Asthma
Asthma is a common chronic inflammatory condition of the airways whose cause is incompletely understood. Symptoms include wheeze, chest tightness, cough and shortness of breath, often worse at night. Asthma commonly starts in childhood between the ages of 3 and 5 years and may either worsen or improve during adolescence. Classically asthma has three characteristics:
 Airflow limitation which is usually reversible spontaneously or with treatment
Airflow limitation which is usually reversible spontaneously or with treatment
 Airway hyperresponsiveness to a wide range of stimuli (see below)
Airway hyperresponsiveness to a wide range of stimuli (see below)
 Bronchial inflammation with T lymphocytes, mast cells, eosinophils with associated plasma exudation, oedema, smooth muscle hypertrophy, matrix deposition, mucus plugging and epithelial damage.
Bronchial inflammation with T lymphocytes, mast cells, eosinophils with associated plasma exudation, oedema, smooth muscle hypertrophy, matrix deposition, mucus plugging and epithelial damage.
In chronic asthma, inflammation may be accompanied by irreversible airflow limitation as a result of airway wall remodelling that may involve large and small airways and mucus impaction.
Prevalence
In many countries, the prevalence of asthma is increasing. This increase is particularly marked in children and young adults where this disease may affect up to 15% of the population. There is also geographical variation, with asthma being commoner in more developed countries. Some of the highest rates are in the UK, New Zealand and Australia, with much lower rates in Far Eastern countries such as China and Malaysia, Africa and Central and Eastern Europe. Long-term follow-up in developing countries suggests that asthma may become more frequent as individuals adopt a more ‘westernized’ lifestyle, but the environmental factors accounting for this remain unknown. Studies of occupational asthma suggest that a large proportion of the workforce (15–20%) may become asthmatic if exposed to potent sensitizers. Worldwide, approximately 300 million people have asthma and this is expected to rise to 400 million by 2025.
Classification
Asthma is a complex disorder of the conducting airways that was often classified into extrinsic and intrinsic asthma but there is considerable overlap.
Extrinsic asthma occurs most frequently in atopic individuals: i.e. those with positive skin-prick reactions to common inhalant allergens such as dust mite, animal danders, pollens and fungi; 90% of children and 70% of adults with persistent asthma have positive skin-prick tests to inhalant allergens. Childhood asthma is often accompanied by eczema (atopic dermatitis) (see p. 1206). Sensitization to chemicals or biological products in the workplace is a frequently overlooked cause of late-onset asthma in adults.
Intrinsic asthma often starts in middle age. Nevertheless, many patients with adult-onset asthma show positive allergen skin tests and on close questioning some of these will give a history of childhood respiratory symptoms suggesting they have extrinsic asthma.
Non-atopic individuals may develop asthma in middle age from extrinsic causes such as sensitization to occupational agents such as toluene diisocyanate, intolerance to non-steroidal anti-inflammatory drugs such as aspirin or because they were given β-adrenoceptor-blocking agents for concurrent hypertension or angina that block the protective effect of endogenous adrenergic agonists. Extrinsic causes must be considered in all cases of asthma and, where possible, avoided.
Aetiology and pathogenesis
The two major factors involved in the development of asthma and many other stimuli that can precipitate attacks are shown in Figure 15.29.
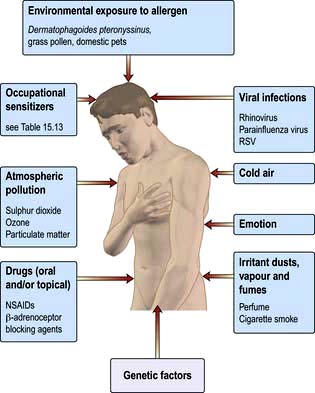
Figure 15.29 Causes and triggers of asthma. RSV, respiratory syncytial virus; NSAIDs, non-steroidal anti-inflammatory drugs.
Atopy and allergy
The term ‘atopy’ was coined in the early twentieth century to describe a group of disorders, including asthma and hayfever, which appeared:
 to have characteristic wealing skin reactions to common allergens in the environment
to have characteristic wealing skin reactions to common allergens in the environment
 to have circulating allergen-specific antibodies (later shown to be IgE).
to have circulating allergen-specific antibodies (later shown to be IgE).
Allergen-specific IgE is present in 30–40% of the UK population, and there is a link between serum IgE levels and both the prevalence of asthma and airway hyperresponsiveness. Genetic and environmental factors affect serum IgE levels.
Genetic
There is no single gene for asthma, but several genes, in combination with environmental factors, appear to influence the development of asthma.
 Genes controlling the production of the cytokines IL-3, IL-4, IL-5, IL-9, IL-13 and GM-CSF – which in turn affect mast and eosinophil cell development and longevity as well as IgE production – are present in a cluster on chromosome 5q31-33 (the IL-4 gene cluster).
Genes controlling the production of the cytokines IL-3, IL-4, IL-5, IL-9, IL-13 and GM-CSF – which in turn affect mast and eosinophil cell development and longevity as well as IgE production – are present in a cluster on chromosome 5q31-33 (the IL-4 gene cluster).
 Polymorphic variation in proteins along the IL-4/-13 signalling pathway is strongly associated with allergy and asthma.
Polymorphic variation in proteins along the IL-4/-13 signalling pathway is strongly associated with allergy and asthma.
 Novel asthma genes identified by positional cloning from whole genome scans are the PHF11 locus on chromosome 2 (that includes genes SETDB2 and RCBTB1) and transcription factors, which are implicated in IgE synthesis and associated more with atopy than asthma.
Novel asthma genes identified by positional cloning from whole genome scans are the PHF11 locus on chromosome 2 (that includes genes SETDB2 and RCBTB1) and transcription factors, which are implicated in IgE synthesis and associated more with atopy than asthma.
 ADAM 33 (a disintegrin and metalloproteinase) on chromosome 20p13 is associated with airway hyperresponsiveness and tissue remodelling.
ADAM 33 (a disintegrin and metalloproteinase) on chromosome 20p13 is associated with airway hyperresponsiveness and tissue remodelling.
 Other genes associated with asthma are those that encode neuropeptide S receptor (GPRA or GPR154) on chromosome 7p15, HLA-G on chromosome 6p21, dipeptidyl peptidase 10 on chromosome 2q14 and ORMDL3, a member of a gene family that encodes transmembrane proteins anchored in the endoplasmic reticulum, on chromosome 17q21.
