Chapter 16 Head and Neck
Diseases of the head and neck range from the common cold to uncommon neoplasms of the nose. Those selected for discussion are assigned, sometimes arbitrarily, to one of the following anatomic sites: (1) oral cavity; (2) upper airways, including the nose, pharynx, larynx, and nasal sinuses; (3) ears; (4) neck; and (5) salivary glands.
ORAL CAVITY
The oral cavity is a fearsome orifice guarded by ranks of upper and lower “horns” (lamentably, quite subject to erosion), demanding constant gratification, and teeming with microorganisms, some of which are potentially harmful. Among the many disorders that affect its various parts, only the more important or frequent conditions involving the teeth and supporting structures, oral mucous membranes, lips, and tongue are considered.
Teeth and Supporting Structures
Teeth contribute to several important functions, including mastication and proper speech. It is useful to briefly review normal dental anatomy before we delve into the common pathologic conditions affecting teeth. As is well known, teeth are firmly implanted in the jaw and are surrounded by the gingival mucosa (Fig. 16-1). The anatomic crown of the tooth projects into the mouth and is covered by enamel, a hard, inert, acellular tissue—the most highly mineralized tissue in the body. The enamel rests upon dentin, which is a specialized form of connective tissue that makes up most of the remaining hard-tissue portion of the tooth. Unlike enamel, dentin is cellular and contains numerous dentinal tubules, which contain the cytoplasmic extensions of odontoblasts. These cells line the interface between the dentin and the pulp and can, when properly stimulated, produce new (secondary) dentin within the interior of the tooth. The pulp chamber itself is surrounded by the dentin and consists of loose connective tissue stroma rich in nerve bundles, lymphatics, and capillaries.
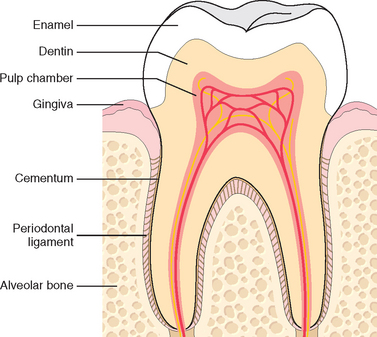
FIGURE 16-1 Schematic representation of the normal dental anatomy and surrounding supporting tissues.
To perform mastication, teeth must not only be composed of hard tissue but must also be firmly attached to the bones of the jaw. If this attachment were excessively firm, chewing would impose sufficient physical stress on the teeth to cause their loss or fracturing. Therefore, in mammals, teeth are attached to the alveolar ridge of the jaws by the periodontal ligament, which provides a strong yet flexible attachment that can withstand the forces of mastication. The periodontal ligament attaches to the alveolar bone of the jaw on one side and to cementum, present on the roots of the teeth, which acts as a “cement” to anchor the periodontal ligament to the tooth.
CARIES (TOOTH DECAY)
Dental caries, caused by focal degradation of the tooth structure, is one of the most common diseases throughout the world and is the most common cause of tooth loss before age 35. Carious lesions are the result of mineral dissolution of tooth structure by acid metabolic end products from bacteria that are present in the oral cavity and are capable of fermenting sugars. Traditionally, the rate of caries has been higher in industrialized countries, where there is ready access to processed foods containing large amounts of carbohydrates. However, global trends may change these demographics. First of all, the rate of caries has markedly dropped in countries such as the United States, where improved oral hygiene and fluoridation of the drinking water has become a standard practice. Fluoride incorporates into the crystalline structure of enamel, forming fluoroapatite, and contributes to resistance to degradation by bacterial acids. Second, with globalization of the world’s economy, increased amounts of processed foods with high carbohydrate content are being imported into developing nations. With these trends, one can expect the rate of caries to increase dramatically in the less-developed world over the next several decades.
GINGIVITIS
Gingiva is the designation of the squamous mucosa in between the teeth and around them. Gingivitis is inflammation of the mucosa and the associated soft tissues. Typically, the development of gingivitis is the result of a lack of proper oral hygiene, leading to an accumulation of dental plaque and calculus. Dental plaque is a sticky, usually colorless, biofilm that builds in between and on the surface of the teeth. It is formed by a complex of the oral bacteria, proteins from the saliva, and desquamated epithelial cells. If plaque continues to build and is not removed, it becomes mineralized to form calculus (tartar). The bacteria in the plaque release acids from sugar-rich foods, which erode the enamel surface of the tooth. Repeated erosions lead to dental caries. Plaque build-up beneath the gumline can cause gingivitis. Chronic gingivitis is characterized by gingival erythema, edema, bleeding, changes in contour, and loss of soft-tissue adaptation to the teeth. Gingivitis occurs at any age but is most prevalent and severe in adolescence (ranging from 40% to 60%), after which the incidence tapers off. It is a reversible disease; therapy is primarily aimed at reducing the accumulation of plaque and calculus via brushing, flossing, and regular dental visits.1
PERIODONTITIS
Periodontitis refers to an inflammatory process that affects the supporting structures of the teeth: periodontal ligaments, alveolar bone, and cementum. With progression, periodontitis can lead to serious sequelae, including the loss of attachment caused by complete destruction of the periodontal ligament and alveolar bone. Loosening and eventual loss of teeth are possible. The pathogenesis of periodontal inflammation is not entirely clear. Until the 1960s it was believed that long-standing gingivitis uniformly progressed to periodontal disease. However, this is no longer thought to be the case. Rather, the development of periodontal disease is now considered to be an independent process, which, for reasons that are still unclear, is associated with a marked shift in the types and proportions of bacteria along the gingiva.2 This shift, along with other environmental conditions such as poor oral hygiene, is believed to be important in the pathogenesis of periodontitis. This view is supported by significant differences in the content of dental plaque in areas of healthy and diseased periodontium. For the most part, facultative gram-positive organisms colonize healthy sites, while plaque within areas of active periodontitis contains anaerobic and microaerophilic gram-negative flora. Although 300 types of bacteria reside in the oral cavity, adult periodontitis is associated primarily with Aggregatibacter (Actinobacillus) actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia.
While it typically presents without any associated disorders, periodontal disease can also be a component of several different systemic diseases, including acquired immunodeficiency syndrome (AIDS), leukemia, Crohn’s disease, diabetes mellitus, Down syndrome, sarcoidosis, and syndromes associated with polymorphonuclear defects (Chédiak-Higashi syndrome, agranulocytosis, and cyclic neutropenia). In addition to being a component of certain systemic diseases, periodontal infections can also be etiologic factors in several important systemic diseases. These include, for example, infective endocarditis, pulmonary and brain abscesses, and adverse pregnancy outcomes.3,4
Inflammatory/Reactive Tumor-like Lesions
Several soft-tissue lesions of the oral cavity, which present as tumor masses or ulcerations, are reactive in nature and represent inflammations induced by irritation or by unknown mechanisms. All suspicious lesions, however, should be examined by biopsy. Reactive nodules of the oral cavity are fairly common and microscopically diverse. The most common fibrous proliferative lesions of the oral cavity include fibroma (61%), peripheral ossifying fibroma (22%), pyogenic granuloma (12%), and peripheral giant-cell granuloma (5%).5 The most common inflammatory/reactive ulcerations of the oral cavity are traumatic and aphthous ulcers.
FIBROUS PROLIFERATIVE LESIONS
The so-called irritation fibroma (Fig. 16-2) primarily occurs in the buccal mucosa along the bite line or at the gingivodental margin. It consists of a nodular mass of fibrous tissue, with few inflammatory cells, covered by squamous mucosa. Treatment is complete surgical excision.
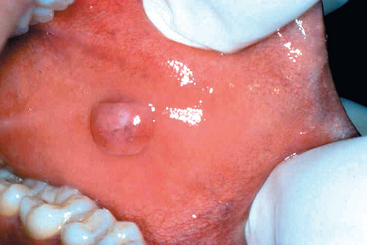
The pyogenic granuloma (Fig. 16-3) is a highly vascular pedunculated lesion, usually occurring in the gingiva of children, young adults, and, commonly, pregnant women (pregnancy tumor). The surface of the lesion is typically ulcerated and red to purple in color. In some cases growth is alarmingly rapid, raising the fear of a malignant neoplasm. Histologically these lesions demonstrate a highly vascular proliferation that is similar to granulation tissue. Because of this histologic picture, pyogenic granulomas are considered by some authorities to be a form of capillary hemangioma (Chapter 11). They either regress, particularly after pregnancy, or undergo fibrous maturation, and they may develop into a peripheral ossifying fibroma. Treatment is complete surgical excision.
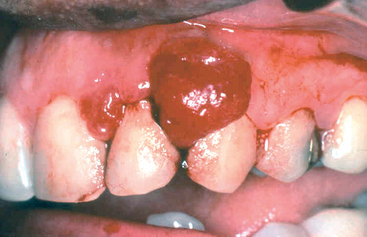
FIGURE 16-3 Pyogenic granuloma. Erythematous, hemorrhagic, and exophytic mass arising from the gingival mucosa.
The peripheral ossifying fibroma is a relatively common growth of the gingiva that is considered to be reactive in nature rather than neoplastic. However, the etiology of the lesion is unknown. Some may arise as a result of the maturation of a long-standing pyogenic granuloma. With a peak incidence in young and teenage females, peripheral ossifying fibromas appear as red, ulcerated, and nodular lesions of the gingiva. They are often mistaken clinically for pyogenic granulomas. Complete surgical excision down to the periosteum is the treatment of choice, since these lesions have a recurrence rate of 15% to 20%.
The peripheral giant cell granuloma is a relatively common lesion of the gingiva. It is generally covered by intact gingival mucosa, but it may be ulcerated. The clinical appearance of peripheral giant-cell granuloma can be similar to that of pyogenic granuloma, but which is generally more bluish purple in color while the pyogenic granuloma is more bright red. Histologically, however, these lesions are distinct. Peripheral giant-cell granuloma is made up of a striking aggregation of multinucleate, foreign body–like giant cells separated by a fibroangiomatous stroma. Although not encapsulated, these lesions are usually well delimited and easily excised. They should be differentiated from central giant-cell granulomas found within the maxilla or the mandible and from the histologically similar but frequently multiple “brown tumors” seen in hyperparathyroidism (Chapter 24).
APHTHOUS ULCERS (CANKER SORES)
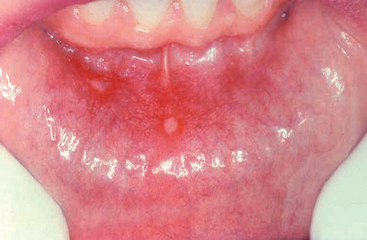
These extremely common superficial ulcerations of the oral mucosa affect up to 40% of the population in the United States.6 They are more common in the first two decades of life, are extremely painful and often recurrent, and tend to be prevalent within certain families.
The lesions appear as single or multiple, shallow, hyperemic ulcerations covered by a thin exudate and rimmed by a narrow zone of erythema (Fig. 16-4). The underlying inflammatory infiltrate is at first largely mononuclear, but secondary bacterial infection introduces numerous neutrophils. The lesions may spontaneously resolve in 7 to 10 days or be stubbornly persistent for weeks. The causation of these lesions is obscure. Most ulcers are more painful than serious and require only symptomatic treatment. Recurrent apthous ulcers may be associated with celiac disease and inflammatory bowel disease.
