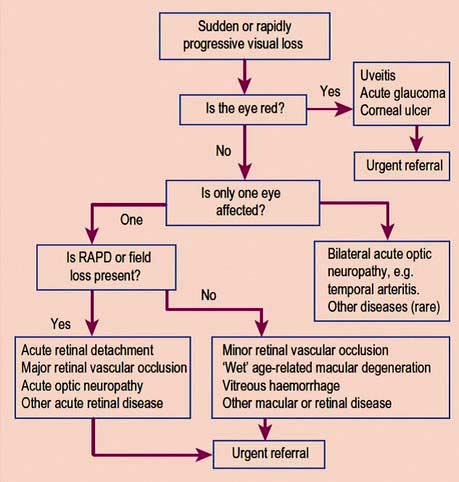
Chapter 21 The special senses
Disorders of the ear, nose and throat
The Ear
Anatomy and physiology
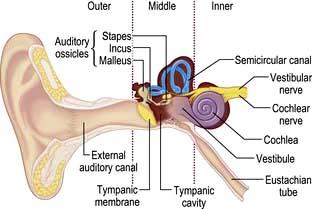
The ear can be divided into three parts: outer, middle and inner (Fig. 21.1).
The outer ear has a skin-lined tube 2.5 cm long leading down to the tympanic membrane (the ear drum). Its outer third is cartilaginous and contains hair, sebaceous and ceruminous glands, but the walls of the inner two-thirds are bony. The outer ear is self-cleaning as the skin is migratory and there are no indications to use cotton wool buds. Wax should only be seen in the outer third.
The middle ear is an air-containing cavity derived from the branchial clefts. It communicates with the mastoid air cells superiorly, and the Eustachian tube connects it to the nasopharynx medially. The Eustachian tube ventilates the middle ear and maintains equal air pressure across the tympanic membrane. It is normally closed but opens via the action of the palatal muscles to allow air entry when swallowing or yawning. A defect in this mechanism, such as with a cleft palate, will prevent air entering the middle ear cleft which may then fill with fluid. Lying within the middle ear cavity are the three ossicles (malleus, incus and stapes) that transmit sound from the tympanic membrane to the inner ear. On the medial wall of the cavity is the horizontal segment of the facial nerve, which can be damaged during surgery or by direct extension of infection in the middle ear.
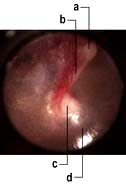
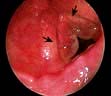
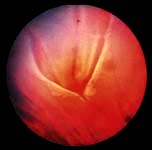
Normal tympanic membrane. a, Lateral process of malleus. b, Long process of malleus. c, Umbo. d, Light reflex.
The inner ear contains the cochlea for hearing and the vestibule and semicircular canals for balance. There is a semicircular canal arranged in each body plane and these are stimulated by rotatory movement. The facial, cochlear and vestibular nerves emerge from the inner ear and run through the internal acoustic meatus to the brainstem (see Fig. 22.7, p. 1076).
Physiology of hearing
The ossicles, in the middle ear, transmit sound waves from the tympanic membrane to the cochlea. They amplify the waves by about 18-fold to compensate for the loss of sound waves moving from the air-filled middle ear to the fluid-filled cochlea. Hair cells in the basilar membrane of the cochlea detect the vibrations and transduce these into nerve impulses which pass to the cochlear nucleus and then eventually to the superior olivary nuclei of both sides; thus lesions central to the cochlear nucleus do not cause unilateral hearing loss.
If the ossicles are diseased, sound can also reach the cochlea by vibration of the temporal bone (bone conduction).
Examination
The pinna and postauricular region should first be examined for scars or swellings. An auroscope is used to examine the external ear canal whilst the pinna is retracted backwards and upwards to straighten the canal. Look for wax, discharge or foreign bodies. The tympanic membrane should always be seen with a light reflex anteroinferiorly. Previous repeated infections may cause a thickened, whitish drum but fluid in the middle ear may show as dullness of the drum. Perforations are marginal or central.
Common disorders
The discharging ear (otorrhoea)
Discharge from the ear is usually due to infection of the outer or middle ear.
This is a diffuse inflammation of the skin of the ear canal. The cause is bacterial, viral or fungal and the patient usually complains of severe pain. Gentle pulling of the pinna is tender and there may be lymphadenopathy of the preauricular nodes.
Examination often reveals debris in the canal which needs to be removed either by gentle mopping or preferably by suction viewed directly under a microscope. In severe cases the canal may be swollen and a view of the tympanic membrane impossible. Any foreign body seen should be removed with great care by trained personnel.
Treatment is with topical antibiotics in the first instance (drops such as dexamethasone 0.05%, framycetin sulphate 0.5%, gramicidin 0.005% drops or hydrocortisone acetate 1%, gentamicin 0.3%, or a spray such as dexamethasone 0.1%, neomycin sulphate 3250 units). If it does not resolve in 3–4 days then microsuction in an ENT department is necessary.
Otitis media can also present with discharge from the middle ear through a perforation of the tympanic membrane. There are no mucous glands in the external ear canal, however; if the discharge is serous, then middle ear pathology is unlikely.
Treatment for the acute case is initially with non-steroidal anti-inflammatory drugs then, if it is not resolving, with systemic antibiotics.
Cholesteatoma
Cholesteatoma is defined as keratinizing squamous epithelium within the middle ear cleft and can present with foul-smelling otorrhoea. Examination shows a defect in the tympanic membrane full of white cheesy material. Mastoid surgery is required to remove this sac of squamous debris as it can erode local structures such as the facial nerve or even extend intracranially.
Hearing loss
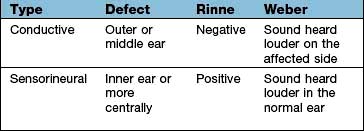
Deafness can be conductive or sensorineural and these can be differentiated at the bedside by the Rinne and the Weber tests (Box 21.1) or with pure-tone audiometry. Conductive hearing loss has many causes (Table 21.1) but wax is the commonest.
| Conductive | Sensorineural |
|---|---|
External meatus |
Congenital |
WaxForeign bodyOtitis externaChronic suppurationDrum |
Pendred’s syndrome (see p. 962) |
Long QT syndrome |
|
Björnstad’s syndrome (pili torti) |
|
End organ |
|
Advancing ageOccupational acoustic traumaMénière’s diseaseDrugs (e.g. gentamicin, furosemide) |
|
Perforation/trauma |
|
Middle ear |
|
Otosclerosis |
|
Eighth nerve lesions |
|
Acoustic neuroma |
|
|
Cranial trauma |
|
Inflammatory lesions: |
|
Tuberculous meningitis |
|
Sarcoidosis |
|
Neurosyphilis |
|
Carcinomatous meningitis |
|
Brainstem lesions (rare) |
|
Multiple sclerosis |
|
Infarction |
 Normally a tuning fork, 512 Hz, will be heard as louder if held next to the ear (i.e. air conduction) than it will if placed on the mastoid bone (Rinne positive).
Normally a tuning fork, 512 Hz, will be heard as louder if held next to the ear (i.e. air conduction) than it will if placed on the mastoid bone (Rinne positive).
 If the tuning fork is perceived louder when placed on the mastoid (i.e. via bone conduction), then a defect in the conducting mechanism of the external or middle ear is present (true Rinne negative).
If the tuning fork is perceived louder when placed on the mastoid (i.e. via bone conduction), then a defect in the conducting mechanism of the external or middle ear is present (true Rinne negative).
A tuning fork placed on the forehead or vertex of a patient with normal hearing (or with symmetrical hearing loss) should be perceived centrally by the patient.
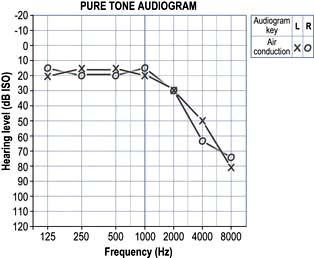
The patient is asked to respond to a series of pure tones presented to each ear, in turn, in a soundproof room. An audiogram is produced (see Fig. 21.2).
Perforated tympanic membrane
This arises from trauma or chronic middle ear disease where recurrent infection results in a permanent defect. Surgical repair is only indicated if the patient is symptomatic with hearing loss or recurrent discharge.
Otitis media
This is an acute inflammation of the middle ear, causing severe pain (otalgia) and conductive hearing loss. This occurs because fluid accumulation in the middle ear impairs sound conduction to the cochlea. Otitis media is often viral in origin, e.g. following a cold, and will settle within 72 hours without antibacterial treatment. In people with systemic features or after 72 hours, a systemic antibiotic, e.g. amoxicillin, should be given, particularly in the under 2 year olds. Topical therapy is of no value. Complications include infection of the mastoid bone.
Acute otitis media can spread to the mastoid area. If there is tenderness and swelling over the mastoid then an urgent ENT opinion should be obtained.
Secretory otitis media with effusion (‘serous otitis media’ or ‘glue ear’)
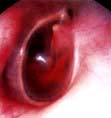
Secretory otitis media (glue ear). Auroscopic view of the tympanic membrane, which is dull with loss of light reflex.
This is common in children because of Eustachian tube dysfunction. The effusion resolves naturally in the majority of cases but can persist giving hearing loss, and it predisposes to recurrent attacks of acute otitis media. A grommet (tympanostomy tube) is inserted into the tympanic membrane and ventilates the middle ear cavity, i.e. takes over the Eustachian tube’s function. Grommets are extruded from the tympanic membrane as it heals (over 6 months to 2 years). Developmental outcomes are not improved by grommet insertions. In most children the middle third of the face grows around the age of 7–14 years and Eustachian tube dysfunction is rare after this.
Otosclerosis
This is usually a hereditary disorder in which new bony deposits occur within the stapes footplate and the cochlea. Characteristically seen in the second and third decades, it is commoner in females and can become worse during pregnancy. The hearing loss may be mixed, and treatment includes a hearing aid or replacement of the fixed stapes with a prosthesis (stapedectomy). The choice of treatment is dependent on the patient. Surgery is an excellent option, with very good success rates in regular stapedectomists’ hands, but it always carries a small risk of a complete hearing loss. Hearing aids, whilst safe, require the patient’s compliance if they are to afford benefit.