Other genes associated with asthma are those that encode neuropeptide S receptor (GPRA or GPR154) on chromosome 7p15, HLA-G on chromosome 6p21, dipeptidyl peptidase 10 on chromosome 2q14 and ORMDL3, a member of a gene family that encodes transmembrane proteins anchored in the endoplasmic reticulum, on chromosome 17q21.
Environmental factors
Early childhood exposure to allergens and maternal smoking has a major influence on IgE production. Much current interest focuses on the role of intestinal bacteria and childhood infections in shaping the immune system in early life. It has been suggested that growing up in a relatively ‘clean’ environment may predispose towards an IgE response to allergens (the ‘hygiene hypothesis’). Conversely, growing up in a ‘dirtier’ environment may allow the immune system to avoid developing allergic responses. Components of bacteria (e.g. lipopolysaccharide endotoxin, immunostimulatory CpG DNA sequences, flagellin), viruses (e.g. SS- and DS-RNA) and fungi (e.g. chiton, a cell wall component) stimulate various toll-like receptors (TLRs) expressed on immune and epithelial cells to direct the immune and inflammatory response away from the allergic (Th2) towards protective (Th1 and Treg) pathways. Th1 immunity is associated with antimicrobial protective immunity whereas regulatory T cells are strongly implicated in tolerance to allergens. Thus early life exposure to inhaled and ingested products of microorganisms, as occurs in livestock farming communities and developing countries, may reduce the subsequent risk of a child becoming allergic and/or developing asthma.
The allergens involved in allergic asthma are similar to those implicated in rhinitis although pollens are relatively less implicated in asthma. Most allergic asthmatics are sensitized to house-dust mite allergens. Cockroach allergy has been implicated in asthma in US inner-city children, while allergens from furry pets (especially cats) are increasingly common causes. The fungal spores from Aspergillus fumigatus cause a range of lung disorders, including asthma (see p. 852). Many allergens, including those from Aspergillus, have intrinsic biological properties, e.g. enzymes with proteolytic function which may increase their sensitizing capacity.
Increased responsiveness of the airways of the lung (airway hyperresponsiveness)
Bronchial hyperresponsiveness (BHR) is a characteristic feature of asthma and can be demonstrated by asking patients to inhale gradually increasing concentrations of histamine or methacholine (bronchial provocation tests). This induces transient airflow limitation in susceptible individuals (approximately 20% of the population); the severity of BHR can be graded according to the provocation dose (PD) or concentration (PC) of the agonist that produces a 20% fall in FEV1 (PD20 FEV1 or PC20 FEV1). Patients with clinical symptoms of asthma respond to very low doses of methacholine, i.e. they have a low PD20 FEV1. BHR can also be assessed by exercise testing or inhalation of cold dry air, mannitol or hypertonic saline. These are indirect tests that release endogenous mediators such as histamine, prostaglandins and leukotrienes which then cause bronchoconstriction. Indirect measures of BHR correlate more closely with symptoms and diurnal peak expiratory flow rate (PEFR) variation than PC20 histamine or methacholine: both are useful in diagnosing asthma if there is doubt and in guiding controller treatment.
Some patients also react to methacholine but at higher doses, e.g. those with:
 attacks of asthma only on extreme exertion, e.g. winter sports enthusiasts
attacks of asthma only on extreme exertion, e.g. winter sports enthusiasts
 wheezing or prolonged periods of coughing following a viral infection
wheezing or prolonged periods of coughing following a viral infection
 seasonal wheeze during the pollen season
seasonal wheeze during the pollen season
 allergic rhinitis, but not complaining of lower respiratory symptoms until specifically questioned
allergic rhinitis, but not complaining of lower respiratory symptoms until specifically questioned
Although the degree of BHR can be influenced by allergic mechanisms (see p. 826 and Fig. 15.32), its pathogenesis and mode of inheritance involve a combination of airway inflammation and tissue remodelling.
Precipitating factors
Occupational sensitizers (Table 15.13)
Over 250 materials encountered at the workplace can cause occupational asthma, which accounts for about 15% of all asthma cases. These are recognized occupational diseases in the UK, and patients in insurable employment are eligible for statutory compensation provided they apply within 10 years of leaving the occupation in which the asthma developed.
Table 15.13 Occupational asthma
| Cause | Source/Occupation |
|---|---|
Low molecular weight (non-IgE related) |
|
Isocyanates |
Polyurethane varnishes |
Industrial coatings |
|
Spray painting |
|
Colophony fumes |
Soldering/welders |
Electronics industry |
|
Wood dust |
|
Drugs |
|
Bleaches and dyes |
|
Complex metal salts, e.g. nickel, platinum, chromium |
|
High molecular weight (IgE related) |
|
Allergens from animals and insects |
Farmers, workers in poultry and seafood processing industry; laboratory workers |
Antibiotics |
Nurses, health industry |
Latex |
Health workers |
Proteolytic enzymes |
Manufacture (but not use) of ‘biological’ washing powders |
Complex salts of platinum |
Metal refining |
Acid anhydrides and polyamine hardening agents |
Industrial coatings |
 Low molecular weight compounds, e.g. reactive chemicals such as isocyanates and acid anhydrides that bond chemically to epithelial cells to activate them as well as provide haptens recognized by T cells.
Low molecular weight compounds, e.g. reactive chemicals such as isocyanates and acid anhydrides that bond chemically to epithelial cells to activate them as well as provide haptens recognized by T cells.
 High molecular weight compounds, e.g. flour, organic dusts and other large protein molecules involving specific IgE antibodies
High molecular weight compounds, e.g. flour, organic dusts and other large protein molecules involving specific IgE antibodies
The risk of developing some forms of occupational asthma increases in smokers. The proportion of employees developing occupational asthma depends primarily upon the level of exposure. Proper enclosure of industrial processes or appropriate ventilation greatly reduces the risk. Atopic individuals develop occupational asthma more rapidly when exposed to agents causing the development of specific IgE antibody. Non-atopic individuals can also develop asthma when exposed to such agents, but after a longer period of exposure.
Nonspecific factors
The characteristic feature of BHR in asthma means that, as well as reacting to specific antigens, the airways will also respond to a wide variety of nonspecific direct and indirect stimuli.
Cold air and exercise
Most asthmatics wheeze after prolonged exercise or inhaling cold dry air. Typically, the attack does not occur while exercising but afterwards. Exercise-induced wheeze is driven by release of histamine, prostaglandins (PGs) and leukotrienes (LTs) from mast cells as well as stimulation of neural reflexes when the epithelial lining fluid of the bronchi becomes hyperosmolar owing to drying and cooling during exercise. The phenomenon can be shown by exercise, cold air and hypertonic (e.g. saline or mannitol) provocation tests.
