chapter 65 Neuromuscular Dysfunction of the Lower Urinary Tract
Objectives
This chapter begins with a summary of the abnormalities of the micturition cycle produced by different types of neuromuscular disease, injury, or dysfunction. The source material for the central and peripheral factors involved in the physiology and pharmacology of lower urinary tract function (and dysfunction) are thoroughly discussed in Chapter 60. Most chronic voiding dysfunctions secondary to neurologic disease or injury are logical, meaning that they can be inferred from a knowledge of the normal physiology and pharmacology and the type(s) and location(s) of the pathologic process(es). Certain secondary factors that can modify the type of voiding dysfunction seen and that, once established, can cause persistence of a filling/storage or voiding/emptying abnormality, even after the initial precipitating factor or factors have disappeared or been corrected, are considered. The specific types of voiding dysfunction that occur secondary to the most common categories of neuromuscular disease, injury, or dysfunction are then described in detail. Ideally, in any such discussion, the expected states of the following parameters should be described (see Chapter 61 for specific definitions of terms):
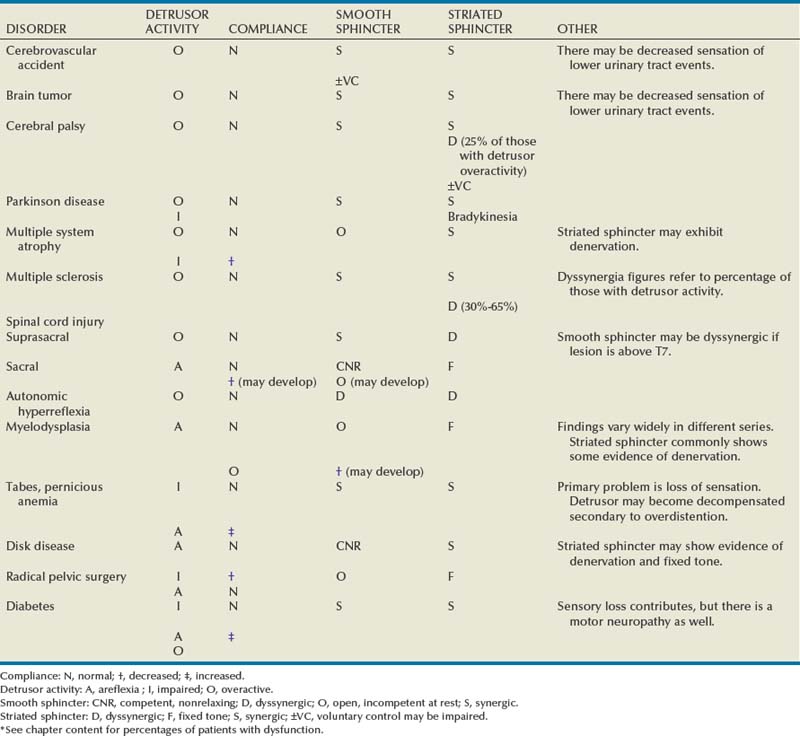
In Table 65–1 an attempt has been made to summarize many of these dysfunctions on the basis of the most common type of abnormal pattern that results from a given disease or injury, insofar as the parameters just listed are concerned. This abbreviated classification is not meant to be all inclusive but simply to indicate that, for the most part, an individual with a specific neurologic abnormality, and voiding dysfunction because of it, will, in general, have the type of dysfunction shown.
Table 65–1 Most Common Patterns of Voiding Dysfunction Seen with Various Types of Neurologic Disease or Injury*
The chapter concludes with a general consideration of the principles that should guide the selection of therapy(ies) for the dysfunctions considered. The individual therapies, and potential consequences thereof, are discussed in great detail in other chapters. The types and management of voiding dysfunction in the pediatric age group are specifically covered in Chapters 127 and 128.
As an apology to others in the field whose works have not been specifically cited or not cited as frequently as they could have been, please note that citations have generally not been chosen, except where noted, because of initial publication or original thinking on a particular subject but primarily because of their review or informational content.
General Patterns of Neuropathic Lower Urinary Tract Dysfunction
Discrete neurologic lesions generally affect the filling/storage and emptying/voiding phases of lower urinary tract function in a relatively consistent fashion. This fashion is dependent on (1) the area(s) of the nervous system affected; (2) the physiologic function(s) and the contents and location of the area(s) affected; and (3) whether the lesion or process is destructive, inflammatory, or irritative. The acute dysfunction produced may differ, for a variety of reasons, from the chronic one.
Key Point: Lesions above the Brainstem
Key Point: Complete Spinal Cord Lesions from Spinal Cord Level T6 to S2
Key Point: Trauma or Disease below Spinal Cord Level S2
Key Point: Interruption of the Peripheral Reflex Arc
Plasticity
When used in the context of the nervous system and the structures it innervates, plasticity refers to the inherent capacity to undergo structural and functional modification. These induced changes can be reflected on a number of levels: (1) structural, (2) metabolic, and (3) neurologic. In addition, the neurologic changes can then be reflected on a number of levels: (1) morphologic, (2) neurochemical, (3) electrical, and (4) organizational. Each of these changes can be studied at a variety of different levels, from investigating the end product (e.g., the clinical manifestations) to the initial molecular correlates and the factors that induce or affect them. The chronic clinical manifestations that we associate with a particular voiding dysfunction may, in fact, be the ultimate results of the phenomena that fall under the rubric “plasticity.” To a certain point, the changes may be reversible. After a certain point the changes may not be reversible, and thus plasticity may account for the persistence of clinical symptoms after the initial stimulus for dysfunction has been removed or corrected. Perhaps the most obvious changes that occur as a result of plasticity are (1) chronic changes in neural organization of the micturition reflex that occur after complete spinal cord transaction above the level of S2 and (2) the changes in peripheral neural organization that occur after damage to or transection of the peripheral parasympathetic innervation of the lower urinary tract. Specific details are contained in the relevant sections of this chapter.
What may be less obvious as a phenomenon related to plasticity are many of the changes that occur subsequent to bladder outlet obstruction. The most obvious changes that occur are those related to muscle and collagen content. However, these are themselves initiated by molecular events that ultimately cause increased contractile protein synthesis and hypertrophic bladder tissue growth (Levin et al, 1995). The initial stimulus might be stretched from overdistention (Cheng et al, 1999) or ischemia, likewise from distention (Chen et al, 1996). Compensation of the bladder smooth muscle cells to initially overcome the increased demand associated with obstruction is associated with alterations in the expression and function of many proteins involved in excitation/contraction coupling and active force generation of bladder smooth muscle (Chacko et al, 1999). Although urodynamic studies reflect obstruction, satisfactory emptying is generally preserved. Changes in the composition of the extracellular matrix occur as well, presumably also caused by an initial stretch stimulus. The ratio of type 3 collagen to type 1 collagen increases, and the localization of type 3 collagen changes as well (within and around some muscle bundle) (Macarak and Howard, 1999). The increase in connective tissue could be related to an increase in certain growth factors emanating from the smooth muscle or to a decrease in the activity of certain metabolic pathways contributing to the breakdown of various forms of collagen (Borer et al, 1999). Ischemia, which itself can be caused by obstruction or atherosclerosis, has also been hypothesized to contribute to remodeling of the extracellular matrix and fibrosis (Azadzoi et al, 1999; Mostwin et al, 2005).
Bladder outlet obstruction has also been postulated to be associated with partial denervation, owing to damage to the intrinsic innervation of the bladder smooth muscle from a combination of pressure and ischemia (Turner and Brading, 1997; Mostwin et al, 2005). With all of these potential adverse changes occurring, it seems almost miraculous that the bladder can maintain its function, but it does for variable periods of time under different circumstances. However, there does come a point when the ability to fill/store and empty is adversely affected, but not necessarily to the same extent. Filling/storage changes seem related primarily to (1) changes in the extracellular matrix, leading to decreased compliance; and (2) to the appearance of phasic bladder overactivity. This overactivity could be myogenic in origin (caused by partial denervation—see Turner and Brading, 1997), or it could be neurogenic and related to another facet of plasticity. Afferent neuroplasticity mediated by nerve growth factors occurs experimentally in response to bladder outlet obstruction, a phenomenon that is inhibited by autoimmunization against nerve growth factor (Steers et al, 1996). The ability to empty can be adversely affected by factors related to neurogenic or myogenic mechanisms. The myogenic mechanisms could include a reversal of the compensatory changes that initially occur (see Chacko et al, 1999) or a breakdown of the structure and function of the proteins that enable the smooth muscle cells to take up, store, and release calcium, affecting the calcium activation of the contractile apparatus (Zderic et al, 1998; Chacko et al, 1999).
Furthermore, these neurogenic changes associated with outflow obstruction may alter the neurotransmitter milieu of the lower urinary tract. In a model of fetal sheep bladder outlet obstruction, ligation of the urachus at midgestation in fetal sheep for 1 month resulted in a shifting of muscarinic, purinergic, and nitrergic mechanisms normally present during fetal development and growth. With outflow obstruction, bladder hypocontractility was induced and contractile forces decreased during stimulated conditions, consistent with denervation and the possibility of atropine resistance. Normal urothelial exerted negative inotropic effects (nitric oxide mediated) were also lost after obstruction. Additionally, loss of compliance resulted in reduced elasticity in the obstructed bladders, consistent with denervation (Thiruchelvam et al, 2003). Other evidence exists suggesting that bladder outlet obstruction alters the milieu of the lower urinary tract. Mirone and coworkers (2007) summarized changes related to bladder outlet obstruction on the ultrastructure of the bladder in prior animal models. These changes include alterations in the cytoskeleton and contractile protein matrix of the bladder, increasing expression of several growth factors and COX-2, swelling and structural destruction of the detrusor mitochondria, augmented extracellular matrix deposition with significant increase of the type III/I collagen ratio, downregulation of matrix metalloproteinases, and upregulation of tissue inhibitors of metalloproteinases, increased afferent neural activity from epithelial cells due to higher density of mechanosensitive epithelial sodium channels, and activation of normal cell and unmyelinated C fibers. All of these factors appear to be related to bladder dysfunction associated with obstruction.
At this time, one cannot reverse certain precipitating factors for the initiation of voiding dysfunction such as spinal cord transection and peripheral nerve injury.
Thus the fact that the changes resulting from the neuroplasticity induced by these insults are permanent is not surprising. However, there are instances in which the initiating “cause” of a particular voiding dysfunction can be removed and yet the symptoms do not entirely disappear. This may be another instance in which neuroplasticity is a major factor. For instance, irritative voiding symptoms fail to disappear in a certain percent of patients with outlet obstruction who undergo surgical correction. Chai and colleagues (1998) found an increased incidence of a positive ice water test in patients with bladder outlet obstruction, indicating the presence of a primitive reflex circuitry capable of mediating an abnormal micturition reflex. Because the ice water test is mediated by C-afferent fibers, the findings support the hypothesis that bladder outlet obstruction is associated with afferent neuroplasticity, detectable in this case by ice water cystometry. Furthermore, persistence of this afferent neural plasticity after relief of the obstruction could account for at least a proportion of the symptomatic treatment failures after urodynamically successful outlet reduction.
Vizzard (1999, 2000a, 2000b, 2000c, 2000d; Qiao and Vizzard, 2004) has written prolifically about various aspects of neuroplasticity, specifically on the occurrence and potential role of such changes in altered lower urinary tract dysfunction after spinal cord injury (SCI) and irritant-induced cystitis. Changes in spinal cord protein expression from retrogradely transported bladder neurotrophic factors could play a role in the neurochemical, electrophysiologic, and organizational properties of the lower urinary tract seen in both of these conditions and could account, in the latter case, for persistence of symptomatic and/or urodynamic abnormalities after the irritating stimulus is removed (e.g., in patients with interstitial cystitis). Those especially interested in this area should consult Vizzard’s articles and associated references.
Disease at or Above the Brainstem
Cerebrovascular Disease
Cerebrovascular Accident (Stroke)
Cerebrovascular accident (CVA) is a common cause of death and one of the most common causes of disability in the United States and Europe. CVA is the most devastating manifestation of cerebrovascular disease, with an annual incidence in the United States that has been variably cited as approximately 550,000 (Blaivas et al, 1998a) and 83 to 160 per 100,000 (Blaivas and Chancellor, 1995a). Approximately one third are fatal, and another third require long-term nursing care (Marinkovic and Badlani, 2001). The prevalence of stroke in persons older than 65 years has been cited as approximately 60 in 1000, and in persons older than 75, 95 per 1000 (Khan et al, 1990). Wyndaele and colleagues (2005) estimate that 1 in 200 individuals will suffer from a CVA. Although CVA is the third leading cause of death in the United States (Marinkovic and Badlani, 2001), approximately 75% of stroke victims survive (Blaivas et al, 1998a) and, of these, only 10% are unimpaired, 40% have a mild residual, 40% have a significant disability, and 10% require institutionalization (Arunable and Badlani, 1993). Thrombosis, occlusion, and hemorrhage are the most common causes of stroke, leading to ischemia and infarction of variably sized areas in the brain, usually around the internal capsule. Marinkovic and Badlani (2001) cite evidence that arterial occlusion is found in 80% of patients.
After the initial acute episode, urinary retention from detrusor areflexia may occur. The neurophysiology of this “cerebral shock” is unclear. After a variable degree of recovery from the neurologic lesion, a fixed deficit may become apparent over a few weeks or months. The most common long-term expression of lower urinary tract dysfunction after CVA is phasic detrusor overactivity (Wein and Barrett, 1988; Khan et al, 1990; Fowler, 1999; Wyndaele et al, 2005). Sensation is variable but is classically described as generally intact, and thus the patient has urgency and frequency with detrusor overactivity. The appropriate response is to try to inhibit the involuntary bladder contraction by forceful voluntary contraction of the striated sphincter. If this can be accomplished, only urgency and frequency result; if not, urgency with incontinence results.
The exact acute and chronic incidence of any voiding dysfunction after CVA, and specifically of incontinence, is not readily discernable. The cited prevalence of urinary incontinence on hospital admission for stroke ranges from 32% to 79%, on discharge from 25% to 28%, and some months later, from 12% to 19% (Brittain et al, 1998). Sakakibara and colleagues (1999), based on their own experience and that of others, estimate that some voiding dysfunction occurs in 20% to 50% of patients with focal brain lesions from tumor and CVA. They cite nocturnal frequency as the most common manifestation, affecting 36% of their patients. Urgency incontinence occurred in 29%, “voiding difficulty” in 25%, urgency without incontinence in 25%, diurnal frequency in 13%, and enuresis in 6%. Acute retention occurred in only 6%. Fowler (1999) cites studies showing that the presence of urinary incontinence within 7 days of a stroke is a more powerful prognostic indicator for poor survival and functional dependence than a depressed level of consciousness. Urinary incontinence at admission was found by Garibella (2003) to have a hazard ratio of 2.8 as a predictor of stroke death at 3 months. Stroke patients who were incontinent had an increased risk of infectious complications and were malnourished, possible confounders of the increased death risk. Patel and colleagues (2001) reported that urinary incontinence was associated with age older than 75 years, dysphagia, visual field defect, and motor weakness. Certain specific types of strokes also appear to be associated with unusual forms of incontinence. Lenticulo-capsular strokes have been noted to be associated with incontinence. Fifty-two percent of patients with strokes in this area of the brain demonstrated poststroke emotional incontinence, which was not related to other aspects of stroke or gender (Kim, 2002). Lesions centrally located in the globus pallidus, especially dorsally located, were more prone to be associated with an emotional incontinence. This area of the brain is noted to have high density of serotonergic fibers, which has been attributed as an explanation as to why this occurs.
Previous descriptions of the voiding dysfunction after CVA have all cited the preponderance of detrusor overactivity with coordinated sphincter activity (Kolominsky-Rabas et al, 2003; Wyndaele et al, 2005). It is difficult to reconcile this with the relatively high incontinence rate that occurs, even considering the probability that a percentage of these patients had an incontinence problem before the CVA. Tsuchida and colleagues (1983) and Khan and colleagues (1990) made early significant contributions in this area by correlating the urodynamic and computed tomographic (CT) pictures after CVA. They reported that patients with lesions in only the basal ganglia or thalamus have normal sphincter function. This means that when an impending involuntary contraction or its onset was sensed, these patients could voluntarily contract the striated sphincter and abort or considerably lessen the effect of an abnormal micturition reflex. The majority of patients with involvement of the cerebral cortex or internal capsule or both were unable to forcefully contract the striated sphincter under these circumstances. Although the authors and others have called this problem “uninhibited relaxation of the sphincter” (Marinkovic and Badlani, 2001), it really is not, but certainly the term does imply that a profound abnormality exists in these patients in the cerebral to corticospinal circuitry that is necessary for voluntary control of the striated sphincter.
Griffiths (2004) has summarized the evidence, obtained by electrical stimulation, positron emission tomography (PET), and functional magnetic resonance imaging (fMRI), implicating Barrington nucleus, the so-called M region, as responsible for coordinated striated sphincter relaxation followed by detrusor contraction (i.e., normal voiding). He summarizes evidence that a second region in the pons—the L region—may be responsible for maintaining striated sphincter tone between voids, although the evidence for this is less convincing. Griffiths (1998), studying the results of single-proton emission computer tomography (SPECT) in a group of geriatric patients with established urinary incontinence, found urge incontinence in approximately 50%; and, in half of these there was reduced sensation of bladder filling, more so in men than in women. True urgency incontinence with reduced bladder sensation was associated with global underperfusion of the cerebral cortex, especially the frontal areas, especially on the right. Thus there are two possible mechanisms for the incontinence associated with involuntary bladder contractions in patients who have sustained a CVA: (1) impaired striated sphincter control and (2) lack of appreciation of bladder filling and impending bladder contraction.
Smooth sphincter activity is generally unaffected (synergic) by CVA. Some authors describe striated sphincter dyssynergia in 5% to 21% of patients with brain disease and voiding dysfunction (Sakakibara et al, 1999). This is incompatible with accepted neural circuitry. The authors of this chapter agree with those who believe that true detrusor–striated sphincter dyssynergia does not occur in this situation. Pseudodyssynergia may indeed occur during urodynamic testing of these patients (Wein and Barrett, 1982). This refers to an electromyographic (EMG) sphincter “flare” during filling cystometry that is secondary to attempted inhibition of an involuntary bladder contraction by voluntary contraction of the striated sphincter. The guarding reflex is generally intact (Siroky and Krane, 1982).
Detrusor hypocontractility or areflexia may persist after CVA. The exact incidence of areflexia as a cause of chronic voiding symptoms after CVA is uncertain. In our patient population, this is rare, but some estimates place it as high as 20% (Arunable and Badlani, 1993). In a group of incontinent male stroke patients, Linsenmeyer and Zorowitz (1992) found 35% had involuntary bladder contractions with urodynamic evidence of bladder outlet obstruction and 6% had detrusor areflexia. In women in this group, 13% had involuntary contraction with a large residual urine volume and 19% had areflexia. Poor flow rates and high residual urine volumes in a male with pre-CVA symptoms of prostatism generally indicate prostatic obstruction, but a full urodynamic evaluation is advisable before committing a patient to mechanical outlet reduction, primarily to exclude detrusor overactivity with impaired contractility as a cause of symptoms.
Key Point: Lower Urinary Tract Dysfunction after CVA
Other important modifying factors should be considered in the case of these patients. This is generally a problem of the elderly, some of whom have preexistent lower urinary tract pathologic processes. Previously, the problems may have been manageable, but the additional difficulty may make the situation intolerable. As Andrews (1994) notes, other aspects of the brain damage can affect general rehabilitation and control of the lower urinary tract dysfunction. These may include cognitive impairment, dysphasia, inappropriate and aggressive behavior, impaired mobility, and low motivation. Finally, the entire voiding dysfunction may be adversely affected by treatment regimens that concentrate on detrusor overactivity alone (e.g., anticholinergic or antispasmodic therapy). Many such patients are depressed, and confusion and disorientation often result, which compounds the problem. Vigorous pharmacologic therapy of detrusor overactivity with agents that cross the blood-brain barrier and inhibit M1 receptor function may make these associated problems of mentation worse.
The underlying basic mechanisms of bladder overactivity after CVA remain unclear. Experimental models of middle cerebral artery occlusion have been described, most recently followed by reperfusion to simulate the clinical condition (Pehrson et al, 2003). Shimizu and colleagues (2003) described the development of a rat model involving an electrolytic lesion of the right basal forebrain. Following at least middle cerebral artery occlusion, the overactivity seems to involve glutaminergic, dopaminergic, and γ-aminobutyric acid (GABA)-ergic innervations (Kanie et al, 2000; Yokoyama et al, 2002). In addition, Fu and colleagues (2004) have shown upregulation of proinflammatory cytokines and neuronal nitric oxide synthetase gene in the spinal cord and bladder. Such findings raise interesting theoretic possibilities for pharmacologic management other than or in addition to antimuscarinic therapy.
Brainstem Stroke
Sakakibara and colleagues (1996b) studied 39 patients with brainstem stroke. Nineteen of these had voiding symptoms. The major problems were nocturnal frequency and voiding difficulty in six, urinary retention in eight, and urinary incontinence in three. Problems were more common after damage from bleeding than from infarction. Symptoms did not occur in those with strictly midbrain lesions but occurred in 18% of patients with medullary stroke and in 35% of patients with pontine lesions. Eleven patients were symptomatic and underwent urodynamic study. Detrusor overactivity was found in 8 of the 11 and low compliance in 1. What was interpreted as striated sphincter dyssynergia was reported in 5 of the 11 patients, and what was called uninhibited sphincter relaxation occurred in 3. The authors concluded that lesions of the dorsolateral pons involving the pontine reticular nucleus, reticular formation, and the locus coeruleus were mainly responsible for the micturition disturbances in patients with brainstem lesions and that these findings corroborated the presence of a pontine micturition center in humans, corresponding to the pontine storage and micturition centers reported in animal studies.
Dementia
Dementia is a poorly understood disease complex involving atrophy and the loss of gray and white matter of the brain, particularly of the frontal lobes. Problems result with memory and the performance of tasks requiring intellectual mentation. Associated conditions include widespread vascular disease, Alzheimer disease, Pick disease, Jakob-Creutzfeldt disease, syphilis, heat trauma, and encephalitis. Alzheimer disease is the principal cause of dementia in the elderly (Wyndaele et al, 2005, 2009). When voiding dysfunction occurs, the result is generally incontinence. It is difficult to ascertain whether the pathophysiology and considerations are similar to those in the stroke patient or whether the incontinence reflects a situation in which the individual has simply lost the awareness of the desirability of voluntary urinary control. Even if the person has voluntary sphincter control, such individuals may void when and where they please, because mentation fails to dictate why they should not. Such activity may be caused by detrusor overactivity or an otherwise normal, but inappropriately timed, micturition reflex. An accurate estimate of the prevalence of dementia-associated incontinence is confounded by the difficulty in distinguishing this from age-related changes in the bladder and from other concomitant diseases, as pointed out by Wyndaele and colleagues (2005, 2009), who cite figures of 30% to 100%. Treatment is obviously difficult without a desire for improvement. Additionally, therapy that inhibits muscarinic brain receptors may be contraindicated in Alzheimer disease if current theories about its etiology are valid (cortical cholinergic loss).
