Myeloproliferative disorders
In these disorders, there is uncontrolled clonal proliferation of one or more of the cell lines in the bone marrow, namely erythroid, myeloid and megakaryocyte lines. Myeloproliferative disorders include polycythaemia vera (PV), essential thrombocythaemia (ET), myelofibrosis (all of which have a JAK-2 molecular lesion) and chronic myeloid leukaemia (CML) (a genetic BCR-ABL lesion). These disorders are grouped together as there can be transition from one disease to another; e.g. PV can lead to myelofibrosis. They may also transform to acute myeloblastic leukaemia. The non-leukaemic myeloproliferative disorders (PV, ET and myelofibrosis) will be discussed in this section. Chronic myeloid leukaemia is described on page 455.
Polycythaemia
Polycythaemia (or erythrocytosis) is defined as an increase in haemoglobin, PCV and red cell count. PCV is a more reliable indicator of polycythaemia than is Hb, which may be disproportionately low in iron deficiency. Polycythaemia can be divided into absolute erythrocytosis where there is a true increase in red cell volume, or relative erythrocytosis where the red cell volume is normal but there is a decrease in the plasma volume (Fig. 8.6).
Absolute erythrocytosis is due to primary polycythaemia (PV) or secondary polycythaemia. Secondary polycythaemia is due to either an appropriate increase in red cells in response to anoxia, or an inappropriate increase associated with tumours, such as a renal carcinoma. The causes of polycythaemia are given in Table 8.15.
Table 8.15 Causes of polycythaemia
Primary |
|
Polycythaemia vera |
|
Mutations in erythropoietin receptor |
|
High-oxygen-affinity haemoglobins |
|
Secondary |
|
Due to an appropriate (hypoxic) increase in erythropoietin |
Due to an inappropriate increase in erythropoietin |
High altitude |
Renal disease–renal cell carcinoma, Wilms’ tumour |
Chronic lung disease |
Hepatocellular carcinoma |
Cardiovascular disease (right-to-left shunt) |
Adrenal tumours |
Sleep apnoea |
Cerebellar haemangioblastoma |
Morbid obesity |
Massive uterine leiomyoma |
Heavy smoking |
Overadministration of erythropoietin |
Increased affinity of haemoglobin, e.g. familial polycythaemia |
Chuvash polycythaemia mutation in von Hippel–Lindau gene |
Relative |
|
Stress or spurious polycythaemia |
|
Dehydration |
|
Burns |
|
Primary polycythaemia: polycythaemia vera (PV)
PV is a clonal stem cell disorder in which there is an alteration in the pluripotent progenitor cell leading to excessive proliferation of erythroid, myeloid and megakaryocytic progenitor cells. Over 95% of patients with PV have acquired mutations of the gene Janus Kinase 2 (JAK2). There is a V617F mutation which causes the substitution of phenylalanine for valine at position 617. JAK2 is a cytoplasmic tyrosine kinase that transduces signals, especially those triggered by haematopoietic growth factors such as erythropoietin, in normal and neoplastic cells. The significance of the discovery is two-fold: first of immediate significance is the clinical utility of the detection of JAK2 mutations for the diagnosis of PV and second is the prospect of the development of new treatments for the myeloproliferative disorders based on targeting JAK2 activity.
Clinical features
The onset is insidious. It usually presents in patients aged over 60 years with tiredness, depression, vertigo, tinnitus and visual disturbance. It should be noted that these symptoms are also common in the normal population over the age of 60 and consequently, PV is easily missed. These features, together with hypertension, angina, intermittent claudication and a tendency to bleed, are suggestive of PV.
Severe itching after a hot bath or when the patient is warm is common. Gout due to increased cell turnover may be a feature, and peptic ulceration occurs in a minority of patients. Thrombosis and haemorrhage are the major complications of PV.
The patient is usually plethoric and has a deep dusky cyanosis. Injection of the conjunctivae is commonly seen. The spleen is palpable in 70% and is useful in distinguishing PV from secondary polycythaemia. The liver is enlarged in 50% of patients.
Diagnosis
Box 8.2 shows the revised WHO criteria for diagnosis in adults. The measurement of red cell and plasma volume is not necessary. There may be a raised serum uric acid, leucocyte alkaline phosphatase and a raised serum vitamin B12 and vitamin B12 binding protein (transcobalamin 1).
