Chapter 6 Pulmonary ventilation
 Pharyngeal and laryngeal muscles display both tonic and phasic contraction to maintain airway patency and to regulate air flow.
Pharyngeal and laryngeal muscles display both tonic and phasic contraction to maintain airway patency and to regulate air flow. The diaphragm, intercostal, and some neck muscles bring about inspiration by a complex combination of actions, these varying with different postures.
The diaphragm, intercostal, and some neck muscles bring about inspiration by a complex combination of actions, these varying with different postures.Breathing consists of rhythmic changes in lung volume brought about by the medullary respiratory neurones described in Chapter 5. Several muscle groups are involved in effecting the change in lung volume. First, muscles of the pharynx and larynx control upper airway resistance; secondly the diaphragm, ribcage, spine and neck muscles bring about inspiration; and finally, muscles of the abdominal wall, ribcage and spine are used when active expiration is required. Many of these muscle groups have common origins and attachments such that their activity is complex and dependent both on each other and many non-respiratory factors including posture, locomotion and voluntary activity.
Upper Airway Muscles
During inspiration through the nose, the pressure in the pharynx must fall below atmospheric by an amount equal to the product of inspiratory gas flow rate and the flow resistance afforded by the nose (see Figure 4.1). This development of only a few kilopascals of sub-atmospheric pressure in the pharynx tends to cause the pharynx to collapse.
Pharyngeal obstruction in response to these pres-sure changes during inspiration is opposed by reflex contraction of pharyngeal dilator muscles during inspiration.1 The afferent side of the reflex arises from mechanoreceptors in the pharynx and larynx. These pressure receptors respond in a graded manner to subatmospheric pressure, and have myelinated afferent fibres to facilitate a rapid response.2,3 Based on the observation that the pharyngeal dilator reflex is less active during sleep (page 270) the reflex pathway is believed to involve higher centres of the brain.4 Nevertheless, the reflex is extremely rapid with both genioglossus and tensor palati electromyographic (EMG) activity increasing less than 50 ms after a negative pressure is applied to the pharynx. This compares with a reaction time for voluntary tongue movements of 190 ms.2 The efferent side of the reflex involves most of the pharyngeal dilator muscles, which display tonic contraction and/or phasic inspiratory activity. Airway diameters are well maintained down to pressures of 1.5 kPa (15 cmH2O) below atmospheric, during active but not passive breathing manoeuvres.5 Pulmonary slowly adapting stretch receptors (page 67) may also be involved in the reflex as the activity of all pharyngeal dilator muscles is inhibited by lung inflation.6
There is no significant narrowing of the airway when changing from the erect to the supine posture in the normal subject.7 Genioglossus EMG activity is increased by 34% in the supine position, presumably to counteract the effect of gravity on the tongue.8 Anatomical considerations suggest that patency of the nasopharynx in the supine position is maintained by tensor palati, palatoglossus and palatopharyngeus, and tonic but not phasic respiratory activity has been detected in levator palati.9 The soft palate tends to fall back against the posterior pharyngeal wall in the supine position without contraction of these muscles.
Failure of the various mechanisms that preserve pharyngeal airway patency may occur in sleep, hypoxia or anaesthesia; their occurrence and prevention are discussed in Chapters 16 and 22.
Laryngeal control of airway resistance. During quiet breathing, movement of the vocal folds is used as a choke for fine control of airway resistance. On inspiration, phasic activity of the posterior cricoarytenoid muscles, acting by rotating the arytenoid cartilages, abducts the vocal cords to minimise resistance.10 A greater effect occurs during expiration, when phasic electrical activity in thyroarytenoid muscles indicates adduction of the vocal cords,13 and therefore an increase in resistance. This may help to prevent collapse of the lower airways (page 49).
Respiratory Muscles of the Trunk
Nomenclature in this area can be confusing with different authors using different terms. The trunk (referred to as chest wall by some studies) may be divided into the ribcage and abdomen. These two compartments are separated by the diaphragm and both are therefore greatly influenced by its activity.
The Diaphragm
The diaphragm is a membranous muscle separating the abdominal cavity and chest, and in adults has a total surface area11 of approximately 900 cm2. It is the most important inspiratory muscle, with motor innervation solely from the phrenic nerves (C3, 4, 5). In comparison with other skeletal muscles, the diaphragm is extremely active. Muscle fibres within the diaphragm can reduce their length by up to 40% between residual volume and total lung capacity,11 and spend 45% of each day contracting, compared with only 14% for the soleus muscle.12 The diaphragm has considerable reserve of function, and unilateral phrenic block causes little decrement of overall ventilatory capacity. Despite the importance of the diaphragm to respiration, bilateral phrenic interruption is still compatible with good ventilatory function.
Mechanics of diaphragmatic function. The origins of the crural part of the diaphragm are the lumbar vertebrae and the arcuate ligaments, whilst the costal parts arise from the lower ribs and xiphisternum. Both parts are inserted into the central tendon. Recent studies of human subjects using MRI scans, illustrated in Figure 6.1, have enabled the in vivo actions of the diaphragm to be better defined.11,13,14 Under normal circumstances, a zone of apposition exists around the outside of the diaphragm where it is in direct contact with the internal aspect of the ribcage, with no lung in between, but the parietal pleura still allowing free movement of the diaphragm. At upright FRC in humans, approximately 55% of the diaphragm surface area is in the zone of apposition.11
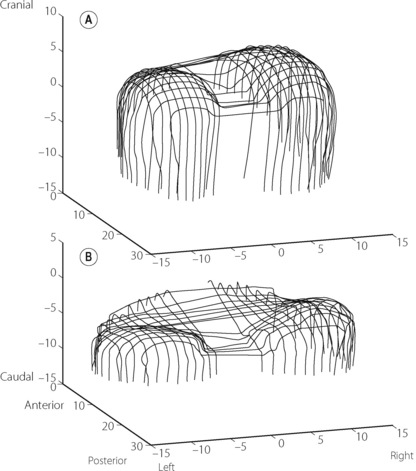
Fig. 6.1 Three-dimensional reconstructions of the human diaphragm at functional residual capacity using fast computed tomography scanning (dimensions in cm). (A) Normal subject showing extensive zone of apposition and normal curvature of the diaphragm domes. (B) Patient with hyperinflated chest as a result of chronic obstructive pulmonary disease (page 411). Note the reduced zone of apposition and the flattened diaphragm domes.
