Myocardium
See the discussion on the myocardium in the section on Responses to Injury.
Disturbances of Growth
Developmental Errors: Congenital Anomalies: The complex events involved in the embryologic development of the heart and great vessels allow substantial opportunities for congenital anomalies to develop (see Fig. 10-1). The functional significance of these anomalies varies widely. Animals with the most extreme defects are unable to survive in utero, and those with the mildest lesions could have no clinical signs of disease during life. However, animals with defects of intermediate severity are most likely to be presented to a veterinarian because of gradually developing signs of cardiac failure, including poor exercise tolerance, cyanosis, and stunted body growth. Ectopia cordis is a congenital development of the heart at an abnormal site outside of the thoracic cavity. In cattle, cases in healthy adult animals have been described in which the heart was located subcutaneously in the caudoventral neck area. The most frequently observed cardiovascular anomalies in domestic animals are listed in Box 10-11.
The causes of congenital cardiovascular anomalies are varied. Most animal species have a low background frequency of spontaneous cardiac malformations. In many species, especially in dogs, these defects are heritable and can be attributed to either single or multiple gene effects. Under experimental conditions, cardiovascular congenital defects can be elicited by exposure of pregnant dams to various chemicals and drugs, physical agents, toxins, or nutritional deficiencies. Chemical compounds implicated include thalidomide, ethanol, salicylates, griseofulvin, and cortisone. Prenatal exposure to x-irradiation or fetal hypoxia can induce defects. Maternal nutritional deficiencies of vitamin A, pantothenic acid, riboflavin, or zinc and excess intake of vitamin A, retinoic acid, or copper can result in cardiovascular anomalies in newborn animals. Infectious diseases have been incriminated, but not confirmed, in cardiovascular defects; they include bluetongue infections in sheep, bovine virus diarrhea in cattle, and parvoviral infections in dogs and cats.
Sites of the major cardiovascular anomalies in the dog are shown in Fig. 10-46.
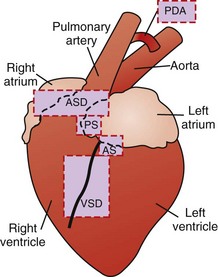
Fig. 10-46 Schematic diagram of the sites of the major cardiovascular anomalies of the dog.
AS, Aortic stenosis; ASD, atrial septal defect; PDA, patent ductus arteriosus; PS, pulmonic stenosis; VSD, ventricular septal defect. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Failure of Closure of Fetal Cardiovascular Shunts:
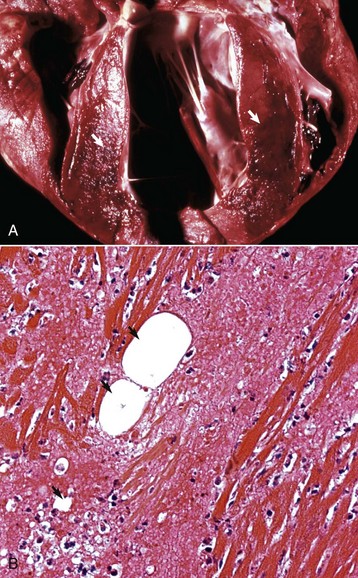
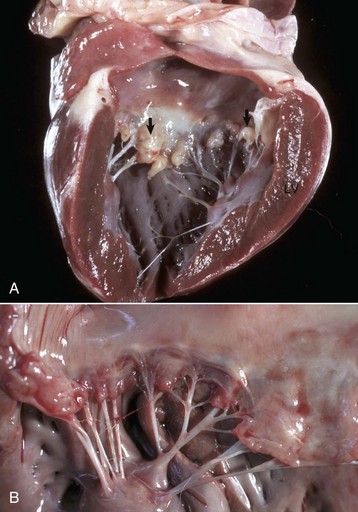
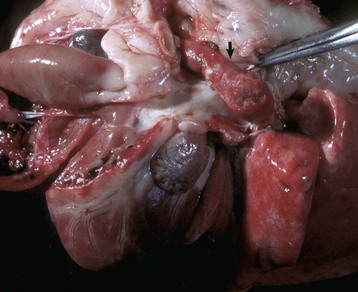
Interventricular Septal Defect: A ventricular septal defect indicates failure of complete development of the interventricular septum and allows the shunting of blood between the ventricles (Fig. 10-47). The defect occurs in many species and more commonly in the upper, membranous portion of the interventricular septum rather than in the lower, muscular septum. Among breeds of dogs, the greatest frequency has been observed in the English bulldog, English springer spaniel, and West Highland white terrier.
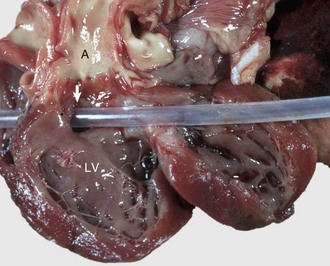
Fig. 10-47 Ventricular septal defect (high defect), heart, opened left side, calf.
Note the large opening in the basal portion of the ventricular septum (arrow) immediately below the aortic valve through which the tube has been passed. A, Aorta; LV, left ventricle. (Courtesy Dr. M. D. McGavin, College of Veterinary Medicine, University of Tennessee.)
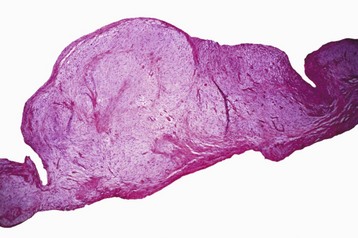
Atrial Septal Defect: An atrial septal defect could represent the failure of closure of the foramen ovale, which is an interatrial septal shunt that allows blood to bypass the lungs of the fetus, or it can be the result of true septal defects at another site because of faulty development of the interatrial septum (Fig. 10-48). Although this defect occurs in all domestic animal species, dog breeds with greatest frequency of this defect are the Boxer, Doberman pinscher, and Samoyed.
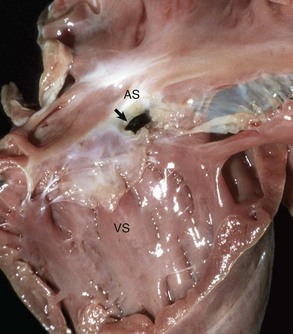
Fig. 10-48 Endocardial cushion defect and tricuspid dysplasia, heart, opened right side, pig.
The endocardial cushion defect (prominent opening [arrow]) can be mistaken as an atrial septal defect but not the location and presence of abnormal valves incorporated in the defect. AS, Atrial septum; VS, Ventricular septum. (Courtesy School of Veterinary Medicine, Purdue University.)
Tetralogy of Fallot: Tetralogy of Fallot is a complicated cardiac anomaly with four lesions (Fig. 10-49). The three primary defects are a ventricular septal defect located high in the septum, pulmonic stenosis (see later discussion), and dextroposition of the aorta (see later discussion). The fourth defect, which develops secondarily, is hypertrophy of the right ventricular myocardium. This complex anomaly is inherited in Keeshond dogs and is frequent in English bulldogs. Cyanosis is often an associated clinical sign. The anomaly is one of the most common cardiac abnormalities seen in hearts of humans (so-called blue babies). In genetic and pathologic studies of Keeshond dogs, the basic defect has been determined to be hypoplasia and malpositioning of the conotruncal septum. Wide variability in the severity of the lesions has been observed. The inheritance pattern in Keeshonds is a simple autosomal locus with partial penetration in heterozygotes and complete penetrance in homozygotes.
