Ervin E. Jones
Following ovulation, the fimbriae of the fallopian tube sweep over the ovarian surface and pick up the oocyte—surrounded by its complement of granulosa cells, the cumulus oophorus, and corona radiata (see Chapter 55)—and deposit it in the fallopian tube. Shortly after ovulation, movements of the cilia and the smooth muscle of the fallopian tube propel the oocyte-cumulus complex toward the uterus.
A man normally deposits 150 to 600 million sperm into the vagina of a woman at the time of ejaculation. Only 50 to 100 of these sperm actually reach the ampullary portion of the fallopian tube, where fertilization normally occurs. However, the sperm get there very quickly, within ~5 minutes of ejaculation. The swimming motion of the sperm alone cannot account for such rapid transport. Forceful contractions of the uterus, cervix, and fallopian tubes propel the sperm into the upper reproductive tract during female orgasm. Prostaglandins in the seminal plasma may induce further contractile activity.
As discussed in Chapter 54, maturation of sperm continues while they are stored in the epididymis. In most species, neither freshly ejaculated sperm cells nor sperm cells that are removed from the epididymis are capable of fertilizing the egg until these cells have undergone further maturation (capacitation) in the female reproductive tract or in the laboratory. Capacitation is a poorly understood physiological process by which spermatozoa acquire the ability to penetrate the zona pellucida of the ovum. The removal or modification of a protective protein coat from the sperm cell membrane appears to be an important molecular event in the process of capacitation. (See Note: Role of a Bicarbonate-Activated Adenylyl Cyclase in Sperm Capacitation)
In women, sperm do not need to pass through the cervix and uterus to achieve capacitation. Successful pregnancy can occur with gamete intrafallopian transfer (GIFT), in which spermatozoa and oocytes are placed directly into the ampulla of the fallopian tube, and also with direct ultrasound-guided intraperitoneal insemination, in which the sperm are deposited in the peritoneal cavity, near the fimbria. Thus, capacitation of sperm in the reproductive tract is not strictly organ specific. As evidenced by the success of in vitro fertilization and embryo transfer (IVF-ET; see the box titled In Vitro Fertilization and Embryo Transfer), capacitation is feasible even if the sperm does not make contact with the female reproductive tract.
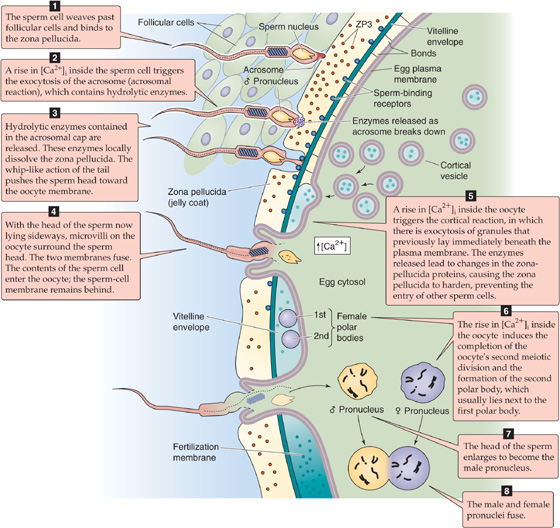
After ovulation, the egg in the fallopian tube is in a semidormant state. If it remains unfertilized, the ripe egg will remain quiescent for some time and eventually degenerates. In the case of fertilization, the sperm normally comes into contact with the oocyte in the ampullary portion of the tube, usually several hours after ovulation. Fertilization causes the egg to awaken (activation), thus initiating a series of morphological and biochemical events that lead to cell division and differentiation. Fertilization occurs in eight steps:
Step 1. The sperm head weaves its way past the follicular cells and attaches to the zona pellucida that surrounds the oocyte (Fig. 56-1). The zona pellucida is composed of three glycoproteins; ZP1 cross-links the filamentous ZP2 and ZP3 into a latticework. Receptors on the plasma membrane of the sperm cell bind to ZP3, thereby initiating a signal transduction cascade.

Figure 56-1 Fertilization. The illustration summarizes the eight steps of fertilization. ZP, zona pellucida.
Step 2. As a result of the sperm-ZP3 interaction, the sperm cell undergoes the acrosomal reaction, a prelude to the migration of the sperm cell through the mucus-like zona pellucida. The acrosome is a unique sperm organelle, essentially a large secretory vesicle, that originates from the Golgi complex in the spermatid (see Chapter 54). The acrosome contains hydrolyzing enzymes that are necessary for the sperm to penetrate the zona pellucida. The acrosome lies in front of and around the anterior two thirds of the sperm nucleus, much like a motorcycle helmet fits over one’s head. During the acrosomal reaction, an increase in [Ca2+]i triggers fusion of the outer acrosomal membrane with the sperm cell’s plasma membrane and results in the exocytosis of most of the acrosomal contents.
Step 3. The spermatozoon penetrates the zona pellucida. One mechanism of this penetration is the action of the acrosomal enzymes. Protease inhibitors can block the penetration of spermatozoa through the zona pellucida. The sperm cell also penetrates the zona pellucida by mechanical action. The sperm head rapidly oscillates about a fulcrum that is situated in the neck region. This rapid, vigorous, rocking action occurs with a frequency of approximately six to eight oscillations per second. The sperm penetrates the zona pellucida at an angle, thus creating a tangential cleavage slit and leaving the sperm head lying sideways against the oocyte membrane. (See Note: Acrosomal Enzymes)
Step 4. The cell membranes of the sperm and the oocyte fuse. Microvilli on the oocyte surface envelop the sperm cell, which probably binds to the oocyte membrane through specific proteins on the surfaces of the two cells. The posterior membrane of the acrosome—which remains part of the sperm cell after the acrosomal reaction—is the first portion of the sperm to fuse with the plasma membrane of the egg. The sperm cell per se does not enter the oocyte. Rather, the cytoplasmic portions of the head and tail enter the oocyte and leaving the sperm cell plasma membrane behind, similar to a snake’s crawling out of its skin.
Step 5. The oocyte undergoes the cortical reaction. As the spermatozoon penetrates the oocyte’s plasma membrane, it initiates formation of inositol 1, 4, 5-triphosphate (IP3) and causes Ca2+ release from internal stores (see Chapter 3) and an increase in [Ca2+]i and [Ca2+]i waves. This rise in [Ca2+]i, in turn, triggers the oocyte’s second meiotic division—discussed later—and the cortical reaction. In the cortical reaction, small electron-dense granules that lie just beneath the plasma membrane fuse with the oocyte’s plasma membrane. Exocytosis of these granules releases enzymes that act on glycoproteins in the zona pellucida and cause them to harden. In the process, polysaccharides are liberated from these glycoproteins. From a teleological perspective, the cortical granule reaction prevents polyspermy. Polyspermic embryos are abnormal because they are polyploid. They do not develop beyond the early cleavage stages. (See Note: Block to Polyspermy)
Step 6. The oocyte completes its second meiotic division. The oocyte, which had been arrested in the prophase of its first meiotic division since fetal life (see Chapter 53), completed its first meiotic division at the time of the surge of luteinizing hormone (LH), which occurred several hours before ovulation (see Chapter 55). The results were the first polar body and a secondary oocyte with a haploid number of duplicated chromosomes (see Fig. 53-1). Before fertilization, this secondary oocyte had begun a second meiotic division, which was arrested in metaphase. The rise in [Ca2+]i inside the oocyte—which the sperm cell triggers, as noted earlier—causes not only the cortical reaction but also the completion of the oocyte’s second meiotic division. One result is the formation of the second polar body, which contains a haploid number of unduplicated maternal chromosomes. The oocyte extrudes the chromosomes of the second polar body, together with a small amount of ooplasm, into a space immediately below the zona pellucida; the second polar body usually lies close to the first polar body. The nucleus of the oocyte also contains a haploid number of unduplicated chromosomes. As its chromosomes decondense, the nucleus of this mature ovum becomes the female pronucleus. (See Note: Meiosis in Males versus Females)
Step 7. The sperm nucleus decondenses and transforms into the male pronucleus, which, like the female pronucleus, contains a haploid number of unduplicated chromosomes (see Fig. 54-6). The cytoplasmic portion of the sperm’s tail degenerates.
Step 8. The male and female pronuclei fuse, to form a new cell, the zygote. The mingling of chromosomes (syngamy) can be considered as the end of fertilization and the beginning of embryonic development. Thus, fertilization results in a conceptus that bears 46 chromosomes, 23 from the maternal gamete and 23 from the paternal gamete. As noted in Chapter 53, fertilization of the ovum by a sperm bearing an X chromosome produces a zygote with XX sex chromosomes; this develops into a female. Fertilization with a Y-bearing sperm produces an XY zygote, which develops into a male. Therefore, chromosomal sex is established at fertilization.
As discussed, the ovum is fertilized in the ampullary portion of the fallopian tube several hours after ovulation (Fig. 56-2), and the conceptus remains in the fallopian tube for ~72 hours, during which time it develops to the morula stage (i.e., a mulberry-shaped solid mass of 12 or more cells), receiving nourishment from fallopian tube secretions. During these 3 days, smooth muscle contractions of the isthmus prevent advancement of the conceptus into the uterus while the endometrium is preparing for implantation. The mechanisms by which the ovum is later propelled through the isthmus of the fallopian tube to the uterus probably include beating of the cilia of the tubal epithelium and contraction of the fallopian tube.
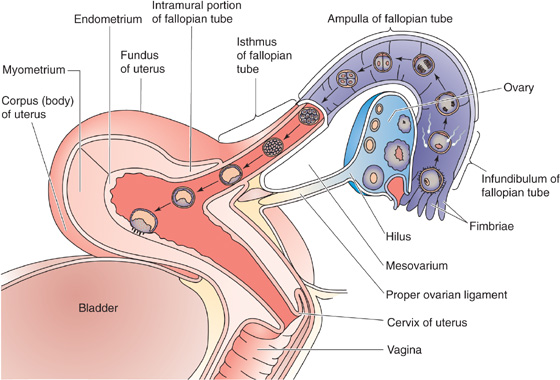
Figure 56-2 Transport of the conceptus to the uterus.
After the morula rapidly moves through the isthmus to the uterine cavity, it floats freely in the lumen of the uterus and transforms into a blastocyst (Fig. 56-2). A blastocyst is a ball-like structure with a fluid-filled inner cavity. Surrounding this cavity is a thin layer of trophoectoderm cells that forms the trophoblast, which develops into a variety of supporting structures, including the amnion, the yolk sac, and the fetal portion of the placenta. On one side of the cavity, attached to the trophoblast, is an inner cell mass, which develops into the embryo proper. The conceptus floats freely in the uterine cavity for ~72 hours before it attaches to the endometrium. Thus, implantation of the human blastocyst normally occurs 6 to 7 days following ovulation. Numerous maturational events occur in the conceptus as it travels to the uterus. The embryo must be prepared to draw nutrients from the endometrium on arrival in the uterine cavity, and the endometrium must be prepared to sustain the implantation of the blastocyst. Because of the specific window in time during which implantation can occur, temporal relationships between embryonic and endometrial maturation assume extreme importance.
