Chapter 5 Nutrition
Introduction
In developing countries, lack of food and poor usage of the available food can result in protein-energy malnutrition (PEM); 50 million pre-school African children have PEM. In developed countries, excess food is available and the most common nutritional problem is obesity.
Diet and disease are interrelated in many ways:
 Excess energy intake contributes to a number of diseases, including ischaemic heart disease and diabetes, particularly when high in animal (saturated) fat content.
Excess energy intake contributes to a number of diseases, including ischaemic heart disease and diabetes, particularly when high in animal (saturated) fat content.
 There is a relationship between food intake and cancer, as found in many epidemiological studies. An excess of energy-rich foods (i.e. fat and sugar containing), often with physical inactivity, plays a role in the development of certain cancers, while diets high in vegetables and fruits reduce the risk of most epithelial cancers. Numerous carcinogens, intentional additions (e.g. nitrates for preserving foods) or accidental contaminants (e.g. moulds producing aflatoxin and fungi) may also be involved in the development of cancer.
There is a relationship between food intake and cancer, as found in many epidemiological studies. An excess of energy-rich foods (i.e. fat and sugar containing), often with physical inactivity, plays a role in the development of certain cancers, while diets high in vegetables and fruits reduce the risk of most epithelial cancers. Numerous carcinogens, intentional additions (e.g. nitrates for preserving foods) or accidental contaminants (e.g. moulds producing aflatoxin and fungi) may also be involved in the development of cancer.
 The proportion of processed foods eaten may affect the development of disease. Some processed convenience foods have a high sugar and fat content and therefore predispose to dental caries and obesity, respectively. They also have a low fibre content, and dietary fibre can help in the prevention of a number of diseases (see p. 199).
The proportion of processed foods eaten may affect the development of disease. Some processed convenience foods have a high sugar and fat content and therefore predispose to dental caries and obesity, respectively. They also have a low fibre content, and dietary fibre can help in the prevention of a number of diseases (see p. 199).
 Long-term undernutrition is implicated in disease by some epidemiological studies, for example low growth rates in utero are associated with high death rates from cardiovascular disease in adult life.
Long-term undernutrition is implicated in disease by some epidemiological studies, for example low growth rates in utero are associated with high death rates from cardiovascular disease in adult life.
In the UK, dietary reference values for food and energy and nutrients are stated as reference nutrient intakes (RNIs), on the basis of data from the Food and Agriculture Organization (FAO-WHO), United Nations University (UNU) expert committee, and elsewhere. The RNI is sufficient or more than sufficient to meet the nutritional needs of 97.5% of healthy people in a population. Most people’s daily requirements are less than this, and so an estimated average requirement (EAR) is also given, which will certainly be adequate for most. A lower reference nutrient intake (LRNI) which fails to meet the requirements of 97.5% of the population is also given. The RNI figures quoted in this chapter are for the age group 19–50 years. These represent values for healthy subjects and are not always appropriate for patients with disease.
Water and electrolyte balance
Water and electrolyte balance is dealt with fully in Chapter 13. About 1 L of water is required in the daily diet to balance insensible losses, but much more is usually drunk, the kidneys being able to excrete large quantities. The daily RNI for sodium is 70 mmol (1.6 g) but daily sodium intake varies in the range 90–440 mmol (2–10 g). These are needlessly high intakes of sodium which are thought by some to play a role in causing hypertension (see p. 778).
Dietary requirements
Energy
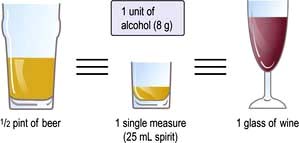
Food is necessary to provide the body with energy (Fig. 5.1). The SI unit of energy is the joule (J), and 1 kJ = 0.239 kcal. The conversion factor of 4.2 kJ, equivalent to 1.00 kcal, is used in clinical nutrition.
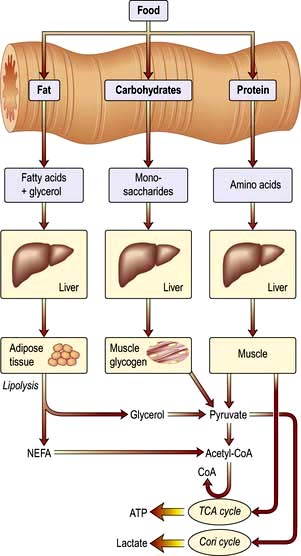
Figure 5.1 The production of energy from the main constituents of food. Alcohol produces up to 5% of total calories, but the variation between individuals is wide. 1 mol of glucose produces 36 mol of ATP. NEFA, non-esterified fatty acids; ATP, adenosine triphosphate; TCA, tricarboxylic acid.
Energy balance
Energy balance is the difference between energy intake and energy expenditure. Weight gain or loss is a simple, but accurate, way of indicating differences in energy balance.
Energy requirements
There are two approaches to assessing energy requirements for subjects who are weight stable and close to energy balance:
Energy intake
This can be estimated from dietary surveys and in the past this has been used to decide daily energy requirements. However, measurement of energy expenditure gives a more accurate assessment of requirements.
Energy expenditure
Daily energy expenditure (Fig. 5.2) is the sum of:
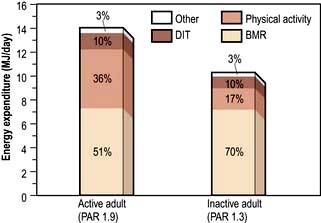
Figure 5.2 Daily energy expenditure in an active and a sedentary 70 kg adult. BMR, basal metabolic rate; DIT, dietary induced thermogenesis; PAR, physical activity ratio.
Total energy expenditure can be measured using a double-labelled water technique. Water containing the stable isotopes 2H and 18O is given orally. As energy is expended carbon dioxide and water are produced. The difference between the rates of loss of the two isotopes is used to calculate the carbon dioxide production, which is then used to calculate energy expenditure. This can be done on urine samples over a 2–3-week period with the subject ambulatory. The technique is accurate, but it is expensive and requires the availability of a mass spectrometer. An alternative tracer technique for measuring total energy expenditure is to estimate CO2 production by isotopic dilution. A subcutaneous infusion of labelled bicarbonate is administered continuously by a minipump, and urine is collected to measure isotopic dilution by urea, which is formed from CO2. Other methods for estimating energy expenditure, such as heart rate monitors or activity monitors, are also available but are less accurate.
Basal metabolic rate. The BMR can be calculated by measuring oxygen consumption and CO2 production, but it is more usually taken from standardized tables (Table 5.1) that only require knowledge of the subject’s age, weight and sex.
Table 5.1 Equations for the prediction of basal metabolic rate (in MJ/day)
| Age range (years) | Equation for predicting BMRa | 95% confidence limits |
|---|---|---|
Men |
|
|
10–17 |
0.0740 × (wt) + 2.754 |
±0.88 |
18–29 |
0.0630 × (wt) + 2.896 |
±1.28 |
30–59 |
0.0480 × (wt) + 3.653 |
±1.40 |
60–74 |
0.0499 × (wt) + 2.930 |
N/A |
75+ |
0.0350 × (wt) + 3.434 |
N/A |
Women |
|
|
10–17 |
0.0560 × (wt) + 2.898 |
±0.94 |
18–29 |
0.0620 × (wt) + 2.036 |
±1.00 |
30–59 |
0.0340 × (wt) + 3.538 |
±0.94 |
60–74 |
0.0386 × (wt) + 2.875 |
N/A |
75+ |
0.0410 × (wt) + 2.610 |
N/A |
Data from Department of Health, 1991. BMR, basal metabolic rate. aBodyweight (wt) in kg.
Physical activity. The physical activity ratio (PAR) is expressed as multiples of the BMR for both occupational and non-occupational activities of varying intensities (Table 5.2).
Table 5.2 Physical activity ratio (PAR) for various activities (expressed as multiples of BMR)
| PAR | |
|---|---|
Occupational activity |
|
Professional/housewife |
1.7 |
Domestic helper/sales person |
2.7 |
Labourer |
3.0 |
Non-occupational activity |
|
Reading/eating |
1.2 |
Household/cooking |
2.1 |
Gardening/golf |
3.7 |
Jogging/swimming/football |
6.9 |
Total daily energy expenditure = BMR × [Time in bed + (Time at work × PAR) + (Non-occupational time × PAR)].
Thus, for example, to determine the daily energy expenditure of a 69-year-old, 50 kg female doctor, with a BMR of 4805 kJ/day spending one-third of a day sleeping, working and engaged in non-occupational activities, the latter at a PAR of 2.1, the following calculation ensues:
In the UK, the estimated ‘average’ daily energy requirement is:
This is at present made up of about 50% carbohydrate, 35% fat, 15% protein ± 5% alcohol. In developing countries, however, carbohydrate may be >75% of the total energy input, and fat <15% of the total energy input.
Energy requirements increase during the growing period, with pregnancy and lactation, and sometimes following infection or trauma. In general, the increased BMR associated with inflammatory or traumatic conditions is counteracted or more than counteracted by a decrease in physical activity, so that total energy requirements are not increased.
In the basal state, energy demands for resting muscle are 20% of the total energy required, abdominal viscera 35–40%, brain 20% and heart 10%. There can be more than a 50-fold increase in muscle energy demands during exercise.
Energy stores
Although virtually all body fat and glycogen are available for oxidation, less than half the protein is available for oxidation. Figure 5.3 shows that fat accounts for the largest reserves of energy in both lean and obese subjects. The size of the stores determines survival during starvation.
Bodyweight
Bodyweight depends on energy balance. Intake depends not only on food availability but also on a number of complex interrelationships that include the stimulus of good food, the role of hunger, metabolic changes (e.g. hypoglycaemia), and the pleasure and habit of eating. Some people are able to keep their bodyweight constant within a few kilograms for many years, but most gradually increase their weight owing to a small but continuous increase of intake over expenditure. A gain or loss of energy of 25–29 MJ (6000–7000 kcal) would respectively increase or decrease bodyweight by approximately 1 kg.
Protein
In the UK, the adult daily RNI for protein is 0.75 g/kg, with protein representing at least 10% of the total energy intake. Most affluent people eat more than this, consuming 80–100 g of protein per day.
The total amount of nitrogen excreted in the urine represents the balance between protein breakdown and synthesis. In order to maintain nitrogen balance, at least 40–50 g of protein are needed. The amount of protein oxidized can be calculated from the amount of nitrogen excreted in the urine over 24 h using the following equation:
Grams of protein required = Urinary nitrogen × 6.25 (most proteins contain about 16% of nitrogen).
In practice, urinary urea is more easily measured and forms 80–90% of the total urinary nitrogen (N). In healthy individuals urinary nitrogen excretion reflects protein intake. However, excretion does not match intake in catabolic conditions (negative N balance) or during growth or repletion following an illness (positive N balance).
Protein contains many amino acids:
 Indispensable (essential): there are nine amino acids that cannot be synthesized and must be provided in the diet: tryptophan, histidine, methionine, threonine, isoleucine, valine, phenylalanine, lysine, leucine.
Indispensable (essential): there are nine amino acids that cannot be synthesized and must be provided in the diet: tryptophan, histidine, methionine, threonine, isoleucine, valine, phenylalanine, lysine, leucine.
 Dispensable (non-essential): amino acids that can be synthesized in the body (some may still be needed in the diet unless adequate amounts of their precursors are available).
Dispensable (non-essential): amino acids that can be synthesized in the body (some may still be needed in the diet unless adequate amounts of their precursors are available).
Animal proteins (e.g. in milk, meat, eggs) contain a good balance of all indispensable amino acids, but many proteins from vegetables are deficient in at least one indispensable amino acid. In developing countries, protein intake derives mainly from vegetable proteins. By combining foodstuffs with different low concentrations of indispensable amino acids (e.g. maize with legumes), protein intake can be adequate provided enough vegetables are available.
Loss of protein from the body (negative N balance) occurs not only because of inadequate protein intake, but also because of inadequate energy intake. When there is loss of energy from the body, more protein is directed towards oxidative pathways and eventually gluconeogenesis for energy.
Role of amino acids
 Glutamine is quantitatively the most significant in the circulation and in inter-organ exchange.
Glutamine is quantitatively the most significant in the circulation and in inter-organ exchange.
 Alanine is released from muscle; it is deaminated and converted into pyruvic acid before entering the citric acid cycle.
Alanine is released from muscle; it is deaminated and converted into pyruvic acid before entering the citric acid cycle.
 Homocysteine is a sulphur-containing amino acid which is derived from methionine in the diet. A raised plasma concentration is an independent risk factor for vascular disease (see p. 728).
Homocysteine is a sulphur-containing amino acid which is derived from methionine in the diet. A raised plasma concentration is an independent risk factor for vascular disease (see p. 728).
 Amino acids are utilized to synthesize products other than protein or urea. For example:
Amino acids are utilized to synthesize products other than protein or urea. For example:
Fat
Dietary fat is chiefly in the form of triglycerides, which are esters of glycerol and free fatty acids. Fatty acids vary in chain length and in saturation (Table 5.3). The hydrogen molecules related to the double bonds can be in the cis or the trans position; most natural fatty acids in food are in the cis position (Box 5.1).
Table 5.3 The main fatty acids in foods
| Fatty acid | No. of carbon atoms : No. of double bonds | Position of double bondsa |
|---|---|---|
Saturated |
|
|
Lauric |
C12:0 |
|
Myristic |
C14:0 |
|
Palmitic |
C16:0 |
|
Stearic |
C18:0 |
|
Monounsaturated |
|
|
Oleic |
C18:1 |
(n-9) |
Elaidic |
C18:1 |
(n-9 trans) |
Polyunsaturated |
|
|
Linoleic |
C18:2 |
(n-6) |
α-Linolenic |
C18:3 |
(n-3) |
Arachidonic |
C20:4 |
(n-6) |
Eicosapentaenoic |
C20:5 |
(n-3) |
Docosahexaenoic |
C22:6 |
(n-3) |
a Positions of the double bonds (designated either n as here or ω) are shown counted from the methyl end of the molecule. All double bonds are in the cis position except that marked trans.
![]() Box 5.1
Box 5.1
Dietary sources of fatty acids
| Type of acid | Sources |
|---|---|
Saturated fatty acids |
Mainly animal fat |
n-6 fatty acids |
Vegetable oils and other plant foods |
n-3 fatty acids |
Vegetable foods, rapeseed oil, fish oils |
trans fatty acids |
Hydrogenated fat or oils, e.g. in margarine, cakes, biscuits |
The essential fatty acids (EFAs) are linoleic and α-linolenic acid, both of which are precursors of prostaglandins. Eicosapentaenoic and docosahexaenoic acid are also necessary, but can be made to a limited extent in the tissues from linoleic and linolenic acid, and thus a dietary supply is not essential.
Synthesis of triglycerides, sterols and phospholipids is very efficient. Even with low-fat diets subcutaneous fat stores can be normal.
Dietary fat provides 37 kJ (9 kcal) of energy per gram. A high-fat intake has been implicated in the causation of:
The data on causation are largely epidemiological and disputed by many. Nevertheless, it is often suggested that the consumption of saturated fatty acids should be reduced, accompanied by an increase in monounsaturated fatty acids (the ‘Mediterranean diet’) or polyunsaturated fatty acids. Any increase in polyunsaturated fats should not, however, exceed 10% of the total food energy, particularly as this requires a big dietary change.
Trans fats (partly hydrogenated fatty acids)
Increased consumption of hydrogenated vegetable and fish oils in margarines has led to increased trans fatty acid consumption. Trans fatty acids (also called trans fats) behave as if they were saturated fatty acids, increasing circulating LDL and decreasing HDL cholesterol concentrations, which in turn increase the risk of cardiovascular disease. In most countries, nutrition labels for all conventional foods and supplements must indicate the trans fatty acid content. The usage of trans fatty acids from partially hydrogenated oils has now been banned in many countries.
Polyunsaturated fatty acids
The n-6 polyunsaturated fatty acids (PUFA) are components of membrane phospholipids, influencing membrane fluidity and ion transport. They also have antiarrhythmic, antithrombotic and anti-inflammatory properties, all of which are potentially helpful in preventing cardiovascular disease.
The n-3 PUFA increase circulating high-density lipoprotein (HDL) cholesterol and lower triglycerides, both of which might reduce cardiovascular risk. Some of the actions of n-3 PUFA are mediated by a range of leukotrienes and eicosanoids, which differ in pattern and functions from those produced from n-6 PUFA.
Epidemiological studies and clinical intervention studies suggest that n-3 PUFA may have effects in the secondary prevention of cardiovascular disease and ‘all-cause mortality’ (e.g. 20–30% reduction in mortality from cardiovascular disease according to some studies). The benefits, which have been noted as early as 4 months after intervention, have been largely attributed to the antiarrhythmic effects of n-3 PUFA, but some work suggests that n-3 PUFA, administered as capsules, can be rapidly incorporated into atheromatous plaques, stabilizing them and preventing rupture. Whether these effects are due directly to n-3 PUFA or other changes in the diet is still debated.
The GISSI Prevention Trial, which followed over 11 000 patients for 3.5 years after a myocardial infarction, administered fish oils (eicosapentaenoic acid, EPA and docosahexaenoic acid, DHA) in the form of capsules and demonstrated a striking benefit in reducing mortality. The effects of vitamin E (300 mg α-tocopherol/day) were also studied, but no benefit was found.
Recommendations for fat intake
The British Nutrition Foundation and the American Heart Association presently recommend a two-fold increase of the current intake of total n-3 PUFA (several fold increase in the intake of fish oils, and a 50% increase in the intake of α-linolenic acid). Implementing this recommendation will mean either a major change in the dietary habits of populations that eat little fish, or ingestion of capsules containing fish oils. Some government agencies have warned of the hazards of eating certain types of fish, which increase the risk of mercury poisoning and possibly other toxicities.
The current recommendations for fat intake for the UK are shown in Box 5.2.
![]() Box 5.2
Box 5.2
Recommended healthy dietary intake
| Dietary component | Approximate amounts given as % of total energy unless otherwise stated | General hints |
|---|---|---|
Total carbohydrate |
55 (55–75) |
Increase fruit, vegetables, beans, pasta, bread |
Free sugar |
10 (<10) |
Decrease sugary drinks |
Protein |
15 (10–15) |
Decrease red meat (see fat below) |
Total fat |
30 (15–30) |
Increase vegetable (including olive oil) and fish oil and decrease animal fat |
Saturated fatty acids |
10 (<10) |
|
Cis-mono unsaturated fatty acids |
20 |
Mainly oleic acid (n-6) |
Cis-polyunsaturated fatty acids |
6 |
Both n-6 and n-3 PUFA |
Approximate amounts |
|
|
Cholesterol |
<300 (<300) mg/day |
Decrease meat and eggs |
Salt |
<6 (<5) g/day |
Decrease prepared meats and do not add extra salt to food |
Total dietary fibre |
30 (>25) g/day |
Increase fruit and vegetables and wholegrain foods |
Values in parentheses are goals for the intake of populations, as given by the WHO (including populations who are already on low-fat diets). Some of the extreme ranges are not realistic short-term goals for developed countries, e.g. 75% of total energy from carbohydrate and 15% fat. When total energy intake is 2500 kcal (10 500 kJ) per day, 55% of intake comes from carbohydrate (344 g, i.e. 1376 kcal (5579 kJ)) and 30% from fat (83 g, i.e. 747 kcal (3137 kJ)).
Cholesterol
Cholesterol is found in all animal products. Eggs are particularly rich in cholesterol, which is virtually absent from plants. The average daily intake in the UK is 300–500 mg. Cholesterol is also synthesized (see p. 306) and only very high or low dietary intakes will significantly affect blood levels.
Essential fatty acid deficiency
Essential fatty acid deficiency may accompany protein-energy malnutrition (PEM), but it has been clearly defined as a clinical entity only in patients on long-term parenteral nutrition given glucose, protein and no fat. Alopecia, thrombocytopenia, anaemia and dermatitis occur within weeks with an increased ratio of triene (n-9) to tetraene (n-6) in plasma fatty acids.
Carbohydrate
Carbohydrates are readily available in the diet, providing 17 kJ (4 kcal) per gram of energy (15.7 kJ (3.75 kcal) per gram monosaccharide equivalent). Carbohydrate intake comprises:
Carbohydrate is cheap compared with other foodstuffs; a great deal is therefore eaten, usually more than required.
Dietary fibre
Dietary fibre, which is largely non-starch polysaccharide (NSP) (entirely NSP according to some authorities), is often removed in the processing of food. This leaves highly refined carbohydrates such as sucrose which contribute to the development of dental caries and obesity. Lignin is included in dietary fibre in some classification systems, but it is not a polysaccharide. It is only a minor component of the human diet.
The principal classes of NSP are:
None of these are digested by gut enzymes. However, NSP is partly broken down in the gastrointestinal tract, mainly by colonic bacteria, producing gas and volatile fatty acids, e.g. butyrate.
All plant food, when unprocessed, contains NSP, so that all unprocessed food eaten will increase the NSP content of the diet. Bran, the fibre from wheat, provides an easy way of adding additional fibre to the diet: it increases faecal bulk and is helpful in the treatment of constipation.
The average daily intake of NSP in the diet is approximately 16 g. NSP deficiency is accepted as an entity by many authorities and it is suggested that the total NSP be increased to up to 30 g daily. This could be achieved by increased consumption of bread, potatoes, fruit and vegetables, with a reduction in sugar intake in order not to increase total calories. Each extra gram of fibre daily adds approximately 3–5 g to the daily stool weight. Pectins and gums have also been added to food to slow down monosaccharide absorption, particularly useful in type 2 diabetes.
