Help the Family Adjust to the Disorder
Once parents learn of the heart defect, they are initially in a period of shock, followed by high anxiety, especially fear of the child’s death. This reaction may occur soon after the child’s birth or at a later period. Whatever its timing, the family needs a period of grief before assimilating the meaning of the defect. Unfortunately, the demands for medical treatment may not allow this, necessitating that the parents be informed of the condition to give informed consent for diagnostic and therapeutic procedures. The nurse can be instrumental in supporting parents in their loss, assessing their level of understanding, supplying information as needed, and helping other members of the health team understand the parents’ reactions.
Severely ill newborns usually remain in the hospital. The nurse can promote parent-infant attachment by encouraging parents to hold, touch, and look at their child and by providing time and privacy to the parents to spend with their newborn. (See Chapter 10 for suggestions for promoting attachment between parents and their hospitalized newborn.)
The effect of a child with a serious heart defect on the family is complex. No members, regardless of how well they adjust, are unaffected. Mothers frequently feel inadequate in their mothering ability because of the more complex care such an infant requires. They may be constantly exhausted from the pressures of caring for this child and the other family members. Likewise, fathers and siblings may feel neglected and resentful, a reaction similar to that in families with children with other chronic conditions. (See Chapter 22.) Often parents do not feel confident leaving the child in the care of anyone else, which affords parents no relief from the constant daily caregiving. This problem can be minimized by gradually teaching someone else (a reliable relative or neighbor) how to care for the child.
The need to maintain discipline and set consistent limits can be difficult for parents. A study by Uzark and Jones (2003) found higher levels of stress in parents of children with heart disease, particularly with regard to limit setting and discipline. Behavior modification techniques using either concrete rewards (e.g., a favorite food) or social reinforcement (e.g., approval) can be effective. However, these techniques are most beneficial if used before the child learns to control the family. Therefore guiding parents toward the need for discipline while the child is in infancy is necessary to prevent later problems.
Another problem that may develop within family relationships is overdependency on the part of the child. This is often a result of parental fear that the child may die and overcompensation through what has been termed benevolent overreaction. (See Chapter 22.) Research has shown no correlation between the severity of the child’s heart defect and maternal anxiety or parental stress (Morelius, Lundh, and Nelson, 2002). The best approach to dealing with this dilemma is prevention. Parents need guidance to recognize the eventual hazards of continuing dependency and protectiveness as the child grows older, and the nurse can assist parents in learning ways to foster optimum development. Unless parents have help to see what activities the child can do, they may focus on physical limitations and encourage dependency. The child needs opportunities for normal social interaction with other children to foster normal social development.
Frequently the unremitting stresses of care—physical exhaustion, financial costs, emotional upset, fear of death, and concern for the child’s future—are not fully appreciated by those caring for the family. Even when the child’s condition is stabilized or corrected, the family may need to make new adjustments in their lifestyle. Introducing them to other families with similarly affected children can help them adjust to the daily stresses* (see Family-Centered Care box).
Educate the Family About the Disorder
Once parents are ready to hear about their child’s heart condition, it is essential that they receive a clear explanation based on their level of understanding. A review of normal cardiac anatomy is helpful before explaining the anatomic defect. A simple diagram, pictures, or a model of the heart can be most helpful in visualizing the heart and the congenital defect. Parents appreciate receiving written information about the specific condition.* Health care professionals should take advantage of subsequent encounters with the family to assess parental understanding of the condition and clarify information as needed.
Different health personnel may convey the same information using different diagrams and medical terms. To prevent this from becoming a problem, the same type of diagram should be used by all, and the parents should write down any unclear terms or ask for clarification. Sometimes it is helpful to provide the family with a glossary of frequently used words for reference.
Parents often use multiple resources to obtain information about their child’s heart defect. Increasingly, families are using the Internet as a source of information. Locating information can be easy with helpful information located at national organizations and large parent support groups.† Parents also find support through contacts with other parents and parent groups. It is important for parents to realize that not all websites offer medically accurate information and that information from other parents may not be applicable to their own situation. Some children with rare, complex heart defects require individualized treatment plans, and general information on the Internet or in books may not apply to them. Parents should talk to their health care team, in particular their cardiologist, about information they have received from other sources.
The nurse must give information to the child in a manner that is appropriate to the child’s developmental age. As the child matures, the level of information is revised to match the child’s new cognitive level. Preschoolers need basic information about what they will experience more than what is actually occurring physiologically. School-age children benefit from a concrete explanation of the defect. Preadolescents and adolescents often appreciate a more detailed description of how the defect affects their heart. Children of all ages need to be able to express their feelings concerning the diagnosis.