GLOSSITIS
Although the designation glossitis implies inflammation of the tongue, it is sometimes applied to the beefy-red tongue encountered in certain deficiency states; this change results from atrophy of the papillae of the tongue and thinning of the mucosa, exposing the underlying vasculature. In some instances the atrophic changes do indeed lead to inflammation and even shallow ulcerations. Such changes may be encountered in deficiencies of vitamin B12 (pernicious anemia), riboflavin, niacin, or pyridoxine. Similar alterations are sometimes encountered with sprue and iron-deficiency anemia, possibly complicated by deficiency in one of the B vitamins. The combination of iron-deficiency anemia, glossitis, and esophageal dysphagia usually related to webs is known as the Plummer-Vinson or Paterson-Kelly syndrome. Glossitis, characterized by ulcerative lesions (sometimes along the lateral borders of the tongue), may also be seen with jagged carious teeth, ill-fitting dentures, and, rarely, with syphilis, inhalation burns, or ingestion of corrosive chemicals.
Infections
The oral mucosa is highly resistant to its indigenous flora, having many defenses, including the competitive suppression of potential pathogens by organisms of low virulence, the elaboration of secretory IgA and other immunoglobulins by submucosal collections of lymphocytes and plasma cells, the antibacterial effects of saliva, and the irrigating effects of food and drink. Nonetheless, any lowering of these defenses, for example, with immunodeficiency or disruption of the microbiologic balance by antibacterial therapy, sets the stage for oral infections. Most of these infections are discussed in Chapter 8, and here we only briefly recapitulate the principal features of the oral lesions.
HERPES SIMPLEX VIRUS INFECTIONS
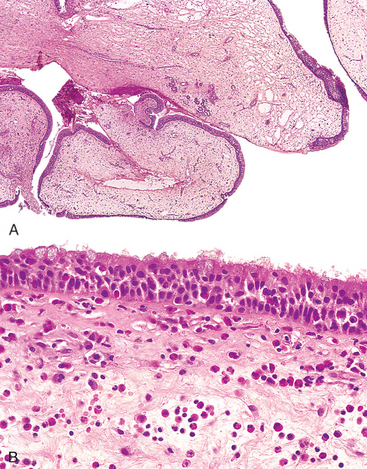
Most orofacial herpetic infections are caused by herpes simplex virus type 1 (HSV-1). However, because of changes in sexual habits, an increase in HSV-2 (genital herpes) has been observed in the oral cavity. Primary HSV infection typically occurs in children age 2 to 4 years, is often asymptomatic, and does not cause significant morbidity. Approximately 10% to 20% of the time, primary infection presents as acute herpetic gingivostomatitis, in which there is an abrupt onset of vesicles and ulcerations throughout the oral cavity, especially in the gingiva. These lesions are also accompanied by lymphadenopathy, fever, anorexia, and irritability.
Morphology. The vesicles range from lesions of a few millimeters to large bullae and are at first filled with a clear, serous fluid, but they often rupture to yield extremely painful, red-rimmed, shallow ulcerations. On microscopic examination there is intracellular and intercellular edema (acantholysis), yielding clefts that may become transformed into macroscopic vesicles. Individual epidermal cells in the margins of the vesicle or lying free within the fluid sometimes develop eosinophilic intranuclear viral inclusions, or several cells may fuse to produce giant cells (multinucleate polykaryons), changes that are demonstrated by the diagnostic Tzanck test, based on microscopic examination of the vesicle fluid. The vesicles and shallow ulcers usually spontaneously clear within 3 to 4 weeks, but the virus treks along the regional nerves and eventually becomes dormant in the local ganglia (e.g., the trigeminal).
The great preponderance of adults harbor latent HSV-1, but in some individuals, usually young adults, the virus becomes reactivated to produce the common but usually mild cold sore. The influences predisposing to activation are poorly understood but are thought to include trauma, allergies, exposure to ultraviolet light, upper respiratory tract infections, pregnancy, menstruation, immunosuppression, and exposure to extremes of temperature.
Recurrent herpetic stomatitis (in contrast to acute gingivostomatitis) occurs either at the site of primary inoculation or in adjacent mucosal areas that are associated with the same ganglion; it takes the form of groups of small (1–3 mm) vesicles. The lips (Herpes labialis), nasal orifices, buccal mucosa, gingiva, and hard palate are the most common locations for recurrent lesions. They resemble those already described in the primary infections but are much more limited in duration, are milder, usually dry up in 4 to 6 days, and heal within a week to 10 days.
OTHER VIRAL INFECTIONS
Additional viral infections that can be seen in the oral cavity as well as the head and neck region include herpes zoster, Epstein-Barr virus (EBV; mononucleosis), cytomegalovirus, enterovirus (herpangina, hand-foot-and-mouth disease, acute lymphonodular pharyngitis), and rubeola (measles).
ORAL CANDIDIASIS (THRUSH)
The many localizations of candidal infection are fully described in Chapter 8, and so this discussion is limited to presentation in the oral cavity. Candidiasis is by far the most common fungal infection in the oral cavity. Candida albicans is a normal component of the oral flora in approximately 50% of the population. Three factors seem to influence the likelihood of a clinical infection: (1) immune status of the individual; (2) the strain of C. albicans present; and (3) the composition of an individual’s oral flora. There are three major clinical forms of oral candidiasis, including pseudo-membranous (thrush), erythematous, and hyperplastic, with several different variations within these groups. Only the pseudo-membranous form, the most common of these, is discussed here. Also known as “thrush,” pseudo-membranous candidiasis typically takes the form of a superficial, curdy, gray to white inflammatory membrane composed of matted organisms enmeshed in a fibrinosuppurative exudate that can be readily scraped off to reveal an underlying erythematous inflammatory base. This fungus causes mischief only in individuals who have some form of immunosuppression, as occurs in patients with diabetes mellitus, organ or bone marrow transplant recipients, those with neutropenia, chemotherapy-induced immunosuppression, or AIDS. In addition, broad-spectrum antibiotics that eliminate or alter the normal bacterial flora of the mouth can also result in the development of oral candidiasis.
DEEP FUNGAL INFECTIONS
In addition to their more common sites of infection, certain deep fungal infections have a significant predilection for the oral cavity and the head and neck region. Such fungi include histoplasmosis, blastomycosis, coccidioidomycosis, cryptococcosis, zygomycosis, and aspergillosis. With an increasing number of patients who are immunocompromised due to diseases such as AIDS or therapies for cancer and organ transplantation, the prevalence of fungal infections of the oral cavity has also increased in recent years.
Oral Manifestations of Systemic Disease
As oral clinicians are at pains to emphasize, the mouth is a part of the body and not merely a gateway for delicacies. Not surprisingly, then, many systemic diseases are associated with oral lesions. In fact, it is not uncommon for oral lesions to be the first sign of some underlying systemic condition. Some of the more common disease associations are cited in Table 16-1, with a few words about the associated oral changes. Only one—hairy leukoplakia—is characterized in more detail.
TABLE 16-1 Oral Manifestantions of Some Systemic Diseases
| Systemic Disease | Associated Oral Changes |
|---|---|
| INFECTIOUS DISEASES | |
| Scarlet fever | Fiery red tongue with prominent papillae (raspberry tongue); white-coated tongue through which hyperemic papillae project (strawberry tongue) |
| Measles | Spotty enanthema in the oral cavity often precedes the skin rash; ulcerations on the buccal mucosa about Stensen duct produce Koplik spots |
| Infectious mononucleosis | Acute pharyngitis and tonsillitis that may cause coating with a gray-white exudative membrane; enlargement of lymph nodes in the neck, palatal petechiae |
| Diphtheria | Characteristic dirty white, fibrinosuppurative, tough, inflammatory membrane over the tonsils and retropharynx |
| Human immunodeficiency virus | Predisposition to opportunistic oral infections, particularly herpesvirus, Candida, and other fungi; oral lesions of Kaposi sarcoma and hairy leukoplakia (described in text) |
| DERMATOLOGIC CONDITIONS* | |
| Lichen planus | Reticulate, lacelike, white keratotic lesions that rarely become bullous and ulcerated; seen in more than 50% of patients with cutaneous lichen planus; rarely, is the sole manifestation |
| Pemphigus | Vesicles and bullae prone to rupture, leaving hyperemic erosions covered with exudates |
| Bullous pemphigoid | Oral lesions resemble macroscopically those of pemphigus but can be differentiated histologically |
| Erythema multiforme | Maculopapular, vesiculobullous eruption that sometimes follows an infection elsewhere, ingestion of drugs, development of cancer, or a collagen vascular disease; when it involves the lips and oral mucosa, it is referred to as Stevens-Johnson syndrome |
| HEMATOLOGIC DISORDERS | |
| Pancytopenia (agranulocytosis, aplastic anemia) | Severe oral infections in the form of gingivitis, pharyngitis, tonsillitis; may extend to produce cellulitis of the neck (Ludwig angina) |
| Leukemia | With depletion of functioning neutrophils, oral lesions may appear like those in pancytopenia |
| Monocytic leukemia | Leukemic infiltration and enlargement of the gingivae, often with accompanying periodontitis |
| MISCELLANEOUS | |
| Melanotic pigmentation | May appear in Addison disease, hemochromatosis, fibrous dysplasia of bone (Albright syndrome), and Peutz-Jegher syndrome (gastrointestinal polyposis) |
| Phenytoin (Dilantin) ingestion | Striking fibrous enlargement of the gingivae |
| Pregnancy | A friable, red, pyogenic granuloma protruding from the gingiva (“pregnancy tumor”) |
| Rendu-Osler-Weber syndrome | Autosomal dominant disorder with multiple congenital aneurysmal telangiectasias beneath mucosal surfaces of the oral cavity and lips |
* See Chapter 25 for details
HAIRY LEUKOPLAKIA
Hairy leukoplakia is a distinctive oral lesion that is usually seen in immunocompromised patients. Approximately 80% of patients with hairy leukoplakia are infected with the human immunodeficiency virus (HIV); the presence of this lesion sometimes calls attention to the existence of HIV infection. However, 20% of lesions are seen in patients who are immunocompromised for other reasons, such as cancer therapy or transplant immunosuppression. Hairy leukoplakia takes the form of white, confluent patches of fluffy (“hairy”), hyperkeratotic thickenings, almost always situated on the lateral border of the tongue. Unlike thrush, the lesion cannot be scraped off. The distinctive microscopic appearance consists of hyperparakeratosis and acanthosis with “balloon cells” in the upper spinous layer. Sometimes there is koilocytosis of the superficial, nucleated epidermal cells, suggesting human papillomavirus (HPV) infection, and HPV transcripts have occasionally been found within the cells. However, EBV is present in most cells and is now accepted as the cause of the condition.7 Sometimes there is superimposed candidal infection on the surface of the lesions, adding to the “hairiness.” In HIV-positive individuals, with hairy leukoplarkia, symptoms of AIDS follow in 2 to 3 years.
Tumors and Precancerous Lesions
Many epithelial and connective tissue tumors of the head and neck region (e.g., papillomas, hemangiomas, lymphomas) also occur elsewhere in the body and are described adequately in other chapters. Therefore, this discussion will consider only oral squamous cell carcinoma and its associated precancerous lesions.
LEUKOPLAKIA AND ERYTHROPLAKIA
As is discussed in more detail below, oral cancers are common worldwide, with a fairly high mortality. Screening and early detection in populations at risk have been proposed to decrease the morbidity and mortality associated with oral cancer.8,9 However, the visual detection of definitive premalignant oral lesions is problematic. This is in stark contrast to skin lesions, where visual screening for melanomas of the skin has been shown to have sensitivity and specificity rates of 93% and 98%.10,11 One explanation for this discrepancy is that the early lesions frequently do not demonstrate any of the clinical characteristics observed in advanced oral cancer such as ulceration, induration, pain, or cervical lymphadenopathy.12 In addition, the clinical presentation of potentially premalignant lesions in the oral cavity is highly heterogeneous. We begin our discussion with two premalignant lesions: leukoplakia and erythroplakia.
