37 GENES, GENETIC DISEASES AND THE ENVIRONMENT
INTRODUCTION
The nature of disease is highly variable. Some diseases are evident from birth, but most develop at a later stage in the life span, due to exposure to infectious agents and environmental factors. Many diseases are caused by exposure to harmful environmental influences, ranging from toxic chemicals such as asbestos in old building materials and heavy metals (such as lead) in paints and contaminated soils, to poorly designed work practices that promote the development of repetitive strain injuries. The government takes action to prevent diseases resulting from such harmful environmental influences by public education and, where necessary, legislation.
Most infectious diseases are acute and transient: the activities of a healthy immune system eliminate the infectious agent and the affected individual recovers from the disease. However, chronic diseases cause symptoms that persist throughout the affected individual’s life and may be directly responsible for their death. The genetic components of disease, known as disease alleles (refer to Chapter 5), are often mutant forms of normal alleles that produce abnormal proteins, which either function incorrectly or fail to function at all (see Figure 37-1A). The absence of normal function leads to disease. For example, cystic fibrosis results from the presence of a defective ion transport protein, and haemophilia is due to the absence of a blood clotting factor. In many instances, diseases arise due to a combination of genetic and environmental factors (see Figure 37-1B and C). Certain combinations of genes can make an individual more likely to develop a disease if they are exposed to particular external influences.
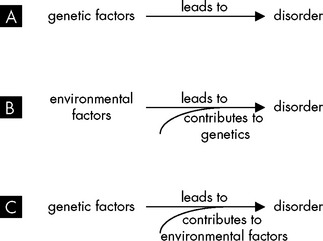
FIGURE 37-1 The contribution of genetics and the environment in the development of disorders.
A A genetic abnormality may lead directly to a disorder, such as an abnormal number of chromosomes. B Environmental factors may lead to development of a disorder, and genetic factors may modify the effects of the environment. C Environmental factors may modify the effects of genes in the development of disease.
Source: Based on Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature Rev Neurosci 2006; 7:583–590.
To illustrate, consider the effects of smoking. Smoking was implicated in the deaths of 14,900 Australians in 2004–2005.1 As you would expect, almost all of those who died were smokers, but the causes of death were quite varied: 40% died of lung cancer, 26% died from chronic obstructive pulmonary disease, 18% died from vascular disease and 10% died from other cancers (stomach, pancreas, kidney, bladder and cervix). Different individuals develop different diseases in response to the same environmental influence (in this case smoking) because their genetic make-up is different. Interestingly, statistical data suggest that smoking actually reduces the risk of death from Parkinson’s disease2 and endometrial cancer.3 However, smoking does not prevent the deaths of all smokers affected with Parkinson’s disease or endometrial cancer — and as a result of smoking these individuals are at increased risk of dying from smoking-related causes. This death toll also includes 56 babies exposed to maternal smoking and 113 non-smoking adults whose deaths from lung cancer or heart disease were attributed to passive (second-hand) smoking. This demonstrates that the individual who ultimately suffers the disease cannot always control lifestyle factors; this is why legislation was introduced to ban smoking in many public venues and workplaces.
In this chapter we consider the genetic and chromosomal abnormalities, as well as the lifestyle and environmental influences, that impact on the appearance of disease. You may already be familiar with some of these concepts through the phrase ‘nature versus nurture’, which asks whether a particular characteristic is evident in a person due to nature (their genetic make-up) or nurture (external influences on the individual, such as nutrition, exercise or the parenting style they experienced). Although some conditions are clearly defined as having a genetic cause, many diseases develop due to a combination of genetic predisposition and environmental influences.
We also investigate the complex relationship among genotype (the genes of the individual), environmental and lifestyle influences, and phenotype (the actual expression of characteristics of the individual, such as the presence of disease). We continue with our focus on diseases that are relatively common in Australia and New Zealand.
STUDYING HEALTH AND DISEASE
Studying populations
Population studies can help identify risk factors that increase the likelihood that an individual will develop a particular disease. Risk factors may be personal characteristics (such as age, sex or blood pressure), behavioural characteristics (such as smoking or participation in exercise activities) or external conditions (such as work environment or home address). To determine relative risk, individuals in the population under study are divided into two groups: those with the characteristic in question and those without. Comparing the incidence of disease between the two groups enables factors that may increase or decrease the risk of developing disease to be identified.
Such data collection is undertaken by a number of government organisations and networks. The Australian Department of Health and Ageing and the New Zealand Ministry of Health, which control the regulatory frameworks within which the healthcare systems operate in each country and coordinate the work of many other government organisations, have a strong focus on promoting healthy lifestyles, preventing disease and developing early intervention strategies.
In 2006–2007, the New Zealand Health Survey, ‘A portrait of health’,4 considered the distribution of a wide range of physical and mental health conditions, risk factors and the use of healthcare services according to parameters such as gender, age group, ethnic group and geographical region. In Australia, similar information is found in the Australian Institute of Health and Welfare’s publication, ‘Australia’s health 2008’.5
In some instances, hospitals collaborate to produce large data sets. For example, since 1999 the Australian and New Zealand Neonatal Network has been collecting data on all high-risk neonates admitted to level II and level II neonatal intensive care units throughout Australia and New Zealand.6 These data are used to identify trends in reasons for admissions, to monitor the performance of participating neonatal intensive care units units, and to contribute to the evaluation of new therapies and treatments.
However, the study of populations only partly explains the development of diseases and disorders. Just because an individual or a particular group in the population has risk factors does not mean that the disease will develop in these people. Rather, the presence of risk factors means that the individual is more likely to develop a disease or disorder compared with individuals without the risk factor. Often we hear stories about elderly people who smoked all their life and did not develop lung cancer or who followed poor dietary choices and did not develop diabetes, cancer or obesity. Yet some people who follow a healthy diet and exercise regularly get heart disease — is all that exercise a waste of time? Such stories abound and often there is confusion about their significance. The reality is that the influence of genes and how they interact with the lifestyle or environment predict the likelihood of disease development. While scientists do not have all the answers as to why the environment influences some genes and not others, there is an increasing body of knowledge that specifically links genetic and environmental aspects to particular diseases. We now look at how genetic and environmental influences on disease can be ascertained.
Studying families
Studying families provides an insight into what diseases are occurring in related sets of individuals. Often, this may indicate a genetic abnormality that is passed from generation to generation. However, it needs to be clarified that just because a condition occurs in a family, it does not necessarily follow that it has a genetic cause. For example, although obesity may occur in a particular family, it may be that the family members all have an unhealthy lifestyle, rather than having a genetic condition that leads to obesity. With that in mind, family studies give us an initial understanding of the types of conditions that are passed through genes.
Human family data are often represented by producing a pedigree, such as the pedigree for Parkinson’s disease shown in Figure 37-2. You will see that each generation occupies one row in the pedigree chart. Females are represented by circles and males by squares, and any individuals whose sex is unknown are represented by diamonds. Affected individuals are indicated by filling the shape with colour, and a diagonal line through a shape indicates that the individual has died.
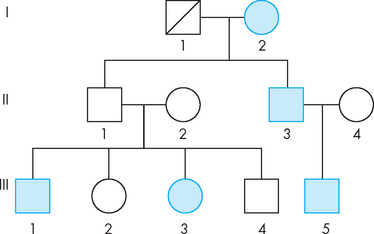
FIGURE 37-2 Family pedigree for Parkinson’s disease, showing the pattern of inheritance in 3 generations.
Note that the mother passed the disease to one of her sons. Her affected son also produced an affected child. Her unaffected son produced 2 affected children. This is consistent with an autosomal recessive pattern of inheritance. The father in generation I is now deceased. Unaffected females =  , unaffected males =
, unaffected males =  . Affected females = •, affected males =
. Affected females = •, affected males =  . Where a male and female are joined by a horizontal line this indicates a partnership, and their offspring are shown in the vertical branching line. The offspring of a partnership in generation I are shown in generation II. Also, those individuals who have joined the family are shown in the second row, as the females who have partnered with the males.
. Where a male and female are joined by a horizontal line this indicates a partnership, and their offspring are shown in the vertical branching line. The offspring of a partnership in generation I are shown in generation II. Also, those individuals who have joined the family are shown in the second row, as the females who have partnered with the males.
While accurate pedigrees provide very useful information, it is often difficult to study patterns of disease within families. The collection of data from several generations is difficult, as family records are often incomplete and may be unreliable. Furthermore, many conditions may have been unrecognised or misdiagnosed in past generations, and paternity itself is sometimes doubtful.
GENETIC CONDITIONS
Genetic conditions are those diseases that are directly attributed to abnormalities in genes, such as having an abnormal number of chromosomes. In Chapter 5 we introduced basic concepts regarding genes and how they are inherited from our parents. Here we explore genetic diseases in order to understand how diseases may be passed through the generations, when linked directly to the DNA, chromosomes or genes. Many genetic diseases are relatively rare in our population and hence have received little focus throughout this text, but we now examine how some of the more common genetic diseases are inherited. The likelihood of these genetic diseases being transmitted from generation to generation can be predicted using Punnett squares and family pedigrees.
If a pair of chromosomes fails to separate at meiosis and migrates as a pair, this will produce gametes with abnormal numbers of particular chromosomes. Recall from Chapter 31 that during a cycle of meiosis, 1 germ cell produces 4 daughter cells, each with a single copy of each chromosome present in normal gametes. If a pair of chromosomes does not separate at meiosis, 1 daughter cell will lack that particular chromosome, while another daughter cell will have 2 copies of that chromosome. The failure of chromosomes to separate is known as non-disjunction and it results in cells with abnormal numbers of chromosomes. This can be seen in Figure 37-3, whereby offspring may have either 3 copies of the chromosome (trisomy) or 1 copy (monosomy), instead of 2 copies as in the normal cell.