(After reference 14 by permission of the authors and the publishers of American Journal of Respiratory and Critical Care Medicine.)
There are many ways by which diaphragm contraction may bring about an increase in lung volume,15 and these are illustrated schematically in Figure 6.2. These may be considered using a ‘piston in a cylinder’ analogy, the trunk representing the cylinder and the diaphragm the piston (Figure 6.2A). Figure 6.2B illustrates the first possible mechanism, involving downward movement of the diaphragm simply by shortening the zone of apposition around the whole cylinder and leaving the dome shape unchanged. This is a pure ‘piston-like’ action and has the advantage of very efficient conversion of diaphragm muscle fibre shortening into changes in lung volume. Figure 6.2C illustrates ‘non-piston-like’ behaviour in which the zone of apposition remains unchanged but an increase in the tension of the diaphragm dome reduces the curvature, so expanding the lung. This is likely to be less efficient than piston-like behaviour because much of the muscle tension developed simply opposes the opposite side of the diaphragm rather than moving the diaphragm downwards, such that in theory, when the diaphragm becomes flat, further contraction will have no effect on lung volume. Finally, Figure 6.2D incorporates both types of behaviour already described but also now includes expansion of the lower ribcage (known as ‘piston in an expanding cylinder’) that occurs with diaphragmatic contraction, particularly in the supine position, and so represents a simple description of the in vivo situation.
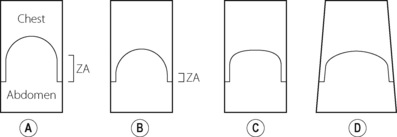
Fig. 6.2 “Piston in a cylinder” analogy of the mechanisms of diaphragm actions on the lung volume. (A) Resting end-expiratory position. (B) Inspiration with pure piston-like behaviour. (C) Inspiration with pure non-piston-like behaviour. (D) Combination of piston-like and non-piston-like behaviour in an expanding cylinder, which equates most closely with inspiration in vivo. ZA, zone of apposition.
In the supine position, diaphragm action is a combination of all the above mechanisms as well as a change in shape involving a tilting and flattening of the diaphragm in the antero-posterior direction.11
Ribcage Muscles16
As already described, the ribcage may be regarded as a cylinder with length governed primarily by the diaphragm and secondarily by flexion and extension of the spine. The cross-sectional area of the cylinder is governed by movement of the ribs. This movement involves mainly rotation of the neck of the rib about the axis of the costovertebral joints, and their shape is such that elevation of the ribs in this way increases both the lateral and anteroposterior diameter of the ribcage. Elevation of the ribs by the intercostal muscles tends to result in a ‘bucket handle’ action, whilst elevation of the anterior ribcage by, for example, the sternomastoid muscle elevating the sternum results in a ‘pump-handle’ type of movement. These two actions tend to occur together, and depend also on other requirements such as posture and upper limb movements. Upper ribs are inserted into the sternum and do not necessarily behave in quite the same way as the lower ‘floating’ ribs, which are inserted into the more flexible costal cartilage.
The intercostal muscles are divided into the external group, fibres of which run in a caudal–ventral direction from their upper rib and are deficient anteriorly, and the less powerful internal group which have fibres running caudal–dorsal from their upper rib and are deficient posteriorly. Internal intercostal muscles of the upper ribcage become thicker anteriorly where they are known as the parasternal intercostal muscles. In 1749, mechanical considerations led Hamberger to suggest that the external intercostals were primarily inspiratory, and the internal intercostals primarily expiratory.17 Though an oversimplification,16 this has generally been confirmed by electromyography. The parasternal portion of the internal intercostals are inspiratory in both humans and animals, and the inspiratory activity of external intercostals, though minimal during quiet breathing, becomes increasingly important during stimulated breathing. Posture plays an important role in intercostal activity in humans. For example, during the rather extreme postural challenge of rotating the trunk, which changes the mechanical properties of the ribs, the respiratory activity of internal and external intercostals is reversed with internal intercostals becoming expiratory and vice versa.18
Scalene muscles are active in inspiration during quiet breathing in humans19 particularly when upright. Their role is to elevate the ribcage and this counteracts the tendency of the diaphragm to cause inward displacement of the upper ribs. Innervation is from C1 to C5.
Accessory muscles. These are silent in normal breathing in humans but as ventilation increases, the inspiratory muscles contract more vigorously and accessory muscles are recruited. Considerable hyperventilation (about 50 1.min−1) or severe increases in respiratory loading are usually present before the accessory muscles become active. Accessory muscles include the sternocleidomastoids, extensors of the vertebral column, pectoralis minor, trapezius and the serrati muscles. Many of these muscles, for example the pectorals, reverse their usual origin/insertion and help to expand the chest, provided the arms and shoulder girdle are fixed by grasping a suitable support.
Abdominal Muscles
With the exception of gas within the bowel lumen, the abdomen is an incompressible volume held between the diaphragm and the abdominal muscles. Contraction of either will cause a corresponding passive displacement of the other. Thus abdominal muscles are generally expiratory.
Rectus abdominis, external oblique, internal oblique and tranversalis muscles are the most important expiratory muscles, whilst the muscles of the pelvic floor have a supportive role. Contraction of these muscles results in an increase in abdominal pressure displacing the diaphragm in a cephalad direction. In addition, their insertion into the costal margin results in a caudad movement of the ribcage, so assisting expiration by opposing the ribcage muscles. Gastric pressure is a valuable index of their activity because their contraction will always cause an increase in intra-abdominal pressure.
In the supine position, the abdominal muscles are normally inactive during quiet breathing and become active only when the minute volume exceeds about 40 1.min−1, in the face of substantial expiratory resistance, during phonation or when making expulsive efforts. When upright, their use in breathing is complicated by their role in the maintenance of posture.
Integration of Respiratory Muscle Activity
Breathing
Figure 6.3 shows the radiographic appearance of the ribcage at residual volume, the normal expiratory level and at maximal inspiration, and illustrates the enormous range of movement within the semi-rigid ribcage. Expiration normally proceeds passively to the functional residual capacity (FRC), which may be considered as the equilibrium position governed by the balance of elastic forces, unless modified by residual end-expiratory tone in certain muscle groups. Inspiration is the active phase, entering the inspiratory capacity but normally leaving a substantial volume unused (the inspiratory reserve volume). Similarly, there is a substantial volume (the expiratory reserve volume) between FRC and the residual volume (see Figure 3.9). By voluntary effort it is possible to effect a satisfactory tidal exchange anywhere within the vital capacity, but the work of breathing is minimal at FRC.
