Adjuvant Analgesics for Postoperative and Other Acute Pain
MANY of the adjuvant analgesics used to treat persistent pain are also used for acute pain. Most are combined with other analgesics as part of a multimodal plan to attack various underlying mechanisms of action (see Chapters 12 and 21 for more on multimodal analgesia). This chapter will present selected adjuvant agents for acute pain, including local anesthetic continuous peripheral nerve block and wound infusion; IV lidocaine; the anticonvulsants gabapentin and pregablin; clonidine; corticosteroids; and ketamine. Antidepressants, which compose another major adjuvant analgesic group, are not discussed here because they have not been shown to be effective for acute pain, including acute experimental pain (Wallace, Barger, Schulteis, 2002), and their delayed onset of analgesia makes them inappropriate for acute pain (Lussier, Portenoy, 2004) (see Chapter 22 for discussion of antidepressantes). See Table V-1, pp. 748-756, at the end of Section V for dosing guidelines and other characteristics of selected adjuvant analgesics.
Continuous Peripheral Nerve Block
For many years, regional anesthesia has been administered via single injection peripheral nerve blocks using a long-acting local anesthetic, such as bupivacaine (Marcaine) or ropivacaine (Naropin), to target a specific nerve or nerve plexus. This technique is highly effective in producing pain relief, but the effect is temporary (Rawal, 2007). The short duration of pain relief of the single-injection technique (4 to 12 hours duration for bupivacaine and ropivacaine) limits its use to a brief period, e.g., during and immediately after surgery (Richman, Liu, Courpas, et al., 2006). However, a relatively new pain management approach, continuous peripheral nerve block (also called perineural regional analgesia), offers an alternative. A continuous peripheral nerve block involves establishment of an initial block followed by the placement of a catheter through which an infusion of local anesthetic is administered continuously, with or without PCA capability. When PCA capability is added, this is referred to as PCRA (patient-controlled regional analgesia) (see dosing regimens that follow). In the acute pain setting, the therapy typically is continued during the first 24 to 72 hours postoperatively, depending on the type of surgery.
The first continuous peripheral nerve blocks were used for surgical pain several decades ago (Ilfeld, Morey, Enneking, 2002a). Over the years, the technique has been perfected, and reports of excellent pain relief, opioid dose-sparing effects, improved rehabilitation, and earlier discharge in the late 1990s sparked an increase in its use (Rawal, Axelsson, Hylander, et al., 1998; Singelyn, Deyaert, Joris, et al., 1998). More recent advances in operator skill and catheters and infusion devices made specifically for continuous peripheral nerve block have resulted in the widespread use of this technique for a variety of types of pain, particularly surgical pain, in both the inpatient and outpatient setting.
Catheter Placement
To place a peripheral nerve catheter, the anesthesiologist inserts a needle into the targeted nerve site, injects incremental doses of local anesthetic to block the desired nerve or nerves, and then threads the catheter through the needle (see Figure 26-1 for the site of lumbar plexus catheter placement). The needle is then removed, the catheter is secured, and the site is dressed. In the inpatient setting, continuous peripheral nerve blocks can be administered by the same infusion devices that are used to administer IV PCA or epidural analgesia. In the outpatient setting, portable disposable pumps (elastomeric or vacuum-driven syringe types) are usually used (Skryabina, Dunn, 2006). These latter pumps offer the benefits of being very small and easily discarded after use, avoiding the need for the patient to return the pump and the hospital to develop a pump tracking system. Some pumps allow patient-controlled capability for breakthrough pain so that PCRA may be administered. Some can be programmed to deliver automated bolus delivery in addition to PCRA capability (Taboada, Rodriguez, Bermudez, et al., 2009). The pumps also have simple mechanisms for stopping the infusion if adverse effects occur or at the end of therapy. Catheters are easily removed by clinicians, patients, or family members (Ilfeld, Enneking, 2002; Rawal, Axelsson, Hylander, et al., 1998; Swenson, Bay, Loose, et al., 2006).

Figure 26-1 Needle entry point for lumbar plexus catheter insertion. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 697, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
There are a number of excellent resources for detailed information on catheters and the placement procedure for continuous peripheral nerve block. For this, the reader is referred to the texts by Cousins, M. J., & Bridenbaugh, P. O. (Eds.). (1998). Neural blockade in clinical anesthesia and management of pain, Philadelphia, Lippincott-Raven; Miller, R. D. (Ed.). (2005). Miller’s anesthesia, ed 6, Philadelphia, Churchill Livingstone; Sinatra, R. S., de Leon-Casasola, O. A., Ginsberg, B., et al. (Eds.). (2007). Acute pain management, Cambridge, NY, Cambridge University Press; Meier, G., & Buttner, J. (2007). Peripheral regional anesthesia: An atlas of anatomy and techniques, ed 2, English translation available, New York, Thieme; and Waldman, S. (2009). Pain review, Philadelphia, Saunders; as well as any one of the research articles cited in this section. Capdevila and colleagues (2009) offer excellent suggestions for proper sterile technique for placement and site dressing. The various pumps used to administer continuous peripheral nerve blocks have been compared and discussed elsewhere (Capdevila, Macaire, Aknin, et al., 2003; Wedel, Horlocker, 2005).
Nurses are referred to their scope of practice as defined by their individual state board of nursing for their role in the administration of continuous peripheral nerve block, and they should see the American Society for Pain Management Nursing’s Position Paper on the Nurse’s Role in the Management and Monitoring of Analgesia by Catheter Techniques for additional guidance (Pasero, Eksterowicz, Primeau, et al., 2007). See also Box 26-1 for guidelines on the care of patients receiving continuous peripheral nerve block.
Research on the Use of Continuous Peripheral Nerve Block
There is now an impressive body of research supporting continuous peripheral nerve block as a primary analgesic technique for postoperative pain, particularly following orthopedic surgery. The following is a list of case reports, reviews, and placebo-controlled research on the use of this method for a variety of types of pain. Note that continuous peripheral nerve blocks have been used for some types of persistent noncancer and cancer-related pain as well.
• Breast surgery (Buckenmaier, Klein, Nielsen, et al., 2003)
• Radical prostatectomy (Ben-David, Swanson, Nelson, et al., 2007)
• Hand surgery (Rawal, Allvin, Axelsson, et al., 2002)
• Wrist, elbow surgery (Grant, Nielsen, Greengrass, et al., 2001; Ilfeld, Morey, Enneking, 2002b)
• Shoulder surgery (Borgeat, Kalberer, Jacob, et al., 2001; Borgeat, Perschak, Bird, et al., 2000; Fredrickson, Ball, Dalgleish, 2008; Gottschalk, Burmeister, Radtke, et al., 2003; Grant, Nielsen, Greengrass, et al., 2001; Hofmann-Kiefer, Eiser, Chappell, et al., 2008; Ilfeld, Enneking, 2002; Ilfeld, Morey, Wright, et al., 2003; Ilfeld, Wright, Enneking, et al., 2005; Mariano, Afra, Loland, et al., 2009; Singelyn, Seguy, Gouverneur, 1999; Stevens, Werdehausen, Golla, et al., 2007)
• Hip surgery (Chelly, 2007; Hebl, Kopp, Ali, et al., 2005; Ilfeld, Ball, Gearen, et al., 2009; Siddiqui, Cepeda, Denman, et al., 2007; Singelyn, Ferrant, Malisse, et al., 2005; Singelyn, Vanderelst, Gouverneur, 2001)
• Knee surgery (Barrington, Olive, Low, et al., 2005; Brodner, Buerkle, Van Aken, et al., 2007; Chelly, Greger, Gebhard, et al., 2001; Dauri, Fabbi, Mariani, et al., 2009; Door, Raya, Long, et al., 2008; Duarte, Fallis, Slonowsky, et al., 2006; Eledjam, Cuvillon, Capdevila, et al., 2002; Fowler, Symons, Sabato, et al., 2008; Grant, Nielsen, Greengrass, et al., 2001; Hayek, Ritchey, Sessler, et al., 2006; Hebl, Kopp, Ali, et al., 2005; Ilfeld, Gearen, Enneking, et al., 2006; Morin, Kratz, Eberhart, et al., 2005; Pulido, Colwell, Hoenecke, et al., 2002; Salinas, Liu, Mulroy, 2006; Syngelyn, Gouverneur, 2000; Zaric, Boysen, Christiansen, et al., 2006)
• Lower extremity distal to knee (Ilfeld, Morey, Wang, et al., 2002)
• Ankle surgery (Grant, Nielsen, Greengrass, et al., 2001; Ilfeld, Loland, Gerancher, et al., 2008; Ilfeld, Thannikary, Morey, et al., 2004)
• Foot surgery (di Benedetto, Casati, Bertini, 2002; Ilfeld, Thannikary, Morey, et al., 2004)
• Amputation (Grant, Nielsen, Greengrass, et al., 2001)
• Multiple combat casualties, amputation (Stojadinovic, Auton, Peoples, et al., 2006)
• Inguinal hernia repair (Schurr, Gordon, Pellino, et al., 2004)
• Cancer-related neuropathic pain (Vranken, van der Vegt, Zuurmond, et al., 2001)
• Burn pain during skin grafting (Cuignet, Pirson, Boughrouph, et al., 2004)
• Complex regional pain syndrome (CRPS Type I) (Wang, Chen, Chang, et al., 2001)
• Trigeminal neuralgia (Umino, Kohase, Ideguchi, et al., 2002)
Inpatient Use
A prospective analysis of nearly 1500 patients who had received continuous peripheral nerve block following inpatient orthopedic procedures found that this approach, supplemented with nonopioid or opioid analgesia as needed, provided effective pain relief in 96.3% of patients, with a very low incidence of complications (Capdevila, Pirat, Bringuier, et al., 2005). The technique is often incorporated into multimodal protocols, with the goal of improving functional outcomes following major orthopedic surgery. A total joint (hip and knee) clinical pathway that utilized a minimally invasive surgical technique and progressive daily recovery goals aggressively addressed pain with continuous peripheral nerve block in addition to preoperative and PRN postoperative opioids and nonopioids (Hebl, Kopp, Ali, et al., 2005). Patients in this study were matched with control patients who underwent the same surgery (non–minimally invasive) in the past 5 years and were managed with conventional opioid-based pain treatment methods. Those who were managed via the pathway had lower pain scores, required 50% less supplemental opioid, and experienced fewer opioid-induced adverse effects, increased ability to ambulate, and a shorter length of hospital stay (2.8 days compared with 5.0 days). There were no local anesthetic or catheter-related complications. Of note, 15% and 0% of those in the control group and the pathway group, respectively, experienced postoperative cognitive dysfunction.
Others have found positive results with this approach. A prospective study randomized patients undergoing total knee arthroplasty via a clinical pathway to receive a single-injection peripheral nerve block or a continuous peripheral nerve block infusion postoperatively and found lower pain scores during physical therapy and reduced supplemental analgesic requirements in those who received continuous peripheral nerve block but no differences in hospital length of stay and long-term functional outcomes (Salinas, Liu, Mulroy, 2006). The authors concluded that analgesia with continuous peripheral nerve block was superior to single-injection nerve block, but suggested that the somewhat disappointing functional results could have been related to the use of clinical pathways that dictate specific daily goals, which may overshadow the ability of improvements in the quality and duration of postoperative analgesia to affect recovery following total knee replacement.
