Schedule for Immunizations
In the United States two organizations, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention and the Committee on Infectious Diseases of the American Academy of Pediatrics, govern the recommendations for immunization policies and procedures. In Canada, recommendations are from the National Advisory Committee on Immunization under the authority of the Minister of Health and Public Health Agency of Canada. The policies of each committee are recommendations, not rules, and they change as a result of advances in the field of immunology. Nurses need to be knowledgeable about the purpose of each organization, view immunization practices in light of the needs of each individual child and the community, and keep informed of the latest advances and changes in policy.
The recommended age for beginning primary immunizations of infants is at birth or within 2 weeks of birth (Figs. 12-13 and 12-14). Children born preterm should receive the full dose of each vaccine at the appropriate chronologic age. A recommended catch-up schedule for children not immunized during infancy is available at the Centers for Disease Control and Prevention website (www.cdc.gov/vaccines/recs/schedules/child-schedule.htm). Table 12-5 describes immunization schedules for Canadian children.
TABLE 12-5
ROUTINE IMMUNIZATION SCHEDULE FOR INFANTS AND CHILDREN: CANADA, 2006
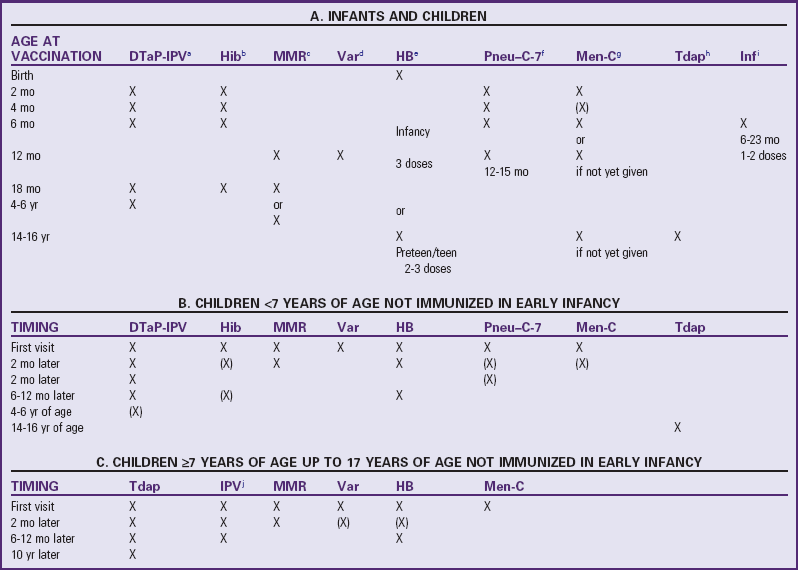
Parentheses imply that these doses may not be required, depending on the age of the child or adult.
aDiphtheria, tetanus, acellular pertussis, and inactivated polio virus vaccine (DTaP-IPV): DTaP-IPV (±Hib) vaccine is the preferred vaccine for all doses in the vaccination series, including completion of the series in children who have received one or more doses of DPT (whole cell) vaccine (e.g., recent immigrants). In schedules A and B, the 4- to 6-yr dose can be omitted if the fourth dose was given after the fourth birthday.
bHaemophilus influenzae type b conjugate vaccine (Hib): The Hib schedule shown is for the Haemophilus b capsular polysaccharide–polyribosylribitol phosphate (PRP) conjugated to tetanus toxoid (PRP-T). For catch up, the number of doses depends on the age at which the schedule is begun. Not usually required past age 5 yr.
cMeasles, mumps, and rubella vaccine (MMR): A second dose of MMR is recommended for children at least 1 mo after the first dose for the purpose of better measles protection. For convenience, options include giving it with the next scheduled vaccination at 18 mo of age or at school entry (4-6 yr) (depending on the provincial/territorial policy) or at any intervening age that is practical. In the catch-up schedule (B), the first dose should not be given until the child is ≥12 mo old. MMR should be given to all susceptible adolescents and adults.
dVaricella vaccine (Var): Children ages 12 mo to 12 yr should receive one dose of varicella vaccine. Susceptible individuals ≥13 yr of age should receive two doses at least 28 days apart.
eHepatitis B vaccine (HB): Hepatitis B vaccine can be routinely given to infants or preadolescents, depending on the provincial/territorial policy. For infants born to chronic carrier mothers, the first dose should be given at birth (with hepatitis B immunoglobulin); otherwise, the first dose can be given at 2 mo of age to fit more conveniently with other routine infant immunization visits. The second dose should be administered at least 1 mo after the first dose, and the third at least 2 mo after the second dose, but these may fit more conveniently into the 4- and 6-mo immunization visits. A two-dose schedule for adolescents is an option.
fPneumococcal conjugate vaccine–7-valent (Pneu–C-7): Recommended for all children <2 yr of age. The recommended schedule depends on the age of the child when vaccination is begun.
gMeningococcal C conjugate vaccine (Men-C): Recommended for children <5 yr of age, adolescents, and young adults. The recommended schedule depends on the age of the individual and the conjugate vaccine used. At least one dose in the primary infant series should be given after 5 mo of age. If the provincial/territorial policy is to give Men-C to persons ≥12 mo of age, one dose is sufficient.
hDiphtheria, tetanus, acellular pertussis vaccine—adult/adolescent formulation (Tdap): A combined adsorbed “adult type” preparation for use in people ≥7 yr of age; contains less diphtheria toxoid and pertussis antigens than preparations given to younger children and is less likely to cause reactions in older people.
iInfluenza vaccine (Inf): Recommended for all children 6-23 mo of age and all persons ≥65 yr of age. Previously unvaccinated children <9 yr of age require two doses of the current season’s vaccine with an interval of ≥4 wk. The second dose within the same season is not required if the child received one or more doses of influenza vaccine during the previous influenza season.
jInactivated polio virus (IPV)
Modified from Public Health Agency of Canada: Canadian immunization guide, ed 7, Ottawa, Ontario, Canada, 2006, The Agency.
