The Child with Musculoskeletal or Articular Dysfunction
On completion of this chapter the reader will be able to:
 Outline a care plan for a child immobilized with an injury or a degenerative disease.
Outline a care plan for a child immobilized with an injury or a degenerative disease.
 Develop a teaching plan for the parents of a child in a cast.
Develop a teaching plan for the parents of a child in a cast.
 Explain the functions of the various types of traction.
Explain the functions of the various types of traction.
 Devise a nursing care plan for a child in traction.
Devise a nursing care plan for a child in traction.
 Differentiate among the various congenital skeletal defects.
Differentiate among the various congenital skeletal defects.
 Design a teaching plan for the parents of a child with a congenital skeletal deformity.
Design a teaching plan for the parents of a child with a congenital skeletal deformity.
 Describe the therapies and nursing care of a child with scoliosis.
Describe the therapies and nursing care of a child with scoliosis.
 Outline a care plan for a child with osteomyelitis.
Outline a care plan for a child with osteomyelitis.
 Differentiate between osteosarcoma and Ewing sarcoma.
Differentiate between osteosarcoma and Ewing sarcoma.
 Describe the nursing care of a child with juvenile idiopathic arthritis.
Describe the nursing care of a child with juvenile idiopathic arthritis.
 Demonstrate an understanding of the management of a child with systemic lupus erythematosus.
Demonstrate an understanding of the management of a child with systemic lupus erythematosus.
THE IMMOBILIZED CHILD
One of the most difficult aspects of illness in children is the immobility it imposes. Children by nature are usually active, and immobility, however temporary, may have lasting effects on the child’s developmental progress. The most frequent reasons for immobility are congenital defects (e.g., spina bifida, arthrogryposis [a generalized immobility of the joints]); degenerative disorders (e.g., muscular dystrophy); and infections or injuries that impair the integumentary system (e.g., severe burns), the musculoskeletal system (e.g., multiple fractures, osteomyelitis), or the neurologic system (e.g., spinal cord injury, polyneuritis, head injury). At times therapies such as traction and spinal fusion are responsible for prolonged immobilization, although the increasing trends in health care are early mobilization and discharge and outpatient treatment.
Physiologic Effects of Immobilization
Many clinical studies, including space program research, have documented predictable consequences that occur after immobilization and the absence of gravitational force. Functional and metabolic responses to restricted movement can be noted in most of the body systems. Each has a direct influence on the child’s growth and development because homeostatic mechanisms thrive on normal use and need feedback to maintain dynamic equilibrium. Inactivity leads to a decrease in the functional capabilities of the whole body as dramatically as the lack of physical exercise leads to muscle weakness.
Disuse from illness, injury, or a sedentary lifestyle can limit function and potentially delay age-appropriate milestones. Most of the pathologic changes that occur during immobilization arise from decreased muscle strength and mass, decreased metabolism, and bone demineralization, which are closely interrelated, with one change leading to or affecting the others. Some results of immobilization are primary and produce a direct effect; other pathophysiologic consequences occur frequently but seem to be more indirect and are therefore secondary effects. Many pathophysiologic changes affect more than one body system, with the primary or secondary effect being demonstrated in both systems.
The major effects of immobilization are outlined briefly in Table 31-1 and are related directly or indirectly to decreased muscle activity, which produces numerous primary changes in the musculoskeletal system with secondary alterations in the cardiovascular, respiratory, metabolic, and renal systems. The musculoskeletal changes that occur during disuse are a result of alterations in the effect of gravity and stress on the muscles, joints, and bones. Muscle disuse leads to tissue breakdown and loss of muscle mass (atrophy). Muscle atrophy causes decreased strength and endurance, which may take weeks or months to restore.
During immobilization a joint contracture begins when the arrangement of collagen, the main structural protein of connective tissues, is altered, resulting in a denser tissue that does not glide as easily. Eventually muscles, tendons, and ligaments can shorten and reduce joint movement, ultimately producing contractures that restrict function. The daily stresses on bone created by motion and weight bearing maintain the balance between bone formation (osteoblastic activity) and bone resorption (osteoclastic activity). During immobilization, increased calcium leaves the bone, causing osteopenia (demineralization of the bones), which may predispose bone to pathologic fractures.
The major musculoskeletal consequences of immobilization are:
 Significant decrease in muscle size, strength, and endurance
Significant decrease in muscle size, strength, and endurance
The larger the portion of the body immobilized and the longer the immobilization, the greater the hazards of immobility.
Psychologic Effects of Immobilization
For children, one of the most difficult aspects of illness is immobilization. Throughout childhood, physical activity is an integral part of daily life and is essential for physical growth and development. It also serves children as an instrument for communication and expression and as a means for learning about and understanding their world. Activity helps them deal with a variety of feelings and impulses and provides a mechanism by which they can exert control over inner tensions. Children respond to anxiety with increased activity. Removal of this power deprives them of necessary input and a natural outlet for their feelings and fantasies. Through movement children also gain sensory input, which provides an essential element for developing and maintaining body image.
When children are immobilized by disease or as part of a treatment regimen, they experience diminished environmental stimuli with a loss of tactile input and an altered perception of themselves and their environment. Sudden or gradual immobilization narrows the amount and variety of environmental stimuli children receive by means of all their senses: touch, sight, hearing, taste, smell, and proprioception (a feeling of where they are in their environment). This sensory deprivation frequently leads to feelings of isolation and boredom and of being forgotten, especially by peers (see Family Focus box).
Physical interference with the activity of infants and young children gives them a feeling of helplessness. Even speech and language skills require sensorimotor activity and experience. For the toddler, exploration and imitative behaviors are essential to developing a sense of autonomy. The preschooler’s expression of initiative is evidenced by the need for vigorous physical activity. The school-age child’s development is strongly influenced by physical achievement and competition. And the adolescent relies on mobility to achieve independence. The quest for mastery at every stage of development is related to mobility.
The monotony of immobilization can lead to sluggish intellectual and psychomotor responses, decreased communication skills, increased fantasizing, and even hallucinations and disorientation. Children are likely to become depressed over loss of ability to function or the marked changes in body image. They may seek the attention of others by reverting to earlier developmental behaviors, such as wanting to be fed, bed-wetting, and baby talk.
Limbs in casts or traction transmit less than normal sensory data. Children who have limited ability to feel others touching them not only experience less tactile stimuli in a physical sense, but are also deprived of warm, loving feelings that arise from being touched. The loss of feeling derived from touch can add to their sense of being isolated and unwanted.
Children may react to immobility by active protest, anger, and aggressive behavior, or they may become quiet, passive, and submissive. They may believe the immobilization is a justified punishment for misbehavior. Children should be allowed to discharge their anger, but it should be within the limits of safety to their self-esteem and not damaging to the integrity of others. For example, providing an object to attack rather than a person or a valued possession is safe and therapeutic. When children are unable to express anger, aggression is often displayed inappropriately through regressive behavior and outbursts of crying or temper tantrums.
Effect on Families
Even brief periods of immobilization may disrupt family function, and catastrophic illness or disability may severely tax their resources and coping abilities. The family’s needs often must be met by the services of a multidisciplinary team, and nurses play a key role in anticipating the services they will need and in coordinating conferences to plan care. In preparation for discharge, home visits are advisable, and home management is frequently planned weeks in advance of the actual discharge, including special considerations for cultural, economic, physical, and psychologic needs. A child with a severe disability is very dependent, and caregivers need rest periods to revitalize themselves. Individual and group counseling is beneficial for solving problems in advance and provides an emotional support system. Parent groups are also helpful and often allow nonthreatening social contact. The families of children with permanent disabilities need long-term resources, since some of the most difficult problems arise as they try to sustain high-quality care for many years (see Chapter 20).
Nursing Care Management
Physical assessment of the child who is immobilized for any number of reasons (illness, treatment, protection) includes a focus not only on the injured part (e.g., fracture, surgical repair), but also on the functioning of other systems that may be affected secondarily—the circulatory, renal, respiratory, muscular, and gastrointestinal systems. With long-term immobilization there may also be neurologic impairment and changes in electrolytes (especially calcium), nitrogen balance, and the general metabolic rate. The psychologic impact of immobilization should also be assessed.
Children who require prolonged total immobility and are unable to move themselves in bed should be placed on a special mattress to prevent skin breakdown. Frequent position changes also help prevent dependent edema and stimulate circulation, respiratory function, gastrointestinal motility, and neurologic sensation. Children at greater risk for skin breakdown include those with prolonged immobilization; orthotic and prosthetic devices, including wheelchairs; and plaster casts (Samaniego, 2003). Additional risk factors include poor nutrition, friction (from bed linen with traction), and moist skin (from urine or perspiration). Nursing care of children at risk includes strategies for preventing skin breakdown when such conditions are present. The Modified Braden Q Scale is a reliable, objective tool that may be used in the assessment for pressure ulcer development in children who are acutely ill or who are at risk for skin breakdown from neurologic conditions and immobilization (Curley, Razmus, Roberts, and others, 2003).
The use of antiembolism stockings or inflatable antiembolism devices may minimize or prevent dependent edema in the lower extremities. The child should be allowed as much activity as possible within the limitations of the illness or treatment; any functional mobility, however minimal, is preferred to total immobility. High-protein, high-calorie foods are encouraged to prevent negative nitrogen balance, which may be difficult to correct by diet, especially if there is anorexia as a result of immobility and decreased gastrointestinal function (decreased motility and possibly constipation). Stimulating the appetite with small servings of attractively arranged, preferred foods may be sufficient. Sometimes, supplementary nasogastric or gastrostomy feedings or intravenous (IV) fluids may be needed, but these are reserved for serious disability in which oral intake is impossible.
Adequate hydration and, when possible, an upright position and remobilization promote bowel and kidney function and help prevent complications in these systems. Children are encouraged to be as active as their condition and restrictive devices allow. This poses few problems for children, whose innate ingenuity and natural inclination toward mobility provide them with the impetus for physical activity. They need the opportunity, the materials or objects to stimulate activity, and the encouragement and participation of others. Those who are unable to move benefit from passive exercise and movement, in consultation with a physical therapist.
Whenever possible, transporting the child by stretcher, wheelchair, stroller, or wagon outside the confines of the room increases environmental stimuli and allows social contact with others. Those confined to wheelchairs have specially designed chairs for increased mobility and independence. While hospitalized, children benefit from same-age visitors, computers, books, interactive video games, and other items brought from their own room at home, all of which help them to function in a more normal way. A play therapist or child life specialist should be consulted for recreational planning. An activity center or tray that slants can be particularly helpful for the child with limited mobility to use for drawing, coloring, writing, and playing with small toys such as trucks and cars. Children are able to express frustration, displeasure, and anger through play activities (see Chapter 21), which is helpful in the child’s recovery. Hospitalized children should be allowed to wear their own clothes (street clothes, especially for preadolescent and adolescent girls) and resume school and preinjury activities. A parent or siblings should be allowed to stay overnight and room in with the hospitalized child to prevent the effects of family disruption from hospitalization. All efforts should be made to minimize family disruption resulting from the hospitalization. Although most of the suggestions discussed relate to hospital care, the same consultations (physical therapist, occupational therapist, child life specialist, speech therapist) and environment may be considered in the home as well to help the child and family achieve independence and normalization (see Chapter 20).
Using dolls, stuffed animals, or puppets to illustrate and explain the restraining (traction, cast) method is a valuable tool for small children. Placing a cast, tubing, or other restraining equipment on the doll offers the child a nonthreatening opportunity to express, through the doll, feelings concerning the restrictions and feelings toward the nurse and other health care providers. The doll or puppet may also be used for teaching the child and family procedures such as IV therapy, conscious sedation, and general anesthesia.
Children typically dislike hospital food, which usually is not tailored to their age. In some institutions food services are geared toward children’s preferences with child-friendly menus and smaller food portions served. Parents and friends are allowed to bring in favorite foods from home or other sources, such as fast food places, provided they meet necessary requirements for the illness. This enables children to have more control over their environment and will decrease resistance to treatments and schedules, which is common behavior evidenced when adults and children are not given any choices in an acute care setting.
One of the most useful interventions to help children cope with immobility is participation in their own care. Self-care to the maximum extent is usually well received by children. They can help plan their daily routine; select their diet (when possible); and choose “street clothes,” including innovative adornment, such as a baseball cap or brightly colored stockings, that expresses their autonomy and individuality. They are encouraged to do as much for themselves as they are able to keep muscles active and their interest alive.
Visits from significant persons, such as family members and friends, offer occasions for emotional support and also provide opportunities for learning how to care for the child. Some privacy is needed, particularly by the adolescent.
For a child with greatly restricted movement (e.g., paraplegic or quadriplegic child, child with a large bilateral hip spica cast), nursing care is often a challenge. These situations require long-term care either in the hospital or at home, but wherever the care occurs, consistent planning and coordination of activities with other health care workers and significant others are vital nursing functions.
With the increased trend toward early mobilization, early discharge, and home health care, many children are discharged home within a few days of hospitalization. Follow-up treatment may take place in the home setting or an outpatient ambulatory facility.
Family Support and Home Care.: The needs of a child with severe disabilities can be complex, and family members require time to assimilate the teachings and demonstrations needed to understand the child’s situation and care. Even the child who is confined on a short-term basis can be a challenge for the family, which is usually unprepared for the problems imposed by the child’s special needs. Home modification is usually needed for facilitating care, especially when it involves traction, large casts, or extended confinement. Suitable child care may be needed for times when all family members work.
Just as in the hospital, the child at home is encouraged to be as independent as possible and to follow a schedule that approximates his or her normal lifestyle as nearly as possible, such as continuing school lessons, regular bedtime, and suitable recreational activities.
TRAUMATIC INJURY
Injuries to the muscles, ligaments, and tendons are common in children (Fig. 31-1). In young children, soft-tissue injury usually results from mishaps during play. In older children and adolescents, participation in sports is a common cause of such injuries.
Contusions
A contusion is damage to the soft tissue, subcutaneous structures, and muscle. The tearing of these tissues and small blood vessels and the inflammatory response lead to hemorrhage, edema, and associated pain when the child attempts to move the injured part. The escape of blood into the tissues is observed as ecchymosis, a black-and-blue discoloration.
Large contusions cause gross swelling, pain, and disability; those sustained while the child is participating in sports usually receive immediate attention from health personnel. The less spectacular, smaller injuries may go unnoticed, allowing continued participation. However, they can become disabling after rest because of pain and muscle spasm. The young athlete is frequently instructed to “work it out” or disregard the pain. Instead of this approach, an assessment of the affected area should be first carried out by a qualified health care worker or certified athletic trainer because further damage to the site may result if the area is severely traumatized. Immediate treatment consists of cold application, as in the treatment of sprains described in the following section. Return to participation is allowed when the strength and range of motion of the affected extremity are equal to those of the opposite extremity or are demonstrated under conditions such as sport-specific tests. Myositis ossificans may occur from deep contusions to the biceps or quadriceps muscles; this condition may result in a restriction of flexibility of the affected limb.
Related to contusions are crush injuries that occur when children slam their fingers (in doors, folding chairs, or equipment) or hit their fingers (as when hammering a nail). A severe crush injury involves the bone, with swelling and bleeding beneath the nail (subungual) and sometimes laceration of the pulp of the distal phalanx. The subungual hematoma can be released by creating a hole at the proximal end of the nail with a battery-operated microcautery device or a heated sterile 18-gauge needle.
Dislocations
Long bones are held in approximation to one another at the joint by ligaments. A dislocation occurs when the force of stress on the ligament is so great as to displace the normal position of the opposing bone ends or the bone end to its socket. The predominant symptom is pain that increases with attempted passive or active movement of the extremity. In dislocations there may be an obvious deformity and inability to move the joint. Children with naturally lax joints are more prone to dislocation of joints. Dislocation of the phalanges is the most common type seen in children, followed by elbow dislocation.
A common injury in young children is subluxation, or partial dislocation, of the radial head, also called pulled elbow or nursemaid’s elbow. In the majority of cases the injury occurs in a child younger than 5 years of age who receives a sudden longitudinal pull or traction at the wrist while the arm is fully extended and the forearm pronated. It usually occurs when an adult or older sibling who is holding the child by the hand or wrist gives a sudden pull or jerk to prevent a fall or attempts to lift the child by pulling the wrist, or when the child pulls away by dropping to the floor or ground. The child often cries, appears anxious, and refuses to use the affected limb. The practitioner manipulates the arm by applying firm finger pressure to the head of the radius, then supinates and flexes the forearm to return the bone structure to normal alignment. A click may be heard or felt, and functional use of the arm returns within minutes. However, the longer the subluxation is present, the longer it takes for the child to recover mobility after treatment. No anesthetic is usually required, but a mild pain reliever such as acetaminophen may be given. In an older child, severe elbow injury or dislocation should be carefully evaluated by a practitioner immediately; likewise, a traumatic elbow injury in the younger child that is not a subluxation should be carefully evaluated.
In children younger than 5 years of age, the hip can be dislocated by a fall. The greatest risk after this injury is the potential loss of blood supply to the head of the femur. Relocation of the hip within 60 minutes after the injury provides the best chance for prevention of damage to the femoral head.
Shoulder dislocations occur most often in older adolescents and are often sports related. Temporary restriction of the joint, with a sling or bandage that secures the arm to the chest in a shoulder dislocation, can provide sufficient comfort and immobilization until medical attention is received.
Simple dislocations should be reduced as soon as possible with the child under conscious sedation and often local anesthesia. Also, anesthetics, such as IV ketamine (Ketalar) and midazolam (Versed), IV propofol (Diprivan), or fentanyl (Sublimaze), can be used to produce partial or complete analgesia. An unreduced dislocation will be complicated by increased swelling, making reduction difficult and increasing the risk of neurovascular problems. Treatment depends on the severity of the injury.
Sprains
A sprain occurs when trauma to a joint is so severe that a ligament is partially or completely torn or stretched by the force created as a joint is twisted or wrenched, often accompanied by damage to associated blood vessels, muscles, tendons, and nerves.
The presence of joint laxity is the most valid indicator of the severity of a sprain. In a severe injury the child complains of the joint “feeling loose” or as if “something is coming apart” and may describe hearing a “snap,” “pop,” or “tearing.” Pain may or may not be the principal subjective symptom, and in some children it may prevent optimal examination of ligamentous instability. There is a rapid onset with swelling, often diffuse, accompanied by immediate disability and appreciable reluctance to use the injured joint.
Strains
A strain is a microscopic tear to the musculotendinous unit and has features in common with sprains. The area is painful to touch and swollen. Most strains are incurred over time rather than suddenly, and the rapidity of the appearance provides clues regarding severity. In general, the more rapidly the strain occurs, the more severe the injury. When the strain involves the muscular portion, there is more bleeding, often palpable soon after injury and before edema obscures the hematoma.
Therapeutic Management
The first minutes to 12 hours is the most critical period for virtually all soft-tissue injuries. Basic principles of managing sprains and other soft-tissue injuries are summarized in the acronyms RICE and ICES:
| Rest | Ice |
| Ice | Compression |
| Compression | Elevation |
| Elevation | Support |
Soft-tissue injuries should be iced immediately. This is best accomplished with crushed ice wrapped in a towel, a screw-top ice bag, or a resealable plastic storage bag. Chemical-activated ice packs are also effective for immediate treatment but are not reusable and must be closely monitored for leakage. A wet elastic wrap, which transfers cold better than dry wrap, is applied to provide compression and to keep the ice pack in place. A cloth barrier should be used between the ice container and the skin to prevent trauma to the tissues. Ice has a rapid cooling effect on tissues and reduces the pain threshold. However, ice should never be applied for more than 30 minutes at a time because of the body’s homeostatic response to cold, which may trigger a decrease in vascularization at the injury site.
