15 5-Hydroxytryptamine and the pharmacology of migraine
Overview
In this chapter, we discuss the role of 5-hydroxytryptamine (5-HT), which functions as a neurotransmitter in the brain and periphery and also as a local hormone. We describe the synthesis, storage and release of 5-HT, the receptor subtypes, its role in the pathophysiology of three important disorders (migraine, carcinoid syndrome and pulmonary hypertension) and the numerous drugs in current use that act wholly or partly on 5-HT receptors.
5-Hydroxytryptamine
This amine, originally detected in extracts of gut (‘enteramine’) and in serum after blood had clotted (’serotonin’) was eventually identified chemically as 5-hydroxytryptamine. Today, the terms ‘5-HT’ and ‘serotonin’ are used interchangeably. 5-HT was subsequently found in the central nervous system (CNS), and shown to function both as a neurotransmitter and as a local hormone in the peripheral vascular system. This chapter deals with the metabolism, distribution and possible physiological roles of 5-HT in the periphery, and with the different types of 5-HT receptor and the drugs that act on them. Further information on the role of 5-HT in the brain, and its relationship to psychiatric disorders and the actions of psychotropic drugs, is presented in Chapters 38, 45 and 46. The use of drugs that modulate 5-HT in the gut is dealt with in Chapter 29.
Distribution, Biosynthesis and Degradation
5-Hydroxytryptamine occurs in the highest concentrations in three organs.
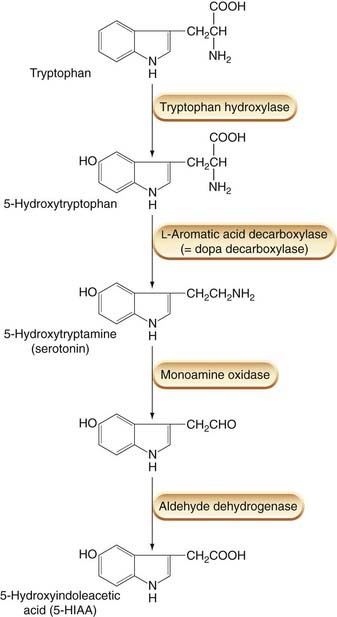
Although 5-HT is present in the diet, most of this is metabolised before entering the bloodstream. Endogenous 5-HT arises from a biosynthetic pathway similar to that which generates noradrenaline (norepinephrine; see Ch. 14), except that the precursor amino acid is tryptophan instead of tyrosine (Fig. 15.1). Tryptophan is converted to 5-hydroxytryptophan (in chromaffin cells and neurons, but not in platelets) by the action of tryptophan hydroxylase, an enzyme confined to 5-HT-producing cells. The 5-hydroxytryptophan is then decarboxylated to 5-HT by a ubiquitous amino acid decarboxylase that also participates in the synthesis of catecholamines (Ch. 14) and histamine (Ch. 17). Platelets (and neurons) possess a high-affinity 5-HT uptake mechanism, and platelets become loaded with 5-HT as they pass through the intestinal circulation, where the local concentration is relatively high. The mechanisms of synthesis, storage, release and reuptake of 5-HT are very similar to those of noradrenaline. Many drugs affect both processes indiscriminately (see Ch. 14), but selective serotonin reuptake inhibitors (SSRIs) have been developed and are important therapeutically as anxiolytics and antidepressants (Chs 43 and 46). 5-HT is often stored in neurons and chromaffin cells as a co-transmitter together with various peptide hormones, such as somatostatin, substance P or vasoactive intestinal polypeptide.
Degradation of 5-HT (Fig. 15.1) occurs mainly through oxidative deamination, catalysed by monoamine oxidase A, followed by oxidation to 5-hydroxyindoleacetic acid (5-HIAA), the pathway being the same as that of noradrenaline catabolism. 5-HIAA is excreted in the urine and serves as an indicator of 5-HT production in the body. This is used, for example, in the diagnosis of carcinoid syndrome (see below).
Pharmacological Effects
The actions of 5-HT are numerous and complex and there is considerable species variation. This complexity reflects a profusion of 5-HT receptor subtypes, which has been revealed in recent years (see below). The main sites of action are as follows.
Gastrointestinal tract
Most 5-HT receptor subtypes (see below) are present in the gut with the exception of those of the 5-HT5/6 family. Only about 10% of 5-HT in the intestine is located in neurons, where it acts as a neurotransmitter, while the remainder is located in the enterochromaffin cells, which act as sensors to transduce information about the state of the gut. The 5-HT is released from enterochromaffin cells into the lamina propria and elsewhere, to stimulate its receptors. The responses observed are very complex and the reader is referred to Beattie & Smith (2008) for a recent comprehensive account. Broadly speaking, 5-HT receptors are present on most neuronal components of the enteric nervous system as well as smooth muscle, secretory and other cells. Their main function is to regulate peristalsis, intestinal motility, secretion and visceral sensitivity.