Help the Family Manage the Illness at Home
Parents are the child’s principal caregivers and need to develop a positive, supportive working relationship with the health care team. Because most children spend the majority of their time at home with episodic trips to the hospital, parents manage their child’s illness on a daily basis. They monitor for signs of illness, give medications and treatments, bring their child to appointments, work with a variety of caregivers, and alert the team to problems. Successful relationships are a partnership between parents and caregivers that is built on mutual trust and respect. Good communication between the family, the cardiology specialists, and the primary care practitioner is essential. As children reach adolescence, they begin to take a larger role in managing their illness and making decisions about their care.
Parents should be aware of the symptoms of their child’s cardiac condition and signs of worsening clinical status. Parents should know how to contact their child’s cardiologist at all times and know what to do in an emergency. Parents of children who may develop HF should be familiar with the symptoms (see p. 1352) and know when to contact the practitioner. Parents of children with cyanosis should be informed about fluid management and hypercyanotic spells (see p. 1362). Parents should have an information sheet with their child’s diagnosis, significant treatments such as surgical procedures, allergies, other health care problems, current medications, and health care providers’ contact numbers available in case of emergencies and to share with other caregivers such as teachers, baby-sitters, or daycare providers.
The family also needs to be knowledgeable regarding the therapeutic management of the disorder and the role that surgery, other procedures, medications, and a healthy lifestyle play in maintaining good health. Medications play a critical role in the management of some cardiac conditions such as arrhythmias and severe HF, in anticoagulation after implantation of artificial valves, and in antirejection treatment after heart transplantation. Some patients must take multiple medications daily for life. Many medications can be dangerous if taken incorrectly and require close monitoring. Teach parents the correct procedure for giving medications and caution them to keep them in a safe area to prevent accidental ingestion (see Family-Centered Care box, p. 1358).
Another area of parental concern is the child’s level of physical activity. Most children do not need to restrict activity, and the best approach is to treat the child normally and allow self-limited activity. Exceptions primarily involve strenuous recreational and competitive sports in children with specific cardiac problems. Discuss activities and exercise restrictions with the child’s cardiologist. Avoid deliberately attempting to prevent crying because it can establish a maladaptive parental pattern of relating to the infant.
Infants and children with CHD require good nutrition. Breast-feeding should be possible for many infants with CHD. Countering a common misconception that breast-feeding would not be possible for these infants because they would get tired or exhibit poor growth, Barbas and Kelleher (2004) found that breast-feeding could be successful with adequate support and education of the mother. Providing adequate nutrition to infants with HF or complex congenital defects is especially difficult due to their high caloric requirements and inability to suck effectively because of fatigue and tachypnea. Instructing parents in feeding methods that decrease the work of the infant and giving high-calorie formula are important interventions (see p. 1359 for a discussion on feeding the infant with HF).
Children with severe cardiac defects are often anorexic. Encouraging them to eat can be a tremendous challenge. Because of the parents’ concern over eating, children learn early to manipulate parents through eating, such as making unrealistic demands for foods that are not available. The nurse advises parents of this potential problem, since prevention yields greater success than intervention. For example, give the child a choice of available high-nutrient foods. Chapter 27 provides suggestions for encouraging sick children to eat.
Infants with heart disease should be immunized according to the current guidelines. Immunization schedules may need to be modified around times of acute illness or surgical procedures (Smith, 2001). Infants and children younger than 2 years of age with unrepaired heart defects, cyanotic lesions, pulmonary hypertension, or a history of prematurity should receive the vaccine for respiratory syncytial virus (RSV) monthly during RSV season (November to April in North America) to prevent RSV infection (American Academy of Pediatrics, 2009). Use of the RSV vaccine palivizumab has been shown to reduce hospitalization due to RSV infection in infants and young children with hemodynamically significant CHD (Feltes, Cabalka, Meissner, et al, 2003). (See Chapter 32.)
Infants and children who have serious heart disease are at risk for developmental delays and there is growing interest in characterizing these outcomes (see Research Focus box). Multiple factors can influence neurodevelopmental outcomes, including genetics (chromosomal abnormalities and microdeletions), family background (parental intelligence quotient [IQ] and socioeconomic status), preoperative factors (including prematurity, cyanosis, shock), intraoperative factors (use of cardiopulmonary bypass, deep hypothermic circulatory arrest), and postoperative factors (hemodynamic instability, hypoxia, acidosis, cardiac arrest, stroke, ischemic events).
Prepare the Child and Family for Invasive Procedures
Chapter 27 provides an extensive discussion of the principles for preparing children for invasive procedures. In 2003 the American Heart Association published a scientific statement, “Recommendations for Preparing Children and Adolescents for Invasive Cardiac Procedures” (LeRoy, Elixson, O’Brien, et al, 2003), that addresses issues specific to the child with heart disease. The reader is referred to these resources for a complete review of the topic. The following discussion highlights some important aspects of preparation for cardiac catheterization and cardiac surgery.