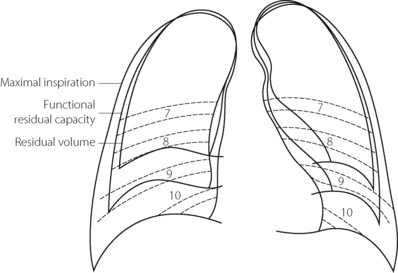
Fig. 6.3 Outlines of chest radiographs of a normal subject at various levels of lung inflation. The numbers refer to ribs as seen in the position of maximal inspiration.
(With thanks to Dr R. L. Marks who was the subject.)
Although we tend to think of the respiratory muscles individually, it is important to remember that they act together in an extraordinarily complex interaction that is influenced by factors including posture, minute volume, respiratory load, disease and anaesthesia. Figure 6.4 illustrates some features of the interaction.18,20
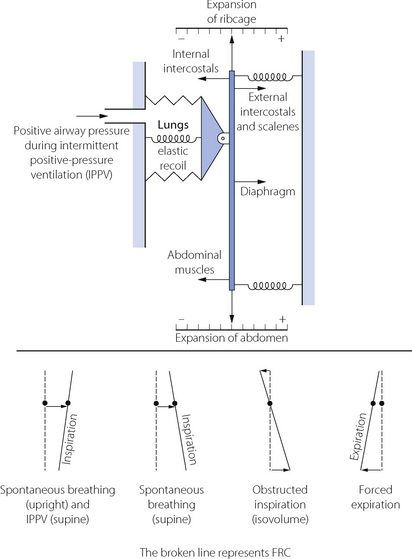
Fig. 6.4 A model of the balance of static and dynamic forces acting on the respiratory system. The central bar, attached to the lungs, is floating freely, held in equilibrium by the elastic forces at the end-expiratory position as shown. It may be displaced by the actions of the various muscles shown, with movement to the right generally indicating inspiration and vice versa. Action of the various inspiratory or expiratory muscles causes changes, not only in the lung volume but also in the inclination of the bar, which represents relative changes in the cross sectional area of the rib cage and abdomen. See text for details.
(Derived from references 20 and 21.)
Inspiration. In Figure 6.4 it can be seen that the ribcage inspiratory muscles (external intercostals and scalenes) and diaphragm act in parallel to inflate the lungs, with posture affecting which muscle group is dominant (see below). In either position, diaphragm activity alone results in a widening of the lower ribcage and an indrawing of the upper ribcage (often seen with spontaneous respiration during general anaesthesia), which must be countered by the intercostal and neck muscles contracting simultaneously.
Expiration. Requires no muscular activity during quiet breathing in the supine position, because the elastic recoil of the lungs provides the energy required, aided by the weight of the abdominal contents pushing the diaphragm in a cephalad direction. In the upright posture and during stimulated ventilation the internal intercostal muscles and the abdominal wall muscles are active in returning the ribcage and diaphragm to the resting position. In extreme hyperventilation, for example following exercise, the expiratory muscles become progressively more important until ventilation assumes a quasi sine wave push-pull pattern.
Separation of Volume Contribution of Ribcage and Abdomen
Konno & Mead originally proposed that the separate contributions to tidal volume of changes in ribcage (RC) and abdominal (AB) compartments could be measured.22 Essentially similar results may be obtained by measuring either anteroposterior distance (magnetometers), circumference (strain gauge) or cross-sectional area (respiratory inductance plethysmography, RIP23). Once initially calibrated to convert measurements of trunk dimensions into volumes, the sum of RC and AB movements correlates well with tidal volume and provides an excellent non-invasive measure of ventilation. RC/(RC + AB) indicates the proportion of tidal volume that can be attributed to expansion of the ribcage (usually expressed as %RC). However, such is the complexity of the muscular system described above that changes in %RC cannot be attributed to changes in the force of contraction of any particular muscle. Figure 6.5 shows RIP traces during normal breathing in different positions.
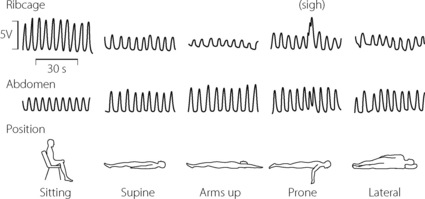
Fig. 6.5 Normal respiratory inductance plethysmography (RIP) traces. The amplitude (in volts) of the RIP signal reflects the cross-sectional area of the rib cage (RC) and abdomen (AB). The sum of the RC and AB signals correlates closely with tidal volume. The figure shows normal breathing in five different positions, demonstrating the predominantly RC contribution when upright and AB contribution in all horizontal positions. Note the spontaneous sigh occurring in the prone position, resulting entirely from rib cage expansion.
(After reference 23 by permission of the publishers of Anesthesia and Analgesia.)
Effect of Posture on Respiratory Muscles
Upright posture, whether standing or sitting, is associated with greater expansion of the ribcage23 such that %RC is around 67% (Figure 6.5). To account for this, increased EMG activity has been demonstrated in the both the scalene and the parasternal intercostals muscles.24
Supine position. When supine, the weight of the abdominal contents pushes the diaphragm upwards, so that in the supine position the diaphragm tends to lie some 4 cm higher, which accords with the reduction in FRC when supine (see Figure 3.10). With the diaphragm higher in the chest, its fibre length is greater and it can therefore contract more effectively, counteracting the tendency to airway closure at the reduced FRC. The dimensions of the ribcage are probably little altered, and the increased diaphragm activity therefore results in a reduced %RC of about 33% in the supine position.23 In the prone and lateral position, RC contribution does not differ significantly from that in the supine position (Figure 6.5).23
Lateral position. In this position (Figure 6.6), only the lower dome of the diaphragm is pushed higher into the chest by the weight of the abdominal contents while the upper dome is flattened. It follows that the lower dome can contract more effectively than the upper, and the ventilation of the lower lung is about twice that of the upper. This is fortunate since gravity causes a preferential perfusion of the lower lung (page 123).
