The Child with Respiratory Dysfunction
General Aspects of Respiratory Tract Infections,
Upper Respiratory Tract Infections
Infections of the Lower Airways
Other Infections of the Respiratory Tract
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
The Child with Disturbance of Oxygen and Carbon Dioxide Exchange, Ch. 31
Compliance, Ch. 27
Controlling Elevated Temperatures, Ch. 27
Family-Centered Care of the Child with Chronic Illness or Disability, Ch. 22
Family-Centered Home Care, Ch. 25
High Risk Related to Disturbed Respiratory Function, Ch. 10
Immunizations, Ch. 12
Infection Control, Ch. 27
Ingestion of Injurious Agents, Ch. 16
Maintaining Healthy Skin, Ch. 27
Pain Assessment; Pain Management, Ch. 7
Physical Examination: Ears, Nose, Mouth and Throat, Chest, Lungs, Ch. 6
Surgical Procedures, Ch. 27
Tobacco (Use), Ch. 21
General Aspects of Respiratory Tract Infections
Infections of the respiratory tract are described according to the areas of involvement. The upper respiratory tract, or upper airway, consists of the oronasopharynx, pharynx, larynx, and upper part of the trachea. The lower respiratory tract consists of the bronchi, bronchioles, and alveoli. The bronchi and bronchioles are the reactive portion of the lower respiratory tract, since they have smooth muscle content and the ability to constrict. Respiratory tract infections spread from one structure to another because of the contiguous nature of the mucous membrane lining the entire tract. Consequently, infections of the respiratory tract involve several areas rather than a single structure, although the effect on one may predominate in any given illness.
Etiology and Characteristics
Respiratory tract infections account for the majority of acute illnesses in children. The age of the child, season, living conditions, and preexisting medical problems influence the cause and course of these infections.
Infectious Agents
The respiratory tract is subject to a wide variety of infective organisms. Most infections are caused by viruses, particularly respiratory syncytial virus (RSV) and the parainfluenza viruses. Other agents involved in primary or secondary invasion include group A β-hemolytic streptococci (GABHS), staphylococci, Haemophilus influenzae, Chlamydia trachomatis, Mycoplasma organisms, and pneumococci.
Age
Healthy full-term infants under age 3 months are presumed to have a lower infection rate because of the protective function of maternal antibodies. The infection rate increases from age 3 to 6 months, the period between the disappearance of maternal antibodies and the infant’s own antibody production. The viral infection rate continues to remain high during the toddler and preschool years. By the time the child reaches 5 years of age, viral respiratory tract infections are less frequent, but the incidence of Mycoplasma pneumoniae and GABHS infections increases. The amount of lymphoid tissue increases throughout middle childhood, and repeated exposure to organisms confers increasing immunity as children grow older.
Some viral agents produce a mild illness in older children but cause severe lower respiratory tract illness or croup in infants. For example, pertussis causes a relatively harmless tracheobronchitis in childhood but is a serious disease in infancy.
Size
Anatomic differences influence the response to respiratory tract infections. The diameter of the airways is smaller in young children and subject to considerable narrowing from edematous mucous membranes and increased production of secretions. (See Fig. 31-5.) In addition, the distance between structures within the tract is shorter in the young child. Therefore organisms move more rapidly down the respiratory tract for more extensive involvement. The relatively short and open eustachian tube in infants and young children allows pathogens easy access to the middle ear.
Resistance
The ability to resist invading organisms depends on several factors. Deficiencies of the immune system place the child at risk for infection. Other conditions that decrease resistance are malnutrition, anemia, fatigue, and chilling of the body. Conditions that weaken defenses of the respiratory tract and predispose a child to infection include allergies (e.g., allergic rhinitis), bronchopulmonary dysplasia (chronic lung disease), asthma, cardiac anomalies that cause pulmonary congestion, and cystic fibrosis (CF). Daycare attendance, especially if the caregivers smoke, also increases the likelihood of infection.
Seasonal Variations
The most common respiratory tract pathogens appear in epidemics during the winter and spring months, but mycoplasmal infections occur more often in autumn and early winter. Infection-related asthma (e.g., asthmatic bronchitis) occurs more frequently during cold weather. Winter and spring are typically RSV season, or the time when children are indoors in close contact and more likely to spread the disease to each other.
Clinical Manifestations
Infants and young children, especially those between 6 months and 3 years of age, react more severely to acute respiratory tract infection than do older children. Young children display a number of generalized signs and symptoms and local manifestations that differ from those seen in older children and adults. Signs and symptoms associated with respiratory tract illnesses are outlined in Box 32-1. See Box 32-2 for components for assessing respiratory function.
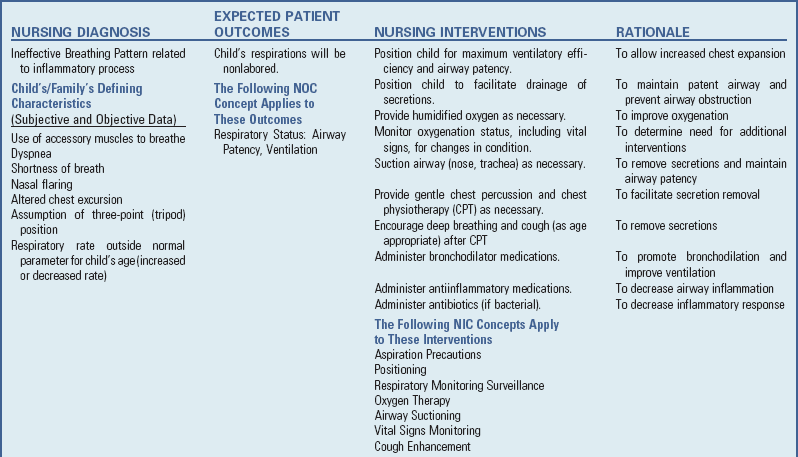
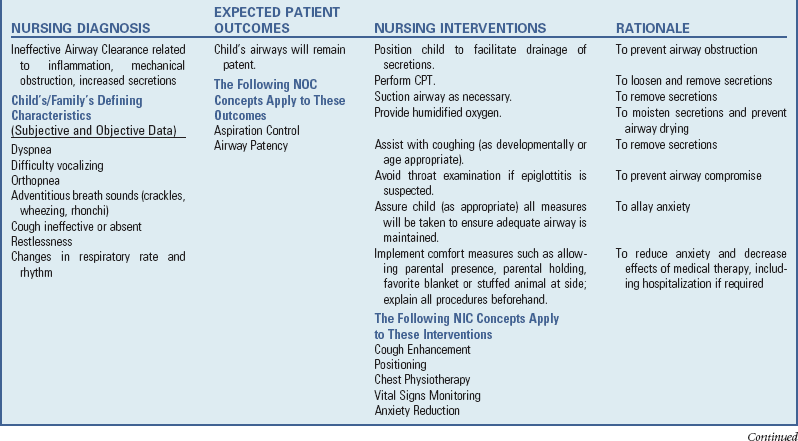
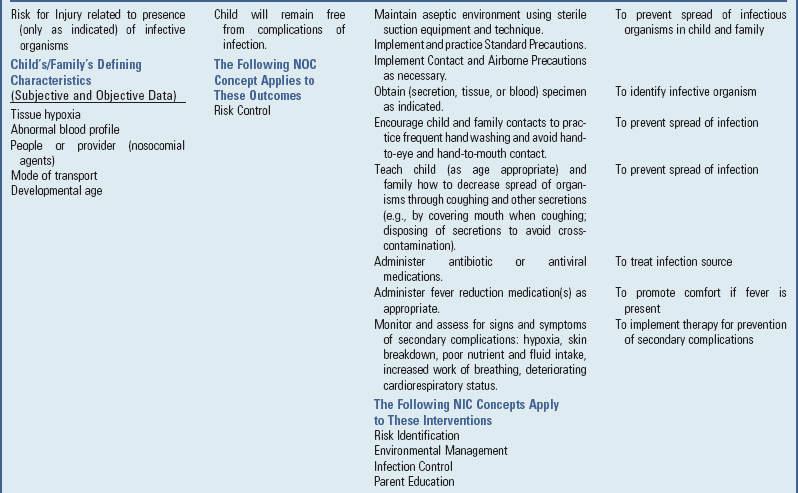
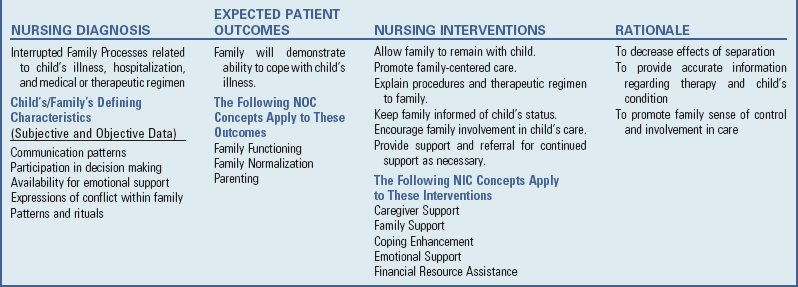
Nursing Care of the Child with a Respiratory Tract Infection
![]() Assessment of the respiratory system follows the guidelines described in Chapter 6 (for nose, mouth and throat, chest, and lungs). The assessment should include heart rate, respiratory rate, depth and rhythm, and hydration status. In addition to these, special attention is given to the observations outlined in Box 32-1, the components in Box 32-2, and assessment of the following:
Assessment of the respiratory system follows the guidelines described in Chapter 6 (for nose, mouth and throat, chest, and lungs). The assessment should include heart rate, respiratory rate, depth and rhythm, and hydration status. In addition to these, special attention is given to the observations outlined in Box 32-1, the components in Box 32-2, and assessment of the following:
![]() Nursing Care Plan—The Child with Acute Respiratory Tract Infection
Nursing Care Plan—The Child with Acute Respiratory Tract Infection
• Respiratory effort (respiratory rate, accessory muscle use, retractions, nasal flaring)
The nursing care of the child with a respiratory tract infection follows established guidelines based on the child’s and family’s individualized needs (see Nursing Care Plan).
Ease Respiratory Efforts
Many acute respiratory tract infections are mild and cause few symptoms. Although children may feel uncomfortable and have a stuffy nose and some mucosal swelling, acute respiratory distress occurs infrequently. The interventions described here are usually sufficient to relieve minor discomfort and ease respiratory efforts. However, children with croup or epiglottitis may develop sufficient swelling to obstruct the airway. These children may require hospitalization for observation and therapy.
Warm or cool mist is a common therapeutic measure for symptomatic relief of respiratory discomfort. The moisture soothes inflamed membranes and is beneficial when there is hoarseness or laryngeal involvement. Mist tents have been used in the hospital for humidifying the air and relieving discomfort but are seldom used in developed countries. The use of steam vaporizers in the home is often discouraged because of the hazards related to their use and limited evidence to support their efficacy. Shallow pans with wide surface areas for evaporation increase humidity, but parents should place them where they do not pose a safety hazard.
A time-honored method (but not evidence based) of producing steam is the shower. Running a shower of hot water into the empty bathtub or open shower stall with the bathroom door closed produces a quick source of steam. Keeping a child in this environment for 10 to 15 minutes may help ease respiratory efforts. A small child can sit on the lap of a parent or other adult. Older children can sit in the bathroom under the supervision of an adult.
Promote Rest
Children who have an acute febrile illness should be encouraged to rest and engage in quiet activities. Most children are self-limiting when febrile and increase activity as the fever subsides. Often children are more likely to stay quiet if they are allowed to lie quietly on a couch where they can watch television or participate in a quiet activity such as coloring or reading a book.
Promote Comfort
Older children are usually able to manage nasal secretions with little difficulty. Instruct parents in the correct administration of nose drops and throat gargles, if ordered. For very young infants, who normally breathe through their noses, an infant nasal aspirator or a rubber ear syringe is helpful in removing nasal secretions before feeding. This practice, in addition to instillation of saline nose drops, may clear nasal passages and promote feeding.
For older children who can tolerate decongestants, vasoconstrictive nose drops may be administered 15 to 20 minutes before feeding and at bedtime. Two drops are instilled, and, because this shrinks only the anterior mucous membranes, two more drops are instilled 5 to 10 minutes later. Phenylephrine (Neo-Synephrine) 0.25% and ephedrine 1% are frequently prescribed. Older cooperative children often prefer nasal sprays. They learn to compress the plastic container at the moment of inspiration. Spray bottles and bottles of nose drops should be used only for one child and only for one illness, since they become easily contaminated with bacteria. To avoid rebound congestion, nose drops or sprays should not be administered for more than 3 days.
Hot or cold applications sometimes provide relief for children with painful cervical adenitis. An ice bag or heating pad applied to the neck decreases the discomfort, but safety precautions must be observed to prevent burns. The ice bag or heating device must be covered, and the heating pad should not be set at high ranges.
Prevent Spread of Infection
Careful hand washing is carried out when caring for children with respiratory tract infections. Children and families should use a tissue or their hand to cover their nose and mouth when they cough or sneeze, dispose of the tissues properly, and wash their hands. Used tissues should be immediately thrown into the wastebasket, not allowed to accumulate in a pile. Children with respiratory tract infections should not share drinking cups, washcloths, or towels.
To decrease contamination with respiratory viruses, wash hands frequently and do not touch eyes or nose with hands.
Parents should try to remove affected children from contact with other children. Parents should also keep affected children out of school or daycare settings to prevent the spread of infection. Ideally, ill children should be isolated in a separate room at the first sign of illness. This may be a problem when living arrangements are crowded and the family has several children. An effort should be made to teach well children to stay away from ill children, to wash their hands frequently, and to avoid eating or drinking from the same utensils or cups.
Reduce Temperature
If the child has a significantly elevated temperature, controlling the fever is important. The parent should know how to take a child’s temperature and read the thermometer accurately. Nurses should not assume that all parents can read a thermometer; those who cannot require instruction.
If the practitioner has prescribed an antipyretic such as ibuprofen or acetaminophen (Tylenol), parents may need help administering the drug. Most parents can read the label and calculate the desired dose, but some may require careful instruction. It is important to emphasize accuracy in both the amount of drug given and the time intervals for drug administration to avoid cumulative effects. Parents should also be cautious of over-the-counter combination “cold” remedies, since these often include acetaminophen. Careful calculation of both the acetaminophen given separately and the acetaminophen in combination medications is necessary to avoid an overdose. To reduce the temperature and minimize the chances of dehydration, encourage cool liquids. (See Controlling Elevated Temperatures, Chapter 27.)
Promote Hydration
Dehydration is a potential complication when children have respiratory tract infections and are febrile or anorexic, especially when vomiting or diarrhea is present. Infants are especially prone to fluid and electrolyte deficits when they have a respiratory illness because a rapid respiratory rate that accompanies such illnesses precludes adequate fluid intake. In addition, the presence of fever increases the total body fluid turnover in infants. If the infant has nasal secretions, this further prevents adequate respiratory effort by blocking the narrow nasal passages when the infant reclines to bottle- or breast-feed and ceases the compensatory mouth breathing effort, thus causing the child to limit intake of fluids. Parents can encourage adequate fluid intake by offering small amounts of favorite fluids (clear liquids if vomiting) at frequent intervals. High-calorie liquids, such as colas, fruit juice drinks, water flavored and sweetened with corn syrup, or similar drinks, help prevent catabolism and dehydration but should be avoided if diarrhea is present. Oral rehydration solutions, such as Infalyte or Pedialyte, are beneficial for infants, and drinks such as sports drinks or those containing electrolytes are appropriate for older children. Fluids should not be forced, and children should not be awakened to take fluids unless the practitioner advises it. Forcing fluids may create the same difficulties as urging unwanted food (discussed below). Gentle persuasion with preferred beverages is usually successful. (See Chapter 28 for instructions on oral hydration.)
For infants who are breast- or bottle-feeding and who have a respiratory illness and secretions, encourage the parent to instill nasal saline drops and suction the passages with a bulb syringe before the feeding. This should alleviate some of the congestion and allow infants to nurse effectively.
To assess their child’s level of hydration (see Chapter 28), advise parents to observe the frequency of voiding and to notify the nurse or practitioner if there is insufficient voiding.
Provide Nutrition
Loss of appetite is characteristic of children with acute infections, and in most cases children can be permitted to determine their own need for food. Urging foods on anorexic children may precipitate nausea and vomiting and cause an aversion to feeding that can extend into the convalescent period and beyond. Many children show no decrease in appetite, and others respond well to foods such as gelatin, Popsicles, soup, and puddings. (See Feeding the Sick Child, Chapter 27.)
