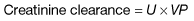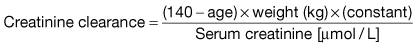Chapter 12 Kidney and urinary tract disease
Functional anatomy
The kidneys are paired organs, 11–14 cm in length in adults, 5–6 cm in width and 3–4 cm in depth. They lie retroperitoneally on either side of the vertebral column at the level of T12 to L3. The renal parenchyma comprises an outer cortex and an inner medulla. The functional unit of the kidney is the nephron, of which each contains approximately one million. Each nephron is made up of a glomerulus, proximal tubule, loop of Henle, distal tubule and collecting duct. The renal capsule and ureters are innervated via T10–12 and L1 nerve roots, and renal pain is felt over the corresponding dermatomes.
Renal arteries and arterioles
Arterial blood is supplied to the kidneys via the renal arteries, which branch off the abdominal aorta, and venous blood is conveyed to the inferior vena cava via the renal veins. Approximately 25% of humans possess dual or multiple renal arteries on one or both sides. The left renal vein is longer than the right and for this reason the left kidney, where possible, is usually chosen for live donor transplant nephrectomy.
The renal artery undergoes a series of divisions within the kidney (Fig. 12.1) forming successively, the interlobar arteries, which run radially to the corticomedullary junction, arcuate arteries, which run circumferentially along the corticomedullary junction, and interlobular arteries, which run radially through the renal cortex towards the surface of the kidney. Afferent glomerular arterioles arise from the interlobular arteries to supply the glomerular capillary bed, which drains into efferent glomerular arterioles. Efferent arterioles from the outer cortical glomeruli drain into a peritubular capillary network within the renal cortex and then into increasingly large and more proximal branches of the renal vein. By contrast, blood from the juxtamedullary glomeruli passes via the vasa recta in the medulla and then turns back towards the area of the cortex from which the vasa recta originated.
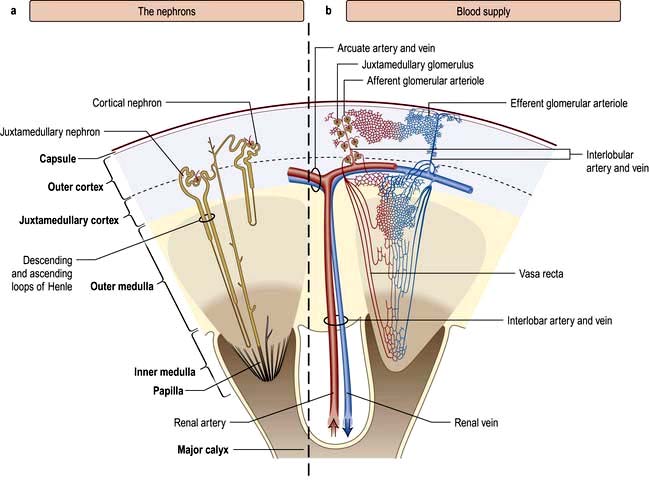
Figure 12.1 Functional anatomy of the kidney. (a) The nephrons. (b) Arterial and venous supply.
(After Standring S (ed) 2008 Gray’s Anatomy, 40th edn. Edinburgh: Churchill Livingstone).
Vasa recta possess fenestrated walls, which facilitates movement of diffusible substances. The collecting ducts merge in the inner medulla to form the ducts of Bellini, which empty at the apices of the papillae into the calyces. The calyces, in common with the renal pelvis, ureter and bladder, are lined with transitional cell epithelium.
The glomerulus
The glomerulus comprises four main cell types:
 Endothelial cells which are fenestrated with 500–1000 Å pores
Endothelial cells which are fenestrated with 500–1000 Å pores
 Visceral epithelial cells (podocytes) which support the delicate glomerular basement membrane by means of an extensive trabecular network (foot processes)
Visceral epithelial cells (podocytes) which support the delicate glomerular basement membrane by means of an extensive trabecular network (foot processes)
 Parietal epithelial cells which cover the Bowman’s capsule
Parietal epithelial cells which cover the Bowman’s capsule
 Mesangial cells (see Fig. 12.11).
Mesangial cells (see Fig. 12.11).
Mesangial cells are believed to be related to macrophages of the reticuloendothelial system and have a phagocytic function and contractile capabilities that can control blood flow and filtration surface area along the glomerular capillaries in response to a host of mediators. They also secrete the mesangial matrix, which provides a skeletal framework for the glomerular capillaries. The glomerular capillary basement membrane lies between the endothelial and the visceral epithelial cells. The latter put out multiple long foot processes which interdigitate with those of adjacent epithelial cells. Together the endothelial cells, basement membrane and epithelial cells form the filtration barrier or sieve (see Fig. 12.12a).
Renal tubules
The renal tubules are lined by epithelial cells, which are cuboidal except in the thin limb of the loop of Henle where they are flat. Proximal tubular cells differ from other cells of the system as they have a luminal brush border. The cortical portion of the collecting ducts contains two cell types with different functions, namely principal cells and intercalated cells (see p. 561). Fibroblast-like cells in the renal cortical interstitium have been shown to produce erythropoietin in response to hypoxia (p. 567).
The juxtaglomerular apparatus
The juxtaglomerular apparatus comprises the macula densa, the extraglomerular mesangium and the terminal portion of the afferent glomerular arteriole (which contains renin-producing granular cells) together with the proximal portion of the efferent arteriole. The macula densa is a plaque of cells containing large, tightly packed cell nuclei (hence the name macula densa; see Fig. 12.2) within the thick ascending limb of the loop of Henle. This anatomical arrangement is such as to allow changes in the renal tubule to influence behaviour of the adjacent glomerulus (tubuloglomerular feedback).
Renal function
Physiology
A conventional diagrammatic representation of the nephron is shown in Figure 12.2a and a physiological version in Figure 12.2b.
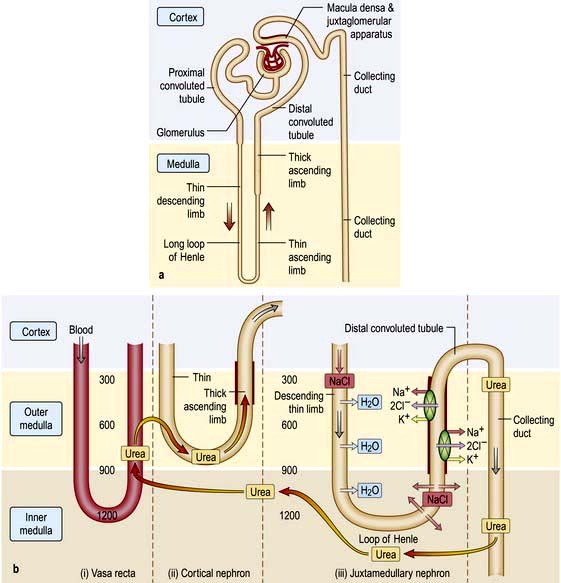
Figure 12.2 (a) Principal parts of the nephron. The point where the distal tubule is in close proximity to its own glomerulus is called the juxtaglomerular apparatus. This contains the macula densa. (b) The countercurrent system. (i) Vasa recta: these vessels descend from the cortex into the medulla and then turn back towards the cortex. (ii) Cortical nephron: these have short descending limbs extending into the outer medulla. (iii) Juxtamedullary nephron: the descending limb dips deeply into the hypertonic inner medulla. Numbers indicate approximate osmolalities.
An essential feature of renal function is that a large volume of blood – 25% of cardiac output or approximately 1300 mL/min – passes through the two million glomeruli.
A hydrostatic pressure gradient of approximately 10 mmHg (a capillary pressure of 45 mmHg minus 10 mmHg of pressure within Bowman’s space and 25 mmHg of plasma oncotic pressure) provides the driving force for ultrafiltration of virtually protein-free and fat-free fluid across the glomerular capillary wall into Bowman’s space and so into the renal tubule (Fig. 12.3).
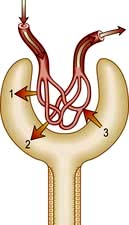
Figure 12.3 Pressures controlling glomerular filtration. (1) Capillary hydrostatic pressure (45 mmHg); (2) hydrostatic pressure in Bowman’s space (10 mmHg); (3) plasma protein oncotic pressure (25 mmHg). Arrows (1, 2, 3) indicate the direction of a pressure gradient.
The ultrafiltration rate (glomerular filtration rate; GFR) varies with age and sex but is approximately 120–130 mL/min per 1.73 m2 surface area in adults. This means that, each day, ultrafiltration of 170–180 L of water and unbound small-molecular-weight constituents of blood occurs. If these large volumes of ultrafiltrate were excreted unchanged as urine, it would be necessary to ingest huge amounts of water and electrolytes to stay in balance. This is avoided by the selective reabsorption of water, essential electrolytes and other blood constituents, such as glucose and amino acids, from the filtrate in transit along the nephron. Thus, 60–80% of filtered water and sodium are reabsorbed in the proximal tubule along with virtually all the potassium, bicarbonate, glucose and amino acids (Fig. 12.2b). Additional water and sodium chloride are reabsorbed more distally, and fine tuning of salt and water balance is achieved in the distal tubules and collecting ducts under the influence of aldosterone and antidiuretic hormone (ADH). The final urine volume is thus 1–2 L daily. Calcium, phosphate and magnesium are also selectively reabsorbed in proportion to the need to maintain a normal electrolyte composition of body fluids.
The urinary excretion of some compounds is more complicated. For example, potassium is freely filtered at the glomerulus, almost completely reabsorbed in the proximal tubule, and secreted in the distal tubule and collecting ducts. A clinical consequence of this is that the ability to eliminate unwanted potassium is less dependent on GFR than is the elimination of urea or creatinine. Other compounds filtered and reabsorbed or secreted to a variable extent include urate, many organic acids and many drugs or their metabolic breakdown products. The more tubular secretion of a compound that occurs, the less dependent elimination is on the GFR; penicillin and cefradine are examples of drugs secreted by the tubules.
Urine concentration and the countercurrent system
Urine is concentrated by a complex interaction between the loops of Henle, the medullary interstitium, medullary blood vessels (vasa recta) and the collecting ducts (see p. 640). The proposed mechanism of urine concentration is termed ‘the countercurrent mechanism’. The countercurrent hypothesis states that: ‘a small difference in osmotic concentration at any point between fluid flowing in opposite directions in two parallel tubes connected in a hairpin manner is multiplied many times along the length of the tubes’. Tubular fluid moves from the renal cortex towards the papillary tip of the medulla via the proximal straight tubule and the thin descending limb of the loop of Henle, which is permeable to water and impermeable to sodium. The tubule then loops back towards the cortex so that the direction of the fluid movement is reversed in the ascending limb, which is impermeable to water but permeable to sodium. This results in a large osmolar concentration difference between the corticomedullary junction and the hairpin loop at the tip of the papilla, and hence countercurrent multiplication. There is an analogy with heat exchangers.
Since the urine that emerges from the proximal tubule is iso-osmotic, the first nephron segment actually involved in urinary concentration is the descending limb of Henle’s loop. There are two types of descending limbs (Fig. 12.2b).
 The short loops originate in superficial and midcortical glomeruli, and turn in the outer medulla. Approximately 85% of nephrons have these.
The short loops originate in superficial and midcortical glomeruli, and turn in the outer medulla. Approximately 85% of nephrons have these.
 The long loops, which originate in the deep cortical and juxtamedullary glomeruli, comprise 15% of nephrons which penetrate the outer medulla up to the tip of the papilla.
The long loops, which originate in the deep cortical and juxtamedullary glomeruli, comprise 15% of nephrons which penetrate the outer medulla up to the tip of the papilla.
Both the ascending limb in the outer and inner medulla and the first part of the distal tubule are impermeable to water and urea. Through the Na+/K+/2Cl− cotransporter, the thick ascending limb actively transports sodium chloride, increasing the interstitial tonicity, resulting in tubular dilution with no net movement of water and urea on account of low permeability. The hypotonic fluid under ADH action undergoes osmotic equilibration with the interstitium in the late distal and the cortical and outer medullary collecting duct, resulting in water removal. Urea concentration in the tubular fluid rises on account of low urea permeability. At the inner medullary collecting duct, which is highly permeable to urea and water, especially in response to ADH, the urea enters the interstitium down its concentration gradient, preserving interstitial hypertonicity and generating high urea concentration in the interstitium.
The hypertonic interstitium pulls water from the descending limb of the loop of Henle, which is relatively impermeable to NaCl and urea. This makes the tubular fluid hypertonic with high NaCl concentration as it arrives at the bend of the loop of Henle. Urea plays a key role in the generation of medullary interstitial hypertonicity. The urea that is reabsorbed into the inner medullary stripe from the terminal inner medullary collecting duct is carried out of this region by ascending vasa recta, which deposit urea into the adjacent descending limb of both short and long loops of Henle, thus recycling the urea to the inner medullary collecting tubule. This process is facilitated by the close anatomical relationship that the hairpin loop of Henle and the vasa recta share.
Glomerular filtration rate (GFR)
In health, the GFR remains remarkably constant owing to intrarenal regulatory mechanisms. In disease (e.g. a reduction in intrarenal blood flow, damage to or loss of glomeruli or obstruction to the free flow of ultrafiltrate along the tubule), the GFR will fall. The ability to eliminate waste material and to regulate the volume and composition of body fluid will decline. This will be manifest as a rise in the plasma urea or creatinine and as a reduction in measured GFR.
The concentration of urea or creatinine in plasma represents the dynamic equilibrium between production and elimination. In healthy subjects there is an enormous reserve of renal excretory function, and serum urea and creatinine do not rise above the normal range until there is a reduction of 50–60% in the GFR. Thereafter, the level of urea depends both on the GFR and its production rate (Table 12.1). The latter is heavily influenced by protein intake and tissue catabolism. The level of creatinine is much less dependent on diet but is more related to age, sex and muscle mass. Once it is elevated, serum creatinine is a better guide to GFR than urea and, in general, measurement of serum creatinine is a good way to monitor further deterioration in the GFR.
Table 12.1 Factors influencing serum urea levels
| Production | Elimination |
|---|---|
Increased by |
Increased by |
High-protein diet |
Elevated GFR, e.g. pregnancy |
Increased catabolism |
|
Surgery |
Decreased by |
Infection |
Glomerular disease |
Trauma |
Reduced renal blood flow |
Corticosteroid therapy |
Hypotension |
Tetracyclines |
Dehydration |
Gastrointestinal bleeding |
Urinary obstruction |
Cancer |
Tubulointerstitial nephritis |
Decreased by |
|
Low-protein diet |
|
Reduced catabolism, e.g. old age |
|
Liver failure |
|
GFR, glomerular filtration rate.
It must be re-emphasized that a normal serum urea or creatinine is not synonymous with a normal GFR.
Measurement of the glomerular filtration rate
Measurement of the GFR is necessary to define the exact level of renal function. It is essential when the serum (plasma) urea or creatinine is within the normal range. The most widely used measurement is the creatinine clearance (Fig. 12.4).
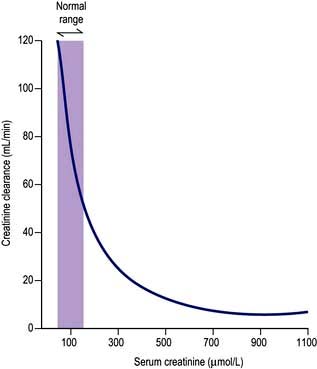
Figure 12.4 Creatinine clearance vs serum creatinine. Note that the serum creatinine does not rise above the normal range until there is a reduction of 50–60% in the glomerular filtration rate (creatinine clearance).
Creatinine clearance is dependent on the fact that daily production of creatinine (principally from muscle cells) is remarkably constant and little affected by protein intake. Serum creatinine and urinary output thus vary very little throughout the day.
Creatinine excretion is, however, by both glomerular filtration and tubular secretion, although at normal serum levels the latter is relatively small.
With progressive renal failure, creatinine clearance may overestimate GFR but, in clinical practice, this is seldom significant.
Given these observations, creatinine clearance, is nevertheless a reasonably accurate measure of GFR – normal or near normal renal function. Urine is collected over 24 h for measurement of urinary creatinine. A single plasma level of creatinine is measured some time during the 24-hour period.
where U = urine concentration of creatinine; V = rate of urine flow in mL/min; P = plasma concentration of creatinine. Normal ranges are 90–140 mL/min in men, 80–125 mL/min in women.
Calculated GFR. Measurement of true GFR is cumbersome, time-consuming and may be inaccurate if 24-hour urine collections are incomplete. Therefore, several formulae have been developed that allow a prediction of creatinine clearance or GFR from serum creatinine and demographics. The Cockcroft–Gault equation for creatinine clearance is shown in Box 12.1.
![]() Box 12.1
Box 12.1
Estimation of glomerular filtration rate (GFR)
Modification of diet in renal disease (MDRD) equation
Calculation of estimated GFR by four variables:
To convert creatinine values in µmol/L to mg/dL multiply by 0.0113.
GFR (mL/min/1.73 m2) = 141 × min (serum creatinine/κ, 1)2 × max (serum creatinine/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum or serum creatinine/κ or 1, and max indicates the maximum of serum creatinine/κ or 1.
A prediction equation has been developed based on the data derived from the Modification of Diet in Renal Disease (MDRD) study in people with chronic kidney disease (CKD) (Box 12.1). This equation is based on age, sex, creatinine and ethnicity. A modification of MDRD equation is used by most chemical pathology laboratories to calculate eGFR but it is less reliable if actual GFR is >60 mL/min and can result in inappropriate referral to renal physicians.
A new equation, the CKD Epidemiology Collaboration (CKD-EPI) equation, uses the same four variables as the MDRD Study equation and is more accurate for estimating GFR, especially at higher GFRs. The improved accuracy is mainly due to a substantial decrease in systematic differences between mGFR and eGFR (bias). The CKD-EPI equation is more accurate than the MDRD study equation overall and across most subgroups. In contrast to the MDRD study equation, eGFR >60 mL/min/1.73 m2 can be reported using the CKD-EPI equation.
All these equations have not, however, been fully validated across all ranges of renal impairment, weights or body mass index (BMI), or ethnic groups; this makes them unreliable in the monitoring of patients with acute or chronic kidney disease while being treated and some clinicians still rely on measured creatinine clearance. In clinical practice, eGFR is reliable enough.
Tubular function
The major function of the tubule is the selective reabsorption or excretion of water and various cations and anions to keep the volume and electrolyte composition of body fluid normal (see Ch. 13).
The active reabsorption from the glomerular filtrate of compounds such as glucose and amino acids also takes place. Within the normal range of blood concentrations these substances are completely reabsorbed by the proximal tubule. However, if blood levels are elevated above the normal range, the amount filtered (filtered load = GFR × plasma concentration) may exceed the maximal absorptive capacity of the tubule and the compound ‘spills over’ into the urine. Examples of this occur with hyperglycaemia in diabetes mellitus or elevated plasma phenylalanine in phenylketonuria.
