Chapter 15 Liver, Gallbladder, and Biliary Tract
See Targeted Therapy available online at studentconsult.com
Contributions of Drs. Jim Crawford and Nelson Fausto to this chapter in earlier editions are gratefully acknowledged.
The Liver
The liver and its companion biliary tree and gallbladder are considered together because of their anatomic proximity and interrelated functions and the overlapping features of some diseases that affect these organs. This chapter focuses primarily on the liver, because it has by far the greater role in normal physiology and is the site of a wide variety of diseases.
Residing at the crossroads between the digestive tract and the rest of the body, the liver has the enormous task of maintaining the body’s metabolic homeostasis. This task includes the processing of dietary amino acids, carbohydrates, lipids, and vitamins; synthesis of serum proteins; and detoxification and excretion into bile of endogenous waste products and xenobiotics. Thus, it is not surprising that the liver is vulnerable to a wide variety of metabolic, toxic, microbial, and circulatory insults. In some instances the disease process is primary to the liver. In others the hepatic involvement is secondary, often to some of the most common diseases in humans, such as heart failure, diabetes, and extrahepatic infections.
The liver has enormous functional reserve, and regeneration occurs in all but the most fulminant of hepatic diseases. Surgical removal of 60% of the liver in a normal person is followed by minimal and transient hepatic impairment, with restoration of most of its mass by regeneration within 4 to 6 weeks. In persons who have sustained massive hepatic necrosis, almost perfect restoration may occur if the patient can survive the metabolic insult of liver failure. The functional reserve and the regenerative capacity of the liver mask to some extent the clinical impact of early liver damage. However, with progression of diffuse disease or disruption of the circulation or bile flow, the consequences of deranged liver function become severe and even life-threatening.
Clinical Syndromes
The major clinical syndromes of liver disease are hepatic failure, cirrhosis, portal hypertension, and cholestasis. These conditions have characteristic clinical manifestations (Table 15–1), and a battery of laboratory tests for their evaluation (Table 15–2), with liver biopsy representing the gold standard for diagnosis.
Table 15–1 Clinical Consequences of Liver Disease
| Characteristic Signs of Severe Hepatic Dysfunction |
| Portal Hypertension Associated with Cirrhosis |
| Complications of Hepatic Failure |
Table 15–2 Laboratory Evaluation of Liver Disease
| Test Category | Serum Measurement* |
|---|---|
| Hepatocyte integrity | Cytosolic hepatocellular enzymes† |
| Serum aspartate aminotransferase (AST) | |
| Serum alanine aminotransferase (ALT) | |
| Serum lactate dehydrogenase (LDH) | |
| Biliary excretory function | Substances secreted in bile† |
| Serum bilirubin | |
| Total: unconjugated plus conjugated | |
| Direct: conjugated only | |
| Delta: covalently linked to albumin | |
| Urine bilirubin | |
| Serum bile acids | |
| Plasma membrane enzymes† (from damage to bile canaliculi) | |
| Serum alkaline phosphatase | |
| Serum γ-glutamyl transpeptidase | |
| Serum 5′-nucleotidase | |
| Hepatocyte function | Proteins secreted into the blood |
| Serum albumin‡ | |
| Prothrombin time† (factors V, VII, X, prothrombin, fibrinogen) | |
| Hepatocyte metabolism | |
| Serum ammonia† | |
| Aminopyrine breath test (hepatic demethylation) | |
| Galactose elimination (intravenous injection) |
* The most commonly performed tests are in italics.
† An elevation indicates liver disease.
‡ A decrease indicates liver disease.
Hepatic Failure
The most severe clinical consequence of liver disease is hepatic failure. It generally develops as the end point of progressive damage to the liver, either through insidious piecemeal destruction of hepatocytes or by repetitive waves of symptomatic parenchymal damage. Less commonly, hepatic failure is the result of sudden, massive destruction. Whatever the sequence, 80% to 90% of hepatic function must be lost before hepatic failure ensues. In many cases, the balance is tipped toward decompensation by intercurrent conditions or events that place demands on the liver. These include systemic infections, electrolyte disturbances, major surgery, heart failure, and gastrointestinal bleeding.
The patterns of injury that cause liver failure fall into three categories:
• Acute liver failure with massive hepatic necrosis. Most often caused by drugs or viral hepatitis, acute liver failure denotes clinical hepatic insufficiency that progresses from onset of symptoms to hepatic encephalopathy within 2 to 3 weeks. A course extending as long as 3 months is called subacute failure. The histologic correlate of acute liver failure is massive hepatic necrosis, whatever the underlying cause. It is an uncommon but life-threatening condition that often necessitates liver transplantation.
• Chronic liver disease. This is the most common route to hepatic failure and is the end point of relentless chronic liver damage. While all structural components of the liver are involved in end-stage chronic liver disease, the processes that initiate and drive chronic damage to the liver can usually be classified as either primarily hepatocytic (or parenchymal), biliary, or vascular. Regardless of the initiating factors, chronic damage to the liver often ends in cirrhosis, as described later.
• Hepatic dysfunction without overt necrosis. Less commonly than the forms described above, hepatocytes may be viable but unable to perform their normal metabolic function. Settings where this is seen most often are mitochondrial injury in Reye syndrome, acute fatty liver of pregnancy, and some drug- or toxin-mediated injuries.
Clinical Features
The clinical manifestations of hepatic failure from chronic liver disease are much the same regardless of the cause of the disease. Jaundice is an almost invariable finding. Impaired hepatic synthesis and secretion of albumin lead to hypoalbuminemia, which predisposes to peripheral edema. Hyperammonemia is attributable to defective hepatic urea cycle function. Signs and symptoms of chronic disease include palmar erythema (a reflection of local vasodilatation) and spider angiomas of the skin. Each angioma is a central, pulsating, dilated arteriole from which small vessels radiate. There may also be impaired estrogen metabolism and consequent hyperestrogenemia, which leads to hypogonadism and gynecomastia in men. Acute liver failure may manifest as jaundice or encephalopathy, but notably absent on physical examination are the other stigmata of chronic liver disease.
Hepatic failure is life-threatening for several reasons. The accumulation of toxic metabolites may have widespread effects and patients are highly susceptible to failure of multiple organ systems. Thus, respiratory failure with pneumonia and sepsis can give rise to renal failure and thus claim the lives of many individuals with hepatic failure. A coagulopathy develops, attributable to impaired hepatic synthesis of blood clotting factors. The resultant bleeding tendency may lead to massive gastrointestinal hemorrhage as well as bleeding elsewhere. Intestinal absorption of blood places a metabolic load on the liver that worsens the severity of hepatic failure.
The outlook with full-blown hepatic failure is particularly grave for persons with chronic liver disease. A rapid downhill course is usual, with death occurring within weeks to a few months in about 80% of cases. About 40% of patients with acute liver failure may recover spontaneously. Liver transplantation in acute or chronic liver failure can be curative, however. Conditions contributing to the extraordinary morbidity and eventual mortality associated with severe liver disease are discussed next.
Jaundice and Cholestasis
Jaundice results from the retention of bile. Hepatic bile formation serves two major functions. First, bile constitutes the primary pathway for the elimination of bilirubin, excess cholesterol, and xenobiotics that are insufficiently water-soluble to be excreted in the urine. Second, secreted bile salts and phospholipid molecules promote emulsification of dietary fat in the lumen of the gut. Bile formation is a complex process and is readily disrupted by a variety of hepatic insults. Thus, jaundice, a yellow discoloration of skin and sclerae (icterus), occurs when systemic retention of bilirubin produces serum levels above 2.0 mg/dL (the normal level in adults is below 1.2 mg/dL). Cholestasis is defined as systemic retention of not only bilirubin but also other solutes eliminated in bile (particularly bile salts and cholesterol).
Bilirubin and Bile Acids
Bilirubin is the end product of heme degradation (Fig. 15–1). Most of the daily production (0.2 to 0.3 g) is derived from breakdown of senescent red cells within mononuclear phagocytes, with the remainder derived primarily from the turnover of hepatic hemoproteins. Excessive destruction of erythroid progenitors in the bone marrow due to intramedullary apoptosis (ineffective erythropoiesis) is an important cause of jaundice in hematologic disorders (Chapter 11). Whatever the source, heme oxygenase oxidizes heme to biliverdin, which is then reduced to bilirubin by biliverdin reductase. Bilirubin thus formed outside the liver in cells of the mononuclear phagocyte system (including the spleen) is released and bound to serum albumin. Hepatocellular processing of bilirubin involves the following sequence:
1 Carrier-mediated uptake at the sinusoidal membrane
2 Cytosolic protein binding and delivery to the endoplasmic reticulum
3 Conjugation with one or two molecules of glucuronic acid by bilirubin uridine diphosphate–glucuronosyltransferase
4 Excretion of the water-soluble, nontoxic bilirubin glucuronides into bile. Most bilirubin glucuronides are deconjugated by gut bacterial β-glucuronidases and degraded to colorless urobilinogens. The urobilinogens and the residue of intact pigment are largely excreted in feces. Approximately 20% of the urobilinogens are reabsorbed in the ileum and colon, returned to the liver, and promptly reexcreted into bile. Conjugated and unconjugated bile acids also are reabsorbed in the ileum and returned to the liver by the enterohepatic circulation.
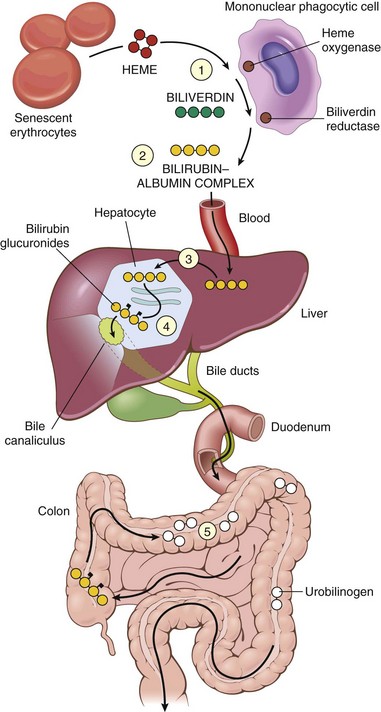
Figure 15–1 Bilirubin metabolism and elimination. 1, Normal bilirubin production (0.2 to 0.3 g/day) is derived primarily from the breakdown of senescent circulating red cells, with a minor contribution from degradation of tissue heme-containing proteins. 2, Extrahepatic bilirubin is bound to serum albumin and delivered to the liver. 3 and 4, Hepatocellular uptake (3) and glucuronidation (4) by glucuronosyltransferase in the hepatocytes generate bilirubin monoglucuronides and diglucuronides, which are water-soluble and readily excreted into bile. 5, Gut bacteria deconjugate the bilirubin and degrade it to colorless urobilinogens. The urobilinogens and the residue of intact pigments are excreted in the feces, with some reabsorption and reexcretion into bile.
![]() Pathogenesis
Pathogenesis
In the normal adult, the rate of systemic bilirubin production is equal to the rates of hepatic uptake, conjugation, and biliary excretion. Jaundice occurs when the equilibrium between bilirubin production and clearance is disrupted; the major responsible disorders are listed in Table 15–3. More than one mechanism may operate to cause jaundice, especially in hepatitis, when both unconjugated and conjugated bilirubin may be produced in excess. In severe disease, bilirubin levels may reach 30 to 40 mg/dL.
Table 15–3 Main Causes of Jaundice
| Predominantly Unconjugated Hyperbilirubinemia |
| Excess Production of Bilirubin |
| Reduced Hepatic Uptake |
| Impaired Bilirubin Conjugation |
| Physiologic jaundice of the newborn |
| Predominantly Conjugated Hyperbilirubinemia |
| Decreased Hepatocellular Excretion |
| Impaired Intra- or Extrahepatic Bile Flow |
| Inflammatory destruction of intrahepatic bile ducts (e.g., primary biliary cirrhosis, primary sclerosing cholangitis, graft-versus-host disease, liver transplantation); gall stones, carcinoma of the pancreas |
Of these various causes of jaundice, the most common are hepatitis, obstruction to the flow of bile (discussed later in this chapter), and hemolytic anemia (Chapter 11). Because the hepatic machinery for conjugating and excreting bilirubin does not fully mature until about 2 weeks of age, almost every newborn develops transient and mild unconjugated hyperbilirubinemia, termed neonatal jaundice or physiologic jaundice of the newborn.
Jaundice also may result from inborn errors of metabolism, including
• Gilbert syndrome, a relatively common (7% of the population), benign, somewhat heterogeneous inherited condition manifesting as mild, fluctuating unconjugated hyperbilirubinemia. The primary cause is decreased hepatic levels of glucuronosyltransferase attributed to a mutation in the encoding gene; polymorphisms in the gene may play a role in the variable expression of this disease. The hyperbilirubinemia is not associated with any morbidity.
• Dubin-Johnson syndrome results from an autosomal recessive defect in the transport protein responsible for hepatocellular excretion of bilirubin glucuronides across the canalicular membrane. Affected persons exhibit conjugated hyperbilirubinemia. Other than having a darkly pigmented liver (from polymerized epinephrine metabolites, not bilirubin) and hepatomegaly, patients are otherwise without functional problems.
Cholestasis, which results from impaired bile flow due to hepatocellular dysfunction or intrahepatic or extrahepatic biliary obstruction, also may manifest as jaundice. However, sometimes pruritus is the presenting symptom, the pathogenesis of which remains obscure. Skin xanthomas (focal accumulations of cholesterol) sometimes appear, the result of hyperlipidemia and impaired excretion of cholesterol. A characteristic laboratory finding is elevated serum alkaline phosphatase, an enzyme present in bile duct epithelium and in the canalicular membrane of hepatocytes. A different alkaline phosphatase isozyme normally is expressed in many other tissues such as bone, and so hepatic origin must be verified. Reduced bile flow also causes intestinal malabsorption including inadequate absorption of the fat-soluble vitamins A, D, and K.
Extrahepatic biliary obstruction frequently is amenable to surgical correction. By contrast, cholestasis caused by diseases of the intrahepatic biliary tree or hepatocellular secretory failure (collectively termed intrahepatic cholestasis) cannot be treated surgically (short of transplantation), and the patient’s condition may be worsened by an operative procedure. Thus, there is some urgency in identifying the cause of jaundice and cholestasis.
![]() Summary
Summary
Jaundice and Cholestasis
• Jaundice occurs when retention of bilirubin leads to serum levels above 2.0 mg/dL.
• Hepatitis and intra- or extrahepatic obstruction of bile flow are the most common causes of jaundice involving the accumulation of conjugated bilirubin.
• Hemolytic anemias are the most common cause of jaundice involving the accumulation of unconjugated bilirubin.
• Cholestasis is the impairment of bile flow resulting in the retention of bilirubin, bile acids, and cholesterol.
• Serum alkaline phosphatase usually is elevated in cholestatic conditions.
Hepatic Encephalopathy
Hepatic encephalopathy may develop rapidly in acute liver failure or insidiously with gradually evolving chronic liver failure from cirrhosis. In either setting, patients with hepatic encephalopathy show a spectrum of brain dysfunction ranging from subtle behavioral abnormalities to marked confusion and stupor, to deep coma and death. These changes may progress over hours or days as, for example, in fulminant hepatic failure or gradually in a person with marginal hepatic function from chronic liver disease. Associated fluctuating neurologic signs include rigidity, hyperreflexia, nonspecific electroencephalographic changes, and, rarely, seizures. Particularly characteristic is asterixis (also called flapping tremor), which is a pattern of nonrhythmic, rapid extension-flexion movements of the head and extremities, best seen when the arms are held in extension with dorsiflexed wrists.
In most instances there are only minor morphologic changes in the brain, such as edema and an astrocytic reaction. Two factors seem to be important in the genesis of this disorder:
• Severe loss of hepatocellular function
• Shunting of blood from portal to systemic circulation around the chronically diseased liver
In the acute setting, an elevation in blood ammonia, which impairs neuronal function and promotes generalized brain edema, seems to be key. In the chronic setting, deranged neurotransmitter production, particularly in monoaminergic, opioidergic, γ-aminobutyric acid (GABA)-ergic, and endocannabanoid systems, leads to neuronal dysfunction.
Cirrhosis
Cirrhosis is among the top 10 causes of death in the Western world. Its major causes include chronic viral infections, alcoholic or nonalcoholic steatohepatitis (NASH), autoimmune diseases affecting hepatocytes and/or bile ducts, and iron overload. Cirrhosis is defined as a diffuse process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules. Its main characteristics by definition are not focal but rather involve most (if not all) of the diseased liver and include
• Fibrous septa in the form of delicate bands or broad scars around multiple adjacent lobules. Long-standing fibrosis generally is irreversible so long as disease persists or if disease-associated vascular shunts are widespread, although regression is possible if the underlying cause of liver disease is reversed.
• Parenchymal nodules, ranging in size from very small (less than 3 mm in diameter—micronodules) to large (over 1 cm—macronodules), encircled by these fibrous bands. Hepatocytes in these nodules derive from two sources: (1) preexistent, long-lived hepatocytes that, by the time cirrhosis is established, display features of replicative senescence; and (2) newly formed hepatocytes capable of replication that are derived from stem/progenitor cells found adjacent to the canals of Hering and small bile ductules—the hepatobiliary stem cell niche. These stem/progenitor cells also give rise to the ductular reactions found at the periphery of most cirrhotic nodules, where parenchyma meets stromal scar, and are accompanied by proliferating endothelial cells, myofibroblasts, and inflammatory cells.
There is no satisfactory classification of cirrhosis save for specification of the presumed underlying etiology. After all known causes have been excluded, about 10% of cases remain, referred to as cryptogenic cirrhosis, although in recent years most of these are recognized as probable “burned-out” NASH. General principles are presented next; the distinguishing features of each form of cirrhosis are discussed subsequently in the relevant disease overview.
![]() Pathogenesis
Pathogenesis
Three processes are central to the pathogenesis of cirrhosis: death of hepatocytes, extracellular matrix deposition, and vascular reorganization.
Changes in the connective tissue and extracellular matrix (ECM) are common to all forms of cirrhosis. In the normal liver, ECM consisting of interstitial collagens (fibril-forming collagen types I, III, V, and XI) is present only in the liver capsule, in portal tracts, and around central veins. The hepatocytes have no true basement membrane; instead, a delicate framework containing type IV collagen and other proteins lies in the space between sinusoidal endothelial cells and hepatocytes (the space of Disse). By contrast, in cirrhosis, types I and III collagen and other ECM components are deposited in the space of Disse (Fig. 15–2).
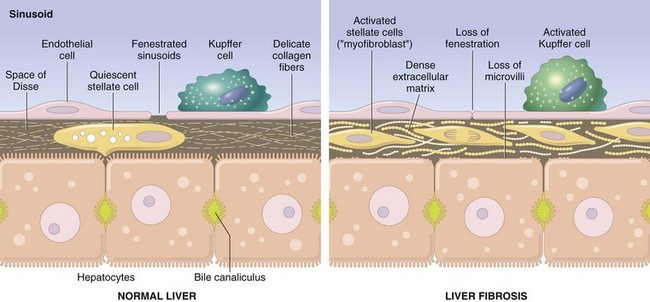
Figure 15–2 Liver fibrosis. In the normal liver, the perisinusoidal space (space of Disse) contains a delicate framework of extracellular matrix components. In liver fibrosis, stellate cells are activated to produce a dense layer of matrix material that is deposited in the perisinusoidal space. Collagen deposition blocks the endothelial fenestrations and prevents the free exchange of materials from the blood. Kuppfer cells also are activated and produce cytokines that are involved in fibrosis. Note that this illustration is not to scale; the space of Disse is actually much narrower than shown.
The major source of excess collagen in cirrhosis are the perisinusoidal stellate cells (formerly known as Ito cells), which lie in the space of Disse. Although they normally function as storage cells for vitamin A, during the development of fibrosis they activate and transform into myofibroblasts. The stimuli for the activation of stellate cells and production of collagen are believed to include reactive oxygen species, growth factors, and cytokines such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and lymphotoxins, which can be produced by damaged hepatocytes or by stimulated Kupffer cells and sinusoidal endothelial cells. Activated stellate cells themselves produce growth factors, cytokines, and chemokines that cause their further proliferation and collagen synthesis—in particular, transforming growth factor-β (TGF-β). Portal fibroblasts probably also participate in some forms of cirrhosis. During the course of chronic liver disease, fibrosis is a dynamic process that involves the synthesis, deposition, and resorption of ECM components, modulated by changing balances between metalloproteases and tissue inhibitors of metalloproteases (Chapter 2). Thus, even in late-stage disease, if the disease process is halted or eliminated, significant remodeling and even restoration of liver function (cirrhotic regression) is possible.
Vascular injuries and changes also play significant roles in remodeling of the liver into a cirrhotic state. Inflammation and thrombosis of portal veins, hepatic arteries, and/or central veins may lead to alternating zones of parenchymal hypoperfusion, with resulting parenchymal atrophy, and hyperperfusion, with overcompensating regeneration. The major vascular lesions that contribute to defects in liver function are loss of sinusoidal endothelial cell fenestrations (Fig. 15–2) and the development of portal vein–hepatic vein and hepatic artery–portal vein vascular shunts. While normal sinusoids have fenestrated endothelial cells that allow free exchange of solutes between plasma and hepatocytes, loss of fenestrations and increased basement membrane formation convert thin-walled sinusoids into higher pressure, fast-flowing vascular channels without such solute exchange. In particular, the movement of proteins (e.g., albumin, clotting factors, lipoproteins) between hepatocytes and the plasma is markedly impaired. These functional changes are aggravated by the loss of microvilli from the hepatocyte surface, further diminishing its transport capacity. Vascular shunts mentioned earlier lead to abnormal vascular pressures in the liver and contribute to hepatic dysfunction and portal hypertension, described later.
The causes of liver cell injury that give rise to cirrhosis are varied and depend on the etiology (viral, alcoholic, drugs). As described earlier, the normal liver cells are replaced by parenchymal nodules derived from long-lived surviving hepatocytes and new cells generated from stem cells. The regenerating liver cells form spherical nodules confined by fibrous septa.