During the middle to late secretory phase of the normal endometrial cycle, the endometrium becomes more vascularized and thickened, and the endometrial glands become tortuous and engorged with secretions. These changes, driven by progesterone from the corpus luteum, peak at ~7 days after ovulation. Additionally, beginning 9 to 10 days after ovulation, a process known as predecidualization begins near the spiral arteries (see Chapter 55). During predecidualization, stromal cells transform into rounded decidual cells, and these cells spread across the superficial layer of the endometrium to make it more compact (zona compacta) and to separate it from the deeper, more spongy layer (zona spongiosa; see Fig. 55-13). If conception fails to occur, the secretory activity of the endometrial glands decreases, followed by regression of the glands 8 to 9 days after ovulation, which is ultimately followed by menstruation.
When pregnancy occurs, the predecidual changes in the endometrium are sustained and extended, thus completing the process of decidualization. The decidua is the specialized endometrium of pregnancy. Its original name was membrana decidua, a term referring to the membranes of the endometrium that are shed following pregnancy, like the leaves of a deciduous tree. Because the degree of decidualization is considerably greater in conception cycles than in nonconception cycles, it is likely that the blastocyst itself promotes decidualization. Indeed, either the presence of the embryo or a traumatic stimulus that mimics the embryo’s invasion of the endometrium induces changes in the endometrium. (See Note: Onset of Decidualization)
The area underneath the implanting embryo becomes the decidua basalis (Fig. 56-3). Other portions of the decidua that become prominent later in pregnancy are the decidua capsularis, which overlies the embryo, and the decidua parietalis, which covers the remainder of the uterine surface. The upper zona compacta layer and the middle zona spongiosa layer of the nonpregnant endometrium are still recognizable in the decidualized endometrium of pregnancy. The glandular epithelium within the zona spongiosa continues its secretory activity during the first trimester. Some of the glands take on a hypersecretory appearance in what has been referred to as the Arias-Stella phenomenon of early pregnancy—named after the pathologist Javier Arias-Stella. Although the decidualized endometrium is most prominent during the first trimester, before the establishment of the definitive placenta, elements of decidualization persist throughout gestation.
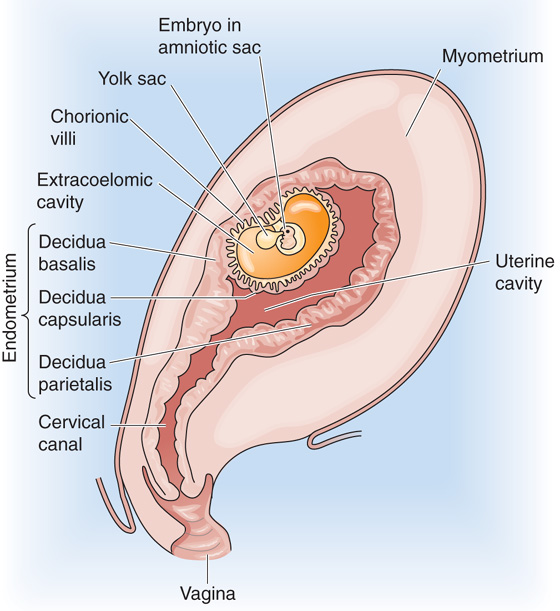
Figure 56-3 The three decidual zones during early embryonic development (~13 to 14 days after fertilization). The figure shows a sagittal section through a pregnant uterus, with the anterior side to the right.
In Vitro Fertilization and Embryo Transfer
IVF is a procedure in which an oocyte is or oocytes are removed from a woman and are then fertilized with sperm under laboratory conditions. Early development of the embryo also proceeds under laboratory conditions. Finally, the physician transfers one or more embryos to the uterine cavity, where the embryo will, one hopes, implant and develop.
Indications. Indications for IVF-ET include disorders that impair the normal meeting of the sperm and the egg in the distal portion of the fallopian tube. In addition to ovulatory dysfunction, these disorders include tubal occlusion, tubal-peritoneal adhesions, endometriosis, or other disease processes of the female peritoneal cavity. In addition, IVF-ET is indicated in some cases of male-factor infertility (abnormalities in male reproductive function) or unexplained infertility.
Ovarian stimulation. Because the success rates are less than 100% for each of the stages of IVF-ET, the physician needs several oocytes, all obtained in a single ovarian cycle. However, a woman normally develops a single dominant follicle each cycle (see Chapter 55). Thus, to obtain the multiple oocytes for IVF-ET, the physician must stimulate the development of multiple follicles in the woman by controlled ovarian hyperstimulation. Although this procedure qualitatively mimics the hormonal control of the normal cycle, the high dose of gonadotropins triggers the development of many follicles. The physician administers some combination of FSH and LH or pure FSH preparation, either intramuscularly or subcutaneously. Because exogenous gonadotropins stimulate the ovaries directly, GnRH analogues (see box in Chapter 55 on therapeutic uses of GnRH) are often used to downregulate the hypothalamic-pituitary axis during controlled ovarian stimulation. One usually administers these GnRH analogues before initiating gonadotropin therapy, primarily to prevent a premature LH surge and ovulation.
Cycle monitoring. After administering the gonadotropins, the physician monitors the simulated follicular growth in the ovaries with sonographic imaging. Size, number, and serial growth of ovarian follicles may be assessed daily or at other appropriate intervals. Serum estradiol levels provide an additional measure of follicular growth and function. When estradiol levels and follicular growth indicate—by established criteria—appropriate folliculogenesis, the physician simulates a natural LH surge by injecting hCG, which is a close relative of LH (see Chapter 55). However, in this case, the simulated LH surge completes the final maturation of multiple follicles and oocytes. As we already know, ovulation usually occurs 36 to 39 hours following the beginning of the LH surge. Thus, the physician plans oocyte retrieval in such a way to allow maximal follicular maturation, but still to harvest the oocytes before ovulation. Thus, retrievals are scheduled for 34 to 36 hours following the administration of hCG.
Oocyte retrieval. The physician retrieves oocytes by aspirating them from individual follicles, under sonographic guidance. With the patient under conscious or unconscious sedation, and after applying a local anesthetic to the posterior vaginal wall, the physician inserts a probe, equipped with a needle guide, into the vagina. After inserting a 16-to 18-gauge needle through the vaginal wall, the specialist aspirates the follicular fluid from each mature follicle and collects it in a test tube containing a small amount of culture medium. The eggs are identified in the follicular fluid, are separated from the fluid and other follicular cells, and are then washed and prepared for insemination. This procedure normally yields 8 to 15 oocytes.
Insemination. The sperm sample is subjected to numerous washes, followed by column chromatography to separate the sperm cells from the other cells and from debris found in the ejaculate. Each egg is inseminated with 50,000 to 300,000 motile sperm in a drop of culture medium and is incubated overnight. Fertilization can usually be detected by the presence of two pronuclei in the egg cytoplasm after 16 to 20 hours. Fertilization rates generally range from 60% to 85%. Embryo development is allowed to continue in vitro for another 48 to 120 hours until embryos are transferred to the uterus.
Among couples whose male partner has very low numbers of motile sperm, high fertilization rates can be achieved using intracytoplasmic sperm injection (ICSI). Micromanipulation techniques are used to inject a sperm cell into the cytoplasm of each egg in vitro. Fertilization rates after ICSI are generally 60% to 70%, or approximately equivalent to conventional insemination in vitro.
Embryo transfer. After culturing the cells for 48 to 120 hours, the physician transfers three to four embryos to the uterus at the four-to eight-cell stage (after 2 days) or fewer embryos at the blastocyst stage (after 5 days). Embryos are selected and are loaded into a thin, flexible catheter, which is inserted into the uterine cavity to the desired depth under ultrasonic guidance. The woman usually receives supplemental progesterone to support implantation and pregnancy. In certain cases, the embryos are transferred to the fallopian tube during laparoscopy. This procedure is referred to as tubal embryo transfer (TET). The rationale for this procedure is that the fallopian tube contributes to the early development of the embryo as it travels down the tube to the uterus.
Success rates. Implantation rates usually range from 8% to 15% per embryo transferred. In the United States, the mean live birth rate per ET procedure is ~33%. Success rates in IVF-ET depend on numerous factors, including age as well as the type and severity of the disease causing infertility.
GIFT. In certain cases of infertility, the physician collects the oocytes and sperm cells in much the same way as described earlier for IVF-ET, but directly transfers the gametes to the fallopian tube, where fertilization occurs. GIFT is accomplished using laparoscopic techniques.
Before the embryo implants in the endometrium and establishes an indirect lifeline between the mother’s blood and its own, it must receive its nourishment from uterine secretions. Following conception, the endometrium is primarily controlled by progesterone, which initially comes from the corpus luteum (see Chapter 55). The uterine glandular epithelium synthesizes and secretes several steroid-dependent proteins (Table 56-1) that are thought to be important for the nourishment, growth, and implantation of the embryo. The endometrium secretes cholesterol, steroids, and various nutrients, including iron and fat-soluble vitamins. It also synthesizes matrix substances, adhesion molecules, and surface receptors for matrix proteins, all of which may be important for implantation.
Table 56-1 Endometrial Proteins, Glycoproteins, and Peptides Secreted by the Endometrial Glands During Pregnancy
Mucins |
Prolactin |
Insulin-like growth factor–binding protein 1 (IGFBP-1) |
Placental protein 14 (PP14) or glycodelin |
Pregnancy-associated endometrial α2-globulin (α2-PEG) |
Endometrial protein 15 |
Fibronectin |
Laminin |
Entactin |
Collagen type IV |
Heparan sulfate |
Proteoglycan |
Integrins |
Albumin |
β Lipoprotein |
Relaxin |
Acidic fibroblast growth factor |
Basic fibroblast growth factor |
Pregnancy-associated plasma protein A (PAPP-A) |
Stress response protein 27 (SRP-27) |
CA-125 |
β Endorphin |
Leu-enkephalin |
Diamine oxidase |
Plasminogen activator (PA) |
Plasminogen activator inhibitor |
Renin |
Progesterone-dependent carbonic anhydrase |
Lactoferrin |
Pinopods appear as small, finger-like protrusions on endometrial cells between day 19 (about the time the embryo would arrive in the uterus) and day 21 (about the time of implantation) of the menstrual cycle; they persist for only 2 to 3 days. Pinopod formation appears to be progesterone dependent, and it is inhibited by estrogens. Pinopods endocytose macromolecules and uterine fluid and absorb most of the fluid in the lumen of the uterus during the early stages of embryo implantation. By removing uterine luminal fluid, the pinopods may allow the embryo and the uterine epithelium to approximate one another more closely. Because apposition and adhesion of the embryo to the uterus are the first events of implantation, the presence and action of pinopods may determine the extent of the implantation window.