The importance of 5-HT in the gut is underlined by the widespread distribution of the serotonin uptake transporter (SERT), which rapidly and efficiently removes released 5-HT, thus limiting its action. Inhibitors of this transporter such as the SSRIs (Ch. 46) may exaggerate the action of 5-HT in the gut, explaining some of the common side effects of these drugs, which include diarrhoea. Back-up transporters have also been identified. Interestingly, there is evidence for genetic defects in this reuptake system in irritable bowel syndrome (Ch. 29), which might explain the rather bewildering symptoms of the disease.
Smooth muscle
In many species (although only to a minor extent in humans), smooth muscle (e.g. uterus and bronchial tree) is contracted by 5-HT.
Blood vessels
The effect of 5-HT on blood vessels depends on various factors, including the size of the vessel, the species and the prevailing sympathetic activity. Large vessels, both arteries and veins, are usually constricted by 5-HT, although the sensitivity varies greatly. This is a direct action on vascular smooth muscle cells, mediated through 5-HT2A receptors (see below). Activation of 5-HT1 receptors causes constriction of large intracranial vessels, dilatation of which contributes to headache (see below). 5-HT can also cause vasodilatation, partly by acting on endothelial cells to release nitric oxide (see Ch. 20) and partly by inhibiting noradrenaline release from sympathetic nerve terminals. If 5-HT is injected intravenously, the blood pressure usually first rises, owing to the constriction of large vessels, and then falls, owing to arteriolar dilatation. 5-HT may play a role in the pathology of pulmonary hypertension.
Platelets
5-HT causes platelet aggregation (see Ch. 24) by acting on 5-HT2A receptors, and the platelets that collect in the vessel release further 5-HT. If the endothelium is intact, 5-HT release from adherent platelets causes vasodilatation, which helps to sustain blood flow; if it is damaged (e.g. by atherosclerosis), 5-HT causes constriction and impairs blood flow further. These effects of platelet-derived 5-HT are thought to be important in vascular disease.
Nerve endings
5-HT stimulates nociceptive (pain-mediating) sensory nerve endings, an effect mediated mainly by 5-HT3 receptors. If injected into the skin, 5-HT causes pain; when given systemically, it elicits a variety of autonomic reflexes through stimulation of afferent fibres in the heart and lungs, which further complicate the cardiovascular response. Nettle stings contain 5-HT among other mediators. 5-HT also inhibits transmitter release from adrenergic neurons in the periphery.
Central nervous system
5-HT excites some neurons and inhibits others; it may also act presynaptically to inhibit transmitter release from nerve terminals. Different receptor subtypes and different membrane mechanisms mediate these effects. The role of 5-HT in the CNS is discussed in Chapter 38.
Actions and functions of 5-hydroxytryptamine ![]()
Classification of 5-HT Receptors
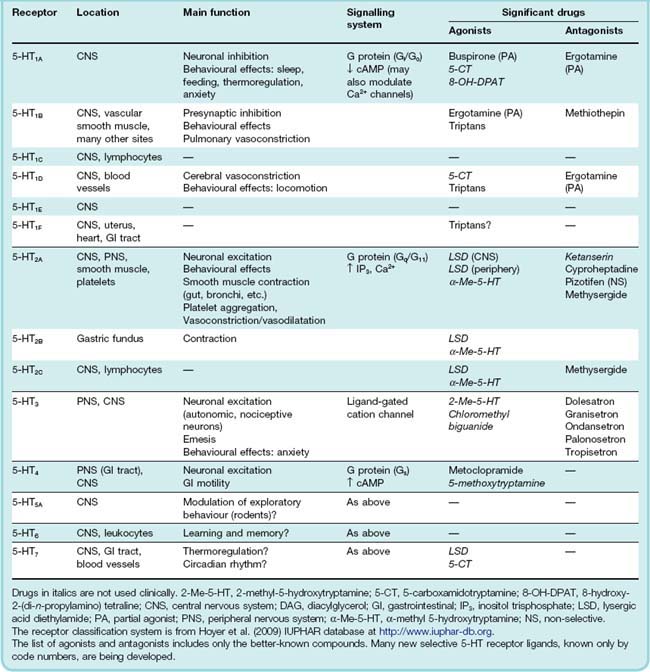
 It was long ago realised that the actions of 5-HT are not all mediated by receptors of the same type, and various pharmacological classifications have come and gone. The current system is summarised in Table 15.1. This classification takes into account sequence data derived from cloning, signal transduction mechanisms and pharmacological specificity as well as the phenotypes of 5-HT receptor ‘knockout’ mice.