Provide Family Support and Home Care
Young children with respiratory tract infections are irritable and difficult to comfort. Therefore the family needs support, encouragement, and practical suggestions concerning comfort measures and administration of medication.
In addition to antipyretics and nose drops, the child may require antibiotic therapy. Parents of children receiving oral antibiotics need to understand the importance of administering the drug regularly and continuing it for the prescribed length of time, regardless of whether the child appears ill.
Also caution parents against giving the child any medications that are not approved by the health practitioner. Adverse effects have occurred in children who have received preparations intended for adults (e.g., some long-acting nose drops and dextromethorphan cough squares [mistaken for candy]). Caution parents about giving the child unprescribed antibiotics left over from a previous illness. Self-medication with unprescribed antibiotics can produce serious side effects, and the likelihood of adverse reactions is increased when medications are administered to children without consulting the practitioner. (See Chapter 27 for administration of medications and teaching parents.)
Upper Respiratory Tract Infections
A number of viruses, usually rhinoviruses, RSV, adenovirus, influenza virus, and parainfluenza virus, cause acute nasopharyngitis (the equivalent of the common cold).
Clinical Manifestations
Symptoms of nasopharyngitis are more severe in infants and children than in adults. Fever is common, especially in young children. Older children have low-grade fevers, which appear early in the illness. In children 3 months to 3 years, fevers occur suddenly and are associated with irritability, restlessness, decreased appetite and fluid intake, and decreased activity. Nasal inflammation may lead to obstruction of passages, producing open-mouth breathing. Vomiting and diarrhea may also be present.
The initial symptoms in older children are dryness and irritation of nasal passages and the pharynx, followed by sneezing, chilly sensations, muscular aches, an irritating nasal discharge, and sometimes cough. Nasal inflammation may lead to obstruction. Continual wiping away of secretions causes skin irritation to nares.
The disease is self-limiting and usually resolves within 4 to 10 days without complications. Occasionally fever recurs and a child (particularly an infant) might experience otitis media (OM), usually early or after the initial phase of nasopharyngitis is past. Pneumonia is less frequent but may be observed in some infants.
Therapeutic Management
Children with nasopharyngitis are managed at home. There is no specific treatment, and effective vaccines are not available. Antipyretics are prescribed for mild fever and discomfort. (See Chapter 27 for management of fever.) Rest is recommended until the child is free of fever for at least 1 day. Decongestants may be prescribed for children over 5 years of age to shrink swollen nasal passages. The decongestants that exert their effect by vasoconstriction are usually less effective when taken orally than when applied topically as nose drops. Because these drugs affect all vascular beds, they should be given with caution to children with diabetes.
Cough suppressants containing dextromethorphan may be prescribed for a dry, hacking cough. Some preparations contain up to 22% alcohol. They should not be administered to young children continuously and must be stored securely away from children.
Recent concerns regarding serious side effects of cough and cold preparations in young children, particularly infants, and lack of convincing evidence that such medications are effective in reducing symptoms, has prompted recommendations by health experts to carefully evaluate the benefits and risks of recommending such preparations for children under 6 years of age (Ryan, Brewer, and Small, 2008).
Antihistamines are largely ineffective in treatment of nasopharyngitis. These drugs have a weak atropine-like effect that dries secretions, but they can cause drowsiness or, paradoxically, have a stimulatory effect on children. There is no support for the usefulness of expectorants, and antibiotics are usually not indicated.
Prevention: Nasopharyngitis is so widespread in the general population that it is impossible to prevent. Children are more susceptible to colds because they have not yet developed resistance to many types of viruses. Very young infants are subject to relatively serious complications; therefore they should be protected from exposure.
Nursing Care Management
A cold is often the parents’ first introduction to an illness in their infants. Most discomfort of nasopharyngitis is related to the nasal obstruction, especially in small infants. Elevating the head of the bed or crib mattress assists with drainage of secretions; suctioning and vaporization may also provide relief. Saline nose drops and gentle suction with a bulb syringe, particularly before feeding, are useful.
Maintaining adequate fluid intake is essential during any infectious process. Although a child’s appetite for solid foods is usually diminished for several days, it is important to offer favorite fluids to prevent dehydration. Fluids can be cool or warm, depending on individual preference.
Because nasopharyngitis is spread from secretions, the best means for prevention is avoiding contact with affected persons. This goal is difficult when large numbers of people are confined in a small area for a long time, such as daycare centers and classrooms. Family members with a cold should try to “keep it to themselves” by carefully disposing of tissues and not sharing towels, glasses, or eating utensils. They should also cover the mouth and nose with tissues when coughing or sneezing; and wash hands thoroughly after nose blowing or sneezing. The most frequent carriers of infection are the human hands, which deposit viruses on doorknobs, faucets, and other everyday objects. Children should wash their hands thoroughly before putting them near their nose, mouth, or eyes.
Family Support: Support and reassurance are important elements of care for families of young children with recurrent upper respiratory tract infections (URIs). Because URIs are so frequent in children less than 3 years of age, families may feel they are on an endless roller coaster of illness. Reassure them that frequent colds are a normal part of childhood and that by 5 years of age, most children will have developed immunity to many viruses. Parents who work outside the home should expect to take time off to care for ill children during the fall and winter months. If the children are cared for routinely in daycare centers, the infection rate will be higher than if they are cared for in the home. Parents should know the signs of respiratory complications and should notify a health professional if any signs of complications appear or if the child does not improve within 2 or 3 days (Box 32-3).
Acute Streptococcal Pharyngitis
GABHS infection of the upper airway (strep throat) is not in itself a serious disease, but affected children are at risk for serious sequelae: acute rheumatic fever, an inflammatory disease of the heart, joints, and central nervous system (see Chapter 34); and acute glomerulonephritis, an acute kidney infection (see Chapter 30). Permanent damage can result from these sequelae, especially acute rheumatic fever. GABHS may also cause skin manifestations including, impetigo and pyoderma.
Scarlet fever may also occur as a result of a strain of group A streptococcus. The clinical manifestations of scarlet fever include pharyngitis and a characteristic erythematous sandpaper-like rash; otherwise scarlet fever shares the same clinical manifestations as those mentioned for GABHS, and treatment and sequelae are the same. Severe scarlet fever is rarely seen in the United States.
Clinical Manifestations
GABHS infection is generally a relatively brief illness that varies in severity from subclinical (no symptoms) to severe toxicity. The onset is often abrupt and characterized by pharyngitis, headache, fever, and abdominal pain. The tonsils and pharynx may be inflamed and covered with exudate (50% to 80% of cases) (Fig. 32-1), which usually appears by the second day of illness. However, streptococcal infections should be suspected in children over the age of 2 years who have pharyngitis even if no exudate is present.
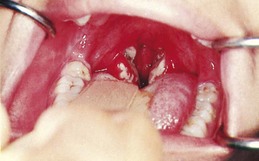
Fig. 32-1 Tonsillitis and pharyngitis. (Courtesy Dr. Edward L. Applebaum, Head, Department of Otolaryngology, University of Illinois Medical Center, Chicago.)
Anterior cervical lymphadenopathy (30% to 50% of cases) usually occurs early, and the nodes are often tender. Pain can be relatively mild to severe enough to make swallowing difficult. Clinical manifestations usually subside in 3 to 5 days unless complicated by sinusitis or parapharyngeal, peritonsillar, or retropharyngeal abscess. Nonsuppurative complications may appear after the onset of GABHS—acute nephritis in about 10 days and rheumatic fever in an average of 18 days.
Children who are GABHS carriers may have a positive throat culture but often experience a coincidental viral illness. Although antibiotic administration is not indicated for most GABHS carriers, some conditions require antibiotic therapy; these are published in the American Academy of Pediatrics (2009b) Red Book. Transmission to others from a carrier is reportedly minimal.
Diagnostic Evaluation
Although 80% to 90% of all cases of acute pharyngitis are viral, a throat culture should be performed to rule out GABHS. Because some children normally harbor streptococci in their throats, a positive culture is not always conclusive evidence of active disease. Most streptococcal infections are short-term illnesses, and antibody (antistreptolysin O) responses appear later than symptoms and are useful only for retrospective diagnosis.
Rapid identification of GABHS with diagnostic test kits is possible in the office or clinic setting. However, because these kits have questionable sensitivity, they are not yet considered a substitute for culture, and a confirmatory throat culture is recommended in patients who have a negative test result with a rapid diagnostic test kit (American Academy of Pediatrics, 2009b).
Therapeutic Management
If streptococcal sore throat infection is present, oral penicillin is prescribed in a dose sufficient to control the acute local manifestations and to maintain an adequate level for at least 10 days to eliminate organisms that might remain to initiate rheumatic fever symptoms. Penicillin does not prevent the development of acute glomerulonephritis in susceptible children. However, it may prevent the spread of a nephrogenic strain of GABHS to others in the family. Penicillin usually produces a prompt response within 24 hours. Some patients require retreatment if the organism is not eradicated.
Intramuscular (IM) penicillin G benzathine is also an appropriate therapy. This drug ensures adequate blood concentrations and avoids the problem of compliance, yet it is painful. Some preparations contain penicillin G procaine as well to decrease the pain. Oral erythromycin is indicated for children allergic to penicillin. Other drugs that have been used to treat GABHS pharyngitis include clarithromycin, azithromycin and clindamycin, oral cephalosporins, amoxicillin, and amoxicillin with clavulanic acid (American Academy of Pediatrics, 2009b).
Nursing Care Management
The nurse often obtains a throat swab for culture and instructs the parents about administering penicillin and analgesics as prescribed. Some children may prefer quiet activities during the acute phase of the illness, whereas others may limit activity only if the temperature is elevated. Cold or warm compresses to the neck may provide relief. In children old enough to cooperate, warm saline gargles offer some relief of throat discomfort. Pain may interfere with oral intake, and the child should not be forced to eat. Instead encourage cool liquids or ice chips, which are usually more acceptable than solids.
Special emphasis is placed on correctly administering oral medication and completing the course of antibiotic therapy. (See Administration of Medication, and Compliance, Chapter 27.) If an antibiotic injection is required, it must be administered deep into a large muscle mass (e.g., the vastus lateralis or ventrogluteal muscle). Parents need to be aware of the residual tenderness. Local applications of heat are helpful in relieving discomfort. (For other atraumatic strategies to reduce injection pain, such as application over the site of EMLA [a eutectic mix of lidocaine and prilocaine]  hours before the injection or LMX4 [lidocaine 4%] 30 minutes beforehand, see Administration of Medication: Intramuscular Administration, Chapter 27.)
hours before the injection or LMX4 [lidocaine 4%] 30 minutes beforehand, see Administration of Medication: Intramuscular Administration, Chapter 27.)
Prevention: No immunization is available for prevention of streptococcal disease. The organism is spread by close contact with affected persons—direct projection of large droplets or physical transfer of respiratory secretions containing the organism. Spread of infection is common in families, classrooms, and daycare centers. Children with streptococcal infection are noninfectious to others 24 hours after initiation of antibiotic therapy. It is generally recommended that children not return to school or daycare until they have been taking antibiotics for a full 24-hour period.
Nurses should remind children with a streptococcal throat infection to discard their toothbrush and replace it with a new one after they have been taking antibiotics for 24 hours.
It is important to know when the organism is epidemic in the community so that families can be alert for symptoms. Directors of daycare centers and school officials should share infectious disease information with parents. Obtaining throat cultures from children who are close family contacts of patients with streptococcal infection is advised.
Tonsillitis
![]() The tonsils are masses of lymphoid tissue located in the pharyngeal cavity. The tonsils filter and protect the respiratory and alimentary tracts from invasion by pathogenic organisms. They also play a role in antibody formation. Although the size of tonsils varies, children generally have larger tonsils than adolescents or adults. This difference is thought to be a protective mechanism, since young children are especially susceptible to URIs.
The tonsils are masses of lymphoid tissue located in the pharyngeal cavity. The tonsils filter and protect the respiratory and alimentary tracts from invasion by pathogenic organisms. They also play a role in antibody formation. Although the size of tonsils varies, children generally have larger tonsils than adolescents or adults. This difference is thought to be a protective mechanism, since young children are especially susceptible to URIs.
Pathophysiology
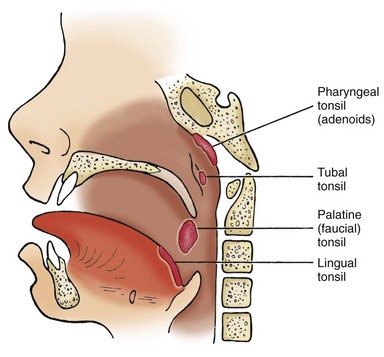
![]() Several pairs of tonsils are part of a mass of lymphoid tissue encircling the nasopharynx and oropharynx, known as the Waldeyer tonsillar ring (Fig. 32-2). The palatine, or faucial, tonsils are located on either side of the oropharynx, behind and below the pillars of the fauces (opening from the mouth). A surface of the palatine tonsils is usually visible during oral examination. The palatine tonsils are those removed during tonsillectomy. The pharyngeal tonsils, also known as the adenoids, are located above the palatine tonsils on the posterior wall of the nasopharynx. Their proximity to the nares and eustachian tubes causes difficulties in instances of inflammation. The lingual tonsils are located at the base of the tongue. The tubal tonsils, found near the posterior nasopharyngeal opening of the eustachian tubes, are not part of the Waldeyer tonsillar ring.
Several pairs of tonsils are part of a mass of lymphoid tissue encircling the nasopharynx and oropharynx, known as the Waldeyer tonsillar ring (Fig. 32-2). The palatine, or faucial, tonsils are located on either side of the oropharynx, behind and below the pillars of the fauces (opening from the mouth). A surface of the palatine tonsils is usually visible during oral examination. The palatine tonsils are those removed during tonsillectomy. The pharyngeal tonsils, also known as the adenoids, are located above the palatine tonsils on the posterior wall of the nasopharynx. Their proximity to the nares and eustachian tubes causes difficulties in instances of inflammation. The lingual tonsils are located at the base of the tongue. The tubal tonsils, found near the posterior nasopharyngeal opening of the eustachian tubes, are not part of the Waldeyer tonsillar ring.
Etiology
Tonsillitis often occurs with pharyngitis. Because of the abundant lymphoid tissue and the frequency of URIs, tonsillitis is a common cause of illness in young children. The causative agent may be viral or bacterial.
Clinical Manifestations
The manifestations of tonsillitis are caused by inflammation. As the palatine tonsils enlarge from edema, they may meet in the midline (kissing tonsils), obstructing the passage of air or food. The child has difficulty swallowing and breathing. When enlargement of the adenoids occurs, the space behind the posterior nares may become blocked, making it difficult or impossible for air to pass from the nose to the throat. As a result, the child breathes through the mouth.
If mouth breathing is continuous, the mucous membranes of the oropharynx become dry and irritated. There may be an offensive mouth odor and impaired senses of taste and smell. Because air cannot be trapped for proper speech sounds, the voice has a nasal and muffled quality. A persistent cough is also common. Because of the proximity of the adenoids to the eustachian tubes, this passageway is frequently blocked by swollen adenoids, interfering with normal drainage and frequently resulting in OM or difficulty hearing.
Therapeutic Management
Medical Treatment: Because the illness is self-limiting, treatment of viral pharyngitis is symptomatic. Throat cultures positive for GABHS infection require antibiotic treatment. It is important to differentiate between viral and streptococcal infection in febrile exudative tonsillitis. Because the majority of infections are of viral origin, early rapid tests can eliminate unnecessary antibiotic administration.
Surgical Treatment: Surgical treatment of chronic tonsillitis is controversial. Except in documented cases of recurrent, frequent streptococcal infection or a history of development of a peritonsillar abscess, tonsillectomy is not indicated in the child who has recurrent pharyngitis.
Tonsillectomy (surgical removal of the palatine tonsils) may be indicated for massive hypertrophy that results in difficulty breathing or eating. Absolute indications are malignancy and obstruction of the airway that result in cor pulmonale. Adenoidectomy (the surgical removal of the adenoids) is recommended for children who have hypertrophied adenoids that obstruct nasal breathing; additional indications for adenoidectomy include recurrent adenoiditis and sinusitis, OM with effusion, airway obstruction and subsequent sleep-disordered breathing, and recurrent rhinorrhea (Benninger and Walner, 2007). The American Academy of Otolaryngology–Head and Neck Surgery (2000) lists a number of indications for tonsillectomy, one of which is three or more infections of the tonsils or adenoids per year despite adequate medical therapy. For some children the effectiveness of tonsillectomy or adenoidectomy is modest and may not justify the risk of surgery. In practice, most primary care providers rely on individualized decision making and do not subscribe to an absolute set of eligibility criteria for these surgical procedures (Paradise, Bluestone, Colborn, et al, 2002) (see Research Focus box).