Presbycusis
This is the commonest cause of deafness. It is a degenerative disorder of the cochlea and is typically seen in old age. It can be due to the loss of outer hair cells (sensory), loss of the ganglion cells (neural), strial atrophy (metabolic) or it can be a mixed picture. Ageing itself does not cause outer hair cell loss but environmental noise toxicity over the years is a major factor. The onset is gradual and the higher frequencies are affected most (Fig. 21.2). Speech has two components: low frequencies (vowels) and high frequencies (consonants). When the consonants are lost, speech loses its intelligibility. Increasing the volume merely increases the low frequencies and the characteristic response of ‘Don’t shout. I’m not deaf!’
A high-frequency-specific hearing aid will do much to ease the frustrations of both the patients and their close contacts.
Noise trauma
Cochlear damage can occur, e.g. when shooting without ear protectors or from industrial noise (see p. 935), and characteristically has a loss at 4 kHz.
Acoustic neuroma
This is a slow-growing benign schwannoma of the vestibular nerve (see p. 1076) which can present with progressive sensorineural hearing loss. Any patient with an asymmetric sensorineural hearing loss or sudden sensorineural hearing loss should be investigated, e.g. with an MRI scan.
Vertigo
Vertigo is usually rotatory when it arises from the ear. The presence of otalgia, otorrhoea, tinnitus or hearing loss suggests an otologic aetiology. Vestibular causes can be classified according to the duration of the vertigo. Common causes are summarized below.
Benign paroxysmal positional vertigo (BPPV)
BPPV is thought to be due to loose otoliths in the semicircular canals, commonly the posterior canal. Positional vertigo is precipitated by head movements, usually to a particular position, and often occurs when turning in bed or on sitting up. The onset is typically sudden and distressing. The vertigo lasts seconds or minutes and the phenomenon becomes less severe on repeated movements (fatigue). There is no serious underlying cause but it sometimes follows vestibular neuronitis (see p. 1079), head injury or ear infection.
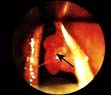
Diagnosis is made on the basis of the history and by the Hallpike manoeuvre (Fig 21.3). A positive Hallpike test confirms BPPV, which can be cured in over 90% of cases by the Epley manoeuvre. This involves gentle but specific manipulation and rotation of the patient’s head to shift the loose otoliths from the semicircular canals.
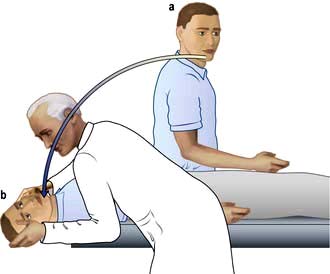
Figure 21.3 Hallpike manoeuvre for diagnosis of benign paroxysmal positional vertigo. This can be done in the outpatient department. (a) The patient sits on a couch, his head is turned towards one ear. (b) The head is supported by the examiner while the patient lies down so that his head is just below the horizontal. Nystagmus (following a latent interval of a few seconds) is commonly noted when the head has been turned towards the affected ear. This can be repeated with the patient’s head turned towards the other ear.
A differential diagnosis is a cerebellar mass, but here positional nystagmus (and vertigo) is immediately apparent (no latent interval) and does not fatigue.
Ménière’s disease
This condition is characterized by recurring episodic rotatory vertigo lasting 30 minutes to a few hours; attacks are recurrent over months or years. Classically, it is associated with a low frequency sensorineural hearing loss, feeling of fullness in the affected ear, loss of balance, tinnitus and vomiting. There is a build-up of endolymphatic fluid in the inner ear, although its precise aetiology is still unclear.
Vestibular sedatives, e.g. cinnarizine, are used in the acute phase. Preventative measures, such as a low-salt diet, betahistine and avoidance of caffeine, are useful. If the disease cannot be controlled, then a chemical labyrinthectomy, perfusing the round window orifice with ototoxic drugs such as gentamicin, is used. Gentamicin destroys the vestibular epithelium; therefore the patient has severe vertigo for around 2 weeks until the body compensates for the lack of vestibular input on that side. The patient will happily trade occasional mild vertigo when the balance system is challenged to the unpredictable, severe and disabling attacks of vertigo of Ménière’s disease.
Labyrinthine or central causes of vertigo (see Table 22.4)
These are managed with vestibular sedatives in the acute phase. Most patients will settle over a few days but continuous true vertigo with nystagmus suggests a central lesion. A patient with a deficit of vestibular function due to viral labyrinthitis or neuronitis should be able to cease vestibular sedatives within 2 weeks; long-term use can give parkinsonian side-effects, delay central compensation and thus prolong the vertigo. Vestibular rehabilitation by a physiotherapist or audiological scientist can speed up the compensation process, although most patients will be able to do this themselves with time.
Tinnitus
This is a sensation of a sound when there is no auditory stimulus. It can occur without hearing loss and results from heightened awareness of neural activity in the auditory pathways. Patients describe a hissing or ringing in their ears and this can cause much distress. It usually does not have a serious cause but vascular malformation, e.g. aneurysms, or vascular tumours can be associated. Tinnitus occurs due to awareness of neural activity in the auditory pathways that our brains are made more conscious of.
The nose
Anatomy and physiology (Fig. 21.4)
The function of the nose is to facilitate smell and respiration:
 Smell is a sensation conveyed by the olfactory epithelium in the roof of the nose. The olfactory epithelium is supplied by the first cranial nerve (see p. 1071).
Smell is a sensation conveyed by the olfactory epithelium in the roof of the nose. The olfactory epithelium is supplied by the first cranial nerve (see p. 1071).
 The nose also filters, moistens and warms inspired air and in doing so assists the normal process of respiration.
The nose also filters, moistens and warms inspired air and in doing so assists the normal process of respiration.
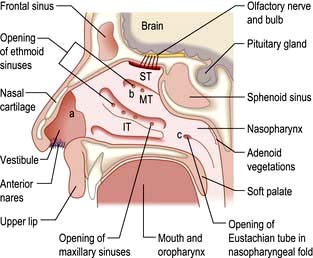
Figure 21.4 The anatomy of the nose in longitudinal section. IT, inferior turbinate; MT, middle turbinate; ST, superior turbinate; a, internal ostium; b, respiratory region; c, choanae.
The external portion of the nose consists of two nasal bones attached to the rest of the facial skeleton and to the upper and lower lateral cartilages. The internal nose is divided by a midline septum that comprises both cartilage and bone. This divides the internal nose in two, from the external nostril to the posterior choanae. The posterior choanae are in continuity with the nasopharynx posteriorly. The paranasal sinuses open into the lateral wall of the nose and are a system of aerated chambers within the facial skeleton.
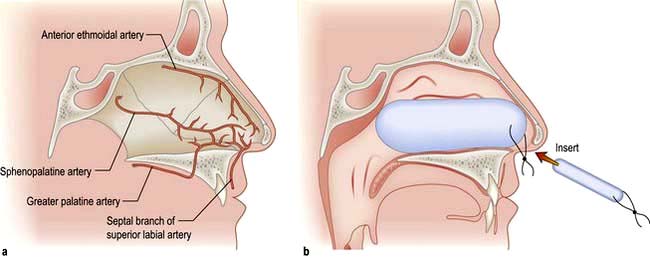
The blood supply of the nose is derived from branches of both the internal and external carotid arteries. The internal carotid artery supplies the upper nose via the anterior and posterior ethmoidal arteries. The external carotid artery supplies the posterior and inferior portion of the nose via the superior labial artery, greater palatine artery and sphenopalatine artery. On the anterior nasal septum is an area of confluence of these vessels (Little’s area) (Fig. 21.5a).
Examination
The anterior part of the nose can be examined using a nasal speculum and light source. Endoscopes are required to examine the nasal cavity and postnasal space.
Common disorders
Epistaxis
Nose bleeds vary in severity from minor to life-threatening. Little’s area (Fig. 21.5a) is a frequent site of nasal haemorrhage. First aid measures should be administered immediately, including external digital compression of the anterior lower portion of the external nose, ice packs and leaning forward. The patient should be asked to avoid swallowing any blood running posteriorly as this causes gastric irritation and then nausea and vomiting.
Not infrequently, small recurrent epistaxes occur and these may require a visit to the emergency clinic for an examination and simple local anaesthetic cautery with a silver nitrate stick. If the bleeding continues profusely then resuscitation in the form of intravenous access, fluid replacement or blood, and oxygen can be administered. If further intervention is necessary, consideration should be given to intranasal cautery of the bleeding vessel, or intranasal packing using a variety of commercially available nasal packs (Fig. 21.5b). In addition to direct treatment of the epistaxis, a cause and appropriate treatment of a cause should be sought (Table 21.2).
Table 21.2 Aetiology of epistaxis
Local |
Idiopathic |
Trauma – foreign bodies, nose-picking and nasal fractures |
|
Iatrogenic – surgery, intranasal steroids |
|
Neoplasm – nasal, paranasal sinus and nasopharyngeal tumours |
|
General |
Anticoagulants |
Coagulation disorders |
|
Hypertension |
|
Osler–Weber–Rendu syndrome (familial haemorrhagic telangiectasia) |
Nasal obstruction
Nasal obstruction is a symptom and not a diagnosis. It can significantly affect a patient’s quality of life. Causes include:
 Rhinitis (see p. 808). If an allergen is identified, then allergen avoidance is the mainstay of treatment. Topical steroids and/or antihistamines can be tried. If severe, then oral antihistamines or referral to an allergy clinic for immunotherapy is warranted.
Rhinitis (see p. 808). If an allergen is identified, then allergen avoidance is the mainstay of treatment. Topical steroids and/or antihistamines can be tried. If severe, then oral antihistamines or referral to an allergy clinic for immunotherapy is warranted.
 Septal deviation. Correction of this deviation can be undertaken surgically.
