Chapter 15 Respiratory disease
Introduction
The main role of the respiratory system is to extract oxygen from the external environment and dispose of waste gases, principally carbon dioxide. This requires the lungs to function as efficient bellows, bringing in fresh air and delivering it to the alveoli, and expelling used air at an appropriate rate. Gas exchange is achieved by exposing thin-walled capillaries to the alveolar gas and matching ventilation to blood flow through the pulmonary capillary bed. In doing this, the lungs expose a large area of tissue, which can be damaged by dusts, gases and infective agents. Host defence is therefore a key priority for the lung and is achieved by a combination of structural and immunological defences.
Structure of the respiratory system
The trachea, bronchi and bronchioles
The trachea is 10–12 cm in length. It lies slightly to the right of the midline and divides at the carina into right and left main bronchi. The carina lies under the junction of the manubrium sterni and the second right costal cartilage. The right main bronchus is more vertical than the left and, hence, inhaled material is more likely to end up in the right lung.
The right main bronchus divides into the upper lobe bronchus and the intermediate bronchus, which further subdivides into the middle and lower lobe bronchi. On the left the main bronchus divides into upper and lower lobe bronchi only. Each lobar bronchus further divides into segmental and subsegmental bronchi. There are about 25 divisions in all between the trachea and the alveoli.
The first seven divisions are bronchi that have:
 walls consisting of cartilage and smooth muscle
walls consisting of cartilage and smooth muscle
 epithelial lining with cilia and goblet cells
epithelial lining with cilia and goblet cells
 submucosal mucus-secreting glands
submucosal mucus-secreting glands
 endocrine cells – Kulchitsky or APUD (amine precursor and uptake decarboxylation) containing 5-hydroxytryptamine.
endocrine cells – Kulchitsky or APUD (amine precursor and uptake decarboxylation) containing 5-hydroxytryptamine.
The next 16–18 divisions are bronchioles that have:
 no cartilage and a muscular layer that progressively becomes thinner
no cartilage and a muscular layer that progressively becomes thinner
 a single layer of ciliated cells but very few goblet cells
a single layer of ciliated cells but very few goblet cells
 granulated Clara cells that produce a surfactant-like substance.
granulated Clara cells that produce a surfactant-like substance.
The ciliated epithelium is a key defence mechanism. Each cell bears approximately 200 cilia beating at 1000 beats per minute in organized waves of contraction. Each cilium consists of nine peripheral parts and two inner longitudinal fibrils in a cytoplasmic matrix (Fig. 15.1). Nexin links join the peripheral pairs. Dynein arms consisting of ATPase protein project towards the adjacent pairs. Bending of the cilia results from a sliding movement between adjacent fibrils powered by an ATP-dependent shearing force developed by the dynein arms (see also p. 22). Absence of dynein arms leads to immotile cilia. Mucus, which contains macrophages, cell debris, inhaled particles and bacteria, is moved by the cilia towards the larynx at about 1.5 cm/min (the ‘mucociliary escalator’, see below).
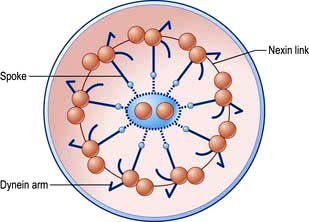
Figure 15.1 Cross-section of a cilium. Nine outer microtubular doublets and two central single microtubules are linked by spokes, nexin links and dynein arms.
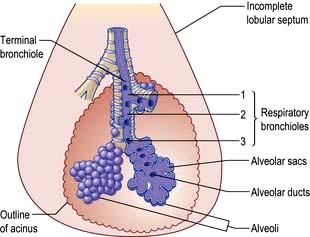
The bronchioles finally divide within the acinus into smaller respiratory bronchioles that have alveoli arising from the surface (Fig. 15.2). Each respiratory bronchiole supplies approximately 200 alveoli via alveolar ducts. The term ‘small airways’ refers to bronchioles of <2 mm; the average lung contains about 30 000 of these.
The alveoli
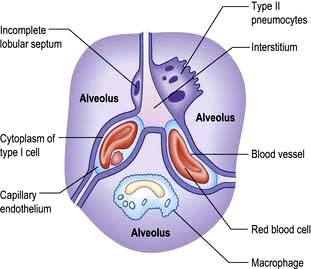
There are approximately 300 million alveoli in each lung. Their total surface area is 40–80 m2. The epithelial lining consists mainly of type I pneumocytes (Fig. 15.3). These cells have an extremely thin layer of cytoplasm, which only offers a thin barrier to gas exchange. Type I cells are connected to each other by tight junctions that limit the movements of fluid in and out of the alveoli. Alveoli are not completely airtight – many have holes in the alveolar wall allowing communication between alveoli of adjoining lobules (pores of Kohn).
Type II pneumocytes are slightly more numerous than type I cells but cover less of the epithelial lining. They are found generally in the borders of the alveolus and contain distinctive lamellar vacuoles, which are the source of surfactant. Type I pneumocytes are derived from type II cells. Large alveolar macrophages are present within the alveoli and assist in defending the lung.
The lungs
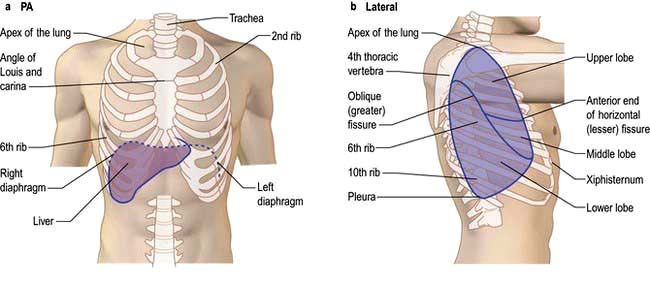
The lungs are separated into lobes by invaginations of the pleura, which are often incomplete. The right lung has three lobes, whereas the left lung has two. The positions of the oblique fissures and the right horizontal fissure are shown in Figure 15.4. The upper lobe lies mainly in front of the lower lobe and therefore physical signs on the right side in the front of the chest are due to lesions of the upper lobe or the middle lobe.
Each lobe is further subdivided into bronchopulmonary segments by fibrous septa that extend inwards from the pleural surface. Each segment receives its own segmental bronchus.
The bronchopulmonary segment is further divided into individual lobules approximately 1 cm in diameter and generally pyramidal in shape, the apex lying towards the bronchiole that supplies it. Within each lobule a terminal bronchus supplies an acinus, and within this structure further divisions of the bronchioles eventually give rise to the alveoli.
The pleura
The pleura is a layer of connective tissue covered by a simple squamous epithelium. The visceral pleura covers the surface of the lung, lines the interlobar fissures, and is continuous at the hilum with the parietal pleura, which lines the inside of the hemithorax. At the hilum the visceral pleura continues alongside the branching bronchial tree for some distance before reflecting back to join the parietal pleura. In health, the pleurae are in apposition apart from a small quantity of lubricating fluid.
The diaphragm
The diaphragm is covered by parietal pleura above and peritoneum below. Its muscle fibres arise from the lower ribs and insert into the central tendon. Motor and sensory nerve fibres go separately to each half of the diaphragm via the phrenic nerves. Fifty per cent of the muscle fibres are of the slow-twitch type with a low glycolytic capacity; they are relatively resistant to fatigue.
Pulmonary vasculature and lymphatics
The lung has a dual blood supply, receiving deoxygenated blood from the right ventricle via the pulmonary artery and oxygenated blood via the bronchial circulation.
The pulmonary artery divides to accompany the bronchi. The arterioles accompanying the respiratory bronchioles are thin-walled and contain little smooth muscle. The pulmonary venules drain laterally to the periphery of the lobules, pass centrally in the interlobular and intersegmental septa, and eventually join to form the four main pulmonary veins.
The bronchial circulation arises from the descending aorta. These bronchial arteries supply tissues down to the level of the respiratory bronchiole. The bronchial veins drain into the pulmonary vein, forming part of the normal physiological shunt.
Lymphatic channels lie in the interstitial space between the alveolar cells and the capillary endothelium of the pulmonary arterioles.
The tracheobronchial lymph nodes are arranged in five main groups: pulmonary, bronchopulmonary, subcarinal, superior tracheobronchial and paratracheal. For all practical purposes these form a continuous network of nodes from the lung substance up to the trachea.
Nerve supply to the lung
The innervation of the lung remains incompletely understood. Parasympathetic and sympathetic fibres (from the vagus and sympathetic chain respectively) accompany the pulmonary arteries and the airways. Airway smooth muscle is innervated by vagal afferents, postganglionic muscarinic vagal efferents and vagally derived non-adrenergic non-cholinergic (NANC) fibres which use a range of neurotransmitters including substance P, neurokinins A and B, calcitonin gene-related peptide, vasoactive intestinal polypeptide and various adenine and guanine phosphates. Three muscarinic receptor subtypes have been identified: M1 receptors on parasympathetic ganglia, a smaller number of M2 receptors on muscarinic nerve terminals, and M3 receptors on airway smooth muscle. The parietal pleura is innervated from intercostal and phrenic nerves but the visceral pleura has no innervation.
Physiology of the respiratory system
The nose
The major functions of nasal breathing are:
About 10 000 L of air are inhaled daily. The relatively low flow rates and turbulence of inspired air in the nose mean that few particles greater than 10 microns (µm) diameter pass through the nose. Particles deposited on the nasal mucosa are removed within 15 minutes, compared with 60–120 days for particles that reach the alveoli. Nasal secretion contains IgA antibodies, lysozyme and interferons. In addition, the cilia of the nasal epithelium move the mucous gel layer rapidly back to the oropharynx where it is swallowed. Bacteria have little chance of settling in the nose. Mucociliary protection is less effective against viral infections because viruses bind to receptors on epithelial cells. The majority of rhinoviruses bind to an adhesion molecule, intercellular adhesion molecule 1 (ICAM-1), which is shared by neutrophils and eosinophils. Many noxious gases, such as SO2, are almost completely removed by nasal breathing.
Breathing
Lung ventilation can be considered in two parts:
 The mechanical process of inspiration and expiration
The mechanical process of inspiration and expiration
 The control of respiration to a level appropriate for metabolic needs.
The control of respiration to a level appropriate for metabolic needs.
Mechanical process
The lungs have an inherent elastic property that causes them to tend to collapse away from the thoracic wall, generating a negative pressure within the pleural space. The strength of this retractive force relates to the volume of the lung: at higher lung volumes the lung is stretched more, and a greater negative intrapleural pressure is generated. Lung compliance is a measure of the relationship between this retractive force and lung volume. At the end of a quiet expiration, the retractive force exerted by the lungs is balanced by the tendency of the thoracic wall to spring outwards. At this point, respiratory muscles are resting. The volume of air remaining in the lung after a quiet expiration is called the functional residual capacity (FRC).
Inspiration from FRC is an active process: a negative intrapleural pressure is created by descent of the diaphragm and movement of the ribs upwards and outwards through contraction of the intercostal muscles. During tidal breathing in healthy individuals, inspiration is almost entirely due to contraction of the diaphragm. More vigorous inspiration requires the use of accessory muscles of ventilation (sternomastoid and scalene muscles). Respiratory muscles are similar to other skeletal muscles but are less prone to fatigue. However, inspiratory muscle fatigue contributes to respiratory failure in patients with severe chronic airflow limitation and in those with primary neurological and muscle disorders.
At rest or during low-level exercise, expiration is passive and results from the natural tendency of the lung to collapse.
Forced expiration involves activation of accessory muscles, chiefly those of the abdominal wall, which help to push up the diaphragm.
The control of respiration
Coordinated respiratory movements result from rhythmical discharges arising in an anatomically ill-defined group of interconnected neurones in the reticular substance of the brainstem, known as the respiratory centre. Motor discharges from the respiratory centre travel via the phrenic and intercostal nerves to the respiratory musculature.
In healthy individuals, the main driver for respiration is the arterial pH, which is closely related to the partial pressure of carbon dioxide in arterial blood. Oxygen levels in arterial blood are usually above the level which triggers respiratory drive. In a typical normal adult at rest:
 The pulmonary blood flow of 5 L/min carries 11 mmol/min (250 mL/min) of oxygen from the lungs to the tissues.
The pulmonary blood flow of 5 L/min carries 11 mmol/min (250 mL/min) of oxygen from the lungs to the tissues.
 Ventilation at about 6 L/min carries 9 mmol/min (200 mL/min) of carbon dioxide out of the body.
Ventilation at about 6 L/min carries 9 mmol/min (200 mL/min) of carbon dioxide out of the body.
 The normal pressure of oxygen in arterial blood (PaO2) is between 11 and 13 kPa.
The normal pressure of oxygen in arterial blood (PaO2) is between 11 and 13 kPa.
 The normal pressure of carbon dioxide in arterial blood (PaCO2) is 4.8–6.0 kPa.
The normal pressure of carbon dioxide in arterial blood (PaCO2) is 4.8–6.0 kPa.
Ventilation is controlled by a combination of neurogenic and chemical factors (Fig. 15.5).
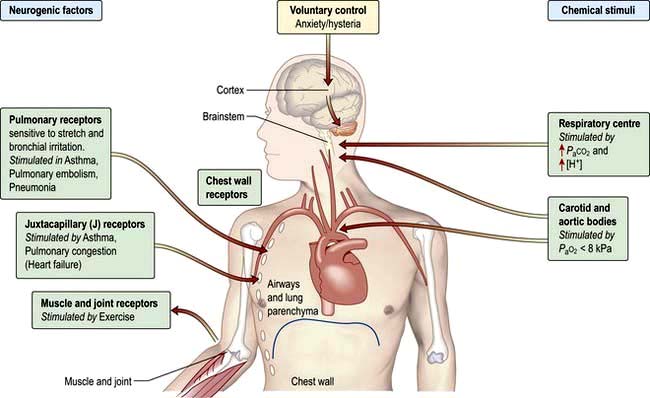
Figure 15.5 Chemical and neurogenic factors in the control of ventilation. The strongest stimulant to ventilation is a rise in PaCO2 which increases [H+] in CSF. Sensitivity to this may be lost in COPD. In these patients hypoxaemia is the chief stimulus to respiratory drive; oxygen treatment may therefore reduce respiratory drive and lead to a further rise in PaCO2. An increase in [H+] due to metabolic acidosis as in diabetic ketoacidosis will increase ventilation with a fall in PaCO2 causing deep sighing (Kussmaul) respiration. The respiratory centre is depressed by severe hypoxaemia and sedatives (e.g. opiates) and stimulated by doxapram, large doses of aspirin and pyrexia. COPD, chronic obstructive pulmonary disease.
Breathlessness on physical exertion is normal and not considered a symptom unless the level of exertion is very light, such as when walking slowly. Surveys of healthy western populations reveal that over 20% of the general population report themselves as breathless on relatively minor exertion. Although breathlessness is a very common symptom, the sensory and neural mechanisms underlying it remain obscure. The sensation of breathlessness is derived from at least three sources:
The airways of the lungs
From the trachea to the periphery, the airways decrease in size but increase in number. Overall, the cross-sectional area available for airflow increases as the total number of airways increases. The airflow rate is greatest in the trachea and slows progressively towards the periphery (since the velocity of airflow depends on the cross-sectional area). In the terminal airways, gas flow occurs solely by diffusion. The resistance to airflow is very low (0.1–0.2 kPa/L in a normal tracheobronchial tree), steadily increasing from the small to the large airways.
Airways expand as the lung volume increases. At full inspiration (total lung capacity, TLC) they are 30–40% larger in calibre than at full expiration (residual volume, RV). In chronic obstructive pulmonary disease (COPD), the small airways are narrowed but this can be partially compensated by breathing closer to TLC.
Control of airway tone
Bronchomotor tone is maintained by vagal efferent nerves and can be reduced by atropine or β-adrenoceptor agonists. Adrenoceptors on the surface of bronchial muscles respond to circulating catecholamines – there is no direct sympathetic innervation. Airway tone shows a circadian rhythm, which is greatest at 04.00 and lowest in the mid-afternoon. Tone can be increased transiently by inhaled stimuli acting on epithelial nerve endings, which trigger reflex bronchoconstriction via the vagus. These stimuli include cigarette smoke, solvents, inert dust and cold air. Airway responsiveness to these stimuli increases following respiratory tract infections even in healthy subjects. In asthma, the airways are very irritable and as the circadian rhythm remains the same, asthmatic symptoms are usually worse in the early morning.
Airflow
Movement of air through the airways results from a difference between atmospheric pressure and the pressure in the alveoli; alveolar pressure is negative in inspiration and positive in expiration. During quiet breathing, the pleural pressure is negative throughout the breathing cycle. With vigorous expiratory efforts (e.g. cough), the pleural pressure becomes positive (up to 10 kPa). This compresses the central airways, but the smaller airways do not close off because the driving pressure for expiratory flow (alveolar pressure) is also increased.
Alveolar pressure (PALV) is equal to the pleural pressure (PPL) plus the elastic recoil pressure (Pel) of the lung.
When there is no airflow (i.e. during a pause in breathing), the tendency of the lungs to collapse (the positive recoil pressure) is exactly balanced by an equivalent negative pleural pressure.
As air flows from the alveoli towards the mouth there is a gradual drop of pressure owing to flow resistance (Fig. 15.6a).
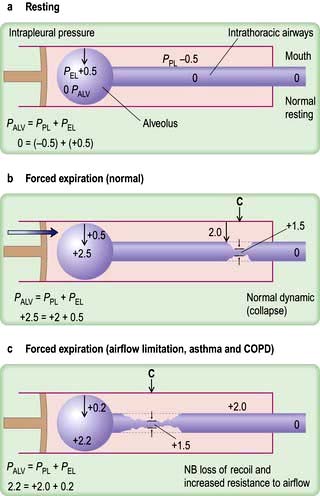
Figure 15.6 Diagrams showing ventilatory forces. (a) During resting at functional residual capacity. (b) During forced expiration in normal subjects. (c) During forced expiration in a patient with COPD. The respiratory system is represented as a piston with a single alveolus and the collapsible part of the airways within the piston (see text). C, collapse point; PALV, alveolar pressure; PEL, elastic recoil pressure; PPL, pleural pressure.
In forced expiration, as mentioned above, the driving pressure raises both the alveolar pressure and the intrapleural pressure. Between the alveolus and the mouth, there is a point (C in Fig. 15.6b) where the airway pressure equals the intrapleural pressure, and the airway collapses. However, this collapse is temporary, as the transient occlusion of the airway results in an increase in pressure behind it (i.e. upstream) and this raises the intra-airway pressure so that the airways open and flow is restored. The airways thus tend to vibrate at this point of ‘dynamic collapse’.
As lung volume decreases during expiration, the elastic recoil pressure of the lungs decreases and the ‘collapse point’ moves upstream (i.e. towards the smaller airways – see Fig. 15.6c). Where there is pathological loss of recoil pressure (as in chronic obstructive pulmonary disease, COPD), the ‘collapse point’ is located even further upstream and causes expiratory flow limitation. The measurement of the forced expiratory volume in 1 second (FEV1) is a useful clinical index of this phenomenon. To compensate, patients with COPD often ‘purse their lips’ in order to increase airway pressure so that their peripheral airways do not collapse. During inspiration, the intrapleural pressure is always less than the intraluminal pressure within the intrathoracic airways, so increasing effort does not limit airflow. Inspiratory flow is limited only by the power of the inspiratory muscles.
Flow-volume loops
The relationship between maximal flow rates and lung volume is demonstrated by the maximal flow-volume (MFV) loop (Fig. 15.7a).
