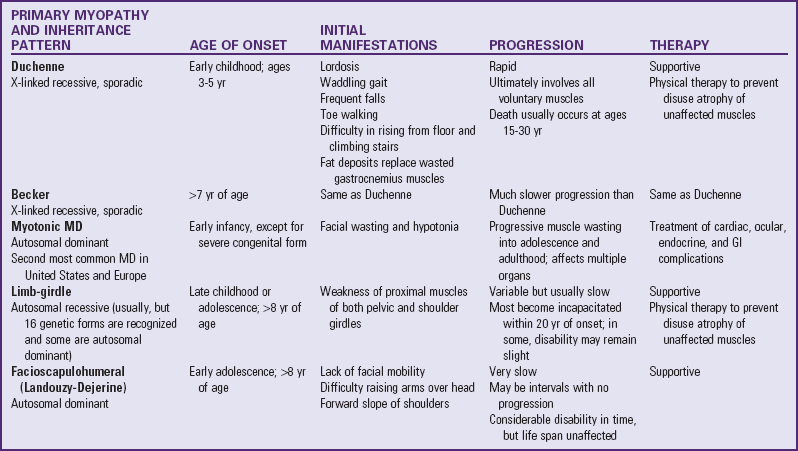
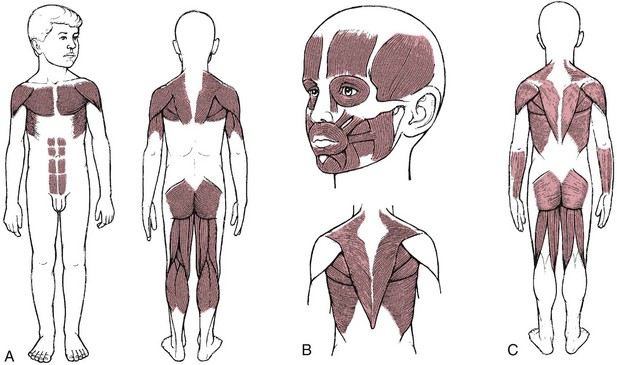
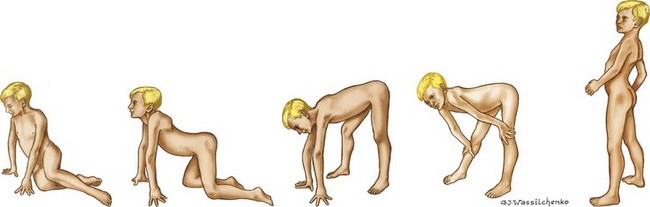
Guillain-Barré Syndrome
![]() Guillain-Barré syndrome (GBS), also known as infectious polyneuritis, is an uncommon acute demyelinating polyneuropathy with a progressive, usually ascending flaccid paralysis. The hallmark of GBS is acute peripheral motor weakness. The paralysis usually occurs approximately 10 days after a nonspecific viral infection (Sarnat, 2007). Several subtypes of GBS include acute inflammatory demyelinating neuropathy, acute motor axonal neuropathy, acute motor sensory axonal neuropathy, and Miller Fisher syndrome. Children are less often affected than adults; among children, those between ages 4 and 10 years have higher susceptibility. The male/female ratio is reported to be 1.5 : 1. Two peak periods with an increased incidence of GBS have been identified: late adolescence and young adulthood.
Guillain-Barré syndrome (GBS), also known as infectious polyneuritis, is an uncommon acute demyelinating polyneuropathy with a progressive, usually ascending flaccid paralysis. The hallmark of GBS is acute peripheral motor weakness. The paralysis usually occurs approximately 10 days after a nonspecific viral infection (Sarnat, 2007). Several subtypes of GBS include acute inflammatory demyelinating neuropathy, acute motor axonal neuropathy, acute motor sensory axonal neuropathy, and Miller Fisher syndrome. Children are less often affected than adults; among children, those between ages 4 and 10 years have higher susceptibility. The male/female ratio is reported to be 1.5 : 1. Two peak periods with an increased incidence of GBS have been identified: late adolescence and young adulthood.
![]() Critical Thinking Exercise—Guillain-Barré Syndrome
Critical Thinking Exercise—Guillain-Barré Syndrome
Congenital GBS is rare yet may occur in the neonatal period and consists of hypotonia, weakness, and decreased or absent reflexes. Maternal neuromuscular disease may or may not be present. Diagnosis is established by the same criteria as in older children, but the symptoms gradually subside over the first few months of life and disappear by 12 months (Sarnat, 2007).
