The major psychotic disorders
schizophrenia and mania
Psychotic disorders
The term psychosis indicates a mental state in which the person affected has lost contact with reality. This is usually experienced as hallucination, delusion or disruption in thought processes often with lack of insight. The most profound ‘functional’ or primary psychotic condition is schizophrenia, but there is a continuum that embraces the so-called schizoaffective disorders. Mania and bipolar disorder can also have psychotic features. Organic disease caused by metabolic disturbance, toxic substances or psychoactive drugs can cause psychosis (Box 21.1).
Schizophrenia
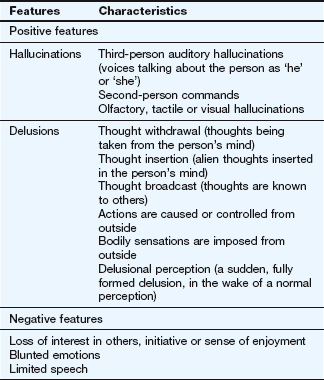
Clinical features of schizophrenia are categorised as positive or negative (Table 21.1), although none are pathognomonic of the disorder. The positive features are disordered versions of thinking, perception, formation of ideas or sense of self. They include hallucinations (false sensory perceptions) and delusions (false beliefs held with absolute certainty and unexplained by the person's socioeconomic background). The negative features, deficits in normal behaviour, are often the most debilitating in the long term. Schizophrenia is more common in males and usually presents relatively early in life. The onset is usually gradual but can be abrupt. Once established, it can have a relapsing or persistent course.
Biological basis of schizophrenia
There is a strong genetic component to schizophrenia with several risk genes that affect early brain development and predispose an individual to developing the condition. Triggers which impact further on neurodevelopment, such as prenatal exposure to viral infections or obstetric complications, probably only lead to the disease in those with a genetic predisposition. Many neurobiological abnormalities have been described in schizophrenia, including disturbances in neuronal numbers and synaptic connections in the cortical, thalamic and hippocampal areas. These disturbances become more marked as the illness progresses, but the heterogenous nature of the disease makes it difficult to determine the precise underlying neuropathology.
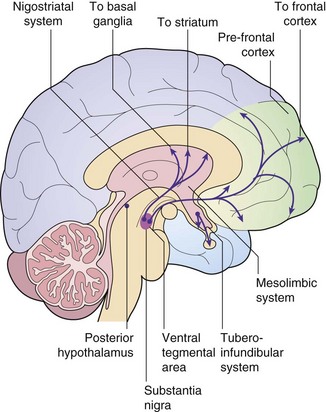
Dopamine–glutamate interactions and their possible involvement in schizophrenia: The limbic region and prefrontal cortex are involved in cognition, emotional memory and the initiation of behaviour. They are regulated by a complex interplay among their neuronal connections, with dopamine and glutamate as important neurotransmitters. Most dopaminergic pathways in the central nervous system (CNS) arise from the substantia nigra (among the basal ganglia) and the ventral tegmental area in the midbrain (Fig. 21.1). One major dopaminergic pathway has its origin in the substantia nigra and projects to γ-aminobutyric acid (GABA)-ergic inhibitory interneurons in the corpus striatum through the nigrostriatal pathway (Ch. 24). This pathway modulates motor and behavioural function through ongoing projections to the thalamus and cortex. The corpus striatum in turn receives glutamatergic inputs from the cortex. Other major pathways connect the ventral tegmental area via the mesolimbic projections to the limbic region (especially the hippocampus) and via the mesocortical projections to the prefrontal cortex (the reward pathway; see Ch. 54). The limbic region also receives cortical afferents.
Several receptors for the neurotransmitter dopamine are found in the brain (see table of receptor families at end of Ch. 1). CNS dopamine receptors belong to two families: D1-like (which includes subtypes D1 and D5 that are coupled to a stimulatory G protein) and D2-like (which includes subtypes D2, D3 and D4 that are coupled to an inhibitory G protein). Postsynaptic D1 and D2 receptor subtypes are found in the dopaminergic pathways in the corpus striatum, limbic system, thalamus and hypothalamus. Postsynaptic D2 receptors are also present in the pituitary. Presynaptic D3 receptors are found on the dopaminergic neuronal terminals in the corpus striatum and limbic system, and their stimulation inhibits dopamine release in these areas. Postsynaptic D4 receptors are found in the limbic system and prefrontal cortex.
Schizophrenia is believed to involve interconnected abnormalities of glutamatergic and dopaminergic neurotransmission. However, it is uncertain whether reduced glutamatergic activity at N-methyl-D-aspartate (NMDA) receptors or increased dopaminergic neurotransmission at D2 receptors in the mesolimbic pathway is the primary abnormality. There are several pieces of evidence that support the involvement of defective glutamatergic and overactive dopaminergic neurotransmission in the genesis of schizophrenia:
 amfetamine-induced dopamine release is greater in people with schizophrenia,
amfetamine-induced dopamine release is greater in people with schizophrenia,
 the dopamine concentration in the corpus striatum is higher in people with schizophrenia,
the dopamine concentration in the corpus striatum is higher in people with schizophrenia,
 blockade of the D2 family of receptors produces antipsychotic effects,
blockade of the D2 family of receptors produces antipsychotic effects,
 increased stimulation of D2-like receptors worsens positive symptoms,
increased stimulation of D2-like receptors worsens positive symptoms,
 glutamate NMDA receptor antagonists (ketamine, phencyclidine) produce positive and negative symptoms, similar to those of schizophrenia,
glutamate NMDA receptor antagonists (ketamine, phencyclidine) produce positive and negative symptoms, similar to those of schizophrenia,
In schizophrenia there is downregulation of D3 and D4 receptors in the prefrontal cortex, which may be responsible for the negative symptoms, and downregulation of D4 receptors in the limbic system. Dysfunction of other neurotransmitter systems utilising serotonin, GABA and neuropeptide Y may also be important in schizophrenia, but their precise roles are as yet unresolved.
