20 THE STRUCTURE AND FUNCTION OF THE MUSCULOSKELETAL SYSTEM
INTRODUCTION
Our knowledge of the musculoskeletal system dates back to the ancient Greeks. The Greek word for bone is osteum and so we have osteology as the study of bones. Many of the terms used to describe bone incorporate the Greek word — for example, bone-destroying cells are known are osteoclasts and bone-building cells are known as osteoblasts. The Greek word for muscle is mysium and this word can be found in the term for the tough outer wrapping of a muscle, the epimysium, while individual muscle cells are called myocytes.
The musculoskeletal system provides shape, support and movement to the body. The bones provide the structure and the joints between them determine the movements possible. Bones are also central to mineral homeostasis. The attachment of muscles on the bones provides a series of levers about the joints, which control the strength or speed of movement. Muscles, as the only contractile tissue within the body, drive the movement. The energy consumed by the muscles also serves to maintain body temperature. We start with an exploration of the skeletal system.
THE STRUCTURE AND FUNCTION OF BONES
Bones give form to the body, support tissues and permit movement by providing points of attachment for muscles. Many bones meet in movable joints that determine the type and extent of movement possible. Bones also protect many of the body’s vital organs. For example, the bones of the skull, thorax and pelvis are hard exterior shields that protect the brain, heart, lungs and reproductive and urinary organs.
The hollow centres of bones are filled with marrow. There are two types of marrow, known as red marrow and yellow marrow, named because of their colour. Red marrow fills the gaps between the trabeculae of spongy bone (the structure of bone is explained later in this chapter) and is the site of blood cell formation. The bones of a newborn infant have only red marrow. As we age, some of the red marrow is replaced by yellow marrow, which forms part of our fat reserve. If required, the yellow marrow can once again become red marrow. In the adult, red marrow normally exists only in the flat bones (the skull, scapulae, sternum and pelvis), the bodies of the vertebrae, the ribs and the proximal ends (closest ends to the body) of the humerus and femur.
Bones also have a crucial role in mineral homeostasis, storing the minerals calcium, phosphate and magnesium, which are essential for cellular and bodily function. Most importantly, calcium is needed for neuron synaptic transmission (see Chapter 6), as well as for haemostasis (blood clotting) and muscle contraction.
Elements of bone tissue
Mature bone is a rigid connective tissue consisting of cells, fibres, a gelatinous (jelly-like) material termed ground substance and large amounts of crystallised minerals, mainly calcium, which give bone its rigidity. Ground substance consists of proteins secreted by chondroblasts (cartilage-forming cells). The structural elements of bone are summarised in Table 20-1.
Table 20-1 THE STRUCTURAL ELEMENTS OF BONE
| STRUCTURAL ELEMENTS | FUNCTION |
|---|---|
| Bone cells | |
| Osteoblasts | Synthesise collagen and proteoglycans (polysaccharide and protein substances): stimulate osteoclast resorptive activity |
| Osteocytes | Maintain bone matrix |
| Osteoclasts | Resorb bone; assist with mineral homeostasis |
| Bone matrix | |
| Collagen fibres | Lend support and tensile strength |
| Proteoglycans | Control transport of ionised materials through matrix |
| Minerals (elements) | |
| Calcium | Crystallises to lend rigidity and compressive strength |
| Phosphate | Regulates vitamin D and thereby promotes mineralisation |
Bone is a very dynamic tissue responding to the stresses that it experiences. The cells within bone enable bone to grow, repair itself and change shape in response to stress. Even mature bone is undergoing a continuous process of synthesis and removal. The fibres in bone are collagen, which gives bone its tensile strength (the ability to resist stretch). The crystallised minerals provide the compressive strength of bone (its hardness). Ground substance acts as a medium for the diffusion of nutrients, oxygen, metabolic wastes and minerals between bone tissue and blood vessels.
Bone formation begins during fetal life with the growth of cartilage or membranes in the shape of each of the required bones. In mature bone, the repair or remodelling of bone begins with the production of an organic matrix by the bone cells. This bone matrix consists of ground substances, collagen and other proteins that take part in bone formation and maintenance.
The next step in bone formation is calcification, in which minerals are deposited and then crystallise. Minerals bind tightly to collagen fibres, producing a composite material with high tensile and compressional strength, allowing bone to withstand pressure and weight-bearing. If the pressure or weight increases, the bone will respond by increasing in size and strength.
Bone cells
Bone contains three types of cells: osteoblasts, osteoclasts and osteocytes (see Figure 20-1). Osteoblasts are the bone-forming cells. Their primary function is to lay down new bone. In the process of calcifying bone the osteoblasts imprison themselves in the newly formed bone and mature to become osteocytes. They help maintain bone by synthesising new bone matrix molecules. Osteoclasts function primarily to resorb (remove) bone.
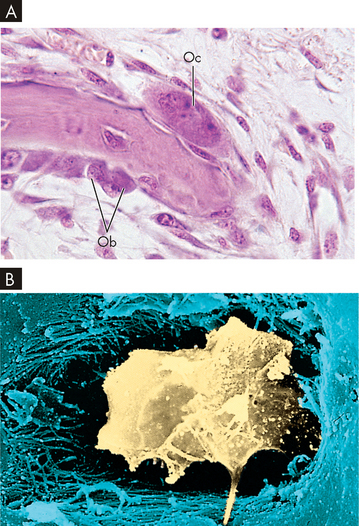
FIGURE 20-1 Cellular constituents of bone.
A Bone-resorbing (osteoclasts) and bone-forming osteoblast cells. Note the multinucleate osteoclast cell (Oc) resorbing bone on the upper surface and smaller osteoblast cells (Ob) on the under surface secreting new osteoid. B Scanning electron micrograph showing an osteocyte within a lacuna. The cell is surrounded by collagen fibres and mineralised bone.
Source: A Patton KT, Thibodeau GA. Anatomy and physiology. 7th edn. St Louis: Mosby; 2010. B Erlandsen S, Magney J. Color atlas of histology. St Louis: Mosby; 1992.
Osteoblasts
An osteoblast is a specialised fibroblast cell derived from stem cells, which produces type I collagen and is the major bone-forming cell. Osteoblasts are responsive to parathyroid hormone and produce osteocalcin (the major non-collagenous protein of the bone matrix) when stimulated by the activated form of vitamin D. Osteoblasts are active on the outer surfaces of bones, where they form a single layer of cells. Osteoblasts bring about new bone formation by their synthesis of osteoid (non-mineralised bone matrix; see Figure 20-1A). Osteoblasts also add minerals to newly formed bone matrix. Stimulation of bone formation and the orderly mineralisation of bone matrix occur by concentrating some of the plasma proteins (growth factors) found in the bone matrix and by facilitating the deposit and exchange of calcium and other ions at the site. Various growth factors (proteins and peptides) are critical components of bone formation, maintenance and remodelling.
Osteoclasts
Osteoclasts are large (typically 20–100 micrometres in diameter; 1 micrometre = 0.001 millimetre) multinucleated cells that develop from the same cells as monocytes and macrophages (see Chapter 12). Osteoclasts are the major resorptive (removal) cells of bone.1 They migrate over bone surfaces to areas that have been prepared and stripped of osteoid by enzymes produced by osteoblasts in the presence of parathyroid hormone (see Chapter 10). Osteoclasts travel over the prepared bone surfaces, creating irregular scalloped cavities known as Howship’s lacunae or resorption bays (see Figure 20-1A) as they resorb bone areas and then dissolve the bone minerals using acid. A specific area of the osteoclast cell membrane forms next to the bone surface and forms multiple infoldings to permit intimate contact with the resorption bay. These infoldings, known as the ruffled border, greatly increase the cells’ surfaces under their scalloped or ruffled borders. Osteoclasts resorb bone by secreting enzymes and hydrochloric acid. Osteoclasts also resorb bone through the action of lysosomes (vacuoles containing digestive enzymes) in their mitochondria.
Once resorption is complete, the osteoclasts loosen from the bone surface under the ruffled border through the action of calcitonin. The osteoclasts then disappear, either by degeneration to the form of their parent cells or through cell movements away from the site, in which the osteoclasts become inactive or resting osteoclasts.
Osteocytes
An osteocyte is a mature osteoblast that is trapped or surrounded in osteoid as it hardens as a result of minerals that enter during calcification (see Figure 20-1B). The osteocyte is within a space in the hardened bone matrix called a lacuna (pool or cavity). Osteocytes are mostly nucleus with a thin layer of non-mineralised osteoid around it, like the egg white surrounding an egg yolk.
The function of osteocytes is not fully understood, but it is known that they synthesise certain matrix molecules, thereby assisting bone calcification. Osteocytes are the most numerous bone cells. They also help concentrate nutrients in the matrix. Osteocytes obtain nutrients from capillaries in the canaliculi, which contain nutrient-rich fluids. Osteocytes help determine bone structure by acting as mechanosensory cells (these specialised cells are responsible for detecting stress and strain) that direct functional adaptation of bone.2 They sense the shape and structure of bone and determine where it is appropriate that bone be formed or resorbed. Osteocytes also prepare the bone for remodelling through the release of enzymes to dissolve the mineralised walls of the lacunae. Through exchanges among these cells, hormone catalysts and minerals, optimal levels of calcium, phosphorus and other minerals are maintained in blood plasma.
Bone matrix
Bone matrix is made of the extracellular elements of bone tissue, specifically collagen fibres, proteins, carbohydrate–protein complexes, ground substance and minerals.
Collagen fibres
Collagen fibres make up the bulk of bone matrix. They are formed as follows:
Proteoglycans
Proteoglycans are large complexes of numerous polysaccharides (carbohydrates) attached to a common protein core. They strengthen bone by forming compression-resistant networks between the collagen fibres. Proteoglycans also control the transport and distribution of electrically charged particles (ions), particularly calcium, through the bone matrix, thereby playing a role in bone calcium deposition and calcification.
Bone minerals
Mineralisation (crystallisation) is the final step in bone formation, after collagen synthesis and fibre formation. Mineralisation has two distinct phases: (1) initiation, where the initial mineral deposit is formed; and (2) growth, where mineral crystals are added to the initial mineral deposits. A series of complex chemical reactions transform calcium and phosphate from a fluid form into the solid hexagonal crystals of hydroxyapatite, which is essential to bone homeostasis.
Types of bone tissue
Bone is composed of two types of bony (osseous) tissue: compact bone (cortical bone) and spongy bone (trabecular or cancellous bone) (see Figure 20-2). Compact bone comprises about 85% of the skeleton; spongy bone makes up the remaining 15%. Both types of bone tissue contain the same structural elements and, with a few exceptions, both compact tissue and spongy tissue are present in every bone. The major difference between the two types of tissue is the organisation of the elements (see Figure 20-3).