Septal deviation. Correction of this deviation can be undertaken surgically.
 Nasal polyps. This condition occurs with inflammation and oedema of the sinus nasal mucosa. This oedematous mucosa prolapses into the nasal cavity and can cause significant nasal obstruction. In allergic rhinitis (see p. 798) the mucosa lining the nasal septum and inferior turbinates are swollen and a dark red or plum colour. Nasal polyps can be identified as glistening swellings which are not tender. Treatment with intranasal steroids helps but if polyps are large or unresponsive to medical treatment then surgery is necessary.
Nasal polyps. This condition occurs with inflammation and oedema of the sinus nasal mucosa. This oedematous mucosa prolapses into the nasal cavity and can cause significant nasal obstruction. In allergic rhinitis (see p. 798) the mucosa lining the nasal septum and inferior turbinates are swollen and a dark red or plum colour. Nasal polyps can be identified as glistening swellings which are not tender. Treatment with intranasal steroids helps but if polyps are large or unresponsive to medical treatment then surgery is necessary.
 Foreign bodies. These are usually seen in children who present with unilateral nasal discharge. Clinical examination of the nose with a light source often reveals the foreign body, which requires removal either in clinic or in theatre with a general anaesthetic.
Foreign bodies. These are usually seen in children who present with unilateral nasal discharge. Clinical examination of the nose with a light source often reveals the foreign body, which requires removal either in clinic or in theatre with a general anaesthetic.
 Sinonasal malignancy. This is extremely rare. The diagnosis must be considered if unusual unilateral symptoms are seen, including nasal obstruction, epistaxis, pain, epiphora, cheek swelling, paraesthesia of the cheek and proptosis of the orbit.
Sinonasal malignancy. This is extremely rare. The diagnosis must be considered if unusual unilateral symptoms are seen, including nasal obstruction, epistaxis, pain, epiphora, cheek swelling, paraesthesia of the cheek and proptosis of the orbit.
Sinusitis
Sinusitis is an infection of the paranasal sinuses that either is bacterial (mainly Streptococcus pneumoniae and Haemophilus influenzae) or is occasionally fungal. It is most commonly associated with an upper respiratory tract infection and can occur with asthma. Symptoms include frontal headache, purulent rhinorrhoea, facial pain with tenderness and fever. It can be confused with a variety of other conditions such as migraine, trigeminal neuralgia and cranial arteritis.
Treatment for a bacterial sinusitis includes nasal decongestants, e.g. xylometazoline, broad-spectrum antibiotics, e.g. co-amoxiclav because H. influenzae can be resistant to amoxicillin, anti-inflammatory therapy with topical corticosteroids such as fluticasone propionate (nasal spray) to reduce mucosal swelling, and steam inhalations.
If the symptoms of sinusitis are recurrent (Box 21.2) or complications such as orbital cellulitis arise, then an ENT opinion is appropriate and a CT scan of the paranasal sinuses is undertaken. Plain sinus X-rays are now rarely used to image the sinuses.
![]() Box 21.2
Box 21.2
Types of sinusitis
Acute |
Symptoms lasting 1 week to 1 month |
Recurrent acute |
>4 episodes of acute sinusitis per year |
Subacute |
Symptoms for 1–3 months |
Chronic |
Symptoms for >3 months |
CT scan of the sinuses or an MRI scan can demonstrate bony landmarks and soft tissue planes.
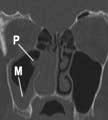
CT of the sinuses showing an inverting papilloma (P) obstructing the right nostril and mucosal thickening (M) of the right maxillary sinus.
Functional endoscopic sinus surgery (FESS) is used for ventilation and drainage of the sinuses.
Anosmia
Olfaction is mainly under the control of cranial nerve I, although irritant, unpleasant nasal sensations are carried by cranial nerves V, IX and X. Anosmia is a complete loss of the sense of smell and hyposmia is a decreased sense of smell:
 A conductive deficit of smell occurs if odorant molecules do not reach the olfactory epithelium high in the nose.
A conductive deficit of smell occurs if odorant molecules do not reach the olfactory epithelium high in the nose.
 A sensorineural loss of smell is incurred if the neural transmission of smell is affected.
A sensorineural loss of smell is incurred if the neural transmission of smell is affected.
 Some conditions predispose to a mixed (conductive and sensorineural) loss of smell.
Some conditions predispose to a mixed (conductive and sensorineural) loss of smell.
The main cause of a loss of smell is nasal obstruction due to upper respiratory infection or nasal polyps. Other causes include sinonasal disease, old age, drug therapy and head injury/trauma. It is difficult to predict the speed and extent of recovery in the latter causes. In many patients anosmia is idiopathic, but before this diagnosis is accepted an assessment of the patient for the possibility of an intranasal tumour or intracranial mass should be undertaken.
Fractured nose
People with a fractured nose present with epistaxis, bruising of the eyes and nasal bridge swelling. Initially, it is often difficult to assess if the bones are deviated, particularly if there is significant swelling. Reduction of the fracture should be undertaken in the first 2 weeks after injury and can be achieved by manipulation. However, if the fracture sets, a more formal rhinoplasty may have to be undertaken at a later stage. The patient should be examined for a head injury and the nose should also be checked for a septal haematoma. This is painful, can cause nasal obstruction, is fluctuant to touch on the nasal septum and requires immediate drainage.
The throat
Anatomy and physiology
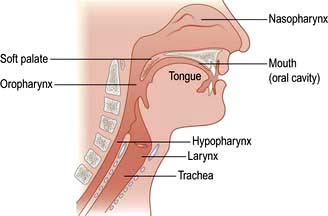
The throat can be considered as the oral cavity, the pharynx and the larynx (Fig. 21.6). The oral cavity extends from the lips to the tonsils. The pharynx can be divided into three areas:
 The nasopharynx: extending from the posterior nasal openings to the soft palate
The nasopharynx: extending from the posterior nasal openings to the soft palate
 The oropharynx: extending from the soft palate to the tip of the epiglottis
The oropharynx: extending from the soft palate to the tip of the epiglottis
 The hypopharynx: extending from the tip of the epiglottis to just below the level of the cricoid cartilage where it is continuous with the oesophagus.
The hypopharynx: extending from the tip of the epiglottis to just below the level of the cricoid cartilage where it is continuous with the oesophagus.
Lying within the hypopharynx is the larynx. This consists of cartilaginous, ligamentous and muscular tissue with the primary function of protecting the distal airway. The pharynx is innervated from the pharyngeal plexus.
In the larynx, there are two vocal cords which abduct (open) during inspiration and adduct (close) to protect the airway and for voice production (phonation). The main nerve supply of the vocal cords comes from the recurrent laryngeal nerves (branches of the vagus nerve) which arise in the neck, but on the left side passes down around the aortic arch and then ascends in the tracheo-oesophageal groove to the larynx.
Normal vocal cords in phonation vibrate between 90 (male) and 180 (female) times per second, giving the voice its pitch or frequency. A healthy voice requires full closure of the vocal cords with a smooth, regular pattern of vibration, and any pathology that prevents full closure will result in air escaping between the vocal cords during phonation and a ‘breathy’ voice.
Examination
Good illumination is essential. Look at teeth, gums, tongue, floor of mouth and oral cavity. Tonsils, soft palate and uvula are easily seen, and a gag reflex (see p. 1080) is present. The remainder of the pharynx and larynx can be inspected with a laryngeal mirror or flexible nasendoscope.
Examination of the neck for lymph nodes and other masses is also performed.
Common disorders
Hoarseness (dysphonia)
There are three essential components for voice production: an air source (the lungs); a vibratory source (the vocal cords); and a resonating chamber (the pharynx, the nasal and oral cavities). Although chest and nasal disorders can affect the voice, the majority of hoarseness is due to laryngeal pathology.
Inflammation which increases the ‘mass’ of the vocal cords will cause the vocal cord frequency to fall, giving a much deeper voice. Thus listening to a patient’s voice can often give a diagnosis before the vocal cords are examined.
Nodules
Nodules (always bilateral and commoner in females) and polyps are found on the free edge of the vocal cord preventing full closure and giving a ‘breathy, harsh’ voice. They are commonly found in professions that rely on their voice for their livelihood, such as teachers, singers and lawyers. They are usually related to poor technique of voice production and can usually be cured with speech therapy. If surgery is needed, great care must be taken to remain in the superficial layers of the vocal cord in order to prevent deep scarring which leaves the voice permanently hoarse.
Reinke’s oedema
This is due to a collection of tissue fluid in the subepithelial layer of the vocal cord. The vocal cord has poor lymphatic drainage, predisposing it to oedema. Reinke’s oedema is associated with irritation of the vocal cords with smoking, voice abuse, acid reflux and very rarely hypothyroidism. Treatment is to remove the irritation in most cases but surgery to incise the cords and reduce the oedema will also allow the voice to return to its normal pitch.
Acute-onset hoarseness
Hoarseness, in a smoker, is a danger sign. Any patient with a hoarse voice for over 6 weeks should be seen by an ENT surgeon. Other red flag symptoms are also to be enquired about and will require urgent laryngoscopy (Emergency Box 21.1). The voice may be deep, harsh and breathy indicating a mass on the vocal cord or it can be weak suggesting a paralysed left vocal cord secondary to mediastinal disease, e.g. bronchial carcinoma.
Early squamous cell carcinoma of the larynx has a good prognosis. Treatment is with carbon dioxide laser resection or radiotherapy. Spread and growth of the tumour can lead to referred otalgia which if significant in its size requires a laryngectomy (removal of the voicebox) with a neck dissection to remove the affected glands in the neck. A patient with a paralysed left vocal cord must have a CT of the neck and chest. Medialization of the paralysed cord to allow contact with the opposite cord can return the voice and give a competent larynx. This can be done under local anaesthesia, giving an immediate result whatever the long-term prognosis of the chest pathology.
