Chapter 16 Pancreas
See Targeted Therapy available online at studentconsult.com
The contributions of those who authored this chapter in previous editions of this book are gratefully acknowledged.
The pancreas has critical endocrine functions, and the exocrine portion of the pancreas is a major source of potent enzymes that are essential for digestion. Diseases affecting the pancreas can be the source of significant morbidity and mortality. Unfortunately, despite its physiologic importance, the retroperitoneal location of the pancreas and the generally vague nature of signs and symptoms associated with its injury or dysfunction allow many pancreatic diseases to progress undiagnosed for extended periods of time; thus, recognition of pancreatic disorders often requires a high degree of suspicion.
The pancreas is a transversely oriented retroperitoneal organ extending from the so-called C loop of the duodenum to the hilum of the spleen. Although the pancreas does not have well-defined anatomic subdivisions, adjacent vessels and ligaments serve to demarcate the organ into a head, body, and tail.
The pancreas gets its name from the Greek pankreas, meaning “all flesh,” and is a complex lobulated organ with distinct endocrine and exocrine elements. The endocrine portion constitutes only 1% to 2% of the pancreas and is composed of about 1 million cell clusters, the islets of Langerhans; these cells secrete insulin, glucagon, and somatostatin. The most significant disorders of the endocrine pancreas are diabetes mellitus and neoplasms; these are described in detail in Chapter 19 and are not discussed further here.
The exocrine pancreas is composed of acinar cells that produce the digestive enzymes, and the ductules and ducts that convey them to the duodenum. The acinar cells are responsible for the synthesis of digestive enzymes, which are mostly made as inactive pro-enzymes that are stored in zymogen granules. When acinar cells are stimulated to secrete, the granules fuse with the apical plasma membrane and release their contents into the central acinar lumen.These secretions are transported to the duodenum through a series of anastomosing ducts.
The epithelial cells lining the ducts also are active participants in pancreatic secretion: The cuboidal cells lining the smaller ductules secrete bicarbonate-rich fluid, while the columnar cells lining the larger ducts produce mucin. The epithelial cells of the larger ducts also express the cystic fibrosis transmembrane conductance regulator (CFTR); aberrant function of this membrane protein affects the viscosity of the pancreatic secretions and has a fundamental role in the pathophysiology of pancreatic disease in persons with cystic fibrosis (Chapter 6).
As described later, autodigestion of the pancreas (e.g., in pancreatitis) can be a catastrophic event. A number of “fail-safe” mechanisms have evolved to minimize the risk of occurrence of this phenomenon:
• A majority of pancreatic enzymes are synthesized as inactive proenzymes and sequestered in membrane-bound zymogen granules, as mentioned above.
• Activation of proenzymes requires conversion of trypsinogen to trypsin by duodenal enteropeptidase (also called enterokinase).
• Trypsin inhibitors (e.g., SPINK1, also known as pancreatic secretory trypsin inhibitor) also are secreted by acinar and ductal cells.
• Trypsin cleaves and inactivates itself, a negative feedback mechanism that normally puts a limit on local levels of activated trypsin.
• Acinar cells are remarkably resistant to the action of activated enzymes such as trypsin, chymotrypsin, and phospholipase A2.
Diseases of the exocrine pancreas include cystic fibrosis, congenital anomalies, acute and chronic pancreatitis, and neoplasms. Cystic fibrosis is discussed in detail in Chapter 6; the other pathologic processes are discussed in the remainder of this chapter.
Congenital Anomalies
Pancreatic development is a complex process involving fusion of dorsal and ventral primordia; subtle deviations in this process frequently give rise to congenital variations in pancreatic anatomy. While most of these do not cause disease per se, variants (especially in ductal anatomy) can present challenges to the endoscopist and the surgeon. For example, failure to recognize idiosyncratic anatomy could result in inadvertent severing of a pancreatic duct during surgery, resulting in pancreatitis.
Agenesis
Very rarely, the pancreas may be totally absent, a condition usually (but not invariably) associated with additional severe malformations that are incompatible with life. Pancreatic duodenal homeobox 1 is a homeodomain transcription factor critical for normal pancreatic development, and mutations of the PDX1 gene, located on chromosomal locus 13q12.1, have been associated with pancreatic agenesis.
Pancreas Divisum
Pancreas divisum is the most common clinically significant congenital pancreatic anomaly, with an incidence of 3% to 10% in autopsy series. It occurs when the duct systems of the fetal pancreatic primordia fail to fuse. As a result, the main pancreatic duct drains only a small portion of the head of the gland, while the bulk of the pancreas (from the dorsal pancreatic primordium) drains through the minor sphincter, which has a narrow opening. As a result of this defect in drainage, persons with pancreas divisum have elevated intraductal pressures throughout most of the pancreas and are at increased risk for chronic pancreatitis.
Annular Pancreas
Annular pancreas is a relatively uncommon variant of pancreatic fusion in which a ring of pancreatic tissue completely encircles the duodenum. It can manifest with signs and symptoms of duodenal obstruction such as gastric distention and vomiting.
Ectopic Pancreas
Aberrantly situated, or ectopic, pancreatic tissue occurs in about 2% of the population; favored sites are the stomach and duodenum, followed by the jejunum, Meckel diverticulum, and ileum. These embryologic rests typically are small (ranging from millimeters to centimeters in diameter) and are located in the submucosa; they are composed of normal pancreatic acini with occasional islets. Though usually incidental and asymptomatic, ectopic pancreas can cause pain from localized inflammation, or—rarely—can cause mucosal bleeding. Approximately 2% of pancreatic neuroendocrine tumors (Chapter 19) arise in ectopic pancreatic tissue.
