Periodontal Diseases
In this textbook of oral and maxillofacial pathology, the discussion of periodontal diseases is limited appropriately in scope. However, several fine textbooks are available on periodontology and can provide the reader with more information on the background, microbiology, clinical presentations, diagnostic procedures, and current therapies used to treat these diseases.
GINGIVITIS
Gingivitis refers to inflammation limited to the soft tissues that surround the teeth. It does not include the inflammatory processes that may extend into the underlying alveolar ridge, periodontal ligament, or cementum. The primary types of gingivitis are listed in Box 4-1. This part of the text concentrates on the plaque-related types. Necrotizing ulcerative gingivitis (NUG), medication-influenced gingivitis, and a specific type of allergic gingivitis (plasma cell gingivitis) are presented later in this chapter. Additional forms of allergic gingivitis are discussed in Chapter 9. The gingivitis associated with specific infections (e.g., herpes simplex, human immunodeficiency virus [HIV]) is discussed in Chapters 5 and Chapter 7. The gingiva is a frequent site of involvement in several of the dermatologic vesiculoerosive diseases; these are well described in Chapter 16.
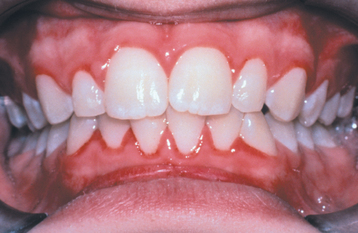
CLINICAL FEATURES: Most cases of gingivitis occur from lack of proper oral hygiene, which leads to the accumulation of dental plaque and calculus; however, many other factors can affect the gingiva’s susceptibility to the oral flora. The frequency of gingivitis is high in all age groups, but its true prevalence is difficult to determine because of the lack of a standardized method of measurement. Clinically detectable inflammatory changes of the gingiva begin in childhood and increase with age. With similar amounts of dental plaque, the severity of gingivitis is greater in adults than in prepubertal children. Around the time of puberty, there is a period of increased susceptibility to gingivitis (puberty gingivitis), with the peak prevalence of involvement occurring between the ages of 9 and 14 years (Fig. 4-1). Between the ages of 11 and 17 years, the frequency declines; then a slow increase is seen until the prevalence approaches 100% in the sixth decade of life.
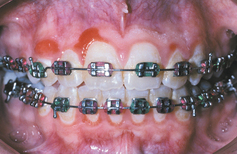
Fig. 4-1 Puberty gingivitis. Erythematous gingivitis that arose at time of initial menses and was slow to respond to local therapy.
In most age groups, females demonstrate a lower frequency of gingivitis than do males (although females have periods of increased susceptibility). This may be due more to better oral hygiene in females than to a physiologic difference between the sexes. In addition to the years of puberty, females exhibit a greater susceptibility to gingivitis when they are exposed to the high levels of progesterone associated with pregnancy or some forms of oral contraceptives. Progesterone appears to increase the permeability of gingival blood vessels, thereby rendering the area more sensitive to bacterial, physical, and chemical irritants.
A number of other systemic factors have been shown to increase the frequency of gingivitis and are listed in Box 4-2. In contrast, smoking and use of many antibiotic drugs, corticosteroid medications, and nonsteroidal antiinflammatory drugs (NSAIDs) have been correlated with a reduced gingival response to plaque. Various local factors that can be related to gingivitis are shown in Box 4-3.
Injury to the gingiva from mastication, oral hygiene techniques, or other habits may result in a breach of the oral mucosa, with secondary infection from the local flora. Most such injuries result in transient areas of erythema. However, if the trauma follows a chronic pattern, then areas of persistently swollen, erythematous gingiva may result. Patients who are mouth breathers or demonstrate incomplete lip closure can display a unique pattern of gingivitis in which the anterior facial gingiva is smooth, swollen, and red (Fig. 4-2).
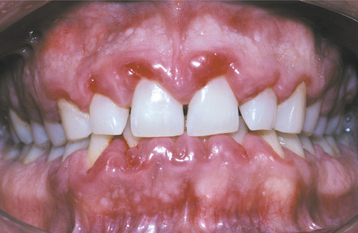
Fig. 4-2 Mouth breathing–related gingivitis. Slick, swollen, and red gingivitis of the anterior facial gingiva secondary to chronic mouth breathing.
In a large group of patients that was controlled for the local and systemic factors, a variety of severity in gingivitis was seen. Susceptibility to plaque-related gingivitis appears to vary within the population, and the individual traits seem to determine the severity of gingivitis, independent of the degree of plaque accumulation. Even after removal of the causative plaque and resolution of the associated gingivitis, individuals identified as being susceptible to gingivitis exhibit measurable differences in gingival crevicular fluid volume from patients who have demonstrated resistance to plaque-related gingivitis. In addition, evidence suggests that susceptibility to gingivitis appears linked to susceptibility to future development of periodontitis. In the future, such research may allow identification of patients who are susceptible to gingivitis and ultimately periodontitis, and appropriate interventions could be instituted.
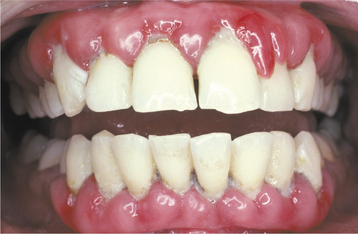
Inflammation of the gingiva may be localized or generalized. The involved area may be diffuse or confined to the free gingival margins (marginal gingivitis) (Fig. 4-3) or the interdental papillae (papillary gingivitis). The earliest signs of gingivitis include a loss of stippling, plus bleeding on gentle probing. Healthy gingiva is coral pink; with inflammation, the involved gingiva becomes light red. With progression, the area becomes redder and edematous. As the process becomes entrenched, the involved gingiva becomes brighter red or magenta; the gingiva often demonstrates margins that may be blunted, receded, or hyperplastic (Fig. 4-4). When chronic inflammation causes significant enlargement because of edema or fibrosis, the process is termed chronic hyperplastic gingivitis (Fig. 4-5). Bleeding occurs easily, and exudate can be seen in the gingival sulcus. A localized tumorlike proliferation of subacutely inflamed granulation tissue, known as a pyogenic granuloma (see page 517), can develop on the gingiva of patients with severe gingivitis (Fig. 4-6).
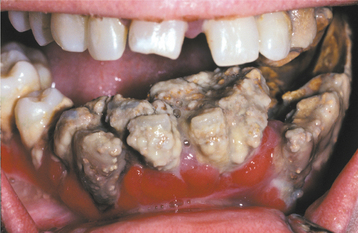
Fig. 4-4 Chronic gingivitis. Bright-red gingiva is blunted, receded, and hyperplastic secondary to a total lack of oral hygiene. Note the extensive calculus buildup.
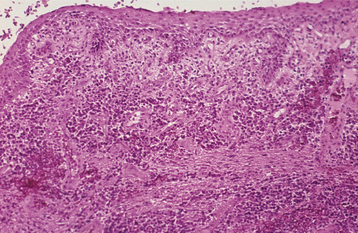
HISTOPATHOLOGIC FEATURES: Incipient gingivitis demonstrates a light inflammatory infiltrate consisting of polymorphonuclear leukocytes that accumulate in the connective tissue adjacent to the sulcular epithelium. With progression, the infiltrate becomes more intense and demonstrates a mixture of lymphocytes, plasma cells, and acute inflammatory cells (Fig. 4-7). Areas of fibrosis, hyperemia, edema, and hemorrhage may be present.
TREATMENT AND PROGNOSIS: Although periodontitis always is preceded by gingivitis, most areas of gingivitis remain stable for years, and the number of affected sites that convert to periodontitis is small. In spite of this, optimal gingival health should be the goal of all clinicians and their patients. In a 26-year study of a cohort receiving state-of-the-art dental care, the prevalence of localized tooth loss increased 46 times in areas associated with gingiva that consistently bled on probing during routine examinations. Even when attachment loss is not evident and the alterations appear restricted to the gingival soft tissues, proactive interventions are recommended to eliminate these areas of persistent pathosis during the early stages of disease.
Treatment of gingivitis consists of elimination (if possible) of any known cause of increased susceptibility and improvement in oral hygiene to decrease the dental plaque responsible for the inflammatory alterations. Most self-administered plaque control programs are ineffective unless periodic professional reinforcement also is provided. A further discussion of dental plaque and its relationship to gingival inflammation is presented in the discussion of periodontitis (see page 168). Research has shown that few individuals have the physical skills and motivation necessary to obtain and maintain ultimate oral hygiene. Mechanical removal of dental plaque can be aided by the use of numerous chemical agents, such as mouth rinses with chlorhexidine or essential oils, or dentifrices containing triclosan with 2.0% Gantrez copolymer. In this vein, studies have evaluated the addition of these chemopreventative agents to typical oral hygiene efforts and shown a statistically significant positive response to these products in controlling plaque and gingivitis. On occasion, hyperplastic and fibrotic gingiva may have to be recontoured surgically to allow total resolution of the pathosis after improvements in hygiene have been made. If the gingivitis does not resolve after improved plaque control and elimination of obvious contributing factors, then the patient should be evaluated for underlying systemic disorders that could be contributing to the process.
NECROTIZING ULCERATIVE GINGIVITIS (VINCENT’S INFECTION; TRENCH MOUTH)
Necrotizing ulcerative gingivitis (NUG) has a distinctive pattern of gingival pathologic changes that have been recognized for hundreds of years. Until recently, the name of this process has been preceded by the term acute (i.e., ANUG); however, several investigators have discontinued the use of this word because there is no chronic form of the disease. In the 1890s the French physician Jean Hyacinthe Vincent identified a fusiform bacterium, Bacillus fusiformis (currently Fusobacterium nucleatum), and a spirochete, Borrelia vincentii, after microscopic examination of plaque samples from affected sites. Vincent believed that the fusiform bacteria were principally responsible for the condition, and the spirochetes mainly were saprophytic opportunists. The spirochete and fusiform bacterium association remains true today, but more sophisticated techniques have implicated Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis, Treponema spp., and Selenomonas spp. Although the association with bacteria is strong, controversial research has suggested that viruses such as cytomegalovirus, Epstein-Barr virus, and herpes simplex may contribute to the onset and progression of the process.
The infection frequently occurs in the presence of psychologic stress. People in military service exhibit an increased frequency of NUG; the disorder was so common in the battlefield trenches during World War I that the nickname trench mouth became well known. Stress-related corticosteroid hormones are thought to alter T4/T8 lymphocyte ratios and may cause the decreased neutrophilic chemotaxis and phagocytic response seen in patients with NUG. Stress-related epinephrine may result in localized ischemia, which predisposes the gingiva to NUG.
In addition to stress, other factors have been related to an increased frequency of NUG:
Immunocompromised status, especially that seen in association with acquired immunodeficiency syndrome (AIDS) (see page 264) and infectious mononucleosis (see page 253), has been related to the development of NUG. The list of predisposing factors clearly supports the association between a depressed systemic immunity and the appearance of the disorder.
CLINICAL FEATURES: NUG may occur at any age; however, when encountered in the United States or Europe, it is seen most frequently in young and middle-aged adults. Several publications have reported a higher frequency in whites. The prevalence in the normal population is less than 0.1%; however, in stressed populations (e.g., military recruits) the frequency increases up to 7%. In developing countries, NUG typically occurs in very young children suffering from malnutrition. Seasonal variations in prevalence have been reported by a number of investigators but have been inconsistent in different areas of the world.
In a classic case of NUG, the interdental papillae are highly inflamed, edematous, and hemorrhagic. Typically, the affected papillae are blunted and demonstrate areas of “punched-out,” craterlike necrosis that are covered with a gray pseudomembrane (Fig. 4-8). Early cases may be missed easily because the ulceration initially involves only the tip of the interdental papilla. A fetid odor, exquisite pain, spontaneous hemorrhage, and accumulations of necrotic debris usually are noted. Although a bad odor is not always noted, its absence in a patient without predisposing factors should raise concern for other pathoses such as gonorrhea (see page 193). Occasional ancillary clinical features include lymphadenopathy, fever, and malaise. The process sometimes can lead to a loss of attachment and the development of associated periodontitis (necrotizing ulcerative periodontitis) or spread to adjacent soft tissue (necrotizing ulcerative mucositis, necrotizing stomatitis) (Fig. 4-9). If the necrotizing infection extends through the mucosa to the skin of the face, then it is typically termed noma (cancrum oris) (see page 201).
