Chapter 2 Pharmacotherapy
Clinical Use of Drugs
Pharmacotherapy refers to the use of drugs for treating or preventing disease, and is sometimes referred to as clinical pharmacology, as distinct from theoretical or experimental pharmacology, in which drugs may be studied to understand their mechanisms of action and effects. In this chapter, we focus on optimising the medical use of drugs, from government policies on drug use, through the roles of health professionals in prescribing, dispensing and administering medicines, to altering and monitoring the person’s responses to drugs.
ACEI angiotensin-converting enzyme inhibitor
ASCEPT Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists
EBM/P evidence-based medicine/practice
MBS Medicare Benefits Schedule
P-list personal preferred list of drugs
PBS Pharmaceutical Benefits Scheme
PHARM Pharmaceutical Health and Rational Use of Medicines
RCCT randomised controlled clinical trial
Quality use of medicines
IN a review of pharmacotherapeutics in Australia in the 20th century, two eminent clinical pharmacologists (Day & Mashford 2001) described the progress in pharmacology up to the 1950s as ‘an impressive story of rapid progress from empiricism towards a rational, biologically based approach to disease’ and since the 1950s as ‘an explosion in the numbers and variety of drugs available and illnesses that are treatable … (and) a marked improvement in general levels of efficacy and tolerability of medicines’. To optimise the use of these new drugs in a rational, clinically- and cost-effective manner, it is important that health professionals understand the decision-making processes before drugs are prescribed or advised, how prescriptions are written and dispensed, the types of formulations in which drugs are administered, the factors that can affect how people respond to drugs, and how drug therapy is monitored to assess the person’s progress—in fact, basically the whole of clinical pharmacology!
Quality use of medicines (QUM) means selecting management options wisely, choosing suitable medicines if a drug is considered advisable and using medicines safely and effectively (see Australia’s National Medicines Policy website). Some professional groups have incorporated a QUM policy within their own charter; for example, the Royal College of Nursing Australia has produced a position statement around Advanced Practice Nurses including their implementation of QUM.
Evidence-based health care
Health professionals generally are encouraged to practise evidence-based health care, commonly referred to as evidence-based medicine (EBM)—the conscientious, explicit and judicious use of current best evidence in making decisions about the medical care of individual patients.1 The practice of EBM (or EBP: evidence-based practice) requires the integration of individual clinical expertise with the best available external clinical evidence from systematic research. This should apply whatever the style of practice, whether with drugs, physiotherapy techniques, orthoptic eye exercises or optometric lens prescriptions, podiatric or dental surgery, nursing care, ambulance and paramedic emergency aid or complementary and alternative remedies.
In the context of drug therapy, the randomised controlled clinical trial (RCCT) has long been accepted as the ‘gold standard’ of evidence; many other types of therapy, which may have been in traditional use for decades or centuries without any evidence of efficacy, are now being subjected to clinical trials (see Chapter 4 and CIB 4-3). Studies in many countries have shown that a large proportion of patients do not receive care based on bestavailable evidence; for example, DUE projects (see later) show there is widespread under-use of oral anticoagulants in people with atrial fibrillation, and conversely over-use of antibiotics in treatment of common cold and acute bronchitis (see Buchan [2007]).
Four levels of evidence
The Australian National Health and Medical Research Council (NHMRC) has classified the strength of evidence on which EBM/EBP is based into four levels, of decreasing value:
Evidence based on clinical experience and descriptive studies, from committees or colleagues, is considered a low form of evidence on which to base clinical decision making. An electronic search of databases such as the Cochrane Collaboration, AustHealth or Medline usually quickly reveals available evidence about a particular treatment. Drug information and prescribing advice in semi-official sources such as the Australian Medicines Handbook or Therapeutic Guidelines is based on the highest-level evidence available. (The term ‘anecdotal evidence’ should be banned as a misnomer—an anecdote can never be relied on as best clinical evidence.)
The evidence–relevance gap
Even with the optimal amount and level of evidence available, the gap between scientific evidence at the population level and what is relevant to a particular patient remains challenging to health professionals. It is impossible for individual clinicians to evaluate the latest research evidence for safety and efficacy of every drug or other medical product. To assist, guidelines produced by independent authorities (such as the Therapeutic Guidelines series) can provide unbiased objective information (see Dartnell et al [2008]).
For example, in the 1990s women were bombarded with an overload of information in the lay press from largescale clinical trials about hormone replacement therapy (HRT) in menopause. Many found it impossible to decide the relevance to their particular situation (suffering symptoms? predisposed to breast cancer? or to colorectal cancer? or cardiovascular disease? want protection against osteoporosis?), and to weigh the short-term benefits of relief of menopausal symptoms and long-term protection from osteoporosis against the possible long-term higher risk of cancers. In fact, the changes in long-term outcome are so small as to be irrelevant for many women (see Neeskens [2002] and CIB 38-2).
Targeting QUM
National Medicines Policies
In 1985 the World Health Organization (WHO) held a conference on the Rational Use of Drugs, and afterwards called on all governments to implement a National Medicinal Drug Policy. Also during the 1980s, many Australian consumer organisations and medical activists campaigned for rationalisation of government policies with respect to medi cines. These initiatives together led to the establishment under the auspices of the PBS of a National Medicines Policy (NMP) to achieve positive health outcomes through QUM. The stated aims of the NMP are:
Some examples of recent studies and programs in QUM include alerting people to the possibility of interactions between prescription medicines and alternative therapies; education about the development of antibiotic-resistant microorganisms and the need to rationalise prescribing of antibiotics; advice that paracetamol is often over-used in treating childhood fevers; and information about the use in arthritis of the new cyclo-oxygenase-2 (COX-2) inhibitors and their adverse effects.
PHARM
Several effective arms to this policy have been implemented. The Australian Pharmaceutical Health and Rational Use of Medicines (PHARM) Committee has placed QUM high in priority on the national health agenda. QUM is considered to mean: (1) selecting management options wisely; (2) choosing suitable medicines if considered necessary; and (3) using medicines safely and effectively. Information sources supported include the journal Australian Prescriber, the Therapeutics Resource and Educational Network for Doctors (TREND), Therapeutic Guidelines and the Australian Medicines Handbook, as well as Consumer Medicine Information (CMI) handouts, which are usually included inside packs of medicines or are available from pharmacists. Barriers to QUM were identified, such as waste or hoarding of medicines, inappropriate demand and poor compliance. Many campaigns on using medicines wisely have been supported at national, state and local levels.
NPS
The Australian National Prescribing Service (NPS) was set up (1998) with representation from doctors and pharmacists to improve health outcomes for all Australians, enhance continuity of QUM programs and coordinate activities influencing prescribing. The NPS has the mission to improve health outcomes for all Australians through QUM. Through its educational activities targeted to doctors, pharmacists, nurses, other health practitioners, consumers and the pharmaceutical industry, the NPS has achieved some significant savings in drug costs. Its computer-based prescribing curriculum is being adopted and implemented by many medical schools, and is seen as providing excellent training of medical students in clinical pharmacology and prescribing of drugs, previously a serious deficit in many programs which led to inadequate prescribing skills in new medical graduates.
New Zealand Medicines Strategy
In New Zealand, the Preferred Medicines List provides guidelines aimed at achieving QUM in the health system in that country (see also CIB 2-2 later). The Pharmacology and Therapeutics Advisory Committee (PTAC) and the Best Practice Advocacy Centre have responsibility for implementing the WHO Medicines Strategy, and are working on developing a New Zealand National Formulary. Primary Health Organisations (PHOs) are funded by District Health Boards to provide essential primary health care services to those people who are enrolled with the PHO (see CIB 2-2). PHARMAC (the Pharmaceutical Management Agency) has responsibility for purchasing medicines and managing supplier contracts under a capped national medicines budget; this has effectively kept down the costs of subsidising drugs compared to the escalating costs in many other developed countries.
Drug usage evaluation
The process of maximising QUM for cost-effective health care, particularly in the hospital context, requires regular monitoring and evaluation of the use of drugs in the institution in order to define patient groups that will best benefit from drugs, to optimise hospital prescribing and to face the implications of spiralling costs and capped budgets. Drug usage evaluation (DUE) teams include clinicians, pharmacologists, pharmacists and nurses interested in QUM in their institution.
Many members of the Clinical Interest Group of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT) have established DUE networks to encourage, review and discuss DUE activities in hospitals; so also have various state chapters of the Society of Hospital Pharmacists of Australia. An interesting study on antibiotic prescribing for community-acquired pneumonia is reviewed in Clinical Interest Box 2-1. Other recent DUE studies have been in the areas of:
Clinical interest box 2-1 A drug usage evaluation case study: antibiotics for pneumonia
The CAPTION project (Community-Acquired Pneumonia: Towards Improving Outcomes Nationally), aimed at improving prescribing of antibiotics for community-acquired pneumonia (CAP) in hospital emergency departments, ran in five Australian states from 2003 to 2005, then was extended to accommodate the new Therapeutic Guidelines: Antibiotics in 2006. Key messages were incorporated into intervention materials such as letters to doctors, wall posters, PowerPoint slides for seminars and stickers with diagnostic hints; these were distributed to participating hospitals and group education and feedback sessions were held. Results were based on audits of use of the Severity Index in diagnosis and prescribing of the appropriate antibiotic (usually penicillins); average length of patient stay and inpatient mortality were documented.
Conclusions from the project showed that the interventions improved management of CAP and rate of appropriate prescribing; however, the techniques used were resource intensive. Participation in the project was seen to improve interdisciplinary working relationships in hospitals and recognition of the role of the pharmacist. Many recommendations were made from the study: on the management of CAP, the methodology of quality improvement, linkages between prescribing in hospitals and the community and the use of ‘social marketing interventions’ in the hospital sector. A major benefit is that a large multicentre DUE such as CAPTION allows hospitals which could not individually undertake such a project to participate, with documented benefits to individuals, staff, patients and hospitals. (See Pulver et al [2009] and www.ciap.health.nsw.gov.au/nswtag/caption.html.)
Such studies have been shown to save not only lives and time but also money. A survey in eight major teaching hospitals across six Australian states looked at changes initiated by clinical pharmacists to drug therapy regimens and patient management (Dooley et al 2003). Altogether, 1399 interventions were documented, evaluated by independent panels and costed. The commonest reasons for intervention were to decrease potential adverse events or morbidity or mortality, and to increase efficacy and symptom control. The most frequent outcomes were avoidance of admissions and procedures, and changes to laboratory monitoring of drug plasma concentrations. Annualised savings at the eight sites totalled over A$4.44 million; for every dollar spent on pharmacist time, $23 were saved. This study demonstrated that routine clinical pharmacist review of inpatient drug therapy is an essential component of QUM programs, and sign ificantly reduces length of hospital stay and potential for readmission.
