![]() Box 4.13
Box 4.13
Lepra reactions following commencement of anti-mycobacterial treatment
| Type 1 | Type 2 (erythema nodosum leprosum) |
|---|---|
Treat with prednisolone |
Treatmenta |
a Thalidomide no longer has any role in the management of lepra reactions.
![]() Box 4.13
Box 4.13
Lepra reactions following commencement of anti-mycobacterial treatment
| Type 1 | Type 2 (erythema nodosum leprosum) |
|---|---|
Treat with prednisolone |
Treatmenta |
a Thalidomide no longer has any role in the management of lepra reactions.
Patient education is essential. Patients should be taught self-care of their anaesthetic hands and feet to prevent ulcers. If ulcers develop, no weight-bearing should be permitted. Cheap canvas shoes with cushioned insoles are protective.
Leprosy should be treated in specialist centres with adequate physiotherapy and occupational therapy support. Surgery and physiotherapy also play a role in the management of trophic ulcers and deformities of the hands, feet and face.
The prevention and control of leprosy depends on rapid treatment of infected patients, particularly those with LL and BL, to decrease the bacterial reservoir.
Anthrax is caused by Bacillus anthracis. The spores of these Gram-positive bacilli are extremely hardy and withstand extremes of temperature and humidity. The organism is capable of toxin production and this property correlates most closely with its virulence. The disease occurs worldwide, but it is most common in Africa and Southern Asia. Transmission is through direct contact with an infected animal; infection is most frequently seen in farmers, butchers and dealers in wool and animal hides. Spores can also be ingested or inhaled. There have been cases in the USA due to the deliberate release of anthrax spores as a bioterrorist weapon (see p. 936).
The incubation period is 1–10 days. Cutaneous anthrax is the most common. The small, erythematous, maculopapular lesion is initially painless. It may subsequently vesiculate and ulcerate, with formation of a central black eschar. The illness is self-limiting in the majority of patients, but occasionally perivesicular oedema and regional lymphadenopathy may be marked and toxaemia can occur.
Inhalational anthrax (woolsorter’s disease) follows inhalation of spores. A febrile illness is accompanied by non-productive cough and retrosternal discomfort; pleural effusions are common. Untreated, the mortality is about 90% and in the bioterrorism cases in the USA it was 45% despite treatment.
Gastrointestinal anthrax is due to consumption of under-cooked, contaminated meat. It presents as severe gastroenteritis; haematemesis and bloody diarrhoea can occur. Toxaemia, shock and death may follow.
The diagnosis is established by demonstrating the organism in smears from cutaneous lesions or by culture of blood and other body fluids. Serological confirmation can be made using ELISAs detecting antibodies to both the organism and a toxin.
Ciprofloxacin is considered the best treatment. In mild cutaneous infections, oral therapy for 2 weeks is adequate but therapy for 60 days was used in the recent outbreaks in the USA. In more severe infections, high doses of intravenous antibiotics are needed, along with appropriate supportive care. A new monoclonal antibody, raxibacumab, has been shown in animal studies to improve survival in inhalation anthrax. Any suspected case should be reported to the relevant authority.
Any infected animal that dies should be burned and the area in which it was housed disinfected. Where animal husbandry is poor, mass vaccination of animals may prevent widespread contamination, but needs to be repeated annually. A human vaccine is available for those at high risk and prophylactic antibiotics may be indicated following exposure. Some countries are establishing public health policies to deal with the deliberate release of anthrax spores.
Buruli ulcer, caused by Mycobacterium ulcerans, is seen in humid rural areas of the tropics, especially in Africa. The mode of transmission is thought to be via infected water bugs living in pools and muddy fields. A small subcutaneous nodule at the site of infection gradually ulcerates, involving subcutaneous tissue, muscle and fascial planes. The ulcers are usually large with undermined edges and markedly necrotic bases due to mycolactone (a toxin produced by the mycobacterium). Smears taken from necrotic tissue generally reveal numerous acid-fast bacilli. Until recently, the only effective treatment was wide surgical excision with skin grafts, but this is often unavailable in areas where the disease is prevalent. Combination therapy with rifampicin and clindamycin has shown significant benefit in some forms of the disease and early evidence suggests that the oral combination of rifampicin and clarithromycin may also work.
These diseases are found in various parts of the tropics and subtropics, mainly in impoverished rural areas. The WHO treated over 50 million cases in the 1950s and 1960s, reducing the prevalence, but subsequently there has been a resurgence of infection. The latest estimate of global prevalence is 2.5 million cases, mainly in South America and Africa (India has recently declared the eradication of yaws). Improvements in sanitation and an increase in living standards will be required to eradicate the diseases completely as organisms are transmitted by bodily contact, usually in children, the organism entering through damaged skin.
Yaws (caused by Treponema pertenue) is the most widespread and common of the endemic treponemal diseases. After an incubation period of weeks or months a primary inflammatory reaction occurs at the inoculation site, from which organisms can be isolated. Dissemination of the organism leads to multiple papular lesions containing treponemes; these skin lesions usually involve the palms and soles. There may also be bone involvement, particularly affecting the long bones and those of the hand.
Approximately 10% of those infected go on to develop late yaws. Bony gummatous lesions may progress to cause gross destruction and disfigurement, particularly of the skull and facial bones, the interphalangeal joints and the long bones. Plantar hyperkeratosis is characteristic. Like syphilis, there may be a latent period between the early and late phases of the disease, but visceral, neurological and cardiovascular problems do not occur.
Bejel is seen in Africa and the Middle East. The causative organism (Treponema endemicum) enters through abrasions in the skin directly or by mouth-to-mouth or skin-to-skin contact indirectly. It differs from venereal syphilis in that a primary lesion is not commonly seen. The late stages resemble syphilis, but cardiological and neu rological manifestations are rare.
Pinta, caused by Treponema carateum, is restricted mainly to Central and South America. It is milder than the other treponematoses and is confined to the skin. The primary lesion is a pruritic red papule, usually on the hand or foot. It may become scaly but never ulcerates and is generally associated with regional lymphadenopathy. In the later stages similar lesions can continue to occur for up to 1 year, associated with generalized lymphadenopathy. Eventually the lesions heal leaving hyperpigmented or depigmented patches.
In endemic areas the diagnosis is usually clinical. The causative organism can be identified from the exudative lesions under dark-ground microscopy. Serological tests for syphilis are positive and do not differentiate between the conditions.
The treatment is with long-acting penicillin (e.g. intramuscular benzathine penicillin, 1.2 million units) given as a single dose. Single dose oral azithromycin gives as good results. Doxycycline is used when penicillin is ineffective or contraindicated.
Trachoma, caused by the intracellular bacterium Chlamydia trachomatis, is the most common cause of blindness in the world. It is estimated that there are 150 million current infections and 6 million people who have been blinded by trachoma. It is a disease of poverty which is found mainly in the tropics and the Middle East: it is entirely preventable. Trachoma commonly occurs in children and is spread by direct transmission or by flies. Isolated infection is probably self-limiting and it is repeated infection which leads to chronic eye disease.
Infection is bilateral and begins in the conjunctiva, with marked follicular inflammation and subsequent scarring. Scarring of the upper eyelid causes entropion, leaving the cornea exposed to further damage with the eyelashes rubbing against it (trichiasis). The corneal scarring that eventually occurs leads to blindness.
Trachoma may also occur as an acute ophthalmic infection in the neonate.
The diagnosis is generally made clinically. However, this is an unreliable indicator of active infection. A newly developed near-patient immunodiagnostic dipstick test may help to better target antibiotic therapy.
Systemic therapy with a single dose of azithromycin 20 mg/kg is the treatment of choice; although tetracycline ointment applied locally each day for 6–8 weeks is effective, compliance is poor. In endemic areas repeated courses of therapy are necessary. Once infection has been controlled, surgery may be required for eyelid reconstruction and for treatment of corneal opacities.
Community health education, improvements in water supply and sanitation (pit latrines) and earlier case reporting could have a substantial impact on disease prevalence. This is reflected in the ‘SAFE’ approach to trachoma: surgery, antibiotics, facial cleanliness, environmental improvement. A WHO target is global eradication by 2020.
FURTHER READING
Britton WJ, Lockwood DNJ. Leprosy. Lancet 2004; 363:1209–1219.
House JI, Avele B, Porco TC et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distribution: a cluster-randomised trial. Lancet 2009; 373:1111–1118.
Johnson PD. Should antibiotics be given for Buruli ulcer? Lancet 2010; 375:618–619.
Cholera is caused by the curved, flagellated Gram-negative bacillus, Vibrio cholerae. The organism is killed by temperatures of 100°C in a few seconds but can survive in ice for up to 6 weeks. One major pathogenic serogroup possesses a somatic antigen (O1) with two biotypes: classical and El Tor. The El Tor biotype replaced the classical biotype as the major cause of the seventh pandemic which began in the 1960s. Infection with the El Tor biotype generally causes milder symptoms, but can still cause severe and life-threatening disease.
The fertile, humid Gangetic plains of West Bengal have traditionally been regarded as the home of cholera. However, a series of pandemics have spread the disease across the world, usually following trade routes. The seventh pandemic currently affects large areas of Asia and sub-Saharan Africa. A new serogroup (O139 Bengal) is responsible for many cases in Bangladesh, India and South-east Asia.
Transmission is by the faeco-oral route. Contaminated water plays a major role in the dissemination of cholera, although contaminated foodstuffs and contact carriers may contribute in epidemics. Achlorhydria or hypochlorhydria facilitates passage of the cholera bacilli into the small intestine. Here they proliferate, elaborating an exotoxin which produces massive secretion of isotonic fluid into the intestinal lumen (see p. 292). Cholera toxin also releases serotonin (5-HT) from enterochromaffin cells in the gut, which activates a neural secretory reflex in the enteric nervous system. This may account for at least 50% of cholera toxin’s secretory activity. V. cholerae also produces other toxins (zona occludens toxin, ZOT and accessory cholera toxin, ACT) which contribute to its pathogenic effect.
The incubation period varies from a few hours to 6 days. The majority of patients with cholera have a mild illness that cannot be distinguished clinically from diarrhoea due to other infective causes. In severe cases, there is abrupt onset of profuse painless diarrhoea, followed by vomiting. As the illness progresses the typical ‘rice water’ stool, flecked with mucus, may be seen. There is massive fluid loss and if this is not replaced the features of hypovolaemic shock (cold clammy skin, tachycardia, hypotension and peripheral cyanosis) and dehydration (sunken eyes, hollow cheeks and a diminished urine output) appear. The patient, though apathetic, is usually lucid. Muscle cramps may be severe. Children may present with convulsions owing to hypoglycaemia.
With adequate treatment the prognosis is good, with a gradual return to normal clinical and biochemical parameters in 1–3 days.
FURTHER READING
Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet 2005; 366:749–762.
Ivers LC, Farmer P, Almazor CP et al. Five complementary interventions to slow cholera: Haiti. Lancet 2010; 376:2048–2051.
Sridhar S. An affordable cholera vaccine: an important step forward. Lancet 2010; 374:1658–1660.
Thaver D, Zaidi AK, Critchley et al. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever). Cochrane Database Syst Rev 2008; 4:CD004530.
This is largely clinical. Examination of freshly passed stools may demonstrate rapidly motile organisms (although this is not diagnostic, as Campylobacter jejuni may give a similar appearance). A rapid dipstick test is now also available. Stool and rectal swabs should be taken for culture to confirm the diagnosis and to establish antibiotic sensitivity. Cholera should always be reported to the appropriate public health authority.
The mainstay of treatment is rehydration and with appropriate and effective rehydration therapy mortality has decreased to less than 1%. Oral rehydration is usually adequate, but intravenous therapy is occasionally required (Fig. 4.29).
Oral rehydration solutions (ORS) are based on the observation that glucose (and other carbohydrates) enhance sodium and water absorption in the small intestine, even in the presence of secretory loss due to toxins. Additions such as amylase-resistant starch to glucose-based ORS have been shown to increase the absorption of fluid. Cereal-based electrolyte solutions have been found to be as effective as sugar/salt ORS and actually reduce stool volume as well as rehydrating. The WHO recommends the use of reduced osmolarity ORS for all types of diarrhoea, although concerns remain about the risk of hyponatraemia. Suitable solutions for rehydration are listed in Box 4.10 (see p. 124).
Immunization is now recommended by the WHO in potential or actual outbreak situations. Live attenuated and killed vaccine (both oral) are available: neither protect against the O139 strain. The best preventative measures, however, are good hygiene and improved sanitation.
Over 17 million new cases of enteric fever occur worldwide, mainly in India and Africa, causing 600 000 deaths per year. Enteric fever is an acute systemic illness characterized by fever, headache and abdominal discomfort. Typhoid, the typical form of enteric fever, is caused by Salmonella typhi. A similar but generally less severe illness known as paratyphoid is due to infection with S. paratyphi A, B or C. Man is the only natural host for S. typhi, which is transmitted in contaminated food or water. The incubation period is 10–14 days.
After ingestion, the bacteria invade the small bowel wall via Peyer’s patches, from where they spread to the regional lymph nodes and then to the blood. The onset of illness is insidious and nonspecific, with intermittent fever, headache and abdominal pain. Physical findings in the early stages include abdominal tenderness, hepatosplenomegaly, lymphadenopathy and a scanty maculopapular rash (‘rose spots’). Without treatment (and occasionally even after treatment) serious complications can arise, usually in the third week of illness. These include meningitis, lobar pneumonia, osteomyelitis, intestinal perforation and intestinal haemorrhage. The fourth week of the illness is characterized by gradual improvement, but in developing countries up to 30% of those infected will die and 10% of untreated survivors will relapse. This compares with a mortality rate of 1–2% in the USA.
After clinical recovery 5–10% of patients will continue to excrete S. typhi for several months: these are termed convalescent carriers. Between 1% and 4% will continue to carry the organism for more than a year: this is chronic carriage. The usual site of carriage is the gall bladder and chronic carriage is associated with the presence of gallstones. However, in parts of the Middle East and Africa where urinary schistosomiasis is prevalent, chronic carriage of S. typhi in the urinary bladder is also common.
The definitive diagnosis of enteric fever requires the culture of S. typhi or S. paratyphi from the patient. Blood culture is positive in most cases in the first 2 weeks. Culture of intestinal secretions, faeces and urine is also used, although care must be taken to distinguish acute infection from chronic carriage. Bone marrow culture is more sensitive than blood culture, but is rarely required except in patients who have already received antibiotics. Leucopenia is common but nonspecific. Serological tests such as the Widal antigen test are of little practical value, are easily misinterpreted and should not be used.
Increasing antibiotic resistance is seen in isolates of S. typhi, especially in the Indian subcontinent. Chloramphenicol, cotrimoxazole and amoxicillin may all still be effective in some cases, but quinolones (e.g. ciprofloxacin 500 mg twice daily) are now the treatment of choice, although increased resistance to these agents is being seen: in such cases azithromycin may be effective. The patient’s temperature may remain elevated for several days after starting antibiotics and this alone is not a sign of treatment failure. Prolonged antibiotic therapy may eliminate the carrier state, but in the presence of gall bladder disease it is rarely effective. Cholecystectomy is not usually justified on clinical or public health grounds.
Tuberculosis is caused by Mycobacterium tuberculosis and occasionally M. bovis or M. africanum. These are slow-growing bacteria and, unlike other mycobacteria, are facultative intracellular organisms. The prevalence of tuberculosis increases with poor social conditions, inadequate nutrition and overcrowding. In developing countries it is most commonly acquired in childhood.
The impact of tuberculosis in the developing world has been magnified in the past 20 years by the emergence of the HIV pandemic (see p. 171) (Fig. 4.30).