It was long ago realised that the actions of 5-HT are not all mediated by receptors of the same type, and various pharmacological classifications have come and gone. The current system is summarised in Table 15.1. This classification takes into account sequence data derived from cloning, signal transduction mechanisms and pharmacological specificity as well as the phenotypes of 5-HT receptor ‘knockout’ mice.
Their diversity is astonishing. Currently, there are some 14 known receptor subtypes (together with an extra gene in mouse). These are divided into seven classes (5-HT1–7), one of which (5-HT3) is a ligand-gated cation channel while the remainder are G-protein-coupled receptors (GPCRs; see Ch. 3). The six GPCR families are further subdivided into some 13 receptor types based on their sequence and pharmacology. Most subtypes are found in all species so far examined, but there are some exceptions (5-HT5B gene is found in mouse but has not been found in humans). The sequences of 5-HT1 and 5-HT2 receptors are highly conserved among species but the 5-HT4–7 receptors are less conserved and are grouped into these families largely on pharmacological grounds.
The most common signalling system appears to be cAMP dependent, but some members (the 5-HT2 subtype) activate phospholipase C to generate phospholipid-derived second messengers (see Ch. 3).
It is not just the sheer numbers of 5-HT receptor genes that is perplexing. Many isoforms have been found, giving rise to four or more variants of some of these receptors. Coupled with this are the polymorphisms which have been reported and which probably contribute to signalling abnormalities found in some types of disease.
With the possible exception of the 5-HT3 family, which are structurally distinct ligand-gated ion channels, the 5-HT receptors are highly promiscuous in their relationships with agonists and antagonists. This makes the pharmacology difficult to interpret in many cases and very difficult to summarise in a meaningful way.
Many transgenic mice lacking functional members of this receptor family have been produced (see for example Bonasera & Tecott, 2000). The functional deficits in such animals are generally quite subtle, suggesting that these receptors may serve to tune, rather than to enable, physiological responses. Table 15.1 gives an overview of the most important receptors. Some of the more significant drug targets include the following.
5-HT1 receptors. Those of pharmacological significance occur mainly in the brain, the subtypes being distinguished on the basis of their regional distribution and their pharmacological specificity. They function mainly as inhibitory presynaptic receptors. The 5-HT1A subtype is particularly important in the brain, in relation to mood and behaviour (see Chs 43, 45, 46) and 5-HT1 ‘knockout’ mice exhibit defects in sleep regulation, learning ability and other CNS functions. Receptor polymorphisms may be associated with increased susceptibility to substance abuse. The 5-HT1B and 5-HT1D subtypes, which are expressed in cerebral blood vessels, are believed to be important in migraine (see below) and are the target for sumatriptan and other triptans, an important group of drugs used to treat acute attacks. Unfortunately, the 5-HT1B receptor is also present in the vasculature of the heart and elsewhere, explaining some of the unwanted effects associated with triptan therapy. The hapless ‘5-HT1C’ receptor—actually the first to be cloned—has been officially declared non-existent, having been ignominiously reclassified as the 5-HT2C receptor when it was found to be linked to inositol trisphosphate production rather than adenylyl cyclase.
5-HT2 receptors. These are present in the CNS but are also particularly important in the periphery. The effects of 5-HT on smooth muscle and platelets, which have been known for many years, are mediated by the 5-HT2A receptor, as are some of the behavioural effects of agents such as lysergic acid diethylamide (LSD; see Table 15.1 and Ch. 47). 5-HT2 receptors are linked to phospholipase C and thus stimulate inositol trisphosphate formation. The 5-HT2A subtype is functionally the most important, the others having a much more limited distribution and functional role. The role of 5-HT2 receptors in normal physiological processes is probably a minor one, but it becomes more prominent in pathological conditions such as asthma and vascular thrombosis (see Chs 24 and 27). Mice lacking the 5-HT2 receptors exhibit defects in colonic motility (5-HT2A), heart defects (5-HT2B) and CNS disorders (5-HT2C).
5-HT3 receptors. 5-HT3 receptors are exceptional in being membrane ion channels (Ch. 3) and cause excitation directly, without involvement of any second messenger. The receptor itself consists of a pentameric assembly of distinct subunits which are designated by further subscript letters (e.g. 5-HT3A–E in humans). 5-HT3 receptors occur mainly in the peripheral nervous system, particularly on nociceptive sensory neurons (see Ch. 41) and on autonomic and enteric neurons, where 5-HT exerts a strong excitatory effect. 5-HT itself evokes pain when injected locally; when given intravenously, it elicits a fine display of autonomic reflexes, which result from excitation of many types of vascular, pulmonary and cardiac sensory nerve fibres. 5-HT3 receptors also occur in the brain, particularly in the area postrema, a region of the medulla involved in the vomiting reflex, and selective 5-HT3 antagonists are used as antiemetic drugs (see Ch. 29). Polymorphisms in the subunits are associated with increased susceptibility to nausea and emesis.