Conversely, inherited or acquired defects in tubular function may lead to incomplete absorption of a normal filtered load, with loss of the compound in the urine (a lowered ‘renal threshold’). This is seen in renal glycosuria, in which there is a genetically determined defect in tubular reabsorption of glucose. It is diagnosed by demonstrating glycosuria in the presence of normal blood glucose levels. Inherited or acquired defects in the tubular reabsorption of amino acids, phosphate, sodium, potassium and calcium also occur, either singly or in combination. Examples include cystinuria and Fanconi’s syndrome (see p. 1040 and Ch. 13). Tubular defects in the reabsorption of water result in nephrogenic diabetes insipidus (p. 992). Under normal circumstances, antidiuretic hormone induces an increase in the permeability of water in the collecting ducts by attachment to receptors with subsequent activation of adenyl cyclase. This then activates a protein kinase, which induces preformed cytoplasmic vesicles containing water channels (termed ‘aquaporins’) to move to and insert into the tubular luminal membrane. This allows water entry into tubular cells down a favourable osmotic gradient. Water then crosses the basolateral membrane and enters the bloodstream. When the effect of ADH wears off, water channels return to the cell cytoplasm (see Fig. 13.5).
Acid-base balance
Tubular function is also critical to the control of acid-base balance. Thus, filtered bicarbonate is largely reabsorbed and hydrogen ions are excreted mainly buffered by phosphate (see p. 698).
Investigation of tubular function in clinical practice
Various tubular mechanisms could theoretically be investigated, but, in clinical practice, tests of tubular function are required less often than glomerular function.
Twenty-four-hour sodium output may be helpful in determining whether a patient is complying with a low-salt diet and in the management of salt-losing nephropathy. Tests of proximal tubular function may be required in the diagnosis of Fanconi’s syndrome or isolated proximal tubular defects (e.g. urate clearance). Bicarbonate, glucose, phosphate and amino acid are all reabsorbed in the proximal tubule. Their presence in the urine is abnormal, and though formal methods of measuring maximal reabsorption are available, they are seldom necessary.
Retinol-binding protein and β2-microglobulin are normally reabsorbed by the proximal tubule, and their urinary excretion is nonspecifically increased by diseases of the proximal tubule.
Two tests of distal tubular function are commonly applied in clinical practice:
These tests are dealt with on page 993 and page 665.
Protein and polypeptide metabolism
The kidney is a major site for the catabolism of many small-molecular-weight proteins and polypeptides, including many hormones such as insulin, parathyroid hormone (PTH) and calcitonin, by endocytosis carried out by the megalin-cubilin complex in the brush border of proximal tubular cells. In chronic kidney disease the metabolic clearance of these substances is reduced and their half-life is prolonged. This accounts, for example, for the reduced insulin requirements of patients with diabetes as their renal function declines.
Drug and toxicant elimination
A substantial fraction of prescription drugs are handled and eliminated by the kidney. Many of these medications (e.g. penicillins, cephalosporins, diuretics, NSAIDs, antivirals and methotrexate) circulate in the plasma as small organic anions. These organic anions, which are often bound to albumin, are actively eliminated by the proximal tubule of the nephron by an organic anion transporter (OAT) system. The OAT system translocates drugs as well as endogenous substances and toxins.
Endocrine function
Renin-angiotensin system
Juxtaglomerular apparatus
The juxtaglomerular apparatus is made up of specialized arteriolar smooth muscle cells that are sited on the afferent glomerular arteriole as it enters the glomerulus. These cells synthesize prorenin, which is cleaved into the active proteolytic enzyme renin. Active renin is then stored in and released from secretory granules. Prorenin is also released in the circulation and comprises 50–90% of circulating renin, but its physiological role remains unclear as it cannot be converted into active renin in the systemic circulation. In the blood, renin converts angiotensinogen, an α2 globulin of hepatic origin, to angiotensin I. Renin release is controlled by:
 Pressure changes in the afferent arteriole
Pressure changes in the afferent arteriole
 Chloride and osmotic concentration in the distal tubule via the macula densa (Fig. 12.2a)
Chloride and osmotic concentration in the distal tubule via the macula densa (Fig. 12.2a)
The renin-angiotensin-aldosterone system is illustrated in Figure 12.5.
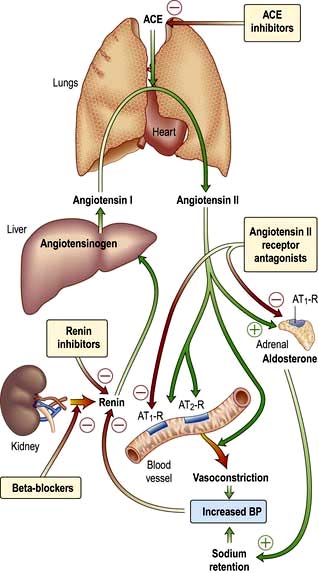
Figure 12.5 The renin-angiotensin-aldosterone system. ACE inhibitors and angiotensin II antagonists can inhibit this system. ACE, angiotensin-converting enzyme.
Angiotensin I is inactive but is further cleaved by angiotensin-converting enzyme (ACE; present in lung and vascular endothelium) into the active peptide, angiotensin II, which has two major actions (mediated by two types of receptor, AT1 and AT2). The AT1 subtype which is found in the heart, blood vessels, kidney, adrenal cortex, lung and brain mediates the vasoconstrictor effect. AT2 is probably involved in vascular growth. Angiotensin II:
 causes rapid, powerful vasoconstriction
causes rapid, powerful vasoconstriction
 stimulates the adrenal zona glomerulosa to increase aldosterone production (over hours or days), leading to sodium and water retention.
stimulates the adrenal zona glomerulosa to increase aldosterone production (over hours or days), leading to sodium and water retention.
Both of these actions will tend to reverse the hypovolaemia or hypotension that is usually responsible for the stimulation of renin release. Angiotensin II promotes renal NaCl and water absorption by direct stimulation of Na+ reabsorption in the early proximal tubule and by increased adrenal aldosterone secretion which enhances Na+ transport in the collecting duct.
In addition to influencing systemic haemodynamics, angiotensin II also regulates GFR. Although it constricts both afferent and efferent arterioles, vasoconstriction of efferent arterioles is three times greater than that of afferent, resulting in increase of glomerular capillary pressure and maintenance of GFR. In addition, angiotensin II constricts mesangial cells, reducing the filtration surface area, and sensitizes the afferent arteriole to the constricting signal of tubuloglomerular feedback (see p. 562). The net result is that angiotensin II has opposing effects on the regulation of GFR: (a) an increase in glomerular pressure and consequent rise in GFR; (b) reduction in renal blood flow and mesangial cell contraction, reducing filtration (see Fig. 12.48). In renal artery stenosis with resultant low perfusion pressure, angiotensin II maintains GFR. However, in cardiac failure and hypertension, GFR may be reduced by angiotensin II.
The renin-angiotensin system can be blocked at several points with renin inhibitors, angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor antagonists (A-IIRA). These are useful agents in treatment of hypertension and heart failure (see p. 782 and p. 719) but have differences in action: ACEIs also block kinin production while A-IIRAs are specific for the AT-II receptors.
Erythropoietin
Erythropoietin (see also p. 374) is the major stimulus for erythropoiesis. It is a glycoprotein produced principally by fibroblast-like cells in the renal interstitium.
 Under hypoxic conditions both the α and β subunits of hypoxia inducible factor 2 (HIF-2) are expressed forming a heterodimer, causing erythropoietin gene transcription via the combined effects of hepatic nuclear factor 4 (HNF-4) and coactivator p300. Erythropoietin, once formed, binds to its receptors on erythroid precursor cells.
Under hypoxic conditions both the α and β subunits of hypoxia inducible factor 2 (HIF-2) are expressed forming a heterodimer, causing erythropoietin gene transcription via the combined effects of hepatic nuclear factor 4 (HNF-4) and coactivator p300. Erythropoietin, once formed, binds to its receptors on erythroid precursor cells.
 Under normal oxygen conditions, only the HIF-2-β subunit is constitutively expressed. The α subunit undergoes proline hydroxylation in the presence of iron and oxygen by prolyl hydroxylase.
Under normal oxygen conditions, only the HIF-2-β subunit is constitutively expressed. The α subunit undergoes proline hydroxylation in the presence of iron and oxygen by prolyl hydroxylase.
 The hydroxylated HIF-2-α subunit binds to von Hippel-Lindau protein and a ubiquitin ligase E3 complex is activated. This leads to ubiquitination (p. 31) and subsequent degradation of HIF-2-α via proteosomes so that no erythropoietin is transcribed. In normoxic conditions HIF-2-α also undergoes asparaginyl hydroxylation which prevents HIF complex from recruiting coactivators. These hydroxylation steps have absolute requirement for molecular oxygen which forms the basis of oxygen sensing.
The hydroxylated HIF-2-α subunit binds to von Hippel-Lindau protein and a ubiquitin ligase E3 complex is activated. This leads to ubiquitination (p. 31) and subsequent degradation of HIF-2-α via proteosomes so that no erythropoietin is transcribed. In normoxic conditions HIF-2-α also undergoes asparaginyl hydroxylation which prevents HIF complex from recruiting coactivators. These hydroxylation steps have absolute requirement for molecular oxygen which forms the basis of oxygen sensing.
Loss of renal substance, with decreased erythropoietin production, results in a normochromic, normocytic anaemia. Conversely, erythropoietin secretion may be increased, with resultant polycythaemia, in people with polycystic renal disease, benign renal cysts or renal cell carcinoma. Recombinant human erythropoietin has been biosynthesized and is available for clinical use, particularly in people with chronic kidney disease (CKD) (see p. 623).
Vitamin D metabolism
Naturally occurring vitamin D (see also p. 622) (cholecalciferol) requires hydroxylation in the liver at position 25 and again by a 1α-hydroxylase enzyme (mitochondrial cytochrome P450) mainly in the distal convoluted tubule, the cortical and inner medullary part of the collecting ducts and the papillary epithelia of the kidney to produce the metabolically active 1,25-dihydroxycholecalciferol (1,25-(OH)2D3). The 1α-hydroxylase activity is increased by high plasma levels of parathyroid hormone (PTH), low phosphate and low 1,25-(OH)2D3. 1,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol are degraded in part by being hydroxylated at position 24 by 24-hydroxylase. The activity of this enzyme is reduced by PTH and increased by 1,25-(OH)2D3 (which therefore promotes its own inactivation).
Reduced 1α-hydroxylase activity in diseased kidneys results in relative deficiency of 1,25-(OH)2D3. As a result, gastrointestinal calcium and to a lesser extent phosphate absorption is reduced and bone mineralization impaired. Receptors for 1,25-(OH)2D3 exist in the parathyroid glands, and reduced occupancy of the receptors by the vitamin alters the set-point for release of PTH in response to a given decrement in plasma calcium concentration. Gut calcium malabsorption, which induces hypocalcaemia, and relative lack of 1,25-(OH)2D3, contribute therefore to the hyperparathyroidism seen regularly in patients with CKD, even of modest degree.
Autocrine function
Endothelins
The endothelins ET-1, ET-2 and ET-3 are a family of similar potent vasoactive peptides that also influence cell proliferation and epithelial solute transport. They do not circulate but act locally. ETs are produced by most types of cells in the kidney. The vascular actions are mediated by two receptors, with ETA (specific for ET-1) mediating vasoconstriction and ETB (responsive to all ETs) causing vasodilatation. Endothelins inhibit sodium and water absorption by suppressing Na+/K+-ATPase and Na+/H+ antiporter activity in the proximal tubule and antagonizing the action of ADH and aldosterone in the collecting duct. Tubular transport actions are mediated by ETB. Endothelins, through vasoconstriction by ETA and salt and water retention via ETB receptors, cause hypertension. Endothelins, mainly through ETA receptors, can also alter cell proliferation and matrix accumulation by increasing tissue inhibitor of metalloproteinase (TIMP), cytokines, fibronectin and collagen. These peptides also stimulate the proliferation of a variety of renal cell types.
Prostaglandins
Prostaglandins are unsaturated, oxygenated fatty acids, derived from the enzymatic metabolism of arachidonic acid, mainly by constitutively expressed cyclo-oxygenase-1 (COX-1) or inducible COX-2 (see Fig. 15.30). COX-1 is highly expressed in the collecting duct, while COX-2 expression is restricted to the macula densa. Both COX isoforms convert arachidonic acid to the same product, the bioactive but unstable prostanoid precursor, prostaglandin H2 (PGH2). PGH2 is converted to:
 PGE2 (formed by PDE2 synthase in the collecting duct, responsible for natriuretic and diuretic effects)
PGE2 (formed by PDE2 synthase in the collecting duct, responsible for natriuretic and diuretic effects)
 PGD2 (undetermined significance, produced in proximal tubule)
PGD2 (undetermined significance, produced in proximal tubule)
 prostacyclin (PGI2) (mainly synthesized in the interstitial and vascular compartment)
prostacyclin (PGI2) (mainly synthesized in the interstitial and vascular compartment)
 thromboxane A2 (vasoconstrictor, mainly synthesized in glomerulus).
thromboxane A2 (vasoconstrictor, mainly synthesized in glomerulus).
They all act through G-coupled transmembrane receptors, maintaining renal blood flow and glomerular filtration rate in the face of reductions induced by vasoconstrictor stimuli such as angiotensin II, catecholamines and α-adrenergic stimulation. In the presence of renal underperfusion, inhibition of prostaglandin synthesis by non-steroidal anti-inflammatory drugs results in a further reduction in GFR, which is sometimes sufficiently severe as to cause acute kidney injury. Renal prostaglandins also have a natriuretic renal tubular effect and antagonize the action of antidiuretic hormone. Renal prostaglandins do not regulate salt and water excretion in normal subjects, but in some circumstances, such as CKD, prostaglandin-induced vasodilatation is involved in maintaining renal blood flow. Patients with CKD are thus vulnerable to further deterioration in renal function on exposure to non-steroidal anti-inflammatory drugs, as are elderly people, in many of whom renal function is compromised by renal vascular disease and/or the effects of ageing upon the kidney. Moreover, in conditions such as volume depletion, which are associated with high renin release (facilitated by prostaglandins), inhibition of prostaglandin synthesis may lead to hyperkalaemia due to hyporeninaemic hypoaldosteronism (since angiotensin II is the main stimulus for aldosterone).
Natriuretic peptides
Natriuretic peptides play a significant role in cardiovascular and fluid homeostasis, but there is no evidence of primary defects in their secretion causing disease.
Atrial natriuretic factors/peptides (ANP)
Atrial natriuretic peptides (ANP), a family of varying length forms, are secreted from atrial granules in response to atrial stretch. They produce marked effects on the kidney, increasing sodium and water excretion and glomerular filtration rate. ANP is also a direct vasodilator, lowering BP; it reduces renin release and aldosterone secretion and consequently inhibits angiotensin II synthesis.
Brain natriuretic peptide (BNP)
Brain natriuretic peptide (BNP) is found in the ventricle as well as the brain and has sequence homology with ANP. Normally its circulating level is 25% less than for ANP but may exceed it in congestive cardiac failure (p. 716).
Nitric oxide and the kidney
Nitric oxide (see Fig. 16.18), a molecular gas, is formed by the action of three isoforms of nitric oxide synthase (NOS; p. 879). The most recognized cellular target of nitric oxide is soluble guanylate cyclase. The stimulation of this enzyme enhances the synthesis of cyclic GMP from GTP. All three isoforms are expressed in the kidney with eNOS in the vascular compartment, nNOS mainly in the macula densa and inner medullary collecting duct, and iNOS in several tubule segments. Nitric oxide mediates the following physiological actions in the kidney:
Investigations
Examination of the urine
Appearance
This is of little value in the differential diagnosis of renal disease except in the diagnosis of haematuria. Overt ‘bloody’ urine is usually unmistakable but should be checked using dipsticks (Stix testing).
Volume
In health, the volume of urine passed is primarily determined by diet and fluid intake. In temperate climates it lies within the range 800–2500 mL per 24 h. The minimum amount passed to stay in fluid balance is determined by the amount of solute – mainly urea and electrolytes – being excreted and the maximum concentrating power of the kidneys would be approximately 650 mL.
In diseases such as CKD or diabetes insipidus, impairment of concentrating ability requires increased volumes of urine to be passed, given the same daily solute output. An increased solute output, such as in glycosuria or increased protein catabolism following surgery, also requires increased urine volumes.
Specific gravity and osmolality
Urine specific gravity is a measure of the weight of dissolved particles in urine, whereas urine osmolality reflects the number of such particles. Usually the relationship between the two is close. Measurement of urine specific gravity or osmolality is required only in the differential diagnosis of oliguric renal failure or the investigation of polyuria or inappropriate ADH secretion. Specific gravity is usually fixed at 1.010 in CKD or acute tubular necrosis as compared to pre-renal acute kidney injury and inappropriate ADH secretion where specific gravity is very high – close to 1.025.
Urinary pH
Measurement of urinary pH is unnecessary except in the investigation and treatment of renal tubular acidosis (see p. 664).
Chemical (Stix) testing
Routine Stix testing of urine for blood, protein and sugar is obligatory in all patients suspected of having renal disease.
Blood
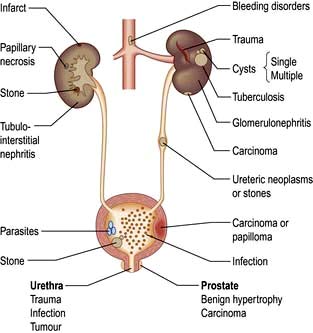
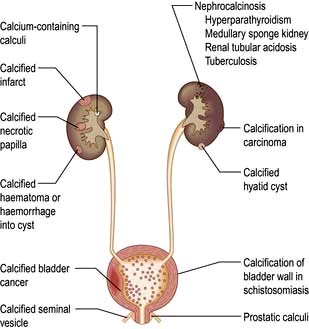
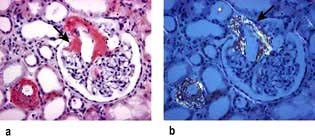
Haematuria may be overt, with bloody urine, or microscopic and found only on chemical testing. A positive Stix test must always be followed by microscopy of fresh urine (with the exception of menstruating women) to confirm the presence of red cells or red-cell casts and so exclude the relatively rare conditions of haemoglobinuria or myoglobinuria. Bleeding may come from any site within the urinary tract (Fig. 12.6):
Protein
Proteinuria is one of the most common signs of renal disease. Detection is primarily by Stix testing. Most reagent strips can detect protein if albuminuria exceeds 300 mg/day. They react primarily with albumin and are relatively insensitive to globulin and Bence Jones proteins.
If proteinuria is confirmed on repeated Stix testing, protein excretion in 24-hour urine collections should be measured. Healthy adults excrete up to 30 mg daily of albumin. Pyrexia, exercise and adoption of the upright posture (postural proteinuria) all increase urinary protein output but are benign.
