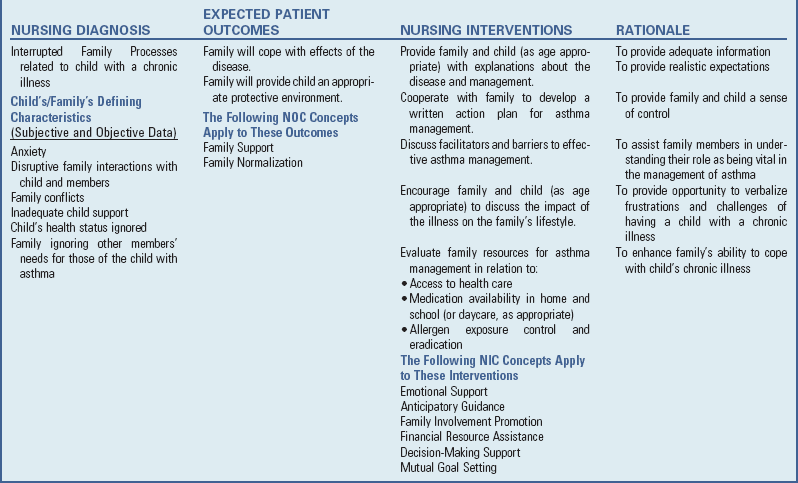
Respiratory Disturbance Caused by Noninfectious Irritants
Foreign Body Ingestion and Aspiration
Small children characteristically explore objects with their hands and mouth and are prone to place FBs into the air passages (nose and mouth). They also place objects such as beads, paper clips, plastic toys, small magnets, or food items in the nose, which can easily be aspirated into the trachea. Small items may also be placed into the external ear canal; small rocks and pebbles appear to be a favorite item for boys, whereas girls prefer colorful beads.
When such objects are placed into the nose or mouth, they can be aspirated into the airway, causing subsequent obstruction. Ingestion or aspiration of an FB can occur at any age but is most common in older infants and children ages 1 to 3 years. Severity depends on the location, type of object aspirated, and extent of obstruction. For example, dry vegetable matter, such as a seed, nut, piece of carrot, or popcorn, that does not dissolve and that may swell when moistened creates a particularly difficult problem. The high fat content of potato chips and peanuts may cause the added risk of lipoid pneumonia. “Fun foods” such as hard candy and hot dog wieners are among the worst offenders. Offending foods, in the order of frequency of aspiration, are hot dog, round candy, peanut or other nut, grape, cookie or biscuit, other meat, carrot, apple, peas, and peanut butter. Round foods are the most frequent offenders. The first four items together make up more than 40% of all aspirated food items. Other items include plastic or glass beads, button or disk batteries, and coins. Objects such as small lithium or cadmium batteries may cause esophageal or tracheal corrosion.
A sharp or irritating object produces irritation and edema. A round, pliable object that does not readily break apart is more likely to occlude an airway than an object with a different shape. A small object may cause little if any pathologic change, whereas an object of sufficient size to obstruct a passage can produce various changes, including atelectasis, emphysema, inflammation, and abscess.
Pathophysiology
Most inhaled FBs lodge in a mainstem or lobar bronchus, a few find their way into more distal portions of the lung field, and the remaining FBs lodge in the trachea. The site is determined by the object’s size, weight, and configuration. For example, heavy objects such as bullets, coins, and nails are more likely to drop into the dependent portions of the tracheobronchial tree. The object may remain in the same location or move in the airway. It can be coughed from a smaller to a larger airway and reaspirated in a different passage—or it might be ejected forcefully into the mouth and subsequently swallowed.
Signs of obstruction caused by an FB in a bronchus are explained by the same mechanisms that control the flow of fluids in pipes (Fig. 32-6). During normal respiration the caliber of bronchi and bronchioles becomes larger during inspiration and smaller during expiration. When a small object partially obstructs a passage, air passes around the obstruction during both inspiration and expiration (bypass valve). In this type of obstruction a wheeze is heard. A somewhat larger obstruction will allow air to enter the distal portion when bronchioles enlarge during inspiration, but when they diminish in caliber during expiration, the lumen becomes occluded and air becomes trapped distal to the obstruction (check valve). This type of obstruction produces obstructive hyperinflation. When there is complete blockage of the bronchus by an FB or by the FB and swollen mucosa, air is unable to move in either direction (stop valve), and the air distal to the obstruction is absorbed, leaving an area of obstruction atelectasis. The right bronchus, with its shorter length and straighter angle, is the usual site of bronchial obstruction.