Elevating the extremity uses gravity to facilitate venous return and reduce edema formation in the damaged area. The point of injury should be kept several inches above the level of the heart for therapy to be effective. Several pillows can be used for elevation. Allowing the extremity to be dependent causes excessive fluid accumulation in the area of injury, delaying healing and causing painful swelling.
Torn ligaments, especially those in the knee, are usually treated by immobilization with a knee immobilizer or range-of-motion brace until the child is able to walk without a limp. Crutches are used for mobility to rest the affected extremity. Passive leg exercises, gradually increased to active ones, are begun as soon as sufficient healing has taken place. Parents and children are cautioned against using any form of liniment or other heat-producing preparation before examination. If the injury requires casting or splinting, the heat generated in the enclosed space can cause extreme discomfort and even tissue damage. In some cases torn knee ligaments are managed with arthroscopy and ligament repair or reconstruction as necessary depending on the extent of the tear, ligaments involved, and child’s age. Surgical reconstruction of the anterior cruciate ligament may be performed in young athletes who wish to continue in active sports (Greene, 2001).
FRACTURES
Bone fractures occur when the resistance of bone against the stress being exerted yields to the stress force. Fractures are a common injury at any age but are more likely to occur in children and older adults. Because childhood is a time of rapid bone growth, the pattern of fractures, problems of diagnosis, and methods of treatment differ in the child and the adult. In children fractures heal much faster than in adults. Consequently, children may not require as long a period of immobilization of the affected extremity as an adult with a fracture.
Fracture injuries in children are most often a result of traumatic incidents at home, at school, in a motor vehicle, or in association with recreational activities. Children’s everyday activities include vigorous play that predisposes them to injury—climbing, falling down, running into immovable objects, skateboarding, and receiving blows to any part of their bodies.
Aside from automobile accidents or falls from heights, true injuries that cause fractures rarely occur in infancy; therefore bone injury in children of that age-group warrants further investigation. In any small child, radiographic evidence of fractures at various stages of healing is, with few exceptions, a result of nonaccidental trauma. Any investigation of fractures in infants, particularly multiple fractures, should include consideration of osteogenesis imperfecta.
The clavicle is probably the bone most frequently broken in childhood, with approximately half of clavicle fractures occurring in children younger than 10 years of age. Common mechanisms of injury include a fall with an outstretched hand or direct trauma to the bone. In neonates, a fractured clavicle may occur with a large newborn and a small maternal pelvis.
Fractures in school-age children are often a result of bicycle-automobile or skateboard injuries. Adolescents are vulnerable to multiple and severe trauma because they are mobile on bicycles, all-terrain vehicles, skateboards, skis, snowboards, and motorcycles and are active in sports.
Epiphyseal (or Physeal) Injuries
The weakest point of long bones is the cartilage growth plate, or epiphyseal plate. Consequently this is a frequent site of damage during trauma. Detection of epiphyseal injuries is sometimes difficult, but critical. Fractures involving the epiphysis or epiphyseal plate present special problems in determining whether bone growth will be affected. Treatment of these fractures may include open reduction and internal fixation to prevent or reduce growth disturbances.
Types of Fractures
A fractured bone consists of fragments—the fragment closer to the midline, or the proximal fragment, and the fragment farther from the midline, or the distal fragment. When fracture fragments are separated, the fracture is complete; when fragments remain attached, the fracture is incomplete. The fracture line can be any of the following:
Transverse—Crosswise, at right angles to the long axis of the bone
Oblique—Slanting but straight, between a horizontal and a perpendicular direction
Spiral—Slanting and circular, twisting around the bone shaft
The twisting of an extremity while the bone is breaking results in a spiral break. If the fracture does not produce a break in the skin, it is a simple, or closed, fracture. Open, or compound, fractures are those with an open wound through which the bone protrudes. If the bone fragments cause damage to other organs or tissues (e.g., the lung, bladder), the injury is said to be a complicated fracture. When small fragments of bone are broken from the fractured shaft and lie in the surrounding tissue, the injury is a comminuted fracture. This type of fracture is rare in children. The types of fractures that are seen most often in children are described in Box 31-1 and in Fig. 31-2.
Immediately after a fracture occurs, the muscles contract and physiologically splint the injured area. This phenomenon accounts for the muscle tightness observed over a fracture site and the deformity that is produced as the muscles pull the bone ends out of alignment. This muscle response must be overcome by traction or complete muscle relaxation (e.g., anesthesia) to realign the distal bone fragment to the proximal bone fragment.
Bone Healing and Remodeling
Bone healing is characteristically rapid in children because of the thickened periosteum and generous blood supply. When there is a break in the continuity of bone, the osteoblasts are stimulated to maximal activity. New bone cells are formed in immense numbers almost immediately after the injury and, in time, are evidenced by a bulging growth of new bone tissue between the fractured bone fragments. This is followed by deposition of calcium salts to form a callus.
Fractures heal in less time in children than in adults. The approximate healing times for a femoral shaft are as follows:
Diagnostic Evaluation
A history is often lacking in childhood injuries. Infants are unable to communicate, and older children seldom volunteer information (even under direct questioning) when the injury occurred during forbidden activities. Unless they are witnesses to the injury, parents may misinterpret what the child is trying to say. In cases of child abuse, parents may give false information to protect themselves.
The child may exhibit the same manifestations seen in adults (Box 31-2). However, often a fracture is remarkably stable because of intact periosteum. The child may even be able to use an affected arm or walk on a fractured leg. Because bones are highly vascular, a soft, pliable hematoma may be felt around the fracture site.
Radiographic examination is the most useful diagnostic tool for assessing skeletal trauma. The calcium deposits in bone make the entire structure radiopaque. Radiographic films are taken after fracture reduction and, in some cases, may be taken during the healing process to determine satisfactory progress.
Therapeutic Management
The majority of children’s fractures heal well, and nonunion is rare. Most fractures are readily reduced by simple traction and immobilization until healing takes place. However, the position of the bone fragments in relation to one another influences the rapidity of healing and the residual deformity. Healing is prompt and complete with end-to-end apposition, but a gap between fragments delays (or prevents) healing. The goals of fracture management are the following:
 To regain alignment and length of the bony fragments (reduction)
To regain alignment and length of the bony fragments (reduction)
 To retain alignment and length (immobilization)
To retain alignment and length (immobilization)
Children with displaced fractures may have immediate reduction and internal fixation with intramedullary nails rather than being immobilized by traction. This practice is more common and holds true for all types of fractures, including femur fractures. The use of traction for fractures in children does vary by institution. The child’s natural tendency to be active is usually sufficient to restore normal mobility, and physical therapy is rarely needed.
Children are most frequently hospitalized for fractures of the femur and the supracondylar area of the distal humerus, which may require internal fixation and pinning; displaced supracondylar fractures in children should be treated surgically (Do and Herrera-Soto, 2003). Fractures of the humerus, which usually result from a fall with the arm in extension, frequently involve the supracondylar portion. These fractures especially place the patient at risk for nerve damage and angulation deformities; therefore most children with a fractured humerus are taken to surgery for either a closed or open reduction with a percutaneous pinning of the fractured bone segments. Preoperatively the fracture is reduced with adequate analgesia and a temporary splint for immobilization.
If simple reductions cannot be achieved or if a neurovascular problem is detected after injury, observation in a hospital is indicated. Severe contusions with profound swelling cannot be treated with a cast, which would act as a tourniquet on the extremity. A badly malaligned fracture may require traction for a period before a cast is applied.
Wrist buckle fractures are common in a child who falls and extends the arm forward to break the fall. There are reports of radius and/or ulna buckle fractures in children treated with a removable splint for 3 to 4 weeks instead of a short arm cast (Plint, Perry, Correll, and others, 2006). The children treated with removable splints had better wrist function, had adequate bone healing, and experienced less inconvenience for bathing compared with the group of children placed in a short arm cast.
The major methods for immobilizing a fracture, casting and traction, are described later.
Nursing Care Management
Nurses are frequently the persons who make the initial assessment of a child with a suspected fracture (see Emergency Treatment box). The child and parents may be frightened and upset, and the child is often in pain. Therefore, if the child is alert and there is no evidence of hemorrhage, the initial nursing interventions are directed toward calming and reassuring the child and parents so that a more extensive assessment can be more easily accomplished.
While remaining calm and speaking in a quiet voice, the nurse can ask the parents and older child to describe what happened. The child may arrive with the limb supported in some manner; if not, careful support or immobilization may be provided to the affected site. In the event that the limb is supported or immobilized, it may be best not to touch the child but to ask him or her to point to the painful area and to wiggle the fingers or toes. By this time the child may feel relatively safe and will allow someone to gently touch the area just enough to feel the pulses and test for sensation. A child’s anxiety is greatly influenced by previous experiences with injury and with health personnel. However, he or she needs to be told what will happen and what to do to help. The affected limb need not be palpated, and it should not be moved unless properly splinted. If the child is at home or if the practitioner is not present to examine the child, some type of splint is applied carefully for transport to the medical facility. Parental anxiety may be heightened by the child’s pain reaction and fear, and possibly by other events surrounding the accident; thus it is important to communicate to the parent that the child will receive the necessary care, including pain management.
THE CHILD IN A CAST
The completeness of the fracture, the type of bone involved, and the amount of weight bearing influence how much of the extremity must be included in the cast to immobilize the fracture site completely. In most cases the joints above and below the fracture are immobilized to eliminate the possibility of movement that might cause displacement at the fracture site. Four major categories of casts are used for fractures: upper extremity to immobilize the wrist or elbow, lower extremity to immobilize the ankle or knee, spinal and cervical to immobilize the spine, and spica casts to immobilize the hip and knee.
The Cast
Casts are constructed from gauze strips and bandages impregnated with plaster of paris or, more commonly, from synthetic lighter weight and water-resistant materials (e.g., fiberglass and polyurethane resin).
Both types of casting produce heat from chemical reaction activated by water immediately after application. Plaster casts mold closely to the body part, take 10 to 72 hours to dry, have a smooth exterior, and are inexpensive. The newer synthetic casting material is lighter, dries in 5 to 30 minutes, permits earlier weight bearing, and is water resistant. The disadvantage of synthetic casting is its inability to mold closely to body parts; its rough exterior, which may scratch surfaces; and increased cost.
Synthetic casts have special advantages for children. They come in different colors and with designs (e.g., cartoons, stripes); and they are lightweight, durable, easy to clean, and relatively water resistant, depending on the type of inner lining used; only those with a Gore-Tex inner lining may be immersed in water without affecting the cast integrity. Bathing with a synthetic cast may be accomplished by covering the cast with a plastic bag; if the synthetic cast gets wet, it should be dried thoroughly. One drawback to immersion is the time necessary to completely dry the cast and padding. Synthetic casts are difficult to write on; a waterproof marker or color markers may be used.
Cast Application.: The child’s developmental age should be considered before the cast is applied. For preschoolers who fear bodily harm and fantasize the loss of an extremity, it may be helpful to use a plastic doll or stuffed animal to explain the procedure beforehand. Toddlers and preschoolers do not have easily defined body boundaries; if an extremity is wrapped in a bandage, cast, or splint, to the young child the extremity ceases to exist. It is also helpful to explain that some synthetic cast material will become warm but will not burn. During the application of the cast, various distraction methods can be used, including discussing favorite pets or activities at school, blowing bubbles, and so forth. In this age-group explanations such as “This will help your arm get better” are futile because the child has no concept of causality.
Before the cast is applied, the extremities are checked for any abrasions, cuts, or other alterations in the skin surface and for the presence of rings or other items that might cause constriction from swelling; such objects are removed. A tube of cloth stockinette or Gore-Tex liner is stretched over the area to be casted, and bony prominences are padded with soft cotton sheeting. Dry rolls of casting material are immersed in a pail of water. The wet rolls are put on in a bandage fashion and molded to the extremity. During application of the plaster cast, the underlying stockinette is pulled over the rough edges of the cast and secured with a layer of wet plaster 1.25 to 2.5 cm (0.5 to 1 inch) below the rim to form a smooth, padded edge to protect the skin.
If the practitioner does not form such a protective edge with stockinette, the rough edges of the plaster cast can be protected by a “petaled” edge. Small pieces approximately 5 to 7.5 cm (2 to 3 inches) long are cut from 2.5- or 3.8-cm (1- or 1.5-inch) wide adhesive tape or moleskin. The edges are rounded with scissors, and these “petals” are placed over the edge of the cast, with each petal slightly overlapping the previous petal to form a smooth, neat edge. It is easier to apply the petal to the underside of the cast first and then bring the loose edge to the front, pressing firmly so that the edges remain securely attached. Synthetic casts usually do not require additional padding on the edges, since they do not crack like plaster material might. However, the padding minimizes irritation and abrasions from the rough edges of the cast.
Nursing Care Management
The complete evaporation of the water from a hip spica cast can take 24 to 48 hours when older types of plaster materials are used. Drying occurs within minutes with fiberglass cast material. The cast must remain uncovered to allow it to dry from the inside out. Turning the child in a plaster cast at least every 2 hours will help to dry a body cast evenly and prevent complications related to immobility. A regular fan or cool-air hair dryer to circulate air may be helpful when the humidity is high.
A wet plaster cast should be supported by a pillow that is covered with plastic and handled by the palms of the hands to prevent indenting the cast, which can create pressure areas. A dry plaster-of-paris cast produces a hollow sound when it is tapped with the finger. If “hot spots” are felt on the cast surface (usually indicating infection beneath the area), this should be reported so that a window can be made in the cast to observe the site.
During the first few hours after a cast is applied, the chief concern is that the extremity may continue to swell to the extent that the cast becomes a tourniquet, shutting off circulation and producing neurovascular complications. To reduce the likelihood of this potential problem, the body part can be elevated, thereby increasing venous return. If edema is excessive, casts are bivalved (i.e., cut to make anterior and posterior halves that are held together with an elastic bandage). The cast and the involved extremity are observed frequently for neurovascular integrity and any signs of compromise. Permanent muscle and tissue damage can occur within 6 to 8 hours.
When an extremity that has sustained an open fracture is casted, a window is often left over the wound area to allow for observation and dressing of the wound. A surgical reduction is usually casted as a closed fracture. For the first few hours after surgery, substantial bleeding may soak through the cast. Periodically the circumscribed bloodstained area should be outlined with a waterproof marker and the time indicated to provide a guide for assessing the amount of bleeding.
Usually the child is discharged to home care after a cast is applied in the emergency department or clinic. Parents need instructions on drying and caring for the plaster cast because it takes longer to dry. Instructions are also given for checking for signs and symptoms that indicate the cast is too tight (see Family-Centered Care box). Parents should also be told to take the child to the health professional for attention if the cast becomes too loose, since a loose cast no longer serves its purpose.
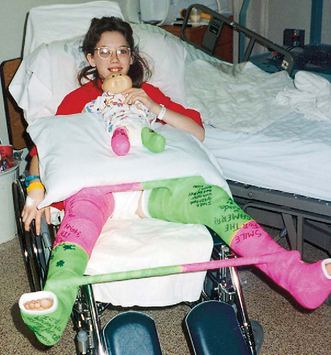
Nurses can help families adapt the child’s home environment to meet the temporary encumbrance of a large cast or one that restricts the child’s mobility (e.g., long leg cast). Home care creates problems of varying magnitudes, especially for children in large casts (e.g., a hip spica [Fig. 31-3]). Commonplace situations become problematic (e.g., transporting a child safely and comfortably in a car). Standard seat belts and car seats may not be readily adapted for use by children in some casts. Specially designed car seats and restraints are available that meet safety requirements. * Alterations to standard car seats to accommodate the cast are not recommended, since the structure may be adversely altered and fail to properly restrain the child.
Parents are taught the proper care of the cast (or orthotic device) and are helped to devise means for maintaining cleanliness. A superabsorbent disposable diaper is tucked beneath the entire perineal opening of the cast. A larger diaper can be applied and fastened over the small diaper and cast to hold the smaller diaper in place. In the event that the larger diaper becomes wet or soiled, it is likely the cast is as well.
For tightly fitting casts, transparent film dressings can be cut into strips as for petaling, and one edge applied to the cast edge and the other directly to the perineum; this forms a continuous, waterproof bridge between the perineum and the cast to prevent leakage. An additional advantage to the use of this transparent dressing is that it keeps both the skin and the cast dry while allowing for observation of skin beneath the dressing.
Older infants and small children may stuff bits of food, small toys, or other items under the cast; parents should be alerted to this possibility so that they can initiate suitable preventive measures.
Feeding the infant in a hip spica cast offers problems in positioning. Very young infants can be fed in the supine position with the head elevated. With the infant’s hips and legs supported on a pillow at the side, the parent can cuddle the infant in his or her arms during feeding. A somewhat similar position can be used for breastfeeding (i.e., with the infant supported on pillows or held in a “football” hold facing the mother with the legs behind her). An alternate position is to hold the infant upright on the caregiver’s lap with the legs of the infant astride the adult’s leg.
Children in spica casts usually find the prone position easier for self-feeding from a small table placed next to the dining table; alternatively, they may manage a semisitting position in bed or in a wheelchair (see Fig. 31-3). The use of a conventional toilet is almost impossible. A bedside toilet can be adapted for use. Small bedpans or other containers offer alternatives for elimination. The nurse may suggest waterproofing methods, by devising plastic wraps, for elimination and showers. Baths are possible only if the plaster cast is kept out of the water and covered to prevent it from becoming wet.
Cast Removal.: Cutting the cast to remove it or to relieve tightness is frequently a frightening experience for children. They fear the sound of the cast cutter and are terrified that their flesh, as well as the cast, will be cut. The oscillating blade vibrates rapidly back and forth and will not cut when placed lightly on the skin. Children have described it as producing a “tickly” sensation. The vibration also generates heat that may be felt by the child. Both these feelings should be explained.
Preparation for the procedure will help reduce anxiety, especially if a trusting relationship has been established between the child and the nurse. Many young children come to regard the cast as part of themselves, which intensifies their fear of removal (Fig. 31-4). They need continual reassurance that all is going well and that their behavior is accepted. After the cast is removed, the parents and child should be given the option of keeping the cast; it may be placed in a plastic bag because of the usual odor. If the cast has been in place for a lengthy period, decreased muscle mass will be noted. The child should be reassured that resuming exercise and routine activities will return function and appearance (provided there was no significant trauma beforehand).
After the cast is removed, the skin surface will be caked with desquamated skin and sebaceous secretions. Simple soaking in a bathtub is usually sufficient for their removal, but it may take several days to eliminate the accumulation completely. Application of oil or lotion may provide comfort. The parents and child should be instructed not to pull or forcibly remove this material with vigorous scrubbing, since it may cause excoriation and bleeding.
THE CHILD IN TRACTION
The ever-changing health care arena has witnessed the demise of many long-term treatments involving lengthy hospitalization; one such change is in the area of traction. Most balanced skeletal traction is applied in children after a severe or complex injury to allow physiologic stability, align bone fragments, and permit closer evaluation of the injured site. Newer technology has produced orthopedic fixation devices that allow partial or full mobility, thus preventing long-term immobilization and its consequences. In many situations, surgical intervention may be carried out within a matter of days; therefore skeletal traction devices described herein may be used infrequently in pediatrics.