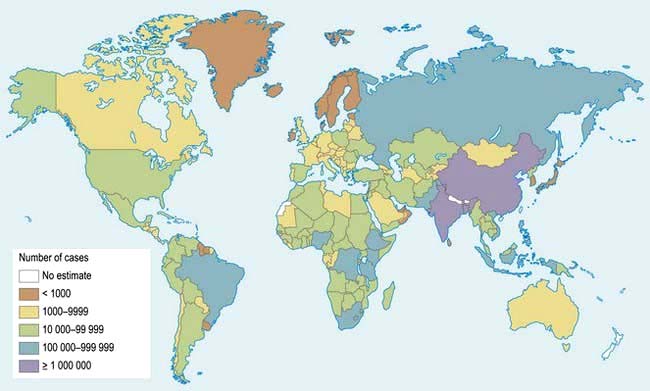
Figure 4.30 Tuberculosis: geographical distribution.
(From Frieden TR, Sterling TR, Munsiff SS et al. Tuberculosis. Lancet 2003; 362:888, with permission from Elsevier.)
Widespread misuse of antibiotics, combined with the breakdown of healthcare systems in parts of Africa, Russia and East Europe, has led to the emergence of drug-resistant tuberculosis. Multidrug-resistant tuberculosis (MDRTB) is caused by bacteria that are resistant to both rifampicin and isoniazid, two drugs which form the mainstay of treatment. It is now widespread in many parts of the world, including Asia, Eastern Europe and Africa. Extensively drug-resistant TB (XDRTB) is additionally resistant to quinolones and injectable second-line agents. MDRTB, and especially XDRTB, are very difficult to treat and carry significant mortality even with the best medical care (see p. 843).
In most people, the initial primary tuberculosis is asymptomatic or causes only a mild illness. The focus of the disease heals.
Occasionally the primary infection progresses locally to a more widespread lesion. Haematogenous spread at this stage may give rise to miliary tuberculosis.
Tuberculosis in the adult is usually the result of reactivation of old disease (post-primary tuberculosis), but primary infection, or more rarely reinfection, also occurs.
Pulmonary tuberculosis is the most common form; this is described on page 839, along with the chemotherapeutic regimens. Tuberculosis also affects other parts of the body.
 The gastrointestinal tract, mainly the ileocaecal area, but occasionally the peritoneum, producing ascites (see p. 269)
The gastrointestinal tract, mainly the ileocaecal area, but occasionally the peritoneum, producing ascites (see p. 269)
 The genitourinary system. The kidneys are most commonly involved, but tuberculosis can also cause painless, craggy swellings in the epididymis and salpingitis, tubal abscesses and infertility in females
The genitourinary system. The kidneys are most commonly involved, but tuberculosis can also cause painless, craggy swellings in the epididymis and salpingitis, tubal abscesses and infertility in females
 The central nervous system, causing tuberculous meningitis and tuberculomas (see p. 1121)
The central nervous system, causing tuberculous meningitis and tuberculomas (see p. 1121)
 The skeletal system, causing septic arthritis and osteomyelitis
The skeletal system, causing septic arthritis and osteomyelitis
 The skin, giving rise to lupus vulgaris
The skin, giving rise to lupus vulgaris
 The eyes, where it can cause choroiditis or iridocyclitis
The eyes, where it can cause choroiditis or iridocyclitis
 The pericardium, producing constrictive pericarditis (see p. 776)
The pericardium, producing constrictive pericarditis (see p. 776)
 The adrenal glands, causing destruction and producing Addison’s disease
The adrenal glands, causing destruction and producing Addison’s disease
 Lymph nodes. This is a common mode of presentation, especially in young adults and children. Any group of lymph nodes may be involved, but hilar and paratracheal lymph nodes are the most common. Initially the nodes are firm and discrete but later they become matted and can suppurate with sinus formation. Scrofula is the term used to describe massive cervical lymph node enlargement with discharging sinuses. Mycobacterial lymph node disease may also be caused by non-tuberculous mycobacteria.
Lymph nodes. This is a common mode of presentation, especially in young adults and children. Any group of lymph nodes may be involved, but hilar and paratracheal lymph nodes are the most common. Initially the nodes are firm and discrete but later they become matted and can suppurate with sinus formation. Scrofula is the term used to describe massive cervical lymph node enlargement with discharging sinuses. Mycobacterial lymph node disease may also be caused by non-tuberculous mycobacteria.
The majority of mycobacterial species are environmental organisms and are rarely pathogenic. Some have been found to cause disease in man, particularly in immunocompromised patients or those with pre-existing chronic lung disease (Table 4.34).
Table 4.34 Non-tuberculous mycobacteria causing disease in man
| Clinical | Common cause | Rare cause |
|---|---|---|
Chronic lung disease |
Mycobacterium avium-intracellulare |
M. malmoense |
M. kansasii |
||
Local lymphadenitis |
M. avium-intracellulare |
M. malmoense |
M. scrofulaceum |
||
Skin and soft tissue infection |
|
|
Fish tank granuloma |
M. marinum |
|
Abscesses, ulcers, sinuses |
M. fortuitum |
M. haemophilum |
M. chelonae |
|
|
Bone and joint infection |
M. kansasii |
M. scrofulaceum |
M. avium-intracellulare |
|
|
Disseminated infection (in HIV) |
M. avium-intracellulare |
|
Plague is caused by Yersinia pestis, a Gram-negative bacillus. Sporadic cases of plague (as well as occasional epidemics) occur worldwide: about 2000 cases per year are reported to the WHO, with a 10% mortality. The majority of cases are seen in sub-Saharan Africa, although the disease is occasionally seen in developed countries in people undertaking outdoor pursuits. The main reservoirs are woodland rodents, which transmit infection to domestic rats (Rattus rattus). The usual vector is the rat flea, Xenopsylla cheopis. These fleas bite humans when there is a sudden decline in the rat population. Occasionally, spread of the organisms may be through infected faeces being rubbed into skin wounds, or through inhalation of droplets.
Four clinical forms are recognized: bubonic, pneumonic, septicaemic and cutaneous.
This is the most common form and occurs in about 90% of infected individuals. The incubation period is 2–7 days. The onset of illness is acute, with high fever, chills, headache, myalgia, nausea, vomiting and, when severe, prostration. This is rapidly followed by the development of lymphadenopathy (buboes), most commonly involving the inguinal region. Characteristically these are matted and tender and suppurate in 1–2 weeks.
This is characterized by the abrupt onset of features of a fulminant pneumonia with bloody sputum, marked respiratory distress, cyanosis and death in almost all affected patients.
This is based on clinical, epidemiological and laboratory findings. Microscopy (on blood or lymph node aspirate) or a rapid antigen detection test can provide a presumptive diagnosis in an appropriate clinical setting. Blood or lymph node culture, or paired serological tests, are required for confirmation.
Treatment is urgent and should be instituted before the results of culture studies are available. The treatment of choice is now gentamicin 1 mg/kg i.v. three times daily for 10 days. Oral doxycycline 500 mg four times daily and chloramphenicol are also effective.
Prevention of plague is largely dependent on the control of the flea population. Outhouses, or huts, should be sprayed with insecticides that are effective against the local flea. During epidemics rodents should not be killed until the fleas are under control, as the fleas will leave dead rodents to bite humans. Tetracycline 500 mg four times daily for 7 days is an effective chemoprophylactic agent. A partially effective formalin-killed vaccine is available for use by travellers to plague-endemic areas.
These conditions are so named because, after apparent recovery from the initial infection, one or more recurrences may occur after a week or more without fever. They are caused by spirochaetes of the genus Borrelia.
Louse-borne relapsing fever (caused by B. recurrentis) is spread by body lice and only humans are affected. Classically it is an epidemic disease of armies and refugees, although it is also endemic in the highlands of Ethiopia, Yemen and Bolivia. Lice are spread from person to person when humans live in close contact in impoverished conditions. Infected lice are crushed by scratching, allowing the spirochaete to penetrate through the skin. Symptoms begin 3–10 days after infection and consist of a high fever of abrupt onset with rigors, generalized myalgia and headache. A petechial or ecchymotic rash may be seen. The general condition then deteriorates, with delirium, hepatosplenomegaly, jaundice, haemorrhagic problems and circulatory collapse. Although complete recovery may occur at this time, the majority experience one or more relapses of diminishing intensity over the weeks following the initial illness. The severity of the illness varies enormously and some cases have only mild symptoms. However, in some epidemics mortality has exceeded 50%.
Tick-borne relapsing fever is caused by B. duttoni and other Borrelia species, spread by soft (argasid) ticks. Rodents are also infected and humans are incidental hosts, acquiring the spirochaete from the saliva of the infected tick. This disease is mainly found in countries where traditional mud huts are the form of shelter and is a common cause of febrile illness in parts of Africa. The illness is generally similar to the louse-borne disease, although neurological involvement is more common.
Spirochaetes can be demonstrated microscopically in the blood during febrile episodes: organisms are more numerous in louse-borne relapsing fever. Treatment is usually with tetracycline or doxycycline (see p. 167). A severe Jarisch–Herxheimer reaction (see p. 167) occurs in many patients, often requiring intensive nursing care and intravenous fluids.
Control of infection relies on elimination of the vector. Ticks live for years and remain infected, passing the infection to their progeny. These reservoirs of infection should be controlled by spraying houses with insecticides and by reducing the number of rodents. Patients infested with lice should be deloused by washing with a suitable insecticide. All clothes must be thoroughly disinfected.
Typhus is the collective name given to a group of diseases caused by Rickettsia species (Table 4.35). Rickettsiae (and the closely related Orientiae) are small intracellular bacteria that are spread to humans by arthropod vectors, including body lice, fleas, hard ticks and larval mites. Rickettsiae inhabit the alimentary tract of these arthropods and the disease is spread to the human host by inoculation of their faeces through broken human skin, generally produced by scratching. Rickettsiae multiply intracellularly and can enter most mammalian cells, although the main lesion produced is a vasculitis due to invasion of endothelial cells of small blood vessels. Multisystem involvement is usual.
Epidemic typhus. The vector of epidemic typhus is the human body louse and like louse-borne relapsing fever, epidemics are associated with war and refugees. Outbreaks have occurred in Africa, Central and South America and Asia.
The incubation period of 1–3 weeks is followed by an abrupt febrile illness associated with profound malaise and generalized myalgia. Headache is severe and there may be conjunctivitis with orbital pain. A measles-like eruption appears around the fifth day. At the end of the first week, signs of meningoencephalitis appear and CNS involvement may progress to coma. At the height of the illness, splenomegaly, pneumonia, myocarditis and gangrene at the peripheries may be evident. Oliguric renal failure occurs in fulminating disease, which is usually fatal. Recovery begins in the third week but is generally slow. The disease may recur many years after the initial attack owing to rickettsiae that lie dormant in lymph nodes. The recrudescence is known as Brill–Zinsser disease. The factors that precipitate recurrence are not clearly defined, although other infections may play a role.
Endemic (murine) typhus. This is an infection of rodents that is inadvertently spread to humans by rat fleas. The disease closely resembles epidemic typhus but is much milder and rarely fatal.
Scrub typhus. Found throughout Asia and the Western Pacific, this disease is spread by larval trombiculid mites (chiggers). An eschar (a black, crusted, necrotic papule) can often be found at the site of the bite. The clinical illness is very variable, ranging from a mild illness to fulminant and potentially fatal disease. The more severe cases resemble epidemic typhus. Unlike other types of typhus the organism is passed on to subsequent generations of mites, which consequently act as both reservoir and vector.
A variety of Rickettsia species, collectively known as the spotted fever group rickettsiae, cause the illnesses known as spotted fevers. In most cases the vector is a hard tick. Although the causative organism and the name of the illness vary from place to place the clinical course is common to all. After an incubation period of 4–10 days, an eschar may develop at the site of the bite in association with regional lymphadenopathy. There is abrupt onset of fever, myalgia and headache, accompanied by a maculopapular rash which may become petechial. Neurological, haematological and cardiovascular complications occur as in epidemic typhus, although these are uncommon.
The diagnosis is generally made on the basis of the history and clinical course of the illness. It can be confirmed serologically or by PCR.
Doxycycline or tetracycline given for 5–7 days is the treatment of choice. Ciprofloxacin is also effective. Doxycycline 200 mg weekly protects against scrub typhus; it is reserved for highly endemic areas. Rifampicin is also used when resistance to tetracycline has occurred. Seriously ill patients need intensive care. Control of typhus is achieved by eradication of the arthropod vectors. Lice and fleas can be eradicated from clothing by insecticides (0.5% malathion or DDT). Control of rodents is necessary in endemic typhus and some of the spotted fevers. Areas of vegetation infested with trombiculid mites can be cleared by chemical spraying from the air. Bites from ticks and mites should be avoided by wearing protective clothing on exposed areas of the body. The likelihood of infection from ticks is related to the duration of feeding and in high-risk areas the body should be inspected twice a day as the bites are painless and any ticks should be removed (see p. 130).
Bartonella spp. are intracellular bacteria closely related to the rickettsiae. A number of human diseases can be caused by these organisms; like rickettsial disease, infection is usually spread from animals via an arthropod vector (Table 4.36).
This disease is restricted mainly to the habitat of its main vector, the sandfly, in the river valleys of the Andes mountains at an altitude of 500–3000 m. Two clinical presentations are seen, which may occur alone or consecutively. Oroya fever is an acute febrile illness causing myalgia, arthralgia, severe headache and confusion, followed by a haemolytic anaemia. Verruga peruana consists of eruptions of reddish-purple haemangiomatous nodules, resembling bacillary angiomatosis. It may follow 4–6 weeks after Oroya fever, or be the presenting feature of infection. Spontaneous resolution may occur over a period of months or years. Carrion’s disease is frequently complicated by superinfection, especially with Salmonella spp.
The diagnosis is made by culturing bacilli from blood or peripheral lesions. Serological tests have been developed but are not widely available.
Treatment with chloramphenicol or tetracycline is very effective in acute disease, but less so in verruga peruana.
These are described on page 116.
Trench fever is caused by Bartonella quintana and transmitted by human body lice. It is mainly seen in refugees and the homeless. It is characterized by cyclical fever (typically every 5 days), chills and headaches, accompanied by myalgia and pretibial pain. The disease is usually self-limiting but it can be treated with erythromycin or doxycycline if symptoms are severe.
Ehrlichiosis and anaplasmosis are infections caused by tick-borne rickettsia-like bacteria. At least three species have been implicated: Ehrlichia chafeensis, which causes human monocytic ehrlichiosis (HME), and E. ewingi and Anaplasma phagocytophilum (formerly known as E. phagocytophilum), which cause human granulocytic ehrlichiosis (HME, also known as human granulocytic anaplasmosis). All cause a rather nonspecific febrile illness with fever, myalgia and headache. Treatment is with doxycycline. The vectors are hard ticks and the main reservoir hosts are deer. As with most tick-borne zoonoses, the avoidance of tick bites and the prompt removal of feeding ticks are the best forms of prevention.
The term melioidosis refers to infections caused by the Gram-negative bacteria Burkholderia pseudomallei. This environmental organism, which is found in soil and surface water, is distributed widely in the tropics and subtropics. The majority of clinical cases of melioidosis occur in South-east Asia. Infection follows inhalation or direct inoculation. More than half of all patients with melioidosis have predisposing underlying disease: it is particularly common in diabetics.
B. pseudomallei causes a wide spectrum of disease and the majority of infections are probably subclinical. Illness may be acute or chronic, localized or disseminated, but one form of the disease may progress to another and individual patients may be difficult to categorize. The most serious form is septicaemic melioidosis, which is often complicated by multiple metastatic abscesses: this is frequently fatal. Serological tests are available, but definitive diagnosis depends on isolating the organism from blood or appropriate tissue. B. pseudomallei has extensive intrinsic antibiotic resistance. The most effective agent is ceftazidime, which is given intravenously for 2–4 weeks; this should be followed by several months of co-amoxiclav to prevent relapses.