Eating a diet rich in plant foods (fruits, vegetables, cereals and whole grain – the main sources of dietary fibre) is generally recommended for general health promotion, including protection against ischaemic heart disease, stroke and certain types of cancers. This has been attributed to a lipid lowering effect, the presence of protective substances, such as vitamin and non-vitamin antioxidants and other vitamins such as folic acid, which is linked to homocysteine metabolism, a risk factor for cardiovascular disease. Fermentation of fibre in the colon may protect against development of colonic cancer. However, associated lifestyle factors such as low physical activity may also help explain some of those associations.
Health promotion
Many chronic diseases – particularly obesity, diabetes mellitus and cardiovascular disease – cause premature mortality and morbidity and are potentially preventable by dietary change. This is a global problem, e.g. obesity affects one in nine adults in the world with the BMI being now similar in high- and middle-income groups. Reduction in salt and fat intake, combined with exercise and stopping smoking, would have a major effect on the health of the population.
Box 5.2 suggests the composition of the ‘ideal healthy diet’. The values given are based on the principle of:
 reducing total fat in the diet, particularly saturated fat
reducing total fat in the diet, particularly saturated fat
 increasing consumption of fish which contain n-3 (or ω-3) polyunsaturated fatty acids
increasing consumption of fish which contain n-3 (or ω-3) polyunsaturated fatty acids
 increasing intake of whole-grain cereals, green and orange vegetables and fruits, leading to an increase in fibre and antioxidants.
increasing intake of whole-grain cereals, green and orange vegetables and fruits, leading to an increase in fibre and antioxidants.
Reductions in dietary sodium and cholesterol have also been suggested. There would be no disadvantage in this, and most studies have suggested some benefit.
Fortification of foods
Fortification of foods with specific nutrients is common. In the UK, margarine and milk are fortified with vitamins A and D, flour with calcium, iron, thiamin and niacin and breakfast cereals with several vitamins and iron. Not all substances used in fortification have nutritive value. For example, Olestra is a polymer of sucrose and six or more triglycerides which has been introduced to combat obesity. It is not absorbed and is therefore used particularly in savoury snack foods (where it has FDA approval) as a ‘fake fat’. Therefore, it results in a reduction in total calories. It has side-effects, e.g. loose stools, abdominal cramps, and its use is being carefully monitored.
Nutrient goals and dietary guidelines
The interests of the individual are often different from those associated with government policy. A distinction needs to be made between nutrient goals and dietary guidelines:
 Nutrient goals refer to the national intakes of nutrients that are considered appropriate for optimal health in the population.
Nutrient goals refer to the national intakes of nutrients that are considered appropriate for optimal health in the population.
 Dietary guidelines refer to the dietary methods used to achieve these goals.
Dietary guidelines refer to the dietary methods used to achieve these goals.
Since dietary habits in different countries vary, dietary guidelines may also differ, even when the nutrient goals are the same. Nutrient goals are based on scientific information that links nutrient intake to disease. Although the information is incomplete, it includes evidence from a wide range of sources, including experimental animal studies, clinical studies and both short-term and long-term epidemiological studies.
FURTHER READING
Beaglehole R, Horton R. Chronic diseases: global action must match global evidence. Lancet 2010; 376:1619–1621.
Elia M, Cummings, JH. Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur J Clin Nutr 2007; (Suppl 1):SO40–SO74.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, protein, and amino acids. Washington, DC: The National Academies Press; 2005.
World Health Organization. Protein and amino acid requirements in human nutrition WHO Technical Report Series 935. Report of a Joint WHO/FAO/UNU Expert Consultation United Nations. Geneva: WHO; 2007.
WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases. Geneva: WHO; 2003.
Zampelas A. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010; 212:34–35.
Protein-energy malnutrition (PEM)
Developed countries
Starvation uncomplicated by disease is relatively uncommon in developed countries, although some degree of undernourishment is seen in very poor areas. Most nutritional problems occurring in the population at large are due to eating wrong combinations of foodstuffs, such as an excess of refined carbohydrate or a diet low in fresh vegetables. Undernourishment associated with disease is common in hospitals and nursing homes, and Table 5.4 gives a list of conditions in which malnutrition is often seen. Surgical complications, with sepsis, are a common cause. Many patients are admitted to hospital undernourished, and a variety of chronic conditions predispose to this state (Table 5.5).
Table 5.4 Common conditions associated with protein-energy malnutrition
| Sepsis | Dementia |
|---|---|
Trauma |
Malignancy |
Surgery, particularly of GI tract with complications |
Any very ill patient |
GI disease, particularly involving the small bowel |
Severe chronic inflammatory diseases |
|
Psychosocial: poverty, social isolation, anorexia nervosa, depression |
Table 5.5 Nutritional consequences of disease and the underlying risk factors (physical/psychosocial problems)
The majority of the weight loss, leading to malnutrition, is due to poor intake secondary to the anorexia associated with the underlying condition. Disease may also contribute by causing malabsorption and increased catabolism, which is mediated by complex changes in cytokines, hormones, side-effects of drugs, and immobility. The elderly are particularly at risk of malnutrition because they often suffer from diseases and psychosocial problems such as social isolation or bereavement (Table 5.5).
Pathophysiology of starvation (Fig. 5.4)
In the first 24 h following low dietary intake, the body relies for energy on the breakdown of hepatic glycogen to glucose. Hepatic glycogen stores are small and therefore gluconeogenesis is soon necessary to maintain glucose levels. Gluconeogenesis takes place mainly from pyruvate, lactate, glycerol and amino acids, especially alanine and glutamine. The majority of protein breakdown takes place in muscle, with eventual loss of muscle bulk.
Lipolysis, the breakdown of the body’s fat stores, also occurs. It is inhibited by insulin, but the level of this hormone falls off as starvation continues. The stored triglyceride is hydrolysed by lipase to glycerol, which is used for gluconeogenesis, and also to non-esterified fatty acids that can be used directly as a fuel or oxidized in the liver to ketone bodies.
Adaptive processes take place as starvation continues, to prevent the body’s available protein being completely utilized. There is a decrease in metabolic rate and total body energy expenditure. Central nervous metabolism changes from glucose as a substrate to ketone bodies. Gluconeogenesis in the liver decreases as does protein breakdown in muscle, both of these processes being inhibited directly by ketone bodies. Most of the energy at this stage comes from adipose tissue, with some gluconeogenesis from amino acids, particularly from alanine in the liver, and glutamine in the kidney.
The metabolic response to prolonged starvation differs between lean and obese individuals. One of the major differences concerns the proportion of energy derived from protein oxidation, which determines the proportion of weight loss from lean tissues. This proportion may be up to three times smaller in obese subjects than lean subjects. It can be regarded as an adaptation which depends on the composition of the initial reserves (Fig. 5.3). This means that deterioration in body function is more rapid in lean subjects. Furthermore, survival time is much less in lean subjects (~2 months), compared to the obese (can be at least several months).
Following trauma or shock, some of the adaptive changes do not take place. Glucocorticoids and cytokines (see below) stimulate the ubiquitin-proteasome pathway in muscle, which is responsible for accelerated proteolysis in muscle in many catabolic illnesses. In starvation, there is a decrease in BMR, while in inflammatory and traumatic disease the BMR is often increased. These changes all result in continuing gluconeogenesis with massive muscle breakdown, and further reduction in survival time.
Regulation of metabolism
Tissue metabolism is regulated by multiple coordinated processes. Some are rapid involving nerves, whilst others are slower involving circulating substrates and hormones. Factors include:
 Circulating substrate concentrations. The uptake and metabolism of ketone bodies, which serve as the major fuel for the brain during prolonged starvation, is primarily determined by the circulatory concentration which can increase up to 5 mmol/L or more. The liver is responsible for producing ketone bodies, the production of which is in turn controlled by the availability of fatty acids derived from adipose tissue. Substrates may also compete with each other for metabolism, for example glucose competes with non-esterified fatty acids for uptake and metabolism in muscle and heart (the glucose-fatty acid cycle) and this is independent of hormones.
Circulating substrate concentrations. The uptake and metabolism of ketone bodies, which serve as the major fuel for the brain during prolonged starvation, is primarily determined by the circulatory concentration which can increase up to 5 mmol/L or more. The liver is responsible for producing ketone bodies, the production of which is in turn controlled by the availability of fatty acids derived from adipose tissue. Substrates may also compete with each other for metabolism, for example glucose competes with non-esterified fatty acids for uptake and metabolism in muscle and heart (the glucose-fatty acid cycle) and this is independent of hormones.
 Blood flow. The delivery of substrates (and other signals) to tissues depends not only on their circulating concentration but also on the blood flow to tissues. In many tissues there is coupling between metabolic activity and blood flow, with arterioles regulating blood flow to the tissue according to demand, e.g. blood flow to muscle increases during exercise.
Blood flow. The delivery of substrates (and other signals) to tissues depends not only on their circulating concentration but also on the blood flow to tissues. In many tissues there is coupling between metabolic activity and blood flow, with arterioles regulating blood flow to the tissue according to demand, e.g. blood flow to muscle increases during exercise.
 Signals. Hormones and other signals, such as cytokines (see below), regulate intracellular metabolism.
Signals. Hormones and other signals, such as cytokines (see below), regulate intracellular metabolism.
Insulin/glucagon ratios in the fed and fasted state
In the fed state, insulin/glucagon ratios are high. Insulin promotes synthesis of glycogen, protein and fat, and inhibits lipolysis and gluconeogenesis.
In the fasted state, insulin/glucagon ratios are low. Glucagon acts mainly on the liver and has no action on muscle. It increases glycogenolysis and gluconeogenesis, as well as increasing ketone body production from fatty acids. It also stimulates lipolysis in adipose tissue. Catecholamines have a similar action to glucagon but also affect muscle metabolism. These agents both act via cyclic adenosine monophosphate (cAMP) to stimulate lipolysis, producing free fatty acids that can then act as a major source of energy.
Proportion of lean to fat tissue
During weight loss uncomplicated by disease, the proportion of lean to fat tissue loss (or proportion of energy derived from protein metabolism) is greater in lean than overweight/obese individuals.
During acute disease, loss of lean tissue, which is associated with protein oxidation, can be particularly rapid. Hormones such as corticosteroids, proinflammatory cytokines and insulin resistance are all involved.
Role of cytokines
The metabolic response to trauma, injury and inflammation depends on the balance between proinflammatory (e.g. tumour necrosis factor, TNF; interleukin-2, IL-2) and anti-inflammatory cytokines (e.g. IL-10), and the production of many of these cytokines is influenced by genetic polymorphisms. Since many chronic diseases, including atherosclerosis, have an inflammatory component, these changes have wide-reaching metabolic implications.
Cytokines such as IL-1, IL-6 and TNF play a significant role in regulating metabolism. In acute diseases they contribute to the catabolic process, glycogenolysis, and acute-phase protein synthesis. TNF, which inhibits lipoprotein lipase, is one of a number of ‘cachexia factors’ in patients with cancer.
It is unclear how these cytokines interact with central feeding pathways to cause anorexia. However, in animal models of both cancer and inflammatory bowel disease, many peripheral and central mediators of appetite are involved. For example, neuropeptide Y levels in the hypothalamus are often inappropriately low, so there is a reduced drive to feeding.
Clinical features
Patients are sometimes seen with loss of weight or malnutrition as the primary symptom (failure to thrive in children). Mostly, however, malnourishment is only seen as an accompaniment of some other disease process, such as malignancy. Severe malnutrition is seen mainly with advanced organic disease or after surgical procedures followed by complications. Three key features which help in the detection of chronic protein-energy malnutrition (PEM) in adults are listed in Box 5.3.
![]() Box 5.3
Box 5.3
Key features in detection of chronic protein-energy malnutrition (PEM) in developed countries
In patients with oedema or dehydration the BMI may be somewhat misleading.
2. Weight loss in previous 3–6 months: >10%, high risk; 5–10%, possible risk; <5% low/no risk of developing PEM.
3. Acute disease effect: diseases that have resulted or are likely to result in no dietary intake for >5 days are associated with a high risk of malnutrition (e.g. prolonged unconsciousness, persistent swallowing problems after a stroke, or prolonged ileus after abdominal surgery).
Other factors that may suggest PEM include:
 History of decreased food intake/loss of appetite
History of decreased food intake/loss of appetite
 Clothes becoming loosely fitting (weight loss) and a general appearance indicating obvious wasting
Clothes becoming loosely fitting (weight loss) and a general appearance indicating obvious wasting
 Physical and psychosocial disturbances likely to have contributed to the weight loss.
Physical and psychosocial disturbances likely to have contributed to the weight loss.
The factors listed in Box 5.3 act as a link between detection and management (Fig. 5.5, the ‘Malnutrition Universal Screening Tool’). If the underlying physical or psychosocial problems are not adequately addressed, treatment may not be successful.
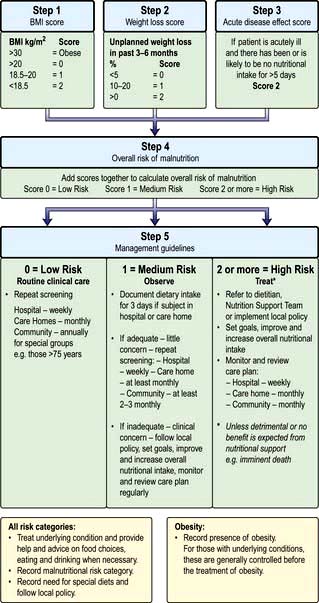
Figure 5.5 ‘Malnutrition Universal Screening Tool’ (‘MUST’)
(with permission from the British Association for Parenteral and Enteral Nutrition (BAPEN), at: http://www.bapen.org.uk).
PEM leads to a depression of the immunological defence mechanism, resulting in a decreased resistance to infection. It also detrimentally affects muscle strength and fatigue, reproductive function (e.g. in anorexia nervosa, which is common in adolescent girls; p. 1188), wound healing, and psychological function (depression, anxiety, hypochondriasis, loss of libido).
In children, growth failure is a key element in the diagnosis of PEM. New WHO standards for optimal growth in children 0–4 years have been adopted by developing and developed countries. They aim to reflect optimal rather than prevailing growth in both developed and developing countries, since they involved a healthy pregnancy and children born to non-smoking, relatively affluent mothers who breast-fed their children exclusively or predominantly for the first 6 months of life. The general principles of management of severe PEM in children are similar in developed and developing countries but resources are required to manage the problems once identified (see p. 205).
Treatment
When malnutrition is obvious and the underlying disease cannot be corrected at once, some form of nutritional support is necessary (see also pp. 221, 223). Nutrition should be given enterally if the gastrointestinal tract is functioning adequately. This can most easily be done by encouraging the patient to eat more often and by giving a high-calorie supplement. If this is not possible, a liquefied diet may be given intragastrically via a fine-bore tube or by a percutaneous endoscopic gastrostomy (PEG). If both of these measures fail, parenteral nutrition is given.
Developing countries
The International Union of Nutritional Sciences, with support from the International Pediatric Association, launched a global Malnutrition Task Force in 2005 to ensure that an integrated system of prevention and treatment of malnutrition is actively supported.
In many areas of the world, people are on the verge of malnutrition due to extreme poverty. In addition, if events such as drought, war or changes in political climate occur, millions suffer from starvation. Although the basic condition of PEM is the same in all parts of the world from whatever cause, malnutrition resulting from long periods of near-total starvation produces unique clinical appearances in children virtually never seen in high-income countries. The term ‘protein-energy malnutrition’ covers the spectrum of clinical conditions seen in adults and children. Children under 5 years may present with the following:
 Kwashiorkor occurs typically in a young child displaced from breast-feeding by a new baby. It is often precipitated by infections such as measles, malaria and diarrhoeal illnesses. The child is apathetic and lethargic with severe anorexia. There is generalized oedema with skin pigmentation and thickening (Fig. 5.6b). The hair is dry, sparse and may become reddish or yellow in colour. The abdomen is distended owing to hepatomegaly and/or ascites. The serum albumin is always low. The exact cause is unknown, but theories related to diet (low in protein, and high in carbohydrate) and free radical damage in the presence of inadequate antioxidant defences have been proposed.
Kwashiorkor occurs typically in a young child displaced from breast-feeding by a new baby. It is often precipitated by infections such as measles, malaria and diarrhoeal illnesses. The child is apathetic and lethargic with severe anorexia. There is generalized oedema with skin pigmentation and thickening (Fig. 5.6b). The hair is dry, sparse and may become reddish or yellow in colour. The abdomen is distended owing to hepatomegaly and/or ascites. The serum albumin is always low. The exact cause is unknown, but theories related to diet (low in protein, and high in carbohydrate) and free radical damage in the presence of inadequate antioxidant defences have been proposed.
 Marasmus is the childhood form of starvation, which is associated with obvious wasting. The child looks emaciated, there is obvious muscle wasting and loss of body fat. There is no oedema. The hair is thin and dry (Fig. 5.6a). The child is not so apathetic or anorexic as with kwashiorkor. Diarrhoea is frequently present and signs of infection must be looked for carefully.
Marasmus is the childhood form of starvation, which is associated with obvious wasting. The child looks emaciated, there is obvious muscle wasting and loss of body fat. There is no oedema. The hair is thin and dry (Fig. 5.6a). The child is not so apathetic or anorexic as with kwashiorkor. Diarrhoea is frequently present and signs of infection must be looked for carefully.
A classification of severe malnutrition by the World Health Organization (WHO) (Table 5.6) makes no distinction between kwashiorkor and marasmus, because their approach to treatment is similar. The WHO classification of chronic undernutrition in children is based on standard deviation (SD) scores. Thus, children with an SD score between −2 and −3 (between 3 and 2 standard deviation scores below the median – corresponding to a value between 0.13 and 2.3 centile) can be regarded as being at moderate risk of undernutrition, and below an SD score of −3, of severe malnutrition. A low weight-for-height is a measure of thinness (wasting when pathological) and a low height-for-age is a measure of shortness (stunting when pathological). Those with oedema and clinical signs of severe malnutrition are classified as having oedematous malnutrition.
Table 5.6 Classification of childhood malnutrition
| Moderate malnutrition | Severe malnutritiona | |
|---|---|---|
Symmetrical oedema |
No |
Yes: oedematous malnutritionb |
Weight-for-height SD score |
−3 to −2 (70–79%)c |
|
Height-for-age SD score |
−3 to −2 (85–89%)c |
<−3 (<85%)c (severe stunting) |
a The diagnoses are not mutually exclusive.
b Older classifications use the terms kwashiorkor and marasmic-kwashiorkor instead.
c Percentage of the median National Centre for Health Statistics/WHO reference.
d Called marasmus (without oedema) in the Wellcome classification and grade II in the Gomez classification.
Starvation in adults may lead to extreme loss of weight depending upon the severity and duration. They may crave for food, are apathetic and complain of cold and weakness with a loss of subcutaneous fat and muscle wasting. The WHO classification is based on body mass index (BMI), with a value <18.5 kg/m2 indicating malnutrition (severe malnutrition if <16.0 kg/m2).
Severely malnourished adults and children are very susceptible to respiratory and gastrointestinal infections, leading to an increased mortality in these groups.
Investigations
These are not always practicable in certain settings in the developing world.
 Stools should be examined for parasitic infestations.
Stools should be examined for parasitic infestations.
 Chest X-ray – tuberculosis is common and is easily missed if a chest X-ray is not performed.
Chest X-ray – tuberculosis is common and is easily missed if a chest X-ray is not performed.
Treatment
Treatment must involve the provision of protein and energy supplements and the control of infection. The approach to treatment of children is described below. Adults do not usually suffer such severe malnutrition, but the same general principles of treatment should be followed.
Resuscitation and stabilization
The severely ill child will require:
 Correction of fluid and electrolyte abnormalities, but intravenous therapy should be avoided if possible because of the danger of fluid overload
Correction of fluid and electrolyte abnormalities, but intravenous therapy should be avoided if possible because of the danger of fluid overload
 Treatment of shock with oxygen
Treatment of shock with oxygen
 Treatment of hypoglycaemia (blood glucose <3 mmol/L), hypothermia (reduce heat loss, and provide additional heat if necessary) and infection (antibiotics) – these often co-exist.
Treatment of hypoglycaemia (blood glucose <3 mmol/L), hypothermia (reduce heat loss, and provide additional heat if necessary) and infection (antibiotics) – these often co-exist.
The standard WHO oral hydration solution has a high sodium and low potassium content and is not suitable for severely malnourished children. Instead, the rehydration solution for malnutrition (ReSoMal) is recommended. It is commercially available but can also be produced by modification of the standard WHO oral hydration solution.
Infection is common (Box 5.4). Diarrhoea is often due to bacterial or protozoal overgrowth; metronidazole is very effective and is often given routinely. Parasites are also common and, as facilities for stool examination are usually not available, mebendazole 100 mg twice daily should be given for 3 days. In high-risk areas, antimalarial therapy is given.
Large doses of vitamin A are also given because deficiency of this vitamin is common. After the initial resuscitation, further stabilization over the next few days is undertaken, as indicated in Table 5.7.
Table 5.7 Timeframe for the management of the child with severe malnutrition (the 10-step approach recommended by the WHO)
Re-feeding
This needs to be planned carefully. During the initial treatment of the acute situation, a balanced diet with sufficient protein and energy is given to maintain a steady state. Large increases in energy can lead to heart failure, circulatory collapse and death (re-feeding syndrome). Initial feeding involves administration of feeds of low osmolarity and low in lactose. WHO recommendations are 100 kcal/kg per day; 1.0–1.5 g protein/kg per day and 130 mL liquid/kg per day (100 mL/kg per day if the child has marked oedema). Attempts should be made to give the feeds slowly and frequently (e.g. 2-hourly during days 1–2; 3-hourly during days 3–5; and 4-hourly thereafter), although anorexia is often a problem and can be exacerbated by excessive feeding. If necessary, fluids and food should be given by nasogastric tube. The child is then gradually weaned to liquids and then solids by mouth. All severely malnourished children have vitamin and mineral deficiencies. Although anaemia is common, the WHO recommends giving iron only after the child develops a good appetite and starts gaining weight, because of concern about making the infection worse (iron is a pro-oxidant). The child should be given daily micronutrient supplements for at least 2 weeks. These should include a multivitamin supplement with folic acid, zinc and copper.
