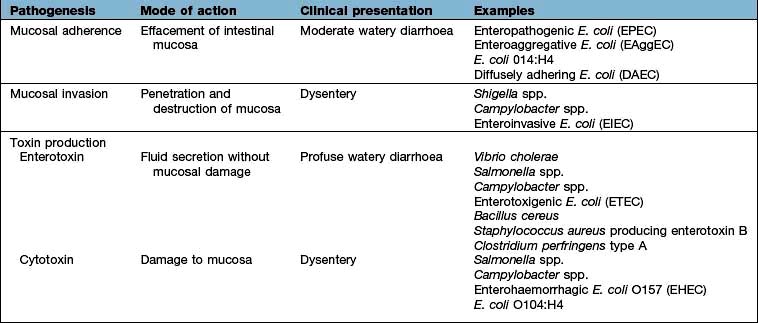
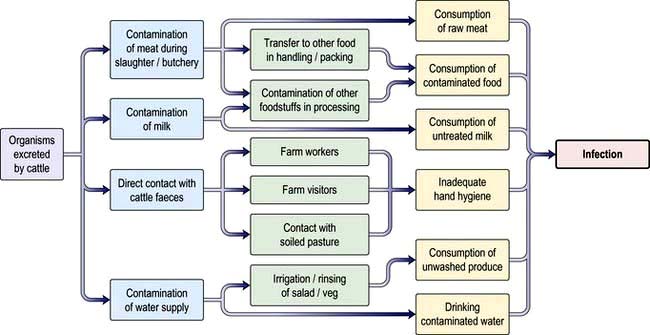
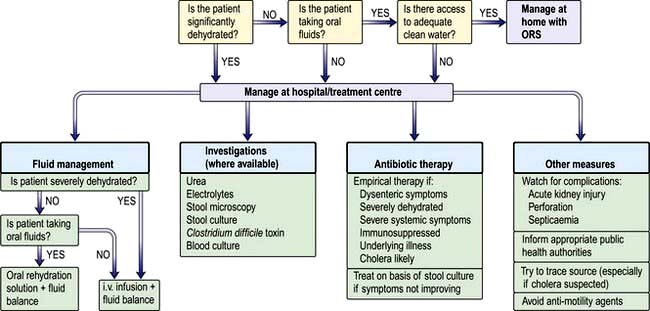
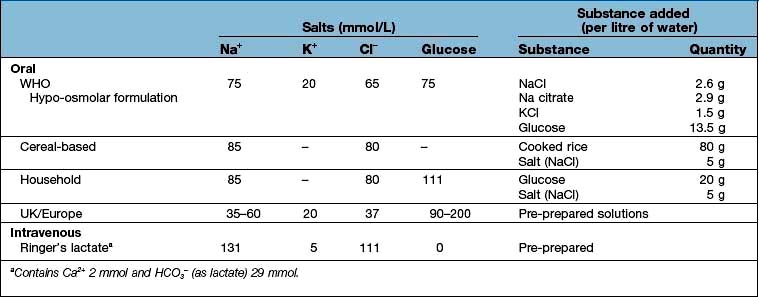
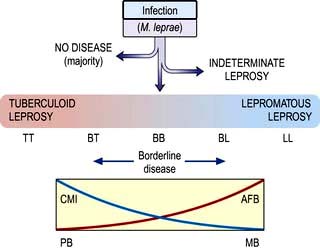
Viral infections
Introduction
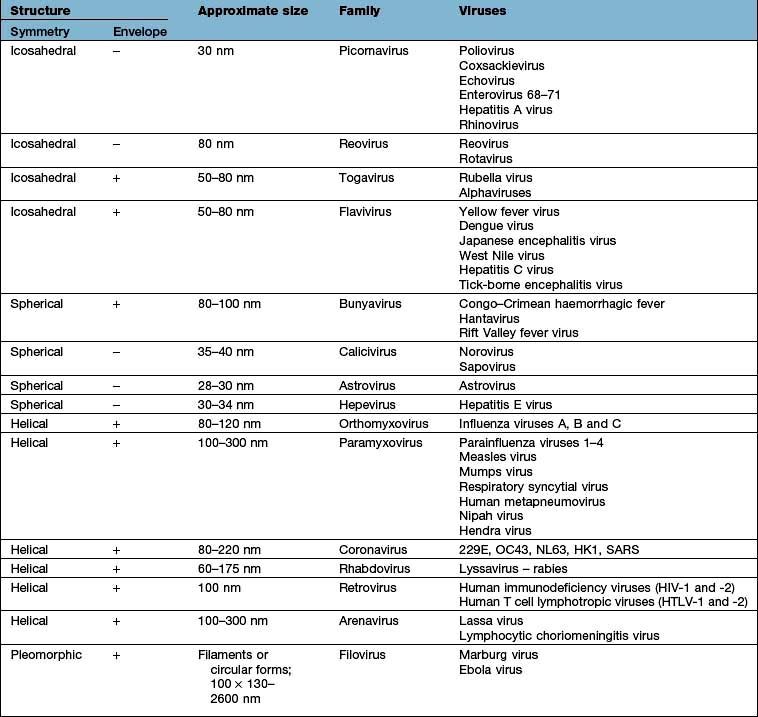
Viruses are much smaller than other infectious agents (see Tables 4.16 and 4.18) and contain either DNA or RNA, not both as in bacteria and other microorganisms. Since they are metabolically inert, they must live intracellularly, using the host cell for synthesis of viral proteins and nucleic acid. Viruses have a central nucleic acid core surrounded by a protein coat that is antigenically unique for a particular virus. The protein coat (capsid) imparts a helical or icosahedral structure to the virus. Some viruses also possess an outer envelope consisting of lipid and protein.
Outcomes of virus infection of a cell
Replication of viruses within a cell may result in sufficient distortion of normal cell function so as to result in cell death – a cytocidal or cytolytic infection. However, acute cell death is not an inevitable consequence of virus infection of a cell. In a chronic, or persistent, infection, virus replication continues throughout the lifespan of the cell, but does not interfere with the normal cellular processes necessary for cell survival. Hepatitis B and C viruses may interact with cells in this way. Some viruses, e.g. the herpesvirus family, are able to go latent within a cell – in such a state, the virus genome is present within the cell, but there is very little, if any, production of viral proteins and no production of mature virus particles. Finally, some viruses are able to transform cells, leading to uncontrolled cell division, e.g. Epstein–Barr virus infection of B lymphocytes, resulting in the generation of an immortal lymphoblastoid cell line.
DNA viruses
Details of the structure, size and classification of human DNA viruses are shown in Table 4.16.
Adenoviruses
Over 50 adenovirus serotypes have been identified as human pathogens, infecting a number of different cell types and therefore resulting in different clinical syndromes. Adenovirus infection commonly presents as an acute pharyngitis and extension of infection to the larynx and trachea in infants may lead to croup. By school age the majority of children show serological evidence of previous infection. Certain subtypes produce an acute conjunctivitis associated with pharyngitis. In adults, adenovirus causes acute follicular conjunctivitis and rarely pneumonia that is clinically similar to that produced by Mycoplasma pneumoniae (see p. 836). Certain adenoviruses (40 and 41) cause gastroenteritis (see p. 104) without respiratory disease and adenovirus infection may be responsible for acute mesenteric lymphadenitis in children and young adults. Mesenteric adenitis due to adenoviruses may lead to intussusception in infants. Infection in an immunocompromised host, e.g. a bone marrow transplant recipient, may result in multisystem failure and fatal disease.
Herpesviruses
Members of the Herpesviridae family are causes of a wide range of human diseases. Details are summarized in Table 4.17. The hallmark of all herpesvirus infections is the ability of the viruses to establish latent (or silent) infections that then persist for the life of the individual.
Herpes simplex virus (HSV) infection
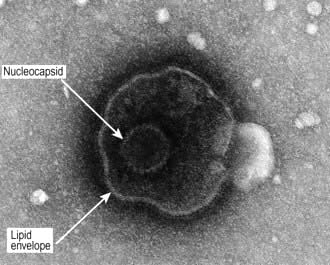
Two types of HSV (Fig. 4.12) have been identified: HSV-1 is the major cause of herpetic stomatitis, herpes labialis (‘cold sore’), keratoconjunctivitis and encephalitis, whereas HSV-2 causes genital herpes and may also be responsible for systemic infection in the immunocompromised host. These divisions, however, are not rigid, for HSV-1 can give rise to genital herpes and HSV-2 can cause infections in the mouth.
HSV-1
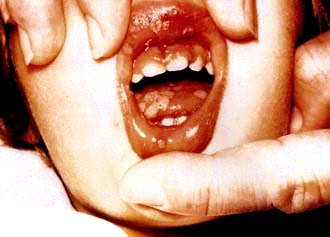
The portal of entry of HSV-1 infection is usually via the mouth or occasionally the skin. The primary infection may go unnoticed or may produce a severe inflammatory reaction with vesicle formation leading to painful ulcers (gingivostomatitis; Fig. 4.13). The virus then remains latent, most commonly in the trigeminal ganglia, but may be reactivated by stress, trauma, febrile illnesses and ultraviolet radiation, producing the recurrent form of the disease known as herpes labialis (‘cold sore’). Approximately 70% of the population is infected with HSV-1 and recurrent infections occur in one-third of individuals. Reactivation often produces localized paraesthesiae in the lip before the appearance of a cold sore.
Complications of HSV-1 infection include transfer to the eye (dendritic ulceration, keratitis), acute encephalitis (see p. 1128), skin infections such as herpetic whitlow and erythema multiforme (see p. 1216).
HSV-2
The clinical features, diagnosis and management of genital herpes are described on page 168. The virus remains latent in the sacral ganglia and during recurrence, can produce a radiculomyelopathy, with pain in the groin, buttocks and upper thighs. Primary anorectal herpes infection is common in men having sex with men (see p. 168). The clinical picture, diagnosis and treatment of genital herpes are described on page 169.
Neonates may develop primary HSV infection following vaginal delivery in the presence of active genital HSV infection in the mother, particularly if the maternal disease is a primary, rather than a recurrent infection. The disease in the baby varies from localized skin lesions (about 10–15%) to widespread visceral disease often with encephalitis, with a poor prognosis. Caesarean section should therefore be performed if active genital HSV infection is present during labour.
Immunocompromised patients such as those receiving intensive cancer chemotherapy or those with the acquired immunodeficiency syndrome (AIDS) may develop disseminated HSV infection involving many of the viscera. In severe cases, death may result from hepatitis and encephalitis. Eczema herpeticum is a serious complication in individuals with eczema, where the non-intact skin allows spread of lesions across large areas and bloodstream access which may lead to herpetic involvement of internal organs.
Humoral antibody develops following primary infection, but mononuclear cell responses probably prevent dissemination of disease.
Varicella zoster virus (VZV) infection
VZV produces two distinct diseases, varicella (chickenpox) and herpes zoster (shingles). The primary infection is chickenpox. It usually occurs in childhood, the virus entering through the mucosa of the upper respiratory tract. In some countries (e.g. the Indian subcontinent), a different epidemiological pattern exists with most infections occurring in adulthood. Chickenpox rarely occurs twice in the same individual. Infectious virus is spread from the throat and from fresh skin lesions by air-borne transmission or direct contact. The period of infectivity in chickenpox extends from 2 days before the appearance of the rash until the skin lesions are all at the crusting stage. Following recovery from chickenpox, the virus remains latent in dorsal root and cranial nerve ganglia. Reactivation of infection then results in shingles.