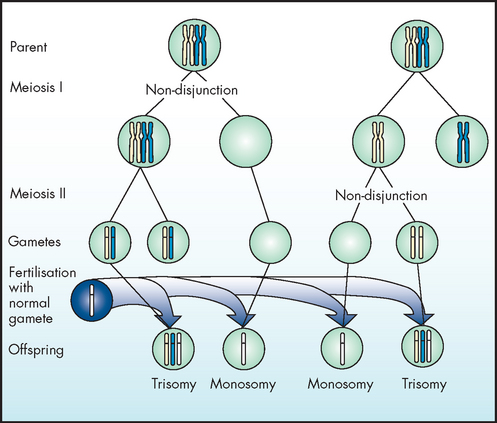
Note that cells with additional or missing chromosomes arise when chromosomes fail to separate and migrate as a pair.
The fusion of gametes with additional or missing chromosomes produces a zygote with an abnormal number of chromosomes. In some instances, non-disjunction occurs in an early mitotic division, producing a zygote with 2 different populations of cells: one with the normal complement of chromosomes and the other with additional or fewer chromosomes.
Non-disjunction of autosomes
Zygotes with a single copy of a particular autosome (any chromosome, other than the sex chromosomes) are not seen: this indicates that at least 2 copies of each autosome are needed for cell survival. Trisomy of all autosomes has been detected in cells from fetuses that have spontaneously aborted. However, trisomy of the smallest chromosomes only is compatible with life. Individuals with trisomy 21 (Down syndrome) have an average life span of 55 years, but infants with trisomy 18 or 13 rarely survive infancy. Trisomy of larger chromosomes does not result in a live birth.7
Down syndrome is the most common cause of intellectual disability; it is also associated with characteristic facial features (see Figure 37-4) and heart malformations. The syndrome has an incidence of approximately 1 in 700 live births in Australia, but the true incidence is higher than this, as a significant proportion of Down syndrome pregnancies are terminated.8,9 The frequency of trisomy 18 is approximately 1 in 3000 births and the frequency of trisomy 13 is approximately 1 in 5000 births.7 These two conditions result in severe heart, kidney and skeletal deformities, and affected infants rarely survive longer than 10 weeks. The frequency of all chromosomal abnormalities, Down syndrome in particular, increases with maternal age, as shown in Table 37-1.
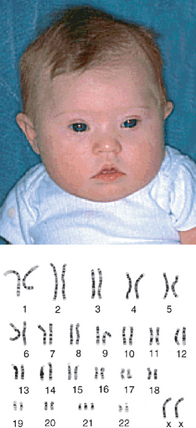
FIGURE 37-4 Trisomy 21 (Down syndrome).
An infant exhibiting characteristic facial features, with karyotype showing 3 copies of chromosome 21.
Source: Katz VL et al. Comprehensive gynecology. 5th edn. St Louis: Mosby; 2007.
Table 37-1 APPROXIMATE RISKS OF CHROMOSOMAL CHANGES ASSOCIATED WITH MATERNAL AGE
| MATERNAL AGE AT DELIVERY (YEARS) | CHANCE OF HAVING A LIVE-BORN BABY WITH DOWN SYNDROME | CHANCE OF HAVING A LIVE-BORN BABY WITH A CHROMOSOMAL ABNORMALITY |
|---|---|---|
| 20–24 years | 1 in 1474 | 1 in 506 |
| 25 years | 1 in 1350 | 1 in 476 |
| 29 years | 1 in 1020 | 1 in 417 |
| 35 years | 1 in 384 | 1 in 179 |
| 40 years | 1 in 112 | 1 in 64 |
| 45 years | 1 in 28 | 1 in 19 |
Source: Cuckle HS, Wald NJ, Thompson SG. Estimating a woman’s risk of having a pregnancy associated with Down syndrome using her age and serum alphafetoprotein level. Br J Obs Gyn 1987; 94:387–402; Hook EB. Rates of chromosomal abnormalities. Obs Gyn 1981; 58:282–285.
Non-disjunction of sex chromosomes
It is estimated that abnormal numbers of sex chromosomes occur in about 1 in 400 male births and 1 in 650 female births.7 These estimates may be unreliable, as many affected individuals have no obvious abnormalities and karyotyping is not routinely performed. If a Y chromosome is present, individuals are classed as males. In the absence of a Y chromosome, individuals are classed as females.
With Turner’s syndrome, affected individuals have a single X chromosome, making a total of 45 chromosomes (rather than the normal 46) per somatic cell. This condition appears in approximately 1 in 2000 births in Australia.7 Females are infertile, with a sexually immature appearance (see Figure 37-5), but normal intelligence. Although this condition is associated with a high spontaneous abortion (miscarriage) rate, it is benign after birth. Oestrogen replacement therapy may be useful to promote the development of secondary sexual characteristics.
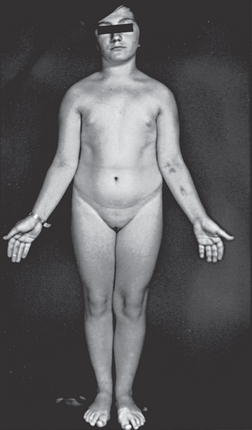
FIGURE 37-5 A 15-year-old female with Turner’s syndrome, exhibiting classical features such as short stature and poor sexual development.
Karyotyping revealed that this individual is a mosaic, with both 45 (XO) and 46 (XX) karyotypes.
Source: Kliegman RM et al. Nelson textbook of pediatrics. 18th edn. Philadelphia: Saunders; 2007.
Additional X chromosomes
Kleinfelter’s syndrome (XXY) occurs in approximately 1 in 500/1000 births.7 Affected individuals present as tall, thin males with enlarged breasts and sparse body hair; they have small testes and produce no sperm. This condition is often not recognised before puberty. After diagnosis, treatment with testosterone helps to promote the development of secondary sexual characteristics.
XXX individuals have a normal female appearance and are usually fertile; many individuals are probably undiagnosed so incidence rates are difficult to determine (estimated at 1 in 1000 births).7
Additional Y chromosomes
Individuals with the XYY karyotype, estimated at 1 in 1000 male births, are typically tall males whose physical appearance is normal. Although many have normal fertility, sperm quality may be reduced. XYY males are overrepresented in the infertile population.10 XYY males have increased risks of learning difficulties and behavioural problems and may show more aggressive or antisocial behaviour than XY males:11 this may be associated with the increased testosterone levels seen in some XYY individuals.
INHERITED DISEASES
Many diseases are associated with specific disease alleles and so they follow the dominant/recessive patterns of inheritance described in Chapter 5. Family studies can be used to determine patterns of inheritance; this information can be used to identify individuals who may carry disease genes and to calculate the likelihood that a couple will produce an affected child.
Autosomal dominant disorders
Dominant disorders arise due to the inheritance of a dominant allele (see Figure 37-6), resulting in the production of a ‘harmful’ protein. A single copy of the gene is sufficient, because any amount of the protein will cause disease. Individuals displaying the dominant phenotype may have 1 or 2 copies of the disease allele, but heterozygotes — with 1 dominant disease allele and 1 normal allele — are most common.
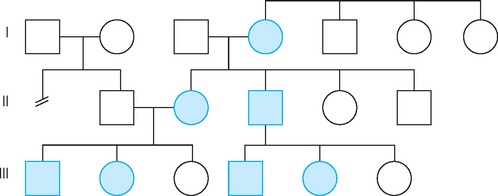
FIGURE 37-6 Pedigree illustrating autosomal dominant inheritance.
Note that the trait appears in every generation; every affected child has an affected parent.
Unaffected females =  , unaffected males =
, unaffected males =  . Affected females = •, affected males =
. Affected females = •, affected males =  .
.
Source: Courtesy of Edith Cheng, MD. Katz VL et al. Comprehensive gynecology. 5th edn. St Louis: Mosby; 2007.
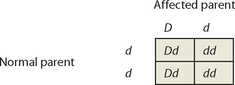
Punnett squares can be used to investigate patterns of inheritance of dominant disorders. We will call the dominant disease allele D, and the recessive normal allele d, and consider the union of a normal unaffected parent and an affected parent. Most commonly, the unaffected parent will have 2 copies of the normal recessive allele (genotype dd) and the affected parent will have 1 copy of the dominant allele and 1 normal allele (genotype Dd).
The Punnett square shows that, on average, about half the children of affected parents will have the disorder. Because the disease allele is present on an autosome (non-sex chromosome), males and females are equally likely to be affected. And because every individual that possesses a copy of the allele will display the associated diseased phenotype, every affected child has an affected parent. These features are clearly seen in the autosomal dominant pedigree (see Figure 37-6).
The collagen disorders provide a good illustration of dominant disease. Collagen is the major structural protein of the body, present in the skin, tendons, ligaments, cartilage and bone. A complete collagen molecule is made up of 3 protein chains, each of which is encoded by a different gene. A defective collagen molecule is formed if any of the 3 collagen protein strands is defective, because the 3 strands are unable to assemble into a functional unit. The presence of a single mutant allele results in the production of defective protein chains that are randomly incorporated into the collagen molecules produced, so a high proportion of collagen molecules produced is defective. Therefore collagen disorders are dominant; every individual that carries a mutant allele will produce a mixture of normal and abnormal collagen molecules.
