chapter 75 Other Therapies for Storage and Emptying Failure
The etiologies of voiding dysfunction are diverse and may occur as a result of neurologic, inflammatory, or infectious disorders. Other factors such as surgical or nonsurgical trauma and aging are also important causes. Obstruction continues to occupy a central role in the development of emptying failure and, when present chronically, may lead to poor bladder compliance and the failure to store. For both filling/storage and emptying disorders, dysfunction may originate primarily from the bladder, sphincter, or a combination of both. The majority of patients with voiding dysfunction will respond favorably to standard therapy (discussed elsewhere in this book). The treatment of complex cases should be individually tailored and may entail a combination of techniques, especially if approached through different mechanisms. Tables 61–3 and 61–4, list the numerous strategies devised to meet this goal, a testament to the complex management of voiding dysfunction. Additional treatments that have been developed for the management of storage and emptying failure are discussed in this chapter. Some of the topics are covered in other areas of this text but require special mention, while other topics are covered nowhere else in this edition. While some of these approaches offer promising results and are deserving of continued follow-up, others have been mentioned for historic reference only.
Methods to Improve Bladder Filling/Urine Storage
Symptoms of urinary frequency, urgency, and/or incontinence are among the most common manifestations of storage failure. These symptoms can be tremendously bothersome and significantly decrease the patient’s quality of life. When severe, many patients will limit their activities and social functions in order to avoid embarrassment from their disorders. Volume accommodation with low pressure, continence, and the absence of involuntary bladder contractions are the goals of management. Treatment is often targeted at decreasing sensory input during filling, inhibition of bladder contractility, and increasing outlet resistance. The following sections discuss methods to facilitate urine storage and promote bladder filling by different means.
Decreasing Spasticity and Increasing Cystometric Capacity
Lower Urinary Tract Denervation, Decentralization, and Neuromodulation
Altering the innervation of the urinary tract is the focus of several strategies for the management of storage failure and is the central mechanism for procedures that vary from sacral rhizotomy to intravesical botulinum toxin. In addition, interventions may involve different levels of the nervous system, such as the spinal cord, nerve roots, or peripheral nerves. Of note, noncentral denervation proximal to the postganglionic neuron is properly termed “decentralization” and because the bladder is innervated by a short-neuron system, almost all denervation procedures outside the central nervous system that we will describe are properly referred to as decentralization. In general, more central procedures would appear more suitable for patients with spinal cord injury (SCI) or neurogenic detrusor overactivity, and although central denervation is theoretically very effective, it may also have a potentially wider range of unintended consequences. Peripheral decentralization is more selective for the bladder and more suitable for idiopathic detrusor overactivity, as other structures innervated by the sacral nerves remain neurologically intact (Madersbacher, 2000). There would seem to be only a small role for most of the procedures discussed in this section, because conservative measures are often sufficient in significantly improving or alleviating storage symptoms in most patients. Although favorable results have been reported with denervation techniques, some of these procedures have been accompanied by significant adverse outcomes that have limited their usefulness. One significant problem with central denervation and decentralization is that neuroplasticity often results in a variable restoration of neurologic function, with an even less desirable result than was present originally in many cases (Madersbacher, 2000). This section will focus on surgical techniques of bladder denervation, both central and peripheral. Sacral neuromodulation is covered in Chapter 70.
Central (Subarachnoid) Block
These procedures, comprise chemical rhizolysis, sacral rhizotomy, and conusectomy, selective anterior sacral rhizotomy, and dorsal rhizotomy are seldom used today and are discussed fully on the Expert Consult website.![]()
Chemical Rhizolysis—Intrathecal Injection of Alcohol/Phenol
Improvements in bladder storage function have been reported following intrathecal injection of neurolytic compounds (Misak et al, 1962; Gibbon, 1966). Urologic applications followed the observation that neurogenic detrusor overactivity was often converted acutely to areflexia in procedures performed primarily for somatic spasticity. The resultant flaccid bladder required clean intermittent catheterization or alternative methods to assist bladder emptying. The obvious disadvantage of this type of procedure is a lack of end-organ selectivity, with unintended motor or sensory loss to other organ systems. Impotence was a common sequela, and residual motor or sensory function was often significantly altered or lost. Further, an areflexic bladder was not always maintained on a long-term basis, and decreased compliance often developed in these patients, resulting in significant storage problems. Significant complications and relative long-term ineffectiveness have precluded continued use of this procedure.