Actinomyces spp. are branching, Gram-positive higher bacteria which are normal mouth and intestine commensals; they are particularly associated with poor mouth hygiene. Actinomyces have a worldwide distribution but are a rare cause of disease in the West.
 Cervicofacial actinomycosis, the most common form, usually occurs following dental infection or extraction. It is often indolent and slowly progressive, associated with little pain and results in induration and localized swelling of the lower part of the mandible. Sinuses and tracts develop with discharge of ‘sulphur’ granules.
Cervicofacial actinomycosis, the most common form, usually occurs following dental infection or extraction. It is often indolent and slowly progressive, associated with little pain and results in induration and localized swelling of the lower part of the mandible. Sinuses and tracts develop with discharge of ‘sulphur’ granules.
 Thoracic actinomycosis follows inhalation of organisms, usually into a previously damaged lung. The clinical picture is not distinctive and is often mistaken for malignancy or tuberculosis. Symptoms such as fever, malaise, chest pain and haemoptysis are present. Empyema occurs in 25% of patients and local extension produces chest-wall sinuses with discharge of ‘sulphur’ granules.
Thoracic actinomycosis follows inhalation of organisms, usually into a previously damaged lung. The clinical picture is not distinctive and is often mistaken for malignancy or tuberculosis. Symptoms such as fever, malaise, chest pain and haemoptysis are present. Empyema occurs in 25% of patients and local extension produces chest-wall sinuses with discharge of ‘sulphur’ granules.
 Abdominal actinomycosis most frequently affects the caecum. Characteristically, a hard indurated mass is felt in the right iliac fossa. Later, sinuses develop. The differential diagnosis includes malignancy, tuberculosis, Crohn’s disease and amoeboma. The incidence of pelvic actinomycosis appears to be increasing with wider use of intrauterine contraceptive devices.
Abdominal actinomycosis most frequently affects the caecum. Characteristically, a hard indurated mass is felt in the right iliac fossa. Later, sinuses develop. The differential diagnosis includes malignancy, tuberculosis, Crohn’s disease and amoeboma. The incidence of pelvic actinomycosis appears to be increasing with wider use of intrauterine contraceptive devices.
Occasionally, actinomycosis becomes disseminated to involve any site.
Diagnosis is by microscopy and culture of the organism. Treatment often involves surgery as well as antibiotics: penicillin is the drug of choice. Intravenous penicillin 2.4 g 4-hourly is given for 4–6 weeks, followed by oral amoxicillin for at least 3–4 months after clinical resolution. Tetracyclines are also effective.
Nocardia spp. are Gram-positive branching bacteria, which are found in soil and decomposing organic matter. N. asteroides and less often N. brasiliensis are the main human pathogens.
Mycetoma is the most common illness. This is a result of local invasion by Nocardia spp. and presents as a painless swelling, usually on the sole of the foot (Madura foot). The swelling of the affected part of the body continues inexorably. Nodules gradually appear which eventually rupture and discharge characteristic ‘grains’, which are colonies of organisms. Systemic symptoms and regional lymphadenopathy are rare. Sinuses may occur several years after the onset of the first symptom. A similar syndrome may be produced by other branching bacteria and also by species of eumycete fungi such as Madurella mycetomi (see p. 142).
Pulmonary disease, which follows inhalation of the organism, presents with cough, fever and haemoptysis: it is usually seen in the immunocompromised. Pleural involvement and empyema occur. In severely immunosuppressed patients, initial pulmonary infection may be followed by disseminated disease.
The diagnosis is often difficult to establish, as Nocardia is not easily detected in sputum cultures or on histological section. Severe pulmonary or disseminated infection may require parenteral treatment. Co-trimoxazole, linezolid, ceftriaxone and amikacin have all been used successfully, but in vitro sensitivities are variable and there is no consensus on the best treatment.
FURTHER READING
Bitam I, Dittmar K, Parola P et al. Fleas and flea-borne diseases. Int J Infect Dis 2010; 14:e667–e676.
Butler T. Plague into the 21st century. Clin Infect Dis 2009; 49:736–742.
Dukes Hamilton C, Sterling T, Blumberg H et al. Extensively drug resistant tuberculosis. Clin Infect Dis 2007; 45:338–342.
Peacock SJ. Melioidosis. Curr Opin Infect Dis 2006; 19:421–428.
Morphologically, fungi can be grouped into three major categories:
 Yeasts and yeast-like fungi, which reproduce by budding
Yeasts and yeast-like fungi, which reproduce by budding
 Moulds, which grow by branching and longitudinal extension of hyphae
Moulds, which grow by branching and longitudinal extension of hyphae
 Dimorphic fungi, which behave as yeasts in the host but as moulds in vitro (e.g. Histoplasma capsulatum and Sporothrix schenckii).
Dimorphic fungi, which behave as yeasts in the host but as moulds in vitro (e.g. Histoplasma capsulatum and Sporothrix schenckii).
Despite the fact that fungi are ubiquitous, systemic fungal infections are relatively rare (in contrast to superficial fungal infections of the skin, nails and orogenital mucosae (see p. 1200)). Systemic mycoses are usually seen in immunocompromised patients and in critical care settings and are becoming more prevalent as this population of patients increases.
Fungal infections are transmitted by inhalation of spores, by contact with the skin, or by direct inoculation. This last can occur through penetrating injuries, injecting drug use, or iatrogenic procedures. Fungi may also produce allergic pulmonary disease. Some fungi such as Candida albicans are human commensals. Diseases are usually divided into systemic, subcutaneous or superficial (Table 4.37).
Table 4.37 Common fungal infections
|
|
Candidiasis is the most common fungal infection in humans and is predominantly caused by Candida albicans although other species of Candida are increasingly recognized. Candida are small asexual fungi. Most species that are pathogenic to humans are normal oropharyngeal and gastrointestinal commensals. Candidiasis is found worldwide.
Any organ in the body can be invaded by candida, but vaginal infection and oral thrush are the most common forms. This latter is seen in the very young, in the elderly, following antibiotic therapy and in those who are immunosuppressed. Candidal oesophagitis presents with painful dysphagia. Cutaneous candidiasis typically occurs in intertriginous areas. It is also a cause of paronychia. Balanitis and vaginal infection are also common (see p. 170).
Dissemination of candidiasis may lead to haematogenous spread, with meningitis, pulmonary involvement, endocarditis or osteomyelitis.
The fungi can be demonstrated in scrapings from infected lesions, tissue secretions or in invasive disease, from blood cultures.
Treatment varies depending on the site and severity of infection. Oral lesions respond to local nystatin or amphotericin B, or systemic fluconazole. For systemic infections, parenteral therapy with amphotericin B, fluconazole, voriconazole or caspofungin is necessary.
Histoplasmosis is caused by Histoplasma capsulatum, a non-encapsulated, dimorphic fungus. Spores can survive in moist soil for several years, particularly when it is enriched by bird and bat droppings. Histoplasmosis occurs worldwide but is only commonly seen in Ohio and the Mississippi river valleys where over 80% of the population have been subclinically exposed. Transmission is mainly by inhalation of the spores, particularly when clearing out attics, barns and bird roosts or exploring caves.
Figure 4.31 summarizes the pathogenesis, main clinical forms and sequelae of Histoplasma infection.
Primary pulmonary histoplasmosis is usually asymptomatic. The only evidence of infection is conversion of a histoplasmin skin test from negative to positive and radiological features similar to those seen with the Ghon primary complex of tuberculosis. Calcification in the lungs, spleen and liver occurs in patients from areas of high endemicity. When symptomatic, primary pulmonary histoplasmosis generally presents as a mild influenza-like illness, with fever, chills, myalgia and cough. The systemic symptoms are pronounced in severe disease.
Complications such as atelectasis, secondary bacterial pneumonia, pleural effusions, erythema nodosum and erythema multiforme also occur.
Chronic pulmonary histoplasmosis is clinically indistinguishable from pulmonary tuberculosis (see p. 839). It is usually seen in American white males over the age of 50 years. The presentation of disseminated histoplasmosis resembles disseminated tuberculosis clinically. Fever, lymphadenopathy, hepatosplenomegaly, weight loss, leucopenia and thrombocytopenia are common. Rarely, features of meningitis, hepatitis, hypoadrenalism, endocarditis and peritonitis may dominate the clinical picture.
Definitive diagnosis is possible by culturing the fungi (e.g. from sputum) or by demonstrating them on histological sections. H. capsulatum glycoprotein can be detected in the urine and serum in those with acute pulmonary and disseminated infection. Antibodies usually develop within 3 weeks of the onset of illness and are best detected by complement-fixation or immunodiffusion (sensitivity of 95% and 90%, respectively).
Only symptomatic acute pulmonary histoplasmosis, chronic histoplasmosis and acute disseminated histoplasmosis require therapy. Itraconazole is effective in mild–moderate disease. Severe infection is treated with intravenous amphotericin B for 1–2 weeks followed by itraconazole for a total of 12 weeks or with voriconazole. Methylprednisolone is recommended in addition for those who develop respiratory complications. Patients with AIDS usually require treatment with parenteral amphotericin B followed by maintenance therapy with itraconazole 200 mg twice daily where HAART is unavailable. Surgical excision of histoplasmomas (pulmonary granuloma due to H. capsulatum) or chronic cavitatory lung lesions and release of adhesions following mediastinitis are often required.
This is caused by Histoplasma duboisii, the spores of which are larger than those of H. capsulatum. Skin lesions (e.g. abscesses, nodules, lymph node involvement and lytic bone lesions) are prominent. Pulmonary lesions do not occur. Treatment is similar to that for H. capsulatum infection.
Aspergillosis is caused by one of several species of dimorphic fungi of the genus Aspergillus. Of these, A. fumigatus is the most common, although A. flavus and A. niger are also recognized. These fungi are ubiquitous in the environment and are commonly found on decaying leaves and trees. Humans are infected by inhalation of the spores. Disease manifestation depends on the dose of the spores inhaled as well as the immune response of the host. Three major forms of the disease are recognized: bronchopulmonary allergic aspergillosis, aspergilloma and invasive aspergillosis (see p. 829).
The diagnosis and treatment are described in more detail in Chapter 15.
Cryptococcosis is caused by the yeast-like fungus Cryptococcus neoformans. It has a worldwide distribution and appears to be spread by birds, especially pigeons, in their droppings. The spores gain entry into the body through the respiratory tract, where they elicit a granulomatous reaction. Pulmonary symptoms are, however, uncommon; meningitis which usually occurs in those with HIV infection or lymphoma is the usual mode of presentation and often develops subacutely. Less commonly, lung cavitation, hilar lymphadenopathy, pleural effusions and occasionally pulmonary fibrosis occur. Skin and bone involvement is rare.
This is established by demonstrating the organisms in appropriately stained tissue sections. A positive latex cryptococcal agglutinin test performed on the CSF is diagnostic of cryptococcosis.
Liposomal amphotericin B alone or in combination with flucytosine for 2 weeks is followed by oral fluconazole 400 mg daily. Therapy should be continued for 8 weeks if meningitis is present. Fluconazole has greater CSF penetration and is used when toxicity is encountered with amphotericin B and flucytosine and as maintenance therapy in immunocompromised patients, especially those with HIV infection (see p. 189).
Coccidioidomycosis is caused by the non-budding spherical form (spherule) of Coccidioides immitis. This is a soil saprophyte and is found in the southern USA, Central America and parts of South America. Humans are infected by inhalation of the thick-walled barrel-shaped spores called arthrospores. Occasionally, epidemics of coccidioidomycosis have been documented following dust storms.
The majority of patients are asymptomatic and the infection is only detected by the conversion of a skin test using coccidioidin (extract from a culture of mycelial growth of C. immitis) from negative to positive. Acute pulmonary coccidioidomycosis presents, after an incubation period of about 10 days, with fever, malaise, cough and expectoration. Erythema nodosum, erythema multiforme, phlyctenular conjunctivitis and, less commonly, pleural effusions may occur. Complete recovery is usual.
Pulmonary cavitation with haemoptysis, pulmonary fibrosis, meningitis, lytic bone lesions, hepatosplenomegaly, skin ulcers and abscesses may occur in severe disease.
The organism can be identified in respiratory secretions and can be cultured in specialist laboratories. Serological tests are also widely used for diagnosis. These include the highly specific latex agglutination and precipitin tests (IgM), which are positive within 2 weeks of infection and decline thereafter. Other tests include complement fixation, ELISA and radioimmunoassay.
A complement-fixation test (IgG) performed on the CSF is diagnostic of coccidioidomycosis meningitis and becomes positive within 4–6 weeks and remains so for many years.
Mild pulmonary infections are self-limiting and require no treatment, but progressive and disseminated disease requires urgent therapy. Ketoconazole, itraconazole or fluconazole for 6 months is the treatment of choice for primary pulmonary disease with more prolonged courses for cavitating or fibronodular disease. Fluconazole in high-dose (600–1000 mg daily) is given for meningitis. Itraconazole provides an alternative. Voriconazole and posaconazole are used for poor responders. Surgical excision of cavitatory pulmonary lesions or localized bone lesions may be necessary.
Blastomycosis is a systemic infection caused by the biphasic fungus Blastomyces dermatitidis. Although initially believed to be confined to certain parts of North America, it has been reported from South America, India and the Middle East.
Blastomycosis primarily involves the skin, where it presents as non-itchy papular lesions that later develop into ulcers with red verrucous margins. The ulcers are initially confined to the exposed parts of the body but later involve the unexposed parts as well. Atrophy and scarring may occur. Pulmonary involvement presents as a solitary lesion resembling a malignancy or gives rise to radiological features similar to the primary complex of tuberculosis. Systemic symptoms such as fever, malaise, cough and weight loss are usually present. Bone lesions are common and present as painful swellings.
The diagnosis is confirmed by demonstrating the organism in histological sections or by culture, although results can be negative in 30–50% of cases. Enzyme immunoassay may be helpful although there is some cross-reactivity of antibodies to blastomyces with histoplasma.
Itraconazole is preferred for treating mild to moderate disease in the immunocompetent for periods up to 6 months. Ketoconazole or fluconazole are also used. In severe or unresponsive disease and in the immunocompromised, amphotericin B is indicated.
FURTHER READING
Horn DL, Neofytos D, Anaissie EJ et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48:1695–1703.
Hsu LY, Ng, ES-T, Koh LP. Common and emerging fungal pulmonary infections. Infect Dis Clin North Am 2010; 24:557–577.
Limper AH et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary critical care patients. Am J Respir Crit Care Med 2011; 183:96–128.
Segal BH, Walsh TJ. Current approaches to the diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med 2006; 173:707–714.
Invasive zygomycosis (mucormycosis) is rare and is caused by several fungi, including Mucor spp., Rhizopus spp. and Absidia spp. It occurs in severely ill patients. The hallmark of the disease is vascular invasion with marked haemorrhagic necrosis.
Rhinocerebral mucormycosis is the most common form. Nasal stuffiness, facial pain and oedema and necrotic, black nasal turbinates are characteristic. It is rare and is mainly seen in diabetics with ketoacidosis.
Subcutaneous zygomycosis presents as a brawny, woody infiltration involving the limbs, neck and trunk and rarely the pharynx and orbital regions in immunosuppressed patients.
Other forms include pulmonary and disseminated infection (immunosuppressed) and gastrointestinal infection (in malnutrition).
Treatment is with amphotericin B and sometimes judicious debridement. Oral saturated potassium iodide has been used in the subcutaneous variety.
Sporotrichosis is caused by the saprophytic fungus Sporothrix schenckii, which is found worldwide. Infection usually follows cutaneous inoculation, at the site of which a reddish, non-tender, maculopapular lesion develops – referred to as ‘plaque sporotrichosis’. Pulmonary involvement and disseminated disease rarely occur.
Treatment with itraconazole 100–200 mg/day for 3–6 months is usually curative.