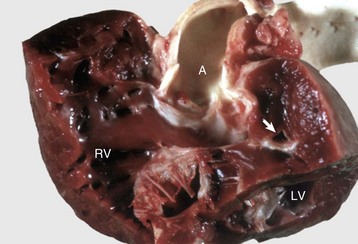
Fig. 10-49 Tetralogy of Fallot, heart, dissected, dog.
Above the large membranous ventricular septal defect is an overlying, straddling aorta (A). There is also severe pulmonic stenosis (arrow) with massive right ventricular hypertrophy. LV, Left ventricle; RV, right ventricle. (Courtesy School of Veterinary Medicine, Purdue University.)
Cardiomyopathies: Primary and secondary cardiomyopathies (Box 10-12) represent important, sometimes inherited, generalized myocardial diseases of either idiopathic or known causation that can result in either hypertrophy or atrophy of the affected myocardium. Primary or idiopathic cardiomyopathies are progressive cardiac diseases. These diseases affect cats, dogs, and cattle and resemble some diseases of humans. These diseases are divided into three morphologic types: hypertrophic, dilated, and restrictive cardiomyopathies. Secondary cardiomyopathies (also termed specific heart muscle diseases) are generalized myocardial diseases of known cause.
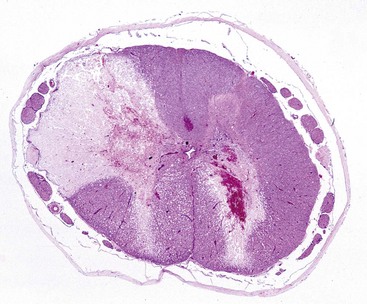
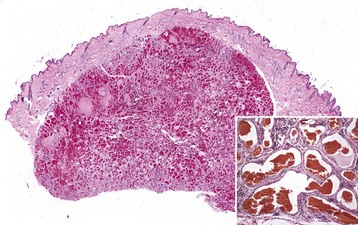
Hypertrophic cardiomyopathy (hypertrophy): Hypertrophic cardiomyopathy occurs frequently in cats, especially in young adult to middle-aged males (1 to 3 years old), and is seen infrequently in dogs, usually affecting males of large breeds. Cats usually have congestive heart failure, and approximately 10% to 20% have posterior paresis from concurrent thromboembolism of the caudal abdominal aorta (“saddle thrombosis”) secondary to left atrial thrombosis. Hypertrophic cardiomyopathy is inherited as an autosomal dominant trait with complete penetrance in Maine coon cats. The occurrence of clusters of cases in Persians, American shorthairs, and ragdoll cat breeds strongly suggests heritability of the disease in some cases. Some cats and dogs die unexpectedly as the only clinical expression of the disease. This clinical presentation also is often seen in humans with the disease. In both cats and dogs, the hearts are enlarged and have prominent hypertrophy of the left ventricle and interventricular septum (Fig. 10-50). The left ventricular cavity is small and the left atrium is dilated. In a few cases, the interventricular septum is disproportionately hypertrophied in relation to the remainder of the myocardium. Microscopically, the lesions of the myocardium are prominent disarrays or disorganizations of myocytes, with interweaving rather than parallel arrangement of fibers (Fig. 10-51; Web Fig. 10-20). Myocyte hypertrophy, various degenerative alterations in myocytes, and interstitial fibrosis also are present.
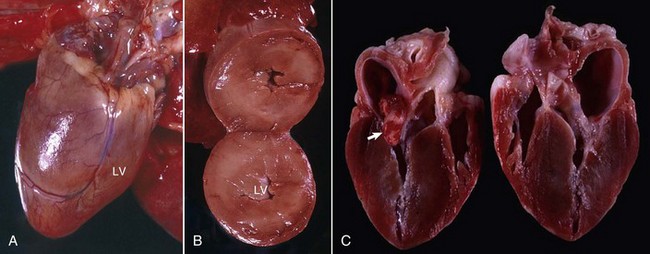
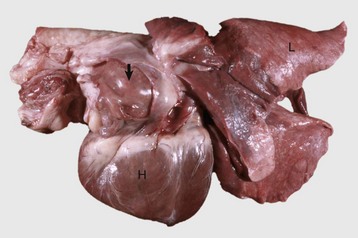
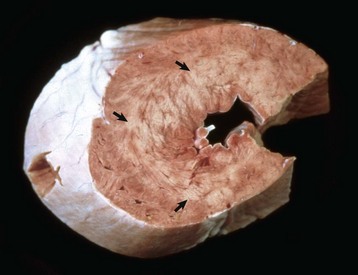
Fig. 10-50 Hypertrophic cardiomyopathy, heart, cat.
A, Note the thickened left ventricular wall (LV). B, The thickened left ventricular free wall and septum have markedly reduced the lumen of the left ventricle (LV). C, There is severe diffuse concentric hypertrophy of the left ventricular free wall, interventricular septum, and papillary muscles. Left atrial dilation is present. There is a fibrotic plaque (arrow) of the midinterventricular septum where the hypertrophied papillary muscle contacts the septum. (A and B courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Ettinger SJ, Feldman EC (eds): Textbook of veterinary internal medicine. Diseases of the dog and cat, vol 2, ed 7, Philadelphia, 2010, Saunders.)
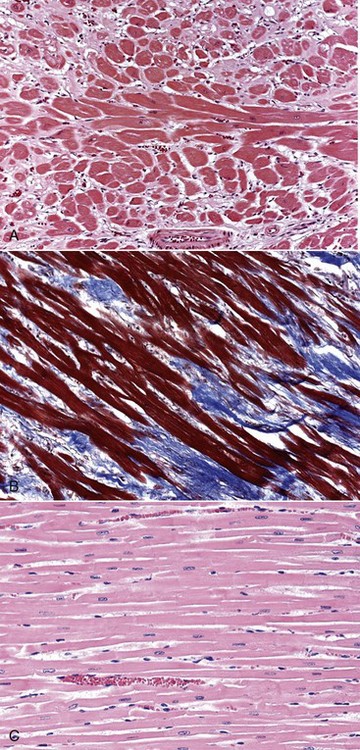
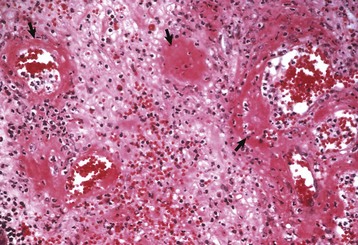
Fig. 10-51 Hypertrophic cardiomyopathy, heart, ventricular myocardium, cat.
A, Cardiac myocytes are hypertrophied and in disarray. H&E stain. B, Masson’s trichrome stain demonstrates abundant amounts of interstitial collagen (blue) produced by fibroblasts. C, Normal cardiac myocytes arranged in parallel bundles. H&E stain. (A and B courtesy Atlantic Veterinary College, University of Prince Edward Island. C courtesy Dr. L. Borst, College of Veterinary Medicine.)