5-HT4 receptors. These occur in the brain, as well as in peripheral organs such as the gastrointestinal tract, bladder and heart. Their main physiological role appears to be in the gastrointestinal tract, where they produce neuronal excitation and mediate the effect of 5-HT in stimulating peristalsis. Mice deficient in the 5-HT4 receptor show a complex phenotype including abnormal feeding behaviour in response to stress.
5-HT5, 5-HT6 and 5-HT7 receptors. Little is known about these receptors. All are present in the CNS as well as other tissues. There are two genes for 5-HT5 isoforms but only one codes for a functional receptor in humans although both may be functional in rodents. At the time of writing, no drugs (other than experimental compounds) are known to act through these receptors although a recent report of selective antagonists at the 5-HT7 receptor may open the way for a detailed examination of the role of this receptor in CNS pathology (Agosti, 2007).
5-Hydroxytryptamine receptors ![]()
Drugs Acting on 5-HT Receptors
Table 15.1 lists some clinically significant agonists and antagonists for the different receptor types. Many are only partly selective. The improved understanding of the location and function of the different receptor subtypes has, however, caused an upsurge of interest in developing compounds with improved receptor selectivity, and useful new drugs are likely to appear in the near future.
Important drugs that act on 5-HT receptors in the periphery include the following:
5-HT is also important as a neurotransmitter in the CNS, and several important antipsychotic and antidepressant drugs owe their actions to effects on these pathways (see Chs 38, 45 and 46). LSD is a relatively non-selective 5-HT receptor agonist or partial agonist, which acts centrally as a potent hallucinogen (see Ch. 47).
Ergot Alkaloids
Ergot alkaloids constitute a hard-to-classify group of drugs that have preoccupied pharmacologists for more than a century. Many of them act on 5-HT receptors, but not selectively, and their actions are complex and diverse.
 Ergot contains many active substances, and it was the study of their pharmacological properties that led Dale to many important discoveries concerning acetylcholine, histamine and catecholamines. Ergot alkaloids occur naturally in a fungus (Claviceps purpurea) that infests cereal crops. Epidemics of ergot poisoning have occurred, and still occur, when contaminated grain is used for food. The symptoms produced include mental disturbances and intensely painful peripheral vasoconstriction leading to gangrene. This came to be known in the Middle Ages as St Anthony’s fire, because it was believed that it could be cured by a visit to the Shrine of St Anthony (which happened to be in an ergot-free region of France).
Ergot contains many active substances, and it was the study of their pharmacological properties that led Dale to many important discoveries concerning acetylcholine, histamine and catecholamines. Ergot alkaloids occur naturally in a fungus (Claviceps purpurea) that infests cereal crops. Epidemics of ergot poisoning have occurred, and still occur, when contaminated grain is used for food. The symptoms produced include mental disturbances and intensely painful peripheral vasoconstriction leading to gangrene. This came to be known in the Middle Ages as St Anthony’s fire, because it was believed that it could be cured by a visit to the Shrine of St Anthony (which happened to be in an ergot-free region of France).
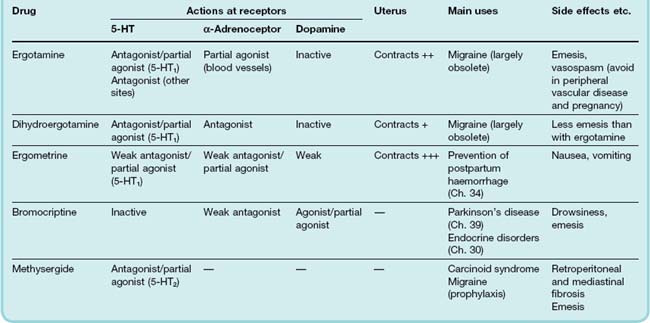
Ergot alkaloids are complex molecules based on lysergic acid (a naturally occurring tetracyclic compound). The important members of the group (Table 15.2) include various naturally occurring and synthetic derivatives with different substituent groups arranged around a basic nucleus. These compounds display many different types of pharmacological action, and it is difficult to discern any clear relationship between chemical structure and pharmacological properties.
Ergot alkaloids ![]()
Actions
Most of the effects of ergot alkaloids appear to be mediated through adrenoceptors, 5-HT or dopamine receptors, although some effects may be produced through other mechanisms. All alkaloids stimulate smooth muscle, some being relatively selective for vascular smooth muscle while others act mainly on the uterus. Ergotamine and dihydroergotamine are, respectively, a partial agonist and an antagonist at α-adrenoceptors. Bromocriptine is an agonist on dopamine receptors, particularly in the CNS (Ch. 38), and methysergide is an antagonist at 5-HT2A receptors.