Nerve Root Procedures
Several variations of sacral rhizotomy exist, however, the goals for all variations are to improve bladder capacity and compliance, abolish autonomic dysreflexia, and improve continence. Denervation may be achieved by sectioning anterior (motor), posterior (sensory), or both sacral roots, converting an overactive bladder into an areflexic one. These procedures are generally performed in association with lumbar or sacral laminectomy and involve the S2 through S5 nerve roots; however, newer, less invasive techniques, such as percutaneous radiofrequency rhizotomy and cryoneurolysis, have been described (Mulcahy et al, 1978; Awad et al, 1987). Typically, intraoperative electrostimulation of prospective nerve roots along with continuous urodynamic monitoring help identify appropriate nerves for transection. Significant side effects may develop following rhizotomy and may affect bladder, bowel, sphincter, sexual, and motor/sensory elements of the lower extremities. With this in mind, patient selection and informed consent is imperative.
Sacral Rhizotomy and Conusectomy
Meirowsky and colleagues (1950) were among the first to observe that SCI patients undergoing rhizotomy for severe somatic spasticity exhibited beneficial effects in bladder management. Subsequently, several reports followed on the use of anterior rhizotomy alone or in combination with posterior rhizotomy for the treatment of bladder hyperactivity (Misak et al, 1962). Initial studies reported increased bladder capacity with more efficient bladder emptying. Likely contributing to these findings was the reduction in urethral resistance, which often resulted from concomitant denervation of the external urinary sphincter (Rockswold et al, 1973). Unfortunately, bladder areflexia rarely persisted, and detrusor overactivity commonly recurred after a short time. Investigators also discovered that bilateral anterior and posterior rhizotomy, or conusectomy, adversely affected the rectum, anal and urethral sphincters, sexual function, and the lower extremities.
Selective Anterior Sacral Rhizotomy
In an attempt to reduce side effects, selective anterior (motor) rhizotomy was introduced with the hope of attaining a more specific response (Rockswold et al, 1973). To enhance clinical efficacy, differential sacral rhizotomy is typically preceded by stimulation and blockade of the individual sacral roots with cystometric and sphincterometric control. In this manner, only motor roots responsible for involuntary contractions are divided. The basis of this technique follows observations by Heimburger and associates (1948) that the anterior S3 nerve root is the dominant motor innervation of the human bladder. Torrens (1985) summarized the collective results of anterior rhizotomy reporting that success ranged from 48% for idiopathic detrusor overactivity to 81% for patients classified as having a “paraplegic bladder.” Unfortunately, the definition of “success” varied substantially among series and among patients. Clearly, when anterior rhizotomy procedures are performed, they should be preceded by urodynamic and urologic evaluation of the effects of selective nerve blocks before proceeding, especially in patients without fixed neurologic disease or injury. Even then, unintended effects on pelvic and lower extremity sensory or motor functions may occur, with disastrous medical and legal sequelae. The role, if any, of anterior rhizotomy procedures within a plan of treatment for detrusor overactivity, still remains to be defined.
Dorsal Rhizotomy
Expanded interest in dorsal (posterior) rhizotomy followed the realization of the importance of afferent stimuli in generating detrusor overactivity. Tanagho and Schmidt (1988), Tanagho and associates (1989), and Brindley (1990) further popularized the idea of sensory deafferentation using dorsal rhizotomy to increase bladder capacity. Similarly, dorsal root ganglionectomy has been reported to increase bladder capacity (McGuire and Savastano, 1984). Interruption of the afferent reflex arc by complete transection of all dorsal roots (sacral or sensory deafferentation) abolishes detrusor overactivity (Brindley, 1994b). Dorsal rhizotomy may also restore continence and may decrease the incidence of autonomic dysreflexia in many instances (Schurch et al, 1998). In addition, striated sphincter dyssynergia is often abolished without altering resting tone (Brindley, 1994a). Madersbacher (2000) stated that deafferentation of the bladder is best achieved using an intradural approach because in this location, motor and sensory fibers can easily be separated. If an intradural procedure is not possible, he believes that a deafferentation at the level of the conus medullaris should then be performed.