Subcutaneous zygomycosis, a disease seen in the tropics, is caused by several filamentous fungi of the Basidiobolus genus. The disease usually remains confined to the subcutaneous tissues and muscle fascia. It presents as a brawny, woody infiltration involving the limbs, neck and trunk. Less commonly, the pharyngeal and orbital regions may be affected in immunocompromised patients and especially those with poorly controlled diabetes mellitus. It is locally erosive and can prove fatal. Amphotericin B is the drug of choice.
Treatment is with saturated potassium iodide solution given orally.
Chromoblastomycosis (chromomycosis) is caused by fungi of various genera including Phialophora, Wangella and Fonsecaea. These are found mainly in tropical and subtropical countries. It presents initially as a small papule, usually at the site of a previous injury. This persists for several months before ulcerating. The lesion later becomes warty and encrusted and gradually spreads. Satellite lesions may be present. Itching is frequent. The drug of choice is amphotericin B in combination with itraconazole or voriconazole. Cryosurgery is used to remove local lesions.
Mycetoma may be due to subcutaneous infection with fungi (Eumycetes spp.) or bacteria (see p. 139). It is largely confined to the tropics. Infection results in local swelling which may discharge through sinuses. Bone involvement may follow.
Treatment consists of surgical debridement, combined with antimicrobials chosen according to the aetiological agent.
Genetic analysis has shown P. jiroveci to be homologous with fungi. Pneumocystis jiroveci disease is almost invariably associated with immunodeficiency states, particularly AIDS and is discussed on page 188.
Dermatophytoses are chronic fungal infections of keratinous structures such as the skin, hair or nails. Trichophyton spp., Microsporum spp., Epidermophyton spp. and Candida spp. can also infect keratinous structures.
Malassezia spp. are found on the scalp and greasy skin and are responsible for seborrhoeic dermatitis, pityriasis versicolor (hypo- or hyperpigmented rash on trunk) and Malassezia folliculitis (itchy rash on back).
Treatment is with topical antifungals or oral ketoconazole if infection is refractory or more extensive.
Protozoa are unicellular eukaryotic organisms. They are more complex than bacteria and belong to the animal kingdom. Although many protozoa are free-living in the environment some have become parasites of vertebrates, including man, often developing complex life cycles involving more than one host species. In order to be transmitted to a new host, some protozoa transform into hardy cyst forms which can survive harsh external conditions. Others are transmitted by an arthropod vector, in which a further replication cycle takes place before infection of a new vertebrate host.
Human malaria is usually caused by one of four species of the genus Plasmodium: P. falciparum, P. vivax, P. ovale, P. malariae. Occasionally other species of malaria usually found in primates can affect man. Malaria probably originated from animal malarias in Central Africa, but was spread around the globe by human migration. Public health measures and changes in land use have eradicated malaria in most developed countries, although the potential for malaria transmission still exists in many areas. Some 400 million people are infected every year and over 1 million die annually; 25 000 international travellers per year are infected.
Malaria is transmitted by the bite of female anopheline mosquitoes. The parasite undergoes a temperature-dependent cycle of development in the gut of the insect and its geographical range therefore depends on the presence of the appropriate mosquito species and an adequate temperature. The disease occurs in endemic or epidemic form throughout the tropics and subtropics except for some areas above 2000 m (Fig. 4.32). Australia, the USA and most of the Mediterranean littoral are also malaria-free. In hyperendemic areas (51–75% rate of parasitaemia or palpable spleen in children 2–9 years of age) and holoendemic areas (>75% rate) where transmission of infection occurs year round, the bulk of the mortality is seen in infants. Those who survive to adulthood acquire significant immunity; low-grade parasitaemia is still present, but causes few symptoms. In mesoendemic areas (11–50%), there is regular seasonal transmission of malaria. Mortality is still mainly seen in infants, but older children and adults may develop chronic ill health due to repeated infections. In hypoendemic areas (0–10%), where infection occurs in occasional epidemics, little immunity is acquired and the whole population is susceptible to severe and fatal disease.
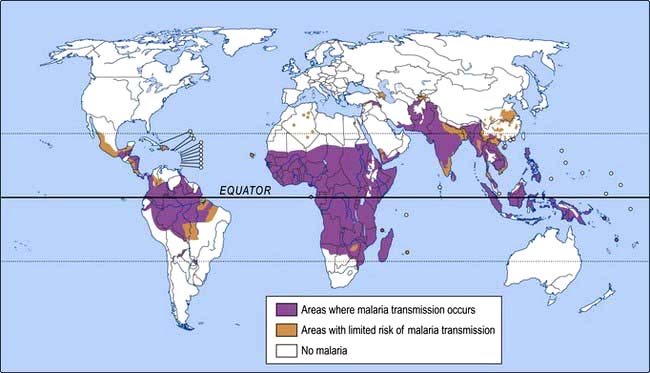
Figure 4.32 Malaria: geographical distribution.
(Reproduced with permission of the World Health Organization.)
Malaria can also be transmitted in contaminated blood transfusions. It has occasionally been seen in injecting drug users sharing needles and as a hospital-acquired infection related to contaminated equipment. Rare cases are acquired outside the tropics when mosquitoes are transported from endemic areas (‘airport malaria’), or when the local mosquito population becomes infected by a returning traveller.
The female mosquito becomes infected after taking a blood meal containing gametocytes, the sexual form of the malarial parasite (Fig. 4.33). The developmental cycle in the mosquito usually takes 7–20 days (depending on temperature), culminating in infective sporozoites migrating to the insect’s salivary glands. The sporozoites are inoculated into a new human host and those which are not destroyed by the immune response are rapidly taken up by the liver. Here they multiply inside hepatocytes as merozoites: this is pre-erythrocytic (or hepatic) sporogeny. After a few days, the infected hepatocytes rupture, releasing merozoites into the blood from where they are rapidly taken up by erythrocytes. In the case of P. vivax and P. ovale, a few parasites remain dormant in the liver as hypnozoites. These may reactivate at any time subsequently, causing relapsing infection.
Inside the red cells, the parasites again multiply, changing from merozoite, to trophozoite, to schizont and finally appearing as 8–24 new merozoites. The erythrocyte ruptures, releasing the merozoites to infect further cells. Each cycle of this process, which is called erythrocytic schizogony, takes about 48 h in P. falciparum, P. vivax and P. ovale and about 72 h in P. malariae. P. vivax and P. ovale mainly attack reticulocytes and young erythrocytes, while P. malariae tends to attack older cells; P. falciparum will parasitize any stage of erythrocyte.
A few merozoites develop not into trophozoites but into gametocytes. These are not released from the red cells until taken up by a feeding mosquito to complete the life cycle.
The pathology of malaria is related to anaemia, cytokine release and in the case of P. falciparum, widespread organ damage due to impaired microcirculation. The anaemia seen in malaria is multifactorial (Table 4.38). In P. falciparum malaria, red cells containing schizonts adhere to the lining of capillaries in the brain, kidneys, gut, liver and other organs. As well as causing mechanical obstruction these schizonts rupture, releasing toxins and stimulating further cytokine release.
Table 4.38 Causes of anaemia in malaria infection
After repeated infections partial immunity develops, allowing the host to tolerate parasitaemia with minimal ill effects. This immunity is largely lost if there is no further infection for a couple of years. Certain genetic traits also confer some immunity to malaria. People who lack the Duffy antigen on the red cell membrane (a common finding in West Africa) are not susceptible to infection with P. vivax. Certain haemoglobinopathies (including sickle cell trait) also give some protection against the severe effects of malaria: this may account for the persistence of these otherwise harmful mutations in tropical countries. Iron deficiency may also have some protective effect. The spleen appears to play a role in controlling infection and splenectomized people are at risk of overwhelming malaria. Some individuals appear to have a genetic predisposition for developing cerebral malaria following infection with P. falciparum. Pregnant women are especially susceptible to severe disease.
Typical malaria is seen in non-immune individuals. This includes children in any area, adults in hypoendemic areas and any visitors from a non-malarious region.
The normal incubation period is 10–21 days, but can be longer. The most common symptom is fever, although malaria may present initially with general malaise, headache, vomiting, or diarrhoea. At first, the fever may be continual or erratic: the classical tertian or quartan fever only appears after some days. The temperature often reaches 41°C and is accompanied by rigors and drenching sweats.
P. vivax or P. ovale infection
The illness is usually relatively mild (although P. vivax can occasionally cause severe disease). Anaemia develops slowly and there may be tender hepatosplenomegaly. Spontaneous recovery usually occurs within 2–6 weeks, but hypnozoites in the liver can cause relapses for many years after infection. Repeated infections often cause chronic ill health due to anaemia and hyperreactive splenomegaly.
This also causes a relatively mild illness, but tends to run a more chronic course. Parasitaemia may persist for years, with or without symptoms. In children, P. malariae infection is associated with glomerulonephritis and nephrotic syndrome.
This causes, in many cases, a self-limiting illness similar to the other types of malaria, although the paroxysms of fever are usually less marked. However, it may also cause serious complications (Box 4.14) and the vast majority of malaria deaths are due to P. falciparum. Patients can deteriorate rapidly and children in particular progress from reasonable health to coma and death within hours. A high parasitaemia (>1% of red cells infected) is an indicator of severe disease, although patients with apparently low parasite levels may also develop complications. Cerebral malaria is marked by diminished consciousness, confusion and convulsions, often progressing to coma and death. Untreated it is universally fatal. Blackwater fever is due to widespread intravascular haemolysis, affecting both parasitized and unparasitized red cells, giving rise to dark urine.
Hyperreactive malarial splenomegaly (tropical splenomegaly syndrome, TSS)
This is seen in older children and adults in areas where malaria is hyperendemic. It is associated with an exaggerated immune response to repeated malaria infections and is characterized by anaemia, massive splenomegaly and elevated IgM levels. Malaria parasites are scanty or absent. TSS usually responds to prolonged treatment with prophylactic antimalarial drugs.
Malaria should be considered in the differential diagnosis of anyone who presents with a febrile illness in, or having recently left, a malarious area. Falciparum malaria is unlikely to present more than 3 months after exposure, even if the patient has been taking prophylaxis, but vivax malaria may cause symptoms for the first time up to a year after leaving a malarious area.
Diagnosis is usually made by identifying parasites on a Giemsa-stained thick or thin blood film (thick films are more difficult to interpret and it may be difficult to speciate the parasite, but they have a higher yield). At least three films should be examined before malaria is declared unlikely. Rapid antigen detection tests are available for near-patient use. In many endemic areas, malaria is overdiagnosed on clinical grounds and a definite diagnosis should be made wherever possible. Serological tests are of no diagnostic value.
Parasitaemia is common in endemic areas and the presence of parasites does not necessarily mean that malaria is the cause of the patient’s symptoms. Further investigation, including a lumbar puncture, may be needed to exclude bacterial infection.
Treatment of uncomplicated malaria. The drug of choice for susceptible parasites is chloroquine (Box 4.15). P. vivax, P. ovale and P. malariae are usually sensitive to this drug, although there is increasing resistance in some strains of P. vivax. Following successful treatment of P. vivax or P. ovale malaria, it is necessary to give a 2- to 3-week course of primaquine (15 mg daily) to eradicate the hepatic hypnozoites and prevent relapse. This drug can precipitate haemolysis in patients with G6PD deficiency (see p. 396).
The artemisinin-based drugs are the most effective treatment for both uncomplicated and severe infections with P. falciparum, in adults and in children. Artemisinin-based combination therapy (ACT) is the recommended oral treatment for uncomplicated falciparum malaria worldwide. These drugs are now quite widely available, partly through the efforts of the Global Fund (www.theglobalfund.org). Five different fixed-dose combinations are recommended by the WHO (Box 4.16): the choice should be based on local resistance to the ‘partner’ drug. Artemisinin derivatives should not be given as monotherapy, to limit resistance which has already occurred in Cambodia. The WHO recommends that a single dose of primaquine should be given as a gametocide, to decrease transmission.
![]() Box 4.16
Box 4.16
Suitable artemisinin combination therapies (ACT) for malaria
Fixed-dose combination tablets available |
|
Artemether-lumefantrine |
4 tablets twice daily for 3 daysa |
Artesunate-amodiaquine |
4 mg/kg per day artesunate for 3 days |
Dihydroartemisinin-piperaquine |
4 mg/kg per day dihydroartemisinin for 3 days |
Available as fixed-dose co-packaged separate tablets |
|
Artesunate-mefloquineb |
4 mg/kg per day artesunate for 3 days |
Artesunate-sulfadoxine-pyrimethaminec |
4 mg/kg per day artesunate for 3 days |
Alternatives where no combination packages are available |
|
Artesunate + clindamycin |
2 mg/kg per day + 10 mg/kg twice daily for 7 days |
Artesunate + doxycycline |
2 mg/kg per day + 3.5 mg/kg per day for 7 days |
aAdult dose: reduce dose by body weight for children; bCombination tablet available soon; cNot suitable for P. vivax or mixed infections. Different fixed-dose combinations available.
Treatment of severe falciparum malaria. Severe malaria, indicated by the presence of any of the complications discussed above, or a parasite count above 1% in a non-immune patient, is a medical emergency (Emergency Box 4.1). Anyone involved in managing patients with malaria should be familiar with the latest WHO guidelines.
 Intravenous artesunate is more effective than intravenous quinine and should be used where available. Absorption from intramuscular injection is less reliable than from intravenous injection.
Intravenous artesunate is more effective than intravenous quinine and should be used where available. Absorption from intramuscular injection is less reliable than from intravenous injection.
 Intensive care facilities may be needed, including mechanical ventilation and dialysis.
Intensive care facilities may be needed, including mechanical ventilation and dialysis.
 Severe anaemia may require transfusion.
Severe anaemia may require transfusion.
 Careful monitoring of fluid balance is essential: both pulmonary oedema and prerenal failure are common.
Careful monitoring of fluid balance is essential: both pulmonary oedema and prerenal failure are common.
 Hypoglycaemia can be induced both by the infection itself and by quinine treatment.
Hypoglycaemia can be induced both by the infection itself and by quinine treatment.
In very heavy infections (parasitaemia >10%), there may be a role for exchange transfusion, if the facilities are available.
SIGNIFICANT WEBSITE
WHO Guidelines for the Treatment of Malaria, 2nd edn. 2010: http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html
FURTHER READING
Dondorp AM, Fanello CI, Hendriksen IC et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT). Lancet 2010; 376:1647–1657.
Smithuis F, Kyaw MK, Phe O et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 2010; 10:673–681.
As with many vector-borne diseases, control of malaria relies on a combination of case treatment, vector eradication and personal protection from vector bites, e.g. insecticide (permethrin) treated nets. Mosquito eradication is usually achieved either by the use of insecticides, house spraying with DDT or by manipulation of the habitat (e.g. marsh drainage). After some initial successes, a WHO campaign to eliminate malaria foundered in the mid-1960s. Since then, the emergence of both parasite resistance to drugs and mosquito resistance to insecticides has rendered the task more difficult. However, malaria is once again a priority for the WHO, which announced a new ‘Roll Back Malaria’ campaign in 1998. This has had good success in some countries who have coordinated programmes, including the use of insecticide-treated bed nets and indoor residual spraying with DDT. A 3-part strategy is now widely endorsed and supported by governments and non-governmental organizations (Box 4.17).
Non-immune travellers to malarious areas should take measures to avoid insect bites, such as using insect repellent (diethyltoluamide, DEET, 20–50% in lotions and sprays) and sleeping under mosquito nets. Antimalarial prophylaxis should also be taken in most cases, although this is never 100% effective (Box 4.18). The precise choice of prophylactic regimen depends both on the individual traveller and on the specific itinerary; further details can be found in National Formularies or from travel advice centres. Despite considerable efforts, there is still no effective vaccine available for malaria.
