27 Respiratory system
Overview
Basic aspects of respiratory physiology (regulation of airway smooth muscle, pulmonary vasculature and glands) are considered as a basis for a discussion of pulmonary disease and its treatment. We devote most of the chapter to asthma, dealing first with pathogenesis and then the main drugs used in its treatment and prevention—inhaled bronchodilators and anti-inflammatory agents. We also discuss chronic obstructive pulmonary disease (COPD). There are short sections on allergic emergencies, surfactants and the treatment of cough. Other important pulmonary diseases, such as bacterial infections (e.g. tuberculosis and acute pneumonias) and malignancies, are addressed in Chapters 50 and 55, respectively, or are not yet amenable to drug treatment (e.g. occupational and interstitial lung diseases). Antihistamines, important in treatment of hay fever, are covered in Chapter 26. Pulmonary hypertension is covered in Chapter 22.
The Physiology of Respiration
Control of Breathing
Respiration is controlled by spontaneous rhythmic discharges from the respiratory centre in the medulla, modulated by input from pontine and higher central nervous system (CNS) centres and vagal afferents from the lungs. Various chemical factors affect the respiratory centre, including the partial pressure of carbon dioxide in arterial blood (PACO2) by an action on medullary chemoreceptors, and of oxygen (PAO2) by an action on the chemoreceptors in the carotid bodies.
Some voluntary control can be superimposed on the automatic regulation of breathing, implying connections between the cortex and the motor neurons innervating the muscles of respiration. Bulbar poliomyelitis and certain lesions in the brain stem result in loss of the automatic regulation of respiration without loss of voluntary regulation.1
Regulation of Musculature, Blood Vessels and Glands of the Airways
Irritant receptors and C fibres respond to chemical irritants and cold air, and also to inflammatory mediators (see below). Efferent pathways controlling the airways include cholinergic parasympathetic nerves and non-noradrenergic non-cholinergic (NANC) inhibitory nerves (see Ch. 12). Inflammatory mediators (see Ch. 17) and NANC bronchoconstrictor mediators also have a role in diseased airways.
The tone of bronchial muscle influences airway resistance, which is also affected by the state of the mucosa and activity of the glands in patients with asthma and bronchitis. Airway resistance can be measured indirectly by instruments that record the volume or flow of forced expiration. FEV1 is the forced expiratory volume in 1 second. The peak expiratory flow rate (PEFR) is the maximal flow (expressed as l/min) after a full inhalation; this is simpler to measure at the bedside than FEV1, which it follows closely.
Efferent Pathways
Autonomic innervation
The autonomic innervation of human airways is reviewed by van der Velden & Hulsmann (1999).
Parasympathetic innervation
Parasympathetic innervation of bronchial smooth muscle predominates. Parasympathetic ganglia are embedded in the walls of the bronchi and bronchioles, and the postganglionic fibres innervate airway smooth muscle, vascular smooth muscle and glands. Three types of muscarinic (M) receptors are present (see Ch. 13, Table 13.2). M3 receptors are pharmacologically the most important. They are found on bronchial smooth muscle and glands, and mediate bronchoconstriction and mucus secretion. M1 receptors are localised in ganglia and on postsynaptic cells, and facilitate nicotinic neurotransmission, whereas M2 receptors are inhibitory autoreceptors mediating negative feedback on acetylcholine release by postganglionic cholinergic nerves. Stimulation of the vagus causes bronchoconstriction—mainly in the larger airways. The possible clinical relevance of the heterogeneity of muscarinic receptors in the airways is discussed below.
A distinct population of NANC nerves (see Ch. 12) also regulates the airways. Bronchodilators released by these nerves include vasoactive intestinal polypeptide (Table 12.2) and nitric oxide (NO; Ch. 20).
Sympathetic innervation
Sympathetic nerves innervate tracheobronchial blood vessels and glands, but not human airway smooth muscle. β-Adrenoceptors are, however, abundantly expressed on human airway smooth muscle (as well as mast cells, epithelium, glands and alveoli) and β agonists relax bronchial smooth muscle, inhibit mediator release from mast cells and increase mucociliary clearance (see below). In humans, β-adrenoceptors in the airways are of the β2 variety.