A website has been set up to collate information on all DUE studies being done in Australia, to prevent duplications and encourage collaborations: see www.qummap.net.au. It is hoped that, as research is implemented, flow-on will occur to maximise QUM in general practice as well as in hospitals.
Modifying drug usage over time
Pharmacopoeias and formularies are in a constant state of change, and health professionals need to keep up to date with current drug information. Some of the influences on evolving drug use are described below, with examples of drugs affected.
New technologies
Until the early 20th century, most drugs were from natural sources such as plants (morphine, cocaine), minerals (iodine, iron) and animals (vaccines, tissue extracts). As chemical industries developed, synthetic and semisynthetic drugs such as antibacterial sulfonamides, thiazide diuretics, volatile general anaesthetics, corticosteroids and oral contraceptives, safe antihypertensives and effective antipsychotic agents became available. Genetic engineering has produced human insulin and other hormones and proteins. Many older, less effective and less safe drugs (such as bromide hypnotics and mercurial diuretics) have become obsolete.
New uses for old drugs
Drugs are sometimes found to have additional uses to those for which they were initially developed. Minoxidil, an antihypertensive agent, was found to cause increased growth of hair and found a new use as a hair restorer. Methylphenidate, originally an appetite suppressant and stimulant, found new application in treating attention deficit hyperactivity disorder.
Better understanding of mechanisms
The discovery of the mechanism of action of aspirin (inhibiting synthesis of prostaglandins) led to further evaluation of its actions. Results of clinical trials showed that subjects receiving aspirin suffered fewer adverse cardiovascular events, demonstrating the antithrombotic actions of aspirin, hence its now widespread prophylactic use against heart attacks and strokes.
Better understanding of aetiology of disease
The evolution of better drugs to treat peptic ulcers followed studies over many decades of the causes of peptic ulcers. First, sedatives were prescribed to reduce stress, then antacids to neutralise gastric acid, then antimuscarinics, histamine H2-receptor antagonists and proton-pump inhibitors to reduce production of acid, and recently antibacterials to reduce infection with Helicobacter pylori were added to the gastroenterologist’s armamentarium. Similarly, rapid advances in understanding of the biochemical pathways involved in control of cell division have led to many new anti-cancer drugs (Chapters 41 and 42).
Old ‘remedies’ proven useless
For centuries syphilis was treated with arsenic- or mercurycontaining compounds, perhaps because no effective treatment was available. These very toxic medicines were used for hundreds of years despite a total lack of efficacy. Only when safe oral antibacterials became available (and the concept of clinical trials developed) did arsenicals and mercurials drop out of pharmacopoeias.
Drug combinations shown to be unjustified
Complex combinations of drugs (galenicals) date back to the time of the ancient Greek physician Galen (CIB 1-3; Figure 1-1). Even into the mid-20th century, doctors often wrote prescriptions for complex mixtures ‘for nerves’ or as ‘tonics’—see an example in Figure 2-3A. Frequently, combinations of antimicrobials or of antihypertensives were formulated together. It is now recognised that it is usually better to prescribe drugs individually, as doses can then be adjusted individually and the drug with the longer half-life does not accumulate and cause toxicity, or the drug with the shorter half-life drop below the therapeutic level.
Changes in popularity of drugs
There is a recognised cycle in popularity of many new drugs (as for many new gadgets): as a drug is developed and marketed—often aggressively to both prescribers and consumers—it rapidly surges in popularity. Adverse reactions inevitably become apparent and its expense is noted, so its use wanes. Then, as rational evidence of the benefits and risks are evaluated, the drug regains a medium but more stable position in the drug usage charts (Figure 2-1).
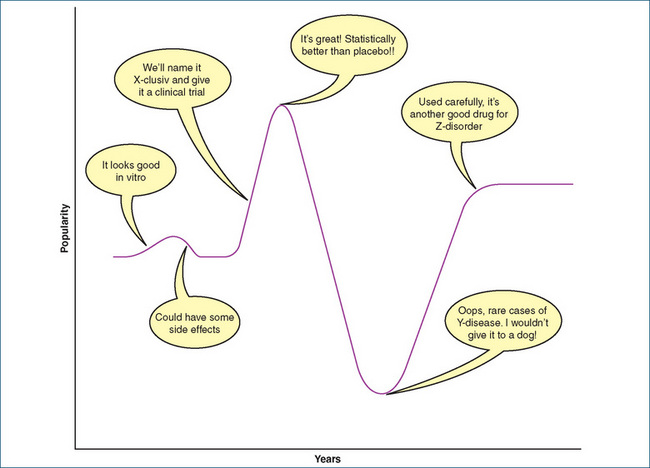
Figure 2-1 The ‘action potential of popularity’ of a new drug
after Figure 1 in Clinical Pharmacology 5th edn, DR Laurence and PN Bennett, Churchill Livingstone, Edinburgh, 1980.
Changes in availability of drugs
There may be a major change in the use of a drug as it is moved between drug schedules (see Chapter 4) and becomes either more or less readily available or expensive. When the histamine H2-receptor antagonists were introduced, they rapidly became very popular and hospital drug budgets blew out. Restrictions were placed on the frequency of their prescribing to rationalise their use and costs. Similarly, when new COX-2 inhibitors (celecoxib, rofecoxib) and statins (simvastatin etc) were introduced in Australia, they were very expensive. Public (and drug company) pressure led to their being subsidised and listed on the PBS, when their use skyrocketed, at great expense to governments (i.e. taxpayers). However, when patent protection for new drug molecules expires, other companies can legally manufacture and market the drug, so competition (hopefully) reduces the price.
Withdrawal of useful drugs
Drug companies may decide to discontinue production and marketing of a useful drug for various reasons: an old drug may become redundant or unprofitable, an old manufacturing process become obsolete, rare unexpected adverse reactions may become apparent, a disease may become much less prevalent or company mergers may bring competitor products into the same ‘house’. However, there can be important implications for patients who may have been stabilised on a particular drug for many years and are intolerant or unresponsive to other drugs. Change to a new drug may cause withdrawal reactions, recurrence of illness or new ADRs or interactions. A coordinated approach between drug companies, government and health professional organisations is required to minimise these problems.
When to use a new drug
The safety and efficacy of new drugs are now determined in RCCTs and the information is soon added to the evidence base for treating certain conditions. Knowledge about the new drug, however, is still quite limited, and postmarketing use of the drug may bring evidence of unusual ADRs and efficacy in subgroups of the population, e.g. pregnant women, children and the elderly. Prescribers then need to consider carefully when they prescribe new drugs. The cautious approach suggests that doctors limit their prescribing of new drugs until post-marketing surveillance provides accumulated data on clinical- and cost-effectiveness in large populations.2 In practice, despite the thousands of drugs in the pharmacopoeias, studies show that most general practitioners prescribe from a limited personal list of less than 100 tried-and-true preferred drugs—their ‘P-list’.
The ‘popularity action potential’ for rofecoxib
A good example of the caution necessary in rapidly prescribing new drugs is the case of the COX-2 inhibitor rofecoxib (Vioxx). This anti-inflammatory agent was introduced in Australia in 1999 after trials in over 5000 patients who had received it for less than 6 months; there was a reported low overall incidence of thromboembolic events. Rofecoxib rapidly became very popular (as was celecoxib) for treatment of painful and inflammatory conditions in patients who might be at risk of gastrointestinal side effects from the older NSAIDs. Meanwhile, analyses of cardiovascular events occurring during larger-scale longer trials and post-marketing use showed that people taking 25 mg rofecoxib daily over a long term had about double the risk of heart attacks and strokes compared to placebo (more details are given in CIB 47-2, and reviews by Jones [2005] and Krumholz et al [2007]). The drug was withdrawn in Australia in 2004; since that time, related COX-2 inhibitors have been closely monitored for similar effects. (Hundreds of patients in Australia have joined legal class actions against the manufacturer of rofecoxib, claiming that the manufacturer knew about the increased risk of cardiovascular events long before the drug was withdrawn. It is reported that, in the year before its withdrawal, the manufacturer Merck reaped revenue of at least US$2.5 billion from sales of Vioxx.)
Pharmacoeconomics
Because of the blow-out in demands for and costs of drugs, no country can provide all the drugs that might be desirable; hence, economic rationalism is essential and decisions must be made to ration drugs and contain costs, if possible without compromising good health care (pharmacoeconomics)—see Table 1-4. Such decisions have both clinical and ethical implications (the latter will be discussed in Chapter 4).
While detailed study of health economics is beyond the scope of this text, some of the issues involved have already been mentioned, such as the costs of developing and providing drugs, the increasing need for drugs by the growing elderly proportion of the population and the increasing demands for ‘lifestyle’ drugs. Health economists need to evaluate many aspects of drug use, including indirect aspects such as savings from shorter hospital admissions, improved quality of life and surgery avoided. Overall, policies such as generic substitution (dispensing the cheaper alternative among medicines considered bioequivalent) and rationalisation of drug policies and QUM help optimise access to essential drugs.
Cost-effectiveness of new drugs
To be listed by the Australian Pharmaceutical Benefits Scheme (PBS) as a subsidised drug, a drug must be proved to be not only safe and effective but also cost-effective, which requires an economic evaluation of the additional cost of any extra benefit over the current standard therapy. Listing as a ‘restricted benefit’ or as ‘authority required to prescribe’ helps limit the PBS use of a drug to those in whom it will be most effective.
For example, a cost-effectiveness analysis of the platelet aggregation inhibitor clopidogrel compared to aspirin in secondary prevention of coronary heart disease showed that clopidogrel alone or in routine combination with aspirin would add a cost of $130,000 (US$, 2002) per quality-adjusted year of life gained3 (see Gaspoz et al [2002]). The Australian approved indications for authority to prescribe clopidogrel list only a few very specific situations in which the drug can be subsidised by the PBS, for example to prevent recurrence of stroke or heart attack in patients who are unable to take aspirin safely. The National Prescribing Service guidelines conclude that ‘clopidogrel has similar efficacy to aspirin plus dipyridamole … low-dose aspirin is a more affordable option for people who do not meet the PBS authority restrictions for clopidogrel’ (NPS News 62, 2009).
Roles of health professionals with respect to drugs
Traditionally, the health professionals most involved with drugs in hospitals were doctors (and dentists), who prescribed them; pharmacists, who dispensed them; and nurses, who administered them. Medicine as a profession in England developed with physicians allowed to prescribe ‘physic’ (medicine) compounded by apothecaries (pharmacists). Apothecaries had developed along with grocers, as they both used scales for weighing. Apothecaries became specialist medicine sellers and used leeches, herbal remedies, pills and potions. Surgeons and barbers were also originally associated because they used the same tools of trade (razors and knives, basins and towels). During the Great Plague (1660s), however, many physicians fled London, leaving apothecaries to care for the sick and dying. After that time, apothecaries gained in popularity and became the general practitioners of the day, even delivering babies. It was not until the 19th century that these roles diverged into those we are familiar with today.
