Chapter 33 Pulmonary surgery
 Surgical resection of lung tissue via a thoracotomy is a routine procedure, used mostly for treating lung cancer, which requires careful assessment of the patient’s physiological reserve.
Surgical resection of lung tissue via a thoracotomy is a routine procedure, used mostly for treating lung cancer, which requires careful assessment of the patient’s physiological reserve. Less invasive surgical techniques such as video-assisted thoracic surgery are increasing rapidly and associated with less physiological disturbance and complications.
Less invasive surgical techniques such as video-assisted thoracic surgery are increasing rapidly and associated with less physiological disturbance and complications. One-lung ventilation is required for many pulmonary surgery procedures and understanding of the physiology involved is vital for its safe use.
One-lung ventilation is required for many pulmonary surgery procedures and understanding of the physiology involved is vital for its safe use.In current clinical practice surgery of the lungs, mediastinum and chest wall is routinely performed, and although still high-risk by modern surgical standards the outcome for most patients is favourable. The physiological disturbances caused during and after pulmonary surgery are immense and in this chapter the effects of the more common pulmonary surgical procedures are outlined.
Physiological Aspects of Common Interventions
Bronchoscopy
Bronchoscopy is performed frequently and allows direct visualisation of the airway and if necessary the collection of washings and biopsies of airway and lung tissue. It may also be used therapeutically to, for example, remove inhaled foreign bodies, resect tumours or place stents to overcome airway obstruction. Two types of bronchoscopy are performed:
Flexible bronchoscopy.1 The flexibility of fibreoptic bronchoscopes allows a view of all the major branches of the tracheobronchial tree with minimal risk of trauma and discomfort for the patient. The procedure can therefore be performed without general anaesthesia, though extensive topical anaesthesia to the airway is required, and most clinicians also provide sedation to relieve the anxiety associated with having a bronchoscopy.2 Hypoxia during a flexible bronchoscopy is common,1 occurring in 17% of patients from one study,2 and supplemental oxygen is therefore normally used. Lung function during bronchoscopy is significantly impaired. Whilst the bronchoscope is in place the functional residual capacity (FRC) is increased by 17%,3 and forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow are all decreased,4 indicating airflow obstruction. These observations are not explained simply by the presence of the bronchoscope in the airway, as the observed airway flow limitation begins after the airway local anaesthetic is applied (before insertion of the bronchoscope) and continues for several minutes after the bronchoscope has been removed, suggesting that a bronchoconstrictor action of the topical anaesthesia is responsible.4 Respiratory depression may also occur during or soon after bronchoscopy, and the causes of this are uncertain but likely to relate either to the sedative drugs or the topical anaesthesia in the airway. The major limitation of flexible bronchoscopy is the size of the instruments that may be passed down the bronchoscope, and though very suitable for visualisation of the airway and obtaining biopsies or washings, for removal of foreign bodies or airway surgery a larger portal for access to the tracheobronchial tree is required.
Rigid bronchoscopy. Straight, rigid, bronchoscopes are available with internal diameters up to 8 mm, and these may be passed into the trachea and a variety of instruments used through the bronchoscope. To see around corners in the bronchial tree 30- and 90-degree angled telescopes are used. With rigid bronchoscopy foreign bodies that are wedged in the airway can be removed, tracheal tumours resected, airway haemorrhage treated, and stents deployed in the trachea or main bronchi to overcome stenosing tumours or airway leaks. The major disadvantage of the technique is the requirement for general anaesthesia, often in a patient with significant respiratory disease.
Ventilation during a rigid bronchoscopy with general anaesthesia is a challenge and four main techniques may be used:5
Thoracoscopy
Insertion of a telescope through the chest wall into the pleural space allows direct inspection of the pleura, lungs, mediastinum and diaphragm to facilitate diagnosis or therapeutic interventions. Three types of thoracoscopy exist:
Thoracotomy
A surgical opening in the chest cavity was first used more than 100 years ago, usually for the treatment of empyema (page 448) and tuberculosis. In current surgical practice the indications for thoracotomy have widened to include surgery of the lungs, major vessels, oesophagus and thoracic spine. In most cases, thoracotomy is performed in the lateral position which has significant effects on respiratory physiology (see below) and through a postero-lateral incision. Thoracotomy includes division of the muscles exterior to the ribcage and entering the pleura, usually through the 5th intercostal space, by separating the intercostal muscles from the rib. The rib adjacent to the thoracotomy is commonly divided or a piece of rib resected to improve access and to minimise rib fractures when the chest wall is retracted.
The effects of thoracotomy on postoperative respiratory function are profound, with significant reductions in chest wall compliance and respiratory muscle activity11 resulting from chest wall oedema, pain, disruption of muscle anatomy, and later in the recovery phase scarring of chest wall tissues. In the first 24 hours following surgery, FVC and FEV1 are only 30–50% of the preoperative volumes, with some evidence that the type of thoracotomy incision used may affect these values.12 Chest wall compliance falls to around 60% of the preoperative value by the third postoperative day before slowly improving.13 At one week after surgery, FVC and FEV1 are around 70–80% of preoperative values, and by this stage the different incisions seem to have little effect on recovery.14,15
Other measures of respiratory muscle strength such as maximum inspiratory and expiratory mouth pressures are also reduced to about half the preoperative values following thoracotomy, and in one study had not returned to normal 12 weeks after surgery.16 The same study showed a rapid return to normal of both measures of muscle function following VATS procedures. Older patients, who have poor respiratory muscle strength relative to younger patients, took longer to recover muscle function following surgery, possibly explaining the greater incidence of pulmonary complications with increasing age. Thoracotomy alone therefore impairs respiratory muscle function to such an extent that ventilation may not be able to keep pace with the extra ventilatory requirements associated with having major surgery, and alveolar hypoventilation can occur along with regional pulmonary collapse and impaired oxygenation. Even in patients less severely affected the ability to cough is always weakened with an increased risk of chest complications.11 For patients who have a lung resection through their thoracotomy, lung compliance is also decreased to about half their preoperative value, compounding the above problems.13
Considering the surgical trauma associated with thoracotomy, it is unsurprising that there is commonly severe pain in the postoperative period. Damage to somatic nerves supplying the skin and chest wall structures is exacerbated by trauma to the visceral nerves supplying the pleura and possibly by involvement of the sympathetic chain in the chest cavity. Because all three of these nerve pathways may be involved, treatment of acute postoperative pain is challenging, with multimodal treatment regimes being required. However, of more significance to the patient is the observation that following a thoracotomy almost half of patients develop a chronic pain syndrome, the pathophysiology of which remains unknown, though genetic and psychological factors are believed to be important contributors.17
Lung Resection
Assessing patient fitness for lung resection.18,19 Lung function is assessed using either the FEV1, or, if the patient has parenchymal lung disease, the diffusing capacity for carbon monoxide (Dlco, page 153). If these are less than 80% of normal predicted values for that patient, an attempt is made to calculate predicted postoperative values based on which anatomical sections of lung need to be removed. Radionucleotide ventilation or perfusion scans or quantitative computerised tomography scans may all be used to measure functional lung units, and are useful techniques as they also show which pathological lung units are already not contributing to overall function. Less invasive is the anatomical method in which the lungs are divided into 19 anatomical segments of equal value, and knowing which segments are to be removed enables estimation of postoperative predicted lung function. For many years a general rule of lung resection was that a predicted postoperative FEV1 of less than 0.8–1.0 l precluded resection, though evidence for this rule is poor. Using an absolute value for FEV1 or Dlco is fraught with difficulties as sex, age and height all affect the normal values, and decisions should now always be based on the values as a percentage of the predicted normal for that patient.