Clinical Features
All forms of cirrhosis may be clinically silent. When symptoms appear, they typically are nonspecific and include anorexia, weight loss, weakness, and, in advanced disease, frank debilitation. Incipient or overt hepatic failure may develop, usually precipitated by imposition of a metabolic load on the liver, as from systemic infection or a gastrointestinal hemorrhage. Most cases of ultimately fatal cirrhosis involve one of the following mechanisms:
![]() Summary
Summary
Cirrhosis
• The three main characteristics of cirrhosis are (1) involvement of most or all of the liver, (2) bridging fibrous septa, and (3) parenchymal nodules containing a mix of senescent and replicating (often stem/progenitor cell-derived) hepatocytes.
• Cirrhosis usually is an end-stage process that may have multiple causes. The most frequent are chronic hepatitis B and C and alcoholic and nonalcoholic steatohepatitis. Less frequent causes are autoimmune and biliary diseases and metabolic conditions such as hemochromatosis.
• The main complications of cirrhosis are related to decreased liver function, portal hypertension, and increased risk for development of hepatocellular carcinoma.
Portal Hypertension
Increased resistance to portal blood flow may develop from prehepatic, intrahepatic, and posthepatic causes (described later). The dominant intrahepatic cause is cirrhosis, accounting for most cases of portal hypertension. Far less frequent are instances of noncirrhotic portal hypertension, such as from schistosomiasis, massive fatty change, diffuse granulomatous diseases (e.g., sarcoidosis, miliary tuberculosis), and diseases affecting the portal microcirculation, exemplified by nodular regenerative hyperplasia.
Portal hypertension in cirrhosis results from increased resistance to portal flow at the level of the sinusoids and compression of central veins by perivenular fibrosis and expanded parenchymal nodules. Anastomoses between the arterial and portal systems in the fibrous bands also contribute to portal hypertension by imposing arterial pressure on the normally low-pressure portal venous system. Another major factor in the causation of portal hypertension is an increase in portal venous blood flow resulting from a hyperdynamic circulation. This is caused by arterial vasodilation in the splanchnic circulation, resulting primarily from increased production of nitric oxide (NO) in the vascular bed. This occurs in response to reduced clearance of bacterial DNA absorbed from the gut that bypasses the Kupffer cells due to intrahepatic shunting of blood from portal to systemic circulation. Bacterial DNA causes increased production of NO. The major clinical consequences are discussed next (Fig. 15–3).
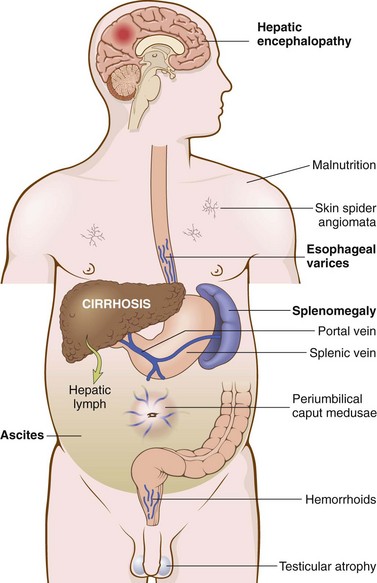
Figure 15–3 Some clinical consequences of portal hypertension in the setting of cirrhosis. The most important manifestations are in bold type.
Ascites
Ascites refers to the collection of excess fluid in the peritoneal cavity. It usually becomes clinically detectable when at least 500 mL have accumulated, but many liters may collect, causing massive abdominal distention. Ascites generally is a serous fluid containing as much as 3 g/dL of protein (largely albumin). More importantly, the serum to ascites albumin gradient is ≥1.1 g/dL. The fluid may contain a scant number of mesothelial cells and mononuclear leukocytes. Influx of neutrophils suggests secondary infection, whereas presence of red cells points to possible disseminated intraabdominal cancer. With long-standing ascites, seepage of peritoneal fluid through transdiaphragmatic lymphatics may produce hydrothorax, more often on the right side.
![]() Pathogenesis
Pathogenesis
The pathogenesis of ascites is complex, involving one or more of the following mechanisms:
• Increased movement of intravascular fluid into the extravascular space of Disse, caused by sinusoidal hypertension and hypoalbuminemia.
• Leakage of fluid from the hepatic interstitium into the peritoneal cavity. Normal thoracic duct lymph flow is 800 to 1000 mL/day. With cirrhosis, hepatic lymphatic flow may approach 20 L/day, exceeding thoracic duct capacity. Hepatic lymph is rich in proteins and low in triglycerides, as reflected in the protein-rich ascitic fluid.
• Renal retention of sodium and water due to secondary hyperaldosteronism (Chapter 3), despite a total body sodium mass greater than normal.
Portosystemic Shunt
With the rise in portal venous pressure, shunts develop wherever the systemic and portal circulations share capillary beds. Principal sites are veins around and within the rectum (manifest as hemorrhoids), the cardioesophageal junction (producing esophagogastric varices), the retroperitoneum, and the falciform ligament of the liver (involving periumbilical and abdominal wall collaterals). Although hemorrhoidal bleeding may occur, it is rarely massive or life-threatening. Much more important are the esophagogastric varices that appear in about 65% of persons with advanced cirrhosis of the liver, causing massive hematemesis and death in some instances (Chapter 14). Rarely, abdominal wall collaterals appear as dilated subcutaneous veins extending outward from the umbilicus (caput medusae).
Splenomegaly
Long-standing congestion may cause congestive splenomegaly. The degree of enlargement varies widely (usually 1000 g or less) and is not necessarily correlated with other features of portal hypertension. Massive splenomegaly may secondarily induce a variety of hematologic abnormalities attributable to hypersplenism (Chapter 11).
Hepatorenal Syndrome
Hepatorenal syndrome generally appears only with severe liver disease and is marked by the development of renal failure without primary abnormalities of the kidneys themselves. Excluded by this definition are concomitant toxic damage to both the liver and the kidney, as may occur in carbon tetrachloride and mushroom poisoning and the copper toxicity of Wilson disease. Also excluded are instances of advanced hepatic failure in which circulatory collapse leads to acute tubular necrosis and renal failure. Kidney function promptly improves if hepatic failure is reversed. Although the exact cause is unknown, evidence points to splanchnic vasodilatation and systemic vasoconstriction, leading to a severe reduction in renal blood flow, particularly to the cortex.
The syndrome is heralded by a drop in urine output and rising blood urea nitrogen and creatinine values. The ability to concentrate urine is retained, producing a hyperosmolar urine devoid of proteins and abnormal sediment that is surprisingly low in sodium (unlike renal tubular necrosis). Renal dialysis or other treatments are at best bridges to the only cure, liver transplantation; however, transplantation recipients with hepatorenal syndrome have a high mortality in the months after the operation.
Portopulmonary Hypertension and Hepatopulmonary Syndrome
Pulmonary dysfunction in chronic liver disease is common and may be life-threatening. Causes of liver injury also may damage the lungs (e.g., α1-antitrypsin deficiency leading to both cirrhosis and emphysema). Ascites, pressing upward on the diaphragm, and pleural effusions associated with portal hypertension can compromise lung capacity. Finally, changes in pulmonary blood flow occurring secondary to hepatic failure may lead to portopulmonary hypertension or hepatopulmonary syndrome.
Portopulmonary hypertension is defined as pulmonary arterial hypertension associated with liver disease or portal hypertension. Although the mechanisms underlying this condition remain obscure, they seem to involve portal hypertension of any cause (cirrhotic or non-cirrhotic) and excessive pulmonary vasoconstriction and vascular remodeling, which eventually lead to right-sided heart failure; the most common clinical manifestations are dyspnea on exertion and clubbing of the fingers, followed by palpitations and chest pain.
Hepatopulmonary syndrome is associated with abnormal intrapulmonary vascular dilatation in combination with increased pulmonary blood flow. Shunting of blood through such dilatations leads to ventilation-perfusion mismatch and reduced oxygen diffusion, thus giving rise to severe arterial hypoxemia with dyspnea and cyanosis. Oxygen supplementation can alleviate these problems early on, though the most severe intrapulmonary vascular dilatation or formation of arteriovenous malformations causes right-to-left shunting that is only partially correctable. Platypnea (easier breathing while lying down as compared to when sitting or standing) and orthodeoxia (fall of arterial blood oxygen with upright posture) are pathognomonic of hepatopulmonary syndrome.
Selected patients with portopulmonary hypertension experience some degree of reversal of disturbed pulmonary function with liver transplantation.
Drug- or Toxin-Induced Liver Disease
As the major drug metabolizing and detoxifying organ in the body, the liver is subject to injury from an enormous array of therapeutic and environmental chemicals. Injury may result from direct toxicity, through hepatic conversion of a xenobiotic to an active toxin, or by immune mechanisms, such as by a drug or a metabolite acting as a hapten to convert a cellular protein into an immunogen.
A diagnosis of drug- or toxin-induced liver disease may be made on the basis of a temporal association of liver damage with drug or toxin exposure and, it is hoped, recovery on removal of the compound(s), combined with exclusion of other potential causes. Exposure to a toxin or therapeutic agent should always be included in the differential diagnosis of any form of liver disease. By far the most important agent that produces toxic liver injury is alcohol; its characteristic histologic (but not clinical) features are shared with nonalcoholic fatty liver disease (NAFLD) and therefore it is discussed in that section.
Drug-induced liver disease is a common condition that may manifest as a mild reaction or, much more seriously, as acute liver failure or chronic liver disease. A large number of drugs and chemicals can produce liver injury (Table 15–4). It is important to keep in mind that compounds other than those normally thought of as drugs or medicines may be to blame; often careful, persistent history taking will uncover exposure to other potential toxins such as herbal remedies, dietary supplements, topical applications (e.g., ointments, perfumes, shampoo), and environmental exposures (e.g., cleaning solvents, pesticides, fertilizers).
Table 15–4 Different Forms of Drug- or Toxin-Induced Hepatic Injury
| Pattern of Injury | Morphologic Findings | Examples of Associated Agents |
|---|---|---|
| Cholestatic | Bland hepatocellular cholestasis, without inflammation | Contraceptive and anabolic steroids; estrogen replacement therapy |
| Cholestatic hepatitis | Cholestasis with lobular inflammation and necrosis; may show bile duct destruction | Numerous antibiotics; phenothiazines |
| Hepatocellular necrosis | Spotty hepatocyte necrosis | Methyldopa, phenytoin |
| Submassive necrosis, zone 3 | Acetaminophen, halothane | |
| Massive necrosis | Isoniazid, phenytoin | |
| Steatosis | Macrovesicular | Ethanol, methotrexate, corticosteroids, total parenteral nutrition |
| Steatohepatitis | Microvesicular, Mallory bodies | Amiodarone, ethanol |
| Fibrosis and cirrhosis | Periportal and pericellular fibrosis | Methotrexate, isoniazid, enalapril |
| Granulomas | Noncaseating epithelioid granulomas | Sulfonamides, numerous other agents |
| Vascular lesions | Sinusoidal obstruction syndrome (venoocclusive disease): obliteration of central veins | High-dose chemotherapy, bush teas |
| Budd-Chiari syndrome | Oral contraceptives | |
| Sinusoidal dilatation | Oral contraceptives, numerous other agents | |
| Peliosis hepatis: blood-filled cavities, not lined by endothelial cells | Anabolic steroids, tamoxifen | |
| Neoplasms | Hepatic adenoma | Oral contraceptives, anabolic steroids |
| Hepatocellular carcinoma | Thorotrast | |
| Cholangiocarcinoma | Thorotrast | |
| Angiosarcoma | Thorotrast, vinyl chloride |
From Washington K: Metabolic and toxic conditions of the liver. In Iacobuzio-Donahue CA, Montgomery EA (eds): Gastrointestinal and Liver Pathology. Philadelphia, Churchill Livingstone, 2005.
Principles of drug and toxic injury are discussed in Chapter 7. Here it suffices to note that drug reactions may be classified as predictable or unpredictable (idiosyncratic). Predictable drug or toxin reactions affect all people in a dose-dependent fashion. Unpredictable reactions depend on individual host variations, particularly the propensity to mount an immune response to drug-related antigen or the rate at which the agent is metabolized. Both classes of injury may be immediate or take weeks to months to develop.
A classic predictable hepatotoxin is acetaminophen, now the most common cause of acute liver failure necessitating transplantation in the United States. The toxic agent is not acetaminophen itself but rather toxic metabolites produced by the cytochrome P-450 system in acinus zone 3 hepatocytes (Fig. 15–4). As these cells die, the zone 2 hepatocytes take over this metabolic function, in turn becoming injured. In severe overdoses the zone of injury extends to the periportal hepatocytes, resulting in fulminant hepatic failure (Fig. 15–5, A and B). While intentional suicidal overdoses are common, so are accidental overdoses. This is because the cytotoxicity is dependent on the activity of the cytochrome P-450 system, which may be upregulated by other agents taken in combination with acetaminophen, such as alcohol (beware acetaminophen as a hangover prophylactic) or codeine in acetaminophen compound tablets.
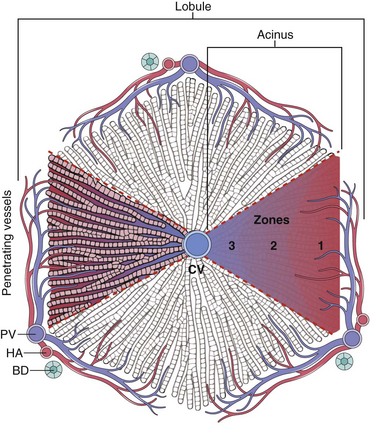
Figure 15–4 Microscopic architecture of the liver parenchyma. Both a lobule and an acinus are represented. The idealized classic lobule is represented as hexagonal centered on a central vein (CV), also known as terminal hepatic venule, and has portal tracts at three of its apices. The portal tracts contain branches of the portal vein (PV), hepatic artery (HA), and the bile duct (BD) system. Regions of the lobule generally are referred to as periportal, midzonal, and centrilobular, according to their proximity to portal spaces and central vein. Another useful way to subdivide the liver architecture is to use the blood supply as a point of reference. Using this approach, triangular acini can be recognized. Acini have at their base branches of portal vessels that penetrate the parenchyma (“penetrating vessels”). On the basis of the distance from the blood supply, the acinus is divided into zones 1 (closest to blood source), 2, and 3 (farthest from blood source).
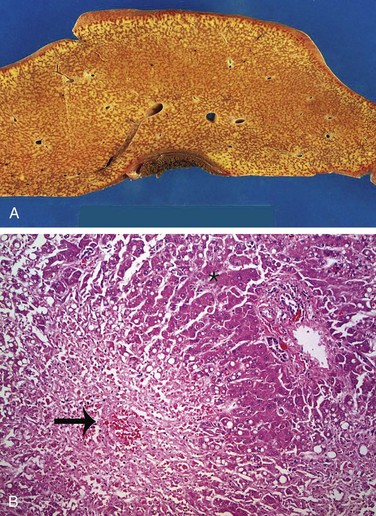
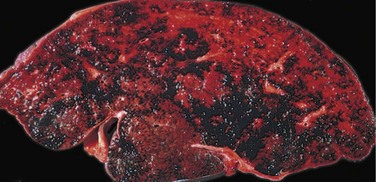
Figure 15–5 A, Massive necrosis, cut section of liver. The liver is small (700 g), bile-stained, soft, and congested. B, Hepatocellular necrosis caused by acetaminophen overdose. Confluent necrosis is seen in the perivenular region (zone 3) (large arrow). There is little inflammation. The residual normal tissue is indicated by the asterisk.
(Courtesy of Dr. Matthew Yeh, University of Washington, Seattle, Washington.)
Examples of drugs that can cause idiosyncratic reactions are chlorpromazine (an agent that causes cholestasis in individuals who metabolize it slowly), halothane (which can cause a fatal immune-mediated hepatitis in some persons exposed to this anesthetic on several occasions), and other drugs such as sulfonamides, α-methyldopa, and allopurinol. Often, idiosyncratic drug or toxin reactions involve a variable combination of direct cytotoxicity and immune-mediated hepatocyte or bile duct destruction. Examples of hepatotoxins are given in each disease-specific category described later.
![]() Summary
Summary
Drug- or Toxin-Induced Liver Disease
• Drug- and toxin-induced liver disease may be predictable (intrinsic) or unpredictable (idiosyncratic).
• Predictable hepatotoxins affect most individuals in a dose-dependent fashion.
• Unpredictable hepatotoxins affect rare persons in an idiosyncratic way, often involving a combination of direct cytotoxicity and immune-mediated injury.
• Every pattern of liver injury can be caused by some toxin or drug; therefore, exposures involving these agents must always be considered in the differential diagnosis.
• In addition to prescription and over-the-counter medications, herbal remedies, dietary supplements, topical applications, and environmental exposures may be responsible for hepatotoxicity.
Acute and Chronic Hepatitis
The terminology of acute and chronic hepatitis can be con-fusing, since the term hepatitis is applied to a number of different diseases and different forms of liver injury. For example, hepatitis is a descriptor for specific histopathologic patterns of hepatocyte injury associated with inflammation and, when chronic, with scarring. Acute and chronic forms of hepatitis are distinguished in part by duration and in part by the pattern of cell injury. Viral hepatitides are also classified on the basis of the causative hepatotropic virus such as hepatitis types A, B, C, D, and E. Because all forms of hepatitis, including those due to the hepatitis viruses as well as autoimmune and drug- and toxin-induced hepatitides, share the same patterns of injury, the general descriptions are presented first, followed by clinicopathologic correlations specific to each cause.
![]() Morphology
Morphology
On gross inspection, liver involved by mild acute hepatitis appears normal or slightly mottled. At the other end of the spectrum, in massive hepatic necrosis the liver may shrink to 500 to 700 g and become transformed into a limp, red organ covered by a wrinkled, baggy capsule. The distribution of liver destruction is extremely capricious: The entire liver may be involved, or only patchy areas affected. On sectioning (Fig. 15–5, A), necrotic areas have a muddy-red, mushy appearance with blotchy bile staining.
If patients survive for more than a week, surviving hepatocytes begin to regenerate (Chapter 2). If the parenchymal framework is preserved, regeneration is orderly and liver architecture is restored. With more massive destruction, regeneration is disorderly, yielding nodular masses of liver cells separated by granulation tissue and, eventually, scar, particularly in patients with a protracted course of submassive necrosis.
The gross appearance of the liver in chronic hepatitis may be normal or include grossly evident focal scarring or, as cirrhosis develops, may feature widespread nodularity surrounded by extensive scarring.
The general microscopic features of acute and chronic hepatitis of all causes are listed in Table 15–5. Unlike most other organ systems in which the distinction between acute and chronic inflammation depends on the predominant type of inflammatory cell—neutrophilic in acute injury, mononuclear in chronic phases—mononuclear infiltrates predominate in all phases of most hepatitic diseases because they all invoke T cell–mediated immunity. Thus, the distinction between acute and chronic hepatitis is based on the pattern of cell injury and severity of inflammation, with acute hepatitis often showing less inflammation and more hepatocyte death than chronic hepatitis.
Table 15–5 Main Morphologic Features of Acute and Chronic Viral Hepatitis
| Acute Hepatitis |
| Gross Changes |
| Enlarged, reddened liver; greenish if cholestatic |
| Parenchymal Changes (Microscopic) |
| Chronic Hepatitis |
HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus.
Both hepatocyte injury and inflammation, while related, can be highly variable depending on etiology and host factors. The hepatocyte injury takes two forms. The first is swelling (ballooning degeneration), producing cells with empty-appearing pale cytoplasm that subsequently rupture and undergo necrosis (cytolysis). The necrotic cells appear to have dropped out, leaving collapsing sinusoidal collagen reticulin framework behind; scavenger macrophages mark sites of dropout. The second pattern of cell death is apoptosis, in which hepatocytes shrink, become intensely eosinophilic, and have fragmented nuclei; effector T cells may be present in the immediate vicinity. When located in the parenchyma away from portal tracts, these features are called lobular hepatitis (Fig. 15–6).
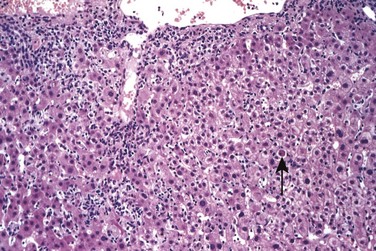
Figure 15–6 Acute viral hepatitis showing disruption of lobular architecture, inflammatory cells in sinusoids, and apoptotic cells (arrow).
In severe cases, confluent necrosis of hepatocytes is seen around central veins (Fig. 15–5, B). In these areas there may be cellular debris, collapsed reticulin fibers, congestion/hemorrhage, and variable inflammation. With increasing severity, central-portal bridging necrosis develops, followed by, even worse, parenchymal collapse. When the injury is overwhelming, massive hepatic necrosis and fulminant liver failure ensue. In occasional cases, the injury is not severe enough to cause death (or necessitate transplantation), and the liver survives, although with abundant scarring that replaces areas of confluent necrosis. In such cases, some patients rapidly develop posthepatitic cirrhosis.
Portal inflammation in acute hepatitis is minimal or absent; dense mononuclear portal infiltrates of variable prominence are the defining lesion of chronic hepatitis (Fig. 15–7). There is often interface hepatitis as well, distinguished from lobular hepatitis by its location at the interface between hepatocellular parenchyma and portal stroma (or scars, when present). The hallmark of severe chronic liver damage is scarring. At first, only portal tracts exhibit fibrosis, but in some patients, with time, fibrous septa—bands of dense scar—will extend between portal tracts. In the most severe cases, continued scarring and nodule formation leads to the development of cirrhosis (Fig 15–8).
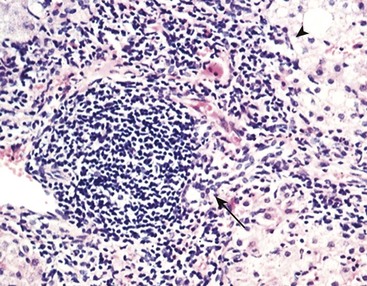
Figure 15–7 Chronic hepatitis showing portal tract expansion by a dense infiltrate of mononuclear cells (arrow) and interface hepatitis with spillover of inflammation into the parenchyma (arrowhead). The prominent lymphoid infiltrate is typical of the cause of disease in this biopsy: chronic hepatitis C.
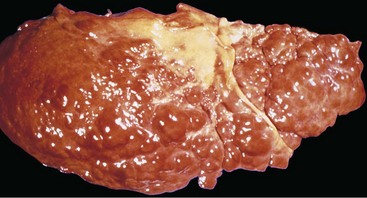
Figure 15–8 Cirrhosis resulting from chronic viral hepatitis. Note the irregular nodularity of the liver surface.