In addition to the autonomic innervation, non-myelinated sensory fibres linked to irritant receptors in the lungs release tachykinins such as substance P, neurokinin A and neurokinin B (see Chs 19 and 41), which act on smooth muscle, secretory and inflammatory cells, producing neurogenic inflammation.
Sensory Receptors and Afferent Pathways
Slowly adapting stretch receptors control respiration via the respiratory centre. Unmyelinated sensory C fibres and rapidly adapting irritant receptors associated with myelinated vagal fibres are also important.
Physical or chemical stimuli, acting on irritant receptors on myelinated fibres in the upper airways and/or C-fibre receptors in the lower airways, cause coughing, bronchoconstriction and mucus secretion. Such stimuli include cold air and irritants such as ammonia, sulfur dioxide, cigarette smoke and the experimental tool capsaicin (Ch. 41), as well as endogenous inflammatory mediators.
Pulmonary Disease and its Treatment
Common symptoms of pulmonary disease include shortness of breath, wheeze, chest pain and cough with or without sputum production or haemoptysis—blood in the sputum. Ideally, treatment is of the underlying disease, but sometimes symptomatic treatment, for example of cough, is all that is possible. The lung is an important target organ of many diseases addressed elsewhere in this book, including infections (Chs 50–54), malignancy (Ch. 55) and occupational and rheumatological diseases; drugs (e.g. amiodarone methotrexate) can damage lung tissue and cause pulmonary fibrosis. Heart failure leads to pulmonary oedema (Ch. 22). Thromboembolic disease (Ch. 24) and pulmonary hypertension (Ch. 22) affect the pulmonary circulation. In this present chapter, we concentrate on two important diseases of the airways: asthma and COPD.
Bronchial Asthma
Asthma is the commonest chronic disease in children in economically developed countries, and is also common in adults. It is increasing in prevalence and severity. It is an inflammatory condition in which there is recurrent reversible airways obstruction in response to irritant stimuli that are too weak to affect non-asthmatic subjects. The obstruction usually causes wheeze and merits drug treatment, although the natural history of asthma includes spontaneous remissions.2 Reversibility of airways obstruction in asthma contrasts with COPD, where the obstruction is either not reversible or at best incompletely reversible by bronchodilators.
Characteristics of Asthma
Asthmatic patients experience intermittent attacks of wheezing, shortness of breath—with difficulty especially in breathing out—and sometimes cough. As explained above, acute attacks are reversible, but the underlying pathological disorder can progress in older patients to a chronic state superficially resembling COPD.
Acute severe asthma (also known as status asthmaticus) is not easily reversed and causes hypoxaemia. Hospitalisation is necessary, as the condition, which can be fatal, requires prompt and energetic treatment.
The term bronchial hyper-reactivity (or hyper-responsiveness) refers to abnormal sensitivity to a wide range of stimuli, such as irritant chemicals, cold air and stimulant drugs, all of which can result in bronchoconstriction. In allergic asthma, these features may be initiated by sensitisation to allergen(s), but, once established, asthma attacks can be triggered by various stimuli such as viral infection, exercise (in which the stimulus may be cold air and/or drying of the airways) and atmospheric pollutants such as sulfur dioxide. Immunological desensitisation to allergens such as pollen or dust mites is popular in some countries but is not superior to conventional inhaled drug treatment.
Pathogenesis of Asthma
The pathogenesis of asthma involves both genetic and environmental factors, and the asthmatic attack itself consists, in many subjects, of two main phases: an immediate and a late (or delayed) phase (see Fig. 27.1).
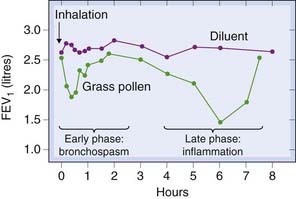
Fig. 27.1 Two phases of asthma demonstrated by the changes in forced expiratory volume in 1 second (FEV1) after inhalation of grass pollen in an allergic subject.
(From Cockcroft D W 1983 Lancet ii: 253.)
Numerous cells and mediators play a part, and the full details of the complex events involved are still a matter of debate (Walter & Holtzman, 2005). The following simplified account is intended to provide a basis for understanding the rational use of drugs in the treatment of asthma.