One of the most common collagen disorders is Marfan’s syndrome,12 which affects the eyes, the skeletal system and the cardiovascular system, producing a wide range of clinical features. Affected individuals are typically tall and thin, with a very wide arm span and extremely flexible joints. Displacement of the lens of the eye is commonly observed; dilatation, then rupture, of the aorta is a common cause of sudden death. Although a tall slender physique is often well suited to sports such as basketball, Marfan’s sufferers are advised against vigorous exercise; some sport associations screen athletes for signs of Marfan’s syndrome. After the 2008 Beijing Olympics it was revealed that Michael Phelps, who won 8 gold medals in swimming for the United States, had been tested for Marfan’s syndrome but cleared. The variable phenotypes observed make definitive diagnosis of Marfan’s syndrome difficult; mild forms of the disease may not be recognised. Estimates range from 1 in 3000 to 1 in 5000 live births, but recent studies suggest that the incidence may be higher.13
Autosomal recessive disorders
In diseases that follow the recessive pattern of inheritance (see Figure 37-7), the dominant allele usually codes for a normal protein and the recessive allele codes for a dysfunctional form of the protein or prevents production of the normal protein. Individuals who have 2 copies of the recessive (defective) allele do not produce any normal protein, so have the disease. Those who have 1 normal allele and 1 defective allele produce half the amount of normal protein, which is often sufficient for normal function, so may ‘carry’ the defective gene without displaying any significant phenotypic changes. In families where the number of offspring is small, the disorder may be carried through several generations before an affected child is born.
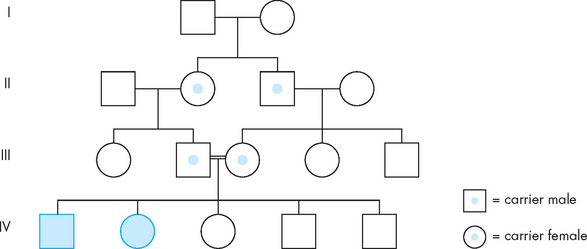
FIGURE 37-7 Pedigree illustrating autosomal recessive inheritance of a rare disorder.
Note that the disorder is not present in every generation. Carriers possess the disease allele but do not display the trait (the carrier status of individuals in generation I is not known). In this example, marriage of first cousins in generation III, both of whom carry the disease allele, has produced affected children. The use of a double line between partners indicates a union between people who are genetically related.
Source: Katz VL et al. Comprehensive gynecology. 5th edn. St Louis: Mosby; 2007.
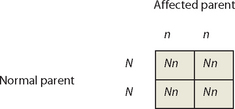
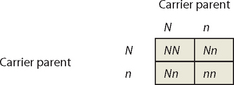
Again, Punnett squares can provide useful information about patterns of inheritance. We will call the normal dominant allele N, and the recessive disease allele n, and consider the union of an affected parent with 2 copies of the recessive disease allele (genotype nn) and an unaffected parent. The unaffected parent may have genotype NN or could carry a single copy of the disease allele (genotype Nn), so 2 alternative scenarios must be considered.
An individual affected with a recessive disorder must have the disease allele on both chromosomes, so must have received a copy of the disease allele from both parents. Therefore, affected parents will produce affected offspring only if their partner is a carrier of the disease allele. If the partner of an affected parent is not a carrier of the disease allele, none of the offspring will be affected, but all of the offspring will be carriers.
Pedigrees for diseases displaying autosomal recessive patterns of inheritance often contain affected children with unaffected parents; the disease appears to ‘skip’ generations. Punnett squares show that 2 unaffected parents can produce an unaffected child if both parents are carriers of the disease allele. Again, males and females are equally likely to be affected.
It has been estimated that each human carries about 30 ‘faulty’ alleles, which are effectively hidden by the presence of the corresponding normal allele. This is responsible for the increased rate (approximately double) of stillbirths, neonatal deaths and congenital malformations observed in offspring of first cousins.14 Marriage of relatives, such as cousins, is certainly ill-advised if the family is known to carry a serious recessive disorder.
Cystic fibrosis is the most common autosomal recessive paediatric disorder in Australia and New Zealand, occurring in approximately 1 in 2800 live births (cystic fibrosis is discussed in Chapter 25).15 However, the less well-known autosomal recessive condition hereditary haemochromatosis is much more common; approximately 1 in 300 Australians will be affected by this condition during their lifetime.7 In this disorder, iron absorbed from food slowly accumulates in organs and joints. Significant liver damage (cirrhosis and cancer) may cause premature death. Symptoms appear in the third or fourth decade for men and even later for women, as menstrual blood loss reduces the iron overload, delaying the onset of symptoms.
Sex-linked disorders
Sex-linked traits are determined by genes carried on the X and Y chromosomes. Because Y-linked disorders are extremely rare, sex-linked disorders are usually X-linked, so the terms sex-linkage and X-linkage are often used interchangeably. X-linked dominant disorders are not common, one example being hypophosphataemia. However, several diseases, including haemophilia, red–green colour blindness and Duchenne’s muscular dystrophy, are X-linked recessive traits.
Because most X-linked traits are recessive and females have 2 X chromosomes, many females carry the genes for X-linked traits without displaying the associated characteristics. The situation in males is very different: as males have 1 X chromosome (and 1 Y chromosome), they have only a single copy of all X-linked genes. Consequently, all males that carry the gene for an X-linked trait will display that trait. Transmission is typically from mothers to sons (see Figure 37-8).
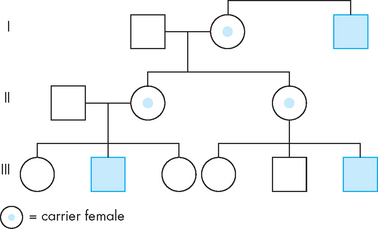
FIGURE 37-8 Pedigree illustrating X-linked recessive inheritance.
Note that the trait may appear to skip a generation. Carrier females do not display the trait, but may pass either the normal X or the affected X chromosome to their offspring. Daughters receiving the affected X chromosome are carriers; sons receiving the affected X chromosome display the trait.
Source: Katz VL et al. Comprehensive gynecology. 5th edn. St Louis: Mosby; 2007.
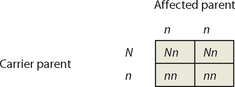
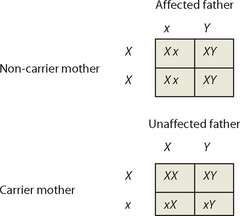
X-linked recessive traits are common in males and rare in females. Punnett squares can be used to explore the most common situations: the union of an affected male with a non-carrier female and the union of a carrier female with an unaffected male. We will use X to represent the normal X chromosome and x to represent the X chromosome carrying the recessive disease allele.
The Punnett squares show that all daughters of affected males will be carriers. Carrier daughters will then pass the trait to (approximately) half of their sons, so that the trait will ‘skip’ generations. Affected males never pass the trait to their sons.
Fragile X syndrome
Fragile X syndrome, an X-linked disorder, is the most common cause of inherited intellectual disability, with a prevalence of approximately 1 in 3500 males.16 Affected individuals present with intellectual disability, delayed development of speech and motor skills, and behavioural problems including attention deficit disorder and autism. Fragile X syndrome is due to changes near the tip of the long arm of the X chromosome that give the chromosome a damaged or ‘fragile’ appearance, hence the name for this syndrome. This region of the X chromosome comprises a short sequence that is repeated many times. About 30 repeats are present in normal individuals, whereas individuals displaying the fragile X trait possess more than 200 repeats. Individuals whose X chromosomes contain 50–200 repeats are usually unaffected and are described as carriers.
Patterns of inheritance of the fragile X syndrome can be unclear, as the number of repeats increases when the X chromosome is passed from mothers to their children (male or female). In contrast, there is no change in the number of repeats when X chromosomes pass from fathers to daughters. As expected for an X-linked condition, the trait is more common in males, but females with 1 ‘fragile’ chromosome may also show symptoms (usually less severe than in males).
GENETIC SCREENING
If a disease is common in a particular family, population or ethnic group, genetic screening may detect carriers of disease alleles. Genetic screening may be performed using a blood sample or other body fluid; the genes of interest are examined in the laboratory to determine whether or not the gene sequence is normal. Genetic screening also includes prenatal tests, whereby a chorionic villus sample is taken from the placenta or amniotic fluid to allow the genes of the fetus to be assessed. In those undergoing in vitro fertilisation (IVF), the genes of the embryo can even be examined prior to implantation of the embryo in the uterus. Both genetic screening and in vitro fertilisation are increasingly being used in contemporary society.
People who are affected (or potentially affected) by particular genetic conditions may undergo genetic counselling, which involves discussions with trained counsellors, who provide information and support. Genetic counselling is most often sought by parents of an affected child, members of a family with a hereditary disease or related individuals (e.g. cousins) who wish to marry. The probability that offspring will be affected can be calculated, but this may be difficult if the pattern of inheritance is not clear or carrier status cannot be ascertained. If the gene sequence of the disease allele is known, specific testing can be performed on DNA isolated from blood or tissue samples. The direct detection of the disease allele provides a definitive diagnosis.
Recessive diseases that are common in particular populations include beta (β)-thalassaemia in Mediterranean populations and sickle cell anaemia in some African populations. Immigration from these regions has increased the incidence of these disorders in Australia and New Zealand.
Although the genes responsible for some diseases have been identified, not all individuals affected with the same disease have the same genetic mutation, so the sequences of disease alleles may vary. For example, the genes associated with cystic fibrosis and Duchenne’s muscular dystrophy are very large and many mutations have been identified; the same disease may be produced by several different mutations. Each mutation must be detected using a specific genetic marker, which highlights the presence of that particular disease allele. To ensure that a specific disease marker has predictive value in a particular family, affected and unaffected members are tested. The marker must be present in all affected family members. Unaffected family members who are carriers of the disease allele (heterozygotes) will possess the genetic marker, but will also test positive for the normal gene sequence.
Prenatal testing can be performed using a chorion biopsy sample obtained at about 8 weeks; fetal sexing, karyotyping and genetic screening can be undertaken. This procedure appears to pose no significant risk to the mother. The risk to the fetus, which is difficult to estimate for high-risk pregnancies that could be expected to abort spontaneously, is approximately 10%. Prenatal diagnosis can be used to determine whether the fetal genotype includes a particular disease allele; often couples will choose to terminate an affected pregnancy if the outcome will be severe. Artificial insemination with donor sperm provides an alternative approach if the disease allele is carried by the father.
