Function
The spleen filters blood and removes foreign particles, bacteria, and erythrocytes that are senescent, have structural membrane abnormalities, or are infected with hemotropic parasites. Its immunologic functions are those of a secondary lymphoid organ and include activation of macrophages to process and present antigen, B lymphocyte proliferation and production of antibody and biologic molecules, and the interaction of T lymphocytes and antigens. In some species, it stores significant quantities of blood (Box 13-6). These functions are best considered on the basis of the two grossly visible components of the splenic parenchyma: red and white pulp and the anatomic systems associated with them: (1) the monocyte-macrophage system, red pulp vascular spaces, and hematopoiesis within the red pulp and (2) the B and T lymphocyte systems within the white pulp.
Monocyte-Macrophage System: Macrophages are located chiefly at two sites in the red pulp of the spleen: red pulp vascular spaces and splenic cords (and in the dog, perisinusoidally). Red pulp vascular spaces are supported by a reticular network, which is a fine meshwork of reticular fibers composed of type III collagen, in which macrophages are dispersed. These macrophages (and those in the reticulum of the marginal zone, which is classified as part of the white pulp) are responsible for phagocytosis of blood-borne foreign material (Fig. 13-40), bacteria, and senescent and/or damaged erythrocytes as in immune-mediated anemias and infections with hemotropic parasites. In the dog, sinusoidal macrophages remove entire erythrocytes (erythrophagocytosis), as well as portions of an erythrocyte’s membrane and cytoplasmic inclusions, such as nuclear remnants like Heinz bodies, by a process called pitting. Indeed, the presence of large numbers of nuclear remnants in erythrocytes in canine blood smears may be indicative of splenic malfunction. The process of removal of senescent erythrocytes normally does not alter the size of the spleen, except when the spleen has to remove large numbers of defective erythrocytes, such as occurs in an acute hemolytic anemia, in which case, the spleen can be markedly enlarged. In nonsinusoidal spleens, macrophages of the splenic cords of the red pulp perform these functions, although the extent and location of the sites of pitting are unclear. The cat’s spleen is deficient in pitting and thus in removal of Heinz bodies, presumably because it has no sinusoids. Also, there are no slits in the red pulp vascular spaces similar to the ones in the sinusoidal wall through which the erythrocytes have to squeeze to return to the circulation, or be either pitted or phagocytosed. Pitting takes place in the feline spleen, but the process is slow.
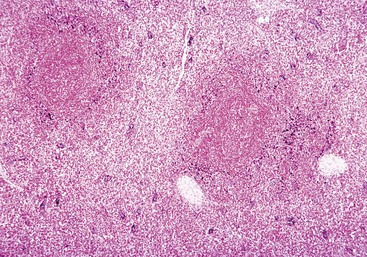
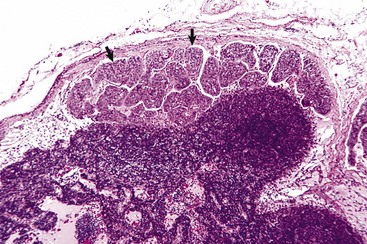
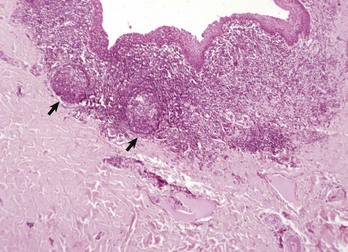
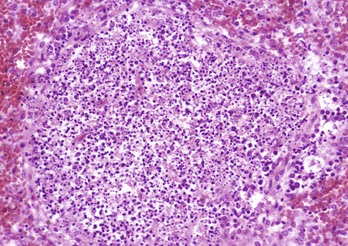
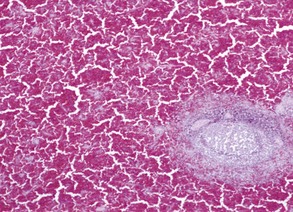
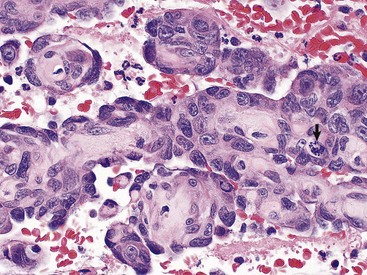
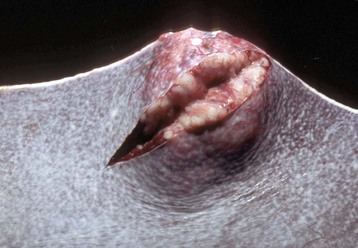
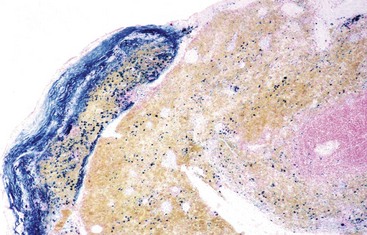
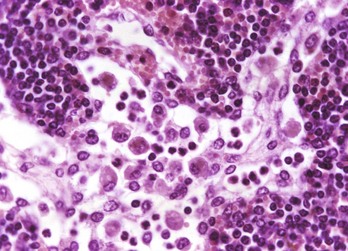
