12 Chemical mediators and the autonomic nervous system
Overview
The network of chemical signals and associated receptors by which cells in the body communicate with one another provides many targets for drug action, and has always been a focus of attention for pharmacologists. Chemical transmission in the peripheral nervous system, and the various ways in which the process can be pharmacologically subverted, are discussed in this chapter. In addition to neurotransmission, we also consider briefly the less clearly defined processes, collectively termed neuromodulation, by which many mediators and drugs exert control over the function of the nervous system. The relative anatomical and physiological simplicity of the peripheral nervous system has made it the proving ground for many important discoveries about chemical transmission, and the same general principles apply to the central nervous system (see Ch. 36). For more detail than is given here, see Cooper et al. (2003), Robertson (2004) and Burnstock (2009).
Historical Aspects
 Studies initiated on the peripheral nervous system have been central to the understanding and classification of many major types of drug action, so it is worth recounting a little history. Excellent accounts are given by Bacq (1975) and Valenstein (2005).
Studies initiated on the peripheral nervous system have been central to the understanding and classification of many major types of drug action, so it is worth recounting a little history. Excellent accounts are given by Bacq (1975) and Valenstein (2005).
Experimental physiology became established as an approach to the understanding of the function of living organisms in the middle of the 19th century. The peripheral nervous system, and particularly the autonomic nervous system, received a great deal of attention. The fact that electrical stimulation of nerves could elicit a whole variety of physiological effects—from blanching of the skin to arrest of the heart—presented a real challenge to comprehension, particularly of the way in which the signal was passed from the nerve to the effector tissue. In 1877, Du Bois-Reymond was the first to put the alternatives clearly: ‘Of known natural processes that might pass on excitation, only two are, in my opinion, worth talking about—either there exists at the boundary of the contractile substance a stimulatory secretion … or the phenomenon is electrical in nature.’ The latter view was generally favoured. In 1869, it had been shown that an exogenous substance, muscarine, could mimic the effects of stimulating the vagus nerve, and that atropine could inhibit the actions both of muscarine and of nerve stimulation. In 1905, Langley showed the same for nicotine and curare acting at the neuromuscular junction. Most physiologists interpreted these phenomena as stimulation and inhibition of the nerve endings, respectively, rather than as evidence for chemical transmission. Hence the suggestion of T R Elliott, in 1904, that adrenaline (epinephrine) might act as a chemical transmitter mediating the actions of the sympathetic nervous system was coolly received, until Langley, the Professor of Physiology at Cambridge and a powerful figure at that time, suggested, a year later, that transmission to skeletal muscle involved the secretion by the nerve terminals of a substance related to nicotine.
One of the key observations for Elliott was that degeneration of sympathetic nerve terminals did not abolish the sensitivity of smooth muscle preparations to adrenaline (which the electrical theory predicted) but actually enhanced it. The hypothesis of chemical transmission was put to direct test in 1907 by Dixon, who tried to show that vagus nerve stimulation released from a dog’s heart into the blood a substance capable of inhibiting another heart. The experiment failed, and the atmosphere of scepticism prevailed.
It was not until 1921, in Germany, that Loewi showed that stimulation of the vagosympathetic trunk connected to an isolated and cannulated frog’s heart could cause the release into the cannula of a substance (‘Vagusstoff’) that, if the cannula fluid was transferred from the first heart to a second, would inhibit the second heart. This is a classic and much-quoted experiment that proved extremely difficult for even Loewi to perform reproducibly. In an autobiographical sketch, Loewi tells us that the idea of chemical transmission arose in a discussion that he had in 1903, but no way of testing it experimentally occurred to him until he dreamed of the appropriate experiment one night in 1920. He wrote some notes of this very important dream in the middle of the night, but in the morning could not read them. The dream obligingly returned the next night and, taking no chances, he went to the laboratory at 3 a.m. and carried out the experiment successfully. Loewi’s experiment may be, and was, criticised on numerous grounds (it could, for example, have been potassium rather than a neurotransmitter that was acting on the recipient heart), but a series of further experiments proved him to be right. His findings can be summarised as follows:
A few years later, in the early 1930s, Dale showed convincingly that acetylcholine was also the transmitter substance at the neuromuscular junction of striated muscle and at autonomic ganglia. One of the keys to Dale’s success lay in the use of highly sensitive bioassays, especially the leech dorsal muscle, for measuring acetylcholine release. Chemical transmission at sympathetic nerve terminals was demonstrated at about the same time as cholinergic transmission and by very similar methods. Cannon and his colleagues at Harvard first showed unequivocally the phenomenon of chemical transmission at sympathetic nerve endings, by experiments in vivo in which tissues made supersensitive to adrenaline by prior sympathetic denervation were shown to respond, after a delay, to the transmitter released by stimulation of the sympathetic nerves to other parts of the body. The chemical identity of the transmitter, tantalisingly like adrenaline but not identical to it, caused confusion for many years, until in 1946 von Euler showed it to be the non-methylated derivative noradrenaline (norepinephrine).
The Autonomic Nervous System
The autonomic nervous system for a long time occupied centre stage in the pharmacology of chemical transmission.