The main pharmacological actions and uses of these drugs are summarised in Table 15.2. As one would expect of drugs with so many actions, their physiological effects are complex and rather poorly understood. Ergotamine, dihydroergotamine and methysergide are discussed here; further information on ergometrine and bromocriptine is given in Chapters 32, 34 and 39.
Vascular effects
When injected into an anaesthetised animal, ergotamine activates α-adrenoceptors, causing vasoconstriction and a sustained rise in blood pressure. At the same time, ergotamine reverses the pressor effect of adrenaline (epinephrine; see Ch. 14). The vasoconstrictor effect of ergotamine is responsible for the peripheral gangrene of St Anthony’s Fire, and probably also for some of the effects of ergot on the CNS. Methysergide and dihydroergotamine have much less vasoconstrictor effect. Methysergide is a potent 5-HT2A receptor antagonist, whereas ergotamine and dihydroergotamine act selectively on 5-HT1 receptors. Although generally classified as antagonists, they show partial agonist activity in some tissues, and this may account for their activity in treating migraine attacks (see below).
Clinical use
The only use of ergotamine is in the treatment of attacks of migraine unresponsive to simple analgesics (see below). Methysergide is occasionally used for migraine prophylaxis, but its main use is in treating the symptoms of carcinoid tumours (see below). All these drugs can be used orally or by injection.
Unwanted effects
Ergotamine often causes nausea and vomiting, and it must be avoided in patients with peripheral vascular disease because of its vasoconstrictor action. Methysergide also causes nausea and vomiting, but its most serious side effect, which considerably restricts its clinical usefulness, is retroperitoneal and mediastinal fibrosis, which impairs the functioning of the gastrointestinal tract, kidneys, heart and lungs. The mechanism of this is unknown, but it is noteworthy that similar fibrotic reactions also occur in carcinoid syndrome (see below), in which there is a high circulating level of 5-HT.
Migraine and Other Clinical Conditions in Which 5-HT Plays a Role
In this section, we discuss three situations in which the peripheral actions of 5-HT are believed to be important, namely migraine, carcinoid syndrome and pulmonary hypertension. The use of 5-HT3 antagonists for treating drug-induced emesis is discussed in Chapter 29. Modulation of 5-HT-mediated transmission in the CNS is an important mechanism of action of antidepressant and antipsychotic drugs (see Chs 38, 43 and 46).
Migraine and Antimigraine Drugs
Migraine1 is a common and debilitating condition affecting 10–15% of people. Although the causes are not well understood, both genetic and environmental factors seem to be important. A ‘textbook’ migraine attack consists of an initial visual disturbance (the aura), in which a flickering pattern, followed by a blind spot (a ‘scintillating scotoma’), progresses gradually across an area of the visual field. This visual disturbance is followed, about 30 minutes later, by a severe throbbing headache, starting unilaterally, often accompanied by photophobia, nausea, vomiting and prostration, which lasts for several hours. In fact, the visual aura occurs only in about 20% of migraine sufferers, although many experience other kinds of premonitory sensation. Sometimes attacks are precipitated by particular foods or by visual stimuli, but more often they occur without obvious cause. In women, migraine may be linked to the menstrual cycle or other reproductive events. It appears that rapidly falling oestrogen levels can precipitate attacks in susceptible subjects.
Pathophysiology
Although controversy abounds and opinions vary, there are three fundamental views of the physiological mechanisms underlying migraine, linking it to primary events in blood vessels, the brain or sensory nerves. The history of these ideas has been reviewed by Eadie (2005).
The classic ‘vascular’ theory, first proposed around 50 years ago by Wolff, implicated an initial humorally-mediated intracerebral vasoconstriction causing the aura, followed by an extracerebral vasodilatation causing the headache. This venerable hypothesis has not, however, been generally supported by more recent blood flow studies involving non-invasive monitoring techniques in patients with migraine (see review by Friberg, 1999). In episodes of migraine with aura, there is indeed a biphasic change in cerebral blood flow (Fig. 15.2), with a reduction of 20–30% preceding the premonitory aura, followed by a highly variable increase of similar magnitude. However, the headache usually begins during the initial vasoconstrictor phase, and blood flow changes of similar magnitude caused by other factors do not produce symptoms. The vasoconstriction starts posteriorly and gradually spreads forwards over the hemisphere, implying a neural rather than a humoral cause. These changes occur only in association with an aura and do not occur in the remaining 80% of migraine sufferers. No consistent blood flow changes are associated with the headache phase itself.