Concerns over the incidence of GBS in those vaccinated for swine influenza in the 1970s prompted a study of the incidence of influenza vaccine–associated GBS in adults. From 1990 through 2003 the incidence of vaccine-associated GBS in adults decreased significantly (fourfold) from 0.17 cases per 100,000 vaccines in 1993 to 1994 to 0.04 per 100,000 vaccines in 2002 to 2003 (Haber, DeStefano, Angulo, et al, 2004). Twenty-two cases of GBS were reported in adolescents ages 11 to 19 years who had received the meningococcal vaccine MCV4 between June 2005 and October 2007. The American Academy of Pediatrics (2009) indicates that there is no conclusive evidence of MCV4 causing GBS, yet suggests that children receive MPSV4 if they have had GBS. The Centers for Disease Control and Prevention notes that since the 1976 swine influenza–associated cases of GBS, there have been no significant increases noted in cases of GBS related to influenza vaccine administration (Fiore, Shay, Broder, et al, 2009). The Centers for Disease Control recommends influenza vaccination for all children ages 6 months to 18 years but cautions against vaccinating persons with GBS or those who have had GBS in the previous 6 weeks.
Pathophysiology
![]() GBS is an immune-mediated disease often associated with a number of viral or bacterial infections or the administration of vaccines. It has been associated with infectious mononucleosis, measles, mumps, Campylobacter jejuni (gastroenteritis), cytomegalovirus, Borrelia burgdorferi (Lyme disease), Epstein-Barr virus, Helicobacter pylori, and Mycoplasma and Pneumocystis infections. Previous infection with C. jejuni is associated with a severe form of GBS.
GBS is an immune-mediated disease often associated with a number of viral or bacterial infections or the administration of vaccines. It has been associated with infectious mononucleosis, measles, mumps, Campylobacter jejuni (gastroenteritis), cytomegalovirus, Borrelia burgdorferi (Lyme disease), Epstein-Barr virus, Helicobacter pylori, and Mycoplasma and Pneumocystis infections. Previous infection with C. jejuni is associated with a severe form of GBS.
![]() Animation—Guillain-Barré Syndrome
Animation—Guillain-Barré Syndrome
Pathologic changes in spinal and cranial nerves consist of inflammation and edema with rapid, segmented demyelination and compression of nerve roots within the dural sheath. Nerve conduction is impaired, producing ascending partial or complete paralysis of muscles innervated by the involved nerves. GBS has three phases (Newswanger and Warren, 2004):
1. Acute or progressive—This phase begins with onset of symptoms and continues until new symptoms stop appearing or deterioration ceases; may last as long as 4 weeks.
2. Plateau—Symptoms remain constant without further deterioration; may last from days to weeks.
3. Recovery—Patient begins to improve and progress to complete recovery; usually lasts a few weeks to a few months.
Clinical Manifestations
A mild influenza-like illness or sore throat usually precedes the paralytic manifestations of GBS. The onset can be rapid, reaching peak activity within 24 hours, or there may be a gradual progression of symptoms over days or weeks. Neurologic symptoms initially involve muscle tenderness that sometimes is accompanied by paresthesia and cramps. Proximal muscle weakness progressing to paralysis usually occurs before distal weakness, and there is a tendency toward symmetric involvement. In most patients paralysis ascends from the lower extremities, often involving the muscles of the trunk and upper extremities and those supplied by cranial nerves. The seventh cranial (facial) nerve is often affected.
Tendon reflexes are depressed or absent, and paralysis is flaccid. Paralysis may involve facial, extraocular, labial, lingual, pharyngeal, and laryngeal muscles. Evidence of intercostal and phrenic nerve involvement includes breathlessness in vocalizations and shallow, irregular respirations. There may be variable degrees of sensory impairment. Most patients complain of muscle tenderness or sensitivity to slight pressure. Lower limb pain and back pain are common in children with GBS. Urinary incontinence or retention and constipation are often present. Abdominal pain and fatigue have also been reported in children with GBS (Lyons, 2008).
Autonomic nervous system disturbances may occur in children and adolescents with severe muscle involvement and respiratory muscle paralysis. These include orthostatic hypotension; hypertension; and vagal responses such as bradycardia, asystole, and heart block (Laskowski-Jones, 2007).
Diagnostic Evaluation
Diagnosis is based on the paralytic manifestations and on EMG. Motor nerve conduction velocities are greatly reduced. Sensory nerve conduction time is often slowed. Cerebrospinal fluid analysis reveals an elevated protein concentration, and normal glucose level, but other laboratory studies are noncontributory. The symmetric nature of the paralysis helps differentiate this disorder from spinal paralytic poliomyelitis, which usually affects sporadic muscles.
Therapeutic Management
Treatment of GBS is primarily supportive. In the acute phase, patients are hospitalized because respiratory and pharyngeal involvement may require assisted ventilation, sometimes with a temporary tracheotomy. Treatment modalities include aggressive ventilatory support, intravenous (IV) administration of immunoglobulin (IVIG), and steroids; plasmapheresis and immunosuppressive drugs may also be used. Plasmapheresis has been shown to decrease the length of recovery in patients with severe GBS yet is expensive, and side effects include hypotension, fever, bleeding disorders, chills, urticaria, and bradycardia. Further evidence reports equal benefits to treatment of GBS with IVIG administration or plasmapheresis; both sped up recovery time in studies reviewed (Hughes and Cornblath, 2005). There is evidence of significant improvement in children with IVIG therapy (versus supportive treatment alone), and IVIG therapy is more cost-effective than plasmapheresis (Harel and Schoenfeld, 2005; Hughes, Raphael, Swan, et al, 2006; Tsai, Wang, Liu, et al, 2007). IVIG is now recommended as the primary treatment of GBS when administered within 2 weeks of diseases onset (Hughes, 2008). Corticosteroids alone do not decrease the symptoms or shorten the duration of the disease.