Basic Anatomy and Physiology
The autonomic nervous system (see Robertson, 2004) consists of three main anatomical divisions: sympathetic, parasympathetic and enteric nervous systems. The sympathetic and parasympathetic systems (Fig. 12.1) provide a link between the central nervous system and peripheral organs. The enteric nervous system comprises the intrinsic nerve plexuses of the gastrointestinal tract, which are closely interconnected with the sympathetic and parasympathetic systems.
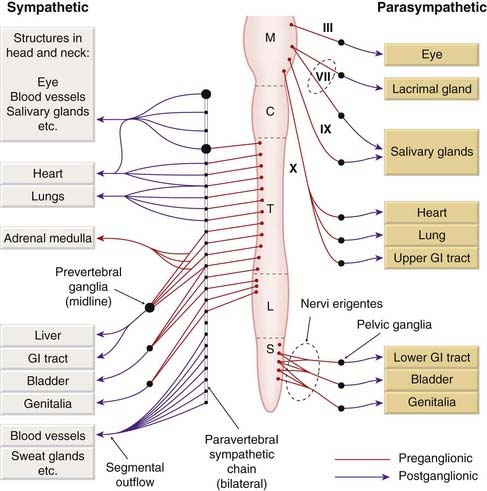
Fig. 12.1 Basic plan of the mammalian autonomic nervous system.
C, cervical; GI, gastrointestinal; L, lumbar; M, medullary; S, sacral; T, thoracic.
The autonomic nervous system conveys all the outputs from the central nervous system to the rest of the body, except for the motor innervation of skeletal muscle. The enteric nervous system has sufficient integrative capabilities to allow it to function independently of the central nervous system, but the sympathetic and parasympathetic systems are agents of the central nervous system and cannot function without it. The autonomic nervous system is largely outside the influence of voluntary control. The main processes that it regulates are:
A degree of autonomic control also affects many other systems, including the kidney, immune system and somatosensory system. The autonomic efferent pathway consists of two neurons arranged in series, whereas in the somatic motor system a single motor neuron connects the central nervous system to the skeletal muscle fibre (Fig.12.2). The two neurons in the autonomic pathway are known, respectively, as preganglionic and postganglionic. In the sympathetic nervous system, the intervening synapses lie in autonomic ganglia, which are outside the central nervous system, and contain the nerve endings of preganglionic fibres and the cell bodies of postganglionic neurons. In parasympathetic pathways, the postganglionic cells are mainly found in the target organs, discrete parasympathetic ganglia (e.g. the ciliary ganglion) being found only in the head and neck.
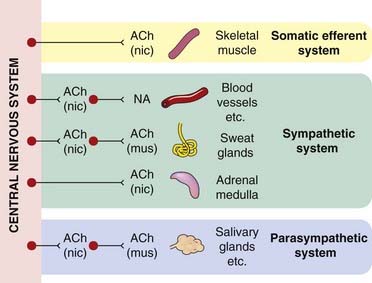
Fig. 12.2 Acetylcholine and noradrenaline as transmitters in the peripheral nervous system.
The two main types of acetylcholine (ACh) receptor, nicotinic (nic) and muscarinic (mus) (see Ch. 13), are indicated. NA, noradrenaline (norepinephrine).
The cell bodies of the sympathetic preganglionic neurons lie in the lateral horn of the grey matter of the thoracic and lumbar segments of the spinal cord, and the fibres leave the spinal cord in the spinal nerves as the thoracolumbar sympathetic outflow. The preganglionic fibres synapse in the paravertebral chains of sympathetic ganglia, lying on either side of the spinal column. These ganglia contain the cell bodies of the postganglionic sympathetic neurons, the axons of which rejoin the spinal nerve. Many of the postganglionic sympathetic fibres reach their peripheral destinations via the branches of the spinal nerves. Others, destined for abdominal and pelvic viscera, have their cell bodies in a group of unpaired prevertebral ganglia in the abdominal cavity. The only exception to the two-neuron arrangement is the innervation of the adrenal medulla. The catecholamine-secreting cells of the adrenal medulla are, in effect, modified postganglionic sympathetic neurons, and the nerves supplying the gland are equivalent to preganglionic fibres.
The parasympathetic nerves emerge from two separate regions of the central nervous system. The cranial outflow consists of preganglionic fibres in certain cranial nerves, namely the oculomotor nerve (carrying parasympathetic fibres destined for the eye), the facial and glossopharyngeal nerves (carrying fibres to the salivary glands and the nasopharynx), and the vagus nerve (carrying fibres to the thoracic and abdominal viscera). The ganglia lie scattered in close relation to the target organs; the postganglionic neurons are very short compared with those of the sympathetic system. Parasympathetic fibres destined for the pelvic and abdominal viscera emerge as the sacral outflow from the spinal cord in a bundle of nerves known as the nervi erigentes (because stimulation of these nerves evokes genital erection—a fact of some importance to those responsible for artificial insemination of livestock). These fibres synapse in a group of scattered pelvic ganglia, whence the short postganglionic fibres run to target tissues such as the bladder, rectum and genitalia. The pelvic ganglia carry both sympathetic and parasympathetic fibres, and the two divisions are not anatomically distinct in this region.