Mania and bipolar disorder
Mania is a disorder of elevated mood that can occur alone (unipolar mania) or is more usually interspersed with episodes of depression (bipolar affective disorder or manic-depressive illness). Mild mania is termed hypomania. Sometimes, the fluctuations of mood are less marked, and the disorder is termed cyclothymia.
The onset of mania can be gradual or sudden, most often between the ages of 15 and 25 years, and varies in severity from mild elation, increased drive and sociability, to grandiose ideas, marked overactivity, overspending and socially embarrassing behaviour. Onset is usually early in adult life. Mania and bipolar disorder have (and share) a stronger genetic component than any other grouping of major psychiatric disorders.
Biological basis of bipolar disorder
The biological basis of bipolar disorder is less well understood than that for unipolar depression. Susceptibility genes have been identified that are shared with those for schizophrenia, but the environmental stressors that result in expression of the disorder are poorly understood. The dysregulation of neuronal function is probably triggered by altered expression of critical neuronal proteins, determined by the genetic predisposition. In bipolar disorder there is increased CNS monoamine neurotransmitter activity (particularly serotonin and dopamine) and reduced acetylcholine and GABA neurotransmission. These may all be important in orchestrating changes in neuronal function within the prefrontal cortex, visual association cortex and limbic circuitry.
The changes in neurotransmitter regulation produce functional disruption in the target neurons. Reduced neuronal levels of brain-derived neurotrophic factor (BDNF) may be important in the genesis of bipolar disorder (see also depression, Ch. 22). BDNF regulates several intracellular signal transduction pathways, and dysregulation of these pathways may produce the neuroplastic changes (especially synaptic plasticity) and neuronal cell loss that are features of bipolar disorder.
Antipsychotic drugs
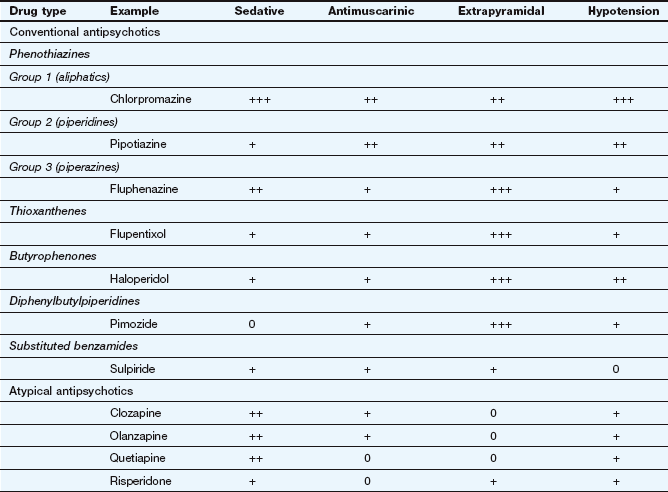
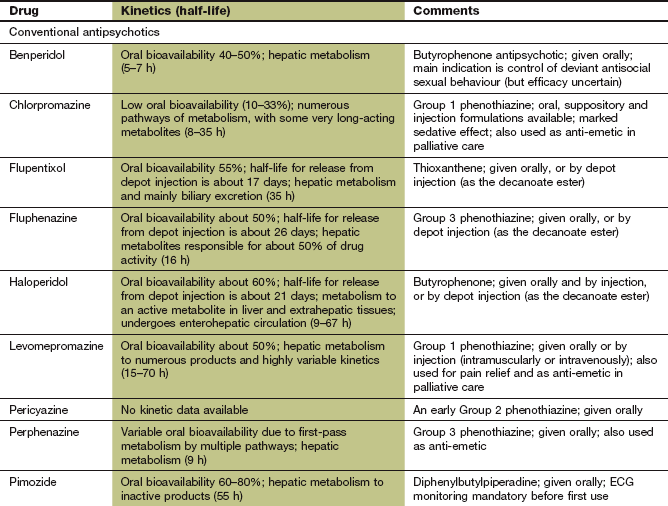
Antipsychotic drugs (also known as neuroleptics or major tranquillizers) have a common mechanism for their beneficial clinical effects, but belong to various chemical classes that differ in their propensity to cause sedation, and antimuscarinic or extrapyramidal effects (Table 21.2). They are commonly considered in two groups that differ in their unwanted effects: the conventional and atypical antipsychotics.
Conventional antipsychotic drugs
Mechanism of action and effects
The antipsychotic action of all conventional (or classical) antipsychotic drugs arises primarily from their antagonism of CNS dopamine D2 receptors in the mesolimbic pathway. High affinity for the family of D2 receptors is a common feature of all conventional antipsychotics, and the affinity of the drug for these receptors correlates well with its effective dose. Conventional antipsychotics have a higher affinity than dopamine for D2 receptors, and dissociate slowly from the receptor. At least 65% D2 receptor occupancy in the mesolimbic system is required for clinical benefit during long-term treatment of psychotic disorders. However, conventional antipsychotics also have D2 receptor antagonist activity in other CNS pathways, and 80% or more D2 receptor occupancy in the striatum will produce extrapyramidal unwanted effects (see below).
Many conventional antipsychotics also block serotonin 5-HT2A and 5-HT2C receptors, actions that may contribute to their clinical effects in suppressing negative symptoms. Antagonist activity at other receptors, including α1-adrenoceptors and histamine H1 receptors, does not influence their efficacy in psychotic illness but can produce unwanted effects (in which respect they resemble tricyclic antidepressants; Ch. 22). The severity of these unwanted effects varies considerably among the different drugs.
