19 Peptides and proteins as mediators
Overview
In this chapter we consider the special features of peptides and proteins, which are ubiquitous chemical mediators, and whose characteristics differ in some important respects from the small molecule mediators discussed in earlier chapters. The field is advancing rapidly through the application of molecular biological techniques, and many clinical applications are in prospect.
Introduction
Pharmacology has traditionally concerned itself with signalling molecules that are of low molecular weight and non-peptide in nature. Since the 1970s, however, it has emerged that peptides and proteins are at least as important—maybe even more so—as signalling molecules. Yet the pharmacological manipulation of peptide signalling is less advanced than that of, say, the cholinergic, adrenergic or 5-hydroxytryptamine systems (Chs 12–14). Pharmacology, one could say, has some catching up to do. In this chapter, we give an overview of the main characteristics of peptides and proteins as mediators and as drugs, bringing out the contrasts between these and non-peptides, and we evaluate the present and possible future use of peptides as therapeutic agents. For reviews, with more detail than can be provided here, see Buckel (1996), Cooper et al. (1996), Hökfelt et al. (2000) and Nestler et al. (2001).
Historical Aspects
 Despite the fact that some peptide mediators were discovered early in the history of our discipline (e.g. substance P was discovered in the 1930s), pharmacology has historically harboured a strong bias towards non-peptides. One reason for this apparently irrational dislike is that, at one time, most drugs were natural (mainly plant) products. Very few were peptides or acted through what we now recognise as peptide signalling systems. A second reason is that the methodology required to study peptides is of more recent origin. The development of high-performance liquid chromatography and solid-phase peptide synthesis, and the use of antibodies for radioimmunoassay and immunocytochemistry, as well as the use of molecular biology, have greatly accelerated the development of the area.
Despite the fact that some peptide mediators were discovered early in the history of our discipline (e.g. substance P was discovered in the 1930s), pharmacology has historically harboured a strong bias towards non-peptides. One reason for this apparently irrational dislike is that, at one time, most drugs were natural (mainly plant) products. Very few were peptides or acted through what we now recognise as peptide signalling systems. A second reason is that the methodology required to study peptides is of more recent origin. The development of high-performance liquid chromatography and solid-phase peptide synthesis, and the use of antibodies for radioimmunoassay and immunocytochemistry, as well as the use of molecular biology, have greatly accelerated the development of the area.
In 1953, du Vigneaud made history, and earned a Nobel Prize, by determining the structure of, and subsequently synthesising, oxytocin, the first peptide mediator to be characterised and the first to be produced commercially for clinical use. The structures of many other mediators, for example substance P, bradykinin and angiotensin, which had been identified as peptides in the 1930s, remained unsolved for many years. While all are small peptides of 11 residues or fewer, determination of their structure, and their total chemical synthesis, was a Herculean effort. The structure of bradykinin was not elucidated until 1960, while that of substance P was published in 1970.
By contrast, the use of contemporary techniques enabled endothelin (a much larger peptide) to be fully characterised, synthesised and cloned within about a year, the complete information being published in a single paper (Yanagisawa et al., 1988). Protein mediators, such as cytokines and growth factors (Ch. 17) containing 50 or more residues are still difficult to synthesise chemically, and major advances must rely largely on molecular biology. The use of recombinant proteins as therapeutic agents—a development driven mainly by the emergent biotechnology industry—is rapidly gaining ground (see Ch. 59). Whereas the discovery of new ‘small molecule’ mediators has virtually dried up, the discovery of new protein and peptide mediators continues apace.
General Principles of Peptide Pharmacology
Structure of Peptides
Peptide and protein mediators generally vary from 3 to about 200 amino acid residues in size (Fig. 19.1), the arbitrary dividing line between peptides and proteins being about 50 residues. For convenience, in this chapter, we use the term peptide to cover both classes. Specific residues in peptides often undergo post-translational modifications, such as C-terminal amidation, glycosylation, acetylation, carboxylation, sulfation or phosphorylation. They also may contain intramolecular disulfide bonds, such that the molecule adopts a cyclic or partially cyclic conformation; or they may comprise two or more separate chains linked by intermolecular disulfide bonds.
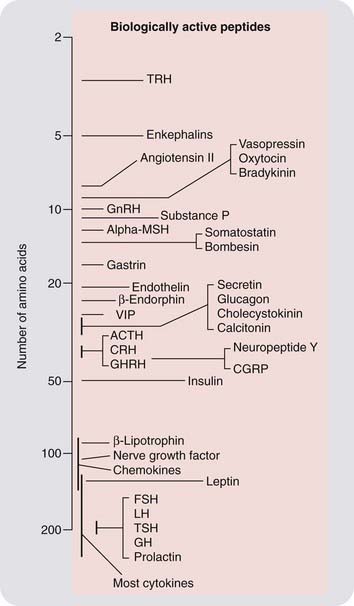
Fig. 19.1 Some typical peptide mediators.