Clinical Manifestations
Initially, an FB in the air passages produces choking, gagging, or coughing, but symptoms depend on the site of obstruction and on the interval between aspiration and presentation. Up to half of all children with FB ingestion may be asymptomatic (Uyemura, 2005). Laryngotracheal obstruction most commonly causes dyspnea, cough, stridor, and hoarseness because of a decreased air entry. Cyanosis may also occur if the obstruction becomes worse. Bronchial obstruction usually produces cough (frequently paroxysmal), wheezing, asymmetric breath sounds, decreased airway entry, and dyspnea.
If the obstruction progresses, the child’s face may become livid, and sometimes the child becomes unconscious and dies of asphyxiation if the object is not removed. If obstruction is partial, hours, days, or even weeks may pass without symptoms after the initial period. Secondary symptoms are related to the anatomic area in which the FB is lodged and are usually caused by a persistent respiratory tract infection located distal to the obstruction. A history of recurrent intractable pneumonia is reason to consider an FB in an airway. Often, by the time secondary symptoms appear, the parents have forgotten the initial episode of coughing and gagging. The most common symptoms observed in children brought to medical attention are stridor, wheezing, sternal retraction, and cough. When an object is lodged in the larynx, the child is unable to speak or breathe.
Diagnostic Evaluation
The diagnosis of FB obstruction is usually suspected on the basis of the history and physical signs. Radiographic examination reveals opaque FBs but is of limited value in localizing vegetable matter and some plastic items. Bronchoscopy is required for a definitive diagnosis of objects in the larynx and trachea. Fluoroscopic examination is valuable in detecting FBs in the bronchi.
On fluoroscopy a check-valve–obstructed lung remains expanded, the diaphragm remains low and fixed on the obstructed side, and the heart and mediastinum shift to the unobstructed side during expiration. In a stop-valve obstruction the heart and mediastinum are drawn to the obstructed side and remain there during both inspiration and expiration. The diaphragm on the obstructed side remains high, whereas that on the unobstructed side moves normally.
The mainstay of diagnosis and management of foreign bodies is endoscopy. If there is doubt about the presence of an FB, endoscopy can be diagnostic and therapeutic. When endoscopy is used to remove an FB, the procedure should be performed by an endoscopist who is experienced and comfortable in caring for children. The endoscopist should also have access to state-of-the-art endoscopy equipment and should perform the procedure in a setting that can accommodate any complication or emergency.
Therapeutic Management
FB aspiration may result in life-threatening airway obstruction, especially in infants because of the small diameters of their airways. Current recommendations for the emergency treatment of the choking child include the use of abdominal thrusts for children over 1 year of age and back blows and chest thrusts for children less than 1 year of age. (See Cardiopulmonary Resuscitation, Chapter 31.) An FB is rarely coughed up spontaneously; therefore it must be removed by endoscopy. Removal of the FB must be done as soon as possible, since the progressive local inflammatory process triggered by the foreign material hampers removal. In addition, a chemical pneumonia soon develops and vegetable matter begins to macerate within a few days, further complicating its removal.
Nursing Care Management
A major role of nurses is to recognize the signs of FB aspiration and implement immediate measures to relieve the obstruction. All persons working with children should be prepared to deal effectively with aspiration of an FB. Choking on food or other material should not be fatal. Two simple procedures—back blows and the abdominal thrust, which can be used by both health professionals and laypersons—can save lives. It is the nurse’s obligation to learn these techniques and teach them to parents and other groups. (See Figs. 31-26 and 31-27.) To aid a child who is choking, nurses need to recognize the signs of distress. Not every child who gags or coughs while eating is truly choking.
Prevention: Small children should not be allowed access to small objects that they might place in their nose or mouth. Anticipatory guidance for parents of small children is essential. Nurses are in a position to teach prevention in a variety of settings (see Community Focus box). They can educate parents singly or in groups about hazards of aspiration in relation to the developmental level of their children and encourage them to teach their children safety. Caution parents about behaviors that their children might imitate (e.g., holding foreign objects, such as pins, nails, and toothpicks, in their lips or mouth). (Chapters 12 and 14 discuss prevention based on the child’s age.)
Foreign Body in the Nose
Children sometimes place foreign objects, such as food (peanuts are a favorite), crayons, small plastic toys, pieces of plastic, beans, beads, erasers, wads of paper, round peas, and small stones, into their nose. An FB is suspected when there is unilateral nasal discharge that is foul smelling, local obstruction with sneezing, mild discomfort, and (rarely) pain. The irritation produces local mucosal swelling if the items increase in size as they absorb moisture (hygroscopic). Signs of obstruction and discomfort may increase with time. Infection usually follows, as evidenced by foul breath and a purulent or bloody discharge from one nostril.