The expected outcomes for preprocedure preparation include reducing anxiety, improving patient cooperation with procedures, enhancing recovery, developing trust with caregivers, and improving long-term emotional and behavioral adjustment following procedures (LeRoy, Elixson, O’Brien, et al, 2003). Important factors to consider in planning preparation strategies are the child’s cognitive developmental level, the child’s previous hospital experiences, the child’s temperament and coping style, the timing of the preparation, and the involvement of the parents. The most beneficial preparation strategies usually combine information giving and training in coping skills such as conscious breathing exercises, distraction techniques, guided imagery, and other behavioral interventions.
Handling preoperative and precatheterization workups on an outpatient basis is common for most elective procedures. Children are then admitted on the morning of the procedure. Preprocedure teaching is often done in the clinic setting or at home, and a tour of the ICU and the inpatient facilities may be added. Children of different ages and developmental levels require different amounts of information and different approaches. Young children should be prepared close in time to the event; older children and adolescents may benefit from teaching several weeks in advance. Include parents in the preparation session to support their child and learn about upcoming events.
The preoperative or precatheterization preparation should include information on the environment, equipment, and procedures that the child will encounter during and following the procedure. The nurse can use many educational techniques, such as verbal and written information, hospital tours, preoperative classes, and picture books or videos. Information about what the child will see, hear, and feel should be included, especially for older children and adolescents. Some of the sensory experiences of being in an ICU or catheterization laboratory include sights (monitors, many people, lots of equipment), sounds (beeping noises, alarms, voices), and sensations (lines and dressings, tape, feelings of discomfort, thirst). Familiar aspects of the environment, like BP cuffs, stethoscopes, or oximeter probes, are reviewed, and new equipment such as monitors, IV lines, and oxygen masks are described. Comforting aspects of the environment are emphasized, such as play areas, chairs for parents, and televisions. Many patients who will be sedated during catheterization or receive narcotic pain relievers after surgery will have minimal recall of that period and will not need detailed information about the equipment or procedures used. Information should be specific to the planned procedure for each patient.
Discuss ways the child can cope with the experience and be helped to recover. For young children, bringing a familiar stuffed animal or comfort object with them will help relieve anxiety, whereas for older children bringing a music player with headphones and favorite recordings to the catheterization laboratory will help distract them during the procedure. Topics to discuss regarding recovery after catheterization include the need to lie still to prevent bleeding at the catheter site, progression of the diet, pain control measures, and monitoring methods. Review the importance of ambulation, coughing and deep breathing, and drinking and eating after surgery, and describe pain management and monitoring routines. Review simple coping strategies for use during painful procedures, including distraction techniques such as counting, blowing, singing, or telling stories.
Children and their families should have a choice about an ICU tour. Exposure to the ICU environment can actually increase anxiety in some children, particularly young children, those with previous hospital experiences, and those who are highly anxious (LeRoy, Elixson, O’Brien, et al, 2003). If a visit to the recovery room and ICU is planned, it should take place when there is minimal activity in the area, when the parents can accompany the child, and when the child is well rested. Usually the day before the procedure is ample time to allow the child to ask questions and to prevent undue fantasizing about the experience. Protect the child from frightening sights in the unit. Equipment that will not be in view postoperatively, such as equipment located behind or below the bed, needs less attention. The child and parents are encouraged to ask questions and to explore further any equipment in the room, but they should not be pushed to assimilate more information than they are able.
Preoperative physical care differs little, if any, from that provided for any other surgery and is discussed in Chapter 27. Assure the child that the parents will be there when the child wakes up. Also allow the parents to accompany the child as far as possible to the operating suite. (See Evidence-Based Practice box, p. 1211.) After all of the equipment and procedures have been explained, it is important to talk about “getting well” and going home.
Provide Postoperative Care
Immediate postoperative care is usually provided by specially trained nurses in the ICU. Performing many of the procedures, such as arterial pressure and CVP monitoring and observations related to vital functions, requires advanced educational training (the reader should refer to critical care texts for further information). However, nurses caring for the child before surgery and during the convalescent period need to be familiar with the major principles of care.
Observe Vital Signs and Arterial and Venous Pressures: Record vital signs frequently, including BP, until the child’s condition is stable. The heart rate and respirations are counted for 1 full minute, compared with the values on the ECG monitor, and recorded with activity. The heart rate is normally increased after surgery. The nurse observes cardiac rhythm and notifies the practitioner of any changes in regularity. Dysrhythmias may occur postoperatively secondary to administration of anesthetics, acid-base and electrolyte imbalance, hypoxia, surgical intervention, or trauma to conduction pathways.
