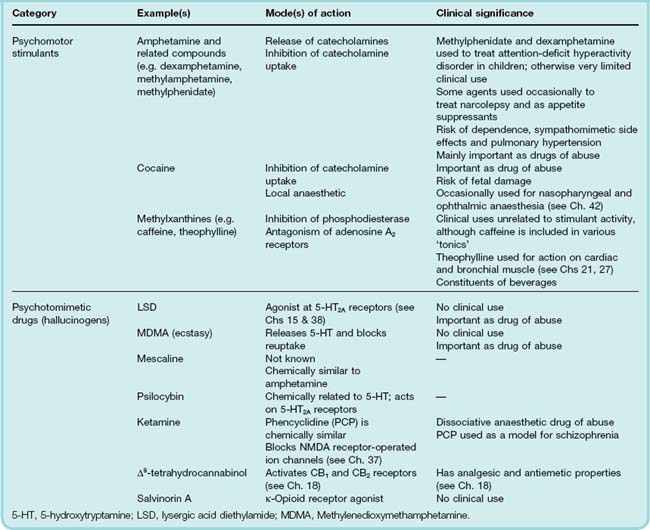
47 CNS stimulants and psychotomimetic drugs
Overview
In this chapter, we describe drugs that have a predominantly stimulant effect on the central nervous system (CNS); these fall into two broad categories:
Drugs in the first category have a marked effect on mental function and behaviour, producing excitement and euphoria, reduced sensation of fatigue, and an increase in motor activity.
Drugs in the second category mainly affect thought patterns and perception, distorting cognition in a complex way.
Table 47.1 summarises the classification of the drugs that are discussed in this chapter.
Several of these drugs have no clinical uses but are used for recreational purposes and as such are recognised as drugs of abuse. This aspect is also discussed in Chapter 48.
Psychomotor Stimulants
Amphetamines and Related Drugs
Amphetamine (speed or billy whizz) and its active dextroisomer dextroamphetamine (dexies), together with methamphetamine (crystal meth or ice) and methylphenidate (better known to many by its trade name Ritalin), comprise a group of drugs with very similar chemical and pharmacological properties (see Fig. 47.1).These drugs act by releasing monoamines, primarily dopamine and noradrenaline, from nerve terminals in the brain (see Seiden et al., 1993; Green et al., 2003). They are substrates for neuronal amine uptake transporters and cause release of these mediators (see Chs 14 and 38) thus producing the acute effects described below. With prolonged use, they are neurotoxic, causing degeneration of amine-containing nerve terminals and eventually cell death. This effect is probably due to the accumulation of reactive metabolites of the parent compounds within the nerve terminals. It has been well documented in experimental animals, and is believed to occur also in humans, possibly accounting for long-term adverse psychological effects in habitual users of amphetamine derivatives.
Further information on the pharmacology, uses and dangers of amphetamines can be found in the monograph by Iversen (2006).
Pharmacological effects
The main central effects of amphetamine-like drugs are:
In addition, amphetamines have peripheral sympathomimetic actions, producing a rise in blood pressure and inhibition of gastrointestinal motility.
In humans, amphetamine causes euphoria; with intravenous injection, this can be so intense as to be described as ‘orgasmic’. Subjects become confident, hyperactive and talkative, and sex drive is said to be enhanced. Fatigue, both physical and mental, is reduced, and amphetamine-like drugs cause marked anorexia, but with continued administration this effect wears off and food intake returns to normal. Rats quickly learn to press a lever in order to obtain a dose of amphetamine—an indication that the drug is rewarding.
Many studies have shown improvement of both mental and physical performance in fatigued, although not in well-rested, subjects (the use of amphetamines in sport is described in Ch. 58). Mental performance is improved for simple tedious tasks much more than for difficult tasks—in animal studies using complex behavioural analysis paradigms, amphetamines are said to make the animals busier rather than brighter! Amphetamines have been used to improve the performance of soldiers, military pilots and others who need to remain alert under extremely fatiguing conditions. They have also been in vogue as a means of helping students to concentrate before and during examinations, but the improvement caused by reduction of fatigue can be offset by the mistakes of overconfidence and a decreased ability to deal with large amounts of information.1
Adverse effects of amphetamines include feelings of anxiety, irritability and restlessness as the body’s energy stores are run down. At high doses, amphetamines may induce panic and paranoia.