Traumatic Brain Injury
Traumatic brain injury has been cited as the most common form of severe neurologic impairment as a result of trauma (Blaivas and Chancellor, 1995a). When voiding dysfunction occurs, there may be an initial period of detrusor areflexia. With lesions above the pontine micturition center, involuntary bladder contractions are the most frequent manifestation of chronic lower urinary tract dysfunction. Coordinated sphincter function is the rule. In patients who have more isolated brainstem injuries with involvement below the pontine micturition center, detrusor striated sphincter dyssynergia may occur in addition.
Chua and colleagues (2003) assessed 66 males and 18 females within 6 weeks of acute traumatic brain injury. Sixty-two percent of patients had urinary incontinence on admission with retention (defined as >100 mL), noted in 9.5%. Sixty-two percent required either indwelling catheters or external collecting devices for urinary maintenance. Urinary incontinence was associated with poor functional status and bilateral lesions, whereas urinary retention was more commonly noted in patients with comorbid diabetes mellitus or fecal impaction. After rehabilitation, 36% remained incontinent (Chua et al, 2003).
Brain Tumor
Both primary and metastatic brain tumors have been reported to be associated with disturbances of bladder function. When dysfunction results, it is related to the localized area involved rather than to the tumor type. The areas that are most frequently involved with associated micturition dysfunction are the superior aspects of the frontal lobe (Blaivas, 1985). When voiding dysfunction occurs, it generally consists of detrusor overactivity and urinary incontinence. These individuals may have a markedly diminished awareness of all lower urinary tract events and, if so, are totally unable to even attempt suppression of the micturition reflex. Smooth and striated sphincter activity is generally synergic. Pseudodyssynergia may occur during urodynamic testing. Fowler (1999) has reviewed the literature on frontal lobe lesions and bladder control. She cites instances of resection of a tumor relieving the micturition symptoms for a period of time, raising the question of whether the phenomenon of tumor-associated bladder overactivity was a positive one (activating some system) rather than a negative one (releasing a system from control). Urinary retention has also been described in patients with space-occupying lesions of the frontal cortex, in the absence of other associated neurologic deficits (Lang et al, 1996). Posterior fossa tumors may be associated with voiding dysfunction (32% to 70%, based on references cited by Fowler, 1999). Retention or difficulty voiding is the rule, with incontinence being rarely reported.
Cerebellar Ataxia
This group of diseases involves pathologic degeneration of the nervous system generally involving the cerebellum, but with a possible extension to the brainstem, spinal cord, and dorsal nerve roots (Leach et al, 1982). Poor coordination, depressed deep tendon reflexes, dysarthria, dysmetria, and choreiform movements result because of the cerebellar involvement. Voiding dysfunction is generally manifested by incontinence, usually associated with detrusor overactivity and sphincter synergia. Retention or high residual urine volume may occur as well. Poor emptying, when present, is most commonly caused by detrusor areflexia, but it may be associated with detrusor–striated sphincter dyssynergia, presumably secondary to spinal cord involvement. Sakakibara and colleagues (1998b) reported micturitional symptoms in 184 patients with spinocerebellar degeneration. Twenty-nine (15.8%) showed stress urinary incontinence. Although 20 of these 29 also had detrusor overactivity and/or low compliance and/or residual urine, 9 had none of these findings. The authors speculate that in some such patients, spinal lesions affecting Onuf nucleus and consequently pudendal nerve function were responsible.
Normal-Pressure Hydrocephalus
This is a disease of progressive dementia and ataxia occurring in patients with normal cerebrospinal fluid pressure and distended cerebral ventricles but with no passage of air over the cerebral convexities by pneumoencephalography (Blaivas, 1985). When voiding dysfunction occurs, it is generally incontinence secondary to detrusor overactivity with sphincter synergia.
Cerebral Palsy
Cerebral palsy (CP) is the name applied to a nonprogressive injury of the brain in the prenatal or perinatal or postnatal period (generally during the first year of life, but some say up to 3 years) that produces neuromuscular disability and/or specific symptom complexes of cerebral dysfunction. The etiology is generally infection or a period of hypoxia. Affected children exhibit delayed gross motor development, abnormal motor performance, altered muscle tone, abnormal posture, and exaggerated reflexes. Most children and adults with only CP have urinary control and what seems to be normal filling/storage and normal emptying. The actual incidence of voiding dysfunction is somewhat vague because the few available series report findings predominantly in those who present with voiding symptoms. Andrews (1994) estimates that a third or more of children with CP are so affected. Roijen and colleagues (2001) surveyed children and adolescents from six rehabilitation centers and cited the prevalence of “primary urinary incontinence” as 23.5%. The most important factors influencing the occurrence of incontinence were spastic tetraplegia and low intellectual capacity. Wyndaele and colleagues (2005, 2009) cite the occurrence of lower urinary tract dysfunction as 36%. When an adult with CP presents with an acute or subacute change in voiding status, however, it is most likely unrelated to CP.
Reid and Borzyskowski (1993) described findings in 27 patients, aged 3 to 20 years, referred for voiding dysfunction. Incontinence (74%), frequency (56%), and urgency (37%) were the most common presenting symptoms, and detrusor overactivity was the most common urodynamic abnormality (87% of those undergoing urodynamics), with 25% of these exhibiting apparent striated sphincter dyssynergia. Mayo (1992) reported on 33 CP patients referred for evaluation, of whom 10 were older than 20 years. Difficulty urinating was the predominant symptom in about half the patients, but half of these also had overactivity and urgency when the bladder was full. The cause of the difficulty in voluntarily initiating micturition was thought to be a problem with relaxing the pelvic floor and not true striated sphincter dyssynergia. Incontinence was the major presenting symptom in the other half, associated in 14 of 16 with detrusor overactivity; all exhibited normal voiding otherwise. Decreased sensation was reported in 17 of 23 patients younger than 20 years of age and in 4 of 10 older than 20. The more serious manifestations such as retention were found only in the adults, prompting the author to suggest that difficulty urinating may progress in adulthood.
Reid and Borzyskowski (1993) note that incontinence can be significantly improved in most CP patients and that, in their experience, intellectual delay is not a barrier to successful management. However, one special problem that is encountered in some of these individuals that makes their management difficult is a severe degree of mental retardation, such that any evaluation or treatment that requires cooperation is virtually impossible. With such individuals, sometimes the best that one can do is to check the upper tracts with renal ultrasonography, measure serum creatinine levels, and obtain an estimate of postvoid residual urine, either by catheterization or ultrasound, and proceed accordingly. In those individuals with CP who exhibit significant dysfunction, the type of damage that one would suspect from the most common urodynamic abnormalities seems to be localized anatomically above the brainstem. This is commonly reflected by phasic detrusor overactivity and coordinated sphincters. However, spinal cord damage can occur, and perhaps this accounts for those individuals with CP who seem to have evidence of striated sphincter dyssynergia or of a more distal type of neural axis lesion.
Parkinson Disease
Parkinson disease (PD) is a neurodegenerative disorder of unknown cause that affects primarily the dopaminergic neurons of the substantia nigra but also heterogeneous populations of neurons elsewhere (Lang and Lozano, 1998). The most important site of pathology is the substantia nigra pars compacta, the origin of the dopaminergic nigrostriatal tract to the caudate nucleus and putamen. Dopamine deficiency in the nigrostriatal pathway accounts for most of the classic clinical motor features of PD. The classic major signs of PD consist of tremor, skeletal rigidity, and bradykinesia, a symptom complex often referred to as parkinsonism. Positron-emission tomography (PET) has shown alteration in brain activation in response to bladder filling in PD. PET revealed changes in nine patients in brain activation associated with detrusor overactivity, specifically in the periaqueductal gray, the supplementary motor area, the cerebellar vermis, the insula, putamen, and thalamus. The most prominent degree of increased activation was noted in the cerebellum, with no change in pons during detrusor overactivity (Kitta et al, 2006).
The role of alterations in dopaminergic receptor subtypes has been assessed in animal models of PD. D-2 agonists and D-1 antagonists appear to result in a reduction of bladder capacity in these models. Brusa and colleagues (2006) studied a group of 87 patients with mild PD who were evaluated by symptomatic change and urodynamics after administration of selective dopaminergic agents. Agents resulting in acute central D-2 stimulation result in bladder capacity and worsened detrusor overactivity, as compared with peripheral dopaminergic antagonists (Brusa et al, 2006). There are causes of parkinsonism other than PD, and in an excellent review, Lang and Lozano (1998) discuss clinical distinguishing features of these other causes of parkinsonism from PD. These other causes consist of (1) multiple system atrophy (MSA: includes striatonigral degeneration, sporadic olivopontocerebellar atrophy, and Shy-Drager syndrome); (2) progressive supranuclear palsy; (3) cortical-basal ganglionic degeneration; (4) so-called vascular parkinsonism; and (5) dementia with Lewy bodies. The combination of asymmetry of symptoms and signs, the presence of a resting tremor, and a good response to levodopa best differentiates PD from parkinsonism produced by other causes, although none of these is individually specific for PD. Wyndaele and colleagues (2005) endorse additional criteria for distinguishing lower urinary tract symptoms caused by MSA from those caused by PD. The following suggest MSA: (1) urinary symptoms precede or present with parkinsonism; (2) urinary incontinence; (3) significant postvoid residual; (4) initial erectile failure; and (5) abnormal striated sphincter electromyogram. Five percent of patients initially diagnosed with PD are found to have Parkinson’s Plus Syndromes. These syndromes are characterized by early dementia and/or falls, symmetric symptoms such as a wide-based gait, normal eye movements, autonomic dysfunction, and marked disability. These variants tend to have a worse prognosis than does idiopathic PD. Urinary function is not well described in this subgroup of patients (Nutt and Wooten, 2005).
The “gold standard” for the diagnosis of PD is the neuropathologic examination. In addition to the characteristic pattern of the loss of selected populations of neurons, there is the presence of degenerating ubiquitin-positive neuronal processes or neurites (Lewy neurites) found in all affected brainstem regions. The Lewy body is an intracytoplasmic eosinophilic hyaline inclusion consistently observed in selectively vulnerable neuronal populations, although these are not specific to PD, being found in small numbers in other neurodegenerative disorders. PD affects both sexes roughly equally. The prevalence is cited as 0.3% of the general population and 3% of people older than 65 years (Lang and Lozano, 1998).
Voiding dysfunction occurs in 35% to 70% of patients with PD (Berger et al, 1990; Sotolongo and Chancellor, 1993; Blaivas et al, 1998a; Wein and Rovner, 1999; Wyndaele et al, 2005, 2009). Preexisting detrusor or outlet abnormalities may be present, and the symptomatology may be affected by various types of treatment for the primary disease. When voiding dysfunction occurs, symptoms generally (50% to 75%) consist of urgency, frequency, nocturia, and urge incontinence. The remainder of patients have “obstructive” symptoms or a combination. The most common urodynamic finding is detrusor overactivity. The pathophysiology of detrusor overactivity most widely proposed (Fowler, 1999) is that the basal ganglia normally have an inhibitory effect on the micturition reflex, which is abolished by the cell loss in the substantia nigra. Whether the dopamine D1 or D2 receptor (or both) are primarily responsible does not seem to have been settled as yet. It has been suggested that loss of inhibitory D1-like receptors causes detrusor overactivity, allowing D2 receptors to facilitate micturition (Andersson and Wein, 2004). The smooth sphincter is synergic. There is some confusion regarding EMG interpretation. Sporadic involuntary activity in the striated sphincter during involuntary bladder contraction has been reported in up to 60% of patients; however, this does not cause obstruction and cannot be termed true dyssynergia, which generally does not occur. Pseudodyssynergia may occur, as well as a delay in striated sphincter relaxation (bradykinesia) at the onset of voluntary micturition, both of which can be urodynamically misinterpreted as true dyssynergia. Impaired detrusor contractility may also occur, either in the form of low amplitude or poorly sustained contractions or a combination. Detrusor areflexia is relatively uncommon in PD.
It should be recognized, however, that many cases of “PD” in the older literature may actually have been MSA, and citations regarding symptoms and urodynamic findings may not therefore be accurate. A good and important example of this is the inference from the publication by Staskin and colleagues (1988) that transurethral prostatectomy (TURP) in the patient with PD is associated with a high incidence of urinary incontinence (because of poor striated sphincter control). Retrospective interpretation (Fowler, 1999, 2001; Wyndaele et al, 2005) has shown that these were patients with MSA and not PD and that TURP should not be contraindicated in patients with PD because external sphincter acontractility is extremely rare in such patients. However, one must be cautious with such patients, and a complete urodynamic or video-urodynamic evaluation is advisable. Poorly sustained bladder contractions, sometimes with slow sphincter relaxation, should make one less optimistic regarding the results of outlet reduction in the male. PD has been shown to impact voiding dysfunction in postradical prostatectomy patients. Routh and colleagues (2006) noted that in a study of retrospective evaluation of 20 patients with PD who had undergone radical prostatectomy that de novo incontinence rate was 24% and was of a minimal nature on the basis of pad utilization. However, the conclusion of the study was that advanced evaluation preoperatively including urodynamic or video-urodynamic evaluation would seem advisable for purposes of counseling given the higher rates of incontinence in this particular population.
Christmas and colleagues (1988) demonstrated that subcutaneous administration of a dopamine receptor agonist (apomorphine) can reliably and rapidly reverse parkinsonian “off” periods (periods of worsening symptoms mainly caused by the timing of previous medication doses and the unpredictable nature of motor fluctuations). By repeating video-urodynamic studies during the motor improvement after administration of apomorphine, bladder outlet obstruction secondary to benign prostatic hyperplasia (BPH) may be able to be distinguished from voiding dysfunction secondary to PD. The authors also point out that apomorphine might be useful in such patients who have severe off-phase voiding dysfunction (e.g., those with disabling nocturnal frequency and incontinence). Voiding dysfunction secondary to PD defies “routine” classification within any system. It is most manifest by storage failure secondary to bladder overactivity, but detailed urodynamic evaluation is mandatory before any but the simplest and most reversible therapy. The therapeutic menus (see Tables 66–3 and 66–4) are perfectly applicable, but the disease itself may impose certain limitations on the use of certain treatments (e.g., limited mobility for rapid toilet access, hand control insufficient for clean intermittent catheterization [CIC]).
Animal models of PD have been developed, using injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or 6-hydroxydopamine into the nigrostriatal pathway (Yoshimura et al, 2003; Andersson and Wein, 2004; Wyndaele et al, 2005).
Subthalamic nucleus deep brain stimulation has been shown to be effective for motor symptoms and dyskinesias in patients with moderate to severe PD. Clinical studies have shown that this type of stimulation improves urinary function in these patients by ameliorating bladder sensation and also improving functional bladder capacity. Herzog and colleagues (2006) studied 11 patients undergoing deep brain stimulation with positron emission tomography assessments of regional cerebral blood flow. Subjects were studied with urodynamics both with stimulation on and stimulation off. At urodynamic capacity, significant increases in anterior cingulate regional blood flow were noted. They were increased when deep brain stimulation was off. At bladder capacity, there was also an increase in lateral frontal cortical blood flow with stimulation off. These results were felt to be indicative of deep brain stimulation improvement of bladder function by the modulation of afferent bladder information to cortical and subcortical areas (Herzog et al, 2006).
Multiple System Atrophy
Multiple system atrophy is a progressive neurodegenerative disease of unknown etiology. The symptoms encompass parkinsonism and cerebellar, autonomic (including urinary and erectile problems), and pyramidal cortical dysfunction in a multitude of combinations. The clinical features and the differentiation from PD are nicely described in a consensus statement by Gilman and colleagues (1999). These investigators advocate a designation of MSA-P if parkinsonian features predominate and one of MSA-C if cerebellar features predominate. Older names such as “striatonigral degeneration,” “sporadic olivopontocerebellar atrophy,” and “Shy-Drager syndrome” (Wein, 2002a) should be discarded in favor of these.
The neurologic lesions of MSA consist of cell loss and gliosis in widespread areas, much more so than with PD, and this more diffuse nature of cell loss probably explains why bladder symptoms may occur earlier than in PD and be more severe and why erection may be affected as well (Kirby et al, 1986; Beck et al, 1994; Chandiramani et al, 1997). Affected areas have been identified in the cerebellum, substantia nigra, globus pallidus, caudate, putamen, inferior olives, intermediolateral columns of the spinal cord, and Onuf nucleus. Males and females are equally affected, with the onset in middle age. MSA is generally progressive and associated with a poor prognosis.
Shy-Drager syndrome has been described in the past as characterized clinically by orthostatic hypotension, anhidrosis, and varying degrees of cerebellar and parkinsonian dysfunction. Voiding and erectile dysfunction are common. Some consider this as late-stage MSA (Chandiramani et al, 1997).
Chandiramani and colleagues (1997) compared the clinical features of 52 patients with probable MSA and 41 patients with PD. Of patients with MSA, 60% had their urinary symptoms precede or present with their symptoms of parkinsonism. Of patients with PD, 94% had been diagnosed for several years before the onset of urinary symptoms. In patients with MSA, urinary incontinence was a significant complaint in 73%, whereas 19% had only frequency and urgency without incontinence. Sixty-six percent of the patients with MSA had a significant postvoid residual volume (100 to 450 mL). In patients with PD, frequency and urgency were the predominant symptoms in 85%, whereas incontinence was the primary complaint in 15%. In only 5 of 32 patients with PD in whom residual urine volume was measured was it significant. Eleven men with MSA underwent TURP. Nine of these had deterioration of their urinary incontinence afterward. All three women with MSA were incontinent after pelvic floor repair. Five men with PD underwent prostatectomy, and three reported a good result. Ninety-three percent of the men with MSA questioned about erectile function reported erectile failure, and in 13 of 27 of these the erectile dysfunction preceded the diagnosis of MSA. Seven of the 21 men with PD had erectile failure, but in all these men the diagnosis of erectile dysfunction followed the diagnosis of PD by 1 to 4 years. Fowler (2001) lists the clinical urogenital criteria favoring a diagnosis of MSA as (1) urinary symptoms precede or present with parkinsonism; (2) male erectile dysfunction precedes or presents with parkinsonism; (3) urinary incontinence; (4) significant postvoid residual; and (5) worsening lower urinary tract dysfunction after urologic surgery.
The initial urinary symptoms of MSA are urgency, frequency, and urgency incontinence, occurring up to 4 years before the diagnosis is made, as does erectile failure. Detrusor overactivity is frequently found, as one would expect from the central nervous system areas affected, but decreased compliance may occur, reflecting distal spinal involvement of the locations of the cell bodies of autonomic neurons innervating the lower urinary tract. As the disease progresses, difficulty in initiating and maintaining voiding may occur, probably from pontine and sacral cord lesions, and this is generally associated with a poor prognosis. Cystourethrography or video-urodynamic studies may reveal an open bladder neck (intrinsic sphincter deficiency), and many patients exhibit evidence of striated sphincter denervation on motor unit electromyography. The smooth and striated sphincter abnormalities predispose women to sphincteric incontinence and make prostatectomy hazardous in men. Berger and coworkers (1990) described a useful urodynamic differentiation of what was termed Shy-Drager syndrome (probable late stage MSA) from PD. Parkinsonian patients with voiding dysfunction generally have detrusor overactivity and normal compliance. An open bladder neck was seen only in patients with “Shy-Drager syndrome,” excluding those patients with PD who have had a prostatectomy. EMG evidence of striated sphincter denervation was seen much more commonly in those diagnosed as having Shy-Drager syndrome.
The treatment of significant voiding dysfunction caused by MSA is difficult and seldom satisfactory. Treatment of detrusor overactivity during filling may worsen problems initiating voluntary micturition or worsen impaired contractility during emptying. Patients generally have sphincteric insufficiency, so an outlet-reducing procedure is rarely indicated. Drug treatment for sphincteric incontinence may further worsen emptying problems. Generally, the goal in these patients is to facilitate storage, and CIC would often be desirable. Unfortunately, patients with advanced disease often are not candidates for CIC.
Diseases Primarily Involving the Spinal Cord
Multiple Sclerosis
Multiple sclerosis (MS) is primarily a disease of young and middle-aged adults with a twofold predilection for women. Litwiller and colleagues (1999) detailed current prevalence rates for MS as 1/1000 Americans, 2/1000 northern Europeans, and 20 to 40/100,000 first-degree relatives of patients with MS. The disease is believed to be immune mediated and is characterized by neural demyelination, generally characterized by axonal sparing, in the brain and spinal cord (Noseworthy et al, 2000). This demyelination causes impairment of saltatory conduction and of conduction velocity in axonal pathways, resulting in various neurologic abnormalities that are subject to exacerbation and remission. Lesions, known as plaques, range from 1 mm to 4 cm and are scattered throughout the white matter of the nervous system (Chancellor and Blaivas, 1993). The demyelinating process most commonly involves the lateral corticospinal (pyramidal) and reticulospinal columns of the cervical spinal cord, and it is thus not surprising that voiding dysfunction and sphincter dysfunction are so common. Autopsy studies have revealed almost constant evidence of demyelination in the cervical spinal cord, but involvement of the lumbar and sacral cord occurs in approximately 40% and 18%, respectively (Blaivas and Kaplan, 1988). Lesions may also occur in the optic nerve and in the cerebral cortex and midbrain, the latter accounting for the intellectual deterioration and/or euphoria that may be seen as well (Kirby, 1994; Noseworthy et al, 2000), ultimately in up to 43% to 65% of patients (Litwiller et al, 1999). A rat model for a demyelinating disease resembling MS has been described using myelin basic protein as an antigen for inducing experimental allergic encephalomyelitis (Mizusawa et al, 2000).
The incidence of voiding dysfunction in MS is related to the disability status. Of patients with MS, 50% to 90% complain of voiding symptoms at some time; the prevalence of incontinence is cited as 37% to 72% (Wyndaele et al, 2005). Litwiller and colleagues (1999), in their excellent review article, cite symptoms of frequency or urgency in 31% to 85% of patients in various series, with corresponding percentages of incontinence as 37% to 72% and of obstructive symptoms with urinary retention in 2% to 52%. Lower urinary tract involvement may constitute the sole initial complaint or be part of the presenting symptom complex in up to 15% of patients, usually in the form of acute urinary retention of “unknown” etiology or as an acute onset of urgency and frequency, secondary to overactivity (Wyndaele et al, 2005).
Detrusor overactivity is the most common urodynamic abnormality detected, occurring in 34% to 99% of cases in reported series (Blaivas and Kaplan, 1988; Chancellor and Blaivas, 1993; Sirls et al, 1994; Litwiller et al, 1999). Of the patients with overactivity, 30% to 65% have coexistent striated sphincter dyssynergia. Impaired detrusor contractility or areflexia may also exist; estimates of prevalence range from 12% to 38% (Wyndaele et al, 2005), a phenomenon that can considerably complicate treatment efforts. Other estimates suggest that 62% of patients with MS have neurogenic detrusor overactivity with bladder outlet obstruction, 25% have neurogenic detrusor overactivity with detrusor sphincter dyssynergia, 20% have detrusor under activity, and 10% have no initial abnormal urodynamic findings. The variability and potential multiplicity of lesions associated with MS, however, prohibit accurate diagnosis on the basis of urodynamics alone (Ukkonen et al, 2004). Bladder dysfunction associated with MS, as compared with women with non-neurogenic detrusor activity, has been shown to demonstrate significant differences. The amplitude of the first overactive contraction is greater in patients with MS as compared with those patients with idiopathic detrusor overactivity, as is the maximal detrusor contraction. The threshold volume at which detrusor overactivity occurs in patients with neurogenic detrusor overactivity, however, is somewhat higher than in those patients with idiopathic detrusor overactivity, which may be related to residual volume and which is noted in patients with detrusor overactivity. Using threshold values for prediction of type of voiding dysfunction, 30 cm of H2O and above was predictive of patients having neurogenic detrusor activity as compared with those who have non-neurogenic detrusor overactivity. These differences in neurogenic detrusor overactivity urodynamic findings may be representative of direct neurogenic influences on the detrusor muscle from abnormal bladder interpolation and/or signaling as compared with non-neurogenic patients (Lemack, 2006).