Congenital Cysts
Congenital cysts probably result from anomalous development of the pancreatic ducts. In polycystic disease, the kidneys, liver, and pancreas can all contain cysts (Chapter 13). Congenital cysts generally are unilocular and range from microscopic to 5 cm in diameter. They are lined by either uniform cuboidal or flattened epithelium and are enclosed in a thin, fibrous capsule. These benign cysts contain clear serous fluid—an important point of distinction from pancreatic cystic neoplasms, which often are mucinous (see further on).
Pancreatitis
Inflammatory disorders of the pancreas range in severity from mild, self-limited disease to life-threatening, widely destructive process, and are accordingly associated with deficits that may be trivial and transient or serious and permanent. In acute pancreatitis, function can return to normal if the underlying cause of inflammation is removed. By contrast, chronic pancreatitis is defined by irreversible destruction of exocrine pancreatic parenchyma.
Acute Pancreatitis
Acute pancreatitis is a reversible inflammatory disorder that varies in severity, ranging from focal edema and fat necrosis to widespread hemorrhagic parenchymal necrosis. Acute pancreatitis is relatively common, with an annual incidence of 10 to 20 per 100,000 people in the Western world. Approximately 80% of cases are attributable to either biliary tract disease or alcoholism (Table 16–1). Roughly 5% of patients with gallstones develop acute pancreatitis, and gallstones are implicated in 35% to 60% of cases overall. Excessive alcohol intake has been reported as a cause of acute pancreatitis at variable rates, from 65% of cases in the United States to 5% or less in the United Kingdom.
Table 16–1 Etiologic Factors in Acute Pancreatitis
| Metabolic |
| Genetic |
| Mutations in the cationic trypsinogen (PRSS1) and trypsin inhibitor (SPINK1) genes |
| Mechanical |
| Vascular |
| Infectious |
* Most common causes in the United States.
Other causes of acute pancreatitis include
• Non–gallstone-related obstruction of the pancreatic ducts (e.g., due to periampullary neoplasms such as pancreatic cancer, pancreas divisum, biliary “sludge,” or parasites, particularly Ascaris lumbricoides and Clonorchis sinensis)
• Medications including anticonvulsants, cancer chemotherapeutic agents, thiazide diuretics, estrogens, and more than 85 others in clinical use
• Infections with mumps virus or coxsackievirus
• Metabolic disorders, including hypertriglyceridemia, hyperparathyroidism, and other hypercalcemic states
• Ischemia due to vascular thrombosis, embolism, vasculitis, or shock
• Trauma, both blunt force and iatrogenic during surgery or endoscopy
• Inherited mutations in genes encoding pancreatic enzymes or their inhibitors (e.g., SPINK1). For example, hereditary pancreatitis is an autosomal dominant disease with 80% penetrance that is characterized by recurrent attacks of severe pancreatitis, usually beginning in childhood. It is caused by mutations in the gene PRSS1, which encodes trypsinogen, the proenzyme of pancreatic trypsin. The pathogenic mutations alter the site through which trypsin cleaves and inactivates itself, abrogating an important negative feedback mechanism. This defect leads not only to the hyperactivation of trypsin, but also to the hyperactivation of many other digestive enzymes that require trypsin cleavage for their activation. As a result of this unbridled protease activity, the pancreas is prone to autodigestion and injury.
Of note, 10% to 20% of cases of acute pancreatitis have no identifiable cause (idiopathic pancreatitis), although a growing body of evidence suggests that many may have an underlying genetic basis.
![]() Morphology
Morphology
The basic alterations in acute pancreatitis are (1) microvascular leakage causing edema, (2) necrosis of fat by lipases, (3) an acute inflammatory reaction, (4) proteolytic destruction of pancreatic parenchyma, and (5) destruction of blood vessels leading to interstitial hemorrhage.
In milder forms, histologic alterations include interstitial edema and focal areas of fat necrosis in the pancreatic substance and peripancreatic fat (Fig. 16–1, A). Fat necrosis results from enzymatic destruction of fat cells; the released fatty acids combine with calcium to form insoluble salts that precipitate in situ.
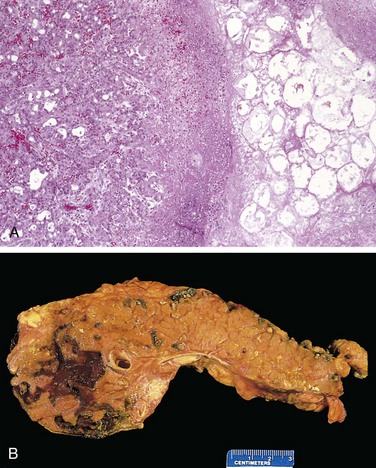
Figure 16–1 Acute pancreatitis. A, The microscopic field shows a region of fat necrosis (right) and focal pancreatic parenchymal necrosis (center). B, The pancreas has been sectioned longitudinally to reveal dark areas of hemorrhage in the pancreatic substance and a focal area of pale fat necrosis in the peripancreatic fat (upper left).
