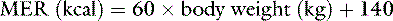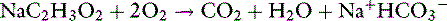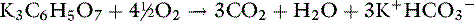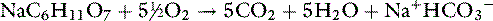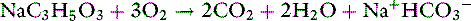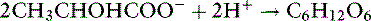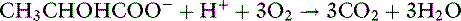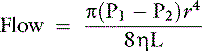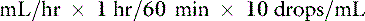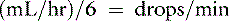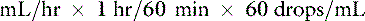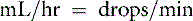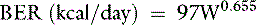Chapter 14 Introduction to Fluid Therapy
She had apparently reached the last moments of earthly existence, and now nothing could injure her—indeed, so entirely was she reduced, that I feared I should be unable to get my apparatus ready ere she expired. Having inserted a tube into the basilic vein, cautiously—anxiously, I watched the effects; ounce after ounce was injected, but no visible change was produced. Still persevering, I thought she began to breathe less laboriously, soon the sharpened features, and sunken eye, and fallen jaw, pale and cold, bearing the manifest impress of death’s signet, began to glow with returning animation; the pulse which had long ceased, returned to the wrist; at first small and quick, by degrees it became more and more distinct, fuller, slower, and firmer, and in the short space of half an hour, when six pints had been injected, she expressed in a firm voice that she was free from all uneasiness, actually became jocular, and fancied that all she needed was a little sleep; her extremities were warm, and every feature bore the aspect of comfort and health.
Thomas Latta, describing the first use of intravenous fluid therapy in a human patient with cholera in a letter to the Lancet, 1832.
Fluid therapy is supportive. The underlying disease process that caused the fluid, electrolyte, and acid-base disturbances in the patient must be diagnosed and treated appropriately. Normal homeostatic mechanisms allow the clinician considerable margin for error in fluid therapy, provided that the heart and kidneys are normal. This is fortunate because estimation of the patient’s fluid deficit is difficult and may be quite inaccurate. The purpose of this chapter is to provide an overview of the principles of fluid therapy. The composition and distribution of body fluids are discussed in Chapter 1, and the technical aspects of vascular access are discussed in Chapter 15. Fluid therapy potentially consists of three phases: resuscitation, rehydration, and maintenance. Most patients in shock (see Chapter 23) require rapid administration of a large volume of crystalloid, colloid, or other fluid to expand the intravascular space and correct perfusion deficits. Dehydrated patients also require sustained administration of crystalloid fluids for 12 to 36 hours to replace fluid losses from the interstitial and intracellular spaces. Patients with normal hydration unable to consume sufficient water to sustain fluid balance require maintenance fluid therapy with crystalloid solutions. In formulating and implementing a fluid therapy plan, eight questions should be considered10,28:
1. Is the patient suffering from a shock syndrome that requires immediate fluid administration?
3. Can the patient consume an adequate volume of water to sustain normal fluid balance?
4. What type of fluid should be given?
5. By what route should the fluid be given?
6. How rapidly should the fluid be given?
Is the patient suffering from a shock syndrome that requires immediate fluid administration?
Shock patients (see Chapter 23) urgently require fluid therapy. The presence of altered mental status and cool extremities in association with tachycardia or severe bradycardia, mucous membrane pallor, prolonged or absent capillary refill time, reduced or absent peripheral pulses, and hypotension are among the most common physical examination findings in patients in shock. Such physical examination findings in association with a compatible clinical history are the basis for the decision to institute a resuscitation phase of fluid therapy. Some forms of shock may be associated with variations in these physical examination findings, and it is crucial to understand the different shock syndromes. (See Chapter 23 for more information on shock.)
The shock syndromes most likely to respond to marked volume expansion of the intravascular space are hypovolemic and distributive shock states. Obstructive forms of shock often respond favorably to moderate volume expansion. Fluid administration is contraindicated in patients with predominantly cardiogenic forms of shock.
Regardless of their underlying disease, severely dehydrated patients can be in shock and require a resuscitation phase of fluid therapy before initiating the rehydration phase. However, not all patients in shock are dehydrated and thus may or may not require a rehydration phase of therapy. The rapidity and volume of loss from both the intravascular and extravascular fluid compartments in conjunction with the extent of any compensatory response will determine whether the patient is in shock or is dehydrated.
Is the patient dehydrated?
The need for a rehydration phase is dependent on the underlying condition of the patient. For surgical patients, there are additional indications for fluid therapy, such as maintenance of venous access for emergencies and establishment of diuresis to maintain renal perfusion during anesthesia (see Chapter 17). For medical patients, the answer to this question depends on an assessment of the animal’s state of hydration. The hydration status of the animal is estimated by careful evaluation of the history, physical examination findings, and the results of a few simple laboratory tests.7,11
In its most narrow sense, dehydration refers to loss of pure water. However, the term dehydration usually is used to include hypotonic, isotonic, and hypertonic fluid losses. The type of dehydration is classified by the tonicity of the fluid remaining in the body (e.g., a hypotonic loss would result in hypertonic dehydration). Isotonic and hypotonic losses are most common in small animal practice. Isotonic fluid loss can result in volume depletion and nonosmotic stimulation of antidiuretic hormone (ADH) release, thus preventing effective excretion of consumed water and resulting in hypotonic dehydration. Types of dehydration are depicted in Figure 3-1 and are discussed in detail in Chapter 3.
Fluid balance
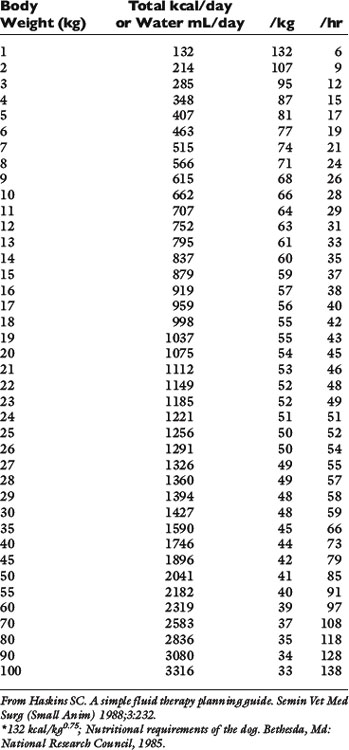
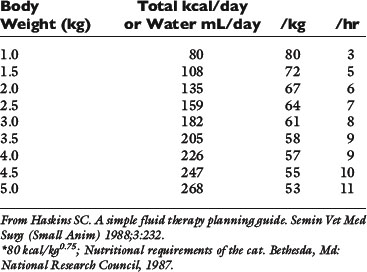
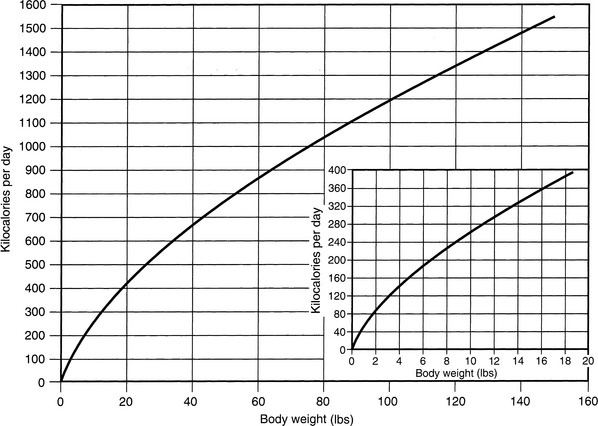
Normal sources of fluid input are water consumed in food, water that is drunk, and water produced in the body as a result of metabolism. Nutrient oxidation produces approximately 0.1 g of water per kilocalorie of energy released.2 Maintenance water and electrolyte needs parallel caloric expenditure,20,22,23 and normal daily losses of water and electrolytes include respiratory, fecal, and urinary losses. Estimated daily caloric and water requirements for dogs and cats are shown in Tables 14-1 and 14-223 and in Figure 14-1.20 Respiratory loss of fluid can be important in dogs because panting has been adapted for thermoregulation in this species. Pyrexic patients also can lose fluid by this route. Normally, cutaneous losses are unimportant in dogs and cats because eccrine sweat glands are limited to the foot pads and do not play an important role in thermoregulation in these species. Sympathetic stimulation as a result of heat stress in the cat may result in increased secretion of saliva, and a small volume of fluid may be lost by this route.
