CHAPTER 7 Immune Receptors and Signal Transduction
The idea that cells may have specific surface receptors that can be triggered by external ligands came from one of the founders of modern immunology. Paul Ehrlich, in his “side chain theory,” published in 1897, conceived of antibodies on the surface of immune cells that recognize antigens and instruct the immune cell to secrete more of the same antibody. Cell surface receptors for hormones were discovered many decades later in the second half of the 20th century but well before the identification of antigen receptors on lymphocytes in the early 1980s.
Cell surface receptors serve two major functions—the induction of intracellular signaling and the adhesion of one cell to another or to the extracellular matrix. Signal transduction broadly refers to the intracellular biochemical responses of cells after the binding of ligands to specific receptors. Most but not all signaling receptors are located on the plasma membrane. Signaling initiated by these receptors typically involves an initial cytosolic phase when the receptor or proteins that interact with the receptor may be post-translationally modified. This often leads to the activation or nuclear translocation of transcription factors that are silent in resting cells, followed by a nuclear phase when transcription factors orchestrate changes in gene expression (Fig. 7-1). Some signal transduction pathways stimulate cell motility or activate granule exocytosis from the cytoplasm independent of a nuclear phase. Signal transduction can result in a number of different consequences for a cell, including acquisition of new functions, induction of differentiation, commitment to a specific lineage, protection from cell death, initiation of proliferative and growth responses, and induction of cell cycle arrest or of death by apoptosis. Antigen receptors on B and T lymphocytes are among the most sophisticated signaling machines known, and they will form a large part of the focus of this chapter.
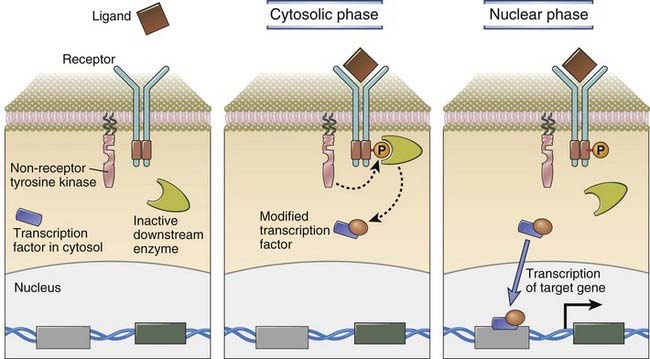
FIGURE 7–1 Signaling from the cell surface involves cytosolic and nuclear phases.
A generic receptor that activates a non-receptor tyrosine kinase after it binds ligand is shown. In the cytosolic signaling phase, the non-receptor kinase phosphorylates a key tyrosine residue on the cytoplasmic tail of the receptor, as a result of which the phosphotyrosine-containing receptor tail is able to recruit a downstream enzyme that is activated once it is recruited. In the cytosolic phase, this activated downstream enzyme post-translationally modifies a specific transcription factor that is located in the cytoplasm. In the nuclear phase, this modified transcription factor enters the nucleus and induces the expression of target genes that all have a binding site in the promoter or in some other regulatory region that can bind to this modified transcription factor and facilitate transcription.
We will initially provide a broad overview of signal transduction, followed by a discussion of signaling mediated by clonally distributed antigen receptors in lymphocytes and by structurally related immune receptors found mainly in cells of the innate immune system. When discussing antigen receptors in T and B cells, we will examine the role of coreceptors in lymphocyte activation, consider signaling through costimulatory receptors in each lymphocyte lineage, and discuss the role of inhibitory receptors in T, B, and NK cells. We will also consider different categories of cytokine receptors and signal transduction mechanisms initiated by these receptors and finally examine the major pathway that leads to the activation of NF-κB, a transcription factor of relevance to both innate and adaptive immunity.
An Overview of Signal Transduction
Receptors that initiate signaling responses are generally integral membrane proteins present on the plasma membrane, where their extracellular domains recognize soluble secreted ligands or structures that are attached to the plasma membrane of a neighboring cell or cells. One distinct category of receptors, nuclear receptors, are actually transcription factors that are functionally activated by lipid-soluble ligands that can easily cross the plasma membrane.
The initiation of signaling from a cell surface receptor may require ligand-induced clustering of the receptor, known as receptor cross-linking, or may involve a conformational alteration of the receptor that is induced by its association with ligand. Both mechanisms of signal initiation typically result in the creation of a novel geometric shape in the cytosolic portion of the receptor that promotes interactions with other signaling molecules. This change in receptor geometry may sometimes result from enzymatic addition of a bulky phosphate residue on a key tyrosine, serine, or threonine side chain on the cytosolic portion of a receptor component or on a distinct adaptor protein. The enzymes that add phosphate groups onto amino acid side chains are called protein kinases. Many of the initiating events in lymphocyte signaling depend on protein kinases that phosphorylate key tyrosine residues, and these enzymes are therefore called protein tyrosine kinases. Other protein kinases that are involved in distinct signaling pathways are serine/threonine kinases, enzymes that phosphorylate protein substrates on serine or threonine residues. Some enzymes activated downstream of signaling receptors phosphorylate lipid substrates; they are therefore known as lipid kinases. For every type of phosphorylation event, there is a specific phosphatase, an enzyme that can remove a phosphate residue and thus modulate signaling. These phosphatases play important, usually inhibitory roles in signal transduction. Phosphorylation of proteins is not the only post-translational modification that drives signal transduction. Many other modifications are known to facilitate signaling events. Some transcription factors as well as histones can be regulated by acetylation and methylation, for instance. A type of modification that we will describe later in this chapter is protein ubiquitination, the addition of ubiquitin molecules that either target proteins for degradation or drive signal transduction in many cells, including lymphocytes. Many important signaling molecules are modified by the addition of lipids that may help localize the protein in the plasma membrane, or sometimes to a specialized region of the plasma membrane that is rich in signaling molecules.
Cellular receptors are grouped into several categories based on the signaling mechanisms they use and the intracellular biochemical pathways they activate (Fig. 7-2):
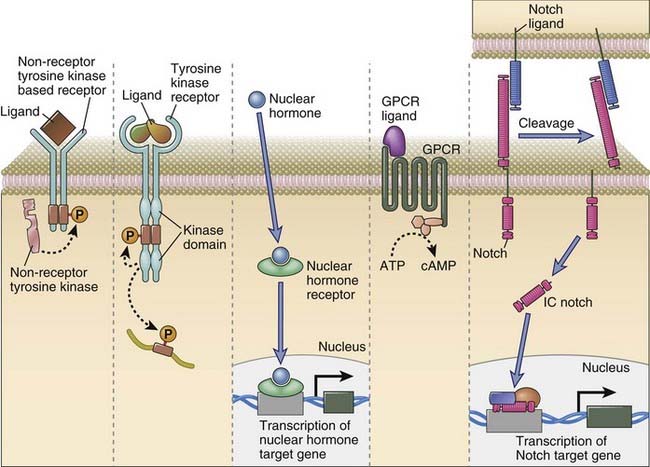
FIGURE 7–2 Major categories of signaling receptors in the immune system.
Depicted here are a receptor that uses a non-receptor tyrosine kinase, a receptor tyrosine kinase, a nuclear receptor that binds its ligand and can then influence transcription, a seven-transmembrane receptor linked to heterotrimeric G proteins, and Notch, which recognizes a ligand on a distinct cell and is cleaved, yielding an intracellular fragment (IC Notch) that can enter the nucleus and influence transcription of specific target genes.
Modular Signaling Proteins and Adaptors
Signaling molecules are often composed of distinct modules, each with a specific binding or catalytic function. The discovery of tyrosine phosphorylation represented a major breakthrough in the study of cellular signaling pathways. It was subsequently discovered that the sequence surrounding specific phosphorylated tyrosine residues contributes to the interaction of tyrosine-phosphorylated proteins with other signaling molecules. An appreciation that signaling molecules contain modules or domains that each have defined functions was obtained from the study of non-receptor tyrosine kinases. The cellular homologue of the transforming protein of the Rous sarcoma virus, called c-Src, is the prototype for an immunologically important family of non-receptor tyrosine kinases known as Src family kinases. c-Src contains unique domains, including Src homology 2 (SH2) and Src homology 3 (SH3) domains described later. It also contains a catalytic tyrosine kinase domain and an N-terminal lipid addition domain that facilitates the covalent addition of a myristic acid molecule to the protein. The myristate helps target Src family kinases to the plasma membrane. The modular structures of three families of tyrosine kinases that are important in the immune system are depicted in Figure 7-3.
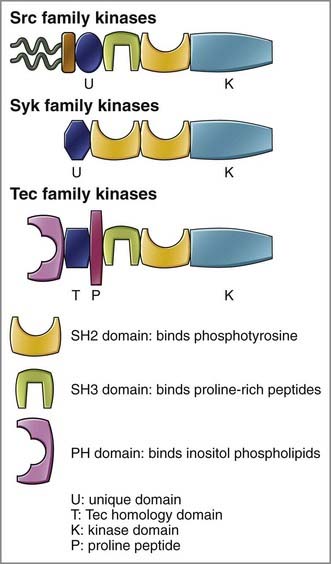
FIGURE 7–3 The modular structure of tyrosine kinases that influence lymphocyte activation.
Modules include SH2 domains that bind specific phosphotyrosine-containing polypeptides, SH3 domains that recognize proline-rich stretches in polypeptides, PH domains that recognize PIP3 or other phosphatidylinositol-derived lipids, and Tec homology domains found in tyrosine kinases of the Tec family. Tyrosine kinase families depicted are the Src family kinases, which include c-Src, Lyn, Fyn, and Lck; the Syk family kinases, which include Syk and ZAP-70; and the Tec family kinases, which include Tec, Btk, and Itk.
SH2 domains are composed of about 100 amino acids folded into a particular conformation, and they recognize specific phosphotyrosine-containing peptides. In antigen receptor signaling, Src family kinases phosphorylate tyrosine residues present in particular motifs in the cytoplasmic tails of proteins that are part of the receptor complex (described later). These phosphotyrosine motifs in the antigen receptor complex are then recognized by SH2 domains present in tyrosine kinases of the Syk family, such as Syk and ZAP-70 (see Fig. 7-3). The recruitment of a Syk family kinase to an antigen receptor by means of a specific SH2 domain–phosphotyrosine interaction is a key step in antigen receptor activation.
SH3 domains are also about 100 amino acids in length, and they help mediate protein-protein interactions by binding to proline-rich stretches in certain proteins. Another type of modular domain, called a pleckstrin homology (PH) domain, can recognize specific phospholipids. The PH domains in a number of signaling molecules including the TEC family tyrosine kinase Btk, recognize phosphatidylinositol trisphosphate (PIP3), a lipid moiety on the inner leaflet of the plasma membrane.
Adaptor proteins function as molecular hubs that physically link different enzymes and promote the assembly of complexes of signaling molecules. Adaptors may be integral membrane proteins like LAT (linker for the activation of T cells) (Fig. 7-4), or they may be cytosolic proteins such as BLNK (B cell linker), SLP-76 (SH2 domain–containing linker protein of 76 kD), and GADS (Grb-2–related adaptor protein downstream of Shc). A typical adaptor may contain a few specific domains that mediate protein-protein interactions, such as SH2 and SH3 domains, among others (there are many more types of modular domains not mentioned here). Adaptors may also contain some proline-rich stretches (that can bind other proteins that contain SH3 domains), and they also often contain critical tyrosine residues that may be phosphorylated by tyrosine kinases. The amino acid residues that are close to a tyrosine moiety that is phosphorylated determine which specific SH2 domains may bind that site. For instance, an adaptor with a YxxM motif (where Y represents tyrosine, M represents methionine, and x refers to any amino acid) will bind an SH2 domain in the lipid kinase phosphatidylinositol 3-kinase (PI3-kinase). The same adaptor protein may recruit a tyrosine kinase with a specific SH3 domain to a proline-rich stretch, and tyrosine phosphorylation of the adaptor may thus result in a tyrosine kinase and PI3-kinase being perched next to each other, resulting in the phosphorylation and activation of PI3-kinase. Signal transduction can therefore be visualized as a kind of social networking phenomenon. An initial signal (tyrosine phosphorylation, for instance) results in proteins being brought close to one another at designated hubs (adaptors), resulting in the activation of specific enzymes that eventually influence the nuclear localization or activity of specific downstream transcription factors or induce other cellular events, such as actin polymerization.
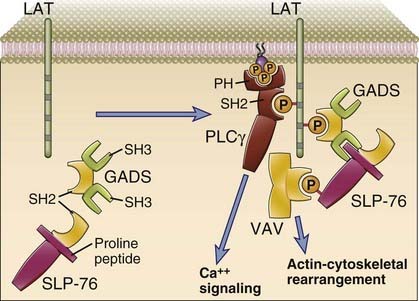
FIGURE 7–4 Selected adaptors that participate in lymphocyte activation.
On the left, LAT, an integral membrane protein that functions as an adaptor, and two cytosolic adaptors, GADS and SLP-76, are shown in a nonactivated T cell. On the right, after T cell activation, LAT is tyrosine phosphorylated and is shown to have recruited PLCγ and the GADS adaptor, both of which contain SH2 domains. A proline-rich amino acid stretch in SLP-76 associates with an SH3 domain of GADS, and tyrosine-phosphorylated SLP-76 recruits Vav.