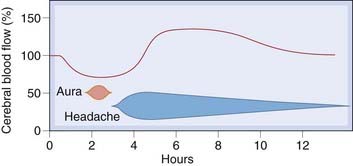
Fig. 15.2 Cerebral blood flow changes during migraine.
(After Olesen et al. 1990 Ann Neurol 28: 791-798.)
The headache originates not in the brain itself, but in extracerebral structures lying within the cranial cavity innervated by nociceptive sensory nerve fibres of the trigeminal pathway, such as the meninges and large arteries. The vascular theory attributes the headache to dilatation in these large arteries. While some studies have shown a unilateral widening of the middle cerebral artery on the same side as the headache sensation, others have shown no clear change. Overall, the evidence for arterial dilatation as a cause of the headache is inconclusive (see Thomsen, 1997).
The ‘brain’ hypothesis (see Lauritzen, 1987) links migraine to the phenomenon of cortical spreading depression. This is a dramatic although poorly understood phenomenon, triggered in experimental animals by local application of K+ to the cortex and also thought to occur in humans after (for example) concussion. This causes an advancing wave of profound neural inhibition, which progresses slowly over the cortical surface at a rate of about 2 mm/min. In the depressed area, the ionic balance is grossly disturbed, with an extremely high extracellular K+ concentration, and the blood flow is reduced. There is strong evidence to suggest that the aura phase of a migraine attack is associated with a wave of spreading depression, although what initiates it remains obscure. However, spreading depression triggered in animal models does not lead to activation or sensitisation of trigeminal afferents (Ebersberger et al., 2001). It is now believed that the aura is associated with spreading depression, but that this is not a necessary step in the pathogenesis of the migraine attack itself.
The ‘inflammation’ hypothesis (see Waeber & Moskowitz, 2005) proposes that activation of trigeminal nerve terminals in the meninges and extracranial vessels is the primary event in a migraine attack. This would cause pain directly and also induce inflammatory changes through the release of neuropeptides and other inflammatory mediators from the sensory nerve terminals (neurogenic inflammation; see Chs 19 and 41). This theory is supported by experiments showing that one such peptide (calcitonin gene-related peptide; see Ch. 19) is released into the meningeal circulation during a migraine attack and that antagonists of this peptide such as telcagepant (in the final stage of clinical trials) are extremely effective in aborting attacks (Farinelli et al., 2008).
These theories are summarised in Figure 15.3. Many variants of these mechanisms have been proposed, but it is noteworthy that none can explain at the biochemical level what initiates a migraine attack or define the underlying abnormality that predisposes particular individuals to suffer such attacks. In some rare types of familial migraine, inherited mutations affecting calcium channels and Na+-K+-ATPase have been found, suggesting that abnormal membrane function may be responsible, but in most forms of migraine there is no clear genetic cause. Whether one inclines to the view that migraine is a vascular disorder, a type of spontaneous concussion, an inflammatory disease or just a bad headache, there are two important factors that implicate 5-HT in its pathogenesis:
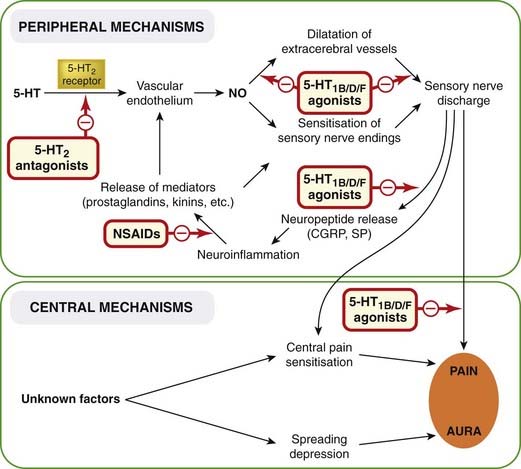
Fig. 15.3 Postulated pathogenesis of migraine.
The initiating event is uncertain but may be an abnormal neuronal discharge set off by emotional or biochemical disturbances. This leads to localised ‘spreading depression’, which causes the aura and may also lead to sensitisation of central pain pathways. In migraine without aura, the primary event is excitation (cause unknown) of nociceptive nerve terminals in the meningeal vessels, leading to the cycle of neurogenic inflammation shown in the upper part of the diagram. 5-HT, 5-hydroxytryptamine; CGRP, calcitonin gene-related peptide; NO, nitric oxide; NSAIDs, non-steroidal anti-inflammatory drugs; SP, substance P.