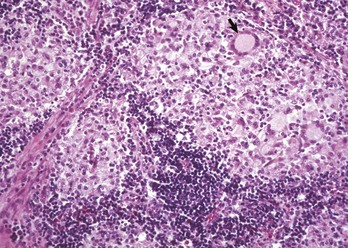
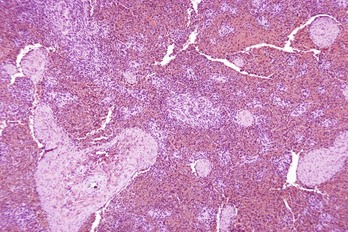
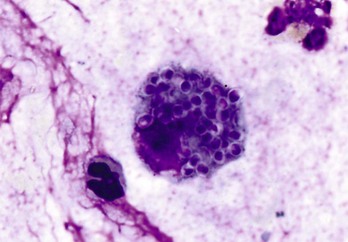
Fig. 13-40 Function of the marginal zone, splenic follicle, white pulp, spleen, calf injected intravenously with micronized carbon particles.
Carbon particles (black pigment) are present in macrophages of the marginal zone. Macrophages of the monocyte-macrophage system phagocytose blood-borne foreign material, bacteria, viruses, and senescent and/or damaged erythrocytes as in immune-mediated anemias and infections with hemotropic parasites. Eosin counterstain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
The sinusoidal, marginal sinus, and splenic cord macrophages are of bone marrow origin. They originate in the bone marrow, circulate in the blood as monocytes and migrate into the spleen. Fixed macrophages elsewhere in the body, in connective tissue, lymph nodes (sinus histiocytes), liver (Kupffer cells), lung (pulmonary intravascular macrophages and pulmonary alveolar macrophages), and brain (resident and perivascular microglial cells) are derived in a similar manner (see Chapters 5, 8, 9, and 14).
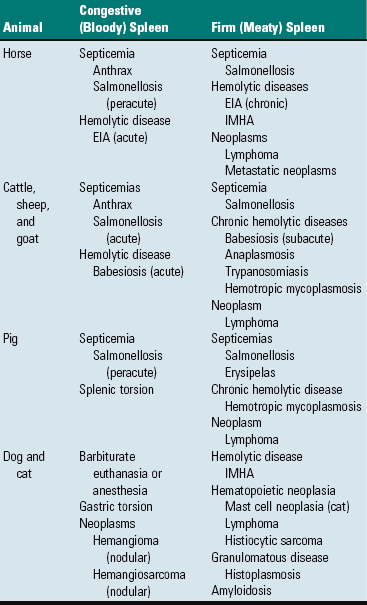
Storage or Defense Spleens: Spleens are classified as either storage or defense spleens, based on whether or not they can store significant volumes of blood. The spleens of domestic animals have both storage and defense functions, but are classified as storage spleens. Their trabeculae and capsules contain smooth muscle, which allows them to expand and contract. Equine, canine, and feline spleens all have considerable capacity. It has been claimed that the canine spleen can store one-third of the dog’s erythrocytes while the animal sleeps and the equine spleen holds one half of the animal’s circulating red cell mass, which is considered an advantage as it reduces the viscosity of the circulating blood. The spleens of ruminants and the pig are intermediate in the amount of smooth muscle and have limited storage capacity. Spleens, whose capsules and trabeculae have a low percentage of smooth muscle and elastic fibers and thus cannot expand and contract (rabbit and human), are designated as defense spleens. Storage spleens expand and contract quickly under the influence of the autonomic nervous system (sympathetic and vagal fibers, which reach the reticular walls of the red pulp vascular spaces; catecholamines; “flight or fight”) and other circulatory perturbations, such as in hypovolemic and/or cardiogenic shock (see the section on Small Spleens). Thus storage spleens may be either grossly enlarged and congested, or small with a wrinkled surface and a dry parenchyma (see the sections on Uniform Splenomegaly and Small Spleens). Also, the spleen responds to septicemia and acute hemotropic parasitic diseases by active hyperemia or acute passive congestion respectively, in the latter case the result of storing infected erythrocytes until they can be phagocytosed. Grossly congested spleens may be so enlarged that foci of white pulp are widely separated, making the white pulp look sparse and thus easily underestimated on gross examination.