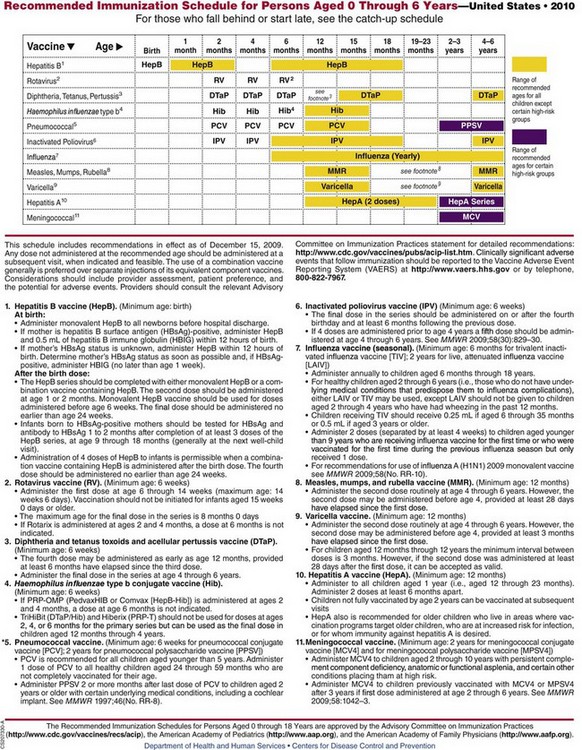
Fig. 12-13 Recommended immunization schedule for persons ages 0 through 6 years. *In March 2010 ACIP changed the recommendation for the pneumococcal vaccine; it is now recommended that PCV13 be administered to all children 2 to 59 months of age. PCV13 should replace PCV 7. (From Centers for Disease Control and Prevention: Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010, MMWR 58[51-52]:1-4, 2010.)

Fig. 12-14 Recommended immunization schedule for persons ages 7 through 18 years. (From Centers for Disease Control and Prevention: Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010, MMWR 58[51-52]:1-4, 2010.)
Children who began primary immunization at the recommended age but fail to receive all the doses do not need to begin the series again but instead receive only the missed doses. For situations in which there is doubt that the child will return for immunization according to the optimum schedule, HBV vaccine (HepB), DTaP, IPV (poliovirus vaccine), MMR, varicella, and Hib vaccines can be administered simultaneously. Parenteral vaccines are given in separate syringes in different injection sites (American Academy of Pediatrics, 2009b).
Recommendations for Routine Immunizations*
Hepatitis B Virus: HBV is a significant pediatric disease because HBV infections that occur during childhood and adolescence can lead to fatal consequences from cirrhosis or liver cancer during adulthood. Up to 90% of infants infected perinatally and 25% to 50% of children infected before age 5 years become HBV carriers. In addition, the incidence of HBV infection increases rapidly during adolescence (American Academy of Pediatrics, 2009b). It is recommended that newborns receive HepB before hospital discharge if the mother is hepatitis B surface antigen (HBsAg) negative. Monovalent HepB should be given as the birth dose, whereas combination vaccine containing HepB may be given for subsequent doses in the series (see also Fig. 12-13, footnote 1). Both full-term and preterm infants born to mothers whose HBsAg status is positive or unknown should receive HepB and hepatitis B immune globulin (HBIG), 0.5 ml, within 12 hours of birth at two different injection sites. Because the immune response to HepB is not optimum in newborns weighing less than 2000 g (4.4 lb), the first HepB dose should be given to such infants at 1 month, as long as the mother’s HBsAg status is negative (American Academy of Pediatrics, 2009b). In the event that the preterm infant is given a dose at birth, the current recommendation is that the infant be given the full series (three additional doses) at 1, 2, and 6 months of age. The American Academy of Pediatrics (2009b) also encourages immunization of all children by age 11 years.
In the late 1990s HepB contained small amounts of mercury (thimerosal) as a preservative, which generated concern regarding possible mercury poisoning in infants and led to a subsequent decrease in HepB immunization rates in newborns. However, a preservative-free HepB (Recombivax HB, pediatric-adolescent formulation) is available, and the Centers for Disease Control and Prevention (2005a) strongly recommend that HepB immunization occur in newborns before discharge from the birth hospital. To date, studies have not found any association between thimerosal in vaccines and neurologic developmental disorders such as autism spectrum disorder (DeStefano, 2007; Heron, Golding, and ALSPAC Study Team, 2004).
The vaccine is given intramuscularly in the vastus lateralis in newborns or in the deltoid for older infants and children. Regardless of age, avoid the dorsogluteal site because it has been associated with low antibody seroconversion rates, indicating a reduced immune response. No data exist regarding the seroconversion when the ventrogluteal site is used. The vaccine can be safely administered simultaneously at a separate site with DTaP, MMR, and Hib vaccines.
Hepatitis A Virus: Hepatitis A has been recognized as a significant child health problem, particularly in communities with unusually high infection rates. HAV is spread by the fecal-oral route and from person-to-person contact, by ingestion of contaminated food or water, and rarely by blood transfusion. The illness has an abrupt onset, with fever, malaise, anorexia, nausea, abdominal discomfort, dark urine, and jaundice being the most common clinical signs of infection. In children under 6 years of age, who represent approximately one third of all cases of hepatitis A, the disease may be asymptomatic, and jaundice is rarely evident.
HepA vaccine is now recommended for all children beginning at age 1 year (i.e., 12 months to 23 months). The second dose in the two-dose series may be administered no sooner than 6 months after the first dose. Since the implementation of widespread childhood HepA vaccination, infection rates among children ages 5 to 14 years have declined significantly (Centers for Disease Control and Prevention, 2006a). For further information, see Fig. 12-13, footnote 10.
Diphtheria: Although cases of diphtheria are rare in the United States, the disease can result in significant morbidity. Respiratory manifestations include respiratory nasopharyngitis or obstructive laryngotracheitis with upper airway obstruction. The cutaneous manifestations of the disease include vaginal, otic, conjunctival, or cutaneous lesions, which are primarily seen in urban homeless persons and in the tropics (American Academy of Pediatrics, 2009b). Administer a single dose of equine antitoxin intravenously to the child with clinical symptoms because of the often fulminant progression of the disease (American Academy of Pediatrics, 2009b). Diphtheria vaccine is commonly administered (1) in combination with tetanus and pertussis vaccines (DTaP) or DTaP and Hib vaccines for children younger than 7 years of age, (2) in combination with a conjugate H. influenzae type B vaccine (see Fig. 12-13), (3) in a combined vaccine with tetanus (DT) for children younger than 7 years of age who have some contraindication to receiving pertussis vaccine, (4) in combination with tetanus and acellular pertussis (Tdap) for children 11 years and older, or (5) as a single antigen when combined antigen preparations are not indicated. Although the diphtheria vaccine does not produce absolute immunity, protective antitoxin persists for 10 years or more when given according to the recommended schedule, and boosters are given every 10 years for life (see discussion below for adolescent diphtheria and acellular pertussis and tetanus toxoid recommendation). Several vaccines contain diphtheria toxoid (Hib, meningococcal, pneumococcal), but this does not confer immunity to the disease.
