The Child with Cardiovascular Dysfunction
Cardiac Structure and Function
Clinical Consequences of Congenital Heart Disease
Classification of Congenital Heart Defects
Defects with Increased Pulmonary Blood Flow
Defects with Decreased Pulmonary Blood Flow
Transposition of the Great Arteries or Transposition of the Great Vessels
Nursing Care of the Child with Congenital Heart Disease and His or Her Family
http://evolve.elsevier.com/wong/ncic
Birth of a Child with a Physical Defect, Ch. 11
Compliance, Ch. 27
Controlling Elevated Temperatures, Ch. 27
Family-Centered Care of the Child with Chronic Illness or Disability, Ch. 22
Family-Centered End-of-Life Care, Ch. 23
Heart (Physical Examination), Ch. 6
High Risk Related to Cardiovascular and Hematologic Complications, Ch. 10
Neonatal Loss, Ch. 10
Pain in Neonates, Ch. 7
Nursing Care of the Surgical Neonate, Ch. 11
Pain Assessment; Pain Management, Ch. 7
Physiologic Measurements, Ch. 6
Preparation for Diagnostic and Therapeutic Procedures, Ch. 27
Skin (Physical Examination), Ch. 6
Surgical Procedures, Ch. 27
Organ or Tissue Donation and Autopsy, Ch. 23
Transplantation (Renal), Ch. 30
Cardiac Structure and Function
![]() Cardiovascular disorders in children are divided into two major groups: congenital cardiac defects and acquired heart disorders. Congenital heart defects are anatomic abnormalities present at birth that result in abnormal cardiac function. The clinical consequences of congenital heart defects fall into two broad categories: heart failure (HF) and hypoxemia. Acquired cardiac disorders refer to disease processes or abnormalities that occur after birth and can be seen in the normal heart or in the presence of congenital heart defects. They result from various factors, including infection, autoimmune responses, environmental factors, and familial tendencies.
Cardiovascular disorders in children are divided into two major groups: congenital cardiac defects and acquired heart disorders. Congenital heart defects are anatomic abnormalities present at birth that result in abnormal cardiac function. The clinical consequences of congenital heart defects fall into two broad categories: heart failure (HF) and hypoxemia. Acquired cardiac disorders refer to disease processes or abnormalities that occur after birth and can be seen in the normal heart or in the presence of congenital heart defects. They result from various factors, including infection, autoimmune responses, environmental factors, and familial tendencies.
![]() Animation—Structure of the Heart
Animation—Structure of the Heart
Understanding the effects of congenital and acquired heart defects requires knowledge of the normal heart’s structure and function, including embryologic development, fetal circulation, and the changes that occur with postnatal growth. Basic cardiac physiology is presented in this section; altered hemodynamics are discussed on p. 1350.
Cardiac Development and Function
The heart is a muscular four-chambered organ whose primary purpose is to pump blood throughout the body. It is located slightly to the left of the sternum in the space between the two pleural cavities, called the mediastinum. The main mass of the heart is formed by the muscular tissue, the myocardium. Lining the inner surface of the myocardium is the endocardium, a thin layer of endothelial tissue. The heart also has its own special covering, a double-walled membrane called the pericardium. Between the two layers is a slight space (pericardial space), which is filled with a few drops of serous fluid (pericardial fluid). These layers provide for frictionless movement of the heart muscle.
The interior of the heart is divided into four chambers. The two upper chambers are called atria; the two bottom chambers are called ventricles. The atria are divided into the right atrium (RA) and the left atrium (LA) by the atrial septum. The ventricles are divided into the right ventricle (RV) and the left ventricle (LV) by the ventricular septum. Located within the heart are four valves, whose main function is to prevent the backflow of blood. The tricuspid valve, so named because it has three leaflets, or cusps, of endocardial tissue projecting into the ventricles, is located between the RA and the RV. The mitral valve has two leaflets and is located between the LA and the LV. Together these two valves are often termed atrioventricular (AV) valves. The valve leaflets are attached to the heart muscle by several cordlike structures called chordae tendineae. The semilunar valves are located in the pulmonary artery (pulmonic valve) and the aorta (aortic valve). Heart sounds (S1 and S2) are related to the vibrations that result during closing of these valves. (See Chapter 6.)
Embryologic Development
The heart and other components of the circulatory system (blood, blood vessels, lymph) begin to develop from the mesoderm during the fourth week of gestation and are completely formed by the eighth week. Cardiac development progresses with the embryo’s increasing nutritional needs.
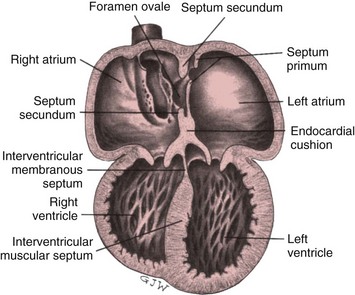
During the third week, two endocardial tubes fuse to become the heart tube. As the tube elongates, it begins to coil to the right (dextro- or d-looping). This looping occurs by approximately the twenty-eighth day, when the heart begins to beat. Concentrations of mesenchymal cells enlarge and cause their lining (endocardium) to bulge into the heart lumen. These internal bulges are called endocardial cushions and eventually merge to divide the heart chambers.
The developing heart tube bulges until it finally lies in the pericardial cavity. The tube remains attached to the pericardium at its cephalic and caudal ends but is free at the midsection. During the fifth week the midcardiac tube grows rapidly and assumes a characteristic convoluted shape with identifiable structures. These structures ultimately give rise to the heart chambers and great vessels and include (1) a common atrium; (2) a common ventricle; (3) the bulbus cordis, which eventually helps form the outflow tracts of the ventricles; (4) the sinus venosus, which develops into the inferior and superior vena cava and coronary sinus; and (5) the truncus arteriosus, which divides into the pulmonary artery and aorta and also gives rise to the aortic arch.
The formation of the heart’s internal structures, particularly the cardiac septa (partitions), takes place almost simultaneously. The atrial septum is formed by the growth of both the septum primum and the septum secundum at about the fourth week of fetal development. Overlapping of the septum primum and septum secundum before fusion results in a temporary flap opening known as the foramen ovale.
The ventricular septum develops from the joining of the muscular and membranous ventricular septa during the fourth to eighth weeks of growth. The muscular septum develops when the right and left ventricular chambers fuse, whereas the membranous septum develops out of an intricate growth of the endocardial cushions, conal cushions, and conotruncal septum (Fig. 34-1). Congenital defects may result if disturbances occur in the formation of various structures during this partitioning process.
Fetal Circulation: ![]() The characteristics of fetal circulation ensure that the most vital organs and tissues receive the maximum concentration of oxygenated blood. The fetal brain requires the highest oxygen concentration. The lungs are essentially nonfunctional, and the liver is only partially functional. Therefore the fetus needs less blood in these organs during fetal life.
The characteristics of fetal circulation ensure that the most vital organs and tissues receive the maximum concentration of oxygenated blood. The fetal brain requires the highest oxygen concentration. The lungs are essentially nonfunctional, and the liver is only partially functional. Therefore the fetus needs less blood in these organs during fetal life.
![]() Anatomy Review—Changes in Circulation at Birth
Anatomy Review—Changes in Circulation at Birth
Blood carrying oxygen and nutritive materials from the placenta enters the fetal system through the umbilicus via the large umbilical vein (Fig. 34-2, A). The blood then travels to the liver, where it divides. Part of the blood enters the portal and hepatic circulation of the liver, and the remainder travels directly to the inferior vena cava (IVC) by way of the ductus venosus. Because of the higher pressure of blood entering the RA from the IVC, it is directed in a straight pathway across the RA and through the foramen ovale to the LA. In this way the better-oxygenated blood enters the LA and LV to be pumped through the aorta to the head and upper extremities. Blood from the head and upper extremities entering the RA from the superior vena cava (SVC) is directed downward through the tricuspid valve into the RV. From there it is pumped through the pulmonary artery, where the major portion is shunted to the descending aorta via the ductus arteriosus. A small amount flows to and from the nonfunctioning fetal lungs. Blood is returned to the placenta from the descending aorta through the two umbilical arteries.
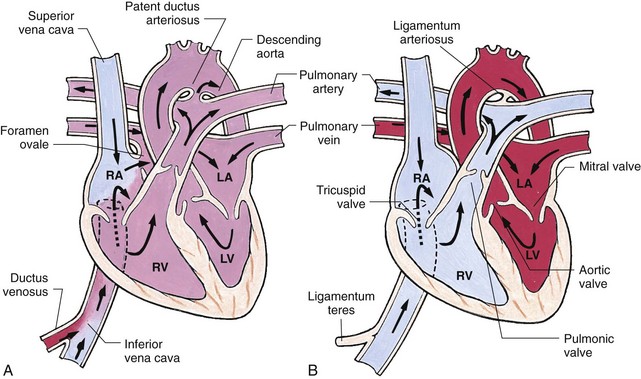
Fig. 34-2 Changes in circulation at birth. A, Prenatal circulation. B, Postnatal circulation. Arrows indicate direction of blood flow. Although four pulmonary veins enter the LA, for simplicity this diagram shows only two. RA, Right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle.
![]() Before birth, the high pulmonary vascular resistance created by the collapsed fetal lung causes greater pressures in the right side of the heart and the pulmonary artery. At the same time, the free-flowing placental circulation and the ductus arteriosus produce a low systemic vascular resistance in the remainder of the fetal vascular system. With the clamping of the umbilical cord and the expansion of the lungs at birth, the hemodynamics of the fetal vascular system undergoes pronounced and abrupt changes. These changes are the direct result of cessation of the placental blood flow and the beginning of lung respiration. Chapter 8 discusses the changes occurring at birth, and Fig. 34-2, B, shows the circulatory changes in the heart.
Before birth, the high pulmonary vascular resistance created by the collapsed fetal lung causes greater pressures in the right side of the heart and the pulmonary artery. At the same time, the free-flowing placental circulation and the ductus arteriosus produce a low systemic vascular resistance in the remainder of the fetal vascular system. With the clamping of the umbilical cord and the expansion of the lungs at birth, the hemodynamics of the fetal vascular system undergoes pronounced and abrupt changes. These changes are the direct result of cessation of the placental blood flow and the beginning of lung respiration. Chapter 8 discusses the changes occurring at birth, and Fig. 34-2, B, shows the circulatory changes in the heart.
Postnatal Development
In infancy the size of the heart in relation to total body size is larger, and the heart occupies a larger space within the mediastinum. The ventricular walls are more or less equal in thickness at birth. With the postnatal rise in systemic vascular resistance, the LV walls become thicker than the walls of the RV, and the pressures on the left side of the heart rise.
Right-sided pressures decrease because the RV is pumping blood to the low-pressure pulmonary bed. An increase in heart size accompanies the adolescent growth spurt, with a resulting increase in blood pressure (BP) and decrease in heart rate. The heart rate at any age shows an inverse relationship to body size. (See inside back cover.)
The arteries and veins elongate to keep pace with expanding body dimensions, and the vessel walls thicken to cope with the increased pressure. The systolic BP after birth is low, reflecting the weaker LV of the neonate. With the developing strength and power of the left side of the heart, the systolic pressure rises rather sharply during the first 6 weeks and continues to rise but at a much slower rate until shortly before puberty, at which point it rises rapidly to adult levels. (See inside back cover.)
Postnatal Circulation: Once the cardiorespiratory system adjusts to the changes necessary to support extrauterine life, the circulation through the heart assumes a pathway that allows for oxygenation of blood by the lungs and delivery of oxygenated blood to the systemic circulation. Blood returning from the body via the SVC and IVC is received in the RA. It flows to the RV through the tricuspid valve. The RV pumps the blood through the pulmonic valve into the pulmonary artery and then to the lungs, where the blood becomes saturated with oxygen. The blood is then returned from the lungs via the pulmonary veins into the LA, where it flows through the mitral valve to the LV, and finally through the aortic valve to the aorta and into the systemic circulation (see Fig. 34-2, B).
Arteries are thicker-walled blood vessels with thin muscular layers that carry highly oxygenated blood away from the heart to the capillary bed, which supplies oxygen and nutrients to the tissues. Veins are thin-walled blood vessels that return desaturated blood to the heart. The arterial system provides resistance to blood flow to maintain BP and circulation. The venous system acts as a collecting system and a reservoir to accommodate changes in circulating blood volume. Both work together to provide equilibrium and maintain BP.
The heart muscle receives its blood supply through the coronary circulation. The right and left coronary arteries, which arise above the aortic valve, supply all of the myocardium. The heart is the first organ to receive blood with each heartbeat; the brain is next. These two organs depend most on adequate oxygen levels for normal function. Coronary veins collect the blood and return it to the RA directly or through the coronary sinus, which drains into the RA. The flow of blood throughout the systemic circulatory system is shown in Fig. 34-3.