Now, again, the roles of health professionals are changing, the boundaries between them are breaking down and many more health professionals are involved with drugs or with people who are taking them; hence the need for all health professionals to know, if not the same depth of pharmacology, at least some of the language and principles of pharmacology. The roles of many of these professionals will be described briefly.4 Specialised aspects, such as details of nursing roles in drug administration and nursing implications of drug therapy, are beyond the scope of this text. In Australia, state and territory governments have historically been responsible for regulating health practitioners; however, national registration guidelines are being developed. Information in Clinical Interest Box 2-2 describes the integration of many health professionals in Primary Health Organisations in New Zealand.
Clinical interest box 2-2 A new zealand health care strategy
The Government in New Zealand introduced a new Health Strategy in 2005. The Primary Health Care Strategy places emphasis on prevention of illness by improving access to, and funding of, primary health care services. The aim is to improve the health of all New Zealanders and promote community involvement in the maintenance of health.
The Primary Health Care Strategy included the formation of Primary Health Organisations (PHOs); these organisations include doctors, nurses, pharmacists and other trained, skilled health professionals (such as Maori health workers, health promotion workers, dieticians, physiotherapists, psychologists and midwives) working together to provide primary health services. Patients need to enrol as members of the PHO, and their visits to the GP are then subsidised by the government. This makes the visit cheaper for the patient and health services more accessible to the public. Children and students at school receive subsidised visits and medicines, and within the PHO structure this has been extended to include 18- to 24-year-olds.
PHOs receive their funding from the local District Health Board (DHB) and are not-for-profit organisations. The funding is based on the numbers and characteristics (e.g. age, sex, ethnicity) of people enrolled with them. That funding pays for:
Not all New Zealanders are members of a PHO, and not all GP practices are PHOs.
Adapted from: http://www.moh.govt.nz/moh.nsf/indexmh/phcs-pho [1 January 2010].
Ambulance and ‘MICA’ paramedics
These specialists in first aid and emergency care are often the first health professionals to respond to situations such as accidents, acute myocardial infarctions (heart attacks), cerebrovascular accidents (strokes) and severe asthma attacks. The roles of standard ambulance paramedics include rapid assessment of a person after medical or trauma emergencies in the pre-hospital setting—especially for central nervous system, cardiovascular and respiratory functions—in order to provide life-saving support and stabilise the condition until the patient is received at a medical facility. Under written standing orders in medical protocols, paramedics can administer drugs, including chewable aspirin and sublingual glyceryl trinitrate for chest pain, IM midazolam for seizures, inhaled methoxyflurane as an analgesic, glucose buccal gel for hypoglycaemic attacks and IM naloxone for opioid overdoses. Paramedics with extra training for the Mobile Intensive Care Ambulance (MICA) service also need to be able to administer more powerful drugs, including anti-arrhythmic agents, cardiac stimulants, bronchodilators, corticosteroids, muscle relax ants and potent analgesics, and to set up intravenous fluid admini stration drips.
Complementary and alternative medicine practitioners
Complementary and alternative medicine (CAM) practitioners use techniques including provision of herbal products, massage, acupuncture, naturopathy, homeopathy and iridology. (These areas are covered in Chapter 3.) It is being increasingly recognised that there can be interactions between practices in Western medicine and CAM, e.g. drug interactions between prescribed drugs and CAM preparations that patients may be taking concurrently, so it is important that such practitioners have some understanding of pharmacology and the potential for problems to occur.
Dentists
Dentists are allowed to prescribe drugs related to their treatment—in particular, antibiotics and analgesics. They frequently administer local anaesthetics during dental procedures to prevent and relieve pain.
Dietitians
Dietitians are particularly involved with the principles of nutrition and food as they relate to health. There is overlap with pharmacology in areas such as parenteral nutrition, dietary supplements and food–drug interactions; dieticians can advise but not actually prescribe.
Doctors
Doctors (medical practitioners) are responsible for diagnosing disease and initiating and monitoring therapy, including prescribing drugs that are not available OTC. Doctors therefore require extensive knowledge of pharmacology in all aspects: actions and mechanisms of drugs (pharmacodynamics), drug handling by the body (pharmacokinetics) and, in particular, clinical aspects, including ADRs, drug interactions, dosages, indications and contraindications for drug use, in all situations and patients, whether children, adults, the elderly or those with concurrent diseases and being administered other therapies. Doctors may specialise in many fields of medicine, such as obstetrics and gynaecology (women’s conditions, pregnancy and childbirth), paediatrics (children), oncology (cancers), anaesthetics etc. Specialists will obviously require particular knowledge of the drugs used in that area. It is said that anaesthetists are the world’s best clinical pharmacologists because, during a surgical operation, they are continually administering many drugs, monitoring the patient’s responses, checking for adverse reactions and drug interactions, altering doses and responding to the clinical situation minute by minute (see CIB 14-5).
The ‘doctor’s bag’ of drugs
Traditionally, doctors visiting patients in the community carried a black bag holding various drugs that the doctor could supply or administer as needed, particularly in emergencies. These ‘doctor’s bag’ supplies may also be important for emergencies or other serious medical conditions that occur in the surgery or office. The PBS still allows doctors to carry such drugs, most of them in parenteral (injectable) form and subsidised by the PBS; choice depends on practice location, conditions likely to be encountered and shelf-life of the formulation. Examples are adrenaline (for cardiac arrest and severe allergic reactions), atropine (for cardiac arrhythmias or poisoning with insecticides), benzylpenicillin (for bacterial infections especially meningococcal), haloperidol (for psychiatric emergencies), frusemide (a potent diuretic drug used in hypertension and acute pulmonary oedema), hydrocortisone (an anti-inflammatory and immunosuppressant agent), lignocaine (anti-arrhythmic and local anaesthetic), glucagon (for hypoglycaemia), morphine (to treat severe pain and pulmonary oedema) and naloxone (an opioid antagonist used to treat overdose with opioids such as heroin). Other non-injectable drugs include soluble aspirin tablets (first-line treatment for anyone suspected of having had a myocardial infarction), diazepam (sedative, antianxiety and anticonvulsant), glyceryl trinitrate spray or patch (for angina or myocardial infarction), salbutamol aerosol (for asthma), methoxyflurane inhalation (for painful procedures on conscious trauma patients) and possibly antiemetics (to prevent vomiting), antibiotics and analgesics. It is suggested that doctors also carry supplies of normal saline and water for injection, as well as a sharps container, disposable gloves and dressing packs. Logbooks of supplies need to be kept, and a system for checking expiry dates implemented (see Baird [2007]).
Health information managers
Health professionals trained in health information management specialise in managing databases in epidemiology and clinical trials data, coding data from patient hospital records, evaluating reports on accreditation and standards and analysing ‘casemix’ information (that related to hospital admissions, patient diagnoses and records, and funding). These health workers need to understand basic concepts in pharmacology and to know the major groups of drugs in order to understand the patient and the medical record and, for example, to be able to code accurately whether a condition is a primary one or an adverse drug reaction, or to understand why treatment of some conditions requires lengthy hospital stays.
Indigenous health workers
Indigenous people throughout the world tend to be disadvantaged across a range of socioeconomic factors, and continue to suffer a greater burden of ill-health than the remainder of the population. As Indigenous groups move from traditional to modern lifestyles, groups rapidly acquire lifestyle diseases such as obesity, cardiovascular disease and type 2 diabetes. In Australia, this is manifest in the average life expectancy of Indigenous people being 17 years less than that of non-Indigenous Australians.
Indigenous Australians (Aboriginal and Torres Strait Islanders) make up approximately 2.4% of the population; of these about 25% live in areas classified as remote or very remote. In the Indigenous Aboriginal communitycontrolled health services in some remote areas of Australia, Indigenous health workers, in collaboration with visiting nurses and pharmacists, provide essential knowledge about health, society and culture. For example, in the Kimberley region of Western Australia, health workers who recognise problems and are familiar with a range of common medicines and their effects play important roles in home medicine reviews, improvement of compliance, help with interviews and communication and information about social circumstances. Greater implementation of Section 100 of the PBS (which provides alternative arrangements for supply and dispensing of medicines for Aboriginal Health Services in remote zones of Australia) would improve access of Indigenous Australians to health services and medicines. (See Couzos and Thiele [2007]; Larkin and Murray [2005]; Stoneman and Taylor [2007].)
Midwives
Midwifery is the area of health care specialising in antenatal care, labour and childbirth; most midwives are registered nurses. Midwifery is generally concerned with normal pregnancy and labour, whereas in difficult or abnormal situations or where medical conditions complicate pregnancy, obstetricians are called in. (Regulations as to prescribing of drugs vary between jurisdictions; local laws should be checked.)
Midwives involved in antenatal care may recommend drugs such as iron and folic acid to treat anaemias. During childbirth, nitrous oxide or oxygen may be administered and, in the absence of a doctor, midwives in some jurisdictions are allowed on phone order to administer one or two doses of pethidine, oxytocin and/or ergometrine for a woman in labour (see Chapter 38), and metoclopramide as an antiemetic. Such doses must be followed up with a written doctor’s prescription within 24 hours. A midwife may top up an epidural anaesthetic if a cannula is in place. The neonate may require oxygen, naloxone for reversal of opiate-induced respiratory depression and vitamin K administered IM.
In New Zealand midwifery registration is an independent registration, so midwives do not necessarily have concurrent registration as nurses. New Zealand midwives work in partnership with women as the lead maternity carer (LMC) in low-risk pregnancies, providing support, care and advice during the antenatal period, labour and up to 6 weeks post partum. Midwives are authorised prescribers under the 1990 Amendments to The Medicines Act and can prescribe medicines for the woman and her baby. There is no separate schedule of medicines from which midwives can prescribe, but Regulation 39 of the Medicines Regulations restricts prescription of any medicine to antenatal, intrapartum and postnatal care (http://www.legislation.govt.nz/). The scope for practice and competency standards for New Zealand midwives can be viewed on the New Zealand Midwifery Council website: http://www.midwiferycouncil.org.nz/index.cfm.
Nurses
Traditionally, nurses have worked in hospitals, community health centres, specialist medical clinics and private practice, and in other areas such as rural and district nursing services and in industry. Nurses are involved, among other roles, in ensuring safe and reliable administration of drugs and in monitoring adverse reactions. In the hospital situation this could include:
Nurses may be the first to recognise adverse reactions such as hypotension, constipation or nausea and vomiting, and have the responsibility to ensure appropriate treatment is instituted. Nurses may refuse to administer a prescribed drug if they feel that the situation has changed and it is not in the best interests of the patient, and have the responsibility to prevent medication errors. Nurses are not allowed to initiate or change prescribed drug therapy or alter labels on drug packs. In an emergency, nurses may implement verbal directions from a doctor to administer a drug, but this must be followed by a written prescription as soon as practicable or, usually, within 24 hours.