Different studies have produced varied results on the association between percentage predicted postoperative FEV1 or Dlco and outcome, but a value of less than 40% of predicted normal is now generally accepted as being associated with an increased mortality and complication rate. For patients in this situation, measurement of preoperative exercise tolerance has the advantage of also including a cardiovascular component to the assessment and may help to further define risks and outcomes. The most objective way of quantifying exercise activity is by measuring Vo2max (page 262). Values of less than 15 ml.min−1.kg−1 are again associated with poor outcome. Clinical measures of exercise tolerance have some value, but these must be performed under supervision as patients’ own reported exercise tolerance is normally greatly exaggerated. Tests which have some limited use in predicting outcome after lung resection include the shuttle test (number of times a patient can walk between two markers 10 m apart), 6-minute walk test (distance able to walk within 6 minutes) and stair climbing (the number of stairs or height of stairs the patient is able to climb20).
Partial lung resection. The magnitude of lung resection operations varies greatly from removal of a small tumour in the lung periphery to a complete pneumonectomy, with the more minor procedures being performed via VATS and the more major via a thoracotomy. Wherever possible, dissection is made between lobes or in intersegmental planes. Great care is required when operating on pulmonary vessels, particularly pulmonary arteries which have thin walls and are easy to damage, resulting in significant haemorrhage that may be difficult to control. The use of staple systems has made the closure of vessels, bronchi and the resection of lung tissue technically much easier and less hazardous than manual suturing of these structures.
Following lung resection, the remaining lung in the hemithorax quickly expands to fill the available space. Two chest drains are usually placed in the chest cavity and connected to underwater seal systems to allow any air or blood to drain. If the remaining lung does not fully expand, a negative pressure of up to 20 cmH2O may be applied to the drains to encourage the lung to expand.
Pneumonectomy. Resection of an entire lung is usually performed for removal of large, central lung tumours (Figure 33.1A). Following pneumonectomy correct management of the empty hemithorax is crucial. If air is drained from the cavity too quickly mediastinal shift will occur which impairs venous drainage to the heart and can cause cardiovascular collapse. The most common practice is therefore to leave no drain in the chest cavity, and monitor the position of the mediastinum daily with a chest x-ray. Alternatively, a chest drain can be placed (Figure 33.1B), but be clamped for most of the time and only released briefly and intermittently to ensure the pressure in the cavity is approximately atmospheric. A more interventional approach is to measure the pressure in the chest cavity and instill or remove air to maintain a pressure of −2 to −4 cmH2O on inspiration and +2 to +4 cmH2O on expiration. Within a few weeks of pneumonectomy the volume of the hemithorax decreases due to a combination of mediastinal shift, elevation of the diaphragm, and contraction of the chest wall, and pleural fluid replaces the air in the chest cavity (Figure 33.1C). Over the ensuing months or years, the fluid volume decreases as the mediastinal shift continues, and the other lung herniates anteriorly or posteriorly across the midline to partially fill the vacated hemithorax. Complete replacement of the fluid is however unusual.
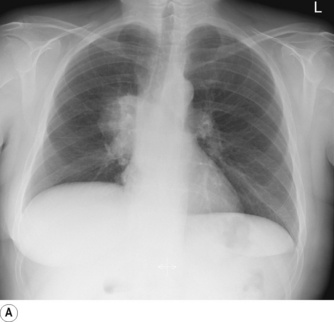
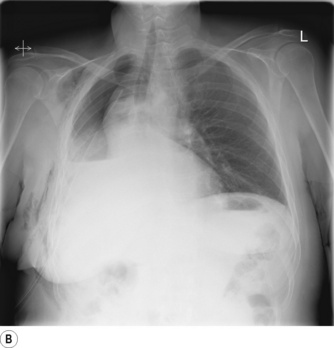
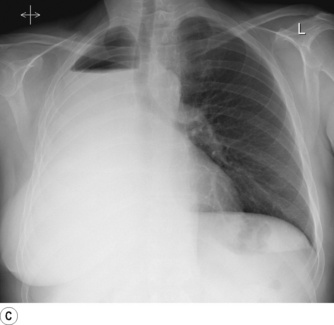
Fig. 33.1 (A) Chest radiograph showing large lung cancer at the right hilum. (B) The same patient 24 hours after a right pneumonectomy. Note the shifted trachea and mediastinum, contracted right thoracic cage, and early accumulation of fluid in the empty hemithorax. (C) One month later, with the empty hemithorax already almost completely filled with fluid and a hyper-expanded left lung.
Recent studies in animals have demonstrated the intriguing phenomenon of ‘neoalveolarisation’ following lung resection.21 Within 20 days of lung resection in mice the number of alveoli in the remaining lung increased by 50%, completely restoring the gas exchanging surface area.22 Neoalveolarisation probably occurs by new alveoli forming in the walls of existing alveolar ducts and respiratory bronchioles, and is so far only described in young animals, as would be expected from the observation that in mammals formation of alveoli is a post-natal process (page 250). It therefore remains only a distant prospect that adult patients will be able to grow new lung tissue after lung resection.
Lung injury following pneumonectomy.23,24 Acute lung injury (Chapter 31) is a serious complication that occurs in the postoperative period in between 2.5% and 9% of pneumonectomies, and more rarely follows smaller lung resections such as lobectomy. Mortality is high, with a quarter of patients dying, though this is an improvement compared with only a few years ago. The pathophysiology of post-pneumonectomy acute lung injury is controversial, with perioperative fluid overload being viewed by many clinicians as the main cause, though the pathophysiology is now better elucidated and far more complex than simply administering excessive volumes of intravenous fluid. High-protein pulmonary oedema develops approximately 24 hours postoperatively, and is believed to result from endothelial cell injury in the pulmonary capillaries. How this initial injury occurs is less clear, though the increased capillary blood flow in the remaining lung is likely to cause stretching of endothelial cells or excessive shear forces in the vessels, both of which may disrupt the inter-cellular junctions. Overdistension of the lung either during surgery with inappropriately large tidal volumes25 or use of PEEP, or following surgery with sub-optimal management of the contralateral chest cavity may all contribute to further disruption of the alveolar–capillary barrier.23 Once this initial lung injury has occurred, many other factors will then affect the severity of the clinical picture and its management, including fluid administration, inspired oxygen levels and the ventilation strategy, all of which should follow the same principles as for the management of ALI whatever the cause (Chapter 31).
Surgery for Chronic Obstructive Pulmonary Disease (COPD)26
Surgical treatment is reserved for patients with severe COPD in whom emphysematous changes predominate. When the airspaces created in emphysema become larger than 1 cm in diameter they are referred to as a ‘bulla’. Nearby bullae can merge and result in extremely large air spaces, occupying up to one-third of the lung volume. Like emphysema, bullae have little effect on gas exchange as both tidal ventilation and blood flow to the bulla are negligible. However, with giant bullae the airspace acts in a similar fashion to a pneumothorax (page 445) and compresses surrounding lung tissue, causing further worsening of airway collapse and subsequent disturbance of gas exchange. In these cases surgical treatment involves ‘bullectomy’, and with careful patient selection this can be a useful operation. Improved surgical techniques led to a resurgence of interest in surgery for COPD and extended the indications to include patients who do not have bullae.
Lung volume reduction surgery (LVRS) involves removing 20–30% of lung volume, to include the most emphysematous areas, and can have impressive results. Improved long-term survival compared with best medical therapy has only been proven in patients with poor exercise capacity and upper lobe emphysema,27,28 and conversely, patients with high exercise capacity and emphysema elsewhere in the lung have a higher mortality following surgery compared with medical treatment. Despite these mixed survival results, in appropriately selected groups of patients LVRS can improve exercise capacity,27,29 lung volumes, quality of life30 and arterial Po2.31 Understanding of the physiological mechanisms leading to clinical improvements remains incomplete. Potential benefits of LVRS include reduced pulmonary collapse adjacent to emphysematous areas, improved elastic recoil of the remaining lung tissue, and better respiratory muscle function, particularly of the diaphragm,32 secondary to reduced hyperinflation (see Figure 6.1).