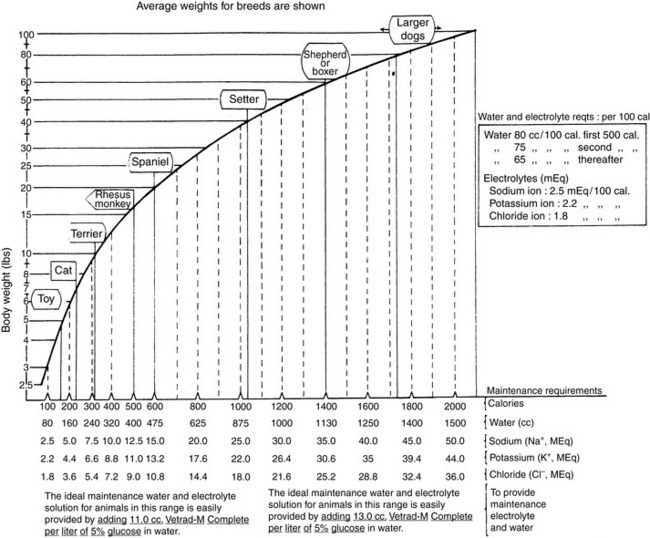
Figure 14-1 Daily water, calorie, and electrolyte requirements for dogs and cats.
(From Harrison JB, Sussman HH, Pickering DE. Fluid and electrolyte therapy in small animals. J Am Vet Med Assoc 1960;137:638.)
In disease states, decreased fluid intake results from anorexia, and increased fluid loss may occur by urinary (e.g., polyuria) and gastrointestinal (e.g., vomiting, diarrhea) routes. Other less common routes of loss include skin (e.g., extensive burns), respiratory tract, and salivary secretions, as described before. Third-space loss of fluid occurs when effective circulating volume is decreased, but the fluid lost remains in the body. Examples include intestinal obstruction, peritonitis, pancreatitis, and effusions or hemorrhage into body cavities. Decreased fluid intake and increased loss often coexist (e.g., anorexia, vomiting, and polyuria in a uremic animal).
History
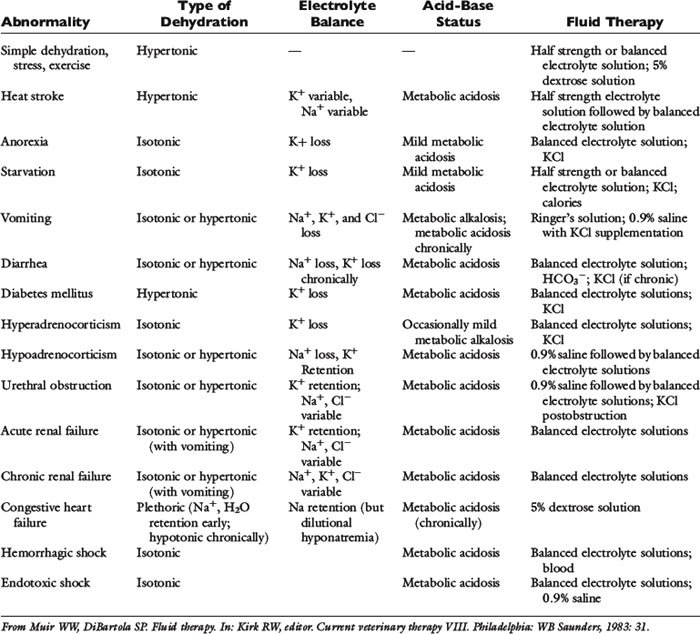
Historical information about the route of fluid loss may suggest the affected fluid compartment or compartments, as well as the patient’s electrolyte and acid-base derangements. The time period over which fluid losses have occurred and an estimate of their magnitude should be determined. Information about food and water consumption, gastrointestinal losses (e.g., vomiting, diarrhea), urinary losses (i.e., polyuria), and traumatic losses (e.g., blood loss, extensive burns) should be obtained from the owner. Excessive insensible water losses (e.g., increased panting, pyrexia) and third-space losses may be determined from the history and physical examination. In addition, the clinician’s knowledge of the suspected disease can aid in predicting the composition of the fluid lost (e.g., vomiting caused by pyloric obstruction leads to loss of hydrogen, chloride, potassium, and sodium ions and development of metabolic alkalosis, whereas small bowel diarrhea typically leads to loss of bicarbonate, chloride, sodium, and potassium ions and development of metabolic acidosis) (Table 14-3).
Physical examination
The physical findings associated with fluid losses of 5% to 15% of body weight vary from no clinically detectable changes (5%) to signs of hypovolemic shock and impending death (15%) (Table 14-4).7,11,20 The clinician may estimate the hydration deficit by evaluating skin turgor or pliability, the moistness of the mucous membranes, the position of the eyes in their orbits, heart rate, character of peripheral pulses, capillary refill time, and extent of peripheral venous distention (e.g., inspection of jugular veins). A decrease in the volume of the interstitial compartment leads to decreased skin turgor and dryness of the mucous membranes. A decrease in plasma volume leads to tachycardia, alterations in peripheral pulses, and collapse of peripheral veins. When these cardiovascular signs are present, the patient is in shock and should be resuscitated promptly before correction of the hydration deficit. Typically, such signs of hypovolemic shock appear with loss of at least 10% to 12% of the patient’s body weight. The fluid deficit in a given patient is difficult to determine with accuracy because of the subjectivity of skin turgor evaluation and the possibility of undetected ongoing (contemporary) losses. Thus a crude clinical estimate of hydration status and the patient’s response to fluid administration become important tools in evaluating the extent of dehydration that was present and in formulating ongoing fluid therapy.
Table 14-4 Physical Findings in Dehydration
| Percent Dehydration | Clinical Signs |
|---|---|
| <5 | Not detectable |
| 5-6 | Subtle loss of skin elasticity |
| 6-8 | Definite delay in return of skin to normal position |
| Slight prolongation of capillary refill time | |
| Eyes possibly sunken in orbits | |
| Possibly dry mucous membranes | |
| 10-12 | Tented skin stands in place |
| Definite prolongation of capillary refill time | |
| Eyes sunken in orbits | |
| Dry mucous membranes | |
| Possibly signs of shock (tachycardia, cool extremities, rapid and weak pulses) | |
| 12-15 | Definite signs of shock |
| Death imminent |
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 33.
Skin turgor is dependent on the amount of subcutaneous fat and elastin and on interstitial volume. Detection of dehydration by skin turgor is dependent on the animal’s skin turgor before dehydration developed, the position of the animal (e.g., standing, recumbent) when the skin is checked, the site used for evaluation, and the amount of subcutaneous fat.19 Skin pliability should be tested over the lumbar region with the dog in a standing position. When evaluated by skin turgor, obese animals may appear well hydrated owing to excessive subcutaneous fat despite being dehydrated. Conversely, emaciated animals and older animals may appear more dehydrated than they actually are because of lack of subcutaneous fat and elastin. A false impression of dehydration also may occur with persistent panting, which may dry the oral mucous membranes. The urinary bladder should be small in a dehydrated animal with normal renal function. A large, urine-filled bladder in a severely dehydrated patient indicates failure of the normal renal concentrating mechanism.