If the blastocyst is to survive, it must avoid rejection by the maternal cellular immune system. It does so by releasing immunosuppressive agents (Table 56-2). The embryo also synthesizes and secretes macromolecules that promote implantation, the development of the placenta, and the maintenance of pregnancy.
Table 56-2 Substances Secreted by the Blastocyst
Immunoregulatory Agents |
Platelet-activating factor (PAF) |
Early pregnancy factor |
Immunosuppressive factor |
PGE2 |
Interleukins 1α, 6, and 8 |
Interferon α Leukemia inhibitory factor |
Colony-stimulating factor |
Human leukocyte antigen 6 |
Fas ligand |
Metalloproteases (facilitate invasion of trophoblast into the endometrium) |
Collagenases: digest collagen types I, II, III, VII, and X |
Gelatinases: two forms, digest collagen type IV and gelatin |
Stromelysins: digest fibronectin, laminin, and collagen types IV, V, and VII |
Serine Proteases (facilitate invasion of trophoblast into the endometrium) |
Other Factors or Actions |
hCG: autocrine growth factor |
Ovum factor |
Early pregnancy factor |
Embryo-derived histamine-releasing factor |
Plasminogen activator and its inhibitors |
Insulin-like growth factor 2 (IGF-2): promotes trophoblast invasiveness |
Estradiol |
β1 Integrin |
Fibroblast growth factor (FGF) |
Transforming growth factor α (TGF-α) |
Inhibins |
Both short-range and long-range embryonic signals may be necessary for implantation, although the nature of some of these signals remains enigmatic. One short-range signal from the blastocyst may stimulate local cborderbackaes in the endometrium at the time of its apposition to the endometrium. A long-range signal that is secreted by the early blastocyst is human chorionic gonadotropin (hCG), which is closely related to LH (see Chapter 55) and sustains the corpus luteum in the presence of rapidly falling levels of maternal LH.
hCG is one of the most important of the factors secreted by the trophoblast of the blastocyst, both before and after implantation. Besides rescuing the corpus luteum, hCG is an autocrine growth factor that promotes trophoblast growth and placental development. hCG levels are high in the area where the trophoblast faces the endometrium. hCG may have a role in the adhesion of the trophoblast to the epithelia of the endometrium, and it also has protease activity.
As noted earlier, the conceptus lies unattached in the uterine cavity for ~72 hours. About halfway through this period (i.e., 5 to 6 days after ovulation), the morula transforms into the blastocyst (Fig. 56-4A). Before the initiation of implantation, the zona pellucida that surrounds the blastocyst degenerates. This process, known as hatching of the embryo, occurs 6 to 7 days after ovulation. Lytic factors in the endometrial cavity appear to be essential for the dissolution of the zona pellucida. The blastocyst probably also participates in the process of zona lysis and hatching; when an unfertilized egg is placed in the uterus under the same conditions, its zona pellucida remains intact. A factor produced by the blastocyst may activate a lytic factor that is derived from a uterine precursor. Plasmin, produced from plasminogen, is a plausible candidate for this uterine factor, because plasmin exhibits a lytic effect on the zona pellucida in vitro, and inhibitors of plasmin block in vitro hatching of rat blastocysts. Implantation occurs in three stages: (1) apposition, (2) adhesion, and (3) invasion.
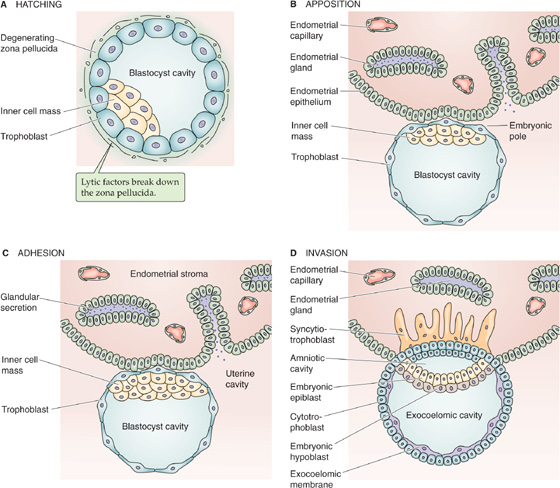
Figure 56-4 Embryo hatching, apposition, adhesion, and invasion.
Apposition The earliest contact between the blastocyst wall, the trophoectoderm, and the endometrial epithelium is a loose connection called apposition (Fig. 56-4B). Apposition usually occurs in a crypt in the endometrium. From the standpoint of the blastocyst, it appears that apposition occurs at a site where the zona pellucida is ruptured or lysed and where it is possible for the cell membranes of the trophoblast to make direct contact with the cell membranes of the endometrium. Although the preimplantation blastocyst is asymmetric, it seems that the entire trophoectoderm has the potential to interact with the endometrium, and the final correct orientation—with the inner cell mass pointing toward the endometrium—occurs by free rotation of the inner cell mass within the sphere of overlying trophoectoderm cells. (See Note: Mechanisms of Apposition)
Adhesion The trophoblast appears to attach to the uterine epithelium through the microvilli of the trophoblast; ligand-receptor interactions are probably involved in adhesion (Fig. 56-4C). The receptors for these ligand-receptor interactions are often members of the integrin family (see Chapter 2) and can be either on the blastocyst or on the endometrium. Integrins are bifunctional integral membrane proteins; on their intracellular side, they interact with the cytoskeleton, whereas on their extracellular side, they have receptors for matrix proteins such as collagen, laminin, fibronectin, and vitronectin. Therefore, ligand-receptor interactions have two possible orientations. For the first, the extracellular surface of the trophoblast has integrins for binding fibronectins, laminin, and collagen type IV. Thus, during implantation, the trophoblast binds to the laminin that is distributed around the stromal (decidual) cells of the endometrium. Fibronectin, a component of the basement membrane, probably guides the implanting embryo (see later) and is subsequently broken down by the trophoblast.
For the second orientation of matrix-integrin interactions, the extracellular surface of the glandular epithelium also expresses integrins on days 20 to 24 of the menstrual cycle, the implantation window (see Chapter 55). The expression of receptors for fibronectin and vitronectin (i.e., integrins) may serve as markers of the endometrial capacity for implantation. Small peptides containing sequences that are homologous to specific sequences of fibronectin block blastocyst attachment and outgrowth on fibronectin.
In addition to the integrin-matrix interactions, another important class of ligand-receptor interactions appears to be between heparin or heparan sulfate proteoglycans (see Chapter 2), which are attached to the surface of the blastocyst and surface receptors on the uterine epithelial cell. These endometrial proteoglycan receptors increase as the time of implantation approaches.
Any of the foregoing ligand-receptor interactions can lead to cytoskeletal changes. Thus, adhesion of the trophoblast through ligand-receptor interactions may dislodge the uterine epithelial cells from their basal lamina and may thereby facilitate access of the trophoblast to the basal lamina for penetration.
Invasion As the blastocyst attaches to the endometrial epithelium, the trophoblastic cells rapidly proliferate, and the trophoblast differentiates into two layers: an inner cytotrophoblast and an outer syncytiotrophoblast (Fig. 56-4D). The syncytiotrophoblast is a multinucleated mass without cellular boundaries. During implantation, long protrusions from the syncytiotrophoblast extend among the uterine epithelial cells. The protrusions dissociate these endometrial cells by secreting tumor necrosis factor α (TNF-α), which interferes with the expression of cadherins (cell adhesion molecules; see Chapter 2) and β-catenin (an intracellular protein that helps to anchor cadherins to the cytoskeleton). The syncytiotrophoblast protrusions then penetrate the basement membrane of the uterine epithelial cells and ultimately reach the uterine stroma. (See Note: Uterine E-Cadherin and β-Catenin at Implantation Site)
The trophoblast secretes several autocrine factors, which appear to stimulate invasion of the endometrial epithelium, as well as proteases (Table 56-2). By degrading the extracellular matrix, metalloproteases and serine proteases may control both the proliferation and the invasion of the trophoblast into the endometrium.
Around the site of penetration of the syncytiotrophoblast, uterine stromal cells take on a polyhedral shape and become laden with lipids and glycogen. These are the decidual cells discussed earlier. The decidual cells degenerate in the region of the invading syncytiotrophoblast and thus provide nutrients to the developing embryo. The blastocyst superficially implants in the zona compacta of the endometrium and eventually becomes completely embedded in the decidua. As the finger-like projections of the syncytiotrophoblast invade the endometrium, they reach the maternal blood supply and represent a primordial form of the chorionic villus of the mature placenta, as discussed in the next section.
Eventually, almost all the materials that are necessary for fetal growth and development move from the maternal circulation to the fetal circulation across the placenta, either by passive diffusion or by active transport. Except for CO2, waste products are largely excreted through the amniotic fluid.
Within the syncytium of the invading syncytiotrophoblast, fluid-filled holes called lacunae develop 8 to 9 days after fertilization (Fig. 56-5A). Twelve to 15 days after fertilization, the finger-like projections of the syncytiotrophoblast finally penetrate the endothelial layer of small veins of the endometrium. Later, these projections also penetrate the small spiral arteries. The result is free communication between the lacunae of the syncytiotrophoblast and the lumina of maternal blood vessels (Fig. 56-5B). Within 12 to 15 days after fertilization, some cytotrophoblasts proliferate and invade the syncytiotrophoblast, to form finger-like projections that are the primary chorionic villi.
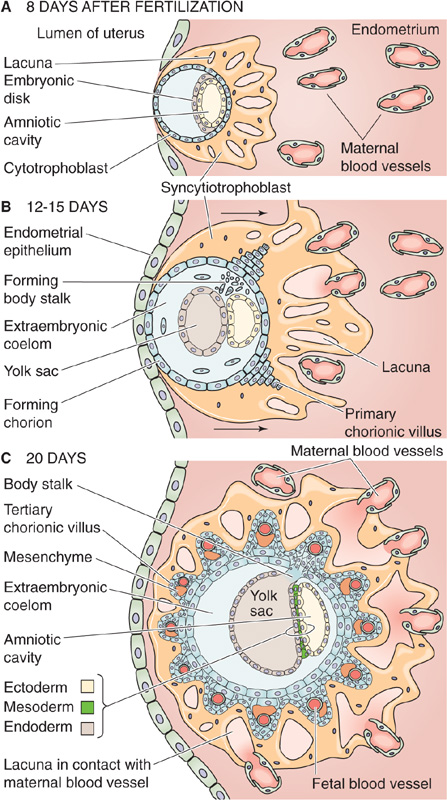
Figure 56-5 Development of the placenta. A, Shortly after the blastocyst has implanted (6 to 7 days after fertilization), the syncytiotrophoblast invades the stroma of the uterus (i.e., the decidua). Within the syncytiotrophoblast are lacunae. B, The invading syncytiotrophoblast breaks through into endometrial veins first, and then later into the arteries, thus creating direct communication between lacunae and maternal vessels. In addition, the proliferation of cytotrophoblasts creates small mounds known as primary chorionic villi. C, The primary chorionic villus continues to grow with the proliferation of cytotrophoblastic cells. In addition, mesenchyme from the extraembryonic coelom invades the villus, to form the secondary chorionic villus. Eventually, these mesenchymal cells form fetal capillaries; at this time, the villus is known as a tertiary chorionic villus. The lacunae also enlarge by merging with one another.