Asthmatics have activated T cells, with a T-helper (Th)2 profile of cytokine production (see Ch. 17 and Table 6.2) in their bronchial mucosa. How these cells are activated is not fully understood, but allergens (Fig. 27.2) are one mechanism. The Th2 cytokines that are released do the following:
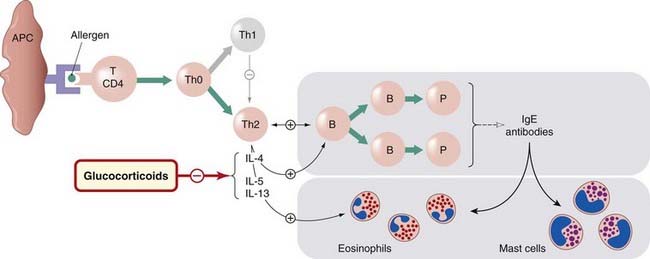
Fig. 27.2 The part played by T lymphocytes in allergic asthma.
In genetically susceptible individuals, allergen (green circle) interacts with dendritic cells and CD4+ T cells, leading to the development of Th0 lymphocytes, which give rise to a clone of Th2 lymphocytes. These then (1) generate a cytokine environment that switches B cells/plasma cells to the production and release of immunoglobulin (Ig)E; (2) generate cytokines, such as interleukin (IL)-5, which promote differentiation and activation of eosinophils; and (3) cytokines (e.g. IL-4 and IL-13) that induce expression of IgE receptors. Glucocorticoids inhibit the action of the cytokines specified. APC, antigen-presenting dendritic cell; B, B cell; P, plasma cell; Th, T-helper cell.
Some asthmatics, in addition to these mechanisms, are also atopic—i.e. they make allergen-specific IgE that binds to mast cells in the airways. Inhaled allergen cross-links IgE molecules on mast cells, triggering degranulation with release of histamine and leukotriene B4, both of which are powerful bronchoconstrictors to which asthmatics are especially sensitive because of their airway hyper-responsiveness. This provides a mechanism for acute exacerbation of asthma in atopic individuals exposed to allergen. The effectiveness of omalizumab (an anti-IgE antibody; see below) serves to emphasise the importance of IgE in the pathogenesis of asthma as well as in other allergic diseases. Noxious gases (e.g. sulfur dioxide, ozone) and airway dehydration can also cause mast cell degranulation.
Clinicians often refer to atopic or ‘extrinsic’ asthma and non-atopic or ‘intrinsic’ asthma; we prefer the terms allergic and non-allergic.
Asthma ![]()
The immediate phase of the asthmatic attack
In allergic asthma, the immediate phase (i.e. the initial response to allergen provocation) occurs abruptly and is mainly caused by spasm of the bronchial smooth muscle. Allergen interaction with mast cell-fixed IgE causes release of histamine, leukotriene B4 and prostaglandin (PG)D2 (Ch. 17).
Other mediators released include IL-4, IL-5, IL-13, macrophage inflammatory protein-1α and tumour necrosis factor (TNF)-α.
Various chemotaxins and chemokines (see Ch. 17) attract leukocytes—particularly eosinophils and mononuclear cells—into the area, setting the stage for the delayed phase (Fig. 27.3).
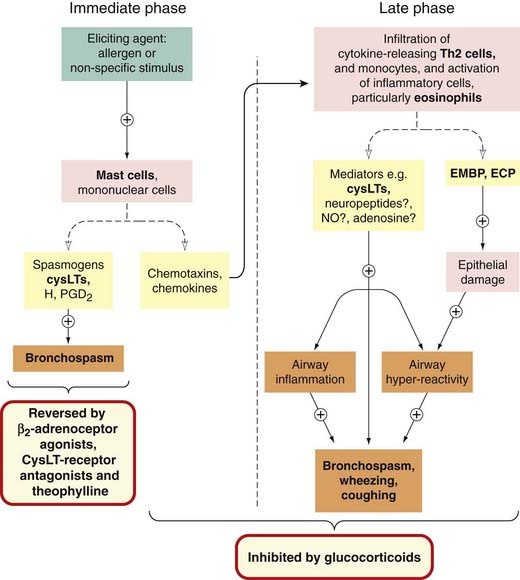
Fig. 27.3 Immediate and late phases of asthma, with the actions of the main drugs.