Body weight recorded on a serial basis traditionally has been thought to be the best indicator of hydration status, especially when fluid loss has been acute and previous body weight has been recorded. Loss of 1 kg of body weight indicates a fluid deficit of 1 L. Unfortunately, previous body weight is often unknown in animals presented for treatment. However, records from previous routine hospital visits may provide this information. Despite conventional reasoning, clinician estimates of hydration in dogs and cats admitted to a veterinary teaching hospital intensive care unit did not reliably predict changes in weight after 24 to 48 hours of fluid therapy.18 Loss of weight in chronic diseases includes loss of muscle mass and fluid loss. An anorexic animal may lose 0.1 to 0.3 kg of body weight per day per 1000 kcal of energy requirement.13 Losses in excess of this amount indicate fluid loss. Another factor that must be considered in evaluating body weight is the possibility of third-space loss. Fluid lost into a third space does not decrease body weight.23
Laboratory findings
The hematocrit or packed cell volume (PCV), total plasma protein concentration (TPP), and urine specific gravity (USG) are simple laboratory tests that can aid in the evaluation of hydration. It is important to obtain these values before initiating fluid therapy. The PCV and TPP should be evaluated together to minimize errors in interpretation. The PCV and TPP increase with all types of fluid losses excluding hemorrhage, whereas serum sodium concentration increases, decreases, or remains unchanged depending on the loss (e.g., hypotonic, hypertonic, isotonic). The effects of the different types of dehydration on the serum sodium concentration are discussed in Chapter 3. Table 14-5 shows possible interpretations of various combinations of PCV and TPP values. The PCV alone may be an unreliable indicator of hemoconcentration in water-deprived dogs, and although TPP increases, test results may not be above the upper limit of the normal range.19 In one study of dogs and cats admitted to an intensive care unit, baseline measurements of PCV and TPP were not abnormally high in animals judged clinically to be dehydrated, and fluid therapy with crystalloids in dogs had no significant effect on PCV, although TPP decreased slightly.18 The USG before fluid therapy is helpful in the preliminary evaluation of renal function. USG should be high (>1.045) in a dehydrated dog or cat if renal function is normal. This may not be true if other disorders affecting renal concentrating ability, such as medullary washout of solute, are present. Furthermore, previous administration of corticosteroids or furosemide can decrease urinary concentrating ability. After fluid therapy has been initiated, USG falls into the isosthenuric range if rehydration has been achieved.
Table 14-5 Interpretation of Hematocrit and Total Plasma Protein Concentrations
| PCV (%) | Total Plasma Proteins (g/dL) | Interpretation |
|---|---|---|
| Increased | Increased | Dehydration |
| Increased | Normal or decreased | Splenic contraction |
| Polycythemia | ||
| Dehydration with preexisting hypoproteinemia | ||
| Normal | Increased | Normal hydration with hyperproteinemia |
| Anemia with dehydration | ||
| Decreased | Increased | Anemia with dehydration |
| Anemia with preexisting hyperproteinemia | ||
| Decreased | Normal | Nonblood loss anemia with normal hydration |
| Normal | Normal | Normal hydration |
| Dehydration with preexisting anemia and hypoproteinemia | ||
| Acute hemorrhage | ||
| Dehydration with secondary compartment shift | ||
| Decreased | Decreased | Blood loss |
| Anemia and hypoproteinemia | ||
| Overhydration |
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 34.
Can the patient consume an adequate volume of water to sustain normal fluid balance?
Hospitalized patients that have been volume resuscitated and rehydrated may not have recovered to the extent that appetite and ability to consume water have returned to normal. Such patients require administration of adequate amounts of fluid to meet their needs. The needs of partially or completely anorexic hospitalized dogs and cats are not well understood. Most predictions about maintenance fluid requirements are extrapolated from studies of normal nonanorexic animals. Absorption of nutrients into the bloodstream and their subsequent metabolism produces solutes that must be excreted in urine. Sensible fluid losses and urine production are decreased during fasting in normal animals because less solute requires excretion. Thus the maintenance fluid requirements of partially or completely anorexic patients are difficult to predict. Typically fluids are administered to veterinary patients in volumes predicated on the needs of animals that are not anorexic. Careful observation of urine production in such patients is warranted. If the patient is expected to have normal urinary concentrating ability and is urinating large volumes of dilute urine frequently, excessive fluid administration may be a contributing factor. Careful reduction of fluid administration and subsequent observation are warranted in such patients.
What type of fluid should be given?
A fluid is said to be balanced if its composition resembles that of extracellular fluid (ECF; e.g., lactated Ringer’s solution, Normosol-R [Abbott Laboratories, Abbott Park, Ill.], Plasma-Lyte 148 [Baxter Healthcare, Deerfield, Ill.]) and unbalanced if it does not (e.g., normal saline). Fluid preparations may be further classified as crystalloids or colloids. Crystalloids are solutions containing electrolyte and nonelectrolyte solutes capable of entering all body fluid compartments (e.g., 5% dextrose, 0.9% saline, lactated Ringer’s solution). Crystalloids exert their effects primarily on the interstitial and intracellular compartments. Colloids are large-molecular-weight substances that are restricted to the plasma compartment in patients with an uncompromised intact endothelium and include plasma, dextrans, hydroxyethyl starch (hetastarch), and hemoglobin-based oxygen-carrying (HBOC) fluids. Colloids exert their primary effect on the intravascular compartment.
Some types of colloids may be used in patients with shock and in those with severe hypoalbuminemia (i.e., albumin <1.5 g/dL). A major limitation to the use of plasma as a colloid is the rapid disappearance of albumin from the vascular space. Dextran 70 is a polymer of glucose that has an average molecular weight of 70,000. Its use in humans has been associated with coagulopathies. Hetastarch has an average molecular weight of 480,000. In humans, coagulopathies also have been associated with the use of hetastarch but typically only when standard dosage recommendations have been exceeded. The main advantages of colloids are that more of the administered solution remains in the plasma compartment and there generally is thought to be less risk of edema in patients with an intact endothelium. Colloids are discussed in detail in Chapter 27.
Crystalloid solutions are equally effective in expanding the plasma compartment, but 2.5 to 3.0 times as much crystalloid solution must be given (compared with a colloid solution) because the crystalloid is distributed to other sites (e.g., interstitial compartment, intracellular compartment).26,27,39 Pulmonary capillaries normally are more permeable to protein, resulting in a higher interstitial concentration of protein and more resistance to leakage of fluid from capillaries.30 Peripheral edema is more likely to occur after crystalloid administration because muscle and subcutaneous capillaries are less permeable to protein.
Crystalloid solutions also can be classified as replacement or maintenance solutions. The composition of replacement solutions (e.g., lactated Ringer’s, Normosol-R, Plasma-Lyte 148) resembles that of ECF (Figure 14-2). Maintenance solutions (e.g., Normosol-M, Plasma-Lyte 56) contain less sodium (40 to 60 mEq/L) and more potassium (15 to 30 mEq/L) than replacement fluids. A simple maintenance solution can be formulated by mixing one part 0.9% NaCl with two parts 5% dextrose and adding 20 mEq KCl per liter of final solution. The approximate composition of such a fluid would be 51 mEq/L sodium, 20 mEq/L potassium, 71 mEq/L chloride, and 33.5 g/L dextrose. It would provide 133 kcal/L and have an osmolality of 328 mOsm/kg. An alternative maintenance solution may be made by mixing one part lactated Ringer’s solution with two parts 5% dextrose and adding 20 mEq KCl per liter of final solution. This solution has the following approximate composition: 43 mEq/L sodium, 21 mEq/L potassium, 56 mEq/L chloride, 1 mEq/L calcium, 9 mEq/L lactate, and 33.5 g/L dextrose. It would provide 133 kcal/L and have an osmolality of 317 mOsm/kg.
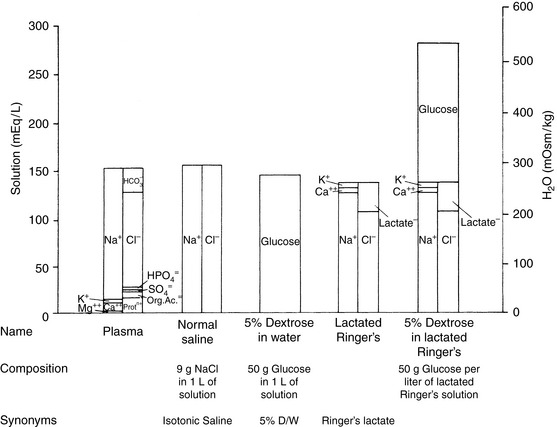
Figure 14-2 Comparison of electrolyte composition of plasma with that of commonly used crystalloid solutions.
(From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 30.)
Another commonly used crystalloid is 5% dextrose. Administering 5% dextrose is equivalent to giving water because the glucose is oxidized to CO2 and water. In fact, the main reason for giving 5% dextrose is to correct a pure water deficit. Except in very small animals, administration of 5% dextrose cannot be relied on to maintain daily caloric needs because 5% dextrose contains only 200 kcal/L. Consider a normal, active 10-kg dog. Its maintenance energy requirement (MER) is approximately 740 kcal:
To provide this number of kilocalories from 5% dextrose (200 kcal/L), almost 4 L of fluid must be administered per day. Such a volume is almost seven times more than the daily maintenance requirement for fluid in this dog. Administration of 4 L of 5% dextrose over a 24-hour period would initiate a diuresis that would impair use of the administered dextrose and cause increased urinary losses of electrolytes.