Clinical features of chickenpox
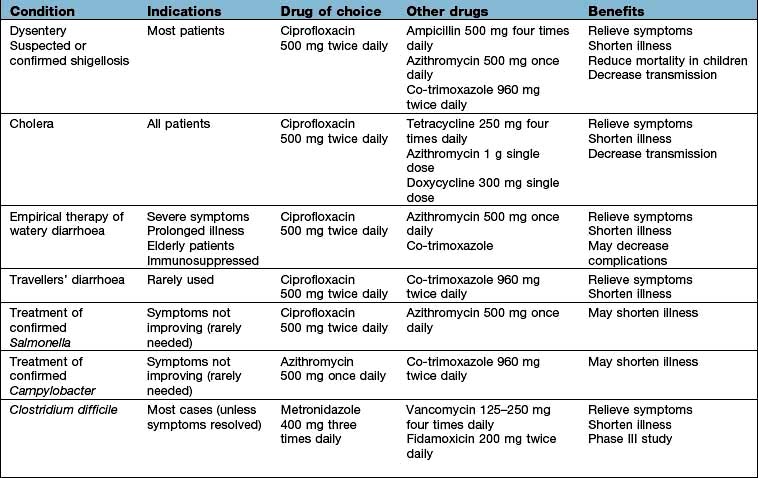
Some 14–21 days after exposure to VZV, a brief prodromal illness of fever, headache and malaise heralds the eruption of chickenpox, characterized by the rapid progression of macules to papules to vesicles to pustules in a matter of hours (Fig. 4.14). In young children, the prodromal illness may be very mild or absent. The illness tends to be more severe in older children and can be debilitating in adults. The lesions occur on the face, scalp and trunk and to a lesser extent, on the extremities. It is characteristic to see skin lesions at all stages of development on the same area of skin. Fever subsides as soon as new lesions cease to appear. Eventually the pustules crust and heal without scarring.
Complications of chickenpox include pneumonia, which generally begins 1–6 days after the skin eruption, and bacterial superinfection of skin lesions. Pneumonia is more common in adults than in children and cigarette smokers are at particular risk. Pulmonary symptoms are usually more striking than the physical findings, although a chest radiograph usually shows diffuse changes throughout both lung fields. CNS involvement occurs in about 1 per 1000 cases and most commonly presents as an acute truncal cerebellar ataxia. The immunocompromised are susceptible to disseminated infection with multiorgan involvement. Women in pregnancy are prone to severe chickenpox and, in addition, there is a risk of intrauterine infection with structural damage to the fetus (if maternal infection is within the first 20 weeks of pregnancy, the risk of varicella embryopathy is 1–2%).
Clinical features of shingles
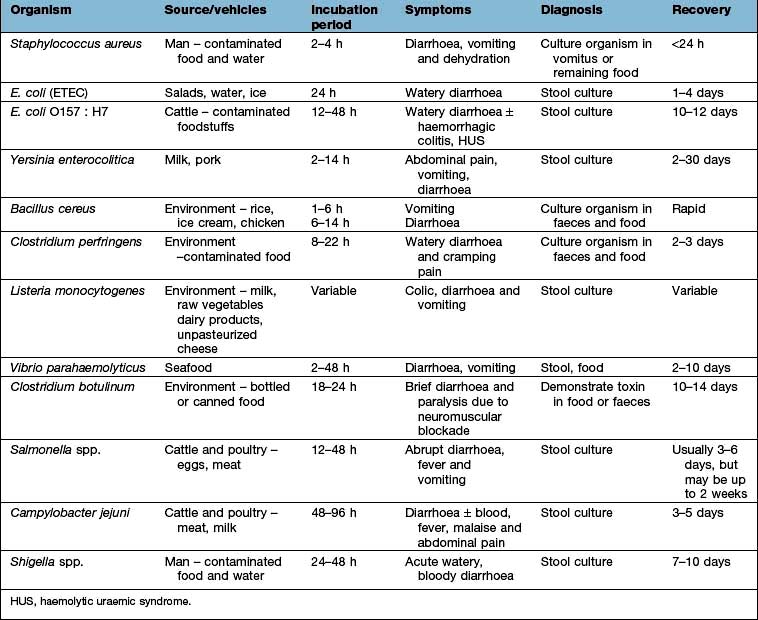
Shingles (see p. 1199) arises from the reactivation of virus latent within the dorsal root or cranial nerve ganglia. It may occur at all ages but is most common in the elderly, producing skin lesions similar to chickenpox, although classically they are unilateral and restricted to a sensory nerve (i.e. dermatomal) distribution (Fig. 4.15). The onset of the rash of shingles is usually preceded by severe dermatomal pain, indicating the involvement of sensory nerves in its pathogenesis. Virus is disseminated from freshly formed vesicles and may cause chickenpox in susceptible contacts.
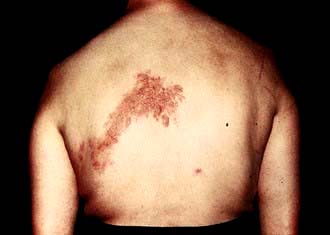
Figure 4.15 Shingles – VZV affecting a dermatome.
(Reproduced with kind permission of Imperial College School of Medicine.)
The commonest complication of shingles is post-herpetic neuralgia (PHN) (see p. 1129).
Diagnosis
The diseases are usually recognized clinically but can be confirmed by detection of VZV DNA within vesicular fluid using PCR, electron microscopy, immunofluorescence or culture of vesicular fluid and by serology.
Prophylaxis and treatment
Chickenpox usually requires no treatment in healthy children and infection results in lifelong immunity. Aciclovir and derivatives are, however, licensed for this indication in the USA, where the argument for treatment is one of health economics, viz. the sooner the child recovers, the sooner the carer can return to work. However, the disease may be fatal in the immunocompromised, who should therefore be offered protection, after exposure to the virus, with zoster-immune globulin (ZIG) and high-dose aciclovir at the first sign of development of the disease.
Anyone with chickenpox who is over the age of 16 years should be given antiviral therapy with aciclovir or a similar drug, if they present within 72 h of onset. Prophylactic ZIG is recommended for susceptible pregnant women exposed to varicella zoster virus and, if chickenpox develops, aciclovir treatment should be given (NB: aciclovir has not been licensed for use in pregnant women). If a woman has chickenpox at term, her baby should be protected by ZIG if delivery occurs within 7 days of the onset of the mother’s rash. An effective live attenuated varicella vaccine is licensed as a routine vaccination of childhood in the USA; it is available on a named-patient basis in the UK and also for susceptible healthcare workers.
Shingles involving motor nerves, e.g. 7th cranial nerve leading to facial palsy, is also treated with aciclovir (or derivatives thereof) as the duration of lesion formation and time to healing can be reduced by early treatment. Aciclovir, valaciclovir and famciclovir have all been shown to reduce the burden of post-herpetic neuralgia when treatment is given in the acute phase. Shingles involving the ophthalmic division of the trigeminal nerve has an associated 50% incidence of acute and chronic ophthalmic complications. Early treatment with aciclovir reduces this to 20% or less. As for chickenpox, all immunocompromised individuals should be given aciclovir at the onset of shingles, no matter how mild the attack appears when it first presents.
Vaccination of all adults over the age of 60, with a dose higher than that used for chickenpox prophylaxis in childhood, reduces shingles-related morbidity and post-herpetic neuralgia and is recommended for all people in the USA.
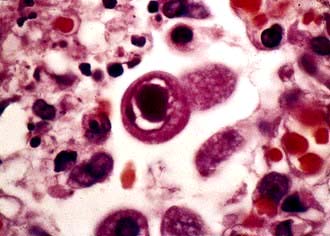
Cytomegalovirus (CMV) infection
Clinically significant CMV infection arises particularly in two patient groups: fetuses who acquire the infection transplacentally and are born congenitally infected and patients who are immunosuppressed, e.g. transplant recipients or patients with HIV infection. As with all herpesviruses, the virus persists for life, usually as a latent infection in which the naked DNA is situated extrachromosomally in the nuclei of the cells in the endothelium of the arterial wall and in T lymphocytes. Over 50% of the adult population have serological evidence of latent CMV infection.
Clinical features
In healthy children and adults, CMV infection is usually asymptomatic but may cause an illness similar to infectious mononucleosis, with fever, occasionally lymphocytosis with atypical lymphocytes and hepatitis with or without jaundice. The Paul–Bunnell test for heterophile antibody is negative. Infection may be spread in saliva (accounting for extensive person-to-person spread in childcare units), sexual intercourse or blood transfusion and transplacentally to the fetus. Disseminated fatal infection with widespread visceral involvement occurs in the immunocompromised (see p. 190) and may cause encephalitis, retinitis, pneumonitis and diffuse involvement of the gastrointestinal tract.
Intrauterine infection may arise from either primary or reactivated maternal infection. CMV is, by far, the commonest congenital infection – in developed countries, such as the UK, 0.3–1% of all babies are born congenitally infected with CMV. Around 5–10% of such babies have severe disease evident at birth, with a poor prognosis. CNS involvement may cause microcephaly and motor disorders. Jaundice and hepatosplenomegaly are common and thrombocytopenia and haemolytic anaemia also occur. Periventricular calcification is seen on skull X-ray. A further 5–10% of infected babies are normal at birth, but developmental abnormalities become apparent later, e.g. sensorineural nerve deafness. The remaining 80–85% of infected babies are normal at birth and develop normally.
Diagnosis
Serological tests can identify latent (IgG) or primary (IgM) infection. However, most infections are now diagnosed by detection and quantification of CMV DNA or RNA using molecular amplification techniques, in blood or other body fluid samples. The virus can also be identified in tissues by the presence of characteristic intranuclear ‘owl’s eye’ inclusions (Fig. 4.16) on histological staining and by direct immunofluorescence. Culture in human embryo fibroblasts is usually slow but diagnosis can be accelerated by immunofluorescent detection of antigen in the cultures.
Treatment
In the immunocompetent, infection is usually self-limiting and no specific treatment is required. In the immunosuppressed, ganciclovir (5 mg/kg twice daily for 14–21 days) reduces retinitis and gastrointestinal damage and can eliminate CMV from blood, urine and respiratory secretions. It is less effective against pneumonitis. In patients who are continually immunocompromised, particularly those with AIDS, maintenance therapy may be necessary. Drug resistance has been reported in AIDS patients and transplant recipients. Bone marrow toxicity is common. Valganciclovir, foscarnet and cidofovir are also available (see p. 93). Treatment of CMV in neonates is difficult, but ganciclovir therapy of infected babies with evident CNS involvement has been shown to improve long-term hearing outcome.
Epstein–Barr virus (EBV) infection
Globally, most individuals are infected with this virus at an early age (0–5 years), at which time clinical symptoms are unusual. Infection at an older age is associated with an acute febrile illness known as infectious mononucleosis (glandular fever), which occurs worldwide in adolescents and young adults. EBV is probably transmitted in saliva and by aerosol.
Clinical features
The predominant symptoms of infectious mononucleosis are fever, headache, malaise and sore throat. Palatal petechiae and a transient macular rash are common, the latter occurring in 90% of patients who have received ampicillin (inappropriately) for the sore throat. Cervical lymphadenopathy, particularly of the posterior cervical nodes, and splenomegaly are characteristic. Mild hepatitis is common, but other complications such as myocarditis, meningitis, encephalitis, mesenteric adenitis and splenic rupture are rare. Splenic rupture occurs in the first 3 weeks of illness and contact sport should be avoided during this period.
Although some young adults remain debilitated and depressed for some months after infection, the evidence for reactivation of latent virus in healthy individuals is controversial, although this is thought to occur in immunocompromised patients.
