Urinary Tract Infections (UTIs)
UTIs include but are not limited to pyelonephritis, ureteritis, cystitis, urethritis, and prostatitis. Infection occasionally is restricted to urine (bacteriuria).104 Identification of the site of a UTI may be difficult, as might be discrimination of the infection as primary or secondary. However, because infection in any region of the urinary tract can be accompanied by or result in infection throughout the tract, terminology inclusive of all sites (i.e., UTI) often is preferred to terms limited to a single site of infection (e.g., upper or lower UTI). However, the site of infection has implications because of potential differences in ease of treatment, including drug distribution. The incidence of UTIs in dogs is higher (estimated at 14%) than that in cats (1% to 3%).104 However, it is not clear whether this takes into account occult infections (see later discussion).
Microbial Targets
Because the urinary tract is a site for which bacterial contamination is common, the size of the inoculum should be considered when identifying target pathogens and the need for treatment. This need increases in the face of emerging antimicrobial resistance. Generally, the urinary tract is sterile above the urethra. Bacteriuria simply refers to the presence of bacteria in the urine. Infection of the urinary tract begins with bacterial adherence to uroepithelial cells of the urinary mucosal surface. The number of CFUs indicative of infection is higher than that for tissues for which contamination is unlikely and is influenced by both the method of sample collection and the gender. In his retrospective evaluating risk factors associated with UTI in dogs (n = 8354) from 1969 to 1995, Ling et al.105 defined clinically important bacteriuria when samples were collected by catheterization as ≥100,000 CFUs/mL in female dogs and ≥10,000 CFUs/mL in males, whereas ≥100,000 CFUs were considered significant in either gender if collected midstream. In an uncontaminated cystocentesis, any growth was considered significant. Other investigators (e.g., Seguin106) have considered growth significant in cystocentesis samples only if CFUs are greater than 1000. This seems to be a reasonable basis for criteria when considering treatment of UTI, as long as the criteria are considered in the context of the circumstances surrounding the infection, including the presence of clinical signs. The presence of three or more different organisms was considered by Ling et al.105 as indicative of contamination regardless of the method of collection and indicated the need to resample and reculture.
Historically, E. coli has been recognized as the predominant cause of UTIs in both dogs and cats (Table 8-9).107,108 Staphylococcus spp. and other gram-positive organisms historically have accounted for 25% of UTIs in dogs. Other cited causative agents in the dog include Proteus, Klebsiella, Enterobacter, and Pseudomonas spp.110 Proteus and Staphylococcus spp. cause urinary alkalinization and as such often are associated with struvite formation in dogs. In the cat, organisms other than E. coli that cause UTIs have included Proteus, Klebsiella, Pasteurella, Enterobacter, Pseudomonas, and Corynebacterium spp.108 Mycoplasma spp. also should be considered as a less common cause of UTIs.111
Table 8-9 Pharmacokinetic and Pharmacodynamic Indices for Treatment of Canine and Feline Escherichia coli Associated with Urinary Tract Infections
KEY POINT 8-22
The need to treat a urinary tract infection should be carefully critiqued. Resolution of the cause of the infection is critical to therapeutic success, including avoidance of resistance.
Enterococcus spp. increasingly is competing with E. coli as the predominant organism associated with UTI. Ling and coworkers105 found that most UTIs were caused by a single agent, with E. coli the organism most commonly isolated (45%; see Table 8-1). The number of animals exhibiting signs associated with UTI was not indicated in the study nor was identification of the UTI as a first occurrence or a recurrence. Although UTIs occurred more frequently in female dogs, the frequency of specific organisms causing UTIs was generally similar between genders, with the exception of Proteus and Enterococcus spp., which were cultured more freqently from females (10.5% and 9.6%, respectively) compared with males (5% and 5% to 6%, respectively). Multiple organism infections were more likely to be found in females than in males. Age did not appear to be an important predictive factor of the most likely pathogen. The majority of infections were associated with ≥100,000 CFUs/mL, whereas approximately 20% of infections, regardless of the number of infecting organisms or the gender of the dog, were characterized by ≤1000 CFUs/mL.
A retrospective study of UTI in dogs admitted to a veterinary teaching hospital and subsequently diagnosed with UTI (n = 240)112 found that E. coli was the causative organism in 50% of the cases. Although the majority of the remaining organisms were gram-negative coliforms (e.g., Proteus, Klebsiella spp.) selected gram-positive organisms (Staphylococcus and Enterococcus spp. being the majority) also were cultured.
The causative agents associated with recurrent UTI (defined later) appears to be similar in that E. coli is most common. However, the causative agents in the remainder may differ. Seguin106 retrospectively examined recurrent UTIs in dogs (n = 441 isolates from 373 positive cultures). E. coli (47%) was the most commonly isolated, followed by Enterococcus spp. (21%). Other organisms included Proteus (7.7%), Klebsiella (5.9%), Staphyloccoccus (5.2%), and Pseudomonas spp. (4.1%). Mixed infections occurred in 17% of the cultures. In a prospective study of dogs receiving glucocorticoids (n = 127), the most common organism isolated was E. coli followed by Enterococcus spp. A retrospective study in cats (n = 141) with diabetes mellitus113 also identified E. coli as the most common organism. However, a study of 123 specimens collected from asymptomatic cats found Enterococcus faecalis to be the most commonly isolated organism (43%), with E. coli the second most common (32%).114 There does not appear to be any information regarding the causative agent associated with infection in different regions of the urinary tract.
Limited information is available regarding the susceptibility of organisms causing UTI. Attention to this issue in clinical studies generally addresses patterns of susceptibility but not the level; however, the latter becomes important to the detection of emerging resistance in those isolates considered susceptible based on CLSI criteria. Boothe and coworkers115 (see Table 7-3) have described the proportion of E. coli isolates susceptible to traditional first-choice antimicrobials, with regional differences apparent. Decreasing susceptibility is likely to limit empirical predictability of those drugs that remain effective. These data underscore the importance of susceptibility methods that allow discernment of different levels of susceptibility. The data also underscore the importance of designing dosing regimens that maximize doses or (for time-dependent drugs) intervals such that resistance might be minimized (see Chapter 6).
In a retrospective study of UTI in dogs receiving glucocorticoids,116 organism susceptibility was recorded (n = 32): trimethorpim–sulfonamide (97%), amoxicillin–clavulanic acid (76%), tetracycline (70%), ampicillin (61%), and cephalexin (52%). Interestingly, only 7% were susceptible to enrofloxacin, but only 6 dogs were receiving enrofloxacin.
Pathophysiology
The clinical signs of UTI vary with the site of infection. As with other body systems, the inflammatory response largely is responsible for the clinical signs of UTI. Bacteriuria can be asymptomatic, detected on urinalysis but causing no clinical signs.109 Acute cystitis can cause dysuria but rarely causes signs of systemic inflammation. Acute pyelonephritis, however, is often associated with signs of systemic inflammation, including fever. As in any body system, evidence of inflammation is not necessarily evidence of infection and the need for antimicrobial therapy. Likewise, absence of inflammation does not rule out bacterial infection.
Possible sources of UTI include ascension from the urethra and hematogenous and lymphatic factors. Ascending infection is by far the most common route of infection, although the kidney is predisposed to develop infection associated with blood-borne organisms. In both humans and dogs the origin of bacteria infecting the urinary tract is generally fecal, with the frequency of infection by a particular strain depending on the virulence of the organism. Pathogens generally travel along the urethra to the bladder. Anatomic deformity and turbulent urine flow may facilitate antegrade movement of organisms toward the bladder. Female patients are more predisposed to ascending infection because of the shorter length of the tract and increased risk of contamination. Once in the bladder, infection can continue to ascend the ureter to the kidney, particularly if vesicoureteral reflux is present.