Tetanus: Three forms of tetanus vaccine—tetanus toxoid, tetanus immunoglobulin (TIG) (human), and tetanus antitoxin (equine antitoxin)—are available; however, tetanus antitoxin is no longer available in the United States. Tetanus toxoid is used for routine primary immunization, usually in one of the combinations listed for diphtheria, and provides protective antitoxin levels for approximately 10 years.
Tetanus and diphtheria toxoids along with acellular pertussis vaccine (Tdap, adolescent formulation) are now recommended for children ages 11 to 12 years who have completed the recommended DTaP/DTP vaccine series but have not received the tetanus (Td) booster dose. Adolescents 13 to 18 years of age who have not received the Td/Tdap booster should receive a single Tdap booster, provided the routine DTaP/DTP childhood immunization series has been previously received. It is recommended that children receive subsequent Td boosters every 10 years (American Academy of Pediatrics, 2009b) (see Fig. 12-13, footnote 3). Boostrix (Tdap) is currently licensed for children 10 to 18 years of age, whereas Adacel (Tdap) is licensed for individuals 11 to 64 years of age.
For wound management, passive immunity is available with TIG. Persons with a history of two previous doses of tetanus toxoid can receive a booster dose of the toxoid. Separate syringes and different sites are used when tetanus toxoid and TIG are given concurrently. Table 12-6 summarizes the recommended procedure for tetanus prophylaxis in wound management.
Table 12-6
GUIDE TO TETANUS PROPHYLAXIS IN ROUTINE WOUND MANAGEMENT

Td, Adult-type diphtheria and tetanus toxoids; TD, pediatric diphtheria and tetanus toxoids; Tdap, tetanus toxoid, reduced diphtheria toxoid, acellular pertussis; TIG, tetanus immunoglobulin.
aSuch as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
bFor children <7 yr old: DTaP (diphtheria-tetanus–acellular pertussis) (or DT [diphtheria-tetanus], if pertussis vaccine is contraindicated) is preferred to tetanus toxoid alone. For persons >7 yr of age, Td is preferred to tetanus toxoid alone.
cTdap is preferred to Td vaccine for adolescents who never have received Tdap vaccine. Td is preferred to tetanus toxoid (TT) vaccine in adolescents who formerly received Tdap vaccine or when Tdap is not available.
dImmune globulin intravenous should be used when TIG is not available
eIf only three doses of fluid toxoid have been received, then a fourth dose of toxoid, preferably an adsorbed toxoid, should be given. Although licensed, fluid tetanus toxoid is rarely used.
fYes, if ≥10 yr since last tetanus-containing dose.
gYes, if >5 yr since last tetanus-containing dose. (More frequent boosters are not needed and can accentuate side effects.)
Data from American Academy of Pediatrics, Committee on Infectious Diseases, Pickering L, editor: 2009 Red book: report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, Ill, 2009, The Academy.
For children over 7 years who require wound prophylaxis, tetanus immunization may be accomplished by administering Td (adult-type diphtheria and tetanus toxoids). If TIG is not available, the equine antitoxin (not available in the United States) may be administered after appropriate testing for sensitivity. The antitoxin is administered in a separate syringe and at a separate intramuscular site if given concurrently with tetanus toxoid.
Pertussis: Pertussis vaccine is recommended for all children 6 weeks through 6 years of age (up to the seventh birthday) who have no neurologic contraindications to its use. Concerns over outbreaks of the disease in the past decade have prompted discussion about vaccinating infants and adults. Many cases of pertussis have occurred in children less than 6 months or persons over 7 years, both groups falling in the category for which pertussis immunization previously was not recommended (Centers for Disease Control and Prevention, 2005c). The tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap) is now recommended at ages 11 to 12 years for children who have completed the DTaP/DTP childhood series. The Tdap is also recommended for adolescents 13 to 18 years old who have not received a tetanus booster (Td) or Tdap dose and have completed the childhood DTaP/DTP series. When the Tdap is used as a booster dose, it may be administered 5 years from the last Td dose or earlier if pertussis immunity is necessary (Centers for Disease Control and Prevention, 2009).
Currently, two forms of pertussis vaccine are available in the United States. The whole-cell pertussis vaccine is prepared from inactivated cells of Bordetella pertussis and contains multiple antigens. In contrast, the acellular pertussis vaccine contains one or more immunogens derived from the B. pertussis organism. The highly purified acellular vaccine is associated with fewer local and systemic reactions than those occurring with the whole-cell vaccine in children of similar age. The acellular pertussis vaccine is recommended by the American Academy of Pediatrics (2009b) for the first three immunizations and is usually given at 2, 4, and 6 months of age with diphtheria and tetanus (DTaP). Several forms of acellular pertussis vaccine are currently licensed for use in infants: Daptacel, Pediarix, Kinrix (DTaP and IPV), and Infanrix (diphtheria, tetanus toxoid, and acellular pertussis conjugate). Pentacel is licensed for use in infants 4 weeks old and older; in addition to acellular pertussis, diphtheria, and tetanus, this vaccine also contains inactivated poliovirus (IPV) and Hib conjugate. Either the acellular or whole-cell vaccine may be given for the fourth and fifth doses, but the acellular is preferred. It is also recommended that the first three DTaP vaccinations be from the same manufacturer. The fourth dose may be from a different manufacturer. The child who has received one or more whole-cell vaccines may complete the series of five with the acellular vaccine.
Health care workers who may be susceptible to pertussis as a result of waning immunity and who have potential exposure to children or adults with pertussis should take the necessary protective precautions against droplet contamination (wear procedural or surgical masks and practice hand washing). The diagnosis of pertussis may be missed or delayed in unvaccinated infants, who often are seen with respiratory distress and apnea without the typical cough (Centers for Disease Control and Prevention, 2005c). Additional guidelines for prevention and treatment of pertussis among health care workers and close contacts are given in the 2009 Red Book: Report of the Committee on Infectious Diseases (American Academy of Pediatrics, 2009b).