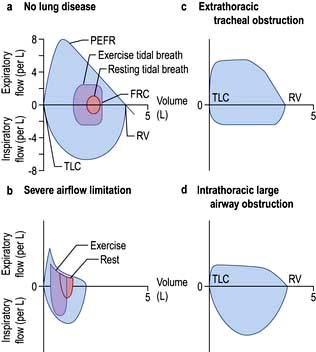
Figure 15.7 (a, b) Maximal flow-volume loops, showing the relationship between maximal flow rates on expiration and inspiration. (a) In a normal subject. (b) In a patient with severe airflow limitation. Flow-volume loops during tidal breathing at rest (starting from the functional residual capacity (FRC)) and during exercise are also shown. The highest flow rates are achieved when forced expiration begins at total lung capacity (TLC) and represent the peak expiratory flow rate (PEFR). As air is blown out of the lung, so the flow rate decreases until no more air can be forced out, a point known as the residual volume (RV). Because inspiratory airflow is only dependent on effort, the shape of the maximal inspiratory flow-volume loop is quite different, and inspiratory flow remains at a high rate throughout the manoeuvre. (c, d) Flow-volume loops of patients with large airway (tracheal) obstruction, showing plateauing of maximal expiratory flow. (c) Extrathoracic tracheal obstruction with a proportionally greater reduction of maximal inspiratory (as opposed to expiratory) flow rate. (d) Intrathoracic large airway obstruction; the expiratory plateau is more pronounced and inspiratory flow rate is less reduced than in (c). In severe airflow limitation the ventilatory demands of exercise cannot be met (cf. a, b), greatly reducing effort tolerance.
In subjects with healthy lungs, maximal flow rates are rarely achieved even during vigorous exercise. However, in patients with severe COPD, limitation of expiratory flow occurs even during tidal breathing at rest (Fig. 15.7b). To increase ventilation these patients have to breathe at higher lung volumes and allow more time for expiration, both of which reduce the tendency for airway collapse. To compensate they increase flow rates during inspiration, where there is relatively less flow limitation.
The volume that can be forced in from the residual volume in 1 second (FIV1) will always be greater than that which can be forced out from TLC in 1 second (FEV1). Thus, the ratio of FEV1 to FIV1 is below 1. The only exception to this occurs when there is significant obstruction to the airways outside the thorax, such as tracheal tumour or retrosternal goitre. Expiratory airway narrowing is prevented by tracheal resistance and expiratory airflow becomes more effort-dependent. During forced inspiration this same resistance causes such negative intraluminal pressure that the trachea is compressed by the surrounding atmospheric pressure. Inspiratory flow thus becomes less effort-dependent, and the ratio of FEV1 to FIV1 exceeds 1. This phenomenon, and the characteristic flow-volume loop, is diagnostic of extrathoracic airways obstruction (Fig. 15.7c).
Ventilation and perfusion relationships
For optimum gas exchange there must be a match between ventilation of the alveoli (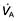 ) and their perfusion (
) and their perfusion (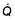 ). However, in reality there is variation in the (
). However, in reality there is variation in the (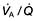 ) ratio in both normal and diseased lungs (Fig. 15.8). In the normal lung both ventilation and perfusion are greater at the bases than at the apices, but the gradient for perfusion is steeper, so the net effect is that ventilation exceeds perfusion towards the apices, while perfusion exceeds ventilation at the bases. Other causes of (
) ratio in both normal and diseased lungs (Fig. 15.8). In the normal lung both ventilation and perfusion are greater at the bases than at the apices, but the gradient for perfusion is steeper, so the net effect is that ventilation exceeds perfusion towards the apices, while perfusion exceeds ventilation at the bases. Other causes of (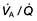 ) mismatch include direct shunting of deoxygenated blood through the lung without passing through alveoli (e.g. the bronchial circulation) and areas of lung that receive no blood (e.g. anatomical deadspace, bullae and areas of underperfusion during acceleration and deceleration, e.g. in aircraft and high performance cars).
) mismatch include direct shunting of deoxygenated blood through the lung without passing through alveoli (e.g. the bronchial circulation) and areas of lung that receive no blood (e.g. anatomical deadspace, bullae and areas of underperfusion during acceleration and deceleration, e.g. in aircraft and high performance cars).
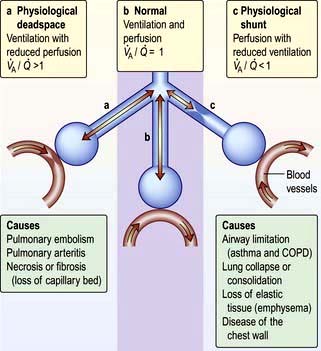
Figure 15.8 Relationships between ventilation and perfusion: a schematic diagram showing the alveolar–capillary interface. The centre (b) shows normal ventilation and perfusion. On the left (a) there is a block in perfusion (physiological deadspace), while on the right (c) there is reduced ventilation (physiological shunting).
An increased physiological shunt results in arterial hypoxaemia since it is not possible to compensate for some of the blood being underoxygenated by increasing ventilation of the well-perfused areas. An increased physiological deadspace just increases the work of breathing and has less impact on blood gases since the normally perfused alveoli are well ventilated. In more advanced disease this compensation cannot occur, leading to increased alveolar and arterial PCO2 (PaCO2), together with hypoxaemia which cannot be compensated by increasing ventilation.
Hypoxaemia occurs more readily than hypercapnia because of the different ways in which oxygen and carbon dioxide are carried in the blood. Carbon dioxide can be considered to be in simple solution in the plasma, the volume carried being proportional to the partial pressure. Oxygen is carried in chemical combination with haemoglobin in the red blood cells, with a non-linear relationship between the volume carried and the partial pressure (Fig. 15.5, p. 341). Alveolar hyperventilation reduces the alveolar PCO2 (PACO2) and diffusion leads to a proportional fall in the carbon dioxide content of the blood (PaCO2). However, as the haemoglobin is already saturated with oxygen, there is no significant increase in the blood oxygen content as a result of increasing the alveolar PO2 through hyperventilation. The hypoxaemia of even a small amount of physiological shunting cannot therefore be compensated for by hyperventilation.
In individuals who have mild degrees of 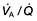 mismatch, the PaO2 and PaCO2 will still be normal at rest. Increasing the requirements for gas exchange by exercise will widen the
mismatch, the PaO2 and PaCO2 will still be normal at rest. Increasing the requirements for gas exchange by exercise will widen the 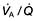 mismatch and the PaO2 will fall.
mismatch and the PaO2 will fall. 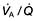 mismatch is by far the most common cause of arterial hypoxaemia.
mismatch is by far the most common cause of arterial hypoxaemia.
Alveolar stability
Pulmonary alveoli are essentially hollow spheres. Surface tension acting at the curved internal surface tends to cause the sphere to decrease in size. The surface tension within the alveoli would make the lungs extremely difficult to distend were it not for the presence of surfactant, an insoluble lipoprotein largely consisting of dipalmitoyl lecithin, which forms a thin monomolecular layer at the air-fluid interface. Surfactant is secreted by type II pneumocytes within the alveolus and reduces surface tension so that alveoli remain stable.
Fluid surfaces covered with surfactant exhibit a phenomenon known as hysteresis; that is, the surface-tension-lowering effect of the surfactant can be improved by a transient increase in the size of the surface area of the alveoli. During quiet breathing, small areas of the lung undergo collapse, but it is possible to re-expand these rapidly by a deep breath; hence the importance of sighs or deep breaths as a feature of normal breathing. Failure of this mechanism, e.g. in patients with fractured ribs – gives rise to patchy basal lung collapse. Surfactant levels may be reduced in a number of diseases that cause damage to the lung (e.g. pneumonia). Lack of surfactant plays a central role in the respiratory distress syndrome of the newborn. Severe reduction in perfusion of the lung impairs surfactant activity and this may explain the characteristic areas of collapse associated with pulmonary embolism.
Defence mechanisms of the respiratory tract
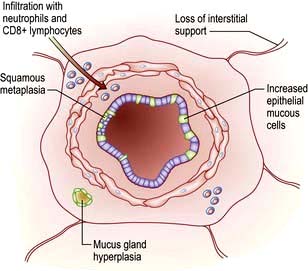
Pulmonary disease often results from a failure of the normal host defence mechanisms of the healthy lung (Fig. 15.9). These can be divided into physical, physiological, humoral and cellular mechanisms.
Physical and physiological mechanisms
Particle removal
Over 90% of particles greater than 10 µm diameter are removed in the nostril or nasopharynx. This includes most pollen grains, which are typically >20 µm in diameter. Particles between 5 and 10 µm become impacted at the carina. Particles smaller than 1 µm tend to remain airborne, thus the particles capable of reaching the deep lung are those in the 1–5 µm range.
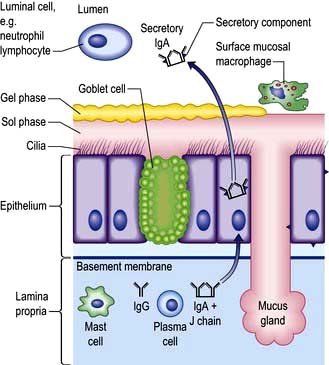
Respiratory tract secretions (Fig. 15.9)
The mucus of the respiratory tract is a gelatinous substance consisting of water and highly glycosylated proteins (mucins). The mucus forms a thick gel that is relatively impermeable to water and floats on a liquid or sol layer found around the cilia of the epithelial cells. The gel layer is secreted from goblet cells and mucous glands as distinct globules that coalesce increasingly in the central airways to form a more or less continuous mucus blanket. In addition to the mucins, the gel contains various antimicrobial molecules (lysozyme, defensins), specific antibodies (IgA) and cytokines, which are secreted by cells in airways and get incorporated into the mucus gel. Bacteria, viruses and other particles get trapped in the mucus and are either inactivated or simply expelled before they can do any damage. Under normal conditions the tips of the cilia engage with the undersurface of the gel phase and by coordinated movement they push the mucus blanket upwards and outwards to the pharynx where it is either swallowed or coughed up. While it only takes 30–60 minutes for mucus to be cleared from the large bronchi, it can be several days before mucus is cleared from respiratory bronchioles. One of the major long-term effects of cigarette smoking is a reduction in mucociliary transport. This contributes to recurrent infection and prolongs contact with carcinogenic material. Air pollutants, local and general anaesthetics and products of bacterial and viral infection also reduce mucociliary clearance.
Congenital defects in mucociliary transport lead to recurrent infections and eventually to bronchiectasis. For example, in the ‘immotile cilia’ syndrome there is an absence of the dynein arms in the cilia themselves, while in cystic fibrosis there is ciliary dyskinesia and abnormally thick mucus.
Humoral and cellular mechanisms
Nonspecific soluble factors
 α-Antitrypsin (α1-antiprotease, see p. 341) in lung secretions is derived from plasma. It inhibits chymotrypsin and trypsin and neutralizes proteases including neutrophil elastase.
α-Antitrypsin (α1-antiprotease, see p. 341) in lung secretions is derived from plasma. It inhibits chymotrypsin and trypsin and neutralizes proteases including neutrophil elastase.
 Antioxidant defences include enzymes such as superoxide dismutase and low-molecular-weight antioxidant molecules (ascorbate, urate) in the epithelial lining fluid. In addition, lung cells are protected by an extensive range of intracellular defences, especially members of the glutathione S-transferase (GST) superfamily.
Antioxidant defences include enzymes such as superoxide dismutase and low-molecular-weight antioxidant molecules (ascorbate, urate) in the epithelial lining fluid. In addition, lung cells are protected by an extensive range of intracellular defences, especially members of the glutathione S-transferase (GST) superfamily.
 Lysozyme is an enzyme found in granulocytes that has bactericidal properties.
Lysozyme is an enzyme found in granulocytes that has bactericidal properties.
 Lactoferrin is synthesized from epithelial cells and neutrophil granulocytes and has bactericidal properties.
Lactoferrin is synthesized from epithelial cells and neutrophil granulocytes and has bactericidal properties.
 Interferons are produced by most cells in response to viral infection and are potent modulators of lymphocyte function.
Interferons are produced by most cells in response to viral infection and are potent modulators of lymphocyte function.
 Complement in secretions is also derived from plasma. In association with antibodies, it plays a major role in cytotoxicity.
Complement in secretions is also derived from plasma. In association with antibodies, it plays a major role in cytotoxicity.
 Surfactant protein A (SPA) is one of four species of surfactant proteins which opsonizes bacteria/particles, enhancing phagocytosis by macrophages.
Surfactant protein A (SPA) is one of four species of surfactant proteins which opsonizes bacteria/particles, enhancing phagocytosis by macrophages.
 Defensins are bactericidal peptides present in the azurophil granules of neutrophils.
Defensins are bactericidal peptides present in the azurophil granules of neutrophils.
 Dimeric secretory IgA targets specific antigens (p. 262).
Dimeric secretory IgA targets specific antigens (p. 262).
Innate and adaptive immunity
These mechanisms act as a defence against microbes, inorganic substances, e.g. asbestos, particulate matter, such as dust, and other antigens. They act by aiding opsonization so that macrophages can better ingest foreign material.
With infection, neutrophils migrate out of pulmonary capillaries into the air spaces and phagocytose and kill microbes with, for example, antimicrobial proteins (lactoferrin), degradative enzymes (elastase) and oxidant radicals. In addition, neutrophil extracellular traps (NET) ensnare and kill extracellular bacteria. Neutrophils also generate a variety of mediators, e.g. TNF-α, IL-1 and chemokines which activate dendritic cells and B cells and produce the T-cell-activating cytokine IL-12. The latter enhances neutrophil-mediated defence during pneumonia. Dendritic cells are antigen presenting cells and are key to the adaptive immune response (p. 58).
Microbes are detected by host cells by pattern recognition receptors, e.g. toll-like receptors. These act via NF-κB transcription factors in the epithelial cells to produce adhesion molecules, chemokines and colony stimulating factors to initiate inflammation. Inflammation is necessary for innate immunity and host defence but can lead to lung damage: there is a fine line between defence and injury.
Symptoms
Runny, blocked nose and sneezing
Nasal symptoms (see also p. 691) are extremely common and both common colds and allergic rhinitis cause ‘runny nose’ (rhinorrhoea), nasal blockage and attacks of sneezing. In allergic rhinitis, symptoms may be intermittent, following contact with pollens or animal danders, or persistent, especially when house-dust mite is the allergen. Colds are frequent during the winter but if the symptoms persist for weeks the patient probably has perennial rhinitis rather than persistent viral infection.
Nasal secretions are usually thin and runny in allergic rhinitis but thicker and discoloured with viral infections. Nose bleeds and blood-stained nasal discharge are common and rarely indicate serious pathology. However, a blood-stained nasal discharge associated with nasal obstruction and pain may be the presenting feature of a nasal tumour (p. 1051). Nasal polyps typically present with nasal blockage and loss of smell.
Cough
Cough (see also p. 822) is the commonest symptom of lower respiratory tract disease. It is caused by mechanical or chemical stimulation of cough receptors in the epithelium of the pharynx, larynx, trachea, bronchi and diaphragm. Afferent receptors go to the cough centre in the medulla where efferent signals are generated to the expiratory musculature. Smokers often have a morning cough with a little sputum. A productive cough is the cardinal feature of chronic bronchitis, while dry coughing, particularly at night, can be a symptom of asthma. Cough also occurs in asthmatics after mild exertion or following forced expiration. Cough can also occur for psychological reasons without any definable pathology.
A worsening cough is the most common presenting symptom of lung cancer. The normal explosive character of the cough is lost when a vocal cord is paralysed, usually as a result of lung cancer infiltrating the left recurrent laryngeal nerve – sometimes termed a bovine cough. Cough can be accompanied by stridor in whooping cough or if there is laryngeal or tracheal obstruction.
Sputum
Approximately 100 mL of mucus is produced daily in a healthy, non-smoking individual. This flows gradually up the airways, through the larynx, and is then swallowed. Excess mucus is expectorated as sputum. Cigarette smoking is the commonest cause of excess mucus production.
Mucoid sputum is clear and white but can contain black specks resulting from the inhalation of carbon. Yellow or green sputum is due to the presence of cellular material, including bronchial epithelial cells, or neutrophil or eosinophil granulocytes. Yellow sputum is not necessarily due to infection, as eosinophils in the sputum, as seen in asthma, can give the same appearance. The production of large quantities of yellow or green sputum is characteristic of bronchiectasis.
Haemoptysis (blood-stained sputum) varies from small streaks of blood to massive bleeding.
 The commonest cause of mild haemoptysis is acute infection, particularly in exacerbations of chronic obstructive pulmonary disease (COPD) but it should not be attributed to this without investigation.
The commonest cause of mild haemoptysis is acute infection, particularly in exacerbations of chronic obstructive pulmonary disease (COPD) but it should not be attributed to this without investigation.
 Other common causes are pulmonary infarction, bronchial carcinoma and tuberculosis.
Other common causes are pulmonary infarction, bronchial carcinoma and tuberculosis.
 In lobar pneumonia, the sputum is usually rusty in appearance rather than frankly blood-stained.
In lobar pneumonia, the sputum is usually rusty in appearance rather than frankly blood-stained.
 Pink, frothy sputum is seen in pulmonary oedema.
Pink, frothy sputum is seen in pulmonary oedema.
 In bronchiectasis, the blood is often mixed with purulent sputum.
In bronchiectasis, the blood is often mixed with purulent sputum.
 Massive haemoptyses (>200 mL of blood in 24 hours) are usually due to bronchiectasis or tuberculosis.
Massive haemoptyses (>200 mL of blood in 24 hours) are usually due to bronchiectasis or tuberculosis.
 Uncommon causes of haemoptyses are idiopathic pulmonary haemosiderosis, Goodpasture’s syndrome, microscopic polyangiitis, trauma, blood disorders and benign tumours.
Uncommon causes of haemoptyses are idiopathic pulmonary haemosiderosis, Goodpasture’s syndrome, microscopic polyangiitis, trauma, blood disorders and benign tumours.
Haemoptysis should always be investigated. Although a diagnosis can often be made from a chest X-ray, a normal chest X-ray does not exclude disease. However, if the chest X-ray is normal, CT scanning and bronchoscopy are only diagnostic in about 5% of patients with haemoptysis.
Firm plugs of sputum may be coughed up by patients suffering from an exacerbation of allergic bronchopulmonary aspergillosis. Sometimes such sputum looks like casts of inflamed bronchi.
Breathlessness
Dyspnoea is a sense of awareness of increased respiratory effort that is unpleasant and that is recognized by the patient as being inappropriate. Patients often complain of tightness in the chest; this must be differentiated from angina.
Breathlessness should be assessed in relation to the patient’s lifestyle. For example, a moderate degree of breathlessness will be totally disabling if the patient has to climb many flights of stairs to reach home.
Orthopnoea (see p. 675) is breathlessness on lying down. While it is classically linked to heart failure, it is partly due to the weight of the abdominal contents pushing the diaphragm up into the thorax. Such patients may also become breathless on bending over.
Tachypnoea and hyperpnoea are, respectively, an increased rate of breathing and an increased level of ventilation. These may be appropriate responses (e.g. during exercise).
Hyperventilation is inappropriate overbreathing. This may occur at rest or on exertion and results in a lowering of the alveolar and arterial PCO2 (see p. 1178).
Paroxysmal nocturnal dyspnoea (see p. 798) is acute episodes of breathlessness at night, typically due to heart failure.
Wheezing
Wheezing is a common complaint and results from airflow limitation due to any cause. The symptom of wheezing is not diagnostic of asthma; other causes include vocal chord dysfunction, bronchiolitis and chronic obstructive pulmonary disease (COPD). Conversely, wheeze may be absent in the early stages of asthma.