Nurse practitioners
In several countries, laws have been changed recently to allow nurses with special expertise and training (including in pharmacology and principles of prescribing) to apply for endorsement as nurse practitioners. The scope of the role is still being established and models are being trialled and evaluated in consultation with doctors and pharmacists.
The Australian Nursing and Midwifery Council (ANMC) has developed a set of National Competency Standards for the Nurse Practitioner (see http://www.anmc.org.au/professional_standards), in which a nurse practitioner is defined as follows:
A nurse practitioner is a registered nurse educated to function autonomously and collaboratively in an advanced and extended clinical role. The role includes assessment and management of clients using nursing knowledge and skills and may include but is not limited to the direct referral of patients to other health care professionals, prescribing medications and ordering diagnostic investigations. The nurse practitioner role is grounded in the nursing profession’s values, knowledge, theories and practice and provides innovative and flexible health care delivery that complements those provided by other health care providers. The scope of practice of the nurse practitioner is determined by the context in which the nurse practitioner is authorised to practise.
It is envisaged that the scheme might include permission for approved nurse practitioners, in their defined area of expertise, to:
In part, this movement is in response to the problems in country areas where there are insufficient doctors. There is at present no single published formulary of drugs that nurse practitioners may prescribe, but the process of diagnosis, drug choice, prescribing, counselling and monitoring therapy would be within the guidelines of the QUM program. Examples of areas in which nurse practitioners may specialise are rural health, diabetes management, drug and alcohol management, geriatric medicine, palliative care and sexual health. It is recognised that, for optimal implementation of the changes, nurse practitioners will also need to be able to access MBS and PBS subsidies for their prescribing, as well as indemnity insurance (see McMillan and Bellchambers [2007]).
In New Zealand, registered Nurse Practitioners™ are classed as designated prescribers under the 1999 Medicine Act Amendments and can prescribe medicines from the schedule of the Medicines (Designated Prescriber: Nurse Practitioners) Regulations (2005) or Schedule 1A of the Misuse of Drugs Regulations (1977) (http://www.legislation.govt.nz/). Prescription of medicines must be within the Nurse Practitioner’s designated area of clinical practice. Under the auspices of the Health Professionals Competence Assurance Act (2003), the New Zealand Nursing Council has responsibility for approval of the scope of practice and registration of Nurse Practitioners. Competency requirements for New Zealand Nurse Practitioners and scopes of practice can be found on the New Zealand Nursing Council website: http://www.nursingcouncil.org.nz/download/68/guidelines-np-sept09.pdf.
Occupational therapists
Occupational therapists work particularly with people who have physical, emotional, psychological or social disorders that affect how they carry out activities of daily living, such as looking after themselves, cooking, driving and job skills. Occupational therapists try to facilitate and rehabilitate the person through the use of activities, group therapy and adaptation of equipment and of the environment. While they do not themselves prescribe or administer drugs, they may need to know the language of pharmacology in order to understand information about the drugs that their patients/clients are taking and how these may affect functioning or cause adverse reactions.
Optometrists and orthoptists
Optometrists specialise in examining the eyes, testing vision and prescribing spectacles (glasses) and contact lenses. Recently, in some Australian states, optometrists have been given the opportunity, after undertaking accredited extra training in pharmacology, to have their registration endorsed so that they can prescribe a limited range of Schedule 4 drugs for optometric use. The list of drugs includes ocular preparations (mainly eye-drops) of antimicrobials (antivirals, antibacterials), local anaesthetics, anti-inflammatory drugs, anti-allergy drugs, drugs to dilate the pupil (mydriatics) and many drugs for treating glaucoma.
Orthoptists generally work with ophthalmologists (doctors specialising in eye disorders) and are involved in the treatment of eye movement disorders such as strabismus (squint) and of people with low vision. Recently the training of orthoptists has been extended to equip them to prescribe spectacles and lenses.
Pharmacists
Pharmacists are specialists in drugs and are involved with their storage, supply and distribution. They generally work in hospital or retail pharmacies and in nursing homes. Some of the many roles of pharmacists are:
Compounding by pharmacists
Traditionally, most of a pharmacist’s work involved compounding of medicines, i.e. the preparation and supply of a single unit of a product, such as an oral mixture, intended for immediate use by a specific consumer. Nowadays virtually all drugs are bought in by a pharmacist from a mass-manufacturing drug company supplier, and the pharmacist selects, dispenses into a suitable container, labels and provides the medicine, with appropriate counselling. However, compounding is still taught as part of the pharmacy school curriculum, and pharmacies are required to maintain basic compounding equipment for this purpose, although they no longer must be able to prepare and sterilise specialised formulations such as eye-drops or parenteral injections. Some compounding is still done, mainly in hospital pharmacies, as many preparations are not available in paediatric formulations or in sugar-free mixtures, or patients may be able to swallow oral solutions but not tablets. Extemporaneously prepared medicines are not subject to control by the Therapeutic Goods Administration (TGA), and so there are risks attached to their preparation and use. The Pharmaceutical Society of Australia has developed professional standards for compounding practice (see Feldschuh [2008]).
Clinical pharmacy
In hospitals, pharmacists also carry out many specialist roles, such as filling and maintaining ward stocks of drugs (imprest cabinets and drug trolleys); preparing sterile parenteral solutions, parenteral nutrition solutions and oncology drugs; and participating in ward rounds, medication history reviews, drug usage evaluations, therapeutic drug monitoring, advice on drug therapy and provision of drug information as specialists in drug therapy. Pharmacists in retail practice often take on responsibilities for medication management services, i.e. overview of the drug therapy of people in nursing homes and domiciliary medication reviews for patients in their own homes.
Physiotherapists
Physiotherapists (also known as physical therapists) deal with problems of movement, muscle coordination and posture, and with impairments caused by physical injury. Many of their patients have neurological, cardiovascular, respiratory or orthopaedic conditions (fractures, soft tissue injuries, vertebral syndromes), have been through surgery or childbirth, have major pain control problems or require rehabilitation after surgery, accidents or neurological damage. Physiotherapists use physical methods of therapy, such as heat, cold, electrical stimulation, exercise, massage and manipulation, electromagnetic radiation and biofeedback, rather than chemical methods (drugs).
Because virtually all their patients are likely to be taking some drugs for their underlying medical conditions or for the problems for which they present for therapy, physiotherapists need to have a good general understanding of the language and principles of pharmacology and, in particular, a thorough knowledge of drugs used in obstetrics, neurological and cardiovascular conditions, asthma and inflammatory conditions and for pain control.
Podiatrists
Podiatrists specialise in disorders of the lower limb, especially of the ankle and foot, and deal with biomechanical, medical, surgical and sports-related problems, especially in diabetes and rheumatology. The drug groups that their patients are likely to be using include cardiovascular drugs, hypoglycaemic agents, anti-inflammatory drugs, analgesics and antimicrobials. Podiatrists are allowed to administer local anaesthetics for pain relief in procedures and surgery involving the foot. Those with extra training in pharmacology and microbiology are allowed in some Australian states to prescribe a limited range of Schedule 4 drugs, such as antimicrobials, anti-inflammatory drugs, analgesics, antianxiety agents and long-acting local anaesthetics.
Prosthetists and orthotists
These health professionals specialise in provision of prostheses (artificial limbs) and orthoses (devices to support limbs). Many of their patients have problems with motor control or poor circulation, especially diabetics, so they need to know about neurological drugs and those used to improve circulation and treat diabetes.
Speech pathologists (speech therapists)
Speech pathologists deal with people who have difficulties with verbal communication, language development and speech, hearing and swallowing. As such, they are not themselves likely to be advising on drug therapy but they may well be dealing with clients who are taking drugs for an underlying clinical problem, such as strokes, other neurological impairments, psychiatric or behavioural disorders. Speech pathologists may therefore find it useful to understand the language of pharmacology and be able to read drug information sources.
Factors modifying responses to drugs
If the same dose of drug (on a mg drug dose per kg body weight basis) is given to similar people—or indeed to the same person on different occasions—their responses are likely to be different. Many factors can modify drug responses and these need to be anticipated by the health professional before prescribing and administering a drug, and also afterwards, when observing responses and monitoring drug therapy. Pharmaceutical factors affecting response, such as the form in which the drug is administered (whether liquid or solid, simple tablet or sustained-release form, patch or ointment etc), are discussed in a later section.
Pharmacokinetic factors
Factors that affect how the body handles drugs, i.e. how the drug is absorbed from its site of administration, distributed around the body in the bloodstream and eliminated by metabolism and excretion, will obviously help determine how much drug is available at any time to act. Pharmacokinetic principles are studied in Chapter 6 and applied in Chapter 8 to dosage regimens and individual and lifespan aspects of drug therapy.
Pharmacokinetic factors affecting drug responses may be influenced by:
Pharmacodynamic factors
Pharmacodynamics refers to what the drug does in the body—its actions and effects, including useful therapeutic effects and adverse reactions, as well as studies of the mechanism of action of the drug at the molecular level. These aspects of pharmacology are discussed in Chapters 5 and 9, and in subsequent chapters under drug groups and in Drug Monographs.
While the mechanism of action of a drug is generally similar in all individuals—unless they happen to have unusual amounts or types of the receptor or other cell component on which the drug acts—there are still factors that can affect pharmacodynamic aspects of an administered drug and hence affect responses. Such factors include tachyphylaxis and desensitisation (rapid decreased effect of a drug) and tolerance (slowly acquired reduction in responsiveness, e.g. in opioid-dependent persons).
Individual and clinical factors
Compliance
Obviously the primary determinant of drug response is whether or not the person takes the drug.5Compliance means following all aspects of a treatment plan. In the context of drug therapy, it implies administering the drug according to the five rights (see Nurses’ roles, above). All the other advice given related to therapy must also be followed, including lifestyle aspects such as diet modification, weight reduction, cessation of smoking and moderation in alcohol intake. This can be demanding and, when many drugs are prescribed, may become a formidable challenge. Poor compliance may involve taking either too little or too much of the drug, or taking it at the wrong time or with other medicines, whether prescribed or OTC.
There are many causes of poor compliance, such as:
The consequences of poor compliance are more serious than just wasted drugs and time. Drug levels in the body may fall below the therapeutic range, leading to inadequate responses and lack of effect, or may rise to potentially toxic levels and cause adverse drug reactions. The doctor cannot properly monitor and adjust therapy if lack of response is due to poor compliance and may waste effort in revising the diagnosis, increasing doses, adding more drugs or sending the patient for more tests. In particular situations, poor compliance with therapy may result in pregnancy (oral contraceptives), convulsions (antiepileptic drugs), strokes or heart attacks (anticoagulant or antithrombotic drugs).
