), if the child is not breathing, continue maintenance of the airway and initiate rescue breathing.
Give Breaths: To ventilate the lungs in the infant (from birth to 1 year of age), place the BVM or operator’s mouth in such a way that both the mouth and the nostrils are covered (Fig. 31-22). Children over 1 year of age are ventilated through the mouth while the nostrils are firmly pinched for airtight contact.
The volume of air in an infant’s lungs is small, and the air passages are considerably smaller, with resistance to flow potentially higher than in adults. The rescuer should deliver small puffs of air and assess the rise of the chest to ensure that overinflation does not occur. A gentle rise of the chest is a sufficient indicator of adequate inflation.
The correct volume for each breath is the volume that causes the chest to rise. If air enters freely and the chest rises, assume the airway is clear. Give breaths slowly with sufficient volume to make the chest rise.
Check Pulse: After an initial two breaths, the health care provider palpates the pulse to ascertain the presence of a heartbeat. The carotid is the most central and accessible artery in children over 1 year of age. However, the infant’s short and often fat neck makes the carotid pulse difficult to palpate. Therefore in the infant it is preferable to use the brachial pulse, located on the inner side of the upper arm midway between the elbow and the shoulder (Fig. 31-23). Absence of a carotid or brachial pulse is considered sufficient indication to begin external cardiac massage. Lay rescuers are not taught to check the pulse but are taught to look for signs of circulation (e.g., normal breathing, coughing, or air movement) in response to rescue breaths.
Perform Chest Compression: External chest compression consists of serial, rhythmic compressions of the chest to maintain circulation to vital organs until the child achieves spontaneous vital signs or ALS can be provided. Chest compressions are always interspersed with ventilation of the lungs. For optimal compressions it is essential that the child’s spine be supported on a firm surface during compressions of the sternum and that sternal pressure is forceful but not traumatic. For a small infant the hard surface can be the rescuer’s hand or forearm, with the palm supporting the infant’s back. Position the child’s head for optimum airway opening using the head tilt–chin lift maneuver. It is essential to prevent overextension of the head of small infants because this tends to close the flexible trachea.
Place the fingers for compression in infants at a point on the lower sternum just below the intersection of the sternum and an imaginary line drawn between the nipples (Fig. 31-24). Apply compressions on the child 1 to 8 years of age to the lower half of the sternum (Fig. 31-25). Sternal compression to infants is applied with two fingers on the sternum, exerting a firm downward thrust; chest compression for children is applied with the heel of one hand or two hands, depending on the child’s size. Current American Heart Association (2005) guidelines include the addition of the two-thumb technique for chest compressions for infants when two health care providers are present. In the two-thumb technique, one of the two rescuers places both thumbs side by side over the lower half of the infant’s sternum; the remaining fingers encircle the infant’s chest and support the back. The two-thumb technique is not taught to lay rescuers and is not practical for the health care provider working alone.
Fig. 31-25 Chest compressions in child: one hand for smaller child (A) and two hands for larger child (B).
Adapt the depth of compression to the child’s size. The location, rate, and depth for children older than 8 years of age are the same as for adults.
Lone-rescuer CPR is continued at the ratio of two breaths to 30 compressions for all ages until signs of recovery appear. These signs include palpable peripheral pulses, return of pupils to normal size, the disappearance of mottling and cyanosis, and possibly return of spontaneous respiration. When two rescuers are present, they should deliver two breaths to each 15 compressions. According to the new guidelines (American Heart Association, 2005), the lay rescuer is not taught two-rescuer CPR. An update to these guidelines is anticipated in 2011.
Administer Medications: Medications are an important adjunct to CPR, especially cardiac arrest, and are used during and after resuscitation in children. Medications are used to (1) correct hypoxemia, (2) increase perfusion pressure during chest compression, (3) stimulate spontaneous or more forceful myocardial contraction, (4) accelerate cardiac rate, (5) correct metabolic acidosis, and (6) suppress ventricular ectopy. Appropriate fluid therapy is initiated immediately in the hospital or by EMS personnel during transport. (See Parenteral Fluid Therapy, Chapter 28; and Shock, Chapter 29.) A complete supply of emergency medications is kept and maintained in all EMS vehicles and on all hospital units. The supply is checked on a regular basis (usually once on each 8- or 12-hour shift). Table 31-6 lists resuscitation medications.