Clinical assessment of chronic hepatitis often requires liver biopsy in addition to clinical and serologic data. Liver biopsy is helpful in confirming the clinical diagnosis, excluding common concomitant conditions (e.g., fatty liver disease, hemochromatosis), assessing histologic features associated with an increased risk of malignancy (e.g., small and large cell change, described later), grading the extent of hepatocyte injury and inflammation, and staging the progression of scarring. Such grading and staging are useful for assessing prognosis and therapeutic options.
Viral Hepatitis
Viral hepatitis is caused mainly by hepatitis viruses A (HAV), B (HBV), C (HCV), D (HDV), and E (HEV). These viruses and their infections have distinct features, which are summarized in Table 15–6.
Hepatitis A Virus
Hepatitis A usually is a benign, self-limited disease with an incubation period of 2 to 6 weeks (average 28 days). HAV does not cause chronic hepatitis or a carrier state. Rarely there is fulminant hepatitis; fatalities occur at a rate of only 0.1%. HAV occurs throughout the world and is endemic in countries with poor hygiene and sanitation, so that most natives of such countries have detectable antibodies to HAV by the age of 10 years. Epidemics are not unusual. The disease tends to be mild or asymptomatic in children, with severe HAV infections occurring mainly in adults.
HAV is spread by ingestion of contaminated water and foods and is shed in the stool for 2 to 3 weeks before and 1 week after the onset of jaundice. HAV is not shed in any significant quantities in saliva, urine, or semen. Close personal contact with an infected person during the period of fecal shedding, with fecal-oral contamination, accounts for most cases and explains the outbreaks in institutional settings such as schools and nurseries. Because HAV viremia is transient, blood-borne transmission of HAV occurs only rarely; therefore, donated blood is not routinely screened for this virus. Waterborne epidemics may occur in developing countries where people live in overcrowded, unsanitary conditions. Among developed countries, sporadic infections may be contracted by the consumption of raw or steamed shellfish (oysters, mussels, clams), which concentrate the virus from seawater contaminated with human sewage.
HAV is a small, nonenveloped, single-stranded RNA picornavirus. It reaches the liver from the intestinal tract after ingestion, replicates in hepatocytes, and is shed in the bile and feces. The virus itself does not seem to be toxic to hepatocytes, and hence the liver injury seems to result from T cell–mediated damage of infected hepatocytes. As depicted in Figure 15–9, immunoglobulin M (IgM) antibodies against HAV appear in blood at the onset of symptoms. Detection of anti-HAV IgM antibody is the best diagnostic marker for the disease; IgG antibody persists beyond convalescence and is the primary defense against reinfection. In the United States, the prevalence of seropositivity increases gradually with age, reaching 40% by the age of 50 years.
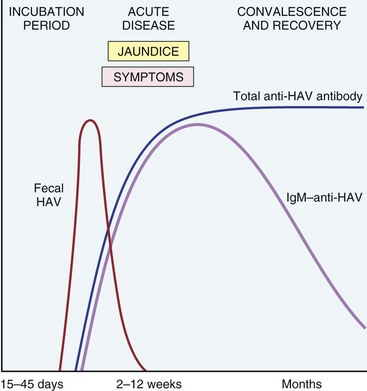
Figure 15–9 The sequence of serologic markers in acute hepatitis A infection. HAV, hepatitis A virus. There are no routinely available tests for IgG anti-HAV; therefore the presence of this antibody is inferred from the difference between total and IgM-HAV.
Measures for the prevention and management of hepatitis A include (1) hygienic practices focused on the disposal of human wastes and personal hygiene; (2) passive immunization with immune serum globulin for persons at high risk for infection (very young, very old, or immunocompromised) after exposure to the virus; and (3) administration of inactivated-virus vaccine given either before exposure (e.g., before travel to endemic areas) or very early after exposure.
Hepatitis B Virus
HBV can produce various clinical syndromes:
• Acute hepatitis with recovery and clearance of the virus
• Fulminant hepatitis with massive liver necrosis
• Nonprogressive chronic hepatitis
HBV-induced chronic liver disease is an important precursor for the development of hepatocellular carcinoma. Figure 15–10 depicts the approximate frequencies of these outcomes.
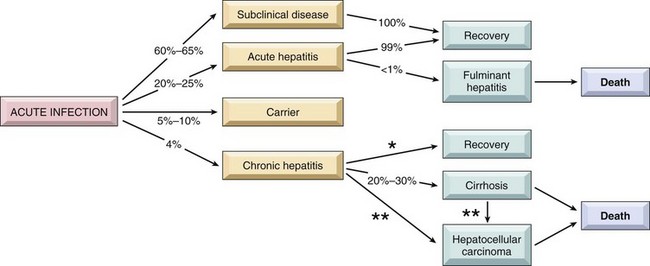
Figure 15–10 The potential outcomes with hepatitis B infection in adults, with their approximate annual frequencies in the United States. *Estimated rate of recovery from chronic hepatitis is 0.5% to 1% per year. **The risk of hepatocellular carcinoma is 0.02% per year for chronic hepatitis B and 2.5% per year when cirrhosis has developed.
Epidemiology and Transmission
Globally, liver disease caused by HBV is an enormous problem, with an estimated 400 million people who are carriers of the virus. It is estimated that HBV will infect more than 2 billion people alive today at some point in their lives. About 80% of all chronic carriers live in Asia and the Western Pacific Rim region, where the prevalence of chronic hepatitis B is more than 10%. In the United States there are approximately 185,000 new infections per year. HBV is found in the blood during the last stages of a prolonged incubation period (4 to 26 weeks) and during active episodes of acute and chronic hepatitis. It also is present in all physiologic and pathologic body fluids, with the exception of stool. HBV is a hardy virus and can withstand extremes of temperature and humidity. Thus, whereas blood and body fluids are the primary vehicles of transmission, virus also may be spread by contact with body secretions such as semen, saliva, sweat, tears, breast milk, and pathologic effusions. In endemic regions, vertical transmission from mother to child during birth constitutes the main mode of transmission. In areas of low prevalence, horizontal transmission via transfusion, blood products, dialysis, needlestick accidents among health care workers, sharing of needles in intravenous drug use, and sexual transmission (homosexual or heterosexual) constitute the primary mechanisms for HBV infection. In one third of patients, the source of infection is unknown. Most HBV infections in adults are cleared, but vertical transmission produces a high rate of persistent infection since infants cannot readily clear the infection. Chronically infected persons are at significantly increased risk for development of hepatocellular carcinoma, explaining the high rate of that malignancy in Asia and Pacific Rim nations.
HBV Structure and Genome
HBV is a member of the Hepadnaviridae, a group of DNA-containing viruses that cause hepatitis in many animal species. HBV replication does not involve the integration of the virus in the DNA of the host cell, but integrated HBV frequently is found in cells. The integrated viruses generally have large deletions and rearrangements and usually become inactive. The genome of HBV is a partially double-stranded circular DNA molecule of 3200 nucleotides that encodes
• The precore/core region of a nucleocapsid “core” protein, the hepatitis B core antigen (HBcAg), and a precore protein designated hepatitis Be antigen (HBeAg). HBcAg is retained in the infected hepatocyte; HBeAg is secreted into blood and is essential for the establishment of persistent infection.
• Envelope glycoprotein, the hepatitis B surface antigen (HBsAg), which may be produced and secreted into the blood in massive amounts. Blood HBsAg is immunogenic.
• A DNA polymerase with an error-prone reverse transcriptase activity that generates mutations in the genomes of replicating virus at a high rate.
• HBV-X protein, which acts as a transcriptional transactivator for many viral and host genes through interaction with various transcription factors. HBV-X is required for viral infectivity and may have a role in the development of hepatocellular carcinoma by regulating p53 degradation and expression (Chapter 6).
Clinical Course
After exposure to the virus, there is a long, asymptomatic incubation period, which may be followed by acute disease (described later) lasting many weeks to months. The natural course of acute disease can be tracked using serum markers (Fig. 15–11):
• HBsAg appears before the onset of symptoms, peaks during overt disease, and then declines to undetectable levels in 3 to 6 months.
• Anti-HBs antibody does not rise until the acute disease is over and usually is not detectable for a few weeks to several months after the disappearance of HBsAg. Anti-HBs may persist for life, conferring immunity; this is the basis for current vaccination strategies using noninfectious HBsAg.
• HBeAg, HBV-DNA, and DNA polymerase appear in serum soon after HBsAg, and all signify active viral replication. Persistence of HBeAg is an important indicator of continued viral replication, infectivity, and probable progression to chronic hepatitis. The appearance of anti-HBe antibodies implies that an acute infection has peaked and is on the wane.
• IgM anti-HBc becomes detectable in serum shortly before the onset of symptoms, concurrent with elevation of serum aminotransferase levels (indicative of hepatocyte destruction). Over a period of months the IgM anti-HBc antibody is replaced by IgG anti-HBc. As in the case of anti-HAV, there is no specific assay for IgG anti-HBc, but its presence is inferred from decline of IgM anti-HBc in the face of rising levels of total anti-HBc.
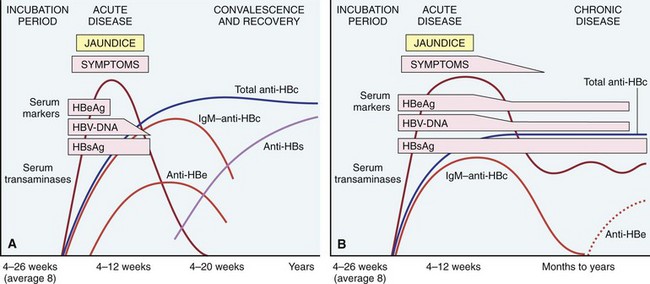
Figure 15–11 The sequence of serologic markers in acute hepatitis B infection. A, Resolution of active infection. B, Progression to chronic infection. See text for abbreviations.
Occasionally, mutated strains of HBV emerge that do not produce HBeAg, but are replication-competent and express HBcAg (more than 30% in the Mediterranean, up to 20% in the United States). In patients infected with such mutated strains, the HBeAg may be low or undetectable despite the presence of HBV viral load. A second ominous development is the emergence of viruses that are resistant to vaccine-induced immunity. For instance, replacement of arginine at amino acid 145 of HBsAg with glycine significantly alters recognition of HBsAg by anti-HBsAg antibodies.
Innate immunity protects the host during the initial phases of the infection, and a strong response by virus-specific CD4+ and CD8+ interferon γ–producing cells is associated with the resolution of acute infection. Current evidence suggests that HBV does not cause direct hepatocyte injury, and hepatocyte damage results from killing of the virus-infected cells by CD8+ cytotoxic T cells.
Hepatitis B can largely be prevented by vaccination and by the screening of donor blood, organs, and tissues. The vaccine is prepared from purified HBsAg produced in yeast. Vaccination induces a protective anti-HBs antibody response in 95% of infants, children, and adolescents. Universal vaccination has been a notable success in countries such as Taiwan and Gambia but unfortunately has not been adopted worldwide.
![]() Morphology
Morphology
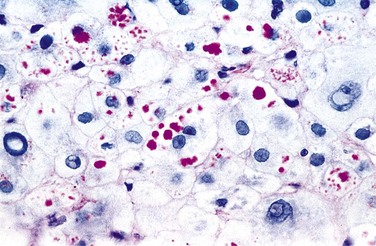
Microscopically, hepatitis B can produce all of the histologic features of acute and chronic hepatitis described earlier, but some liver biopsy specimens also display a particular morphologic feature that is nearly diagnostic, the ground glass cell (Fig. 15–12). In chronic HBV infection, some hepatocytes may have viral genomes integrated into the host genome. If, by chance, the surface antigen gene integrates into a host genomic site adjacent to an active promoter, then the cell is converted into a factory for surface antigen production. Usually in such cells full viral replication does not take place. Since surface antigen can only exit the cell as part of intact viral particles, the antigen just accumulates in these cells, creating a large cytoplasmic inclusion consisting of endoplasmic reticulum stuffed with surface antigen that has a fine, smoothly granular appearance similar to that of ground glass.
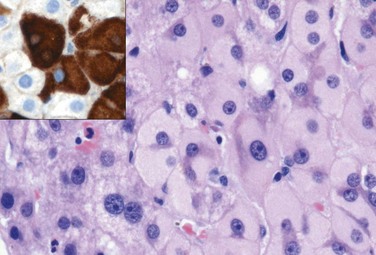
Figure 15-12 Ground-glass hepatocytes in chronic hepatitis B, caused by accumulation of HBsAg in cytoplasm, have large, pale, finely granular, pink cytoplasmic inclusions on hematoxylin-eosin staining; immunostaining (inset) confirms that the endoplasmic reticulum is ballooned with surface antigen (brown). HBsAg, hepatitis B surface antigen.
Hepatitis C Virus
Epidemiology and Transmission
HCV also is a major cause of liver disease. The worldwide carrier rate is estimated at 175 million persons (a 3% prevalence rate, ranging widely from 0.1% to 12%, depending on the country). Persistent chronic infection exists in 3 to 4 million persons in the United States, where the number of newly acquired HCV infections per year dropped from 180,000 in the mid-1980s to about 19,000 in 2006. This welcome change resulted from the marked reduction in transfusion-associated hepatitis C (as a result of screening procedures) and a decline of infections in intravenous drug abusers (related to practices motivated by fear of human immunodeficiency virus infection). However, the death rate from HCV will continue to climb for 20 to 25 years, because of the decades-long lag time between acute infection and liver failure. The major route of transmission is through blood inoculation, with intravenous drug use accounting for at least 60% of cases in the United States. Transmission by blood products is now fortunately rare, accounting for only 4% of all acute HCV infections. Occupational exposure among health care workers accounts for another 4% of cases. The rates of sexual transmission and vertical transmission are low. Infections of unknown origin account for 9% to 27% of cases. HCV infection has a much higher rate than HBV of progression to chronic disease and eventual cirrhosis (Fig. 15–13). In fact, hepatitis C is the condition that most frequently necessitates liver transplantation in the United States.
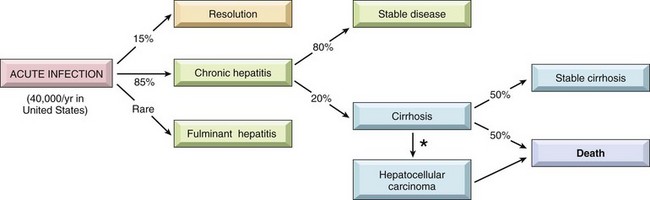
Figure 15–13 The potential outcomes of hepatitis C infection in adults, with their approximate annual frequencies in the United States. The population estimates are for newly detected infection; because of the decades-long lag time for progression from acute infection to cirrhosis, the actual annual death rate from hepatitis C is about 10,000 per year and exceeded 22,000 deaths per year by 2008. *The risk of hepatocellular carcinoma is 1% to 4% per year.
Viral Structure and Genome
HCV is a positive-sense single-stranded RNA virus belonging to the family Flaviviridae. It contains highly conserved 5′- and 3′-terminal regions that flank a single open reading frame of nearly 9500 nucleotides that encode structural and nonstructural proteins. HCV is subclassified into six genotypes, based on the genetic sequence. Moreover, because of the poor fidelity of RNA replication, an infected person may carry many HCV variants, called quasispecies. The relationships between quasispecies and disease progression are being investigated, but it seems that high multiplicity of quasispecies is associated with worse prognosis. In addition, this variability seriously hampers efforts to develop an HCV vaccine.
Clinical Course
The incubation period for hepatitis C ranges from 2 to 26 weeks, with a mean of 6 to 12 weeks. Acute hepatitis C is asymptomatic in 75% of affected persons and is easily missed. Thus, not much is known about this phase of the disease. HCV RNA can be detected in blood within days to 8 weeks depending on the inoculum size. Elevations of serum aminotransferases occur in 2 to 12 weeks. Although neutralizing anti-HCV antibodies develop within weeks to a few months, they do not confer effective immunity (Fig. 15–14). Strong immune responses involving CD4+ and CD8+ cells are associated with self-limited HCV infections, but it is not known why only a minority of persons are capable of clearing HCV infection.
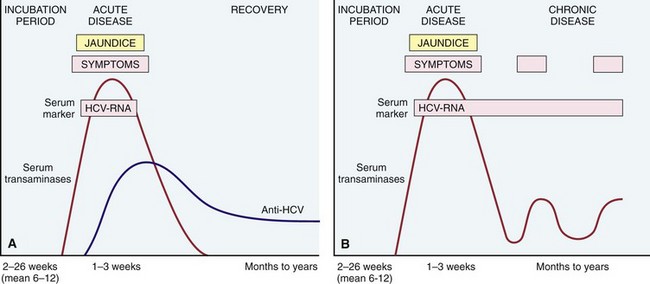
Figure 15–14 Sequence of serologic markers for hepatitis C. A, Acute infection with resolution. B, Progression to chronic infection. See text for abbreviations.
In persistent infection, circulating HCV-RNA is detectable, and aminotransferases show episodic elevations, or continuous elevation with fluctuating levels. In a small percentage of affected persons, aminotransferase levels are normal even though abnormal liver histology persists. Increased enzyme activity may occur in the absence of clinical symptoms, presumably reflecting recurrent bouts of hepatocyte necrosis. Persistent infection is the hallmark of HCV infection, occurring in 80% to 85% of patients with subclinical or asymptomatic acute infection (Fig. 15–13). Cirrhosis develops in 20% of persistently infected persons: It can be present at the time of diagnosis or may take up to 20 years to develop. Alternatively, patients may have documented chronic HCV infection for decades, without progressing to cirrhosis. Fulminant hepatitis is rare. Hepatitis C confers a significantly increased risk for hepatocellular carcinoma.
![]() Morphology
Morphology
Microscopically, chronic hepatitis C displays the typical features of chronic hepatitis described above, but has some distinctive, common associated findings: (1) fatty change, resulting either from altered lipid metabolism in infected hepatocytes, or insulin resistance and the so-called metabolic syndrome (described later); (2) lymphoid infiltrates in portal tracts, sometimes with fully formed lymphoid follicles (Fig. 15–7); and (3) bile duct injury, which may be related to direct infection of cholangiocytes by the virus.
Hepatitis D Virus
Also called delta hepatitis virus, HDV is a unique RNA virus that is replication-defective, causing infection only when it is encapsulated by HBsAg. Thus, although taxonomically distinct from HBV, HDV is absolutely dependent on HBV coinfection for multiplication. Delta hepatitis arises in two settings: (1) acute coinfection after exposure to serum containing both HDV and HBV and (2) superinfection of a chronic carrier of HBV with a new inoculum of HDV. In coinfections, HBV infection must first be established before HBsAg is made in sufficient amounts for production of HDV virions. Most coinfected persons clear the viruses and recover completely. By contrast, in most superinfected persons there is an acceleration of hepatitis, progressing to more severe chronic hepatitis 4 to 7 weeks later.
Infection by HDV is worldwide, with prevalence rates ranging from 8% among HBsAg carriers in southern Italy to as high as 40% in Africa and the Middle East. Surprisingly, HDV infection is uncommon in Southeast Asia and China, areas in which HBV infection is endemic. Periodic epidemic outbreaks have occurred in subtropical areas of Peru, Colombia, and Venezuela. In the United States, HDV infection is largely restricted to drug addicts and persons receiving multiple transfusions (e.g., hemophiliacs), who have prevalence rates of 1% to 10%.
HDV RNA and the HDV antigen (HDV Ag) are detectable in the blood and liver just before and in the early days of acute symptomatic disease. IgM anti-HDV antibody is the most reliable indicator of recent HDV exposure, as it is present at high titers only transiently in the immediate post-infection period. Acute coinfection by HDV and HBV is best indicated by detection of IgM against both HDV Ag and HBcAg (denoting new infection with HBV). With chronic delta hepatitis arising from HDV superinfection, HBsAg is present in serum, and anti-HDV antibodies (IgM and IgG) persist in low titer for months or longer.
Hepatitis E Virus
HEV hepatitis is an enterically transmitted, waterborne infection occurring primarily beyond the years of infancy. HEV is endemic in India where it is caused by fecal contamination of drinking water. Prevalence rates of anti-HEV IgG antibodies approach 40% in the Indian population. Epidemics have been reported from Asia, sub-Saharan Africa, and Mexico. Sporadic infection seems to be uncommon; it is seen mainly in travelers and accounts for more than 50% of cases of sporadic acute viral hepatitis in India. In most cases, the disease is self-limited; HEV is not associated with chronic liver disease or persistent viremia. A characteristic feature of the infection is the high mortality rate among pregnant women, approaching 20%. The average incubation period after exposure is 6 weeks (range, 2 to 8 weeks).
HEV is a nonenveloped, single-stranded RNA hepevirus. A specific antigen, HEV Ag, can be identified in the cytoplasm of hepatocytes during active infection. Virus can be detected in stools, and anti-HEV IgG and IgM antibodies are detectable in serum.
Clinical Features and Outcomes for Viral Hepatitis
A number of clinical syndromes may develop after exposure to hepatitis viruses:
• Asymptomatic acute infection: serologic evidence only
• Acute hepatitis: anicteric or icteric
• Fulminant hepatitis: submassive to massive hepatic necrosis with acute liver failure
• Chronic hepatitis: with or without progression to cirrhosis
• Chronic carrier state: asymptomatic without apparent disease
Not all of the hepatotropic viruses provoke each of these clinical syndromes (Table 15–6). As already mentioned, viral persistence and development of chronic disease are much more common after HCV infection than for HBV infection. Because other infectious or noninfectious causes, particularly drugs and toxins, can lead to essentially identical syndromes, serologic studies are critical for the diagnosis of viral hepatitis and identification of virus types.
Presented next are brief summaries of clinical outcomes with viral hepatitis.
Asymptomatic Infection
Not surprisingly, patients with asymptomatic infection are identified only incidentally on the basis of minimally elevated serum aminotransferases or after the fact by the presence of antiviral antibodies.
Acute Viral Hepatitis
Any one of the hepatotropic viruses can cause acute viral hepatitis. Acute infections are easily detected for HBV infections but are only rarely diagnosed for HCV. Although the following description is based mostly on HBV infections, acute viral hepatitis, whatever the agent, can be divided into four phases: (1) incubation period, (2) symptomatic preicteric phase, (3) symptomatic icteric phase (with jaundice and scleral icterus), and (4) convalescence.