Studies have shown that good compliance is the exception rather than the rule and, despite Hippocrates’ warning, that doctors are not good at predicting which patients are likely to be good or poor compliers. Ways of assessing compliance include careful counts of tablets or other dose forms remaining after a specified period (but patients can get cunning in flushing away the tablets they should have taken) and assays (measurements) of drug levels in blood samples—see later section, ‘Therapeutic Drug Monitoring’.
It must be recognised that people have a right to autonomy in their own medical care and may justifiably refuse to take drugs for good reasons. However, it is important that the prescribers be made aware if drugs are not being taken as directed, so that allowances can be made and doses altered or different drugs substituted as appropriate.
Drug interactions
After a person has been stabilised on repeated doses of a drug, the responses to the drug may be affected by interactions with any other drug taken, including non-prescribed OTC drugs and CAM therapies, as well as other ingested compounds such as food and drinks. As a person takes, or a patient is prescribed, more and more drugs, the possibility of drug interactions rises exponentially. The topic of drug interactions is discussed in Chapter 9 and in individual Drug Monographs where clinically relevant. There are exhaustive lists of common drug interactions in reference texts such as the Australian Medicines Handbook (Appendix), MIMS Annual (Index to Drug Interactions Table) and Avery’s Drug Treatment (Appendix B: Guide to Clinically More Important Drug Interactions).
Drug interactions may involve either the pharmacokinetics of the drugs involved (e.g. monoamine oxidase inhibitors inhibiting the metabolism of many other drugs) or pharmacodynamic aspects (e.g. antihistamines and alcohol having additive CNS-depressant effects, or β-blockers used for hypertension and β2-agonists used for asthma having opposing effects on receptors).
Polypharmacy
Polypharmacy is defined as ‘the concurrent use of multiple medications’, usually five or more drugs including prescribed, OTC and CAM. In the clinical context, it has the connotation of implying prescription and use of too many or unnecessary drugs, or use at frequencies greater than therapeutically essential. Polypharmacy is thus a situation in which multiple drug interactions can occur and is potentially harmful to the patient.
An Australian National Health Survey (1995, reviewed in NPS Newsletter 13, 2000) found that 10.7 million Australians—almost 60% of the population—were taking prescribed or OTC medications (excluding CAM therapies) at any one time. If CAM had been included, the proportion would have been much higher. Of those using at least one medication, 14.5% were taking four or more drugs and 4.6% were taking six or more. In persons over 75 years, the proportions were about 40% and 17%, respectively. This very high reliance on drugs (Australia has been called ‘the overmedicated society’) means that many people are at high risk of adverse drug reactions and drug interactions, and older people particularly are at risk of falls (see Hilmer [2008]).
Polypharmacy is not the sole responsibility of prescribing doctors; various health professionals can initiate the steps recommended for managing and avoiding polypharmacy:
A case involving polypharmacy, and a successful resolution of the problems, is described in Clinical Interest Box 2-3.
Clinical interest box 2-3 Clues in the medicine cabinet
Doctor D was called with a request that a home visit be made to an elderly woman living at home: ‘Mother is going downhill … someone has to come around and sort things out.’ The doctor visited Mrs X, armed with the files on her medical history, which revealed many years of hypertension, angina, heart failure, a heart attack, osteoarthritis, depression, obesity and surgical repair of a hiatus (oesophageal) hernia. Most recently she had been diagnosed with heart failure and peripheral vascular disease, and doses of digoxin and frusemide had been increased.
Mrs X’s current complaints were of worsening dyspnoea (difficulty in breathing) and ankle swelling, poor circulation in the legs and cold feet, cramps, anorexia and nausea and postural hypotension (dizziness when standing up). On examination she was unwell: pale, with pitting oedema, irregular pulse with atrial fibrillation, high blood pressure (180/95) and bilateral pulmonary crepitations (crackling noises indicating abnormal fluid in the lungs). Possible causes of her problems were myocardial infarction (heart attack), renal failure, digitalis toxicity, hypokalaemia, anaemia and drug interactions.
A tactful request to view the medicine cabinet was greeted with relief by Mrs X’s daughter, and proved ‘a revelation almost beyond belief’. The hoarded contents included:
Mrs X was confused about her medicines (not surprisingly, as several had no instructions for administration) but thought she took most of them. Review of the many potential drug interactions and ADRs revealed that several of the drugs are contraindicated in heart failure, the anti-inflammatory agents could be exacerbating fluid retention, potassium supplementation was probably inadequate for two potassium-depleting diuretics and digitalis toxicity could be contributing to arrhythmias and gastrointestinal upsets.
Most of the medicine cabinet was cleared out and several tests ordered to monitor digoxin levels (high), potassium (low), renal function and haemoglobin (normal). With fluid restriction and weight reduction after stabilisation on therapy, Mrs X was maintained on digoxin ( the previous dose), amiloride plus hydrochlorothiazide (one potassium-conserving and one potassium-depleting diuretic) and paracetamol as necessary for pain. Her blood pressure was monitored carefully but required no medication.
the previous dose), amiloride plus hydrochlorothiazide (one potassium-conserving and one potassium-depleting diuretic) and paracetamol as necessary for pain. Her blood pressure was monitored carefully but required no medication.
The case highlights many problems of polypharmacy, including:
Source:Murtagh 1992; used with permission.
Placebo effect
The Latin word placebo literally means ‘I will please’. In the pharmacological context, it refers to a harmless or inactive preparation prescribed to satisfy a patient who does not require an active drug. In a clinical trial, it is formulated to look identical to the active drug under trial, to maintain ‘double blinding’, so that neither subject nor clinician knows which treatment group the subject is in.
Patients and subjects in trials frequently appear to respond to placebos, with therapeutic or adverse effects. This ‘placebo response’ may be a significant but temporary alteration in the person’s condition, due to the person’s expectations or other unexplained psychological effect. Factors possibly inducing the response include the relationship between the patient and the health professional, the wish to be seen to respond, response to the increased care and attention and aspects such as the colour and taste of the dose form or pain on injection (no gain without pain).
Drug prescriptions and formulations
Prescriptions
A prescription (script) is a written direction for the preparation and administration of a drug for a specified person, containing the names and quantities of the active ingredients; there are legal requirements for a valid prescription (described later).
Prescriptions are thought to date back to the earliest known records of medical practice: in the ancient Babylonian civilisation, medical information on clay tablets recorded symptoms of illness, lists of pharmaceutical ingredients and directions for compounding (i.e. the prescription), and invocations to the gods for healing. The prescription sign—sometimes shown in typeface as Rx—may derive from the Egyptian character for the Eye of Horus, the symbol of good fortune and healing, or relate to the Roman god Jupiter. Another suggestion is that Rx is short for the Latin word ‘recipe’, meaning the direction ‘take …’, instructing the pharmacist to take the following chemicals and compound them into a formulation.
Decisions to be made before prescribing
Any therapeutic intervention—whether administering a drug, implementing a physiotherapeutic electrotherapy program, carrying out a dental or podiatric surgical procedure, altering a person’s diet or administering a CAM therapy such as acupuncture, herbal remedy or massage—will interfere with the person’s body systems, either physically or chemically. The first priority before carrying out any intervention must be based on the advice of Hippocrates: FIRST DO NO HARM. Then there are many questions that need to be answered, consciously or intuitively, before intervening and, in the context of drug therapy, the rest of this book attempts to provide useful answers to these questions. (The following list was presented in cartoon format in the textbook of clinical pharmacology by Sweeney [1990], and is reproduced here with permission in Figure 2-2; see also review by Aronson [2006].)

Figure 2-2 Questions to ask and answer when prescribing a drug
from Sweeney [1990]; used with permission.
What is the problem?
The question is: ‘What is going wrong here?’ A full health history may take at least 30 minutes to complete and should list all the patient’s current problems, including medications, past drug history, allergies, ADRs and interactions, relevant family history, use of social drugs such as alcohol and tobacco and CAM therapies, and all treatment modalities being used. For effective treatment the starting point is the problems specified in terms of pathophysiology or altered anatomy or psychology, not necessarily a proven diagnosis.
Is there a drug-based solution?
There may not be a drug-based solution to the problem—not all medical problems are currently treatable with pharmacotherapy. It is important to attempt to identify what changes need to be brought about in the person’s functioning and whether drug treatment can cure the condition or improve symptoms. Practitioners should keep an open mind here and consider all modalities—surgery, nursing care, physiotherapy, podiatry, lifestyle changes (e.g. diet, exercise, stress levels), psychotherapy, CAM methods and combinations of therapies, as well as drug treatment.
If so, which drug?
Assuming that there are safe, effective drugs available to treat the problem, there are many decisions to be made:
What does the drug do and how does it act?
What is known about the drug’s pharmacodynamics? What actions does it have and what is the mechanism—does it affect receptors, enzymes, ion channels, transport processes? How will this affect the person’s problems? What do we not know that could be important? What are the common ADRs and potential drug interactions? Checking a drug monograph for the drug is helpful here.
How long will the patient be treated?
This requires knowing the usual course of the condition and prognosis. Will the patient get better after a few days of treatment (e.g. after an acute infection), might there be ongoing relapses and remissions (as with multiple sclerosis) or will the condition progress relentlessly (as do diabetes and dementia)? Are there long-term effects of the drug?
How will you monitor therapy?
The patient’s progress must be monitored to evaluate effects of the therapy. It is possible to monitor several parameters (see later section on therapeutic drug monitoring):
How much drug should be given?
What dose is being prescribed, for what effect, and is it appropriate? Doses need to be individualised so do not feel you have to rely on memory—look it up! Pharmacokinetic principles will determine the frequency of dosing and possibly the appropriate route. The drug’s therapeutic index will determine how critical the exact dose is. The route may determine the formulation or there may be choices: if oral, will it be tablets, capsules, a mixture, a sustained-release form?
What is special about this patient?
If the patient is not the standard 70-kg fully functioning adult, what is the patient’s age and weight? Is the person particularly susceptible to adverse effects? Might a woman patient be pregnant or breastfeeding? How effective are the liver and kidney functions likely to be? (These affect pharmacokinetic parameters such as protein binding and drug elimination, and are discussed in Chapters 6–8). Are there other concurrent medical conditions? Is the drug contraindicated in any of these situations? Are there relevant socio-cultural aspects relating to values, beliefs, cultural differences or restricted income that might affect compliance or responses to therapy? Is the person taking any other medications, whether prescribed, OTC or CAM? If so, what drug interactions are possible or clinically significant?
Can you write (or dispense, or administer) the prescription?
Are the ‘five rights’ (patient, drug, dose, route, time) right? Does the prescription seem appropriate? Does it conform with PBS and institutional requirements and QUM guidelines? Are the instructions to the patient adequate and correct? (See later under ‘Prescriptions’.)
Are there any warnings for the patient or staff?
