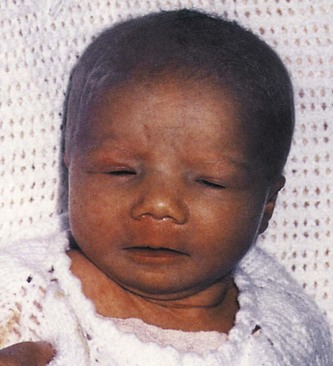
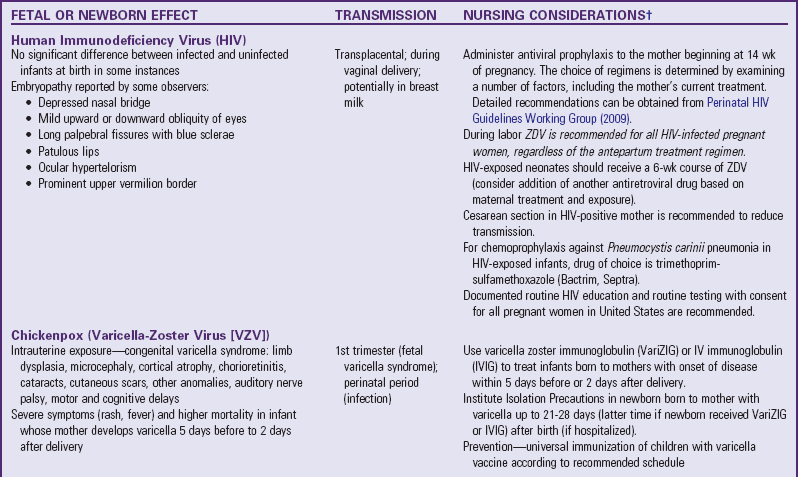
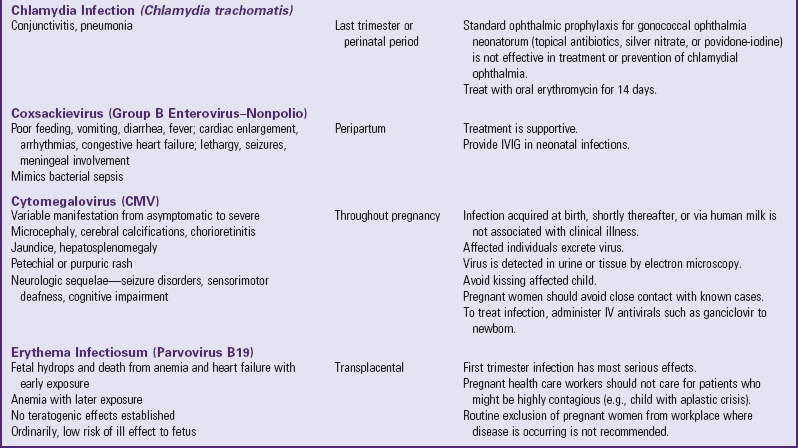
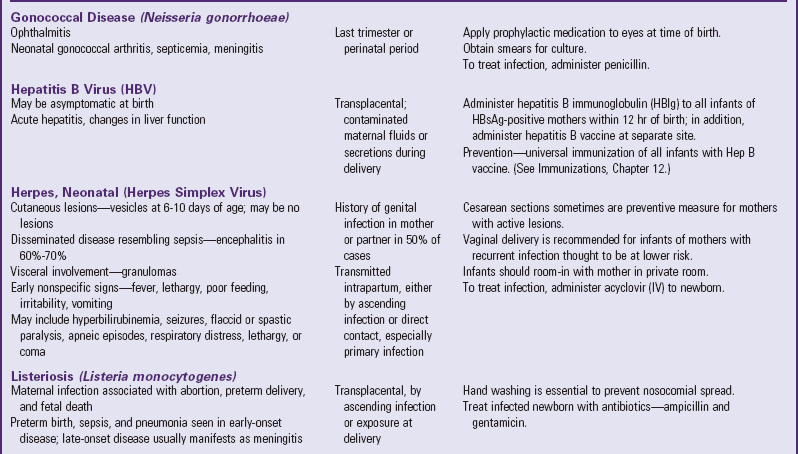
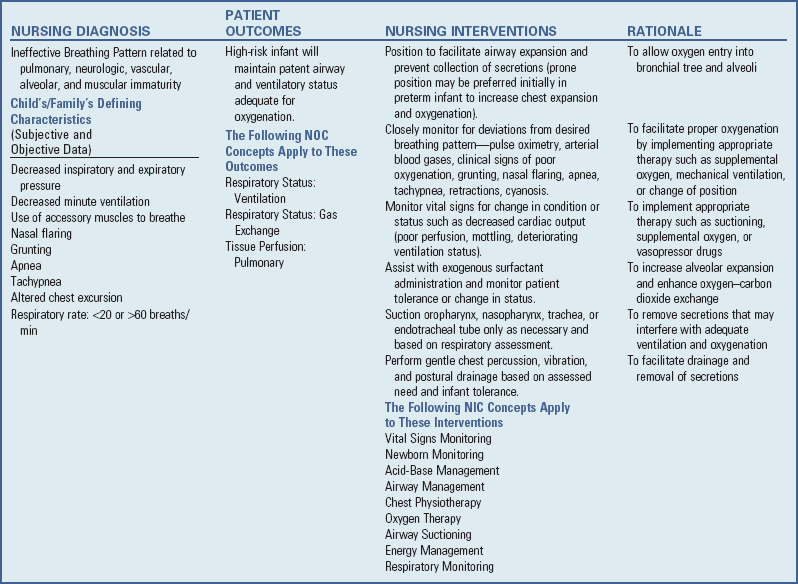
Clinical Manifestations
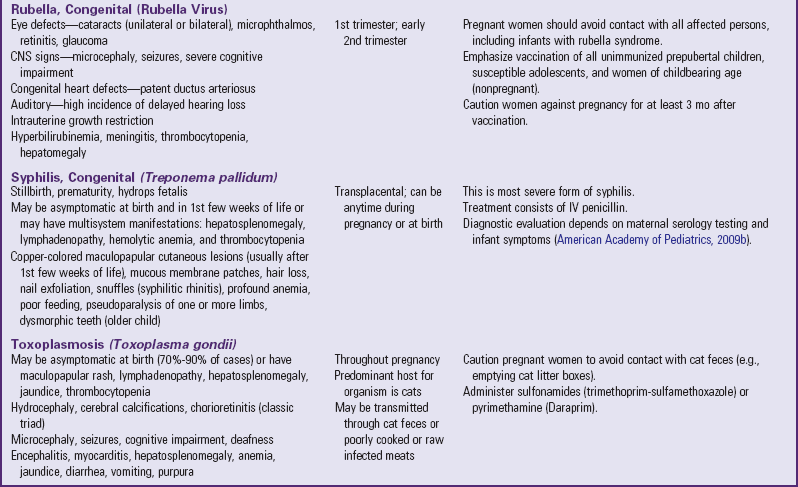
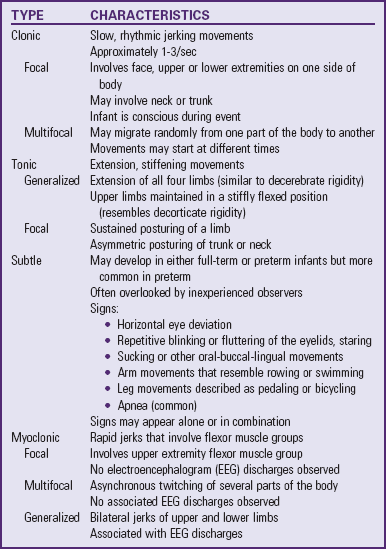
Infants with RDS can develop respiratory distress either acutely or over a period of hours, depending on the acuity of pulmonary immaturity, associated illness factors, and gestational maturity. The observable signs produced by the pulmonary changes usually begin to appear in infants who apparently achieve normal breathing and color soon after birth. In a matter of a few hours, breathing gradually becomes more rapid (>60 breaths/min). Infants may display retractions—suprasternal or substernal, and supracostal, subcostal, or intercostal—which result from a compliant chest wall. Weak chest wall muscles and the highly cartilaginous rib structure produce an abnormally elastic rib cage, resulting in indrawing, or retraction, of the skin between the ribs. During this early period the infant’s color may remain satisfactory, and auscultation reveals air entry. Some of the criteria for evaluating respiratory distress in infants are illustrated in Fig. 10-14.