Drugs used for migraine ![]()
Acute attack
Prophylaxis
Antimigraine Drugs
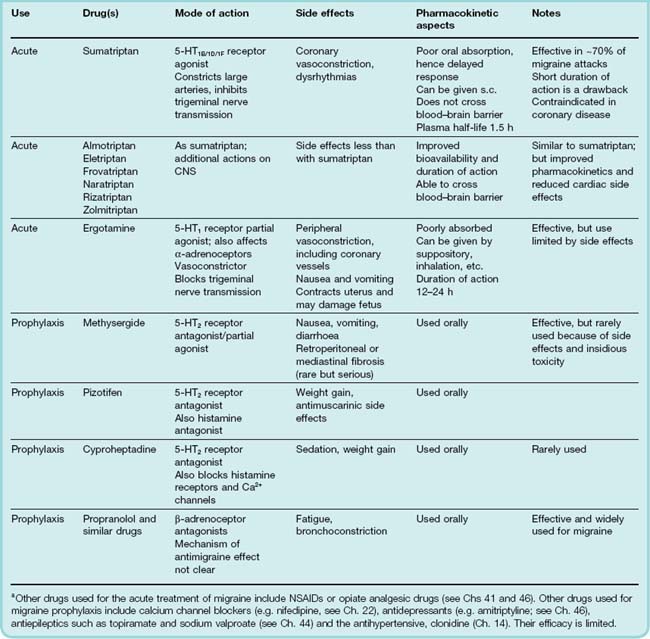
The main drugs currently used to treat migraine are summarised in Table 15.3, and their postulated sites of action are shown in Figure 15.3. It is important to distinguish between drugs used therapeutically to treat acute attacks of migraine (appropriate when the attacks are fairly infrequent) and drugs that are used prophylactically. Apart from 5-HT2 receptor antagonists, the drugs used prophylactically are a mixed bag, and their mechanism of action is poorly understood.
The most important agents for the treatment of acute attacks are currently the triptans. These are 5-HT1 agonists, and are usually classified as 5-HT1B/1D agonists, largely because it is difficult to distinguish between actions at these two receptors. However, selective high-affinity 5-HT1D subtype agonists have proved disappointing in the clinic, arguing against a role for this subtype. Recently, the 5-HT1F receptor has been cloned and has been found to bind sumatriptan with high affinity (Agosti, 2007) suggesting another potential target. This is significant because one of the drawbacks to triptan therapy has always been the vasoconstriction caused in other peripheral vascular beds including the heart. If drugs acting through the 5-HT1F receptor are found to have antimigraine properties, this may prove a useful way forward for drug developers.
Carcinoid Syndrome
Carcinoid syndrome (see Creutzfeld & Stockmann, 1987) is a rare disorder associated with malignant tumours of enterochromaffin cells, which usually arise in the small intestine and metastasise to the liver. These tumours secrete a variety of chemical mediators: 5-HT is the most important, but neuropeptides such as substance P (Ch. 19), and other agents such as prostaglandins and bradykinin (Ch. 17), are also produced. The release of these substances into the bloodstream results in several unpleasant symptoms, including flushing, diarrhoea, bronchoconstriction and hypotension, which may cause dizziness or fainting. Fibrotic stenosis of heart valves, which can result in cardiac failure, also occurs. It is reminiscent of retroperitoneal and mediastinal fibrosis, which are adverse effects of methysergide (see above), and appears to be related to overproduction of 5-HT.
The syndrome is readily diagnosed by measuring the urinary excretion of the main metabolite of 5-HT, 5-HIAA. Excretion in the disease may increase 20-fold and is raised even during periods when the tumour is asymptomatic. 5-HT2 antagonists, such as cyproheptadine, are effective in controlling some of the symptoms of carcinoid syndrome. A complementary therapeutic approach is to use octreotide (a long-acting analogue of somatostatin), which suppresses hormone secretion from neuroendocrine, including carcinoid, cells (see Ch. 32).
Pulmonary Hypertension
Pulmonary hypertension (see also Ch. 27) is an extremely serious disease characterised by the progressive remodelling of the pulmonary vascular tree. This leads to an inexorable rise in pulmonary arterial pressure which, if untreated (and treatment is difficult), inevitably leads to right heart failure and death. The role of 5-HT in this pathology was suggested by the fact that at least one form of the condition was precipitated by the use of appetite suppressants (e.g. dexfenfluramine) that were at one time widely prescribed as ‘weight loss’ or ‘slimming’ aids. These drugs apparently blocked SERT and since 5-HT promotes the growth and proliferation of pulmonary arterial smooth muscle cells and also produces a net vasoconstrictor effect in this vascular bed, the hypothesis seemed reasonable.
Since it was first mooted, however, this hypothesis has been overturned in the face of apparently conflicting data, reborn in the light of emerging facts on SERT polymorphisms and undergone several important changes of emphasis. The bottom line is that pulmonary hypertension is still considered to be a disease in which 5-HT plays an important role and which therefore may become a target for novel drug development. The interested reader is referred to MacLean (2007) for an accessible account of the current thinking in this area, and to Chapter 27, where this topic is also covered.