ACTH, adrenocorticotrophic hormone; Alpha-MSH, α-melanocyte-stimulating hormone; CGRP, calcitonin gene-related peptide; CRH, corticotrophin-releasing hormone; FSH, follicle-stimulating hormone; GH, growth hormone; GHRH, growth hormone-releasing hormone; GnRH, gonadotrophin-releasing hormone; LH, luteinising hormone; TRH, thyrotrophin-releasing hormone; TSH, thyroid-stimulating hormone; VIP, vasoactive intestinal peptide.
It is difficult to determine the conformation of peptides in solution because they are so flexible, and peptides of fewer than about 40 residues have proved difficult to crystallise, precluding the use of X-ray diffraction methods to study their conformation (although some other techniques, such as nuclear magnetic resonance, have proved helpful). Larger proteins adopt more restricted conformations, but because of their size they generally interact with multiple sites on their receptors. To envisage peptides fitting into a receptor site in a precise ‘lock and key’ mode is to imagine that you can unlock your front door with a length of cooked spaghetti. Such problems have greatly impeded the rational design of non-peptide analogues (peptidomimetics) that mimic the action of peptides at their receptors. The use of random screening methods has (somewhat to the chagrin of the rationalists) nevertheless led in recent years to the discovery of many non-peptide antagonists—although few agonists—for peptide receptors (see below; Betancur et al., 1997—an exception is the opioid field; see Ch. 41).
Types of Peptide Mediator
Peptide mediators that are secreted by cells and act on surface receptors of the same or other cells can be very broadly divided into four groups:
Some important examples of peptide and protein mediators are shown in Figure 19.1.
Role of Molecular Biology
 Because peptide structures are represented directly in the genome, molecular biology has been the key to most of the recent advances in knowledge. It is used in many ways, as in the following examples.
Because peptide structures are represented directly in the genome, molecular biology has been the key to most of the recent advances in knowledge. It is used in many ways, as in the following examples.
Peptides in the Nervous System: Comparison with Conventional Transmitters
The abundance of neuropeptides in the brain and elsewhere became evident in the 1970–1980s, and new examples are still emerging. In most respects, neuropeptide-mediated transmission resembles transmission by ‘conventional’ non-peptide mediators; the mechanisms for peptide storage and release (summarised in Fig. 19.2) and the receptor mechanisms through which their effects are produced are essentially the same in both cases. One difference is that the vesicles are loaded with peptide precursors in the cell body, the active peptides being generated within the vesicles as they move to the nerve terminals. Following exocytosis, the vesicles cannot be reloaded in situ but must instead be replaced with new preloaded vesicles. Transmitter turnover is therefore less rapid than with conventional mediators, and recapture of the released transmitter does not occur.
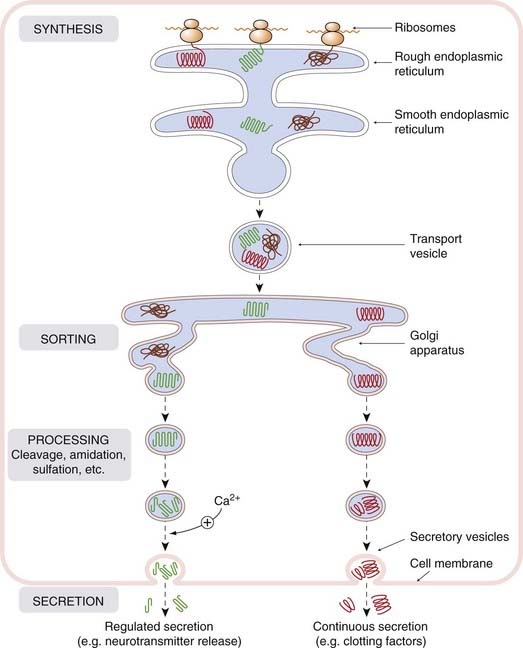
Fig. 19.2 Cellular mechanisms for peptide synthesis and release.
Proteins synthesised by ribosomes are threaded through the membrane of the rough endoplasmic reticulum, from where they are conveyed in transport vesicles to the Golgi apparatus. Here, they are sorted and packaged into secretory vesicles. Processing (cleavage, glycosylation, amidation, sulfation, etc.) occurs within the transport and secretory vesicles, and the products are released from the cell by exocytosis. Constitutive secretion (e.g. of plasma proteins and clotting factors by liver cells) occurs continuously, and little material is stored in secretory vesicles. Regulated secretion (e.g. of neuropeptides or cytokines) occurs in response to increased intracellular Ca2+ or other intracellular signals, and material is typically stored in significant amounts in secretory vesicles awaiting release.
As with other chemical mediators, the effects of peptides may be excitatory or inhibitory, pre- or postsynaptic, and exerted over short or long distances from the site of release. There are, however, certain monopolies of function between peptide and non-peptide mediators. For example, endogenous peptides rarely activate ligand-gated ion channels,1 and therefore do not function as fast neurotransmitters in the manner of non-peptides such as acetylcholine, glutamate, glycine or GABA (see Chs 13 and 37). Instead, they serve (as do many non-peptides) mainly as neuromodulators, by activating G-protein-coupled receptors. In contrast, the ligands for tyrosine kinase-linked receptors are all peptides or proteins.