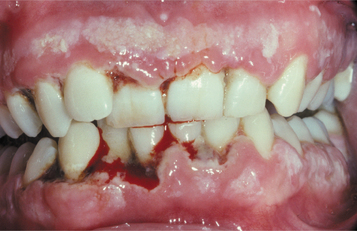
Fig. 4-8 Necrotizing ulcerative gingivitis (NUG). Gingiva is friable and hemorrhagic with necrosis of the interdental papillae.
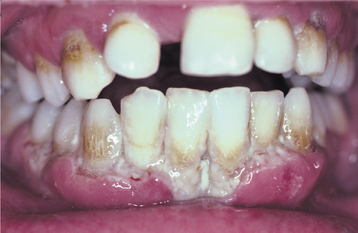
Fig. 4-9 Necrotizing ulcerative mucositis. Gingiva exhibits epithelial necrosis that has extended between the adjacent interdental papillae and apically to the alveolar mucosa junction.
Several investigators have suggested that NUG, necrotizing ulcerative periodontitis, and necrotizing stomatitis are one disease process termed necrotizing gingivostomatitis. Evidence presented by numerous authors has shown the diseases to be similar clinically, histopathologically, and bacteriologically, with the only differences being underlying systemic factors and anatomic extension of the necrosis.
HISTOPATHOLOGIC FEATURES: The histopathologic features of NUG are not specific. Typically, affected gingival papillae demonstrate surface ulceration that is covered by a thickened fibrinopurulent membrane. The underlying lamina propria demonstrates an intense acute or mixed inflammatory infiltrate and extensive hyperemia. In nonulcerated affected epithelium, often a loss of the typical surface keratinization occurs. Necrotic material and extensive bacterial colonization often are included in the material submitted for microscopic examination.
TREATMENT AND PROGNOSIS: In contrast to most forms of periodontal disease, NUG typically demonstrates quick resolution after removal of the bacterial challenge. Even with conservative therapy, regeneration of the affected gingiva is normally seen. The affected area is treated best with débridement by scaling, curettage, or ultrasonic instrumentation (except when contraindicated, as in HIV-positive patients). Topical or local anesthetic often is required before the clinician can débride the tissues adequately. Frequent rinses with chlorhexidine, warm saltwater, or diluted hydrogen peroxide are beneficial in increasing the therapeutic response. Antibiotic medications (metronidazole and penicillin have been suggested as the drugs of choice) are a useful adjunct, especially in the presence of fever or lymphadenopathy.
Treatment should include instructions on oral hygiene and patient motivation; identification and resolution of any predisposing factors also are advantageous. Supportive therapy (e.g., rest, appropriate fluid intake, soft nutritious diet) often improves the clinical response. Follow-up appointments are necessary to reinforce the home care instructions and to rule out a recurrence of the process. In cases resistant to treatment, further evaluation to rule out HIV infection or infectious mononucleosis is prudent.
The clinician must be ever vigilant in the search for other signs and symptoms of immunosuppression. Subtle palatal candidiasis or HIV-related oral hairy leukoplakia (see page 268) can be overlooked easily in a patient with NUG. Appropriate attention must be directed toward the oral soft tissue examination, especially in patients with infections such as NUG that are related to immunosuppression. In addition, a thorough investigation of underlying causes of immunosuppression should be performed on patients whose conditions are resistant to normal therapy.
PLASMA CELL GINGIVITIS (ATYPICAL GINGIVOSTOMATITIS)
A distinctive pattern of gingival inflammation, plasma cell gingivitis, was brought to the attention of health care practitioners during the late 1960s and early 1970s. A rash of cases occurred during that time, and most appear to have been related to a hypersensitivity to a component of chewing gum. Since that time, the number of cases has dwindled, but similar gingival alterations are reported occasionally.
Although the association with chewing gum has decreased, allergy still is responsible for many reported cases. A brand of herbal toothpaste, a specific type of mint candy, and peppers used for cooking have all been implicated in more recent reports. The list of allergens appears to be variable, and a thorough evaluation often is required to rule out an allergic cause.
CLINICAL FEATURES: Patients with plasma cell gingivitis experience a rapid onset of sore mouth, which often is intensified by dentifrices and hot or spicy foods. The entire free and attached gingiva demonstrates a diffuse enlargement with bright erythema and loss of normal stippling (Fig. 4-10). Extension onto the palate can occur, and edentulous areas typically exhibit less intense changes. On occasion, a similar localized gingival and vestibular alteration can occur from topical placement of a material that elicits a similar plasmacytic inflammatory reaction.
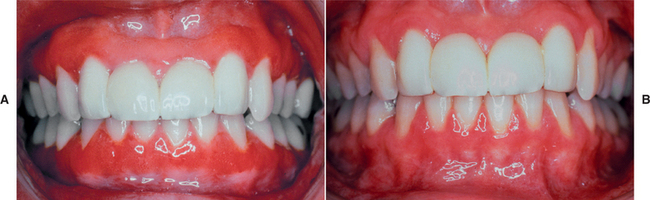
Fig. 4-10 Plasma cell gingivitis. A, Diffuse, bright-red enlargement of the free and attached gingiva. B, Same patient as depicted in A after elimination of the inciting allergen.
Additional sites of involvement may be seen, or the changes may be localized to the gingiva. In the chewing gum–related cases of the early 1970s, involvement of the lips and tongue was typical. The lips were dry, atrophic, occasionally fissured, and angular cheilitis was frequent. Tongue involvement resulted in erythematous enlargement with furrows, mild crenation, and loss of the typical dorsal coating.
More recent reports have described lesions often isolated to the gingiva without the classic lip and tongue involvement seen in the past. A larger percentage of these cases are idiopathic, and occasional extraoral involvement of sites such as the supraglottic region occurs.
HISTOPATHOLOGIC FEATURES: The cases of classic plasma cell gingivitis of the 1970s demonstrated psoriasiform hyperplasia and spongiosis of the surface epithelium, with intense exocytosis and neutrophilic microabscesses. The underlying lamina propria contains numerous dilated vascular channels and an extremely dense chronic inflammatory infiltrate that is composed predominantly of plasma cells (Fig. 4-11). The more recent cases are similar but often demonstrate less involvement of the surface epithelium and a less dense underlying plasmacytic infiltrate.
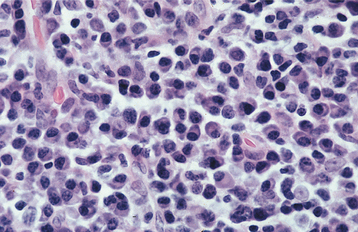
Fig. 4-11 Plasma cell gingivitis. High-power photomicrograph exhibiting a dense inflammatory infiltrate consisting predominantly of plasma cells with scattered lymphocytes.
Investigation of the clonality of the plasma cell infiltrate may be necessary to rule out the possibility of a monoclonal plasma cell neoplasm. All allergic and idiopathic cases of plasma cell gingivitis demonstrate a polyclonal mixture of plasma cells and a normal profile on plasma immunoelectrophoresis.
TREATMENT AND PROGNOSIS: All patients with plasma cell gingivitis should be instructed to keep a complete dietary history, with records of everything taken into the mouth (e.g., foods, dentifrice, mouthwash, tobacco, alcohol, chewing gum, candy, medications). Possible allergens should be eliminated in an attempt to discover the underlying cause. If an easy answer is not apparent, then extensive allergy testing and an elimination diet can be undertaken.
Many patients in whom no underlying cause could be discovered have been treated with topical or systemic immunosuppressive medications, with variable results. Betamethasone rinses, fluocinonide gel, topical triamcinolone, and topical fusidic acid are several of the reported choices. In spite of all the evaluations and therapeutic interventions, some patients do not respond to treatment and no cause for the disease can be identified.
GRANULOMATOUS GINGIVITIS
The discovery of unexplained granulomatous inflammation in a gingival biopsy specimen is termed granulomatous gingivitis and represents a diagnostic challenge for the pathologist, referring clinician, and patient. The pathologist must rule out histologically distinctive granulomatous diseases and specific granulomatous infectious processes (e.g., foreign material, deep fungal infections, acid-fast bacteria) (see Chapters 5 and Chapter 6). The clinician must search for signs and symptoms of local and systemic granulomatous diseases (e.g., Crohn’s disease, sarcoidosis, chronic granulomatous disease, Wegener’s granulomatosis) (see Chapters 9 and Chapter 17), and the patient must endure and pay for these evaluations. Even after a costly workup, some patients who have localized areas of granulomatous inflammation of the gingiva have no signs or symptoms of any of the previously mentioned disorders.
Several investigators have reported granulomatous gingival lesions caused by the introduction of dental materials into the connective tissue deep to the sulcular epithelium. As more cases have been reported, it has become evident that the associated inflammatory reaction often is not granulomatous and may mimic gingival lichen planus or create a nonspecific pattern of chronic or subacute mucositis. These lesions have been termed foreign body gingivitis and are thought to arise when damage to the sulcular epithelium during restorative or oral hygiene procedures allows the introduction of foreign material into the gingival tissues. When the material is obvious on light microscopy, the association between the gingival pathosis and the material can be made easily. Often the material is smaller than 1 μm in diameter and is so fine that it could be overlooked.
In a review of 85 cases of foreign body gingivitis, energy-dispersive radiographic microanalysis revealed 21 different elements embedded with the gingival soft tissues. The most common elements were silver, aluminum, silicon, tin, sulfur, copper, calcium, phosphorus, and iron. Elements compatible with fine particles of amalgam dust were identified most often. Particles consistent with corundum or silica also were common (sandpaper disks, polishing paste, toothpaste, and possibly restorative filling material). Materials implicated less frequently include dust from tungsten carbide burs, composite material, endodontic sealer components, and temporary cement. This investigation demonstrates that the presumed causative agents are diverse and can originate from a wide variety of dental materials.
CLINICAL FEATURES: Both foreign body gingivitis and nonspecific granulomatous gingivitis may occur at any age; however, they are most frequently encountered in adulthood. The lesions may be solitary or multifocal, typically with a diameter less than 2 cm. The affected areas appear as red or red-and-white macules, which most frequently involve the interdental papillae but also may occur along the marginal gingiva (Figs. 4-12 and 4-13). Pain or sensitivity is a common finding, and the lesions persist despite conventional therapy and rigorous oral hygiene. The process can be seen adjacent to clinically normal teeth or next to teeth with restorations.
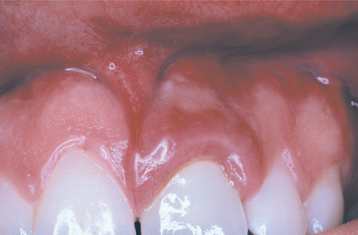
Fig. 4-12 Granulomatous gingivitis. Localized enlarged and erythematous gingiva associated with the maxillary left central incisor. The alterations developed shortly after placement of a porcelain-fused-to-metal (PFM) full crown and were not responsive to conservative local therapy. (Courtesy of Dr. Timothy L. Gutierrez.)
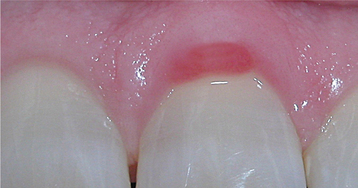
Fig. 4-13 Foreign body gingivitis. Isolated area of marginal gingivitis. (Courtesy of Dr. Ronald Godat.)
Frequently, foreign body gingivitis creates areas of erythematous and atrophic mucositis that closely resemble gingival lichen planus. In many cases the causative foreign material is not obvious during review of submitted biopsy specimens. A good clinicopathologic correlation often is beneficial in arriving at the correct diagnosis. A diagnosis of gingival lichen planus should be viewed with suspicion in a patient who does not have extragingival involvement or if the gingival changes are somewhat localized and nonmigrating. In such cases a request to the pathologist for a thorough search for foreign material is prudent.
HISTOPATHOLOGIC FEATURES: A biopsy specimen of granulomatous gingivitis demonstrates focal collections of histiocytes intermixed with an intense lymphocytic infiltrate (Fig. 4-14). On occasion, well-formed histiocytic granulomas with multinucleated giant cells are seen. Special stains for organisms should be negative. If foreign material is detected, then the clinician can render a diagnosis of a foreign body reaction (rather than the more nonspecific term, granulomatous gingivitis). In some cases, however, the foreign material may be too fine to be detected.
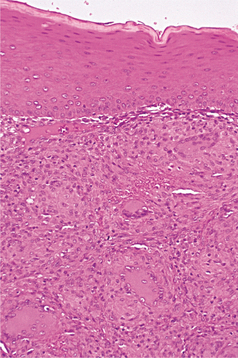
Fig. 4-14 Granulomatous gingivitis. Focal collection of histiocytes, lymphocytes, and multinucleated giant cells within the superficial lamina propria of the gingiva.