The Immune Receptor Family
Immune receptors are a unique family of receptor complexes typically made up of integral membrane proteins of the immunoglobulin (Ig) superfamily that are involved in ligand recognition, associated with other transmembrane signaling proteins that have unique tyrosine-containing motifs in their cytoplasmic tails. Whereas the signaling components are generally separate proteins from those involved in ligand recognition, in a few members of the family, the receptor consists of a single chain in which the extracellular domain is involved in ligand recognition and the cytoplasmic tail contains tyrosine residues that contribute to signaling. The signaling proteins of the immune receptor family are often positioned close to non-receptor tyrosine kinases of the Src family. The latter also possess N-terminal lipid anchors that tether them to the inner leaflet of the plasma membrane. The cytoplasmic tyrosine-containing motifs on the signaling proteins of the immune receptor family are generally one of two different types. ITAMs (immunoreceptor tyrosine-based activating motifs) are found on receptors involved in cell activation and have the sequence YxxL/I(x)6-8YxxL/I, where Y represents a tyrosine residue, L represents leucine, I represents isoleucine, and x refers to any amino acid. ITAM motifs can be phosphorylated on both tyrosine residues that are present in this motif by Src family kinases when immune receptors are activated. Tyrosine-phosphorylated ITAMs recruit a distinct tyrosine kinase of the Syk/ZAP-70 family, which contains tandem SH2 domains that each bind to one of the two phosphorylated YxxL/I motifs of the ITAM. Binding of Syk (or ZAP-70) to a phospho-ITAM results in a conformational change in this kinase and its activation. The activated Syk or ZAP-70 kinase then drives immune cell activation. Some immune receptors inhibit cellular responses, and signaling chains in these receptors may contain a slightly different tyrosine-containing motif that is called an ITIM (immunoreceptor tyrosine-based inhibitory motif), which has the consensus sequence V/L/IxYxxL, where V refers to valine. Phosphorylated ITIMs recruit tyrosine or inositol lipid phosphatases, enzymes that remove phosphate residues from phosphotyrosine moieties or from certain lipid phosphates and thus counteract ITAM-based immune receptor activation.
Members of the immune receptor family include antigen receptors on both B cells and T cells, the IgE receptor on mast cells, and activating and inhibitory Fc receptors on innate immune cells and B lymphocytes (Fig. 7-5). ITAMs are found in the cytoplasmic tails of several immune receptor complexes that are involved in signal transduction, including the ζ chain and CD3 proteins of the T cell receptor (TCR) complex, Igα and Igβ proteins associated with membrane Ig molecules (the antigen receptors) of B cells, and components of several Fc receptors and of the NKG2D activating receptor on natural killer (NK) cells (see Chapter 4). ITIM-containing inhibitory receptors include CD22, FcγRIIB, and several inhibitory NK cell receptors.
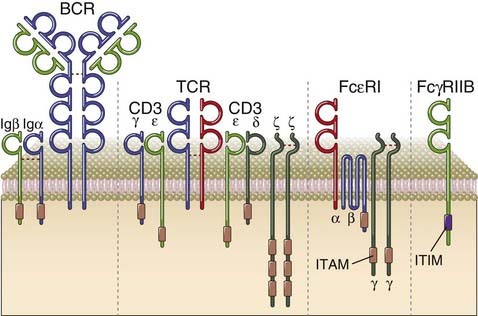
FIGURE 7–5 Selected members of the immune receptor family.
Four selected members of the immune receptor family are depicted. Typically, immune receptors that activate immune cells have separate chains for recognition and associated chains that contain cytosolic ITAMs. Examples shown here include the B cell receptor (BCR), the T cell receptor (TCR), and the high-affinity receptor for IgE (FcεRI). Inhibitory receptors in the immune system typically have ITIM motifs on the cytosolic portion of the same chain that uses its extracellular domain for ligand recognition. The inhibitory receptor shown, FcγRIIB, is found on B cells and myeloid cells.
General Features of Antigen Receptor Signaling
Signaling downstream of the T and B cell antigen receptors is characterized by a similar sequence of events, consisting of the following.
This sequence of events is described in more detail in the context of T cell and B cell receptor signaling later in the chapter.
Alterations in the strength of TCR and B cell receptor (BCR) signaling influence the fates of lymphocytes during their development and activation. In other words, the presence of different numbers of activated signaling molecules induced by antigen-ligated receptors is interpreted differently by lymphocytes. For instance, during maturation of T cells in the thymus, weak antigen receptor signals are required for positive selection, the process that preserves useful cells by matching coreceptors to the appropriate MHC molecules, and gradations of signal strength may determine positive selection of developing T cells into the CD4 or CD8 lineage (see Chapter 8). In contrast, strong antigen receptor signals during maturation may contribute to lymphocyte death by apoptosis. The strength of TCR and BCR signaling may also differentially influence the type of immune response that is generated by a given antigen.
Antigen receptor signaling is fine-tuned and modulated by three mechanisms that are unique to this class of receptors.
In addition, antigen receptor signals may, in some circumstances, cooperate with signals from receptors, known as costimulatory receptors, that add yet another level of control to the process of lymphocyte activation. Costimulatory receptors provide “second signals” for lymphocytes (antigen recognition provides the first signal) and ensure that immune responses are optimally triggered by infectious pathogens and substances that mimic microbes. Unlike coreceptors, costimulatory receptors do not recognize components of the same ligands as do antigen receptors; signal outputs downstream of costimulatory receptors are integrated with the signals derived from the antigen receptor, and these signals cooperate to fully activate lymphocytes. The prototypic costimulatory receptor is CD28 on T cells, which is activated by the costimulatory molecules B7-1 and B7-2 (CD80 and CD86), ligands induced on antigen-presenting cells (APCs) as a result of their exposure to microbes (see Chapter 9).
The T Cell Receptor Complex and T Cell Signaling
The TCR was discovered in the early 1980s, around the same time that the structure of major histocompatibility complex (MHC) molecules associated with peptides, the ligands for T cells, was being defined (see Chapter 6). A number of separate approaches were used to molecularly identify the TCR. One approach depended on the identification of genes that were expressed specifically in T cells and that also could be shown to have undergone a gene rearrangement event specifically in these cells (a characteristic feature of antigen receptor genes, described in Chapter 8). The first gene thus identified was homologous to Ig genes and proved to be a chain of the hetero-dimeric γδ TCR. In another approach, clonal populations of T cells were created and monoclonal antibodies were generated against different T cell clones. Monoclonal antibodies that each recognized only a specific T cell clone were identified. These clonotype-specific antibodies identified a chain of the TCR. In yet another study, one chain of the TCR was identified serendipitously, when sequencing of a T cell–specific library of cDNAs unexpectedly revealed a novel gene with homology to immunoglobulins. We now know that the TCR is similar to antibodies, but there are important differences between these two types of antigen receptors (Table 7-1).
TABLE 7–1 Properties of Lymphocyte Antigen Receptors: T Cell Receptor and Immunoglobulins
| T Cell Receptor (TCR) | Immunoglobulin (Ig) | |
|---|---|---|
| Components | α and β chains | Heavy and light chains |
| Number of Ig domains | One V domain and one C domain in each chain | Heavy chain: one V domain, three or four C domains Light chain: one V domain and one C domain |
| Number of CDRs | Three in each chain for antigen binding | Three in each chain |
| Associated signaling molecules | CD3 and ζ | Igα and Igβ |
| Affinity for antigen (Kd) | 10−5-10−7 M | 10−7-10−11 M (secreted Ig) |
| Changes after cellular activation | ||
| Production of secreted form | No | Yes |
| Isotope switching | No | Yes |
| Somatic mutations | No | Yes |
The Structure of the T Cell Receptor for Antigen
The antigen receptor of MHC-restricted CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs) is a heterodimer consisting of two transmembrane polypeptide chains, designated TCR α and β, covalently linked to each other by a disulfide bridge between extracellular cysteine residues (Fig. 7-6). These T cells are called αβ T cells. A less common type of TCR, found on γδ T cells, is composed of TCR γ and δ chains. Each TCR α and β chain consists of one Ig-like N-terminal variable (V) domain, one Ig-like constant (C) domain, a hydrophobic transmembrane region, and a short cytoplasmic region. Thus, the extracellular portion of the TCR αβ heterodimer is structurally similar to the antigen-binding fragment (Fab) of an Ig molecule, which is made up of the V and C regions of a light chain and the V region and one C region of a heavy chain (see Chapter 5).
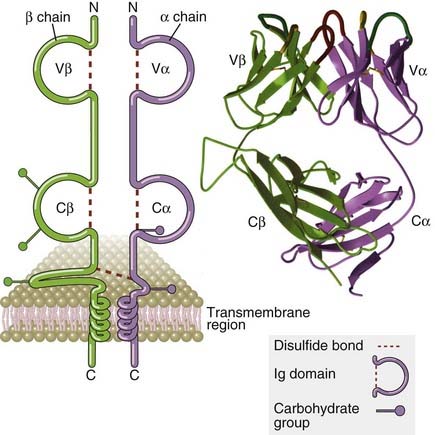
FIGURE 7–6 Structure of the T cell receptor.
The schematic diagram of the αβ TCR (left) shows the domains of a typical TCR specific for a peptide-MHC complex. The antigen-binding portion of the TCR is formed by the Vβ and Vα domains. The ribbon diagram (right) shows the structure of the extracellular portion of a TCR as revealed by x-ray crystallography. The hypervariable segment loops that form the peptide-MHC binding site are at the top.
(Modified from Bjorkman PJ. MHC restriction in three dimensions: a view of T cell receptor/ligand interactions. Cell 89:167-170, 1997. Copyright Cell Press.)
The V regions of the TCR α and β chains contain short stretches of amino acids where the variability between different TCRs is concentrated, and these form the hypervariable or complementarity-determining regions (CDRs). Three CDRs in the α chain and three similar regions in the β chain together form the part of the TCR that specifically recognizes peptide-MHC complexes (Fig. 7-7). The β chain V domain contains a fourth hypervariable region that does not appear to participate in antigen recognition but is the binding site for microbial products called superantigens (see Chapter 15). Each TCR chain, like Ig heavy and light chains, is encoded by multiple gene segments that undergo somatic rearrangements during the maturation of the T lymphocytes (see Chapter 8).
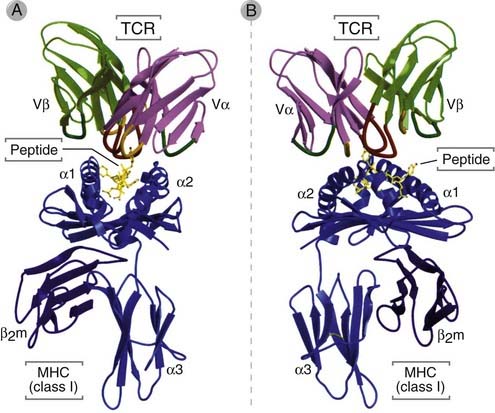
FIGURE 7–7 Binding of a TCR to a peptide-MHC complex.
The V domains of a TCR are shown interacting with a human class I MHC molecule, HLA-A2, presenting a viral peptide (in yellow). A is a front view and B is a side view of the x-ray crystal structure of the trimolecular MHC-peptide-TCR complex.
(From Bjorkman PJ. MHC restriction in three dimensions: a view of T cell receptor/ligand interactions. Cell 89:167-170, 1997. Copyright Cell Press.)
The C regions of both α and β chains continue into short hinge regions, which contain cysteine residues that contribute to a disulfide bond linking the two chains. The hinge is followed by hydrophobic transmembrane portions, an unusual feature of which is the presence of positively charged amino acid residues, including a lysine residue (in the α chain) or a lysine and an arginine residue (in the β chain). These residues interact with negatively charged residues present in the transmembrane portions of other polypeptides (those of the CD3 complex and ζ) that are part of the TCR complex. Both TCR α and β chains have carboxyl-terminal cytoplasmic tails that are 5 to 12 amino acids long. Like membrane Ig on B cells (see later), these cytoplasmic regions are too small to transduce signals, and specific molecules physically associated with the TCR serve the signal-transducing functions of this antigen receptor complex.
The CD3 and ζ proteins are noncovalently associated with the TCR αβ heterodimer, and when the TCR recognizes antigen, these associated proteins transduce the signals that lead to T cell activation. The components of the TCR complex are illustrated in Figures 7-8 and 7-9. The CD3 proteins and the ζ chain are identical in all T cells regardless of specificity, which is consistent with their role in signaling and not in antigen recognition. The CD3 proteins are required not only for signaling in T cells but for surface expression of the functionally complete receptor complex on T cells.
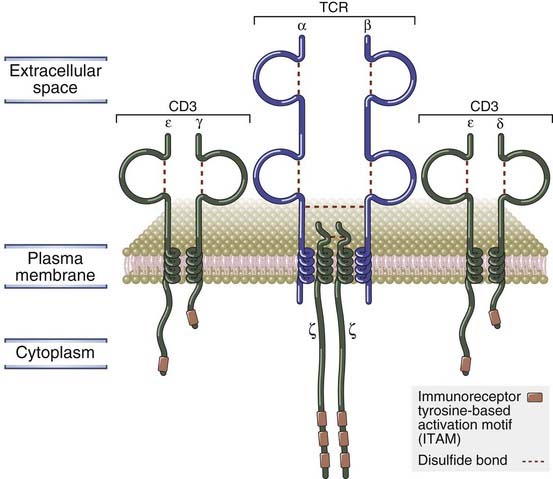
FIGURE 7–8 Components of the TCR complex.