Prenatal testing can have dramatic effects. One particular study involved a Sardinian population, where 13% of the population carry a recessive allele for β-thalassaemia. Prior to the availability of prenatal testing, many families with a history of this disease curtailed reproduction and about 70% of pregnancies were terminated. When prenatal testing became available, providing reliable information about the outcome of a pregnancy, most couples resumed ‘normal’ reproductive behaviour and the pregnancy termination rate fell to about 30%. Prior to prenatal screening, the incidence of β-thalassaemia was 1 in 250 live births, but after prenatal screening was introduced the incidence fell to 1 in 4000 live births.17
Cystic fibrosis is a rare disease (incidence of 1 in 2800 in Australia and New Zealand). It is estimated that 1 in 25 individuals of European ancestry carry the cystic fibrosis gene, but most have no family history of the disease. A recent Australian study investigated the effectiveness of routine testing for cystic fibrosis carrier status.18 In the study, 1000 couples contemplating pregnancy or in the early stages of pregnancy were offered a test to determine their status as cystic fibrosis carriers; all accepted the offer. Four couples were identified in which both partners were carrying the cystic fibrosis mutation and therefore they had a high risk of having a child with the disease; two couples had a family history of cystic fibrosis and two couples did not. All four couples chose actions to avoid having a child with cystic fibrosis, either by a combination of IVF and genetic testing before implantation or by chorion biopsy and termination of affected embryos.
CONGENITAL ABNORMALITIES
Congenital abnormalities range from minor defects, such as webbing between the fingers and toes, to major malformations, such as cleft palate and neural tube defects. Some congenital malformations have a clear genetic basis. For example, brachydactyly (short fingers and toes) and polydactyly (extra fingers and toes) are inherited disorders associated with specific alleles. Chromosomal imbalances, such as the duplication or deletion of entire chromosomes (or large chromosome segments), may produce multiple malformations that are extremely severe, often causing spontaneous miscarriage or death in the perinatal period.
In addition to these genetic influences, congenital abnormalities include developmental and structural defects that may be caused directly by changes in the uterine environment at particular stages of fetal growth. Therefore, these abnormalities represent a group of disorders where the interaction between genes and the environment may be seen on a relatively simple level. Depending on the environment, the fetus may be more or less likely to develop a particular disease. The mother’s lifestyle or environmental exposure can have a profound influence on the developing fetus, and hence the influence of the environment on the genes of the fetus is quite apparent.
Agents that interfere with normal embryonic or fetal development are known as teratogens. They include drugs, chemicals, infectious agents (often viruses) and sources of ionising radiation, such as X-rays. Often, the timing of exposure to the teratogenic agent determines whether abnormalities will arise in the fetus. The first trimester, especially the first 6 weeks after conception, is a critical period of fetal development; however, many women are not aware of their pregnancy at this early stage. Therefore, precautions are often applied to all women of child-bearing age, rather than only to those who are known to be pregnant.
The incidence of many congenital disorders can be reduced by appropriate preventive actions; these are a public health priority. For example, congenital rubella syndrome (hearing loss, eye and heart defects) is a common outcome if mothers contract rubella in the first trimester of pregnancy. Rubella vaccination programs (MMR: measles, mumps and rubella vaccine in Australia and New Zealand) have essentially eliminated this condition in developed nations, although a small number of cases are attributed to vaccine failure.19
Neural tube defects
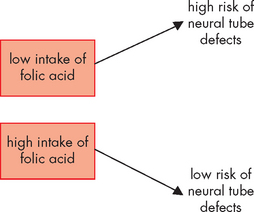
Increased intake of folic acid around the period of conception has been shown to significantly reduce the incidence of neural tube defects, including spina bifida; this is an example where the appropriate environment, namely adequate levels of folic acid, can influence the genetic appearance (see Figure 37-9). However, public education programs to encourage women of child-bearing age to voluntarily increase their folic acid intake have not had the desired outcomes.20 Folic acid is particularly abundant in green leafy vegetables and our modern diets tend to have low quantities of these vegetables. Mandatory fortification of bread-making flour with folic acid commenced in Australia in September 2009 and will commence in New Zealand from 2012.
Fetal alcohol syndrome
Alcohol is known to induce developmental abnormalities in the fetus; the outcomes vary according to the stage of pregnancy and the amount of alcohol consumed. Key features of fetal alcohol syndrome include mental retardation, microcephaly (reduced head size and poor brain development), characteristic facial features (see Figure 37-10) and behavioural problems. Babies of binge drinkers are most at risk of having this syndrome; alcohol consumption in the first 8 weeks of pregnancy has the most serious effect on brain and craniofacial development.21 Children who have been exposed to alcohol during pregnancy, but do not have diagnostic features of fetal alcohol syndrome, are more likely to display reduced IQ, learning difficulties and aggressive behaviour than children who were not exposed to alcohol during pregnancy.22 This disorder represents a powerful influence between the genetics of the fetus and the environment of intrauterine life, based on the mother’s lifestyle.
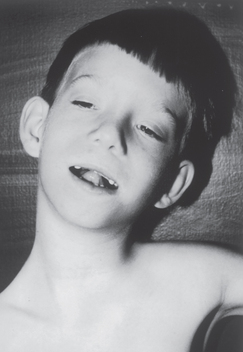
FIGURE 37-10 Characteristic facial features of a child affected by fetal alcohol syndrome.
Note the short, downslanting eye openings, sunken nasal bridge, short upturned nose, long smooth philtrum (midline groove from below the nose to the upper lip) and thin upper lip.
Source: Keane JF et al. Nadas’ pediatric cardiology. 2nd edn. Philadelphia: Saunders; 2006.
In addition, a fetus with a genetic variation on the ADH gene may be more susceptible to intrauterine exposure to alcohol. Therefore, both the fetal genes and the fetal environment within the uterus appear to be important in the potential development of fetal alcohol syndrome (see Figure 37-11). It also appears that there is an interaction between genes and the environment in this condition.23
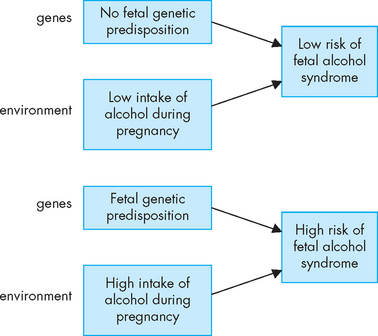
FIGURE 37-11 Risk of developing fetal alcohol syndrome.
A combination of both the genetic predisposition in the fetus and the maternal environment of exposure to alcohol can influence the development of fetal alcohol syndrome.
Fetal alcohol syndrome is often unrecognised or misdiagnosed; it is estimated that the prevalence in Australia is approximately 1 in 1000 live births.24 Early diagnosis allows for appropriate intervention, which is essential to reduce the societal and economic impact of this disorder.
Phenylketonuria
In phenylketonuria (PKU), an accumulation of the amino acid phenylalanine within the brain can result in mental retardation. However, the extent of this retardation is determined by the amount of phenylalanine that is consumed and a diet low in this amino acid can minimise the effects of the disease.23 Because of the importance of early diagnosis, this condition is assessed during the routine newborn screening program (discussed in Chapter 5). Therefore, even though this disease has a genetic basis, it is the environment that determines the severity.
MULTIFACTORIAL INHERITANCE
Many traits, such as height, weight and IQ, are controlled by several genes, so are described as polygenic traits. Interactions among the controlling genes produce a broad range of phenotypes. Many diseases are also controlled by several genes, so do not follow the simple dominant/recessive patterns described above. For single gene disorders, the presence or absence of the disease allele determines whether or not an individual has a particular disease. For diseases controlled by several genes, the interactions among the controlling genes often determines an individual’s susceptibility to developing disease; whether or not disease then develops is determined by environmental factors (see Figure 37-12). In this section, we explore a number of common conditions where there appears to be a role for both genetics and the environment in determining those who develop the disease. It is becoming more apparent that for many diseases, there is a complex relationship between genetics and the environment.
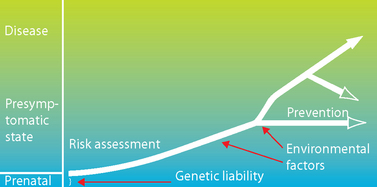
FIGURE 37-12 Model for multifactorial disorders.
Everyone inherits some genetic liability for disease risk, but this genetic predisposition may not produce disease on its own. Over time, exposure to environmental risk factors leads to the development of a disease state. Preventative measures can reduce risk. Identifying the genes responsible for disease risk can lead to treatments and can be used to identify those for whom preventative strategies may be of benefit.
Source: Based on Kliegman RM et al. Nelson textbook of pediatrics. 18th edn. Philadelphia: Saunders; 2007.
Traits that are determined by a combination of environmental factors and several genes are known as multifactorial traits. Many diseases, including diabetes (types 1 and 2), schizophrenia, coronary heart disease and many forms of cancer, are multifactorial. They are associated with particular genotypes, which means there is a genetic predisposition in some individuals. If several genes are involved in the development of a particular condition, they may interact with each other to influence the progression of disease. An individual with only one or two genetic tendencies for a disease may be less likely to develop the disease as quickly as someone with several genetic tendencies. Therefore, gene–gene interactions can influence the likelihood of disease development. For most diseases, some genes have already been identified and it is anticipated that further research will reveal additional genes.
Furthermore, development of the disease is often triggered by lifestyle or environmental factors. For most diseases, several environmental risk factors have been defined, and it is likely that increasing numbers of environmental factors will become evident with further research. A combination of several genes, as well as several environmental triggers, makes the actual development of the condition complex and difficult to predict (see Figure 37-13). It is the interaction between genes and the environment that is of particular interest to scientists who are researching the causes and underlying pathophysiology of disease.