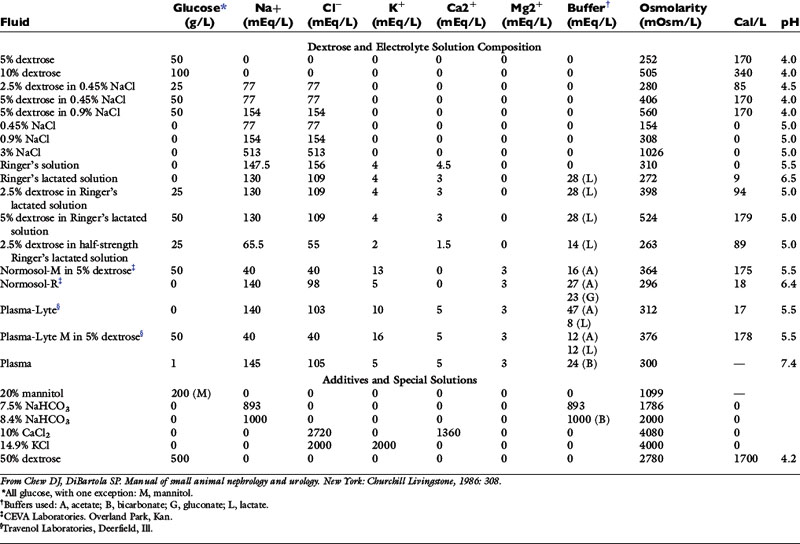
The veterinary practitioner can manage most animals requiring fluid therapy with a limited number of crystalloid and additive solutions. The most useful crystalloid solutions for routine use are a balanced replacement solution (e.g., lactated Ringer’s solution, Normosol-R, Plasma-Lyte 148), 0.9% saline, and 5% dextrose in water. The solute composition of these fluids is compared with that of ECF in Figure 14-2, and the electrolyte composition of several commercially available solutions is summarized in Table 14-6.
Supplementation of crystalloid solutions with KCl may be necessary when body fluid losses include large amounts of potassium. An empirical scale has been devised to estimate the amount of potassium to add to parenterally administered fluids (Table 14-7).17 This protocol has not been evaluated experimentally in dogs or cats but has been used successfully in clinical veterinary patients during the past 30 years. Potassium supplementation is discussed in Chapter 5.
Table 14-7 Sliding Scale for Potassium Supplementation
| Serum Potassium (mEq/L) | mEq KCl to Add to 250 mL Fluid | Maximal Fluid Infusion Rate* (mL/kg/hr) |
|---|---|---|
| <2.0 | 20 | 6 |
| 2.1-2.5 | 15 | 8 |
| 2.6-3.0 | 10 | 12 |
| 3.1-3.5 | 7 | 16 |
* So as not to exceed 0.5 mEq/kg/hr.
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 38.
Other additive solutions include 50% dextrose, calcium chloride, calcium gluconate, potassium phosphate, 8.4% sodium bicarbonate, and water-soluble B vitamins. Thiamine supplementation may be particularly important in cats because their requirement for this vitamin may be higher than that of dogs. Phosphate rarely is used as an additive but often is required in patients with diabetic ketoacidosis during insulin therapy.40 Phosphate supplementation is discussed in Chapter 7. Theoretically, sodium bicarbonate should not be added to solutions containing calcium (e.g., lactated Ringer’s solution, Plasmalyte-R) because of the risk of forming insoluble calcium carbonate crystals. Despite this concern, no adverse consequences have been observed when small amounts of sodium bicarbonate have been added to lactated Ringer’s solution.22,23
When additives are used, the clinician must keep in mind that the final osmolality of the fluid may be higher than anticipated. The final osmolality may be approximated by adding the number of milliequivalents per liter of electrolyte and millimoles per liter of nonelectrolyte solutes found in the solution. The final osmolality of the solution also may differ depending on how the solution was formulated. For example, if 500 mL of lactated Ringer’s solution is mixed with 500 mL of 5% dextrose to create a replacement solution with 2.5% dextrose, the resulting solution has an approximate osmolality of 275 mOsm/kg (virtually the same as that of lactated Ringer’s solution). Conversely, if 50 mL of 50% dextrose is added to 1 L of lactated Ringer’s solution, the resulting solution contains 2.5% dextrose but has an approximate osmolality of 391 mOsm/kg, which is substantially higher.
The choice of fluid to administer is dependent on the nature of the disease process and the composition of the fluid lost. Underlying acid-base and electrolyte disturbances should be taken into consideration when choosing the type of fluid to administer. The clinician should attempt to replace losses with a fluid that is similar in volume and electrolyte composition to that which has been lost from the body (see Table 14-3). If clinical assessment of the patient suggests a fluid-responsive type of shock, the resuscitation phase of fluid therapy should be instituted. If the patient has abnormally low oncotic pressure or an underlying disease condition for which a low-volume resuscitation strategy may prove advantageous, synthetic colloids should be considered as the primary fluid choice for resuscitation (see Chapters 23 and 27). If neither of these considerations applies, resuscitation with a balanced crystalloid solution is indicated. If there are no clinical signs of hypovolemia, the hydration deficit and maintenance needs may be combined and administered during the next 24 hours.
Persistent vomiting caused by pyloric obstruction would be expected to result in losses of hydrochloric acid, potassium, sodium, and water, potentially producing hypokalemia, hypochloremia, and metabolic alkalosis. The initial fluid of choice in this setting is 0.9% NaCl with 20 to 30 mEq KCl per liter. Except in the case of vomiting of stomach contents, lactated Ringer’s is a good first choice for fluid therapy while awaiting laboratory results. Normal saline (0.9% NaCl) is less ideal because it is not a balanced solution. It contains chloride in greater concentration than body fluids (154 mEq/L versus 110 mEq/L in dogs and 120 mEq/L in cats), and as a result of displacement of bicarbonate with chloride in ECF and initiation of natriuresis, it has a mild acidifying effect.32 Examples of fluid therapy in specific diseases are listed in Table 14-3.
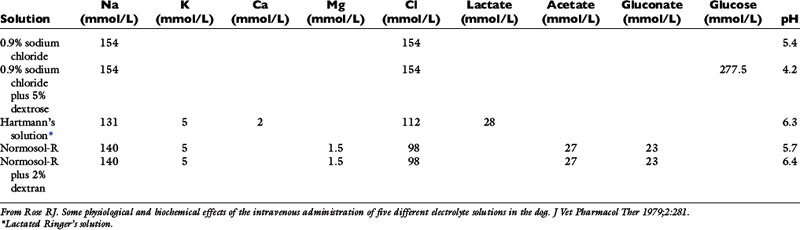
In one study, five different solutions were administered to unanesthetized dogs during a 1-hour period: 0.9% NaCl, 0.9% NaCl with 5% dextrose, lactated Ringer’s solution, Normosol-R, and Normosol-R with 2% dextran.32 The approximate composition of these fluids is presented in Table 14-8. The fluids were warmed to body temperature, and no decreases in rectal temperature were observed. Laboratory variables were measured after 1 hour of infusion. Fluids were administered at 76 mL/kg/hr except for Normosol-R with dextran, which was administered at a rate of 31.5 mL/kg/hr.
Most of the fluids increased heart rate, diastolic arterial pressure, and central venous pressure (CVP), and all of them decreased hematocrit, hemoglobin, and total protein concentrations by 21% to 25%. All solutions except for Normosol-R and Normosol-R with 2% dextran caused an increase in serum chloride concentration, and the saline solutions decreased pH and bicarbonate concentration. All solutions except Normosol-R caused a decrease in serum potassium concentration. The causes of the decreased serum potassium concentrations in these dogs presumably included dilution and increased distal tubular flow rate with enhanced urinary excretion of potassium. The presence of 5% dextrose in two of the solutions resulted in significantly lower serum potassium concentrations, suggesting movement of potassium into cells with glucose.