Because E. coli is consistently the most common organism associated with UTI, a focus on its pathophysiology is warranted. E. coli can be identified based on the presence of selected antigens that serve as a basis for serotyping: O, or somatic (more than 140 serotypes); K, or capsular (which may be associated with more virulence factors); and H, or flagella (necessary for ascending infection and renal invasion). E. coli is also represented by a number of pathogenic strains based on the presence of specific virulence factors that facilitate infection. Strains are broadly categorized as intestinal or extraintestinal; the latter group generally is recognized to originate from intestinal strains that subsequently acquire virulence factors that facilitate extra-intestinal survival. Among the extraintestinal strains are uropathogenic E. coli (UPEC) represented by many different serotypes.109 Initial infection by UPEC depends on adherence to uroepithelial cells (e.g., pili or fimbriae). Type 1 pili are among the most important virulence factors facilitating invasion, adherence, and persistence of E. coli infections; similar factors are associated with infection by Proteus spp. and Klebsiella spp.117b Toxins are released; examples include hemolysin, causing uroepithelial cell death (thus providing nutrients for UPEC) and cytotoxic necrotizing factor, which stimulates apotosis and inflammatory cell chemotaxis. Other virulence factors act to scavenge environmental iron necessary for extra-intestinal survival of the bacteria (e.g., enterobactin, which itself is inactivated by lipocalin 2, a bacteriostatic factor secreted by host leukocytes); others impair the hosts’ defensive ability to scavenge iron. Factors also facilitate uroepithelial cell penetration by E. coli; rapid intra-epithelial proliferation of microbes is accompanied by biofilm formation. Exfoliation of surface uroepithelial cells that occurs with urination is an important host protective mechanism but may facilitate recurrence as infected uroepithelial cells are exposed from the deeper layers. Inflammatory cells respond to cell destruction.
Much of the understanding associated with UPEC pathogenesis comes from contrasting it to a UPEC strain that is associated with much less pathogenicity, causing asymptomatic bacturia (ASB; strain 83792). This strain has been used prophylactically to infect the bladder of humans afflicted with UTI associated with more pathogenic UPEC; this unique approach to treatment is currently undergoing clinical trials.117c
Understanding the role of virulence factors in UTI is a prelude to identifying therapies and alternative antimicrobials. Among the factors targeted are adherence factors, in part because of their critically important role in initial infection. E. coli contains adhesins that bind to the glycolipid receptors. Mannose-containing receptors are present on most Enterobacteriaceae. On entry into the lower urinary tract E. coli organisms associated with canine UTI appear to adhere primarily through these mannose-sensitive adhesins, initiating colonization. In contrast, mannose-resistant fimbriae and other adhesins appear to be critical for colonization of the renal structures. Binding between receptor and adhesin changes the receptor-bearing cell. The severity of a UTI may be correlated with the degree of adherence to uroepithelial cells. Organisms causing acute pyelonephritis in human patients are characterized by higher adherence compared with organisms causing asymptomatic bacteria.117 The interaction between microbe and receptors may offer a target for treatment. Treatment with mannose or similar molecules has been proposed to block the receptors, thus reducing adherence.117 However, while this may decrease infection in the bladder, some of the most pathogenic E. coli do not recognize mannose (e.g, UPEC associated with renal infection). Further, interaction between the pathogen and the mannose receptor may be important in the initiation of host defense. Cranberry juice extract contains proanthocyanidins that appear to block uroepithelial cell adherance (Type pili) receptors, presumably precluding bacterial adherence. Several antimicrobials interfere with bacterial expression of fimbrial adhesins and thus may prevent bacterial attachment and colonization. Examples include penicillin, ampicillin, amoxicillin, and streptomycin. Once-daily administration of antimicrobials at reduced (one half to as little as one eighth) doses also may be able to prevent UTIs because of interference with fimbrial expression or formation.119 The role of fosfomycin for this indication requires further development. Among the adherence factors, biofilm has a profound influence on UTI, particularly persistent or recurrent UTI caused by E. coli.117a,b Although bioifilm clearly contributes to infections associated with urinary catheters, its role in chronic UTI is less appreciated. Biofilm physically and functionally contributes to antimicrobial resistance. A crystalline-based biofilm produced in association with urease production (and urinary alkalinization) appears to contribute to, and indeed, be necessary for the formation of struvite crystals. Use of compounds such as iodoacetamide (IDA) and N -ethyl maleimide (NEM) that inhibit enzymes necessary for the formation of the biofilm matrix of these crystials is an area of investigation.
Predisposing Factors
A number of microbial factors increase the risk of UTI.104 Siqueira and coworkers118 have described virulence factors associated with E. coli isolated from dogs with UTI (n = 51) and pyometra (n = 52) and feces of healthy dogs (n = 55). These include but are not limited to other microbial antigens, production of toxins such as hemolysin or urease, and the mucoid polysaccharide capsules (e.g., Pseudomona spp.). Urease producers (Staphylococcus, Proteus, or Mycoplasma spp.) increase the risk of struvite urolithiasis, predominantly in dogs.
Other host factors increase the risk of UTI. Anatomic predispositions to infection include perineal urethrostomy (particularly in cats). Bacterial infection is rarely the initial cause of disease of the lower urinary tract in cats, but development of infection is a common sequela.108 The role of viruses in feline lower urinary tract disease has been reviewed.120 Viruses that have been isolated from the urinary tract of cats with spontaneous disease include feline calicivirus, bovine herpesvirus 4, and feline syncytium-forming virus.121 Other potential uropathogens include mycoplasmas and ureaplasmas.111,121 Risk factors have been described in cats with diabetes mellitus, with infection present in 18 of 141 cats.113 Hyperthyroidism also is a risk factor. Immunosuppressive therapy is a risk factor, as has been demonstrated prospectively in dogs, (n = 127) receiving glucocorticoids (prednisolone or methylprednisolone): 18% were positive (compared with a matched control set of dogs not receiving glucocorticoids) on at least one culture.116
Recurrent UTIs reflect either persistence or relapse of the infection or reinfections.106,109 Persistent or relapsing infections reflect therapeutic failure, whereas reinfection reflects a new bacterial species or strain following a period of urine sterility (i.e., negative urine culture). Superinfection refers to infection with a different organism that emerges during treatment of the original organism. Discriminating among persistence, relapse, or reinfection is difficult. Drazenovich and coworkers122 used pulsed-field gel electrophoresis (PGFE) to pulsotype E. coli isolates associated with persistent UTI in dogs in an attempt to discriminate between recurrence and reinfection. Interestingly, of the E. coli in their study (n = 12 dogs, 47 isolates), only two dogs had the same PFGE genetic pattern (pulsotype); further, few virulence factors were identified among infecting isolates. Frietag and coworkers123 studied whether antimicrobial susceptibility profiles could predict a recurrent infection as persistent or relapsing, versus reinfection, with pulsotypes as the determining factor. Susceptibility was effective only 58% of the time in predicting the pattern of recurrence. However, the study group included only five cats (17 isolates); the interval between diagnosis ranged from 6 weeks to 2.5 years. Of the 17 isolates, 9 unique isolates were identified, with no more than 2 from each cat, but susceptibility patterns differed within and between clones. Because PGFE is based largely on chromosomal DNA (i.e., does not include plasmid DNA) and detects the presence of the gene but not its expression, pulsotypes may not be the most appropriate standard on which to base recurrence patterns.