Fig. 10-14 Criteria for evaluating respiratory distress. (Modified from Silvermann WA, Anderson DH: A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate, and necropsy findings among premature infants, Pediatrics 17:1, 1956.)
Within a few hours, respiratory distress becomes more obvious. The respiratory rate continues to increase (to 80 to 120 breaths/min), and breathing becomes more labored. It is significant to note that infants increase the rate rather than the depth of respiration when in distress. Substernal retractions become more pronounced as the diaphragm works hard in an attempt to fill collapsed air sacs. Fine inspiratory crackles can be heard over both lungs, and there is an audible expiratory grunt. This grunting, a useful mechanism observed in the earlier stages of RDS, serves to increase end-expiratory pressure in the lungs, thus maintaining alveolar expansion and allowing gas exchange for an additional brief period. Flaring of the nares is also a sign that accompanies tachypnea, grunting, and retractions in respiratory distress. Central cyanosis (a bluish discoloration of oral mucous membranes and generalized body cyanosis) is a late and serious sign of respiratory distress. Initially supplemental oxygen may eliminate cyanosis. The use of pulse oximetry and arterial blood gas sampling obviates the necessity for dependence on color to determine oxygen requirements.
Severe RDS is often associated with a shocklike state, as manifested by diminished cardiac inflow and low arterial blood pressure. As a result of extreme pulmonary immaturity, decreased glycogen stores, and lack of accessory muscles, the ELBW and VLBW infant may have severe RDS at birth, bypassing the aforementioned steps in the development of RDS.
Infants with RDS who are treated with exogenous surfactant have a good chance for recovery. Complications of RDS include those described as complications of positive pressure ventilation (see p. 353). Associated complications (of prematurity and RDS) include PDA and congestive heart failure, retinopathy of prematurity, IVH, BPD, NEC, and neurologic sequelae.
Diagnostic Evaluation
Laboratory data are nonspecific, and the abnormalities observed are identical to those observed in numerous biochemical abnormalities of the newborn (i.e., the findings of hypoxemia, hypercapnia, and acidosis). Specific tests are used to determine complicating factors, such as blood glucose (to test for hypoglycemia), blood gas measurements for serum pH (to test for acidosis), and Pao2 (to test for hypoxia). Pulse oximetry is an important component for determining hypoxia. Other special examinations may be used to diagnose or rule out complications.
Radiographic findings characteristic of RDS include (1) a diffuse granular pattern over both lung fields that resembles ground glass and represents alveolar atelectasis and (2) dark streaks, or air bronchograms, within the ground glass areas that represent dilated, air-filled bronchioles. It is important to distinguish between RDS and pneumonia in infants with respiratory distress.
Prenatal Diagnosis: Fetal lung maturity depends on gestational age and maternal illnesses. Problems such as maternal diabetes delay fetal lung maturation, whereas fetuses exposed to chronic stress (intrauterine growth restriction [IUGR], drug exposure) often have more mature lungs. Antenatal administration of glucocorticoids enhances fetal lung maturity, especially when combined with postnatal surfactant administration (Hintz, Poole, Wright, et al, 2005; Baud, 2004).
Functional maturity of the fetal lung can be determined by using surfactant phospholipids in amniotic fluid as indicators of maturity. The most commonly tested is the lecithin/sphingomyelin (L/S) ratio, which represents the relationship between these two lipids during gestation. Phospholipids are synthesized by fetal alveolar cells, and the concentrations in amniotic fluid change during gestation. Initially there is more sphingomyelin, but at approximately 32 to 33 weeks the concentrations become equal; sphingomyelin then diminishes and lecithin increases significantly until the fetus has developed sufficient surfactant to maintain alveolar stability at approximately 35 weeks. An L/S ratio of 2 : 1 in nondiabetic mothers indicates virtually no risk of RDS.
Other key surfactant compounds (also phospholipids) that are needed to stabilize surfactant are phosphatidylcholine (PC) and phosphatidylglycerol (PG). Without these compounds, lecithin is not functional as a surfactant. Concentrations of PC parallel those of lecithin, peaking at 35 weeks and then gradually decreasing. At 36 weeks PG appears in amniotic fluid and increases until term. By measuring these phospholipids—L/S ratio, PC, and PG—the clinician can estimate the maturity of the lungs with a high degree of accuracy. Other, less commonly used methods have been devised to provide rapid, inexpensive, and accurate measures of lung maturity. These include the “shake” or “bubble” test, in which stable foam or bubbles form when amniotic fluid is shaken in the presence of ethanol, and the tap test, in which abundant bubbles appear in a test tube of amniotic fluid with 6N-hydrochloric acid and diethyl ether.