In experimental animals, the behavioural effects of amphetamines are produced by the release of catecholamines in the brain. Thus pretreatment with 6-hydroxydopamine, which depletes the brain of both noradrenaline and dopamine, abolishes the effect of amphetamine, as does pretreatment with α-methyltyrosine, an inhibitor of catecholamine biosynthesis (see Ch. 14). Similarly, monoamine oxidase inhibitors (see Ch. 46) potentiate the effects of amphetamine, presumably by blocking metabolism. Interestingly, reserpine, which inhibits vesicular storage of catecholamines (see Ch. 14), does not block the behavioural effects of amphetamine. This is probably because amphetamine releases cytosolic rather than vesicular catecholamines (see Ch. 14). The behavioural effects of amphetamine are due mainly to release of dopamine rather than noradrenaline. The evidence for this is that destruction of the central noradrenergic bundle does not affect locomotor stimulation produced by amphetamine, whereas destruction of the dopamine-containing nucleus accumbens (see Ch. 38) or administration of antipsychotic drugs that antagonise dopamine (see Ch. 45) inhibit both locomotor and rewarding responses.
Chronic use, tolerance and dependence
If amphetamine is taken repeatedly over the course of a few days, which occurs when users seek to maintain the euphoric ‘high’ that a single dose produces, a state of ‘amphetamine psychosis’ can develop, which closely resembles an acute schizophrenic attack (see Ch. 45), with hallucinations accompanied by paranoid symptoms and aggressive behaviour. At the same time, repetitive stereotyped behaviour may develop (e.g. polishing shoes or stringing beads). The close similarity of this condition to schizophrenia, and the effectiveness of antipsychotic drugs in controlling it, is consistent with the dopamine theory of schizophrenia (see Ch. 45). When the drug is stopped after a few days, there is usually a period of deep sleep and on awakening the subject feels lethargic, depressed, anxious (sometimes even suicidal) and hungry. Even a single dose of amphetamine, insufficient to cause psychotic symptoms, usually leaves the subject later feeling tired and depressed. These after-effects may be the result of depletion of the normal stores of dopamine and noradrenaline, but the evidence for this is not clear-cut.
Tolerance develops rapidly to euphoric and anorexic effects of amphetamine, but more slowly to the other effects (locomotor stimulation, stereotyped behaviour and peripheral sympathomimetic action).
Dependence on amphetamine appears to be a consequence of the unpleasant after-effects that it produces and to the insistent memory of euphoria, which leads to a desire for a repeat dose. There is no clear-cut physical withdrawal syndrome such as occurs with opioids. It is estimated that only about 5% of users progress to full dependence, the usual pattern being that the dose is increased as tolerance develops, and then uncontrolled ‘binges’ occur in which the user takes the drug repeatedly over a period of a day or more, remaining continuously intoxicated. Large doses may be consumed in such binges, with a high risk of acute toxicity, and the demand for the drug displaces all other considerations.
Experimental animals, given unlimited access to amphetamine, take it in such large amounts that they die from the cardiovascular effects within a few days. Given limited amounts, they too develop a binge pattern of dependence.
Pharmacokinetic aspects
Amphetamine is readily absorbed from the gastrointestinal tract, but to increase the intensity of the hit it can be snorted or injected. In crystal form, the free base of methamphetamine can be ignited and smoked in a manner similar to crack cocaine (see below). Amphetamine freely penetrates the blood–brain barrier. It does this more readily than other indirectly acting sympathomimetic amines such as ephedrine or tyramine (Ch. 14), which probably explains why it produces more marked central effects than those drugs. Amphetamine is mainly excreted unchanged in the urine, and the rate of excretion is increased when the urine is made more acidic (see Ch. 9). The plasma half-life of amphetamine varies from about 5 h to 20–30 h, depending on urine flow and urinary pH.