Although the object is usually situated anteriorly, unskilled attempts at removal may move it further posteriorly. Removal should occur as soon as possible to prevent the risk of aspiration and local tissue necrosis. Removal usually occurs easily with either forceps or suction. In some cases mild sedation may be necessary.
Aspiration Pneumonia
Aspiration pneumonia occurs when food, secretions, inert materials, volatile compounds, or liquids enter the lung and cause inflammation and a chemical pneumonitis. Many conditions increase the risk of aspiration (Box 32-15). Aspiration of fluid or food substances is particularly hazardous in the child who has difficulty swallowing or is unable to swallow because of paralysis, weakness, debility, congenital anomalies such as cleft palate or tracheoesophageal fistula, or absent cough reflex (unconsciousness) or who is force fed, especially while crying or breathing rapidly.
Clinical signs of the aspiration of oral secretions may not be distinguishable from other forms of acute bacterial pneumonia. For example, if vegetable matter has been aspirated, manifestations may not appear for several weeks after the event. Classic symptoms include an increasing cough or fever with foul-smelling sputum, deteriorating chest radiographs, and other signs of lower airway involvement. These deviations may persist for weeks, however, while the child starts to feel better.
Hydrocarbon Pneumonia
Children frequently develop pneumonia secondary to the ingestion of various forms of hydrocarbons, such as kerosene, gasoline, solvents, lighter fluid, furniture polish, and mineral oil. Petroleum distillates are generally impure substances contaminated with heavy metals or other toxic chemicals that cause systemic, as well as local, effects. Many hydrocarbons are made from petroleum and are found in the home or garage.
Hydrocarbons are usually packaged in attractive containers, and some have a pleasant aroma; consequently, they are frequently ingested accidentally by young children. On average, children swallow less than 30 ml (often about 3 to 4 ml). They begin coughing severely and swallow no more. Although central nervous system abnormalities, gastrointestinal irritation, cardiomyopathy, and renal toxicity can occur, the most serious complication is pneumonitis.
Distillates that have high volatility (evaporate quickly), decreased viscosity (thinner solution), and low surface tension are more likely to be aspirated and produce respiratory complications. Decreased viscosity enhances penetration into distal airways. Lower surface tension facilitates spread over a larger area of lung surface. Consequently, ingestion of lighter fluid, kerosene, or gasoline is more likely to cause a pathologic condition than substances that have high viscosity (e.g., petroleum jelly, tar, or lubricating oil).
Pathogenesis: The severity of the lung injury depends on the pH of the aspirated material, the presence of bacteria, and the volatility and viscosity of the substance. Irritation from aspiration during swallowing, vomiting, or gastric lavage may also cause pulmonary involvement. Pathologic changes include signs of inflammation (edema, hyperemia, infiltration of polymorphonuclear cells); vascular thrombosis and hemorrhage; and necrosis of bronchial, bronchiolar, and alveolar tissues. Other reactions are bronchospasm, atelectasis, emphysema, pulmonary hemorrhage, necrosis, surfactant impairment, and pulmonary edema. Even in small amounts, hydrocarbons spread over the surface of tissues and the lungs and interfere with gas exchange. Aspiration of inert fluids may not produce a chemical or bacterial pneumonia, but these fluids can decrease lung compliance and cause hypoxemia.
Clinical Manifestations: Acid aspiration may produce immediate pulmonary symptoms that worsen over the first 24 hours. Coughing and vomiting, which occur almost immediately after ingestion, contribute to the aspiration. Central nervous system symptoms include agitation, restlessness, confusion, drowsiness, and coma. The temperature is elevated (37.8° to 40° C [100° to 104° F]). (See Ingestion of Injurious Agents, Chapter 16.)
After swallowing, coughing, and choking, the child becomes short of breath, and older children complain of dyspnea. There are varying degrees of cyanosis, tachycardia, tachypnea, nasal flaring, and retractions. Intercostal retractions, grunting, cough, and fever may appear within 30 minutes or be delayed for a few hours. Localized areas of dullness are felt on percussion, and moderately intense wheezes and crackles are heard. Severe injury causes hemoptysis, pulmonary edema, severe cyanosis, and death within 24 hours of aspiration.