Stridor
Stridor or noisy breathing can be divided into:
 Inspiratory: obstruction is at the level of the vocal cords or above
Inspiratory: obstruction is at the level of the vocal cords or above
 Mixed (both inspiratory and expiratory): obstruction is in the subglottis or extrathoracic trachea
Mixed (both inspiratory and expiratory): obstruction is in the subglottis or extrathoracic trachea
 Expiratory: obstruction is in the intrathoracic trachea or distal airways.
Expiratory: obstruction is in the intrathoracic trachea or distal airways.
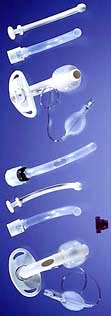
All people with stridor, both paediatric and adult, are potentially at risk of asphyxiation and should be investigated fully. Severe stridor may be an indication for either intubation or a tracheostomy (Table 21.3).
Table 21.3 Indications for tracheostomy
 Cuffed or uncuffed. A high-volume, low-pressure cuff is used to prevent aspiration and to allow positive-pressure ventilation.
Cuffed or uncuffed. A high-volume, low-pressure cuff is used to prevent aspiration and to allow positive-pressure ventilation.
 Fenestrated or unfenestrated. A fenestrated cuff has a small hole on the greater curvature of the tube (both outer and inner) allowing air to escape upwards to the vocal cords and therefore the patient can speak. This tube often has a valve which allows air to enter from the stoma but closes on expiration, directing the air through the fenestration.
Fenestrated or unfenestrated. A fenestrated cuff has a small hole on the greater curvature of the tube (both outer and inner) allowing air to escape upwards to the vocal cords and therefore the patient can speak. This tube often has a valve which allows air to enter from the stoma but closes on expiration, directing the air through the fenestration.
Most long-term tracheostomy tubes have an inner and outer tube. The inner tube fits inside the outer tube and projects beyond its lower end. A major problem with a tracheostomy tube is crusting of its distal end with dried secretions, and this arrangement allows the inner tube to be removed, cleaned and replaced as frequently as required, without disrupting the outer tube.
When to decannulate a patient is often a difficult issue if laryngeal competence is unclear. Movement of the vocal cords requires an ENT examination but owing to the risk of aspiration, a speech therapist’s opinion can also be useful. The tracheostomy tube itself can also give problems due to compression of the oesophagus with a cuffed tube and by preventing the larynx from rising during normal swallowing.
Tonsillitis and pharyngitis
Viral infections of the throat are common and, although many practitioners are under pressure from the patient to give antibiotics, the vast majority are usually self-limiting, settling with bed rest, analgesia and encouraging fluid intake. Fungal infections, usually candidiasis, are uncommon and may indicate an immunocompromised patient or undiagnosed diabetes.
Tonsillitis
Tonsillitis, with a good history of pyrexia, dysphagia, lymphadenopathy and severe malaise, is usually bacterial with β-haemolytic streptococcus the commonest organism.
Glandular fever (see p. 99)
This can also present with tonsillitis. Although clinically the tonsils have a white exudate, there is often a petechial rash on the soft palate and an accompanying lymphadenopathy.
Quinsy
Quinsy is a collection of pus outside the capsule of the tonsil usually located adjacent to its superior pole. The patient often has trismus making examination difficult but the pus pushes the uvula across the midline to the opposite side. The area is usually hyperaemic and smooth but unilateral tonsil ulceration is more likely to be a malignancy. In either case urgent referral to an ENT specialist is essential.
Indications for a tonsillectomy are shown in Table 21.4. This is carried out under a general anaesthetic and current surgical techniques include diathermy dissection, laser excision and coblation (using an ultrasonic dissecting probe). There are strong advocates for each technique and much will depend on the individual surgeon’s preference. Some departments now carry out tonsillectomy as a day case procedure as most reactionary bleeding will occur within the first 8 hours postoperatively.
Table 21.4 Indications for tonsillectomy
Snoring
Snoring is due to vibration of soft tissue above the level of the larynx. It is a common condition (50% of 50-year-old males will snore to some extent) and can be considered to be related to obstruction of three potential areas: the nose, the palate or/and the hypopharynx (see Fig. 15.26).
The Epworth questionnaire (see Table 15.11) can assist in the discrimination of sleep apnoea from simple snoring. People with a history of habitual, non-positional, heroic (can be heard through a wall) snoring require a full ENT examination and can be investigated by sleep nasendoscopy in which a sedated, snoring patient has a flexible nasendoscope inserted to identify the source of vibration.
Nasal pathology such as polyps can be removed surgically with good results and most patients will benefit from lifestyle changes such as weight loss. Stiffening or shortening the soft palate via surgery, often using a laser, can help for palatal snorers but hypopharyngeal snorers require either a dental prosthesis at night to hold the mandible forward or continuous positive airway pressure (CPAP) via a mask (see p. 818).
SIGNIFICANT WEBSITE
Interactive version of the Epworth sleepiness scale: www.britishsnoring.co.uk/sleep_apnoea/epworth_sleepiness_scale.php
FURTHER READING
Dhillon RS, East CA. Ear, Nose and Throat and Head and Neck Surgery, 3rd edn. Edinburgh: Churchill Livingstone; 2006.
Roland NJ, McRae RD, McCombe AW. Key Topics in Otolaryngology, 2nd edn. London: Bios Scientific Publishers; 2001.
Warner G, Burgess A, Patel S et al. Otolaryngology and Head and Neck Surgery, 1st edn. Oxford: Oxford University Press; 2009.
Dysphagia
Difficulty in swallowing is a common symptom but can be the presenting feature of carcinoma of the pharynx and therefore requires investigation. Dysphagia (see p. 237) occurs because of any lesion between the throat and stomach. The two conditions described here are the ones usually dealt with by ENT departments. Gastroenterology departments see causes further down the gullet.
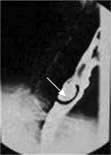
A pharyngeal pouch is a herniation of mucosa through the fibres of the inferior pharyngeal constrictor muscle (cricopharyngeus) (Fig. 21.7a). An area of weakness known as Killian’s dehiscence allows a pulsion diverticulum to form. This will collect food, which may regurgitate into the mouth or even down to the lungs at night with secondary pneumonia. Diagnosis is made with a barium swallow and treatment is surgical, either via an external approach through the neck where the pouch is excised or more commonly endoscopically with stapling of the party wall (Fig. 21.7b).
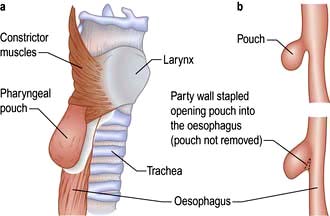
Figure 21.7 Pharyngeal pouch. (a) Position of pharyngeal pouch. (b) Stapling of the party wall between oesophagus and pouch.
Foreign bodies in the pharynx can be divided into three general categories: soft food bolus, coins (smooth), bones (sharp). Soft food bolus can be initially treated conservatively with muscle relaxants for 24 hours. Impacted coins should be removed at the earliest opportunity but sharp objects require emergency removal to avoid perforation of the muscle wall.
If the patient perceives the foreign body to be to one side, then it should be above the cricopharyngeus and an ENT examination will locate it; common areas are the tonsillar fossae, base of tongue, posterior pharyngeal wall and valleculae. Radiology will identify coins, and it can be a clinical decision to see whether a coin will pass down to the stomach, in which case no further treatment is required as it will exit naturally. Some departments advocate the use of a metal detector to monitor the position of the coin in the patient, who is usually a child or has a mental disorder. Fish can be divided into those with a bony skeleton (teleosts) and those with a cartilaginous skeleton (elasmobranchs), and therefore radiology is useful only in some cases. Radiology can also identify air in the cervical oesophagus indicating a radiolucent foreign body lying distally. A soft tissue lateral neck radiograph is the investigation of choice to delineate some of the features above.
Globus pharyngeus
This is a functional disorder and is not a true dysphagia. It is a condition with classic symptoms of an intermittent sensation of a lump in the throat. This is perceived to be in the midline at the level of the cricoid cartilage and is worse when swallowing saliva; indeed it often disappears when ingesting food or liquids. ENT examination is clear and normal laryngeal mobility can be felt when gently rocking the larynx across the postcricoid tissues. A contrast swallow will show not only the structures below the pharynx but also assess the swallowing dynamically. Any suspicious area will require an endoscopy with biopsy.
Disorders of the eye
Most of the major and common types of eye disease are covered below. However, diabetic eye disease and hypertensive eye disease are discussed elsewhere.
Applied anatomy and physiology
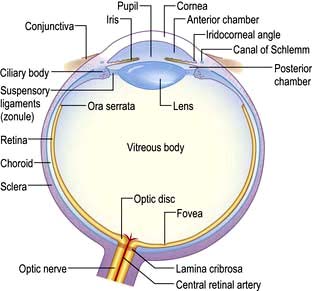
The average length of the human eye is 24 mm. It is essentially made up of two segments:
 The anterior, smaller segment is transparent and coated by the cornea; its radius is approximately 8 mm
The anterior, smaller segment is transparent and coated by the cornea; its radius is approximately 8 mm
 The larger posterior segment is coated by the opaque sclera and is approximately 12 mm in radius.
The larger posterior segment is coated by the opaque sclera and is approximately 12 mm in radius.
It is the cornea and the sclera that give the mechanical strength and shape to the exposed surface of the eye.
The cornea occupies the central aspect of the globe and is one of the most richly innervated tissues in the body. This clear, transparent and avascular structure, measuring 12 mm horizontally and 11 mm vertically, provides 78% of the focusing power of the eye. The eyelids prevent the cornea from drying and becoming an irregular surface by distributing the tear film over the surface of the globe with each blink.
Anatomically the cornea is made up of five layers:
The endothelial cells lining the inner surface of the cornea are responsible for maintaining the clarity of the cornea by continuously pumping fluid out of the tissue. Any factor which alters the function of these cells will result in corneal oedema and cause blurred vision.
The sclera is a white opaque structure covering four-fifths of the globe and is continuous with the cornea at the limbus. The six extraocular muscles responsible for eye movements are attached to the sclera and the optic nerve perforates it posteriorly.