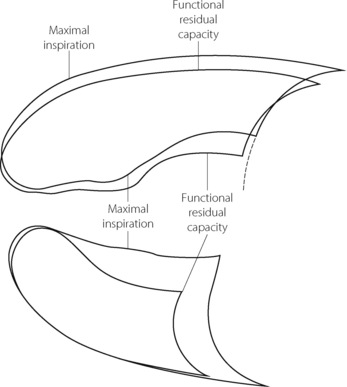
Fig. 6.6 Radiographic outlines of the lungs at two levels of lung volume in a conscious subject during spontaneous breathing in the lateral position (right side down). This is the same subject as in Figure 6.3: comparison will show that, in the lateral position at FRC, the lower lung is close to residual volume while the upper lung is close to inspiratory capacity. The diaphragm therefore lies much more cephalad in the lower half of the chest. Both these factors contribute to the greater volume changes that occur in the lower lung during inspiration.
Chemoreceptor Activation
In animals, clear differences have been demonstrated in the respiratory muscle response to hyperventilation induced either by hypoxia or hypercapnia. For an equivalent minute volume, hypoxia stimulates mostly inspiratory muscles whilst hypercapnia stimulates both inspiratory and expiratory groups. Similar responses occur in humans, with diaphragm EMG activity increasing in response to both hypercapnia and hypoxia, but more rapidly in the latter, and expiratory muscle activity increasing almost exclusively during hypercapnic hyperventilation.24 Hyperventilation in response to hypercapnia in the supine position results in a small increase in RC contribution (7% per kPa Pco2 or 1% per mmHg Pco2).25
Neuronal Control of Respiratory Muscles
The respiratory muscles, in common with other skeletal muscles, have their tension controlled by a servo mechanism mediated by muscle spindles. They appear to play a more important role in the intercostal muscles than in the diaphragm but muscle spindles do exist in both. Their function is largely inferred from knowledge of their well established role in other skeletal muscles not concerned with respiration.
Two types of cell can be distinguished in the motor neurone pool of the anterior horn cell. The alpha motoneurone has a thick efferent fibre (12–20 μm diameter) and passes in the ventral root directly to the neuromuscular junction of the muscle fibre (Figure 6.7A). The gamma motor neurone has a thin efferent fibre (2–8 μm), which also passes in the ventral root, but terminates in the intrafusal fibres of the muscle spindle. Contraction of the intrafusal fibres alone (without overall shortening of the muscle) increases the tension in the central part of the spindle (the nuclear bag), causing stimulation of the annulospiral endings. Impulses so generated are then transmitted via fibres that lie in the dorsal root to reach the anterior horn where they have an excitatory effect on the alpha motor neurones. Using this system, an efferent impulse transmitted by the gamma system causes reflex contraction of the main muscle mass by means of an arc through the annulospiral afferent and the alpha motor neurone. Thus contraction of the whole muscle may be controlled entirely by efferents travelling in the gamma fibres and this is believed to occur in relation to breathing.26
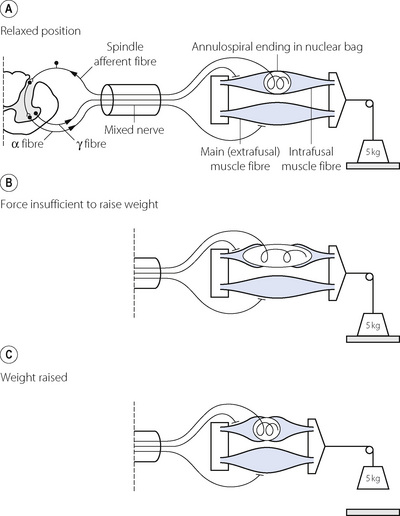
Fig. 6.7 Diagrammatic representation of the servo mechanism mediated by the muscle spindles. (A) The resting state with muscle and intrafusal fibres of spindle relaxed. (B) The muscle is attempting to lift the weight following discharge of both alpha and gamma systems. The force developed by the muscle is insufficient: the weight is not lifted and the muscle cannot shorten. However, the intrafusal fibres are able to shorten and stretch the annulospiral endings in the nuclear bag of the spindle. Afferent discharge causes increased excitation of the motor neurone pool in the anterior horn. (C) Alpha discharge is augmented and the weight is finally lifted by the more powerful contraction of the muscle. When the weight is lifted, the tension on the nuclear bag is relieved and the afferent discharge from the spindle ceases. This series of diagrams relates to the lifting of a weight but it is thought that similar action of spindles is brought into play when inspiratory muscles contract against increased airways resistance.
Alternatively, muscle contraction may in the first instance result from discharge of both the alpha and gamma motoneurones. If shortening of the muscle is unopposed, main (extrafusal) and intrafusal fibres will contract together and the tension in the nuclear bag of the spindle will be unchanged. If, however, the shortening of the muscle is opposed, the intrafusal fibres will shorten more than the extrafusal fibres, causing the nuclear bag to be stretched (Figure 6.7B). The consequent stimulation of the annulospiral endings results in afferent activity that raises the excitatory state of the motor neurones, causing the main muscle fibres to increase their tension until the resistance is overcome, allowing the muscle to shorten and the tension in the nuclear bag of the spindle to be reduced (Figure 6.7C).
By this mechanism, fine control of muscle contraction is possible. The message from the upper motor neurone is in the form: ‘muscles should contract with whatever force may be found necessary to effect such and such a shortening’, and not simply: ‘muscles should contract with such and such a force’. The former message is typical of input into a servo system and far more satisfactory when the load is not known in advance.
For respiratory muscles, a servo system is very advantageous. The message conveyed by the efferent tract from the inspiratory neurones of the medulla would be in the form: ‘inspiratory muscles should contract with whatever force may be necessary to effect a required change in length (corresponding to a certain tidal volume)’ and not simply: ‘inspiratory muscles should contract with such and such a force’. A servo system also provides an excellent mechanism for rapid response to sudden changes in airway resistance. The nature and magnitude of the response of the inspiratory muscles to added resistance to breathing is described in Chapter 4, and the immediate effectiveness of the response is easily explicable in terms of muscle spindles.