Causes of recurrent UTI (defined as 2 or more in a 6-month period) were retrospectively studied in dogs (n = 100).106 The median age was 7.7 years. Persistence (defined as same organism and same susceptibility pattern; 42%) and reinfection (different isolate; 50%) were equally responsible for recurrence. Superinfections were identified in 2% of dogs; it is not clear how many animals were cultured while on antimicrobial therapy; the proportion may be higher if superinfections are prospectively sought. Dogs younger than 3 years of age were at greater risk, whereas dogs older than 10 years were associated with a decreased risk of recurrence. This may reflect, in younger dogs, anatomic or other underlying causes being more important to recurrence. Females were at greater risk than males, and sporting dogs, nonsporting dogs, and hounds were at risk compared with other breeds. E. coli (56%) and Enterococcus spp. (21%) were the most common organisms. Multiple isolates were present in 18% of infections. An underlying cause (or causes) was found in 71 of the dogs; correction occurred in approximately 25 of these. Disorders include abnormal micturition, anatomic defects, altered urothelium (i.e., tumors or uroliths), altered urine composition (hypoadrenocorticism or diabetes mellitus), and impaired immunity (e.g., chemotherapy, hyperadrenocorticism). Dogs treated without removing the underlying cause of disease were more likely to be considered poorly controlled (74.5%), with duration of a disease-free period being less than 8 weeks. Correction of underlying disease or therapy intended to prevent reinfection (i.e., low-dose, long-term antimicrobials) was associated with better control. Over 29% of the infections were associated with multidrug resistant microbes, with abnormal micturition more commonly associated with resistant organisms. Interestingly, 87% of the dogs in which relapse occurred were initially presented for a problem other than UTI, and 50% of the dogs studied were asymptomatic for UTI. Sediments in 15% of the dogs were not indicative of infection, and these isolates in particular tended to be resistant. The authors concluded that culture and susceptibility testing may be indicated in the presence of a predisposing disorder.
The need for preemptively culturing urine in animals predisposed to infection was supported by the prospective findings of Torres and others116 and Bailiff and others113 Torres and others116 studied UTI in dogs (n = 127) receiving glucocorticoids; 18% were positive (compared with a matched control set of dogs not receiving glucocorticoids) on at least one culture, although no dog had clinical signs of UTI. However, pyuria or bacturia predicted positive culture 90% and 95% of the time, respectively. The most common organism isolated was E. coli, followed by Enterococcus spp. Superinfection was present in 6 of 52 dogs whose urine was cultured while receiving glucocorticoids; each dog was receiving cephalexin (22 mg/kg bid by mouth), and the cultured isolate was resistant to cephlexin. Bailiff et al.113 retrospectively studied 141 cats with diabetes mellitus. Of these, 13% also had UTI, with E. coli the most common organism. Risk factors were female and low body weight; no treatment-related risk factors were identified, including level of diabetic control, or glucosuria.
KEY POINT 8-23
Culture and susceptibility testing should be strongly considered as the basis of therapy in any urinary tract infection that is not occurring for the first time.
Occult UTIs are not limited only to animals with underlying disease; it is possible that “asymptomatic bacteria” is a more appropriate term. Occult UTI appears to occur more commonly than previously thought in cats.114 In a study of 123 specimens collected from asymptomatic cats, 38% were positive. Positive cultures were more prevalent in older female cats. In contrast to most studies, E. faecalis was the most commonly isolated organism (43%), with E. coli the second most common (32%).
Other factors may contribute to recurrence or reinfection. Urinary tract catheterization contributes to an increased risk of UTI in an experimental model of male feline cystitis.124 Catheterization is a common cause of ascension of bacteria from the urethra to the bladder. In human patients one catheterization results in infection in 1% of patients, and infection develops in most, if not all, patients within 3 to 4 days of placement of an indwelling, open-drainage catheter system.109 A study of infection in cats after perineal urethrostomy found that while the surgical procedure does not predispose the cat to recurrent bacterial UTI, surgical alteration of the urethral surface coupled with underlying uropathy may increase the risk and thus prevalence of ascending infection.125 Catheters should be used only in cats for which obstruction is likely if catheterization is not performed.126 Urinary calculi will contribute to canine and feline lower UTIs. One study reports growth of bacteria in urine or calculi of 41% of cats with urinary calculi.127 Staphylococcus spp. were responsible for most (45%) of these infections. Pyometra may serve as a source of reinfecting organisms,128 as might tumors (e.g., transmissible venereal tumors).129
Resistant Urinary Tract Infection
Resistant microbes, and particularly E. coli, are increasingly common causes of UTI (Figure 8-5; see also Table 7-3). In a prospective study of E. coli isolates, the majority of which were associated with UTI in dogs (n = 240) at a teaching hospital, Boothe and coworkers115 found the rate of resistance of E. coli to common first-choice antimicrobials limited empirical selection of an appropriate antimicrobial at that hospital (Figure 8-5). The percentage of organisms resistant to first-choice drugs exceeded 50% for ampicillin (e.g., amoxicillin) and was 40% or more for drugs considered relatively invulnerable resistant to beta-lactam resistance (i.e., amoxicillin–clavulanic acid and cephalothin). Moreover, 40% of the E. coli organisms also were resistant to trimethoprim–sulfamethoxazole. Disconcertingly, 40% of organisms were resistant to fluoroquinolones, the first choice for complicated infections, and 50% were resistant to extended-spectrum penicillins (carbenicillin, piperacillin, and ticarcillin). Indeed, the only drugs to which E. coli was predictably susceptible were nitrofurantoin and the aminoglycosides, particularly amikacin. Third-generation cephalosporins ceftiofur and particularly ceftazidime also exhibited susceptibility, although extended-spectrum beta-lactamases (see Chapter 7) were not tested. Several factors were associated with UTI resistance in these organisms, with antimicrobial therapy within the past 5 days and duration of hospital stay being the most important. The incidence of resistance in this retrospective study is in contrast to earlier reports of UTI resistance. For example, in a 2001 study, E. coli isolates (the majority being UTI) cultured from dogs were susceptible to norfloxacin (90%), enrofloxacin (87.5%), gentamicin (90.7%), and amikacin (85.9%).130 The proportion of E. coli classified as resistant to amoxicillin or amoxicillin–clavulanic acid is likely to increase in the next few years because CLSI has lowered the breakpoints for these two drugs (along with cephalexin). Other investigators have reported E. coli resistance, particularly to fluoroquinolone. In a study by the author of E. coli organisms (n = 50)—most of which were isolated from the urine—the MIC90 for the fluroquinolones was greater than 64 μg/mL, suggesting high-level multistep resistance.131 Multidrug-resistant E. coli has emerged as a cause of nosocomial infections in dogs132 and UTI in canine critical care patients.106,133 Data generated by the author suggest that resistance to fluoroquinolones is multidrug in nature, reflecting not only mutations in topoisomerases but also induction of efflux pumps.134 E. coli is not the only organism associated with UTI to which resistance has emerged in clinical cases. Enterococcus resistance in particular has emerged, probably reflecting its presence as the major gram-positive aerobe in the gastrointestinal tract, although its role as a uropathogen is not clear.