The term leukoplakia is defined by the World Health Organization as “a white patch or plaque that cannot be scraped off and cannot be characterized clinically or pathologically as any other disease.” Simply put, if a white lesion in the oral cavity can be given a specific diagnosis it is not a leukoplakia. This clinical term is reserved for lesions that are present in the oral cavity for no apparent reason. As such, white patches caused by entities such as lichen planus and candidiasis are not leukoplakias. Approximately 3% of the world’s population have leukoplakic lesions, and somewhere between 5% and 25% of these lesions are premalignant.13 Thus, until it is proved otherwise via histologic evaluation, all leukoplakias must be considered precancerous.
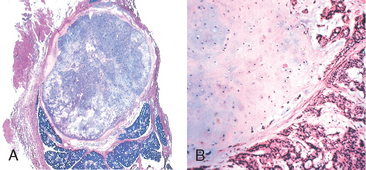
Related to leukoplakia, but much less common and much more ominous, is erythroplakia. It represents a red, velvety, possibly eroded area within the oral cavity that usually remains level with or may be slightly depressed in relation to the surrounding mucosa (Fig. 16-5). The epithelium in such lesions tends to be markedly atypical, incurring a much higher risk of malignant transformation than that seen with leukoplakia. Intermediate forms are occasionally encountered that have the characteristics of both leukoplakia and erythroplakia, termed speckled leukoerythroplakia.
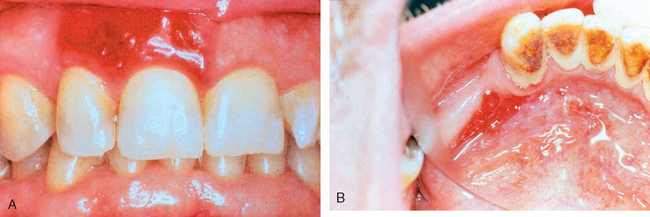
FIGURE 16-5 Erythroplakia. A, Lesion of the maxillary gingiva. B, Red lesion of the mandibular alveolar ridge. Biopsy of both lesions revealed carcinoma in situ.
Both leukoplakia and erythroplakia may be seen in adults at any age, but they are usually found between ages 40 and 70, with a 2 : 1 male preponderance. Although these lesions have multifactorial origins, the use of tobacco (cigarettes, pipes, cigars, and chewing tobacco) is the most common antecedent.
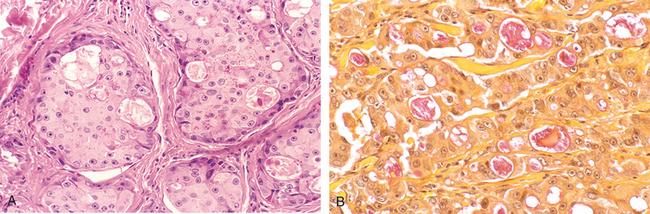
Morphology. Leukoplakias may occur anywhere in the oral cavity (favored locations are buccal mucosa, floor of the mouth, ventral surface of the tongue, palate, and gingiva). They appear as solitary or multiple white patches or plaques, often with sharply demarcated borders. They may be slightly thickened and smooth or wrinkled and fissured, or they may appear as raised, sometimes corrugated, verrucous plaques (Fig. 16-6). On histologic examination they present a spectrum of epithelial changes ranging from hyperkeratosis overlying a thickened, acanthotic but orderly mucosal epithelium to lesions with markedly dysplastic changes sometimes merging into carcinoma in situ (Fig. 16-7). The more dysplastic or anaplastic the lesion, the more likely that a subjacent inflammatory infiltrate of lymphocytes and macrophages will be present.
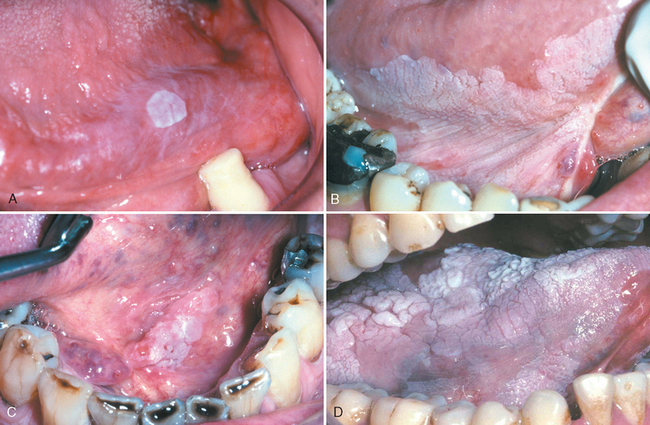
FIGURE 16-6 Leukoplakia. Clinical appearance of leukoplakias is highly variable and can range from (A) smooth and thin with well-demarcated borders, (B) diffuse and thick, (C) irregular with a granular surface, to (D) diffuse and corrugated.
(Courtesy of Drs. Neville, Damm, Allen, Bouquot [eds], Oral and Maxillofacial Pathology. Philadelphia, WB Saunders, 2008.)
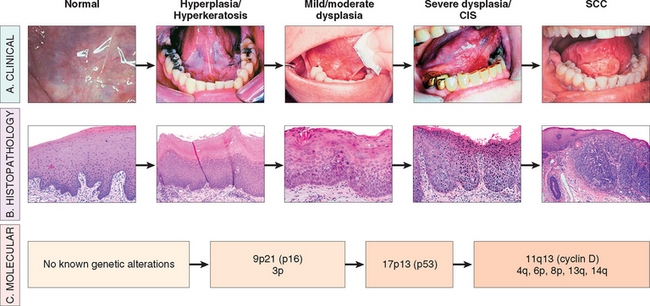
FIGURE 16-7 Clinical, histologic, and molecular progression of oral cancer. A, The typical clinical progression of oral cancer. B, The histologic progression of squamous epithelium from normal, to hyperkeratosis, to mild/moderate dysplasia, to severe dysplasia, to cancer. C, The sites of the most common genetic alterations identified as important for cancer development. CIS, carcinoma in situ; SCC, squamous cell carcinoma.
(Clinical photographs courtesy of Sol Silverman, MD, from Silverman S: Oral Cancer. Hamilton, Ontario, Canada, BD Dekker, 2003.)
The histologic changes in erythroplakia only rarely consist of orderly epidermal maturation; virtually all (approximately 90%) disclose superficial erosions with dysplasia, carcinoma in situ, or already developed carcinoma in the surrounding margins. Often, an intense subepithelial inflammatory reaction with vascular dilation is seen that likely contributes to the reddish clinical appearance.
SQUAMOUS CELL CARCINOMA
At least 95% of cancers of the head and neck are squamous cell carcinomas (HNSCCs), arising most commonly in the oral cavity. The remainder includes adenocarcinomas (of salivary gland origin), melanomas, various carcinomas, and other rarities. Biologically, squamous cell carcinomas in the oral cavity are fairly similar to those elsewhere in the head and neck, hence they are described together here. Features that apply to squamous cell cancer at specific sites in the head and neck are mentioned in the following discussion. Laryngeal squamous cell cancers are described later.
HNSCC is an aggressive epithelial malignancy that is the sixth most common neoplasm in the world today. At current rates, approximately 45,000 cases in the United States and more than 650,000 cases worldwide will be diagnosed each year.14,15-17 Despite numerous advances in treatment taking advantage of the most recent protocols for surgery, radiation therapy, and chemotherapy, the overall long-term survival has remained at less than 50% for the past 50 years.16 The 5-year survival rate of early-stage oral cancer is approximately 80%, while survival drops to 19% for late-stage disease. This dismal outlook is due to several factors, including the fact that oral cancer is often diagnosed when the disease has already reached an advanced stage. In addition, the frequent development of multiple primary tumors markedly decreases survival. The rate of second primary tumors in these patients has been reported to be 3% to 7% per year, which is higher than for any other malignancy.18,19 This observation has led to the concept of “field cancerization.” It is postulated that multiple individual primary tumors develop independently in the upper aerodigestive tract as a result of years of chronic exposure of the mucosa to carcinogens.20,21 Because of such field cancerization, an individual who is fortunate to live 5 years after the initial primary tumor has up to a 35% chance of developing at least one new primary tumor within that period of time. The occurrence of new primary tumors can be particularly devastating for individuals whose initial lesions were small. The 5-year survival rate for the first primary tumor is considerably better than 50%, but in such individuals, second primary tumors are the most common cause of death.22 Therefore, the early detection of all premalignant lesions is critical for the long-term survival of these patients.
Pathogenesis.
The pathogenesis of squamous cell carcinoma is multifactorial. Within North America and Europe it has classically been considered to be a disease of middle-aged men who have been chronic abusers of smoked tobacco and alcohol. The risk is magnified in those who smoke as well as consume alochol. Not unexpectedly, therefore, and concurrent with increased cigarette usage, the incidence of oral cancer in women is on the rise.
It is now known that at least 50% of oropharyngeal cancers, particularly those involving the tonsils, the base of the tongue, and the oropharynx harbor oncogenic variants of HPV.23 It is predicted that the incidence of HPV-associated HNSCC will surpass that of cervical cancer in the next decade, in part because the anatomic sites of origin (tonsillar crypts, base of tongue, and oropharynx) are not readily accessible or amenable to cytologic screening (unlike the cervix). It should be noted, however, that patients with HPV-positive HNSCC do better than those with HPV-negative tumors. The HPV vaccine, which is protective against cervical cancer, offers hope to stem the tide of HPV-associated HNSCC, although it is not yet approved for this use.
There is increasing epidemiologic evidence that a family history of head and neck cancer is a risk factor for the disease, thought to be due to inherited genomic instability.24 Finally, actinic radiation (sunlight) and, particularly, pipe smoking are known predisposing influences for cancer of the lower lip. Outside of North America and Europe, a major regional predisposing influence is the chewing of betel quid and paan in India and parts of Asia. The betel quid is a “witches’ brew” that contains several ingredients such as areca nut, slaked lime, and tobacco, which are wrapped in a betel leaf. While protracted irritation from ill-fitting dentures, jagged teeth, or chronic infections is no longer thought to be a direct antecedent to oral cancer, chronic irritation of the mucosa could act as a “promoter” of cancer in much the same way as alcohol does.
The incidence of oral cancer in individuals under age 40 who have no known risk factors has been on the rise for the past several years.25-27 The pathogenesis of this group of patients who are nonsmokers and not infected with HPV is unknown.
Molecular Biology of Squamous Cell Carcinoma.
Like all epithelial neoplasms, the development of squamous cell carcinoma is thought to be a multi-step process involving the sequential activation of oncogenes and inactivation of tumor suppressor genes in a clonal population of cells. Several genetic alterations, some definitively identified and some inferred from tumor-specific chromosomal alterations, have been found in HNSCC. While not all of the specific mutations required for progression have been delineated, a working molecular model has been established (see Fig. 16-7). The first change is the loss of chromosomal regions of 3p and 9p21.28 Loss of heterozygosity (LOH) in conjunction with promoter hypermethylation at this locus results in the inactivation of the p16 gene, an inhibitor of cyclin-dependent kinase (Chapter 7). This alteration is associated with the transition from normal to hyperplasia/hyperkeratosis and occurs before the development of histologic atypia, thus underscoring the histologic limitations for early diagnosis. Subsequent LOH at 17p with mutation of the p53 tumor suppressor gene is associated with progression to dysplasia.29 Recently it has been demonstrated that gross genomic alterations as well as deletions on 4q, 6p, 8p, 11q, 13q, and 14q may act as predictors of progression to frank malignancy.30 Ultimately, amplification and overexpression of the cyclin D1 gene (located on chromosome 11q13), which constitutively activates cell cycle progression, is a common late event. Data suggest that alterations of this gene confer the ability to invade in certain clones.31,32
However, while this model is a good working draft of the molecular changes involved in development of HNSCC, it is incomplete. First, while some of the gross genomic alterations correlate with genes known to be important in HNSCC (such as p16, p53, and CyclinD1), many of the specific genes are still unknown. Second, this model does not take into account alterations in genes such as the epidermal growth factor receptor (EFGR), which is overexpressed in a high percentage of HNSCC and has been successfully targeted in the treatment of this disease. Finally, as indicated above, it is increasingly clear that HNSCC is a heterogeneous disease in terms of etiology and its molecular mechanisms of development.