Clinical use
Attention-deficit hyperactivity disorder (ADHD)
The main use of amphetamines is in the treatment of ADHD, particularly in children. Methylphenidate is most commonly used, at doses lower than those causing euphoria and other undesired effects. ADHD is a common condition in children—estimated as occurring in up to 9% of youth—whose incessant overactivity and very limited attention span disrupt their education and social development. The efficacy of amphetamines has been confirmed in many controlled trials. Disorders of dopamine pathways are suspected to underlie ADHD symptomatology, but the mechanism of action of amphetamines is unclear.
Other drug treatments for ADHD include the noradrenaline reuptake inhibitor, atomoxetine, and α2 adrenoceptor agonists such as clonidine and guanfacine. The amine uptake inhibitor, modafinil, is not approved for paediatric use but may be effective in adult ADHD.
Narcolepsy
This is a disabling condition, probably a form of epilepsy, in which the patient suddenly and unpredictably falls asleep at frequent intervals during the day. Amphetamine is helpful but not completely effective. Modafinil is also effective in reducing the need for sleep and is becoming increasingly popular as a lifestyle drug (see Ch. 58) with students and young professionals. Sodium oxybate, the sodium salt of γ-hydroxybutyrate (see Ch. 37), is a CNS depressant that paradoxically is licensed for the treatment of narcolepsy with cataplexy (abrupt onset of paralysis of variable extent often triggered by emotion, sometimes with ‘frozen’ posture). The drug is frequently abused and is taxing to take correctly (on retiring and 2–4 hours later—an alarm clock is obligatory!); it is prescribed by specialists in sleep disorders.
Unwanted effects
The limited clinical usefulness of amphetamine is offset by its many unwanted effects, including hypertension, insomnia, anorexia, tremors, risk of exacerbating schizophrenia and risk of dependence. Cerebral haemorrhage has also been reported after amphetamine use, possibly the result of acutely raised blood pressure. There is evidence that habitual use of amphetamines is associated with long-term psychological effects of many kinds, including psychotic symptoms, anxiety, depression and cognitive impairment. The evidence in man is not conclusive, but taken in conjunction with animal data, it suggests that amphetamines can cause long-term damage.
Amphetamines ![]()
Cocaine
Cocaine (see Streatfeild, 2002) is found in the leaves of the South American shrub, coca. These leaves are used for their stimulant properties by natives of South America, particularly those in mountainous areas, who use it to reduce fatigue during work at high altitude.
Considerable mystical significance was attached to the powers of cocaine to boost the flagging human spirit, and Freud tested it extensively on his patients and his family, publishing an influential monograph in 1884 advocating its use as a psychostimulant.2 Freud’s ophthalmologist colleague, Köller, obtained supplies of the drug and discovered its local anaesthetic action (Ch. 42), but the psychostimulant effects of cocaine have not proved to be clinically useful. On the other hand, they led to it becoming a widespread drug of abuse in Western countries. The mechanisms and treatment of cocaine abuse are discussed in Chapter 48.
Pharmacological effects
Cocaine binds to and inhibits the transporters responsible for the uptake of dopamine and noradrenaline into nerve terminals (see Chs 14 and 38), thereby enhancing the peripheral effects of sympathetic nerve activity and producing a marked psychomotor stimulant effect.
In humans, cocaine produces euphoria, garrulousness, increased motor activity and a magnification of pleasure. Users feel alert, energetic and physically strong and believe they have enhanced mental capabilities. Its effects resemble those of amphetamines, although it has less tendency to produce stereotyped behaviour, delusions, hallucinations and paranoia. With excessive dosage, tremors and convulsions, followed by respiratory and vasomotor depression, may occur. The peripheral sympathomimetic actions lead to tachycardia, vasoconstriction and an increase in blood pressure. Body temperature may increase, owing to the increased motor activity coupled with reduced heat loss.