The enteric nervous system (reviewed by Goyal & Hirano, 1996) consists of the neurons whose cell bodies lie in the intramural plexuses in the wall of the intestine. It is estimated that there are more cells in this system than in the spinal cord, and functionally they do not fit simply into the sympathetic/parasympathetic classification. Incoming nerves from both the sympathetic and the parasympathetic systems terminate on enteric neurons, as well as running directly to smooth muscle, glands and blood vessels. Some enteric neurons function as mechanoreceptors or chemoreceptors, providing local reflex pathways that can control gastrointestinal function without external inputs. The enteric nervous system is pharmacologically more complex than the sympathetic or parasympathetic systems, involving many neuropeptide and other transmitters (such as 5-hydroxytryptamine, nitric oxide and ATP).
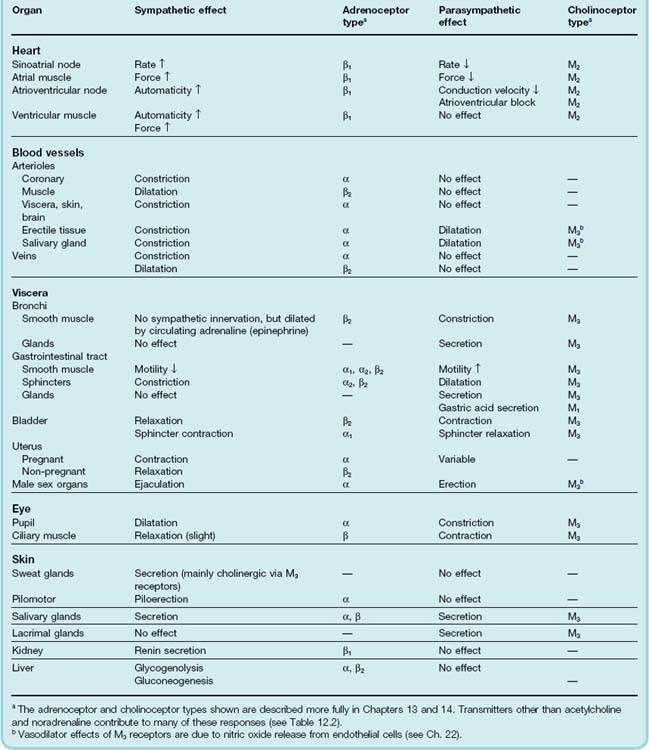
In some places (e.g. in the visceral smooth muscle of the gut and bladder, and in the heart), the sympathetic and the parasympathetic systems produce opposite effects, but there are others where only one division of the autonomic system operates. The sweat glands and most blood vessels, for example, have only a sympathetic innervation, whereas the ciliary muscle of the eye has only a parasympathetic innervation. Bronchial smooth muscle has only a parasympathetic (constrictor) innervation (although its tone is highly sensitive to circulating adrenaline—acting probably to inhibit the constrictor innervation rather than on the smooth muscle directly). Resistance arteries (see Ch. 22) have a sympathetic vasoconstrictor innervation but no parasympathetic innervation; instead, the constrictor tone is opposed by a background release of nitric oxide from the endothelial cells (see Ch. 20). There are other examples, such as the salivary glands, where the two systems produce similar, rather than opposing, effects.
It is therefore a mistake to think of the sympathetic and parasympathetic systems simply as physiological opponents. Each serves its own physiological function and can be more or less active in a particular organ or tissue according to the need of the moment. Cannon rightly emphasised the general role of the sympathetic system in evoking ‘fight or flight’ reactions in an emergency, but emergencies are rare for most animals. In everyday life, the autonomic nervous system functions continuously to control specific local functions, such as adjustments to postural changes, exercise or ambient temperature (see Jänig & McLachlan, 1992). The popular concept of a continuum from the extreme ‘rest and digest’ state (parasympathetic active, sympathetic quiescent) to the extreme emergency fight or flight state (sympathetic active, parasympathetic quiescent) is an oversimplification.
Table 12.1 lists some of the more important autonomic responses in humans.
Basic anatomy of the autonomic nervous system ![]()
Physiology of the autonomic nervous system ![]()
Transmitters in the Autonomic Nervous System
The two main neurotransmitters that operate in the autonomic system are acetylcholine and noradrenaline, whose sites of action are shown diagrammatically in Figure 12.2. This diagram also shows the type of postsynaptic receptor with which the transmitters interact at the different sites (discussed more fully in Chs 13 and 14). Some general rules apply:
Acetylcholine and noradrenaline are the grandees among autonomic transmitters, and are central to understanding autonomic pharmacology. However, many other chemical mediators are also released by autonomic neurons (see below), and their functional significance is gradually becoming clearer.
Transmitters of the autonomic nervous system ![]()
Some General Principles of Chemical Transmission
The essential processes in chemical transmission—the release of mediators, and their interaction with receptors on target cells—are described in Chapters 4 and 3, respectively. Here we consider some general characteristics of chemical transmission of particular relevance to pharmacology. Many of these principles apply also to the central nervous system and are taken up again in Chapter 36.
Dale’s Principle
 Dale’s principle, advanced in 1934, states, in its modern form: ‘A mature neuron releases the same transmitter (or transmitters) at all of its synapses.’ Dale considered it unlikely that a single neuron could store and release different transmitters at different nerve terminals, and his view was supported by physiological and neurochemical evidence. It is now known, however, that there are situations where different transmitters are released from different terminals of the same neuron. Further, most neurons release more than one transmitter (see Co-transmission, below) and may change their transmitter repertoire, for example during development or in response to injury. Moreover (see Fig. 4.12), the balance of the cocktail of mediators released by a nerve terminal can vary with stimulus conditions, and in response to presynaptic modulators. Dale’s principle was, of course, framed long before these complexities were discovered, and it has probably now outlived its usefulness, although purists seem curiously reluctant to let it go.