Generally, the smooth sphincter is synergic. Chancellor and Blaivas (1993) reviewed urodynamic findings in multiple series of patients with MS and voiding dysfunction and summarized the incidence of three basic patterns: (1) detrusor overactivity, striated sphincter synergia 26% to 50% (average 38%); (2) detrusor overactivity, striated sphincter dyssynergia 24% to 46% (average, 29%); and (3) detrusor areflexia 19% to 40% (average, 26%). Litwiller and colleagues (1999) tabularized 22 references, reporting approximately the same ranges. It is also possible to see relative degrees of sphincteric flaccidity caused by MS, a phenomenon cited as occurring in less than 15% of patients (Litwiller et al, 1999); this finding could predispose and contribute to sphincteric incontinence. De Ridder and colleagues (1998) reported weakness of pelvic floor contraction in nearly all 30 women they studied with MS. Spasticity of the pelvic floor was present in all patients with striated sphincter dyssynergia but in none with detrusor overactivity alone.
Because sensation is frequently intact in these patients, one must be careful to distinguish urodynamic pseudodyssynergia from true striated sphincter dyssynergia. Blaivas and colleagues (1981) subcategorized true striated sphincter dyssynergia in patients with MS and identified some varieties that are more worrisome than others. For instance, in a female with MS, a brief period of striated sphincter dyssynergia during detrusor contraction but one that does not result in excessive intravesical pressure during voiding, substantial residual urine volume, or secondary detrusor hypertrophy may be relatively inconsequential, whereas those varieties that are more sustained—resulting in high bladder pressures of long duration—are most associated with urologic complications. Giannantoni and colleagues (1998) reviewed 116 of their patients and likewise concluded that there was a significant relationship between the maximum amplitude of the involuntary bladder contractions and upper urinary tract deterioration. Chancellor and Blaivas (1993) emphasized what they believed were the most important parameters predisposing patients with MS to significant urologic complications: (1) striated sphincter dyssynergia in men; (2) high detrusor filling pressure (>40 cm H2O); and (3) an indwelling catheter. Interestingly, however, Wyndaele’s committee (Wyndaele et al, 2005) concluded that progressive neurologic disease, MS included, rarely causes upper urinary tract damage, even when, in MS, severe spasticity and disability exist. The reason for this is unknown, but they believe that the situation and concerns with respect to this are unlike those for SCI.
Key Point: Multiple Sclerosis
Aggressive and anticipatory medical management can obviate most significant complications. Sirls and colleagues (1994) reported that only 7% (the author calculated 10.4%) of their patients required surgical intervention because of failure of aggressive medical management and that none developed hydronephrosis on such therapy. The regimens used were (1) drugs to decrease detrusor overactivity plus CIC (57%); (2) such drugs alone (13%); (3) CIC alone (15%); and (4) behavioral therapy. A significant proportion of patients with MS with and without new symptoms will develop changes in their detrusor compliance and urodynamic pattern (Ciancio et al, 2001). Caution should therefore be exercised in recommending irreversible therapeutic options. No factors appear to be predictive of upper tract changes in MS. In a 4-year follow-up of multiple sclerosis patients, 113 patients were evaluated, of whom 66 patients had both urodynamic and renal ultrasound testing. Sixteen percent (11 patients) had abnormal ultrasound findings. The most significant finding was minor caliectasis, which was of no clinical significance. Neither creatinine nor urodynamic findings were associated with the abnormal renal ultrasound findings (Lemack et al, 2005). Surgical intervention for multiple sclerosis appears to be diminishing with improved pharmacologic management and the realization of the alternating neurologic picture of lower urinary dysfunction associated with MS (Ukkonen et al, 2004).
No consensus on optimal bladder management has identified for multiple sclerosis, and most commonly, management is predicated on symptomatic and urodynamic findings. According to consensus agreement, De Ridder and colleagues (2005) concluded that in early multiple sclerosis, anticholinergics and intermittent catheterization were considered to be cornerstones of therapy. For advanced MS (with an EDSS > 7), specific guidelines remain lacking. In general, crédé voiding or Valsalva voiding are contraindicated, especially in the presence of detrusor sphincter dyssynergia. They further recommended that indwelling catheters be reserved for patients for whom all other possible treatments have failed. In the approximately 30% of patients with MS using indwelling urinary catheters, the suprapubic route is the preferred route in both men and women. This form of management is considered reasonable for that subpopulation, as long as long-term follow-up is continued (De Ridder et al, 2005).
Spinal Cord Injury
Epidemiology, Morbidity, General Concepts
SCI may occur as a consequence of acts of violence, fracture, or dislocation of the spinal column secondary to motor vehicle, diving accidents or falls, vascular injuries or repair, infection, disk prolapse, or sudden or severe hyperextension from other causes. Altered lower urinary tract and sexual function frequently occur secondary to SCI and significantly affect quality of life; SCI patients are at risk urologically for urinary tract infection, sepsis, upper and lower urinary tract deterioration, upper and lower urinary tract calculi, autonomic hyperreflexia (dysreflexia), skin complications, and depression (which can complicate their urologic management). Failure to properly address the lower urinary tract dysfunction can lead to significant morbidity and mortality. There is great variation in urologic practice regarding initial evaluation, follow-up, and surveillance among spinal injury units (Bycroft et al, 2004), a problem that Boone (2004) properly attributes to a lack of evidence-based decision making.
Complete anatomic transection of the spinal cord is rare, and the degree of neurologic deficit varies with the level and severity of the injury. Spinal column (bone) segments are numbered by the vertebral level, and these have a different relationship to the spinal cord segmental level at different locations. One must be careful to specify cord or column level when discussing SCI. The sacral spinal cord begins at about spinal column level T12 to L1. The spinal cord terminates in the cauda equina at approximately the spinal column level of L2. Multiple level injuries may occur, and, even with a single initial injury, cord damage may not be confined to a single cord segment and may extend cephalad, caudad, or both.
Stover and Fine (1987) reviewed the epidemiology and other general aspects of SCI. The annual rate was reported as 30 to 32 new SCIs per million persons at risk in the United States; the prevalence was approximately 906 per million. This coincides roughly with the estimate by DeVivo (1997) of approximately 10,000 new cases of SCI in the United States yearly and an estimate of 12,000 per year by Rabchevsky and Smith (2001). DeVivo (1997) reported the most common mechanisms of injury, as collected by the National Spinal Cord Injury Statistical Center, as motor vehicle accidents (35.9%), violence (29.5%), falls (20.3%), and sports-related injuries (7.3%). Males account for 71% to 80% of patients with SCI. The average age at injury is 31.5 years. Children comprise 3% to 5% of all patients with SCI (Generao et al, 2004). Stover and Fine (1987) reported that neurologically incomplete quadriplegics constituted the largest group of SCI patients at the time of hospital admission (28%), followed by complete paraplegics (26%), complete quadriplegics (24%), and incomplete paraplegics (18%). This seems to have changed little during the 1990s. The majority occur at or above the T12 spinal column (vertebral) level.
Although earlier data (Hackler, 1977) indicated that renal disease was the major cause of death, at least in the paraplegic patient, a retrospective study of more than 5000 patients who sustained SCI between 1973 and 1980 revealed that the leading causes of death at that time were pneumonia, septicemia, heart disease, accidents, and suicide (Stover and Fine, 1987; Soden et al, 2000). These figures seemingly indicate a distinct improvement in the urologic care of these patients. Impaired mobility is commonly noted in the spinal cord–injured patient and may substantially affect urinary habit and incontinence (Biering-Sorensen et al, 2004). Urologic phenomena also figure prominently in chronic SCI. Within 10 years after SCI, approximately 7% of patients with spinal cord will develop an initial kidney stone with the greatest risk occurring during the first 3 months after injury. Ninety-eight percent of these stones will be apatite or struvite in composition. There appear to be two time frames for stone formation in this population, one being the acute phase associated with immobilization and immobilization hypercalciuria. The other is a more chronic phase stone formation period, usually associated with chronic catheter management years after injury and predominately involving the lower tract (Post and Noreau, 2005).
Controlled and coordinated lower urinary tract function depends on an intact neural axis. Bladder contractility and the occurrence of reflex contractions depend on an intact sacral spinal cord and its afferent and efferent connections (see Chapter 61).
Key Point: Spinal Cord Injury
An impressive amount of literature is continuously building on the neurobiology of the spinal cord and its acute and chronic alteration after SCI. These topics are not specifically considered in detail here, nor are the ramifications of this information relative to potential improvement of spinal cord function after injury by stem cell implant or reinnervation. Earlier reviews can be found by Olson (1997), Fawcett (1998), and Kakulas (1999); later ones were presented by Rabchevsky and Smith (2001) (this also includes a discussion of pathophysiology and experimental models), Cao and colleagues (2002) (stem cell repair), Fawcett (2002) (repair of SCI), Rossi and Cattaneo (2002) (stem cell therapy), Mitsui and colleagues (2003) (stem cell repair), Kakulas (2004) (neuropathology and natural history of the spinal cord changes), and Livshits and colleagues (2004) (reinnervation). Sexual and reproductive dysfunction in the patient with SCI is a topic that deserves much attention in the overall rehabilitation plan. Pertinent general and specific concepts of sexual and reproductive dysfunction and their normalization in this special group of patients can be found in Chapters 23, 27 (male), and 30 (female). Other excellent reviews on the specifics of sexual function in SCI can be found by Bennett and colleagues (1988), Stone and MacDermott (1989), Smith and Bodner (1993), and Biering-Sorensen and Sonksen (2001), and on infertility by Linsenmeyer and Perkash (1991), Rajaskaran and Monga (1999), and Rutkowski and colleagues (1999).
Spinal Shock
After a significant SCI, a period of decreased excitability of spinal cord segments at and below the level of the lesion occurs, referred to as “spinal shock.” There is absent somatic reflex activity and flaccid muscle paralysis below this level. Although classic teaching refers to generalized areflexia below the level of the lesion for days to months, Thomas and O’Flynn (1994) confirm that the most peripheral somatic reflexes of the sacral cord segments (the anal and bulbocavernosus reflexes) may never disappear or, if they do, may return within minutes or hours of the injury. However, functions proximal to the level of the injury may be depressed as well (Atkinson and Atkinson, 1996). Although the course of spinal shock is well known, the actual phenomenon remains poorly understood, with little or no basic research evident recently.
Spinal shock includes a suppression of autonomic activity, as well as somatic activity, and the bladder is acontractile and areflexic. Radiologically, the bladder has a smooth contour with no evidence of trabeculation. The bladder neck is generally closed and competent unless there has been prior surgery or in some cases of thoracolumbar and presumably sympathetic injury (Sullivan and Yalla, 1992). The smooth sphincter mechanism seems to be functional. Some EMG activity may be recorded from the striated sphincter, and the maximum urethral closure pressure is lower than normal but still maintained at the level of the external sphincter zone; however, the normal guarding reflex (striated sphincter response during filling) is absent and there is no voluntary control (Fam and Yalla, 1988). Because sphincter tone exists, urinary incontinence generally does not result unless there is gross overdistention with overflow. In evolving lesions, every attempt should be made to preserve as low a bladder storage pressure as possible and to avoid any measures that might impair this. Urinary retention is the rule, and catheterization is necessary to circumvent this problem. Although virtually all would agree that CIC is an excellent method of management during this period and advocate its use, Lloyd and colleagues (1986) reported their own experience and cite that of others that indicate no differences in outcome when a small-bore Foley catheter or suprapubic tube is used at this stage.
If the distal spinal cord is intact but is simply isolated from higher centers, there is generally a return of reflex detrusor contractility. At first, such reflex activity is poorly sustained and produces only low-pressure changes, but the strength and duration of such involuntary contractions increase, producing involuntary voiding, usually with incomplete bladder emptying. This return of reflex bladder activity is generally manifested by involuntary voiding between catheterizations and occurs along with the recovery of lower extremity deep tendon reflexes. Spinal shock generally lasts 6 to 12 weeks in complete suprasacral spinal cord lesions but may last up to 1 or 2 years. It may last a shorter period of time in incomplete suprasacral lesions and only a few days in some patients.
Suprasacral Spinal Cord Injury
There is no unanimous agreement on the neurobiology of the development of reflex bladder contraction in response to bladder distention after suprasacral SCI. de Groat and colleagues (1997) have studied this phenomenon and related events extensively in cats and listed four potential mechanisms for the recovery of such micturition and the development of C-fiber afferent evoked bladder reflexes (see also the description by Yoshimura and Chancellor in Chapter 60): (1) elimination of bulbospinal inhibitory pathways; (2) strengthening of existing synapses or formation of new synaptic connections from axonal sprouting in the spinal cord; (3) changes in synthesis, release, or actions of neurotransmitters, and (4) alterations in afferent input (afferent axonal sprouting) from peripheral organs. Recent reports of specific alterations in animal models are summarized by Morrison and colleagues (2005) as (1) increased sensitivity of C-fiber afferents, possibly involving nerve growth factor; (2) enlargement of dorsal root ganglion cells; (3) increased electrical excitability of afferents associated with a shift in expression of sodium channels from a high-threshold tetrodotoxin-resistant type to a low-threshold tetrodotoxin-sensitive type. Other findings possibly related to the development of lower urinary tract dysfunction after SCI have been reported as (1) increased concentrations of glutamate, glycine, and taurine (Smith et al, 2002); (2) disruption of bladder epithelium barrier function (Apodaca et al, 2003); (3) change from low affinity M1 to high affinity M3 receptors at prejunctional cholinergic nerve endings (Somogyi et al, 2003); (4) increased release of adenosine triphosphate from bladder urothelium (Khera et al, 2004); (5) increased spinal cord nerve growth factor (Seki et al, 2004); and (6) alterations in smooth muscle myosin heavy chain gene expression (Wilson et al, 2005). Recently, in murine models of acute SCI, nicotinic or purinergic receptor mechanisms have been shown to be the primary mechanism for ATP release as atropine has been shown to be only partially effective in stimulating ATP release (predominantly a muscarinic receptor phenomenon in the absence of injury).These findings further indicate a change in receptor-mediated bladder activity associated with SCI (Salas et al, 2007).
The characteristic pattern that results when a patient has a complete lesion above the sacral spinal cord is detrusor overactivity, smooth sphincter synergia (with lesions below the sympathetic outflow), and striated sphincter dyssynergia (Sullivan and Yalla, 1992; Thomas and O’Flynn, 1994; Chancellor and Blaivas, 1995b). Neurologic examination shows spasticity of skeletal muscle distal to the lesion, hyperreflexic deep tendon reflexes, and abnormal plantar responses. There is impairment of superficial and deep sensation. Figures 65-1 to 65-3 typify the cystourethrographic and urodynamic patterns. The guarding reflex is absent or weak in most patients with a complete suprasacral SCI. In incomplete lesions the reflex is often preserved but variable (Morrison et al, 2005).
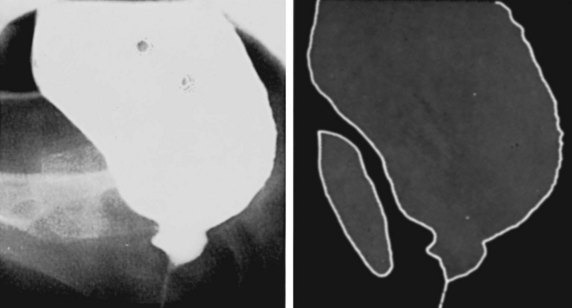
Figure 65–1 Cystourethrogram in a 19-year-old woman with detrusor–striated sphincter dyssynergia secondary to a complete spinal cord injury at vertebral level T11. Image was taken during an involuntary bladder contraction with exaggerated bladder neck opening caused by the obstruction below.
(From Nordling J, Olesen KP. Basic urographic and cystourethrographic patterns. In: Pollack HM, editor. Clinical urography. Philadelphia: WB Saunders; 1990, p. 1953.)
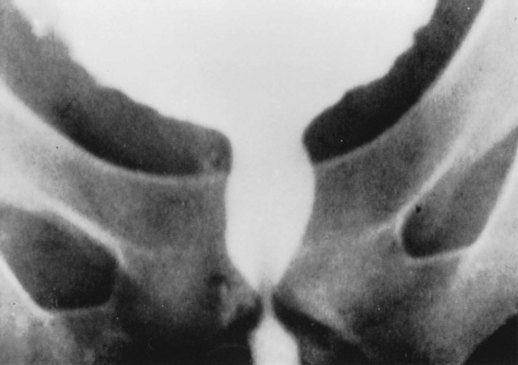
Figure 65–2 Typical cystourethrographic configuration of a synergic smooth sphincter and a dyssynergic striated sphincter in a man during a bladder contraction.
(From Nanninga JB: Radiological appearances following surgery for neuromuscular diseases affecting the urinary tract. In: Pollack HM, editor. Clinical urography. Philadelphia: WB Saunders; 1990, p. 2003.)
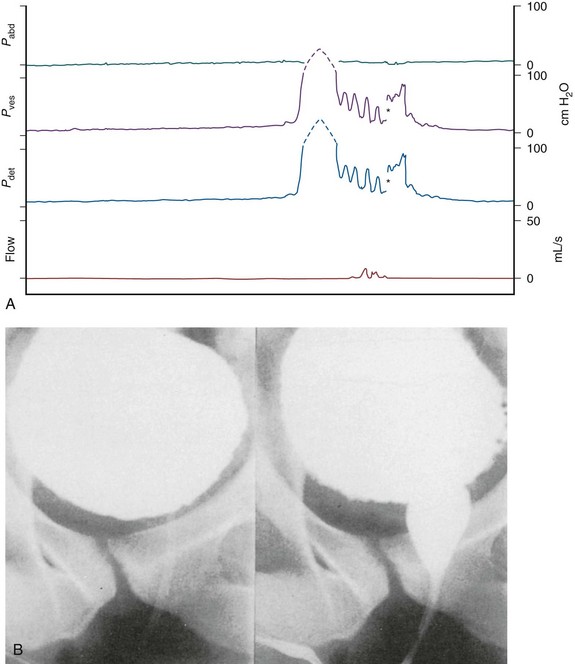
Figure 65–3 Video images in B at corresponding points of the urodynamic tracings in A. Detrusor hyperreflexia (Pdet 150 cm H2O), synergic bladder neck, dyssynergic striated sphincter. The asterisk represents a range change from a scale of 0 to 100 cm H2O.
(From Lawrence WT, Thomas DC. Urodynamic techniques in the neurologic patient. In: O’Reilly PH, George NJR, Weiss RM, editors. Diagnostic techniques in urology. Philadelphia: WB Saunders; 1990. p. 360.)
The striated sphincter dyssynergia causes a functional obstruction with poor emptying and high detrusor pressure. In an effort to subclassify detrusor sphincter dyssynergia, Karsenty and colleagues (2005) retrospectively evaluated video-urodynamic recordings of patients with complete SCI with untreated neurogenic overactive bladder and detrusor sphincter dyssynergia. They identified two time periods within the tracings, with Delay A being defined as that period between the onset of external urethral sphincter (EUS) pressure increase and the subsequent onset of bladder pressure increase. Delay B was defined as the period between the onset of urethral sphincter pressure increase and the moment at which bladder pressure increase reached a level of 10 cm H2O or greater above the baseline value. The recordings of 20 patients were assessed, with the Delay A timeframe found to be significant in 16 of 20 patients, with a meantime for delay of 2.2 seconds. There was a positive association between this delay and the completeness of the SCI and the presence of continuous DSD on electromyogram. Delay B was positive in all patients with a mean delay time of 7.6 seconds. The authors concluded that EUS contraction starts before the onset of bladder contraction in most patients with coexistent SCI and detrusor sphincter dyssynergia. Occasionally, incomplete bladder emptying may result from what seems to be a poorly sustained or absent detrusor contraction. This seems to occur more commonly in lesions close to the conus medullaris than with more cephalad lesions. This may result from a second occult lesion or may be caused by locally functioning reflex arcs, which result in detrusor inhibition from strong striated pelvic floor muscle contraction, or to a loss of higher center–mediated detrusor facilitation, which normally occurs after the initial increase in pressure during a bladder contraction (Thomas and O’Flynn, 1994). Once reflex voiding is established, it can be initiated or reinforced by the stimulation of certain dermatomes, as by tapping the suprapubic area. The urodynamic and upper tract consequences of the striated sphincter dyssynergia vary with severity (generally worse in complete lesions than in incomplete ones), duration (continuous contraction during detrusor activity is worse than intermittent contraction), and anatomy (male is worse than female) (Linsenmeyer et al, 1998).
The type of dyssynergia also appears to be associated with degree of injury and potential for progression of dysfunction. Schurch and colleagues (2005) evaluated 105 male patients with SCI to determine if neurologic examination could predict the type of detrusor sphincter dyssynergia present. A correlation was noted between type of dyssynergia and completeness of spinal cord lesion. More commonly, patients with incomplete sensory and motor lesions presented with type 1 DSD. Complete sensory and motor lesions were more commonly associated with either type 2 or type 3 detrusor sphincter dyssynergia. There was no correlation, however, noted between the type of DSD and the lesion level. At chronic follow-up DSD type was noted to evolve from type 2 or type 3 DSD in approximately a quarter of the studied patients, whereas 65% of patients remained stable with the same type of DSD at follow-up (Schurch et al, 2005).
Key Point: Management of Patients with Suprasacral Spinal Cord Injury
Sacral Spinal Cord Injury
After the patient recovers from spinal shock, there is generally a depression of deep tendon reflexes below the level of a complete lesion with varying degrees of flaccid paralysis. Sensation is generally absent below the lesion level. Detrusor areflexia with high or normal compliance is the common initial result, but decreased compliance may develop, a change seen in some neurologic lesions at or distal to the sacral spinal cord that most likely represents a complex response to neurologic decentralization probably involving reorganization and plasticity of neural pathways (Fam and Yalla, 1988; de Groat et al, 1997; Blaivas et al, 1998b). There is surprisingly little consensus on the evolution of the appearance or function of the bladder neck or smooth sphincter area after sacral spinal cord damage. The classic outlet findings are described as a competent but nonrelaxing smooth sphincter and a striated sphincter that retains some fixed tone but is not under voluntary control. Closure pressures are decreased in both areas (Sullivan and Yalla, 1992; Thomas and O’Flynn, 1994). However, the late appearance of the bladder neck may be “open” (Kaplan et al, 1991). Attempted voiding by straining or Credé’s maneuver results in “obstruction” at the bladder neck (if closed) or at the distal sphincter area by fixed sphincter tone (Fam and Yalla, 1988; Thomas and O’Flynn, 1994). Figure 65–4 illustrates the typical cystourographic and urodynamic pictures of the late phases of such a complete lesion.