Polio: An all-IPV (inactivated poliovirus vaccine) schedule for routine childhood polio vaccination is now recommended for children in the United States. All children should receive four doses of IPV at 2 months, 4 months, 6 to 18 months, and 4 to 6 years of age (American Academy of Pediatrics, 2009b).
The change from the exclusive use of oral polio vaccine (OPV) to the exclusive use of IPV is related to the rare risk of vaccine-associated polio paralysis (VAPP) from OPV. The exclusive use of IPV eliminates the risk of VAPP but is associated with an increased number of injections and increased cost. Since IPV usage was instituted in the United States in 2000, no new indigenously acquired cases of VAPP have occurred. Pediarix is a combination vaccine containing DTaP, hepatitis B, and IPV. This may be used as the primary immunization beginning at 2 months of age. Kinrix may be used for the fourth booster dose of IPV and fifth booster dose of DTaP at 4 to 6 years of age (American Academy of Pediatrics, 2009b).
Measles: The measles (rubeola) vaccine is given at 12 to 15 months of age. During the course of measles outbreaks, the vaccine can be given any time after 6 months of age, followed by a second inoculation after age 12 months. The second measles immunization is recommended at 4 to 6 years of age (at school entry) but may be given earlier provided that 4 weeks have elapsed since the administration of the previous dose. Revaccination should occur by 11 to 12 years of age if the measles vaccine was not administered at school entry (4 to 6 years). Any child who is vaccinated before 12 months of age should receive two additional doses beginning at 12 to 15 months and separated by at least 4 weeks (American Academy of Pediatrics, 2009b). Revaccination should include all individuals born after 1956 who have not received two doses of measles vaccine after 12 months of age. Individuals born before this date are thought to be immune from exposure to natural measles virus. Because of the continuing occurrence of measles in older children and young adults, identify potentially susceptible adolescents and young adults and immunize them if two doses of measles vaccine have not been administered previously or the person had a confirmed case of the illness.
The MMRV vaccine (measles, mumps, rubella, and varicella) is an attenuated live virus vaccine and may be given to children 12 months to 12 years of age concurrent with other vaccines. Recent concerns for increased risk of febrile seizures in children 12 months to 23 months of age after administration of MMRV has prompted the ACIP (Centers for Disease Control and Prevention, 2008b) to remove its recommendation for MMRV being the preferred vaccine (versus separate injections of MMR and varicella vaccines) (American Academy of Pediatrics, 2009b).
Vitamin A supplementation has been effective in decreasing the morbidity and mortality associated with measles in developing countries. A Cochrane review of studies wherein a single dose of vitamin A was administered to children with measles found no decrease in mortality. However, children with measles under the age of 2 years who received two doses of vitamin A (200,000 international units) on consecutive days did have decreased mortality rates and a reduced rate of pneumonia-specific mortality (Huiming, Chaomin, and Meng, 2005).
Mumps: Mumps virus vaccine is recommended for children at 12 to 15 months of age and is typically given in combination with measles and rubella. It should not be administered to infants younger than 12 months because persisting maternal antibodies can interfere with the immune response. Because of continued occurrence of the disease, especially in children 10 to 19 years of age, mumps immunization is recommended for all individuals born after 1957 who may be susceptible to mumps (i.e., those who have no history of having had the disease or vaccine and who have no laboratory evidence of immunity).
Rubella: Rubella is a relatively mild infection in children, but in a pregnant woman the actual infection presents serious risks to the developing fetus. Therefore the aim of rubella immunization is actually protection of the unborn child rather than the recipient of the immunization.
Rubella immunization is recommended for all children at 12 to 15 months of age and is administered in a combined form with measles and mumps vaccine. Increased emphasis should also be placed on vaccinating all unimmunized prepubertal children and susceptible adolescents and adult women in the childbearing age-group. Because the live attenuated virus may cross the placenta and theoretically present a risk to the developing fetus, rubella vaccine is currently not given to any pregnant woman. Although this is standard practice, current evidence from women who received the vaccine while pregnant and delivered unaffected offspring indicates that the risk to the fetus is negligible. In addition, there is no reported danger of administering rubella vaccine to a child if the mother is pregnant.
Haemophilus influenzae Type b: Hib conjugate vaccines protect against a number of serious infections caused by Hib, especially bacterial meningitis, epiglottitis, bacterial pneumonia, septic arthritis, and sepsis (Hib is not associated with the viruses that cause influenza, or “flu”). Hib vaccines that are currently available include PedvaxHIB, Pentacel, and Comvax, which are combination vaccines; HibTITER; and ActHIB. Pentacel is described in the previous section on Pertussis. These conjugate vaccines connect Hib to a nontoxic form of another organism, such as meningococcal protein or diphtheria protein. There is no antibody response to these nontoxic proteins, but they significantly improve the antibody response to Hib, especially in infants. The use of combination vaccines provides equivalent immunogenicity and decreases the number of injections an infant receives. However, it is important that they be given to the appropriate-age child.
The 2009 Centers for Disease Control and Prevention immunization guidelines indicate there is limited data for administering the HiB vaccine to persons 5 years and older; however, the guidelines suggest that children with sickle cell disease, leukemia, or human immunodeficiency virus (HIV) infection, or children who have had a splenectomy, may benefit from one dose of the Hib vaccine (Centers for Disease Control and Prevention, 2009).
When possible, the Hib conjugate vaccine used at the first vaccination should be used for all subsequent vaccinations in the primary series. All Hib vaccines are administered by intramuscular injection using a separate syringe and at a site separate from any concurrent vaccinations.
The use of meningococcal and diphtheria proteins in combination vaccines does not mean the child has received adequate immunization for meningococcal or diphtheria illnesses. The child must be given the appropriate vaccine for that specific disease.
Varicella: Administration of the cell-free live-attenuated varicella vaccine is recommended for any susceptible child (one who lacks proof of varicella vaccination or has a reliable history of varicella infection). A single dose of 0.5 ml should be given by subcutaneous injection. The first dose of varicella vaccine is recommended for children ages 12 to 15 months, and to ensure adequate protection a second varicella vaccine is recommended for children at 4 to 6 years of age. The second varicella vaccine may be administered before 4 years of age as long as a period of 3 months occurs between the first and second doses. Children 13 years of age or older who are susceptible should receive two doses administered at least 4 weeks apart. Children in the same age-group who have received only one previous varicella vaccine should receive a second varicella vaccine. MMRV is not licensed for use in children ages 13 years or older (American Academy of Pediatrics, 2009b). The American Academy of Pediatrics (2009b) reports that the two-dose regimen was adopted to protect children who did not have adequate protection with one dose, not because of waning immunity to the vaccine.