![]() Box 4.18
Box 4.18
Malaria prophylaxis for adult travellers
| Area visited | Prophylactic regimen | Alternative |
|---|---|---|
No chloroquine resistance |
Chloroquine 300 mg weekly |
Proguanil 200 mg daily |
Limited chloroquine resistance |
Chloroquine 300 mg weekly |
Doxycycline 100 mg daily |
plus |
or |
|
Proguanil 200 mg daily |
Malarone 1 tablet daily |
|
|
or |
|
|
Mefloquine 250 mg weekly |
|
Significant chloroquine resistance |
Mefloquine 250 mg weekly |
Doxycycline 100 mg daily |
|
or |
|
|
Malarone 1 tablet daily |
Sleeping sickness is caused by trypanosomes transmitted to humans by the bite of the tsetse fly (genus Glossina). It is endemic in a belt across sub-Saharan Africa, extending to about 14°N and 20°S: this marks the natural range of the tsetse fly. Two subspecies of trypanosome cause human sleeping sickness: Trypanosoma bruceigambiense (‘Gambian sleeping sickness’) and T. b. rhodesiense (‘Rhodesian sleeping sickness’).
Sleeping sickness due to T. b. gambiense is found from Uganda in Central Africa, west to Senegal and south as far as Angola. Man is the major reservoir and infection is transmitted by riverine Glossina species (e.g. G. palpalis).
Sleeping sickness due to T. b. rhodesiense occurs in East and Central Africa from Ethiopia to Botswana. It is a zoonosis of both wild and domestic animals. In endemic situations it is maintained in game animals and transmitted by savanna flies such as G. morsitans. Epidemics are usually related to cattle and the vectors are riverine flies.
Political upheavals during the 1990s disrupted established treatment and control programmes, resulting in major epidemics in the Republic of Angola, the Democratic Republic of Congo (DRC) and Uganda. By 1997 as many as 500 000 people were affected by sleeping sickness. A concerted control programme has brought this number down to below 30 000, most of which are in DRC and the Central African Republic.
Tsetse flies bite during the day and unlike most arthropod vectors both males and females take blood meals. An infected insect may deposit metacyclic trypomastigotes (the infective form of the parasite) into the subcutaneous tissue. These cause local inflammation (‘trypanosomal chancre’) and regional lymphadenopathy. Within 2–3 weeks the organisms invade the bloodstream, subsequently spreading to all parts of the body including the brain.
T. b. gambiense causes a chronic, slowly progressive illness. Episodes of fever and lymphadenopathy occur over months or years and hepatosplenomegaly may develop. Eventually infection reaches the central nervous system, causing headache, behavioural changes, confusion and daytime somnolence. As the disease progresses patients may develop tremors, ataxia, convulsions and hemiplegias; eventually coma and death supervene. Histologically there is a lymphocytic meningoencephalitis, with scattered trypanosomes visible in the brain substance.
T. b. rhodesiense sleeping sickness is a much more acute disease. Early systemic features may include myocarditis, hepatitis and serous effusions and patients can die before the onset of CNS disease. If they survive, cerebral involvement occurs within weeks of infection and is rapidly progressive.
Trypanosomes may be seen on Giemsa-stained smears of thick or thin blood films, or of lymph node aspirate. Blood films are usually positive in T. b. rhodesiense, but may be negative in T. b. gambiense: concentration techniques may increase the yield. Serological tests are useful for screening for infection: the card agglutination test for trypanosomiasis (CATT) is a robust and easy-to-use field assay. Examination of cerebrospinal fluid is essential in patients with evidence of trypanosomal infection. CNS involvement causes lymphocytosis and elevated protein in the CSF and parasites may be seen in concentrated specimens.
The treatment of sleeping sickness had remained largely unchanged for more than 40 years, but there have been recent improvements in the management of T. b. gambiense infection. In both forms, treatment is usually effective if given before the onset of CNS involvement (Box 4.19). A single dose of suramin should be given to patients with parasitaemia prior to lumbar puncture, to avoid inoculation into the CSF. The treatment of choice for 2nd stage (CNS) disease in T. b. gambiense is a combination of eflornithine and nifurtimox, a therapy introduced in 2009 and provided free via the WHO. Melarsoprol remains the only treatment for CNS infection with T. b. rhodesiense. It is extremely toxic: 2–10% of patients develop an acute encephalopathy, with a 50–75% mortality; peripheral neuropathy and hepatorenal toxicity are also common. Between 3% and 6% of patients relapse following melarsoprol treatment.
![]() Box 4.19
Box 4.19
Drugs used in the treatment of African trypanosomiasis
| T. b. gambiense | T. b. rhodesiense | |
|---|---|---|
Stage 1 |
Pentamidine |
Suramina |
Stage 2 (CNS) |
Eflornithine + nifurtimox |
Melarsoprol |
Eflornithine monotherapy |
|
|
|
(melarsoprol) |
|
SIGNIFICANT WEBSITES
WHO African trypanosomiasis website: http://www.who.int/topics/trypanosomiasis_african/en/
Chagas’ disease is widely distributed in rural areas of South and Central America, where up to 10 million people are infected. It is caused by Trypanosoma cruzi, which is transmitted to humans in the faeces of bloodsucking reduviid bugs (also called cone-nose or assassin bugs). Faeces infected with T. cruzi trypomastigotes are rubbed in through skin abrasions, mucosa or conjunctiva. The bugs, which live in mud or thatch buildings, feed on a variety of vertebrate hosts (e.g. rats, opossums) at night, defecating as they do so.
The parasites spread in the bloodstream, before entering host cells and multiplying. Cell rupture releases them back into the circulation, where they can be taken up by a feeding bug. Further multiplication takes place in the insect gut, completing the trypanosome life cycle. Human infection can also occur via contaminated blood transfusion, or occasionally by transplacental spread.
Acute infection, which usually occurs in children, often passes unnoticed. A firm reddish papule is sometimes seen at the site of entry, associated with regional lymphadenopathy. In the case of conjunctival infection there is swelling of the eyelid, which may close the eye (Romaña’s sign). There may be fever, lymphadenopathy, hepatosplenomegaly and rarely meningoencephalitis. Acute Chagas’ disease is occasionally fatal in infants, but normally there is full recovery within a few weeks or months.
Chronic Chagas’ disease. 10–30% of people go on to develop chronic Chagas’ disease after a latent period of many years. The pathogenesis of this is unclear: it is possibly due to an autoimmune response triggered by the initial infection, although recent evidence has thrown doubt on this mechanism. The heart is commonly affected, with conduction abnormalities, arrhythmias, aneurysm formation and cardiac dilatation. Gastrointestinal involvement leads to progressive dilatation of parts of the gastrointestinal tract: this commonly results in megaoesophagus (causing dysphagia and aspiration pneumonia) and megacolon (causing severe constipation).
Trypanosomes may be seen on a stained blood film during the acute illness. In chronic disease, parasites may be detected by xenodiagnosis: infection-free reduviid bugs are allowed to feed on the patient and the insect gut subsequently examined for parasites. Serological tests can detect both acute and chronic Chagas’ disease.
Nifurtimox and benznidazole are the main drugs used in Chagas’ disease. Both are highly effective in acute infection, with a cure rate of over 90%, but much less so in chronic disease. They are relatively toxic with adverse reactions in up to 40% of patients and new drugs are urgently needed. Antiarrhythmic drugs and pacemakers may be needed in cardiac disease and surgical treatment is sometimes needed for gastrointestinal complications.
In the long term, prevention of Chagas’ disease relies on improved housing and living conditions. In the interim, local vector control programmes may be effective and the countries of the ‘Southern Cone’ of South America run a successful joint programme to control the disease by spraying houses with insecticide. Impregnated bed nets with pyrethroid and insect repellents should be used.
This group of diseases is caused by protozoa of the genus Leishmania, which are transmitted by the bite of the female phlebotomine sandfly (Table 4.39). Leishmaniasis is seen in localized areas of Africa, Asia (particularly India and Bangladesh), Europe, the Middle East and South and Central America. Certain parasite species are specific to each geographical area. The clinical picture is dependent on the species of parasite and on the host’s cell-mediated immune response. Asymptomatic infection, in which the parasite is suppressed or eradicated by a strong immune response, is common in endemic areas, as demonstrated by a high incidence of positive leishmanin skin tests. Symptomatic infection may be confined to the skin (sometimes with spread to the mucous membranes) or widely disseminated throughout the body (visceral leishmaniasis). Relapse of previously asymptomatic infection is seen in patients who become immunocompromised, especially those with HIV infection.
Table 4.39 Leishmania species causing visceral and cutaneous disease in man
| Species complex | Species | |
|---|---|---|
Visceral leishmania |
L. donovani |
L. donovani |
L. infantum |
||
L. chagasi |
||
Cutaneous leishmania |
L. tropica |
L. tropica |
L. major |
L. major |
|
L. aethiopica |
L. aethiopica |
|
L. mexicana |
L. mexicana |
|
L. amazonensis |
||
L. garnhami |
||
L. pifanoi |
||
L. venezuelensis |
||
L. braziliensis |
L. braziliensis |
|
L. guyanensis |
||
L. panamanensis |
||
L. peruviana |
||
Mucocutaneous leishmania |
|
L. braziliensis |
In some areas, leishmania is primarily zoonotic, whereas in others, man is the main reservoir of infection. In the vertebrate host the parasites are found as oval amastigotes (Leishman–Donovan bodies). These multiply inside the macrophages and cells of the reticuloendothelial system and are then released into the circulation as the cells rupture. Parasites are taken into the gut of a feeding sandfly (genus Phlebotomus in the Old World, genus Lutzomyia in the New World), where they develop into the flagellate promastigote form. These migrate to the salivary glands of the insect, where they can be inoculated into a new host.
Visceral leishmaniasis (kala azar) is caused by L. donovani, L. infantum, or L. chagasi and is prevalent in localized areas of Asia, Africa, the Mediterranean littoral and South America. In parts of India, where man is the main host, the disease occurs in epidemics. In most other areas it is endemic and it is mainly children and visitors to the area who are at risk. The main animal reservoirs in Europe and Asia are dogs and foxes, while in Africa it is carried by various rodents.
The incubation period is usually 1–2 months, but may be several years. The onset of symptoms is insidious and the patient may feel quite well despite markedly abnormal physical findings. Fever is common and although usually low-grade, it may be high and intermittent. The liver and especially the spleen, become enlarged; lymphadenopathy is common in African kala azar. The skin becomes rough and pigmented. If the disease is not treated, profound pancytopenia develops and the patient becomes wasted and immunosuppressed. Death usually occurs within a year and is normally due to bacterial infection or uncontrolled bleeding.
Specific diagnosis is made by demonstrating the parasite in stained smears of aspirates of bone marrow, lymph node, spleen or liver. The organism can also be cultured from these specimens. Specific serological tests are positive in 95% of cases. Pancytopenia, hypoalbuminaemia and hypergamma-globulinaemia are common. The leishmanin skin test is negative, indicating a poor cell-mediated immune response.
The most widely used drugs for visceral leishmaniasis are the pentavalent antimony salts (e.g. sodium stibogluconate and meglumine antimoniate). Resistance to antimony salts is increasing and relapses may occur following treatment. Intravenous amphotericin B (preferably liposomal, which may be curative as a single dose treatment) is effective but expensive; intramuscular paromomycin is cheaper and also has a good cure rate. An oral drug, miltefosine, has been shown in India to be highly effective and may replace older therapies. There is currently considerable interest in the use of combination therapy to shorten treatment courses and limit resistance.
Successful treatment may be followed in a small proportion of patients by a skin eruption called post-kala azar dermal leishmaniasis (PKDL). It starts as a macular maculopapular nodular rash which spreads over the body. It is most often seen in the Sudan and India. There have been reports of PKDL responding to miltefosine.
Cutaneous leishmaniasis is caused by a number of geographically localized species, which may be zoonotic or anthroponotic. Following a sandfly bite, leishmania amastigotes multiply in dermal macrophages. The local response depends on the species of leishmania, the size of the inoculum and the host immune response. Single or multiple painless nodules occur on exposed areas within 1 week to 3 months following the bite. These enlarge and ulcerate with a characteristic erythematous raised border. An overlying crust may develop. The lesions heal slowly over months or years, sometimes leaving a disfiguring scar.
L. major and L. tropica are found in Russia and Eastern Europe, the Middle East, Central Asia, the Mediterranean littoral and sub-Saharan Africa. The reservoir for L. major is desert rodents, while L. tropica has a mainly urban distribution with dogs and humans as reservoirs. L. aethiopica is found in the highlands of Ethiopia and Kenya, where the animal reservoir is the hyrax. The skin lesions usually heal spontaneously with scarring: this may take a year or more in the case of L. tropica. Leishmaniasis recidivans is a rare chronic relapsing form caused by L. tropica.
L. mexicana is found predominantly in Mexico, Guatemala, Brazil, Venezuela and Panama; infection usually runs a benign course with spontaneous healing within 6 months. L. braziliensis infections (which are seen throughout tropical South America) also usually heal spontaneously, but may take longer.
L. mexicana amazonensis and L. aethiopica may occasionally cause diffuse cutaneous leishmaniasis. This is rare and is characterized by diffuse infiltration of the skin by Leishman–Donovan bodies. Visceral lesions are absent.
The diagnosis can often be made clinically in a patient who has been in an endemic area. Giemsa stain on a split-skin smear will demonstrate leishmania parasites in 80% of cases. Biopsy tissue from the edge of the lesion can be examined histologically and parasites identified by PCR; culture is less often successful. The leishmanin skin test is positive in over 90% of cases, but does not distinguish between active and resolved infection. Serology is unhelpful.
Small lesions usually require no treatment. Large lesions or those in cosmetically sensitive sites can sometimes be treated locally, by curettage, cryotherapy or topical antiparasitic agents. In other cases, systemic treatment (as for visceral leishmaniasis) is required.
FURTHER READING
Sundar S, Sinha PK, Rai M et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 2011; 377:477–486.
van Griensven J, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet 2011; 377:443–444.
Mucocutaneous leishmaniasis occurs in 3–10% of infections with L. b. braziliensis and is commonest in Bolivia and Peru. The cutaneous sores are followed months or years later by indurated or ulcerating lesions affecting mucosa or cartilage, typically on the lips or nose (‘espundia’). The condition can remain static, or there may be progression over months or years affecting the nasopharynx, uvula, palate and upper airways.
Biopsies usually show only very scanty organisms, although parasites can be detected by PCR; serological tests are frequently positive.
Amphotericin B is the treatment of choice if available, although systemic antimonial compounds are widely used; miltefosine may also be effective. Relapses are common following treatment. Patients may die because of secondary bacterial infection, or occasionally laryngeal obstruction.
Prevention of leishmaniasis relies on control of vectors and/or reservoirs of infection. Insecticide spraying, control of host animals and treating infected humans may all be helpful. Personal protection against sandfly bites is also necessary, especially in travellers visiting endemic areas. Sandflies are poor fliers and sleeping off the ground helps prevent bites.
Toxoplasmosis is caused by the intracellular protozoan parasite Toxoplasma gondii. The sexual form of the parasite lives in the gut of the definitive host, the cat, where it produces oocysts. After a period of maturing in the environment, these oocysts become the source of infection for secondary hosts which may ingest them. In the secondary hosts (which include man, cattle, sheep, pigs, rodents and birds), there is disseminated infection. Following a successful immune response the infection is controlled, but dormant parasites remain encysted in host tissue for many years. The life cycle is completed when carnivorous felines eat infected animal tissue. Humans are infected either from contaminated cat faeces, or by eating undercooked infected meat; transplacental infection may also occur.
Toxoplasmosis is common: seroprevalence in adults in the UK is about 25%, rising to 90% in some parts of Europe. Most infections are asymptomatic or trivial. Symptomatic patients usually present with lymphadenopathy, mainly in the head and neck. There may be fever, myalgia and general malaise; occasionally there are more severe manifestations including hepatitis, pneumonia, myocarditis and choroidoretinitis. Lymphadenopathy and fatigue can sometimes persist for months after the initial infection.