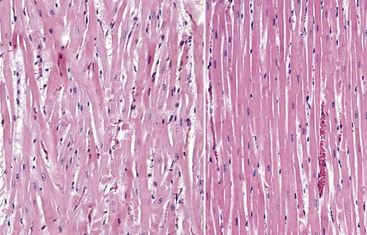
Web Fig. 10-20 Hypertrophic cardiomyopathy, heart, ventricular myocardium, cat.
A, Note the pattern of interwoven cardiac myocytes, indicating myofiber disarray, and the hypertrophic myocytes (compare with Figs. 10-20 and 10-51). The number of fibroblasts is increased in the interstitium. H&E stain. B, Normal cardiac myocytes arranged in parallel bundles. (Courtesy Dr. L Borst, College of Veterinary Medicine, University of Illinois.)
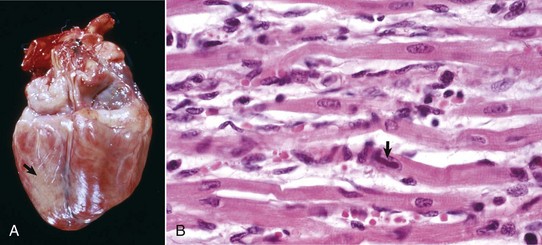
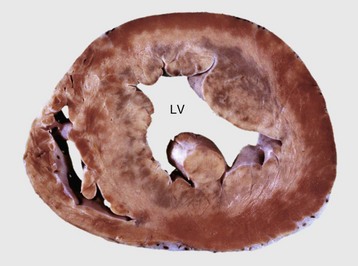
Dilated or congestive cardiomyopathy: Dilated or congestive cardiomyopathy is an important cause of congestive heart failure in cats and dogs. Many affected cats and some dogs have low tissue concentrations of taurine, and supplementation of cats with taurine has reversed the clinical signs of cardiac failure. Routine taurine supplementation of feline commercial diets has resulted in a dramatic reduction in cases of dilated cardiomyopathy. Taurine-deficient foxes also develop cardiac failure. Cattle with dilated cardiomyopathy in Switzerland and Japan have an autosomal recessive mode of inheritance.
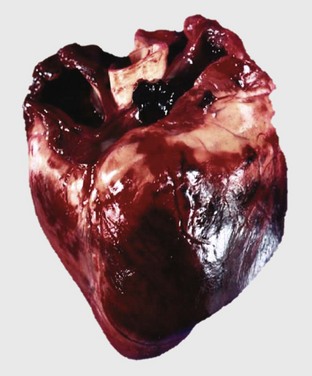
Affected cats often are middle-aged males, and affected dogs often are males of large breeds such as Doberman pinschers, Portuguese water dogs, dalmatians, Scottish deerhounds, Irish wolfhounds, Saint Bernards, Afghan hounds, Newfoundland dogs, Old English sheepdogs, Great Danes, and boxers, although smaller breeds, such as English cocker spaniels, may be affected. The disease often has a familial pattern in the affected breeds and appears to be inherited as an autosomal recessive or X-linked recessive trait. Some cats also develop aortic thromboembolism. At necropsy, lesions of congestive heart failure are present and the hearts are rounded because of biventricular dilation (Figs. 10-52 and 10-53). The dilated cardiac chambers often have a diffusely white, thickened endocardium. Microscopic and ultrastructural alterations are nonspecific, can be either mild or absent, and may include interstitial fibrosis and fatty infiltration and changes of myocyte degeneration, including the occurrence of so-called attenuated wavy fibers.
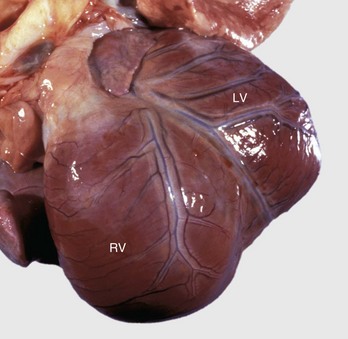
Fig. 10-52 Dilated (congestive) cardiomyopathy, heart, left ventricle (LV) and right ventricle (RV), dog.
Biventricular dilation has resulted in the heart having a double apex. (Courtesy Dr. T. Boosinger, College of Veterinary Medicine, Auburn University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
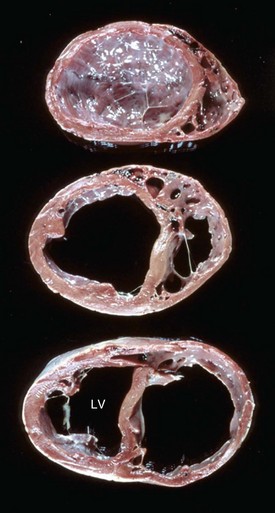
Fig. 10-53 Dilated (congestive) cardiomyopathy, heart, ventricles, cross-section, dog.
The left ventricle (LV) and right ventricle have thin walls, dilated chambers, and white fibrotic endocardium. (Courtesy Dr. Y. Niyo, College of Veterinary Medicine, Iowa State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Restrictive cardiomyopathy: Restrictive cardiomyopathy occurs infrequently. It occurs in cats as one of two types of endocardial lesions that result in impaired ventricular filling. In one type, the left ventricular endocardium has diffuse notable fibrosis. Available evidence suggests that the fibrotic lesion is preceded by endomyocarditis. The second type results from excessive moderator bands that traverse the left ventricular cavity. Other examples of restrictive cardiomyopathy in animals include endocardial fibrosis in certain strains of aged rats and congenital endocardial fibroelastosis in Burmese cats (Fig. 10-54).
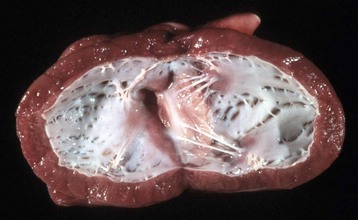
Fig. 10-54 Subendocardial fibroelastosis, heart, left ventricle, dog.
The endocardium is opaque because increased amounts of collagen and elastic fibers were deposited in the subendocardium secondary to turbulence of blood flow within the ventricles. This dog had a persistent ductus arteriosus. This lesion may have a hereditary basis in Burmese cats and is often a sequela to turbulence within ventricles in cardiac disease. (Courtesy College of Veterinary Medicine, University of Illinois.)
Molecular mechanisms of hereditary cardiomyopathies: Our understanding of the molecular mechanisms of the hereditary cardiomyopathies is developing rapidly. In humans with familial hypertrophic cardiomyopathy inherited in an autosomal dominant manner, a variety of single-gene mutations have been documented. The mutations affect genes that encode sarcomere proteins of cardiac myocytes. Altered cardiac proteins include cardiac α- and β-myosin heavy chain, cardiac troponin T and I, and Ca-tropomyosin, actin, titin, ventricular myosin essential light chain, ventricular myosin regulatory light chain, and cardiac myosin-binding protein C. It remains unclear how these mutant proteins result in functional and structural alterations of cardiac muscle cells. However, recent studies suggest that shortening of telomeres (structures that cap the ends of chromosomes) triggers apoptosis of cardiac muscle cells and may explain the end-stage finding of myocardial fibrosis in heart failure of various causes, including cardiomyopathy. Some similar gene mutations and altered proteins were recently discovered in the various heritable cardiomyopathies of animals (e.g. cardiac myosin-binding protein C in Maine Coon cats and Ragdoll cats with hypertrophic cardiomyopathy). Also, a portion of cases of dilated cardiomyopathy in humans appears to be inherited. In these patients, alterations in several myocytic proteins, including dystrophin, actin, desmin, troponin T, β-myosin heavy chain, lamin, and taffazin, and alterations in the cardiac calcium regulating protein phospholamban have been documented.