Pleurodesis33
Pleurodesis describes a variety of procedures, all of which aim to induce adhesions between the visceral and parietal pleura. The two most common indications are pneumothorax that has failed to respond to conservative management (page 456) or palliation of malignant pleural effusion. Though the preferred technique varies with the indication, the success of any pleurodesis depends upon inducing inflammation in the pleura whilst simultaneously ensuring the two pleural layers are closely apposed, so allowing the normal inflammation and tissue repair processes to cause scarring in the pleural space. Apposition of the pleura is usually achieved by using a pleural drain, but if required an inflammatory reaction in the pleura can be initiated by various means. A pleurectomy may be performed, with the parietal pleura being simply stripped from the inside of the chest wall, or a less traumatic technique is pleural abrasion in which the pleura is rubbed with a dry gauze or other abrasive surface. Alternatively, sclerosants can be instilled into the pleural cavity, including antibiotics (e.g. doxycycline), antiseptics (e.g. iodopovidone), anticancer drugs or minerals such as talc. Talc pleurodesis is the most common technique, and the talc may either be instilled as a slurry through a small pleural catheter to avoid surgical intervention, or as a dry powder (poudrage) via a surgical approach. Recent work has identified the importance of the particle size of talc in the development of adverse effects from talc pleurodesis.34 Talc particles have been found to enter the lung parenchyma or systemic circulation following pleurodesis, risking the development of pulmonary fibrosis or systemic inflammation respectively. Use of particle sizes greater than 5 μm reduces the complication rate, presumably because the talc particles are unable to pass through the similarly-sized stoma in the pleura (page 445) to gain access to the lymphatics and circulation.
Obliterating the pleura by these techniques may be expected to cause long-term impairment of lung function, but after an initial decline immediately after the procedure, total lung capacity returns to normal approximately 6 months later.35 This is in keeping with the bizarre observation, first discovered in the 1700s, that elephants have no pleural space with connective tissue binding their lungs tightly to the inside of the chest wall, with no apparent long-term ill effects for the species.36
One-Lung Ventilation
Many of the surgical procedures already described will be facilitated by apnoea of the operative lung during surgery. These, and other indications for one-lung ventilation (OLV), are shown in Table 33.1. Indications are divided into absolute, where without OLV the patient’s life is at risk, and relative, when OLV will help manage the patient’s condition but is not mandatory.
Table 33.1 Indications for one-lung ventilation37
| ABSOLUTE INDICATIONS | RELATIVE INDICATIONS |
|---|---|
| Isolation of lung to avoid cross contamination: Lung abscess Massive haemorrhage Unilateral ventilation: Bronchopleural fistula Giant lung cyst or bulla Tracheobronchial tree disruption |
Surgical exposure: Thoracic aortic surgery Pneumonectomy Lung resection Thoracoscopy Oesophagectomy Thoracic spine surgery |
| Intensive care: Life threatening hypoxia from unilateral lung disease |
Intensive care: Severe hypoxia from unilateral lung disease |
Lung Isolation Techniques
Isolation of one lung requires knowledge of some anatomical features of the large airways. First, the angle at which the right and left main bronchi branch from the trachea is highly variable, being on average 25° from the vertical for the right main bronchus and 45° for the left (Figure 33.2). There is however wide individual variation in these angles both in health and disease,38 but the right main bronchus is almost always at the less acute angle. Second, the distance between the carina and the first segmental bronchus is normally 5 cm in the left main bronchus and only 2.5 cm in the right, making occlusion of the right upper lobe a possibility when a cuffed tube is in the right main bronchus.
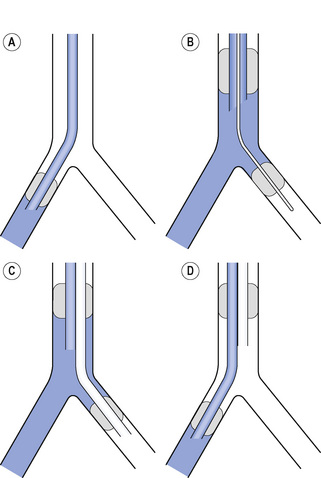
Fig. 33.2 Four methods of achieving one-lung ventilation of the right lung. (A) Single lumen endobronchial tube in the right main bronchus. (B) Bronchial blocker passed through a standard tracheal tube into the left main bronchus. (C) Left sided double-lumen tube with ventilation via the tracheal lumen. (D) Right sided double-lumen tube with ventilation via the bronchial lumen. In each case the blue area shows where ventilation is occurring.
There are three options available to isolate one lung:37
Physiology of One-lung Ventilation39,40
Despite OLV now being a routine technique in many situations, hypoxia still occurs commonly. A detailed understanding of the physiology of OLV is therefore vital if a logical approach to management is to be adopted. First, the factors that influence lung function during OLV will be considered.
Patient position
Though OLV may be required in a supine patient, the lateral position is most commonly used for thoracic surgery and this position significantly affects ventilation and perfusion of the lungs. The loss of muscle tone in the chest wall and diaphragm associated with general anaesthesia (page 333) causes gravity to affect the volumes of the left and right hemithoraces. The volume of the dependent lung is decreased by the weight of the mediastinum above and by cephalad movement of the diaphragm from the weight of the abdominal contents. Table 8.1 (page 120) shows the distribution of FRC and ventilation in the left and right lungs in both lateral positions when anaesthetised. FRC in the dependent lung is approximately one litre less than the non-dependent lung, and inevitably, under general anaesthesia, atelectasis forms in the dependent lung (Figure 33.3).41 ‘Breaking’ the operating table to open the intercostal space on the operative side of the chest will further compress the dependent lung.
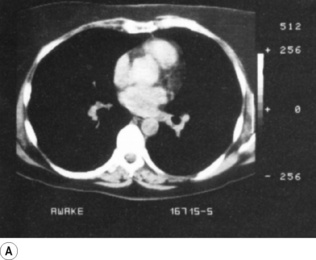
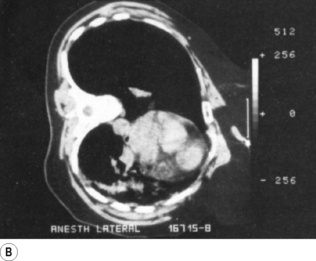
Fig. 33.3 Computerised tomography scans during anaesthesia in the lateral position. (A) Patient awake in the supine position. (B) Patient anaesthetised and turned into the left lateral position. Note the position of the mediastinum and the atelectasis in the left lung.
Scars kindly provided by Prof. G. Hedenstierna.
These changes in lung volume affect the position of each lung on a regional compliance curve. In a spontaneously breathing awake patient in the lateral position, the lower lung is on a steeper part of the compliance curve than the non-dependent lung and therefore receives more ventilation (Figure 33.4A). Dependent lung ventilation is also enhanced by the cephalad movement of the lower diaphragm increasing its efficiency. When anaesthetised, paralysed and ventilated in the lateral position the effect of the diaphragm position is lost, and the reduction of FRC for both lungs causes the non-dependent lung to reside in the steep middle part of a compliance curve and it is this lung that now receives approximately 60% of ventilation (Figure 33.4B). Due to the larger volume of the right lung, these considerations are affected by which side the patient is on, with a larger differential FRC and ventilation when the left lung is dependent. Perfusion of the dependent lung is always greater than the non-dependent lung in the lateral position, and this differential is mostly uninfluenced by anaesthesia and paralysis. Thus the ventilation/perfusion ratios of the lung are well matched when in the lateral position and awake, but anaesthesia and artificial ventilation in this position results in significant mismatch with greater ventilation of the non-dependent lung associated with greater perfusion of the dependent lung.