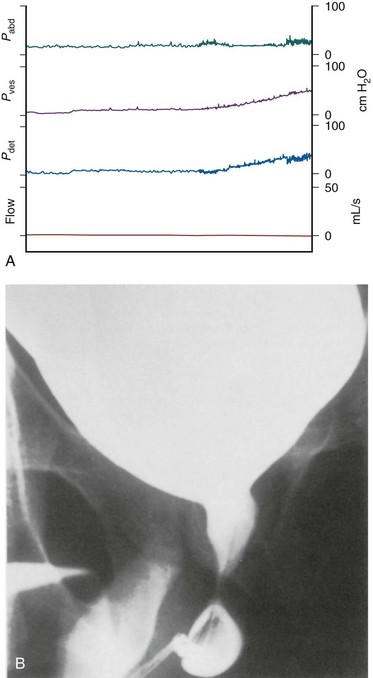
Figure 65–4 Simultaneous video (B) and urodynamic study (A) from a 28-year-old man whose bladder has been filled with 420 mL of contrast material. There is low compliance; the bladder neck is incompetent; and with straining the distal sphincter mechanism does not open—a pattern often seen in sacral spinal cord or efferent nerve root injury or disease.
(From Lawrence WT, Thomas DC. Urodynamic techniques in the neurologic patient. In: O’Reilly PH, George NJR, Weiss RM, editors. Diagnostic techniques in urology. Philadelphia: WB Saunders; 1990, p. 362.)
Neurologic and Urodynamic Correlation
Although generally correct, the correlation between somatic neurologic findings and urodynamic findings in suprasacral and sacral SCI patients is not exact. A number of factors should be considered in this regard. First, whether a lesion is complete or incomplete is sometimes a matter of definition, and a complete lesion, somatically speaking, may not translate into a complete lesion autonomically and vice versa. Multiple injuries may actually exist at different levels, even though what is seen somatically may reflect a single level of injury. Even considering these situations, however, all such discrepancies are not explained. This is further demonstrated in patients with incomplete lesions. Patki and colleagues (2006) assessed a group of 43 men and 21 women with incomplete SCI (ASA grades D and E) during a 2-year period. In this group, 40 patients who were initially assessed as having a bladder not at risk for deterioration ultimately experienced deterioration requiring clean, intermittent catheterization. Conversely, 5 of 20 patients, who initially required clean intermittent catheterization, no longer required this with time. In chronic follow-up, 68% of patients continued to have abnormal urodynamic findings and 37% of patients required a change in urologic management in the absence of perceptible change in neurologic status, indicating the potential of continued bladder changes in the absence of other detectable neurologic disease (Patki et al, 2006).
Key Point: Management of Patients with Sacral Cord Injury
In a classic article, Blaivas (1982) correlated clinical and urodynamic data from 550 patients with voiding dysfunction. In 155 patients with complete and incomplete suprasacral neurologic lesions, physiologically normal voiding was reported in 41%. Detrusor–striated sphincter dyssynergia was demonstrated in 34%, and, surprisingly (and seemingly paradoxically), detrusor areflexia was noted in 25%. Other authors have noted detrusor areflexia with suprasacral SCI or disease, and the causes have been hypothesized as a coexistent distal spinal cord lesion or a disordered integration of afferent activity at the sacral root or cord level (Light et al, 1985; Beric and Light, 1992). Detrusor–striated sphincter dyssynergia was reported in 45% of 119 patients with suprasacral spinal cord lesions. None of 36 patients with supraspinal neurologic lesions had striated sphincter dyssynergia. These data certainly support prior conclusions that (1) coordinated voiding is regulated by neurologic centers above the spinal cord and (2) a diagnosis of striated sphincter dyssynergia implies a neurologic lesion that interrupts the neural axis between the pontine-mesencephalic reticular formation and the sacral spinal cord. All 27 patients with neurologic lesions above the pons and who were able to void did so synergistically—with relaxation of the striated sphincter preceding detrusor contraction. Twenty of these patients had detrusor overactivity, but 12 of these 20 had voluntary control of the striated sphincter, supporting a thesis of separate neural pathways governing voluntary control of the bladder and of the periurethral striated musculature. Most of these patients with detrusor overactivity secondary to lesions above the pons were able to voluntarily contract the striated sphincter, but without abolishing bladder contraction. This seems to indicate that the inhibition of bladder contraction by pudendal motor activity is not merely a simple sacral reflex but, rather, a complex neurologic event. Twenty-two of these patients had evidence of either sacral or infrasacral neurologic impairment of bladder function with suprasacral control of striated sphincter function or vice versa; this provides a clinical correlate to the separate anatomic locations of the parasympathetic motor nucleus and the pudendal nucleus in the sacral spinal cord (see Chapter 60).
A subsequent study from the same center analyzed the results of urodynamic evaluation in 489 consecutive patients with either congenital or acquired SCI or disease and correlated these with the diagnosed neurologic deficit (Kaplan et al, 1991). Although there was a general correlation between the neurologic level of injury and the expected vesicourethral function, it was neither absolute nor specific. Twenty of 117 cervical lesions exhibited detrusor areflexia; 42 of 156 lumbar lesions, detrusor–striated sphincter dyssynergia; and 26 of 84 sacral lesions, either detrusor overactivity or detrusor–striated sphincter dyssynergia. The patients were further classified on the basis of the integrity of the sacral dermatomes (intact sacral reflexes or not). This helps to explain some, but not all, of the apparent discrepancies. Of the patients with suprasacral cord lesions who had detrusor areflexia, 84% also had abnormal sacral cord signs (absent bulbocavernosus reflex, lax anal sphincter tone, or sphincter EMG abnormalities indicative of lower motor neuron degeneration). All suprasacral cord lesion patients who had no evidence of sacral cord involvement had either detrusor overactivity or detrusor striated sphincter dyssynergia.
Patients were also classified according to the three most common neurologic causes for their lesion: trauma, myelomeningocele, and spinal stenosis. Of the 284 trauma patients, all with thoracic cord lesions had either detrusor overactivity or detrusor striated dyssynergia and negative sacral cord signs. In contrast, in patients with traumatic lesions affecting other parts of the spinal cord, there was a wide distribution of both urodynamic and sacral cord sign findings. For example, of patients with traumatic lumbar cord injury, 38% had detrusor areflexia and positive sacral cord signs, 25% had detrusor–striated sphincter dyssynergia and negative sacral cord signs, 25% had detrusor overactivity and negative sacral cord signs, and 14% had either detrusor overactivity or detrusor–striated sphincter dyssynergia and negative sacral cord signs. Of 25 patients with lumbar myelomeningocele, 20 had either detrusor areflexia or detrusor–striated sphincter dyssynergia. All patients with lumbar myelomeningocele and detrusor areflexia had positive sacral cord signs. Of 48 patients with sacral myelomeningocele, 37 had detrusor areflexia and 35 had positive sacral cord signs. Of 54 patients with spinal stenosis, all those with cervical and thoracic cord lesions had either detrusor overactivity or detrusor–striated sphincter dyssynergia and negative sacral cord signs. Patients with a lumbar cord stenosis had no consistent pattern of detrusor activity or sacral cord signs. An open bladder neck at rest was found in 21 patients. All had either lumbar or sacral SCI. Sixteen of these had sacral cord lesions and detrusor areflexia. Decreased bladder compliance was noted in 54 patients, 41 of whom had sacral cord injury and 43 of whom had detrusor areflexia.
Koh et al (2006) reported urodynamic evaluation of five patients with myelomeningocele who underwent prenatal closure of the spinal dysraphism as compared with a large group of 88 patients who underwent postnatal closure. All five of the reported patients had lower lumbosacral lesions. They noted that there is no spontaneous electromyographic activity in the five prenatally intervened children as compared with only 39% identification in the postnatal group. Additionally, all five patients in the prenatal cohort exhibited detrusor overactivity as compared with 38% in the postnatal group. These findings were similar both at baseline and at 1 year. The finding of the complete innervation of the EUS suggested the need for close postnatal surveillance to exclude the possibility of tethering of the spinal cord and other events postsurgical intervention. The value of intervention in these patients has been noted in patients undergoing detethering. In a study of 120 children with myelomeningocele, Abrahamsson and colleagues (2007) identified 20 who underwent cord detethering at a median age of 8 years. Preintervention and postintervention urodynamic evaluation was performed (median of 5 months preoperatively and a median of 12 months postoperatively). Of the 20 that underwent detethering in this group, 7 had improvement of their voiding dysfunction to a milder level of dysfunction with 1 experiencing deterioration. Six patients actually experienced deterioration of bladder dysfunction before detethering, all of whom returned to a less significant level postoperatively. Ten patients had no change in bladder function on urodynamics after intervention, one of whom actually deteriorated on urodynamic findings. The overall 35% improvement underscored the need for regular evaluation of bladder function in these children. Metcalfe and colleagues (2006) reported 36 patients who underwent sectioning of the filum terminale as management of tethered cord. They estimated 0.4% of pediatric clinic visits in the urology department resulted in neurosurgical referral for the potential exclusion of occult tethered cord. Symptoms included daytime incontinence in 83% of the patients and 47% with encopresis. Preintervention urodynamics showed a mean of a 55% reduction of expected bladder capacity in the affected patients. Clinical improvement was noted in 72% of patients undergoing intervention with 42% becoming clinically continent. Additionally, 88% of cases noted improvement in bowel symptomatology including 53% of patients noting improvement in encopresis after 3 months of surgery. Urodynamic improvement was likewise documented in 57% of patients on the basis of improved bladder capacity. No specific factors were identified that would improve surgical success on the basis of either urodynamic findings or symptomatic presentation. In yet another cohort of tethered cord patients (Guerra et al, 2006), 24 patients (10 male, 14 female) between the ages of 1 month and 12 years were treated for this condition. Diurnal incontinence was noted in 50% and constipation was noted in 42%. Incontinence resolved in all but 1 patient postoperatively with improvement in constipation in 4 of 10 patients with preoperative constipation. Forty-eight percent of children had resolution of urodynamic abnormalities postoperatively with 10 of 17 with detrusor overactivity normalizing and 7 remaining unchanged. Four patients with either diminished bladder capacity or low compliance did not improve postoperatively after intervention on urodynamics. Similarly, two of three patients with normal preoperative urodynamic evaluation developed detrusor overactivity and/or poor compliance postoperatively. They found there was no relationship between the level of conus on magnetic resonance imaging and predictive findings of urodynamics. The authors did raise concern regarding the potential for decompensation even after surgery.
Kessler and colleagues (2006) assessed 123 patients with myelomeningocele retrospectively on the basis of urodynamic patterns. The patients were classified into four categories: detrusor overactivity with overactive sphincter (43 patients), overactive detrusor and underactive or acontractile sphincter (37 patients), underactive acontractile detrusor with overactive spastic sphincter (8 patients), and underactive acontractile detrusor and underactive acontractile sphincter (35 patients). Patients were assessed for urinary incontinence and the need for adjunctive incontinence surgery between the four groups. At mean follow-up of 10 years, social continence was highest in groups one and three (86% and 87%, respectively) and lowest in groups two and four (57% and 74% respectively). There was statistical significance between interventions among the groups. The study concluded that initial urodynamics were useful in counseling families regarding the ultimate status of urinary incontinence in patients with myelomeningocele including rates of social continence.
Wyndaele (1997) correlated the neurologic and urodynamic data in 92 patients with SCI and came to the same general conclusion: Although there was a general correlation between the neurologic level of injury and the expected vesicourethral function, it was neither absolute nor specific, especially in the group of patients with paraplegia resulting from spinal cord lesions at column level T10 to L2. With reference to this latter group, Pesce and colleagues (1997) reported on 46 patients with complete SCIs from vertebral lesions between T11 and L2. Fifty percent of the patients had detrusor areflexia, and 50% had overactivity; 16 of the latter group also had striated sphincter dyssynergia. Of 22 patients with lesions above vertebral level L1, 8% showed areflexia and 14 showed overactivity, of whom 9 demonstrated sphincter dyssynergia. Of 9 patients with a lesion between T12 and L1, 3 showed detrusor areflexia and 6 overactivity, of whom 4 showed striated sphincter dyssynergia. Of 15 patients with a lesion at L1 or lower, 3% showed detrusor overactivity and striated sphincter dyssynergia.
Weld and Dmochowski (2000) agreed that the correlation between somatic neurologic findings or spinal imaging studies and urodynamic findings in patients with SCI is not exact, based on their review of 243 post-traumatic SCI patients who had complete spinal CT or MRI studies. It should be noted, however, that their correlation between level of injury and urodynamic findings was in fact better than those previously reported, most likely because of the more exact determination of the level of injury and the detection of multiple levels of injury, attributable to the precision of the radiologic imaging studies. Of 196 patients with suprasacral injuries, 94.9% demonstrated overactivity and/or striated sphincter dyssynergia, 41.8% had low bladder compliance (defined as 12.5 mL/cm H2O), and 40.3% had detrusor leak point pressures greater than 40 cm H2O. Of the 14 patients with sacral injuries, 85.7% demonstrated detrusor areflexia; 78.6%, low compliance; and 85.7%, high leak point pressures. Of 33 patients with combined suprasacral and sacral injuries, 68% demonstrated detrusor overactivity and/or detrusor dyssynergia; 27%, areflexia; 58%, low compliance; and 61%, high leak point pressures.
The degree and magnitude of SCI also impacts the overall management of these patients. Patki and colleagues (2006) assessed a group of 43 men and 21 women with incomplete SCI (ASA grades D and E) during a 2-year period. In this group, 40 patients who were initially assessed as having a bladder not at risk for deterioration ultimately experienced deterioration requiring clean, intermittent catheterization. Conversely, 5 of 20 patients, who initially required clean intermittent catheterization, no longer required this with time. In chronic follow-up, 68% of patients persisted with abnormal urodynamic findings, and 37% of patients required a change in urologic management in the absence of perceptible neurologic disease, indicating the potential of continued bladder changes in the absence of other detectable neurologic disease. Other factors such as underlying histology may also contribute to upper tract deterioration. Ozkan studied a group of patients undergoing augmentation for neurogenic detrusor overactivity, requiring augmentation cystoplasty. Patients underwent perioperative full-thickness bladder biopsies during the augmentation cystoplasty. A relationship between the degree of and severity of detrusor fibrosis was noted to be a significant risk factor of upper tract deterioration. Additionally, leak-point pressures of greater than 75 cm of water were also found to be consistent with upper tract deterioration (Ozkan et al, 2006).
All these data make the point, as cogently as possible, that management of the urinary tract in such patients must be based on urodynamic principles and findings rather than inferences from the neurologic history and evaluation. Similarly, although the information regarding “classic” complete lesions is for the most part valid, one should not make neurologic conclusions solely on the basis of urodynamic findings.
Autonomic Hyperreflexia
First described by Guttmann and Whitteridge in 1947, autonomic hyperreflexia (autonomic dysreflexia) is a potentially fatal emergency unique to the SCI patient. Excellent sources of information include the reviews by Trop and Bennett (1991), Vaidyanathan and colleagues (1998), and Karlsson (1999). Autonomic hyperreflexia represents an acute massive disordered autonomic (primarily sympathetic) response to specific stimuli in patients with SCI above the cord level of T6 to T8 (the sympathetic outflow). It is more common in cervical (60%) than thoracic (20%) injuries. Onset after injury is variable (usually soon after spinal shock) but may be up to years after injury. Distal cord viability is a prerequisite.
Symptomatically, autonomic hyperreflexia is a syndrome of exaggerated sympathetic activity in response to stimuli below the level of the lesion. The symptoms are pounding headache, hypertension, and flushing of the face and body above the level of the lesion with sweating. Bradycardia is a usual accompaniment, although tachycardia or arrhythmia may be present. Hypertension may be of varying severity, from causing a mild headache before the occurrence of voiding to life-threatening cerebral hemorrhage or seizure. The stimuli for this exaggerated response commonly arise from the bladder or rectum and generally involve distention, although other stimuli from these areas can be precipitating. Precipitation may be the result of simple lower urinary tract instrumentation, tube change, catheter obstruction, or clot retention; and, in such cases, the symptoms resolve quickly if the stimulus is withdrawn. Other causes or exacerbating factors may include other upper or lower urinary tract pathology (e.g., calculi), gastrointestinal pathology, long bone fracture, sexual activity, electrocoagulation, and decubiti.
Striated sphincter dyssynergia invariably occurs, and smooth sphincter dyssynergia is generally a part of the syndrome as well, at least in males. The pathophysiology is that of a nociceptive stimulation via afferent impulses that ascend through the cord and elicit reflex motor outflow, causing arteriolar, pilomotor, and pelvic visceral spasm and sweating. Normally, the reflexes would be inhibited by secondary output from the medulla, but because of the SCI this does not occur below the lesion level. Vaidyanathan and colleagues (1998) emphasized that the SCI disrupts control of the sympathetic preganglionic neurons because bulbospinal input has been lost, and the remaining regulation is accomplished by spinal circuits consisting of dorsal root afferent and spinal interneurons. Karlsson (1999), however, points out that the underlying pathogenic mechanisms may not be as simple as they first appear. The amplitude of the blood pressure reaction indicates involvement of a large vascular bed, perhaps larger than that of the skin and skeletal muscle. It may be that the splanchnic vascular bed is involved as well, either from the standpoint of active vasoconstriction or simply from a lack of the ability to exhibit compensatory vasodilatation. Afferent and efferent plasticity in the sympathetic nervous system may also be involved.
Ideally, any endoscopic procedure in susceptible patients should be done under spinal anesthesia or carefully monitored general anesthesia. Acutely, the hemodynamic effects of this syndrome may be managed with β- and/or α-adrenergic blocking agents. Ganglionic blockers had previously been the mainstay of treatment (Wein, 2002a), but their usage has essentially been abandoned. Sublingual nifedipine had been reported to be capable of alleviating this syndrome when given during cystoscopy (10 to 20 mg) and of preventing it when given orally 30 minutes before cystoscopy (10 mg) (Dykstra et al, 1987). The rationale was that this presumably prevented smooth muscle contraction through its calcium antagonist properties and thereby prevented the increase in peripheral vascular resistance normally seen with sympathetic stimulation. The use of sublingual nifedipine, however, has been prohibited in the authors’ particular medical center and doubtless in some others as well. It has been reported that the sublingual absorption of nifedipine is negligible and that the favorable therapeutic results obtained from such administration are probably caused by swallowing the drug (van Harten et al, 1987). These particular authors believe that if a fast onset of action of nifedipine is desired, the patient should be instructed to bite the capsule and swallow the contents with water, thereby rapidly achieving therapeutic plasma levels of the drug. Before electroejaculation, Steinberger and colleagues (1990) recommended oral prophylaxis with 20 mg of nifedipine, finding this markedly lowered pressure rises during treatment. Captopril, hydralazine, or diazoxide are still occasionally recommended but are less advantageous. Other rapidly acting agents have been reported to be beneficial. Many anesthesiologists recommend labetalol (Bycroft et al, 2005).
Chancellor and colleagues (1994) reported on the use of terazosin (a selective α1-adrenergic blocker) for long-term management (3-month study) and prophylaxis. A nightly dose of 5 mg reduced severity, whereas erectile function and blood pressure were unchanged. Vaidyanathan and colleagues (1998) affirmed the success of the prophylactic use of terazosin. They treated 18 adults with tetraplegia and 3 with paraplegia with graduated increasing doses of the drug, ultimately varying from 1 to 10 mg daily, and reported complete subsidence of dysreflexic symptoms in all patients. Only one patient, a tetraplegic, required discontinuation of the drug because of persistent dizziness. Such prophylaxis may be particularly important in view of the fact that significant elevations in blood pressure can occur without other symptoms of autonomic hyperreflexia (Linsenmeyer et al, 1996). Similar salubrious results have also been reported with prazosin as prophylaxis for this condition (Bycroft et al, 2005).
Prophylaxis, however, does not eliminate the need for careful monitoring during provocative procedures. There are patients with severe dysreflexia that is intractable to oral prophylaxis and correction by urologic procedures. For these unfortunate individuals, a number of neurologic ablative procedures have been used—sympathectomy, sacral neurectomy, sacral rhizotomy, cordectomy, and dorsal root ganglionectomy (Trop and Bennett, 1991). Hohenfellner and colleagues (2001) advocate sacral bladder denervation by sacral rhizotomy as a moderately invasive, relatively low-risk procedure that, along with intermittent catheterization, produces good results in refractory patients.
Vesicoureteral Reflux
Surprisingly little is written about VUR in the SCI patient. The reported incidence varies between 17% and 25% of such patients (Thomas and Lucas, 1990) and is more common in those with suprasacral SCI. Contributing factors include (1) elevated intravesical pressure during filling and emptying and (2) infection. Persistent reflux can lead to chronic renal damage and may be an important factor in the long-term survival of SCI patients. In the series of SCI patients reported by Hackler and colleagues (1965), persistent reflux was present in 60% of patients of those dying of renal disease. In patients with only transient reflux over a 5- to 15-year period, the urogram was normal in 83% and calyceal changes were only minimal. It should be noted, however, that high storage and voiding pressures without reflux can be responsible for renal damage (McGuire, 1984; Vega and Pascual, 2001). Ku and colleagues (2005) studied 179 men undergoing annual follow-up for SCI. The average duration of follow-up after injury was 29 years. The incidence of reflux in this group was 15.1%. Thirty-four percent of the patients were diagnosed with pyelonephritis at some point during their course of follow-up, and 24.6% developed renal stones. Thirty-two percent experienced upper tract deterioration as manifested by hydronephrosis. Upper tract degeneration was more prevalent in those managed with urethral catheters (51%) than in those managed with either spontaneous voiding or intermittent catheterization. Urethral catheter drainage was found to be inferior to all other forms of bladder drainage in terms of protection of the upper urinary tract, suggesting the inadvisability of urethral catheterization chronically in these patients (Ku et al, 2005).
The best initial treatment for reflux in a patient with voiding dysfunction secondary to neurologic disease or injury is to normalize lower urinary tract urodynamics as much as possible. Depending on the clinical circumstances, this may be by pharmacotherapy, urethral dilatation (in the myelomeningocele patient), neuromodulation, deafferentiation, augmentation cystoplasty, or sphincterotomy (Flood et al, 1994; Perkash et al, 1998). If this fails, the question of whether to operate on such patients for correction of the reflux or to correct the reflux while performing another procedure (e.g., augmentation cystoplasty) is not an easy one because correction of reflux in an often thickened bladder may not be an easy task.
Transureteroureterostomy for unilateral reflux is feasible, but even experienced urologists have had difficulties with ureteral calculi trapping, recurrent reflux, and obstruction at the vesicoureteral junction following such procedures in this difficult group of patients (Van Arsdalen and Hackler, 1983). Submucosal trigonal injection of bulking substances may add a new dimension to the treatment of this difficult problem.
One must remember the potential artifact that significant reflux can introduce into urodynamic studies. Measured bladder capacity appears artifactually large, and measured pressures at given inflow volumes may appear lower than after reflux correction. The apparent significance of detrusor overactivity may thus be underestimated.