Microalbuminuria
Normal individuals excrete less than 20 µg of albumin per minute (30 mg in 24 hours). Dipsticks, however, detect albumin only in a concentration above 200 µg (300 mg per 24 hours if urine volume is normal). An albumin excretion between these two levels – called microalbuminuria – is an early indicator of diabetic glomerular disease and systemic endothelial dysfunction and is a useful prognostic marker for future cardiovascular events.
Timed 24-hour urinary excretion rates provide the most precise measure of microalbuminuria. However, in clinical practice it is more convenient, practical and relatively accurate to test for microalbuminuria using either random or early morning urine samples in which albumin concentration is related to urinary creatinine concentration. Generally an albumin:creatinine ratio (ACR) of 2.5:20 corresponds to albuminuria of 30–300 mg daily respectively. Kits are available to test for microalbuminuria. The urinary total protein:creatinine ratio (PCR) is also used for monitoring patients with CKD of various etiologies in their clinical practice. It is relatively cheap and identifies patients whose proteinuria is of tubular rather than glomerular origin. ACR or PCR levels independently predict all-cause and cardiovascular mortality in the general population in addition to better risk stratifications of patients with CKD for future renal outcomes.
Glucose
Renal glycosuria is uncommon, so that a positive test for glucose always requires exclusion of diabetes mellitus.
Bacteriuria
Dipstick tests for bacteriuria are based on the detection of nitrite produced from the reduction of urinary nitrate by bacteria and also for the detection of leucocyte esterase, an enzyme specific for neutrophils. Although each test on its own has limitations, a positive reaction with both tests has a high predictive value for urinary tract infection (p. 593).
Microscopy
Urine microscopy should be carried out in all patients suspected of having renal disease, on a ‘clean’ sample of mid-stream urine. The presence of numerous skin squames suggests a contaminated, poorly collected sample that cannot be properly interpreted.
If a clean sample of urine cannot be obtained, suprapubic aspiration is required in suspected urinary tract infections, particularly in children.
 White blood cells. The presence of ≥10 WBCs/mm3 in fresh unspun mid-stream urine samples is abnormal and indicates an inflammatory reaction within the urinary tract such as urinary tract infection (UTI), stones, tubulointerstitial nephritis, papillary necrosis, tuberculosis and interstitial cystitis.
White blood cells. The presence of ≥10 WBCs/mm3 in fresh unspun mid-stream urine samples is abnormal and indicates an inflammatory reaction within the urinary tract such as urinary tract infection (UTI), stones, tubulointerstitial nephritis, papillary necrosis, tuberculosis and interstitial cystitis.
 Red cells. The presence of one or more red cells per cubic millimetre in unspun urine samples results in a positive Stix test for blood and is abnormal.
Red cells. The presence of one or more red cells per cubic millimetre in unspun urine samples results in a positive Stix test for blood and is abnormal.
 Casts are cylindrical bodies, moulded in the shape of the distal tubular lumen, and may be hyaline, granular or cellular. Coarse granular casts occur with pathological proteinuria in glomerular and tubular disease. Red-cell casts – even if only single – always indicate renal disease. White cell casts may be seen in acute pyelonephritis. They may be confused with the tubular cell casts that occur in patients with acute tubular necrosis.
Casts are cylindrical bodies, moulded in the shape of the distal tubular lumen, and may be hyaline, granular or cellular. Coarse granular casts occur with pathological proteinuria in glomerular and tubular disease. Red-cell casts – even if only single – always indicate renal disease. White cell casts may be seen in acute pyelonephritis. They may be confused with the tubular cell casts that occur in patients with acute tubular necrosis.
 Bacteria, see page 593. Always culture urine prior to starting antibiotic therapy for sensitivities. Stix testing for blood or protein is of no value in the diagnosis of a UTI as both can be absent in the urine of many people with bacteriuria.
Bacteria, see page 593. Always culture urine prior to starting antibiotic therapy for sensitivities. Stix testing for blood or protein is of no value in the diagnosis of a UTI as both can be absent in the urine of many people with bacteriuria.
Blood and quantitative tests
The use of serum urea, creatinine and GFR as measures of renal function is discussed on page 564. Other quantitative tests of disturbed renal function are described under the relevant disorders, as are diagnostic tests, e.g. ANCA, immunofluorescence and complement.
Imaging techniques
Plain X-ray
A plain radiograph of the abdomen is valuable to identify renal calcification or radiodense calculi in the kidney, renal pelvis, line of the ureters or bladder (Fig. 12.7).
Ultrasonography
Ultrasonography of the kidneys and bladder has the advantage over X-ray techniques of avoiding ionizing radiation and intravascular contrast medium. In renal diagnosis it is the imaging method of choice for:
 Renal measurement and for renal biopsy or other interventional procedures
Renal measurement and for renal biopsy or other interventional procedures
 Checking for pelvicalyceal dilatation as an indication of renal obstruction when chronic renal obstruction is suspected. (In suspected acute ureteric obstruction, unenhanced spiral CT is the method of choice.)
Checking for pelvicalyceal dilatation as an indication of renal obstruction when chronic renal obstruction is suspected. (In suspected acute ureteric obstruction, unenhanced spiral CT is the method of choice.)
 Characterizing renal masses as cystic or solid
Characterizing renal masses as cystic or solid
 Diagnosing polycystic kidney disease
Diagnosing polycystic kidney disease
 Detecting intrarenal and/or perinephric fluid (e.g. pus, blood)
Detecting intrarenal and/or perinephric fluid (e.g. pus, blood)
 Demonstrating renal arterial perfusion or detecting renal vein thrombosis using Doppler. Doppler ultrasonography (duplex) has the advantage of being non-invasive and is based on the principle that, when incident sound waves are reflected from a moving structure, their frequency is shifted by an amount proportional to the velocity of the reflector (e.g. an RBC); this shift can be quantified and displayed as a spectral Doppler scan or colour overlay (colour Doppler). However, duplex imaging is limited by central obesity, bowel gas and certain body habitus characteristics. Moreover, it is technically demanding, highly operator dependent, and is not universally available. It is at best a screening initial investigation and always requires confirmation by more reliable imaging techniques (CTA/MRA see below) if renal stenosis is suspected
Demonstrating renal arterial perfusion or detecting renal vein thrombosis using Doppler. Doppler ultrasonography (duplex) has the advantage of being non-invasive and is based on the principle that, when incident sound waves are reflected from a moving structure, their frequency is shifted by an amount proportional to the velocity of the reflector (e.g. an RBC); this shift can be quantified and displayed as a spectral Doppler scan or colour overlay (colour Doppler). However, duplex imaging is limited by central obesity, bowel gas and certain body habitus characteristics. Moreover, it is technically demanding, highly operator dependent, and is not universally available. It is at best a screening initial investigation and always requires confirmation by more reliable imaging techniques (CTA/MRA see below) if renal stenosis is suspected
 Measurement of bladder wall thickness in a distended bladder and to check for bladder tumours and stones. A scan obtained after voiding allows bladder emptying to be assessed.
Measurement of bladder wall thickness in a distended bladder and to check for bladder tumours and stones. A scan obtained after voiding allows bladder emptying to be assessed.
The disadvantages of using ultrasonography to assess the urinary tract are:
 It does not show detailed pelvicalyceal anatomy
It does not show detailed pelvicalyceal anatomy
 It does not fully visualize the normal adult ureter
It does not fully visualize the normal adult ureter
 It may miss small renal calculi and does not detect the majority of ureteric calculi
It may miss small renal calculi and does not detect the majority of ureteric calculi
In people with suspected benign prostatic hypertrophy, examination of the bladder before and after voiding, with measurement of the prostate, and examination of the kidneys to check for pelvicalyceal dilatation suffice. If prostate cancer is suspected, more detailed ultrasound examination of the prostate with a transrectal transducer, usually with transrectal prostate biopsy, is necessary.
Computed tomography (CT)
Computed tomography is used as a first-line investigation in cases of suspected ureteric colic. Multislice detector CT has both improved image resolution and allows reconstruction of the imaging data in a variety of planes. CT is also used to:
 Characterize renal masses which are indeterminate at ultrasonography
Characterize renal masses which are indeterminate at ultrasonography
 Detect ‘lucent’ calculi (low-density calculi which are lucent on plain films, e.g. uric acid stones, are well seen on CT)
Detect ‘lucent’ calculi (low-density calculi which are lucent on plain films, e.g. uric acid stones, are well seen on CT)
 Evaluate the retroperitoneum for tumours, retroperitoneal fibrosis (periaortitis) and other causes of ureteric obstruction
Evaluate the retroperitoneum for tumours, retroperitoneal fibrosis (periaortitis) and other causes of ureteric obstruction
 Visualize the renal arteries and veins by CT angiography
Visualize the renal arteries and veins by CT angiography
 Stage bladder and prostate tumours (MRI is increasingly used instead to stage prostate cancer).
Stage bladder and prostate tumours (MRI is increasingly used instead to stage prostate cancer).
Disadvantages include radiation and contrast nephrotoxicity (p. 614).
The use of unenhanced CT in suspected ureteric colic permits diagnosis of causes of pain other than calculi more readily than does urography.
Magnetic resonance imaging (MRI)
 To characterize renal masses as an alternative to CT
To characterize renal masses as an alternative to CT
 To stage renal, prostate and bladder cancer
To stage renal, prostate and bladder cancer
 To demonstrate the renal arteries by magnetic resonance angiography with gadolinium as contrast medium. In experienced hands its sensitivity and specificity approaches renal angiography.
To demonstrate the renal arteries by magnetic resonance angiography with gadolinium as contrast medium. In experienced hands its sensitivity and specificity approaches renal angiography.
Magnetic resonance urography is preferred over intravenous urography (IVU) in patients with chronic urolithiasis or intrinsic or extrinsic ureteric tumour, and in paediatric uroradiology. Gadolinium is used as contrast medium and is less nephrotoxic than iodine-containing agents used in IVU. However, the Federal Drug Administration (FDA) advises not using gadolinium in patients with renal insufficiency because of development of nephrogenic systemic fibrosis (pp. 1220 and 598).
Excretion urography
Excretion urography (also known as IVU or intravenous pyelography, IVP) has largely been replaced by ultrasonography and CT scanning.
Antegrade pyelography
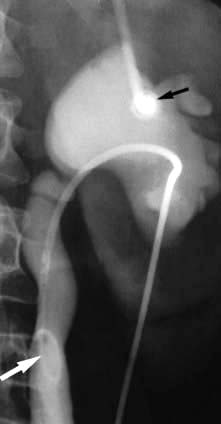
Antegrade pyelography (Fig. 12.8) involves percutaneous puncture of a pelvicalyceal system with a needle and the injection of contrast medium to outline the pelvicalyceal system and ureter to the level of obstruction. It is used when ultrasonography has shown a dilated pelvicalyceal system in a patient with suspected obstruction. Antegrade pyelography is the preliminary to percutaneous placing of a drainage catheter or ureteric stent in the obstructed pelvicalyceal system (percutaneous nephrostomy).
Retrograde pyelography
Following cystoscopy, preferably under screening control, a catheter is either impacted in the ureteral orifice or passed a short distance up the ureter, and contrast medium is injected. Retrograde pyelography is mainly used to investigate lesions of the ureter and to define the lower level of ureteral obstruction shown on CT or ultrasound plus antegrade studies. It is invasive, commonly requires a general anaesthetic, and may result in the introduction of infection.
Micturating cystourethrography (MCU)
This involves catheterization and the instillation of contrast medium into the bladder. The catheter is then removed and the patient screened during voiding to check for vesicoureteric reflux and to study the urethra and bladder emptying. It is used in children with recurrent infection (see p. 592).
MCU is not an appropriate investigation in adults because vesicoureteric reflux tends to disappear by the time adulthood is reached. The presence or absence of vesicoureteric reflux may also be investigated by scintigraphy (see below).
Aortography or renal arteriography
Conventional or digital subtraction angiography (DSA) is used. The latter allows the use of smaller doses of contrast medium which can be injected via a central venous catheter (venous DSA) or via a fine transfemoral arterial catheter (arterial DSA). Angiography is mainly used to define extrarenal or intrarenal arterial disease. Magnetic resonance angiography and multislice CT angiography are used (Fig. 12.9). Complications include cholesterol embolizations (p. 599) and contrast-induced kidney damage (contrast nephropathy).
Renal scintigraphy
Renal scintigraphy using a gamma camera is divided into:
 dynamic studies in which the function of the kidney is examined serially over a period of time, most often using a radiopharmaceutical excreted by glomerular filtration
dynamic studies in which the function of the kidney is examined serially over a period of time, most often using a radiopharmaceutical excreted by glomerular filtration
 static studies involving imaging of tracer that is taken up and retained by the renal tubule.
static studies involving imaging of tracer that is taken up and retained by the renal tubule.
Dynamic scintigraphy
The radiopharmaceutical technetium-labelled diethylenetriaminepenta-acetic acid, [99mTc]DTPA, is excreted by glomerular filtration. Dimercaptosuccinic acid labelled with technetium (99mTc-DMSA) is filtered by the glomerulus and then bound to proximal tubular cells. Mercapto-acetyltriglycine (MAG3) labelled with technetium (99mTc) is excreted by renal tubular secretion. Following venous injection of a bolus of tracer, emissions from the kidney can be recorded by gamma camera. This information allows examination of blood perfusion of the kidney, uptake of tracer as a result of glomerular filtration, transit of tracer through the kidney, and the outflow of tracer-containing urine from the collecting system.
Renal blood flow. Dynamic studies can be used to investigate people in whom renal artery stenosis is suspected as a cause for hypertension and patients with severe oliguria (post-traumatic, post-aortic surgery, or after a kidney transplant) to establish whether, and to what extent, there is renal perfusion. In patients with unilateral renal artery stenosis there is, typically, a slowed and reduced uptake of tracer with delay in reaching a peak. Studies carried out before and after administration of an ACE inhibitor may demonstrate a fall in uptake that is suggestive of functional arterial stenosis. Both false-positive and false-negative results occur, particularly in patients with CKD, and renal arteriography remains the ‘gold standard’ in the diagnosis of renal artery stenosis. In patients with total renal artery occlusion, no kidney uptake of tracers is observed.
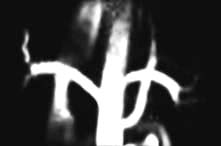
Investigation of obstruction. Renal scintigraphy provides functional evidence of obstruction. After injection usually of [99mTc]MAG3 a rise in resistance to flow in the pelvis or ureter prolongs the parenchymal transit of tracer and there is usually a delay in emptying the pelvis. On whole-kidney renograms, the time-activity curve fails to fall after an initial peak, or continues to rise (Fig. 12.10).
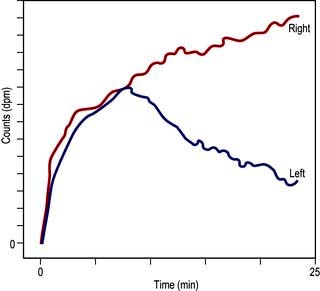
Figure 12.10 Dynamic scintigram. Note the progressive rise of the right kidney curve to a plateau (in contrast to the normal left kidney curve) owing to urinary tract obstruction on the right side.
When the possibility of obstruction is suspected, a dynamic renal scintigram is performed with diuresis. Furosemide (0.5 mg/kg, adult dose 40 mg) is given intravenously about 18–20 min into the study. Time-activity curves show an immediate fall after furosemide in the absence of obstruction but the retention of activity in the pelvis persists in the presence of obstruction. A decision as to whether conservative surgery or nephrectomy should be carried out in unilateral obstruction is facilitated by renographic assessment of the contribution of each kidney.
At the end of dynamic studies, bladder emptying may be investigated and any postmicturition residual urine measured.
Glomerular filtration rate. This is discussed on page 564.
Static scintigraphy
This is usually performed using [99mTc]DMSA (dimercaptosuccinic acid), which is taken up by tubular cells. Uptake is proportional to renal function.
Relative renal function. Function is normally evenly divided between the kidneys, with a range of 45–55%. Static studies are particularly useful in unilateral renal disease, where the relative uptake of the two kidneys can be calculated.
Kidney visualization. Normal kidneys show a uniform uptake with a smooth renal outline. Scars can be identified as photon-deficient ‘bites’. Static scintigraphy is of considerable value in identifying ectopic kidneys or ‘pseudotumours’ of the kidneys (i.e. normally functioning renal tissue abnormally placed within the kidney).
Localization of infection. The use of citrate labelled with gallium-67 or isotopically labelled leucocytes that are taken up by inflammatory tissue may be of value in defining localized infection, such as renal abscesses or infection within a renal cyst.
Transcutaneous renal biopsy (Practical Box 12.1)
Renal biopsy is carried out under ultrasound control in specialized centres and requires interpretation by an experienced pathologist. Renal biopsy is helpful in the investigation of the nephritic and nephrotic syndromes, acute and CKD, haematuria after urological investigations and renal graft dysfunction. Native renal biopsy material must be examined by conventional histochemical staining, by electron microscopy, and by immunoperoxidase or immunofluorescence. Techniques like in situ hybridization and polymerase chain reaction analysis are also widely used in renal biopsy specimens.
The complications of transcutaneous renal biopsy are shown in Table 12.2.
Table 12.2 Complications of transcutaneous renal biopsy
Glomerular diseases
A glomerulus consists of a collection of capillaries which come from the afferent arteriole and are confined within the urinary space (Bowman’s capsule); this is continuous with the proximal tubule. The capillaries are partially attached to mesangium, a continuation of the arteriolar wall consisting of mesangial cells and the matrix. The free wall of glomerular capillaries (across which filtration takes place) consists of basement membrane covered by visceral epithelial cells with individual foot processes and lined by endothelial cells (Fig. 12.11). The normal thickness of the basement membrane is about 250–300 nm. The spaces between foot processes, with diameters of 20–60 nm, are called filtration pores, by which filtered fluid reaches the urinary space. The endothelial cells on the luminal aspect of the basement membrane are fenestrated (diameter 70–100 nm). The basement membrane is arranged in three zones: lamina rara externa, lamina rara densa and lamina rara interna, and is composed of type IV collagen and negatively charged proteoglycans (heparan sulphate).
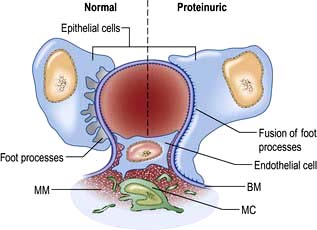
Figure 12.11 Diagram showing a normal (left) and proteinuric (right) glomerulus. A capillary loop showing normal glomerular morphology on the left with an epithelial cell with pseudopodia (foot processes). In the proteinuric diagram (right) there is fusion of the foot processes characteristic of many diseases. BM, basement membrane; MC, mesangial cell; MM, mesangial matrix.