CysLTs, cysteinyl leukotrienes (leukotrienes C4 and D4); ECP, eosinophil cationic protein; EMBP, eosinophil major basic protein; H, histamine; iNO, induced nitric oxide.
(For more detail of the Th2-derived cytokines and chemokines, see Ch. 17 and Fig. 6.4.)
The late phase
The late phase or delayed response (see Figs 27.1 and 27.3) may be nocturnal. It is, in essence, a progressing inflammatory reaction, initiation of which occurred during the first phase, the influx of Th2 lymphocytes being of particular importance. The inflammatory cells include activated eosinophils. These release cysteinyl leukotrienes, interleukins IL-3, IL-5 and IL-8, and the toxic proteins, eosinophil cationic protein, major basic protein and eosinophil-derived neurotoxin. These play an important part in the events of the late phase, the toxic proteins causing damage and loss of epithelium (see, for example, Larche et al., 2003; Kay, 2005). Other putative mediators of the inflammatory process in the delayed phase are adenosine (acting on the A1 receptor; see Ch. 16), induced NO (see Ch. 20) and neuropeptides (see Ch. 19).
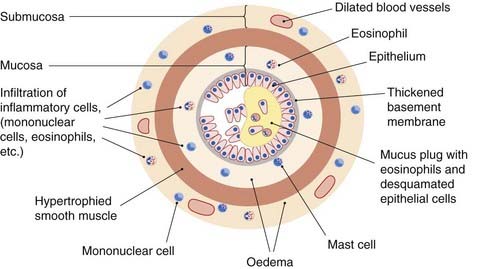
Growth factors released from inflammatory cells act on smooth muscle cells, causing hypertrophy and hyperplasia, and the smooth muscle can itself release proinflammatory mediators and autocrine growth factors (Chs 5 and 17). Figure 27.4 shows schematically the changes that take place in the bronchioles. Epithelial cell loss means that irritant receptors and C fibres are more accessible to irritant stimuli—an important mechanism of bronchial hyper-reactivity.
‘Aspirin-sensitive’ asthma
 Non-steroidal anti-inflammatory drugs (NSAIDs), especially aspirin, can precipitate asthma in sensitive individuals. Such aspirin-sensitive asthma is relatively uncommon (< 10% of asthmatic subjects), and is often associated with nasal polyps. Individuals sensitive to one NSAID are usually also sensitive to other chemically unrelated cyclo-oxygenase (COX) inhibitors, including sometimes paracetamol (Ch. 26). Abnormal leukotriene production and sensitivity are implicated. Patients with aspirin-sensitive asthma produce more cysteinyl leukotriene and have greater airway hyper-responsiveness to inhaled cysteinyl leukotrienes than aspirin-tolerant asthmatics. Such airway hyper-responsiveness reflects elevated expression of cysteinyl leukotriene receptors on inflammatory cells, and this is downregulated by aspirin desensitisation (Sousa et al., 2002). In addition, aspirin and similar drugs directly activate eosinophils and mast cells in these patients through IgE-independent mechanisms.
Non-steroidal anti-inflammatory drugs (NSAIDs), especially aspirin, can precipitate asthma in sensitive individuals. Such aspirin-sensitive asthma is relatively uncommon (< 10% of asthmatic subjects), and is often associated with nasal polyps. Individuals sensitive to one NSAID are usually also sensitive to other chemically unrelated cyclo-oxygenase (COX) inhibitors, including sometimes paracetamol (Ch. 26). Abnormal leukotriene production and sensitivity are implicated. Patients with aspirin-sensitive asthma produce more cysteinyl leukotriene and have greater airway hyper-responsiveness to inhaled cysteinyl leukotrienes than aspirin-tolerant asthmatics. Such airway hyper-responsiveness reflects elevated expression of cysteinyl leukotriene receptors on inflammatory cells, and this is downregulated by aspirin desensitisation (Sousa et al., 2002). In addition, aspirin and similar drugs directly activate eosinophils and mast cells in these patients through IgE-independent mechanisms.
Drugs Used to Treat and Prevent Asthma
There are two categories of antiasthma drugs: bronchodilators and anti-inflammatory agents. Bronchodilators reverse the bronchospasm of the immediate phase; anti-inflammatory agents inhibit or prevent the inflammatory components of both phases (Fig. 27.3). These two categories are not mutually exclusive: some drugs classified as bronchodilators also have some anti-inflammatory effect.