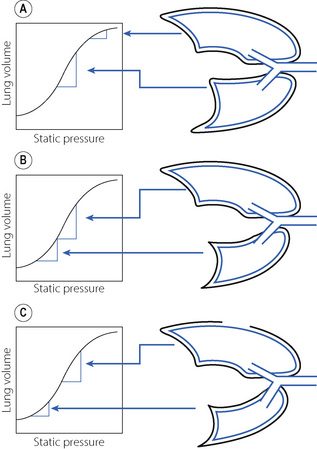
Fig. 33.4 Schematic representation of lung volumes and regional compliance in the right lateral position. (A) Awake patient, spontaneously breathing. The non-dependent (left) lung has a higher FRC than in the supine position so is at a less favourable part of the compliance curve and the dependent (right) lung will receive relatively more ventilation. (B) Anaesthetised and paralysed patient in the same position. The loss of muscle activity in the diaphragm and chest wall reduces FRC in both lungs and the weight of the mediastinum compresses the dependent lung. This changes their position on the compliance curve, and the non-dependent (left) lung is now the better ventilated. (C) Same situation with the chest open. Loss of the negative pleural pressure of the non-dependent lung causes further mediastinal shift, further compromising the function of the dependent lung. In the left lateral position the same physiological changes occur, but the impact is greater when the smaller left lung is the dependent one.
Ceasing ventilation of the non-dependent lung therefore removes the better ventilated lung, leaving the challenge of ventilating the low volume, low-compliance dependent lung, but also removes the larger part of the 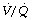 mismatch.
mismatch.
The Open Chest
Allowing air to enter the pleural space of the non-dependent lung will exacerbate the changes described so far. The negative intrapleural pressure of the upper hemithorax in effect holds up the mediastinum, and when this is lost the full weight of the heart and other mediastinal structures further compresses the dependent lung (Figure 33.4C). This effect will occur whether the pleura is open to the atmosphere, or if gases have been insufflated into the chest cavity to facilitate surgery, and the effect of a positive intrapleural pressure, as sometimes used in thoracoscopic surgery, will be even more significant. With a thoracotomy, the compliance of the chest wall is in effect removed, and only lung compliance (page 27) will determine ventilation of the non-dependent lung, which will be free to expand to a large volume if ventilation of both lungs continues.
Perfusion of the non-ventilated lung
Once ventilation of the non-dependent lung ceases, matching of ventilation to perfusion becomes almost entirely dependent on the amount of blood flowing through the apnoeic non-dependent lung. Factors affecting pulmonary vascular resistance are described in detail in Chapter 7 and include passive factors such as gravity and lung volume, and active control of pulmonary blood vessel size. During OLV the effect of gravity depends on patient position: in the lateral position perfusion of the upper lung will be reduced, but this will not be the case if OLV is used in the supine position. As a result of this relatively high blood flow in the supine position oxygenation is more often impaired than during OLV in the lateral position.42 Pulmonary vascular resistance (PVR) is minimal at FRC (see Figure 7.4) so a small reduction in pulmonary blood will occur when the non-ventilated lung collapses towards residual volume. Surgical manipulation of the lung is also likely to reduce its blood flow either by distorting and so occluding pulmonary vasculature or by direct clamping of pulmonary vessels as part of the surgical procedure.
Of the many mechanisms involved in active control of PVR (see Table 7.1) it is hypoxic pulmonary vasoconstriction (HPV, page 108) that is believed to be the most important determinant of pulmonary blood flow in the non-ventilated lung. The 40–50% of cardiac output that would be expected to flow through the non-ventilated lung is believed to be reduced to 20–30% by hypoxic pulmonary vasoconstriction.39 The passive increase in PVR as a result of reduced lung volume is thought to be small in comparison with the effect of HPV – if during OLV the non-ventilated lung is reinflated and ventilated with nitrogen the blood flow to the lung is unaffected, but performing the same manoeuvre with oxygen increases the blood flow to normal.43 How much HPV occurs is influenced by both alveolar and mixed venous Po2 (see Figure 7.8), so changes in cardiac output or oxygen consumption that will affect venous Po2 may influence blood flow to the non-ventilated lung. A fall in mixed venous Po2 enhances the HPV response, and an abnormally high mixed venous Po2 may have the opposite effect as oxygen diffuses from the alveolar capillary blood into the non-ventilated alveoli.43
The effect of general anaesthesia on HPV has been controversial for some years (see page 346).39,44 Results from in-vitro or animal studies have shown that all inhalational anaesthetic agents, including nitrous oxide, cause some inhibition of HPV whilst propofol and fentanyl have been shown to have no effect. Translating these observations into clinical practice is problematic due to the numerous other factors that may affect HPV such as:
 Cardiac output, which is reduced by most general anaesthetic drugs. A fall in cardiac output will reduce blood flow through both shunt and normal regions of lung (page 134) and decrease the mixed venous Po2, which will affect HPV as described above.
Cardiac output, which is reduced by most general anaesthetic drugs. A fall in cardiac output will reduce blood flow through both shunt and normal regions of lung (page 134) and decrease the mixed venous Po2, which will affect HPV as described above. Individual variation in the HPV response is large and difficult to predict. Evidence for this is seen at high altitude, where varying degrees of HPV between individuals affects their likelihood of developing pulmonary oedema (page 286).
Individual variation in the HPV response is large and difficult to predict. Evidence for this is seen at high altitude, where varying degrees of HPV between individuals affects their likelihood of developing pulmonary oedema (page 286). Non-anaesthetic drugs, particularly vasoactive drugs. Vasodilators such as calcium channel blockers (which are commonly taken by patients having thoracic surgery) are known to attenuate the HPV response. Conversely, routinely used vasoconstrictors such as phenylephrine may preferentially constrict pulmonary vessels in normoxic lung regions.39
Non-anaesthetic drugs, particularly vasoactive drugs. Vasodilators such as calcium channel blockers (which are commonly taken by patients having thoracic surgery) are known to attenuate the HPV response. Conversely, routinely used vasoconstrictors such as phenylephrine may preferentially constrict pulmonary vessels in normoxic lung regions.39
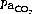 and alkalosis. Hypocapnia or metabolic alkalosis may attenuate HPV whilst hypercapnia and acidosis have the opposite effect. Thus any abnormality of
and alkalosis. Hypocapnia or metabolic alkalosis may attenuate HPV whilst hypercapnia and acidosis have the opposite effect. Thus any abnormality of 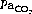 or acid–base balance may adversely affect relative blood flow between the two lungs during OLV.
or acid–base balance may adversely affect relative blood flow between the two lungs during OLV. Epidural analgesia is widely used during thoracic surgery and has been described as enhancing the HPV response, but this observation is also likely to be indirectly mediated by changes in the systemic circulation.45
Epidural analgesia is widely used during thoracic surgery and has been described as enhancing the HPV response, but this observation is also likely to be indirectly mediated by changes in the systemic circulation.45 Temperature affects HPV, with animal studies showing an attenuated response during hypothermia and vice versa.46
Temperature affects HPV, with animal studies showing an attenuated response during hypothermia and vice versa.46Clinical studies of OLV using various anaesthetic agents have failed to show any consistent differences between anaesthetic techniques. Inhalational anaesthetic agents used at clinically appropriate doses of around 1 MAC produce similar degrees of impaired oxygenation as intravenous anaesthetic techniques,39 particularly if depth of anaesthesia with the two techniques is equivalent.47 Thoracic epidural anaesthesia has also been shown to have no effect on oxygenation during OLV irrespective of whether the technique is used with intravenous or inhalational general anaesthesia.48
Pharmacological enhancement of HPV can be used to improve oxygenation during OLV. Inhaled nitric oxide (page 111) may be used to improve blood flow to the ventilated lung, though the expected improvement in oxygenation only seems to occur in patients who are already hypoxic or who have existing pulmonary hypertension.49 Almitrine is a systemically administered peripheral chemoreceptor agonist that can enhance HPV and so improve oxygenation during OLV.50 Its effects are dose-dependent, and if the dose is too high then generalised pulmonary vasoconstriction occurs, rather than only in hypoxic regions, and pulmonary hypertension and a greater shunt fraction occurs. These two potential therapeutic techniques are therefore not yet in routine clinical use.