Clinical improvement with antipsychotic drugs develops slowly, despite an immediate antagonist action at dopamine receptors. There is increasing evidence that these drugs modulate complex intracellular pathways that affect neuroplasticity. This leads to changes in synaptic connections in areas of the brain known to be involved in psychotic illness which may be important for their long-term benefit. Clinically useful effects produced by antipsychotic drugs include the following.
 A depressant action on conditioned responses and emotional responsiveness: in psychoses this is particularly helpful for the management of thought disorders, abnormalities of perception and delusional beliefs.
A depressant action on conditioned responses and emotional responsiveness: in psychoses this is particularly helpful for the management of thought disorders, abnormalities of perception and delusional beliefs.
 A sedative action, which is useful for the treatment of restlessness and confusion: sensory input into the reticular activating system is reduced by inhibition of collateral fibres from the lemniscal pathways. Spontaneous activity is preserved but arousal stimuli produce less response.
A sedative action, which is useful for the treatment of restlessness and confusion: sensory input into the reticular activating system is reduced by inhibition of collateral fibres from the lemniscal pathways. Spontaneous activity is preserved but arousal stimuli produce less response.
 An anti-emetic effect through dopamine receptor antagonist activity at the chemoreceptor trigger zone (CTZ), which is useful to treat vomiting, such as that associated with drugs (e.g. cytotoxics, opioid analgesics) and uraemia: some antipsychotic drugs are also effective in motion sickness, through muscarinic receptor blockade (Ch. 32).
An anti-emetic effect through dopamine receptor antagonist activity at the chemoreceptor trigger zone (CTZ), which is useful to treat vomiting, such as that associated with drugs (e.g. cytotoxics, opioid analgesics) and uraemia: some antipsychotic drugs are also effective in motion sickness, through muscarinic receptor blockade (Ch. 32).
 Antihistamine activity produced by histamine H1-receptor antagonism can be used for treatment of allergic reactions (Ch. 39).
Antihistamine activity produced by histamine H1-receptor antagonism can be used for treatment of allergic reactions (Ch. 39).
Pharmacokinetics
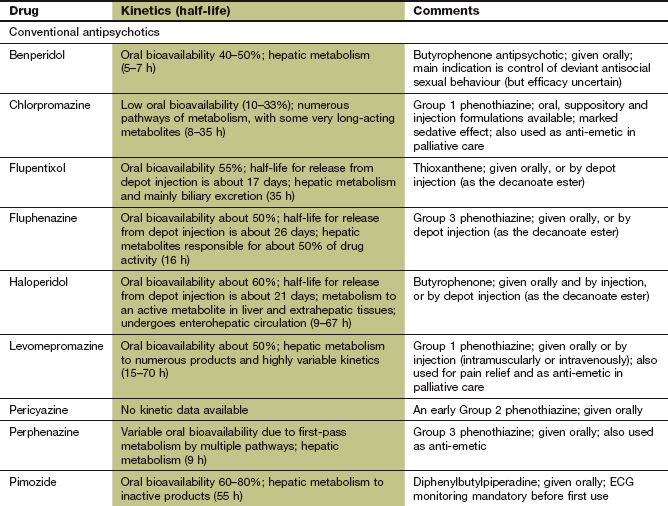
Conventional antipsychotics are rapidly absorbed from the gut but most undergo extensive first-pass metabolism. For some drugs, the plasma concentrations of active drug (including metabolites) can vary up to 10-fold among individuals, but there is not a close relationship between plasma drug concentration and clinical response. Elimination is by metabolism in the liver. Several antipsychotic drugs, such as chlorpromazine, haloperidol, perphenazine and zuclopenthixol, are metabolised predominantly by the polymorphic enzyme CYP2D6. There is a relationship between the steady-state plasma concentrations of these drugs (and therefore propensity to unwanted effects) and the CYP2D6 genotype (Ch. 2). Sulpiride does not undergo first-pass metabolism and is largely eliminated unchanged by the kidney. The half-lives of the antipsychotics vary widely; for example, that of sulpiride is 6–8 h, while the half-life of pimozide is very long, at 2 days. Some antipsychotics, such as chlorpromazine and haloperidol, can be given by intramuscular injection for more rapid onset of action.
Since adherence to treatment is often poor in psychotic disorders, depot formulations of many antipsychotics have been developed. They are given by intramuscular injection as a prodrug – which is the active compound esterified to a long-chain fatty acid and dissolved in a vegetable oil – that slowly releases the drug for between 1 and 12 weeks (depending on the formulation). When given as a depot preparation, or by deep intramuscular injection, the doses used are smaller than those for oral treatment, due to the absence of first-pass metabolism. The half-lives given in the Compendium at the end of this chapter do not reflect the slow absorption rate-limited half-life of the depot form (see Ch. 2). Examples of depot preparations are flupentixol decanoate and zuclopenthixol decanoate.
Unwanted effects
The antipsychotic drugs differ mainly in the degree of associated or unwanted effects (Table 21.2).
 Extrapyramidal effects arise from D2 receptor blockade in the nigrostriatal pathways, and take various forms: acute dystonias (tongue protrusion, torticollis, oculogyric crisis) are most common after the first dose or first few doses in children and young adults. Akathisia (restlessness) usually follows large initial doses, while parkinsonism has a gradual onset over several weeks usually in adults or the elderly. Extrapyramidal effects (Ch. 24) occur in more than half of those being treated with conventional antipsychotics, but are usually reversible if the drug is stopped. With prolonged use (several months to years) and especially in the elderly, tardive dyskinesias or tardive dystonias can develop. These consist of choreoathetoid and repetitive orofacial movements which often persist when the drug is withdrawn. Their aetiology is uncertain: upregulation of D2 receptors may contribute, but damage to inhibitory GABAergic neurons and/or dysfunction in other neurotransmitter pathways is probably involved. Extrapyramidal effects are most common with piperazine phenothiazines (such as prochlorperazine), the butyrophenones (such as haloperidol) and depot preparations (Table 21.2).