Patients have the right to get as much medical information as they want. In particular, they need to know about their condition, why a drug is being prescribed and for how long, whether it is to treat the disease or to relieve symptoms and how and when to take the medicine. Patients need to be warned about possible significant adverse reactions, how to recognise them and what to do if they occur, drug and food (and alcohol) interactions and what to do if they miss a dose. Printed consumer product information should be included in the drug package with all relevant details. Patients’ carers, both family and nursing staff, may also need warnings about some adverse reactions to help them in their roles.
Prescription orders
A prescription is written by a licensed prescriber (medical practitioner, dentist or veterinary surgeon)6 and may present in two formats: on a prescription form or an institutional order sheet (Figures 2-3A–C). Prescriptions must comply with legal formats, e.g. as laid down in the Australian Drugs, Poisons and Controlled Substances Act, and Regulations 1981. Prescriptions are then dispensed (filled) by a registered pharmacist.

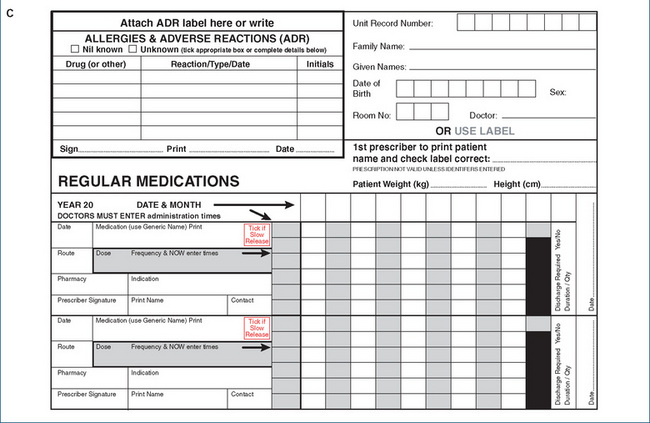
Figure 2-3 Typical prescriptions. A An example of a prescription from the early 20th century. B A typical current Pharmaceutical Benefits Scheme prescription; names and details have been changed. C Part of a typical medication therapy chart from a patient’s hospital record (also known as the patient’s drug chart). (© Commonwealth of Australia; reproduced with permission.)
Traditionally, a prescription had four main sections:
Prescriptions
A prescription must be clear, concise and correct. It has elements that can be correlated with the five nursing rights of medication administration: the patient’s name, address and age (right patient); date written; generic or proprietary drug name (right drug); drug dose, strength and dosage form (right dose); route of administration (right route); dosage instructions or frequency of administration (right time); and bears the signature, name, address and contact number of the prescriber. The number of times the prescription can be repeated should also be specified.
All the elements should be clearly written to avoid any chance of error; for example, if there is any chance that ‘μg’ might be read as ‘mg’, then the word ‘microgram’ should be written in full or abbreviated to mcg. Formerly, it was required that prescriptions be written indelibly in the prescriber’s own handwriting, but now they can be computer-generated and printed. All efforts must be made to prevent forgery of details or of entire prescriptions. Only accepted abbreviations should be used (see below). If any confusion or doubt exists, the prescriber is contacted for clarification.
It is suggested that, for good prescribing practice, prescribers should have a finite list of ‘personal preferred drugs’ (their P-list), with which they are very familiar, and feel confident in their ability to evaluate new information and prescribe wisely.
Telephone orders and standing orders
Drugs are also sometimes prescribed by doctors as ‘telephone orders’ or standing orders if the doctor cannot be present at the time. A doctor may telephone a drug prescription through to a pharmacist, who is legally entitled to dispense the prescription, and to a nurse entitled to administer it on the oral instructions. The doctor must then, as soon as is practicable, write out the drug order on a prescription pad, sign it and post or deliver it to the pharmacy or use the hospital drug chart. A faxed prescription copy can confirm an oral order but is not legally acceptable, as the signature has to be original, i.e. in the doctor’s handwriting; the pharmacist must see the original prescription before releasing the drug to the client.
‘Standing orders’ are sometimes left by doctors as ongoing prescriptions in a hospital, nursing home or residential care setting. These have no legal validity unless properly written, dated and signed as for any normal prescription. Repeating prescriptions without clinical review of the patient brings the risks of unnecessary and/or unsafe drug use.
Hospital drug charts
Prescriptions in hospitals are usually written on a drug chart or medication therapy chart (Figure 2-3C). These can become quite complicated and may run to many pages in a patient’s medical record, as patients in hospital are frequently prescribed 10–15 drugs during one stay. In a typical chart, the users are instructed:
This form also has sections for general medications and for admission and discharge drugs (those the patient was using when admitted, and those prescribed when discharged).
Specialised medication charts are also available to record phone orders, comments on administration discrepancies and errors, IV orders (covering fluids administered, drip rates and additives), diabetes management and fluid balance. There are also forms designed for recording other aspects of health care, such as for physiotherapy and podiatry progress notes, nursing admission data, neurological observations, patient’s consent to treatment, delivery and neonatal records and residential care information.
Instructions and abbreviations in prescriptions
A clearly written drug order specifies the conditions for drug administration; for example, a routine order means that the drug is administered until discontinued by the prescriber; a prn (pro re nata = when necessary) order is administered only when needed by the patient, while a stat (statim = immediately) order is for immediate administration of one dose of medication.
Many abbreviations and symbols are used in drug ordering (see Table 2-1 for a list of commonly used forms). Use of abbreviations can lead to potentially serious errors in administration; only those shown asterisked (Table 2-1) are recommended by the Australian Medicines Handbook. Drug names should never be abbreviated; if there is any possibility of confusion, all words should be written in full: ‘If in doubt, write it out!’
Table 2-1 Common abbreviations and symbols in prescriptions*
| Abbreviation | Unabbreviated | Form meaning |
| *ac | Ante cibum | Before meals |
| ad lib | Ad libitum | Freely |
| am | Ante meridiem | Morning |
| *aq | Aqua | Water, aqueous |
| *bd, bid | Bis die, bis in die | Twice each day |
| c | Cum | With |
| *cap | Capsule | Capsule |
| cc, cm3 | Cubic centimetre | Cubic centimetre (=1 mL) |
| D5W | Dextrose 5% water | 5% Dextrose in water (5 g/100 mL) |
| DW | Distilled water | Water purified by condensation from steam |
| EC | Enteric-coated | Tablet or capsule formulation whose coating prevents dissolution until reaching the small intestine |
| elix | Elixir | Elixir |
| *g, gm | Gram | 1000 milligrams |
| gtt | Gutta | Drop |
| h, hr | Hora | Hour |
| hs | Hora somni | At bedtime |
| IA | Intra-arterial | Into an artery or arteriole |
| ID | Intradermal | Into the skin |
| *IM | Intramuscular | Into muscle |
| *inj | Injection | Injection |
| IT | Intrathecal | Into the subarachnoid space |
| IU | International Unit | Unit of pharmacological activity for a particular drug, as defined by an international convention |
| *IV | Intravenous | Into a vein |
| IVPB | IV piggyback | Secondary IV line |
| kg | Kilogram | 1000 grams |
| KVO | Keep vein open | Very slow infusion rate |
| L | Litre | Litre (1000 cm3) |
| M | Mitte | Send, supply |
| *mane | Morning | In the morning |
| μg, mcg | Microgram | One millionth of a gram |
| *mg | Milligram | One thousandth of a gram |
| mEq | Milliequivalent | One thousandth of the gram equivalent weight of a solute in an electrolyte solution mist Mistura Mixture |
| *mL | Millilitre | One thousandth of a litre, 1 cm3 |
| NG | Nasogastric | Into the stomach via the nose |
| *nocte | At night | At night |
| NS | Normal saline | 0.9% Sodium chloride solution |
| oc | Oculorum | Eye |
| os | Os | Mouth |
| OTC | Over-the-counter | Non-prescription drug |
| otic | Otikos | The ear |
| *pc | Post cibum | After meals |
| pm | Post meridiem | After noon |
| PO | Per os | By mouth, orally |
| PR | Per rectum | Into the rectum |
| *prn | Pro re nata | According to necessity (lit. for the thing [i.e. need] having arisen) |
| PV | Per vagina | Into the vagina |
| q | Quaque | Every |
| *qd, qid | Quater in die | Four times a day |
| qh | Quaque hora | Every hour |
| q4h, qqh | Quaque quarta hora | Every 4 hours |
| qs | Quantum satis | Suffficient quantity |
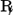 |
Receipt, recipe | Take (or dispense, provide) |
| s | Sine | Without |
| SC | Subcutaneous | Into subcutaneous tissue |
| Sig. | Signature | Label, instructions |
| SL | Sub linguam | Under the tongue |
| SOS | Si opus sit | If necessary |
| ss | Semis | A half |
| *stat | Statim | At once |
| *tab | Tablet | Tablet |
| tbsp | Tablespoon | Tablespoon (15 mL) |
| *tid or TDS | Ter in die | Three times a day |
| TO | Telephone order | Order received over the telephone |
| top | Topically | Applied to the skin |
| tsp | Teaspoon | Teaspoon (4 or 5 mL) |
| U | Unit | A dose measure for insulin, heparin |
| ung | Unguentum | Ointment |
| VO | Verbal order | Order received verbally |
| × | Times | As in two times a week |
* Only the abbreviations marked with an asterisk are approved by the Australian Medicines Handbook.
Electronic prescribing
General practitioners (GPs) in Australia have been encouraged to adopt the use of computers in their practices. A study in 2000 showed that about 65% of GPs used electronic prescribing packages. While there are some disadvantages (initial cost, information overload, invasion of patient’s privacy, intrusiveness of advertising), the advantages are many:
There are risks that drug company advertisements included in prescribing packages will influence the prescriber’s choice of drugs.
Unsuccessful prescribing
Underprescribing
The most common type of sub-optimal prescribing is underprescribing, where drugs are not prescribed despite guidelines recommending them, or doses are subtherapeutic. Elderly people in particular are often not treated optimally; for example, ACE inhibitors and statins are used less effectively than recommended.
Inappropriate prescribing
Overprescribing (e.g. various NSAIDs for different conditions in one patient) and irrational prescribing (such as antibiotics for viral infections, or cough suppressants in productive cough) also contribute to unsuccessful prescribing. Prescribing errors occur frequently, particularly when busy prescribers in hospitals or general practice fail to consider all the ‘questions to answer’ before prescribing.
Teaching prescribing
Medical students (often in retrospect) have said that not enough time is devoted to teaching prescribing in medical programs. Whereas all dental students have practice in injecting local anaesthetics and filling and extracting teeth, and podiatry students in carrying out invasive procedures under local anaesthesia, it is possible for a medical student to graduate without ever having written a prescription (or removed an appendix).
A review of prescribing practice and teaching recommends the following steps (see Aronson [2006]):
Formulations of drugs (pharmaceutics)
Depending on the route by which a drug is administered (e.g. whether by mouth, topically onto the skin, by injection, into the eye or ear), different drug dosage forms are possible or appropriate. Pharmaceutics is the science of formulating drugs into different types of preparation, e.g. tablets, ointments, injectable solutions or eye-drops. It also includes study of the ways in which various drug forms influence pharmacokinetic and pharmacodynamic activities of the active drug. Formulating a drug into an appropriate dose-form is an important role of pharmacists, mainly carried out now by choosing a formulation prepared in a drug company’s laboratories or factories.
