36 Chemical transmission and drug action in the central nervous system
Overview
Brain function is the single most important aspect of physiology that defines the difference between humans and other species. Disorders of brain function, whether primary or secondary to malfunction of other systems, are a major concern of human society, and a field in which pharmacological intervention plays a key role. In this chapter, we introduce some basic principles of neuropharmacology that underlie much of the material in the rest of this section.
Introduction
There are two reasons why understanding the action of drugs on the central nervous system (CNS) presents a particularly challenging problem. The first is that centrally acting drugs are of special significance to humankind. Not only are they of major therapeutic importance,1 but they are also the drugs that humans most commonly administer to themselves for non-medical reasons (e.g. alcohol, tea and coffee, cannabis, nicotine, opioids, amphetamines and so on). The second reason is that the CNS is functionally far more complex than any other system in the body, and this makes the understanding of drug effects very much more difficult. The relationship between the behaviour of individual cells and that of the organ as a whole is far less direct in the brain than in other organs. Currently, the links between a drug’s action at the biochemical and cellular level and its effects on brain function remain largely mysterious. Functional brain imaging is beginning to reveal relationships between brain activity in specific regions and mental function, and this tool is being used increasingly to probe drug effects. Nevertheless, the fairly gross (millimetre scale) resolution currently achievable with imaging methods is far from being able to reveal events at the level of individual neurons and synapses. Despite sustained progress in understanding the cellular and biochemical effects produced by centrally acting drugs, and the increasing use of brain imaging to study brain function and drug effects, the gulf between our understanding of drug action at the cellular level and at the functional and behavioural level remains, for the most part, very wide.
In some instances, our understanding of brain function and how drugs alter it is more advanced. Thus, the relationship between dopaminergic pathways in the extrapyramidal system and the effects of drugs in alleviating or exacerbating the symptoms of Parkinson’s disease (see Ch. 39) is clear cut. Many CNS drugs are used to treat psychiatric disorders that are defined according to their symptomatology rather than on the basis of causative factors or clinical signs and investigations. What is called ‘schizophrenia’ or ‘depression’ on the basis of particular symptoms is likely to consist of several distinct disorders caused by different mechanisms and responding to drugs in different ways. Much effort is going into pinning down the biological basis of psychiatric disorders—a necessary step to improve the design of better drugs for clinical use—but the task is daunting and progress is slow.
In this chapter, we outline the general principles governing the action of drugs on the CNS. Most neuroactive drugs work by interfering with the chemical signals that underlie brain function, and the next two chapters discuss the major CNS transmitter systems and the ways in which drugs affect them. In Chapter 39, we focus on neurodegenerative diseases, and the remaining chapters in this section deal with the main classes of neuroactive drugs that are currently in use.
Background information will be found in neurobiology textbooks such as Kandel et al. (2000) and Bear et al. (2006), and in texts on neuropharmacology such as Nestler et al. (2008) and Iversen et al. (2009).
Chemical Signalling in the Nervous System
The brain (like every other organ in the body!) is basically a chemical machine; it controls the main functions of a higher animal across timescales ranging from milliseconds (e.g. returning a 100 mph tennis serve) to years (e.g. remembering how to ride a bicycle).2 The chemical signalling mechanisms cover a correspondingly wide dynamic range, as summarised, in a very general way, in Figure 36.1. Currently, we understand much about drug effects on events at the fast end of the spectrum—synaptic transmission and neuromodulation—but much less about long-term adaptive processes, although it is quite evident that the latter are of great importance for the neurological and psychiatric disorders that are susceptible to drug treatment.
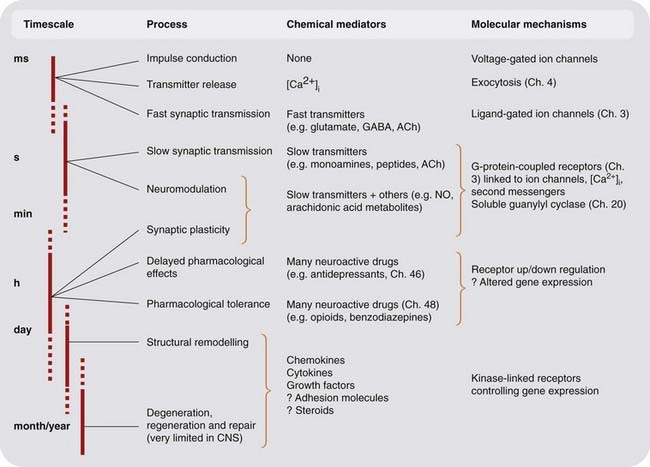
Fig. 36.1 Chemical signalling in the nervous system.