Extrapyramidal effects arise from D2 receptor blockade in the nigrostriatal pathways, and take various forms: acute dystonias (tongue protrusion, torticollis, oculogyric crisis) are most common after the first dose or first few doses in children and young adults. Akathisia (restlessness) usually follows large initial doses, while parkinsonism has a gradual onset over several weeks usually in adults or the elderly. Extrapyramidal effects (Ch. 24) occur in more than half of those being treated with conventional antipsychotics, but are usually reversible if the drug is stopped. With prolonged use (several months to years) and especially in the elderly, tardive dyskinesias or tardive dystonias can develop. These consist of choreoathetoid and repetitive orofacial movements which often persist when the drug is withdrawn. Their aetiology is uncertain: upregulation of D2 receptors may contribute, but damage to inhibitory GABAergic neurons and/or dysfunction in other neurotransmitter pathways is probably involved. Extrapyramidal effects are most common with piperazine phenothiazines (such as prochlorperazine), the butyrophenones (such as haloperidol) and depot preparations (Table 21.2).
 Drowsiness and cognitive impairment can occur as a result of histamine and dopamine receptor antagonism.
Drowsiness and cognitive impairment can occur as a result of histamine and dopamine receptor antagonism.
 Galactorrhoea, with gynaecomastia, amenorrhoea in women, impotence in men and reduced bone mineral density. These can arise when more than 70% D2 receptor occupancy in hypothalamic pathways produces hyperprolactinaemia and reduced gonadotrophin secretion. Antimuscarinic activity and α1-adrenoceptor antagonism also contribute.
Galactorrhoea, with gynaecomastia, amenorrhoea in women, impotence in men and reduced bone mineral density. These can arise when more than 70% D2 receptor occupancy in hypothalamic pathways produces hyperprolactinaemia and reduced gonadotrophin secretion. Antimuscarinic activity and α1-adrenoceptor antagonism also contribute.
 Antimuscarinic effects: peripheral antimuscarinic actions include dry mouth, constipation, micturition difficulties, blurred vision and reduced sexual arousal (Ch. 4). CNS muscarinic receptor blockade predisposes to acute confusional states.
Antimuscarinic effects: peripheral antimuscarinic actions include dry mouth, constipation, micturition difficulties, blurred vision and reduced sexual arousal (Ch. 4). CNS muscarinic receptor blockade predisposes to acute confusional states.
 Postural hypotension, nasal stuffiness and impaired erection and ejaculation in men due to α1-adrenoceptor antagonism.
Postural hypotension, nasal stuffiness and impaired erection and ejaculation in men due to α1-adrenoceptor antagonism.
 Hypothermia as a consequence of depressed hypothalamic function. Altered serotonergic neuronal activity may be responsible.
Hypothermia as a consequence of depressed hypothalamic function. Altered serotonergic neuronal activity may be responsible.
 Hypersensitivity reactions include cholestatic jaundice, skin reactions and bone marrow depression.
Hypersensitivity reactions include cholestatic jaundice, skin reactions and bone marrow depression.
 Weight gain, with an increased risk of insulin resistance and glucose intolerance.
Weight gain, with an increased risk of insulin resistance and glucose intolerance.
 Gastrointestinal disturbances.
Gastrointestinal disturbances.
 Prolongation of the Q–T interval on the electrocardiogram, a particular problem with pimozide, predisposes to ventricular arrhythmias (Ch. 8).
Prolongation of the Q–T interval on the electrocardiogram, a particular problem with pimozide, predisposes to ventricular arrhythmias (Ch. 8).
 Neuroleptic malignant syndrome is a rare genetically determined disorder caused by a polymorphism in the D2 receptor and consequent abnormal dopamine receptor antagonist activity in the corpus striatum and hypothalamus. In the presence of this polymorphism, antipsychotic drugs produce high fever, muscle rigidity, autonomic instability with hypertension, urinary incontinence and sweating, and altered consciousness. Immediate withdrawal of the antipsychotic and treatment with dantrolene or a dopamine receptor agonist (Ch. 24) may be life-saving. Symptoms can take up to 1 week to subside, or longer after a depot preparation. Cautious reintroduction of an antipsychotic may be possible without recurrence, but at least 2 weeks should be allowed after symptoms of the syndrome have resolved.
Neuroleptic malignant syndrome is a rare genetically determined disorder caused by a polymorphism in the D2 receptor and consequent abnormal dopamine receptor antagonist activity in the corpus striatum and hypothalamus. In the presence of this polymorphism, antipsychotic drugs produce high fever, muscle rigidity, autonomic instability with hypertension, urinary incontinence and sweating, and altered consciousness. Immediate withdrawal of the antipsychotic and treatment with dantrolene or a dopamine receptor agonist (Ch. 24) may be life-saving. Symptoms can take up to 1 week to subside, or longer after a depot preparation. Cautious reintroduction of an antipsychotic may be possible without recurrence, but at least 2 weeks should be allowed after symptoms of the syndrome have resolved.
 Sudden withdrawal after long-term use can produce nausea, vomiting, anorexia, diarrhoea, sweating, myalgia, paraesthesia, insomnia and agitation. These symptoms usually subside within 2 weeks.
Sudden withdrawal after long-term use can produce nausea, vomiting, anorexia, diarrhoea, sweating, myalgia, paraesthesia, insomnia and agitation. These symptoms usually subside within 2 weeks.