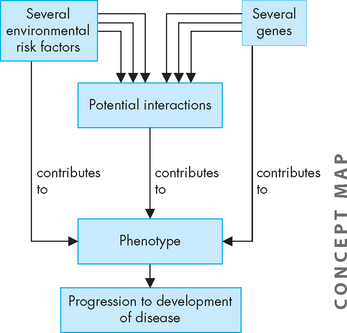
FIGURE 37-13 The complex nature of environmental and genetic factors in disease development.
Each risk factor on its own may contribute to disease development. In addition, there may be interactions between multiple risk factors, which can also contribute to disease. In most cases, the interaction between the genetic and environmental factors remains largely unknown.
Heritability measures the relative contribution of genetics to the development of a particular trait. If heritability = 1, the trait is completely determined by genotype; if heritability = 0, the trait is completely determined by environment and has no genetic component. Phenotypes with intermediate heritability values, such as IQ (heritability = approximately 0.5), are influenced by both genotype and environmental factors. As we explain below, the influence of genotype and environmental factors is poorly understood for many common chronic conditions.
It can be difficult to determine the relative contributions of genetics and environment to disease as individual families are both genetically related and share the same environment, but studying twins can provide useful insights. Monozygotic twins, commonly known as identical twins, are derived from a single embryo, so are genetically identical. Dizygotic (fraternal) twins develop from 2 independently fertilised ova; they are genetically equivalent to siblings, so their genetic similarity is approximately 50%. If twins are raised in the same household, under the same conditions, their environment is very similar, although never truly identical. If a trait is determined by genes alone (heritability = 1), then both members of a pair of monozygotic twins would be expected to display the trait, while dizygotic twins would share the trait approximately half the time (perhaps less in opposite-sex twin pairs). If a trait is determined by environment alone (heritability = 0), then both monozygotic and dizygotic twins would be expected to display the trait. Therefore, studying twins can help determine whether a trait is determined by genetic or environmental components.
The Australian Twin Registry was established in the 1970s and maintains a database representing more than 35,000 sets of twins (and other multiple birth sets). The Registry facilitates scientific research, recruiting suitable members for approved studies that aim to improve the health and wellbeing of all Australians.25
CONDITIONS ARISING FROM GENETIC AND ENVIRONMENTAL FACTORS
It was suggested more than 100 years ago that diseases arise not just due to genetic or environmental causes, but also as a result of an interaction between genes and the environment.26 Given that only several environmental risk factors and even fewer genes have been associated with each disease, a detailed knowledge about the potential interactions between the two remains relatively incomplete.
In considering the interaction between genes and the environment, we focus on the diseases that have the greatest impact on our community. These diseases tend to be chronic, take many years to develop and hence are more often seen in older people — for example, cancer, diabetes and coronary heart disease. Due to the wide range of exposure to environmental influences over a person’s life prior to disease becoming evident, it is difficult to define all lifestyle factors that may have contributed to the development of disease. However, in many cases at least a few risk factors have been reasonably well documented.
Cardiovascular disease
Coronary heart disease
Coronary heart disease (blockage of the coronary arteries, causing angina and myocardial infarctions; see Chapter 23) is the leading individual cause of registered deaths in Australia and New Zealand — 16% of deaths in Australia in 2007;27 21% of deaths in New Zealand in 2006.28 Approximately 1 in 20 New Zealand adults have been diagnosed with coronary heart disease: 4% experience angina and 3% have had a myocardial infarction requiring hospitalisation.
Coronary heart disease is a very complex condition. Whether disease develops, and the severity of the disease, is determined by the combined interaction of many genes and environmental factors. An individual’s genotype determines their general predisposition to coronary heart disease; and environmental factors such as activity levels, diet and smoking can determine whether disease develops. As seen in Figure 37-14, where an individual has more genetic risk factors and more environmental risk factors, they are most likely to develop coronary heart disease while young.29 The interaction between these genes and environmental factors is being further investigated.
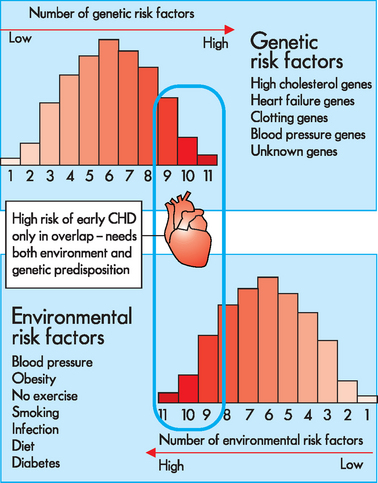
FIGURE 37-14 Model for gene–environment interaction.
This model proposes that each individual occupies a position on the genetic risk spectrum, depending upon how many risk-increasing gene variants have been inherited. Similarly, individuals are exposed to a range of environmental challenges depending on lifestyle choices. This theory proposes that risk of CHD occurs only when an individual at high genetic risk enters a high-risk environment, and that genetic risk factors and environmental risk factors alone will not trigger a CHD event. CHD = coronary heart disease.
Source: Based on Talmud PJ. Gene-environment interaction and its impact on coronary heart disease risk. Nutr Metab Cardiovasc Dis 2007; 17:148–152.
Studies of twins have clearly indicated that genes play a significant role in the development of coronary heart disease, but the number of genes potentially involved makes data interpretation difficult.30 Interestingly, these studies suggest that the genetic component is stronger in females than in males (heritability = 0.6 and 0.26, respectively). Genes associated with increased risk of coronary heart disease include genes involved in the metabolism of lipids (especially cholesterol), the maintenance of veins and arteries, blood clotting and inflammation.
Familial hypercholesterolaemia
Familial hypercholesterolaemia is a dominant disorder associated with approximately 5% of myocardial infarctions in people under 60 years of age.31 The disorder arises due to a mutation in the gene that produces LDL (low density lipoprotein) receptors, which play an essential role in cholesterol uptake by cells. If LDL receptors are faulty or absent, cholesterol is not taken up by cells and continues to circulate in plasma; high plasma cholesterol promotes the development and progression of atherosclerosis. Individuals with 2 copies of the familial hypercholesterolaemia allele are rare and unlikely to survive past 30 years of age. However, about 1 in 500 individuals carry 1 copy of the familial hypercholesterolaemia allele (and 1 normal gene) and so have about half the number of normal LDL receptors; therefore, plasma cholesterol levels in those who are untreated are about twice the normal level. Approximately 50% of males with a single copy of the familial hypercholesterolaemia allele will die of myocardial infarction before 60 years of age; the disease progresses more slowly in females, where the corresponding percentage is 15%. Restricting dietary intake of cholesterol alone is ineffective for these individuals; therapeutic strategies involve drugs that reduce liver–cholesterol synthesis (statins), prevent cholesterol absorption and promote cholesterol excretion.
Hypertension
Hypertension is a disorder that is a significant co-morbidity factor for diseases such as coronary heart disease, stroke and diabetes mellitus. It is now common in Australia and New Zealand (see Chapter 23). As yet, it is unclear which genes are the main contributors to primary hypertension, although more than 20 have been implied.32 However, only 30% of hypertension is hereditary33 and it is evident that hypertension results from a combination of genetic susceptibility and lifestyle factors.32
Obesity
Obesity has widespread impact in our community — in Australia and New Zealand, almost two-thirds of the population are above their ideal weight range and are defined as being either overweight or obese.4,34 In Chapter 35 we discussed modifiable lifestyle factors for obesity. There is substantial evidence that indicates the importance of these risk factors in the development, treatment and prevention of obesity. The potential role of genetics in obesity is still being understood.
There are some rare monogenetic causes of obesity in humans, including abnormalities associated with leptin or the leptin receptor.35 Such abnormalities are an example of genetic conditions that may alter energy balance through interactions between leptin and the hypothalamus or influence brain development relating to the hypothalamus.36 Prader-Willis syndrome is another example of a monogenetic cause of obesity, with a chromosomal abnormality that relates to obesity, short stature and other effects including mental retardation.37 However, these genetic abnormalities appear to be rare and are not related to obesity in most individuals.
There is evidence that genetic factors involved in obesity are polygenetic — that is, several genetic factors may be involved.38,39 Genetic factors that appear to be important include the β3-adrenergic receptor gene,35 as this receptor is found on adipose cells. Overall, it is possible that there are some major obesity genes, with other minor obesity genes having a lesser contribution to individual obesity.
Interestingly, in a study of minority groups from a rural area in China (Xinjiang), mainly herdsmen and peasants, almost 50% of the population were above their ideal weight.40 It may be said that these people have a strong genetic link to obesity, as their environmental influences would be substantially different from the urbanised experience in cities in developed countries. However, although these peasants are isolated from large cities, their diets are rich in alcohol, salty food and meat. Therefore, these data may contribute to our understanding of the role of these risk factors in obesity.
As with other chronic diseases, there is evidence that both genetics and environmental factors are important in obesity (see Box 37-1). Obesity genes may be involved in gene–gene interactions, such as the β3-adrenergic receptor genes, which can disrupt mitochondrial energy usage. Furthermore, there are gene–environment interactions, such as the β3-adrenergic receptor genes influencing physical activity.35 Thus a genetic abnormality may lead to altered adipose cell physiology, thereby contributing to obesity. A gene–environment interaction has been suggested relating a genetic component (PPAR; peroxisome-proliferated activator receptor) to the proportion of polyunsaturated fat to saturated fat.37 Gene–environment interactions in obesity are in the early stages of being understood and future research may clarify this relationship.