Contraindications to either tonsillectomy or adenoidectomy are (1) cleft palate, since both tonsils help minimize escape of air during speech; (2) acute infections at the time of surgery because the locally inflamed tissues increase the risk of bleeding; and (3) uncontrolled systemic diseases or blood dyscrasias.
Generally, removal of the tonsils should not occur until after 3 or 4 years of age because of the problem of excessive blood loss in young children and the possibility of regrowth or hypertrophy of lymphoid tissue. The tubal and lingual tonsils often enlarge to compensate for the lost lymphoid tissue, resulting in continued pharyngeal and eustachian tube obstruction.
Nursing Care Management
Nursing care of the child with tonsillitis involves providing comfort and minimizing activities or interventions that precipitate bleeding. A soft to liquid diet is generally preferred. A cool-mist vaporizer keeps the mucous membranes moist during periods of mouth breathing. Warm saltwater gargles, throat lozenges, and analgesic-antipyretic drugs such as acetaminophen are useful to promote comfort. Often opioids are needed to reduce pain for the child to drink. Combination nonopioid and opioid elixirs such as acetaminophen with codeine or with hydrocodone (Lortab) relieve pain and should be given routinely every 4 hours.
If surgery is needed, the child requires the same psychologic preparation and physical care as for any procedure. (See Chapters 26 and 27.) The following discussion focuses on specific nursing care for tonsillectomy and adenoidectomy (T&A), although both procedures may not be performed.
The nurse takes a complete history, with special notation of any bleeding tendencies because the operative site is highly vascular. Baseline vital signs are important for postoperative monitoring and observation. Signs of any URI are noted and reported, and bleeding and clotting times may be obtained with the usual laboratory work requests. During physical assessment the presence of any loose teeth is noted. (See Surgical Procedures, Chapter 27.)
After the surgery, until they are fully awake, children are placed on the abdomen or side to facilitate drainage of secretions. Suctioning is performed carefully to avoid trauma to the oropharynx. When alert, children may prefer sitting up, although they should remain in bed for the remainder of the day. They are discouraged from coughing frequently, clearing their throat, blowing their nose, or any activities that could aggravate the operative site.
Some secretions, particularly dried blood from surgery, are common. Inspect all secretions and vomitus for evidence of fresh bleeding (some blood-tinged mucus is expected). Dark brown (old) blood is usually present in the emesis, as well as in the nose and between the teeth. If parents are not prepared for this, they may be frightened at a time when they need to be calm and reassuring.
The throat is very sore after surgery. An ice collar may provide relief, but many children find it bothersome and refuse to use it. Most children experience moderate pain after a T&A and need pain medication for at least the first 24 hours. Analgesics may need to be given intravenously to avoid the oral route. Because pain is continuous, analgesics should be administered at regular intervals. Local anesthetics, such as tetracaine lollipops or ice pops, and antiemetics, such as ondansetron (Zofran) may be administered postoperatively. (See Pain Management, Chapter 7.)
Food and fluid are restricted until children are fully alert with no signs of hemorrhage. Cool water, crushed ice, flavored ice pops, or diluted fruit juice is given, and fluids with a red or brown color are avoided to distinguish fresh or old blood in emesis from the ingested liquid. Straws should be avoided, since these may damage the surgical site and cause subsequent bleeding. Citrus juice may cause discomfort and is usually poorly tolerated. Milk, ice cream, or pudding is not usually offered until clear fluids are retained because milk products coat the mouth and throat, causing the child to clear the throat, which may initiate bleeding.
Children often begin soft foods, particularly gelatin, cooked fruits, sherbet, soup, and mashed potatoes, on the first or second postoperative day or as the child tolerates feeding. The pain from surgery often inhibits intake, reinforcing the need for adequate pain control.
Postoperative hemorrhage is unusual but can occur. The nurse observes the throat directly for evidence of bleeding, using a good source of light and, if necessary, carefully inserting a tongue depressor. Other signs of hemorrhage are tachycardia, pallor, frequent clearing of the throat or swallowing by a younger child, and vomiting of bright red blood. Restlessness, an indication of hemorrhage, may be difficult to differentiate from general discomfort after surgery. Decreasing blood pressure is a much later sign of shock. A cream-colored membrane is often visible on the tonsillar bed postoperatively; reassure parents this is an expected finding.
Surgery may be required to cauterize or ligate a bleeding vessel. Airway obstruction may also occur as a result of edema or accumulated secretions and is indicated by signs of respiratory distress, such as stridor, drooling, restlessness, agitation, increasing respiratory rate, and progressive cyanosis. Suction equipment and oxygen should be available after tonsillectomy.
Family Support and Home Care: Discharge instructions include (1) avoiding foods that are irritating or highly seasoned, (2) avoiding the use of gargles or vigorous toothbrushing, (3) discouraging the child from coughing or clearing the throat or putting objects in the mouth, (4) using analgesics or an ice collar for pain, and (5) limiting activity to decrease the potential for bleeding. Hemorrhage may occur up to 10 days after surgery as a result of tissue sloughing from the healing process. Any sign of bleeding warrants immediate medical attention. Objectionable mouth odor and slight ear pain with a low-grade fever are common for a few days postoperatively. However, persistent severe earache, fever, or cough requires medical evaluation. Most children are ready to resume normal activity within 1 to 2 weeks after the operation.
Most children are admitted to a same-day surgery or ambulatory surgery unit and discharged home after a recovery period. T&A often represents the first hospitalization experience for the child and family. Because the surgery is usually an elective procedure, there is ample opportunity to prepare both children and parents for this event. Both need reassurance about what to expect at the time of admission, before and after surgery, and at discharge. Children are informed about postoperative discomfort and reassured that they will be able to talk. Some children believe the procedure will immediately “make the throat all better” and are dismayed to find that it still hurts after the surgery.
Infectious Mononucleosis
![]() Infectious mononucleosis is an acute, self-limiting infectious disease that is common among young people under 25 years of age. The disease is characterized by an increase in the mononuclear elements of the blood and by symptoms of an infectious process. The course is usually mild but occasionally can be severe or, rarely, accompanied by serious complications.
Infectious mononucleosis is an acute, self-limiting infectious disease that is common among young people under 25 years of age. The disease is characterized by an increase in the mononuclear elements of the blood and by symptoms of an infectious process. The course is usually mild but occasionally can be severe or, rarely, accompanied by serious complications.
Etiology and Pathophysiology
The herpeslike Epstein-Barr virus is the principal cause of infectious mononucleosis. It appears in both sporadic and epidemic forms, but the sporadic cases are more common. The virus is believed to be transmitted by direct contact with oral secretions, blood transfusion, or transplantation. It is mildly contagious, and the period of communicability is unknown. There is evidence that the virus is spread through sexual contact, especially when multiple partners are involved (Rimsza and Kirk, 2005). The incubation period after exposure in adolescents is estimated to be 30 to 50 days (American Academy of Pediatrics, 2009b).
Clinical Manifestations
Symptoms of infectious mononucleosis appear anywhere from 10 days to 6 weeks after exposure and may be acute or insidious. The common presenting symptoms vary greatly in type, severity, and duration. The characteristics of the disease are malaise, sore throat, and fever with generalized lymphadenopathy and splenomegaly that may persist for several months. Often the symptoms appear insidiously with fatigue, lack of energy, and sore throat. The child’s chief complaint is difficulty in maintaining the usual level of activity. This is often attributed to lack of sleep or a URI. In many instances the manifestations never arouse enough concern to bring the affected individual to medical attention. The clinical manifestations of infectious mononucleosis are usually less severe (often subclinical or unapparent) and the recovery phase is shorter in younger children than in older children and young adults. Many young children do not develop all the expected clinical and laboratory findings. Often a complication is the only or the presenting symptom.
A skin rash that involves a discrete macular eruption (most prominent over the trunk) is present in some cases and is often associated with the administration of ampicillin or amoxicillin. Other symptoms include headache, epistaxis, and a severe sore throat. The tonsils may be enlarged, reddened, and sometimes covered with a diphtheria-like membrane. In some cases airway compromise may occur with tonsillar swelling, requiring careful airway management, corticosteroids, humidified air, and intravenous (IV) hydration (Jenson, 2007). In about half the cases the spleen is enlarged. Splenic hemorrhage or rupture may occur but is usually related to trauma (Jenson, 2007). The extensive mononuclear infiltration produces symptoms related to any body tissue, and the clinical picture can resemble that of many conditions, including neurologic manifestations and cardiac involvement.
Diagnostic Evaluation
The diagnosis is established on the basis of clinical manifestations, increase in atypical leukocytes in a peripheral blood smear, and a positive heterophil agglutination test. Differential diagnosis depends on the clinical symptoms present. For example, the pharyngitis may simulate symptoms of diphtheria and streptococcal pharyngitis. Lymphadenopathy, fever, malaise, central nervous system manifestations, and skin eruptions may be similar to symptoms seen in a variety of conditions. The leukocyte count may be normal or low, but usually lymphocytic leukocytosis develops.
The heterophil antibody test determines the extent to which the patient’s serum will agglutinate sheep red blood cells. In infectious mononucleosis a titer of 1:160 is considered diagnostic, although a rising titer during the earlier stages is the best indicator. Because young children have a lower rate of heterophil antibody responses, the diagnosis may be overlooked in this group.
The spot test (Monospot) is a slide test of high specificity for the diagnosis of infectious mononucleosis. It is rapid, sensitive, inexpensive, and easy to perform, and it has the advantage that it can detect significant agglutinins at lower levels, thus permitting earlier diagnosis. Blood is usually obtained for the test by finger puncture and is placed on special paper. If the blood agglutinates, forming fragments or clumps, the test is positive for the infection.
Therapeutic Management
No specific treatment exists for infectious mononucleosis. Common symptoms are ordinarily relieved by simple remedies. A mild analgesic is usually sufficient to relieve the bothersome symptoms of headache, fever, and malaise. Bed rest is encouraged for fatigue but is not imposed for any specified time. Affected children and adolescents should regulate activities according to their own tolerance unless complicating factors are present. If the spleen is enlarged, children should avoid activities in which they might receive a blow to the abdomen or chest.
A short course of corticosteroids may assist in decreasing some of the complications (e.g., airway obstruction) of the illness. Administration of ampicillin or amoxicillin frequently precipitates a maculopapular rash in affected persons (80% of cases); therefore their use is contraindicated. Gargles; hot drinks; analgesic or anesthetic troches; or analgesics, including opioids, can relieve a sore throat. Although corticosteroids have been used to treat respiratory distress from tonsillar hypertrophy, hemolytic anemia, thrombocytopenia, and neurologic complications, the routine use of steroids is not recommended (American Academy of Pediatrics, 2009b).
Prognosis: The course of infectious mononucleosis is self-limiting and usually uncomplicated. Contrary to popular belief, mononucleosis is not necessarily a difficult, prolonged, or disabling disease, and the prognosis is generally good. Acute symptoms usually disappear within 7 to 10 days, and the persistent fatigue subsides within 2 to 4 weeks. A number of affected children or adolescents may need to restrict activities for 2 to 3 months; the disease rarely extends for longer periods.
Complications are uncommon but can be serious and require appropriate management. Neurologic complications occur in some outbreaks and vary in severity and outcome. These include seizures; ataxia; and perceptual distortions of shapes, spatial relationships, and sizes. Other complications include pneumonitis, myocarditis, hemolytic anemia, thrombocytopenia, and ruptured spleen. Rarely Reye syndrome or Guillain-Barré syndrome may develop following the acute phase of the illness (Jenson, 2007). Some evidence indicates a depressed cellular immune reactivity during the course of the disease and for some time afterward. Thus it is best to avoid live vaccines until several months after recovery.
Nursing Care Management
Direct nursing responsibilities toward providing comfort measures to relieve the symptoms and toward helping affected children and adolescents and their families determine appropriate activities for the stage of the disease and their interests. Airway assessment for impending obstruction during the acute phase of the illness is imperative. The adolescent with infectious mononucleosis may not be able to swallow secretions and may be in considerable pain. The child or adolescent is encouraged to increase clear fluid intake and decrease solid foods that may exacerbate the pain. In addition, the nurse should encourage the administration of age-appropriate antipyretics and encourage the affected individual to curtail activities that are strenuous until splenomegaly is resolved. Pain medications in elixir form such as acetaminophen with codeine or hydrocodone may be required during the acute phase so the adolescent can swallow liquids. Make every effort to prevent a secondary infection by counseling the adolescent to limit exposure to persons outside the family, especially during the acute phase of illness.
Influenza
Three of the orthomyxoviruses, which are antigenically distinct, cause the influenza, or “flu”: types A and B, which cause epidemic disease, and type C, which is unimportant epidemiologically. The viruses undergo significant changes from time to time. Major changes that occur at intervals of usually 5 to 10 years are called antigenic shift; minor variations within the same subtypes, antigenic drift, occur almost annually. Consequently, antigenic drift can alter the virus sufficiently to result in susceptibility of individuals to a type for which they were previously immunized or infected.
The disease is spread from one individual to another by direct contact (large-droplet infection) or by articles recently contaminated by nasopharyngeal secretions. There is no predilection for a specific age-group, but attack rates are highest in young children who have not had previous contact with a strain. It is frequently most severe in infants and older adults. During epidemics, infection among school-age children is believed to be a major source of transmission in a community. Influenza is more common during the winter months.
The disease has a 1- to 4-day incubation period, and affected persons are most infectious for 24 hours before and after the onset of symptoms. The virus has a peculiar affinity for epithelial cells of the respiratory tract mucosa, where it destroys ciliated epithelium with metaplastic hyperplasia of the tracheal and bronchial epithelium with associated edema. The alveoli may also become distended with a hyaline-like material. The viruses can be isolated from nasopharyngeal secretions early after the onset of infection, and serologic tests identify the type by complement fixation or the subgroups by hemagglutination inhibition.
H1N1 (swine flu) is a subtype of influenza type A. The current pandemic of H1N1 caused significant morbidity and mortality, particularly in Mexico and the United States. The signs and symptoms of H1N1 flu are the same as those mentioned below for influenza. A pandemic is defined by the World Health Organization as the spread of a new disease to which the population has little or no immunity and that spreads rapidly from human to human. In response to the 2009 H1N1 pandemic, the World Health Organization (2009) recommends that those infected with the virus be given either oseltamivir (Tamiflu) or zanamivir; a few isolated cases of H1N1 flu resistant to oseltamivir have been reported, but these are not believed to represent a significant threat. In the United States there are two vaccinations for H1N1: a live attenuated H1N1 influenza virus (LAIV) vaccine given intranasally, and an inactivated influenza (H1N1) monovalent vaccine given intramuscularly (Centers for Disease Control and Prevention, 2009b). Targeted candidates to receive the first supplies of vaccine available included pregnant women, persons ages 6 months to 24 years, health care and emergency workers, persons living with or providing care for infants less than 6 months of age, and persons ages 25 to 64 years who have medical conditions that place them at higher risk for influenza-related complications (Centers for Disease Control and Prevention, 2009b). The most updated information on the status of this disease may be found at the Centers for Disease Control and Prevention and World Health Organization websites: www.cdc.gov and www.who.int/csr/disease/swineflu/en/index.html.
Clinical Manifestations
The manifestations of influenza may be subclinical, mild, moderate, or severe. In most cases the throat and nasal mucosa are dry, and there is a dry cough and a tendency toward hoarseness. A sudden onset of fever and chills is accompanied by flushed face, photophobia, myalgia, hyperesthesia, and sometimes prostration. Subglottal croup is common, especially in infants. The symptoms last 4 or 5 days. Complications include severe viral pneumonia (often hemorrhagic); encephalitis; and secondary bacterial infections, such as OM, sinusitis, or pneumonia.
Therapeutic Management
Uncomplicated influenza in children usually requires only symptomatic treatment: acetaminophen or ibuprofen for fever and sufficient fluids to maintain hydration. Amantadine hydrochloride (Symmetrel) has been effective in reducing symptoms associated with type A disease if administered within 24 to 48 hours after their onset; the symptoms associated with influenza are reportedly shortened by 24 hours but the drug does not “cure” the disease. It is ineffective against type B or C influenza or other viral diseases. It should not be given to children under 1 year of age but is recommended for unvaccinated high-risk children. Since early 2006, however, there has been an increase in influenza strains resistant to amantadine, and thus the neuraminidase inhibitors oseltamivir and zanamivir have been recommended for influenza treatment (American Academy of Pediatrics, 2009b). A small number of influenza strains are resistant to oseltamivir.