Serum sodium concentrations were similar despite differences in the sodium concentrations of the various fluids, demonstrating effective natriuresis in normal dogs receiving sodium-containing crystalloid solutions. Serum chloride concentration increased with administration of the saline solutions containing 154 mEq/L chloride, and mild metabolic acidosis developed. Serum chloride concentration also increased slightly with administration of lactated Ringer’s solution (112 mEq/L chloride), but there was no change in acid-base balance. The increased serum chloride concentration and alterations in acid-base balance could have resulted from decreased reabsorption of bicarbonate with sodium in the kidney during natriuresis and decreased strong ion difference.37,38 Expansion acidosis is an unlikely explanation because all fluids administered presumably expanded the ECF volume.
Anions such as acetate, gluconate, and lactate are added to crystalloid solutions as a source of base because their oxidative metabolism in the body yields bicarbonate. The alkalinizing effect of the metabolism of these anions and that of citrate is as follows:
Most lactate is produced in muscle and gut and metabolized to either glucose (via cytosolic gluconeogenesis) or CO2 and water (via mitochondrial oxidation) in the liver. Normally, gluconeogenesis predominates. Acetate is metabolized primarily in muscle. The alkalinizing effect of these anions is delayed because of the requirement for metabolism. In one study, equivalent doses of acetate, bicarbonate, and lactate had similar alkalinizing effects in anesthetized dogs 45 minutes after infusion.21 The effect of bicarbonate occurred earliest because metabolism was not necessary.
Lactate originally was introduced for the treatment of acidosis because of technical difficulties in preparation of bicarbonate solutions suitable for intravenous use.5,36 These technical difficulties have been overcome, but crystalloid solutions containing lactate as a source of base (e.g., lactated Ringer’s solution) still are widely used for fluid therapy in clinical practice. Most patients treated with lactate-containing replacement solutions respond well, probably as a result of ECF volume expansion and improved tissue perfusion.
Whether it is converted to glucose or oxidized to CO2 and water, the metabolism of lactate consumes hydrogen ions and has an alkalinizing effect:
There has been some concern that lactate in lactated Ringer’s solution may be harmful to patients with poor tissue perfusion and severe metabolic acidosis (pH, <7.1 to 7.2). Administration of lactate as a salt cannot contribute directly to metabolic acidosis. Rather, the ability of the liver to metabolize lactate and the potentially detrimental effect of lactate on myocardial contractility have been debated. During severe hypoxia, increased lactate production in gut and muscle and decreased hepatic extraction of lactate led to progressive lactic acidosis. In moderate metabolic acidosis, administration of lactated Ringer’s solution probably is beneficial because any tendency toward lactate accumulation is likely to be offset by improved hepatic perfusion and oxygen delivery as a result of ECF volume expansion.
Newer commercially available balanced crystalloid solutions contain approximately twice the amount of bicarbonate precursors when compared with lactated Ringer’s solution. As a result, these solutions generally are thought to be more efficient than lactated Ringer’s solution in treatment of metabolic acidosis, provided that metabolic conversion of the precursors to bicarbonate occurs quickly. There is some concern that such fluids may contribute to the development of metabolic alkalosis, but this does not occur in animals with relatively normal renal function because the kidneys can efficiently excrete the excess bicarbonate.
Crystalloid solutions with preservatives must be avoided in cats. Benzoic acid derivatives (e.g., benzyl alcohol, methylparaben, propylparaben, ethylparaben) have been added to some solutions for their antimicrobial effect. Clinical signs in cats receiving fluids with such preservatives have included behavioral changes, hypersalivation, ataxia, muscle fasciculations, seizures, dilated nonresponsive pupils, coma, and death.3,9,33 Young cats may be at increased risk for these complications.
By what route should fluids be given?
The route of fluid therapy depends on the nature of the clinical disorder, its severity, and its duration.
Intravenous
The intravenous route is preferred when the patient is very ill, when there has been severe fluid loss, or when the fluid loss has been acute. This route also is used during anesthesia to maintain renal perfusion and vascular access for emergencies. The intravenous route provides rapid dispersion of water and electrolytes and allows precise dosage. A large volume can be given rapidly, and hypertonic fluids can be given safely via a large vein. This route requires vascular access and close monitoring during infusion to avoid complications such as overhydration, infection, thrombosis, phlebitis, embolism, and impaired fluid delivery (e.g., obstruction of the catheter by a change in the patient’s limb position).
The veins available for vascular access include the jugular, cephalic, lateral saphenous, and femoral veins. There are advantages and disadvantages of each, but the jugular vein is most useful because it allows delivery of large volumes, administration of hypertonic or potentially irritating solutions, measurement of CVP, and repeated venous blood sampling. The cephalic vein also is commonly used, but fluid delivery can be hindered by flexion of the elbow, and extremely hypertonic or irritating solutions should not be used. Intravenous catheter function and the catheter-skin interface should be monitored routinely to detect complications. Catheters that remain clean and free of complications need not be replaced at some routine interval. The types of catheters used and their placement are discussed in Chapter 15.
Subcutaneous
The subcutaneous route is convenient for maintenance fluid therapy in small dogs and cats. The subcutaneous space in dogs and cats can accommodate relatively large volumes of fluid, and potassium can be used in concentrations up to 30 to 35 mEq/L without irritation.13 Approximately 10 mL/kg or 50 to 200 mL may be administered per site.34 Fluid is administered under the skin along the back from the area of the scapulae to the lumbar region. Volume overload is unlikely to occur when fluids are administered subcutaneously in patients with no underlying cardiac insufficiency. Furthermore, some owners can use subcutaneous administration to give fluids at home to animals with chronic disease problems (e.g., chronic renal failure).
The subcutaneous route is not adequate for patients with acute and severe losses (e.g., shock) and is not recommended for extremely dehydrated or hypothermic animals because peripheral vasoconstriction may reduce absorption and dispersion of the administered fluid in these settings. The volume that may be given is limited by skin elasticity, and this route is not useful in larger animals requiring large volumes of fluids. Irritating or hypertonic solutions must not be used subcutaneously; only isotonic fluids are recommended. Isotonic fluids containing bicarbonate precursors other than lactate also are not recommended for subcutaneous administration. Although not harmful, they appear to cause mild local discomfort and are not well tolerated by veterinary patients. The subcutaneous administration of 5% dextrose in water should be avoided because equilibration of ECF with a pool of electrolyte-free solution may lead to temporary aggravation of electrolyte imbalance.
Oral
The oral route is most physiologic, and fluids with a wide variety of compositions may be given. Oral fluid therapy is useful for administering hypertonic fluids with high caloric density. Fluid can be administered rapidly with minimal adverse effects, and caloric needs can be met. However, this route should not be used in the presence of gastrointestinal dysfunction (e.g., vomiting, diarrhea). The oral route also is inadequate in animals that have had acute or extensive fluid losses because dispersion and use of the administered fluid and electrolytes are not sufficiently rapid. In anorexic animals without vomiting or diarrhea, fluid can be administered orally using a number of different techniques (e.g., nasogastric tube, esophagostomy tube, gastrostomy tube).
Intraperitoneal
Intraperitoneal administration of fluid allows moderately rapid absorption of large volumes. Only isotonic fluids can be used because administration of hypertonic fluids results in further contraction of the extracellular compartment as water enters the peritoneal space by osmosis. Peritonitis also is a potential complication of this route. The intraperitoneal route is not used commonly except to perform peritoneal dialysis as described in Chapter 28.
Intraosseous (intramedullary)
The intraosseous, or intramedullary, route is useful in very young or small animals in which venous access is difficult. The procedure has been available for many years6 and has received renewed attention.14,16,29 This route provides rapid vascular access via bone marrow sinusoids and medullary venous channels and allows rapid dispersion of fluid. The bone marrow does not collapse when the patient is hypovolemic, and access to the marrow is simple. For some clinicians, this technique may be accomplished more rapidly than performing a venous cutdown. Sites that can be used for intraosseous administration of fluid include the tibial tuberosity, trochanteric fossa of the femur, wing of the ilium, and greater tubercle of the humerus. The periosteum should be anesthetized by infiltration with 1% lidocaine solution to avoid pain during needle placement. The potential risks include osteomyelitis and pain on administration of fluid. However, pain was not observed clinically in two studies.16,29
How rapidly may fluids be given?