Neoplastic Transformation: Various primary and secondary neoplasms develop either in or near the heart. Primary neoplasms include rhabdomyoma, rhabdomyosarcoma, schwannoma, and hemangiosarcoma. Rhabdomyomas and rhabdomyosarcomas are rare in animals and form white to gray nodules in the myocardium that often project into the cardiac chambers. Congenital rhabdomyomatosis in pigs and guinea pigs is a nonneoplastic hamartoma (i.e., malformation often resembling a neoplasm that is composed of an overgrowth of mature cells and tissues that normally occur in the affected organ). Single or multiple, pale, poorly circumscribed areas are scattered in the myocardium and are composed of large glycogen-laden myocytes.
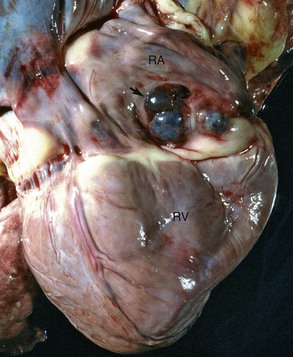
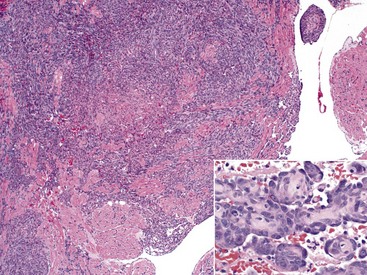
Malignant lymphoma (lymphosarcoma) is the most common secondary neoplasm occurring in the heart and often causes lesions in the hearts of cattle, which can be severe enough to cause death from cardiac failure. Cardiac lesions may be present in dogs and cats with malignant lymphoma. The neoplastic cell infiltration can be diffuse or nodular and involve the myocardium, endocardium, and pericardium. Lymphomatous tissue appears as white masses that may resemble deposits of fat (Fig. 10-55). Microscopically, extensive infiltrations of neoplastic lymphocytes are present between myocytes (Fig. 10-56). Other neoplasms, such as malignant melanomas, occasionally have metastatic lesions in the heart.
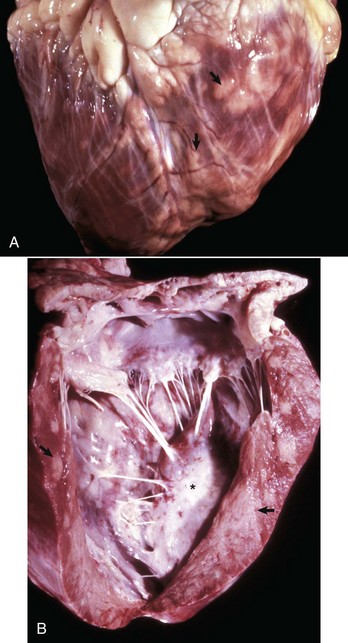
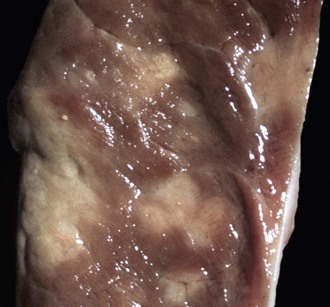
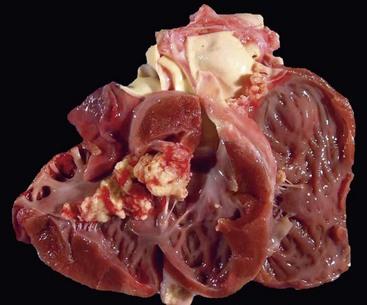
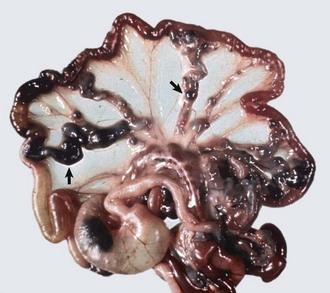
Fig. 10-55 Lymphosarcoma, heart, myocardium, cow.
A, Sites of infiltrating neoplastic lymphocytes in the ventricular myocardium are evident as numerous white areas and nodules (arrows). B, Similar white areas of tumor are visible in the section of the left ventricular wall (arrows) and subendocardially (asterisk) in the ventricular septum. (Courtesy College of Veterinary Medicine, University of Illinois.)
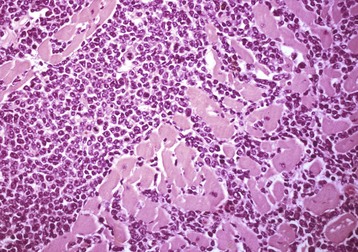
Fig. 10-56 Lymphosarcoma, heart, section of myocardium, cow.
Neoplastic lymphocytes have extensively infiltrated between the cardiomyocytes. Extensive infiltration can result in myocyte atrophy and loss. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
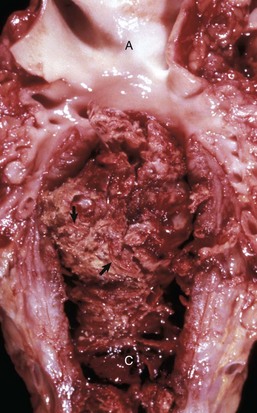
Heart-based tumors are primary neoplasms of extracardiac tissues in dogs and rarely cats. They arise at the base of the heart and can produce vascular obstruction and cardiac failure. The most common neoplasm arising at this location is the aortic body tumor or paraganglioma (chemodectoma), but occasionally, ectopic thyroid or parathyroid tissue gives origin to neoplasms in this area. The aortic body is a chemoreceptor organ. In some cases, aortic body tumors become large, white, firm masses that surround and compress the great vessels and atria (Fig. 10-57). Brachycephalic dog breeds are most frequently affected. Microscopically, the neoplastic cells are polyhedral with vacuolated cytoplasm and are supported by a fine connective tissue stroma (see Fig. 12-55).
Cell Degeneration and Death
Toxicoses: The mechanisms of cardiotoxicities include (1) exaggerated pharmacologic action of drugs acting on cardiovascular tissues, (2) exposure to substances that depress myocardial function, (3) direct injury of cardiac muscle cells by chemicals, and (4) hypersensitivity reactions.
White snakeroot–induced myocardial degeneration: See the discussion on white snakeroot–induced myocardial degeneration in the section on Disorders of Horses.
Ionophore-induced myocardial degeneration: See the discussion on ionophore-induced myocardial degeneration in the section on Disorders of Horses.
Gossypol-induced myocardial degeneration: See the discussion on gossypol-induced myocardial degeneration in the section on Disorders of Ruminants.