Zanamivir and rimantadine have been approved for the treatment of flu symptoms in children under 18 years of age. Both medications must also be started within 48 hours of symptom onset. Zanamivir is an inhaled medication effective for type A and B influenza. The drug is taken twice daily for 5 days and is administered by a specially designed oral inhaler (Diskhaler). Zanamivir cannot be used for children less than 7 years of age except for specific prophylaxis indications in children ages 5 years and above (US Food and Drug Administration, 2009). Zanamivir is recommended for persons ages 7 years and above who have been exposed to H1N1 in 2009. A fourth drug, oseltamivir, is a neuroaminidase inhibitor that may be administered orally for 5 days to children over 3 months (and adults) to decrease the flu symptoms. As with other antiviral drugs, this must be taken within 2 days of the onset of symptoms. It is effective for types A and B influenza (American Academy of Pediatrics, 2009b). Bronchospasm and a decline in lung function can occur when zanamivir is used in patients with underlying airway disease such as asthma or chronic obstructive pulmonary disease. Rimantadine is effective only for type A virus; this drug is taken orally in tablet or syrup twice daily for 7 days. Rimantadine cannot be used for children less than 1 year of age. Children with influenza (or other similar viruses) should not receive aspirin because of its possible link with Reye syndrome.
Prevention: The influenza vaccine is now recommended annually for children 6 months to 18 years (completed). Influenza vaccine (trivalent inactivated influenza vaccine [TIV]) may be given to any healthy children 6 months old and older. The vaccine may be given simultaneously with other vaccines but at a separate site. TIV is administered yearly because different strains of influenza are used each year in the manufacture of the vaccine.
LAIV is an acceptable alternative to the IM trivalent vaccine in specific age-groups. Either TIV or LAIV may be given to healthy, nonpregnant persons ages 2 to 49 years (American Academy of Pediatrics, 2009b). Yearly influenza vaccine should be administered to children ages 6 to 59 months with medical conditions that place them at risk for influenza-related complications (including asthma, cardiac disease, human immunodeficiency virus [HIV], diabetes, and sickle cell disease) and to health care workers. (See Immunizations, Chapter 12.)
Nursing Care Management
Nursing care is the same as for any child with a URI, including helping the family implement measures to relieve symptoms. The greatest danger to affected children is development of a secondary infection. Prolonged fever or appearance of fever during early convalescence is a sign of secondary bacterial infection and should be reported to the practitioner for antibiotic therapy. In addition to the measures mentioned previously, nursing care of the child with influenza includes educating the parents regarding the prevention of the spread of the disease to other individuals, especially those who are at higher risk for complications, and educating the parents about the use of antiinfluenza medications. Parents are informed about the nature of antiviral drugs in regards to symptom management. Parents may also ask the practitioner to prescribe an antibiotic for the influenza, not understanding that these are ineffective against viral infections; indiscriminate use of antibiotics may lead to increased resistance to common antibiotics. The nurse should also educate parents regarding yearly influenza immunization and its effectiveness at decreasing morbidity among children.
Otitis Media
OM is one of the most prevalent illnesses of early childhood. Its incidence is highest in the winter months. Many cases of bacterial OM are preceded by a viral respiratory tract infection. The two viruses most likely to precipitate OM are RSV and influenza. Most episodes of acute otitis media (AOM) occur in the first 24 months of life, but the incidence decreases with age, except for a small increase at age 5 or 6 years when children enter school. OM occurs infrequently in children older than 7 years of age. Preschool-age boys are affected more frequently than preschool-age girls. Children who have siblings or parents with a history of chronic OM have a higher incidence of OM. Out-of-home daycare is a significant risk factor for OM.
Children living in households with many members (especially smokers) are more likely to have OM than those living with fewer persons. Passive smoking increases the risk of persistent middle ear effusion by enhancing attachment of the pathogens that cause otitis to the respiratory epithelium in the middle ear space, prolonging the inflammatory response, and impeding drainage through the eustachian tube (American Academy of Pediatrics, 2004a). Family socioeconomic status and extent of exposure to other children are the two most important identifiable risk factors for the occurrence of OM (American Academy of Pediatrics 2004a; Kershner, 2007).
OM has been defined in a variety of ways. The standard terminology is given in Box 32-4, and AOM and OM with effusion (OME) guidelines have been published (American Academy of Pediatrics, 2004a, 2004b).
Etiology
AOM is frequently caused by Streptococcus pneumoniae, H. influenzae, and Moraxella catarrhalis. The two viruses most likely to precipitate OM are RSV and influenza, although the adenoviruses, metapneumoviruses, and rhinoviruses also cause a significant number of URIs and OM. The etiology of the noninfectious type is unknown, although it is frequently the result of blocked eustachian tubes from the edema of URIs, allergic rhinitis, or hypertrophic adenoids. Chronic OM is frequently an extension of an acute episode.
A relationship has been observed between the incidence of OM and infant feeding methods. Infants fed breast milk have a lower incidence of OM compared with formula-fed infants. Breast-feeding may protect infants against respiratory viruses and allergy because it contains secretory immunoglobulin A, which limits the exposure of the eustachian tube and middle ear mucosa to microbial pathogens and foreign proteins. Reflux of milk up the eustachian tubes is less likely in breast-fed infants because of the semivertical positioning during breast-feeding compared with bottle-feeding.
Pathophysiology
OM is primarily a result of a dysfunctioning eustachian tube. The eustachian tube is part of a contiguous system composed of the nares, nasopharynx, eustachian tube, middle ear, and mastoid antrum and air cells. Eustachian tubes have three functions relative to the middle ear: (1) protection of the middle ear from nasopharyngeal secretions, (2) drainage of secretions produced in the middle ear into the nasopharynx, and (3) ventilation of the middle ear to equalize air pressure within the middle ear and atmospheric pressure in the external ear canal and replenishment of oxygen that has been absorbed.
Mechanical or functional obstruction of the eustachian tube causes accumulation of secretions in the middle ear. Infection or allergy can cause intrinsic obstruction. Extrinsic obstruction is usually a result of enlarged adenoids or nasopharyngeal tumors. Persistent collapse of the tube during swallowing can cause functional obstruction associated with decreased stiffness or an inefficient opening mechanism. Eustachian tube obstruction results in negative middle ear pressure and, if persistent, produces a transudative middle ear effusion. Sustained negative pressure and impaired ciliary transport within the tube inhibit drainage. When the passage is not totally obstructed, contamination of the middle ear can take place by reflux, aspiration, or insufflation during crying, sneezing, nose blowing, and swallowing when the nose is obstructed.
Several factors predispose infants and young children to development of OM (Box 32-5 and Fig. 32-3).
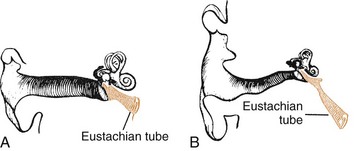
Fig. 32-3 Comparison of anatomic position of eustachian tube in a child (A) and an adult (B). Eustachian tube is shorter, wider, straighter, and more horizontal in a child than in an adult.
Complications: The consequences of prolonged middle ear disorders can be either functional or structural. The principal functional consequence is hearing loss, although loss in most children is conductive in nature and mild in severity. The causes of hearing loss are negative middle ear pressure, effusion in the middle ear, or structural damage to the tympanic membrane. However, the most feared consequence of hearing loss is its adverse effect on development of speech, language, and cognition. Children who have prolonged periods of middle ear effusion perform less well on speech and language tests than those who have few or no middle ear diseases.
Structural complications or sequelae involve primarily the tympanic membrane. Tympanic membrane retraction or retraction pockets occur in areas of low tensile strength or atrophic segments of the drum head when continued negative middle ear pressure draws the tympanic membrane inward. This retraction may result in impaired sound transmission, perforation of the thinned-out areas, or infection in the pockets and, later, cholesteatoma.
Tympanosclerosis (eardrum scarring) is the deposition of hyaline material into the fibrous layer of the tympanic membrane. It often occurs in children with inflammatory middle ear disease or those with repeated tympanoplasty tube placement. Eardrum perforation is a common complication in AOM and often accompanies chronic disease. Persistent perforation is a complication of tympanostomy tube placement. Surgery is required to close some perforations.
Adhesive OM (glue ear) is a thickening of the mucous membrane by proliferation of fibrous tissue that can cause fixation of the ossicles with a resultant hearing loss. Chronic suppurative OM, an inflammation of the middle ear and mastoid, is evidenced by perforation and discharge (otorrhea) for up to 6 weeks’ duration. Labyrinthitis, infection of the inner ear, and mastoiditis, infection of the mastoid sinus, are rare since the advent of antibiotic therapy. Meningitis and other suppurative intracranial conditions are possible complications of extension of infection from the middle ear or mastoid. However, these complications occur infrequently when adequate antibiotic therapy is implemented.
Cholesteatoma is the least common but most potentially dangerous sequela of OME. A cholesteatoma forms when the keratinizing, stratified, squamous epithelial cell lining desquamates to form scales that accumulate within the middle ear space. As it enlarges, the cholesteatoma erodes all structures it encounters, especially bone, destroying the ossicles and gaining entry to the inner ear and meninges. Clinical signs are a foul-smelling, grayish yellow discharge; sometimes pain; and permanent, progressive hearing loss. Treatment is surgical excision of the entire cholesteatoma.
Clinical Manifestations
As purulent fluid accumulates in the small space of the middle ear chamber, pain results from the pressure on surrounding structures. Infants become irritable and indicate their discomfort by holding or pulling at their ears and rolling their head from side to side. Young children usually verbally complain of the pain. A temperature as high as 40° C (104° F) is common, and postauricular and cervical lymph glands may be enlarged. Rhinorrhea, vomiting, diarrhea, and signs of concurrent respiratory tract or pharyngeal infection may also be present. Loss of appetite typically occurs, and sucking or chewing tends to aggravate the pain. In children with OME, exudate accumulates and pressure increases, with the potential for tympanic membrane rupture.
As a result of rupture, there is immediate relief of pain, a gradual decrease in temperature, and the presence of purulent discharge in the external auditory canal.
Severe pain or fever is usually absent in OME, and the child may not appear ill. Instead there is a feeling of “fullness” in the ear, a popping sensation during swallowing, and a feeling of “motion” in the ear if air is present above the level of fluid. Because chronic serous OM is the most frequent cause of conductive hearing loss in young children, audiometry may reveal deficient hearing.
Diagnostic Evaluation
Careful assessment of tympanic membrane mobility with a pneumatic otoscope is essential to differentiate AOM from OME (American Academy of Pediatrics, 2004a). If an accumulation of cerumen prevents adequate visualization of the tympanic membrane, the cerumen should be removed before inspection of the membrane. A diagnosis of AOM is made if visual inspection of the tympanic membrane reveals a purulent, discolored effusion and a bulging or full, opacified, or very reddened immobile membrane. Some practitioners also consider the presence of acute onset of less than 48 hours of ear pain with the preceding criteria to be a diagnostic factor in AOM (Powers, 2007). An immobile tympanic membrane or an orange-discolored membrane indicates OME. Clinical symptoms of otitis are also helpful in making the diagnosis. In AOM, symptoms such as acute ear pain, fever, and a bulging yellow or red tympanic membrane are usually present. In OME these symptoms may be absent, and other nonspecific symptoms such as rhinitis, cough, or diarrhea are often present.
Several tests provide an assessment of mobility of the tympanic membrane. Chapter 6, under Ears, discusses pneumatic otoscopy and tympanometry. Acoustic reflectometry measures the level of sound transmitted and reflected from the middle ear to a microphone located in a probe tip placed against the ear canal opening and directed toward the tympanic membrane. The information provides a measure of canal length and presence of effusion. The greater the cancellation of transmitted sound by reflected sound, the greater the probability of middle ear effusion.
Therapeutic Management: Acute Otitis Media
Treatment for AOM is one of the most common reasons for antibiotic use in the ambulatory setting. However, recent concerns about drug-resistant S. pneumoniae and other drug-resistant strains have led infectious disease authorities to recommend careful and judicious use of antibiotics for treatment of this illness. Current literature indicates that waiting up to 72 hours for spontaneous resolution is safe and appropriate management of AOM in healthy infants over 6 months and children (American Academy of Pediatrics, 2004a; Bhetwal and McConaghy, 2007). Furthermore some reviews of the treatment of AOM reveal no clear evidence that antibiotics improve outcomes in children younger than 2 years of age with uncomplicated AOM. However, the watchful waiting approach is not recommended for children younger than 2 years who have persistent acute symptoms of fever and severe ear pain (Kershner, 2007). In addition, all cases of AOM in infants younger than 6 months of age should be treated with antibiotics because of the infant’s immature immune system and the potential for infection with bacteria other than the three most common organisms found in older infants and children with AOM.
When antibiotics are necessary, oral amoxicillin in high doses (80 to 90 mg/kg/day, divided twice daily) is the treatment of choice for initial episodes of AOM in children who have not received antibiotics within the past month (American Academy of Pediatrics, 2004a; Bhetwal and McConaghy, 2007; Pichichero and Casey, 2005). The recommendation for the duration of antibiotic therapy is 10 days for severe AOM; for children with mild to moderate disease and those who are 6 years and older, a 5- to 7-day course is adequate (American Academy of Pediatrics, 2009b).
Second-line antibiotics used to treat OM include amoxicillin-clavulanate (Augmentin); azithromycin; and cephalosporins such as cefdinir, cefuroxime, and cefpodoxime. IM ceftriaxone is used if the causative organism is a highly resistant pneumococcus or if there is noncompliance with the therapy. An important consideration with the use of single-dose IM injections is the pain involved in this therapy. One strategy to minimize pain at the injection site is to reconstitute the cephalosporin with 1% lidocaine (without epinephrine). The use of steroids, decongestants, and antihistamines to treat AOM is not recommended.
Supportive care or symptomatic treatment of AOM includes treating the fever and pain. For fever or discomfort associated with OM, analgesic-antipyretic drugs such as acetaminophen or ibuprofen (ibuprofen only if >6 months of age) may be given. Topical pain relief is recommended by external application of heat or cold, or the practitioner may prescribe topical pain relief drops such as benzocaine drops. Antibiotic ear drops have no value in treating AOM.
Children with AOM should be seen after antibiotic therapy is complete to evaluate the effectiveness of the treatment and to identify potential complications, such as effusion or hearing impairment.
Myringotomy, a surgical incision of the eardrum, may be necessary to alleviate the severe pain of AOM. A myringotomy is also performed to drain infected middle ear fluid in the presence of complications (mastoiditis, labyrinthitis, or facial paralysis) or to allow purulent middle ear fluid to drain into the ear canal for culture. A minimally invasive laser-assisted myringotomy procedure may be performed in outpatient settings. These procedures should only be performed by ear, nose, and throat (ENT) specialists.
Therapeutic Management: Recurrent Otitis Media
Therapy for recurrent AOM has included chemoprophylaxis with long-term antibiotic therapy, immunotherapy, and surgery. Children receiving long-term antibiotic therapy should be evaluated once a month to detect any evidence of effusion. Any acute infection during prophylaxis is treated with an alternate antibiotic regimen.
Tympanostomy tube placement and adenoidectomy are surgical procedures that may be done to treat recurrent OM. Tympanostomy tubes are pressure-equalizer (PE) tubes or grommets that facilitate continued drainage of fluid and allow ventilation of the middle ear. Adenoidectomy is not recommended for treatment of AOM and is performed only in children with recurrent AOM or chronic OME with postnasal obstruction, adenoiditis, or chronic sinusitis.
Therapeutic Management: Otitis Media with Effusion
In some children, residual middle ear effusions remain after episodes of AOM. Management options for OM with residual effusion include observation, antibiotics alone, or a combination of antibiotic and corticosteroid therapy. Antibiotics are not required for initial treatment of OME but may be indicated for children with persistent effusion for more than 3 months (American Academy of Pediatrics, 2004a). It has been estimated that avoiding unnecessary treatment of OME with antibiotics would save millions of courses of antibiotics each year (American Academy of Pediatrics, 2004a).
Some children have fluid that persists in the middle ear for weeks or months. OME is frequently associated with mild to moderate hearing impairment. The major goal of therapy is to establish and maintain an aerated middle ear that is free of fluid with a normal mucosa and ultimately to achieve normal hearing.