Chest pain
The most common type of chest pain reported in respiratory disease is a localized sharp pain, often termed pleuritic. It is made worse by deep breathing or coughing and the patient can usually localize it. Localized anterior chest pain with tenderness of a costochondral junction is caused by costochondritis. Shoulder tip pain suggests irritation of the diaphragmatic pleura, while central chest pain radiating to the neck and arms is likely to be cardiac. Retrosternal soreness is associated with tracheitis, while malignant invasion of the chest wall causes a constant, severe, dull pain.
Examination of the respiratory system
The chest
Examination of the chest
Inspection
Assess mental alertness, cyanosis, breathlessness at rest, use of accessory muscles, any deformity or scars on the chest and movement on both sides. CO2 intoxication causes coarse tremor or flap of the outstretched hands. Prominent veins on the chest may imply obstruction of the superior vena cava.
Cyanosis (see p. 676) is a dusky colour of the skin and mucous membranes, due to the presence of more than 50 g/L of desaturated haemoglobin. When due to central causes, cyanosis is visible on the tongue (especially the underside) and lips. Patients with central cyanosis will also be cyanosed peripherally. Peripheral cyanosis without central cyanosis is caused by a reduced peripheral circulation and is noted on the fingernails and skin of the extremities with associated coolness of the skin.
Finger clubbing is present when the normal angle between the base of the nail and the nail fold is lost. The base of the nail is fluctuant owing to increased vascularity, and there is an increased curvature of the nail in all directions, with expansion of the end of the digit. Some causes of clubbing are given in Table 15.1. Clubbing is not a feature of uncomplicated COPD.
Table 15.1 Some causes of finger clubbing
Palpation and percussion
Check the position of the trachea and apex beat. Examine the supraclavicular fossa for enlarged lymph nodes. The distance between the sternal notch and the cricoid cartilage (three to four finger breadths in full expiration) is reduced in patients with severe airflow limitation. Check chest expansion. A tape measure is used if precise or serial measurements are needed, e.g. in ankylosing spondylitis. Local discomfort over the sternochondral joints suggests costochondritis. In rib fractures, compression of the chest laterally and anteroposteriorly produces localized pain. On percussion, liver dullness is usually detected anteriorly at the level of the sixth rib. Liver and cardiac dullness disappear when lungs are over-inflated (Table 15.2).
Auscultation
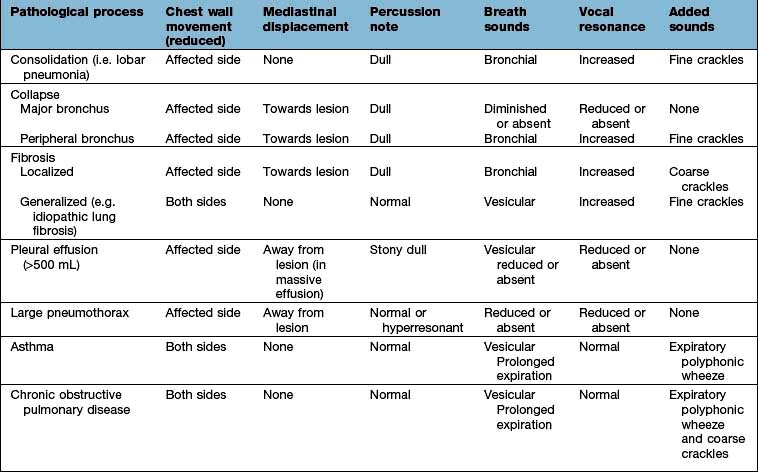
Ask the patient to take deep breaths through the mouth. Inspiration should be more prolonged than expiration. Normal breath sounds are caused by turbulent flow in the larynx and sound harsher anteriorly over the upper lobes (particularly on the right). Healthy lungs filter out most of the high-frequency component, and the resulting sounds are called vesicular.
If the lung is consolidated or collapsed, the high-frequency hissing components of breath are not attenuated, and can be heard as ‘bronchial breathing’. Similar sounds may be heard over areas of localized fibrosis or bronchiectasis. Bronchial breathing is accompanied by whispering pectoriloquy (whispered, high-pitched sounds can be heard distinctly through a stethoscope).
Added sounds
Wheeze. Wheeze results from vibrations in the collapsible part of the airways when apposition occurs as a result of the flow-limiting mechanisms. Wheeze is usually heard during expiration and is commonly but not invariably present in asthma and in chronic obstructive pulmonary disease. In acute severe asthma wheeze may not be heard, as airflow may be insufficient to generate the sound. Wheezes may be monophonic (single large airway obstruction) or polyphonic (narrowing of many small airways). An end-inspiratory wheeze or ‘squeak’ may be heard in obliterative bronchiolitis.
Crackles. These brief crackling sounds are probably produced by opening of previously closed bronchioles – early inspiratory crackles are associated with diffuse airflow limitation, while late inspiratory crackles are characteristically heard in pulmonary oedema, lung fibrosis and bronchiectasis.
Pleural rub. A creaking or groaning sound that is usually well localized. It indicates inflammation and roughening of the pleural surfaces, which normally glide silently over one another.
Vocal resonance. Healthy lung attenuates high-frequency notes, as compared to the lower-pitched components of speech. Consolidated lung has the reverse effect, transmitting high frequencies well; the spoken word then takes on a bleating quality. Whispered (and therefore high-pitched) speech can be clearly heard over consolidated areas, as compared to healthy lung. Low-frequency sounds such as ‘ninety-nine’ are well transmitted across healthy lung to produce vibration that can be felt over the chest wall. Consolidated lung transmits these low-frequency noises less well, and pleural fluid severely dampens or obliterates the vibrations altogether. Tactile vocal fremitus is the palpation of this vibration, usually by placing the edge of the hand on the chest wall. For all practical purposes this duplicates the assessment of vocal resonance and is not routinely performed as part of the chest examination.
Cardiovascular system examination (p. 676) gives additional information about the lungs.
Investigation of respiratory disease
Imaging
Radiology is essential in investigating most chest symptoms. Some diseases such as tuberculosis or lung cancer may be undetectable on clinical examination but are obvious on the chest X-ray. Conversely, asthma or chronic bronchitis may be associated with a normal chest X-ray. Always try to get previous films for comparison.
Chest X-ray
 Centring of the film. The distance between each clavicular head and the spinal processes should be equal
Centring of the film. The distance between each clavicular head and the spinal processes should be equal
 Penetration (check film is not too dark)
Penetration (check film is not too dark)
 View. Routine films are taken postero-anterior (PA), i.e. the film is placed in front of the patient with the X-ray source behind. Anteroposterior (AP) films are taken only in very ill patients who are unable to stand up or be taken to the radiology department; the cardiac outline appears bigger and the scapulae cannot be moved out of the way. Lateral chest X-rays were often performed in the past to localize pathology, but CT scans have replaced these.
View. Routine films are taken postero-anterior (PA), i.e. the film is placed in front of the patient with the X-ray source behind. Anteroposterior (AP) films are taken only in very ill patients who are unable to stand up or be taken to the radiology department; the cardiac outline appears bigger and the scapulae cannot be moved out of the way. Lateral chest X-rays were often performed in the past to localize pathology, but CT scans have replaced these.
X-ray abnormalities
Collapse and consolidation
Simple pneumonia is easy to recognize (see Fig. 15.33) but look carefully for any evidence of collapse (Fig. 15.10, Table 15.3). Loss of volume or crowding of the ribs are the best indicators of lobar collapse. The lung lobes collapse in characteristic directions. The lower lobes collapse downwards and towards the mediastinum, the left upper lobe collapses forwards against the anterior chest wall, while the right upper lobe collapses upwards and inwards, forming the appearance of an arch over the remaining lung. The right middle lobe collapses anteriorly and inward, obscuring the right heart border. If a whole lung collapses, the mediastinum will shift towards the side of the collapse. Uncomplicated consolidation does not cause mediastinal shift or loss of lung volume, so any of these features should raise the suspicion of an endobronchial obstruction.
Figure 15.10 Collapse of the left upper lobe. Chest X-ray showing triangular shadow in the left upper zone, next to the mediastinum.
Table 15.3 Causes of collapse of the lung
Pleural effusion
Pleural effusions (see Fig. 15.45) need to be larger than 500 mL to cause much more than blunting of the costophrenic angle. On an erect film they produce a characteristic shadow with a curved upper edge rising into the axilla. If very large, the whole of one hemithorax may be opaque, with mediastinal shift away from the effusion.
Fibrosis
Localized fibrosis causes streaky shadowing, and the accompanying loss of lung volume causes mediastinal structures to move to the same side. More generalized fibrosis can lead to a honeycomb appearance (see p. 849), seen as diffuse shadows containing multiple circular translucencies a few millimetres in diameter.
Round shadows
Lung cancer is the commonest cause of large round shadows but many other causes are recognized (Table 15.4).
Table 15.4 Causes of round shadows (>3 cm) in the lung
Miliary mottling
This term, derived from the Latin for millet, describes numerous minute opacities, 1–3 mm in size. The commonest causes are tuberculosis, pneumoconiosis, sarcoidosis, idiopathic pulmonary fibrosis and pulmonary oedema (see Fig. 14.15), although pulmonary oedema is usually perihilar and accompanied by larger, fluffy shadows. Pulmonary microlithiasis is a rare but striking cause of miliary mottling.
Computed tomography
CT provides excellent images of the lungs and mediastinal structures (Fig. 15.11). Narrow slice, high-resolution CT scans show the lung parenchyma well, while thicker slice staging CT scans are used for diagnosis of malignant disease. Mediastinal structures are shown more clearly after injecting intravenous contrast medium.
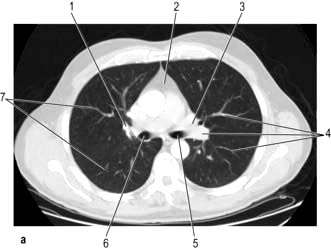
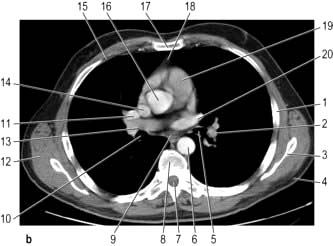
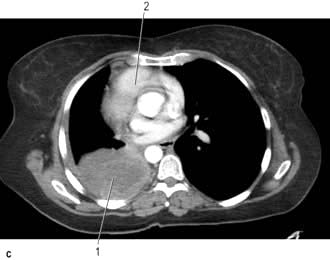
Figure 15.11 CT scans of the lung. (a) Lung setting – showing normal lung markings. 1, right hilum; 2, mediastinum; 3, left hilum; 4, lung vessels; 5, left main bronchus; 6, right main bronchus; 7, peripheral lung vessels. (b) Mediastinal (soft tissue) setting – showing normal mediastinal structures following intravenous contrast enhancement. 1, rib; 2, descending left pulmonary artery; 3, scapula; 4, subcutaneous fat; 5, left main bronchus; 6, descending aorta; 7, spinal canal; 8, vertebral body; 9, oesophagus; 10, right main bronchus; 11, right pulmonary artery; 12, muscle; 13, right superior pulmonary vein; 14, superior vena cava; 15, costal cartilage; 16, ascending aorta; 17, sternum; 18, thymic remnant; 19, pulmonary trunk; 20, left superior pulmonary vein. (c) Post-contrast scan showing large right upper zone carcinoma with enlarged lymph nodes in the mediastinum surrounding the trachea.
CT is essential in staging bronchial carcinoma by demonstrating tumour size, nodal involvement, metastases and invasion of mediastinum, pleura or chest wall. CT-guided needle biopsy allows samples to be obtained from peripheral masses. Staging scans should assess liver and adrenals, which are common sites for metastatic disease.
High-resolution CT (HRCT) scanning samples lung parenchyma with 1–2 mm thickness scans at 10–20 mm intervals and are used to assess diffuse inflammatory and infective parenchymal processes. It is valuable in:
 Evaluating diffuse disease of the lung parenchyma, including sarcoidosis, hypersensitivity pneumonitis, occupational lung disease, and any other form of interstitial pulmonary fibrosis.
Evaluating diffuse disease of the lung parenchyma, including sarcoidosis, hypersensitivity pneumonitis, occupational lung disease, and any other form of interstitial pulmonary fibrosis.
 Diagnosis of bronchiectasis. HRCT has a sensitivity and specificity >90%.
Diagnosis of bronchiectasis. HRCT has a sensitivity and specificity >90%.
 Distinguishing emphysema from diffuse parenchymal lung disease or pulmonary vascular disease as a cause of a low gas transfer factor with otherwise normal lung function.
Distinguishing emphysema from diffuse parenchymal lung disease or pulmonary vascular disease as a cause of a low gas transfer factor with otherwise normal lung function.
 Suspected opportunistic lung infection in immunocompromised patients
Suspected opportunistic lung infection in immunocompromised patients
Multi-slice CT scanners can produce detailed images in two or three dimensions in any plane. This detail is particularly useful for the detection of pulmonary emboli. Pulmonary nodules and airway disease are more easily defined and the technique makes HRCT less necessary.
Magnetic resonance imaging
MRI is less valuable than CT in assessing the lung parenchyma. In the mediastinum, MRI with ECG-gating allows accurate imaging of the heart and aortic aneurysms, and MRI has been used in staging lung cancer, for assessing tumour invasion in the mediastinum, chest wall and at the lung apex, because it produces good images in the sagittal and coronal planes. Vascular structures can be clearly differentiated as flowing blood produces a signal void on MRI.
Positron emission tomography (PET)
Tumours take up labelled fluorodeoxyglucose (FDG), which emits positrons that can be imaged and helps to differentiate benign from malignant tumours. In bronchial carcinoma, PET scanning combined with CT is now the investigation of choice for assessing lymph nodes and metastatic disease.
Scintigraphic imaging
Isotopic lung scans were used widely for the detection of pulmonary emboli but are now performed less often owing to widespread use of D-dimer measurements and CT pulmonary angiography.
Perfusion scan
Macro-aggregated human albumin labelled with technetium-99m (99mTc) is injected intravenously. The particles impact in pulmonary capillaries, where they remain for a few hours. A gamma camera is then used to detect the deposition of the particles. The resultant pattern indicates the distribution of pulmonary blood flow; cold areas occur where there is defective blood flow (e.g. in pulmonary emboli).
Ventilation–perfusion scan
Xenon-133 gas is inhaled and its distribution is detected at the same time as the perfusion scan. Areas affected by pulmonary embolism will have reduced perfusion relative to ventilation (see Chapter 14). Other lung diseases (e.g. asthma or pneumonia) impair both ventilation and perfusion. Unfortunately, a pulmonary embolus can affect the lung substance (e.g. atelectasis) leading to reduced ventilation. Nevertheless, this is a better technique than perfusion scan alone.
Ultrasound (USS)
Ultrasound is useful for diagnosing and aspirating small pleural effusions, and for the safe placement of intercostal drains. Ultrasound guided biopsy is used for lung masses that abut the pleura, but ultrasound is not useful for lung parenchymal disease as ultrasound energy is scattered by air. Endobronchial ultrasound is helpful.
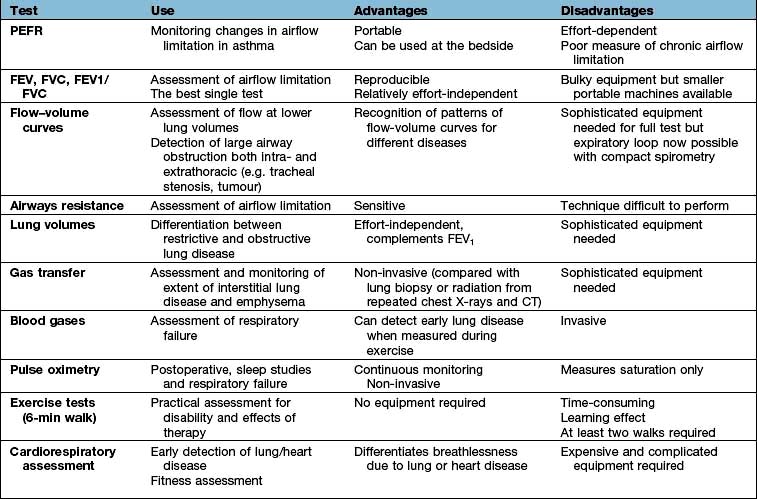
Respiratory function tests (Table 15.5)
In clinical practice, airflow limitation can be assessed by relatively simple tests that have good intra-subject repeatability. Results must be compared with predicted values for healthy subjects as normal ranges vary with sex, age and height. Moreover, there is considerable variation between healthy individuals of the same size and age; the standard deviation for the peak expiratory flow rate is approximately 50 L/min, and for the FEV1 it is approximately 0.4 L. Repeated measurements of lung function are useful for assessing the progression of disease in individual patients.
Tests of ventilatory function
These tests are used mainly to assess the degree of airflow limitation during expiration.
Spirometry
The patient takes a maximum inspiration followed by a forced expiration (for as long as possible) into the spirometer. The spirometer measures the one second forced expiratory volume (FEV1) and the total volume of exhaled gas (forced vital capacity, FVC). Both FEV1 and FVC are related to height, age and sex (Fig. 15.12).
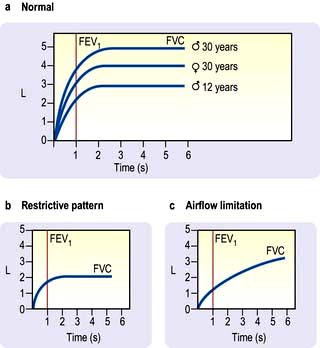
Figure 15.12 Spirometry. Volume–time curves showing (a) normal patterns for age and sex, (b) restrictive pattern (FEV1 and FVC reduced), (c) airflow limitation (FEV1 only reduced).
In airflow limitation, the FEV1 is reduced as a percentage of FVC. In normal health the FEV1/FVC ratio is around 75%. With increasing airflow limitation FEV1 falls proportionately more than FVC, so the FEV1/FVC ratio is reduced. With restrictive lung disease FEV1 and FVC are reduced proportionately and the FEV1/FVC ratio remains normal or may even increase because of enhanced elastic recoil.
In chronic airflow limitation (particularly in COPD and asthma) the total lung capacity (TLC) is usually increased, yet there is nearly always some reduction in the FVC. This is due to collapse of small airways causing obstruction to airflow before the normal residual volume (RV) is reached. This trapping of air within the lung is a characteristic feature of these diseases.
Peak expiratory flow rate (PEFR)
This is an extremely simple and cheap test. Subjects take a full inspiration to total lung capacity and then blow out forcefully into the peak flow meter (Fig. 15.13). The best of three attempts is recorded.
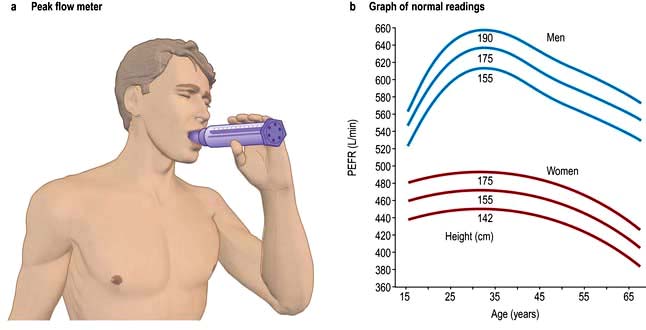
Figure 15.13 Peak flow measurements. (a) Peak flow meter: the lips should be tight around the mouthpiece. (b) Graph of normal readings for men and women.
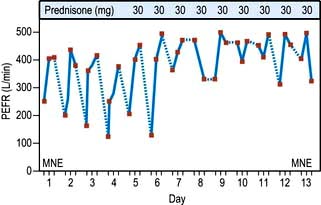
Although reproducible, PEFR is mainly dependent on the flow rate in larger airways and it may be falsely reassuring in patients with moderate airflow limitation. PEFR is mainly used to diagnose asthma, and to monitor exacerbations of asthma and response to treatment. Regular measurements of peak flow rates on waking, during the afternoon, and before going to bed demonstrate the wide diurnal variations in airflow limitation that characterize asthma and allow objective assessment of response to treatment (Fig. 15.14).