Chemotherapeutic agent-induced myocardial degeneration: Cardiotoxicity has emerged as a significant clinical entity in veterinary medicine in recent years with the growing use of antineoplastic drugs in small animal practice and the widespread use of growth promotants in ruminants (see Fig. 10-69).
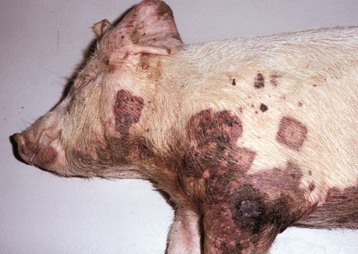
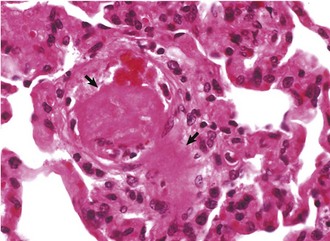
Myocardial Necrosis and Mineralization: Myocardial necrosis and mineralization can result from a number of causes, including nutritional deficiencies, chemical and plant toxins, ischemia, metabolic disorders, heritable diseases, and physical injuries (see Box 10-5). From this large list of causes of myocardial injury, some of the most frequently observed current examples are ionophore toxicity in horses and ruminants, vitamin E–selenium deficiency in the young of all species, “heart-brain syndrome” of dogs (see Fig. 10-83), anthracycline toxicity in dogs, and gossypol toxicosis in pigs. In various localized areas throughout the world, numerous deaths in ruminants have resulted from consumption of poisonous plants such as Acacia georginae and Dichapetalum cymosum.
Mulberry Heart Disease: See the discussion of mulberry heart disease in the section on Disorders of Pigs.
Brain-Heart Syndrome: See the discussion on brain-heart syndrome in Chapter 14.
Inflammation
Myocarditis: See the discussion on inflammation in the section on Myocardium, Responses to Injury.
Bacterial Septicemias: See the discussion on bacterial septicemias in Chapter 4.
Cardiac Conduction System
See the discussion on the cardiac conduction system in the section on Responses to Injury.
Disturbances of Growth
Schwannomas: Schwannomas involve cardiac nerves in cattle and appear as single or multiple white nodules detected as incidental findings at slaughter (see Fig. 14-115).
Cell Degeneration and Death
Conduction system diseases have been described mainly in dogs and horses, probably because clinical cardiac evaluations are done most frequently in these species. Secondary conduction system disorders result from myocardial disease (inflammation, neoplasia, or degeneration) near the conduction system. Specific presumably inherited diseases in dogs include (1) syncope in pug dogs with lesions of the bundle of His; (2) intermittent sinus arrest in deaf dalmatian dogs, presumably associated with lesions in the sinus node; (3) sinoatrial syncope (sick sinus dysfunction) in female miniature schnauzers, West Highland white terriers, cocker spaniels, and dachshunds; (4) inherited ventricular arrhythmia and sudden unexpected death in German shepherds, and (5) widespread conduction pathology in Alaskan sled dogs that died suddenly and unexpectedly in a race. Other arrhythmias in dogs and horses are atrial fibrillation and heart block. Dogs with atrial fibrillation often have concurrent congestive heart failure and have atrial dilation with AV valve insufficiency, but most horses live a normal or near-normal lifespan, may respond to cardioversion, and at necropsy have atrial myocardial fibrosis. Heart block of the first degree (delay of impulse through AV node), second degree (intermittent failure to conduct through the AV node with dropped beats), and third degree (complete) has been associated with myocardial lesions, such as areas of scarring, in horses and dogs. Second-degree heart block is considered to be a normal phenomenon in horses.
Persistent atrial standstill (silent atria, AV myopathy) is a progressive cardiac disease of English springer spaniels and cats characterized by notable atrial dilation and fibrosis.
Atrial fibrillation occurs in cattle in association with right atrial dilation and fibrosis and alterations in the SA mode. Also, sudden (unexpected) cardiac death is described in racehorses with right atrial myocardial fibrosis, fibrosis of the upper ventricular septum, and arteriosclerosis of intramyocardial arteries.
Endocardium and Heart Valves
See the discussion of the endocardium and heart valves in the section on Responses to Injury. The major types of AV valvular diseases are shown in Fig. 10-58.
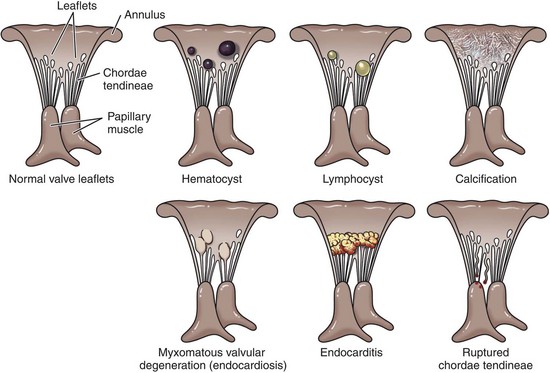
Fig. 10-58 Schematic diagram of the major types of cardiac atrioventricular valvular disease. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Disturbances of Growth
Developmental Errors: Congenital Anomalies:
Failure of Normal Valvular Development:
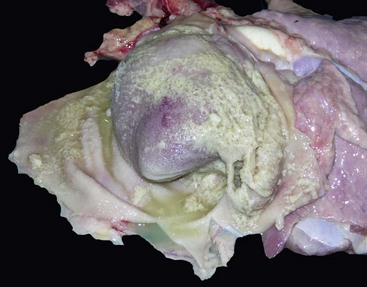
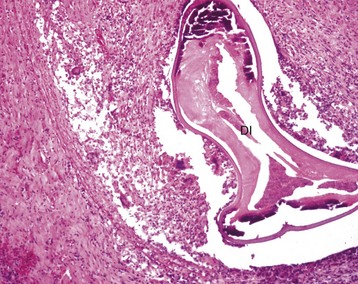
Pulmonic stenosis: Pulmonic stenosis has been recognized as a frequently occurring anomaly in dogs and is inherited in the beagle (Fig. 10-59). Other breeds in which this lesion is frequent are basset hound, boxer, Chihuahua, Chow chow, cocker spaniel, English bulldog, Labrador retriever, mastiff, Newfoundland, Samoyed, schnauzer, and terrier. Several types of valvular lesions have been described and include formation of a circumferential band of fibrous or muscular tissue beneath the valve (subvalvular stenosis) or malformation of the valve (valvular stenosis), with a small central orifice in a dome of thickened valvular tissue. Notable concentric hypertrophy (see the discussion on hypertrophy in the section on Disturbances of Growth, Myocardium, Responses to Injury) of the right ventricle develops from the resulting pressure overload.
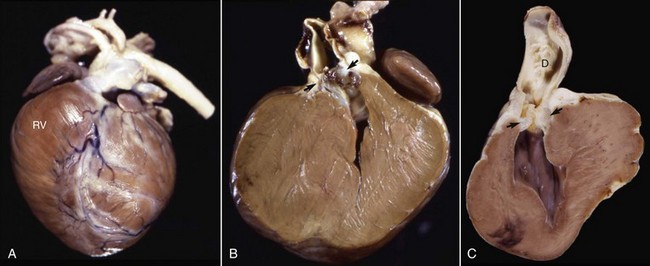
Fig. 10-59 Pulmonic stenosis, heart, pulmonary artery, dogs.