Box 37-1 THE ROLE OF GENETICS AND ENVIRONMENTAL FACTORS IN OBESITY
According to one study: ‘… obesity is a multifactorial disease that is influenced by genetic and environmental factors. An inherited component to body weight accounts for 40% to 70% of an individual’s predisposition to obesity … Therefore, weight gain is caused by dietary and lifestyle choices on a background of genetic susceptibility. Most genes that contribute to this predisposition are still unknown, but the discovery and characterization of single gene defects that cause severe human obesity has provided some insight into the hereditary nature of body weight.’
Source: Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin N Am 2008; 37:733–751.
Metabolic syndrome
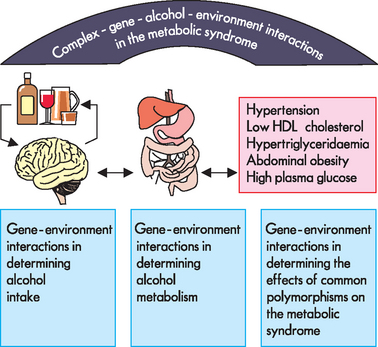
Metabolic syndrome is related to obesity, diabetes mellitus, cardiovascular disease and renal disorders. There is evidence that gene–environment interactions may be significant in its development. One factor that is important in the development of this syndrome is alcohol intake. It has been proposed that gene–environment interactions may underlie alcohol intake, alcohol metabolism and hence development of this syndrome.41 As shown in Figure 37-15, there may be an interaction between genetics and the environment through a number of avenues that lead to this disorder. This highlights the complexity of the relationships among genetic and environmental factors.
Diabetes mellitus
Diabetes mellitus is characterised by hyperglycaemia and accompanying glycosuria (see Chapter 35 to review this disease). Type 1 diabetes is an autoimmune disease in which the insulin-producing beta cells of the pancreas are destroyed; it accounts for approximately 10% of diabetes cases in Australia.42 At least 7 different genetic locations, distributed across 6 chromosomes, have been found to have a role in determining an individual’s susceptibility to developing type 1 diabetes.43 These loci include the insulin gene itself and genes that control its expression; the HLA genes (see Chapter 15), which have a well-defined role in the development of autoimmune disease; and other genes linked to autoimmune disease states. About half of the genetic susceptibility to type 1 diabetes is associated with the inheritance of particular HLA alleles. The remaining 6 genes determine the balance of an individual’s susceptibility to type 1 diabetes, but no single one of these 6 genes makes a significant contribution to disease risk.44
While the genotype determines an individual’s predisposition to developing type 1 diabetes, disease onset is triggered by environmental factors, which are not yet clear. Viral infections, in particular congenital rubella infection, have been implicated in the development of type 1 diabetes. Exposure to protein allergens, such as cow’s milk proteins, may also trigger disease onset. It has been noted that disease is more common in cold climates. Overall, it appears that there is a genetic likelihood of developing type 1 diabetes, with an environmental influence (as yet poorly understood) that triggers the commencement of this disease.
Type 2 diabetes is much more common and is often associated with obesity. Approximately 4.5% of adults in Australia42 and New Zealand have been diagnosed with type 2 diabetes, rising to 14% of people aged over 65.4 These individuals produce insulin, but the amount is reduced and/or its activity is compromised, so removal of glucose from the bloodstream is inefficient. To date, 18 susceptibility genes scattered throughout the genome have been shown to be associated with type 2 diabetes.45 One important gene is the PPAR-gamma gene (peroxisome-proliferated activator receptor gamma gene), which is involved in the processing of dietary fats. While the biological role of many of these genes is not yet clear, analysis indicates that most affect insulin secretion, rather than insulin action.
Environmental triggers include obesity, a sedentary lifestyle, high blood pressure and high blood cholesterol. The interaction between genes and lifestyle, although not fully understood, appears to be significant in development of type 2 diabetes.46 For example, there is evidence that a genetic abnormality in the PPAR-gamma gene can interact with different dietary fats to influence insulin resistance, BMI and type 2 diabetes.47–49 4 6
Unlike for type 1 diabetes, there is strong evidence indicating the significance of environmental or lifestyle factors in the development and progression of type 2 diabetes. On the other hand, the genetic influence on the development of type 1 diabetes appears to be clear, whereas the role of genetics is less certain in the development of type 2 diabetes.
It is difficult to determine the heritability of diabetes. Studies involving identical twins have shown that if one twin has diabetes, the likelihood that the second twin will develop diabetes is approximately 50% for type 1 diabetes and 60–80% for type 2.50 This might suggest that type 2 diabetes has a stronger genetic component than type 1. However, it is difficult to separate the genetic effects from those of the environment, as identical twins usually have very similar lifestyles, and lifestyles that can increase the risk of type 2 diabetes are likely to be shared by twins.
Therefore, twin studies alone cannot distinguish between genetic and environmental causes, particularly if the twins adopt a similar lifestyle. So, although a disease may be seen among different family members, it does not necessarily mean that it is a genetic condition, as their lifestyle and environment may be similar as well as their genes.
Some patients with diabetes develop chronic kidney disease and end-stage kidney disease. In these patients, there appears to be an interaction between genetics and environmental factors.51 It has been suggested that the toxic internal environment of these patients with kidney disease (due to uraemia) may actually make them more susceptible to any genetic predisposition52 — this would demonstrate a strong interaction between genes and the environment.
Cancers
When all cancers from different organ systems are added together, cancer may be considered the leading cause of death in Australia and New Zealand, causing 18% of deaths in Australia in 200727 and 29% of deaths in New Zealand in 2006.4 (Note though that because the cancers in different organs are different, they are not usually summed in health statistics.) Cancers typically arise due to genetic changes that occur within specific cells and tissues during an individual’s lifetime. Both genetic and environmental factors contribute to the development of cancers.
For some cancers, environmental factors play a major role. For example, chemicals present in cigarette smoke alter DNA bases, altering the genes and leading to lung cancer. UV exposure causes genetic mutations, leading to melanoma (which is also why exposure to X-rays is carefully limited). Skin cancers are much more prevalent in people with fair skin,53 and represent a type of condition in which the environmental risk (sunlight exposure) interacts differently depending on whether the person is genetically fair-skinned or darker-skinned. In this case, the gene–environment interaction is relatively simple to understand, even without having detailed knowledge of the genes involved.
However, twin and family studies indicate that a predisposition towards particular types of cancer is inherited,54 although for most cancers the specific genes involved are not yet known.55 There is evidence that environmental factors are responsible for at least 65% of cancer cases.56
Breast cancer is the most common cancer among women in Australia and New Zealand (refer to Figure 36-29) and accounts for 10% of all new cancers diagnosed worldwide each year.57 A range of environmental and behavioural factors are associated with the development of breast cancer, including diet, alcohol intake, use of oral contraceptives and short periods/absence of breastfeeding. Many of these contribute to the major risk factor for breast cancer: exposure to oestrogen. Consistent with the prevailing patterns of diet, childbearing and breastfeeding, breast cancer incidence rates in Australia and New Zealand are among the highest in the world.
Mutations in two genes, BRCA1 and BRCA2, have been shown to strongly increase an individual’s predisposition towards breast and ovarian cancers.58 Compared with a woman without these mutations, a woman with the BRCA1 or BRCA2 mutation is 5 times more likely to develop breast cancer and 10 times more likely to develop ovarian cancer. Approximately 6% of males carrying the BRCA2 mutation also develop breast cancer. Individuals carrying the BRCA mutations are encouraged to undergo regular screening; some affected individuals elect to have breast tissue removed before breast cancer develops.
Both genetic and environmental influences also appear to be important in colorectal cancer. The most common genetic alteration in this disease is mutation of the p53 tumour-suppressor gene, which protects against cancers. In one study, patients with colorectal cancer who had this genetic abnormality also had a higher intake of red meat and alcohol.59 However, the interaction between the abnormal gene and dietary intake remains unclear.
One theme that may be relevant in the progression of cancers involves disturbance to the regular circadian rhythm (the sleep–wake cycle; refer to Chapter 6). Clock genes, which are important in regulating this cycle, are actually tumour-suppressor genes and it may be that people whose circadian rhythm is disrupted are more prone to cancer development. In addition, some chemotherapy drugs used to treat colon cancer actually may be more effective at different times during the circadian rhythm,60 which suggests a powerful environmental influence for this cancer. Studies are needed to determine whether regulating the circadian clocks of these patients can improve their quality of life60 — this would be a promising example of an interaction between genes and the environment.
Although pancreatic cancer has a relatively low incidence rate in Australia and New Zealand, it has a high mortality rate (see Chapter 36). It has been estimated that genetic factors account for 5–10% of cases of pancreatic cancer.61 Several genes have been identified, including the familial pancreatic cancer gene PALB2, the cystic fibrosis CFTR gene, the hereditary pancreatitis genes PRSS1 and SPINK1 and the hereditary breast cancer gene BRCA2. Lifestyle and environmental contributing factors include tobacco smoking, obesity, diabetes mellitus and chronic pancreatitis.61 In order to assess the risk of an individual developing pancreatic cancer, it may be useful to consider a combination of DNA analysis, family pedigree and lifestyle factors.61,62
Ultimately, there appears to be roles for both genetic and environmental factors in the development of cancers (see Figure 37-16).63 The complex interaction between them can influence the progression of cells from being normal, to being susceptible to becoming cancerous, to finally becoming cancerous.
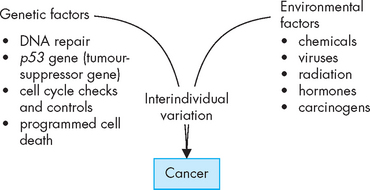
FIGURE 37-16 Both genetic and environmental factors can have a significant role in the development of cancer.
The interaction between genetics and the environment, as well as the variation or effects that may be unique to each individual, can influence the development of cancer.
Source: Based on Shields PG, Harris CC. Cancer risk and low-penetrance susceptibility genes in gene–environment interactions. J Clin Oncol 2000; 18:2309–2315.