Following primary infection, EBV remains latent in resting memory B lymphocytes. It has been shown in vitro that of nearly 100 viral genes expressed during replication, approximately only 10 are expressed in the latently infected B cells.
Severe, often fatal infectious mononucleosis may result from a rare X-linked lymphoproliferative syndrome affecting young boys. Those who survive have an increased risk of hypogammaglobulinaemia and/or lymphoma.
EBV is the cause of oral hairy leucoplakia in AIDS patients and is intimately linked to the generation of a number of malignancies, including Burkitt’s lymphoma, undifferentiated nasopharyngeal carcinoma, post-transplant lymphoma, the immunoblastic lymphoma of AIDS patients, some forms of Hodgkin’s lymphoma and gastric cancer. Different levels of expression of EBV latency genes occur in these proliferative conditions caused by the virus and various co-factors are also involved in their pathogenesis, e.g. in Burkitt’s lymphoma, the commonest tumour of childhood in sub-Saharan Africa, epidemiological evidence points to an interplay between EBV infection and the presence of hyperendemic (i.e. present all year-round) malaria. EBV is a cause of haemophagocytic lymphohistiocytosis (HLH) (see p. 379). HLH is an uncommon condition presenting with fever, rash, jaundice, hepatosplenomaly and enlarged lymph nodes. Blood tests show cytopenia, a high ferritin and the bone marrow shows haemophagocytosis. It can be primary (inherited) or secondary, e.g. EBV, and has a high mortality.
Diagnosis
EBV infection should be strongly suspected if atypical mononuclear cells (activated CD8 positive T lymphocytes) are found in the peripheral blood. It can be confirmed during the second week of infection by a positive Paul–Bunnell reaction, which detects heterophile antibodies (IgM) that agglutinate sheep erythrocytes, in around 90% of cases. False positives can occur in other conditions such as viral hepatitis, Hodgkin’s lymphoma and acute leukaemia. The Monospot test is a sensitive and easily performed screening test for heterophile antibodies. Specific EBV IgM antibodies indicate recent infection by the virus. Clinically similar illnesses are produced by CMV, toxoplasmosis and acute HIV infection (the so-called seroconversion illness) but these can be distinguished serologically.
Treatment
The majority of cases require no specific treatment and recovery is rapid. Corticosteroid therapy is advised when there is neurological involvement (e.g. encephalitis, meningitis, Guillain–Barré syndrome), when there is marked thrombocytopenia or haemolysis, or when the tonsillar enlargement is so marked as to cause respiratory obstruction.
Human herpesvirus type 6 (HHV-6)
This human herpesvirus infects CD4+ T lymphocytes, occurs worldwide and exists as a latent infection in over 85% of the adult population. It is spread by contact with oral secretions. The virus causes roseola infantum (exanthem subitum), which presents as a high fever followed by generalized macular rash in infants. HHV-6 is a common cause of febrile convulsions and aseptic meningitis or encephalitis occur as rare complications. Reactivation in the immunocompromised may lead to severe pneumonia.
Human herpesvirus type 7 (HHV-7)
This virus is similar to HHV-6 in being a T lymphotropic herpesvirus. It is also present as a latent infection in over 85% of the adult population and it is known to infect CD4+ helper T cells by using the CD4 antigen (the main receptor employed by HIV). The full spectrum of disease due to HHV-7 has not yet been fully characterized, but, like HHV-6, it may cause roseola infantum in infants.
Human herpesvirus type 8 (Kaposi’s sarcoma-associated herpesvirus)
This human herpesvirus is strongly associated with the aetiology of all forms of Kaposi’s sarcoma. Antibody prevalence is high in those with tumours but relatively low in the general population of most industrialized countries. High rates of infection (>50% population) have been described in central and southern Africa and this matches the geographic distribution of classical Kaposi’s sarcoma before the era of AIDS. HHV-8 is transmitted sexually and through exposure to blood from needle sharing. It is thought that salivary transmission may be the predominant route in Africa. HHV-8 RNA transcripts have been detected in Kaposi’s sarcoma cells and in circulating mononuclear cells from patients with the tumour. This virus also has an aetiological role in two rare lymphoproliferative diseases – multicentric Castleman’s disease (a disorder of the plasma cell type) and primary effusion lymphoma (body-cavity-based lymphoma), which is characterized by pleural, pericardial or peritoneal lymphomatous effusions in the absence of a solid tumour mass.
Papovaviruses
This virus family originally comprised the papilloma, polyoma and vacuolar viruses, although the polyomaviruses have now been reclassified into a separate family. These viruses tend to produce chronic infections, often with evidence of latency. They are capable of inducing neoplasia in some animal species and were among the first viruses to be implicated in tumorigenesis. Human papillomaviruses, of which there are at least 100 types, are responsible for the common skin and genital warts and certain types (mainly 16 and 18) are the cause of carcinoma of the cervix and some oral cancers (type 16). The realization that cancer of the cervix is an infectious disease has led to the development of papillomavirus vaccines, which have been shown to prevent disease associated with the high-risk HPV types 16 and 18 and have recently been licensed for use in the USA, Australia and Europe. The current recommendations in many countries are for vaccination of all girls at age 9–14 years. It may also be sensible to vaccinate boys, but this strategy is much less cost-effective. (For genital warts see page 169.)
The human BK virus, a polyomavirus, is generally found in immunocompromised individuals and may be detected in the urine of 15–40% of renal transplant patients. Rarely, this is associated with impairment of function of the transplanted kidney – BK nephropathy. JC virus, also a polyomavirus, is the cause of progressive multifocal leucoencephalopathy (PML), which presents as dementia in the immunocompromised and is due to progressive cerebral destruction resulting from accumulation of the virus in brain tissue. WU and KI polyomaviruses have been recently identified. These may be associated with respiratory tract infections in young children. Merkel cell polyomavirus has been identified in the malignant tissue of the rare Merkel cell carcinoma of the skin.
Erythroviruses
Human erythrovirus B19 causes erythema infectiosum (fifth disease), a common infection in schoolchildren. The rash is typically on the face (the ‘slapped-cheek’ appearance). The patient is well and the rash can recur over weeks or months. Asymptomatic infection occurs in 20% of children. Nonspecific respiratory tract illness is another common manifestation of infection. Moderately severe self-limiting polyarthropathy (see p. 519) is common if infection occurs in adulthood, especially in women. Aplastic crisis may occur in patients with chronic haemolysis (e.g. sickle cell disease). Chronic infection with anaemia occurs in immunocompromised subjects. Hydrops fetalis (3% risk) and spontaneous abortion (9% risk) may result from infection during the first and second trimesters of pregnancy.
Bocavirus is a recently identified erythrovirus, which accounts for around 3–5% of respiratory tract infections in young children.
Poxviruses
Smallpox (variola)
This disease was eradicated in 1977 following an aggressive vaccination policy and careful detection of new cases coordinated by the World Health Organization. Its possible use in bioterrorism has resulted in the reintroduction of smallpox vaccination in some countries (see p. 935).
Monkeypox
This is a rare zoonosis that occurs in small villages in the tropical rainforests in several countries of West and Central Africa. Its clinical effects, including a generalized vesicular rash, are indistinguishable from smallpox, but person-to-person transmission is unusual. Most infections occur in children who have not been vaccinated against smallpox. Disease can be severe, with mortality rates of 10–15% in unvaccinated individuals. Serological surveys indicate that several species of squirrel are likely to represent the animal reservoir. The virus was introduced into the USA in 2003 via West African small mammals illegally imported as pets. Widespread infection of prairie dogs resulted and there were 37 laboratory confirmed cases in humans, only two of which suffered complications (keratitis, encephalopathy).
Cowpox
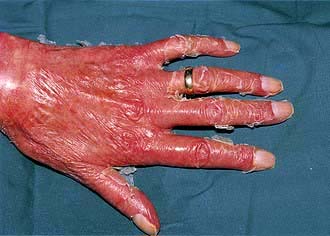
Cowpox produces large vesicles which are classically on the hands in those in contact with infected cows. The lesions are associated with regional lymphadenitis and fever. Cowpox virus has been found in a range of species including domestic and wild cats and the reservoir is thought to exist in a range of rodents.
Vaccinia virus
This is a laboratory virus and does not occur in nature in either humans or animals. Its origins are uncertain but it has been invaluable in its use as the vaccine to prevent smallpox. Vaccination is now not recommended except for laboratory personnel handling certain poxviruses for experimental purposes or in contingency planning to manage a deliberate release of smallpox virus. It is being assessed experimentally as a possible carrier for new vaccines.
Orf
This poxvirus causes contagious pustular dermatitis in sheep and hand lesions in humans (see p. 1199).
Hepadna viruses
These partially double-stranded DNA viruses infect a number of species. The representative virus from this family which infects humans, hepatitis B virus, is discussed, along with other hepatitis viruses, on page 317.
RNA viruses (Table 4.18)
Picornaviruses (PICO = small)
This is a large family of small RNA viruses, which includes the enteroviruses and rhinoviruses which infect humans and also hepatitis A virus (see p. 316). The term enterovirus refers to the enteric means of spread of these viruses, i.e. via the faeco-oral route. The enteroviruses include poliovirus types 1–3, Cxsackie A and B viruses, echoviruses and enteroviruses (EV) 68–71. There are several newly described EVs yet to be officially classified.
Poliovirus infection (poliomyelitis)
Poliomyelitis occurs when a susceptible individual is infected with poliovirus type 1, 2 or 3. These viruses have a propensity for the nervous system, especially the anterior horn cells of the spinal cord and cranial motor neurones. Poliomyelitis was found worldwide but its incidence has decreased dramatically following improvements in sanitation, hygiene and the widespread use of polio vaccines. Spread is usually via the faeco-oral route, as the virus is excreted in the faeces.
Clinical features
The incubation period is 7–14 days. Although polio is essentially a disease of childhood, no age is exempt. The clinical manifestations vary considerably. The commonest outcome (95% of individuals) is asymptomatic seroconversion.
 Abortive poliomyelitis occurs in approximately 4–5% of cases, characterized by the presence of fever, sore throat and myalgia. The illness is self-limiting and of short duration.
Abortive poliomyelitis occurs in approximately 4–5% of cases, characterized by the presence of fever, sore throat and myalgia. The illness is self-limiting and of short duration.
 Non-paralytic poliomyelitis (poliovirus meningitis) has features of abortive poliomyelitis as well as signs of meningeal irritation, but recovery is complete.
Non-paralytic poliomyelitis (poliovirus meningitis) has features of abortive poliomyelitis as well as signs of meningeal irritation, but recovery is complete.
 Paralytic poliomyelitis occurs in approximately 0.1% of infected children (1.3% of adults). Factors predisposing to the development of paralysis include male sex, exercise early in the illness, trauma, surgery or intramuscular injection, which localize the paralysis, and recent tonsillectomy (bulbar poliomyelitis).
Paralytic poliomyelitis occurs in approximately 0.1% of infected children (1.3% of adults). Factors predisposing to the development of paralysis include male sex, exercise early in the illness, trauma, surgery or intramuscular injection, which localize the paralysis, and recent tonsillectomy (bulbar poliomyelitis).
The paralytic form of the disease follows about 4–5 days after an initial illness simulating abortive poliomyelitis. Meningeal irritation and muscle pain recur and are followed by the onset of asymmetric flaccid paralysis without sensory involvement. The paralysis is usually confined to the lower limbs in children under 5 years of age and the upper limbs in older children, whereas in adults it manifests as paraplegia or quadriplegia.