Experimental animals rapidly learn to press a lever to self-administer cocaine and will consume toxic amounts of the drug if access is not limited. In transgenic mice lacking the D2 receptor, the enhanced locomotor effects of cocaine are reduced, but surprisingly self-administration of cocaine is increased, in contrast to what is found with other self-administered drugs such as ethanol and morphine (see De Mei et al., 2009).
Chronic use, dependence and tolerance
Cocaine undoubtedly causes strong psychological dependence (see Ch. 48), but there is some debate about whether or not its continued use induces tolerance and physical dependence. Users may increase their intake of the drug but this may reflect a desire for an increased effect rather than the development of tolerance. In experimental animals, sensitisation (the opposite of tolerance) can be observed but the relevance of this to the situation in humans is unclear (see Bradberry, 2007). Like amphetamine, cocaine produces no clear-cut withdrawal syndrome but depression, dysphoria and fatigue may be experienced following the initial stimulant effect. Withdrawal of cocaine after administration for a few days causes a marked deterioration of motor performance and learned behaviour, which are restored by resuming dosage with the drug. Cocaine induces psychological dependence where users crave the drug’s euphoric and stimulatory effects. The cellular mechanisms underlying craving and pharmacological approaches to reduce craving are discussed in Chapter 48. The pattern of dependence, evolving from occasional use through escalating dosage to compulsive binges, is similar to that seen with amphetamines.
Pharmacokinetic aspects
Cocaine is readily absorbed by many routes. For many years, illicit supplies have consisted of the hydrochloride salt, which could be given by nasal inhalation or intravenously. The latter route produces an intense and immediate euphoria, whereas nasal inhalation produces a less dramatic sensation and also tends to cause atrophy and necrosis of the nasal mucosa and septum.
Cocaine use increased dramatically when the free-base form (‘crack’) became available as a street drug. When an aqueous solution of cocaine hydrochloride is heated with sodium bicarbonate, then free-base cocaine, water, CO2 and NaCl are produced. The free-base cocaine is insoluble in water, precipitates out and can then be rolled into ‘rocks’ of crack. Free-base cocaine vaporises at around 90°C, much lower than the melting point of cocaine hydrochloride (190°C) which burns rather than vaporises. Thus crack can be smoked, with the uncharged free-base being rapidly absorbed across the large surface area of the alveolae, giving rise to a greater CNS effect than that obtained by snorting cocaine. Indeed, the effect is nearly as rapid as that of intravenous administration, with less inconvenience and social stigma. The social, economic and even political consequences of this small change in formulation have been far-reaching.
The duration of its stimulant effect, about 30 min, is much shorter than that of amphetamine. It is rapidly metabolised in the liver.
A cocaine metabolite is deposited in hair, and analysis of its content along the hair shaft allows the pattern of cocaine consumption to be monitored, a technique that has revealed a much higher incidence of cocaine use than was voluntarily reported. Cocaine exposure in utero can be estimated from analysis of the hair of neonates.
Cocaine is still occasionally used topically as a local anaesthetic, mainly in ophthalmology and minor nose and throat surgery, but has no other clinical uses. It is a valuable pharmacological tool for the study of catecholamine release and reuptake, because of its relatively specific action in blocking noradrenaline and dopamine uptake.
Adverse effects
Toxic effects occur commonly in cocaine abusers. The main acute dangers are serious cardiovascular events (cardiac dysrhythmias, aortic dissection, and myocardial or cerebral infarction or haemorrhage). Progressive myocardial damage can lead to heart failure, even in the absence of a history of acute cardiac effects.
Cocaine can severely impair brain development in utero (see Volpe, 1992). The brain size is significantly reduced in babies exposed to cocaine in pregnancy, and neurological and limb malformations are increased. The incidence of ischaemic and haemorrhagic brain lesions, and of sudden infant death, is also higher in cocaine-exposed babies. Interpretation of the data is difficult because many cocaine abusers also take other illicit drugs that may affect fetal development, but the probability is that cocaine is highly detrimental.