Atmospheric pollution and irritant dusts, vapours and fumes
Many patients with asthma experience worsening of symptoms on contact with tobacco smoke, car exhaust fumes, solvents, strong perfumes or high concentrations of dust in the atmosphere. Major epidemics have been recorded when large amounts of allergens are released into the air, e.g. soybean dust in Barcelona. Asthma exacerbations increase during summer and winter air pollution episodes associated with climatic temperature inversions. Epidemics of asthma have occurred in the presence of high concentrations of ozone, particulates and NO2 in the summer and particulates, NO2 and SO2 in the winter.
Diet
Increased intakes of fresh fruit and vegetables have been shown to be protective, possibly owing to the increased intake of antioxidants or other protective molecules such as flavonoids. Genetic variation in antioxidant enzymes is associated with more severe asthma.
Emotion
It is well known that emotional factors may influence asthma both acutely and chronically, but there is no evidence that patients with the disease are any more psychologically disturbed than their non-asthmatic peers. An asthma attack is a frightening experience, especially when of sudden and unexpected onset. Patients at special risk of life-threatening attacks are understandably anxious.
Drugs
Non-steroid anti-inflammatory drugs (NSAIDs). NSAIDs, particularly aspirin and propionic acid derivatives, e.g. indometacin and ibuprofen, are implicated in triggering asthma in approximately 5% of patients. NSAID intolerance is especially prevalent in those with both nasal polyps and asthma and is not infrequently associated with rhinitis and flushing on drug exposure. NSAIDs inhibit arachidonic acid metabolism via the cyclo-oxygenase (COX) pathway, preventing the synthesis of certain prostaglandins. In aspirin-intolerant asthma there is reduced production of PGE2 which, in a sub-proportion of genetically susceptible subjects, induces the overproduction of cysteinyl leukotrienes by eosinophils, mast cells and macrophages. In such patients there is evidence for genetic polymorphisms involving the enzymes and receptors of the leukotriene generating pathway (Fig. 15.30). Interestingly, asthma in intolerant patients is not precipitated by COX-2 inhibitors, indicating that it is blockade of the COX-1 isoenzyme that is linked to impaired PGE2 production.
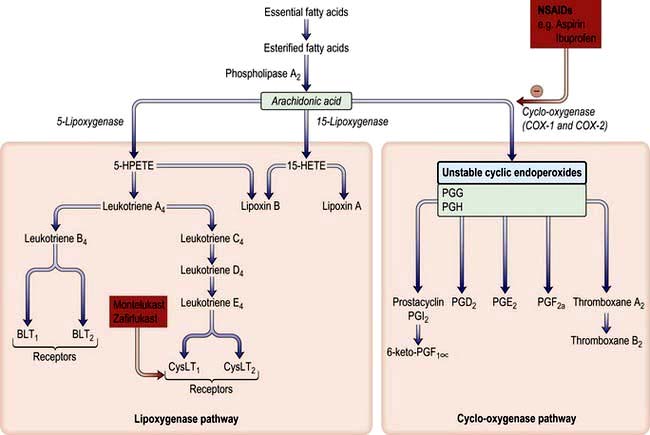
Figure 15.30 Arachidonic acid metabolism and the effect of drugs. The sites of action of NSAIDs (e.g. aspirin, ibuprofen) are shown. The enzyme cyclo-oxygenase occurs in three isoforms, COX-1 (constitutive), COX-2 (inducible) and COX-3 (in brain). PG, prostaglandin; BLT, B leukotriene receptor; CysLT, cysteinyl leukotriene receptor.
Beta-blockers. The airways have a direct parasympathetic innervation that tends to produce bronchoconstriction. There is no direct sympathetic innervation of the smooth muscle of the bronchi, and antagonism of parasympathetically induced bronchoconstriction is critically dependent upon circulating epinephrine (adrenaline) acting through β2-receptors on the surface of smooth muscle cells. Inhibition of this effect by β-adrenoceptor-blocking drugs such as propranolol leads to bronchoconstriction and airflow limitation, but only in asthmatic subjects. Selective β1-adrenergic-blocking drugs such as atenolol may still induce attacks of asthma; ideally alternative drugs should be used to treat hypertension or angina in asthmatic patients.
Allergen-induced asthma
The experimental inhalation of allergen by atopic asthmatic individuals leads to the development of different types of reaction, as illustrated in Figure 15.31. Much of our knowledge of asthma mechanisms comes from studies of allergen inhalation, but it must always be remembered this is only a model of the disease.
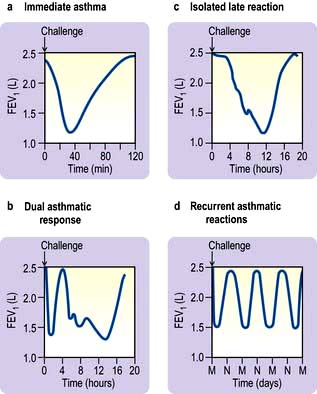
Figure 15.31 Different types of asthmatic reactions following challenge with allergen. M, midnight; N, noon.
Immediate asthma (early reaction). Airflow limitation begins within minutes of contact with the allergen, reaches its maximum in 15–20 minutes and subsides by 1 hour.
Dual and late-phase reactions. Following an immediate reaction many asthmatics develop a more prolonged and sustained attack of airflow limitation that responds less well to inhalation of bronchodilator drugs such as salbutamol. Isolated late-phase reactions with no preceding immediate response can occur after the inhalation of some occupational sensitizers such as isocyanates. BHR increases during and for several weeks after the exposure, which may explain persisting symptoms after allergen exposure.
Pathogenesis
The pathogenesis of asthma is complex and not fully understood. It involves a number of cells, mediators, nerves and vascular leakage that can be activated by several different mechanisms, including exposure to allergens (Fig. 15.32). The varying clinical severity and chronicity of asthma is dependent on an interplay between airway inflammation and airway wall remodelling. The inflammatory component is driven by Th2-type T lymphocytes which facilitate IgE synthesis through production of IL-4 and eosinophilic inflammation through IL-5 (Fig. 15.32). However, as the disease becomes more severe and chronic and loses its sensitivity to corticosteroids, there is greater evidence of a Th1 response with release of mediators such as TNF-α and associated tissue damage, mucous metaplasia and aberrant epithelial and mesenchymal repair.