According to the American Academy of Pediatrics (2009b), children who have received two doses of the varicella vaccine are one third less likely to have breakthrough illness in the first 10 years of immunization in comparison with those who have received one dose. Children who do contract varicella after immunization reportedly have milder cases with fewer vesicles, lower degree of fever, and faster recovery. Antibodies persist for at least 8 years (American Academy of Pediatrics, 2009b).
Keep the vaccine frozen in the lyophilic form (stable particles that readily go into solution) and use it within 30 minutes of being reconstituted to ensure viral potency.
Varicella vaccine may be administered simultaneously with MMR. However, separate syringes and injection sites should be used. If they are not administered simultaneously, the interval between administration of varicella vaccine and MMR should be at least 1 month. Varicella vaccine may also be given simultaneously with DTaP, IPV, HepB, or Hib (American Academy of Pediatrics, 2009b).
Pneumococcal: A seven-valent Streptococcus pneumoniae conjugate vaccine (PCV7, or Prevnar) has been used for children under 2 years of age since 2000. In February 2010 the Food and Drug Administration approved a new 13-valent pneumococcal vaccine, Prevnar 13, which replaces PCV7 (Prevnar). PCV13 was approved for children 6 weeks to 71 months of age (Centers for Disease Control and Prevention, 2010). Streptococcal pneumococci are responsible for a number of bacterial infections in children under 2 years, which may cause serious morbidity and mortality. Among these are generalized infections such as septicemia and bacterial meningitis or localized infections such as otitis media, sinusitis, and pneumonia. These illnesses are particularly problematic in children who attend daycare facilities (the incidence in daycare children is two to three times higher than in children not attending out-of-home daycare) and in those who are immunocompromised.
The vaccine is administered at 2, 4, and 6 months, with a fourth dose at 12 to 15 months of age. Children 7 to 11 months old may receive three doses as long as they are 6 to 8 weeks apart and a fourth dose at 12 to 15 months. Children 12 to 23 months who have not been immunized with the pneumococcal vaccine may be given two doses, 6 to 8 weeks apart. PCV13 is also recommended for all children under 24 months and in older children (24 to 71 months) with sickle cell disease; functional or anatomic asplenia; nephrotic syndrome or chronic renal failure; conditions associated with immunosuppression, such as solid organ transplantation, drug therapy, or cytoreduction therapy (including long-term systemic corticosteroid therapy); diabetes mellitus; cochlear implants; congenital immunodeficiency; HIV infection; cerebrospinal fluid leaks; chronic cardiovascular disease (e.g., congestive heart failure or cardiomyopathy); chronic pulmonary disease (e.g., emphysema or cystic fibrosis, but not asthma); chronic liver disease (e.g., cirrhosis); or exposure to living environments or social settings in which the risk of invasive pneumococcal disease or its complications is very high (e.g., Alaskan Native, African-American, and certain Native American populations) (American Academy of Pediatrics, 2009b). Low-birth-weight infants (≤1500 g [3.3 lb]) should receive the vaccine when they reach a chronologic age of 6 to 8 weeks regardless of calculated gestational age. The PCV7 vaccine may be administered in conjunction with all other immunizations in a separate syringe and at a separate intramuscular site.
The PPV (pneumococcal polysaccharide [23-valent] vaccine) is not recommended for children younger than 24 months who do not have one of the high-risk conditions described previously.
Influenza: The influenza vaccine is now recommended annually for children 6 months to 18 years. Influenza vaccine (trivalent inactivated influenza vaccine [TIV]) may be given to any healthy children 6 months old and older. Children who have a reported anaphylactic hypersensitivity to eggs should not receive the vaccine. The vaccine is administered in early fall before the flu season begins and is repeated yearly for ongoing protection. The intramuscular vaccine is administered as two separate doses 4 weeks apart in first-time recipients under the age of 9 years. The dose is 0.25 ml for children ages 6 to 35 months and 0.5 ml for children 3 years and above. The vaccine may be given simultaneously with other vaccines but at a separate site. The vaccine is administered yearly because different strains of influenza are used each year in the manufacture of the vaccine.
The live attenuated influenza vaccine (LAIV) is an acceptable alternative to the intramuscular trivalent vaccine in specific age-groups. The vaccine is given nasally as two doses at least 28 days apart in healthy persons ages 2 to 49 years. Although it is an alternative to the injection, it costs more and may not be covered by insurance companies. Either TIV or LAIV may be given to healthy, nonpregnant persons ages 2 to 49 years (American Academy of Pediatrics, 2009c). Yearly influenza vaccine should be administered to health care workers and to children ages 6 to 59 months with medical conditions (including asthma, cardiac disease, HIV, diabetes, and sickle cell disease) that place them at risk for influenza-related complications.
The H1N1 virus (swine flu) is a subtype of influenza type A. Previous outbreaks of H1N1 influenza occurred in 1918, and the mortality rates were significant both in the United States and worldwide (American Academy of Pediatrics, 2009b). The pandemic of H1N1 caused significant morbidity and mortality worldwide, but particularly in Mexico and the United States. Antigenic shift occurs when influenza A viruses undergo significant changes that result in new infection subtypes; such is the case in the current pandemic. The signs and symptoms of H1N1 flu are the same as those mentioned below for influenza. Oseltamivir (Tamiflu) has been approved for infants 3 months of age and older who are symptomatic and in infants 0 to 3 months if the practitioner feels it is absolutely critical to the child’s well being. In the United States the 2011-2012 winter influenza vaccines (LAIV and TIV) contain protection against the H1N1 influenza strain as well as other influenza strains. The most updated information on the status of this disease may be found at the websites for the Centers for Disease Control and Prevention (www.cdc.gov) and the World Health Organization (www.who.int/csr/disease/swineflu/en/index.html).