Filtration barrier (slit diaphragm) (Fig. 12.12)
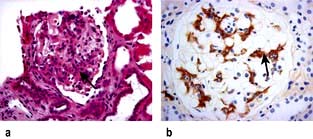
The glomerular filtration barrier consists of the fenestrated endothelium, the glomerular basement membrane and the terminally differentiated visceral epithelial cells known as podocytes. Podocytes dictate the size-selective nature of the filtration barrier. They attach to the glomerular basement membrane by foot processes via adhesion molecules, e.g. α3β1 and dystroglycans. Adjacent podocytes are joined laterally via their foot process by slit diaphragms which bridge across the filtration slits. The various proteins comprising the slit diaphragm include nephrin, CD2AP (CD2-associated protein), canonical TRPC6 (transient receptor potential channel 6), podocin, P-cadherin, α- and β-catenin, ZO-1 (zonula occludens-1). These co-localize within the subcellular domain to function as a molecular sieve. These proteins, in addition to providing structural support to the cytoskeletal proteins like filamentous actin, also have signalling functions in order to maintain the normal function of podocytes. Abnormalities in any of these proteins result in the breakdown of the filtration barrier with consequent torrential leak of macromolecules.
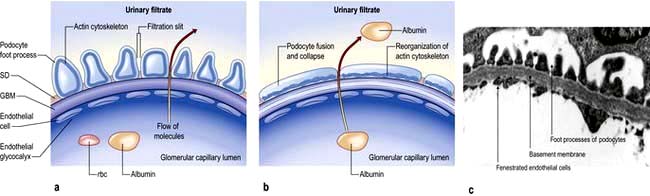
Figure 12.12 The glomerular filtration barrier. (a) Blood enters the glomerular capillaries and is filtered across the endothelium and the glomerular basement membrane (GBM) and through the filtration slits between podocyte foot processes to produce the primary urine filtrate. In healthy glomeruli, this barrier restricts the passage of macromolecules. The proteins which form the slit diaphragm (SD) are essential for the normal functioning of the filtration barrier. (b) Loss of these proteins, either genetically or acquired, leads to foot process effacement and breakdown of the barrier and leakage of albumin. (c) Electron micrograph of normal filtration barrier.
(a, b Adapted from Quaggin S. 2007 Sizing up sialic acid in glomerular disease. Journal of Clinical Investigation 117:1480–1483.)
Podocyte changes
The podocyte structure (see above) is maintained by actin which supports the cytoskeleton (Fig. 12.12). A rearrangement of the fluid actin cytoskeleton leads to foot process effacement (flattening) with a leak of albumin while its reversal (and stabilization) decreases proteinuria.
The cytoskeleton can be altered by abnormalities of the above cytoskeletal proteins, e.g. alpha-actinin-4, which causes hereditary focal segmental glomerular sclerosis, injury to the slit diaphragm proteins, changes in the glomerular basement membrane or by direct injury to the podocytes by, e.g. viral infection, drugs, toxins or the local activation of the renin–angiotensin system.
Glomerulopathies
Glomerulopathies are the third most common cause of endstage kidney disease (ESKD) (after diabetes and hypertension) in Europe and the USA, accounting for some 10–15% of such patients.
Glomerulopathy (GN) is a general term for a group of disorders in which:
 there is primarily an immunologically mediated injury to glomeruli, although renal interstitial damage is a regular accompaniment
there is primarily an immunologically mediated injury to glomeruli, although renal interstitial damage is a regular accompaniment
 the kidneys are involved symmetrically
the kidneys are involved symmetrically
 secondary mechanisms of glomerular injury come into play following an initial immune insult such as fibrin deposition, platelet aggregation, neutrophil infiltration and free radical-induced damage
secondary mechanisms of glomerular injury come into play following an initial immune insult such as fibrin deposition, platelet aggregation, neutrophil infiltration and free radical-induced damage
 renal lesions may be part of a generalized disease (e.g. systemic lupus erythematosus, SLE).
renal lesions may be part of a generalized disease (e.g. systemic lupus erythematosus, SLE).
Pathogenesis
GN is considered to be an immunologically mediated disorder with involvement of cellular immunity (T lymphocytes, macrophages/dendritic cells), humoral immunity (antibodies, immune complexes, complement) and other inflammatory mediators (including cytokines, chemokines and the coagulation cascade). The immune response can be directed against known target antigens, particularly when GN complicates infections, neoplasia or drugs. More frequently the underlying antigenic target is unknown. Primary GN may occur in genetically susceptible individuals following an environmental insult. The genetic susceptibility is usually determined by major histocompatibility complex (HLA) genes (e.g. HLA-A1, B8, DR2, DR3). The environmental factors may be drugs (e.g. hydralazine), chemicals (e.g. gold, silica, hydrocarbons) or infectious agents. The known predisposing factors are discussed in more detail below. The physical evidence of immune reactions is indicated by the presence of circulating autoantibodies and/or abnormalities in serum complement and glomerular deposition of antibodies, immune complexes, complement and fibrin.
Pathological terms in glomerular disease
The most commonly used terms are:
 Focal: some, but not all, glomeruli show the lesion
Focal: some, but not all, glomeruli show the lesion
 Diffuse (global): most of the glomeruli (>75%) contain the lesion
Diffuse (global): most of the glomeruli (>75%) contain the lesion
 Segmental: only a part of the glomerulus is affected (most focal lesions are also segmental, e.g. focal segmental glomerulosclerosis)
Segmental: only a part of the glomerulus is affected (most focal lesions are also segmental, e.g. focal segmental glomerulosclerosis)
 Proliferative: an increase in cell numbers due to hyperplasia of one or more of the resident glomerular cells with or without inflammation
Proliferative: an increase in cell numbers due to hyperplasia of one or more of the resident glomerular cells with or without inflammation
 Membrane alterations: capillary wall thickening due to deposition of immune deposits or alterations in basement membrane
Membrane alterations: capillary wall thickening due to deposition of immune deposits or alterations in basement membrane
 Crescent formation: epithelial cell proliferation with mononuclear cell infiltration in Bowman’s space.
Crescent formation: epithelial cell proliferation with mononuclear cell infiltration in Bowman’s space.
Classification of glomerulopathies
There is no complete correlation between the histopathological types of GN and the clinical features of disease. Glomerular diseases have been classified in numerous ways. Here they are organized and discussed as they relate to four major glomerular syndromes:
 Nephrotic syndrome – massive proteinuria (>3.5 g/day), hypoalbuminaemia, oedema, lipiduria and hyperlipidaemia.
Nephrotic syndrome – massive proteinuria (>3.5 g/day), hypoalbuminaemia, oedema, lipiduria and hyperlipidaemia.
 Acute glomerulonephritis (acute nephritic syndrome) – abrupt onset of glomerular haematuria (RBC casts or dysmorphic RBC), non-nephrotic range proteinuria, oedema, hypertension and transient renal impairment.
Acute glomerulonephritis (acute nephritic syndrome) – abrupt onset of glomerular haematuria (RBC casts or dysmorphic RBC), non-nephrotic range proteinuria, oedema, hypertension and transient renal impairment.
 Rapidly progressive glomerulonephritis – features of acute nephritis, focal necrosis with or without crescents and rapidly progressive renal failure over weeks.
Rapidly progressive glomerulonephritis – features of acute nephritis, focal necrosis with or without crescents and rapidly progressive renal failure over weeks.
Certain types of GN, particularly those that are a part of a systemic disease, can present as more than one syndrome, e.g. lupus nephritis, cryoglobulinaemia, and Henoch–Schönlein purpura. They are usually associated with the nephrotic syndrome and will be discussed below. Investigation of glomerular diseases is shown in Table 12.3.
Table 12.3 Investigation of glomerular diseases
| Investigations | Positive findings |
|---|---|
Urine microscopy |
Red cells, red-cell casts |
Urinary protein |
Nephrotic or sub-nephrotic range proteinuria |
Serum urea |
May be elevated |
Serum creatinine |
May be elevated |
Culture (throat swab, discharge from ear, swab from inflamed skin) |
Nephritogenic organism (not always) |
Antistreptolysin-O titre |
Elevated in post-streptococcal nephritis |
C3 and C4 levels |
May be reduced |
Antinuclear antibody |
Present in significant titre in systemic lupus erythematosus |
ANCA |
Positive in some vasculitis |
Anti-GBM |
Positive in Goodpasture’s syndrome |
Cryoglobulins |
Increased in cryoglobulinaemia |
Creatinine clearance |
Normal or reduced |
Chest X-ray |
Cardiomegaly, pulmonary oedema (not always) |
Renal imaging |
Usually normal |
Renal biopsy |
Any glomerulopathy |
Nephrotic syndrome
Pathophysiology
Hypoalbuminaemia. Urinary protein loss of the order 3.5 g daily or more in an adult is required to cause hypoalbuminaemia. The normal dietary protein intake in the UK is around 70 g daily and the normal liver can synthesize albumin at a rate of 10–12 g daily. How then does a urinary protein loss of the order of 3.5 g daily result in hypoalbuminaemia? This can be partly explained by increased catabolism of reabsorbed albumin in the proximal tubules during the nephrotic syndrome even though actual albumin synthesis rate is increased. However, in addition, dietary intake of protein increases albuminuria, so that the plasma albumin concentration tends to decrease during consumption of a high-protein diet. If the increase in urinary albumin excretion that follows dietary augmentation is prevented by administration of ACE inhibitors (ACEI), a high-protein diet causes an increase in plasma albumin concentration in the nephrotic syndrome. Therefore, to maximize serum albumin concentration in nephrotic patients, a reduction in urinary albumin excretion with an ACEI is always necessary.
Proteinuria. The mechanism of the proteinuria is complex. It occurs partly because structural damage to the glomerular basement membrane leads to an increase in the size and number of pores, allowing passage of more and larger molecules. Electrical charge is also involved in glomerular permeability. Fixed negatively charged components are present in the glomerular capillary wall, which repel negatively charged protein molecules. Reduction of this fixed charge occurs in glomerular disease and appears to be a key factor in the genesis of heavy proteinuria.
Hyperlipidaemia. The characteristic disorder is an increase in the low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), and/or intermediate-density lipoprotein (IDL) fractions, but no change or decrease in HDL. This results in an increase in the LDL/HDL cholesterol ratio. Hyperlipidaemia is the consequence of increased synthesis of lipoproteins (such as apolipoprotein B, C-III lipoprotein (a)), as a direct consequence of a low plasma albumin. There is also a reduced clearance of the principal triglycerides bearing lipoprotein (chylomicrons and VLDL) in direct response to albuminuria.
Oedema in hypoalbuminaemia. See Chapter 13 (p. 643).
Management
General measures
 Initial treatment should be with dietary sodium restriction and a thiazide diuretic (e.g. bendroflumethiazide 5 mg daily). Unresponsive patients require furosemide 40–120 mg daily with the addition of amiloride (5 mg daily), with the serum potassium concentration monitored regularly. Nephrotic patients may malabsorb diuretics (as well as other drugs) owing to gut mucosal oedema, and parenteral administration is then required initially. Patients are sometimes hypovolaemic, and moderate oedema may have to be accepted in order to avoid postural hypotension.
Initial treatment should be with dietary sodium restriction and a thiazide diuretic (e.g. bendroflumethiazide 5 mg daily). Unresponsive patients require furosemide 40–120 mg daily with the addition of amiloride (5 mg daily), with the serum potassium concentration monitored regularly. Nephrotic patients may malabsorb diuretics (as well as other drugs) owing to gut mucosal oedema, and parenteral administration is then required initially. Patients are sometimes hypovolaemic, and moderate oedema may have to be accepted in order to avoid postural hypotension.
 Normal protein intake is advisable. A high-protein diet (80–90 g protein daily) increases proteinuria and can be harmful in the long term.
Normal protein intake is advisable. A high-protein diet (80–90 g protein daily) increases proteinuria and can be harmful in the long term.
 Albumin infusion produces only a transient effect. It is only given to patients who are diuretic-resistant and those with oliguria and uraemia in the absence of severe glomerular damage, e.g. in minimal-change nephropathy. Albumin infusion is combined with diuretic therapy and diuresis often continues with diuretic treatment alone.
Albumin infusion produces only a transient effect. It is only given to patients who are diuretic-resistant and those with oliguria and uraemia in the absence of severe glomerular damage, e.g. in minimal-change nephropathy. Albumin infusion is combined with diuretic therapy and diuresis often continues with diuretic treatment alone.
 Hypercoagulable states predispose to venous thrombosis. The hypercoagulable state is due to loss of clotting factors (e.g. antithrombin) in the urine and an increase in hepatic production of fibrinogen. Prolonged bed rest should therefore be avoided as thromboembolism is very common in the nephrotic syndrome. In the absence of any contraindication, long-term prophylactic anticoagulation is desirable. If renal vein thrombosis occurs, permanent anticoagulation is required.
Hypercoagulable states predispose to venous thrombosis. The hypercoagulable state is due to loss of clotting factors (e.g. antithrombin) in the urine and an increase in hepatic production of fibrinogen. Prolonged bed rest should therefore be avoided as thromboembolism is very common in the nephrotic syndrome. In the absence of any contraindication, long-term prophylactic anticoagulation is desirable. If renal vein thrombosis occurs, permanent anticoagulation is required.
 Sepsis is a major cause of death in nephrotic patients. The increased susceptibility to infection is partly due to loss of immunoglobulin in the urine. Pneumococcal infections are particularly common and pneumococcal vaccine should be given. Early detection and aggressive treatment of infections, rather than long-term antibiotic prophylaxis, is the best approach.
Sepsis is a major cause of death in nephrotic patients. The increased susceptibility to infection is partly due to loss of immunoglobulin in the urine. Pneumococcal infections are particularly common and pneumococcal vaccine should be given. Early detection and aggressive treatment of infections, rather than long-term antibiotic prophylaxis, is the best approach.
 Lipid abnormalities are responsible for an increase in the risk of cardiovascular disease in patients with proteinuria. Treatment of hypercholesterolaemia starts with an HMG-CoA reductase inhibitor.
Lipid abnormalities are responsible for an increase in the risk of cardiovascular disease in patients with proteinuria. Treatment of hypercholesterolaemia starts with an HMG-CoA reductase inhibitor.
 ACE inhibitors and/or angiotensin II receptor antagonists (AIIRA) are used for their antiproteinuric properties in all types of GN. These groups of drugs reduce proteinuria by lowering glomerular capillary filtration pressure; the blood pressure and renal function should be monitored regularly.
ACE inhibitors and/or angiotensin II receptor antagonists (AIIRA) are used for their antiproteinuric properties in all types of GN. These groups of drugs reduce proteinuria by lowering glomerular capillary filtration pressure; the blood pressure and renal function should be monitored regularly.
Specific measures
The aim is to reverse the abnormal urinary protein leak. These measures are discussed in detail below.
Table 12.4 shows the glomerular lesions commonly associated with the nephrotic syndrome. These are divided into diseases with or without RBC casts (active or bland urine sediments). Each of these entities occurs as a primary renal lesion or as a secondary component of a systemic disease.
Table 12.4 Glomerulopathies associated with the nephrotic syndrome
Nephrotic syndrome with ‘bland’ urine sediments
Minimal-change glomerular lesion (minimal-change nephropathy, minimal-change disease, MCD)
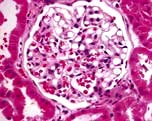
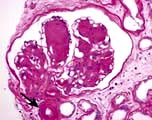
In this condition the glomeruli appear normal on light microscopy (Fig. 12.13). The only abnormality seen on electron microscopy is fusion of the foot processes of epithelial cells (podocytes) (Fig. 12.12b; p. 573). This is a nonspecific finding and is seen in many conditions associated with proteinuria, e.g. focal segmental glomerulosclerosis (FSGS). Neither immune complexes nor anti-GBM antibody can be demonstrated by immunofluorescence.
However, an explanation for the proteinuria is that immature differentiating CD34 stem cells rather than mature T lymphocytes are responsible for the pathogenesis of minimal change nephropathy. Other factors that may have an effect on the podocytes include IL-13, the production of vascular endothelial growth factor (VEGF), or the upregulation of vascular angiopoietin-like-4 (ANGPTL4) secreted by the podocytes.
Many drugs have been implicated in MCD, e.g. NSAIDs, lithium, antibiotics (cephalosporins, rifampicin, ampicillin), bisphosphonates and sulfasalazine. Atopy is present in 30% of cases of MCD and allergic reactions can trigger the nephrotic syndrome. Infections, e.g. HCV, HIV and TB, are rarer causes.
Clinical features
Minimal-change nephropathy is most common in children, particularly males, accounting for the large majority of cases of nephrotic syndrome (proteinuria is usually highly selective) in childhood. Oedema is present and in children this may be facial. The condition accounts for 20–25% of cases of adult nephrotic syndrome. It is often regarded as a condition that does not lead to CKD (but see FSGS, below).
Management
High-dose corticosteroid therapy with prednisolone 60 mg/m2 daily (up to a maximum of 80 mg/day) for a maximum of 4–6 weeks followed by 40 mg/m2 every other day for a further 4–6 weeks corrects the urinary protein leak in more than 95% of children. Response rates in adults are significantly lower and response may occur only after many months (12 weeks with daily steroid therapy and 12 weeks of maintenance with alternate-day therapy). Spontaneous remission also occurs and steroid therapy should, in general, be withheld if urinary protein loss is insufficient to cause hypoalbuminaemia or oedema.
In children, two-thirds subsequently relapse and further courses of corticosteroids are required. One-third of these children regularly relapse on steroid withdrawal, so that cyclophosphamide should be added after repeat induction with steroids. A course of cyclophosphamide 1.5–2.0 mg/kg daily is given for 8–12 weeks with concomitant prednisolone 7.5–15 mg/day. This increases the likelihood of long-term remission. Steroid unresponsive patients may also respond to cyclophosphamide. No more than two courses of cyclophosphamide should be prescribed in children because of the risk of side-effects, which include azoospermia.
In both children and adults, if remission lasts for 4 years after steroid therapy, further relapse is very rare.
An alternative to cyclophosphamide is ciclosporin 3–5 mg/kg per day, which is effective but must be continued long term to prevent relapse on stopping treatment. The antiproteinuric effect of ciclosporin is normally attributed to its immunosuppressive action but it may result from the stabilization of the actin cytoskeleton in kidney podocytes. Ciclosporin inhibits the calcineurin-mediated dephosphorylation of synaptopodin (a regulator of actin cytoskeleton) and protects it from cathepsin L-mediated degradation. These results have shed new light on the role of calcineurin signaling in proteinuric kidney diseases. Excretory function and ciclosporin blood levels (recommended trough levels 80–150 ng/mL) must be monitored regularly, as ciclosporin is potentially nephrotoxic.
In corticosteroid-dependent children, the anthelminthic agent levamisole 2.5 mg/kg to a maximum of 150 mg on alternate days is useful in maintenance of remission but its mode of action is unexplained.
Congenital nephrotic syndrome
Congenital nephrotic syndrome (Finnish type) is an autosomal recessively inherited disorder due to mutations in the gene coding for a transmembrane protein, nephrin, that occurs with a frequency of 1 per 8200 live births in Finland. Its loss of function results in massive proteinuria shortly after birth; these patients usually have an enlarged placenta. This disorder can be diagnosed in utero; increased α-fetoprotein in amniotic fluid is a common feature. The microscopic features of the kidney are varied. Some glomeruli are small and infantile, whereas others are enlarged, more mature and have diffuse mesangial hypercellularity. Because of the massive proteinuria, some tubules develop microcysts and are dilated. On electron microscopy, complete effacement of the foot processes of visceral epithelial cells is observed. This condition is characterized by relentless progression to ESKD.