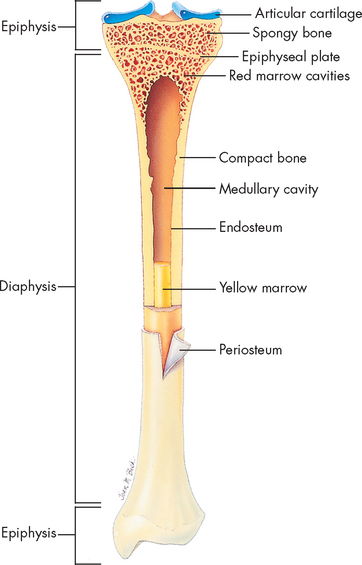
FIGURE 20-2 Cross-section of bone.
Longitudinal section of long bone (tibia) showing spongy (cancellous) and compact bone.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
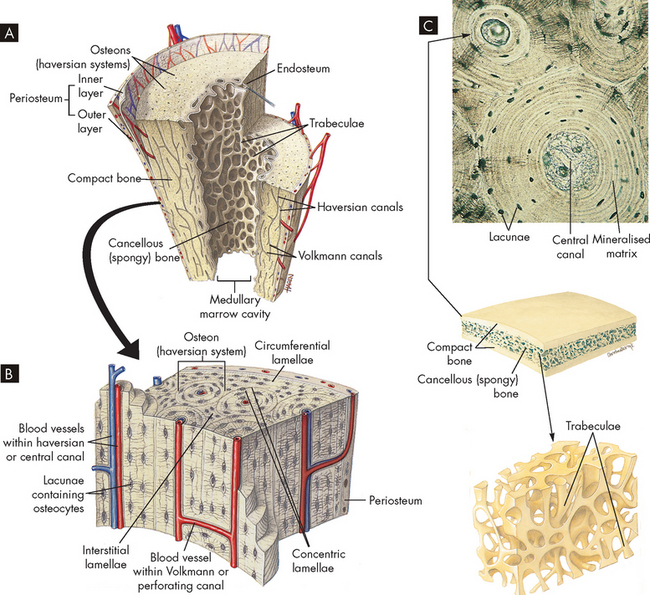
FIGURE 20-3 The structure of compact and spongy bone.
A Longitudinal section of a long bone showing both cancellous and compact bone. B A magnified view of compact bone. C Section of a flat bone. Outer layers of compact bone surround spongy bone. Fine structure of compact and spongy bone is shown in the middle.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Compact bone is highly organised, solid and extremely strong. The basic structural unit in compact bone is the haversian system (also known as an osteon) (see Figure 20-3). Each haversian system is made up of the following:
Spongy bone is less complex and lacks haversian systems. In spongy bone, the lamellae are not arranged in concentric layers but in plates or bars termed trabeculae (hence the name trabecular bone), which branch and unite with one another to form an irregular meshwork. The pattern of the network is determined by the direction of stress on the particular bone. The spaces between the trabeculae are filled with red bone marrow. The osteocyte-containing lacunae are distributed between the trabeculae and interconnected by canaliculi. Capillaries pass through the marrow to nourish the osteocytes.
All bones are covered with a double-layered connective tissue called the periosteum. The outer layer of the periosteum contains blood vessels and nerves, some of which penetrate to the inner structures of the bone through channels called Volkmann canals (see Figure 20-3). The inner layer of the periosteum is anchored to the bone by collagenous fibres that penetrate the bone.
Characteristics of bone
The 206 bones of the human skeleton are distributed between the axial skeleton and the appendicular skeleton: 80 bones are in the axial skeleton, making up the skull, vertebral column and thorax; the other 126 bones are in the appendicular skeleton, making up the upper and lower extremities, the shoulder girdle (pectoral girdle) and the pelvic girdle (os coxae) (see Figure 20-4). The skeleton contributes approximately 14% of an adult’s body weight.
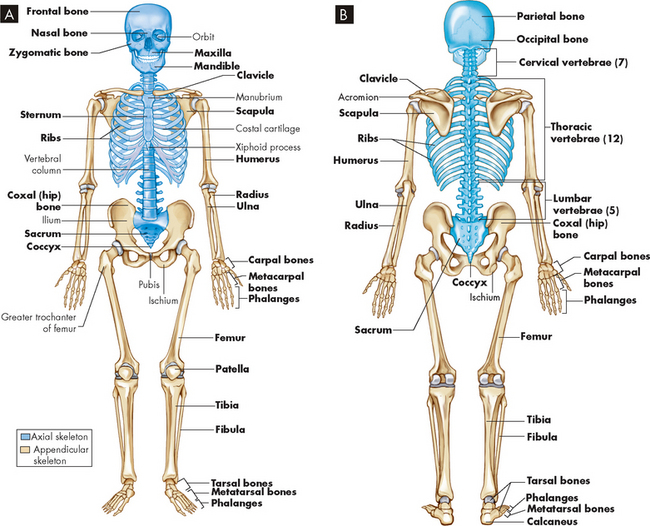
FIGURE 20-4 A Anterior view of skeleton. B Posterior view of the skeleton. Axial skeleton in blue; appendicular skeleton in tan.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Bones can be classified by shape as long, flat, short (cuboidal) or irregular (see Figure 20-5). Long bones are longer than they are wide and consist of a narrow tubular midportion (diaphysis) that merges into a broader neck (metaphysis) and a broad end (epiphysis) (see Figure 20-2).
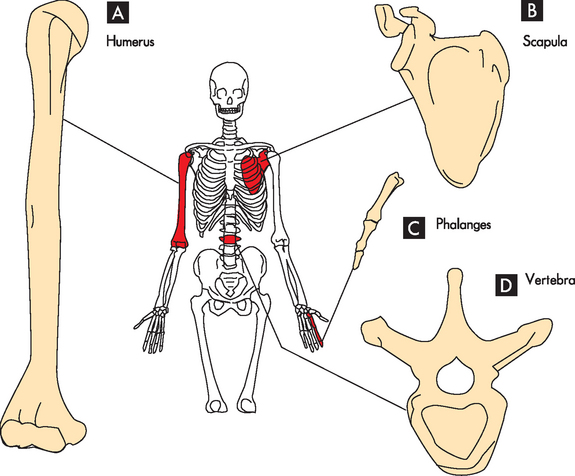
A Long bones (humerus); B flat bones (scapula); C short bones (phalanx); and D irregular bones (vertebra).
Source: Based on Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The diaphysis consists of a shaft of thick, rigid compact bone that resists bending forces. Contained within the diaphysis is the elongated marrow (medullary) cavity. The marrow cavity of the diaphysis contains primarily fatty tissue, which is referred to as yellow marrow. During times of stress the yellow marrow assists red bone marrow in haematopoiesis (blood cell production). The yellow marrow cavity of the diaphysis is continuous with marrow cavities in the spongy bone of the metaphysis and diaphysis. The marrow contained within the epiphysis is red because it contains primarily blood-forming tissue (see Chapter 16). A layer of connective tissue, the endosteum, lines the outer surfaces of both types of marrow cavity.
The broadness of the epiphysis allows weight-bearing to be distributed over a wide area. The epiphysis is made of spongy bone covered by a thin layer of compact bone. In a child, the epiphysis is separated from the metaphysis by a cartilaginous growth plate (epiphyseal plate). This growth plate allows the bone to increase in length. After puberty, the epiphyseal plate calcifies and the epiphysis and metaphysis merge. Therefore, further elongation of bones during growth is no longer possible. By adulthood, the line of demarcation between the epiphysis and metaphysis is undetectable. However, the epiphyseal plate can be clearly seen on X-rays and can be used to determine rates of bone growth.
In flat bones, such as the ribs and scapulae, two plates of compact bone are roughly parallel to each other. Between the compact bone plates is a layer of spongy bone. Short bones, such as the bones of the wrist and ankle, are often cuboidal in shape. They consist of spongy bone covered by a thin layer of compact bone. Irregular bones, such as the vertebrae, mandibles and other facial bones, have various shapes that include thin and thick segments. The thin part of an irregular bone consists of two plates of compact bone with spongy bone in between. The thick part consists of spongy bone surrounded by a layer of compact bone.
Maintenance of bone integrity
Remodelling
The internal structure of bone is maintained by remodelling, a three-phase process in which existing bone is resorbed and new bone is laid down to replace it.
 In phase 1 (activation), a stimulus (e.g. hormone, drug, vitamin, physical stressor) activates the production of osteoclasts.
In phase 1 (activation), a stimulus (e.g. hormone, drug, vitamin, physical stressor) activates the production of osteoclasts. In phase 2 (resorption), the osteoclasts gradually resorb bone, leaving behind an elongated cavity termed a resorption cavity. The resorption cavity in compact bone follows the longitudinal axis of the haversian system, whereas the resorption cavity in spongy bone parallels the surface of the trabeculae.
In phase 2 (resorption), the osteoclasts gradually resorb bone, leaving behind an elongated cavity termed a resorption cavity. The resorption cavity in compact bone follows the longitudinal axis of the haversian system, whereas the resorption cavity in spongy bone parallels the surface of the trabeculae. In phase 3 (formation), new bone (termed secondary bone) is laid down by osteoblasts lining the walls of the resorption cavity. Successive layers (lamellae) in compact bone are laid down, until the resorption cavity is reduced to a narrow haversian canal around a blood vessel. In this way, old haversian systems are destroyed and new haversian systems are formed. New trabeculae are formed in spongy bone.
In phase 3 (formation), new bone (termed secondary bone) is laid down by osteoblasts lining the walls of the resorption cavity. Successive layers (lamellae) in compact bone are laid down, until the resorption cavity is reduced to a narrow haversian canal around a blood vessel. In this way, old haversian systems are destroyed and new haversian systems are formed. New trabeculae are formed in spongy bone.Repair
The remodelling process can repair microscopic bone injuries, but gross injuries such as fractures and surgical wounds (osteotomies) heal by the same stages as soft-tissue injuries, except that new bone, instead of scar tissue, is the final result (see Chapter 13). The stages of bone wound healing are listed here and shown in Figure 20-6:
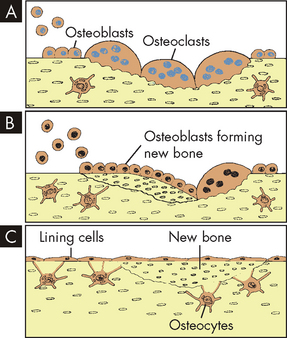
In the remodelling sequence, bone sections are removed by bone-resorbing cells (osteoclasts) and replaced with a new section laid down by the bone-forming cells, osteoblasts. The cells work in response to signals generated in that environment. Only osteoclasts mediate the first phase of remodelling. They are activated, scoop out bone, A, and resorb it; then the work of the osteoblasts begins, B. They form new bone that replaces bone removed by the resorption process, C.