Dependence, the main psychological adverse effect of amphetamines and cocaine, has potentially severe effects on quality of life (Ch. 48).
Methylxanthines
Various beverages, particularly tea, coffee and cocoa, contain methylxanthines, to which they owe their mild central stimulant effects. The main compounds responsible are caffeine and theophylline. The nuts of the cola plant also contain caffeine, which is present in cola-flavoured soft drinks. However, the most important sources, by far, are coffee and tea, which account for more than 90% of caffeine consumption. A cup of instant coffee or strong tea contains 50–70 mg of caffeine, while filter coffee contains about twice as much. Among adults in tea- and coffee-drinking countries, the average daily caffeine consumption is about 200 mg. Further information on the pharmacology and toxicology of caffeine is presented by Fredholm et al. (1999).
Pharmacological effects
Methylxanthines have the following major pharmacological actions:
The latter two effects resemble those of β-adrenoceptor stimulation (see Chs 14, 21 and 27). This is thought to be because methylxanthines (especially theophylline) inhibit phosphodiesterase, which is responsible for the intracellular metabolism of cAMP (Ch. 3). They thus increase intracellular cAMP and produce effects that mimic those of mediators that stimulate adenylyl cyclase. Methylxanthines also antagonise many of the effects of adenosine, acting on both A1 and A2 receptors (see Ch. 16). Transgenic mice lacking functional A2 receptors are abnormally active and aggressive, and fail to show increased motor activity in response to caffeine (Ledent et al., 1997), suggesting that antagonism at A2 receptors accounts for part, at least, of its CNS stimulant action. Caffeine also sensitises ryanodine receptors (see Ch. 4) but this effect occurs at higher concentrations (> 10 mmol/l) than those achieved by recreational intake of caffeine. The concentration of caffeine reached in plasma and brain after two or three cups of strong coffee—about 100 µmol/l—is sufficient to produce appreciable adenosine receptor block and a small degree of phosphodiesterase inhibition. The diuretic effect probably results from vasodilatation of the afferent glomerular arteriole, causing an increased glomerular filtration rate.
Caffeine and theophylline have very similar stimulant effects on the CNS. Human subjects experience a reduction of fatigue, with improved concentration and a clearer flow of thought. This is confirmed by objective studies, which have shown that caffeine reduces reaction time and produces an increase in the speed at which simple calculations can be performed (although without much improvement in accuracy). Performance at motor tasks, such as typing and simulated driving, is also improved, particularly in fatigued subjects. Mental tasks, such as syllable learning, association tests and so on, are also facilitated by moderate doses (up to about 200 mg of caffeine, or about two cups of coffee) but impaired by larger doses. Insomnia is common. By comparison with amphetamines, methylxanthines produce less locomotor stimulation and do not induce euphoria, stereotyped behaviour patterns or a psychotic state, but their effects on fatigue and mental function are similar.
Tolerance and habituation develop to a small extent, but much less than with amphetamines, and withdrawal effects are slight. Caffeine is not self-administered by animals, and it cannot be classified as a dependence-producing drug.
Clinical use and unwanted effects
There are few clinical uses for caffeine. It is included with aspirin in some preparations for treating headaches and other aches and pains, and with ergotamine in some antimigraine preparations, the object being to produce a mildly agreeable sense of alertness. Theophylline (formulated as aminophylline) is used mainly as a bronchodilator in treating severe asthmatic attacks (see Ch. 27). Caffeine has few unwanted side effects and is safe even in very large doses. In vitro tests show that it has mutagenic activity, and large doses are teratogenic in animals. However, epidemiological studies have shown no evidence of carcinogenic or teratogenic effects of tea or coffee drinking in humans.
Methylxanthines ![]()
Other Stimulants
Arecoline, a cholinergic agonist, is a mild stimulant contained in the betel nut. Its use is widespread in India, Thailand, Indonesia and other Asian cultures. Arecoline improves learning and memory.