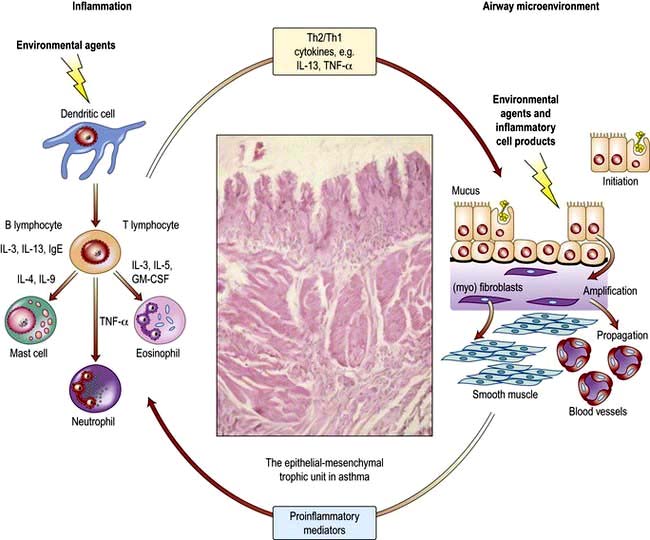
Figure 15.32 Inflammatory and remodelling responses in asthma with activation of the epithelial mesenchymal trophic unit. Epithelial damage alters the set point for communication between bronchial epithelium and underlying mesenchymal cells, leading to myofibroblast activation, an increase in mesenchymal volume, and induction of structural changes throughout airway wall.
(Adapted from Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006; 368: 780–793, with permission from Elsevier.)
Inflammation
Several key cells are involved in the inflammatory response that characterizes all types of asthma.
Mast cells (see also p. 53). These are increased in the epithelium, smooth muscle and mucous glands in asthma and release powerful preformed and newly generated mediators that act on smooth muscle, small blood vessels, mucus-secreting cells and sensory nerves, such as histamine, tryptase, PGD2 and cysteinyl leukotrienes, which cause the immediate asthmatic reaction. Mast cells are inhibited by sodium cromoglycate and β2 agonists, which may partly explain their ability to prevent acute bronchoconstriction triggered by indirect challenges. Mast cells also release an array of cytokines, chemokines and growth factors that contribute to the late asthmatic response and more chronic aspects of asthma.
Eosinophils. These are found in large numbers in the bronchial wall and secretions of asthmatics. They are attracted to the airways by the eosinophilopoietic cytokines IL-3, IL-5 and GM-CSF as well as by chemokines which act on type 3 C-C chemokine receptors (CCR-3) (i.e. eotaxin, RANTES, MCP-1, MCP-3 and MCP-4). These mediators also prime eosinophils for enhanced mediator secretion. When activated, eosinophils release LTC4, and basic proteins such as major basic protein (MBP), eosinophil cationic protein (ECP) and eosinophils peroxidase (EPX) that are toxic to epithelial cells. Both the number and activation of eosinophils are rapidly decreased by corticosteroids. Sputum eosinophilia is of diagnostic help as well as providing a biomarker of response to therapy
Dendritic cells and lymphocytes. These cells are abundant in the mucous membranes of the airways and the alveoli. Dendritic cells have a role in the initial uptake and presentation of allergens to lymphocytes. T helper lymphocytes (CD4+) show evidence of activation (Fig. 15.32) and the release of their cytokines plays a key part in the migration and activation of mast cells (IL-3, IL-4, IL-9 and IL-13) and eosinophils (IL-3, IL-5, GM-CSF). In addition, production of IL-4 and IL-13 helps maintain the proallergic Th2 phenotype, favouring switching of antibody production by B lymphocytes to IgE. In mild/moderate asthma there is selective upregulation of Th2 T cells with reduced evidence of the Th1 phenotype (producing gamma-interferon, TNF-α and IL-2), although Th1 cells are more prominent in more severe disease. This polarization is mediated by dendritic cells and involves a combination of antigen presentation, co-stimulation and exposure to polarizing cytokines. The activity of both macrophages and lymphocytes is influenced by corticosteroids but not β2-adrenoceptor agonists.
Remodelling
A characteristic feature of chronic asthma is an alteration of structure and functions of the formed elements of the airways. Together, these structural changes interact with inflammatory cells and mediators to cause the characteristic features of the disease. Deposition of matrix proteins, swelling and cellular infiltration expand the submucosa beneath the epithelium so that for a given degree of smooth muscle shortening there is excess airway narrowing. Swelling outside the smooth muscle layer spreads the retractile forces exerted by the surrounding alveoli over a greater surface area so that the airways close more easily. Several factors contribute to these changes.
The epithelium. In asthma the epithelium of the conducting airways is stressed and damaged with loss of ciliated columnar cells. Metaplasia occurs with a resultant increase in the number and activity of mucus-secreting goblet cells. The epithelium is a major source of mediators, cytokines and growth factors that enhance inflammation and promote tissue remodelling (Fig. 15.32). Damage and activation of the epithelium make it more vulnerable to infection by common respiratory viruses (e.g. rhinovirus, coronavirus) and to the effects of air pollutants. Increased production of nitric oxide (NO), due to the increased expression of inducible NO synthase, is a feature of epithelial damage and activation. Measurement of exhaled NO is proving useful as a non-invasive test of continuing inflammation (p. 829).
Epithelial basement membrane. A pathognomonic feature of asthma is the deposition of repair collagens (types I, III and V) and proteoglycans in the lamina reticularis beneath the basement membrane. This, along with the deposition of other matrix proteins such as laminin, tenascin and fibronectin, causes the appearance of a thickened basement membrane observed by light microscopy in asthma. This collagen deposition reflects activation of an underlying sheath of fibroblasts that transform into contractile myofibroblasts which also have an increased capacity to secrete matrix. Aberrant signalling between the epithelium and underlying myofibroblasts is thought to be the principal cause of airway wall remodelling, since the cells are prolific producers of a range of tissue growth factors such as epidermal growth factor (EGF), transforming growth factor (TGF)-α and -β, connective tissue-derived growth factor (CTGF), platelet-derived growth factor (PDGF), endothelin (ET), insulin-like growth factors (IGF), nerve growth factors and vascular endothelial growth factors (Fig. 15.32). The same interaction between epithelium and mesenchymal tissues is central to branching morphogenesis in the developing fetal lung. It has been suggested that these mechanisms are reactivated in asthma, but instead of causing airway growth and branching, they lead to thickening of the airway wall (remodelling, Fig. 15.32). Increased deposition of collagens, proteoglycans and matrix proteins creates a microenvironment which encourages ongoing inflammation since these molecules also possess cell-signalling functions, which aid cell movement, prolong inflammatory cell survival and prime them for mediator secretion.