The TCR complex of MHC-restricted T cells consists of the αβ TCR noncovalently linked to the CD3 and ζ proteins. The association of these proteins with one another is mediated by charged residues in their transmembrane regions, which are not shown.
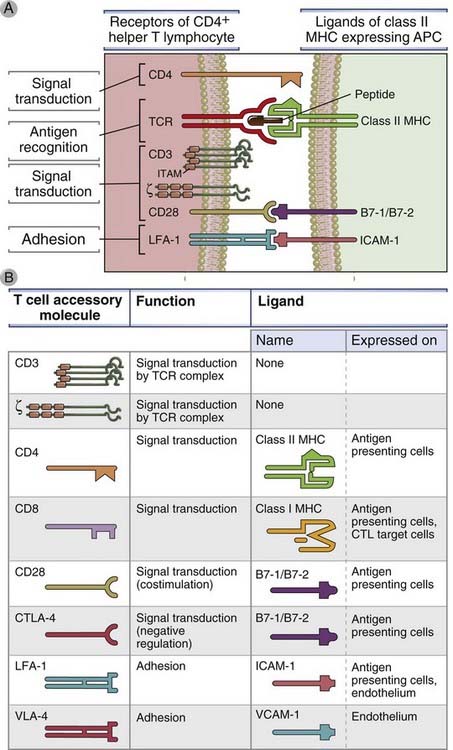
FIGURE 7–9 Ligand-receptor pairs involved in T cell activation.
A, The major surface molecules of CD4+ T cells involved in the activation of these cells (the receptors) and the molecules on APCs (the ligands) recognized by the receptors are shown. CD8+ T cells use most of the same molecules, except that the TCR recognizes peptide–class I MHC complexes, and the coreceptor is CD8, which recognizes class I MHC. Immunoreceptor tyrosine-based activation motifs (ITAMs) are the regions of signaling proteins that are phosphorylated on tyrosine residues and become docking sites for other signaling molecules. CD3 is composed of three polypeptide chains, named γ, δ, and ε, arranged in two pairs (γε and δε); we show CD3 as three protein chains. B, The important properties of the major “accessory” molecules of T cells, so called because they participate in responses to antigens but are not the receptors for antigen, are summarized. CTLA-4 (CD152) is a receptor for B7 molecules that delivers inhibitory signals; its role in shutting off T cell responses is described in Chapter 9. VLA molecules are integrins involved in leukocyte binding to endothelium (see Chapter 3). APC, antigen-presenting cell; ICAM-1, intercellular adhesion molecule 1; LFA-1, leukocyte function-associated antigen 1; MHC, major histocompatibility complex; TCR, T cell receptor; VLA, very late antigen.
The CD3 γ, δ, and ε proteins are homologous to each other. The N-terminal extracellular regions of the γ, δ, and ε chains each contains a single Ig-like domain, and therefore these three proteins are members of the Ig superfamily. The transmembrane segments of all three CD3 chains contain a negatively charged aspartic acid residue that binds to positively charged residues in the transmembrane domains of the TCR α and β chains. Each TCR complex contains one TCR αβ heterodimer associated with one CD3 γε heterodimer, one CD3 δε heterodimer, and one disulfide-linked ζζ homodimer.
The cytoplasmic domains of the CD3 γ, δ, and ε proteins range from 44 to 81 amino acid residues in length, and each of these domains contains one ITAM. The ζ chain has a short extracellular region of nine amino acids, a transmembrane region containing a negatively charged aspartic acid residue (similar to the CD3 chains), and a long cytoplasmic region (113 amino acids) that contains three ITAMs. It is normally expressed as a homodimer. The ζ chain is also associated with signaling receptors on lymphocytes other than T cells, such as the Fcγ receptor (FcγRIII) of NK cells.
Signal Initiation by the T Cell Receptor
Ligation of the TCR by MHC-peptide ligands results in the clustering of coreceptors with the antigen receptor and phosphorylation of ITAM tyrosine residues. Phosphorylation of ITAM tyrosines initiates signal transduction and the activation of downstream tyrosine kinases, which in turn phosphorylate tyrosine residues on other adaptor proteins. The subsequent steps in signal transduction are generated by the specific recruitment of key enzymes that each initiate distinct downstream signaling pathways.
It is thought that the TCR, like other immune receptors, is activated when multiple receptor molecules are brought together by binding to adjacent antigenic epitopes. However, cross-linking of the TCR poses a challenge because the induction of receptor clustering would require a high density of identical MHC-peptide complexes on APCs, and APCs generally express very few peptide-MHC complexes, perhaps as few as 100 per cell, that may be recognized by a given TCR (see Chapter 6). How, then, is the signal from the TCR initiated? It is known that antigen recognition by the TCR induces ITAM phosphorylation by active Src family kinases, but the actual mechanism of signal initiation remains to be conclusively determined. There is growing evidence that ITAMs in the TCR complex are “folded” and unavailable before the TCR recognizes antigen. Recognition of MHC-peptide complexes may induce a conformational change in the TCR, making the ITAMs associated with the linked CD3 or ζ chains available for tyrosine phosphorylation by Src family kinases. Alternatively, the activity of Src family kinases may be enhanced after receptor ligation (Fig. 7-10). The CD4 and CD8 coreceptors (described next) greatly facilitate the activation process by bringing Lck (which is loosely associated with the tail of the coreceptor proteins) close to the CD3 and ζ ITAMs (see Fig. 7-10). Eventually, a relatively stable interface is formed between the T cell and the APC, and this interface is known as the immunologic synapse (discussed later).
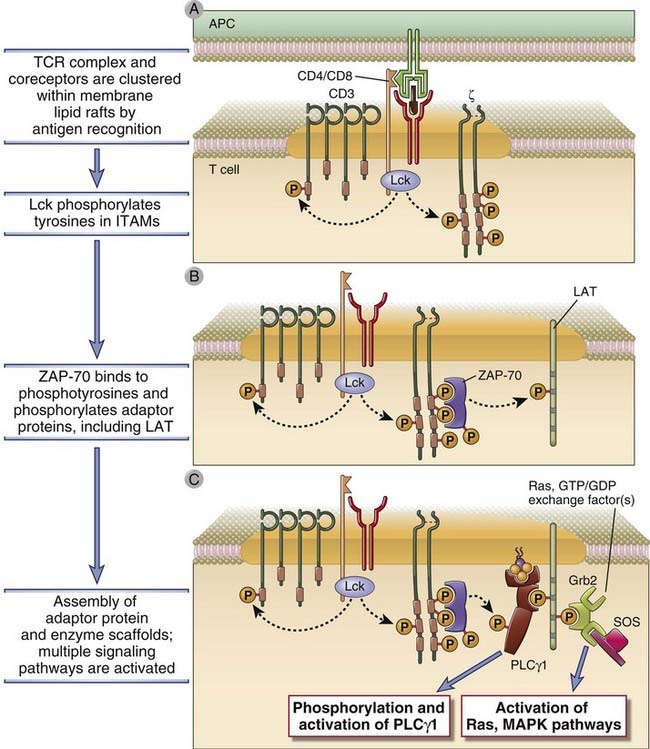
FIGURE 7–10 Early tyrosine phosphorylation events in T cell activation.
On antigen recognition, there is clustering of TCR complexes with coreceptors (CD4, in this case). CD4-associated Lck becomes active and phosphorylates tyrosines in the ITAMs of CD3 and ζ chains (A). ZAP-70 binds to the phosphotyrosines of the ζ chains and is itself phosphorylated and activated. (The illustration shows one ZAP-70 molecule binding to two phosphotyrosines of one ITAM in the ζ chain, but it is likely that initiation of a T cell response requires the assembly of multiple ZAP-70 molecules on each ζ chain.) Active ZAP-70 then phosphorylates tyrosines on various adaptor molecules, such as LAT (B). The adaptors become docking sites for cellular enzymes such as PLCγ1 and exchange factors that activate Ras and other small G proteins upstream of MAP kinases (C), and these enzymes activate various cellular responses.
The Role of the CD4 and CD8 Coreceptors in T Cell Activation
CD4 and CD8 are T cell coreceptors that bind to nonpolymorphic regions of MHC molecules and facilitate signaling by the TCR complex during T cell activation (see Fig. 7-9). These proteins are called coreceptors because they bind to MHC molecules and thus recognize a part of the same ligand (peptide-MHC complexes) that interacts with the TCR. Mature αβ T cells express either CD4 or CD8, but not both. CD8 and CD4 interact with class I and class II MHC molecules, respectively, and are responsible for the class I or class II MHC restriction of these subsets of T cells (see Fig. 7-9 and Chapter 6).
CD4 and CD8 are transmembrane glycoprotein members of the Ig superfamily (Fig. 7-11). CD4 is expressed as a monomer on the surface of peripheral T cells and thymocytes and is also present on mononuclear phagocytes and some dendritic cells. It is the receptor on T cells for the envelope protein of the human immunodeficiency virus. CD4 has four extracellular Ig-like domains, a hydrophobic transmembrane region, and a highly basic cytoplasmic tail 38 amino acids long. The two N-terminal Ig-like domains of the CD4 protein bind to the nonpolymorphic β2 domain of the class II MHC molecule.
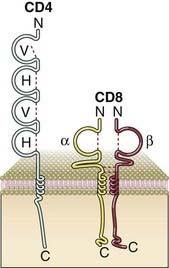
FIGURE 7–11 A schematic view of the structure of the CD4 and CD8 coreceptors.
The CD4 protein is an integral membrane monomer consisting of four extracellular Ig domains, a transmembrane domain, and a cytoplasmic tail. The CD8 protein is either a disulfide-linked αβ integral membrane heterodimer or a disulfide linked αα homodimer (not shown). Each chain has a single extracellular Ig domain. The cytoplasmic portions of both CD4 and CD8 can associate with Lck (not shown).
Most CD8 molecules exist as disulfide-linked heterodimers composed of two related chains called CD8α and CD8β (see Fig. 7-11). Both the α chain and the β chain have a single extracellular Ig domain, a hydrophobic transmembrane region, and a highly basic cytoplasmic tail about 25 amino acids long. The Ig domain of CD8 binds to the nonpolymorphic α3 domain of class I MHC molecules. Some T cells express CD8 αα homodimers, but this different form appears to function like the more common CD8 αβ heterodimers. These homodimers are also present on a subset of murine dendritic cells (see Chapter 6).
The cytoplasmic tails of both CD4 and CD8 bind the Src family kinase Lck. The ability of these coreceptors to bind to MHC molecules helps these proteins to be drawn adjacent to the TCR that contacts the same MHC-peptide complex on the APC. As a result, on the cytosolic face of the membrane, Lck is drawn very close to the ITAMs in CD3 and ζ proteins and phosphorylates the ITAMs, thus facilitating the subsequent recruitment and activation of the kinase ZAP-70.
Activation of Tyrosine Kinases and a Lipid Kinase During T Cell Activation
Phosphorylation of residues in proteins and lipids plays a central role in the transduction of signals from the TCR complex and coreceptors. Within seconds of TCR ligation, many of the tyrosine residues within the ITAMs of the CD3 and ζ chains become phosphorylated (see Fig.7-10). In addition to coreceptor-associated Lck, another Src family kinase that is found in physical association with the TCR complex is CD3-associated Fyn, and it may play a role similar to that of Lck. Knockout mice lacking Lck show some defects in T cell development, and double knockout mice lacking both Lck and Fyn show even more severe defects.
The tyrosine-phosphorylated ITAMs in the ζ chain become “docking sites” for the Syk family tyrosine kinase called ZAP-70 (ζ-associated protein of 70 kD). ZAP-70 contains two SH2 domains that can bind to ITAM phosphotyrosines. Each ITAM has two tyrosine residues, and both of these must be phosphorylated to provide a docking site for one ZAP-70 molecule. The bound ZAP-70 becomes a substrate for the adjacent Lck, which phosphorylates specific tyrosine residues of ZAP-70. As a result, ZAP-70 acquires its own tyrosine kinase activity and is then able to phosphorylate a number of other cytoplasmic signaling molecules. A critical threshold of ZAP-70 activity may be needed before downstream signaling events will proceed, and this threshold is achieved by the recruitment of multiple ZAP-70 molecules to the phosphorylated ITAMs on the ζ chains and on CD3 tails.
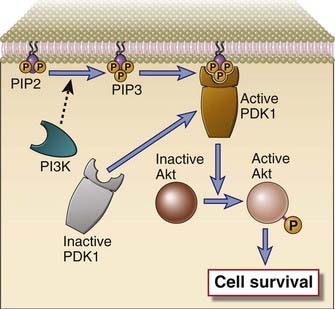
Another signaling pathway in T cells involves the activation of PI3-kinase, which phosphorylates a specific membrane-associated inositol lipid (Fig. 7-12). This enzyme is recruited to the TCR complex and associated adaptor proteins and generates phosphatidylinositol trisphosphate (PIP3) from membrane phosphatidylinositol bisphosphate (PIP2) on the inner leaflet of the plasma membrane. Certain signaling proteins in the cytosol have specialized PH domains that have an affinity for PIP3, and as a result, PH domain–containing proteins can bind to the inside of the cell membrane only when PIP3 is generated. Examples of PH domain–containing proteins include kinases such as Itk in T cells and Btk in B cells. Another important PIP3-dependent kinase is PDK1, which is required for the phosphorylation and activation of an important downstream kinase called Akt. Activated Akt phosphorylates crucial targets and contributes to cell survival in a number of ways. Phosphorylation by Akt leads to the inactivation of two proapoptotic members of the Bcl-2 family, BAD and BAX. Akt also inactivates a Forkhead family transcription factor that induces the expression of Fas ligand, and this kinase also targets caspase-9 for degradation.