The speed with which bone heals depends on the severity of the bone disruption; the type and amount of bone tissue that needs to be replaced (spongy bone heals faster); blood and oxygen supply to the site; the presence of growth and thyroid hormones, insulin, vitamins and other nutrients; the presence of systemic disease; the effects of ageing; and effective treatment, including immobilisation and the prevention of complications such as infection. In general, however, haematoma formation occurs within hours of fracture or surgery, formation of procallus by osteoblasts within days, callus formation within weeks, and replacement and contour modelling within years — up to 4 years in some cases.
THE STRUCTURE AND FUNCTION OF JOINTS
The site where two or more bones are attached is called an articulation, or more commonly joint (see Figure 20-7). The primary function of joints is to provide stability and mobility to the skeleton. Whether a joint provides stability or mobility depends on its location and its structure. Generally, joints that stabilise the skeleton have a simpler structure than those that enable the skeleton to move. Most joints provide both stability and mobility to some degree (see Figure 20-8).
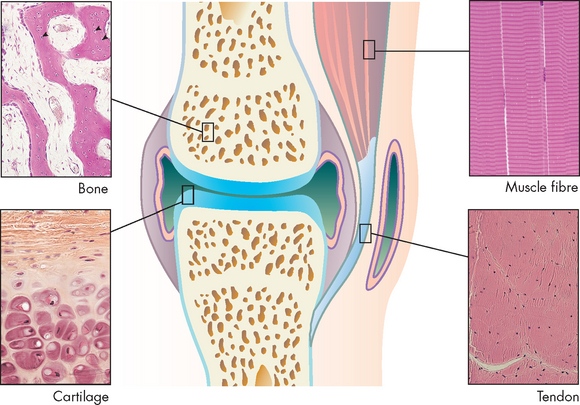
FIGURE 20-7 Main tissues of a joint.
Source: Micrographs from Gartner LP, Hiatt JL. Color textbook of histology. 3rd edn. Philadelphia: Saunders; 2007.
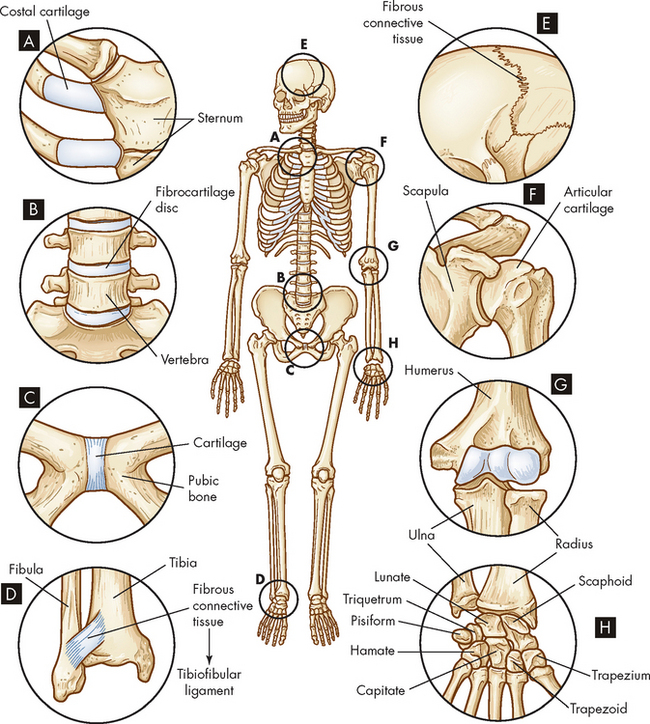
Cartilaginous (amphiarthrodial) joints, which are slightly movable, include a synchondrosis that attaches ribs to costal cartilage, A, a symphysis that connects vertebrae, B, and the symphysis that connects the two pubic bones, C. Fibrous (synarthrodial) joints, which are immovable, include the syndesmosis between the tibia and fibula, D, and sutures that connect the skull bones and the gomphosis (not shown), which holds teeth in their sockets, E. The synovial joints include the spheroid type at the shoulder, F, the hinge type at the elbow, G, and the gliding joints of the hand, H.
Joints are classified based on the degree of movement they permit or on the connecting tissues that hold them together. Based on movement, joints are classified as synarthroses (immovable joints), amphiarthroses (slightly movable joints) or diarthroses (freely movable joints) (see Figure 20-8). On the basis of connective structures, joints are classified broadly as fibrous, cartilaginous or synovial. Each of these three structural classifications can be subdivided according to the shape and contour of the articulating surfaces (ends) of the bones and the type of motion the joint permits. Clinically, most problems occur with the cartilaginous intervertebral joints and the synovial joints. It will aid your understanding of joint problems to study the anatomy of each of these joints, as outlined below.
Fibrous joints
A joint in which bone is united directly to bone by fibrous connective tissue is called a fibrous joint. These joints have no joint cavity and allow little, if any, movement. Fibrous joints are subdivided into three types:
 A suture has a thin layer of dense fibrous tissue that binds together interlocking flat bones in the skulls of young children. Sutures form an extremely tight union that permits no motion. By adulthood, the fibrous tissue has been replaced by bone.
A suture has a thin layer of dense fibrous tissue that binds together interlocking flat bones in the skulls of young children. Sutures form an extremely tight union that permits no motion. By adulthood, the fibrous tissue has been replaced by bone. A syndesmosis is a joint in which the two bony surfaces are united by a ligament or membrane. The fibres of ligaments are flexible and stretch, permitting a limited amount of movement. The paired bones of the lower arm (radius and ulna) and the lower leg (tibia and fibula) and their ligaments are syndesmotic joints.
A syndesmosis is a joint in which the two bony surfaces are united by a ligament or membrane. The fibres of ligaments are flexible and stretch, permitting a limited amount of movement. The paired bones of the lower arm (radius and ulna) and the lower leg (tibia and fibula) and their ligaments are syndesmotic joints.Cartilaginous joints
There are two types of cartilaginous joints:
 A symphysis is a cartilaginous joint in which bones are united by a pad or disc of fibrocartilage. A thin layer of hyaline cartilage usually covers the articulating surfaces of these two bones and the thick pad of fibrocartilage acts as a shock absorber and stabiliser. Examples of symphyses are the symphysis pubis, which joins the two pubic bones in the pelvis, and the intervertebral discs, which join the bodies of the vertebrae.
A symphysis is a cartilaginous joint in which bones are united by a pad or disc of fibrocartilage. A thin layer of hyaline cartilage usually covers the articulating surfaces of these two bones and the thick pad of fibrocartilage acts as a shock absorber and stabiliser. Examples of symphyses are the symphysis pubis, which joins the two pubic bones in the pelvis, and the intervertebral discs, which join the bodies of the vertebrae. A synchondrosis is a joint in which hyaline cartilage, rather than fibrocartilage, connects the two bones. The joints between the ribs and the sternum are synchondroses. The hyaline cartilage of these joints is called costal cartilage. Slight movement at the synchondroses between the ribs and the sternum allows the chest to move outwards and upwards during breathing.
A synchondrosis is a joint in which hyaline cartilage, rather than fibrocartilage, connects the two bones. The joints between the ribs and the sternum are synchondroses. The hyaline cartilage of these joints is called costal cartilage. Slight movement at the synchondroses between the ribs and the sternum allows the chest to move outwards and upwards during breathing.Synovial joints
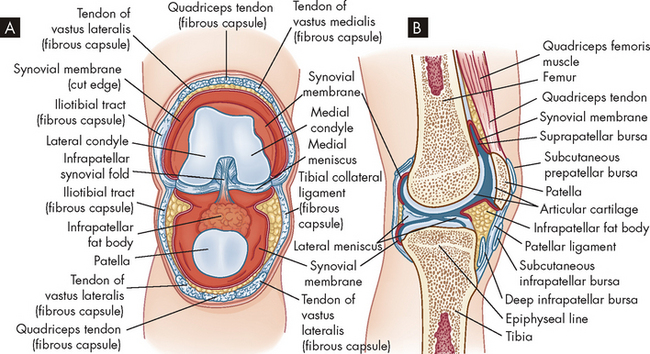
Synovial joints (diarthroses) are the most movable and the most complex joints in the body (see Figure 20-9). They incorporate a fluid-filled capsule (joint capsule) surrounding the articulating surfaces. Because injury of joints usually involves damage to some structure associated with the synovial joint, below we look more closely at the individual parts of these joints.
Joint capsule
The joint capsule comprises fibrous connective tissue that covers the ends of bones where they meet in a joint; perforating fibres firmly attach the proximal and distal capsule to the periosteum, and ligaments and tendons may also reinforce the capsule. The capsule is composed of parallel, interlacing bundles of dense, white fibrous tissue richly supplied with nerves, blood vessels and lymphatic vessels. In addition, nerves in and around the joint capsule are sensitive to the rate and direction of motion, compression, tension, vibration and pain.
Synovial membrane
The synovial membrane is a smooth, delicate membrane that lines all the interior of the joint capsule except for the joint (articular) surfaces. The membrane is organised into folds around the joint that allow movement. Some cells within the membrane ingest and remove (phagocytose) bacteria (see Chapter 13) and particles of debris in the joint cavity; others secrete a substance that gives synovial fluid its viscous quality. The synovial membrane is richly supplied with blood and lymphatic vessels and is capable of rapid repair and regeneration.
Joint cavity
The joint cavity (also called joint space) is an enclosed, fluid-filled space between the articulating surfaces of two bones. It enables two bones to move ‘against’ one another and is surrounded by synovial membrane and filled with synovial fluid.
Synovial fluid
Synovial fluid arises from plasma from blood vessels that lubricates the joint surfaces, nourishes the pad of the articular cartilage and covers the ends of the bones. The fluid also contains free-floating synovial cells and various leucocytes (white blood cells; see Chapter 16) that phagocytose joint debris and microorganisms. This is very important in maintaining an infection-free environment.
Articular cartilage
Articular cartilage is a layer of hyaline cartilage that covers the end of each bone; it may be thick or thin, depending on the size of the joint, the fit of the two bone ends, and the amount of weight and shearing force the joint normally withstands. The function of articular cartilage is to reduce friction in the joint and to distribute the forces of weight-bearing. Articular cartilage is composed of a few cartilage cells (chondrocytes) and a matrix of collagen and glycoprotein, but is mostly water. Individual water molecules rapidly enter or exit the articular cartilage to contribute to the resiliency of the tissue. It is important to note that the articular cartilage has no blood supply and also lacks the layer of cells capable of repairing or rebuilding cartilage. Therefore, damage to the articular cartilage heals very slowly.