Urinary Tract Infection
Urinary tract infection (UTI) is relatively common in patients with SCI. Fifty-seven percent of patients with SCI experience UTI or bacteriuria in the first year after initial hospitalization (Morton et al, 2002). Recurrent infections may be a manifestation of upper or lower tract calculi, symptomatic or silent pyelonephritis, or lower urinary tract dysfunction causing persistent residual urine. In conjunction with poor urodynamic function, UTI can lead to high morbidity, poor quality of life, and decreased life expectancy in patients with SCI (Sauerwein, 2002). The use of antibiotics in SCI patients remains a topic of discussion. Virtually all who have written on the subject agree that bacteriuria should be treated only when signs or symptoms present, a consensus reached by the National Institute on Disability and Rehabilitation Research Group (1992) (Penders et al, 2003). Biering-Sorensen and colleagues (2001) authored a nicely organized, well-written, and referenced article on the subject. They recommended the following: (1) treat bacteriuria only if symptomatic; (2) use antimicrobial agents, if possible, with little or no impact on normal flora; (3) treat at least 5 days; those with reinfection or relapse, treat 7 to 14 days; (4) repair structural and functional risk factors; (5) use prophylaxis only in those with recurrent UTI when no underlying cause can be found and especially if the upper tracts are dilated; (6) do not use antibiotics to prevent UTI in patients with an indwelling catheter. They regard the use of prophylactic antibiotics in patients on CIC as controversial.
Morton and colleagues (2002) concluded from a meta-analysis of 15 controlled trials that prophylactic antibiotics were not generally helpful but added that they could not exclude a clinically important effect, especially in those who had recurrent UTI that limited their functioning and well-being. Sauerwein (2002) recommends beginning antibiotic prophylaxis in patients on CIC and stopping after 1 year if there has been one or fewer UTIs. Some (Burns et al, 2001) believe that significant pyuria, defined as 8 to 10 or more white blood cells per high-power field, connotes tissue invasion and is an indication for antibiotic treatment. Evidence-based data in this area are sparse.
Spinal Cord Injury in Women
There are many aspects of management of the lower urinary tract affected by SCI that are specific to women (Yang and Cardenas, 2001). McColl (2002) summarized some of these. The problem of osteoporosis places women at a much increased risk of fracture. The symptoms of menopause (e.g., hot flashes) may be difficult to distinguish from those of autonomic dysreflexia. Incontinence and UTI become worse with age in women in the general population and particularly in those with SCI. Body composition of SCI women shows deficient protein and bone mass and excess fat, predisposing them to an increased incidence of fractures and the risk of skin breakdown. The incidence of SCI is highest among young men and older women. Psychologic outcomes (e.g., depression) have been shown to improve after 30 years’ duration of disability. Women injured at a modal age of 60 are unlikely to enjoy this benefit.
Special difficulty in this category of patients is encountered because of the lack of an appropriate external collecting device. Suitable bladder reservoir function can usually be achieved either pharmacologically or surgically, and paraplegic women can generally master CIC. Although a few quadriplegic women can be trained to self-CIC, for the majority there is no practical alternative to indwelling catheterization (Lindan et al, 1987). McGuire and Savastano (1986) point out that indwelling catheter drainage on a long-term basis in women may not be, however, as is sometimes written, well tolerated because significant incontinence around the catheter and upper tract changes may develop.
For those SCI female patients who can assume CIC or who have around-the-clock medical or family care, creation of adequate bladder reservoir function is reasonable. For those not in this category, the alternatives are limited and difficult. Bennett and colleagues (1995) compared the incidence of major complications in a group of female SCI patients who were managed long term by (1) CIC; (2) reflex voiding and incontinence padding; and (3) an indwelling catheter. There were 10 major complications in the 25 patients in group 2 and 58 in the 22 patients in group 3, compared with only 4 major complications in group 1. Singh and Thomas (1997) looked at the results of treatment in a group of female tetraplegics. Twenty-three of 27 patients with complete lesions wound up using an indwelling catheter, 3 underwent diversion, and in 1 patient the caregiver performed CIC. In 20 patients with incomplete lesions, all with poor functional recovery, 14 had permanent indwelling catheters, 3 were able to perform CIC, and 3 used reflex voiding by triggering. Of 37 patients with incomplete lesions with good functional recovery, only 3 required indwelling catheterization; 4 used CIC, and most were able to use reflex voiding by triggering. The authors noted also that 55% of the women with permanent catheters had bladder calculi, 35% had leakage around the catheter, and 33% had recurrent symptomatic infection. Although upper tract changes were seen in only 5%, it is obvious that the authors consider that, for the most part, female patients with voiding dysfunction secondary to cervical SCI who exhibit poor functional recovery represent urologic failures of management.
Spinal Cord Injury and Bladder Cancer
The development of carcinoma of the bladder in 6 of 59 patients with SCIs who had long-term indwelling catheters was reported by Kaufman and colleagues (1977). All were squamous cell lesions. Four of these patients had no obvious tumors visible at endoscopy, and the diagnosis was made by bladder biopsy. Five of these patients also had transitional cell elements in their tumor. Broecker and colleagues (1981) surveyed 81 consecutive SCI patients with an indwelling urinary catheter for more than 10 years. Although the investigators did not find frank carcinoma in any patients, they found squamous metaplasia of the bladder in 11 and leukoplakia in 1. Locke and colleagues (1985) noted two cases of squamous cell carcinoma of the bladder in 25 consecutive SCI patients catheterized for a minimum of 10 years. Bickel and colleagues (1991) reported eight cases of bladder cancer in SCI men, although the denominator was uncertain. Four of the men had been managed by indwelling catheterization for 7, 10, 14, and 19 years, respectively. All of these four men had transitional cell carcinoma, whereas in the other four, there were two cases of transitional and two of squamous cell carcinoma. In Chao and colleagues’ series (1993), 6 patients developed bladder cancer, 3 of whom had indwelling catheters (of a total of 32) and 3 of whom (of 41) did not. Stonehill and colleagues (1996) retrospectively reviewed all bladder tumors in their SCI patients for 7 years and compared these with matched controls. They found 17 malignant and 2 benign bladder tumors. Indwelling catheters and a history of bladder calculi were statistically significant risk factors.
Hess and colleagues (2003) reported their own series and summarized one view of the literature regarding SCI and bladder cancer: (1) the relative risk in SCI patients is 16% to 28% greater than the general population; (2) the overall incidence is 2.3% to 10%; (3) there is a higher proportion of squamous cell than transitional cell carcinoma; (4) the prevalence peaks at an earlier age than in the general population; (5) diagnosis is made at a more advanced stage; (6) risk factors include chronic indwelling catheterization, bladder stones, and chronic UTI; (7) neither cystoscopy nor cytology is an entirely reliable diagnostic tool; and (8) those with multiple risk factors should have a more aggressive evaluation.
Tempering this are reports by Pannek (2002), who reviewed the data from 43,561 SCI patients in three countries and concluded the incidence of bladder cancer is comparable with that of the general population, but more than 60% of those affected presented initially with muscle invasive disease. Chronic indwelling catheters and persistent or recurrent UTI are suggested as the risk factors rather than the SCI itself. Subramonian and colleagues (2004) reported similar conclusions regarding age-standardized incidence of bladder cancer relative to the general population. Seventy-five percent of the affected patients in the series had indwelling catheters for 18 to 32 years.
The incidence of bladder cancer in MS patients with indwelling catheters is estimated at 0.29%, as compared with 0.004% in the general population of females and 0.018% in the population of males. The incidence of bladder cancer in SCI patients has been recorded to range from 0.27% to 9.6%; however, in larger series, the overall incidence is 0.27% to 0.37%. The natural history of bladder cancer is thought to be more highly aggressive in neurogenic patients and is responsible for 0.3% to 2.8% of known deaths in the SCI population.
Follow-Up
Linsenmeyer and Culkin (1999) reported the American Paraplegic Society (APS) Guidelines for urologic care of SCI. Annual follow-up is recommended for the first 5 to 10 years after injury. If the patient is doing well, then follow-up every other year is advised. Upper and lower tract evaluation should be done initially and yearly for 5 to 10 years, then every other year. Burns and colleagues (2001) recommended at least plain films and nuclear renal scans, with a decrease of more than 20% in renal plasma flow warranting further investigation. Urodynamic evaluation was recommended by the APS at the same intervals as upper and lower tract screening. Cystoscopy was recommended annually in those with an indwelling catheter.
Cervical Myelopathy
Cervical myelopathy is generally caused by compression, secondary to either spondylosis, ossification of the posterior longitudinal ligament, or cervical disk herniation (Sakakibara et al, 1995a; Mochida et al, 1996). Sakakibara and colleagues (1995a) studied 128 affected patients, of whom 95 had voiding symptoms, 61 had irritative symptoms, 71 had obstructive symptoms, and 25 had urinary incontinence. Urodynamic studies revealed involuntary bladder contractions in 61 patients and detrusor sphincter dyssynergia (DESD) in 22. Mochida and colleagues (1996), on the other hand, reported on 60 patients undergoing surgery for cervical myelopathy, 22 of whom (37%) were found to have neuropathic bladder dysfunction on urodynamic study. Of these, 9 (41%) were found to have detrusor overactivity but 13 (59%) were characterized as having an underactive detrusor. Because this is at odds with what one would expect with only cervical spinal cord pathology, it reinforces the need for urodynamic study to optimally guide therapy in patients with neurogenic bladder.
Acute Transverse Myelitis
Acute transverse myelitis is a rapidly developing condition with motor, sensory, and sphincter abnormalities, generally with a well-defined upper sensory limit and no signs of spinal cord compression or other neurologic disease (Kalita et al, 2002). It may result from a variety of mechanisms—parainfectious, autoimmune, vascular, or demyelinating (Ganeson and Borzyskowski, 2001). The condition usually stabilizes within 2 to 4 weeks, is not progressive afterward, and is followed by variable recovery with some residual neurologic deficits. Although recovery is more variable, and the prognosis, in general, is more favorable, the development and nature of voiding dysfunction has been reported to be similar, level by level, to that of SCI (Sakakibara et al, 1995b). Kalita and colleagues (2002), however, reported on 18 patients with acute transverse myelitis whose 6-month outcome included persistent retention in 6 and storage symptoms in 10, of whom 5 had emptying problems as well; 2 patients had regained normal voiding. In the acute state, urodynamics showed an areflexic or contractile bladder in 10, detrusor overactivity with poor compliance in 2, and DESD in 3. Seventeen had presented in urinary retention. As in SCI, because the activity of the bladder and outlet during filling/storage and emptying/voiding does not always correspond to what they “should” be, based on the level of pathology, urodynamic studies are necessary to guide irreversible therapy.
Neurospinal Dysraphism
These topics are covered primarily in Chapters 127 and 128. However, certain considerations regarding the adult with these abnormalities should be mentioned. Spinal dysraphism refers to the malformation of the vertebral arches and, commonly, malformation of the neural tube. The term includes spina bifida occulta, which involves only a bony (vertebral) arch defect, and spina bifida cystica (aperta), which involves a bony defect and a neural tube (spinal cord) defect. The two primary subclasses of spina bifida cystica are myelomeningocele (the nerve roots or portions of the spinal cord have evaginated beyond the vertebral bodies) and meningoceles (contain only a herniated meningeal sac with no neural elements). If fatty tissue is present in the sac in either, the prefix “lipo-” is added (Churchill et al, 2001). Myelomeningocele accounts for more than 90% of spina bifida cystica and is the most devastating condition in terms of sequelae. Of myelomeningoceles, 2% are cervical, 5% thoracic, 26% lumbar, 47% lumbosacral, and 20% sacral. The level(s) of the lesion correlates poorly with urodynamic findings (Churchill et al, 2001). Myelomeningocele occurs in approximately 1/1000 live births (Wyndaele et al, 2005). The incidence of lower urinary tract dysfunction is not absolutely documented, but most studies suggest an incidence of greater than 90% (Wyndaele et al, 2005).
McGuire and Denil (1991) and Woodhouse (2005) point out that, secondary to progress in the overall care of children with myelodysplasia, urologic dysfunction often becomes a problem of the adolescent or adult with this disease. In McGuire and Denil’s (1991) experience, the “typical” myelodysplastic patient shows an areflexic bladder with an open bladder neck. The bladder generally fills until the resting residual fixed external sphincter pressure is reached, and then leakage occurs. Stress incontinence occurs also, related to changes in intra-abdominal pressure. A small percentage (10% to 15%) of patients demonstrate detrusor striated sphincter dyssynergia, but these individuals show normal bladder neck function that, if detrusor reflex activity is controlled, is associated with continence. After puberty, most authors report that most myelodysplastic patients note an improvement in continence, but, at that age and after, they are less inclined than children to tolerate any degree of incontinence. In adult patients, the problems encountered in myelodysplastic children still exist but are often compounded by prior surgery, upper tract dysfunction, and one form of urinary diversion or another.
In adult females, the treatment strategy is generally to increase urethral sphincter efficiency without causing a major enough increase in urethral closing pressure that will result in a change in bladder compliance (McGuire and Denil, 1991). Periurethral injection therapy to achieve continence may replace the pubovaginal sling and artificial sphincter in this circumstance. These authors also point out that continence in adult male myelodysplastic individuals follows the same general rules as in females, and injectable materials may give good results in this group as well. When the urethra is widely dilated and somewhat rigid, and neither procedure alone will provide sufficient coaptation, it may be possible to combine a “prostatic sling” with periurethral collagen injection. Dry individuals, of course, will be on intermittent self-catheterization.
“Classic” may imply different urodynamic findings to different groups of clinicians. Nowhere is the failure of a neurologic examination to predict urodynamic behavior more obvious than in patients with myelomeningocele.
Van Gool and colleagues (2001) summarized the urodynamic findings in 188 children with myelomeningocele and categorized these into five groups. Seven percent had normal detrusor and sphincter activity; 11% had detrusor overactivity and an inactive sphincter; 45% had detrusor overactivity and an overactive sphincter; 23% had an inactive detrusor and inactive sphincter; and 14% had an inactive detrusor and an overactive sphincter. Sakakibara and colleagues (2003a) reported on urodynamic results in 16 adult patients with myelomeningocele. Detrusor overactivity was found in 38%, low compliance in 81%, impaired bladder sensation in 25%, DESD in 50%, low maximum urethral pressure in 56%, and silent sphincter electromyogram in 25%. Webster and colleagues (1986) urodynamically classified a large number of myelomeningocele patients as follows: 62% had detrusor overactivity, whereas 38% had detrusor areflexia, with 30 of 34 of these having low compliance with high terminal filling pressure. Striated sphincter behavior was characterized as follows: true detrusor–striated sphincter dyssynergia in 15%, an apparently innervated but fixed nonrelaxing sphincter in 15%, and some evidence of striated sphincter denervation in 69%.
It is clear, however, that whatever the pattern of voiding dysfunction in the adult, all authors would agree that a prime directive of therapy is still the avoidance of high storage pressures (McGuire and Denil, 1991; Persun et al, 1999; Woodhouse, 2005).
Voiding dysfunction secondary to occult spinal dysraphism may not present in childhood, and such patients may be referred as adults for symptoms as mundane as incontinence or recurrent urinary infection. Jakobsen and colleagues (1985) reported seven such patients ranging in age from 10 to 51 years. Delayed diagnosis of such voiding dysfunction was also reported by Yip and colleagues (1985). There was no characteristic abnormality, and the specific dysfunction is dependent on the level and extent of the neurologic injury.
The urologic rehabilitation of patients with spinal dysraphism relies primarily on medical management with the selective use of augmentation or urinary diversion after failure thereof. However, surgery does not necessarily yield superior results. In a review of 421 patients managed for spina bifida, 45% were medically treated, either with intermittent catheterization, spontaneous voiding, or no specific method utilized. Two-hundred and thirty patients were surgically treated. Overall incontinence episodes were higher in the surgical versus medical management group. These results may have been reflective of the aggressiveness of management, as well as the severity of disease (Lemelle et al, 2006).
Recently, neural rerouting has been proposed as a potential option for some of these individuals. Ziao and colleagues have performed microanastomosis of the fifth lumbar ventral root to the third sacral ventral root in order to bypass low-level spina bifida injury. Initial improvements in bladder compliance and urinary incontinence were noted in patients and paralleled similar findings in spinal cord patients (Joseph, 2005).
Tethered cord syndrome (TCS) is defined as a stretch-induced functional disorder of the spinal cord with its caudal part anchored by inelastic structures. Vertical movement is restricted. The anchoring structures can include scar from prior surgery, fibrous or fibroadipose filum terminale, a bony septum, or tumor (Yamada et al, 2004a, 2004b). Adults with TCS can be divided into those with a prior history of spinal dysraphism with a previously stabilized neurologic status who present with subtle progression in adulthood and those without associated spinal dysraphism who present with new subtle neurologic symptoms (Yamada et al, 2004a, 2004b). Symptoms can include back pain, leg weakness, foot deformity, scoliosis, sensory loss, and bowel or lower urinary tract dysfunction (Phuong et al, 2002). TCS is reported to occur in 3% to 15% of patients with myelomeningocele. There is no typical dysfunction in TCS, and treatment must be based on contemporary urodynamic evaluation. Voiding dysfunction may not be present until the teenage years or later (Kaplan and Blaivas, 1988; Husmann, 1995). Giddens and colleagues (1999) point out that, whereas children often develop symptoms of tethered cord after growth spurts, in adults presenting symptomatology often follows activities that stretch the spine such as sports or motor vehicle accidents. In adults, urologic presentation can include irritative voiding symptoms, incontinence, or retention. These authors reported neurourologic and urodynamic findings in a group of adult patients. At presentation, urgency (67%) and urge incontinence (50%) were the most common findings. Pretreatment urodynamics in 18 patients revealed detrusor overactivity in 13 (72%), DESD in 4 (22%), decreased sensation in 4 (22%), decreased compliance in 3 (17%), and what was called a “hypocontractile” detrusor in 2 (11%). It is interesting that postoperative urodynamic findings were improved in only 4 patients (29%) and unchanged in 10 (71%). Steinbok and colleagues (2007) assessed eight children undergoing section of the filum inducing the tethered cord and compared them with seven children who had abnormal urodynamic findings and did not undergo filum release. Clinical improvement occurred in seven of the eight children at a mean follow-up of 3 years with improved urodynamics in four of seven children tested after surgery. These improvements were also associated with nonurologic functional improvements (motor leg function). At a mean follow-up of 3 years in the nonsurgical group, two patients had urologic improvement but three patients required surgical intervention and five had persistence of nonurologic symptoms. Section of the cord appeared to improve function as compared with conservative management (Steinbok et al, 2007).
Tabes Dorsalis, Pernicious Anemia
Although syphilitic myelopathy is disappearing as a major neurologic problem, involvement of the spinal cord dorsal columns and posterior sacral roots can result in a loss of bladder sensation and large residual urine volumes and therefore be a cause of “sensory neurogenic bladder” (see Chapter 61). Although this represents the “classic” tabetic bladder (Wheeler et al, 1986), Hattori and colleagues (1990) reported on some patients with only tabes as an obvious cause of their voiding dysfunction who had low compliance or detrusor overactivity. Another spinal cord cause of the classic “sensory bladder” is the now uncommon pernicious anemia that produced this disorder by virtue of subacute combined degeneration of the dorsolateral columns of the spinal cord.
Poliomyelitis
When seen, voiding dysfunction in polio is that of a typical “motor neurogenic bladder” (see Chapter 61) with urinary retention, detrusor areflexia, and intact sensation. The reported incidence of voiding dysfunction in patients with polio was described as ranging from 4% to 42% by Bors and Comarr (1971).
Disease Distal to the Spinal Cord
Disk Disease
Goldman and Appell (2000a) nicely summarize the anatomic and neurologic considerations applicable to voiding dysfunction from lumbar disk disease. In the adult, the sacral segments of the spinal cord are at the level of the L1 and L2 vertebral bodies. In this distal end of the spinal cord, commonly called the conus medullaris, the spinal cord segments are named for the vertebral body at which the nerve roots exit the spinal canal. Thus although the sacral spinal cord segment is located at vertebral segment L1, its nerve roots run in the subarachnoid space posterior to the L2 to L5 vertebral bodies until reaching the S1 vertebral body, at which point they exit the canal. Thus all of the sacral nerves that originate at the L1 and L2 spinal column levels run posterior to the lumbar vertebral bodies until they reach their appropriate site of exit from the spinal canal. This group of nerve roots running at the distal end of the spinal cord is commonly referred to as the cauda equina.
Usually, disk prolapse is in a posterolateral direction, which does not affect the majority of the cauda equina. However, in 1% to 15% of the cases (Goldman and Appell, 2000a), central disk prolapse occurs and compression of the cauda equina may result. Thus disk prolapse anywhere in the lumbar spine could interfere with the parasympathetic and somatic innervation of the lower urinary tract, striated sphincter and other pelvic floor musculature, and afferent activity from the bladder and affected somatic segments to the spinal cord. Most disk protrusions compress the spinal roots in the L4-L5 or L5-S1 vertebral interspaces. Voiding dysfunction may result and, when present, generally occurs with the usual clinical manifestations of low back pain radiating in a girdle-like fashion along the involved spinal root areas. Examination may reveal reflex and sensory loss consistent with nerve root compression. The most characteristic findings on physical examination are sensory loss in the perineum or perianal area (S2-S4 dermatomes), sensory loss on the lateral foot (S1-S2 dermatomes), or both.
Goldman and Appell (2000a) also reviewed the literature on voiding dysfunction associated with lumbar disk disease. The incidence of such dysfunction in their review ranged from 27% to 92%. The true incidence is a bit difficult to ascertain because many reported series on this subject describe findings only in patients who present with voiding dysfunction. Bartolin and colleagues (1998) reported on findings in 114 patients with lumbar disk protrusion who were prospectively studied. They found detrusor areflexia in 31 (27.2%) and normal detrusor activity in the remaining 83. All 31 patients with detrusor areflexia reported difficulty voiding with straining. Patients with voiding dysfunction generally presented with these symptoms or in urinary retention. The most consistent urodynamic finding was that of a normally compliant areflexic bladder associated with normal innervation or findings of incomplete denervation of the perineal floor musculature. In a later report, Bartolin and colleagues (2002) describe findings in 122 patients with lumbar disk protrusion. Detrusor areflexia was found in 32 (26%) and normal bladder urodynamic findings in 90 (74%). All with areflexia complained of difficulty voiding; 8 could not void at all, 14 had an interrupted flow, and 10 had a continuous but low flow. Occasionally, patients may show detrusor overactivity, attributed to irritation of the nerve roots (O’Flynn et al, 1992).
The detrusor areflexia associated with lumbar disk protrusion shows a lower incidence of decreased compliance than in the voiding dysfunction caused by myelomeningocele. Sandri and colleagues (1987) offered two possible explanations for this difference: (1) the effect of the disk represents a more incomplete lesion of the preganglionic parasympathetic fibers and (2) the lesion is more sensory than motor, implying that the decreased compliance seen with the type of neural lesion in myelomeningocele is primarily caused by injury of the preganglionic parasympathetic motor fibers to the bladder.
Laminectomy may not improve bladder function, and prelaminectomy urodynamic evaluation is therefore desirable because it may be difficult postoperatively in these cases to separate causation of voiding dysfunction owing to the disk sequelae from changes secondary to the surgery. Bartolin and colleagues (1999) reported on the results of surgery in a group of patients with lumbar disk protrusion. Of 27 patients with detrusor areflexia preoperatively, detrusor activity returned to normal in only 6. The patients were studied up to a year after surgery. Of the 71 patients with normal urodynamic findings preoperatively, 4 developed detrusor overactivity and 3 developed detrusor areflexia postoperatively. The medicolegal implications of a presurgical and postsurgical urodynamic evaluation are obvious.