Congenital toxoplasmosis may also be asymptomatic, but can produce serious disease. Clinical manifestations include microcephaly, hydrocephalus, encephalitis, convulsions and mental retardation. Choroidoretinitis is common; occasionally this may be the only feature.
Immunocompromised patients, especially those with HIV infection, are at risk of serious infections with T. gondii. In acquired immunodeficiency states this is usually due to reactivation of latent disease (see p. 189).
Diagnosis is usually made serologically. IgG antibodies detectable by the Sabin–Feldman dye test remain positive for years; acute infection can be confirmed by demonstrating a rising titre of specific IgM.
Acquired toxoplasmosis in an immunocompetent host rarely requires treatment. In those with severe disease (especially eye involvement) sulfadiazine 2–4 g daily and pyrimethamine 25 mg daily are given for 4 weeks, along with folinic acid. The management of pregnant women with toxoplasmosis aims to decrease the risk of fetal complications. However, there is little good evidence that giving spiramycin either alone or in combination with sulfadiazine (which is the recommended treatment) has any significant effect on the frequency or severity of fetal damage. Infected infants should be treated from birth. The treatment of toxoplasmosis in HIV-positive patients is covered on page 189.
Babesiosis is a tick-borne parasitic disease, diagnosed most commonly in North America and Europe. It is a zoonosis of rodents and cattle and is occasionally transmitted to humans: infection is more common and more severe in those who are immunocompromised following splenectomy. The causative organisms are the plasmodium-like Babesia microti (rodents) and B. divergens (cattle).
The incubation period averages 10 days. In patients with normal splenic function, the illness is usually mild. In splenectomized individuals, systemic symptoms are more pronounced and haemolysis is associated with haemoglobinuria, jaundice and acute kidney injury (AKI). Examination of a peripheral blood smear may reveal the characteristic plasmodium-like organisms.
The standard treatment of severe babesiosis is a combination of quinine 650 mg and clindamycin 600 mg orally three times daily for 7 days. Atovaquone and azithromycin plus doxycycline is used for persistent or relapsing disease.
The major gastrointestinal parasites of man are shown in Table 4.40.
Table 4.40 Pathogenic human intestinal protozoa
Amoebiasis is caused by Entamoeba histolytica. The organism formerly known as E. histolytica is known to consist of three distinct species: E. histolytica, which is pathogenic, E. dispar, which is non-pathogenic, and E. moshkovskii, which is of uncertain significance. Cysts of the three species are identical, but can be distinguished by molecular techniques after culture of the trophozoite. E. histolytica can be distinguished from all other amoebae and from other intestinal protozoa, by microscopic appearance. Amoebiasis occurs worldwide, although much higher incidence rates are found in the tropics and subtropics.
The organism exists both as a motile trophozoite and as a cyst that can survive outside the body. Cysts are transmitted by ingestion of contaminated food or water, or spread directly by person-to-person contact. Trophozoites emerge from the cysts in the small intestine and then pass on to the colon, where they multiply.
It is believed that many individuals can carry the pathogen without obvious evidence of clinical disease (asymptomatic cyst passers). However, this may be due in some cases to the misidentification of non-pathogenic E. dispar as E. histolytica and it is not clear how often true E. histolytica infection is symptomless. In affected people E. histolytica trophozoites invade the colonic epithelium, probably with the aid of their own cytotoxins and proteolytic enzymes. The parasites continue to multiply and finally frank ulceration of the mucosa occurs. If penetration continues, trophozoites may enter the portal vein, via which they reach the liver and cause intrahepatic abscesses. This invasive form of the disease is serious and may even be fatal.
The incubation period of intestinal amoebiasis is highly variable and may be as short as a few days or as long as several months. The usual course is chronic, with mild intermittent diarrhoea and abdominal discomfort. This may progress to bloody diarrhoea with mucus and is sometimes accompanied by systemic symptoms such as headache, nausea and anorexia. Less commonly, infection may present as acute amoebic dysentery, resembling bacillary dysentery or acute ulcerative colitis.
Complications are unusual, but include toxic dilatation of the colon, chronic infection with stricture formation, severe haemorrhage, amoeboma and amoebic liver abscess. Amoebic liver abscesses often develop in the absence of a recent episode of colitis. Tender hepatomegaly, a high swinging fever and profound malaise are characteristic, although early in the course of the disease both symptoms and signs may be minimal. The clinical features are described in more detail on page 345.
Microscopic examination of fresh stool or colonic exudate obtained at sigmoidoscopy is the simplest way of diagnosing colonic amoebic infection. To confirm the diagnosis motile trophozoites containing red blood cells must be identified: the presence of amoebic cysts alone does not imply disease. Sigmoidoscopy and barium enema examination may show colonic ulceration but are rarely diagnostic.
The amoebic fluorescent antibody test is positive in at least 90% of patients with liver abscess and in 60–70% with active colitis. Seropositivity is low in asymptomatic cyst passers.
Metronidazole 800 mg three times daily for 5 days is given in amoebic colitis; a lower dose (400 mg three times daily for 5 days) is usually adequate in liver abscess. Tinidazole is also effective. After treatment of the invasive disease, the bowel should be cleared of parasites with a luminal amoebicide such as diloxanide furoate.
Amoebiasis is difficult to eradicate because of the substantial human reservoir of infection. The only progress will be through improved standards of hygiene, sanitation and better access to clean water. Cysts are destroyed by boiling, but chlorine and iodine sterilizing tablets are not always effective.
Giardia intestinalis is a flagellate (Fig. 4.34) that is found worldwide. It causes small intestinal disease, with diarrhoea and malabsorption. Prevalence is high in many developing countries and it is the most common parasitic infection in travellers returning to the UK. In certain parts of Europe and in some rural areas of North America, large water-borne epidemics have been reported. Person-to-person spread may occur in day nurseries and residential institutions. The organism exists both as a trophozoite and a cyst, the latter being the form in which it is transmitted.
Figure 4.34 Giardia intestinalis on small intestinal mucosa.
(Courtesy of Dr A Phillips, Department of Electron Microscopy, Royal Free Hospital, London.)
The organism sometimes colonizes the small intestine and may remain there without causing detriment to the host. In other cases, severe malabsorption may occur which is thought to be related to morphological damage to the small intestine. The changes in villous architecture are usually mild partial villous atrophy; subtotal villous atrophy is rare. The mechanism by which the parasite causes alteration in mucosal architecture and produces diarrhoea and intestinal malabsorption is unknown: there is evidence that the morphological damage is immune-mediated. Bacterial overgrowth has also been found in association with giardiasis and may contribute to fat malabsorption.
Many individuals excreting Giardia cysts have no symptoms. Others become ill within 1–3 weeks after ingesting cysts: symptoms include diarrhoea, often watery in the early stage of the illness, nausea, anorexia and abdominal discomfort and bloating. In most people affected these symptoms resolve after a few days, but in some they persist. Stools may then become paler, with the characteristic features of steatorrhoea. If the illness is prolonged, weight loss, which can be marked, occurs. Chronic giardiasis frequently seen in developing countries can result in growth retardation in children.
Both cysts and trophozoites can be found in the stool, but negative stool examination does not exclude the diagnosis since the parasite may be excreted at irregular intervals. The parasite can also be seen in duodenal aspirates (obtained either at endoscopy or with a luminal capsule) and in histological sections of jejunal mucosa.
Metronidazole 2 g as a single dose on three successive days will cure the majority of infections, although sometimes a second or third course is necessary. Alternative drugs include tinidazole, mepacrine and albendazole. Preventative measures are similar to those outlined above for E. histolytica.
This organism is found worldwide, cattle being the major natural reservoir. It has also been demonstrated in supplies of drinking water in the UK. The parasite is able to reproduce both sexually and asexually; it is transmitted by oocysts excreted in the faeces.
In healthy individuals cryptosporidiosis is a self-limiting illness. Acute watery diarrhoea is associated with fever and general malaise lasting for 7–10 days. In immunocompromised patients, especially those with HIV, diarrhoea is severe and intractable (see p. 189).
Diagnosis is usually made by faecal microscopy, although the parasite can also be detected in intestinal biopsies. There is no reliable treatment, although nitazoxanide may be of benefit.
Balantidium coli is the only ciliate that produces clinically significant infection in humans. It is found throughout the tropics, particularly in Central and South America, Iran, Papua New Guinea and the Philippines. It is usually carried by pigs and infection is most common in those communities that live in close association with swine. Its life cycle is identical to that of E. histolytica. B. coli causes diarrhoea and sometimes a dysenteric illness with invasion of the distal ileal and colonic mucosa. Trophozoites rather than cysts are found in the stool. Treatment is with tetracycline or metronidazole.
B. hominis is a strictly anaerobic protozoan pathogen that inhabits the colon. The pathogenicity for humans remains controversial despite many studies indicating response to chemotherapy.
Cyclospora cayetanensis, a coccidian protozoal parasite, was originally recognized as a cause of diarrhoea in travellers to Nepal. It has been detected in stool specimens from immunocompetent and immunodeficient people worldwide. Infection is usually self-limiting, but can be treated with co-trimoxazole.
Protozoa of the phylum Microsporea can cause diarrhoea in patients with HIV/AIDS (see p. 190).
Worm infections are very common in developing countries, causing much disease in both humans and domestic animals. Worms are frequently imported into industrialized countries. The most common human helminth infections are listed in Table 4.41. Three in particular – ascariasis, hookworm and trichuriasis – are included in a list of 13 ‘neglected tropical diseases’, which the WHO has identified as causing major disability among the poorest people in the world.
Table 4.41 Helminths commonly infecting man
| Helminth | Common name/disease caused | |
|---|---|---|
Nematodes (roundworms) |
|
|
Tissue-dwelling worms |
Wuchereria bancrofti |
Filariasis |
Brugia malayi/timori |
Filariasis |
|
Loa loa |
Loiasis |
|
Onchocerca volvulus |
River blindness |
|
Dracunculus medinensis |
Dracunculiasis |
|
Mansonella perstans |
Mansonellosis |
|
Intestinal human nematodes |
Enterobius vermicularis |
Threadworm |
Ascaris lumbricoides |
Roundworm |
|
Trichuris trichiura |
Whipworm |
|
Necator americanus |
Hookworm |
|
Ancylostoma duodenale |
Hookworm |
|
Strongyloides stercoralis |
Strongyloidosis |
|
Zoonotic nematodes |
Toxocara canis |
Toxocariasis |
Trichinella spiralis |
Trichinellosis |
|
Trematodes (flukes) |
|
|
Blood flukes |
Schistosoma species |
Schistosomiasis |
Lung flukes |
Paragonimus species |
Paragonimiasis |
Intestinal/hepatic flukes |
Fasciolopsis buski |
|
Fasciola hepatica |
|
|
Clonorchis sinensis |
|
|
Opisthorchis felineus |
|
|
Cestodes (tapeworms) |
|
|
Intestinal adult worms |
Taenia saginata |
Beef tapeworm |
Taenia solium |
Pork tapeworm |
|
Diphyllobothrium latum |
Fish tapeworm |
|
Hymenolepis nana |
Dwarf tapeworm |
|
Larval tissue cysts |
Taenia solium |
Cysticercosis |
Echinococcus granulosus |
Hydatid disease |
|
Echinococcus multilocularis |
Hydatid disease |
|
Spirometra mansoni |
Sparganosis |
Helminths are the largest internal human parasite. They reproduce sexually, generating millions of eggs or larvae. Nematodes and trematodes have a mouth and intestinal tract, while cestodes absorb nutrients directly through the outer tegument. All worms are motile, although once the adults are established in their definitive site, they rarely migrate further. Adult helminths may be very long-lived: up to 30 years in the case of the schistosomes.
Many helminths have developed complex life cycles, involving more than one host. Both primary and intermediate hosts are often highly specific to a particular species of worm. In some cases of human infection man is the primary host, while in others humans are a nonspecific intermediary or are coincidentally infected. Multiple infections with different helminths are common in endemic areas. Mass treatment programmes, in which one or more anthelminthic drugs are given on a regular (usually annual) basis, are used to keep the total worm load down (Table 4.42).
Table 4.42 Drugs used in mass treatment
| Drug | Infection |
|---|---|
Diethylcarbamazine (DEC) |
Loiaisis |
Filariasis |
|
Ivermectin |
Loiaisis |
Filariasis |
|
Onchocerciasis |
|
Strongyloidiasis |
|
Albendazole |
Filariasis (with DEC) |
Intestinal helminths |
|
Praziquantel |
Schistosomiasis |
Human infections can be divided into:
 Tissue-dwelling worms, including the filarial worms and the Guinea worm Dracunculus medinensis
Tissue-dwelling worms, including the filarial worms and the Guinea worm Dracunculus medinensis
 Human intestinal worms, including the human hookworms, the common roundworm (Ascaris lumbricoides) and Strongyloides stercoralis, which are the most common helminthic parasites of man. The adult worms live in the human gut and do not usually invade tissues, but many species have a complex life cycle involving a migratory larval stage
Human intestinal worms, including the human hookworms, the common roundworm (Ascaris lumbricoides) and Strongyloides stercoralis, which are the most common helminthic parasites of man. The adult worms live in the human gut and do not usually invade tissues, but many species have a complex life cycle involving a migratory larval stage
 Zoonotic nematodes, which accidentally infect man and are not able to complete their normal life cycle. They often become ‘trapped’ in the tissues, causing a potentially severe local inflammatory response.
Zoonotic nematodes, which accidentally infect man and are not able to complete their normal life cycle. They often become ‘trapped’ in the tissues, causing a potentially severe local inflammatory response.
Several nematodes belonging to the superfamily Filarioidea can infect humans. The adult worms are long and threadlike, ranging from 2 cm to 50 cm in length; females are generally much larger than males. Larval stages are inoculated into humans by various species of biting flies (each specific to a particular parasite). The adult worms which develop from these larvae mate, producing millions of offspring (microfilariae), which migrate in the blood or skin. These are ingested by feeding flies, in which the remainder of the life cycle takes place. Disease, which may be caused by either the adult worms or by microfilariae, is caused by host immune response to the parasite and is characterized by massive eosinophilia. Adult worms are long-lived (10–15 years) and reinfection is common, so that disease tends to be chronic and progressive.
Lymphatic filariasis, which may be caused by different species of filarial worm, has a scattered distribution in the tropics and subtropics (Table 4.43). More than 1 billion people in developing countries are at risk. Wuchereria bancrofti is transmitted to man by a number of mosquito species, mainly Culex fatigans. Adult female worms (which are 5–10 cm long) live in the lymphatics, releasing large numbers of microfilariae into the blood. Generally this occurs at night, coinciding with the nocturnal feeding pattern of C. fatigans. Non-periodic forms of W. bancrofti, transmitted by day-biting species of mosquito, are found in the South Pacific. Brugia malayi (and the closely related B. timori) are very similar to W. bancrofti, exhibiting the same nocturnal periodicity. The usual vectors are mosquitoes of the genus Mansonia, although other mosquitoes have been implicated.
Many filarial worms co-exist with symbiotic Wolbachia bacteria, which are in themselves a cause of inflammation in the human host.
Following the bite of an infected mosquito, the larvae enter the lymphatics and are carried to regional lymph nodes. Here, they grow and mature for 6–18 months.