Fig. 34-3 Diagram showing serially connected pulmonary and systemic circulatory systems and how to trace the flow of blood. Right heart chambers propel unoxygenated blood through the pulmonary circulation, and the left side of the heart propels oxygenated blood through the systemic circulation. See Fig. 34-2 for abbreviations. (From McCance KL, Huether SE: Pathophysiology: the biological basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
Conduction System: ![]() To maintain an orderly and effective pumping action, the heart has a specialized electrical conduction system. Electrical impulses generated within the heart initiate the mechanical contraction that leads to the circulation of blood. Although all myocardial cells are capable of developing an action potential and depolarizing without external stimulation, certain specialized cells make up the heart’s normal conduction system. These structures include the following:
To maintain an orderly and effective pumping action, the heart has a specialized electrical conduction system. Electrical impulses generated within the heart initiate the mechanical contraction that leads to the circulation of blood. Although all myocardial cells are capable of developing an action potential and depolarizing without external stimulation, certain specialized cells make up the heart’s normal conduction system. These structures include the following:
![]() Anatomy Review—Conduction System of the Heart
Anatomy Review—Conduction System of the Heart
Sinoatrial (SA) node, located within the RA wall near the opening of the SVC
AV node, also located within the RA but near the lower end of the septum
AV bundle (bundle of His), which extends from the AV node along each side of the interventricular septum and then divides into right and left bundle branches
Purkinje fibers, which extend from the AV bundle into the walls of the ventricles
The SA node is normally the heart’s pacemaker and initiates an impulse. The impulse spreads from the SA node throughout the atria to cause depolarization. As the atria depolarize, impulses spread to the AV node to conduct to the ventricles. The AV node is the major pathway by which the impulses from the atria can be transmitted to the ventricles. The impulses then spread to the AV bundle and Purkinje fibers to cause simultaneous depolarization of the ventricles.
A cardiac cycle is composed of sequential contraction (systole) and relaxation (diastole) of both the atria and the ventricles. First, the atria contract, ejecting blood into the relaxed ventricles. Then, as the atria relax, the ventricles contract to eject blood into the pulmonary artery and aorta. During diastole, blood enters the atria from the systemic and pulmonary veins, which completes one cardiac cycle.
Basic Cardiac Physiology
The heart is basically a complex pump, ejecting blood throughout the body. The heart and lungs function together to deliver oxygen to the tissues and remove waste products such as carbon dioxide. The primary function of the cardiopulmonary system is to provide effective oxygen transport to meet the body’s metabolic needs. To perform this function, the heart must maintain an adequate cardiac output. By definition, cardiac output is the volume of blood ejected by the heart in 1 minute. This is calculated by multiplying the heart rate (number of beats per minute) by the stroke volume. Stroke volume is the amount of blood ejected by the heart in any one contraction. Three factors influence stroke volume: preload, afterload, and contractility.
The autonomic nervous system influences heart rate. The sympathetic fibers increase heart rate, and the parasympathetic fibers, acting through the vagus nerve, decrease heart rate. Levels of circulating catecholamines and other hormones also influence heart rate. Generally, an increase in heart rate increases cardiac output, and a decrease or irregularity in heart rate (bradycardia, dysrhythmia) impairs cardiac output. However, a very fast heart rate shortens diastole and impairs coronary artery perfusion, which causes eventual impairment of cardiac muscle function.
In simple terms, preload is the volume of blood returning to the heart, or the circulating blood volume. In physiologic terms, preload refers to myocardial fiber length. If the amount of blood delivered to the heart increases, then the myocardial fibers lengthen, and a greater amount of blood is pumped out of the heart. The circulating blood volume is easiest to assess clinically using the central venous pressure (CVP).
Afterload refers to the resistance against which the ventricles must pump when ejecting blood (ventricular ejection). Conditions that make it more difficult for the heart to pump blood forward into the circulation (e.g., severe hypertension) increase the afterload. Afterload is determined by several complex factors, primarily the relative resistances of the systemic circulation (systemic vascular resistance) and the pulmonary circulation (pulmonary vascular resistance). Clinically, in the absence of hemodynamic monitoring, measurement of arterial BP gives some indication of afterload. Higher BP indicates greater afterload.
Contractility refers to the efficiency of myocardial fiber shortening, or the ability of the cardiac muscle to act as an efficient pump. There is no simple bedside technique to assess contractility, although an echocardiogram may be useful. Contractility is often inferred in clinical practice. Assessments of peripheral tissue perfusion (pulses, warmth of extremities, and capillary refill) and urinary output can be helpful. Decreased contractility is suspected if the extremities are cool with thready pulses and urinary output is diminished. Certain states are known to depress contractility (e.g., hypoxia, acidosis). Adequate systemic perfusion depends on an appropriate heart rate, adequate circulating blood volume, efficient pump function, appropriate systemic and pulmonary vascular resistances, capillary permeability, and tissue utilization of oxygen. The body makes frequent adjustments in the various determinants of cardiac output to maintain a steady state. It often decreases after cardiac surgery in the early postoperative period.
Several clinical examples are useful to illustrate these principles. The Starling law (Frank-Starling curve) demonstrates that an increase in ventricular end-diastolic volume (caused by an increased preload) somewhat increases stroke volume. Because the myocardial fibers can stretch only to a certain point and still function effectively, however, any increase in volume beyond this point impairs cardiac output. When decreased cardiac output results from decreased preload (e.g., in hypovolemia due to blood loss), treatment involves providing volume, either with intravenous (IV) fluids or blood products. If decreased cardiac output results from a dramatic increase in afterload (e.g., severe hypertension) that increases the myocardial workload, treatment involves reducing afterload with vasodilating drugs. Medications such as digoxin (Lanoxin) or IV inotropic agents such as dopamine (Intropin) or dobutamine (Dobutrex) enhance contractility. Adjustments in heart rate are the most common response to changes in cardiac output. The heart rate is slowest during sleep and can more than double with strenuous physical exercise.
Assessment of Cardiac Function
Taking an accurate health history is an important first step in assessing an infant or child for possible heart disease. Parents may have specific concerns, such as poor feeding or fast breathing in their infant or the inability of their 7-year-old to keep up with his friends on the soccer field. Other parents may not realize that their child has a medical problem; the child may always have been pale and a fussy baby.
Asking details about the mother’s health history, pregnancy, and birth history are important in assessing infants. Mothers with chronic health conditions, such as diabetes or lupus erythematosus, are more likely to have infants with heart disease. Some medications, such as phenytoin (Dilantin), are teratogenic to the fetus. Maternal alcohol use or illicit drug use increases the risk of congenital heart defects. Exposure to infection, such as rubella, early in pregnancy may result in congenital anomalies. Infants with low birth weight because of intrauterine growth restriction are more likely to have congenital anomalies. High-birth-weight infants, often offspring of diabetic mothers, also have an increased incidence of heart disease.
A detailed family history is also important. There is an increased incidence of congenital cardiac defects if either parent or a sibling has a heart defect. Some diseases, such as Marfan syndrome and hypertrophic cardiomyopathy, are hereditary. A family history of frequent fetal loss, sudden infant death, and sudden death in adults may indicate heart disease. Congenital heart defects occur in many disorders such as Down syndrome and Turner syndrome.
The health history of an infant should include details about feeding patterns, weight gain, and development. Feeding difficulties accompanied by fatigue, rapid breathing, and sweating with feeds and poor weight gain are common in infants with heart disease. Discuss the incidence of respiratory infections and breathing problems. Report the onset and frequency of color changes, particularly cyanosis.
With older children and adolescents, history taking should include questions about exercise tolerance and activities, edema and respiratory problems, chest pain, palpitations, and neurologic problems such as fainting or headaches. Recent infections or toxic exposures may precede the development of heart diseases such as cardiomyopathy or rheumatic fever.
In all patients, a review of all other health problems and the presence of other congenital anomalies is important. All medications taken, including over-the-counter medications and herbal supplements, should be reviewed, since prolonged or incorrect use of many medications can cause cardiac symptoms.
Physical Examination
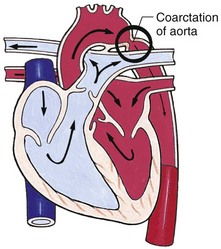
Assessment of vital signs is helpful in screening patients for diseases of the cardiovascular system. A normal pulse rate varies with age. The younger the patient, the faster the rate. A heart rate that is abnormally fast (tachycardia) or abnormally slow (bradycardia) may indicate cardiac disease. It is important to note that an acceleration of the heart rate with inspiration is normal. A fast respiratory rate (tachypnea) may indicate HF. Hypertension is diagnosed by serial BP measurements. Differences in BP between the upper and lower extremities may indicate coarctation of the aorta (see Box 34-5).
Several aspects of physical examination may yield evidence of heart disease. (See Chapter 6 for a general discussion of physical assessment of the heart.) During inspection perform a general assessment of skin color (particularly the presence of cyanosis), position of comfort, and overall nutritional status. During palpation establish the point of maximum intensity and the apical impulse, because they may offer clues to the position of the heart. Note the presence of a thrill, a soft vibration over the heart that reflects the transmitted sound of a heart murmur. Assess the quality of chest activity (“active precordium”), quality and symmetry of all pulses, warmth of extremities, and presence or absence of edema. Locating the hepatic and splenic borders for evidence of organ enlargement is also important.
![]() Auscultation of heart sounds begins with assessment of heart rate and rhythm. The normal heart sounds S1 and S2 are auscultated, and the normal physiologic splitting of S2 is noted. This splitting is caused by the normal closure of the aortic valve before the pulmonic valve. The presence of additional heart sounds, such as a gallop or a murmur, is noted. Auscultation of lung sounds, in particular crackles, wheezing, grunting, or decreased or absent breath sounds in some areas, is also important in the assessment of cardiovascular disease.
Auscultation of heart sounds begins with assessment of heart rate and rhythm. The normal heart sounds S1 and S2 are auscultated, and the normal physiologic splitting of S2 is noted. This splitting is caused by the normal closure of the aortic valve before the pulmonic valve. The presence of additional heart sounds, such as a gallop or a murmur, is noted. Auscultation of lung sounds, in particular crackles, wheezing, grunting, or decreased or absent breath sounds in some areas, is also important in the assessment of cardiovascular disease.
Murmurs are heart sounds that reflect the flow of blood within the heart. They may occur in either systole or diastole or in both (a continuous murmur). They may reflect blood flow through a normal heart (particularly during periods of increased cardiac output such as fever, anemia, or rapid growth) or indicate abnormalities within the heart or the great arteries. (See Chapter 6 for a more detailed discussion of heart murmurs.) About 80% of children have an innocent murmur of one type at some point during childhood (Park, 2008). Innocent murmurs are present in infants and children with normal cardiac anatomy and heart function.
Tests of Cardiac Function
A variety of invasive and noninvasive tests may be employed in the diagnosis of heart disease. Table 34-1 briefly outlines cardiac diagnostic procedures. The more frequently conducted tests are described here.
TABLE 34-1
PROCEDURES FOR CARDIAC DIAGNOSIS
| PROCEDURE | DESCRIPTION |
| Chest radiography (x-ray) | Provides information on heart size and pulmonary blood flow patterns |
| Electrocardiography (ECG) | Graphic measure of electrical activity of heart |
| Holter monitoring | 24-hr continuous ECG recording used to assess dysrhythmias |
| Echocardiography | Uses high-frequency sound waves obtained by a transducer to produce an image of cardiac structures |
| Transthoracic | Done with transducer on chest |
| M-mode | Provides one-dimensional graphic view to estimate ventricular size and function |
| Two-dimensional | Provides real-time, cross-sectional views of heart to identify cardiac structures and cardiac anatomy |
| Doppler | Shows blood flow patterns and pressure gradients across structures |
| Fetal | Images fetal heart in utero |
| Transesophageal | Uses transducer placed in esophagus behind heart to obtain images of posterior heart structures or improve views in patients with poor images from chest approach |
| Cardiac catheterization | Uses radiopaque catheters placed in a peripheral blood vessel and advanced into heart to measure pressures and oxygen levels in heart chambers and visualize heart structures and blood flow patterns |
| Hemodynamics | Measures pressures and oxygen saturations in heart chambers |
| Angiography | Involves injection of contrast material to illuminate heart structures and blood flow patterns |
| Biopsy | Employs special catheter to remove tiny samples of heart muscle for microscopic evaluation; used in assessing infection, inflammation, or muscle dysfunction disorders and to evaluate for rejection after heart transplantation |
| Electrophysiologic study | Employs special catheters with electrodes to record electrical activity from within heart; used to diagnose rhythm disturbances |
| Exercise stress test | Monitors heart rate, blood pressure, ECG, and oxygen consumption at rest and during progressive exercise on a treadmill or bicycle |
| Cardiac magnetic resonance imaging | Noninvasive imaging technique; allows evaluation of vascular anatomy outside of heart (e.g., coarctation of the aorta, vascular rings) and estimation of ventricular mass and volume |
Radiologic Imaging
A chest x-ray examination is the most frequently ordered radiologic test for children with suspected cardiac problems. A chest film provides a permanent record of (1) the heart’s size and configuration, its chambers, and the great vessels; and (2) the pattern of blood flow, especially in pulmonary vessels. Fluoroscopy is used mainly in conjunction with cardiac catheterization.