In summary, the similarities in function between peptide and non-peptide mediators are more striking than the differences. The main difference stems from the fact that peptides, being gene products, represent variations on a single theme—a linear string of amino acids. Such sequences are much more susceptible to evolutionary change than are the structures of non-peptide mediators, and the number of known peptide mediators now greatly exceeds that of non-peptides. As Iversen pointed out in 1983: ‘almost overnight, the number of putative transmitters in the mammalian nervous system has jumped from the ten or so monoamine and amino acid candidates to more than 40’. Since then, no new monoamine transmitters have appeared, but there are at least another 60 peptides.
The role of peptides as co-transmitters is discussed in Chapter 12. Two well-documented examples (reviewed by Lundberg, 1996) are the parasympathetic nerves innervating the salivary glands (where the secretory response is produced by acetylcholine and the vasodilatation partly by vasoactive intestinal peptide) and the sympathetic innervation to many tissues, which releases the vasoconstrictor neuropeptide Y in addition to noradrenaline (norepinephrine).
The distinction between neuropeptides and peripherally acting hormones is useful but not absolute. Thus the incretins and insulin (Ch. 30), angiotensin, atrial natriuretic peptide (Chs 21 and 22) and oxytocin (Ch. 34) are best known as hormones that are formed, released and act in the periphery. They are, however, also found in the brain, although their role there is uncertain. Similarly, endothelin (Ch. 22) was first discovered in blood vessels but is now known to occur extensively in the brain as well.
Multiple Physiological Roles of Peptides
 In common with many non-peptide mediators, such as noradrenaline, dopamine, 5-hydroxytryptamine or acetylcholine, the same peptides may function as mediators in several different organs, and intriguingly often appear to subserve some coordinated physiological function. For example, angiotensin acts on the cells of the hypothalamus to release antidiuretic hormone (vasopressin), which in turn causes water retention. Angiotensin also acts elsewhere in the brain to promote drinking behaviour and to increase blood pressure by activation of the sympathetic system; in addition, it releases aldosterone, which causes salt and water retention and acts directly to constrict blood vessels. Each of these effects plays a part in the overall response of the body to water deprivation and reduced circulating volume. There are other examples of what appears to be an orchestrated functional response produced by the various actions of a single mediator, but there are many more examples where the multiple effects seem just to be exactly that—multiple effects.
In common with many non-peptide mediators, such as noradrenaline, dopamine, 5-hydroxytryptamine or acetylcholine, the same peptides may function as mediators in several different organs, and intriguingly often appear to subserve some coordinated physiological function. For example, angiotensin acts on the cells of the hypothalamus to release antidiuretic hormone (vasopressin), which in turn causes water retention. Angiotensin also acts elsewhere in the brain to promote drinking behaviour and to increase blood pressure by activation of the sympathetic system; in addition, it releases aldosterone, which causes salt and water retention and acts directly to constrict blood vessels. Each of these effects plays a part in the overall response of the body to water deprivation and reduced circulating volume. There are other examples of what appears to be an orchestrated functional response produced by the various actions of a single mediator, but there are many more examples where the multiple effects seem just to be exactly that—multiple effects.
So far, the stream of new information about neuropeptides since the 1970s has led to few useful generalisations about their functional role, and surprisingly few new drugs—with the exception of antihypertensive drugs acting on the renin–angiotensin system (see Ch. 22), most of which are peptidomimetics. For whatever reason, peptide pharmacology has proved to be something of a graveyard for drug discovery projects. For example, substance P antagonists were confidently expected to be effective analgesic drugs based on copious data from animal studies, but proved to have no analgesic activity in humans, although one such drug, aprepitant, has been found to have a role in preventing vomiting caused by cisplatin-based cytotoxic chemotherapy (Ch. 55). They also have unexpected anxiolytic properties.
Structure and function of peptide mediators ![]()
Biosynthesis and Regulation of Peptides
Peptide structure is, of course, directly coded in the genome, in a manner that the structure of (say) acetylcholine is not, so intracellular manufacture is simpler. Peptide synthesis (Fig. 19.3) begins with the manufacture of a precursor protein in which the peptide sequence is embedded, along with specific proteolytic enzymes that excise the active peptide, a process of sculpture rather than synthesis. The precursor protein is packaged into vesicles at the point of synthesis, and the active peptide is formed in situ ready for release (Fig. 19.2). Thus there is no need for specialised biosynthetic pathways, or for the uptake or recapturing mechanisms, that are important for the synthesis and release of non-peptide mediators.