Smooth muscle. Another prominent feature of asthma is hyperplasia of the helical bands of airway smooth muscle. In addition to increasing in amount, the smooth muscle alters in function so it contracts more easily and stays contracted because of a change in actin–myosin cross-link cycling. These changes allow asthmatic airways to contract too much and too easily at the least provocation. Asthmatic smooth muscle also secretes a wide range of cytokines, chemokines and growth factors that help sustain the chronic inflammatory response. The asthma gene ADAM33 has been implicated in driving increased airway smooth muscle and other features of remodelling through increased availability of growth factors.
Nerves. Neural reflexes, both central and peripheral, contribute to the irritability of asthmatic airways. Central reflexes involve stimulation of nerve endings in the epithelium and submucosa with transmission of impulses via the spinal cord and brain back down to the airways where release of acetylcholine from nerve endings stimulates M3 receptors on smooth muscle causing contraction. Local neural reflexes involve antidromic neurotransmission and the release of a variety of neuropeptides. Some of these are smooth muscle contractants (substance P, neurokinin A), some are vasoconstrictors (e.g. calcitonin gene-related peptide, CGRP) and some vasodilators (e.g. neuropeptide Y, vasoactive intestinal polypeptide). A polymorphism of the neuropeptide S receptor (GPR 154) is associated with asthma susceptibility. Bradykinin generated by tissue and serum proteolytic enzymes (including mast cell tryptase and tissue kallikrein) is also a potent stimulus of local neural reflexes involving (nonmyelinated) nerve fibres.
Clinical features
The principal symptoms of asthma are wheezing attacks and episodic shortness of breath. Symptoms are usually worst during the night, especially in uncontrolled disease. Cough is a frequent symptom that sometimes predominates, especially in children in whom nocturnal cough can be a presenting feature. Attacks vary greatly in frequency and duration. Some patients only have one or two attacks a year that last for a few hours, while others have attacks lasting for weeks. Some patients have chronic persistent symptoms, on top of which there are fluctuations. Attacks may be precipitated by a wide range of triggers (Fig. 15.29). Asthma is a major cause of impaired quality of life with impact on work and recreational, as well as physical activities, and emotions.
Investigations
There is no single satisfactory diagnostic test for all patients with asthma.
Lung function tests
Peak expiratory flow rate (PEFR) measurements on waking, prior to taking a bronchodilator and before bed after a bronchodilator, are particularly useful in demonstrating the variable airflow limitation that characterizes the disease (Fig. 15.14). The diurnal variation in PEFR is a good measure of asthma activity and is of help in the longer-term assessment of the patient’s disease and its response to treatment.
Spirometry is useful, especially in assessing reversibility. Asthma can be diagnosed by demonstrating a greater than 15% improvement in FEV1 or PEFR following the inhalation of a bronchodilator. However, there may be less reversibility when asthma is in remission or in severe chronic asthma when little reversibility can be demonstrated or if the patient is already being treated with long-acting bronchodilators.
Exercise tests
These have been widely used in the diagnosis of asthma in children. Ideally, the child should run for 6 minutes on a treadmill at a workload sufficient to increase the heart rate above 160 beats per minute. Alternative methods use cold air challenge, isocapnic hyperventilation (forced overbreathing with artificially maintained PACO2) or aerosol challenge with hypertonic solutions. A negative test does not automatically rule out asthma.
Histamine or methacholine bronchial provocation test (see p. 824)
This test indicates the presence of airway hyperresponsiveness, a feature found in most asthmatics, and can be particularly useful in investigating those patients whose main symptom is cough. The test should not be performed on individuals who have poor lung function (FEV1 <1.5 L) or a history of ‘brittle’ asthma. In children, controlled exercise testing as a measure of BHR is often easier to perform.
Trial of corticosteroids
All patients who present with severe airflow limitation should undergo a formal trial of corticosteroids. Prednisolone 30 mg orally should be given daily for 2 weeks with lung function measured before and immediately after the course. A substantial improvement in FEV1 (>15%) confirms the presence of a reversible element and indicates that the administration of inhaled steroids will prove beneficial to the patient. If the trial is for ≤2 weeks, the oral corticosteroid can be withdrawn without tailing off the dose, and should be replaced by inhaled corticosteroids in those who have responded.
Exhaled nitric oxide (NO)
A measure of airway inflammation and an index of corticosteroid response; used in children to assess the efficacy of corticosteroids.
Blood and sputum tests
Patients with asthma sometimes have increased numbers of eosinophils in peripheral blood (>0.4 × 109/L) but sputum eosinophilis is a more specific diagnostic finding.
Chest X-ray
There are no diagnostic features of asthma on the chest X-ray, although overinflation is characteristic during an acute episode or in chronic severe disease. A chest X-ray may be helpful in excluding a pneumothorax, which can occur as a complication, or in detecting the pulmonary infiltrates associated with allergic bronchopulmonary aspergillosis.
Management
 Restore normal or best possible lung function
Restore normal or best possible lung function
 Reduce the risk of severe attacks
Reduce the risk of severe attacks
 Patient and family education about asthma
Patient and family education about asthma
 Patient and family participation in treatment
Patient and family participation in treatment
 Avoidance of identified causes where possible
Avoidance of identified causes where possible
 Use of the lowest effective doses of convenient medications to minimize short-term and long-term side-effects.
Use of the lowest effective doses of convenient medications to minimize short-term and long-term side-effects.
Many asthmatics join self-help groups whose aim in order to improve their understanding of the disease and to foster self-confidence and fitness.
Control of extrinsic factors
Measures must be taken to avoid causative allergens such as pets, moulds and certain foodstuffs (see allergic rhinitis), particularly in childhood. Avoidance of house-dust mite is very difficult. There is little evidence for the effectiveness of current physical or chemical measures to control house-dust mite levels. The use of covers for bedding and changes to living accommodation has no beneficial effect on outcomes. Active and passive smoking should be avoided, as should beta-blockers in either tablet or eye drop form. Individuals intolerant to aspirin should avoid NSAIDs, although they may tolerate COX-2 inhibitors. Other agents (e.g. preservatives and colouring materials such as tartrazine) should be avoided if shown to be a causative factor. About one-third of individuals sensitized to occupational agents may be cured if they are kept permanently away from exposure. The remaining two-thirds will continue to have symptoms, and in half of these the symptoms may be as severe as when exposed to materials at work, especially if they were symptomatic for a long time before the diagnosis was made.