Dale’s principle, advanced in 1934, states, in its modern form: ‘A mature neuron releases the same transmitter (or transmitters) at all of its synapses.’ Dale considered it unlikely that a single neuron could store and release different transmitters at different nerve terminals, and his view was supported by physiological and neurochemical evidence. It is now known, however, that there are situations where different transmitters are released from different terminals of the same neuron. Further, most neurons release more than one transmitter (see Co-transmission, below) and may change their transmitter repertoire, for example during development or in response to injury. Moreover (see Fig. 4.12), the balance of the cocktail of mediators released by a nerve terminal can vary with stimulus conditions, and in response to presynaptic modulators. Dale’s principle was, of course, framed long before these complexities were discovered, and it has probably now outlived its usefulness, although purists seem curiously reluctant to let it go.
Denervation Supersensitivity
It is known, mainly from the work of Cannon on the sympathetic system, that if a nerve is cut and its terminals allowed to degenerate, the structure supplied by it becomes supersensitive to the transmitter substance released by the terminals. Thus skeletal muscle, which normally responds to injected acetylcholine only if a large dose is given directly into the arterial blood supply, will, after denervation, respond by contracture to much smaller amounts. Other organs, such as salivary glands and blood vessels, show similar supersensitivity to acetylcholine and noradrenaline when the postganglionic nerves degenerate, and there is evidence that pathways in the central nervous system show the same phenomenon.
 Several mechanisms contribute to denervation supersensitivity, and the extent and mechanism of the phenomenon varies from organ to organ. Reported mechanisms include the following (see Luis & Noel, 2009).
Several mechanisms contribute to denervation supersensitivity, and the extent and mechanism of the phenomenon varies from organ to organ. Reported mechanisms include the following (see Luis & Noel, 2009).
Supersensitivity can occur, but is less marked, when transmission is interrupted by processes other than nerve section. Pharmacological block of ganglionic transmission, for example, if sustained for a few days, causes some degree of supersensitivity of the target organs, and long-term blockade of postsynaptic receptors also causes receptors to proliferate, leaving the cell supersensitive when the blocking agent is removed. Phenomena such as this are of importance in the central nervous system, where such supersensitivity can cause ‘rebound’ effects when drugs that impair synaptic transmission are given for some time and then discontinued.
Presynaptic Modulation
The presynaptic terminals that synthesise and release transmitter in response to electrical activity in the nerve fibre are often themselves sensitive to transmitter substances and to other substances that may be produced locally in tissues (for review see Boehm & Kubista, 2002). Such presynaptic effects most commonly act to inhibit transmitter release, but may enhance it. Figure 12.3A shows the inhibitory effect of adrenaline on the release of acetylcholine (evoked by electrical stimulation) from the postganglionic parasympathetic nerve terminals of the intestine. The release of noradrenaline from nearby sympathetic nerve terminals can also inhibit release of acetylcholine. Noradrenergic and cholinergic nerve terminals often lie close together in the myenteric plexus, so the opposing effects of the sympathetic and parasympathetic systems result not only from the opposite effects of the two transmitters on the smooth muscle cells, but also from the inhibition of acetylcholine release by noradrenaline acting on the parasympathetic nerve terminals. A similar situation of mutual presynaptic inhibition exists in the heart, where noradrenaline inhibits acetylcholine release, as in the myenteric plexus, and acetylcholine also inhibits noradrenaline release. These are examples of heterotropic interactions, where one neurotransmitter affects the release of another. Homotropic interactions also occur, where the transmitter, by binding to presynaptic autoreceptors, affects the nerve terminals from which it is being released. This type of autoinhibitory feedback acts powerfully at noradrenergic nerve terminals (see Starke et al., 1989). Figure 12.3B shows that in normal mice, noradrenaline release from the hippocampus increases only slightly as the number of stimulus trains increases from 1 to 64. In transgenic mice lacking a specific type of presynaptic α2 adrenoceptor (see Ch. 14), the amount released by the longer stimulus train is greatly increased, though the amount released by a single stimulus is unaffected. This is because with one or a few stimuli, there is no opportunity for autoinhibitory feedback to develop, whereas with longer trains the inhibition operates powerfully. A similar autoinhibitory feedback occurs with many transmitters, including acetylcholine and 5-hydroxytryptamine.
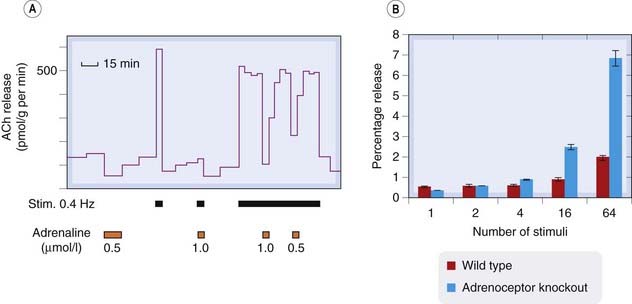
Fig. 12.3 Examples of presynaptic inhibition.