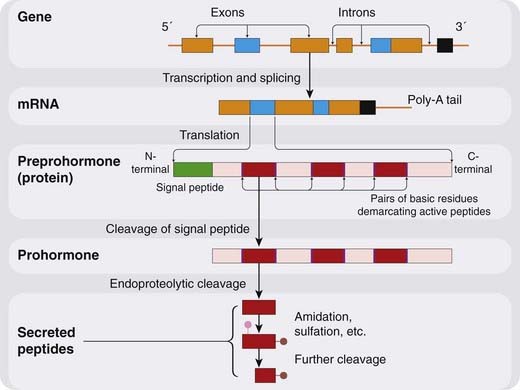
Fig. 19.3 Synthesis of a peptide mediator.
The coding regions of the gene (exons) are transcribed and spliced to give rise to mRNA, segments of which (blue) are translated to produce preprohormones. Cleavage of the N-terminal signal peptide produces the prohormone, from which endopeptidases excise peptide fragments. These may be active as such, or they may undergo further post-translational processing (amidation etc.).
Peptide Precursors
The precursor protein, or preprohormone, usually 100–250 residues in length, consists of an N-terminal signal sequence (peptide), followed by a variable stretch of unknown function, and a peptide-containing region in which several copies of active peptide fragments may be contained. Often, several different peptides are found within one precursor, but sometimes there is only one in multiple copies. An extreme example occurs in the invertebrate Aplysia, in which the precursor contains 28 copies of the same short peptide. The signal peptide, which is strongly hydrophobic, facilitates insertion of the protein into the endoplasmic reticulum and is then cleaved off at an early stage, yielding the prohormone.
The active peptides are usually demarcated within the prohormone sequence by pairs of basic amino acids (Lys-Lys or Lys-Arg), which are cleavage points for the trypsin-like proteases that release the peptides. This endoproteolytic cleavage generally occurs in the Golgi apparatus or the secretory vesicles. The enzymes responsible are known as prohormone convertases, of which two subtypes (PC1 and PC2) have been studied in detail (see Cullinan et al., 1991). Scrutiny of the prohormone sequence often reveals likely cleavage points that demarcate unknown peptides. In some cases (e.g. CGRP; see below), new peptide mediators have been discovered in this way, but there are many examples where no function has yet been assigned. Whether these peptides are, like strangers at a funeral, waiting to declare their purpose or merely functionless relics, remains a mystery. There are also large stretches of the prohormone sequence of unknown function lying between the active peptide fragments.
The abundance of mRNA coding for particular preprohormones, which reflects the level of gene expression, is very sensitive to physiological conditions, and this type of transcriptional control is one of the main mechanisms by which peptide expression and release are regulated over the medium to long term. Inflammation, for example, increases the expression, and hence the release, of various cytokines by immune cells (see Ch. 16). Sensory neurons respond to peripheral inflammation by increased expression of tachykinins, which is important in the genesis of inflammatory pain (see Ch. 41).
Diversity Within Peptide Families
 Peptides commonly occur in families with similar or related sequences and actions. Opioid peptides (see Ch. 41) provide a good example of the representation of such a family at the genomic level. Opioid peptides, defined as peptides with opiate-like pharmacological effects, are coded by three distinct genes whose products are, respectively, prepro-opiomelanocortin (POMC), preproenkephalin and preprodynorphin. Each of these precursors contains the sequences of a number of opioid peptides (Fig. 19.4). Hughes and Kosterlitz, who discovered the enkephalins in 1975, noticed that the sequence of met-enkephalin is contained within that of a pituitary hormone, β-lipotrophin. About this time, three other peptides with morphine-like actions were discovered, α-, β- and γ-endorphin, which also were contained within the β-lipotrophin molecule. It was then found that the enkephalins actually come from the other gene products, proenkephalin and prodynorphin, POMC itself serving as a source of adrenocorticotrophic hormone (ACTH), melanocyte-stimulating hormones (MSH) and β-endorphin, but not of enkephalins.
Peptides commonly occur in families with similar or related sequences and actions. Opioid peptides (see Ch. 41) provide a good example of the representation of such a family at the genomic level. Opioid peptides, defined as peptides with opiate-like pharmacological effects, are coded by three distinct genes whose products are, respectively, prepro-opiomelanocortin (POMC), preproenkephalin and preprodynorphin. Each of these precursors contains the sequences of a number of opioid peptides (Fig. 19.4). Hughes and Kosterlitz, who discovered the enkephalins in 1975, noticed that the sequence of met-enkephalin is contained within that of a pituitary hormone, β-lipotrophin. About this time, three other peptides with morphine-like actions were discovered, α-, β- and γ-endorphin, which also were contained within the β-lipotrophin molecule. It was then found that the enkephalins actually come from the other gene products, proenkephalin and prodynorphin, POMC itself serving as a source of adrenocorticotrophic hormone (ACTH), melanocyte-stimulating hormones (MSH) and β-endorphin, but not of enkephalins.