Cauda equina syndrome is a term applied to the clinical picture of perineal sensory loss with loss of voluntary control of both anal and urethral sphincter and of sexual responsiveness. This can occur not only secondary to disk disease (severe central posterior disk protrusion) but to other spinal canal pathologic processes as well. Yamanishi and colleagues (2003) place the incidence of cauda equina syndrome at 1% to 5% of all prolapsed lumbar disks. They reported on eight patients undergoing emergency corrective surgery for this entity. All had an acontractile detrusor with no bladder sensation, and four of seven had an inactive sphincter electromyogram. Follow-up urodynamics showed that all continued with an acontractile detrusor, three had normal EMG activity, and three had EMG activity but with denervation potentials in two and low activity in two.
Spinal Stenosis
Spinal stenosis is a term applied to any narrowing of the spinal canal, nerve root canals, or intervertebral foramina. It may be congenital, developmental, or acquired. Compression of the nerve roots or cord by such a problem may lead to neuronal damage, ischemia, or edema. Spinal stenosis may occur without disk prolapse. Symptoms may range from those consequent to cervical spinal cord compression to a cauda equina syndrome, with corresponding urodynamic findings (Smith and Woodside, 1988). Back and lower extremity pain, cramping, and paresthesias related to exercise and relieved by rest are the classic symptoms of lumbar stenosis caused by lumbar spondylosis and are believed to result from a sacral nerve root ischemia. The urodynamic findings are dependent on the level and the amount of spinal cord or nerve root damage. Deen and colleagues (1994) reported subjective improvement in more than 50% of such patients with bladder dysfunction who were treated by decompressive laminectomy. In cervical spondylitic spinal stenosis, detrusor overactivity or underactivity may occur, depending on whether the primary pathologic process affecting the micturition neural axis is compression of the inhibitory reticulospinal tracts or myelopathy in the posterior funiculus, which carries proprioceptive sensation (Tammela et al, 1992). Because there is no consistent pattern of dysfunction with any type of spinal stenosis, urodynamic studies again are the cornerstone of rational therapy. Podnar and colleagues (2006) assessed 65 cauda equina patients with neuro-urologic examination, electromyography, and urodynamics. Severe LUT dysfunction was noted in 14% of women and 15% of men, whereas moderate dysfunction was noted in 27% of men and 46% of women. Incomplete emptying was the most common symptom (>90%), and urinary incontinence was next with 56% of men and 71% of women. Reduced capacity was noted in 9% and 15%, respectively. Poor detrusor contractility was noted in 59% of men and 85% of women. Using multiple linear regression, perianal sensory loss and female gender had the most significant positive predictive value for urinary incontinence.
Radical Pelvic Surgery
Voiding dysfunction after pelvic plexus injury occurs most commonly after abdominoperineal resection and radical hysterectomy. The true incidence of neurogenic vesicourethral dysfunction after various types of pelvic surgery is unknown because there are few prospectively studied series of patients with preoperative and postoperative urodynamic evaluation. The incidence has been estimated to range from 20% to 68% of patients after abdominoperineal resection, 16% to 80% after radical hysterectomy, 20% to 25% after anterior resection, and 10% to 20% after proctocolectomy (Blaivas and Chancellor, 1995b). These are estimates drawn from past literature, and the current incidence is most likely significantly lower owing to the use of nerve-sparing techniques during these types of pelvic surgery. It has been estimated, however, that in 15% to 20% of affected individuals, the voiding dysfunction is permanent (McGuire, 1984; Mundy, 1984). The injury may occur consequent to denervation or neurologic decentralization, tethering of the nerves or encasement in scar, direct bladder or urethral trauma, or bladder devascularization. Adjuvant treatment such as chemotherapy or irradiation may play a role as well. The type of voiding dysfunction that occurs is dependent on the specific nerves involved, the degree of injury, and any pattern of reinnervation or altered innervation that results over time (see Chapter 60 and the previous section on neuroplasticity).
Literature on the effects of parasympathetic decentralization on neuromorphology and neuropharmacology of the lower urinary tract in many animal models is abundant (Wein and Barrett, 1988). Parasympathetic decentralization has been reported to lead to a marked increase in adrenergic innervation of the bladder in some experimental models, with the resultant conversion of the usual β (relaxant) response of the bladder body in response to sympathetic stimulation to an α (contractile) effect (Sundin et al, 1977). Hanno and colleagues (1988) confirmed that, in the cat model, parasympathetic decentralization does result in adrenergic hyperinnervation of the detrusor but that pelvic plexus neurectomy alone or parasympathetic decentralization plus hypogastric neurectomy yields no detectable increase in adrenergic innervation. In their experimental model, decentralization did result in synaptic reorganization in bladder wall ganglia with new cholinergic excitatory inputs from the hypogastric nerves. Koyanagi was the first to call attention to what he referred to as supersensitivity of the urethra to α-adrenergic stimulation in a similar group of patients with neurologic decentralization of the lower urinary tract, implying a similar change in adrenergic receptor function in the urethra after parasympathetic decentralization (Koyanagi et al, 1988). Nordling and colleagues (1981) described a similar change in females after radical hysterectomy and ascribed this change to damage to the sympathetic innervation of the lower urinary tract.
When permanent voiding dysfunction occurs after radical pelvic surgery, the pattern is generally one of a failure of voluntary bladder contraction, or impaired bladder contractility, with obstruction by what seems urodynamically to be residual fixed striated sphincter tone, which is not subject to voluntarily induced relaxation. Often, the smooth sphincter area is open and nonfunctional. Whether this appearance of the bladder neck/proximal urethra is caused by parasympathetic damage or terminal sympathetic damage or whether it results from the hydrodynamic effects of obstruction at the level of the striated sphincter is debated and unknown. Decreased compliance is common in these patients, and this, with the “obstruction” caused by fixed residual striated sphincter tone, results in both storage and emptying failure. These patients often experience leaking across the distal sphincter area and, in addition, are unable to empty the bladder because although intravesical pressure may be increased, there is nothing that approximates a true bladder contraction. The patient often presents with urinary incontinence that is characteristically most manifest with increases in intra-abdominal pressure. This is usually most obvious in females because the prostatic bulk in males often masks an equivalent deficit in urethral closure function. Alternatively, patients may present with variable degrees of urinary retention.
Urodynamic studies may show decreased compliance, poor proximal urethral closure function, loss of voluntary control of the striated sphincter, and a positive bethanechol supersensitivity test, findings similar to those in Figure 65–4. Upper tract risk factors are related to intravesical pressure and the detrusor leak point pressure, and the therapeutic goal is always low-pressure storage with periodic emptying. The temptation to perform a prostatectomy should be avoided unless a clear demonstration of outlet obstruction at this level is possible. Otherwise, prostatectomy simply decreases urethral sphincter function and thereby may result in the occurrence or worsening of sphincteric urinary incontinence. Most of these dysfunctions will be transient, and the temptation to “do something” other than perform CIC initially after surgery in these patients, especially in those with little or no preexistent history of voiding dysfunction, cannot be too strongly criticized. Our general practice in such patients is to discharge them on CIC and then have them return for a full urodynamic evaluation at a later date. Six to 12 months may elapse before detrusor function returns to an acceptable level (Blaivas and Chancellor, 1995b). Many of the changes after radical pelvic surgery are similar to those seen in sacral cord injury or disease. Sislow and Mayo (1990), in an excellent study on decreased bladder compliance after decentralization, noted a higher prevalence of this finding in patients who had undergone radical pelvic surgery than in those who had sustained conus medullaris or cauda equina injury.
Finally, to answer questions as to whether some types of nonradical pelvic surgery such as simple hysterectomy are ultimately responsible for filling/storage or emptying abnormalities on the basis of neurologic damage, more series that include sophisticated preoperative and early and late postoperative urodynamic evaluation are necessary.
Herpesvirus Infections
Invasion of the sacral dorsal root ganglia and posterior nerve roots with herpes zoster virus may produce urinary retention and detrusor areflexia days to weeks after the other primary viral manifestations (Ryttov et al, 1985). Generally, painful cutaneous eruptions secondary to the virus are also present, but initially there may be just fever and malaise with perineal and thigh paresthesias and obstipation. Urinary incontinence secondary to detrusor overactivity may also occur, but the pathophysiology is uncertain. It may be related to nerve root irritation, inflammation of the meninges or spinal cord, or “zoster cystitis” (Broseta et al, 1993). Cystoscopy may reveal vesicles in the bladder mucosa similar to those seen on the skin. Spontaneous resolution generally occurs in 1 to 2 months. Broseta and colleagues (1993) reported on 57 patients with herpes zoster infection whose records were reviewed retrospectively. Fifteen (26%) of these patients showed urologic manifestations, but in only two of these did frank urinary retention occur. Three patients demonstrated urinary incontinence; urodynamically, all of those showed involuntary bladder contractions. Ten patients demonstrated irritative voiding symptoms with dysuria and frequency.
Chen and colleagues (2002) reported on the incidence of voiding dysfunction in 423 patients admitted with a diagnosis of herpes zoster. Seventeen (4%) had voiding dysfunction. Excluding those with cranial rather than spinal nerve involvement, the incidence was 8.8%; when only lumbosacral dermatome patients were considered, the figure rose to 28.6%. The authors subdivided the bladder disorders into three types. The most common is associated with a herpetic cystitis. Patients in this group may present with dysuria, frequency, retention, pyuria, or hematuria. Twelve of the 17 affected patients (71%) were in this group; they presented with dysuria and frequency or retention. The second type is neuritis associated, presumably affecting the sacral motor neurons. Four of the 17 (24%) were in this group and presented in urinary retention and with a “flaccid bladder.” Myelitis-associated voiding dysfunction is the third type, associated with spinal cord involvement and presenting with detrusor overactivity. One of their patients was in this group. All patients regained a normal or “balanced bladder” within 8 weeks, and no major urologic sequelae were noted.
Urinary retention has also been reported to occur in association with anogenital herpes simplex virus infection. Caplan and colleagues (1977) reported 11 such patients with the typical clinical picture of herpes genitalis, all of whom developed urinary retention 2 to 7 days after the genital eruption. Hemrika and colleagues (1986) reported three such patients, each of which showed pleocytosis of the cerebrospinal fluid, a finding that they believed was indicative of central nervous system involvement. They termed the coexistence of bilateral involvement of the sacral nerve roots of rapid onset accompanied by sphincteric incontinence with cerebrospinal fluid pleocytosis the Elsberg syndrome and tabulated 47 such cases reported before their article. Haanpaa and Paavonen (2004) added two cases, in which both patients had transient urinary retention but developed chronic neuropathic pain in the sacral area. The lower urinary tract dysfunction was transient, as with herpes zoster.
Diabetes Mellitus
Diabetes is the most common cause of peripheral neuropathy in Europe and North America. The exact prevalence of diabetes in the United States is somewhere between 1% and 6%, depending on whether one includes only diagnosed patients or diagnosed and undiagnosed patients and on what fasting blood glucose criteria one uses for inclusion (the higher estimate of prevalence represents a recent reduction in blood glucose criteria to 126 mg/dL) (Chancellor and Blaivas, 1995a; Goldman and Appell, 2000b). The clinical spectrum of voiding dysfunction thought to be caused by diabetes has been well reviewed by a number of authors, each of whom has cited the same historical articles up to their date of publication and added their personal experience at that time (Kaplan and Blaivas, 1988; Kaplan and Te, 1992; Beck et al, 1994; Chancellor and Blaivas, 1995a; Kaplan et al, 1995; Wein and Rovner, 1999; Goldman and Appell, 2000b). The exact incidence of voiding dysfunction caused by diabetes is, however, uncertain. Unselected patients generally do not complain of bladder symptoms. If specifically questioned, anywhere from 5% to 59% of patients with diabetes report symptoms of voiding dysfunction. However, the symptoms may or may not be caused by just the diabetes. In trying to come to conclusions regarding the incidence and types of voiding dysfunction specifically from diabetes, one has to carefully discriminate between articles that consider patients referred for voiding symptoms versus those that have evaluated unselected patients from a population known to have diabetes.
Key Point: Diabetes Mellitus
Current evidence points to both a sensory and a motor neuropathy as being involved in the pathogenesis, the motor aspect per se contributing to the impaired detrusor contractility. The typically described classic urodynamic findings include impaired bladder sensation, increased cystometric capacity, decreased bladder contractility, impaired uroflow, and, later, increased residual urine volume. The main differential diagnosis, at least in men, is generally bladder outlet obstruction because both conditions commonly produce a low flow rate. Pressure/flow urodynamic studies easily differentiate the two. Smooth or striated sphincter dyssynergia is generally not seen in classic diabetic cystopathy, but these diagnoses can easily be erroneously made on a poor or incomplete urodynamic study—voiding may involve abdominal straining, which will produce an interference EMG pattern (pseudo-dyssynergia), and abdominal straining alone will not open the bladder neck area.
Other articles have appeared, however, suggesting that this “classic” diabetic cystopathy may not be the predominant form of lower urinary tract dysfunction. Starer and Libow (1990) reported on a group of 23 elderly diabetic nursing home patients who presented with symptoms of urinary dysfunction. In these 19 women and 4 men, 61% had involuntary bladder contractions, 17% had voluntary contractions of decreased magnitude, 13% had normal detrusor contractility, and 9% were unable to initiate a detrusor contraction at all. Kaplan and colleagues (1995) reported on another group of patients with diabetes referred because of voiding symptoms. Fifty-five percent were found to have involuntary bladder contractions; 23%, impaired detrusor contractility; 10%, detrusor areflexia; and 11%, “indeterminate findings.” In the 42 patients in this group with sacral cord neurologic signs, 50% had impaired detrusor contractility and 24% had detrusor areflexia. Chancellor and Blaivas (1995a) detailed the urodynamic findings in 43 diabetic patients at Chancellor’s institution: 33% had involuntary bladder contractions with normal contractility; 23% had involuntary bladder contractions with impaired contractility but were able to void; 9% had impaired bladder contractility alone but were able to void; 23% had detrusor areflexia; and only 12% had a normal urodynamic study. Ueda and colleagues (1997) also found involuntary bladder contractions in a moderate percentage of diabetic patients (25%) but noted that all these patients had a history of cerebrovascular disease and that no patient had involuntary bladder contractions who did not have such a history. Although it is obvious that some (or even many) of the patients with diabetes who exhibited involuntary bladder contractions may have had factors other than diabetes to account for their bladder overactivity, the importance of urodynamic study in diabetic patients before the institution of therapy cannot be overemphasized.
A variety of animal models have been able to replicate the diabetic state; however, different models demonstrate different patterns of disease expression. Alloxan-induced diabetic rats demonstrated detrusor overactivity. Sucrose-fed rats demonstrated normal bladder contractions. Streptozotocin-induced diabetic rats demonstrate pyuria more commonly (raising the question of an inflammatory component). Therefore the type of model used should be considered when attempting to interpret experimental findings (Yoshimura et al, 2005).
Liu and Daneshgari (2005) concluded that bladders of diabetic and diuretic rats weighed more than control animals and that diabetes and diuresis caused a significant increase in overall fluid intake, urine output, and bladder size. An increased response after field stimulation was noted in both diabetic and diuretic conditions, and a reduced response to cholinergic activity was noted as compared with controls. The authors noted this finding indicated the possibility of neurogenically mediated bladder contraction in the diabetic rat (Liu and Daneshgari, 2005). Numerous articles describe potential pathophysiologic mechanisms that could account for the various types of voiding dysfunction seen in diabetes. Clark and Lee (1995) describe the basic mechanism of interference with physiologic mechanisms as increases in blood glucose increasing the intercellular accumulation of both glucose and its subsequent metabolic products. Hyperglycemia is then proposed to lead to microvascular and neurologic complications, the neurologic sequelae ultimately resulting in a loss of myelinated and unmyelinated fibers, wallerian degeneration, and blunted nerve fiber reproduction and function. The proposed mechanisms include increased accumulation of polyols (sorbitol) from glucose through the aldolase-reductase pathway, inhibiting both glomerular and neural synthesis of myoinositol. The decrease in myoinositol synthesis depresses phosphoinositide metabolism, decreasing Na+,K+-ATPase activity. Hyperglycemia also leads to the formation of advanced glycosylation end products, inhibition of the formation of which in animals has been shown to improve response to functional and structural abnormalities of peripheral nerves.
Nerve growth factor (NGF) and its primary receptor p75NTR expression have been shown to be altered in rodent diabetic models. Tong and Cheng (2005) evaluated NGF levels in streptozotocin-treated rats by means of ELISA expressions for MRNAs of NGF and its primary receptor. NGF levels were found to be significantly lower in streptozotocin-induced diabetic rats, as compared with controls for both NGF and its primary receptor. In a treatment group managed with insulin, with normal glycemia, NGF levels and expressions of NGF, MRNAs, and primary receptor MRNAs were normalized.
Another aspect of diabetic bladder dysfunction may occur at the organelle level. In rodent models, morphologic changes in chronic diabetes have also included mitochondrial abnormalities, especially in the urothelium. Increased collagen was noted to be deposited in capillary walls, with an interruption or extensive widening of the gap junctions between myocytes in the bladder muscularis. Reduced mitochondrial counts were noted in the urothelium and bladder muscle. Degenerated nerve fibers and myelin bodies were identified between myocytes with increased collagen, and mast cells inflammatory aggregates were noted in the stroma of diabetic rats as compared with nondiabetic controls. The duration of diabetes appeared to amplify these changes, and the authors concluded that diabetic changes were a time-dependent phenomenon (Rizk et al, 2006).
In addition to this overall hypothesis, there are tantalizing “chunks” of data from various investigators that may or may not prove to be involved in the pathogenesis of voiding dysfunction secondary to diabetes. In streptozotocin-induced diabetic rats, Hashitani and Suzuki (1996) report reduced spontaneous spike activity, failure of neuromuscular transmission, reduced potency of the smooth muscle sodium-potassium pump, and the development of a postjunctional, muscarinic supersensitivity, but without alteration of the adenosine triphosphate receptor sensitivity. Tong and colleagues (1999) reported an upregulation of M2 receptor protein in the bladder of streptozotocin-induced diabetic rats and an upregulation of M2 receptor protein in bladder body tissue (Tong and Juei-Tang, 2002). Presumably, this could be related to detrusor overactivity and conceivably account for a differential effect of various anticholinergic agents, depending on their receptor specificity. Mumtaz and colleagues (1999) reported an impairment of nitric oxide–mediated urethral smooth muscle relaxation in alloxan-induced diabetic rabbits along with a significant impairment of noradrenergic, noncholinergic, nerve-mediated relaxation in this area. They hypothesize that nitric oxide may be functionally inactive and/or unavailable in this type of diabetes, and this lack may contribute to non–BPH-related outlet obstruction in patients with long-term diabetes and to detrusor overactivity, although it is a bit unclear as to how changes in only the outlet might influence overactivity. Gupta and colleagues (1996) and Gupta and Wein (1999) suggested that diabetes diminishes sodium pump activity, thereby inhibiting agonist-induced contractions in bladder smooth muscle by an increase in intracellular sodium concentration, the latter acting to diminish calcium influx. In abstract form, Chacko’s group (Su et al, 2004) has hypothesized a translocation of protein kinase C isoforms as being involved in decreased detrusor contractility in alloxan-induced diabetes in rabbits. Cardozo and colleagues (2002) reported enhancement of bladder contractions to substance P and des-Arg-BK in a streptozotocin model. Sasaki and colleagues (2002) described decreased nerve growth factor in bladder tissue and L6 to S1 dorsal root ganglia. In alloxan-induced diabetic animals, Khan and colleagues (2002) noted decreased apoptosis of bladder urothelial cells. In this same model, Su and colleagues (2004) reported increased myosin light chain phosphorylation and decreased sensitivity to activator calcium; Changolkar and colleagues (2005) reported decreased smooth muscle force associated with increased lipid peroxides and sorbitol and an overexpression of aldolase reductase and polyol pathway activation. Another interesting pathophysiologic explanation is that attributed to autoimmune phenomena. In a murine model, antibody-mediated bladder dysfunction in type 1 diabetes has been identified. In diabetic mice, anti–voltage-gated calcium channel antibodies induce urodynamic findings compatible with overactive bladder such as phasic detrusor contractions and also loss of bladder wall compliance. This autoimmune dysfunction was reversed by the administration of agonists for voltage-gated calcium channels. The possible reversibility of this phenomenon remains intriguing for early-stage treatment (Wan et al, 2007).
Early institution of timed voiding will avoid the proportion of the impaired detrusor contractility from chronic distention and detrusor decompensation. Experimental studies are currently directed at inhibiting the proposed mechanisms by which hyperglycemia produces neuropathy (Clark and Lee, 1995). Ayan and colleagues (1999) cite references showing that intensive therapy for diabetes can slow its progression and slow the development of abnormal autonomic tests. As a corollary, they showed on a short-term basis in alloxan-induced diabetic rabbits that insulin therapy prevented the urodynamic (increased bladder capacity and compliance) and histopathologic changes seen in a similar but non–insulin-treated group of animals. Insulin reversed most of the changes reported by Cardozo and colleagues (2002).
Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) is an inflammatory demyelinating disorder of the peripheral somatic and autonomic nervous system that may be life threatening. It is described as a recognizable clinical entity characterized by rapidly evolving symmetrical limb weakness, loss of tendon reflexes, absent or mild sensory signs, and variable autonomic dysfunctions (Hahn, 1998). It results from aberrant immune responses directed against peripheral nerve components (Hartung et al, 1995; Hahn, 1998). It is triggered by a preceding bacterial or viral infection, with the immune responses directed toward the infecting organisms cross-reacting with neural tissues. The immune reactions against Schwann cell surface membrane or myelin result in acute inflammatory demyelinating neuropathy (accounting for 85% of cases), whereas reactions against axonal membrane components cause acute motor-sensory axonal neuropathy, accounting for 15% of cases. Two thirds of patients report an antecedent acute infectious illness, most commonly a respiratory tract infection or gastroenteritis, that has resolved by the time the neurologic symptoms begin. Several anecdotal case reports have linked GBS to vaccination by temporal association alone. About 75% of cases reach their nadir within 2 weeks and 94% within 4 weeks. After a brief plateau phase, improvement begins, with gradual resolution of paralysis over weeks to months. The outcome is generally favorable, but Hahn (1998) quotes a mortality rate of 5% to 8% despite the most aggressive management. Autonomic neuropathy is a common complication. Cardiac arrhythmia; hypertension and hypotension; and bowel, bladder, and sexual dysfunction may occur. The prevalence of lower urinary tract dysfunction has been reported as ranging from 25% to more than 80% (Wyndaele et al, 2005). Zochodne (1994) reviewed multiple series and reported a urinary retention rate of 11% to 30%. Sakakibara and colleagues (1997a) reported 7 of 28 patients with voiding dysfunction. Of these, three had transient urinary retention; two had urgency, nocturia, and urge incontinence; one had stress incontinence; and one had voiding difficulty, otherwise unexplained. Many of these patients will have an indwelling catheter to monitor output while in the intensive care unit. Otherwise, their voiding dysfunction should be managed by reversible therapy (e.g., CIC, anticholinergic therapy) while waiting and hoping for resolution.
