16 Purines
Overview
In this chapter we describe the role of purine nucleosides and nucleotides as chemical mediators subserving a wide range of functions. The mechanisms responsible for their synthesis and release are considered, as well as the various receptors on which they act, and drugs that affect purinergic signalling.
Introduction
Nucleosides (especially adenosine) and nucleotides (especially ADP and ATP) will already be familiar to you because of their crucial role in DNA/RNA synthesis and energy metabolism, but it may come as a surprise to learn that they also produce a wide range of unrelated pharmacological effects. Interest in this field probably began with the observation in 1929 that adenosine injected into anaesthetised animals caused bradycardia, hypotension, vasodilatation and inhibition of intestinal movements. Since then, it has become clear that purines participate in many physiological control mechanisms, including the regulation of coronary flow and myocardial function (Chs 21 and 22), platelet aggregation and immune responses (Chs 17 and 24), as well as neurotransmission in both the central and peripheral nervous system (Chs 12 and 38).
There is, therefore, increasing interest in purine pharmacology and the potential role of purinergic agents in the treatment of pain and a variety of disorders, particularly of thrombotic and respiratory origin. The full complexity of purinergic control systems, and their importance in many pathophysiological mechanisms, is only now emerging, and the therapeutic relevance of the various receptor subtypes is still being unravelled.1 There is no doubt that drugs affecting these systems will assume growing significance but, recognising that the overall picture is far from complete, we will focus our discussion on a few prominent areas.
Figure 16.1 summarises the mechanisms by which purines are released and interconverted, and the main receptor types on which they act.
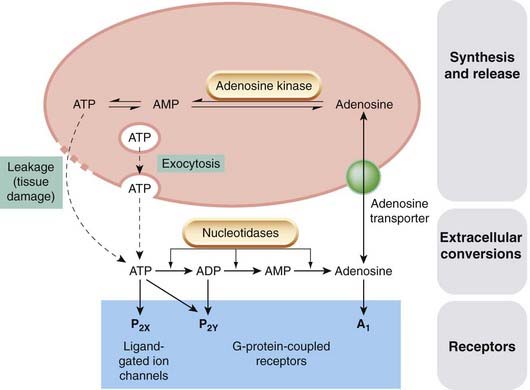
Fig. 16.1 Purines as mediators.
ATP (and, in platelets, ADP) is stored in vesicles and released by exocytosis. It is also present in the cytosol of all cells, from which large quantities may be released by cellular damage. Adenosine is present in the cytosol of all cells, and is taken up and released via a specific membrane transporter. When released, ATP and ADP are rapidly converted to adenosine by the action of tissue nucleotidases.
Purinergic Receptors
Purines exert their biological actions through three families of receptors. Table 16.1 lists these and summarises what is known about their signalling systems, their endogenous ligands and antagonists of pharmacological interest. It should be noted, however, that the family of purinergic receptors continues to grow and their pharmacology can be confusing. In part, this is because nucleotides are rapidly degraded by ecto-enzymes and there is also evidence of interconversion by phosphate exchange. Thus it is possible to envisage a situation where ATP may produce effects at all three receptor subclasses depending upon the extent of its enzymatic hydrolysis.
The three main types of purine receptor are:
The subtypes in each family may be distinguished on the basis of their molecular structure as well as their agonist and antagonist selectivity. The latter has usually been determined by the use of groups of experimental compounds with varying degrees of receptor selectivity and need not concern us here. The P2Y group is particularly problematic: several receptors have been cloned on the basis of homology with other family members, but their ligands have yet to be identified (in other words they are ‘orphan receptors’). In addition, some members of this family also recognise pyrimidines such as UTP and UDP as well as purines, and as such are sometimes classed as pyrimidinoceptors. Little is known about the role of pyrimidines in cell signalling.
With the exception of platelet P2Y12 antagonists such as clopidogrel, there are so far few therapeutic agents that act on these receptors, and we will confine this account to some prominent and interesting aspects; the reading list provides further information.
Purines as mediators ![]()
Adenosine as a Mediator
The simplest of the purines, adenosine is found in biological fluids throughout the body. It exists free in the cytosol of all cells and is transported in and out mainly by a membrane transporter. Little is known about the way in which this is controlled but the extracellular concentrations are usually quite low compared with intracellular levels. Adenosine in tissues comes partly from this intracellular source and partly from extracellular hydrolysis of released ATP or ADP (Fig. 16.1).