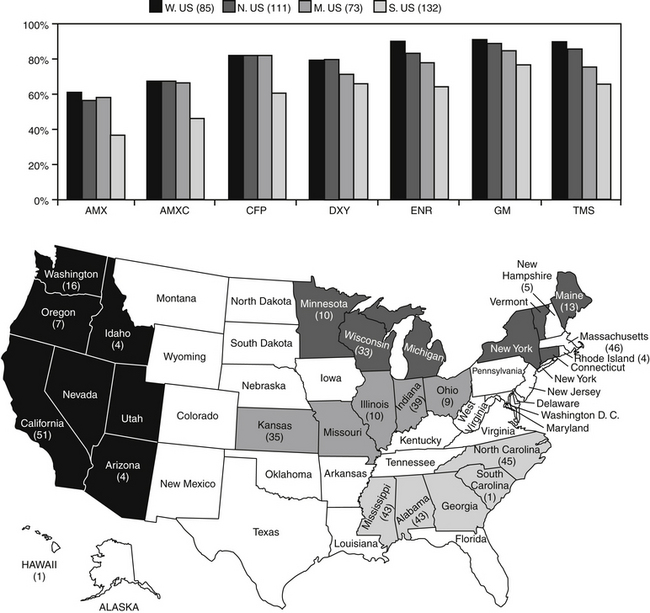
Figure 8-5 Percentage of canine and feline pathogenic Escherichia coli (n = 401) collected during May through September 2005 susceptible to selected antimicrobials. Resistance differed by regions (regions delineated in map), with the south characterized by the lowest level of susceptibility for each drug. AMX, Amoxicillin; AMXC, amoxicillin–clavulanic acid; CFP, cefpodoxime; DXY, doxycycline; ENR, enrofloxacin; GM, gentamicin; TMS, trimethoprim sulfonamide.
Although in vitro resistance may not necessarily predict in vivo failure, evidence in human medicine suggests otherwise. As in dogs, E. coli, is the most prevalent uropathogen in humans. However, antimicrobial resistance to first-choice drugs, trimethoprim–sulfonamethoxazole (TMP-SMX) and ampicillin, often exceeds 30% in humans. In women with UTI caused by E. coli characterized by in vitro resistance to TMP-SMX and subsequently treated with the combination drug, approximately 50% were bacteriologic failures and another 40% clinical failures.135 Previous antimicrobial therapy profoundly affected the likelihood of resistance, with the risk greater if the antimicrobial of interest has been used. Again, in women the most important independent risk factors for TMP-SMX resistance in nonhospitalized cases was use of the antimicrobial within the past 3 months. Those who had taken any antimicrobial were more than twice as likely to be infected with a resistant isolate; use of TMP-SMX within the past 2 weeks was associated with a sixteenfold greater risk of infection with a resistant isolate.135 Interestingly, trimethoprim by itself results in cure rates that are similar to combination with sulfamethoxazole; because it is associated with fewer side effects, it is the preferred drug for treatment of uncomplicated UTI by some.135
Urinary catheterization is not only a recognized risk factor for UTI but also for antimicrobial resistance. Catheterization has resulted in bacteriuria in previous bacteria-free urine and has been associated with changes in urine microflora, as well as increased resistance.136 Although aseptic techniques will reduce the risk of infection, infection is not prevented. The risk of persistent UTI in cats with experimentally induced cystitis was increased with catheter placement, despite the use of a closed system of urine drainage.110 The risk of infection can be correlated with duration of catheter placement, with the risk being reduced in patients catheterized for less than 3 days.137 Previous antimicrobial therapy is likely to contribute to the risk: resistance in dogs catheterized more than 5 days was strongly associated with the advent of resistant microrganisms.115 Catheter type influences the risk of infection, probably because of its impact on biofilm formation (see Chapter 6) and bacterial swarming.138 However, isolation of organisms within microcosms associated with biofilm and subsequently isolated from urine collected from the catheter—or from the catheter tip itself—does not necessarily indicate infection137; The incidence of resistance in E. coli collected from catheters tips is greater than that collected by cystocentesis112 If infection is present, the causative organism should be identified based on cystocentesis, or urine collected from the passage of a fresh, sterile catheter. However, catheterization should be kept to a minimum. Intermittent catheterization in spinal patients offers unique challenges. In humans, risk of resistance was related to frequency of catheterization (three times was associated with a greater risk than six times per day) and bladder overfilling (overfilling increased risk); previous indwelling catheterization also increased the risk. Trauma to the urethera during catheterization in itself did not increase the risk of infection, but the development of “false passages” or strictures resulting from repeat trauma did. Hydrophilic catheters appeared to reduce the risk of infection. Antimicrobials should be used judiciously in spinal patients. Whereas symptomatic infections are treated, asymptomatic bacteriuria is not necessarily treated; not surprisingly, long-term prophylactic antimicrobial therapy is associated with an increased risk of infection. As such, antimicrobials therapy tends to be limited to treatment of symptomatic infections or prophylaxis during initial (short-term) catheterization.139 The efficacy of antimicrobial infusion at the end of catheterization is not clear, with studies generating conflicting results. However, in contrast to multiple systemic dosing, single local infusion of high concentrations of drug is less likely to lead to resistance and potentially might do less harm than systemic therapy. Local intravesicular infusion of an antimicrobial might be considered of inducing minimal risk to resistance.
Difficulties encountered in the successful treatment of UTI, and particularly multidrug-resistant UTI, mandate that approaches be taken to prevent resistance, which includes preventing infection. Because previous use of antimicrobials consistently is a major predictor of emerging resistance, the question of the need to treat should be the first consideration for all infections. If reasonable alternatives exist to antimicrobial drug therapy, they might be considered first or in addition to antimicrobial therapy.
Prevention of Urinary Tract Infection
A number of host factors prevent or limit bacterial infection in the bladder.109 Among the host factors important in preventing infection are normal micturition, normal anatomic barriers, systemic immunocompetence, mucosal defense barriers, and the inherent antibacterial properties of urine. Recurring infections are most likely to reflect failed host defenses, whether originating spontaneously or iatrogenically (e.g., immunosuppressive drugs).106 Support of factors can be targeted with adjuvant therapies instead of antimicrobial treatment, when possible, or to facilitate antimicrobial therapy, particularly in the patient at risk for recurrent infections.
The decision to treat or not treat an infection might take into account the size of the inoculum (i.e., CFU/mL of urine) but should also take into account host factors (e.g., clinical signs, history, ability to control underlying cause, contributing factors, previous response to therapy). The location of infection may play a major role in determining the need for therapy. Whereas asymptomatic bacteriuria will often resolve or become self-limiting if left untreated, bacterial pyelonephritis is likely to progress.104,117 Virulence testing or serotyping eventually may be helpful in the decision to treat or not treat. The normal flora of the vulva and prepuce may be an important host defense mechanism against infecting microorganisms of the urinary tract. Normal flora may prevent colonization by pathogenic organisms or disrupt metabolism of pathogens. Secretory antibodies may coat infecting organisms, preventing adherence, and reduced antibody production may promote infection. Mucus may have other antibacterial effects. In the bladder, mucosal secretion of surface mucopolysaccharides is important to host defense by preventing attachment of bacteria. Destruction of this layer facilitates infection. Treatment with sulfonated glycosaminoglycans intraluminally may coat the uroepithelium and thus provide a barrier to bacterial adherence. Administration of carbenoxolone (a licorice derivative) stimulates secretion of mucosal polysaccharide and (in rabbits and humans) increases the clearance of E. coli infection. In the bladder, the composition of urine can affect bacterial growth. Urine concentration (unless extreme) is not likely to affect bacteria (bacteria are generally hypertonic compared with their environment), but high concentrations of urea or other compounds or low pH may impair bacterial growth. The addition of prostatic fluid inhibits bacterial growth. Exceptions include selected Staphylococcus and Proteus spp., which are relatively resistant to the antibacterial effects of urea.109 Tamm–Horsfall protein secreted by the cells of the ascending loop of Henle binds to E. coli by way of mannose-containing side chains and, as such, probably acts as a urinary bacterial defense mechanism. Other factors that help prevent or reduce bacterial infection include frequent urination, a small residual urine volume in the bladder, and rapid urination.
Therapy
The Need for Drug Therapy
The presence of bacteriuria is not necessarily an indication of the need for antimicrobial therapy (Figure 8-6). Antimicrobial therapy should be used only when reasonable evidence of infection exists. Bacterial UTI occurs much less frequently in cats than in dogs, and even the presence of clinical signs indicative of cystitis should not be interpreted as a need for antimicrobial drug therapy.