Electrocardiography
Electrocardiography measures the electrical activity of the heart and records it on graph paper in the electrocardiogram (ECG). This allows the evaluation of the sequence and magnitude of the electrical impulses generated by the heart (Fig. 34-4). The normal ECG consists of:
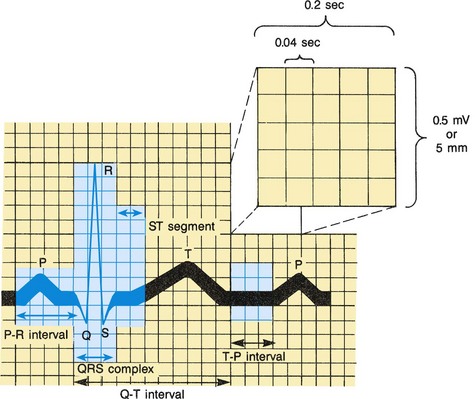
Fig. 34-4 Normal electrocardiogram pattern. Inset (upper right) shows conventional time and voltage or amplitude (height) calibrations.
P wave—Represents the spread of the impulse over the atria (atrial depolarization). The sinus node’s electrical activity is not represented in the ECG.
P-R interval—Represents the time that elapses from the beginning of atrial depolarization to the beginning of ventricular depolarization. It is termed P-R instead of P-Q because the Q wave is frequently absent.
QRS complex—Represents ventricular depolarization. It is actually composed of three separate waves—the Q, the R, and the S—that result from the currents generated when the ventricles depolarize before their contraction.
T wave—Represents ventricular repolarization.
Q-T interval—Represents ventricular depolarization and repolarization. This interval varies with heart rate; the faster the rate, the shorter the Q-T interval. Therefore in children this interval is normally shorter than in adults.
ST segment—Represents the time that the ventricles are in the absolute refractory period, the period between ventricular depolarization and repolarization.
Information supplied by an ECG includes heart rate and rhythm and indications of conduction abnormalities, muscular damage (ischemia), hypertrophy, electrolyte imbalance, effects of various drugs, and pericardial disease. The ECG gives no direct information about the mechanical performance of the heart as a pump.
Special uses of the ECG include (1) continuous ambulatory monitoring, which employs a Holter monitor, a transistorized tape recorder attached to chest leads; and (2) exercise stress testing, in which the ECG is monitored during controlled exercise, usually on a treadmill.
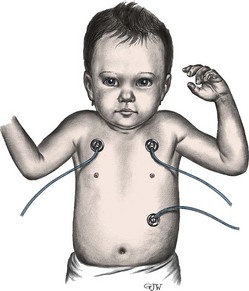
An ECG is taken by placing leads or electrodes on the skin to transmit electrical impulses back to a recording machine. Usually the electrodes are attached to the extremities and chest with an adhesive, such as hydrogel, or with a suction bulb. An electrolyte lubricant is placed between the skin and the lead to increase conductivity. Chest leads must be positioned correctly, since even minor misplacement can cause considerable inaccuracy in the recording. The standard adult ECG is measured using 12 leads (six limb leads and six chest leads). The standard pediatric ECG is measured using 15 leads, with leads added on the right side of the chest and on the left lateral chest area. Although all ECG testing is painless, the leads can be frightening. Children old enough to understand benefit from an explanation of the procedure. The child must remain still for the standard ECG; infants and young children may be more cooperative if they can rest in the parent’s lap during the procedure.
Bedside ECG cardiac monitoring is commonly used in pediatrics, especially in the care of children with heart disease. The bedside monitor provides valuable information about heart rate and rhythm through a graphic display of the ECG tracing and a digital display. An alarm can be set with parameters matched to individual patient requirements and will sound if the heart rate is above or below the set parameters. Gelfoam electrodes are commonly used and are placed on the right side of the chest (above the level of the heart) and on the left side of the chest; a ground electrode is placed on the abdomen (Fig. 34-5). Electrodes should be changed every 1 or 2 days because they irritate the skin. Bedside monitors are an adjunct to patient care and should never be substituted for direct assessment and auscultation of heart sounds. The nurse should assess the patient, not the monitor.
Holter recording is often used when a child is having daily symptoms of a potential arrhythmia. The Holter monitor records the heart rhythm continuously for 24 to 72 hours, using electrodes that are attached to the child’s chest. The rhythm is recorded with a cassette tape and then sent to a pediatric cardiologist to interpret. Also instruct the child and the parents to keep a diary of activities so that any correlation between activity and any rhythm event may be interpreted (Park, 2008).
Echocardiography
Echocardiography is one of the most frequently used procedures for detecting cardiac dysfunction in children. Recent improvements in echocardiographic techniques have made it possible to confirm the diagnosis of many congenital heart defects without resorting to cardiac catheterization as in the past. Many defects can now be diagnosed prenatally with fetal echocardiography, and the number of infants diagnosed with heart defects in utero is rising.
Echocardiography involves the use of ultra-high-frequency sound waves to produce an image of the heart’s structure. A transducer placed directly on the chest wall delivers repetitive pulses of ultrasound and processes the returned signals (echoes).
There are two types of transthoracic echocardiography. Motion-mode (M-mode) echocardiography provides a one-dimensional view of the heart and is useful in determining its size, the presence or absence of structures, and their relationship to one another. Two-dimensional (2-D), or cross-sectional, echocardiography provides information about spatial relationships between structures. Pulse, or continuous Doppler, echocardiography is primarily a velocity-sensing system and is generally used with 2-D “echo” to provide information about volume flow rate. Depending on the type of test, the nurse can obtain information regarding the integrity of septa, chamber size, and position and contractility. The nurse can also obtain information about the presence, position, size, and function of the valves, velocity of blood flow, and relationship between and size of the great vessels.
Although the test is noninvasive, painless, and associated with no known side effects, it can be stressful for children. The child must lie quietly in the standard echocardiographic positions; crying, nursing, or sitting up often leads to diagnostic errors or omissions. Therefore infants and young children may need a mild sedative (see Preoperative Sedation, p. 1007); older children benefit from psychologic preparation for the test. The distraction of a videotape is often helpful.
Transesophageal echocardiography can provide information in cases in which it is difficult to obtain information using the transthoracic approach. A transducer is passed into the esophagus to an area behind the atria. This procedure is more complicated than external echocardiography and may require intubation to protect the airway of smaller children. Patients require IV sedation before this test. Transesophageal echocardiography is frequently used in the operating room to assess for residual problems before the patient comes off cardiopulmonary bypass. Its use has reduced morbidity and mortality following surgical repair (Smallhorn, 2002).
Cardiac Magnetic Resonance Imaging
When echocardiography may be limiting, especially in the case of a child that may have poor acoustic windows or difficult and complex structures that are difficult to visualize by ultrasound alone, cardiac magnetic resonance imaging (MRI) is often used to define the unresolved anatomic pathways. The MRI can often be performed in place of a historic cardiac catheterization to obtain true three-dimensional angiography (Wood, 2006). In today’s practice, cardiac MRI is increasingly used in conjunction with other imaging modalities for assessment of cardiac anatomy, measurement of blood flow, and evaluation of myocardial perfusion and viability (Prakash, Powell, Krishnamurthy, et al, 2004). Although MRI is noninvasive, children under the age of 7 often require anesthesia, deep sedation, or conscious sedation. The patient’s developmental age and maturity are often the primary consideration in these choices, but length of the anticipated procedure and previous experience with such procedures should be considered (Geva and Van der Velde, 2006).
Cardiac Catheterization
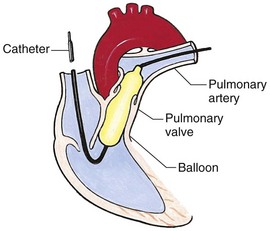
![]() The most invasive diagnostic procedure is cardiac catheterization, in which a radiopaque catheter is inserted through a peripheral blood vessel into the heart. It is usually combined with angiography (angiocardiography), in which a radiopaque contrast material is injected through the catheter and into the circulation. Cardiac catheterization provides information regarding:
The most invasive diagnostic procedure is cardiac catheterization, in which a radiopaque catheter is inserted through a peripheral blood vessel into the heart. It is usually combined with angiography (angiocardiography), in which a radiopaque contrast material is injected through the catheter and into the circulation. Cardiac catheterization provides information regarding:
![]() Animation—Catheter Placement, Umbilical Vein
Animation—Catheter Placement, Umbilical Vein
![]() Critical Thinking Exercise—Cardiac Catheterization
Critical Thinking Exercise—Cardiac Catheterization
• Oxygen saturation of blood within the chambers and great vessels
• Pressure changes within these structures
• Cardiac output or stroke volume (the amount of blood pumped out of the LV into the aorta with each contraction)
• Anatomic abnormalities, such as septal defects or obstruction to flow
Cardiac catheterization may be performed for diagnostic, interventional, or electrophysiologic purposes. The two main types of diagnostic cardiac catheterization are (1) right-sided, or venous, catheterization, in which the catheter is introduced from a vein into the RA; and (2) left-sided, or arterial, catheterization, in which the catheter is threaded by way of a systemic artery retrograde into the aorta and LV, or from a right-sided approach across the LA by means of a septal puncture or through an existing abnormal septal opening. In children the most common method is right-sided catheterization, since septal defects permit entry into the left side of the heart. The number of diagnostic catheterizations is decreasing as improvements in echocardiography and other noninvasive imaging methods allow more accurate diagnosis.
The catheter is usually introduced through a percutaneous puncture into the femoral vein (the catheter is threaded over a guide wire inserted through a large-bore needle). Rarely, a cutdown procedure is needed to gain access to the vein. This approach is associated with an increased risk of infection, hemorrhage, and obstruction. Once the vessel is entered, the catheter is guided through the heart with the aid of fluoroscopy. As the tubing is advanced, the child may feel pressure at the insertion site and vasospasm (fluttering) of the small vessels. Once the catheter is within the heart, blood samples and pressure readings are taken for analysis. Then contrast material may be injected and films taken of the dilution and circulation of the material. As the contrast medium is administered, the child may experience warmth, nausea, vomiting, restlessness, or headache.
Interventional cardiac catheterization is the use of a catheter to treat heart disease, such as use of a balloon catheter to dilate narrowed valves and vessels and catheter delivery of devices to close some simple defects. It has rapidly expanded in the last 20 years as new techniques, devices, and applications have been developed. It has replaced surgical treatment for some congenital heart defects, such as isolated valvular pulmonary artery stenosis, and has become an alternate therapy for others, such as patent ductus arteriosus, some atrial septal defects, and some types of ventricular septal defects. Complications are more common during interventional catheterizations and include balloon or device damage to vessels or valves and damage to structures due to thrombus or embolization (Lock, 2006).
Electrophysiologic studies are increasingly being used to evaluate and treat dysrhythmias. Diagnostic electrophysiologic catheterization employs catheters with tiny electrodes that record the heart’s electrical impulses directly from the conduction system. Interventional electrophysiologic catheterization uses radiofrequency ablation to destroy accessory pathways, which cause some tachydysrhythmias.
Nursing Care Management: Cardiac catheterization has become a routine procedure and may be done on an outpatient basis. Catheterization is not without risks, however, especially in neonates and seriously ill infants and children. Possible complications include acute hemorrhage from the entry site (more likely with interventional procedures because larger catheters are used), low-grade fever, nausea, vomiting, loss of a pulse in the catheterized extremity (usually transient, resulting from a clot, hematoma, or intimal tear), and transient dysrhythmias (generally catheter induced). Therefore it is essential that the nurse employ good nursing judgment and physical assessment before and after the procedure.
Preprocedural Care: A complete nursing assessment is necessary to ensure the safety of the procedure and minimize complications. This assessment should include an accurate measurement of height (essential to correct catheter selection) and weight. Obtaining a history of allergic reactions is important, since some of the contrast agents are iodine based. Specific attention to signs and symptoms of infection is crucial. Severe diaper rash may be a reason to cancel the procedure if femoral access is required. Because assessment of pedal pulses is important after catheterization, the nurse should assess and mark pulse locations (dorsalis pedis, posterior tibial) before the child goes to the catheterization room. Clearly document the presence and quality of pulses in both feet. Also record baseline oxygen saturation in children with cyanosis.
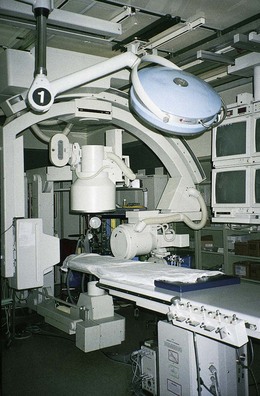
Preparing the child for the procedure is the joint responsibility of the physician, nurse, and parents. School-age children and adolescents benefit from a description of the catheterization laboratory (Fig. 34-6) and a chronologic explanation of the procedure emphasizing what they will see, feel, and hear. Preparation materials such as picture books or videotapes or tours of the catheterization laboratory may be helpful. Preparation should be geared to the child’s developmental level. (See further discussion on p. 1376 and in Chapter 27.) The child’s caregivers often benefit from the same explanations. Additional information, such as the expected length of the catheterization procedure, description of the child’s appearance after catheterization, and usual postprocedural care, should be outlined.
Methods of sedation vary among institutions and may include oral or IV medications. (See Preoperative Sedation, p. 1007.) General anesthesia is usually unnecessary except in selected interventional procedures. Typically the child is allowed nothing by mouth (NPO) before catheterization, although polycythemic infants and children may require IV fluids to prevent dehydration, and neonates may need dextrose solution for up to 2 hours before the procedure to prevent hypoglycemia. Usually the morning dose of all oral medications is withheld, although this is clarified beforehand with the practitioner.