Other ventilatory function tests
Measurement of airways resistance in a body box (plethysmograph) is more sensitive but the equipment is expensive and the necessary manoeuvres are too exhausting for many patients with chronic airflow limitation.
Flow-volume loops
Plotting flow rates against expired volume (flow-volume loops, see Fig. 15.7) shows the site of airflow limitation within the lung. At the start of expiration from TLC, maximum resistance is from the large airways, and this affects the flow rate for the first 25% of the curve. As air is exhaled, lung volume reduces and the flow rate becomes dependent on the resistance of smaller airways. In chronic obstructive pulmonary disease (COPD), where the disease mainly affects the smaller airways, expiratory flow rates at 50% or 25% of the vital capacity are disproportionately reduced when compared with flow rates at larger lung volumes. Flow-volume loops will also show obstruction of large airways, e.g. tracheal narrowing due to tumour or retrosternal goitre.
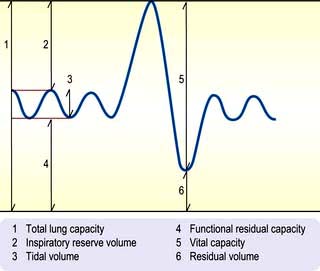
Lung volumes
The subdivisions of lung volume are shown in Figure 15.15. Tidal volume and vital capacity can be measured using a simple spirometer, but alternative techniques are needed to measure TLC and RV. TLC is measured by inhaling air containing a known concentration of helium and measuring its dilution in the exhaled air. RV can be calculated by subtracting the vital capacity from the TLC.
TLC measurements using this technique are inaccurate if there are large cystic spaces in the lung, because helium cannot diffuse into them. Under these circumstances the thoracic gas volume can be measured more accurately using a body plethysmograph. The difference between measurements made by these two methods shows the extent of non-communicating air space within the lungs.
Transfer factor
To measure the efficiency of gas transfer across the alveolar-capillary membrane carbon monoxide is used as a surrogate, since its diffusion rate is similar to oxygen. A low concentration of carbon monoxide is inhaled and the rate of absorption calculated. In normal lungs the transfer factor accurately reflects the diffusing capacity of the lungs for oxygen and depends on the thickness of the alveolar-capillary membrane. In lung disease the diffusing capacity (DCO) is also affected by the ventilation–perfusion relationship. To control for differences in lung volume, the uptake of carbon monoxide is expressed relative to lung volume as a transfer coefficient (KCO).
Gas transfer is reduced in patients with severe degrees of emphysema and fibrosis, but also in heart failure and anaemia. Although relatively nonspecific, gas transfer is particularly useful in the detection and monitoring of diseases affecting the lung parenchyma (e.g. idiopathic pulmonary fibrosis, sarcoidosis, asbestosis).
Measurement of blood gases
This technique is described on page 891.
Measurement of the partial pressures of oxygen and carbon dioxide in arterial blood is essential in managing respiratory failure and severe asthma, when repeated measurements are often the best guide to therapy.
Peripheral oxygen saturation (SpO2) can be continuously measured using an oximeter with either ear or finger probes. Pulse oximetry has become an essential part of routine monitoring of patients in hospital and clinics. It is also useful in exercise testing and reduces the need to measure arterial blood gases.
Haematological and biochemical tests
 Haemoglobin, to detect anaemia or polycythaemia
Haemoglobin, to detect anaemia or polycythaemia
 Packed cell volume (secondary polycythaemia occurs with chronic hypoxia)
Packed cell volume (secondary polycythaemia occurs with chronic hypoxia)
 Routine biochemistry (often disturbed in carcinoma and infection)
Routine biochemistry (often disturbed in carcinoma and infection)
 D-dimer to detect intravascular coagulation. A negative test makes pulmonary embolism very unlikely.
D-dimer to detect intravascular coagulation. A negative test makes pulmonary embolism very unlikely.
Other blood investigations sometimes required include α1-antitrypsin levels, Aspergillus antibodies, viral and mycoplasma serology, autoantibody profiles and specific IgE measurements.
Sputum
Sputum should be inspected for colour:
 Yellowish-green indicates inflammation (infection or allergy)
Yellowish-green indicates inflammation (infection or allergy)
 Blood suggests neoplasm or pulmonary infarct (see haemoptysis, p. 798).
Blood suggests neoplasm or pulmonary infarct (see haemoptysis, p. 798).
Microbiological studies (e.g. Gram-stain and culture) are rarely helpful in upper respiratory tract infections or in acute or chronic bronchitis. They are of value in:
Sputum cytology
This is useful in the diagnosis of bronchial carcinoma and asthma. Its advantages are speed, cheapness and its non-invasive nature.
However, not everyone can produce sputum and a reliable cytologist is needed. Sputum can be induced by inhalation of nebulized hypertonic saline (5%). Better samples can be obtained by bronchoscopy and bronchial washings (see p. 797).
Exercise tests
The predominant symptom in respiratory medicine is breathlessness. The degree of disability produced by breathlessness can be assessed by asking the patient to walk for 6 minutes along a measured track. This has been shown to be a reproducible and useful test once the patient has undergone an initial training walk to overcome the learning effect. Additional information can be obtained by using pulse oximetry during exercise to assess desaturation.
More sophisticated cardiopulmonary exercise tests are useful in investigating unexplained breathlessness. Measurement of uptake of oxygen (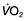 ), work performed, heart rate and blood pressure together with serial ECGs allows the following:
), work performed, heart rate and blood pressure together with serial ECGs allows the following:
Pleural aspiration
Diagnostic aspiration is necessary for all but very small effusions. Nowadays this is usually done under ultrasound guidance, using full aspetic precautions. A needle attached to a 20 mL syringe is inserted under local anaesthesia through an intercostal space towards the top of an area of dullness. Fluid is withdrawn and the presence of any blood is noted. Samples are sent for protein estimation, lactate dehydrogenase (LDH), cytology and bacteriological examination, including culture and Ziehl–Neelsen/auramine stain for tuberculosis. Large amounts of fluid can be aspirated through a large-bore needle to help relieve extreme breathlessness.
Pleural biopsy
Pleural biopsy used to be performed at the bedside, but is now generally done under direct vision using video-assisted thoracoscopy.
Intercostal drainage
This is carried out when large effusions are present, producing severe breathlessness, or for drainage of an empyema (see Practical Box 15.1). Drains should be inserted with ultrasound guidance. Pleurodesis is performed for recurrent/malignant effusion.
![]() Practical Box 15.1
Practical Box 15.1
Intercostal drainage
 Explain to the patient the nature of the procedure.
Explain to the patient the nature of the procedure.
Mediastinoscopy and scalene node biopsy
Mediastinoscopy is used in the diagnosis of mediastinal masses and in staging nodal disease in carcinoma of the bronchus. An incision is made just above the sternum and a mediastinoscope inserted by blunt dissection.
Fibreoptic bronchoscopy
See Practical Box 15.2 and Fig. 15.16.
![]() Practical Box 15.2
Practical Box 15.2
Fibreoptic bronchoscopy
This enables the direct visualization of the bronchial tree as far as the subsegmental bronchi under a local anaesthetic. Informed written consent should be obtained after explaining the nature of the procedure.
Indications
 Lesions requiring biopsy seen on chest X-ray
Lesions requiring biopsy seen on chest X-ray
 Positive sputum cytology for malignant cells with no chest X-ray abnormality
Positive sputum cytology for malignant cells with no chest X-ray abnormality
 Collection of bronchial secretions for bacteriology, especially tuberculosis
Collection of bronchial secretions for bacteriology, especially tuberculosis
 Recurrent laryngeal nerve paralysis of unknown aetiology
Recurrent laryngeal nerve paralysis of unknown aetiology
 Infiltrative lung disease (to obtain a transbronchial biopsy)
Infiltrative lung disease (to obtain a transbronchial biopsy)
 Investigation of collapsed lobes or segments and aspiration of mucus plugs.
Investigation of collapsed lobes or segments and aspiration of mucus plugs.
Disadvantages
 All patients require sedation to tolerate the procedure.
All patients require sedation to tolerate the procedure.
 Minor and transient cardiac dysrhythmias occur in up to 40% of patients on passage of the bronchoscope through the larynx. Monitoring is required.
Minor and transient cardiac dysrhythmias occur in up to 40% of patients on passage of the bronchoscope through the larynx. Monitoring is required.
 Oxygen supplementation is required in patients with PaO2 below 8 kPa.
Oxygen supplementation is required in patients with PaO2 below 8 kPa.
 Fibreoptic bronchoscopy should be performed with care in the very sick, and transbronchial biopsies avoided in ventilated patients owing to the increased risk of pneumothorax.
Fibreoptic bronchoscopy should be performed with care in the very sick, and transbronchial biopsies avoided in ventilated patients owing to the increased risk of pneumothorax.
 Massive bleeding may occur after biopsy of vascular lesions or carcinoid tumours. Rigid bronchoscopy may be required to allow adequate access to the bleeding point for haemostasis.
Massive bleeding may occur after biopsy of vascular lesions or carcinoid tumours. Rigid bronchoscopy may be required to allow adequate access to the bleeding point for haemostasis.
Under local anaesthesia and sedation, the central airways can be visualized down to subsegmental level and biopsies taken for histology. More distal lesions may be sampled by washing or blind brushing. Diffuse inflammatory and infective lung processes may be sampled by bronchoalveolar lavage and transbronchial biopsy. The yield is best in sarcoidosis, lymphangitis carcinomatosa and hypersensitivity pneumonitis. Other fibrotic lung diseases usually yield non-diagnostic samples so it may be more relevant to proceed directly to open or thoracoscopic lung biopsy. Endoscopic bronchoscopic ultrasound enables direct sampling of lymph nodes for diagnostic staging of lung cancer.
Video-assisted thoracoscopic (VATS) lung biopsy
This technique has largely replaced open thoracotomy when a lung biopsy is required (p. 850).
Skin-prick tests
Allergen solutions are placed on the skin (usually the volar surface of the forearm) and the epidermis is broken using a 1 mm tipped lancet. A separate lancet should be used for each allergen. If the patient is sensitive to the allergen a wheal develops. The wheal diameter is measured after 10 minutes. A wheal of at least 3 mm diameter is regarded as positive provided that the control test is negative. The results should always be interpreted in the light of the history. Skin tests are not affected by bronchodilators or corticosteroids but antihistamines should be discontinued at least 48 hours before testing.
Smoking and air pollution
Smoking
Prevalence
Cigarette smoking is declining in the western world. In 1974 in the UK, 51% of men and 41% of women smoked cigarettes – nearly half the adult population. Now 22% of men and 21% of women aged 16 years and over smoke. The highest rates are in women aged 20–24, 31% of whom smoke, and men aged 25–34, 30% of whom smoke. The highest rates of cigarette consumption per capita are in Greece, Russia and parts of Eastern Europe. In global terms the USA ranks 39th and the UK is now down to 65th, close to the rates in Sweden and Malaysia. Smoking continues to increase in many developing countries, particularly among women.
Toxic effects
Cigarette smoke contains polycyclic aromatic hydrocarbons and nitrosamines, which are potent carcinogens and mutagens in animals. It causes release of enzymes from neutrophil granulocytes and macrophages that are capable of destroying elastin and leading to lung damage. Pulmonary epithelial permeability increases even in symptomless cigarette smokers, and correlates with the concentration of carboxyhaemoglobin in blood. This altered permeability may allow easier access for carcinogens.
The dangers
Cigarette smoking is addictive and harmful to health (Table 15.6). People usually start smoking in adolescence for psychosocial reasons and, once they smoke regularly, the pharmacological properties of nicotine encourage persistence, by their effect on the smoker’s mood. Very few cigarette smokers (<2%) can limit themselves to occasional or intermittent smoking.
Table 15.6 The dangers of cigarette smoking
|
|
Significant dose–response relationships exist between cigarette consumption, airway inflammation (Table 15.7) and lung cancer mortality. Sputum production and airflow limitation increase with daily cigarette consumption, and effort tolerance decreases. Smoking 20 cigarettes daily for 20 years increases the lifetime risk of lung cancer by about 10 times compared to a lifelong non-smoker. Smoking and asbestos exposure are synergistic risk factors for lung cancer, with a combined risk of about 90 times that of unexposed non-smokers.
Table 15.7 Effects of smoking on the lung
|
|
Cigarette smokers who change to cigars or pipe-smoking can reduce their risk of lung cancer. However, pipe and cigar smokers remain at greater risk of lung cancer than lifelong non-smokers or former smokers.
Environmental tobacco smoke (‘passive smoking’) has been shown to increase the frequency and severity of asthma attacks in children and may also increase the incidence of asthma. It is also associated with a small but definite increase in lung cancer. Worldwide, second hand smoke was estimated to affect 40% of children, 33% of non-smoking males and 35% of non-smoking females in 2004. This caused a 1% worldwide mortality and 0.7% of the total worldwide burden of disease in DALYs (disability-adjusted life years).
FURTHER READING
Gu Dongfeng, Kelly TN, Wu Xigui et al. Mortality attributable to smoking in China. N Engl J Med 2009; 360:150–159.
Hales S, Howden-Chapman P. Effects of air pollution on health. BMJ 2007; 335:314–315.
Lippmann M. Health effects of airborne particulate matter. N Engl J Med 2007; 57:2395–2397.
Oberg M, Jaakkola MS, Woodward A et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 372:139–46.
Oncken C. Nicotine replacement for smoking cessation during pregnancy. N Engl J Med 2012; 366:846–847.
WHO. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005. Copenhagen: World Health Organization; 2005.
Stopping smoking
If the entire population could be persuaded to stop smoking, the effect on healthcare use would be enormous. National campaigns, bans on advertising and a substantial increase in the cost of cigarettes are the best ways of achieving this at the population level. Smoking bans in the workplace, pubs and public spaces have also helped. Meanwhile, active encouragement to stop smoking remains a useful approach for individuals. Smokers who want to stop should have access to smoking cessation clinics to provide behavioural support. Nicotine replacement therapy (NRT) and bupropion are effective aids to smoking cessation in those smoking >10 cigarettes/day. Both should only be used in smokers who commit to a target stop date, and the initial prescription should be for 2 weeks beyond the target stop date. NRT is the preferred choice; there is no evidence that combined therapy offers any advantage. Therapy should be changed after 3 months if abstinence is not achieved.
Varenicline is an oral partial agonist on the α4β2 subtype of the nicotinic acetylcholine receptor. It stimulates the nicotine receptor and reduces withdrawal symptoms and also the craving for cigarettes. A 12-week course increases the chance of stopping smoking four times; its main side-effects are nausea and sometimes severe depression. Cytisine, which has high affinity for the same receptor, also aids smoking cessation.
Air pollution and epidemiology
Atmospheric air pollution, due to the burning of coal for energy and heat, has been a feature of urban living in developed countries for at least two centuries. It consists of black smoke and sulphur dioxide (SO2). Air pollution of this type peaked in the 1950s in the UK, until legislation led to restrictions on coal burning. Such pollution remains common in some parts of Eastern Europe and Russia and is increasing in newly industrialized countries (especially India and China). The combustion of hydrocarbon fuels in motor vehicles has led to new forms of air pollution, consisting of primary pollutants such as nitrogen oxides (NO and NO2), diesel particulates, polyaromatic hydrocarbons and ozone, a secondary pollutant generated by photochemical reactions in the atmosphere (ozone levels are highest in sunny, rural areas). Levels of NO2 can be high in poorly ventilated kitchens and living rooms where gas is used for cooking and in fires.
Particulate matter consists of coarse particles (10–2.5 µm in aerodynamic diameter), produced by construction work and farming, and fine particles (<2.5 µm) generated from burning fossil fuels. Fine particulates (PM2.5) remain airborne for long periods and are carried into rural areas. Several respiratory and cardiac problems are exacerbated by these very small particles.
The WHO global air-quality guidelines suggest 24 hour values of <25 µg/m3 for PM25 for the short term and 10 µg/m3 in the long term. In Europe, 70% of the particulates present in urban air result from the combustion of diesel fuel, providing a background concentration of 3–5 µg/m3. The WHO estimates that air pollution causes 800 000 premature deaths worldwide every year.
Deaths from respiratory and cardiovascular disease occur mainly in older populations; air pollution mainly causes bronchitis in children. Pollution from motor vehicles has been linked to increased hospital admissions, reduced lung function in children and younger adults and an increase in lung cancer (polyaromatic hydrocarbons).
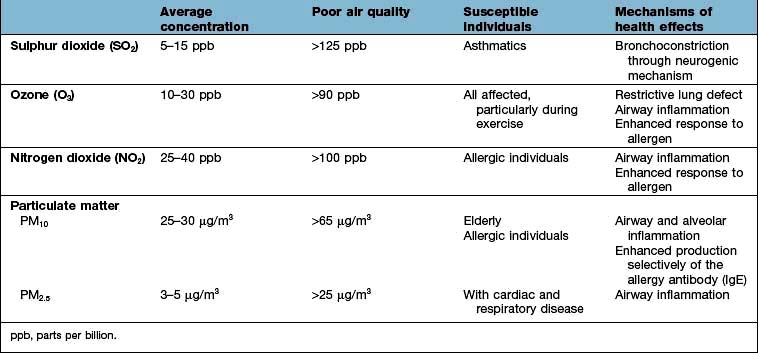
Although it has been proposed that air pollution may cause asthma and other allergic diseases, there is no current evidence for this (Table 15.8). However, air pollution does adversely affect lung development in teenage children, while both NO2 and ozone enhance the nasal and lung airway responses to inhaled allergen in people with established allergic disease.
Management
When air quality is poor, asthmatics are advised to avoid exercising outdoors and to increase their anti-inflammatory medication (i.e. inhaled corticosteroids).
Short- and long-term measures are required to reduce air pollution, particularly diesel particulates (which are predicted to increase as more diesel engines are used). Such measures include increased motor engine efficiency, catalytic converters, diesel particulate traps and decreased reliance on cars and trucks.
Diseases of the upper respiratory tract
The common cold (acute coryza)
This highly infectious illness causes a mild systemic upset and prominent nasal symptoms. It is due to infection by a wide range of respiratory viruses, of which the rhinoviruses are the most common. Other common cold viruses include coronaviruses and adenoviruses. Infectivity from close personal contact (nasal mucus on hands) or droplets is high in the early stages of the infection, and spread is facilitated by overcrowding and poor ventilation. There are at least 100 different antigenic strains of rhinovirus, making it difficult for the immune system to confer protection. On average, individuals suffer two to three colds per year, but the incidence lessens with age, presumably as a result of accumulating immunity to different virus strains. The incubation period varies from 12 hours to 5 days.
The clinical features are tiredness, slight pyrexia, malaise and a sore nose and pharynx. Sneezing and profuse, watery nasal discharge are followed by thick mucopurulent secretions which may persist for up to a week. Secondary bacterial infection occurs only in a minority of cases.
Rhinitis
Rhinitis is defined clinically as sneezing attacks, nasal discharge or blockage occurring for more than an hour on most days:
 For a limited period of the year (seasonal or intermittent rhinitis)
For a limited period of the year (seasonal or intermittent rhinitis)
 Throughout the whole year (perennial or persistent rhinitis).
Throughout the whole year (perennial or persistent rhinitis).
Seasonal rhinitis
This is the commonest allergic disorder. It is often called ‘hayfever’ but as this implies that only grass pollen is responsible, it is better described as seasonal (or intermittent) allergic rhinitis. Worldwide prevalence rates vary from 2% to 20%. Prevalence is maximal in the 2nd decade, and up to 30% of UK teenagers and young adults are affected each June and July.