Purposes of Traction
The six primary purposes of traction for reduction of fractures are:
1. To fatigue the involved muscles and reduce muscle spasm so that bones can be realigned
2. To position the distal and proximal bone ends in desired realignment to promote satisfactory bone healing
3. To immobilize the fracture site until realignment has been achieved and sufficient healing has taken place to permit casting or splinting
4. To help prevent or improve contracture deformity
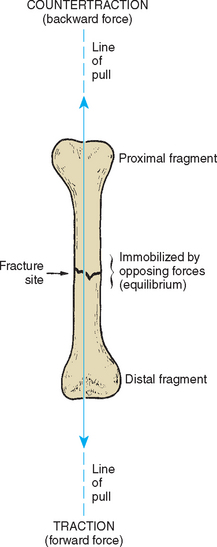
The three essential components of traction management are traction, countertraction, and friction (Fig. 31-5). To reduce or realign a fracture site, traction (forward force) is produced by attaching weight to the distal bone fragment; body weight provides countertraction (backward force); and the patient’s contact with the bed constitutes the frictional force. These forces are used to align the distal and proximal bone fragments by adjusting the line of pull upward or downward and adducting or abducting the extremity.
To attain equilibrium, the amount of forward force is adjusted by adding weight to or subtracting weight from the traction, or countertraction can be increased by elevating the foot of the bed to create a greater gravitational pull to the backward force.
The all-or-none law, characteristic of muscle contractibility, influences the complete relaxation. When muscles are stretched, muscle spasm ceases, which permits the realignment of the bone ends. The continuous maintenance of traction is important during this phase because releasing the traction allows the muscle’s normal contracting ability to again cause a malpositioning of the bone ends.
The realignment of the fragments is a gradual process that is achieved more rapidly in infants, who have limited muscle tone, than in muscular teenagers. The desired line of pull and callus formation are checked periodically by radiographic examination. The traction pull to some degree immobilizes the fracture site; however, adjunctive immobilizing devices such as splints or casts are sometimes used with skeletal traction. Immobilization with traction will be maintained until the bone ends are in satisfactory realignment, after which a less confining type of immobilization’a cast, pins, or external stabilization device—will be applied.
Types of Traction (General)
The pull needed for traction can be applied to the distal bone fragment in several ways (Box 31-3). The type of traction applied is determined primarily by the child’s age, the condition of the soft tissues, and the type and degree of displacement of the fracture. Fractures most commonly treated by application of traction are those involving the femur and vertebrae. The major types of traction for specific fractures are briefly discussed in the following sections.
Upper Extremity Traction
The use of upper extremity traction in children is uncommon. Newer surgical techniques allow for early mobilization and optimal results without traction. Nursing care of the child with upper extremity traction is the same as that for lower extremity traction, which is discussed below.
Lower Extremity Traction
The frequent site for a femoral fracture is in the middle third of the shaft. With this fracture there may be significant overriding but minimal displacement. In a fracture in the lower third of the shaft, the pull of the gastrocnemius muscle causes the distal fragment to become downwardly displaced.
Fractures of the femur can often be reduced with immediate application of a hip spica cast in young children. When traction is required, several types may be used, based on the initial assessment.
Bryant traction is a type of running traction in which the pull is in only one direction. Skin traction is applied to the legs, which are flexed at a 90-degree angle at the hips. The child’s trunk (with buttocks raised slightly off the bed) provides countertraction.
Buck extension (Fig. 31-6) is a type of traction with the legs in an extended position. Except for fracture cases, turning from side to side with care is permitted to maintain the involved leg in alignment. Buck extension is used primarily for short-term immobilization, preoperatively with dislocated hips, for correcting contractures, or for bone deformities such as Legg-Calvé-Perthes disease. Buck traction may be accomplished with either skin straps or a special foam boot designed for traction.
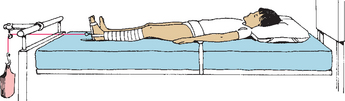
FIG. 31-6 Buck extension traction. (Redrawn from Hilt NE, Schmitt EW: Pediatric orthopedic nursing, St Louis, 1975, Mosby.)
Russell traction uses skin traction on the lower leg and a padded sling under the knee. Two lines of pull, one along the longitudinal line of the lower leg and one perpendicular to the leg, are produced. This combination of pulls allows realignment of the lower extremity and immobilizes the hip and knee in a flexed position. The hip flexion must be kept at the prescribed angle to prevent fracture malalignment, since there is no direct support under the fracture and the skin traction may slip. Special nursing measures include carefully checking the position of the traction so that the amount of desired hip flexion is maintained and damage to the common peroneal nerve under the knee does not produce footdrop.
A common skeletal traction is 90-degree–90-degree traction (90-90 traction). The lower leg is supported by a boot cast or a calf sling, and a skeletal Steinmann pin or Kirschner wire is placed in the distal fragment of the femur, resulting in a 90-degree angle at both the hip and the knee (Fig. 31-7). From a nursing standpoint, this traction facilitates position changes, toileting, and prevention of complications related to traction.
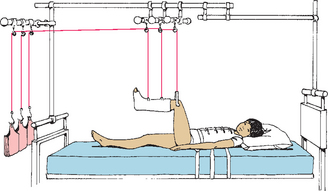
FIG. 31-7 Ninety-ninety traction. (Redrawn from Hilt NE, Schmitt EW: Pediatric orthopedic nursing, St Louis, 1975, Mosby.)
Balanced suspension traction may be used with or without skin or skeletal traction. Unless used with another traction, the balanced suspension merely suspends the leg in a desired flexed position to relax the hip and hamstring muscles and does not exert any traction directly on a body part. A Thomas splint extends from the groin to midair above the foot, and a Pearson attachment supports the lower leg. Towels or pieces of felt covered with stockinette are clipped or pinned to the splints for leg support. When the child is lifted off the bed, the traction lifts with the child without loss of alignment. This traction requires careful checking of splints and ropes to make certain that no slippage or fraying has occurred. The traction is of great value in an older and heavier child when it is essential to lift the patient for care.
Cervical Traction
The cervical area is a vulnerable site for flexion or extension injuries to muscle, vertebrae, or the spinal cord. Cervical muscle trauma without other complications is treated with a cervical hard collar to relieve the weight of the head from the fracture site. When a child displaces or fractures a cervical vertebra, it may be necessary to reduce and immobilize the site with cervical skeletal traction. The spinal cord runs through the intravertebral canal, and dislocation or fracture of the vertebrae can also cause spinal cord injury. Nursing assessment of neurologic function is essential to prevent further injury during the application and use of cervical skeletal traction.
Most cervical traction is accomplished with the use of a halo brace or halo vest (Fig. 31-8, A). This device consists of a steel halo attached to the head by four screws inserted into the outer skull; several rigid bars connect the halo to a vest that is worn around the chest, thus providing greater mobility of the rest of the body while avoiding cervical spinal motion altogether. If the injury has been limited to a vertebral fracture without neurologic deficit, a halo brace can be applied to permit earlier ambulation.
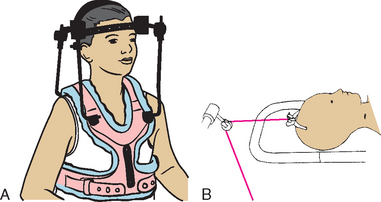
FIG. 31-8 A, Halo vest. B, Crutchfield tong traction. (B, Redrawn from Hilt NE, Schmitt EW: Pediatric orthopedic nursing, St Louis, 1975, Mosby.)
Cervical traction may also be accomplished by the insertion of Crutchfield, Barton, or Gardner-Wells tongs through burr holes in the skull, with weights attached to the hyperextended head (Fig. 31-8, B). As the neck muscles fatigue with constant traction pull, the vertebral bodies gradually separate so that the cord is no longer pinched between the vertebrae. Immobilization until fracture healing or surgical fixation can occur is an essential goal of cervical traction.
Nursing Care Management
To assess the child in traction, it is essential to know the purpose for which the traction is applied and to understand the basic principles of traction. Regular assessment of both the child and the traction apparatus is required (see Nursing Care Guidelines box). Many of the nursing problems associated with a child in traction are related to immobility. Modifying the child’s diet, encouraging fluids, and offering a mild laxative may be necessary to prevent constipation.
When indicated by the attending practitioner, the nurse may remove nonadhesive skin traction. In these cases intermittent traction is periodically released and reapplied as ordered. A child may have several types of traction at one time, and each one must be assessed separately to avoid problems.
In addition to routine skin observation and care, the child in skeletal traction will need special skin care at the pin site according to hospital policy or practitioner preference. Pin sites should be frequently assessed and cleaned to prevent infection; after the first 48 to 72 hours pin site care may be performed once daily or weekly for mechanically stable pins (Holmes, Brown, and Pin Site Care Expert Panel, 2005). Use of a 2 mg/ml chlorhexidine solution has been proposed as best-practice care for skeletal pin sites by the National Association of Orthopaedic Nurses (Holmes, Brown, and Pin Site Care Expert Panel, 2005). Before the child’s discharge, the family is taught pin site care, including how to observe for infection or pin instability, using a return demonstration method. A pressure reduction device, such as a special air mattress, decreases the chance of skin breakdown.
When the child is first placed in traction, increased discomfort is common as a result of the traction pull fatiguing the muscle. It has been determined that orthopedic conditions are associated with a higher-than-average number of painful events and a higher percentage of bodily symptoms than other common conditions. IV opioids, including analgesics and muscle relaxants, help during this phase of care and should be administered liberally.
The specific nursing responsibilities for the patient in traction are outlined in the Nursing Care Guidelines box (p. 1120).
DISTRACTION
Unlike traction, which helps bones realign and fuse properly, distraction is the process of separating opposing bone to encourage regeneration of new bone in the created space. Distraction can also be used when limbs are of unequal lengths and new bone is needed to elongate the shorter limb.
External Fixation
The Ilizarov external fixator (IEF) is a common external fixation device. The IEF uses a system of wires, rings, and telescoping rods that permits limb lengthening to occur by manual distraction (Fig. 31-9). In addition to lengthening bones, the device can be used to correct angular or rotational defects or to immobilize fractures. The device is attached surgically by securing a series of external full or half rings to the bone with wires. External telescoping rods connect the rings to each other. Manual distraction is accomplished by manipulating the rods to increase the distance between the rings. A percutaneous osteotomy is performed when the device is applied to create a “false” growth plate. A special osteotomy or corticotomy involves cutting only the cortex of the bone while preserving its blood supply, bone marrow, endosteum, and periosteum. Capillary blood flow to the transected area is essential for proper bone growth. Cut bone ends typically grow at a rate of 1 cm (0.4 inches) per month. The IEF can result in up to a 15-cm (6-inch) gain in length.
Nursing Care Management
Success of the IEF depends on the child’s and family’s cooperation; therefore before surgery they must be fully informed of the appearance of the device, how it accomplishes bone growth and limits bone mobility, alterations in activities, and home and follow-up care. Children are involved in learning to adjust the device to accomplish distraction. Children and parents should be instructed in pin care, including observation for infection and loosening of the pins. Cleaning routines for the pin sites vary among practitioners but should not traumatize the skin.
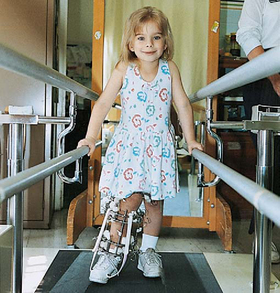
Children who participate actively in their care report less discomfort. Because the device is external, the child and family need to be prepared for the reactions of others and assisted in camouflaging the device with appropriate apparel, such as wide-legged pants that close with self-adhering fasteners around the device. A loose sock or stockinette may also be used over the device to decrease public awareness. Partial weight bearing is allowed, and the child learns to walk with crutches. Alterations in activity include modifications at school and in physical education. Full weight bearing is not allowed until the distraction is completed and bone consolidation has occurred. Follow-up care is essential to maintain appropriate distraction until the desired limb length is achieved. The device is removed surgically after the bone has consolidated, and the child may need to use crutches or have a cast for 4 to 6 weeks after removal.
AMPUTATION
A child may be born with the congenital absence of an extremity, have a traumatic loss of an extremity, or need a surgical amputation for a pathologic condition such as osteosarcoma (see p. 1136). With today’s surgical technology and the quick thinking of bystanders who save a traumatically amputated body part, some children have had fingers and arms sewn back on with variable degrees of functional use regained.
Surgical amputation or the surgical repair of a permanently severed limb focuses on constructing an adequately nourished stump. A smooth, healthy, padded stump, free of nerve endings, is important in prosthesis fitting and subsequent ambulation. In some situations in which there is no vascular or neurologic deficit, a cast is applied to the stump immediately after the procedure, and a pylon, metal extension, and artificial foot are attached so that the patient can walk on the temporary prosthesis within a few hours.
Nursing Care Management
Stump shaping is done postoperatively with special elastic bandaging using a figure-eight bandage, which applies pressure in a cone-shaped fashion. This technique decreases stump edema, controls hemorrhage, and aids in developing desired contours so that the child will bear weight on the posterior aspect of the skin flap rather than on the end of the stump. Stump elevation may be used during the first 24 hours, but after this time the extremity should not be left in this position, since contractures in the proximal joint will develop and seriously hamper ambulation. Monitoring proper body alignment will further decrease the risk of flexion contractures.
For older children and adolescents, arm exercises, bed pushups, and prosthesis-training programs using parallel bars help build up the arm muscles necessary for walking with crutches. Full range-of-motion exercises of joints above the amputation must be performed several times daily, using active and isotonic exercises. Young children are spontaneously active and require little encouragement.
Depending on the child’s age, children or their parents will need to learn stump hygiene, including carefully washing with soap and water every day and checking for skin irritation, breakdown, or infection. A tube of stockinette or powder is used to slide the prosthesis on more easily. Skin must be checked carefully every time the prosthesis is removed, and prosthesis tolerance time must be adjusted to prevent skin breakdown.
For children who have had an amputation, phantom limb sensation is an expected experience because the nerve-brain connections are still present. Gradually these sensations fade, although in many amputees they persist for years. Preoperative discussion of this phenomenon will aid a child in understanding these “unusual feelings” and not hiding the experiences from others. Limb pain, especially pain that increases with ambulation, should be evaluated for the possibility of a neuroma at the free nerve endings in the stump, or other problems such as a poorly fitting prosthesis or joint instability.
CONGENITAL DEFECTS
Some skeletal defects may be diagnosed at birth or within days, weeks, or months after birth. In other cases the deviation may be difficult to detect without careful inspection. Therefore it is imperative that nurses become acquainted with signs of these defects and understand the principles of therapy in order to direct others in the care and management of these children.
DEVELOPMENTAL DYSPLASIA OF THE HIP
The broad term developmental dysplasia of the hip (DDH) describes a spectrum of disorders related to abnormal development of the hip that may occur at any time during fetal life, infancy, or childhood. A change in terminology from congenital hip dysplasia and congenital dislocation of the hip to DDH more properly reflects a variety of hip abnormalities in which there is a shallow acetabulum, subluxation, or dislocation.
The incidence of hip instability of some kind is approximately 10 per 1000 live births. The incidence of frank dislocation or a dislocatable hip is 1 per 1000 live births (Wall, 2000), and approximately 16% to 25% of infants with DDH are born breech (Hosalkar, Horn, Friedman and others, 2007). The left hip is involved in 60% of cases, the right hip in 20%, and both hips in 20%. Sixty percent of the patients are girls. Caucasian children have a higher incidence of developmental dysplasia than other groups (Maher, Salmond, and Pellino, 2002).
Pathophysiology
The cause of DDH is unknown, but certain factors such as gender, birth order, family history, intrauterine position, delivery type, joint laxity, and postnatal positioning are believed to affect the risk of DDH. Predisposing factors associated with DDH may be divided into three broad categories: (1) physiologic factors, which include maternal hormone secretion and intrauterine positioning; (2) mechanical factors, which involve breech presentation, multiple fetus, oligohydramnios, and large infant size (other mechanical factors may include continued maintenance of the hips in adduction and extension that will in time cause a dislocation); and (3) genetic factors, which entail a higher incidence (6%) of DDH in siblings of affected infants and an even greater incidence (36%) of recurrence if a sibling and one parent were affected.
Some experts categorize DDH into two major groups: (1) typical, in which the infant is neurologically intact; and (2) teratologic, which involves a neuromuscular defect such as arthrogryposis or myelodysplasia. The teratologic forms usually occur in utero and are much less common.
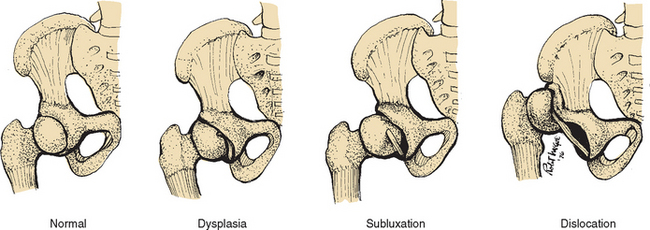
Three degrees of DDH are illustrated in Fig. 31-10:
1. Acetabular dysplasia (or preluxation)—This is the mildest form of DDH, in which there is neither subluxation nor dislocation. There is a delay in acetabular development evidenced by osseous hypoplasia of the acetabular roof that is oblique and shallow, although the cartilaginous roof is comparatively intact. The femoral head remains in the acetabulum.
2. Subluxation—The largest percentage of DDH, subluxation, implies incomplete dislocation of the hip and is sometimes regarded as an intermediate state in the development from primary dysplasia to complete dislocation. The femoral head remains in contact with the acetabulum, but a stretched capsule and ligamentum teres cause the head of the femur to be partially displaced. Pressure on the cartilaginous roof inhibits ossification and produces a flattening of the socket.
3. Dislocation—The femoral head loses contact with the acetabulum and is displaced posteriorly and superiorly over the fibrocartilaginous rim. The ligamentum teres is elongated and taut.
Factors related to infant handling are indicated in the Cultural Awareness box.
Diagnostic Evaluation
DDH is often not detected at the initial examination after birth; thus all infants should be carefully monitored for hip dysplasia at follow-up visits throughout the first year of life. In the newborn period dysplasia usually appears as hip joint laxity rather than as outright dislocation (Fig. 31-11). Subluxation and the tendency to dislocate can be demonstrated by the Ortolani or Barlow tests (see Fig. 31-11, B, C, and D). The Ortolani and Barlow tests are most reliable from birth to 2 or 3 months of age. With the Barlow test the thighs are adducted, whereas the Ortolani test involves abducting the thighs to test for hip subluxation or dislocation (Seidel, Ball, Dains, and others, 2006). Other signs of DDH are shortening of the limb on the affected side (see Fig. 31-11, C), asymmetric thigh and gluteal folds (see Fig 31-11, A), and broadening of the perineum (in bilateral dislocation) (Box 31-4).