Other inherited nephrotic syndromes involve mutations in other genes that encode podocyte proteins such as podocin, α-actinin-4 and Wilms’ tumour suppressor gene.
Focal segmental glomerulosclerosis (FSGS)
Clinical features
This disease of unknown aetiology usually presents as massive proteinuria (usually non-selective), haematuria, hypertension and renal impairment. People with nephrotic syndrome are often resistant to steroid therapy. All age groups are affected. It usually recurs in transplanted kidneys, sometimes within days of transplantation, particularly in patients with aggressive renal disease.
Aetiology. A circulating permeability factor causes the increased protein leak; plasma from patients increases membrane permeability in isolated glomeruli. Kidneys transplanted into murine models of FSGS develop the lesion, but kidneys from FSGS-prone mice transplanted to a normal strain are protected. Removal of this factor by plasmapheresis results in transient amelioration of proteinuria. The identity of the permeability factor remains unknown but recent findings suggest that cardiotrophin-like cytokine-1 and/or soluble urokinase receptor are likely candidates in FSGS. In addition upregulation of CD80 in podocytes has a major role in the co-stimulatory immune response pathway. Anti-CD80 antibodies, used in renal transplantation, are likely to be used in FSGS in the future.
Pathology
This glomerulopathy is defined primarily by its appearance on light microscopy. Segmental glomerulosclerosis is seen, which later progresses to global sclerosis. The deep glomeruli at the corticomedullary junction are affected first. These may be missed on transcutaneous biopsy, leading to a mistaken diagnosis of a minimal-change glomerular lesion. A pathogenetic link may exist between minimal-change nephropathy (p. 575) and focal glomerulosclerosis, as a proportion of cases classified as having the former condition develop progressive CKD, which is unusual. Immunofluorescence shows deposits of C3 and IgM in affected portions of the glomerulus. The other glomeruli are usually enlarged but may be of normal size. In some patients, mesangial hypercellularity is a feature. Focal tubular atrophy and interstitial fibrosis are invariably present. Electron microscopic findings mirror light microscopic features with capillary obliteration by hyaline deposits (mesangial matrix and basement membrane material) and lipids. The other glomeruli exhibit primarily foot process effacement, occasionally in a patchy distribution.
Five histological variants of FSGS exist:
 In classic FSGS (Fig. 12.14a) the involved glomeruli show sclerotic segments in any location of the glomerulus.
In classic FSGS (Fig. 12.14a) the involved glomeruli show sclerotic segments in any location of the glomerulus.
 The glomerular tip lesion is characterized by segmental sclerosis, at the tubular pole of all the affected glomeruli at a very early stage (tip FSGS) (Fig. 12.14b). Capillaries contain foam cells, and overlying visceral epithelial cells are enlarged and adherent to the most proximal portion of proximal tubules. These patients have a more favourable response to steroids and run a more benign course.
The glomerular tip lesion is characterized by segmental sclerosis, at the tubular pole of all the affected glomeruli at a very early stage (tip FSGS) (Fig. 12.14b). Capillaries contain foam cells, and overlying visceral epithelial cells are enlarged and adherent to the most proximal portion of proximal tubules. These patients have a more favourable response to steroids and run a more benign course.
 In collapsing FSGS (Fig. 12.14c) the visceral cells are usually enlarged and coarsely vacuolated with wrinkled and collapsed capillary walls. These features indicate a severe lesion, with a corresponding progressive clinical course of the disease. Collapsing FSGS is commonly seen in young blacks with human immunodeficiency virus (HIV) infection or disease and is known as HIV-associated nephropathy (HIVAN) (see p. 93).
In collapsing FSGS (Fig. 12.14c) the visceral cells are usually enlarged and coarsely vacuolated with wrinkled and collapsed capillary walls. These features indicate a severe lesion, with a corresponding progressive clinical course of the disease. Collapsing FSGS is commonly seen in young blacks with human immunodeficiency virus (HIV) infection or disease and is known as HIV-associated nephropathy (HIVAN) (see p. 93).
 The perihilar variant (Fig. 12.14d) consists of perihilar sclerosis and hyalinosis in more than 50% of segmentally sclerotic glomeruli. It is frequently observed with secondary FGS due to processes associated with increased glomerular capillary pressure and declining renal mass.
The perihilar variant (Fig. 12.14d) consists of perihilar sclerosis and hyalinosis in more than 50% of segmentally sclerotic glomeruli. It is frequently observed with secondary FGS due to processes associated with increased glomerular capillary pressure and declining renal mass.
 The cellular variant (Fig. 12.14e) is characterized by at least one glomerulus with segmental endocapillary hypercellularity that occludes the capillary lumen. Other glomeruli may exhibit findings consistent with classic FGS.
The cellular variant (Fig. 12.14e) is characterized by at least one glomerulus with segmental endocapillary hypercellularity that occludes the capillary lumen. Other glomeruli may exhibit findings consistent with classic FGS.
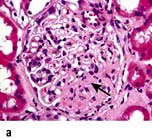
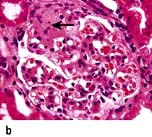

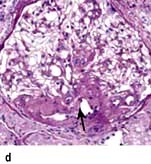
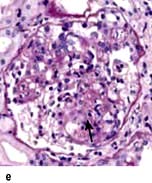
Figure 12.14 Focal segmental glomerulosclerosis (FSGS). (a) Classic FSGS showing sclerotic segments (arrow) in glomerulus. (b) Glomerular tip lesions showing segmental sclerosis (arrow) at pole of glomerulus. (c) Collapsing FSGS (arrow). (d) FSGS perihilar variant. (e) FSGS cellular variant.
The tip and collapsing variants have to be excluded histologically to make a diagnosis of the cellular variant. Patients with this variant can have severe proteinuria.
Similar glomerular changes are seen as a secondary phenomenon when the number of functioning nephrons is reduced for any reasons (e.g. nephrectomy, hypertension, gross obesity, ischaemia, sickle nephropathy, reflux nephropathy, chronic allograft nephropathy, IgA nephropathy and scarring following renal vasculitis), leading to the hypothesis that FSGS results from overloading (glomerular hyperfiltration) of the remaining nephrons.
Secondary forms are also caused by mutations in specific podocyte genes. Some viruses, e.g. HIV type 1, erythrovirus B19, cytomegalovirus, Epstein–Barr virus and the simian virus 40, are associated with FSGS. Drugs such as heroin, all interferons, anabolic steroids, lithium sirolimus, pamidronate and calcineurin inhibitors, e.g. ciclosporin, can also cause FSGS.
The cause of the primary form is unknown but could be due to circulating permeability factors mentioned above.
Treatment
 Prednisolone 0.5–2 mg/kg per day is used in most patients and continued for 6 months before the patient is considered resistant to therapy, which is common.
Prednisolone 0.5–2 mg/kg per day is used in most patients and continued for 6 months before the patient is considered resistant to therapy, which is common.
 Ciclosporin at doses to maintain serum trough levels at 150–300 ng/mL may be effective in reducing or stopping urinary protein excretion. Relapse after reducing or stopping ciclosporin is very common so that long-term use is required.
Ciclosporin at doses to maintain serum trough levels at 150–300 ng/mL may be effective in reducing or stopping urinary protein excretion. Relapse after reducing or stopping ciclosporin is very common so that long-term use is required.
 Cyclophosphamide, chlorambucil or azathioprine are used for second-line therapy in adults. In patients with FSGS with mesangial hypercellularity and tip lesion, cyclophosphamide 1–1.5 mg/kg per day with 60 mg of prednisolone for 3–6 months followed by prednisolone and azathioprine can be used as maintenance therapy.
Cyclophosphamide, chlorambucil or azathioprine are used for second-line therapy in adults. In patients with FSGS with mesangial hypercellularity and tip lesion, cyclophosphamide 1–1.5 mg/kg per day with 60 mg of prednisolone for 3–6 months followed by prednisolone and azathioprine can be used as maintenance therapy.
About 50% of patients progress to ESKD within 10 years of diagnosis, particularly those who are resistant to therapy. The recurrence of this renal lesion following renal transplantation is very high with poor renal prognosis. Plasmapheresis or immunoabsorption has been the mainstay of treatment in patients with post-transplant recurrence but with modest success.
Membranous glomerulopathy
Clinical features
This condition occurs mainly in adults, predominantly in males. People present with asymptomatic proteinuria or frank nephrotic syndrome. Microscopic haematuria, hypertension and/or renal impairment may accompany the nephrotic syndrome. As in all nephritides, hypertension and a greater degree of renal impairment are poor prognostic signs. In membranous GN almost half of the patients undergo spontaneous or therapy-related remission. However, eventually about 40% develop CKD, usually in association with persistent nephrotic range proteinuria. Younger people, females and those with asymptomatic proteinuria of modest degree at the time of presentation do best.
Aetiopathogenesis
An identical glomerular histological picture is seen in the primary or idiopathic form (which comprises 75% of the cases) and also when membranous GN is secondary to drugs (e.g. penicillamine, gold, NSAIDs, probenecid, mercury, captopril), autoimmune disease (e.g. SLE, thyroiditis), infectious disease (e.g. hepatitis B, hepatitis C, schistosomiasis, Plasmodium malariae), neoplasia (e.g. carcinoma of lung, colon, stomach, breast and lymphoma) and other causes (e.g. sarcoidosis, kidney transplantation, sickle cell disease). At all stages, immunofluorescence shows the presence of uniform granular capillary wall deposits of IgG and complement C3. In the early stage the deposits are small and can be missed on light microscopy. Electron microscopy reveals small electron-dense deposits in the subepithelial aspects of the capillary walls. In the intermediate stage the deposits are encircled by basement membrane, which gives an appearance of spikes of basement membrane perpendicular to the basement membrane on silver staining. Late in the disease the deposits are completely surrounded by basement membrane and are undergoing resorption, which appears as uniform thickening of the capillary basement membrane on light microscopy (Fig. 12.15).
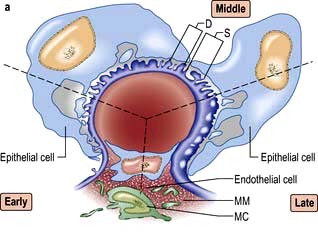
Figure 12.15 Membranous glomerulopathy. (a) Schematic representation of membranous nephropathy showing the early, middle and late stages. D, subepithelial deposits; MC, mesangial cell; MM, mesangial matrix; S, spikes. (After: Marsh FP. Postgraduate Nephrology. Oxford: Butterworth Heinemann; 1985.) (b) Light microscopy of membranous nephropathy showing thickened basement membrane (arrow). (c) Silver stain showing spikes (arrow) in membranous nephropathy.
An animal model (Heymann nephritis) in which the morphological appearances closely resemble the human condition can be induced in susceptible rats by immunization with renal autoantigens such as the brush border component of proximal tubular cells, megalin (gp330). However, the target autoantigen in humans can not be megalin as it is absent from human podocytes.
A majority of patients with idiopathic membranous nephropathy have been found to have IgG4 type autoantibodies against phospholipase receptor A2 (PLA2R), a glycoprotein protein constituent of normal glomeruli. PLA2R is present in normal human podocytes and in immune deposits in patients with idiopathic membranous nephropathy, indicating that PLA2R could be a major autoantigen in this disease; it is linked to HLA-DQA1. Specific IgG4 autoantibodies to anti-aldose reductase (AR) and anti-manganese superoxide dismutase (SOD2) have also been found in the sera and glomeruli of patients with membranous nephropathy but not in other renal pathologies or normal kidney. This suggests that AR and SOD2 could be additional renal autoantigens of human membranous nephropathy under certain clinical circumstances.
FURTHER READING
Fogō AB. Milk and membranous nephropathy. N Engl J Med 2011; 364:2154–2159.
Prunotto M, Carnevali ML, Candiano G et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 2010; 21:507–519.
Stone JH et al. Mechanisms of disease: IgG4-related disease. N Engl J Med 2012; 366:539–551.
Treatment
There is no consensus on therapies for all cases but, in general, patients with moderate to heavy proteinuria are treated:
 Oral high-dose corticosteroids and azathioprine are not associated with any significant benefits.
Oral high-dose corticosteroids and azathioprine are not associated with any significant benefits.
 The alkylating agents, cyclophosphamide and chlorambucil, are both effective in the management of membranous GN but are reserved for patients with severe or prolonged nephrosis (i.e. proteinuria >6 g/day for >6 months), renal insufficiency and hypertension. There is a high likelihood of progression to ESKD.
The alkylating agents, cyclophosphamide and chlorambucil, are both effective in the management of membranous GN but are reserved for patients with severe or prolonged nephrosis (i.e. proteinuria >6 g/day for >6 months), renal insufficiency and hypertension. There is a high likelihood of progression to ESKD.
 Chlorambucil (0.2 mg/kg per day in months 2, 4 and 6 alternating with oral prednisolone 0.4 mg/kg per day in months 1, 3 and 5) or cyclophosphamide (1.5–2.5 mg/kg per day for 6–12 months with 1 mg/kg per day of oral prednisolone on alternate days for the first 2 months) are equally effective.
Chlorambucil (0.2 mg/kg per day in months 2, 4 and 6 alternating with oral prednisolone 0.4 mg/kg per day in months 1, 3 and 5) or cyclophosphamide (1.5–2.5 mg/kg per day for 6–12 months with 1 mg/kg per day of oral prednisolone on alternate days for the first 2 months) are equally effective.
 Ciclosporin and mycophenolate with oral steroids may become the agents of choice.
Ciclosporin and mycophenolate with oral steroids may become the agents of choice.
 Anti-CD20 antibodies (rituximab, which ablates B lymphocytes) have been shown to improve renal function, reduce proteinuria and increase the serum albumin; no significant adverse affects have been shown in the short term.
Anti-CD20 antibodies (rituximab, which ablates B lymphocytes) have been shown to improve renal function, reduce proteinuria and increase the serum albumin; no significant adverse affects have been shown in the short term.
Amyloidosis (see p. 1042)
Amyloidosis is an acquired or inherited disorder of protein folding, in which normally soluble proteins or fragments are deposited extracellularly as abnormal insoluble fibrils causing progressive organ dysfunction and death.
Pathology
On light microscopy, eosinophilic deposits are seen in the mesangium, capillary loops and arteriolar walls. Staining with Congo red renders these deposits pink and they show green birefringence under polarized light (Fig. 12.16). Immunofluorescence is unhelpful, but on electron microscopy the characteristic fibrils of amyloid can be seen. Amyloid consisting of immunoglobulin light chains (AL amyloid) can be identified by immunohistochemistry in only 40% of the cases as compared to almost 100% of patients with protein found in secondary amyloid (AA amyloid). Amyloid A (AA) amyloidosis, also referred to as secondary amyloidosis, is a rare but serious complication of chronic inflammatory diseases and chronic infections.
Diagnosis and treatment
The diagnosis can often be made clinically when features of amyloidosis are present elsewhere. On imaging, the kidneys are often large. Scintigraphy with radiolabelled serum amyloid P (SAP), a technique for quantitatively imaging amyloid deposits in vivo, is used to detect the rate of regression or progression of amyloidosis over a period of time (p. 1043). Renal biopsy is necessary in all suspected cases of renal involvement.
Treatment. Treatments that reduce production of the amyloidogenic protein can improve organ function and survival in immunoglobulin-light-chain-related (AL) amyloidosis and hereditary transthyretin-associated (ATTR) amyloidosis (see p. 1042).
In AA amyloidosis, production of serum amyloid A can sometimes be decreased by treatment of the underlying inflammatory condition but cannot be completely suppressed. A new class of drug, eprodisate (see p. 1043), has shown modest success in patients with renal amyloidosis.
Renoprotective measures should be started (p. 622). The success of dialysis and kidney transplantation is dependent upon the extent of amyloid deposition in extrarenal sites, especially the heart.
Diabetic nephropathy
Diabetic renal disease is the leading cause of ESKD in the western world. People with type 1 and type 2 diabetes (see p. 1025) have equivalent rates of proteinuria, azotaemia, and ultimately ESKD. Both types of diabetes show strong similarities in their rate of renal functional deterioration, and onset of co-morbid complications.
Pathology
The kidneys enlarge initially and there is glomerular hyperfiltration (GFR >150 mL/min). The major early histological lesions seen are glomerular basement membrane thickening and mesangial expansion. Moreover, progressive depletion of podocytes (p. 573) from the filtration barrier due to either apoptosis or detachment and resulting podocyturia appears to be a very early ultrastructural change. Later, glomerulosclerosis develops with nodules (Kimmelstiel–Wilson lesion) and hyaline deposits in the glomerular arterioles (Fig. 12.17). It has recently been shown that the mesangial expansion and the hyalinosis are partly due to amylin (beta islet specific amyloid protein) deposits. These later changes are associated with heavy proteinuria. The lesions seen in type 1 are also seen in type 2.
The Renal Pathology Society has developed a consensus classification combining type 1 and type 2 diabetic nephropathies (Table 12.5). This discriminates lesions by various degrees of severity for use in international clinical practice.
Table 12.5 Renal Pathology Society Classification of Type 1 and 2 diabetic nephropathy
| Class | Name |
|---|---|
I |
Isolated glomerular basement membrane thickening (>395 nm in females, >430 nm in males). No evidence of mesangial expansion, mesangial matrix increase, or global glomerulosclerosis involving >50% of glomeruli |
IIa |
Mild mesangial expansion |
IIb |
Severe mesangial expansion (in a severe lesion >25% of the total mesangium contains areas of expansion larger than the mean area of a capillary lumen) |
III |
Nodular intercapillary glomerulosclerosis (≥1 Kimmelstiel–Wilson lesion(s)) and <50% global glomerulosclerosis |
IV |
Advanced diabetic glomerulosclerosis and >50% global glomerulosclerosis |
The pathophysiology is discussed on page 1025.
Treatment
Lifestyle changes (cessation of smoking and increase in exercise), hypertension, poor metabolic regulation and hyperlidaemia should be addressed in every diabetic. Microalbuminuria is a reason to start treatment with ACE inhibitors or an angiotensin II receptor antagonist (AIIRA) in either type of diabetes, regardless of blood pressure elevation. Like other kidney diseases, however, nearly the entire course of renal injury in diabetes is clinically silent. The timing of medical intervention during this silent phase (see Box 12.6) is renoprotective, as judged by slowed loss of glomerular filtration. Despite intensified metabolic control and antihypertension treatment in patients with diabetes, a substantial number still go on to develop ESKD. In a randomized controlled trial, the addition of paricalcitol (a selective activator of the vitamin D receptor) to treatment with ACE inhibitors reduced albuminuria (a surrogate marker of progressive renal disease) in patients with type 2 diabetes. Paricalcitol worked best in patients with a high sodium intake in their diet, who respond poorly to ACE inhibitor and ARB therapy.