Management of One-Lung Ventilation
The aim of ventilation during OLV is to maintain arterial Po2 and Pco2 as near normal as possible and is achieved by maintaining adequate alveolar ventilation while minimising the amount of shunt through the non-ventilated lung. Understanding of the physiology already described allows a logical approach to management.
Artificial ventilation during OLV
If alveolar minute volume during OLV is maintained at similar values as when ventilating two lungs, then CO2 elimination is also maintained, though achieving adequate alveolar ventilation, particularly in diseased lungs, may be a significant challenge. The traditional technique is to use smaller tidal volumes than two lung ventilation at a faster respiratory rate, the latter being adjusted to achieve normal end-tidal or arterial Pco2 values. Reducing tidal volume increases the anatomical dead space (page 130) so significant increases in respiratory rate may be needed to maintain alveolar ventilation. If respiratory rate is too fast, intrinsic PEEP (page 476) may occur and cause overexpansion of the dependent lung, increasing airway pressures and reducing blood flow through the dependent lung leading to a worsening of shunt and oxygenation. This is a particular risk in patients with increased airway resistance of any cause. The optimum size of tidal volume to use for OLV remains controversial.51,52 Use of a standard two lung ventilation value of 10–15 ml.kg−1 will commonly lead to unacceptably high airway pressures, may over-distend alveoli (potentially damaging the lung), cause increased pulmonary vascular resistance (so increasing shunt) and contribute to postoperative lung injury.25 If small tidal volumes are used such as 5–8 ml.kg−1 then alveolar ventilation will be difficult to maintain and atelectasis more likely to occur. Finally, the addition of PEEP to the ventilated lung during OLV seems like a logical response to its reduced lung volume and propensity to develop atelectasis, but PEEP will also increase the pulmonary vascular resistance of the dependent lung and potentially worsen the shunt. Thus numerous studies have reported conflicting results concerning the benefit of dependent lung PEEP on oxygenation during OLV.39
So tidal volume, respiratory rate and PEEP each have opposing and undesirable effects at extreme values, risking either inadequate alveolar ventilation with hypoxia or hypercapnia at one extreme, or lung damage that may result in acute lung injury of the ventilated lung at the other. Suggested optimal ventilator settings for OLV have followed the debate regarding ventilation in patients with acute lung injury, who share the challenge of having only a small functional lung (page 457). The suggested ‘protective ventilation’ strategy requires low tidal volumes of 6–8 ml.kg−1 used in conjunction with low levels of PEEP (5 cmH2O), and has been shown to improve oxygenation and reduce the systemic inflammatory response associated with OLV.53 In addition, this strategy may be more effective with pressure controlled ventilation compared with volume controlled ventilation.54 Finally, when calculating the required tidal volume an a per kg basis, calculated lean body weight should be used in obese patients rather than total weight. Lung size is more closely related to height rather than weight, and application of a ‘per kg’ tidal volume in some of today’s very obese patients will risk volutrauma to the ventilated lung.
Use of a high inspired oxygen fraction (Fio2) during OLV is routine to maximise oxygenation of blood in areas of lung with low, but greater than zero, 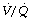 ratios. Use of 100% oxygen may risk encouraging atelectasis formation in a lung with an already reduced lung volume (page 336). Conversely, as described above, achievement of a high mixed venous Po2 may reduce pulmonary vascular resistance in the ventilated lung and so reduce shunt fraction.
ratios. Use of 100% oxygen may risk encouraging atelectasis formation in a lung with an already reduced lung volume (page 336). Conversely, as described above, achievement of a high mixed venous Po2 may reduce pulmonary vascular resistance in the ventilated lung and so reduce shunt fraction.
As for two-lung ventilation (page 336) a recruitment manoeuvre can be performed during OLV to re-expand atelectasis in the dependent lung, a strategy that has been shown to decrease dead space and improve oxygenation.55 The recruitment manoeuvre described for OLV involves volume controlled ventilation with a respiratory rate of 12 breaths per minute and inspiratory time of 50%. Every 5 breaths the tidal volume and PEEP are then increased to achieve peak inspiratory pressures and PEEP values (respectively) of 30/10, 35/15 and 40/20, these last settings being maintained for 10 breaths before reducing the settings in the same stepwise manner.
Management of the non-ventilated lung39
In some patients no action needs to be taken with the non-ventilated lung which can be allowed to collapse, following which gas exchange will remain acceptable. Sadly this is often not the case. Given that the most likely cause of hypoxia during OLV is shunt through the non-ventilated lung the first approach should be to minimise this blood flow. Ventilation with 100% oxygen before isolating the lung causes it to collapse more quickly, and although in theory this will delay the onset of HPV there were no adverse effects on oxygenation 10 minutes after lung isolation in a clinical study.56 As described above, encouraging the surgeon to manipulate the lung, or if appropriate to clamp the pulmonary vessels, are direct ways of reducing non-ventilated lung perfusion. Facilitating effective HPV by avoiding the various factors described above that attenuate the response should be routine practice. The second approach is to accept that shunt through the non-ventilated lung is inevitable and to oxygenate this blood by apnoeic oxygenation. Insufflation of a few litres per minute of oxygen at zero end-expiratory pressure (ZEEP) may be effective, but care must be taken to ensure there is a route for gas to flow back out of the non-ventilated lung to avoid lung expansion or even barotrauma.
Using ZEEP, the lung will continue to collapse and the oxygen will therefore not gain access to those areas of lung where the shunt is occurring. A more effective technique is to apply CPAP to the non-ventilated lung which delivers 100% oxygen, limits the maximum pressure that can be attained in the lung, and reduces the amount of lung collapse that occurs. Applying 5–10 cmH2O of CPAP has been shown to not inflate the lung sufficiently to interfere with surgery and to be very effective at improving oxygenation. The timing of the application of CPAP may be important as it will be less effective if lung collapse has already occurred, in which case, provided the surgery permits, the lung may be briefly reinflated and the CPAP applied during the deflation phase.
Summary of the Clinical Management of OLV
Prior to commencing OLV initial ventilator settings:
 pressure controlled ventilation, with inflation pressure adjusted to achieve tidal volume of 6–8 ml.kg−1
pressure controlled ventilation, with inflation pressure adjusted to achieve tidal volume of 6–8 ml.kg−1 establish that the double lumen tube position is still correct and that ventilation of the non-ventilated lung is still occurring with the required gas mixture
establish that the double lumen tube position is still correct and that ventilation of the non-ventilated lung is still occurring with the required gas mixtureLung Transplantation
Transplantation of a human lung was first performed in 1963,57 but in the years following this few patients survived for longer than a month. In the early 1980s improved immunosuppression led to a resurgence of interest and the technique has now become an established form of treatment. The function of a transplanted lung is important for the well-being of the recipient, but also furthers our understanding of certain fundamental issues of pulmonary physiology. The subject has been reviewed recently.58-63
Clinical Aspects64
Indications
Patients who are considered for transplant have severe respiratory disease, and are receiving optimal therapy, but still have a life expectancy of less than 2–3 years. Uncontrolled respiratory infection, significant disease of other organs, continued smoking or an age in excess of 55–65 years are normally contraindications. Precise selection criteria for recipients vary between transplant centres and with the respiratory disease, but in general patients referred for transplant have a forced expiratory volume in one second (FEV1) of less than 30% predicted, resting hypoxia, hypercapnia and commonly pulmonary hypertension. The indications for lung transplant are shown in Table 33.2, where it can be seen that COPD remains the most common.