Meningococcal: Invasive meningococcal disease continues to be the cause of high morbidity in children in the United States. Infants younger than 1 year of age are particularly susceptible, yet the highest fatalities occur in adolescents (approximately 20%). There is also evidence that the risk of meningococcal infections is high in college freshmen living in dormitories. Meningococcal infections are also responsible for significant morbidities, including limb or digit amputation, skin scarring, hearing loss, and neurologic disabilities (American Academy of Pediatrics, 2009b).
Neisseria meningitidis is the leading cause of bacterial meningitis in the United States. It is now recommended that children 2 to 10 years old at increased risk for meningococcal disease receive either the quadrivalent conjugate vaccine MCV4 (Menactra) or MenACY-CRM (Menveo). Adolescents who received a single meningococcal vaccine prior to the age of 16 years should receive a single booster of meningococcal quadrivalent vaccine (Centers for Disease Control and Prevention, 2011). Children in certain high-risk groups such as those with terminal complement component deficiency, anatomic or functional asplenia, or HIV and children who travel to or reside in countries where N. meningitidis is hyperendemic or epidemic should also receive one of the quadrivalent meningococcal vaccines (American Academy of Pediatrics, 2009b). Children and adolescents 11 to 18 years of age should receive a single immunization of MCV4. Others at high risk who should receive MCV4 include college freshmen living in dormitories and military recruits (American Academy of Pediatrics, 2009b).
Persons who are at high risk for the disease and previously received MPSV4 3 or more years previously should be vaccinated with MCV4. The vaccine protects against meningococcal disease caused by serogroups A, C, Y, and W-135. MCV4 is administered as an intramuscular injection (0.5 ml) and may be administered in conjunction with other vaccines in a separate syringe and at a separate site. Immunization with MCV4 is contraindicated in persons with hypersensitivity to any components of the vaccine, including diphtheria toxoid, and to rubber latex (part of vial stopper).
Reports of an association between MCV4 (Menactra) and cases of Guillain-Barré syndrome in vaccinated persons ages 11 to 19 years of age have been addressed. Onset of symptoms occurred within 2 to 23 days of vaccination. A preliminary survey by the Centers for Disease Control and Prevention (2006b) indicates there are insufficient data to change the 2005 recommendation for adolescents, college freshmen residing in dormitories, and other high-risk populations.
Recommendations for Selected Immunizations
Two additional vaccines are recommended for children and adolescents at high risk for particular diseases. Two rotavirus vaccines, RotaTeq and Rotarix, have been licensed by the U.S. Food and Drug Administration for distribution in the United States. Rotavirus is one of the leading causes of severe diarrhea in infants and young children. RotaTeq is licensed for administration to infants at 6 to 12 weeks of age, with two additional doses administered at 4- to 10-week intervals but not after 32 weeks of age. The dose is 2 ml, and the product must be protected from light until administration (American Academy of Pediatrics, 2009b). Rotarix (1 ml) may be administered beginning at 6 weeks of age with a second dose at least 4 weeks after the first dose, but before 24 weeks of age. Both vaccines are administered orally.
A quadrivalent human papillomavirus (HPV) vaccine, Gardasil, has been approved and is recommended for female children and adolescents to prevent HPV-related cervical cancer. The vaccine is administered intramuscularly in three separate doses; the first dose in the series may be given at 11 to 12 years of age (minimum age 9 years), the second dose is administered 2 months after the first, and the third dose is given 6 months after the first dose (Centers for Disease Control and Prevention, 2007b). (See Fig. 12-14, footnote 2.)
Immunizations that may be used in older children and adolescents in the future and that are being evaluated include vaccines for preventing diseases such as herpes simplex virus, human cytomegalovirus, and Epstein-Barr virus. Others, such as the rabies vaccine, are discussed elsewhere in this text.
Reactions
Vaccines for routine immunizations are among the safest and most reliable drugs available. However, minor side effects do occur after many of the immunizations, and, rarely, a serious reaction may result from the vaccine. A number of inactive components are incorporated in vaccines to enhance their effectiveness and safety. Some of these components include preservatives, stabilizers, adjuvants, antibiotics (e.g., neomycin), and purified culture medium proteins (e.g., egg) to enhance effectiveness. A child may react to the preservative in the vaccine rather than the vaccine component; an example of this is the hepatitis B vaccine, which is prepared from yeast cultures. Yeast hypersensitivity therefore would preclude one from receiving that particular vaccine without consulting an allergist. Trace amounts of neomycin are used to decrease bacterial growth within certain vaccine preparations, and persons with documented anaphylactic reactions to neomycin should avoid those vaccines. Most vaccine preparations now contain vial stoppers with a synthetic rubber to prevent latex allergy reactions. In the event that an individual has a severe reaction to a vaccine and subsequent immunizations are required, an allergist may be consulted to determine the best course of action.
Some vaccines contain a preservative, thimerosal, which contains ethylmercury. Concerns regarding possible mercury poisoning in the 1990s prompted many to put off vaccination of infants and small children for fear of childhood developmental problems such as autism. A number of manufacturers have since stopped producing vaccines containing thimerosal. No local hypersensitivity reactions to thimerosal have been recorded, and studies on thimerosal and the potential link to autism or any other pervasive developmental disorder failed to establish a causal relationship between the two (DeStefano, 2007; Hviid, Stellfeld, Wohlfahrt, et al, 2003; Parker, Schwartz, Todd, et al, 2004). The Institute of Medicine (2004), following an in-depth 3-year study, issued a report concluding that there is no link between autism and the MMR vaccine or vaccines containing the preservative thimerosal. The H1N1 vaccines do not contain any additives such as thimerosal.
With inactivated antigens, such as DTaP, side effects are most likely to occur within a few hours or days of administration and are usually limited to local tenderness, erythema, and swelling at the injection site; low-grade fever; and behavioral changes (drowsiness, fretfulness, eating less, prolonged or unusual cry). Local reactions tend to be less severe when a needle of sufficient length to deposit the vaccine in the muscle is used (see Atraumatic Care box). Rarely, more severe reactions may occur, especially with pertussis and varicella. Reactions to DTaP tend to be more severe if they occurred with a previous immunization.
Hib vaccine is one of the safest vaccines available but may be associated with low-grade fever and mild local reactions at the site of injection, which resolve rapidly.
Unlike the inactivated antigens, live attenuated virus vaccines such as MMR multiply for days or weeks, and unfavorable reactions and vaccine-associated disorders can occur up to 30 to 60 days later. These reactions are usually mild, although reactions to rubella tend to be more troublesome in older children and adults.