FURTHER READING
Tervaert TW, Mooyaart AL, Amann K et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21:556–563.
de Zeeuw D, Agarwal R, Amdahl M et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010; 376:1543–1551.
Nephrotic syndrome with ‘active’ urine sediments (mixed nephrotic/nephritic)
Mesangiocapillary (membranoproliferative) glomerulonephritis (MCGN)
This uncommon lesion has three subtypes with similar clinical presentations: the nephrotic syndrome, haematuria, hypertension and renal impairment. They also have similar microscopic findings although the pathogenesis may be different. Electron microscopy defines:
 Type 1 MCGN. There is mesangial cell proliferation, with mainly subendothelial immune deposition and apparent splitting of the capillary basement membrane, giving a ‘tram-line’ effect. It can be associated with persistently reduced plasma levels of C3 and normal levels of C4 due to activation of the complement cascade by the classical pathway. It is often idiopathic but occurs with chronic infection (abscesses, infective endocarditis, infected ventriculoperitoneal shunt) or cryoglobulinaemia secondary to hepatitis C infections (Fig. 12.18a).
Type 1 MCGN. There is mesangial cell proliferation, with mainly subendothelial immune deposition and apparent splitting of the capillary basement membrane, giving a ‘tram-line’ effect. It can be associated with persistently reduced plasma levels of C3 and normal levels of C4 due to activation of the complement cascade by the classical pathway. It is often idiopathic but occurs with chronic infection (abscesses, infective endocarditis, infected ventriculoperitoneal shunt) or cryoglobulinaemia secondary to hepatitis C infections (Fig. 12.18a).
 Type 2 MCGN. There is mesangial cell proliferation with electron-dense, linear intramembranous deposits that usually stain for C3 only (Fig. 12.18b). This type may be idiopathic or be associated with partial lipodystrophy (loss of subcutaneous fat on face and upper trunk). MCGN affects young adults. These patients have low C3 levels as in type 1 but this is due to the activation of the alternative pathway of the complement cascade; they also have autoantibodies to C3 convertase enzyme.
Type 2 MCGN. There is mesangial cell proliferation with electron-dense, linear intramembranous deposits that usually stain for C3 only (Fig. 12.18b). This type may be idiopathic or be associated with partial lipodystrophy (loss of subcutaneous fat on face and upper trunk). MCGN affects young adults. These patients have low C3 levels as in type 1 but this is due to the activation of the alternative pathway of the complement cascade; they also have autoantibodies to C3 convertase enzyme.
 Type 3 MCGN has features of both types 1 and 2. Complement activation appears to be via the final common pathway of the cascade.
Type 3 MCGN has features of both types 1 and 2. Complement activation appears to be via the final common pathway of the cascade.
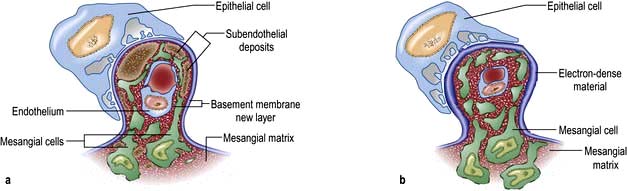
Figure 12.18 Mesangiocapillary glomerulonephritis (MCGN). (a) Type 1 MCGN showing expanded mesangial matrix and mesangial cells, thickened capillary wall, large subendothelial deposits and formation of a new layer of basement membrane (tram-line effect). (b) Type 2 MCGN. This shows a variable glomerular appearance; very electron-dense material has replaced Bowman’s capsule, tubular basement membrane and part of the capillary. There is some proliferation of mesangial cells.
(After: Marsh FP. Postgraduate Nephrology. Oxford: Butterworth Heinemann; 1985.)
Most patients eventually go on to develop ESKD over several years. Type 2 MCGN recurs in virtually 100% of renal transplant patients but recurrence is less common in type 1 (25%). However, recurrence does not interfere with long-term graft function.
Management
In idiopathic MCGN (all age groups) with normal renal function, non-nephrotic range proteinuria, no specific therapy is required. Follow-up every 4 months, with specific attention to blood pressure control, is required.
In children with the nephritic syndrome and/or impaired renal function, a trial of steroids is warranted (alternate-day prednisolone 40 mg/m2 for a period of 6–12 months). If no benefit is seen, this treatment is discontinued. Regular follow-up with control of blood pressure, use of agents to reduce proteinuria and correction of lipid abnormalities is necessary.
In adults with the nephritic syndrome and/or renal impairment, aspirin (325 mg) or dipyridamole (75–100 mg) daily or a combination of the two, should be given for 6–12 months. Again, if no benefits are seen, the treatment should be stopped. Treatment to slow the rate of progression of CKD is instituted (p. 622).
Mesangial proliferative GN (IgM nephropathy, C1q nephropathy)
In addition to minimal-change disease, there are two other disorders that usually present with heavy proteinuria with only minor changes on light microscopy.
IgM nephropathy is characterized by increased mesangial cellularity in most of the glomeruli, associated with granular immune deposits (IgM and complement) in the mesangial regions. People present with episodic or persistent haematuria with the nephrotic syndrome. Unlike minimal-change disease, the prognosis is not uniformly good, as steroid response is only 50%. Between 10% and 30% develop progressive renal insufficiency with evidence of secondary FSGS (p. 576) on repeat biopsy. A trial of cyclophosphamide with prednisolone is used with persistent nephrotic syndrome, particularly with a rising plasma creatinine concentration.
C1q nephropathy is very similar to IgM nephropathy in presenting features and microscopic appearance with the exception of C1q deposits in the mesangium. Sometimes it is misdiagnosed as lupus nephritis, particularly in people with negative serology (so-called ‘seronegative lupus’). The distinguishing features are intense C1q staining and absence of tubuloreticular inclusions (attributable to high circulating α-interferon) on electron microscopy. Only some people are steroid dependent. Progression to CKD is, as in most glomerular diseases, most likely to occur in people with heavy proteinuria and renal insufficiency.
Systemic lupus erythematosus (lupus glomerulonephritis)
Overt renal disease occurs in at least one-third of SLE patients and, of these, 25% reach end-stage CKD within 10 years (see also p. 536). Histologically, almost all patients will have changes. Box 12.2 shows the progression of histological findings and the clinical picture from classes I to VI.
![]() Box 12.2
Box 12.2
Classification of lupus nephritis
Class I – Minimal mesangial lupus nephritis (LN), with immune deposits but normal on light microscopy. Asymptomatic.
Class II – Mesangial proliferative LN with mesangial hypercellularity and matrix expansion. Clinically, mild renal disease.
Class III – Focal LN (involving <50% of glomeruli) with subdivisions for active or chronic lesions. Subepithelial deposits seen. Clinically have haematuria and proteinuria; 10–20% of all LN.
Class IV – Diffuse LN (involving >50% of glomeruli) (Fig. 12.19) classified by the presence of segmental and global lesions as well as active and chronic lesions. Subendothelial deposits are present. Clinically, there is progression to the nephrotic syndrome, hypertension and renal insufficiency. Most common and most severe form of LN.
Class V – Membranous LN affects 10–20% of patients. Can occur in combination with III or IV. Good prognosis.
Class VI – Advanced sclerosing LN (≥90% globally sclerosed glomeruli without residual activity). This represents the advanced stages of the above, as well as healing. Immunosuppressive therapy is unlikely to help as it is ‘inactive’. Progressive CKD.
Modified from International Society of Nephrology/Renal Pathological Society 2004.
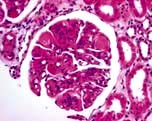
Figure 12.19 Lupus nephritis type IV – a diffuse proliferative nephritis. There is proliferation of endothelial and mesangial cells.
Serial renal biopsies show that in approximately 25% of patients, histological appearances change from one class to another during the interbiopsy interval. Immune deposits in the glomeruli and mesangium are characteristic of SLE (tubuloreticular structure in glomerular endothelial cells) and stain positive for IgG, IgM, IgA and the complement components C3, C1q and C4 on immunofluorescence.
Pathophysiology
SLE is now known to be an autoantigen-driven, T-cell-dependent and B-cell-mediated autoimmune disorder (p. 535). Lupus nephritis typically has circulating autoantibodies to cellular antigens (particularly anti-dsDNA, anti-Ro) and complement activation which leads to reduced serum levels of C3, C4, and particularly C1q. C1q is the first component of the classical pathway of the complement cascade (see p. 51) and is involved in the activation of complement and clearance of self-antigens generated during apoptosis. Anti-C1q antibodies may help in distinguishing a renal from a non-renal relapse. However, not all autoantibodies are pathogenic to the kidney. These nephrogenic antibodies have specific physicochemical characteristics and correlate well with the pattern of renal injury. DNA was thought to be the inciting autoantigen, but nucleosomes (structures comprising DNA and histone, generated during apoptosis) are the most likely autoantigen. Nucleosome-specific T cells, antinucleosome antibodies and nephritogenic immune complexes are generated. Positively charged histone components of the nucleosome bind to the negatively charged heparan sulphate (within the glomerular basement membrane) inciting an inflammatory reaction and resulting in mesangial cell proliferation, mesangial matrix expansion and inflammatory leucocytes. Other pathogenic mechanisms include infarction of glomerular segments, thrombotic microangiopathy, vasculitis and glomerular sclerosis.
Although humoral responses are the main effector mediators of lupus nephritis, IgE autoantibodies, basophils and type 2 helper (Th2) cells are also involved. IgE-containing immune complexes trigger circulating basophils (thought to play a role in SLE) to home in on secondary lymphoid organs and express MHC class II. In secondary lymphoid organs, these activated basophils secrete interleukin-4 and thus promote Th2 cell differentiation. Th2 cells, in cooperation with basophils, enhance B-cell differentiation and survival, and the production of autoreactive antibodies. The immune complexes in which these auto reactive antibodies are present are subsequently deposited in glomeruli and most likely cause lupus nephritis.
The extraglomerular features of lupus nephritis include tubulointerstitial nephritis (75% of patients), renal vein thrombosis and renal artery stenosis. Thrombotic manifestations are associated with autoantibodies to phospholipids (anticardiolipin or lupus anticoagulant) (p. 538).
Management
Initial treatment depends on the clinical presentation but hypertension and oedema should always be treated. A definite histopathological diagnosis is required. Type I requires no treatment. Type II usually runs a benign course but some patients are treated with steroids.
 There have been a number of clinical trials with immunosuppressive agents have been trialled in types III, IV and V which are the most severe form of lupus nephritis. Outcomes of these types are affected by ethnicity, clinical characteristics, irreversible damage (on renal biopsy), initial response to treatment and the future frequency of renal flares.
There have been a number of clinical trials with immunosuppressive agents have been trialled in types III, IV and V which are the most severe form of lupus nephritis. Outcomes of these types are affected by ethnicity, clinical characteristics, irreversible damage (on renal biopsy), initial response to treatment and the future frequency of renal flares.
 Steroids and high-dose intravenous cyclophosphamide or mycophenolate mofetil (MMF) are usually used for induction. In white populations, low-dose cyclophosphamide is a good alternative to high-dose cyclophosphamide as it is similarly effective and associated with less toxicity.
Steroids and high-dose intravenous cyclophosphamide or mycophenolate mofetil (MMF) are usually used for induction. In white populations, low-dose cyclophosphamide is a good alternative to high-dose cyclophosphamide as it is similarly effective and associated with less toxicity.
 Mycophenolate mofetil is as effective as high-dose intravenous cyclophosphamide in the induction phase with a similar safety profile but cyclophosphamide may be inferior to MMF in black and Hispanic people.
Mycophenolate mofetil is as effective as high-dose intravenous cyclophosphamide in the induction phase with a similar safety profile but cyclophosphamide may be inferior to MMF in black and Hispanic people.
 Most patients respond to induction therapy. Remission is maintained with MMF (superior to azathioprine) and azathioprine, which is similar in effectiveness to ciclosporin in reducing the risk of relapse.
Most patients respond to induction therapy. Remission is maintained with MMF (superior to azathioprine) and azathioprine, which is similar in effectiveness to ciclosporin in reducing the risk of relapse.
 B cell depletion with rituximab (anti-CD20) has been used in some patients with favourable results over the short term. However, controlled trials have not shown consistent results. It might be useful in severe, refractory lupus nephritis.
B cell depletion with rituximab (anti-CD20) has been used in some patients with favourable results over the short term. However, controlled trials have not shown consistent results. It might be useful in severe, refractory lupus nephritis.
Prognosis
Treatment leading to the normalization of proteinuria, hypertension and renal dysfunction indicates a good prognosis. The prognosis is better in patients with types I, II and V. Glomerulosclerosis (type VI) usually predicts end-stage renal disease (p. 581).
Cryoglobulinaemic renal disease
Cryoglobulins (CG) are immunoglobulins and complement components, which precipitate reversibly in the cold. Three types are recognized:
Type I: the cryoprecipitable immunoglobulin is a single monoclonal type, as is found in multiple myeloma and lymphoproliferative disorders.
Types II and III cryoglobulinaemias are mixed types. In each, a polyclonal IgG antigen is bound to an antiglobulin. In type II, the antiglobulin component, which is usually of the IgM or IgA class with rheumatoid factor activity, is monoclonal, while in type III it is polyclonal. Type II CGs account for 40–60% cases, while 40–50% of all CG cases are of type III.
Glomerular disease is more common in type II than in type III cryoglobulinaemia. In approximately 30% of these ‘mixed’ cryoglobulinaemias, no underlying or associated disease is found (essential cryoglobulinaemia). Recognized associations include viral infections (hepatitis B and C, HIV, cytomegalovirus, Epstein–Barr infection), fungal and spirochaetal infections, malaria and infective endocarditis and autoimmune rheumatic diseases (SLE, rheumatoid arthritis and Sjögren’s syndrome). Glomerular pathological changes resemble MCGN (Fig. 12.18).
Presentation is usually in the 4th or 5th decades of life, and women are more frequently affected than men. Systemic features include purpura, arthralgia, leg ulcers, Raynaud’s phenomenon, evidence of systemic vasculitis, a polyneuropathy and hepatic involvement. The glomerular disease presents typically as asymptomatic proteinuria, microscopic haematuria or both, but presentation with an acute nephritic and nephrotic syndrome (commonest presentation) or features of CKD also occurs.
A reduction in concentration of early complement components with an elevation of later components, detection of CGs, monoclonal gammopathy, rheumatoid factor, autoantibodies and antiviral antibodies or mRNA of hepatitis C, depending on the associated disorder, is seen.
Spontaneous remission occurs in about one-third of cases and approximately one-third pursue an indolent course. Corticosteroid and/or immunosuppressive therapy with cyclophosphamide may be of benefit, but evaluation of treatment is difficult owing to the rarity of the disease and the occurrence of spontaneous remissions. Intensive plasma exchange or cryofiltration has been used in selected cases. Interferon with ribavirin reduces the viraemia in hepatitis C but does not influence the cryoglobulinaemia. Uncontrolled studies of the anti-CD20 chimeric monoclonal antibody rituximab, which depletes B cells, appear promising, reporting improvement in general manifestations as well as glomerulonephritis.
Henoch–Schönlein syndrome (purpura)
This clinical syndrome comprises a characteristic skin rash, abdominal colic, joint pain and glomerulonephritis. Approximately 30–70% have clinical evidence of renal disease with haematuria and/or proteinuria. The renal disease is usually mild but the nephrotic syndrome and acute kidney injury can occur. The renal lesion is a focal segmental proliferative glomerulonephritis, sometimes with mesangial hypercellularity. In the more severe cases, epithelial crescents may be present. Immunoglobulin deposition is mainly IgA in the glomerular mesangium distribution, similar to IgA nephropathy. There is no treatment of proven benefit; steroid therapy is ineffective. Treatment is usually supportive but with crescentic GN aggressive immunosuppression has been tried with variable outcome.
Idiopathic fibrillary glomerulopathy
In this rare condition, characteristic microfibrillary structures are seen in the mesangium and glomerular capillary wall on electron microscopy that are clearly different from those seen in amyloidosis; the fibrils are larger than those in amyloidosis (20–30 vs 10 nm diameter) and do not stain with Congo red. The median age at presentation is approximately 45 years (range 10–80 years). People present with proteinuria, mostly in the nephrotic range (60%), and microscopic haematuria (70%), hypertension and CKD (50%) that may progress rapidly: 40–50% of patients develop ESKD within 2–6 years.
No treatment is known to be of benefit, although isolated instances of an apparent response to corticosteroid and immunosuppressive therapy have been reported.
Immunotactoid glomerulopathy
In this disorder, microtubules which are much larger (30–40 nm diameter) than the fibrils in fibrillary glomerulopathy are seen on electron microscopy. The majority of patients have circulating paraproteins, or monoclonal immunoglobulin deposition is seen in the glomeruli on immunofluorescence microscopy. A lymphoproliferative disease is the underlying cause in over 50% of cases. The clinical presentation and course are similar to fibrillary glomerulopathy. Complete or partial remission of the nephrotic syndrome can be achieved by various chemotherapeutic agents in over 80% of patients.
Fibronectin glomerulopathy
This is also a form of glomerulonephritis due to fibrillar deposits which, unlike amyloidosis but like fibrillary glomerulonephritis and immunotactoid glomerulopathy, is Congo red staining negative. It is inherited as an autosomal dominant disorder and is associated with the massive deposition of fibronectin, a large dimeric glycoprotein consisting of two similar subunits (approximately 250 kDa in weight). The possible genetic abnormality in this disorder is a loss of function mutation in uteroglobin.
Fibronectin glomerulopathy is extremely rare and was originally described only in Caucasians of European descent. An Asian family with this disease has since been reported. There are as yet no known cases in black or Hispanic people. It presents with varying degrees of proteinuria seen first between the ages of 20 and 40. This is followed by hypertension, microscopic haematuria, and slow progression to endstage renal disease in most patients. Diagnosis is confirmed by renal biopsy which demonstrates enlarged glomeruli with minimal proliferation and massive Congo red negative fibrillary deposits in the capillary walls.
Treatment includes nonspecific measures such as adequate blood pressure control by blockade of the renin-angiotensin system. ESKD patients are treated with dialysis, and recurrence of disease following renal transplant has been noted.
Acute glomerulonephritis (acute nephritic syndrome) (Table 12.6)
 Haematuria (macroscopic or microscopic) – red-cell casts are typically seen on urine microscopy
Haematuria (macroscopic or microscopic) – red-cell casts are typically seen on urine microscopy
Table 12.6 Diseases commonly associated with the acute nephritic syndrome
|
Post-streptococcal glomerulonephritis Non-streptococcal post-infectious glomerulonephritis, e.g. Staphylococcus, pneumococcus, Legionella, syphilis, mumps, varicella, hepatitis B and C, echovirus, Epstein–Barr virus, toxoplasmosis, malaria, schistosomiasis, trichinosis Systemic lupus erythematosus (see p. 509) Henoch–Schönlein syndrome (see p. 582) Cryoglobulinaemia (see p. 581) |
The histological pattern is characterized by cellular proliferation (mesangial and endothelial) and inflammatory cell infiltration (neutrophils, macrophages).