Mental illness
Although some studies point to significant interactions between genes and the environment in mental illness, other studies have not shown an interaction.23 This is one important field where additional research is necessary to provide further information on the development of these conditions.
There is evidence that major depression is heritable, and that the heritability of schizophrenia and bipolar disorder is actually higher (that is, they are both more likely to be passed through the family).64 Interestingly, the relevant environmental factors — such as parenting style and socioeconomic factors — are not so important to the population, but are important to the individual’s risk of developing depression. It has been concluded that depression is a result of both genetic and environmental influences.64 Similarly, while genetics seem to contribute to 30–40% of the likelihood of suicidal behaviour, there appear to be complex interactions between genes and environmental risk factors in these individuals.65
There is even evidence that genetics may influence the ability to control cravings relating to addictive behaviours such as cigarette smoking and drinking alcohol, as those with a particular genotype (DRD4 long gene for the dopamine receptor) exhibit stronger cravings.66 This type of research may be useful in the future in determining the interaction between genes and the environment. Ultimately, it is the interaction between genes and the environment that will lead to improved knowledge about diseases.
Interestingly, genes can actually influence an individual’s behaviour or lifestyle. One example is the gene involved in the metabolism of alcohol, ALDH2 (alcohol dehydrogenase).23 This gene may be involved in addictive behaviour. Individuals with abnormalities associated with the ALDH2 gene cannot metabolise alcohol efficiently and hence they experience an unpleasant facial flushing, nausea and headache associated with alcohol consumption. Individuals who are homozygous for abnormalities in this gene (and hence have low levels of alcohol dehydrogenase to metabolise alcohol) usually have a relatively low intake of alcohol. In turn, their lower consumption of alcohol can actually lower the development of other alcohol-related disorders, particularly cancers.23,67 Hence, the genes influence the environment, which in turn influence the development of other diseases.
Some mental health conditions, such as posttraumatic stress disorder, mood disorders and anxiety disorders, are strongly linked to the stress response. There is evidence that the individual variability in the stress response, mediated through the hypothalamic-pituitary-adrenal axis (see Chapter 34), may actually be influenced by both genes and the environment. For example, two genes that are involved are CRHR1 and FKBP5, while environmental factors may include both positive and negative life experiences.68 Socioeconomic status can also contribute to the developing stress response, due to experiences with nutrition, poverty, housing and healthcare factors.69 Furthermore, the interactions between these genes and the environment early in life may influence the stress response in later life.68,69
THE RELATIVE IMPORTANCE OF GENETIC AND ENVIRONMENTAL CONTRIBUTIONS TO DISEASE
The links between the pathogenesis of disease and environmental factors are extremely complicated. A person with a genetic abnormality (or predisposition) towards a particular disease may develop the disease young in life, seemingly regardless of lifestyle choices or environmental exposure to risks (see Figure 37-17A). This may apply to cancers in some people, where genetic links are already known, such as in some cases of breast cancer and colorectal cancer; often, those who develop these cancers very young in life are seen to have a particular gene for the cancer. If someone with this genetic mutation undertakes lifestyle choices that lower the risk of that disease, they may delay the onset of the disease until much later. This is because in some circumstances the conditions that the cells are exposed to can slow down the progression towards becoming abnormal.
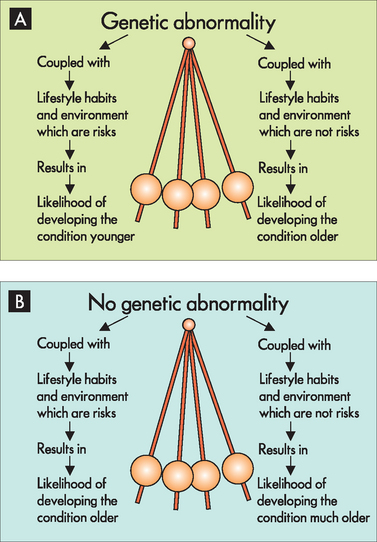
FIGURE 37-17 The likelihood of developing diseases that have both genetic and environmental influences.
A In an individual who has a genetic abnormality towards a particular disease, environmental factors that are consistent with development of that disease increase the likelihood of it being seen at a younger age. On the other hand, if the same individual were to avoid environmental factors related to that disease, it may only become apparent later in life. B In an individual who does not have genetic abnormalities for a specific disease, exposure to environmental factors for that disease may still result in the development of the disease. If that same individual were to avoid the relevant environmental factors, then the likelihood is that the disease may not be seen until much later in life.
An individual without a genetic susceptibility for a disease may be considered much less likely overall to develop the disease or to develop the disease later in life. The important point to remember is that each passing year of the individual’s life increases the amount of time in which the cells have been exposed to risk factors. Some individuals have greater environmental risks than others and may therefore develop the disease at a younger age (see Figure 37-17B). For example, an individual who follows a lifestyle that is reasonably consistent with developing coronary heart disease may experience their first episode of angina in their 20s. While it may be tempting (and perhaps easier) to suggest that this case could be due to a genetic cause, because it occurred at such a young age, the importance of lifestyle factors cannot be ignored. On the other hand, someone without a genetic predisposition who also avoids associated risk factors is not guaranteed to be disease-free. However, they are less likely to develop the disease younger in life.
Thus, although environmental factors may increase or decrease the risk of developing a disease, there are no certainties. This means that undertaking a low-risk lifestyle (such as not smoking) is not a guarantee of avoiding a disease, particularly if there is a strong genetic likelihood. An individual may develop cancer in their 50s after having led a healthy lifestyle. The sceptic may think that a healthy lifestyle was fruitless, because they still developed the cancer. However, it is now becoming evident that lifestyle choices may actually prevent the development of cancer earlier in life, so the avoidance of risk factors slows the progression of disease.
In addition, the influence of environmental factors may well be proportional to the extent of exposure to those factors. For instance, it is unlikely that smoking a handful of cigarettes over your lifetime will cause lung cancer, but moderate levels of smoking over a few years increase the risk and heavy smoking over a longer period, such as a packet a day for 30 years (known as 30 pack years), increases the risk substantially.
Scientific and medical understanding of both genes and risk factors is constantly improving. Some people may have a genetic abnormality for a disease that has not yet actually been discovered. As a result, no genetic test is available and so a genetic link may not be made at this point in time, although it may become apparent in the future. Similarly, some diseases have well-defined risk factors, while other risk factors may be inconclusive in the scientific and medical literature at this stage. An example is the link between high-voltage power lines and the development of cancers, particularly childhood leukaemias. Although some studies indicate a strong link, others have found no sufficient evidence to support this link. It is likely that other risk factors for particular diseases will become more apparent with increased research.
It is clear that there are roles for both genes and environmental factors relating to the main chronic diseases that impact our community. According to WHO: ‘Genes define opportunities for health and susceptibility to disease, while environmental factors determine which susceptible individuals will develop illness.’70 It is our understanding of the interaction between genes and environmental factors that remains relatively preliminary at this point. This interaction may fall into one of the models shown in Table 37-2.71 However, to obtain adequate data on the interactions between these two factors, mass samplings of patient DNA and documentation of environmental factors would be required.
Table 37-2 FOUR BASIC MODELS OF GENE–ENVIRONMENT INTERACTION
| Model 1 | Neither genotype nor environment alone increases the risk of disease; rather, the risk is increased by the combined effect. |
| Model 2 | Genotype exacerbates an already known genetic factor. |
| Model 3 | The environmental risk factor exacerbates an already known genetic factor. |
| Model 4 | Genetic and environmental risk factors both influence the risk, but in combination they greatly influence the risk of disease. |
Source: Giarelli E, Jacobs LA. Modifying cancer risk factors: the gene-environment interaction. Seminars in Oncology Nursing 2005; 21.
THE PREVENTION OF DISEASE
While there appears to be a complex relationship between genes and lifestyles in the development of disease, protective lifestyles may also interact with genes in the prevention of disease. For example, there is some evidence that exercise may offer slightly different degrees of protection from disease depending on genes.72 Thus, some individuals may obtain a greater benefit from exercise than others (see Figure 37-18). Approximately 15 different genes have been identified in this relationship, including alterations in the ACE gene (angiotensin-converting enzyme gene, involved in the renin-angiotensin-aldosterone system) and the ADRB2 gene (the β-adrenergic receptor gene associated with the sympathetic nervous system). Regardless of individual variability, it is important to remember that there are still substantial benefits to be gained from exercise, particularly relating to conditions such as obesity, hypertension and coronary heart disease.
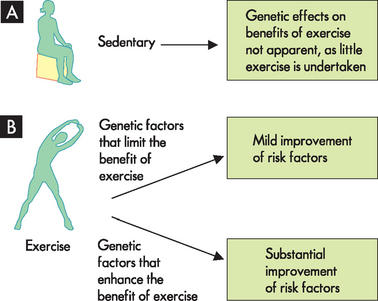
FIGURE 37-18 The effects of genetic factors may influence the benefits of exercise.
A In the sedentary individual, any genetic effects on the benefits of exercise would be minimal. B Some active individuals may reap greater benefits from exercise, based on their genes.
Source: Based on Mori M et al. Genetic basis of inter-individual variability in the effects of exercise on the alleviation of lifestyle-related diseases. J Physiol 2009; 587:5577–5784.
The future of disease prevention and treatment
If genes associated with multifactorial disorders can be clearly identified, population screening could be used to identify individuals with ‘high-risk’ genotypes. Those who are predisposed to develop a particular disease could then be advised to take appropriate action to control relevant environmental factors or to take other preventive measures.
The Human Genome Project has identified the human genome sequence, the complete genetic information on humans. The project was a collaborative effort involving scientific teams from every continent and was completed in April 2003. Mapping the human genome has provided detailed chromosome maps that are assisting researchers in their quest to identify disease genes. (Remember that a gene is a sequence of DNA, so mapping even one gene involves determining substantial amounts of DNA; see Chapter 5.) The sequence of the human genome shows that 99% of the DNA base sequence is identical for all individuals; it is the remaining 1% that makes us interesting! Clearly, these components of DNA cannot easily be mapped for every individual.