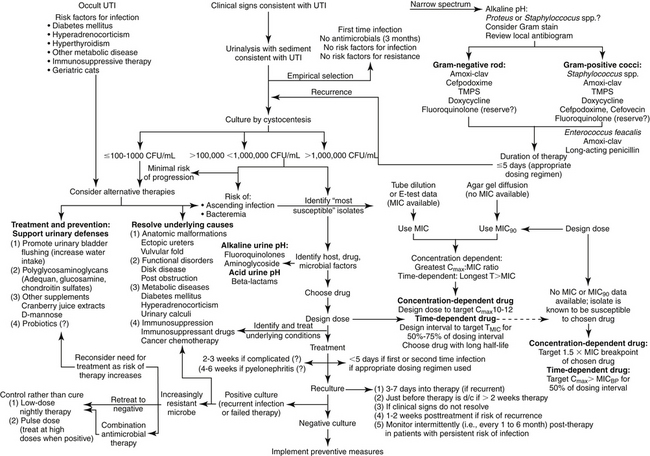
Figure 8-6 Suggested algorithm for treatment of urinary tract infections in dogs or cats. Treatment should be begun with assessing the need to treat with antimicrobial drugs (upper left) versus alternative therapies. Empirical treatment (upper right) should be pursued only for uncomplicated infections, including animals not recently exposed to antimicrobials (this may include any household member or pet). Culture and susceptibility data (lower right) should be used to both select drug and design the dose; in its absence, population pharmacodynamic data (MIC90) can be used if the identity of the infecting microbe is known. Treament of recurring infections (lower left) requires a series of reassessments, including recultures. With continued recurrence, the need for continued therapy should be balanced with the risk of therapy to the patient; prophylactic therapy might be considered to prevent recurrence once the urine has been cleared of infection (far lower left).
In humans, to prevent resistance, treatment generally is not indicated in asymptomatic bacteriuria except under certain conditions in which the patient is at risk, such as during pregnancy or invasive surgical procedures.140 Likewise, for veterinary patients the risk of emerging resistance must be weighed against the risk of failing to treat. If the patient is one for which aminoglycoside therapy is inadvisable, the need for treatment should be even more closely examined. With Enterococcus bacteriuria, treatment should not be implemented unless the need is clear. Clearly, if the decision is made to treat a UTI, therapy must be aggressive, designed to kill invading pathogens as well as emerging mutants as rapidly as possible. Non-antimicrobial alternatives should be considered in lieu of or in addition to antimicrobial therapy.
The Sequelae of Drug Therapy
The traditional goal of drug therapy for UTI is to eliminate bacteriuria in animals exhibiting clinical signs and urinalyses consistent with UTI. However, four sequelae of antimicrobial therapy may occur.104,109 Cure can be defined as negative urine cultures during and after (usually 1 to 2 weeks) antimicrobial therapy. Quantitative bacterial counts should decrease within 48 hours after initiation of an appropriate antimicrobial. Cure does not rule out the possibility of reinfection. UTIs may reflect first-time or recurrent infections. Chronic UTI is often used to refer to persistence of infection. The previously defined terms recurrence, persistence, and relapse often are used interchangeably when referring to UTIs. Persistence, or recurrence, can refer to presence of significant or low numbers of bacteria after 48 hours of therapy. If the numbers are significant, antimicrobial resistance or insufficient drug concentrations (e.g., improper dose, poor oral absorption, poor renal elimination) should be suspected. If numbers are very low, a continuous source of bacteria in the urinary tract (e.g., urinary calculi, prostate, kidney) or contamination from the lower urinary tract might be suspected.109 In such cases, cultures can identify persistent organisms after therapy has been discontinued. An appropriate approach would be classification of recurrent or persistent infections into three categories. Relapse occurs when the same organism causes infection 1 to 2 weeks after therapy has been discontinued. Relapse generally occurs within 1 to 2 weeks of cessation of therapy and may reflect either a very deep-seated infection or an abnormality of the urinary tract (e.g., structural, renal, or prostatic infection). The presence of a different organism is considered reinfection (i.e., a new infection). A new infection also can occur by the same organism located outside of the urinary tract. Generally, reinfection occurs more than 1 to 2 weeks after cessation of therapy. Superinfection may also occur and reflects infection with an additional organism during the course of antimicrobial treatment.104 Evidence of persistent or relapsing infection or superinfection should lead to more aggressive therapy and to the use of bactericidal rather than static drugs. Among the common causes of complications associated with UTI, antimicrobial resistance, unidentified underlying disease, and inappropriate dosing regimen should be considered.
Identification of the Target
In all but simple UTI, culture is indicated. Culture is recommended in patients whose history includes exposure to antimicrobial therapy within the last 3 months; exposure may include any household member, including other pets. The more complicated the infection or the greater the patient is at risk for resistance to emerge, the more important it is to base therapy on susceptibility data. In human patients diagnosis of UTI in asymptomatic patients is. based on at least two clean-catch midstream urine collections. The same organism should be present in significant (see previous discussion) amounts in both cultures. A single culture is sufficient in the presence of symptoms. Urinary cultures should be the basis of antimicrobial selection in complicated infections (e.g., reinfection or relapse, history of antimicrobial use in the past 4 to 6 weeks)104 or if the infection represents a risk to the patient’s health. Infection after recent urinary catheterization also should lead to culture collection. Increasingly, culturing at the outset, even in simple, first-time infections, may become prudent.
Quantitative urine culture (i.e., colony counts) should be implemented to facilitate discrimination of harmless bacterial contaminations (e.g., from the urethra) from pathogenic organisms (see previous discussion). Bacterial counts of more than 105 CFU are clearly indicative of infection regardless of the method of collection, whereas counts between 103 and 105 organisms are considered suspect if not collected by cystocentesis or if collected from female dogs. Counts of less than 1000 CFU should lead to a second culture and, in the absence of clinical signs or mitigating circumstances, consideration of alternative therapies. Methods have been described for culture procedures performed in practice.104 In addition to the method of collection, consideration must be given to sample handling. Samples should be kept refrigerated if the time from collection to processing by the lab is anticipated to exceed 12 hours, unless an appropriate amount of preservative is added. A viable alternative for submission of urine samples that will be in transit longer than 12 hours is collection and subsequent submission using a “paddle” apparatus (e.g., UriCult®).
Antimicrobial Selection
Presumably, a drug that is renally excreted should be selected for treatment of UTIs. Urinary concentrations of such drugs often surpass serum concentrations (up to 300-fold), which is particularly helpful for concentration-dependent drugs (Table 8-10). Susceptibility data do not take these higher concentrations into account, but this may be appropriate. Several caveats must be recognized when basing antimicrobial selection on renal elimination and anticipation of high urine drug concentrations:
Table 8-10 Mean Urine Concentrations for Antimicrobials Used to Treat Urinary Tract Infections in Dogs
KEY POINT 8-25
The design of the dosing regimen for treatment of a urinary tract infection should be based on plasma, not urine, drug concentrations.
A prudent approach for treatment of UTI is choice of a renally excreted drug but dosing based on drug concentrations achieved in plasma. Data from a few prospective studies, including animal model studies and human patient–based clinical trials, generally demonstrate that breakpoints based on plasma drug concentrations rather than urine appear to moderately predict outcomes associated with treatment of UTI.45 For example, maximum efficacy of aminopenicillins in treating UTI in human clinical trials is achieved when plasma drug concentrations are maintained above the MIC for 30 hours or more.271 Accordingly, CLSI interpretive criteria for infections in other tissues are relevant to UTI. Drugs that are not eliminated in urine might be characterized by lower concentrations in urine compared with tissues but still can be beneficial for UTI if dosing regimens are appropriate. Some drugs used to treat UTIs (e.g., indanyl–carbenicillin, nitrofurantoin) are recognized not to achieve effective concentrations in other tissues, and therefore use in areas other than the urinary bladder should be done cautiously.