The number of patients awaiting transplant exceeds the number of donors. In recent years the number of donor organs available has remained static while the number of candidates for lung transplants has risen rapidly.62 As a result, median waiting time for an organ to become available has increased, and many patients still die while on the waiting list. Cadaveric donor lungs are taken from patients less than 65 years of age with limited smoking history and no evidence of lung disease. Using current selection criteria only about 15% of organ donors are suitable for lung donation. Strategies to improve the number of lung transplants being performed include living-related lobar transplants (see below), extending donor selection criteria using more objective tests of lung function,66 or using non-heartbeating donors.67 This last approach potentially offers unique advantages for lung donation, as oxygenation of the donor lung after cessation of circulation can conserve lung function for up to an hour, and in future this may be extended further by ex-vivo ventilation and perfusion of the lung.62
Types of transplant
Donor and recipient chest sizes are matched. With current organ preservation solutions, lung transplants must be performed within 6–8 hours of organ removal.
Single lung transplant is the simplest procedure. The recipient’s pneumonectomy is undertaken via a thoracotomy using one-lung ventilation (page 497), which presents a significant challenge in these patients.68 The donor lung is implanted, with anastomoses of the main bronchus, the left or right pulmonary artery and a ring of left atrium containing both pulmonary veins of one side. Cardiopulmonary bypass is required in some cases, particularly those patients with preoperative pulmonary hypertension who are at risk of right-sided heart failure during one-lung ventilation.
Bilateral lung transplant comes in two forms. Double-lung transplant performed at a single operation is a more complex procedure for which sternotomy and cardiopulmonary bypass are required. The donor lungs are implanted with anastomoses of either the trachea or both bronchi, the main pulmonary artery and the posterior part of the left atrium containing all four pulmonary veins. A simpler alternative is to transplant two lungs sequentially (termed a double single-lung transplant) through bilateral thoracotomies, and this has now almost completely replaced double-lung transplant.
Heart–lung transplant was originally used for patients with primary pulmonary hypertension and Eisenmenger’s syndrome, and continues to be the operation of choice for the latter (Table 33.2). Total cardiopulmonary bypass is, of course, essential and the anastomoses involve the right atrium, the aorta and the trachea. The complexity and complication rates of heart–lung transplant are high, and, wherever possible, alternative procedures are now preferred, leading to a decline in the number of heart–lung transplant procedures being performed.65
Choice of operation depends on the indication for the transplant, and types of surgery performed are shown in Table 33.2. Single-lung transplantation is favoured, partly because mortality may be lower following this operation, but also because each suitable donor can be used to transplant two recipients. Congenital heart disease commonly requires heart–lung transplant, whilst diseases associated with pulmonary hypertension ideally need either heart–lung transplant or bilateral lung transplant to normalise pulmonary arterial pressure. Lung disease alone is satisfactorily treated with single-lung transplant.
Living-related lung transplants are carried out at several centres in the world.69,70 Left or right lower lobes of the donor relative are transplanted into the whole hemithorax of the recipient, so the technique is only suitable for children or small adults. The same selection criteria apply as for cadaveric transplantation, so CF patients must have bilateral transplants and therefore two related donors. Survival figures are at least comparable with other forms of lung transplantation, and evidence is emerging of better survival in paediatric lung transplants when living-related, rather than cadaveric, organs are used.67 The technique is in its infancy, and offers theoretical benefits in the availability of organs and attenuated organ rejection, but the ethical issues for donors are substantial.
Outcome following transplant
The actuarial survival of lung transplant recipients is shown in Figure 33.5. Given the nature of the surgery it is not surprising that there is significant perioperative and early postoperative mortality. Thereafter, mortality rates are low when consideration is given to the two-year predicted survival of recipients prior to transplant.
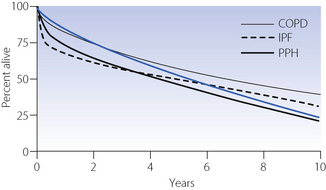
Fig. 33.5 Actuarial survival following lung transplantation for the most common indications. The blue line shows results for all lung transplants; the other lines show results for individual diseases as indicated. Data are from the Registry of the International Society for Heart and Lung Transplantation,65 and include transplants performed between 1990 and 2006. COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; PPH, primary pulmonary hypertension.
After lung transplantation FEV1 is initially poor due to the effects of the surgery, but then shows a gradual improvement, reaching a peak 3–6 months after surgery. From pre-transplant values of 20–30% of predicted normal, recipients of a single-lung transplant achieve values of 50–60% and patients receiving bilateral-lung transplant typically have normal values. These improvements in ventilatory performance contribute to the huge improvement in quality of life following lung transplant.
Exercise performance depends on many factors, which, in addition to pulmonary function, include circulation, condition of the voluntary muscles, motivation and freedom from pain on exertion. Improvement in performance does occur following lung transplantation, but exercise limitation remains common, with maximal oxygen uptake (page 262) of about half normal. There is no evidence that this limitation results from poor pulmonary function, and a muscular origin is more likely,71 possibly related to myopathy induced by immunosuppressant drugs.
Rejection
Acute rejection occurs following activation of cytotoxic T-lymphocytes by helper T-cells which ‘recognise’ the foreign tissue. This form of rejection occurs in about 15% of patients and presents as acute lung injury (Chapter 31) within 72 hours of the transplant operation. Treatment involves escalation of immunosuppressive therapy and supportive management as for other forms of lung injury. Recovery of the transplanted lung may occur, but mortality from acute rejection is high.
Chronic rejection in the lung manifests itself as obliterative bronchiolitis syndrome, the origin of which is not clear but which occurs in up to half of patients, normally more than a year after transplantation. Detection of chronic rejection is problematic because in the early stages of acute rejection it is difficult to distinguish rejection from infection on clinical evidence. Both conditions feature arterial hypoxaemia, pyrexia, leukocytosis, dyspnoea and reduced exercise capacity. These changes are followed by a decrease in diffusing capacity and FEV1, and later by perihilar infiltration or graft opacification on the chest radiograph. Bronchiolitis obliterans, as the name suggests, causes significant air-flow limitation; the FEV1 is used as a screening test and also to stage the degree of rejection.
Except in the case of identical twins, survival of the transplanted lung depends on immunosuppression.72 Current therapy involves immunosuppression by three groups of drugs:
Physiological Effects of Lung Transplant
Transplantation inevitably disrupts the nerve supply, lymphatics and the bronchial circulation. The condition of the recipient is further compromised by immunosuppressive therapy.
The denervated lung. The transplanted lung has no afferent or efferent innervation and there is, as yet, no evidence that re-innervation occurs in patients.73 However, in dogs, vagal stimulation has been observed to cause bronchoconstriction 3–6 months after lung reimplantation,74 and sympathetic re-innervation has been demonstrated after 45 months.75
In Chapter 5, attention was paid to the weakness of the Hering–Breuer reflex in humans. It was therefore to be expected that denervation of the lung, with block of pulmonary baroreceptor input to the medulla, would have minimal effect on the respiratory rhythm. This is in contrast to the dog and most other laboratory animals, in whom vagal block is known to cause slow deep breathing. Bilateral vagal block in human volunteers was already known to leave the respiratory rhythm virtually unchanged, and it was therefore no great surprise when it was shown that bilateral lung transplant had no significant effect on the respiratory rate and rhythm in patients, after the early postoperative period.76 Breathing during sleep is also normal.77
Bronchial hypersensitivity, i.e. enhanced sensitivity to the bronchoconstrictor effects of inhaled methacholine or histamine can be demonstrated after heart-lung transplantation.78 This is thought to be due to hypersensitivity of receptors in airway smooth muscle, following denervation of the predominantly constrictor autonomic supply, though not all studies have demonstrated this.73 In spite of these findings, airway hyper-responsiveness is rarely a problem in transplanted patients.
The cough reflex, in response to afferents arising from below the level of the tracheal or bronchial anastomosis, is permanently lost after lung transplantation.79 Following single-lung transplant, the remaining diseased lung will continue to stimulate coughing, which will facilitate clearance of secretions from the transplanted lung. Similarly, a bilateral single-lung transplant will be preferable to a double-lung transplant, as the former will maintain intact the potent carinal cough reflex. The abnormality in cough reflex is a major contributor to lung infection following transplant, along with altered mucous clearance as described below.