Knowledge of the mediators and mechanisms becomes sparser as we move from the rapid events of synaptic transmission to the slower ones involving remodelling and alterations of gene expression. ACh, acetylcholine; CNS, central nervous system; NO, nitric oxide.
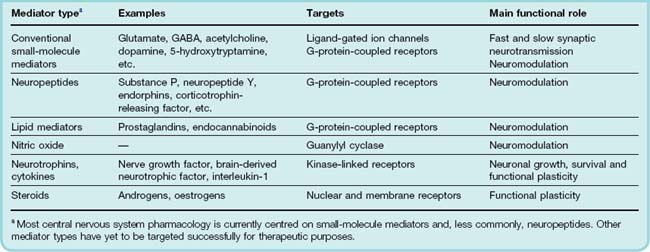
The original concept of neurotransmission envisaged a substance released by one neuron and acting rapidly, briefly and at short range on the membrane of an adjacent (postsynaptic) neuron, causing excitation or inhibition. The principles outlined in Chapter 12 apply to the central as well as the peripheral nervous system. It is now clear that chemical mediators within the brain can produce slow and long-lasting effects; that they can act rather diffusely, at a considerable distance from their site of release; and that they can produce diverse effects, for example on transmitter synthesis, on the expression of neurotransmitter receptors and on neuronal morphology, in addition to affecting the ionic conductance of the postsynaptic cell membrane. The term neuromodulator is often used to denote a mediator, the actions of which do not conform to the original neurotransmitter concept. The term is not clearly defined, and it covers not only the diffusely acting neuropeptide mediators, but also mediators such as nitric oxide (NO) and arachidonic acid metabolites, which are not stored and released like conventional neurotransmitters, and may come from non-neuronal cells, particularly glia, as well as neurons. In general, neuromodulation relates to synaptic plasticity, including short-term physiological events such as the regulation of presynaptic transmitter release or postsynaptic excitability. Longer-term neurotrophic effects are involved in regulating the growth and morphology of neurons, as well as their functional properties. Table 36.1 summarises the types of chemical mediator that operate in the CNS.
Glial cells, particularly astrocytes, which are the main non-neuronal cells in the CNS and outnumber neurons by 10 to 1, also play an important signalling role. Once thought of mainly as housekeeping cells, whose function was merely to look after the fastidious neurons, they are increasingly seen as ‘inexcitable neurons’ with a major communications role (see Barres, 2008), albeit on a slower timescale than that of neuronal communication. These cells express a range of receptors and transporters similar to those present in neurons, and also release a wide variety of mediators, including glutamate, D-serine, lipid mediators and growth factors. They respond to chemical signals from neurons, and also from neighbouring astrocytes and microglial cells (the CNS equivalent of macrophages, which function much like inflammatory cells in peripheral tissues). Electrical coupling between astrocytes causes them often to respond in concert in a particular brain region, thus controlling the chemical environment in which the neurons operate. Although they do not conduct action potentials, and do not send signals to other parts of the body, astrocytes are otherwise very similar to neurons and play a crucial communication role within the brain. Because they are difficult to study in situ, however, our knowledge of how they function, and how they respond to drugs, is still fragmentary. It is an area to watch closely.
Chemical transmission in the central nervous system ![]()
Targets for Drug Action
 To recapitulate what was discussed in Chapters 2 and 3, neuroactive drugs act on one of four types of target proteins, namely ion channels, receptors, enzymes and transport proteins. Of the four main receptor families—ionotropic receptors, G-protein-coupled receptors, kinase-linked receptors and nuclear receptors—current drugs target mainly the first two.
To recapitulate what was discussed in Chapters 2 and 3, neuroactive drugs act on one of four types of target proteins, namely ion channels, receptors, enzymes and transport proteins. Of the four main receptor families—ionotropic receptors, G-protein-coupled receptors, kinase-linked receptors and nuclear receptors—current drugs target mainly the first two.
In the last two or three decades, knowledge about these targets in the CNS has accumulated rapidly, particularly as follows.
Drug Action in the Central Nervous System
As we have already emphasised, the molecular and cellular mechanisms underlying drug action in the CNS and in the periphery are essentially similar. Understanding how drugs affect brain function is, however, made difficult by several factors. One is the complexity of neuronal interconnections in the brain—the wiring diagram. Figure 36.2 illustrates in a schematic way the kind of interconnections that typically exist for, say, a noradrenergic neuron in the locus coeruleus (see Ch. 38), shown as neuron 1 in the diagram, releasing transmitter a at its terminals. Release of a affects neuron 2 (which releases transmitter b), and also affects neuron 1 by direct feedback and, indirectly, by affecting presynaptic inputs impinging on neuron 1. The firing pattern of neuron 2 also affects the system, partly through interneuronal connections (neuron 3, releasing transmitter c). Even at this grossly oversimplified level, the effects on the system of blocking or enhancing the release or actions of one or other of the transmitters are difficult to predict, and will depend greatly on the relative strength of the various excitatory and inhibitory synaptic connections, and on external inputs (x and y in the diagram). Added to this complexity at the level of neuronal interconnections is the influence of glial cells, mentioned above.