Recruitment and Modification of Adaptor Proteins
Activated ZAP-70 phosphorylates several adaptor proteins that are able to bind signaling molecules (see Fig. 7-10). A key early event in T cell activation is the ZAP-70–mediated tyrosine phosphorylation of adaptor proteins such as SLP-76 and LAT. Phosphorylated LAT directly binds PLCγ1, a key enzyme in T cell activation (discussed later), and coordinates the recruitment of several other adaptor proteins, including SLP-76, GADS, and Grb-2, to the cluster of TCR and TCR-associated proteins, sometimes referred to as the signalosome. Thus, LAT serves to bring a variety of downstream components of TCR signaling pathways close to their upstream activators. Because the function of many of these adaptors depends on their tyrosine phosphorylation by active ZAP-70, only antigen recognition (the physiologic stimulus for ZAP-70 activation) triggers the signal transduction pathways that lead to functional T cell responses.
Formation of the Immunologic Synapse
When the TCR complex recognizes MHC-associated peptides on an APC, several T cell surface proteins and intracellular signaling molecules are rapidly mobilized to the site of T cell–APC contact (Fig. 7-13). This region of physical contact between the T cell and the APC forms a bull’s-eye–like structure that is called an immunologic synapse or a supramolecular activation cluster (SMAC). The T cell molecules that are rapidly mobilized to the center of the synapse include the TCR complex (the TCR, CD3, and ζ chains), CD4 or CD8 coreceptors, receptors for costimulators (such as CD28), enzymes such as PKC-θ, and adaptor proteins that associate with the cytoplasmic tails of the transmembrane receptors. At this portion of the synapse, called the c-SMAC (for central supramolecular activation cluster), the distance between the T cell plasma membrane and that of the APC is about 15 nm. Integrins remain at the periphery of the synapse, where they function to stabilize the binding of the T cell to the APC, forming the peripheral portion of the SMAC called the p-SMAC. In this outer part of the synapse, the two membranes are about 40 nm apart. Many signaling molecules found in synapses are initially localized to regions of the plasma membrane that have a lipid content different from the rest of the cell membrane and are called lipid rafts or glycolipid-enriched microdomains. TCR and costimulatory receptor signaling is initiated in these rafts, and signaling initiates cytoskeletal rearrangements that allow rafts to coalesce and form the immunologic synapse.
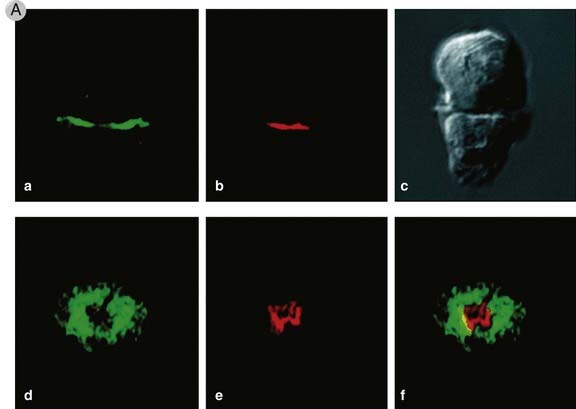
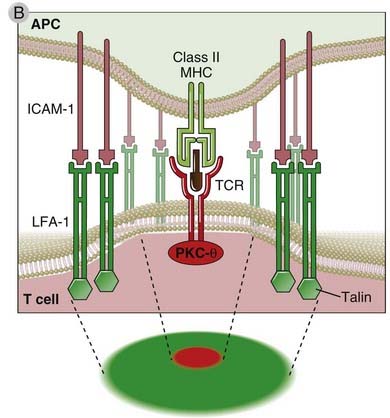
FIGURE 7–13 The immunologic synapse.
A, This figure shows two views of the immunologic synapse in a T cell–APC conjugate (shown as a Nomarski image in panel c). Talin, a protein that associates with the cytoplasmic tail of the LFA-1 integrin, was revealed by an antibody labeled with a green fluorescent dye, and PKC-θ, which associates with the TCR complex, was visualized by antibodies conjugated to a red fluorescent dye. In panels a and b, a two-dimensional optical section of the cell contact site along the x-y axis is shown, revealing the central location of PKC-θ and the peripheral location of talin, both in the T cell. In panels d-f, a three-dimensional view of the entire region of cell-cell contact along the x-z axis is provided. Note, again, the central location of PKC-θ and the peripheral accumulation of talin.
(Reprinted with permission of Macmillan Publishers Ltd. from Monks CRF, BA Freiburg, H Kupfer, N Sciaky, and A Kupfer. Three dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86, copyright 1998.)
B, A schematic view of the synapse, showing talin and LFA-1 in the p-SMAC (green) and PKC-θ and the TCR in the c-SMAC (red).
Immunologic synapses may serve a number of functions during and after T cell activation.
MAP Kinase Signaling Pathways in T Lymphocytes
Small guanine nucleotide–binding proteins (G proteins) activated by antigen recognition stimulate at least three different mitogen-activated protein (MAP) kinases, which in turn activate distinct transcription factors. G proteins are involved in diverse activation responses in different cell types. Two major members of this family activated downstream of the TCR are Ras and Rac. Each activates a different component or set of transcription factors, and together they mediate many cellular responses of T cells.
The mechanism of Ras activation in T cells involves the adaptor proteins LAT and Grb-2 (Fig. 7-14). When LAT is phosphorylated by ZAP-70 at the site of TCR clustering, it serves as the docking site for the SH2 domain of Grb-2. Once attached to LAT, Grb-2 recruits the Ras GTP/GDP exchange factor called SOS (so named because it is the mammalian homologue of a Drosophila protein called son of sevenless) to the plasma membrane. SOS catalyzes GTP for GDP exchange on Ras. This generates the GTP-bound form of Ras (written as Ras·GTP), which then activates a “MAP kinase” cascade of three kinases, the first two of which phosphorylate and activate the next kinase in the cascade. The last kinase in the cascade initiated by Ras is a MAP kinase called ERK. Ras·GTP activates a kinase called c-Raf, which then activates a dual-specificity kinase that phosphorylates ERK on closely spaced threonine and tyrosine residues. This dual-specificity kinase is an example of a MAP kinase kinase (a kinase that activates a MAP kinase). The activated ERK MAP kinase translocates to the nucleus and phosphorylates a protein called Elk, and phosphorylated Elk stimulates transcription of c-Fos, a component of the activation protein 1 (AP-1) transcription factor.
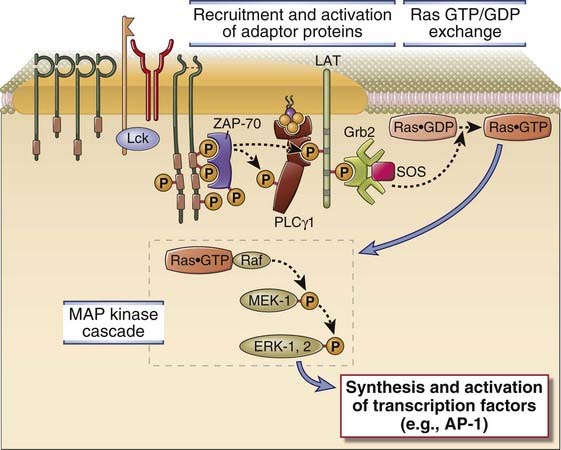
FIGURE 7–14 The Ras-MAP kinase pathway in T cell activation.
ZAP-70 that is activated by antigen recognition phosphorylates membrane-associated adaptor proteins (such as LAT), which then bind another adaptor, Grb-2, that provides a docking site for the GTP/GDP exchange factor SOS. SOS converts Ras·GDP to Ras·GTP. Ras·GTP activates a cascade of enzymes, which culminates in the activation of the MAP kinase ERK. A parallel Rac-dependent pathway generates another active MAP kinase, JNK (not shown).
The activities of ERK and JNK are eventually shut off by the action of dual-specificity protein tyrosine/threonine phosphatases. These phosphatases are induced or activated by ERK and JNK themselves, providing a negative feedback mechanism to terminate T cell activation.
Calcium- and PKC-Mediated Signaling Pathways in T Lymphocytes
TCR signaling leads to the activation of the γ1 isoform of the enzyme phospholipase C (PLCγ1), and the products of PLCγ1-mediated hydrolysis of membrane lipids activate enzymes that induce specific transcription factors in T cells (Fig. 7-15). PLCγ1 is a cytosolic enzyme specific for inositol phospholipids that is recruited to the plasma membrane by tyrosine-phosphorylated LAT within minutes of ligand binding to the TCR. Here, the enzyme is phosphorylated by ZAP-70 and by other kinases, such as the Tec family kinase called Itk. Phosphorylated PLCγ1 catalyzes the hydrolysis of a plasma membrane phospholipid called PIP2, generating two breakdown products, the soluble sugar triphosphate, inositol 1,4,5-trisphosphate (IP3), and membrane-bound diacylglycerol (DAG). IP3 and DAG then activate two distinct downstream signaling pathways in T cells.
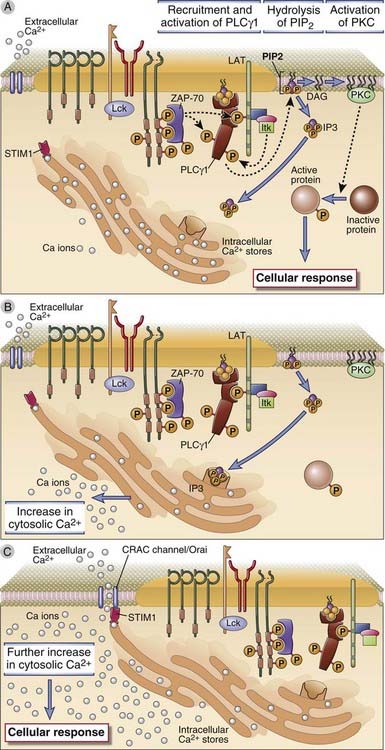
FIGURE 7–15 T cell signaling downstream of PLCγ1.
A, The LAT adaptor protein that is phosphorylated on T cell activation binds the cytosolic enzyme PLCγ1, which is phosphorylated by ZAP-70 and other kinases, such as Itk, and activated. Active PLCγ1 hydrolyzes membrane PIP2 to generate IP3, which stimulates an increase in cytosolic calcium, and DAG, which activates the enzyme PKC. B, Depletion of endoplasmic reticulum calcium is sensed by STIM1. C, STIM1 which induces the opening of the CRAC channel that facilitates entry of extracellular calcium into the cytosol. Orai is a component of the CRAC channel. Increased cytosolic calcium and PKC then activate various transcription factors, leading to cellular responses.
IP3 produces a rapid increase in cytosolic free calcium within minutes after T cell activation. IP3 diffuses through the cytosol to the endoplasmic reticulum, where it binds to its receptor, a ligand-gated calcium channel, and stimulates release of membrane-sequestered calcium stores. The released calcium causes a rapid rise (during a few minutes) in the cytosolic free calcium ion concentration, from a resting level of about 100 nM to a peak of 600 to 1000 nM. The depletion of endoplasmic reticulum calcium is sensed by an endoplasmic reticulum membrane protein called STIM1, which activates a “store-operated” plasma membrane ion channel called a CRAC (calcium release–activated calcium) channel. The result is an influx of extracellular calcium that sustains cytosolic levels at about 300 to 400 nM for more than an hour. A key component of the CRAC channel is a protein called Orai, which was discovered as a gene that is defective in a rare human immunodeficiency disease. Cytosolic free calcium acts as a signaling molecule by binding to a ubiquitous calcium-dependent regulatory protein called calmodulin. Calcium-calmodulin complexes activate several enzymes, including a protein serine/threonine phosphatase called calcineurin that is important for transcription factor activation, as discussed later.
Diacylglycerol (DAG), the second breakdown product of PIP2, is a membrane-bound lipid that activates the enzyme protein kinase C (PKC). There are several isoforms of PKC that participate in the generation of active transcription factors, discussed later. The combination of elevated free cytosolic calcium and DAG activates certain isoforms of membrane-associated PKC by inducing a conformational change that makes the catalytic site of the kinase accessible to its substrates. Numerous downstream proteins are phosphorylated by PKC. The PKC-θ isoform localizes to the immunologic synapse and is involved in the activation and nuclear translocation of the nuclear factor κB (NF-κB) transcription factor. Pathways of NF-κB activation are discussed later in this chapter.