Outpatient Use
The phenomenal growth in ambulatory surgery is described as one of the most significant changes in surgical practice in the past two decades (Rawal, 2007). However, patients in this setting are discharged rapidly following painful surgery, usually with a prescription for an oral nonopioid-opioid analgesic, and their postoperative pain may be poorly controlled. A systematic review revealed that 45% of patients undergoing ambulatory surgery experience moderate to severe pain and a high incidence of other distressing symptoms, such as nausea (17%) and sedation (42%), during the first 48 hours postoperatively (Wu, Berenholtz, Pronovost, et al., 2002).
Numerous studies have demonstrated the value of continuous peripheral nerve block for a variety of ambulatory surgical procedures (Richman, Liu, Courpas et al., 2006). Randomized controlled trials report excellent pain control with minimal adverse effects and complications, and patients and their families are able to manage the infusions at home without difficulty (Evans, Nielsen, Tucker, et al., 2009; Ilfeld, Morey, Enneking, 2002a, 2002b, 2002c; Ilfeld, Morey, Wright, et al., 2003; Rawal, Allvin, Axelsson, et al., 2002; Swenson, Bay, Loose, et al., 2006).
An observational study described the outcomes of 620 patients who received continuous peripheral nerve blocks in the home setting following ambulatory surgery (Swenson, Bay, Loose, et al., 2006). The patients underwent a variety of different ambulatory surgical procedures, and all received continuous peripheral nerve blocks with a fixed infusion of 5 mL of bupivacaine 0.25% (without PCRA capability) and a prescription of a nonopioid-opioid analgesic for breakthrough pain. A very small number (1.1%) experienced inadequate pain control, usually related to catheter problems. Two patients had complications, both following popliteal fossa catheterization—one developed CRPS and one experienced weakness and sensory loss; both resolved within weeks. A small number of patients (26 patients, 4.2%) required additional interventions, such as for inadequate pain control, additional patient teaching, and pump malfunction. Anesthesiologists were able to manage many of the problems by providing telephone instructions.
A randomized controlled trial of patients undergoing anterior cruciate ligament repair demonstrated that continuous femoral nerve block (4-day) infusion, as part of a multimodal plan that included standardized spinal anesthesia and NSAID and ketamine administration, significantly reduced pain scores for 7 days postoperatively, compared with placebo infusion (Williams, Kentor, Vogt, et al., 2006). However, analysis of patient-reported health status and functional outcomes between 7 days and 12 weeks found no differences among the groups (Williams, Dang, Bost, et al., 2009).
Efficacy Compared with Other Analgesic Approaches
A meta-analysis of research (19 randomized controlled trials, 603 patients) comparing postoperative opioid analgesia (variety of agents and routes) and continuous peripheral nerve block (variety of catheter locations and regimens) concluded that the latter provided superior analgesia (approximately a 50% VAS score reduction) at all evaluation time periods through postoperative day 3, fewer adverse effects (e.g., sedation, nausea), and higher patient satisfaction compared with opioid-based analgesic approaches (Richman, Liu, Courpas, et al., 2006). A later randomized controlled study comparing IV PCA alone with continuous peripheral nerve block plus IV PCA following hip arthroplasty observed similar findings (Siddiqui, Cepeda, Denman, et al., 2007). Those who received a continuous lumbar plexus block combined with IV PCA had improved analgesia, reduced opioid requirements and adverse effects, and enhanced patient satisfaction. One patient in the IV PCA-only group experienced clinically significant respiratory depression and required ventilator support for 6 hours. One patient in the continuous nerve block group developed quadricep weakness, which was discovered 4 weeks following surgery. Although the cause of the deficit was not determined, its occurrence led the researchers to speculate that it could have been related to the use of regional anesthesia in the presence of anticoagulation (see following discussion of adverse effects). A later study comparing IV PCA and continuous peripheral nerve block after open shoulder surgery reported better analgesia with the block but no differences in functional outcomes (Hofmann-Kiefer, Eiser, Chappell, et al., 2008).
Epidural analgesia has long been a first-line approach for the management of pain after total knee replacement. A meta-analysis of research comparing peripheral nerve block with epidural analgesia did not distinguish between single-injection and continuous peripheral nerve blocks and emphasized the urgent need for more research comparing the two techniques; the researchers concluded that peripheral nerve blocks appear to represent the best balance between analgesia and adverse effects for major knee surgery (Fowler, Symons, Sabato, et al., 2008). Patients undergoing knee replacement in one study were randomized to receive continuous epidural (ropivacaine + fentanyl) or continuous peripheral nerve block (femoral nerve = ropivacaine + fentanyl; sciatic nerve = ropivacaine) (Zaric, Boysen, Christansen, et al., 2006). Adverse effects, such as sedation and nausea, were more common in the epidural group, but pain was equally well-controlled in both groups, and there were no differences in mobilization and other rehabilitation outcomes or length of hospital stay between the two groups. Another study concluded that IV PCA morphine, continuous femoral nerve block, and continuous epidural analgesia provided similar pain relief, rehabilitation, and hospital stay but recommended continuous femoral nerve block as the best choice following hip arthroplasty given its more favorable adverse effect profile (Singelyn, Ferrant, Malisse, et al., 2005) (see Chapter 17 for IV PCA and Chapter 15 for epidural analgesia). An interesting study used an aggressive multimodal plan that included preoperative and postoperative nonopioid and opioid analgesics; preoperative pericapsular ropivacaine, steroid, and morphine; and epidural anesthesia for patients undergoing total knee arthroplasty (Dorr, Raya, Long, et al., 2008). Patients were given continuous femoral nerve block (N = 35) or continuous epidural analgesia (N = 35) postoperatively. Those receiving continuous peripheral nerve block consumed less oral opioid; walking distance on day 0 and day 1 was better for patients with epidural analgesia; length of stay was comparable between the groups; and adverse effects and complications were minimal in both groups. No patients had ileus or respiratory depression, which the researchers attributed to the avoidance of parenteral opioids in almost all patients in the study.
Other methods of local anesthetic administration have been compared as well. A prospective, randomized study of patients undergoing anterior cruciate ligament repair found that continuous femoral nerve block produced better pain relief during rest and activity and greater nonopioid and opioid dose-sparing effects compared with intraarticular and wound infusions of local anesthetic (see later in the chapter for these methods) (Dauri, Fabbi, Mariani, et al., 2009).
Dosing and Administration Regimens
Continuous peripheral nerve blocks are administered via three basic regimens: continuous infusion only (basal rate only), PCRA with basal rate, and PCRA with bolus doses only (Boezaart, 2006). Similar to PCA by other routes of administration, PCRA allows patients to take an active role in the management of their pain and individualize therapy to meet their unique analgesic needs. Some clinicians prefer to use PCRA with a basal rate rather than use a basal rate-only mode for continuous peripheral nerve block because it allows the lowest effective continuous infusion dose (Ilfeld, Morey, Enneking, 2002b, 2004; Ilfeld, Thannikary, Morey, et al., 2004). Practice varies widely, however (see later in the chapter).
As mentioned, automated bolus doses have been administered in addition to PCRA capability. A study randomized 50 patients to receive a continuous popliteal nerve block by automated bolus doses or a continuous infusion; both had PCRA capability (Taboada, Rodriguez, Bermudez, et al., 2009). The quality of analgesia and need for rescue analgesia were similar among the two groups, but those who received automated bolus doses required fewer PCRA doses and consumed less local anesthetic.
A variety of multimodal strategies are used with continuous peripheral nerve block therapy. Nonopioids, such as acetaminophen and an NSAID, and/or anticonvulsants, such as gabapentin or pregablin (see later in this chapter), may be provided in scheduled doses. IV (opioid) PCA (usually without basal rate) is sometimes used to provide supplemental analgesia for patients receiving continuous peripheral nerve block by basal rate only (i.e., without PCRA capability). Alternately, oral opioid or nonopioid-opioid combination analgesics are provided as needed for breakthrough pain. These are sometimes administered in scheduled doses to prevent breakthrough pain. Supplemental analgesia is also provided prior to ambulation and physical therapy to maximize comfort during these painful activities; when PCRA is used, patients are taught to self-administer a dose prior to these activities to improve their ability to participate.
The most commonly used local anesthetics and concentrations for continuous peripheral nerve block are ropivacaine 0.1% to 0.2% and bupivacaine 0.0625% to 0.125%. (The initial block is established with incremental doses of a higher concentration, usually of the same local anesthetic.) Some clinicians report efficacy with higher concentrations, e.g., a fixed infusion of 0.25% bupivacaine at 5 mL/h (Swenson, Bay, Loose, et al., 2006). One study found that 0.1% ropivacaine provided ineffective analgesia and 0.2% and 0.3% ropivacaine provided similarly effective analgesia (Brodner, Buerkle, Van Aken, et al., 2007). There were no adverse effects among the various concentrations, and the researchers suggested 0.2% concentration at an initial infusion rate of 15 mL/h. Clonidine is sometimes added to peripheral nerve block infusions to enhance the duration and effectiveness of the local anesthetic (Ilfeld, Morey, Enneking, 2003) (see Chapter 22 for more on clonidine).
Randomized studies have evaluated various dosing strategies in an attempt to determine an optimal regimen. It appears to vary depending on type of surgery and location of catheter. One compared 0.2% ropivacaine administered by: (1) a basal rate of 12 mL/h plus a low PCRA bolus dose of 0.05 mL (designated the basal group), (2) a basal rate of 8 mL/h plus a PCRA bolus dose of 4 mL (designated the basal-bolus group), or (3) a low basal rate of 0.3 mL/h plus a large PCRA bolus dose of 9.9 mL (designated the bolus group) in patients following foot or ankle surgery (Ilfeld, Thannikary, Morey, et al., 2004). PCRA bolus doses were available once every hour. The patients in the bolus group (PCRA only) had a higher incidence of inadequate pain control, increased supplemental opioid requirements, and more sleep disturbances compared with the basal groups. The basal-bolus group experienced the best overall outcome. The same mode was recommended following upper extremity surgery at or distal to the elbow (Ileld, Morey, Enneking, 2004). However, another group of researchers found no benefit with the addition of a ropivacaine basal rate, and they recommended PCRA bolus-only mode after major knee surgery (Eledjam, Cuvillon, Capdevila, et al., 2002). Others have conducted similar studies with bupivacaine 0.125%, and recommended PCRA bolus-only following total knee replacement (Singelyn, Gouverneur, 2000) and hip replacement (Singelyn, Vanderelst, Gouverneur, 2001) and a basal rate plus PCRA boluses for shoulder surgery (Singelyn, Seguy, Gouverneur, 1999).
The relationship between concentration and volume has also been studied and varies depending on catheter location. One study recommended a more concentrated solution (0.4% versus 0.2% of ropivacaine) in a smaller volume (4 mL/h versus 8 mL/h) for popliteal sciatic nerve block (Ilfeld, Loland, Gerancher, et al., 2008). Another recommended further research but suggested that a lower concentration in a larger volume provided superior analgesia for interscalene nerve blocks (Le, Loland, Mariano, et al., 2008) and for infraclavicular nerve blocks (Ilfeld, Le, Ramjohn, et al., 2009).
The duration of continuous peripheral nerve block therapy depends on many factors, including type of surgical procedure, location of catheter, and patient status (e.g., NPO, chest tubes still in place). A review of the data of nearly 1500 patients who received continuous peripheral nerve block for inpatient orthopedic surgeries revealed a median duration of 56 hours (Capdevila, Pirat, Bringuier, et al., 2005). Generally, the duration ranges from 24 to 72 hours. A secondary analysis of a double-blind, randomized controlled study found that extending the duration of therapy to 96 hours (4 days) had no effect on patients’ functional status and well-being between 7 days and 1 year following knee arthroplasty (Ilfeld, Meyer, Le, et al., 2009).