In more severe forms, such as acute necrotizing pancreatitis, necrosis of pancreatic tissue affects acinar and ductal tissues as well as the islets of Langerhans; vascular damage causes hemorrhage into the parenchyma of the pancreas. Macroscopically, the pancreas exhibits red-black hemorrhagic areas interspersed with foci of yellow-white, chalky fat necrosis (Fig. 16–1, B). Fat necrosis also can occur in extrapancreatic fat, including the omentum and bowel mesentery, and even outside the abdominal cavity (e.g., in subcutaneous fat). In most cases the peritoneum contains a serous, slightly turbid, brown-tinged fluid with globules of fat (derived from enzymatically digested adipose tissue). In the most severe form, hemorrhagic pancreatitis, extensive parenchymal necrosis is accompanied by diffuse hemorrhage within the substance of the gland.
![]() Pathogenesis
Pathogenesis
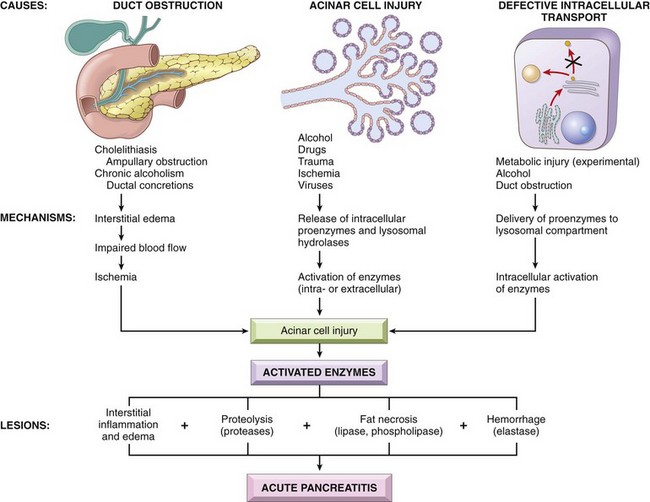
The histologic changes seen in acute pancreatitis strongly suggest autodigestion of the pancreatic substance by inappropriately activated pancreatic enzymes. As described previously, the zymogen forms of pancreatic enzymes must be enzymatically cleaved to be activated; trypsin is central in this process, so activation of trypsin is a critical triggering event in acute pancreatitis. If trypsin is inappropriately generated from its proenzyme trypsinogen, it can activate itself as well as other proenzymes (e.g., phospholipases and elastases) that can then take part in the process of autodigestion. Trypsin also converts prekallikrein to its activated form, thus sparking the kinin system, and, by activation of factor XII (Hageman factor), also sets in motion the clotting and complement systems (Chapter 2). Three pathways can incite the initial enzyme activation that may lead to acute pancreatitis (Fig. 16–2):
• Pancreatic duct obstruction. Impaction of a gallstone or biliary sludge, or extrinsic compression of the ductal system by a mass blocks ductal flow, increases intraductal pressure, and allows accumulation of an enzyme-rich interstitial fluid. Since lipase is secreted in an active form, local fat necrosis may result. Injured tissues, periacinar myofibroblasts, and leukocytes then release pro-inflammatory cytokines that promote local inflammation and interstitial edema through a leaky microvasculature. Edema further compromises local blood flow, causing vascular insufficiency and ischemic injury to acinar cells.
• Primary acinar cell injury. This pathogenic mechanism comes into play in acute pancreatitis caused by ischemia, viral infections (e.g., mumps), drugs, and direct trauma to the pancreas.
• Defective intracellular transport of proenzymes within acinar cells. In normal acinar cells, digestive enzymes intended for zymogen granules (and eventually extracellular release) and hydrolytic enzymes destined for lysosomes are transported in discrete pathways after synthesis in the endoplasmic reticulum. However, at least in some animal models of metabolic injury, pancreatic proenzymes and lysosomal hydrolases become packaged together. This results in proenzyme activation, lysosomal rupture (action of phospholipases), and local release of activated enzymes. The role of this mechanism in human acute pancreatitis is not clear.
Alcohol consumption may cause pancreatitis by several mechanisms. Alcohol transiently increases pancreatic exocrine secretion and contraction of the sphincter of Oddi (the muscle regulating the tone at the ampulla of Vater). Alcohol also has direct toxic effects on acinar cells, including induction of oxidative stress in acinar cells, which leads to membrane damage (see below). Finally, chronic alcohol ingestion results in the secretion of protein-rich pancreatic fluid, which leads to the deposition of inspissated protein plugs and obstruction of small pancreatic ducts.
Clinical Features
Abdominal pain is the cardinal manifestation of acute pancreatitis. Its severity varies from mild and uncomfortable to severe and incapacitating. Suspected acute pancreatitis is diagnosed primarily by the presence of elevated plasma levels of amylase and lipase and the exclusion of other causes of abdominal pain. In 80% of cases acute pancreatitis is mild and self limiting; the remaining 20% develop severe disease.
Full-blown acute pancreatitis constitutes a medical emergency of the first magnitude. Affected persons usually experience the sudden calamitous onset of an “acute abdomen” with pain, abdominal guarding, and the ominous absence of bowel sounds. Characteristically, the pain is constant and intense and often is referred to the upper back; it must be differentiated from pain of other causes such as perforated peptic ulcer, biliary colic, acute cholecystitis with rupture, and occlusion of mesenteric vessels with infarction of the bowel.
The manifestations of severe acute pancreatitis are attributable to systemic release of digestive enzymes and explosive activation of the inflammatory response. The initial clinical evaluation may reveal leukocytosis, disseminated intravascular coagulation (Chapter 11), acute respiratory distress syndrome (due to alveolar capillary injury) (Chapter 12), and diffuse fat necrosis. Peripheral vascular collapse (shock) can rapidly ensue as a result of increased microvascular permeability and resultant hypovolemia, compounded by endotoxemia (from breakdown of the barriers between gastrointestinal flora and the bloodstream), and renal failure due to acute tubular necrosis (Chapter 13).