Another test currently being used to evaluate fetal lung maturity is the TDx Fetal Lung Maturity (FLM) assay, which determines PG levels in amniotic fluid or neonatal tracheal aspirates. The FLM test is faster than L/S ratio determination (<1 hour versus 4 to 5 hours) and is reported to predict the absence of RDS with greater accuracy; a level of 50 or more is predictive of fetal lung maturity (Fantz, Powell, Karon, et al, 2002). TDx FLM may also be used in the postnatal period to determine the presence of RDS as a result of surfactant deficiency by collecting tracheal aspirate samples (Parvin, Kaplan, Chapman, et al, 2005).
Lamellar bodies, representing the storage form of surfactant, are found in amniotic fluid in increasing quantities with the advancement of gestational age and lung maturity. A quantitative count of lamellar bodies has been reported to be as accurate as the L/S ratio in determining fetal lung maturity. The count can be obtained faster than the L/S ratio, thus making it clinically appealing (Neerhof, Dohnal, Ashwood, et al, 2001; Wijnberger, Huisjes, Voorbij, et al, 2001).
Therapeutic Management
The treatment of RDS includes all the general measures required for any preterm infant, as well as those instituted to correct imbalances. The supportive measures most crucial to a favorable outcome are (1) maintain adequate ventilation and oxygenation with an oxygen hood, continuous positive airway pressure (CPAP), or mechanical ventilation; (2) maintain acid-base balance; (3) maintain a neutral thermal environment; (4) maintain adequate tissue perfusion and oxygenation; (5) prevent hypotension; and (6) maintain adequate hydration and electrolyte status. Nipple and gavage feedings are avoided in any situation that creates a marked increase in respiratory rate because of the greater hazards of aspiration.
Surfactant: The administration of exogenous surfactant to preterm neonates with RDS has become an accepted and common therapy in most neonatal centers worldwide. Numerous clinical trials involving the administration of exogenous surfactant to infants with or at high risk for RDS demonstrate improvements in blood gas values and ventilator settings, decreased incidence of pulmonary air leaks, decreased deaths from RDS, and an overall decreased infant mortality rate (Halliday, 2003; Soll, 2000, American Academy of Pediatrics, 2008). Exogenous surfactant comes from a natural source (e.g., porcine or bovine) or from the production of artificial surfactant. Commercially available surfactant products include beractant (Survanta), a bovine surfactant; and poractant alfa (Curosurf), a porcine surfactant.
Studies have shown mixed results in comparing one surfactant product with another. One study found fewer complications and earlier improvement with natural (versus synthetic) surfactant use (Soll and Blanco, 2001). Moya, Gadzinowski, Bancalari, and colleagues (2005) found that an investigational synthetic surfactant, lucinactant, mimics the action of human surfactant protein-B (SP-B), and it was more effective than beractant and colfosceril palmitate at reducing the RDS-related mortality rates by 14 days of life. BPD was significantly less common in infants at 36 weeks postmenstrual age who had received lucinactant.
Additional benefits of surfactant replacement therapy include decreased oxygen requirements and mean airway pressure (MAP) within hours of administration and an overall decrease in the incidence of pulmonary air leaks. To date, long-term improvement in the decrease of BPD and IVH has not been evidenced in all surfactant clinical trials.
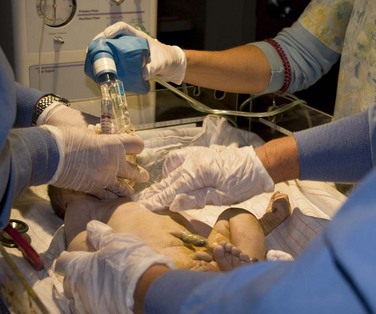
Complications seen with surfactant administration include pulmonary hemorrhage and mucus plugging. Additional studies investigating the potential benefits of surfactant in infants with meconium aspiration found a reduction in the severity of respiratory illness and subsequent requirement of ECMO support (El Shahed, Dargaville, Ohlsson, et al, 2007). Other studies continue to investigate the potential benefits of exogenous surfactant for the treatment of infectious pneumonia and lung hypoplasia concomitant with congenital diaphragmatic hernia (Wiswell, 2001). Acute RDS/ALI may also respond favorably to surfactant administration (see Acute Respiratory Distress Syndrome/Acute Lung Injury, Chapter 32). Surfactant may be administered at birth as a prophylactic treatment of RDS or later in the course of RDS as a rescue treatment. Studies found improved clinical outcomes and fewer adverse effects when surfactant is administered prophylactically to infants at risk for developing RDS (American Academy of Pediatrics, 2008). Surfactant is administered via the ET tube directly into the infant’s trachea (Fig. 10-15); the exact number of doses (single versus multiple) that is most effective has yet to be determined.