Adverse Effects and Complications
The adverse effects of local anesthetics delivered by continuous peripheral nerve block are similar to those by other routes of administration (see Box 26-1 and Chapter 15). Intravascular catheter migration is rare but has been reported (Capdevila, Pirat, Bringuier, et al., 2005) and would produce signs of local anesthetic toxicity, such as metallic taste, perioral numbness, and tinnitus. Patients receiving this technique should be evaluated systematically for these signs, and those who receive continuous peripheral nerve block in the home setting must be given verbal and written instructions that include the signs and symptoms of adverse effects and what to do if detected (see pp. 757-758 at the end of Section V). Intravascular injection and undetected early signs of local anesthetic toxicity can progress to cardiovascular collapse. Lipid emulsion has been used to successfully resuscitate several patients from cardiac arrest from local anesthetic-induced cardiotoxicity (Clark, 2008); however, the effectiveness of this treatment depends on the type of local anesthetic administered. That is, intralipid treatment appears to be effective for bupivacaine-induced but not ropivacaine- or mepivacaine-induced cardiac arrest (Espinet, Emmerton, 2009; Zausig, Zink, Keil, et al., 2009). Large doses of epinephrine are also reported to be required for reversal of bupivacaine-induced cardiotoxicity (Mulroy, 2002).
All local anesthetics produce both motor and sensory blockade (Wilson, 2009). The ability to participate in physical therapy early and effectively during the postoperative course for orthopedic patients is critical. Despite the administration of low concentrations of local anesthetic, motor block can present a problem, but adjustments in dose and technique, multimodal approaches, and the use of assistive devices have contributed to improved outcomes in these patients (Dorr, Raya, Long et al., 2008; Ilfeld, Gearden, Enneking, et al., 2006; Wilson, 2009) (see later discussion of Local Infiltration Analgesia).
As mentioned, opioids and nonopioids are often used for breakthrough pain during continuous peripheral nerve block therapy; however, the significant dose-sparing effects of the therapy reduce the incidence of adverse effects associated with these other analgesics, such as GI disturbances from nonopioids, and nausea, sedation, and respiratory depression from opioids. Research suggests that there is less cognitive dysfunction with this technique as well, which has implications particularly in older adult patients (Hebl, Kopp, Ali, et al., 2005). Still, patients must be assessed for these adverse effects whenever these other analgesics are co-administered with the therapy (see Chapters 6 and 19).
Complications are rare with continuous peripheral nerve blocks (Capdevila, Pirat, Bringuier, et al., 2005; Cuvillon, Ripart, Lalourcey, et al., 2001; Swenson, Bay, Loose, et al., 2006) and are thought to be less common than with single-injection blocks (Boezaart, 2006). Transient or permanent nerve injury can be caused by the nerve block as well as by inadvertent intraoperative or postoperative pressure applied to a nerve or postoperatively from traction injury (Boezaart, 2006) or hematoma compression (Siddiqui, Cepeda, Denman, et al., 2007). Transient nerve damage has been reported most often with interscalene blocks (Capdevila, Pirat, Bringuier, et al., 2005) and axillary blocks (Boezaart, 2006). Low incidences of persistent sensory blockade (3%), persistent motor blockade (2.2%), and paresthesias or dysesthesias (1.5%) during the postoperative period were described in one prospective analysis (Capdevila, Pirat, Bringuier, et al., 2005). All resolved without sequelae.
The use of anticoagulation therapy has significantly improved morbidity and mortality following some major surgeries, such as total knee or hip replacement, but this practice has presented a challenge to those who must find strategies that provide both effective and safe management of the associated pain. Peripheral nerve blocks provide exceptional pain relief but are not without a low risk (0.019% to 1.7%) of nerve damage and deficits from complications (Meier, Buttner, 2007), such as hematoma formation and compression (Siddiqui, Cepeda, Denman, et al., 2007). It is essential that patients be regularly assessed for signs of compression syndrome, such as changes in skin color in the affected area indicating poor circulation or increased numbness, tingling, or weakness in an affected extremity, and to be aware that these may be masked by the effects of local anesthetics (see Box 26-1). The reader is referred to the American Society of Anesthesiologists (ASA) guideline for the use of regional anesthetic techniques in anticoagulated patients, which describes the safe use of peripheral nerve blocks in these patients (Horlocker, Wedel, Benzon, et al., 2003) (see Chapter 15 and Table 15-8 on p. 439).
As with any indwelling catheter technique, there is a risk of infection and localized inflammation with continuous peripheral nerve blocks. Risk factors include a stay in the ICU with a possible association with catheter placement in patients who have sustained traumatic injury, duration of the indwelling catheter for greater than 48 hours, site of catheter (e.g., higher risk with femoral than with popliteal), male sex, and absence of antibiotic prophylaxis (Capdevila, Bringuier, Borgeat, 2009). Capdevila and colleagues (2009) recommend the same maximal sterile precautions used for epidural cathether placement to anesthesia providers placing peripheral nerve catheters (see Chapter 15). A prospective study of the experiences of nearly 1500 patients who had received continuous peripheral nerve blocks provided bacterial analysis of the catheters placed in 68% of the patients (Capdevila, Pirat, Bringuier et al., 2005). Positive bacterial colonization occurred in 28.7% of the cases, most often from the staphylococcus species (staphylococcus epidermidis [61%], gram-negative bacillus [21.6%], and staphylococcus aureus [17.6%]). The reader is referred to this study for a breakdown of the incidence and type of bacteria depending on type of peripheral nerve block. One patient in this study experienced symptomology from a staphylococcus aureus infection that resolved with antibiotic treatment. Another study found a 57% rate of bacterial colonization (primarily staphylococcus epidermidis) of femoral catheters; three transitory bacteremias occurred but resolved with catheter removal and no antibiotics (Cuvillon, Ripart, Llaourcey, et al., 2001). The authors recommended close monitoring of patients for signs of infection but did not recommend systematic bacterial analysis of catheters (see Box 26-1). Subcutaneous tunneling of the catheter is suggested as a means of further reducing risk, but well-controlled research is needed to confirm the effectiveness of this approach for this purpose (Compere, Legrand, Guitard, et al., 2009).
Technical difficulties in placement and maintaining the placement of catheters are reported in nearly every study but are relatively rare (Capdevila, Pirat, Bringuier, et al., 2005; Swenson, Bay, Loose, et al., 2006). As with any therapy that utilizes infusion devices, equipment malfunction is described as well.
Continuous Local Anesthetic Wound Infusion
Continuous local anesthetic wound infusions involve the surgeon’s placement of a catheter subcutaneously into the surgical wound at the end of the surgical procedure to be used for continuous infusion of local anesthetics, such as bupivacaine or ropivacaine, to control postoperative pain (Rawal, 2007). For some orthopedic surgeries, the wound catheter may be placed intraarticularly (Axelsson, Gupta, Johanzon, et al., 2008; Vintar, Rawal, Veselko, 2005). Intraarticular local anesthetics have also been administered by single injections (Convery, Milligan, Quinn, et al., 2001; Moiniche, Mikkelsen, Wetterslev, et al., 1999; Muittari, Kirvela, 1998; Ng, Nordstrom, Axelsson, et al., 2006). Single-dose intra-wound local anesthetic instillation has been used as well (Goldstein, Grimault, Henique, et al., 2000; Labaille, Mazoit, Paqueron, et al., 2002; Ng, Swami, Smith, et al., 2002). Just as with continuous peripheral nerve blocks, supplemental opioid or nonopioid-opioid combination analgesics must be provided in addition to these therapies.
The same analgesic infusion devices used for continuous peripheral nerve blocks can be used for continuous wound infusions. Advantages of this modality include that it is a simple and efficient technique and is less labor-intensive and expensive than some of the other methods used to manage postoperative pain, such as epidural analgesia and peripheral nerve blocks. A quantitative and qualitative systematic review of randomized controlled trials conducted between 1983 and 2006 showed that continuous local anesthetic wound infusions produced effective analgesia with a consistent and significant opioid dose-sparing effect, which was linked to a 30% to 80% reduction in postoperative nausea and vomiting (Liu, Richman, Thirlby, et al., 2006). Other findings were high patient satisfaction, reduced length of hospital stay, a low incidence of technical failure (1%), and no cases of local anesthetic toxicity. There were similar wound infection rates among those who received wound infusion and those who did not. In contrast, a more recent systematic review of the literature on local anesthetic infiltration for major abdominal and orthopedic surgeries concluded that a variety of techniques are used to administer continuous wound infusions, effectiveness is inconsistent, and none of the techniques described thus far in the literature could be recommended for routine use (Dahl, Moiniche, 2009).The reader is referred to this review and the review by Liu and colleagues (2006) for the many studies that have been conducted using this technique. Some of the research and surgical procedures for which the technique has been used are listed below.
• Shoulder repair (Axelsson, Gupta, Johanzon, et al., 2008; Axelsson, Nordenson, Johanzon, et al., 2003; Barber, Herbert, 2002; Gottschalk, Burmeister, Radtke, et al., 2003; Park, Lee, Kim, et al., 2002)
• Hip surgery (Bianconi, Ferraro, Traina, et al., 2003)
• Knee surgery (Alford, Fadale, 2003; Bianconi, Ferraro, Traina, et al., 2003; Vintar, Rawal, Veselko, 2005)
• Iliac crest bone graft (Blumenthal, Dullenkopf, Rentsch, et al., 2005; Singh, Samartzis, Strom, et al., 2005)
• Thoracotomy (Karakaya, Baris, Ozkan, et al., 2004; Tetik, Islamoglu, Ayan, et al., 2004)
• Mastectomy (Sidiropoulou, Buonomo, Fabbi, et al., 2008)
• Coronary bypass, cardiac surgery (Dowling, Thielmeier, Ghaly, et al., 2003; Magnano, Montalbano, Lamarra, et al., 2005; White, Rawal, Latham, et al., 2003)
• Inguinal hernia (Lau, Patil, Lee, 2003; LeBlanc, Bellanger, Rhynes, et al., 2005; Sanchez, Waxman, Tatevossian, et al., 2004)
• Colectomy (Baig, Zmora, Derdemezi, 2006)
• Cesarean section (Fredman, Shapiro, Zohar, et al., 2000; Givens, Lipscomb, Meyer, 2002)
• Abdominal hysterectomy (Gupta, Perniola, Axelsson, et al., 2004; Leong, Lo, Chiu, 2002)
• Major abdominal surgeries, e.g., colectomy, gastrectomy, cholecystectomy (Fredman, Zohar, Tarabykin, et al., 2001)
Local Infiltration Analgesia
The concept of local infiltration analgesia is based on the long history of infiltration of wounds with single injections of local anesthetics, but it differs in that local infiltration analgesia administers boluses of local anesthetic in a systematic fashion via a catheter placed in the wound prior to wound closure (Wilson, 2009). The administration of local infiltration analgesia was first introduced as part of a fast track program for lower limb surgery with a goal of aggressive ambulation in the immediate postoperative period and early discharge (Kerr, Kohan, 2008). Patients in this study (N = 325) underwent hip resurfacing or total hip or total knee arthroplasty and were given systematic infiltrations of ropivacaine, ketorolac, and epinephrine via single injections followed by a catheter placed in a manner that would ensure delivery of the mixture to the joint, tissue planes, and under the wound. (The reader is referred to the publication of this study [Kerr, Kohan, 2008] for direction for catheter placement depending on surgical procedure.) The researchers used other measures to localize the local anesthetic mixture to the surgical site and prevent motor blockade, such as extremity splinting, cooling, and compression. Before removal of the catheter at 15 to 20 hours postoperatively, it was reinjected with 15 mL of the local anesthetic mixture. Oral nonopioid and IV opioid supplemental analgesia was provided as needed. More than 80% of the patients in each group reported pain ratings of 3/10 or less at 4 hours (time of highest pain score) during both rest and mobilization; mean time to mobilization ranged from 9 (hip resurfacing) to 13 (knee arthroplasty) hours; 89%, 51%, and 41% of the patients receiving hip resurfacing, knee arthroplasty, and hip arthroplasty, respectively, were discharged directly home after a 1-night stay; and there were no serious adverse effects or complications. A systematic review of the literature on local anesthetic infiltration techniques for major abdominal and orthopedic surgeries emphasized the need for more research into local infiltration analgesia and discussed the difficulties in concluding effectiveness (i.e., many other variables must be factored in when a multimodal approach such as was described in the above study is used) (Dahl, Moiniche, 2009).