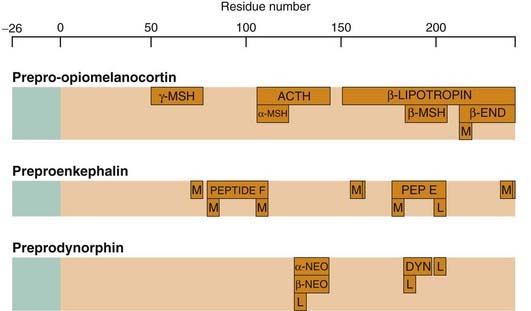
Structures of the three opioid precursor proteins, showing the location of opioid and other peptides within the sequence. These embedded peptides are bounded by pairs of basic amino acids, which form points of attack for enzymatic cleavage. The signal peptide sequence is shown in green. β-END, β-endorphin; ACTH, adrenocorticotrophic hormone; DYN, dynorphin; L, leucine enkephalin; M, methionine enkephalin; MSH, melanocyte-stimulating hormone; NEO, neoendorphin.
The expression of the precursor proteins varies greatly in different tissues and brain areas. For example, POMC and its peptide products are found mainly in the pituitary and hypothalamus, whereas endorphin, met-enkephalin, leu-enkephalin and dynorphin are more widely distributed. In the spinal cord, dynorphin occurs mainly in interneurons, while the enkephalins are found mainly in long descending pathways from the midbrain to the dorsal horn. Opioid peptides are also produced by many non-neuronal cells, including endocrine and exocrine glands and cells of the immune system, as well as in brain areas distinct from those involved in nociception, and correspondingly they play a regulatory role in many different physiological systems, as reflected in the rather complex pharmacological properties of opiate drugs.
Diversity of members of a peptide family can also arise by gene splicing or during post-translational processing of the prohormone.
Gene splicing as a source of peptide diversity
 Genes contain coding regions (exons) interspersed with non-coding regions (introns), and when the gene is transcribed, RNA (heterologous nuclear RNA; hnRNA) is spliced to remove the introns and some of the exons, forming the final mRNA that is translated. Control of the splicing process allows a measure of cellular control over the peptides that are produced. Good examples of this are calcitonin/CGRP and substance P/neurokinin A.
Genes contain coding regions (exons) interspersed with non-coding regions (introns), and when the gene is transcribed, RNA (heterologous nuclear RNA; hnRNA) is spliced to remove the introns and some of the exons, forming the final mRNA that is translated. Control of the splicing process allows a measure of cellular control over the peptides that are produced. Good examples of this are calcitonin/CGRP and substance P/neurokinin A.
The calcitonin gene codes for calcitonin itself (Ch. 35) and also for a completely dissimilar peptide, CGRP. Alternative splicing allows cells to produce either procalcitonin (expressed in thyroid cells) or pro-CGRP (expressed in many neurons) from the same gene. Substance P and neurokinin A are two closely related tachykinins belonging to the same family, and are encoded on the same gene. Alternative splicing results in the production of two precursor proteins; one of these includes both peptides, the other includes only substance P. The ratio of the two varies widely between tissues, which correspondingly produce either one or both peptides. The control of the splicing process is not well understood.
Post-translational modifications as a source of peptide diversity
 Many peptides, such as tachykinins and peptides related to ACTH (see Ch. 32), must undergo enzymatic amidation at the C-terminus to acquire full biological activity. Tissues may also generate peptides of varying length from the same primary sequence by the action of specific peptidases that cut the chain at different points. For example, procholecystokinin (pro-CCK) contains the sequences of at least five CCK-like peptides ranging in length from 4 to 58 amino acid residues, all with the same C-terminal sequence. CCK itself (33 residues) is the main peptide produced by the intestine, whereas the brain produces mainly CCK-8. The opioid precursor, prodynorphin, similarly gives rise to several peptides with a common terminal sequence, the proportions of which vary in different tissues and in different neurons in the brain. In some cases (e.g. the inflammatory mediator bradykinin; Ch. 17), peptide cleavage occurring after release generates a new active peptide (des-Arg9-bradykinin), which acts on a different receptor, both peptides contributing differently to the inflammatory response.
Many peptides, such as tachykinins and peptides related to ACTH (see Ch. 32), must undergo enzymatic amidation at the C-terminus to acquire full biological activity. Tissues may also generate peptides of varying length from the same primary sequence by the action of specific peptidases that cut the chain at different points. For example, procholecystokinin (pro-CCK) contains the sequences of at least five CCK-like peptides ranging in length from 4 to 58 amino acid residues, all with the same C-terminal sequence. CCK itself (33 residues) is the main peptide produced by the intestine, whereas the brain produces mainly CCK-8. The opioid precursor, prodynorphin, similarly gives rise to several peptides with a common terminal sequence, the proportions of which vary in different tissues and in different neurons in the brain. In some cases (e.g. the inflammatory mediator bradykinin; Ch. 17), peptide cleavage occurring after release generates a new active peptide (des-Arg9-bradykinin), which acts on a different receptor, both peptides contributing differently to the inflammatory response.
In some cases cyclic peptides may be produced. This is often seen in plant and fungal tissues and some of the products are pharmacologically important (e.g. ciclosporin; Ch. 26).