Morphology. Squamous cell carcinoma may arise anywhere in the oral cavity, but the favored locations are the ventral surface of the tongue, floor of the mouth, lower lip, soft palate, and gingiva (Fig. 16-8). The malignancies themselves are typically preceded by the presence of premalignant lesions that can be very heterogeneous in presentation (see above).
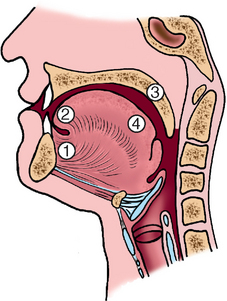
FIGURE 16-8 Schematic representation of the sites of origin of squamous cell carcinoma of the oral cavity, in numerical order of frequency.
In the early stages, cancers of the oral cavity appear either as raised, firm, pearly plaques or as irregular, roughened, or verrucous areas of mucosal thickening, possibly mistaken for leukoplakia. Either pattern may be superimposed on a background of apparent leukoplakia or erythroplakia. As these lesions enlarge, they typically create ulcerated and protruding masses that have irregular and indurated (rolled) borders.
On histologic examination, these cancers begin as dysplastic lesions, which may or may not progress to full-thickness dysplasia (carcinoma in situ) before invading the underlying connective tissue stroma (Fig. 16-7). This difference in progression should be contrasted with cervical cancer (Chapter 22), in which, typically, full-thickness dysplasia, representing carcinoma in situ, develops before invasion. Squamous cell carcinomas range from well-differentiated keratinizing neoplasms to anaplastic, sometimes sarcomatoid, tumors, and from slowly to rapidly growing lesions. However, the degree of histologic differentiation, as determined by the relative degree of keratinization, is not correlated with behavior. As a group these tumors tend to infiltrate locally before they metastasize to other sites. The routes of extension depend on the primary site. The favored sites of local metastasis are the cervical lymph nodes, while the most common sites of distant metastasis are mediastinal lymph nodes, lungs, liver, and bones. Unfortunately, such distant metastases are often occult at the time of discovery of the primary lesion.
Odontogenic Cysts and Tumors
In contrast to the rest of the skeleton, epithelial-lined cysts are quite common in the jaws. The overwhelming majority of these cysts are derived from remnants of odontogenic epithelium present within the jaws. In general, these cysts are subclassified as either inflammatory or developmental (Table 16-2). Only the most common of these lesions are described below.
TABLE 16-2 Histologic Classification of Odontogenic Cysts
| 1. INFLAMMATORY |
| 2. DEVELOPMENTAL |
The dentigerous cyst is defined as a cyst that originates around the crown of an unerupted tooth and is thought to be the result of a degeneration of the dental follicle. Radiographically, they are unilocular lesions and are most often associated with impacted third molar (wisdom) teeth. Histologically they are lined by a thin layer of stratified squamous epithelium. Often, there is a very dense chronic inflammatory cell infiltrate in the connective tissue stroma. Complete removal of the lesion is curative. This is important, since incomplete excision may result in recurrence or, very rarely, neoplastic transformation into an ameloblastoma or a squamous cell carcinoma.
The odontogenic keratocyst (OKC) is an important entity to differentiate from other odontogenic cysts because it is locally aggressive and has a high rate of recurrence. OKCs can be seen at any age but are most often diagnosed in patients between ages 10 and 40. They occur most commonly in males within the posterior mandible. Radiographically, OKCs present as well-defined unilocular or multilocular radiolucencies. Histologically, the cyst lining consists of a thin layer of parakeratinized or orthokeratinized stratified squamous epithelium with a prominent basal cell layer and a corrugated appearance of the epithelial surface. Treatment requires aggressive and complete removal of the lesion, because recurrence rates for inadequately removed lesions can reach 60%. Multiple OKCs may occur; these patients should be evaluated for nevoid basal cell carcinoma syndrome (Gorlin syndrome), which, as we shall see, is related to mutations in the tumor suppressor gene PTCH located on chromosome 9q22 (Chapter 25).
The periapical cyst, in contrast to the developmental cysts described above, is inflammatory in origin. These are extremely common lesions found at the apex of teeth. They develop as a result of long-standing pulpitis, which may be caused by advanced carious lesions or by trauma to the tooth in question. The inflammatory process may result in necrosis of the pulpal tissue, which can traverse the length of the root and exit the apex of the tooth into the surrounding alveolar bone, giving rise to a periapical abscess. Over time, like any chronic inflammatory process, a lesion with granulation tissue (with or without an epithelial lining) may develop. While the term periapical granuloma is not the most appropriate terminology (as the lesion does not show true granulomatous inflammation), old terminology, like bad habits, is difficult to shed. Periapical inflammatory lesions persist as a result of the continued presence of bacteria or other offensive agents in the area. Successful treatment, therefore, necessitates the complete removal of offending material and appropriate restoration of the tooth or extraction.
Odontogenic tumors are a complex group of lesions with diverse histologic appearances and clinical behavior.33 Some are true neoplasms (both benign and malignant), while others are more likely hamartomas. Odontogenic tumors are derived from odontogenic epithelium, ectomesenchyme, or both (Table 16-3). The two most common and clinically significant tumors are:
TABLE 16-3 Histologic Classification of Odontogenic Tumors
| 1. TUMORS OF ODONTOGENIC EPITHELIUM |
| Benign |
| Ameloblastoma |
| Calcifying epithelial odontogenic tumor (Pindborg tumor) |
| Squamous odontogenic tumor |
| Malignant |
| Ameloblastic carcinoma |
| Malignant ameloblastoma |
| Clear-cell odontogenic carcinoma |
| 2. TUMORS OF ODONTOGENIC ECTOMESENCHYME |
| Odontogenic fibroma |
| Odontogenic myxoma |
| Cementoblastoma |
| 3. TUMORS OF ODONTOGENIC EPITHELIUM AND ECTOMESENCHYME |
| Benign |
| Ameloblastic fibroma |
| Ameloblastic fibro-odontoma |
| Ameloblastic fibrosarcoma |
| Adenomatoid odontogenic tumor |
| Odontoameloblastoma |
| Complex odontoma |
| Compound odontoma |
| Malignant |
| Ameloblastic fibrosarcoma |
UPPER AIRWAYS
The term upper airways is used here to include the nose, pharynx, and larynx and their related parts. Disorders of these structures are among the most common afflictions of humans, but fortunately the overwhelming majority are more nuisances than threats.
Nose
Inflammatory diseases, mostly in the form of the common cold, as everyone knows, are the most common disorders of the nose and accessory air sinuses. Most of these inflammatory conditions are viral in origin, but they are often complicated by superimposed bacterial infections having considerably greater significance. Much less common are a few destructive inflammatory nasal diseases and tumors primary in the nasal cavity or paranasal sinuses.
INFLAMMATIONS
Infectious Rhinitis.
Infectious rhinitis, the more elegant way of saying “common cold,” is in most instances caused by one or more viruses. Major offenders are adenoviruses, echoviruses, and rhinoviruses. They evoke a profuse catarrhal discharge that is familiar to all and the bane of the kindergarten teacher. During the initial acute stages, the nasal mucosa is thickened, edematous, and red; the nasal cavities are narrowed; and the turbinates are enlarged. These changes may extend, producing a concomitant pharyngotonsillitis. Secondary bacterial infection enhances the inflammatory reaction and produces an essentially mucopurulent or sometimes frankly suppurative exudate. But as all have learned from experience, these infections soon clear up—as the saying goes, in a week if treated but in 7 days if ignored.
Allergic Rhinitis.
Allergic rhinitis (hay fever) is initiated by hypersensitivity reactions to one of a large group of allergens, most commonly the plant pollens, fungi, animal allergens, and dust mites.34 It affects 20% of the US population. As is the case with asthma, allergic rhinitis is an IgE–mediated immune reaction with an early- and late-phase response (see “Immediate (Type I) Hypersensitivity” in Chapter 6). The allergic reaction is characterized by marked mucosal edema, redness, and mucus secretion, accompanied by a leukocytic infiltration in which eosinophils are prominent.
Nasal Polyps.
Recurrent attacks of rhinitis may eventually lead to focal protrusions of the mucosa, producing so-called nasal polyps, which may reach 3 to 4 cm in length. On histologic examination these polyps consist of edematous mucosa having a loose stroma, often harboring hyperplastic or cystic mucous glands, infiltrated with a variety of inflammatory cells, including neutrophils, eosinophils, and plasma cells with occasional clusters of lymphocytes (Fig. 16-9). In the absence of bacterial infection, the mucosal covering of these polyps is intact, but with chronicity it may become ulcerated or infected. When multiple or large, the polyps may encroach on the airway and impair sinus drainage. Although the features of nasal polyps point to an allergic etiology, most people with nasal polyps are not atopic, and only 0.5% of atopic patients develop polyps.35
Chronic Rhinitis.
Chronic rhinitis is a sequel to repeated attacks of acute rhinitis, either microbial or allergic in origin, with the eventual development of superimposed bacterial infection. A deviated nasal septum or nasal polyps with impaired drainage of secretions contribute to the likelihood of microbial invasion. Frequently, there is superficial desquamation or ulceration of the mucosal epithelium and a variable inflammatory infiltrate of neutrophils, lymphocytes, and plasma cells subjacent to the epithelium. These suppurative infections sometimes extend into the air sinuses.
Sinusitis.
Acute sinusitis is most commonly preceded by acute or chronic rhinitis, but maxillary sinusitis occasionally arises by extension of a periapical infection through the bony floor of the sinus. The offending agents are usually inhabitants of the oral cavity, and the inflammatory reaction is entirely nonspecific. Impairment of drainage of the sinus by inflammatory edema of the mucosa is an important contributor to the process and, when complete, may impound the suppurative exudate, producing empyema of the sinus. Obstruction of the outflow, most often from the frontal and less commonly from the anterior ethmoid sinuses, occasionally leads to an accumulation of mucous secretions in the absence of direct bacterial invasion, producing a so-called mucocele. Acute sinusitis may, in time, give rise to chronic sinusitis, particularly when there is interference with drainage. There is usually a mixed microbial flora, largely of normal inhabitants of the oral cavity. Particularly severe forms of chronic sinusitis are caused by fungi (e.g., mucormycosis), especially in diabetics. Uncommonly, sinusitis is a component of Kartagener syndrome, which also includes bronchiectasis and situs inversus (Chapter 15). All these features are secondary to defective ciliary action. Although most instances of chronic sinusitis are more uncomfortable than disabling or serious, the infections have the potential of spreading into the orbit or of penetrating into the surrounding bone to give rise to osteomyelitis or spreading into the cranial vault, causing septic thrombophlebitis of a dural venous sinus.
NECROTIZING LESIONS OF THE NOSE AND UPPER AIRWAYS
Necrotizing ulcerating lesions of the nose and upper respiratory tract may be produced by
Nasopharynx
Although the nasopharyngeal mucosa, related lymphoid structures, and glands may be involved in a wide variety of specific infections (e.g., diphtheria, infectious mononucleosis) and by neoplasms, the only disorders mentioned here are nonspecific inflammations; tumors are discussed separately.