With further development, mesenchymal cells from the extraembryonic mesoderm invade the primary chorionic villi, which now are known as secondary chorionic villi. Eventually, these mesenchymal cells form fetal blood vessels de novo, at which point the villi are known as tertiary chorionic villi (Fig. 56-5C). Continued differentiation and amplification of the surface area of the fetal tissue protruding into the maternal blood create mature chorionic villi. The outer surface of each villus is lined with a very thin layer of syncytiotrophoblast, which has prominent microvilli (brush border) that face the maternal blood. Under the syncytiotrophoblast lie sparse cytotrophoblasts, mesenchyme, and fetal blood vessels. The lacunae, filled with maternal blood, eventually merge with one another, to create one massive, intercommunicating intervillous space (Fig. 56-6). The fetal villi protruding into this space resemble a thick forest of trees arising from the chorionic plate, which is the analogue of the soil from which the trees sprout. Thus, in the mature placenta, fetal blood is separated from maternal blood only by the fetal capillary endothelium, some mesenchyme and cytotrophoblasts, and a thin layer of syncytiotrophoblast.
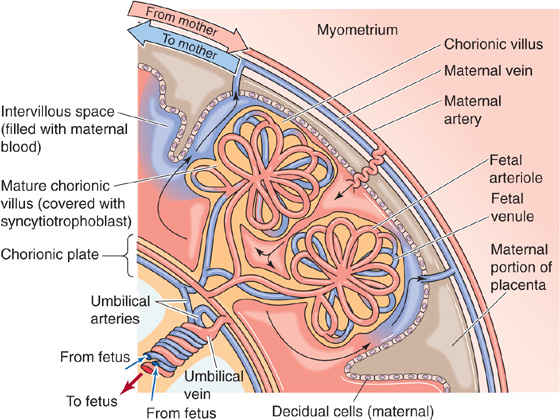
Figure 56-6 The mature placenta. With further development beyond that shown in Figure 56-5C, the outer surface of the mature chorionic villus is covered with a thin layer of syncytiotrophoblast. Under this are cytotrophoblasts, mesenchyme, and fetal blood vessels. The lacunae into which the villi project gradually merge into one massive intervillous space. Maternal blood is trapped in this intervillous space, between the endometrium on the maternal side and the villi on the fetal side. In the mature placenta, as shown here, “spiral” arteries from the mother empty directly into the intervillous space, which is drained by maternal veins. The villi look like a thick forest of trees arising from the chorionic plate, which is the analogue of the soil from which the trees sprout.
Maternal Blood Flow The maternal arterial blood is discharged from ~120 spiral arteries; these arteries may have multiple openings, not all of which need be open at the same time. Blood enters in pulsatile spurts through the wall of the uterus and moves in discrete streams into the intervillous space toward the chorionic plate (Fig. 56-6). Small lakes of blood near the chorionic plate dissipate the force of the arterial spurts and reduce blood velocity. The maternal blood spreads laterally and then reverses direction and cascades over the closely packed villi. Blood flow slows even more, to allow adequate time for exchange. After bathing the chorionic villi, the maternal blood drains through venous orifices in the basal plate, enters the larger maternal placental veins, and ultimately flows into the uterine and other pelvic veins. No capillaries are present between the maternal arterioles and venules; the intervillous space is the functional capillary. Because the intervillous spaces are very narrow, and the arterial and venous orifices are randomly scattered over the entire base of the placenta, the maternal blood moves efficiently among the chorionic villi and avoids arteriovenous shunts.
The spiral arteries are generally perpendicular, and the veins are generally parallel to the uterine wall. Thus, because of both the geometry of the maternal blood vessels and the difference between maternal arterial and venous pressure, the uterine contractions that occur periodically during pregnancy, as well as during delivery, attenuate arterial inflow and completely interrupt venous drainage. Thus, the volume of blood in the intervillous space actually increases, to provide continual, albeit reduced, exchange. The principal factors that regulate the flow of maternal blood in the intervillous space are maternal arterial blood pressure, intra-uterine pressure, and the pattern of uterine contraction.
Fetal Blood Flow The fetal blood originates from two umbilical arteries. Unlike systemic arteries after birth, umbilical arteries carry deoxygenated blood. As these umbilical arteries approach the placenta, they branch repeatedly beneath the amnion, penetrate the chorionic plate, and then branch again within the chorionic villi, to form a capillary network. Blood that has obtained a significantly higher O2 and nutrient content returns to the fetus from the placenta through the single umbilical vein.
The amniotic fluid that fills the amniotic cavity serves two important functions. First, it serves as a mechanical buffer and thus protects the fetus from external, physical insults. Second, it serves as a mechanism by which the fetus excretes many waste products. The water in the amniotic fluid turns over at least once a day. After the fetal kidneys mature (10 to 12 weeks), the renal excretions of the fetus are the major source of amniotic fluid production (~75%); pulmonary secretions account for the rest. Fluid removal occurs through the actions of the fetal gastrointestinal tract (~55%), amnion (~30%), and lungs (~15%).
The placenta is the major lifeline between the mother and the fetus. It provides nutrients and O2 to the fetus, and it removes CO2 and certain waste products from the fetus.
O2 and CO2 Transport The maternal blood coming into the intervillous space has a gas composition similar to that of systemic arterial blood: a PO2 of ~100 mm Hg (Table 56-3), a PCO2 of ~40 mm Hg, and a pH of 7.40. However, the diffusion of O2 from the maternal blood into the chorionic villi of the fetus causes the PO2 of blood in the intervillous space to fall, so the average PO2 is 30 to 35 mm Hg. Given the O2 dissociation curve of maternal (i.e., adult) hemoglobin (Hb), this PO2 translates to an O2 saturation of ~65%. The PO2 of blood in the umbilical vein is even less. Despite the relatively low PO2 of the maternal blood in the intervillous space, the fetus does not suffer from a lack of O2. Because fetal Hb has a much higher affinity for O2 than does maternal Hb, the fetal Hb can extract O2 from the maternal Hb (see Chapter 29). Thus, a PO2 of 30 to 35 mm Hg, which yields an Hb saturation of ~65% in the intervillous space in the mother’s blood, produces an Hb saturation of ~85% in the umbilical vein of the fetus (Table 56-3), assuming that the O2 fully equilibrates between intervillous and fetal blood. Other mechanisms of ensuring adequate fetal oxygenation include the relatively high cardiac output per unit body weight of the fetus and the increasing O2-carrying capacity of fetal blood late in pregnancy as the Hb concentration rises to a level 50% higher than that of the adult.
Table 56-3 Maternal and Fetal Oxygen Levels
Site |
PO2 (mm Hg) |
Hemoglobin Saturation |
Maternal Values |
|
|
Uterine artery |
100 |
97.5% |
Intervillous space |
30-35 |
57%-67% |
Uterine vein |
30 |
57% |
Fetal Values |
|
|
Umbilical arteries |
23 |
60.5% |
Umbilical vein |
30 |
85.5% |
The transfer of CO2 from the fetus to the mother is driven by a concentration gradient between the blood in the umbilical arteries and that in the intervillous space. Near the end of pregnancy, the PCO2 in the umbilical arteries is ~48 mm Hg, and the PCO2 in the intervillous space is ~43 mm Hg, a gradient of ~5 mm Hg. The fetal blood also has a somewhat lower affinity for CO2 than does maternal blood, thus favoring the transfer of CO2 from the fetus to mother.
Other Solutes Various other solutes besides O2 and CO2 move across the placenta between the mother and the fetus and avail themselves of numerous transport mechanisms. Some of these solutes, such as the waste products urea and creatinine, probably move passively from fetus to mother. The lipid-soluble steroid hormones shuttle among the mother, the placenta, and the fetus by simple diffusion. Glucose moves from the mother to the fetus by facilitated diffusion, and amino acids move by secondary active transport (see Chapter 5). The placenta also transports several other essential substances, such as vitamins and minerals, that are needed for fetal growth and development. Many substances are present in the fetal circulation at concentrations higher than in the maternal blood, and they must be actively transported against concentration or electrochemical gradients. The necessary energy (i.e., ATP) is derived from glycolysis and the citric acid cycle, for which the enzymes are present in the human placenta at term. Also present are the enzymes for the pentose phosphate pathway, an alternative pathway for the oxidation of glucose, which provides the NADPH that is necessary for several synthetic pathways that require reducing equivalents in the human placenta at term.
The placenta takes up large molecules from the mother through receptor-mediated endocytosis (see Chapter 2). The uptake of substances such as low-density lipoproteins (LDL), transferrin, hormones (e.g., insulin), and antibodies (e.g., immunoglobulin G) increases throughout gestation until just before birth.
The placenta plays a key role in steroid synthesis, which is discussed in the next major section. In addition, the placenta manufactures numerous amines, polypeptides (including peptide hormones and neuropeptides), proteins, glycoproteins, and steroids (Table 56-4). Among these peptides are the placental variants of all known releasing hormones, which are produced by the hypothalamus (see Chapter 47). These placental releasing hormones may act in a paracrine fashion, controlling the release of local placental hormones, or they may enter the maternal or fetal circulations. In addition, several proteases are also present in the placenta. Although the placenta synthesizes a wide variety of substances, the significance of many of these substances is not clear.
Table 56-4 Hormones Made by the Placenta
Peptide Hormones and Neuropeptides |
hCG |
Thyrotropin (thyroid-stimulating hormone [TSH]) |
Placental-variant growth hormone |
hCS1 and hCS2, also known as hPL (hPL1 and hPL2) |
Placental proteins PP12 and PP14 |
TRH |
Corticotropin-releasing hormone (CRH) |
Growth hormone–releasing hormone (GHRH) |
GnRH |
Substance P |
Neurotensin |
Somatostatin |
Neuropeptide Y |
ACTH-related peptide |
The inhibins |
Steroid Hormones |
Progesterone |
Estrone |
Estradiol |
Estriol |
The most important placental peptide hormone is hCG. In the developing blastocyst, and later in the mature placenta, the syncytiotrophoblast cells synthesize hCG, perhaps under the direction of progesterone and estrogens. The placenta also produces two human chorionic somatomammotropins, hCS1 and hCS2, also called human placental lactogen (hPL). hCS1 and hCS2 are polypeptide hormones structurally related to growth hormone (GH) and placental-variant GH, as well as to prolactin (PRL; see Table 48-1). They play a role in the conversion of glucose to fatty acids and ketones, thus coordinating the fuel economy of the fetoplacental unit. The fetus and placenta use fatty acids and ketones as energy sources and store them as fuels in preparation for the early neonatal period, when a considerable reservoir of energy is necessary for the transition from intra-uterine life to life outside the uterus. hCS1 and hCS2 also promote the development of maternal mammary glands during pregnancy.