Postprocedural Care: Patients may recover from the catheterization procedure in a recovery unit or in their hospital rooms. Some may require care in the intensive care unit (ICU). Patients are usually placed on a cardiac monitor and a pulse oximeter for the first few hours after catheterization.
The most important nursing responsibility is observation of the following for signs of complications:
• Pulses, especially below the catheterization site, for equality and symmetry (Pulse distal to the site may be weaker for the first few hours after catheterization but should gradually increase in strength.)
• Temperature and color of the affected extremity, since coolness or blanching may indicate arterial obstruction
• Vital signs, which may be taken as frequently as every 15 minutes, with special emphasis on the heart rate, which is counted for 1 full minute for evidence of dysrhythmias or bradycardia
• BP, especially for hypotension, which may indicate hemorrhage from cardiac perforation or bleeding at the site of initial catheterization
• Dressing, for evidence of bleeding or hematoma formation in the femoral or antecubital area
• Fluid intake, both IV and oral, to ensure adequate hydration (Blood loss in the catheterization laboratory, the child’s preprocedure NPO status, and diuretic actions of contrast material used during the procedure put the child at risk for hypovolemia and dehydration.)
Infants are particularly at risk for hypoglycemia. They should receive dextrose-containing IV fluids, and blood glucose levels should be checked.
Depending on hospital policy, the child may remain in bed with the affected extremity maintained straight for 4 to 6 hours after venous catheterization and 6 to 8 hours after arterial catheterization to facilitate healing of the cannulated vessel. If younger children have difficulty complying, they can be held in the parent’s lap with the leg maintained in the correct position. The child’s usual diet can be resumed as soon as tolerated, beginning with sips of clear liquids and advancing as the condition allows it. Generally there is only slight discomfort at the percutaneous site. Acetaminophen (Tylenol), with or without codeine, or ibuprofen, can be given for pain. The catheterization site is covered with an occlusive waterproof pressure dressing (usually a foam tape dressing tightly applied) to prevent bleeding and contamination that could cause infection. The dressing is left on until the next day. Home care instructions are listed in the Family-Centered Care box.
Congenital Heart Disease
The incidence of congenital heart disease (CHD) in children is approximately 5 to 8 in 1000 live births (Park, 2008). About 2 to 3 in 1000 infants are symptomatic during the first year of life with significant heart disease that requires treatment (Hoffman and Kaplan, 2002). CHD is the major cause of death (other than prematurity) in the first year of life. Although there are more than 35 well-recognized defects, the most common heart anomaly is ventricular septal defect (Box 34-1).
The exact cause of most congenital cardiac defects is unknown. Most are thought to be a result of multiple factors—a complex interaction of genetic and environmental influences. The tremendous amount of information being discovered through molecular biology and the Human Genome Project will likely increase our understanding of the genetic causes of congenital heart defects.
Some risk factors are known to be associated with increased incidence of congenital heart defects. Maternal risk factors include chronic illnesses such as diabetes or poorly controlled phenylketonuria, alcohol consumption, and exposure to environmental toxins and infections. Family history of a cardiac defect in a parent or sibling increases the likelihood of a cardiac anomaly. The risk of CHD increases if a first-degree relative (parent or sibling) is affected. The familial risk is higher with left-sided obstructive lesions.
Congenital heart anomalies are often associated with chromosomal abnormalities, specific syndromes, or congenital defects in other body systems (see Research Focus box). Down syndrome (trisomy 21) and trisomy 13 and 18 are highly correlated with congenital heart defects. Syndromes associated with heart defects include Noonan syndrome (pulmonic valve anomalies and cardiomyopathy), Williams syndrome (aortic and pulmonic stenosis), and Holt-Oram syndrome (upper limb anomalies and atrial septal defect). Extracardiac defects such as tracheoesophageal fistula, renal abnormalities, and diaphragmatic hernia are seen in association with heart anomalies.
Altered Hemodynamics
If the physiology of heart defects is to be understood, the role of pressure gradients, flow, and resistance within the circulation must be reviewed. Blood flows because of pressure gradients in different parts of the body and because of the heart’s pumping action. Like any fluid, blood flows from an area of high pressure to one of low pressure and takes the path of least resistance. The rate of flow is directly proportional to the pressure gradient (i.e., the higher the pressure gradient, the greater the rate of flow) and inversely proportional to the resistance (i.e., the higher the resistance, the lower the rate of flow). However, increased resistance does not always decrease flow. If the proximal cardiac chamber can increase the driving pressure proportionally, flow can remain unchanged.
Normally the pressure on the right side of the heart is lower than that on the left side, and the resistance in the pulmonary circulation is less than that in the systemic circulation. Likewise, vessels entering or exiting these chambers have corresponding pressures (e.g., lower pressure in the pulmonary artery and higher pressure in the aorta). Therefore, if an abnormal connection exists between the heart chambers, such as a septal defect, blood flows from an area of higher pressure (left side) to one of lower pressure (right side). This directional flow of blood is termed a left-to-right shunt. If the opening is small, the amount of blood shunted to the atrium or ventricle may be minimal.
An understanding of saturations within the heart is also helpful in understanding CHD. The blood returning to the heart via the great veins (the SVC and the IVC) should have the lowest oxygen saturation because the tissues should have extracted oxygen, leaving the venous blood desaturated. Saturations in the RA, RV, and pulmonary artery should be equal. Blood returning from the lungs to the heart through the pulmonary veins should be fully saturated, the most oxygen-rich blood in the body. Saturations on the left side of the heart should all be equal, with fully saturated blood entering the aorta and first supplying the heart muscle through the coronary arteries and then supplying the brain (Fig. 34-7). Normally, saturated blood circulates separately from desaturated blood. Depending on the type of defect, saturated and desaturated blood may be mixed. The amount of mixed blood that reaches the systemic circulation is a significant feature of several cardiac anomalies, and varying degrees of hypoxemia and cyanosis result.
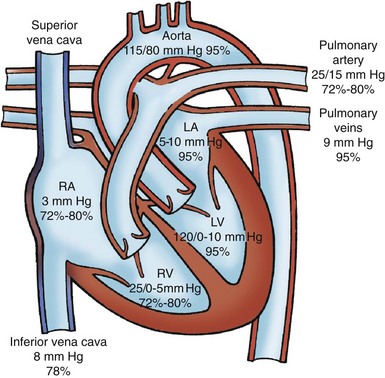
Fig. 34-7 Normal chamber pressures (mm Hg) and oxygen saturations (Sao2) in cardiac chambers and great arteries. For simplicity, only two of the four pulmonary veins are shown. See Fig. 34-2 for abbreviations.
Clinical Consequences of Congenital Heart Disease
Depending on the severity of the cardiac defect and the altered hemodynamics, two principal clinical consequences can occur: HF and hypoxemia. Defects that result in left-to-right shunting of blood cause symptoms of HF. Defects that result in decreased pulmonary blood flow cause cyanosis. The conditions can occur alone or together. Pulmonary hypertension, an uncommon condition, may occur as a result of congenital heart defects and is included in this section, although some cases may occur from unknown causes. Nursing care plays a critical role in the early identification and supportive management of these conditions.
Heart Failure
![]() HF is the inability of the heart to pump an adequate amount of blood to the systemic circulation at normal filling pressures to meet the body’s metabolic demands. Causes of HF can be classified in terms of the following changes:
HF is the inability of the heart to pump an adequate amount of blood to the systemic circulation at normal filling pressures to meet the body’s metabolic demands. Causes of HF can be classified in terms of the following changes:
![]() Nursing Care Plan—The Child with Heart Failure
Nursing Care Plan—The Child with Heart Failure
• Volume overload, especially with left-to-right shunts that may cause the RV to hypertrophy to compensate for the additional blood volume
• Pressure overload, primarily resulting from obstructive lesions, such as valvular stenosis or coarctation of the aorta
• Decreased contractility, primarily decreased contractility of the myocardium, caused by factors such as cardiomyopathy or myocardial ischemia from severe anemia or asphyxia; heart block; acidemia; and low levels of potassium, glucose, calcium, or magnesium
• High cardiac output demands, in which the body’s need for oxygenated blood exceeds the heart’s cardiac output (even though the volume may be normal), such as in sepsis, hyperthyroidism, and severe anemia
The etiology of heart failure varies according to the age of onset; HF occurs in children with both CHD and normal cardiac structure (Box 34-2). HF occurs most frequently secondary to congenital heart defects in which structural abnormalities result in an increased volume load or increased pressure load on the ventricles. For example, septal defects can cause large left-to-right shunts, which result in a volume load on the RV. Obstruction to flow out of the LV, such as narrowing of the aorta (coarctation of the aorta), can cause increased pressure inside the ventricle. HF can also be a result of an excessive workload on a normal myocardium. Myocardial failure, in which the contractility of the heart muscle is impaired, can result from cardiomyopathy, drugs, electrolyte imbalances, dysrhythmias, and other causes. Diseases in other organ systems, particularly the lungs, also can cause HF. Obstructive changes in the lungs result in increased pulmonary vascular resistance, which increases the RV workload. In time, the right side of the heart has difficulty pumping blood forward to the lungs, becomes dilated, and hypertrophies; then signs and symptoms of right-sided heart failure are seen. Cor pulmonale is the term for HF resulting from obstructive lung diseases such as cystic fibrosis or bronchopulmonary dysplasia.
Pathophysiology
Theoretically, heart failure may be divided into two types: right-sided failure and left-sided failure. In right-sided failure, RV function is reduced. RV end-diastolic pressure rises, causing increased CVP and systemic venous engorgement. Systemic venous hypertension causes hepatomegaly and may cause edema in the extremities. In left-sided failure, LV dysfunction occurs and LV end-diastolic pressure rises; the result is increased pressure in the LA and also in the pulmonary veins. The lungs become congested with blood, which leads to elevated pulmonary pressures and pulmonary edema.
Although each type of heart failure produces different signs and symptoms, clinically it is unusual to observe solely right- or left-sided failure in children. Because each side of the heart depends on adequate functioning of the other side, failure of one chamber causes a reciprocal change in the opposite chamber.
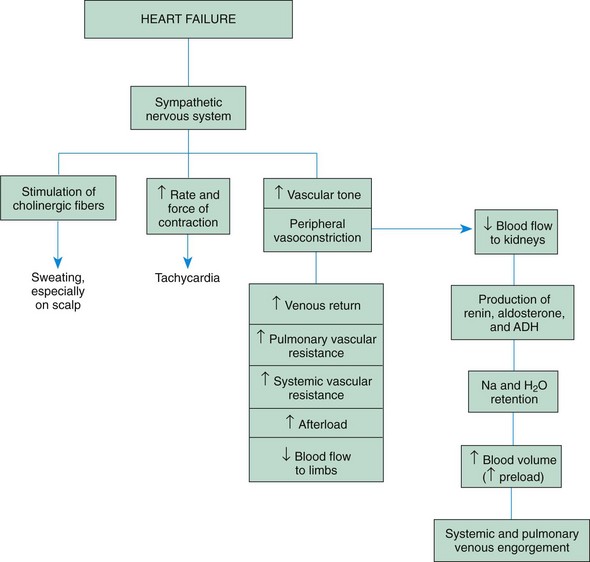
Compensatory Mechanisms: The heart initially tries to meet the body’s demand for increased cardiac output through several compensatory mechanisms called the cardiac reserve. These include hypertrophy and dilation of the cardiac muscle and stimulation of the sympathetic nervous system (Fig. 34-8).
Hypertrophy and Dilation of the Cardiac Muscle: In response to the need to increase cardiac output, the cardiac muscle hypertrophies, developing greater tension. It is able to generate increased pressure within the ventricle, pumping blood out of the heart at a higher pressure. Also, the cardiac muscle can dilate and increase the stretch of its fibers, which increases the force of contraction. However, both hypertrophy and dilation have potentially negative effects. Hypertrophy may result in decreased ventricular compliance over time. When compliance decreases, a higher filling pressure is required to produce the same stroke volume. The increased muscle mass impairs oxygenation to the heart muscle. Beyond a certain amount of dilation, the force of contraction decreases and the heart fails. (See discussion of the Starling law, p. 1344.)
Stimulation of the Sympathetic Nervous System: When cardiac output begins to fall, stretch receptors and baroreceptors in the blood vessels stimulate the sympathetic nervous system, releasing catecholamines. Catecholamines increase the force and rate of myocardial contraction, as manifested by tachycardia. They cause peripheral vasoconstriction, which results in increased systemic vascular resistance; increased venous return; and reduced blood flow to the limbs, viscera, and kidneys. Sympathetic cholinergic fibers cause sweating.
Although initially successful in increasing cardiac output, prolonged sympathetic stimulation also has negative effects. By shortening the diastolic period, tachycardia increases oxygen consumption by the heart muscle, eliminates the heart’s resting phase, and impairs coronary artery perfusion. A continued increase in systemic vascular resistance increases the afterload on the heart muscle, which requires extra work by the heart muscle and reduces systemic blood flow.
The renal system is particularly sensitive to reductions in blood flow and renal perfusion, which activate the renin-angiotensin-aldosterone mechanism. Renin-angiotensin secretion causes vasoconstriction and leads to an increase in aldosterone secretion, which causes retention of salt and water. Retention of salt and water causes an increase in preload. Although at first helpful to the failing heart, the sodium and water retention becomes excessive, resulting in signs of systemic venous congestion and fluid overload.