Cathinone and cathine are the active ingredients in the khat shrub. Chewing the leaves is popular in parts of Africa such as Ethiopia and Somalia and its use is spreading through immigrant populations in Western countries.
Nitrites such as amyl nitrite (see Ch. 21) produce a rush as heart rate increases and blood rushes to the head. Headache, dizziness, nausea and a feeling of light-headedness as well as a slowing of time are experienced. Sexual pleasure may be enhanced.
Psychotomimetic Drugs
Psychotomimetic drugs (also referred to as psychedelic or hallucinogenic drugs) affect thought, perception and mood, without causing marked psychomotor stimulation or depression (see Nichols, 2004). Thoughts and perceptions tend to become distorted and dream-like, rather than being merely sharpened or dulled, and the change in mood is likewise more complex than a simple shift in the direction of euphoria or depression. Importantly, psychotomimetic drugs do not cause dependence, even though their psychological effects overlap those of highly addictive major psychostimulants such as cocaine and amphetamines.
Psychotomimetic drugs include the following:
LSD, Psilocybin and Mescaline
LSD is an exceptionally potent psychotomimetic drug capable of producing strong effects in humans in doses less than 1 µg/kg. It is a chemical derivative of lysergic acid, which occurs in the cereal fungus ergot (see Ch. 15), and was first synthesised by Hoffman in 1943. Hoffman deliberately swallowed about 250 µg of LSD (the threshold dose is now known to be around 20 µg) and wrote 30 years later of the experience: ‘the faces of those around me appeared as grotesque coloured masks … marked motoric unrest, alternating with paralysis … heavy feeling in the head, limbs and entire body, as if they were filled with lead … clear recognition of my condition, in which state I sometimes observed, in the manner of an independent observer, that I shouted half insanely.’ These effects lasted for a few hours, after which Hoffman fell asleep, ‘and awoke next morning feeling perfectly well’. Apart from these dramatic psychological effects, LSD has few physiological effects.
Mescaline, which is derived from a Mexican cactus and has been known as a hallucinogenic agent for many centuries, was made famous by Aldous Huxley in The Doors of Perception. It is chemically related to amphetamine.
Psilocybin is obtained from fungi (colloquially known as magic mushrooms). The effects of taking psilocybin are similar to those experienced with LSD.
Pharmacological effects
The main effects of these drugs are on mental function, most notably an alteration of perception in such a way that sights and sounds appear distorted and fantastic. Hallucinations—visual, auditory, tactile or olfactory—also occur, and sensory modalities may become confused, so that sounds are perceived as visions. Thought processes tend to become illogical and disconnected, but subjects retain insight into the fact that their disturbance is drug induced, and generally find the experience exhilarating. Occasionally, especially if the user is already anxious, LSD produces a syndrome that is extremely disturbing (the ‘bad trip’), in which the hallucinatory experience takes on a menacing quality and may be accompanied by paranoid delusions. Furthermore, ‘flashbacks’ of the hallucinatory experience have been reported weeks or months later.
LSD acts on various 5-HT-receptor subtypes (see Chs 15 and 38); its psychotomimetic effects are thought to be mediated mainly by its 5-HT2A receptor agonist actions (see Nichols, 2004). It inhibits the firing of 5-HT-containing neurons in the raphe nuclei (see Ch. 38), apparently by acting as an agonist on the inhibitory autoreceptors of these cells. The significance of this response to its psychotomimetic effects is unclear. Psilocybin is dephosphorylated to psilocin which is an agonist at several 5-HT receptors including the 5-HT2A receptor. The mechanism of action of mescaline is less well defined. There are contradictory reports about its activity at 5-HT2A receptors. It has also been reported to act as an inhibitor of monoamine transport.
The main effects of psychotomimetic drugs are subjective, so it is not surprising that animal tests that reliably predict psychotomimetic activity in humans have not been devised.3
Dependence and adverse effects
Psychotomimetic agents are largely not self-administered by experimental animals. Indeed, in contrast to most of the drugs that are widely abused by humans, they have aversive rather than reinforcing properties in behavioural tests. Tolerance to their effects develops quite quickly, but there is no physical withdrawal syndrome in animals or humans.