IV Lidocaine
As discussed earlier in Chapter 23, IV lidocaine is an option for refractory persistent neuropathic pain. It is also occasionally used for acute pain treatment. One of its primary benefits is its ability to reduce postoperative GI complications (Wright, Durieux, Groves, 2008). Early research demonstrated that IV lidocaine was effective in reducing postoperative ileus (Rimback, Cassuto, Tollesson, 1990), a major cause of morbidity after surgery (Kehlet, 2005) (see Chapter 19 for more on ileus). This effect is a primary reason for the occasional use of IV lidocaine in the perioperative setting as part of a multimodal pain treatment plan. It is usually combined with a nonopioid, such as ketorolac or celecoxib, and an opioid. A meta-analysis of eight studies involving patients who underwent abdominal surgery revealed that IV lidocaine compared with placebo was associated with better pain control as well as decreases in the duration of ileus, nausea and vomiting, and length of hospital stay (Marret, Rolin, Beaussier, et al., 2008). Although more research is needed, it has also been shown to attenuate surgery-induced suppression of immune function (Yardeni, Beilin, Mayburd, et al., 2009). Perioperative IV lidocaine reduced opioid requirement but did not reduce time to discharge in ambulatory surgery patients (McKay, Gottschalk, Ploppa, et al., 2009). More research and clinical experience are needed to define the role of IV lidocaine in this setting (Wu, Liu, 2009) (see Chapter 23 for mechanisms of action and adverse effects of IV lidocaine and Box 26-2 for administration guidelines).
A randomized controlled study administered a preoperative loading bolus dose of IV lidocaine (1.5 mg/kg) followed by a 4-hour lidocaine infusion (2 mg/minute) after skin closure or a placebo bolus and 4-hour placebo infusion for colorectal surgery (Herroeder, Pecher, Schonherr, et al., 2007). Return of bowel function and other GI parameters were significantly better and length of hospital stay was shorter for those who received IV lidocaine, but pain relief and opioid consumption did not differ between the two groups. Serum lidocaine levels were acceptable for all patients except one who presented with a peak value of 5.8 mcg/mL after lidocaine bolus (see later in the chapter for a discussion of serum lidocaine levels). All patients who were started on the lidocaine infusion finished it without adverse cardiac effects. Other studies have shown similar positive results with IV lidocaine (Groudine, Fisher, Kaufman, et al., 1998; Koppert, Weigand, Neumann, et al., 2004). Co-administration of the alpha2-adrenergic agonist dextromethorphan (see Chapter 23) and IV lidocaine demonstrated a synergistic effect and resulted in better pain relief and improved bowel function compared with placebo after laparoscopic cholecystectomy (Wu, Borel, Lee, et al., 2005).
In contrast, a randomized, placebo-controlled study showed a preoperative bolus of lidocaine (1.5 mg/kg) followed by an intraoperative lidocaine infusion (1.5 mg/kg/h) had no effect on postoperative pain control, opioid consumption, or hip flexion following hip arthroplasty (Martin, Cherif, Gentili, et al., 2008). However, another randomized controlled study used a more aggressive treatment protocol that consisted of a preoperative IV lidocaine bolus (1.5 mg/kg) followed by intraoperative (2 mg/kg/h) and 24-hour postoperative (1.33 mg/kg/h) lidocaine infusions or placebo administration in the same manner to patients undergoing colectomy (Kaba, Laurent, Detroz, et al., 2007). Compared with placebo, the patients who received lidocaine had significantly better results in consumption of opioid, pain and fatigue scores, return of bowel function, and hospital stay. Serum lidocaine levels remained well below toxic levels during infusion and for 24 hours after. Although lidocaine did not inhibit the stress response in this study, obvious major benefits to patient outcomes were attained. The researchers pointed out that a more profound blockade (e.g., epidural analgesia) may be required to alter stress response. Thoracic epidural lidocaine has been shown to produce better pain relief, less opioid consumption, and an earlier return of bowel function compared with IV lidocaine following colon surgery (Kuo, Jao, Chen, et al., 2006), but studies comparing the two therapies for effects on stress response could not be found.
Anecdotal experience suggests that IV lidocaine may be efficacious for burn pain, but a Cochrane Collaboration Review showed no significant benefits of systemic lidocaine compared with placebo and other pain therapies for this type of pain (Wasiak, Cleland, 2007). A lack of effectiveness for burn pain may be related to inadequate dosing. It has been noted that serum lidocaine levels higher than 4 mcg/mL are required to relieve thermally-evoked pain (Kingery, 1997). Monitoring serum lidocaine levels during therapy to determine optimal dose may be helpful for some types of pain (see Chapter 23).
Dosing and Serum Lidocaine Levels
Published IV lidocaine dosing regimens have varied (see Box 26-2). Most studies have produced good results with a preoperative bolus of 1.5 to 3 mg/kg followed by a continuous infusion of 1.33 to 3 mg/kg/h (Kaba, Laurent, Detroz, et al., 2007; Koppert, Weigand, Neumann, et al., 2004; Kuo, Jao, Chen, et al., 2006) or 2 to 3 mg/min (Groudine, Fisher, Kaufman, et al., 1998; Herroeder, Pecher, Schonherr, et al., 2007). If administered, intraoperative infusions of 2 mg/kg/h (Kaba, Laurent, Detroz, et al., 2007) and 2 to 3 mg/min have been used (Gordon, 2008; Herroeder, Pecher, Schonherr, et al., 2007; Wu, Borel, Lee, et al., 2005). Bolus doses are usually administered over a few minutes (e.g., 10 minutes). The length of infusion time varies widely among studies, with some administered only through surgery and others lasting between 1 hour and 24 hours postoperatively (Marret, Rolin, Beaussier, et al., 2008). Bolus doses and infusions should be administered via an infusion device to help ensure accurate dose delivery.
Cardiac antiarrhythmic activity and toxicity are associated with lidocaine serum levels of 1.5 mcg/mL and 5 mcg/mL, respectively (Lema, 1996; Groudine, Fisher, Kaufman, et al., 1998). Concentrations below toxicity levels (range 1.3 to 4.6 mcg/mL) have been reported in postoperative studies with the dosing previously described (Groudine, Fisher, Kaufman, et al., 1998; Kaba, Laurent, Detroz et al., 2007; Koppert, Weigand, Neumann, et al., 2004). One postoperative study reported that serum levels were within a range of 1.1 to 4.2 mcg/mL during IV lidocaine infusion in all of the patients except one who had a peak level of 5.8 mcg/mL following a lidocaine bolus (Herroeder, Pecher, Schonherr, et al., 2007). See Table V-1 on pp. 748-756 for dose selection for persistent neuropathic pain.
Anticonvulsants
The anticonvulsants gabapentin and pregabalin are first-line analgesics for neuropathic pain (see Chapter 23) and are increasingly being added to postoperative pain treatment plans. They have been shown to improve analgesia, allow lower doses of other analgesics, and help to prevent persistent neuropathic postsurgical pain syndromes (Dauri, Faria, Gatti, et al., 2009). Following is a discussion of the use of these two drugs for acute pain. See Chapter 23 for detailed discussion of their mechanisms of action and adverse effects.
Gabapentin
Several years before gabapentin was used in the clinical setting for acute pain treatment, it was studied in volunteers for its effects in an acute inflammatory pain model (Werner, Perkins, Holte, et al., 2001). The subjects in this study were randomized to receive placebo or 1200 mg of gabapentin 3 hours before delivery of a first-degree experimental thermal injury. Gabapentin diminished both the decrease in mechanical pain threshold in the burn area and the secondary hyperalgesia. Another study in healthy volunteers using heat-capsaicin sensitization showed similar results (Dirks, Petersen, Rowbotham, et al., 2002). The researchers in both groups concluded that gabapentin might have clinical potential in the treatment of acute pain disorders involving neuronal sensitization.
Animal research has shown that gabapentinoids work synergistically with the nonopioid naproxen to reverse peripheral inflammation and hyperalgesia (Hurley, Chatterjea, Feng, et al., 2002). Although more human research is needed to define the association between the gabapentinoids and other analgesics, these studies support a role for gabapentin (and pregabalin) in acute pain management. The practice of adding gabapentin to multimodal pain treatment plans to improve postoperative analgesia and prevent persistent neuropathic postsurgical pain is increasing (Ho, Gan, Habib, 2006; Tiippana, Hamunen, Kontinen, et al., 2007) and should be considered for other types of acute inflammatory pain as well (Gray, Williams, Cramond, 2008).
A meta-analysis of 18 studies concluded that gabapentin improved postoperative analgesia at rest and movement, and reduced opioid dose requirements and opioid-induced adverse effects (Peng, Wijeysundera, Li, 2007). Several other reviews have yielded similar conclusions (Gilron, 2006; Ho, Gan, Habib, 2006; Hurley, Cohen, Williams, et al., 2006; Seib, Paul, 2006; Tiippana, Hamunen, Kontinen, et al., 2007). All have called for more research with larger numbers of patients to establish optimal dose and titration for acute pain. Nonanalgesic effects, such as anxiolysis, and the impact of dizziness and sedation require further study as well (Peng, Wijeysundera, Li, 2007).
Numerous randomized controlled trials were identified for the first procedure-specific review of the literature on perioperative gabapentin (Mathiesen, Moiniche, Dahl, 2007). Surgical procedures included in this review were abdominal hysterectomy, breast surgery, spinal surgery, laparoscopic surgeries, orthopedic surgeries, ear-nose-throat surgeries, and others. Of these, only abdominal hysterectomy and spinal surgery offered enough studies to perform a quantitative procedure-specific analysis, which underscores the need for more research. The researchers concluded that perioperative use of gabapentin improved pain control and produced a significant opioid dose-paring effect for both abdominal hysterectomy and spinal surgery.
A meta-analysis of 18 clinical trials that combined gabapentin with other analgesics or placebo was undertaken to evaluate analgesic consumption as an efficacy outcome measure of analgesics (McQuay, Poon, Derry, et al., 2008). The authors concluded that it was difficult to precisely measure treatment gains by adding gabapentin to opioid or combination analgesic therapy and called for further analysis of analgesic consumption as a measure of analgesic efficacy in clinical trials. Nonetheless, the use of gabapentin preoperatively (400 mg to 1200 mg) and postoperatively (400 to 600 mg every 6 to 8 hours for at least 24 hours) is gaining favor as a therapeutic option for postoperative pain control (see dose selection). Following is a list of the research, case reports, and reviews on the use of gabapentin for acute pain.