Laboratory findings include markedly elevated serum amylase during the first 24 hours, followed (within 72 to 96 hours) by rising serum lipase levels. Hypocalcemia can result from precipitation of calcium in areas of fat necrosis; if persistent, it is a poor prognostic sign. The enlarged inflamed pancreas can be visualized by computed tomography (CT) or magnetic resonance imaging (MRI).
The crux of the management of acute pancreatitis is supportive therapy (e.g., maintaining blood pressure and alleviating pain) and “resting” the pancreas by total restriction of food and fluids. In 40% to 60% of cases of acute necrotizing pancreatitis, the necrotic debris becomes infected, usually by gram-negative organisms from the alimentary tract, further complicating the clinical course. Although most persons with acute pancreatitis eventually recover, some 5% die from shock during the first week of illness; acute respiratory distress syndrome and acute renal failure are ominous complications. In surviving patients, sequelae include sterile or infected pancreatic “abscesses” or pancreatic pseudocysts.
Pancreatic Pseudocysts
A common sequela of acute pancreatitis (and in particular, alcoholic pancreatitis) is a pancreatic pseudocyst. Liquefied areas of necrotic pancreatic tissue become walled off by fibrous tissue to form a cystic space, lacking an epithelial lining (hence the designation pseudo). The cyst contents are rich in pancreatic enzymes, and a laboratory assessment of the cyst aspirate can be diagnostic. Pseudocysts account for approximately 75% of all pancreatic cysts. While many pseudocysts spontaneously resolve, they can become secondarily infected, and larger pseudocysts can compress or even perforate into adjacent structures.
![]() Morphology
Morphology
Pseudocysts usually are solitary; they commonly are attached to the surface of the gland and involve peripancreatic tissues such as the lesser omental sac or the retroperitoneum between the stomach and transverse colon or liver (Fig. 16–3, A). They can range in diameter from 2 cm to 30 cm. Since pseudocysts form by walling off areas of hemorrhagic fat necrosis, they typically are composed of necrotic debris encased by fibrous walls of granulation tissue lacking an epithelial lining (Fig. 16–3, B).
Chronic Pancreatitis
Chronic pancreatitis is characterized by long-standing inflammation, fibrosis, and destruction of the exocrine pancreas; in its late stages, the endocrine parenchyma also is lost. Although chronic pancreatitis can result from recurrent bouts of acute pancreatitis, the chief distinction between acute and chronic pancreatitis is the irreversible impairment in pancreatic function in the latter. The prevalence of chronic pancreatitis is difficult to determine but probably ranges between 0.04% and 5% of the U.S. population. By far the most common cause of chronic pancreatitis is long-term alcohol abuse; middle-aged men constitute the bulk of patients in this etiologic group. Less common causes of chronic pancreatitis include
• Long-standing pancreatic duct obstruction (e.g., by pseudocysts, calculi, neoplasms, or pancreas divisum)
• Tropical pancreatitis, a poorly characterized heterogeneous disorder seen in Africa and Asia, with a subset of cases having a genetic basis
• Hereditary pancreatitis due to mutations in the pancreatic trypsinogen gene (PRRS1) (see Table 16–1 earlier), or the SPINK1 gene encoding a trypsin inhibitor
• Chronic pancreatitis associated with CFTR mutations. As discussed in detail in Chapter 6, cystic fibrosis is caused by mutations in the CFTR gene; the CFTR protein also is expressed in pancreatic ductal epithelium, and CFTR mutations decrease bicarbonate secretion and increase the viscosity of the secretions, thereby promoting protein plugging.
As many as 40% of persons with chronic pancreatitis have no recognizable predisposing factors. As with acute pancreatitis, however, a growing number of these “idiopathic” cases are associated with inherited mutations in genes important for normal pancreatic exocrine function. For example, genetic testing reveals that 25% to 30% of patients with “idiopathic” pancreatitis harbor germline mutations in the CFTR gene, albeit distinct from the ones that lead to classic multisystem cystic fibrosis (Chapter 6).
![]() Morphology
Morphology
Chronic pancreatitis is characterized by parenchymal fibrosis, reduced number and size of acini, and variable dilation of the pancreatic ducts; there is a relative sparing of the islets of Langerhans (Fig. 16–4, A). Acinar loss is a constant feature, usually with a chronic inflammatory infiltrate around remaining lobules and ducts. The ductal epithelium may be atrophied or hyperplastic or exhibit squamous metaplasia, and ductal concretions may be noted (Fig. 16–4, B). The remaining islets of Langerhans become embedded in the sclerotic tissue and may fuse and appear enlarged; eventually they also disappear. On gross evaluation, the gland is hard, sometimes with extremely dilated ducts and visible calcified concretions.
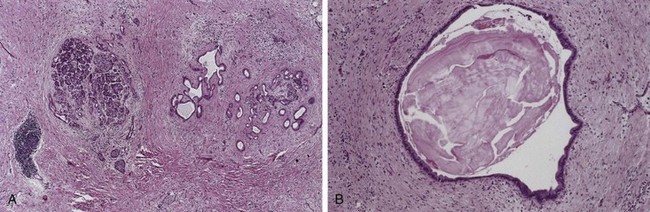
Figure 16–4 Chronic pancreatitis. A, Extensive fibrosis and atrophy has left only residual islets (left) and ducts (right), with a sprinkling of chronic inflammatory cells and acinar tissue. B, A higher-power view demonstrating dilated ducts with inspissated eosinophilic concretions in a patient with alcoholic chronic pancreatitis.