Drug treatment
The mainstay of asthma therapy is the use of therapeutic agents delivered as aerosols or powders directly into the lungs (see Practical Box 15.3). The advantages of this method of administration are that drugs are delivered direct to the airways and first-pass metabolism in the liver is avoided; thus lower doses are necessary and systemic unwanted effects are minimized.
![]() Practical Box 15.3
Practical Box 15.3
Inhaled therapy
Patients should be taught how to use inhalers and their technique checked regularly.
Use of a metered-dose inhaler
2. The patient exhales to functional residual capacity (not residual volume), i.e. normal expiration.
3. The aerosol nozzle is placed to the open mouth.
4. The patient simultaneously inhales rapidly and activates the aerosol.
6. The breath is held for 10 seconds if possible. Even with good technique, only 15% of the contents is inhaled and 85% is deposited on the wall of the pharynx and ultimately swallowed.
Spacers
These are plastic cones or spheres inserted between the patient’s mouth and the inhaler. Some inhalers have a built-in spacer extension. These are designed to reduce particle velocity so that less drug is deposited in the mouth. Spacers also diminish the need for coordination between aerosol activation and inhalation. They are useful in children and in the elderly and reduce the risk of candidiasis.
Both national and international guidelines have been published on the stepwise treatment of asthma (Box 15.3), based on three principles:
 Asthma self-management with regular asthma monitoring using PEF meters and individual treatment plans that are discussed with each patient and written down.
Asthma self-management with regular asthma monitoring using PEF meters and individual treatment plans that are discussed with each patient and written down.
 The appreciation that asthma is an inflammatory disease and that anti-inflammatory (controller) therapy should be started even in mild cases.
The appreciation that asthma is an inflammatory disease and that anti-inflammatory (controller) therapy should be started even in mild cases.
 Use of short-acting inhaled bronchodilators (e.g. salbutamol and terbutaline) only to relieve breakthrough symptoms. Increased use of bronchodilator treatment to relieve increasing symptoms is an indication of deteriorating disease.
Use of short-acting inhaled bronchodilators (e.g. salbutamol and terbutaline) only to relieve breakthrough symptoms. Increased use of bronchodilator treatment to relieve increasing symptoms is an indication of deteriorating disease.
![]() Box 15.3
Box 15.3
The stepwise management of asthma
| Step | PEFR | Treatment |
|---|---|---|
1. Occasional symptoms; less frequent than daily |
100% predicted |
As-required short-acting β2 agonists |
2. Daily symptoms |
<80% predicted |
Regular inhaled preventer therapy: |
3. Severe symptoms |
50–80% predicted |
Inhaled corticosteroids and long-acting inhaled β2 agonist |
4. Severe symptoms uncontrolled with high-dose inhaled corticosteroids |
50–80% predicted |
High-dose inhaled corticosteroid and regular bronchodilators |
5. Severe symptoms deteriorating |
≤50% predicted |
Regular oral corticosteroids |
6. Severe symptoms deteriorating in spite of prednisolone |
≤30% predicted |
Hospital admission |
Short-acting bronchodilator treatment taken at any step on an as-required basis.
A list of drugs used in asthma is shown in Box 15.4. These are given in a stepwise fashion as indicated in Box 15.3.
![]() Box 15.4
Box 15.4
Drugs used in asthma
Steroid-sparing agents
 Anti-IgE monoclonal antibody – omalizumab
Anti-IgE monoclonal antibody – omalizumab
 Etanercept (p. 72), infliximab, lebrikizumab
Etanercept (p. 72), infliximab, lebrikizumab
Once asthma is brought under control, for at least 2–3 months, the drug regimen should be reassessed in order to reduce the dosage of inhaled steroids.
β2-Adrenoceptor agonists
The most widely used bronchodilator preparations contain β2-adrenoceptor agonists that are selective for the respiratory tract and do not stimulate the β1 adrenoceptors of the myocardium. These drugs are potent bronchodilators because they relax the bronchial smooth muscle. They are very effective in relieving symptoms but do little for the underlying airways inflammation. Their usage is as follows:
 Mildest asthmatics with intermittent attacks. Only these people should rely on bronchodilator treatment alone. Short-acting β agonists (SABAs) such as salbutamol (100 µg), (called albuterol in the USA), or terbutaline (250 µg) should be prescribed as ‘two puffs as required’. Some patients use nebulizers at home for self-administration of salbutamol or terbutaline. Such treatment is effective, but patients should not rely on repeated home administration of nebulized β2-adrenoceptor agonists for worsening asthma, and should seek medical advice urgently if their condition does not improve. Excessive use of SABAs was linked to two epidemics of asthma mortality in the 1960s and 1980s.
Mildest asthmatics with intermittent attacks. Only these people should rely on bronchodilator treatment alone. Short-acting β agonists (SABAs) such as salbutamol (100 µg), (called albuterol in the USA), or terbutaline (250 µg) should be prescribed as ‘two puffs as required’. Some patients use nebulizers at home for self-administration of salbutamol or terbutaline. Such treatment is effective, but patients should not rely on repeated home administration of nebulized β2-adrenoceptor agonists for worsening asthma, and should seek medical advice urgently if their condition does not improve. Excessive use of SABAs was linked to two epidemics of asthma mortality in the 1960s and 1980s.
 SABAs can be taken at any step, as and when required from step 1 to step 5 (see above).
SABAs can be taken at any step, as and when required from step 1 to step 5 (see above).
 Poorly controlled asthmatics on standard doses of inhaled steroids. These patients require salmeterol or formoterol, which are highly selective and potent long-acting β2-adrenoceptor agonists (LABAs) effective by inhalation for up to 12 hours, thereby reducing the need for administration to once or twice daily. LABAs improve symptoms and lung function and reduce exacerbations in patients. They should never be used alone but always in combination with an inhaled corticosteroid. Increasingly, these drugs are administered as fixed-dose combinations with corticosteroids (salmeterol/fluticasone and formoterol/budesonide) in the same inhaler (step 3).