[A] Inhibitory effect of adrenaline on acetylcholine (ACh) release from postganglionic parasympathetic nerves in the guinea pig ileum. The intramural nerves were stimulated electrically where indicated, and the ACh released into the bathing fluid determined by bioassay. Adrenaline strongly inhibits ACh release. [B] Noradrenaline release from mouse hippocampal slices in response to trains of electrical stimuli. Blue bars show normal (wild type) mice. Red bars show α2 adrenoceptor knockout mice. The lack of presynaptic autoinhibition in the knockout mice results in a large increase in release with a long stimulus train, but does not affect release by fewer than four stimuli, because the autoinhibition takes a few seconds to develop.
([A] From Vizi E S 1979 Prog Neurobiol 12: 181. [B] Redrawn from Trendelenburg et al. 2001 Naunyn Schmiedeberg’s Arch Pharmacol 364: 117–130.)
In both the noradrenergic and cholinergic systems, the presynaptic autoreceptors are pharmacologically distinct from the postsynaptic receptors (see Chs 13 and 14), and there are drugs that act selectively, as agonists or antagonists, on the pre- or postsynaptic receptors.
Cholinergic and noradrenergic nerve terminals respond not only to acetylcholine and noradrenaline, as described above, but also to other substances that are released as co-transmitters, such as ATP and neuropeptide Y (NPY), or derived from other sources, including nitric oxide, prostaglandins, adenosine, dopamine, 5-hydroxytryptamine, GABA, opioid peptides, endocannabinoids and many other substances. The physiological role and pharmacological significance of these various interactions is still unclear (see review by Vizi, 2001), but the description of the autonomic nervous system represented in Figure 12.2 is undoubtedly oversimplified. Figure 12.4 shows some of the main presynaptic interactions between autonomic neurons, and summarises the many chemical influences that regulate transmitter release from noradrenergic neurons.
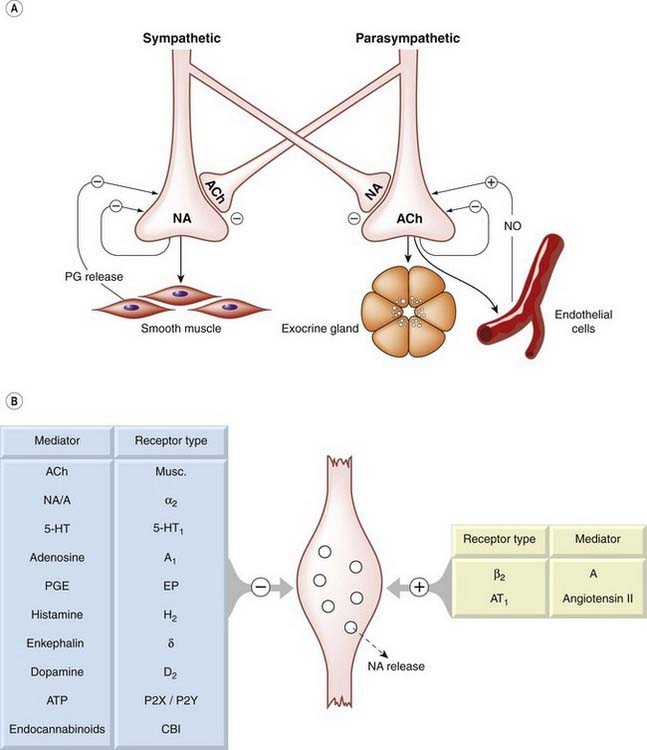
Fig. 12.4 Presynaptic regulation of transmitter release from noradrenergic and cholinergic nerve terminals.
[A] Postulated homotropic and heterotropic interactions between sympathetic and parasympathetic nerves. [B] Some of the known inhibitory and facilitatory influences on noradrenaline release from sympathetic nerve endings. 5-HT, 5-hydroxytryptamine; A, adrenaline; ACh, acetylcholine; NA, noradrenaline; NO, nitric oxide; PG, prostaglandin; PGE, prostaglandin E.
Presynaptic receptors regulate transmitter release mainly by affecting Ca2+ entry into the nerve terminal (see Ch. 4), but also by other mechanisms (see Kubista & Boehm, 2006). Most presynaptic receptors are of the G-protein-coupled type (see Ch. 3), which control the function of calcium channels and potassium channels either through second messengers that regulate the state of phosphorylation of the channel proteins, or by a direct interaction of G-proteins with the channels. Transmitter release is inhibited when calcium channel opening is inhibited, or when potassium channel opening is increased (see Ch. 4); in many cases, both mechanisms operate simultaneously. Presynaptic regulation by receptors linked directly to ion channels (ionotropic receptors; see Ch. 3) rather than to G-proteins also occurs (see Kubista & Boehm, 2006). Nicotinic acetylcholine receptors (nAChRs) are particularly important in this respect. They can either facilitate or inhibit the release of other transmitters, such as glutamate (see Ch. 36), and most of the nAChRs expressed in the central nervous system are located presynaptically. Another example is the GABAA receptor, whose action is to inhibit transmitter release (see Chs 4 and 37). Other ionotropic receptors, such as those activated by ATP and 5-hydroxytryptamine (Chs 15 and 16), may have similar effects on transmitter release.