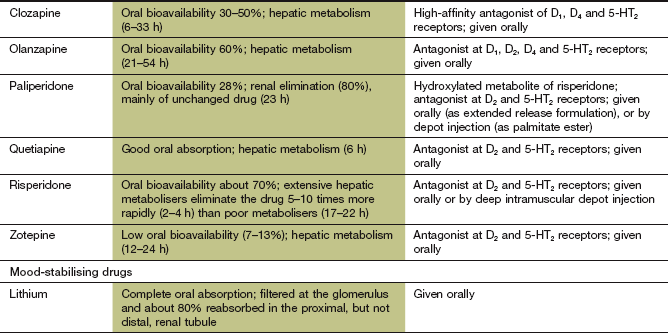
Atypical antipsychotic drugs
Mechanism of action and effects
The antipsychotic action of atypical antipsychotic drugs, like that of conventional antipsychotics, arises primarily from blockade of CNS dopamine D2 receptors in mesolimbic pathways. However, the atypical antipsychotic drugs have a lower affinity for D2 receptors than dopamine and transient receptor occupancy. Since the receptor occupancy is much less than that of conventional antipsychotics, they are less likely to produce extrapyramidal movement disorders at usual doses (Ch. 24). Antagonist activity at serotonin 5-HT2 receptors may contribute to their antipsychotic action, particularly in improving the negative features such as apathy and blunted emotions.
 Aripiprazole has partial agonist activity at the D2 and D3 receptors, which limits the degree of receptor antagonism. It is also a partial agonist at 5-HT1A and 5-HT2C receptors, but an antagonist at 5-HT2A receptors, and has moderate antagonist activity at histamine H1 receptors and α-adrenoceptors.
Aripiprazole has partial agonist activity at the D2 and D3 receptors, which limits the degree of receptor antagonism. It is also a partial agonist at 5-HT1A and 5-HT2C receptors, but an antagonist at 5-HT2A receptors, and has moderate antagonist activity at histamine H1 receptors and α-adrenoceptors.
 Clozapine is a relatively weak D2 receptor antagonist with selective cortical receptor occupancy, and shows greater antagonist activity at D1 and D4 receptors. It has a much higher affinity for and antagonist activity at serotonin 5-HT2A and 5-HT2C receptors, α1-adrenoceptors, H1 histamine receptors and muscarinic receptors.
Clozapine is a relatively weak D2 receptor antagonist with selective cortical receptor occupancy, and shows greater antagonist activity at D1 and D4 receptors. It has a much higher affinity for and antagonist activity at serotonin 5-HT2A and 5-HT2C receptors, α1-adrenoceptors, H1 histamine receptors and muscarinic receptors.
 Olanzapine has a similar profile to clozapine, with additional antagonist activity at serotonin 5-HT3 receptors.
Olanzapine has a similar profile to clozapine, with additional antagonist activity at serotonin 5-HT3 receptors.
 Quetiapine has moderate affinity for D2 receptors, and is an antagonist at 5-HT1A, 5-HT2A and 5-HT2C receptors. It also has antagonist activity at α1- and α2-adrenoceptors and histamine H1 receptors.
Quetiapine has moderate affinity for D2 receptors, and is an antagonist at 5-HT1A, 5-HT2A and 5-HT2C receptors. It also has antagonist activity at α1- and α2-adrenoceptors and histamine H1 receptors.
 Risperidone has higher-affinity for D2 and D4 receptors, with dose-dependent limbic selectivity. It also has antagonist activity at several 5-HT1 and 5-HT2 receptor subtypes, α1- and α2-adrenoceptors and histamine H1 receptors. It does not bind to muscarinic receptors.
Risperidone has higher-affinity for D2 and D4 receptors, with dose-dependent limbic selectivity. It also has antagonist activity at several 5-HT1 and 5-HT2 receptor subtypes, α1- and α2-adrenoceptors and histamine H1 receptors. It does not bind to muscarinic receptors.
Adherence to treatment with atypical antipsychotics is greater than for conventional antipsychotics, probably as a result of less marked unwanted effects, which may explain their apparently greater efficacy. Clozapine, however, is uniquely superior to all other drugs for treatment of refractory schizophrenia.
Pharmacokinetics
Atypical antipsychotics are rapidly absorbed from the gut and most undergo extensive first-pass metabolism to inactive metabolites. The half-lives of the atypical antipsychotics vary widely. Some atypical antipsychotics, such as olanzapine and risperidone, can be given in a depot formulation.
Unwanted effects
The atypical antipsychotic drugs show some differences from conventional antipsychotics in their unwanted effects (Table 21.2).
 Extrapyramidal effects are less likely to be caused by atypical antipsychotics, except at high dosages, when the risk is similar to conventional antipsychotics.
Extrapyramidal effects are less likely to be caused by atypical antipsychotics, except at high dosages, when the risk is similar to conventional antipsychotics.
 Drowsiness and cognitive impairment are less marked than with conventional antipsychotics. Risperidone can cause insomnia and agitation.
Drowsiness and cognitive impairment are less marked than with conventional antipsychotics. Risperidone can cause insomnia and agitation.
 Galactorrhoea and sexual dysfunction are less common with most atypical antipsychotics, except risperidone.
Galactorrhoea and sexual dysfunction are less common with most atypical antipsychotics, except risperidone.
 Antimuscarinic effects are uncommon with atypical antipsychotics.
Antimuscarinic effects are uncommon with atypical antipsychotics.
 Postural hypotension, especially during initial dose titration with clozapine and quetiapine.
Postural hypotension, especially during initial dose titration with clozapine and quetiapine.
 Reduced seizure threshold with clozapine.
Reduced seizure threshold with clozapine.
 Agranulocytosis is a particular problem with clozapine (1–2% risk); regular blood tests are mandatory during treatment with this drug.
Agranulocytosis is a particular problem with clozapine (1–2% risk); regular blood tests are mandatory during treatment with this drug.
 Weight gain with clozapine and olanzapine.
Weight gain with clozapine and olanzapine.
 Hyperglycaemia is more common than with conventional antipsychotics.
Hyperglycaemia is more common than with conventional antipsychotics.
Mood-stabilising drugs for bipolar disorder
Mechanism of action
The mechanism of action of lithium is not well understood, but it has multiple effects in the CNS.