References and Further Reading
Agosti R.M. 5HT1F- and 5HT7-receptor agonists for the treatment of migraines. CNS Neurol. Disord. Drug Targets. 2007;6:235-237. (Describes cutting-edge research in the field of migraine treatment utilising agonists at newly cloned 5-HT receptors)
Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083-1152. (Useful general review focusing on CNS)
Beattie D.T., Smith J.A. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch. Pharmacol.. 2008;377:181-203. (Very comprehensive and up-to-date review dealing with a complex topic. Easy to read)
Bonasera S.J., Tecott L.H. Mouse models of serotonin receptor function: towards a genetic dissection of serotonin systems Pharmacol. Ther. 88:2000:133-142 (Review of studies on transgenic mice lacking 5-HT1 or 5-HT2 receptors; shows how difficult it can be to interpret such experiments)
Branchek T.A., Blackburn T.P. 5-HT6 receptors as emerging targets for drug discovery. Annu. Rev. Pharmacol. Toxicol.. 2000;40:319-334. (Emphasises future therapeutic opportunities)
Farinelli I., Missori S., Martelletti P. Proinflammatory mediators and migraine pathogenesis: moving towards CGRP as a target for a novel therapeutic class. Expert Rev. Neurother.. 2008;8:1347-1354.
Gershon M.D. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004;20(Suppl. 7):3-14.
Kroeze W.K., Kristiansen K., Roth B.L. Molecular biology of serotonin receptors structure and function at the molecular level. Curr. Top. Med. Chem.. 2002;2:507-528.
Spiller R. Serotonergic agents and the irritable bowel syndrome: what goes wrong? Curr. Opin. Pharmacol. 8:2008:709-714 (A very interesting account of the development—and withdrawl—of 5-HT3/4 antagonists in irritable bowel syndrome and a discussion of the role of SERT polymorphisms in the disease. Illustrates the type of problems encountered when trying to develop useful drugs that act at 5-HT receptors)
Migraine and other pathologies
Creutzfeld W., Stockmann F. Carcinoids and carcinoid syndrome. Am. J. Med.. 1987;82(Suppl. 58):4-16.
Eadie M.J. The pathogenesis of migraine—17th to early 20th century understandings. J. Clin. Neurosci.. 2005;12:383-388. (Fascinating account of the historical development of theories of causes of migraine. Good if you are interested in the history of medicine!)
Ebersberger A., Schaible H.-G., Averbeck B., et al. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann. Neurol.. 2001;49:7-13. (Their conclusion is that there is no connection—spreading depression does not produce inflammation or affect sensory neurons)
Edvinsson L., editor. Migraine and headache pathophysiology. London: Martin Dunitz, 1999. (Collected articles summarising current, and often conflicting, views on the mechanism of migraine)
Friberg L. Migraine pathophysiology, its relation to cerebral haemodynamic changes. In: Edvinsson L., editor. Migraine and headache pathophysiology. London: Martin Dunitz, 1999. (Useful summary of findings in a controversial area)
Goadsby P.J. Can we develop neurally acting drugs for the treatment of migraine? Nat. Rev. Drug. Discov.. 2005;4:741-750. (Useful review of the causes and treatments of migraine)
Lauritzen M. Cerebral blood flow in migraine and cortical spreading depression Acta. Neurol. Scand. Suppl. 113:1987:140 (Review of clinical measurements of cerebral blood flow in migraine, which overturn earlier hypotheses)
MacLean M.R. Pulmonary hypertension and the serotonin hypothesis: where are we now? Int. J. Clin. Pract. Suppl.:2007:27-31 (Easy-to-read account of current thinking on the role of 5-HT receptors in pulmonary hypertension by a leading researcher in the field)
Thomsen L.L. Investigations into the role of nitric oxide and the large intracranial arteries in migraine headache. Cephalalgia. 1997;17:873-895. (Revisits the old vascular theory of migraine in the light of recent advances in the nitric oxide field)
Villalon C.M., Centurion D., Valdivia L.F., et al. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr. Vasc. Pharmacol.. 2003;1:71-84.
Waeber C., Moskowitz M.A. Migraine as an inflammatory disorder. Neurology. 2005;64:S9-S15. (Useful review of the ‘inflammation’ hypothesis of migraine)
Green A.R., editor. Neuropharmacology of serotonin. Oxford: Oxford University Press, 1985. (Useful—if dated—compilation of articles on 5-HT pharmacology)
Sjoerdsma A.G. Starting with serotonin: how a high-rolling father of drug discovery repeatedly beat the odds. Silver Spring MD: Improbable Books; 2008.
1The word is apparently of French origin and is probably a corruption of hemicrania, the Latin name for the disease.