Muscle Fibre Subtypes27
Respiratory muscles, like all skeletal muscle, contain different types of muscle fibre classified according to which isoform of myosin heavy chain (MHC) is expressed. Table 6.1 shows the three fibre types known to exist in human respiratory muscles and their contractile and biochemical features. Which isoform of MHC is expressed in a muscle fibre determines the velocity of contraction (Table 6.1). Different isoforms of enzymes involved in muscle relaxation also exist in the different fibre types, and so influence the rate at which relaxation occurs, and therefore the ability of the cell to maintain a tetanic contraction. Type I fibres contract and relax slowly, but can maintain tension for long periods using aerobic metabolic pathways, and are fatigue resistant. In contrast, type IIb fibres rely mainly on glycolytic metabolic pathways for energy supply, contraction is quicker and stronger in bursts of activity, and they fatigue easily. Type IIa fibres have properties intermediate between these two extreme fibre types. The proportions of different fibre types in a muscle therefore reveals the sort of work normally undertaken by the muscle, for example in muscles mainly involved in maintaining posture, type I fibres predominate, whilst in those requiring intermittent activity such as hand muscles type IIa or IIb fibres predominate.
Table 6.1 Properties of muscle fibre types found in human respiratory muscle, and their relative proportions in normal and pathological situations27,28,29
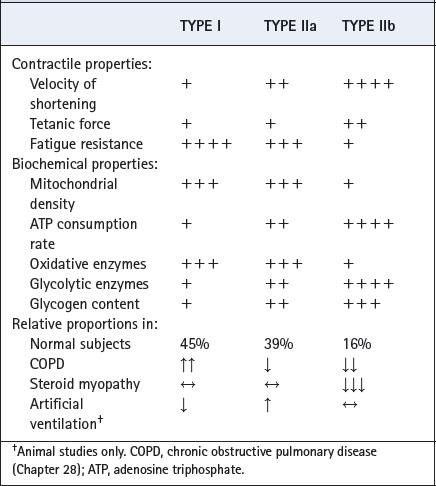
Relative proportions of the different fibre types in human respiratory muscle are shown in Table 6.1, but it is unclear which types of fibre are responsible for different respiratory muscle activities. In animal respiratory muscles, which tend to have less type II fibres than humans, both eupnoeic and stimulated breathing can be achieved solely by using type I fibres, and type II fibres are only required for expulsive efforts such as sneezing and coughing.12 A high proportion of type I fibres (45% in human diaphragm) indicates that they are probably responsible for both posture and respiration in humans, and that type II fibres are again only required for expulsive efforts and active movements such as running, jumping etc. Respiratory disease, drugs and artificial ventilation all cause changes in the relative proportions of different fibre types in respiratory muscles (Table 6.1).
Respiratory Muscle Fatigue and Disuse27,30
The diaphragm, like other striated muscles, is subject to fatigue – defined as an inability to sustain tension with repeated activity.30 For non-respiratory skeletal muscle, fatigue may be ‘central’ – that is, the subject is not trying hard enough (either consciously or subconsciously) – but this is unlikely to be significant in respiration because subjects with respiratory failure usually have a high central respiratory drive. Peripheral fatigue occurs when the frequency of motor nerve action potentials becomes chronically increased in an attempt to increase muscle tension. Eventually, when working against an unsustainable load, striated muscle shows a progressive loss of the high frequency component of the EMG relative to lower frequencies. Finally, relaxation of the muscle fibre, the energy-requiring part of contraction, becomes excessively prolonged and the muscle is unable to respond to the next action potential in order to generate the required tension. In the diaphragm, resistive loads less than 40% of maximum may be sustained indefinitely, but loads greater than 40% of maximum can only be sustained for a short time.31
Blood supply to respiratory muscles may be important in fatigue.32 Animal studies have shown that increased cardiac output and diaphragmatic blood flow (stimulated with noradrenaline) augment the contractility of fatigued diaphragm. In addition, patients with severe congestive cardiac failure, and therefore low cardiac output, have weakened respiratory muscles compared with matched controls, despite having similar muscle strength in the arms.33 The high rate of activity of respiratory muscles seems to leave them more susceptible to weakness in the face of reduced oxygen supply when compared with other muscles, a situation that often causes problems in intensive care when trying to wean patients from artificial ventilation before their cardiovascular function is adequate (page 474).
Effect of Disuse12,34,35
The diaphragm may be rested by artificial ventilation with or without neuromuscular blockade, and the effect on diaphragmatic performance is clearly important. After only 18 hours of mechanical ventilation there are histological and gene-expression changes indicating muscle fibre atrophy,36 and within days diaphragm strength is substantially reduced (Table 6.1).27 Extrapolating these results to all artificially ventilated patients is difficult, as there are numerous factors affecting respiratory muscle strength in critically ill patients.35 Even so, it seems safe to assume that complete inactivity of the normally very active respiratory muscles will be detrimental to their function, and recent developments in artificial ventilation have mostly focussed on supporting, rather than replacing, activity of the patient’s respiratory muscles (Chapter 32).
The Work of Breathing
When expiration is passive during quiet breathing, the work of breathing is performed entirely by the inspiratory muscles. Approximately half of this work is dissipated during inspiration as heat in overcoming the frictional forces opposing inspiration. The other half is stored as potential energy in the deformed elastic tissues of lungs and chest wall. This potential energy is thus available as the source of energy for expiration and is then dissipated as heat in overcoming the frictional forces resisting expiration. Energy stored in deformed elastic tissue thus permits the work of expiration to be transferred to the inspiratory muscles. This remains true with moderate increases of either inspiratory or expiratory resistance, lung volume and therefore elastic recoil being increased in the latter condition (page 54).
The actual work performed by the respiratory muscles is very small in the healthy resting subject. Under these circumstances the oxygen consumption of the respiratory muscles is only about 3 ml.min−1 or less than 2% of the metabolic rate. Furthermore, the efficiency of the respiratory muscles is only about 10%. The efficiency is further reduced in many forms of respiratory disease, certain deformities, pregnancy and when the minute volume is increased (Figure 6.8). When maximal ventilation is approached, the efficiency falls to such a low level that additional oxygen made available by further increases in ventilation will be entirely consumed by the respiratory muscles.
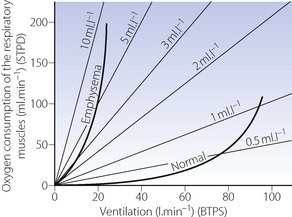
Fig. 6.8 Oxygen consumption of the respiratory muscles plotted against minute volume of respiration. The isopleths indicate the oxygen cost of breathing in millilitres of oxygen consumed per litre of minute volume. The curve obtained from the normal subject shows the low oxygen cost of breathing up to a minute volume of 70 l.min−1. Thereafter the oxygen cost rises steeply. In a patient with chronic obstructive pulmonary disease the oxygen cost of breathing is not only much higher at the resting minute volume but also rises more steeply as ventilation increases. At a minute volume of 20 l.min−1, the respiratory muscles are consuming 200 ml of oxygen per minute, and a further increase of ventilation would consume more oxygen than it would make available to the rest of the body.