• Craniotomy (Ture, Sayin, Karlikaya, et al., 2009)
• Abdominal hysterectomy (Dierking, Duedahl, Rasmussen, et al., 2004; Fassoulaki, Stamatakis, Petropoulos, et al., 2006; Gilron, Orr, Tu, et al., 2005; Mathiesen, Moiniche, Dahl, 2007; Sen, Sizlan, Yanarates, et al., 2009; Tiippana, Hamunen, Kontinen, et al., 2007; Turan, White, Karamanlioglu, et al., 2006)
• Vaginal hysterectomy (Rorarius, Mennander, Suominen, et al., 2004)
• Mastectomy and lumpectomy (Dirks, Fredensborg, Christensen, et al., 2002; Mathiesen, Moiniche, Dahl, 2007; Tiippana, Hamunen, Kontinen, et al., 2007)
• Spinal surgeries; laminectomy (Mathiesen, Moiniche, Dahl, 2007; Pandey, Navkar, Giri, et al., 2005; Pandey, Sahay, Gupta, et al., 2004; Radhakrishnan, Bithal, Chaturvedi, 2005; Turan, Karamanlioglu, Memis, et al., 2004; Van Elstraete, Tirault, Lebrun, et al., 2008)
• Laparoscopic cholecystectomy (Gilron, Orr, Tu, et al., 2009; Pandey, Priye, Ambesh, et al., 2004; Pandey, Priye, Singh, et al., 2004)
• Nephrectomy (Pandey, Singhal, Kumar, et al., 2005)
• Breast surgery (Dirks, Fredensborg, Christensen, et al., 2002; Fassoulaki, Patris, Sarantopoulos, et al., 2002; Fassoulaki, Triga, Melemeni, et al., 2005)
• Hand surgery (enhanced IV regional anesthesia) (Turan, White, Karamanlioglu, et al., 2007)
• Hip surgery (Rasmussen, Mathiesen, Dierking, et al., 2009)
• Knee surgery (Clarke, Pereira, Kennedy, et al., 2009; Dietrich, Kinney, Pulido, et al., 2009; Menigaux, Adam, Guignard, et al., 2005)
• Lower limb scar revision, skin graft (Turan, Kaya, Karamanlioglu, et al., 2006)
• Shoulder surgery (Adam, Menigaux, Sessler, et al., 2006)
• Ear-nose-throat surgery (Turan, Memis, Karamanlioglu, et al., 2004)
• Thyroidectomy (Al-Mujadi, A-Refai, Katzarov, et al., 2006; Brogly, Wattier, Andrieu, et al., 2008)
• Varicocele (Koc, Memis, Sut, 2007)
• Acute neuropathic burn pain (Gray, Williams, Cramond, 2008)
Prevention of Persistent Neuropathic Postsurgical Pain
The use of gabapentin preoperatively may be justified on the empirical basis that this drug can potentially reduce the likelihood of acute pain leading to persistent pain (Gilron, 2006; Tiippana, Hamunen, Kontinen et al., 2007) (see Section I for more on the incidence and underlying mechanisms of persistent postsurgical pain). With this goal in mind, it has been administered to patients undergoing surgical procedures that pose considerable risk for the development of persistent neuropathic postsurgical pain syndromes (e.g., thoracotomy, mastectomy, hernia repair, limb amputation, abdominal hysterectomy, and cholecystectomy).
A randomized controlled trial of patients undergoing thyroidectomy found that preoperative gabapentin (1200 mg) did not impact the quality of immediate postoperative pain or analgesic intake; however, it was associated with a significant reduction in the incidence of persistent neuropathic pain at a 6-month evaluation (Brogly, Wattier, Andrieu, et al., 2008). Another study found similar results, with a reduction in the incidence of persistent post–abdominal surgery pain (Fassoulaki, Stamatakis, Petropoulos, et al., 2006). In this study, treatment involved 400 mg of gabapentin or placebo given every 6 hours, beginning 18 hours preoperatively and continuing for 5 days.
An interesting blinded study randomized 50 patients undergoing cancer breast surgery to receive placebo or a multimodal treatment plan (Fassoulaki, Triga, Melemeni, et al., 2005). The treatment group received gabapentin 400 mg preoperatively and every 6 hours for 8 postoperative days, EMLA (eutectic mixture of local anesthetics) cream applied to the surgical wound area on the day of surgery and for 3 postoperative days, and intraoperative irrigations of ropivacaine 0.75% to the brachial plexus and adjacent intercostal spaces. The control group received placebo oral capsules, placebo cream, and saline irrigations administered as described for the treatment group. Those who received the multimodal treatment had better postoperative pain control and consumed less supplemental analgesia. A major finding was a significant reduction in persistent pain in the multimodal treatment group. Whereas 82% and 57% of those in the control group reported pain at 3 and 6 months, respectively, 45% and 30% of those in the treatment group reported persistent pain at 3 and 6 months, respectively. A later study found similar results with ropivacaine 0.75% wound irrigation and 400 mg gabapentin every 6 hours for 7 days postoperatively in women undergoing abdominal hysterectomy (Fassoulaki, Melemeni, Stamatakis, et al., 2007). An interesting placebo-controlled study compared preoperative gabapentin and ketamine and found that both drugs improved pain control and reduced opioid consumption; however, gabapentin, but not ketamine, prevented persistent pain in the first 6 months after abdominal hysterectomy (Sen, Sizlan, Yanarates, et al., 2009).
Not all studies have produced positive results with regard to prevention of persistent neuropathic postsurgical pain with gabapentin. One trial randomized 75 patients to receive gabapentin (1200 mg/day for 10 days) or the oral local anesthetic mexiletine (600 mg/day for 10 days) or placebo after cancer breast surgery (Fassoulaki, Patris, Sarantopoulos, et al., 2002). Those who were given gabapentin or mexiletine experienced less pain, reduced acetaminophen intake, and a 50% reduction in opioid requirement during the postoperative period compared with those who received placebo. Although burning pain was less frequent at 3 months in those who were given gabapentin or mexiletine, the incidence of persistent pain, its intensity, and the need for analgesia was similar among the three groups. A randomized controlled trial in which gradually increased doses (to 2400 mg/day) of gabapentin were administered for 30 days following elective lower amputation failed to demonstrate an effect on the incidence of phantom limb pain (Nikolajsen, Finnerup, Kramp, et al., 2006). More research with well-designed patient groups (surgical procedure and adjuvant therapy), more detailed follow-up evaluations, and sufficient numbers of patients has been encouraged (Kehlet, 2006).
Dose Selection
There is no consensus on an optimal perioperative gabapentin dosing regimen, and they vary widely in studies. An extensive review of the research on the use of gabapentin for postoperative pain treatment described both single- and multiple-dose regimens (Ho, Gan, Habib, 2006). Generally, preoperative doses range between 400 mg and 1200 mg. Postoperative doses are typically 400 mg to 600 mg administered every 6 to 8 hours. The duration of postoperative administration also varies widely and ranges from 24 hours to several days. The reader is referred to the systematic reviews that describe the various studies and dosing regimens (Gilron, 2006; Ho, Gan, Habib, 2006; Hurley, Cohen, Williams, et al., 2006; Peng, Wijeysundera, Li, 2007; Seib, Paul, 2006; Tiippana, Hamunen, Kontinen, et al., 2007).
Pregabalin
As a newer anticonvulsant, more research is needed to fully evaluate the effects of pregabalin on postoperative and other types of acute pain. Although a 2007 systematic review concluded that gabapentin and pregabalin effectively reduce postoperative pain, the researchers identified only one randomized controlled trial on the use of pregabalin for acute pain (Tiippana, Hamunen, Kontinen, et al., 2007). Patients in this study were randomized to receive placebo, ibuprofen (400 mg), pregabalin (50 mg), or pregabalin (300 mg) following third-molar extraction (Hill, Balkenohl, Thomas, et al., 2001). Onset of analgesia was rapid for pregabalin (23.5 minutes) but slightly slower than ibuprofen (16 minutes). Those who received the larger 300 mg dose of pregabalin or ibuprofen experienced superior pain relief compared with placebo. Pregabalin at the 50 mg dose did not differ from placebo in outcome parameters. Pregabalin 300 mg exhibited a significantly longer duration of analgesia compared with ibuprofen, pregabalin 50 mg, and placebo. The group who received pregabalin 300 mg had the highest patient global impression scores but also experienced adverse effects (e.g., dizziness, sedation, vomiting) most frequently (48%).
A randomized, placebo-controlled trial in which a single dose of 100 mg of pregabalin was administered preoperatively to women undergoing minor gynecologic surgery failed to show any reduction in postoperative pain or improvement in quality of recovery (Paech, Goy, Chua, et al., 2007). Given the findings of the previous study, in which 50 mg was essentially equivalent to placebo (Hill, Balkenohl, Thomas, et al., 2001), the lack of positive results in this study may have been due to the administration of too low a dose.
Patients undergoing upper or lower limb amputation were randomized to receive placebo or 300 mg or 600 mg of pregabalin 2 hours before surgery, followed by placebo or 300 mg or 600 mg of pregabalin 8, 16, and 24 hours after the preoperative dose (Azer, Abdelhalim, Elsayed, 2006). Both pregabalin doses significantly reduced pain and supplemental morphine consumption compared with placebo. The most common adverse effect was sedation, but this was not statistically significant. A single preoperative dose of pregabalin has been shown to produce similar opioid-dose sparing effects (Murcia Sanchez, Orts Castro, Perez Doblado, et al., 2006).
Another study of 91 women undergoing laparoscopic hysterectomy used the maximum recommended pregabalin dose (600 mg) and found that the drug produced a dose-dependent opioid dose-sparing effect, but the incidence of headache, dizziness, and blurred vision was highest with this dose compared with diazepam 10 mg and pregabalin 300 mg (Jokela, Ahonen, Tallgren, et al., 2008). A dose-ranging study randomized 108 patients undergoing a variety of outpatient surgical procedures to receive placebo or pregabalin 75, 150, or 300 mg 60 to 90 minutes prior to surgery (White, Tufanogullari, Taylor, et al., 2009). There were no differences in anxiety scores, pain scores, patient satisfaction, tolerance of normal diet, or return of bowel activity among the four groups. Sedation scores were higher and reports of dizziness more common in those who received 300 mg of pregabalin; some patients were reported to be difficult to arouse in this group. Length of hospital stay was also longer in this group, but this did not reach statistical significance. Others found similar results in patients following laparoscopic cholecystectomy (Chang, Lee, Kim, et al., 2009).
Pregabalin was studied as a component of a multimodal analgesia treatment plan but with disappointing results. Patients (N = 116) undergoing abdominal hysterectomy were randomized to receive preoperative acetaminophen (1000 mg) plus placebo alone, or with pregablin (300 mg), or with pregabalin (300 mg) plus dexamethasone (8 mg) (Mathieson, Rasmussen, Dierking, et al., 2009). Morphine consumption and pain scores at rest and activity did not differ among the groups. The incidence of nausea was significantly lower in the group that received dexamethasone, and vomiting was less in both of the groups that received pregabalin. Other adverse effects did not differ among the groups. An earlier placebo-controlled study by these researchers used the same protocol but without acetaminophen in patients undergoing hip arthroplasty (N = 120) and reported a reduction in morphine consumption but no differences in pain scores, no reduction in nausea and vomiting, and increased sedation in those who received pregabalin (Mathiesen, Jacobsen, Holm, et al., 2008).
Like gabapentin, pregabalin may have a role in the prevention of persistent postsurgical pain. A prospective, randomized controlled trial (N = 240) administered placebo or 300 mg of pregabalin preoperatively and 150 mg twice daily postoperatively through day 10; 75 mg twice daily on days 11 and 12; and 50 mg twice daily on days 13 and 14 to patients undergoing total knee arthroplasty; all patients received epidural analgesia postoperatively (Buvanendran, Kroin, Della Valle, et al., 2010). Those who received pregablin experienced significantly less persistent neuropathic postsurgical pain at 6 months than placebo (0% and 5.2%, respectively). Other positive results in those who received pregabalin in this study were greater knee flexion, less epidural analgesia consumption, better sleep, and faster time to meeting discharge criteria; however, the patients who received pregabalin also experienced more sedation and confusion on the day of surgery and the first postoperative day compared with those who received placebo.