Placement of tympanostomy tubes is recommended after a total of 4 to 6 months of bilateral effusion with a bilateral hearing deficit (American Academy of Pediatrics, 2004b). This therapy allows for mechanical drainage of the fluid, which promotes healing of the membrane and prevents scar formation and loss of elasticity. The primary objective is to allow the eustachian tube a period of recovery while the surgically placed tube performs its functions. The surgery is relatively benign; however, sometimes the tubes become plugged and they often require reinsertion. Complications of repeated or long-term tube placement are tympanosclerosis, localized or diffuse atrophy of the membrane, persistent perforation, or, rarely, cholesteatoma. Myringotomy with or without insertion of PE tubes should not be performed for initial management of OME, but may be recommended for children who have recurrent episodes of OME with a long cumulative duration. A Cochrane review concluded that tympanostomy tubes had a significant effect on decreasing the incidence of AOM in the first 6 months after insertion (McDonald, Langston Hewer, and Nunez, 2008).
Tonsillectomy, either alone or with adenoidectomy, is not considered an effective treatment of OME (American Academy of Pediatrics, 2004b). According to guidelines published by the Agency for Healthcare Research and Quality,* steroids are not recommended for treatment of OME in children of any age.
Prevention
Routine immunization with the pneumococcal conjugate vaccine PCV 7 (Prevnar) has reduced the incidence of AOM in some infants and children (American Academy of Pediatrics, 2009b). In 2010 the FDA approved a new conjugate vaccine, Prevnar 13, which replaces Prevnar. The vaccine is administered as a four-dose series beginning at 2 months of age; infants and children who have started the series with Prevnar may complete the series with Prevnar 13 (Centers for Disease Control and Prevention, 2010).
Parents can reduce risk factors for AOM by breast-feeding infants for at least the first 6 months of life, avoiding propping the bottle, decreasing or discontinuing pacifier use after 6 months, and preventing exposure to tobacco smoke (American Academy of Pediatrics, 2004a).
Prognosis
Most cases of OM resolve eventually. However, hearing loss, typically conductive, is a common complication of OM. The degree of hearing loss can vary from none to severe. Although conductive hearing loss is most often associated with OM, sensorineural hearing loss may also be present, especially in severe forms of chronic or recurrent OM, because of the passage of toxic products from fluids into the cochlea through the tympanic membrane. The longer the fluid is present, the greater the sensorineural hearing loss. Children who are prone to OM should be referred to a pediatric otolaryngologist and possibly a pediatric allergist for identification and treatment of the cause of their eustachian tube dysfunction. They should also be referred to a speech and language pathologist for primary prevention counseling. In addition, the child should ideally be monitored by an audiologist to evaluate the adequacy of hearing.
Nursing Care Management
Nursing objectives for the child with AOM include (1) relieving pain, (2) facilitating drainage when possible, (3) preventing complications or recurrence, (4) educating the family in care of the child, and (5) providing emotional support to the child and family.
Analgesics are helpful to reduce severe earache. High fever, particularly in infants, should be reduced with antipyretic drugs. An advantage of using ibuprofen rather than acetaminophen is its longer duration of action (about 6 hours), especially for nighttime comfort. Ibuprofen is only indicated for those over 6 months of age. For more severe pain, the Centers for Disease Control and Prevention and American Academy of Pediatrics guidelines recommend a stronger analgesic such as codeine. The application of heat may reduce pain in some children but may aggravate discomfort in others. Local heat should be placed over the ear while the child lies on the affected side. This position also facilitates drainage of the exudate if the eardrum has ruptured or if myringotomy was performed.
If the ear is draining, the external canal may be cleaned with sterile cotton swabs or pledgets coupled with topical antibiotic treatment. If ear wicks or lightly rolled sterile gauze packs are placed in the ear after surgical treatment, they should be loose enough to allow accumulated drainage to flow out of the ear; otherwise the infection may be transferred to the mastoid process. Parents should keep these wicks dry during shampoos or baths. Occasionally drainage is so profuse that the pinna and surrounding skin become excoriated from exudate. Frequent cleansing and application of various moisture barriers (e.g., Aloe Vesta Protective Ointment, Proshield Plus Skin Protectant), zinc oxide, or petrolatum jelly (e.g., Vaseline) can prevent this.
Parents require anticipatory guidance regarding temporary hearing loss that accompanies OM. For example, they may need to speak louder, at closer proximity, and while facing the child. They are reminded that the child is not ignoring them. The child may not be able to localize where a sound is coming from because awareness and understanding of speech are reduced either unilaterally or bilaterally, depending on the degree of hearing deficit. The schoolteacher may also need to place the child closer to the front of the class if hearing has been impaired or if the teacher believes the child is not hearing all of the information being given in class. The family should also be aware of possible behavioral changes with hearing loss, including inattentiveness to or lack of awareness of environmental sounds; requests for repetition in conversation or mishearing of content; softer or louder voice than usual; poor attention span and fidgety behavior when in a group listening situation (e.g., classroom); aggressiveness and low frustration tolerance because of frequent communication breakdowns; and impaired speech and language skills. Persistent difficulty in hearing beyond the acute stage should be evaluated.
Preventing recurrence requires adequate parent education regarding antibiotic therapy. Because the symptoms of pain and fever usually subside within 24 to 48 hours, nurses must emphasize that, although the child may appear well, the infection is not completely eradicated until all the prescribed medication is taken. It is important to stress the potential complications of OM, especially hearing loss, which can be prevented with adequate treatment and follow-up care. (See Administration of Medication, and Compliance, Chapter 27.)
Tympanostomy tubes may allow water to enter the middle ear, but recommendations for earplugs are inconsistent. Research indicates that swimming without earplugs poses a slightly increased risk of infection (Goldstein, Mandel, Kurs-Lasky, et al, 2005). Moreover, lake water is contaminated, and wearing earplugs while swimming in a lake prevents total flooding of the external canal. Parents should keep bathwater and shampoo water out of the ear, if possible, since soap reduces the surface tension of water and facilitates entry through the tube. Parents should be aware of the appearance of a grommet (usually a tiny, white, plastic spool-shaped tube) so they can recognize it if it falls out. They are reassured that this is normal and requires no immediate intervention, although they should notify the practitioner.
Parents sometimes ask about preventing ear discomfort in their infants during ascent or descent of an airplane. During ascent, air in the middle ear expands, but decompression takes place through a normal eustachian tube. If the tissues are congested with a URI, the passage of air may be blocked. A nasal mucosa–shrinking spray or oral decongestant before the trip may be helpful. During descent, the air within the middle ear decreases as atmospheric pressure increases. Swallowing is the simplest and most effective method for inflating the middle ear on descent; therefore feeding or offering a pacifier to infants during descent is beneficial.
Reducing the chances of OM is possible with simple measures, such as sitting or holding an infant upright for feedings. Propping bottles is discouraged to avoid pooling of milk while the child is in the supine position and to encourage human contact during feeding. Eliminating tobacco smoke and known allergens is also recommended. Forceful nose blowing during a URI is discouraged to avoid forcing organisms to ascend through the eustachian tube. Early detection of middle ear effusion is essential in prevention of complications. Infants and preschool children should be screened for effusion, and all schoolchildren, especially those with learning disabilities, should be tested for middle ear effusion. Frequent audiologic evaluations, medical consultation, and education of parents and children are advised when middle ear effusion is detected.
Otitis Externa
Infections of the external ear result from normal ear flora (Staphylococcus epidermidis and Corynebacterium organisms) that assume pathogenic characteristics under conditions of excessive wetness or dryness. Ordinarily the external ear canal is protected by a waxy, water-repellent coating composed of highly viscid secretions of the sebaceous glands and the watery, pigmented secretions of apocrine glands in combination with exfoliated surface cells. Inflammation occurs when this environment is altered by swimming, bathing, or increased environmental humidity; by infection, dermatoses, or insufficient cerumen; or by trauma from a foreign body (FB) or a finger.
Secondary invasion of foreign pathogens also occurs. In addition to the resident flora, the offending agents can be Pseudomonas aeruginosa (most common), Enterobacter aerogenes, Proteus mirabilis, Klebsiella pneumoniae, streptococci, and fungi such as Candida and Aspergillus organisms. The ear canal becomes irritated, and maceration takes place.
The predominant symptom of external ear infection, or swimmer’s ear, is ear pain accentuated by manipulation of the pinna, especially pressure on the tragus. The pain often appears to be out of proportion to the degree of inflammation. Conductive hearing loss may be present as a result of the edema, secretions, and accumulation of debris within the canal. Edema, erythema, a cheesy green-blue-gray discharge, and tenderness appear as the infection progresses. The external canal may be so tender and swollen that visualization is difficult. There may be fever. In advanced cases the pain is intense, constant, and aggravated by jaw motion or ear manipulation.
Therapeutic objectives include relief of pain, edema, and itching and restoration of normal flora, cerumen, and canal epithelium. Analgesics are prescribed for pain. Debris is removed with gentle suction and wisps of cotton on metal cotton carriers. Otic preparations containing neomycin with either colistin or polymyxin and corticosteroids are instilled in the canal. A gauze wick may be inserted if edema is present to facilitate the medication reaching the site of inflammation. The wick is removed after swelling and pain have subsided, but the drops are continued for at least 3 days after relief of pain. The best management for external ear inflammation is prevention.
Nursing Care Management
Nurses can teach parents or patients simple steps to prevent recurrent infections. Children should limit their stay in the water to less than an hour, if possible, and ears should dry completely (1 to 2 hours) before entering the water again. The ear canal can also be dried with a small tuft of cotton (not a swab). Placing a combination of white vinegar and rubbing alcohol (50:50) in both ear canals on arising, at bedtime, and at the end of each swim is effective in preventing recurrence. A 2% acetic acid solution may also be used. The solution should remain in the canal for 5 minutes. Caution children not to pick at the ears with a pencil, cotton swab, bobby pin, or other object, which can injure or infect the ear canal.
Croup Syndromes
Croup is a general term applied to a group of symptoms characterized by hoarseness, a resonant cough described as “barking” or “brassy” (croupy), varying degrees of inspiratory stridor, and varying degrees of respiratory distress resulting from swelling or obstruction in the region of the larynx. Acute infections of the larynx are of greater importance in infants and small children than they are in older children because of the increased incidence in children in this age-group and the smaller diameter of the airway, which renders it subject to significantly greater narrowing with the same degree of inflammation (Fig. 32-4). With widespread immunization programs aimed at preventing H. influenzae type b, most cases of croup in the United States are attributed to viruses, namely parainfluenza virus, influenza types A and B, adenovirus, RSV, and measles (Roosevelt, 2007).
Croup is a common respiratory disease of childhood and occurs more often in boys than in girls. The number of croup cases increases in the late autumn through early winter months and occurs primarily in children 6 months to 3 years of age. Hospitalization may be necessary for some children with croup, and a small percentage of hospitalized children require intubation.
Croup syndromes affect to varying degrees the larynx, trachea, and bronchi. However, laryngeal involvement often dominates the clinical picture because of the severe effects on the voice and breathing. Croup syndromes are usually described according to the primary anatomic area affected (i.e., epiglottitis [or supraglottitis], laryngitis, laryngotracheobronchitis [LTB], and tracheitis). In general, LTB tends to occur in very young children, whereas epiglottitis is more characteristic of older children. Table 32-1 gives a comparison of croup syndromes.
Because croup is one of the most benign conditions causing upper airway obstruction, it is vitally important to correctly identify it and distinguish the type of croup syndrome or condition (i.e., spasmodic croup or LTB as opposed to a potentially life-threatening condition such as epiglottitis, bacterial tracheitis, FB aspiration, or a peritonsillar abscess). The key differences between LTB and epiglottitis are the absence of cough, the presence of dysphagia, and the high degree of toxicity in children with epiglottitis. Children with epiglottitis usually look worse than they sound, in contrast to children with LTB, who sound worse than they look (see Critical Thinking Exercise).
Acute Epiglottitis
![]() Acute epiglottitis, or acute supraglottitis, is a serious obstructive inflammatory process that occurs principally in children between 2 and 5 years of age but can occur from infancy to adulthood. The disorder is a medical emergency and requires immediate medical attention. The obstruction is supraglottic, as opposed to the subglottic obstruction of laryngitis. LTB and epiglottitis do not occur together.
Acute epiglottitis, or acute supraglottitis, is a serious obstructive inflammatory process that occurs principally in children between 2 and 5 years of age but can occur from infancy to adulthood. The disorder is a medical emergency and requires immediate medical attention. The obstruction is supraglottic, as opposed to the subglottic obstruction of laryngitis. LTB and epiglottitis do not occur together.
Clinical Manifestations
The onset of epiglottitis is abrupt, less often preceded by cold symptoms and more often by a sore throat. It can rapidly progress to severe respiratory distress. The child usually goes to bed asymptomatic to awaken later complaining of sore throat and pain on swallowing. The child has a fever and appears sicker than clinical findings suggest. The child insists on sitting upright and leaning forward (tripod position), with the chin thrust out, mouth open, and tongue protruding. Drooling of saliva is common because of the difficulty or pain on swallowing and excessive secretions.
The child is irritable and extremely restless and has an apprehensive and frightened expression. The voice is thick and muffled, with a froglike croaking sound on inspiration. The child is not hoarse. Suprasternal and substernal retractions may be visible. The child seldom struggles to breathe, and slow, quiet breathing provides better air exchange. The sallow color of mild hypoxia may progress to frank cyanosis if treatment is delayed. The throat is red and inflamed, and a distinctive, large, cherry red, edematous epiglottis is visible on careful throat inspection. Throat inspection should only be performed when emergency resuscitation personnel and equipment are available.
Therapeutic Management
Epiglottitis may develop suddenly, with respiratory obstruction appearing rapidly. Progressive obstruction leads to hypoxia, hypercapnia, and acidosis followed by decreased muscular tone; reduced level of consciousness; and, when obstruction becomes more or less complete, sudden death. A presumptive diagnosis of epiglottitis constitutes an emergency.
The child is best transported while sitting in a parent’s lap to reduce distress. Examination of the throat with a tongue depressor is contraindicated until experienced personnel and equipment are at hand in the event that the examination precipitates further or complete obstruction. Immediate intubation or tracheotomy may need to be performed.
When a lateral neck radiograph of the soft tissues is indicated, the same experienced personnel should accompany the child to the radiology department. For a young child who is likely to become more agitated by the procedure, it is preferable that the child not be transported but remain on the parent’s lap in the examination area during portable radiology.
Nasotracheal intubation or tracheotomy is usually considered for the child with epiglottitis with severe respiratory distress. It is recommended that the intubation or tracheotomy and any invasive procedure, such as starting an IV infusion, be performed in an area where emergency airway maintenance can be easily and quickly accomplished. Humidified oxygen is administered as necessary either via mask in older children or as blow-by in younger children to avoid further agitation. Whether or not there is an artificial airway, the child requires intensive observation by experienced personnel. The epiglottal swelling usually decreases after 24 hours of antibiotic therapy, and the epiglottis is near normal by the third day.
Children with suspected bacterial epiglottitis are given antibiotics intravenously, followed by oral administration to complete a 7- to 10-day course. The use of corticosteroids for reducing edema may be beneficial during the early hours of treatment. Most intubated children have a course of corticosteroids for 24 hours before extubation.
Prevention: The American Academy of Pediatrics (2009b) recommends that all children, beginning at 2 months of age, receive the H. influenzae type b conjugate vaccine. Since administration of the vaccine has become a routine part of the immunization schedule, the incidence of epiglottitis has declined. Patients now tend to be older and have disease caused by viral agents. (See Immunizations, Chapter 12.)
Nursing Care Management
Epiglottitis is a serious and frightening disease for the child, family, and health professionals. It is important to act quickly but calmly and provide support without unduly increasing anxiety. The child is allowed to remain in the position that provides the most comfort and security, and parents are reassured that everything possible is being done to obtain relief for their child.
Acute care of the child is the same as that described for the child with acute respiratory distress and artificial airways in Chapter 31. Continuous monitoring of respiratory status, including pulse oximetry (or blood gases if the patient is intubated), is part of nursing observations, and the IV infusion is maintained. (See Chapter 28.)
Acute Laryngitis
Acute infectious laryngitis is a common illness in older children and adolescents. Infants and smaller children experience more generalized involvement. (See next section on LTB.) Viruses are the usual causative agents, and the principal complaint is hoarseness, which may be accompanied by other upper respiratory symptoms (e.g., coryza, sore throat, nasal congestion) and systemic manifestations (e.g., fever, headache, myalgia, malaise). Other complaints vary with the infecting virus. For example, adenoviruses and influenza viruses are responsible for more systemic involvement; parainfluenza virus, rhinoviruses, and RSV cause more mild illness.