In the previously mentioned review of 85 cases of foreign body gingivitis, granulomatous inflammation was present in approximately 20%. In the remainder, the inflammatory infiltrate was dominated by lymphocytes, intermixed with plasma cells and macrophages (Fig. 4-15). In some cases, neutrophils were noted along with the chronic inflammatory cellular infiltrate. In a small number of cases, prominent fibrosis was associated with the foreign material. Not infrequently, the mucositis was lichenoid, with degeneration of the basal cell layer of the epithelium and a superficial bandlike inflammatory cell infiltrate in the superficial lamina propria. Because the immune reaction in lichen planus tends to be composed primarily of lymphocytes, the presence of significant numbers of plasma cells, histiocytes, or neutrophils in the absence of plaque-related gingivitis should suggest a thorough search for subtle foreign material.
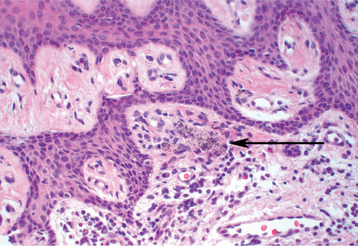
Fig. 4-15 Foreign body gingivitis. Particles of pigmented foreign material (arrow) intermixed with lymphocytes and plasma cells. This biopsy was obtained from the patient depicted in Fig. 4-13.
In the majority of cases, the foreign material appears as black or brown-black granules. In many cases, colorless, translucent crystal structures are noted and may be intermixed with black granules. These crystals often are difficult to detect unless viewed under polarized light. To ensure that any foreign material is not an artifact introduced during processing, it should be present in multiple sections.
TREATMENT AND PROGNOSIS: When all the histopathologic and clinical investigations have been performed, the final differential diagnosis of granulomatous gingivitis is usually narrowed down to a localized form of orofacial granulomatosis (see page 341) or a foreign body reaction. Without definitive demonstration of foreign material, a complete physical evaluation for diseases known to be associated with orofacial granulomatosis is mandatory. On occasion, patients with orofacial granulomatosis initially have gingival lesions but eventually develop more widespread manifestations such as cheilitis granulomatosa, cobblestone buccal mucosa, or vestibular linear hyperplastic folds.
Surgical excision of the affected tissue is the therapy of choice for those cases related to foreign material. In persistently atrophic or erosive areas of foreign body gingivitis, overlaying the damaged area with a graft from a healthy gingival donor site may be a better option than complete excision. In an attempt to prevent future introduction of iatrogenic foreign material, it appears appropriate to follow certain guidelines:
• The clinician should use extreme care when trimming restorations or using abrasive instruments close to gingival margins.
• Air abrasion (i.e., sandblasting) should be used cautiously.
• Dental prophylaxis should be delayed for 2 days after scaling, root planing, and curettage procedures.
Patients who do not respond to surgical removal and have recurrences of granulomatous gingivitis despite cautious dental care probably should be classified as having orofacial granulomatosis and managed accordingly.
DESQUAMATIVE GINGIVITIS
Most clinicians use the term desquamative gingivitis to describe gingival epithelium that spontaneously sloughs or can be removed with minor manipulation. The process most likely represents a manifestation of one of several different vesiculoerosive diseases. Histopathologic and immunologic investigations of this condition reveal that most patients exhibit features that are diagnostic of pemphigoid or lichen planus. Other diagnoses that are made less frequently include linear IgA disease, pemphigus vulgaris, epidermolysis bullosa acquisita, systemic lupus erythematosus (SLE), chronic ulcerative stomatitis, and paraneoplastic pemphigus. The gingival manifestations of these mucosal and dermatologic diseases are described in greater detail in Chapter 16, so further discussion here is not warranted.
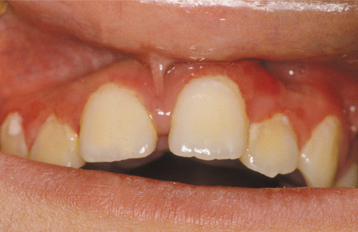
DRUG-RELATED GINGIVAL HYPERPLASIA (DRUG-RELATED GINGIVAL OVERGROWTH)
Drug-related gingival hyperplasia refers to an abnormal growth of the gingival tissues secondary to use of a systemic medication. The term is a misnomer because neither the epithelium nor the cells within the connective tissue exhibit either hyperplasia or hypertrophy. The increased gingival size is due to an increased amount of extracellular matrix, predominantly collagen. Therefore, several authors designate the alteration as medication-associated gingival enlargement or gingival overgrowth. These designations are further supported by investigators who have suggested the gingival changes arise from interference with normal intracellular collagen degradation. It is known that gingival collagen constantly undergoes physiologic remodeling, and the process must be tightly controlled to maintain a constant volume of the gingival tissues. Investigators have suggested that cyclosporine, phenytoin, and nifedipine are all associated with calcium deregulation, which disrupts the normal collagen phagocytosis and remodeling process. If this is true, then the increased collagen does not occur from hyperplasia but from impaired collagen degradation and remodeling.
A list of medications reported to be associated with gingival hyperplasia is provided in Box 4-4. Of these medications, a strong association has been noted only with cyclosporine (Fig. 4-16), phenytoin, and nifedipine (Fig. 4-17). In the remainder, the prevalence is much lower or the association is weak or anecdotal. As new drugs have been developed, the list of offending medications has grown. When cyclosporine and nifedipine are used concurrently, the severity of the associated hyperplasia often is increased (Fig. 4-18).
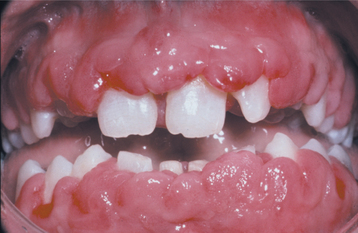
Fig. 4-16 Cyclosporine-related gingival hyperplasia. Diffuse, erythematous, and fibrotic gingival hyperplasia.
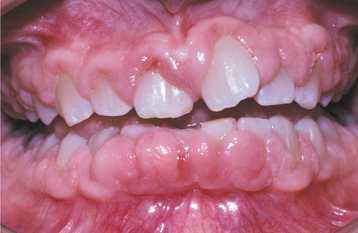
Fig. 4-17 Nifedipine-related gingival hyperplasia. Diffuse, fibrotic gingival hyperplasia after 1 month of intensive oral hygiene. Significant erythema, edema, and increased enlargement were present before intervention.
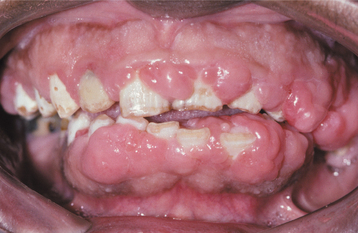
Fig. 4-18 Cyclosporine- and nifedipine-related gingival hyperplasia. Dramatic gingival hyperplasia in a patient using two drugs associated with gingival enlargement.
The prevalence of these hyperplasias varies widely; however, as reported in one critical review of the literature, the prevalence related to use of phenytoin is approximately 50%. Cyclosporine and nifedipine each produce significant changes in about 25% of patients treated. Whether there is a relationship between the particular dose and the risk or severity of the hyperplasia is a controversial issue. Investigators have suggested that susceptibility to cyclosporine gingival hyperplasia is associated with certain histocompatibility antigen (HLA) types, whereas other HLA types appear to protect against hyperplasia. Whether similar correlations exist for the other forms of medication-associated gingival hyperplasia is unknown.
The degree of gingival enlargement appears to be related significantly to the patient’s susceptibility and the level of oral hygiene. In observations of patients with excellent oral hygiene, gingival overgrowth (as ascertained by pseudopocket formation) is reduced dramatically or not present. Even with good oral hygiene, however, some degree of gingival enlargement can be discovered in susceptible individuals, although in many cases the changes are difficult to detect. Rigorous oral hygiene often can limit the severity to clinically insignificant levels. Of the medications discussed, cyclosporine appears to be the least responsive to the institution of a rigorous program of oral hygiene; even with this medication, however, the elimination of gingival inflammation results in noticeable clinical improvement. In addition, the degree of drug-associated gingival hyperplasia appears to be markedly higher in smokers.
CLINICAL FEATURES: Because young patients use phenytoin most often, the gingival hyperplasia it induces is primarily a problem in people younger than age 25. Cases related to the calcium channel blockers occur mainly in middle-aged or older adults. Cyclosporine is used over a broad age range, and this correlates with the age of reported hyperplasia. A greater risk for gingival hyperplasia occurs when the drug is used in children, especially adolescents. No sex or race predilection is present.
After 1 to 3 months of drug use, the enlargements originate in the interdental papillae and spread across the tooth surfaces (Fig. 4-19). The anterior and facial segments are the most frequently involved areas. In extensive cases, the hyperplastic gingiva can cover a portion (or all) of the crowns of many of the involved teeth (Figs. 4-20 and 4-21). Extension lingually and occlusally can interfere with speech and mastication. In one report, significant lingual expansion of the gingiva resulted in tongue displacement and respiratory distress. Edentulous areas are generally not affected, but significant hyperplasia under poorly maintained dentures and around implants has been noted (Fig. 4-22).
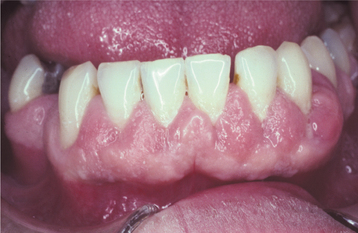
Fig. 4-19 Mild phenytoin-related gingival hyperplasia. Gingival enlargement present predominantly in the interdental papillae.
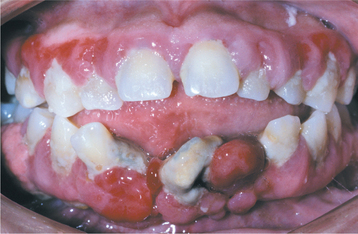
Fig. 4-20 Phenytoin-related gingival hyperplasia. Significant erythematous gingival hyperplasia is covering portions of the crowns of numerous teeth.
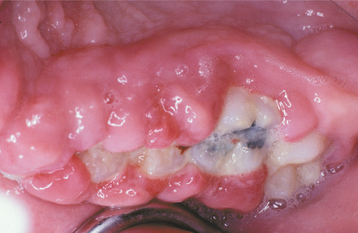
Fig. 4-21 Phenytoin-related gingival hyperplasia. Significant gingival hyperplasia almost totally covers the crowns of the posterior maxillary dentition. (Courtesy of Dr. Ann Drummond and Dr. Timothy Johnson.)
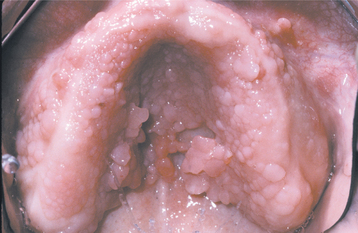
Fig. 4-22 Phenytoin-related palatal hyperplasia. Extensive hyperplasia of palatal mucosa in an edentulous patient with poor denture hygiene.
Nongingival soft tissue growths that resemble pyogenic granulomas have been reported in allogenic bone marrow transplant recipients who are receiving cyclosporine for graft-versus-host disease (GVHD) (Fig. 4-23). It is thought that cyclosporine triggers the proliferations in areas chronically inflamed by GVHD.
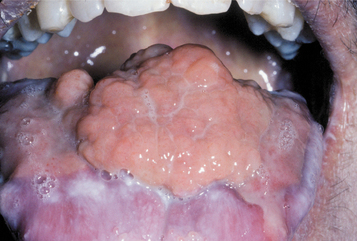
Fig. 4-23 Nongingival cyclosporine hyperplasia. Exophytic and granulomatous-appearing mass of the dorsal surface of the tongue that arose in a bone marrow transplant patient who was receiving cyclosporine for graft-versus-host disease (GVHD).
In the absence of inflammation, the enlarged gingiva is normal in color and firm, with a surface that may be smooth, stippled, or granular. With inflammation, the affected gingiva often becomes dark red and edematous, with a surface that is friable, bleeds easily, and occasionally is ulcerated. Pyogenic granuloma-like enlargements occasionally are seen in the presence of heavy inflammation.