Diagnosis
The diagnosis is a clinical one. Distinction from Guillain–Barré syndrome is easily made by the absence of sensory involvement and the asymmetrical nature of the paralysis in poliomyelitis. Laboratory confirmation and distinction between the wild virus and vaccine strains is achieved by genome detection techniques, virus culture, neutralization and temperature marker tests.
Treatment
Treatment is supportive. Bed rest is essential during the early course of the illness. Respiratory support with intermittent positive-pressure respiration is required if the muscles of respiration are involved. Once the acute phase of the illness has subsided, occupational therapy, physiotherapy and occasionally surgery have roles in patient rehabilitation.
Prevention and control
Immunization (Box 4.6) has dramatically decreased the prevalence of this disease worldwide and global eradication of the virus, coordinated by the World Health Organization, is within reach. The virus remains endemic in only four countries: Nigeria, India, Pakistan and Afghanistan. However, there have been outbreaks in West Africa where cultural taboos have disrupted the polio vaccination campaign and also in the Sudan, with spread of disease into neighbouring countries. New preparations of inactivated IM poliovirus vaccine (IPV) have greater potency than the original Salk IPV. The greater reliability of IPV in hot climates and the scientific and ethical problems of continuing to use oral polio vaccine (OPV) in countries free from poliomyelitis, mean that IPV has replaced OPV in the routine immunization schedules in many countries.
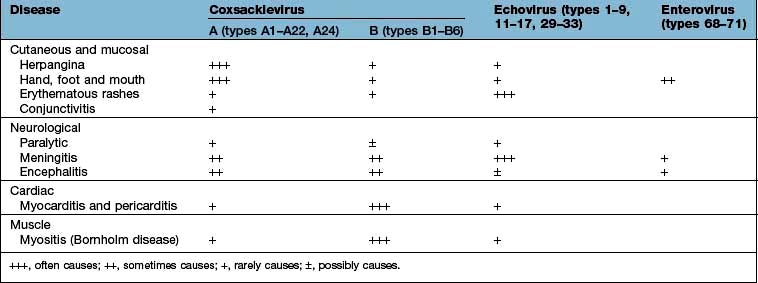
Coxsackievirus, echovirus and other enterovirus infections
These viruses are also spread by the faeco-oral route. They each have a number of different types and are responsible for a broad spectrum of disease involving the skin and mucous membranes, muscles, nerves, the heart (Table 4.19) and, rarely, other organs, such as the liver and pancreas. They are frequently associated with pyrexial illnesses and are the most common cause of aseptic meningitis.
This disease is mainly caused by Coxsackie A viruses and presents with a vesicular eruption on the fauces, palate and uvula. The lesions evolve into ulcers. The illness is usually associated with fever and headache but is short-lived, recovery occurring within a few days.
This disease is mainly caused by Coxsackievirus A16 or A10. It is also the main feature of infection with enterovirus (EV)71. Oral lesions are similar to those seen in herpangina but may be more extensive in the oropharynx. Vesicles and a maculopapular eruption also appear, typically on the palms of the hands and the soles of the feet, but also on other parts of the body. This infection commonly affects children. Recovery occurs within a week.
Other enteroviruses in addition to poliovirus can cause a broad range of neurological disease, including meningitis, encephalitis and a paralytic disease similar to poliomyelitis. EV71 has particular predilection for neuroinvasion. Thus, in epidemics of EV71 infection, a variety of serious neuromotor syndromes arise, albeit in a minority of those infected.
Enterovirus infection is a cause of acute myocarditis and pericarditis, from which, in general, there is complete recovery. However, these viruses can also cause chronic congestive cardiomyopathy and, rarely, constrictive pericarditis.
Skeletal muscle involvement, particularly of the intercostal muscles, is a feature of Bornholm disease, a febrile illness usually due to Coxsackievirus B. The pain may be of such an intensity as to mimic pleurisy or an acute abdomen. The infection affects both children and adults and may be complicated by meningitis or cardiac involvement.
Rhinovirus infection
Rhinoviruses are responsible for the common cold (see p. 808). Chimpanzees and humans are the only species to develop the common cold. ICAM-1 is a cellular receptor for rhinoviruses and only these two species have the specific binding domain. Peak incidence rates occur in the colder months, especially spring and autumn. There are multiple rhinovirus immunotypes (>100), which makes vaccine control impracticable. In contrast to enteroviruses, which replicate at 37°C, rhinoviruses grow best at 33°C (the temperature of the upper respiratory tract), which explains the localized disease characteristic of common colds.
Reoviruses
Reoviruses are a large family of viruses with double-stranded RNA segmented genomes.
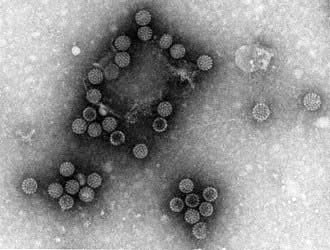
Rotavirus infection
Rotavirus (Latin rota = wheel) is so named because of its electron microscopic appearance with a characteristic circular outline with radiating spokes (Fig. 4.17). It is responsible worldwide for both sporadic cases and epidemics of diarrhoea and is the commonest cause of childhood diarrhoea. More than 500 000 infected children under the age of 5 years are estimated to die annually in resource-deprived countries, compared with 75–150 in the USA. The prevalence is higher during the winter months in non-tropical areas. Asymptomatic infections are common and bottle-fed babies are more likely to be symptomatic than those that are breast-fed.
Adults may become infected with rotavirus but symptoms are usually mild or absent. The virus may, however, cause diarrhoea in immunosuppressed adults, or outbreaks in patients on care of the elderly wards.
Clinical features
The illness is characterized by vomiting, fever, diarrhoea and the metabolic consequences of water and electrolyte loss.
Diagnosis and differential diagnosis
The diagnosis can be established by PCR for genome detection, or ELISA for the detection of rotavirus antigen in faeces and by electron microscopy of faeces. Histology of the jejunal mucosa in children shows shortening of the villi, with crypt hyperplasia and mononuclear cell infiltration of the lamina propria.
Treatment and prevention
Treatment is directed at overcoming the effects of water and electrolyte imbalance with adequate oral rehydration therapy and, when indicated, intravenous fluids (see Box 4.10). Antibiotics should not be prescribed. A controlled trial in Egypt in children with rotavirus diarrhoea demonstrated faster recovery (31 h vs 75 h) in those given nitazoxanide, a broad-spectrum anti-infective agent, for 3 days compared with placebo.
Despite a major setback when the first licensed rotavirus vaccine was rapidly withdrawn from the market in 1999 following reports of increased rates of intussusception, two new vaccines have been developed and are now used in many countries. Both are live vaccines. One vaccine contains an attenuated human strain with the relevant antigens being P[8] and G1, while another is based on a bovine parent strain and comprises five single-gene reassortants each containing a human-strain outer capsid gene encoding the most common human antigenic types (P[8] and G1–4).
Caliciviruses
This extensive virus family, named after the cup-shaped (Latin calyx = cup) indentations on their viral surface seen by electron microscopy, contains four genera, two of which, the noroviruses and sapoviruses, infect humans and cause gastroenteritis.
Norovirus is the major cause of acute non-bacterial gastroenteritis, causing outbreaks in nursing homes, hospitals, schools, leisure centres, restaurants and cruise ships. Transmission is mostly faeco-oral with outbreaks suggesting a common source, such as food and water and fomites. Aerosol transmission also occurs and noroviruses can be detected in vomit. Illness is usually self-limiting (12–48 hours) and mild, consisting of nausea, headache and abdominal cramps, followed by diarrhoea and vomiting, which may be the only feature (winter vomiting). Diagnosis is by demonstration of viral nucleic acid or antigen in diarrhoeal faeces. Treatment is with oral rehydration solutions (ORS). Prevention can be difficult but handwashing and good hygienic food preparation is required.
Sapovirus causes gastroenteritis, mainly in children.
Other viruses associated with gastroenteritis are shown in Table 4.20.
Table 4.20 Viruses associated with gastroenteritis
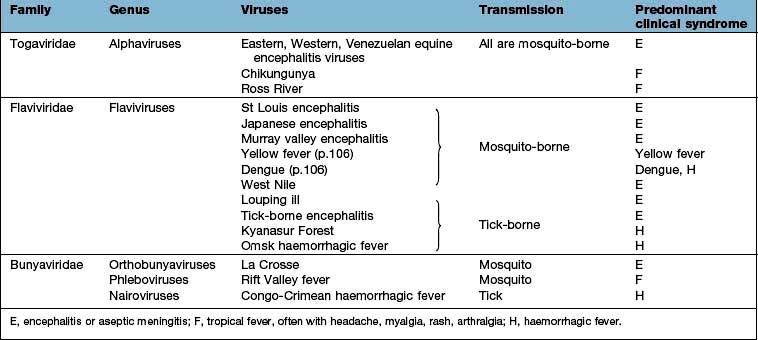
Togaviruses
This family comprises two genera: the rubiviruses, which include rubella virus; and the alphaviruses, which include some of the arthropod-borne viruses (see Box 4.7 and Table 4.21).
![]() Box 4.7
Box 4.7
Arbovirus (arthropod-borne) infection
Defining features of arboviruses
 Zoonotic viruses, transmitted through the bites of insects, especially mosquitoes and ticks.
Zoonotic viruses, transmitted through the bites of insects, especially mosquitoes and ticks.
 >385 viruses are classified as arboviruses.
>385 viruses are classified as arboviruses.
 Most are members of the Togavirus, Flavivirus and Bunyavirus families (see Table 4.21).
Most are members of the Togavirus, Flavivirus and Bunyavirus families (see Table 4.21).
 Culex, Aedes and Anopheles mosquitoes account for transmission of the majority of these viruses.
Culex, Aedes and Anopheles mosquitoes account for transmission of the majority of these viruses.
Clinical features of arbovirus infection
 Most arbovirus diseases are generally mild; epidemics are frequent and when these occur, the mortality is high.
Most arbovirus diseases are generally mild; epidemics are frequent and when these occur, the mortality is high.
 In general, short incubation period (<10 days). Common features include a biphasic illness, pyrexia, conjunctival suffusion, a rash, retro-orbital pain, myalgia and arthralgia. Lymphadenopathy is seen in dengue.
In general, short incubation period (<10 days). Common features include a biphasic illness, pyrexia, conjunctival suffusion, a rash, retro-orbital pain, myalgia and arthralgia. Lymphadenopathy is seen in dengue.
 Haemorrhage (from increased vascular permeability, capillary fragility, consumptive coagulopathy) is a feature of some arbovirus infections (see Table 4.23).
Haemorrhage (from increased vascular permeability, capillary fragility, consumptive coagulopathy) is a feature of some arbovirus infections (see Table 4.23).
 Encephalitis resulting from cerebral invasion may be prominent in some arbovirus fevers.
Encephalitis resulting from cerebral invasion may be prominent in some arbovirus fevers.
Rubella
Rubella (‘German measles’) is caused by a spherical, enveloped RNA virus which is easily killed by heat and ultraviolet light. While the disease can occur sporadically, epidemics are not uncommon. It has a worldwide distribution. Spread of the virus is via droplets; maximum infectivity occurs before and during the time the rash is present.
Clinical features
The incubation period is 14–21 days, averaging 18 days. The clinical features are largely determined by age, with symptoms being mild or absent in children under 5 years.
During the prodrome, the patient may develop malaise and fever and mild conjunctivitis and lymphadenopathy involving particularly the suboccipital, postauricular and posterior cervical groups of lymph nodes. Small petechial lesions on the soft palate (Forchheimer spots) are suggestive but not diagnostic. Splenomegaly may be present.