At least hourly, auscultate the lungs for breath sounds. Diminished or absent breath sounds may indicate an area of atelectasis, pleural effusion, or pneumothorax. All such cases require further assessment. Auscultation guides the nurse’s selective use of postural drainage and percussion to those pulmonary lobes most in need. It also allows a more objective evaluation of effective ventilation.
Temperature changes are typical during the early postoperative period. Hypothermia is expected immediately after surgery due to hypothermia procedures, effects of anesthesia, and loss of body heat to the cool environment. During this period the child is kept warm to prevent additional heat loss. Infants may be placed under radiant heat warmers. During the next 24 to 48 hours the body temperature may rise to 37.8° C (100° F) or slightly higher as part of the inflammatory response to tissue trauma. After this period an elevated temperature is most likely a sign of infection and warrants immediate investigation for probable cause.
Intraarterial monitoring of BP is almost always done following open-heart surgery. Residual vasoconstriction after cardiopulmonary bypass makes indirect BP readings less reliable, and intraarterial monitoring permits continuous rather than intermittent observation. A catheter is passed into the radial artery or the dorsalis pedis or posterior tibial artery, and the other end is attached to an electronic monitoring system, which provides a continuous recording of the BP. The intraarterial line is maintained with a low-rate, constant infusion of heparinized saline to prevent clotting. Continuous BP readings are compared with those taken indirectly using a sphygmomanometer or oscillometric device (Dinamap). A discrepancy between the two may indicate a change in peripheral vascular resistance, a malfunction in the electronic device, or human error in using the wrong-size BP cuff. The nurse also observes for potential complications of intraarterial monitoring, such as arterial thrombosis, infection, air emboli, or blood loss through the catheter. Prevention of each of these hazards is similar to care for any other type of infusion line.
The intraarterial line is maintained with a low-rate, constant infusion of heparinized saline to prevent clotting. The amount of irrigant is recorded as intake fluid. The dressing at the site is changed daily.
Several IV lines are inserted preoperatively: a peripheral IV to give fluids and medications and a CVP line that is usually inserted in a large vessel in the neck. Intracardiac monitoring lines are placed intraoperatively in the RA, LA, or pulmonary artery. Intracardiac lines allow assessment of pressures inside the cardiac chambers, which give vital information on blood volume, cardiac output, ventricular function, pulmonary artery pressures, and responses to drug therapy in the immediate postoperative period. The RA and CVP lines may also be used to infuse fluids and medications. LA lines and pulmonary artery lines are used with more complex repairs. Intracardiac lines are used only in the ICU, although CVP lines may remain for use as a central IV line outside the ICU. All lines must be cared for using strict aseptic technique to prevent infection. Patients must be carefully assessed for bleeding at the time of line removal. See critical care texts for a more complete discussion of intracardiac lines.
Maintain Respiratory Status: Infants usually require mechanical ventilation in the immediate postoperative period. Children may be extubated in the operating room or in the first few postoperative hours, especially if cardiopulmonary bypass was not required. When weaning and extubation are completed, oxygen is delivered by mask, hood, or nasal cannula and is humidified to prevent drying of mucosa. Encourage the child to turn and deep breathe at least hourly. Every means is employed to enhance ventilation and decrease pain, such as splinting of the operative site and use of analgesics.
Suctioning is performed only as needed and is done carefully to avoid vagal stimulation (which can trigger cardiac dysrhythmias) and laryngospasm, especially in infants. Suctioning is intermittent and is maintained for no more than 5 seconds to prevent depleting the oxygen supply. Supplemental oxygen is administered with a manual resuscitation bag before and after the procedure to prevent hypoxia. The heart rate is monitored after suctioning to detect changes in rhythm or rate, especially bradycardia. The child should always be positioned facing the nurse to permit assessment of the child’s color and tolerance of the procedure.
Chest tubes may be inserted into the pleural or mediastinal space during surgery or in the immediate postoperative period to remove secretions and air and allow reexpansion of the lung. The chest tube is attached to a disposable water-seal drainage system. The underwater drainage prevents air from traveling up the tube into the pleural space and causing pneumothorax. Nursing considerations include (1) do not interrupt water-seal drainage unless the chest tube is clamped, (2) check for tube patency (fluctuation in the water-seal chamber), and (3) maintain sterility.
Check drainage hourly for color and quantity. Immediately postoperatively the drainage may be bright red, but afterward it should be serous. The largest volume of drainage occurs in the first 12 to 24 hours, and drainage is greater after extensive heart surgery.
Chest radiographs are taken when the tubes are inserted to check their location and after they are removed to evaluate the inflation of the lungs. Chest tubes are usually removed on the first to third postoperative day when drainage has diminished.