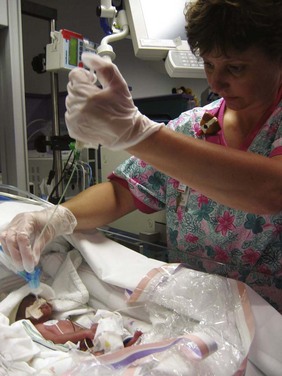
Fig. 10-15 Exogenous surfactant administration to infant on mechanical ventilation. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Nursing responsibilities with surfactant administration include assistance in the delivery of the product, collection and monitoring of arterial blood gases, scrupulous monitoring of oxygenation, and assessment of the infant’s tolerance of the procedure. Once surfactant is absorbed, there is usually an increase in respiratory compliance that requires adjustment of ventilator settings to decrease MAP and prevent overinflation or hyperoxemia. Suctioning is usually delayed for an hour or so (depending on the type of surfactant, delivery system, and unit protocol) to allow maximum effects to occur. Current research is investigating the possibility of delivering an aerosolized surfactant. This method would decrease the problems associated with current delivery systems (contamination of the airway, interruption of mechanical ventilation, and loss of the drug in the ET tubing from reflux).
Oxygen Therapy: The goals of oxygen therapy are to provide adequate oxygen to the tissues, prevent lactic acid accumulation resulting from hypoxia, and, at the same time, avoid the potentially negative effects of oxygen toxicity. Numerous methods have been devised to improve oxygenation (Table 10-8). All require that the gas be warmed and humidified before entering the respiratory tract. If the infant does not require ventilatory assistance, oxygen can be given via a plastic hood placed over the head to supply variable concentrations of humidified oxygen. (See Oxygen Therapy, Chapter 31.) If oxygen saturation of blood cannot be maintained at a satisfactory level and the carbon dioxide level (Paco2) rises, infants will require ventilatory assistance.
TABLE 10-8
COMMON METHODS FOR ASSISTED VENTILATION IN NEONATAL RESPIRATORY DISTRESS
*Also referred to as conventional ventilation (versus HFOV).
Oxygen should be administered judiciously to preterm infants being stabilized in labor and delivery and for oxygenation maintenance in the NICU. Much attention has focused recently on high oxygen concentration and the effect of free oxygen radicals on the development of conditions such as NEC, BPD, and ROP. The current Neonatal Resuscitation Program guidelines recommend the use of oxygen concentrations between 21% and 100% in order to achieve an oxygen saturation of approximately 90% (American Academy of Pediatrics and American Heart Association, 2006). Finer and Leone (2009) recommend oxygen delivery to maintain an Spo2 of 83% to 93% in preterm infants to decrease morbidity and mortality associated with liberal oxygen usage and high oxygen concentrations. Research indicates that optimal target ranges for maintaining adequate oxygenation while preventing ROP and BPD or other conditions is as yet unknown (Askie, Henderson-Smart, and Ko, 2009).