Rehabilitation
Gradually, as the child improves, more energy can be given, and during rehabilitation weight gain is achieved by providing extra energy and protein (‘catch-up weight gain’). Children who have been severely ill need constant attention right through the convalescent period, as often home conditions are poor and feeds are refused. Sensory stimulation and emotional support is a major component of management during both the stabilization and rehabilitation phases. The treatment of underlying chronic infective conditions such as HIV, malaria and tuberculosis is also necessary.
Care setting
There are not enough hospitals or therapeutic feeding centres to cope with the malnutrition problem (even acute malnutrition problems), which emphasizes the need for outpatient and community based programmes, although these require investment and time to build to full capacity. These may involve the use of ready-to-use therapeutic foods, such as energy-dense pastes with minerals and vitamins, without the need to add water, which could potentially contaminate the food.
Prognosis
Children with extreme malnutrition have a mortality of over 50%. By careful management, this can be reduced significantly to less than 10%, depending on the availability of facilities and trained staff. Treatment of underlying disease is essential. Brain development takes place in the first years of life, a time when severe PEM frequently occurs. There is evidence that intellectual impairment and behavioural abnormalities occur in severely affected children. Physical growth is also impaired. Probably both of these effects can be alleviated if it is possible to maintain a high standard of living with a good diet and freedom from infection over a long period.
Prevention
Prevention of PEM depends not only on adequate nutrients being available but also on education of both governments and individuals in the importance of good nutrition and immunization (Box 5.5). Short-term programmes are useful for acute shortages of food, but long-term programmes involving improved agriculture are equally necessary. Bad feeding practices and infections are more prevalent than actual shortage of food in many areas of the world. However, good surveillance is necessary to avoid periods of famine.
Food supplements (and additional vitamins) should be given to ‘at-risk’ groups by adding high-energy food (e.g. milk powder, meat concentrates) to the diet. Pregnancy and lactation are times of high energy requirement and supplements have been shown to be beneficial.
FURTHER READING
Bhutta ZA. Addressing severe malnutrition where it matters. Lancet 2009; 374:94–96.
Collins S, Dent N, Binns P et al. Management of severe acute malnutrition in children. Lancet 2006; 368:1992–2000.
Collins S, Sadler K, Dent N et al. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull 2006; 27:S49–S82.
Elia M, Russell CA, Stratton RJ. Malnutrition in the UK: Policies to address the problem. Proc Nutr Soc 2010; 69:470–476.
Kerac M, Egan R, Mayer S et al. New WHO growth standards: roll-out needs more resources. Lancet 2009; 374:100–102.
Stratton RJ, Elia M. Encouraging appropriate, evidence-based use of oral nutritional supplements. Proc Nutr Soc 2010; 69:477–487.
Stratton RJ, Elia M. A review of reviews: a new look at the evidence for oral nutritional supplements in clinical practice. Clin Nutr Suppl 2007; 2:5–23.
Wright CM, Williams AF, Ellman D et al. Using the new UK-WHO growth charts. BMJ 2010; 340:647–650. (The charts and supporting materials can be downloaded from: www.growthcharts.rcpch.ac.uk)
Vitamins
Deficiencies due to inadequate intake associated with PEM (Table 5.8) are commonly seen in the developing countries. This is not, however, invariable. For example, vitamin A deficiency is not seen in Jamaica, but is common in PEM in Hyderabad, India. In the West, deficiency of vitamins is less common but prominent in the specific groups shown in Table 5.9. The widespread use of vitamins as ‘tonics’ is unnecessary and should be discouraged. Toxicity from excess fat-soluble vitamins is occasionally seen.
Table 5.8 Fat-soluble and water-soluble vitamins: UK reference nutrient intake (RNI) and lower reference nutrient intake (LRNI) for men aged 19–50 yearsa
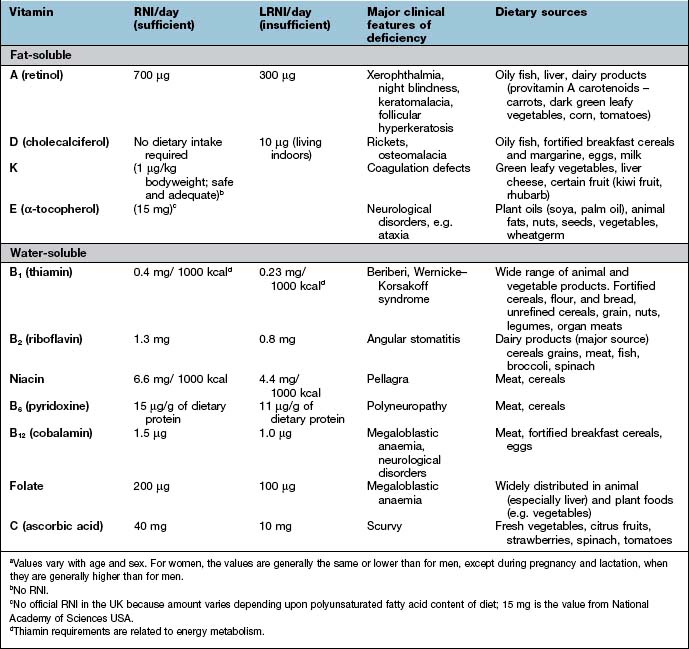
Table 5.9 Some causes of vitamin deficiency in developed countries
Fat-soluble vitamins
Vitamin A
Vitamin A (retinol) is part of the family of retinoids which is present in food and the body as esters combined with long-chain fatty acids. The richest food source is liver, but it is also found in milk, butter, cheese, egg yolks and fish oils. Retinol or carotene is added to margarine in the UK and other countries.
Beta-carotene is the main carotenoid found in green vegetables, carrots and other yellow and red fruits. Other carotenoids, lycopene and lutein, are probably of little quantitative importance as dietary precursors of vitamin A.
Beta-carotene is cleaved in the intestinal mucosa by carotene dioxygenase, yielding retinaldehyde which can be reduced to retinol. Between a quarter and a third of dietary vitamin A in the UK is derived from retinoids. Nutritionally, 6 µg of β-carotene is equivalent to 1 µg of preformed retinol; vitamin A activity in the diet is given as retinol equivalents.
Function
Retinol is stored in the liver and is transported in plasma bound to an α-globulin, retinol-binding protein (RBP). Vitamin A has several metabolic roles:
 Retinaldehyde in its cis form is found in the opsin proteins in the rods (rhodopsin) and cones (iodopsin) of the retina (p. 1055). Light causes retinaldehyde to change to its trans isomer, and this leads to changes in membrane potentials that are transmitted to the brain.
Retinaldehyde in its cis form is found in the opsin proteins in the rods (rhodopsin) and cones (iodopsin) of the retina (p. 1055). Light causes retinaldehyde to change to its trans isomer, and this leads to changes in membrane potentials that are transmitted to the brain.
 Retinol and retinoic acid are involved in the control of cell proliferation and differentiation.
Retinol and retinoic acid are involved in the control of cell proliferation and differentiation.
 Retinyl phosphate is a cofactor in the synthesis of most glycoproteins containing mannose.
Retinyl phosphate is a cofactor in the synthesis of most glycoproteins containing mannose.
Vitamin A deficiency
Worldwide, vitamin A deficiency and xerophthalmia (see below) is the major cause of blindness in young children despite intensive preventative programmes.
Xerophthalmia has been classified by the WHO (Table 5.10). Impaired adaptation followed by night blindness is the first effect. There is dryness and thickening of the conjunctiva and the cornea (xerophthalmia occurs as a result of keratinization). Bitot’s spots – white plaques of keratinized epithelial cells – are found on the conjunctiva of young children with vitamin A deficiency. These spots can, however, be seen without vitamin A deficiency, possibly caused by exposure. Corneal softening, ulceration and dissolution (keratomalacia) eventually occur, superimposed infection is a frequent accompaniment and both lead to blindness. In PEM, retinol-binding protein along with other proteins is reduced. This suggests vitamin A deficiency, although body stores are not necessarily reduced.
Table 5.10 Classification of xerophthalmia by ocular signs
| Ocular signs | Classification |
|---|---|
Night blindness |
XN |
Conjunctival xerosis |
XIA |
Bitot’s spot |
X2 |
Corneal xerosis |
X2 |
Corneal ulceration/keratomalacia < |
X3A |
Corneal ulceration/keratomalacia > |
X3B |
Corneal scar |
XS |
Xerophthalmic fundus |
XF |
From WHO/UNICEF/IVACG 1988. X, xerophthalmia.
Vitamin A in malnourished children
Vitamin A supplementation (single oral dose of 60 mg retinol palmitate) appears to improve morbidity and mortality from measles. It has also been suggested that a similar supplementation reduces morbidity and/or mortality from diarrhoeal diseases and respiratory infections and improves growth. Despite low circulating concentrations of vitamin A in HIV-infected individuals, supplementation of HIV-infected pregnant women does not appear to reduce the risk of mother-to-child transmission of HIV.
Diagnosis
In parts of the world where deficiency is common, diagnosis is made on the basis of the clinical features, and deficiency should always be suspected if any degree of malnutrition is present. Blood levels of vitamin A will usually be low, but the best guide to the diagnosis is a response to replacement therapy.
Treatment
Urgent treatment with retinol palmitate 30 mg orally should be given on two successive days. In the presence of vomiting and diarrhoea, 30 mg of vitamin A is given intramuscularly. Associated malnutrition must be treated, and superadded bacterial infection should be treated with antibiotics. Referral for specialist ophthalmic treatment is necessary in severe cases.
Prevention
Most western diets contain enough dairy products and green vegetables, but vitamin A is added to foodstuffs (e.g. margarine) in some countries. Vitamin A is not destroyed by cooking.
In some developing countries, vitamin A supplements are given at the time the child attends for measles vaccination. Food fortification programmes are another approach. Education of the population is necessary and people should be encouraged to grow their own vegetables. In particular, pregnant women and children should be encouraged to eat green vegetables and yellow fruits.
Other effects of vitamin A
In a chronically malnourished population maternal repletions with vitamin A before, during and after pregnancy may improve lung function in the offspring at 9–13 years. It may also reduce maternal mortality. The effect of β-carotene in cardiovascular and other diseases is discussed below in the section entitled ‘Dietary antioxidants’ (p. 211). Retinoic acid and some synthetic retinoids are used in dermatology (p. 1213).
Possible adverse effects
 High intakes of vitamin A. Chronic ingestion of retinol can cause liver and bone damage, hair loss, double vision, vomiting, headaches and other abnormalities. Single doses of 300 mg in adults or 100 mg in children can be harmful.
High intakes of vitamin A. Chronic ingestion of retinol can cause liver and bone damage, hair loss, double vision, vomiting, headaches and other abnormalities. Single doses of 300 mg in adults or 100 mg in children can be harmful.
 Retinol is teratogenic. The incidence of birth defects in infants is high with vitamin A intakes of >3 mg a day during pregnancy. In pregnancy, extra vitamin A or consumption of liver is not recommended in the UK. However, β-carotene is not toxic.
Retinol is teratogenic. The incidence of birth defects in infants is high with vitamin A intakes of >3 mg a day during pregnancy. In pregnancy, extra vitamin A or consumption of liver is not recommended in the UK. However, β-carotene is not toxic.
Vitamin D
Vitamin D is discussed in more detail in Chapter 11, where the most common manifestations of deficiency are discussed (bone and calcium disorders, Chapter 24; rickets and osteomalacia, Chapter 24). Vitamin D receptors are distributed widely in human tissues, but their function in many non-musculoskeletal tissues still remains poorly understood. Vitamin D status has been linked to a wide range of diseases, including:
 Cardiovascular (ischaemic heart disease, heart failure, hypertension)
Cardiovascular (ischaemic heart disease, heart failure, hypertension)
 Respiratory (chest infections)
Respiratory (chest infections)
 Renal (progression of renal disease)
Renal (progression of renal disease)
 Endocrinological (type 1 and type 2 diabetes)
Endocrinological (type 1 and type 2 diabetes)
 Neuropsychiatric disorders (depression, cognitive deficits)
Neuropsychiatric disorders (depression, cognitive deficits)
 Cancer (e.g. prostate, breast, colon) and mortality from various causes.
Cancer (e.g. prostate, breast, colon) and mortality from various causes.
It has therefore been suggested that vitamin D may have a role in global health, and not just the health of the musculoskeletal system. Studies of the relationship between vitamin D status and risk for these conditions has led to different definitions of various levels for adequate status, implying that there are different requirements for vitamin D in different diseases. However, randomized controlled trials (RCTs) of vitamin D supplementation have not been as promising in averting some of these conditions as might have been anticipated from the observational relationships.
Vitamin K
Vitamin K is found as phylloquinone (vitamin K1) in green leafy vegetables, dairy products, rapeseed and soya bean oils. Intestinal bacteria can synthesize the other major form of vitamin K, menaquinone (vitamin K2), in the terminal ileum and colon. Vitamin K is absorbed in a similar manner to other fat-soluble substances in the upper small gut. Some menaquinones must also be absorbed as this is the major form found in the human liver.
Function
Vitamin K is a cofactor necessary for the production not only of blood clotting factors (II, VII, IX and X, and other proteins involved in coagulation; Chapter 20), but also for proteins necessary in the formation of bone.
Vitamin K is a cofactor for the post-translational carboxylation of specific protein-bound glutamate residues in γ-carboxyglutamate (Gla). Gla residues bind calcium ions to phospholipid templates, and this action on factors II, VII, IX and X and on proteins C and S, is necessary for coagulation to take place.
Bone osteoblasts contain three vitamin K-dependent proteins, osteocalcin, matrix Gla protein and protein S, which have a role in bone matrix formation. Osteocalcin contains three Gla residues which bind tightly to the hydroxyapatite matrix depending on the degree of carboxylation; this leads to bone mineralization. There is, however, no convincing evidence that vitamin K deficiency or antagonism affects bone other than rapidly growing bone.
Vitamin K deficiency
Vitamin K deficiency results in inadequate synthesis of clotting factors (p. 423), which leads to an increase in the prothrombin time and haemorrhage. Deficiency occurs in the following circumstances:
The newborn
Deficiency occurs in the newborn owing to:
 poor placental transfer of vitamin K
poor placental transfer of vitamin K
 little vitamin K in breast milk
little vitamin K in breast milk
 no hepatic stores of menaquinone (no intestinal bacteria in the neonate).
no hepatic stores of menaquinone (no intestinal bacteria in the neonate).
Deficiency leads to a haemorrhagic disease of the newborn, which can be prevented by prophylactic vitamin K. Vitamin K (phytomenadione 1 mg, i.m.) is given to all neonates after risks have been discussed with parents and consent obtained.
Cholestatic jaundice
When bile flow into the intestine is interrupted, malabsorption of vitamin K occurs as no bile salts are available to facilitate absorption and the prothrombin time increases. This can be corrected by giving 10 mg of phytomenadione intramuscularly. (Note that an increased prothrombin time because of liver disease does not respond to vitamin K injection, there being no shortage of vitamin K, just bad liver function.) In patients with chronic cholestasis (e.g. primary biliary cirrhosis) oral therapy using a water-soluble preparation, menadiol sodium phosphate 10 mg daily, is used.
Concomitant vitamin K antagonists
Oral anticoagulants, e.g. warfarin, antagonize vitamin K (p. 428). Antibacterial drugs also interfere with the bacterial synthesis of vitamin K.
Vitamin E
Vitamin E includes eight naturally occurring compounds divided into tocopherols and tocotrienols. The most active compound and the most widely available in food is the natural isomer d- (or RRR) α-tocopherol, which accounts for 90% of vitamin E in the human body. Vegetables and seed oils, including soya bean, saffron, sunflower, cereals and nuts, are the main sources. Animal products are poor sources of the vitamin. Vitamin E is absorbed with fat, transported in the blood largely in low-density lipoproteins (LDL).
An individual’s vitamin E requirement depends on the intake of polyunsaturated fatty acids (PUFAs). Since this varies widely, no daily requirement is given in the UK. The requirement stated in the USA is approximately 7–10 mg/day, but average diets contain much more than this. If PUFAs are taken in large amounts, more vitamin E is required.
Function
The biological activity of vitamin E results principally from its antioxidant properties. In biological membranes, it contributes to membrane stability. It protects cellular structures against damage from a number of highly reactive oxygen species, including hydrogen peroxide, superoxide and other oxygen radicals. Vitamin E may also affect cell proliferation and growth.
Vitamin E deficiency
The first deficiency to be demonstrated was a haemolytic anaemia described in premature infants. Infant formulations now contain vitamin E.
Deficiency is seen only in children with abetalipoproteinaemia (p. 270) and in patients on long-term parenteral nutrition. The severe neurological deficit (gross ataxia) can be prevented by vitamin E injections.
Plasma or serum levels of α-tocopherol can be measured and should be corrected for the level of plasma lipids by expressing the value as milligrams per milligram of plasma lipid.
Epidemiological data and clinical trials
Animals fed an atherogenic diet supplemented with α-tocopherol develop many fewer new atheromatous lesions than those fed an atherogenic diet alone; there may be regression of existing lesions.
There is also evidence for vitamin E intake and blood α-tocopherol levels as an independent risk factor for the development of ischaemic heart disease (IHD) in healthy, well-nourished individuals eating a western diet. This has been shown in comparisons of different communities in the WHO ‘MONICA’ observational study.
Randomized trials involving vitamin E supplementation have produced conflicting results, possibly due to factors such as short duration of treatment, use of suboptimal doses or without the concurrent administration of vitamin C. There are very few trials to assess the role of vitamin E in prevention of peripheral vascular disease and for cancer prevention.
Water-soluble vitamins
Water-soluble vitamins are non-toxic and relatively cheap and can therefore be given in large amounts if a deficiency is possible. The daily requirements of water-soluble vitamins are given in Table 5.8.
Thiamin (vitamin B1)
Function
Thiamin diphosphate, often called thiamin pyrophosphate (TPP), is an essential cofactor, particularly in carbohydrate metabolism.
TPP is involved in the oxidative decarboxylation of acetyl CoA in mitochondria. In formation of acetyl CoA (from pyruvate) and in the Krebs cycle, TPP is the key enzyme for the decarboxylation of α-ketoglutarate to succinyl CoA. TPP is also the cofactor for transketolase, a key enzyme in the hexose monophosphate shunt.
Thiamin is found in many foodstuffs, including cereals, grains, beans, nuts, as well as pork and duck. It is often added to food (e.g. in cereals) in developed countries. The dietary requirement (see Table 5.8) depends on energy intake, more being required if the diet is high in carbohydrates.
Following absorption, thiamin is found in all body tissues, the majority being in the liver. Body stores are small and signs of deficiency quickly develop with inadequate intake.
There is no evidence that a high oral intake is dangerous, but ataxia has been reported after high parenteral therapy.
Thiamin deficiency
 as beriberi, where the only food consumed is polished rice
as beriberi, where the only food consumed is polished rice
 in chronic alcohol-dependent patients who are consuming virtually no food at all
in chronic alcohol-dependent patients who are consuming virtually no food at all
 in starved patients (e.g. with carcinoma of the stomach), and in severe prolonged hyperemesis gravidarum, anorexia nervosa and prolonged total starvation in healthy subjects (e.g. fasts for political reasons). It can also occur in patients given parenteral nutrition with little or no thiamine as large doses of glucose increase requirements of thiamin and can precipitate deficiency, e.g. during re-feeding.
in starved patients (e.g. with carcinoma of the stomach), and in severe prolonged hyperemesis gravidarum, anorexia nervosa and prolonged total starvation in healthy subjects (e.g. fasts for political reasons). It can also occur in patients given parenteral nutrition with little or no thiamine as large doses of glucose increase requirements of thiamin and can precipitate deficiency, e.g. during re-feeding.
Beriberi
This is now confined to the poorest areas of South-east Asia. It can be prevented by eating undermilled or par-boiled rice, or by fortification of rice with thiamine. The prevention of beriberi needs a general increase in overall food consumption so that the staple diet is varied and contains legumes and pulses, which contain a large amount of thiamin. There are two main clinical types of beriberi which, surprisingly, only rarely occur together.
 Dry beriberi usually presents insidiously with a symmetrical polyneuropathy. The initial symptoms are heaviness and stiffness of the legs, followed by weakness, numbness, and pins and needles. The ankle jerk reflexes are lost and eventually all the signs of polyneuropathy that may involve the trunk and arms are found (p. 1147). Cerebral involvement occurs, producing the picture of the Wernicke–Korsakoff syndrome (p. 1147). In endemic areas, mild symptoms and signs may be present for years without unduly affecting the patient.
Dry beriberi usually presents insidiously with a symmetrical polyneuropathy. The initial symptoms are heaviness and stiffness of the legs, followed by weakness, numbness, and pins and needles. The ankle jerk reflexes are lost and eventually all the signs of polyneuropathy that may involve the trunk and arms are found (p. 1147). Cerebral involvement occurs, producing the picture of the Wernicke–Korsakoff syndrome (p. 1147). In endemic areas, mild symptoms and signs may be present for years without unduly affecting the patient.