Contraindications and Precautions
Nurses need to be aware of the reasons for withholding immunizations—both for the child’s safety in terms of avoiding reactions and for the child’s maximum benefit from receiving the vaccine. Unfounded fears and lack of knowledge regarding contraindications can needlessly prevent a child from gaining protection from life-threatening diseases. Issues that have surfaced regarding vaccines include the misconception that administering combination vaccines may overload the child’s immune system. The combined vaccines have undergone rigorous study in relation to side effects and immunogenicity rates following administration. Give parents appropriate information regarding vaccine safety, benefits, and risks so they can make informed decisions regarding vaccinations for their children. The advantage of widespread information on television and the Internet is that it is readily available at any given moment. The disadvantage is that some of this information may be incorrect, incomplete, or misleading and may influence parents to make decisions that could harm their children’s health. Parents may also receive information regarding vaccines from antivaccine groups, which advocate changes in the mass vaccination system in the United States. At times such groups may publicize information related to extremely rare events occurring after a child is immunized.
The general contraindication for all immunizations is a severe febrile illness. This precaution avoids adding the risk of adverse side effects from the vaccine to an already ill child or mistakenly identifying a symptom of the disease as having been caused by the vaccine. The presence of minor illnesses such as the common cold is not a contraindication. Live virus vaccines are generally not administered to anyone with an altered immune system, since multiplication of the virus may be enhanced, causing a severe vaccine-induced illness.
Another contraindication to live virus vaccines (MMR and varicella) is the presence of recently acquired passive immunity through blood transfusions, immunoglobulin, or maternal antibodies. Administration of MMR and varicella should be postponed for a minimum of 3 months after passive immunization with immunoglobulins and blood transfusions (except washed RBCs, which do not interfere with the immune response). Suggested intervals between administration of immunoglobulin preparations and MMR and varicella depend on the type of immune product and dosage. If the vaccine and immunoglobulin are given simultaneously because of imminent exposure to disease, the two preparations are injected at sites far from each other. Vaccination should be repeated after the suggested intervals unless there is serologic evidence of antibody production.
Pregnancy is a contraindication to MMR vaccines, although the risk of fetal damage is primarily theoretic. Breast-feeding is not a contraindication for any vaccine.
A final contraindication is a known allergic response to a previously administered vaccine or a substance in the vaccine. MMR vaccines contain minute amounts of neomycin; measles and mumps vaccines, which are grown on chick embryo tissue cultures, are not believed to contain significant amounts of egg cross-reacting proteins. Therefore only a history of anaphylactic reaction to neomycin, gelatin, or the vaccine itself is considered a contraindication to their use. To identify the rare child who may not be able to receive the vaccines, take a careful allergy history. (See Nursing Care Guidelines box, “Taking an Allergy History,” Chapter 6.) If the child has a history of anaphylaxis, report this to the practitioner before administering the vaccine. Contact dermatitis in reaction to neomycin is not considered a contraindication to immunization. Evidence indicates that children who are egg-sensitive are not at increased risk for untoward reactions to MMR vaccine. Furthermore, skin testing of egg-allergic children with vaccine has failed to predict immediate hypersensitivity reactions (American Academy of Pediatrics, 2009b).
A family history of seizures, allergies to duck meat or duck feathers, and a family history of SIDS are not considered contraindications to receiving childhood vaccines (American Academy of Pediatrics, 2009b).
Nurses are at the forefront in providing parents with appropriate information regarding childhood immunization benefits, contraindications, and side effects and the effects of nonvaccination on the child’s health. Some suggestions for communicating with parents about the benefits of immunizations in childhood include (portions adapted from Coyer, 2002; Fredrickson, Davis, and Bocchini, 2001; Rosenthal, 2004):
• Provide accurate and user-friendly information on vaccines (the necessity for each one, the disease each prevents, potential adverse effects).
• Realize that the parent is expressing concern for the child’s health.
• Acknowledge the parent’s concerns in a genuine, empathetic manner.
• Be knowledgeable about the benefits of individual vaccines, the common adverse effects, and how to minimize those effects.
• Give the parent the vaccine information statement (VIS) beforehand and be prepared to answer any questions that may arise.
• Help the parent make an informed decision regarding the administration of each vaccine.
• Be flexible and provide parents options regarding the administration of multiple vaccines, especially in infants, who must receive multiple injections at 2, 4, and 6 months (i.e., allow parents to space the vaccinations at different visits to decrease the total number of injections at each visit; make provisions for office visits for immunization purposes only [does not incur a practitioner fee except for administration of vaccine], provided the child is healthy).
• Involve the parent in minimizing the potential adverse effects of the vaccine (e.g., administering an appropriate dose of acetaminophen 45 minutes before administering the vaccine [as warranted]; applying a topical analgesic such as lidocaine-prilocaine [EMLA] or 4% lidocaine [LMX4] to the injection sites before going to the administration site [see Atraumatic Care box, p. 505]; following up to check on the child if untoward reactions have occurred in the past or parent is especially anxious about the child’s well-being).
Administration
The principal precautions in administering immunizations include proper storage of the vaccine to protect its potency and institution of recommended procedures for injection. The nurse must be familiar with the manufacturer’s directions for storage and reconstitution of the vaccine. For example, if the vaccine is to be refrigerated, it should be stored on a center shelf, not in the door, where frequent temperature increases from opening the refrigerator can alter the vaccine’s potency. For protection against light the vial can be wrapped in aluminum foil. Periodic checks are established to ensure that no vaccine is used after its expiration date.
The DTP (or DTaP) vaccines contain an adjuvant to retain the antigen at the injection site and prolong the stimulatory effect. Because subcutaneous or intracutaneous injection of the adjuvant can cause local irritation, inflammation, or abscess formation, excellent intramuscular injection technique must be used (see Atraumatic Care box, p. 505).
The total series requires several injections, and every attempt is made to rotate the sites and administer the injections as painlessly as possible. (See discussion on intramuscular injections, Chapter 27.) When two or more injections are given at separate sites, the order of injections is arbitrary. Some practitioners suggest injecting the less painful one first. Some believe this is DTP (or DTaP), whereas others suggest the MMR or Hib vaccine. Still others advocate injecting at two sites simultaneously (requires two operators) (see Research Focus box).