In addition to its secretory functions, the placenta also stores vast amounts of proteins, polypeptides, glycogen, and iron. Many of these stored substances can be used at times of poor maternal nutrition and also during the transition from intrauterine to extrauterine life.
Following ovulation during a normal or nonconception cycle, the cells of the ovarian follicle functionally transform into luteal cells, which produce mainly progesterone, but also estrogens (see Chapter 55). However, the corpus luteum has a finite life span, which lasts only ~12 days before it begins its demise in the presence of declining LH levels. As a consequence of luteal demise, levels of both progesterone and estrogens decline.
In contrast, during pregnancy, maternal levels of progesterone and estrogens (estradiol, estrone, estriol) all increase and reach concentrations substantially higher than those achieved during a normal menstrual cycle (Fig. 56-7). These elevated levels are necessary for maintaining pregnancy. For example, progesterone reduces uterine motility and inhibits propagation of contractions. How are these elevated levels of female steroids achieved? Early in the first trimester, hCG that is manufactured by the syncytiotrophoblast rescues the corpus luteum, which is the major source of progesterone and estrogens. This function of the corpus luteum in the ovary continues well into early pregnancy. However, by itself, the corpus luteum is not adequate to generate the very high steroid levels characteristic of late pregnancy. The developing placenta itself augments its production of progesterone and estrogens, so by 8 weeks of gestation, the placenta has become the major source of these steroids. The placenta continues to produce large quantities of estrogens, progestins, and other hormones throughout gestation. Estriol, which is not important in nonpregnant women, is a major estrogen during pregnancy (Fig. 56-7).
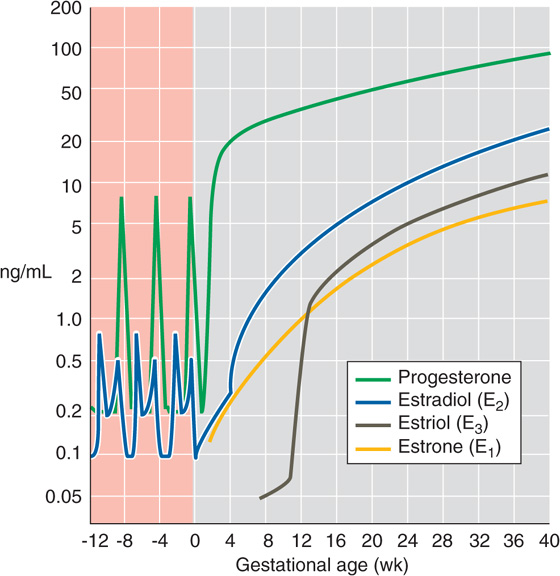
Figure 56-7 Maternal levels of progesterone and the estrogens just before and during pregnancy. The y-axis scale is logarithmic. The zero point on the x-axis is the time of fertilization. The progesterone spikes near −8 and −4 weeks refer to the two menstrual cycles before the one that resulted in the pregnancy. (Data from Wilson JD, Foster DW, Kronenberg HM, Larsen PR [eds]: Williams Textbook of Endocrinology, 9th ed. Philadelphia: WB Saunders, 1998.)
Although it emerges as the major source of progesterone and estrogens (Table 56-5), the placenta cannot synthesize these hormones by itself; it requires the assistance of both mother and fetus. This joint effort in steroid biosynthesis has led to the concept of the maternal-placental-fetal unit. Figure 56-8—which resembles the maps describing the synthesis of glucocorticoids, mineralocorticoids (see Fig. 50-2), male steroids (see Fig. 54-5), and female steroids (see Fig. 55-10, later)—illustrates the pathways used by the maternal-placental-fetal unit to synthesize progesterone and the estrogens. Figure 56-9 summarizes the exchange of synthetic intermediates among the three members of the maternal-placental-fetal unit.
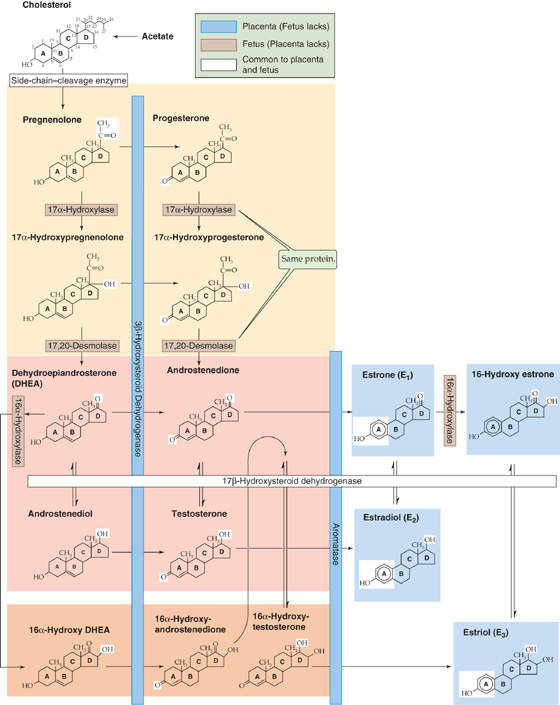
Figure 56-8 Synthesis of progesterone and the estrogens by the maternal-placental-fetal unit. Individual enzymes are shown in the horizontal and vertical boxes. See Figures 50-2, 54-5, and 55-9 for cellular localizations of enzymes. Chemical groups modified by each enzyme are highlighted in the reaction products. The fetus lacks 3β-hydroxysteroid dehydrogenase (3β-HSD) and aromatase (P-450arom), shown on the blue background. Placenta lacks 17α-hydroxylase and 17, 20-desmolase activity (contributed by the same protein, P-450c17) and 16α-hydroxylase, shown on the brown background. The blue and brown color coding of enzymes distinguishes fetus from placenta, whereas color coding in previous steroidogenesis figures indicates subcellular localization.
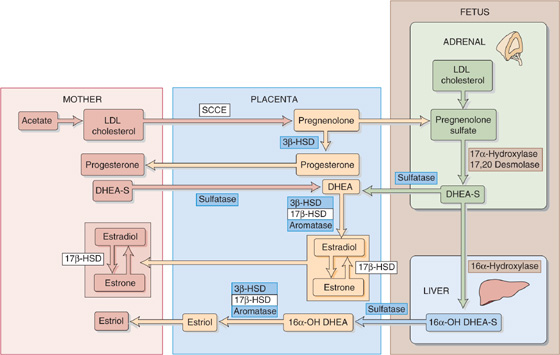
Figure 56-9 The interactions of the maternal-placental-fetal unit. The details of the enzymatic reactions are provided in Figure 56-8. SCCE is the side-chain–cleavage enzyme; the S in DHEA-S and 16α-OH DHEA-S represents sulfate. 17β-HSD, 17β-hydroxysteroid dehydrogenase.
Table 56-5 Roles of the Mother, Placenta, and Fetus in Steroid Biosynthesis
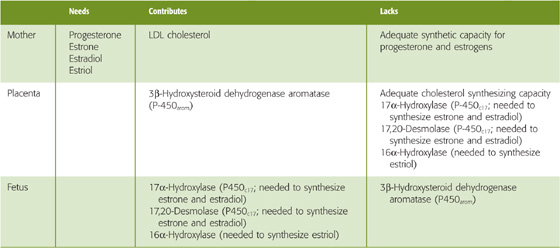
Unlike the corpus luteum, which manufactures progesterone, estrone, and estradiol early in pregnancy (see Chapter 55), the placenta is an imperfect endocrine organ. First, the placenta cannot manufacture adequate cholesterol, the precursor for steroid synthesis. Second, the placenta lacks two crucial enzymes that are needed for synthesizing estrone and estradiol. Third, the placenta lacks a third enzyme that is needed to synthesize estriol. The enzymes missing from the placenta are listed in Table 56-5, and they also are indicated with a brown background in Figures 56-8 and 56-9.
The maternal-placental-fetal unit overcomes these placental shortcomings in two ways. First, the mother supplies most of the cholesterol as LDL particles (see Chapter 46). With this supply of maternal cholesterol, the placenta can generate large amounts of progesterone and can export it to the mother, thus solving the problem of maintaining maternal progesterone levels after the corpus luteum becomes inadequate. Second, the fetal adrenal gland and liver supply the three enzymes lacking in the placenta. The fetal adrenal glands are up to this metabolic task; at term, these glands are as large as those of an adult.
The fetus does not synthesize estrogens without assistance, for two reasons. First, it cannot, because the fetus lacks the enzymes that catalyze the last two steps in the production of estrone, the precursor of estradiol. These two enzymes are also necessary to synthesize estriol. The enzymes missing from the fetus are listed in Table 56-5, and they also are indicated with a blue background in Figures 56-8 and 56-9. Second, the fetus should not synthesize estrogens without assistance. If the fetus were to carry out the complete, classic biosynthesis of progesterone and the estrogens, it would expose itself to dangerously high levels of hormones that are needed not by the fetus, but by the mother.
The fetus and its placenta use three strategies to extricate themselves from this conundrum. First, because the fetus lacks the two enzymes noted earlier, it never makes anything beyond dehydroepiandrosterone (DHEA) and 16α-hydroxy-DHEA (Fig. 56-8). In particular, the fetus cannot make progesterone or any of the three key estrogens. Second, the placenta is a massive sink for the weak androgens that the fetus does synthesize, thus preventing the masculinization of female fetuses. Third, the fetus conjugates the necessary steroid intermediates to sulfate, which greatly reduces their biological activity (Fig. 56-9). Thus, as pregnenolone moves from the placenta to the fetus, it is sulfated. The products of fetal pregnenolone metabolism are also sulfated (DHEA-S and 16α-hydroxy-DHEA-S) as long as they reside inside the fetus. It is only when DHEA-S and 16α-hydroxy-DHEA-S finally move to the placenta that a sulfatase removes the sulfate groups, and thus the placenta can complete the process of steroidogenesis and can export the hormones to the mother.