(After reference 37 by permission of the Journal of Applied Physiology.)
Units of Measurement of Work
Work is performed when a force moves its point of application, and the work is equal to the product of force and distance moved. Similarly, work is performed when force is applied to the plunger of a syringe raising the pressure of gas contained therein. In this case the work is equal to the product of the mean pressure and the change in volume, or alternatively the product of the mean volume and the change in pressure. The units of work are identical whether the product is force × distance or pressure × volume. A multiplicity of units have been used for measuring work and are listed in Appendix A.
Power is a measure of the rate at which work is being (or can be) performed. The term ‘work of breathing’, as it is normally used and when expressed in watts, is thus a misnomer because we are referring to the rate at which work is being performed and power is the correct term. ‘Work of breathing’ would be appropriate for a single event such as one breath, and joules would then be the appropriate units.
Dissipation of the Work of Breathing
The work of breathing overcomes two main sources of impedance. The first is the elastic recoil of the lungs and chest wall (Chapter 3) and the second is the non-elastic resistance to gas flow (Chapter 4).
Work against elastic recoil. When an elastic body is deformed, no work is dissipated as heat and all work is stored as potential energy. Figure 6.9A shows a section of the alveolar pressure/volume plot for the total respiratory system, showing only the straight part of the curve from near FRC (see Figure 3.7). As the lungs are inflated, the plot forms the hypotenuse of a triangle, whose area represents the work done against elastic resistance. The area of the triangle (half the base times the height) will thus equal either half the tidal volume times the pressure change or the mean pressure times the volume change. Either product has the units of work or energy (joules) and represents the potential energy available for expiration. In Figure 6.9B, the pressure/volume curve is flatter, indicating stiffer or less compliant lungs. For the same tidal volume, the area of the triangle is increased. This indicates the greater amount of work performed against elastic resistance and the greater potential energy available for expiration.
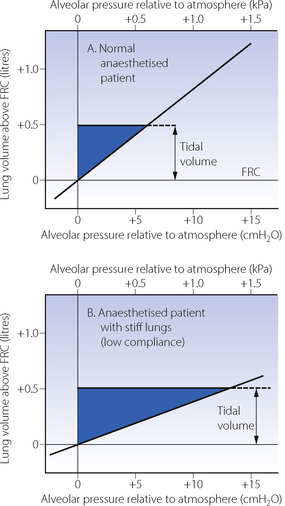
Fig. 6.9 Work of breathing against elastic resistance during passive inflation. The lines show pressure/volume plots of the lungs of anaesthetised patients (conscious subjects are shown in Figure 3.8). The length of the pressure/volume curve covered during inspiration forms the hypotenuse of a right-angled triangle whose area equals the work performed against elastic resistance. Note that the area is greater when the pressure/volume curve is flatter (indicating stiffer or less compliant lungs).
Work against resistance to gas flow. Frictional resistance was ignored in Figure 6.9. Additional pressure is required to overcome frictional resistance to gas flow that is reflected in the mouth pressure, which, during inspiration, is above the alveolar pressure by the driving pressure required to overcome frictional resistance. When mouth pressure is plotted as in Figure 6.10, the inspiratory curve is bowed to the right and the darker shaded area to the right of the pressure volume curve indicates the additional work performed in overcoming inspiratory frictional resistance. Figure 6.10B represents a patient with increased airway resistance. The expiratory curve, not shown in Figure 6.10, would be bowed to the left as the mouth-to-alveolar pressure gradient is reversed during expiration.
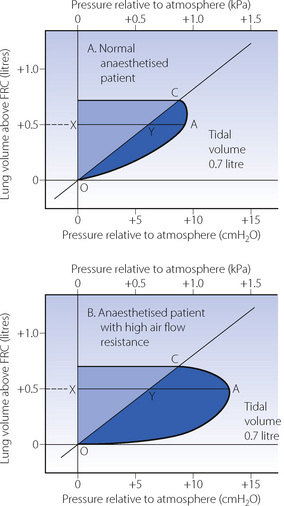
Fig. 6.10 Work of breathing against air flow resistance during passive inflation. The sloping line OYC is the alveolar pressure/volume curve. The curve OAC is the mouth pressure/volume curve during inflation of the lungs. The darker shaded area indicates the work of inspiration performed against air flow resistance. This work is increased in the patient with high resistance (B). At the point when 500 ml gas has entered the patient, XY represents the pressure distending the lungs, while YA represents the pressure overcoming air flow resistance. XA is the inflation pressure at that moment. The lighter shaded areas represent the work done against elastic resistance (see Figure 6.9).
The Minimal Work of Breathing
For a constant minute volume, the work performed against elastic resistance is increased when breathing is slow and deep. Conversely, the work performed against air flow resistance is increased when breathing is rapid and shallow. If the two components are summated and the total work is plotted against respiratory frequency, it will be found that there is an optimal frequency at which the total work of breathing is minimal (Figure 6.11). If there is increased elastic resistance (as in patients with pulmonary fibrosis), the optimal frequency is increased, while in the presence of increased air flow resistance the optimal frequency is decreased. Humans and animals tend to select a respiratory frequency close to that which minimises respiratory work. This applies to different species, different age groups and also to pathological conditions.
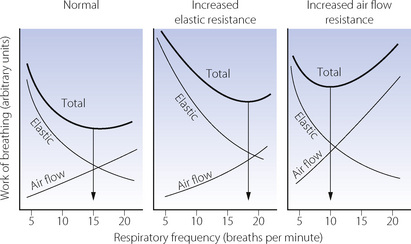
Fig. 6.11 Minimal work of breathing. The diagrams show the work done against elastic and air flow resistance separately and summated to indicate the total work of breathing at different respiratory frequencies. The total work of breathing has a minimum value at about 15 breaths per minute under normal circumstances. For the same minute volume, minimum work is performed at higher frequencies with stiff (less compliant) lungs and at lower frequencies when the air flow resistance is increased.