How best to use these drugs to treat asthma is complex. A guideline (see www.brit-thoracic.org.uk, updated in 2009) specifies five therapeutic steps for adults and children with chronic asthma. Very mild disease may be controlled with short-acting bronchodilator alone (step 1), but if patients need this more than once a day, a regular inhaled corticosteroid should be added (step 2). If the asthma remains uncontrolled, the next step is to add a long-acting bronchodilator (salmeterol or formoterol); this minimises the need for increased doses of inhaled corticosteroid (step 3). Theophylline and leukotriene antagonists, such as montelukast, also exert a corticosteroid-sparing effect, but this is less reliable. One or other is added in for patients with more severe asthma who remain symptomatic and/or the dose of inhaled corticosteroid increased to the maximum recommended (step 4). If the patient’s condition is still poorly controlled, it may be necessary to add a regular oral corticosteroid (e.g. prednisolone)—step 5. Corticosteroids are the mainstay of therapy because they are the only asthma drugs that potently inhibit T-cell activation, and thus the inflammatory response, in the asthmatic airways. Cromoglicate (see below) has only a weak effect and is now seldom used.
Bronchodilators
The main drugs used as bronchodilators are β2-adrenoceptor agonists; others include theophylline, cysteinyl leukotriene receptor antagonists and muscarinic receptor antagonists.
β-Adrenoceptor agonists
The β2-adrenoceptor agonists are dealt with in Chapter 14. Their primary effect in asthma is to dilate the bronchi by a direct action on the β2 adrenoceptors of smooth muscle. Being physiological antagonists of bronchoconstrictors (see Ch. 2), they relax bronchial muscle whatever the spasmogens involved. They also inhibit mediator release from mast cells and TNF-α release from monocytes, and increase mucus clearance by an action on cilia.
The β2-adrenoceptor agonists are usually given by inhalation of aerosol, powder or nebulised solution (i.e. solution that has been converted into a cloud or mist of fine droplets), but some may be given orally or by injection. A metered-dose inhaler is used for aerosol preparations.
Two categories of β2-adrenoceptor agonists are used in asthma.
Unwanted effects
The unwanted effects of β2-adrenoceptor agonists result from systemic absorption and are given in Chapter 14. In the context of their use in asthma, the commonest adverse effect is tremor; other unwanted effects include tachycardia and cardiac dysrhythmia.
Xanthine drugs (see Chs 15 and 45)
Theophylline (1,3-dimethylxanthine), which is also used as theophylline ethylenediamine (known as aminophylline), is the main therapeutic drug of this class, and has long been used as a bronchodilator.3 Here we consider it in the context of respiratory disease, its only current therapeutic use.
Mechanism of action
The mechanism of theophylline is still unclear. The relaxant effect on smooth muscle has been attributed to inhibition of phosphodiesterase (PDE) isoenzymes, with resultant increase in cAMP and/or cGMP (see Fig. 4.10). However, the concentrations necessary to inhibit the isolated enzymes exceed the therapeutic range of plasma concentrations.
Competitive antagonism of adenosine at adenosine A1 and A2 receptors (Ch. 16) may contribute, but the PDE inhibitor enprofylline, which is a potent bronchodilator, is not an adenosine antagonist.
Type IV PDE is implicated in inflammatory cells (see below), and methylxanthines may have some anti-inflammatory effect. (Roflumilast, a type IV PDE inhibitor, is mentioned below in the context of COPD.)
Theophylline activates histone deacetylase (HDAC) and may thereby reverse resistance to the anti-inflammatory effects of corticosteroids (Barnes, 2006).
Methylxanthines stimulate the CNS (Ch. 47) and respiratory stimulation may be beneficial in patients with COPD and reduced respiration evidenced by a tendency to retain CO2 (see below).
Unwanted effects
When theophylline is used in asthma, its other actions (CNS, cardiovascular, gastrointestinal and diuretic) result in unwanted side effects (e.g. insomnia, nervousness). The therapeutic plasma concentration range is 30–100 µmol/l, and adverse effects are common with concentrations greater than 110 µmol/l; thus, there is a relatively narrow therapeutic window. Serious cardiovascular and CNS effects can occur when the plasma concentration exceeds 200 µmol/l. The most serious cardiovascular effect is dysrhythmia (especially during intravenous administration of aminophylline), which can be fatal. Seizures can occur with theophylline concentrations at or slightly above the upper limit of the therapeutic range, and can be fatal in patients with impaired respiration due to severe asthma. Monitoring the concentration of theophylline in plasma is useful for optimising the dose.