The conjunctiva covers the anterior surface of the sclera. This richly vascularized and innervated mucous membrane stretches from the limbus over the anterior sclera (where it is called the bulbar conjunctiva) and is then reflected onto the undersurface of the upper and lower lids (the tarsal conjunctiva). The area of conjunctival reflection under the lids makes up the upper and lower fornix.
The anterior chamber is the space between the cornea and the iris, and is filled with aqueous humour (Fig. 21.8). This fluid is produced by the ciliary body (2 µL/min) and provides nutrients and oxygen to the avascular cornea. The outflow of aqueous humour is through the trabecular meshwork and canal of Schlemm adjacent to the limbus. Any factor which impedes its outflow will increase the intraocular pressure. The upper range of normal for intraocular pressure is 21 mmHg.
The uveal tract is made up of the iris anteriorly, the ciliary body and the choroid.
The iris is the coloured part of the eye under the transparent cornea. The muscles of the iris diaphragm regulate the size of the pupil, thereby controlling the amount of light entering the eye. The muscles of the ciliary body control the accommodation of the lens and the secretory epithelium produces the aqueous humour (see above). The highly vascular choroid lines the inner aspect of the sclera and upon this lies the retina.
The lens lies immediately posterior to the pupil and anterior to the vitreous humour. It is a transparent biconvex structure and is responsible for 22% of the refractive power of the eye. By changing its shape it can alter its refractive power and help to focus objects at different distances from the eye. By the fourth decade of life this ability to change shape starts to decline and with time the lens starts to become less transparent and cataracts begin to develop.
The vitreous humour fills the cavity between the retina and the lens.
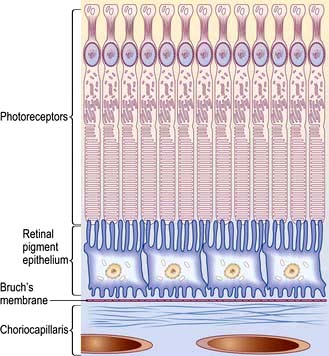
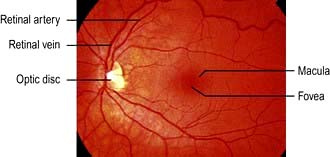
The retina is a multi-layered structure. The metabolically active region of the retina is represented in Figure 21.9. There are two types of photoreceptors in the retina, rods and cones. There are approximately 6 million cones mainly confined to the macula and these are responsible for detailed central vision and colour vision. The peripheral retina has around 125 million rods that are responsible for peripheral vision. The axons of the ganglion cells form the optic nerve (or disc) of the eye (Fig. 21.10).
The blood supply to the eye is via the ophthalmic artery and, in particular, the central retinal artery is responsible for supplying the inner retinal layers. Venous return is through the central retinal and ophthalmic veins. Local lymphatic drainage is to the preauricular and submental nodes.
The sensory innervation of the eye is through the trigeminal (V) nerve. The six extraocular muscles are supplied by different nerves.
 Oculomotor (III) nerve: medial, superior, inferior rectus and inferior oblique
Oculomotor (III) nerve: medial, superior, inferior rectus and inferior oblique
The oculomotor (III) nerve also supplies the upper lid and indirectly the pupil (parasympathetic fibres are attached to it). The facial (VII) nerve supplies the orbicularis and other muscles of facial expression.
History and examination
A careful and detailed history gives most of the facts needed to make a working diagnosis. The eye has limited mechanisms by which it can convey a diseased state. Common symptoms include alteration in visual acuity, redness, pain, discharge and photophobia.
It is essential to adopt a systematic approach to the examination of the eye. Different approaches and instruments are necessary for examination of the lids, anterior and posterior segments as well as extraocular movements. It is vital that an accurate visual acuity is recorded in all people with an eye problem. The Snellen eye chart is the most commonly used, but use of the LogMAR chart (logarithm of the Minimum Angle of Resolution) is increasing, largely due to its necessity in studies or research. Unlike the Snellen and other visual acuity charts, the LogMAR chart has equal graduation between the letters on a line as well as the space between lines. There is also a fixed number of letters, five, on each line. Research done using a logarithmic progression in size of letters on a test chart gives most accurate visual acuity measurement.
Visual acuity
The visual acuity of each eye is recorded in two ways: distance visual acuity and near visual acuity. Distance vision is measured in Snellen letters or, ever more commonly, in LogMAR letters or figures of different sizes, constructed to subtend 5′ (5 min) of arc at the nodal point to give a value of visual acuity. The recording is given as an expression of the line of letters which can be discerned at a particular distance, usually 6 metres (or 20 feet). For example 6/60, where 6 equals the distance of the chart from the eye in metres and 60 equals the distance at which the letter subtends 5′ at the nodal point.
Refractive errors
The eye projects a sharp and focused image onto the retina. Refractive errors refer to any abnormality in the focusing mechanism of the eye and not to any opacity in the system such as a corneal or retinal scar.
The refraction of light in emmetropic (normal), myopic (short-sighted, negative lenses will correct) and hypermetropic (long-sighted, positive lenses will correct) eyes is shown in Figure 21.11.
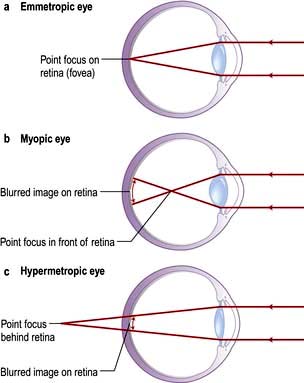
Figure 21.11 Refraction. (a) Emmetropic (normal). (b) Myopic (short-sighted). (c) Hypermetropic (long-sighted).
Astigmatism is a retractive error of the eye in which there is a different degree of refraction in the different meridians of curvature. It may be myopic in one plane and hypermetropic or emmetropic in the other plane. In this situation the front surface of the eye is more rugby ball shaped than football shaped.
Presbyopia is the term used to describe the normal ageing of the lens and leads to a change in the refractive state of the eye. As the lens ages it becomes less able to alter its curvature and this causes difficulty with near vision, especially reading.
Treatment
Errors of refraction can be corrected by using spectacles or contact lenses. The latter often results in better quality vision, but carries the risk of infection. They may be the only option in some refractive states such as keratoconus, a degenerative disorder of the eye in which structural changes within the cornea cause it to thin and become a more conical shape than its normal gradual curve. A number of surgical techniques can correct these errors of refraction, with varying degrees of accuracy. The most popular method is to use an excimer laser to re-profile the corneal curvature (PRK, LASIK, LASEK). The laser either removes corneal tissue centrally to flatten the cornea in myopia or it removes tissue from the peripheral cornea to steepen it in hypermetropia.
Disorders of the lids
The lids afford protection to the eyes and help to distribute the tear film over the front surface of the globe. Excess tears are drained via the punctae and lacrimal system to the nose (Fig. 21.12). Malposition of the lids, factors which affect blinking or lacrimal drainage, can all cause problems.
Entropion. The lid margin rolls inwards so that the lashes are against the globe (Fig. 21.13a). The lashes act as a foreign body and cause irritation, leading to a red eye which can mimic conjunctivitis. Occasionally the constant rubbing of lashes against the cornea causes an abrasion. The commonest cause is ageing and surgery is usually required.
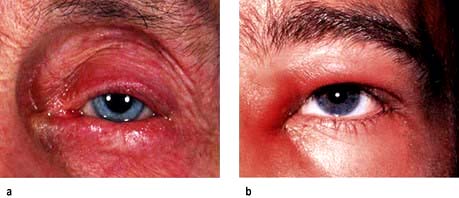
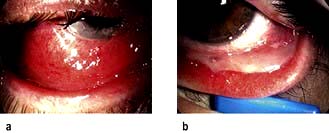
Figure 21.13 Disorders of the eyelid. (a) Lid entropion. The lower lid appears inverted. (b) Acute dacryocystitis showing a lump on the side of the nose.
Ectropion. The lid margin rolls outwards and is not apposed to the globe. As a result the lacrimal puncta is not in the correct anatomical position to drain tears and patients usually complain of a watery eye. Underlying factors include age, VII nerve palsy and cicatricial skin conditions. Surgery is usually required.
Dacryocystitis. Patients who have inflammation of the lacrimal sac usually present with a painful lump at the side of the nose adjacent to the lower lid (Fig. 21.13b). This should be treated with oral broad-spectrum antibiotics such as cefalexin, and patients should be watched carefully for signs of cellulitis. All patients should be referred to the ophthalmologist as some have an underlying mucocele or dilated sac, and will require surgery.
Blepharitis. This is an extremely common condition where inflammation of the lid margins may involve the lashes and lash follicles (Fig. 21.14a) resulting in styes, or inflammation and blockage of meibomian glands (Fig. 21.14b) leading to chalazion (Fig. 21.14c). Common underlying causes of blepharitis include meibomian gland dysfunction, seborrhoea and Staphylococcus aureus infection. Patients can be asymptomatic or complain of itchy, burning eyes because of tear film instability resulting from meibomian gland dysfunction. Staphylococcus aureus is frequently responsible for chronic blepharo-conjunctivitis and some patients may develop keratitis in the cornea (Fig. 21.15).
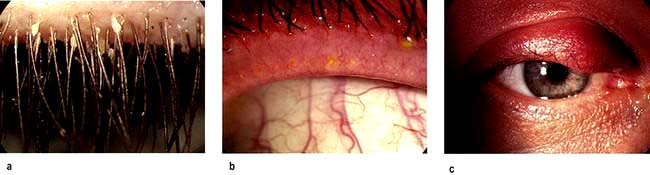
Figure 21.14 Blepharitis. (a) Crusty and scaly deposits on the lashes and lash bases. (b) Upper lid showing meibomian glands plugged with oily secretions. (c) Blockage of the meibomian glands leads to swelling of the lid (chalazion).