HISTOPATHOLOGIC FEATURES: The exact histopathologic changes that occur in people with drug-induced gingival hyperplasia are difficult to ascertain because of variations in the techniques of investigation. In spite of this, most controlled microscopic examinations of hyperplastic gingival tissues removed from lesions caused by phenytoin or the dihydropyridines reveal redundant tissue of apparently normal composition. Those cases related to cyclosporine use demonstrate an increased amount of collagen per unit volume, with a normal density of fibroblasts.
The overlying surface epithelium may demonstrate elongation of the rete ridges, with long extensions into the underlying lamina propria. In patients with secondary inflammation, there is increased vascularity and a chronic inflammatory cellular infiltrate that most frequently consists of lymphocytes and plasma cells. In patients with pyogenic granuloma-like overgrowths, the proliferations often demonstrate an increased vascularity and significant subacute inflammation.
TREATMENT AND PROGNOSIS: Discontinuation of the offending medication by the attending physician often results in cessation, and possibly some regression, of the gingival enlargement; even substitution of one medication for another may be beneficial. If the patient’s response allows drug substitution, then cyclosporine can be replaced with tacrolimus; phenytoin with carbamazepine, lamotrigine, gabapentin, sulthiame, topiramate, or valproic acid; and nifedipine with isradipine or atenolol. Often the response to medication substitution is not immediate. Unless the degree of hyperplasia dramatically affects aesthetics and function, allowing at least 6 to 12 months between discontinuation of the offending medication and the decision whether to proceed with surgical therapy is recommended. If the drug use is mandatory, then professional cleaning, frequent reevaluations, and home plaque control are important. Antiplaque agents, such as chlorhexidine, have been beneficial in the prevention of plaque buildup and the associated gingival hyperplasia.
Systemic or topical folic acid has been shown to ameliorate the gingival hyperplasia in some cases. In addition, several authors have documented significant resolution of cyclosporine-related gingival hyperplasia after a short course of metronidazole or azithromycin. Although the mechanism is not clear, it appears these antibiotic medications can inhibit proliferation of collagen fibers along with their antimicrobial abilities. Azithromycin also may be beneficial in resolving gingival hyperplasia related to nifedipine and phenytoin.
Although gingival hyperplasia is associated with increased probing depths, some investigators do not believe this necessarily leads to exaggerated attachment loss or an increased loss of teeth. Therefore, some clinicians exercise watchful waiting and do not perform invasive therapy without evidence of attachment loss, inappropriate aesthetics, or disruption of speech or mastication. When objectionable alterations are noted and all other interventions fail to achieve significant resolution, eradication of the excess gingival tissues remains the treatment of choice. This can be achieved by surgical removal, by chemosurgical techniques, by means of electrosurgery, or by use of a carbon dioxide laser. Histopathologic examination of all excised tissue is mandatory to confirm the diagnosis. Recurrence is not uncommon, especially in patients with inadequate oral hygiene. Although recurrences may arise in as little as 3 months, most surgical results are maintained for at least 12 months.
GINGIVAL FIBROMATOSIS (FIBROMATOSIS GINGIVAE; ELEPHANTIASIS GINGIVAE)
Gingival fibromatosis is a slowly progressive gingival enlargement caused by a collagenous overgrowth of the gingival fibrous connective tissue. In spite of the name, this disorder bears no relationship to the hypercellular and neoplastic fibromatoses that can occur in soft tissue and bone (see pages 515 and page 658).
Gingival fibromatosis may be familial or idiopathic. Other findings sometimes seen in conjunction with gingival fibromatosis include hypertrichosis (Fig. 4-24), generalized aggressive periodontitis, epilepsy, mental retardation, sensorineural deafness, hypothyroidism, chondrodystrophia, and growth hormone deficiency. The familial variations may occur as an isolated finding or in association with one of several hereditary syndromes (e.g., Zimmermann-Laband, Murray-Puretic-Drescher, Rutherfurd, multiple hamartoma [see page 760], Cross, Ramon, Jones, prune belly).
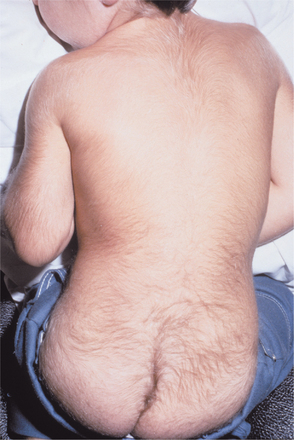
Fig. 4-24 Hypertrichosis in association with gingival fibromatosis. Dramatically increased body hair of the back and buttocks in a patient with gingival fibromatosis. (Courtesy of Dr. George Blozis.)
In most cases of isolated gingival fibromatosis, an autosomal dominant pattern of inheritance is seen; however, autosomal recessive examples also have been noted. Incomplete penetrance and variable expressivity are seen. Even in cases with similar patterns of inheritance, genetic heterogeneity of gingival fibromatosis has been noted and confirms that this alteration represents a group of clinically similar disorders. In the autosomal dominant pattern of isolated gingival fibromatosis, one of three different mutations, GINGF (HGF1), GINGF2 (HGF2), and GINGF3 (HGF3), have been documented and correlates respectively to chromosomes 2p21, 5q13-q22, and 2p22.3-p23.3. Of the three defined loci, only the SOS1 (son of sevenless-1) gene associated with the GINGF locus has been identified. In studies of another kindred with hereditary gingival fibromatosis and hypertrichosis, this presentation was not linked to either mutation known at that time (GINGF or GINGF1), further confirming the genetic heterogeneity in this hereditary gingival fibromatosis.
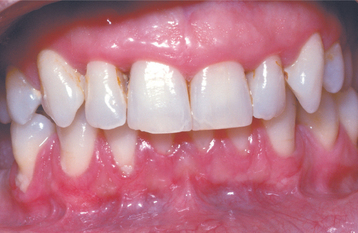
CLINICAL FEATURES: In most instances, the enlargement begins before age 20 and often is correlated with the eruption of the deciduous or permanent teeth (Fig. 4-25). Most investigators believe that the presence of teeth probably is necessary for the condition to occur. After the process has begun, it can overgrow the associated teeth and even interfere with lip closure. Failure or delay in eruption of subsequent teeth may be evident (Fig. 4-26). In some instances, a tooth may have erupted into a normal position, but the fibrous connective tissue continues to cover the crown and prevent visualization.
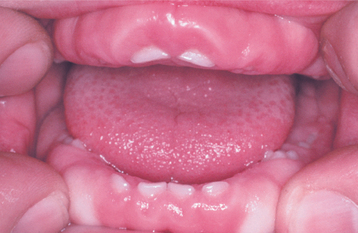
Fig. 4-25 Gingival fibromatosis. A young child with cheeks retracted by the parent. Note erythematous gingival hyperplasia arising in association with erupting deciduous dentition. (Courtesy of Dr. George Blozis.)
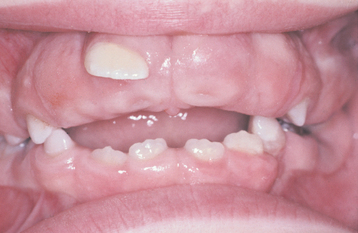
Fig. 4-26 Gingival fibromatosis. Significant fibrotic gingival hyperplasia with resultant delayed eruption of numerous teeth. (From Neville BW, Damm DD, White DK, Waldron CA: Color atlas of clinical oral pathology, Philadelphia, 1991, Lea & Febiger.) Lea & Febiger
The gingival changes may be generalized or localized to one or more quadrants. Either jaw may be involved, but the maxilla is affected more frequently and demonstrates a greater degree of enlargement. Palatal surfaces are typically increased in thickness more than the buccal side. Typically, extension past the alveolar mucosal junction into the mucobuccal fold is not seen, but palatal extensions can cause significant distortion of the contour of the palate and, at times, almost can meet in the midline.
In localized cases, the hyperplasia may involve a group of teeth and remain stable or, at a later date, may extend to other segments of one or both jaws. One distinctive and not uncommon pattern involves the posterior maxillary alveolar ridge. In this pattern, the hyperplastic tissue forms bilaterally symmetrical en-largements that extend posteriorly and palatally from the posterior alveolar ridges (Fig. 4-27). Less commonly, the overgrowth also may be isolated to the facial gingiva of the lower molars.
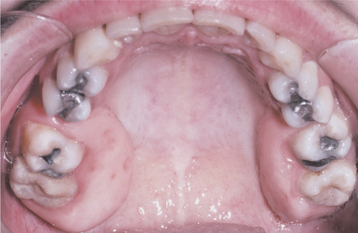
Fig. 4-27 Localized gingival fibromatosis. Bilateral and symmetrical fibrotic enlargements of the palatal surfaces of the posterior maxillary alveolar ridges.
The gingiva is firm, normal in color, and covered by a surface that is smooth or finely stippled. In older patients, the surface may develop numerous papillary projections. The frenular attachments may appear to divide the gingival tissues of the alveolar ridge into lobules. Associated clinical problems include poor aesthetics, prolonged retention of deciduous teeth, abnormal occlusion, inadequate lip closure, and difficulty in eating and speaking.
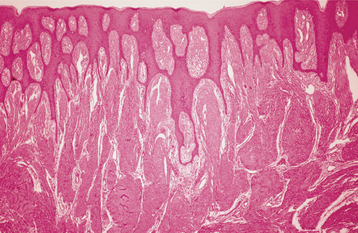
HISTOPATHOLOGIC FEATURES: The enlargements of gingival fibromatosis consist of dense hypocellular, hypovascular collagenous tissue, which forms numerous interlacing bundles that appear to run in all directions. The surface epithelium often exhibits long, thin rete ridges that extend deeply into the underlying fibrous connective tissue (Fig. 4-28). Inflammation is absent to mild. On occasion, scattered islands of odontogenic epithelium, foci of dystrophic calcification, or areas of osseous metaplasia may be seen. Electron microscopic examination demonstrates a mixture of both fibroblasts and myofibroblast-like cells.
TREATMENT AND PROGNOSIS: Conservative treatment consists of gingivectomy in conjunction with a rigorous program of oral hygiene. Follow-up is recommended because there is a tendency for recurrence within a few years. Investigators have suggested that the frequency of recurrence is less if gingivectomy is delayed until after full eruption of the permanent dentition. In severe cases, selective extraction of teeth (and gingivectomy) often is required to achieve a normal gingival morphology.
PERIODONTITIS
Periodontitis refers to an inflammation of the gingival tissues in association with some loss of both the attachment of the periodontal ligament and bony support. With progressive loss of attachment, significant destruction of the periodontal ligament and adjacent alveolar bone can occur. Apical migration of the crevicular epithelium along the root surface results in the formation of periodontal pockets. Loosening and eventual loss of teeth are possible.
For more than a century, the presence of the disease has been correlated with the accumulation of dental plaque on the tooth and under the gingiva. Despite this, current evidence suggests that dental plaque is part of the natural human microflora. In some patients with extensive dental plaque, destructive lesions of the periodontium do not develop. Many investigators now believe that periodontitis occurs not from the mere presence of dental plaque but as a result of shifts in the proportions of bacterial species in the plaque, possibly related to changes in the dentogingival environment (e.g., a soft diet or a highly fermentable carbohydrate content diet).
Dramatic differences exist in the content of dental plaque in areas of healthy and diseased periodontium. Healthy sites are colonized primarily by facultative gram-positive organisms, such as actinomycetes and streptococci; plaque within areas of active periodontitis contains anaerobic and microaerophilic gram-negative flora. Of the more than 500 types of bacteria that may reside in the oral cavity, only a few have been related to periodontitis, and the specific types often correlate with the clinical patterns of periodontitis. Chronic periodontitis is associated strongly with Actinobacillus actinomycetemcomitans, Tannerella forsythensis (formerly Bacteroides forsythus), and Porphyromonas gingivalis. Additional organisms frequently thought to be involved include Prevotella intermedia, Campylobacter rectus, Treponema denticola, and Fusobacterium nucleatum. Although controversial, some investigators also have suggested that human cytomegalovirus and other herpesviruses could play a contributing role.
The pathogenic organisms exist in an organized community termed a biofilm. Bacteria growing in biofilms are relatively protected from normal host defenses and exhibit an increased resistance to locally or systemically administered antibiotic medications. Lipopolysaccharides released from the biofilms are thought to trigger release of catabolic inflammatory mediators that lead to the loss of attachment. Mechanical disruption of this organized bacterial biofilm may be an important factor associated with successful treatment of periodontitis.