Proper patient selection is paramount in selecting patients for this procedure because dorsal rhizotomy of the S2 through S5 nerve roots abolishes reflex erections, reflex ejaculation, and sacral sensation, and it can reduce reflex defecation. Partial or selective procedures should be considered only in patients who retain some sensation or have excellent reflex erections that they wish to preserve. Selective dorsal rhizotomy of only a few nerve roots is not as effective as procedures that transect all dorsal roots from S2 to S5, because the former procedure often results in incompletely blocked reflex impulses and persistent detrusor overactivity (Sauerwein, 1990).
Complete and long-term resolution of detrusor overactivity has been attained with complete sensory deafferentation (Madersbacher, 2000; Kutzenberger et al, 2005). Koldewijn and associates (1994) reported on the effects of intradural bilateral posterior root rhizotomies from S2 to S5 with implantation of an anterior root stimulator in a group of patients with suprasacral SCI. All showed persistent detrusor areflexia afterward, although two required subsequent secondary rhizotomy at the level of the conus. Madersbacher (2000) treated 65 tetraplegic or paraplegic patients with post-SCI reflex urinary incontinence resistant to all other means of conservative treatment. Continence was achieved in 90% of these patients.
Complete deafferentation produces detrusor areflexia that requires clean intermittent catheterization (CIC) or alternative methods to empty the bladder. Insertion of an anterior root stimulator, to electrically induce voiding, is often performed concurrently with dorsal rhizotomy procedures (Seif et al, 2004). A significant potential shortcoming of anterior root stimulation is simultaneous, unphysiologic contraction of the bladder and urethral sphincter, which may result in clinically significant detrusor sphincter dyssynergia (DSD) or increased intravesical pressures during voiding (Schumacher et al, 1999). Anterior root stimulators may also be used to induce erections. Patient satisfaction rates following combined sacral deafferentation and anterior root stimulation has been reported to be very high (Kutzenberger et al, 2005). For more on anterior nerve root stimulation and related procedures, see Chapter 70.
Sacral Neuromodulation
Sacral neuromodulation is currently the most commonly used surgical procedure to treat patients with refractory urgency-frequency or urgency incontinence. For more information on the indications, procedural aspects, treatment results, and complications of sacral neuromodulation, see Chapter 70.
Peripheral, Perivesical, and Intravesical Bladder Denervation
Transvaginal Denervation
A number of procedures fall into the category of attempts to achieve a peripheral denervation. The exact nature of these denervations is unclear and may involve interruption of sensory, motor, or both types of innervation. As evident from neuroanatomic considerations, at best, such attempts achieve primarily neurologic decentralization and, at most, partial peripheral denervation. Although many authors report relatively high success rates for these procedures, it is unclear why they are not used more frequently. Because they are primarily used for detrusor overactivity, it is likely that many clinicians use nonsurgical methods first and are often successful in managing detrusor overactivity with fewer side effects. Also, because these procedures are uncommonly performed, many clinicians are unfamiliar with how to perform the procedure, and the success rate is likely much lower than when performed by clinicians who commonly perform these procedures. Lastly, many clinicians have begun to use neuromodulation or botulinum toxin injections to treat refractory overactive bladder, leaving the role of transvaginal denervation in question.
Regarding the literature on transvaginal denervation, in many articles, there is little description of what “success” actually means. In addition, there are few studies with long-term follow-up, and “postoperative assessment” often means a few months of follow-up. A comprehensive summary of the surgical treatment of detrusor overactivity by peripheral denervation was reported by Mundy in 1985; however, many reports have been published since that time.
Transvaginal partial denervation of the bladder was originally described by Ingelman-Sunberg in 1959. The original procedure, which was an extensive denervation, was used mostly for the treatment of refractory urge-urinary incontinence, and in the originator’s hands, success rates of up to 80% were achieved. Mundy (1985) reported that other investigators found success in 50% to 65% of cases, and Torrens (1985) noted that “the technique has not found favor with other workers.”
More recently, Cespedes and associates (1996) described McGuire’s modification (limited lateral vaginal dissections) of the Ingelman-Sundberg procedure in 25 women with urinary urgency incontinence and detrusor overactivity who had failed behavioral and medical therapy (Fig. 75–1). Transvaginal local anesthesia was used as a test to determine which patients would benefit from the procedure, with significant improvement of urgency symptoms considered a positive test. Twenty-five patients were found to have a positive test; the number of patients unsuccessfully tested was not reported. Sixteen (64%) of these patients were cured of urgency incontinence at a mean follow-up of 14.8 months. Of the 16 patients cured, 9 patients required one medication, and 2 required two medications. Madersbacher (2000) comments that a follow-up period of 15 months is too short to determine long-term effects.