Pharmacokinetic aspects
Theophylline is given orally as a sustained-release preparation. Aminophylline can be given by slow intravenous injection of a loading dose followed by intravenous infusion.
Theophylline is well absorbed from the gastrointestinal tract. It is metabolised by P450 enzymes in the liver; the mean elimination half-life is approximately 8 h in adults but there is wide inter-individual variation. The half-life is increased in liver disease, cardiac failure and viral infections, and is decreased in heavy cigarette smokers (as a result of enzyme induction). Unwanted drug interactions are clinically important: its plasma concentration is decreased by drugs that induce P450 enzymes (including rifampicin, phenytoin and carbamazepine). The concentration is increased by drugs that inhibit P450 enzymes, such as erythromycin, clarithromycin, ciprofloxacin, diltiazem and fluconazole. This is important in view of the narrow therapeutic window; antibiotics such as clarithromycin are often started when asthmatics are hospitalised because of a severe attack precipitated by a chest infection, and if the dose of theophylline is unaltered, severe toxicity can result.
Muscarinic receptor antagonists
Muscarinic receptor antagonists are dealt with in Chapter 13. The main compound used as a bronchodilator is ipratropium. Tiotropium is also available; it is a longer-acting drug used in maintenance treatment of COPD (see below). Ipratropium is seldom used on a regular basis in asthma but can be useful for cough caused by irritant stimuli in such patients.
Ipratropium is a quaternary derivative of N-isopropylatropine. It does not discriminate between muscarinic receptor subtypes (see Ch. 13), and it is possible that its blockade of M2 autoreceptors on the cholinergic nerves increases acetylcholine release and reduces the effectiveness of its antagonism at the M3 receptors on smooth muscle. It is not particularly effective against allergen challenge, but it inhibits the augmentation of mucus secretion that occurs in asthma and may increase the mucociliary clearance of bronchial secretions. It has no effect on the late inflammatory phase of asthma.
Ipratropium is given by aerosol inhalation. As a quaternary nitrogen compound, it is highly polar and is not well absorbed into the circulation (Ch. 8), limiting systemic effects. The maximum effect occurs approximately 30 min after inhalation and persists for 3–5 h. It has few unwanted effects and is, in general, safe and well tolerated. It can be used with β2-adrenoceptor agonists. See the clinical box, above, for clinical uses.
Clinical use of inhaled muscarinic receptor antagonists (e.g. ipratropium) ![]()
Cysteinyl leukotriene receptor antagonists
Two receptors for cysteinyl leukotrienes (LTC4, LTD4 and LTE4) have been cloned, CysLT1 and CysLT2 (see Ch. 17), and both are expressed in respiratory mucosa and infiltrating inflammatory cells, but the functional significance of each is unclear. The ‘lukast’ drugs (montelukast and zafirlukast) antagonise only CysLT1.
Lukasts reduce acute reactions to aspirin in sensitive patients, but have not been shown to be particularly effective for aspirin-sensitive asthma (see above) in the clinic. They inhibit exercise-induced asthma and decrease both early and late responses to inhaled allergen. They relax the airways in mild asthma but are less effective than salbutamol, with which their action is additive. They reduce sputum eosinophilia, but there is no clear evidence that they modify the underlying inflammatory process in chronic asthma.
The lukasts are taken by mouth, in combination with an inhaled corticosteroid. They are generally well tolerated, adverse effects consisting mainly of headache and gastrointestinal disturbances.
Histamine H1-receptor antagonists
Although mast cell mediators play a part in the immediate phase of allergic asthma (Fig. 27.3) and in some types of exercise-induced asthma, histamine H1-receptor antagonists have no routine place in therapy, although they may be modestly effective in mild atopic asthma, especially when this is precipitated by acute histamine release in patients with concomitant allergy such as severe hay fever.