Acute Laryngotracheobronchitis
LTB is the most common type of croup experienced by children admitted for hospitalization and primarily affects children less than 5 years of age. Organisms responsible for LTB are the parainfluenza virus type 1, followed by parainfluenza virus types 3 and 2, RSV, influenza types A and B, measles and M. pneumoniae. The illness is usually preceded by a URI, which gradually descends to adjacent structures. It is characterized by the gradual onset of low-grade fever, and the parents often report that the child went to bed and later awoke with a barky, brassy cough and at times inspiratory stridor. Symptoms are typically worse at night, and agitation and crying tend to exacerbate the symptoms.
Inflammation of the mucosa lining the larynx and trachea causes a narrowing of the airway. When the airway is significantly narrowed, the child struggles to inhale air past the obstruction and into the lungs, producing the characteristic inspiratory stridor and suprasternal retractions. Other classic manifestations include cough and hoarseness. Respiratory distress in infants and toddlers may be manifested by nasal flaring, intercostal retractions, tachypnea, and continuous stridor. The typical child with LTB is a toddler who develops the classic barking or seal-like cough and acute stridor after several days of coryza. The degree of respiratory distress varies; hypoxia and decreased oxygen saturations are observed primarily when complete airway obstruction is imminent. Radiographs are not helpful in establishing the diagnosis of LTB.
Therapeutic Management
The major objective in medical management of infectious LTB is maintaining an airway and providing for adequate respiratory exchange. Children with mild croup (no stridor at rest) are managed at home. Parents need to learn the signs of respiratory distress so that they can call professional help if needed. Children whose symptoms progressively get worse should receive medical attention.
Cool mist provides relief for most children, although there is no substantial evidence to its efficacy. A cool-air vaporizer can be used at home. In the hospital mist may be provided with a face mask or as blow-by. Controversy surrounds the use of mist therapy to treat croup. Studies have failed to demonstrate any improvement in subglottic edema with mist therapy (Moore and Little, 2006).
The cool-temperature therapy modalities assist by constricting edematous blood vessels. In the home environment, suggestions to provide cool air include taking the child outside to breathe in cool night air, using a cold-water vaporizer or humidifier, standing in front of the open freezer, and taking the child to a cool basement or garage. Although these are often recommended, there is no evidence to unconditionally support their use.
Nebulized epinephrine (racemic epinephrine) is now used in children with croup that is not alleviated with cool mist. The α-adrenergic effects cause mucosal vasoconstriction and subsequent decreased subglottic edema. The onset of action is rapid. Peak effect is observed in less than 2 hours. Additional doses may be administered every 20 to 30 minutes as needed. Close observation of patients receiving nebulized racemic epinephrine is critical to detect the reappearance of symptoms, monitor the response to therapy, and note any deterioration in respiratory status. There is evidence that use of l-epinephrine is just as effective as racemic epinephrine but without the side effects of tachycardia and hypertension (Roosevelt, 2007). The patient who has received racemic epinephrine for croup should be observed for 2 to 3 hours for any visible signs of respiratory distress.
The use of corticosteroids is beneficial because the antiinflammatory effects decrease subglottic edema. Oral steroids are effective in the treatment of croup. IM dexamethasone may be given to children who are unable to tolerate oral dosing. Nebulized budesonide may be administered in conjunction with IM dexamethasone. A single dose of oral corticosteroid has shown to decrease hospitalizations and the need for multiple racemic epinephrine treatments in children with mild croup (Roosevelt, 2007). The onset of action is clinically detectable as early as 6 hours after administration, with continued improvement over a period of 12 to 24 hours.
In severe cases of LTB the administration of a mixture of helium and oxygen (heliox) may reduce the work of breathing and relieve the airway obstruction. Because helium has a lower density than room air, it forms a respirable gas (with oxygen) that reduces airway turbulence. The use of heliox, however, has not proved to be more effective than standard treatments and is currently not recommended as a standard management for croup (Myers, 2006; Vorwek and Coats, 2008).
It is essential to allow children with mild croup to continue to drink beverages they like and to encourage parents to use comforting measures with their child (e.g., holding, rocking, walking, singing). If the child is unable to take oral fluids, IV fluid therapy may be indicated.
Nursing Care Management
The most important nursing function in the care of children with LTB is continuous, vigilant observation and accurate assessment of respiratory status. Cardiac, respiratory, and pulse oximetry monitoring supplement visual observations. Changes in therapy are frequently based on nurses’ observations and assessment of a child’s status, response to therapy, and tolerance of procedures. The trend away from early intubation of children with LTB emphasizes the importance of nursing observation and the ability to recognize impending respiratory failure so that intubation can be implemented without delay. Intubation equipment and bag and valve mask equipment should be readily accessible and taken with the child during transport to other areas (e.g., pediatric intensive care if intubation and further observation are required).
To conserve energy, children are given every opportunity to rest. Infants or small children find that being placed on a face mask, coughing, having laryngeal spasms, and needing IV therapy are additional sources of distress. Infants and small children prefer sitting upright, and most want to be held. Children need the security of the parent’s presence. Because crying increases respiratory distress and hypoxia, the nurse needs to assess a child’s individual tolerance for these therapies. An extremely fussy child may do better when held in the parent’s lap with cool mist directed toward the child’s face.
The rapid progression of croup, the alarming sound of the cough and stridor, and the child’s apprehensive behavior and ill appearance combine to create a frightening experience for the parents. They need reassurance regarding the child’s progress and an explanation of treatments. The family should be allowed to remain with the child as much as possible, especially when this decreases the child’s distress.
Fortunately, as the crisis subsides and the child responds to therapy, breathing becomes easier and recovery is generally prompt. Home care after discharge includes continued humidity, adequate hydration, and nourishment. Encourage parents to ask questions about home care and preparation for discharge.
Acute Spasmodic Laryngitis
Acute spasmodic laryngitis (spasmodic croup, “midnight croup,” or “twilight croup”) is distinct from laryngitis and LTB and characterized by paroxysmal attacks of laryngeal obstruction that occur chiefly at night. Signs of inflammation are absent or mild, and there is often a history of previous attacks lasting for 2 to 5 days followed by uneventful recovery. It usually affects children ages 1 to 3 years. Some children appear to be predisposed to the condition; allergy and psychogenic factors contribute to some cases.
The child goes to bed well or with some mild respiratory symptoms but awakes suddenly with characteristic barking, metallic cough; hoarseness; noisy inspirations; and restlessness. The child appears anxious and frightened. Excitement can aggravate dyspnea. However, there is no fever, the attack subsides in a few hours, and the child appears well the next day with the exception of slight hoarseness.
Therapeutic and Nursing Care Management
Children with spasmodic croup are managed at home. Cool mist is recommended for the child’s room. Warm mist provided by steam from hot running water in a closed bathroom may be helpful. Humidification may help, but warm temperatures will not relieve the constriction. Sometimes sudden exposure to cold air relieves the spasm (as when the child is taken out into the night air to see the practitioner). Parents are usually advised to have the child sleep in humidified air until the cough has subsided to prevent subsequent episodes. Children with moderately severe symptoms may be hospitalized for observation and therapy with cool mist and racemic epinephrine, as for LTB. Patients may respond to corticosteroid therapy. The disease is usually self-limiting.
Bacterial Tracheitis
Bacterial tracheitis, an infection of the mucosa of the upper trachea, is a distinct entity with features of both croup and epiglottitis. The disease is more common in children younger than 3 years and may be a serious cause of airway obstruction—severe enough to cause respiratory arrest. It is believed to be a complication of LTB, and although Staphylococcus aureus is the most frequent organism responsible, M. catarrhalis, S. pneumoniae, and H. influenzae have also been implicated.
Many of the manifestations of bacterial tracheitis are similar to those of LTB but are unresponsive to LTB therapy. There is a history of previous URI with croupy cough, stridor unaffected by position, toxicity, absence of drooling, and high fever. A prominent manifestation is the production of thick, purulent tracheal secretions. Respiratory difficulties are secondary to these copious secretions. Children with this condition may develop a life-threatening upper airway obstruction, respiratory failure, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction (Hopkins, Lahiri, Salerno, et al, 2006).
Therapeutic and Nursing Care Management
Bacterial tracheitis requires vigorous management with antipyretics and antibiotics. Many children require endotracheal intubation and mechanical ventilation; patients are closely monitored for impending respiratory failure if not intubated. Early recognition to prevent life-threatening airway obstruction is essential.
Infections of the Lower Airways
The reactive portion of the lower respiratory tract includes the bronchi and bronchioles in children. Cartilaginous support of the large airways is not fully developed until adolescence. Consequently, the smooth muscle in these structures represents a major factor in constriction of the airway, particularly in the bronchioles, that portion that extends from the bronchi to the alveoli.
Table 32-2 compares some of the major features of bronchial and bronchiolar infections.
TABLE 32-2
COMPARISON OF CONDITIONS AFFECTING THE BRONCHI
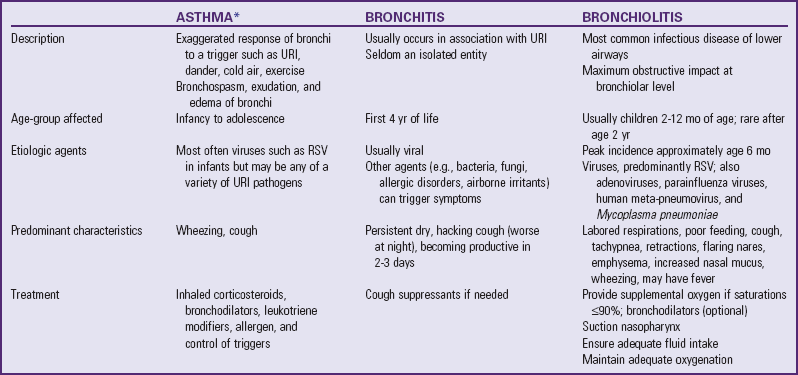
RSV, Respiratory syncytial virus; URI, upper respiratory tract infection.
*See Asthma, p. 1263.
Bronchitis
Bronchitis (sometimes referred to as tracheobronchitis) is an inflammation of the large airways (trachea and bronchi), which is frequently associated with a URI. Viral agents are the primary cause of the disease, although M. pneumoniae is a common cause in children older than 6 years of age. The condition is characterized by a dry, hacking, and nonproductive cough that is worse at night and becomes productive in 2 or 3 days.
Bronchitis is a mild, self-limiting disease that requires only symptomatic treatment, including analgesics, antipyretics, and humidity. Cough suppressants may be useful to allow rest but can interfere with clearance of secretions. Most patients recover uneventfully in 5 to 10 days.
Respiratory Syncytial Virus and Bronchiolitis
![]() Bronchiolitis is an acute viral infection with maximum effect at the bronchiolar level. The infection occurs primarily in winter and spring. Although most cases of bronchiolitis are caused by RSV, adenoviruses and parainfluenza viruses are also implicated; recently, human meta-pneumovirus has also been associated with bronchiolitis in children. By age 3 years most children have been infected at least once. Reinfection with RSV may occur in as many as three fourths of affected children in the second year of life; the antibody response to the virus is inadequate to protect against subsequent reinfection (Robinson, 2008). RSV affects males more than females, it occurs less frequently in breast-fed infants, and it has a higher rate in children in crowded living conditions (Goodman, 2007). RSV infection is the most frequent cause of hospitalization in children less than 1 year old. In addition, severe RSV infections in the first year of life represent a significant risk factor for the development of asthma up to age 13 (Chávez-Bueno, Mejías, Jafri, et al, 2005). The precise link between RSV and asthma is unknown, but genetic predisposition to inflammation has been suggested (Mailaparambil, Grychtol, and Heinzmann, 2009; Thomsen, van der Sluis, Stensballe, et al, 2009). RSV infection may also occur in children older than 1 year who have a chronic or serious disabling illness and in preterm infants. Hospitalization may occur in 1% to 3% of infants with RSV infection (Mcintosh, 2007). It is important to note that not all infants and children with RSV will develop a lower respiratory tract infection (Goodman, 2007).
Bronchiolitis is an acute viral infection with maximum effect at the bronchiolar level. The infection occurs primarily in winter and spring. Although most cases of bronchiolitis are caused by RSV, adenoviruses and parainfluenza viruses are also implicated; recently, human meta-pneumovirus has also been associated with bronchiolitis in children. By age 3 years most children have been infected at least once. Reinfection with RSV may occur in as many as three fourths of affected children in the second year of life; the antibody response to the virus is inadequate to protect against subsequent reinfection (Robinson, 2008). RSV affects males more than females, it occurs less frequently in breast-fed infants, and it has a higher rate in children in crowded living conditions (Goodman, 2007). RSV infection is the most frequent cause of hospitalization in children less than 1 year old. In addition, severe RSV infections in the first year of life represent a significant risk factor for the development of asthma up to age 13 (Chávez-Bueno, Mejías, Jafri, et al, 2005). The precise link between RSV and asthma is unknown, but genetic predisposition to inflammation has been suggested (Mailaparambil, Grychtol, and Heinzmann, 2009; Thomsen, van der Sluis, Stensballe, et al, 2009). RSV infection may also occur in children older than 1 year who have a chronic or serious disabling illness and in preterm infants. Hospitalization may occur in 1% to 3% of infants with RSV infection (Mcintosh, 2007). It is important to note that not all infants and children with RSV will develop a lower respiratory tract infection (Goodman, 2007).
![]() Nursing Care Plan—The Infant with Bronchiolitis and Respiratory Syncytial Virus (RSV) Infection
Nursing Care Plan—The Infant with Bronchiolitis and Respiratory Syncytial Virus (RSV) Infection
Etiology
RSV is a paramyxovirus containing a single strand of ribonucleic acid and is related to parainfluenza virus. RSV strains have two major subgroups: A (the more virulent) and B. More children develop bronchiolitis and pneumonia from RSV subgroup A infections than from subgroup B infections during major outbreaks. The disease usually begins in the fall, reaches a peak during the winter, and then decreases during the spring. In tropical regions, peaks of activity are less pronounced, and outbreaks tend to occur in rainy seasons.
Pathophysiology
RSV affects the epithelial cells of the respiratory tract. The ciliated cells swell, protrude into the lumen, and lose their cilia. RSV produces a fusion of the infected cell membrane with cell membranes of adjacent epithelial cells, thus forming a giant cell with multiple nuclei. At the cellular level this fusion results in multinucleated masses of protoplasm, or syncytia.
The bronchiolar mucosa swells, and lumina are subsequently filled with mucus and exudate. The walls of the bronchi and bronchioles are infiltrated with inflammatory cells, and peribronchiolar interstitial pneumonitis is usually present. Because luminal epithelial cells are shed into the bronchioles when they die, the lumina are frequently obstructed, particularly on expiration. The varying degrees of obstruction produced in small air passages lead to hyperinflation, obstructive emphysema resulting from partial obstruction, and patchy areas of atelectasis. Dilation of bronchial passages on inspiration allows sufficient space for intake of air, but narrowing of the passages on expiration prevents air from leaving the lungs. Thus air is trapped distal to the obstruction and causes progressive overinflation (emphysema).
Transmission
The transmission of RSV is predominantly through direct contact with respiratory secretions, mainly as a result of inoculation from hand to eye, nose, or other mucous membranes. It can also occur by direct inoculation by large-particle aerosols or by self-inoculation from contaminated fomites (Mcintosh, 2007). RSV in secretions can survive for hours on countertops, gloves, paper tissues, and cloth, and for half an hour on skin; it remains infectious when transferred from hands or objects. There is no documentation of distant spread of RSV by small-particle aerosols (airborne transmission).
Clinical Manifestations
The younger the infant, the greater the likelihood that severe lower respiratory tract disease requiring hospitalization will occur. The peak incidence for RSV is 2 to 7 months of age, but reinfection with RSV is common at all ages, with the highest rates being reported in children who attend a daycare center. The severity of RSV tends to diminish with age and repeated infections.
The illness usually begins with a URI after an incubation of about 5 to 8 days. Symptoms such as rhinorrhea and low-grade fever often appear first. OM and conjunctivitis may also be present. In time a cough may develop. If the disease progresses, it becomes a lower respiratory tract infection and manifests typical symptoms (Box 32-6). Infants may have several days of URI symptoms or no symptoms except slight lethargy, poor feeding, or irritability.