Poiseuille’s law governs the flow of fluids through a catheter:
where P1 − P2 represents the pressure differential on the fluid, η is the viscosity of the fluid, r is the radius of the catheter, and L is the length of the catheter. Thus the diameter of the catheter is of primary importance in establishing a rapid rate of flow. The choice of catheter length sometimes is affected by factors other than flow rate (e.g., use of jugular catheters to monitor CVP). In a study of gravity flow of lactated Ringer’s solution, in vivo flow rates averaged 7% less than in vitro flow rates, presumably because of tissue pressure.15 Fluid flow rate increased by 50% when the pressure differential was increased by raising the fluid bag from 0.91 to 1.75 m. Flow rate increased linearly with increasing catheter radius rather than geometrically as predicted by Poiseuille’s law.
The rate of fluid administration is dictated by the magnitude and rapidity of the fluid loss. The patient with fluid-responsive shock syndrome requires aggressive fluid administration. Fluid administration rates may vary, depending on the type of fluid or combination of types that has been chosen. One approach is to calculate a “shock fluid dose” and administer it as rapidly as possible in divided aliquots until a stable and sustainable cardiovascular endpoint has been achieved (see Chapter 23). Clinical evaluation of the patient should occur after administration of each aliquot using a “titrate to effect” approach. The shock dosage of synthetic colloids is 20 mL/kg for dogs and 10 to 15 mL/kg for cats. The shock dosage of isotonic crystalloids is 80 to 90 mL/kg for dogs and 40 to 60 mL/kg for cats. In experimental studies, crystalloid fluids administered at 90 mL/kg/hr did not cause pulmonary edema in normal dogs and cats.4,8
Anesthetized cats receiving lactated Ringer’s solution at a rate of 225 mL/kg for 1 hour developed serous nasal discharge, chemosis, ascites, diarrhea, and fluid exudation from catheter sites. At necropsy, these cats had ascites, pancreatic edema, and accumulation of free fluid in the trachea. Body temperature decreased and CVP and left atrial pressure increased in cats receiving 225 mL/kg/hr, whereas hematocrit, total protein concentration, and colloidal osmotic pressure decreased in cats receiving both 90 and 225 mL/kg/hr.4
Lactated Ringer’s solution was administered to unanesthetized, dehydrated dogs at rates of 90, 225, and 360 mL/kg for 1 hour.8 At rates of 90 and 225 mL/kg/hr, some dogs had serous nasal discharge, mild coughing, and slight chemosis. At 360 mL/kg/hr, marked serous nasal discharge, restlessness, coughing, dyspnea, pulmonary crackles, ascites, polyuria, chemosis, protrusion of eyes, and diarrhea were observed. These signs resolved when fluid administration was discontinued. Hematocrit, TPP, and serum potassium concentration decreased during fluid administration. In this study, body temperature decreased despite the fact that fluids were warmed to 37° C. Serum sodium concentration remained unchanged, but pulse rate, respiratory rate, and systemic arterial pressure increased slightly. Pulmonary capillary wedge pressure (PWP) and CVP increased, and these measurements correlated well with one another. It was concluded that lactated Ringer’s solution at 90 mL/kg/hr was tolerated safely. CVP should be monitored if fluids must be administered at rates in excess of 90 mL/kg/hr.
Contemporary losses must also be considered when adjusting the rate of fluid administration. Severe ongoing losses (e.g., vomiting and diarrhea in a patient with acute gastroenteritis) may necessitate rapid administration to keep pace with contemporary fluid loss. When fluids are given rapidly, it is necessary to monitor cardiovascular and renal function.
It usually is not necessary or desirable to replace the hydration deficit rapidly in chronic disease states. Instead, the hydration deficit may be calculated, the daily maintenance requirement of fluid added to this amount, and the total volume administered over 24 hours.35 Ongoing or contemporary losses also must be considered and taken into consideration when estimating the patient’s fluid requirements for a 24-hour period. This approach allows adequate time for equilibration of fluid and electrolytes with the intracellular compartment and avoids potential complications (e.g., edema or effusion related to increased hydrostatic pressure, diuresis, and loss of administered electrolytes in urine). It is the method most commonly used for medical patients at the Ohio State University Veterinary Teaching Hospital.
Whenever possible, intravascular volume deficits should be replaced before anesthesia and surgery. Ideally, such patients also should be rehydrated depending on the urgency of their underlying condition. During induction and maintenance of anesthesia, prevention of hypovolemia, and maintenance of renal perfusion are essential. Induction of diuresis in this setting may be an important factor in prevention of intraoperative acute renal failure. A basal fluid administration rate of 5 to 10 mL/kg/hr is recommended during anesthesia and surgery. During major surgery (e.g., exploratory laparotomy, thoracotomy), fluid administration at twice this basal rate is recommended. Fluid therapy during anesthesia and surgery is discussed in more detail in Chapter 17.
Most administration sets designed for adult human patients deliver 10 to 20 drops/mL, whereas pediatric administration sets deliver 60 drops/mL.35 This information is used to calculate the drip rate:
or
or
Fluid orders should be written so that the volume to be administered is recorded as mL/day, mL/hr, and drops/min. This allows personnel to detect errors in calculations. The clinician should not assume that the animal has received the volume of fluid ordered, and the volume actually received should be noted in the record by nursing personnel. All additives should be clearly listed on the bottle, and adhesive labels for this purpose are available (Figure 14-3). Infusion pumps are available for clinical use (e.g., Heska, Baxter) and provide a highly accurate record of the volume infused (Figure 14-4). These pumps also have alarm systems that can alert personnel when flow is obstructed. The availability of affordable electronic fluid pumps has resulted in widespread incorporation of such equipment into veterinary practice. Although use of infusion pumps makes fluid administration safer and more accurate, the equipment must be used appropriately, maintained in good working order, and tested regularly for accuracy. Mistakes in fluid administration still can occur as a consequence of human error or equipment failure. For practices that do not routinely use electronic fluid pumps, several management practices may assist in accurately and safely delivering fluid therapy. A strip of adhesive tape can be attached to the bottle and marked appropriately to provide a quick visual estimate of the volume of fluid received (Figure 14-5). In the Buretrol system (Baxter, Deerfield, Ill.), a reservoir allows a predetermined volume of fluid to be delivered over a given period (Figure 14-6). This approach prevents infusion of excessive volumes of fluid to small animals. The technical aspects of fluid therapy are discussed in detail in Chapter 15.
Figure 14-3 Adhesive label for fluid additives.
(From Chew DJ. Parenteral fluid therapy. In: Sherding RG, editor. The cat: diseases and clinical management. New York: Churchill Livingstone, 1989: 50.)

Figure 14-4 A and B, Fluid infusion pumps. A, Baxter Flo-Gard 6200 Volumetric Infusion Pump (Baxter Health Care, Deerfield Ill.). B, Medex Medfusion 2010 Syringe Infusion Pump (Medex, Carlsbad Calif.).
How much fluid should be given?
The purpose of fluid therapy is to increase tissue perfusion, repair fluid deficits, supply daily fluid needs, and replace ongoing losses. It has been emphasized: “the aim of therapy is not to administer fluids but to induce positive fluid balance.”31
Components of fluid therapy
The volume requirements of patients with fluid-responsive shock syndromes can vary widely. Ultimately, the goal of reestablishing widespread effective tissue perfusion should dictate the volume of fluid administered. In general, the same cardiovascular parameters used to characterize the patient’s shock syndrome should return to normal or to the extent they are able to do so given the limitations of the patient’s underlying disease condition. For example, a severely dehydrated dog with tachycardia, pale mucous membranes, prolonged capillary refill time, and hypotension should receive a volume of fluid sufficient to return these cardiovascular parameters to normal, and these parameters should not deviate from normal when the rate of fluid administration is decreased and rehydration of the patient begun. In patients not experiencing ongoing loss of fluids from the intravascular compartment or other more complex cardiovascular derangements, response to fluid resuscitation should be rapid and complete.
The initial assessment of hydration determines the volume of fluid needed to replace the hydration deficit (replacement requirement).12,20 The hydration deficit is calculated as the percentage dehydration (estimated by physical examination) times the patient’s body weight in kilograms. The resultant value is the fluid deficit in liters. During the rehydration phase of therapy, this volume is administered for 24 hours in conjunction with maintenance fluid requirements and replacement of ongoing or contemporary losses that are occurring.