Therapeutic Management: Inducing the child to vomit is contraindicated because of the renewed danger of aspiration. Hydrocarbons are readily absorbed by the gastrointestinal tract and excreted by the lungs. Bronchitis or pneumonia usually develops early (within the first 24 hours) but may be delayed. Recovery from pulmonary involvement occurs in most instances despite a severe clinical course. Death is generally the result of hepatic failure complicated by pulmonary factors. Treatment is the same as for any lower respiratory tract inflammation and consists of high humidity, supplemental oxygen, hydration, and treatment of any secondary infection. Endotracheal intubation may be required if the child develops respiratory failure.
Lipoid Pneumonia
Oily substances aspirated into the respiratory passages initially cause an interstitial proliferative inflammation that may include an exudative pneumonia. The next stage involves a diffuse, chronic, proliferative fibrosis that is often complicated by acute bronchopneumonia. The final stage features multiple localized nodules or tumorlike paraffinomas. There are no characteristic manifestations. Cough is usually present, and dyspnea occurs in severe cases. Secondary bronchopneumonia is common. The outcome depends on the extent of pulmonary damage, the general condition of the infant or child, and discontinuation of the oily inhalation. No specific treatment exists.
Powder Inhalation
A significant number of infants suffer talcum powder aspiration. Commercial talcum powder is predominantly a mixture of talc (hydrous magnesium silicate) and other silicates. Severe respiratory distress occurs immediately as a result of an inflammatory reaction in small bronchioles initiated by deep inhalation of the extremely light powder. (See Chapter 12 for further discussion of powder inhalation.)
Nursing Care Management
Care of the child with aspiration pneumonia is the same as that described for the child with pneumonia from other causes. However, the major focus of nursing care is on prevention of aspiration. Proper feeding techniques should be carried out for weak, debilitated, and uncooperative children, and measures should be taken to prevent aspiration of any material that might enter the nasopharynx. Nasogastric tubes used for feedings are checked before the initiation of bolus feedings; continuous nasogastric tube feedings are also evaluated periodically for proper tube placement. Children who are at risk for swallowing difficulties as a result of illness, physical debilitation, anesthesia, or sedation are kept NPO (nothing by mouth) until they can properly swallow fluids effectively. The child who is at risk for vomiting and incapable of protecting the airway should be positioned in a side-lying recovery position. (See Fig. 31-28.)
Oily nose drops and oil-based vitamin preparations are not appropriate for infants and small children. Solvents, lighter fluid, and other hydrocarbon substances should be kept away from older infants and small children, who are likely to put anything in their mouth and who may be attracted by the slightly sweet smell.
Talcum powder should not be used. If used, careful application (placing it on the caregiver’s hand and then on the child’s skin) and proper storage are essential.
Acute Respiratory Distress Syndrome and Acute Lung Injury
ARDS occurs in children and adults and has been associated with clinical conditions and injuries such as sepsis, trauma, viral pneumonia, fat emboli, drug overdose, reperfusion injury after lung transplantation, smoke inhalation, and near-drowning. ARDS is characterized by respiratory distress and hypoxemia that occur within 72 hours of a serious injury or surgery in a person with previously normal lungs. ALI is said to involve a spectrum of inflammatory disease responses to a precipitating event, with ARDS being the more severe form of ALI (Frye, 2005; Randolph, 2009).
ARDS and ALI cause acute respiratory failure and account for significant morbidity and mortality in critically ill patients. Acute pulmonary inflammation with alveolar capillary membrane destruction results in significant hypoxemia, and mechanical ventilation is often required. ARDS is the most severe in the spectrum of illnesses in relation to the degree of hypoxemia. Hypoxemia is expressed as the ratio of partial pressure of oxygen (Pao2) to fraction of inspired oxygen (Fio2), or P/F ratio, with the P/F ratio for ALI being 300 or less and the P/F ratio for ARDS being 200 or less. Both ALI and ARDS demonstrate radiographic evidence of bilateral alveolar infiltrates without evidence of left-sided heart failure (Rice and Bernard, 2006).
The hallmark of ARDS is increased permeability of the alveolocapillary membrane that results in pulmonary edema. During the acute phase of ARDS, the alveolocapillary membrane is damaged, with an increasing pulmonary capillary permeability and resulting interstitial edema. Later stages are characterized by pneumocyte and fibrin infiltration of the alveoli, with the start of either the healing process or fibrosis. When fibrosis occurs, the child may demonstrate respiratory distress and the need for mechanical ventilation. In ARDS the lungs become stiff as a result of surfactant inactivation, gas diffusion is impaired, and eventually bronchiolar mucosal swelling and congestive atelectasis occur. The net effect is decreased functional residual capacity, pulmonary hypertension, and increased intrapulmonary right-to-left shunting of pulmonary blood flow. Surfactant secretion is reduced, and the atelectasis and fluid-filled alveoli provide an excellent medium for bacterial growth (Fig. 32-7).