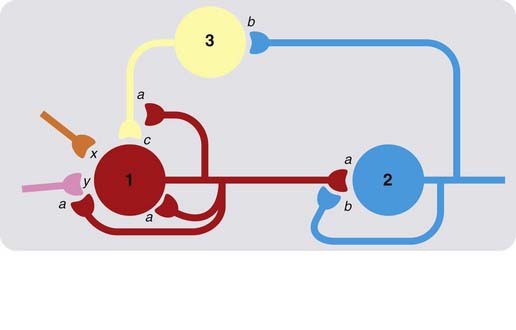
Fig. 36.2 Simplified scheme of neuronal interconnections in the central nervous system.
Neurons 1, 2 and 3 are shown releasing transmitters a, b and c, respectively, which may be excitatory or inhibitory. Boutons of neuron 1 terminate on neuron 2, but also on neuron 1 itself, and on presynaptic terminals of other neurons that make synaptic connections with neuron 1. Neuron 2 also feeds back on neuron 1 via interneuron 3. Transmitters (x and y) released by other neurons are also shown impinging on neuron 1. Even with such a simple network, the effects of drug-induced interference with specific transmitter systems can be difficult to predict.
A further important complicating factor is that a range of secondary, adaptive responses is generally set in train by any drug-induced perturbation of the system. Typically, an increase in transmitter release, or interference with transmitter reuptake, is countered by inhibition of transmitter synthesis, enhanced transporter expression or decreased receptor expression. These changes, which involve altered gene expression, generally take time (hours, days or weeks) to develop and are not evident in acute pharmacological experiments.
In the clinical situation, the effects of psychotropic drugs often take weeks to develop, so it is likely that they reflect the adaptive responses rather than the immediate pharmacodynamic effects of the drug. This is well documented for antidepressant drugs (Ch. 46) and some antipsychotic drugs (Ch. 45). The development of dependence on drugs such as opioids, benzodiazepines and psychostimulants is similarly gradual (Ch. 48). Thus, one has to take into account not only the primary interaction of the drug with its target, but also the secondary response of the brain to this primary effect; it is often the secondary response, rather than the primary effect, which leads to clinical benefit.
Blood–Brain Barrier
 A further important factor in CNS pharmacology is the existence of the blood–brain barrier (see Ch. 8), penetration of which requires molecules to traverse the vascular endothelial cells rather than going between them. In general, only small non-polar molecules can diffuse passively across cell membranes. Some neuroactive drugs penetrate the blood–brain barrier in this way, but many do so via transporters, which either facilitate entry into the brain or diminish it by pumping the compound from the endothelial cell interior back into the bloodstream. Drugs that gain entry in this way include L-dopa (Ch. 39), valproate (Ch. 44) and various sedative histamine antagonists (Ch. 17). Active extrusion of drugs from the brain occurs via P-glycoprotein, an ATP-driven drug efflux transporter, and related transporter proteins (see Ch. 8). Drugs that are excluded from the brain include many antibacterial and anticancer drugs while the brain concentrations of some CNS-acting drugs—including certain opioid, antidepressant, antipsychotic and antiepileptic drugs—may be limited by active extrusion from the brain see (Linnet & Ejsing, 2008). In addition, variations in the activity of efflux transporters between individuals due to levels of expression or genetic variations and the potential for drug interactions at the level of these transporters due to inhibition or induction of activity by other drugs is becoming an important consideration.
A further important factor in CNS pharmacology is the existence of the blood–brain barrier (see Ch. 8), penetration of which requires molecules to traverse the vascular endothelial cells rather than going between them. In general, only small non-polar molecules can diffuse passively across cell membranes. Some neuroactive drugs penetrate the blood–brain barrier in this way, but many do so via transporters, which either facilitate entry into the brain or diminish it by pumping the compound from the endothelial cell interior back into the bloodstream. Drugs that gain entry in this way include L-dopa (Ch. 39), valproate (Ch. 44) and various sedative histamine antagonists (Ch. 17). Active extrusion of drugs from the brain occurs via P-glycoprotein, an ATP-driven drug efflux transporter, and related transporter proteins (see Ch. 8). Drugs that are excluded from the brain include many antibacterial and anticancer drugs while the brain concentrations of some CNS-acting drugs—including certain opioid, antidepressant, antipsychotic and antiepileptic drugs—may be limited by active extrusion from the brain see (Linnet & Ejsing, 2008). In addition, variations in the activity of efflux transporters between individuals due to levels of expression or genetic variations and the potential for drug interactions at the level of these transporters due to inhibition or induction of activity by other drugs is becoming an important consideration.