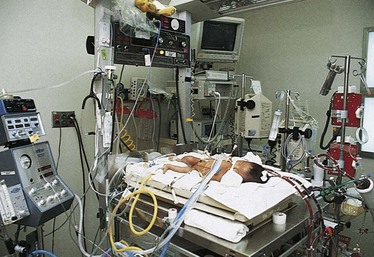
CPAP, the application of 3 to 8 cm H2O (positive) pressure to the airway, uses the infant’s spontaneous respiration to improve oxygenation by helping prevent alveolar collapse and increasing diffusion time. CPAP may be delivered via fitted face mask, nasal prongs, or an ET tube (Fig. 10-16). Ventilation with CPAP is done entirely by the infant. If oxygenation is not improved and the infant requires assisted ventilation, intermittent mandatory ventilation (IMV) is used with positive end-expiratory pressure (PEEP). This allows infants to breathe at their own rate but provides positive pressure with end-expiratory pressure to prevent alveolar collapse and overcome airway resistance. Additional components involved in IMV are peak inspiratory pressure (PIP) and rate (number of breaths per minute). The PIP is the maximum amount of positive pressure applied to the infant on inspiration. The total amount of pressure transmitted to the airway throughout an entire respiratory cycle is called the mean airway pressure (MAP). Increasing MAP in infants with severe RDS correlates positively with improved oxygenation by maintaining functional residual capacity and overcoming the resistive forces of the atelectatic lung. The MAP is affected by changes in the PEEP, PIP, and inspiratory/expiratory ratio. Although MAP is now recognized as the major determinant of oxygenation, this does not imply that simply increasing MAP alone will automatically improve oxygenation (Wood, 2003).
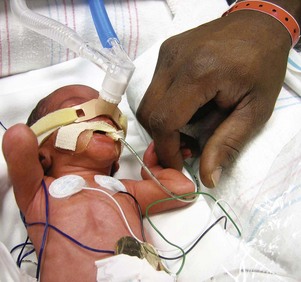
Fig. 10-16 Infant on nasal continuous positive airway pressure with father’s finger in hand. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Improved technology has made available to preterm or sick neonates a form of mechanical ventilatory assistance previously used in adults: synchronized intermittent mandatory ventilation (SIMV). With this method breaths delivered by the ventilator are synchronized to the onset of spontaneous infant breaths. The net effect is to produce full respiratory synchrony rather than asynchronous respiratory efforts (commonly called “fighting the ventilator”) that are believed to significantly impede the ability to adequately oxygenate infants without sedation or muscle paralysis. With SIMV, the operator sets the number of breaths per minute delivered by the ventilator, and the patient may breathe spontaneously between mechanical breaths. In the “assist-control” mode a mechanical breath is delivered each time a spontaneous respiration is detected; the “control” mode includes the delivery of a mechanical breath at a regular rate if the patient fails to initiate a spontaneous respiration. Additional benefits of SIMV are improved oxygenation, decreased incidence of pulmonary air leaks (pneumothorax), and decreased time on mechanical ventilation (Greenough, Dimitriou, Prendergast, et al, 2008).
If adequate oxygenation cannot be maintained and hypercarbia persists, infants may benefit from one of the two high-frequency ventilation (HFV) modalities. HFV delivers gas at very rapid rates to provide adequate minute volumes using lower proximal airway pressures by way of high-frequency oscillatory ventilation (HFOV) or high-frequency jet ventilation (HFJV). HFV was initially recommended for intractable respiratory failure, especially for infants with pulmonary air leaks and PIE. More recently, many clinicians are recommending earlier use of HFOV to prevent volutrauma to the lungs of very preterm infants (Ventre and Arnold, 2004).
Volutrauma is believed to be a key factor in the development of BPD. Courtney, Durand, Asselin, and colleagues (2002) reported that HFOV was associated with improved survival and a decreased need for supplemental oxygen at 36 weeks of postmenstrual age. HFJV is most often used in the treatment of full-term infants with meconium aspiration, persistent pulmonary hypertension, or air leak syndromes. The Cochrane review of HFJV use in preterm infants with RDS reports a similar benefit to that of HFOV in terms of pulmonary outcomes but cautions that sufficient studies have not been done to recommend the use of HFJV in preterm infants (Bhuta and Henderson-Smart, 2000).
Complications of Positive Pressure Ventilation: Although lifesaving, mechanical ventilation is not without hazards. Positive pressure introduced by mechanical apparatus has caused an increased incidence of air leaks that produce complications, such as PIE, pneumothorax, and pneumomediastinum (see p. 358). The avoidance of intubation and mechanical ventilation reduces the incidence of BPD (Verder, Bohlin, Kamper, et al, 2009). Other complications directly related to positive pressure include various problems associated with intubation, such as nasal, tracheal, or pharyngeal perforation; stenosis; inflammation; palatal grooves; subglottic stenosis; tube obstruction; and infection.