Autoimmune pancreatitis (AIP) is a distinct form of chronic pancreatitis that is characterized by one of two morphologic patterns: (1) striking infiltration of the pancreas by lymphoplasmacytic cells, many of which are positive for IgG4, accompanied by a “swirling” fibrosis and venulitis (lymphoplasmacytic sclerosing pancreatitis), or (2) a duct-centric mixed infiltrate composed of neutrophils, lymphocytes and plasma cells, often obliterating the ductal epithelium (idiopathic duct centric pancreatitis). IgG4-related autoimmune pancreatitis is a multisystem disease and may be one manifestation of IgG4-associated fibrosing disorders (Chapter 4). Recognition of autoimmune pancreatitis in both its forms is important, because it can mimic pancreatic cancer and also because it responds to steroid therapy.
![]() Pathogenesis
Pathogenesis
Although the pathogenesis of chronic pancreatitis is not well defined, several hypotheses are proposed:
• Ductal obstruction by concretions. Many of the inciting agents in chronic pancreatitis (e.g., alcohol) increase the protein concentration of pancreatic secretions, and these proteins can form ductal plugs.
• Toxic-metabolic. Toxins, including alcohol and its metabolites, can exert a direct toxic effect on acinar cells, leading to lipid accumulation, acinar cell loss, and eventually parenchymal fibrosis.
• Oxidative stress. Alcohol-induced oxidative stress may generate free radicals in acinar cells, leading to membrane damage (Chapter 1), and subsequent expression of chemokines like interleukin-8 (IL-8), which recruits mononuclear inflammatory cells. Oxidative stress also promotes the fusion of lysosomes and zymogen granules with resulting acinar cell necrosis, inflammation, and fibrosis.
In contrast with acute pancreatitis, a variety of profibrogenic cytokines, such as transforming growth factor-β (TGF-β), connective tissue growth factor, and platelet-derived growth factor, are secreted in chronic pancreatitis. These cytokines induce the activation and proliferation of periacinar myofibroblasts (“pancreatic stellate cells”), which deposit collagen and are instrumental in the pathogenesis of fibrosis.
Clinical Features
Chronic pancreatitis manifests in several different ways. It may announce itself with repeated bouts of jaundice, vague indigestion, or persistent or recurrent abdominal and back pain, or it may be entirely silent until pancreatic insufficiency and diabetes mellitus develop (the latter as a consequence of islet destruction). Attacks can be precipitated by alcohol abuse, overeating (increases demand on pancreatic secretions), or opiates or other drugs that increase the muscle tone of the sphincter of Oddi.
The diagnosis of chronic pancreatitis requires a high degree of clinical suspicion. During an attack of abdominal pain, there may be mild fever and modest elevations of serum amylase. In end-stage disease, however, acinar destruction may be so advanced that enzyme elevations are absent. Gallstone-induced obstruction may manifest as jaundice or elevated levels of serum alkaline phosphatase. A very helpful finding is visualization of calcifications within the pancreas by CT or ultrasonography. Weight loss and hypoalbuminemic edema from malabsorption caused by pancreatic exocrine insufficiency also can point to the disease.
Although chronic pancreatitis is usually not acutely life-threatening, the long-term outlook is poor, with a 50% mortality rate over 20 to 25 years. Severe pancreatic exocrine insufficiency and chronic malabsorption may develop, as can diabetes mellitus. In other patients, severe chronic pain may dominate the clinical picture. Pancreatic pseudocysts (described earlier) develop in about 10% of patients. Persons with hereditary pancreatitis have a 40% lifetime risk of developing pancreatic cancer. The degree to which other forms of chronic pancreatitis contribute to cancer development is unclear.
![]() Summary
Summary
Pancreatitis
• Acute pancreatitis is characterized by inflammation and reversible parenchymal damage that ranges from focal edema and fat necrosis to widespread parenchymal necrosis and hemorrhage; the clinical presentation varies widely, from mild abdominal pain to rapidly fatal vascular collapse.
• Chronic pancreatitis is characterized by irreversible parenchymal damage and scar formation; clinical presentations include chronic malabsorption (due to pancreatic exocrine insufficiency) and diabetes mellitus (due to islet cell loss).
• Both entities share similar pathogenic mechanisms, and indeed recurrent acute pancreatitis can result in chronic pancreatitis. Ductal obstruction and long-term alcohol abuse are the most common causes in both forms. Inappropriate activation of pancreatic digestive enzymes (due to mutations in genes encoding trypsinogen or trypsin inhibitors) and primary acinar injury (due to toxins, infections, ischemia, or trauma) also cause pancreatitis.
Pancreatic Neoplasms
Pancreatic exocrine neoplasms can be cystic or solid. Some tumors are benign, while others are among the most lethal of all malignancies.
Cystic Neoplasms
Only 5% to 15% of all pancreatic cysts are neoplastic; these constitute less than 5% of all pancreatic neoplasms. Some of these are entirely benign (e.g., serous cystadenoma); others, such as mucinous cystic neoplasms, can be benign or malignant.