There has been much concern over reports that LSD and other psychotomimetic drugs, as well as causing potentially dangerous bad trips, can lead to more persistent mental disorder (see Abraham & Aldridge, 1993). Unexpected flashbacks can be very disturbing. Also, there are recorded instances in which altered perception and hallucinations have lasted for up to 3 weeks following a single dose of LSD, and of precipitation of attacks in schizophrenic patients.
MDMA (Ecstasy)
MDMA (3,4-methylenedioxymethamphetamine) is widely used as a ‘party drug’ because of the euphoria, loss of inhibitions and energy surge that it induces. It is a stimulant drug which also has mild hallucinogenic effects. The experience of taking the drug has been likened to taking amphetamine and weak LSD.
Pharmacological effects
Although it is an amphetamine derivative (Fig. 47.1), it affects monoamine function in a different manner from the amphetamines (see Green et al., 2003; Morton, 2005; Iversen, 2006). It inhibits monoamine transporters, principally the 5-HT transporter, and also releases 5-HT, the net effect being a large increase in free 5-HT in certain brain regions, followed by depletion. Similar but smaller changes occur in relation to dopamine and noradrenaline. Simplistically, the effects on 5-HT function determine the psychotomimetic effects, while dopamine and noradrenaline changes account for the initial euphoria and later rebound dysphoria. Although not addictive, MDMA carries serious risks, both acute and long term.
Sudden illness and death can occur even after small doses of MDMA. This can be due to several factors:
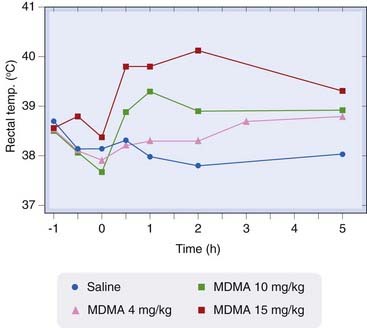
Fig. 47.2 A single injection of MDMA causes a dose-related increase in body temperature in rats.
Drug administered at time zero (Reproduced with permission from Green et al., 2004.)
The after-effects of MDMA persist for a few days and comprise depression, anxiety, irritability and increased aggression—the ‘mid-week blues’. There is also evidence of long-term deleterious effects on memory and cognitive function in heavy MDMA users. In animal studies, MDMA can cause degeneration of 5-HT and dopamine neurons, but whether this occurs in humans is uncertain (see Morton, 2005).
Illicit ‘ecstasy’ tablets and powder are sometimes contaminated or entirely substituted with para-methoxyamphetamine which produces similar behavioural effects but which may be more dangerous to the user. Another related drug is 4-bromo-2,5-dimethoxyphenethylamine (2CB).
Ketamine and Phencyclidine
Ketamine (‘Special K’) is a dissociative anaesthetic (Ch. 40) now also used as a recreational drug. An analogue, phencyclidine (PCP, ‘Angel dust’), was a popular hallucinogen in the 1970s but its use has declined. These drugs produce a feeling of euphoria. At higher doses they cause hallucinations and a feeling of detachment, disorientation and numbness. PCP was reported to cause psychotic episodes and is used in experimental animals to produce a model of schizophrenia (see Ch. 45 and Morris et al., 2005).
Pharmacological effects
Their main pharmacological effect is block of the NMDA receptor channel (see Ch. 37). This was at one time mistakenly described as ‘acting at σ opioid receptors’. Long-term regular use of ketamine can result in severe bladder pain through an as yet unknown mechanism. Combination of ketamine with depressant drugs such as alcohol, barbiturates and heroin can result in dangerous overdose.
Other Psychotomimetic Drugs
Salvinorin A is a hallucinogenic agent contained in the American sage plant Salvia divinorum, a member of the mint family. It was originally used by the Mazatecs in Mexico; in recent years its use has spread and it has become known as herbal ecstasy. It is a κ-opioid receptor agonist (see Ch. 41).4 At high doses, delirium may be produced.