Intra-articular menisci
Intra-articular menisci are fibrocartilage structures found in some joints. In the knee the meniscus extends partway through the joint with free borders on the medial and lateral articular surfaces. The meniscus of the knee may be torn as a result of injury. The disrupted or detached portion of the meniscus may interfere with movement of the joint, causing pain, and may lock the joint or cause it to ‘give way’. Injuries of this type are usually repaired by laparoscopic surgery and involve removing the torn or free cartilage. After surgery the meniscus may grow back to the same shape as originally.
The movement of synovial joints
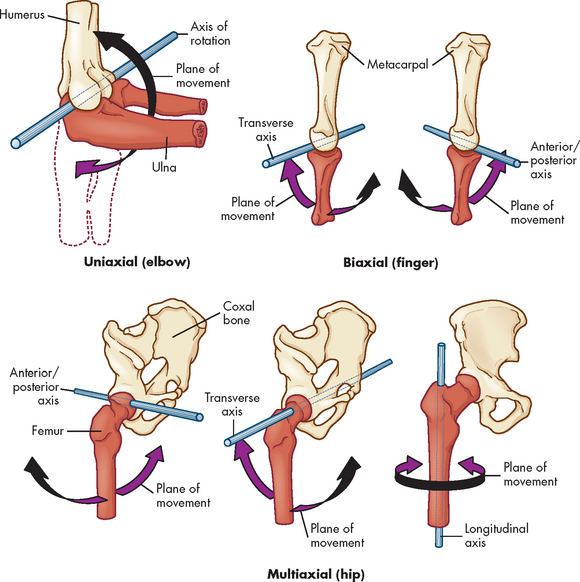
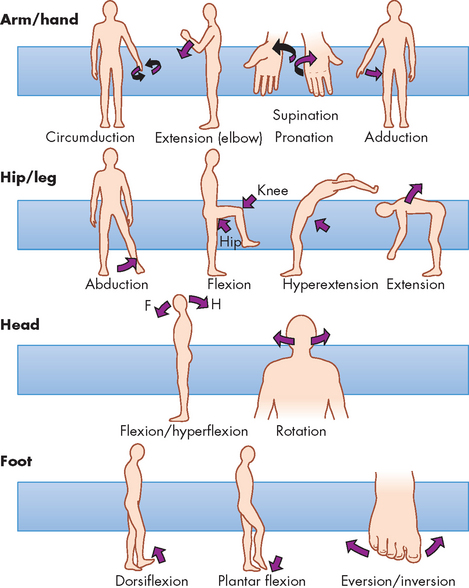
Synovial joints are described as uniaxial, biaxial or multiaxial according to the shape of the bone ends and the type of movement occurring at the joint (see Figure 20-10). Usually, one of the bones is stable and serves as an axis for the motion of the other bone. The body movements made possible by various synovial joints are either circular or angular (see Figure 20-11).
THE STRUCTURE AND FUNCTION OF SKELETAL MUSCLES
Life would not be possible without the contractile function of the skeletal muscles. They enable us to sit, walk, run, stand, speak and breathe. The total muscle mass makes up approximately 40% of an adult’s body weight and about 50% of a child’s body weight. Interestingly, muscle is mostly composed of water (accounting for about 75% of the weight), protein (20%), and organic and inorganic compounds (5%). One-third of all protein stores for energy and metabolism are contained in muscle.
We begin by looking at some features of complete muscles, before examining how muscular function is organised. Then we discuss muscular function at the cellular level — an understanding of muscle function at this level will aid your comprehension of muscular dysfunction outlined in Chapter 21. Finally, we describe some of the clinical issues related to muscle.
Whole muscle
There are more than 600 skeletal muscles in the human body (see Figure 20-12). Of these, many have common names as well as scientific ones. For example, the muscles of the upper arm are known as the biceps (biceps brachii) and triceps (triceps brachii), and those of the thigh are the known as the quadriceps (a group of muscles, the rectus femoris, and the three vastus muscles, the medialis, intermedius and lateralis) and the hamstrings (biceps femoris, semimembranosus and semitendinosus). The abdominals or ‘abs’ (rectus abdominis) support the abdomen, and the ‘pecs’ (pectoralis major) enable movement of the arm back towards the body. The group of muscles that close the opening of the true pelvis are the pelvic floor muscles (bulbocavernosus, ischiocavernosus, transverse perineal, levator ani, and urethral and anal sphincters). These latter muscles become disturbed in the process of childbirth and after birth it is particularly important for mothers to perform pelvic floor exercises.
In addition, many muscles are named according to the bone that they overlay. Thus the frontalis muscle is over the frontal bone, the temporalis muscle overlies the temporal bone and the occipitalis muscle is located over the occipital bone (note that the appropriate lobes of the brain also carry these names; see Chapter 6).
The body’s muscles vary dramatically in size and shape. They range from 2 cm to 60 cm in length and are shaped according to function:
 Pennate muscles are broad, flat and slightly fan-shaped, with fibres running at an angle to the muscle’s long axis. The multipennate deltoid muscle, which flexes and extends the arm, is a good example of a muscle shaped according to its function.
Pennate muscles are broad, flat and slightly fan-shaped, with fibres running at an angle to the muscle’s long axis. The multipennate deltoid muscle, which flexes and extends the arm, is a good example of a muscle shaped according to its function.Each skeletal muscle is a separate organ, encased in a three-part connective tissue framework called fascia. The layers of connective tissue protect the muscle fibres, attach the muscle to bones and provide a structure for a network of nerve fibres, blood vessels and lymphatic channels. The layers are as follows:
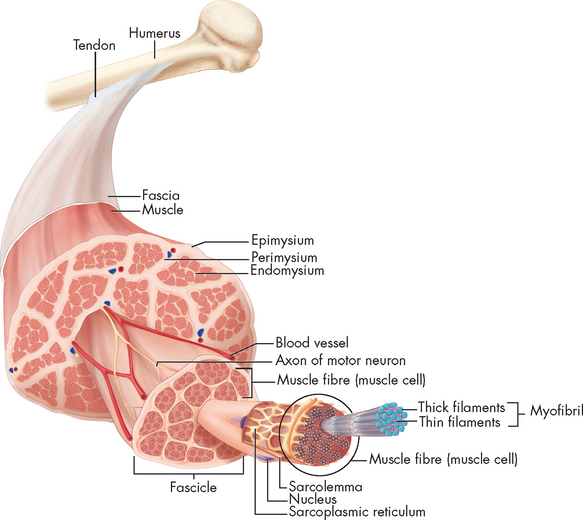
FIGURE 20-13 Cross-section of skeletal muscle showing muscle fibres and their coverings.
Note that the connective tissue coverings, the epimysium, perimysium and endomysium, are continuous with each other and with the tendon. The muscle fibres are held together by the perimysium in groups called fascicles.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Each of these connective tissue layers extends beyond the individual muscle fibres and together they form the tendon (see Figure 20-13). Thus each muscle fibre is ultimately attached to the origin (non-moving end) at one end of the muscle and to the insertion (the point the muscle is attached to that moves when the muscle contracts) at the other. Tendons allow short muscles to exert power on a distant joint, whereas a thick muscle would interfere with the ability of the joint to move freely.
The ligaments, tendons and fascia are made up of connective tissue that, because of its high tensile strength and elasticity, also buffers the limbs from the effects of sudden strains or changes in speed. The rapid recovery necessary for strenuous exercise is supported by the elastic property of muscle and its connective tissue.
Skeletal muscle has been termed voluntary (controlled directly by the nervous system) or striated (having a striped pattern when viewed under a light microscope). Components that are visible on gross inspection of the whole muscle include the motor and sensory nerve fibres. These function together with the muscle, innervating portions of it supplying sensory information to the central nervous system and providing the electrical impulses needed for motor function.
Motor unit
The motor unit enables a variation in strength and precision of contraction for different muscles. A motor unit is defined as a single motor neuron and the muscle fibres that it innervates (see Figure 20-14). The cell body of the lower motor neuron is located in the anterior horn of the spinal cord; its axon runs through a peripheral nerve to synapse with the muscle fibres it excites. Thus the motor unit may be considered as the functional unit of skeletal muscle.
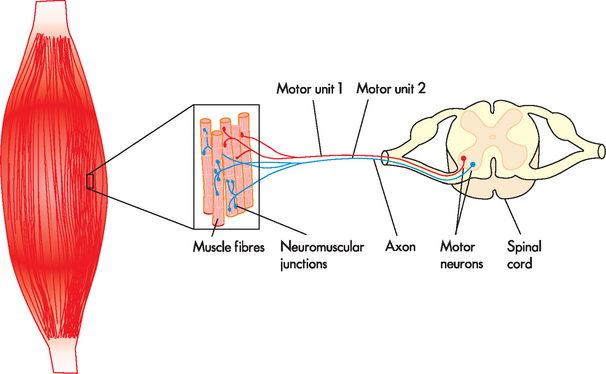
FIGURE 20-14 Motor units of a muscle.
Each motor unit consists of a motor neuron and all the muscle fibres (cells) supplied by the neuron and its axon branches.
The number of motor units per individual muscle varies greatly. In the calf, for example, one motor neuron innervates approximately 2000 muscle fibres, out of a total of 1,200,000 muscle fibres. This is a high innervation ratio of muscle fibres to neurons and it contrasts markedly with the low innervation ratio in the laryngeal muscles (involved in vocal production). There, 2 to 3 muscle fibres constitute each motor unit. The innervation ratio can be of great functional significance. The greater the innervation ratio of a particular muscle, the greater its endurance — thus, higher innervation ratios prevent fatigue. Lower innervation ratios allow for precision of movement.
Sensory receptors
Although muscles function as effector organs, they also contain sensory receptors and are involved in sending different signals to the central nervous system. Among these receptors are the muscle spindles and Golgi tendon organs. Spindles are mechanoreceptors that lie parallel to muscle fibres and respond to muscle stretching. They are the receptors involved with the muscle stretch reflex (the reflex arc is described in Chapter 6) and the maintenance of normal muscle tone. Golgi tendon organs are dendrites that terminate and branch to tendons near the neuromuscular junction. They have an inhibitory effect on overexertion of muscle contraction. In this way the Golgi tendon organs protect the muscles from rupture. Together the muscle spindles, Golgi tendon organs and free nerve endings provide a means of reporting changes in muscle length, tension, velocity and tone.