Measurement of Ventilation
Volume may be measured either directly or by the continuous integration of instantaneous gas flow rate (Figure 6.12).
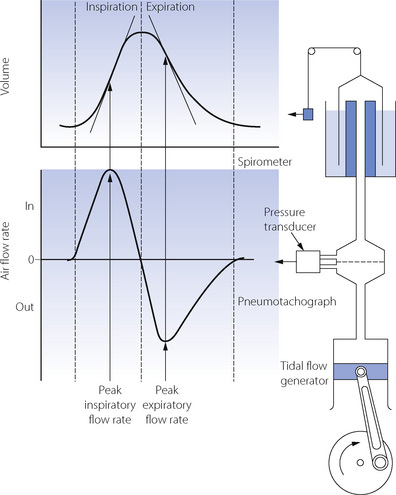
Fig. 6.12 Relationship between volume and flow rate. The upper graph shows volume plotted against time; this type of tracing is obtained from a spirometer. The lower graph shows instantaneous air flow rate plotted against time; this type of tracing is obtained from a pneumotachograph. At any instant, the flow-rate trace indicates the slope of the volume trace, while the volume trace indicates the cumulative area under the flow-rate trace. Flow is the differential of volume; volume is the integral of flow rate. Differentiation of the spirometer trace gives a ‘pneumotachogram’; integration of the pneumotachogram gives a ‘spirometer’ trace.
Direct Measurement of Respired Volumes38
Inspiratory and expiratory tidal volumes (and therefore minute volume) may be markedly different, and the difference is important in calculations of gas exchange. The normal respiratory exchange ratio of about 0.8 means that inspiratory minute volume is about 50 ml larger than expiratory minute volume in the resting subject. Much larger differences can arise during exercise and during uptake or wash-out of an inert gas such as nitrogen or, to a greater extent, nitrous oxide.
Water-sealed spirometers provide the reference met-hod for the measurement of ventilation (Figure 6.12), and may be precisely calibrated by water displacement. They provide negligible resistance to breathing and, by suitable design, may have a satisfactory frequency response up to very high respiratory frequencies.
Dry spirometers are hinged bellows, usually with electronic displays of both volume and instantaneous flow rate. Their accuracy approaches that of a water-filled spirometer and they are far more convenient in use.
Impellers and turbines. The best known of these instruments is the respirometer developed by Wright in 1955.39 The mechanism is entirely mechanical with indication of volume on a dial but the output may be converted to an electrical signal to indicate either tidal volume or minute volume. In general the respirometer is accurate and tends to read low at low minute volumes and high at high minute volumes; departure from normality is thus exaggerated and the instrument is essentially safe.
Respiratory inductance plethysmography. Reference has been made above (page 87) to this method of measurement of cross-sectional area of ribcage and abdominal compartments. The sum of these signals correlates well with lung volume and, following calibration against a spirometer, changes in the summated signals provide a very useful non-invasive method of measuring ventilation, uninfluenced by the presence of a mouthpiece or mask, and feasible during sleep (Figure 6.5).
Measurement of Ventilatory Volumes by Integration of Instantaneous Gas Flow Rate
Electronics have made measurement of ventilatory volumes by integration of instantaneous flow rate a widespread technique in clinical environments. There are many methods for measuring rapidly changing gas flow rates of which the original was pneumotachography. This employs measurement of the pressure gradient across a laminar resistance, which ensures that the pressure drop is directly proportional to flow rate. This is illustrated in Figure 6.12 where the resistance is a wire mesh screen. It is necessary to take precautions to prevent errors due to different gas composition and temperature, and to prevent condensation of moisture on the screen. The pressure drop need not exceed a few millimetres of water and the volume can be very small. The pneumotachograph should not therefore interfere with respiration.
Most ventilators and anaesthetic machines currently in use can measure respiratory volumes. A pneumotachograph or electronic turbine system is used, normally on the expired limb of the breathing system, and designed to be of very low resistance to allow measurements during spontaneous respiration. In this way, each expired tidal volume may be measured from which respiratory rate and minute volume can be derived, and a useful method of detecting apnoea or disconnection is therefore provided.
Measurement of Ventilatory Capacity38,40
Measurement of ventilatory capacity is the most commonly performed test of respiratory function. The ratio of ventilatory capacity to actual ventilation is a measure of ventilatory reserve and of the comfort of breathing.
Maximal Voluntary Ventilation (MVV)
Also referred to as maximal breathing capacity, MBC is defined as the maximum minute volume of ventilation that the subject can maintain for 12–15 s. In the normal subject MVV is about 15–20 times the resting minute volume. The subject simply breathes in and out of a spirometer without the need for removal of carbon dioxide; although simple, the test is exhausting to perform and is now seldom used. The average fit young male adult should have an MBC of about 170 l.min−1 but normal values depend upon body size, age and sex.
Forced Expiration
A more practical test of ventilatory capacity is the forced expiratory volume in 1 second (FEV1) which is the maximal volume exhaled in the first second starting from a maximal inspiration. A simple spirometer is all that is required. It is far more convenient to perform than the MVV and less exhausting for the patient. It correlates well with the MVV, which is normally about 35 times the FEV1. A variety of similar measurements relating to a single forced expiration are described, including forced expiratory volume in 0.5 s or 0.75 s, which may be useful in children, or forced expiratory volume in 6 s which is a surrogate for forced vital capacity.40
Peak Expiratory Flow Rate
Most convenient of all the indirect tests of ventilatory capacity is the peak expiratory flow rate. This can be measured with simple and inexpensive hand-held devices, and is an easy method for assessing large airway calibre. Interpretation of measurements of maximal expirations may be misleading. It should be remembered that these tests measure active expiration, which plays no part in normal breathing. They are most commonly performed as a measure of airway obstruction and are extensively used in patients with asthma and chronic obstructive airway disease. However, the results also depend on many other factors, including chest restriction, motivation and muscular power. The measurements may also be inhibited by pain. A more specific indication of airway resistance is the ratio of FEV1 to vital capacity, which should exceed 75% in the normal subject.
Assessment of the Respiratory Muscles41
Severe abnormalities of muscle function may be assessed by simple observation of spontaneous breathing. During inspiration, paradoxical movements of the trunk may occur such as inward displacement of the abdominal wall (diaphragm failure) or inward movement of the upper chest (intercostal failure). Fluoroscopy or ultrasound imaging of the diaphragm provides a more subtle form of observation, and is helpful in detecting phrenic nerve damage, particularly if unilateral when the body surface changes will be less obvious.