Clinical Manifestations
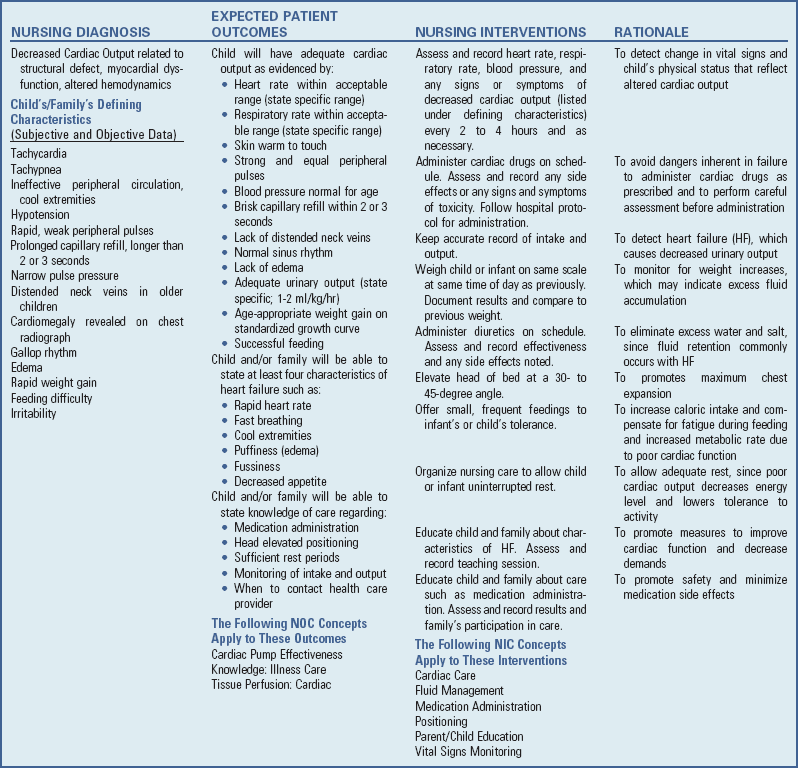
As the capacity of the compensatory mechanisms is exceeded, the child exhibits signs of HF because of decreased myocardial contraction, increased preload, and increased afterload. The signs and symptoms of HF can be divided into three groups: (1) impaired myocardial function, (2) pulmonary congestion, and (3) systemic venous congestion (Box 34-3). Because these hemodynamic changes occur from different causes and at differing times, the clinical presentation may vary among children.
Impaired Myocardial Function: One of the earliest signs of HF is tachycardia (sleeping heart rate >160 beats/min in infants) as a direct result of sympathetic stimulation. Heart rate is elevated even during rest but becomes extremely rapid with the slightest exertion. Ventricular dilation and excess preload result in extra heart sounds S3 and S4, referred to as gallop rhythm. Diaphoresis often occurs, especially on the head during exertion. Children are easily fatigued, have poor exercise tolerance, and are often irritable. Decreased cardiac output results in poor perfusion, manifested by cold extremities, weak pulses, slow capillary refill, low BP, and mottled skin. Extreme pallor or duskiness is an ominous sign.
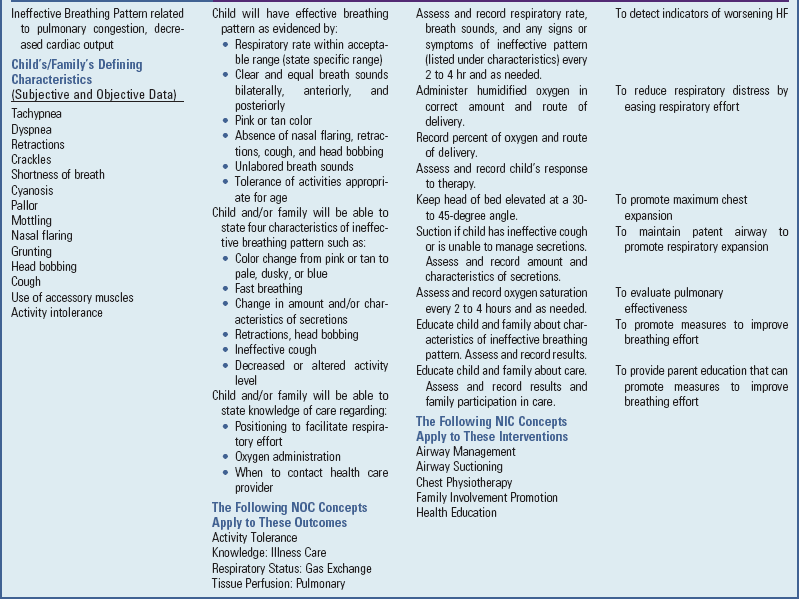
Pulmonary Congestion: Tachypnea (respiratory rate >60 breaths/min in infants) occurs in response to decreased lung compliance (ability to expand). Tachypnea can lead to hypoxemia because oxygen does not reach the alveoli for gas exchange in adequate amounts with fast breathing rates. Mild cyanosis results from impaired gas exchange and is relieved by oxygen administration. Dyspnea is caused by a decrease in the distensibility of the lungs. Inability to feed with resultant poor weight gain is primarily a result of tachypnea and dyspnea on exertion. Costal retractions occur as the pliable chest wall in the infant is drawn inward during attempts to ventilate the noncompliant lungs. Initially dyspnea may be evident only on exertion, but it may progress to the point that even slight activity results in labored breathing. In infants dyspnea at rest is a prominent sign and may be accompanied by flaring nares.
As the LV fails, blood volume and pressure increase in the LA, pulmonary veins, and lungs. Eventually the pulmonary capillary pressure exceeds the plasma osmotic pressure, which forces fluid into the interstitial space and finally causes pulmonary edema. Increased interstitial lung water also decreases the compliance of the lungs and increases the work of breathing.
Orthopnea (dyspnea in the recumbent position) is caused by increased blood flow to the heart and lungs from the extremities. It is relieved by sitting up, since then blood pools in the lower extremities, which decreases venous return. In addition, this position decreases pressure from the abdominal organs on the diaphragm. In infants orthopnea may be evident in the inability to lie supine and the desire to be held upright.
Edema of the bronchial mucosa may produce wheezing from obstruction to airflow. Mucosal swelling and irritation result in a persistent, dry, hacking cough. As pulmonary edema increases, the cough may be productive due to increased secretions. Pressure on the laryngeal nerve results in hoarseness. A late sign of heart failure is gasping and grunting respirations.
Infants with HF have an increased metabolic rate and require additional caloric intake to grow. The work of the heart and breathing demands all of the infant’s energy, leaving little for normal activity. As a result of poor weight gain and activity intolerance, infants with HF demonstrate developmental delays. Because of the physical energy and strength needed to sit up, pull to stand, and walk, these infants are delayed most in gross motor activities. The fine motor, social, and cognitive aspects of development seem less impaired. Following surgical correction, most children catch up to their peers with time. Older children with severe HF have decreased exercise tolerance and persistent developmental delays.
Systemic Venous Congestion: Systemic venous congestion from right-sided failure results in increased pressure and pooling of blood in the venous circulation. Hepatomegaly occurs from pooling of blood in the portal circulation and transudation of fluid into the hepatic tissues. The liver may be tender on palpation, and its size is an indication of the course of heart failure.
Edema develops as the sodium and water retention causes systemic vascular pressure to rise. The earliest sign is weight gain. However, as additional fluid accumulates, it leads to swelling of soft tissue that is dependent and favors the flow of gravity, such as the sacral area and scrotum (when recumbent) and loose periorbital tissues. In infants edema is usually generalized and difficult to detect. Gross fluid accumulation may produce ascites and pleural effusions.
Distended neck and peripheral veins result from consistently elevated CVP. Normally neck and hand veins collapse when the head or hands are raised above the level of the heart, because the blood drains by gravity back to the heart. When the venous pressure is high, however, it slows venous return, which causes the veins to remain distended. Distended neck veins are difficult to detect in the short, fat necks of infants and are usually observed only in older children.
Diagnostic Evaluation
Diagnosis is made on the basis of clinical symptoms such as tachypnea and tachycardia at rest, dyspnea, retractions, activity intolerance (especially during feeding in infants), weight gain caused by fluid retention, and hepatomegaly. A chest x-ray film demonstrates cardiomegaly and increased pulmonary vascular markings due to increased pulmonary blood flow. Signs of ventricular hypertrophy appear on the ECG. Echocardiography is performed to determine the cause of HF, such as a congenital heart defect or poor ventricular function.
Therapeutic Management
The goals of treatment are to (1) improve cardiac function (increase contractility and decrease afterload), (2) remove accumulated fluid and sodium (decrease preload), (3) decrease cardiac demands, and (4) improve tissue oxygenation and decrease oxygen consumption. For most infants diagnosed with HF the cause is a congenital heart defect. Infants are stabilized on medical therapy and then referred for surgical repair. Today many children are being surgically repaired in the neonatal and early infancy stages before the onset of HF symptoms (Margossian, 2008). In infants who do manifest these symptoms, medical and nutrition management are optimized preoperatively. For children newly diagnosed with HF the cause may be worsening ventricular function following a previous cardiac repair, cardiomyopathy, arrhythmia, or other condition. In addition to management of HF, the underlying cause is treated if possible.
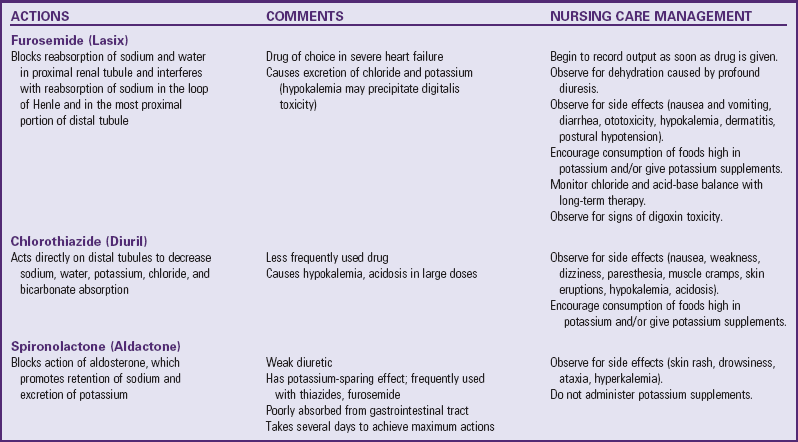
Improve Cardiac Function: Two groups of drugs are used to enhance myocardial function in HF: (1) digitalis glycosides (digoxin), which improve contractility; and (2) angiotensin-converting enzyme (ACE) inhibitors, which reduce the afterload on the heart and thus make it easier for the heart to pump.
Digitalis has three major actions: (1) it increases the force of contraction (positive inotropic), (2) it decreases the heart rate (negative chronotropic) and slows the conduction of impulses through the AV node (negative dromotropic), and (3) it indirectly enhances diuresis by increasing renal perfusion. The beneficial effects are increased cardiac output, decreased heart size, decreased venous pressure, and relief of edema. Digoxin is used less often in the preoperative patient with shunt and volume overload lesions, since the contractility in this population is typically normal. However, for the patient with worsening ventricular function after a previous cardiac repair, digoxin continues to be used in conjunction with ACE inhibitors, diuretics, and beta blockers.
In pediatrics, digoxin is used because of its rapid onset of action and decreased risk of toxicity as a result of its relatively short half-life (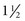 days) compared with other digitalis preparations. It is available as an elixir (50 mcg/ml) for oral administration or in a parenteral preparation (0.1 mg/ml). For infants the dosage is often calculated in micrograms (1000 mcg = 1 mg).
days) compared with other digitalis preparations. It is available as an elixir (50 mcg/ml) for oral administration or in a parenteral preparation (0.1 mg/ml). For infants the dosage is often calculated in micrograms (1000 mcg = 1 mg). ![]() Because digoxin has a very narrow margin of safety, the dosage must be calculated exactly. Premature infants are more sensitive to digoxin and require smaller dosages because their impaired renal excretion causes the drug to accumulate in the blood faster than in full-term infants and children.
Because digoxin has a very narrow margin of safety, the dosage must be calculated exactly. Premature infants are more sensitive to digoxin and require smaller dosages because their impaired renal excretion causes the drug to accumulate in the blood faster than in full-term infants and children.
![]() Critical Thinking Exercise—Digoxin Administration
Critical Thinking Exercise—Digoxin Administration
Treatment involves administration of a digitalizing dose, given intravenously or orally in divided doses over 24 hours to bring the child’s serum digoxin level into the therapeutic range. A maintenance dose, usually one eighth of the digitalizing dose, is given orally twice a day to maintain blood levels (Table 34-2).
TABLE 34-2
ORAL DIGOXIN DOSAGE IN INFANTS AND CHILDREN*
| AGE | TOTAL DIGITALIZING DOSE† | DAILY MAINTENANCE DOSE‡ |
| Premature infant | 20 | 5 |
| Full-term infant | 30 | 8-10 |
| <2 yr | 40-50 | 10-12 |
| >2 yr | 30 | 8-10 |
*Dosage in mcg/kg of body weight.