Post-streptococcal glomerulonephritis (PSGN)
The patient, usually a child, suffers a streptococcal infection 1–3 weeks before the onset of the acute nephritic syndrome. Streptococcal throat infection, otitis media or cellulitis can all be responsible. The infecting organism is a Lancefield group A β-haemolytic streptococcus of a nephritogenic type. The latent interval between the infection and development of symptoms and signs of renal involvement reflects the time taken for immune complex formation and deposition and glomerular injury to occur. PSGN is now rare in developed countries. Renal biopsy shows diffuse, florid, acute inflammation in the glomerulus (without necrosis but occasionally cellular crescents), with neutrophils and deposition of immunoglobulin (IgG) and complement (Fig. 12.20). Ultrastructural findings are those of electron-dense deposits, characteristically but not solely in the subepithelial aspects of the capillary walls. Endothelial cells often are swollen. Similar biopsy findings may be seen in non-streptococcal post-infectious glomerulonephritis (Table 12.6).
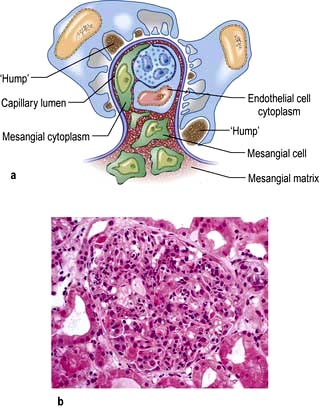
Figure 12.20 Post-streptococcal glomerulonephritis. (a) Diagram showing large aggregates of immune material (humps) in the extracapillary area. There is an increase in mesangial matrix and mesangial cells with occlusion of the capillary lumen by endothelial cell cytoplasm, leucocytes and mesangial cell cytoplasm. (b) Light microscopy showing acute inflammation of the glomerulus with neutrophils.
(After: Marsh FP. Postgraduate Nephrology. Oxford: Butterworth Heinemann; 1985.)
Management
The acute phase should be treated with antihypertensives, diuretics, salt restriction and dialysis as necessary. If recovery is slow, corticosteroids may be helpful. The prognosis is usually good in children. A small number of adults develop hypertension and/or CKD later in life. Therefore in older patients, an annual blood pressure check and measurement of serum creatinine are required. Evidence in support of long-term penicillin prophylaxis after the development of glomerulonephritis is lacking. In non-streptococcal post-infectious glomerulonephritis, prognosis is equally good if the underlying infection is eradicated.
Glomerulonephritis with infective endocarditis
GN occurs rarely in patients with infective endocarditis (usually i.v. drug users). It usually manifests itself as the acute nephritic syndrome. A similar presentation is in patients with infected ventriculoperitoneal shunt (shunt nephritis). Microscopic appearances resemble post-infectious GN, but lesions are usually focal and segmental. Crescentic GN with acute kidney injury has been described, particularly with Staphylococcus aureus infection. Appropriate antibiotic therapy or surgical eradication of infection in fulminant cases (embolic infarct of the kidney) usually results in a return of normal renal function.
Glomerulonephritis associated with visceral abscesses (mainly pulmonary)
Clinical and microscopic features are indistinguishable from post-infectious GN, but usually complement levels are normal and immune deposits are absent on biopsy. Antibiotic therapy and surgical drainage of the abscess result in complete recovery of renal function in approximately 50% of patients.
Asymptomatic urinary abnormalities
A variety of renal lesions may present as either isolated proteinuria or haematuria, alone or with proteinuria.
Isolated proteinuria without haematuria in asymptomatic patients is usually an incidental finding. It is usually in the sub-nephrotic range without an active urine sediment and there is normal renal function. Over 50% of these patients have postural proteinuria. The outcome of isolated proteinuria (postural or non-postural) is excellent in the majority of patients, with a gradual decline in proteinuria. Occasionally, it may be an early sign of a serious glomerular lesion such as membranous GN, IgA nephropathy, FSGS, diabetic nephropathy or amyloidosis. Moreover, mild proteinuria may accompany a febrile illness, congestive heart failure or infectious diseases with no clinical renal significance.
Haematuria with or without sub-nephrotic range proteinuria in an asymptomatic patient may lead to early discovery of potentially serious glomerular disease such as SLE, Henoch–Schönlein purpura, post-infectious GN or idiopathic hypercalciuria in children. Asymptomatic haematuria is also the primary presenting manifestation of a number of specific glomerular diseases discussed below.
IgA nephropathy (Fig. 12.21)
This disease has replaced post-streptococcal glomerulonephritis as the commonest form of glomerulonephritis worldwide. Demographic and family studies support the existence of a genetic contribution to the pathogenesis of IgA nephropathy, but results from genetic association studies of candidate genes are inconsistent. In a genome-wide analysis study conducted in European patients showed a strong association on chromosome 6p in the region of the MHC/DQ and HLA-B loci. These results suggest that the HLA region contains the strongest common susceptibility alleles that predispose to IgA nephropathy in the European population.
Histology
There is a focal and segmental proliferative glomerulonephritis with mesangial deposits of polymeric IgA1. In some cases IgG, IgM and C3 are also seen in the glomerular mesangium.
A new Oxford histological classification for IgA nephropathy is shown in Table 12.7. The features have prognostic significance and it is recommended that they be taken into account for predicting outcome independent of the clinical features both at the time of presentation and during follow-up.
Table 12.7 Oxford histological classification for IgA nephropathy
| Histological variable | Class | |
|---|---|---|
Mesangial hypercellularity |
Average mesangial hypercellularitya >0.5 |
M1 |
Average mesangial hypercellularity <0.5 |
M0 |
|
Segmental glomerulosclerosis |
Part of the glomerular tuft is involved in sclerosis |
S1 |
No segmental glomerulosclerosis |
S0 |
|
Endocapillary hypercellularity |
Hypercellularity present and results in lumina narrowing |
E1 |
No hypercellularity |
E0 |
|
Tubular atrophy/interstitial fibrosis |
Percentage of cortical area involved: |
|
>50 |
T2 |
|
26–50 |
T1 |
|
0–25 |
T0 |
a Mesangial hypercellularity is scored zero (0) for glomeruli with <4 mesangial cells per mesangial area; 1 for those with 4–5 cells; 2 for 6–7 cells; and 3 for ≥8 cells. Scores obtained for all glomeruli are then averaged.
Pathogenesis
The disease may be a result of an exaggerated bone marrow and tonsillar IgA1 immune response to viral or other antigens and is associated with an abnormality in O-linked galactosylation in the hinge region of the IgA1 molecule. Functional abnormalities of two IgA receptors – CD89 expressed on blood myeloid cells and the transferring receptor (CD71) on mesangial cells – are seen. IgA nephropathy is due to circulating immune complexes composed of a glycan-specific IgG and a galactose-deficient IgA1 antibody which deposit in the glomerular mesangium and induce the mesangioproliferative glomerulonephritis characteristic of IgA nephropathy. Removal of the complexes by bacterial proteases results in attenuation of IgA nephropathy. Available evidence suggests that glycan-specific autoantibodies rather than IgA1 itself may play a key role in the pathogenesis.
Up to 50% of patients exhibit elevated serum IgA (polyclonal) concentration. Superimposed crescent formation is frequent, particularly following macroscopic haematuria due to upper respiratory tract infection.
Several diseases are associated with IgA deposits, including Henoch–Schönlein purpura, chronic liver disease, malignancies (especially carcinoma of bronchus), seronegative spondyloarthritis, coeliac disease, mycosis fungoides and psoriasis.
Clinical presentation
IgA nephropathy tends to occur in children and young males. They present with asymptomatic microscopic haematuria or recurrent macroscopic haematuria sometimes following an upper respiratory or gastrointestinal viral infection. Proteinuria occurs and 5% can be nephrotic. The prognosis is usually good, especially in those with normal blood pressure, normal renal function and absence of proteinuria at presentation. Surprisingly, recurrent macroscopic haematuria is a good prognostic sign, although this may be due to ‘lead-time bias’ (p. 436), as patients with overt haematuria come to medical attention at an earlier stage of their illness. The risk of eventual development of ESKD is about 25% in those with proteinuria of more than 1 g per day, elevated serum creatinine, hypertension, ACE gene polymorphism (DD isoform) and tubulointerstitial fibrosis on renal biopsy.
Management
Patients with proteinuria over 1–3 g/day, mild glomerular changes only and preserved renal function should be treated with steroids. Steroids reduce proteinuria and stabilize renal function. Addition of azathioprine to steroids does not confer additional benefits. The combination of cyclophosphamide, dipyridamole and warfarin should not be used, nor should ciclosporin. In patients with progressive disease (eGFR <60 mL/min) fish oil or prednisolone with cyclophosphamide for 3 months followed by maintenance with prednisolone and azathioprine may be tried. A tonsillectomy can reduce proteinuria and haematuria in those patients with recurrent tonsillitis. All patients, with or without hypertension and proteinuria, should receive a combination of ACE inhibitor and angiotensin II receptor antagonist which enhances reduction of proteinuria and preservation of renal function. Mesangial IgA deposits are commonly found in the allografts of transplanted patients but loss of graft function as a result is uncommon.
Alport’s syndrome
Alport’s syndrome is a rare condition characterized by an hereditary nephritis with haematuria, proteinuria (<1–2 g/day), progressive kidney disease and high-frequency nerve deafness. Approximately 15% of cases may have ocular abnormalities such as bilateral anterior lenticonus and macular and perimacular retinal flecks. In about 85% of patients with Alport’s syndrome there is X-linked inheritance of a mutation in the COL4α5 gene encoding the COL4α5 collagen chain. In female carriers, penetrance is variable and depends on the type of mutation or degree of mosaicism following hybridization of the X chromosome. Patients with autosomal recessive or dominant modes of inheritance have also been described with mutations in COL4α3 or COL4α4 genes. In families with stromal cell tumours there is an additional mutation in the COL4α6 gene.
Mutations present in Alport’s syndrome that produce post-translational defects in α3, α4 and α5 chains result in incorrect assembly or folding of monomers; such defective monomers are rapidly degraded. These mutations arrest the normal developmental switch and cause the persistence of embryonic α1, α1 and α2 networks in glomerular basement membrane. These networks are more susceptible to endo-proteolysis and oxidative stress than the α3, α4 and α5 network. Over time, patients with Alport’s syndrome probably become more sensitive to selective basement membrane proteolysis, which may explain why their glomerular membranes thicken unevenly, split and ultimately deteriorate.
The primary glomerular filtration barrier of the glomerular capillary consists of the basement membrane and the outer slit diaphragm formed between adjacent podocytes. Loss of slit function causes massive proteinuria (congenital nephrotic syndrome, p. 576) but deterioration of glomerular basement membrane produces only mild proteinuria. The mild proteinuria in Alport’s syndrome is the result of glomerular sclerosis, rather than primary loss of slit pores. In pedigrees with a history of CKD, disease progresses from concomitant interstitial fibrosis, macrophage and lymphocyte infiltration secondary to tubular basement disruption and transdifferentiation of epithelial mesenchymal cells to fibroblasts. This fibrogenic response destroys renal architecture. The renal histology characteristically shows split basement membrane. In some patients with Alport’s syndrome and carriers, thin basement membrane, as seen in benign familial haematuria, is the only abnormality detected on histology. For this reason, the boundary between Alport’s and benign familial haematuria has become increasingly vague.
Management
The disease is progressive and accounts for some 5% of cases of ESKD in childhood or adolescence. Patients with mild CKD can be treated with ACE inhibitors to attenuate proteinuria. Exciting experimental evidence suggests that mesenchymal stem cells can transdifferentiate into podocytes and repair basement abnormalities and slow the rate of progression. Anti-GBM antibody does not adhere normally to the glomerular basement membrane of affected individuals but development of crescentic glomerulonephritis in the transplanted kidney due to anti-GBM alloantibody is a well-recognized complication.
Thin glomerular basement membrane disease
This condition is inherited as an autosomal dominant and typically presents with persistent microscopic glomerular haematuria (RBC casts or dysmorphic RBCs). The diagnosis is made by renal biopsy, which shows thinning of the glomerular capillary basement membrane on electron microscopy. The condition was underdiagnosed and is much commoner than previously believed. The prognosis for renal function is usually very good but some patients develop renal insufficiency over decades. The cause of renal impairment in this condition is not known but may be due to secondary FSGS or concomitant IgA nephropathy. Misdiagnosis occurs with Alport’s syndrome which shares similar histological features. No treatment is of known benefit.
C3 glomerulonephritis/complement factor H-related protein 5 (CFHR5) nephropathy
This newly described familial renal disease mainly affects people of Cypriot origin and is an autosomal dominant trait. It is characterized by persistent microscopic hematuria, synpharyngitic macroscopic hematuria and progressive CKD culminating in ESKD.
Typically there is isolated glomerular accumulation of complement component 3 (C3) with variable degrees of glomerular inflammation. Patients have a heterozygous duplication in the CFHR5 gene; however, how this results in renal disease is not understood. Recurrence after renal transplantation suggests that CFHR5 protein derived from the new kidney cannot prevent the development of CFHR5 nephropathy.
CFHR5 nephropathy should be included in the differential diagnosis of familial haematuria and kidney disease, particularly if renal biopsy shows C3 deposition in the kidney. Even though IgA nephropathy with microscopic haematuria accompanied by macroscopic haematuria after an upper respiratory tract infection remains highly prevalent, CFHR5 nephropathy shares these features.
Standard CKD intervention is the only therapy which can be offered to these patients like in any other form of progressive kidney disease.
Rapidly progressive glomerulonephritis (RPGN)
RPGN is a syndrome with glomerular haematuria (RBC casts or dysmorphic RBCs), rapidly developing acute kidney failure over weeks to months and focal glomerular necrosis (Fig. 12.22) with or without glomerular crescent development on renal biopsy. The ‘crescent’ is an aggregate of macrophages and epithelial cells in Bowman’s space (Fig. 12.22). RPGN can develop with immune deposits (anti-GBM or immune complex type, e.g. SLE) or without immune deposits (pauci-immune, e.g. anti-PR3 and or anti-MPO-ANCA positive vasculitides). It can also develop as an idiopathic primary glomerular disease or can be superimposed on secondary glomerular diseases such as IgA nephropathy, membranous GN and post-infective GN. The classification used here is based on the immunofluorescence information obtained from renal histology (Table 12.8), viz linear, granular and negative immunofluorescent patterns.
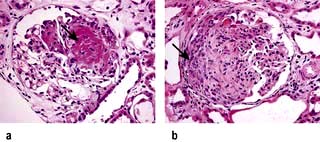
Figure 12.22 Rapidly progressive glomerulonephritis (RPGN). Arrows show ‘crescents’ with aggregates of macrophages and epithelial cells in Bowman’s space. (a) Focal necrotizing glomerulonephritis. (b) Crescentic glomerulonephritis.
Table 12.8 Types of rapidly progressive glomerulonephritis (RPGN)
|
Linear immunofluorescent pattern (Fig. 11.23a) Granular immunofluorescent pattern (immune complex-mediated RPGN) (Fig. 11.23b) |
Anti-GBM glomerulonephritis (Fig. 12.23a)
Anti-GBM glomerulonephritis, characterized by linear capillary loop staining with IgG and C3 and extensive crescent formation, accounts for 15–20% of all cases of RPGN, although overall it accounts for less than 5% of all forms of glomerulonephritis. This condition is rare, with an incidence of 1 per 2 million in the general population. About two-thirds of these patients have Goodpasture’s syndrome with associated lung haemorrhage (p. 850). The remainder have a renal restricted anti-GBM RPGN, which is seen in patients over 50 years and affects both genders equally.
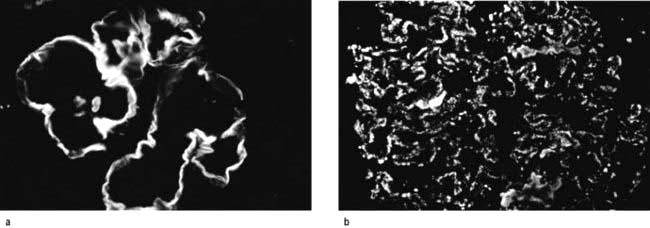
Figure 12.23 Immunofluorescence. (a) Showing antiglomerular basement membrane antibody (anti-GBM) deposition in a linear pattern typical of Goodpasture’s syndrome. (b) Showing immune complex deposition in a diffuse granular pattern.
Anti-GBM antibodies (detected by ELISA) are present in serum and are directed against the non-collagenous (NCl) component of α3 (IV) collagen of basement membrane. This target antigen must be present as a component of the native α3, α4, α5 (IV) network of selected basement membrane in order for pulmonary and renal disease to develop. Consequently, there are no known cases of anti-GBM glomerulonephritis in patients with Alport’s syndrome (p. 584). However, the α3, α4, α5 (IV) network is also a target for anti-GBM alloantibodies in Alport’s syndrome (see this chapter) post-transplantation glomerulonephritis, which occurs in 3–5% of patients with Alport’s syndrome and results in allograft loss. Alport’s post-transplantation nephritis is mediated by the deposition of alloantibodies to the α3NC1 and α5NC1 domains in response to the ‘foreign’ α3, α4, α5 (IV) collagen network that is absent in the patient’s own kidneys with Alport’s syndrome but present in the renal allograft. Alloantibodies in Alport’s syndrome patients bind to epitopes in intact cross-linked α345NC1 hexamers. In contrast, autoantibodies in Goodpasture’s syndrome bind to native cross-linked α345NC1 hexamers only if dissociated first to unmask hidden epitopes.
Anti-GBM RPGN is restricted by the major histocompatibility complex; HLA-DRB1*1501 and HLA-DRB1*1502 alleles increase susceptibility, whereas HLA-DR7 and HLA-DR1 are protective. The thymus expresses α3 (IV) NCl peptides that can eliminate autoreactive CD4+ helper T cells, but a few such cells escape deletion and are kept in check by circulating regulatory cells (Treg). Breakdown of this peripheral tolerance (the mechanism of which is unknown) results in these autoreactive CD4+ cells producing anti-GBM antibodies. These antibodies are very specific as shown by the fact that antibodies against α1, α1 and α2 NCl domains do not cause RPGN. Since the α3 (IV) NCl epitope is hidden within the α3, α4 and α5 (IV) promoter, it is presumed that an environmental factor, such as exposure to hydrocarbons or tobacco smoke, is required in order to reveal cryptic epitopes to the immune system.
The mechanism of renal injury is complex. When anti-GBM antibody binds basement membrane it activates complement and proteases and results in disruption of the filtration barrier and Bowman’s capsule, causing proteinuria and the formation of crescents. Crescent formation is facilitated by interleukin-12 and γ-interferon which are produced by resident and infiltrating inflammatory cells.