Muscle fibres
Each muscle fibre is a single muscle cell, cylindrical in structure and surrounded by a membrane capable of excitation and impulse propagation. The muscle fibre contains bundles of myofibrils, the fibre’s functional subunits, in a parallel arrangement along the longitudinal axis of the muscle (see Figure 20-15). At birth, the muscle fibres have completed development from precursor cells called myoblasts. Myoblasts are the main cells responsible for muscle growth and regeneration. Myoblasts are termed satellite cells when in a dormant state.3
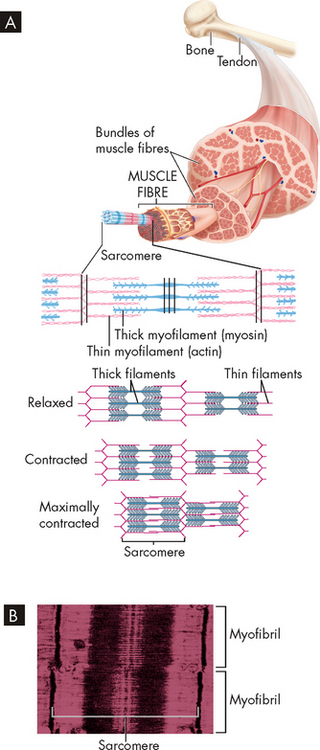
FIGURE 20-15 Skeletal muscle structure.
A Each muscle has many muscle fibres, each containing many bundles of thick and thin myofilaments. These are arranged to form adjacent segments called sarcomeres. During contraction, the thin filaments are pulled towards the centre of each sarcomere, thereby shortening the whole muscle. B This electron micrograph shows that the overlapping thick and thin filaments within each sarcomere create a pattern of dark striations in the muscle.
Source: Based on Thibodeau GA, Patton KT. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
Different types of muscle fibres are named due to their appearance: red muscle (containing the oxygen-carrying protein myoglobin, which is a red colour) and white muscle (lacking myoglobin). They are also named according to their metabolic processes: slow-twitch fibres depend on aerobic oxidative metabolism, and fast-twitch fibres use the anaerobic glycolytic pathway for rapid energy conversion (these pathways are discussed in Chapter 3). Muscle fibres are further grouped into type I (red, slow twitch) and type II (white, fast twitch).
The motor neurons supplying the different muscle fibre types have different axon diameters and conduction velocities. Type I fibres are supplied by the smallest, slowest conducting neurons, whereas type II fibres are supplied by larger, faster conducting neurons. To highlight this, we can compare the composition of fibre types in different athletes: sprinters tend to have more fast-twitch fibres than slow-twitch fibres in their leg muscles, whereas endurance runners have more slow-twitch fibres than fast-twitch fibres in their leg muscles.4 Table 20-2 describes the specific characteristics of type I and type II fibres. In addition to the type of fibres, the diameter of the axon is directly related to the speed of conduction (see Chapter 6).
Table 20-2 CHARACTERISTICS OF MUSCLE FIBRES
| CHARACTERISTICS | TYPE I (RED) | TYPE II (WHITE) |
|---|---|---|
| Anatomical location | Deep axial portion of surface muscle | Surface portion of surface muscle |
| Contraction speed | Slow | Fast |
| Motor neuron type | Type I, α | Type II, A |
| Firing frequency | Low, long duration | Rapid, short duration |
| Resistance to fatigue | High | Low |
| Myoglobin | High | Low |
| Capillary supply | Profuse | Intermediate to sparse |
| Metabolism | Oxidative | Glycolysis |
| Mitochondria | Many | Few |
| Creatine kinase | Cardiac type | Fast, skeletal |
| Example (most muscles are mixed) | Greater proportion of slow-contracting fibres in soleus | Greater proportion of fast-contracting fibres in laryngeal and ocular muscles |
| Glycogen content | Low | High |
| Intensity of contraction | Low | High |
| Aerobic metabolic capacity | High | Low |
| Fibre diameter | Small | Large |
Source: Spence AP, Mason EE. Human anatomy and physiology. 4th edn. St Paul, Minn: West Publishing; 1992.
Staining of muscle biopsy specimens cut transversely across the muscle provides a characteristic chequerboard appearance that shows an equal distribution of fibre types throughout the muscle. The existence of the different muscle fibre types in an individual muscle enables it to contract with a low force for a very long time (think how long you can walk) and also produce maximum force for a short time (how long can you maintain a sprint?). Nevertheless, some muscles contain proportionally more of one fibre type than another. The postural muscles have more type I fibres, allowing them the high resistance to fatigue that is necessary to maintain the same position for extended periods. The ocular muscles have more type II muscle fibres, allowing them to respond rapidly to visual changes.
The number of muscle fibres varies according to location. Large muscles, such as the gastrocnemius in the calf, have more fibres (1,200,000) than smaller muscles, such as the lumbrical muscles in your hands (10,000). The diameter of muscle fibres also varies. The closely packed polygons (muscle fibres) are small (10–20 micrometres) until puberty, when they attain the normal adult diameter of 40–80 micrometres. Females usually have smaller diameter fibres than males. Muscle fibres in small muscles, such as the ocular muscles, are approximately 15 micrometres in diameter; while in larger, more proximal muscles they are 40 micrometres. Only type II fibres are capable of hypertrophy (increasing cell size in response to increased functional demand; see Chapter 4). Thus body builders who train by doing few repetitions of near maximal force (5–8 repetitions using at least 80% of maximal force) over time develop large muscles (hypertrophied), while endurance athletes who train for long periods with less force do not develop hypertrophied muscles but instead develop enhanced capability of type I muscle.
The major components of the muscle fibre include the cell membrane (sarcolemma and basement membrane), myofibrils, transverse tubules (T tubules), sarcoplasmic reticulum (called endoplasmic reticulum in other cells), sarcoplasm (cytoplasm) and mitochondria (see Figure 20-16). Muscles receive inputs from neurons to initiate contraction, at the neuron–muscle synapse known as the neuromuscular junction. At the motor nerve end plate, where the nerve impulse is transmitted, the sarcolemma forms the highly convoluted synaptic cleft. The sarcolemma is made up of lipid molecules and proteins. The proteins perform normal cellular functions, such as transport of nutrients and protein synthesis (production). They also provide the sodium–potassium pump and, importantly, include the cell’s cholinergic receptor (so named because it binds acetylcholine). This allows acetylcholine to bind, when released from the neuron, to initiate muscle contraction.
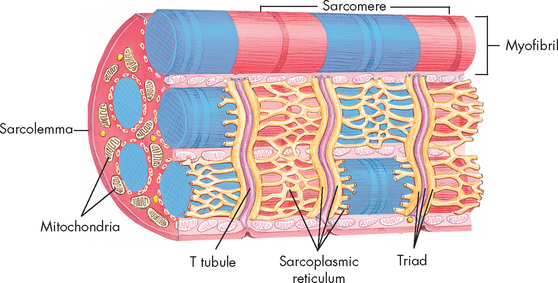
FIGURE 20-16 Skeletal muscle cell.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Contained within the sarcolemma is the sarcoplasm, the cytoplasm of the muscle cell containing the intracellular components that are common to all cells (see Chapter 3). The multiple nuclei of skeletal muscle fibres lie in the sarcoplasm, adjacent to the inside surface of the sarcolemma. The sarcoplasm provides a matrix that surrounds the myofibrils. It contains numerous enzymes and proteins that are responsible for the cell’s energy production, protein synthesis and oxygen storage. The multiple mitochondria house enzyme systems for energy production. Many other structures are present in the sarcoplasm. The ribosomes are primarily composed of RNA and participate in the process of protein synthesis. Glycogen granules (stores of glucose), myoglobin (a red pigment similar to haemoglobin that stores oxygen) and lipid droplets are suspended in the sarcoplasmic matrix. These are all important to muscle metabolism.
Unique to the muscle is the sarcotubular system, a network that includes the transverse tubules and the sarcoplasmic reticulum, which crosses the interior of the cell. In the muscle cells, the sarcoplasmic reticulum is involved in calcium transport, which initiates muscle contraction at the sarcomere, a portion of the myofibril. The sarcoplasmic reticulum is composed of tubules that run parallel to the myofibrils. The longitudinal tubules are termed sarcotubules. The transverse tubules, which are closely associated with the sarcotubules, run across the sarcoplasm and communicate with the extracellular space. Together, the tubules of this membrane system allow for intracellular calcium uptake, regulation, release during muscle contraction and storage during muscle relaxation.
Soft-tissue repair
Developments in identifying musculoskeletal progenitor cells (myogenic stem cells) have opened up a new realm of tissue repair. It may soon be much easier to manage difficult problems involving severe tendon or muscle injuries. Mesenchymal progenitor cells are programmed to form fat, bone, muscle, tendon and cartilage. Transcriptional factors, including the transforming growth factor beta superfamily and other related intracellular proteins, transmit signals from surface cells to the nucleus.
One group of intracellular proteins known as Smads has been shown to induce osteoblast formation, and appear to promote and enhance skeletal muscle differentiation and assist in the formation of tendon cells. Satellite cells found in muscle are capable of tissue regeneration and have been grown in culture. Because they are already established as muscle cells, satellite cells form muscle more precisely than even myogenic cells.
These discoveries present the real opportunity to engineer new tissues that can successfully treat conditions such as muscular dystrophy, severe traumatic injuries, burns and anatomic defects. The regeneration and formation of new tissue holds great promise for the fields of orthopaedics, plastic surgery, trauma and rehabilitation.
Source: Bach AD et al. Skeletal muscle tissue engineering. J Cell Mol Med 2004; 8(4):413–422; Towler DA, Gelberman RH. The alchemy of tendon repair: a primer for the (S)mad scientist. J Clin Investig 2006; 116(4):863–866.
Myofibrils, sarcomeres and myofilaments
Myofibrils are the functional units of muscle contraction. They are the most abundant subcellular muscle component, equalling 85–90% of the total volume. On cross-section, they are seen to be irregular polygons with a mean diameter of between 1 and 2 micrometres. Myofibrils are densely packed — there are up to thousands in each muscle fibre. Within each myofibril are sarcomeres, the contractile units of the muscle cell. Sarcomeres are arranged in parallel and appear at regular intervals (see Figure 20-15). They are normally between 1.6 and 2.2 micrometres long. Sarcomeres are made up of hundreds of rod-like structures called myofilaments. These are divided into thick and thin myofilaments, which contain the proteins actin, myosin, tropomyosin and troponin (see Table 20-3). The thick myofilament is made up of myosin, while the thin myofilament is composed mainly of actin, with tropomyosin and troponin attached. The reason why the thin myofilament is composed of three proteins and the thick myofilament only one lies in the size of the proteins. Myosin has two subunits that resemble a golf club shaft bundled together and appears quick thick. In contrast, actin, tropomyosin and troponin strands twist around each other and appear thin. The importance of these proteins will become evident when we discuss muscle contraction later in the chapter. These proteins refract light differently, such that actin appears light and myosin appears dark. As a result, the muscle cells appear striped or striated.