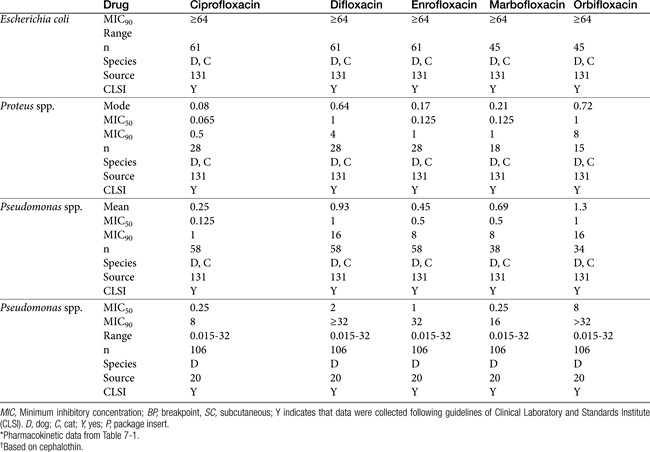
As resistance to E. coli and other uropathogens increases, empirical therapy for UTI increasingly will be limited (Table 8-9). Caution is recommended because of the recent recognition of the growing incidence of antimicrobial resistance. Among the organisms frequently causing UTIs in dogs and cats, E. coli, Proteus spp., P. aeruginosa, and Enterobacter spp. are among the organisms that vary widely in their susceptibility pattern and for which empirical therapy is more risky (Table 8-11; see also Table 7-3Table 7-3). Empirical therapy, if it is to be pursued, is indicated only for uncomplicated infection—that is, those patients in which no underlying structural, neurologic, or functional abnormality can be identified.104 The absence of previous antimicrobial therapy should also be interpreted as uncomplicated. Once relapse occurs (see later discussion), an infection should no longer be considered uncomplicated.
Table 8-11 Susceptibility Data (μg/mL) for Drugs for Gram-Negative Pathogens Collected from Antimicrobial-Free Dogs and Cats∗
Attention should be paid to the pH of the urine compared with the pKa of the chosen drug. In the presence of an alkaline pH, weakly basic antimicrobials might be considered (aminoglycosides, and fluoroquinolones, with the latter being amphoteric but more effective in an alkaline urine). Because urease producers may alkalinize the urine, drugs targeting such organisms (e.g., Proteus, Staphyloccocus, and some Klebsiella spp.) should be selected. In the presence of an acidic urinary pH (perhaps caused by E. coli), weakly acid drugs (e.g., penicillins, cephalosporins, potentiated sulfonamides) might be better empirical selections. However, if the drug is highly concentrated in the urine, even a predominantly ionized drug (e.g., a weak base in an acidic environment), may be sufficiently un-ionized to ensure effective concentrations.
Infections uncomplicated by previous antimicrobial therapy or in regions for which resistance has minimally emerged may respond to empiric therapy. E. coli or Enterobacter spp. traditionally have been considered responsive to trimethoprim–sulfadiazine, third-generation cephalosporins (cefpodoxime, cefovecin), or amoxicillin–clavulanic acid combinations. Among these, penicillins may be preferred because of their effects of fimbriae. Approximately 60% of E. coli are resistant to cephalexin. Klebsiella, Proteus, and Staphylococcus spp. are likely to respond to a first- or third-generation cephalosporin or to amoxicillin–clavulanic acid. Amoxicillin or ampicillin (preferably combined with a beta-lactamase protector) should be effective if a decision is made to treat Enterococcus spp. (see Tables 7-9, 8-3, and 8-6). However, for Enterobacteriaceae in particular, integration of pharmacokinetics and pharmacodynamics raise concerns regarding efficacy for drugs with short elimination half-lives, particularly if infection involves tissue other than the urinary bladder. In the absence of an MIC, the more stringent MIC90 (see Tables 8-9 and 8-11) and Cmax from package inserts or the literature (see Table 7-1) can be used to design dosing regimens. The highest end of the dosing regimen is indicated, particulary for at-risk patients. For selected concentration-dependent drugs, Cmax:MIC should exceed 10 to 12, and for time-dependent drugs, T>MIC should be at least 50% if not more of the dosing interval (generally, initial target concentrations should exceed the MIC by at least two to four fold (see Table 8-9). For time-dependent drugs, those with longer half-lives might be preferred (e.g., cefovecin and cefpodoxime). Although the package insert for cefovecin indicates that MIC90 concentrations for E. coli (1 μg/mL) are not achieved after a recommended dose of 8 mg/kg subcutaneously, predicted unbound plasma drug concentrations will be maintained at or above this concentration for 3 days. Cefovecin is approved for treatment of canine E. coli UTI in Canada at 8 mg/kg. Note that neither cefovecin nor cefpodoxime are effective against Enterococcus spp., indicating that the Gram-staining characteristics of the infecting organism might facilitate proper antimicrobial choice for empirical treatment of a UTI in dogs or cats. Because resistance in E. coli toward fluoroquinolones with rare exception is associated with MDR, their use for treatment of UTI should be considered second tier and ideally, be based on culture and susceptibility testing. Care should be taken using the fluoroquinolones for Enterococcus spp., for which efficacy can be variable. Boothe et al demonsrated that for E. coli, when using the MIC90 for fluoroquinolones, the Cmax:MIC90 failed to reach ≥10—as is suggested for concentration-dependent drugs—even with the highest dose for each drug. (see Tables 7-12 and 8-9 for additional MIC data).131 However, for isolates that are known to be susceptible to ciprofloxacin, enrofloxacin, and marbofloxacin reached the target Cmax:MIC90 at the high (but not low) dose. Even with susceptibility data that provide MIC, because the tested concentrations are close to the breakpoint MICs, isolates considered susceptible to fluoroquinolones may have already developed low-level resistance, increasing the risk of emergent resistance in at-risk patients.142 Certainly an “I” to any one fluoroquinolone should be interpreted as emerging resistance to others. Isolates with MIC approaching the breakpoint should be treated with alternative drugs, combination drugs, or “added doses” (e.g., high dose, twice daily). Marbofloxacin is preferred for cats at risk for retinal adversities, particularly if dosing twice daily. Although ciprofloxacin is more potent against gram-negative isolates, dosing should be increased to compensate for differences in oral bioavailability and oral dosing should not be used in cats. Fluoroquinolones are also effective against Mycoplasma and Ureaplasma spp.
A summary of treatment of UTI in humans might offer some guidance for empirical therapy in dogs or cats. In women with risk factors for infection with resistant bacteria, or in the setting of a high prevalence of TMP/SMX resistance, a fluoroquinolone or nitrofurantoin is recommended for empirical treatment. The goal of treatment is eradication of infection using shorter courses of therapy (i.e., 3 days) with once-daily dosing of a selected drug or a single dose of a particularly efficacious antimicrobial.141 The role of nitrofurantoin is increasing for treatment of UTI in women, particularly in the presence of increasing antimicrobial resistance to other urinary antimicrobials. Resistance among uropathogens to nitrofurantoin generally is consistently at a low level despite its use for 5 decades. An advantage to nitrofurantoin is its minimal effect on the normal gut flora. Consequently, selection pressure for antimicrobial resistance is reduced compared with other antimicrobials.135 Further, nitrofurantoin does not share cross-resistance with more commonly prescribed antimicrobials, and its use is justified from a public health perspective as a fluoroquinolone-sparing agent. For example, single-dose ciprofloxacin prophylaxis increased the prevalence of ciprofloxacin-resistant fecal E. coli from 3% to 12%.135 After treatment with ciprofloxacin for prostatitis, 50% resulted in post-treatment fecal colonization with quinolone-resistant E. coli genetically distinct from the prostatic infection. Indeed, in humans, although flouroquinolones are effective as short-course therapy for acute cystitis, widespread empirical use is discouraged because of potential promotion of resistance.140 An exception is made for acute (nonobstructive) pyelonephritis but only if culture results direct continuing therapy.