Coincident with or after replacement of the animal’s hydration deficit, the maintenance fluid requirement must be administered.12,20 The maintenance fluid requirement is the volume needed per day to keep the animal in balance (i.e., no net change in body water). Daily fluid requirements (milliliters per kilogram per day) parallel energy requirements (kilocalories per kilogram per day).20,22,23
The basal energy requirement (BER) is that of a resting animal in a thermoneutral environment 12 to 18 hours after eating.24 In dogs, BER is not a linear function of body weight but rather is related to body surface area by the following equation1:
where W is body weight in kilograms. This relationship is plotted in Figure 14-7 so that BER may be determined from body weight.
The maintenance energy requirement (MER) is that of a moderately active adult animal in a nonthermoneutral environment. The MER in sedentary animals is approximately 1.5 to 2.0 BER.
In domestic cats, the relationship of basal heat production to body weight is almost linear because of the small size and relatively narrow normal range of body weight in this species.25 Based on available data, BER in cats may be estimated as 50 to 60 kcal/kg/day. However, the question remains whether daily energy requirements approximate daily fluid requirements. Daily fluid requirements of anorexic dogs and cats in a hospital environment and the relationship of these fluid requirements to the daily urinary solute load are areas deserving future clinical study.
At the Ohio State University Veterinary Teaching Hospital, the maintenance fluid requirement for dogs and cats is determined from reference charts that use the above formulas to calculate accurate daily fluid requirements based on caloric needs. Although estimates of 40 to 60 mL/kg/day frequently are used to calculate maintenance fluid requirements, it is important to recognize that such estimates are only accurate for some veterinary patients. Cats, very small dogs, and very large dogs are not well served by the use of such estimates, and these patients likely will benefit from more accurate assessment of their fluid requirements. Approximately two thirds of the maintenance requirement represents sensible (i.e., easy to measure) losses of fluid (urine output), and one third represents insensible (i.e., difficult to measure) losses (primarily fecal and respiratory water loss). Thus daily maintenance for a 10-kg dog may be 600 mL, with 400 mL representing sensible loss and 200 mL representing insensible loss.
Some clinicians multiply maintenance fluid requirements by some factor between 1 and 3 to estimate a patient’s 24-hour fluid needs. Assuming 60 mL/kg/day to represent the maintenance rate of fluid administration, the information in Table 14-9 can be used to quickly determine the implied hydration deficit and actual rate of fluid administration using this approach.
Table 14-9 Maintenance and Dehydration Fluid Volume Requirements*
| Maintenance (M) + Dehydration (%) | mL/kg/day | Factor ×Maintenance |
|---|---|---|
| M + 1 | 70 | 1.17 |
| M + 2 | 80 | 1.33 |
| M + 3 | 90 | 1.50 |
| M + 4 | 100 | 1.67 |
| M + 5 | 110 | 1.83 |
| M + 6 | 120 | 2.00 |
| M + 7 | 130 | 2.17 |
| M + 8 | 140 | 2.33 |
| M + 9 | 150 | 2.50 |
| M + 10 | 160 | 2.67 |
* Maintenance defined as 60 mL/kg/day.
From Chew DJ, Kohn CW, DiBartola SP. Disorders of fluid balance and fluid therapy. In: Fenner WR, editor. Quick reference to veterinary medicine, 2nd ed. Philadelphia: JB Lippincott, 1991: 570.
In addition to the hydration deficit (replacement requirement) and maintenance requirement, contemporary (ongoing) losses must be considered. These are not always easily determined or quantitated in small animals but can be very important in fluid therapy. An attempt should be made to estimate ongoing losses, which may include losses related to vomiting, diarrhea, polyuria, large wounds or burns, drains, peritoneal or pleural losses, panting, fever, and blood loss. During surgical procedures, careful attention should be given to the amount of blood lost, drying of exposed tissues, and effusions removed by suction. Blood lost at surgery should be estimated, and 3 mL of crystalloid solution should be administered for each milliliter of blood lost. Each 4 × 4-inch gauze sponge, when saturated with blood, represents a blood loss of 15 mL.28 Contemporary losses must be estimated and carefully replaced along with the maintenance volume of fluid. Box 14-1 summarizes the components of fluid therapy and their calculation.
Box 14-1 Calculation of Replacement Requirement (Hydration Deficit)
1. Hydration deficit (replacement requirement)
2. Maintenance requirement (40-60 mL/kg/day)
3. Contemporary (ongoing) losses (e.g., vomiting, diarrhea, polyuria)
From Muir WW, DiBartola SP. Fluid therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983: 35.
* 500 mL = 1 lb.
Failure to achieve rehydration
Repeated assessment of the patient by observation of clinical signs and determinations of body weight, urine output, PCV, TPP, and USG is mandatory in making appropriate readjustments of fluid therapy. Reasons for failure to achieve satisfactory rehydration include calculation errors, underestimation of the initial hydration deficit, contemporary losses larger than first appreciated (e.g., vomiting, diarrhea), infusion of fluid at an excessively rapid rate with consequent diuresis and obligatory urinary loss of fluid and electrolytes, administered fluid not reaching the extracellular compartment (e.g., technical problems with the intravenous catheter, third-space loss), sensible losses larger than appreciated (e.g., polyuria), and insensible losses larger than appreciated (e.g., panting, fever). Failure to achieve successful hydration is an indication to increase the volume of fluid administered if the heart and kidneys are functioning adequately. As a rule, the daily fluid volume may be increased by an amount equivalent to 5% of body weight if the initial infusion fails to restore hydration. Finally, the possibility must be considered that the animal was not dehydrated at presentation (e.g., abnormal skin turgor related to old age or emaciation). This should be considered if the animal does not gain weight despite several days of fluid therapy.
Monitoring fluid therapy
It is important to remember that the hydration deficit as estimated by history and physical examination is only an estimate, and fluid therapy must be tailored to physical (e.g., body weight) and laboratory (e.g., PCV, TPP) findings during the first few days of fluid therapy.
Physical and laboratory findings
A complete physical examination, including evaluation of skin turgor and careful thoracic auscultation, should be performed once or twice daily for animals receiving fluid therapy. Hematocrit, TPP, and body weight should be monitored. Serial body weight has been considered one of the most important variables to follow, and animals receiving continuous fluid therapy should be weighed once or twice daily using the same scale. A gain or loss of 1 kg can be considered an excess or deficit of 1 L of fluid because lean body mass is not quickly gained or lost. A dehydrated patient should gain weight as rehydration is achieved, and afterward weight should remain relatively constant. However, weight may increase without restoration of effective circulating volume in patients with severe third-space losses. Despite these traditional principles, one study of dogs and cats hospitalized in an intensive care unit showed that clinical estimates of dehydration did not reliably predict changes in body weight after 24 to 48 hours of fluid therapy.18
Urine output
The clinician should observe the animal’s urine output carefully after fluid therapy has begun. Oliguria should be strongly suspected in patients with acute renal failure, especially those with possible ethylene glycol ingestion.
Urine output should be monitored when fluids are administered intravenously at a rapid rate and renal function is in question. Normal urine output is 1 to 2 mL/kg/hr. As the patient becomes rehydrated, physiologic oliguria should resolve, and urine output should increase while USG decreases. If oliguria that was present at admission persists after the hydration deficit has been replaced, it is prudent to divide daily fluid therapy into six 4-hour intervals if the status of renal function is uncertain. The calculated insensible volume plus a volume equal to the urine output of the previous 4 hours is administered during each 4-hour period (known as measuring “ins and outs”). The risk of overhydration is minimized, and fluid therapy keeps pace with urine output even if oliguria is present when this technique is used. If oliguria persists, an increase in the daily fluid volume by an amount equal to 5% of body weight is justified on the assumption that the initial clinical estimate of dehydration was inaccurate. If oliguria does not respond to mild volume expansion, administration of increased volumes of fluid may result in pulmonary edema.
Central venous pressure
Measurement of CVP with a jugular catheter positioned at the level of the right atrium allows the cardiovascular response to fluid administration to be monitored. Normal CVP is 0 to 3 cm H2O. CVP increases from below normal into the normal range when fluids are administered to a dehydrated animal. A progressive increase in CVP above normal during fluid therapy is an indication to decrease the rate of fluid administration or to stop fluid therapy temporarily. A sudden and sustained increase in CVP may indicate failure of the cardiovascular system to handle the fluid load effectively and could result in pulmonary edema caused by left-sided heart failure. In addition to the volume of fluid administered, other factors that may affect CVP include heart rate, vascular capacity, and cardiac contractility. A reduction in any of these three parameters could cause an increase in CVP.