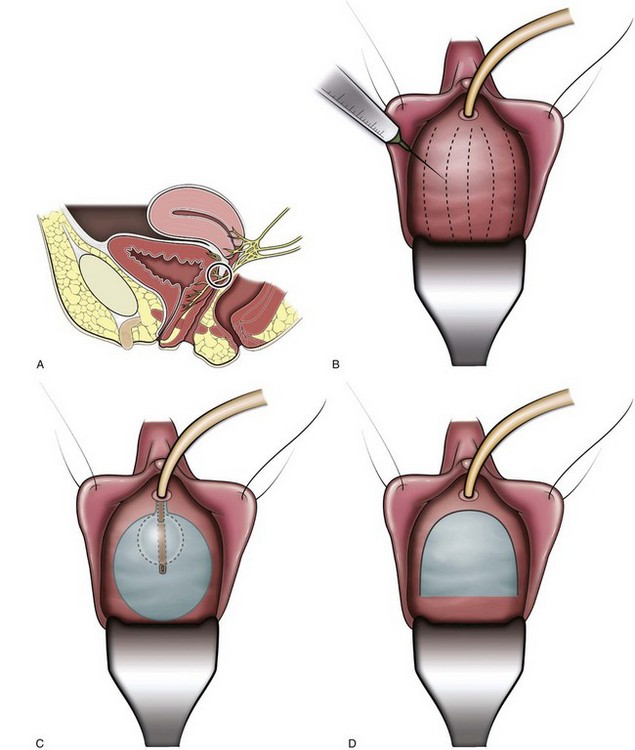
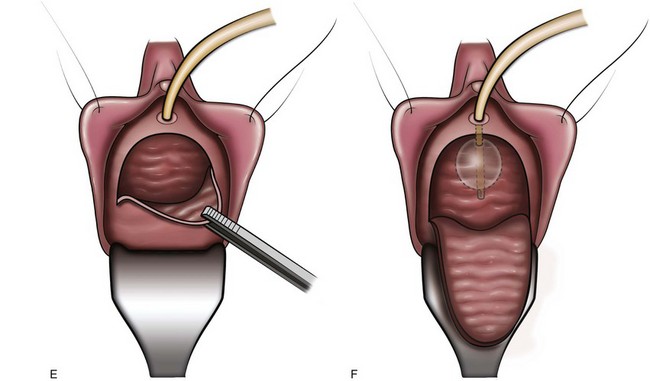
Figure 75–1 Terminal pelvic nerve branches enter the bladder near the trigone. A, To assess preoperatively in clinic whether the patient would benefit from the procedure, local anesthetic (0.25% bupivacaine) is injected subtrigonally to block the nerves which would be denervated by the operative procedure. B, Normal saline is injected submucosally to facilitate dissection of the vaginal mucosa off the bladder. C, Position of the trigone outlined by a catheter balloon inflated to 30 mL. D, Inverted-”U” vaginal incision is made. E, Dissection of the vaginal mucosa and deeper tissues off the underlying bladder. F, Completed dissection.
Long-term follow-up was reported by Westney and associates (2002), who retrospectively reviewed 28 patients with refractory urgency incontinence, having undergone the modified procedure with a mean follow-up of 44.1 months. Of these, 68% were cured (54%) or improved (14%). Based on these results and the prior series, the authors concluded that the modified Ingelman-Sundberg procedure is an efficacious, minimally invasive option for this difficult-to-treat patient population.
Bladder Transection and Peripheral Chemical Neurolysis
These procedures are seldom used today and are fully discussed on the Expert Consult website.![]()
Bladder transection involves a complete circumferential division of the full thickness of the bladder wall at a level just above the ureteric orifices, although Mundy (1985) believed that only the posterior part of the transection was important. Initial encouraging reports in the early 1970s were followed by longer-term reviews of larger series, all reporting success rates in excess of 50% for at least detrusor overactivity, using varying criteria. Mundy (1985) reviewed his large experience with transection in patients with detrusor instability and reported that of 104 patients with a follow-up for 1 to 5 years, 74% were cured, 14% were improved, and 12% were failures. Between 20 and 32 months, 10% of the group initially judged to have a satisfactory response suffered a relapse, giving a long-term subjective success rate of 65%. Only 35% of those who claimed to be symptomatically cured reverted to urodynamically stable detrusor behavior, however. Crooks and colleagues (1995) reported on the results of open bladder transection in 12 patients with urinary frequency and nocturia unresponsive to more conservative measures. Three patients were cured, 7 were better, and 2 had failures. Interestingly, 6 patients with preoperative enuresis were cured of at least this symptom by the procedure.