Virtually all cells express one or more A-receptors and so adenosine produces many pharmacological effects, both in the periphery and in the CNS. Based on its ability to inhibit cell function and thus minimise the metabolic requirements of cells, one of its functions may be as an ‘acute’ protective agent that is released immediately when tissue integrity is threatened (e.g. by coronary or cerebral ischaemia; see Chs 21 and 39). Under less extreme conditions, variations in adenosine release may play a role in controlling blood flow and (through effects on the carotid bodies) respiration, matching them to the metabolic needs of the tissues.
Adenosine and the Cardiovasular System
Adenosine inhibits cardiac conduction and it is likely that all four of the adenosine receptors are involved in this effect. Because of this, adenosine itself may be used as a drug, being given as an intravenous bolus injection to terminate supraventricular tachycardia (Ch. 21). In this respect it is safer than alternatives such as β-adrenoceptor antagonists or verapamil, because of its short duration of action: it is destroyed or taken up within a few seconds of intravenous administration. Longer-lasting analogues have been discovered that also show greater receptor selectivity. Adenosine uptake is blocked (and thus its action prolonged) by dipyridamole, a vasodilator and antiplatelet drug (see Ch. 24).
Adenosine and Asthma
Adenosine receptors are found on all the cell types involved in asthma and the overall pharmacology is complex. However, by acting through its A1 receptor, adenosine promotes mediator release from mast cells, and causes enhanced mucus secretion, bronchoconstriction and leukocyte activation. Methylxanthines, especially analogues of theophylline (Ch. 27), are adenosine receptor antagonists. Theophylline has been used for the treatment of asthma and part of its beneficial activity may be ascribed to its antagonism of the A1 receptor; however, methylxanthines also increase cAMP by inhibiting phosphodiesterase, which contributes to their pharmacological actions independently of adenosine receptor antagonism. Certain derivatives of theophylline are claimed to show greater selectivity for adenosine receptors over phosphodiesterase. In contrast to the A1 receptor, activation of the A2A subtype exerts a largely protective and anti-inflammatory effect.
Activation of the A2B receptor also promotes mast cell mediator release, while the role of the A3 receptor has yet to be fully elucidated. Recent thinking therefore suggests that an antagonist of the A1 and A2B receptor or an agonist of the A2A receptor would represent a significant therapeutic advance (see Brown et al., 2008).
Adenosine in the CNS
Acting through A1 and A2A receptors, adenosine has an inhibitory effect on many CNS neurons, and the stimulation experienced after consumption of methylxanthines such as caffeine (see Ch. 47) occurs partly as a result of block of these receptors.
ADP as a Mediator
ADP is usually stored in vesicles in cells. When released, it exerts its biological effects predominantly through the P2Y family of receptors.
ADP and Platelets
The secretory vesicles of blood platelets store both ATP and ADP in high concentrations, and release them when the platelets are activated (see Chs 23 and 24). One of the many effects of ADP is to promote platelet aggregation, so this system provides positive feedback—an important mechanism for controlling this process. The receptor involved is P2Y12. Clopidogrel, prasugrel and the earlier agent, ticlopidine (not available in the UK), are P2Y12 antagonists and exert their antiaggregating effects through this mechanism (Ch. 24).
ATP as a Mediator
ATP exerts its action primarily through the P2X receptors. The extracellular domain of these multimeric receptors can bind three molecules of ATP. When activated, the receptor gates the cation-selective ion channels that trigger ongoing intracellular signalling. The other actions of ATP in mammals are mediated through the P2Y receptors. Suramin (a drug originally developed to treat trypanasome infections) and an experimental compound PPADS antagonise ATP and have broad-spectrum inhibitory activity at most P2X and P2Y receptors. ATP is present in all cells in millimolar concentrations and is released, independently of exocytosis, if the cells are damaged (e.g. by ischaemia). ATP released from cells is rapidly dephosphorylated by a range of tissue-specific nucleotidases, producing ADP and adenosine (Fig. 16.1), both of which produce a wide variety of receptor-mediated effects. The role of intracellular ATP in controlling membrane potassium channels, which is important in the control of vascular smooth muscle (Ch. 22) and of insulin secretion (Ch. 30), is quite distinct from its transmitter function.