![]() Box 8.2
Box 8.2
Polycythaemia vera (PV) – modified from revised WHO criteria for
Minor criteria
 Bone marrow biopsy, showing hypercellularity for age with trilineage growth (panmyelosis) with prominent erythroid, granulocytic and megakaryocytic proliferation
Bone marrow biopsy, showing hypercellularity for age with trilineage growth (panmyelosis) with prominent erythroid, granulocytic and megakaryocytic proliferation
 Serum erythropoietin level below the reference range for normal
Serum erythropoietin level below the reference range for normal
 Endogenous erythroid colony (EEC) formation in vitroa
Endogenous erythroid colony (EEC) formation in vitroa
Diagnosis requires the presence of both major criteria and one minor criterion or the presence of the first major criterion together with two minor criteria.
a EEC. This is not routinely available but colony formation in the absence of exogenous erythropoietin in vitro is 100% specific and sensitive in patients without previous treatment. (This research was originally published in: Tefferi A, Thiele J, Orazi A et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 2007; 110:1092–1097. ©American Society of Hematology.)
Course and management
Treatment is designed to maintain a normal blood count and to prevent the complications of the disease, particularly thromboses and haemorrhage. Treatment is aimed at keeping the PCV below 0.45 L/L and the platelet count below 400 × 109/L. There are three types of specific treatment:
 Venesection. The removal of 400–500 mL weekly will successfully relieve many of the symptoms of PV. Iron deficiency limits erythropoiesis. Venesection is often used as the sole treatment and other therapy is reserved to control the thrombocytosis. The aim is to maintain a packed cell volume (PVC) of <0.45 L/L.
Venesection. The removal of 400–500 mL weekly will successfully relieve many of the symptoms of PV. Iron deficiency limits erythropoiesis. Venesection is often used as the sole treatment and other therapy is reserved to control the thrombocytosis. The aim is to maintain a packed cell volume (PVC) of <0.45 L/L.
 Chemotherapy. Continuous or intermittent treatment with hydroxycarbamide (hydroxyurea) is used frequently because of the ease of controlling thrombocytosis and general safety in comparison to the alkylating agents such as busulfan, which carry an increased risk of acute leukaemia. Low-dose intermittent busulfan may be more convenient for elderly people, and this must be weighed against the potential risk of long-term complications.
Chemotherapy. Continuous or intermittent treatment with hydroxycarbamide (hydroxyurea) is used frequently because of the ease of controlling thrombocytosis and general safety in comparison to the alkylating agents such as busulfan, which carry an increased risk of acute leukaemia. Low-dose intermittent busulfan may be more convenient for elderly people, and this must be weighed against the potential risk of long-term complications.
 Low-dose aspirin 100 mg daily with the above treatments is used for patients with recurrent thrombotic episodes.
Low-dose aspirin 100 mg daily with the above treatments is used for patients with recurrent thrombotic episodes.
 Anagrelide inhibits megakaryocyte differentiation and is useful for thrombolysis.
Anagrelide inhibits megakaryocyte differentiation and is useful for thrombolysis.
General treatment
Radioactive 32P is only given to patients over 70 years because of the increased risk of transformation to acute leukaemia. Allopurinol is given to block uric acid production. The pruritus is lessened by avoiding very hot baths. H1-receptor antagonists have largely proved unsuccessful in relieving distressing pruritus, but H2-receptor antagonists such as cimetidine are occasionally effective.
Surgery. Polycythaemia should be controlled before surgery. Patients with uncontrolled PV have a high operative risk; 75% of patients have severe haemorrhage following surgery and 30% of these patients die. In an emergency, reduction of the haematocrit by venesection and appropriate fluid replacement must be carried out.
Secondary polycythaemias
Many high-oxygen affinity haemoglobin mutants (HOAHM) have been described which lead to increased oxygen affinity but decreased oxygen delivery to the tissues, resulting in compensatory polycythaemia. A congenital autosomal recessive disorder (Chuvasch polycythaemia) is due to a defect in the oxygen-sensing erythropoietin production pathway caused by a mutation of the von Hippel–Lindau (VHL) gene, resulting in an increased production of erythropoietin.
The causes of secondary polycythaemias are shown in Table 8.15.
Serum erythropoietin (EPO) levels are normal or raised in secondary polycythaemia. Rarely the discovery of a high EPO level may be the clue to the presence of an EPO secreting tumour.
The treatment is that of the precipitating factor; e.g. renal or posterior fossa tumours need to be resected. The commonest cause is heavy smoking, which can produce as much as 10% carboxyhaemoglobin and this can produce polycythaemia because of a reduction in the oxygen-carrying capacity of the blood. Heavy smokers also often have respiratory disease.
Complications of secondary polycythaemia are similar to those seen in PV, including thrombosis, haemorrhage and cardiac failure, but the complications due to myeloproliferative disease such as progression to myelofibrosis or acute leukaemia do not develop. Venesection may be symptomatically helpful in the hypoxic patient, particularly if the PCV is above 0.55 L/L.