Once the lower airway is involved, classic manifestations include signs of altered air exchange, such as wheezing, retractions, crackles, dyspnea, tachypnea, and diminished breath sounds. Pneumonia may occur in conjunction with RSV bronchiolitis. Apnea is a complication of bronchiolitis and is more common in term infants less than 1 month old, in preterm infants with a postconceptual age less than 48 weeks, and in infants with a previous history of apnea (Seiden and Scarfone, 2009).
Diagnostic Evaluation
Because RSV infection may be manifested as a URI, it is often difficult to identify the specific etiologic agent by clinical criteria alone. The most difficult distinction is between RSV and asthma, since both conditions involve the lower airway and have similar symptoms.
Identification has been simplified by the development of tests done on nasal or nasopharyngeal secretions, using either rapid immunofluorescent antibody–direct fluorescent antibody staining (DFA) or enzyme-linked immunosorbent assay (ELISA) techniques for RSV antigen detection. The more traditional viral culture is becoming obsolete, since it takes several days to get a result. Other simultaneous infections may occur with RSV. The infant should be carefully evaluated for the presence of urinary tract infection, meningitis, and bacteremia; antibiotics are prescribed for a coexisting bacterial infection (Sorce, 2009).
Therapeutic Management
Uncomplicated cases of bronchiolitis are treated symptomatically with supplemental oxygen as required, adequate fluid intake, airway maintenance, and medications. Most children with bronchiolitis can be managed at home. Hospitalization is usually recommended for children with respiratory distress or those who cannot maintain adequate hydration. Other reasons for hospitalization include complicating conditions, such as underlying lung or heart disease (e.g., prematurity), or caregiver inability to provide adequate care during illness. The child who is tachypneic or apneic, has marked retractions, seems listless, or has a history of poor fluid intake should be admitted. Pneumonia and electrolyte imbalance are commonly seen in infants who are hospitalized with RSV infection.
The American Academy of Pediatrics practice parameter (2006) recommends the use of supplemental oxygen if the infant fails to maintain a consistent oxygen saturation of at least 90% after nasal suctioning and repositioning. Routine chest physiotherapy (CPT) is not recommended; infants with abundant nasal secretions benefit from periodic suctioning. Fluids by mouth may be contraindicated because of tachypnea, weakness, and fatigue. Therefore IV fluids are preferred until the acute stage of the disease has passed. Nasogastric fluids may be required if the infant is unable to tolerate oral fluids and a peripheral IV is difficult to establish.
Clinical assessments, noninvasive oxygen monitoring, and in severe cases, blood gas values guide therapy. Medical therapy for bronchiolitis is primarily supportive and aimed at decreasing airway hyperresonance and inflammation and promoting adequate fluid intake. Bronchodilators may provide short-term benefits, yet overall significant improvement in the child’s condition is not always appreciable. A short acting β-agonist bronchodilator may be given as a test dose initially; if no improvement occurs, the medication is discontinued. Approximately 50% of infants with RSV lower airway infection and obstruction respond to a short acting β-agonist. Some centers use racemic epinephrine to produce modest improvement in ventilation status. The use of 3% nebulized (hypertonic) saline is associated with an increase in mucociliary clearance in children with RSV (Sorce, 2009) and has shown to be effective in treating RSV in a few small studies (Seiden and Scarfone, 2009).
The use of systemic corticosteroids is controversial but may be used in some centers. Antibiotics are not part of the treatment of RSV unless there is a coexisting bacterial infection such as OM (American Academy of Pediatrics, 2006). Additional treatment recommendations in the American Academy of Pediatrics practice guideline (2006) are to encourage breast-feeding; avoid passive tobacco smoke exposure; and promote preventive measures, including hand washing and the administration of palivizumab (Synagis) to high-risk infants.
Ribavirin, an antiviral agent (synthetic nucleoside analog), is the only specific therapy approved for hospitalized children. However, use of this drug in infants with RSV is controversial because of concerns about the high cost, aerosol route of administration, potential toxic effects among exposed health care personnel, and conflicting results of efficacy trials (American Academy of Pediatrics, 2006; Chávez-Bueno, Mejías, Jafri, et al, 2005; Ventre and Randolph, 2007).
The only product available in the United States for prevention of RSV is palivizumab, a monoclonal antibody, which is given monthly in an IM injection. According to the American Academy of Pediatrics practice guideline (2006), candidates for palivizumab include infants born before 32 weeks of gestation who required medical therapy such as supplemental oxygen or mechanical ventilation. Infants and children younger than 2 years of age with bronchopulmonary dysplasia who have received medical therapy (supplemental oxygen, bronchodilator, diuretic, or corticosteroid therapy) for the condition within 6 months before the anticipated RSV season may benefit from palivizumab prophylaxis. Children with more severe bronchopulmonary dysplasia may benefit from palivizumab prophylaxis for two RSV seasons. Children with severe immunodeficiencies (e.g., severe combined immunodeficiency or acquired immunodeficiency syndrome) may also benefit from prophylaxis. Infants and children younger than 2 years of age with hemodynamically significant congenital heart disease benefit from five monthly IM injections of palivizumab. Prophylaxis for RSV should be initiated at the onset of the RSV season and terminated at the end of the season (November to March). Additional age and condition recommendations are outlined in the American Academy of Pediatrics practice guideline (2006). At the time of this writing, RSV prophylaxis is only available for this subset of infants; other children may be at risk for acquiring the illness but do not qualify for palivizumab prophylaxis, which costs approximately $725 per dose. In addition, some children acquire the illness despite palivizumab prophylaxis (Sorce, 2009).
A second-generation monoclonal antibody, motavizumab, is currently undergoing phase III clinical trials; this drug is reported to be more effective in the prevention of RSV than palivizumab (DeVincenzo, 2008).
Nursing Care Management
Children admitted to the hospital with suspected RSV infection may need separate rooms or rooms with other RSV-infected children. Use Contact and Standard Precautions, including hand washing, not touching the nasal mucosa or conjunctiva, and using gloves and gowns when entering the patient’s room. Other isolation procedures of potential benefit are those aimed at diminishing the number of hospital personnel, visitors, and uninfected children in contact with the child. In some cases visitors, especially children, may be screened for illness before being allowed to visit high-risk infants. Another measure is to make patient assignments so that nurses assigned to children with RSV are not caring for other patients who are considered high risk.
Infants with RSV often have copious nasal secretions, making breathing and nursing or bottle-feeding difficult. This engenders concerns that the child will lose weight or stop breast-feeding altogether. Encourage breast-feeding mothers to pump their milk and store appropriately for later use. (See Chapter 8.) Parents should learn how to instill normal saline drops into the nares and suction the mucus with a bulb syringe before feedings and before bedtime so the child may eat and rest better; unfortunately no medications appropriate for infants can help with these symptoms. To address the issue of decreased fluid intake, parents may offer small amounts of clear fluids, 5 to 10 ml at a time, with a medication syringe every 10 minutes or so. Infants may cough or vomit as the secretions settle in the stomach and make them prone to emesis of such secretions.
The nurse aims additional interventions at monitoring oxygenation with pulse oximetry, ensuring bronchodilator therapy is optimized by using a small mask for delivery (versus blow-by), monitoring IV fluids administered, monitoring fever and administering antipyretics, and providing information for the parent regarding the infant’s status. Inform the parents that the infant’s cough may persist for a few weeks.
The critically ill infant with RSV usually is placed in the pediatric intensive care unit for continuous monitoring of respiratory status, cardiac output, and maintenance of adequate systemic pressure. IV fluids, antibiotics, mechanical ventilation, and inotropes are often required in the unstable child. Parents and family members need emotional support and information regarding the child’s status during this crisis.
The unpredictability of the infant’s individual response to the disease compounds parental anxiety when they hear about children who had serious morbidity or died from RSV. However, in most cases the infant recovers quickly from the disease and resumes normal daily activities, including fluid intake. Such infants are at risk for further episodes of wheezing that may or may not involve an RSV infection. Reinfection with the virus may occur in the same season, but subsequent infections are not as severe as the first.
Pneumonia
Pneumonia, an inflammation of the pulmonary parenchyma, is common in childhood, occurring more frequently in infancy and early childhood. Clinically, pneumonia may occur either as a primary disease or as a complication of another illness.
Pneumonia can be classified according to morphology, etiologic agent, or clinical form. Although morphologic classification is typically used (Box 32-7), the most useful classification is based on the etiologic agent (i.e., viral, bacterial, mycoplasmal, or aspiration of foreign substances) (Box 32-8). The causative agent is usually introduced into the lungs through inhalation or from the bloodstream. Pneumonia may be caused by histomycosis, coccidioidomycosis, and other fungi. Other terms that describe pneumonias are hemorrhagic, fibrinous, and necrotizing. Pneumonitis is a localized acute inflammation of the lung without the toxemia associated with lobar pneumonia.
The clinical manifestations of pneumonia vary depending on the etiologic agent, the child’s age, the child’s systemic reaction to the infection, the extent of the lesions, and the degree of bronchial and bronchiolar obstruction. The clinical history, the child’s age, the general health history, the physical examination, radiography, and the laboratory examination can help identify the etiologic agent.
Viral Pneumonia
Viral pneumonias occur more frequently than bacterial pneumonias and are seen in children of all age-groups. They are often associated with viral URIs, and the pathologic changes involve interstitial pneumonitis with inflammation of the mucosa and the walls of bronchi and bronchioles. Viruses that cause pneumonia include RSV in infants and parainfluenza, influenza, human meta-pneumovirus, and adenovirus in older children. There are few clinical symptoms to distinguish between the responsible organisms, and only laboratory examination can differentiate between specific viruses.
Clinical Manifestations
The onset may be acute or insidious, and symptoms vary from mild fever, slight cough, and malaise to high fever, severe cough, and fatigue. Early in the illness, the cough is likely to be unproductive or productive of small amounts of whitish sputum. Breath sounds may include a few wheezes or fine crackles. Radiography reveals diffuse or patchy infiltration with a peribronchial distribution.
Therapeutic and Nursing Care Management
The prognosis is generally good, although viral infections of the respiratory tract render the affected child more susceptible to secondary bacterial invasion. Treatment is usually symptomatic and includes measures to promote oxygenation and comfort, such as oxygen administration, CPT and postural drainage, antipyretics for fever management, fluid intake, and family support. Although some authorities recommend antimicrobial therapy in the hope of reducing or preventing secondary bacterial infection, it is usually reserved for children in whom the presence of such infection is demonstrated by appropriate cultures.
Primary Atypical Pneumonia
Atypical pneumonia refers to pneumonia that is caused by pathogens other than the traditionally most common and readily cultured bacteria (e.g., S. pneumoniae). In the category of atypical pneumonias, M. pneumoniae and Chlamydia pneumoniae are the most common causes of community-acquired pneumonia in children 5 years old or older (Rafei and Lichenstein, 2006). It occurs principally in the fall and winter months and is more prevalent in crowded living conditions.
Clinical Manifestations
The onset may be sudden or insidious and is usually accompanied by general systemic symptoms, including fever, chills (in older children), headache, malaise, anorexia, and muscle pain (myalgia). These symptoms are followed by rhinitis; sore throat; and a dry, hacking cough. The cough, initially nonproductive, produces seromucoid sputum that later becomes mucopurulent or blood streaked. The degree of fever varies widely, from several days to 2 weeks. Dyspnea occurs infrequently.
Radiographic examination reveals evidence of pneumonia before physical signs are apparent. There may be fine crepitant crackles over various areas of the lung fields, but consolidation is usually not demonstrated. The pathologic process consists of interstitial round cell infiltration and edema of alveolar septa and varying distribution of areas of inflammation, necrosis, and ulceration of the mucosal lining of bronchi and bronchioles. Areas of consolidation and emphysema are present.
Bacterial Pneumonia
![]() Bacterial pneumonia is often a serious infection. The pathogenetic mechanisms involved are often aspiration or hematogenous dissemination. The cause varies depending on the child’s age, underlying illness, and degree of immunosuppression or immunocompetence.
Bacterial pneumonia is often a serious infection. The pathogenetic mechanisms involved are often aspiration or hematogenous dissemination. The cause varies depending on the child’s age, underlying illness, and degree of immunosuppression or immunocompetence.
Etiology and Epidemiology
S. pneumoniae is the most common bacterial pathogen responsible for community-acquired pneumonia in both children and adults (Rafei and Lichenstein, 2006). Other bacteria that cause pneumonia in children are group A streptococci, S. aureus, M. catarrhalis, M. pneumoniae, and C. pneumoniae.
Beyond the neonatal period, bacterial pneumonias display distinct clinical patterns that facilitate their differentiation from other forms of pneumonia. The onset of illness is abrupt and generally follows a viral infection that disturbs the natural defense mechanisms of the upper respiratory tract. In the 3-month to 5-year age-group, S. pneumoniae, M. catarrhalis, and group A streptococci are common causes. H. influenzae type b is causing fewer infections because of the Hib vaccine. S. aureus pneumonia is also now rarely seen in infants and toddlers.
Clinical Manifestations
The child with bacterial pneumonia usually appears ill. Symptoms include fever, malaise, rapid and shallow respirations, cough, and chest pain. The older child may complain of headache, chills, abdominal pain, chest pain, or meningeal symptoms (meningism) (Box 32-9). Respiratory distress may or may not be present. In some cases the only finding is an increased respiratory rate. The pain of pneumonia may be referred to the abdomen and confused with appendicitis.
Infants and young children develop more severe symptoms than older children. Cyanosis and apnea are common, and the parent may report the infant’s activity and eating pattern was decreased for a few days. Additional clinical manifestations in infants include abrupt fever, vomiting, diarrhea, and abdominal distention (Sectish and Prober, 2007). Because pneumonia in newborns carries a high morbidity and mortality rate, suspect bacterial infection in all neonates with respiratory symptoms.
Initially, the cough is usually hacking and nonproductive, and breath sounds are diminished or heard as scattered crackles. When consolidation is present, breath sounds may be tubular in quality with no adventitious noises. As the infection resolves, coarse crackles and wheezing are heard, and the cough becomes productive with purulent sputum.
Staphylococcal pneumonia is rare but particularly progressive and must be treated aggressively. The onset is rapid, with rapid deterioration. Conjunctivitis and furuncles are signs of a probable staphylococcal infection.
Diagnostic Evaluation
The key to a preliminary diagnosis is finding pulmonary infiltrates on radiographic examination, usually revealing lobar consolidation and, in some severe cases, pleural effusion. Laboratory studies include Gram stain and culture of sputum in older children, nasopharyngeal specimens, blood cultures, and lung aspiration and biopsy. The white blood cell count may be elevated, but it may be normal for infants with staphylococcal disease. Children with streptococcal disease usually have an elevated antistreptolysin O titer. The infant or child with recurrent pneumonia should be further evaluated for CF or an immunodeficiency disease. Diagnostic evaluation should include ruling out aspiration pneumonia as a potential cause.
Therapeutic Management
Antimicrobial therapy has significantly reduced the morbidity and mortality from bacterial pneumonia. Oral amoxicillin is widely used for outpatient management of infants and children younger than 5 years of age. Patients incompletely immunized against H. influenzae should receive amoxicillin-clavulanate or a second-generation cephalosporin (cefuroxime, cefadroxil). Erythromycin is the drug of choice for older children and adolescents because of its activity against M. pneumoniae. In the hospital, medications are given parenterally for rapid action and maximum effect. IV cefuroxime, cefotaxime, and ceftriaxone are considered the primary antibacterial agents for bacterial pneumonia in the hospitalized child (Sectish and Prober, 2007). Parenteral or oral erythromycin should be added for children older than 5 years of age until M. pneumoniae is ruled out. CPT with postural drainage may be helpful in clearing secretions in some cases.
Most older children with pneumonia can be treated at home, especially if the condition is recognized and treatment initiated early. Antibiotic therapy, rest, liberal oral intake of fluid, and administration of antipyretics for fever are the principal therapeutic measures. Hospitalization is indicated when pleural effusion or empyema accompanies the disease, when compliance with therapy is estimated to be poor, in infants less than 1 month old, and when there are chronic illnesses such as congenital heart disease or bronchopulmonary dysplasia (Rafei and Lichenstein, 2006). Pneumonia in the infant or young child may also require hospitalization because the course of illness is variable and complications are more common in very young patients. In addition, IV fluid administration is frequently necessary, and oxygen may be required if the child is in respiratory distress.