Removal of chest tubes can be an uncomfortable, frightening experience (see Atraumatic Care box). Warn children that they will feel a sharp, momentary pain. After the suture is cut, the tubes are quickly pulled out at the end of full inspiration in the extubated patient to prevent intake of air into the pleural cavity. (In the intubated patient, the tubes are pulled out on inspiration, since the lungs are stented open with the positive pressure ventilation.) A purse-string suture (placed when the tubes were inserted) is pulled tight to close the opening. A petrolatum-covered gauze dressing is immediately applied over the wound and securely taped to the skin on all four sides so that an airtight seal is formed. The dressing is checked for signs of drainage. It is removed the next day. Breath sounds are auscultated, since pneumothorax is a possible complication of chest tube removal. A chest x-ray film is usually obtained after removal to assess for pneumothorax or pleural effusion.
Provide Maximum Rest: After heart surgery maximum rest should be provided to decrease the workload of the heart and promote healing. Nursing care is planned according to the child’s usual activity and sleep patterns. The simplest way to ensure individualized, efficient, high-quality care is to plan at the beginning of the shift the nursing procedures to be done. Identify periods of rest. Share the schedule with parents to allow them to visit at the most advantageous times, such as after a rest period when no special treatments are anticipated.
Provide Comfort: Heart surgery is both painful and frightening for children, and providing comfort is a primary nursing concern. Several incisions are used for heart surgery. A median sternotomy following the sternum down the center of the chest is most common. A ministernotomy opens the lower sternum. A thoracotomy incision is most uncomfortable because it goes through muscle tissue. It allows access to the side of the chest through an incision that runs from under the arm around the back to the scapula.
Adequate pain control decreases postoperative complications such as atelectasis, pneumonia, and deep vein thrombosis by improving coughing and ambulation. Pain level is now considered the fifth vital sign. Many pain assessment tools are available for infants and children of different ages. (See Pain Assessment, Chapter 7.)
Continuous IV infusion of opioids, particularly morphine and fentanyl, is a safe and effective method of pain control. Patient-controlled analgesia may be used with children old enough to understand the concept (Macfadyen and Buckmaster, 1999). Epidural morphine is another option. Children receiving opioid infusions for a prolonged period are weaned slowly from the medication to prevent withdrawal symptoms. Nonsteroidal antiinflammatory drugs (NSAIDs) such as IV ketorolac (Toradol) or oral ibuprofen may be used to provide relief of moderate postoperative pain.
Most patients need IV analgesics for pain control during the 24- to 48-hour postoperative period. After lines and tubes have been removed and when patients are tolerating oral fluids, pain may be controlled with oral narcotics such as codeine or oxycodone, often combined with acetaminophen or an oral NSAID such as ibuprofen, or with acetaminophen alone. As noted earlier, thoracotomy incisions are usually more painful than sternotomies because the incision is through muscle. Higgins, Turley, Harr, and colleagues (1999) have found that round-the-clock use of acetaminophen or ibuprofen to augment narcotics is advantageous in providing pain relief after heart surgery. Acetaminophen or ibuprofen alone is usually adequate for pain control after discharge. (See Pain Management, Chapter 7.)
In addition to providing pharmacologic pain control, make every effort to minimize the discomfort of procedures by other means, such as by placing a firm pillow or favorite stuffed animal against the chest incision during coughing and performing treatments after pain medication is given, preferably at a time that coincides with the drug’s peak effect. Employ nonpharmacologic measures to lessen the perception of pain, and encourage parents to comfort their child as much as possible. (See Pain Management, Chapter 7.)
Monitor Fluids: Intake and output of all fluids must be accurately calculated. Intake is primarily IV fluids; however, the nurse also needs to keep a record of fluid used to flush the arterial and CVP lines or to dilute medications. Monitoring of output includes hourly recordings of urine (usually a Foley catheter is inserted and attached to a closed collecting device), drainage from chest and nasogastric tubes, and blood drawn for analysis. Urine is analyzed for specific gravity to evaluate the kidneys’ concentrating ability and to assess the body’s degree of hydration. Renal failure is a potential risk from a transient period of low cardiac output.
During open-heart surgery, the cardiopulmonary pump is primed with a large volume of fluid (usually electrolyte solution), which may greatly dilute the patient’s blood. The large amount of fluid also diffuses into the interstitial spaces, causing total-body edema and pulmonary edema. Patients return from the operating room with fluid overload. Fluids are restricted to less than maintenance level during the first postoperative day, and drugs are used to promote diuresis. A return to maintenance fluid levels then occurs over the next few days as patients resume normal nutrition. Electrolyte levels are closely monitored because electrolyte imbalances, especially hypokalemia, are a common result of diuresis and fluid shifts, and electrolytes may need replacement.