Peak infectivity, attributed to the presence of circulating infectious viral particles, occurs during the last asymptomatic days of the incubation period and the early days of acute symptoms. The preicteric phase is marked by nonspecific, constitutional symptoms. Malaise is followed in a few days by general fatigability, nausea, and loss of appetite. Weight loss, low-grade fever, headaches, muscle and joint aches, vomiting, and diarrhea are inconstant symptoms. About 10% of patients with acute hepatitis B develop a serum sickness–like syndrome consisting of fever, rash, and arthralgias, attributed to circulating immune complexes. The hepatitis-related origin of all of these symptoms is suggested by elevated serum aminotransferase levels. Physical examination reveals a mildly enlarged, tender liver. In some patients the nonspecific symptoms are more severe, with higher fever, shaking chills, and headache, sometimes accompanied by right upper quadrant pain and tender liver enlargement. Surprisingly, as jaundice appears and these patients enter the icteric phase, other symptoms abate. The jaundice is caused predominantly by conjugated hyperbilirubinemia, which produces dark-colored urine. With hepatocellular damage and consequent defect in bilirubin conjugation, unconjugated hyperbilirubinemia also can occur. The stools may become light-colored, and the retention of bile salts may cause pruritus. An icteric phase is usual in adults (but not children) infected with HAV, present in about half of the cases involving HBV, and absent in most cases of HCV infection. In a few weeks to perhaps several months, the jaundice and most of the other systemic symptoms clear as convalescence begins.
Fulminant Hepatitis
In a very small proportion of patients with acute hepatitis A, B, D, or E, acute liver failure may result from massive hepatic necrosis. (With the exception of immunosuppressed individuals, HCV almost never causes acute liver failure). Cases with a more protracted course of several weeks or months usually are referred to as “subacute hepatic necrosis”; the liver shows both massive necrosis and regenerative hyperplasia. As discussed later, drugs and chemicals also may cause massive hepatic necrosis.
Chronic Hepatitis
Chronic hepatitis is defined by the presence of symptomatic, biochemical, or serologic evidence of continuing or relapsing hepatic disease for more than 6 months, with histologically documented inflammation and necrosis. Although the hepatitis viruses are responsible for most cases, there are many causes of chronic hepatitis (described later), such as autoimmunity, drugs and toxins, Wilson disease, and α1-antitrypsin (AAT) deficiency.
Etiology rather than the histologic pattern is the most important determinant of the probability of developing progressive chronic hepatitis. In particular, HCV is notorious for causing a chronic hepatitis evolving to cirrhosis (Fig. 15–13), regardless of histologic features at the time of initial evaluation.
The clinical features of chronic hepatitis are highly variable and are not predictive of outcome. In some patients, the only signs of chronic disease are persistent elevations of serum aminotransferase levels. The most common overt symptoms are fatigue and, less commonly, malaise, loss of appetite, and bouts of mild jaundice. Physical findings are few, the most common being spider angiomas, palmar erythema, mild hepatomegaly, and hepatic tenderness. Laboratory studies may reveal prolongation of the prothrombin time and, in some instances, hypergammaglobulinemia, hyperbilirubinemia, and mildly elevated alkaline phosphatase levels. Occasionally in cases of HBV and HCV infection, circulating antibody-antigen complexes produce immune-complex disease in the form of vasculitis (subcutaneous or visceral) (Chapter 9) and glomerulonephritis (Chapter 13). Cryoglobulinemia is found in as many as 50% of patients with hepatitis C.
The clinical course is highly variable. Persons with hepatitis C may experience spontaneous remission or may have indolent disease without progression for years. Conversely, some patients have rapidly progressive disease and develop cirrhosis within a few years. The major causes of death in patients with chronic hepatitis relate to cirrhosis—namely, liver failure, hepatic encephalopathy, massive hematemesis from esophageal varices, and hepatocellular carcinoma.
The Carrier State
A carrier is an asymptomatic person who harbors and therefore can transmit an organism. With hepatotropic viruses, carriers are those who
• Harbor one of the viruses but are free of symptoms or of significant histologic hepatitis on liver biopsy
• Have liver damage evident on biopsy (e.g., only mild necroinflammatory activity and scarring that remains in the early, noncirrhotic stages) but are essentially free of symptoms or disability
Both types of carriers constitute reservoirs of infection. HBV infection early in life, particularly through vertical transmission during childbirth, produces a carrier state 90% to 95% of the time. By contrast, only 1% to 10% of HBV infections acquired in adulthood yield a carrier state. Persons with impaired immunity are particularly likely to become carriers. The situation is less clear with HDV, although there is a well-defined low risk of posttransfusion hepatitis D, indicative of a carrier state in conjunction with HBV infection. From 0.2% to 0.6% of the general U.S. population is estimated to carry HCV.
Other Viral Infections of the Liver
• Epstein-Barr virus (EBV) infection may cause a mild hepatitis during the acute phase of infectious mononucleosis.
• Cytomegalovirus infection, particularly in the newborn or immunocompromised, can cause the typical cytomegalic changes of that virus in almost any cell of the liver, including hepatocytes, cholangiocytes, and endothelial cells.
• Herpes simplex may infect hepatocytes in newborns or the immunosuppressed, leading to the appearance of the characteristic cytopathic changes and hepatic necrosis.
• Yellow fever, which has been a major and serious cause of hepatitis in tropical countries, causes hepatocyte apoptosis, which can be extensive. The apoptotic hepatocytes are intensely eosinophilic and are referred to as Councilman bodies after the pathologist who first described them. Infrequently, in children and immunosuppressed persons, hepatitis may be caused by rubella virus, adenovirus, or enterovirus infections.
![]() Summary
Summary
Viral Hepatitis
• In the alphabet of hepatotropic viruses, some easy mnemonic devices may be useful:
 Only the consonants (hepatitis B, C, D) have the potential to cause chronic disease (C for consonant and for chronic).
Only the consonants (hepatitis B, C, D) have the potential to cause chronic disease (C for consonant and for chronic). Hepatitis B can be transmitted by blood, birthing, and “bonking” (as they say in the United Kingdom).
Hepatitis B can be transmitted by blood, birthing, and “bonking” (as they say in the United Kingdom). Hepatitis C is the single virus that is more often chronic than not (almost never detected acutely; 85% or more of patients develop chronic hepatitis, 20% of whom will develop cirrhosis).
Hepatitis C is the single virus that is more often chronic than not (almost never detected acutely; 85% or more of patients develop chronic hepatitis, 20% of whom will develop cirrhosis). Hepatitis D, the delta agent, is a defective virus, requiring hepatitis B coinfection for its own capacity to infect and replicate.
Hepatitis D, the delta agent, is a defective virus, requiring hepatitis B coinfection for its own capacity to infect and replicate.• The inflammatory cells in both acute and chronic viral hepatitis are mainly T cells; it is the pattern of injury that is different, not the nature of the infiltrate.
• Biopsy assessment in chronic viral hepatitis is most important for grading and staging of disease, which are used to decide whether a patient undergoes often arduous antiviral treatments.
• Patients with long-standing HBV or HCV infections are at increased risk for the development of hepatocellular carcinomas, even in the absence of established cirrhosis.
Autoimmune Hepatitis
Autoimmune hepatitis is a chronic disorder associated with histologic features that may be indistinguishable from chronic viral hepatitis. This disease may run an indolent or a severe course and typically responds dramatically to immunosuppressive therapy. Salient features include
• Absence of serologic evidence of viral infection
• Elevated serum IgG (levels greater than 2.5 g/dL)
• High titers of autoantibodies in 80% of cases
• The presence of other forms of autoimmune diseases, seen in up to 60% of patients, including rheumatoid arthritis, thyroiditis, Sjögren syndrome, and ulcerative colitis
Autoimmune hepatitis can be divided into subtypes on the basis of the autoantibodies produced, but the relevance of this classification to clinical management is unclear. Most patients are found to have circulating antinuclear antibodies, anti–smooth muscle antibodies, liver/kidney microsomal antibody, and/or anti–soluble liver/pancreas antigen. These antibodies can be detected by immunofluorescence or enzyme-linked immunosorbent assays. The main effectors of cell damage in autoimmune hepatitis are believed to be CD4+ helper cells. Autoimmune hepatitis may manifest as mild to severe chronic hepatitis. Response to immunosuppressive therapy usually is dramatic, although a full remission of disease is unusual. The overall risk of cirrhosis, the main cause of death, is 5%.
![]() Morphology
Morphology
Although autoimmune hepatitis shares patterns of injury with acute or chronic viral hepatitis, the time course of histologic progression differs. In viral hepatitis, fibrosis typically follows years or decades of slowly accumulating parenchymal injury, whereas in autoimmune hepatitis, there appears to be an early phase of severe cell injury and inflammation followed by rapid scarring. Of interest, and for unclear reasons, this early wave of hepatocyte damage and necrosis usually is subclinical. Clinical evolution correlates with a limited number of histologic patterns:
• Very severe hepatocyte injury associated with widespread confluent necrosis
• Marked inflammation concurrent with advanced scarring
• Burned-out cirrhosis, associated with little ongoing cell injury or inflammation. This last category was once the most common finding at diagnosis, but heightened clinical awareness of autoimmune hepatitis specifically has led to increasingly earlier diagnosis. Of note, the mononuclear infiltrate in autoimmune hepatitis frequently has abundant plasma cells.
Drug/Toxin-Mediated Injury Mimicking Hepatitis
Many drugs have effects that can mimic the features of acute or chronic viral or autoimmune hepatitis.
• As already described, acetaminophen toxicity is one of the leading causes of acute failure leading to liver transplantation. The histologic features may be indistinguishable from those of fulminant acute hepatitis A or hepatitis B.
• Isoniazid is an example of an idiosyncratic hepatotoxin that can cause a chronic hepatitis precisely mimicking chronic viral hepatitis that may or may not resolve on removal of the instigating agent.
• Other drugs (e.g., minocyclin and nitrofurantoin) or toxins can induce an autoimmune hepatitis with all of the clinical and histologic features typical of that disease: autoantibodies, elevated IgG, and plasma cell–rich hepatic infiltrates. Such cases sometimes respond to treatment with immunosuppression but occasionally do not, progressing to cirrhosis despite withdrawal of the inciting agent.
Alcoholic and Nonalcoholic Fatty Liver Disease
Alcohol is a well-known cause of fatty liver disease in adults, and can manifest histologically as steatosis, steatohepatitis, and cirrhosis. In recent years it has become evident that another entity, the so-called nonalcoholic fatty liver disease (NAFLD), can mimic the entire spectrum of hepatic changes typically associated with alcohol abuse. NAFLD (described in more detail later) is associated with insulin resistance, obesity, diabetes mellitus, hypertension, and dyslipidemias, collectively called the metabolic syndrome. Since the morphologic changes of alcoholic and nonalcoholic fatty liver disease are indistinguishable, they are described together, followed by the distinctive clinical features of each of the entities.
![]() Morphology
Morphology
Three categories of liver alterations are observed in fatty liver disease. They can be present in any combination: steatosis (fatty change), hepatitis (alcoholic or steatohepatitis), and fibrosis.
Hepatocellular Steatosis. Hepatocellular fat accumulation typically begins in centrilobular hepatocytes. The lipid droplets range from small (microvesicular) to large (macrovesicular), the largest filling and expanding the cell and displacing the nucleus. As steatosis becomes more extensive, the lipid accumulation spreads outward from the central vein to hepatocytes in the midlobule and then the periportal regions (Fig. 15–15). Macroscopically, the fatty liver with widespread steatosis is large (weighing 4 to 6 kg or more), soft, yellow, and greasy.
Figure 15–15 Fatty liver disease. Macrovesicular steatosis is most prominent around the central vein and extends outward to the portal tracts with increasing severity. The intracytoplasmic fat is seen as clear vacuoles. Some fibrosis (stained blue) is present in a characteristic perisinusoidal “chicken wire fence” pattern. (Masson trichrome stain.)
(Courtesy of Dr. Elizabeth Brunt, Washington University in St. Louis, St. Louis, Missouri.)
Steatohepatitis. These changes typically are more pronounced with alcohol use than in NAFLD, but can be seen with variable degrees of prominence in fatty liver disease of any cause:
• Hepatocyte ballooning. Single or scattered foci of cells undergo swelling and necrosis; as with steatosis, these features are most prominent in the centrilobular regions.
• Mallory-Denk bodies. These consist of tangled skeins of intermediate filaments (including ubiquitinated keratins 8 and 18) and are visible as eosinophilic cytoplasmic inclusions in degenerating hepatocytes (Fig. 15–16).
• Neutrophil infiltration. Predominantly neutrophilic infiltration may permeate the lobule and accumulate around degenerating hepatocytes, particularly those containing Mallory-Denk bodies. Lymphocytes and macrophages also may be seen in portal tracts or parenchyma (Fig. 15–16, A and B).
Figure 15-16 A, Alcoholic hepatitis with clustered inflammatory cells marking the site of a necrotic hepatocyte. A Mallory-Denk body is present in another hepatocyte (arrow). B, Steatohepatitis with many ballooned hepatocytes (arrowheads) containing prominent Mallory-Denk bodies; clusters of inflammatory cells are also seen; inset shows immunostaining for keratins 8 and 18 (brown), with most hepatocytes, including those with fat vacuoles, showing normal cytoplasmic staining, but in the ballooned cell (dotted line), the keratins are collapsed into the Mallory-Denk body, leaving the cytoplasm “empty.”
(Courtesy of Dr. Elizabeth Brunt, Washington University in St. Louis, St. Louis, Missouri.)
Steatohepatitis with fibrosis. Fatty liver disease of all kinds has a distinctive pattern of scarring. Like the other changes, fibrosis appears first in the centrilobular region as central vein sclerosis. Perisinusoidal scar appears next in the space of Disse of the centrilobular region and then spreads outward, encircling individual or small clusters of hepatocytes in a chicken wire fence pattern (Fig. 15–15). These tendrils of fibrosis eventually link to portal tracts and then begin to condense to create central-portal fibrous septa. As these become more prominent, the liver takes on a nodular, cirrhotic appearance. Because in most cases of fatty liver disease the underlying cause persists, the continual subdivision of established nodules by new, perisinusoidal scarring leads to a classic micronodular or Laennec cirrhosis. Early in the course, the liver is yellow-tan, fatty, and enlarged. However, with persistent damage, over the course of years the liver is transformed into a brown, shrunken, nonfatty organ composed of cirrhotic nodules that are usually less than 0.3 cm in diameter—smaller than is typical for most chronic viral hepatitis (Fig. 15–17). The end-stage cirrhotic liver may enter into a “burned-out” phase devoid of fatty change and other typical features (Fig. 15–18). A majority of cases of cryptogenic cirrhosis, without clear etiology, are now recognized as “burned-out” NASH.
Alcoholic Liver Disease
Excessive ethanol consumption causes more than 60% of chronic liver disease in Western countries and accounts for 40% to 50% of deaths due to cirrhosis. The following statistics attest to the magnitude of the problem in the United States:
• More than 10 million Americans are alcoholics.
• Alcohol abuse is the fifth leading cause of death, being responsible for 80,000 to 85,000 deaths annually. Of these deaths, 20,000 are attributable directly to end-stage cirrhosis; another 10,000 to 12,000 are the result of automobile accidents.
• From 25% to 30% of hospitalized patients have problems related to alcohol abuse.
Chronic alcohol consumption has many adverse effects (Chapter 7). Among the most important are the distinctive, overlapping forms of alcohol-related fatty liver disease already described: (1) hepatic steatosis, (2) alcoholic hepatitis, and (3) fibrosis and cirrhosis, collectively referred to as alcoholic liver disease (Fig. 15–19).
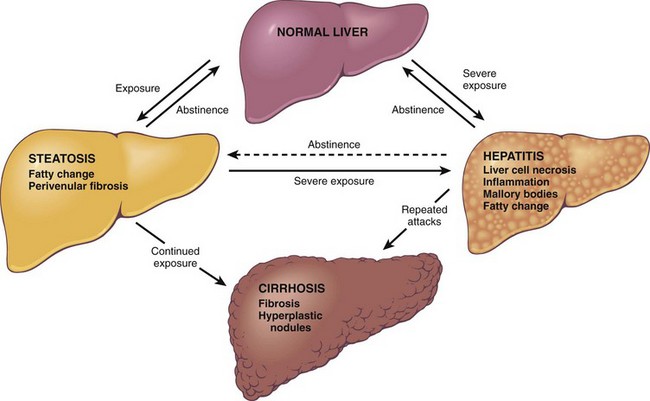
Figure 15–19 Alcoholic liver disease. The interrelationships among hepatic steatosis, alcoholic hepatitis, and alcoholic cirrhosis are shown, along with a depiction of key morphologic features at the microscopic level. As stated in the text, it should be noted that steatosis, alcoholic hepatitis, and cirrhosis may also develop independently and not along a continuum.
From 90% to 100% of heavy drinkers develop fatty liver (i.e., hepatic steatosis), and of those, 10% to 35% develop alcoholic hepatitis, whereas only 8% to 20% of chronic alcoholics develop cirrhosis. Steatosis, alcoholic hepatitis, and fibrosis may develop independently, so they do not necessarily represent a continuum of changes. Hepatocellular carcinoma arises in 10% to 20% of patients with alcoholic cirrhosis.
![]() Pathogenesis
Pathogenesis
Short-term ingestion of as much as 80 g of ethanol per day (5–6 beers or 8–9 ounces of 80-proof liquor) generally produces mild, reversible hepatic changes, such as fatty liver. Chronic intake of 40 to 80 g/day is considered a borderline risk factor for severe injury. For reasons that may relate to decreased gastric metabolism of ethanol and differences in body composition, women are more susceptible than men to hepatic injury. It seems that how often and what one drinks may affect the risk of liver disease development. For example, binge drinking causes more liver injury than that associated with steady, lower level consumption. Individual, possibly genetic risk factors must exist, but no reliable markers of susceptibility are known. In the absence of a clear understanding of the factors that influence liver damage, no safe upper limit for alcohol consumption can be proposed.
The metabolism of ethanol by the alcohol dehydrogenase and microsomal ethanol-oxidizing system is discussed in Chapter 7. As mentioned, the induction of cytochrome P-450 by chronic alcohol use leads to augmented transformation of other drugs to toxic metabolites. In particular, this effect can accelerate the metabolism of acetaminophen into highly toxic metabolites and increase the risk of liver injury even with therapeutic doses. Discussed next are the detrimental effects of alcohol and its byproducts on hepatocellular function.
Hepatocellular steatosis results from several mechanisms: (1) shunting of substrates away from catabolism and toward lipid biosynthesis, because of the generation of excess reduced nicotinamide-adenine dinucleotide resulting from metabolism of ethanol by alcohol dehydrogenase and acetaldehyde dehydrogenase; (2) impaired assembly and secretion of lipoproteins; and (3) increased peripheral catabolism of fat.
The causes of alcoholic hepatitis are uncertain, but it may stem from one or more of the following toxic by products of ethanol and its metabolites:
• Acetaldehyde (a major metabolite of ethanol) induces lipid peroxidation and acetaldehyde-protein adduct formation, which may disrupt cytoskeleton and membrane function.
• Alcohol directly affects cytoskeleton organization (as illustrated by Mallory-Denk bodies), mitochondrial function, and membrane fluidity.
• Reactive oxygen species generated during oxidation of ethanol by the microsomal ethanol oxidizing system react with and damage membranes and proteins. Reactive oxygen species also are produced by neutrophils, which infiltrate areas of hepatocyte necrosis.
• Cytokine-mediated inflammation and cell injury is a major feature of alcoholic hepatitis and alcoholic liver disease in general. TNF is considered to be the main effector of injury; IL-1, IL-6, and IL-8 may also contribute. The main stimuli for the production of cytokines in alcoholic liver disease are the reactive oxygen species, mentioned earlier, and microbial products (e.g., endotoxin) derived from gut bacteria.
Because generation of acetaldehyde and free radicals is maximal in the centrilobular region, this region is most susceptible to toxic injury. Pericellular fibrosis and sinusoidal fibrosis develop in this area of the lobule. Concurrent viral hepatitis, particularly hepatitis C, is a major accelerator of liver disease in alcoholics. The prevalence of hepatitis C among persons with alcoholic disease is about 30% (and vice versa).
For unknown reasons, cirrhosis develops in only a small fraction of chronic alcoholics. With complete abstinence, some regression of scar can be seen in all cases, and the micronodular liver transforms, with regeneration, into a macronodular cirrhotic organ; rarely, there is regression of cirrhosis altogether.
Clinical Features
Hepatic steatosis may be innocuous or give rise to hepatomegaly with mild elevations of serum bilirubin and alkaline phosphatase. Severe hepatic compromise is unusual. Alcohol withdrawal and the provision of an adequate diet are sufficient treatment.
It is estimated that 15 to 20 years of excessive drinking are necessary to develop alcoholic cirrhosis, but alcoholic hepatitis can occur after just weeks or months of consistent use. The onset is typically acute and often follows a bout of particularly heavy drinking. Symptoms and laboratory abnormalities may range from minimal to severe. Most patients present with malaise, anorexia, weight loss, upper abdominal discomfort, tender hepatomegaly, and fever. Typical laboratory findings include hyperbilirubinemia, elevated alkaline phosphatase, and neutrophilic leukocytosis. Serum alanine aminotransferase and aspartate aminotransferase are elevated but usually remain below 500 U/mL. The outlook is unpredictable; each bout of hepatitis carries about a 10% to 20% risk of death. With repeated bouts, cirrhosis appears in about one third of patients within a few years; alcoholic hepatitis also may be superimposed on cirrhosis.
The manifestations of alcoholic cirrhosis are similar to those of other forms of cirrhosis, presented earlier. Commonly, the first signs of cirrhosis relate to complications of portal hypertension. The stigmata of cirrhosis (e.g., an abdomen grossly distended with ascites, wasted extremities, caput medusae) may be the presenting features. Alternatively, a patient may first present with life-threatening variceal hemorrhage or hepatic encephalopathy. In other cases, insidious onset of malaise, weakness, weight loss, and loss of appetite precede the appearance of jaundice, ascites, and peripheral edema. Laboratory findings reflect the developing hepatic disease, with elevated serum aminotransferase, hyperbilirubinemia, variable elevation of alkaline phosphatase, hypoproteinemia (globulins, albumin, and clotting factors), and anemia. Finally, cirrhosis may be clinically silent, discovered only at autopsy or when stress such as infection or trauma tips the balance toward hepatic insufficiency. In chronic alcoholics, alcohol may become a major caloric source in the diet, displacing other nutrients and leading to malnutrition and vitamin deficiencies (e.g., thiamine, vitamin B12). Compounding these effects is impaired digestive function, primarily related to chronic gastric and intestinal mucosal damage and pancreatitis.