Serous Cystadenomas
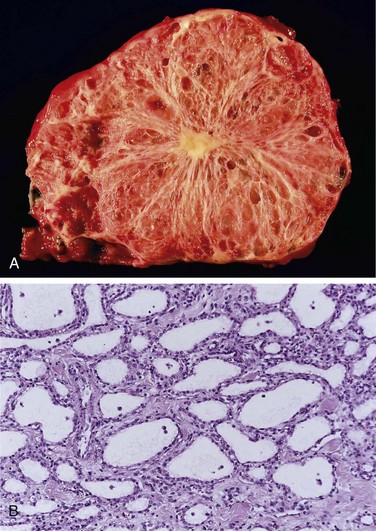
Serous cystadenomas account for approximately 25% of all pancreatic cystic neoplasms; they are composed of glycogen-rich cuboidal cells surrounding small cysts containing clear, straw-colored fluid (Fig. 16–5). The tumors typically manifest in the seventh decade of life with nonspecific symptoms such as abdominal pain; the female-to-male ratio is 2 : 1. These tumors are almost uniformly benign, and surgical resection is curative in the vast majority of patients. Most serous cystadenomas carry somatic mutations of the von Hippel-Lindau (VHL) tumor suppressor gene, the product of which binds to hypoxia-inducible factor 1 alpha (HIF1alpha) and results in its degradation (Chapter 5).
Mucinous Cystic Neoplasms
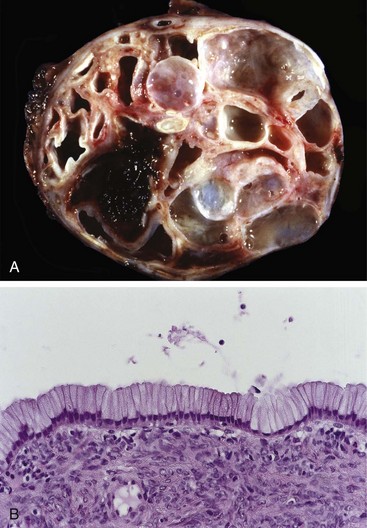
Close to 95% of mucinous cystic neoplasms arise in women, usually in the body or tail of the pancreas, and manifest as painless, slow-growing masses. The cystic spaces are filled with thick, tenacious mucin, and the cysts are lined by a columnar mucinous epithelium with an associated densely cellular stroma resembling that of the ovary (Fig. 16–6). Based on the degree of cytologic and architectural atypia in the lining epithelium, noninvasive mucinous cystic neoplasms are classified as harboring low-grade, moderate, or severe dysplasia. Up to one third of these cysts can be associated with an invasive adenocarcinoma. Distal pancreatectomy for noninvasive cysts typically is curative, even in the setting of severe dysplasia.
Intraductal Papillary Mucinous Neoplasms
Intraductal papillary mucinous neoplasms (IPMNs) are mucin-producing intraductal neoplasms. In contrast with mucinous cystic neoplasms, IPMNs occur more frequently in men than in women and more frequently involve the head of the pancreas. IPMNs arise in the main pancreatic ducts, or one of its major branch ducts, and lack the cellular stroma seen in mucinous cystic neoplasms (Fig. 16–7). As with mucinous cystic neoplasms, the epithelia of noninvasive IPMNs harbor various grades of dysplasia, and a subset of lesions is associated with an invasive adenocarcinoma component. Notably, up to two thirds of IPMNs harbor oncogenic mutations of GNAS on chromosome 20q13, which encodes the alpha subunit of a stimulatory G-protein, Gs (Chapter 19). Constitutive activation of this G-protein is predicted to result in an intracellular cascade that promotes cell proliferation.
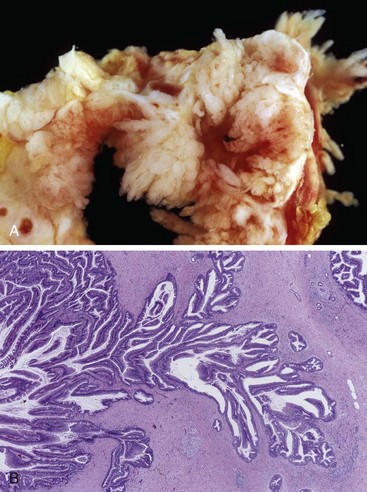
Figure 16–7 Intraductal papillary mucinous neoplasm. A, Cross-section through the head of the pancreas showing a prominent papillary neoplasm distending the main pancreatic duct. B, The papillary mucinous neoplasm involved the main pancreatic duct (left) and is extending down into the smaller ducts and ductules (right).
Pancreatic Carcinoma
Infiltrating ductal adenocarcinoma of the pancreas (more commonly referred to as “pancreatic cancer”) is the fourth leading cause of cancer death in the United States, preceded only by lung, colon, and breast cancers. Although it is substantially less common than the other three malignancies, pancreatic carcinoma is near the top of the list of killers because it carries one of the highest mortality rates. Over 44,000 Americans were diagnosed with pancreatic cancer in 2010, and virtually all will die of it; the 5-year survival rate is dismal—less than 5%. Sadly, Ralph Steinman, one of the 2011 Nobel Laureates in physiology or medicine died of pancreatic cancer, three days before the announcement of his award.