 Lithium has complex effects on the generation of intracellular second messengers in cortical neuronal pathways. It attenuates the function of Gs proteins coupled to adenylyl cyclase but increases basal adenylyl cyclase activity, effects that alter cAMP synthesis. Lithium also inhibits intracellular inositol monophosphatase, and therefore interferes with substrate generation for second messengers involved in phosphoinositide pathway signalling. These actions affect several monoaminergic and cholinergic systems in the CNS. The overall action of lithium may be to stabilise intracellular signalling by enhancing basal activity but decreasing maximum activity.
Lithium has complex effects on the generation of intracellular second messengers in cortical neuronal pathways. It attenuates the function of Gs proteins coupled to adenylyl cyclase but increases basal adenylyl cyclase activity, effects that alter cAMP synthesis. Lithium also inhibits intracellular inositol monophosphatase, and therefore interferes with substrate generation for second messengers involved in phosphoinositide pathway signalling. These actions affect several monoaminergic and cholinergic systems in the CNS. The overall action of lithium may be to stabilise intracellular signalling by enhancing basal activity but decreasing maximum activity.
 It suppresses pro-apoptotic genes and increases expression of anti-apoptotic genes, with consequent neuroprotection. Lithium inhibits the multifunctional enzyme glucose synthase kinase-3 (GSK-3), a regulator of many signal transduction pathways that are involved in neuronal apoptosis. Inhibition of the activity of the pro-apoptotic enzyme caspase-3 by lithium also confers neuroprotection.
It suppresses pro-apoptotic genes and increases expression of anti-apoptotic genes, with consequent neuroprotection. Lithium inhibits the multifunctional enzyme glucose synthase kinase-3 (GSK-3), a regulator of many signal transduction pathways that are involved in neuronal apoptosis. Inhibition of the activity of the pro-apoptotic enzyme caspase-3 by lithium also confers neuroprotection.
 Increased neurogenesis has been found in the hippocampus after lithium treatment, which may be one consequence of the complex changes in intracellular signalling.
Increased neurogenesis has been found in the hippocampus after lithium treatment, which may be one consequence of the complex changes in intracellular signalling.
Pharmacokinetics
Lithium is given as a salt (e.g. carbonate, citrate), which is rapidly absorbed from the gut. To avoid high peak plasma concentrations (which are associated with unwanted effects), modified-release formulations are normally used. Lithium is widely distributed in the body but enters the brain slowly. It is selectively concentrated in bone and the thyroid gland. Excretion is by glomerular filtration, with 80% reabsorbed in the proximal tubule by the same mechanism as Na+ although, unlike Na+, lithium is not reabsorbed from more distal parts of the kidney. When the body is depleted of salt and water, for example by vomiting or diarrhoea, then enhanced reabsorption of Na+ in the proximal tubule is accompanied by enhanced lithium reabsorption, which can produce acute toxicity. Lithium has a long half-life of about 1 day. Lithium has a narrow therapeutic index, and regular monitoring of serum concentrations is mandatory at least every 3 months during long-term treatment. The serum concentration should be measured 12 h after dosing, so that the absorption and distribution phases are completed, with the aim of maintaining a therapeutic plasma lithium concentration between 0.4 and 1.0 mmol⋅L−1.
Unwanted effects
 Nausea and diarrhoea can occur even at low plasma concentrations.
Nausea and diarrhoea can occur even at low plasma concentrations.
 CNS effects, including tremor, giddiness, ataxia, dysarthria and mild cognitive and memory impairment.
CNS effects, including tremor, giddiness, ataxia, dysarthria and mild cognitive and memory impairment.
 Hypothyroidism can be caused by interference with thyroxine synthesis during long-term treatment. Thyroid function should be monitored every 6 months
Hypothyroidism can be caused by interference with thyroxine synthesis during long-term treatment. Thyroid function should be monitored every 6 months
 Reduced responsiveness of the distal renal tubule to antidiuretic hormone (ADH), which can produce a reversible nephrogenic diabetes insipidus with polyuria and consequent polydipsia.
Reduced responsiveness of the distal renal tubule to antidiuretic hormone (ADH), which can produce a reversible nephrogenic diabetes insipidus with polyuria and consequent polydipsia.
 Overdosage usually produces symptoms with serum lithium concentrations above 1.5 mmol⋅L−1. Severe toxicity (serum lithium concentration above 2.0 mmol⋅L−1) can lead to coma, convulsions and profound hypotension with oliguria.
Overdosage usually produces symptoms with serum lithium concentrations above 1.5 mmol⋅L−1. Severe toxicity (serum lithium concentration above 2.0 mmol⋅L−1) can lead to coma, convulsions and profound hypotension with oliguria.
Drug interactions
Diuretics can reduce lithium excretion by producing dehydration (see pharmacokinetics above). This is most marked with thiazides (Ch. 14) because of their prolonged action. Angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists (Ch. 6) and some non-steroidal anti-inflammatory drugs (Ch. 29) also reduce the excretion of lithium. The risk of extrapyramidal effects may be increased when lithium is prescribed concurrently with antipsychotic drugs.
Anticonvulsants
Mechanism of action in bipolar disorder
The mode of action of the anticonvulsants carbamazepine, lamotrigine and sodium valproate in mania may be related to facilitation of GABAergic inhibitory neurotransmission and consequent modulation of excitatory glutamatergic neurons. Like lithium, anticonvulsants affect cAMP-mediated intracellular events, inhibit the phosphoinositide signalling pathway and activate neuroprotective anti-apoptotic genes. They also stimulate hippocampal neurogenesis. Anticonvulsant (antiepileptic) drugs are discussed in Chapter 23.