Ventilation-perfusion (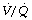 ) relationships. Bilateral lung or heart-lung transplants usually result in normal
) relationships. Bilateral lung or heart-lung transplants usually result in normal 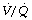 relationships, but following single-lung transplant the situation is more complex. For most indications, including COPD, the single transplanted lung receives the majority of pulmonary ventilation (60–80% of the total) and a similar proportion of pulmonary blood flow, and so
relationships, but following single-lung transplant the situation is more complex. For most indications, including COPD, the single transplanted lung receives the majority of pulmonary ventilation (60–80% of the total) and a similar proportion of pulmonary blood flow, and so 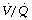 relationships are acceptable, though not normal.80,81 However, following single-lung transplant for primary pulmonary hypertension, ventilation to the two lungs remains approximately equal whilst the majority of blood flow (often > 80%) is to the transplanted lung. This
relationships are acceptable, though not normal.80,81 However, following single-lung transplant for primary pulmonary hypertension, ventilation to the two lungs remains approximately equal whilst the majority of blood flow (often > 80%) is to the transplanted lung. This 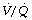 mismatch fortunately has little effect on arterial oxygenation at rest. During exercise in patients with a single-lung transplant, the already high blood flow to the transplanted lung seems not to increase further, and the normal recruitment of apical pulmonary capillaries (page 103) cannot be demonstrated.80
mismatch fortunately has little effect on arterial oxygenation at rest. During exercise in patients with a single-lung transplant, the already high blood flow to the transplanted lung seems not to increase further, and the normal recruitment of apical pulmonary capillaries (page 103) cannot be demonstrated.80
Hypoxic pulmonary vasoconstriction seems to be an entirely local mechanism and, as might be expected, has been shown to persist in the human transplanted lung,82 though some studies have demonstrated abnormalities, particularly in patients with pulmonary hypertension.80,81
Mucociliary clearance. Mucociliary clearance is defective after transplantation.83 The cause seems to be defective production of mucus, rather than changes in the frequency of cilial beat. This, together with the absent cough reflex below the line of the airway anastomosis, means that the patient is at a disadvantage in clearing secretions. Side effects of immunosuppression compound these changes and lead to enhanced susceptibility to infection of the transplanted lung. Though these factors clearly do not preclude long-term survival of the graft, one-quarter of deaths following lung transplantation result from infection.
References
1. Honeybourne D, Bab J, Bowie P, et al. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(suppl 1):1-21.
2. Putinati S, Ballerin L, Corbetta L, Trevisani L, Potena A. Patient satisfaction with conscious sedation for bronchoscopy. Chest. 1999;115:1437-1440.
3. Matsushima Y, Jones RL, King EG, Moysa G, Alton JD. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984;86:184-188.
4. Peacock AJ, Benson-Mitchell R, Godfrey R. Effect of fibreoptic bronchoscopy on pulmonary function. Thorax. 1990;45:38-41.
5. Ehrenwerth J, Brull SJ. Anesthesia for thoracic diagnostic procedures. In Kaplan JA, Slinger PD, editors: Thoracic Anesthesia, 3rd ed., Philadelphia: Churchill Livingstone, 2003.
6. Jardine AD, Harrison MJ, Healy TEJ. Automatic flow interruption bronchoscope: A laboratory study. Br J Anaesth.. 1975;47:385-389.
7. Tassi GF, Davies RJ, Noppen M. Advanced techniques in medical thoracoscopy. Eur Respir J. 2006;28:1051-1059.
8. Krasna MJ. Thoracoscopic sympathectomy: a standardized approach to therapy for hyperhidrosis. Ann Thorac Surg.. 2008;85:S764-S767.
9. Ojimba TA, Cameron AE. Drawbacks of endoscopic thoracic sympathectomy. Br J Surg.. 2004;91:264-269.
10. Flores RM, Alam N. Video-assisted thoracic surgery (VATS) lobectomy, open thoracotomy, and the robot for lung cancer. Ann Thorac Surg.. 2008;85:S710-S715.
11. Bolton JWR, Weiman DS. Physiology of lung resection. Clin Chest Med.. 1993;14:293-303.
12. Lemmer JH, Gomez MN, Symreng T, Ross AF, Rossi NP. Limited lateral thoracotomy. Arch Surg.. 1990;125:873-877.
13. Peters RM, Wellons HA, Htwe TM, Hill C. Total compliance and work of breathing after thoracotomy. J Thorac Cardiovasc Surg.. 1969;57:348-355.
14. Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg.. 1991;101:394-401.
15. Akçali Y, Demir H, Tezcan B. The effect of standard posterolateral versus muscle-sparing thoracotomy on multiple parameters. Ann Thorac Surg.. 2003;76:1050-1054.
16. Nomori H, Horio H, Fuyuno G, Kobayashi R, Yashima H. Respiratory muscle strength after lung resection with special reference to age and procedures of thoracotomy. Eur J Cardiothorac Surg.. 1996;10:352-358.
17. Shaw A, Keefe FJ. Genetic and environmental determinants of post-thoracotomy pain syndrome. Curr Opin Anaesthesiol.. 2008;21:8-11.
18. Gene L, Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery. ACCP evidenced-based clinical practice guidelines, 2nd ed. Chest. 2007;132:161S-177S.
19. van Tilburg PMB, Stam H, Hoogsteden HC, van Klaveren RJ. Pre-operative pulmonary evaluation of lung cancer patients: a review of the literature. Eur Respir J. 2009;33:1206-1215.
20. Brunelli A, Al Refai M, Monteverde M, Borri A, Salati M, Fianchini A. Stair climbing test predicts cardiopulmonary complications after lung resection. Chest. 2002;121:1106-1110.
*21. Weibel ER. How to make an alveolus. Eur Respir J. 2008;31:483-485.
22. Fehrenbach H, Voswinckel R, Michl V, et al. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2008;31:515-522.
*23. Slinger PD. Postpneumonectomy pulmonary edema. Good news and bad news. Anesthesiol.. 2006;105:2-5.
24. Slinger PD. Perioperative fluid management for thoracic surgery: the puzzle of postpneumonectomy pulmonary edema. J Cardiothorac Vasc Anesth.. 1995;9:442-451.
25. Fernández-Pérez ER, Keegan MT, Brown DR, Hubmayr RD, Gajic O. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiol.. 2006;105:14-18.
26. Meyers BF, Patterson GA. Chronic obstructive pulmonary disease 10: Bullectomy, lung volume reduction surgery, and transplantation for patients with chronic obstructive pulmonary disease. Thorax. 2003;58:634-638.
27. Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema, and the National Emphysema Treatment Trial Research Group. N Engl J Med., 348. 2003: 2059-2073.
28. Lenfant C. Will lung volume reduction surgery be widely applied? Ann Thorac Surg.. 2006;82:385-387.
29. Criner GJ, Belt P, Sternberg AL, et al. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest. 2009;135:1268-1279.
30. Miller JD, Malthaner RA, Goldsmith CH, et al. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thoracic Surg.. 2006;81:314-320.
31. Snyder ML, Goss CH, Neradilek B, et al. Changes in arterial oxygenation and self-reported oxygen use after lung volume reduction surgery, and the National Emphysema Treatment Trial Research Group. Am J Respir Crit Care Med., 178. 2008: 339-345.
32. Gorman RB, McKenzie DK, Butler JE, Tolman JF, Gandevia SC. Diaphragm length and neural drive after lung volume reduction surgery. Am J Respir Crit Care Med.. 2005;172:1259-1266.
33. Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc.. 2008;83:235-250.
34. Noppen M. Who’s (still) afraid of talc? Eur Respir J. 2007;29:619-621.
35. Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax. 1997;52:329-332.
36. West JB. Snorkel breathing in the elephant explains the unique anatomy of its pleura. Respir Physiol.. 2001;126:1-8.
37. Wilson WC, Benumof JL. Anesthesia for thoracic surgery. In Miller RD, editor: Miller’s Anesthesia, 6th ed., Philadelphia: Churchill Livingstone, 2005.