 Wet beriberi causes oedema. Initially this is of the legs, but it can extend to involve the whole body, with ascites and pleural effusions. The peripheral oedema may mask the accompanying features of dry beriberi.
Wet beriberi causes oedema. Initially this is of the legs, but it can extend to involve the whole body, with ascites and pleural effusions. The peripheral oedema may mask the accompanying features of dry beriberi.
Thiamin deficiency impairs pyruvate dehydrogenase with accumulation of lactate and pyruvate, producing peripheral vasodilatation and eventually oedema. The heart muscle is also affected and heart failure occurs, causing a further increase in the oedema. Initially there are warm extremities, a full, fast, bounding pulse and a raised venous pressure (‘high-output state’), but eventually heart failure advances and a poor cardiac output ensues. The electrocardiogram may show conduction defects.
Infantile beriberi occurs, usually acutely, in breast-fed babies at approximately 3 months of age. The mothers show no signs of thiamin deficiency but presumably their body stores must be virtually nil. The infant becomes anorexic, develops oedema and has some degree of aphonia. Tachycardia and tachypnoea develop and, unless treatment is instituted, death occurs quickly.
Diagnosis
In endemic areas, the diagnosis of beriberi should always be suspected and if in doubt treatment with thiamine should be instituted. A rapid disappearance of oedema after thiamine (50 mg i.m.) is diagnostic. Other causes of oedema must be considered (e.g. renal or liver disease), and the polyneuropathy is indistinguishable from that due to other causes. The diagnosis is confirmed by measurement of the circulating thiaminconcentration or transketolase activity in red cells using fresh heparinized blood.
Treatment
Thiamine 50 mg i.m. is given for 3 days, followed by 50 mg of thiamine daily by mouth. The response in wet beriberi occurs in hours, giving dramatic improvement, but in dry beriberi improvement is often slow to occur. In most cases all the B vitamins are given because of multiple deficiency. Infantile beriberi is treated by giving thiamine to the mother, which is then passed on to the infant via the breast milk.
Thiamin deficiency in people with alcohol dependence or acute illness
In the developed world, alcohol-dependent people and those with severe acute illness receiving high-carbohydrate infusions without vitamins are the only major groups to suffer from thiamin deficiency. Rarely, they develop wet beriberi, which must be distinguished from alcoholic cardiomyopathy. More usually, however, thiamin deficiency presents with polyneuropathy or with the Wernicke–Korsakoff syndrome.
This syndrome, which consists of dementia, ataxia, varying ophthalmoplegia and nystagmus (see p. 1147), presents acutely and should be suspected in all heavy drinkers. If treated promptly it is reversible; if left it becomes irreversible. It is a major cause of dementia in the USA.
Urgent treatment with thiamine 250 mg i.m. or i.v. infusion once daily is given for 3 days, often combined with other B-complex vitamins. Anaphylaxis can occur. Thiamine must always be given before any intravenous glucose infusion.
Riboflavin
Riboflavin is widely distributed throughout all plant and animal cells. Good sources are dairy products, offal and leafy vegetables. Riboflavin is not destroyed appreciably by cooking, but is destroyed by sunlight. Riboflavin is a flavo-protein that is a cofactor for many oxidative reactions in the cell.
There is no definite deficiency, although many communities have low dietary intakes. Studies in volunteers taking a low riboflavin diet have produced:
 angular stomatitis or cheilosis (fissuring at the corners of the mouth)
angular stomatitis or cheilosis (fissuring at the corners of the mouth)
 seborrhoeic dermatitis, particularly involving the face (around the nose) and the scrotum or vulva.
seborrhoeic dermatitis, particularly involving the face (around the nose) and the scrotum or vulva.
Conjunctivitis with vascularization of the cornea and opacity of the lens has also been described. It is probable, however, that many of the above features are due to multiple deficiencies rather than the riboflavin itself.
Riboflavin 5 mg daily can be tried for the above conditions, usually given as the vitamin B complex.
Niacin
This is the generic name for the two chemical forms, nicotinic acid and nicotinamide, the latter being found in the two pyridine nucleotides, nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Both act as hydrogen acceptors in many oxidative reactions, and in their reduced forms (NADH and NADPH) act as hydrogen donors in reductive reactions. Many oxidative steps in the production of energy require NAD, and NADP.
Niacin is found in many foodstuffs, including plants, meat (particularly offal) and fish. Niacin is lost by removing bran from cereals but is added to processed cereals and white bread in many countries.
Niacin can be synthesized in humans from tryptophan, 60 mg of tryptophan being converted to 1 mg of niacin. The amount of niacin in food is given as the ‘niacin equivalent’, which is equal to the amount of niacin plus one-60th of the tryptophan content. Eggs and cheese contain tryptophan.
Kynureninase and kynurenine hydroxylase, key enzymes in the conversion of tryptophan to nicotinic acid, are both B6 and riboflavin dependent, and deficiency of these B vitamins can also produce pellagra.
Pellagra
This is rare and is found in people who eat virtually only maize, for example in parts of Africa. Maize contains niacin in the form of niacytin, which is biologically unavailable, and has a low content of tryptophan. In Central America, pellagra has always been rare because maize (for the cooking of tortillas) is soaked overnight in calcium hydroxide, which releases niacin. Many of the features of pellagra can be explained purely by niacin deficiency; but some are probably due to multiple deficiencies, including deficiencies of proteins and of other vitamins.
Clinical features
The classical features are of dermatitis, diarrhoea and dementia. Although this is an easily remembered triad, not all features are always present and the mental changes are not a true dementia.
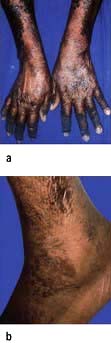
Pellagra. (a) Hyperpigmentation with desquamation of the dorsal aspects of the hands and forearms. (b) Hyperpigmented desquamation of the distal lower extremity. Note the shiny, shellac-like appearance on the lateral ankle.
(From: Bolognia J, Jorrizzo J, Rapini R (eds). Dermatology, 2nd edn. St Louis: Mosby; 2008: Fig. 51.9, with permission.)
 Dermatitis. In the areas of skin exposed to sunlight, initially there is redness followed by cracks with occasional ulceration. Chronic thickening, dryness and pigmentation develop. The lesions are always symmetrical and often affect the dorsal surfaces of the hands. The perianal skin and vulva are frequently involved. Casal’s necklace or collar is the term given to the skin lesion around the neck, which is confined to this area by the clothes worn.
Dermatitis. In the areas of skin exposed to sunlight, initially there is redness followed by cracks with occasional ulceration. Chronic thickening, dryness and pigmentation develop. The lesions are always symmetrical and often affect the dorsal surfaces of the hands. The perianal skin and vulva are frequently involved. Casal’s necklace or collar is the term given to the skin lesion around the neck, which is confined to this area by the clothes worn.
 Diarrhoea. This is often a feature but constipation is occasionally seen. Other gastrointestinal manifestations include a painful, red, raw tongue, glossitis and angular stomatitis. Recurring mouth infections occur.
Diarrhoea. This is often a feature but constipation is occasionally seen. Other gastrointestinal manifestations include a painful, red, raw tongue, glossitis and angular stomatitis. Recurring mouth infections occur.
 Dementia. This occurs in chronic disease. In milder cases there are symptoms of depression, apathy and sometimes thought disorders. Tremor and an encephalopathy frequently occur. Hallucinations and acute psychosis are also seen with more severe cases.
Dementia. This occurs in chronic disease. In milder cases there are symptoms of depression, apathy and sometimes thought disorders. Tremor and an encephalopathy frequently occur. Hallucinations and acute psychosis are also seen with more severe cases.
Pellagra may also occur in the following circumstances:
 Isoniazid therapy can lead to a deficiency of vitamin B6, which is needed for the synthesis of nicotinamide from tryptophan. Vitamin B6 is now given concomitantly with isoniazid.
Isoniazid therapy can lead to a deficiency of vitamin B6, which is needed for the synthesis of nicotinamide from tryptophan. Vitamin B6 is now given concomitantly with isoniazid.
 In Hartnup’s disease, a rare inborn error, in which basic amino acids including tryptophan are not absorbed by the gut. There is also loss of this amino acid in the urine.
In Hartnup’s disease, a rare inborn error, in which basic amino acids including tryptophan are not absorbed by the gut. There is also loss of this amino acid in the urine.
 In generalized malabsorption (rare).
In generalized malabsorption (rare).
 In alcohol-dependent patients who eat little.
In alcohol-dependent patients who eat little.
 Very low protein diets given for renal disease or taken as a food fad.
Very low protein diets given for renal disease or taken as a food fad.
 In the carcinoid syndrome and phaeochromocytomas, tryptophan metabolism is diverted away from the formation of nicotinamide to form amines.
In the carcinoid syndrome and phaeochromocytomas, tryptophan metabolism is diverted away from the formation of nicotinamide to form amines.
Diagnosis and treatment
In endemic areas, this is based on the clinical features, remembering that other vitamin deficiencies can produce similar changes (e.g. angular stomatitis). Nicotinamide (approximately 300 mg daily by mouth), with a maintenance dose of 50 mg daily is given with dramatic improvement in the skin and diarrhoea. Mostly, however, vitamin B complex is given, as other deficiencies are often present.
An increase in the protein content of the diet and treatment of malnutrition and other vitamin deficiencies is essential.
Vitamin B6
Vitamin B6 exists as pyridoxine, pyridoxal and pyridoxamine, and is found widely in plant and animal foodstuffs. Pyridoxal phosphate is a cofactor in the metabolism of many amino acids. Dietary deficiency is extremely rare. Some drugs (e.g. isoniazid, hydralazine and penicillamine) interact with pyridoxal phosphate, producing B6 deficiency. The polyneuropathy occurring after isoniazid usually responds to vitamin B6.
Sideroblastic anaemia may respond to vitamin B6 (see p. 380).
A polyneuropathy has occurred after high doses (>200 mg) given over many months. Vitamin B6 is used for premenstrual tension: a daily dose of 10 mg should not be exceeded.
Biotin and pantothenic acid
Biotin is involved in a number of carboxylase reactions. It occurs in many foodstuffs and the dietary requirement is small. Deficiency is extremely rare and is confined to a few people who consume raw eggs, which contain an antagonist (avidin) to biotin. It has also been reported in patients receiving long-term parenteral nutrition without adequate amounts of biotin. It causes a dermatitis that responds to biotin replacements.
Pantothenic acid is widely distributed in all foods and deficiency in humans has not been described.
Vitamin C
Ascorbic acid is a powerful reducing agent controlling the redox potential within cells. It is involved in the hydroxylation of proline to hydroxyproline, which is necessary for the formation of collagen. The failure of this biochemical pathway in vitamin C deficiency accounts for virtually all of the clinical effects seen.
Humans, along with a few other animals (e.g. primates and the guinea-pig), are unusual in not being able to synthesize ascorbic acid from glucose.
Vitamin C is present in all fresh fruit and vegetables. Unfortunately, ascorbic acid is easily leached out of vegetables when they are placed in water and it is also oxidized to dehydro-ascorbic acid during cooking or exposure to copper or alkalis. Potatoes are a good source as many people eat a lot of them, but vitamin C is lost during storage.
It has been suggested that ascorbic acid in high dosage (1–2 g daily) will prevent the common cold. While there is some scientific support for this, clinical trials have shown no significant effect. Vitamin C supplements have also been advocated to prevent atherosclerosis and cancer, but again a clear benefit has not been demonstrated.
Vitamin C deficiency is seen mainly in infants fed boiled milk and in the elderly and single people who do not eat vegetables. In the UK, it is also seen in Asians eating only rice and chapattis and in food faddists.
Scurvy
In adults, the early symptoms of vitamin C deficiency may be nonspecific, with weakness and muscle pain. Other features are shown in Table 5.11. Parafollicular haemorrhages and corkscrew hairs occur. In infantile scurvy, there is irritability, painful legs, anaemia and characteristic subperiosteal haemorrhages, particularly into the ends of long bones.
Table 5.11 Clinical features of vitamin C deficiency (scurvy)
Diagnosis
The anaemia is usually hypochromic but occasionally, a normochromic or megaloblastic anaemia is seen. The type of anaemia depends on whether iron deficiency (owing to decreased absorption or loss due to haemorrhage) or folate deficiency (folate being largely found in green vegetables) is present.
Plasma ascorbic acid is very low in obvious deficiency and a vitamin C level of <11 µmol/L (0.2 mg/100 mL) indicates vitamin C deficiency. The leucocyte-platelet layer (buffy coat) of centrifuged blood corresponds to vitamin C concentrations in other tissues. The normal level of leucocyte ascorbate is 1.1–2.8 pmol/106 cells.
Treatment
Initially the patient is given 250 mg of ascorbic acid daily and encouraged to eat fresh fruit and vegetables. Subsequently, 40 mg daily will maintain a normal exchangeable body pool of about 900 mg (5.1 mmol).
Prevention
Orange juice should be given to bottle-fed infants. The intake of breast-fed infants depends on the mother’s diet. In the elderly, eating adequate fruit and vegetables is the best way to avoid scurvy. Careful surveillance of the elderly, particularly those who live alone, is necessary. Ascorbic acid supplements should only be necessary occasionally.
Vitamin B12 and folate
These are dealt with on page 381 and daily requirements are shown in Table 5.8.
Folate. In many developed countries, up to 15% of the population have a partial deficiency of 5,10-methylene tetrahydrofolate reductase, a key folate-metabolizing enzyme. This is due to a point mutation and is associated with an increase in neural tube defects and hyperhomocysteinaemia, which has been linked to cardiovascular disease. Autoantibodies against folate receptors have been found in serum from women who have had a pregnancy complicated by neural tube defects. However, the role of this in the pathogenesis is unclear.
In the USA and some other countries, enriched cereals are fortified with 1.4 mg/kg grain of folic acid to increase daily requirements.
Dietary antioxidants
Free radicals are generated during inflammatory processes, radiotherapy, smoking, and during the course of a wide range of diseases. They may cause uncontrolled damage of multiple cellular components, the most sensitive of which are unsaturated lipids, proteins and DNA, and they also disrupt the normal replication process. They have been implicated as a cause of a wide range of diseases, including malignant, acute inflammatory and traumatic diseases, cardiovascular disease, neurodegenerative conditions such as Alzheimer’s disease, senile macular degeneration, and cataract. The defence against uncontrolled damage by free radicals is provided by antioxidant enzymes (e.g. catalase, superoxide dismutase) and antioxidants, which may be endogenous (e.g. glutathione) or exogenous (e.g. vitamins C and E, carotenoids). A possible causal link between lack of antioxidants and cardiovascular disease has emerged from epidemiological studies although several RCTs have not confirmed this.
Epidemiological studies
Dietary intake
 A high intake of fruits and vegetables has been linked to reduced risk of heart disease, cerebrovascular disease and total cardiovascular morbidity and mortality.
A high intake of fruits and vegetables has been linked to reduced risk of heart disease, cerebrovascular disease and total cardiovascular morbidity and mortality.
 A high intake of nuts (rich in vitamin E) and dietary components, e.g. red wine, onions, apples (rich in flavonoids), which are strong scavengers of free radicals, has also been linked to reduced risk of cardiovascular disease.
A high intake of nuts (rich in vitamin E) and dietary components, e.g. red wine, onions, apples (rich in flavonoids), which are strong scavengers of free radicals, has also been linked to reduced risk of cardiovascular disease.
 The seasonal variation in cardiovascular disease, which is higher in winter, has been related to decreased intake of fresh fruit and vegetables in winter.
The seasonal variation in cardiovascular disease, which is higher in winter, has been related to decreased intake of fresh fruit and vegetables in winter.
 The decline in cardiovascular disease in the USA since the 1950s has been associated with a simultaneous increase in the intake of fresh fruit and vegetables.
The decline in cardiovascular disease in the USA since the 1950s has been associated with a simultaneous increase in the intake of fresh fruit and vegetables.
Status of antioxidant nutrients
The level of antioxidant nutrients in the circulation has been reported to be inversely related to cardiovascular morbidity and mortality, extent of atherosclerosis assessed by intra-arterial ultrasound, and clinical signs of ischaemic heart disease. The tissue content of lycopene, a marker of vegetable intake, has been reported to be low in patients with myocardial infarction.
Antioxidants, especially vitamin E, have been shown to prevent the initiation and progression of atherosclerotic disease in animals. They also reduce the oxidation of low-density lipoprotein (LDL) in the arterial wall in vitro. Oxidation of LDL is an initial event in the atherosclerotic process (p. 725). However, these epidemiological studies show an association rather than a causal link and RCTs comparing the antioxidant against a control group are necessary.
Randomized controlled trials (RCT) (see also p. 905). The results of such trials have been formally evaluated through a series of systematic reviews and meta-analyses.
 For primary or secondary prevention of cardiovascular disease, intervention with β-carotene, α-tocopherol (vitamin E) and ascorbic acid (vitamin C) has demonstrated no significant benefit.
For primary or secondary prevention of cardiovascular disease, intervention with β-carotene, α-tocopherol (vitamin E) and ascorbic acid (vitamin C) has demonstrated no significant benefit.
 Vitamin E or β-carotene given in, e.g. stroke and fatal and non-fatal myocardial infarction, has also not yielded benefits.
Vitamin E or β-carotene given in, e.g. stroke and fatal and non-fatal myocardial infarction, has also not yielded benefits.
 There is a report of increased risk of intracerebral and subarachnoid haemorrhage in healthy individuals receiving carotene and α-tocopherol.
There is a report of increased risk of intracerebral and subarachnoid haemorrhage in healthy individuals receiving carotene and α-tocopherol.
 A meta-analysis has shown a small but significant overall increased risk of cardiovascular death and all-cause mortality in individuals treated with β-carotene (compared to the control group).
A meta-analysis has shown a small but significant overall increased risk of cardiovascular death and all-cause mortality in individuals treated with β-carotene (compared to the control group).
 An increased risk of developing lung cancer by administering large doses of β-carotene to subjects with a history of heavy smoking.
An increased risk of developing lung cancer by administering large doses of β-carotene to subjects with a history of heavy smoking.
 Although administration of antioxidant nutrients has been proposed in a wide range of acute (e.g. critical illness, pancreatitis) and chronic diseases, the evidence base from RCTs is generally not strong.
Although administration of antioxidant nutrients has been proposed in a wide range of acute (e.g. critical illness, pancreatitis) and chronic diseases, the evidence base from RCTs is generally not strong.
 In some cases, improvement in indices of free radical damage had been demonstrated (e.g. in acute inflammatory conditions), but with little evidence of clinical benefit.
In some cases, improvement in indices of free radical damage had been demonstrated (e.g. in acute inflammatory conditions), but with little evidence of clinical benefit.
Epidemiological studies are also confounded by other associated variables, e.g. eating a low-fat diet or undertaking more exercise. The latter may be more valuable in the causal pathway than the intake of antioxidants. Diets rich in fresh fruit and vegetables also contain a range of antioxidants that were not tested in the clinical trials. Therefore, the results of large-scale RCTs using various combinations and doses of antioxidant nutrients are awaited. In the meantime, the policy of encouraging ‘healthy’ behaviour, which includes increased physical activity and a varied diet rich in fresh fruit and vegetables, and nuts, is still generally recommended both for the population as a whole and for those at risk of cardiovascular disease.
Homocysteine, cardiovascular disease and B vitamins
The circulating concentration of the amino acid homocysteine is an independent risk factor for cardiovascular disease (p. 728). A high concentration is related to ischaemic heart disease, stroke, thrombosis, pulmonary embolism, coronary artery stenosis, and heart failure. The strength of the association is similar to smoking or hyperlipidaemia.
Proposed mechanisms, based on experimental evidence, by which homocysteine detrimentally affects vascular function, include:
 the direct damaging effects of homocysteine on endothelial cells of blood vessels
the direct damaging effects of homocysteine on endothelial cells of blood vessels
Homocysteine is not found in food, but results from metabolism within the body which depends on folic acid, vitamin B12 and pyridoxine (vitamin B6) (Fig. 5.7). Deficiency of one or more of these vitamins is common in the elderly, which would increase the concentration of homocysteine. If an elevated homocysteine concentration was causally linked to cardiovascular disease then it should be possible to lower the risk by administering one or more of these vitamins to lower the homocysteine concentration. However, several recent studies suggest that lowering homocysteine concentrations in this way does not reduce the risk of cardiovascular disease.
FURTHER READING
Clarke R, Halsey J, Lewington S et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 2010; 170:1622–1631.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, protein, and amino acids. Washington DC: The National Academies Press; 2005.
Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington DC: The National Academies Press; 2000.
Minerals
A number of minerals have been shown to be essential in animals, and an increasing number of deficiency syndromes are becoming recognized in humans. Long-term total parenteral nutrition allowed trace element deficiency to be studied in controlled conditions; now trace elements are always added to long-term parenteral nutrition regimens. It is highly probable that trace-element deficiency is also a frequent accompaniment of all PEM states, but this is difficult to study because of multiple deficiencies. Sodium, potassium, magnesium and chloride are discussed in Chapter 13. Reference nutrient intake (RNI) values are shown in Table 5.12.