INFLAMMATIONS
Pharyngitis and tonsillitis are frequent in the usual viral upper respiratory infections. Most commonly implicated are the multitudinous rhinoviruses, echoviruses, and adenoviruses, and, less frequently, respiratory syncytial viruses and the various strains of influenza virus. In the usual case, there is reddening and slight edema of the nasopharyngeal mucosa, with reactive enlargement of the related lymphoid structures. Bacterial infections may be superimposed on these viral involvements, or may be primary invaders. The most common offenders are the β-hemolytic streptococci, but sometimes Staphylococcus aureus or other pathogens may be implicated. The inflamed nasopharyngeal mucosa may be covered by an exudative membrane (pseudo-membrane), and the nasopalatine and palatine tonsils may be enlarged and covered by exudate. A typical appearance is of enlarged, reddened tonsils (due to reactive lymphoid hyperplasia) dotted by pinpoints of exudate emanating from the tonsillar crypts, so-called follicular tonsillitis.
The major importance of streptococcal “sore throats” lies in the possible development of late sequelae, such as, rheumatic fever (Chapter 12) and glomerulonephritis (Chapter 20). Whether recurrent episodes of acute tonsillitis favor the development of chronic tonsillitis (true chronic tonsillitis is extremely rare) is open to debate, but the result is residual enlargement of the lymphoid tissue, inviting the tender mercies of the otolaryngologist.
Tumors of the Nose, Sinuses, and Nasopharynx
Tumors in these locations are infrequent but include the entire category of mesenchymal and epithelial neoplasms.36,37 Brief mention is made of some distinctive types.
Nasopharyngeal Angiofibroma.
This is a highly vascular tumor that occurs almost exclusively in adolescent males. Despite its benign nature, it may cause serious clinical problems because of its tendency to bleed profusely during surgery.
Sinonasal (Schneiderian) Papilloma.
These are benign neoplasms arising from the sinonasal mucosa and are composed of squamous or columnar epithelium. Although their etiology is still unproven, HPV types 6 and 11 have been identified in the lesions. These lesions occur in three forms: exophytic (most common), inverted (most important biologically), and cylindrical. Because of its uniquely aggressive biologic behavior, only inverted papilloma is discussed here. Inverted papillomas are benign but locally aggressive neoplasms occurring in both the nose and the paranasal sinuses. As the name implies, the papillomatous proliferation of squamous epithelium, instead of producing an exophytic growth (like the septal and cylindrical papillomas), extends into the mucosa, that is, it is inverted (Fig. 16-10). If not adequately excised, it has a high rate of recurrence, with the potentially serious complication of invasion of the orbit or cranial vault; rarely, frank carcinoma may also develop.
Olfactory Neuroblastoma (Esthesioneuroblastoma).
These are uncommon, malignant tumors composed of small round cells, resembling neuroblasts, which form lobular nests encircled by vascularized connective tissue. They arise most often superiorly and laterally in the nose from the neuroendocrine cells dispersed in the olfactory mucosa. The differential diagnosis of these neoplasms includes all other small-cell tumors (Chapter 10), such as lymphoma, Ewing sarcoma, and embryonal rhabdomyosarcoma.38 The cells are of neuroendocrine origin and thus exhibit membrane-bound secretory granules on electron microscopy and express neuron-specific enolase, synaptophysin, CD56, and chromogranin by immunohistochemistry. While the name implies that they are primitive neuroectodermal tumors, many do not share the 11;22 translocation or fusion-gene products typical of Ewing sarcoma of bone (Chapter 26) and other primitive neuroectodermal tumors. Some of these tumors reveal trisomy 8. Depending on the stage and grade of a particular neoplasm, combinations of surgery, radiation therapy, and chemotherapy yield 5-year survival rates of 40% to 90%.39
Nasopharyngeal Carcinoma.
This tumor is characterized by a distinctive geographic distribution, a close anatomic relationship to lymphoid tissue, and an association with EBV infection.40 The nomenclature for nasopharyngeal carcinomas is in constant flux. However, at present the disease is thought to take one of three patterns: (1) keratinizing squamous cell carcinomas, (2) nonkeratinizing squamous cell carcinomas, and (3) undifferentiated carcinomas that have an abundant non-neoplastic, lymphocytic infiltrate. The last pattern has often been called lymphoepithelioma. However, while widely used in clinical practice, this highly descriptive term should be avoided.
Three factors affect the origins of these neoplasms: (1) heredity, (2) age, and (3) infection with EBV. Nasopharyngeal carcinomas are particularly common in some parts of Africa, where they are the most frequent childhood cancer. In contrast, in southern China, they are very common in adults but rarely occur in children. In the United States they are rare in both adults and children. In addition to EBV infection, diets high in nitrosamines, such as fermented foods and salted fish, as well as other environmental factors such as smoking and chemical fumes, have been linked to the disease. Components of the EBV genome such as EBNA-1 can be identified in the tumor epithelial cells (not the lymphocytes) of most undifferentiated and nonkeratinizing squamous cell nasopharyngeal carcinomas, particularly when in situ hybridization is performed.41
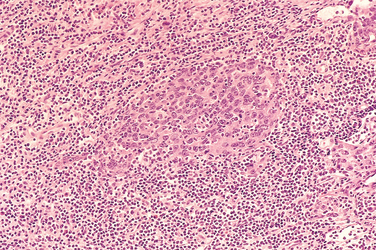
Morphology. On histologic examination, the keratinizing and nonkeratinizing squamous cell lesions resemble usual well-differentiated and poorly differentiated squamous cell carcinomas arising in other locations. The undifferentiated variant is composed of large epithelial cells with oval or round vesicular nuclei, prominent nucleoli, and indistinct cell borders disposed in a syncytium-like array (Fig. 16-11). Admixed with the epithelial cells are abundant, mature, normal-appearing lymphocytes, which are predominantly T cells.
Primary nasopharyngeal carcinomas are often clinically occult for long periods, and present as metastases in the cervical lymph nodes in as many as 70% of the patients. Radiotherapy is the standard modality of treatment, yielding 50% to 70% 3-year survival rate. The undifferentiated carcinoma is the most radiosensitive and the keratinizing squamous cell carcinoma is the least radiosensitive.
Larynx
The most common disorder that affects the larynx is inflammation. Tumors are uncommon but are amenable to resection, though often at the price of loss of natural voice.
INFLAMMATIONS
Laryngitis may occur as the sole manifestation of allergic, viral, bacterial, or chemical insult, but it is more commonly part of a generalized upper respiratory tract infection or the result of heavy exposure to environmental toxins such as tobacco smoke. It may also occur in association with gastroesophageal reflux due to the irritating effect of gastric contents. The larynx may also be affected in systemic infections, such as tuberculosis and diphtheria. Although most infections are self-limited, they may at times be serious, especially in infancy or childhood, when mucosal congestion, exudation, or edema may cause laryngeal obstruction. In particular, laryngoepiglottitis, caused by respiratory syncitial virus, Haemophilus influenzae, or β-hemolytic streptococci in infants and young children with their small airways, may induce such sudden swelling of the epiglottis and vocal cords that a potentially lethal medical emergency is created. This form of disease is uncommon in adults because of the larger size of the larynx and the stronger accessory muscles of respiration. Croup is the name given to laryngotracheobronchitis in children, in which the inflammatory narrowing of the airway produces the inspiratory stridor so frightening to parents. The most common form of laryngitis, encountered in heavy smokers, predisposes to squamous epithelial metaplasia and sometimes overt carcinoma.
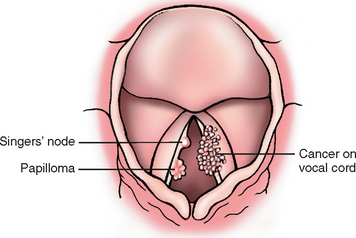
REACTIVE NODULES (VOCAL CORD NODULES AND POLYPS)
Reactive nodules, also called polyps, sometimes develop on the vocal cords, most often in heavy smokers or in individuals who impose great strain on their vocal cords (singers’ nodules) (Fig. 16-12). By convention, singers’ nodules are bilateral lesions and polyps are unilateral. Adults, predominantly men, are most often affected. These nodules are smooth, rounded, sessile or pedunculated excrescences, generally only a few millimeters in the greatest dimension, located usually on the true vocal cords. They are typically covered by squamous epithelium that may become keratotic, hyperplastic, or even slightly dysplastic. The core of the nodule is a loose myxoid connective tissue that may be variably fibrotic or punctuated by numerous vascular channels. When nodules on opposing vocal cords impinge on each other, the mucosa may undergo ulceration. Because of their strategic location and accompanying inflammation, they characteristically change the character of the voice and often cause progressive hoarseness. They virtually never give rise to cancers.
SQUAMOUS PAPILLOMA AND PAPILLOMATOSIS
Laryngeal squamous papillomas are benign neoplasms, usually located on the true vocal cords, that form soft, raspberry-like excrescences rarely more than 1 cm in diameter (Fig. 16-12). On histologic examination, the papillomas are made up of multiple slender, finger-like projections supported by central fibrovascular cores and covered by an orderly stratified squamous epithelium. When the papillomas are on the free edge of the vocal cord, trauma may lead to ulceration that can be accompanied by hemoptysis.
Papillomas are usually single in adults but are often multiple in children, in whom they are referred to as juvenile laryngeal papillomatosis.42 However, multiple recurring papillomas also occur in adults. The lesions are caused by HPV types 6 and 11. They do not become malignant, but frequently recur. They often spontaneously regress at puberty, but some affected patients endure numerous surgeries before this occurs. Cancerous transformation is rare.
CARCINOMA OF THE LARYNX
Sequence of Hyperplasia-Dysplasia-Carcinoma.
A spectrum of epithelial alterations is seen in the larynx. They range from hyperplasia, atypical hyperplasia, dysplasia, carcinoma in situ, to invasive carcinoma.43 Macroscopically, the epithelial changes vary from smooth, white or reddened focal thickenings, sometimes roughened by keratosis, to irregular verrucous or ulcerated white-pink lesions that are similar in appearance to carcinoma.
There are all gradations of epithelial hyperplasia of the true vocal cords, and the likelihood of the development of an overt carcinoma is directly proportional to the level of atypia when the lesion is first seen. Orderly hyperplasias have almost no potential for malignant transformation, but the risk rises to 1% to 2% during the span of 5 to 10 years with mild dysplasia and 5% to 10% with severe dysplasia. Only histologic evaluation can determine the gravity of the changes.
The various changes described are most often related to tobacco smoke, the risk being proportional to the level of exposure. Indeed, up to the point of frank cancer, the changes often regress after cessation of smoking. Alcohol is also clearly a risk factor. Together smoking and alochol increase the risk substantially. Other factors that may contribute to increased risk include nutritional factors, exposure to asbestos, irradiation, and infection with HPV.44,45
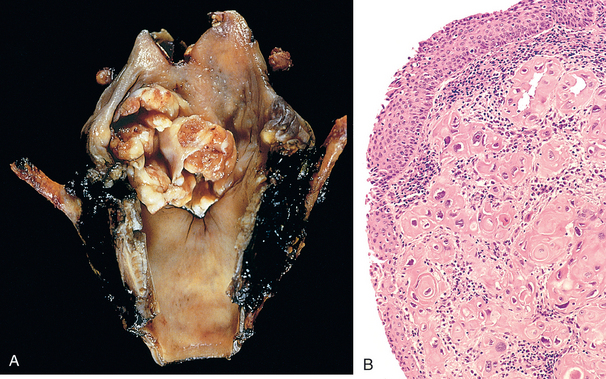
Morphology. About 95% of laryngeal carcinomas are typical squamous cell tumors. The tumor usually develops directly on the vocal cords, but it may arise above or below the cords, on the epiglottis or aryepiglottic folds, or in the pyriform sinuses. Those confined within the larynx proper are termed intrinsic, whereas those that arise or extend outside the larynx are called extrinsic. Squamous cell carcinomas of the larynx follow the growth pattern of other squamous cell carcinomas. They begin as in situ lesions that later appear as pearly gray, wrinkled plaques on the mucosal surface, ultimately ulcerating and fungating (Fig. 16-13). The degree of anaplasia of the laryngeal tumors is highly variable. Sometimes massive tumor giant cells and multiple bizarre mitotic figures are seen. As expected with lesions arising from recurrent exposure to environmental carcinogens, adjacent mucosa may demonstrate squamous cell hyperplasia with foci of dysplasia or even carcinoma in situ.