The long-term outlook for alcoholic patients with liver disease is variable. The most important aspect of treatment is abstinence from alcohol. The 5-year survival rate approaches 90% in abstainers who are free of jaundice, ascites, or hematemesis but drops to 50% to 60% in persons who continue to imbibe. Among those with end-stage alcoholic liver disease, the immediate causes of death are
• Massive gastrointestinal hemorrhage
• Intercurrent infection (to which affected persons are predisposed)
![]() Summary
Summary
Alcoholic Liver Disease
• Alcoholic liver disease has three main manifestations: hepatic steatosis, alcoholic hepatitis, and cirrhosis, which may occur alone or in combination.
• Consumption of 50 to 60 g/day of alcohol is considered to be the threshold for the development of alcoholic liver disease.
• Cirrhosis typically develops after 10 to 15 years of drinking or more, but only occurs in a small proportion of chronic alcoholics; alcoholic cirrhosis has the same morphologic and clinical features as cirrhosis caused by viral hepatitis.
• The multiple pathologic effects of alcohol include changes in lipid metabolism, decreased export of lipoproteins, and cell injury caused by reactive oxygen species and cytokines.
Nonalcoholic Fatty Liver Disease (NAFLD)
NAFLD is a common condition in which fatty liver disease develops in persons who do not drink alcohol. The liver can show any of the three types of changes described earlier (steatosis, steatohepatitis, and cirrhosis). The term nonalcoholic steatohepatitis (NASH) is used to describe overt clinical features of liver injury, such as elevated transaminases and histologic features of hepatitis already described. NAFLD is consistently associated with insulin resistance and the metabolic syndrome (described below). Other commonly associated abnormalities are:
• Type 2 diabetes (or family history of the condition)
• Obesity, primarily central obesity (body mass index greater than 30 kg/m2 in whites and greater than 25 kg/m2 in Asians)
• Dyslipidemia (hypertriglyceridemia, low high-density lipoprotein cholesterol, high low-density lipoprotein cholesterol)
![]() Pathogenesis
Pathogenesis
Metabolic syndrome is defined as having at least two of the following: obesity, insulin resistance, dyslipidemia, and hypertension. In patients with metabolic syndrome, the presence of type 2 diabetes and obesity is the best predictor of severe fibrosis and disease progression. Insulin resistance results in the accumulation of triglycerides in hepatocytes by at least three mechanisms:
• Impaired oxidation of fatty acids
• Increased synthesis and uptake of fatty acids
• Decreased hepatic secretion of very-low-density lipoprotein cholesterol
Fat-laden hepatocytes are highly sensitive to lipid peroxidation products generated by oxidative stress, which can damage mitochondrial and plasma membranes, causing apoptosis. Either as a consequence of oxidative stress or through release from visceral adipose tissue, levels of TNF, IL-6, and of the MCP-1 chemokine increase, contributing to liver damage and inflammation. Important roles are emerging for adiponectin and leptin in regulating these processes as well.
NAFLD is the most common cause of incidental elevation of serum transaminases. Most persons with steatosis are asymptomatic; patients with active steatohepatitis or fibrosis may also be asymptomatic, but some may have fatigue, malaise, right upper quadrant discomfort, or more severe symptoms of chronic liver disease. Liver biopsy is required for diagnosis. Fortunately, the frequency of progression from steatosis to active steatohepatitis and then from active steatohepatitis to cirrhosis seems to be low. Nevertheless, NAFLD is considered to be a significant contributor to the pathogenesis of “cryptogenic” cirrhosis.
Because they share common features, the incidence of coronary artery disease is increased in patients with NAFLD. Current therapy of NAFLD is directed toward obesity reduction and decrease in insulin resistance. Clinical trials of pioglitazone, a stimulator of the transcription factor PPAR-γ, which modulates the expression of insulin-sensitive genes, in nondiabetic patients with biopsy-proven steatohepatitis demonstrated a significant benefit as evidenced by reversal of NAFLD histologic changes. Lifestyle modifications (diet and exercise) appear to be the most effective form of treatment.
Pediatric NAFLD is becoming an increasing problem as metabolic syndrome and NAFLD approach epidemic proportions. In children, the appearance of the histologic injuries is somewhat different: Inflammation and scarring tend to be more prominent in the portal tracts and periportal regions, and mononuclear infiltrates rather than neutrophilic infiltrates predominate.
![]() Summary
Summary
Nonalcoholic Fatty Liver Disease
• Nonalcoholic fatty liver disease (NAFLD) is associated with the metabolic syndrome, obesity, type 2 diabetes, and dyslipidemia and/or hypertension.
• NAFLD may show all the changes associated with alcoholic liver disease: steatosis, steatohepatitis (NASH), and cirrhosis, although the features of steatohepatitis (such as hepatocyte ballooning, Mallory-Denk bodies, and neutrophilic infiltration) often are less prominent than they are in alcohol-related injury.
• Pediatric NAFLD is increasingly being recognized as the obesity epidemic spreads to pediatric age groups, although its histologic pattern differs somewhat from that seen in adults.
Drug/Toxin-Mediated Injury with Steatosis
Steatosis has a wide array of causes, including alcohol abuse, total parenteral nutrition, amiodarone, and methotrexate. A special category meriting emphasis involves mitochondrial injury leading to diffuse, hepatocellular microvesicular steatosis, usually associated with severe and potentially fatal acute liver dysfunction. The classic example is Reye syndrome, a rare disease that primarily affects children younger than 4 years of age who have a viral illness. The onset is heralded by pernicious vomiting and accompanied by irritability or lethargy and hepatomegaly. Serum bilirubin, ammonia, and aminotransferase levels are essentially normal at presentation. Although most patients recover, about 25% progress to coma, accompanied by elevations in the serum levels of bilirubin, aminotransferases, and particularly ammonia. Death occurs from progressive neurologic deterioration or liver failure. Survivors of more serious illness may be left with permanent neurologic impairments.
Reye syndrome has been associated with aspirin administration during viral illnesses; while there is no definitive evidence that salicylates play a causal role in this disorder, as a precaution aspirin is contraindicated in children and teenagers with febrile illnesses. Agents that are known to cause similar mitochondrial dysfunction include tetracycline and valproate, as well as toxins in unripe ackee fruit, popular in Jamaica. Highly active antiretroviral therapy (HAART) regimens used for HIV disease also may cause the same histologic injuries, but, for unclear reasons, these patients are spared significant morbidity.
![]() Morphology
Morphology
The key pathologic finding is hepatocellular microvesicular steatosis. Electron microscopy of hepatocellular mitochondria reveals pleomorphic enlargement and electron lucency of the matrices, with disruption of cristae and loss of dense bodies. Specifically with Reye syndrome, cerebral edema usually is present. Astrocytes are swollen and mitochondrial changes similar to those seen in the liver may develop. Inflammation is notably absent, as is any evidence of viral infection. Skeletal muscles, kidneys, and heart also may reveal microvesicular fatty change and mitochondrial alterations, although these changes are more subtle than those in the liver.
Cholestatic Liver Diseases
Cholestatic liver diseases include entities that lead to primary hepatocellular dysfunction (e.g. neonatal cholestasis, drug cholestasis, sepsis) or to bile duct injuries, including some that are mechanical (e.g., obstruction of large ducts by stones or tumor) or inflammatory (such as autoimmune diseases). To be clear, however, bile duct diseases cannot be neatly divided into intrahepatic or extrahepatic, because diseases may affect both intra- and extrahepatic segments, and exclusively extrahepatic biliary disorders may still cause secondary changes within the liver. We will first discuss the important cholestatic liver diseases, a heterogeneous group of disorders that have as their hallmark clinical signs and symptoms of cholestasis; biliary obstruction is considered under diseases of the extrahepatic biliary tree.
Neonatal Cholestasis
As mentioned earlier, mild transient elevations in serum unconjugated bilirubin are common in normal newborns. Prolonged conjugated hyperbilirubinemia that lasts beyond the first 14 days of life is termed neonatal cholestasis. The major causes are extrahepatic biliary atresia, discussed later, and a variety of other disorders collectively referred to as neonatal hepatitis. Neonatal hepatitis is not a specific entity, nor are the disorders necessarily inflammatory. Instead, the finding of neonatal cholestasis should evoke a diligent search for recognizable toxic, metabolic, and infectious liver diseases. With greater awareness of etiology and better diagnostic tools, idiopathic neonatal hepatitis constitutes only 10% to 15% of cases of neonatal hepatitis.
Clinical presentation of infants with any form of neonatal cholestasis is fairly typical, with jaundice, dark urine, light or acholic stools, and hepatomegaly. Variable degrees of hepatic synthetic defects, such as hypoprothrombinemia, may be present. Differentiation between the two most common causes of neonatal cholestasis—extrahepatic atresia and idiopathic hepatitis—assumes great importance, because definitive treatment of biliary atresia requires surgical intervention, whereas surgery may adversely affect the clinical course in a child with idiopathic neonatal hepatitis. Fortunately, discrimination between these diseases can be made in about 90% of cases using clinical data and liver biopsy.
Cholestasis of Sepsis
Sepsis may affect the liver in many ways. These include direct effects of intrahepatic bacterial infection (e.g., abscess formation or bacterial cholangitis) and ischemia caused by septic shock (particularly when the liver is cirrhotic) or in response to circulating microbial products. The latter is most likely to lead to the cholestasis of sepsis, particularly when the systemic infection is due to gram-negative organisms.
The most common alteration is canalicular cholestasis, with bile plugs within predominantly centrilobular canaliculi (Fig. 15–20, A). Histologic findings include prominent activated Kupffer cells and mild portal inflammation, but hepatocyte necrosis is scant or absent. Ductular cholestasis is a more ominous finding, wherein canals of Hering and bile ductules at the interface of portal tracts and parenchyma become dilated and contain prominent bile plugs (Fig. 15–20, B). This change, which is not a typical feature of biliary obstruction, often accompanies or even precedes the development of septic shock.
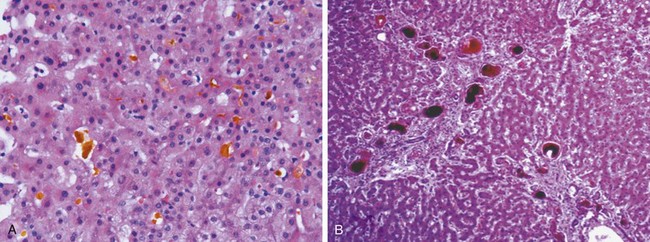
Figure 15–20 A, Cholestasis of sepsis. Prominent bile plugs are present in dilated canaliculi in the centrilobular region. B, Ductular cholestasis. Large, dark bile concretions within markedly dilated canals of Hering and ductules at the portal-parenchymal interface. This feature, indicative of current or impending severe sepsis, is related to endotoxemia.
(B, Courtesy of Dr. Jay Lefkowitch, Columbia University, New York.)
Primary Biliary Cirrhosis
Primary biliary cirrhosis (PBC) is a chronic, progressive, and sometimes fatal cholestatic liver disease characterized by destruction of intrahepatic bile ducts, portal inflammation and scarring, and the development of cirrhosis and liver failure over years to decades (Table 15–7). The cardinal feature of PBC is a nonsuppurative destruction of small and medium-sized intrahepatic bile ducts. PBC is primarily a disease of middle-aged women, with peak incidence between the ages of 40 and 50 years. The name is a bit of a misnomer, because end-stage PBC is not always cirrhotic. Some patients may die or undergo transplantation because of severe portal hypertension in the absence of fully established cirrhosis, although others, usually displaying severe intractable cholestasis, are fully cirrhotic.
Table 15–7 Main Features of Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis
| Parameter | Primary Biliary Cirrhosis | Primary Sclerosing Cholangitis |
|---|---|---|
| Age | Median age 50 years (30–70) | Median age 30 years |
| Gender | 90% female | 70% male |
| Clinical course | Progressive | Unpredictable but progressive |
| Associated conditions | Sjögren syndrome (70%) | Inflammatory bowel disease (70%) |
| Scleroderma (5%) | Pancreatitis (≤25%) | |
| Thyroid disease (20%) | Idiopathic fibrosing diseases (retroperitoneal fibrosis) | |
| Serology | 95% AMA-positive | 0–5% AMA-positive (low titer) |
| 20% ANA-positive | 6% ANA-positive | |
| 40% ANCA-positive | 65% ANCA-positive | |
| Radiology | Normal | Strictures and beading of large bile ducts; pruning of smaller ducts |
| Duct lesion | Florid duct lesions and loss of small ducts only | Inflammatory destruction of extrahepatic and large intrahepatic ducts; fibrotic obliteration of medium and small intrahepatic ducts |
AMA, antimitochondrial antibody; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody.
![]() Pathogenesis
Pathogenesis
More than 90% of persons with PBC demonstrate high titers of autoantibodies directed against several mitochondrial acid dehydrogenases. It is still unclear why the immune response is focused on these enzymes and why intrahepatic bile ducts are the targets of the immune attack. Recent evidence suggests that exposure to certain xenobiotics may modify mitochondrial proteins, providing one possible trigger for the immune response.
Clinical Course
The onset of PBC is insidious, but the disease often comes to attention while the patient is still asymptomatic through discovery of an elevated serum alkaline phosphatase in routine laboratory tests. The diagnosis is usually made by demonstration of antimitochondrial antibodies and typical biopsy findings. Patients often present with pruritus and usually prove to have advanced disease.
Untreated patients progress to hepatic decompensation associated with portal hypertension, with variceal bleeding and hepatic encephalopathy, over a period of 2 or more decades. However, early treatment with oral ursodeoxycholic acid dramatically improves the disease course, greatly slowing progression. Its mechanisms of action remain unclear, although they appear to include inhibition of apoptosis of biliary epithelium and inhibition of immune responses. Hyperbilirubinemia is a late feature and usually signifies incipient hepatic decompensation. Associated extrahepatic conditions include the Sjögren syndrome of dry eyes and dry mouth, scleroderma, thyroiditis, rheumatoid arthritis, Raynaud phenomenon, and celiac disease.
![]() Morphology
Morphology
The morphology of PBC is most revealing in the precirrhotic stage. Interlobular bile ducts are actively destroyed by lymphocytic or plasmacytic inflammation with or without granulomas (the florid duct lesion) (Fig. 15–21). Some biopsy specimens, however, do not have active destructive lesions and show only portal tracts lacking bile ducts. Portal tracts upstream from the damaged bile ducts show bile ductular proliferation, and inflammation and necrosis of the adjacent periportal parenchyma. This leads to the development of portal-portal septal fibrosis (Fig. 15–22). Mild interface and lobular hepatitis also may be present. The features sometimes overlap with those of autoimmune hepatitis; this overlap syndrome is diagnosed only when the hepatitic component is very prominent and there are serologic findings typical for autoimmune hepatitis.
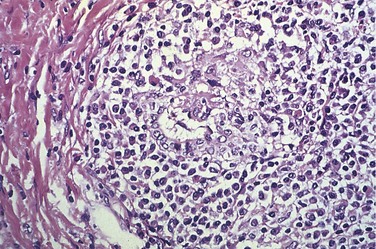
Figure 15–21 Primary biliary cirrhosis. A portal tract is markedly expanded by an infiltrate of lymphocytes and plasma cells. Note the granulomatous reaction to a bile duct undergoing destruction (florid duct lesion).
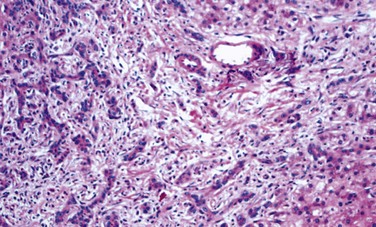
Figure 15–22 An example of ductular reaction in a fibrotic septum.
(Courtesy of Dr. Matthew Yeh, University of Washington, Seattle, Washington.)
Two paths to end-stage liver disease are recognized, both taking years or decades to evolve. Some patients develop prominent portal hypertension; on histologic examination these livers may display widespread nodularity without the surrounding scar tissue seen in cirrhosis—a feature called nodular regenerative hyperplasia. Such livers often are larger than normal and may show a vague nodularity that differs from the obvious nodules of cirrhosis. Why these hepatocytic changes occur in a disease that appears to be primarily biliary in nature is not understood.
Other patients follow a classical path with increasingly widespread duct loss leading to cirrhosis and profound cholestasis. The bile accumulation in such chronic cholestasis is not centrilobular, as in drug-induced or sepsis-associated cholestatic syndromes, but is periportal/periseptal and associated with feathery degeneration marked by ballooned, bile-stained hepatocytes, often with prominent Mallory-Denk bodies. These cells, while morphologically similar to those seen in alcoholic hepatitis, differ by their periportal rather than centrilobular location. Such end-stage livers show cirrhosis and vivid green discoloration, matching the patient’s general icteric state (Fig. 15–23). Because there is little hepatocyte loss, the regenerative nodular hyperplasia often leads to hepatomegaly early in the disease course, a point of distinction from the shrunken livers of chronic hepatitis or alcoholism.
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) is a chronic cholestatic disorder, characterized by progressive fibrosis and destruction of extrahepatic and intrahepatic bile ducts of all sizes (Table 15–7). Because the changes in the ducts are patchy, cholangiography, performed endoscopically or with the aid of magnetic resonance imaging (MRI), shows a characteristic beading in the affected segments of the biliary tree due to narrow strictures alternating with normal sized or dilated ducts. PSC commonly is seen in association with inflammatory bowel disease (Chapter 15), particularly ulcerative colitis, which coexists in approximately 70% of affected patients. Conversely, the prevalence of PSC among persons with ulcerative colitis is about 4%. The disorder tends to occur in the third through fifth decades of life, most often after development of inflammatory bowel disease. Males are affected more often than females, in a ratio of 2 : 1.
The cause of PSC is unknown. The association with ulcerative colitis, linkage with certain HLA-DR alleles, and presence of antinuclear cytoplasmic antibodies with a perinuclear localization (Chapter 10) in 80% of cases all suggest that this is an immunologically mediated disease.
![]() Morphology
Morphology
The characteristic features of PSC are different in the extrahepatic and large intrahepatic ducts than in the smaller ducts. The largest ducts have chronic inflammation with superimposed acute inflammation, very similar to the mucosal lesions of ulcerative colitis. These inflamed areas lead to narrowing of the larger ducts either because edema and inflammation narrows the lumen or because of subsequent scarring. The smaller ducts, however, often have little in the way of inflammation and show a striking circumferential fibrosis often referred to as onion skinning around an increasingly atrophic duct lumen (Fig. 15–24). Eventually the lumen disappears altogether, leaving just a dense button of scar tissue, the virtually diagnostic tombstone scar. Because the likelihood of sampling such smaller duct lesions on a random needle biopsy is minuscule, diagnosis depends not on biopsy but on radiologic imaging of the extrahepatic and largest intrahepatic ducts.
Figure 15–24 Primary sclerosing cholangitis. A bile duct undergoing degeneration is entrapped in a dense, “onion-skin” concentric scar.
In response to duct loss, as in PBC, bile ductular proliferation, portal-portal septal fibrosis, and cirrhosis follow. The end-stage liver is therefore usually cirrhotic and green. Biliary intraepithelial neoplasia may appear and be a harbinger of cholangiocarcinoma, a fatal complication seen in a minority of patients.
Clinical Course
Asymptomatic patients may come to attention due to persistently elevated levels of serum alkaline phosphatase. Other patients present acutely with symptoms related to acute cholangitis—infection complicating previously asymptomatic biliary strictures—such as fever, right upper quadrant tenderness, and sometimes acute jaundice. Patients who present at later stages may have progressive fatigue, pruritus, and chronic jaundice. PSC generally has a protracted course over many years. Cholangiocarcinoma develops in 10% to 15% of patients with PSC, with a median time from diagnosis to malignant transformation of 5 years.
Drug/Toxin-Induced Cholestasis
Cholestasis is a common feature of drug- or toxin-induced hepatic injury and may be seen alone or in combination with hepatitic features. When hepatocyte injury is minimal it is referred to as bland cholestasis, which is characterized by canalicular bile plugs and/or mid-centrilobular hepatocyte swelling and cytoplasmic bile pigment accumulation. Cholestatic hepatitis, in which features of cholestasis (elevated serum bilirubin and alkaline phosphatase, canalicular and hepatocellular cholestasis) and of hepatitis (elevated serum transaminases, lobular and interface hepatitis) coexist, is a particularly telling indication of drug- or toxin-induced injury. Common or well-known causes of such injury include C17 alkylated anabolic or contraceptive steroids, total parenteral nutrition, and antibiotics. Mimics of PBC and PSC, associated with not only cholestasis but actual loss of bile ducts, include liver injuries caused by chlorpromazine, amitryptyline, and organic arsenicals.
Inherited Metabolic Diseases
Although there are a relatively large number of inherited metabolic liver diseases, in this section only some common and selected conditions are discussed: hemochromatosis, Wilson disease, and AAT deficiency.
Hemochromatosis
Hereditary hemochromatosis refers to genetic disorders characterized by excessive accumulation of body iron, most of which is deposited in the liver, pancreas, and heart. At least four genetic variants of hereditary hemochromatosis are recognized. The most common form is an autosomal recessive disease of adult onset caused by mutations in the HFE gene. Acquired forms of iron accumulation from known sources of excess iron are called secondary iron overload. Among the most important are multiple transfusions, ineffective erythropoiesis (as in β-thalassemia and myelodysplastic syndromes), and increased iron intake.
As discussed in Chapter 11, the total body iron pool ranges from 2 to 6 gm in normal adults; about 0.5 gm is stored in the liver, 98% of which is in hepatocytes. In hereditary hemochromatosis, iron accumulates over the lifetime of the affected person from excessive intestinal absorption. Total iron accumulation may exceed 50 gm, over one third of which is found in the liver. Fully developed cases show (1) cirrhosis (seen in all patients), (2) diabetes mellitus (in 75% to 80% of patients), and (3) skin pigmentation (in 75% to 80%).