Poorly controlled asthmatics on standard doses of inhaled steroids. These patients require salmeterol or formoterol, which are highly selective and potent long-acting β2-adrenoceptor agonists (LABAs) effective by inhalation for up to 12 hours, thereby reducing the need for administration to once or twice daily. LABAs improve symptoms and lung function and reduce exacerbations in patients. They should never be used alone but always in combination with an inhaled corticosteroid. Increasingly, these drugs are administered as fixed-dose combinations with corticosteroids (salmeterol/fluticasone and formoterol/budesonide) in the same inhaler (step 3).
To help those who cannot coordinate activation of the aerosol and inhalation, several breath-activated or dry powder devices have been developed.
Antimuscarinic bronchodilators
Muscarinic receptors are found in the respiratory tract; large airways contain mainly M3 receptors whereas the peripheral lung tissue contains M3 and M1 receptors (see p. 793). Non-selective muscarinic antagonists – ipratropium bromide (20–40 µg three or four times daily) or oxitropium bromide (200 µg twice daily) – by aerosol inhalation can be useful during asthma exacerbations, but they are less useful in stable asthma.
Anti-inflammatory drugs
Sodium cromoglycate and nedocromil sodium prevent activation of many inflammatory cells, particularly mast cells, eosinophils and epithelial cells, but not lymphocytes, by blocking a specific chloride channel which in turn prevents calcium influx. These drugs are effective in patients with milder asthma (step 2) but have fallen out of favour in recent years.
Inhaled corticosteroids
All patients who have regular persistent symptoms (even mild symptoms) need regular treatment with inhaled corticosteroids delivered in a stepwise fashion (from step 2 upwards) or as a high dose followed by a reduction to maintenance levels. Beclometasone dipropionate (BDP) is the most widely used inhaled steroid and is available in doses of 50, 100, 200 and 250 µg per puff. Other inhaled steroids include budesonide, fluticasone, mometasone and triamcinolone.
Much of the inhaled dose does not reach the lung but is either swallowed or exhaled. Deposition in the lung varies between 10% and 25% depending on inhaler technique and the technical characteristics of the aerosol device. Drug which is deposited in the airways reaches the systemic circulation directly, through the bronchial circulation, while any drug that is swallowed has to pass through the liver before it can reach the systemic circulation. Gram for gram, fluticasone and mometasone are more potent than beclometasone with considerably less systemic bioavailability, owing to their greater sensitivity to hepatic metabolism. Absorption of beclometasone and budesonide does not seem to present a risk at doses up to 800 µg/day, but fluticasone or mometasone may be preferred because of their lower bioavailability when high-dose inhaled steroids are needed. The dose-response curve for inhaled corticosteroids is flat beyond 800 µg beclometasone or equivalent, and in patients with moderate asthma who are taking this daily, addition of a LABA is more effective than doubling the dose of inhaled corticosteroid.
Unwanted effects of inhaled corticosteroids include oral candidiasis (5% patients), and hoarseness. Subcapsular cataract formation is rare but can occur in the elderly. Osteoporosis is less likely than with oral steroids but can occur with high-dose inhaled corticosteroids (beclometasone or budesonide >800 µg daily). In children, inhaled corticosteroids at doses >400 µg daily have been shown to retard short-term growth, but final heights are not affected. Inhaled corticosteroid use should be stepped down once asthma comes under control (p. 830). Candidiasis and GI absorption can be reduced by using spacers, mouthwashing and teeth cleaning after use. Inhaled corticosteroids that are esterified in the lung, thereby reducing systemic effects, are also used (e.g. ciclesonide 80 µg daily).
Asthmatic patients who smoke are less responsive to inhaled corticosteroids due to induction of a range of genes and proteins in their respiratory epithelium. Assistance with smoking cessation should be offered, and additional therapy, e.g. with leukotriene receptor antagonists or theophylline, is required.
A functional GLCCI1 variant is associated with a decreased response to inhaled corticosteroids.
Many patients with anything more than mild/moderate asthma benefit from combination LABA/corticosteroid therapy and there is some evidence that the two drugs interact therapeutically.
Oral corticosteroids and steroid-sparing agents
Oral corticosteroids are needed for individuals not controlled on inhaled corticosteroids (step 5). The dose should be kept as low as possible to minimize side-effects. The effect of short-term treatment with prednisolone 30 mg daily is shown in Figure 15.14 (p. 804). Some patients require continuing treatment with oral corticosteroids. Several studies suggest that treatment with low doses of methotrexate (15 mg weekly) can significantly reduce the dose of prednisolone needed to control the disease in some patients, and ciclosporin also improves lung function in some steroid-dependent asthmatics. Several other steroid-sparing strategies including ciclosporin and immunoglobulin have also been tried, but with varying success.
Cysteinyl leukotriene receptor antagonists (LTRAs)
This class of anti-asthma therapy targets one of the principal asthma mediators by inhibiting the cysteinyl LT1 receptor. A second receptor (cyst LT2) has been identified on inflammatory cells. Montelukast, pranlukast (only available in South-east Asia) and zafirlukast are given orally and are effective in a subpopulation of patients. However, it is not possible to predict which individuals will benefit: a 4-week trial of LTRA therapy is recommended before a decision is made to continue or stop. LTRAs should be tried in any patient who is not controlled on low to medium doses of inhaled steroids (step 2). Their action is additive to that of long-acting β2 agonists. LTRAs are particularly useful in patients with aspirin-intolerant asthma, in those patients requiring high-dose inhaled or oral corticosteroids and in asthmatic smokers. Because these drugs are orally active they are helpful in asthma combined with rhinitis and in young children with asthma and/or virus-associated wheezing.
Monoclonal antibodies
Omalizumab, a recombinant humanized monoclonal antibody directed against IgE, chelates free IgE and downregulate the number and activity of mast cells and basophils. It is given subcutaneously every 2–4 weeks, depending on total serum IgE level and body weight. Although expensive, it is cost-effective in patients with frequent exacerbations requiring hospital admission. Proof-of-concept trials have shown that anti-TNF therapy (infliximab or etanercept) may be helpful in severe corticosteroid-refractory asthma. There is still a need to examine other biological agents as potential new controller therapies for the 5–10% of patients with severe disease, who account for a high proportion of the health costs of asthma.
Lebrikizumab, a monoclonal antibody to IL-3, showed improvement in lung function in one recent study.