Management of psychotic disorders
Acute psychotic symptoms such as hallucinations and delusions can be controlled relatively rapidly with an antipsychotic drug such as haloperidol or chlorpromazine. The initial sedative actions of these drugs can be particularly helpful. However, reductions in thought disturbance, withdrawal and apathy are delayed and the clinical improvement is gradual over several weeks of treatment. Atypical antipsychotics should be considered in preference to conventional antipsychotics:
 when choosing first-line treatment for newly diagnosed schizophrenia,
when choosing first-line treatment for newly diagnosed schizophrenia,
 if there are unacceptable unwanted effects with a conventional drug,
if there are unacceptable unwanted effects with a conventional drug,
 during an acute schizophrenic episode when discussion with the person is not possible.
during an acute schizophrenic episode when discussion with the person is not possible.
Treatment for schizophrenia is not curative and long-term maintenance therapy is usually required to prevent relapse. The optimal duration of this treatment is determined by the number of acute episodes, and is usually at least 2–5 years. Intermittent treatment that is only given for relapses is associated with a higher overall relapse rate of 50–80%, compared with 25–40% in those taking prophylactic therapy. The relapse rate is lower with atypical antipsychotics compared to conventional antipsychotics. Adherence to maintenance treatment is often poor in schizophrenia, and can be improved by depot injections given every 1–4 weeks. Continuous antipsychotic treatment provides relief of symptoms for more than 70% of people with schizophrenia but resistance to conventional antipsychotics is particularly common if negative symptoms predominate.
Atypical antipsychotic drugs produce greater relief of negative symptoms than the conventional antipsychotic drugs, although this may be due to better adherence to treatment. There is limited evidence to support the concurrent use of a selective serotonin reuptake inhibitor (SSRI; Ch. 22) with an atypical antipsychotic drug for those whose negative symptoms do not respond to the antipsychotic drug alone. Clozapine is the only antipsychotic drug that is effective in treatment resistance (incomplete recovery), but close monitoring is required because of the 1–2% risk of agranulocytosis. Clozapine should always be tried if symptoms have failed to respond to two antipsychotic drugs, at least one of which should be an atypical drug, each given for 6–8 weeks. Between 30 and 50% of those who are resistant to other treatments will respond to clozapine.
Various psychological treatments to improve social skills are important as an adjunct to drug treatment and should be provided along with social support.
Management of mania and bipolar disorder
When symptoms of acute mania are mild or moderate, they can usually be controlled by lithium, although the therapeutic effect may be delayed for at least a week. For this reason, a benzodiazepine (Ch. 20) is usually given as well for the first 7 days. The anticonvulsant sodium valproate is an effective alternative to lithium for the acute phase, and its sedative action produces a response in 1–4 days when used alone. Carbamazepine can be used, but has a delayed onset of action and is given initially with a benzodiazepine.
If manic symptoms are more severe it is usually necessary to give an antipsychotic drug in combination with lithium, carbamazepine or sodium valproate and perhaps initially a benzodiazepine. Conventional antipsychotic drugs are only recommended for short-term use, because of their extrapyramidal unwanted effects, and an atypical drug such as olanzapine, quetiapine or risperidone is usually preferred.
Depression in bipolar disorder is often treated with a combination of lithium and an antidepressant. However, the response to antidepressant therapy is less satisfactory than with unipolar depression, and there is a risk of provoking a switch to mania. There is limited evidence that mania is less likely to be provoked by an SSRI than by a tricyclic antidepressant (Ch. 22). Alternative treatments include the atypical antipsychotic quetiapine, a combination of olanzapine with the SSRI fluoxetine, or the mood-stabilising anticonvulsant lamotrigine, which have all been reported to be effective for bipolar depression.
Treatment should be continued for at least two years from the last episode of mania. If a person with bipolar disorder has had at least two episodes of either mania or depression in five years then prophylactic therapy is recommended for at least five years. The optimal duration of prophylactic therapy is unknown. If a decision is made to discontinue treatment then gradual withdrawal is recommended to reduce the risk of relapse, especially of mania. Lithium is the conventional treatment of choice for prophylaxis (although the full prophylactic effect may not be apparent for 6–12 months), but carbamazepine or lamotrigine are equally effective. There is less evidence to support the use of sodium valproate, which is usually reserved for those who do not tolerate first-line treatments, or for when these are ineffective.
Electroconvulsive therapy is used for refractory episodes of both mania and depression, and has a much more rapid action than drug therapy. As for schizophrenia, psychological treatments are an important adjunct to drug therapy in bipolar disorder.
True/false questions
1. It may take several weeks for the full beneficial effects of antipsychotics to be seen.
2. The positive symptoms of schizophrenia (e.g. delusions) are more readily controlled than negative symptoms (e.g. withdrawal).
3. There is a close correlation between plasma levels of chlorpromazine and its antipsychotic effect.
4. Antipsychotics are effective in treating about 70% of people with schizophrenia.
5. Some antipsychotic drugs can be given in a depot preparation injected at 6-monthly intervals.
6. The atypical antipsychotic drugs have relatively low affinity for dopamine D2 receptors.
7. The atypical antipsychotic clozapine has greater antimuscarinic activity than chlorpromazine.
8. Clozapine causes agranulocytosis.
9. The atypical antipsychotic drugs have relatively few effects on the extrapyramidal system.
10. Group 2 phenothiazines cause fewer extrapyramidal symptoms than other conventional antipsychotics.
11. Lithium interferes with thyroxine synthesis.
12. Lithium is reabsorbed through the distal convoluted tubule in the kidney.
One-best-answer (OBA) question
Choose the most accurate statement about antipsychotic drugs.
Extended-matching-item questions
Choose one mechanism from A to E below most likely to underlie each of the drug effects 1–5:
Case-based questions
A 25-year-old man (Mr PS) with schizophrenia has been treated with high-dose oral chlorpromazine for 2 years. His main symptoms of auditory hallucinations and delusional thoughts (‘The people in the flat above are broadcasting my thoughts on their radio’) had improved, but he remained socially withdrawn and apathetic and described a number of problems, including feeling very tired, faintness on standing up, dry mouth, sexual problems, blurred vision, occasional difficulty with fine control of movement and weight gain. Mr PS has had two severe relapses requiring hospitalisation within the last 18 months and is vague on whether he always takes his medication as directed.