Because allergic reactions can occur after injection of vaccines, take the appropriate precautions. (See Nursing Alert on anaphylactic reaction, Chapter 36.)
One of the most important features of injecting vaccines is adequate penetration of the muscle for deposition of the drug intramuscularly, not subcutaneously. In two studies, the use of longer needles in administering vaccines to a group of infants significantly decreased the incidence of localized edema and tenderness (Diggle and Deeks, 2000; Diggle, Deeks, and Pollard, 2006) (see Evidence-Based Practice box).
Because nurses often administer vaccines, they may have the responsibility for adequately informing parents of the nature, prevalence, and risks of the disease; the type of immunization product to be used; the expected benefits and risk of side effects of the vaccine; and the need for accurate immunization records. Referring to immunizations as “baby shots” and limiting the discussion to vague statements about the vaccines are unacceptable practices.
Another important nursing responsibility is accurate documentation. Each child should have an immunization record for parents to keep, especially for families who move frequently. Although immunization rates have increased significantly, health professionals should use every opportunity to encourage complete immunization of all children (see Community Focus box). Blank immunization records may be downloaded from a number of websites, including the Immunization Action Coalition (www.immunize.org), which has vaccine information and records in a number of languages.
Document the following information on the medical record: day, month, and year of administration; manufacturer and lot number of vaccine; and name, address, and title of the person administering the vaccine. Additional data to record are the site and route of administration and evidence that the parent or legal guardian gave informed consent before the immunization was administered. Report any adverse reactions after the administration of a vaccine to the Vaccine Adverse Event Reporting System (www.vaers.hhs.gov; 800-822-7967).
An additional source of vaccine information that must be given to parents (as required by the National Childhood Vaccine Injury Act, 1986) before the administration of vaccines is the VIS for the particular vaccine being administered. Practitioners are required by law to fully inform families of the risks and benefits of the vaccines. VISs are designed to provide updated information to the adult vaccinee or parents or legal guardians of children being vaccinated regarding the risks and benefits of each vaccine. The practitioner should answer questions regarding the information in the VISs. VISs are available for the following vaccines: anthrax, tetanus, diphtheria, pertussis, MMR, IPV, HPV, varicella, Hib, H1N1 influenza, influenza, meningococcal, pneumococcal, rabies, rotavirus, shingles, smallpox, yellow fever, Japanese encephalitis, typhoid, and hepatitis A and B. An updated VIS should be provided to the primary caregiver, and documentation in the patient’s chart should include the VIS title and the VIS publication date. VISs are available from state or local health departments, the Immunization Action Coalition (www.immunize.org/vis), and the Centers for Disease Control and Prevention (http://www.cdc.gov/vaccines/pubs/vis/default.htm; 800-232-4636).
In response to the concerns of manufacturers, practitioners, and parents of children with serious vaccine-associated injuries, the National Childhood Vaccine Injury Act of 1986 and the Vaccine Compensation Amendments of 1987 were passed. These laws are designed to provide fair compensation for children who are inadvertently injured and provide greater protection from liability for vaccine manufacturers and providers. (See American Academy of Pediatrics, 2009b, for further details of this program.)
Bioterrorism and Vaccines: A number of events, including those of September 11, 2001, have precipitated changes in family’s lives across the United States. The threats of anthrax and smallpox germ warfare have prompted public safety and health care officials to reevaluate disasters and threats to the general population’s health. Children are aware of media stories discussing potential threats and may have concerns and fears regarding their personal, family’s, and friends’ safety and health. A common theme expressed among adolescents after the September 11 attack and various wide-scale shootings in high schools was concern and fear for personal safety and the general fear of the unknown outcome in the event of another attack in their community or school.
Nurses are in a position to help families deal effectively with children’s fears and concerns related to events that may affect their mental and physical health. It is not within the scope of this text to discuss the many strategies for counseling children about natural and man-made disasters, but it is important to be knowledgeable about health issues related to vaccines should an event occur that requires wide-scale inoculation of children and adults.
The American Academy of Pediatrics offers a number of resources regarding disasters (www.aap.org/disasters/resources.cfm), including a free Family Readiness Kit that may help parents discuss disaster issues with children (www.aap.org/family/frk/frkit.htm). Starr (2002) provides a number of strategies for helping children of different ages cope with disaster and numerous excellent resources for parents and nurses helping children.
Nurses working with children may use these resources and others to help families and children become knowledgeable about disasters and vaccines developed for the protection of children and adults in the event of exposure to toxic agents.
 inch).
inch). to 1 inch) for deltoid, or 25 to 32 mm (1 to
to 1 inch) for deltoid, or 25 to 32 mm (1 to 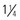 inches) for vastus lateralis (
inches) for vastus lateralis (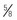 -inch) needle (
-inch) needle (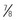 to 1 inch) needle can be used in infants 4 months and older. The
to 1 inch) needle can be used in infants 4 months and older. The 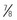 - to
- to 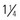 -inch) needle in the deltoid if muscle size is adequate; a minimum of a 25-mm long needle is recommended for anterolateral thigh injection in toddlers. Both the
-inch) needle in the deltoid if muscle size is adequate; a minimum of a 25-mm long needle is recommended for anterolateral thigh injection in toddlers. Both the 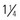 -inch) needle for toddlers, and 38- to 51-mm (
-inch) needle for toddlers, and 38- to 51-mm (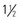 - to 2-inch) needle for older children; preterm and small emaciated infants may require a shorter needle (16 to 25 mm [
- to 2-inch) needle for older children; preterm and small emaciated infants may require a shorter needle (16 to 25 mm [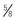 to 1 inch]) based on weight and muscle mass size.
to 1 inch]) based on weight and muscle mass size.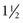 minutes. In a similar study of infants treated for accidents, the bed was commonly listed as being involved, whereas car seat at 2 months of age and stairs at 12 months were reported to be the cause of the accidental injury (
minutes. In a similar study of infants treated for accidents, the bed was commonly listed as being involved, whereas car seat at 2 months of age and stairs at 12 months were reported to be the cause of the accidental injury (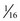 inch. However, the safety of any extension is questionable. Decorative extensions need to be removed from cribs. Ideally, information regarding correct crib design should be given prenatally, before parents have purchased or borrowed a crib.
inch. However, the safety of any extension is questionable. Decorative extensions need to be removed from cribs. Ideally, information regarding correct crib design should be given prenatally, before parents have purchased or borrowed a crib.