Postsynaptic Modulation
Chemical mediators often act on postsynaptic structures, including neurons, smooth muscle cells, cardiac muscle cells, etc., in such a way that their excitability or spontaneous firing pattern is altered. In many cases, as with presynaptic modulation, this is caused by changes in calcium and/or potassium channel function mediated by a second messenger. We give only a few examples here.
The pre- and postsynaptic effects described above are often described as neuromodulation, because the mediator acts to increase or decrease the efficacy of synaptic transmission without participating directly as a transmitter. Many neuropeptides, for example, affect membrane ion channels in such a way as to increase or decrease excitability and thus control the firing pattern of the cell. Neuromodulation1 is loosely defined but, in general, involves slower processes (taking seconds to days) than neurotransmission (which occurs in milliseconds), and operates through cascades of intracellular messengers (Ch. 3) rather than directly on ligand-gated ion channels.
Transmitters other than Acetylcholine and Noradrenaline
As mentioned above, acetylcholine or noradrenaline are not the only autonomic transmitters. The rather grudging realisation that this was so dawned many years ago when it was noticed that autonomic transmission in many organs could not be completely blocked by drugs that abolish responses to these transmitters. The dismal but tenacious term non-adrenergic non-cholinergic (NANC) transmission was coined. Later, fluorescence and immunocytochemical methods showed that neurons, including autonomic neurons, contain many potential transmitters, often several in the same cell. Compounds now known to function as NANC transmitters include ATP, vasoactive intestinal peptide (VIP), NPY and nitric oxide (see Fig. 12.5 and Table 12.2), which function at postganglionic nerve terminals, as well as substance P, 5-hydroxytryptamine, GABA and dopamine, which play a role in ganglionic transmission (see Lundberg, 1996, for a comprehensive review).
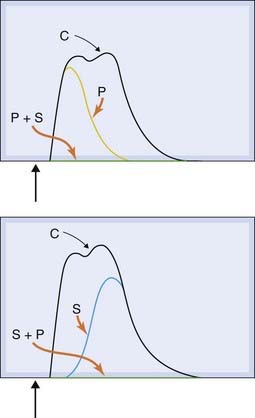
Fig. 12.5 Noradrenaline/ATP co-transmission in the guinea pig vas deferens.
Contractions of the tissue are shown in response to a single electrical stimulus causing excitation of sympathetic nerve endings. With no blocking drugs present, a twin-peaked response is produced (C). The early peak is selectively abolished by the ATP antagonist suramin (S), while the late peak is blocked by the α1-adrenoceptor antagonist prazosin (P). The response is completely eliminated when both drugs are present.
(Reproduced with permission from von Kugelglen & Starke 1991 Trends Pharmacol Sci 12: 319–324.)
Table 12.2 Examples of non-adrenergic non-cholinergic transmitters and co-transmitters in the peripheral nervous system
| Transmitter | Location | Function |
|---|---|---|
| Non-peptides | ||
| ATP | Postganglionic sympathetic neurons | Fast depolarisation/contraction of smooth muscle cells (e.g. blood vessels, vas deferens) |
| GABA, 5-hydroxytryptamine | Enteric neurons | Peristaltic reflex |
| Dopamine | Some sympathetic neurons (e.g. kidney) | Vasodilatation |
| Nitric oxide | Pelvic nerves | Erection |
| Gastric nerves | Gastric emptying | |
| Peptides | ||
| Neuropeptide Y | Postganglionic sympathetic neurons | Facilitates constrictor action of noradrenaline; inhibits noradrenaline release (e.g. blood vessels) |
| Vasoactive intestinal peptide (VIP) | Parasympathetic nerves to salivary glands | Vasodilatation; co-transmitter with acetylcholine |
| NANC innervation of airways smooth muscle | Bronchodilatation | |
| Gonadotrophin-releasing hormone | Sympathetic ganglia | Slow depolarisation; co-transmitter with acetylcholine |
| Substance P | Sympathetic ganglia, enteric neurons | Slow depolarisation; co-transmitter with acetylcholine |
| Calcitonin gene-related peptide | Non-myelinated sensory neurons | Vasodilatation; vascular leakage; neurogenic inflammation |
NANC, non-adrenergic non-cholinergic.
Co-Transmission
It is probably the rule rather than the exception that neurons release more than one transmitter or modulator (see Kupfermann, 1991; Lundberg, 1996), each of which interacts with specific receptors and produces effects, often both pre- and postsynaptically. The example of noradrenaline/ATP co-transmission at the sympathetic nerve endings is shown in Figure 12.5, and the best-studied examples and mechanisms are summarised in Table 12.2 and Figures 12.6 and 12.7.
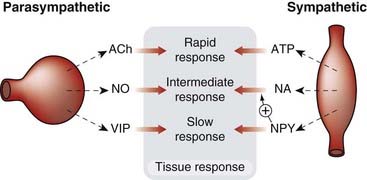
Fig. 12.6 The main co-transmitters at postganglionic parasympathetic and sympathetic neurons.
The different mediators generally give rise to fast, intermediate and slow responses of the target organ. ACh, acetylcholine; ATP, adenosine triphosphate; NA, noradrenaline; NO, nitric oxide; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide.