The Frank-Starling curve (Figure 14-8) relates stroke volume (SV) to left ventricular end-diastolic pressure (LVEDP). If there is no obstruction across the mitral valve, left atrial pressure (LAP) should equal LVEDP, a measure of cardiac function. PWP measured with a Swan-Ganz catheter is an estimate of LAP. Generally, there is a direct relationship between right atrial pressure (RAP) and LAP. Thus measuring CVP gives an indirect indication of LVEDP. Without cardiac dysfunction, CVP correlates well with PWP and LAP. In one study, pulmonary artery diastolic pressure and PWP increased before CVP in dogs receiving an infusion of lactated Ringer’s solution.8
Complications of fluid therapy
Signs of overhydration occur when fluid is administered too rapidly. These may include serous nasal discharge, chemosis, restlessness, shivering, tachycardia, cough, tachypnea, dyspnea, pulmonary crackles and edema, ascites, polyuria, exophthalmos, diarrhea, and vomiting.8 Expected laboratory abnormalities include a reduction in PCV and TPP and an increase in body weight.
When the intravenous route is chosen for fluid therapy, the clinician has made a commitment to careful, aseptic catheter placement and proper maintenance (see Chapter 15). The animal should be checked daily for cleanliness of the catheter site, local pain or swelling, fever, or cardiac murmurs. If any of these signs are observed, the catheter should be removed, its tip cultured, the patient started on appropriate antibiotic therapy, and a new catheter placed in another vein. Complications related to catheter placement include bacterial endocarditis, thrombophlebitis, thromboembolism, and migration of a catheter fragment. When not in use, the catheter should be irrigated with a small volume (<1 mL) of a solution containing between 1 and 5 U of heparin per milliliter of 0.9% NaCl (“heparinized saline”). The complications of fluid therapy are discussed further in Chapter 16.
When should fluid therapy be discontinued?
Ideally, fluid therapy is discontinued when hydration is restored and when the animal can maintain fluid balance on its own by oral intake of food and water. As the animal recovers, fluid therapy usually is tapered by decreasing the volume of fluid administered by 25% to 50% per day. If an animal remains anorexic for more than 3 to 5 days, enteral or parenteral nutritional therapy must be considered. Parenteral nutrition is discussed in Chapter 25.
1 Abrams J.T. The nutrition of the dog. In: Rechcigl M., editor. CRC handbook series in nutrition and food. Section G: diets, culture media, and food supplements. Boca Raton, Fla: CRC Press; 1977:1.
2 Anderson R.S. Water balance in the dog and cat. J Small Anim Pract. 1982;23:588.
3 Bedford P.G.C., Clark E.G.C. Experimental benzoic acid poisoning in the cat. Vet Rec. 1972;90:53.
4 Bjorling D.E., Rawlings C.A. Relationship of intravenous administration of Ringer’s lactate solution to pulmonary edema in halothane-anesthetized cats. Am J Vet Res. 1983;44:1000.
5 Cohen R.D., Simpson R. Lactate metabolism. Anesthesiology. 1975;43:661.
6 Corley E.A. Intramedullary transfusion in small animals. J Am Vet Med Assoc. 1963;142:1005.
7 Cornelius L.M. Fluid therapy in small animal practice. J Am Vet Med Assoc. 1980;176:110.
8 Cornelius L.M., Finco D.R., Culver D.H. Physiologic effects of rapid infusion of Ringer’s lactate solution into dogs. Am J Vet Res. 1978;39:1185.
9 Cullison R.F., Menard P.D., Buck W.B. Toxicosis in cats from the use of benzyl alcohol in lactated Ringer’s solution. J Am Vet Med Assoc. 1983;182:61.
10 DiBartola S.P. Disorders of fluid, acid-base, and electrolyte balance. In: Sherding R.G., editor. Medical emergencies (contemporary issues in small animal practice). New York: Churchill Livingstone; 1985:137.
11 Finco D.R. Fluid therapy-detecting deviations from normal. J Am Anim Hosp Assoc. 1972;8:155.
12 Finco D.R. A scheme for fluid therapy in the dog and cat. J Am Anim Hosp Assoc. 1972;8:178.
13 Finco D.R. Fluid therapy. In: Kirk R.W., editor. Current veterinary therapy VI. Philadelphia: WB Saunders; 1977:8.
14 Fiser D.H. Intraosseous infusion. N Engl J Med. 1989;322:1579.
15 Fulton R.B., Hauptman J.G. In vitro and in vivo rates of fluid flow through catheters in peripheral veins of dogs. J Am Vet Med Assoc. 1991;198:1622.
16 Garvey M.S. Fluid and electrolyte balance in critical patients. Vet Clin North Am Small Anim Pract. 1989;19:1021.
17 Greene R.W., Scott R.C. Lower urinary tract disease. In: Ettinger S.J., editor. Textbook of veterinary internal medicine. Philadelphia: WB Saunders; 1975:1572.
18 Hansen B., DeFrancesco T. Relationship between hydration estimate and body weight change after fluid therapy in critically ill dogs and cats. J Vet Emerg Crit Care. 2002;12:235.
19 Hardy R.M., Osborne C.A. Water deprivation test in the dog: maximal normal values. J Am Vet Med Assoc. 1979;174:479.
20 Harrison J.B., Sussman H.H., Pickering D.E. Fluid and electrolyte therapy in small animals. J Am Vet Med Assoc. 1960;137:637.
21 Hartsfield S.M., Thurmon J.C., Corbin J.E., et al. Effects of sodium acetate, bicarbonate and lactate on acid-base status in anesthetized dogs. J Vet Pharmacol Ther. 1981;4:51.
22 Haskins S.C. Fluid and electrolyte therapy. Compend Contin Educ Pract Vet. 1984;6:244.
23 Haskins S.C. A simple fluid therapy planning guide. Semin Vet Med Surg. 1988;3:227.
24 Kleiber M. The fire of life. Huntington, NY: Robert E. Krieger Publishing Co; 1975.
25 McDonald M.L., Rogers Q.R., Morris J.G. Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr. 1984;4:521.
26 Monafo W. Expensive salt water. Surgery. 1981;89:525.
27 Moss G.S., Lowe R.J., Jilek J., et al. Colloid or crystalloid in the resuscitation of hemorrhagic shock: a controlled clinical trial. Surgery. 1981;89:434.
28 Muir W.W., DiBartola S.P. Fluid therapy. In: Kirk R.W., editor. Current veterinary therapy VIII. Philadelphia: WB Saunders; 1983:28.
29 Otto C.M., McCal-Kauffman G., Crowe D.T. Intraosseous infusion of fluids and therapeutics. Compend Contin Educ Pract Vet. 1989;11:421.
30 Rose B.D. Clinical physiology of acid base and electrolyte disorders. New York: McGraw-Hill. 1994:455.
31 Rose B.D. Clinical physiology of acid base and electrolyte disorders. New York: McGraw-Hill. 1994:409.
32 Rose R.J. Some physiological and biochemical effects of the intravenous administration of five different electrolyte solutions in the dog. J Vet Pharmacol Ther. 1979;2:279.
33 Ryan C.P. Toxicity associated with lactated Ringer’s solution containing preservatives. J Am Vet Med Assoc. 1982;12:7.
34 Schaer M. General principles of fluid therapy in small animal medicine. Vet Clin North Am Small Anim Pract. 1989;19:203.
35 Schall W.D. General principles of fluid therapy. Vet Clin North Am. 1982;12:453.
36 Schwartz W.B., Waters W.C. Lactate versus bicarbonate. Am J Med. 1962;32:831.
37 Stewart P.A. How to understand acid-base. New York: Elsevier; 1981.
38 Stewart P.A. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444.
39 Virgilio R.W., Rice C.L., Smith D.E., et al. Crystalloid versus colloid resuscitation: is one better? Surgery. 1979;85:129.
40 Willard M.D., Zerbe C.A., Schall W.D., et al. Severe hypophosphatemia associated with diabetes mellitus in six dogs and one cat. J Am Vet Med Assoc. 1987;190:1007.