Miscellaneous Neurologic Diseases Causing Voiding Dysfunction
Lyme Disease
The associated neurologic symptoms of Lyme disease (neuroborreliosis) fall broadly into three syndromes: (1) encephalopathy, (2) polyneuropathy, and (3) leukoencephalitis. The Lyme spirochete can also (rarely) invade the bladder itself. Chancellor and colleagues (1993) described seven patients who also had lower urinary tract dysfunction. Five had detrusor overactivity, none had dyssynergia, and two had detrusor areflexia. In two women, urinary retention was the presenting symptom of Lyme disease. Other subjective symptoms noted were urgency, frequency, nocturia, and urge incontinence. Follow-up at 6 months to 2 years after treatment revealed residual urgency and frequency in three patients.
Hereditary Spastic Paraplegia
Hereditary spastic paraplegia is a genetically transmitted disorder, generally autosomal dominant, less commonly autosomal recessive, and rarely sex linked. There is a pattern of central demyelination with axon loss and progressive lower extremity spasticity generally with muscle weakness. Bushman and colleagues (1993) reported three patients, two of whom had detrusor overactivity (one with striated sphincter dyssynergia), one who had significantly decreased compliance, and one who was urodynamically normal except for a high maximum urethral pressure of uncertain significance.
Jensen and colleagues (1998) reported the voiding characteristics of 11 patients with autosomal dominant pure spastic paraplegia linked to chromosome 2p21-p24. These were culled from six of eight families with the disorder, the authors commenting that lower urinary tract symptoms were present in 16 of 44 definitely affected family members. One patient had an indwelling catheter, and thus an accurate description of symptomatology was not possible. For the other patients, urinary urgency and frequency were the dominant complaints, with 6 of these 10 patients regularly experiencing urgency incontinence. Urodynamically, three patients showed detrusor overactivity, six demonstrated normal detrusor activity, and one demonstrated “hyporeflexia” with delayed first sensation. Satisfactory sphincter EMG recordings were obtained from seven patients, and all were normal. Postvoid residual urine volumes were raised in 8 of 10 patients. The bulbocavernosus reflex was absent in six patients and was truly normal in only one. The authors commented that the frequency of urinary symptoms in their patients (36%) correlated well with other reports in the literature. The authors proposed that the lower urinary tract symptoms (and bowel and sexual dysfunction) in patients with this disorder are caused by a combination of somatic and autonomic nervous system involvement, supporting a multisystem involvement.
Tropical Spastic Paraparesis
Tropical spastic paraparesis is primarily a spinal cord myelopathy caused by a retrovirus (human T-cell leukemia virus 1 [HTLV-1]) similar to HIV. Progressive lower limb weakness and back pain are typically the primary complaints, but voiding dysfunction occurs in up to 60% of those affected (Walton and Kaplan, 1993). Eardley and colleagues (1991) studied six such patients with voiding dysfunction. Two had detrusor areflexia, and three had overactivity, one of whom with dyssynergia. Walton and Kaplan (1993) found four of five consecutive patients had detrusor overactivity and striated sphincter dyssynergia, whereas one had overactivity and synergia. The type of voiding dysfunction depends on whether the damage is primarily to the descending spinal tracts, to the sacral nuclei, or to the sacral outflow. The disease must be distinguished from other myelopathic conditions associated with voiding dysfunction such as MS.
Acquired Immunodeficiency Syndrome
Infection with HIV can affect both the central and peripheral nervous systems, and so it is not unexpected that symptoms of lower urinary tract dysfunction occur, although there is some disagreement as to the overall prevalence of such symptoms in this population. Khan and colleagues (1992) were among the first to report on the types of voiding dysfunctions seen in patients with AIDS. They reported 11 such patients. Urinary retention occurred in six. On urodynamic evaluation, three had detrusor overactivity, four had detrusor areflexia, two had a hypocontractile detrusor, and two had outlet obstruction secondary to BPH. Hermieu and colleagues (1996) prospectively studied 39 patients with HIV infection and voiding symptoms. Clinical symptoms included frequency, urgency, and incontinence in 41% of patients, acute urinary retention in 28%, dysuria and frequency in 18%, and decreased flow in 13%. Seventeen of the patients had involuntary bladder contractions; of these, eight had striated sphincter dyssynergia or a lack of sphincter relaxation during micturition, five patients had detrusor areflexia, four patients had what was termed a “hypertonic urethra,” three had “hypersensitivity,” and five patients had a normal urodynamic evaluation. The authors characterized the voiding dysfunctions broadly as inability to void in 41% of the cases and frequency/urgency/incontinence in 41%. The authors comment that the appearance of neurogenic voiding disturbances heralds a poor prognosis. Eighteen HIV-positive patients, 13 with AIDS, who presented with voiding dysfunction were urodynamically characterized by Kane and colleagues (1996). Chief presenting complaints were daytime frequency in eight patients; retention in three; nocturia in two; and having to strain to void, feeling incompletely empty, incontinence, split stream, and groin pain in one each. Five patients (28%) showed detrusor overactivity, five showed DESD, and one (6%) showed detrusor areflexia. The authors remarked that there was a correlation between cytomegalovirus infection, polyradiculopathy, and detrusor areflexia, a phenomenon reported by others as well. Other common causes of voiding dysfunction exist as well because four patients had outlet obstruction secondary to BPH and in one patient it was secondary to urethral stricture. In another study, Lima and colleagues (2002) evaluated a group of 26 men with HIV-induced bladder dysfunction. Eighty percent of those patients demonstrated detrusor overactivity, and 34% had detrusor-sphincter dyssynergia. These findings are indicative of the myopathy associated with HIV (Lima et al, 2002). Additionally, impaired micturition in the HIV complex may be due to infestations (cerebral toxoplasmosis or viral encephalitis), demyelination disorders, cytomegalovirus, polyradiculopathy, and AIDS-related dementia. Thirty to forty percent of patients with AIDS will develop neurologic involvement with 20% presenting initially with some neurologic symptom. In those patients presenting with urologic symptoms, an underlying neurologic cause is present in approximately 60% of those patients, and 90% of those patients will have already developed an AIDS-associated neoplasm or infestation. Detrusor-sphincter dyssynergia, poor bladder contractility, detrusor overactivity, and bladder outlet obstruction have all been reported in this population. AIDS-related neurogenic voiding dysfunction is associated with a poor outcome, with a mortality rate of approximately 40% within 8 months of development of urologic symptoms (Heyns and Fisher, 2005). How common are voiding problems overall in patients with HIV infection and AIDS? Gyrtrup and colleagues (1995) prospectively investigated voiding function in 77 men and 4 women with HIV infection or AIDS consecutively attending an outpatient clinic. Eight of these (10%) had moderate subjective voiding problems, whereas two (2%) had severe problems. The authors believed that in only 4% of patients did the nature of the disturbance warrant urodynamic examination and concluded that urinary voiding symptoms are only a modest problem overall in an HIV/AIDS population. They concluded that neuropathic bladder dysfunction is rare and occurs mostly in the late stages of the disease.
Acute Disseminated Encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is an acute inflammatory demyelinating disorder of the central nervous system of unknown etiology. It is also sometimes known as parainfectious or postinfectious encephalomyelitis. The sites of lesions are multifocal and can include the cerebral white matter, cerebellum, brainstem, and spinal cord. The records of 11 patients with ADEM were reviewed and commented on by Sakakibara and colleagues (1996a). Nine patients had presented in urinary retention; the other two had urgency, frequency, nocturia, and difficulty voiding, one of whom had enuresis and urgency incontinence as well. The urodynamic findings are difficult to correlate with the presenting symptoms because in four of the patients the studies were done considerably after the onset of the disease. During the follow-up period, seven of the nine patients who originally had retention became able to urinate. Five had difficulty voiding, and four developed irritative symptoms. Six of the patients ultimately had a near-complete neurologic recovery, but voiding symptoms persisted in three. The authors concluded that the supranuclear and nuclear types of pelvic and pudendal nerve dysfunction were primarily responsible for the micturitional disturbances in patients with this disease and that voiding dysfunction was common in these patients.
Syringomyelia
Syringomyelia is a chronic disorder of the spinal cord characterized by dissociated sensory loss and brachial amyotrophy. It usually affects the cervical spinal cord but can extend caudally. Voiding dysfunction has been reported in 9% to 25% of patients. Fourteen patients with syringomyelia were studied urologically by Sakakibara and colleagues (1996d). Eleven of these had urinary symptoms: difficulty voiding in eight, retention in three, nocturnal and daytime frequency in three, incontinence in two, and urgency and enuresis in one. The urinary symptoms appeared from 2 months to 13 years after the initial neurologic symptoms. Urodynamic studies revealed detrusor overactivity in seven, detrusor-sphincter dyssynergia in four, detrusor areflexia in four, and “uninhibited sphincter relaxation” in two. It is interesting that motor unit EMG recordings disclosed findings compatible with denervation of the striated sphincter in five of six patients. The authors concluded that both supranuclear and nuclear types of peripheral autonomic and somatic nerve dysfunction are responsible for the voiding dysfunctions seen. It is also interesting that the micturitional status gradually improved in four of six patients after syringosubarachnoid shunts.
Schistosomal Myelopathy
In addition to causing voiding dysfunction secondary to bladder neck obstruction and impaired muscle contractility as a result of infiltration of the bladder smooth muscle itself, schistosomiasis can rarely cause spinal cord involvement, either as a granulomatous intrathecal mass or as an acute transverse myelitis (Razdan et al, 1997). Two such patients presented with urinary incontinence as their chief urologic complaint. Both had detrusor overactivity: one without dyssynergia and with minimal motor weakness and the other with striated sphincter dyssynergia and a T11 sensory level. It was believed that the findings in the former patient were characteristic of a partial spinal cord or cerebral lesion and that the second patient had a suprasacral transverse myelopathy. In the first patient, the urinary symptoms developed approximately 2 months after exposure and after the development of systemic symptoms; in the second case, symptoms developed some 5 years after the initial diagnosis. Gomes and colleagues (2002) reviewed the records of 14 patients with schistosomal myelopathy referred for voiding dysfunction. Of five patients with acute disease, three presented with retention and two had incontinence. Urodynamics were performed in three of these. Two (one in retention, one with hesitancy and incontinence) demonstrated detrusor areflexia, and one (in retention) showed detrusor overactivity and striated sphincter dyssynergia. Of the 9 chronic patients, 5 showed detrusor overactivity with dyssynergia, 1 with decreased compliance; 2 showed overactivity with synergic sphincters; and 25 showed detrusor areflexia, 1 with decreased compliance.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a disease in which there is widespread inflammatory change in the connective tissues and small vessels of the skin and systemic organs, probably autoimmune in origin (Hahn cited by Sakakibara et al, 2003b). The prevalence of nervous system involvement is cited from 18% to 75%. Myelopathy occurs in 1% to 3% of SLE patients. Sakakibara and colleagues (2003b) reported on eight patients with SLE who presented with voiding dysfunction, six with voiding difficulty (two in retention) and four with urinary incontinence. Five exhibited decreased flow, three had increased residual urine, five had detrusor overactivity, five had impaired detrusor contractility, four had detrusor–striated sphincter dyssynergia, and two of four exhibited abnormal striated sphincter EMG potentials. Sensation was impaired in two patients. Although three patients had subacute encephalomyelopathy (one subacute myelopathy, four chronic myelopathy), the lower urinary tract dysfunction seemed related mainly to the myelopathy. Yu and colleagues (2003) studied 152 women with lupus. Urodynamics and an AUA-7 symptom index were administered and compared with 227 age-matched healthy women. A significantly higher percentage of women with SLE experienced emptying and storage dysfunction with higher symptom scores in the lupus group, as compared with the control group. There was a correlation between symptomatic presentation and Lupus index, but no correlation with disease duration. However, there was a significant relationship between symptom degree and magnitude of symptoms and central nervous system involvement in the lupus group. The lupus patients more commonly manifested a diminished cystometric capacity and a decreased flow rate.
Reflex Sympathetic Dystrophy
Reflex sympathetic dystrophy is a disabling syndrome characterized by severe pain with autonomic changes such as vasomotor disturbances. The condition usually follows a traumatic injury. The exact etiology and pathogenesis are unclear. The prevalence of voiding dysfunction in patients with reflex sympathetic dystrophy is unknown, but it must be more than a rare occurrence because Chancellor and colleagues (1996) were able to collect 20 consecutive patients with neurologically verified reflex sympathetic dystrophy who were referred for voiding symptoms, none of which existed before the initial trauma that induced the reflex sympathetic dystrophy. Seven of the patients presented with urinary retention, although some of these had had various types of surgery designed to treat the symptoms of the dystrophy. Five presented with urgency incontinence, one of whom also had stress incontinence. Six had urgency as a primary complaint, one had daytime frequency, and one had severe nocturia. Detrusor overactivity was demonstrated in eight patients, DESD in one, detrusor areflexia in eight, and hypersensitivity on filling in three. Because the authors excluded patients with acute development of voiding dysfunctions after back surgery and with herniated disks and included only those in whom voiding symptoms developed concurrently with progressive symptoms of reflex sympathetic dystrophy, one must conclude that significant lower urinary tract dysfunction can develop as a direct result of or an association with this problem, although the cause-and-effect relationship is unknown.
Other Conditions
A number of other conditions have been associated with voiding dysfunction, most of which have been reported as individual cases or small groups of cases. These are associated with neurologic symptoms typical of central and/or peripheral neural involvement. The interested reader is referred to relevant articles on amyloidosis (Sakamoto and Wheeler, 1997), adult polyglucosan body disease (Gray et al, 1988), Behçet disease (Theodorou et al, 1999; Saito and Miyagawa, 2000; Sakakibara et al, 2000), neurofibromatosis (Brownlee et al, 1998), spinal muscular atrophy (Von Gontard et al, 2001), Duchenne muscular dystrophy (MacLeod et al, 2003), and familial dysautonomia (Saini et al, 2003).
Miscellaneous Conditions Definitely, Probably, or Possibly Related to Neuromuscular Dysfunction
Detrusor Sphincter Dyssynergia
Dyssynergia refers to the kinesiologic disassociation of two groups of muscles that generally work in harmony. Sphincter dyssynergia refers to an involuntary contraction or lack of relaxation of either the striated sphincter (the striated muscle surrounding the proximal urethra and the striated muscle that forms a part of the urethra for a variable distance from the “urogenital diaphragm” to the bladder neck) or the smooth sphincter (the smooth muscle of the bladder neck and proximal urethra). Detrusor sphincter dyssynergia, unless specified otherwise, refers to dyssynergia of the striated sphincter and is sometimes abbreviated DSD or DESD. This is discussed in Chapter 61 and in the earlier parts of this chapter, especially in the section on SCI. It is discussed as a separate entity here as well to emphasize its importance in terms of recognition and proper management in patients with neurogenic voiding dysfunction.
True DESD should exist only in patients who have an abnormality in pathways between the sacral spinal cord and the brainstem pontine micturition center, generally caused by neurologic injury or disease (Blaivas, 1982; Rudy, 1993; Chancellor and Rivas, 1995; Wein and Rovner, 1999). The diagnosis of DESD should be suspected in any patient with a neurologic lesion in this area. Common causes include traumatic SCI, multiple sclerosis, and the various forms of transverse myelitis. Conversely, in patients without such a lesion, this diagnosis should always be viewed with skepticism, and, without such apparent pathology, such a patient deserves exhaustive study to exclude a neural diagnosis. One exception to this precept is in infants and children with dysfunctional voiding or the Hinman syndrome (see later).
Blaivas and colleagues (1981) have described three main types of DESD. In type 1, there is concomitant increase in both detrusor pressure and EMG activity; at the peak of the detrusor contraction, the sphincter suddenly relaxes and unobstructed voiding occurs. In type 2, there are sporadic contractions of the striated sphincter throughout the detrusor contraction. In type 3, there is a crescendo-decrescendo pattern of sphincter contraction that results in outlet obstruction throughout the entire detrusor contraction. Schurch and colleagues (2005) have correlated neurologic status and DESD type after SCI. Those with an incomplete sensory and motor lesion generally present with type 1 DESD; those with complete sensory and motor lesions present with type 2 and type 3. Weld and colleagues (2000) prefer to classify DESD as intermittent or continuous but note that in their experience the clinical significance of DESD type is not crucial because both types require urodynamic surveillance and expedient treatment to minimize complications. No significant association between type and level of injury was found. Continuous DESD was more associated with complete injuries.
Sphincter EMG activity that increases simultaneously with intravesical or detrusor pressure does not always indicate true DESD, however. These other instances are referred to as pseudodyssynergia (Wein and Barrett, 1982), and such a misdiagnosis may be accompanied by adverse therapeutic consequences. Common causes of pseudodyssynergia include (1) abdominal straining to either initiate or augment a bladder contraction or in response to discomfort and (2) attempted inhibition of a bladder contraction either because of its involuntary nature or because of discomfort. Rudy (1993) has reported that pseudodyssynergia can reliably be differentiated from true DESD urodynamically by analyzing the patterns of detrusor and EMG activity, but others have not always found this to be the case.
Without proper treatment, more than 50% of men with DESD will develop significant complications such as VUR, upper tract deterioration, urolithiasis, urosepsis, and ureterovesical obstruction (Chancellor and Rivas, 1995). In women, these complications are much less common, probably because of the decreased detrusor pressures generated. Using Blaivas’ categorization, type 1 DESD is generally managed with observation alone unless there is persistent reflux, hydronephrosis, or autonomic hyperreflexia. Types 2 and 3 are generally treated. In assessing success or failure of treatment, Kim and colleagues (1998) have used bladder leak point pressure greater than 40 cm H2O as an indicator of the failure of at least sphincterotomy, because in their experience there was a significantly higher incidence of upper tract damage and persistent DESD in such patients. This most likely applies to other treatments as well. Therapy for DESD is designed to either eliminate or significantly lessen the abnormal sphincter activity or to circumvent it. Oral medical therapy directed toward the striated sphincter has not enjoyed wide success, and the most common current approaches are (1) CIC (usually combined with therapy to control detrusor overactivity), (2) sphincterotomy, (3) stent placement across the sphincter, (4) injection of botulinum toxin into the sphincter, (5) continuous catheterization, and (6) urinary diversion.
Experimental small animal models of DESD have been reported by Burnett and colleagues (1997) in mice with targeted deletion of the gene for neuronal nitric oxide synthase and by Cheng and colleagues (1997) by instillation of cold water into the urinary bladder of rats.
Dysfunctional Voiding
This subject is more extensively considered in Chapters 127-128 but is mentioned here because individuals with a history of unexplained lower urinary tract dysfunction symptomatology may not present to the urologist or be definitively diagnosed with this entity until adulthood. This syndrome, also described by various authors as non-neurogenic neurogenic bladder, occult voiding dysfunction, occult neuropathic bladder, learned voiding dysfunction, and the Hinman syndrome, presents the unusual circumstance of what appears urodynamically to be involuntary obstruction at the striated sphincter level existing in the absence of demonstrable neurologic disease (Hinman, 1986). It is difficult to prove urodynamically that an individual has this entity, and it should further be noted that the diagnoses in many of the patients reported have been made on the basis of only history, isolated flowmetry, isolated measurements of total intravesical pressure, and pelvic floor EMG activity (Wein and Barrett, 1988). I believe unequivocal demonstration of this entity requires pressure-flow-EMG evidence of bladder emptying occurring simultaneously with involuntary striated sphincter contraction in the absence of any element of abdominal straining, either in an attempt to augment bladder contraction or as a response to discomfort during urination. Such reports do exist and confirm the existence of this syndrome. The etiology is uncertain and may represent a persistent transitional phase in the development of micturitional control or persistence of a reaction phase to the stimulus of lower urinary tract discomfort during voiding, long after the initial problem that caused this has disappeared (Jorgensen et al, 1982).
Bladder Neck Dysfunction
Bladder neck dysfunction is defined here as an incomplete opening of the bladder neck during voluntary or involuntary voiding. It has also been referred to as smooth sphincter dyssynergia, proximal urethral obstruction, primary bladder neck obstruction, and dysfunctional bladder neck. The term smooth sphincter dyssynergia or proximal sphincter dyssynergia is generally used when referring to this urodynamic finding in an individual with autonomic hyperreflexia. In male patients with autonomic hyperreflexia, the neurologic pathophysiology is clear. The term bladder neck dysfunction more often refers to a poorly understood non-neurogenic condition first described over a century ago but first fully characterized by Turner-Warwick and colleagues in 1973. The dysfunction is found almost exclusively in young and middle-aged men, and characteristically they complain of long-standing voiding/emptying (obstructive) and filling/storage (irritative) symptoms (Webster et al, 1980; Norlen and Blaivas, 1986; Wein and Barrett, 1988; Trockman et al, 1996; Yamanishi et al, 1997). These patients have often been seen by many urologists and have been diagnosed as having psychogenic voiding dysfunction because of a normal prostate on rectal examination, a negligible residual urine volume, and a normal endoscopic bladder appearance. The differential diagnosis also includes anatomic bladder neck contracture, BPH, dysfunctional voiding, prostatitis/prostatosis, neurogenic dysfunction, and low pressure/low flow (see later). Objective evidence of outlet obstruction in these patients is easily obtainable by urodynamic study. Once obstruction is diagnosed, it can be localized at the level of the bladder neck by video-urodynamic study, cystourethrography during a bladder contraction, or micturitional urethral profilometry (see Chapter 62). The diagnosis may also be made indirectly by the urodynamic findings of outlet obstruction in the typical clinical situation in the absence of urethral stricture, prostatic enlargement, and striated sphincter dyssynergia. Involuntary bladder contractions or decreased compliance may occur. Noble and colleagues (1994) cite the incidence as 50%, but this seems high. Trockman and colleagues (1996) quote it as 34%.
The exact cause of this problem is unknown. Some have proposed that there is an abnormal arrangement of musculature in the bladder neck region, so coordinated detrusor contractions cause bladder neck narrowing instead of the normal funneling (Bates et al, 1975). The occurrence of this problem in young, anxious, and high-strung individuals and its partial relief by α-adrenergic blocking agents have prompted some to speculate that it may in some way be related to sympathetic hyperactivity. When prostatic enlargement develops in individuals with this problem, a double obstruction results, to which Turner-Warwick (1984) has applied the term trapped prostate. The lobes of the prostate cannot expand the bladder neck and therefore expand into the urethra. A patient so affected generally has a lifelong history of voiding dysfunction that has gone relatively unnoticed because he has always accepted this as normal, and exacerbation of these symptoms occurs during a relatively short and early period of prostatic enlargement. Although α-adrenergic blocking agents provide improvement in some patients with bladder neck dysfunction, definitive relief in the male is best achieved by bladder neck incision. In patients with this and a trapped prostate, marked relief is generally effected by a “small” prostatic resection or ablation that includes the bladder neck or a transurethral incision of the bladder neck and prostate. Such patients often note afterward that they have “never” voided as well as after their treatment.