Carcinoma of the larynx manifests itself clinically by persistent hoarseness. At presentation, about 60% of these cancers are confined to the larynx. The prognosis for these is better than for those tumors that have spread into adjacent structures. Later, laryngeal tumors may produce pain, dysphagia, and hemoptysis. Patients are also extremely vulnerable to secondary infection of the ulcerating lesion. With surgery, irradiation, or combination therapy, many patients can be cured, but about one third die of the disease. The usual causes of death are infection of the distal respiratory passages or widespread metastases and cachexia.
EARS
Although disorders of the ear rarely shorten life, many impair its quality. The most common aural disorders, in descending order of frequency, are (1) acute and chronic otitis (most often involving the middle ear and mastoid), sometimes leading to a cholesteatoma; (2) symptomatic otosclerosis; (3) aural polyps; (4) labyrinthitis; (5) carcinomas, largely of the external ear; and (6) paragangliomas, found mostly in the middle ear. Only those conditions that have distinctive morphologic features (save for labyrinthitis) are described. Paragangliomas are discussed later.
Inflammatory Lesions
Inflammations of the ear—otitis media, acute or chronic—occur mostly in infants and children. These lesions are typically viral in nature and produce a serous exudate but may become suppurative with superimposed bacterial infection. The most common offenders are Streptococcus pneumoniae, non-typeable H. influenzae, and Moraxella catarrhalis.46
Repeated bouts of acute otitis media with failure of resolution lead to chronic disease. The causative agents of chronic disease are usually Pseudomonas aeruginosa, Staphylococcus aureus, or a fungus; sometimes a mixed flora is the cause. Chronic infection has the potential to perforate the eardrum, encroach on the ossicles or labyrinth, spread into the mastoid spaces, and even penetrate into the cranial vault to produce a temporal cerebritis or abscess. Otitis media in the diabetic person, when caused by P. aeruginosa, is especially aggressive and spreads widely causing destructive necrotizing otitis media.
Cholesteatomas, associated with chronic otitis media, are not neoplasms, nor do they always contain cholesterol. Rather, they are cystic lesions 1 to 4 cm in diameter, lined by keratinizing squamous epithelium or metaplastic mucus-secreting epithelium, and filled with amorphous debris (derived largely from desquamated epithelium). Sometimes they contain spicules of cholesterol. The precise events involved in their development are not clear, but it is proposed that chronic inflammation and perforation of the eardrum with ingrowth of the squamous epithelium or metaplasia of the secretory epithelial lining of the middle ear are responsible for the formation of a squamous cell nest that becomes cystic. A chronic inflammatory reaction surrounds the keratinous cyst. Sometimes, the cyst ruptures, increasing the inflammatory reaction and inducing the formation of giant cells that enclose partially necrotic squames and other particulate debris. These lesions, by progressive enlargement, can erode into the ossicles, the labyrinth, the adjacent bone, or the surrounding soft tissue and sometimes produce visible neck masses.
Otosclerosis
As the name implies, otosclerosis refers to abnormal bone deposition in the middle ear about the rim of the oval window into which the footplate of the stapes fits. Both ears are usually affected. At first there is fibrous ankylosis of the footplate, followed in time by bony overgrowth anchoring it into the oval window. The degree of immobilization governs the severity of the hearing loss. This condition usually begins in the early decades of life; minimal degrees of this derangement are exceedingly common in the United States in young to middle-aged adults, but fortunately more severe symptomatic otosclerosis is relatively uncommon. In most instances it is familial, following autosomal dominant transmission with variable penetrance. The basis for the osseous overgrowth is completely obscure, but it appears to represent uncoupling of normal bone resorption and bone formation. Thus, it begins with bone resorption, followed by fibrosis and vascularization of the temporal bone in the immediate vicinity of the oval window, in time replaced by dense new bone anchoring the footplate of the stapes. In most instances the process is slowly progressive over the span of decades, leading eventually to marked hearing loss.
Tumors
A large variety of epithelial and mesenchymal tumors that arise in the ear—external, middle, internal—all are rare save for basal cell or squamous cell carcinomas of the pinna (external ear). These carcinomas tend to occur in elderly men and are thought to be associated with actinic radiation. By contrast, those within the canal tend to be squamous cell carcinomas, which occur in middle-aged to elderly women and are not associated with sun exposure. Wherever they arise they morphologically resemble their counterparts in other skin locations, beginning as papules that extend and eventually erode and invade locally. Basal cell and squamous cell lesions of the pinna are locally invasive but they rarely spread. Squamous cell carcinomas arising in the external canal may invade the cranial cavity or metastasize to regional nodes accounting for a 5-year mortality of about 50%.
NECK
Most of the conditions that involve the neck are described elsewhere (e.g., squamous cell and basal cell carcinomas of the skin, melanomas, lymphomas), or they are a component of a systemic disorder (e.g., generalized rashes, the lymphadenopathy of infectious mononucleosis or tonsillitis). What remains for consideration here are a few uncommon lesions unique to the neck.
Branchial Cyst (Cervical Lymphoepithelial Cyst)
These benign cysts usually appear on the upper lateral aspect of the neck along the sternocleidomastoid muscle. The vast majority of these cysts are thought to arise from remnants of the second branchial arch and are most commonly observed in young adults between the ages of 20 and 40. Clinically, the cysts are well circumscribed, 2 to 5 cm in diameter, with fibrous walls usually lined by stratified squamous or pseudostratified columnar epithelium. The cyst wall typically contains lymphoid tissue with prominent germinal centers. The cystic contents may be clear, watery to mucinous fluid or may contain desquamated, granular cellular debris. The cysts enlarge slowly, are rarely the site of malignant transformation, and generally are readily excised. Similar lesions sometimes appear in the parotid gland or in the oral cavity beneath the tongue.
Thyroglossal Duct Cyst
Embryologically, the thyroid anlage begins in the region of the foramen cecum at the base of the tongue; as the gland develops it descends to its definitive location in the anterior neck. Remnants of this developmental tract may persist, producing cysts, 1 to 4 cm in diameter, which may be lined by stratified squamous epithelium, when located near the base of the tongue, or by pseudostratified columnar epithelium in lower locations. Transitional patterns are also encountered. The connective tissue wall of the cyst may harbor lymphoid aggregates or remnants of recognizable thyroid tissue. The treatment is excision. Malignant transformation within the lining epithelium has been reported but is rare.
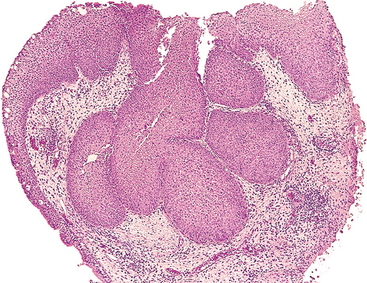
Paraganglioma (Carotid Body Tumor)
Paraganglia are clusters of neuroendocrine cells associated with the sympathetic and parasympathetic nervous systems. As a result, these neoplasms can be seen throughout various regions of the body. While the most common location of these tumors is within the adrenal medulla, where they give rise to pheochromocytomas (Chapter 24), approximately 70% of extra-adrenal paragangliomas occur in the head and neck region.47 The pathogenesis of paragangliomas is not fully understood. However, alterations in genes encoding subunits of succinate oxidoreductase, an enzyme involved in mitochondrial respiration, have been reported in both hereditary and spontaneous paragangliomas. Paragangliomas typically develop in two locations:
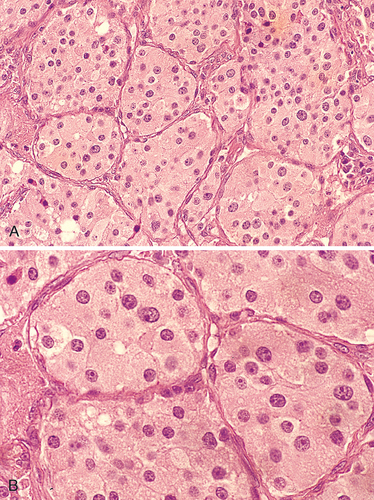
Morphology. The carotid body tumor is a prototype of a parasympathetic paraganglioma. It rarely exceeds 6 cm in diameter and arises close to or envelops the bifurcation of the common carotid artery. The tumor tissue is red-pink to brown. The microscopic features of all paragangliomas, wherever they arise, are remarkably uniform. They are chiefly composed of nests (Zellballen) of round to oval chief cells (neuroectodermal in origin) that are surrounded by delicate vascular septae. The tumor cells contain abundant, clear or granular, eosinophilic cytoplasm and uniform, round to ovoid, sometimes vesicular, nuclei (Fig. 16-14).48 In most tumors there is little cellular pleomorphism, and mitoses are scant. The chief cells stain strongly for neuroendocrine markers such as chromogranin, synaptophysin, neuron-specific enolase, CD56, and CD57. In addition, there is a supporting network of spindle-shaped stromal cells, collectively called sustentacular cells, that are positive for S-100 protein. Electron microscopy often discloses well-demarcated neuroendocrine granules in paravertebral tumors, but their number can be highly variable and they tend to be scant in nonfunctioning tumors.
Carotid body tumors (and paragangliomas in general) are rare. They are slow-growing and painless masses that usually arise in the fifth and sixth decades of life. They commonly occur singly and sporadically but may be familial, with autosomal dominant transmission in the multiple endocrine neoplasia 2 syndrome (Chapter 24); in this setting they are often multiple and sometimes bilaterally symmetric. Carotid body tumors frequently recur after incomplete resection and, despite their benign appearance, may metastasize to regional lymph nodes and distant sites. About 50% ultimately prove fatal, largely because of infiltrative growth. Unfortunately, it is almost impossible histologically to judge the clinical course of a carotid body tumor—mitoses, pleomorphism, and even vascular invasion are not reliable indicators.47
SALIVARY GLANDS
There are three major salivary glands—parotid, submandibular, and sublingual—as well as innumerable minor salivary glands distributed throughout the mucosa of the oral cavity. All these glands are subject to inflammation or to the development of neoplasms.
Xerostomia
Xerostomia is defined as a dry mouth resulting from a decrease in the production of saliva. Its incidence among various populations has been reported to be as high as 29%.48 It is a major feature of the autoimmune disorder Sjögren syndrome, in which it is usually accompanied by dry eyes (Chapter 6). A lack of salivary secretions is also a major complication of radiation therapy. However, xerostomia is most frequently observed as a result of many commonly prescribed classes of medications including: anticholinergic, antidepressant/antipsychotic, diuretic, antihypertensive, sedative, muscle relaxant, analgesic, and antihistamine agents.48-50 The oral cavity may merely reveal dry mucosa and/or atrophy of the papillae of the tongue, with fissuring and ulcerations, or in Sjögren syndrome, concomitant inflammatory enlargement of the salivary glands. Complications of xerostomia include increased rates of dental caries, candidiasis, as well as difficulty in swallowing and speaking.