Although the human genome sequence is known, the position of many genes is still uncertain. Present estimates are that the human genome contains about 30,000 genes, but the function of half of these genes remains unknown. The average gene size is 3000 bases, but some genes are much larger. For example, the dystrophin gene, associated with Duchenne’s and other muscular dystrophies, covers approximately 2.4 million bases on the X chromosome. The enormous size of this gene means that it is highly prone to mutations, many of which involve deletions of gene segments.
Considerable research effort has been focused on determining the position of genes on chromosomes and identifying disease markers. At present, more than a million single nucleotide polymorphisms (sites where single base variations are observed) have been identified. These sites are associated with variability between individuals and with genetic diseases. Family studies may identify single nucleotide polymorphisms linked to particular diseases and hence help to pinpoint relevant genes. Once disease genes are located and their sequence is known, targeted therapies may be developed:
If research support continues at vigorous levels, we imagine that genome science will soon begin revealing the mysteries of hereditary factors in heart disease, cancer, diabetes, schizophrenia and a host of other conditions. Genomics holds the promise of ‘individualized medicine’, tailoring prescribing practices and management of patients to each person’s genetic profile. These revelations should also lead us to develop and target drugs in a rational fashion to genes, protein pathways and networks shown to be involved in primary disease pathogenesis. Furthermore, a better understanding of the genetic factors that influence susceptibility and/or response to various infectious diseases could have an enormous impact on health in the developing world.73
The potential benefits of mapping the full human genome include the improved ability to diagnose, treat and even prevent diseases in the future. Treatment of chronic disease that may be modified according to genes would allow treatment to be individualised — targeted to the patient, depending on their genes. As an example, someone with the slow variant of the gene UGT1A6 metabolises aspirin more slowly and so this drug appears to have a greater benefit in the prevention of colorectal polyps.53 Aspirin use and dosage could be modified depending on the individual’s variant of the UGT1A6 gene, thereby targeting treatment to match genetics. Population-level screening programs could be introduced to allow early identification and management of genetic conditions.
To conclude, by understanding the function of genes and how genetics are inherited using methods such as family studies and population studies we can define the importance of genetics in disease (see Figure 37-19). In addition, environmental factors are increasingly becoming better understood in their role in disease development. Overall, there is a role for both genetic and environmental factors in the development of disease, although the interaction between them is complex and not completely understood for many conditions. Even if all genes for a particular disease were defined, the ability to use genetic treatments as suggested by the Human Genome Project is still some distance away in the future.
FIGURE 37-19 The relationship between studying families and populations, genetics and environmental factors in the development of disease.
For now, individuals who wish to reduce their likelihood of succumbing to disease may wish to construct a pedigree showing the diseases of family members, which may indicate genetic susceptibilities, as well as known genetic factors such as the skin colour of parents and grandparents. Environmental factors could also be added, such as those who smoke, have high lipid diets, whose lifestyle is relatively sedentary or spend time in the sun. Most chronic diseases are preventable, in that appropriate lifestyle choices can substantially decrease the risk of disease development (see Box 37-2). Individuals may modify the known (and possible) lifestyle or environmental risk factors, as well as considering preventive medications (such as aspirin) to lower the risks and thereby maintain homeostasis for a longer period of time and potentially delay the onset of disease.
Box 37-2 MOST CHRONIC DISEASES ARE PREVENTABLE
‘Chronic diseases are largely preventable diseases. Although more basic research may be needed on some aspects of the mechanisms that link diet to health, the currently available scientific evidence provides a sufficiently strong and plausible basis to justify taking action now. Beyond the appropriate medical treatment for those already affected, the public health approach of primary prevention is considered to be the most cost-effective, affordable and sustainable course of action to cope with the chronic disease epidemic worldwide. The adoption of a common risk-factor approach to chronic disease prevention is a major development in the thinking behind an integrated health policy. Sometimes chronic diseases are considered communicable at the risk factor level. Modern dietary patterns and physical activity patterns are risk behaviours that travel across countries and are transferable from one population to another like an infectious disease, affecting disease patterns globally.’
Source: Diet, nutrition and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series 916. Geneva: World Health Organization; 2003.
Genetic conditions
Inherited diseases
 In dominant disorders, a single copy of the disease allele is sufficient to cause disease. In family pedigrees, the disorder appears in every generation; affected individuals have an affected parent.
In dominant disorders, a single copy of the disease allele is sufficient to cause disease. In family pedigrees, the disorder appears in every generation; affected individuals have an affected parent. For recessive disorders, 2 copies of the disease allele are required to cause disease; individuals carrying 1 copy of the disease allele often display no symptoms. In family pedigrees, the disorder ‘skips’ generations; affected individuals often have unaffected parents.
For recessive disorders, 2 copies of the disease allele are required to cause disease; individuals carrying 1 copy of the disease allele often display no symptoms. In family pedigrees, the disorder ‘skips’ generations; affected individuals often have unaffected parents. The X chromosome carries several recessive disease alleles. Females, with two X chromosomes, may carry X-linked recessive traits; males, with a single X chromosome, display X-linked recessive traits. In family pedigrees, the disorder ‘skips’ generations and appears almost exclusively in males; affected females are very rare.
The X chromosome carries several recessive disease alleles. Females, with two X chromosomes, may carry X-linked recessive traits; males, with a single X chromosome, display X-linked recessive traits. In family pedigrees, the disorder ‘skips’ generations and appears almost exclusively in males; affected females are very rare.Genetic screening
Congenital abnormalities
Multifactorial inheritance
 Polygenic traits are controlled by several genes, so do not follow simple dominant/recessive patterns of inheritance.
Polygenic traits are controlled by several genes, so do not follow simple dominant/recessive patterns of inheritance. Multifactorial traits are influenced by several genes and by environmental factors; twin studies are useful in determining the relative contributions of genes and environment.
Multifactorial traits are influenced by several genes and by environmental factors; twin studies are useful in determining the relative contributions of genes and environment.Conditions arising from genetic and environmental factors
 The development of most chronic diseases may be influenced by both genetic factors and environmental factors. Determining which genes are involved and which environmental factors are relevant are topics of ongoing research. The interactions between genetics and environmental factors may also be revealed with future research.
The development of most chronic diseases may be influenced by both genetic factors and environmental factors. Determining which genes are involved and which environmental factors are relevant are topics of ongoing research. The interactions between genetics and environmental factors may also be revealed with future research. There is strong evidence for the role of both genetics and environmental factors in coronary heart disease; genetic influences may be more important in females than in males.
There is strong evidence for the role of both genetics and environmental factors in coronary heart disease; genetic influences may be more important in females than in males. Familial hypercholesterolaemia may arise from a dominant genetic abnormality; considerable modification of the environment, including pharmacological treatment, is necessary.
Familial hypercholesterolaemia may arise from a dominant genetic abnormality; considerable modification of the environment, including pharmacological treatment, is necessary. Only 30% of hypertension may be hereditary; the remaining 70% is likely to be caused by the environment.
Only 30% of hypertension may be hereditary; the remaining 70% is likely to be caused by the environment. Approximately 40–70% of cases of obesity may result from heredity, with the rest the result of environmental factors. Rare genetic causes of obesity include those associated with leptin.
Approximately 40–70% of cases of obesity may result from heredity, with the rest the result of environmental factors. Rare genetic causes of obesity include those associated with leptin. Several genetic causes have been determined for type 1 diabetes. Environmental factors, currently poorly understood, may trigger these genetic abnormalities and lead to the development of type 1 diabetes.
Several genetic causes have been determined for type 1 diabetes. Environmental factors, currently poorly understood, may trigger these genetic abnormalities and lead to the development of type 1 diabetes. Genetic factors may be important in the development of type 2 diabetes; however, it is well known that environmental factors are implicated in its development.
Genetic factors may be important in the development of type 2 diabetes; however, it is well known that environmental factors are implicated in its development. Cancers occur due to abnormalities in the genes, but these abnormalities may arise due to a combination of genetic and environmental factors. Some cancers (such as some types of breast cancer and colorectal cancer) are related to well-defined genes. For most people, environmental factors are critical in the development of cancer.
Cancers occur due to abnormalities in the genes, but these abnormalities may arise due to a combination of genetic and environmental factors. Some cancers (such as some types of breast cancer and colorectal cancer) are related to well-defined genes. For most people, environmental factors are critical in the development of cancer.The relative importance of genetic and environmental contributions to disease
 An individual with a genetic susceptibility and who experiences unfavourable environmental factors is likely to develop a disease early in life; however, if the same individual experiences mainly protective environmental factors, the disease may not develop until later in life.
An individual with a genetic susceptibility and who experiences unfavourable environmental factors is likely to develop a disease early in life; however, if the same individual experiences mainly protective environmental factors, the disease may not develop until later in life.The prevention of disease
 Complex relationships appear to be involved in not only the development of disease, but also protection from disease.
Complex relationships appear to be involved in not only the development of disease, but also protection from disease. Identification of individuals with genetic susceptibility may lead to personalised prevention and treatment options.
Identification of individuals with genetic susceptibility may lead to personalised prevention and treatment options. The Human Genome Project is an important step in understanding the human genome and may assist in targeting future prevention and treatment strategies.
The Human Genome Project is an important step in understanding the human genome and may assist in targeting future prevention and treatment strategies.Felicity is 29 years old and recently married. She had an older brother, now deceased, who suffered from Duchenne’s muscular dystrophy, an X-linked recessive disorder. Although Felicity would like to start a family, she is quite worried that she might have a child affected with Duchenne’s muscular dystrophy and she therefore seeks genetic counselling.