A, Closed heart, and B, sectioned heart. Note the prominent concentric right ventricular (RV) hypertrophy resulting from pressure overload. The orifice of the pulmonic valve (arrows) is markedly narrowed. C, sectioned heart, there is poststenotic dilation (D) of the pulmonary artery with irregular intimal thickenings (jet lesions). (Courtesy Atlantic Veterinary College, University of Prince Edward Island.)
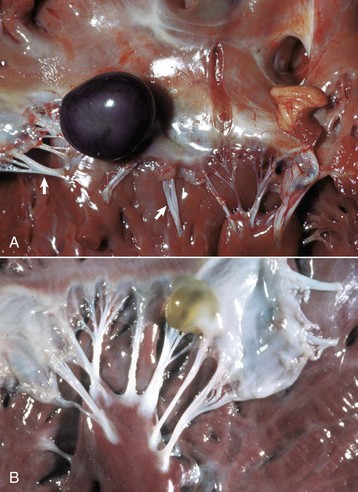
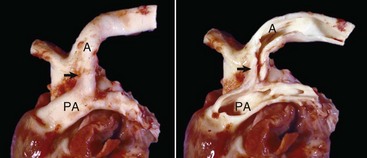
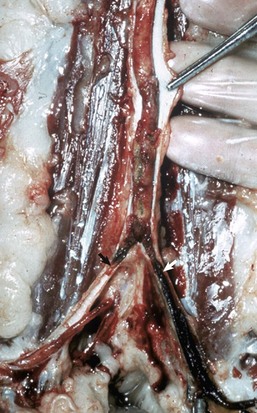
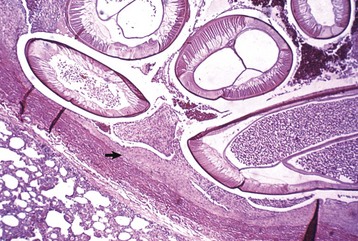
Aortic and subaortic stenoses: True stenoses of the aortic valve are uncommon. Subaortic stenosis is a cardiac anomaly frequently observed in pigs and dogs. It apparently is inherited in Newfoundland, boxer, and German shepherd dogs (Fig. 10-60). The lesion is also observed in the German shorthair pointer, golden retriever, Great Dane, Rottweiler, Samoyed, and bull terrier breeds. In clinical cases, the stenosis is produced by the presence of a thick zone of endocardial fibrous tissue that encircles the left ventricular outflow tract below the valve. In mild cases, often subclinical, the lesion is limited to white nodules on the ventricular septum immediately below the valve. Microscopically, the altered endocardial tissue can contain proliferated mesenchymal cells, mucinous ground substance, and foci of metaplastic cartilage. Other cardiac lesions develop as a result of the altered left ventricular outflow; these include left ventricular concentric hypertrophy, disseminated foci of myocardial necrosis, fibrosis in the inner left ventricular wall, and thickening of the walls of intramyocardial arteries.
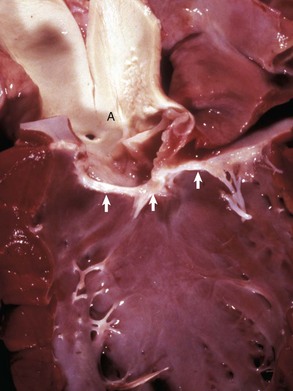
Fig. 10-60 Subaortic stenosis, heart, opened left side, dog.
A thick, white, broad band of fibrous connective tissue (arrows) encircles the left ventricular outflow tract below the aortic valve. The force of the blood ejected through the stenotic lesion is responsible for the “jet lesions” in the overlying aorta (A) (right half: roughened surface; left half: dilation [note the gray area]). (Courtesy College of Veterinary Medicine, University of Illinois.)
Valvular dysplasias: endocardial cushion defects: Other valvular developmental anomalies include endocardial cushion defects (persistent AV canal) in pigs, sheep, and cats; mitral dysplasia in cats and dogs; and tricuspid dysplasia in cats and dogs (see Fig. 10-48). Tricuspid dysplasia is inherited as an autosomal dominant trait with reduced penetrance in Labrador retrievers. The tricuspid valve is abnormal with shortened and thickened chordae tendineae resulting in an incompetent valve and right-sided heart failure.
Endocardial fibroelastosis: Endocardial fibroelastosis in animals was historically recognized as a primary cardiac defect in Burmese and Siamese cats. Affected animals had prominent, white, thickened endocardium, especially of the left ventricle, because of the proliferation of fibroelastic tissue (see Fig. 10-54). Endocardial fibroelastosis is a reaction of the endocardium to hypoxia and often associated with heart disease, which results in dilated cardiac chambers. It is unclear whether this is a true congenital anomaly or a response to left atrial dilation.
Valvular hematomas: See the discussion on valvular hematomas in the section on Disorders of Ruminants.
Valvular lymphocysts: See the discussion on valvular lymphocysts in the section on Disorders of Ruminants.
Cell Degeneration and Death
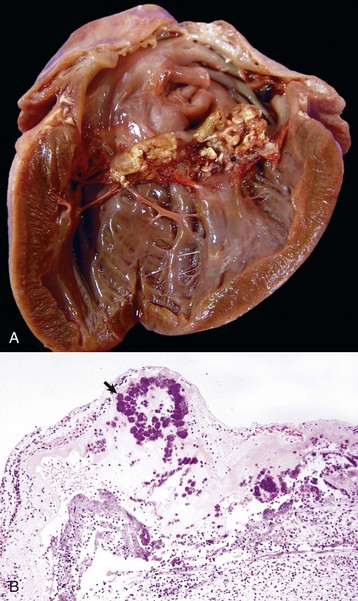
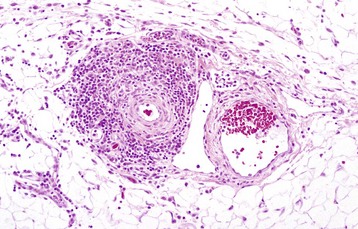
Uremic Endocarditis: Uremic endocarditis is most commonly a disorder in dogs that follows acute or repeated episodes of uremia. These episodes cause ulcerative endocarditis (injury of the endothelium) of the left atrium that is resolved via healing characterized by fibrosis, with or without mineralization and chronically dilated atria (Fig. 10-61).
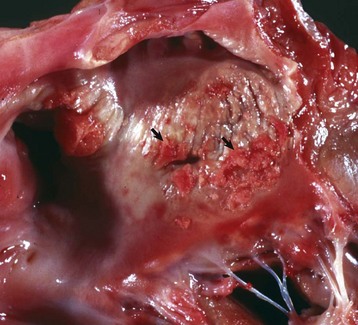
Fig. 10-61 Ulcerative endocarditis (uremia), heart, endocardium of left atrium, dog.
Note the white-red, thick, wrinkled area (arrows) of endocarditis, mineralization, and fibrous tissue (scar) formation caused by uremia in this dog with chronic renal failure. (Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Myxomatous Valvular Degeneration (Valvular Endocardiosis): Degenerative changes in the valves are frequently seen in older dogs and the process that leads to this lesion is termed myxomatous valvular degeneration (valvular endocardiosis). Also see the section on Disorders of Dogs; also see Fig. 10-84).