Treatment of blepharitis. Lid hygiene is the mainstay of treatment as it helps to reduce the bacterial load and unblock meibomian glands. A short course of topical chloramphenicol or fusidic acid is useful in chronic cases but in severe cases or cases where acne rosacea is suspected, oral doxycycline may be required. Some patients are left with a lump once the acute inflammatory phase has subsided. Most of these patients find the lump, or chalazion, cosmetically unacceptable and require incision and curettage. People with keratitis should be referred to the ophthalmologist for topical steroids.
Conjunctivitis
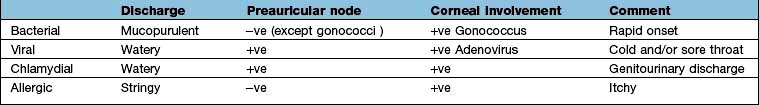
The commonest cause of a red eye, inflammation of the conjunctiva can arise from a number of causes, with viral, bacterial and allergic being the commonest. Common features in all types include soreness, redness and discharge, and in general the visual acuity is good. History should include the speed of onset of the inflammation, the colour and consistency of the discharge, whether the eye is itchy, and if there has been a recent history of a cold or sore throat. In the neonate it is vital to exclude gonococcal or chlamydial conjunctivitis associated with maternal sexually transmitted infection. The differential diagnosis of conjunctivitis is shown in Table 21.5.
Bacterial conjunctivitis
Bacterial conjunctivitis is uncommon, making up 5% of all cases of conjunctivitis. In the vast majority of patients it causes a sore or gritty eye in the presence of good vision. Bacterial conjunctivitis is invariably bilateral and should be suspected when conjunctival inflammation is associated with a purulent discharge.
Clinical features
Gonococcal conjunctivitis should be suspected when the onset of symptoms is rapid, the discharge is copious, and ocular inflammation includes chemosis (conjunctival oedema) and lid oedema. Gonococci are a cause of conjunctivitis giving rise to a palpable preauricular node. Less acute or subacute purulent conjunctivitis with moderate discharge can be attributed to organisms such as Haemophilus influenzae and Streptococcus pneumoniae. Chronic conjunctivitis is usually associated with mild conjunctival injection and scant purulent discharge. Common organisms include Staphylococcus aureus and Moraxella lacunata.
Treatment
Prompt treatment with oral and topical penicillin is given in gonococcal conjunctivitis to ensure a reduced rate of corneal perforation. A Gram-stain of the conjunctival swab can quickly confirm the presence of diplococci. Gonococcal conjunctivitis is a notifiable disease in the UK. Empirical treatment for both subacute and chronic conjunctivitis involves a topical broad-spectrum antibiotic such as chloramphenicol. Swabs should be taken if these cases do not respond to this initial treatment. Antibiotic resistance is increasing.
Chlamydial conjunctivitis
Chlamydia trachomatis (see p. 164) is seen in developed countries as a sexually transmitted infection that is most prevalent in sexually active adolescents and young adults. Direct or indirect contact with genital secretions is the usual route of infections but shared eye cosmetics can also be involved. Neonatal chlamydial conjunctivitis is a notifiable disease in the UK and should be suspected in newborns with a red eye. Mothers should be asked about sexually transmitted infections.
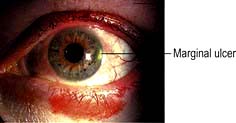
Trachoma caused by the same organism, but not usually sexually transmitted, is found mainly in the tropics and the Middle East and is a very common cause of blindness in the world (see p. 133). Chronic conjunctival inflammation causes progressive scarring, trichiasis, entropion and subsequent corneal scarring which leads to severe visual impairment or blindness from corneal opacification or ulceration.
Clinical features
The onset of symptoms is slow, and patients may complain of mild discomfort for weeks. In these cases the red eye is associated with a scanty mucopurulent discharge and a palpable preauricular lymph node. In chronic cases it is not unusual to see superior corneal vascularization. In neonates the onset of the red eye is typically around 2 weeks after birth, whereas gonococcal conjunctivitis occurs within days of birth. Conjunctival swabs should be taken and a nucleic acid amplification test (NAAT) performed (p. 163) prior to commencement of treatment.
Viral conjunctivitis
Adenoviral conjunctivitis
This is highly contagious and can cause epidemics in communities. Transmission is through direct or indirect contact with infected individuals. The onset of symptoms may be preceded by a cold or flu-like symptoms. Inflammation is commonly associated with chemosis, lid oedema and a palpable preauricular lymph node. Some patients develop a membrane on the tarsal conjunctiva (Fig. 21.16) and haemorrhage on the bulbar conjunctiva. Viral conjunctivitis can cause deterioration in visual acuity owing to corneal involvement (focal areas of inflammation). In 50% of the patients the conjunctivitis is unilateral.
Treatment
The condition is largely self-limiting in the majority of cases. Lubricants together with a cold compress can be soothing for patients. Strict hygiene and keeping towels separate from the rest of the household goes a long way towards reducing the spread of the infection. In people with corneal involvement or intense conjunctival inflammation, topical steroids are indicated.
Herpes simplex conjunctivitis
Primary ocular herpes simplex conjunctivitis is typically unilateral. It usually causes a palpable preauricular lymph node and cutaneous vesicles develop on the eyelids and the skin around the eyes in the majority of patients. Over 50% of these patients develop a dendritic corneal ulcer (Fig. 21.17). The organism responsible for this condition is the herpes simplex virus (HSV), which is usually HSV-1 but HSV-2 can give rise to ocular infection.
Molluscum contagiosum conjunctivitis
This is typically unilateral and produces a red eye that generally goes unrecognized and comes to the forefront because patients fail to improve and the cornea starts to become involved. On close inspection, pearly umbilicated nodules, filled with the DNA poxvirus, can be seen on the lid margin.
Phthiriasis palpebrarum
Phthiriasis palpebrarum is an eyelid infestation caused by Phthirus pubis, or crab lice. Infestation of the cilia and eyelid is rare. It leads to blepharitis with marked conjunctival inflammation, preauricular lymphadenopathy, and rarely secondary infection at the site of lice bite.
Allergic conjunctivitis
There are five main types of allergic conjunctivitis: seasonal, perennial, vernal, atopic and giant papillary. Both seasonal and perennial allergic conjunctivitis are acute allergic conjunctival disorders. Symptoms include itching and pink to reddish eyes. These two eye conditions are mediated by mast cells and can be easily treated with cold compresses, eyewashes with tear substitutes, and avoidance of allergens. The last three are difficult to treat, chronic and can be sight-threatening. They should be referred to an ophthalmologist.
Seasonal/perennial conjunctivitis
Seasonal allergic conjunctivitis and perennial conjunctivitis, affecting 20% of the general population in the UK, is an allergic reaction to grass, pollen and fungal spores and occurs mainly in spring and summer. Perennial allergic conjunctivitis occurs all year round but peaks in the autumn and includes allergens such as house-dust mites.
The main symptoms include itching, redness, soreness, watering and a stringy discharge. Occasionally the conjunctiva may become so hyperaemic that chemosis results. This is usually associated with swollen lids.
Treatment
Reducing the allergen load (reducing dust, p. 825) is helpful. Medical treatment includes the use of antihistamine drops such as azelastine and emedastine together with topical mast cell-stabilizing agents such as sodium cromoglicate and nedocromil. Olopatadine (twice daily) has dual action and is very effective. Corticosteroid drops should be avoided. Oral antihistamines help the itching.
Corneal disorders
Trauma
Corneal abrasions
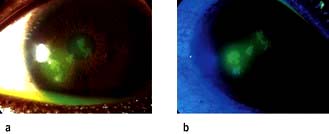
Trauma resulting in the removal of a focal area of epithelium on the cornea is very common. Abrasions usually occur when the eye is accidentally poked with a finger, a foreign body (FB) flies into the eye or something brushes against the eye.
Clinical features
Symptoms include severe pain, due to exposure of the corneal nerve endings, lacrimation and inability to open the eye (blepharospasm). Blinking and eye movement can aggravate the pain and foreign body sensation. The visual acuity is usually reduced. Most cases will need topical anaesthetic drops such as oxybuprocaine or tetracaine to be administered before it is possible to examine the eye. The cornea should be inspected with a blue light after instillation of fluorescein drops. The orange dye will stain the area of the abrasion. Under blue light the abrasion lights up as green. Occasionally FBs can lodge on the undersurface of the upper lid and give rise to linear vertical abrasions. Eversion of the upper lid is necessary in all cases of abrasions (Fig. 21.18).
Corneal foreign body
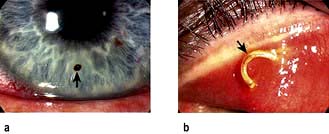
Occasionally when something flies into the eye it gets stuck on the cornea (Fig. 21.19a). It may be associated with lacrimation and photophobia. Examination is best attempted following instillation of a topical anaesthetic and should include everting the upper lid (Fig. 21.19b). Corneal foreign bodies can usually be seen directly with a white light.
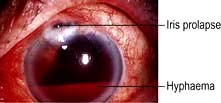
High-velocity trauma
In cases of high-velocity trauma corneal perforation or intraocular FB should be suspected. Examination may show a corneal laceration with or without the FB embedded in the cornea. The FB may be present on the iris or in the lens or vitreous. Other clues of a penetrating injury include a large subconjunctival haemorrhage, a flat anterior chamber with low intraocular pressure, or the presence of blood in the anterior chamber (hyphaema). Urgent referral to the ophthalmologist is mandatory, ensuring that no drops are instilled into the eye and that a plastic shield has been placed over the eye to minimize further risk of trauma.
Blunt trauma usually results in periorbital bruising and gross lid oedema, which can make examination to exclude perforating injury difficult. These patients should be referred to the ophthalmologist for a detailed ocular examination to exclude a perforation, retinal detachment or a traumatic hyphaema (Fig. 21.20).
Keratitis
This is a general term to describe corneal inflammation. Common causes include herpes simplex virus, contact lens-associated infection and blepharitis. Symptoms include the sensation of a foreign body or pain (depending on the size and depth of the ulcer), photophobia and lacrimation. Vision is reduced if the ulcer affects the visual axis.