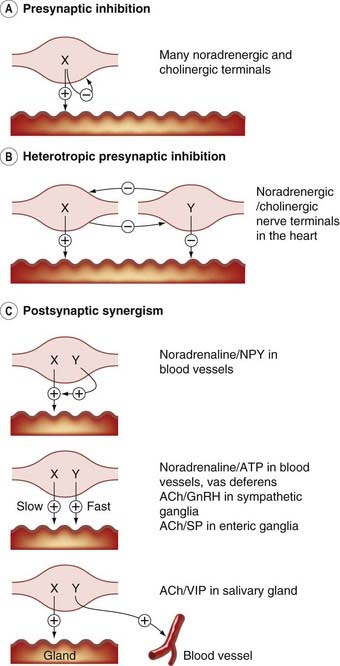
Fig. 12.7 Co-transmission and neuromodulation—some examples.
[A] Presynaptic inhibition. [B] Heterotropic presynaptic inhibition. [C] Postsynaptic synergism. ACh, acetylcholine; ATP, adenosine triphosphate; GnRH, gonadotrophin-releasing hormone (luteinising hormone-releasing hormone); NPY, neuropeptide Y; SP, substance P; VIP, vasoactive intestinal peptide.
What, one might well ask, could be the functional advantage of co-transmission, compared with a single transmitter acting on various different receptors? The possible advantages include the following.
Neuromodulation and presynaptic interactions ![]()
Termination of Transmitter Action
Chemically transmitting synapses other than the peptidergic variety (Ch. 19) invariably incorporate a mechanism for disposing rapidly of the released transmitter, so that its action remains brief and localised. At cholinergic synapses (Ch. 13), the released acetylcholine is inactivated very rapidly in the synaptic cleft by acetylcholinesterase. In most other cases (see Fig. 12.8), transmitter action is terminated by active reuptake into the presynaptic nerve, or into supporting cells such as glia. Such reuptake depends on transporter proteins, each being specific for a particular transmitter. The major class (Na+-Cl− co-transporters), whose molecular structure and function are well understood (see Nelson, 1998; Torres et al., 2003, Gether et al., 2006), consists of a family of membrane proteins, each possessing 12 transmembrane helices. Different members of the family show selectivity for each of the main monoamine transmitters (e.g. the noradrenaline [norepinephrine] transporter, NET, the serotonin transporter, SERT, which transports 5-hydroxytryptamine and the dopamine transporter, DAT). These transporters are important targets for psychoactive drugs, particularly antidepressants (Ch. 46), anxiolytic drugs (Ch. 43) and stimulants (Ch. 47). Transporters for glycine and GABA belong to the same family.
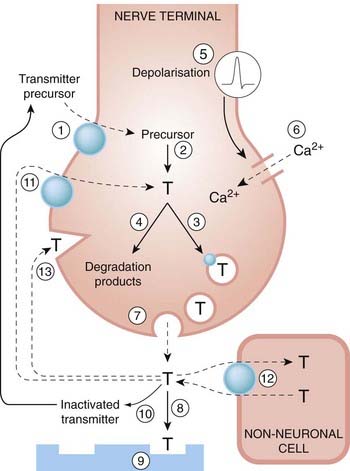
Fig. 12.8 The main processes involved in synthesis, storage and release of amine and amino acid transmitters.
1, Uptake of precursors; 2, synthesis of transmitter; 3, uptake/transport of transmitter into vesicles; 4, degradation of surplus transmitter; 5, depolarisation by propagated action potential; 6, influx of Ca2+ in response to depolarisation; 7, release of transmitter by exocytosis; 8, diffusion to postsynaptic membrane; 9, interaction with postsynaptic receptors; 10, inactivation of transmitter; 11, reuptake of transmitter or degradation products by nerve terminals; 12, uptake of transmitter by non-neuronal cells; and 13, interaction with presynaptic receptors. The transporters (11 and 12) can release transmitter under certain conditions by working in reverse. These processes are well characterised for many transmitters (e.g. acetylcholine, monoamines, amino acids, ATP). Peptide mediators (see Ch. 19) differ in that they may be synthesised and packaged in the cell body rather than the terminals.
Vesicular transporters (Ch. 4), which load synaptic vesicles with transmitter molecules, are closely related to the membrane transporters. Membrane transporters usually act as co-transporters of Na+, Cl− and transmitter molecules, and it is the inwardly directed ‘downhill’ gradient for Na+ that provides the energy for the inward ‘uphill’ movement of the transmitter. The simultaneous transport of ions along with the transmitter means that the process generates a net current across the membrane, which can be measured directly and used to monitor the transport process. Very similar mechanisms are responsible for other physiological transport processes, such as glucose uptake (Ch. 30) and renal tubular transport of amino acids. Because it is the electrochemical gradient for sodium that drives the inward transport of transmitter molecules, a reduction of this gradient can reduce or even reverse the flow of transmitter. This is probably not important under normal conditions, but when the nerve terminals are depolarised or abnormally loaded with sodium (e.g. in ischaemic conditions), the resulting non-vesicular release of transmitter (and inhibition of the normal synaptic reuptake mechanism) may play a significant role in the effects of ischaemia on tissues such as heart and brain (see Chs 21 and 39). Studies with transgenic ‘knockout’ mice (see Torres et al., 2003) show that the store of releasable transmitter is substantially depleted in animals lacking the membrane transporter, showing that synthesis is unable to maintain the store if the recapture mechanism is disabled. As with receptors (see Ch. 3), many genetic polymorphisms of transporter genes occur in humans, which raised hopes of finding associations with various neurological, cardiovascular and psychiatric disorders. But despite intensive research efforts, the links have so far remained elusive (see Lin & Madras, 2006).