Inflammation (Sialadenitis)
Sialadenitis may be of traumatic, viral, bacterial, or autoimmune origin. Mucoceles are the most common type of inflammatory salivary gland lesion. The most common form of viral sialadenitis is mumps, in which the major salivary glands, particularly the parotids, are affected (Chapter 8). Other glands (e.g., the pancreas and testes) may also be involved. Autoimmune disease underlies the inflammatory salivary gland changes of Sjögren syndrome, discussed in Chapter 6. In this condition the widespread involvement of the salivary glands and the mucus-secreting glands of the mucosa induces xerostomia. Associated involvement of the lacrimal glands produces dry eyes—keratoconjunctivitis sicca.
Mucocele.
This is the most common lesion of the salivary glands. It results from either blockage or rupture of a salivary gland duct, with consequent leakage of saliva into the surrounding connective tissue stroma. Mucoceles are most often found on the lower lip and are the result of trauma (Fig. 16-15A). As such, they are typically seen in toddlers and young adults as well as the geriatric population (as a result of falling). Clinically, they present as fluctuant swellings of the lower lip and have a blue translucent hue to them. Patients may report a history of changes in the size of the lesion, particularly in association with meals. Histologically, mucoceles demonstrate a cystlike space that is lined by inflammatory granulation tissue or by fibrous connective tissue. The cystic spaces are filled with mucin as well as inflammatory cells, particularly macrophages (Fig. 16-15B). Complete excision of the cyst with the minor salivary gland lobule of origin is required. Incomplete excision can result in recurrence.
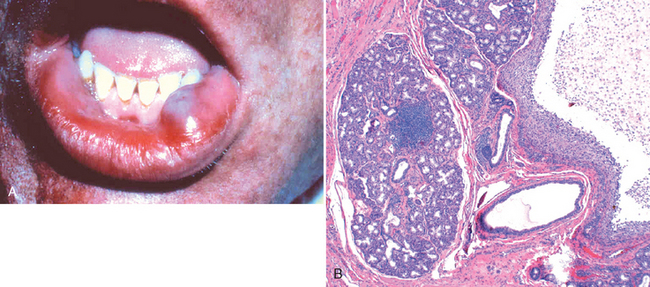
FIGURE 16-15 Mucocele. A, Fluctuant fluid-filled lesion on the lower lip subsequent to trauma. B, Cystlike cavity filled with mucinous material and lined by organizing granulation tissue.
A ranula is histologically identical to a mucocele. However, this term is reserved for mucoceles that arise when the duct of the sublingual gland has been damaged. A ranula can become extremely large and develop into a “plunging ranula” when it dissects its way through the connective tissue stroma connecting the two bellies of the mylohyoid muscle.
Sialolithiasis and Nonspecific Sialadenitis.
Nonspecific bacterial sialadenitis, most often involving the major salivary glands, particularly the submandibular glands, is a common condition, usually secondary to ductal obstruction produced by stones (sialolithiasis). The common offenders are S. aureus and Streptococcus viridans. The stone formation is sometimes related to obstruction of the orifices of the salivary glands by impacted food debris or by edema about the orifice after some injury. Frequently, the stones are of obscure origin. Dehydration and decreased secretory function may also predispose to secondary bacterial invasion, as sometimes occurs in patients receiving long-term phenothiazines that suppress salivary secretion. Dehydration with decreased secretion may lead to the development of bacterial suppurative parotitis in elderly patients with a recent history of major thoracic or abdominal surgery.
Whatever the origin, the obstructive process and bacterial invasion lead to a nonspecific inflammation of the affected glands that may be largely interstitial or, when induced by staphylococcal or other pyogens, may be associated with overt suppurative necrosis and abscess formation. Unilateral involvement of a single gland is the rule. The inflammatory involvement causes painful enlargement and sometimes a purulent ductal discharge.
Neoplasms
Despite their relatively simple morphology, the salivary glands give rise to no fewer than 30 histologically distinct tumors.51-53 A classification and the relative incidence of benign and malignant tumors is shown in Table 16-4; not included are the rare benign and malignant mesenchymal neoplasms.
TABLE 16-4 Histologic Classification and Incidence of Benign and Malignant Tumors of the Salivary Glands
| Benign | Malignant |
|---|---|
| Pleomorphic adenoma (50%) (mixed tumor) | Mucoepidermoid carcinoma (15%) |
| Warthin tumor (5% to 10%) | Adenocarcinoma (NOS) (10%) |
| Oncocytoma (1%) | Acinic cell carcinoma (5%) |
| Other adenomas (5% to 10%) | Adenoid cystic carcinoma (5%) |
| Basal cell adenoma | Malignant mixed tumor (3% to 5%) |
| Canalicular adenoma | Squamous cell carcinoma (1%) |
| Ductal papillomas | Other carcinomas (2%) |
NOS, not otherwise specified.
Data from Ellis GL, Auclair PL: Tumors of the Salivary Glands. Atlas of Tumor Pathology, Third Series. Washington, DC, Armed Forces Institute of Pathology, 1996.
As indicated in Table 16-4, a small number of neoplasms makes up more than 90% of salivary gland tumors, and so our discussion is restricted to these. Overall, these neoplasms are relatively uncommon and represent less than 2% of all tumors in humans. About 65% to 80% arise within the parotid, 10% in the submandibular gland, and the remainder in the minor salivary glands, including the sublingual glands. Approximately 15% to 30% of tumors in the parotid glands are malignant. In contrast, approximately 40% of submandibular, 50% of minor salivary gland, and 70% to 90% of sublingual tumors are cancerous. The likelihood, then, of a salivary gland tumor being malignant is more or less inversely proportional to the size of the gland.
These tumors usually occur in adults, with a slight female predominance, but about 5% occur in children younger than age 16 years. For unknown reasons, Warthin tumors occur much more often in males than in females. The benign tumors most often appear in the fifth to seventh decades of life. The malignant ones tend, on average, to appear somewhat later. Whatever the histologic pattern, neoplasms in the parotid glands produce distinctive swellings in front of and below the ear. In general, when they are first diagnosed, both benign and malignant lesions range from 4 to 6 cm in diameter and are mobile on palpation except in the case of neglected malignant tumors. Although benign tumors are known to have been present usually for many months to several years before coming to clinical attention, cancers seem to demand attention more promptly, probably because of their more rapid growth. Ultimately, however, there are no reliable criteria to differentiate, on clinical grounds, the benign from the malignant lesions, and morphologic evaluation is necessary.
PLEOMORPHIC ADENOMA
Because of their remarkable histologic diversity, these neoplasms have also been called mixed tumors. They represent about 60% of tumors in the parotid, are less common in the submandibular glands, and are relatively rare in the minor salivary glands. They are benign tumors that consist of a mixture of ductal (epithelial) and myoepithelial cells, and therefore they show both epithelial and mesenchymal differentiation. They reveal epithelial elements dispersed throughout the matrix along with varying degrees of myxoid, hyaline, chondroid (cartilaginous), and even osseous tissue. In some tumors the epithelial elements predominate; in others they are present only in widely dispersed foci.
Little is known about the origins of these neoplasms, except that radiation exposure increases the risk. Equally uncertain is the histogenesis of the various components. A currently popular view is that all neoplastic elements, including those that appear mesenchymal, are of either myoepithelial or ductal reserve cell origin (hence the designation pleomorphic adenoma).
Morphology. Most pleomorphic adenomas present as rounded, well-demarcated masses rarely exceeding 6 cm in the greatest dimension (Fig. 16-16). Although they are encapsulated, in some locations (particularly the palate) the capsule is not fully developed, and expansile growth produces protrusions into the surrounding gland, rendering enucleation of the tumor hazardous. The cut surface is gray-white with myxoid and blue translucent areas of chondroid (cartilage-like).
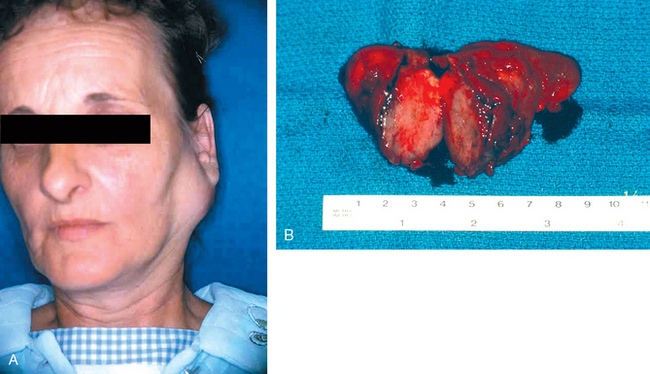
FIGURE 16-16 Pleomorphic adenoma. A, Slowly enlarging neoplasm in the parotid gland of many years duration. B, The bisected, sharply circumscribed, yellow-white tumor can be seen surrounded by normal salivary gland tissue.
The dominant histologic feature is the great heterogeneity mentioned. The epithelial elements resembling ductal cells or myoepithelial cells are arranged in duct formations, acini, irregular tubules, strands, or sheets of cells. These elements are typically dispersed within a mesenchyme-like background of loose myxoid tissue containing islands of chondroid and, rarely, foci of bone (Fig. 16-17). Sometimes the epithelial cells form well-developed ducts lined by cuboidal to columnar cells with an underlying layer of deeply chromatic, small myoepithelial cells. In other instances there may be strands or sheets of myoepithelial cells. Islands of well-differentiated squamous epithelium may also be present. In most cases there is no epithelial dysplasia or evident mitotic activity. There is no difference in biologic behavior between the tumors composed largely of epithelial elements and those composed largely of seemingly mesenchymal elements.
Clinical Features.
These tumors present as painless, slow-growing, mobile discrete masses within the parotid (Fig. 16-16) or submandibular areas or in the buccal cavity. The recurrence rate (perhaps months to years later) with parotidectomy is about 4% but, with simple enucleation, approaches 25%. This high recurrence is because of failure to recognize minute protrusions from the main mass, into the surrounding tissue, at the time of surgery.
A carcinoma arising in a pleomorphic adenoma is referred to variously as a carcinoma ex pleomorphic adenoma or a malignant mixed tumor. The incidence of malignant transformation increases with the duration of the tumor, being about 2% for tumors present less than 5 years and almost 10% for those of more than 15 years’ duration. The cancer usually takes the form of an adenocarcinoma or undifferentiated carcinoma, and often it virtually completely overgrows the last vestiges of the preexisting pleomorphic adenoma; but to substantiate the diagnosis of carcinoma ex pleomorphic adenoma, recognizable traces of the latter must be found. Regrettably, these cancers, when they appear, are among the most aggressive of all salivary gland malignant neoplasms, producing mortality rates of 30% to 50% at 5 years.
WARTHIN TUMOR (PAPILLARY CYSTADENOMA LYMPHOMATOSUM)
This curious benign neoplasm with its intimidating histologic name is the second most common salivary gland neoplasm. It arises almost exclusively in the parotid gland (the only tumor virtually restricted to the parotid) and occurs more commonly in males than in females, usually in the fifth to seventh decades of life. About 10% are multifocal and 10% bilateral. Smokers have eight times the risk of nonsmokers for developing these tumors.