‘Relative’ or ‘apparent’ polycythaemia (Gaisböck’s syndrome)
This condition was originally thought to be stress-induced. The red cell volume is normal, but as the result of a decreased plasma volume, there is a relative polycythaemia. ‘Relative’ polycythaemia is more common than PV and occurs in middle-aged men, particularly in smokers who are obese and hypertensive. The condition may present with cardiovascular problems such as myocardial or cerebral ischaemia. For this reason, it may be justifiable to venesect the patient. Smoking should be stopped.
Essential thrombocythaemia
Essential thrombocythaemia (ET) is a myeloproliferative disorder closely related to PV. Patients have normal Hb levels and WBC but elevated platelet counts. At diagnosis the platelet count will usually be >600 × 109/L, and may be as high as 2000 × 109/L or rarely even higher. ET presents either symptomatically with thromboembolic or less commonly bleeding problems or incidentally (e.g. at a routine medical check).
The diagnosis of ET is not straightforward as there is no global gold standard test. The JAK2 mutation tests (see PV) are useful in that the gene is mutated in about half of all cases of ET, confirming a myeloproliferative disorder. For the remaining 50% of patient with a normal JAK2 gene, clinical assessment and observation over a period of time are required. As a generalization a person with a very high platelet count (>1000 × 109/L) who is clinically normal with good health will most likely prove to have ET. In a patient with a lower platelet count, e.g. 600 × 109/L, and in poor health the diagnosis can be more difficult. Other disorders which may give rise to reactive high platelet counts include autoimmune rheumatic disorders and malignancy. Individuals who have been splenectomized (for any reason, including trauma) sometimes have high platelet counts.
Treatment
Treatment is with hydroxycarbamide (hydroxyurea), anagrelide or busulfan to control the platelet count to less than 400 × 109/L.
α-Interferon is also effective; it is administered by subcutaneous injection. ET may eventually transform into PV, myelofibrosis or acute leukaemia, but the disease may not progress for many years.
Myelofibrosis
Myelofibrosis is a very debilitating chronic myeloproliferative neoplasm. It may be primary or develop late in the course of essential thrombocythaemia or polycythaemia vera. There is clonal proliferation of stem cells and myeloid metaplasia in the liver, spleen and other organs. Increased fibrosis in the bone marrow is caused by hyperplasia of abnormal megakaryocytes which release fibroblast-stimulating factors such as platelet-derived growth factor.
Clinical features
The disease presents insidiously with lethargy, weakness and weight loss. Patients often complain of a ‘fullness’ in the upper abdomen due to splenomegaly. Severe pain related to respiration may indicate perisplenitis secondary to splenic infarction, and bone pain and attacks of gout can complicate the illness. Bruising and bleeding occur because of thrombocytopenia or abnormal platelet function. Other physical signs include anaemia, fever and massive splenomegaly (for other causes, see p. 406).
Investigations
 Anaemia with leucoerythroblastic features is present (see p. 413). Poikilocytes and red cells with characteristic tear-drop forms are seen. The WBC count may be over 100 × 109/L, and the differential WBC count may be very similar to that seen in chronic myeloid leukaemia (CML); later leucopenia may develop.
Anaemia with leucoerythroblastic features is present (see p. 413). Poikilocytes and red cells with characteristic tear-drop forms are seen. The WBC count may be over 100 × 109/L, and the differential WBC count may be very similar to that seen in chronic myeloid leukaemia (CML); later leucopenia may develop.
 The platelet count may be very high, but in later stages, thrombocytopenia occurs.
The platelet count may be very high, but in later stages, thrombocytopenia occurs.
 Bone marrow aspiration is often unsuccessful and this gives a clue to the presence of the condition. A bone marrow trephine is necessary to show the markedly increased fibrosis. Increased numbers of megakaryocytes may be seen.
Bone marrow aspiration is often unsuccessful and this gives a clue to the presence of the condition. A bone marrow trephine is necessary to show the markedly increased fibrosis. Increased numbers of megakaryocytes may be seen.
 The Philadelphia chromosome is absent; this helps to distinguish myelofibrosis from most cases of CML.
The Philadelphia chromosome is absent; this helps to distinguish myelofibrosis from most cases of CML.
 JAK2 mutation is present in approximately half of the cases.
JAK2 mutation is present in approximately half of the cases.