Prognosis: The prognosis for pneumonia is generally good, with rapid recovery when it is recognized and treated early. The course of staphylococcal pneumonia is generally prolonged. The prognosis varies with the length of the illness before treatment, although early recognition and treatment are usually beneficial.
Prevention: The use of the pneumococcal conjugate vaccine (PCV 13; Prevnar 13) is recommended for infants and children younger than 23 months to be administered at 2, 4, 6, and between 12 and 15 months. The four-dose series may be completed with Prevnar 13 if PCV 7 was given for any of the previous doses, as long as a total of four doses are administered. Prevnar 13 is also recommended for children aged 60 to 71 months with underlying medical conditions who are at high risk for the development of pneumococcal disease or complications (Centers for Disease Control and Prevention, 2010). Studies have demonstrated a decrease in pneumococcal pneumonia in children younger than 24 months. (See Immunizations, Chapter 12.)
Complications: At present the classic features and clinical course of pneumonia are rarely seen because of early and vigorous antibiotic and supportive therapy. However, some children, especially infants, with staphylococcal pneumonia develop empyema, pyopneumothorax, or tension pneumothorax (Fig. 32-5). AOM and pleural effusion are common in children with pneumococcal pneumonia (Box 32-10).
Fig. 32-5 Pneumothorax. Air in the pleural space causes the lung to collapse around the hilus and may push mediastinal contents (heart and great vessels) toward the other lung. (From McCance KL, Huether SE: Pathophysiology: the biological basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
When fluid is either suspected or identified by radiograph in the pleural cavity, a needle aspiration or thoracentesis is performed. Nonpurulent effusions do not require surgical drainage.
Continuous closed chest drainage may be instituted with a complicated pleural effusion. Closed drainage is continued until drainage fluid is free of pathogens, which rarely requires more than 5 to 7 days. Additional therapies for empyema may involve the instillation of antibiotic into the pleural space via the chest tube, instillation of intrapleural fibrinolytics such as urokinase or streptokinase, or video-assisted thoracoscopy (Ranganathan and Sonnappa, 2009; Bergelson, Shah, and Zaouitis, 2008).
Thoracentesis: Dyspnea resulting from pressure from fluid accumulation in the pleural cavity requires removal by thoracentesis. Thoracentesis is also performed to obtain fluid for culture or to instill antibiotics directly into the pleural cavity. Nursing responsibilities include obtaining and setting up equipment, preparing the child physically and psychologically, monitoring the sedated child’s vital signs during the procedure and recovery, and assisting with the procedure. If continuous closed chest drainage is anticipated, this equipment should also be available. Procedural sedation may be performed using a number of drugs singly or in combination (ketamine, propofol, fentanyl, morphine, midazolam) to provide adequate anxiolysis and analgesia (Meredith, O’Keefe, and Galwankar, 2008). Additional nursing responsibilities include documenting the patient’s tolerance of procedure and managing pain after the procedure.
In addition, the nurse makes the child comfortable and records observations and physical and emotional responses, the amount and description of the fluid obtained, and any medication instilled. Specimens are sent to the laboratory for culture. Continuous closed chest drainage is managed according to the same protocol as for the child with a thoracotomy. (See Chapter 34.)
Nursing Care Management
Nursing care of the child with pneumonia is primarily supportive and symptomatic but necessitates thorough respiratory assessment and administration of supplemental oxygen (as required) and antibiotics. The child’s respiratory rate and status, oxygenation, general disposition, and level of activity are frequently assessed. If the cough is disturbing, the use of antitussives, especially before rest times and meals, is often helpful. To prevent dehydration, fluids are frequently administered intravenously during the acute phase. Oral fluids, if allowed, are given cautiously to avoid aspiration and to decrease the possibility of aggravating a fatiguing cough.
Nursing care of the child with a chest tube requires close attention to respiratory status, as noted previously; the chest tube and drainage device used are monitored for proper function (i.e., drainage is not impeded, vacuum setting is correct, tubing is free of kinks, dressing covering chest tube insertion site is intact, water seal is maintained [if used], and chest tube remains in place). Movement in bed and ambulation with a chest tube are encouraged according to the child’s respiratory status, but children often require a mild analgesic such as acetaminophen.
If needed, supplemental oxygen may be administered by nasal cannula; newborns may receive oxygen via a plastic hood. Children are usually more comfortable in a semierect position but should be allowed to determine the position of comfort. Control fever by cooling the environment and administering antipyretic drugs as prescribed. Temperature is monitored regularly to detect a rise that might trigger a febrile seizure.
Monitor vital signs and oxygenation to assess the progress of the disease and to detect early signs of complications. Children with ineffectual cough or those with difficulty handling secretions, especially infants, require suctioning to maintain a patent airway. A simple bulb suction syringe is usually sufficient for clearing the nares and nasopharynx of infants, but mechanical suction should be readily available if needed. Older children can usually handle secretions without assistance. Postural drainage and CPT are generally prescribed every 4 hours or more often, depending on the child’s condition.
The hospitalized child may be apprehensive, and the treatments and tests are frightening and stress producing. It is important to involve the entire family in the care as appropriate and to encourage questions and facilitate effective communication. Reducing the child’s anxiety, apprehension, and psychologic distress leads to relaxation and decreased respiratory efforts. Easing respiratory efforts further reduces the child’s apprehension. Encouraging the presence of the caregiver provides the child with a source of comfort and support.
Chlamydial Pneumonia
C. trachomatis, an intracellular microorganism similar to gram-negative bacteria, is responsible for one of the most common sexually transmitted infections. Newborn infants acquire pulmonary infection from their mothers via ascending infection just before or in the process of birth.
Chlamydial pneumonia is usually an afebrile illness that occurs between 2 and 19 weeks after delivery (American Academy of Pediatrics, 2009b). It is also characterized by a persistent cough, tachypnea, and sometimes rales. Radiographs show nonspecific abnormalities. Oral azithromycin given for 5 days is the treatment of choice; alternatively erythromycin base or ethylsuccinate is administered for 14 days (American Academy of Pediatrics, 2009b). Nursing care is the same as for any infant with pneumonia.
Other Infections of the Respiratory Tract
Pertussis, or whooping cough, is an acute respiratory tract infection caused by Bordetella pertussis that occurs primarily in children younger than 4 years of age who have not been immunized. It is highly contagious and is particularly threatening in young infants, who have a higher morbidity and mortality rate. Infants less than 6 months of age may not come in to the practitioner with the typical cough; in this age-group, apnea is a common presenting manifestation (American Academy of Pediatrics, 2009b). Likewise older children often manifest the disease with a persistent cough and the absence of the characteristic whoop. (See Table 16-1 for signs, symptoms, and management of pertussis.) The incidence is highest in the spring and summer months, and a single attack confers lifetime immunity.
The resurgence of pertussis in the United States, particularly among children 10 years old and older, has prompted concerns over the long-term effects of the pertussis vaccine. Consequently a new booster vaccine for pertussis has been approved for children. Boostrix contains acellular pertussis, diphtheria toxoid, and tetanus toxoid and is indicated as a booster for children ages 10 to 18 years (American Academy of Pediatrics, 2009b); one additional acellular pertussis vaccine (Adacel) has now been approved for persons ages 11 to 64 years. (See also Immunizations, Chapter 12.) Parapertussis (Bordetella parapertussis) causes pertussis but cases are milder than those caused by B. pertussis; parapertussis is more common in Eastern and Western Europe than in the United States (Long, 2007).
Tuberculosis
Since 1993 the incidence of tuberculosis (TB) has steadily declined significantly in the United States, yet it remains high in certain populations. According to the Centers for Disease Control and Prevention (2009a), preliminary data from 2008 show the lowest rate of TB since 1953—4.2 cases per 100,000 population. The rate of TB among foreign-born immigrants was 10 times higher than those with the United States as country of origin. In recent years, foreign-born children have accounted for more than one fourth of newly diagnosed cases of TB in children 14 years of age or younger in the United States (American Academy of Pediatrics, 2009b). Four states—Florida, New York, Texas, and California—combined accounted for more than 50% of all cases of TB reported in 2008 (Centers for Disease Control and Prevention, 2009a). TB is a significant factor in the mortality of persons infected with HIV. Global estimates show that approximately 2 million persons, including 500,000 children, die each year from TB disease (Ranganathan and Sonnappa, 2009).
Etiology
TB is caused by Mycobacterium tuberculosis, an acid-fast bacillus not readily decolorized by acids after staining. Children are susceptible to the human (M. tuberculosis) and the bovine (Mycobacterium bovis) organisms. In parts of the world where TB in cattle is not controlled or milk is not pasteurized, the bovine type is a common source of infection.
Although the causative agent for TB is the tubercle bacillus, other factors influence the degree to which the organism produces an altered state in the host. These include heredity (resistance to the infection may be genetically transmitted), gender (higher rates in adolescent girls), age (lower resistance in infants, higher incidence during adolescence), stress (emotional or physical), nutritional state, and intercurrent infection (especially HIV, measles, and pertussis). Children with HIV infection have an increased incidence of TB disease, and all children with TB should be tested for HIV (Box 32-11).
Pathophysiology
The source of infection in children is usually an infected member of the household or any frequent visitor to the household, such as a baby-sitter or domestic worker. Transmission of M. tuberculosis occurs when the child inhales microdroplets (usually 1 to 5 mm in size) into the respiratory tract after someone has coughed or sneezed. Although the lung is the most frequent portal of entry in humans, the organism M. bovis can be ingested via infected milk (unpasteurized milk or fresh cheese). When the M. tuberculosis droplet is inhaled, it passes down the bronchial tree, implants in either a bronchiole or alveolus, and starts to multiply. M. bovis typically causes cervical lymphadenitis, meningitis, and intestinal TB disease and is more common in adults and children from countries where M. bovis is prevalent (American Academy of Pediatrics, 2009b).
Epithelial cells surround and encapsulate the multiplying bacilli in an attempt to wall off the invading organisms, thus forming the typical tubercle. During the inflammatory process, some bacilli leave the focal area and are carried to the regional lymph nodes that drain the area; as a result, the child develops a fever. Radiographic examinations may be positive if such tests are performed when the child is known to have been exposed. The tuberculin skin test is positive.
Extension of the primary lesion at the original site causes progressive tissue destruction as it spreads within the lung, discharges material from foci to other areas of the lungs (e.g., bronchi or pleura), or produces pneumonia. Erosion of blood vessels by the primary lesion can cause widespread dissemination of the tubercle bacillus to near and distant sites (miliary TB). Organisms deposited in the upper lung zones, bones, kidneys, and brain may find favorable environments for growth, but organs and tissue such as bone marrow, liver, and spleen appear to inhibit multiplication of the bacilli.
Extrapulmonary TB may be manifested as superior lymphadenitis, meningitis, or osteoarthritis and may appear in the middle ear and mastoid and on the skin (American Academy of Pediatrics, 2009b; Feja and Saiman, 2005). With the exception of meningitis, treatment for extrapulmonary TB may be the same drug regimen as for pulmonary TB. Renal TB is rare in children but may occur in adolescents.
For children not immunosuppressed or immunocompromised, a strong cell-mediated immune response provides specific immunity that usually limits further multiplication of the bacilli. These children remain asymptomatic, and the lesions usually heal. TB infection is manifested by a positive skin test only. In a small percentage of persons with newly acquired TB, replication of the organism continues and TB disease occurs, as evidenced by a positive chest radiograph, positive sputum culture, and signs of disease.
Clinical Manifestations
Clinical manifestations of pulmonary TB in children are extremely variable. The disease may be asymptomatic or produce a broad range of symptoms, including general responses such as fever, malaise, anorexia, and weight loss or more specific symptoms related to the site of infection (e.g., lungs, bone, brain, kidneys). Lung disease may or may not include cough (which progresses slowly over weeks to months), aching pain and tightness in the chest, and (rarely) hemoptysis.
As increasing amounts of lung tissue become involved, the respiratory rate increases, the lung on the affected side does not expand as well as the other, auscultation reveals diminished breath sounds and crackles, and there is dullness to percussion. In children (usually infants) who are unable to contain the spread of infection, the fever persists; the generalized symptoms are manifest; and the patient develops pallor, anemia, weakness, and weight loss.
Diagnostic Evaluation
Diagnosis is based on information derived from physical examination, history, reaction to a tuberculin test, organism cultures, and radiographic examinations. In addition, it must be determined if the lesion is in the active, quiescent, or healed stage.
History: Symptoms generally do not contribute significantly to a diagnosis. A history of possible contact with a person known to be infected or subsequently found to be infected is helpful. All contacts of an affected child are examined for the disease.
Tuberculin Test: The tuberculin skin test (TST) is the most important indicator of whether a child has been infected with the tubercle bacillus. The standard dose of purified protein derivative (PPD) is 5 tuberculin units in 0.1 ml of solution, which is administered using a 27-gauge needle and a 1-ml syringe intradermally into the volar aspect of the forearm. Creation of a visible wheal is crucial to accurate testing. A primary infection initiates a hypersensitivity reaction to the protein fraction of the tubercle bacillus, which can be detected 2 to 10 weeks after the infection. In the past, multiple puncture tests such as the tine test were used, but these tests have significant problems and are no longer recommended (Selekman, 2006).
The American Academy of Pediatrics (2009b) has recommended a change in TB screening procedures. It no longer recommends universal testing of all children for TB. Now a targeted testing method is used, wherein only children and adolescents at high risk for contracting the disease and those patients at risk for progression to TB disease are screened. A risk factor questionnaire has been developed to facilitate screening pediatric populations at high risk. Factors included on the questionnaire include a close association with persons having latent TB infection (LTBI) or active disease, foreign birth, or foreign travel (Pediatric Tuberculosis Collaborative Group, 2004; the entire questionnaire is available at this reference). Recommendations for TB skin testing of children are given in Box 32-12.
The QuantiFERON-TB Gold and T-SPOT TB are tests of interferon quantification (interferon gamma release assay [IGRA]) used in the diagnosis of TB in adults; however, their use in children is limited, especially in those under 5 years of age. The IGRA tests may be helpful in confirming the presence of TB disease in a child who has received BCG (bacille Calmette-Guérin, a vaccine containing bovine bacilli with reduced virulence) and has a borderline positive or negative TST (American Academy of Pediatrics, 2009b; Ranganathan and Sonnappa, 2009).
The tuberculin is injected intradermally with the bevel of the needle pointing upward. A wheal 6 to 10 mm in diameter should form between the layers of the skin when the solution is injected properly. If the wheal does not form, the procedure is repeated. The volar or dorsal surface of the forearm is the usual injection site. The reaction to the skin test is determined in 48 to 72 hours; reactions occurring after 72 hours should be measured and considered the result. The size of the transverse diameter of induration, not the erythema, is measured. The diameter transverse to the long axis of the forearm is the only one standardized for measurement purposes (American Academy of Pediatrics, 2009b).
Guidelines for interpreting the TST are listed in Box 32-13. The American Academy of Pediatrics (2009b) and the Pediatric Tuberculosis Collaborative Group (2004) recommend that TST results be read only by a health care professional.
A positive TST reaction indicates that the person has been infected and has developed sensitivity to the protein of the tubercle bacillus; it does not, however, confirm the presence of active disease. Once individuals react positively, they will always react positively. A positive reaction in a previously negative reactor indicates that the person has been infected since the last test.
A negative reaction does not exclude the presence of LTBI or active disease. Children with immunosuppression, concurrent viral infection (e.g., measles, varicella, influenza), HIV, disseminated TB disease, and recent TB infection may have decreased TST reactivity. Several factors can produce false-negative results (Box 32-14). Tuberculin testing should not be carried out at the same time as measles immunization. Viral interference from the measles vaccine may cause a false-negative reaction. Prompt radiographic evaluation of all children with a positive TST is recommended.
A finding of LTBI indicates infection in a person who has a positive TST, no physical findings of disease, and normal chest radiograph findings. The American Academy of Pediatrics (2009b) states that a diagnosis of LTBI or TB disease in a young child represents a public health sentinel event indicating recent transmission of the M. tuberculosis organism. The term tuberculosis disease is used when a child has clinical symptoms or radiographic manifestations caused by the M. tuberculosis organism.
Bacteriologic Examination: A definitive diagnosis is made by demonstrating the presence of mycobacteria in culture. The organism is identified from microscopic examination of properly prepared and stained smears from early-morning gastric washings or from sputum, pleural fluid, urine, spinal fluid, draining lymph nodes, and other body fluids. Induced sputum and gastric lavage sputum specimens are often obtained for culture from children who are unable to expectorate a sputum specimen.