Digoxin is the only oral inotropic agent generally available for infants and children, although other oral inotropic agents are being used in clinical trials in adults. For patients with severe HF, IV inotropic agents such as dopamine or milrinone are used to improve contractility. They are generally given in ICU settings.
Another group of drugs used in the treatment of HF, the ACE inhibitors, inhibits the normal function of the renin-angiotensin system in the kidney. The production of renin triggers the production of angiotensin I and angiotensin II, which cause vasoconstriction and aldosterone secretion. The ACE inhibitors block the conversion of angiotensin I to angiotensin II so that, instead of vasoconstriction, vasodilation occurs. Vasodilation results in decreased pulmonary and systemic vascular resistance, decreased BP, a reduction in afterload, and decreased RA and LA pressures. It also reduces the secretion of aldosterone, which reduces preload by preventing volume expansion from fluid retention and decreases the risk of hypokalemia. Renal blood flow is improved, which enhances diuresis.
ACE inhibitors are employed frequently in pediatrics, but their use in children has not been extensively studied. Common medications in this class are captopril (Capoten), given three times a day; enalapril (Vasotec), given twice a day; and lisinopril, given once daily. The principal side effects of ACE inhibitors are hypotension, renal dysfunction, and cough. Captopril may also have some immune-based side effects, including fever and allergic reactions. Because enalapril has the same principal side effects but fewer immune-based side effects, patients may be switched from one preparation to the other (see Box 34-12) (Phillips and Somers, 2000).
Beta blockers, specifically metoprolol and carvedilol (Coreg), are the newest medications to be added to the treatment of some children with chronic HF. The α- and β-adrenergic receptors are blocked, causing decreased heart rate, decreased BP, and vasodilation. In addition, beta blockers have antiarrhythmic effects, coronary artery vasodilatory effects, and negative chronotropic effects (Moffett and Chang, 2006). Carvedilol has been shown to decrease morbidity and mortality in some adults with heart failure and is being used selectively in children. When added to standard heart failure therapy, it improved symptoms and LV function in a recent multicenter study (Bruns, Chrisant, Lamour, et al, 2001). Side effects included dizziness, headache, and hypotension.
Cardiac resynchronization therapy (CRT) using biventricular pacing is an effective treatment in adult patients with heart failure and is beginning to be applied in the pediatric population. With pharmacologic therapies discussed above, CRT has the potential to improve cardiac function in this group of patients. The causes for heart failure in the young are more varied and often include patients with a single ventricle, making CRT more challenging. Initial studies of CRT in this population demonstrate improved outcomes in those with adequate follow-up (Cecchin, Frangini, Brown, et al, 2009; Dubin, Janousek, Rhee, et al, 2005).
Remove Accumulated Fluid and Sodium: Treatment to remove accumulated fluid and sodium consists of administration of diuretics, possible fluid restriction, and possible sodium restriction. Diuretics are the mainstay of therapy to eliminate excess water and salt and to prevent reaccumulation. The most commonly used agents are listed in Table 34-3. Because furosemide (Lasix) and the thiazides cause loss of potassium, the child receives potassium supplements and rich dietary sources of the electrolyte (see Evidence-Based Practice box).
Fluid restriction may be required in the acute stages of HF and must be carefully calculated to avoid dehydrating the child, especially if cyanotic CHD and significant polycythemia are present. Infants rarely need fluid restriction, since HF makes feeding so difficult that they struggle to take maintenance fluids.
Sodium-restricted diets are used less often in children than in adults to control HF because of their potential negative effects on the child’s appetite and ultimate growth. If salt intake is restricted, the diet usually focuses on avoiding additional table salt and highly salted foods. Low-salt formulas are available but are used infrequently, since infants need a normal sodium source to offset the sodium depletion of chronic diuretic therapy. Most infant formulas have slightly more sodium than does breast milk.
Decrease Cardiac Demands: To lessen the workload on the heart, metabolic needs are minimized by (1) providing a neutral thermal environment to prevent cold stress in infants, (2) treating any existing infections, (3) reducing the effort of breathing (by placement in semi-Fowler position), (4) using medication to sedate an irritable child, and (5) providing for rest and decreasing environmental stimuli.
Improve Tissue Oxygenation: All the preceding measures serve to increase tissue oxygenation, either by improving myocardial function or by lessening tissue oxygen demands. In addition, supplemental cool humidified oxygen may be administered to increase the amount of available oxygen during inspiration. Oxygen administration is especially helpful in patients with pulmonary edema, intercurrent respiratory tract infections, and increased pulmonary vascular resistance (oxygen is a vasodilator that decreases pulmonary vascular resistance).
An oxygen hood, face tent, or nasal cannula is used to deliver supplemental oxygen. Nasal cannulas are ideal for long-term oxygen administration because the child can be ambulatory and can easily eat and drink. Cool humidification is necessary to counteract the drying effect of oxygen. The amount of cool humidity is carefully regulated to prevent chilling.
Nursing Care Management
Infants or children with HF may be acutely ill, and some may require intensive care until their symptoms improve. Expert nursing care is essential to reduce the cardiac demands that strain the failing heart muscle (see Nursing Care Plan, p. 1360). During this time the child and family require emotional support; for some children severe HF represents end-stage cardiac disease.
Although the objectives of nursing care are the same, interventions for infants are different from those for older children.
Assist in Measures to Improve Cardiac Function: The nurse’s responsibility in administering digoxin includes calculating and giving the correct dosage and observing for signs of toxicity. The child’s apical pulse is always checked before administering digoxin. As a rule, the drug is not given if the pulse is below 90 to 110 beats/min in infants and young children or below 70 beats/min in older children (the cutoff point for adults is 60 beats/min). However, because the pulse rate varies in children in different age-groups, the written drug order should specify at what heart rate the drug is withheld. The nurse should also use judgment in evaluating the pulse rate. If it is significantly lower than the previous recording, the dose should be withheld until the practitioner is notified.
The apical rate is measured because a pulse deficit (radial pulse rate lower than apical) may be present with decreased cardiac output. The pulse is auscultated for 1 full minute to evaluate alterations in rhythm. If the child is monitored by ECG, a rhythm strip is obtained and attached to the chart for rate and rhythm analysis, such as abnormal lengthening of the P-R interval (>50% increase over the predigitalization interval) and dysrhythmias.
Digoxin is a potentially dangerous drug because the margin of safety between therapeutic, toxic, and lethal doses is very narrow. Many toxic responses are extensions of its therapeutic effects. Therefore the nurse must be vigilant for signs of toxicity when administering digoxin. The most common signs of digoxin toxicity in infants and children are bradycardia (although other dysrhythmias may occur), anorexia, nausea, and vomiting. Although vomiting should alert the nurse to observe for other evidence of cardiac toxicity, one episode of vomiting does not warrant cessation of the drug, since vomiting from other causes frequently occurs, especially in infants. Vomiting associated with digoxin toxicity is often unrelated to feedings, and infants are usually less interested in feeding and show a recent decrease in oral intake. When in doubt regarding what caused the vomiting and whether another dose of digoxin should be given, the nurse should seek the practitioner’s advice before administering the next dose. When concerned about possible digoxin toxicity, the nurse should check the digoxin drug level.
Other extracardiac signs of toxicity are neurologic and visual disturbances, which are extremely difficult to identify in children and consequently are of little value in assessing toxicity in infants.
Because digoxin toxicity can occur from accidental overdose, great care must be taken in properly calculating and measuring the dosage. When converting milligrams to micrograms to milliliters, the nurse carefully checks the placement of the decimal point, since an error causes a significant change in dosage. For example, 0.1 mg is 10 times the dosage of 0.01 mg.
If digoxin toxicity occurs, especially as a result of a drug overdose, withhold all subsequent doses. The nurse monitors the child closely for dysrhythmias, which are treated appropriately if they occur. Digoxin immune Fab fragments are used as an antidote to digoxin in cases of severe digitalis toxicity. Because of the long half-life of digoxin (18 to 35 hours in infants and children with normal renal function; longer in those with renal impairment and in premature infants and adults), it may be several days before the blood level returns to normal (Taketomo, Hodding, and Kraus, 2009).
These same principles are taught to parents in preparation for the child’s discharge, although the correct dose in milliliters is usually specified on the container, which reduces potential errors in calculation. The nurse observes the parent measuring the elixir in the dropper and stresses that the level mark is the meniscus of the fluid observed at eye level. Other instructions for administering digoxin are listed in the Family-Centered Care box. Nurses should also advise parents of the signs of digoxin toxicity.
Reduce Afterload: For patients receiving ACE inhibitors for afterload reduction, the nurse should carefully monitor BP before and after dose administration, observe for symptoms of hypotension, and notify the practitioner if BP is low. Monitor serum electrolyte levels. Because ACE inhibitors also block the action of aldosterone, they act as potassium-sparing agents. Most patients do not need potassium supplements or spironolactone while receiving these medications. Numerous medications affecting the kidney can potentiate renal dysfunction, so children taking multiple diuretics along with an ACE inhibitor require careful assessment.
Decrease Cardiac Demands: The infant requires rest and conservation of energy for feeding. Make every effort to organize nursing activities to allow for uninterrupted periods of sleep. Whenever possible, encourage parents to stay with their infant to provide the holding, rocking, and cuddling that help children sleep more soundly. To minimize disturbing the infant, changing bed linen and complete bathing are done only when necessary. Plan feeding to accommodate the infant’s sleep and wake patterns. The child is fed when hungry, such as when sucking on fists, rather than when crying for a bottle, since the stress of crying exhausts the limited energy supply. Because infants with HF tire easily and may sleep through feedings, smaller feedings every 3 hours may be helpful. Gavage feedings may be instituted to provide adequate nutrition and allow the infant to rest.
Make every effort to minimize unnecessary stress. With infants this primarily involves preserving the parent-child relationship and meeting the infant’s needs to reduce frustration. Older children need an explanation of what is happening to them to decrease anxiety about their illness and necessary treatments, such as cardiac monitoring, oxygen administration, and medications. Outlining a plan for the day, preparing the child for tests and procedures, providing quiet activities, and providing adequate rest periods are all helpful interventions with older children. Some infants and children require sedation during the acute phase of illness to allow them to rest.
Carefully monitor temperature for hyperthermia (a sign of infection) or hypothermia (loss of heat to ambient air). Report fevers, since infection must be treated promptly. Fever increases oxygen demands and is poorly tolerated. If body temperature is low, keep the child warm with additional blankets or a radiant heater. Maintaining body temperature is important for children who are receiving cool, humidified oxygen and for children who tend to be diaphoretic, losing heat via evaporation.
Prevent skin breakdown from edema with frequent change of position and the use of pressure-relieving or pressure-reducing mattresses or beds. The skin, especially over the sacrum, is checked for evidence of redness from pressure.
Reduce Respiratory Distress: Careful assessment, positioning, and oxygen administration can reduce respiratory distress. Respirations are counted for 1 full minute during a resting state. Any evidence of increased respiratory distress is reported, since this may indicate worsening heart failure.
Position infants to encourage maximum chest expansion, with the head of the bed elevated. They should sit up in an infant seat or be held at a 45-degree angle. Children prefer to sleep on several pillows and remain in a semi-Fowler or high Fowler position during waking hours. Shirts and diapers are pinned loosely to allow maximum chest expansion. Safety restraints, such as those used with infant seats, are applied low on the abdomen and loosely enough to provide safety and maximum expansion.
The infant or child is often given humidified supplemental oxygen via an oxygen hood or tent, nasal cannula, or mask. The child’s response to oxygen therapy is carefully evaluated by noting the respiratory rate; ease of respiration; color; and especially oxygen saturations, as measured by oximetry.
Respiratory tract infections can exacerbate HF and should be appropriately treated and prevented if possible. The child should be protected from persons with respiratory tract infections and should have a noninfectious roommate. Practice good hand-washing technique before and after caring for any hospitalized child. Antibiotics may be given to combat respiratory tract infection. The nurse ensures that the drug is given at equal intervals over a 24-hour period to maintain high blood levels of the antibiotic.
Maintain Nutritional Status: Meeting the nutritional needs of infants with HF or serious cardiac defects is a nursing challenge. The metabolic rate of these infants is greater because of poor cardiac function and increased heart and respiratory rates. Their caloric needs are greater than those of the average infant because of their increased metabolic rate, yet fatigue limits their ability to take in adequate calories. Feeding a fragile infant with serious CHD is similar to exercise in an adult, and the infant often does not have the energy or cardiac reserve to do extra work. The nurse seeks measures to enable the infant to feed easily without excess fatigue and to increase the caloric density of the formula.
The infant should be well rested before feeding and fed soon after awakening so as not to expend energy on crying. A 3-hour feeding schedule works well for many infants. (Feeding every 2 hours does not provide enough rest between feedings, and a 4-hour schedule requires an increased volume of feeding, which many infants are unable to take.) The feeding schedule should be individualized to the infant’s needs. Use of a soft preemie nipple or a regular nipple slit to enlarge the opening decreases the energy expenditure of the infant while sucking. Infants should be well supported and fed in a semiupright position. The infant may need to rest frequently and may need to have the jaw and cheeks stroked to encourage sucking. Generally, giving an infant about a half hour to complete a feeding is reasonable. Prolonging the feeding time can exhaust the infant and decrease the rest period between feedings.