These studies provide information similar to that obtained about gabapentin. Some, but not all, indicated benefit for treatment in the acute pain setting, and dose effects are likely to be important. Overall, more research is needed to determine the role of the gabapentinoids as adjuvant agents in multimodal postoperative pain treatment plans.
Clonidine
In the perioperative setting, the alpha2-adrenergic agonist clonidine has been used via multiple routes of administration to enhance pain relief, produce an opioid dose-sparing effect, and prolong anesthesia (Buvandendran, Kroin, 2007). Studies have suggested that it can reduce dose requirements for both general and IV anesthesia (Moss, Glick, 2005). When used for pain or anesthesia, it must be appreciated that hemodynamic effects of both spinal and systemic clonidine begin within 30 minutes of administration, reach a peak at 1 to 3 hours, and last approximately 6 to 8 hours after injection. Bioavailability by the oral route is almost 100%, and half-life ranges from 6 to 24 hours (Westfall, Westfall, 2006). A meta-analysis of randomized controlled trials comparing the various alpha2-adrenergic agonists (e.g., clonidine, dexmedetomidine) with controls concluded that the alpha2-adrenergic agonists reduce mortality and myocardial infarction after vascular surgery, and that during cardiac surgery, they may reduce ischemia and have effects on mortality and myocardial infarction (Wijeysundera, Naik, Beattie, 2003).
Dexmedetomidine (Precedex), another alpha2-adrenergic agonist, is used primarily for perioperative management and sedation in the critically ill. Similar to clonidine, dexmedetomidine has been shown to act synergistically with opioids, exert an opioid dose-sparing effect, and enhance anesthesia (Pandharipande, Ely, Maze, 2006). This drug is discussed in more detail in Chapter 27. (See Chapter 22 for underlying mechanisms and adverse effects of the alpha2-adrenergic agonists and Table 26-1 for a summary of the efficacy and adverse effects specifically with perioperative use of clonidine and dexmedetomidine.)
Table 26-1
Perioperative Use of Alpha2-Adrenergic Agonists: Efficacy and Major Adverse Effects
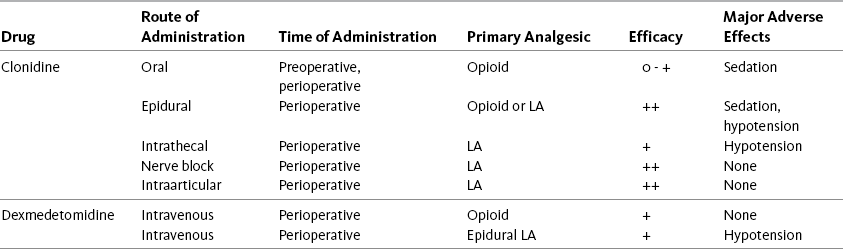
This table shows the efficacy and major adverse effects of some of the alpha2-adrenergic agonists used in the perioperative setting.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 711, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Intraspinal clonidine has been shown to work synergistically with opioids to produce improved postoperative pain outcomes. A randomized controlled trial of 85 patients undergoing coronary artery bypass surgery administered intrathecal morphine with or without clonidine (Nader, Li, Dosluoglu, et al., 2009). Those who received clonidine experienced a significant morphine dose-sparing effect, lower pain intensity, and shorter ventilation time. A trend toward increased vasopressin use was noted in the clonidine group, but this was not statistically significant. Intrathecal remifentanil and clonidine produced similar results in this same high-risk population (Lena, Balarac, Lena, et al., 2008). Another randomized controlled trial administered an epidural infusion of clonidine or placebo to patients following decompressive spinal surgery; all patients received IV PCA morphine postoperatively (Farmery, Wilson-MacDonald, 2009). Those who received clonidine used less morphine and experienced less nausea than those who received placebo. Other studies have shown similar positive results (Paech, Pavy, Orlikowski, et al., 2004; Schug, Saunders, Kurowski, et al., 2006). Compared with intrathecal morphine alone, the addition of clonidine to intrathecal morphine resulted in less intraoperative sufentanil, a longer time to first request for analgesia, and improved analgesia with rest and coughing in patients following radical prostatectomy (Andrieu, Roth, Ousmane, et al., 2009).
Timing of administration may be an important factor in determining efficacy of intraspinal clonidine. Epidural clonidine administered before abdominal surgery resulted in a greater opioid dose-sparing effect, better pain scores at 6 and 24 hours, and less sedation than epidural clonidine administered at the end of surgery (Persec, Persec, Bukovic, et al., 2007).
Other research has combined clonidine with an opioid and local anesthetic intraspinally (Forster, Rosenberg, 2004; Huang, Lin, Huh, et al., 2007; Schug, Saunders, Kurowski, et al., 2006). A systematic review of 22 randomized controlled trials concluded that clonidine prolonged the analgesic action and sensory and motor blockade of a variety of intrathecal local anesthetics in a dose-dependent manner, but was unable to establish the optimal dose of clonidine for these effects (Elia, Culebras, Mazza, et al., 2008) (see Chapter 15 for more on intraspinal clonidine).
An abundance of research has shown the benefits of adding clonidine to local anesthetic peripheral nerve blockade. An early systematic review of 24 studies concluded that clonidine as an adjunct to brachial plexus block produced improved analgesia and minimal adverse effects in single doses up to 150 mcg (Murphy, McCartney, Chan, 2000). A later review of 27 studies found that clonidine improved the duration of anesthesia and analgesia of continuous local anesthetic peripheral block, again with a low incidence of adverse effects in single doses up to 150 mcg (McCartney, Duggan, Apatu, 2007).
Randomized controlled trials have shown that intraarticular administration of clonidine alone (Gentili, Enel, Szymskiewicz, et al., 2001) or a combination of clonidine, opioids, and local anesthetics (Armellin, Nardacchione, Ori, 2008; Joshi, Reuben, Kilaru, et al., 2000) improved pain relief and reduced supplemental analgesic intake following knee surgery.
Systemic Clonidine
Systemic (oral and IV) clonidine has also been administered in the perioperative setting. Preoperative oral clonidine produced an anxiolytic effect and reduced postoperative morphine consumption by 30% in one study (Caumo, Levandovski, Hidalgo, 2009). An interesting study administered oral clonidine (2 mcg/kg) or placebo to patients with obstructive sleep apnea the night before ear-nose-throat surgery (Pawlik, Hansen, Waldhauser, et al., 2005). Those who received clonidine had significantly lower pain scores and consumed significantly less opioid postoperatively than those who received placebo. Another important finding was that minimal postoperative oxygen saturation was lower in the placebo group than in the clonidine group. Similar effects have been noted with the use of IV clonidine in this high-risk population. One study randomly assigned morbidly obese patients undergoing bariatric surgery to receive a preoperative IV infusion of clonidine and ketamine or standard anesthesia protocol and demonstrated reduced analgesic requirements and earlier extubation in those who received clonidine and ketamine (Sollazzi, Modesti, Vitale, et al., 2009). Given the need to find methods that allow reduced opioid doses in morbidly obese patients, who may be at higher risk for postoperative respiratory depression (ASA, 2006), the results of these studies of systemic clonidine are noteworthy (see Chapter 19 for more on postoperative respiratory depression).
Clonidine has also been added to opioids for IV PCA administration (Lehman, 2005). Patients (N = 60) undergoing lower abdominal surgery were randomized to receive a 20-minute IV infusion of clonidine (4 mcg/kg) at the end of surgery followed by postoperative PCA bolus doses of morphine (1 mg) plus clonidine (20 mcg) or a placebo infusion followed by postoperative PCA bolus doses of morphine (1 mg) alone (Jeffs, Hall, Morris, 2002). Although morphine consumption was similar between the groups, those who received clonidine had better pain relief during the first 12 hours postoperatively, less nausea during the first 24 hours, and were more satisfied with their pain relief.
Finally, IV clonidine has been used to effectively prevent and treat postanesthesia shivering (Kranke, Eberhart, Roewer, et al., 2004). A randomized controlled trial found that 100% of patients who received a single dose of 150 mcg of IV clonidine administered in the postanesthesia care unit (PACU) stopped shivering; a single dose of 25 mg of meperidine, which is the most common treatment for shivering in the PACU, stopped shivering in 18 of 20 patients with 2 patients requiring a second dose to cease shivering (Schwarzkopf, Hoff, Hartmann, et al., 2001).
Prevention of Persistent Neuropathic Postsurgical Pain
Clonidine may have a role in preventing persistent neuropathic postsurgical pain as well. A randomized controlled study found that an intraoperative intrathecal injection of clonidine (300 mcg) was more effective than an intraoperative intrathecal injection of bupivacaine or placebo in influencing several postoperative outcomes in patients undergoing colonic surgery (De Kock, Lavand’homme, Waterloos, 2005). Patients in the clonidine group had a longer time to first request for analgesia, lower pain scores and supplemental analgesic consumption, and a lower incidence of persistent pain at 6 and 12 months following colonic surgery compared with those in the other groups. No adverse events occurred in any of the groups. Intraoperative hemodynamic adverse effects, e.g., hypotension and bradycardia, occurred most often in the clonidine group but were corrected without incident. No one experienced excessive sedation.
Corticosteroids
Corticosteroids, particularly dexamethasone (Decadron), have been suggested as adjuvant agents in multimodal treatment plans for some types of postoperative pain (Buvanendran, Kroin, 2007; Gilron, 2004; Salerno, Hermann, 2006) (see Chapter 22 for underlying mechanisms and adverse effects). In experimental research in healthy volunteers, IV dexamethasone was not found to reduce inflammatory changes and pain when administered 2 hours prior to burn injury (Werner, Lassen, Kehlet, 2002). This finding supports the clinical impression that the drug does not provide sufficient analgesia acutely to be designated a primary analgesic. Nonetheless, it may contribute to positive outcomes when combined with other analgesics for acute pain. For example, a single dose of 40 mg IV dexamethasone administered as part of a multimodal treatment plan reduced pain with movement post–hip arthroplasty (Kardash, Beng, Tessler, et al., 2008), and 0.1 mg/kg dexamethasone IV given preoperatively enhanced intrathecal opioid analgesia following abdominal surgery (Movafegh, Soroush, Navi, et al., 2007).
Corticosteroids have a well-established role in the prevention and treatment of postoperative nausea and vomiting (PONV). Doses of 4 to 8 mg of dexamethasone are widely recommended for prevention of PONV (Fujii, Nakayama, 2007, 2008; Gan, Meyer, Apfel, et al., 2007; Golembiewski, Tokumaru, 2006) (see Chapter 19 for more on PONV).
A randomized controlled trial compared intraarticular dexamethasone and intraarticular morphine and found that both reduced pain for 5 days following knee surgery in patients with persistent osteoarthritis (Stein, Yassouridis, Szopko, et al., 1999). Further research is required to establish the efficacy of intraarticular corticosteroids for postoperative pain control (Lavelle, Lavelle, Lavelle, 2007).
N-Methyl-d-Aspartate (NMDA) Receptor Antagonists
The NMDA receptor antagonists, dextromethorphan and ketamine, were discussed in Chapter 23 for the treatment of persistent neuropathic pain. The discussion here will focus on their use for acute and postoperative pain. (See Chapter 23 for mechanisms of action and adverse effects.)