Herpes simplex keratitis
Corneal epithelial cells infected with the virus eventually undergo lysis and form an ulcer which is typically dendritic in shape (Fig. 21.17). The ulcer stains with fluorescein and can be observed easily with a blue light. Topical immunosuppression, e.g. steroid drops, or systemic immunosuppression, e.g. AIDS, can lead to the centrifugal spread of the virus such that the ulcer increases in area and is referred to as a geographic ulcer. Recurrent attacks of HSV keratitis can be triggered by ultraviolet light, stress and menstruation. All these factors are responsible for activating the virus, which normally lies dormant in the ganglion of the Vth nerve.
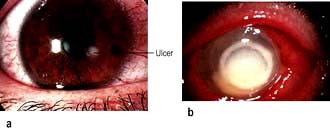
Contact lens-related keratitis
A small number of contact lens wearers develop infective corneal ulcers which are potentially sight-threatening (Fig. 21.21). The organisms usually responsible include Gram-positive and Gram-negative bacteria. Patients should be referred to an ophthalmologist for scraping of the ulcer and commencement of antibiotic treatment.
Corneal dystrophy
Fuchs’ corneal dystrophy is a genetically associated degenerative disorder leading to corneal oedema and vision loss. The gene is TCF4. It affects both eyes, is commoner in females, and is of gradual onset leading to blindness in the 40–60 age group. There is an accumulation of deposits (guttae) in the cornea with thickening of Descemet’s membrane. Treatment is by corneal transplantation.
Cataracts
Cataract (Fig. 21.22a,b) is by far the commonest cause of preventable blindness in the world with an effective surgical treatment. In the UK, approximately 250 000 cataract operations are performed each year, making it the commonest surgical procedure.
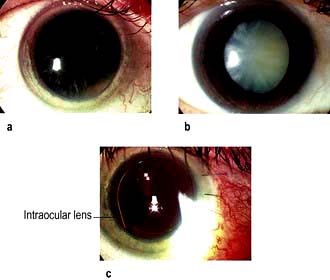
Figure 21.22 Cataracts. (a) Early cataract. (b) White mature cataract. (c) Artificial intraocular lens following phacoemulsification.
Aetiology
Age-related opacification of the lens (cataract) is the commonest cause of visual impairment with 30% of people over 65 years having visual acuities below that required for driving (Snellen acuity less than 6/12). The common causes of cataracts are summarized in Table 21.6.
Table 21.6 Cataracts: aetiology
Congenital |
Maternal infection |
Familial |
|
Age |
Elderly |
Metabolic |
Diabetes, galactosaemia, hypocalcaemia, Wilson’s disease |
Drug-induced |
Corticosteroids, phenothiazines, miotics, amiodarone |
Traumatic |
Post-intraocular surgery |
Inflammatory |
Uveitis |
Disease associated |
Down’s syndrome |
Dystrophia myotonica |
|
Lowe’s syndrome |
In young patients, familial or congenital causes should be excluded. Any history of ocular inflammation is noted. Cataracts diagnosed in infants demand urgent referral to the ophthalmologist in order to minimize the subsequent development of amblyopia.
Clinical features
Gradual painless deterioration of vision is the commonest symptom. Other symptoms are dependent upon the type of cataract, for example a posterior capsular type would lead to glare and problems with night driving. Early changes in the lens are correctable by spectacles but eventually the opacification needs surgical intervention.
Investigations
Blood glucose, serum calcium and liver biochemistry should be measured to diagnose metabolic disorders.
Treatment
Small incision extracapsular or phacoemulsification cataract extraction with the insertion of an intraocular lens is the treatment of choice (Fig. 21.22c).
Glaucoma
This is a group of diseases in which the pressure inside the eye is sufficiently elevated to cause optic nerve damage and result in visual field defects (Fig. 21.23). Normal intraocular pressure (IOP) is 10–21 mmHg. Some types of glaucoma can result in an IOP exceeding 70 mmHg. Glaucoma is the second commonest cause of blindness worldwide and the third commonest cause of blind registration in the UK.
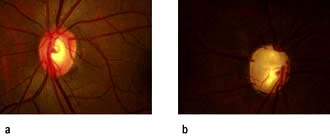
Figure 21.23 (a) Normal optic disc and (b) glaucomatous optic disc. The central cup is enlarged and deepened.
Primary open-angle glaucoma (POAG)
This is the commonest form of glaucoma. High intraocular pressures result from reduced outflow of aqueous humour through the trabecular meshwork. Common risk factors include age (0.02% of 40-year-olds versus 10% of 80-year-olds), race (black Africans are at five times greater risk than whites), positive family history and myopia.
POAG causes a gradual, insidious, painless loss of peripheral visual field. It is initially asymptomatic and the central vision remains good until the end-stage of the disease. Usually glaucoma is identified during a routine ophthalmic examination. Diagnosis is only made if the IOP is measured. The optic disc is inspected and shows an enlarged cup with a thin neuroretinal rim. Visual fields are performed and show a normal blind spot with scotomas.
Treatment
Treatment aims to reduce the IOP, either by reducing aqueous production or by increasing aqueous drainage:
 Beta-blockers such as timolol, carteolol and levobunolol reduce aqueous production and are the commonest prescribed topical agents. These drugs are contraindicated in people with chronic obstructive pulmonary disease, asthma or heart block.
Beta-blockers such as timolol, carteolol and levobunolol reduce aqueous production and are the commonest prescribed topical agents. These drugs are contraindicated in people with chronic obstructive pulmonary disease, asthma or heart block.
 Prostaglandin analogues such as latanoprost and travoprost, increase aqueous outflow and are also used (alone or in combination with beta-blockers) for POAG as they can reduce IOP by 30%.
Prostaglandin analogues such as latanoprost and travoprost, increase aqueous outflow and are also used (alone or in combination with beta-blockers) for POAG as they can reduce IOP by 30%.
 Carbonic anhydrase inhibitors such as acetazolamide reduce aqueous production and are available in both topical and oral form. In its oral form acetazolamide is the most potent drug for reducing ocular pressure. It should not be used in patients who have a sulphonamide allergy.
Carbonic anhydrase inhibitors such as acetazolamide reduce aqueous production and are available in both topical and oral form. In its oral form acetazolamide is the most potent drug for reducing ocular pressure. It should not be used in patients who have a sulphonamide allergy.
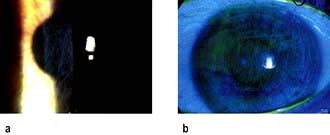
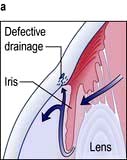
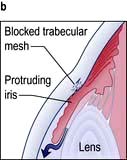
Acute angle-closure glaucoma (AACG)
This is an ophthalmic emergency. There is a sudden rise in intraocular pressure to levels greater than 50 mmHg. This occurs due to reduced aqueous drainage as a result of the ageing lens pushing the iris forward against the trabecular meshwork. People most at risk of developing AACG are those with shallow anterior chambers such as hypermetropes and women. The attack is more likely to occur under reduced light conditions when the pupil is dilated.
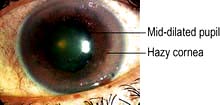
Red eye and other signs and symptoms
AACG causes sudden onset of a red painful eye and blurred vision. Patients become unwell with nausea and vomiting and complain of headache and severe ocular pain. The eye is injected, tender and feels hard. The cornea is hazy and the pupil is semi-dilated (Fig. 21.24). Table 21.7 shows the differential diagnosis of the acute red eye. Emergency Box 21.2 shows features that require urgent referral to an ophthalmologist.
Treatment
Prompt treatment is required to preserve sight and includes:
 Intravenous acetazolamide 500 mg (provided there are no contraindications) to reduce IOP, and
Intravenous acetazolamide 500 mg (provided there are no contraindications) to reduce IOP, and
 Instillation of pilocarpine 4% drops to constrict the pupil to improve aqueous outflow and prevent iris adhesion to the trabecular meshwork.
Instillation of pilocarpine 4% drops to constrict the pupil to improve aqueous outflow and prevent iris adhesion to the trabecular meshwork.
Other topical drops such as beta-blockers and prostaglandin analogues can also be instilled if available, provided there are no contraindications. Analgesia and antiemetics are given as required.
Patients must be referred to an ophthalmologist immediately so that reduction in IOP can be monitored and other agents such as oral glycerol or i.v. mannitol can be administered to non-responding patients. Definitive treatment involves making a hole in the periphery of the iris of both eyes either by laser or surgically.
Uveitis
Uveitis is inflammation of the uveal tract, which includes the iris, ciliary body and choroid. Inflammation confined to the anterior segment of the eye (in front of the iris) is referred to as iritis or anterior uveitis, that involving the ciliary body is referred to as intermediate uveitis whilst inflammation of the choroid is termed posterior uveitis. If all three regions are involved then the term panuveitis is used. For posterior uveitis, referral to an ophthalmologist is required.
The most common symptoms of uveitis are blurred vision, pain, redness, photophobia and floaters. Each symptom is determined by the location of the inflammation such that photophobia and pain are common features of iritis whilst floaters are commonly seen with posterior uveitis.
Uveitis is commonly encountered with ankylosing spondylitis and positive HLA-B27 (see p. 527), arthritis, inflammatory bowel disease, sarcoid, tuberculosis, syphilis, toxoplasmosis, Behçet’s syndrome, lymphoma and viruses such as herpes, cytomegalovirus and HIV infection. In a number of patients no cause is found (idiopathic uveitis).
Anterior uveitis (iritis)
The classic presentation entails a triad of eye symptoms: redness, pain and photophobia. Vision can be normal or blurred depending on the degree of inflammation. The eye can be generally red or the injection can be localized to the limbus. The anterior chamber shows features consistent with inflammation including cells, keratic precipitates (KP) on the corneal endothelium, fibrin or hypopyon (pus), and the pupil may have adhered to the lens (posterior synechiae) (Fig. 21.25). The IOP may be normal or raised either due to cells clogging up the trabecular meshwork or posterior synechiae causing aqueous to build up behind the iris and force the iris against the trabecular meshwork and so reduce aqueous drainage.