TABLE 31-6
DRUGS FOR PEDIATRIC CARDIOPULMONARY RESUSCITATION
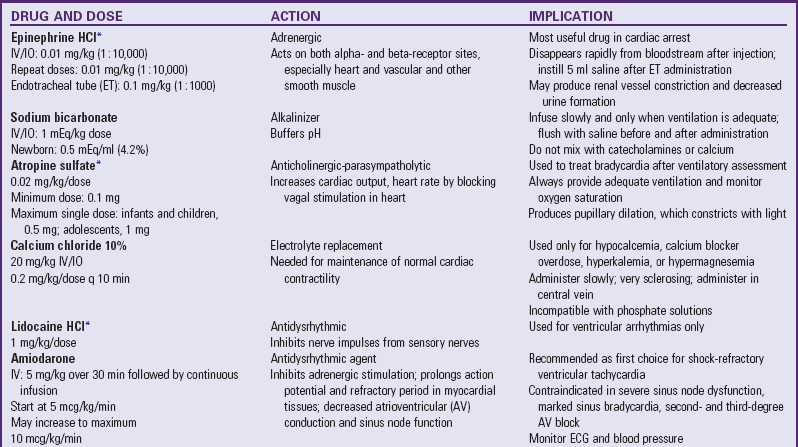
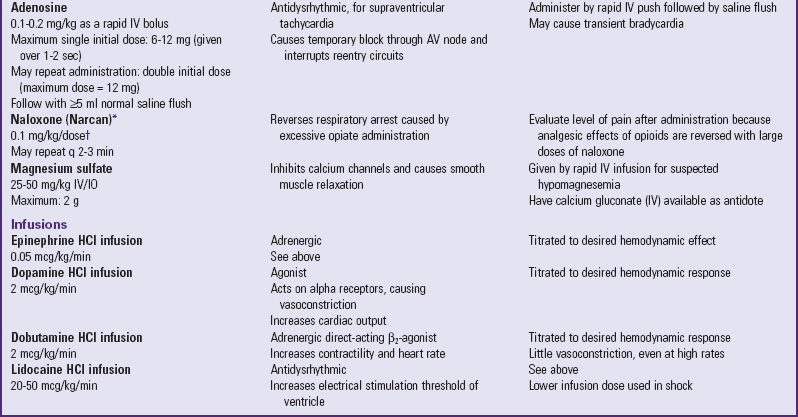
ECG, Electrocardiogram; IO, intraosseous; IV, intravenous.
*These drugs may be administered via ET tube if IV/IO is not available; IV/IO is the preferred route.
†Dose of naloxone to reverse respiratory depression without reversing analgesia from opioids is 0.5 mcg/kg in children <40 kg (88 lb) (American Pain Society, 1999).
When administering drugs during CPR (or a “code”), use a saline flush between medications to prevent drug interactions. Document all drugs, dosages, and the time and route of administration.
Airway Obstruction
Attempts at clearing the airway should be considered for (1) children in whom aspiration of an FB is witnessed or strongly suspected; and (2) unconscious, nonbreathing children whose airways remain obstructed despite the usual maneuvers to open them. When aspiration is strongly suspected, encourage the child to continue coughing as long as the cough remains forceful.
In a conscious choking child, attempt to relieve the obstruction only if:
Infants
A combination of back blows (over the spine between the shoulder blades) and chest thrusts (on the sternum, in the same location as for chest compressions) is recommended to relieve the FB obstruction in infants (Fig. 31-26). Place a choking infant face down over the rescuer’s arm with the head supported and lower than the trunk. For additional support the rescuer should support the arm firmly against the thigh. Deliver up to five quick, sharp, back blows between the infant’s shoulder blades with the heel of the rescuer’s hand. Use less force than would be applied to an adult. After delivery of the back blows, place the free hand flat on the infant’s back so that the infant is “sandwiched” between the two hands, making certain the neck and chin are well supported. While maintaining support with the infant’s head lower than the trunk, turn the infant and place the infant supine on the rescuer’s thigh, and apply up to five quick downward chest thrusts in rapid succession in the same location as external chest compressions described for CPR. Continue back blows and chest thrusts until the object is removed or the infant becomes unconscious.
Children
A series of subdiaphragmatic abdominal thrusts (Heimlich maneuver) is recommended for children older than 1 year of age. The maneuver creates an artificial cough that forces air, and with it the FB, out of the airway. The procedure is carried out with the child in a standing, sitting, or lying position (Fig. 31-27). In the conscious choking child, upward thrusts are delivered to the upper abdomen with the fisted hand at a point just below the rib cage. To prevent damage to the internal organs, the rescuer’s hands should not touch the xiphoid process of the sternum or the lower margins of the ribs. Repeat up to five thrusts in rapid succession until the FB is expelled.
It is neither necessary nor desirable to squeeze or compress the arms during the procedure. It is not a punch or a bear hug. The child may vomit after relief of the obstruction and should be positioned to prevent aspiration. After breathing is restored, the child should receive medical attention and be assessed for complications.