Dextromethorphan
Dextromethorphan has been administered by the oral or parenteral (IM, IV) routes with opioids and NSAIDs to enhance analgesia after surgery. The drug is available in cough suppressant products only in the United States. Some formulations are combined with alcohol and other additives, and, at present, the most concentrated source is in a 30 mg/5 mL suspension (e.g., Delsym 12-Hour). The lack of an IV formulation is problematic in postoperative patients who are often unable to take oral medications. This, in addition to unpredictable efficacy and no clear benefit over other analgesics, has resulted in limited use of dextromethorphan in the postoperative setting in the United States; however, it is used in countries where other formulations are available.
A systematic review of 28 randomized, placebo-controlled trials concluded that although there is potential for dextromethorphan to be a safe adjuvant to opioid analgesia for postoperative pain, the extant research has demonstrated inconsistent opioid dose-sparing and analgesic effects (Duedahl, Romsing, Moiniche, et al., 2006). Parenteral dextromethorphan produced more consistent reductions in opioid requirements than oral formulations, and adverse effects were minimal with the drug. Benefits appear to be greater when dextromethorphan is initiated before rather than after surgical incision (Helmy, Bali, 2001).
One study randomized patients (N = 100) undergoing abdominal hysterectomy to receive 30 mg of oral dextromethorphan or oral placebo preoperatively and three times during the first 24 hours after surgery (Chau-In, Sukmuan, Ngamsangsirisapt, et al., 2007). IV PCA morphine was administered postoperatively. Those who received dextromethorphan consumed less opioid intraoperatively and in the PACU and had significantly lower pain scores and a longer time to first analgesic use in the PACU, but there were no differences in pain scores, analgesic requirements, or adverse effects at later postoperative evaluations (6 and 24 hours). Others found similar disappointing results when the drug was added to a multimodal plan that included intrathecal morphine (Choi, Kliffer, Douglas, 2003).
Large doses of dextromethorphan were evaluated in a two-part randomized controlled trial of patients following knee surgery (Wadhwa, Clarke, Goodchild, et al., 2001). The first part of the study was undertaken to determine a maximum tolerated oral dextromethorphan dose, which was accomplished by administering titrated doses in the PACU. Some patients experienced unacceptable adverse effects of slurred speech and sedation followed by deep sedation, and one patient experienced frightening hallucinations with doses of 800 mg. This led the researchers to identify 750 mg as the maximum tolerated oral dextromethorphan dose. The second phase of the study randomized another group of patients undergoing knee surgery to receive 400 mg of dextromethorphan preoperatively followed by 200 mg at 8 and 16 hours postoperatively, or placebo at the same intervals. Those who received dextromethorphan consumed 30% less morphine but experienced no other benefits. Another important finding was that both the preoperative and postoperative doses of dextromethorphan were associated with a significantly high incidence of postoperative nausea. A placebo-controlled trial that administered preoperative dextromethorphan (150 mg) also found no benefits except a 30% reduction in opioid requirements but only for the first 3 hours postoperatively in patients following total abdominal hysterectomy (Ilkjaer, Bach, Nielsen, et al., 2000). This lower dose most likely contributed to a similar adverse effect profile compared with placebo. There were no differences between the placebo and dextromethorphan groups in hyperalgesia at 24 hours and 3 months postoperatively.
A different dosing regimen led to better results in a double-blind, placebo-controlled study in patients (N = 120) undergoing lower body bone and soft-tissue cancer surgery (Weinbroum, Bender, Nirkin, et al., 2004). The patients in this study had preoperative pain that was well controlled with NSAIDs. They were randomized to receive 90 mg of oral dextromethorphan or placebo 90 minutes before surgery and 90 mg daily for two days postoperatively. These patients were further randomized to one of two methods of postoperative pain control: IV PCA or patient-controlled epidural analgesia (PCEA). All patients were given the same general anesthetic approach for surgery. Overall, the patients who received IV PCA had the worst pain, sedation, and well-being scores. Analgesic consumption and pain and sedation scores were lowest and well-being scores the highest in the patients receiving PCEA and dextromethorphan. Other important benefits were that those given dextromethorphan ambulated earlier and experienced a two- to three-fold lower incidence of adverse effects compared with those who were given placebo. Lower pain scores, shorter time to first analgesic request, lower analgesic requirements, and earlier bowel function were also found with preincisional IM dextromethorphan (40 mg) in patients undergoing colon surgery (Yeh, Jao, Huh, et al., 2005).
In summary, until further research is conducted and more IV and oral formulations are available in the United States, the use of dextromethorphan cannot be recommended for acute pain management.
Ketamine
Ketamine has been administered in low (subanesthetic) doses for the treatment of postoperative pain and other types of acute pain. A low dose of ketamine is defined as an IV or epidural bolus less than 1 mg/kg and an infusion of 1.2 mg/kg/h (DeKock, Lavand’homme, 2007). Subanesthetic doses help to diminish the concerning psychotomimetic effects that can occur with higher ketamine doses. Ketamine may also have a role in treating acute opioid-related hyperalgesia and tolerance (Edrich, Friedrich, Eltzschig, et al., 2004; Gurfinkel, Czeiger, Douvdevani, et al., 2006; Svenson, Abernathy, 2007), and it has been studied for prevention of persistent neuropathic postsurgical pain syndromes (Remerand, Le Tendre, Baud, et al., 2009).
Although ketamine has been given alone for acute pain, the existing data suggest that its best use may be in combination with other analgesics, particularly opioids, to produce a dose-sparing effect. The versatility of routes by which it can be administered is an advantage, along with the fact that it does not produce respiratory depression. The primary routes of administration for perioperative ketamine are IV and epidural. The nurse has an important role in the administration and monitoring of ketamine (see Chapter 23 for mechanism of action and adverse effects and Box 23-1 on pp. 675-677 for administration guidelines).
Research on the use of ketamine for acute postoperative pain is more abundant and favorable than it is for persistent pain. An extensive review of the literature evaluated the use of ketamine as an adjuvant to opioid analgesia (Subramaniam, Subramaniam, Steinbrook, 2004). Only studies that compared an opioid alone with an opioid plus ketamine were included in the review. Nearly as many trials found improvement (20) as no improvement (17) in analgesia with the addition of ketamine. However, a later Cochrane Collaboration Review concluded that perioperative ketamine in subanesthetic doses reduces opioid requirements and PONV during the first 24 hours after surgery, and adverse effects at the doses used in this setting were found to be mild or absent (Bell, Dahl, Moore, et al., 2006). Another review found similar benefits (Elia, Tramer, 2005).
IV Ketamine
Practice varies considerably with IV ketamine use in the perioperative setting. It has been administered preoperatively, intraoperatively, and postoperatively via single bolus technique, continuous infusion, and added to IV PCA regimens. As part of a multimodal analgesic treatment plan, IV ketamine administered pre-incision, and then again postoperatively, produced a morphine dose-sparing effect, facilitated rehabilitation at 1 month, and was associated with reduced postoperative persistent pain at 6 months following total hip arthroplasty (Remerand, Le Tendre, Baud, et al., 2009). A low-dose ketamine infusion initiated immediately after anesthesia induction and completed before the end of surgery decreased opioid requirements both during and after open colorectal surgery (Guignard, Coste, Costes, et al., 2002). A combination approach whereby ketamine was administered intraoperatively followed by a postoperative infusion for 2 days resulted in significantly better pain control and less morphine consumption and postoperative nausea compared with just intraoperative ketamine or placebo (Zakine, Samarcq, Lorne, et al., 2008). A randomized, placebo-controlled study administered a single dose of 0.15 mg/kg ketamine or placebo IV 10 minutes after anesthesia induction for anterior cruciate ligament repair (Menigaux, Fletcher, Dupont, et al., 2000). Those who received ketamine consumed lower opioid doses and had improved mobilization postoperatively compared with those who received placebo. A systematic review concluded that there are no differences in postoperative effects based on the timing of ketamine administration (Subramaniam, Subramaniam, Steinbrook, 2004).
The addition of ketamine to IV PCA after surgery has produced excellent results and significant opioid dose-sparing effects in some studies (Guillou, Tanguy, Seguin, et al., 2003; Michelet, Guervilly Helaine, et al., 2007; Nesher, Ekstein, Paz, et al., 2009; Yamauchi, Asano, Watanabe, et al., 2008); however, a systematic review concluded that, although its addition to IV PCA therapy does not increase adverse effects, it does not improve analgesia either (Subramaniam, Subramaniam, Steinbrook, 2004). A fixed PCA combination, as occurs when the drugs are combined in the same drug reservoir, should be avoided in opioid-tolerant patients who may require large and rapidly increased opioid doses to control pain. This could result in unnecessarily high ketamine doses and a subsequent increase in adverse psychotomimetic effects (De Kock, Lavand’homme, 2007).
Continuous IV infusion of ketamine as an adjunct to opioid therapy produced somewhat better results in the previously mentioned review (Subramaniam, Subramaniam, Steinbrook, 2004). It was suggested that the small infrequent doses that are administered via PCA may be less effective than a continuous infusion, which produces a steady state. Three trials showed no difference, but four showed improvement when continuous ketamine was added to a postoperative opioid treatment plan; the research was strongest for the use of this method of delivery following major abdominal surgery.
Ketamine and Other Therapies
Ketamine has also been administered as part of a multimodal postoperative plan that included midazolam (Versed) (Wang, Xie, Wang, 2006) and tramadol (Webb, Skinner, Leong, et al., 2007) and also has been found to work synergistically with propofol to produce significant reductions in postoperative opioid requirement (Badrinath, Avramov, Shadrick, et al., 2000; Kakinohana, Higa, Sasara, et al., 2004). One study found that intraoperative co-administration of ketamine with propofol attenuated hypoventilation and produced positive mood effects without perceptual changes after ambulatory surgery (Mortero, Clark, Tolan, et al., 2001). The researchers suggested that ketamine might facilitate earlier recovery of postoperative cognition. In another study, patients with preexisting major depression who were undergoing orthopedic surgery were induced with propofol and fentanyl plus ketamine or placebo (Kudoh, Takahira, Katagai, et al., 2002). Those who received ketamine had significantly lower scores in pain, depressed mood, suicidal tendencies, somatic anxiety, and hypochondriasis. It has also been shown to attenuate delirium in patients following cardiac surgery with cardiopulmonary bypass (Hudetz, Patterson, Iqbal, et al., 2009).
IV ketamine has been administered with opioid-local anesthetic epidural analgesia and produced excellent results. A randomized controlled trial administered an intraoperative IV ketamine or placebo bolus and infusion and postoperative epidural morphine-bupivacaine analgesia to patients who underwent renal surgery (Kararmaz, Kaya, Karaman, et al., 2003). Both resting and coughing pain scores were lower, analgesic consumption was less, time to first analgesia was longer, and incidence of adverse effects was lower in those who received ketamine. Others have found this same synergistic effect between IV ketamine and epidural opioid–local anesthetic infusions (Suzuki, Haraguti, Sugimoto, et al., 2006).
Epidural Ketamine
Ketamine is not approved in the United States for epidural administration because of a lack of availability of a preservative-free ketamine and concerns about neurotoxicity from the preservative chlorbutanol and from the drug itself (Wilson, Nimmo, Fleetwood-Walker, et al., 2008). Nevertheless, its administration by the epidural route has been studied extensively, and a systematic review reported impressive results when administered by a single bolus technique or added to a continuous infusion with PCEA (Subramaniam, Subramaniam, Steinbrook, 2004). Systemic (not epidural) ketamine reduces hyperalgesia (see the paragraphs that follow) and appears to be more effective than epidural ketamine; therefore, the parenteral (IV) route of administration is the preferred route for postoperative pain treatment (De Kock, Lavand’homme, 2007).