The presence of pathogenic bacteria is essential but insufficient to produce periodontitis. Although mild-to-moderate periodontitis is present in the majority of adults, only 10% to 15% of the population develops severe, generalized disease. The variation of susceptibility to periodontitis appears related to genetic influences on host response, with 50% of the risk for chronic periodontitis attributed to heredity, 20% to tobacco abuse, and another 20% to colonization by specific pathogenic bacteria.
The classification of periodontitis, as delineated by the American Academy of Periodontology, is listed in Box 4-5. In 1999 this classification underwent significant revision, with consolidation of many previously distinct disorders. The concept of “early-onset periodontitis” and all of its subdivisions has been reclassi fied as aggressive periodontitis. The following text concentrates on the chronic form of periodontitis; a later section discusses the aggressive forms of periodontitis. From this list it should be clear that periodontitis represents a heterogeneous group of disorders.
Periodontitis associated with systemic disease is not rare, and Box 4-6 lists many of the disorders that may be associated with a premature loss of periodontal attachment. Necrotizing ulcerative periodontitis (NUP) represents the loss of attachment that often occurs in association with necrotizing ulcerative gingivitis (NUG) (see page 157). This form has been correlated with aggressive invasion by a number of spirochetes and Prevotella intermedia.
CLINICAL AND RADIOGRAPHIC FEATURES:
CHRONIC PERIODONTITIS: With the decline in caries, chronic periodontitis has become the primary cause of tooth loss in patients older than 35 years of age. A national survey found that 44% of adults in the United States had attachment loss of 3 μm or more in at least one site. The disorder demonstrates an increased prevalence in males, although researchers believe that much of this effect is related to poorer oral hygiene and dental-visit behavior. In addition, an increased prevalence of chronic periodontitis is associated with the following:
Local factors also may predispose patients to isolated periodontal defects; these include tooth shape and alignment, presence and quality of dental restorations, poor interdental contact, calculus formation, subgingival dental caries, traumatic occlusion, and abnormal alveolar bone or gingival anatomy.
Conversely, it appears that the presence of significant periodontitis may place patients at risk for an increased prevalence or greater severity of certain medical disorders. Although controversial, increasing evidence links periodontitis with an elevated risk for coronary artery disease, stroke, progressive diabetes mellitus, respiratory diseases, and delivery of low–birth weight babies. If true, then it is unclear if these associations are due to dissemination of triggering host inflammatory mediators or the spread of bacteria or their related toxins. Although strong direct associations have been documented, the epidemiology is difficult to interpret because of the additional risk factors associated with both conditions. For example, cardiovascular disease has been related to periodontitis, but the nature of this association is cloudy because both are strongly associated with smoking. Numerous interventional studies are ongoing to investigate the possible reduction in these medical disorders secondary to elimination and control of periodontitis.
In chronic periodontitis, no abnormalities of the immune system are found. Periodontitis begins in youth and early adulthood, takes years to decades to progress, and includes cyclic patterns of exacerbation and remission. The assumption that periodontitis is a disease of aging has been challenged, and most believe the increased periodontal destruction observed in older adults reflects a lifetime of disease accumulation rather than an age-specific disease.
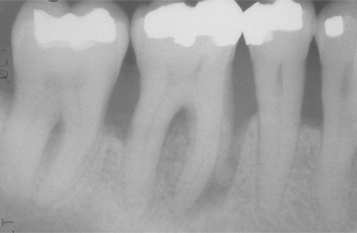
In patients with periodontitis, gingivitis is present and precedes the development of significant periodontal lesions. Although many sites may demonstrate gingivitis and few progress to attachment loss, lifelong local measures directed against sites of gingivitis represent an effective approach for prevention of chronic periodontitis. As loss of attachment occurs, blunting and apical positioning of the gingival margins typically are present (Fig. 4-29). Periodontal disease is present when a loss of attachment can be demonstrated through the use of a periodontal probe. In the absence of significant gingival hyperplasia, a measurement of pocket depths greater than 3 to 4 μm indicates destruction of the periodontal ligament and resorption of adjacent alveolar bone; however, clinical attachment loss is the best measurement of accumulated periodontal destruction and represents the diagnostic gold standard. High-quality dental radiographs exhibit a decreased vertical height of the bone surrounding the affected teeth (Fig. 4-30).With advanced bone loss, tooth mobility is present.
NECROTIZING ULCERATIVE PERIODONTITIS: NUP presents similarly to NUG (see page 157), but it also demonstrates loss of clinical attachment and alveolar bone. This destructive form of periodontitis may arise within a zone of preexisting periodontitis, or it may represent a sequela of a single or multiple episodes of NUG. Many believe that NUG and NUP represent different stages of the same infection. Patients affected with this pattern frequently are younger than most patients affected with chronic periodontitis and often demonstrate immunosuppression or malnutrition.
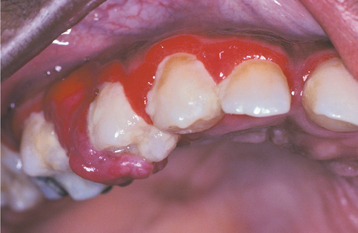
PERIODONTAL ABSCESS: A periodontal abscess (Figs. 4-31 and 4-32) is a localized purulent infection of the gingiva with involvement of the adjacent periodontal attachment and alveolar bone. On occasion, an abscess may be localized to the marginal or interdental gingiva without involvement of the adjacent periodontal ligament or alveolar bone. This lesion is termed a gingival abscess and often is secondary to plaque or foreign material that has become entrapped in the gingival sulcus.
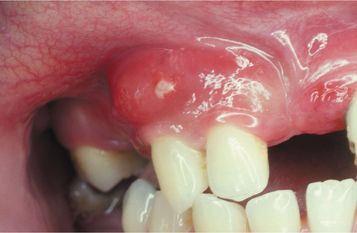
Fig. 4-31 Periodontal abscess. Localized erythematous gingival enlargement with central purulent drainage.
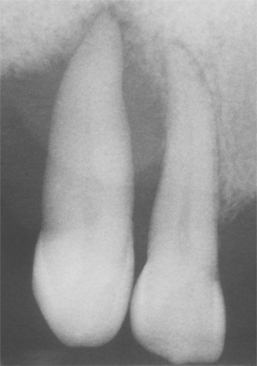
Fig. 4-32 Periodontal abscess. Same patient as depicted in Fig. 4-31. Note extensive loss of bone support associated with the maxillary cuspid.
A periodontal abscess often arises in a preexisting periodontal lesion and usually is precipitated by alterations in the subgingival flora, host resistance, or both. Factors frequently associated with abscess formation are closure of the entrance into a periodontal pocket, furcation involvement, or diabetes. Many cases arise in patients actively undergoing periodontal therapy, perhaps because of incomplete removal of deep calculus with microbial penetration of the soft tissue surrounding the pocket or premature sealing of the coronal opening to the pocket. Other factors involved less frequently are trauma and anatomic dental anomalies, such as enamel pearls (see page 93) and dens invaginatus (see page 90). Most cases arise in adults; periodontal abscesses in children are rare and most frequently the result of a foreign body that has been introduced into previously healthy periodontal tissues.
A periodontal abscess appears as a zone of gingival enlargement along the lateral aspect of a tooth. The involved gingiva may be erythematous and edematous, with a slick, red surface, or it may be hemorrhagic, with a dark-red coloration (Fig. 4-33). Common symptoms include the following:
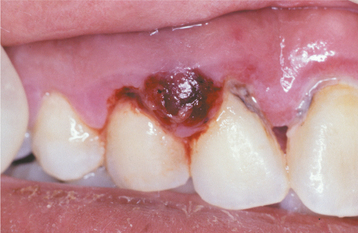
Fig. 4-33 Periodontal abscess. Dark-red and hemorrhagic enlargement of the interdental papilla between the maxillary right lateral incisor and cuspid.
• Extreme sensitivity to palpation of the affected gingiva
Probing or gentle pressure on the affected gingiva often results in the expression of pus from the sulcus. The abscess may drain through an overlying sinus tract. With drainage, the abscess becomes asymptomatic but can demonstrate acute exacerbations if the mucosa heals over and the pressure builds again. Radiographs often demonstrate bone loss associated with the previous periodontal defect or additional radiolucency secondary to the current acute process. In some cases, the infection can spread into the periapical region and create a combined periodontal-endodontic lesion.
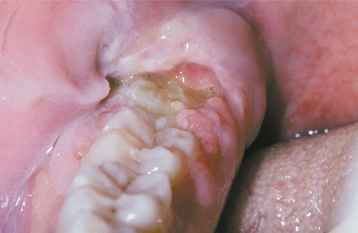
PERICORONITIS: Pericoronitis is an inflammatory process that arises within the tissues surrounding the crown of a partially erupted tooth. The inflammatory reaction often arises when food debris and bacteria are present beneath the gingival flap overlying the crown. Other predisposing factors include stress and upper respiratory infections, especially tonsillitis or pharyngitis.
These gingival flaps can exhibit long periods of chronic inflammation without symptoms. If the debris and bacteria become entrapped deep within the gingival flap, then abscess formation develops. Abscess development is seen most frequently in association with the mandibular third molars, and the predominant symptoms are extreme pain in the area, a foul taste, and inability to close the jaws. The pain may radiate to the throat, ear, or floor of the mouth. The affected area is erythematous and edematous, and the patient often has lymphadenopathy, fever, leukocytosis, and malaise (Fig. 4-34). NUG-like necrosis may develop in areas of persistent pericoronitis.
HISTOPATHOLOGIC FEATURES: When soft tissue from areas of periodontitis is examined microscopically, gingivitis is present and the crevicular epithelium lining the pocket is hyperplastic, with extensive exocytosis of acute inflammatory cells. The adjacent connective tissue exhibits an increased vascularity and contains an inflammatory cellular infiltrate consisting predominantly of lymphocytes and plasma cells, but with a variable number of polymorphonuclear leukocytes. Frequently, large colonies of microorganisms, representing plaque and calculus, are noted.
PERIODONTITIS: Initial attention must be directed toward elimination of any existing risk factors. Even with appropriate treatment and improved oral hygiene, many patients fail to respond to therapy unless certain factors (e.g., smoking, inadequately controlled diabetes) are eliminated. Once these influences have been managed, the treatment of periodontitis is directed toward stopping the loss of attachment. The foremost goal of this process is the elimination of the pathogenic bacterial plaque. Scaling, root planing, and curettage can be used to treat early periodontal lesions. In deeper pockets, a surgical flap may be required to gain access to the tooth for necessary débridement. At this time, the underlying bone may be recontoured (if necessary) to aid in the resolution of the periodontal pocket.
In some bony defects, regeneration of the attachment can be attempted through interdental denudation or the placement of autogenous bone grafts, allografts, or alloplastic materials. Often these grafts are used in conjunction with materials such as polytetrafluoroethylene in an attempt to achieve guided tissue regeneration in moderate-to-advanced periodontal defects.
Because of the chronic nature of periodontitis, antibiotic medications are not generally used except in patients who do not respond to conventional therapy. Inappropriate use of antibiotic agents can lead to overgrowth of potentially pathogenic organisms and development of bacterial drug resistance. When required, tetracycline or metronidazole are used most frequently. The choice of antibiotic medication always should be guided by microbiologic analysis with susceptibility testing. Several studies also suggest that NSAIDs may help slow the progression of bone loss in some cases of destructive periodontitis.
Several forms of local antibiotic delivery have been developed. The antibiotic drugs are placed directly into sites of refractory periodontitis and consist of gels, ointments, nonresorbable fibers, and resorbable polymers. These antibiotic agents represent an adjunct to scaling and root planing and should be limited to sites that are resistant to conventional therapy alone. Although short-term benefits have been demonstrated, many investigations have revealed limited long-term positive effects when compared with scaling and root planing without antibiotic medications.
In many cases the prognosis for chronic periodontitis correlates directly with the patient’s desire to maintain oral health. Long-term studies show that periodontal health can be maintained after appropriate periodontal therapy if a program of rigorous oral hygiene and professional care is established. Professional scaling and root planing modify the composition of the plaque microflora so that pathogenic plaques are converted to those with bacterial types normally found in healthy mouths. Bacterial morphotypes return to pretreatment levels 42 days after professional prophylaxis, but pathogenic complexes capable of inducing attachment loss require approximately 3 months to be reestablished functionally. In patients with less-than-optimal oral hygiene or with isolated defects that cannot be self-cleaned, a loss of attachment can be prevented if professional scaling and root planing are performed at 3-month intervals.