Anti-Inflammatory Agents
Glucocorticoids
Glucocorticoids (see Ch. 30) are the main drugs used for their anti-inflammatory action in asthma. They are not bronchodilators, but prevent the progression of chronic asthma and are effective in acute severe asthma (see below).4
Actions and mechanism
The basis of the anti-inflammatory action of glucocorticoids is discussed in Chapter 32. An important action, of relevance for asthma, is that they decrease formation of cytokines, in particular the Th2 cytokines that recruit and activate eosinophils and are responsible for promoting the production of IgE and the expression of IgE receptors. Glucocorticoids also inhibit the generation of the vasodilators PGE2 and PGI2, by inhibiting induction of COX-2 (Fig. 17.1). By inducing annexin-1,5 they could inhibit production of leukotrienes and platelet-activating factor, although there is currently no direct evidence that annexin-1 is involved in the therapeutic action of glucocorticoids in human asthma.
Corticosteroids inhibit the allergen-induced influx of eosinophils into the lung. Glucocorticoids upregulate β2-adrenoceptors, decrease microvascular permeability and indirectly reduce mediator release from eosinophils by inhibiting the production of cytokines (e.g. IL-5 and granulocyte-macrophage colony stimulating factor) that activate eosinophils. Reduced synthesis of IL-3 (the cytokine that regulates mast cell production) may explain why long-term steroid treatment eventually reduces the number of mast cells in the respiratory mucosa, and hence suppresses the early-phase response to allergens and exercise.
Glucocorticoids are sometimes ineffective, even in high doses, for reasons that are incompletely understood (reviewed by Adcock & Ito, 2004). Many individual mechanisms could contribute to glucocorticoid resistance. The phenomenon has been linked to the number of glucocorticoid receptors, but in some situations other mechanisms are clearly in play—for example, reduced activity of histone deacetylase (HDAC) may be important in cigarette smokers (see below).
The main compounds used are beclometasone, budesonide, fluticasone, mometasone and ciclesonide. These are given by inhalation with a metered-dose or dry powder inhaler, the full effect on bronchial hyper-responsiveness being attained only after weeks or months of therapy.
Unwanted effects
Serious unwanted effects are uncommon with inhaled steroids. Oropharyngeal candidiasis (thrush; Ch. 52) can occur (T lymphocytes are important in protection against fungal infection), as can sore throat and croaky voice, but use of ‘spacing’ devices, which decrease oropharyngeal deposition of the drug and increase airway deposition, reduces these problems. Regular high doses can produce some adrenal suppression, particularly in children, and necessitate carrying a ‘steroid card’ (Ch. 32). This is less likely with fluticasone, mometasone and ciclesonide, as these drugs are poorly absorbed from the gastrointestinal tract and undergo almost complete presystemic metabolism. The unwanted effects of oral glucocorticoids are given in Chapter 32 and Figure 32.7.
Clinical use of glucocorticoids in asthma ![]()
Cromoglicate and nedocromil
These two drugs, of similar chemical structure and properties, are now hardly used for the treatment of asthma. Although very safe, they have only weak anti-inflammatory effects and short duration of action. They are given by inhalation as aerosols or dry powders, and can be also be used topically for allergic conjunctivitis or rhinitis. They are not bronchodilators, having no direct effects on smooth muscle, nor do they inhibit the actions of any of the known smooth muscle stimulants. Given prophylactically, they reduce both the immediate- and late-phase asthmatic responses and reduce bronchial hyper-reactivity.
Their mechanism of action is not fully understood. Cromoglicate is a ‘mast cell stabiliser’, preventing histamine release from mast cells. However, this is not the basis of its action in asthma, because compounds that are more potent than cromoglicate at inhibiting mast cell histamine release are ineffective against asthma.
Cromoglicate depresses the exaggerated neuronal reflexes that are triggered by stimulation of the ‘irritant receptors’; it suppresses the response of sensory C fibres to capsaicin and may inhibit the release of T-cell cytokines. Various other effects, of uncertain importance, on the inflammatory cells and mediators involved in asthma have been described.
Anti-IgE treatment
Omalizumab is a humanised monoclonal anti-IgE antibody. It is effective in patients with allergic asthma as well as in allergic rhinitis. It is of considerable theoretical interest (see review by Holgate et al., 2005), but it is expensive and its place in therapeutics is unclear.