Vital capacity (VC, Figure 3.9) is accepted as the best ‘bedside’ monitor of respiratory muscle function, particularly when performed supine.41 Performance of a VC manoeuvre requires patient cooperation and coordination, and a single low reading is non-specific. However, repeated measurement allows the observation of a trend in VC to be followed, and a 25% reduction is unequivocally abnormal. In spite of the many causes of a reduced VC, this method of assessing respiratory muscle function is very useful for monitoring the development of progressive muscle weakness in conditions such as myasthenia gravis and Guillain–Barré syndrome (page 395).
Pressure measurements, when breathing against an imposed resistance, are used to assess both inspiratory and expiratory muscle strength. All require some patient compliance and involve a degree of respiratory discomfort so these tests, though more specific than VC for respiratory muscle function, are not widely used. Mouth pressure may be measured whilst a slow inspiration or expiration is performed against a moderate respiratory resistance, or mouth pressure may be measured during a rapid ‘sniff’ procedure in which the nasal airway acts as the resistance.
References
1. Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol. 2008;586:4283-4294.
2. Horner RL, Innes JA, Guz A. Reflex pharyngeal dilator muscle activation by stimuli of negative airway pressure in awake man. Sleep. 1993;16(suppl 8):S85-S86.
*3. Widdicombe J. Airway receptors. Respir Physiol. 2001;125:3-15.
4. Remmers JE. Wagging the tongue and guarding the airway. Reflex control of the genioglossus. Am J Respir Crit Care Med. 2001;164:2013-2015.
5. Wheatley JR, Kelley WT, Tully A, Engel LA. Pressure-diameter relationships in the upper airway in awake supine subjects. J Appl Physiol. 1991;70:2242-2251.
6. Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol. 2006;101:609-617.
7. Yildirim N, Fitzpatrick MF, Whyte KF, Jalleh R, Wightman AJA, Douglas NJ. The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1991;144:845-847.
8. Douglas NJ, Jan MA, Yildirim N, Warren PM, Drummond GB. Effect of posture and breathing route on genioglossal EMG activity in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1993;148:1341-1345.
9. Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol. 1991;70:2574-2581.
10. Brancatisano TP, Dodd DS, Engel LA. Respiratory activity of posterior cricoarytenoid muscle and vocal cords in humans. J Appl Physiol. 1984;57:1143-1149.
*11. Gauthier AP, Verbanck S, Estenne M, Segebarth C, Macklem PT, Paiva M. Three-dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J Appl Physiol. 1994;76:495-506.
12. Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641-659.
13. Loring SH. Invited editorial on “Three-dimensional reconstruc-tion of the in vivo human diaphragm shape at different lung volumes.”. J Appl Physiol. 1994;79:493-494.
14. Cassart M, Pettiaux N, Gevenois PA, Paiva M, Estenne M. Effect of chronic hyperinflation on diaphragm length and surface area. Am J Respir Crit Care Med. 1997;156:504-508.
15. Petroll WM, Knight H, Rochester DF. A model approach to assess diaphragmatic volume displacement. J Appl Physiol. 1990;69:2175-2182.
*16. De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717-756.
17. Hamberger GE. De Respirirationis Mechanismo et Usu Genuino. Jena: 1749.
18. Rimmer KP, Ford GT, Whitelaw WA. Interaction between postural and respiratory control of human intercostal muscles. J Appl Physiol. 1995;79:1556-1561.
19. Hudson AL, Gandevia SC, Butler JE. The effect of lung volume on the co-ordinated recruitment of scalene and sternomastoid muscles in humans. J Physiol. 2007;584:261-270.
20. Hillman DR, Finucane KE. A model of the respiratory pump. J Appl Physiol. 1987;63:951-961.
21. Drummond GB. Chest wall movements in anaesthesia. Eur J Anaesthiol. 1989;6:161-196.
22. Konno K, Mead J. Measurement of the separate volume changes of ribcage and abdomen during breathing. J Appl Physiol. 1967;22:407-422.
23. Lumb AB, Nunn JF. Respiratory function and ribcage contribution to ventilation in body positions commonly used during anaesthesia. Anesth Analg. 1991;73:422-426.
24. Xie S, Takasaki Y, Popkin J, Orr D, Bradley TD. Chemical and postural influence on scalene and diaphragmatic activation in humans. J Appl Physiol. 1991;70:658-664.
25. Lumb AB, Nunn JF. Ribcage contribution to CO2 response during rebreathing and steady state methods. Respir Physiol. 1991;85:97-110.
26. Robson JG. The respiratory centres and their responses. In: Evans FT, Gray TC, editors. Modern Trends in Anaesthesia – 3. London: Butterworths, 1967.
*27. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10-48.
28. Gayan-Ramirez G, Decramer M. Effects of mechanical ventilation on diaphragm function and biology. Eur Respir J. 2002;20:1579-1586.
29. Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799-1806.
30. Moxham J. Respiratory muscle fatigue: mechanisms, evaluation and therapy. Br J Anaesth. 1990;65:43-53.
31. Roussos C, Macklem PT. Diaphragmatic fatigue in man. J Appl Physiol. 1977;43:189-197.
32. Fujii Y, Toyooka H, Amaha K. Diaphragmatic fatigue and its recovery are influenced by cardiac output. J Anaesth. 1991;5:17-23.
33. Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive cardiac failure. Chest. 1990;98:1091-1094.
34. Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. N Engl J Med. 2009;358:1392-1394.
35. Callahan LA. Invited editorial on “Acquired respiratory muscle weakness in critically ill patients: what is the role of mechanical ventilation-induced diaphragm dysfunction?”. J Appl Physiol. 2009;106:360-361.
36. Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327-1335.
37. Campbell EJM, Westlake EK, Cherniak RM. Simple methods of estimating oxygen consumption and the efficiency of the muscles of breathing. J Appl Physiol. 1957;11:303-308.
38. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
39. Wright BM. A respiratory anemometer. J Physiol. 1955;127:25P.
40. Cotes JE, Chinn DJ, Miller MR. Lung function. Physiology, measurement and application in medicine. Oxford: Blackwell Publishing; 2006.
41. Polkey MI, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax. 1995;50:1131-1135.