Peptide Trafficking and Secretion
The basic mechanisms by which peptides are synthesised, packaged into vesicles, processed and secreted are summarised in Figure 19.2 (see review by Perone et al., 1997). Two secretory pathways exist, for constitutive and regulated secretion, respectively. Constitutively secreted proteins (e.g. plasma proteins, some clotting factors) are not stored in appreciable amounts, and secretion is coupled to synthesis. Regulated secretion is, as with many hormones and transmitters, controlled mainly by intracellular Ca2+ (see Ch. 4), and peptides awaiting release are stored in cytoplasmic vesicles. Specific protein–protein interactions appear to be responsible for the sorting of different proteins into different vesicles, and for their selective release. Identification of the specific ‘trafficking’ proteins involved in particular secretory pathways may yield novel drug targets for the selective control of secretion, but the prospect is still some way off, and conventional receptor-based pharmacology will be the basis for shorter-term therapeutic developments.
Biosynthesis and release of peptides ![]()
Peptide Antagonists
Although selective antagonists are available for the great majority of non-peptide receptors, only a few peptide antagonists are so far in clinical use, although their therapeutic potential is considerable (see Betancur et al., 1997). Substitution into endogenous peptides of unnatural amino acids, such as D-amino acids, sometimes produces excellent antagonists. This strategy was successful in the case of substance P, angiotensin and bradykinin. However, for reasons discussed below, such peptide antagonists are of little use therapeutically, so effort has been channelled instead into discovering non-peptides that bind to peptide receptors. In a few cases, ‘peptoids’ have been produced by modifying the peptide backbone, while retaining as far as possible the disposition of the side-chain groups that are responsible for binding to the receptor. Such compounds have been developed as antagonists for several peptide receptors (e.g. CCK and neuropeptide Y). In other cases, random screening of large compound libraries has succeeded where rational approaches failed, resulting in highly potent and selective antagonists, some of which are in use, or under development, as therapeutic agents. The most important peptide receptor antagonists in clinical use, all of them non-peptides, include:
Antagonists for many other peptides, including bradykinin, substance P, CGRP, corticotrophin-releasing factor, neuropeptide Y, neurotensin, oxytocin, antidiuretic hormone and somatostatin, have been discovered but, with some notable exceptions (e.g. the oxytocin antagonist atosiban; see Ch. 34, and the substance P antagonist aprepitant; see Ch. 55), have not yet been developed for clinical use. Details can be found in Alexander et al. (2006) and in the review by Betancur et al. (1997).
 Few, if any, agonists at peptide receptors have been discovered by random screening, and morphine-like compounds are probably the most important clinical examples of non-peptide agonists at peptide receptors. It is becoming increasingly clear, however, that some peptide receptors are ‘promiscuous’, in that they can bind both peptide and non-peptide ligands. A recent example is that of the formyl peptide receptor (FPR) family of G-protein-coupled receptors, one member of which (FPRL1/ALX) recognises a whole range of molecular species including the bacterial tripeptide fMLP, several endogenous anti-inflammatory proteins and peptides including annexin A1 as well as the anti-inflammatory lipid lipoxin A4 (Ch. 16). Binding of these ligands probably occurs at different receptor domains but, in an additional twist, this receptor can also recognise proinflammatory substances such as serum amyloid A and correctly transduce the appropriate signal to the cell (see Ye et al., 2009). How this occurs and what makes non-peptides chemically recognisable by peptide receptors is incompletely understood, much to the frustration of medicinal chemists who would dearly like to be able to design such compounds de novo. There remain many peptide mediators for which no antagonists are known, but strenuous efforts are being made to fill this gap in the hope of developing new therapeutic agents.
Few, if any, agonists at peptide receptors have been discovered by random screening, and morphine-like compounds are probably the most important clinical examples of non-peptide agonists at peptide receptors. It is becoming increasingly clear, however, that some peptide receptors are ‘promiscuous’, in that they can bind both peptide and non-peptide ligands. A recent example is that of the formyl peptide receptor (FPR) family of G-protein-coupled receptors, one member of which (FPRL1/ALX) recognises a whole range of molecular species including the bacterial tripeptide fMLP, several endogenous anti-inflammatory proteins and peptides including annexin A1 as well as the anti-inflammatory lipid lipoxin A4 (Ch. 16). Binding of these ligands probably occurs at different receptor domains but, in an additional twist, this receptor can also recognise proinflammatory substances such as serum amyloid A and correctly transduce the appropriate signal to the cell (see Ye et al., 2009). How this occurs and what makes non-peptides chemically recognisable by peptide receptors is incompletely understood, much to the frustration of medicinal chemists who would dearly like to be able to design such compounds de novo. There remain many peptide mediators for which no antagonists are known, but strenuous efforts are being made to fill this gap in the hope of developing new therapeutic agents.
Not surprisingly, it has proved easier to find synthetic compounds that block receptors for small peptides (e.g. most neuropeptides), which have only a few points of attachment, than for large peptides and proteins (e.g. cytokines and growth factors), which can interact with the receptor at many points. These receptors are not easily fooled by small molecules, and efforts to target them therapeutically rely on protein-based approaches (see below).