The mean duration of pregnancy is ~266 days (38 weeks) from the time of ovulation or 280 days (40 weeks) from the first day of the last menstrual period. During this time, the mother experiences numerous and profound adaptive changes in her cardiovascular system, fluid volume, respiration, fuel metabolism, and nutrition. These orderly changes reflect the effects of various hormones, as well as the increase in the size of the pregnant uterus.
The maternal blood volume starts to increase during the first trimester, expands rapidly during the second trimester, rises at a much lower rate during the third trimester, and finally achieves a plateau during the last several weeks of pregnancy. Maternal blood volume may have increased by as much as 45% near term in singleton pregnancies and up to 75% to 100% in twin or triplet pregnancies. The ultimate increase in blood volume results from an increase in the volume of both the plasma and erythrocytes. However, the rise in plasma volume begins earlier and is ultimately greater (~50%) than the rise in total erythrocyte volume (~33%). A proposed mechanism for the increase in plasma volume is that elevated progesterone and estrogens cause a vasodilation that decreases peripheral vascular resistance and thus renal perfusion. One mechanism of the vasodilation is refractoriness to the pressor effects of angiotensin II. The renin-angiotensin-aldosterone axis responds by increasing aldosterone, which augments renal reabsorption of salt and water. In addition, pregnancy causes a leftward shift of the relationship between arginine vasopressin (AVP) release and plasma osmolality (see Chapter 41). Immediately after the delivery of the placenta, with the attendant decreases in progesterone and estrogen levels, the mother commences vigorous diuresis.
The increase in blood volume is needed to meet the demands of the enlarged pregnant uterus with its greatly hypertrophied vascular system. It also protects mother and fetus against the deleterious effects of impaired venous return in the supine and erect positions, and it safeguards the mother against the adverse effects of the blood loss associated with parturition.
Cardiac output increases appreciably during the first trimester of pregnancy (by 35% to 40%), but it increases only slightly during the second and third trimesters (~45% at term). The increase in cardiac output, which reflects mainly an increase in stroke volume but also heart rate, is highly targeted. Renal blood flow increases 40%. Uterine blood flow rises from just 1% to 15% of cardiac output. Blood flow to the heart (to support increased cardiac output), skin (to increase heat radiation), and breasts (to support mammary development) also increases. However, no change occurs in blood flow to the brain, gut, or skeleton. The increase in cardiac output with physical activity is greater in pregnant women (for most of the pregnancy) than it is in nonpregnant women.
Despite the large increase in plasma volume, mean arterial pressure (MAP) usually decreases during midpregnancy and then rises during the third trimester, although it normally remains at or lower than normal. The reason for this initial fall in MAP is a decrease in peripheral vascular resistance, possibly reflecting—in part—the aforementioned vasodilating effects of progesterone and estradiol.
Posture has a major effect on cardiac output (see Chapter 25). In late pregnancy, cardiac output is typically higher when the mother is in the lateral recumbent position than when she is in the supine position. In the supine position, the fundus of the enlarged uterus rests on the inferior vena cava near L5, thereby impeding venous return to the heart.
During pregnancy, hormonal and mechanical factors lead to several anatomical changes that have the net effect of increasing alveolar ventilation. The level of the diaphragm rises ~4 cm, probably reflecting the relaxing effects of progesterone on the diaphragm muscle and fascia. At the same time, the costovertebral angle widens appreciably as the transverse diameter of the thoracic cage increases ~2 cm. Although these two cborderbackaes have opposite effects on the residual volume (RV) of air in the lungs (see Chapter 27), the elevation of the diaphragm dominates, thus causing a net decrease in RV and functional residual capacity (FRC). Vital capacity (VC), maximal pulmonary ventilation, and pulmonary compliance do not cborderbackae appreciably. Total pulmonary resistance falls, thereby facilitating airflow. Because of the increased size of the abdominal contents during pregnancy, the abdominal muscles are less effective in aiding forced expirations.
Although pregnancy has little effect on respiratory rate, it increases tidal volume (VT) markedly—by ~40%—and thereby increases alveolar ventilation ( A; see Chapter 30). These increases in VT and
A; see Chapter 30). These increases in VT and  A are some of the earliest physiological cborderbackaes during pregnancy, beginning 6 weeks after fertilization. They may reflect, at least in part, a direct stimulatory effect of progesterone and, to a lesser extent, estrogen on the medullary respiratory centers. The physiological effect of the increased
A are some of the earliest physiological cborderbackaes during pregnancy, beginning 6 weeks after fertilization. They may reflect, at least in part, a direct stimulatory effect of progesterone and, to a lesser extent, estrogen on the medullary respiratory centers. The physiological effect of the increased 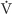 A during pregnancy is a fall in maternal arterial PCO2, which typically decreases from a value before pregnancy of ~40 to ~32 mm Hg, despite the net increase in CO2 production that reflects fetal metabolism. A side effect is mild respiratory alkalosis for which the kidneys compensate by lowering plasma [HCO−3] modestly (see Chapter 28).
A during pregnancy is a fall in maternal arterial PCO2, which typically decreases from a value before pregnancy of ~40 to ~32 mm Hg, despite the net increase in CO2 production that reflects fetal metabolism. A side effect is mild respiratory alkalosis for which the kidneys compensate by lowering plasma [HCO−3] modestly (see Chapter 28).
During pregnancy, an additional 30 g of protein will be needed each day to meet the demand of the growing fetus, placenta, uterus, and breasts, as well as the increased maternal blood volume. Most protein should come from animal sources, such as meat, milk, eggs, cheese, poultry, and fish, because these foods furnish amino acids in optimal combinations.
Almost any diet that includes iodized salt and adequate caloric intake to support the pregnancy also contains enough minerals, except iron (see Table 45-3). Pregnancy necessitates a net gain of ~800 mg of circulating iron to support the expanding maternal Hb mass, the placenta, and the fetus. Most of this iron is used during the latter half of pregnancy. A nonpregnant woman of reproductive age needs to absorb ~1.5 mg/day of iron in a diet that contains 15 to 20 mg/day (see Chapter 45). In contrast, during pregnancy, the average required iron uptake rises to ~7 mg/day. Very few women have adequate iron stores to supply this amount of iron, and a typical diet seldom contains sufficient iron. Thus, the recommended supplementation of elemental iron is 60 mg/day, taken in the form of a simple ferrous iron salt.
Maternal folate requirements increase significantly during pregnancy, in part reflecting an increased demand for producing blood cells. This increased demand can lead to lowered plasma folate levels or, in extreme cases, to maternal megaloblastic anemia (see Chapter 45). Folate deficiency may cause neural tube defects in the developing fetus. Because oral supplementation of 400 to 800 μg/day of folic acid produces a vigorous hematologic response in pregnant women with severe megaloblastic anemia, this dose would almost certainly provide very effective prophylaxis.
The recommended weight gain during a singleton pregnancy for a woman with a normal ratio of weight to height (i.e., body mass index) is 11.5 to 16 kg. This number is higher for women with a low body mass index. A weight gain of 14 kg would include 5 kg for intrauterine contents—the fetus (3.3 kg), placenta and membranes (0.7 kg), and amniotic fluid (1 kg). The maternal contribution of 9 kg would include increases in the weight of the uterus (0.7 kg), the blood (1.3 kg), and the breasts (2.0 kg), as well as adipose tissue and interstitial fluid (5.0 kg). The interstitial fluid expansion may be partly the result of increased venous pressure created by the large pregnant uterus and, as noted earlier, partly caused by aldosterone-dependent Na+ retention.
For a woman whose weight is normal before pregnancy, a weight gain in the recommended range correlates well with a favorable outcome of the pregnancy. Most pregnant women can achieve an adequate weight gain by eating—according to appetite—a diet adequate in calories, protein, minerals, and vitamins. Seldom, if ever, should maternal weight gain be deliberately restricted to less than this level. Failure to gain weight is an ominous sign; birth weight parallels maternal weight, and neonatal mortality rises with low birth weight, particularly for babies weighing less than 2500 g.
Throughout most of pregnancy, the uterus is quiescent. Both progesterone and relaxin may promote this inactivity. Weak and irregular uterine contractions occur throughout the last month of pregnancy. Eventually, a series of regular, rhythmic, and forceful contractions develops to facilitate thinning and dilation of the cervix—the obstetric definition of labor (Table 56-6). These contractions may last for several hours, a day, or even longer and may eventually result in the expulsion of the fetus, placenta, and membranes. Although not all the factors leading to the initiation of labor are known, endocrine, paracrine, and mechanical stretching of the uterus all play a role. Once labor is initiated, it is sustained by a series of positive feedback mechanisms.
Table 56-6 Stages of Labor
In rabbits, withdrawal of progesterone, made primarily in the placenta, results in prompt evacuation of the uterus; administration of progesterone delays the onset of labor. However, most human studies have failed to provide evidence that progesterone levels fall before the onset of labor. Nonetheless, it appears that progesterone plays an important role in maintaining the length of gestation in primates.
Other studies point to the importance of the fetal hypothalamic-pituitary-adrenal axis in the preparation for, or initiation of, parturition. In the fetal lamb, transection of the hypothalamic portal vessels prolongs gestation. In the human, an equivalent disruption of the fetal hypothalamic-pituitary-adrenal axis occurs in anencephalic fetuses, in which the cerebral hemispheres are absent and the rest of the brain is severely malformed. Indeed, gestation is prolonged in human pregnancies with anencephalic fetuses.
Infusing adrenocorticotropic hormone (ACTH) into fetal lambs with intact adrenal glands, or directly infusing cortisol, causes premature parturition. Although the theory that cortisol plays a role in initiating parturition remains attractive, several naturally occurring instances of failure of cortisol production in the human fetus do not prolong gestation. As discussed in the next section, prostaglandins appear to play a crucial role in the initiation of labor.
Whereas hormones (particularly oxytocin [OT]) and paracrine factors (particularly prostaglandins) play an important role in stimulating the uterine contractions that sustain labor, only the prostaglandins are believed to have a key role in the initiation of labor.
Prostaglandins The uterus, the placenta, and the fetal membranes synthesize and release prostaglandins (see Chapter 3). Prostaglandins from the uterine decidual cells, particularly prostaglandins F2 and E2 (PGF2α and PGE2), act by a paracrine mechanism on uterine smooth muscle cells. OT (see later) stimulates uterine decidual cells to increase their PGF2α synthesis. Arachidonic acid, the precursor of prostaglandins, is present in very high concentrations in the fetal membranes near term.
Prostaglandins have three major effects. First, prostaglandins strongly stimulate the contraction of uterine smooth muscle cells. Second, PGF2α potentiates the contractions induced by OT by promoting formation of gap junctions between uterine smooth muscle cells; estradiol also increases the number of gap junctions (see Chapter 9). These gap junctions permit synchronous contraction of the uterine smooth muscle cells, reminiscent of the contraction of the ventricles of the heart. Third, prostaglandins also cause softening, dilatation, and thinning (effacement) of the cervix, which occurs early during labor. This softening is akin to an inflammatory reaction in that it is associated with an invasion by polymorphonuclear leukocytes (e.g., neutrophils). Because of these effects, prostaglandins are used to induce labor and delivery in certain clinical settings.