Ketamine for Other Types of Acute Pain
Ketamine has shown good results for treatment of other types of acute pain as well. A retrospective study of 40 patients transported by a regional aeromedical program found IV ketamine to be an ideal drug in the prehospital setting (Svenson, Abernathy, 2007). Ketamine provided effective sedation and improved analgesia for burn pain and trauma pain that was refractory to opioid and sedative treatment in combative patients. Other benefits were maintenance of ventilatory response in patients with a difficult airway, and hemodynamic stability in patients who were hypotensive or had experienced hypotension with propofol sedation. A multicenter clinical trial randomized trauma patients with severe acute pain admitted to the emergency department to receive an IV ketamine or placebo infusion over 10 minutes followed by titration to comfort with IV morphine (Galinski, Dolveck, Combes, et al., 2007). Pain scores were similar, but morphine consumption was significantly less in those who received ketamine.
Animal research has shown that ketamine can interfere with the inflammatory cascade associated with burn injury and can improve survival in the presence of sepsis (Gurfinkel, Czeiger, Douvdevani, et al., 2006). As discussed, it can also produce effective analgesia for burn pain that is refractory to high doses of opioid analgesics (Svenson, Abernathy, 2007). A case report described long-term use of ketamine for sedation and analgesia in a patient with second- to third-degree facial and neck burns (Edrich, Friedrich, Eltzschig, et al., 2004). Prior to the addition of ketamine, the patient was receiving large doses of diazepam, midazolam, propofol, hydromorphone, and fentanyl without adequate sedation and comfort. He also developed bowel obstruction. The addition of a ketamine infusion (2.7 mg/kg/h) allowed tapering of opioid and sedative doses within 12 hours and eventual resolution of the severe ileus. The patient maintained good pain control and denied any psychotomimetic effects, was tapered from ketamine over a 2-day period, and was discharged after 37 days in the ICU.
Secondary Hyperalgesia and Central Sensitization
The role of the NMDA receptor in central sensitization suggests that NMDA receptor antagonists may be able to reduce the CNS changes following acute trauma that result in spreading or persistent pain (see Section I). Following surgical incision or similar tissue injury, there is primary hyperalgesia (immediate hypersensitivity and pain at the site of injury) and secondary hyperalgesia (pain felt in uninjured adjacent tissues). Secondary hyperalgesia may be related to the changes associated with NMDA-mediated central sensitization. Ketamine’s effect on experimental central sensitization suggests that it may be a drug that could be used to reduce the clinical phenomena associated with this pathophysiology. Studies show conflicting results, and more research is needed to identify underlying mechanisms and best prevention regimens (Duale, Sibaud, Guastella, et al., 2008; Wilson, Nimmo, Fleetwood-Walker, et al., 2008). It is possible that systemic (not epidural) therapy is needed to obtain these putative benefits (De Kock, Lavand’homme, Waterloos, 2001). If so, systemic (i.e., IV) ketamine would be the preferred route of administration as part of a balanced multimodal perioperative pain treatment plan (De Kock, Lavand’homme, 2007).
Opioid-Induced Hyperalgesia
Opioid-induced hyperalgesia (OIH) is a paradoxical situation in which increasing doses of opioid result in increasing sensitivity to pain (Compton, 2008) (see Chapter 11 for more on OIH). It is easily demonstrated in animal models and is thought to be an uncommon but potentially serious consequence of opioid administration (Angst, Clark, 2006; Chu, Angst, Clark, 2008). It is closely related to tolerance, in which increasing doses of opioid are needed to provide the same level of pain relief (Angst, Clark, 2006). Tolerance involves decreased sensitivity to opioids as a result of opioid exposure, whereas OIH represents increased sensitivity to pain in response to opioid exposure (DuPen, Shen, Ersek, 2007). OIH can be demonstrated in animals after minimal opioid exposure, but it is very rarely considered a phenomenon of interest during short-term opioid use in the acute pain setting.
OIH is suspected when increasing doses (usually high, rapidly escalating doses) of opioid fail to “catch up” with escalating pain; pain and skin sensitivity may occur at sites remote from the original pain (Chu, Angst, Clark, 2008; Mitra, 2008). There may be diffuse pain, and diffuse allodynia (Compton, 2008; Chu, Angst, Clark, 2008; Mitra, 2008), and this syndrome may be associated with agitation and tremulousness.
To assess the possible presence of OIH, other reasons for failure of the opioid to relieve pain must be ruled out. These include increase in pain pathology, opioid tolerance, opioid withdrawal, pseudoaddiction, and opioid addiction (Compton, 2008) (see Table 11-3, p. 296, for a brief description of these conditions compared with OIH). If the conditions outlined in Table 11-3 have been ruled out, OIH should be considered.
Several strategies have been suggested for the treatment of OIH. Though more research and clinical experience are needed, there may be a role for the NMDA receptor antagonists, particularly ketamine, when OIH occurs during pain treatment (De Kock, Lavand’homme, 2007). When added to the treatment plan, dramatic reductions in opioid dose have been reported (Edrich, Friedrich, Eltzschig, et al., 2004; Gurfinkel, Czeiger, Douvdevani, et al., 2006; Svenson, Abernathy, 2007).
Indications
The NMDA receptor antagonists are not first-line analgesics for any type of pain but may be considered in patients with severe pain refractory to first-line analgesics. Low-dose perioperative ketamine should be considered in patients at high risk for persistent neuropathic postsurgical pain, such as those undergoing hernia repair, thoracotomy, mastectomy, or elective limb amputation. A trial of a subanesthetic dose of ketamine may be appropriate in patients who are strongly suspected of having OIH. The decision to use NMDA receptor antagonists should be made by experienced pain management clinicians or anesthesiologists who are aware of their indications, patient populations who may benefit most, dosing regimens, administration techniques, adverse effects, and monitoring parameters to ensure patient safety.
Dose Selection
There are no consistent dosing recommendations for dextromethorphan. A preoperative dose of 90 mg administered 90 minutes before surgery then 90 mg daily for 2 days postoperatively produced good results in one study (Weinbroum, Bender, Nirkin, et al., 2004). On the other hand, another study found reduced opioid consumption but no other benefits with 400 mg of dextromethorphan preoperatively followed by 200 mg at 8 and 16 hours postoperatively (Wadhwa, Clarke, Goodchild, et al., 2001). An initial arm of this study established a maximum tolerated oral dose of 750 mg in the PACU.
Dosing guidelines have not been established for IV ketamine either. Typically treatment of acute pain is initiated with very low (subanesthetic) doses and titrated according to response (pain relief and adverse effects). IV ketamine doses of 0.2 to 0.5 mg/kg are reported to produce analgesia (Svenson, Abernathy, 2007). Severe trauma-related acute pain was treated in one study with an IV ketamine bolus dose of 0.2 mg/kg over a 10-minute period and IV morphine titration to comfort with an initial IV morphine dose of 0.1 mg/kg followed by 3 mg of IV morphine every 5 minutes (Galinski, Dolveck, Combes, et al., 2007). Although this ketamine protocol produced a significant morphine dose-sparing effect, pain scores were not significantly different than those who received a placebo bolus infusion and the same morphine titration protocol in this study.
The reader is referred to systematic reviews that provide the range of doses for perioperative ketamine that were effective and safe in research (e.g., Bell, Dahl, Moore, et al., 2006; Subramaniam, Subramaniam, Steinbrook, 2004). In general, intraoperative IV ketamine dosing recommendations are to give a 0.5 mg/kg bolus followed by a 5 mcg/kg/min infusion until skin closure then a 2 mcg/kg/min infusion for 48 hours (De Kock, Lavand’homme, 2007). Others have found good results with similar dosing (Guillou, Tanguy, Seguin, et al., 2003). Still others recommend a wider intraoperative dose range, i.e., 0.5 to 2 mg/kg at induction of general anesthesia followed by PRN or low-dose infusion depending on general anesthetic agent (Reves, Glass, Lubarsky, et al., 2005). Ketamine infusions should be administered via an infusion device that does not allow free flow to ensure accurate dose delivery.
It is believed that higher ketamine doses are no better than lower doses in providing pain relief. One early study found no significant differences in pain relief between IV doses of 5, 10, and 20 mg/h in older adults following surgery, and effects such as dreaming increased with increased doses (Edwards, Fletcher, Cole, et al., 1993).
The drug has been administered with the opioid in the same drug reservoir for IV PCA or has been infused concurrently but via a separate infusion. A novel study analyzed 12 combinations of morphine and ketamine for IV PCA and found a 1:1 ratio of morphine to ketamine and a lockout (delay) interval of 8 minutes to be optimal (Sveticic, Gentilini, Eichenberger, et al., 2003). However, a fixed combination is not recommended and is particularly important to avoid in opioid-tolerant patients who may require large and rapidly increased opioid doses to control pain. A fixed combination in this case could result in unnecessarily high ketamine doses and a subsequent increase in adverse psychotomimetic effects (De Kock, Lavand’homme, 2007).
There are no guidelines for epidural ketamine dosing. A randomized controlled trial administered 3.3 mg/kg/L ketamine in combination with 0.125% bupivacaine epidurally at 15 mL/h to patients prior to limb amputation; infusion rates were adjusted (range 10 to 20 mL/h) and bolus doses (10 to 15 mL) were administered postoperatively to provide optimal pain control (Wilson, Nimmo, Fleetwood-Walker, et al., 2008). The researchers described their previous experience with frequent and safe administration of 2 to 4 mg/h of epidural ketamine and noted that 500 mcg/kg or higher epidural ketamine bolus doses were associated with excessive sedation. Another study administered a single epidural ketamine bolus dose of 1 mg/kg combined with 50 mcg/kg of morphine and found no significant difference in pain or adverse effects compared with epidural placebo combined with 50 mcg/kg of morphine (Subramaniam, Subramaniam, Pawar, et al., 2001). A randomized controlled trial found that low epidural ketamine doses of 0.5 mg/kg provided better analgesia compared with low IV ketamine doses of 0.5 mg/kg or placebo (Xie, Wang, Liu, et al., 2003).
Oral ketamine is rarely used in the postoperative setting, but following a well-tolerated subcutaneous test dose of 0.1 mg/kg of ketamine, 50 mg of oral ketamine given twice daily in juice was added to other analgesics to treat an opioid-tolerant patient with severe refractory acute postoperative pain following spinal fusion (Friedman, Jallo, Young, 2001). Pain was stabilized at a pain rating of 3/10, and significant reductions in opioid were possible. Pain ratings remained stable after the ketamine was discontinued on the day of discharge 12 days after surgery.
As mentioned, the undesirable effects of ketamine have led some experts to recommend its prescription and management only by those skilled in pain management and anesthesiology (Bell, 2009; Cvrcek, 2008). Palliative care specialists are also among those knowledgeable in the safe administration of the drug. Some hospitals restrict the administration of ketamine to specific clinical areas, such as ICU, palliative care, and the emergency department. Nurses should be aware of their scope of practice, state board of nursing’s opinions, and institutional policies and procedures regarding the administration and monitoring of agents such as ketamine. See Box 23-1 on pp. 675-677 for guidelines on ketamine administration.
Conclusion
Many of the adjuvant analgesics can be combined with other analgesics as part of a multimodal plan for the treatment of acute pain. Among the most common are local anesthetics given by the IV or perineural routes of administration, gabapentinoids, clonidine, and ketamine. Each of these agents has a different underlying mechanism of action, and many work synergistically with other analgesics to produce significant dose-sparing effects.