Adult worms produce allergic lymphangitis. The clinical picture depends on the individual immune response, which in turn may depend on factors such as age at first exposure. In endemic areas many people have asymptomatic infection. Sometimes early infection is marked by bouts of fever accompanied by pain, tenderness and erythema along the course of affected lymphatics. Involvement of the spermatic cord and epididymis are common in Bancroftian filariasis. These acute attacks subside spontaneously in a few days, but usually recur. Recurrent episodes cause intermittent lymphatic obstruction, which in time can become fibrotic and irreversible. Obstructed lymphatics may rupture, causing cellulitis and further fibrosis; there may also be chylous pleural effusions and ascites. Over time, there is progressive enlargement, coarsening and fissuring of the skin, leading to the classical appearances of elephantiasis. The limbs or scrotum may become hugely swollen. Eventually, the adult worms will die, but the lymphatic obstruction remains and tissue damage continues. Elephantiasis takes many years to develop and is only seen in association with recurrent infection in endemic areas.
Occasionally the predominant features of filarial infection are pulmonary. Microfilariae become trapped in the pulmonary capillaries, generating intense local allergic response. The resulting pneumonitis causes cough, fever, weight loss and shifting radiological changes, associated with a high peripheral eosinophil count. This is known as tropical pulmonary eosinophilia (see p. 853).
The clinical picture in established disease is usually diagnostic, although similar lymphatic damage may occasionally be caused by silicates absorbed through the feet from volcanic soil (podoconiosis). Parasitological diagnosis has traditionally relied on detecting microfilariae in blood films or skin snips, but rapid and sensitive near-patient antigen detection tests are now available.
Diethylcarbamazine (DEC) kills both adult worms and microfilariae. Serious allergic responses may occur as the parasites are killed and particular care is needed when using DEC in areas endemic for loiasis. Mass treatment programmes using combinations of DEC, ivermectin and albendazole to target various helminthic infections are used in many parts of the world: the exact regimens depend on local situations (see ‘Further Reading’). Over 500 million people have already received such treatment. There is currently much interest in using doxycycline to kill the symbiotic Wolbachia bacteria, without which the adult worm will eventually die. However, the best way of incorporating this into the overall management strategy remains unclear.
Loiasis is found in the humid forests of West and Central Africa. The causative parasite, Loa loa, is a small (3–7 cm) filarial worm, which is found in the subcutaneous tissues. The microfilariae circulate in the blood during the day, but cause no direct symptoms. The vectors are day-biting flies of the genus Chrysops.
Adult worms migrate around the body in subcutaneous tissue planes. Worms may be present for years, frequently without causing symptoms. From time to time localized, tender, hot, soft tissue swellings occur due to hypersensitivity (Calabar swellings) often near to a joint. These are produced in response to the passage of a worm and usually subside over a few days or weeks. There may also be more generalized urticaria and pruritus. Occasionally, a worm may be seen crossing the eye under the conjunctiva; they may also enter retro-orbital tissue, causing severe pain. Short-term residents of endemic areas often have more severe manifestations of the disease.
Microfilariae may be seen on stained blood films, although these are often negative. Serological tests are relatively insensitive and cross-react with other microfilariae. There is usually massive eosinophilia. DEC may cause severe allergic reactions associated with parasite killing and is being replaced by newer agents. Ivermectin in single doses of 200–400 µg/kg is effective: it may occasionally cause severe reactions. Albendazole, which causes a more gradual reduction in microfilarial load, may be preferable in heavily-infected patients. Mass treatment with either DEC or ivermectin can decrease the transmission of infection, but the mainstay of prevention is vector avoidance and control.
Onchocerciasis (river blindness) affects 37 million people worldwide, of whom 250 000 are blind and 500 000 visually impaired; most of these are in West and Central Africa, with small foci in the Yemen and Central and South America. It is the result of infection with Onchocerca volvulus. Infection is transmitted by day-biting flies of the genus Simulium.
Infection occurs when larvae are inoculated into humans by the bite of an infected fly. The worms mature in 2–4 months and can live for more than 15 years. Adult worms, which can reach lengths of 50 cm (although <0.5 mm in diameter), live in the subcutaneous tissues. They may form fibrotic nodules, especially over bony prominences and sites of trauma. Huge numbers of microfilariae are distributed in the skin and may invade the eyes. Live microfilariae cause relatively little harm, but dead parasites may cause severe allergic reactions, with hyaline necrosis and loss of tissue collagen and elastin. In the eye a similar process causes conjunctivitis, sclerosing keratitis, uveitis and secondary glaucoma. Choroidoretinitis is also occasionally seen.
Symptoms usually start about a year after infection. Initially, there is generalized pruritus, with urticaria and fleeting oedema. Subcutaneous nodules (which can be detected by ultrasound) start to appear and in dark-skinned individuals, hypo- and hyperpigmentation from excoriation and inflammatory changes. Over time more chronic inflammatory changes appear, with roughened, inelastic skin. Superficial lymph nodes become enlarged and in the groin may hang down in loose folds of skin (‘hanging groin’). Eye disease, which is associated with chronic heavy infection, usually first manifests as itching and conjunctival irritation. This gradually progresses to more extensive eye disease and eventually to blindness.
In endemic areas, the diagnosis can often be made clinically, especially if supported by finding eosinophilia on a blood film. In order to identify parasites, skin snips taken from the iliac crest or shoulder are placed in saline under a cover slip. After 4 hours, microscopy will show microfilariae wriggling free on the slide. If this is negative, DEC can be applied topically under an occlusive dressing: this will provoke an allergic rash in the majority of infected people (modified Mazzotti reaction) but this is not routinely performed as it is unpleasant. Slit-lamp examination of the eyes may reveal the microfilariae. Rapid serological tests are being developed.
Ivermectin, in a single dose of 150 µg/kg, kills microfilariae and prevents their return for 6–12 months. There is little effect on adult worms, so annual (or more frequent) retreatment is needed. In patients co-infected with Loa loa, ivermectin may occasionally induce severe allergic reactions, including a toxic encephalopathy.
Since 1974, the WHO Onchocerciasis Control Programme has had a considerable impact on onchocerciasis in West Africa. A combination of vector control measures and, more recently, mass treatment with ivermectin, has led to a decrease in both infection rates and progression to serious disease. Humans are the only host but measures are required over a long period because of the longevity of the worm (10–15 years).
Infection with the Guinea worm, Dracunculus medinensis, occurs when water fleas (copepods) containing the parasite larvae are swallowed in contaminated drinking water. Ingested larvae mature and the female worm, which can reach over 1 metre in length, migrates through connective and subcutaneous tissue for 9–18 months before surfacing on the skin. The uterus of the worm ruptures, releasing larvae which are ingested by the small crustacean water fleas and the cycle is completed.
The diagnosis is clinical. The traditional treatment, extracting the worm over several days by winding it round a stick, is probably still the most effective. The worm should not be damaged. Antibiotics may be needed to control secondary infection.
Water fleas (and thus infective larvae) can be removed from drinking water by chemical treatment or by simple filtration. Large-scale eradication programmes have been in place for several years and the number of reported cases has fallen from over 3 million in 1985, to 4619 in 2008. The disease is now confined to small areas of Africa, mainly in Sudan and Ghana. Man is the only host of D. medinensis and it should therefore be possible to completely eradicate this parasite.
Adult intestinal nematodes (also sometimes referred to as soil-transmitted helminths, or geohelminths) live in the human gut. There are two main types of life cycle, both including a soil-based stage. In some cases, infection is spread by ingestion of eggs (which often require a period of maturation in the environment), while in others, the eggs hatch in the soil and larvae penetrate directly through the skin of a new host. Ascaris lumbricoides larvae invade the duodenum and enter the venous system, via which they reach the lungs. They are eventually expectorated and swallowed, entering the intestine where they complete their maturation. Strongyloides is also unusual, in that it is the only nematode that is able to complete its life cycle in humans. Larvae may hatch before leaving the colon and so are able to reinfect the host by penetrating the intestinal wall and entering the venous system.
Ascaris lumbricoides is a pale yellow worm, 20–35 cm in length (Fig. 4.35). It is found worldwide but is particularly common in poor rural communities, where there is heavy faecal contamination of the immediate environment. Larvae migrate through the tissues to the lungs before being expectorated and swallowed; adult worms are found in the small intestine. Ova are deposited in faeces and require a 2–4-month maturation in the soil before they are infective.
Infection is usually asymptomatic, although heavy infections are associated with nausea, vomiting, abdominal discomfort and anorexia. Worms can sometimes obstruct the small intestine, the most common site being at the ileocaecal valve. They may also occasionally invade the appendix, causing acute appendicitis, or the bile duct, resulting in biliary obstruction and suppurative cholangitis. Larvae in the lung may produce pulmonary eosinophilia. Heavy infection in children, especially those who are already malnourished, may have significant effects on nutrition and development. Serious morbidity and mortality are rare in ascariasis, but the huge number of people infected means that on a global basis roundworm infection causes a significant burden of disease, especially in children.
Ascaris eggs can be identified in the stool and occasionally adult worms emerge from the mouth or the anus. They may also be seen on barium enema studies. Appropriate drug treatments are shown in Box 4.20. Very rarely, surgical or endoscopic intervention may be required for intestinal or biliary obstruction.
E. vermicularis is a small (2–12 mm) worm, which is common throughout the world. Larval development takes place mainly in the small intestine and adult worms are normally found in the colon. The gravid female deposits eggs around the anus causing intense itching, especially at night. Unlike A. lumbricoides, the eggs do not require a maturation period in soil and infection is often directly transmitted from anus to mouth via the hands. Eggs may also be deposited on clothing and bed linen and are subsequently either ingested or inhaled. Apart from discomfort and local excoriation, infection is usually harmless.
Ova can be collected either using a moistened perianal swab, or by applying adhesive cellophane tape to the perianal skin. They can then be identified by microscopy.
The most commonly used drugs are mebendazole and piperazine (Box 4.20). However, isolated treatment of an affected person is often ineffective. Other family members (especially small children) may also need to be treated and the whole family should be given advice about personal hygiene. Two courses of treatment 2 weeks apart may break the cycle of autoinfection.
Infections with whipworm are common worldwide, especially in poor communities with inadequate sanitation. Adult worms, which are 3–5 cm long, inhabit the terminal ileum and caecum, although in heavy infection they are found throughout the large bowel. The head of the worm is embedded in the intestinal mucosa. Ova are deposited in the faeces and require a maturation period of 3–4 weeks in the soil before becoming infective.
Infection is usually asymptomatic, but mucosal damage can occasionally be so severe that there is colonic ulceration, dysentery or rectal prolapse.
Diagnosis is made by finding ova on stool microscopy, or occasionally by seeing adult worms on sigmoidoscopy. Drug treatment is shown in Box 4.20.
Hookworm infections, caused by the human hookworms Ancylostoma duodenale and Necator americanus, are found worldwide. They are relatively rare in developed countries, but very common in areas with poor sanitation and hygiene: overall about 25% of the world’s population is affected. Hookworm infection is a major contributing factor to anaemia in the tropics. A. duodenale is found mainly in East Asia, North Africa and the Mediterranean, while N. americanus is the predominant species in South and Central America, South-east Asia and sub-Saharan Africa.
Adult worms (which are about 1 cm long) live in the duodenum and upper jejunum, where they are often found in large numbers. They attach firmly to the mucosa using the buccal plate, feeding on blood. Eggs passed in the faeces develop in warm moist soil, producing infective filariform larvae. These penetrate directly through the skin of a new host and are carried in the bloodstream to the lungs. Having crossed into the alveoli, the parasites are expectorated and then swallowed, thus arriving at their definitive home.
Local irritation as the larvae penetrate the skin (‘ground itch’) may be followed by transient pulmonary signs and symptoms, often accompanied by eosinophilia. Light infections, especially in a well-nourished person, are often asymptomatic. Heavier worm loads may be associated with epigastric pain and nausea, resembling peptic ulcer disease. Chronic heavy infection, particularly on a background of malnourishment, may cause iron deficiency anaemia and hypoproteinaemia. Heavy infection in children is associated with delays in physical and mental development.
The diagnosis is made by finding eggs on faecal microscopy. In infections heavy enough to cause anaemia these will be present in large numbers. The aim of treatment in endemic areas is reduction of worm burden rather than complete eradication: albendazole given as a single dose is the best drug (Box 4.20). The WHO is promoting mass treatment programmes for school-children in many parts of the world, together with treatment for schistosomiasis where appropriate.
Strongyloides stercoralis is a small (2 mm long) worm which lives in the small intestine. It is found in many parts of the tropics and subtropics and is especially common in Asia. Eggs hatch in the bowel and larvae are found in the stool. Usually these are non-infective rhabditiform larvae, which require a further period of maturation in the soil before they can infect a new host, but sometimes this maturation can occur in the large bowel. Infective filariform larvae can therefore penetrate directly through the perianal skin, reinfecting the host. In this way, autoinfection may continue for years or even decades. Some war veterans who were imprisoned in the Far East during the Second World War have been found to have active strongyloidiasis over 50 years later. After skin penetration the life cycle is similar to that of the hookworm, except that the adult worms may burrow into the intestinal mucosa, causing a local inflammatory response.
S. stercoralis, following skin penetration, causes a similar local dermatitis to hookworm. In autoinfection this manifests as a migratory linear weal around the buttocks and lower abdomen (cutaneous larva currens). In heavy infections damage to the small intestinal mucosa can cause malabsorption, diarrhoea and even perforation. There is usually a persistent eosinophilia.
In patients who are immunosuppressed (e.g. by corticosteroid therapy or intercurrent illness) filariform larvae may penetrate directly through the bowel wall in huge numbers, causing an overwhelming and usually fatal generalized infection (the strongyloidiasis hyperinfestation syndrome). This condition is often complicated by a Gram-negative septicaemia due to bowel organisms.
A number of nematodes which are principally parasites of animals may also affect man. The most common are described below.
The normal hosts of Trichinella spiralis, the cause of trichinosis, include pigs, bears and warthogs. Man is infected by eating undercooked meat from these animals. Ingested larvae mature in the small intestine, where adults release new larvae which penetrate the bowel wall and migrate through the tissues. Eventually, these larvae encyst in striated muscle.
Light infections are usually asymptomatic. Heavier loads of worms produce gastrointestinal symptoms as the adults establish themselves in the small intestine, followed by systemic symptoms as the larvae invade. The latter include fever, oedema and myalgia. Massive infection may occasionally be fatal, but usually the symptoms subside once the larvae encyst.
The diagnosis can usually be made from the clinical picture, associated eosinophilia and serological tests. If necessary it can be confirmed by muscle biopsy a few weeks after infection. Albendazole (20 mg/kg for 7 days) given early in the course of the illness will kill the adult worms and decrease the load of larvae reaching the tissues. Analgesia and steroids may be needed for symptomatic relief.
Eggs of the dog roundworm, Toxocara canis, are occasionally ingested by humans, especially children. The eggs hatch and the larvae penetrate the small intestinal wall and enter the mesenteric circulation, but are then unable to complete their life cycle in a ‘foreign’ host. Many are held up in the capillaries of the liver, where they generate a granulomatous response, but some may migrate into other tissues including lungs, striated muscle, heart, brain and eye. In most cases, infection is asymptomatic and the larvae die without causing serious problems. In heavy infections, there may be generalized symptoms (fever and urticaria) and eosinophilia, as well as focal signs related to the migration of the parasites. Pulmonary involvement may cause bronchospasm and chest X-ray changes. Ocular infection may produce a granulomatous swelling mimicking a retinoblastoma, while cardiac or neurological involvement is occasionally fatal. Rarely, larvae survive in the tissues for many years, causing symptoms long after infection.
Isolation of the larvae is difficult and the diagnosis is usually made serologically. Albendazole 400 mg daily (5–10 mg/kg in children) for a week is the most effective treatment.
CLM is caused by the larvae of the non-human hookworms Ancylostoma braziliense and A. caninum. Like human hookworms, these hatch in warm moist soil and then penetrate the skin. In man they are unable to complete a normal life cycle and instead migrate under the skin for days or weeks until they eventually die. The wandering of the larva is accompanied by a clearly defined, serpiginous, itchy rash, which progresses at the rate of about 1 cm per day. There are usually no systemic symptoms. The diagnosis is purely clinical. Single larvae may be treated with a 15% solution of topical tiabendazole; multiple lesions require systemic therapy with a single dose of albendazole 400 mg or ivermectin 150–200 µg/kg.