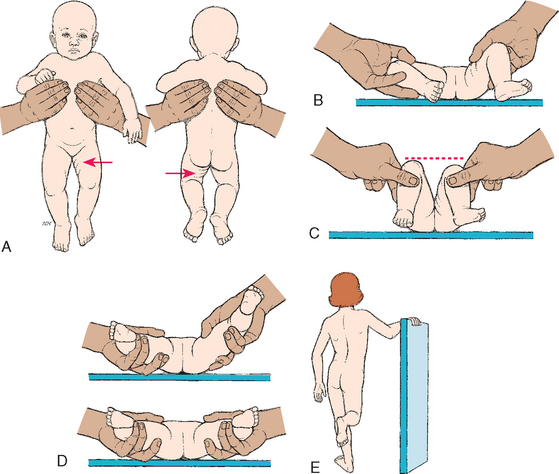
FIG. 31-11 Signs of developmental dysplasia of the hip. A, Asymmetry of gluteal and thigh folds with shortening of the thigh (Galeazzi sign). B, Limited hip abduction, as seen in flexion (Ortolani test). C, Apparent shortening of the femur, as indicated by the level of the knees in flexion (Allis sign). D, Ortolani test with femoral head moving in and out of acetabulum (in infants 1 to 2 months old). E, Positive Trendelenburg sign with lordosis (if child is weight bearing).
Radiographic examination in early infancy is not reliable, since ossification of the femoral head does not normally take place until the third to sixth month of life. However, the cartilaginous head can be visualized directly by ultrasonography. Widespread newborn screening with ultrasound has been proposed; however, numerous studies reveal this approach has a high rate of false positives and subsequent overtreatment. Therefore ultrasound is recommended as an adjunct to other diagnostic procedures (American Academy of Pediatrics, 2000a). In infants older than age 4 months and in children, radiographic examination is useful in confirming the diagnosis. An upward slope in the roof of the acetabulum (the acetabular angle) greater than 40 degrees with upward and outward displacement of the femoral head is a frequent finding in older children. Computed tomography (CT) scan may be useful to assess the position of the femoral head relative to the acetabulum after closed reduction and casting. The American Academy of Pediatrics (2000a) has published extensive clinical guidelines for the early detection of DDH.
Therapeutic Management
Treatment is begun as soon as the condition is recognized, since early intervention is more favorable to the restoration of normal bony architecture and function. The longer treatment is delayed, the more severe the deformity, the more difficult the treatment, and the less favorable the prognosis. The treatment varies with the child’s age and the extent of the dysplasia. The goal of treatment is to obtain and maintain a safe, congruent position of the hip joint to promote normal hip joint development.
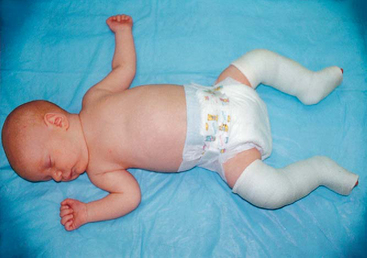
Newborn to Age 6 Months.: The hip joint is maintained by dynamic splinting in a safe position with the proximal femur centered in the acetabulum in an attitude of flexion. Of the numerous devices available, the Pavlik harness is the most widely used, and with time, motion, and gravity, the hip works into a more abducted, reduced position (Fig. 31-12). The harness is worn continuously until the hip is proved stable on clinical and radiographic examination, usually in about 3 to 5 months.
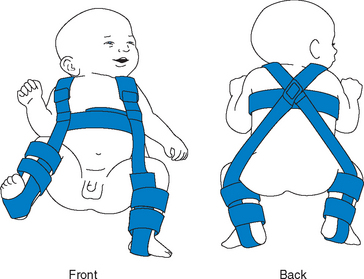
FIG. 31-12 Child in Pavlik harness. (From Ball JW: Mosby’s pediatric patient teaching guides, St Louis, 1998, Mosby.)
When adduction contracture is present, other devices (such as skin traction) are used to slowly and gently stretch the hip to full abduction, after which wide abduction is maintained until stability is attained. When there is difficulty in maintaining stable reduction, a hip spica cast is applied and changed periodically to accommodate the child’s growth. After 3 to 6 months, sufficient stability is acquired to allow transfer to a removable protective abduction brace. The duration of treatment depends on development of the acetabulum but is usually accomplished within the first year.
Ages 6 to 18 Months.: In this age-group the dislocation is not recognized until the child begins to stand and walk, when attendant shortening of the limb and contractures of hip adductor and flexor muscles become apparent. Gradual reduction by traction is used for approximately 3 weeks. An individualized home traction program may be developed for the child preoperatively to decrease the length of hospitalization and maintain the home environment. The child then undergoes an attempted closed reduction of the hip using general anesthesia; if the hip is not reducible, an open reduction is performed. After reduction, the child is placed in a hip spica cast for 2 to 4 months until the hip is stable, at which time a flexion-abduction brace is applied.
Older Child.: Correction of the hip deformity in older children is inherently more difficult than in the preceding age-groups, since secondary adaptive changes and other etiologic factors (such as juvenile arthritis or nonambulatory cerebral palsy) complicate the condition. Operative reduction, which may involve preoperative traction, tenotomy of contracted muscles, and any one of several innominate osteotomy procedures designed to construct an acetabular roof, is usually required. After cast removal and before weight bearing is permitted, range-of-motion exercises help restore movement. Successful reduction and reconstruction become increasingly difficult after the age of 4 years and are usually impossible or inadvisable in children older than 6 years of age because of severe shortening and contracture of muscles and deformity of the femoral and acetabular structures.
Nursing Care Management
Nurses are in a unique position to detect DDH in early infancy. During the infant assessment process and routine nurturing activities, the hips and extremities are inspected for any deviations from normal. These observations are reported to the attending practitioner, and the ambulatory child who displays a limp or an unusual gait should be referred for evaluation. This may indicate an orthopedic or neurologic problem. Nonambulatory children with cerebral palsy should also be assessed for evidence of dislocation.
The major nursing problems in the care of an infant or child in a cast or other device are related to maintenance of the device and adaptation of nurturing activities to meet the patient’s needs. Generally, treatment and follow-up care of these children are carried out in a clinic, practitioner’s office, or outpatient unit.
The primary nursing goal is teaching parents to apply and maintain the reduction device. The Pavlik harness allows for easy handling of the infant and usually produces less apprehension in the parent than heavy braces and casts. Because of the infant’s rapid growth, the straps should be checked in the beginning of therapy every week for possible adjustments (Hart, Albright, Rebello, and others, 2006). It is important that parents understand the correct use of the appliance, which may or may not allow for its removal during bathing. Unbuckling or removing the harness is determined individually on the basis of the family’s level of understanding and the degree of hip deformity. Parents are instructed not to adjust the harness without medical supervision. The child should be examined by the practitioner before any adjustment is attempted to make certain the hips are in correct placement before the harness is resecured.
Skin care is an important aspect of the care of an infant in a harness. The following instructions for preventing skin breakdown are stressed:
 Always put an undershirt (or a shirt with extensions that close at the crotch) under the chest straps, and put knee socks under the foot and leg pieces to prevent the straps from rubbing the skin.
Always put an undershirt (or a shirt with extensions that close at the crotch) under the chest straps, and put knee socks under the foot and leg pieces to prevent the straps from rubbing the skin.
 Check frequently (at least two or three times a day) for red areas under the straps and the clothing.
Check frequently (at least two or three times a day) for red areas under the straps and the clothing.
 Gently massage healthy skin under the straps once a day to stimulate circulation. In general, avoid lotions and powders because they can cake and irritate the skin.
Gently massage healthy skin under the straps once a day to stimulate circulation. In general, avoid lotions and powders because they can cake and irritate the skin.
Parents are encouraged to hold the infant with a harness and continue care and nurturing activities. The nurse can assist by being available for parents’ questions about the necessary adaptations to daily care to decrease the parent’s anxiety and possible feelings about the child being hurt by routine caring.
Casts and orthotic devices (braces) offer more challenging nursing and caregiver problems, since they cannot be removed for routine care, although sometimes a brace may be removed for bathing. Care of an infant or small child with a cast requires nursing innovation to reduce irritation and to maintain cleanliness of both the child and the cast, particularly in the diaper area. (see p. 1115 for care of the child in a cast.)
It is important for nurses, parents, and other caregivers to understand that children in corrective devices need to be involved in all the activities of any child in the same age-group. Confinement in a cast or appliance should not exclude children from family (or unit) activities. They can be held astride the lap for comfort and transported to areas of activity. The child may be allowed to walk in a cast or orthotic device. An adapted wheelchair, stroller, or scooter can offer mobility to the older infant or child.
CONGENITAL CLUBFOOT
Congenital clubfoot is a complex deformity of the ankle and foot that includes forefoot adduction, midfoot supination, hindfoot varus, and ankle equinus. Deformities of the foot and ankle are described according to the position of the ankle and foot. The more common positions involve the following variations:
Talipes varus—An inversion, or bending inward
Talipes valgus—An eversion, or bending outward
Talipes equinus—Plantar flexion, in which the toes are lower than the heel
Talipes calcaneus—Dorsiflexion, in which the toes are higher than the heel
Most cases of clubfoot are a combination of these positions, and the most frequently occurring type of clubfoot (approximately 95% of cases) is the composite deformity talipes equinovarus (TEV), in which the foot is pointed downward and inward in varying degrees of severity (Fig. 31-13). Unilateral clubfoot may occur as an isolated defect or in association with other disorders or syndromes, such as chromosomal aberrations, arthrogryposis, cerebral palsy, or spina bifida.
FIG. 31-13 Bilateral congenital talipes equinovarus (congenital clubfoot) in 2-month-old infant. (From Zitelli BJ, Davis HW: Atlas of pediatric physical diagnosis, ed 4, St Louis, 2002, Mosby.)
The incidence of clubfoot in the general population is 1 to 2 per 1000 live births, with boys affected twice as often as girls. Bilateral clubfeet occur in 50% of the cases (Gilmore and Thompson, 2003). The precise cause of clubfoot is unknown. Some authorities attribute the defect to abnormal positioning and restricted movement in utero, although the evidence is not conclusive. Other experts implicate arrested or abnormal embryonic development. Arrested development during this early stage tends to result in a rigid deformity, whereas mechanical pressures from intrauterine positioning are likely causes of more flexible deformities.
Classification
Clubfoot may be further divided into three categories: (1) positional clubfoot (also called transitional, mild, or postural clubfoot), which is believed to occur primarily from intrauterine crowding and responds to simple stretching and casting; (2) syndromic (or teratologic) clubfoot, which is associated with other congenital anomalies such as myelomeningocele or arthrogryposis and is a more severe form of clubfoot that is often resistant to treatment; and (3) congenital clubfoot, also referred to as idiopathic, which may occur in an otherwise normal child and has a wide range of rigidity and prognosis.
The mild, or postural, clubfoot may correct spontaneously or may require passive exercise or serial casting. There is no bony abnormality, but there may be tightness and shortening of the soft tissues medially and posteriorly. The teratologic clubfoot usually requires surgical correction and has a high incidence of recurrence. The congenital idiopathic clubfoot, or “true clubfoot,” almost always requires surgical intervention because there is bony abnormality.
Diagnostic Evaluation
The deformity is readily apparent and easily detected prenatally through ultrasonography or at birth. However, it must be differentiated from some positional deformities that can be passively corrected or overcorrected. Paralytic changes in the lower extremity of children with neuromuscular involvement often produce equinovarus deformity. An increased risk of hip dysplasia is associated with clubfoot deformities.
Therapeutic Management
The goal of treatment for clubfoot is to achieve a painless, plantigrade, and stable foot. Treatment of clubfoot involves three stages: (1) correction of the deformity, (2) maintenance of the correction until normal muscle balance is regained, and (3) follow-up observation to avert possible recurrence of the deformity. Some feet respond to treatment readily; some respond only to prolonged, vigorous, and sustained efforts; and the improvement in others remains disappointing even with maximal effort on the part of all concerned.
Serial casting is begun shortly after birth, before discharge from the nursery. Successive casts allow for gradual stretching of skin and tight structures on the medial side of the foot (Fig. 31-14). Manipulation and casting are repeated frequently (every week) to accommodate the rapid growth of early infancy. The extremity or extremities are casted until maximum correction is achieved, usually within 8 to 12 weeks. A Denis Browne splint may be used to manage feet that correct with casting and manipulation. A radiograph or ultrasound is then evaluated to see the relationship of the bones to each other. Failure to achieve normal alignment by 3 months indicates the need for surgical intervention. Current practice involves a percutaneous heel cord release at approximately 2 to 3 months of age followed by serial casting. This has decreased the number of children requiring more extensive surgery between 6 and 12 months of age. The foot (or feet) is immobilized postoperatively for approximately 6 to 12 weeks, and the child is allowed to walk after the cast is removed (Gilmore and Thompson, 2003).
Nursing Care Management
Nursing care of the child with clubfoot is the same as for any child who has a cast (see p. 1115). Because the child will spend considerable time in a corrective device, nursing care plans include both long-term and short-term goals. Conscientious observation of the skin and circulation is particularly important in young infants because of their rapid growth rate. Because treatment and follow-up care are handled in the orthopedist’s office, clinic, or outpatient department, parent education and support are important in nursing care of these children.
Parents need to understand the overall treatment program, the importance of regular cast changes, and the role they play in the long-term effectiveness of the therapy. Reinforcing and clarifying the orthopedist’s explanations and instructions, teaching parents about care of the cast or appliance (including vigilant observation for potential problems), and encouraging parents to facilitate normal development within the limitations imposed by the deformity or therapy are all part of nursing responsibilities.
METATARSUS ADDUCTUS (VARUS)
Metatarsus adductus, or metatarsus varus, is probably the most common congenital foot deformity. In most instances it is a result of abnormal intrauterine positioning, particularly in the firstborn child, and is usually detected at birth. The deformity is characterized by medial adduction of the toes and forefoot, frequently in association with inversion, and by convexity of the lateral border of the foot. Metatarsus adductus may be divided into three categories: type I, in which the forefoot is flexible and corrects easily with manipulation; type II, in which there is only partial flexibility in the forefoot and it corrects passively past neutral position but only to neutral position with active manipulation; and type III, in which the forefoot is rigid and will not stretch to neutral position with manipulation (Gilmore and Thompson, 2003). Unlike TEV, with which it is often confused, the angulation occurs at the tarsometatarsal joint, whereas the heel and ankle remain in a neutral position. Ankle range of motion is normal. This deformity often causes a pigeon-toed gait in the child.
Management depends on the rigidity and type of the deformity. With types I and II, correction can usually be accomplished by gentle manipulation and passive stretching of the foot, which the parent is taught to perform. Repeated and consistent stretching is continued for the first 6 weeks, after which the treatment is based on the flexibility of the foot. With type III, the child will usually require serial manipulation and casting to correct the defect. Casting is performed every 1 to 2 weeks for 6 to 8 weeks, after which a corrective shoe or orthosis may be used. Surgical correction is rarely required for the condition (Gilmore and Thompson, 2003).
Nursing Care Management
The nursing role primarily involves identifying the defect, so that early therapy and instruction of the parents can be initiated. The nurse teaches the parents how to hold the heel firmly and to stretch only the forefoot; otherwise, undue force on the heel may produce a valgus deformity. If casting or orthosis is required, the nurse instructs the parents in cast care and observation of the corrective device (see p. 1115).
SKELETAL LIMB DEFICIENCY
Congenital limb deficiencies, or reduction malformations, are manifested by a variety of degrees of loss of functional capacity. They are characterized by underdevelopment of skeletal elements of the extremities. The range of malformation can extend from minor defects of the digits to serious abnormalities, such as amelia, absence of an entire extremity; or meromelia, partial absence of an extremity, which includes phocomelia (seal limbs), the interposed deficiency of long bones with relatively good development of hands and feet attached at or near the shoulder or the hips. Most reduction defects are primary defects of development of the limb, but prenatal destruction of the limb can occur, such as full or partial amputation of a limb in utero from constriction of an amniotic band (amniotic band syndrome).
Pathophysiology
Limb deficiencies can be attributed to both heredity and environment and can originate at any stage of limb development. Formation of limbs may be suppressed at the time of limb bud formation, or there may be interference in later stages of differentiation and growth. Heredity appears to play a prominent role, and prenatal environmental insults have been implicated in a number of cases, such as the well-publicized thalidomide tragedy of the 1950s and early 1960s, which demonstrated a clear relationship between the time of exposure of the pregnant woman to the antiemetic drug and the presence and type of limb deformity in the newborn. There still are many drugs that may have similar teratogenic effects in the first trimester of pregnancy; therefore medication administration during this period should be carefully evaluated by the practitioner. Unfortunately, during this period, the woman may not realize her pregnant condition unless the event is highly anticipated, and inadvertent consumption of harmful medications may occur. Deletion or shortening of digits or limbs may also be associated with chorionic villus sampling, especially before 10 to 12 weeks of gestation; however, the incidence and relationship remain uncertain.
Therapeutic Management
Children with congenital limb deficiencies should be fitted with prosthetic devices whenever possible, and the devices should be applied at the earliest possible stage of development in an attempt to match the infant’s motor readiness. This favors natural progression of prosthetic use. For example, an infant with an upper extremity deficiency is fitted with a simple passive device, such as a mitten prosthesis, to encourage limb exploration, sitting (with the extremities needed for support), and bilateral hand activities.
Lower limb prostheses are applied when the infant begins sitting up and can maintain balance. In preparation for prosthetic devices, surgical modification may be necessary to ensure the most favorable use of the device, since severe deformity can interfere with its effective use. Phocomelic digits are preserved for controlling switches of externally powered appliances in upper extremities. Digits (in both upper and lower extremities) provide the child with surfaces for tactile exploration and stimulation. Prostheses are replaced to accommodate the child’s growth and increasing capabilities.
Nursing Care Management
Prosthetic application training and habilitation are most successfully carried out in a center that specializes in meeting the special needs of these children, especially very young children and those with amputations or missing limbs. Management involves a prosthetist, who specializes in the development, fitting, and maintenance of prosthetic limbs, and other health care workers such as physical and occupational therapists. Parents need special attention and support and are encouraged to assist the child in making age-commensurate adjustments to the environment. Although these children need assistance, overprotection may produce overdependence, with later maladjustment to school and other situations.
OSTEOGENESIS IMPERFECTA
Osteogenesis imperfecta (OI) is the most common osteoporosis syndrome in childhood. OI is a heterogeneous, autosomal dominant disorder characterized by fractures and bone deformity. There are at least five types of OI, which accounts for significant disease variability. Clinical features may include varying degrees of bone fragility, deformity, and fracture; blue sclerae; hearing loss; and dentinogenesis imperfecta (hypoplastic discolored teeth). Although inheritance follows an autosomal dominant pattern in most cases, rare autosomal recessive inheritance exists (Box 31-5).
Most types of OI have defects in the COL1A1 or COL1A2 genes, which code for polypeptide chains in type 1 procollagen, a precursor of type 1 collagen, a major structural component of bone. The error results in faulty bone mineralization, abnormal bone architecture, and increased susceptibility to fracture.
OI has several classifications based on clinical features and patterns of inheritance (see Box 31-5). Clinically, type I is the most common, with wide variability of bone fragility; some affected family members have significant deformity and disability, whereas others lead agile, active lives. Type II variants are the most severe and are considered lethal in infancy. Type III OI is characterized by multiple fractures, bone deformity, and severe disability; affected individuals rarely live to 30 years of age. Type IV is similar to type I with blue or white sclerae. Another variant, or type V, has been described in which those affected have a hyperplastic callus, a radiodense metaphyseal band, and calcification of the interosseous membrane of the forearm; no collagen mutations are noted in this group (Marini, 2007). A type VI has been described with a characteristic mineralization defect, which does not respond to pamidronate therapy as do types I to V (Land, Rauch, Travers, and others, 2007). Children affected with this type have no dental involvement and normal sclerae; a bone biopsy is the only way to establish a diagnosis because of the similarities to other types.