Fluid requirements are based on the child’s weight and body surface area. The child is weighed daily, preferably in the morning, using the same scale and in similar clothing. The child is usually given nothing by mouth for the first 24 hours. Oral fluids are usually withheld until the child is extubated. Patients begin taking clear liquids when bowel sounds are heard and advance slowly to a regular diet. Nausea and vomiting are common in the first few days after surgery, likely a side effect of anesthesia and analgesics. Providing adequate nutrition, ideally by oral intake, becomes important by the fourth or fifth postoperative day. Consider nasogastric tube feedings or parenteral nutrition for patients who are unable to tolerate oral feedings.
Plan for Progressive Activity: Fatigue and weakness are common after heart surgery. However, moderate activity is essential to prevent pulmonary and vascular complications. Initially, turning, coughing, and deep breathing are sufficient to promote respiratory expansion. Passive range-of-motion exercises, especially to the lower extremities, are instituted to prevent venous stasis.
A progressive schedule of ambulation and activity is planned, based on the child’s preoperative activity patterns and postoperative cardiovascular and pulmonary function. Provide the child with toys to encourage movement. It is important to plan the activity at times when the child is well rested, is comfortable (usually has had analgesic medication), and is not scheduled for any strenuous procedure or treatment immediately afterward.
Ambulation is initiated early, usually by the second postoperative day, after extubation and when many lines and tubes have been removed. Patients progress from sitting on the edge of the bed and dangling the legs to standing up and to sitting in a chair while being assisted and assessed by the nursing staff. Carefully monitor the heart rate and respirations to assess the degree of cardiac demand imposed by each activity. Tachycardia, dyspnea, cyanosis, desaturation, progressive fatigue, or dysrhythmias indicate the need to limit further energy expenditure. After ambulation a rest period is scheduled.
Observe for Complications of Heart Surgery: Several complications can occur after heart surgery, most of which are related to open-heart surgery and the use of cardiopulmonary bypass. Many of the procedures discussed in the preceding paragraphs are aimed at preventing these problems. Only those that have not already been discussed are included here. A serious complication, infective endocarditis (bacterial), is discussed on p. 1382.
Cardiac Changes: Preoperatively the workload of the heart is increased because of the abnormal hemodynamics caused by the congenital defect. In the initial postoperative period the heart is under increased stress because of the effects of surgery and the use of the heart-lung machine. In some cases cardiac function can actually be worse in the early postoperative period despite repair of the congenital defect. HF, hypoxia, low cardiac output, dysrhythmias, and tamponade are all potential postoperative problems.
HF may occur postoperatively because of excessive pulmonary blood flow or fluid overload (see p. 1354 for assessment and management of HF). Hypoxia may occur because of inadequate pulmonary blood flow or because of respiratory problems. Rapid assessment of the causes of hypoxia and appropriate interventions to improve ventilation and perfusion are vital, since hypoxia can rapidly lead to acidosis, which can impair ventricular function.
Low cardiac output syndrome and decreased peripheral perfusion can occur from hypothermia or inability of the LV to maintain systemic circulation. It affects up to 25% of infants and young children after cardiac surgery (Hoffman, Wernovsky, Atz, et al, 2003). The most important signs of adequate peripheral perfusion are rapid capillary refill, good skin color, warm extremities, and strong pulses. Indications of low cardiac output are similar to signs of shock (i.e., decreased BP, decreased pulse pressure, cool extremities, metabolic acidosis, and oliguria). Low cardiac output states are aggressively treated with IV inotropic medications such as dopamine, dobutamine, and milrinone. Milrinone, widely used in pediatrics, has also been shown to prevent low cardiac output syndrome (Hoffman, Wernovsky, Atz, et al, 2003). If maximum medical therapy is failing, cardiac assist methods such as extracorporeal membrane oxygenation or a ventricular assist device may be used in some centers under certain circumstances. Mortality is higher than 50% for patients who require mechanical support. Patients who have recovery of ventricular function within 2 to 3 days and have a short period of support have the best outcomes (Craig, Smith, and Fineman, 2001).
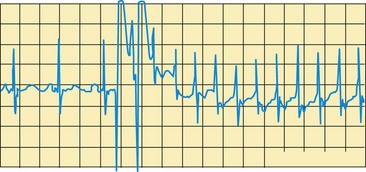
Dysrhythmias are common in the early postoperative period and can result from electrolyte imbalance, especially hypokalemia, and surgical intervention to the septum or myocardium. The heart rate and rhythm are carefully monitored by observing the ECG pattern and by counting the apical pulse for 1 full minute. In some children, a faster than normal rate may be required to maintain an adequate cardiac output in the postoperative period, and a slower than normal rhythm can impair cardiac output. Epicardial pacing wires may be inserted during surgery for managing cardiac dysrhythmias postoperatively.