Table 5.12 Daily reference nutrient intake (RNI) values for some elements
| Element | Daily RNI | Dietary sources |
|---|---|---|
Sodium |
1.6 g (70 mmol) |
Mostly in processed food (e.g. meat products, bread cereal) but added salt contributes |
Chloride |
2.5 g (70 mmol) |
As for sodium |
Potassium |
3.5 g (90 mmol) |
Vegetables, fruit, juices, meat and milk |
Calcium |
700 mg (17.5 mmol)a |
In many foodstuffs; two-thirds of intake comes from milk and milk products, and only 5% from vegetablesb |
Phosphate |
550 mg (17.5 mmol) |
All natural foods e.g. milk, meat, bread, cereals, |
Magnesium |
300 mg (12.3 mmol) for men |
Milk bread, cereal products, potatoes and other vegetables |
270 mg (10.9 mmol) for women |
||
Iron |
160 µmol (8.7 mg) for men |
Meat, bread, flour, cereal products, potatoes and vegetables |
260 µmol (14.8 mg) for women |
||
Copper |
1.2 mg (19 µmol) |
Shellfish, legumes, cereals and nuts |
Zinc |
9.5 mg (145 µmol) for men |
Widely available in food |
7 mg (110 µmol) for women |
||
Iodine |
140 µg (1.1 µmol) |
Milk, meat and seafoods |
Fluoride |
None |
Little fluoride in food except seafish and tea (tea provides 70% of daily intake) |
Selenium |
75 µg (0.9 µmol) for men |
Cereals, fish, meat, cheese, eggs, milk |
60 µg (0.8 µmol) for women |
a UK value; a substantially higher value is recommended in the USA.
b In the UK, most flour is fortified.
Iron
Iron deficiency (see also p. 379) is common worldwide, affecting both developing and developed countries. It is particularly prevalent in women of reproductive age. Dietary iron overload is seen in South African men who cook and brew in iron pots.
Copper
Deficiency
Menkes’ kinky hair syndrome is a rare condition caused by malabsorption of copper. The Menkes’ disease gene (ATP7A) encodes a copper-transporting ATPase and has a homology to the gene in Wilson’s disease. Infants with this sex-linked recessive abnormality develop growth failure, mental retardation, bone lesions and brittle hair. Anaemia and neutropenia also occur. This condition, which serves as a model for copper deficiency, supports the idea that some of the clinical features seen in PEM are due to copper deficiency. Breast and cow’s milk are low in copper, and supplementation is occasionally necessary when first treating PEM.
Zinc
Zinc is involved in many metabolic pathways, often acting as a coenzyme; it is essential for the synthesis of RNA and DNA.
Deficiency
Acrodermatitis enteropathica is an inherited disorder caused by malabsorption of zinc. Infants develop growth retardation, severe diarrhoea, hair loss and a skin rash, which can occur anywhere on the body, but most often around the mouth, genitalia and hands (a similar rash occurs in adults suffering from zinc deficiency due to other causes (see below). There are also associated Candida and bacterial infections. This condition provides a model for zinc deficiency. Zinc supplementation results in a complete cure. Zinc deficiency probably also plays a role in PEM and in many diseases in children in the developing world. Zinc supplementation has been shown to be of some benefit in, for example, the prevention of diarrhoeal diseases and acute respiratory infections; it also improves growth.
(From: Bolognia J, Jorrizzo J, Rapini R (eds). Dermatology, 2nd edn. St Louis: Mosby; 2008: Fig. 51.11A, with permission.)
Zinc levels have also been shown to be low in some patients with malabsorption or skin disease, and in patients with AIDS, but the exact role of zinc in these situations is disputed. Zinc has low toxicity, but high zinc levels from water stored in galvanized containers interfere with iron and copper absorption. Conversely, administration of copper or iron to treat deficiencies such as iron deficiency anaemia can precipitate zinc deficiency. Wound healing is impaired with moderate zinc deficiency and is improved by zinc supplements. Impaired taste and smell, hair loss and night blindness are also features of severe zinc deficiency.
Iodine
Iodine exists in foodstuffs as inorganic iodides which are efficiently absorbed. Iodine is a constituent of the thyroid hormones (p. 960).
Deficiency
Many areas throughout the world lack iodine in the soil, and so iodine deficiency, which impairs brain development, is a WHO priority. Two billion people (one-third children) worldwide have insufficient iodine intake. Endemic goitre occurs in remote areas where the daily intake is below 70 µg, and in those parts 1–5% of babies are born with cretinism. In these areas, iodized oil should be given intramuscularly to all reproductive women every 3–5 years. Salt iodization is now used in many countries and is a simple, cost-effective way to prevent deficiency.
Fluoride
In areas where the level of fluoride in drinking water is less than 1 p.p.m. (0.7–1.2 mg/L), dental caries is relatively more prevalent. Fluoridation of the water provides 1–2 mg daily, resulting in a reduction of about 50% of tooth decay in children. There is little fluoride in food. Fluoride-containing toothpaste may add up to 2 mg/day.
Excessive fluoride intake in areas where the water fluoride level is above 3 mg/L can result in fluorosis, in which there is infiltration into the enamel of the teeth, producing pitting and discoloration.
Selenium
Clinical deficiency of selenium is rare except in areas of China where Keshan disease, a selenium-responsive cardiomyopathy, occurs. Selenium deficiency may also cause a myopathy. Toxicity has been described with very high intakes.
Calcium
Calcium absorption (see also p. 513) from the gastrointestinal tract is vitamin D-dependent. Some 99% of body calcium is in the skeleton.
Increased calcium is required in pregnancy and lactation, when dietary intake must be increased. Calcium deficiency is usually due to vitamin D deficiency.
Phosphate
Phosphates (see also p. 519) are present in all natural foods, and dietary deficiency has not been described. Patients taking large amounts of aluminium hydroxide can, however, develop phosphate deficiency owing to binding in the gut lumen. It can also be seen in total parenteral nutrition. Symptoms include anorexia, weakness and osteoporosis.
Other trace elements
The possible significance of cadmium, chromium, cobalt, manganese, molybdenum, nickel and vanadium is shown in Table 5.13.
Table 5.13 Other trace elements (see text)
| Element | Deficiency |
|---|---|
Cadmium |
? |
Chromium |
Glucose intolerance |
Cobalt |
Anaemia (vitamin B12) |
Manganese |
Skin rash, ? osteoporosis,? mood |
Molybdenum |
? (case study involving parenteral nutrition) |
Nickel |
? Animals only |
Vanadium |
? |
FURTHER READING
Checkley W, West KP, Wise RA et al. Maternal vitamin supplementation and lung function in offspring. N Engl J Med 2010; 1784–1794.
Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, chromium, copper, iodine, iron, manganese, molybdenum, silicon, vanadium, and zinc. Washington DC: Institute of Medicine; 2001.
Pearce SHS, Cheetam TD. Diagnosis and management of vitamin D deficiency. BMJ 2010; 340:141–147.
Pitta AG, Chung M, Trikalinos T et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010; 152:307–314.
Nutrition and ageing
Many animal studies have shown that life expectancy can be extended by restricting food intake. It is, however, not known whether the ageing process in humans can be altered by nutrition.
The ageing process
The process of ageing is not well understood. While wear and tear may play a role, it is an insufficient explanation for the causation of ageing. The ‘programmed’ theories depend on inbuilt biological clocks that regulate lifespan, and involve genes that are responsible for controlling signals that influence various body systems. The ‘error’ theories involve environmental stressors that induce damage (e.g. mitochondrial DNA damage or cross-linking).
The search for a single cause of ageing, e.g. a single gene defect, has been replaced by the view that ageing is a complex multifactorial process that involves an interaction between genetic, environmental and stochastic (random damage to essential molecules) causes. The following theories have been suggested:
Molecular theories
 Gene regulation: ageing, for example, results from changes in expression of genes that regulate both development and ageing. An insulin-like signalling pathway has been linked to the lifespan of worms, flies and mice (activation of a transcription factor in response to reduced insulin-like signalling prolongs lifespan)
Gene regulation: ageing, for example, results from changes in expression of genes that regulate both development and ageing. An insulin-like signalling pathway has been linked to the lifespan of worms, flies and mice (activation of a transcription factor in response to reduced insulin-like signalling prolongs lifespan)
 Codon restriction: inadequate mRNA translation resulting from inadequate decoding of codons in mRNA
Codon restriction: inadequate mRNA translation resulting from inadequate decoding of codons in mRNA
 Error catastrophe: errors in gene expression result in abnormal proteins
Error catastrophe: errors in gene expression result in abnormal proteins
 Somatic mutation: cumulative molecular damage mainly to genetic material
Somatic mutation: cumulative molecular damage mainly to genetic material
 Dysdifferentiation: cumulative random molecular damage detrimentally affects gene expression.
Dysdifferentiation: cumulative random molecular damage detrimentally affects gene expression.
Mutations in genes encoding lamin A are found in fibroblasts of elderly people and in progeria syndromes.
Cellular theories
 Cellular senescence-telomere: an increase in senescent cells occurs from:
Cellular senescence-telomere: an increase in senescent cells occurs from:
 Free radical: production of free radicals during oxidative metabolism, which damages fat, protein and DNA.
Free radical: production of free radicals during oxidative metabolism, which damages fat, protein and DNA.
 Wear and tear: cumulative damage from normal injury/stress which is unable to repair itself.
Wear and tear: cumulative damage from normal injury/stress which is unable to repair itself.
 Apoptosis theory: programmed cell death due to genetic events.
Apoptosis theory: programmed cell death due to genetic events.
System theories
These theories involve loss in the function of neuroendocrine or immune systems with consequent age-related physiological changes and an increase in autoimmunity.
Whole body metabolism and energy expenditure theory proposes that there is a fixed limit to the cumulative energy expenditure and metabolism during a lifetime, so that if this limit is reached quickly the lifespan is short. Energy restriction in rodents reduces energy expenditure and prolongs lifespan, but there is a lack of studies in primates or humans.
Evolutionary theories
 Cumulative mutation: mutations that accumulate during a lifetime act in older age rather than during the active reproductive period (for which there is evolutionary selection), producing pathology and senescence. The theory was initially based on the observation that Huntington’s disease, a dominant lethal mutation which typically manifests itself between 35 and 55 years, allows affected individuals to reproduce.
Cumulative mutation: mutations that accumulate during a lifetime act in older age rather than during the active reproductive period (for which there is evolutionary selection), producing pathology and senescence. The theory was initially based on the observation that Huntington’s disease, a dominant lethal mutation which typically manifests itself between 35 and 55 years, allows affected individuals to reproduce.
 Disposable soma: the somatic body is maintained to ensure reproductive success, after which it is disposable. Factors that may enhance reproductive success may have detrimental effects on ageing – a possible example being androgen secretion, which may be beneficial to reproduction but potentially detrimental with development of prostatic cancer and cardiovascular disease in later life.
Disposable soma: the somatic body is maintained to ensure reproductive success, after which it is disposable. Factors that may enhance reproductive success may have detrimental effects on ageing – a possible example being androgen secretion, which may be beneficial to reproduction but potentially detrimental with development of prostatic cancer and cardiovascular disease in later life.
Nutritional components of theories of ageing
Several of the theories above have strong nutritional components. Disability and dependency in older humans are at least partly due to poor nutrition, and correction of deficiencies or nutrient imbalances can prevent the decline in function from falling below the disability threshold (Fig. 5.8). In this way, some loss of function may be prevented or reversed, especially if other measures, such as physical activity, which increases muscle mass and strength, are undertaken.
Early origins of health and disease in older adults
A low birth weight (and/or length) is associated with reduced height, as well as reduced mass and fat-free mass in adult life. These relationships are independent of genetic factors: the smaller of identical twins becomes a shorter and lighter adult.
Relationships have also been reported between growth of the fetus and a variety of diseases and risk factors for disease in adults and older people. These include cardiovascular disease (especially ischaemic heart disease), hypertension and diabetes, and even obesity and fat distribution. However, the strength of association for some of these conditions is weak. Animal studies involving dietary modifications (e.g. protein and zinc, even within the normal range) during pregnancy or in early postnatal life have clearly demonstrated effects, such as hypertension. The effects can persist, not only through the lifetime of the offspring, but also through to their offspring.
The extent to which these findings apply to humans is uncertain, and the mechanisms are poorly understood. Since relationships have been reported between cardiovascular disease in old age and growth in the first few years of life, as well as starvation during puberty, it is likely that cumulative environmental stresses, including nutritional stress, from the time of implantation of the fertilized egg, to fetal and postnatal growth and development, and into adult life, summate to produce an overall disease risk (Fig. 5.8).
Nutritional requirements in the elderly
These are qualitatively similar to the requirements of younger adults: the diet should contain approximately the same proportions of nutrients, and essential nutrients are still required. However, the RNIs stated earlier (p. 195) are intended for healthy people without disease; specific requirements in disease, which is common in older people, are less well-defined.
Maintenance of physical activity continues to be necessary for overall health, regardless of age. However, energy expenditure by the elderly is less, so they have a lower energy requirement. For people aged 60 and above, irrespective of age, the daily energy requirement has been set to be approximately 1.5 × BMR. Because they have reduced fat-free mass, from an average of 60 kg to 50 kg in men and from 40 kg to 35 kg in women, their BMR is reduced.
Nutritional deficits in the elderly are common and may be due to many factors, such as dental problems, lack of cooking skills (particularly in widowers), depression and lack of motivation. Significant malnourishment in developed countries is usually secondary to social problems or disease. In elderly people who are in institutions, multiple nutrient deficiencies are common. Vitamin D supplements may be required because often elderly people do not go into the sunlight. Owing to the high prevalence of osteoporosis in elderly people, increased daily calcium intake (1–1.5 g/day) is often recommended.
FURTHER READING
Artandi SE. Telomeres, telomerase, and human disease. N Engl J Med 2006; 355:1195–1197.
Cox LS, Faragher RG. From old organisms to new molecules: integrative biology and therapeutic targets in accelerated human ageing. Cell Mol Life Sci 2007; 64:2620–2641.
Flatt T, Schmidt PS. Integrating evolutionary and molecular genetics of aging. Biochim Biophys Acta 2009;1790:951–962.
Gluckman PD, Hanson MA, Cooper C. Effect of in utero and early life conditions on adult health and disease. N Engl J Med 2008; 359:61–73.
Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res 2006; 40:1230–1238.
Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 2010; 48:642–655.
Vina J, Borras C, Miquel J. Theories of ageing. IUBMB Life 2007; 59:249–254.
Obesity
Obesity is almost invariable in developed countries and almost all people accumulate some fat as they get older. The World Health Organization acknowledges that obesity (BMI >30 kg/m2) is a worldwide problem which also affects many developing countries. Obesity implies an excess storage of fat, and this can most easily be detected by looking at the undressed patient. Not all obese people eat more than the average person, but all obviously eat more than they need.
The present obesity epidemic is mainly due to changes in lifestyle behaviour (although genetic factors may be involved in some individuals). There has been a trebling in the prevalence of obesity in the UK over the last three decades as well as a vast increase in developing countries. The growing obesity problem in humans has affected children, adults and older people. Clinical and public health interventions require a multi-level approach, e.g. by altering the cumulative environmental experience during the lifespan. Strategies to prevent and treat obesity in children can influence obesity in adults, and this in turn influences obesity in old age. Ultimately, all depend on changing energy balance through effects on food intake and/or energy expenditure.
Most patients suffer from simple obesity, but in certain conditions, obesity is an associated feature (Table 5.14). Even in the latter situation, the intake of calories must have exceeded energy expenditure over a prolonged period of time. Hormonal imbalance is often incriminated in women (e.g. postmenopause or when taking contraceptive pills), but most weight gain in such cases is usually small and due to water retention.
Table 5.14 Conditions in which obesity is an associated feature
Suggested mechanisms
Genetic and environmental factors
These have always been difficult to separate when studying obesity, but there is little doubt that the recent obesity ‘epidemic’, which has developed over a few decades, is predominantly due to changes in lifestyle (various environmental factors) and unlikely to be due to rapid changes in the gene pool over this period of time. This is consistent with the view that evolution during times of limited food resources has tended to defend more against undernutrition than overnutrition. However, observational studies in both monozygotic and dizygotic twins, reared together or apart, suggest that strong genetic influences account for the difference in BMI later in life, and that the influence of the childhood environment is weaker. These observations also showed that weight gain did not occur in all pairs of twins, suggesting that environmental factors operate.
A search for genetic factors led to the identification of a putative gene, first in the obese (ob ob) mouse and now in humans. The ob gene was shown to be expressed solely in both white and brown adipose tissue. The ob gene is found on chromosome 7 and produces a 16 kDa protein called leptin. In the ob ob mouse, a mutation in the ob gene leads to production of a non-functioning protein. Administration of normal leptin to these obese mice reduces food intake and corrects the obesity. A similar situation has been described in a very rare genetic condition causing obesity in humans, in which leptin is not expressed.
In massively obese subjects, leptin mRNA in subcutaneous adipose tissue is 80% higher than in controls. Plasma levels of leptin are also very high, correlating with the BMI. Weight loss due to food restriction decreases plasma levels of leptin. However, in contrast to the ob ob mouse, the leptin structure is normal, and abnormalities in leptin are not the prime cause of human obesity.
Leptin secreted from fat cells was thought to act as a feedback mechanism between the adipose tissue and the brain, acting as a ‘lipostat’ (adipostat), controlling fat stores by regulating hunger and satiety (see below). However, many other signals are involved and the human genome map has identified hundreds of genes that correlate with the presence of obesity. It is also interesting that obesity is largely restricted to humans and animals that are either domesticated or in zoos.
Food intake
Many factors related to the home environment, such as finance and the availability of sweets and snacks, will affect food intake. Some individuals eat more during periods of heavy exercise or during pregnancy and are unable to get back to their former eating habits. The increase in obesity in social class 5 can usually be related to the type of food consumed (i.e. food containing sugar and fat). Psychological factors and how food is presented may override complex biochemical interactions.
It has been shown that obese patients eat more than they admit to eating, and over the years, a very small daily excess of intake over expenditure can lead to a large accumulation of fat. For example, a 44 kJ (10.5 kcal) daily excess would lead to a 10 kg weight gain over 20 years.
Control of appetite
Appetite is the desire to eat and this usually initiates food intake. Following a meal, satiation occurs. This depends on gastric and duodenal distension and the release of many substances peripherally and centrally.
Following a meal, cholecystokinin (CCK), bombesin, glucagon-like peptide 1 (GLP-1), enterostatin, and somatostatin are released from the small intestine, and glucagon and insulin from the pancreas. All of these hormones have been implicated in the control of satiety. Centrally, the hypothalamus – particularly the lateral hypothalamic area, and paraventricular and arcuate nuclei – plays a key role in integrating signals involved in appetite and bodyweight regulation (Fig. 5.9). There are two main pathways in the arcuate nucleus (Fig. 5.9):
 The central appetite-stimulating (orexigenic) pathway in the ventromedial part of the arcuate nucleus, which expresses NPY (neuropeptide Y) and AgRP (agouti-pathway) related protein). Animal studies suggest that this pathway also decreases energy expenditure.
The central appetite-stimulating (orexigenic) pathway in the ventromedial part of the arcuate nucleus, which expresses NPY (neuropeptide Y) and AgRP (agouti-pathway) related protein). Animal studies suggest that this pathway also decreases energy expenditure.
 The central appetite-suppressing (anorexigenic pathway or leptin-melanocortin pathway) in the dorsolateral part of the arcuate nucleus, which expresses POMC/CART (pro-opiomelanocortin/cocaine-and-amphetamine-regulated transcript). In this pathway, α-MSH (α-melanocyte-stimulating hormone), formed by cleavage of POMC by PC1 (prohormone convertase), exerts its appetite-suppressing effect via the Mc4R (melanocortin-4 receptors) in areas of the brain that regulate food intake and autonomic activity. Animal studies suggest that this pathway also increases energy expenditure.
The central appetite-suppressing (anorexigenic pathway or leptin-melanocortin pathway) in the dorsolateral part of the arcuate nucleus, which expresses POMC/CART (pro-opiomelanocortin/cocaine-and-amphetamine-regulated transcript). In this pathway, α-MSH (α-melanocyte-stimulating hormone), formed by cleavage of POMC by PC1 (prohormone convertase), exerts its appetite-suppressing effect via the Mc4R (melanocortin-4 receptors) in areas of the brain that regulate food intake and autonomic activity. Animal studies suggest that this pathway also increases energy expenditure.
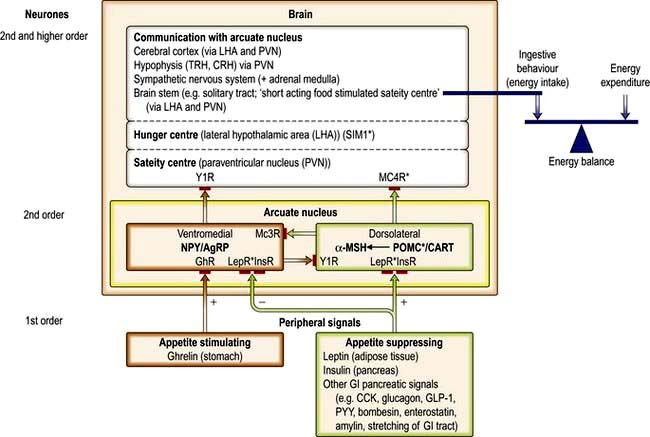
Figure 5.9 Peripheral signals and central pathways involved in the control of food intake. The stimulatory (orange) and suppressive (green) signals and pathways are shown. In the arcuate nucleus POMC is converted to melanocortins, including α-MSH, through the action of prohormone convertase. The solid red areas represent receptors for a variety of signals (see list below). Asterisks (*) indicate mutations that have resulted in human obesity. Receptors: GhR, ghrelin receptor; LepR, leptin receptor; InsR, insulin receptor; Mc3R, melanocortin 3 receptor; Mc4R, melanocortin 4 receptor; Y1R, Y1 subtype of neuropeptide Y (NPY) receptor. Other abbreviations: CCK, cholecystokinin; CRH, corticotrophin-releasing hormone; GLP-1, glucagon-like peptide; LHA, lateral hypothalamic area; α-MSH, α-melanocyte-stimulating hormone; NPY/AgRP, neuropeptide Y/agouti-related protein; POMC/CART, pro-opiomelanocortin/cocaine-and-amphetamine-regulated transcript; PVN, paraventricular nucleus; PYY, peptide YY; TRH, thyrotrophin-releasing hormone.