The eruptive or exanthematous phase usually occurs within 7 days of the initial symptoms. The rash first appears on the forehead and then spreads to involve the trunk and limbs. It is pinkish red, macular and discrete, although some of these lesions may coalesce (Fig. 4.18). It usually fades by the second day and rarely persists beyond 3 days after its appearance.
Complications
Complications are rare. They include superadded pulmonary bacterial infection, arthralgia (commoner in females), haemorrhagic manifestations due to thrombocytopenia, encephalitis and the congenital rubella syndrome. Rubella affects the fetuses of up to 80% of all women who contract the infection during the 1st trimester of pregnancy. The incidence of congenital abnormalities diminishes in the 2nd trimester and no ill-effects result from infection in the 3rd trimester.
Congenital rubella syndrome is characterized by the presence of fetal cardiac malformations, especially patent ductus arteriosus and ventricular septal defect, eye lesions (especially cataracts), microcephaly, mental retardation and deafness. Hepatosplenomegaly, myocarditis, interstitial pneumonia and metaphyseal bone lesions also occur.
Diagnosis and treatment
The diagnosis is clinical, but laboratory diagnosis is essential (especially in pregnancy) to distinguish the illness from other virus infections (e.g. erythrovirus B19, echovirus) and drug rashes. This is achieved by the detection of rubella-specific IgM by ELISA in an acute serum sample, preferably confirmed by the demonstration of IgG seroconversion (or a rising titre of IgG) in a subsequent sample taken 14 days later. Viral genome can be detected in throat swabs (or oral fluid samples), urine and, in the case of intrauterine infection, the products of conception.
Prevention
Human immunoglobulin can decrease the symptoms of this already mild illness, but does not prevent the teratogenic effects. Several live attenuated rubella vaccines have been used with great success in preventing this illness and these have been successfully combined with measles and mumps vaccines in the MMR vaccine. The side-effects of vaccination have been dramatically decreased by using vaccines prepared in human embryonic fibroblast cultures (RA 27/3 vaccine). Use of the vaccine is contraindicated during pregnancy or if there is a likelihood of pregnancy within 3 months of immunization. Inadvertent use of the vaccine during pregnancy has not, however, revealed a risk of teratogenicity.
Alphaviruses
The 29 viruses of this group are all transmitted by mosquitoes; eight result in human disease (see Box 4.7 and Table 4.21). These viruses are globally distributed and tend to acquire their names from the location where they were first isolated (such as Ross River, Eastern, Venezuelan and Western equine encephalitis viruses) or by the local expression for a major symptom caused by the virus (such as chikungunya, meaning ‘doubled up’). Infection is characterized by fever, headache, maculopapular skin rash, arthralgia, myalgia and sometimes encephalitis.
Major epidemics of chikungunya were reported in India, Sri Lanka and islands in the Indian Ocean (including Reunion, Mauritius and the Seychelles) in 2005 and 2006, with at least 1 million cases and several hundred deaths. The severity of these epidemics is possibly due to a viral strain with mutations resulting in a higher neurovirulence. Several European countries reported cases of chikungunya in returning travellers – including 133 cases in the UK in 2006. The virus may now populate the local Aedes mosquito via increased numbers of travellers who may import virus into countries where it has not previously been described, e.g. an outbreak in Italy in 2007 involved over 150 cases with one death and two locally-acquired confirmed cases were reported from the French mainland in 2010.
Flaviviruses
There are 60 viruses in this group, some of which are transmitted by ticks and others by mosquitoes. Hepatitis C virus (see p. 323) is also a flavivirus.
Yellow fever
Yellow fever, caused by a flavivirus, is an illness of widely varying severity. It is confined to Africa (90% of cases) and South America between latitudes 15°N and 15°S. For poorly understood reasons, yellow fever has not been reported from Asia, despite the fact that climatic conditions are suitable and the vector, Aedes aegypti, is common. The infection is transmitted in the wild by A. africanus in Africa and the Haemagogus species in South and Central America. Extension of infection to humans (via the mosquito from monkeys) leads to the occurrence of ‘jungle’ yellow fever. A. aegypti, a domestic mosquito which lives in close relationship to humans, is responsible for human-to-human transmission in urban areas (urban yellow fever). Once infected, a mosquito remains so for life.
Clinical features
The incubation period is 3–6 days. Mild infection is indistinguishable from other viral fevers such as influenza or dengue.
Three phases in the severe (classical) illness are recognized. Initially, the patient presents with a high fever of acute onset, usually 39–40°C, which then returns to normal in 4–5 days. During this time, headache is prominent. Retrobulbar pain, myalgia, arthralgia, a flushed face and suffused conjunctivae are common. Epigastric discomfort and vomiting are present when the illness is severe. Relative bradycardia (Faget’s sign) is present from the second day of illness. The patient then makes an apparent recovery and feels well for several days. Following this ‘phase of calm’, the patient again develops increasing fever, deepening jaundice and hepatomegaly. Ecchymosis, bleeding from the gums, haematemesis and melaena may occur. Coma, which is usually a result of uraemia or haemorrhagic shock, occurs for a few hours preceding death. The mortality rate is up to 40% in severe cases. The pathology of the liver shows mid-zone necrosis and eosinophilic degeneration of hepatocytes (Councilman bodies) (see p. 316).
Diagnosis and treatment
The diagnosis is established by a history of travel and vaccination status and by isolation of the virus (when possible) from blood during the first 3 days of illness. Serodiagnosis is possible, but in endemic areas cross-reactivity with other flaviviruses is a problem.
Treatment is supportive. Bed rest (under mosquito nets), analgesics and maintenance of fluid and electrolyte balance are required.
Prevention and control
Yellow fever is an internationally notifiable disease. It is easily prevented using the attenuated 17D chick embryo vaccine but concerns over safety have arisen because of infection with the 17D virus. Vaccination is not recommended for children under 9 months or immunosuppressed patients, unless there are compelling reasons. For the purposes of international certification, immunization is valid for 10 years, but protection lasts much longer than this and probably for life. The WHO Expanded Programme of Immunization includes yellow fever vaccination in endemic areas.
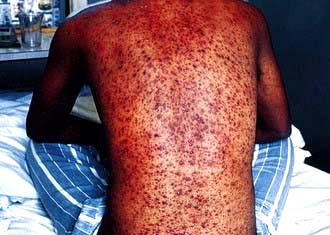
Dengue
This is the commonest arthropod-borne viral infection in humans: over 100 million cases occur every year in the tropics, with over 10 000 deaths from dengue haemorrhagic fever. Dengue is caused by a flavivirus and is found mainly in Asia, South America and Africa, although it has been reported from the USA and, more recently, in Italy.
Four different antigenic varieties of dengue virus are recognized and all are transmitted by the daytime-biting A. aegypti, which breeds in standing water in refuse dumps in inner cities. A. albopictus is a less common transmitter. Humans are infective during the first 3 days of the illness (the viraemic stage). Mosquitoes become infective about 2 weeks after feeding on an infected individual and remain so for life. The disease is usually endemic. Heterotypic immunity between serotypes after the illness is partial and lasts only a few months, although homotype immunity is lifelong.
Clinical features
The incubation period is 5–6 days following the mosquito bite. Asymptomatic or mild infections are common. Two clinical forms are recognized (Fig. 4.19).
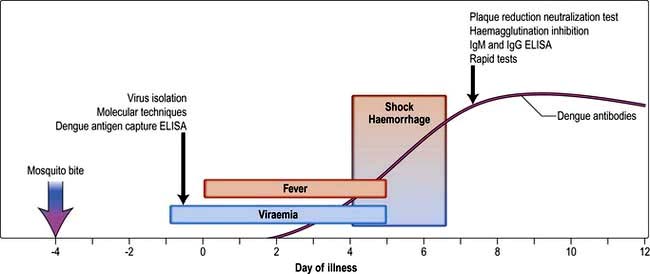
Figure 4.19 Dengue infection – course and timing of diagnosis.
(Reprinted from Halstead SB. Dengue. Lancet 2007; 370:1644–1652, with permission from Elsevier.)
Classic dengue fever is characterized by the abrupt onset of fever, malaise, headache, facial flushing, retrobulbar pain which worsens on eye movements, conjunctival suffusion and severe backache, which is a prominent symptom. Lymphadenopathy, petechiae on the soft palate and transient, morbilliform skin rashes may also appear on the limbs with subsequent spread to involve the trunk. Desquamation occurs subsequently. Cough is uncommon. The fever subsides after 3–4 days, the temperature returns to normal for a couple of days and then the fever returns, together with the features already mentioned, but milder. This biphasic or ‘saddleback’ pattern is considered characteristic. Severe fatigue, a feeling of being unwell and depression are common for several weeks after the fever has subsided.
Dengue haemorrhagic fever (DHF)
Dengue haemorrhagic fever is a severe form of dengue fever and is believed to be the result of two or more sequential infections with different dengue serotypes. It is characterized by the capillary leak syndrome, thrombocytopenia, haemorrhage, hypotension and shock. It is characteristically a disease of children, occurring most commonly in South-east Asia. The disease has a mild start, often with symptoms of an upper respiratory tract infection. This is then followed by the abrupt onset of shock and haemorrhage into the skin and ear, epistaxis, haematemesis and melaena known as the dengue shock syndrome. This has a mortality of up to 44%. Serum complement levels are depressed and there is laboratory evidence of a consumptive coagulopathy.
Diagnosis and treatment
 Isolation of dengue virus by tissue culture, or detection of viral RNA by PCR in sera obtained during the first few days of illness is diagnostic.
Isolation of dengue virus by tissue culture, or detection of viral RNA by PCR in sera obtained during the first few days of illness is diagnostic.
 Detection of virus-specific IgM antibodies, or of rising IgG titres in sequential serum samples, haemagglutination inhibition, ‘ELISA’ or complement-fixation assays confirm the diagnosis.
Detection of virus-specific IgM antibodies, or of rising IgG titres in sequential serum samples, haemagglutination inhibition, ‘ELISA’ or complement-fixation assays confirm the diagnosis.
Treatment is supportive with analgesics and adequate fluid replacement. Corticosteroids are of no benefit and convalescence can be slow. In DHF, blood transfusion may be necessary.
Prevention
Travellers should be advised to sleep under impregnated nets but this is not very effective as the mosquito bites in daytime. Topical insect repellents should be used. Adult mosquitoes should be destroyed by sprays and breeding sites, e.g. small stagnant water pools, should be eradicated. There is no effective vaccine yet although some are being trialled.
Japanese encephalitis
Japanese encephalitis is a mosquito-borne encephalitis caused by a flavivirus. It has been reported most frequently from the rice-growing countries of South-east Asia and the Far East. Culex tritaeniorhynchus is the usual vector and this feeds mainly on pigs as well as birds such as herons and sparrows. Humans are accidental hosts.
As with other viral infections, the clinical manifestations are variable. The incubation period is 5–15 days. Most infections are asymptomatic. When disease arises, the onset is heralded by severe rigors. Fever, headache and malaise last 1–6 days. Weight loss is prominent. In the acute encephalitic stage, the fever is high (38–41°C), neck rigidity occurs and neurological signs such as altered consciousness, hemiparesis and convulsions develop. Mental deterioration occurs over a period of 3–4 days and culminates in coma. Mortality varies from 7% to 40% and is higher in children. Residual neurological defects such as deafness, emotional lability and hemiparesis occur in about 70% of patients who have had CNS involvement. Convalescence is prolonged. Antibody detection in serum and CSF by IgM capture ELISA is a useful rapid diagnostic test. Vaccines containing formalin-inactivated viruses derived from mouse brain are effective and available. Treatment is supportive.