![]() Pathogenesis
Pathogenesis
The total body content of iron is tightly regulated, such that the limited daily losses of iron are matched by gastrointestinal absorption since there is no excretory pathway for excess absorbed iron. In hereditary hemochromatosis there is a defect in the regulation of intestinal absorption of dietary iron, leading to net iron accumulation of 0.5 to 1.0 g/year. The hereditary hemochromatosis gene, responsible for the most common form of this disorder, is called HFE. It is located on the short arm of chromosome 6. It encodes a protein that is similar in structure to MHC class I proteins. The role of HFE in regulating iron uptake is complex and not fully understood. Expression of the mutated HFE protein on small intestinal enterocytes leads to inappropriately upregulated absorption of iron and its binding to transferrin, the major iron carrying molecule in blood. But HFE is not the whole story, and its interactions with other proteins, particularly hepcidin, produced by the liver, form a web of iron metabolic control networks that are only recently becoming elucidated.
It appears that HFE and the other genes involved in less common forms of hereditary hemochromatosis all regulate the levels of hepcidin, the iron hormone produced by the liver. Hepcidin normally down-regulates the efflux of iron from the intestines and macrophages into the plasma and inhibits iron absorption. When hepcidin levels are reduced there is increased iron absorption. Mice in whom the hepcidin gene is deleted develop iron overload resembling hemochromatosis, and mice that overexpress hepcidin develop severe iron deficiency, thus establishing the central role of hepcidin in regulating iron absorption. As might be expected, hepcidin levels are reduced in all currently known genetic forms of hemochromatosis, including the most common form caused by mutations in the HFE gene. The interconnections between the functions of these various genes and hepcidin synthesis are still being elucidated.
Hereditary hemochromatosis manifests typically after 20 gm of storage iron has accumulated. Regardless of source, excessive iron seems to be directly toxic to tissues by the following mechanisms:
![]() Morphology
Morphology
The morphologic changes in hereditary hemochromatosis are all responses to the deposition of hemosiderin in the following organs (in decreasing order of severity): liver, pancreas, myocardium, pituitary, adrenal, thyroid and parathyroid glands, joints, and skin. In the liver, iron becomes evident first as golden-yellow hemosiderin granules in the cytoplasm of periportal hepatocytes, which stain blue with the Prussian blue stain (Fig. 15–25). With increasing iron load, there is progressive involvement of the rest of the lobule, along with bile duct epithelium and Kupffer cells. Iron is a direct hepatotoxin, and inflammation is characteristically absent. At this stage, the liver typically is slightly larger than normal, dense, and chocolate brown. Fibrous septa develop slowly, linking portal tracts to each other and leading ultimately to cirrhosis in an intensely pigmented (very dark brown to black) liver.
Figure 15–25 Hereditary hemochromatosis. In this Prussian blue–stained histologic section, hepatocellular iron appears blue. The parenchymal architecture is normal.
In normal persons the iron content of unfixed liver tissue is less than 1000 µg/g dry weight. Adult patients with clinically evident iron overload of hereditary hemochromatosis exhibit greater than 10,000 µg/g dry weight of iron; hepatic iron concentrations in excess of 22,000 µg/g dry weight are associated with the development of fibrosis and cirrhosis.
The pancreas becomes intensely pigmented, acquires diffuse interstitial fibrosis, and may show some parenchymal atrophy. Hemosiderin is found in the acinar and the islet cells and sometimes in the interstitial fibrous stroma. The heart often is enlarged, with hemosiderin granules within the myocardial fibers. The pigmentation may induce a striking brown coloration of the myocardium. A delicate interstitial fibrosis may appear. Although skin pigmentation is partially attributable to hemosiderin deposition in dermal macrophages and fibroblasts, most of the coloration results from increased epidermal melanin production. The combination of these pigments renders the skin slate-gray. With hemosiderin deposition in the joint synovial linings, an acute synovitis may develop. There is also excessive deposition of calcium pyrophosphate, which damages the articular cartilage and sometimes produces disabling polyarthritis, referred to as pseudogout. The testes may be small and atrophic but usually are not discolored.
Clinical Features
Males predominate (in a ratio of 5 to 7 : 1), with slightly earlier clinical presentation, partly because physiologic iron loss (with menstruation or pregnancy) retards iron accumulation in women. In the most common forms, caused by HFE mutations, symptoms usually first appear in the fifth and sixth decades of life in men and later in women. With population screening it has become clear that even homozygous carriers of the most common HFE mutation (C282Y) have variable penetrance, so that disease progression is unpredictable.
The principal manifestations include hepatomegaly, skin pigmentation (particularly in sun-exposed areas), deranged glucose homeostasis or frank diabetes mellitus from destruction of pancreatic islets, cardiac dysfunction (arrhythmias, cardiomyopathy), and atypical arthritis. In some patients the presenting complaint is loss of libido and impotence. The classic clinical triad of cirrhosis with hepatomegaly, skin pigmentation, and diabetes mellitus may not develop until late in the course of the disease. Death may result from cirrhosis, hepatocellular carcinoma, or cardiac disease. Treatment of iron overload does not remove the risk for development of hepatocellular carcinoma, because of the cumulative, irreversible oxidative damage to DNA produced by iron. The risk for hepatocellular carcinoma development in patients with hemochromatosis is 200 times higher than in normal populations.
Fortunately, hereditary hemochromatosis can be diagnosed long before irreversible tissue damage has occurred. Screening involves demonstration of very high levels of serum iron and ferritin, exclusion of secondary causes of iron overload, and liver biopsy if indicated. Also important is screening of relatives of probands for the causative mutations. The natural course of the disease can be substantially altered by a variety of interventions, mainly phlebotomy and the use of iron chelators to decrease total body iron. Patients diagnosed in the subclinical, precirrhotic stage and treated by regular phlebotomy have a normal life expectancy. Heterozygotes may show a mild increase in iron absorption and accumulation.
Wilson Disease
This autosomal recessive disorder is marked by the accumulation of toxic levels of copper in many tissues and organs, principally the liver, brain, and eye. The cause is loss-of-function mutations in the ATP7B gene, more than 300 of which have been identified. This gene, located on chromosome 13, encodes an ATPase metal ion transporter that localizes to the Golgi region of hepatocytes. About 1 in 100 people are asymptomatic carriers, and the incidence of the disease is approximately 1 per 30,000 population; thus, it is much less common than hereditary hemochromatosis.
![]() Pathogenesis
Pathogenesis
Normal copper physiology involves the following sequence:
1 Absorption of ingested copper (2 to 5 mg/day)
2 Plasma transport in complex with albumin
3 Hepatocellular uptake, followed by binding to an α2-globulin (apoceruloplasmin) to form ceruloplasmin
4 Secretion of ceruloplasmin-bound copper into plasma, where it accounts for 90% to 95% of plasma copper
5 Hepatic uptake of desialylated, senescent ceruloplasmin from the plasma, followed by lysosomal degradation and secretion of free copper into bile
In Wilson disease, the initial steps of copper absorption and transport to the liver are normal. However, without ATP7B activity, copper cannot be passed on to apoceruloplasmin and therefore cannot be excreted into bile, the primary route for copper elimination from the body. Copper thus accumulates progressively in hepatocytes, apparently causing toxic injury by a three-step mechanism: (1) promoting the formation of free radicals, (2) binding to sulfhydryl groups of cellular proteins, and (3) displacing other metals in hepatic metalloenzymes.
Usually by the age of 5 years, copper begins to escape from the overloaded, damaged hepatocytes into the circulation. Free copper generates oxidants that can lead to red cell hemolysis. It also is deposited in many other tissues, such as the brain, cornea, kidneys, bones, joints, and parathyroid glands, where it also produces damage through the same mechanisms that injure hepatocytes. Concomitantly, urinary excretion of copper increases markedly.
![]() Morphology
Morphology
The liver often bears the brunt of injury in Wilson disease, with protean hepatic changes ranging from relatively minor to massive damage and mimicking many other diseases. Thus, Wilson disease may mimic fatty liver disease, with mild to moderate steatosis, steatohepatitis, and even similar patterns of scarring. Patterns of acute hepatitis and chronic hepatitis simulate those of viral hepatitis. The acute hepatitis–like injury, in its most severe form, may manifest as fulminant hepatic failure. The chronic hepatitis–like picture may co-exist with features of fatty liver disease, including the mononuclear infiltrates and lobular-interface hepatitis of the former, with the steatosis, hepatocyte ballooning, and Mallory-Denk bodies of the latter. With progression of chronic hepatitis, cirrhosis develops. Excess copper deposition can often be demonstrated by special stains (e.g., rhodanine stain for copper, orcein stain for copper-associated protein). Because copper also accumulates in chronic obstructive cholestasis, and because histologic analysis cannot reliably distinguish Wilson disease from other causes of liver disease, demonstration of hepatic copper content in excess of 250 µg/g dry weight is most helpful for making a diagnosis.
In the brain, toxic injury primarily affects the basal ganglia, particularly the putamen, which demonstrates atrophy and even cavitation. Nearly all patients with neurologic involvement develop eye lesions called Kayser-Fleischer rings (green to brown deposits of copper in Descemet membrane in the limbus of the cornea)—hence the alternative designation of this condition as hepatolenticular degeneration.
Clinical Features
The age at onset and the clinical presentation of Wilson disease are extremely variable, but the disorder rarely manifests before the age of 6 years or in elderly persons. The most common presentation is acute or chronic liver disease. Neuropsychiatric manifestations, including mild behavioral changes, frank psychosis, or a Parkinson disease–like syndrome, are the initial features in most of the remaining cases. Unlike nearly all other forms of cirrhosis, hepatocellular carcinoma is quite uncommon in Wilson disease. Demonstration of Kayser-Fleischer rings or of markedly elevated hepatic copper levels in a person with low serum ceruloplasmin strongly favors the diagnosis. Early recognition and long-term therapy with copper chelators (such as d-penicillamine) and zinc salts (which lower copper uptake from the gut) have dramatically altered the usual progressive downhill course.
α1-Antitrypsin Deficiency
α1-Antitrypsin (AAT) deficiency is an autosomal recessive disorder marked by abnormally low serum levels of this protease inhibitor. The major function of AAT is the inhibition of proteases, particularly neutrophil elastase released at sites of inflammation. AAT deficiency leads to pulmonary emphysema, because a relative lack of this protein permits the unrestrained activity of tissue-destructive proteases (Chapter 12). Hepatic disease results from retention of mutant AAT in the liver.
![]() Pathogenesis
Pathogenesis
AAT is a small (394–amino acid) plasma glycoprotein synthesized predominantly by hepatocytes. The AAT gene, located on human chromosome 14, is very polymorphic, and at least 75 forms have been identified. Most allelic variants produce normal or mildly reduced levels of serum AAT. However, homozygotes for the Z allele (PiZZ genotype) have circulating AAT levels that are only 10% of normal levels. AAT alleles are autosomal codominant, and consequently PiMZ heterozygotes have intermediate plasma levels of AAT. The PiZ polypeptide contains a single amino acid substitution that results in misfolding of the nascent polypeptide in the hepatocyte endoplasmic reticulum. Because the mutant protein cannot be secreted by the hepatocyte, it accumulates in the endoplasmic reticulum and triggers the so-called unfolded protein response, which can lead to induction of apoptosis (Chapter 1). Curiously, all persons with the PiZZ genotype accumulate AAT in the liver, but only 8% to 20% develop significant liver damage. This manifestation may be related to a genetic tendency in which susceptible persons are less able to degrade accumulated AAT protein within hepatocytes.
![]() Morphology
Morphology
Hepatocytes in AAT deficiency contain round to oval cytoplasmic globules composed of retained AAT, a glycoprotein that is strongly positive in a periodic acid–Schiff stain (Fig. 15–26). By electron microscopy they lie within smooth, and sometimes rough, endoplasmic reticulum. Hepatic injury associated with PiZZ homozygosity may range from marked cholestasis with hepatocyte necrosis in newborns, to childhood cirrhosis, to a smoldering chronic hepatitis or cirrhosis that becomes apparent only late in life.
Clinical Course
Of all newborns with AAT deficiency, 10% to 20% exhibit cholestasis. In older children, adolescents, and adults, presenting symptoms may be related to chronic hepatitis, cirrhosis, or pulmonary disease. The disease may remain silent until cirrhosis appears in middle to later life. Hepatocellular carcinoma develops in 2% to 3% of adults with PiZZ genotype, usually but not always in the setting of cirrhosis. The treatment and cure for the severe hepatic disease are orthotopic liver transplantation.
![]() Summary
Summary
Inherited Metabolic Diseases
• Hemochromatosis is characterized by accumulation of iron in liver, pancreas, heart, pituitary gland, joints and other tissues. It is usually caused by mutations in the HFE gene, which encodes a protein that influences intestinal iron uptake.
• Wilson disease is the result of accumulation of copper in the liver, brain, and eyes; it is caused by a mutation in the metal ion transporter ATP7B.
• α1-Antitrypsin (AAT) deficiency in persons of PiZZ genotype causes pulmonary emphysema (due to increased elastase activity) and liver injury (caused by the accumulation of misfolded AAT).
Circulatory Disorders
In view of the enormous flow of blood through the liver, it is not surprising that circulatory disturbances have considerable impact on the liver. These disorders can be grouped according to whether blood flow into, through, or from the liver is impaired (Fig. 15–27).
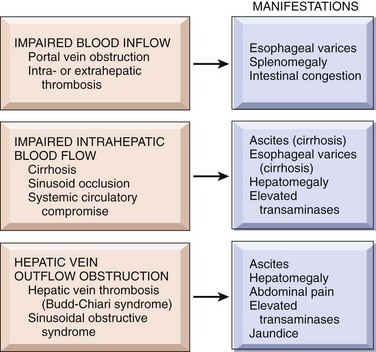
Figure 15–27 Hepatic circulatory disorders. The forms and clinical manifestations of compromised blood flow are contrasted.
Impaired Blood Flow into the Liver
Hepatic Artery Inflow
Liver infarcts are rare, thanks to the double blood supply to the liver. Interruption of the main hepatic artery does not always produce ischemic necrosis of the organ, because retrograde arterial flow through accessory vessels and the portal venous supply may sustain the liver parenchyma. The one exception is hepatic artery thrombosis in the transplanted liver, which generally leads to loss of the organ. Thrombosis or compression of an intrahepatic branch of the hepatic artery by polyarteritis nodosa (Chapter 9), embolism, neoplasia, or sepsis may result in a localized parenchymal infarct.
Portal Vein Obstruction and Thrombosis
Extrahepatic blockage of the portal vein may be slow in developing and well tolerated or may be a catastrophic and potentially lethal event; most cases fall somewhere in between. Occlusion of the portal vein or its major branches typically produces abdominal pain and, in most instances, ascites and other manifestations of portal hypertension like esophageal varices prone to rupture. Acute impairment of visceral blood flow leads to profound congestion and bowel infarction. Extrahepatic portal vein obstruction may arise from the following:
• Peritoneal sepsis (e.g., acute diverticulitis or appendicitis leading to pylephlebitis in the splanchnic circulation)
• Pancreatitis initiating splenic vein thrombosis that propagates into the portal vein
• Thrombogenic diseases and postsurgical thromboses
• Vascular invasion by primary or secondary cancer in the liver that progressively occludes portal inflow to the liver (extensions of hepatocellular carcinoma can even occlude the main portal vein)
• Banti syndrome, in which subclinical thrombosis of the portal vein (as from neonatal omphalitis or umbilical vein catheterization) produces a fibrotic, partially recanalized vascular channel presenting as splenomegaly or esophageal varices years after the occlusive event
• Cirrhosis, although not an extrahepatic cause of obstruction, reduces the flow of blood in the portal veins and hence provides a substratum for extrahepatic portal vein thrombosis.
Intrahepatic thrombosis of a portal vein radicle, when acute, does not cause ischemic infarction but instead results in a sharply demarcated area of red-blue discoloration (so-called infarct of Zahn). There is no necrosis; the only morphologic changes are hepatocellular atrophy and marked congestion of distended sinusoids. Hepatoportal sclerosis is a chronic, usually idiopathic (perhaps autoimmune), generally bland condition of progressive portal tract sclerosis leading to impaired portal vein inflow. Identified causes include myeloproliferative disorders associated with hypercoagulability, peritonitis, or exposure to arsenicals.
Impaired Blood Flow Through the Liver
The most common intrahepatic cause of portal blood flow obstruction is cirrhosis, as described earlier. In addition, physical occlusion of the sinusoids occurs in a small but important group of diseases. In sickle cell disease, the hepatic sinusoids may become obstructed by sickled red cells, leading to panlobular parenchymal necrosis. Disseminated intravascular coagulation may cause occlusion of sinusoids. This blockage usually is inconsequential except in eclampsia, in which periportal sinusoidal occlusion and parenchymal necrosis may occur. Subsequent suffusion of blood under the capsule may precipitate a fatal intra-abdominal hemorrhage.
Passive Congestion and Centrilobular Necrosis
Passive congestion and centrilobular necrosis are hepatic manifestations of systemic circulatory disorders that constitute a morphologic continuum. Right-sided cardiac decompensation leads to passive congestion of the liver and, if persistent, can cause centrilobular necrosis and perivenular fibrosis in the areas of necrosis. In most instances, the only clinical evidence of centrilobular necrosis is a small elevation in serum aminotransferase levels, although some patients have hyperbilirubinemia and elevated alkaline phosphatase.
![]() Morphology
Morphology
In right-sided cardiac failure, the liver is slightly enlarged, tense, and cyanotic, with rounded edges. Microscopic examination reveals congestion of centrilobular sinusoids. Over time, centrilobular hepatocytes become atrophic, resulting in markedly attenuated liver cell cords. An uncommon complication of sustained, chronic, severe congestive heart failure is so-called cardiac sclerosis. The pattern of liver fibrosis is distinctive, inasmuch as it is mostly centrilobular. Rarely bridging fibrous septa and cirrhosis develop.
Left-sided cardiac failure or shock may lead to hepatic hypoperfusion and hypoxia. In this instance, the two areas most dependent on arterial flow (receiving little in the way of portal vein nutrients)—namely, centrilobular hepatocytes and bile ducts—undergo ischemic necrosis. The combination of left-sided hypoperfusion and right-sided retrograde congestion acts synergistically to generate a distinctive lesion, centrilobular hemorrhagic necrosis (Fig. 15–28). The liver takes on a variegated mottled appearance, reflecting hemorrhage and necrosis in the centrilobular regions, alternating with pale midzonal areas, known traditionally as the nutmeg liver because of its similarity to the cut surface of a nutmeg.
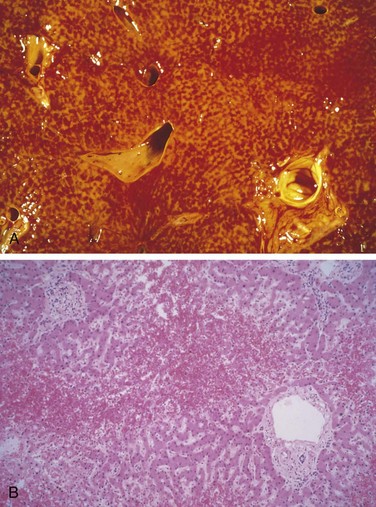
Figure 15–28 Centrilobular hemorrhagic necrosis (nutmeg liver). A, The cut liver section, in which major blood vessels are visible, is notable for a variegated mottled red appearance, representing hemorrhage in the centrilobular regions of the parenchyma. B, On microscopic examination, the centrilobular region is suffused with red blood cells, and hepatocytes are not readily visible. Portal tracts and the periportal parenchyma are intact.
Hepatic Vein Outflow Obstruction
Hepatic Vein Thrombosis (Budd-Chiari Syndrome)
The Budd-Chiari syndrome results from the thrombosis one or more major hepatic veins and is characterized by hepatomegaly, ascites, and abdominal pain. Hepatic vein thrombosis is associated with myeloproliferative disorders (especially polycythemia vera), pregnancy, the postpartum state, the use of oral contraceptives, paroxysmal nocturnal hemoglobinuria, and intra-abdominal cancers, particularly hepatocellular carcinoma. All of these conditions produce thrombotic tendencies or, in the case of liver cancers, sluggish blood flow. Some cases are caused by mechanical obstruction to blood outflow, as by a massive intrahepatic abscess or parasitic cyst, or by obstruction of the inferior vena cava at the level of the hepatic veins by thrombus or tumor. About 10% of cases are idiopathic.
![]() Morphology
Morphology
With acutely developing thrombosis of the major hepatic veins or inferior vena cava, the liver is swollen and red-purple, with a tense capsule (Fig. 15–29). On microscopic examination, severe centrilobular congestion and necrosis are apparent in affected hepatic parenchyma. In instances in which the thrombosis develops more slowly, centrilobular fibrosis is seen. The major veins may contain completely or incompletely occlusive fresh thrombi or, in chronic cases, organized adherent thrombi.
Mortality attributable to untreated acute Budd-Chiari syndrome is high. Prompt surgical creation of a portosystemic venous shunt permits reverse flow through the portal vein and improves the prognosis considerably; direct dilation of caval obstruction may be possible during angiography. The chronic form of the syndrome is far less grave, and more than two thirds of the patients are alive after 5 years.
Sinusoidal Obstruction Syndrome
Originally described in Jamaican drinkers of “bush tea” containing pyrrolizidine alkaloid, sinusoidal obstruction syndrome formerly was known as venoocclusive disease. The new designation more specifically indicates that the condition is caused by toxic injury to sinusoidal endothelium. Damaged endothelial cells slough, leading to formation of thrombi that block sinusoidal flow. Endothelial damage allows red cells to spill into the space of Disse, and also causes proliferation of stellate cells and fibrosis of terminal branches of the hepatic vein (Fig. 15–30). Sinusoidal obstruction syndrome now occurs primarily in the first 20 to 30 days after bone marrow transplantation, affecting 20% of recipients. The sinusoidal injury is believed to be caused by chemotherapeutic agents such as cyclophosphamide, actinomycin D, and mithramycin, and by total body radiation, used in pre- or posttransplantation regimens. The clinical presentation resembles the Budd-Chiari syndrome and varies, ranging from mild to severe. Severe sinusoidal obstruction syndrome that does not resolve after 3 months of treatment can be fatal.