Table 20-3 CONTRACTILE PROTEINS OF SKELETAL MUSCLE FIBRILS
| NAME | APPROXIMATE PERCENTAGE OF MYOFIBRIL PROTEIN | FUNCTION |
|---|---|---|
| Myosin | 50–55% | Contraction; breaks down ATP and develops tension |
| Actin | 20% | Contraction; interacts with myosin |
| Troponin | 7% | Regulatory protein; in presence of calcium, promotes actin–myosin activation |
| Tropomyosin | 5–7% | Regulatory and structural function; links filaments, controls filament length |
Source: Buckwater JA, Einhorn TA, Simon SR (eds). Orthopaedic basic science. 2nd edn. Chicago: American Academy of Orthopaedic Surgeons; 2000; Costa ML et al. Desmin: molecular interactions and punitive functions of the muscle intermediate filament protein. Braz J Med Biol Res 2004; 37(12):1819–1830; Liu X, Pollack GH. Stepwise sliding of single actin and myosin filaments. Biophysical J 2004; 86:353–358.
Non-protein constituents of muscle
Substances such as nitrogen, creatine, creatinine (a breakdown product of creatine), phosphocreatine (an energy-storing compound), purines, uric acid and amino acids all serve in the complex process of muscle metabolism. Energy is provided by glycogen and its derivatives.
Creatine metabolism and creatinine metabolism have been used to measure muscle mass. Plasma creatine is taken up by muscle and converted into the high-energy phosphate compound phosphocreatine by the enzyme creatine kinase. Creatinine is formed constantly in muscle from creatine. Plasma levels of creatinine are increased in muscle wasting diseases (because production of creatinine is increased) and kidney disease (because excretion of creatinine is reduced). (Tests for plasma creatinine are discussed in Chapter 28.)
Components of muscle function
The ultimate function of muscle is to contract. Muscle contraction occurs on the molecular level and leads to the observable phenomenon of muscle movement.
Neuromuscular junction
The area where the nerve fibre meets the muscle cell is referred to as the neuromuscular junction (see Figure 20-17). The motor neuron (efferent neuron that innervates muscle) travels to the motor unit, where it connects to the sarcolemma of the muscle cell. It is important to understand that the end of the neuron, referred to as the presynaptic membrane, does not physically touch the sarcolemma of the muscle cell. Basically, there is a synapse between these two structures, which is a space called the synaptic cleft. The sarcolemma side is referred to as the postsynaptic membrane and when stimulated it causes muscle contraction. Muscle contraction occurs because the electrical signal (the action potential in the neuron; review using Chapter 6) is converted to a chemical signal, which travels across the synaptic cleft to interface with receptors on the postsynaptic membrane. This triggers the generation of another electrical signal or action potential in the sarcolemma, resulting in muscle contraction. We now examine in detail how this arises.
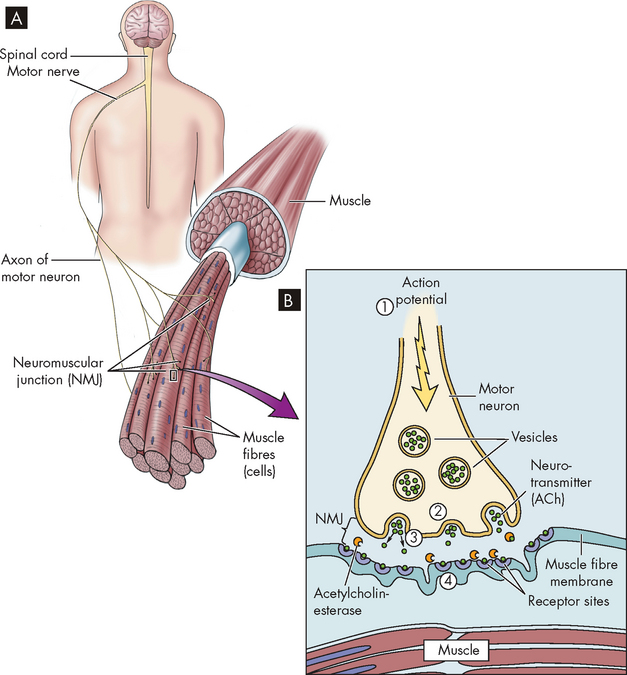
FIGURE 20-17 The arrangement of muscle fibres and the neuromuscular junction.
A Muscle fibres and motor neurons. B The four steps in the transmission of the signal at the neuromuscular junction. (1) The action potential is transmitted to the end of the motor neuron. (2) The acetylcholine-filled vesicles move the presynaptic membrane. (3) The vesicles release acetylcholine into the synapse. (4) Acetylcholine binds to the postsynaptic membrane and causes depolarisation.
Source: Based on Herlihy B. The human body in health and illness, 3rd edn. St Louis: Saunders; 2007.
Muscle contraction at the molecular level
The four steps of muscle contraction are (1) excitation, (2) coupling, (3) contraction and (4) relaxation. The process involves the movement of ions across the plasma membrane and through the sarcotubular system. The muscle fibre is an excitable tissue. At rest, an electric charge of −90 mV is continually maintained across the sarcolemma. This resting potential, generated by the presence of sodium ions outside the cell and potassium ions inside the cell against their respective concentration gradients, is created and maintained by the energy-requiring activity of the sodium–potassium pump (see Chapter 3).
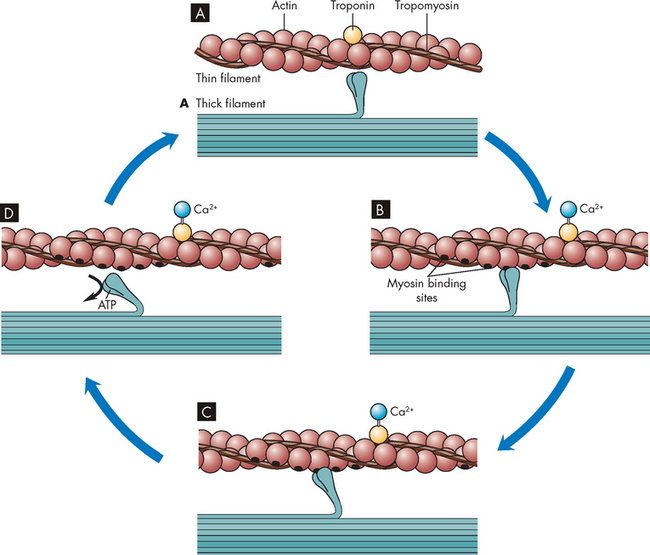
FIGURE 20-18 The cross-bridge cycle of muscle contraction.
A The myosin head has a high affinity for actin but cannot bind because the actin-binding sites are not accessible. B When calcium enters the cell it binds to troponin, and the tropomyosin-blocking protein moves to allow myosin to bind actin, forming a cross-bridge. C The act of binding changes the shape of myosin and slides the actin along. D When a new molecule of ATP binds to the myosin, it changes and releases from the actin.
Source: Based on Copstead L-EC, Banasik JL. Pathophysiology. 4th edn. St Louis: Saunders; 2010.
Muscle metabolism
As an energetic tissue, skeletal muscle requires a constant supply of ATP and phosphocreatine. These substances are necessary to fuel the complex processes of muscular function, driving the detachment of the myosin heads from the actin during contraction and transporting calcium ions back into the sarcoplasmic reticulum to enable relaxation. Another internal process of the muscular system that requires ATP is protein synthesis, which replenishes muscle constituents and accommodates growth and repair. The rate of protein synthesis is related to hormone levels (particularly insulin), amino acid substrates and overall nutritional status. At rest, the rate of ATP formation from glucose is sufficient to maintain internal processes, given normal nutritional status. During activity, the need for ATP increases 100-fold. Activity lasting longer than 5 seconds expends the available stored ATP and phosphocreatine.
Stored glycogen and blood glucose are converted anaerobically to sustain brief, intense activity without increasing the demand for oxygen. Anaerobic glycolysis (see Chapter 3) is much less efficient than aerobic metabolism, using six to eight times more glucose to produce the same amount of ATP. With increased activity, such as very intense exercise or with ischaemia, lactic acid concentrations increase, causing a decrease in muscle pH. This short-term mechanism buys time by allowing ATP formation in spite of inadequate energy stores or oxygen supply. When the anaerobic threshold is reached and more oxygen is required, physiological changes occur, including an increase in lactic acid and increases in oxygen consumption, heart rate, breath rate and muscle blood flow.
Strenuous exercise requires oxygen, which activates aerobic metabolism to form ATP. Maximal exercise increases oxygen uptake by 15 to 20 times over the resting state. When these systems become exhausted or inadequate to respond to the need for ATP, fatigue and weakness finally force the muscle to reduce activity.
One other factor that changes energy requirements is muscle fibre type. Type II fibres rely on anaerobic glycolytic metabolism and fatigue readily. Type I fibres can resist fatigue for longer periods because of their capacity for oxidative metabolism.
Muscle mechanics
The amount of tension a muscle fibre can develop depends on the number of cross-bridges formed. In addition, muscle fibres and motor units contract according to the all-or-none principle, meaning that they have two states, either contraction (on) or relaxation (off). The nervous system is able to control the force of contraction of a complete muscle by employing more (or fewer) motor units. As the requirement for force increases, more and more motor units are stimulated — this process is known as recruitment. The nervous system is also able to alter the frequency of stimulation of individual motor units, a process known as repetitive discharge.
Recruitment and repetitive discharge of motor units allow the muscle to activate the number of motor units needed to generate the desired force. The total force developed is the sum of the force generated by each motor unit. As the strength, speed and duration of stimuli increase, the summation of contractions reaches a critical frequency called tetanus, beyond which further contraction cannot occur.
Other variables, such as fibre type, innervation ratio, muscle temperature and muscle shape, influence the efficiency of muscular contraction. The two muscle fibre types differ in their responses to electrical activity. Speed of contraction and tetanus are achieved more rapidly in type II (white fast-twitch) than in type I (red slow-twitch) muscle fibres. Low innervation ratios promote control and coordination, whereas high ratios promote strength and endurance. Muscles work best at normal body temperature, approximately 37°C. Finally, muscles with a large cross-sectional area, such as the fan-shaped pennate muscles, develop greater contractile forces than smaller diameter muscles.