So far, we have described several signal transduction pathways initiated by ligand binding to the TCR that result in the activation of different types of enzymes: small G protein–MAP kinase pathways leading to activation of kinases such as ERK and JNK; a PLCγ1-calcium–dependent pathway leading to activation of the phosphatase calcineurin; and a DAG-dependent pathway leading to activation of PKC. Each of these pathways contributes to the expression of genes encoding proteins needed for T cell clonal expansion, differentiation, and effector functions. In the following section, we describe the mechanisms by which these different signaling pathways stimulate the transcription of various genes in T cells.
Activation of Transcription Factors That Regulate T Cell Gene Expression
The enzymes generated by TCR signaling activate transcription factors that bind to regulatory regions of numerous genes in T cells and thereby enhance transcription of these genes (Fig. 7-16). Much of our understanding of the transcriptional regulation of genes in T cells is based on analyses of cytokine gene expression. The transcriptional regulation of most cytokine genes in T cells is controlled by the binding of transcription factors to nucleotide sequences in the promoter and enhancer regions of these genes. For instance, the IL-2 promoter, located 5′ of the coding exons of this gene, contains a segment of approximately 300 base pairs in which are located binding sites for several different transcription factors. All these sites must be occupied by transcription factors for maximal transcription of the IL-2 gene. Different transcription factors are activated by different cytoplasmic signal transduction pathways, and the requirement for multiple transcription factors accounts for the need to activate many signaling pathways after antigen recognition. It is likely that the same principles are true for many genes in T cells, including genes encoding cytokine receptors and effector molecules, although different genes may be responsive to different combinations of transcription factors.
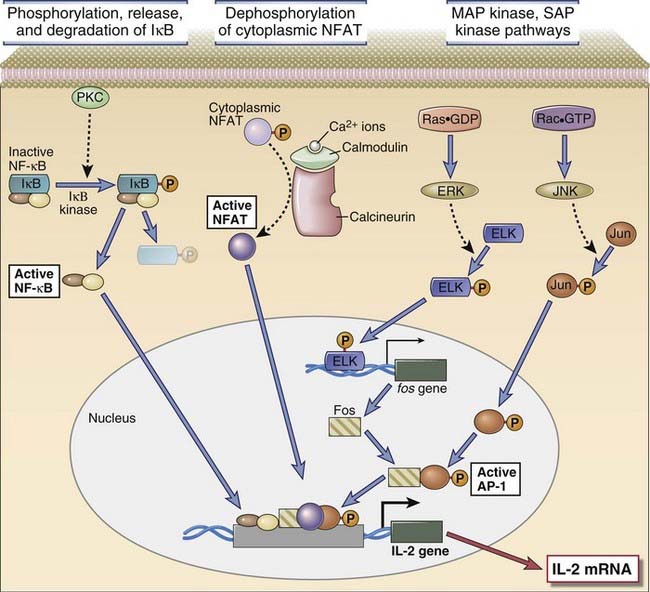
FIGURE 7–16 Activation of transcription factors in T cells.
Multiple signaling pathways converge in antigen-stimulated T cells to generate transcription factors that stimulate expression of various genes (in this case, the IL-2 gene). The calcium-calmodulin pathway activates NFAT, and the Ras and Rac pathways generate the two components of AP-1. Less is known about the link between TCR signals and NF-κB activation. (NF-κB is shown as a complex of two subunits, which in T cells are typically the p50 and p65 proteins, named for their molecular sizes in kilodaltons.) PKC is important in T cell activation, and the PKC-θ isoform is particularly important in activating NF-κB. These transcription factors function coordinately to regulate gene expression. Note also that the various signaling pathways are shown as activating unique transcription factors, but there may be considerable overlap, and each pathway may play a role in the activation of multiple transcription factors.
Three transcription factors that are activated in T cells by antigen recognition and appear to be critical for most T cell responses are nuclear factor of activated T cells (NFAT), AP-1, and NF-κB.
The mechanism of activation of NFAT was discovered indirectly by studies of the mechanism of action of the immunosuppressive drug cyclosporine (see Chapter 16). This drug and the functionally similar compound, FK506, are natural products of fungi and are widely used therapeutic agents to treat allograft rejection. They function largely by blocking T cell cytokine gene transcription. Cyclosporine binds to a cytosolic protein called cyclophilin, and FK506 binds to a protein called FK506-binding protein (FKBP). Cyclophilin and FKBP are also called immunophilins. Cyclosporine-cyclophilin complexes and FK506-FKBP complexes bind to and inhibit calcineurin and thereby block translocation of NFAT into the nucleus.
The links between different signaling proteins, activation of transcription factors, and functional responses of T cells are often difficult to establish because there are complex and incompletely understood interactions between signaling pathways. Also, for the sake of simplicity, we often discuss signaling in the context of linear pathways, but it is likely that this does not reflect the more complex and interconnected reality. Finally, we have focused on selected pathways to illustrate how antigen recognition may lead to biochemical alterations, but it is clear that many other signaling molecules are also involved in antigen-induced lymphocyte activation.
Modulation of T Cell Signaling by Protein Tyrosine Phosphatases
Tyrosine phosphatases remove phosphate moieties from tyrosine residues on proteins and generally inhibit TCR signaling. Two tyrosine phosphatases that serve an important inhibitory role in lymphocytes and other hematopoietic cells are called SHP-1 and SHP-2 (for SH2 domain–containing phosphatases 1 and 2). Inhibitory phosphatases are typically recruited by inhibitory receptors that are induced after a lymphocyte has been activated by tyrosine kinases. These phosphatases inhibit signal transduction by removing phosphates from tyrosine residues in key signaling molecules and thus functionally antagonize tyrosine kinases. Another inhibitory phosphatase that does not act on phosphoproteins but rather is specific for an inositol phospholipid is called SHIP (SH2 domain–containing inositol phosphatase). Like SHP-1 and SHP-2, SHIP binds to phosphorylated ITIM sequences on specific inhibitory receptors. SHIP removes a phosphate group from PIP3, a phospholipid in the inner leaflet of the plasma membrane, and thus antagonizes PI3-kinase signaling in lymphocytes.
Although most phosphatases attenuate lymphocyte signaling, one tyrosine phosphatase, CD45, facilitates lymphocyte activation. The CD45 protein is a receptor tyrosine phosphatase expressed in all hematopoietic cells. It is an integral membrane protein whose cytoplasmic tail contains tandem protein tyrosine phosphatase domains. CD45 dephosphorylates inhibitory tyrosine residues in the Src family kinases Lck and Fyn and thus contributes to the generation of active kinases.
Costimulatory Receptors of T Cells
Costimulatory signals are delivered by receptors that recognize ligands that are induced on APCs by microbes and cooperate with TCR signals to augment signaling and activate T cells. The two-signal hypothesis for T cell activation was introduced in Chapter 1. TCR signaling aided by coreceptors drives the T cell’s response to foreign structures. In immunologic jargon, this response by the TCR to MHC and peptide on an APC is referred to as signal 1. T cells are fully activated only when a foreign peptide is recognized in the context of the activation of the innate immune system by a pathogen or some other cause of inflammation. Costimulatory ligands represent the danger signals (or signal 2) induced on antigen-presenting cells by microbes. “Foreignness” must combine with “danger” for optimal T cell activation to occur.
The CD28 Family of Costimulatory Receptors
The best defined costimulators for T lymphocytes are a pair of related proteins, called B7-1 (CD80) and B7-2 (CD86), which are expressed on activated dendritic cells, macrophages, and B lymphocytes. The CD28 molecule on T cells is the principal costimulatory receptor for delivery of second signals for T cell activation. The biologic roles of the B7 and CD28 proteins are considered in more detail in Chapter 9.
Another important activating member of the CD28 family is a receptor called ICOS (inducible costimulator), which plays an important role in T follicular helper cell development and will be discussed in Chapters 9 and 11.
The CD2/SLAM Family of Costimulatory Receptors
Although the best studied and most prominent family of costimulatory receptors on T cells is the CD28 family, other proteins also contribute to optimal T cell activation and differentiation. One important family of proteins that plays a role in the activation of T cells and NK cells is a group of proteins structurally related to a receptor called CD2 (Fig. 7-17). CD2 is a glycoprotein present on more than 90% of mature T cells, on 50% to 70% of thymocytes, and on NK cells. The molecule contains two extracellular Ig domains, a hydrophobic transmembrane region, and a long (116 amino acid residues) cytoplasmic tail. The principal ligand for CD2 in humans is a molecule called leukocyte function-associated antigen 3 (LFA-3, or CD58), also a member of the CD2 family. LFA-3 is expressed on a wide variety of hematopoietic and nonhematopoietic cells, either as an integral membrane protein or as a phosphatidylinositol-anchored membrane molecule. In mice, the principal ligand for CD2 is CD48, which is also a member of the CD2 family and is distinct from but structurally similar to LFA-3.
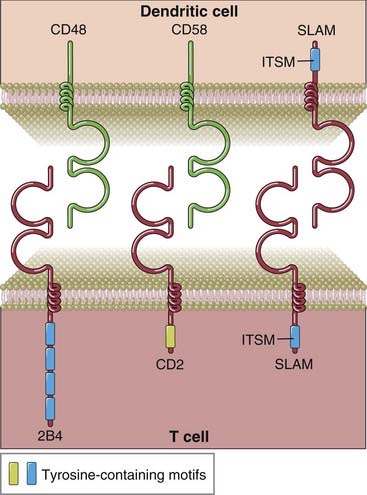
FIGURE 7–17 Selected costimulatory receptors of the CD2 family and their ligands.
2B4, CD2, and SLAM contain two extracellular Ig-like domains, and their cytoplasmic tails also contain tyrosine-containing motifs. The tyrosine-based motif in the tail regions of SLAM and SLAM family members such as 2B4 is called an ITSM and binds to SAP or SAP-like proteins (not shown).
CD2 functions both as an intercellular adhesion molecule and as a signal transducer. Some anti-CD2 antibodies increase cytokine secretion by and proliferation of human T cells cultured with anti-TCR/CD3 antibodies, indicating that CD2 signals can enhance TCR-triggered T cell responses. Some anti-CD2 antibodies block conjugate formation between T cells and other LFA-3–expressing cells, indicating that CD2 binding to LFA-3 also promotes cell-cell adhesion. Such antibodies inhibit both CTL activity and antigen-dependent helper T cell responses. Knockout mice lacking both CD28 and CD2 have more profound defects in T cell responses than do mice lacking either molecule alone. This indicates that CD28 and CD2 may compensate for each other, an example of the redundancy of costimulatory receptors of T cells. On the basis of such findings, anti-CD2 antibodies are currently being tested for their efficacy in psoriasis.
A distinct subgroup of the CD2 family of proteins is known as the SLAM (signaling lymphocytic activation molecule) family. SLAM, like all members of the CD2 family, is an integral membrane protein that contains two extracellular Ig domains and a relatively long cytoplasmic tail. The cytoplasmic tail of SLAM, but not of CD2, contains a specific tyrosine-based motif, TxYxxV/I (where T is a threonine residue, Y is a tyrosine residue, V is a valine, I is an isoleucine, and x is any amino acid), known as an immunoreceptor tyrosine-based switch motif (ITSM) that is distinct from the ITAM and ITIM motifs found in other activating and inhibitory receptors. It is called a switch motif because in some receptors, this motif can orchestrate a “switch” from the binding of a tyrosine phosphatase, SHP-2, in the absence of an adaptor to the binding of other enzymes in the presence of an adaptor called SAP (SLAM-associated protein), thus potentially mediating a change from an inhibitory to an activating function.
The extracellular Ig domains of SLAM are involved in homophilic interactions. SLAM on a T cell can interact with SLAM on a dendritic cell and, as a result, the cytoplasmic tail of SLAM may deliver signals to T cells. The ITSM motif binds to SAP, and the latter forms a bridge between SLAM and Fyn (a Src family kinase that is also physically linked to CD3 proteins in T cells). SLAM and other members of the SLAM family function as costimulatory receptors in T cells, NK cells, and some B cells. As we shall discuss in Chapter 20, mutations in the SH2D1A gene encoding SAP are the cause of a disease called the X-linked lymphoproliferative syndrome (XLP).
An important member of the SLAM family in NK cells, CD8+ T cells, and γδ T cells is called 2B4 (see Fig. 7-17). 2B4 recognizes a known ligand for CD2 called CD48. Like SLAM, the cytoplasmic tail of 2B4 contains ITSM motifs, binds to the SAP adaptor protein, and signals by recruiting Fyn. Defective 2B4 signaling may contribute in a major way to the immune deficit in patients with the X-linked lymphoproliferative syndrome.
The B Lymphocyte Antigen Receptor Complex
The B lymphocyte antigen receptor is a transmembrane form of an antibody molecule associated with two signaling chains. The structure of antibodies was described in detail in Chapter 5. Here we will focus on some salient features of the membrane forms of Ig and their associated proteins and discuss how they deliver signals to B cells. Because the signaling pathways are similar to those in T cells, we will summarize these without much detail. However, there are both similarities and significant differences between B and T cell antigen receptors (see Table 7-1).