Antibiotics
Although wheezing frequently occurs in infective exacerbations of COPD, there is little evidence that antibiotics are helpful in managing patients with asthma. During acute exacerbations, yellow or green sputum containing eosinophils and bronchial epithelial cells may be coughed up. This is normally due to viral rather than bacterial infection and antibiotics are not required. Occasionally, mycoplasma and Chlamydia infections can cause chronic relapsing asthma and macrolide antimicrobials may be helpful if a bacterial diagnosis has been established by culture or serology.
Asthma attack
Although these may occur spontaneously, asthma exacerbations are most commonly caused by lack of treatment adherence, respiratory virus infections associated with the common cold, and exposure to allergen or triggering drug, e.g. an NSAID. Whenever possible, patients should have a written personalized plan that they can implement in anticipation of or at the start of an exacerbation that includes the early use of a short course of oral corticosteroids. If the PEFR is >150 L/min, patients may improve dramatically on nebulized therapy and may not require hospital admission. Their regular treatment should be increased, to include treatment for 2 weeks with 30–60 mg of prednisolone followed by substitution by an inhaled corticosteroid preparation. Short courses of oral prednisolone can be stopped abruptly without tailing down the dose.
Acute severe asthma
The term acute severe asthma is used to mean an exacerbation of asthma that has not been controlled by the use of standard medication.
Patients with acute severe asthma typically have:
 the inability to complete a sentence in one breath
the inability to complete a sentence in one breath
 a respiratory rate of ≥25 breaths/min
a respiratory rate of ≥25 breaths/min
 Tachycardia ≥110 beats/min (pulsus paradoxus is not useful as it is only present in 45% of cases)
Tachycardia ≥110 beats/min (pulsus paradoxus is not useful as it is only present in 45% of cases)
Features of life-threatening attacks are:
 A silent chest, cyanosis or feeble respiratory effort
A silent chest, cyanosis or feeble respiratory effort
 PEFR <30% of predicted normal or best (approximately 150 L/min in adults).
PEFR <30% of predicted normal or best (approximately 150 L/min in adults).
Arterial blood gases should always be measured in asthmatic patients requiring admission to hospital, with particular attention paid to the PaCO2. Pulse oximetry is useful in monitoring oxygen saturation during the admission and can reduce the need for repeated arterial puncture. Features suggesting very severe life-threatening attacks are:
Treatment (Emergency Box 15.2) consists of nebulized short-acting bronchodilators; nebulized antimuscarinics (e.g. ipratropium bromide) are also helpful. A chest X-ray is useful to exclude pneumothorax and other causes of dyspnoea. Intravenous hydrocortisone is useful, and in very severe cases, β2-adrenoceptor agonists and/or magnesium sulphate are also given intravenously. Oral prednisolone (40–60 mg daily) should be given orally. Ventilation is required for patients who deteriorate despite this initial regimen.
![]() Emergency Box 15.2
Emergency Box 15.2
Treatment of severe asthma
At home
1. The patient is assessed. Tachycardia, a high respiratory rate and inability to speak in sentences indicate a severe attack.
2. If the PEFR is <150 L/min (in adults), an ambulance should be called. (All doctors should carry peak flow meters.)
3. Nebulized salbutamol 5 mg or terbutaline 10 mg is administered.
4. Hydrocortisone sodium succinate 200 mg i.v. is given.
At hospital
3. The PEFR is measured using a low-reading peak flow meter, as an ordinary meter measures only from 60 L/min upwards.
4. Nebulized salbutamol 5 mg or terbutaline 10 mg is repeated and administered 4-hourly.
5. Add nebulized ipratropium bromide 0.5 mg to nebulized salbutamol/terbutaline.
6. Hydrocortisone 200 mg i.v. is given 4-hourly for 24 hours.
7. Prednisolone is continued at 60 mg orally daily for 2 weeks.
8. Arterial blood gases are measured; if the PaCO2 is >7 kPa, ventilation may be required.
9. A chest X-ray is performed to exclude pneumothorax.
10. One of the following intravenous infusions is given if no improvement is seen:
Depending on progress, patients may go home after receiving nebulized therapy. More severe cases should be kept in hospital for 2–5 days with regular monitoring of oxygen saturation and peak flow rates. Downstream assessment of patients admitted with asthma should address trigger factors and aim to reduce the risk of readmission. Bronchial thermoplasty is a novel approach for moderate to severe persistent asthma is. This bronchoscopic procedure reduces the mass of airway smooth muscle, reducing bronchoconstriction, and is being evaluated.
Management of catastrophic sudden severe asthma (brittle asthma)
A small minority of patients with asthma suffer sudden life-threatening attacks despite being well controlled between attacks. These attacks may occur within hours or even minutes, and can cause sudden death. Such patients require a carefully worked out management plan agreed by patient, primary care physician hospital emergency services and the respiratory physician, which may include:
 Optimization of standard therapy
Optimization of standard therapy
 Emergency supplies of medications at home, in the car and at work
Emergency supplies of medications at home, in the car and at work
 Oxygen and resuscitation equipment at home and at work
Oxygen and resuscitation equipment at home and at work
 Nebulized β2-adrenoceptor agonists at home and at work
Nebulized β2-adrenoceptor agonists at home and at work
 Self-injectable adrenaline (epinephrine): two autoinjectors of 0.3 mg adrenaline at home, at work and to be carried by the patient at all times
Self-injectable adrenaline (epinephrine): two autoinjectors of 0.3 mg adrenaline at home, at work and to be carried by the patient at all times
On developing wheeze, the patient should attend the nearest hospital immediately. Direct admission to intensive care may be required.
Prognosis of asthma
Although asthma often improves in children as they reach their teens, the disease frequently returns in the 2nd, 3rd and 4th decades. In the past, the data indicating a natural decrease in asthma through teenage years have led to childhood asthma being treated as an episodic disorder. However, airway inflammation is present continuously from an early age and usually persists even if the symptoms resolve. Moreover, airways remodelling accelerates the process of decline in lung function over time. This has led to a reappraisal of the treatment strategy for asthma, mandating the early use of controller drugs and environmental measures from the time asthma is first diagnosed.
FURTHER READING
British Thoracic Society. British guidelines on the management of asthma. Thorax 2008; 63(SIV):1–121.
Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355:2226–2235.
Holgate ST, Polosa R. The mechanisms, diagnosis and management of severe asthma in adults. Lancet 2006; 368:780–793.
SIGNIFICANT WEBSITE
Scottish Intercollegiate Guidelines Network. Guideline No. 101. British Guideline on the Management of Asthma. 2011 update: http://www.sign.ac.uk/guidelines/fulltext/101/index.html
 blood glucose
blood glucose