West Nile virus
In 1999, West Nile virus was first recognized in the western hemisphere (New York, USA) having been previously reported in Africa, Asia and parts of Europe. By the end of 2009, the US outbreak had resulted in over 25 000 human cases and over 1100 deaths. The vast majority of infections are asymptomatic. In a minority of cases, infection presents as a febrile illness, with a maculopapular rash, with 1% resulting in severe encephalitis. Disease severity and mortality is age-related, being greatest in the elderly. The primary hosts of infection are birds. It is spread by mosquitoes and may also infect humans and horses. It can also be transmitted by blood transfusions, breast-feeding and organ donation from an infected individual. Diagnosis is by genome detection in appropriate samples, or specialized serology for the detection of IgM virus-specific antibodies.
Tick-borne encephalitis virus (TBEV)
This flavivirus (actually a series of closely related viruses) is transmitted by Ixodes spp. ticks. It occurs in an area extending from Western Europe to Japan. The tick is the main reservoir for the virus, which is transmitted when it feeds on mice and other rodents.
The disease starts 4–28 days after a bite from an infected tick and is biphasic in 80% of patients. Fever, malaise, headache and fatigue are followed, after a symptom-free period of about 7 days, by encephalitis. There may be associated limb paralysis which is due to anterior horn cell involvement mainly of the cervical region. Cranial nerve involvement also occurs. TBEV IgM and IgG antibodies are present and the virus can be detected in blood by RT-PCR. Overall mortality is about 1% (but can be considerably higher for certain strains) but 30% have impairment in neurological function with persistent paralysis in 6%. A preventative vaccine is available.
Bunyaviruses
Bunyaviruses belong to a large family of more than 200 viruses, grouped into a number of genera, most of which are arthropod-borne.
Congo-Crimean haemorrhagic fever
This widespread disease, caused by a virus of the nairovirus genus of bunyaviruses, is found mainly in Asia and Africa. The primary hosts are cattle and hares and the vectors are the Hyalomma ticks. Following an incubation period of 3–6 days there is an influenza-like illness with fever and haemorrhagic manifestations. The mortality is 10–50%.
Hantaviruses
Hantaviruses belong to the Hantavirus genus of bunyaviruses and are enzootic viruses of wild rodents which are spread by aerosolized excreta and not by insect vectors. The most severe form of this infection is Korean haemorrhagic fever (or haemorrhagic fever with renal syndrome, HFRS). This condition has a mortality of 5–10% and is characterized by fever, shock and haemorrhage followed by an oliguric phase. Milder forms of the disease are associated with related viruses (e.g. Puumala virus) and may present as nephropathia epidemica, an acute fever with renal involvement. It is seen in Scandinavia and in other European countries in people who have been in contact with bank voles. In the USA, a Hantavirus (transmitted by the deer mouse) termed Sin Nombre was identified as the cause of outbreaks of acute respiratory disease (Hantavirus pulmonary syndrome, HPS) in adults. Other Hantavirus types and rodent vector systems have been associated with this syndrome.
Diagnosis of Hantavirus infection is made by an ELISA technique for specific antibodies.
Rift Valley fever
Rift Valley fever, caused by a virus from the phlebovirus genus of bunyaviruses, is primarily an acute febrile illness of livestock: sheep, goats and camels. It is found in southern and eastern Africa. The vector in East Africa is Culex pipiens and in southern Africa, Aedes caballus, but it can be transmitted by the bite of an infected animal. Following an incubation period of 3–6 days, the patient has an acute febrile illness that is difficult to distinguish clinically from other viral fevers. The temperature pattern is usually biphasic. The initial febrile illness lasts 2–4 days and is followed by a remission and a second febrile episode. Complications are indicative of severe infection and include retinopathy, meningoencephalitis, haemorrhagic manifestations and hepatic necrosis. Mortality approaches 50% in severe forms of the illness. Treatment is supportive. Animals can be vaccinated.
Orthomyxoviruses
Influenza
Three types of influenza virus are recognized: A, B and C, distinguishable by the nature of their internal proteins. The influenza virus is a spherical or filamentous enveloped virus. Haemagglutinin (H), a surface glycoprotein, aids attachment of the virus to the surface of susceptible host cells at specific receptor sites. Cell penetration, probably by pinocytosis and release of replicated viruses from the cell surface effected by budding through the cell membrane, is facilitated by the action of the enzyme neuraminidase (N) which is also present on the viral envelope. Sixteen H subtypes (H1–H16) and nine N subtypes (N1–N9) have been identified for influenza A viruses but only H1, H2, H3 and N1 and N2 have established stable lineages in the human population since 1918.
 Influenza A is generally responsible for pandemics and epidemics.
Influenza A is generally responsible for pandemics and epidemics.
 Influenza B often causes smaller or localized and milder outbreaks, such as in camps or schools. There are no subtypes of influenza B.
Influenza B often causes smaller or localized and milder outbreaks, such as in camps or schools. There are no subtypes of influenza B.
Antigenic shift generates new influenza A subtypes, which emerge at irregular intervals and give rise to influenza pandemics. Possible mechanisms include:
1. Genetic reassortment of the RNA of the virus (which is arranged in eight segments) with that of an avian influenza virus; this requires co-infection of a host with both human and avian viruses. The pig is one animal in which this may occur. Alternatively, humans may act as the mixing vessel.
2. Trans-species transmission of an avian influenza virus to humans. Viruses transmitted in this way are usually not well adapted to growth in their new host, but adaptation may occur as a result of spontaneous mutations, leading to the emergence of a pandemic strain.
Antigenic drift (minor changes in influenza A and B viruses) results from point mutations leading to amino acid changes in the two surface glycoproteins, haemagglutinin and neuraminidase, which induce humoral immunity. This enables the virus to evade previously induced immune responses and is the process whereby annual influenza epidemics arise.
Thus, changes due to antigenic shift or drift render the individual’s immune response less able to combat the new variant.
The most serious pandemic of influenza, caused by influenza A/H1N1, occurred in 1918 and was associated with more than 20 million deaths worldwide. In 1957, antigenic shift led to the appearance of influenza A/H2N2, which caused a worldwide pandemic. A further pandemic occurred in 1968 with the emergence of Hong Kong influenza A/H3N2 and minor antigenic drifts have caused outbreaks around the world ever since. In 1976, influenza A H1N1 reappeared, most likely as a result of accidental release from a laboratory and has since co-circulated with A/H3N2 and B viruses. In 1997, avian influenza A/H5N1 viruses were first isolated from humans, raising the spectre of another pandemic. As of July 2010, over 500 sporadic human A/H5N1 infections have been reported from 15 countries, mostly in Asia (Indonesia, China and Vietnam) and almost always arising from direct contact with infected chickens, with a mortality of >50%. However, while this virus is highly pathogenic to humans, due to the induction of a cytokine storm within the lungs, it still has not evolved to replicate well in human cells and human-to-human spread is unusual. However, anxieties remain that either genetic reassortment will occur in a human co-infected with human A/H1N1 or A/H3N2 viruses, or adaptive mutations will occur within infected human hosts, such that a truly pandemic strain will emerge.
In April 2009, a novel influenza A virus (H1N1) was identified in patients with severe respiratory illness in Mexico and North America. The virus quickly spread across the world, with the WHO declaring an official pandemic on 11 June 2009. The virus was the end product of several reassortments between pre-existing swine, avian and human virus lineages, with the swine H1 protein showing around 20% amino acid sequence divergence from previously circulating human seasonal H1N1 influenza viruses. The virus is variably referred to as ‘swine H1N1’, ‘2009 H1N1’ or ‘H1N1v’, where ‘v’ stands for variant. Although unquestionably highly transmissible (with estimates of millions of infections worldwide within 1 year), this pandemic virus was (perhaps fortunately), not especially virulent. Most infections occurred in children – adults over 50 years of age had evidence of pre-existing protective immunity. A minority of infections resulted in serious disease, with an estimated symptomatic case-fatality ratio of 0.04% (equating to around 500 deaths) in the UK. Worldwide, around 20 000 deaths in laboratory confirmed cases have been reported to the WHO. Risk factors for serious disease included pre-existing underlying medical conditions, age <5 years, obesity and pregnancy. The pandemic was declared officially over by the WHO in August 2010, with the virus now expected to behave as a normal seasonal influenza virus, replacing the previously circulating A H1N1 virus.
Purified haemagglutinin and neuraminidase from recently circulating strains of influenza A and B viruses are incorporated in current vaccines.
Sporadic cases of influenza and outbreaks among groups of people living in a confined environment are frequent. The incidence increases during the winter months. Spread is mainly by droplet infection but fomites and direct contact have also been implicated.
The clinical features, diagnosis, treatment and prophylaxis of influenza are discussed on page 811.
Paramyxoviruses
These are a heterogeneous group of enveloped viruses with negative single-stranded RNA genomes of varying size that are responsible for a variety of infections.
Parainfluenza
Parainfluenza is caused by the parainfluenza viruses types I–IV; these have a worldwide distribution and cause acute respiratory disease. Type IV appears to be less virulent than the other types and has been linked only to mild upper respiratory diseases in children and adults.
Parainfluenza is essentially a disease of children and presents with features similar to the common cold. When severe, a brassy cough with inspiratory stridor and features of laryngotracheitis (croup) are present. Fever usually lasts for 2–3 days and may be more prolonged if pneumonia develops. The development of croup is due to sub-mucosal oedema and consequent airway obstruction in the subglottic region. This may lead to cyanosis, subcostal and intercostal recession and progressive airway obstruction. Infection in the immunocompromised is usually prolonged and may be severe. Treatment is supportive with oxygen, humidification and sedation when required. The role of steroids and the antiviral agent ribavirin is controversial.
Measles (rubeola)
Measles is a highly communicable disease that occurs worldwide. With the introduction of aggressive immunization policies, the incidence of measles has fallen dramatically in the West but there are an estimated 0.2 million deaths annually due to measles infection worldwide, mostly in Africa and South-east Asia, with mortality being highest in children younger than 12 months of age. It is spread by droplet infection and the period of infectivity is from 4 days before until 2 days after the onset of the rash.
Clinical features
The incubation period is 8–14 days. Two distinct phases of the disease can be recognized.
 The pre-eruptive and catarrhal stage. This is the stage of viraemia and viral dissemination. Malaise, fever, rhinorrhoea, cough, conjunctival suffusion and the pathognomonic Koplik’s spots are present during this stage. Koplik’s spots are small, greyish, irregular lesions surrounded by an erythematous base and are found in greatest numbers on the buccal mucous membrane opposite the second molar tooth. They occur a day or two before the onset of the rash.
The pre-eruptive and catarrhal stage. This is the stage of viraemia and viral dissemination. Malaise, fever, rhinorrhoea, cough, conjunctival suffusion and the pathognomonic Koplik’s spots are present during this stage. Koplik’s spots are small, greyish, irregular lesions surrounded by an erythematous base and are found in greatest numbers on the buccal mucous membrane opposite the second molar tooth. They occur a day or two before the onset of the rash.
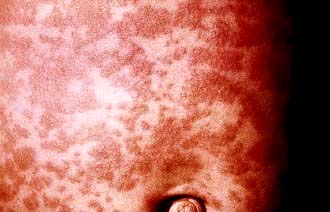
 The eruptive or exanthematous stage. This is characterized by the presence of a maculopapular rash that initially occurs on the face, chiefly the forehead, and then spreads rapidly to involve the rest of the body (Fig. 4.20). At first, the rash is discrete but later it may become confluent and patchy, especially on the face and neck. It fades in about 1 week and leaves behind a brownish discoloration.