Many clinicians believe that the average individual is neither motivated nor sufficiently effective in maintaining the level of plaque control necessary to prevent periodontal disease. In these cases, supplementing normal hygiene techniques with regular professional cleaning and interventions such as counterrotational electric toothbrushes with automatic timers, antimicrobial mouth rinses (e.g., essential oils, chlorhexidine), and toothpastes containing triclosan with 2% Gantrez copolymer may prove beneficial. Other interventions occasionally recommended include medications to temper the host response (e.g., NSAIDs, doxycycline at subantimicrobial dose).
Destructive periodontal disease that is nonresponsive to normal therapy in compliant patients is termed refractory periodontitis. In such cases the patient should be reevaluated closely for any predisposing risk factors (such as smoking) or systemic diseases known to be associated with an increased prevalence of periodontitis. Subgingival microbial cultures can be obtained to assist in selection of an appropriate antibiotic intervention. Antimicrobial therapy may be combined with more frequent periodontal maintenance therapy and stronger reinforcement of the patient’s oral hygiene techniques.
Investigators are beginning to discover genetic markers for those patients who are at risk for developing severe, progressive periodontitis. Many envision a future in which patients are evaluated for the presence of these markers, with susceptible individuals monitored closely for early colonization by periodontal pathogens. If colonization is detected, then it could be eliminated easily and inexpensively. Attempts at vaccine development have been hindered by the multifactorial nature of periodontitis combined with the complexity of the bacterial biofilms.
NECROTIZING ULCERATIVE PERIODONTITIS: Once any underlying influence (e.g., immunosuppression, malnutrition) has been resolved, NUP often responds well to irrigation, débridement of the necrotic areas, effective oral hygiene measures, and administration of systemic antibiotic medications. Failure to respond to standard therapy mandates a thorough physical evaluation to rule out the possibility of an underlying disease.
PERIODONTAL ABSCESS: A gingival or periodontal abscess is treated by drainage through the sulcus or by an incision through the overlying mucosa. Thorough cleansing of the area with removal of all foreign material, plaque, and calculus should be performed. Penicillin or other antibiotic drugs are prescribed when a fever is present. Analgesic agents are prescribed, and the patient receives a soft diet, is told to use warm saltwater rinses, and is instructed to return each day until the symptoms have resolved. After the acute phase has passed, the patient is treated for any underlying chronic pathologic periodontal condition.
PERICORONITIS: Acute pericoronitis is treated with gentle antiseptic lavage under the gingival flap to remove gross food debris and bacteria. Systemic antibiotic agents are used if a fever or general symptoms are noted. The patient is instructed to use warm saltwater rinses and to return in 24 hours. Once the acute phase has subsided, the tooth can be extracted if long-term maintenance is contraindicated. If tooth retention is desirable, then the overlying gingival flap is removed surgically, followed by elimination of all food debris and bacterial colonies by thorough curettage.
AGGRESSIVE PERIODONTITIS
Although periodontitis is much more frequent in older adults, it also can be a significant problem in children and young adults. Before the 1999 reclassification by the American Academy of Periodontology, destructive periodontal disease in younger patients was termed early-onset periodontitis and subdivided into prepubertal, localized juvenile, generalized juvenile, and rapidly progressing forms of periodontitis. The “early-onset” designation was discontinued during the 1999 workshop because the term was deemed too restrictive. Many argued that this pattern of periodontitis can occur at any age and is not restricted to patients younger than 35 years old. It was agreed that an appropriate classification system should not be based on age but should consider primarily the clinical, radiographic, historical, and laboratory findings.
The 1999 workshop concluded that the most logical classification system should not be age dependent or require knowledge of rates of progression. In general, the new designation of localized aggressive periodontitis replaces the older term, localized juvenile periodontitis, whereas generalized aggressive periodontitis supersedes generalized juvenile periodontitis. The pattern previously designated as prepubertal periodontitis has been associated with a systemic leukocyte dysfunction termed leukocyte adhesion syndrome. This disease currently is classified as one of the forms of periodontitis presenting as a manifestation of a systemic disease.
By definition, aggressive periodontitis occurs in otherwise healthy people; there should be no association with a systemic disease process. In keeping with this definition, the diagnosis is one of exclusion, and all systemic disorders known to be related to premature loss of attachment (see Box 4-6) should be ruled out before the definitive diagnosis is made.
In contrast to chronic disease, aggressive periodontitis appears to be correlated with one or more deficiencies in the immune response, rather than with inappropriate accumulations of plaque and calculus. Researchers believe that aggressive periodontitis represents a number of different pathoses that have been grouped together because of similar clinical presentations. Suspected pathogens that are commonly found in these diseases include Actinobacillus actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, and a variety of other less common organisms. The response to therapy often hinges on the successful elimination of these organisms. As mentioned in the discussion of periodontitis (see page 168), an association with a number of viruses has been suggested but disputed by others.
The majority of patients with aggressive periodontitis have a demonstrable neutrophil dysfunction but without systemic manifestations. In the localized variant, a number of affected patients demonstrate a specific defect of bactericidal activity toward A. actinomycetemcomitans. Although this is a controver-sial topic, several investigators have suggested that aggressive periodontitis requires specific bacterial flora and the presence of a selective immune dysfunction that allows these pathogens to flourish. This unique pattern of immune alteration may explain the failure to defend appropriately against certain periodontal pathogens without exhibiting systemic signs of immunodeficiency.
Familial aggregation of patients with aggressive periodontitis is noted and suggests an underlying genetic foundation, which may be transmitted in some families as an autosomal dominant trait with reduced penetrance (some patients can harbor mutation without clinical evidence of disease). In all likelihood, aggressive periodontitis is genetically heterogeneous, meaning the mutation of any one of several different gene loci can result in the disease; however, only one of these causative mutations is identified within a kindred. With this knowledge, it would not be surprising to encounter variations in inheritance patterns in different geographic locations and ethnic groups.
CLINICAL AND RADIOGRAPHIC FEATURES:
LOCALIZED AGGRESSIVE PERIODONTITIS: As previously stated, aggressive periodontitis can be localized or generalized. One large study of children aged 5 to 17 years in the United States demonstrated a prevalence of 0.53% for the localized form and 0.13% for the generalized variant. Localized aggressive periodontitis typically begins around the ages of 11 to 13 years and has a strong familial tendency. The following specific features have been delineated by the American Academy of Periodontology:
• Robust serum antibody response to infecting agents
• Attachment loss localized to the first molars and incisors, with involvement of no more than two teeth other than the first molars and incisors
This form may appear to localize around the first molars and the incisors, possibly because these teeth have been erupted for the longest duration (Fig. 4-35). In numerous clinical studies, minimal supragingival plaque or calculus has been documented; however, this finding has been disputed. The rate of bone destruction is three to five times faster than that seen in chronic periodontitis.
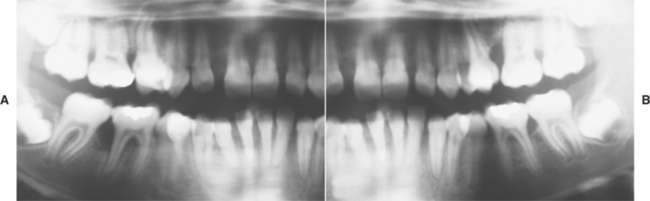
Fig. 4-35 Localized aggressive periodontitis. A, Loss of bone support in the area of the first molars and incisors of both maxillary and mandibular right quadrants in a 14-year-old patient. B, Left quadrants of the same patient depicted in A. Note the similar pattern of bone loss in the area of the first molars and incisors.
In the first molar regions, radiographs reveal vertical bone resorption that often is bilateral and symmetrical. In classic cases an arc-shaped zone of bone loss extends from the distal aspect of the second bicuspid to the mesial aspect of the second molar. Similar involvement is apparent around the anterior teeth. Tooth migration and mobility are common. If untreated, then the process often continues until the teeth are exfoliated. In about one third of patients affected with localized aggressive periodontitis, progression to more generalized disease occurs.
Of all the pathogens in dental plaque, A. actinomycetemcomitans appears to be predominant in localized aggressive periodontitis. This bacterium is present in disease sites in more than 90% of cases. Its ability to invade gingival tissue has created difficulties in mechanical eradication. Knowledge of its importance to the disease process has led to remarkable advances in therapy.
GENERALIZED AGGRESSIVE PERIODONTITIS: Generalized aggressive periodontitis may not represent a distinct disease entity but, rather, may occur in a collection of young adults with advanced periodontal disease. Many cases may represent localized aggressive periodontitis that has become more generalized with time; other cases initially demonstrate generalized disease. As with the localized variant, a significant percentage of cases demonstrate neutrophil dysfunction. The American Academy of Periodontology recognizes the following features:
• Usually diagnosed in patients younger than 30 years old but may occur at any age
• Poor serum antibody response to infecting agents
• Pronounced episodic destruction of periodontal attachment and alveolar bone
• Generalized loss of attachment that must affect at least three teeth other than the first molars and incisors
Most affected patients are between the ages of 12 and 32. In contrast to many examples of the localized variant, heavy plaque, calculus, and marked gingival inflammation may be present. Compared with the localized variant, more teeth are affected and the bone loss is not restricted to specific areas of the jaws.
Although the localized pattern is associated primarily with A. actinomycetemcomitans, the pathogens active in the generalized variant are more complex, more closely aligned to chronic periodontitis, and also involve organisms such as Prevotella intermedia, Porphyromonas gingivalis, Tannerella forsythensis, Fusobacterium nucleatum, Campylobacter rectus, and various spirochetes. In patients whose disease progresses from the localized to generalized pattern, the associated periodontal pathogens often become more diverse as the patient ages and the disease becomes more widespread.
HISTOPATHOLOGIC FEATURES: The microscopic examination of granulation tissue removed from sites of aggressive periodontitis does not differ dramatically from that seen in chronic periodontitis. In spite of this, initial histopathologic examination of the material removed from active sites of disease is mandatory to rule out the possibility of other disease processes, such as Langerhans cell disease (see page 590). Even when the attachment loss presents in a classic localized pattern, systemic disease cannot be eliminated without an examination of tissue. The definitive diagnosis centers on the clinical, radiographic, histopathologic, and microbiologic findings, combined with the family history and leukocyte function tests.
TREATMENT AND PROGNOSIS: Unlike the treatment used for patients with chronic periodontitis, scaling and root planing alone do not stop progression of aggressive periodontitis. The defects in leukocyte function, in addition to the invasive capabilities of the involved pathogenic organisms, mandate the use of antibiotics in combination with mechanical removal of subgingival plaque and inflamed periodontal tissues. Although tetracycline, amoxicillin and clavulanate potassium, minocycline, and erythromycin can be used in selected patients, the combination of high-dose (500 mg three times per day) amoxicillin and metronidazole has been shown to be most effective in controlling the involved periodontal pathogens, especially A. actinomycetemcomitans. Therapy often is predicated on microbiologic testing to ensure selection of the most appropriate antimicrobial agent. Some investigators have claimed better results if the scaling and root planing are completed within a 24-hour period, rather than treating a quadrant at a time over an extended period. Reinfection of previously cleaned areas by organisms from untreated sites is thought to worsen the response to therapy.
A reevaluation with professional prophylaxis is performed once a month for 6 months and then every 3 months thereafter. Specimens for anaerobic cultures are obtained at each 3-month recall. Patients with refractory disease or significant colonization by pathogenic organisms receive additional courses of appropriate antibiotics. Long-term follow-up is mandatory because of the possibility of reinfection or incomplete elimination of the organisms. The presence of deep residual pockets is associated with disease progression. In such circumstances, periodontal surgery often is performed to eliminate these defects. This intervention is directed at any pocket consistently deeper than 5 μm and typically is performed after 2 to 6 months of nonsurgical therapy.
Dental practitioners should alert proband patients with aggressive periodontitis of the possible genetic transmission of the disease process. In general, patients diagnosed with localized aggressive periodontitis typically exhibit relatively stable disease, whereas those initially diagnosed with generalized involvement often continue to lose periodontal attachment and teeth. Patients who smoke and those who present for therapy with advanced clinical attachment loss tend to demonstrate a worse prognosis and respond less reliably to therapy.
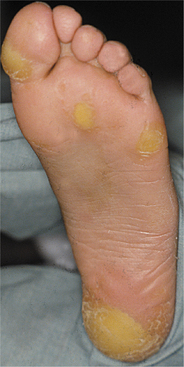
PAPILLON-LEFÈVRE SYNDROME
In 1924, Papillon and Lefévre initially described the syndrome that bears their names. This autosomal recessive disorder predominantly demonstrates oral and dermatologic manifestations; similar dermatologic changes can be seen in the absence of oral findings (keratoderma palmoplantar of Unna-Thost syndrome and Meleda disease). Because of the autosomal recessive inheritance pattern, the parents typically are not affected; consanguinity is noted in approximately one third of cases. The predominant oral finding is accelerated periodontitis that appears to be caused by defects in neutrophil function and multiple immune-mediated mechanisms.