The success of the technique is primarily a result of the obstruction occurring at the end of a maximum respiration. The victim is most likely to choke on food during inspiration; therefore the tidal volume plus expiratory reserve volume is present in the lungs. When pressure is exerted on the diaphragm by the maneuver, the food bolus is ejected with considerable force by this trapped air.
If the victim is breathing or resumes effective breathing after emergency interventions, place in the recovery position: move the head, shoulders, and torso simultaneously and turn onto the side. The leg not in contact with the ground may be bent and the knee moved forward to stabilize the victim (Fig. 31-28). Do not move the victim in any way if trauma is suspected, and do not place the child in the recovery position if rescue breathing or CPR is required.
Key Points
• The major functions of the respiratory tract are to distribute air and exchange gases to supply cells with oxygen and to remove carbon dioxide.
• Several anatomic features predispose infants and young children to airway obstruction and atelectasis: they have less alveolar surface for gas exchange, narrowly branching peripheral airways become easily obstructed, and lack of collateral pathways inhibits ventilation beyond obstructed units.
• Gas exchange depends on the amount and composition of gases inhaled, thickness of the alveolar wall, adequacy of circulation to the alveoli, and substances within the alveoli that prevent their inflation or gas exchange.
• The amount of oxygen that diffuses into the blood depends on a pressure gradient between alveolar air and capillary blood, the total functional surface area of the alveolocapillary membrane, minute volume, and alveolar ventilation.
• Defense mechanisms of the respiratory tract include the lymphatic system, mucus secretions, ciliary action, epiglottis, cough reflex, tracheobronchial dynamics, body position changes, and humoral defenses.
• Complete assessment of respiratory function involves a detailed history, physical examination, pulmonary function tests, radiography, and blood gas determination.
• Pulse oximetry is a noninvasive method of determining the oxygen saturation in the blood. One limitation of the technology is that it does not identify dangerously high oxygen levels.
• Improvement in respiratory function may be accomplished with measures such as oxygen therapy, positioning, humidification, aerosol therapy, and artificial ventilation.
• Always humidify oxygen when administering it to children.
• CPT is useful for patients with increased sputum production but is contraindicated for some.
• Implications for possible intubation include airway obstruction, respiratory arrest, neuromuscular compromise or paralysis, and hypoxemia.
• Respiratory failure is the inability of the respiratory system to maintain adequate oxygenation of the blood, with or without carbon dioxide retention.
• Management of respiratory failure is to provide oxygen, maintain ventilation, apply appropriate therapy, and anticipate complications.
• ET and tracheostomy suctioning involves premeasured insertion of the catheter, application of suction for 3 or 4 seconds when withdrawing the catheter, and supplemental oxygen before and after suctioning.
• Occlusion of the ET and tracheostomy tube is life threatening; therefore equipment for replacing a tube must always be available.
• Pediatric CPR includes five cycles (about 2 minutes) of ventilations (two) and compressions (30) before summoning emergency help.
• Automatic external defibrillators are safe to use in children 1 year and older in an arrest situation where there is no pulse.
• Choking and respiratory failure are respiratory emergencies that require immediate intervention.
• Use abdominal thrusts in children in whom FB obstruction is witnessed or strongly suspected. Use a combination of back blows and chest thrusts for infants with FB obstruction.
• In a conscious choking child, make attempts to relieve the obstruction only if the child is unable to make any sounds, the cough becomes ineffective, or the child has increasing respiratory difficulty with stridor.
References
American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(24 Suppl 1):IV–166.
American Pain Society. Principles of analgesic use in the treatment of acute pain and chronic cancer pain, ed 4. Skokie, Ill: The Society; 1999.
Atkins, DL, Everson-Stewart, S, Sears, GK, et al. Epidemiology and outcome from out-of-hospital cardiac arrest in children: the resuscitation Outcomes Consortium Epistry–Cardiac Arrest. Circulation. 2009;119(11):1484–1491.
Bilan, N, Behbahan, AG, Khosroshahi, AJ. Validity of venous blood gas analysis for diagnosis of acid-base imbalance in children admitted to paediatric intensive care unit. World J Pediatr. 2008;4(2):114–117.
Boat, TF, Green, TP. Chronic or recurrent respiratory symptoms. In Behrman RE, Kliegman RM, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Bottor, LT. Rapid sequence intubation in the neonate. Adv Neonat Care. 2009;9(3):111–117.
Brinker, D. Sedation and comfort issues in the ventilated infant and child. Crit Care Nurs Clin North Am. 2004;16:365–377.
Celik, SA, Kanan, N. A current conflict: use of isotonic sodium chloride solution on endotracheal suctioning in critically ill patients. Dimens Crit Care Nurs. 2006;25(1):11–14.