Bladder Outlet Obstruction in Women
The female counterpart of non-neurogenic bladder neck dysfunction in men does exist, although it is rare. Bladder outlet obstruction in women in general is uncommon. Dionko and colleagues (1984) were among the first to clearly define this entity in women on the basis of video-urodynamic studies. Nitti and colleagues (1999) evaluated the video-urodynamic studies of 261 of 331 women who underwent multichannel studies for non-neurogenic voiding dysfunction. They defined bladder outlet obstruction as radiographic evidence of obstruction between the bladder neck and the distal urethra in the presence of a sustained detrusor contraction of any magnitude, which is usually associated with reduced or delayed urinary flow rate. Obstruction at the level of the bladder neck was diagnosed when the bladder neck was closed or narrowed during voiding. Obstruction of the urethra was diagnosed as a discrete area of narrowing, associated with proximal dilatation. Strict pressure-flow criteria were not used to classify cases as obstructed or not obstructed. Using these criteria they found 76, or 23%, of their cases to be obstructed. Of those obstructed, only 16% (12 patients) were diagnosed as having primary bladder neck obstruction (the counterpart to non-neurogenic bladder neck dysfunction in the male). Thirty-three percent of the cases of obstruction were caused by dysfunctional voiding (see earlier for description), 28% by cystocele, 14% by obstruction created by prior incontinence surgery, 4% by urethral stricture, 3% by uterine prolapse, and 1% each by urethral diverticulum and rectocele. Groutz and colleagues (2000) reviewed their urodynamic database of 587 consecutive women referred for evaluation of voiding symptoms. They defined obstruction as a persistent, low, noninvasive maximum flow rate less than 12 mL/sec on repeated study combined with a detrusor pressure at maximum measured flow rate of more than 20 cm H2O in a pressure-flow study. Only 38 women (6.5%) met these criteria of bladder outlet obstruction. Of those, only 8% (three women) were characterized as having primary bladder neck obstruction. Twenty-six percent (10 women) had obstruction on the basis of prior anti-incontinence surgery, 24% because of severe genital prolapse, 13% because of urethral stricture, 5% because of dysfunctional voiding, 5% because of true detrusor striated sphincter dyssynergia, and 3% because of urethral diverticulum. In 16%, there was no definable etiology. Most authors would agree that surgical treatment of this problem in women should be approached with caution because sphincteric incontinence is a significant risk. Smith and Appell (2006) commented on the importance of urodynamics in distinguishing dysfunctional voiding versus bladder neck dysfunction. They commented on the importance of evaluation inclusive of symptom assessment, uninstrumented uroflow patterns, simultaneous urodynamic and EMG assessment of voiding, as well as the addition of fluoroscopy to assess function of the bladder neck during voiding. They further stressed multidisciplinary therapy including pelvic floor therapy (biofeedback), behavioral modification, and the addition of pharmacotherapy. Neuromodulation may have a role in select patients, as well as rare surgical intervention for primary bladder neck dysfunction.
Low-Pressure/Low-Flow Voiding in Younger Men: Bashful Bladder
Low-pressure/low-flow voiding can be the result of a number of causes, most notably a decompensating detrusor (generally from bladder outlet obstruction—see Chapters 62 and 92) or as a part of the syndrome known as detrusor hyperactivity with impaired contractility (DHIC—see Chapters 62 and 76). When this occurs in a young man, it is generally characterized by frequency, hesitancy, and a poor stream. The entity is readily demonstrated on urodynamic assessment and with no coexisting endoscopic abnormality. The patient usually notes marked hesitancy when attempting to initiate micturition in the presence of others, and some have therefore described this condition as an “anxious bladder” or a “bashful bladder.” The estimate of the incidence of this problem in younger male patients referred for urodynamic assessment varies between 6% (Barnes et al, 1985) and 19% (George and Slade, 1979).
Barnes and colleagues (1985) suggested that these men are psychologically unusual but in the direction of being obsessional rather than anxious. They suggest that these individuals have a lifelong tendency to overcontrol the process of micturition and are thus vulnerable to lower urinary tract symptoms under stress, and they recommend that a behavioral modification program be considered. Rosario and colleagues (2000) performed ambulatory urodynamic studies on 40 consecutive symptomatic men with a mean international prostate symptom score of 19 who were unable to “perform” during conventional video-urodynamic study. They concluded that a surgically correctable cause of the symptoms could be found in about 20% of men, but only in those older than 40 years. They believed therefore that the contribution of ambulatory urodynamic monitoring in such cases in men younger than 40 years was negligible. They also stated that they thought evidence from the literature suggested that a significant proportion of such nonobstructed cases would respond to drug therapy or behavioral therapy. The authors’ experiences have been similar to those of others who have stated that, in the younger nonobstructed male with this condition, neither empirical pharmacologic treatment nor transurethral surgery has had any consistent beneficial effect.
Urinary Retention; the Fowler Syndrome in Young Women
Urinary retention is encountered fairly commonly by the urologist, especially in adult men secondary to anatomic obstruction from benign prostatic enlargement. Although unusual, urinary retention in women is not rare. As in the male, the potential causes are classically cited as neurologic, pharmacologic, anatomic, myopathic, functional, and psychogenic. A particularly excellent review of the various causes of urinary retention in women along with an algorithm for evaluation and treatment has been published by Smith and colleagues (1999).
The Fowler syndrome (Fowler et al, 1988; Noble et al, 1994; Fowler, 1999, 2007; Swinn and Fowler, 2001) refers to a syndrome of urinary retention in young women in the absence of overt neurologic disease. The typical history is that of a woman younger than 30 years of age who has found herself unable to void for a day or more with no urinary urgency but increasing lower abdominal discomfort. A bladder capacity of more than 1 L with no sensation of urgency is necessary for the diagnosis. There are no neurologic or laboratory features to support a diagnosis of any neurologic disease. MRI of the brain and the entire spinal cord is normal. On concentric needle electrode examination of the striated muscle of the urethral sphincter, however, Fowler and colleagues described a unique EMG abnormality. This abnormal activity, localized to the urethral sphincter, consists of a type of activity that would be expected to cause inappropriate contraction of the muscle. Sphincter activity consists of two components: complex repetitive discharges and decelerating bursts. This abnormal activity impairs sphincter relaxation. These patients often have polycystic ovaries, raising the possibility that the activity is linked in some way to impaired muscle membrane stability, allowing direct spread of electrical impulses throughout the muscle, owing possibly to a hormonal abnormality; and thus the disorder may possibly be the manifestation of a focal, hormonally dependent “channelopathy.” This would explain why the condition is seen only in premenopausal women. Efforts to treat this condition by hormonal manipulation, pharmacologic therapy, or injections of botulinum toxin have been unsuccessful. This condition is highly responsive to neuromodulation (success rate approaching 70%) even in women who have been in retention for many months or years.
Kavia and colleagues (2006) assessed a large group of women (247) for assessment of either complete (42%) or partial (58%) retention. Completeness was defined as inability to void whatsoever. These women had previously been diagnosed with abnormality of the striated urethral sphincter electromyogram (Fowler syndrome), which led to the referral. These women were assessed with a combination of urethral profilometry (71%), transvaginal ultrasonographic measurement of sphincter volume (57%), and sphincter electromyogram (39%). Those patients with complete retention showed a significant difference in sphincter volume between those who were EMG positive, as opposed to those who were EMG negative. The authors concluded that on the basis of this, the most common diagnosis for women referred for urinary retention was Fowler syndrome, which led to the potential use of neuromodulation for those patients.
The urodynamic problem is detrusor acontractility. The same EMG abnormality, however, is found sometimes in women with obstructed voiding. This type of EMG activity is not uncommon (Fitzgerald and colleagues [2000] cite an incidence of 8% in a series of women undergoing routine urodynamic and EMG studies), but its correlation with complete retention is relatively rare.
Neuromodulation has been advocated for Fowler syndrome (nonobstructive urinary retention in women). De Ridder and colleagues (2007) reported 62 women who underwent sacral nerve stimulation, 30 of whom had findings compatible with Fowler syndrome and 32 with idiopathic retention. Somatoform disorder was found in 26% of the Fowler’s patients and 43.8% of the idiopathic group. Depression was also higher in the Fowler syndrome group (30% vs. 18.8%). Neither of these findings, however, had correlation with outcome. Nine patients with Fowler syndrome as compared with 19 without failed neuromodulation. The authors concluded that Fowler syndrome was actually a positive predictive factor for SNS response in patients with female urinary retention. They did advocate preimplant psychologic testing including the patient health questionnaire; however, there was no correlation with outcomes in this population.
Postoperative Urinary Retention
Postoperative urinary retention is a well-recognized but poorly understood event. Its incidence is generally quoted overall as 4% to 25%. It occurs more frequently after lower urinary tract, perineal, gynecologic, and anorectal surgery. In the placebo arms of four trials of α-adrenergic blocker prophylaxis after these types of surgery, the incidence of postoperative retention ranged from 18.8% to 57% (Velanovich, 1992). Contributing factors, which are not mutually exclusive, include the following eight factors:
Anesthesia and analgesia can contribute to factors 2, 3, 4, and 6. The idea of a nociceptive inhibitory reflex, initiated by pain or discomfort, is an attractive one because a sympathetic efferent limb could directly affect factors 4, 5, and 6 (see Chapter 60).
Bladder decompression for 18 to 24 hours postoperatively decreased the incidence of retention in patients undergoing joint replacement surgery by 52% versus 27% (Michelson et al, 1988) and 65% versus 0% (Carpiniello et al, 1988), compared with CIC. The incidence of urinary infection with continuous catheterization was no different in the Michelson and colleagues’ study (15% vs. 11%) and was less in the Carpiniello and colleagues’ study (16% vs. 43%), in which straight catheterization was carried out in the recovery room as well. The avoidance of acute bladder overdistention to prevent postoperative urinary retention is supported by the experimental observation of a reduced bladder response to sacral neural stimulation during overdistention (>80% reduction) and, as well, after overdistention (19% reduction) (Bross et al, 1999).
α-Adrenergic blockade with phenoxybenzamine historically has seemed effective prophylactically in decreasing the incidence of postoperative retention. Velanovich (1992) performed a meta-analysis on the use of phenoxybenzamine (only randomized placebo-controlled studies) and concluded that this agent reduced the occurrence by 29.1%. In a retrospective review of colorectal patients treated with and without phenoxybenzamine, Goldman and colleagues (1988) found a 54.7% incidence of retention in patients not given this agent versus a 19.2% incidence in those who were. The regimen for those not catheterized preoperatively was 10 mg orally the evening before and 1 hour before surgery, 2 hours after, and 10 mg twice daily for 3 days. For those who were catheterized before the procedure, the regimen was 10 mg twice daily, initiated the day before catheter removal. The mechanism of action is uncertain. If an inhibitory nociceptive reflex is initiated, and this is similar to the sympathetic reflex elicited by bladder filling (see Chapter 60), the mechanism is multifactorial. Alternatively, the drug may act only on the outlet to decrease resistance, which may be pathologically increased by anxiety, pain, and other factors related to surgery. Whether other α-adrenergic blockers are as effective is uncertain (Cataldo and Senagore, 1991).
Hyperthyroidism
Patients with thyrotoxicosis often present with symptoms caused by sympathetic overactivity and autonomic nervous system imbalance. Goswami and colleagues (1997) reported that 12 of 30 patients (40%) experienced the onset of voiding symptoms 1 to 6 months after the onset of the symptoms of thyrotoxicosis. Four of these patients had enuresis. Urodynamic studies were done in five patients; all had reduced flow rates, and four had a significant postvoid residual, three of whom had an enlarged bladder capacity and increased perineal EMG activity during voiding. The voiding dysfunction and urodynamic abnormalities resolved after resolution of the hyperthyroidism. The bladder symptoms were more common in females than in males. The authors hypothesize that increased β-adrenergic activity in thyrotoxicosis is responsible for a reduced flow rate and increased bladder capacity because of the inhibitory β-adrenergic activity on detrusor muscle contractility. Stoffer (1988) cited a much lower incidence of voiding dysfunction in thyrotoxicosis: a 7% prevalence of urgency and hesitancy and a 1% prevalence of enuresis.
Schizophrenia
Bonney and colleagues (1997) proposed that a significant subset of schizophrenic patients have involuntary bladder contractions secondary to brain pathology. In a previous study (Gupta et al, 1995), the same group demonstrated involuntary bladder contractions in 4 of 10 evaluable patients with schizophrenia who were referred for voiding dysfunction or incontinence. All of these patients had significant childhood incontinence, urge incontinence, bedwetting, and a small bladder capacity. In the later report (Bonney et al, 1997) the prevalence of urinary incontinence and related symptoms in a group of chronic schizophrenic patients was compared with a group of comparatively hospitalized patients with mood disorders. There was a significant difference in the prevalence of urge incontinence (34% vs. 17%) and bedwetting (46% vs. 20%). There were no significant differences in urinary urgency, overall voiding dysfunction, fecal incontinence, or sexual dysfunction. The hypothesis of a neurobiologic correlate between schizophrenic patients and the occurrence of involuntary bladder contractions in these patients is an intriguing one.
Gastroparesis
Gastroparesis is a condition characterized by symptoms from impaired transit of intraluminal gastric contents into the duodenum but in the absence of mechanical obstruction. It may be caused by diabetes, occur after gastric surgery, or be idiopathic. Goldman and Dmochowski (1997) characterized the voiding dysfunction of 17 patients with gastroparesis who were referred for voiding symptoms, 10 of whom had idiopathic gastroparesis and in 7 of whom the condition was secondary to diabetes. Seven patients had abnormal detrusor contraction and delayed sensation, five had poor detrusor function and normal sensation, three had normal detrusor function and poor sensation, and two had normal detrusor contraction and sensation. There was no difference between the occurrence of the dysfunctions in the two groups. Predominant symptoms were urinary frequency in 7 and difficulty emptying in 10. Patients with idiopathic gastroparesis were more likely to note difficulty emptying (70%), whereas those with diabetic gastroparesis were more likely to have urinary frequency (71%). The authors postulated an association between idiopathic gastroparesis and bladder dysfunction and proposed that a common autonomic neuropathic syndrome may account for the bladder dysfunction in both the idiopathic and the diabetic forms of this syndrome.
Myasthenia Gravis
Any neuromuscular disease that affects the tone of the smooth or striated muscle of the distal sphincter mechanism can predispose an individual patient to a greater chance of urinary incontinence after even a well-performed transurethral or open prostatectomy. Myasthenia gravis is an autoimmune disease caused by autoantibodies to acetylcholine nicotinic receptors. This leads to neuromuscular blockade and hence to weakness in a variety of striated muscle groups. The incidence of incontinence after prostatectomy is indeed greatly increased in patients with this disease (Greene et al, 1974; Khan and Bhola, 1989). Sandler and colleagues (1998), in addition, reviewed three cases of de novo voiding dysfunction in patients with myasthenia gravis (one woman with intrinsic sphincter deficiency, poor pelvic muscle contractility, and detrusor hyperreflexia; one male with detrusor hyporeflexia complaining of urgency and incontinence; and one young woman with an acontractile bladder) and added a personal report of a fourth patient with urinary retention from detrusor areflexia. They hypothesize that such autonomic dysfunction in a patient with myasthenia might indicate a unique subset with a worse prognosis.
Isaacs Syndrome
Isaacs syndrome is a rare neurologic disorder characterized by continuous muscle contraction, fasciculations, myokymia, excessive sweating, and elevated creatinine kinase level. It is shown to be secondary to antibodies possibly directed against potassium channels on peripheral nerves and is associated with peripheral neuropathy, autoimmune diseases, malignancies, and endocrine disorders. Tiguert and colleagues (1999) present a case with urinary retention associated with a picture of acute demyelinating neuropathy. Their patient was in painful urinary and fecal retention; the urinary retention was presumed to be caused by spasm of the periurethral striated sphincter, diagnosed by an inability to pass a catheter beyond this. Rectal sphincter spasm was also diagnosed. The condition was treated with plasmapheresis and pharmacologic agents to relax skeletal muscle. Suprapubic drainage was instituted. The condition subsided, and normal urinary function was ultimately restored.
Wernicke Encephalopathy
Wernicke encephalopathy is a rare but well-documented condition caused by a deficiency in thiamine (vitamin B1) in both alcoholic and nonalcoholic populations. Pathologic lesions are characteristically distributed periventricularly at the levels of the third and fourth ventricles including the mammillary body, medial thalamic nucleus, hypothalamus, superior cerebellar vermis, periaqueductal gray matter, and midbrain tegmentum. The two major clinical manifestations of thiamine deficiency involve the cardiovascular and neurologic systems. The latter manifests generally as peripheral neuropathy, the condition being known as Wernicke encephalopathy. The initial symptoms of the polyneuropathy range from simply burning feet to muscle weakness. Sakakibara and colleagues (1997b) report a case of a pregnant woman with multiple neurologic manifestations of central and peripheral neuropathy and with urge incontinence, manifested urodynamically by involuntary bladder contractions and a decreased bladder volume. Resolution of the urinary symptoms occurred after thiamine replacement. The authors hypothesize that lesions in the medial thalamic-hypothalamic area and periaqueductal gray matter were primarily responsible for the micturitional disturbance. Tjandra and Janknegt (1997) reported a case of a chronic alcoholic male with seemingly isolated erectile and voiding dysfunction, the latter consisting of complaints referable to emptying, correlated with flowmetry during a prolonged void with a peak flow rate of 6.4 mL/sec with an interrupted pattern, suggesting poor detrusor contractility. The erectile dysfunction was determined to be neurogenic. Both resolved with thiamine replacement.
Systemic Sclerosis (Scleroderma)
Scleroderma is a disease of the connective tissue characterized by thickening and fibrosis of the skin, abnormalities of the small arteries, and involvement of the gastrointestinal tract, heart, lung, and kidneys. The pathogenesis is unknown but thought to be caused by overexpression of the collagen gene DNA, contributing to excessive production of collagen in these patients. Lazzeri and colleagues (1995) report the urodynamic assessment and histologic evaluation of nine such women, of whom five had hesitancy; four, decreased stream; two, frequency and nocturia; and two, suprapubic pain. Four patients had detrusor areflexia, one of whom also had decreased compliance. Another patient with decreased stream also had decreased compliance. Three of the patients with areflexia demonstrated collagen accumulation on histologic examination of bladder biopsies. The authors review five literature reports of various aspects of lower urinary tract function and histology in patients with scleroderma but fail to find a consistent pattern in these. They hypothesize that in their patients the areflexia resulted from impaired neurologic modulation owing to the histologic changes in the detrusor tissue.
Minervini and colleagues (1998), conversely, carried out urodynamic and bladder morphologic evaluations in 23 female patients with systemic sclerosis, 9 of whom complained of urinary symptoms, and found urodynamic alterations in only 3 cases. They were unable to correlate voiding symptoms, urodynamic changes, and the degree of bladder wall fibrosis or visceral involvement. Evidence of autonomic nervous system dysfunction was found outside the urinary tract in 13 of these patients. The authors speculate that voiding dysfunction, when it occurs, could be caused by the fibrotic replacement of bladder smooth muscle, but they did not exclude some degree of autonomic dysfunction as well.
Ehlers-Danlos Syndrome
Ehlers-Danlos syndrome refers to a heterogeneous group of disorders characterized by inherited abnormalities of connective tissue. The main clinical manifestations are skin fragility, skin hyperextensibility, and joint mobility. More than 10 subtypes of the syndrome have been defined on the basis of clinical, genetic, and biochemical criteria. Bladder diverticula have been associated with this disorder, with operative repair characterized by an increased recurrence rate over that which would ordinarily be expected. Deveaud and colleagues (1999) reviewed the literature on this subject and reported on the intensive study of one such patient with a large left-sided diverticulum that did not empty, along with a greatly enlarged bladder capacity and residual urine. Simultaneously, they reported a second patient without Ehlers-Danlos syndrome who presented with a urinary tract infection and left pyelonephritis. This patient also reported decreased force of stream, and evaluation disclosed left VUR with a left (presumably congenital) periureteral diverticulum. The diverticulum enlarged with voiding, and the patient left a large postvoid residual. Both patients were treated surgically (successfully). The authors thought the tissue from the nonperiureteral diverticulum was more closely related to the pathophysiology of Ehlers-Danlos syndrome, noting the tissue from that diverticulum to be more compliant, and attributed this to changes in the extracellular matrix protein caused by the Ehlers-Danlos syndrome. Nonspecific joint hypermobility may also be associated with voiding dysfunction. Manning and colleagues (2003) prospectively evaluated 1000 women referred for urodynamic evaluation and noted benign joint hypermobility to be associated with increasing bowel dysfunction and a higher degree of urinary tract symptomatology. Also noted was a significant childhood history for both of these phenomena. Generalized joint hypermobility (GJH) has been associated with non-neurogenic bladder dysfunction in children. de Kort and colleagues (2003) reported 89 children with GJH and found that 19% of males, as compared to 4% of control, had constipation. Fecal soiling also occurred more commonly in the GJH group (34% vs. 18%). In girls, day and night urinary incontinence was more prevalent in the joint hypermobility group (38% and 14%, respectively), as compared with controls (13% and 2%). Urinary tract infections also tended to occur more commonly in joint hypermobility with 24% being noted, as compared with 11% in the control group. The generalized hypermobility is a mild form of a genetic, inheritable connective-tissue disease, which is related to Ehlers-Danlos syndrome, Marfan syndrome, and osteogenesis imperfecta. Hypermobility in these children has been reported in multiple organ systems. Additionally, an increased incidence of genitourinary prolapse in women with joint hypermobility has been attributed to connective tissue deficit.
Myotonic Dystrophy
Myotonic dystrophy is an autosomal dominant hereditary multiorgan disease characterized by myotonia and distal muscle atrophy, and in later stages by cataracts, endocrine disturbances, mental retardation or dementia, testicular atrophy and infertility, progressive frontal alopecia, and disturbances in cardiac conduction. Although myotonic activity has not been found in the sphincter or pelvic floor, many patients appear to have voiding complaints. Bernstein and colleagues (1992) reported on 10 patients, 8 of whom had urinary complaints by history (4 infrequent voiders, 1 with urge and stress incontinence, 1 with urge and urge incontinence, 1 with slight urgency without incontinence, and 1 with obstructive symptoms only in the morning). There were no characteristic urodynamic patterns observed, and urodynamic findings did not correlate particularly well with symptoms. Sakakibara and colleagues (1995b) also reported lower urinary tract symptoms in such patients, but the message seems to be that there is no characteristic pattern of dysfunction, and thus such patients need to be characterized urodynamically before making any assumptions regarding therapy based on symptoms alone.
Corticobasalar Degeneration
Corticobasalar degeneration is a rare neurodegenerative disorder of the corticobasalar tracts in the cerebral cortex and basal ganglia, most likely in the supranuclear parasympathetic system. The disorder tends to have a unilateral predominance. Cortical, extrapyramidal, long-tract, and urinary symptoms are commonly noted in this disease process. Sakakibara and colleagues (2004b) assessed 10 patients with this disorder compared with 11 age-matched controls. As compared with controls, the degeneration patients had more common urinary symptoms (80% of study group). Urinary symptoms usually appeared within 1 to 3 years after onset of the disease and became more common with longer disease duration. Nocturnal frequency tended to be the initial urinary symptom, followed by incontinence, urgency, and frequency. Urodynamic findings included decreased bladder capacity, detrusor overactivity (most commonly), detrusor hypocontractility, and low compliance in individual patients. Detrusor sphincter dyssynergia was not noted (Sakakibara et al, 2004b).