As we shall see in subsequent chapters, both membrane and vesicular transporters are targets for various drug effects, and defining the physiological role and pharmacological properties of these molecules is the focus of much current research.
Basic Steps in Neurochemical Transmission: Sites of Drug Action
Figure 12.8 summarises the main processes that occur in a classical chemically transmitting synapse, and provides a useful basis for understanding the actions of the many different classes of drug, discussed in later chapters, that act by facilitating or blocking neurochemical transmission.
All the steps shown in Figure 12.8 (except for transmitter diffusion, step 8) can be influenced by drugs. For example, the enzymes involved in synthesis or inactivation of the transmitter can be inhibited, as can the transport systems responsible for the neuronal and vesicular uptake of the transmitter or its precursor. The actions of the great majority of drugs that act on the peripheral nervous system (Chs 13 and 14) and the central nervous system fit into this general scheme.
References and Further Reading
Bacq Z.M. Chemical transmission of nerve impulses: a historical sketch. Oxford: Pergamon Press; 1975. (Lively account of the history of the discovery of chemical transmission)
Burnstock G. Autonomic neurotransmission: 60 years since Sir Henry Dale. Ann. Rev. Pharmacol.. 2009;49:1-30. (Elegant and well-illustrated account of many of the topics discussed in this chapter. Recommended)
Cooper J.C., Bloom F.E., Roth R.H. The biochemical basis of neuropharmacology, eighth ed. New York: Oxford University Press; 2003. (Excellent general account covering a broad area of neuropharmacology)
Goyal R.K., Hirano I. The enteric nervous system. N. Engl. J. Med.. 1996;334:1106-1115. (Excellent review article)
Jänig W., McLachlan E.M. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci.. 1992;15:475-481. (Short article emphasising that the sympathetic system is far from being an all-or-none alarm system)
Luis E.M.Q., Noel F. Mechanisms of adaptive supersensitivity in vas deferens. Auton. Neurosci.. 2009;146:38-46. (Summarises mechanisms contributing to denervation supersensitivity in a typical organ supplied by the sympathetic nervous system)
Robertson D.W., editor. Primer on the autonomic nervous system. New York: Academic Press, 2004. (An excellent comprehensive textbook on all aspects, including pharmacology, of the autonomic nervous system. By no means elementary despite its title)
Valenstein E.S. The war of the soups and the sparks. New York: Columbia University Press; 2005. (Readable and informative account of origins of the theory of chemical transmission)
Boehm S., Kubista H. Fine tuning of sympathetic transmitter release via ionotropic and metabotropic receptors. Pharm. Rev.. 2002;54:43-99. (Comprehensive review of presynaptic modulation, focusing on sympathetic neurons though mechanisms are widespread)
Gonçalves J., Bueltmann R., Driessen B. Opposite modulation of cotransmitter release in guinea-pig vas deferens: increase of noradrenaline and decrease of ATP release by activation of prejunctional β-receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 1996;353:184-192. (Shows that presynaptic regulation can affect specific transmitters in different ways)
Kubista H., Boehm S. Molecular mechanisms underlying the modulation of exocytotic noradrenaline release via presynaptic receptors. Pharm. Ther.. 2006;112:213-242. (Describes the wide variety of mechanisms by which presynaptic receptors affect transmitter release)
Starke K., Gothert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev.. 1989;69:864-989. (Comprehensive review article)
Jan Y.N., Jan L.Y. A LHRH-like peptidergic neurotransmitter capable of ‘action at a distance’ in autonomic ganglia. Trends Neurosci.. 1983;6:320-325. (Electrophysiological analysis of co-transmission)
Kupfermann I. Functional studies of cotransmission. Physiol. Rev.. 1991;71:683-732. (Good review article)
Lundberg J.M. Pharmacology of co-transmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev.. 1996;48:114-192. (Detailed and informative review article)
Gether U., Andersen P.H., Larsson O.M., et al. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol. Sci.. 2006;27:375-383. (Useful short review article)
Lin Z., Madras B.K. Human genetics and pharmacology of monoamine transporters. In: Sitte, H.H., Freissmuth, M. (Eds.), Neurotransmitter transporters. Handb. Exp. Pharmacol. 175:2006:327-371 (Summarises evidence of linkage between transporter polymorphisms and human disease—complex and unclear so far)
Nelson N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem.. 1998;71:1785-1803. (Review article describing the molecular characteristics of the different families of neurotransporters)
Torres G.E., Gainetdinov R.R., Caron M.G. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci.. 2003;4:13-25. (Describes molecular, physiological and pharmacological aspects of transporters)
Vizi E.S. Role of high-affinity receptors and membrane transporters in non-synaptic communication and drug action in the central nervous system. Pharmacol. Rev.. 2001;52:63-89. (Comprehensive review on pharmacological relevance of presynaptic receptors and transporters; useful for reference)
1Recently, this term has been used to embrace a range of experimental therapeutic approaches based on nerve stimulation techniques, which have been claimed to be effective in a variety of neurological disorders such as bladder dysfunction, epilepsy and depression.