Structure of the B Cell Receptor for Antigen
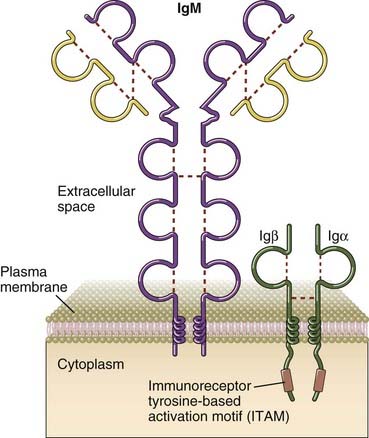
Membrane IgM and IgD, the antigen receptors of naive B cells, have short cytoplasmic tails consisting of only three amino acids (lysine, valine, and lysine). These tails are too small to transduce signals generated after the recognition of antigen. Ig-mediated signals are transduced by two other molecules, called Igα and Igβ, that are disulfide linked to one another and are expressed in B cells noncovalently associated with membrane Ig (Fig. 7-18). These proteins each contain an ITAM motif in their cytoplasmic tails, are required for the transport of membrane Ig molecules to the cell surface, and together with membrane Ig form the B cell receptor (BCR) complex. B cell receptor complexes in class-switched B cells, including memory B cells, contain membrane immunoglobulins that may be of the IgG, IgA, or IgE classes (see Chapter 11).
Signal Initiation by the B Cell Receptor
Signal initiation by antigens occurs by cross-linking of the BCR and is facilitated by the coreceptor for the BCR. It is thought that cross-linking of membrane Ig by multivalent antigens brings Src family kinases together and, by promoting their physical interaction, fully activates these enzymes, enabling them then to phosphorylate the tyrosine residues on the ITAMs of Igα and Igβ. It is also possible that as in T cells, antigen binding facilitates a conformational change in BCR-associated ITAMs, making them accessible to already active Src family kinases that modify ITAM tyrosines, but there is at present no firm evidence to support such a model. The phosphorylation of ITAM tyrosine residues triggers all subsequent signaling events downstream of the BCR (Fig. 7-19). Cross-linked Ig receptors enter lipid rafts, where many adaptor proteins and signaling molecules are concentrated. Igα and Igβ are loosely connected to Src family tyrosine kinases such as Lyn, Fyn, and Blk, and these enzymes are also linked by lipid anchors to the inside of the plasma membrane. The phosphorylation of the tyrosine residues in the ITAMs of Igα and Igβ provides a docking site for the tandem SH2 domains of the Syk tyrosine kinase. Syk is activated when it associates with phosphorylated tyrosines of ITAMs and may itself be phosphorylated on specific tyrosine residues by BCR-associated Src family kinases, leading to further activation. If the antigen is monovalent and incapable of cross-linking multiple Ig molecules, some signaling may nevertheless occur, but additional activation by helper T cells may be necessary to fully activate B cells, as discussed in Chapter 11.
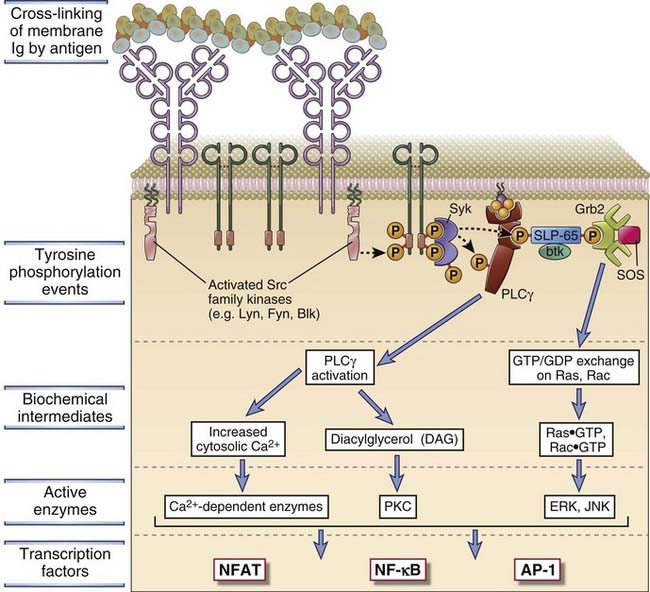
FIGURE 7–19 Signal transduction by the BCR complex.
Antigen-induced cross-linking of membrane Ig on B cells leads to clustering and activation of Src family tyrosine kinases and tyrosine phosphorylation of the ITAMs in the cytoplasmic tails of the Igα and Igβ molecules. This leads to docking of Syk and subsequent tyrosine phosphorylation events as depicted. Several signaling cascades follow these events, as shown, leading to the activation of several transcription factors. These signal transduction pathways are similar to those described in T cells.
Role of the CR2/CD21 Complement Receptor as a Coreceptor for B Cells
The activation of B cells is enhanced by signals that are provided by complement proteins and the CD21 coreceptor complex, which link innate immunity to the adaptive humoral immune response (Fig. 7-20). The complement system consists of a collection of plasma proteins that are activated either by binding to antigen-complexed antibody molecules (the classical pathway) or by binding directly to some microbial surfaces and polysaccharides in the absence of antibodies (the alternative and lectin pathways) (see Chapters 4 and 12). Thus, polysaccharides and other microbial components may activate the complement system directly, during innate immune responses. Proteins and other antigens that do not activate complement directly may be bound by preexisting antibodies or by antibodies produced early in the response, and these antigen-antibody complexes activate complement by the classical pathway. Recall that complement activation results in the proteolytic cleavage of complement proteins. The key component of the system is a protein called C3, and its cleavage results in the production of a molecule called C3b that binds covalently to the microbe or antigen-antibody complex. C3b is further degraded into a fragment called C3d, which remains bound to the microbial surface or on the antigen-antibody complex. B lymphocytes express a receptor for C3d that is called the type 2 complement receptor (CR2, or CD21). The complex of C3d and antigen or C3d and antigen-antibody complex binds to B cells, with the membrane Ig recognizing antigen and CR2 recognizing the bound C3d (see Fig. 7-20).
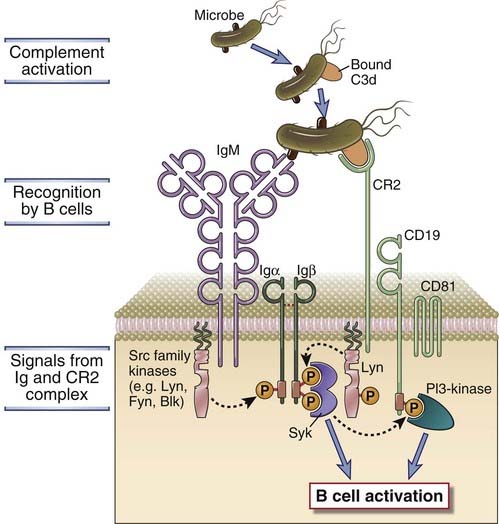
FIGURE 7–20 Role of complement in B cell activation.
B cells express a complex of the CR2 complement receptor, CD19, and CD81. Microbial antigens that have bound the complement fragment C3d can simultaneously engage both the CR2 molecule and the membrane Ig on the surface of a B cell. This leads to the initiation of signaling cascades from both the BCR complex and the CR2 complex, because of which the response to C3d-antigen complexes is greatly enhanced compared with the response to antigen alone.
CR2 is expressed on mature B cells as a complex with two other membrane proteins, CD19 and CD81 (also called TAPA-1). The CR2-CD19-CD81 complex is often called the B cell coreceptor complex because CR2 binds to antigens through attached C3d at the same time that membrane Ig binds directly to the antigen. Binding of C3d to the B cell complement receptor brings CD19 in proximity to BCR-associated kinases, and the cytoplasmic tail of CD19 rapidly becomes tyrosine phosphorylated. Phosphorylation of the tail of CD19 results in the efficient recruitment of Lyn, a Src family kinase, that can amplify BCR signaling by greatly enhancing the phosphorylation of ITAM tyrosines in Igα and Igβ. Phosphorylated CD19 also activates other signaling pathways, notably one dependent on the enzyme PI3-kinase, which in turn further augment signaling initiated by antigen binding to membrane Ig. PI3-kinase is required for the activation of Btk and PLCγ2 because these enzymes must bind to PIP3 on the inner leaflet of the plasma membrane to be fully activated, in a manner analogous to that shown in Figure 7-12. The net result of coreceptor activation is that the response of the antigen-stimulated B cell is greatly enhanced.
Signaling Pathways Downstream of the B Cell Receptor
After antigen binding to the BCR, Syk and other tyrosine kinases activate numerous downstream signaling pathways that are regulated by adaptor proteins (see Fig. 7-19). The cross-linking of the BCR or the activation of the BCR by a coreceptor-dependent mechanism results in ITAM phosphorylation and recruitment of Syk to the ITAM, followed by the activation of this dual SH2 domain–containing kinase. Activated Syk phosphorylates critical tyrosine residues on adaptor proteins such as SLP-65 (SH2-binding leukocyte phosphoprotein of 65 kD, also called BLNK, B cell linker protein). This facilitates the recruitment to these adaptor proteins of other SH2 domain– and phosphotyrosine-binding (PTB) domain–containing enzymes, including guanine nucleotide exchange proteins that can separately activate Ras and Rac, PLCγ2, and the Btk tyrosine kinase, among others. Recruitment facilitates the activation of these downstream effectors, each generally contributing to the activation of a distinct signaling pathway.
These signaling cascades ultimately lead to the activation of a number of transcription factors that induce the expression of genes whose products are required for functional responses of B cells. Some of the transcription factors that are activated by antigen receptor–mediated signal transduction in B cells are Fos (downstream of Ras and ERK activation), JunB (downstream of Rac and JNK activation), and NF-κB (downstream of Btk, PLCγ2, and PKC-β activation). These were discussed earlier when we described T cell signaling pathways. These and other transcription factors, many not mentioned here, are involved in stimulating proliferation and differentiation of B cells (see Chapter 11).
As in T cells, our knowledge of antigen-induced signaling pathways in B cells and their links with subsequent functional responses is incomplete. We have described some of these pathways to illustrate the main features, but others may play important roles in B cell activation. The same signaling pathways are used by membrane IgM and IgD on naive B cells and by IgG, IgA, and IgE on B cells that have undergone isotype switching because all these membrane isotypes associate with Igα and Igβ.
The Attenuation of Immune Receptor Signaling
Activation of lymphocytes has to be tightly controlled to limit immune responses against microbes for avoidance of “collateral damage” to host tissues. In addition, the immune system needs mechanisms that will prevent reactions against self antigens. We will describe the biology of these control mechanisms in later chapters, notably Chapter 14. Attenuation of signaling is essential to prevent uncontrolled inflammation and lymphoproliferation. Here we discuss the biochemical mechanisms that serve to limit and terminate lymphocyte activation.
Inhibitory signaling in lymphocytes is mediated primarily by inhibitory receptors and also by enzymes known as E3 ubiquitin ligases that mark certain signaling molecules for degradation. Inhibitory receptors typically recruit and activate phosphatases that counter signaling events induced by antigen receptors (Fig. 7-21). The functional responses of all cells are regulated by a balance between stimulatory and inhibitory signals, and we will first describe, from a broad mechanistic standpoint, how inhibitory receptors may function in NK cells, T cells, and B cells. We will then describe how ubiquitin E3 ligases may attenuate signaling in lymphocytes. The biologic relevance of signal attenuation through inhibitory receptors in NK cells, T cells, and B cells is addressed in Chapters 4, 9, and 11, respectively.
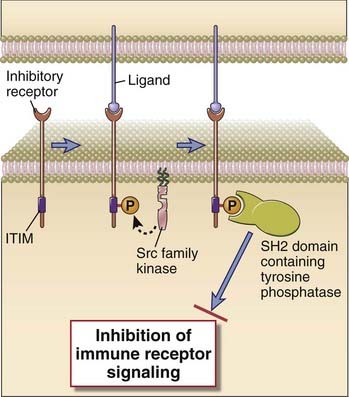
FIGURE 7–21 Inhibitory signaling in lymphocytes.
A schematic depiction is provided of an inhibitory receptor with an extracellular ligand-binding domain and a cytosolic ITIM motif. Ligand binding results in phosphorylation of the ITIM tyrosine by a Src family kinase, followed by recruitment of an SH2 domain–containing tyrosine phosphatase that can attenuate immune receptor signaling.
Inhibitory Receptors in NK Cells, B Cells, and T Cells
Most but not all inhibitory receptors in the immune system contain cytosolically oriented ITIM motifs that can recruit SH2 domain–containing phosphatases and thus attenuate signaling in a broadly similar manner (see Fig 7-21). Inhibitory receptors play key roles in NK cells, T cells, and B cells as well as in other cells of innate immunity.
In human NK cells, the key inhibitory receptors can broadly be divided into three groups: KIRs or killer Ig-like receptors (see Chapter 4); ILT (Ig-like transcript) family proteins that are closely related to KIRs; and C-type lectins, the major one being a heterodimer consisting of the NKG2A C-type lectin and CD94. These inhibitory receptors are not restricted to NK cells and may also be present on some activated T cells. KIRs contain extracellular Ig domains that can recognize class I HLA molecules, and a subset of these receptors contains cytosolic ITIM motifs. ILT-2, part of an evolutionarily older family of inhibitory receptors than KIRs, also has extracellular Ig domains that bind HLA class I and cytosolic ITIM motifs. The CD94/NKG2A dimer binds to an atypical class I MHC molecule called HLA-E, and the NKG2A chain of this dimer contains cytosolic ITIM motifs.