Nasal irritation, sneezing and watery rhinorrhoea are the most troublesome symptoms, but many also suffer from itching of the eyes and soft palate and occasionally even itching of the ears because of the common innervation of the pharyngeal mucosa and the ear. In addition, approximately 20% suffer from seasonal wheezing. The common seasonal allergens are shown in Figure 15.17. Since pollination of plants that give rise to high pollen counts varies from country to country, seasonal rhinoconjunctivitis and accompanying wheeze may occur at different times of year in different regions.
Perennial rhinitis
Patients with perennial rhinitis rarely have symptoms that affect the eyes or throat. Half have symptoms predominantly of sneezing and watery rhinorrhoea, whilst the other half complain mostly of nasal blockage. The patient may lose the sense of smell and taste. Sinusitis occurs in about 50% of cases, due to mucosal swelling that obstructs drainage from the sinuses. Perennial rhinitis is most frequent in the 2nd and 3rd decades, decreasing with age, and can be divided into four main types.
Perennial allergic rhinitis
The commonest cause is allergy to the faecal particles of the house-dust mite Dermatophagoides pteronyssinus or D. farinae; these are under 0.5 mm in size, invisible to the naked eye (Fig. 15.18), and found in dust throughout the house, particularly in older, damp dwellings. Mites live off desquamated human skin scales and the highest concentrations (4000 mites/g of surface dust) are found in human bedding. Their faecal particles are approximately 20 µm in diameter (Fig. 15.18), and impact in the nose rather than the lungs, unless the patient breathes through their mouth.
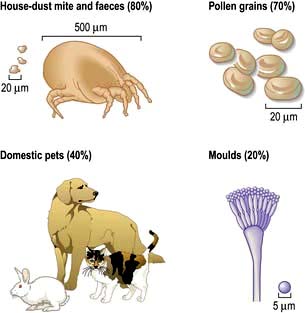
Figure 15.18 Common allergens causing allergic rhinitis and asthma. The house-dust mite, faeces of house-dust mites, pollen grains, domestic pets and moulds. Percentages are those of positive skin-prick tests to these allergens in patients with allergic rhinitis.
The next most common allergens come from domestic pets (especially cats) and are proteins derived from urine or saliva spread over the surface of the animal as well as skin protein. Allergy to urinary protein from small mammals is a major cause of morbidity among laboratory workers.
Industrial dust, vapours and fumes cause occupationally related perennial rhinitis more often than asthma.
The presence of perennial rhinitis makes the nose more reactive to nonspecific stimuli such as cigarette smoke, washing powders, household detergents, strong perfumes and traffic fumes. Although patients often think they are allergic to these stimuli, these are irritant responses and do not involve antibodies.
Perennial non-allergic rhinitis with eosinophilia
No extrinsic allergic cause can be identified, either from the history or on skin testing, but eosinophilic granulocytes are present in nasal secretions. Most of these patients are intolerant of aspirin/NSAIDs.
Vasomotor rhinitis
These patients with perennial rhinitis have no demonstrable allergy or nasal eosinophilia. Watery secretions and nasal congestion are triggered by, for example, cold air, smoke, perfume, newsprint, possibly because of an imbalance of the autonomic nerves controlling the erectile tissue (sinusoids) in the nasal mucosa.
Nasal polyps
These are round, smooth, soft, semi-translucent, pale or yellow, glistening structures attached to the sinus mucosa by a relatively narrow stalk or pedicle, occurring in patients with allergic or vasomotor rhinitis. The mechanism(s) of their formation is not known. They contain mast cells, eosinophils and mononuclear cells in large numbers and cause nasal obstruction, loss of smell and taste, and mouth breathing, but rarely sneezing, since the mucosa of the polyp is largely denervated.
Pathogenesis
Sneezing, increased secretion and changes in mucosal blood flow are mediated both by efferent nerve fibres and by released mediators (see p. 827). Mucus production results largely from parasympathetic stimulation. Blood vessels are under both sympathetic and parasympathetic control. Sympathetic fibres maintain tonic contraction of blood vessels, keeping the sinusoids of the nose partially constricted with good nasal patency. Stimulation of the parasympathetic system dilates these blood vessels. This stimulation varies spontaneously in a cyclical fashion so that air intake alternates slowly over several hours from one nostril to the other. The erectile cavernous nasal sinusoids can be influenced by emotion, which, in turn, can affect nasal patency.
B cells produce IgE antibody against the allergen. IgE binds to mast cells via high affinity cell surface receptors, causing degranulation and release of histamine, proteases (tryptase, chymase), prostaglandins (PGDs), cysteinyl leukotrienes (LTC4, LTD4, LTE4), and cytokines. This causes the acute symptoms of sneezing, itch, rhinorrhoea and nasal congestion. Sneezing results from stimulation of afferent nerve endings (mostly via histamine) and begins within minutes of the allergen entering the nose. This is followed by nasal exudation and secretion and eventually nasal blockage peaking 15–20 minutes after contact with the allergen. These latter symptoms are driven by increased epithelial permeability, mostly due to histamine.
Additionally, allergens are also presented to T cells via antigen presenting cells (dendritic cells). This causes a release of IL-4 and IL-13 which further stimulate the B cells and also IL-5, IL-9 and GM-CSF, switching from a Th1 to a Th2 response to activate and recruit eosinophils, basophils, neutrophils and T lymphocytes. These cause chronic swelling and irritation, leading to nasal obstruction, hyper-reactivity and anosmia.
Investigations and diagnosis
The allergic factors causing rhinitis can usually be identified from the history. Skin-prick testing is used to support the history. A positive test does not necessarily mean that the particular allergen producing the wheal causes the respiratory disease. However, if there is a compatible clinical history, a causative role is likely. Allergen-specific IgE antibodies can be measured in blood but such tests are much more expensive than skin tests and should only be used in patients who cannot be skin tested for some reason (e.g. dermatographism, active eczema or inability to stop antihistamines for 3 days before skin tests).
Treatment
Allergen avoidance
Removal of a household pet or total enclosure of industrial processes releasing sensitizing agents can lead to cure of rhinitis and, indeed, asthma.
Pollen avoidance is impossible. Contact may be diminished by wearing sunglasses, driving with the car windows shut, avoiding walks in the countryside (particularly in the late afternoon when the number of pollen grains is highest at ground level), and keeping the bedroom window shut at night. These measures are rarely sufficient in themselves to control symptoms. Exposure to pollen is generally lower in coastal regions, where sea breezes keep pollen grains inland.
The house-dust mite infests most areas of the house, but particularly the bedroom. Mite allergen exposure can be reduced by enclosing bedding in fabric specifically designed to prevent the passage of mite allergen, while allowing water vapour through. This is both comfortable and reduces symptoms. Acaricides are less effective and cannot be recommended. Increased room ventilation and reduced soft furnishings including carpets, curtains and soft toys are all helpful in reducing the mite load. However, a meta-analysis has questioned the value of house-dust mite control measures in asthma (p. 829).
H1 antihistamines
Antihistamines remain the most common therapy for rhinitis, and many can be purchased directly over the counter in the UK. They are particularly effective against sneezing and itching of the eyes and palate, but are less effective against rhinorrhoea and have little influence on nasal blockage. First-generation antihistamines (chlorphenamine, hydroxyzine) cause sedation and loss of concentration in all patients (including those who are not aware of the problem) and should no longer be used. Second-generation drugs such as loratadine (10 mg once daily), desloratadine (5 mg daily), cetirizine (10 mg daily), and fexofenadine (120 mg daily) are at least as potent and they do not cause sedation.
Decongestants
Drugs with sympathomimetic activity (α-adrenergic agents) are widely used to treat nasal obstruction. They may be taken orally or more commonly as nasal drops or sprays (e.g. ephedrine nasal drops). Xylometazoline and oxymetazoline are widely used because they have a prolonged action and tachyphylaxis does not develop. Secondary nasal hyperaemia can occur some hours later as a rebound effect and rhinitis medicamentosa can develop if patients take increasing quantities of decongestant to overcome this phenomenon. Although local decongestants are an effective treatment for vasomotor rhinitis, patients must be warned about rebound nasal obstruction and should use the drugs carefully. Usually, such preparations should be prescribed for only a limited period to open the nasal airways and allow better access for other local therapy, such as topical corticosteroids.
Anti-inflammatory drugs
Sodium cromoglycate and nedocromil sodium act by blocking an intracellular chloride channel and influence mast cell and eosinophil activation and nerve function. Topical sodium cromoglycate and nedocromil sodium can be very effective in allergic conjunctivitis but are of limited value in allergic rhinitis.
Corticosteroids
The most effective treatment for rhinitis is a topical corticosteroid preparation (e.g. beclometasone, fluticasone propionate, fluticasone furoate or mometasone furoate spray). Topical steroids should be started before the beginning of seasonal symptoms. The combination of a topical corticosteroid with a non-sedating antihistamine taken regularly is particularly effective. Patients should be carefully instructed in how to use the nasal steroid device to achieve optimal drug deposition. In selected cases, an α-adrenergic agonist may help to decongest the nose prior to taking the topical corticosteroid. Patients often worry about possible side-effects: nasal steroids can cause epistaxis, but the amount used is insufficient to cause systemic effects.
If other therapy has failed, seasonal and perennial rhinitis respond readily to a short course (max. 2 weeks) of treatment with oral prednisolone 5–10 mg daily. Nasal polyps may respond to oral corticosteroids and their recurrence may be prevented by continuous use of topical corticosteroids.
Immunotherapy
This is used for patients with seasonal allergic rhinitis who have not responded to the above. Both oral and injectable vaccines are available (p. 69). Other forms of desensitizing vaccines are under development.
Pharyngitis
The most common viruses causing pharyngitis are adenoviruses, of which there are about 32 serotypes. Endemic adenovirus infection causes the common sore throat, in which the oropharynx and soft palate are reddened and the tonsils are inflamed and swollen. Within 1–2 days the tonsillar lymph nodes enlarge. Occasionally, localized epidemics due to adenovirus serotype 8 occur, particularly in schools during summer, with episodes of fever, conjunctivitis, pharyngitis and lymphadenitis of the neck glands. The disease is self-limiting and only requires symptomatic treatment without antibiotics.
Over several decades, the proportion of sore throats due to bacterial infections, e.g. haemolytic streptococcus, has fallen. Many different pathogens have been implicated in pharyngitis but most do not require specific treatment. Persistent and severe tonsillitis should be treated with phenoxymethylpenicillin 500 mg four times a day or cefaclor 250 mg three times daily. Amoxicillin and ampicillin should be avoided if there is a possibility of infectious mononucleosis (p. 100).
Acute laryngotracheobronchitis
Acute laryngitis is an occasional but striking complication of upper respiratory tract infections, particularly those caused by parainfluenza viruses and measles. The condition is most severe in children under the age of 3 years. Inflammatory oedema extends to the vocal cords and the epiglottis, causing narrowing of the airway; there may be associated tracheitis or tracheobronchitis. The voice becomes hoarse, with a barking cough (croup) and there is audible stridor. Progressive airways obstruction may occur, with recession of the soft tissue of the neck and abdomen during inspiration and, in severe cases, central cyanosis. Steam inhalations are not helpful. Nebulized adrenaline (epinephrine) gives short-term relief. Oral or i.m. corticosteroids (e.g. dexamethasone) should be given with oxygen and adequate fluids. If steroids are used, endotracheal intubation is rarely necessary. Rarely, a tracheostomy is required.
Acute epiglottitis
H. influenzae type b (Hib) can cause life-threatening infection of the epiglottis, usually in children under 5 years of age. The child becomes extremely ill with a high fever, and severe airflow obstruction may rapidly occur. This is a life-threatening emergency and requires urgent endotracheal intubation and intravenous antibiotics (e.g. ceftazidime 25–150 mg/kg). Chloramphenicol (50–100 mg/kg) can also be used. The epiglottis, which is red and swollen, should not be inspected until facilities to maintain the airways are available.
Other manifestations of Hib infection are meningitis, septic arthritis and osteomyelitis. A highly effective vaccine is now available, which is given to infants at 2, 3 and 4 months, with their primary immunizations against diphtheria, tetanus and pertussis (DTP). This programme has reduced death rates from Hib infections virtually to zero in many countries.
Influenza (see also p. 108)
The influenza virus belongs to the orthomyxovirus group and exists in two main forms, A and B. Influenza B is associated with localized outbreaks of mild disease, whereas influenza A causes worldwide pandemics (see p. 108).
Clinical features
The incubation period of influenza is usually 1–3 days. The illness starts abruptly with a fever, shivering and generalized aching in the limbs. This is associated with severe headache, soreness of the throat and a dry cough that can persist for several weeks. Diarrhoea occurs in 70% of cases of H5N1. Influenza infection can be followed by a prolonged period of debility and depression that may take weeks or months to clear.
Complications
Secondary bacterial infection is common following influenza virus infection, particularly with Strep. pneumoniae and H. influenzae. Secondary pneumonia caused by Staph. aureus is rarer, but more serious, and carries a mortality of up to 20%. Post-infectious encephalomyelitis is rare after influenza infection.
Diagnosis and treatment
Laboratory diagnosis is not usually necessary, but a definitive diagnosis can be established by demonstrating a four-fold increase in complement-fixing antibody or the haemagglutinin antibody measured at onset and after 1–2 weeks or by demonstrating the virus in throat or nasal secretions.
Treatment is by bed rest and paracetamol, with antibiotics to prevent secondary infection in those with chronic bronchitis, cardiac or renal disease.
Neuraminidase inhibitors help to shorten the duration of symptoms in patients with influenza, if given within 48 h of the first symptom. The cost-benefit of zanamivir and oseltamivir remains unproven but these are currently recommended in the UK for patients with suspected influenza over the age of 65 and ‘at-risk’ adults, as part of a strategy to reduce admissions to hospital when influenza is circulating in the community.
Prophylaxis
Protection by influenza vaccines is only effective in about 70% of people and only lasts for about a year. Influenza vaccine should not be given to individuals who are allergic to egg protein as some are manufactured in chick embryos. New vaccines have to be prepared to cover each change in viral antigenicity and are therefore in limited supply at the start of an epidemic. Nevertheless, routine vaccination is recommended for all individuals over 65 years of age and also for younger people with chronic heart disease, chronic lung disease (including asthma), chronic kidney disease, diabetes mellitus and those who are immunosuppressed. During pandemics, key hospital and health service personnel should also be vaccinated.
Inhalation of foreign bodies
Children inhale foreign bodies, e.g. peanuts, more commonly than do adults. In adults, inhalation may occur after excess alcohol or under general anaesthesia (loose teeth or dentures).
When the foreign body is large it may impact in the trachea. The person chokes and then becomes silent; death ensues unless the material is quickly removed (see Emergency Box 15.1).
![]() Emergency Box 15.1
Emergency Box 15.1
Treatment of inhaled foreign bodies (Heimlich manoeuvre)
More often, impaction occurs in the right main bronchus and produces:
Diseases of the lower respiratory tract
Lower respiratory tract infection accounts for approximately 10% of the worldwide burden of morbidity and mortality. Some 75% of all antibiotic usage is for these diseases, despite the fact that they are mainly due to viruses.
Acute bronchitis
Acute bronchitis in previously healthy subjects is often viral. Bacterial infection with Strep. pneumoniae or H. influenzae is a common sequel to viral infections, and is more likely to occur in cigarette smokers or people with chronic obstructive pulmonary disease (COPD).
The illness begins with an irritating, non-productive cough, together with discomfort behind the sternum. There may be associated chest tightness, wheezing and shortness of breath. Later the cough becomes productive, with yellow or green sputum. There is a mild fever and a neutrophil leucocytosis; wheeze with occasional crackles can be heard on auscultation. In otherwise healthy adults the disease improves spontaneously in 4–8 days without the patient becoming seriously ill.
Antibiotics are often given (e.g. amoxicillin 250 mg three times daily), but it is not known whether this hastens recovery in otherwise healthy individuals.
Chronic obstructive pulmonary disease (COPD)
COPD is predicted to become the third most common cause of death and fifth most common cause of disability worldwide by 2020.
The term COPD was introduced to bring together a variety of clinical syndromes associated with airflow obstruction and destruction of the lung parenchyma. The older terms ‘chronic obstructive airways disease’ and ‘chronic obstructive lung disease’ are synonymous with COPD. Prior to 1979, patients with COPD were often classified by symptoms (chronic bronchitis, chronic asthma), by pathological changes (emphysema) or by physiological correlates (pink puffers, blue bloaters). Recognition that these entities overlapped and often co-existed led to introduction of the term COPD.
COPD is associated with a number of co-morbidities, e.g. ischaemic heart disease, hypertension, diabetes, heart failure and cancer, suggesting that it may be part of a generalized systemic inflammatory process.
Epidemiology and aetiology
COPD is caused by long-term exposure to toxic particles and gases. In developed countries, cigarette smoking accounts for over 90% of cases. In developing countries other factors, such as the inhalation of smoke from biomass fuels used in heating and cooking in poorly ventilated areas, are also implicated. However, only 10–20% of heavy smokers develop COPD, indicating individual susceptibility. The development of COPD is proportional to the number of cigarettes smoked per day; the risk of death from COPD in patients smoking 30 cigarettes daily is 20 times that of a non-smoker. Autopsy studies have shown substantial numbers of centri-acinar emphysematous spaces in the lungs of 50% of British smokers over the age of 60 years independent of whether significant respiratory disease was diagnosed before death.
Climate and air pollution are lesser causes of COPD, but mortality from COPD increases dramatically during periods of heavy atmospheric pollution (p. 341). Urbanization, social class and occupation may also play a part in aetiology, but these effects are difficult to separate from that of smoking. Some animal studies suggest that diet could be a risk factor for COPD, but this has not been proven in humans.
The socioeconomic burden of COPD is considerable. In the UK, COPD causes approximately 18 million lost working days annually for men and 2.1 million lost working days for women, accounting for approximately 7% of all days of absence from work due to sickness. Nevertheless, the number of COPD admissions to UK hospitals has been falling steadily over the last 30 years.
Pathophysiology
The most consistent pathological finding in COPD is increased numbers of mucus-secreting goblet cells in the bronchial mucosa, especially in the larger bronchi (Fig. 15.19). In more advanced cases, the bronchi become overtly inflamed and pus is seen in the lumen.
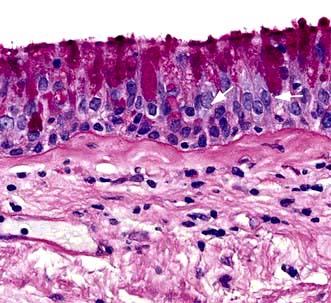
Figure 15.19 COPD. Section of bronchial mucosa stained for mucus glands by PAS showing increase in mucus-secreting goblet cells.
(Courtesy of Dr J Wilson and Dr S Wilson, University of Southampton.)
Microscopically, there is infiltration of the walls of the bronchi and bronchioles with acute and chronic inflammatory cells; lymphoid follicles may develop in severe disease. In contrast to asthma, the lymphocytic infiltrate is predominantly CD8+. The epithelial layer may become ulcerated and, with time, squamous epithelium replaces the columnar cells. The inflammation is followed by scarring and thickening of the walls which narrows the small airways (Fig. 15.20).
The small airways are particularly affected early in the disease, initially without the development of any significant breathlessness. This initial inflammation of the small airways is reversible and accounts for the improvement in airway function if smoking is stopped early. In later stages the inflammation continues, even if smoking is stopped.