Nitric Oxide: Inhaled nitric oxide (NO) has emerged as a significant treatment modality for neonates with conditions that cause persistent pulmonary hypertension, pulmonary vasoconstriction, and subsequent acidosis and severe hypoxia. Infants with conditions such as MAS, pneumonia, sepsis, and congenital diaphragmatic hernia with pulmonary hypoplasia often require intervention in an attempt to reverse pulmonary hypertension. NO is a colorless, highly diffusible gas that causes smooth muscle relaxation and reduces pulmonary vasoconstriction and subsequent pulmonary hypertension when inhaled into the lungs. NO may be administered through the ventilator circuit and blended with oxygen. It attaches readily to hemoglobin and is thus deactivated so that systemic vasculature is not affected. NO is toxic in large quantities, but the amount required to induce pulmonary vasculature relaxation (6 to 20 ppm) is well below toxic levels.
Studies of term and near-term infants being treated with NO for respiratory failure have been positive (Finer and Barrington, 2006). In many cases reversal of persistent pulmonary hypertension of the newborn (PPHN) without ECMO has been achieved in infants with MAS, RDS, perioperative congenital heart disease, and sepsis (Konduri, 2004). One exception is the study of newborns with congenital diaphragmatic hernia who required ECMO after NO and whose morbidity and mortality were not significantly improved with inhaled NO (Field, 2005; Finer and Barrington, 2006). Surfactant replacement therapy may be performed in combination with inhaled NO therapy in infants with inadequate pulmonary maturity. Nursing care of the infant receiving inhaled NO is the same as for the newborn with PPHN; continuous assessment of respiratory status and response to treatment is essential.
The use of NO for preterm infants remains controversial. Some studies have proposed a role for NO in the treatment of RDS and respiratory failure in these infants, whereas others suggest no benefit (Barrington and Finer, 2006; Field, 2005; Hascoet, Fresson, Claris, et al, 2005; Mercier, Olivier, Loron, et al, 2009).
Medical Therapies: The treatment of the infant with RDS requires the establishment of one or more IV lines to maintain hydration and nutrition, monitor arterial blood gases, and administer medications. Systemic antibiotics may be administered during the acute phase if sepsis is suspected (see Sepsis, p. 362). The administration of morphine or fentanyl for pain and sedation is individualized according to the infant’s response to illness. Caffeine may be administered to treat apnea and to prepare for weaning VLBW and ELBW infants from mechanical ventilation. Inotropes such as dopamine and dobutamine may be required to support the infant’s systemic blood pressure and maintain effective cardiac output during the acute phase of illness.
Prevention: The most successful approach to prevention of RDS is prevention of preterm delivery, especially elective early delivery and cesarean section. Improved methods for assessing the maturity of the fetal lung by amniocentesis, although not a routine procedure, allow a reasonable prediction of adequate surfactant formation (see Diagnostic Evaluation, p. 350). Because estimation of a delivery date can be miscalculated by as much as 1 month, such tests are particularly valuable when scheduling an elective cesarean section. Studies indicate that the combination of maternal glucocorticoid administration before delivery and surfactant administration postnatally has a synergistic effect on neonatal lungs, with the net result being a decrease in infant mortality, incidence of IVH, pulmonary air leaks, and problems with PIE and RDS (Dudell and Stoll, 2007; Halliday, 2005).
Nursing Care Management
Care of infants with RDS involves all the observations and interventions previously described for high-risk infants. In addition, the nurse is concerned with the complex problems related to respiratory therapy and the constant threat of hypoxemia and acidosis that complicates the care of patients in respiratory difficulty.
The respiratory therapist, an important member of the neonatal intensive care team, is often responsible for maintenance and regulation of respiratory equipment. Nevertheless, nurses should understand the equipment and be able to recognize when it is not functioning correctly. The most essential nursing function is to observe and assess the infant’s response to therapy. Continuous monitoring and close observation are mandatory because an infant’s status can change rapidly and because oxygen concentration and ventilation parameters are prescribed according to the infant’s blood gas measurements, tcPo2, and pulse oximetry readings.
Changes in oxygen concentration are based on these observations. The nurse determines the amount of oxygen administered, expressed as the fraction of inspired air (Fio2), on an individual basis according to pulse oximetry and/or direct or indirect measurement of arterial oxygen concentration. Capillary samples collected from the heel (see Chapter 27 for procedure) are useful for pH and Paco2 determinations but not for oxygenation status. Continuous pulse oximetry readings are recorded at least hourly or more often as required. Blood sampling is performed after ventilator changes for the acutely ill infant and thereafter when clinically indicated.