Pathophysiology Review
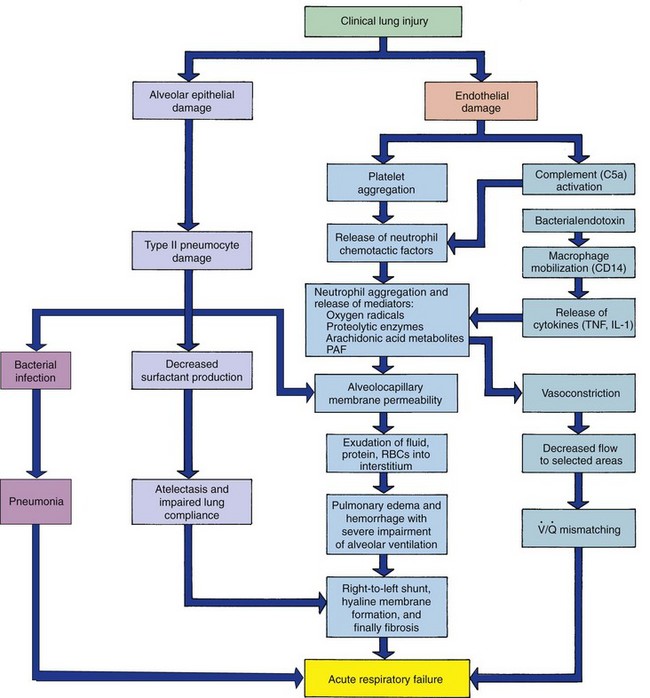
Fig. 32-7 Pathogenesis of acute respiratory distress syndrome. IL-1, Interleukin-1; PAF, platelet-activating factor; RBCs, red blood cells; TNF, tumor necrosis factor; V/Q, ventilation-perfusion. (From McCance KL, Huether SE: Pathophysiology: the biological basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
The criteria for diagnosis of ARDS in children are an acute antecedent illness or injury, acute respiratory distress or failure, severe arterial hypoxemia (see above) unresponsive to oxygen therapy alone, no evidence of left atrial hypertension, and diffuse bilateral infiltrates evidenced on chest radiography (Randolph, 2009). The child with ARDS may first demonstrate only symptoms caused by an injury or infection, but as the condition deteriorates, hyperventilation, tachypnea, increasing respiratory effort, cyanosis, and decreasing oxygen saturation occur. In one study severe sepsis with pneumonia as the primary infection focus was the leading cause of ALI (Zimmerman, Akhtar, Caldwell, et al, 2009).
Therapeutic Management
Treatment involves supportive measures such as maintenance of adequate oxygenation and pulmonary perfusion, treatment of infection (or the precipitating cause), maintenance of adequate cardiac output and vascular volume, hydration, adequate nutritional support, comfort measures, prevention of complications such as gastrointestinal ulceration and aspiration, and psychologic support. Prone positioning may be used to improve oxygenation, but studies have not demonstrated prone positioning to decrease the total number of days on mechanical ventilation; in addition this requires close communication and coordination among the health care team (Curley, Hibberd, Fineman, et al, 2005; Frye, 2005). Definitive therapy is directed toward improvement of oxygenation. The use of endotracheal intubation, positive end-expiratory pressure, and low tidal volume may be required to ensure maximum oxygen delivery by increasing functional residual capacity, reducing intrapulmonary shunting, and reducing pulmonary fluid. Ventilation with low tidal volume (6 ml/kg of ideal body weight) has been associated with lower mortality rates in children with ALI (Albuali, Singh, Fraser, et al, 2007; Hanson and Flori, 2006).
Additional supportive strategies in the treatment of ARDS in children include the use of permissive hypercapnia, inhaled nitric oxide, exogenous surfactant administration, high-frequency ventilation, partial liquid ventilation, and extracorporeal life support (extracorporeal membrane oxygenation, or ECMO). Exogenous surfactant therapy has increased oxygenation status in infants and children with ARDS-ALI and decreased disease severity (Willson, Chess, and Notter, 2008). Once the underlying cause is identified, specific treatment (e.g., antibiotics for infection) is initiated.