Many drugs are available in different formulations. This variety assists the prescriber in choosing a formulation that is best suited to the individual patient and route of administration, and according to whether it is intended that the drug act locally or be absorbed into the systemic circulation. Table 2-2 shows a comprehensive listing of various forms of drug preparations, with a brief description of each form. The routes of drug administration and the ways in which the route affects absorption of drugs are discussed in Chapter 6. More details of formulations specific to particular drug groups or routes of administration are given in the relevant chapters, as follows:
Table 2-2 Various drug formulations
| PREPARATIONS FOR ORAL USE
Liquid
• Emulsion (two-phase system of two immiscible liquids, with fine droplets of one phase dispersed in the other by action of an emulsifying agent)
|
| PREPARATIONS FOR PARENTERAL USE (INJECTIONS) |
| INTRAVENOUS INFUSIONS (CONTAINER SUSPENDED ON HANGER AT BEDSIDE OR IN PUMP)
• Glass bottles, flexible collapsible plastic bags, or semi-rigid plastic containers in sizes from 100 to 1000 mL used for continuous infusion of fluid replacement with or without drug
• Heparin lock or angiocath (a port site for direct administration of intermittent IV medications without the need for a primary IV solution)
• Intermittent IV infusions (usually a small secondary IV set to which drug is added; it runs as a ‘piggyback’ to the primary IV infusion; see Figure 2-4)
|
| PREPARATIONS FOR TOPICAL USE
• Cream (semi-solid emulsion that contains a drug incorporated into both an aqueous and an oily base)
• Lotion (liquid preparation that can be protective, emollient, cooling, astringent, antipruritic, cleansing etc)
|
| PREPARATIONS FOR USE ON MUCOUS MEMBRANES
• Aqueous solutions of medications, usually for topical action but occasionally for systemic effects, including enemas, douches, mouthwashes, nasal and throat sprays and gargles
• Aerosol sprays, nebulisers and inhalers (deliver aqueous solutions of drug in fine droplet form, or very finely dispersed powders, to the target membrane, e.g. bronchodilators to the bronchial tree)
• Drops (aqueous solutions, with or without gelling agent to prolong retention time; used for eyes, ears or nose)
|
| MISCELLANEOUS DRUG DELIVERY SYSTEMS
• Intradermal implant (sterile pellet or rod containing a small deposit of drug for insertion into a dermal pocket, designed to allow drug to leach slowly into tissue; usually for administration of hormones such as testosterone or oestradiol)
|
Formulations for oral administration
An oral drug may appear in solid form (e.g. tablet, capsule or powder) or in liquid form (solution, elixir or suspension). Disintegration of the solid dose form must occur before dissolution, a process by which a drug goes into solution and becomes available for absorption. The form of the drug dose is important because the more finely divided a solid is, the more rapid the rate of dissolution, and the more readily the compound crosses cell membranes and is absorbed into the systemic circulation to be circulated to the site where it acts. Oral drugs in liquid form are therefore more rapidly available for gastrointestinal absorption than those in solid form.
Tablets
The oral route of administration is by far the most common: about three-quarters of all drugs prescribed are administered orally and, of these, about 60% are in tablet form. Tablets are compressed mixtures of active drug with various other chemicals, called excipients, which assist in the formulation. For example, pharmacologically inert chemicals may be present as diluents (fillers), binders, adhesives, disintegrants, lubricants, flavours, colours, sweeteners or absorbents. Thus the active drug may make up only a very small fraction of the total tablet weight. The weight quoted for the tablet (e.g. aspirin tablets commonly 300 mg) refers to the average amount of active drug in the tablet; the whole tablet will actually weigh considerably more.
Tablets may appear as simple white discs or may be multilayered or coated with a film7 to mask an unpleasant taste. Some tablets are prepared for sublingual administration (dissolved under the tongue) or as effervescent tablets that fizz and dissolve in water so that they are easier to take.
Tablets may also be available for use by a pharmacist or scientist in preparing solutions of particular strengths.
The rate of release of active drug from a tablet—and thus the drug concentration in the circulation and at the active site—can be manipulated by pharmaceutical processing. Finely ground powders are more quickly dissolved than are coarse powders or granules. It is possible to combine an active drug with a resin or other substance from which it is slowly released, to delay absorption (sustained-release [SR], or controlled-release [CR] preparations). In addition, it is possible to prepare a tablet with a coating that offers relative resistance to the digestive action of stomach contents (enteric coating, EC). Enteric coatings on drugs are used:
Parenteral dose forms
Parenteral administration
Parenteral administration of drugs is the administration of drugs by injection and is the most rapid form of systemic therapy. The intravenous (IV) route, in which the drug is injected directly into the circulation, avoids any delay during absorption. Other parenteral routes include intradermal (into the skin), intramuscular, intra-arterial and subcutaneous (into the fatty tissue under the skin); specialist techniques for local anaesthetics include the epidural (= extradural) and intrathecal routes—see Figure 14-10.
Equipment and solutions
Because any injection is an invasive procedure with the potential for infection being introduced, drugs formulated as solutions for parenteral administration must be sterile, filtered, particle-free and preferably isotonic with body solutions (i.e. with normal saline, 0.9% sodium chloride solution) and buffered to body pH. Solutions that are acidic or alkaline, hypertonic or contain irritant drugs can damage tissues into which they are injected. If solid particles are injected, they can cause granulomas, ischaemia or phlebitis.
Usually, parenteral solutions consist of the drug dissolved in an aqueous solution, but in some cases a drug that is very insoluble in water and which must be administered parenterally, such as the general anaesthetic propofol (Drug Monograph 14–3), can be formulated in an oily emulsion suitable for injecting.
Solutions for injection are usually presented in glass ampoules or bottles or plastic bags. The equipment for delivery of a drug by an IV infusion is complicated (Figure 2-4) and may include sterile tubing, an in-line filter and drip chamber, clamps to adjust the flow rate and a cannula for insertion into the vein. Most institutions will have guidelines as to the use of such IV sets, with lists of appropriate infusion solutions and possible admixtures (agents added to IV fluids). Compatibilities of drugs with parenteral drug solutions are dependent on many chemical and physical factors. The general rule is that, unless an admixture is specifically allowed, it should not be made, unless a hospital pharmacist or drug information centre has approved the addition. (As always, if in doubt, don’t!)
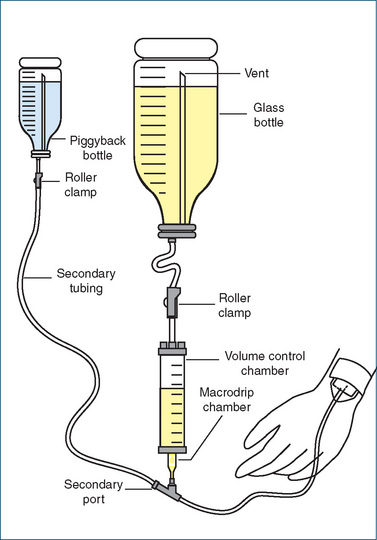
Figure 2-4 Diagram of a typical intravenous infusion set-up, showing a secondary ‘piggyback’ bottle with a reservoir of drug in IV fluid; the main solution is now most commonly contained in a plastic pack.
For example, it is recommended that morphine sulfate (a powerful pain-reliever) can be injected during infusion with dextrose, saline, dextrose/saline or Hartmann’s solution, and mixed in a syringe with metoclopramide (an antiemetic) or bupivacaine (a long-acting local anaesthetic), but must not be mixed in a syringe or pack with aminophylline, flucloxacillin, phenytoin or several other listed drugs (see Murney [2008]).
Formulations for children
Formulations suitable for children pose a special challenge to pharmacists and drug companies: very young children cannot swallow tablets or capsules so require oral solutions; the unpleasant taste of some drugs may need to be masked by sweeteners or flavours; however, sugary mixtures can encourage dental caries and be problematic for management of diabetes (see CIB 36-6). More unusual drug-delivery systems such as patches for transdermal absorption or dispersible tablets may not have been clinically trialled in children (see review by Nunn and Williams [2005]). One success story in formulation of drugs for children is that of the fentanyl ‘lollipop’, an applicator for self-administration of the opioid analgesic by rubbing on the inside cheek mucosa; these are manufactured in a wide range of strengths including doses suitable for children with severe pain.
Therapeutic drug monitoring
To paraphrase an old legal maxim: therapy must not only be done, it must be seen to be done. Therapy can be monitored in various ways, including simply observing the patient’s progress and changes in signs and symptoms. In the specific case of checking on results of drug therapy, there are three main ways of carrying out therapeutic drug monitoring (TDM)—observing and/or measuring therapeutic effects, adverse drug reactions or levels of the drug in the body. The purpose of TDM is to provide evidence on which to base rational adjustment of dosage, to enhance efficacy, reduce toxicity or assist with diagnosis.
Indications for monitoring
The main indications for TDM, i.e. the situations in which it is considered useful, include:
Drugs commonly monitored
Methods of monitoring therapy
Monitoring of therapeutic effects
The useful effects of drugs can be monitored by measuring various physiological parameters. Examples include measuring blood pressure in a patient receiving an antihypertensive agent, measuring blood clotting times with anticoagulant drug therapy and gauging the effects of hypoglycaemic agents by measuring the patient’s blood glucose levels.
Monitoring of adverse reactions
Particularly in the case of drugs with a low therapeutic index, the adverse reactions may limit the level to which the dose can be increased. Cytotoxic therapy is frequently monitored by checking the patient’s white blood cell count; if this falls too low, the patient is at risk of overwhelming infection. The white cell count is monitored during a course of chemotherapy; only when it has recovered sufficiently is another course of chemotherapy instituted. The aminoglycoside antibiotics can cause severe damage to the kidneys and to hearing, so patients who require these drugs may have their hearing and renal function monitored. A common adverse reaction to the non-steroidal antiinflammatory drugs is dyspepsia and exacerbation of peptic ulcers. A way to monitor this effect might be for the prescriber to advise the patient: ‘Stop taking the drug if you start to feel sick, and let me know’.
Monitoring plasma concentration of drug
TDM refers most commonly to assay (measurement) of the concentration of the drug in a sample of the patient’s blood plasma. For drugs that require frequent monitoring, automated drug screens are available: a blood sample will be taken and sent to the clinical pharmacology or biochemistry laboratory for the drug levels to be assayed.