In infants with RDS who are acutely ill or extremely preterm, an umbilical arterial catheter (UAC) may be used to draw arterial blood for monitoring oxygenation. This method, although initially invasive and therefore performed by the practitioner with sterile precautions, allows for blood sampling without repeated peripheral arterial punctures. The catheter is inserted via one of the umbilical arteries to the premeasured desired position (either at the level of the diaphragm, T6-10, or between L3-4) and rests in the descending aorta. Continuous arterial pressure monitoring may be carried out with an “in-line” transducer. Practices vary regarding medication administration via a UAC. The nurse is aware of the potential hazards associated with these catheters (infection, hemorrhage, thrombus formation and subsequent vessel occlusion, arterial vasospasm) and implements monitoring and observation strategies to promptly intervene should complications occur (see Hydration, p. 322). An umbilical venous catheter (UVC) may be used separately or in conjunction with the UAC, depending on the severity of the infant’s illness, the fluid requirements, and preferred medical practice.
Mucus may collect in the respiratory tract as a result of the infant’s pulmonary condition. Secretions interfere with gas flow and may obstruct the passages, including the ET tube. Suctioning should occur only when necessary and should be based on individual infant assessment, which includes auscultation of the chest, evidence of decreased oxygenation, excess moisture in the ET tube, or increased infant irritability. When nasopharyngeal passages, the trachea, or the ET tube is being suctioned, insert the catheter gently but quickly; intermittent suction is applied as the catheter is withdrawn. It is imperative that the catheter obstruct the airway for no more than 5 seconds, since continuous suction removes air from the lungs along with the mucus. It is recommended that, where possible, an in-line suction device be used on infants who are acutely ill and who do not tolerate any procedure without profound decreases in oxygen saturation, blood pressure, and heart rate. The purpose of suctioning an artificial airway is to maintain patency of that airway, not the bronchi. Suction applied beyond the ET tube can cause traumatic lesions of the trachea.
Research indicates that suctioning to a point where the catheter meets resistance and is then withdrawn causes trauma to the tracheobronchial wall. To remove secretions without damage to the tracheobronchial mucosa, the suction catheter is premeasured and inserted to a predetermined depth to avoid extension beyond the ET tube. The practice of suctioning patients on mechanical ventilation has undergone close scrutiny in recent years; further studies are needed to validate this practice and to determine the best methods for maintaining a patent airway without compromising the patient’s well-being.
The most advantageous positions for facilitating an infant’s open airway are with the infant on the side with the head supported in alignment by a small folded blanket or with the infant on the back, positioned to keep the neck slightly extended. With the head in the “sniffing” position, the trachea is opened to its maximum; hyperextension reduces the tracheal diameter in neonates (see Therapeutic Positioning, p. 336). The pulse oximeter is observed before, during, and after suctioning to provide an ongoing assessment of oxygenation status and to prevent hypoxemia.
Inspection of the skin is part of routine infant assessment. Position changes and the use of gel mattresses are helpful in guarding against skin breakdown.
Mouth care is especially important when infants are receiving nothing by mouth, and the problem is often aggravated by the drying effect of oxygen therapy. The nurse can prevent drying and cracking by good oral hygiene using sterile water. Irritation to the nares or mouth that occurs from appliances used to administer oxygen may be reduced by the use of a water-soluble ointment (see Skin Care, p. 329; see Nursing Care Plan).
Nursing care of an infant with RDS is demanding. Pay meticulous attention to subtle changes in the infant’s oxygenation status. The importance of attention to detail cannot be overemphasized, particularly in regard to medication administration.
 to 4 hours, especially in infants of mothers with poorly controlled diabetes. Precipitous drops in blood glucose levels can cause serious neurologic damage or death. The birth defects observed in IDMs are thought to occur as a result of multifactorial teratogenic factors, rather than hyperglycemia alone (
to 4 hours, especially in infants of mothers with poorly controlled diabetes. Precipitous drops in blood glucose levels can cause serious neurologic damage or death. The birth defects observed in IDMs are thought to occur as a result of multifactorial teratogenic factors, rather than hyperglycemia alone (