Proteins and Peptides as Drugs
Many proteins, including hormones, antibodies, decoy receptors, cytokines, enzymes and clotting factors, are registered for use as therapeutic agents in specific conditions; they are mainly given by injection but occasionally by other routes (see Table 19.1). Many of the proteins currently in therapeutic use are functional human proteins prepared by recombinant technology, which are used to supplement the action of endogenous mediators. Although their preparation requires advanced technology, such proteins are relatively straightforward to develop as drugs, because they rarely cause toxicity (although some may be immunogenic) and have a more predictable therapeutic effect than synthetic drugs.
Table 19.1 Some peptide and protein drugs
| Drug | Use | Route |
|---|---|---|
| Peptides | ||
| Captopril/enalapril (peptide related) | Hypertension, heart failure (Ch. 22) | Oral |
| ADH, desmopressin and lypressin | Diabetes insipidus (Ch. 30) | Intranasal, injection |
| Oxytocin | Induction of labour (Ch. 34) | Injection |
| GnRH analogues (e.g. buserelin) | Infertility, suppression of ovulation (Ch. 34), prostate and breast tumours | Intranasal, injection |
| ACTH | Diagnosis of adrenal insufficiency (Ch. 32) | Injection |
| TSH/TRH | Diagnosis of thyroid disease (Ch. 33) | Injection |
| Calcitonin | Paget’s disease of bone (Ch. 35) | Intranasal, injection |
| Insulin | Diabetes (Ch. 30) | Injection |
| Somatostatin, octreotide | Acromegaly, gastrointestinal tract tumours (Ch. 32) | Intranasal, injection |
| Growth hormone | Dwarfism (Ch. 32) | Injection |
| Ciclosporin | Immunosuppression (Ch. 26) | Oral |
| F(ab) fragment | Digoxin overdose | Injection |
| Proteins | ||
| Streptokinase, TPA | Thromboembolism (Ch. 24) | Injection |
| Asparaginase | Tumour chemotherapy (Ch. 55) | Injection |
| DNAase | Cystic fibrosis (Ch. 27) | Inhalation |
| Glucocerebrosidase | Gaucher’s disease | Injection |
| Interferons | Tumour chemotherapy (Chs 17 and 55), multiple sclerosis (Ch. 39) | Injection |
| Erythropoietin, GMCF, etc. | Anaemia (Ch. 25) | Injection |
| Clotting factors | Clotting disorders (Ch. 24) | Injection |
| Monoclonal antibodies (e.g. TNF-α) | Inflammatory diseases (Chs 6, 26) | Injection, infusion |
| Antibodies, vaccines, etc. | Infectious diseases | Injection, oral |
| Enfurvitide | HIV infection (Ch. 51) | Injection |
ACTH, adrenocorticotrophic hormone; ADH, antidiuretic hormone; GMCF, granulocyte colony-stimulating factor; GnRH, gonadotrophin-releasing hormone; TNF-α, tumour necrosis factor-α; TPA, tissue plasminogen activator; TRH, thyrotrophin-releasing hormone; TSH, thyroid-stimulating hormone.
While clearly different from conventional drugs in many ways, the same principles of pharmacodynamics apply to proteins and peptides although their pharmacokinetic properties are usually radically different from those of their small-molecule cousins, largely because of the way that they are metabolised (see Lin, 2009, for a good discussion of this point).
‘Designer proteins’—genetically engineered variants of natural proteins—for specific purposes are already a reality (see Ch. 59). Examples include ‘humanised antibodies’ and fusion proteins consisting of an antibody (targeted, for example, at a tumour antigen) or a peptide (e.g. bombesin or somatostatin, which bind to receptors on tumour cells) linked to a toxin (such as ricin or diphtheria toxin) to kill the target cells (see Ch. 55). Many ingenious ideas are being explored, and some prophets anticipate the dawn of a new era of therapeutics, as the hegemony of small-molecule therapeutics begins to fade. Pharmacologists, needless to say, are somewhat sceptical, but nobody can afford to ignore the potential of biotechnology-based therapeutics in the future. A full discussion of this exciting area is provided in Ch. 59.
Smaller peptides are used therapeutically mainly when there is simply no viable alternative (e.g. insulin and its designer variants, Ch. 30) but, in general, peptides make bad drugs. There are several reasons for this:
A list of some important therapeutic proteins and peptides is given in Table 19.1.
Concluding Remarks
The physiology and pharmacology of peptides—particularly neuropeptides—has stimulated a formidable corpus of research since the early 1980s, and the flow of data continues unabated. With more than a dozen major families of peptides, and a host of minor players, it is beyond the scope of this book to cover them individually or in detail. Instead, we will introduce information on peptide pharmacology wherever it has relevance to the physiology and pharmacology under discussion. Examples are bradykinin (Ch. 17) and monoclonal antibodies (Chs 26 and 59) in inflammation; endothelins and angiotensin in cardiovascular regulation (Ch. 22); tachykinins in asthma (Ch. 27); tachykinins and opioid peptides in nociception (Ch. 41); and leptin, neuropeptide Y and orexins in obesity (Ch. 31). Useful general accounts of peptide pharmacology include Sherman et al. (1989), Cooper et al. (1996), Hökfelt (1991), Hökfelt et al. (2000) and Nestler et al. (2001).