Hematopoietic Tissue: Although the liver is the primary site of hematopoiesis in the developing fetus, the spleen also makes a minor contribution. Shortly before or after birth, depending on the species, hematopoiesis ceases in the liver and spleen and the bone marrow becomes the primary hematopoietic organ. However, often under conditions of severe demand, for example in a severe and prolonged anemia, this increased demand can be met partly through reactivation of splenic hematopoiesis. This outcome is called extramedullary hematopoiesis (EMH). It is also an incidental finding in splenic nodular hyperplasia (see the section on Nodular Spleens with Firm Consistency).
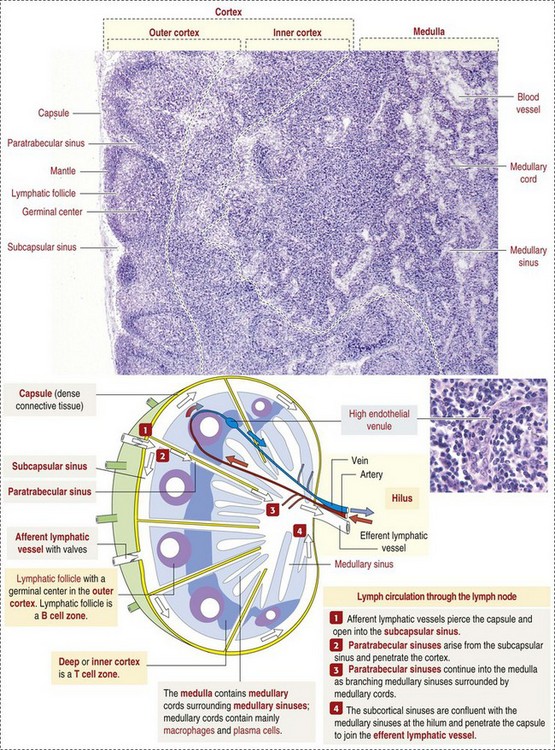
White Pulp: White pulp is grossly visible on the cut surface as distinct foci of grayish lymphoid tissue. Each focus consists of a periarteriolar lymphoid sheath and a splenic nodule surrounded by a marginal zone. There is considerable confusion regarding the terminology of splenic nodules or follicles. Histology books avoid the use of “lymphoid” as in “lymphoid nodule” or “lymphoid follicle”. Terms used include “lymphoid-like follicle,” “splenic follicle,” “splenic nodule” and even just “white pulp.” However, the pathology literature frequently uses the term “splenic lymphatic (or lymphoid) follicle”. This chapter uses the terms splenic nodule and splenic lymphoid follicle.
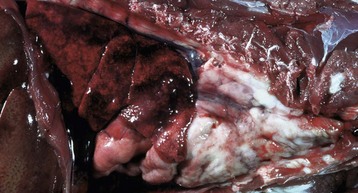
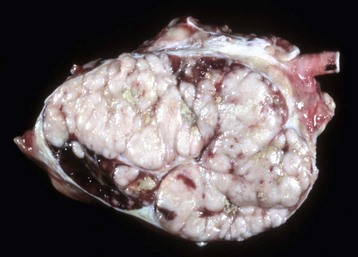
Normally, foci of white pulp are small and may not be visible on gross examination of a cross section of spleen. However, if nodules are enlarged either by lymphoid hyperplasia or by a neoplastic process (e.g., lymphoma), these foci initially become visible on the cut surface, as 0.5- to 1.0-mm white dots scattered through the red pulp. Also, in animals with storage spleens, the distention of the red pulp from stored blood may separate white pulp, making it look sparse.