Beta-lactams and fosfomycin are also considered second-line (to TMP-SMX) agents for empirical treatment of cystitis in humans. Fosfomycin may be a reasonable first choice for treatment of UTI in dogs if data support its use for treatment of canine UTI. Advantages to fosfomycin tromethamine justify consideration of its use in dogs. Despite many years of use in humans, it is characterized by an extremely low incidence of resistance. Indeed, canine and feline isolates expressing MDR maintain susceptibility to fosfomycin.135a Further, pharmacokinetics in dogs support its use for treatment of UTI. Its role for treatment of UTI in dogs is emerging; a prudent approach might be as a second- or third-tier drug because of its importance in human medicine. In general, drugs metabolized by the liver (chloramphenicol) or excreted in the bile (macrolides, clindamycin) do not achieve high concentrations in the urine and should generally be avoided for treatment of UTI. Doxycycline might be appropriate if susceptibility data is available, although concentrations achieved in urine might be bacteriostatic.
Duration of Therapy
The “test for cure”104 (or perhaps, more appropriately, “response”) can be based on a second culture 3 to 5 days into therapy. Cure should be anticipated only if the organism count is less than 100 per mL of urine. Urine culture a second time just before discontinuation of therapy has been recommended,104 particularly if antimicrobial prophylaxis is to be implemented. The duration for successful treatment of uncomplicated lower UTIs might be as short as 3 to 5 days, particularly if doses are designed to target all infecting isolates, including those with the highest MICs.104 Such an approach is more likely to be successful if high doses and appropriate intervals are chosen. Treatment may need to be extended, however, if infection occurs anywhere other than the uroepithelium. Traditionally, a 10- to 14-day therapeutic regimen has been recommended for the first episode of therapy; the evidence for this recommendation is limited and clinical trials demonstrating appropriate duration of therapy are needed. Shorter-term antimicrobial therapy (a single high dose) has proved effective for female human patients with a lower UTI. Drugs that have been used successfully in humans for short-term dosing include trimethoprim–sulfonamide combinations, aminoglycosides, selected cephalosporins, and fluoroquinolones. Both single-dose and 3-day antimicrobial treatment regimens have been studied with dogs receiving amikacin and a trimethoprim–sulfonamide combination. Therapy was not uniformly successful, suggesting that caution should be used with this treatment regimen.143 However, these studies were implemented before understanding the importance of reaching targeted PDI (i.e., Cmax:MIC > 10-12 and T>MIC for more than 50% of the dosing interval). Factors that should preclude single-dose antimicrobial therapy for a lower UTI include recurrence, historical poor response to single-dose therapy, underlying predisposing factors to a UTI (including structural abnormalities or metabolic disorders, such as diabetes mellitus, and hyperadrenocorticism), and either pyelonephritis or symptoms of a UTI that have occurred for more than 7 days.
For infections that reflect a relapse, the duration of therapy should be at least 2 weeks; however, for human patients suffering from a relapse, a higher cure rate occurred with a 6-week course of therapy. For animals a duration of 4 to 6 weeks is recommended.104 The duration also might be based on the cause of the relapse and whether the underlying cause is curable and treated. Because relapse is likely to occur shortly after antimicrobial therapy is discontinued, cultures should be collected again 7 to 10 days after cessation of therapy to detect the recurrence. In general, regardless of the sequence of UTI, doses should always maximize efficacy and avoidance of resistance. A new antimicrobial should be selected if infection occurs more than 10 days after cessation of therapy;104 as more time elapses between cessation of therapy and the presence of bacteriuria, the more likely it is that reinfection is the cause of recurrence.
In the event of relapse after 6 weeks of therapy, 6 months of therapy or more may be necessary. A 3- to 6-month duration of therapy may be indicated for animals. However, clinical trials are lacking to document these recommendations as well. Greater care must be taken, in the selection of antimicrobials for longer-term therapy, with special consideration to toxicity. Drugs that are used for long-term therapy in human patients include amoxicillin, cephalexin, trimethoprim–sulfonamide combination, or a fluorinated quinolone. Cultures should be repeated monthly, and as long as significant bacteria are not present, the drug need not be changed. Should relapse occur after a drug is discontinued, the same drug or a new drug should be administered for a longer course of therapy. Long-term therapy may be particularly important for animals in which renal parenchymal damage is a risk. As with first-time infection, and perhaps more so, the need for treatment must be balanced with the disadvantages of treatment (or the risks of not treating should be balanced with the benefits of treating).
Clinical Trials
Despite the frequency of UTI in dogs and cats, the number of clinical trials examining therapy are limited. Pradofloxacin (n = 27 dogs; 5 mg/kg) has been compared with doxycycline (n = 23; 5 mg/kg load, followed by 2.5 mg/kg bid for 2 doses, then 2.5 mg qd) and amoxicillin–clavulanic acid (n = 28; 62.5 mg/kg bid for 10 days) in cats with clinical signs of UTI.144 Cats were not randomly assigned; assignment to treatment group was based on susceptibility; if more than one drug was designated susceptible, animals were assigned to keep groups balanced. All cats receiving pradofloxacin responded, with 13% and 10%, respectively, of the doxycycline and amoxicillin–clavulanic acid groups remaining infected after treatment. However, the proportion of responders was not different among treatment groups, probably reflecting small sample size.
Efficacy of cefovecin (8 mg/kg, administered subcutaneously) was compared with that of cephalexin for treatment of canine (n = 129) UTI.145 Efficacy was based on elimination of the pretreatment uropathogen. Exclusion criteria included local or systemic antimicrobials within the previous 14 days, or short- (7 days) or long-acting (30 days) glucocorticoids. The study was implemented as a multicenter, blinded, randomized study, with cephalexin (15 mg/kg, administered orally bid) serving as the positive control. Animals were treated for 14 days. The most common uropathogen was E. coli (60%), followed by P. mirabilis (12%) and S. intermedius (12%). As would be expected on the basis of the cephalexin MIC90 of E. coli, the overall cure rate for animals infected with E. coli was 79% in the cefovecin group compared with 36% for cephalexin. Bacteria were eliminated in 59% of cefovecin-treated dogs compared with 35% of cephalexin-treated dogs. Of the infecting isolates, 90.5% of E. coli infections were eradicated compared with 53% of E. coli infections for cephalexin. Of infections caused by S. intermedius, 6 of 6 were eliminated by cefovecin, compared with 7 of 10 for cephalexin; for Proteus mirabilius, 9 of 9 were eliminated by cefovecin, compared with 5 of 7 for cephalexin. Interestingly, although 319 dogs with clinical signs of UTI were evaluated for inclusion, only 137 (43%) actually were bacteriuric.