Peptides and proteins as drugs ![]()
References and Further Reading
Alexander S.P., Mathie A., Peters J.A. Guide to receptors and channels, 2nd ed. Br. J. Pharmacol.. 2006;147(Suppl. 3):S1-S168. Comprehensive summary of receptors, including peptide receptors, and compounds that act on them
Banks W.A. The CNS as a target for peptides and peptide-based drugs. Expert Opin. Drug. Deliv.. 2006;3:707-712. Getting useful peptide drugs into the CNS is problematic and this paper reviews some of the major issues
Betancur C., Azzi M., Rostene W. Nonpeptide antagonists of neuropeptide receptors. Trends Pharmacol. Sci.. 1997;18:372-386. Describes success in finding non-peptide antagonists—for a long time elusive—and their possible therapeutic uses
Bruckdorfer T., Marder O., Albericio F. From production of peptides in milligram amounts for research to multi-tons quantities for drugs of the future. Curr. Pharm. Biotechnol.. 2004;5:29-43. Deals with the considerable technical problems in scaling up the synthesis of peptide drugs, and gives the example of enfurvitide, the anti-AIDS drug that was the first peptide to be produced in multi-ton amounts; it is a remarkable story, even if you are not a chemical engineering geek
Buckel P. Recombinant proteins for therapy. Trends Pharmacol. Sci.. 1996;17:450-456. Good account of therapeutic proteins
Civelli O., Nothacker H.-P., Saito Y, et al. Novel neurotransmitters as natural ligands of orphan G-protein-coupled receptors. Trends Neurosci.. 2001;24:230-237. Describes how new peptide mediators have been discovered by screening orphan receptors
Cooper J.R., Bloom F.E., Roth R.H. Biochemical basis of neuropharmacology. New York: Oxford University Press; 1996. Excellent standard textbook
Cullinan W.E., Day N.C., Schafer M.K., et al. Neuroanatomical and functional studies of peptide precursor-processing enzymes. Enzyme. 1991;45:285-300. Review of enzyme mechanisms involved in neuropeptide processing
Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867-879. Excellent overview by a neuropeptide pioneer
Hökfelt T., Broberger C., Xu Z.-Q.D., et al. Neuropeptides—an overview. Neuropharmacology. 2000;39:1337-1356. Excellent summary of developments at the millennium
Lin J.H. Pharmacokinetics of biotech drugs: peptides, proteins and monoclonal antibodies. Curr. Drug. Metab. 2009. Epub ahead of print. (Excellent discussion of the factors that regulate the pharmacokinetics of protein and peptide drugs as opposed to small-molecule inhibitors)
Lundberg J.M. Pharmacology of co-transmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev.. 1996;48:114-192.
Meunier J.-C., Mollereau C., Toll L., et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532-535. Describes the discovery of an opioid-like peptide ligand for a hitherto ‘orphan’ receptor
Mizejewski G.J. Peptides as receptor ligand drugs and their relationship to G-coupled signal transduction. Expert Opin. Investig. Drugs. 2001;10:1063-1073. Useful general review of peptide therapeutics, dealing with many issues including the advantages and disadvantages of peptides as drugs and their potential use in anticancer therapy
Nestler E.J., Hyman S.E., Malenka R.C. Molecular neuropharmacology. New York: McGraw-Hill; 2001. Good modern textbook
Okuda-Ashitaka E., Ito S. Nocistatin: a novel neuropeptide encoded by the gene for the nociceptin/orphanin FQ precursors. Peptides. 2000;21:1101-1109.
Perone M.J., Windeatt S., Castro M.G. Intracellular trafficking of prohormones and proneuropeptides: cell type-specific sorting and targeting. Exp. Physiol.. 1997;82:609-628. Excellent review of mechanisms by which cells manage to avoid getting their many neuropeptides confused
Sherman T.G., Akil H., Watson S.J. The molecular biology of neuropeptides. Disc. Neurosci.. 1989;6:1-58. General review
Siemens J., Zhou S., Piskorowski R., et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208-212. Definitely not for arachnophobes!
Yanagisawa M., Kurihara H., Kimura S., et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411-415. The discovery of endothelin—a remarkable tour de force
Ye R.D., Boulay F., Wang J.M., et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev.. 2009;61:119-161. Heavyweight review that describes the biology of the FPR receptor family which includes examples of ‘promiscuous’ G-protein-coupled receptors that can recognise peptides, proteins and lipids as ligands
1But there are non-physiological exceptions. Some spider venom peptides, for example, produce pain by activating the ion-channel linked capsaicin receptor TRPV1.