Monitoring plasma concentration is most useful for drugs with a low therapeutic index, for which a welldefined therapeutic range has been established. Drugs commonly monitored this way include digoxin, lithium, perhexiline, anticonvulsants, antiarrhythmics, anticoagulants, immunosuppressants, toxic antibiotics and paracetamol if an overdose is suspected. There are limitations to the usefulness of drug concentration results, and doctors are advised to ‘treat the individual patient and not the laboratory value’ (Ghiculescu 2008).
Procedures for monitoring by plasma concentration
The drug concentration measured will be compared to the therapeutic range (published values of the concentration of the drug in plasma at steady state during effective therapy [Css]). This is reached after about five half-lives during regular dosing; the plasma drug concentration will fluctuate between peaks and troughs—see Figure 8-6. Therapeutic ranges are valid for about 80% of the population and are a good guide to expected plasma concentration of the drug. Trough levels (the lowest level likely to be in blood, measured by taking a blood sample immediately before the next dose) are usually assayed. In some situations, e.g. to avoid toxic levels, peak levels are measured at the time of maximum plasma concentration.
Drug concentration is measured by highly accurate and specific techniques such as immunoassay, highperformance liquid chromatography (HPLC), gas chromatography or flame photometry.
Typical procedure
The usual procedure for TDM is:
If the therapy appears well controlled, further measurements may be taken 6–8 weeks later and again at 6–12-monthly intervals.
Drug screens
Drug screens are a particular example of TDM by drug blood levels, in which samples taken from patients are screened for the presence of a range of drugs, usually for medicolegal reasons. They may be carried out to detect drugs of abuse, drugs possibly taken in overdoses or, in the forensic context, to detect poisons. Almost any body fluid can be screened, as well as solid tissues such as hair, bone or nails. False-positive results may arise from interference from other drugs that have been appropriately taken, and false-negatives because of levels below the detection limit, owing to inappropriate sampling time or insufficiently sensitive methods. Some toxic substances that are rapidly metabolised, including insulin, succinylcholine and potassium, may be undetectable by any method after a delay (see CIB 2-4).
Clinical interest box 2-4 A late drug screen solves nothing
A classic unsolved mystery in Australian police files is the ‘Bogle–Chandler case’, in which two bodies were found on the banks of the Lane Cove River in Sydney on the morning of 1 January 1963 after a New Year’s Eve party. Apparently the man and woman had died from a poison, but which poison was never identified. The case generated huge interest as it involved a prominent CSIRO scientist, his colleague’s wife, society parties and drugs, a possible unidentified witness/perpetrator and a mysterious poison.
There have been many theories as to the cause of the deaths, including poisoning by natural substances such as insulin and potassium which would have been unidentifiable by the time drug screening tests were done some days later. Another theory is that hydrogen sulfide gas from industrial pollution accumulated around the river banks during the still night—with the couple being so unaware of their surroundings as not to smell ‘rotten-egg gas’. The case remains so fascinating that it rates several pages in Wikipedia, plus its own website (http://www.boglechandler.com/).
Key points
 With the enormous range of drugs available and the increasing costs of new drugs, it is essential that quality use of medicines be encouraged; government policies on prescribing, schemes limiting availability of drugs, educational bodies disseminating objective information and studies of drug usage in hospitals all contribute to costeffective clinical pharmacotherapy.
With the enormous range of drugs available and the increasing costs of new drugs, it is essential that quality use of medicines be encouraged; government policies on prescribing, schemes limiting availability of drugs, educational bodies disseminating objective information and studies of drug usage in hospitals all contribute to costeffective clinical pharmacotherapy. Effective use of drugs in the clinical situation requires thorough knowledge of all aspects of the drugs and understanding of the patient’s condition as it relates to how the drug is affected by the body and how the body responds to the drug.
Effective use of drugs in the clinical situation requires thorough knowledge of all aspects of the drugs and understanding of the patient’s condition as it relates to how the drug is affected by the body and how the body responds to the drug. Many factors may affect how a person responds to drugs, including pharmacodynamic and pharmacokinetic aspects; in the clinical situation, compliance, drug interactions, polypharmacy and placebo effects are particularly important.
Many factors may affect how a person responds to drugs, including pharmacodynamic and pharmacokinetic aspects; in the clinical situation, compliance, drug interactions, polypharmacy and placebo effects are particularly important. Prescriptions are legally regulated documents, with specific legal requirements for format; abbreviations in prescriptions should be used only cautiously to avoid confusion.
Prescriptions are legally regulated documents, with specific legal requirements for format; abbreviations in prescriptions should be used only cautiously to avoid confusion.Review exercises
References and Further reading
Aronson J.K. A prescription for better prescribing. British Journal of Clinical Pharmacology. 2006;61(5):487-491.
Australian Medicines Handbook 2009. Adelaide: AMH, 2009.
Baird A. Drugs for the doctor’s bag. Australian Prescriber. 2007;30(6):143-146.
Barber N. Extended prescribing rights—the UK experience. Australian Prescriber. 2009:118-119.
Braun L., Cohen M. Herbs and Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Churchill Livingstone-Elsevier; 2007.
Buchan H. Turning knowledge into action. Australian Prescriber. 2007;30(5):114-115.
Burridge N., editor. Australian Injectable Drugs Handbook, 4th edn., Melbourne: Society of Hospital Pharmacists of Australia, 2008.
Couzos S., Thiele D.D. The International Covenant on Economic, Social and Cultural Rights and the right to health: is Australia meeting its obligations to Aboriginal peoples? Medical Journal of Australia. 2007;186:522-524.
Dartnell J., Hemming M., Collier J., Ollenschlaeger G. Putting evidence into context: some advice for guideline writers. Evidence Based Nursing. 2008;11:6-8.
Dawes M., Davies P., Gray A., Mant J., Seers K., Snowball R. Evidence-Based Practice: A Primer for Health Care Professionals, 2nd edn. Edinburgh: Elsevier Churchill Livingstone; 2005.
Day R.O., Mashford M.L. Pharmacotherapeutics: what a difference five decades make!. Medical Journal of Australia. 2001;174(1):48-51.
Disabled Men’s Association of Australia. Medical Prescriptions (for all diseases and ailments). Melbourne: Disabled Men’s Association, c. 1929.
Dooley M.J., Allen K.M., Doecke C.J., et al. A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. British Journal of Clinical Pharmacology. 2003;57(4):513-521.
Feldschuh M. Compounding in community pharmacy. Australian Prescriber. 2008;31(2):30-31.
Gaspoz J.-M., Coxson P.G., Goldman P.A., et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. New England Journal of Medicine. 2002;346(23):1800-1806.
Ghiculescu R.A. Therapeutic drug monitoring: which drugs, why, when and how to do it. Australian Prescriber. 2008;31(2):42-44.
Gilbert A., Roughead L., Sansom L. I’ve missed a dose; what should I do? Australian Prescriber. 2002;25(1):16-18.
Hilmer S. The dilemma of polypharmacy. Australian Prescriber. 2008;31(1):2-3.
Hopkins H., Wade T., Weir D. ‘Take as directed’, whatever that means. Australian Prescriber. 2000;23(5):103-104.
Jones S.C. Relative thromboembolic risks associated with COX-2 inhibitors. Annals of Pharmacotherapy. 2005;39(7):1249-1259.
Krumholz H.M., Ross J.S., Presler A.H., Egilman D.S. What have we learnt from Vioxx? British Medical Journal. 2007;334:120-123.
Larkin C., Murray R. Assisting Aboriginal patients with medication management. Australian Prescriber. 2005;28(5):123-125.
Lexchin J. Are new drugs as good as they claim to be? Australian Prescriber. 2004;27(1):2-3.
McMillan M., Bellchambers H. Nurse prescribing: adding value to the consumer experience. Australian Prescriber. 2007;30(1):2-3.
Murney P. To mix or not to mix: compatibilities of parenteral drug solutions. Australian Prescriber. 2008;31(4):98-101.
Murtagh J. Cautionary Tales: Authentic Case Histories from Medical Practice. Sydney: McGraw-Hill; 1992.
Neeskens P. The evidence–relevance gap: the example of hormone replacement therapy. Australian Prescriber. 2002;25(3):60-62.
Nunn T., Williams J. Formulation of medicines for children. British Journal of Clincial Pharmacology. 2005;59(6):674-676.
Pharmacy Board of Victoria. Guidelines for Good Pharmaceutical Practice 2002. Melbourne: Pharmacy Board of Victoria; 2002.
Phillips S. The National Prescribing Service and Australian Prescriber. Australian Prescriber. 2002;25(2):26-27.
Pulver L.K., Tett S.E., Coombes J. The Queensland experience of participation in a national drug use evaluation project. Community-Acquired Pneumonia Towards Improving Outcomes Nationally (CAPTION). BMC Pulmonary Medicine. 2009;9:38.
Shargel L., Mutnick A.H., Souney P.F., et al. Comprehensive Pharmacy Review, 3rd edn. Baltimore: Williams & Wilkins; 1997.
Smith A. Competency for new prescribers. Australian Prescriber. 2007;30(3):58-59.
Stoneman J., Taylor S.J. Improving access to medicines in urban, regional and rural Aboriginal communities: is expansion of Section 100 the answer? Rural and Remote Health. 2007;7(2):738.
Sweeney G. Clinical Pharmacology: A Conceptual Approach. New York: Churchill Livingstone; 1990.
Therapeutic Guidelines Groups. Therapeutic Guidelines: Antibiotic. Version 13 [and other titles in the Therapeutic Guidelines series]. Melbourne: Therapeutic Guidelines, 2006.
Various authors. Adopting best evidence in practice. Medical Journal of Australia. 2004;180(6):S43. (Suppl) and following 11 articles
Youngson R.M. Collins Dictionary: Medicine, 2nd edn. Glasgow: HarperCollins; 1998.
Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT): www.ascept.org/
Australia’s National Medicines Policy (NMP): www.health.gov.au/internet/main/publishing.nsf/Content/National+Medicines+Policy-2
Australian Nursing & Midwifery Council: www.anmc.org.au/professional_standards
Cochrane Library: www.cochrane.org
Indigenous health workers information webpage: www.healthinfonet.ecu.edu.au/health-systems/health-workers
New Zealand Primary Health Care & Organisations (PHO): www.moh.govt.nz/primaryhealthcare
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
NPS News 62: www.nps.org.au/health_professionals/publications/nps_news/current/nps_news_62
Therapeutic Advisory Group Inc: www.ciap.health.nsw.gov.au/nswtag/
Society of Hospital Pharmacists of Australia: www.shpa.org.au/scripts/cgiip.exe/WService=SHPA/ccms.r?PageId=3
Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP): www.tga.gov.au/ndpsc/susdp.htm#susdp
Royal College of Nursing Australia: www.rcna.org.au/
Therapeutic Goods Administration (TGA): www.tga.gov.au
Victorian QUM and Drug Usage Evaluation Unit: www.health.vic.gov.au/qum/partnerships/vdueg.htm
More weblinks at: evolve.elsevier.com/AU/Bryant/pharmacology/