Prognosis: In spite of advances in understanding and treating ARDS-ALI, childhood mortality rates of 18% to 49% have been reported (Albuali, Singh, Fraser, et al, 2007; Randolph, 2009), and prolonged respiratory failure requiring an average of 10 to 16 days on mechanical ventilation is common (Randolph, 2009). The precipitating disorder influences the outcome. The worst prognosis is associated with profound hypoxemia, uncontrolled sepsis, bone marrow transplantation, cancer, and multisystem involvement with hepatic failure. Children who recover may have persistent cough and exertional dyspnea.
Nursing Care Management
The child with ARDS is cared for in intensive care during the acute stages of illness. Nursing care involves close monitoring of oxygenation and respiratory status, cardiac output, perfusion, fluid and electrolyte balance, and renal function (urinary output). Blood gas analysis and pulse oximetry are important evaluation tools. Parenteral and enteral nutritional support is often required because of the length of the acute phase of the illness. Medications are administered to reduce pulmonary fluid, decrease pulmonary hypertension, and treat the underlying cause (e.g., antibiotics for sepsis). Nursing management also includes managing pain, monitoring the effects of the numerous parenteral fluids and drugs used to stabilize the child, and monitoring for changes in the child’s hemodynamic status. Most children with ARDS require invasive monitoring via a central venous line and possibly a pulmonary artery catheter to monitor oxygenation and administer medications. The nursing care of the child with ARDS also involves close observation of skin condition and prevention of breakdown, passive range of motion for prevention of muscle atrophy and contractures, and nutritional support.
Respiratory distress is a frightening situation for both the child and the parents, and attention to their psychologic needs is a major element in the care of these children. The child is often sedated during the acute phase of the illness, and weaning from sedation requires close monitoring for anxiety reduction and comfort. Because the mortality rate of ARDS is high, keep the family informed of the child’s status, progression through the various stages of the illness, and, as appropriate, the possibility of death. (See Chapter 23.)
Smoke Inhalation Injury
A number of noxious substances that may be inhaled are toxic to humans. They are primarily products of incomplete combustion and cause more deaths from fires than flame injuries do. The severity of the injury depends on the nature of the substances generated by the material being burned, whether the victim is confined in a closed space, and the duration of contact with the smoke.
General Aspects
Possible inhalation injury is suspected when there is a history of flames in a closed space, whether or not burns are present. Sooty material around the nose or in the sputum; singed nasal hairs; or mucosal burns of the nose, lips, mouth, or throat are all signs that the affected person requires observation for possible pulmonary injury from inhalants. A hoarse voice and cough are further evidence of airway involvement, and increased inspiratory and expiratory stridor indicates severe damage to the upper passages. Signs of respiratory distress also include tachypnea; tachycardia; and diminished or abnormal breath sounds, including crackles and wheezes.
Three distinct syndromes of pulmonary complications may occur in the child suffering from inhalation injury: (1) early carbon monoxide poisoning, airway obstruction, and pulmonary edema; (2) ARDS occurring at 24 to 48 hours, or later in some cases; and (3) late complications of bronchopneumonia, and pulmonary emboli (Antoon and Donovan, 2007). Strangulation may also occur from the cervical eschar secondary to a severe burn.
Smoke inhalation causes three different types of injury: heat, local chemical, and systemic.
Heat Injury: Heat causes thermal injury to the upper airway, but because air has low specific heat, the injury goes no farther than the upper airway. Reflex closure of the glottis prevents injury to the lower airway. Heat may reach the middle airway occasionally, but it rarely penetrates to the lungs.
Chemical Injury: The combustion of materials such as clothing, furniture, and floor coverings can generate a wide variety of gases. Acids, alkalis, and their precursors in smoke can produce chemical burns. These substances can be carried deep into the respiratory tract, including the lower respiratory tract, in the form of insoluble gases. Soluble gases tend to dissolve in the upper respiratory tract.
Synthetic materials are especially toxic, producing gases such as oxides of sulfur and nitrogen, acetaldehyde, formaldehyde, hydrocyanic acid, and chlorine. Heated plastics are the source of extremely toxic vapors, including chlorine and hydrochloric acid from polyvinylchloride, and hydrocarbons, aldehydes, ketones, and acids from polyethylene. Irritant gases such as nitrous oxide or carbon dioxide combine with water in the lungs to form corrosive acids. Aldehydes cause denaturation of proteins, cellular damage, and edema of pulmonary tissues. Chemical burns to the airways are similar to burns on the skin, except they are painless because the tracheobronchial tree is relatively insensitive to pain.
Inhalation of small amounts of noxious irritants produces alveolar and bronchiolar damage that can lead to obstructive bronchiolitis. Severe exposure causes further injury, including alveolocapillary damage with hemorrhage, necrotizing bronchiolitis, and inhibited secretion of surfactant, with resultant atelectasis.