Morphology. Most Warthin tumors are round to oval, encapsulated masses, 2 to 5 cm in diameter, usually arising in the superficial parotid gland, where they are readily palpable. Transection reveals a pale gray surface punctuated by narrow cystic or cleftlike spaces filled with a mucinous or serous secretion. On microscopic examination these spaces are lined by a double layer of neoplastic epithelial cells resting on a dense lymphoid stroma sometimes bearing germinal centers (Fig. 16-18). The spaces are frequently narrowed by polypoid projections of the lymphoepithelial elements. The double layer of lining cells is distinctive; it consists of a surface palisade of columnar cells having an abundant, finely granular, eosinophilic cytoplasm, that imparts an oncocytic appearance, which rests on a layer of cuboidal to polygonal cells. Oncocytes are epithelial cells stuffed with mitochondria, which impart the granular appearance to the cytoplasm. Secretory cells are dispersed in the columnar cell layer, accounting for the secretions within the cystically dilated lumens. On occasion, there are foci of squamous metaplasia.
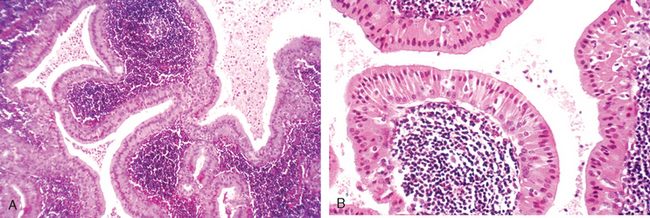
FIGURE 16-18 Warthin tumor. A, Low-power view showing epithelial and lymphoid elements. Note the follicular germinal center beneath the epithelium. B, Cystic spaces separate lobules of neoplastic epithelium consisting of a double layer of eosinophilic epithelial cells based on a reactive lymphoid stroma.
The histogenesis of these tumors has long been disputed. The occasional finding of small salivary gland rests in lymph nodes in the neck suggests that these tumors arise from the aberrant incorporation of similar inclusion-bearing lymphoid tissue in the parotids. Indeed, rarely, Warthin tumors have arisen within cervical lymph nodes, a finding that should not be mistaken for metastases. These neoplasms are benign, with recurrence rates of only 2% after resection.
MUCOEPIDERMOID CARCINOMA
These neoplasms are composed of variable mixtures of squamous cells, mucus-secreting cells, and intermediate cells. They represent about 15% of all salivary gland tumors, and while they occur mainly (60% to 70%) in the parotids, they account for a large fraction of salivary gland neoplasms in the other glands, particularly the minor salivary glands. In more than half the cases, this tumor is associated with a balanced (11;19) (q21;p13) chromosomal translocation that creates a fusion gene composed of portions of the MECT1 and MAML2 genes. The MECT1-MAML2 gene is believed to play a key role in the genesis of this tumor, possibly by perturbing the notch and cAMP-dependent signaling pathways.54,55 Overall, they are the most common form of primary malignant tumor of the salivary glands.
Morphology. Mucoepidermoid carcinomas can grow as large as 8 cm in diameter and although they are apparently circumscribed, they lack well-defined capsules and are often infiltrative at the margins. Pale and gray-white on transection, they frequently contain small, mucin-containing cysts. The basic histologic pattern is that of cords, sheets, or cystic configurations of squamous, mucous, or intermediate cells. The hybrid cell types often have squamous features, with small to large mucus-filled vacuoles, best seen when highlighted with mucin stains (Fig. 16-19). The tumor cells may be regular and benign appearing or, alternatively, highly anaplastic and unmistakably malignant. Accordingly, mucoepidermoid carcinomas are subclassified into low, intermediate, or high grade.
The clinical course and prognosis depend on the grade of the neoplasm. Low-grade tumors may invade locally and recur in about 15% of cases, but only rarely do they metastasize and so yield a 5-year survival rate of more than 90%. By contrast, high-grade neoplasms and, to a lesser extent, intermediate-grade tumors are invasive and difficult to excise and so recur in about 25% to 30% of cases and, in 30% of cases, metastasize to distant sites. The 5-year survival rate in patients with these tumors is only 50%.
OTHER SALIVARY GLAND TUMORS
Two less common neoplasms merit brief description: adenoid cystic carcinoma and acinic cell tumor.
Adenoid cystic carcinoma is a relatively uncommon tumor, which in approximately 50% of cases is found in the minor salivary glands (in particular the palate). Among the major salivary glands, the parotid and submandibular glands are the most common locations. Similar neoplasms have been reported in the nose, sinuses, and upper airways and elsewhere.
Morphology. In gross appearance, they are generally small, poorly encapsulated, infiltrative, gray-pink lesions. On histologic evaluation, they are composed of small cells having dark, compact nuclei and scant cytoplasm. These cells tend to be disposed in tubular, solid, or cribriform patterns reminiscent of cylindromas arising in the adnexa of the skin. The spaces between the tumor cells are often filled with a hyaline material thought to represent excess basement membrane (Fig. 16-20A). Other less common histologic patterns have been designated as tubular and solid variants.
Although slow growing, adenoid cystic carcinomas are relentless and unpredictable tumors with a tendency to invade perineural spaces (Fig. 16-20B), and they are stubbornly recurrent. Eventually, 50% or more disseminate widely to distant sites such as bone, liver, and brain, sometimes decades after attempted removal. Thus, although the 5-year survival rate is about 60% to 70%, it drops to about 30% at 10 years and 15% at 15 years. Neoplasms arising in the minor salivary glands have, on average, a poorer prognosis than those primary in the parotids.
The acinic cell tumor is composed of cells resembling the normal serous acinar cells of salivary glands. They are relatively uncommon, representing only 2% to 3% of salivary gland tumors. Most arise in the parotids; the remainder arise in the submandibular glands. They rarely involve the minor glands, which normally have only a scant number of serous cells. Like Warthin tumor, they are sometimes bilateral or multicentric. They are generally small, discrete lesions that may appear encapsulated. On histologic examination, they reveal a variable architecture and cell morphology. Most characteristically, the cells have clear cytoplasm but the cells are sometimes solid and at other times vacuolated. The cells are disposed in sheets or microcystic, glandular, follicular, or papillary patterns. There is usually little anaplasia and few mitoses, but some tumors are occasionally slightly more pleomorphic.
The clinical course of these neoplasms is somewhat dependent on the level of pleomorphism. Overall, recurrence after resection is uncommon, but about 10% to 15% of these neoplasms metastasize to lymph nodes. The survival rate is in the range of 90% at 5 years and 60% at 20 years.
1 Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J Periodontol. 2001;72:1790.
2 Research, Science and Therapy Committee of the American Academy of Periodontology. Epidemiology of periodontal disease. J Periodontol. 2005;76:1406.
3 Kinane D. Periodontal disease and health: Consensus report of the sixth European workshop on Periodontology. J Clin Periodontol. 2008;35:333.
4 Persson GR, Persson RE. Cardiovascular disease and periodontitis: an update on the associations and risk. J Clin Periodontol. 2008;35:362.
5 Cawson RA, et al, editors. Lucas’s Pathology of Tumors of the Oral Tissues. London: Churchill Livingstone, 1998.
6 Scully C. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc. 2003;134:200.
7 Hille JJ, et al. Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. Oral Dis. 2002;8:161.
8 Silverman S. Early diagnosis of oral cancer. Cancer. 1988;62:1796.
9 Shugars DC, Patton LL. Detecting, diagnosing, and preventing oral cancer. Nurse Pract. 1997;22:109.
10 Whited JD, Grichnick JM. Does this patient have a mole or a melanoma? JAMA. 1998;279:696.
11 Rampen FH, et al. False-negative findings in skin cancer and melanoma screening. J Am Acad Dermatol. 1995;33:59.
12 Mashberg A, Feldman LJ. Clinical criteria for identifying early oral and oropharyngeal carcinoma: erythroplasia revisted. Am J Surg. 1988;156:273.
13 Neville BW, et al, editors. Oral and Maxillofacial Pathology. Philadelphia: WB Saunders, 2008.
14 Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71.
15 Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74.
16 Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143.
17 Pai SI, Westia WH. Molecular pathology of head and neck cancer: implication for diagnosis and treatment. Ann Rev Pathol Mech Dis. 2009;4:49.
18 Anderson WF, et al. Secondary chemoprevention of upper aerodigestive tract tumors. Semin Oncol. 2001;28:106.
19 Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14.
20 Slaughter DP, et al. “Field cancerization” in oral stratified squamous epithelium. Cancer. 1953;6:962.
21 Braakhuis BJM, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727.
22 Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: the overshadowing threat for patients with early-stage disease. Int J Radiat Oncol Biol Phys. 1989;17:691.
23 Chaturvedi AK, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. JCO. 2008;26:612.
24 Jefferies S, Foulkes WD. Genetic mechanisms in squamous cell carcinoma of the head and neck. Oral Oncol. 2001;37:115.
25 Koch WM, et al. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109:1544.
26 Lingen MW, et al. Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5–9. Head Neck. 2000;22:328.
27 Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128:268.
28 Mao L, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682.
29 Boyle JO, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53:4477.
30 Rosin MP, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357.
31 Michalides R, et al. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995;55:975.
32 Izzo JG, et al. Dysregulated cyclin D1 expression early in head and neck tumorigenesis: in vivo evidence for an association with subsequent gene amplification. Oncogene. 1998;17:2313.
33 El-Mofty S, editor. Odontogenic tumors. Semin Diagn Pathol. 1999;16:269.
34 Pinto JM, Naclerio RM. Environmental and allergic factors in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:25.
35 Slavin RG. Nasal polyps and sinusitis. JAMA. 1997;278:1845.
36 Thompson LDR. Malignant neoplasms of the nasal cavity, paranasal sinuses, and nasopharynx. In: Thompson LDR, editor. Head and Neck Pathology. London: Churchill Livingtone; 2006:155-213.
37 Perez-Ordonez B, Huvos AG. Nonsquamous lesions of the nasal cavity, paranasal sinuses and nasopharynx. In: Gnepp DR, editor. Diagnostic Surgical Pathology of the Head and Neck. Philadelphia: W.B. Saunders; 2001:79-139.
38 Meyers LL, Oxford LE. Differential diagnosis and treatment options in paranasal sinus cancers. Surg Oncol Clin N Am. 2004;13:167.
39 Klepin HD, et al. Esthesioneuroblastoma. Curr Treat Options Oncol. 2005;6:509.
40 Rabb-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431.
41 Thompson LDR. Malignant neoplasms of the nasal cavity, paranasal sinuses, and nasopharynx. In: Thompson LDR, editor. Head and Neck Pathology. Philadelphia: Churchill Livingstone; 2006:155-215.
42 Juvenile recurrent respiratory papillomatosis: still a mystery disease with difficult management. Head Neck. 2007;29:155.
43 Kristt D, et al. The spectrum of laryngeal neoplasia: the pathologist’s view. Pathol Res Pract. 2002;198:709.
44 Loyo M, Pai SI. The molecular genetics of laryngeal cancer. Otolaryngol Clin North Am. 2008;41:657.
45 Hobbs CG, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:259.
46 Rovers MM, et al. Otitis media. Lancet. 2004;363:465.
47 Capella C, et al. Histopathology, cytology, and cytochemistry of pheochromocytomas and paragangliomas including chemodectomas. Pathol Res Pract. 1988;186:176.
48 Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61.
49 Ciancio SG. Medications’ impact on oral health. J Am Dent Assoc. 2004;135:1440.
50 Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9:165.
51 Ellis GL, et al. Surgical Pathology of Salivary Glands. Philadelphia: WB Saunders, 1991.
52 Ellis GL, Auclair PL. Tumors of the salivary glands. In: Atlas of Tumor Pathology, Third Series, Fascicle 17. Washington, DC: Armed Forces Institute of Pathology; 1996.
53 Dardick I. Color Atlas/Text of Salivary Gland Pathology. New York: Igaku-Shoin, 1996.
54 Tonon G, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208.
55 Coxon A, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65:7137.