Prophylaxis
Long-term prophylaxis can be implemented for patients at risk for recurrence. Prophylaxis (by definition) can occur only after the infection has been eradicated. The use of low doses of antimicrobials in the presence of bacteriuria is likely to lead to the generation of resistant organisms and is contraindicated. Thus prophylactic antimicrobial therapy of UTIs is indicated for reinfection but not relapse (the latter suggests that the organism was never completely eradicated). The antimicrobial chosen for long-term prophylaxis should be both safe and inexpensive. Trimethoprim–sulfonamide combinations (monitor for immune-mediated reactions), nitrofurantoin, and fluoroquinolones are examples. However, neurologic side effects with nitrofurantoin may preclude its use, particularly long term. Fosfomycin might also fill this role. The dose for prophylaxis is generally reduced to 30% to 50% of the full dose.104 Subtherapeutic concentrations of drugs often are sufficiently inhibitory to prevent infection of the uroepithelium. The drug should be administered at night to maximize contact of the drug with the urinary tract. Intermittent urine cultures (monthly) are indicated to detect breakthrough infections in animals receiving long-term antimicrobial prophylaxis. Negative cultures for 6 to 9 months or more may indicate that prophylaxis is no longer necessary.
Adjuvant Therapy
Diuresis has been advocated in the treatment of UTIs in humans. Advantages include rapid dilution of bacteria, removal of infected urine, and subsequent rapid reduction of bacterial counts. In patients with pyelonephritis, an added advantage may be enhanced host defenses: medullary hypertonicity inhibits leukocyte migration, and high ammonia concentration inactivates complement. In the presence of vesicoureteral reflux, however, diuresis may increase the risk of acute urinary retention.
Use of drugs to modify urinary pH may facilitate the antibacterial effects of urine. The presence of ionizable organic acids (hippuric and gamma-hydroxybutyric acid) in an acidic pH may enhance the antibacterial activity of the urine. Antibacterial activity may be increased by ingestion of cranberry juice (if urinary pH is acidic), which contains precursors of hippuric acid. Cranberry juice extract also should be considered because of its potential ability to block adherence receptors. Methenamine, available as a hippuric acid or mandelate salt, releases formaldehyde at a urinary pH of 5.5 or less, which also can increase antibacterial activity of urine.
Local urinary analgesics, such as phenazopyridine, rarely are indicated for the management of UTIs. Dysuria is most likely to respond to appropriate antimicrobial therapy. These drugs cause methemoglobinemia and are contraindicated in cats.
Drugs or nutraceutical products that enhance polysulfated glycosaminoglycan synthesis (e.g., Adequan, pentosan polysulfate, glucosamine, chondroitin sulfate) might be considered for patients with complicated UTI. Such products may cover or help repair the uroepithelium, thus decreasing bacterial adherence. Gunn-Moore and Shenoy146 prospectively studied the effects of 60 days oral n-acetyl glucosamine in cats (n = 40) with feline idiopathic cystitis using a randomized, double-blinded, placebo controlled study. Response was based on owner assessment. Both groups improved significantly, with 26 of 40 cats suffering recurrences. Although the power of the study was large, the size of the placebo response limited the ability to detect a significant difference between treatment groups. Improvement in both groups was potentially attributed to owners, most of whom changed the cats to moist rather than dry diets. The quality of the glucosamine was not addressed. Wallius and Tidholm147 studied the effects of polysulfated glycosaminoglycans (PGAGs) in cats (n = 19) with clinical signs indicative of cystitis but culture negative. Cats were randomly assigned to receive either saline placebo or 3 mg/kg PGAGs on days 1, 2, 5, and 10. Assessment was based on owner perceptions of improvement. A treatment effect could not be detected because clinical signs resolved in essentially all cats (save one from each group). As with the previous study, the authors of this study could not conclude that PGAGs were not beneficial for treatment of inflammation, including that associated with UTI. Treatment with mannose or similar molecules may block mannose receptors, thus reducing adherence.119
The use of probiotics in the treatment of UTIs in human patients was reviewed by Lenoir-Wijnkoop.148 In general, Lactobacillus spp. are most commonly recommended for treatment of UTI. However, selected species are likely to emerge as more effective than others and microbiota may need to originate from the target species. The use of probiotics in the treatment of renal oxalate stones in human patients was also reviewed by Lenoir-Wijnkoop and coworkers.148 The absence of Oxalobacter formigenes from fecal microbiota increases the risk of kidney stones. Although no study has demonstrated an association between probiotic therapy and decreased renal stones, both animal and human studies have documented that O. formigenes can become established in the gastrointestinal tract, and establishment reduces urinary oxalate concentrations.
Pyelonephritis
Treatment of pyelonephritis may require hospitalization with intravenous fluid administration. Oral antimicrobial therapy is acceptable for mild to moderate cases as long as oral therapy is tolerated well. Because renal dysfunction can be life threatening, antimicrobial selection should ultimately be based on culture and susceptibility data. Therapy can be initiated empirically; however, resistance among E. coli organisms should lead to selections with known susceptibility. Combination therapy should be considered. Urine is not likely to be concentrated, thus added attention must be paid to ensure that effective concentrations reach the target tissue. Pyelonephritis can be associated with bacteremia, particularly gram-negative bacteremia. Clinical signs indicative of severe, life-threatening infection should lead to parenteral antimicrobial therapy with predictably effective drugs (e.g., aminoglycosides, fluoroquinolones, extended-spectrum beta-lactams, and third-generation cephalosporins known to not be extended-spectrum beta-lactam producers). Combination therapy also should be strongly considered. The high concentration of antimicrobial that facilitates treatment of the lower urinary tract (bladder and lower) may not occur in pyelonephritis; thus close attention must be paid to using sufficiently high doses and frequent dosing. Drugs whose efficacy is dependent on a hypotonic environment (compared with the target organism), such as beta-lactams, fosfomycin, or vancomycin, may be less effective in the face of medullary hypertonicity, although this may be less than normal in the face of pyelonephritis. Nonetheless, if drug combinations are used, at least one of the two drugs should not target cell walls. As with infection lower in the tract, bacterial numbers should decrease dramatically within the first 48 hours of treatment. For uncomplicated pyelonephritis, 14 days of therapy may be sufficient of treatment. Cultures should be repeated as previously indicated during and within 1 to 2 weeks of discontinuation of therapy. Complications such as abscessation may require surgical intervention and longer-term therapy.
Prostatitis
Pathophysiology
Bacterial prostatitis can present as either an acute or chronic infection. One does not necessarily precede or follow the other. Among the causes of prostatic infection, ascending infection and urine reflux appear to be most likely. Acute prostatitis often is accompanied by fever, pain, and symptoms typical of a UTI. Palpation of the prostate reveals tenderness, swelling, and (potentially) a fluctuant surface (Figure 8-7). Care should be taken when palpating the prostate so that the risk of bacteremia is minimized. Chronic bacterial prostatitis most commonly is caused by gram-negative coliforms, with E. coli being most common, followed by Klebsiella spp., Enterobacter spp., P. mirabilis, and S. aureus.
Antimicrobial Selection and Use
Antimicrobial penetration into the noninflamed canine prostate is limited. Drugs that are basic and lipid soluble appear to diffuse through tissues best, including macrolides (e.g., erythromycin and presumably azithromycin and clarithromycin), fluoroquinolones, and trimethoprim–sulfonamide combinations. The intense inflammatory response that accompanies acute prostatitis facilitates antimicrobial movement into the prostate,109 although high doses should be used to ensure adequate concentrations at the site of infection. Parenteral antimicrobials based on culture (prostatic fluid or urine) should be used in the presence of life-threatening infection; otherwise, oral therapy is acceptable. Duration of therapy should be at least 4 weeks to minimize the risk of progression to chronic prostatitis. Prostatic abscessation generally requires surgical intervention. Chronic prostatitis in human patients is difficult to cure unless infected tissue is surgically excised. Chronic prostatitis generally results in relapse. Therapy, when successful, generally requires 1 to 2 months of therapy. Antimicrobials that penetrate the noninflamed prostate (e.g., fluoroquinolones, trimethoprim–sulfonamide combinations, or macrolides) should be selected.109
Adjuvant therapy for the treatment of prostatitis includes stool softeners and, if indicated, analgesics. Neutering should be considered.