Systemic Injury: Gases that are nontoxic to the airways (e.g., carbon monoxide, hydrogen cyanide) can cause injury and death by interfering with or inhibiting cellular respiration. Carbon monoxide is a colorless, odorless gas with an affinity for hemoglobin 230 times greater than that of oxygen. When carbon monoxide enters the bloodstream, it readily binds with hemoglobin to form carboxyhemoglobin (COHb). Because carbon monoxide combines more readily and is released less readily than oxygen, very low levels of tissue oxygen must be reached before appreciable amounts of oxygen are released from the hemoglobin. Therefore tissue hypoxia reaches dangerous levels before oxygen is available to meet tissue needs.
Accidental carbon monoxide poisoning is most often a result of exposure to fumes from heaters or smoke from structural fires, although poorly ventilated recreational vehicles with improperly operated or maintained gas lamps or stoves and cooking in underventilated areas with charcoal grills or hibachis are also frequent causes. Intentional carbon monoxide poisoning may occur in an attempted suicide in the vehicle parked in a closed garage for a long period. Accidental carbon monoxide poisoning may also occur in a vehicle with inadequate vented exhaust which leaks into the vehicle’s passenger (or closed truck bed) compartment. Carbon monoxide is produced by incomplete combustion of carbon or carbonaceous material, such as wood or charcoal.
The signs and symptoms of carbon monoxide poisoning are secondary to tissue hypoxia and vary with the level of COHb. Mild manifestations include headache, visual disturbances, irritability, and nausea, whereas more severe intoxication causes confusion, hallucinations, ataxia, and coma. Carbon monoxide may increase cerebral blood flow, increase cerebral capillary permeability, and increase cerebrospinal fluid pressure, all of which contribute to the central nervous system signs observed. The bright, cherry red lips and skin often described are less common than pallor and cyanosis.
Therapeutic Management
The treatment of children with smoke toxicity is largely symptomatic. The most widely accepted treatment is placing the child on humidified 100% oxygen as quickly as possible and monitoring for signs of respiratory distress and impending failure. Blood gases are drawn to determine baseline arterial blood gases and COHb levels. Arterial oxygen partial pressure may be within normal limits unless there is marked respiratory depression. If carbon monoxide poisoning is confirmed, 100% oxygen is continued until COHb levels fall to the nontoxic range of about 10%.
Respiratory distress may occur early in the course of smoke inhalation as a result of hypoxia, or patients who are breathing well on admission may suddenly develop respiratory distress. Therefore endotracheal intubation equipment should be readily available. Transient edema of the airways can occur at any level in the tracheobronchial tree. Assessment and localization of the obstruction should be accomplished before severe swelling of the head, neck, or oropharynx occurs. Intubation is often necessary when (1) severe burns in the area of the nose, mouth, and face increase the likelihood of developing oropharyngeal edema and obstruction; (2) vocal cord edema causes obstruction; (3) the patient has difficulty handling secretions; and (4) progressive respiratory distress requires artificial ventilation. Controversy surrounds tracheotomy, but many prefer this procedure when the obstruction is proximal to the larynx and reserve nasotracheal intubation for lower tract involvement.
Pulmonary care may be facilitated by bronchodilators, inhaled corticosteroids, humidification, and CPT to enhance the removal of necrotic material, minimize bronchoconstriction, and avoid atelectasis. Bronchoscopy may be needed to clear heavy secretions.
Carbon monoxide is excreted primarily through the lungs. Treatment of carbon monoxide intoxication with 100% oxygen via nonrebreathing face mask reduces the COHb level by one half in 40 to 60 minutes. Hyperbaric oxygen may be required for severe carbon monoxide poisoning (COHb level >25% in children) (Rodgers, Condurache, Reed, et al, 2007).
Nursing Care Management
Nursing care of the child with inhalation injury is the same as that for any child with respiratory distress. The initial goal is to maintain a patent airway and effective ventilation status; endotracheal intubation may be required early depending on the patient’s respiratory status and the progression of airway and pulmonary edema. (See also Respiratory Failure, Chapter 31.) In the acute phase take vital signs, oxygenation, work of breathing, and other respiratory assessments frequently, and carefully observe and maintain the pulmonary status. The administration of nebulized bronchodilators, humidified oxygen, and inhaled corticosteroids is often part of the nursing care if a respiratory specialist is not available. CPT is also an important part of the therapeutic program. IV fluids are often required to maintain adequate hydration if the patient’s respiratory status is deteriorating. Fluid requirements for children experiencing inhalation injury are greater than for those with surface burns alone. However, one concern is the development of pulmonary edema; therefore accurate monitoring of intake and output is essential. For the patient requiring mechanical ventilation, the nurse monitors respiratory status and ensures airway patency is maintained. Such patients may be mildly sedated initially; once they are alert, the nurse reassures the patients of the temporary nature of the inability to speak, effectively manages any pain they are experiencing, and implements comfort measures.