Prostaglandins may physiologically initiate labor. Both PGF2α and PGE2 evoke myometrial contractions at any stage of gestation, regardless of the route of administration. The levels of prostaglandins or their metabolic products naturally increase in the blood and amniotic fluid just before and during labor. Arachidonic acid instilled into the amniotic cavity causes the uterus to contract and to expel its contents. Aspirin, which inhibits the enzyme cyclooxygenase (see Chapter 3), reduces the formation of PGF2α and PGE2, thus inhibiting labor and prolonging gestation.
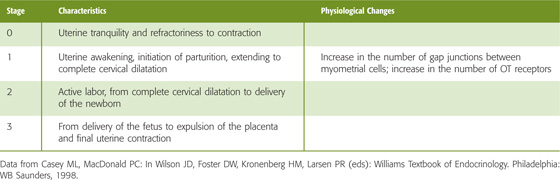
Oxytocin The nonapeptide OT is closely related to AVP (Fig. 56-10). The two hormones apparently evolved from vasotocin, the single neurohypophyseal hormone in nonmammalian vertebrates. OT and AVP, which both differ from vasotocin by a single amino acid, are synthesized in the cell bodies of the neurons in the supraoptic and paraventricular nuclei of the hypothalamus. Both OT and AVP then move by fast axonal transport to the posterior pituitary gland, where they are stored in the nerve terminals until they are released in response to the appropriate stimuli. Both OT and AVP are closely associated with—and released with—peptides known as neurophysins.
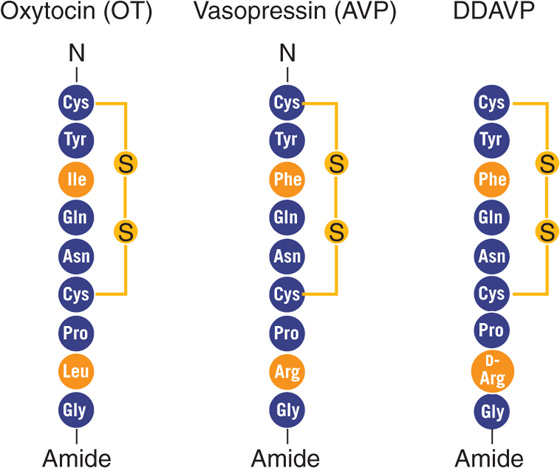
Figure 56-10 Comparison of structures of OT and AVP. DDAVP is a synthetic AVP in which the N-terminal Cys is deaminated and L-Arg at position 8 is replaced with D-Arg (see the box on diabetes insipidus in Chapter 38).
Circulating OT binds to Gαq-coupled OT receptors on the plasma membrane of uterine smooth muscle cells; this process triggers the phospholipase C cascade (see Chapter 3). Presumably, formation of IP3 leads to Ca2+ release from internal stores and to an increase in [Ca2+]i. The rise in [Ca2+]i activates calmodulin, which stimulates myosin light-chain kinase to phosphorylate the regulatory light chain and to cause contraction of uterine smooth muscle cells (see Chapter 9) and increased intrauterine pressure. OT also binds to a receptor on decidual cells, thereby stimulating PGF2α production, as discussed earlier.
Estrogen increases the number of OT receptors in the myometrial and decidual tissue of pregnant women. The uterus actually remains insensitive to OT until ~20 weeks’ gestation, at which time the number of OT receptors increases progressively to 80-fold higher than baseline values by ~36 weeks’ gestation, plateaus just before labor, then rises again to 200-fold during early labor. The time course of the expression of OT receptors may account for the increase in spontaneous myometrial contractions even in the absence of increased plasma OT levels. Whereas the uterus is sensitive to OT only at the end of pregnancy, it is susceptible to prostaglandins throughout pregnancy.
Although the prevailing view is that OT of maternal origin is not involved in initiating labor in humans, maternal OT may help to maintain labor. OT of fetal origin, which moves to the maternal circulation, could be involved in the onset of labor, because fetal plasma OT levels rise during the first stage of labor (Table 56-6). However, infusing OT at pharmacological doses into the circulation of the fetal lamb only stimulates uterine contractions. Therefore, normal levels of fetal OT probably have little influence on labor.
Once labor is initiated (stage 1), maternal OT is released in bursts, and the frequency of these bursts increases as labor progresses. The primary stimulus for the release of maternal OT appears to be distention of the cervix; this effect is known as the Ferguson reflex. OT is an important stimulator of myometrial contraction late in labor. During the second stage of labor, OT release may play a synergistic role in the expulsion of the fetus by virtue of its ability to stimulate prostaglandin release.
During the third stage of labor, uterine contractions induced by OT are also important for constricting uterine blood vessels at the site where the placenta used to be, thus promoting hemostasis (i.e., blood coagulation). Basal maternal plasma OT levels are unchanged after delivery. Fetal plasma OT levels are higher after vaginal delivery than after delivery by cesarean section, presumably because the maternal OT triggered by the Ferguson reflex crosses the placenta into the fetus.
Relaxin This 48–amino acid polypeptide hormone, structurally related to insulin, is produced by the corpus luteum, the placenta, and the decidua. Relaxin may play a role in keeping the uterus in a quiet state during pregnancy. Production and release of relaxin increase during labor, when relaxin may soften and may thus help to dilate the cervix.
Mechanical Factors Mechanical stretch placed on the uterine muscle may lead to the rhythmic contractions of labor. Thus, the increase in the size of the uterine contents to a critical level may stimulate uterine contractions, thereby leading to initiation of labor.
Positive Feedback Once labor is initiated, several positive feedback loops involving prostaglandins and OT help to sustain it. First, uterine contractions stimulate prostaglandin release, which itself increases the intensity of uterine contractions. Second, uterine activity stretches the cervix, thus stimulating OT release through the Ferguson reflex. Because OT stimulates further uterine contractions, these contractions become self-perpetuating.
Almost immediately following delivery of the newborn, marked changes occur in the endocrine status of the mother. During pregnancy, many hormones are secreted in massive quantities. Estrogens are mitogenic, causing considerable hypertrophy of the uterine muscle cells during gestation. As the levels of these hormones fall abruptly, stimulation ceases, and uterine smooth muscle cells decrease in size. The vasculature of the uterus regresses, and blood flow to the uterus is significantly curtailed, thus leading to further involution of this organ.
The fundamental secretory unit of the breast (Fig. 56-11A) is the alveolus (Fig. 56-11B, C), which is surrounded by contractile myoepithelial cells and adipose cells. These alveoli are organized into lobules, each of which drains into a ductule. Groups of 15 to 20 ductules drain into a duct, which widens at the ampulla—a small reservoir. The lactiferous duct carries the secretions to the outside.
Figure 56-11 Cross section of the breasts and milk production. A, The breast consists of a series of secretory lobules, which empty into ductules. The ductules from 15 to 20 lobules combine into a duct, which widens at the ampulla—a small reservoir. The lactiferous duct carries the secretions to the outside. B, The lobule is made up of many alveoli, the fundamental secretory units. C, Each alveolus consists of secretory epithelial cells (alveolar cells) that actually secrete the milk, as well as contractile myoepithelial cells, which are, in turn, surrounded by adipose cells. D, The alveolar cell secretes the components of milk through five pathways.
Breast development at puberty depends on several hormones, but primarily on the estrogens and progesterone. During pregnancy, gradual increases in levels of PRL and hCS, as well as very high levels of estrogens and progesterone, lead to full development of the breasts.
As summarized in Table 56-7, hormones affecting the breast are mammogenic (promoting the proliferation of alveolar and duct cells), lactogenic (promoting initiation of milk production by alveolar cells), galactokinetic (promoting contraction of myoepithelial cells, and thus milk ejection), or galactopoietic (maintaining milk production after it has been established).
Table 56-7 Hormones Affecting the Mammary Gland During Pregnancy and Breast-Feeding
Mammogenic Hormones (promote cell proliferation) |
Lobuloalveolar Growth |
Estrogen |
Growth hormone (IGF-1) |
Cortisol |
Prolactin |
Relaxin? |
Ductal Growth |
Estrogen |
Growth hormone |
Cortisol |
Relaxin |
Lactogenic Hormones (promote initiation of milk production by alveolar cells) |
Prolactin |
hCS (or hPL) |
Cortisol |
Insulin (IGF-1) |
Thyroid hormones |
Growth hormone? |
Withdrawal of estrogens and progesterone |
Galactokinetic Hormones (promote contraction of myoepithelial cells and thus milk ejection) |
OT |
AVP (1% to 20% as powerful as OT) |
Galactopoietic Hormones (maintain milk production after it has been established) |
PRL (primary) |
Cortisol and other metabolic hormones (permissive) |
IGF-1, insulin-like growth factor type 1.
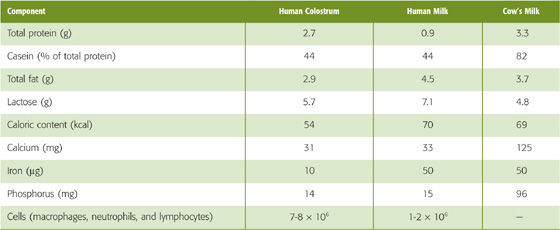
Milk is an emulsion of fats in an aqueous solution containing sugar (lactose), proteins (lactalbumin and casein), and several cations (K+, Ca2+, and Na+) and anions (Cl− and phosphate). The composition of human milk differs from that of human colostrum (the thin, yellowish, milk-like substance secreted during the first several days after parturition) and cow’s milk (Table 56-8). Cow’s milk has nearly three times more protein than human milk, almost exclusively a result of its much higher casein concentration. It also has a higher electrolyte content. The difference in composition between human milk and cow’s milk is important because a newborn, with his or her delicate gastrointestinal tract, may not tolerate the more concentrated cow’s milk.
Table 56-8 Composition of Human Colostrum, Human Milk, and Cow’s Milk (per Deciliter of Fluid)
The epithelial cells in the alveoli of the mammary gland secrete the complex mixture of constituents that make up milk by five major routes (Fig. 56-11D):
1. Secretory pathway. The milk proteins lactalbumin and casein are synthesized in the endoplasmic reticulum and are sorted to the Golgi apparatus (see Chapter 2). Here alveolar cells add Ca2+ and phosphate to the lumen. Lactose synthetase in the lumen of the Golgi catalyzes synthesis of lactose, the major carbohydrate. Lactose synthetase has two components, a galactosyl transferase and lactalbumin, both made in the endoplasmic reticulum. Water enters the secretory vesicle by osmosis. Finally, exocytosis discharges the contents of the vesicle into the lumen of the alveolus.