Splenic white pulp is organized around branches of the splenic artery, the central arteriole, in the form of PALS, which is populated primarily by T lymphocytes (see Fig. 13-38). Primary splenic follicles are located eccentrically in PALS and are primarily comprised of B lymphocytes. When exposed to antigen, the latter develop germinal centers characteristic of secondary lymphoid follicles (see the section on Lymph Nodes).
The marginal zone is the third component of the splenic white pulp. It is located at the interface of the white and red pulp and surrounds the marginal sinus, which is immediately adjacent to the follicle. The marginal sinus is supplied with blood by radial branches of the central artery, which drain into the marginal sinus (see Fig. 13-38) and is the portal of entry into the spleen for recirculating B and T lymphocytes. The marginal zone consists of macrophages, dendritic cells, and T and B lymphocytes and is an important transit area for B and T lymphocytes, which after they leave the circulation, enter the marginal zone. From here, T lymphocytes migrate to the PALS. Those B lymphocytes that recognize antigens for which they have been programmed are activated and enter the follicle and proliferate to form plasmablasts. Dendritic cells in the marginal zone capture blood-borne antigens, process them, and preset them to the follicular lymphocytes.
Macrophages in the white pulp or marginal zone are phenotypically distinct from those in the red pulp. The latter function primarily to actively phagocytose particles, senescent erythrocytes, erythrocytes containing hemotropic parasites (e.g., Babesia spp.), and pathogenic bacteria and fungi and thus are responsible for “filtering” the blood. Many of the marginal zone macrophages are involved in phagocytosis and processing of antigen. However, some marginal zone macrophages actively phagocytose particulate matter in the blood (see Fig. 13-40) and bacteria in septicemias.
Responses to injury
The responses of the spleen to injury (Box 13-7) include acute inflammation, hyperplasia of the monocyte-macrophage system, hyperplasia of lymphoid tissues, atrophy of lymphoid tissues, storage of blood or contraction to expel reserve blood, and neoplasia. These responses are reflections of the spleen’s three chief anatomic components: (1) monocyte-macrophage system, (2) lymphoid (immune) system, and (3) vasculature, chiefly the red pulp vascular spaces and their supporting reticular stroma (reticulum). Because the spleen is examined on the basis of red and white pulp, this approach is used in the following description.
Monocyte-Macrophage System: The distribution and function of macrophages in the spleen is described above under Structure and Function. These interactions are complex and their relationships to both innate and adaptive immunity, as well as cell turnover, are areas of intense study (see Chapter 5). The extent of phagocytosis by macrophages in the red pulp vascular spaces, which are attached to the reticulum forming the walls lining these spaces, splenic cords, and marginal sinuses, depends on the order in which they receive blood. In most species, the marginal sinus is the first, and consequently, particles and bacteria tend to be more concentrated here initially (see Fig. 13-40). However, again there are species differences, for example, in the cat, the marginal sinus is small and PAMS play a larger role in phagocytosis.
The spleen is able to mount a very strong response to blood-borne pathogens. In immunized rabbits injected intravenously with pneumococci, the blood was cleared of 98% of those bacteria within 15 minutes and 100% of an inoculum of 1 billion bacteria was removed from the blood within an hour. Also, the rabbit spleen removed 10 times the number of bacteria per gram as the liver. When 1 billion pneumococci per pound of body weight were injected into the splenic artery of a dog over a 5-minute period, all bacteria were removed from the blood in 65 minutes. After splenectomy, blood-borne organisms multiply rapidly and are disseminated widely in the body and may cause “overwhelming postsplenectomy infection.” It has also been shown that the phagocytic function of the spleen is critical in the control of plasmodium in humans with malaria and in cattle with babesiosis. To facilitate filtering, all of the blood in the body passes through the spleen at least once a day. In the dog, the blood flow and transit time depend on whether the spleen is contracted or distended. Blood flow is slower in the distended spleen, apparently because blood flows through the red pulp vascular spaces, rather than through the sinusoids as it does in the contracted canine spleen.