Further progression of the airways disease leads to progressive squamous cell metaplasia, and fibrosis of the bronchial walls. The physiological consequence of these changes is the development of airflow limitation. If the airway narrowing is combined with emphysema (causing loss of the elastic recoil of the lung with collapse of small airways during expiration) the resulting airflow limitation is even more severe.
Emphysema is defined as abnormal, permanent enlargement of air spaces distal to the terminal bronchiole, accompanied by destruction of their walls and without obvious fibrosis. Recent evidence suggests that the enlargement of the distal air spaces (i.e. emphysema) is a secondary result of small airway inflammation and destruction. It is classified according to the site of damage:
 Centri-acinar emphysema. Distension and damage of lung tissue is concentrated around the respiratory bronchioles, whilst the more distal alveolar ducts and alveoli tend to be well preserved. This form of emphysema is extremely common; when of modest extent, it is not necessarily associated with disability. Severe centri-acinar emphysema is associated with substantial airflow limitation.
Centri-acinar emphysema. Distension and damage of lung tissue is concentrated around the respiratory bronchioles, whilst the more distal alveolar ducts and alveoli tend to be well preserved. This form of emphysema is extremely common; when of modest extent, it is not necessarily associated with disability. Severe centri-acinar emphysema is associated with substantial airflow limitation.
 Pan-acinar emphysema. This is less common. Distension and destruction affect the whole acinus, and in severe cases the lung is just a collection of bullae. Severe airflow limitation and
Pan-acinar emphysema. This is less common. Distension and destruction affect the whole acinus, and in severe cases the lung is just a collection of bullae. Severe airflow limitation and 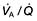 mismatch occur. α1-Antitrypsin deficiency is associated with this type of emphysema (see p. 341).
mismatch occur. α1-Antitrypsin deficiency is associated with this type of emphysema (see p. 341).
 Irregular emphysema. There is scarring and damage affecting the lung parenchyma patchily independent of acinar structure.
Irregular emphysema. There is scarring and damage affecting the lung parenchyma patchily independent of acinar structure.
Emphysema leads to expiratory airflow limitation and air trapping. The loss of lung elastic recoil results in an increase in TLC. Premature closure of airways limits expiratory flow while the loss of alveoli decreases capacity for gas transfer.
 mismatch is partly due to damage and mucus plugging of smaller airways from chronic inflammation, and partly due to rapid closure of smaller airways in expiration owing to loss of elastic support. The mismatch leads to a fall in PaO2 and increased work of respiration.
mismatch is partly due to damage and mucus plugging of smaller airways from chronic inflammation, and partly due to rapid closure of smaller airways in expiration owing to loss of elastic support. The mismatch leads to a fall in PaO2 and increased work of respiration.
CO2 excretion is less affected by  mismatch and many patients have low normal PaCO2 values due to increasing alveolar ventilation in an attempt to correct their hypoxia. Other patients fail to maintain their respiratory effort and then their PaCO2 levels increase. In the short term, this rise in CO2 leads to stimulation of respiration but in the longer term, these patients often become insensitive to CO2 and come to depend on hypoxaemia to drive their ventilation. Such patients appear less breathless and because of renal hypoxia they start to retain fluid and increase erythrocyte production (leading eventually to polycythaemia). In consequence they become bloated, plethoric and cyanosed, the typical appearance of the ‘blue bloater’. Attempts to abolish hypoxaemia by administering oxygen can make the situation much worse by decreasing respiratory drive in these patients who depend on hypoxia to drive their ventilation.
mismatch and many patients have low normal PaCO2 values due to increasing alveolar ventilation in an attempt to correct their hypoxia. Other patients fail to maintain their respiratory effort and then their PaCO2 levels increase. In the short term, this rise in CO2 leads to stimulation of respiration but in the longer term, these patients often become insensitive to CO2 and come to depend on hypoxaemia to drive their ventilation. Such patients appear less breathless and because of renal hypoxia they start to retain fluid and increase erythrocyte production (leading eventually to polycythaemia). In consequence they become bloated, plethoric and cyanosed, the typical appearance of the ‘blue bloater’. Attempts to abolish hypoxaemia by administering oxygen can make the situation much worse by decreasing respiratory drive in these patients who depend on hypoxia to drive their ventilation.
The classic Fletcher and Peto studies (Fig. 15.21) show that there is a loss of 50 mL per year in FEV1 in patients with COPD compared with 20 mL per year in healthy people. A recent study has shown a 40 mL loss per year but only in 38% of the patients studied. Biomarkers to indicate the rate of decline have been unhelpful although CC16 (Clare cell secretory protein 16) was shown to be a reasonable indicator in a study.
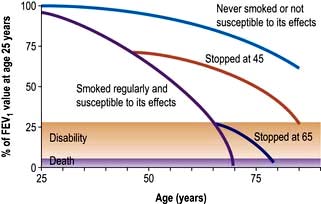
Figure 15.21 Influence of smoking on airflow limitation.
(From Fletcher CM, Peto R. The natural history of chronic airflow abstruction. British Medical Journal 1977; 1:1645.)
In summary, three mechanisms have been suggested for this limitation of airflow in small airways (<2 mm in diameter).
 Loss of elasticity and alveolar attachments of airways due to emphysema. This reduces the elastic recoil and the airways collapse during expiration.
Loss of elasticity and alveolar attachments of airways due to emphysema. This reduces the elastic recoil and the airways collapse during expiration.
 Inflammation and scarring cause the small airways to narrow.
Inflammation and scarring cause the small airways to narrow.
Each of these narrows the small airways and causes air trapping leading to hyperinflation of the lungs,  mismatch, increased work of breathing and breathlessness.
mismatch, increased work of breathing and breathlessness.
Pathogenesis
Cigarette smoking
Bronchoalveolar lavage and biopsies of the airways of smokers show increased numbers of neutrophil granulocytes. These granulocytes can release elastases and proteases, which may help to produce emphysema. It has been suggested that an imbalance between protease and antiprotease activity causes the damage. α1-Antitrypsin is a major serum antiprotease which can be inactivated by cigarette smoke (see below).
Mucous gland hypertrophy in the larger airways is thought to be a direct response to persistent irritation resulting from the inhalation of cigarette smoke. The smoke has an adverse effect on surfactant, favouring overdistension of the lungs.
Infections
Patients with COPD cope badly with respiratory infections, which are often the precipitating cause of acute exacerbations of the disease. However, it is less clear whether infection is responsible for the progressive airflow limitation that characterizes disabling COPD. Prompt use of antibiotics and routine vaccinations against influenza and pneumococci are appropriate.
α1-Antitrypsin deficiency
α1-Antitrypsin (see also p. 341) is a proteinase inhibitor which is produced in the liver, secreted into the blood and diffuses into the lung. Here it inhibits proteolytic enzymes such as neutrophil elastase, which are capable of destroying alveolar wall connective tissue.
More than 75 alleles of the α1-antitrypsin gene have been described. The three main phenotypes are MM (normal), MZ (heterozygous deficiency) and ZZ (homozygous deficiency). About 1 child in 5000 in Britain is born with the homozygous deficiency, but not all develop chest disease. Those who do develop breathlessness under the age of 40 years have radiographic evidence of basal emphysema and are usually, but not always, cigarette smokers. Hereditary deficiency of α1-antitrypsin accounts for about 2% of emphysema cases. A small minority develop liver disease (see p. 341).
Clinical features
Symptoms
The characteristic symptoms of COPD are productive cough with white or clear sputum, wheeze and breathlessness, usually following many years of a smoker’s cough. Colds seem to ‘settle on the chest’ and frequent infective exacerbations occur, with purulent sputum. Symptoms can be worsened by cold, foggy weather and atmospheric pollution. With advanced disease, breathlessness is severe even after mild exercise such as getting dressed. Apart from the pulmonary features, there are systemic effects including hypertension, osteoporosis, depression, weight loss and reduced muscle mass with general weakness.
Signs
In mild COPD, there may be no signs or just quiet wheezes throughout the chest. In severe disease, the patient is tachypnoeic, with prolonged expiration. The accessory muscles of respiration are used and there may be intercostal indrawing on inspiration and pursing of the lips on expiration (see p. 792). Chest expansion is poor, the lungs are hyperinflated, and there is loss of the normal cardiac and liver dullness.
Patients who remain responsive to CO2 are usually breathless and rarely cyanosed. Heart failure and oedema are rare features except as terminal events. In contrast, patients who become insensitive to CO2 are often oedematous and cyanosed but not particularly breathless. Those with hypercapnia may have peripheral vasodilatation, a bounding pulse, and when the PaCO2 is above about 10 kPa, a coarse flapping tremor of the outstretched hands. Severe hypercapnia causes confusion and progressive drowsiness. Papilloedema may be present but this is neither specific nor sensitive as a diagnostic feature.
Respiratory failure
The later stages of COPD are characterized by the development of respiratory failure. For practical purposes this is said to occur when there is either a PaO2 <8 kPa (60 mmHg) or a PaCO2 >7 kPa (55 mmHg).
Chronic alveolar hypoxia and hypercapnia leads to constriction of the pulmonary arterioles and pulmonary hypertension. Cardiac output is normal or increased but salt and fluid retention occurs as a result of renal hypoxia.
Pulmonary hypertension (cor pulmonale)
Patients with advanced COPD may develop cor pulmonale, which is defined as symptoms and signs of fluid overload secondary to lung disease. The fluid retention and peripheral oedema is due to failure of excretion of sodium and water by the hypoxic kidney rather than heart failure. It is characterized by pulmonary hypertension and right ventricular hypertrophy. On examination, the patient is centrally cyanosed (owing to the lung disease) and later becomes more breathless and develops ankle oedema. Initially there may be a prominent parasternal heave, due to right ventricular hypertrophy, and a loud pulmonary second sound. In very severe pulmonary hypertension, the pulmonary valve becomes incompetent. With severe fluid overload, tricuspid incompetence may develop with a greatly elevated jugular venous pressure (JVP), ascites and upper abdominal discomfort due to liver swelling.
Diagnosis
This is usually clinical (Table 15.9) and based on a history of breathlessness and sputum production in a chronic smoker. In the absence of a history of cigarette smoking asthma is a more likely explanation unless there is a family history suggesting α1-antitrypsin deficiency.
Table 15.9 Classification of severity of Airflow limitation in COPD (2011)
| Gold stage | FEV1 / FVC | % Predicted |
|---|---|---|
1. Mild |
<70% |
≥80% |
2. Moderate |
<70% |
<80% |
3. Severe |
<70% |
<50% |
4. Very severe |
<70% |
<30% |
Modified from Global Institute for Chronic Obstructive Lung Disease, 2011. www.goldcopd.com.
No individual clinical feature is diagnostic. The patient may have signs of hyperinflation and typical pursed lip respiration. There may be signs of overinflation of the lungs (e.g. loss of liver dullness on percussion), but this also occurs in other diseases such as asthma. Conversely, centri-acinar emphysema may be present without signs of overinflation. The chest may become ‘barrel-shaped’ but this can also result from osteoporosis of the spine in older men without emphysema.
Investigations
 Lung function tests show evidence of airflow limitation (see Figs 15.7 and 15.12). The FEV1: FVC ratio is reduced and the PEFR is low. In many patients the airflow limitation is partly reversible (usually a change in FEV1 of <15%), and it can be difficult to distinguish between COPD and asthma. Lung volumes may be normal or increased; carbon monoxide gas transfer factor is low when significant emphysema is present.
Lung function tests show evidence of airflow limitation (see Figs 15.7 and 15.12). The FEV1: FVC ratio is reduced and the PEFR is low. In many patients the airflow limitation is partly reversible (usually a change in FEV1 of <15%), and it can be difficult to distinguish between COPD and asthma. Lung volumes may be normal or increased; carbon monoxide gas transfer factor is low when significant emphysema is present.
 Chest X-ray is often normal, even when the disease is advanced. The classic features are overinflation of the lungs with low, flattened diaphragms, and sometimes the presence of large bullae. Blood vessels may be ‘pruned’ with large proximal vessels and relatively little blood visible in the peripheral lung fields.
Chest X-ray is often normal, even when the disease is advanced. The classic features are overinflation of the lungs with low, flattened diaphragms, and sometimes the presence of large bullae. Blood vessels may be ‘pruned’ with large proximal vessels and relatively little blood visible in the peripheral lung fields.
 High-resolution CT scans are useful, particularly when the plain chest X-ray is normal.
High-resolution CT scans are useful, particularly when the plain chest X-ray is normal.
 Haemoglobin level and PCV can be elevated as a result of persistent hypoxaemia (secondary polycythaemia, see p. 404).
Haemoglobin level and PCV can be elevated as a result of persistent hypoxaemia (secondary polycythaemia, see p. 404).
 Blood gases are often normal at rest, but patients desaturate on exercise. In more advanced cases there is resting hypoxaemia and there may also be hypercapnia.
Blood gases are often normal at rest, but patients desaturate on exercise. In more advanced cases there is resting hypoxaemia and there may also be hypercapnia.
 Sputum examination is not useful in ordinary cases. Strep. pneumoniae and H. influenzae are the only common organisms to produce acute exacerbations. Occasionally, Moraxella catarrhalis may cause infective exacerbations.
Sputum examination is not useful in ordinary cases. Strep. pneumoniae and H. influenzae are the only common organisms to produce acute exacerbations. Occasionally, Moraxella catarrhalis may cause infective exacerbations.
 Electrocardiogram is often normal. In advanced pulmonary hypotension the P wave is tall (P pulmonale) and there may be right bundle branch block and evidence of right ventricular hypertrophy (see p. 764).
Electrocardiogram is often normal. In advanced pulmonary hypotension the P wave is tall (P pulmonale) and there may be right bundle branch block and evidence of right ventricular hypertrophy (see p. 764).
 Echocardiogram is useful to assess cardiac function where there is disproportionate dyspnoea.
Echocardiogram is useful to assess cardiac function where there is disproportionate dyspnoea.
 α1-Antitrypsin levels and genotype are worth measuring in premature disease or lifelong non-smokers.
α1-Antitrypsin levels and genotype are worth measuring in premature disease or lifelong non-smokers.
FURTHER READING
Barnes PJ. Small airways in COPD. N Engl J Med 2004; 350:2635–2637.
Niewoehner DE. Outpatient management of severe COPD. N Engl J Med 2012; 362:1407–1416.
Qaseem A, Snow V, Shekelle P et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A practice guideline from the American College of Physicians. Ann Intern Med 2007; 147:633–638.
Management
See Figure 15.22 for management strategies.
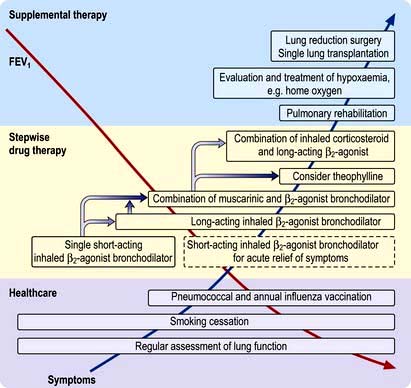
Figure 15.22 Algorithm for the treatment of COPD. The various components of management are shown as the FEV1 decreases and the symptoms become more severe.
(After Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 2004; 350:2689–2697.
Smoking cessation
The single most useful measure is to persuade the patient to stop smoking. Even in advanced disease this may slow down the rate of deterioration and prolong the time before disability and death occur (see Fig. 15.22). Smoke from burning biomass fuels in poorly ventilated homes should also be reduced or get to non-smoking facilities if possible.
Drug therapy
This is used both for the short-term management of exacerbations and for the long-term relief of symptoms. Many of the drugs used are similar to those used in asthma (see p. 829).
Bronchodilators
 β-Adrenergic agonists. Many patients with mild COPD feel less breathless after inhaling a β-adrenergic agonist such as salbutamol (200 µg every 4–6 hours). In more severe airway limitation (moderate and severe COPD), a long-acting β2 agonist should be used, e.g. formoterol 12 µg powder inhaled twice daily or salmeterol 50 µg twice daily. Indacaterol 150–300 µg daily is also effective.
β-Adrenergic agonists. Many patients with mild COPD feel less breathless after inhaling a β-adrenergic agonist such as salbutamol (200 µg every 4–6 hours). In more severe airway limitation (moderate and severe COPD), a long-acting β2 agonist should be used, e.g. formoterol 12 µg powder inhaled twice daily or salmeterol 50 µg twice daily. Indacaterol 150–300 µg daily is also effective.
 Antimuscarinic drugs. More prolonged and greater bronchodilatation is achieved with antimuscarinic agents: tiotropium (long-acting) (18 µg daily), ipratropium (40 µg four times daily) or oxitropium (200 µg twice daily). Tiotropium improves function and quality of life but does not affect the decline in FEV1. Compound bronchodilators, a selective β2 agonist and an antimuscarinic agent, are used. If patients find inhalers difficult to use, spacer devices improve delivery. Objective evidence of improvement in peak flow rate or FEV1 may be small and decisions to continue or stop therapy are based mainly on the patient’s reported symptoms.
Antimuscarinic drugs. More prolonged and greater bronchodilatation is achieved with antimuscarinic agents: tiotropium (long-acting) (18 µg daily), ipratropium (40 µg four times daily) or oxitropium (200 µg twice daily). Tiotropium improves function and quality of life but does not affect the decline in FEV1. Compound bronchodilators, a selective β2 agonist and an antimuscarinic agent, are used. If patients find inhalers difficult to use, spacer devices improve delivery. Objective evidence of improvement in peak flow rate or FEV1 may be small and decisions to continue or stop therapy are based mainly on the patient’s reported symptoms.
 Theophyllines. Long-acting preparations of theophylline are of little benefit in COPD.
Theophyllines. Long-acting preparations of theophylline are of little benefit in COPD.
Phosphodiesterase type 4 inhibitors
Roflumilast is an inhibitor with anti-inflammatory properties. It is used as an adjunct to bronchodilators for the maintenance treatment of COPD patients.
Corticosteroids
In symptomatic patients with moderate/severe COPD, a trial of corticosteroids is always indicated, since a proportion of patients have a large, unsuspected, reversible element to their disease and airway function may improve considerably. Prednisolone 30 mg daily should be given for 2 weeks, with measurements of lung function before and after the treatment period. If there is objective evidence of a substantial degree of improvement in airflow limitation (FEV1 increase >15%), prednisolone should be discontinued and replaced by inhaled corticosteroids (beclometasone 40 µg twice daily in the first instance, adjusted according to response). The long-term value of regular inhaled corticosteroids in all patients with COPD has not been proven.
Combination of a corticosteroid with a long-acting β2 agonist may protect against lung function decline but does not improve overall mortality. High-dose inhaled steroids are not advised as they cause increased rates of respiratory infection
Antibiotics
Prompt antibiotic treatment shortens exacerbations and should always be given in acute episodes as it may prevent hospital admission and further lung damage. Patients can be given antibiotics to keep at home to start as soon as their sputum turns yellow or green. Although amoxicillin-resistant H. influenzae is increasing worldwide (occurring in about 20% of isolates from sputum) it is not a serious clinical problem. Resistance to cefaclor (500 mg 8-hourly) or cefixime (400 mg once daily) is significantly less frequent; co-amoxiclav is a useful alternative.
Long-term treatment with antibiotics remains controversial. They were once thought to be of no value, but eradication of infection and keeping the lower respiratory tract free of bacteria may help to prevent deterioration in lung function.
Antimucolytic agents
These reduce sputum viscosity and can reduce the number of acute exacerbations. A 4-week trial of carbocysteine 2.25 g daily can be tried.
Diuretic therapy (see p. 644)
This is necessary for all oedematous patients. Daily weights should be recorded during acute inpatient episodes.
Oxygen therapy
Two controlled trials (chiefly in males) have shown improved survival with the continuous administration of oxygen at 2 L/min via nasal prongs to achieve an oxygen saturation of greater than 90% for large proportions of the day and night. Survival curves from these two studies are shown in Figure 15.23.