![]() Pathogenesis
Pathogenesis
Like all cancers, pancreatic cancer arises as a consequence of inherited and acquired mutations in cancer-associated genes. In a pattern analogous to that seen in the multistep progression of colon cancer (Chapter 5), there is a progressive accumulation of genetic changes in pancreatic epithelium as it proceeds from non-neoplastic, to noninvasive precursor lesions, to invasive carcinoma (Fig. 16–8). While both intraductal papillary mucinous neoplasms and mucinous cystic neoplasms can progress to invasive adenocarcinoma and are thus considered bona fide precursors of cancer (as noted earlier), the most common antecedent lesions of pancreatic cancer arise in small ducts and ductules, and are called pancreatic intraepithelial neoplasias (PanINs). Evidence in favor of the precursor relationship of PanINs to frank malignancy includes the fact that these microscopic lesions often are found adjacent to infiltrating carcinomas and the two share a number of genetic alterations. Moreover, the epithelial cells in PanINs show dramatic telomere shortening, potentially predisposing these lesions to accumulating additional chromosomal abnormalities on their way to becoming invasive carcinoma.
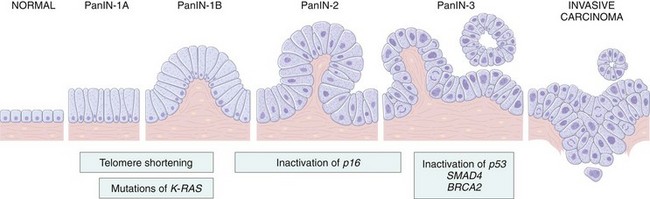
Figure 16–8 Progression model for the development of pancreatic cancer. It is postulated that telomere shortening and mutations of the oncogene K-RAS occur at early stages, inactivation of the p16 tumor suppressor gene occurs at intermediate stages, and the inactivation of the TP53, SMAD4, and BRCA2 tumor suppressor genes occurs at late stages. Note that while there is a general temporal sequence of changes, the accumulation of multiple mutations is more important than their occurrence in a specific order. PanIN, pancreatic intraepithelial neoplasm. The numbers following the labels on the top refer to stages in the development of PanINs.
(Modified from Maitra A, Hruban RH: Pancreatic cancer. Annu Rev Pathol Mech Dis 3:157, 2008.)
The recent sequencing of the pancreatic cancer genome has confirmed that four genes are most commonly affected by somatic mutations in this neoplasm: KRAS, CDKNA2A/p16, SMAD4, and TP53:
• KRAS is the most frequently altered oncogene in pancreatic cancer; it is activated by a point mutation in 80% to 90% of cases. These mutations impair the intrinsic GTPase activity of the Kras protein so that it is constitutively active. In turn, Kras activates a number of intracellular signaling pathways (“Kras effectors”) that promote carcinogenesis (Chapter 5).
• The p16 (CDKN2A) gene is the most frequently inactivated tumor suppressor gene in pancreatic cancer, being turned off in 95% of cases. The p16 protein has a critical role in cell cycle control; inactivation removes an important checkpoint.
• The SMAD4 tumor suppressor gene is inactivated in 55% of pancreatic cancers and only rarely in other tumors; it codes for a protein that plays an important role in signal transduction downstream of the transforming growth factor-β receptor.
• Inactivation of the TP53 tumor suppressor gene occurs in 50% to 70% of pancreatic cancers. Its gene product, p53, acts both to enforce cell cycle checkpoints and as an inducer of apoptosis or senescence (Chapter 5).
• Mutations of VHL or GNAS, found in aforementioned pancreatic cysts, have not been described in ductal adenocarcinomas, and provide a likely basis for the widely different histopathology and natural history of these lesions.
What causes these molecular changes is unknown. Pancreatic cancer is primarily a disease of the elderly population, with 80% of cases occurring between the ages of 60 and 80. The strongest environmental influence is smoking, which doubles the risk. Chronic pancreatitis and diabetes mellitus are also both associated with an increased risk of pancreatic cancer. It is difficult to sort out whether chronic pancreatitis is the cause of pancreatic cancer or an effect of the disease, since small pancreatic cancers can block the pancreatic duct and thereby produce chronic pancreatitis. On the other hand, as discussed in Chapter 5, chronic inflammation is now considered an enabler of malignancy. Likewise, the basis of the association of diabetes mellitus with pancreatic cancer is also unclear, since diabetes can occur as a consequence of pancreatic cancer, and in fact, new-onset diabetes in an elderly patient may be the first sign of this malignancy. Familial clustering of pancreatic cancer has been reported, and a growing number of inherited genetic defects are now recognized that increase pancreatic cancer risk. For example, germline mutations of the familial breast/ovarian cancer gene BRCA2 are seen in approximately 10% of cases arising in persons of Ashkenazi Jewish heritage.
![]() Morphology
Morphology
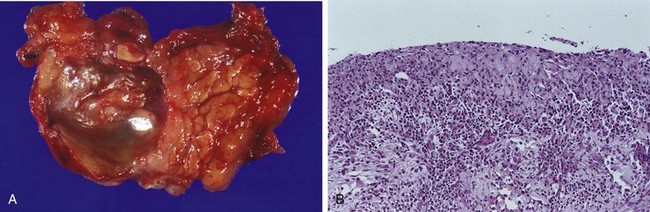
Approximately 60% of pancreatic cancers arise in the head of the gland, 15% in the body, and 5% in the tail; in the remaining 20%, the neoplasm diffusely involves the entire organ. Carcinomas of the pancreas usually are hard, gray-white, stellate, poorly defined masses (Fig. 16–9, A).