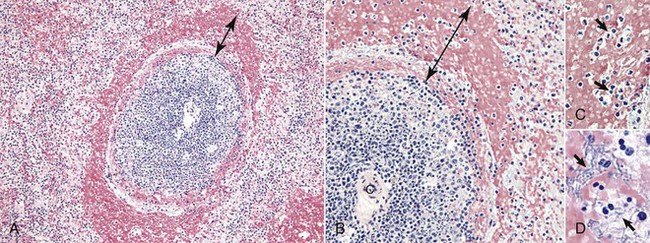
Sometimes in septicemias, the number of pathogenic bacteria arriving via the circulation can be so large that it exceeds the capacity of the splenic defenses to overcome them immediately. The result is acute splenic congestion and active hyperemia, followed by focal necrosis and or inflammation and a grossly enlarged and congested spleen. In the case of pyogenic bacteria, there may be neutrophils, other inflammatory cells, and bacteria throughout the red pulp. The marginal zone at the interface of the red and white pulp responds similarly to the red pulp vascular spaces and can be the initial site of response to blood-borne antigens and bacteria delivered by the radial branches of the central artery. As a result, it can be congested and in later stages (after only a few hours with highly pathogenic organisms) contain a diffuse spread or focal aggregates of neutrophils and macrophages. Histologically, the congestion forms a concentric ring, which may be incomplete, around the circumference of the splenic nodule (see the section on Anthrax).
Red pulp macrophages also proliferate in chronic hemolytic diseases in which there is a prolonged need to phagocytose erythrocytes and in chronic splenic congestion, which is usually a result of portal or splenic vein hypertension. The result is widened splenic cords. Unlike in humans, hepatic cirrhosis in animals does not cause chronic splenic congestion.
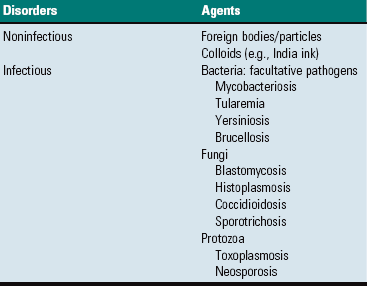
Macrophages in the red pulp (splenic cords and walls and lumens of red pulp vascular spaces) and marginal sinus also proliferate in response to fungi and facultative intracellular pathogens (e.g., Mycobacterium bovis) arriving hematogenously in the spleen. Their number may be augmented by monocytes recruited from the blood to form granulomatous inflammation. This inflammatory response can be diffuse (e.g. blastomycosis) or focal (tuberculosis).
White Pulp: The major system of the white pulp is the lymphoid system (PALS, splenic follicle), and the marginal zone, which lies at the junction of the red and white pulp but is not visible grossly.
The responses of the white pulp to injury are as follows:
• Activation or lymphocytolysis, necrosis, and reduction in size or disappearance (atrophy) of the follicle’s germinal center. These are followed by atrophy of the splenic follicles and ultimately atrophy of the lymph node.
• Phagocytosis of antibody, particulate material and microorganisms, macrophage hyperplasia (see the section on Monocyte-Macrophage System).
Splenic follicular hyperplasia is a response to antigenic stimuli and results in the formation of secondary follicles and consequently an increase in the size of the white pulp, which may be visibly enlarged. Splenic lymphoid follicles, lymphoid follicles in lymph nodes, and diffuse and nodular lymphatic tissue have a well-established sequence of morphologic changes, chiefly in the germinal center, which are helpful in tracking the stage of the B lymphocyte response. These sequential changes are similar to those described later in the Lymph Nodes section on Responses to Injury.
Splenic follicular atrophy occurs in response to lack of antigenic stimulation or from regression after antigenic stimulation has ceased, and from the effects of toxins, antineoplastic chemotherapeutic agents, microorganisms such as viruses and bacteria, radiation, malnutrition, wasting/cachectic diseases, and aging (see Box 13-5). The follicles are depleted of lymphocytes and with time, germinal centers and follicles disappear. The amount of the total lymphoid tissue is reduced and the spleen may be smaller.
Monocyte-Macrophage System: The response of the monocyte-macrophage system in the marginal sinus and marginal zone to injury is phagocytosis and proliferation. Proliferation also occurs in the viral disease porcine PMWS where there is proliferation of both follicular macrophages and those in the deep cortex (see the section on Disorders of Pigs).