Genetic studies of patients with Papillon-Lefévre syndrome have mapped the major gene locus to chromosome 11q14-q21 and revealed mutation and loss of function of the cathepsin C gene. This gene is important in the structural growth and development of the skin and is critical for appropriate immune response of myeloid and lymphoid cells. Researchers believe that the loss of appropriate function of the cathepsin C gene results in an altered immune response to infection. In addition, the altered gene may affect the integrity of the junctional epithelium surrounding the tooth.
A closely related disease, Haim-Munk syndrome, also exhibits palmoplantar keratosis, progressive periodontal disease, recurrent skin infections, and several skeletal malformations. In this syndrome, the skin manifestations are more severe and the periodontal disease is milder. Studies have demonstrated that Haim-Munk syndrome and many examples of prepubertal periodontitis also exhibit mutation of the cathepsin C gene and represent allelic variants of the mutated gene responsible for Papillon-Lefévre syndrome.
CLINICAL AND RADIOGRAPHIC FEATURES: Papillon-Lefévre syndrome exhibits a prevalence of one to four per million people in the population, and carriers are thought to be present in two to four per thousand persons. In most cases, the dermatologic manifestations become clinically evident in the first 3 years of life. Diffuse transgredient (first occurs on the palms and soles and then spreads to the dorsa of the hands and feet) palmar-plantar keratosis develops, with occasional reports of diffuse follicular hyperkeratosis, nail dystrophy, hyperhidrosis, and keratosis on the elbows and knees (Fig. 4-36). Other less common sites of involvement include the legs, thighs, dorsal surface of the fingers and toes, and (rarely) the trunk. Although the appearance of the dermatologic manifestations is variable, the lesions typically present as white, light-yellow, brown, or red plaques and patches that develop crusts, cracks, or deep fissures. Some patients describe worsening in the winter, and others describe keratotic desquamation, which may be confused with psoriasis.
The oral manifestations consist of dramatically advanced periodontitis that is seen in both the deciduous and the permanent dentitions and develops soon after the eruption of the teeth. Extensive hyperplastic and hemorrhagic gingivitis is seen (Fig. 4-37). A rapid loss of attachment occurs, with the teeth soon lacking osseous support and radiographically appearing to float in the soft tissue (Fig. 4-38). Without aggressive therapy, the loss of the dentition is inevitable. Mobility and migration of the teeth are observed consistently, and mastication often is painful because of the lack of support. The teeth spontaneously exfoliate or are removed because of sensitivity during function. This process prematurely eliminates the deciduous dentition; with eruption of the permanent teeth, the destructive pattern is duplicated. When the teeth are absent, the alveolar mucosa is normal in appearance.
Fig. 4-38 Papillon-Lefévre syndrome. Multifocal sites of bone loss in all four quadrants. (From Giansanti JS, Hrabak RP, Waldron CA: Palmoplantar hyperkeratosis and concomitant periodontal destruction [Papillon-Lefévre syndrome], Oral Surg Oral Med Oral Pathol 36:40, 1973.)
Although other pathogenic bacteria have been isolated from sites of active disease, Actinobacillus actinomycetemcomitans has been related directly to the periodontal destruction. Although a hereditary component exists and leukocyte dysfunction can be demonstrated, it appears that an infection with a specific, potent bacterium, such as A. actinomycetemcomitans, must be present for the periodontal component to develop. Interestingly, one investigation documented the development of appropriate peripheral leukocyte function after successful resolution of the pathogenic organisms responsible for the periodontitis. This indicates that the leukocyte dysfunction may be induced by infection with A. actinomycetemcomitans (possibly secondary to generated leukotoxins).
In addition to the dermatologic and oral manifestations, numerous investigators have documented less frequent findings. Retardation of somatic development and ectopic calcifications of the falx cerebri and choroid plexus have been reported, in addition to an increased susceptibility to infections beyond the oral cavity. Pyoderma, furunculosis, pneumonia, hepatic abscesses, and other infections have been documented.
HISTOPATHOLOGIC FEATURES: Once again, the histopathologic features of Papillon-Lefévre syndrome resemble those seen in chronic periodontitis and are not specific. Submitted tissue often contains hyperplastic crevicular epithelium with exocytosis. The underlying connective tissue exhibits increased vascularity and a mixed inflammatory cellular infiltrate consisting predominantly of polymorphonuclear leukocytes, lymphocytes, histiocytes, and plasma cells. Initially, histopathologic examination is recommended to rule out other pathologic causes of the periodontal destruction.
TREATMENT AND PROGNOSIS: The most successful treatment of the skin lesions has been retinoid (e.g., etretinate) administration, which has resulted in remarkable improvement with complete clearance in the majority of patients. Surprisingly, a few authors have reported improvement of the associated periodontal disease during periods of retinoid use, but others have disputed this claim. Possible adverse reactions caused by retinoid administration include angular cheilitis, dry lips, hair loss, arthralgia, tendinous and ligamentous calcifications, and teratogenicity. In an attempt to avoid these drug-related adverse reactions, patients with mild dermatologic manifestations often are treated with topical lubricants, keratolytic agents (salicylic or lactic acid), corticosteroid agents, or antibiotics medications.
Attempts at resolution of the periodontal disease often have been frustrating. In spite of extensive periodontal therapy and antibiotic agents, in many patients the disease progresses until all teeth are lost. However, several investigators have reported a cessation of attachment loss, and two different treatment approaches have been used.
Despite the use of numerous antibiotic medications, several reports document a difficulty in resolution of the infection associated with teeth that already exhibit attachment loss. In some of the cases, all of the periodontally involved deciduous teeth were extracted, followed by a period of edentulousness with antibiotic treatment in an attempt to remove the causative pathogens. Tetracycline was successful in preventing the redevelopment of periodontitis in the permanent teeth after the extractions, as well as in the resolution of the infection in the deciduous dentition. However, penicillin, erythromycin, metronidazole, and tetracycline were all unsuccessful in resolving active sites of periodontitis.
The second approach revolves around direct attack against A. actinomycetemcomitans. In numerous studies of patients with destructive periodontitis associated with A. actinomycetemcomitans, therapy with high-dose amoxicillin and metronidazole has proven effective when combined with high patient compliance and strong supportive periodontal therapy. A variety of other regimens have been used with variable success in different geographic regions, suggesting the possibility of inconsistent effectiveness related to local patterns of antibiotic resistance. It appears clear that elimination of A. actinomycetemcomitans and continued control of plaque and calculus are mandatory, but the antibiotic agent best suited for this task can vary.
Through the use of mechanical plaque control and appropriate antibiotic medications directed toward A. actinomycetemcomitans, the course of the disease might be altered. The progression of attachment loss is slowed dramatically, and the teeth that erupt after the initiation of therapy do not develop periodontal destruction. Rigorous oral hygiene, chlorhexidine mouth rinses, frequent professional prophylaxis, and periodic appropriate antibiotic therapy are necessary for long-term maintenance.
BIBLIOGRAPHY
Newman, MG, Takei, H, Carranza, FA, et al. Clinical periodontology, ed 10. Philadelphia: WB Saunders, 2006.
American Academy of Periodontology. Parameter on plaque-induced gingivitis. J Periodontol. 2000;71(suppl):851–852.
American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol. 2001;72:1790–1800.
Fransson, C, Berglundh, T, Lindhe, J. The effects of age on the development of gingivitis. Clinical, microbiological and histological findings. J Clin Periodontol. 1996;23:379–385.
Gunsolley, JC. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc. 2006;137:1649–1657.
Löe, H, Theilade, E, Jensen, SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187.
Löe, H, Theilade, E, Jensen, SB, et al. Experimental gingivitis in man. 3. Influence of antibiotics on gingival plaque development. J Periodontol Res. 1967;2:282–289.
Moskow, BS, Polson, AM. Histologic studies on the extension of the inflammatory infiltrate in human periodontitis. J Clin Periodontol. 1991;18:534–542.
Schätzle, M, Löe, H, Lang, NP, et al. The clinical course of chronic periodontitis. IV. Gingival inflammation as a risk factor in tooth mortality. J Clin Periodontol. 2004;31:1122–1127.
Tatakis, DN, Trombelli, L. Modulation of clinical expression of plaque-induced gingivitis. I. Background review and rationale. J Clin Periodontol. 2004;31:229–238.
Theilade, E, Wright, WH, Jensen, SB, et al. Experimental gingivitis in man. II. A longitudinal clinical and bacteriologically investigation. J Periodontol Res. 1966;1:1–13.
Trombelli, L. Susceptibility to gingivitis: a way to predict periodontal disease? Oral Health Prev Dent. 2004;2(suppl 1):265–269.
Trombelli, L, Scapoli, C, Orlandini, E, et al. Modualtion of clinical expression of plaque-induced gingivitis. III. Response of “high responders” and “low responders” to therapy. J Clin Periodontol. 2004;31:253–259.
Necrotizing Ulcerative Gingivitis
American Academy of Periodontology. Parameter on acute periodontal diseases. J Periodontol. 2000;71(suppl):863–866.
Arendorf, TM, Bredekamp, B, Cloete, C-A. Seasonal variation of acute necrotising ulcerative gingivitis in South Africans. Oral Dis. 2001;7:150–154.
Contreras, A, Falkler, WA, Jr., Enwonwu, C, et al. Human herpesviridae in acute necrotizing ulcerative gingivitis in children in Nigeria. Oral Microbiol Immuol. 1997;12:259–265.
Hartnett, AC, Shiloah, J. The treatment of acute necrotizing ulcerative gingivitis. Quintessence Int. 1991;22:95–100.
Horning, GM. Necrotizing gingivostomatitis-NUG to noma. Compend Contin Educ Dent. 1996;17:951–962.
Horning, GM, Cohen, ME. Necrotizing ulcerative gingivitis, periodontitis, and stomatitis: clinical staging and predisposing factors. J Periodontol. 1995;66:990–998.
Jiménez, LM, Duque, FL, Baer, PN, et al. Necrotizing ulcerative periodontal diseases in children and young adults in Medellín, Columbia, 1965-2000. J Int Acad Periodontol. 2005;7:55–63.
Johnson, BD, Engel, D. Acute necrotizing ulcerative gingivitis: a review of diagnosis, etiology and treatment. J Periodontol. 1986;57:141–150.
Rowland, RW. Necrotizing ulcerative gingivitis. Ann Periodontol. 1999;4:65–73.
Wade, DN, Kerns, DG. Acute necrotizing ulcerative gingivitis-periodontitis: a literature review. Mil Med. 1998;5:337–342.
Kerr, DA, McClatchey, KD, Regezi, JA. Allergic gingivostomatitis (due to gum chewing). J Periodontol. 1971;42:709–712.
Kerr, DA, McClatchey, KD, Regezi, JA. Idiopathic gingivostomatitis: cheilitis, glossitis, gingivitis syndrome: atypical gingivostomatitis plasma-cell gingivitis, plasmacytosis of gingiva. Oral Surg Oral Med Oral Pathol. 1971;32:402–423.
Lubow, RM, Cooley, RL, Hartman, KS, et al. Plasma-cell gingivostomatitis: report of a case. J Periodontol. 1984;55:235–241.
MacLeod, RL, Ellis, JE. Plasma cell gingivitis related to the use of herbal toothpaste. Br Dent J. 1989;166:375–376.
Mahler, V, Hornstein, OP. Plasma cell gingivitis: treatment with 2% fusidic acid. J Am Acad Dermatol. 1996;34:145–146.
Marker, P, Krogdahl, A. Plasma cell gingivitis apparently related to the use of khat: report of a case. Br Dent J. 2002;192:311–313.
Owings, JR. An atypical gingivostomatitis: report of four cases. J Periodontol. 1969;40:538–542.
Perry, HO, Deffner, NF, Sheridan, PJ. Atypical gingivostomatitis: nineteen cases. Arch Dermatol. 1973;107:872–878.
Serio, FG, Siegel, MA. Plasma cell gingivitis of unusual origin: report of a case. J Periodontol. 1991;62:390–393.
Silverman, S, Jr., Lozada, F. An epilogue to plasma-cell gingivostomatitis (allergic gingivostomatitis). Oral Surg Oral Med Oral Pathol. 1977;43:211–217.