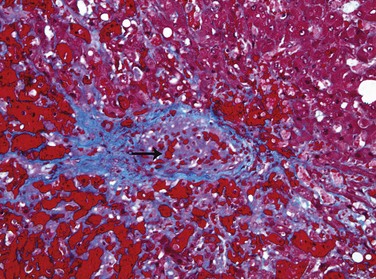
Figure 15–30 Sinusoidal obstruction syndrome (formerly known as venoocclusive disease).
A central vein is occluded by cells and newly formed collagen (arrow). There is also fibrosis in the sinusoidal spaces. Fibrous tissue is stained blue by the Masson trichrome stain.
(Courtesy of Dr. Matthew Yeh, University of Washington, Seattle, Washington.)
![]() Summary
Summary
Circulatory Disorders
• Circulatory disorders of the liver can be caused by impaired blood inflow, defects in intrahepatic blood flow, and obstruction of blood outflow.
• Portal vein obstruction by intra- or extrahepatic thrombosis may cause portal hypertension, esophageal varices, and ascites.
• The most common cause of impaired intrahepatic blood flow is cirrhosis.
• Conditions of obstruction of blood outflow include hepatic vein thrombosis (Budd-Chiari syndrome) and sinusoidal obstruction syndrome, previously known as venoocclusive disease.
Other Inflammatory and Infectious Diseases
Liver Abscesses
In developing countries liver abscesses are common; most result from parasitic infections, such as amebic and (less commonly) other protozoal and helminthic organisms. In developed countries parasitic liver abscesses are more rare. In the Western world, bacterial abscesses are more common, representing a complication of an infection elsewhere. The organisms reach the liver through one of the following pathways:
• Ascending infection in the biliary tract (ascending cholangitis)
• Vascular seeding, either portal or arterial, predominantly from the gastrointestinal tract
Debilitating disease with immune deficiency is a common setting—for example, extreme old age, immunosuppression, or cancer chemotherapy with marrow failure.
Pyogenic (bacterial) hepatic abscesses may occur as solitary or multiple lesions, ranging in size from millimeters to massive lesions many centimeters in diameter. Many pathogens can cause pyogenic liver abscess and often more than one organism is involved. The most common bacterial agents are E. coli, Klebsiella pneumoniiae, Proteus spp., pseudomonas, and Streptococcus milleri. Increasingly Candida spp. are also being isolated. Because of the polymicrobial nature of the abscesses, identification of the organisms is important. Gross and microscopic features are those of any pyogenic abscess: liquefactive necrosis with abundant neutrophils.
Liver abscesses are associated with fever and, in many instances, with right upper quadrant pain and tender hepatomegaly. Jaundice may result if there is biliary obstruction. Antibiotic therapy along with percutaneous or surgical drainage is often necessary. If the treatment is delayed, particularly in persons with serious coexistent disease, the mortality rate with large liver abscesses ranges from 30% to 90%. With early recognition and appropriate management, as many as 90% of patients may survive.
Granulomatous Disease
Granulomas are common in the liver, being present in as many as 10% of liver biopsy specimens. They may reflect systemic disease or be specific to the liver. Granulomatous disease may be classified into four etiologic categories:
• “See the cause,” in which infectious agents can be visualized with special stains, such as fungal or acid-fast organisms
• “Know the cause,” in which organisms are not seen but a previous diagnosis (e.g., known tuberculosis, systemic sarcoidosis, duct destructive lesions in known primary biliary cirrhosis) explains the lesions
• “Suspect the cause,” in which the appearance of the granulomas or clinicopathologic correlation points to a likely underlying disorder, such as caseous necrosis, strongly suggestive of infection, or eosinophilia, which points to parasites or drug- or toxin-induced injury
• “Don’t know the cause,” accounting for approximately 10% of hepatic granulomas, most of which are incidental findings of no clinical import
Because of their infiltrative nature, granulomatous diseases of the liver give rise to intrahepatic cholestasis with elevations of alkaline phosphatase and γ-glutamyl transpeptidase.
Tumors and Hepatic Nodules
The liver and lungs share the dubious distinction of being the visceral organs most often involved by metastatic cancers. Indeed, the most common hepatic neoplasms are metastatic carcinomas, with colon, lung, and breast heading the list of the primary sites. Primary hepatic malignancies are almost all hepatocellular carcinomas, which show a dramatic geographic variation in incidence throughout the world, as discussed later. Two other types of primary liver cancers, hepatoblastoma (a childhood hepatocellular tumor) and angiosarcoma (a tumor of blood vessels associated with exposure to vinyl chloride and arsenic), are too rare to merit further discussion here.
Hepatic masses come to attention for a variety of reasons. They may generate epigastric fullness and discomfort or be detected by routine physical examination. Radiographic studies for other indications may pick up incidental liver masses.
Benign Tumors
The most common benign lesions of the liver are cavernous hemangiomas, which are identical to those occurring in other parts of the body (Chapter 9). These well-circumscribed lesions consist of endothelial cell–lined vascular channels and intervening stroma. They appear as discrete red-blue, soft nodules, usually less than 2 cm in diameter, often directly beneath the capsule. Their chief clinical significance lies in the importance of not mistaking them for metastatic tumors; blind percutaneous needle biopsy may cause severe intra-abdominal bleeding.
Von Meyenburg complexes are another quite common benign finding in the liver. These are presumed to be congenital bile duct hamartomas and are usually isolated or present in small numbers. They are composed of bile duct–like structures separated by bland, densely collagenized stroma. These lesions have no malignant potential, but when multiple may indicate the presence of fibropolycystic disease of the liver, which may occur in association with certain forms of polycystic kidney disease (Chapter 13).
Focal Nodular Hyperplasia
Focal nodular hyperplasia (FNH) is found most often in otherwise normal livers. It is a localized, well-demarcated, but poorly encapsulated lesion, consisting of hyperplastic hepatocyte nodules with a central, stellate fibrous scar. It is not a true neoplasm but rather represents a response to abnormal vascular flow through a congenital or acquired vascular anomaly that gives rise to alternating areas of parenchymal regeneration and atrophy. FNH may range in size from 1 cm to many centimeters across. It usually is an incidental finding, most commonly in women of reproductive age, in whom it may grow in response to estrogens, including those found in contraceptive pills. These lesions carry no risk for malignancy but may cause symptoms by pressing on the liver capsule.
Hepatic Adenoma
Hepatic adenoma is a benign hepatocellular neoplasm that usually occurs in women of child-bearing age who have used oral contraceptive pills, and it may regress on discontinuance of hormone use. These lesions usually are well-demarcated but unencapsulated tumors that may be pale, yellow-tan, or bile-stained and up to 30 cm in diameter (Fig. 15–31). On histologic examination, hepatic adenomas are composed of sheets and cords of cells that may resemble normal hepatocytes or have minimal variation in cell and nuclear size. Portal tracts are absent; instead, prominent arterial vessels and draining veins are distributed throughout. Liver cell adenomas are significant for three reasons: (1) when they manifest as an intrahepatic mass, they may be mistaken for the more ominous hepatocellular carcinoma; (2) subcapsular adenomas are at risk for rupture, particularly during pregnancy (under estrogenic stimulation), causing life-threatening intraabdominal hemorrhage; and (3) although malignant transformation is unusual, adenomas harboring β-catenin mutations carry a risk of developing into hepatocellular carcinoma.
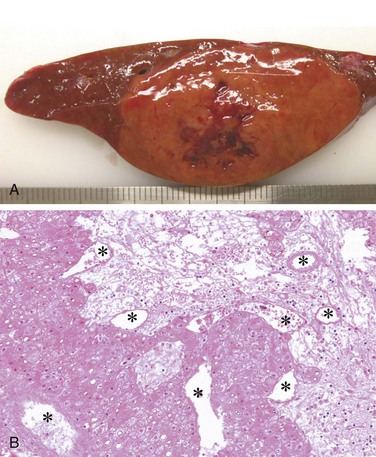
A, Surgically resected specimen showing a discrete mass underneath the liver capsule with hemorrhagic necrosis (dark red areas). B, Photomicrograph showing adenoma, with cords of normal-appearing hepatocytes, absence of portal tracts, and prominent neovascularization (asterisks). A large zone of infarcted tumor is present.
(A, Courtesy of Dr. Paulette Bioulac-Sage, University of Bordeaux, Bordeaux, France.)
Molecular studies now classify these lesions into three somewhat discrete types:
• 35% to 40% of adenomas have biallelic inactivation through somatic (90%) or germline (10%) mutations of either the HNF1A gene (encoding a hepatocyte transcription factor) or the CYP1B1 gene (encoding cytochrome P-450). These adenomas are most common in women, sometimes associated with oral contraceptive use, and are often yellow from marked steatosis. They carry little risk for malignant transformation.
• 10% to 15% of adenomas have activating mutations of β-catenin. These have a high risk for malignant transformation, are most common in men and may be related to anabolic steroid use and possibly nonalcoholic fatty liver disease.
• More than 50% of adenomas are inflammatory, being associated with increased expression of acute phase reactant proteins such as serum amyloid A and C-reactive protein in the tumor and sometimes in the serum. About 10% of these tumors also have activating mutations of β-catenin and may undergo malignant transformation. This type is most common in women and is often associated with obesity and fatty liver disease. Morphologically, this subgroup may be identical to FNH, and the distinction may be made by demonstrating an inflammatory phenotype (such as by expression of serum amyloid A protein, SAA).
Precursor Lesions of Hepatocellular Carcinoma
While hepatocellular adenomas sometimes can be premalignant, they represent a rare pathway to hepatocellular malignancy. Far more common precursors are cellular changes and nodular lesions that are found in the setting of chronic liver disease, particularly chronic viral hepatitis, alcoholic liver disease, and metabolic diseases such as AAT deficiency and hereditary hemochromatosis. Usually they are found in late-stage disease, in particular when cirrhosis is already established. However, while it has often been said that cirrhosis itself is premalignant, this is imprecise: The processes that lead to cirrhosis and to malignant transformation usually take years to decades and run parallel to each other, rather than in sequence.
Cellular Dysplasia
Two forms of hepatocellular dysplasia are recognized, both of which are most common in the setting of chronic viral hepatitis. Large cell change consists of scattered hepatocytes, usually in periportal or periseptal regions, that are larger than normal hepatocytes and have pleomorphic, often multiple nuclei (Fig. 15–32, A). Although morphologically atypical, these cells are not believed to be on the pathway to malignant transformation but rather are considered a marker of molecular changes stemming from chronic injury that predispose other morphologically normal hepatocytes to undergo malignant transformation. Small cell change is characterized by smaller-than-normal hepatocytes with normal-sized, often hyperchromatic, oval or angulated nuclei. Small cell change may appear anywhere in the hepatic lobule, often with formation of vaguely nodular clusters. This form of dysplasia is considered to be directly premalignant (Fig. 15–32, B).
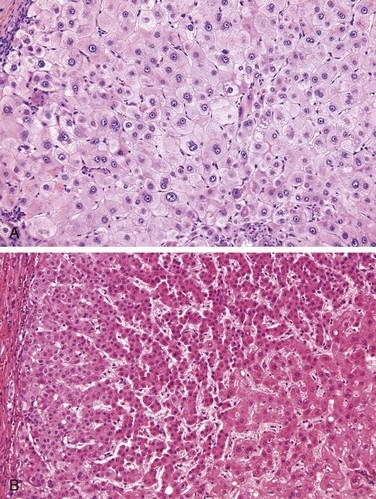
Figure 15–32 A, Large cell change. Very large hepatocytes with very large, often atypical nuclei are scattered among normal-size hepatocytes with round, typical nuclei. B, Small cell change (SCC). Normal-appearing hepatocytes are in the lower right corner. SCC is indicated by smaller than normal hepatocytes with thickened liver cell plates, and high nuclear : cytoplasmic ratio.
(A and B, Courtesy of Dr. Young Nyun Park, Yonsei Medical College, Seoul, South Korea.)
Dysplastic Nodules
Dysplastic nodules probably represent the major pathway to hepatocellular carcinoma in chronic liver disease. In cirrhotic livers, dysplastic nodules are distinguished by their larger size: Most cirrhotic nodules range from 0.3 to 0.8 cm, but dysplastic nodules often are 1 to 2 cm across (Fig. 15–33, A). These are neoplastic growths that encompass many adjacent hepatic lobules, without displacing all of the portal tracts. These lesions are at high risk for malignant transformation and in fact sometimes contain overtly malignant subnodules (Fig. 15–33, B).
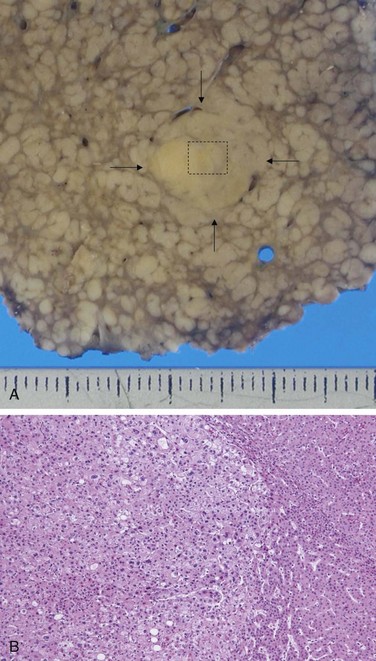
Figure 15–33 A, Hepatitis C–related cirrhosis with a distinctively large nodule (arrows). Nodule-in-nodule growth in this dysplastic nodule suggests a high grade lesion. B, Histologically the region within the box in A shows a well-differentiated hepatocellular carcinoma (HCC) (right side) and a subnodule of moderately differentiated HCC within it (center, left).
(A and B, Courtesy of Dr. Masamichi Kojiro, Kurume University, Kurume, Japan.)
Hepatocellular Carcinomas
Epidemiology
Worldwide, hepatocellular carcinoma, HCC (also known erroneously as hepatoma) accounts for approximately 5.4% of all cancers, but its incidence varies widely in different parts of the world. More than 85% of cases occur in countries with high rates of chronic HBV infection. The highest incidences of HCC are found in Asian countries (southeast China, Korea, Taiwan) and sub-Saharan African countries in which HBV is transmitted vertically, and, as already discussed, the carrier state starts in infancy. Moreover, many of these populations are exposed to aflatoxin, which when combined with HBV infection increases the risk of hepatocellular carcinoma dramatically over that in noninfected, nonexposed populations; depending on the study, the estimated increase in HCC ranges from 23-fold to 216-fold.The peak incidence of hepatocellular carcinoma in these areas is between 20 and 40 years of age, and in almost 50% of cases, the tumor appears in the absence of cirrhosis. In Western countries the incidence of hepatocellular carcinoma is rapidly increasing, largely owing to the hepatitis C epidemic. It tripled in the United States in recent decades, but it is still 8-fold to 30-fold lower than the incidence in some Asian countries. In Western populations, hepatocellular carcinoma rarely manifests before the age of 60, and in almost 90% of cases, tumors develop in the setting of cirrhosis. There is a pronounced male preponderance throughout the world, about 3 : 1 in low-incidence areas and as high as 8 : 1 in high-incidence areas.
![]() Pathogenesis
Pathogenesis
Several general factors relevant to the pathogenesis of HCC are discussed in Chapter 5. Only a few points merit emphasis here.
• Three major etiologic associations have been established: infection with HBV or HCV, alcoholic cirrhosis, and aflatoxin exposure. In the U.S., NAFLD is increasingly becoming an important risk factor for hepatocellular cancer. Other associated conditions include hemochromatosis, α1-antitrypsin deficiency, and tyrosinemia.
• Many variables, including age, gender, chemicals, viruses, hormones, alcohol, and nutrition, interact in the development of HCC. For example, the disease most likely to give rise to this tumor is hereditary tyrosinemia, a rare disorder in which almost 40% of patients develop this tumor.
• In many parts of the world, including Japan and Central Europe, chronic HCV infection is the most important risk factor in the development of liver cancer.
• In certain regions of the world, such as China and southern Africa, especially Mozambique, where HBV is endemic, high-level exposure to dietary aflatoxins derived from the fungus Aspergillus flavus is common. These carcinogenic toxins are found in “moldy” grains and peanuts. Aflatoxin can bind covalently with cellular DNA, resulting in mutations in genes such as TP53.
Despite the detailed knowledge about the etiologic agents of HCC, the pathogenesis of the tumor is still uncertain. In most cases, it develops from small-cell, high-grade dysplastic nodules in cirrhotic livers. In keeping with this, these nodules may be monoclonal and may contain chromosomal aberrations similar to those seen in HCCs. The cell of origin of HCC has been the subject of considerable debate. It seems that the tumors may arise from both mature hepatocytes and progenitor cells (known as ductular cells or oval cells). Distinguishing high-grade dysplastic nodules from early HCC is difficult even in biopsies, because there are no molecular markers specific for these stages. An important criterion is nodule vascularization, visualized by imaging, which is almost always a clear indication of malignancy.
An almost universal feature of hepatocellular carcinoma is the presence of structural and numeric chromosomal abnormalities indicative of genomic instability. The precise origin of genomic instability in this tumor is not known, but several factors seem to be most important:
• Inflammation and regeneration, seen in all forms of chronic hepatitis, are believed to be main contributors to acquired mutations in genomic DNA.
• Acquired mutations in specific oncogenes (such as β-catenin) and tumor suppressors (such as TP53) contribute to dysregulated growth and further increases in genomic instability.
• Acquired defects in DNA repair, particularly those in repair systems for double-stranded DNA breaks, also perpetuate DNA damage and may cause chromosomal defects.
Neither HBV nor HCV contains oncogenes. The already mentioned HBV-X gene may have some oncogenic potential (Chapter 5). The tumorigenic capacity of these viruses probably relates primarily to their capacity to cause chronic inflammation and increased cell turnover.
![]() Morphology
Morphology
HCC may appear grossly as (1) a unifocal, usually massive tumor; (2) a multifocal tumor made of nodules of variable size; or (3) a diffusely infiltrative cancer, permeating widely and sometimes involving the entire liver, blending imperceptibly into the cirrhotic background. Particularly in the latter two patterns, it may be difficult to radiologically distinguish regenerative cirrhotic nodules from neoplasms of similar size. Discrete tumor masses usually are yellow-white, punctuated sometimes by bile staining and areas of hemorrhage or necrosis. HCC has a strong propensity for vascular invasion. Extensive intrahepatic metastases are characteristic, and occasionally snakelike masses of tumor invade the portal vein (with occlusion of the portal circulation) or inferior vena cava, extending even into the right side of the heart.
On histologic examination, HCCs range from well-differentiated lesions that reproduce hepatocytes arranged in cords, trabeculae or glandular patterns (Fig. 15–34), to poorly differentiated lesions, often composed of large, multinucleate anaplastic giant cells. In the better-differentiated variants, globules of bile may be found within the cytoplasm of cells and in pseudocanaliculi between cells. Acidophilic hyaline inclusions within the cytoplasm may be present, resembling Mallory bodies. There is little stroma in most hepatocellular carcinomas, explaining their soft consistency.
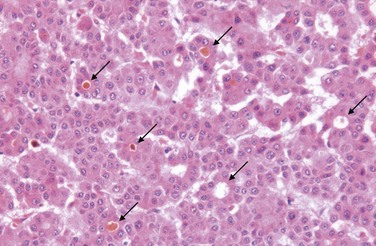
Figure 15–34 Well-differentiated hepatocellular carcinoma has distortions of normal structures: Liver cell plates are markedly widened, and frequent “pseudoacinar” structures (arrows)—abnormal bile canaliculi—often contain bile.
A distinctive clinicopathologic variant of HCC is the fibrolamellar carcinoma. It occurs in young male and female adults (20 to 40 years of age) with equal incidence and has no association with cirrhosis or other risk factors. It usually consists of a single tumor with fibrous bands coursing through it, superficially resembling focal nodular hyperplasia. The fibrolamellar variant has a better prognosis than that of the other, more common variants.
Clinical Features
Although HCC may manifest with silent hepatomegaly, it is more often encountered in persons with symptomatic cirrhosis of the liver. In these persons, a rapid increase in liver size, sudden worsening of ascites, or the appearance of bloody ascites, fever, and pain call attention to the development of a tumor. There are no good serologic screening tests for hepatocellular carcinoma. The most commonly used marker is serum alpha-fetoprotein level, but it rises only with advanced tumors and only in 50% of patients. Furthermore, false-positive results are obtained in yolk-sac tumors, and many non-neoplastic conditions such as cirrhosis, chronic hepatitis, normal pregnancy, and massive liver necrosis. Hence the test is neither specific nor sensitive. Radiologic screening of patients with cirrhosis at 6-month intervals, looking for dysplastic nodules or early, small hepatocellular carcinomas, is the current clinical frontier.
The overall prognosis with advanced HCC is grim. Resection or ablation may be curative for a single small lesion (most often those with the uncommon fibrolamellar variant), but does not prevent de novo emergence of new HCCs in a chronically diseased liver. Transplantation can be curative, however. Without resection, median survival is 7 months. Recent clinical trials have shown that treatment with sorafenib, a broad-spectrum tyrosine kinase inhibitor, provides some benefit to those with advanced disease. In some countries such as Taiwan, HBV immunization programs have lowered the incidence of HCC substantially, proving that preventive measures can alleviate the terrible toll taken by this disease in endemic regions.
![]() Summary
Summary
Liver Tumors
• The most common malignant tumors of the liver are metastatic carcinomas, most often from colon, lung, and breast.
• The main primary malignancy is hepatocellular carcinoma. It is common in regions of Asia and Africa, and its incidence is increasing in the United States.
• The main etiologic agents for hepatocellular carcinoma are hepatitis B and C, alcoholic cirrhosis, hemochromatosis, and, more rarely, tyrosinemia and α1-antitrypsin (AAT) deficiency.
• In the Western population, about 90% of hepatocellular carcinomas develop in cirrhotic livers; in Asia, almost 50% of cases develop in noncirrhotic livers.
• The chronic inflammation and cellular regeneration associated with viral hepatitis may be predisposing factors for the development of carcinomas.
• Hepatocellular carcinomas may be unifocal or multifocal, tend to invade blood vessels, and recapitulate normal liver architecture to varying degrees.