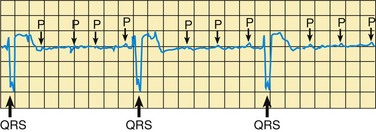
![]() Cardiac tamponade is compression of the heart by blood and other effusion (clots) in the pericardial sac, which severely restricts the normal heart movement. Signs include rising and equalizing RA and LA filling pressures, narrowing pulse pressure, tachycardia, dyspnea, apprehension, and an abrupt stop to chest tube drainage from mediastinal tubes. The nurse immediately reports any evidence of this potentially fatal complication. An echocardiogram confirms the diagnosis. Treatment consists of prompt pericardiocentesis to remove the blood or fluid. If active hemorrhage and coagulopathy are present, steps are taken to enhance blood clotting.
Cardiac tamponade is compression of the heart by blood and other effusion (clots) in the pericardial sac, which severely restricts the normal heart movement. Signs include rising and equalizing RA and LA filling pressures, narrowing pulse pressure, tachycardia, dyspnea, apprehension, and an abrupt stop to chest tube drainage from mediastinal tubes. The nurse immediately reports any evidence of this potentially fatal complication. An echocardiogram confirms the diagnosis. Treatment consists of prompt pericardiocentesis to remove the blood or fluid. If active hemorrhage and coagulopathy are present, steps are taken to enhance blood clotting.
Pulmonary Changes: Areas of atelectasis are common immediately after surgery as a result of deflation of the lung during cardiopulmonary bypass. Other pulmonary complications include pneumothorax, especially caused by faulty chest tubes; pulmonary edema from increased pulmonary blood flow or heart failure; and pleural effusion caused by persistent venous congestion. Signs of pneumothorax are persistent decreased breath sounds, sudden dyspnea, tachycardia, rapid shallow respirations, cyanosis, and sometimes sharp chest pain. Signs of pulmonary edema are tachypnea, rales, wheezing, moist dyspneic respirations, tachycardia, cyanosis, and restlessness. Signs and symptoms of pleural effusions include increased respiratory rate, vomiting, decreased breath sounds, fatigue, irritability, and decreased oxygen saturation. Chest radiography is important in the accurate diagnosis of pulmonary complications and is done frequently postoperatively.
Neurologic Changes: Neurologic complications such as seizures, strokes, cerebral edema, and hypoxic or ischemic brain injury are uncommon after open-heart surgery but can be devastating when they occur. Nurses are alert to the possibility of neurologic symptoms and perform ongoing neurologic assessments, including evaluation of the equality of strength and reflexes in both extremities for evidence of paralysis; pupil size, equality, reaction to light, and accommodation; and the child’s orientation to the environment. The nurse also observes for focal or generalized seizure activity. Any evidence of cerebral damage is reported immediately. Further neurologic evaluation and management are needed for all abnormalities.
Seizures are the most common neurologic condition, seen most often in infants. Longer periods of deep hypothermic cardiopulmonary bypass (>40 minutes), sometimes needed in complex repairs on neonates, have been associated with an increased risk of seizure activity and later developmental delays (Wypij, Newberger, Rappaport, et al, 2003). Significant improvements in cardiopulmonary bypass techniques, arterial filters, and equipment and a better understanding of neuroprotection during heart surgery have resulted in a reduced incidence of seizures, movement disorders, and coma (Menasche, duPlessis, Wessel, et al, 2002).
Infection: All patients are at risk for infections postoperatively; especially vulnerable are infants, those with poor cardiac function, and those who require multiple invasive lines and procedures for a prolonged period. Prophylactic antibiotics are given for the first 1 or 2 days. All dressings are applied and changed using aseptic technique. Good hand washing, careful use of aseptic technique when placing and accessing lines, and close attention to surgical wounds are all important to prevent infection. Monitor patients closely for fever and signs of infection. Monitor all IV sites for signs of infection or phlebitis. Appropriate treatment is instituted if an infection is identified.
Hematologic Changes: While passing through the heart-lung machine, blood is exposed to substantial trauma because of mechanical action and direct contact with oxygen, foreign substances, and massive doses of anticoagulants. The result of mechanical trauma is red blood cell hemolysis and potential renal tubular necrosis. Heparinization of the blood during extracorporeal circulation can result in clotting abnormalities from decreased thrombin and prothrombin levels, decreased levels of platelets, and altered platelet aggregation.
Hemolysis of red blood cells leads to blood loss and anemia, which may require packed red blood cell transfusion. The nurse monitors results of complete blood counts to identify the severity of the hemolysis. All urine is tested for blood. If transfusions are required, the child is closely observed for signs of reaction and fluid overload. (See Table 35-2.) The need to measure urinary output hourly has already been discussed.
Because blood-clotting mechanisms are affected, signs of hemorrhage, especially bleeding from the chest tubes and a fall in arterial and venous pressures, are important observations. Hemorrhage is more likely to occur in patients who undergo repair of cyanotic heart defects because of the associated physiologic thrombocytopenia.