2. Transcellular endocytosis and exocytosis. The basolateral membrane takes up maternal immunoglobulins by receptor-mediated endocytosis (see Chapter 2). Following transcellular transport of these vesicles to the apical membrane, the cell secretes these immunoglobulins (primarily IgA) by exocytosis. The gastrointestinal tract of the infant takes up these immunoglobulins (see Chapter 45), which are important for conferring immunity before the infant’s own immune system matures.
3. Lipid pathway. Epithelial cells synthesize short-chain fatty acids. However, the longer chain fatty acids (>16 carbons) that predominate in milk originate primarily from the diet or from fat stores. The fatty acids form into lipid droplets and move to the apical membrane. As the apical membrane surrounds the droplets and pinches off, it secretes the milk lipids into the lumen in a membrane-bound sac.
4. Transcellular salt and water transport. Various transport processes at the apical and basolateral membranes move small electrolytes from the interstitial fluid into the lumen of the alveolus. Water follows an osmotic gradient generated primarily by lactose (present at a final concentration of ~200 mM) and, to a lesser extent, by the electrolytes.
5. Paracellular pathway. Salt and water can also move into the lumen of the alveolus through the tight junctions (see Chapter 5). In addition, cells, primarily leukocytes, squeeze between cells and enter the milk.
PRL is a polypeptide hormone that is structurally related to GH, placental-variant GH, and hCS1 and hCS2 (see Table 48-1). Like GH, PRL is made and released in the anterior pituitary; however, lactotrophs rather than somatotrophs, are responsible for PRL synthesis. Another difference is that whereas GH-releasing hormone stimulates somatotrophs to release GH, dopamine (DA) inhibits the release of PRL from lactotrophs. Thus, the removal of inhibition promotes PRL release.
The actions of PRL on the mammary glands (Table 56-7) include the promotion of mammary growth (mammogenic effect), the initiation of milk secretion (lactogenic effect), and the maintenance of milk production once it has been established (galactopoietic effect). Although the initiation of lactation requires the coordinated action of several hormones, PRL is the classic lactogenic hormone. Initiating milk production also necessitates the abrupt fall in estrogens and progesterone that accompanies parturition. PRL is also the primary hormone responsible for maintaining milk production once it has been initiated.
PRL binds to a tyrosine kinase–associated receptor (see Chapter 3) in the same family of receptors as the GH receptor. PRL receptors, which have equal affinities for GH, are present in tissues such as breast, ovary, and liver. Presumably through pathways initiated by protein phosphorylation at tyrosine residues, PRL stimulates transcription of the genes that encode several milk proteins, including lactalbumin and casein.
Suckling is the most powerful physiological stimulus for PRL release. Nipple stimulation triggers PRL secretion through an afferent neural pathway through the spinal cord, thereby inhibiting dopaminergic neurons in the median eminence of the hypothalamus (Fig. 56-12). Because DA normally inhibits PRL release from the lactotrophs, it is called a PRL-inhibitory factor (PIF). Thus, because suckling decreases DA delivery through the portal vessels, it relieves the inhibition on the lactotrophs in the anterior pituitary and stimulates bursts of PRL release. Treating women with DA-receptor agonists rapidly inhibits PRL secretion and milk production.
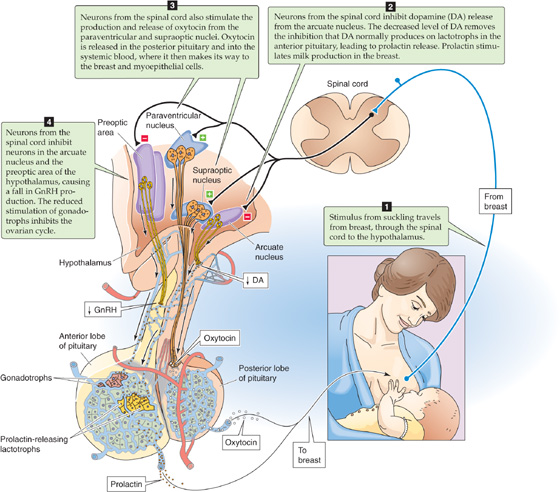
Figure 56-12 Effect of suckling on the release of PRL, OT, and GnRH. Suckling has four effects. First, it stimulates sensory nerves, which carry the signal from the breast to the spinal cord, where the nerves synapse with neurons that carry the signal to the brain. Second, in the arcuate nucleus of the hypothalamus, the afferent input from the nipple inhibits neurons that release DA. DA normally travels through the hypothalamic-portal system to the anterior pituitary, where it inhibits PRL release by lactotrophs. Thus, inhibition of DA release leads to an increase in PRL release. Third, in the supraoptic and paraventricular nuclei of the hypothalamus, the afferent input from the nipple triggers the production and release of OT in the posterior pituitary. Fourth, in the preoptic area and arcuate nucleus, the afferent input from the nipple inhibits GnRH release. GnRH normally travels through the hypothalamic-portal system to the anterior pituitary, where it stimulates the synthesis and release of FSH and LH. Thus, inhibiting GnRH release curbs FSH and LH release and thereby inhibits the ovarian cycle.
Several factors act as PRL-releasing factors (PRFs): thyrotropin-releasing hormone (TRH), angiotensin II, substance P, β endorphin, and AVP. In the rat, suckling stimulates the release of TRH from the hypothalamus. In lactating women, TRH leads to increased milk production. Estradiol modulates PRL release in two ways. First, estradiol increases the sensitivity of the lactotroph to stimulation by TRH. Second, estradiol decreases the sensitivity of the lactotroph to inhibition by DA.
During the first 3 weeks of the neonatal period, maternal PRL levels remain tonically elevated. Thereafter, PRL levels decrease to a constant baseline level higher than that observed in women who are not pregnant. If the mother does not nurse her young, PRL levels generally fall to nonpregnant levels after 1 to 2 weeks. If the mother does breast-feed, increased PRL secretion is maintained for as long as suckling continues. Suckling causes episodic increases in PRL secretion with each feeding, thus producing peaks in PRL levels superimposed on the elevated baseline PRL levels. After the infant completes a session of nursing, PRL levels return to their elevated baseline and remain there until the infant nurses again.
OT, which can promote uterine contraction, also enhances milk ejection by stimulating the contraction of the network of myoepithelial cells surrounding the alveoli and ducts of the breast (galactokinetic effect). Nursing can sometimes cause uterine cramps. During nursing, suckling stimulates nerve endings in the nipple and triggers rapid bursts of OT release (Fig. 56-12). This neurogenic reflex is transmitted through the spinal cord, the midbrain, and the hypothalamus, where it stimulates neurons in the paraventricular and supraoptic nuclei that release OT from their nerve endings in the posterior pituitary. From the posterior pituitary, OT enters the systemic circulation and eventually reaches the myoepithelial cells arranged longitudinally on the lactiferous ducts and around the alveoli in the breast (Fig. 56-11C, D). Because these cells have OT receptors, OT causes them to contract by mechanisms similar to those for the contraction of uterine smooth muscle, described earlier. The result is to promote the release of pre-existing milk after 40 to 60 seconds, a process known as the let-down reflex.
In addition to the suckling stimulus, many different psychic stimuli emanating from the infant, as well as neuroendocrine factors, also promote OT release. The site or sound of an infant may trigger milk let-down, a phenomenon observed in many mammals. Thus, the posterior pituitary releases OT episodically even in anticipation of suckling. This psychogenic reflex is suppressed when fear, anger, or other stresses are encountered, and the results are inhibition of OT release and suppression of milk outflow.
Lactation generally inhibits cyclic ovulatory function. Suckling likely reduces the release of gonadotropin-releasing hormone (GnRH) by neurons in the arcuate nucleus and the preoptic area of the hypothalamus (Fig. 56-12). Normally, GnRH travels through the portal vessels to the gonadotrophs in the anterior pituitary. Thus, the decreased GnRH release induced by suckling reduces the secretion of follicle-stimulating hormone (FSH) and LH and has a negative effect on ovarian function. As a result, breast-feeding delays ovulation and normal menstrual cycles. However, if the mother continues to nurse her infant for a prolonged period, ovulatory cycles will eventually resume. Suckling intensity and frequency, which decrease with the introduction of supplementary foods to the infant, determine the duration of anovulation and amenorrhea in well-nourished women. In breast-feeding women in Bangladesh, the period of anovulation averages 18 to 24 months. If the mother does not nurse her child after delivery, ovulatory cycles resume, on average, 8 to 10 weeks after delivery, with a range of up to 18 weeks.
Books and Reviews
Casey ML, MacDonald PC: Endocrine changes of pregnancy. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, eds. Williams Textbook of Endocrinology. Philadelphia: WB Saunders; 1998.
Fuchs A-R: Physiology and endocrinology of lactation. In: Gabbe SG, Niebyl JR, Simpson JL, (eds): Obstetrics: Normal and Problem Pregnancies, 3rd ed. New York: Churchill Livingstone, 1996, 137-157.
Lamberts SWJ, Macleod RM: Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev 1990; 70:279-318.
Moore TR, Reiter RC, Rebar RW, Baker VV (eds): Gynecology and Obstetrics: A Longitudinal Approach. New York: Churchill Livingstone, 1993.
Ramsey EM, Eonner MW: Placental Vasculature and Circulation. Philadelphia: WB Saunders, 1980.
Stulc J: Placental transfer of inorganic ions and water. Physiol Rev 1997; 77:805-836.
Vonderhaar BK, Ziska SE: Hormonal regulation of milk protein gene expression. Annu Rev Physiol 1989; 51:641-652.
Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds): Williams Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders, 1998.
Yen SSC, Jaffe RB (eds): Reproductive Endocrinology. Philadelphia: WB Saunders; 1986.
Journal Articles
Fuch AR, Fuchs F, Husslein P, et al: Oxytocin receptors and human parturition: A dual role for oxytocin in the initiation of labor. Science 1982; 215:1396-1398.
Goebelsmann U, Jaffe RB: Oestriol metabolism in pregnant women. Acta Endocrinol 1971; 66:679-693.
Liggins GC: Initiation of parturition. Br Med Bull 1979; 35:145-150.
Perez A, Vela P, Masnick GS, Potter RG: First ovulation after childbirth: The effect of breast feeding. Am J Obstet Gynecol 1972; 114:1041-1047.
Tabibzadeh S, Babaknia A: The signals and molecular pathways involved in implantation, a symbiotic interaction between blastocyst and endometrium involving adhesion and tissue invasion. Hum Reprod 1995; 10:1579-1602.
Wigglesworth JS: Vascular organization of the human placenta. Nature 1967; 216:1120-1121.
Wilkening RB, Meschia G: Fetal oxygen uptake, oxygenation, and acid-base balance as a function of uterine blood flow. Am J Physiol 1983; 244:H749-H755.