Tyrosine residues on the ITIMs of these and other inhibitory receptors can be phosphorylated by Src family kinases linked to lymphocyte activation and, as described earlier, recruit SH2 domain–containing tyrosine phosphatases such as SHP-1 and SHP-2 and an SH2 domain–containing inositol phosphatase called SHIP. SHP-1 and SHP-2 attenuate tyrosine kinase–initiated signaling from activating receptors in NK cells as well as from the BCR and TCR in B and T cells, respectively. SHIP removes phosphate moieties from PIP3 and thus inhibits PI3-kinase activity in lymphocytes, NK cells, and innate immune cells.
The prototypical inhibitory receptor of the CD28 family, CTLA-4 (also called CD152), has the ability to inhibit T cell responses induced on activated T cells and has a higher affinity than CD28 for B7 proteins. CTLA-4 is involved in the maintenance of unresponsiveness (tolerance) to self antigens and is discussed in this context in Chapter 14. Another inhibitory receptor of the same family is called PD-1 (programmed death 1), and this is also discussed in Chapter 14. CTLA-4 contains a tyrosine-containing motif in its tail that may be inhibitory; PD-1 contains cytosolic ITIM and ITSM motifs, and its cytosolic tail is critical for the initiation of inhibitory signals. The key inhibitory receptors in B cells include FcγRIIB and CD22/Siglec-2. FcγRIIB, an important attenuator of signaling in activated B cells as well as in dendritic cells and macrophages, can bind IgG-containing immune complexes through extracellular Ig domains. It primarily recruits SHIP and antagonizes PI3-kinase signaling. This receptor dampens B cell activation in the latter part of a humoral immune response and will be discussed in more detail in Chapter 11.
E3 Ubiquitin Ligases and the Degradation of Signaling Proteins
One of the major ways of degrading cytosolic and nuclear proteins involves the covalent attachment of ubiquitin residues to these proteins. Although ubiquitination of proteins is frequently linked to the degradation of these proteins in proteasomes, proteins can be ubiquitinated in a number of ways, each form of ubiquitination serving a very different function. In the context of signal transduction, different types of ubiquitination mediate signal attenuation on the one hand and signal generation on the other.
Ubiquitination was briefly discussed in Chapter 6 in the context of class I MHC–based antigen processing and presentation. Ubiquitin is a 76–amino acid protein that is activated in an ATP-dependent fashion by an E1 enzyme, then “carried” by an E2 enzyme, and transferred to lysine residues on specific substrates that are recognized by specific E3 ubiquitin ligases. In many cases, after the C terminus of a ubiquitin moiety is covalently linked to a lysine residue on a target protein, the C-terminal ends of subsequent ubiquitin moieties may be covalently attached to lysine residues on the preceding ubiquitin to generate a polyubiquitin chain. The geometric shape of the polyubiquitin chain is very different, depending on which specific lysine residue on the preceding ubiquitin molecule in the chain is the site for covalent binding of the next ubiquitin molecule, and the shape of the ubiquitin chain has important functional consequences. If lysine in position 48 of the first ubiquitin moiety forms an isopeptide bond with the C terminus of the next ubiquitin and so on, a lysine-48 type of ubiquitin chain will be generated that can be recognized by the proteasomal cap, and the protein will be targeted for degradation in the proteasome. Some E3 ligases generate a different type of polyubiquitin chain called a lysine-63 type of chain, which does not target proteins for degradation but instead generates a structure for latching the marked proteins onto other specific proteins; this is important in NF-κB signaling, as discussed later. For some functions, in particular targeting membrane proteins to lysosomes rather than to proteasomes, only a single ubiquitin moiety may need to be attached to a protein target.
Several E3 ligases are found in T cells; some of them are involved in signal activation and others in signal attenuation. The prototype of E3 ligases involved in terminating T cell responses is Cbl-b, but several others serve similar functions. Recruitment of Cbl-b to the TCR complex and associated adaptor proteins leads to the monoubiquitination, endocytosis, and lysosomal degradation of the TCR complex, and this may be a mechanism for the attenuation of TCR signaling (Fig. 7-22). CD28 signals block the inhibitory activity of Cbl-b, and this is one mechanism by which costimulation augments TCR signals. In knockout mice lacking Cbl-b, the T cells respond to antigen even without CD28-mediated costimulation and produce abnormally high amounts of IL-2. These mice develop autoimmunity as a result of the enhanced activation of their T cells.
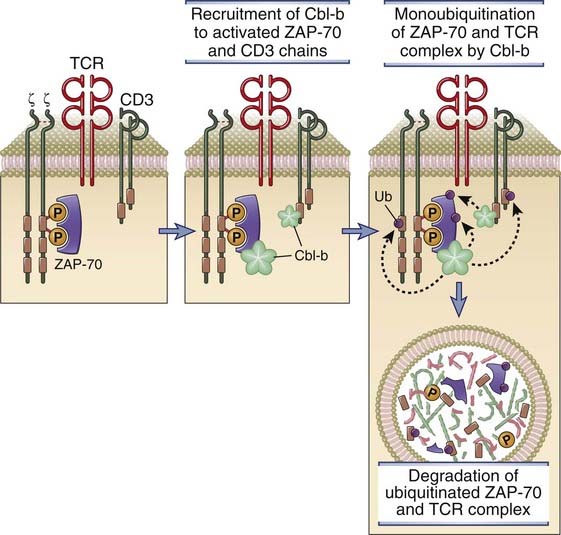
FIGURE 7–22 Role of the ubiquitin ligase Cbl-b in terminating T cell responses.
Cbl-b is recruited to the TCR complex, where it facilitates the monoubiquitination of CD3, ZAP-70, and other proteins of the TCR complex. These proteins are targeted for proteolytic degradation in lysosomes and other organelles (not shown).
Cytokine Receptors and Signaling
Cytokines, the secreted “messenger molecules” of the immune system, were introduced in Chapter 1 and discussed in Chapter 4 in the context of innate immunity; their roles in adaptive T cell–mediated immune responses will be described in Chapters 9 and 10. Here we will describe receptors for cytokines and their mechanisms of signaling.
All cytokine receptors consist of one or more transmembrane proteins whose extracellular portions are responsible for cytokine binding and whose cytoplasmic portions are responsible for initiation of intracellular signaling pathways. For most cytokine receptors, these signaling pathways are typically activated by ligand-induced receptor clustering, bringing together the cytoplasmic portions of two or more receptor molecules, thus inducing the activity of unique non-receptor tyrosine kinases. In the case of the TNF receptor family of cytokine receptors, preformed receptor trimers apparently undergo a conformational change after contacting their cognate trimeric ligands.
Classes of Cytokine Receptors
The most widely used classification of cytokine receptors is based on structural homologies of the extracellular cytokine-binding domains and shared intracellular signaling mechanisms (Fig. 7-23). Signaling through type I and type II cytokine receptors occurs by a similar mechanism, known as JAK-STAT signaling, that is described in more detail later. Cytokine receptors of the TNF receptor family activate a number of pathways, a prominent one being the NF-κB pathway, which will also be considered in detail later. Signaling through the IL-1R and the TLR families uses a common cytoplasmic domain, and a major component downstream is ubiquitin E3 ligase–dependent activation of the NF-κB pathway. Chemokines, which are chemotactic cytokines, activate a large subfamily of receptors and have been discussed in Chapter 3. Chemokine receptors are seven-transmembrane GPCRs described in the early part of this chapter and are not elaborated on here.
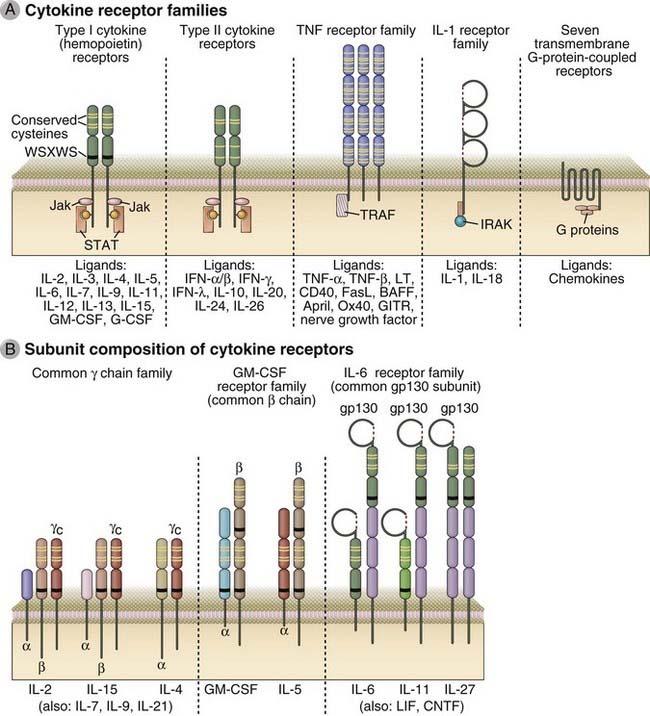
FIGURE 7–23 Structure of cytokine receptors.
A, Receptors for different cytokines are classified into families on the basis of conserved extracellular domain structures and signaling mechanisms. The cytokines or other ligands that bind to each receptor family are listed below the schematic drawings. WSXWS, tryptophan-serine-X-tryptophan-serine. B, Groups of cytokine receptors share identical or highly homologous subunit chains. Selected examples of cytokine receptors in each group are shown.
Type I Cytokine Receptors (Hematopoietin Receptor Family)
Type I cytokine receptors are dimers or trimers that typically consist of unique ligand-binding chains and one or more signal-transducing chains, which are often shared by receptors for different cytokines. These chains contain one or two domains with a conserved pair of cysteine residues and a membrane proximal peptide stretch containing a tryptophan-serine-X-tryptophan-serine (WSXWS) motif, where X is any amino acid (Fig. 7-23A). The conserved sequences of the receptors form structures that bind cytokines that have four α-helical bundles and are referred to as type I cytokines, but the specificity for individual cytokines is determined by amino acid residues that vary from one receptor to another. This receptor family can be divided into subgroups based on structural homologies or the use of shared signaling polypeptides (Fig. 7-23B). One group contains a signaling component called the common γ chain (CD132); in this group are the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. A distinct subgroup of type I receptors includes receptors that share a common β chain (CD131) subunit. This subgroup includes the receptors for IL-3, IL-5, and GM-CSF. Another subgroup of receptors uses the gp130 signaling component, and this includes the receptors for IL-6, IL-11, and IL-27. All the type I cytokine receptors engage JAK-STAT signaling pathways.
Type II Cytokine Receptors (Interferon Receptor Family)
The type II receptors are similar to type I receptors by virtue of possessing two extracellular domains with conserved cysteines, but type II receptors do not contain the WSXWS motif. These receptors consist of one ligand-binding polypeptide chain and one signal-transducing chain. All the type II cytokine receptors, like the type I receptors, engage JAK-STAT signaling pathways. This family includes receptors for type I and type II interferons and for IL-10, IL-20, and IL-26.
TNF Receptor Family
These receptors are part of a large family of preformed trimers (some of which are not considered cytokine receptors) with conserved cysteine-rich extracellular domains and shared intracellular signaling mechanisms that typically stimulate gene expression but in some cases induce apoptosis. Some important receptors of this family, most of which will be discussed in other chapters in their biologic contexts, include the TNF receptors TNFRI and TNFRII, the CD40 protein, Fas, the lymphotoxin receptor, and the BAFF receptor family. The ligands for these receptors also form trimers. Some of these ligands are membrane bound, whereas others are soluble.
Binding of the ligands to the preformed trimeric receptors typically induces a conformational change and recruits adaptor proteins to the receptor complex. These adaptors in turn recruit enzymes that include both E3 ubiquitin ligases, which mediate nondegradatory polyubiquitination, and protein kinases, which initiate downstream signaling. In the case of the TNF receptor illustrated in Figure 7-24, the receptor recruits the adaptor protein TRADD (TNF receptor–associated death domain), and TRADD in turn can recruit proteins called TRAFs (TNF receptor associated factors), which possess a unique type of E3 ligase activity that will be discussed in the section on NF-κB signaling. The type I TNF receptor (there are two different receptors for TNF) and Fas (CD95) can also recruit adaptors that induce the activation of caspase-8, and these receptors, in certain cells, can thereby induce apoptosis.
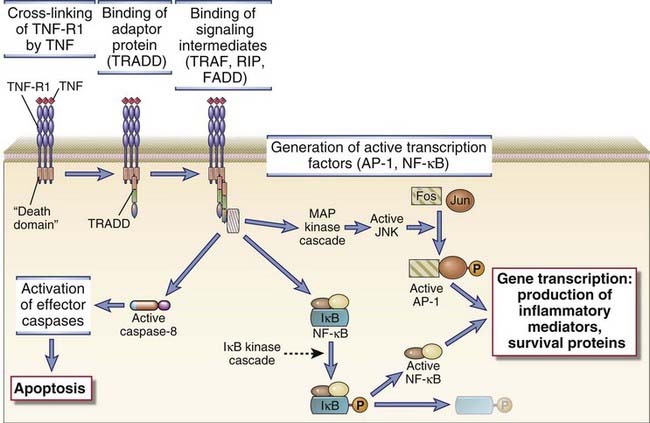
FIGURE 7–24 Signaling through the TNF receptor can result in NF-κB and MAP kinase activation or in the induction of apoptotic death.