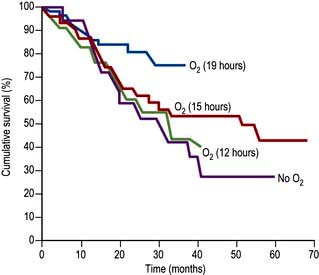
Figure 15.23 Cumulative survival curves for patients receiving oxygen. Oxygen doses are in hours per day.
Only 30% of those not receiving long-term oxygen therapy survived for more than 5 years. A fall in pulmonary artery pressure was achieved if oxygen was given for 15 hours daily, but substantial improvement in mortality was only achieved by the administration of oxygen for 19 hours daily. These results suggest that long-term continuous domiciliary oxygen therapy will benefit patients who have:
 PaO2 of <7.3 kPa (55 mmHg) when breathing air. Measurements should be taken on two occasions at least 3 weeks apart after appropriate bronchodilator therapy (Box 15.1).
PaO2 of <7.3 kPa (55 mmHg) when breathing air. Measurements should be taken on two occasions at least 3 weeks apart after appropriate bronchodilator therapy (Box 15.1).
 PaO2 7.3–8 kPa with secondary polycythaemia, nocturnal hypoxaemia, peripheral oedema or evidence of pulmonary hypertension.
PaO2 7.3–8 kPa with secondary polycythaemia, nocturnal hypoxaemia, peripheral oedema or evidence of pulmonary hypertension.
 Carboxyhaemoglobin of <3% (i.e. patients who have stopped smoking).
Carboxyhaemoglobin of <3% (i.e. patients who have stopped smoking).
![]() Box 15.1
Box 15.1
Guidelines for domiciliary oxygen (Royal College of Physicians, 1999)
 Chronic obstructive pulmonary disease with PaO2 <7.3 kPa when breathing air during a period of clinical stability
Chronic obstructive pulmonary disease with PaO2 <7.3 kPa when breathing air during a period of clinical stability
 Chronic obstructive pulmonary disease with PaO2 7.3–8 kPa in the presence of secondary polycythaemia, nocturnal hypoxaemia, peripheral oedema or evidence of pulmonary hypertension
Chronic obstructive pulmonary disease with PaO2 7.3–8 kPa in the presence of secondary polycythaemia, nocturnal hypoxaemia, peripheral oedema or evidence of pulmonary hypertension
 Severe chronic asthma with a PaCO2 <7.3 kPa or persistent disabling breathlessness
Severe chronic asthma with a PaCO2 <7.3 kPa or persistent disabling breathlessness
 Diffuse lung disease with PaO2 <8 kPa and in patients with PaO2 >8 kPa with disabling dyspnoea
Diffuse lung disease with PaO2 <8 kPa and in patients with PaO2 >8 kPa with disabling dyspnoea
 Cystic fibrosis when PaO2 <7.3 kPa or if PaO2 7.3–8 kPa in the presence of secondary polycythaemia, nocturnal hypoxaemia, pulmonary hypertension or peripheral oedema
Cystic fibrosis when PaO2 <7.3 kPa or if PaO2 7.3–8 kPa in the presence of secondary polycythaemia, nocturnal hypoxaemia, pulmonary hypertension or peripheral oedema
 Pulmonary hypertension without parenchymal lung involvement when PaO2 <8 kPa
Pulmonary hypertension without parenchymal lung involvement when PaO2 <8 kPa
 Neuromuscular or skeletal disorders, after specialist assessment
Neuromuscular or skeletal disorders, after specialist assessment
 Obstructive sleep apnoea despite continuous positive airways pressure therapy, after specialist assessment
Obstructive sleep apnoea despite continuous positive airways pressure therapy, after specialist assessment
 Pulmonary malignancy or other terminal disease with disabling dyspnoea
Pulmonary malignancy or other terminal disease with disabling dyspnoea
 Heart failure with daytime PaO2 <7.3 kPa (on air) or with nocturnal hypoxaemia
Heart failure with daytime PaO2 <7.3 kPa (on air) or with nocturnal hypoxaemia
Domiciliary oxygen is best provided by using an oxygen concentrator, which is considerably cheaper than using oxygen cylinders.
Nocturnal hypoxia
COPD patients with severe arterial hypoxaemia may experience profound nocturnal hypoxaemia which may drop the PaO2 as low as 2.5 kPa (19 mmHg), particularly during the rapid eye movement (REM) phase of sleep.
Because patients with COPD are already hypoxic, the fall in PaO2 produces a much larger fall in oxygen saturation (owing to the shape of the oxygen-haemoglobin dissociation curve) and desaturation of up to 50% occurs. The mechanism is alveolar hypoventilation due to:
 Inhibition of intercostal and accessory muscles in REM sleep
Inhibition of intercostal and accessory muscles in REM sleep
 Shallow breathing in REM sleep, which reduces ventilation, particularly in severe COPD
Shallow breathing in REM sleep, which reduces ventilation, particularly in severe COPD
 An increase in upper airway resistance because of a reduction in muscle tone.
An increase in upper airway resistance because of a reduction in muscle tone.
These nocturnal hypoxaemic episodes are associated with a further rise in pulmonary arterial pressure owing to vasoconstriction. Most deaths in patients with COPD occur at night, possibly from cardiac arrhythmias due to hypoxaemia. Secondary polycythaemia may be exacerbated by the severe nocturnal hypoxaemia.
Each episode of desaturation is usually terminated by arousal from sleep, so the amount of normal sleep is reduced and patients suffer from daytime sleepiness.
Treatment. Patients with arterial hypoxaemia should not be given sleeping tablets, as these will further depress respiratory drive. Treatment is with nocturnal administration of oxygen and ventilatory support.
Non-invasive positive-pressure ventilation can be administered with a tightly fitting nasal mask and bilevel positive airway pressure (BiPAP) – inspiratory to provide inspiratory assistance and expiratory to prevent alveolar closure, each adjusted independently. This improves ventilation during sleep and allows respiratory muscles to rest at night. BiPAP helps prevent hypoxaemic damage at night in COPD but it does not improve daytime respiratory function, respiratory muscle strength, exercise tolerance or breathlessness.
Pulmonary rehabilitation
Exercise training can modestly increase exercise capacity with a diminished sense of breathlessness and improved general wellbeing. Regular training periods can be instituted at home; climbing stairs or walking fixed distances can be combined with regular clinic visits for encouragement. Breathing exercises are probably of less value. Quality-of-life can be improved by a multidisciplinary approach involving physiotherapy, exercise and education, although this does not alter life expectancy or the rate of decline in lung function; stopping smoking is still the most useful thing patients can do to help themselves. Nutritional advice, psychological, social and behavioural interventions are also helpful.
Additional measures
 Vaccines. Patients with COPD should receive a single dose of the polyvalent pneumococcal polysaccharide vaccine and yearly influenza vaccinations.
Vaccines. Patients with COPD should receive a single dose of the polyvalent pneumococcal polysaccharide vaccine and yearly influenza vaccinations.
 α1-Antitrypsin replacement. Weekly or monthly infusions of α1-antitrypsin have been recommended for patients with serum levels below 310 mg/L and abnormal lung function. Whether this modifies the long-term progression of the disease remains to be determined.
α1-Antitrypsin replacement. Weekly or monthly infusions of α1-antitrypsin have been recommended for patients with serum levels below 310 mg/L and abnormal lung function. Whether this modifies the long-term progression of the disease remains to be determined.
 Heart failure should be treated (p. 718).
Heart failure should be treated (p. 718).
 Secondary polycythaemia requires venesection if the PCV is >55%.
Secondary polycythaemia requires venesection if the PCV is >55%.
 Pulmonary hypertension can be partially relieved by the use of oral β-adrenergic agonists such as salbutamol (4 mg three times daily), but the long-term value is unknown.
Pulmonary hypertension can be partially relieved by the use of oral β-adrenergic agonists such as salbutamol (4 mg three times daily), but the long-term value is unknown.
 The sensation of breathlessness can be reduced by either promethazine 125 mg daily or dihydrocodeine 1 mg/kg by mouth. Although opiates are the most effective treatment for intractable breathlessness they depress ventilation and carry the risk of increasing respiratory failure.
The sensation of breathlessness can be reduced by either promethazine 125 mg daily or dihydrocodeine 1 mg/kg by mouth. Although opiates are the most effective treatment for intractable breathlessness they depress ventilation and carry the risk of increasing respiratory failure.
 Antileukotriene agents have been tried but are rarely effective (p. 831).
Antileukotriene agents have been tried but are rarely effective (p. 831).
 Air travel. Commercial aircraft are pressurized to the equivalent of 2000–2400 m altitude. In healthy people, this causes PaO2 to fall from 13.5 to 10 kPa, causing a trivial 3% drop in oxygen saturation, but patients with moderate COPD may desaturate significantly. The desaturation associated with air travel can be simulated by breathing 15% oxygen at sea level. Patients whose saturation drops below 85% within 15 minutes should be advised to contact their airline to request supplemental oxygen during flight.
Air travel. Commercial aircraft are pressurized to the equivalent of 2000–2400 m altitude. In healthy people, this causes PaO2 to fall from 13.5 to 10 kPa, causing a trivial 3% drop in oxygen saturation, but patients with moderate COPD may desaturate significantly. The desaturation associated with air travel can be simulated by breathing 15% oxygen at sea level. Patients whose saturation drops below 85% within 15 minutes should be advised to contact their airline to request supplemental oxygen during flight.
 Surgery. Some patients have large emphysematous bullae which reduce lung capacity. Surgical bullectomy can enable adjacent areas of collapsed lung to re-expand and start functioning again. In addition, carefully selected patients with severe COPD (FEV1 <1 L) have been treated with lung volume reduction surgery. This increases elastic recoil, which reduces the expiratory collapse of the airway and decreases expiratory airflow limitation. It also enables the diaphragm to work at a better mechanical advantage. Initial studies suggested that ventilation was improved and patients felt less breathless, although mortality was unchanged. However, a controlled trial in severe emphysema found increased mortality and no improvement in the patients’ condition. Single lung transplantation (see p. 822) is used for end-stage emphysema, with 3-year survival rates of 75%. The principal benefit is improved quality of life but it does not extend survival.
Surgery. Some patients have large emphysematous bullae which reduce lung capacity. Surgical bullectomy can enable adjacent areas of collapsed lung to re-expand and start functioning again. In addition, carefully selected patients with severe COPD (FEV1 <1 L) have been treated with lung volume reduction surgery. This increases elastic recoil, which reduces the expiratory collapse of the airway and decreases expiratory airflow limitation. It also enables the diaphragm to work at a better mechanical advantage. Initial studies suggested that ventilation was improved and patients felt less breathless, although mortality was unchanged. However, a controlled trial in severe emphysema found increased mortality and no improvement in the patients’ condition. Single lung transplantation (see p. 822) is used for end-stage emphysema, with 3-year survival rates of 75%. The principal benefit is improved quality of life but it does not extend survival.
Acute respiratory failure in COPD
 Oxygen therapy. COPD is by far the commonest cause of respiratory failure (Fig. 15.24). In managing respiratory failure the main goal is to improve the PaO2 by continuous oxygen therapy. In type II respiratory failure the PaCO2 is elevated and the patient is dependent on hypoxic drive. In this setting, giving additional oxygen will nearly always cause a further rise in PaCO2. Small increases in PaCO2 can be tolerated but the pH should not be allowed to fall below 7.25; if it does, increased ventilation must be achieved either by artificial ventilation or by using a respiratory stimulant. In COPD exacerbations, a fixed-percentage mask (Venturi mask; Fig. 15.25) is used to deliver controlled concentrations of oxygen. Initially, 24% oxygen is given, and the concentration of inspired oxygen can be gradually increased provided the PaCO2 does not rise unacceptably.
Oxygen therapy. COPD is by far the commonest cause of respiratory failure (Fig. 15.24). In managing respiratory failure the main goal is to improve the PaO2 by continuous oxygen therapy. In type II respiratory failure the PaCO2 is elevated and the patient is dependent on hypoxic drive. In this setting, giving additional oxygen will nearly always cause a further rise in PaCO2. Small increases in PaCO2 can be tolerated but the pH should not be allowed to fall below 7.25; if it does, increased ventilation must be achieved either by artificial ventilation or by using a respiratory stimulant. In COPD exacerbations, a fixed-percentage mask (Venturi mask; Fig. 15.25) is used to deliver controlled concentrations of oxygen. Initially, 24% oxygen is given, and the concentration of inspired oxygen can be gradually increased provided the PaCO2 does not rise unacceptably.
 Removal of retained secretions. Patients should be encouraged to cough up secretions. Physiotherapy is helpful. If this fails, secretions can be aspirated by bronchoscopy or via an endotracheal tube
Removal of retained secretions. Patients should be encouraged to cough up secretions. Physiotherapy is helpful. If this fails, secretions can be aspirated by bronchoscopy or via an endotracheal tube
 Respiratory support (see p. 895). Non-invasive ventilatory techniques can be very helpful in avoiding the need for endotracheal intubation. The best current technique uses tight-fitting facial masks to deliver bilevel positive airway pressure ventilatory support (BiPAP). Assisted ventilation with an endotracheal tube is occasionally used for patients with COPD with severe respiratory failure but only when there is a clear precipitating factor and the overall prognosis is reasonable. Assessing the likelihood of reversibility in an acute setting can present a difficult ethical problem.
Respiratory support (see p. 895). Non-invasive ventilatory techniques can be very helpful in avoiding the need for endotracheal intubation. The best current technique uses tight-fitting facial masks to deliver bilevel positive airway pressure ventilatory support (BiPAP). Assisted ventilation with an endotracheal tube is occasionally used for patients with COPD with severe respiratory failure but only when there is a clear precipitating factor and the overall prognosis is reasonable. Assessing the likelihood of reversibility in an acute setting can present a difficult ethical problem.
 Respiratory stimulants. Respiratory stimulants such as doxapram were widely used in the past, but have fallen out of favour due to improvements in non-invasive ventilation.
Respiratory stimulants. Respiratory stimulants such as doxapram were widely used in the past, but have fallen out of favour due to improvements in non-invasive ventilation.
 Corticosteroids, antibiotics and bronchodilators should be administered in the acute phase of respiratory failure but decisions on long-term use should wait until the patient has recovered (see above).
Corticosteroids, antibiotics and bronchodilators should be administered in the acute phase of respiratory failure but decisions on long-term use should wait until the patient has recovered (see above).
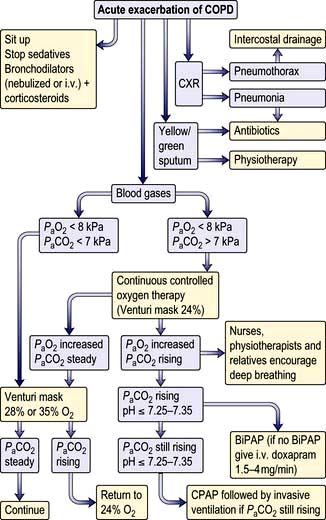
Figure 15.24 Algorithm for the treatment of respiratory failure in COPD. LVF, left ventricular failure; CPAP, continuous positive airway pressure; BiPAP, bilevel positive airway pressure; CXR, chest X-ray.
Figure 15.25 ‘Fixed-performance’ device for administration of oxygen to spontaneously breathing patients (Venturi mask). Oxygen is delivered through the injector of the Venturi mask at a given flow rate. A fixed amount of air is entrapped and the inspired oxygen can be predicted accurately. Masks are available to deliver 24%, 28% and 35% oxygen.
Prognosis of COPD
Predictors of a poor prognosis are increasing age and worsening airflow limitation, i.e. decreasing FEV1. A predictive index (BODE: Body mass index, degree of airflow Obstruction, Dyspnoea and Exercise capacity) is shown in Box 15.2.
![]() Box 15.2
Box 15.2
BODE index (body mass index, degree of airflow obstruction, dyspnoea and exercise capacity)
Scores on the modified Medical Research Council (MMRC) dyspnoea scale range from 0 to 4, with a score of 4 indicating that the patient is breathless when dressing.
A patient with a BODE index of 0–2 has a 4-year mortality rate of 10%, compared with an 80% rate in one with a BODE index of 7–10.
Obstructive sleep apnoea
OSA affects 1–2% of the population and occurs most often in overweight middle-aged men. It can occur in children, particularly those with enlarged tonsils. The major symptoms and their frequency are listed in Table 15.10. During sleep, activity of the respiratory muscles is reduced, especially during REM sleep when the diaphragm is virtually the only active muscle. Apnoeas occur when the airway at the back of the throat is sucked closed when breathing in during sleep. When awake, this tendency is overcome by the action of opening muscles of the upper airway, the genioglossus and palatal muscles, but these become hypotonic during sleep (Fig. 15.26). Partial narrowing results in snoring, complete occlusion causes apnoea and critical narrowing causes hypopnoeas. Apnoea leads to hypoxia and increasingly strenuous respiratory efforts until the patient overcomes the resistance. The combination of the central hypoxic stimulation and the effort to overcome obstruction wakes the patient from sleep. These awakenings are so brief that the patient remains unaware of them but may be woken hundreds of times per night leading to sleep deprivation, especially a reduction in REM sleep, with consequent daytime sleepiness and impaired intellectual performance. Contributory factors are obesity, narrow pharyngeal opening and co-existent COPD.
Table 15.10 Symptoms of obstructive sleep apnoea
| (%) | |
|---|---|
Loud snoring |
95 |
Daytime sleepiness |
90 |
Unrefreshed sleep |
40 |
Restless sleep |
40 |
Morning headache |
30 |
Nocturnal choking |
30 |
Reduced libido |
20 |
Morning drunkenness |
5 |
Ankle swelling |
5 |
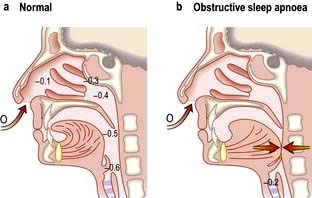
Figure 15.26 Section through head, showing pressure changes (in kPa) in (a) the normal situation and (b) obstructive sleep apnoea. There is a pressure drop during inspiration as air is sucked through the turbinates. In patients with obstructive sleep apnoea this is sufficient to collapse the pharynx, obstructing inspiration.
Correctable factors occur in about one-third of cases and include:
 Encroachment on pharynx – obesity, acromegaly, enlarged tonsils
Encroachment on pharynx – obesity, acromegaly, enlarged tonsils
 Nasal obstruction – nasal deformities, rhinitis, polyps, adenoids
Nasal obstruction – nasal deformities, rhinitis, polyps, adenoids
 Respiratory depressant drugs – alcohol, sedatives, strong analgesics.
Respiratory depressant drugs – alcohol, sedatives, strong analgesics.
Diagnosis
Relatives often provide a good history of the snore-silence-snore cycle. The Epworth Sleepiness Scale (Table 15.11) helps discriminate OSA from simple snoring. The diagnosis is supported by overnight pulse oximetry performed at home. Characteristically, oxygen saturation falls in a cyclical manner giving a sawtooth appearance to the tracing. If oximetry is negative or equivocal, inpatient assessment with oximetry and video-recording is indicated, preferably in a room specifically adapted for sleep studies. Full polysomnographic studies are rarely needed for clinical diagnosis but are useful in research labs. These involve oximetry, direct measurements of thoracic and abdominal movement to assess breathing, and electroencephalography to record patterns of sleep and arousal. Some centres also measure oronasal airflow.