Therapeutic Management
The treatment for OI is primarily supportive, although patients and families are optimistic about new research advances. Bone marrow transplant for severe OI was first reported in 1999 with positive results; however, this is still considered an experimental treatment. The use of bisphosphonate therapy with IV pamidronate to promote increased bone density and prevent fractures has become standard therapy for many children with OI.
The goals of a rehabilitative approach to management are directed to preventing (1) positional contractures and deformities, (2) muscle weakness and osteoporosis, and (3) malalignment of lower extremity joints prohibiting weight bearing.
Lightweight braces and splints help support limbs, prevent fractures, and aid in ambulation. Physical therapy helps prevent disuse osteoporosis and strengthens muscles, which in turn improves bone density.
Surgery is sometimes used to help treat the manifestations of the disease. Surgical techniques are used to correct deformities that interfere with bracing, standing, or walking. For the child with recurrent fractures, inserting an intramedullary rod provides stability to bones.
Nursing Care Management
Infants and children with this disorder require careful handling to prevent fractures. They must be supported when they are being turned, positioned, moved, and held. Even changing a diaper may cause a fracture in severely affected infants. These children should never be held by the ankles when being diapered but should be gently lifted by the buttocks or supported with pillows.
Both parents and the affected child need education regarding the child’s limitations and guidelines in planning suitable activities that promote optimal development and protect the child from harm. Realistic occupational planning and genetic counseling are part of the long-term goals of care. Educational materials and information can be obtained from the Osteogenesis Imperfecta Foundation,** which also has a network that can put a family in contact with other families with a similar problem.
OI is a differential diagnosis that must be ruled out in the event of multiple fractures that could be attributed to nonaccidental injury. A detailed history, no evidence of associated soft-tissue injury, and the presence of other symptoms related to OI help to determine the diagnosis.
ACQUIRED DEFECTS
Legg-Calvé-Perthes disease, sometimes called coxa plana or osteochondritis deformans juvenilis, is a self-limiting disorder in which there is aseptic necrosis of the femoral head. The disease affects children ages 2 to 12 years, but most cases occur in boys between 4 and 8 years of age as an isolated event. In approximately 10% of cases the involvement is bilateral; most of the affected children have a skeletal age significantly below their chronologic age (Hosalkar, Horn, Friedman and others, 2007). The male/female ratio is 4:1 or 5:1. Caucasian children are affected 10 times more frequently than African-American children.
Pathophysiology
The cause of the disease is unknown, but there is a disturbance of circulation to the femoral capital epiphysis that produces an ischemic aseptic necrosis of the femoral head. During middle childhood, circulation to the femoral epiphysis is more tenuous than at other ages and can become obstructed by trauma, inflammation, coagulation defects, and a variety of other causes. The pathologic events seem to take place in four stages (Box 31-6). The entire process may encompass as little as 18 months or continue for several years. The reformed femoral head may be severely altered or appear entirely normal.
Clinical Manifestations and Diagnostic Evaluation
The onset of Legg-Calvé-Perthes disease is usually insidious, and the history may reveal only intermittent appearance of a limp on the affected side or a symptom complex, including hip soreness, ache, or stiffness, which can be constant or intermittent. The parents may report seeing the child limping, and the limp becomes more pronounced with increased activity. The pain may be experienced in the hip, along the entire thigh, or in the vicinity of the knee joint. The pain and limp are usually most evident on arising and at the end of a long day of activities. The pain is usually accompanied by joint dysfunction and limited range of motion. There may be a vague history of trauma. The diagnosis is established by radiographic examination, with the definitive diagnosis being magnetic resonance imaging (MRI), which demonstrates osteonecrosis.
Therapeutic Management
Because deformity occurs early in the disease process, the aims of treatment are to eliminate hip irritability; restore and maintain adequate range of hip motion; prevent capital femoral epiphyseal collapse, extrusion, or subluxation; and ensure a well-rounded femoral head at the time of healing. Treatment varies according to the child’s age at the time of diagnosis and the appearance of the femoral head vasculature and position within the acetabulum. Nonsurgical containment of the femoral head may be accomplished with abduction casts, whereas a pelvic or femoral osteotomy may be used to contain the femoral head. Activity causes microfractures of the soft ischemic epiphysis, which tend to induce synovitis, stiffness, and adductor contracture. The initial therapy is rest and non—weight bearing, which helps reduce inflammation and restore motion. Later, active motion is encouraged. In some cases traction is applied to stretch tight adductor muscles.
Containment can be accomplished in several ways. One is the use of non—weight-bearing devices, such as an abduction brace, leg casts, or a leather harness sling, which prevent weight bearing on the affected limb. Another includes the use of various weight-bearing appliances, such as abduction-ambulation braces or casts after a period of bed rest and traction. A third option consists of surgical reconstruction and containment procedures. Conservative therapy must be continued for 2 to 4 years, although braces constructed from lightweight materials allow the child to maintain a nearly normal activity level. Surgical correction, although subjecting the child to additional risks (e.g., from anesthesia, infection, blood transfusion), returns the child to normal activities in 3 to 4 months. The use of home traction has also been explored.
The disease is self-limiting, but the ultimate outcome of therapy depends on early and efficient treatment and the child’s age at the onset of the disorder. Younger children (5 years and under), whose epiphyses are more cartilaginous, have the best prognosis for complete recovery. Children 10 years and older have a significant risk for degenerative arthritis, especially with femoral head deformity at the time of diagnosis. The later the diagnosis is made, the more femoral damage will have occurred before treatment is implemented. In most cases, with good patient compliance with the prescribed regimen, the prognosis is excellent.
Nursing Care Management
Nurses may be the first health professionals to identify affected children and to refer them for medical evaluation. They are also persons on whom the child and the family can rely to help them understand and adjust to the therapeutic measures. Because most of the child’s care is conducted on an outpatient basis, the major emphasis of nursing care is teaching the family the care and management of the corrective appliance selected for therapy. The family needs to learn the purpose, function, application, and care of the corrective device and the importance of compliance to achieve the desired outcome (see Family Focus box).
One of the most difficult aspects associated with the disorder is coping with a normally active child who feels well but must remain relatively inactive during periods where non—weight bearing is required. Suitable activities must be devised to meet the needs of a child in the process of developing a sense of initiative or industry. Activities that meet the creative urges are well received.
SLIPPED CAPITAL FEMORAL EPIPHYSIS
Slipped capital femoral epiphysis (SCFE), or coxa vara, refers to the spontaneous displacement of the proximal femoral epiphysis in a posterior and inferior direction. It develops most frequently shortly before or during accelerated growth and the onset of puberty (children between the ages of 10 and 16 years; median age 13 for boys, 12 for girls) and is most frequently observed in males and obese children. Bilateral involvement occurs in up to 40% to 50% of cases (Greene, 2001).
Pathophysiology
Most cases of SCFE are idiopathic, although it can be associated with endocrine disorders, growth hormone therapy, renal osteodystrophy, and radiotherapy. The cause of idiopathic SCFE is multifactorial and includes obesity, physeal architecture and orientation, and pubertal hormone changes that affect physeal strength. Although obesity stresses the physeal plate, SCFE can also occur in children who are not obese. Radiographs show medial displacement of the epiphysis and uncovered upper portion of the femoral neck adjacent to the physis. There is a widened growth plate and irregular metaphysis. The capital femoral epiphysis remains in the acetabulum, but the femoral neck slips, deforming the femoral head and stretching blood vessels to the epiphysis.
Diagnostic Evaluation
The disorder is suspected when an adolescent or preadolescent youngster displays clinical signs or complains of hip, thigh, or knee pain (Box 31-7). The diagnosis is confirmed by anteroposterior and frog-leg radiographic examination.
Therapeutic Management
Treatment goals are to prevent further slippage and restore function (Perron, Miller, and Brady, 2002). Once the diagnosis is established, the child should be made completely non—weight bearing to prevent further slippage. Surgical treatment varies with the degree of displacement. Traditional methods included presurgery bed rest and traction followed by surgical pinning; surgical pinning involves the placement of a single pin or multiple pins and screws, or osteotomy for deformity correction if needed. Postsurgical care includes non—weight bearing with crutch ambulation until acceptable, painless range of motion is achieved. SCFE is an emergency and requires early diagnosis and treatment to increase the likelihood of a satisfactory cure.
KYPHOSIS AND LORDOSIS
The spine, consisting of numerous segments, can acquire deformity curves of three types: kyphosis, lordosis, and scoliosis (Fig. 31-15). Kyphosis is an abnormally increased convex angulation in the curvature of the thoracic spine (see Fig. 31-15, B). It can occur secondary to disease processes such as tuberculosis, chronic arthritis, osteodystrophy, or compression fractures of the thoracic spine. The most common form of kyphosis is postural. Children, especially during the time when skeletal growth outpaces growth of muscle, are prone to exaggeration of a normal kyphosis. They assume abnormal sitting and standing positions. Scheuermann kyphosis is a thoracic curve greater than 45 degrees with wedging greater than 5 degrees of at least three adjacent vertebral bodies and vertebral irregularity.
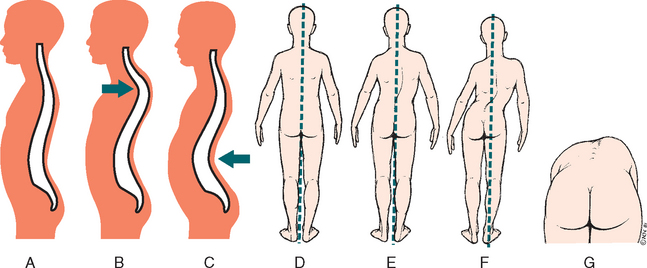
FIG. 31-15 Defects of spinal column. A, Normal spine. B, Kyphosis. C, Lordosis. D, Normal spine in balance. E, Mild scoliosis in balance. F, Severe scoliosis not in balance. G, Rib hump and flank asymmetry seen in flexion caused by rotary component. (Redrawn from Hilt NE, Schmitt EW: Pediatric orthopedic nursing, St Louis, 1975, Mosby.)
Postural kyphosis is almost always accompanied by a compensatory postural lordosis, an abnormally exaggerated concave lumbar curvature. Treatment of kyphosis consists of exercises to strengthen shoulder and abdominal muscles and bracing for more marked deformity. With adolescents, who are self-conscious about their appearance, the best approach is to emphasize the cosmetic value of corrective therapy and to place the responsibility on the adolescent for carrying out an exercise program at home with regular visits to and assessments by a therapist. Treatment with a brace may be indicated until skeletal maturity, and surgical fusion may be considered for severe, painful, or progressive thoracic curves such as Scheuermann kyphosis.
Lordosis is an accentuation of the cervical or lumbar curvature beyond physiologic limits (see Fig. 31-15, C). It may be a secondary complication of a disease process, a result of trauma, or idiopathic. It is often seen in association with flexion contractures of the hip, scoliosis, obesity, DDH, and SCFE. During the pubertal growth spurt, lordosis of varying degrees is observed in teenagers, especially girls. In obese children the weight of the abdominal fat alters the center of gravity, causing a compensatory lordosis. Unlike kyphosis, severe lordosis is usually accompanied by pain.
Treatment involves management of the predisposing cause when possible, such as weight loss and correction of deformities. Postural exercises or support garments are helpful in relieving symptoms in some cases; however, these do not usually effect a permanent cure.
IDIOPATHIC SCOLIOSIS
Idiopathic scoliosis is a complex spinal deformity in three planes, usually involving lateral curvature, spinal rotation causing rib asymmetry, and thoracic hypokyphosis. It is the most common spinal deformity and can be further classified according to age of onset: infantile, at birth or up to 3 years of age; juvenile, which develops during childhood; or, most commonly, adolescent, which develops during the growth spurt of early adolescence.
Idiopathic scoliosis can be caused by a number of conditions and may occur alone or in association with other diseases, particularly neuromuscular conditions. In most cases, however, there is no apparent cause, hence the name idiopathic scoliosis. There appears to be a genetic component to the etiology of idiopathic scoliosis; however, the exact relationship has yet to be established. The following section is limited to a discussion of adolescent idiopathic scoliosis.
Idiopathic scoliosis is most noticeable during the preadolescent growth spurt. Parents frequently bring a child for follow-up on an abnormal school scoliosis screening or because of ill-fitting clothes, such as poorly fitting slacks. School screening is somewhat controversial, since there are no controlled studies to demonstrate improved outcomes and a reported number of false positives lead to referrals (Bunnell, 2005); however, many experts suggest that school screening has increased public and professional awareness of scoliosis and has decreased the number of significant cases of serious deformity (Newton and Wenger, 2001). The American Academy of Pediatrics (2000b) recommends scoliosis screening at the time of primary practitioner visits in all preadolescent and adolescent children.
Diagnostic Evaluation
Observation is performed behind an undressed (in undergarments), standing child, noting asymmetry of shoulder height, scapular or flank shape, or hip height and alignment. When the child bends forward at the waist (the Adams test) with hanging arms, asymmetry of the ribs and flanks may be noted. A scoliometer is also used in the initial screening to measure truncal rotation (also measured by the Adams test). Often a primary curve and a compensatory curve will place the head in alignment with the gluteal cleft. However, in the uncompensated curve the head and hips are not aligned (see Fig. 31-15, E and F). (See Spine, Chapter 6, for additional information.) Definitive diagnosis is made by radiographs of the child in the standing position and use of the Cobb technique (standard measurement of angle curvature), which establishes the degree of curvature. The Risser scale is used to evaluate skeletal maturity on the radiographs; the scale assists in making a determination of the likely progression of the spinal angulature as the child’s bones mature. Not all spinal curvatures are scoliosis. A curve of less than 10 degrees is considered a postural variation. Curves of less than 20 degrees are mild and, if nonprogressive, do not require treatment.
Therapeutic Management
Current management options include observation with regular clinical and radiographic evaluation, orthotic intervention (bracing), and surgical spinal fusion. Treatment decisions are based on the magnitude, location, and type of curve; the child’s age and skeletal maturity; and any underlying or contributing disease process.
Bracing and Exercise.: For many curves in the growing child and adolescent, bracing may be the treatment of choice. It is important to realize that bracing is not curative, but that it may slow the progression of the curvature to allow skeletal growth and maturity. The two most common types of bracing are (1) the Boston and Wilmington braces, which are underarm orthoses customized from prefabricated plastic shells, with corrective forces for each patient using lateral pads and decreasing lumbar lordosis, and (2) a TLSO (thoracolumbosacral orthosis), which is an underarm orthosis made of plastic that is custom molded to the body and then shaped to correct or hold the deformity (Fig. 31-16). The Milwaukee brace, which is an individually adapted brace that includes a neck ring, is rarely used in scoliosis but is sometimes used in the treatment of kyphosis. The Charleston nighttime bending brace is worn only when the child is in bed because it prevents walking because of the severity of the trunk bend. Bracing, although used as the gold standard treatment for mild to moderate curvatures, has not proved to be entirely effective in the treatment of scoliosis (Newton and Wenger, 2001), and some retrospective studies show only slight variation in outcomes in relation to the type of brace used. Compliance in wearing the brace is difficult because of the child’s age and preoccupation with body image and appearance.
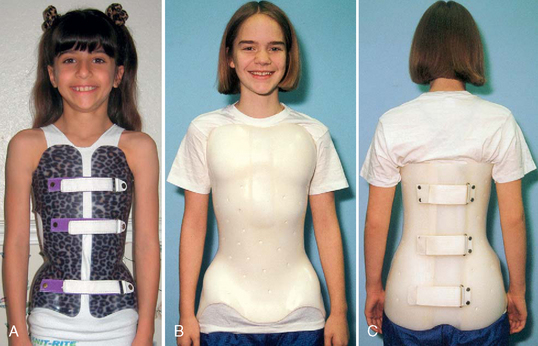
FIG. 31-16 A, Standard thoracolumbosacral orthotic (TLSO) brace for idiopathic scoliosis. Brace may be decorated to make it more acceptable to adolescents. B, Variation of a standard TLSO brace that fastens in the back to provide needed support. C, Posterior view of same brace.
Exercises alone and chiropractic treatment are rarely of value for managing scoliosis; transcutaneous electrical nerve stimulation has also proved to be an ineffective treatment for this condition. Exercises are of benefit when used in conjunction with bracing to maintain and strengthen spinal and abdominal muscles during treatment.
Surgical Management.: Surgical intervention may be required for correction of severe curves (usually 40 degrees or more). The degree of curvature and the cause inform the decision for surgery. Bracing and exercise have been universally disappointing in curves greater than 40 degrees, and paralytic and congenital curves, which will eventually progress, are best treated with early surgical stabilization if the child’s health status will allow major surgery. The child’s age and location of the curvature influence the decision for surgery, and any progressive or severe curve that does not respond to more conservative orthotic measures requires surgical correction. Difficulties with balance or seating, respiratory excursion, or pain are also considered.
The surgical technique consists of realignment and straightening with internal fixation and instrumentation combined with bony fusion (arthrodesis) of the realigned spine. The goals of surgical intervention are to correct the curvatures on the sagittal and coronal planes and to have a solid, pain-free fusion in a well-balanced torso, with maximum mobility of the remaining spinal segments.
Many instrumentation systems are available, including Harrington, Dwyer, Zielke, Luque, Cotrel-Dubousset, Isola, TSRH (Texas Scottish Rite Hospital), and Moss Miami. Selection of the system is individualized according to the patient’s needs and surgeon’s preference. Posterior or anterior approaches can be used.
The Harrington system, the first internal spinal instrumentation device, consists of distraction and compression rods, hooks, and nuts. The posterior elements are decorticated, and bone from the iliac crest or donor bone is placed across the vertebrae to provide fusion. Postoperatively the child is log-rolled to prevent spinal motion and a molded plastic jacket is used to stabilize the spine until the fusion is solid.
The Luque-rod segmental spinal instrumentation provides segmental stability with the use of wires and L-shaped rods. By way of a posterior approach, the wires are threaded beneath the lamina of each vertebra and tightened around the rods resting along the transverse processes to stabilize the spine. Bone from the iliac crest or donor bone is used to fuse the spine. The advantage of this method is that the patient can be mobile within a few days and requires no postoperative immobilization. The disadvantage is the risk of nerve damage.
The Cotrel-Dubousset instrumentation combines the Harrington and L-rod approaches by using bilateral rods and hooks at many sites. Anterior approaches using the Dwyer or Zielke instrumentation involve screws into the vertebral bodies connected by a cable or rod. These systems require postoperative immobilization with a custom-fitted plastic jacket.
Nursing Care Management
Treatment for scoliosis extends over a significant portion of the affected child’s period of growth. In adolescents this period is the one in which their identity, both physical and psychologic, is formed. The identification of scoliosis as a “deformity,” in combination with unattractive appliances and a significant surgical procedure, can have a negative effect on the already fragile adolescent body image. The adolescent and family require excellent nursing care not only for physical needs to be met, but also for psychologic needs associated with the diagnosis, surgery, postoperative recovery, and eventual rehabilitation (Slote, 2002). Although adolescents with scoliosis are encouraged to participate in most peer activities, necessary therapeutic modifications are likely to make them feel different and apart. Nursing care of the adolescent who is facing scoliosis surgery, potential social isolation, pain, and uncertainty, not to mention misunderstood emotions and body image issues, must be evaluated from the adolescent’s perspective to be successful in meeting the individual’s needs (Napierkowski, 2007).