DMT (dimethyltryptamine) and DOM (2,5-dimethoxy-4-methylamphetamine) are synthetic hallucinogenic drugs that produce effects similar to LSD.
Muscarinic receptor antagonists (see Chs 13 and 36), hyoscine, hyoscyamine and atropine are contained in various plants, including henbane and mandrake. Consumption can cause hallucinations, drowsiness and disorientation.
Ibogaine is contained in the root bark of iboga shrubs in Africa, South America and Australia. At high doses, it is hallucinogenic. Users have reported experiencing a reduced desire to take other drugs such as cocaine and heroin leading to ibogaine being investigated as a potential treatment for drug craving (see Ch. 48).
Psychotomimetic drugs ![]()
References and Further Reading
Courtwright D.T. Forces of habit: drugs and the making of the modern world. Cambridge: Harvard University Press; 2001. (A lively historical account of habit-forming drugs)
Bradberry C.W. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705-717.
De Mei C., Ramos M., Litaka C., Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin. Pharmacol.. 2009;9:53-58.
Fredholm B.B., Battig K., Holmes J., et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev.. 1999;51:83-133. (Comprehensive review article covering pharmacological, behavioural and social aspects)
Iversen L.L. Speed, ecstasy, ritalin. The science of amfetamines. Oxford and New York: Oxford University Press; 2006. (Authoritative book on all aspects of the properties, use and abuse of amfetamines)
Ledent C., Vaugeois J.M., Schiffmann S.N., et al Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor Nature 388:1997:674-678 (Study of transgenic mice, showing loss of stimulant effects of caffeine in mice lacking A2 receptors)
Seiden L.S., Sabol K.E., Ricaurte G.A. Amfetamine: effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol,. 1993;33:639-677.
Streatfeild D. Cocaine: a definitive history. Derby, PA: Diane Publishing Co.; 2002.
Volpe J.J. Effect of cocaine on the fetus. N. Engl. J. Med.. 1992;327:399-407.
Abraham H.D., Aldridge A.M. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
Green A.R., Mechan A.O., Elliott J.M., et al. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). Pharm. Rev.. 2003;55:463-508.
Green A.R., O’Shea E., Colado I. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur. J. Pharmacol.. 2004;500:3-13.
Morris B.J., Cochran S.M., Pratt J.A. PCP: from pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol.. 2005;5:101-106. (Review arguing that NMDA channel block by phencyclidine closely models human schizophrenia)
Morton J. Ecstasy: pharmacology and neurotoxicity. Curr. Opin. Pharmacol.. 2005;5:79-86. (Useful short review focusing on adverse effects of MDMA)
Nichols D.E. Hallucinogens Pharmacol. Ther. 101:2004:131-181 (Comprehensive review article focusing on 5-HT2A receptors as the target of psychotomimetic drugs)
1Pay heed to the awful warning of the medical student who, it is said, having taken copious amounts of dextroamphetamine, left the examination hall in confident mood, having spent 3 hours writing his name over and over again.
2In the 1860s, a Corsican pharmacist, Mariani, devised cocaine-containing beverages, Vin Mariani and Thé Mariani, which were sold very successfully as tonics. Imitators soon moved in, and Thé Mariani became the forerunner of Coca-Cola. In 1903, cocaine was removed from Coca-Cola because of its growing association with addiction and criminality (see Courtwright, 2001, for a lively account).
3One of the more bizarre tests involves spiders, whose normal elegantly symmetrical webs become jumbled and erratic if the animals are treated with LSD. It is worth searching the web for ‘spiders LSD’ to see images.
4In Phase 1 clinical trials of synthetic κ agonists as potential analgesic agents, the drugs were reported to induce a feeling of dysphoria. Perhaps the ‘normal’ volunteers in those trials were disturbed by the hallucinations they probably experienced. Interesting then that a naturally occurring κ agonist has now become a recreational drug.