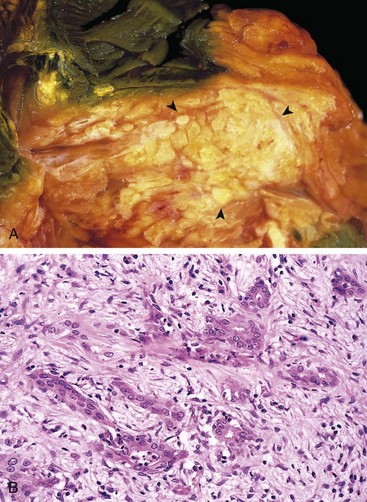
Figure 16–9 Carcinoma of the pancreas. A, A cross-section through the head of the pancreas and adjacent common bile duct showing both an ill-defined mass in the pancreatic substance (arrowheads) and the green discoloration of the duct resulting from total obstruction of bile flow. B, Poorly formed glands are present in a densely fibrotic (desmoplastic) stroma within the pancreatic substance.
The vast majority of carcinomas are ductal adenocarcinomas, recapitulating to some degree the normal duct epithelium by forming glands and secreting mucin. Two features are characteristic of pancreatic cancer: It is highly invasive (even “early” invasive pancreatic cancers invade peripancreatic tissues extensively), and it elicits an intense non-neoplastic host reaction composed of fibroblasts, lymphocytes, and extracellular matrix (desmoplastic response).
Most carcinomas of the head of the pancreas obstruct the distal common bile duct as it courses through the head of the pancreas. In 50% of such cases, there is marked distention of the biliary tree, and patients typically exhibit jaundice. In marked contrast, carcinomas of the body and tail of the pancreas do not impinge on the biliary tract and hence remain silent for some time. They may be quite large and widely disseminated by the time they are discovered. Pancreatic cancers often extend through the retroperitoneal space, entrapping adjacent nerves (thus, accounting for the pain), and occasionally invade the spleen, adrenals, vertebral column, transverse colon, and stomach. Peripancreatic, gastric, mesenteric, omental, and portahepatic lymph nodes frequently are involved, and the liver often is enlarged as a consequence of metastatic deposits. Distant metastases may occur, principally to the lungs and bones.
On microscopic examination, pancreatic carcinoma usually is a moderately to poorly differentiated adenocarcinoma forming abortive tubular structures or cell clusters and exhibiting an aggressive, deeply infiltrative growth pattern (Fig. 16–9, B). Dense stromal fibrosis accompanies tumor invasion, and there is a proclivity for perineural invasion within and beyond the organ. Lymphatic invasion also is commonly seen.
Less common variants of pancreatic cancer include adenosquamous carcinomas with focal squamous differentiation in addition to glandular differentiation; and undifferentiated carcinomas with osteoclast-like giant cells of monocytic lineage intermixed within the neoplasm.
Clinical Features
Carcinomas of the pancreas typically remain silent until their extension impinges on some other structure. Pain usually is the first symptom, but by that point these cancers are often beyond cure. Obstructive jaundice can be associated with carcinoma in the head of the pancreas, but it rarely draws attention to the cancer soon enough for timely intervention. Weight loss, anorexia, and generalized malaise and weakness are manifestations of advanced disease. Migratory thrombophlebitis (Trousseau syndrome) occurs in about 10% of patients and is attributable to the elaboration of platelet-aggregating factors and pro-coagulants from the tumor or its necrotic products (Chapter 3).
The clinical course of pancreatic carcinoma is rapidly progressive and distressingly brief. Less than 20% of pancreatic cancers are resectable at the time of diagnosis. It has long been recognized that there is a profound need for biomarkers capable of detecting early, potentially curable, pancreatic cancers. Although serum levels of many enzymes and antigens (e.g., carcinoembryonic and CA19-9 antigens) are elevated, these markers are neither specific nor sensitive enough to be useful for screening. Several imaging techniques, such as endoscopic ultrasonography and high-resolution CT scans, are helpful for investigation in cases of suspected cancer but are not useful as screening tests.
![]() Summary
Summary
Pancreatic Neoplasms
• Pancreatic cancer probably arises from noninvasive precursor lesions (most commonly, PanINs), developing by progressive accumulation of characteristic mutations of oncogenes (e.g., KRAS) and tumor suppressor genes (e.g., CDKN2A/p16, TP53, and SMAD4).
• Typically, these neoplasms are ductal adenocarcinomas that produce an intense desmoplastic response.
• Most pancreatic cancers are diagnosed at an advanced stage, accounting for the high mortality rate.
• Obstructive jaundice is a feature of carcinoma of the head of the pancreas; many patients also experience debilitating pain.
Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2009;10:63. [A comprehensive review on the basic science aspects of chronic pancreatitis, including genetic causes.]
DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2009;25:454. [A clinically oriented review on the natural history and management of chronic pancreatitis.]
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605. [An outstanding clinically oriented update on pancreatic cancer, including newly emerging molecular targets for therapy.]
Matthaei H, et al. Cystic precursors to invasive pancreatic cancer. Nat Rev. Gastroenterol Hepatol. 2011;8:141. [A comprehensive review on pancreatic cysts and their clinical relevance.]
Sand J, Nordback I. Acute pancreatitis: risk of recurrence and late consequences of the disease. Nat Rev Gastroenterol Hepatol. 2009;6:470. [An outstanding review on the late complications of acute pancreatitis, such as pseudocysts, and the progression to chronic pancreatic insufficiency.]
Sugumar A, Chari ST. Autoimmune pancreatitis. J Gastroenterol Hepatol. 2011;26:1368. [An excellent update on this newly emerging form of chronic pancreatitis, including a discussion of the major subtypes.]
Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607. [A state of the art clinical and pathologic review on pancreatic cancer.]
Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131. [An excellent review on the pathogenesis of pancreatitis in the setting of alcohol and nicotine exposure, including the contribution of genetic susceptibility.]