Ligation of the type I TNF receptor results in the recruitment of an adaptor protein called TRADD, which in turn can activate TRAF molecules (E3 ubiquitin ligase) and the RIP1 kinase. Downstream consequences include the activation of the NF-κB pathway and the JNK MAP kinase pathway or the induction of apoptotic death.
IL-1/TLR Family
The receptors of this family share a conserved cytosolic sequence, called the Toll-like/IL-1 receptor (TIR) domain, and engage similar signal transduction pathways that induce new gene transcription. Toll-like receptor (TLR) signaling has been considered in Chapter 4. Briefly, engagement of the IL-1R or of TLRs results in receptor dimerization and the recruitment of one or more of four known TIR domain–containing adaptors to the TIR domain of the cytoplasmic tail of the receptor. The adaptors link TLRs to different members of the IRAK (IL-1R–associated kinase) family. IRAKs can in turn link adaptors to TRAF6, an E3 ubiquitin ligase required for NF-κB activation. Other pathways activated by TLR signaling include MAP kinase activation and the phosphorylation of IRF3 and IRF7, transcriptional inducers of type I interferons. The latter aspect of TLR signaling has been considered in the context of the antiviral state in Chapter 4. In a general sense, the MAL/MyD88 adaptor pair links TLRs to the early induction of NF-κB signaling and to MAP kinase activation, whereas the TRAM/TRIF adaptor pair leads to the delayed activation of NF-κB and the activation of IRF3. TLR4, for instance, activates MAL/Myd88 signaling initially from the cell surface and TRAM/TRIF signaling subsequently after receptor endocytosis. The mechanisms connecting IL-1R/TLR signaling and NF-κB activation are discussed below.
JAK-STAT Signaling
Cytokine receptors of the type I and type II receptor families engage signal transduction pathways that involve non-receptor tyrosine kinases called Janus kinases or JAKs and transcription factors called signal transducers and activators of transcription (STATs). The discovery of the JAK-STAT pathways came from biochemical and genetic analyses of interferon signaling. There are four known Janus kinases (JAK1-3 and TYK2) and seven STATs (STAT1-4, 5a, 5b, and 6).
The sequence of events in the JAK-STAT signaling pathways is now well defined (Fig. 7-25). Inactive JAK enzymes are noncovalently attached to the cytoplasmic domains of type I and type II cytokine receptors. When two receptor molecules are brought together by binding of a cytokine molecule, the receptor-associated JAKs are activated and phosphorylate tyrosine residues in the cytoplasmic portions of the clustered receptors. Some of these phosphotyrosine moieties of the receptors are then recognized and bind to Src homology 2 (SH2) domains of monomeric cytosolic STAT proteins. The STAT proteins are thus brought close to JAKs and are phosphorylated by the receptor-associated kinases. The SH2 domain of one STAT monomer is able to bind to a phosphotyrosine residue on an adjacent STAT protein. The STAT dimers that are generated migrate to the nucleus, where they bind to specific DNA sequences in the promoter regions of cytokine-responsive genes and activate gene transcription.
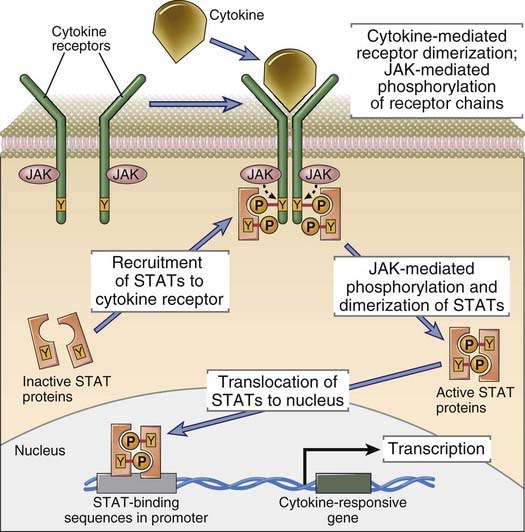
FIGURE 7–25 Type I and type II cytokines induce JAK-STAT signaling.
Ligation of receptors for type I and type II cytokines results in the activation of an associated JAK tyrosine kinase, the phosphorylation of the receptor tail, and the recruitment of an SH2 domain–containing activator of transcription (STAT) to the receptor. The recruited STAT is activated by JAK phosphorylation, dimerizes, enters the nucleus, and turns on the expression of cytokine target genes.
An intriguing question is how the specificity of responses to many different cytokines is achieved, given the limited numbers of JAKs and STATs used by the various cytokine receptors. The likely answer is that unique amino acid sequences in the different cytokine receptors provide the scaffolding for specifically binding, and thereby activating, different combinations of JAKs and STATs. The SH2 domains of different STAT proteins selectively bind to phosphotyrosines and flanking residues of different cytokine receptors. This is largely responsible for the activation of particular STATs by various cytokine receptors and therefore for the specificity of cytokine signaling. Several type I and type II cytokine receptors are heterodimers of two different polypeptide chains, each of which binds a different JAK. Furthermore, two different STATs may heterodimerize on phosphorylation. Therefore, there is a significant amount of combinatorial diversity in the signaling that can be generated from a limited number of JAK and STAT proteins.
In addition, cytokines activate signaling pathways and transcription factors other than STATs. For instance, the IL-2 receptor β chain activates Ras-dependent MAP kinase pathways that may be involved in gene transcription and growth stimulation. Other cytokine receptors may similarly activate other signaling pathways in concert with the JAK-STAT pathways to elicit biologic responses to the cytokines.
Several mechanisms of negative regulation of JAK-STAT pathways have been identified. Proteins called suppressors of cytokine signaling (SOCS) can be identified by the presence of an SH2 domain and a conserved 40–amino acid C-terminal region called a SOCS box. SOCS proteins serve as adaptors for multisubunit E3 ligase activity. They can bind to activated STATs and JAKs, and the tightly associated E3 ligases ubiquitinate the JAKs and STATs, thus targeting them for proteasomal degradation. SOCS protein levels can be regulated by TLR ligands, by cytokines themselves, and by other stimuli. In this way, SOCS serve as negative feedback regulators of the cytokine-mediated activation of cells. Other inhibitors of JAK-STAT signaling include tyrosine phosphatases, such as SHP-1 and SHP-2, which can dephosphorylate and therefore deactivate JAK molecules. Another family of inhibitory proteins, called protein inhibitors of activated STAT (PIAS), were originally defined as negative regulators of STATs. PIAS proteins bind phosphorylated STATs and prevent their interaction with DNA. It is now known that PIAS proteins also interact with and block the function of other transcription factors associated with cytokine signaling, including NF-κB and SMADs (transcription factors downstream of members of the TGF-β receptor family).
Pathways of NF-κB Activation
NF-κB is a transcription factor that plays a central role in inflammation, lymphocyte activation, cell survival, and the formation of secondary lymphoid organs. It is also an important player in lymphocyte development and in the pathogenesis of many cancers, including malignant neoplasms derived from activated lymphocytes. NF-κB is activated by many cytokine and TLR stimuli and by antigen recognition and is discussed here as the prototype of a transcription factor with fundamental roles in innate and adaptive immunity.
There are five NF-κB proteins. The domain that is common to all NF-κB proteins is a DNA-binding domain called a Rel homology domain. For a transcription factor to be active, it must both bind DNA and contain an activation domain that can facilitate transcriptional initiation. Three NF-κB proteins have both Rel homology domains and activation domains. These are p65/RelA, RelB, and c-Rel. NF-κB1/p50 and NF-κB2/p52 proteins contain a DNA-binding Rel homology domain but lack activation domains. NF-κB1 typically forms active heterodimers with p65/RelA or with c-Rel, and these heterodimers are typically considered “canonical” NF-κB heterodimers (Fig. 7-26). Canonical NF-κB heterodimers reside in the cytosol bound to an inhibitor of NF-κB called IκBα. Canonical NF-κB heterodimers are activated by a number of signaling receptors that drive inflammation or lymphocyte activation.
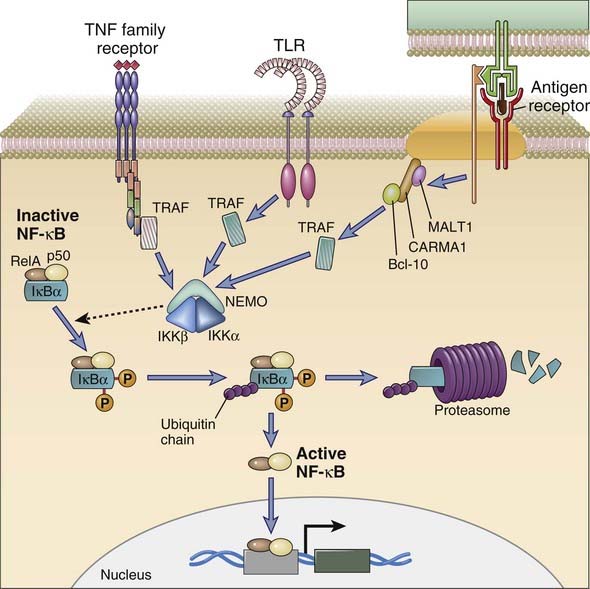
FIGURE 7–26 The canonical NF-κB pathway.
Antigen receptors activate specific PKCs that activate the CARMA1/Bcl-10/MALT1 complex, which in turn contributes to the induction of a TRAF E3 ligase that can polyubiquitinate NEMO/IKKγ, a component of the IκB kinase (IKK) complex, forming lysine-63–linked ubiquitin chains. This leads to the phosphorylation and activation of IKKβ by an upstream kinase. IKKβ phosphorylates the inhibitor of NF-κB (IκBα) and targets it for lysine-48 polyubiquitination and proteasomal degradation. Degradation of IκBα leads to the entry of active NF-κB into the nucleus. TLRs, members of the IL-1R family, and many members of the TNF receptor family activate TRAF family members that can activate this pathway.
As we have noted earlier in this chapter, TLRs, the BCR, the TCR, and many cytokine receptors of the TNF and IL-1R family activate NF-κB, and we will examine the common pathway involved in activating canonical NF-κB signaling. This NF-κB pathway induces the tagging and degradation of IκBα, allowing the unfettered heterodimeric NF-κB transcription factor to migrate into the nucleus. Most receptors that activate NF-κB do so by inducing this pathway. Two very different types of polyubiquitination events are required for canonical NF-κB activation. There are a few common steps in the canonical pathway that apply to all upstream signal inputs.
We have discussed earlier how TCR and BCR signaling contributes to the activation of PKC-θ and PKC-β, respectively. These PKCs can phosphorylate a protein called CARMA1 that forms a complex with two proteins called Bcl-10 and MALT1. The CARMA1/MALT1/Bcl-10 complex can contribute to the activation of a lysine-63 type of ubiquitin E3 ligase called TRAF6. Active TRAF6 can activate TAK1 and also add a lysine-63 ubiquitin chain to NEMO, thus facilitating the activation of IKKβ. TLRs and the IL-1R also activate TRAF6 to initiate IKK activation. Many members of the TNF receptor family, including the TNF receptor and CD40, can activate canonical NF-κB signaling through the activation of other TRAF proteins such as TRAF2, TRAF3, and TRAF5.
Heterodimers of NF-κB2 and RelB make up a “non-canonical” form of NF-κB, and these heterodimers are activated by a separate signaling pathway that is particularly important for lymphoid organ biogenesis and the survival of naive B lymphocytes. The two key receptors that induce the non-canonical or alternative NF-κB pathway, the LTβR (lymphotoxin β receptor) and the BAFFR (BAFF receptor), activate an IKK-like complex that contains IKKα homodimers. This leads to ubiquitination and degradation of a part of the NF-κB2–RelB dimer and release of the active protein.
Summary
Call ME, Wucherpfennig KW. Common themes in the assembly and architecture of activating immune receptors. Nature Reviews Immunology. 2007;7:841-850.
Cannons JL. SLAM family receptors and SAP adaptors in immunity. Annual Review of Immunology. 29, 2011.
Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annual Review of Immunology. 2009;27:693-733.
Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annual Review of Immunology. 2010;28:343-365.
T Cell Receptor Structure and Signaling
Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annual Review of Immunology. 2008;26:233-259.
Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annual Review of Immunology. 2010;28:79-105.
Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nature Immunology. 2006;7:25-32.
Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual Review of Immunology. 2010;28:491-533.
Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133-139.
Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annual Review of Immunology. 2006;24:419-466.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual Review of Immunology. 2009;27:591-619.
van der Merwe P, Dushek O. Mechanisms for T cell receptor triggering. Nature Reviews Immunology. 2011;11:47-55.
Weil R, Israel A. Deciphering the pathway from the TCR to NF-κB. Cell Death and Differentiation. 2006;13:826-833.
B Cell Receptor Structure and Signaling
Harwood NE, Batista FD. Early events in B cell activation. Annual Review of Immunology. 2010;28:185-210.
Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annual Review of Immunology. 2010;28:21-55.
Signal Attenuation in Lymphocytes
Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nature Reviews. Immunology. 2008;8:699-712.
Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annual Review of Immunology. 2007;25:473-523.
Smith KG, Clatworthy MR. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nature Reviews Immunology. 2010;10:328-343.
Sun SC. Deubiquitylation and regulation of the immune response. Nature Reviews Immunology. 2008;8:501-511.