Treatment
This consists of general supportive measures such as blood transfusion, folic acid, analgesics and allopurinol. If the spleen becomes very large and painful, and transfusion requirements are high, it may be advisable to perform splenectomy. Splenectomy may also result in relief of severe thrombocytopenia. Treatment for myelofibrosis is often difficult but an estimation of prognosis from a prognostic scoring system is a good basis to start planning a treatment strategy for the individual patient. This may range from observation alone in those with the best prognosis to drug treatment to allogeneic stem cell transplantation. A new and very promising development in the treatment of myelofibrosis is the targeted therapy with JAK inhibitors. Ruxolitinib is being used in trials and has shown benefit.
Myelodysplasia
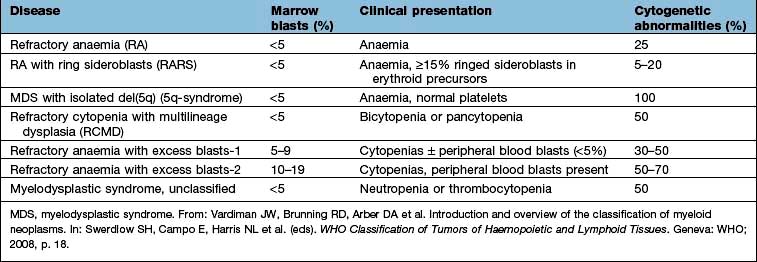
Myelodysplasia (MDS) describes a group of acquired bone marrow disorders that are due to a defect in stem cells. They are characterized by increasing bone marrow failure with quantitative and qualitative abnormalities of all three myeloid cell lines (red cells, granulocyte/monocytes and platelets). The natural history of MDS is variable, but there is a high morbidity and mortality owing to bone marrow failure, and transformation into acute myeloblastic leukaemia occurs in about 30% of cases. WHO classification of the myelodysplastic syndrome is shown in Table 8.16.
Somatic point mutations are commonly seen. A poor survival is seen in those carrying mutations in TP53, E2H2, ETV6, RUNX1 and ASXL1.
Clinical and laboratory features
MDS occurs mainly in the elderly, and presents with symptoms of anaemia, infection or bleeding due to pancytopenia. Serial blood counts show evidence of increasing bone marrow failure with anaemia, neutropenia, monocytosis and thrombocytopenia, either alone or in combination. By contrast, in chronic myelomonocytic leukaemias (CMML), monocytes are >1 × 109/L and the WBC count may be >100 × 109/L.
The bone marrow usually shows increased cellularity despite the pancytopenia. Dyserythropoiesis is present, and granulocyte precursors and megakaryocytes also have abnormal morphology. Ring sideroblasts are present in some types. Table 8.16 shows the classification and the clinical presentation.
Management
Patients with <5% blasts in the bone marrow are usually managed conservatively with red cell and platelet transfusions and antibiotics for infections, as they are needed. Haemopoietic growth factors (e.g. erythropoietin, G-CSF) may be useful in some patients.
Patients with >5% blasts have a less favourable prognosis, and a number of treatment options are available:
 Supportive care only is suitable for elderly patients with other medical problems.
Supportive care only is suitable for elderly patients with other medical problems.
 ‘Gentle’ chemotherapy (low-dose or single-agent, e.g. azacytidine) may be useful in patients with high WBC counts.
‘Gentle’ chemotherapy (low-dose or single-agent, e.g. azacytidine) may be useful in patients with high WBC counts.
 Intensive chemotherapy schedules used for acute myeloblastic leukaemia (see p. 454) may be tried in patients under the age of 60, but the remission rate is less, and prolonged pancytopenia may occur owing to poor haemopoietic regeneration because of the defect in stem cells.
Intensive chemotherapy schedules used for acute myeloblastic leukaemia (see p. 454) may be tried in patients under the age of 60, but the remission rate is less, and prolonged pancytopenia may occur owing to poor haemopoietic regeneration because of the defect in stem cells.
 Lenalidomide (a thalidomide analogue) has been proven to be remarkably successful in the treatment of early stage myelodysplasia with a chromosome 5q deletion (the 5q– syndrome). Avoid use in women of child-bearing age.
Lenalidomide (a thalidomide analogue) has been proven to be remarkably successful in the treatment of early stage myelodysplasia with a chromosome 5q deletion (the 5q– syndrome). Avoid use in women of child-bearing age.
 Bone marrow transplantation offers the hope of cure in the small proportion of MDS patients who are under the age of 50 and who have an HLA-identical sibling or an unrelated HLA-matched donor.
Bone marrow transplantation offers the hope of cure in the small proportion of MDS patients who are under the age of 50 and who have an HLA-identical sibling or an unrelated HLA-matched donor.