These pathways interact with each other and feed into the lateral hypothalamus, which communicates with other parts of the brain, and influence the autonomic nervous system and ingestive behaviour. These central pathways are in turn influenced by a variety of peripheral signals which can also be classified as appetite stimulating or appetite suppressing.
 Peripheral appetite-suppressing signals: Leptin and insulin act centrally to activate the appetite-suppressing pathway (while also inhibiting the appetite-stimulating pathway). Since these hormones circulate in proportion to adipose tissue mass, they can be regarded as long-term signals, although they probably also modulate short-term signals (insulin also responds acutely to meal ingestion). Peptide YY (PYY) is produced by the L cells of the large bowel and distal small bowel in proportion to the energy ingested. The release of this rapidly responsive (short-acting) signal begins shortly after food intake, suggesting that the initial response involves neural pathways, before ingested nutrients reach the site of PYY production. PYY is thought to reduce appetite, at least partly through inhibition of the appetite-stimulating pathway (NPY/AgRP-expressing neurones). There are a large number of other peripheral appetite suppressing signals, including glucagon-like peptide 1 (GLP-1) and oxyntomodulin, which, like PYY, are produced by the gut in a nutrient dependent manner.
Peripheral appetite-suppressing signals: Leptin and insulin act centrally to activate the appetite-suppressing pathway (while also inhibiting the appetite-stimulating pathway). Since these hormones circulate in proportion to adipose tissue mass, they can be regarded as long-term signals, although they probably also modulate short-term signals (insulin also responds acutely to meal ingestion). Peptide YY (PYY) is produced by the L cells of the large bowel and distal small bowel in proportion to the energy ingested. The release of this rapidly responsive (short-acting) signal begins shortly after food intake, suggesting that the initial response involves neural pathways, before ingested nutrients reach the site of PYY production. PYY is thought to reduce appetite, at least partly through inhibition of the appetite-stimulating pathway (NPY/AgRP-expressing neurones). There are a large number of other peripheral appetite suppressing signals, including glucagon-like peptide 1 (GLP-1) and oxyntomodulin, which, like PYY, are produced by the gut in a nutrient dependent manner.
 Peripheral appetite-stimulating signals: Ghrelin is a 28-amino-acetylated peptide produced by the oxyntic cells of the fundus of the stomach. It is the first known gastrointestinal tract peptide that stimulates appetite by activating the central appetite-stimulating pathway. The circulatory concentration is high before a meal and is reduced rapidly by ingestion of a meal or glucose (cf. peptide YY, which increases after a meal). It may also act as a long-term signal, as its circulating concentration in weight-stable individuals is inversely related to BMI over a wide range (cf. insulin and leptin which are positively related to BMI, see below). It is also increased in several situations in which there is a negative energy balance, e.g. long-term exercise, very low-calorie diets, anorexia nervosa and both cancer and cardiac cachexia (an exception is vertical banded gastric bypass surgery, where its concentration is low rather than high). Recent studies suggest that another peptide, obestatin, produced by the same gene that encodes ghrelin, counteracts the increase in food intake induced by ghrelin.
Peripheral appetite-stimulating signals: Ghrelin is a 28-amino-acetylated peptide produced by the oxyntic cells of the fundus of the stomach. It is the first known gastrointestinal tract peptide that stimulates appetite by activating the central appetite-stimulating pathway. The circulatory concentration is high before a meal and is reduced rapidly by ingestion of a meal or glucose (cf. peptide YY, which increases after a meal). It may also act as a long-term signal, as its circulating concentration in weight-stable individuals is inversely related to BMI over a wide range (cf. insulin and leptin which are positively related to BMI, see below). It is also increased in several situations in which there is a negative energy balance, e.g. long-term exercise, very low-calorie diets, anorexia nervosa and both cancer and cardiac cachexia (an exception is vertical banded gastric bypass surgery, where its concentration is low rather than high). Recent studies suggest that another peptide, obestatin, produced by the same gene that encodes ghrelin, counteracts the increase in food intake induced by ghrelin.
The single gene mutations affecting this pathway in humans, e.g. leptin, leptin receptor, POMC, Mc4R, PC1 and SIM1, are rare and recessive, with the exception of the Mc4R, which is common and dominant with incomplete penetrance. It appears that the Mc4R mutation accounts for 2–6% of human obesity. Affected individuals are obese without disturbances in pituitary function or resting energy expenditure, although children tend to be tall. However, these mutations are of little significance as obesity is predominantly polygenic in origin (the human obesity gene map has already identified several hundreds of candidate genes).
 Another system, the endocannabinoid system, is involved in both central and peripheral regulation of food intake and control of energy balance. There are two receptors: endocannabinoid in the brain and CB2 in the periphery. CB1 receptors are located in the cerebral cortex, cerebellum and hippocampus.
Another system, the endocannabinoid system, is involved in both central and peripheral regulation of food intake and control of energy balance. There are two receptors: endocannabinoid in the brain and CB2 in the periphery. CB1 receptors are located in the cerebral cortex, cerebellum and hippocampus.
The control of appetite is extremely complex. For example, if one considers only one signal, i.e. leptin, there can be leptin resistance where obese individuals have high circulating leptin but with no reduction of appetite. In contrast, in acute starvation, leptin concentrations decrease to lower levels than expected from the prevailing adipose tissue mass. It is known that cytokines, such as TNF and IL-2, which are elevated in a wide range of inflammatory and traumatic conditions, also suppress appetite, although the exact pathways involved are not entirely clear. Finally, there is a range of transmitters in the central nervous system that appear to affect appetite:
Energy expenditure
Basal metabolic rate (BMR). BMR in obese subjects is higher than in lean subjects, which is not surprising since obesity is associated with an increase in lean body mass.
Physical activity. Obese patients tend to expend more energy during physical activity as they have a larger mass to move. On the other hand, many obese patients decrease their amount of physical activity. The energy expended on walking at 3 miles/hour is only 15.5 kJ/min (3.7 kcal/min) and therefore, a mild to moderate increase in physical activity plays only a small part in losing weight. Nevertheless, because increased body fat develops insidiously over many years, any change in energy balance is helpful.
Thermogenesis
About 10% of ingested energy is dissipated as heat and is unconnected with physical activity. This dietary induced thermogenesis has been reported to be lower in obese and post-obese subjects than in lean subjects. This would tend to favour energy deposition in obesity and those predisposed to obesity. However, other reports have identified no difference in dietary induced thermogenesis between lean and obese subjects.
Brown adipose tissue in animals, when stimulated by cold or food, dissipates the energy derived from ingested food into heat. This can be a major component of overall energy balance in small mammals but the effect is likely to be very small, and of doubtful clinical significance in adult humans, even though brown adipose tissue is found in humans. β3-adrenergic receptors are the principal receptors mediating catecholamine-stimulated lipolysis in brown adipose tissue and to a lesser extent at other sites. Drugs with β3-adrenergic activities have been developed, but side-effects have limited their use.
Morbidity and mortality
Obese patients are at risk of early death, mainly from diabetes, coronary heart disease and cerebrovascular disease. The greater the obesity, the higher the morbidity and mortality rates. For example, men who are 10% overweight have a 13% increased risk of death, while the increase in mortality for those 20% overweight is 25%. The rise is less in women, and in men over 65, obesity is not an independent risk factor. Weight reduction reduces this mortality and therefore should be strongly encouraged. The benefits are probably greater in more obese subjects (Table 5.15).
Table 5.15 Potential benefits that may result from the loss of 10 kg in patients who are initially 100 kg and suffer from co-morbidities
Mortality |
20–25% fall in total mortality |
30–40% fall in diabetes-related deaths |
|
40–50% fall in obesity-related cancer deaths |
|
Blood pressure |
Fall of about 10 mmHg (systolic and diastolic) |
Diabetes |
Reduces risk of developing diabetes by >50% |
30–50% fall in fasting blood glucose |
|
15% fall in HbA1c |
|
Serum lipids |
10% fall in total cholesterol |
15% fall in LDL cholesterol |
|
30% fall in triglycerides |
|
8% increase in HDL cholesterol |
Clinical features
Most patients recognize their own problems, although often they are unaware of the main foods that cause obesity. Many symptoms are related to psychological problems or social pressures, such as the woman who cannot find fashionable clothes to wear.
The degree of obesity can be assessed by comparison with tables of ideal weight for height, from the BMI (Box 5.6), and by measuring skinfold thickness. The latter should be measured over the middle of the triceps muscle; normal values are 20 mm in a man and 30 mm in a woman. A central distribution of body fat (a waist/hip circumference ratio of >1.0 in men and >0.9 in women) is associated with a higher risk of morbidity and mortality than is a more peripheral distribution of body fat (waist/hip ratio <0.85 in men and <0.75 in women). This is because fat located centrally, especially inside the abdomen, is more sensitive to lipolytic stimuli, with the result that the abnormalities in circulating lipids are more severe.
![]() Box 5.6
Box 5.6
Ranges of body mass index (BMI) used to classify degrees of overweight and associated risk of co-morbidities
| WHO classification | BMI (kg/m2) | Risk of co-morbidities |
|---|---|---|
Overweight |
25–30 |
Mildly increased |
Obese |
>30 |
|
Class I |
30–35 |
Moderate |
Class II |
35–40 |
Severe |
Class III |
>40 |
Very severe |
Table 5.16 shows the conditions and complications that are associated with obesity. The relationship between cardiovascular disease (hypertension or ischaemic heart disease), hyperlipidaemia, smoking, physical exercise and obesity is complex. Difficulties arise in interpreting mortality figures because of the number of factors involved. Many studies do not differentiate between the types of physical exercise taken or take into account the cuff-size artefact in the measurement of blood pressure (an artefact will occur if a large cuff is not used in patients with a large arm). Nevertheless, obesity almost certainly plays a part in all of these diseases and should be treated. An exception is that stopping smoking, even if accompanied by weight gain, is more beneficial than any of the other factors. Physical fitness is also helpful, and there is some evidence to suggest that a fit obese person may have similar or even lower cardiovascular risk than a leaner unfit person.
Table 5.16 Conditions and complications associated with obesity
Metabolic syndrome
There are two classification systems which are shown in Table 5.17. The differences are:
 A large waist is an absolute requirement for the International Diabetes Federation (IDF), but not in the ATP III NCEP.
A large waist is an absolute requirement for the International Diabetes Federation (IDF), but not in the ATP III NCEP.
 The IDF criteria use lower cut-off values for waist circumference (close to values of people with a BMI of 25 kg/m2) and lower fasting blood glucose concentrations.
The IDF criteria use lower cut-off values for waist circumference (close to values of people with a BMI of 25 kg/m2) and lower fasting blood glucose concentrations.
Table 5.17 Classification systems for metabolic syndrome: ATP III of the National Cholesterol Education Programme (NCEP) and International Diabetes Federation (IDF)
| Risk factor | ATP III NCEP (any 3 of the 5 features) | International Diabetes Federation (large waist + any other 2 features) |
|---|---|---|
Waist circumference |
|
|
Men |
>102 cm (40 in) |
>94 cm (37 in) |
Women |
>88 cm (35 in) |
>80 cm (35 in) |
Triglycerides |
>1.7 mmol/L (150 mg/dL) |
1.7 mmol/L (150 mg/dL) |
HDL cholesterol |
|
|
Men |
<1.03 mmol/L (40 mg/dL) |
<1.03 mmol/L (40 mg/dL) |
Women |
<1.29 mmol/L (50 mg/dL) |
<1.29 mmol/L (50 mg/dL) |
Blood pressure |
>130/85 mmHg |
>130/85 mmHg |
Fasting glucose |
>5.6 mmol/L (100 mg/dL) |
>5.6 mmol/L (100 mg/dL) |
ATP III, Adult Treatment Panel 3.
This means that the prevalence of metabolic syndrome will be higher using the IDF criteria and the IDF criteria will identify at-risk patients at an earlier stage. This could lead to further investigations following on from the initial screening, and earlier institution of preventative as well as therapeutic measures. Other classification systems also exist, e.g. using BMI (an overall measure of obesity) instead of waist circumference (a measure of central obesity, which is more likely to be associated insulin resistance).
Overweight/central obesity and insulin resistance, which causes glucose and lipid disturbances, seem to form the basis of many features of the metabolic syndrome. Early treatment of obesity and the metabolic syndrome can avoid development of clinical diabetes and its complications.
The metabolic syndrome is a combination of risk factors (Table 5.17). Its overall role in the prediction of the risk of cardiovascular disease has been questioned as the sum of the combined risk factors in the syndrome does not offer more than the individual factors added together.
Treatment
Dietary control
This largely depends on a reduction in calorie intake. The most common diets allow a daily intake of approximately 4200 kJ (1000 kcal), although this may need to be nearer 6300 kJ (1500 kcal) for someone engaged in physical work. Very low calorie diets are also advocated by some, usually over shorter periods of time, but unless they are accompanied by changes in lifestyle, weight regain is likely. Patients must realize that prolonged dieting is necessary for large amounts of fat to be lost. Furthermore, a permanent change in eating habits is required to maintain the new low weight. It is relatively easy for most people to lose the first few kilograms, but long-term success in moderate obesity is poor (no more than 10%). Most obese people oscillate in weight; they often regain the lost weight, but many manage to lose weight again. This ‘cycling’ in bodyweight may play a role in the development of coronary artery disease.
Many dietary regimens aim to produce a weight loss of approximately 1 kg/week. Weight loss will be greater initially owing to accompanying protein and glycogen breakdown and consequent water loss. After 3–4 weeks, further weight loss may be very small because only adipose tissue is broken down and there is less accompanying water loss.
Patients must understand the principles of energy intake and expenditure, and the best results are obtained in educated, well-motivated patients. Constant supervision by healthcare professionals, by close relatives or through membership of a slimming club helps to encourage compliance. It is essential to establish realistic aims. A 10% weight loss, which is regarded by some as a ‘success’ (see Table 5.15), is a realistic initial aim.
An increase in exercise will increase energy expenditure and should be encouraged – provided there is no contraindication – since weight control is usually not achieved without exercise. The effects of exercise are complex and not entirely understood. However, exercise alone will usually produce little long-term benefit. On the other hand there is evidence to suggest that in combination with dietary therapy, it can prevent weight being regained. In addition, regular exercise (30 min daily) will improve general health.
The diet should contain adequate amounts of protein, vitamins and trace elements (Box 5.7).
![]() Box 5.7
Box 5.7
Constituents of a diet for weight loss
 4200 kJ (1000 kcal) per day made up of >50 g protein, approximately 100 g of carbohydrate and 40 g of fat
4200 kJ (1000 kcal) per day made up of >50 g protein, approximately 100 g of carbohydrate and 40 g of fat
 Carbohydrate should be in the form of complex carbohydrates such as vegetables and fruit rather than simple sugars
Carbohydrate should be in the form of complex carbohydrates such as vegetables and fruit rather than simple sugars
 Alcohol should be discouraged (contains 29 kJ/g (7 kcal/g)): it can be substituted for other foods in the diet, but it often reduces the willpower
Alcohol should be discouraged (contains 29 kJ/g (7 kcal/g)): it can be substituted for other foods in the diet, but it often reduces the willpower
 With a varied diet, vitamin and mineral intake will be adequate and supplements are not necessary.
With a varied diet, vitamin and mineral intake will be adequate and supplements are not necessary.
A balanced diet, attractively presented, is of much greater value and safer than any of the slimming regimens often advertised in magazines.
A wide range of diets are available, including low-fat or low-carbohydrate diets, and some suit certain individuals better than others. The following general statements can be made about them:
 All low-calorie diets produce loss of bodyweight and fat, irrespective of dietary composition. Short-term weight loss is faster on low-carbohydrate diets, as a result of greater loss of body water, which is regained after the end of dietary therapy.
All low-calorie diets produce loss of bodyweight and fat, irrespective of dietary composition. Short-term weight loss is faster on low-carbohydrate diets, as a result of greater loss of body water, which is regained after the end of dietary therapy.
 Very low-fat diets are often low in vitamins E, B12, and zinc. Very low-carbohydrate diets may be nutritionally inadequate, and may lead to deficiencies.
Very low-fat diets are often low in vitamins E, B12, and zinc. Very low-carbohydrate diets may be nutritionally inadequate, and may lead to deficiencies.
 Low-fat diets decrease LDL triglycerides and increase HDL, whereas low-carbohydrate diets produce a greater decrease in HDL and triglyceride, with no change in LDL.
Low-fat diets decrease LDL triglycerides and increase HDL, whereas low-carbohydrate diets produce a greater decrease in HDL and triglyceride, with no change in LDL.
 There are some potential long-term concerns with low-carbohydrate diets (high in fat and protein), including increased risk of osteoporosis, renal stones and atheroma (due to high saturated fat, high trans fat and cholesterol and the lack of fruits, vegetables and whole grains) but long-term studies are lacking.
There are some potential long-term concerns with low-carbohydrate diets (high in fat and protein), including increased risk of osteoporosis, renal stones and atheroma (due to high saturated fat, high trans fat and cholesterol and the lack of fruits, vegetables and whole grains) but long-term studies are lacking.
 Low-energy-density diets, often bulky and rich in fibre and complex carbohydrates, may be more satiating but they are often less palatable than high-energy-dense diets, which may affect long-term compliance.
Low-energy-density diets, often bulky and rich in fibre and complex carbohydrates, may be more satiating but they are often less palatable than high-energy-dense diets, which may affect long-term compliance.
 Liquids, e.g. soft drinks, appear to be less satiating than solid foods.
Liquids, e.g. soft drinks, appear to be less satiating than solid foods.
 A recent study has shown that Mediterranean and low-carbohydrate diets are as effective as a low-fat diet for weight loss.
A recent study has shown that Mediterranean and low-carbohydrate diets are as effective as a low-fat diet for weight loss.
Behavioural modification
The aim of behavioural modification is to encourage the patient to take personal responsibility for changing lifestyle, which will determine dietary habits and physical activity. Family therapy may also be useful, especially when it involves obese children. It can be time-consuming and expensive. Cognitive behavioural therapy is even more time-consuming and expensive.
Drug therapy
Drugs can be used in the short term (up to 3 months) as an adjunct to the dietary regimen, but they do not substitute for strict dieting.
Centrally acting drugs
 Drugs acting on both serotoninergic and noradrenergic pathways, e.g. sibutramine (now withdrawn in Europe due to side-effects). Other drugs, such as lorcaserin, a selective 5-HT2C receptor agonist, are being evaluated.
Drugs acting on both serotoninergic and noradrenergic pathways, e.g. sibutramine (now withdrawn in Europe due to side-effects). Other drugs, such as lorcaserin, a selective 5-HT2C receptor agonist, are being evaluated.
 Cannabinoid-1 receptor blockers, e.g. rimonabant (now withdrawn due to depression/suicide risk), acting on the endocannabinoid system.
Cannabinoid-1 receptor blockers, e.g. rimonabant (now withdrawn due to depression/suicide risk), acting on the endocannabinoid system.
 Drugs acting on the noradrenergic pathways do suppress appetite but all have been withdrawn, at least in the UK, because of cardiovascular side-effects.
Drugs acting on the noradrenergic pathways do suppress appetite but all have been withdrawn, at least in the UK, because of cardiovascular side-effects.
Peripherally acting drugs
 Orlistat is an inhibitor of pancreatic and gastric lipases. It reduces dietary fat absorption and aids weight loss. Weight regain occurs after the drug is stopped. It has been used continuously in a large-scale trial for up to 2 years. The patients complain of diarrhoea during treatment and to avoid this, take a low-fat diet resulting in weight loss.
Orlistat is an inhibitor of pancreatic and gastric lipases. It reduces dietary fat absorption and aids weight loss. Weight regain occurs after the drug is stopped. It has been used continuously in a large-scale trial for up to 2 years. The patients complain of diarrhoea during treatment and to avoid this, take a low-fat diet resulting in weight loss.
 Glucagon-like peptide 1 (GLP-1) suppresses appetite and injections have been used to treat obesity (Fig. 5.9) and type 2 diabetes mellitus (p. 1011).
Glucagon-like peptide 1 (GLP-1) suppresses appetite and injections have been used to treat obesity (Fig. 5.9) and type 2 diabetes mellitus (p. 1011).
A systematic review of long-term pharmacotherapy concluded that there was a paucity of long-term studies with anti-obesity agents, and that in weight loss trials of 1-year duration, appear to be only modestly effective in promoting weight loss (about 3–4 kg greater weight loss, respectively than the control group). Other randomized trials show that a combination of lifestyle modification and pharmacotherapy produce greater weight loss than either treatment alone, but the withdrawal of several anti-obesity drugs suggests that a pharmacotherapeutic magic bullet to treat obesity without substantial short-term and long-term effects is not yet available although the search continues. Alternative forms of treatment should be considered (unless obesity and its risks are accepted as part of modern society).
Surgical treatment
Surgery is used in some cases of morbid obesity (BMI >35 kg/m2) or patients with a BMI >30 kg/m2 and obesity related complications, after conventional medical treatments have failed. It can be used as a first-line option for individuals with a BMI >50 kg/m2. Fitness for surgery should be checked, especially in older people. A variety of gastrointestinal surgical procedures have been used. They fall into three main groups (Fig. 5.10):
Figure 5.10 Examples of surgery procedures to treat morbid obesity. (a) Restrictive procedure: gastric banding with subcutaneous port attached to anterior abdominal wall so that fluid can be injected into the adjustable band around the upper stomach. (b) Restrictive plus malabsorptive procedures: Roux-en-Y gastric bypass, in which food bypasses through a small stomach pouch and bypasses the proximal small bowel.