The initial length of a muscle and the range of shortening that occur when the muscle contracts also determine the force it can generate. The maximal strength that a muscle can generate is directly related to the length of the muscle fibres before contraction. This is referred to as the length–tension relationship (see Figure 20-19). This is important to understand, because if the muscle is stretched too much, the myosin has nothing to bind to and muscle contraction will either not occur or be very weak. Conversely, if the muscle is shortened, the actin and myosin (sarcomere) are compressed and no tension can be developed. For instance, the long fusiform muscles (elongated muscles that are spindle-shaped, such as the biceps) have a greater range of shortening and can contract up to 57% of their resting length. Pre-stretching a muscle will increase the force and speed with which it can contract (in the heart this effect is known as the Frank-Starling mechanism; see Chapter 22). If an athlete completes two vertical jumps, the first by crouching and holding the crouch for 5 seconds before jumping, and the second by crouching and immediately jumping, they will achieve a higher jump on the second attempt. The length–tension concept is particularly important in cardiac muscle contraction, which is explained in Chapter 22. This is crucial to your understanding of why the heart muscle cannot generate an adequate contraction during heart failure, which is discussed in detail in Chapter 23.
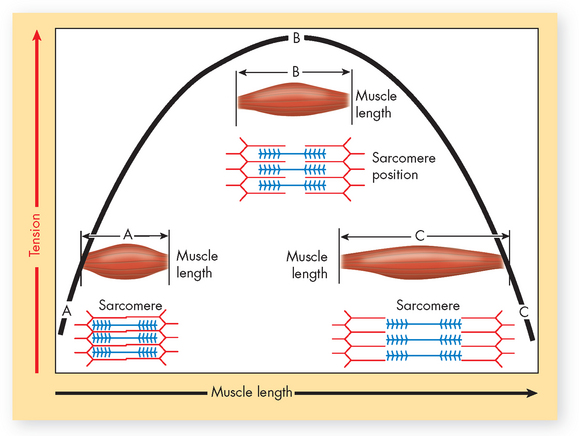
FIGURE 20-19 The length–tension relationship.
As this graph of muscle tension shows, the maximum strength that a muscle can develop is directly related to the initial length of its fibres. At a short initial length the sarcomeres are already compressed, and thus the muscle cannot develop much tension (position A). Conversely, the thick and thin myofilaments are too far apart in an overstretched muscle to generate much tension (position C). Maximum tension can be generated only when the muscle has been stretched to a moderate, optimal length (position B).
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Types of muscle contraction
During isometric contraction (same length), the muscle maintains constant length as tension is increased (see Figure 20-20). Isometric contraction occurs, for example, when the arm or leg is pushed against an immovable object. The muscle contracts, but the limb does not move.
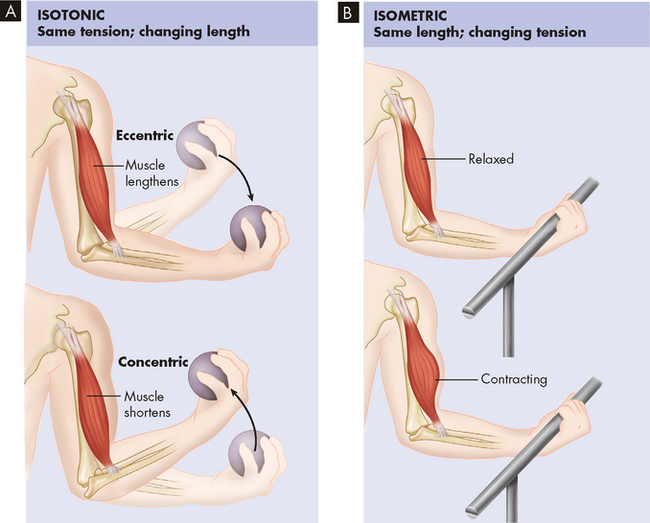
FIGURE 20-20 Isotonic and isometric contraction.
A In isotonic contraction, the muscle shortens, producing movement. B In isometric contraction, the muscle pulls forcefully against a load but does not shorten.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
During isotonic contraction (same tension), the muscle maintains a constant tension as it moves. Isotonic contractions can be eccentric (lengthening) or concentric (shortening). Positive work is accomplished during concentric contraction and energy is released to exert force or lift a weight. In contrast, during eccentric contraction the muscle lengthens and absorbs energy. Eccentric contraction requires less energy to accomplish and often results in the development of pain and stiffness after unaccustomed exercise. The pain is known as delayed-onset muscle soreness (DOMS) and can be produced easily by running downhill or doing a type of exercise that you have not done for a lengthy period of time.
The movement of muscle groups
Muscles do not act alone but in groups, often under automatic control. When a muscle contracts and acts as a prime mover (agonist), the muscle that opposes it (antagonist) relaxes. This is easily tested by holding the right arm in the horizontal position in front of the body, then bending the elbow while feeling the biceps in the front and the triceps in the back with the other hand. The biceps is firm and the triceps is soft. When the elbow is extended, the biceps is soft and the triceps firm. Completing this movement causes the agonist and antagonist to change automatically; only the movement is commanded, not the alternate contraction and relaxation of the specific muscle groups.
Other associated actions may be seen during walking; as the foot leaves the ground, the paravertebral and gluteal muscles on the opposite sides of the body contract to maintain balance. An individual will notice the loss of the associated muscle’s action when paralysis offsets this process and decreases balance. If a person is partially paralysed, difficulty in maintaining balance is noticeable.
The clinical relevance of skeletal muscle
 For some clinical procedures where muscle contraction is unwanted it is necessary to paralyse the appropriate muscle. The receptors in the neuromuscular junction that are a subgroup of cholinergic receptors known as nicotinic receptors (so-called because they are stimulated by nicotine) are the targets of two different classes of paralysing drugs. One class is known as depolarising, because the drug (suxamethonium) attaches to the receptor, causing the muscle fibre to depolarise (contract). You may notice fasciculations (small, involuntary contractions of parts of the muscle without any coordination) when using this drug. The drug remains attached to the receptor longer than acetylcholine (about 8 minutes) and is not degraded by the enzyme acetylcholine esterase (the enzyme that normally breaks down acetylcholine). Because of a wide range of contraindications this class of drug is typically only used in emergency situations, such as an emergency intubation. The other class is known as non-depolarising, because the muscle fibre does not depolarise. The drug (pancuronium or vecuronium) binds to the receptor and blocks the acetylcholine from binding. Non-depolarising drugs have varying speed of onset and length of action and are typically used for open bowel or laparoscopic surgery.
For some clinical procedures where muscle contraction is unwanted it is necessary to paralyse the appropriate muscle. The receptors in the neuromuscular junction that are a subgroup of cholinergic receptors known as nicotinic receptors (so-called because they are stimulated by nicotine) are the targets of two different classes of paralysing drugs. One class is known as depolarising, because the drug (suxamethonium) attaches to the receptor, causing the muscle fibre to depolarise (contract). You may notice fasciculations (small, involuntary contractions of parts of the muscle without any coordination) when using this drug. The drug remains attached to the receptor longer than acetylcholine (about 8 minutes) and is not degraded by the enzyme acetylcholine esterase (the enzyme that normally breaks down acetylcholine). Because of a wide range of contraindications this class of drug is typically only used in emergency situations, such as an emergency intubation. The other class is known as non-depolarising, because the muscle fibre does not depolarise. The drug (pancuronium or vecuronium) binds to the receptor and blocks the acetylcholine from binding. Non-depolarising drugs have varying speed of onset and length of action and are typically used for open bowel or laparoscopic surgery. Various medications are injected into muscle. Generally a larger amount of medication may be injected intramuscularly than subcutaneously. Because the muscles are situated below the skin and subcutaneous fat, a longer needle is required. The speed of onset of drug action depends on the blood supply of the muscle but is slower than the intravenous route and the effect is longer lasting. Intramuscular sites include the upper arm (deltoid), thigh (vastus lateralis) and buttocks (ventrogluteal and dorsogluteal) — when the gluteal muscles are used, the site should be in the upper outer quadrant to avoid the sciatic nerve. The most common complications of muscle injections are due to damaging a nerve or accidental injection into a blood vessel (which can be avoided by a brief aspiration after the needle is inserted to check whether any blood enters the syringe).
Various medications are injected into muscle. Generally a larger amount of medication may be injected intramuscularly than subcutaneously. Because the muscles are situated below the skin and subcutaneous fat, a longer needle is required. The speed of onset of drug action depends on the blood supply of the muscle but is slower than the intravenous route and the effect is longer lasting. Intramuscular sites include the upper arm (deltoid), thigh (vastus lateralis) and buttocks (ventrogluteal and dorsogluteal) — when the gluteal muscles are used, the site should be in the upper outer quadrant to avoid the sciatic nerve. The most common complications of muscle injections are due to damaging a nerve or accidental injection into a blood vessel (which can be avoided by a brief aspiration after the needle is inserted to check whether any blood enters the syringe). The bacteria Clostridium botulinum, which may be present in canned or smoked food, causes the disease botulism after eating the contaminated food. The bacteria produce a toxin that prevents the release of acetylcholine into the neuromuscular junction, leading to a potentially fatal paralysis. The use of this toxin as Botox for cosmetic use and the treatment of cerebral palsy is discussed in Chapter 9.
The bacteria Clostridium botulinum, which may be present in canned or smoked food, causes the disease botulism after eating the contaminated food. The bacteria produce a toxin that prevents the release of acetylcholine into the neuromuscular junction, leading to a potentially fatal paralysis. The use of this toxin as Botox for cosmetic use and the treatment of cerebral palsy is discussed in Chapter 9. When a person dies the ATP supply in the muscles becomes exhausted. As a result, all the cellular processes relying on ATP (such as the sodium–potassium pump and calcium pump, which reclaim calcium ions to the sarcoplasmic reticulum) cease to function. The result is liberation of calcium ions into the muscle fibres, which then contract. Without ATP the cross-bridges cannot detach and so the muscles lock in the contracted state. Every muscle is affected and consequently the person’s body becomes very rigid. This is known as rigor mortis. The dead person will remain in this state until lysosomal enzymes (released from all the cells as they lyse) break down the myosin approximately 36–62 hours after death.