Management
This is based on counteracting the factors involved in the pathogenesis and employs:
 Plasma exchange to remove circulating antibodies
Plasma exchange to remove circulating antibodies
 Steroids to suppress inflammation from antibody already deposited in the tissue
Steroids to suppress inflammation from antibody already deposited in the tissue
The prognosis is directly related to the extent of glomerular damage (measured by percentage of crescents, serum creatinine and need for dialysis) at the initiation of treatment. When oliguria occurs or serum creatinine rises above 600–700 µmol/L, renal failure is usually irreversible. Once the active disease is treated, this condition, unlike other autoimmune diseases, does not follow a remitting/relapsing course. Furthermore, if left untreated, autoantibodies diminish spontaneously within 3 years and autoreactive T cells cannot be detected in the convalescent patients. This is suggestive of re-establishment of peripheral tolerance which coincides with re-emergence of regulatory CD25+ cells in the peripheral blood; these play a key role in inhibiting the autoimmune response. The emergence and persistence of these regulatory cells may underlie the ‘single hit’ nature of this condition.
ANCA-positive vasculitides (see also p. 544)
Inflammation and necrosis of the blood vessel wall occurs in many primary vasculitic disorders. Wegener’s granulomatosis, microscopic polyangiitis and Churg–Strauss syndrome are described as small vessel vasculitides and are commonly associated with antineutrophil cytoplasm antibodies (ANCA). These diseases share common pathology with focal necrotizing lesions, which affect many different vessels and organs; in the lungs, a capillaritis may cause lung haemorrhage; within the glomerulus of the kidney, crescentic GN and/or focal necrotizing lesions (FNGN) may cause acute kidney injury (Fig. 12.22); in the dermis, a purpuric rash or vasculitic (Fig. 12.24) ulceration. Wegener’s and Churg–Strauss syndrome may have additional granulomatous lesions.
Renal histology is regarded as a ‘gold standard’ for the diagnosis and prognostication of ANCA-associated GN. A consensus group proposed a new classification around four general categories of lesions:
 Focal ( ≥50% normal glomeruli that are not affected by the disease process)
Focal ( ≥50% normal glomeruli that are not affected by the disease process)
 Crescentic ( ≥50% of glomeruli with cellular crescents)
Crescentic ( ≥50% of glomeruli with cellular crescents)
 Mixed (a heterogeneous glomerular phenotype wherein no glomerular feature predominates)
Mixed (a heterogeneous glomerular phenotype wherein no glomerular feature predominates)
This system has been shown to have a prognostic value for 1- and 5-year renal outcomes. It is believed that it will aid in the prognostication of patients at the time of diagnosis and facilitate uniform reporting between centers. This classification at some point might also provide a means to guide therapy.
Pathogenesis
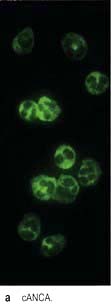
ANCA positive glomerulonephritis – immunofluorescence.
(From Schrier RW 1999. The Schrier Atlas of Diseases of the Kidney Vol IV: Systemic Diseases and the Kidney. Wiley-Blackwell, with permission).
There are two forms of ANCA (p. 544), viz PR3-ANCA (cANCA) and MPO-ANCA (pANCA). If ELISA and indirect immunofluorescence techniques are combined, diagnostic specificity is 99%. Testing for antineutrophil cytoplasmic antibodies should be accompanied by appropriate tests of autoantibodies directed against DNA and the glomerular basement membrane antigen. The simultaneous occurrence of ANCA and anti-GMB antibody is well documented; such patients tend to follow the natural history of Goodpasture’s syndrome. Variations in the ANCA titres have been used in the assessment of disease activity.
 PR3-ANCA positivity is found in the large majority (>90%) of patients with active Wegener’s granulomatosis and in up to 50% of patients with microscopic polyangiitis.
PR3-ANCA positivity is found in the large majority (>90%) of patients with active Wegener’s granulomatosis and in up to 50% of patients with microscopic polyangiitis.
 Anti-MPO positivity is present in the majority of patients with idiopathic crescentic glomerulonephritis and in a variable number of cases of microscopic polyangiitis. There is some evidence to suggest that ANCA are pathogenic and not just markers of disease; for example, development of drug-induced ANCA is associated with vasculitic lesions in humans. Churg–Strauss syndrome may have either anti-MPO- or anti-PR3-ANCA.
Anti-MPO positivity is present in the majority of patients with idiopathic crescentic glomerulonephritis and in a variable number of cases of microscopic polyangiitis. There is some evidence to suggest that ANCA are pathogenic and not just markers of disease; for example, development of drug-induced ANCA is associated with vasculitic lesions in humans. Churg–Strauss syndrome may have either anti-MPO- or anti-PR3-ANCA.
 Positivity for both types of ANCA antibodies occurs in up to 10% of patients who have a variable clinical course but a worse renal outcome.
Positivity for both types of ANCA antibodies occurs in up to 10% of patients who have a variable clinical course but a worse renal outcome.
 Drugs (e.g. propylthiouracil, hydralazine, minocycline, penicillamine) may induce vasculitides associated with ANCA. Most patients reported with drug-induced ANCA-associated vasculitis have MPO-ANCA, often in very high titres. In addition to MPO-ANCA, most also have antibodies to elastase or to lactoferrin. A relatively small number have PR3-ANCA. Many cases of drug-induced ANCA-associated vasculitis present with constitutional symptoms, arthralgias/arthritis, and cutaneous vasculitis. However, the full range of clinical features associated with ANCA, including crescentic GN and lung haemorrhage, can also occur.
Drugs (e.g. propylthiouracil, hydralazine, minocycline, penicillamine) may induce vasculitides associated with ANCA. Most patients reported with drug-induced ANCA-associated vasculitis have MPO-ANCA, often in very high titres. In addition to MPO-ANCA, most also have antibodies to elastase or to lactoferrin. A relatively small number have PR3-ANCA. Many cases of drug-induced ANCA-associated vasculitis present with constitutional symptoms, arthralgias/arthritis, and cutaneous vasculitis. However, the full range of clinical features associated with ANCA, including crescentic GN and lung haemorrhage, can also occur.
Both ANCA autoantigens are present in immature neutrophil granules. In contrast to the normally silenced state of these two genes in mature neutrophils of healthy subjects, PR3 and MPO are aberrantly expressed in mature neutrophils of patients with ANCA vasculitis due to unsilencing of both antigens because of an epigenetic modifications.
 Autoimmunity. It is unclear how and why autoimmunity causes the formation of ANCA antibodies. Patients with anti-PR3 also have autoantibodies to a peptide translated from the antisense DNA strand of PR-3 (complementary PR-3; cPR-3) or to a mimetic of this peptide. This suggests that autoimmunity can be initiated through an immune response against a peptide that is antisense or complementary to the autoantigen, which then induces anti-idiotypic antibodies (autoantibodies) that cross-react with the autoantigen.
Autoimmunity. It is unclear how and why autoimmunity causes the formation of ANCA antibodies. Patients with anti-PR3 also have autoantibodies to a peptide translated from the antisense DNA strand of PR-3 (complementary PR-3; cPR-3) or to a mimetic of this peptide. This suggests that autoimmunity can be initiated through an immune response against a peptide that is antisense or complementary to the autoantigen, which then induces anti-idiotypic antibodies (autoantibodies) that cross-react with the autoantigen.
 A recent study has shown that infection by fimbriated bacteria (Gram-negative pathogens such as Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis) can trigger, due to molecular mimicry, a cross-reactive autoimmunity to lysosomal membrane protein 2 (LAMP-2), a glycosylated membrane protein which is co-localized with PR3 and MPO in the intracellular vesicles of neutrophils.
A recent study has shown that infection by fimbriated bacteria (Gram-negative pathogens such as Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis) can trigger, due to molecular mimicry, a cross-reactive autoimmunity to lysosomal membrane protein 2 (LAMP-2), a glycosylated membrane protein which is co-localized with PR3 and MPO in the intracellular vesicles of neutrophils.
There may be multiple factors that contribute to the initiation of an ANCA autoimmune response and the induction of injury by ANCA, such as genetic predisposition (α1-antitrypsin deficiency; Pi-Z allele) and environmental factors (e.g. silica exposure, viral infection, Staph. aureus infection) can result in high local or systemic pro-inflammatory cytokines such as tumour necrosis factor (TNF).
Treatment
The sooner treatment is instituted the more chance there is of recovery of renal function.
 Corticosteroids and cyclophosphamide are of benefit: high-dose oral prednisolone (maximum 80 mg/day reducing over time to 15 mg/day by 3 months) and cyclophosphamide (2 mg/kg per day, adjusted for age, renal function and prevailing WBC count). Intravenous pulse, rather than daily oral, cyclophosphamide is associated with an equivalent response with better side-effect profile but is associated with higher relapse rate. The best indicators of prognosis are pulmonary haemorrhage and severity of renal failure at presentation.
Corticosteroids and cyclophosphamide are of benefit: high-dose oral prednisolone (maximum 80 mg/day reducing over time to 15 mg/day by 3 months) and cyclophosphamide (2 mg/kg per day, adjusted for age, renal function and prevailing WBC count). Intravenous pulse, rather than daily oral, cyclophosphamide is associated with an equivalent response with better side-effect profile but is associated with higher relapse rate. The best indicators of prognosis are pulmonary haemorrhage and severity of renal failure at presentation.
 Patients who present with fulminant disease need intensification of immunosuppression with adjuvant plasma exchanges (7 × 3–4 L over 14 days) or intravenous pulse methyl prednisolone (1 g/day for 3 consecutive days). Plasma exchange appeared to have better outcome than pulse methyl prednisolone in one study.
Patients who present with fulminant disease need intensification of immunosuppression with adjuvant plasma exchanges (7 × 3–4 L over 14 days) or intravenous pulse methyl prednisolone (1 g/day for 3 consecutive days). Plasma exchange appeared to have better outcome than pulse methyl prednisolone in one study.
 Once remission has been achieved, azathioprine should be substituted for cyclophosphamide. In cases of intolerance to azathioprine or cyclophosphamide, mycophenolate or methotrexate has been tried with some success.
Once remission has been achieved, azathioprine should be substituted for cyclophosphamide. In cases of intolerance to azathioprine or cyclophosphamide, mycophenolate or methotrexate has been tried with some success.
 Colonization of the upper respiratory tract with Staph. aureus increases the risk of relapse, and treatment with sulfamethoxazole/trimethoprim reduces the relapse rate.
Colonization of the upper respiratory tract with Staph. aureus increases the risk of relapse, and treatment with sulfamethoxazole/trimethoprim reduces the relapse rate.
 Relapse after complete cessation of immunosuppressive therapy has been observed relatively frequently, and therefore long-term, albeit relatively low-dose, immunosuppression is necessary.
Relapse after complete cessation of immunosuppressive therapy has been observed relatively frequently, and therefore long-term, albeit relatively low-dose, immunosuppression is necessary.
 Intravenous immunoglobulin (anti-thymocyte globulin, ATG, directed against activated T lymphocytes causes lymphopenia), lymphocyte-depleting anti-CD52 (campath-IH) antibodies, and anti-TNF therapy have shown promise in the treatment of severe and drug-resistant cases as induction therapy. However, an anti-TNF agent, etanercept, has been ineffective as a sole agent for maintenance.
Intravenous immunoglobulin (anti-thymocyte globulin, ATG, directed against activated T lymphocytes causes lymphopenia), lymphocyte-depleting anti-CD52 (campath-IH) antibodies, and anti-TNF therapy have shown promise in the treatment of severe and drug-resistant cases as induction therapy. However, an anti-TNF agent, etanercept, has been ineffective as a sole agent for maintenance.
 Two studies have shown that rituximab is equally effective compared to cyclophosphamide for inducing remission in ANCA-associated vasculitides in the short term (6–12 months) with similar adverse event rates. Rituximab may be a therapeutic option in those patients who cannot tolerate cyclophosphamide, and patients whose disease is poorly controlled who relapse while on cyclophosphamide.
Two studies have shown that rituximab is equally effective compared to cyclophosphamide for inducing remission in ANCA-associated vasculitides in the short term (6–12 months) with similar adverse event rates. Rituximab may be a therapeutic option in those patients who cannot tolerate cyclophosphamide, and patients whose disease is poorly controlled who relapse while on cyclophosphamide.
 Up to 25% of patients with PR3-ANCA harbour antibodies against human plasminogen and/or tissue plasminogen activator. Their presence has been correlated with venous thromboembolic events and fibrinoid necrotic glomerular lesions, suggesting functional interference with fibrinolysis. However, a formal role for anticoagulation in patients with ANCA-associated GN remains uncertain.
Up to 25% of patients with PR3-ANCA harbour antibodies against human plasminogen and/or tissue plasminogen activator. Their presence has been correlated with venous thromboembolic events and fibrinoid necrotic glomerular lesions, suggesting functional interference with fibrinolysis. However, a formal role for anticoagulation in patients with ANCA-associated GN remains uncertain.
FURTHER READING
Berden AE, Ferrario F, Hagen EC et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21:1628–1636.
Jayne D. Challenges in the management of microscopic polyangiitis: past, present and future. Curr Opin Rheumatol 2008; 20(1):3–9.
Jennette JC, Falk RJ. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr Opin Rheumatol 2008; 20(1):55–60.
Other glomerular disorders
HIV-associated nephropathy (HIVAN)
A number of renal lesions have been described in association with HIV infection (see p. 177). These include glomerulonephritis of various histological types and the haemolytic uraemic syndrome. The most common (80–90%) histological abnormality is a focal glomerulosclerosis (FGS).
HIV-associated FGS
A characteristic ‘collapsed’ appearance of glomeruli is often seen on light microscopy similar to that seen in other causes of focal segmental glomerulosclerosis (see Fig. 12.14c). In HIVAN many visceral epithelial cells (podocytes) are enlarged, hyperplastic, coarsely vacuolated, contain protein absorption droplets and overlie capillaries with varying degrees of wrinkling and collapse of the walls. It is associated with loss of podocyte-specific markers such as Wilms’ tumour factor and synaptopodin due to HIV-1 infection of podocytes of patients with HIVAN. HIVAN has striking predilection; over 90% of patients are black. Clinically HIVAN presents with proteinuria in the nephrotic range, oedema and a ‘bland’ urine. Hypertension is unusual. If untreated, patients go on to CKD which can be rapid in progression.
IgA may be an integral feature of HIV-1 infection, as is IgA nephropathy. In this setting, HIV antigen may be a part of the glomerular immune complexes and circulating immune complexes.
Highly active antiretroviral therapy (HAART) may result in stabilization of renal function and prevention of progression to ESKD (efficacy 23%) and HIV-associated mortality in patients with ESKD. A cyclin-dependent kinase inhibitor, roscovitine, has been successfully used in the treatment of experimental HIVAN.
Fabry’s disease
This is the result of deficiency of the enzyme α-galactosidase with accumulation of sphingolipids in many cells. In the kidney, accumulation of sphingolipids especially affects podocytes, which on light microscopy appear enlarged and vacuolated. Ultrastructurally, these inclusion bodies appear as zebra or myeloid bodies representing sphingolipids. Similar appearances have been described in patients taking chloroquine, hydroxychloroquine and amantadine because these can cause hyperlipidosis. These structures can also be found in endothelial, mesangial, and arterial and arteriolar smooth muscle cells. The most common renal manifestation is proteinuria and progressive CKD.
Treatment (see p. 1034).
Sickle nephropathy
Sickle disease or trait is complicated relatively commonly by papillary sclerosis or necrosis, nephrogenic diabetes insipidus and incomplete renal tubular acidosis. Glomerular lesions are rare and can sometimes be traced to hepatitis B or C infection acquired through repeated blood transfusions. Occasionally, proteinuria or nephrotic syndrome with progressive renal insufficiency is seen without prior infection. The rare glomerular lesion is that of membranous GN or membranoproliferative GN with IgG deposits. No form of effective therapy is known.
Glomerulopathy associated with pre-eclampsia
The glomerular lesion of pre-eclampsia is characterized by marked endothelial swelling and obliteration of capillary lumina. Fibrinogen-fibrin deposits may be found in the mesangium. The renal lesion may not be reversible and 30% of patients have changes for ≥6 months. Patients who have had pre-eclampsia are more likely to develop hypertension in subsequent pregnancies. Severe proteinuria may occur during the course of pre-eclampsia and from time to time, produce features of nephrotic syndrome. Ordinarily, proteinuria disappears after delivery.
In severe cases, associated with cortical necrosis, there may be microangiopathic haemolytic anaemia. Vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) play a key role in the development of the placenta.
Relative deficiency of either factor can theoretically cause implantation abnormalities normally seen in pre-eclampsia. A soluble fms-like tyrosine kinase (sFlt1) receptor also called VEGF-receptor, which is an antagonist of PLGF and specifically of VEGF, is upregulated in the placenta of patients with pre-eclampsia. High circulating levels of these receptors antagonize angiopoeitic factors and cause endothelial dysfunction. Excessive free radical generation in the placenta of pre-eclamptic patients is due to upregulation of NADPH oxidase activity caused by generation of an angiotensin II receptor agonist antibody in some patients.
Paraneoplastic glomerulonephritis
A rare complication of malignancy, paraneoplastic glomerulonephritis is usually misdiagnosed as idiopathic glomerulonephritis. Such misdiagnosis can subject patients to potentially harmful, ineffective therapy.
The pathology of paraneoplastic glomerulonephritis varies depending on types of malignancy.
 Thymoma or Hodgkin’s lymphoma: polarization of the immune response toward a T-helper-2 profile and possibly excessive production of interleukin-13 may lead to the development of minimal change disease or focal associated membranoproliferative glomerulonephritis and membranous nephropathy
Thymoma or Hodgkin’s lymphoma: polarization of the immune response toward a T-helper-2 profile and possibly excessive production of interleukin-13 may lead to the development of minimal change disease or focal associated membranoproliferative glomerulonephritis and membranous nephropathy
 B cell lymphoma and leukaemia are related to the presence of monoclonal immunoglobulin, cryoglobulin, and possibly hepatitis C virus infection
B cell lymphoma and leukaemia are related to the presence of monoclonal immunoglobulin, cryoglobulin, and possibly hepatitis C virus infection
 Polycythaemia vera, essential thrombocythemia or primary myelofibrosis: severe thrombocytosis may induce focal segmental glomerulosclerosis, possibly due to elevated levels of platelet derived growth factor
Polycythaemia vera, essential thrombocythemia or primary myelofibrosis: severe thrombocytosis may induce focal segmental glomerulosclerosis, possibly due to elevated levels of platelet derived growth factor
 Myelodysplastic syndromes: autoimmunity causes a variety of glomerulonephritides
Myelodysplastic syndromes: autoimmunity causes a variety of glomerulonephritides
 Epithelial carcinoma: the presence of glomerular inflammatory cells, and subepithelial immune IgG1- and IgG2-containing complexes are usually present and may aid in the diagnosis of paraneoplastic membranous nephropathy.
Epithelial carcinoma: the presence of glomerular inflammatory cells, and subepithelial immune IgG1- and IgG2-containing complexes are usually present and may aid in the diagnosis of paraneoplastic membranous nephropathy.