(Courtesy of Mr Amit Patel.)
Restrictive procedures, which restrict the ability to eat (e.g. adjustable gastric banding, vertical banded gastroplasty and sleeve gastroplasty).
Malabsorptive procedures, which reduce the ability to absorb nutrients (e.g. biliopancreatic diversion and Roux-en-Y gastric bypass). The malabsorptive procedures cause nutrient deficiencies, malnutrition and in some cases, anastomotic leaks and the dumping syndrome (e.g. with the duodenal switch).
Restrictive plus malabsorptive procedures (e.g. duodenal switch, Roux-en-Y gastric bypass, intragastric balloon).
The procedures all have advantages and disadvantages, and there is controversy about the procedure of choice for specific groups of patients. The restrictive procedures are more straightforward than the complex bypass procedures. The adjustable gastric banding procedure, although attractive in concept, especially since it can be undertaken laparoscopically with a lower perioperative mortality (<0.3%) than the other procedures (~1%), can be associated with erosion and slippage of the band, as well problems with the port, making repeat operations a frequent requirement (>10% of cases). The sleeve gastrectomy is associated with heartburn and greater risk of weight regain, but a biliary pancreatic diversion (duodenal switch) can be added at a later time.
There is a need to carefully monitor nutrient status with blood tests and provide supplements of vitamins and minerals (including iron and calcium). Weight loss following the combined restrictive and malabsorptive procedures tends to be greater than with either procedure alone.
A systematic analysis of several bariatric surgical procedures concluded that, in comparison to non-surgical treatments, they produced significantly more weight loss (23–37 kg), which was maintained to 8 years and associated with improvement in quality of life and co-morbidities.
Liposuction, the removal of large amounts of fat by suction (liposuction), does not deal with the underlying problem and weight regain frequently occurs. There appears to be no reduction in cardiovascular risk factors with the procedure.
Prevention
Preventing obesity must always be the goal because most obese people find it difficult to maintain any weight loss they have managed to achieve. All health professionals must be aware of the dangers of obesity and encourage children, young as well as older adults, from gaining too much weight. A small gain each year over a long period produces an obese individual for whom treatment is difficult. Public health policies should consider creation of public places to encourage physical activity and fitness, education about the benefits of losing weight or not gaining it, through healthy eating and physical activity, and changes in food composition (alternatives to high-fat, high-energy-dense foods).
Since the present obesity epidemic has resulted from lifestyle changes, it is appropriate to promote lifestyle changes, not only as the first-line therapy for most overweight and obese individuals, but also in the prevention of overweight and obesity. Lifestyle modification would involve changes in the amount of time watching television and using computers, use of bicycle paths, dietary changes and educational activities of patients and public, parents and children. To prevent long-term weight gain after any of the therapies discussed above, each therapy should be part of a package that involves lifestyle modification.
FURTHER READING
Eckel RH. Nonsurgical management of obesity in adults. N Engl J Med 2008; 358:1941–1950.
Eckel, RH, Alberti KG, Grund SM. The metabolic syndrome. Lancet 2010; 375:181–183.
Franks P, Hanson RL, Knowler WC et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010; 362:485–493.
Han JC, Lawler DA, Kimm SY. Childhood obesity. Lancet 2010; 375:1737–1748.
Ingelfinger J. Bariatric surgery in adolescents. N Engl J Med 2011; 365:1365–1367.
Leff DR, Heath D. Surgery for obesity in adulthood. BMJ 2009: 339:b3402. doi: 10.1136/bmj.b3402.
National Institute for Health and Clinical Excellence. Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. Clinical Guideline 43; 2006. Also at: www.nice.org.uk
Robinson MK. Surgical treatment of obesity – weighing the facts. N Engl J Med 2010; 361:520–521.
Shai I, Schwarz Fuchs D, Henkin Y et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008; 359:229–241.
Tharakan G, Tan T, Bloom S. Emerging therapies in the treatment of ‘diabesity’: beyond GLP-1. Trends Pharmacol Sci 2011; 32:8–15.
Nutritional support in the hospital patient
Nutritional support is recognized as being necessary in many hospitalized patients. The pathophysiology and hallmarks of malnutrition have been described earlier (p. 200); here the forms of nutritional support that are available are discussed, along with special nutritional requirements in some diseases.
Principles
Some form of nutritional supplementation is required in those patients who cannot eat, should not eat, will not eat or cannot eat enough. All patients should be screened for malnutrition on admission and the findings linked to a care plan, preferably under the supervision of a trained multidisciplinary team. The Council of Europe has produced 10 key characteristics of good nutritional care in hospital (see: bapen.org.uk). Plans are discussed with patients and consent is taken for any invasive procedure (e.g. nasogastric tube, parenteral nutrition). If the patient is unable to give consent, the healthcare team should act in the patient’s best interest, taking into account previously expressed wishes of the patient and views of the family. It is usually necessary to provide nutritional support for:
 all severely malnourished patients on admission to hospital
all severely malnourished patients on admission to hospital
 moderately malnourished patients who, because of their physical illness, are not expected to eat for more than 3–5 days
moderately malnourished patients who, because of their physical illness, are not expected to eat for more than 3–5 days
 normally nourished patients expected not to eat for more than 5 days or to eat less than half their intake for more than 8–10 days.
normally nourished patients expected not to eat for more than 5 days or to eat less than half their intake for more than 8–10 days.
Enteral rather than parenteral nutrition should be used if the gastrointestinal tract is functioning normally.
In re-feeding syndrome, the shifts of water and electrolytes that occur after parenteral and enteral nutrition can be life-threatening. Carbohydrate intake stimulates insulin release which leads to cellular uptake of phosphate, potassium and magnesium. Complications include hypophosphataemia, hypokalaemia, hypomagnesaemia and fluid overload because of sodium retention (decreased renal excretion of sodium and water). Patients who have eaten little or nothing for more than 5 days should initially receive no more than 50% of their energy requirements (NICE guidelines).
Nutritional requirements for adults
 Water. Typical requirements are ~2–3 L/day. Increased requirements occur in patients with large-output fistulae, nasogastric aspirates and diarrhoea. Reduced requirements occur in patients with oedema, hepatic failure, renal failure (oliguric and not dialysed) and brain oedema.
Water. Typical requirements are ~2–3 L/day. Increased requirements occur in patients with large-output fistulae, nasogastric aspirates and diarrhoea. Reduced requirements occur in patients with oedema, hepatic failure, renal failure (oliguric and not dialysed) and brain oedema.
 Energy. Typical requirements are ~7.5–10.0 MJ/day (1800–2400 kcal/day). Disease increases resting energy expenditure but decreases physical activity. Extra energy is given for repletion and reduced energy for obesity.
Energy. Typical requirements are ~7.5–10.0 MJ/day (1800–2400 kcal/day). Disease increases resting energy expenditure but decreases physical activity. Extra energy is given for repletion and reduced energy for obesity.
 Protein. Typically 10–15 g N/day (62–95 g protein/day) or 0.15–0.25 g N/kg per day (0.94–1.56 g protein/kg per day). Extra protein may be needed in severely catabolic conditions, such as extensive burns.
Protein. Typically 10–15 g N/day (62–95 g protein/day) or 0.15–0.25 g N/kg per day (0.94–1.56 g protein/kg per day). Extra protein may be needed in severely catabolic conditions, such as extensive burns.
 Major minerals. Typical requirements for sodium and potassium are 60–100 mmol/day. Increased requirements occur in patients with gastrointestinal effluents. The excretion of these minerals in various effluents can provide an indication of the additional requirements (see Table 13.10). Low requirements may be necessary in those with fluid overload (or patients with hypernatraemia and hyperkalaemia). The requirements of calcium and magnesium are higher for enteral than for parenteral nutrition because only a proportion of these minerals is absorbed by the gut.
Major minerals. Typical requirements for sodium and potassium are 60–100 mmol/day. Increased requirements occur in patients with gastrointestinal effluents. The excretion of these minerals in various effluents can provide an indication of the additional requirements (see Table 13.10). Low requirements may be necessary in those with fluid overload (or patients with hypernatraemia and hyperkalaemia). The requirements of calcium and magnesium are higher for enteral than for parenteral nutrition because only a proportion of these minerals is absorbed by the gut.
 Trace elements. For trace elements such as iodide, fluoride and selenium that are well absorbed, the requirements for enteral and parenteral nutrition are similar. For other trace elements, such as iron, zinc, manganese and chromium, the requirements for parenteral nutrition are substantially lower than for enteral nutrition (Fig. 5.11).
Trace elements. For trace elements such as iodide, fluoride and selenium that are well absorbed, the requirements for enteral and parenteral nutrition are similar. For other trace elements, such as iron, zinc, manganese and chromium, the requirements for parenteral nutrition are substantially lower than for enteral nutrition (Fig. 5.11).
 Vitamins. Many vitamins are given in greater quantities in patients receiving parenteral nutrition than in those receiving enteral nutrition (Fig. 5.12). This is because patients on parenteral nutrition may have increased requirements, partly because of severe disease, partly because they may already have depleted pools of vitamins, and partly because some vitamins degrade during storage. Vitamin K is usually absent from parenteral nutrition regimens and therefore it may need to be administered separately.
Vitamins. Many vitamins are given in greater quantities in patients receiving parenteral nutrition than in those receiving enteral nutrition (Fig. 5.12). This is because patients on parenteral nutrition may have increased requirements, partly because of severe disease, partly because they may already have depleted pools of vitamins, and partly because some vitamins degrade during storage. Vitamin K is usually absent from parenteral nutrition regimens and therefore it may need to be administered separately.
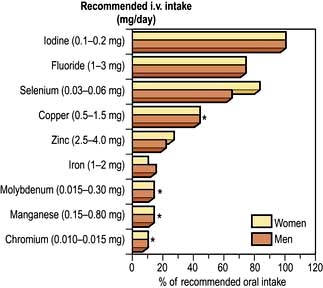
Figure 5.11 Trace elements. Recommended intravenous intake in absolute values and as a percentage of recommended oral intake. *Trace elements for which there is too little information to establish a recommended value for dietary oral intake; the midpoint of estimated safe and adequate oral intake is shown.
Enteral nutrition (EN)
Feeds can be given by various routes:
 By mouth (food can be supplemented with solid or liquid supplements with multiple benefits).
By mouth (food can be supplemented with solid or liquid supplements with multiple benefits).
 By fine-bore nasogastric tube (see Practical Box 5.1)
By fine-bore nasogastric tube (see Practical Box 5.1)
 Percutaneous endoscopic gastrostomy (PEG) is useful for patients who need enteral nutrition for a prolonged period (e.g. >30 days), such as those with swallowing problems following a head injury or in elderly people after a stroke. A catheter is placed percutaneously into the stomach under endoscopic control (Fig. 5.13).
Percutaneous endoscopic gastrostomy (PEG) is useful for patients who need enteral nutrition for a prolonged period (e.g. >30 days), such as those with swallowing problems following a head injury or in elderly people after a stroke. A catheter is placed percutaneously into the stomach under endoscopic control (Fig. 5.13).
 With needle catheter jejunostomy, a fine catheter is inserted into the jejunum at laparotomy and brought out through the abdominal wall.
With needle catheter jejunostomy, a fine catheter is inserted into the jejunum at laparotomy and brought out through the abdominal wall.
![]() Practical Box 5.1
Practical Box 5.1
Enteral feeding via nasogastric tube
The procedure should be explained to the patient and consent taken.
Diet formulation (Table 5.18)
A polymeric diet with whole protein and fat can be used, except in patients with severely impaired gastrointestinal function who may require a predigested (i.e. elemental) diet. In these patients, the nitrogen source is purified low-molecular-weight peptides or amino acid mixtures, with sometimes the fat being given partly as medium-chain triglycerides.
Table 5.18 Standard enteric diet, providing 8.4 MJ/day (= 2000 kcal)
Management
Daily amounts of diet vary between 1.5 and 2.5 L, but small amounts are started in patients with suspected poor gastric emptying and severe malnutrition (to avoid the re-feeding syndrome).
Hypercatabolic patients require a high supply of nitrogen (15 g daily) and often will not achieve positive nitrogen balance until the primary injury is resolved.
The success of enteral feeding depends on careful supervision of the patient, with monitoring of weight, biochemistry and diet charts.
Parenteral nutrition
Peripheral parenteral nutrition
Specially formulated mixtures for peripheral use are available, with a low osmolality (<800 mosmol/L) and containing lipid emulsions. Heparin and corticosteroids can be added to the infusion and local application of glyceryl trinitrate patches reduces the occurrence of thrombophlebitis and prolongs catheter life.
 A peripheral cannula can be inserted into a mid-arm vein (20 cm) and can be left for up to 5 days.
A peripheral cannula can be inserted into a mid-arm vein (20 cm) and can be left for up to 5 days.
 A longer (60 cm) peripherally inserted central catheter (PICC) inserted into an antecubital fossa vein has its distal end lying in a central vein; here there is less risk of thrombophlebitis and hyperosmolar solutions can be given. With careful management, PICCs can be used for up to about a month.
A longer (60 cm) peripherally inserted central catheter (PICC) inserted into an antecubital fossa vein has its distal end lying in a central vein; here there is less risk of thrombophlebitis and hyperosmolar solutions can be given. With careful management, PICCs can be used for up to about a month.
Peripheral parenteral nutrition is often preferred initially, allowing time to consider the necessity for having to insert a central venous catheter.
Parenteral nutrition via a central venous catheter (PN) (see Practical Box 5.2)
A silicone catheter is placed into a central vein, usually using the infraclavicular approach to the subclavian vein. The skin-entry site should be dressed carefully and not disturbed unless there is a suggestion of catheter-related sepsis.
![]() Practical Box 5.2
Practical Box 5.2
Central catheter placement for parenteral nutrition
This should be performed only by experienced clinicians under aseptic conditions in an operating theatre.
Give an explanation and obtain consent from the patient.
 The patient is placed supine with 5° of head-down tilt to avoid air embolism.
The patient is placed supine with 5° of head-down tilt to avoid air embolism.
 The skin below the midpoint of the right clavicle is infiltrated with 1–2% lidocaine and a 1 cm skin incision is made.
The skin below the midpoint of the right clavicle is infiltrated with 1–2% lidocaine and a 1 cm skin incision is made.
 A 20-gauge needle on a syringe is inserted beneath the clavicle and first rib and angled towards the tip of a finger held in the suprasternal notch.
A 20-gauge needle on a syringe is inserted beneath the clavicle and first rib and angled towards the tip of a finger held in the suprasternal notch.
 When blood is aspirated freely, the needle is used as a guide to insert the cannula through the skin incision and into the subclavian vein.
When blood is aspirated freely, the needle is used as a guide to insert the cannula through the skin incision and into the subclavian vein.
 The catheter is advanced so that its tip lies in the distal part of the superior vena cava.
The catheter is advanced so that its tip lies in the distal part of the superior vena cava.
 A skin tunnel is created under local anaesthetic using an introducer inserted through a point about 10 cm below and medial to the incision and passed upwards to the incision.
A skin tunnel is created under local anaesthetic using an introducer inserted through a point about 10 cm below and medial to the incision and passed upwards to the incision.
 The proximal end of the catheter (with hub removed) is passed backwards through the introducer to emerge 10 cm below the clavicle, where it is sutured to the chest wall.
The proximal end of the catheter (with hub removed) is passed backwards through the introducer to emerge 10 cm below the clavicle, where it is sutured to the chest wall.
Complications of catheter placement include central vein thrombosis, pneumothorax and embolism, but one of the commonest problems is catheter-related sepsis. Organisms, mainly staphylococci, enter along the side of the catheter, leading to septicaemia. Sepsis can be prevented by careful and sterile placement of the catheter, by not removing the dressing over the catheter entry site, and by not giving other substances (e.g. blood products, antibiotics) via the central vein catheter.
Sepsis should be suspected if the patient develops fever and leucocytosis. In two-thirds of cases, organisms can be grown from the catheter tip after removal. Treatment involves removal of the catheter and appropriate systemic antibiotics.
Nutrients
With PN, it is possible to provide sufficient nitrogen for protein synthesis and calories to meet energy requirements. Electrolytes, vitamins and trace elements are also necessary. All of these substances are infused simultaneously.
Nitrogen source
Most patients receive at least 11–15 g N/day, in the form of synthetic L-amino acids.
Energy source
This is provided by glucose, with additional calories provided by a fat emulsion. Fat infusions provide a greater number of calories in a smaller volume than can be provided by carbohydrate. Fat infusions are not hypertonic and they also prevent essential fatty acid deficiency.
Essential fatty acid deficiency has been reported in long-term parenteral nutritional regimens without fat emulsions. It causes a scaly skin, hair loss and a delay in healing.
Electrolytes, vitamins and trace elements
Initially, the electrolyte status should be monitored on a daily basis and electrolyte solutions given as appropriate. Fat- and water-soluble vitamins and minerals including trace elements should be given routinely (see Figs 5.12, 5.13).
Administration and monitoring
Peripheral parenteral nutrition. Administered via 3-L bags over 24 hours, with the constituents being premixed under sterile conditions by the pharmacy. Table 5.19 shows the composition which provides 9 g of nitrogen and 1700 calories in 24 h.
Table 5.19 Examples of parenteral nutrition regimens
Peripheral: all mixed in 3-L bags and infused over 24 hours |
||
Nitrogen |
L-amino acids 9 g/L |
1 L |
Energy |
Glucose 20% |
1 L |
Lipid 20% |
0.5 L |
|
+ Trace elements, electrolytes, and water-soluble and fat-soluble vitamins, heparin 1000 UL and hydrocortisone 100 mg; insulin is added if required. Nitrogen 9 g, non-protein calories 7206 kJ (1700 kcal) |
||
Central: all mixed in 3-L bags and infused over 24 hours |
||
Nitrogen |
L-amino acids 14 g/L |
1 L |
Energy |
Glucose 50% |
0.5 L |
Glucose 20% |
0.5 L |
|
+ Lipid 10% as either Intralipid or Lipofundin |
0.5 L |
|
Fractionated soya oil 100 g/L, Soya oil 50 g, medium-chain triglycerides 50 g/L |
||
+ Electrolytes, water-soluble vitamins, fat-soluble vitamins, trace elements, heparin and insulin may be added if required. Nitrogen 14 g, non-protein calories 9305 kJ (2250 kcal) |
||
Central venous PN regimen. Most hospitals now use premixed 3-L bags. A standard parenteral nutrition regimen which provides 14 g of nitrogen and 2250 calories over 24 hours is also given in Table 5.19. Monitoring includes:
 Blood tests. Daily plasma electrolytes and glucose (at least initially). Twice weekly FBC, liver biochemistry and function, calcium, phosphate, and magnesium, zinc and triglycerides weekly.
Blood tests. Daily plasma electrolytes and glucose (at least initially). Twice weekly FBC, liver biochemistry and function, calcium, phosphate, and magnesium, zinc and triglycerides weekly.
 Nutritional status. Weekly weight and skinfold thickness if appropriate callipers are available. Daily weight changes reflect changes in fluid balance.
Nutritional status. Weekly weight and skinfold thickness if appropriate callipers are available. Daily weight changes reflect changes in fluid balance.
 Nitrogen balance (p. 197) assessment, but this requires complete collections of urine.
Nitrogen balance (p. 197) assessment, but this requires complete collections of urine.
Complications
 Mechanical: insertional trauma and catheter-related (see above)
Mechanical: insertional trauma and catheter-related (see above)
 Metabolic, e.g. hyperglycaemia (insulin therapy is usually necessary), fluid and electrolyte disturbances, hypercalcaemia, nutrient deficiencies (if inadequately provided)
Metabolic, e.g. hyperglycaemia (insulin therapy is usually necessary), fluid and electrolyte disturbances, hypercalcaemia, nutrient deficiencies (if inadequately provided)
 Organ or tissue dysfunction, e.g. abnormal liver dysfunction, respiratory distress and metabolic bone disease
Organ or tissue dysfunction, e.g. abnormal liver dysfunction, respiratory distress and metabolic bone disease
 Others, e.g. rare allergic reactions to lipid, and psychological disturbances.
Others, e.g. rare allergic reactions to lipid, and psychological disturbances.
The need for major improvements in the practice of parenteral nutrition in British hospitals has been emphasized by the National Confidential Enquiry into Patient Outcome and Death (NCEPOD). This included need for improvements at every level: assessment, monitoring and follow-up, including appropriate care of lines to avoid catheter-related sepsis and documentation.
Nutritional support in the home patient
In both high- and low-income countries, there is considerably more undernutrition in the community than in hospital. However, the principles of care are very similar: detection of malnutrition and the underlying risk factors; treatment of underlying disease processes and disabilities; correction of specific nutrient deficiencies and provision of appropriate nutritional support. This typically begins with dietary advice, and may involve the provision of ‘meals on wheels’ by social services. A systematic review of the use of nutritional supplements in the community came to the following conclusions:
 Supplements are generally of more value in patients with a BMI <20 kg/m2 and children with growth failure (weight for height <85% of ideal) than in those with better anthropometric indices. They are likely to be of little or no value in patients with little weight loss and a BMI >20 kg/m2. The supplemental energy intake in such subjects largely replaces oral food intake.
Supplements are generally of more value in patients with a BMI <20 kg/m2 and children with growth failure (weight for height <85% of ideal) than in those with better anthropometric indices. They are likely to be of little or no value in patients with little weight loss and a BMI >20 kg/m2. The supplemental energy intake in such subjects largely replaces oral food intake.