Disorders of the Gallbladder and the Extrahepatic Biliary Tract
Disorders of the gallbladder and biliary tract affect a large proportion of the world’s population. Cholelithiasis (gallstones) accounts for more than 95% of these diseases. About 2% of the United States federal health budget is spent on cholelithiasis and its complications. In this section, gallbladder diseases (cholelithiasis and cholecystitis) are discussed first, followed by consideration of some disorders of the extrahepatic bile ducts. It should be kept in mind that lesions of the extrahepatic biliary tract may extend to intrahepatic bile ducts, and that tumors of the biliary tract (cholangiocarcinomas, described later) may have intra- or extrahepatic locations.
Gallbladder Diseases
Cholelithiasis (Gallstones)
Gallstones afflict 10% to 20% of adults residing in Western countries in the Northern Hemisphere, 20% to 40% in Latin American countries, and only 3% to 4% in Asian countries. In the United States, about 1 million new cases of gallstones are diagnosed annually, and two thirds of persons so affected undergo surgery, with retrieval of as much as 25 to 50 million tons of stones! There are two main types of gallstones: cholesterol stones, containing crystalline cholesterol monohydrate (80% of stones in the West), and pigment stones, made of bilirubin calcium salts.
![]() Pathogenesis
Pathogenesis
Bile formation is the only significant pathway for elimination of excess cholesterol from the body, either as free cholesterol or as bile salts. Cholesterol is rendered water-soluble by aggregation with bile salts and lecithins. When cholesterol concentrations exceed the solubilizing capacity of bile (supersaturation), cholesterol can no longer remain dispersed and crystallizes out of solution. Cholesterol gallstone formation is enhanced by hypomobility of the gallbladder (stasis), which promotes nucleation, and by mucus hypersecretion, with consequent trapping of the crystals, thereby enhancing their aggregation into stones.
Formation of pigment stones is more likely in the presence of unconjugated bilirubin in the biliary tree, as occurs in hemolytic anemias and infections of the biliary tract. The precipitates are primarily insoluble calcium bilirubinate salts.
The major risk factors for gallstones are listed in Table 15–8. Up to 80% of people with gallstones, however, have no identifiable risk factors other than age and gender. Some elaboration on these risk factors follows:
• Age and gender. The prevalence of gallstones increases throughout life. In the United States, less than 5% to 6% of the population younger than age 40 has stones, in contrast with 25% to 30% of those older than 80 years. The prevalence in women of all ages is about twice as high as in men.
• Ethnic and geographic. Cholesterol gallstone prevalence approaches 50% to 75% in certain Native American populations—the Pima, Hopi, and Navajos—whereas pigment stones are rare; the prevalence seems to be related to biliary cholesterol hypersecretion.
• Heredity. In addition to ethnicity, a positive family history imparts increased risk, as do a variety of inborn errors of metabolism such as those associated with impaired bile salt synthesis and secretion.
• Environment. Estrogenic influences, including oral contraceptives and pregnancy, increase hepatic cholesterol uptake and synthesis, leading to excess biliary secretion of cholesterol. Obesity, rapid weight loss, and treatment with the hypocholesterolemic agent clofibrate also are strongly associated with increased biliary cholesterol secretion.
• Acquired disorders. Any condition in which gallbladder motility is reduced predisposes to gallstones, such as pregnancy, rapid weight loss, and spinal cord injury. In most cases, however, gallbladder hypomotility is present without obvious cause.
Table 15–8 Risk Factors for Gallstones
| Cholesterol Stones |
| Pigment Stones |
![]() Morphology
Morphology
Cholesterol stones arise exclusively in the gallbladder and consist of 50% to 100% cholesterol. Pure cholesterol stones are pale yellow; increasing proportions of calcium carbonate, phosphates, and bilirubin impart gray-white to black discoloration (Fig. 15–35). They are ovoid and firm; they can occur singly, but most often there are several, with faceted surfaces resulting from their apposition. Most cholesterol stones are radiolucent, although as many as 20% may have sufficient calcium carbonate to be radiopaque.
Figure 15–35 Cholesterol gallstones.
Mechanical manipulation during laparoscopic cholecystectomy has caused fragmentation of several cholesterol gallstones, revealing interiors that are pigmented because of entrapped bile pigments. The gallbladder mucosa is reddened and irregular as a result of coexistent acute and chronic cholecystitis.
Pigment stones may arise anywhere in the biliary tree and are classified into black and brown stones. In general, black pigment stones are found in sterile gallbladder bile, while brown stones are found in infected intrahepatic or extrahepatic ducts. The stones contain calcium salts of unconjugated bilirubin and lesser amounts of other calcium salts, mucin glycoproteins, and cholesterol. Black stones are usually small in size, fragile to the touch, and numerous (Fig. 15–36). Brown stones tend to be single or few in number and to have a soft, greasy, soaplike consistency that results from the presence of retained fatty acid salts released by the action of bacterial phospholipases on biliary lecithins. Because of calcium carbonates and phosphates, 50% to 75% of black stones are radiopaque. Brown stones, which contain calcium soaps, are radiolucent.
Clinical Features
70% to 80% of individuals with gallstones remain asymptomatic throughout life, with the risk of symptoms diminishing over time. In the unfortunate minority, however, the symptoms are striking. There is usually pain, often excruciating, which typically localizes to the right upper quadrant or epigastric region and can be constant or, less commonly, spasmodic. Such “biliary” pain is caused by gallbladder or biliary tree obstruction, or by inflammation of the gallbladder itself. More severe complications include empyema, perforation, fistulas, inflammation of the biliary tree, and obstructive cholestasis or pancreatitis. The larger the calculi, the less likely they are to enter the cystic or common ducts to produce obstruction; it is the very small stones, or “gravel,” that are more dangerous. Occasionally a large stone may erode directly into an adjacent loop of small bowel, generating intestinal obstruction (gallstone ileus).
Cholecystitis
Inflammation of the gallbladder may be acute, chronic, or acute superimposed on chronic, and almost always occurs in association with gallstones. In the United States, cholecystitis is one of the most common indications for abdominal surgery. Its epidemiologic distribution closely parallels that of gallstones.
![]() Morphology
Morphology
In acute cholecystitis, the gallbladder usually is enlarged and tense, and it assumes a bright red or blotchy, violaceous color, the latter imparted by subserosal hemorrhages. The serosa frequently is covered by a fibrinous, or in severe cases, fibrinopurulent exudate. In 90% of cases, stones are present, often obstructing the neck of the gallbladder or the cystic duct. The gallbladder lumen is filled with cloudy or turbid bile that may contain fibrin, blood, and frank pus. When the contained exudate is mostly pus, the condition is referred to as empyema of the gallbladder. In mild cases the gallbladder wall is thickened, edematous, and hyperemic. In more severe cases the gallbladder is transformed into a green-black necrotic organ—a condition termed gangrenous cholecystitis. On histologic examination, the inflammatory reactions are not distinctive and consist of the usual patterns of acute inflammation (i.e., edema, leukocytic infiltration, vascular congestion, frank abscess formation, or gangrenous necrosis).
The morphologic changes in chronic cholecystitis are extremely variable and sometimes subtle. The mere presence of stones within the gallbladder, even in the absence of acute inflammation, often is taken as sufficient justification for the diagnosis. The gallbladder may be contracted, of normal size, or enlarged. Mucosal ulcerations are infrequent; the submucosa and subserosa often are thickened from fibrosis. In the absence of superimposed acute cholecystitis, mural lymphocytes are the only signs of inflammation.
Acute Calculous Cholecystitis
Acute inflammation of a gallbladder that contains stones is termed acute calculous cholecystitis and is precipitated by obstruction of the gallbladder neck or cystic duct. It is the most common major complication of gallstones and the most common reason for emergency cholecystectomy. Manifestations of obstruction may appear with remarkable suddenness and constitute a surgical emergency. In some cases, however, symptoms may be mild and resolve without medical intervention.
Acute calculous cholecystitis is initially the result of chemical irritation and inflammation of the gallbladder wall in the setting of obstruction to bile outflow. The action of phospholipases derived from the mucosa hydrolyzes biliary lecithin to lysolecithin, which is toxic to the mucosa. The normally protective glycoprotein mucous layer is disrupted, exposing the mucosal epithelium to the direct detergent action of bile salts. Prostaglandins released within the wall of the distended gallbladder contribute to mucosal and mural inflammation. Distention and increased intraluminal pressure also may compromise blood flow to the mucosa. These events occur in the absence of bacterial infection; only later may bacterial contamination develop.
Acute Acalculous Cholecystitis
Between 5% and 12% of gallbladders removed for acute cholecystitis contain no gallstones. Most cases occur in seriously ill patients. Some of the most common predisposing insults are
Other contributing factors include dehydration, gallbladder stasis and sludging, vascular compromise, and, ultimately, bacterial contamination.
Chronic Cholecystitis
Chronic cholecystitis may be the sequel to repeated bouts of acute cholecystitis, but in most instances it develops without any history of acute attacks. Like acute cholecystitis it is almost always associated with gallstones. However, gallstones do not seem to have a direct role in the initiation of inflammation or the development of pain, because chronic acalculous cholecystitis causes symptoms and morphologic alterations similar to those seen in the calculous form. Rather, supersaturation of bile predisposes the patient to both chronic inflammation and, in most instances, stone formation. Microorganisms, usually E. coli and enterococci, can be cultured from the bile in only about one third of cases. Unlike acute calculous cholecystitis, stone obstruction of gallbladder outflow in chronic cholecystitis is not a requisite. Most gallbladders removed at elective surgery for gallstones show features of chronic cholecystitis, making it likely that biliary symptoms emerge after long-term coexistence of gallstones and low-grade inflammation.
Clinical Features
Acute calculous cholecystitis presents with biliary pain that lasts for more than 6 hours. The pain is severe, usually steady, upper abdominal in location, and often radiates to the right shoulder. Fever, nausea, leukocytosis, and prostration are classic; the presence of conjugated hyperbilirubinemia suggests obstruction of the common bile duct. The right subcostal region is markedly tender and rigid as a result of spasm of the abdominal muscles; occasionally a tender, distended gallbladder can be palpated. Mild attacks usually subside spontaneously over 1 to 10 days; however, recurrence is common. Approximately 25% of symptomatic patients are sufficiently ill to require surgical intervention.
Symptoms arising from acute acalculous cholecystitis usually are obscured by the generally severe clinical condition of the patient. The diagnosis therefore rests on keeping this possibility in mind.
Chronic cholecystitis does not have the striking manifestations of the acute forms and is usually characterized by recurrent attacks of steady epigastric or right upper quadrant pain. Nausea, vomiting, and intolerance for fatty foods are frequent accompaniments.
The diagnosis of acute cholecystitis usually is based on the detection of gallstones by ultrasonography, typically accompanied by evidence of a thickened gallbladder wall. Chronic cholecystitis, on the other hand, is a pathologic diagnosis based on the examination of the resected gall-bladder. Attention to this disorder is important because of the potential for the following serious complications:
• Bacterial superinfection with cholangitis or sepsis
• Gallbladder perforation and local abscess formation
• Gallbladder rupture with diffuse peritonitis
• Biliary enteric (cholecystenteric) fistula, with drainage of bile into adjacent organs, entry of air and bacteria into the biliary tree, and potentially gallstone-induced intestinal obstruction (ileus)
• Aggravation of preexisting medical illness, with cardiac, pulmonary, renal, or liver decompensation
Disorders of Extrahepatic Bile Ducts
Choledocholithiasis and Cholangitis
Choledocholithiasis and cholangitis are considered together because these conditions frequently go hand in hand. Choledocholithiasis is the presence of stones within the biliary tree. In Western nations, almost all stones are derived from the gallbladder; in Asia, there is a much higher incidence of primary ductal and intrahepatic, usually pigmented, stone formation. Choledocholithiasis may not immediately obstruct major bile ducts; asymptomatic stones are found in about 10% of patients at the time of surgical cholecystectomy. Symptoms may develop because of (1) biliary obstruction, (2) cholangitis, (3) hepatic abscess, (4) chronic liver disease with secondary biliary cirrhosis, or (5) acute calculous cholecystitis.
Cholangitis is the term used for acute inflammation of the wall of bile ducts, almost always caused by bacterial infection of the normally sterile lumen. It can result from any lesion obstructing bile flow, most commonly choledocholithiasis, and also from surgery involving the biliary tree. Other causes include tumors, indwelling stents or catheters, acute pancreatitis, and benign strictures. Bacteria most likely enter the biliary tract through the sphincter of Oddi, rather than by the hematogenous route. Ascending cholangitis refers to the propensity of bacteria, once within the biliary tree, to infect intrahepatic biliary ducts. The usual pathogens are E. coli, Klebsiella, Enterococci, Clostridium, and Bacteroides. Two or more organisms are found in half of the cases. In some world populations, parasitic cholangitis is a significant problem. Causative organisms include Fasciola hepatica or schistosomiasis in Latin America and the Near East, Clonorchis sinensis or Opisthorchis viverrini in the Far East, and cryptosporidiosis in persons with acquired immunodeficiency syndrome.
Bacterial cholangitis usually produces fever, chills, abdominal pain, and jaundice. The most severe form of cholangitis is suppurative cholangitis, in which purulent bile fills and distends bile ducts, with an attendant risk of liver abscess formation. Because sepsis rather than cholestasis is the predominant risk in cholangitic patients, prompt diagnosis and intervention are imperative.
Secondary Biliary Cirrhosis
Prolonged obstruction of the extrahepatic biliary tree results in profound damage to the liver itself. The most common cause of obstruction is extrahepatic cholelithiasis. Other obstructive conditions include biliary atresia (discussed later on), malignancies of the biliary tree and head of the pancreas, and strictures resulting from previous surgical procedures. The initial morphologic features of cholestasis were described earlier and are entirely reversible with correction of the obstruction. However, secondary inflammation resulting from biliary obstruction initiates periportal fibrogenesis, which eventually leads to scarring and nodule formation, generating secondary biliary cirrhosis.
Biliary Atresia
Biliary atresia is a major cause of neonatal cholestasis, accounting for one third of the cases of cholestasis in infants and occurring in approximately 1 in 10,000 live births. Biliary atresia is defined as a complete obstruction of bile flow caused by destruction or absence of all or part of the extrahepatic bile ducts. It is the most frequent cause of death from liver disease in early childhood and accounts for more than half of the referrals of children for liver transplantation.
The salient features of biliary atresia include
• Inflammation and fibrosing stricture of the hepatic or common bile ducts
• Inflammation of major intrahepatic bile ducts, with progressive destruction of the intrahepatic biliary tree
• Florid features of biliary obstruction on liver biopsy (i.e., ductular reaction, portal tract edema and fibrosis, and parenchymal cholestasis)
• Periportal fibrosis and cirrhosis within 3 to 6 months of birth
Clinical Course
Infants with biliary atresia present with neonatal cholestasis; there is a slight female predominance. Affected infants have normal birth weights and postnatal weight gain. Stools become acholic as the disease evolves. Laboratory findings do not distinguish between biliary atresia and intrahepatic cholestasis, but a liver biopsy provides evidence of bile duct obstruction in 90% of cases of biliary atresia. Liver transplantation is the definitive treatment. Without surgical intervention, death usually occurs within 2 years of birth.
![]() Summary
Summary
Diseases of the Gallbladder and Extrahepatic Bile Ducts
• Gallbladder diseases include cholelithiasis and acute and chronic cholecystitis.
• Gallstone formation is a common condition in Western countries. The great majority of the gallstones are cholesterol stones. Pigmented stones containing bilirubin and calcium are most common in Asian countries.
• Risk factors for the development of cholesterol stones are advancing age, female gender, estrogen use, obesity, and heredity.
• Cholecystitis almost always occurs in association with cholelithiasis, although in about 10% of cases it occurs in the absence of gallstones.
• Acute calculous cholecystitis is the most common reason for emergency cholecystectomy.
• Obstructive lesions of the extrahepatic bile ducts in adults can give rise to ascending infection (cholangitis) and secondary biliary cirrhosis.
• Infants born with congenital biliary atresia present with neonatal cholestasis and require liver transplantation for cure.
Tumors
Carcinoma of the Gallbladder
Although uncommon, carcinoma of the gallbladder is the most frequent malignant tumor of the biliary tract. It is 2 to 6 times more common in women and occurs most frequently in the seventh decade of life. Carcinoma of the gallbladder is more frequent in the populations of Mexico and Chile, presumably due to the higher incidence of gallstone disease in these regions. In the United States the incidence is highest in Hispanics and Native Americans. Only rarely is it discovered at a resectable stage, and the mean 5-year survival rate is a dismal 5%. Gallstones are present in 60% to 90% of cases. In Asia, where pyogenic and parasitic diseases of the biliary tree are more common, gallstones are less important. Presumably, gallbladders containing stones or infectious agents develop cancer as a result of recurrent trauma and chronic inflammation. The role of carcinogenic derivatives of bile acids is unclear.
![]() Morphology
Morphology
Cancers of the gallbladder may exhibit exophytic or infiltrating growth patterns. The infiltrating pattern is more common and usually appears as a poorly defined area of diffuse thickening and induration of the gallbladder wall that may cover several square centimeters or involve the entire gallbladder. These tumors are scirrhous and very firm. The exophytic pattern grows into the lumen as an irregular, cauliflower-like mass but at the same time also invades the underlying wall (Fig. 15–37). Most are adenocarcinomas, which may be papillary or poorly differentiated. About 5% are squamous cell carcinomas or demonstrate adenosquamous differentiation, and rare neuroendocrine tumors also occur. By the time gallbladder cancers are discovered, most have invaded the liver or have spread to the bile ducts or to the portal hepatic lymph nodes.
Clinical Features
Preoperative diagnosis of carcinoma of the gallbladder is the exception, being reported in less than 20% of patients. Onset of symptoms is insidious, and presenting manifestations typically are indistinguishable from those associated with cholelithiasis: abdominal pain, jaundice, anorexia, and nausea and vomiting. The fortunate person develops early obstruction and acute cholecystitis or undergoes cholecystectomy for coexistent symptomatic gallstones before the tumor spreads to other sites.
Cholangiocarcinomas
Cholangiocarcinomas are adenocarcinomas that arise from cholangiocytes lining the intrahepatic and extrahepatic biliary ducts. Extrahepatic cholangiocarcinomas constitute approximately two thirds of these tumors and may develop at the hilum (known as Klatskin tumors) or more distally in the biliary tree. Cholangiocarcinomas occur mostly in persons of 50 to 70 years of age. Because both intra- and extrahepatic cholangiocarcinomas generally are asymptomatic until they reach an advanced stage, the prognosis is poor, and most patients have unresectable tumors. Risk factors include primary sclerosing cholangitis, fibropolycystic diseases of the biliary tree, and infestation by Clonorchis sinensis or Opisthorchis viverrini.
All risk factors for cholangiocarcinomas cause chronic cholestasis and inflammation, which presumably promote the occurrence of somatic mutations in cholangiocytes. Several consistent genetic changes have been noted in these tumors, including activating mutations in the KRAS and BRAF oncogenes and loss-of-function mutations in the TP53 tumor suppressor gene.
![]() Morphology
Morphology
Cholangiocarcinomas are typical adenocarcinomas with more or less well-formed glands often accompanied by abundant fibrous stroma (desmoplasia) yielding a firm, gritty consistency (Fig. 15–38). Bile pigment and hyaline inclusions are absent from the tumor cells, while intracellular mucin may be prominent.
Figure 15–38 Cholangiocarcinoma.
A, Massive neoplasm in the right lobe and widespread intrahepatic metastases. B, Tumor cells forming glandular structures surrounded by dense sclerotic stroma.
Because partial or complete obstruction of bile ducts rapidly leads to jaundice, extrahepatic biliary tumors tend to be relatively small at the time of diagnosis, whereas intrahepatic tumors may cause symptoms only when much of the liver is replaced by tumor. Cholangiocarcinomas may spread to extrahepatic sites such as regional lymph nodes, lungs, bones, and adrenal glands. Invasion along peribiliary nerves is another route of spread to the abdomen. Cholangiocarcinoma has a greater propensity for extrahepatic spread than does hepatocellular carcinoma.
Clinical Features
Intrahepatic cholangiocarcinoma may be manifested by the presence of a liver mass and nonspecific signs and symptoms such as weight loss, pain, anorexia, and ascites. Symptoms and signs arising from extrahepatic cholangiocarcinomas (jaundice, acholic stools, nausea and vomiting, and weight loss) result from biliary obstruction. Commonly associated findings include elevated serum levels of alkaline phosphatase and aminotransferases. Surgical resection is the only treatment available, but in a large majority of cases is not curative. Transplantation is contraindicated. Mean survival times range from 6 to 18 months, regardless of whether aggressive resection or palliative surgery is performed.
Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep. 2011;13:56.
Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet. 2010;376:190.
Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Focal nodular hyperplasia, hepatocellular adenomas: past, present, future. Gastroenterol Clin Biol. 2010;34:355. [From the pioneers of the new, molecular diagnostics of benign liver tumors.]
Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev. Gastroenterol Hepatol. 2010;7:195. [As authoritative as one can be on the topic.]
Chun LJ, Tong MJ, Busuttil RW, et al. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342. [About the most common cause of acute liver failure leading to transplantation.]
Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655. [As authoritative as one can be on the topic.]
Gatto M, Alvaro D. New insights on cholangiocarcinoma. World J Gastrointest Oncol. 2010;2:136.
Gouw ASW, Clouston AD, Theise ND. Ductular reactions in human livers: diversity at the interface. Hepatology. 2011;54:1853. [A review of ductular reactions, the stem cell response of human livers in all liver diseases, that are related to mechanisms of regeneration, fibrogenesis and neoplasia.]
Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481.
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma. Hepatology. 2009;49:658. [A good example of how change comes to medicine, individual efforts combining, over years, to achieve a new consensus.]
Joyce MA, Tyrrell DL. The cell biology of hepatitis C virus. Microbes Infect. 2010;12:263.
Lai M, Liaw YF. Chronic hepatitis B: past, present, and future. Clin Liver Dis. 2010;14:531.
Lagana SM, Moreira RK, Lefkowitch JH. Hepatic granulomas: pathogenesis and differential diagnosis. Clin Liver Dis. 2010;14:605.
Paumgartner G. Biliary physiology and disease: reflections of a physician-scientist. Hepatology. 2010;51:1095. [How bench top work comes to exert an impact on clinical medicine, sometimes, slowly, over decades.]
Perrault M, Pécheur EI. The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership. Biochem J. 2009;423:303.
Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393.
Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745.
Schilsky ML. Wilson disease: current status and the future. Biochimie. 2009;91:1278.