Drug action in the central nervous system ![]()
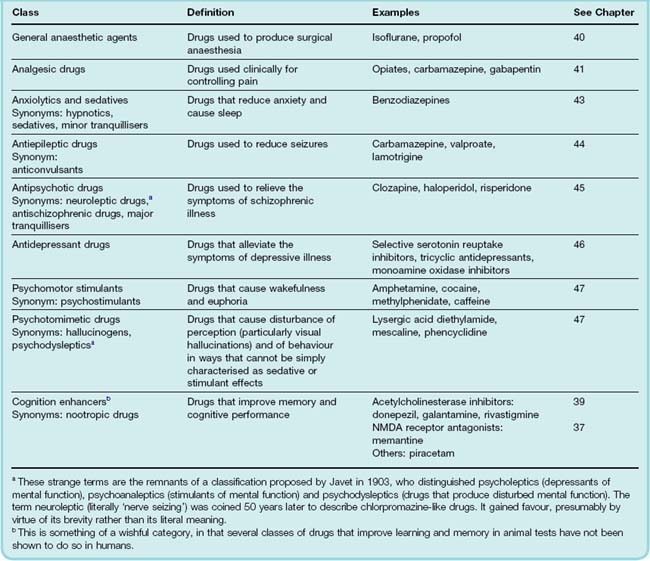
The Classification of Psychotropic Drugs
Psychotropic drugs are defined as those that affect mood and behaviour. Because these indices of brain function are difficult to define and measure, there is no consistent basis for classifying psychotropic drugs. Instead, we find a confusing mêlée of terms relating to chemical structure (benzodiazepines, butyrophenones, etc.), biochemical target (monoamine oxidase inhibitors, serotonin reuptake inhibitors, etc.), behavioural effect (hallucinogens, psychomotor stimulants) or clinical use (antidepressants, antipsychotic agents, antiepileptic drugs, etc.), together with a number of indefinable rogue categories (atypical antipsychotic drugs, nootropic drugs) thrown in for good measure.
However, grumbling about terminology is fruitless. The general classification in Table 36.2 is based on that suggested in 1967 by the World Health Organization; although flawed, it provides a basis for the material presented later (Chs 37–48).
Also, some drugs defy classification in this scheme, for example lithium (see Ch. 46), which is used in the treatment of manic depressive psychosis, and ketamine (see Ch. 40), which is classed as a dissociative anaesthetic but produces psychotropic effects rather similar to those produced by phencyclidine.
In practice, the use of drugs in psychiatric illness frequently cuts across specific therapeutic categories. For example, it is common for antipsychotic drugs to be used as ‘tranquillisers’ to control extremely anxious or unruly patients, or to treat severe depression. Antidepressant drugs are often used to treat anxiety (Ch. 43) and neuropathic pain (Ch. 41), and certain psychostimulants are of proven efficacy for treating hyperactive children. Here we will adhere to the conventional pharmacological categories, but it needs to be emphasised that in clinical use these distinctions are often disregarded.
References and Further Reading
Barañano D.E., Ferris C.D., Snyder S.H. Atypical neural messengers Trends Neurosci. 24:2001:99-106 (Short review on some established mediators, such as nitric oxide, and some speculative ones, such as carbon monoxide and D-serine)
Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430-440. (Extensive review of the role of glial cells in brain development, function and disease)
Bear M.F., Connors B.W., Paradiso M.A. Neuroscience: exploring the brain, third ed. Baltimore: Lippincott, Williams & Wilkins; 2006. (Comprehensive textbook on neuroscience)
Iversen L.L., Iversen S.D., Bloom F.E., Roth R.H. Introduction to neuropsychopharmacology. New York: Oxford University Press; 2009. (Excellent and readable account focusing on basic rather than clinical aspects)
Kandel E., Schwartz J.H., Jessell T.M. Principles of neural science, fourth ed. New York: Elsevier; 2000. (Excellent and detailed standard text on neurobiology—little emphasis on pharmacology)
Linnet K., Ejsing T.B. A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur. Neuropsychopharmacol. 2008;18:157-169. (Review of how P-glycoprotein can limit the brain concentration of antidepressant and antipsychotic drugs)
Nestler E.J., Hyman S.E., Malenka R.C. Molecular neuropharmacology, second ed. New York: McGraw-Hill; 2008. (Good modern textbook)
1In Britain in 2008/2009, 145 million prescriptions (about 20% of all prescriptions), costing £1.7 billion, were for CNS drugs as defined by the British National Formulary. This amounted to over two per person across the whole population.
2Memory of drug names and the basic facts of pharmacology seems to come somewhere in the middle of this range (skewed towards the short end).