1. True. Acute psychotic symptoms may respond relatively rapidly but further gradual improvement is seen over several weeks.
2. True. Negative symptoms are more difficult to treat, but may respond better to atypical antipsychotics.
3. False. The plasma levels of chlorpromazine are highly variable and do not correlate with clinical effect.
4. True. Schizophrenia is well controlled in about 70% of people taking continuous antipsychotic drug therapy.
5. False. Depot injections are usually given at 1- to 4-week intervals, depending on the drug dose and formulation.
6. True. The atypical antipsychotics have fewer extrapyramidal (movement) than the conventional drugs.
7. True. The atypical antipsychotics have lower affinity for D2 receptors, and more transient D2 receptor occupancy, than conventional antipsychotics.
8. False. The atypical antipsychotics such as clozapine have less antimuscarinic activity than most phenothiazines.
9. True. The 1–2% risk of agranulocytosis with clozapine necessitates regular blood monitoring.
10. True. Compared to other classical antipsychotic drugs, Group 2 phenothiazines have relatively high antimuscarinic activity, which may reduce unwanted extrapyramidal effects (see use of antimuscarinic drugs in parkinsonism, Ch. 24).
11. True. Hypothyroidism can occur with use of lithium and thyroid function should be monitored.
12. False. Lithium is reabsorbed through the proximal convoluted tubule in the kidney, at the same site that Na+ is absorbed.
OBA answer
A Incorrect. Adherence is higher with atypical drugs, probably as a result of fewer unwanted effects.
B Incorrect. The risk of extrapyramidal effects is increased with depot injections.
C Correct. Dopamine receptor agonists reverse D2 receptor blockade in neuroleptic malignant syndrome.
D Incorrect. Antipsychotics do not cause nausea and some are used in the treatment of nausea and vomiting.
Extended-matching-item answers
1. Answer D is correct. Serotonin (5-HT) receptor antagonism is most likely to contribute to antipsychotic activity.
2. Answer C is correct. Blockade of α1-adrenoceptors on blood vessels produces hypotension.
3. Answer E is correct. Blockade of histamine H1 receptors in the CNS causes sedation.
4. Answer B is correct. Antimuscarinic effects include constipation, dry mouth, blurred vision and problems with micturition.
5. Answer A is correct. Antagonism of nigrostriatal pathway dopamine D2 receptors produces extrapyramidal disorders of movement.
Case-based answers
A Approaches include the possible use of a depot preparation and stressing the importance of support from the patient's GP and family in maintaining adherence. A principal cause of poor adherence is the unwanted effects of antipsychotic therapy. Since unwanted effects vary widely from drug to drug, the choice of drug may have a major impact on adherence.
B Mr PS may benefit from a different conventional antipsychotic drug, such as another phenothazine that causes less sedation and fewer antimuscarinic effects than chlorpromazine, but extrapyramidal effects may be worse. An atypical drug may be more appropriate and Mr PS's adherence may increase as a result of fewer unwanted effects. Atypical antipsychotics may also be more effective on Mr PS's negative symptoms of apathy and withdrawal. Both positive and negative features may be particularly resistant in a proportion of people with schizophrenia; clozapine may be effective in unresponsive patients after failure of two or more antipsychotic drugs, one of which is an atypical drug, but the risk of blood disorders with clozapine makes blood monitoring mandatory.
Altamura, AC, Sassella, F, Santini, A, et al. Intramuscular preparations of antipsychotics. Drugs. 2003;63:493–512.
Belmaker, RH. Bipolar disorder. N Engl J Med. 2004;351:476–486.
Cipriani, A, Barbui, C, Salanti, G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta–analysis. Lancet. 2011;378:1306–1315.
Frye, MA. Bipolar disorder – a focus on depression. N Engl J Med. 2011;364:51–59.
Geddes, J, Freemantle, N, Harrison, P, Bebbington, P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321:1371–1376.
Hartling, L, Abou-Setta, AM, Dursun, S. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. JAMA. 2012;157:498–511.
Harwood, AJ, Agam, G. Search for a common mechanism of action of mood stabilizers. Biochem Pharmacol. 2003;66:179–189.
Horacek, J, Bubenikov-Valesova, V, Kopecek, M, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409.
Kane, JM, Correll, CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–1124.
Laruelle, M, Kegele, S, Abi-Darham, A. Glutamate dopamine and schizophrenia. From pathology to treatment. Ann NY Acad Sci. 2003;1003:138–158.
Laruelle, M, Frankle, WG, Narenran, R, et al. Mechanism of action of antipsychotic drugs: from dopamine D2 receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2007;27(Suppl A):S16–S24.
Li, X, Ketter, TA, Frye, MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord. 2002;69:1–14.
Miyamoto, S, Duncan, GE, Marx, CE, et al. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104.
Möller, H-J. Management of the negative symptoms of schizophrenia. CNS Drugs. 2003;17:793–823.
Mueser, KT, McGurk, SR. Schizophrenia. Lancet. 2004;363:2063–2072.
Müller-Oerlinghausen, B, Berghöfer, A, Bauer, M. Bipolar disorder. Lancet. 2002;359:241–247.
Picchioni, MM, Murray, RM. Schizophrenia. BMJ. 2007;335:91–95.
Rochon, PA, Stukel, TA, Sykora, A, et al. Atypical antipsychotics and parkinsonism. Arch Intern Med. 2005;165:1882–1888.
Van Os, J, Kapur, S. Schizophrenia. Lancet. 2009;374:635–645.