When a child or adolescent first faces the prospect of a prolonged period in a brace, jacket, or other device, the therapy program and the nature of the device must be explained thoroughly to both the child and the parents so that they will understand the anticipated results, how the appliance corrects the defect, the freedoms and constraints imposed by the device, and what they can do to help achieve the desired goal. Management involves the skills and services of a team of specialists, including the orthopedist, physical therapist, orthotist (a specialist in fitting orthopedic braces), nurse, social worker, and sometimes a thoracic or pulmonary specialist.
It is difficult for a child or adolescent to be restricted at any phase of development, but the adolescent needs continual positive reinforcement, encouragement, and as much independence as can be safely assumed during this time. Adolescents appreciate guidance and assistance regarding anticipated problems, such as selection of clothing and participation in social activities. Socialization with peers is strongly encouraged, and every effort is expended to help the adolescent feel attractive and worthwhile.
Preoperative Care.: The preoperative workup usually involves a radiographic series, including bending and traction films, pulmonary function studies, and a number of routine laboratory studies (including prothrombin, partial thromboplastin, and bleeding times; blood count; electrolyte levels; urinalysis and urine culture; and blood levels of any medications). Because spinal surgery usually involves considerable blood loss, several options are considered preoperatively to maintain or replace blood volume. These options include autologous blood donations obtained from the patient before the surgery; intraoperative blood salvage; intraoperative hemodilution; erythropoietin administration; and controlled induced hypotension, which must be carefully monitored at all times to prevent physiologic instability (Newton and Wenger, 2001).
Surgery for spinal fusion is complex, and often adolescents who require the procedure because of idiopathic scoliosis are not familiar with medical terms, procedures, or experiences. Preoperative teaching is critical for the adolescent to be able to cooperate and participate in his or her treatment and recovery. Because the surgery is extensive, the patient is taught how to manage his or her own patient-controlled analgesia (PCA) pump; how to log-roll; and the use and function of other equipment, such as a chest tube (for anterior repair) and Foley catheter. It is recommended that the child or adolescent bring a favorite toy (age dependent) or personal items such as a favorite stuffed animal, laptop computer (for web surfing and e-mails), movie player, MP3 player, or portable compact disc player for postoperative use. Meeting with a peer who has undergone a similar surgery is also valuable (Slote, 2002).
Postoperative Care.: After surgery, patients are monitored in an acute care setting and log-rolled when changing position to prevent damage to the fusion and instrumentation. Skin care is important, and pressure-relieving mattresses or beds may be needed to prevent pressure wounds (see Maintaining Healthy Skin, Chapter 22).
In addition to the usual postoperative assessments—of wound, circulation, and vital signs—the neurologic status of the patient’s extremities requires special attention. Prompt recognition of any neurologic impairment is imperative because delayed paralysis may develop that requires surgical intervention. The most common postoperative problems after spinal fusion include neurologic injury or spinal cord injury, hypotension from acute blood loss, wound infection, delayed neurologic injury, and implanted hardware complications (Newton and Wenger, 2001). The child usually has considerable pain for the first few days after surgery and requires frequent administration of pain medication, preferably IV opioids administered on a regular schedule. For children able to understand the concept, PCA is recommended (see Pain Assessment; Pain Management, Chapter 7).
In most cases the patient begins ambulation as soon as possible, depending on the instrumentation used—generally by the second or third postoperative day. They are discharged by 1 week, depending on the surgical approach. In addition to pain management, the patient is evaluated for skin integrity, adequate urinary output, fluid and electrolyte balance, and ileus (Slote, 2002). The latter may be particularly distressful for the adolescent who is self-conscious. Discharge planning should include a timetable for follow-up with the practitioner and resumption of regular activities.
The patient may start physical therapy as soon as he or she is able, beginning with range-of-motion exercises on the first postoperative day and many of the activities of daily living in the following days. Self-care, such as washing and eating, is always encouraged. Throughout the hospitalization age-appropriate activities and contact with family and friends are important parts of nursing care and planning (see Immobilization, p. 1107).
The family is encouraged to become involved in the patient’s care to facilitate the transition from hospital to home management. An organization that provides education and services to both families and professionals is the National Scoliosis Foundation.* The American Academy of Orthopaedic Surgeons† and Scoliosis Research Society,‡ an organization of physicians and scientists, have published an excellent book, Scoliosis, and the Scoliosis Research Society has educational information available on its website.
INFECTIONS OF BONES AND JOINTS
Osteomyelitis, an infectious process in the bone, can occur at any age but most frequently is seen in children 10 years of age or younger. Staphylococcus aureus is the most common causative organism. Acute hematogenous osteomyelitis results when a bloodborne bacterium causes an infection in the bone. Common foci include infected lesions, upper respiratory tract infections, otitis media, tonsillitis, abscessed teeth, pyelonephritis, and infected burns. Exogenous osteomyelitis is acquired from direct inoculation of the bone from a puncture wound, open fracture, surgical contamination, or adjacent tissue infection. Subacute osteomyelitis has a longer course and may be caused by less virulent microbes with a walled-off abscess or Brodie abscess, typically in the proximal or distal tibia. Chronic osteomyelitis is a progression of acute osteomyelitis and is characterized by dead bone, bone loss, and drainage and sinus tracts.
Generally, healthy bone is not likely to become infected. Factors that contribute to infection include inoculation with a large number of organisms, presence of a foreign body, bone injury, high virulence of an organism, immunosuppression, and malnutrition; certain types and locations of bone are also more vulnerable to infection.
Typically children with acute hematogenous osteomyelitis are seen with a 2- to 7-day history of pain, warmth, tenderness, and decreased range of motion in the affected limb, along with systemic symptoms of fever, irritability, and lethargy (Box 31-8). Symptoms often resemble those observed in other diseases involving bones such as arthritis or leukemia.
Pathophysiology
Osteomyelitis can be acquired exogenously by direct inoculation of bone during trauma or surgery; the hand and foot are commons sites. Hematogenous osteomyelitis is seeded by organisms from a preexisting infection such as tonsillitis or impetigo or from a contiguous source such as an adjacent infected bone or joint. Hematogenous osteomyelitis usually occurs in the metaphyses of long bones such as the femur or tibia. The infecting organism travels from the site of infection to the small end-artery capillary loops in the bone metaphyses, causing obstruction and initiating infection, with complications of bone destruction and abscess formation. In infants the diagnosis is challenging because of difficulty localizing symptoms and the increased likelihood of multiple bone involvement.
Diagnostic Evaluation
Organism identification and antibiotic susceptibility testing are essential for effective therapy. Cultures of aspirated subperiosteal pus along with cultures of blood, joint fluid, and infected skin samples should be obtained. Bone biopsy is indicated if blood culture results and radiographic findings are not consistent with osteomyelitis. Supporting evidence for osteomyelitis includes leukocytosis and elevated erythrocyte sedimentation rate. Radiographic signs, except for soft-tissue swelling, are evident only after 2 to 3 weeks. A three-phase technetium bone scan can show areas of increased blood flow, such as occurs in early stages in infected bone, and is useful in locating multiple sites; however, it is not a diagnostic test. CT can detect bone destruction, and MRI provides anatomic details useful in delineating the area of involvement, especially if surgical intervention is planned. Sometimes the osteomyelitis may be unrecognized if it occurs as a complication of a severe toxic and debilitating disease.
Therapeutic Management
After culture specimens are obtained, empiric therapy is started with IV antibiotics covering the mostly likely organisms. For S. aureus, nafcillin or clindamycin is generally used; methicillin-resistant S. aureus may require vancomycin. When the infective agent is identified, administration of the appropriate antibiotic is initiated and continued for at least 4 weeks, but the length of therapy is determined by the duration of the symptoms, the response to treatment, and the sensitivity of the organism. In selected cases oral antibiotic therapy may follow a shorter IV course. Because of the prolonged duration of high-dose antibiotic therapy, it is important to monitor for hematologic, renal, hepatic, ototoxic, and other potential side effects.
Surgery may be indicated if there is no response to specific antibiotic therapy, persistent soft-tissue abscess is seen, or the infection spreads to the joint. Opinions differ regarding surgical intervention, but many advocate sequestrectomy and surgical drainage to decompress the metaphyseal space before pus erupts and spreads to the subperiosteal space, forming abscesses that strip the periosteum from bone or form draining sinuses. When these complications occur, a chronic infection usually persists. When surgical drainage is carried out, polyethylene tubes are placed in the wound; one tube instills an antibiotic solution directly into the infected area by gravity, and the other, connected to a suction apparatus, provides drainage.
Nursing Care Management
During the acute phase of illness any movement of the affected limb will cause discomfort; therefore the child is positioned comfortably with the affected limb supported. Moving and turning are carried out carefully and gently to minimize pain. The child may require pain medication or sedation. Vital signs are taken and recorded frequently, and measures are implemented to reduce a significant temperature elevation.
Antibiotic therapy requires careful observation and monitoring of the IV equipment and site. Because more than one antibiotic is usually administered, the compatibility of the drugs is determined and care is taken to avoid mixing incompatible drugs. For long-term antibiotic therapy, an intermittent infusion device or peripherally inserted central catheter is used. Antibiotic therapy is often continued at home.
Standard precautions are put in effect for children with open wounds, depending on the institution’s policies. The wound is managed according to the practitioner’s directions. Administration of antibiotic solution directly into the wound is most efficiently accomplished using a regular infusion setup that is prepared and regulated in the same manner as for any IV infusion. Intake and output are measured and recorded, and the character of both the wound and drainage is noted. The amount and character of drainage on the wound dressing are also noted.
Casts are sometimes used for immobilization, and, if so, routine cast care is carried out. The extremity is examined for sensation, circulation, and pain, and the area over the inflammation is usually left open for observation. The affected area, casted or uncasted, is assessed for color, swelling, heat, and tenderness.
The child usually has a poor appetite and may be prone to vomiting. The appetite returns as the acute symptoms recede. During convalescence, adequate nutrition must be maintained to aid healing and formation of new bone.
When the acute stage subsides, children begin to feel better, appetite improves, and they become interested in their surroundings and relationships. They wish to move about in bed and are allowed to do so. However, weight bearing on the affected limb is not permitted until healing is well under way in order to avoid pathologic fractures. Provision of diversional and constructive activities becomes an important nursing intervention. Children are usually confined to bed for some time after the acute phase but may be allowed to move about on a stretcher or in a wheelchair when isolation and bed rest are no longer necessary. At this stage the continuous IV infusion may be replaced by a heparin lock system to allow greater freedom.
As the infection subsides, physical therapy is instituted to ensure restoration of optimum function. The child is usually discharged on a regimen of oral antibiotics, and progress is followed closely for some time.
SEPTIC ARTHRITIS
Septic arthritis is a bacterial infection in the joint. It usually results from hematogenous spread or from direct extension of an adjacent cellulitis or osteomyelitis. Direct inoculation from trauma accounts for 15% to 20% of septic arthritis cases. The most common causative organism is S. aureus. Community-acquired methicillin-resistant S. aureus is commonly a cause of septic arthritis (Gutierrez, 2005). In addition to S. aureus, pathogens seen in neonates include group B streptococci, Escherichia coli, and Candida albicans. In children 2 months to 5 years of age, S. aureus, Streptococcus pyogenes, Streptococcus pneumoniae, and Kingella kingae are the primary organisms causing infection, whereas children older than 5 years are more likely to be infected by S. aureus and S. pyogenes; sexually active adolescents may be infected by Neisseria gonorrhoeae (Gutierrez, 2005).
Knees, hips, ankles, and elbows are the most common joints affected. Clinical manifestations include severe joint pain, swelling, warmth of overlying tissue, and occasionally erythema. The child is resistant to any joint movement. Features of systemic illness such as fever, malaise, headache, nausea, vomiting, and irritability may also be present.
Therapeutic Management and Nursing Care Management
The affected joint is aspirated and the specimen evaluated by Gram stain; culturing (including separate cultures for Haemophilus influenzae and N. gonorrhoeae); and determination of leukocyte count and glucose, lactate, and protein levels. An infection involving the hip, however, is considered a surgical emergency to prevent compromised blood supply to the head of the femur (Lampe, 2007). In addition, blood culture should be performed, and complete blood count with differential and erythrocyte sedimentation rate or C-reactive protein level should be obtained. Early radiographic findings are limited to soft-tissue swelling but may reveal a foreign body, and such films always provide a baseline for comparison. Technetium scans reveal areas of increased blood flow but will not differentiate between sites. MRI and CT scans provide more detailed images of cartilage loss, joint narrowing, erosions, and ankylosis of progressive disease.
Surgical intervention may also be required if there was a penetrating wound or a possible involvement of a foreign object. Physical therapy may be initiated for the child who is immobilized in a cast or traction to prevent flexion contractures.
Treatment is IV antibiotic therapy based on Gram stain results and the clinical presentation. The benefits of serial aspirations to demonstrate sterility of synovial fluid and reduce pressure or pain are controversial. Pain management is an important aspect of nursing care, particularly with involvement of a large joint such as the hip. Additional nursing care is the same as for osteomyelitis.
SKELETAL TUBERCULOSIS
In children tubercular infection of the bones and joints is acquired by lymphohematogenous spread at the time of primary infection. Occasionally it is from chronic pulmonary tuberculosis. Skeletal tubercular infection is not common in the United States but should be considered in communities with high tuberculosis case rates. The infection is most likely to involve the vertebrae, causing tubercular spondylitis. If the infection is progressive, it causes Pott disease with destruction of the vertebral bodies and results in kyphosis. Symptoms are insidious. The child may report persistent or intermittent pain. Other findings include joint swelling and stiffness; fever and weight loss are not common. Tubercular arthritis can also affect single joints such as a knee or hip and tends to cause severe destruction of adjacent bone. Infection in the fingers causes spina ventosa, a tuberculous dactylitis.
As with pulmonary tuberculosis, the index case should be located. A family and environmental history needs to be obtained and tuberculin skin tests (TSTs) performed. Results of TSTs are positive for the majority of children with tuberculous arthritis; however, the results are not diagnostic, and the clinical and laboratory features do not differentiate tubercular arthritis from a nontubercular septic arthritis. Diagnosis requires isolation of Mycobacterium tuberculosis from the site. Patients with the susceptible organism start treatment with combined antituberculosis chemotherapy (isoniazid, rifampin, and pyrazinamide); directly observed therapy (DOT) is preferred. (See also Chapter 23.)
BONE AND SOFT-TISSUE TUMORS
Malignant bone tumors represent less than 5% of all malignant neoplasms, but 85% of all primary malignant bone tumors in children are either osteogenic sarcoma or Ewing sarcoma. The peak ages during childhood are 15 to 19 years. The sexes are affected equally until puberty, at which time the ratio approaches 2:1 in favor of males. This propensity for males with a peak incidence during adolescence is thought to result from the accelerated growth rate of osseous tissue.
Neoplastic disease can arise from any tissues involved in bone growth, such as osteoid matrix, bone marrow elements, fat, blood and lymph vessels, nerve sheath, and cartilage. They have several characteristics in common, which are discussed in the following sections. Specific information about each tumor is then presented.
Most malignant bone tumors produce localized pain in the affected site, which may be severe or dull and may be attributed to trauma or the vague complaint of “growing pains.” The pain is often relieved by a flexed position, which relaxes the muscles overlying the stretched periosteum. Frequently it draws attention when the child limps, curtails physical activity, or is unable to hold heavy objects (Box 31-9).
Diagnostic Evaluation
Diagnosis begins with a thorough history and physical examination. A primary objective is to rule out causes such as trauma or infection. Careful questioning regarding pain is essential in determining the duration and rate of tumor growth. Physical assessment focuses on functional status of the affected area; signs of inflammation; size of the mass; involvement of regional lymph nodes; and any systemic indication of generalized malignancy, such as anemia, weight loss, and frequent infection.
Definitive diagnosis is based on radiologic studies, such as CT to determine the extent of the lesion; MRI to assess soft tissue, tumor boundaries, and nerve and vessel involvement; radioisotope bone scans to evaluate metastasis; and either needle or surgical bone biopsy to determine the histologic pattern. Radiologic findings are characteristic for each type of tumor. In osteogenic sarcoma, needlelike new bone formation growing at right angles to the diaphysis (shaft) produces a “sunburst” appearance. In Ewing sarcoma the deposits of new bone in layers under the periosteum produce an “onion skin” appearance. In both types of bone tumors, soft-tissue infiltration may be apparent.
At present there is no reliable biochemical test for bone cancers. Elevated alkaline phosphatase levels may occur in osteoid tumors. Several tests may be done for differential diagnosis in terms of secondary bone metastasis from Wilms tumor, neuroblastoma, retinoblastoma, rhabdomyosarcoma, lymphoma, or leukemia. Lung computerized tomography is usually a standard procedure, since pulmonary metastasis is the most common complication of primary bone tumors. Bone marrow aspiration is helpful in diagnosing Ewing sarcoma in the rare event that the child has bone marrow metastasis.
OSTEOSARCOMA
Osteosarcoma (osteogenic sarcoma) is the most common bone cancer in children. Its peak incidence is between 10 and 25 years of age (Link, Gebhardt, and Myers, 2006). It presumably arises from bone-forming mesenchyme, which gives rise to malignant osteoid tissue. Most primary tumor sites are in the metaphysis (wider part of the shaft, adjacent to the epiphyseal growth plate) of long bones, especially in the lower extremities. More than half occur in the femur, particularly the distal portion, with the rest involving the humerus, tibia, pelvis, jaw, and phalanges.
Therapeutic Management
Optimum treatment of osteosarcoma is surgery and chemotherapy. The surgical approach consists of surgical biopsy followed by either limb salvage or amputation. Depending on the tumor site, surgery includes amputation of the affected extremity at least 7.5 cm (3 inches) above the proximal tumor margin or above the joint proximal to the involved bone. With tumors of the distal femur, preservation of the hip joint may be possible. Other procedures include an above-the-knee amputation for tumors of the tibia or fibula, a hemipelvectomy for tumors of the innominate (hip) bone, and a forequarter amputation (removal of arm, scapula, and portion of the clavicle on the affected side) for tumors of the upper humerus.
The other surgical approach for selected patients is the limb salvage procedure, which involves en bloc resection of the primary tumor with prosthetic replacement of the involved bone. For example, with osteosarcoma of the distal femur, a total femur and joint replacement is performed. Frequently children undergoing a limb salvage procedure will receive preoperative chemotherapy in an attempt to decrease the tumor size and make surgery more manageable (Link, Gebhardt, and Myers, 2006).
Chemotherapy plays a vital role in treatment of osteosarcoma. Antineoplastic drugs, such as high-dose methotrexate with citrovorum factor rescue, doxorubicin, bleomycin, actinomycin D, cyclophosphamide, ifosfamide, and cisplatin, may be administered singly or in combination and may be employed both before and after surgery. When pulmonary metastasis is found, thoracotomy and chemotherapy have resulted in prolonged survival and potential cure. These combined-modality approaches have significantly improved the prognosis in osteosarcoma to approximately 85% in nonmetastatic patients (Lanzkowsky, 2000). New trials have recently been completed using muramyl tripeptide phosphatidylethanolamine to eradicate micrometastases by stimulating macrophages to kill tumor cells not eliminated by chemotherapy (Link, Gebhardt, and Myers, 2006).