Medications that may be administered during the acute phase include a low-molecular-weight heparin to prevent deep vein thrombosis (DVT), a mild laxative or stool softener to prevent constipation, pain medication such as acetaminophen, and a histamine-antagonist to prevent stress ulcer formation. Chronic neuropathic pain following GBS may be treated with gabapentin, which is reported to be more effective than carbamazepine (Sarnat, 2007).
Rehabilitation after the acute phase may involve physical therapy, occupational therapy, and speech therapy. Additional consideration should be given to problems of general weakness and retraining for toileting and feeding (Lyons, 2008).
Prognosis: Recovery usually begins within 2 to 3 weeks, and most patients regain full muscle strength. The recovery of muscle strength progresses in the reverse order of onset of paralysis, with lower extremity strength being the last to recover. There are few long-term outcome studies in children, but in one study 23% of the children had a residual weakness in at least one muscle group (Vajsar, Fehlings, and Stephens, 2003). Poor prognosis with subsequent residual effects in children is reportedly associated with cranial nerve involvement, extensive disability at time of presentation, and intubation (Sarnat, 2007).
The rate of recovery is usually related to the degree of involvement, which may extend from a few weeks to months. The greater the degree of paralysis, the longer the recovery phase.
Nursing Care Management
Nursing care is essentially supportive and is the same as that required for the child with immobilization and respiratory compromise. The emphasis of care is on close observation to assess the extent of paralysis and on prevention of complications, including aspiration, atelectasis, DVT, pressure ulcer, fear and anxiety, autonomic dysfunction, and pain.
During the acute phase of the disease the nurse should carefully observe the child’s condition for possible difficulty in swallowing and respiratory involvement. Closely monitor the child’s respiratory function, and keep the oxygen source, appropriate-sized insufflation bag and mask, endotracheal intubation and suctioning equipment, tracheotomy tray, and vasoconstrictor drugs available. Monitor vital signs frequently, including neurologic signs and level of consciousness. For the child who develops respiratory impairment, the care is the same as that for any child with respiratory distress requiring mechanical ventilation.
Respiratory care, should intubation be required, requires close monitoring of oxygenation status (usually by pulse oximetry and sometimes arterial blood gases), maintenance of an open airway with suctioning, and postural changes to prevent pneumonia. Children with oral and pharyngeal involvement may be fed via a nasogastric or gastrostomy tube to ensure adequate feeding. Immobilization, which occurs with GBS, decreases gastrointestinal function; therefore attention to problems such as decreased gastric emptying, constipation, and feeding residuals require nursing assessment and appropriate collaborative interventions. Temporary urinary catheterization may be required; urinary retention is not uncommon, and appropriate assessment of urinary output is vital. Sensory impairment and paralysis in the lower extremities make the child susceptible to skin breakdown; therefore attention should be given to meticulous skin care. Passive range-of-motion exercises and application of orthoses to prevent muscle contractures are important when paralysis is present. Prevention of DVT is accomplished with pneumatic compression (antiembolism) devices, administration of a low-molecular-weight heparin, and early mobilization and ambulation. Autonomic dysfunction may be life threatening; thus close monitoring of vital signs in the acute phase is essential.
A key to recovery in the child with GBS is the prevention of muscle and joint contractures, so passive range-of-motion exercises must be carried out routinely to maintain vital function. Although the child may have a generalized paralysis, cognitive function remains intact; therefore it is important for nursing care to involve communication with the child regarding procedures and treatments that may be frightening, especially if mechanical ventilation is required. Encourage parents to talk to the child and make eye and physical contact as much as possible to reassure the child during the illness.
Pain management is crucial in the care of children with GBS. Although neuromuscular impairment may make pain perception more difficult to accurately evaluate, use objective pain scales. Carbamazepine and gabapentin may be used to manage neuropathic pain in patients with GBS.
Physical therapy may be limited to passive range-of-motion exercises during the evolving phase of the disease. Later, as the disease stabilizes and recovery begins, an active physical therapy program is implemented to prevent contracture deformities and facilitate muscle recovery. This may include active exercise, gait training, and bracing.
Throughout the course of the illness, child and parent support is paramount. The usual rapidity of the paralysis and the long recovery period greatly tax the emotional reserves of all family members. The parents and child benefit from repeated reassurance that recovery is occurring and from realistic information regarding the possibility of permanent disability. In the event of a residual disability, the family needs assistance in accepting and adjusting to the loss of function. (See Chapter 22.) The GBS/CIDP Foundation International* is a nonprofit organization devoted to support, education, and research. It provides families with support from recovered persons, publishes informational literature and a newsletter, and maintains a list of practitioners experienced with the disease.


 Forrest Ave., Narberth, PA 19072; 610-667-0131, 866-224-3301;
Forrest Ave., Narberth, PA 19072; 610-667-0131, 866-224-3301;