Centers for Disease Control and Prevention. Guidelines for preventing health-care-associated pneumonia, 2003. MMWR Recomm Rep. 2004;53(RR03):1–36.
Curley, MA, Hibberd, PL, Fineman, LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):248–250.
Curley, MAQ, Moloney-Harmon, PA. Critical care nursing of infants and children, ed 2. Philadelphia: Saunders; 2001.
Dudell, GG, Stoll, BJ. Respiratory tract disorders. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Engleman, SG, Turnage-Carrier, C. Tolerance of the Passy-Muir speaking valve™ in infants and children less than 2 years of age. Pediatr Nurs. 1997;23:571–573.
Fauroux, B, Guillemot, N, Aubertin, G, et al. Physiologic benefits of mechanical insufflations-exsufflation in children with neuromuscular diseases. Chest. 2008;133(1):161–168.
Gardner, DL, Shirland, L. Evidence-based guideline for suctioning the intubated neonate and infant. Neonat Netw. 2009;28(5):281–302.
Halm, MA, Krisko-Hagel, K. Instilling normal saline with suctioning: beneficial technique or potentially harmful sacred cow? Am J Crit Care. 2008;17(5):469–472.
Haddad, GG. Congenital central hypoventilation syndrome (Ondine curse. In Behrman RE, Kliegman RM, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Homnick, DN. Mechanical insufflations-exsufflation for airway mucus clearance. Respir Care. 2007;52(10):1296–1305.
Ireton, J. Tracheostomy suction: a protocol for practice. Paediatr Nurs. 2007;19(10):14–18.
Kaut, K, Turcott, JC, Lavery, M. Passy-Muir speaking valve. Dimens Crit Care Nurs. 1996;15:298–306.
Kennedy, JD, Martin, AJ. Chronic respiratory failure and neuromuscular disease. Pediatr Clin North Am. 2009;56(1):261–273.
Marks, JH. Pulmonary care of children and adolescents with developmental disabilities. Pediatr Clin North Am. 2008;55(6):1299–1314.
McCance, KL, Huether, SE. Pathophysiology: the biological basis for disease in adults and children, ed 6. St Louis: Mosby; 2010.
McIlwaine, M. Chest physical therapy breathing techniques and exercise in children with CF. Paediatr Respir Rev. 2007;8(1):8–16.
Morrow, BM, Argent, AC. A comprehensive review of pediatric endotracheal suctioning: effects, indications, and clinical practice. Pediatr Crit Care Med. 2008;9(5):465–477.
Myers, BA. Wound management. Upper Saddle River, NJ: Pearson/Prentice Hall; 2008.
Naylor, JM, McLean, A, Chow, CM, et al. A modified postural drainage position produces less cardiovascular stress than a head-down position in patients with severe heart disease: a quasi-experimental study. Aust J Physiother. 2006;52(3):201–209.
Paul-Allen, J, Ostrow, CL. Survey of nursing practices with closed-system suctioning. Am J Crit Care. 2000;9(1):9–17.
Ridling, DA, Martin, LD, Bratton, SL, et al. Endotracheal suctioning with or without instillation of isotonic sodium chloride in critically ill children. Am J Crit Care. 2003;12(3):212–219.
Rogovik, A, Goldman, R. Permissive hypercapnia. Emerg Med Clin North Am. 2008;26(4):941–952.
Rotta, AT, Wiryawan, B. Respiratory emergencies in children. Respir Care. 2003;48(3):248–260.
Sherman, JM, Davis, S, Albamonte-Petrick, S, et al. Care of the child with a tracheostomy: official statement of the American Thoracic Society. Am J Respir Crit Care Med. 2000;161(1):297–308.
Sole, ML, Byers, JF, Ludy, JE, et al. A multisite survey of suctioning techniques and airway management practices. Am J Crit Care. 2003;12(3):220–230.
Topjian, AA, Nadkarni, VM, Berg, RA. Cardiopulmonary resuscitation in children. Curr Opin Crit Care. 2009;15(3):203–208.
Watts, KD, Goodman, DM. Wheezing, bronchiolitis, and bronchitis. In Behrman RE, Kliegman RM, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Wilson, JR, Mills, JG, Prather, ID, et al. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18(7):373–378.
Yildizdas, D, Yapicioglu, H, Yilmaz, HL, et al. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child. 2004;89(2):176–180.
Young, KD, Gausche-Hill, M, McClung, CD, et al. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114(1):157–164.
Zander, J, Hazinski, MF. Pulmonary disorders. In Hazinski MF, ed.: Nursing care of the critically ill child, ed 2, St Louis: Mosby, 1992.