Nursing Care Management
Nursing care depends on the type of surgical approach. Obviously the family may have more difficulty adjusting to an amputation than a limb salvage procedure. In either instance, preparation of the child and family is critical. Straightforward honesty is essential in gaining the child’s cooperation and trust. The diagnosis of cancer should not be disguised with falsehoods such as “infection.” To accept the need for radical surgery, the child must be aware of the lack of alternatives for treatment. Although the responsibility of telling the child is generally left to the physician, the nurse should be present at the discussion or be aware of exactly what is said. The child should be told a few days before surgery to allow him or her time to think about the diagnosis and consequent treatment and to ask questions.
Sometimes children have many questions about the prosthesis, limitations on physical ability, and prognosis in terms of cure. At other times they react with silence or with a calm manner that belies their concern and fear. Either response must be accepted, since it is part of the grieving process of a loss. For those who desire information, it may be helpful to introduce them to another amputee before surgery or to show them pictures of the prosthesis.* However, the nurse must be careful not to overwhelm children with information. A sound approach is to answer questions without offering additional information. For those who do not pursue additional information, the nurse expresses a willingness to talk.
The child is also informed of the need for chemotherapy and its side effects before surgery. Caution must be exercised about offering too much information at one time. It is wise to discuss hair loss with an emphasis on positive aspects, such as wearing a wig. Because bone tumors affect adolescents and young adults, it is not unusual for them to become angry over all the radical body alterations.
If an amputation is performed, the child is usually fitted with a temporary prosthesis immediately after surgery, which permits early functioning and fosters psychologic adjustment. If this is not done, the child requires stump care, which is the same as for any amputee. A permanent prosthesis is usually fitted within 6 to 8 weeks. During hospitalization the child begins physical therapy to become proficient in the use and care of the device.
Phantom limb pain may develop after amputation. This symptom is characterized by sensations such as tingling, itching, and, more frequently, pain felt in the amputated limb. The child and family need to know that the sensations are real, not imagined. Amitriptyline (Elavil) has been used successfully in children to decrease the pain (Olsson, 1999).
Discharge planning must begin early in the postoperative period. Once the child has begun physical therapy, the nurse should consult with the therapist and practitioner to evaluate the child’s physical and emotional readiness to reenter school. It is an opportune time to involve a community nurse in the child’s home care. Every effort is made to promote normalcy and gradual resumption of realistic preamputation activities.* Role-playing in anticipation of such experiences is beneficial in preparing the child for the inevitable confrontation by others. Environmental barriers, such as stairs, are assessed in terms of the accessibility in the school and home, especially because the child may need to use crutches or a wheelchair before complete healing and prosthetic competency are achieved.
The nurse encourages the child to select clothing that best camouflages the prosthesis, such as pants or long-sleeved shirts. Well-fitted prostheses are so natural looking that girls can usually wear sheer stockings without revealing the device. Emphasizing feminine or masculine apparel helps the child regain a feeling of self-identity. Even during the postoperative period, encouraging the child to wear blue jeans and a T-shirt may distract attention from the deformity and focus it on familiar aspects of appearance.
The family and child need much support in adjusting not only to a life-threatening diagnosis but also to alteration in body form and function. Because loss of a limb entails a grieving process, those caring for the child need to recognize that the reactions of anger and depression are normal and necessary. Often parents view the anger as a direct affront to them for allowing the amputation to occur, or they see the depression as rejection. These are not personal attacks but the child’s attempts to cope with a loss.
EWING SARCOMA (PRIMITIVE NEUROECTODERMAL TUMOR)
Ewing sarcoma, classified as a primitive neuroectodermal tumor (PNET), is the second most common malignant bone tumor (after osteosarcoma) in childhood. Ewing sarcoma arises in the marrow spaces of the bone rather than from osseous tissue. The tumor originates in the shaft of long and trunk bones, most often affecting the femur, tibia, fibula, humerus, ulna, vertebrae, scapula, ribs, pelvic bones, and skull (Link, Gebhardt, and Myers, 2006). It occurs almost exclusively in individuals under age 30, with the majority being between 4 and 25 years of age.
Therapeutic Management
Surgical amputation is not routinely recommended but may be considered when the results of radiotherapy render the extremity useless or deformed (e.g., from restricted growth in young children). The treatment of choice is intensive irradiation of the involved bone combined with chemotherapy. A widely used drug regimen includes vincristine, actinomycin D, cyclophosphamide or ifosfamide, etoposide, and doxorubicin. The addition of ifosfamide and etoposide has increased the 3-year survival to 80% (Lanzkowsky, 2000).
Nursing Care Management
The psychologic adjustment to Ewing sarcoma is typically less traumatic than it is to osteosarcoma because of the preservation of the affected limb. Many families accept the diagnosis with relief in knowing that this type of bone cancer does not necessitate amputation, and initially they may not be aware of the damaging effects on the irradiated site. Consequently they need preparation for the various diagnostic tests, including bone marrow aspiration and surgical biopsy, and adequate explanation of the treatment regimen. High-dose radiotherapy often causes a skin reaction of dry or moist desquamation followed by hyperpigmentation. The child should wear loose-fitting clothes over the irradiated area to minimize additional skin irritation. Because of increased sensitivity, the area is protected from sunlight and sudden changes in temperature, such as from heating pads or ice packs. The child is encouraged to use the extremity as tolerated. Occasionally the physical therapist may plan an active exercise program to preserve maximum function.
The child needs the same considerations for adjusting to the effects of chemotherapy as any other patient with cancer. The drug regimen usually results in hair loss, severe nausea and vomiting, peripheral neuropathy, and possibly cardiotoxicity. Every effort should be made to outline a treatment plan that allows the child maximum resumption of a normal lifestyle and activities (Kline and Sevier, 2003). (See also Nursing Care Plan: The Child with Cancer, Chapter 26.)
RHABDOMYOSARCOMA
Soft-tissue sarcomas are the fourth most common type of solid tumors in children. These malignant neoplasms originate from undifferentiated mesenchymal cells in muscles, tendons, bursae, and fascia, or in fibrous, connective, lymphatic, or vascular tissue. They derive their name from the specific tissue(s) of origin, such as myosarcoma (myo, muscle). Rhabdomyosarcoma (rhabdo, striated) is the most common soft-tissue sarcoma in children. Striated (skeletal) muscle is found almost anywhere in the body, so these tumors occur in many sites, the most common of which are the head and neck, especially the orbit. The disease occurs in children in all age-groups but is most common in children younger than 5 years of age. Its incidence is approximately 8.5 per 1 million for Caucasian children but only 4.0 per 1 million for African-American children in the age-group, from 2 to 19 years (Lanzkowsky, 2000). Rhabdomyosarcoma arises from embryonic mesenchyme.
The initial signs and symptoms are related to the site of the tumor and compression of adjacent organs (Box 31-10). Some tumor locations, particularly the orbit, produce symptoms early in the course of the illness and contribute to rapid diagnosis and an improved prognosis. Other tumors, such as those of the retroperitoneal area, produce no symptoms until they are large, invasive, and widely metastasized. Unfortunately, many of the signs and symptoms attributable to rhabdomyosarcoma are vague and frequently suggest a common childhood illness, such as “earache” or “runny nose.” In some instances a primary tumor site is never identified.
Diagnostic Evaluation
Diagnosis begins with a careful examination of the head and neck area, particularly palpation of a nontender, hard mass. The nasopharynx and oropharynx are inspected for any evidence of a visible mass. Radiographic studies to isolate a tumor site are performed, accompanied by chest x-ray examinations, CT, MRI, bone surveys, and bone marrow aspiration to rule out metastasis. A lumbar puncture is indicated for head and neck tumors to examine the cerebrospinal fluid for malignant cells. An excisional biopsy is done to confirm the histologic type.
Careful staging is extremely important for planning treatment and determining the prognosis. The Intergroup Rhabdomyosarcoma Study has established clinical staging (Wexler, Meyer, and Helman, 2006).
With the change in treatment from radical surgery or radiotherapy to a multimodal approach, survival rates for all stages have increased considerably. Five-year survival rates are approximately 65% (Lanzkowsky, 2000; Wexler, Meyer, and Helman, 2006). Data suggest that children who remain disease free for 2 years are probably cured; however, if relapse occurs, the prognosis for long-term survival is extremely poor.
Therapeutic Management
Because this tumor is highly malignant, with metastasis frequently occurring at the time of diagnosis, aggressive multimodal therapy is recommended. In the past, radical surgical removal of the tumor was the treatment of choice, but with improved survival from combined chemotherapy and irradiation, surgery plays a lesser role. Complete removal of the primary tumor is advocated whenever possible. However, only biopsy is required in certain tumor locations, such as those of the orbit, when followed by irradiation and chemotherapy. This is a fortunate change, since it avoids the devastating effects of enucleation, amputation, or pelvic exenteration.
High-dose irradiation to the primary tumor is recommended, except in group I tumors. Chemotherapy plays a major role in treatment of all groups. Drugs that are cytotoxic for rhabdomyosarcoma are vincristine, actinomycin D, ifosfamide, cisplatin, carboplatin, etoposide, cyclophosphamide, topotecan, melphalan, and doxorubicin, which are administered for 1 to 2 years, depending on the stage of the disease (Lanzkowsky, 2000).
Nursing Care Management
The nursing responsibilities are similar to those for other types of cancer, especially the solid tumors when surgery is employed. Specific objectives include (1) careful assessment for signs of the tumor, especially during well-child examinations; (2) preparation of the child and family for the multiple diagnostic tests, and (3) supportive care during each stage of multimodal therapy (Kline and Sevier, 2003).
DISORDERS OF JOINTS
JUVENILE IDIOPATHIC ARTHRITIS (JUVENILE RHEUMATOID ARTHRITIS)
Juvenile idiopathic arthritis (JIA) is a new name replacing juvenile rheumatoid arthritis (JRA) in the research literature and more slowly in clinical practice. The JRA nomenclature revision to JIA was due in part to the minimally applicable reference to “rheumatoid” in JRA. Only a small percentage of children have a positive rheumatoid factor, yet the name burdens the family with images of adult disfiguring rheumatoid arthritis. Furthermore, the JRA classification system focused more on disease at onset vs disease progression, which is more important (Warren, Perez, Curry, and others, 2001).
Semantics aside, JIA is a chronic autoimmune inflammatory disease causing inflammation of joints and other tissue with an unknown cause. JIA starts before age 16 years with peak onset between 1 and 3 years of age. Twice as many girls as boys are affected. The incidence is reported to be approximately 13.9 per 100,000 children per year among Caucasian children, with an overall prevalence of approximately 113 per 100,000 children (Miller and Cassidy, 2007). The cause is unknown, but two factors are hypothesized: immunogenic susceptibility and an environmental or external trigger such as a virus (e.g., rubella, Epstein-Barr virus, parvovirus B19) (Miller and Cassidy, 2007).
Pathophysiology
The disease process is characterized by chronic inflammation of the synovium with joint effusion and eventual erosion, destruction, and fibrosis of the articular cartilage. Adhesions between joint surfaces and ankylosis of joints occur if the inflammatory process persists.
Clinical Manifestations
The outcome of JIA is variable and unpredictable. The disease, even in severe forms, is rarely life threatening but can cause significant disability. The arthritis tends to wax and wane and eventually becomes inactive in approximately 70% of the cases; however, these children may have severe or minimal joint damage remaining when active arthritis abates. Approximately 30% of the children will have progressive arthritis into adulthood. Their arthritis can cause significant joint deformity and functional disability, requiring medication, physical therapy, and perhaps future joint replacement. Chronic and acute uveitis can cause permanent vision loss if undiagnosed and not aggressively treated.
Classification of Juvenile Idiopathic Arthritis
JIA is not a single disease, but a heterogeneous group of diseases. The universal Durban classification of JIA, revised and published in 1998, lists several disease categories, each with its own set of criteria and exclusions, which continue to be revised (Petty, Southwood, Manners, and others, 2004; Petty, Southwood, Baum, and others, 1998):
Systemic arthritis is arthritis in one or more joints associated with at least 2 weeks of fever, rash, lymphadenopathy, hepatosplenomegaly, and serositis.
Oligoarthritis is arthritis in one to four joints for the first 6 months of disease. It is subdivided to persistent oligoarthritis if it remains in four joints or less or becomes extended oligoarthritis if it involves more than four joints after 6 months.
Polyarthritis rheumatoid factor negative affects five or more joints in the first 6 months with a negative rheumatoid factor.
Polyarthritis rheumatoid factor positive also affects five or more joints in first 6 months, but these children have a positive rheumatoid factor.
Psoriatic arthritis is arthritis with psoriasis or an associated dactylitis, nail pitting, or onycholysis or psoriasis in a first-degree relative.
Enthesitis-related arthritis is arthritis and/or enthesitis (inflammation at the tendon insertion site) associated with at least two of the following: sacroiliac or lumbosacral pain, HLA-B27 antigen, arthritis in male older than 6 years, acute anterior uveitis, inflammatory bowel disease, Reiter syndrome, or acute anterior uveitis in a first-degree relative.
The JIA classification is replacing the JRA classification, which had three subtypes: pauciarticular, polyarticular, and systemic JRA.
Diagnostic Evaluation
JIA is a diagnosis of exclusion; there are no definitive tests. Classifications are based on the clinical criteria of age of onset before 16 years, arthritis in one or more joints for 6 weeks or longer, and exclusion of other causes. Laboratory tests may provide supporting evidence of disease. Sedimentation rate may or may not be elevated. Leukocytosis is frequently present during exacerbations of systemic JIA. Antinuclear antibodies are common in JIA but are not specific for arthritis; however, they help identify children who are at greater risk for uveitis. Plain radiographs are the best initial imaging studies and may show soft-tissue swelling and joint space widening from increased synovial fluid in the joint. Later films can reveal osteoporosis, narrow joint space, erosions, subluxation, and ankylosis. A slit lamp eye examination is necessary to diagnosis uveitis, inflammation in the anterior chamber of the eye, which is most common in antinuclear antibody—positive young girls with oligoarthritis. Routine examinations are necessary for early diagnosis and treatment to avoid sight-threatening disease (Kump, Castañeda, Androudi, and others, 2006).
Therapeutic Management
There is no cure for JIA. The major goals of therapy are to control pain, preserve joint range of motion and function, minimize effects of inflammation such as joint deformity, and promote normal growth and development. Outpatient care is the mainstay of therapy; lengthy hospitalizations are infrequent in this era of managed care. The treatment plan can be exhaustive and intrusive for the child and family, including medications, physical and occupational therapy, ophthalmologic slit lamp examinations, splints, comfort measures, dietary management, school modifications, and psychosocial support.
Medications.: Many arthritis medications are available, and most are effective in suppressing the inflammatory process and relieving pain. These drugs may be given alone or in combination and are prescribed in a stepwise manner dependent on disease response to each level.
Nonsteroidal antiinflammatory drugs (NSAIDs) are the first drugs used. Naproxen, ibuprofen, and tolmetin are approved for use in children. They are effective with few common side effects other than gastrointestinal irritation and bruising; with naproxen, skin fragility is a possible side effect. NSAIDs must be taken with food. Aspirin, once the drug of choice, has been replaced by NSAIDs because they have fewer side effects and easier administration schedules.
Methotrexate is the second-line medication used in children who have failed with NSAIDs alone. It is started in combination with an NSAID. It is effective, with acceptable toxicity, which requires monitoring of complete blood cell counts and liver functions. Patient education about possible side effects, including discussions with teens about birth defects and avoiding alcohol, is essential.
Corticosteroids are potent immunosuppressives used for life-threatening complications, incapacitating arthritis, and uveitis. They are administered at the lowest effective dosage for the briefest period and discontinued on a tapering schedule. They may be administered orally, as intraarticular joint injections, as IV infusions, or in eye drop form for uveitis. A single intraarticular injection may provide effective relief for children with pauciarticular disease unresponsive to NSAIDs (Padeh and Passwell, 1998). Prolonged use of systemic steroids is associated with significant side effects, including Cushing syndrome, osteoporosis, increased infection risk, glucose intolerance, cataracts, and growth suppression.
Tumor Necrosis Factor Inhibition.: Etanercept is a tumor necrosis factor-α receptor blocker and an effective drug for children with JIA unresponsive to methotrexate (Lovell, Giannini, Reiff, and others, 2003). It is given once or twice a week via subcutaneous injections. Possible side effects include transient allergic reaction at the injection site, increased infection risk, and rare reports of demyelinating disease and pancytopenia. The risk of malignancy is unknown. Parents and patients should be informed that biologic drugs are new therapies and more will be learned about potential side effects in the postmarketing period.
Slow-acting antirheumatic drugs (SAARDs) may require months to be effective and typically work in combination with NSAIDs. SAARDs include sulfasalazine, hydroxychloroquine, gold, and δ-penicillamine. SAARDs are used less often because methotrexate has been recognized as second-line therapy.
Physical and Occupational Therapy.: Programs of physical management are individualized for each child and designed to reach the ultimate goal: preserving function or preventing deformity. Physical therapy is directed toward specific joints, focusing on strengthening muscles, mobilizing restricted joint motion, and preventing or correcting deformities. Occupational therapy assumes responsibility for generalized mobility and performance of activities of daily living.
General treatment or maintenance programs vary; physical therapists may be involved several times weekly to monthly in management of a home program, or their visits may be limited to infrequent review of the home program for compliance, effectiveness, and need. Normal activities of daily living and the child’s natural tendency to be active are usually sufficient to maintain muscle strength and joint mobility.
Exercising in a pool is excellent therapy, since it allows freedom of movement with support and minimal gravitational pull. If there is pain on motion, a hot pack or warm bath before therapy may help.
Practitioners may recommend nighttime splinting to help minimize pain and reduce flexion deformity. Joints most frequently splinted are the knees, wrists, and hands. Positioning during rest is also important. The child rests on a firm mattress with no pillow or a very low one and has no support under the knee. Loss of extension in the knee, hip, and wrist causes special problems and requires vigilance to detect the earliest signs of involvement and vigorous attention to prevent deformity with specialized passive stretching, positioning, and resting splints.
Nursing Care Management
Nursing the child with JIA involves assessment of the child’s general health, the status of involved joints, and the child’s emotional response to all ramifications of the disease—discomfort, physical restrictions, therapies, and self-concept.
The effects of JIA are manifest in every aspect of the child’s life, including physical activities, social experiences, and personality development. Although children with severe disease may have more physical barriers to overcome, studies show that emotional and behavioral functioning is most closely linked with maternal depression and parental distress, not with physical disability (Frank, Hagglund, Schopp, and others, 1998). Nursing interventions to support the parents may foster successful adaptation for the entire family. Parental concerns about the disease prognosis, financial and insurance issues, spouse and sibling relationships, and job and schedule conflicts must all be addressed. Referral to social workers, counselors, or support groups may be needed.
Relieve Pain.: The pain of JIA is related to several aspects of the disease: disease severity, functional status, individual pain threshold, family variables, and psychologic adjustment. The aim is to provide as much relief as possible with medication and other therapies to help children tolerate the pain and cope as effectively as possible. Opioid administration is not a routine therapy for the chronic pain of JIA. Nonpharmacologic modalities such as behavioral therapy and relaxation techniques have proved effective in modifying pain perception (see Pain Management, Chapter 7) and activities that aggravate pain. Opioid analgesics are typically avoided in juvenile arthritis; however, for children immobilized with refractory pain, short-term opioid analgesics can be part of a comprehensive plan that uses multiple pain relief techniques (Connelly and Schanberg, 2006).