When a person dies the ATP supply in the muscles becomes exhausted. As a result, all the cellular processes relying on ATP (such as the sodium–potassium pump and calcium pump, which reclaim calcium ions to the sarcoplasmic reticulum) cease to function. The result is liberation of calcium ions into the muscle fibres, which then contract. Without ATP the cross-bridges cannot detach and so the muscles lock in the contracted state. Every muscle is affected and consequently the person’s body becomes very rigid. This is known as rigor mortis. The dead person will remain in this state until lysosomal enzymes (released from all the cells as they lyse) break down the myosin approximately 36–62 hours after death.AGEING AND THE MUSCULOSKELETAL SYSTEM
Ageing of bones
Ageing is accompanied by the loss of bone tissue. Bones become less dense, less strong and more brittle with increasing age. The bone remodelling cycle takes longer to complete and the rate of mineralisation also slows.
As women age, they experience loss of bone density, accelerated by the rapid bone loss that occurs during early menopause from increased osteoclastic bone resorption. By age 70 years, susceptible women have, on the average, lost 50% of their peripheral cortical bone mass. Bone mass losses to such an extent lead to deformity, pain, stiffness and high risk for fractures.
Men experience bone loss also, but at later ages and much slower rates (about 3% of bone mass per decade) than women (about 8% of bone mass per decade). Also, initial bone masses in men are approximately 30% higher than in women; therefore, bone loss in men causes less risk of disability than for women. Men’s peak bone mass is related to their race, heredity, hormonal factors, physical activity and calcium intake during childhood.
Bone loss in both sexes is related to smoking, calcium deficiency, alcohol intake and physical inactivity. Bone mass can be gained in healthy young women up to the third decade through physical, weight-bearing activity, intake of dietary calcium and other minerals, and use of oral contraceptives. Height is also lost with ageing because of intervertebral disc degeneration and, sometimes, osteoporotic spinal fractures.
Stem cells in the bone marrow perform less efficiently, predisposing older persons to acute and chronic illnesses. Such illnesses cause weakness and confusion and may increase the risk of injury or falling.
Ageing of joints
With ageing, cartilage becomes more rigid, fragile and susceptible to fibrillation because of more cross-linking of collagen and elastin, decreasing water content in the cartilage ground substance and decreasing concentrations of glycosaminoglycans. Decreased range of motion of the joint is related to the changes in ligaments and muscles. Bones in joints develop evidence of osteoporosis with fewer trabeculae and thinner, less dense bones, making them prone to fractures. Intervertebral disc spaces decrease in height. The rate of loss of height accelerates at age 70 years and beyond. Tendons shrink and harden.
Ageing of muscles
The function of skeletal muscle depends on many influences that are affected by ageing, including the nervous, vascular and endocrine systems. In the young child, the development of muscle tissue depends greatly on continuing neurodevelopmental maturation. Muscle fibre composition in adults does not change until late in life, but the variation among individuals increases with age. Muscle function remains trainable even into advanced age. Maintaining musculoskeletal fitness at any age can improve overall health.6 Muscle diseases have a definite association with specific age groups. Muscular dystrophies occur in children, and muscle disabilities related to rheumatic diseases usually occur in advancing age.
Age-related loss in skeletal muscle is referred to as sarcopenia and is a direct cause of the age-related decrease in muscle strength. As the body ages, muscle mass and strength decline slowly; thus, strength is maintained into the 50s, with a slow decline in dynamic and isometric strength evident after age 70 years. Type II fibres also decrease. There is reduced RNA synthesis, loss of mitochondrial function7 and reduction in the size of motor units. The regenerative function of muscle tissue remains normal in ageing individuals. As much as 30–40% of skeletal muscle mass and strength may be lost from the third to ninth decades. Muscle fatigue may also contribute to loss of function with ageing.8 Sarcopenia is thought to be secondary to progressive neuromuscular changes and diminishing anabolic hormones.
Maximal oxygen intake declines with age. The basal metabolic rate is reduced and lean body mass decreases in the aged population.
The structure and function of bones
 Bones provide support and protection for the body’s tissues and organs, are important in mineral homeostasis and are the source of all blood cells.
Bones provide support and protection for the body’s tissues and organs, are important in mineral homeostasis and are the source of all blood cells. Bone formation begins with the production of an inorganic matrix by bone cells. Bone minerals crystallise in and around collagen fibres in the matrix, giving bone its characteristic hardness and strength.
Bone formation begins with the production of an inorganic matrix by bone cells. Bone minerals crystallise in and around collagen fibres in the matrix, giving bone its characteristic hardness and strength. Bone tissue is continuously being resorbed and synthesised by osteoclasts and osteoblasts in a process known as remodelling.
Bone tissue is continuously being resorbed and synthesised by osteoclasts and osteoblasts in a process known as remodelling. Bones in the body are made up of compact bone tissue and spongy bone tissue. Compact bone is highly organised into haversian systems that consist of concentric layers of crystallised matrix surrounding a central canal that contains blood vessels and nerves. Dispersed throughout the concentric layers of crystallised matrix are small spaces containing osteocytes. Smaller canals, called canaliculi, interconnect the osteocyte-containing spaces. The crystallised matrix in spongy bone is arranged in bars or plates. Spaces containing osteocytes are dispersed between the bars or plates and interconnected by canaliculi.
Bones in the body are made up of compact bone tissue and spongy bone tissue. Compact bone is highly organised into haversian systems that consist of concentric layers of crystallised matrix surrounding a central canal that contains blood vessels and nerves. Dispersed throughout the concentric layers of crystallised matrix are small spaces containing osteocytes. Smaller canals, called canaliculi, interconnect the osteocyte-containing spaces. The crystallised matrix in spongy bone is arranged in bars or plates. Spaces containing osteocytes are dispersed between the bars or plates and interconnected by canaliculi. There are 206 bones in the body divided into the axial skeleton and appendicular skeleton. Bones are classified by their shape as long, short, flat or irregular. Long bones have a broad end (epiphysis), broad neck (metaphysis) and narrow midportion (diaphysis), which contains the medullary cavity.
There are 206 bones in the body divided into the axial skeleton and appendicular skeleton. Bones are classified by their shape as long, short, flat or irregular. Long bones have a broad end (epiphysis), broad neck (metaphysis) and narrow midportion (diaphysis), which contains the medullary cavity. Bone injuries are repaired in stages. Haematoma formation provides the fibrin framework for the formation and organisation of granulation tissue. The granulation tissue provides a cartilage model for the formation and crystallisation of bone matrix. Remodelling restores the original shape and size to the injured bone.
Bone injuries are repaired in stages. Haematoma formation provides the fibrin framework for the formation and organisation of granulation tissue. The granulation tissue provides a cartilage model for the formation and crystallisation of bone matrix. Remodelling restores the original shape and size to the injured bone.The structure and function of joints
 Joints are classified as synarthroses, amphiarthroses or diarthroses, depending on the degree of movement they allow.
Joints are classified as synarthroses, amphiarthroses or diarthroses, depending on the degree of movement they allow. Joints are also classified by the type of connecting tissue holding them together. Fibrous joints are connected by dense fibrous tissue, ligaments or membranes. Cartilaginous joints are connected by fibrocartilage or hyaline cartilage. Synovial joints are connected by a fibrous joint capsule. Within the capsule is a small fluid-filled space. The fluid in the space nourishes the articular cartilage, which covers the ends of the bones meeting in the synovial joint.
Joints are also classified by the type of connecting tissue holding them together. Fibrous joints are connected by dense fibrous tissue, ligaments or membranes. Cartilaginous joints are connected by fibrocartilage or hyaline cartilage. Synovial joints are connected by a fibrous joint capsule. Within the capsule is a small fluid-filled space. The fluid in the space nourishes the articular cartilage, which covers the ends of the bones meeting in the synovial joint.The structure and function of skeletal muscles
 Whole muscles vary in size (from 2 cm to 60 cm) and shape (fusiform, pennate). They are encased in a three-part connective tissue framework. The fundamental concept of muscle function is the motor unit, defined as those muscle fibres innervated by a single motor neuron.
Whole muscles vary in size (from 2 cm to 60 cm) and shape (fusiform, pennate). They are encased in a three-part connective tissue framework. The fundamental concept of muscle function is the motor unit, defined as those muscle fibres innervated by a single motor neuron. Muscle fibres contain bundles of myofibrils arranged in parallel along the longitudinal axis and include the muscle membrane, myofibrils, sarcotubular system, aqueous sarcoplasm and mitochondria. There are two types of muscle fibres: type I and type II.
Muscle fibres contain bundles of myofibrils arranged in parallel along the longitudinal axis and include the muscle membrane, myofibrils, sarcotubular system, aqueous sarcoplasm and mitochondria. There are two types of muscle fibres: type I and type II. Myofibrils and myofilaments contain the major muscle proteins, actin and myosin, which interact to form cross-bridges during muscle contraction. The non-protein muscle constituents provide an energy source for contraction and regulate protein synthesis, enzyme systems and membrane stabilisation.
Myofibrils and myofilaments contain the major muscle proteins, actin and myosin, which interact to form cross-bridges during muscle contraction. The non-protein muscle constituents provide an energy source for contraction and regulate protein synthesis, enzyme systems and membrane stabilisation. Muscle strength is graded by the all-or-none principle and recruitment. Speed of contraction is affected by several factors: muscle fibre type, temperature, stretch and weight of the load.
Muscle strength is graded by the all-or-none principle and recruitment. Speed of contraction is affected by several factors: muscle fibre type, temperature, stretch and weight of the load.Ageing and the musculoskeletal system
 The major effect of ageing on the skeletal system is loss of bone mass. For females loss of bone mass begins with menopause. Weight-bearing exercise and adequate calcium intake before menopause will maximise the length of time before bone mass–related health problems occur. Men experience bone loss later and at a slower rate than women.
The major effect of ageing on the skeletal system is loss of bone mass. For females loss of bone mass begins with menopause. Weight-bearing exercise and adequate calcium intake before menopause will maximise the length of time before bone mass–related health problems occur. Men experience bone loss later and at a slower rate than women.Two months ago 55-year-old Piri was invited by his 16-year-old grandson, Whiremu, to a local skateboard park. Whiremu is a promising skateboarder and he was keen to show his grandfather what he could do. Piri had done a bit of skateboarding in his youth and after watching his grandson for some time, he decided that he would demonstrate what he could do. But after only a couple of tricks, Piri attempted a difficult manoeuvre and fell towards his right side. He hit the concrete, using his right hand to break the fall. He was taken to the local Accident and Emergency Department where an X-ray showed a break in the proximal ulna. His arm was set in a plaster cast from the armpit to the wrist. He was told he would be in the cast for 6 weeks. Two weeks ago, when the cast was removed, Piri’s right arm was noticeably smaller than his left.
Answer the following questions using terminology that Piri’s whanau (extended family) will understand.