ATP as a Neurotransmitter
The idea that such a workaday metabolite as ATP might be a member of the neurotransmitter elite was resisted for a long time, but is now firmly established. ATP is a transmitter in the periphery, both as a primary mediator and as a co-transmitter in noradrenergic nerve terminals. P2X2, P2X4 and P2X6 are the predominant receptor subtypes expressed in neurons. P2X1 predominates in smooth muscle.
The nucleotide is contained in synaptic vesicles of both adrenergic and cholinergic neurons, and it accounts for many of the actions produced by stimulation of autonomic nerves that are not caused by acetylcholine or noradrenaline (see Ch. 12). These effects include the relaxation of intestinal smooth muscle evoked by sympathetic stimulation, and contraction of the bladder produced by parasympathetic nerves. Burnstock and his colleagues have shown that ATP is released on nerve stimulation in a Ca2+-dependent fashion, and that exogenous ATP, in general, mimics the effects of nerve stimulation in various preparations. ATP functions as a conventional ‘fast’ transmitter in the CNS and in autonomic ganglia.
Adenosine, produced following hydrolysis of ATP, exerts presynaptic inhibitory effects on the release of excitatory transmitters in the CNS and periphery.
ATP in Nociception
ATP causes pain when injected, as a result of activation of P2X2 and/or P2X3 receptors in afferent neurons involved in the transduction of nociception (see Ch. 41). Oddly, perhaps, the same receptors seem to be involved in taste perception on the tongue. Elsewhere in the CNS, P2X4 receptors on microglia may be important in the development of neuropathic pain.
ATP in Inflammation
The P2X7 receptor is widely distributed on cells of the immune system, and ATP, apparently acting through this receptor, causes the release from macrophages and mast cells of cytokines and other mediators of the inflammatory response. Mice in which the receptor is deleted by genetic techniques show a reduced capacity to develop chronic inflammation.
Future Prospects
While it is true that few currently available drugs act through purinergic receptors when compared, for example, with 5-HT receptors discussed in Chapter 15, the area as a whole holds promise for future therapeutic exploitation, particularly in the treatment of asthma (Brown et al., 2008), pain (Liu et al., 2005; Burnstock, 2006) and gastrointestinal disorders (Burnstock, 2008), provided compounds with sufficient receptor selectivity can be found.
References and Further Reading
(A note of caution: the nomenclature of these receptors has changed several times and this can make for difficulties when reading some older papers. For the latest version of the nomenclature, always refer to http://www.iuphar-db.org.)
Brown R.A., Spina D., Page C.P. Adenosine receptors and asthma. Br. J. Pharmacol.. 2008;153(Suppl. 1):S446-S456. (Excellent review of the pharmacology of adenosine in the lung. Very accessible)
Brundege J.M., Dunwiddie T.V. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv. Pharmacol.. 1997;39:353-391. (Good review article)
Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin. Med.. 2002;2:45-53.
Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol. Ther.. 2006;110:433-454. (This paper, and the reviews that follow by the same author, cover various aspects of purinergic signalling and its therapeutic application)
Burnstock G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut. 2008;57:1193-1194.
Cunha R.A. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int.. 2001;38:107-125. (Speculative review on the functions of adenosine in the nervous system)
Fredholm B.B., Arslan G., Halldner L., et al. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2001;362:364-374. (General review article)
Illes P., Klotz K.-N., Lohse M.J. Signalling by extracellular nucleotides and nucleosides. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2000;362:295-298. (Introductory article in a series of useful reviews on purinergic mechanisms in the same issue)
Khakh B.S., North R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527-532. (Excellent and very readable review on P2X receptors. Recommended)
Klotz K.-N. Adenosine receptors and their ligands. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2000;362:382-391. (Account of known agonists and antagonists at adenosine receptors)
Liu X.J., Salter M.W. Purines and pain mechanisms: recent developments. Curr. Opin. Investig. Drugs. 2005;6:65-75.
Stone T.W. Purines and neuroprotection. Adv. Exp. Med. Biol.. 2002;513:249-280.
Surprenant A., North R.A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol.. 2009;71:333-359. (Extremely comprehensive review of P2X receptor biology if you are interested in following up the latest cutting-edge thinking)
von Kügelglen I., Wetter A. Molecular pharmacology of P2Y receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2000;362:310-323.
1Indeed, a journal, Purinergic Signalling, devoted exclusively to these issues was launched recently.