38. Karabulut N. CT assessment of tracheal carinal angle and its determinants. Br J Radiol.. 2005;78:787-790.
39. Triantafillou AN, Benumof JL, Lecamwasam HS. Physiology of the lateral decubitus position, the open chest, and one-lung ventilation. In Kaplan JA, Slinger PD, editors: Thoracic anesthesia, 3rd ed., Philadelphia: Churchill Livingstone, 2003.
40. Szegedi LL. Pathophysiology of one-lung ventilation. Anesthesiol Clin North America. 2001;19:435-453.
41. Klingstedt C, Hedenstierna G, Baehrendtz S, et al. Ventilation-perfusion relationships and atelectasis formation in the supine and lateral positions during conventional mechanical and differential ventilation. Acta Anaesthesiol Scand.. 1990;34:421-429.
42. Watanabe S, Noguchi E, Yamada S, Hamada N, Kano T. Sequential changes of arterial oxygen tension in the supine position during one-lung ventilation. Anesth Analg.. 2000;90:28-34.
43. Benumof JL. Mechanism of decreased blood flow to atelectactic lung. J Appl Physiol.. 1979;46:1047-1048.
44. Nagendran J, Stewart K, Hoskinson M, Archer SL. An anesthesiologist’s guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anesthesiol.. 2006;19:34-43.
45. Ishibe Y, Shiokawa Y, Umeda T, Uno H, Nakamura M, Izumi T. The effect of thoracic epidural anesthesia on hypoxic pulmonary vasoconstriction in dogs: an analysis of the pressure-flow curve. Anesth Analg.. 1996;82:1049-1055.
46. Benumof JL, Wahrenbrock EA. Dependency of hypoxic pulmonary vasoconstriction on temperature. J Appl Physiol.. 1977;42:56-58.
47. Pruszkowski O, Dalibon N, Moutafis M, et al. Effects of propofol vs sevoflurane on arterial oxygenation during one-lung ventilation. Br J Anaes.. 2007;98:539-544.
48. Özcan PE, Sentürk M, Sungur Ulke Z, et al. Effects of thoracic epidural anaesthesia on pulmonary venous admixture and oxygenation during one-lung ventilation. Acta Anaesthesiol Scand.. 2007;51:1117-1122.
49. Rocca GD, Passariello M, Coccia C, et al. Inhaled nitric oxide administration during one-lung ventilation in patients undergoing thoracic surgery. J Cardiothorac Vasc Anesth.. 2001;15:218-223.
50. Dalibon N, Moutafis M, Liu N, Law-Koune J-D, Monsel S, Fischler M. Treatment of hypoxemia during one-lung ventilation using intravenous almitrine. Anesth Analg.. 2004;98:590-594.
51. Slinger P. Pro: Low tidal volume is indicated during one-lung ventilation. Anesth Analg.. 2006;103:268-270.
52. Gal T. Con: Low tidal volumes are indicated during one-lung ventilation. Anesth Analg.. 2006;103:271-273.
53. Michelet P, D’Journo X-B, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy. Anesthesiol.. 2006;105:911-919.
54. Sentürk M, Dilek A, Çamci E, et al. Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressure-controlled one-lung ventilation. J Cardiothorac Vasc Anesth.. 2005;19:71-75.
55. Tusman G, Böhm SH, Sipmann FS, Maisch S. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg.. 2004;98:1604-1609.
56. Ko R, McRae K, Darling G, et al. The use of air in the inspired gas mixture during two-lung ventilation delays lung collapse during one-lung ventilation. Anesth Analg.. 2009;108:1092-1096.
57. Hardy JD, Webb WR, Dalton ML, Walker GR. Lung homotransplantation in man. JAMA. 1963;186:1065-1074.
58. Bracken CA, Gurkowski MA, Naples JJ. Lung transplantation: Historical perspectives, current concepts, and anesthetic considerations. J Cardiothorac Vasc Anesth.. 1997;11:220-241.
59. DeMeo DL, Ginns LC. Lung transplantation at the turn of the century. Annu Rev Med.. 2001;52:185-201.
60. Snyder LD, Palmer SM. Quality, quantity, or both?: Life after lung transplantation. Chest. 2005;128:1086-1087.
61. Pierson RN. Lung transplantation: Current status and challenges. Transplantation. 2006;81:1609-1615.
*62. Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med.. 2005;172:944-955.
63. Corris PA, Christie JD. Update in transplantation 2006. Am J Respir Crit Care Med.. 2007;175:432-435.
64. Lama VN. Update in lung Transplantation 2008. Am J Respir Crit Care Med.. 2009;179:759-764.
65. Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth official adult lung and heart/lung transplantation report – 2008. J Heart Lung Transplant.. 2008;27:957-969.
66. Chang AC, Orens JB. Are there more lungs available than currently meet the eye? Am J Respir Crit Care Med.. 2006;174:624-625.
67. de Perrot M, Weder W, Patterson GA, Keshavjee S. Strategies to increase limited donor resources. Eur Respir J. 2004;23:477-482.
68. Singh H, Bossard RF. Perioperative anaesthetic considerations for patients undergoing lung transplantation. Can J Anaesth.. 1997;44:284-299.
69. Dark JH. Lung: living related transplantation. Br Med Bull.. 1997;53:892-903.
70. Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg.. 2004;127:114-122.
*71. Reinsma GD, ten Hacken NHT, Grevink RG, van der Bij W, Koëter GH, van Weert E. Limiting factors of exercise performance 1 year after lung transplantation. J Heart Lung Transplant.. 2006;25:1310-1316.
72. Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J. 2004;23:159-171.
73. Stretton CD, Mak JCW, Belvisi MG, Yacoub MH, Barnes PJ. Cholinergic control of human airways in vitro following extrinsic denervation of the human respiratory tract by heart-lung transplantation. Am Rev Respir Dis.. 1990;142:1030-1033.
74. Edmunds LH, Graf PD, Nadel JA. Reinnervation of reimplanted canine lung. J Appl Physiol.. 1971;31:722-727.
75. Lall A, Graf PD, Nadel JA, Edmunds LH. Adrenergic reinnervation of the reimplanted dog lung. J Appl Physiol.. 1973;35:439-442.
76. Shaw IH, Kirk AJB, Conacher ID. Anaesthesia for patients with transplanted hearts and lungs undergoing non-cardiac surgery. Br J Anaesth.. 1991;67:772-781.
77. Sanders MH, Costantino JP, Owens GR, et al. Breathing during wakefulness and sleep after human heart-lung transplantation. Am Rev Respir Dis.. 1989;140:45-51.
78. Glanville AR, Burke CM, Theodore J, et al. Bronchial hyper-responsiveness after human cardiopulmonary transplantation. Clin Sci.. 1987;73:299-303.
79. Higenbottam T, Jackson M, Woolman P, Lowry R, Wallwork J. The cough response to ultrasonically nebulized distilled water in heart-lung transplantation patients. Am Rev Respir Dis.. 1989;140:58-61.
80. Ross DJ, Waters PF, Waxman AD, Koerner SK, Mohsenifar Z. Regional distribution of lung perfusion and ventilation at rest and during steady-state exercise after unilateral lung transplantation. Chest. 1993;104:130-135.
81. Kuni CC, Ducret RP, Nakhleh RE, Boudreau RJ. Reverse mismatch between perfusion and aerosol ventilation in transplanted lungs. Clin Nucl Med.. 1993;18:313-317.
82. Robin ED, Theodore J, Burke CM, et al. Hypoxic pulmonary vasconstriction persists in the human transplanted lung. Clin Sci.. 1987;72:283-287.
83. Herve P, Silbert D, Cerrina J, Simonneau G, Dartevelle P. Impairment of bronchial mucociliary clearance in long-term survivors of heart/lung and double-lung transplantation, and the Paris-Sud Lung Transplant Group. Chest, 103. 1993: 59-63.