Endocardial Mineralization: Endocardial mineralization occurs from intake of excessive amounts of vitamin D and from intoxication by calcinogenic plants (Cestrum diurnum, Trisetum flavescens, Solanum malacoxylon, Solanum torvum) that contain vitamin D analogs. These plant-induced syndromes of cattle have been called by different names in various areas of the world, such as “Manchester wasting disease” in Jamaica, “enzootic calcinosis” in Europe, “Naalehu disease” in Hawaii, “enteque seco” in Argentina, and “espichamento” in Brazil. Multiple, large, white, rough, firm plaques of mineralized fibroelastic tissue are present in the endocardium and intima of large elastic arteries. Fibrosis, with or without mineralization, occurs in chronically dilated hearts, in hearts of debilitated cattle with Johne’s disease (see Figs. 10-32 and 10-39), in dogs with healed lesions of left atrial ulcerative endocarditis associated with a prior uremic episode (see Fig. 10-61), and in the so-called jet lesions produced by the trauma of refluxed blood in valvular insufficiencies.
Inflammation
Valvular Endocarditis: See the discussion on vegetative valvular and mural endocarditis in the section on Inflammation, Endocardium, Responses to Injury, as well as the discussion on valvular endocarditis in Chapter 3.
Blood and Lymphatic Vascular Systems
See the discussion on blood and lymphatic vascular systems in the section on Responses to Injury. The major arterial diseases are shown in Fig. 10-62.
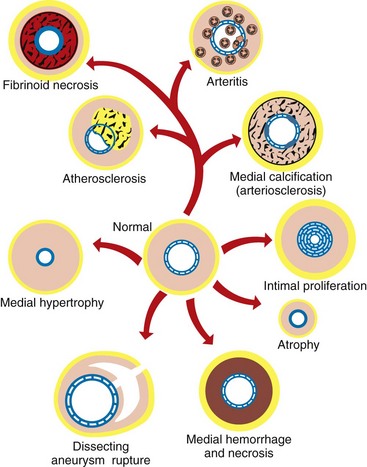
Fig. 10-62 Schematic diagram of the major arterial diseases. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Blood Vessels
Effusions: See the discussion on effusions in the section on Disturbances of Circulation, Pericardium and Epicardium, Responses to Injury and Disorders of Domestic Animals.
Aortic rupture and rupture of large arteries: Aortic rupture and rupture of large arteries can be the sequela of severe trauma or occur spontaneously (see Figs. 10-34 and 10-35). Sudden rupture of the ascending aorta or pulmonary artery near the pulmonic valve in horses is associated with notable exertion and severe trauma to the ventral thorax from falling. Death ensues rapidly from cardiac tamponade, as the tear is in that portion of the aorta or pulmonary artery within the pericardial sac. In horses, the internal carotid artery can rupture into the adjacent guttural pouch, with subsequent epistaxis. This is a consequence of deep mycotic infection of the guttural pouch. Rupture of the middle uterine artery may occur during parturition in mares and with uterine torsion or prolapse in cows. Aortic rupture, with or without dissection, is an important cause of death in male turkeys. The most common vascular diseases with rupture are listed in Box 10-13.
Gastric Dilation and Volvulus: See the discussion on gastric dilation and volvulus in Chapter 7.
Developmental Errors: Congenital Anomalies:
Failure of closure of fetal cardiovascular shunts:
Patent ductus arteriosus: See the discussion of patent ductus arteriosus in the section on Diseases of Dogs.
Portacaval shunts: Portacaval shunts occur in animals, particularly in the dog. The normal flow from the portal vein is diverted, either partially or completely, to the systemic circulation, thus bypassing the liver (Fig. 10-63). Normal hepatic detoxification of portal flow is incomplete and may result in neurologic signs and elevated circulating bile acids. The resulting nervous system syndrome is termed hepatic encephalopathy. Specifically, the shunts represent retained fetal vascular structures, as in persistent ductus venosus, or arise from prominent dilation of various portosystemic shunts that normally are quite small vessels. See Chapter 8 on diseases of the liver for further details.
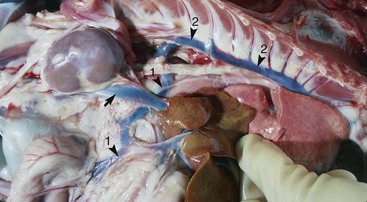
Fig. 10-63 Portacaval shunt, dog.
Note that the branch of the portal vein (arrowhead 1) passes under the caudal vena cava (arrow) and anastomoses with the azygous vein (arrowhead 2). The azygous vein returns the blood to the caudal vena cava near the heart and thus this blood and its ammoniacal and protein metabolites are shunted away from processing to blood urea nitrogen (BUN) in the liver. The liver is normal color but extremely small, which is typical of these types of shunts (see Chapter 8). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Malpositioning of great vessels:
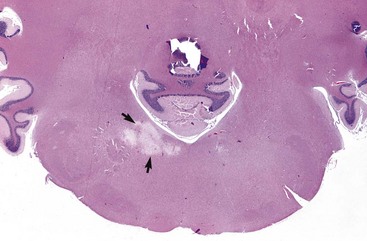
Persistent right aortic arch: Persistent right aortic arch occurs in dogs; German shepherd, Irish setter, and Great Dane dogs are predisposed (Fig. 10-64). This defect arises because the right fourth aortic arch, rather than the normal left fourth aortic arch, develops and ascends on the right side of the midline so that the ligamentum arteriosum forms a vascular ring over the esophagus and trachea. This arrangement eventually results in esophageal obstruction and proximal dilation (megaesophagus), which often results in aspiration pneumonia as the animal matures and consumes solid feed.
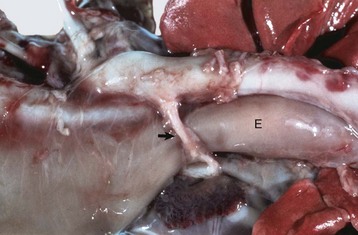
Fig. 10-64 Persistent right aortic arch, ligamentum arteriosum, megaesophagus, calf.
During embryogenesis the aorta was formed from the right aortic arch instead of the left one, thus the aorta is now on the right. For the ligamentum arteriosum (arrow) to connect the aorta with the pulmonary artery, it has to pass dorsally over the esophagus and trachea. The ligamentum, together with the aorta and pulmonary artery, form a vascular ring that constricts the esophagus (E), which is dilated cranial to the constriction. (Courtesy Dr. S. Snyder, College of Veterinary Medicine, Colorado State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Transposition of the aorta and pulmonary artery: Transposition of the aorta and pulmonary artery are severe anomalies, of which there are several types. In complete transposition, the aorta serves as the outflow from the right ventricle and the pulmonary artery is the left ventricle primary outflow. Other congenital anomalies, including ventricular septal defect, often accompany this anomaly.
Vascular Melanosis: See the discussion on vascular melanosis in Chapters 1 and 2.
Neoplastic Transformation: See the discussion on neoplastic transformation in the section on Disorders of Dogs.