Promote General Health.: The child’s general health must be considered. A well-balanced diet with sufficient calories to maintain growth is essential. If the child is relatively inactive, caloric intake needs to match energy needs to avoid excessive weight gain, which places additional stress on affected joints. Sleep and rest are essential for children with JIA. Some children will require rest during the day; however, daytime napping that interferes with nighttime sleepiness should be avoided. A bedtime routine that involves comfort measures can help induce sleep. A firm mattress, heated water bed, electric blanket, or sleeping bag helps provide warmth, comfort, and rest. Nighttime splints needed to maintain range of motion might initially be a source of bedtime conflict. The family needs to be instructed on how to use the splint appropriately; the splint should not be painful or impede sleep. Behavior modification programs that reward splint and exercise compliance may be helpful in reducing compliance barriers. Well-child care to assess growth, development, and immunization requirements needs to be coordinated between the primary care provider and the rheumatologist. Common childhood illnesses, such as upper respiratory tract infections, may cause arthritis to worsen; consequently, medical attention must be sought quickly for relatively minor illness to prevent arthritis flares. Effective communication between the family, the primary care provider, and the rheumatology team is essential for care coordination.
Children are encouraged to attend school, even on days when there may be some pain or discomfort. The school nurse’s aid is enlisted so that a child is permitted to take the prescribed medication at school and to arrange for rest in the nurse’s office during the day. Split days or half days may help a child remain involved in school. Permitting the child to come to school late allows time to gain joint movement and reduces the time at school to avoid exhaustion. It is important that the child attend school to learn skills and engage in social interaction, especially if the JIA continues to limit physical skills. Arranging for two sets of textbooks eliminates the need to carry books to and from school, thus reducing discomfort and difficulty walking. A formal school hearing may be necessary to obtain an individualized education plan, ensured by public law, which includes intensive school modifications.
Facilitate Compliance.: The child and family are involved in the therapeutic plan. They need to know the purpose and correct use of any splints and appliances and the medication regimen. The family is instructed regarding administration of medications and the value of a regular schedule of administration to maintain a satisfactory drug level in the body. They need to know that NSAIDs should not be given on an empty stomach and to be alert for signs of medication toxicity. If evidence of drug toxicity is noted, the family is instructed to notify the health professional and follow that person’s instructions.
Encourage Heat and Exercise.: Heat has been shown to be beneficial to children with arthritis. Moist heat is best for relieving pain and stiffness, and the most efficient and practical method is in the bathtub with warm water. In some cases a daily whirlpool bath, paraffin bath, or hot packs may be used as needed for temporary relief of acute swelling and pain. Hot packs are easily applied using a bath towel wrung out after being immersed in hot water or heated in a microwave oven, applied to the area, and covered with plastic for 20 minutes. Commercial pads that warm in only a few minutes in the microwave are also available. Painful hands or feet can be immersed in a pan of warm water for 10 minutes two or three times daily in addition to tub baths.
Pool therapy is the easiest method for exercising a large number of joints. Swimming activities strengthen muscles and maintain mobility in larger joints. Very small children who are frightened of the water can carry out their exercises in the bathtub. Small children love to splash, kick, and throw things in the water. Remember, adult supervision is necessary for all water activities.
Activities of daily living provide satisfactory exercise for older children to maintain maximal mobility with minimal pain. These children are encouraged in their efforts to be independent and patiently allowed to dress and groom themselves, to assume daily tasks, and to care for their belongings. It is often difficult for children to manipulate buttons, comb or brush hair, and turn faucets, but unless there is an acute flare, parents and other caregivers should not offer assistance. In addition, children should learn and understand why others do not help them. Many helpful devices, such as self-adhering fasteners, tongs for manipulating difficult items, and grab bars installed in bathrooms for safety, can be used to facilitate tasks. A raised (higher) toilet seat often makes the difference between dependent and independent toileting, since weak quadriceps muscles and sore knees inhibit the ability to raise the body from a low sitting position.
A child’s natural affinity for play offers many opportunities for incorporating therapeutic exercises. Throwing or kicking a ball and riding a tricycle (with the seat raised to achieve maximum leg extension) are excellent moving and stretching exercises for a very young child whose daily living activities are physically limited.
An effective approach to beginning the day’s activities is to awaken children early to give them their medication and then to allow them to sleep for an hour. On arising, children take a hot bath (or shower) and perform a simple ritual of limbering-up exercises, after which they commence the activities of the day, such as going to school. Exercise, heat, and rest are spaced throughout the remainder of the day according to the child’s individual needs and schedules. Parents are instructed in exercises that meet the child’s needs.
The Arthritis Foundation* and the American Juvenile Arthritis Organization* provide services for both parents and professionals. Nurses should refer families to these agencies as an added resource.
Support Child and Family.: JIA affects every aspect of life for the child and family. Physical limitations may interfere with self-care, school participation, and recreational activities. The intensive treatment plan, including multiple medications, physical therapy, comfort measures, and medical appointments, is intrusive and disruptive to the parents’ work schedule and the family routine. To prevent isolation and foster independence, the family is encouraged to pursue their normal activities. Unfortunately, the adaptations necessary to make that occur take resourcefulness and commitment from all family members. At diagnosis and throughout the span of JIA, it is essential to recognize signs of stress and counterproductive coping and provide the necessary support to maximize adaptation. The problems and needs of these families are discussed in Chapter 18, and the reader is directed to that chapter for guidance in planning care.
SYSTEMIC LUPUS ERYTHEMATOSUS
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disease of the connective tissues and blood vessels characterized by inflammation in potentially any body tissue. Its course and symptoms are variable and unpredictable, with mild to life-threatening complications. In addition to SLE, there are other forms of lupus, such as neonatal lupus, which occurs when maternal autoantibodies cross the placenta and cause transient lupuslike symptoms in the newborn, with the potential serious complication of heart block. The remaining discussion focuses on SLE.
Recent reports suggest that survival rates in children with SLE have significantly improved; 5-year survival rates are said to be almost 100%, and 10-year survival rates are close to 90% (Ravelli, Ruperto, and Martini, 2005). SLE is more common in girls, with an approximate 5:1 female-to-male ratio, and typically occurs between the ages of 10 and 19 years. There is a familial tendency, although many newly diagnosed patients are unaware of other affected family members. SLE has been reported in all cultures, but within the United States there has been a disproportionately higher incidence in African-American, Asian, and Hispanic children.
The cause of SLE is not known. It appears to result from a complex interaction of genetics with an unidentified trigger that activates the disease. Suspected triggers include exposure to ultraviolet light, estrogen, pregnancy, infections, and drugs. Genetic predisposition to SLE is evidenced in an increased concordance rate in twins (tenfold), increased incidence within family members (10% to 16%), and increased frequency of certain gene alleles in population-based studies.
Clinical Manifestations and Diagnostic Evaluation
The child with SLE may have any clinical manifestation with mild to life-threatening severity (Box 31-11). The diagnosis is established when 4 of the 11 diagnostic criteria are met (Box 31-12). Kidney involvement heralds progressive disease and the need for rigorous therapeutic management.
Therapeutic Management
The goal of treatment is to ensure the child’s health by balancing the medications necessary to avoid exacerbation and complications while preventing or minimizing treatment-associated morbidity. Therapy involves the use of specific medications and general supportive care. The drugs used to control inflammation are corticosteroids administered in doses sufficient to control inflammation, then tapered to the lowest suppressive dose. Other drugs include antimalarial preparations, which are useful for rash and arthritis; NSAIDs, which relieve muscle and joint inflammation; and immunosuppressive agents, such as cyclophosphamide, for renal and central nervous system disease. Antihypertensives, aspirin, and antibiotics are just a few of the additional drugs that may be necessary to treat or avoid complications.
General supportive care includes sufficient nutrition, sleep and rest, and exercise. Exposure to the sun and ultraviolet B (UVB) light is limited because of its association with SLE exacerbation.
Nursing Care Management
The principal nursing goal is to help the child and family positively adjust to the disease and therapy. The child and family must learn to recognize subtle signs of disease exacerbation and potential complications of medication therapy and to communicate these concerns to their care provider. Consequently, patient and family education is an ongoing process initiated at diagnosis and tailored to the patient’s individual needs. Referral to a social worker, psychologist, or support group may help the child and family make a successful adjustment. Support groups are associated with the Lupus Foundation of America* and the Arthritis Foundation.†
Key issues include therapy compliance; body-image problems associated with rash, hair loss, and steroid therapy; school attendance; vocational activities; social relationships; sexual activity; and pregnancy. (See Chapter 18 for a discussion on adjusting to a chronic illness.) Specific instructions for avoiding exposure to the sun and UVB light, such as using sunscreens, wearing sun-resistant clothing, and altering outdoor activities, must be provided with great sensitivity to ensure compliance while minimizing the associated feeling of being different from peers (see Sunburn, Chapter 30). Patients need to be instructed to maintain regular medical supervision and seek attention quickly during illness or before elective surgical procedures, such as dental extraction, because of potential needs for increased steroids or prophylactic antibiotics. People with SLE should carry medical identification for their disease and steroid dependence.
REFERENCES
American Academy of Pediatrics. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4):896–905.
American Academy of Pediatrics. School health assessments. Pediatrics 2000;105(4):875–877. [reaffirmed 2006]. Pediatrics. 2006;118(3):1266–1267.
Bunnell, WP. Selective screening for scoliosis. Clin Orthop Relat Res. 2005;434:40–45.
Connelly, M, Schanberg, L. Opioid therapy for the treatment of refractory pain in children with juvenile rheumatoid arthritis. Natl Clin Pract Rheumatol. 2006;2(12):636–637.
Curley, MA, Razmus, IS, Roberts, KE, et al. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nurs Res. 2003;52(1):22–33.
Do, T, Herrera-Soto, J. Elbow injuries in children. Curr Opin Pediatr. 2003;15(1):68–73.
Frank, RG, Hagglund, KJ, Schopp, LH, et al. Disease and family contributors to adaptation in juvenile rheumatoid arthritis and juvenile diabetes. Arthritis Care Res. 1998;11(3):166–176.
Gilmore, A, Thompson, GH. Common childhood foot deformities. Consult Pediatr. 2003;2(2):63–71.
Greene WB, ed. Essentials of musculoskeletal care, ed 2, Rosemont, Ill: American Academy of Orthopaedic Surgeons, 2001.
Gutierrez, K. Bone and joint infections in children. Pediatr Clin North Am. 2005;52(3):779–794.
Hart, ES, Albright, MB, Rebello, GN, et al. Developmental dysplasia of the hip: nursing implications and anticipatory guidance for parents. Orthop Nurs. 2006;25(2):100–109.
Holmes, SB, Brown, SJ, Pin Site Care Expert Panel. Skeletal pin site care: National Association of Orthopaedic Nurses guidelines for orthopaedic nursing. Orthop Nurs. 2005;24(2):99–107.
Hosalkar, HS, Horn, D, Friedman, JE, et al. The hip. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Kline, NE, Sevier, N. Solid tumors in children. J Pediatr Nurs. 2003;18(2):96–102.
Kump, LI, Castañeda, RA, Androudi, SN, et al. Visual outcomes in children with juvenile idiopathic arthritis—associated uveitis. Ophthalmology. 2006;113(10):1874–1877.
Lampe, RM. Osteomyelitis and suppurative arthritis. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Land, C, Rauch, F, Travers, R, et al. Osteogenesis imperfecta type VI in childhood and adolescence: effects of cyclical intravenous pamidronate treatment. Bone. 2007;40(3):638–644.
Lanzkowsky, P. Manual of pediatric hematology and oncology. San Diego: Academic Press, 2000.
Link, MP, Gebhardt, MC, Myers, PA. Osteosarcoma. In Pizzo PA, Poplack DG, eds.: Principles and practices of pediatric oncology, ed 5, Philadelphia: Lippincott, 2006.
Lovell, DJ, Giannini, EH, Reiff, A, et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48(1):218–226.
Maher, AB, Salmond, SW, Pellino, TA. Orthopaedic nursing, ed 3. Philadelphia: Saunders, 2002.
Marini, JC. Osteogenesis imperfect. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
McIlvain-Simpson, G, Singsen, B. Decreasing morning stiffness. Small Talk. 1997;3(6):8.
Miller, ML, Cassidy, JT. Juvenile rheumatoid arthritis. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Napierkowski, DB. Scoliosis: a case study in an adolescent boy. Orthop Nurs. 2007;26(3):147–153.
Newton, PO, Wenger, DR. Idiopathic and congenital scoliosis. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s pediatric orthopaedics. Philadelphia: Williams & Wilkins, 2001.
Olsson, GL. Neuropathic pain in children. In: McGrath PJ, Finley GA, eds. Chronic and recurrent pain in children and adolescents. Seattle: IASP Press, 1999.
Padeh, S, Passwell, P. Intraarticular corticosteroid injection in the management of children with chronic arthritis. Arthritis Rheum. 1998;41(7):1210–1214.
Perron, AD, Miller, MD, Brady, WJ. Orthopedic pitfalls in the ED: slipped capital femoral epiphysis. Am J Emerg Med. 2002;20(5):484–487.
Petty, RE, Southwood, TR, Baum, J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25(10):1991–1994.
Petty, RE, Southwood, TR, Manners, P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis, second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392.
Plint, AC, Perry, JJ, Correll, R, et al. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691–697.
Ravelli, A, Ruperto, N, Martini, A. Outcome in juvenile onset lupus erythematosus. Curr Opin Rheumatol. 2005;17(5):568–573.
Samaniego, IA. A sore spot in pediatrics: risk factors for pressure ulcers. Pediatr Nurs. 2003;29(4):278–283.
Seidel, HM, Ball, JW, Dains, JE, et al. Mosby’s guide to physical examination, ed 5. St Louis: Mosby, 2006.
Slote, RJ. Psychological effects of caring for the adolescent undergoing spinal fusion for scoliosis. Orthop Nurs. 2002;21(6):19–28.
Wall, EJ. Practical primary pediatric orthopaedics. Nurs Clin North Am. 2000;35(1):95–113.
Warren, RW, Perez, MD, Curry, MR, et al. Juvenile idiopathic arthritis (juvenile rheumatoid arthritis). In: Koopman WJ, ed. Arthritis and allied conditions. Philadelphia: Lippincott Williams & Wilkins, 2001.
Wexler, LH, Meyer, WH, Helman, LJ. Rhabdomyosarcoma and the undifferentiated sarcomas. In Pizzo PA, Poplack DG, eds.: Principles and practices of pediatric oncology, ed 5, Philadelphia: Lippincott, 2006.
*For information on specially adapted molded-plastic chairs for children who have spica casts, contact Britax Hippo by SnugSeat, (800) 336-7684, http://www.snugseat.com. The E-Z-On vest is a special safety harness for larger children with poor trunk control. Additional safety restraints and a listing of distributors are available from SafetyBeltSafe U.S.A., http://www.carseat.org; (800) 745-SAFE; Spanish: (800) 747-SANO.
*804 W. Diamond Ave., Suite 210, Gaithersburg, MD 20878; (800) 981-2663; http://www.oif.org.
*Five Cabot Place, Stoughton, MA 02072; (800) 673-6922; http://www.scoliosis.org.
†6300 N. River Road, Rosemont, IL 60018-4262; (847) 823-7186; http://www.aaos.org.
‡611 E. Wells St., Suite 1100, Milwaukee, WI 53202; (414) 289-9107; http://www.srs.org.
*Information about prostheses can be obtained from the National Amputation Foundation, 40 Church St., Malverne, NY 11565; (516) 887-3600; http://www.nationalamputation.org.
**Information about special programs for children with amputations is available from the Candlelighters Childhood Cancer Foundation, PO Box 498, Kensington, MD 20895; (800) 366-2223, (301) 962-3520; fax: (301) 962-3521; http://www.candlelighters.org.
*PO Box 7669, Atlanta, GA 30357-0669; (800) 283-7800; http://www.arthritis.org. In Canada, the Arthritis Society may be contacted for locations of all local Canadian province offices: http://www.arthritis.ca.
*2000 L St. NW, Suite 710, Washington, DC 20036; (202) 349-1155, (800) 558-0121; http://www.lupus.org.
 IN TEXT
IN TEXT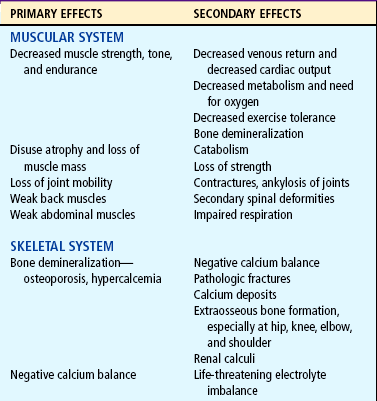
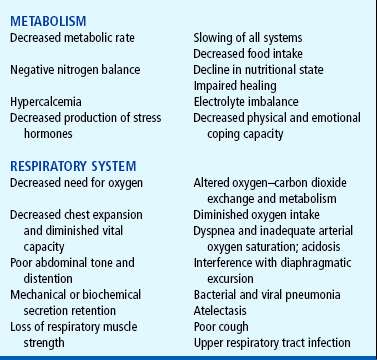
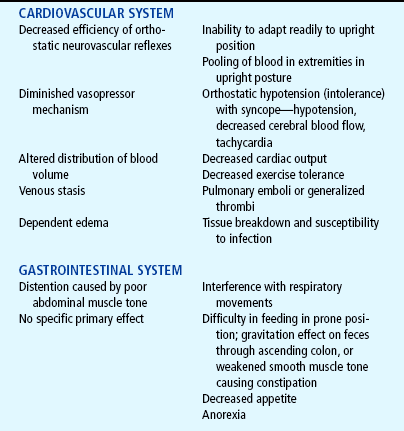
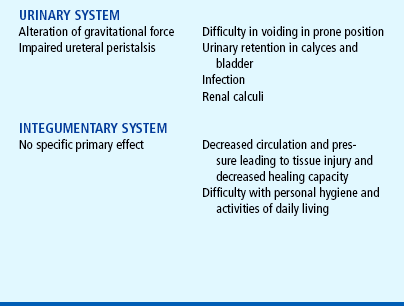
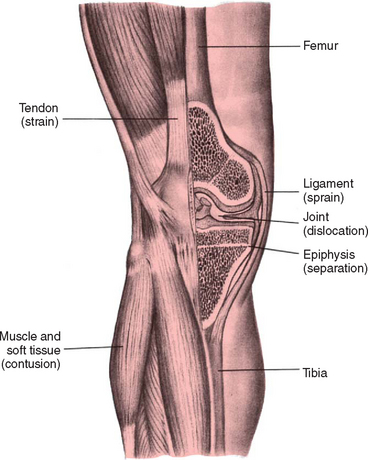
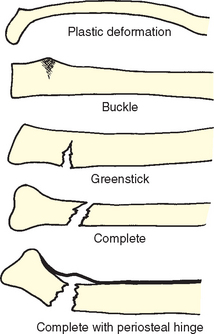
 FAMILY-CENTERED CARE
FAMILY-CENTERED CARE