Table 15.11 Epworth sleepiness scale
How likely are you to doze off or fall asleep in the following situations, in contrast to just feeling tired? This refers to your usual way of life in recent time. Even if you have not done some of these things recently, try to work out how they would have affected you. Use the following scale to choose the most appropriate number for each situation. |
|
0 = would never doze |
|
1 = slight chance of dozing |
|
2 = moderate chance of dozing |
|
3 = high chance of dozing |
|
Situation |
Chance of dozing |
Sitting and reading |
_______________ |
Watching TV |
_______________ |
Sitting and inactive in a public place (theatre or meeting) |
_______________ |
As a passenger in a car for an hour without a break |
_______________ |
Lying down to rest in the afternoon when circumstances permit |
_______________ |
Sitting and talking to someone |
_______________ |
Sitting quietly after lunch (without alcohol) |
_______________ |
In a car, while stopped for a few minutes in the traffic |
_______________ |
TOTAL |
_______________ |
Normal 5 ± 4 |
|
The diagnosis of sleep apnoea/hypopnoea is confirmed if there are more than 10–15 apnoeas or hypopnoeas in any 1 hour of sleep. There is, however, overlap with central sleep apnoea (see p. 1069).
Management
Management consists of correction of treatable factors (see above) with, if necessary, nasal continuous positive airway pressure (CPAP) delivered by a nasal mask during sleep. Such systems raise the pressure in the pharynx by about 1 kPa, keeping the walls apart. Nasal CPAP improves symptoms, quality of life, daytime alertness and survival. However, up to 50% of OSA patients cannot tolerate CPAP. Modafinil (a CNS stimulant) is a useful short-term alternative.
Bronchiectasis
This term describes abnormal and permanently dilated airways. Bronchial walls become inflamed, thickened and irreversibly damaged. The mucociliary transport mechanism is impaired and frequent bacterial infections ensue. Clinically, the disease is characterized by productive cough with large amounts of discoloured sputum, and dilated, thickened bronchi, detected on CT.
Aetiology
Cystic fibrosis is the most common cause in developed countries. For other causes see Table 15.12.
Table 15.12 Causes of bronchiectasis
Clinical features
Patients with mild bronchiectasis only produce yellow or green sputum after an infection. Localized areas of the lung may be particularly affected, in which case sputum production will vary with position. As the condition worsens, patients suffer persistent halitosis, recurrent febrile episodes with malaise, and episodes of pneumonia. Clubbing occurs, and coarse crackles can be heard over the infected areas, usually the lung bases. When the condition is severe there is continuous production of foul-smelling, thick, khaki-coloured sputum. Haemoptysis can occur either as blood-stained sputum or as a massive haemorrhage. Breathlessness may result from airflow limitation.
Investigations
 Chest X-ray may show dilated bronchi with thickened bronchial walls and sometimes multiple cysts containing fluid, but can be normal.
Chest X-ray may show dilated bronchi with thickened bronchial walls and sometimes multiple cysts containing fluid, but can be normal.
 High-resolution CT scanning (see p. 791) reveals thickened, dilated bronchi and cysts at the end of the bronchioles (Fig. 15.27). Characteristically the airways are larger than their associated blood vessels.
High-resolution CT scanning (see p. 791) reveals thickened, dilated bronchi and cysts at the end of the bronchioles (Fig. 15.27). Characteristically the airways are larger than their associated blood vessels.
 Sputum examination and culture are essential for adequate treatment. The major pathogens are Staph. aureus, Pseudomonas aeruginosa, H. influenzae and anaerobes. Other pathogens include Strep. pneumoniae and Klebsiella pneumoniae. Aspergillus fumigatus can be isolated from 10% of sputum specimens in cystic fibrosis, but the role of this organism in producing infection is uncertain. Mycobacterium avium-intracellulare complex (MAI) is being increasingly found.
Sputum examination and culture are essential for adequate treatment. The major pathogens are Staph. aureus, Pseudomonas aeruginosa, H. influenzae and anaerobes. Other pathogens include Strep. pneumoniae and Klebsiella pneumoniae. Aspergillus fumigatus can be isolated from 10% of sputum specimens in cystic fibrosis, but the role of this organism in producing infection is uncertain. Mycobacterium avium-intracellulare complex (MAI) is being increasingly found.
 Sinus X-rays. 30% of bronchiectasis patients also have rhinosinusitis.
Sinus X-rays. 30% of bronchiectasis patients also have rhinosinusitis.
 Serum immunoglobulins. Up to 10% of adults with bronchiectasis have antibody class or subclass deficiency (mainly IgA). Some have normal antibody class levels but fail to respond to respiratory pathogens. Responses to Hib and pneumococcal vaccines may be impaired.
Serum immunoglobulins. Up to 10% of adults with bronchiectasis have antibody class or subclass deficiency (mainly IgA). Some have normal antibody class levels but fail to respond to respiratory pathogens. Responses to Hib and pneumococcal vaccines may be impaired.
 Sweat electrolytes – if cystic fibrosis is suspected (see p. 821).
Sweat electrolytes – if cystic fibrosis is suspected (see p. 821).
 Mucociliary clearance (nasal clearance of saccharin). A 1 mm cube of saccharin is placed on the inferior turbinate and the time to taste measured (normally <30 minutes).
Mucociliary clearance (nasal clearance of saccharin). A 1 mm cube of saccharin is placed on the inferior turbinate and the time to taste measured (normally <30 minutes).
Treatment
Postural drainage
Postural drainage is valuable. Patients must be trained by physiotherapists to tip themselves so that the affected lobe(s) are uppermost at least three times daily for 10–20 minutes. Most patients find that lying over the side of the bed with head and thorax down is effective.
Antibiotics
Experience from the treatment of cystic fibrosis suggests that bronchopulmonary infections need to be eradicated if progression of the disease is to be halted. In mild cases, intermittent chemotherapy with cefaclor 500 mg three times daily or ciprofloxacin 500 mg twice daily may be sufficient. Flucloxacillin 500 mg 6-hourly is needed if Staph. aureus is isolated.
If the sputum remains yellow or green despite regular physiotherapy and intermittent chemotherapy, or if lung function deteriorates despite treatment with bronchodilators, it is likely that there is infection with Pseudomonas aeruginosa. Treatment requires parenteral or aerosol chemotherapy at regular 3-month intervals. Ceftazidime 2 g intravenously 8-hourly or by inhalation (1 g twice daily) has been shown to be effective. Ciprofloxacin 750 mg twice daily orally may work in the short term, but resistance can develop rapidly. High sputum levels of some antibiotics, e.g. colistin or tobramycin, can be achieved by inhalation.
Treatment for aspergillus is described on page 852 and MAI on page 192.
Complications
The incidence of complications has fallen with antibiotic therapy. Pneumonia, pneumothorax, empyema and metastatic cerebral abscess can occur. Severe, life-threatening haemoptysis can also occur, particularly in patients with cystic fibrosis.
Massive haemoptysis originates from the high-pressure systemic bronchial arteries and has a mortality of 25%. Other causes of massive haemoptysis are pulmonary tuberculosis (most common), aspergilloma, lung abscess and primary and secondary malignant tumours.
Treatment of the haemoptysis consists of bed rest and antibiotics, when most stop bleeding. Blood transfusion is given if required. Urgent fibreoptic bronchoscopy is occasionally necessary to detect the source of bleeding. If the haemoptysis does not settle rapidly, the treatment of choice is bronchial artery embolization. Surgical resection may be required if embolization fails.
Prognosis
The advent of effective antibiotic therapy has greatly improved the prognosis. Ultimately, most patients with severe bronchiectasis will develop respiratory failure or cor pulmonale, but those with mild disease have normal life expectancy. Of the pathogens that cause infective episodes, the most difficult to eradicate are Pseudomonas aeruginosa, Aspergillus fumigatus and MAI.
Cystic fibrosis
In cystic fibrosis (CF) there is an alteration in the viscosity and tenacity of mucus produced at epithelial surfaces. The classical form of the syndrome includes bronchopulmonary infection and pancreatic insufficiency, with a high sweat sodium and chloride concentration. It is an autosomal recessive inherited disorder with a carrier frequency in Caucasians of 1 in 22 (see p. 43). There is a gene mutation on the long arm of chromosome 7 in the region of 7q31.2. The commonest abnormality is a specific deletion at position 508 in the amino acid sequence [ΔF508] – which results in a defect in a transmembrane regulator protein (see p. 44). This is the cystic fibrosis transmembrane conductance regulator (CFTR), which is a critical chloride channel (Fig. 15.28). The mutation alters the secondary and tertiary structure of the protein, leading to a failure of opening of the chloride channel in response to elevated cyclic AMP in epithelial cells. This results in decreased excretion of chloride into the airway lumen and an increased reabsorption of sodium into the epithelial cells. With less excretion of salt there is less excretion of water and increased viscosity and tenacity of airway secretions. A possible reason for the high salt content of sweat is that there is a CFTR-independent mechanism of chloride secretion in the sweat gland with an impaired reabsorption of sodium chloride in the distal end of the duct. Many genetic variants are known. The frequency of λF508 mutation in CF is 70% in the USA and UK, under 50% in southern Europe and 30% in Ashkenazi families. A further mutation G551D-CFTR reaches the cell surface but fails to open VX-770 allows it to open momentarily (see p. 811)
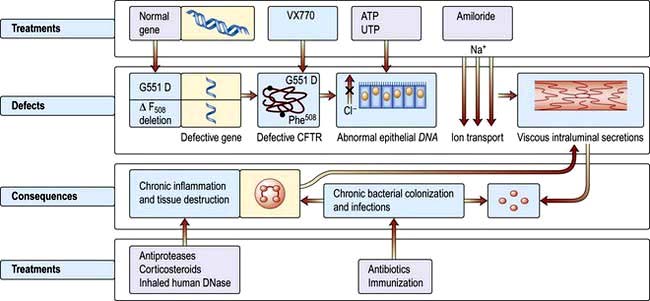
Figure 15.28 Cystic fibrosis. Abnormalities and possible therapies (see text). CFTR, cystic fibrosis transmembrane conductance regulator. Ph508 phenylalanine deletion in λ F508. G551D, glycine replaced by aspartate.
Clinical features
Respiratory effects
Although the lungs of babies born with CF are structurally normal at birth, frequent respiratory infections soon develop and are often the presenting feature. CF is the commonest cause of recurrent bronchopulmonary infection in childhood, and is a major cause in early adult life. Sinusitis is almost universal and nasal polyps are common. Breathlessness and haemoptysis occur in the later stages as airflow limitation and bronchiectasis develop. Spontaneous pneumothorax may occur. Respiratory failure and cor pulmonale eventually develop.
Gastrointestinal effects
About 85% of patients have symptomatic steatorrhoea owing to pancreatic dysfunction (see p. 366). Children may be born with meconium ileus owing to the viscid consistency of meconium in CF; later in life they may develop the meconium ileus equivalent syndrome, a form of small intestinal obstruction unique to CF. Cholesterol gallstones occur with increased frequency. Cirrhosis develops in about 5% of older patients and there are increased incidences of peptic ulceration and gastrointestinal malignancy.
Nutritional effects
Many patients suffer from malnutrition due to a combination of malabsorption and maldigestion. Poor nutrition is associated with increased risk of pulmonary sepsis.
Other features
Puberty and skeletal maturity are delayed in most CF patients. Males are almost always infertile owing to failure of development of the vas deferens and epididymis. Females are able to conceive, but often develop secondary amenorrhoea as the disease progresses. Arthropathy and diabetes mellitus occur in 11% of adults with CF.
Diagnosis
The diagnosis of CF in older children and adults is based on the clinical history and:
 a family history of the disease
a family history of the disease
 a high sweat sodium concentration over 60 mmol/L (meticulous technique by laboratories performing regular sweat analysis is essential, but the test is still difficult to interpret in adults)
a high sweat sodium concentration over 60 mmol/L (meticulous technique by laboratories performing regular sweat analysis is essential, but the test is still difficult to interpret in adults)
 blood DNA analysis of gene defect
blood DNA analysis of gene defect
 radiology showing features seen in bronchiectasis (see p. 819)
radiology showing features seen in bronchiectasis (see p. 819)
 absent vas deferens and epididymis
absent vas deferens and epididymis
 blood immunoreactive trypsin levels – these are not useful diagnostically but may be useful in screening.
blood immunoreactive trypsin levels – these are not useful diagnostically but may be useful in screening.
Treatment
CF patients should be managed by multidisciplinary specialized teams. The overall care involves education to improve their quality of life, good nutrition and the prompt treatment of exacerbations to avoid hospitalization.
 General care should include stopping smoking, vaccination with influenza and pneumococcal vaccines and pulmonary rehabilitation (p. 817).
General care should include stopping smoking, vaccination with influenza and pneumococcal vaccines and pulmonary rehabilitation (p. 817).
 Oxygen therapy should be given as necessary (Box 15.1).
Oxygen therapy should be given as necessary (Box 15.1).
 Antibiotic treatment for respiratory infections is as described under bronchiectasis on page 820.
Antibiotic treatment for respiratory infections is as described under bronchiectasis on page 820.
 Seventy per cent of adults with CF have Pseudomonas infection in their sputum. Nebulized anti-pseudomonal antibiotic therapy improves lung function and decreases the risk of infective exacerbations and hospitalization.
Seventy per cent of adults with CF have Pseudomonas infection in their sputum. Nebulized anti-pseudomonal antibiotic therapy improves lung function and decreases the risk of infective exacerbations and hospitalization.
 Drug therapy: β2 agonists (p. 830) and inhaled corticosteroids (p. 831) may provide symptomatic relief but have no effect on long-term survival.
Drug therapy: β2 agonists (p. 830) and inhaled corticosteroids (p. 831) may provide symptomatic relief but have no effect on long-term survival.
 Airway clearance. Inhalation of recombinant DNAse (dornase alfa 2.5 mg daily) has been shown to improve FEV1 by 20% in some patients. Hypertonic saline by inhalation (in concentrations of up to 7%) gives short-term benefit. Amiloride, which inhibits sodium transport, has been used but no overall benefit has been shown in meta-analysis. Acetylcysteine has been shown in vitro to liquefy CF sputum by cleaving disulfide bonds in mucus glycoproteins but clinical studies have been disappointing.
Airway clearance. Inhalation of recombinant DNAse (dornase alfa 2.5 mg daily) has been shown to improve FEV1 by 20% in some patients. Hypertonic saline by inhalation (in concentrations of up to 7%) gives short-term benefit. Amiloride, which inhibits sodium transport, has been used but no overall benefit has been shown in meta-analysis. Acetylcysteine has been shown in vitro to liquefy CF sputum by cleaving disulfide bonds in mucus glycoproteins but clinical studies have been disappointing.
 Non-invasive ventilation (p. 895) improves symptoms in chronic respiratory failure but there is no evidence of a survival benefit. It acts as a bridge to lung transplantation (p. 822).
Non-invasive ventilation (p. 895) improves symptoms in chronic respiratory failure but there is no evidence of a survival benefit. It acts as a bridge to lung transplantation (p. 822).
 Treatment for pancreatic insufficiency (p. 366) and malnutrition (p. 203).
Treatment for pancreatic insufficiency (p. 366) and malnutrition (p. 203).
 Human experimental studies have been conducted on the delivery to the epithelium of the normal CFTR gene using, as a vector, a replication-deficient adenovirus containing normal human CFTR complementary DNA which is trophic for epithelial cells. Gentamicin can suppress premature termination codons, and nasal administration has been shown to correct the physiological abnormality. These studies are in their early stages.
Human experimental studies have been conducted on the delivery to the epithelium of the normal CFTR gene using, as a vector, a replication-deficient adenovirus containing normal human CFTR complementary DNA which is trophic for epithelial cells. Gentamicin can suppress premature termination codons, and nasal administration has been shown to correct the physiological abnormality. These studies are in their early stages.
 Targeted genetic therapy – see page 44.
Targeted genetic therapy – see page 44.
Prognosis and screening
Almost all CF patients develop progressive respiratory failure but the prognosis has improved considerably over the years. Some 90% of children now survive into their teens and the median survival for those born after 1990 is estimated at 40 years. A major problem is sputum infection with Burkholderia cepacia which is associated with accelerated disease and rapid death. Multiple antibiotic resistance is common and spread occurs from person to person. Strategies to limit transmission include rigid segregation of both inpatients and outpatients and advice to CF sufferers not to socialize together. Sadly, groups formed for mutual support and education have had to be dissolved, leading to considerable distress.
Genetic screening is available for the 20 commonest mutations and this identifies 85–95% of carriers. Screening for the carrier state should be offered to persons or couples with a family history of CF, together with counselling (see p. 43).
Chronic cough (see p. 798)
Pathological coughing results from two mechanisms:
 Stimulation of sensory nerves in the epithelium by secretions, foreign bodies, cigarette smoke and tumours
Stimulation of sensory nerves in the epithelium by secretions, foreign bodies, cigarette smoke and tumours
 Sensitization of the cough reflex with abnormal increase in the sensitivity of the cough receptors.
Sensitization of the cough reflex with abnormal increase in the sensitivity of the cough receptors.
Sensitization of the cough reflex can be demonstrated by inhalation of capsaicin or saline solution and presents clinically as a persistent tickling sensation in the throat with paroxysms of coughing induced by changes in air temperature, aerosol sprays, perfumes and cigarette smoke. It is found in association with viral infections, oesophageal reflux, post-nasal drip, cough-variant asthma, and in 15% of patients taking angiotensin-converting enzyme (ACE) inhibitors. The association with ACE inhibitors implicates neuropeptides, prostaglandins E2 and F2 and bradykinin as causes of the cough. In some patients no cause can be found (idiopathic cough). In the absence of chest X-ray abnormalities, possible investigations include:
 ENT examination (p. 1051) and sinus CT for post-nasal drip
ENT examination (p. 1051) and sinus CT for post-nasal drip
 Lung function tests and histamine bronchial provocation testing (p. 829) for cough-variant asthma
Lung function tests and histamine bronchial provocation testing (p. 829) for cough-variant asthma
 Ambulatory oesophageal ph monitoring and manometry for oesophageal reflux
Ambulatory oesophageal ph monitoring and manometry for oesophageal reflux
 Fibreoptic bronchoscopy for inhaled foreign body or tumour
Fibreoptic bronchoscopy for inhaled foreign body or tumour
 ECG, echocardiography and exercise testing and impedance for cardiac causes
ECG, echocardiography and exercise testing and impedance for cardiac causes
Symptomatic management of unexplained cough can be difficult. Morphine depresses the sensitized cough reflex but its unwanted effects limit its long-term use. Dihydrocodeine linctus may help some patients. Demulcent preparations and cough sweets only provide temporary relief. Patients who cough while taking ACE inhibitors should switch to an angiotensin-II receptor antagonist, e.g. losartan (see p. 719), which does not block bradykinin breakdown.
Lung and heart-lung transplantation
Indications and donor selection
The main diseases treated by transplantation are:
Patients selected for transplantation are usually under 60 years with a life expectancy of less than 18 months, no underlying cancer and no serious systemic disease.
Donor organs are taken from donors under 40 years, with good cardiac and lung function, and chest measurements slightly smaller than those of the recipient. Matching for ABO blood group is essential, but rhesus blood group compatibility is not essential. Since donor material is limited, single lung transplantation is preferred to double lung or heart-lung transplantation and can be successfully undertaken in pulmonary fibrosis, pulmonary hypertension and emphysema. Bilateral lung transplantation is required in infective conditions to prevent spillover of bacteria from the diseased lung to a single lung transplant. Eisenmenger’s syndrome requires heart-lung transplant.
Complications and their treatment
Prognosis
Several studies show a major improvement in overall quality of life. One year survival rates have improved with a yearly mortality rate of about 10%. Death is mainly due to bronchiolitis. Actual survival varies with the original diagnosis but the median survival is approximately four years.