The eruptive or exanthematous stage. This is characterized by the presence of a maculopapular rash that initially occurs on the face, chiefly the forehead, and then spreads rapidly to involve the rest of the body (Fig. 4.20). At first, the rash is discrete but later it may become confluent and patchy, especially on the face and neck. It fades in about 1 week and leaves behind a brownish discoloration.
The most feared complication in an immunocompetent child is acute measles encephalitis, with an incidence of 1/1000 to 1/5000 cases of measles. This is post-infectious, i.e. virus is not present in the brain and the encephalitis presumably arises through an aberrant cross-reaction of the host immune response to infection. Prognosis is poor, with a high mortality (30%) and severe residual damage in survivors.
Measles carries a high mortality in the malnourished and in those who have other diseases. Complications are common in such individuals and include bacterial pneumonia, bronchitis, otitis media and gastroenteritis. Less commonly, myocarditis, hepatitis and encephalomyelitis may occur. In those who are malnourished or those with defective cell-mediated immunity, the classical maculopapular rash may not develop and widespread desquamation may occur. The virus also causes the rare condition subacute sclerosing panencephalitis, which may follow measles infection occurring early in life (<18 months of age). Persistence of the virus with reactivation pre-puberty results in accumulation of virus in the brain, progressive mental deterioration and a fatal outcome (see p. 1082).
Maternal measles, unlike rubella, does not cause fetal abnormalities. It is, however, associated with spontaneous abortions and premature delivery.
Diagnosis and treatment
Most cases of measles are diagnosed clinically but detection of measles-specific IgM in blood or oral fluid, or genome or antigen detection from nasopharyngeal aspirates or throat swabs should be used to confirm the diagnosis.
Treatment is supportive. Antibiotics are indicated only if secondary bacterial infection occurs.
Prevention
A previous attack of measles confers a high degree of immunity and second attacks are uncommon. Normal human immunoglobulin given within 5 days of exposure effectively aborts an attack of measles. It is indicated for previously unimmunized children below 3 years of age, during pregnancy and in those with debilitating disease.
Active immunization. Children in the UK are immunized with the combined mumps-measles-rubella (MMR) vaccine (Box 4.6). In developing countries, the first measles vaccination is given at 9 months.
Mumps
Mumps is the result of infection with a paramyxovirus. It is spread by droplet infection, by direct contact or through fomites. Humans are the only known natural hosts. The peak period of infectivity is 2–3 days before the onset of the parotitis and for 3 days afterwards.
Clinical features
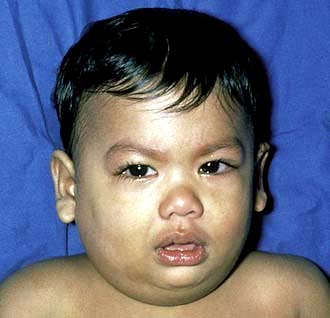
The incubation period averages 18 days. Although no age is exempt, it is primarily a disease of school-aged children and young adults; it is uncommon before the age of 2 years. The prodromal symptoms are nonspecific and include fever, malaise, headache and anorexia. This is usually followed by severe pain over the parotid glands, with either unilateral or bilateral parotid swelling (Fig. 4.21). These enlarged glands obscure the angle of the mandible and may elevate the ear lobe, which does not occur in cervical lymph node enlargement. Trismus due to pain is common at this stage. Submandibular gland involvement occurs less frequently.
Complications
CNS involvement is the most common extrasalivary gland manifestation of mumps. Clinical meningitis occurs in 5% of all infected patients and 30% of patients with CNS involvement have no evidence of parotid gland involvement.
Epididymo-orchitis develops in about one-third of patients who develop mumps after puberty. Bilateral testicular involvement results in sterility in only a small percentage of these patients. Pancreatitis, oophoritis, myocarditis, mastitis, hepatitis and polyarthritis may also occur.
Diagnosis and treatment
The diagnosis of mumps is on the basis of the clinical features. In doubtful cases, serological demonstration of a mumps-specific IgM response in an acute blood or oral fluid sample is diagnostic. Virus can be isolated in cell culture, or identified by genome or antigen detection assays, from saliva, throat swab, urine and CSF.
Treatment is supportive. Attention should be given to adequate nutrition and mouth care. Analgesics should be used to relieve pain.
Prevention
Active immunization. Children in the UK are immunized with the MMR vaccine (Box 4.6) and the mumps vaccine is given in most developing countries. Vaccination is contraindicated in immunosuppressed individuals, during pregnancy, or in those with severe febrile illnesses.
Respiratory syncytial virus infection
Respiratory syncytial virus (RSV) is a paramyxovirus that causes many respiratory infections in epidemics each winter. It is a common cause of bronchiolitis in infants, complicated by pneumonia in approximately 10% of cases. The infection normally starts with upper respiratory symptoms. After an interval of 1–3 days a cough and low-grade fever may develop. The onset of bronchiolitis is characterized by dyspnoea and hyperexpansion of the chest with subcostal and intercostal recession. The disease may be severe and potentially fatal in babies with underlying cardiac, respiratory (including prematurity) or immunodeficiency disease. RSV infection has been associated with the occurrence of sudden infant death syndrome (SIDS). The virus undergoes antigenic drift and, consequently, reinfection occurs throughout life. RSV is occasionally the cause of outbreaks of influenza-like illness or pneumonia in the elderly and in the immunocompromised.
Transfer of infection between children in hospital commonly occurs unless infected patients are isolated or cohorted. Meticulous attention to handwashing and other infection control measures reduces the risk of transmission by staff members.
Diagnosis and treatment
Genome detection or immunofluorescence on nasopharyngeal aspirates, virus culture and serology are the usual ways of confirming the diagnosis.
Treatment is generally supportive, but aerosolized ribavirin can be given to severe cases, particularly those with underlying cardiac or respiratory disease.
Prevention
No vaccine is currently available for RSV but high-risk children (including those with bronchopulmonary dysplasia and congenital heart disease) can be protected against severe disease by monthly administration of either a hyperimmune globulin against RSV, or a humanized monoclonal antibody (palivizumab) during the winter months (see this chapter).
Metapneumovirus
Human metapneumovirus (hMPV) causes approximately 10% of lower respiratory tract infections in infants and young children. Infection is clinically indistinguishable from that caused by RSV.
Hendra and Nipah viruses
Hendra virus (formerly called equine morbillivirus) and Nipah virus are zoonotic viruses that have caused disease in humans who have been in contact with infected animals (horses and pigs, respectively). The viruses are named after the locations where they were first isolated, Hendra in Australia and Nipah in Malaysia, and both are classified as paramyxoviruses. Hendra virus has caused severe respiratory distress in horses and humans and Nipah virus caused a major outbreak of viral encephalitis (265 cases and 105 deaths) in Malaysia between September 1998 and April 1999. Treatment of these conditions is largely supportive, although there is some evidence that early treatment with ribavirin may reduce the severity of the diseases.
Coronaviruses
Human coronaviruses were first isolated in the mid-1960s and the majority of isolates (related to the reference strains 229E and OC43) have been associated with common colds. In November 2002, an apparently new viral disease occurred in China and this spread rapidly in other parts of the Far East and thence across the world.
This disease, known as ‘severe acute respiratory syndrome’ (SARS), of which bronchopneumonia is a major feature, is caused by a previously unknown coronavirus (SARS-CoV). Similarity of this virus to coronaviruses isolated from civet cats, raccoons and ferret badgers indicates the likelihood that SARS is a zoonotic disease. Bats are the likely host species for this virus.
The epidemic was finally brought under control in the summer of 2003, by which time there had been >8000 cases with approximately 800 deaths. In 2004 and 2005, two new coronavirus infections of humans were described – NL63 and HKU1. These are associated with upper respiratory tract symptomatology, such as the common cold.
Rhabdoviruses
Rabies
Rabies is a major problem in some countries, with an estimated 55 000 deaths per year worldwide. Established infection is almost invariably fatal. It is caused by a genotype 1, single-stranded RNA virus of the Lyssavirus genus. The rabies virus is bullet-shaped and has spike-like structures arising from its surface containing glycoproteins that cause the host to produce neutralizing, haemagglutination-inhibiting antibodies. The virus has a marked affinity for nervous tissue and the salivary glands. It exists in two major epidemiological settings:
 Urban rabies is most frequently transmitted to humans through rabid dogs and, less frequently, cats.
Urban rabies is most frequently transmitted to humans through rabid dogs and, less frequently, cats.
 Sylvan (wild) rabies is maintained in the wild by a host of animal reservoirs such as foxes, skunks, jackals, mongooses and bats.
Sylvan (wild) rabies is maintained in the wild by a host of animal reservoirs such as foxes, skunks, jackals, mongooses and bats.
With the exception of Australia, New Zealand and the Antarctic, human rabies has been reported from all continents. Transmission is usually through the bite of an infected animal. However, the percentage of rabid bites leading to clinical disease ranges from 10% (on the legs) to 80% (on the head). Other forms of transmission, if they occur, are rare.
Virus replicates in the muscle cells near the entry wound. It penetrates the nerve endings and travels in the axoplasm to the spinal cord and brain. In the CNS the virus again proliferates before spreading to the salivary glands, lungs, kidneys and other organs via the autonomic nerves.
There have been only six recorded cases of survival from clinical rabies.
Clinical features
The incubation period is variable, ranging from a few weeks to several years; on average it is 1–3 months. In general, bites on the head, face and neck have a shorter incubation period than those elsewhere. In humans, two distinct clinical varieties of rabies are recognized:
The only characteristic feature in the prodromal period is the presence of pain and tingling at the site of the initial wound. Fever, malaise and headache are also present. About 10 days later, marked anxiety and agitation or depressive features develop. Hallucinations, bizarre behaviour and paralysis may also occur. Hyperexcitability, the hallmark of this form of rabies, is precipitated by auditory or visual stimuli. Hydrophobia (fear of water) is present in 50% of patients and is due to severe pharyngeal spasms on attempting to eat or drink. Aerophobia (fear of air) is considered pathognomonic of rabies. Examination reveals hyperreflexia, spasticity and evidence of sympathetic overactivity indicated by pupillary dilatation and diaphoresis.
The patient goes on to develop convulsions, respiratory paralysis and cardiac arrhythmias. Death usually occurs in 10–14 days.
Diagnosis
The diagnosis of rabies is generally made clinically. Skin-punch biopsies are used to detect antigen with an immunofluorescent antibody test on frozen section. Viral RNA can be isolated using the reverse transcription polymerase chain reaction (RT-PCR). Isolation of viruses from saliva or the presence of antibodies in blood or CSF may establish the diagnosis. The corneal smear test is not recommended as it is unreliable. The classic Negri bodies are detected at post-mortem in 90% of all patients with rabies; these are eosinophilic, cytoplasmic, ovoid bodies, 2–10 nm in diameter, seen in greatest numbers in the neurones of the hippocampus and the cerebellum. The diagnosis should be made pathologically on the biting animal using RT-PCR, immunofluorescence assay (IFA) or tissue culture of the brain.
Treatment
Once the CNS disease is established, therapy is symptomatic, as death is virtually inevitable. The patient should be nursed in a quiet, darkened room. Nutritional, respiratory and cardiovascular support may be necessary.
Drugs such as morphine, diazepam and chlorpromazine should be used liberally in patients who are excitable.
Prevention
Pre-exposure prophylaxis. This is given to individuals with a high risk of contracting rabies, such as laboratory workers, animal handlers and veterinarians. Three doses (1.0 mL) of human diploid (HDCV) or chick embryo cell vaccine given by deep subcutaneous or intramuscular injection on days 0, 7 and 28 provide effective immunity. A reinforcing dose is given after 12 months and additional reinforcing doses are given every 3–5 years depending on the risk of exposure. Vaccines of nervous-tissue origin are still used in some parts of the world. These, however, are associated with significant side-effects and are best avoided if HDCV is available.