In addition to the observation and management of the physical aspects of inhalation injury, the nurse also deals with the psychologic needs of a frightened child and distraught parents. Parents need support; reassurance; and information about their child’s condition, treatment, and progress.
Environmental Tobacco Smoke Exposure
Numerous investigations indicate that parental smoking is an important cause of morbidity in children. Children exposed to passive or environmental tobacco smoke have an increased number of respiratory illnesses, increased respiratory symptoms (e.g., cough, sputum, and wheezing), and reduced performance on pulmonary function tests (PFTs). AOM and OME are also increased in children who have smoking parents. Indoor exposure to environmental tobacco smoke has been linked to asthma in children (Morkjaroenpong, Rand, Butz, et al, 2002). Among children with asthma, there is an association between parental cigarette smoking and asthma exacerbations, trips to the emergency department, medication use, and impaired recovery after hospitalization for acute asthma. Children exposed to second-hand smoke experience more wheezing episodes as infants. Maternal cigarette smoking is associated with increased respiratory symptoms and illnesses in children; decreased fetal growth; increased deliveries of low-birth-weight, preterm, and stillborn infants; and a greater incidence of sudden infant death syndrome (SIDS). Antenatal maternal smoking has emerged as a significant risk factor for SIDS (American Academy of Pediatrics, 2005). The risk for diagnosis of early-onset asthma in the first 3 years of life is associated with in utero exposure to maternal smoking; grandmaternal smoking was also associated with an increased risk of early-onset asthma in the grandchild even if the mother did not smoke during pregnancy (Li, Langholz, Salam, et al, 2005). Exposure to tobacco smoke during childhood may also contribute to the development of bronchopulmonary dysplasia in the adult.
State, federal, and local governments have enacted legislation prohibiting smoking in public and work places. Children experience more second-hand smoke exposure in the home setting than anywhere else. The impact of tobacco smoke also contributes to an increase in childhood deaths attributed to residential fires in households where adults smoke. The financial impact of second-hand smoke exposure is significant. The Centers for Disease Control and Prevention (2008) estimates that from 2001 to 2004 the average annual smoking-attributable health care expenditures were $96 billion.
Recently concern has grown regarding third-hand tobacco exposure—the tobacco toxins that remain in the environment long after the smoker has stopped smoking. Such toxins may be found in elevators, cars, and homes and on clothing. The 2006 surgeon general’s report concluded there is no risk-free level of tobacco exposure because of the residual effects of the chemical toxins in tobacco (Surgeon General Report, 2006). The effect of such chemicals on the developing child’s brain are under evaluation.
The amount of passive smoke exposure in infants and children is directly related to the presence of smoking parents and the number of smokers in a household. Past studies that have measured passive smoke exposure have analyzed the amount of cotinine in a child’s urine (Winkelstein, Tarzian, and Wood, 1997; Olivieri, Bodini, Peroni, et al, 2006). Cotinine, a by-product of nicotine, is considered a valid biochemical marker for environmental smoke exposure. Urinary cotinine levels are increased in children who live in homes with smokers, and these levels increase proportionately with the number of smokers in the home. Cotinine levels have also been used to document exposure to passive smoke in the fetus and newborn.
Nursing Care Management
Passive smoke exposure during childhood may also contribute to the development of bronchopulmonary dysplasia in adulthood, and some have attributed infertility in adults to exposure to second-hand smoke as children. Nurses and other health care professionals need to include assessments of passive smoke exposure in all children, especially those with respiratory illnesses. In families where smokers refuse to quit, house rules should be established for reducing smoke in the child’s environment. Nurses should also inform caregivers of the health hazards of children’s exposure to tobacco smoke*; set an example for children and families; and become advocates for “no smoking” ordinances in public places, prohibition of advertising tobacco products in the media, and inclusion of health warnings of sidestream smoke on tobacco products. Nurses also have an important role in offering tobacco smoking cessation counseling and in teaching such classes in the community at large. The role of the nurse in promoting smoking cessation among adolescents is discussed in Chapter 21.






 inches) from the mouth or
inches) from the mouth or