 Supplements may be of value in weight-losing patients (e.g. >10% weight loss compared to pre-illness) with a BMI >20 kg/m2, and in children with deteriorating growth performance without chronic protein-energy undernutrition.
Supplements may be of value in weight-losing patients (e.g. >10% weight loss compared to pre-illness) with a BMI >20 kg/m2, and in children with deteriorating growth performance without chronic protein-energy undernutrition.
 The functional benefits vary according to the patient group. In patients with chronic obstructive airways disease the observed functional benefits were increased respiratory muscle strength, increase in handgrip strength, and an increase in walking distance/duration of exercise. In the elderly the benefits were reduced number of falls, or increase in activities of daily living, and reduced pressure sore surface area. In patients with HIV/AIDS there were changes in immunological function and improved cognition. Patients with liver disease experienced a lower incidence of severe infections and had a lower frequency of hospitalization.
The functional benefits vary according to the patient group. In patients with chronic obstructive airways disease the observed functional benefits were increased respiratory muscle strength, increase in handgrip strength, and an increase in walking distance/duration of exercise. In the elderly the benefits were reduced number of falls, or increase in activities of daily living, and reduced pressure sore surface area. In patients with HIV/AIDS there were changes in immunological function and improved cognition. Patients with liver disease experienced a lower incidence of severe infections and had a lower frequency of hospitalization.
 Acceptability and compliance are likely to be better when a choice of supplements (of type, flavour, consistency) and schedule is decided in conjunction with the patient and/or carer. Changes in these may be necessary when there is a change in patterns of daily activities, disease status, and ‘taste fatigue’ with prolonged use of the same supplement.
Acceptability and compliance are likely to be better when a choice of supplements (of type, flavour, consistency) and schedule is decided in conjunction with the patient and/or carer. Changes in these may be necessary when there is a change in patterns of daily activities, disease status, and ‘taste fatigue’ with prolonged use of the same supplement.
 Nutritional counselling and monitoring are recommended before and after the start of supplements (see below).
Nutritional counselling and monitoring are recommended before and after the start of supplements (see below).
Some patients receive enteral tube feeding or parenteral nutrition at home. At any one point in time in developed countries enteral tube feeding occurs more frequently at home than in hospital.
Home enteral nutrition
In adults, the commonest reason for starting home tube feeding is for swallowing difficulties. This involves patients with neurological disorders, such as motor neurone disease, multiple sclerosis and Parkinson’s disease, but the commonest single diagnosis is cerebrovascular disease. Approximately 2% of patients who have had a stroke in the UK receive home enteral tube feeding (HETF). However, in a British Nutrition survey of patients with these disorders (apart from Parkinson’s), only 15% in total were able to return to oral feeding after 1 year.
The swallowing capabilities of patients should be assessed regularly in order to avoid unnecessary tube feeding. The patients and/or carers should have adequate training, contacts with appropriate health professions, and a reliable delivery service for feeds and ancillary equipment. They should also be clear about how to manage simple problems associated with the feeding tube, which is usually a gastrostomy tube rather than a nasogastric tube.
Home parenteral nutrition
Home parenteral nutrition is practised much less frequently, usually under the supervision of specialist centres. The potential value of intestinal transplantation in patients with long-term intestinal failure is still being assessed.
FURTHER READING
Kurien M, McAlindon ME, Westaby D et al. Percutaneous endoscopic gastrostomy (PEG) feeding. BMJ 2010; 340:1136.
Mehanna HM, Moledina J, Travis J. Re-feeding syndrome. BMJ 2008; 336:1495–1498.
National Institute for Health and Clinical Excellence. Nutrition support in adults. Clinical Guideline 32; 2006. Also at: www.nice.org.uk
Singer P, Berger MM, Van den Berghe G et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 2009; 28:387–400.
Smith T, Elia M. Artificial nutrition support in hospital: indications and complications. Clin Med 2006; 6:457–460.
Stewart JA, Nason DG, Smith N et al. A mixed bag. An Enquiry into the care of hospital patients receiving parenteral nutrition. A report by the National Confidential Enquiry into Patient Outcome and Death (NCEPOD). London: NCEPOD; 2010.
Stratton RJ, Green CJ, Elia M. Disease-related malnutrition. An evidence-based approach to treatment. Oxford: CABI Publishing (CAB International); 2003.
Zaloga GP. Parenteral nutrition in adult inpatients with functioning gastrointestinal tracts: assessment of outcomes. Lancet 2006; 367:1101–1111.
Food allergy and food intolerance
Many people ascribe their various symptoms to food, and many such sufferers are seen and started on exclusion diets. The scientific evidence that food does harm is in most instances weak, although adverse reactions to food certainly exist. These can be divided into those that involve immune mechanisms (food allergy) and those that do not (food intolerance).
Food allergy
Food allergy, which is estimated to affect up to about 5% of young children and about 1–2% of adults, may be IgE mediated or non-IgE mediated (T-cell mediated). The IgE-mediated reactions tend to occur early after a food challenge (within minutes to an hour). Adults tend to be allergic to fish, shellfish and peanuts, while children tend to be allergic to cow’s milk, egg white, wheat and soy. Peanuts are very allergenic and peanut allergy persists throughout life. The following conditions can result from food allergy:
 Acute hypersensitivity. An example is urticaria, vomiting or diarrhoea after eating nuts, strawberries or shellfish. These IgE-mediated reactions do not usually produce clinical problems as the patients have already learned to avoid the suspected food. Inadvertent ingestion of the incriminating food can sometimes occur, leading to angioneurotic oedema (p. 1211).
Acute hypersensitivity. An example is urticaria, vomiting or diarrhoea after eating nuts, strawberries or shellfish. These IgE-mediated reactions do not usually produce clinical problems as the patients have already learned to avoid the suspected food. Inadvertent ingestion of the incriminating food can sometimes occur, leading to angioneurotic oedema (p. 1211).
 Eczema and asthma. These tend to affect young children and are often due to egg and are IgE mediated.
Eczema and asthma. These tend to affect young children and are often due to egg and are IgE mediated.
 Rhinitis and asthma. These have been produced by foods such as milk and chocolate, mainly in atopic subjects.
Rhinitis and asthma. These have been produced by foods such as milk and chocolate, mainly in atopic subjects.
 Chronic urticaria. This has been treated successfully by an exclusion diet.
Chronic urticaria. This has been treated successfully by an exclusion diet.
 Food-sensitive enteropathy. This may manifest itself as coeliac disease (gluten (wheat) sensitive enteropathy) and cow’s milk enteropathy (in infants), and is T-cell mediated.
Food-sensitive enteropathy. This may manifest itself as coeliac disease (gluten (wheat) sensitive enteropathy) and cow’s milk enteropathy (in infants), and is T-cell mediated.
Food intolerance
 Migraine. This sometimes follows the intake of foods such as chocolate, cheese and alcohol, which are rich in certain amines, such as tyramine. Patients on monoamine oxidase inhibitors, which are involved in the metabolism of these amines, are particularly vulnerable.
Migraine. This sometimes follows the intake of foods such as chocolate, cheese and alcohol, which are rich in certain amines, such as tyramine. Patients on monoamine oxidase inhibitors, which are involved in the metabolism of these amines, are particularly vulnerable.
 Irritable bowel syndrome. In some patients, this seems to be related to ingestion of certain food items, such as wheat, but the mechanisms are not clearly defined.
Irritable bowel syndrome. In some patients, this seems to be related to ingestion of certain food items, such as wheat, but the mechanisms are not clearly defined.
 Chinese restaurant syndrome. Monosodium glutamate, a flavour enhancer used in cooking Chinese food, may produce dizziness, faintness, nausea, sweating and chest pains.
Chinese restaurant syndrome. Monosodium glutamate, a flavour enhancer used in cooking Chinese food, may produce dizziness, faintness, nausea, sweating and chest pains.
 Lactose intolerance. Patients develop abdominal bloating and diarrhoea following ingestion of lactose, which is present in milk (p. 264). This is probably the commonest form of food intolerance worldwide, and may be genetic in origin.
Lactose intolerance. Patients develop abdominal bloating and diarrhoea following ingestion of lactose, which is present in milk (p. 264). This is probably the commonest form of food intolerance worldwide, and may be genetic in origin.
 Phenylketonuria. This can also be classified as a form of food intolerance, and is due to lack of phenylalanine hydroxylase, which is necessary for the metabolism of phenylalanine present in dietary protein.
Phenylketonuria. This can also be classified as a form of food intolerance, and is due to lack of phenylalanine hydroxylase, which is necessary for the metabolism of phenylalanine present in dietary protein.
A number of other inborn errors of metabolism can also be regarded as forms of food intolerance.
Food intolerance may be due to a constituent of food (e.g. the histamine in mackerel or canned food or the tyramine in cheeses); chemical mediators released by food (e.g. histamine may be released by tomatoes or strawberries); or toxic chemicals found in food (e.g. the food additive tartrazine). Many other additives and compounds with certain E numbers have been implicated as causing reactions, but the evidence is poor.
There is little or no evidence to suggest that diseases such as arthritis, behaviour and affective disorders and Crohn’s disease are due to ingestion of a particular food. Multiple vague symptoms such as tiredness or malaise are also not due to food allergy. Most of the patients in this group are suffering from a psychiatric disorder (p. 1185).
Management
 A careful history may help to delineate the causative agent, particularly when the effects are immediate.
A careful history may help to delineate the causative agent, particularly when the effects are immediate.
 Skin-prick testing with allergen and measurement in the serum of antigen or antibodies have not correlated with symptoms and are usually misleading. ‘Fringe’ techniques such as hair analysis, although widely advertised, are of no value.
Skin-prick testing with allergen and measurement in the serum of antigen or antibodies have not correlated with symptoms and are usually misleading. ‘Fringe’ techniques such as hair analysis, although widely advertised, are of no value.
 Diagnostic exclusion diets are sometimes used, but they are time-consuming. They can occasionally be of value in identifying a particular food causing problems.
Diagnostic exclusion diets are sometimes used, but they are time-consuming. They can occasionally be of value in identifying a particular food causing problems.
 Dietary challenge consists of the food and the test being given sublingually or by inhalation in an attempt to reproduce the symptoms. Again, this may be helpful in a few cases.
Dietary challenge consists of the food and the test being given sublingually or by inhalation in an attempt to reproduce the symptoms. Again, this may be helpful in a few cases.
 Most people who have acute reactions to food realize it and stop the food, and do not require medical attention. In the remainder of patients, a small minority seem to be helped by modifying their diet, but there is no good scientific evidence to support these exclusion diets.
Most people who have acute reactions to food realize it and stop the food, and do not require medical attention. In the remainder of patients, a small minority seem to be helped by modifying their diet, but there is no good scientific evidence to support these exclusion diets.
FURTHER READING
Hare ND, Fasano MB. Clinical manifestations of food allergy: differentiating true allergy from food intolerance. Postgrad Med 2008; 120:E01–E05.
Lack. G. Clincal practice. Food allergy. N Engl J Med 2008; 359:1252–1260.
Montalto M, Santoro L, D’Onofrio F et al. Adverse reactions to food: allergies and intolerances. Dig Dis 2008; 26:96–103.
Prescott SL, Bouygue GR, Videky D et al. Avoidance or exposure to foods in prevention and treatment of food allergy? Curr Opin Allergy Clin Immunol 2010; 10:258–266.
Alcohol
Although alcohol is not a nutrient, it is consumed in large quantities all over the world. In many countries, alcohol consumption is becoming a major medical and social problem (see p. 1163). It increases morbidity and mortality in a variety of ways, including effects on heart disease, stroke, cancers, liver and neurological/psychiatric problems, and it is associated with nutritional deficiencies and abnormal metabolism of drugs.
Ethanol (ethyl alcohol) is oxidized, in the steps shown in Box 5.8, to acetaldehyde. Acetaldehyde is then converted to acetate, 90% in the liver mitochondria. Acetate is released into the blood and oxidized by peripheral tissues to carbon dioxide and water.
![]() Box 5.8
Box 5.8
The main pathways of ethanol oxidization
 The liver microsomal enzyme oxidizing system (MEOS) including the specific P450 enzyme, CYP2EI, which is induced by ethanol:
The liver microsomal enzyme oxidizing system (MEOS) including the specific P450 enzyme, CYP2EI, which is induced by ethanol:
Alcohol dehydrogenases are found in many tissues and it has been suggested that enzymes present in the gastric mucosa may contribute substantially to ethanol metabolism.
Ethanol itself produces 29.3 kJ/g (7 kcal/g), but many alcoholic drinks also contain sugar, which increases their calorific value. For example, one pint of beer provides about 1045 kJ (250 kcal), so the heavy drinker will be unable to lose weight if he or she continues to drink.
Effects of excess alcohol consumption
Excess consumption of alcohol leads to two major problems, both of which can be present in the same patient:
 Alcohol dependence syndrome (p. 1182)
Alcohol dependence syndrome (p. 1182)
Each unit of alcohol (defined as one half pint of normal beer, one single measure of spirit or one small glass of wine) contains 8 g of ethanol (Fig. 5.14). All the long-term effects of excess alcohol consumption are due to excess ethanol, irrespective of the type of alcoholic beverage, i.e. beer and spirits are no different in their long-term effects. Short-term effects, such as hangovers, depend on additional substances, particularly other alcohols such as isoamyl alcohol, which are known as congeners. Brandy and bourbon contain the highest percentage of congeners.
The amount of alcohol that produces damage varies and not everyone who drinks heavily will suffer physical damage. For example, only 20% of people who drink heavily develop cirrhosis of the liver. The effect of alcohol on different organs of the body is not the same; in some patients, the liver is affected, in others, the brain or muscle. The differences may be genetically determined.
Thiamin deficiency contributes to both neurological (confusion, Wernicke–Korsakoff syndrome; see p. 1091) and some of the non-neurological manifestations (cardiomyopathy). Susceptibility to damage of different organs is variable and the figures given in Box 5.9 are given only as a guide to sensible drinking. Heavy persistent drinkers for many years are at greater risk than heavy sporadic drinkers.
![]() Box 5.9
Box 5.9
Guide to sensible drinking of alcohol
Remember
 Health can be damaged without being ‘drunk’. Regular heavy intake is more harmful than occasional binges
Health can be damaged without being ‘drunk’. Regular heavy intake is more harmful than occasional binges
 Do not drink to ‘drown your sorrows’
Do not drink to ‘drown your sorrows’
 In the UK, the drink-before-driving limit of alcohol in the blood is 800 mg/L (80 mg%)
In the UK, the drink-before-driving limit of alcohol in the blood is 800 mg/L (80 mg%)
 One unit of alcohol is eliminated per hour, therefore spread drinking time
One unit of alcohol is eliminated per hour, therefore spread drinking time
 Food decreases absorption and therefore results in a lower blood alcohol level
Food decreases absorption and therefore results in a lower blood alcohol level
 4–5 units are sufficient to put the blood alcohol level over the legal driving limit in a 70 kg man (less in a lighter person).
4–5 units are sufficient to put the blood alcohol level over the legal driving limit in a 70 kg man (less in a lighter person).
Liver disease
In general, the effects of a given intake of alcohol seem to be worse in women. The following figures are for men and should be reduced by 50% for women:
Alcohol consumption in pregnancy
Women are advised not to drink alcohol at all during pregnancy because even small amounts of alcohol consumed can lead to ‘small babies’. The fetal alcohol syndrome is characterized by mental retardation, dysmorphic features and growth impairment; it occurs in fetuses of alcohol-dependent women.
A summary of the physical effects of alcohol is given in Table 5.20. Details of these diseases are discussed in the relevant chapters. The effects of alcohol withdrawal are discussed on page 1183.
Table 5.20 Physical effects of excess alcohol consumption
|
|
FURTHER READING
Braglehole R, Bonita R. Alcohol and global health priority. Lancet 2009; 373:2173–2174.
Casswell S, Thamarangsi T. Alchohol and global health 3. Reducing harm from alcohol: call to action. Lancet 2009; 373:2247–2257.
Guo R, Ren J. Alcohol and acetaldehyde in public health: from marvel to menace. International Journal of Environmental Research and Public Health 2010; 7:1285–1301.
Schuckit A. Alcohol use disorders. Lancet 2009; 373:492–501.
Ahima RS. Nutrition, obesity and metabolism. Gastroenterology (Special Issue). 2007;132:2085–2275.
Gibney MJ, Elia M, Ljungqvist O, et al. Clinical Nutrition. Oxford: Blackwell Science; 2006.
National Institute for Health and Clinical Excellence. Nutrition support in adults. Clinical Guideline. 2006;32. www.nice.org.uk
National Institute for Health and Clinical Excellence. Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. Clinical Guideline 2006;43.www.nice.org.uk
UK information on food composition and dietary surveys.
http://www.who.int/nutgrowthdb/
World Health Organization site, provides information on worldwide nutritional issues, resources and research
WHO recommendations and intervention programmes for nutrient-related diseases.
Food and Agriculture Organization (FAO) – autonomous body within the United Nations, aims to improve health through nutrition and agricultural productivity, especially in rural populations.
International Food Information Council (IFIC) – non-profit organization providing access to health and nutrition resources to improve communication of health and nutrition information to consumers.
http://www.ama-assn.org/ama/pub/category/10931.html
American Medical Association: Assessment and management of adult obesity
http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/profmats.htm
National Heart, Lung and Blood Institute: Aim for a healthy weight
Health Development Agency: Management of obesity and overweight
American Journal of Clinical Nutrition
International Journal of Obesity
http://www.nutritionsociety.org/publications/nutrition-society-journals/british-journal-of-nutrition
http://www.nutritioncare.org/wcontent.aspx?id=172
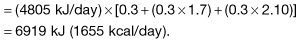
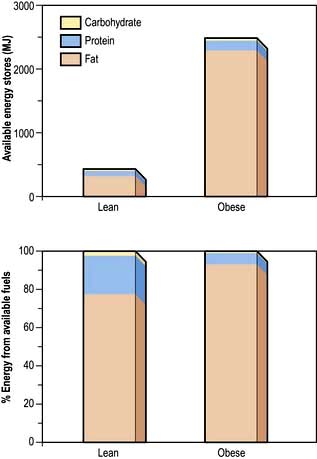
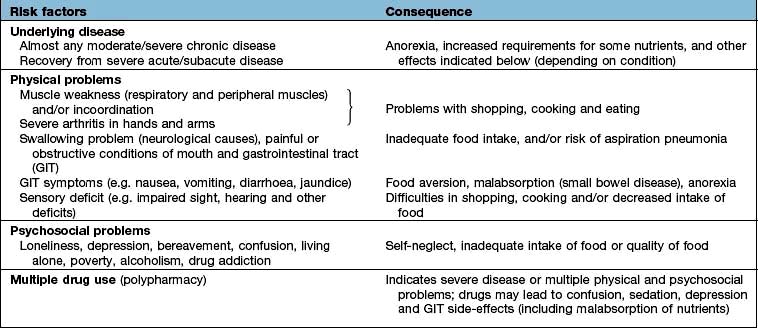
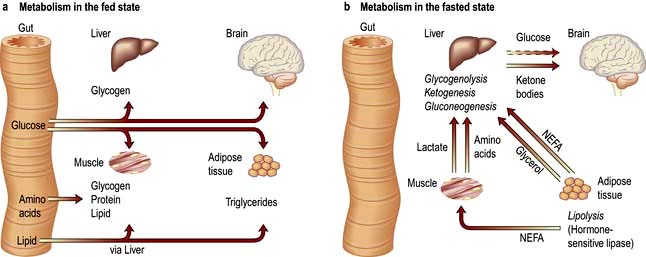
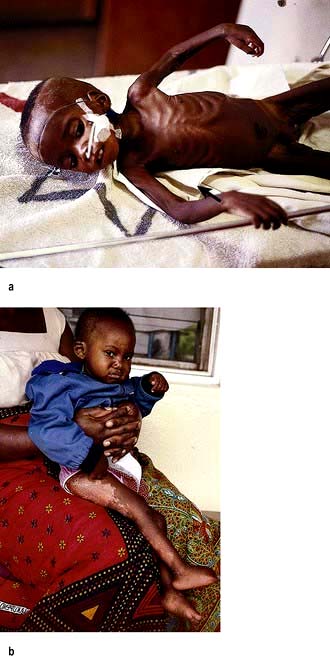
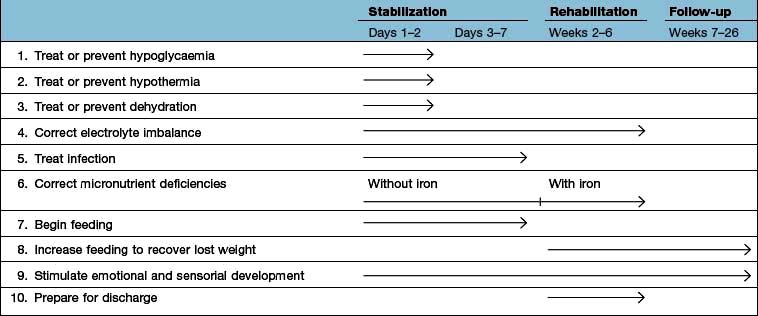
 corneal surface
corneal surface corneal surface
corneal surface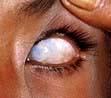
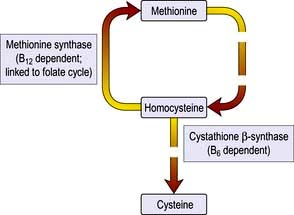
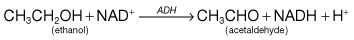 [1]
[1]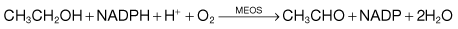 [2]
[2]