Sollecito, TP, Greenberg, MS. Plasma cell gingivitis: report of two cases. Oral Surg Oral Med Oral Pathol. 1992;73:690–693.
Timms, MS, Sloan, P. Association of supraglottic and gingival idiopathic plasmacytosis. Oral Surg Oral Med Oral Pathol. 1991;71:451–453.
Daley, TD, Wysocki, GP. Foreign body gingivitis: an iatrogenic disease? Oral Surg Oral Med Oral Pathol. 1990;69:708–712.
Gordon, SC, Daley, TD. Foreign body gingivitis: clinical and microscopic features of 61 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:562–570.
Gordon, SC, Daley, TD. Foreign body gingivitis: identification of the foreign material by energy-dispersive x-ray microanalysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:571–576.
Gravitis, K, Daley, TD, Lochhead, MA. Management of patients with foreign body gingivitis: report of 2 cases with histologic findings. J Can Dent Assoc. 2005;71:105–109.
Koppang, HS, Roushan, A, Srafilzadeh, A, et al. Foreign body gingival lesions: distribution, morphology, identification by x-ray energy dispersive analysis and possible origin of foreign material. J Oral Pathol Med. 2007;36:161–172.
Lombardi, T, Kuffer, R, Dubrez, B. Polishing-paste-induced silica granuloma of the gingiva. Dermatology. 2001;203:177–179.
Mignogna, MD, Fedele, S, LoRusso, L, et al. Orofacial granulomatosis with gingival onset. J Clin Periodontol. 2001;28:692–696.
Drug-Related Gingival Hyperplasia
American Academy of Periodontology. Informational paper. Drug-associated gingival enlargement. J Periodontol. 2004;75:1424–1431.
Botha, PJ. Drug-induced gingival hyperplasia and its management—a literature review. J Dent Assoc S Afr. 1997;52:659–664.
Brunet, L, Miranda, J, Farré, M, et al. Gingival enlargement induced by drugs. Drug Saf. 1996;15:219–231.
Butler, RT, Kalkwarf, KL, Kaldahl, WB. Drug-induced gingival hyperplasia: phenytoin, cyclosporine, and nifedipine. J Am Dent Assoc. 1987;114:56–60.
Camargo, PM, Melnick, PR, Pirih, FQM, et al. Treatment of drug-induced gingival enlargement: aesthetic and functional considerations. Periodontol 2000. 2001;27:131–138.
Desai, P, Silver, JG. Drug-induced gingival enlargements. J Can Dent Assoc. 1998;64:263–268.
Dongari, A, McDonnell, HT, Langlais, RP. Drug-induced gingival overgrowth. Oral Surg Oral Med Oral Pathol. 1993;76:543–548.
Eggerath, J, English, H, Leichter, JW. Drug-associated gingival enlargement: case report and review of aetiology, management and evidenced-based outcomes of treatment. J N Z Soc Periodontol. 2005;88:7–14.
Hall, EE. Prevention and treatment considerations in patients with drug-induced gingival enlargement. Curr Opin Periodontol. 1997;4:59–63.
Hassell, TM, Hefti, AF. Drug-induced gingival overgrowth: old problem, new problem. Crit Rev Oral Biol Med. 1991;2:103–137.
McCulloch, CA. Drug-induced fibrosis: interference with intracellular collagen degradation pathway. Curr Opin Drug Discov Devel. 2004;7:720–724.
Meisel, P, Schwahn, C, John, U, et al. Calcium antagonists and deep gingival pockets in the population-based SHIP study. Br J Clin Pharmacol. 2005;60:552–559.
Woo, S-B, Allen, CM, Orden, A, et al. Non-gingival soft tissue overgrowths after allogeneic marrow transplantation. Bone Marrow Transplant. 1996;17:1127–1132.
Coletta, RD, Graner, E. Hereditary gingival fibromatosis: a systematic review. J Periodontol. 2006;77:753–764.
Hart, TC, Zhang, Y, Gorry, MC, et al. A mutation in the SOS1 gene causes hereditary gingival fibromatosis type 1. Am J Hum Genet. 2002;70:943–954.
Jorgenson, RJ, Cocker, ME. Variation in the inheritance and expression of gingival fibromatosis. J Periodontol. 1974;45:472–477.
Mangino, M, Pizzuti, A, Dallapiccola, B, et al. Hereditary gingival fibromatosis (HGF) with hypertrichosis is unlinked to the HGF1 and HGF2 loci. Am J Med Genet. 2003;116A:312–314.
Rushton, MA. Hereditary or idiopathic hyperplasia of the gums. Dent Pract Dent Rec. 1957;7:136–146.
Shashi, V, Pallos, D, Pettenati, MJ, et al. Genetic heterogeneity of gingival fibromatosis on chromosome 2p. J Med Genet. 1999;36:683–686.
Takagi, M, Yamamoto, H, Mega, H, et al. Heterogeneity in the gingival fibromatoses. Cancer. 1991;68:2202–2212.
Xiao, S, Bu, L, Zhu, L, et al. A new locus for hereditary gingival fibromatosis (GINGF2) maps to 5q13-q22. Genomics. 2001;74:180–185.
Ye, X, Shi, L, Cheng, Y, et al. A novel locus for autosomal dominant hereditary gingival fibromatosis, GINGF3, maps to chromosome 2q22.3-p23.3. Clin Genet. 2005;68:239–244.
Albandar, JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206.
American Academy of Periodontology. Position paper. Supportive periodontal therapy (SPT). J Periodontol. 1998;69:502–506.
American Academy of Periodontology. Parameter on acute periodontal diseases. J Periodontol. 2000;71:863–866.
American Academy of Periodontology. Parameter on “refractory” periodontitis. J Periodontol. 2000;71:859–860.
American Academy of Periodontology. Informational paper. Modulation of the host response in periodontal therapy. J Periodontol. 2003;73:460–470.
American Academy of Periodontology. Position paper. Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–1247.
American Academy of Periodontology. Informational paper. Implications of genetic technology for the management of periodontal diseases. J Periodontol. 2005;76:850–857.
American Academy of Periodontology. Position paper. Epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419.
Armitage, G. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6.
Bataineh, AB, Al Qudah, MA. The predisposing factors of pericoronitis of mandibular third molars in a Jordanian population. Quintessence Int. 2003;34:227–231.
Cappuyns, I, Gugerli, P, Mombelli, A. Viruses in periodontal disease—a review. Oral Dis. 2005;11:219–229.
Corbet, EF. Diagnosis of acute periodontal lesions. Periodontol 2000. 2004;34:204–216.
Gaggl, AJ, Rainer, H, Grund, E, et al. Local oxygen therapy for treating acute necrotizing periodontal disease in smokers. J Periodontol. 2006;77:31–38.
Greenstein, G. Periodontal response to mechanical non-surgical therapy: a review. J Periodontol. 1992;63:118–130.
Dentino, AR, Kassab, MW, Renner, EJ. Prevention of periodontal diseases. Dent Clin North Am. 2005;49:573–594.
Ismail, AI, Morrison, EC, Burt, BA, et al. Natural history of periodontal disease in adults: findings from the Tecumseh periodontal disease study, 1959-87. J Dent Res. 1990;69:430–435.
Kinane, DF, Hart, TC. Genes and genetic polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449.
Lindhe, J, Nyman, S. Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol. 1984;11:504–514.
Loos, BG, John, RP, Laine, ML. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J Clin Periodontol. 2005;32(suppl 6):159–179.
Meng, HX. Periodontal abscess. Ann Periodontol. 1999;4:79–82.
Minsk, L. Diagnosis and treatment of acute periodontal conditions. Compend Contin Educ Dent. 2006;27:8–11.
Newman, HN. Plaque and chronic inflammatory periodontal disease: a question of ecology. J Clin Periodontol. 1990;17:533–541.
Novak, MJ. Necrotizing ulcerative periodontitis. Ann Periodontol. 1999;4:74–77.
Preshaw, PM, Seymour, RA, Heasman, PA. Current concepts in periodontal pathogenesis. Dent Update. 2004;21:570–578.
Rees, JS, Midda, M. Update on periodontology: 1. Current concepts in the histopathology of periodontal disease. Dent Update. 1991;18:418–422.
Scannapieco, FA. Systemic effects of periodontal diseases. Den Clin North Am. 2005;49:533–550.
Slots, J. Herpesviruses in periodontal diseases. Periodontol 2000. 2005;38:33–62.
Tatakis, DN, Kumar, PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516.
Tonetti, MS, D’Aiuto, F, Nibali, L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;35:911–920.
Albandar, JM, Brown, LJ, Genco, RJ, et al. Clinical classification of periodontitis in adolescents and young adults. J Periodontol. 1997;68:545–555.
American Academy of Periodontology. Parameter on aggressive periodontitis. J Periodontol. 2000;71:867–869.
American Academy of Periodontology. Position paper. Periodontal diseases of children and adolescents. J Periodontol. 2003;74:1696–1704.
Armitage, GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6.
Donly, KJ, Ashkenazi, M. Juvenile periodontitis: a review of pathogenesis, diagnosis and treatment. J Clin Pediatr Dent. 1992;16:73–78.
Guerrero, A, Griffiths, GS, Nibali, L, et al. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol. 2005;32:1096–1107.
Hughes, FJ, Syed, M, Koshy, B, et al. Prognostic factors in the treatment of generalized aggressive periodontitis: I. Clinical features and initial outcome. J Clin Periodontol. 2006;33:663–670.
Kamma, JJ, Slots, J. Herpesviral-bacterial interactions in aggressive periodontitis. J Clin Periodontol. 2003;30:420–426.
Kinane, DF, Hart, TC. Genes and genetic polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449.
Lindhe, J, Liljenberg, B. Treatment of localized juvenile periodontitis: results after 5 years. J Clin Periodontol. 1984;11:399–410.
Löe, H, Brown, LJ. Early onset periodontitis in the United States of America. J Periodontol. 1991;62:608–616.
Mongardini, C, van Steenberghe, D, Dekeyser, C, et al. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Long-term clinical observations. J Periodontol. 1999;70:632–645.
Novak, MJ, Novak, KF. Early-onset periodontitis. Curr Opin Periodontol. 1996;3:45–58.
Tonetti, MS, Mombelli, A. Early-onset periodontitis. Ann Periodontol. 1999;4:39–52.
Xajigeorgiou, C, Sakellari, D, Slini, T, et al. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006;33:254–264.
Ahuja, V, Shin, RH, Mudgil, A, et al. Papillon-Lefèvre syndrome: a successful outcome. J Periodontol. 2005;76:1996–2001.
Gorlin, RJ, Sedano, H, Anderson, VE. The syndrome of palmar-plantar hyperkeratosis and premature destruction of the teeth: a clinical and genetic analysis of the Papillon-Lefèvre syndrome. J Pediatr. 1964;65:895–908.
Hart, TC, Hart, PS, Bowden, DW, et al. Mutations of the cathepsin C gene are responsible for Papillon-Lefèvre syndrome. J Med Genet. 1999;36:881–887.
Hart, TC, Hart, PS, Michalec, M, et al. Haim-Munk syndrome and Papillon-Lefèvre syndrome are allelic mutations in cathepsin C. J Med Genet. 2000;37:88–94.
Hattab, FN, Amin, WM. Papillon-Lefèvre syndrome with albinism: a review of the literature and report of 2 brothers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:709–716.
Hewitt, C, McCormick, D, Linden, G. The role of cathepsin C in Papillon-Lefèvre syndrome, prepubertal periodontitis, and aggressive periodontitis. Hum Mutat. 2004;23:222–228.
Lundgren, T, Crossner, C-G, Twetman, S, et al. Systemic retinoid medication and periodontal health in patients with Papillon-Lefèvre syndrome. J Clin Periodontol. 1996;23:176–179.
Noack, B, Görgens, H, Hoffmann, TH, et al. Novel mutations in the cathepsin C gene in patients with pre-pubertal aggressive periodontitis and Papillon-Lefèvre syndrome. J Dent Res. 2004;83:368–370.
Pacheco, JJ, Coelho, C, Salazar, F, et al. Treatment of Papillon-Lefèvre syndrome periodontitis. J Clin Periodontol. 2002;29:370–374.
Wiebe, CB, Häkkinen, L, Putnins, EE, et al. Successful periodontal maintenance of a case with Papillon-Lefèvre syndrome: 12-year follow-up and review of the literature. J Periodontol. 2001;72:824–830.