Cell Degeneration and Death: Toxicants that affect vessels are listed in Web Box 10-1.
Vitamin E–Selenium Deficiency: See the discussion on vitamin E–selenium deficiency in the section on Disorders of Pigs.
Omphalophlebitis (“Navel Ill”): Omphalophlebitis (“navel ill”) is inflammation of the umbilical vein that often occurs in neonatal farm animals because of bacterial contamination of the umbilicus immediately after parturition. Bacteria from this site can cause septicemia, suppurative polyarthritis, hepatic abscesses (the umbilical vein drains into the liver), and umbilical abscesses.
Jugular Thrombophlebitis: Jugular thrombophlebitis may be associated with indwelling jugular catheters and is reported to be increased with several concurrent disease conditions such as hypoproteinemia, salmonellosis, endotoxemia, and large intestinal disease (see Fig. 10-43).
Lymphatic Vessels
Rupture of the thoracic duct: Rupture of the thoracic duct, either as a result of trauma or from spontaneous disruption, causes chylothorax in dogs and cats (see Fig. 9-101). However, many cases of chylothorax occur without injury to the thoracic duct and have been attributed to lesions that interfere with central venous return or produce obstruction of the thoracic duct (right-sided heart failure, neoplasms, granulomas, cranial vena cava thrombosis, or dirofilariasis) or that are idiopathic.
Developmental Errors: Congenital Anomalies:
Lymphangiectasia: Lymphangiectasia is dilation of lymphatic vessels. The cause may be a congenital anomaly (Fig. 10-65) or caused by obstruction of lymph drainage by invading masses of malignant neoplasms or inflammation (Fig. 10-66).
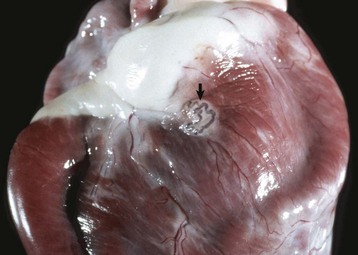
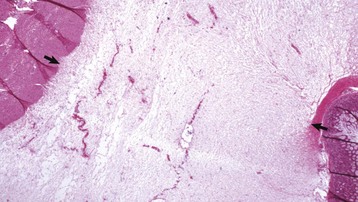
Fig. 10-65 Congenital lymphangiectasia, epicardium, young horse.
Note the tortuous appearance of the epicardial lymphatic vessel (arrow). In congenital lymphangiectasia, lymphatic vessels fail to make connections with other vessels or are obstructed because of anomalous development. (Courtesy College of Veterinary Medicine, University of Illinois.)
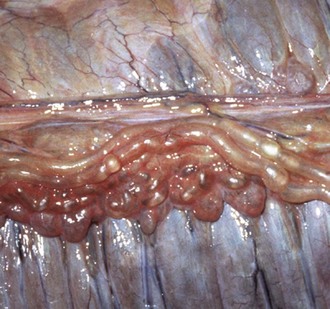
Fig. 10-66 Acquired lymphangiectasia, lymphoma (lymphosarcoma), mesoccolon, horse.
Note the distended lymphatics on the serosal surface of the large colon, the result of impeded lymph flow through the colic lymph nodes, caused by compression of their cortical and medullary sinuses by proliferating neoplastic lymphocytes. (Courtesy College of Veterinary Medicine, University of Illinois.)
Hereditary lymphedema: Hereditary lymphedema has been described in dogs, Ayrshire and Angus calves, and pigs. Affected animals have prominent subcutaneous edema that, in calves, often causes severe swelling of the tips of the ears. Interference with lymph drainage results from defective development of the lymphatic vessels that are aplastic or hypoplastic.
Neoplastic Transformation: Lymphangioma is a rare benign neoplasm composed of lymphatic channels. Lymphangiosarcoma, the malignant counterpart, occurs more often than the benign neoplasm. Vascular spaces formed by neoplastic lymphatic endothelial cells contain lymph rather than blood. Lymphatic vessels are frequently invaded by primary carcinomas and are a common route of metastasis (see the section on Disorders of Dogs).
Inflammation: The most common diseases with thrombosis and embolism are listed in Box 10-14.
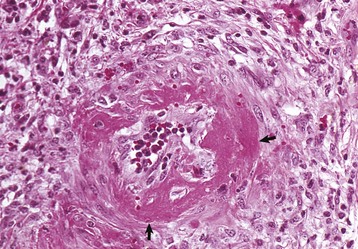
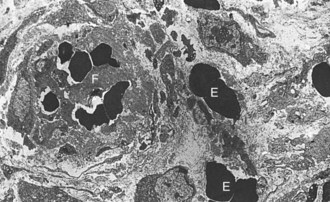
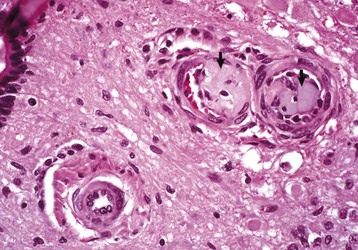
Lymphangitis: Lymphangitis is a feature of many diseases (see Box 10-8). The affected vessels are often located in the distal limbs and are thick, cordlike structures (Fig. 10-67). Lymphedema can be present. Nodular suppurative lesions of lymphangitis often ulcerate and discharge pus onto the surface of the skin. In Johne’s disease, the mesenteric lymphatic vessels are often prominent because of granulomatous lymphangitis, an extension of the enteric infection producing a granulomatous enteritis and lymphangitis (Fig. 10-68).
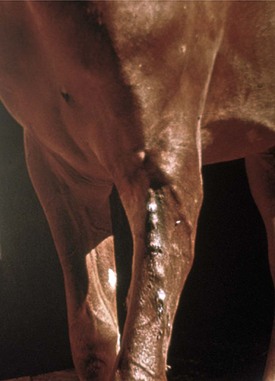
Fig. 10-67 Lymphangitis, forelimb, lymphatic vessels, horse.
Note the multiple swellings (cordlike) of the afferent lymphatics in the skin. These lymphatics lie in the subcutis and empty into the caudal superficial cervical (prescapular) lymph node. (Courtesy School of Veterinary Medicine, Purdue University.)
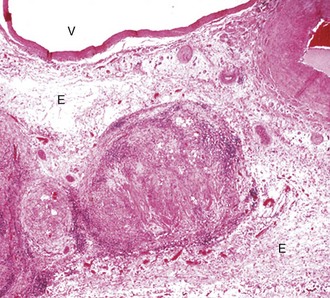
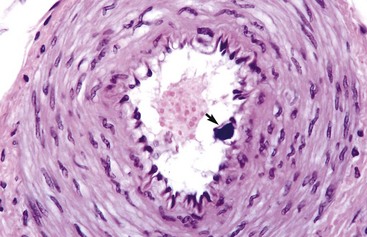
Fig. 10-68 Granulomatous lymphangitis, Johne’s disease, mesenteric lymphatic vessel, sheep.
The lymphatic is occluded by a fibrinous thrombus secondary to the destruction of the endothelium by inflammatory cells including macrophages. Early proliferating fibrous tissue and extensive edema (E) surround the lymphatic vessel. The adjacent artery (upper right) and vein (V) are unaffected. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)