Infants with feeding difficulties are often gavage fed using a nasogastric tube to supplement oral feedings and ensure adequate caloric intake. If they are stressed and fatigued, in respiratory distress, or tachypneic at 80 to 100 breaths/min, oral feedings may be withheld and all nutrition given by gavage feedings. Gavage feedings are usually a temporary measure until the infant’s medical status improves and nutritional needs can be met through oral feedings. Some infants with severe HF, neurologic deficits, or significant gastroesophageal reflux may need placement of a gastrostomy tube to allow adequate nutrition.
The caloric density of formulas is frequently increased by concentration and then the addition of Polycose (or, less commonly, corn oil or medium-chain triglycerides oil). Infant formulas provide 20 kcal/oz, and the use of additives can increase the calories to 30 kcal/oz or more. This allows the infant to obtain more calories despite intake of a smaller volume of formula. The caloric density of the formula must be increased slowly (by 2 kcal/oz/day) to prevent diarrhea or formula intolerance. Encourage breast-feeding mothers to provide the infant with alternating feedings of breast milk and high-calorie formula. Some lactating mothers prefer to feed the child expressed breast milk that has been fortified with Similac or Enfamil powder, Polycose, or corn oil to increase caloric intake. A supplemental nurser may also be helpful. A diet plan specific to the individual infant’s needs is calculated and prescribed by the nutritionist in collaboration with the other health care personnel. The nurse needs to reinforce this information with the parents as necessary.
Assist in Measures to Promote Fluid Loss: When diuretics are given, the nurse records fluid intake and output and monitors body weight at the same time each day to evaluate the benefit of the drug. Because profound diuresis may cause dehydration and electrolyte imbalance (loss of sodium, potassium, chloride, bicarbonate), the nurse observes for signs indicating either complication, as well as signs and symptoms suggesting reactions to the drugs. Give diuretics early in the day to children who are toilet trained to avoid the need to urinate at night. If potassium-losing diuretics are given, the nurse encourages consumption of foods high in potassium, such as bananas, oranges, whole grains, legumes, and leafy vegetables, and administers prescribed supplements.
Fluid restriction is rarely necessary in infants because of their difficulty in feeding. However, if fluids are restricted, the nurse plans fluid intake schedules for a 24-hour period, allowing for administration of most fluids during waking hours. With toddlers and preschoolers it is psychologically advantageous to give small amounts of liquid in small cups so that the containers appear full. Suitable containers are decorated medicine cups, small paper cups, doll-sized teacups, and measuring cups. It is also important to avoid leaving extra fluids at the bedside, since older children may help themselves to additional servings. Placing them in charge of recording fluid intake will help gain their cooperation.
If salt intake is to be limited, the nurse discusses food sources of sodium with the family and discourages their bringing salt-containing treats to the child. At mealtime check the child’s tray to make sure the appropriate diet is provided.
Support the Child and Family: HF is a serious complication of heart disease. Parents and older children are usually acutely aware of the critical nature of the condition. Because stress places additional demands on cardiac function, the nurse should focus on reducing anxiety through anticipatory preparation, frequent communication with the parents regarding the child’s progress, and constant reassurance that everything possible is being done.
Home care involves many of the same interventions discussed later under Plan for Discharge and Home Care (see p. 1381). The nurse teaches the family about the medications that need to be administered and alerts them to the signs of worsening HF that require medical attention, such as increased sweating, decreased urinary output (noted in fewer wet diapers or infrequent use of the toilet), or poor feeding. Compliance can be a major issue, since patients are often prescribed multiple medications, and the medication regimen can change frequently. Facilitate the family’s adherence to the medication schedule by adapting the schedule to their usual home routines, avoiding medication administration at night, making it as simple as possible, and providing charts or visual aids to help them remember when to give medications. (See Chapter 27.) Written instructions regarding correct administration of digitalis (digoxin) are essential (see Family-Centered Care box, p. 1358), including an explanation regarding signs of toxicity.
If HF is the end stage of a severe heart defect, the nurse cares for the child the same as for any child who is terminally ill, using the principles discussed in Chapter 23 (see Nursing Care Plan).
Hypoxemia
Hypoxemia refers to an arterial oxygen tension (or pressure, Pao2) that is lower than normal and can be identified by measuring arterial oxygen saturation (Sao2) or Pao2 to detect decreased levels. Hypoxia is a reduction in tissue oxygenation that is caused by low Sao2 and Pao2 and results in impaired cellular processes. Cyanosis is a blue discoloration of the mucous membranes, skin, and nail beds of the child with reduced oxygen saturation. It results from the presence of deoxygenated hemoglobin (hemoglobin not bound to oxygen) at a concentration of 5 g/dl of blood or more. Cyanosis is usually apparent when Sao2 is 85% or lower. Determination of cyanosis is subjective. Its appearance can vary depending on skin pigment, quality of light, color of the room, and clothing worn by the child. The presence of cyanosis may not accurately reflect arterial hypoxemia, since both Sao2 and the amount of circulating hemoglobin are involved. Children with severe anemia may not be cyanotic despite severe hypoxemia, since the hemoglobin level may be too low to produce the characteristic blue color. Conversely, patients with polycythemia may appear cyanotic despite a near-normal Pao2.
Altered Hemodynamics
Heart defects that cause hypoxemia and cyanosis are those that allow desaturated venous blood (blue blood) to enter the systemic circulation without passing through the lungs. Three types of defect cause cyanosis in infants. The first involves severe obstruction to pulmonary blood flow and blood shunting from the right side to the left side of the heart, or right-to-left shunting. Tetralogy of Fallot is the most common example. The second is mixing of arterial and venous blood within the chambers of the heart itself; a single ventricle is an example. The third defect, transposition of the great arteries, presents a unique situation in which the pulmonary and systemic circulations are parallel rather than in sequence. Fully oxygenated blood returns to the lungs, and desaturated blood returns to the body. Newborns with transposition of the great arteries depend on intracardiac mixing from a patent foramen ovale, septal defect, or ductus arteriosus to allow oxygenation.
Infants and children with some complex cardiac anomalies can be both hypoxemic and cyanotic and have symptoms of HF. Defects resulting in one functional ventricle, hypoplastic left heart syndrome, and transposition of the great arteries with a ventricular septal defect are examples.
Clinical Manifestations
Over time, two physiologic changes occur in the body in response to chronic hypoxemia: polycythemia and clubbing. Persistent hypoxemia stimulates erythropoiesis, which results in polycythemia, an increased number of red blood cells. Theoretically a greater number of red blood cells increase the oxygen-carrying capacity of the blood. However, this increased red blood cell formation may result in anemia if iron is not readily available for the formation of hemoglobin. In addition, polycythemia increases the viscosity of the blood, and platelets and other coagulation factors tend to be crowded out. These hematologic changes increase the likelihood of postoperative bleeding. Clubbing, a thickening and flattening of the tips of the fingers and toes, is thought to occur because of chronic tissue hypoxemia and polycythemia (Fig. 34-9).
Infants with mild hypoxemia may be asymptomatic except for cyanosis and exhibit near-normal growth and development. Those with more severe hypoxemia may exhibit fatigue with feeding, poor weight gain, tachypnea, and dyspnea. Flaccidity is usually a sign of severe cardiovascular compromise. Children who are cyanotic from birth are generally smaller than their peers, exhibit poor weight gain, have dyspnea on exertion, fatigue easily, and have poor exercise tolerance.
Severe hypoxemia resulting in tissue hypoxia is manifested by clinical deterioration and signs of poor perfusion. The infant is pale and dusky with increased cyanosis; cool to the touch with diminished pulses; and lethargic with signs of respiratory distress, including hyperpnea and gasping respirations. Tissue hypoxia causes metabolic acidosis, which leads to hyperventilation and a rapidly worsening clinical course unless prompt treatment is instituted.
Hypercyanotic spells, also referred to as blue spells or tet spells because they are often seen in infants with tetralogy of Fallot, may occur in any child whose heart defect includes obstruction to pulmonary blood flow and communication between the ventricles (see Fig. 34-12). The infant becomes acutely cyanotic and hyperpneic because when sudden infundibular spasms decreases pulmonary blood flow and increases right-to-left shunting (the proposed mechanism in tetralogy of Fallot). This then leads to hypoxia. With other anomalies, an increase in oxygen requirements, which the infant is unable to meet, may cause a spell. Hypoxia causes acidosis, which further increases pulmonary vascular resistance; this, in turn, further decreases pulmonary blood flow. Thus a vicious cycle ensues. Spells, rarely seen before 2 months of age, occur most frequently in the first year of life and more often in the morning, and they may be preceded by feeding, crying, or defecation. Because profound hypoxemia causes cerebral hypoxia, hypercyanotic spells require prompt assessment and treatment to prevent brain damage or possibly death.
Patients with persistent right-to-left shunts as a result of CHD are at risk for significant neurologic complications. Polycythemia and the resultant increased viscosity of the blood increase the risk of thromboembolic events. Small blood clots in the venous system reach the right side of the heart and can enter the systemic circulation and travel to the brain through the right-to-left shunt. The presence of poor ventricular function and atrial arrhythmias further increases the risk of cerebrovascular accidents (CVAs), or strokes. More common in patients with severe cyanosis, thromboembolic events may occur spontaneously but often follow an acute febrile illness; a hypoxic spell; cardiac catheterization; or cardiac surgery, including the Fontan procedure with fenestration (see Box 34-6).
Patients who are cyanotic, especially those with systemic-to-pulmonary shunts, are at increased risk of bacterial endocarditis (BE; infective) (see p. 1382).
Negative developmental consequences, particularly in the area of motor and cognitive development, may occur from chronic hypoxemia. Fifty percent of postnatal brain growth takes place in the first year of life, so chronic hypoxemia, poor growth, and poor nutrition during this period can have significant adverse effects. In addition, the risks of CVA; periods of profound cyanosis and hypoxia during hypercyanotic spells; and multiple surgeries, hospitalizations, and cardiac catheterizations significantly increase the possibility of neurologic insult resulting in developmental delays, a danger that increases with each year of life. The desire to minimize these risks is an important factor in the trend toward early corrective surgical repair of cyanotic defects in infancy.
Diagnostic Evaluation
Cyanosis in the newborn can be a result of cardiac, pulmonary, metabolic, or hematologic disease, although cardiac and pulmonary causes occur most often. To distinguish between the two, a hyperoxia test may be helpful. The infant is placed in a 100% oxygen environment, and blood parameters are monitored. A Pao2 of 100 mm Hg or higher suggests lung disease, and a Pao2 lower than 100 mm Hg suggests cardiac disease (the problem is related to inadequate perfusion of the pulmonary bed) (Park, 2008). An accurate history, a chest radiograph (demonstrating reduced pulmonary blood flow), and especially an echocardiogram contribute to the diagnosis of cyanotic heart disease.
Therapeutic Management
Newborns generally exhibit cyanosis within the first few days of life as the ductus arteriosus, which provided pulmonary blood flow, begins to close. Prostaglandin E1, which causes vasodilation and smooth muscle relaxation and thus increases dilation and patency of the ductus arteriosus, is administered intravenously to reestablish pulmonary blood flow. The use of prostaglandins has been lifesaving for infants with ductus-dependent cardiac defects. The increase in oxygenation allows the infant’s condition to be stabilized and a complete diagnostic evaluation to be performed before further treatment is needed.
Hypercyanotic spells occur suddenly, and prompt recognition and treatment are essential. In the hospital setting, spells are often seen during blood drawing or IV line insertion, when the child is highly agitated, or following cardiac catheterization. Treatment of a hypercyanotic spell is outlined in the Nursing Care Guidelines box. Placing an infant in the knee-chest position reduces the venous return from the legs (which is desaturated) and increases systemic vascular resistance, which diverts more blood into the pulmonary artery (Fig. 34-10). Morphine, administered subcutaneously or through an existing IV line, is helpful in reducing infundibular spasm. A spell may indicate the need for prompt surgical treatment. In rare cases, propranolol (Inderal) may be given in the interim to prevent infundibular spasm.
The cyanotic infant or child is well hydrated to keep the hematocrit and blood viscosity within acceptable limits to reduce the risk of CVA. Fevers are carefully evaluated, since bacteremia can result in bacterial endocarditis. Monitor the infant closely for anemia because of the risk of CVAs and the reduced arterial oxygen-carrying capacity that occurs. Iron supplementation and possibly blood transfusion are used as needed. Older children and adolescents may require serial phlebotomy to reduce blood viscosity and minimize the risk of CVA. The goal is to reduce the hematocrit to approximately 60% by removing small aliquots of blood and replacing blood with normal saline or other IV solutions to maintain intravascular volume. This procedure is a temporary measure but may relieve symptoms of dyspnea, headache, and malaise for short periods and can be repeated every 1 or 2 months if polycythemia is severe.
Respiratory tract infections or reduced pulmonary function from any cause can worsen hypoxemia in the cyanotic child. Aggressive pulmonary hygiene, chest physiotherapy, administration of antibiotics, and use of oxygen to improve arterial saturations are important interventions.