Trematodes (flukes) are flat leaf-shaped worms. They have complex life cycles, often involving fresh water snails and intermediate mammalian hosts. Disease is caused by the inflammatory response to eggs or to the adult worms.
Schistosomiasis affects over 200 million people in the tropics and subtropics, mostly in sub-Saharan Africa. Chronic infection causes significant morbidity and after malaria it has the most socioeconomic impact of any parasitic disease. Schistosomiasis is largely a disease of the rural poor, but has also been associated with major development projects such as dams and irrigation schemes.
There are three species of schistosome which commonly cause disease in man: Schistosoma mansoni, S. haematobium and S. japonicum. S. mekongi and S. intercalatum also affect man but have very restricted distribution (Fig. 4.36). Eggs are passed in the urine or faeces of an infected person and hatch in fresh water to release the miracidia. These ciliated organisms penetrate the tissue of the intermediate host, a species of water snail specific to each species of schistosome. After multiplying in the snail, large numbers of fork-tailed cercariae are released back into the water, where they can survive for 2–3 days. During this time, the cercariae can penetrate the skin or mucous membranes of the definitive host, man. Transforming into schistosomulae, they pass through the lungs before reaching the portal vein, where they mature into adult worms (the male is about 20 mm long and the female a little larger). Worms pair in the portal vein before migrating to their final destination: mesenteric veins in the case of S. mansoni and S. japonicum and the vesicular plexus for S. haematobium. Here, they may remain for many years, producing vast numbers of eggs. The majority of these are released in urine or faeces, but a small number become embedded in the bladder or bowel wall and a few are carried in the circulation to the liver or other distant sites.
The pathology of schistosome infection varies with species and stage of infection. In the early stages, there may be local and systemic allergic reactions to the migrating parasites. As eggs start to be deposited there may be a local inflammatory response in the bowel or bladder, while ectopic eggs may produce granulomatous lesions anywhere in the body. Chronic heavy infection, in which large numbers of eggs accumulate in the tissues, leads to fibrosis, calcification and in some cases, dysplasia and malignant change. Morbidity and mortality are related to duration of infection and worm load, as well as to the species of parasite. Children in endemic areas tend to have the heaviest worm load, because of both increased exposure to infection and differences in the immune response between adults and children.
Cercarial penetration of the skin may cause local dermatitis (‘swimmer’s itch’). After a symptom-free period of 3–4 weeks, systemic allergic features may develop, including fever, rash, myalgia and pneumonitis (Katayama fever). These allergic phenomena are common in non-immune travellers, but are rarely seen in local populations, who are usually exposed to infection from early childhood onwards. If infection is sufficiently heavy, symptoms from egg deposition may start to appear 2–3 months after infection.
S. haematobium infection (bilharzia). The earliest symptom is usually painless terminal haematuria. As bladder inflammation progresses there is increased urinary frequency and groin pain. Obstructive uropathy develops, leading to hydronephrosis, chronic kidney disease and recurrent urinary infection. There is a strong association between chronic urinary schistosomiasis and squamous cell bladder carcinoma. The genitalia may also be affected and ectopic eggs may cause pulmonary or neurological disease.
S. mansoni usually affects the large bowel. Early disease produces superficial mucosal changes, accompanied by blood-stained diarrhoea. Later the mucosal damage becomes more marked, with the formation of rectal polyps, deeper ulceration and eventually fibrosis and stricture formation. Ectopic eggs are carried to the liver, where they cause an intense granulomatous response. Hepatitis is followed by progressive periportal fibrosis, leading to portal hypertension, oesophageal varices and splenomegaly (see p. 345). Hepatocellular function is usually well preserved.
S. japonicum, unlike the other species, infects numerous other mammals apart from man. It is similar to S. mansoni, but infects both large and small bowel and produces a greater number of eggs. Disease therefore tends to be more severe and rapidly progressive. Hepatic involvement is more common and neurological involvement is seen in about 5% of cases.
Schistosomiasis is suggested by relevant symptoms following fresh water exposure in an endemic area. In the early allergic stages, the diagnosis can only be made clinically. When egg deposition has started, the characteristic eggs (with a terminal spine in the case of S. haematobium and a lateral spine in the other species) can be detected on microscopy. In S. haematobium infection, the best specimen for examination is a filtered mid-day urine sample. Parasites may also be found in semen and in rectal snip preparations. S. mansoni and S. japonicum eggs can usually be found in faeces or in a rectal snip. Serological tests are available and may be useful in the diagnosis of travellers returning from endemic areas, although the test may not become positive for 12 weeks after infection: a parasitological diagnosis should always be made if possible. In chronic disease, X-rays, ultrasound examinations and endoscopy may show abnormalities of the bowel or urinary tract, although these are nonspecific. Liver biopsy may show the characteristic periportal fibrosis.
The aim of treatment in endemic areas is to decrease the worm load and therefore minimize the chronic effects of egg deposition. It may not always be possible (or even desirable) to eradicate adult worms completely and reinfection is common. However, a 90% reduction in egg output has been achieved in mass treatment programmes and in light infections where there is no risk of re-exposure the drugs are usually curative. The most widely used is praziquantel (Table 4.44), which is effective against all species of schistosome, well-tolerated and reasonably cheap.
Table 4.44 Treatment of trematode infections
| Parasite | Drug and dose |
|---|---|
Schistosoma mansoni |
Praziquantel 40 mg/kg single dosea |
S. haematobium |
Praziquantel 40 mg/kg single dosea |
S. japonicum |
Praziquantel 60 mg/kg single dosea |
Paragonimus spp. |
Praziquantel 25 mg/kg 8-hourly for 3 days |
Clonorchis sinensis |
Praziquantel 25 mg/kg 8-hourly for 1–3 daysc |
Opisthorchis spp. |
Praziquantel 25 mg/kg 8-hourly for 1–3 daysc |
Fasciolopsis buski |
Praziquantel 25 mg/kg 8-hourly for 1 day |
Fasciola hepatica |
Triclabendazole 10 mg/kg single doseb |
Prevention of schistosomiasis is difficult and relies on a combination of approaches. Mass treatment of the population (especially children) will decrease the egg load in the community. Health education programmes, the provision of latrines and access to a safe water supply should decrease contact with infected water. Attempts to eradicate the snail host have generally been unsuccessful, although manmade bodies of water can often be made less ‘snail-friendly’. Travellers should be advised to avoid potentially infected water.
Many flukes infect man via ingestion of an intermediate host, often fresh water fish.
Over 20 million people are infected with lung flukes of the genus Paragonimus. The adult worms (of which the major species is P. westermani) live in the lungs, producing eggs which are expectorated or swallowed and passed in the faeces. Miracidia emerging from the eggs penetrate the first intermediate host, a fresh water snail. Larvae released from the snail seek out the second intermediate host, fresh water crustacea, in which they encyst as metacercariae. Humans and other mammalian hosts become infected after consuming uncooked shellfish. Cercariae penetrate the small intestinal wall and migrate directly from the peritoneum to the lungs across the diaphragm. Having established themselves in the lung, the adult worms may survive for 20 years.
The common clinical features are fever, cough and mild haemoptysis. In heavy infections the disease may progress, sometimes mimicking pneumonia or pulmonary tuberculosis. Ectopic worms may cause signs in the abdomen or the brain.
The diagnosis is made by detection of ova on sputum or stool microscopy. Radiological appearances are variable and nonspecific. Treatment is with praziquantel and prevention involves avoidance of inadequately cooked shellfish.
The human liver flukes, Clonorchis sinensis, Opisthorchis felineus and O. viverrini, are almost entirely confined to East and South-east Asia, where they infect more than 20 million people. Adults live in the bile ducts, releasing eggs into the faeces. The parasite requires two intermediate hosts, a fresh water snail and a fish, and humans are infected by consumption of raw fish. The cycle is completed when excysted worms migrate from the small intestine into the bile ducts.
Infection is often asymptomatic, but may be associated with cholangitis and biliary carcinoma. The diagnosis is made by identifying eggs on stool microscopy. Treatment is with praziquantel and infection can be avoided by cooking fish adequately.
Man can also be infected with a variety of animal flukes, notably the liver fluke Fasciola hepatica and the intestinal fluke Fasciolopsis buski. Both require a water snail as an intermediate host; cercariae encyst on aquatic vegetation and then are consumed by animals or man. After ingestion, F. hepatica penetrates the intestinal wall before migrating to the liver: during this stage it causes systemic allergic symptoms. After reaching the bile ducts, it causes similar problems to those of the other liver flukes. F. buski does not migrate after it excysts and causes mainly bowel symptoms.
Cestodes (tapeworms) are ribbon-shaped worms, which vary from a few millimetres to several metres in length. Adult worms live in the human intestine, where they attach to the epithelium using suckers on the anterior portion (scolex). From the scolex arises a series of progressively developing segments, called proglottids. The mature distal segments contain eggs, which may either be released directly into the faeces, or are carried out with an intact detached proglottid. The eggs are consumed by intermediate hosts, after which they hatch into larvae (oncospheres). These penetrate the intestinal wall of the host (pig or cattle) and encyst in the tissues. Man ingests the cysts in undercooked meat and the cycle is completed when the parasites excyst in the stomach and develop into adult worms in the small intestine. Infections are usually solitary, but several adult tapeworms may co-exist. The exceptions to this life cycle are the dwarf tapeworm, Hymenolepis nana, which has no intermediate host and is transmitted from person to person by the faeco-oral route and Taenia solium, which produces cysticercosis (see below).
T. saginata, the beef tapeworm, may reach a length of several metres. It is common in all countries where undercooked beef is eaten. The adult worm causes few if any symptoms. Infection is usually discovered when proglottids are found in faeces or on underclothing, often causing considerable anxiety. Ova may also be seen on stool microscopy. Infection can be cleared with a single dose of praziquantel (10 mg/kg). It can be prevented by careful meat inspection, or by thorough cooking of beef.
T. solium, the pork tapeworm, is generally smaller than T. saginata, although it can still reach 6 metres in length. It is particularly common in South America, South Africa, China and parts of South-east Asia. As with T. saginata, infection is usually asymptomatic. The ova of the two species are identical, but the proglottids can be distinguished on inspection.
Pork tapeworm infection is acquired by eating uncooked pork. Treatment is with praziquantel or niclosamide. There is no evidence that drug treatment should be accompanied by a purgative, as was previously believed.
Cysticercosis is caused by ingestion of cysts rather than the adult worm and follows the ingestion of eggs from contaminated food and water. Faeco-oral autoinfection can occur but is rare. Patients with tapeworms do not usually develop cysticercosis and patients with cysticercosis do not usually harbour tapeworms. Following the ingestion of eggs, the larvae are liberated, penetrate the intestinal wall and are carried to various parts of the body where they develop into cysticerci. These are cysts, 0.5–1 cm in diameter, containing the scolex of a new adult worm. Common sites for cysticerci include subcutaneous tissue, skeletal muscle and brain.
Superficial cysts may be felt under the skin, but usually cause no significant symptoms. Cysts in the brain can cause a variety of problems including epilepsy, personality change, hydrocephalus and focal neurological signs (see p. 1130). These may only appear many years after infection.
Muscle cysts tend to calcify and are often visible on X-rays. Cutaneous cysts can be excised and examined. Brain cysts are less prone to calcification and are often only seen on CT or MRI scan. Serological tests may support the diagnosis.
The role of anthelminthics in cysticercosis remains controversial. Even in neurocysticercosis there is little evidence of benefit, although symptomatic patients with viable neurocysts should probably be treated. Albendazole 15 mg/kg daily for 8–20 days is the drug of choice; the alternative is praziquantel 50 mg/kg daily (in divided doses) for 15 days.
Successful treatment is accompanied by increased local inflammation and corticosteroids should be given during and after the course of anthelminthic. Prevention of cysticercosis depends on good hygiene, as well as on the eradication of human T. solium infection.
Anticonvulsants should be given for epilepsy and surgery may be indicated if there is hydrocephalus (see p. 1115).
Infection with the fish tapeworm, D. latum, is common in northern Europe and Japan, owing to the consumption of raw fish. The adult worm reaches a length of several metres, but like the other tapeworms usually causes no symptoms. A megaloblastic anaemia (due to competitive utilization of B12 by the parasite) may occur. Diagnosis and treatment are the same as for Taenia species.
Hydatid disease occurs when humans become an intermediate host of the dog tapeworm, Echinococcus granulosus. The adult worm lives in the gut of domestic and wild canines and the larval stages are usually found in sheep, cattle and camels. Man may become infected either from direct contact with dogs, or from food or water contaminated with dog faeces. After ingestion the parasites excyst, penetrate the small intestine wall and are carried to the liver and other organs in the bloodstream. A slow-growing, thick-walled cyst is formed, inside which further larval stages of the parasite develop. The life cycle cannot be completed unless the cyst is eaten by a dog. Hydatid disease is prevalent in areas where dogs are used in the control of livestock, especially sheep. It is common in Australia, Argentina, the Middle East and parts of East Africa.
Symptoms depend mainly on the site of the cyst. The liver is the most common organ affected (60%), followed by the lung (20%), kidneys (3%), brain (1%) and bone (1%). The symptoms are those of a slowly growing benign tumour. Pressure on the bile ducts may cause jaundice. Rupture into the abdominal cavity, pleural cavity or biliary tree may occur. In the latter situation, intermittent jaundice, abdominal pain and fever associated with eosinophilia result. A cyst rupturing into a bronchus may result in its expectoration and spontaneous cure, but if secondary infection supervenes a chronic pulmonary abscess will form. Focal seizures can occur if cysts are present in the brain. Renal involvement produces lumbar pain and haematuria. Calcification of the cyst occurs in about 40% of cases.
A related parasite of foxes, E. multilocularis, causes a similar but more severe infection, alveolar hydatid disease. These cysts are invasive and metastases may occur.
The diagnosis and treatment of hydatid liver disease are described on page 345.
Arthropods, which include the arachnid ticks and mites as well as insects, may be responsible for human disease in several ways.
Local lesions may be caused by hypersensitivity to allergens in arthropod saliva. This common reaction, known as papular urticaria, is nonspecific and is seen in the majority of people in response to the bite of a variety of blood-sucking arthropods including mosquitoes, bugs, ticks, lice and mites. Occasionally, tick bites may cause a more severe systemic allergic response, especially in previously sensitized individuals.
Most of these parasites alight on man only to feed, but some species of lice live in very close proximity to the skin: body lice in clothing and head and pubic lice on human hairs (see Chapter 24).
Other ectoparasites are actually resident within the skin, causing more specific local lesions.
Jiggers is due to infection with the jigger flea, Tunga penetrans, and is common throughout South America and Africa. The pregnant female flea burrows into the sole of the foot, often between the toes. The egg sac grows to about 0.5 cm in size, before the eggs are discharged onto the ground. The main danger is bacterial infection or tetanus. The flea should be removed with a needle or scalpel and the area kept clean until it heals.
Myiasis is caused by invasion of human tissue by the larva of certain flies, principally the Tumbu fly, Cordylobia anthropophaga (found in sub-Saharan Africa), and the human botfly, Dermatobia hominis (Central and South America). The larvae, which hatch from eggs laid on laundry and linen, burrow into the skin to form boil-like lesions: a central breathing orifice may be visible. Again, the main risk is secondary infection. It is not always easy to extract the larva: covering it with petroleum jelly may bring it up in search of air.
Many arthropods can cause local or systemic illness through envenoming, i.e. injection of venom.
The main role of arthropods in causing human disease is as vectors of parasitic and viral infections. Some of these infections are shown in Table 4.4 and discussed in detail elsewhere.