Post-exposure prophylaxis. The wound should be cleaned carefully and thoroughly with soap and water and left open. Human rabies immunoglobulin should be given immediately (20 IU/kg); half should be injected around the area of the wound and the other half should be given intramuscularly. Five 1.0 mL doses of HDCV should be given intramuscularly: the first dose is given on day 0 and is followed by injections on days 3, 7, 14 and 28. Reaction to the vaccine is uncommon.
Domestic animals should be vaccinated if there is any risk of rabies in the country. In the UK, control has been by quarantine of imported animals for 6 months and no indigenous case of rabies has been reported for many years. The Pet Travel Scheme (PETS) recently introduced enables certain pet animals to enter or re-enter Great Britain without quarantine if they come from qualifying countries via designated routes, are carried by authorized transport companies and meet the conditions of the scheme. Wild animals in ‘at risk’ countries must be handled with great care.
Retroviruses
Retroviruses (Table 4.22) are distinguished from other RNA viruses by their ability to replicate through a DNA intermediate using the enzyme reverse transcriptase.
Table 4.22 Human lymphotropic retroviruses
| Sub-family | Virus | Disease |
|---|---|---|
Lentivirus |
HIV-1 |
AIDS |
HIV-2 |
AIDS |
|
Oncovirus |
HTLV-1a |
Adult T-cell leukaemia/lymphoma |
Tropical spastic paraparesis |
||
HTLV-2 |
Myelopathy |
a HTLV, human T-cell lymphotropic virus.
HIV-1 and the related virus, HIV-2, are further classified as lentiviruses (‘slow’ viruses) because of their slowly progressive clinical effects.
HIV-1 and HIV-2 are discussed on page 173.
HTLV-1 causes adult T-cell leukaemia/lymphoma and tropical spastic paraparesis.
Arenaviruses
Arenaviruses are pleomorphic, round or oval viruses with diameters ranging from 50 to 300 nm. The virion surface has club-shaped projections and the virus itself contains a variable number of characteristic electron-dense granules that represent residual, non-functional host ribosomes. Arenaviruses are responsible for Lassa fever and also for lymphocytic choriomeningitis, Argentinian and Bolivian haemorrhagic fevers.
Lassa fever
This illness was first documented in the town of Lassa, Nigeria, in 1969 and is confined to sub-Saharan West Africa (Nigeria, Liberia and Sierra Leone). The multimammate rat, Mastomys natalensis, is known to be the reservoir. Humans are infected by ingesting foods contaminated by rat urine or saliva containing the virus. Person-to-person spread by body fluids also occurs. Only 10–30% of infections are symptomatic.
Clinical features
The incubation period is 7–18 days. The disease is insidious in onset and is characterized by fever, myalgia, severe backache, malaise and headache. A transient maculopapular rash may be present. A sore throat, pharyngitis and lymphadenopathy occur in over 50% of patients. In severe cases, epistaxis and gastrointestinal bleeding may occur; hence the classification of Lassa fever as a viral haemorrhagic fever. The fever usually lasts 1–3 weeks and recovery within 1 month of the onset of illness is usual. However, death occurs in 15–20% of hospitalized patients, usually from irreversible hypovolaemic shock.
Diagnosis
The diagnosis is established by serial serological tests (including the Lassa virus-specific IgM titre) or by genome detection by means of RT-PCR in throat swab, serum or urine.
Treatment
Treatment is supportive. In addition, clinical benefit and reduction in mortality can be achieved with ribavirin therapy, if given in the first week.
In non-endemic countries, strict isolation procedures should be used, the patient ideally being nursed in a flexible-film isolator. Specialized units for the management of Lassa fever and other haemorrhagic fevers have been established in the UK. As Lassa fever virus and other causes of haemorrhagic fever (Marburg/Ebola and Congo-Crimean haemorrhagic fever viruses, Table 4.23) have been transmitted from patients to staff in healthcare situations, great care should be taken in handling specimens and clinical material from these patients.
Table 4.23 Viral infections associated with haemorrhagic manifestationsa
|
|
a Most of these are arboviruses. Some (e.g. Hantavirus, Lassa fever) have a rodent vector. The source and transmission route of filoviruses is not known.
Lymphocytic choriomeningitis (LCM)
This infection is a zoonosis, the natural reservoir of LCM virus being the house mouse. Infection is characterized by:
Occasionally, a more severe form occurs, with encephalitis leading to disturbance of consciousness.
This illness is generally self-limiting and requires no specific treatment.
Filoviruses
Marburg virus disease and Ebola virus disease
These severe, haemorrhagic, febrile illnesses are discussed together because their clinical manifestations are similar. The diseases are named after Marburg in Germany and the Ebola River region in the Sudan and Zaire where these viruses first appeared. The natural reservoir for these viruses has not been identified and the precise mode of spread from one individual to another has not been elucidated.
Epidemics have occurred periodically in recent years, mainly in sub-Saharan Africa. The mortality from Marburg and Ebola has ranged from 25% to 90% and recovery is slow in those who survive.
The illness is characterized by the acute onset of severe headache, severe myalgia and high fever, followed by prostration. On about the fifth day of illness a non-pruritic maculopapular rash develops on the face and then spreads to the rest of the body. Diarrhoea is profuse and is associated with abdominal cramps and vomiting. Haematemesis, melaena or haemoptysis may occur between the 7th and 16th day. Hepatosplenomegaly and facial oedema are usually present. In Ebola virus disease, chest pain and a dry cough are prominent symptoms.
Treatment is symptomatic. Convalescent human serum may decrease the severity of the attack.
Postviral/chronic fatigue syndrome (see also p. 1162)
Many viral infections have been implicated aetiologically, including EBV, Coxsackie B viruses, echoviruses, CMV and hepatitis A virus. Non-viral causes such as allergy to Candida spp. have also been proposed. Only a minority of patients have an identifiable precipitating infectious illness. Reports of identification of an infectious retrovirus, xenotropic murine leukaemia virus-related virus (XMRV), in the peripheral blood of a high percentage of patients with CFS (but not in the blood of healthy controls) in late 2009, generated considerable excitement among both patients and their carers. However, a number of independent investigators have failed to confirm these findings, which remain unproven.
transmissible spongiform encephalopathies (TSE or prion diseases)
Transmissible spongiform encephalopathies are caused by the accumulation in the nervous system of a protein, termed a ‘prion’, which is an abnormal isoform PrPSc of a normal, host protein (PrPc).
Although familial forms of prion disease are known to exist, these conditions can be transmissible, particularly if brain tissue enters another host. There is no convincing evidence for the presence of nucleic acid in association with prions; thus these agents cannot be considered orthodox viruses and it is the abnormal prion protein itself that is infectious and can trigger a conversion of the normal protein into the atypical isoform. After infection, a long incubation period is followed by CNS degeneration associated with dementia or ataxia, which invariably leads to death. Histology of the brain reveals spongiform change with an accumulation of the abnormal prion protein in the form of amyloid plaques.
The human prion diseases are Creutzfeldt–Jakob disease, including the sporadic, familial, iatrogenic and variant forms of the disease, Gerstmann–Straussler–Scheinker syndrome, fatal familial insomnia and kuru.
 Creutzfeldt–Jakob disease (CJD) usually occurs sporadically worldwide with an annual incidence of one per million of the population. Although, in most cases, the epidemiology remains obscure, transmission to others has occurred as a result of administration of human cadaveric growth hormone or gonadotrophin, from dura mater and corneal grafting and in neurosurgery from reuse of contaminated instruments and electrodes (iatrogenic CJD).
Creutzfeldt–Jakob disease (CJD) usually occurs sporadically worldwide with an annual incidence of one per million of the population. Although, in most cases, the epidemiology remains obscure, transmission to others has occurred as a result of administration of human cadaveric growth hormone or gonadotrophin, from dura mater and corneal grafting and in neurosurgery from reuse of contaminated instruments and electrodes (iatrogenic CJD).
 Variant CJD. In the UK, knowledge that large numbers of cattle with the prion disease, bovine spongiform encephalopathy (BSE) had gone into the human food chain, led to enhanced surveillance for emergence of the disease in humans. The evidence is convincing, based on transmission studies in mice and on glycosylation patterns of prion proteins, that this has occurred and, to date, there have been approximately 170 confirmed and suspected cases of variant CJD (human BSE) in the UK and 40 in the rest of the world. In contrast to sporadic CJD, which presents with dementia at a mean age of onset of 60 years, variant CJD presents with ataxia, dementia, myoclonus and chorea at a mean age of onset of 29 years. The epidemic curve of vCJD in the UK is shown in Figure 4.22.
Variant CJD. In the UK, knowledge that large numbers of cattle with the prion disease, bovine spongiform encephalopathy (BSE) had gone into the human food chain, led to enhanced surveillance for emergence of the disease in humans. The evidence is convincing, based on transmission studies in mice and on glycosylation patterns of prion proteins, that this has occurred and, to date, there have been approximately 170 confirmed and suspected cases of variant CJD (human BSE) in the UK and 40 in the rest of the world. In contrast to sporadic CJD, which presents with dementia at a mean age of onset of 60 years, variant CJD presents with ataxia, dementia, myoclonus and chorea at a mean age of onset of 29 years. The epidemic curve of vCJD in the UK is shown in Figure 4.22.
 Gerstmann–Straussler–Scheinker syndrome and fatal familial insomnia are rare prion diseases, usually occurring in families with a positive history. The pattern of inheritance is autosomal dominant with some degree of variable penetrance. The gene encoding PrPC in these familes often contains mutations.
Gerstmann–Straussler–Scheinker syndrome and fatal familial insomnia are rare prion diseases, usually occurring in families with a positive history. The pattern of inheritance is autosomal dominant with some degree of variable penetrance. The gene encoding PrPC in these familes often contains mutations.
 Kuru was described and characterized in the Fore highlanders in NE New Guinea. Transmission was associated with ritualistic cannibalism of deceased relatives. With the cessation of cannibalism by 1960, the disease has gradually diminished and recent cases had all been exposed to the agent before 1960.
Kuru was described and characterized in the Fore highlanders in NE New Guinea. Transmission was associated with ritualistic cannibalism of deceased relatives. With the cessation of cannibalism by 1960, the disease has gradually diminished and recent cases had all been exposed to the agent before 1960.
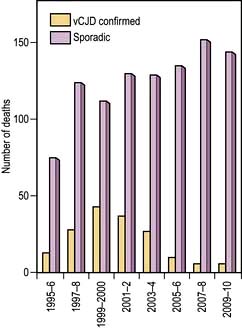
Figure 4.22 Creutzfeldt–Jakob disease. Deaths of confirmed variant and sporadic cases of CJD (to early Dec. 2010) in the UK.
(Courtesy of Professor JW Ironside, Director National CJD Surveillance Unit, University of Edinburgh.)
The infectious agents of prion disease have remarkable characteristics. In the infected host there is no evidence of inflammatory, cytokine or immune reactions. The agent is highly resistant to decontamination and infectivity is not reliably destroyed by autoclaving or by treatment with formaldehyde and most other gas or liquid disinfectants. It is very resistant to γ-irradiation. Autoclaving at a high temperature (134–137°C for 18 min) is used for decontamination of instruments and hypochlorite (20 000 p.p.m. available chlorine) or 1 molar sodium hydroxide are used for liquid disinfection. Uncertainty about the reliability of any methods for safe decontamination of surgical instruments has necessitated the introduction of guidelines for patient management.