Normally the filter and bubble trap on the heart-lung machine remove air emboli, tiny clots, fat debris, and organisms from the arterialized (oxygenated) blood before its return to the body. However, the entry of impure blood into the systemic circulation can cause fat embolism, thromboembolism, and infection anywhere in the body and, most important, in the brain.
Postpericardiotomy Syndrome: The postpericardiotomy syndrome of fever, leukocytosis, pericardial friction rub, or pericardial and pleural effusion can occur anytime the pericardium is opened, either in the immediate postoperative period or after surgery, typically around day 7 to 21. The cause is unknown, although etiologic theories include viral infection, autoimmune response to myocardial tissue, and a reaction to blood in the pericardium. The syndrome is self-limiting and is treated with rest, salicylates, NSAIDs, and sometimes steroids. Pericardiocentesis or pleurocentesis may be needed to treat large effusions.
Provide Emotional Support
Children may become depressed after surgery. This is thought to be caused by preoperative anxiety, postoperative psychologic and physiologic stress, and sensory overstimulation. Typically the child’s disposition improves on leaving the ICU. (See Chapter 26.)
Children may also be angry and uncooperative after surgery as a response to the physical pain and to the loss of control imposed by the surgery and treatments. They need an opportunity to express feelings, either verbally or through activity. Nurses can praise children for their efforts to cooperate and should refrain from expecting too much courage or bravery. Children often regress in their behavior during the stress of surgery and hospitalization. Children also may express feelings of anger or rejection toward parents. The nurse must reassure parents that this is normal and that with continued support the anger will subside.
The nurse can support the parents by being available to provide information and explaining all the procedures to them. The first few postoperative days are particularly difficult because parents see their child in pain and realize the potential risks from surgery. They often are overwhelmed by the physical environment of the ICU and feel useless because they can do so little for their child. The nurse can minimize such feelings by including parents in caregiving activities and comfort and play activities; by providing information about the child’s condition; and by being sensitive to their emotional and physical needs. The importance of their presence in making the child feel more secure is stressed, even if they do not provide physical care.
Plan for Discharge and Home Care
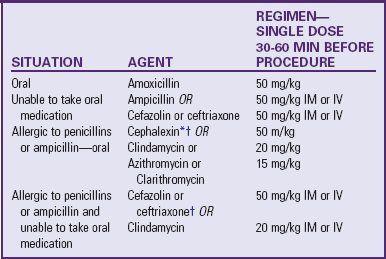
Assessment of discharge needs should begin at admission so parents and health care providers have ample time to plan for a safe discharge and arrange for necessary equipment and supports. The family needs verbal and written instructions on medication, nutrition, activity restrictions, wound care, pain management, and signs and symptoms of infection or complications. Other discussion topics may include return to school and work, special medication teaching for warfarin (Coumadin) or other drugs that require detailed home management, and infective endocarditis (subacute bacterial endocarditis [SBE]) prophylaxis. Referrals to community agencies may be necessary to assist parents in the transition from hospital to home and to reinforce the teaching (see Family-Centered Care box).
The parents also need clear instructions on when to seek medical care for complications and how to contact the health care provider. Follow-up with the cardiologist, usually within 2 weeks, and with the primary care provider is also arranged before discharge. Encourage parents to keep an updated summary of their child’s diagnosis, surgical procedures, allergies, medications, health care providers with contact information, and other health problems readily available for emergencies and to share this summary with school personnel, baby-sitters, and others. Appropriate medical identification, such as a MedicAlert bracelet, is indicated for children with a pacemaker or a heart transplant and for those receiving anticoagulation therapy or antidysrhythmic medication.
The nurse also discusses common behavior disturbances that may occur after discharge, such as nightmares, sleep disturbances, separation anxiety, and overdependence. A supportive, consistent response is essential to allow the child to overcome the surgical experience. The child may work out feelings and fears through therapeutic play, and this should be encouraged.
Although surgical correction of heart defects has improved dramatically, it is still not possible to totally repair many complex anomalies. Some repairs require several operations over a period of years. For many children, repeat procedures are required to replace conduits or valves or to manage complications such as restenosis. Consequently, the long-term prognosis is uncertain, and full recovery is not always possible. For these families, close medical follow-up and continued emotional support are essential.
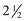 to 3 years of age, since the child must remain still for up to 1 hour to obtain adequate visualization of the coronary arteries and cardiac structures and function.
to 3 years of age, since the child must remain still for up to 1 hour to obtain adequate visualization of the coronary arteries and cardiac structures and function.
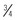 cup/day at age 1 year to 3 cups/day for a 14- to 18-year-old boy.
cup/day at age 1 year to 3 cups/day for a 14- to 18-year-old boy.