Palliative Surgery: Severely hypoxemic newborns with cardiac defects not initially amenable to corrective repair may undergo a palliative surgical procedure to establish a shunt. The shunt serves the same purpose as the ductus arteriosus: to increase blood flow to the lungs through a systemic artery–to–pulmonary artery connection. Currently, a modified Blalock-Taussig operation, in which a Gore-Tex or Impra tube graft is placed to create a communication between the right or left subclavian artery and the pulmonary artery on the same side, is the preferred procedure. The original Blalock-Taussig shunt procedure directly anastomosed the subclavian artery to the pulmonary artery to provide pulmonary blood flow and was the first operation devised for patients with cyanotic heart disease. Because of the higher resistance in the systemic circulation, blood flows from the subclavian artery to the pulmonary artery and to the lungs for oxygenation. The small diameter of the subclavian artery and the shunt (often 3.5 or 4 mm) automatically restricts the volume of blood flow to the pulmonary artery, which prevents severe pulmonary overcirculation and HF. Table 34-4 outlines the most commonly performed shunt procedures today (Fig. 34-11). Corrective surgical repair is always preferred to a palliative shunt procedure if it can be performed at low risk. Corrective techniques are described in the discussion of the particular cardiac defect.
After a shunt procedure, assess the infant for signs of increased or decreased pulmonary blood flow. If the shunt is too small or has narrowed, the newborn may remain severely hypoxemic, with oxygen saturations below 70%. Surgical revision of the shunt or placement of an additional shunt may be needed. More often, the shunt is too large and the pulmonary blood flow may be excessive, resulting in signs and symptoms of HF and oxygen saturations above 85%. The infant may require digoxin and diuretic therapy (see discussion of HF). Most surgeons place infants on low-dose aspirin therapy for several months to prevent platelet aggregation and subsequent narrowing of the shunt. Acute cyanosis and signs of tissue hypoxia may occur if the shunt is occluded and pulmonary blood flow is severely limited; shunt occlusion is a medical emergency. The presence of prosthetic material puts patients at risk for infective (bacterial) endocarditis.
Nursing Care Management
The general appearance of infants and children with significant cyanosis poses unique concerns. Blue lips and fingernails are obvious signs of the hidden cardiac defect. Clubbing and small, thin stature in older children further indicate severe heart disease. Body image concerns are important. These children are often teased about their appearance and singled out as different. Adolescents are especially concerned about their body image, and cyanosis can become a particular issue for them. Many children, when asked what surgery will do, reply, “Make me pink.” Their joy and excitement after surgery are evident when they see their pink fingers. Accentuating the normal and positive and being careful not to call attention to their cyanosis are helpful interventions. Meeting other children who are cyanotic in the clinic or hospital reassures them that they are not the only ones who are blue.
Parents are often fearful of their child’s bluish color because cyanosis is usually associated with lack of oxygen and severe illness (see Critical Thinking Exercise). They also must deal with comments from relatives, friends, and strangers in the community about their child’s abnormal color. They need a simple explanation of hypoxemia and cyanosis and reassurance that cyanosis does not imply a lack of oxygen to the brain. Their questions and fears need to be addressed in a calm, supportive manner, and positive aspects of their child’s growth and development must be emphasized. Teach parents the treatment for hypercyanotic spells. (See Nursing Care Guidelines box, p. 1363.)
Dehydration must be prevented in hypoxemic children because it increases the risk of CVAs. Fluid status is carefully monitored through accurate intake and output and daily weight measurements. Maintenance fluid therapy is the minimum requirement; supplemental fluids should be readily available, and gavage feeding or IV hydration is given to children unable to take in adequate fluids orally. Fever, vomiting, and diarrhea can cause dehydration and require prompt treatment. Instruct parents in the importance of adequate fluid intake and measures to prevent dehydration. An oral electrolyte solution such as Pedialyte should be available at home in the event that the infant is unable to tolerate the usual formula. The practitioner should be notified of fever, vomiting, diarrhea, or other problems.
Preventive measures and accurate assessment of respiratory infection are important nursing considerations. Any compromise in pulmonary function increases the infant’s hypoxemia. Good hand washing and protection from individuals with an obvious respiratory tract infection are important. Aggressive pulmonary hygiene, treatment with antibiotics or antiviral agents as indicated, and delivery of supplemental oxygen to decrease hypoxemia are necessary measures. Infants may need to be gavage fed or given parenteral nutrition if respiratory distress prevents oral feeding.
Classification of Congenital Heart Defects
Congenital heart defects have been classified into several categories. Traditionally a physical characteristic, cyanosis, has been used as the distinguishing feature, so that the anomalies have been divided into acyanotic and cyanotic defects. In clinical practice this system is problematic, since children with acyanotic defects may develop cyanosis. Also, more often, those with cyanotic defects may be pink and have more clinical signs of HF. Because of the complexity of many defects and the variability of their clinical manifestations, the cyanotic-acyanotic classification system has proven to be inadequate and misleading.
A more useful classification system is based on hemodynamic characteristics, or movements involved in the circulation of blood. The defining characteristic is blood flow patterns: (1) increased pulmonary blood flow; (2) decreased pulmonary blood flow; (3) obstruction to blood flow out of the heart; and (4) mixed blood flow, in which saturated and desaturated blood mix within the heart or great arteries. Fig. 34-12 outlines both classification systems.
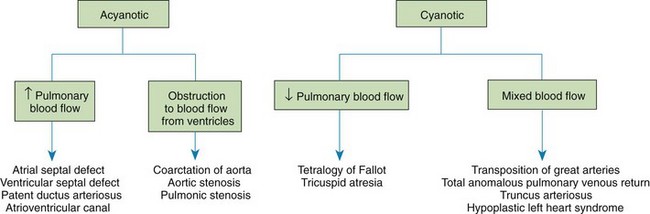
Fig. 34-12 Comparison of acyanotic-cyanotic and hemodynamic classification systems for congenital heart disease.
With the hemodynamic classification system, the clinical manifestations of each group are more uniform and predictable. Defects that allow blood flow from the high-pressure left side of the heart to the lower-pressure right side (left-to-right shunt) result in increased pulmonary blood flow and cause HF. Obstructive defects impede blood flow out of the ventricles; obstruction on the left side of the heart results in HF, whereas severe obstruction on the right side causes cyanosis. Defects that cause decreased pulmonary blood flow result in cyanosis. Mixed lesions present a variable clinical picture based on the degree of mixing and amount of pulmonary blood flow; hypoxemia (with or without cyanosis) and HF usually occur together. (For more detailed explanations, see discussions of specific defects later in this chapter.)
More than 35 types of congenital heart defect have been identified, and some patients have multiple defects. Although some defects are common and fairly uniform, like atrial septal defects or pulmonic stenosis, others are uncommon and highly variable, like single-ventricle anomalies. In a review by Hoffman and Kaplan (2002), defects are categorized as mild, moderate, or severe. Mild defects are found in the largest number of patients, many of who are asymptomatic and may not require treatment. Mild defects include small patent ductus arteriosus, small ventricular septal defects, and mild pulmonic stenosis. Moderate defects include mild aortic stenosis, moderate pulmonic stenosis, coarctation of the aorta, atrial septal defects, and ventricular septal defects. Most patients with moderate defects are symptomatic during childhood and require treatment. Severe defects include all cyanotic heart disease and other complex defects such as AV canal, critical aortic stenosis, critical coarctation of the aorta, and complex ventricular septal defects. Most patients with these defects are identified during the newborn period or early infancy, are severely ill, and require surgical treatment. The clinical presentation and management of the most common defects are outlined in the following sections and in Boxes 34-4 to 34-7.
The outcomes of surgical treatment for patients with moderate to severe disease are variable. Patient risk factors for increased morbidity and mortality include prematurity or low birth weight, a genetic syndrome, multiple cardiac defects, a noncardiac congenital anomaly, and age at the time of surgery (neonates are a higher-risk group). For example, aortic stenosis and coarctation presenting in the first week of life are more severe and carry a higher mortality than if they manifest at 1 year of age. Outcomes for surgical repair of similar congenital heart defects also vary among treatment centers. The most recent mortality rates and statistics on incidence of CHD from the American Heart Association are found in Box 34-1. Individual center results may be better or worse than those listed. In general, the outcomes of surgical procedures have steadily improved, with mortality rates for many severe defects below 10%, and the incidence of complications and length of hospital stay have declined.
Defects with Increased Pulmonary Blood Flow
![]() In cardiac defects with increased pulmonary blood flow, intracardiac communications along the septum or an abnormal connection between the great arteries allows blood to flow from the high-pressure left side of the heart to the lower-pressure right side of the heart (Fig. 34-13). Increased blood volume on the right side of the heart increases pulmonary blood flow at the expense of systemic blood flow. Clinically patients demonstrate signs and symptoms of HF. Atrial and ventricular septal defects and patent ductus arteriosus are typical anomalies in this group (Box 34-4).
In cardiac defects with increased pulmonary blood flow, intracardiac communications along the septum or an abnormal connection between the great arteries allows blood to flow from the high-pressure left side of the heart to the lower-pressure right side of the heart (Fig. 34-13). Increased blood volume on the right side of the heart increases pulmonary blood flow at the expense of systemic blood flow. Clinically patients demonstrate signs and symptoms of HF. Atrial and ventricular septal defects and patent ductus arteriosus are typical anomalies in this group (Box 34-4).
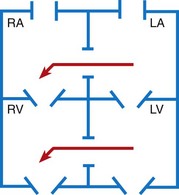
Fig. 34-13 Hemodynamics in defects with increased pulmonary blood flow. See Fig. 34-2 for abbreviations.
Obstructive Defects
Obstructive defects are those in which blood exiting the heart meets an area of anatomic narrowing (stenosis), which causes obstruction to blood flow. The pressure in the ventricle and in the great artery before the obstruction is increased, and the pressure in the area beyond the obstruction is decreased. The location of the narrowing is usually near the valve (Fig. 34-14):
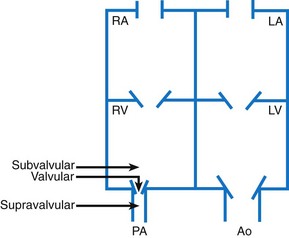
Fig. 34-14 Obstruction to ventricular ejection can occur at the valvular level (shown), below the valve (subvalvular), or above the valve (supravalvular). Pulmonic stenosis is shown here. Ao, Aorta; PA, pulmonary artery. See Fig. 34-2 for other abbreviations.
Valvular—Narrowing at the site of the valve itself
Subvalvular—Narrowing in the ventricle below the valve (also referred to as the ventricular outflow tract)
Coarctation of the aorta (narrowing of the aortic arch), aortic stenosis, and pulmonic stenosis are typical defects in this group (Box 34-5). Hemodynamically there is a pressure load on the ventricle and decreased cardiac output. Clinically infants and children exhibit signs of HF. Children with mild obstruction may be asymptomatic. Rarely, as in severe pulmonic stenosis, hypoxemia may occur.
Defects with Decreased Pulmonary Blood Flow
In defects with decreased pulmonary blood flow, there is obstruction of pulmonary blood flow and an anatomic defect (atrial septal defect or ventricular septal defect) between the right and left sides of the heart (Fig. 34-15). Because blood has difficulty exiting the right side of the heart via the pulmonary artery, pressure on the right side increases, exceeding left-side pressure. This allows desaturated blood to shunt right to left, which causes desaturation in the left side of the heart and in the systemic circulation. Clinically these patients are hypoxemic and usually appear cyanotic. Tetralogy of Fallot and tricuspid atresia are the more common defects in this group (Box 34-6).
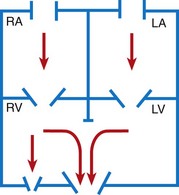
Fig. 34-15 Hemodynamic defects with decreased pulmonary blood flow. See Fig. 34-2 for abbreviations.
Mixed Defects
Many complex cardiac anomalies are classified together in the mixed category (Box 34-7), since survival in the postnatal period depends on mixing of blood from the pulmonary and systemic circulations within the heart chambers. Hemodynamically, fully saturated systemic blood mixes with the desaturated pulmonary blood, which causes a relative desaturation of the systemic blood. Pulmonary congestion occurs because the differences in pulmonary artery pressure and aortic pressure favor pulmonary blood flow. Cardiac output decreases because of a volume load on the ventricle. Clinically these patients have a variable picture that combines some degree of desaturation (although cyanosis is not always visible) and signs of HF. Some defects, such as transposition of the great arteries, cause severe cyanosis in the first days of life and later cause HF. Others, such as truncus arteriosus, cause severe HF in the first weeks of life and mild desaturation.
Nursing Care of the Child with Congenital Heart Disease and His or Her Family
When a child is born with a severe cardiac anomaly, parents face the immense psychologic and physical tasks of adjusting to the birth of a child with special needs. Family issues and nursing interventions to support the family are similar to those described in Chapters 11 and 22. The following discussion concerns primarily (1) interactions with the family of an infant who has a serious heart defect and requires home care before definitive repair, and (2) preparation and care of the child and family when invasive procedures (catheterization and surgery) are performed. For nursing care related to the child with hypoxemia and HF, the reader should refer to earlier discussions of these topics.
Nursing care of the child with a congenital heart defect begins as soon as the diagnosis is suspected. Prenatal diagnosis of congenital heart defects is becoming increasingly frequent. New demands are being placed on nurses to counsel and support families as they prepare for the birth of these infants.