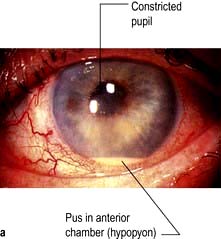
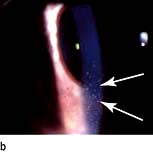
Figure 21.25 Anterior uveitis. (a) Acute with hypopyon. (b) Showing keratic precipitates on the corneal endothelium.
Treatment
This consists of reducing inflammation with the use of topical steroids such as dexamethasone 0.1% and dilating the pupil with cyclopentolate 1% to prevent formation of posterior synechiae. Dilatation also allows fundoscopy to exclude posterior segment involvement. If the IOP is raised, this is treated with topical beta-blockers, prostaglandin analogues, or oral or i.v. acetazolamide. Referral should be made to the ophthalmologist.
Disorders of the retina
Central retinal vein occlusion (CRVO)
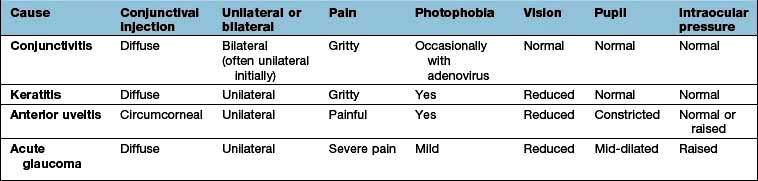
This usually leads to profound sudden painless loss of vision with thrombosis of the central retinal vein at or posterior to the lamina cribrosa where the optic nerve exits the globe. The thrombus causes obstruction to the outflow of blood leading to a rise in intravascular pressure. This results in dilated veins, retinal haemorrhage, cotton wool spots, and abnormal leakage of fluid from vessels resulting in retinal oedema (Fig. 21.26). In severe cases, an afferent papillary defect is present and this suggests the ischaemic variant.
Predisposing factors include increasing age, hypertension and cardiovascular disease, diabetes, glaucoma, and in the younger age group, blood dyscrasias and vasculitis.
Treatment
Treatment of any underlying medical condition is mandatory. Referral to an ophthalmologist is essential to monitor the eye, as some patients can develop retinal ischaemia with resulting neovascularization of the retina and iris. Panretinal photocoagulation should be commenced if there is neovascularization, and intravitreal steroid or anti-vascular endothelial growth factor (anti-VEGF) therapy is also used if there is macular oedema. Patients who develop iris neovascularization, rubeosis, are at risk of developing rubeotic glaucoma.
Central retinal artery occlusion (CRAO)
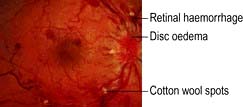
This results in sudden painless severe loss of vision. Retinal arterial occlusion results in infarction of the inner two-thirds of the retina. The arteries become narrow and the retina becomes opaque and oedematous. A cherry red spot is seen at the fovea because the choroidal vasculature shows up through the thinnest part of the retina (Fig. 21.27). An afferent papillary defect is usually present.
Arteriosclerosis-related thrombosis is the most common cause of CRAO. Emboli from atheromas and diseased heart valves are other causes. Giant cell arteritis (see p. 543) must be excluded.
Treatment
CRAO is an ophthalmic emergency since studies have shown that irreversible retinal damage occurs after 90 minutes of onset. Ocular massage and 500 mg i.v. acetazolamide help to reduce ocular pressure and may help in dislodging the emboli. Breathing into a paper bag allows a build-up of carbon dioxide which acts as a vasodilator and so helps in dislodging the emboli. Other options include making a corneal paracentesis to drain off some aqueous humour, thereby reducing the intraocular pressure.
People with CRAO should have a thorough medical evaluation to determine the aetiology of the emboli or thrombus. Some patients may present with transient loss of vision or amaurosis fugax (see p. 1098). All people with CRAO and amaurosis fugax should be started on oral aspirin if it is not medically contraindicated.
Retinal detachment
This causes a painless progressive visual field loss. The shadow corresponds to the area of detached retina. If the detachment affects the macula, central vision will be lost. Following a tear in the retina, fluid collects in the potential space between the sensory retina and the pigment epithelium (Fig. 21.28). Patients usually report a sudden onset of floaters often associated with flashes of light (photopsia) prior to the detachment. These patients should be referred to an ophthalmologist for a detailed fundal examination.
Retinitis pigmentosa
This is a common chronic inherited degenerative disease of the retina which can be primary or part of a syndrome and leads to blindness. There is constriction of the peripheral vision leading to tunnel vision and progressive loss of night vision.
Ophthalmoscopy shows bone spicule deposits and attenuated retinal vessels. Several genes are implicated.
There is no treatment but high-dose vitamin A supplementation may slow progression. Gene therapy is being investigated.
Age-related macular degeneration (AMD)
This is the commonest cause of visual impairment in patients over 50 years in the western world, and blind registration in this age group. It affects 10% of people over 65 years and 30% over 80 years. Mutations in various genes have been reported; fibulin 5, complement factor H, Arg 80 Gly variant of complement C3.
The cause is unknown but suggested risk factors include increasing age, smoking, hypertension, hypercholesterolaemia and ultraviolet exposure.
 Non-exudative (dry) macular degeneration describes a painless and progressive loss of central vision. With age, lipofuscin deposits (drusen) are found between the retinal pigment epithelium (RPE) and Bruch’s membrane (see Fig. 21.9 and Fig. 21.29a). Drusen may be hard or soft and there may be focal RPE detachment. Not all people with these changes will be affected visually but some develop distortion and blurring of their central vision. Extensive atrophy of RPE can occur (geographic atrophy).
Non-exudative (dry) macular degeneration describes a painless and progressive loss of central vision. With age, lipofuscin deposits (drusen) are found between the retinal pigment epithelium (RPE) and Bruch’s membrane (see Fig. 21.9 and Fig. 21.29a). Drusen may be hard or soft and there may be focal RPE detachment. Not all people with these changes will be affected visually but some develop distortion and blurring of their central vision. Extensive atrophy of RPE can occur (geographic atrophy).
 Exudative (wet) AMD (10% of cases) occurs with the development of abnormal subfoveal choroidal neovascularization in the region of the macula and causes severe central visual loss (Fig. 21.29b).
Exudative (wet) AMD (10% of cases) occurs with the development of abnormal subfoveal choroidal neovascularization in the region of the macula and causes severe central visual loss (Fig. 21.29b).
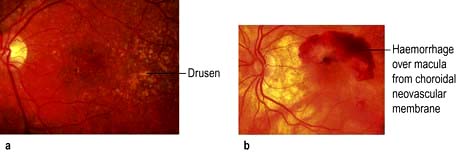
Figure 21.29 Age-related macular degeneration. (a) With deposition of material (drusen) over the macula. (b) With choroidal neovascularization.
Treatment
The Age-Related Eye Disease Study (AREDS) has shown that vitamins C and E, β-carotene, zinc and copper slow progression of the disease. People with central distortion or with frank macular pathology should be referred urgently to the ophthalmologist for assessment of treatment.
Antivascular endothelial growth factors (anti-VEGF), such as ranibizumab and bevacizumab, are given by intravitreal injections with great success, although the latter is more expensive. The treatment course should be commenced as a matter of urgency as vision is maintained in up to 95% of patients and improves in approximately a third. Monthly monitoring with optical coherence tomography (OCT) is recommended (Fig. 21.30). Laser treatment and photodynamic therapy with verteporfin were the treatment of choice in the past for the treatment of wet AMD, but now have limited roles.
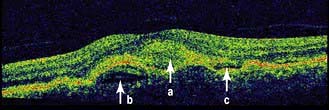
Figure 21.30 Age-related macular degeneration seen by optical coherence tomography. a, Area of thickened retina; b, pigment epithelial detachment; c, subretinal fluid.
Severe visual loss is possible and low-vision aids, such as magnifying glasses, may help to improve a patient’s independence.
Visual loss
Every patient with unexplained sudden visual loss requires ophthalmic referral. The initial history and examination are summarized in Emergency Box 21.3.
![]() Emergency Box 21.3
Emergency Box 21.3
The initial history and examination in the patient presenting with sudden loss of vision
RAPD, relative afferent papillary defect.
(After: Pane A, Simcock P. Practical Ophthalmology. Edinburgh: Elsevier Churchill Livingstone; 2005.)
The common causes of blindness are similar across the world (Box 21.3). In developing countries, trachoma due to Chlamydia trachomatis (see p. 164) is also a major cause, accounting for 10% of global blindness, as is onchocerciasis (river blindness, due to Onchocerca volvulus – see p. 154), which accounts for blindness in about 1 million people, although this is decreasing with treatment. In leprosy, 70% of patients have ocular involvement, and blindness occurs in 5–10% of these. Ocular involvement is common in cerebral malaria (see p. 144), although loss of vision is rare.
![]() Box 21.3 Loss of vision
Box 21.3 Loss of vision
summary
| Painless loss of vision | Painful loss of vision |
|---|---|
Cataract |
Acute angle-closure glaucoma |
Open-angle glaucoma |
Giant cell arteritis |
Retinal detachment |
Optic neuritis |
Central retinal vein occlusion |
Uveitis |
Central retinal artery occlusion |
|
Diabetic retinopathy |
|
Vitreous haemorrhage |
|
Posterior uveitis |
|
Age-related macular degeneration |
|
Optic nerve compression |
|
Cerebral vascular disease |
|
HIV infection can produce uveitis but the major problem is severe opportunistic infection of the eye when the CD4 count falls (see p. 178) and HAART is not available.
Vitamin A deficiency and xerophthalmia affects millions each year; the WHO classification of xerophthalmia by ocular signs is shown in Table 5.10 (see p. 206).
WHO lists the commonest causes of blindness across the world as cataract, glaucoma, acute macular degeneration, corneal opacity, diabetic retinopathy and infections from bacteria or parasites.