Parsons and coworkers (1984) described endoscopic bladder transection in patients with phasic detrusor hyperactivity. Their early results were encouraging, but Lucas and Thomas (1987) reported essentially no change in 14 of 18 patients with intractable detrusor overactivity treated by this technique. Two achieved complete symptomatic relief, and 2 more were rendered continent but with the complaint of urgency and nocturia. Hasan and associates (1995) reported on the results of endoscopic bladder transection in 50 patients with urinary frequency, urgency, and urgency incontinence refractory to anticholinergic therapy. Subjective assessment revealed an overall symptomatic improvement in 12%, while objective urodynamic analysis demonstrated no significant improvement. Overall, it is unclear whether bladder transection is a viable procedure for patients with a refractory overactive bladder (OAB); however, if attempted, open transection appears to be superior to endoscopic transection.
Chemical Neurolysis
Chemical neurolysis has been used in an attempt to reproduce the results of the surgical approaches outlined above but in a minimally invasive manner. Mundy (1985) and Torrens (1985) credit the original technique, using transvesical infiltration of the pelvic plexus with phenol, to Ewing and colleagues (1983), who reported successful treatment of 19 of 24 patients with multiple sclerosis (MS). The potential risks of this procedure were found to be urinary retention and vaginal fistula. Blackford and associates (1984) reported a satisfactory response in 82% of women with refractory neurogenic detrusor overactivity and in 69% of women with detrusor overactivity who were more than 55 years old, but in only 14% of females younger than 55 years old. Cameron-Strange and Millard (1988) reported a 70% success rate in 11 patients with neurogenic detrusor overactivity secondary to MS. Although they achieved a 58% success rate in 29 patients with detrusor overactivity, Wall and Stanton (1989) reported only a 29% significant response rate to therapy in a mixed group of 28 females with urinary urgency incontinence. Chapple and coworkers (1991) reported success in only 2 of 18 patients with idiopathic detrusor overactivity, who were observed for 6 months, and in 2 of 6 with neurogenic detrusor overactivity. Two fistulas resulted, one vesicoureterovaginal and one vesicovaginal. They concluded, on reviewing their results and those of others, that subtrigonal phenol should be used in such cases only when no other treatment is possible.
Intravesical Botulinum Toxin Injections
One of the more recent innovations is the use of botulinum toxin to chemically denervate the detrusor musculature. Originally used in spinal cord injury patients to denervate the external sphincter, it has recently found much success in patients with refractory overactive bladder. The exact mechanism and the site within the nervous system by which botulinum toxin exerts its effects is largely unknown. A further discussion of the possible mechanisms and site of action at which botulinum toxin exerts its effects is covered in Chapter 60, and its clinical use in the management of detrusor overactivity is covered in Chapter 68.
Acupuncture
The use of acupuncture as a therapeutic treatment modality is described in Chinese medical texts as early as 300 BC. Acupuncture is a form of somatic sensory stimulation using a variety of procedures that involve stimulation of specific anatomical locations by insertion of thin, solid, metallic needles into the skin. In the classic forms of acupuncture, needles are stimulated manually to achieve the desired effects. In more modern forms of acupuncture, heat or electrical stimulation may be provided by the addition of an energy source or be performed needleless, as in laser acupuncture. Indeed, McGuire and coworkers’ (1983) developed transcutaneous posterior tibial nerve stimulation for bladder inhibition that was based on the acupuncture points. The use of acupuncture has been described for a number of urologic symptoms, including urinary urgency, frequency, incontinence, nonobstructive urinary retention, enuresis, and neurogenic detrusor overactivity. Acupuncture is generally a well-tolerated procedure, and although side effects are generally uncommon and minimal in nature, treatment may occasionally result in localized pain, hematoma, infection, and even organ puncture. Several treatment sessions are usually required.
There has been much interest in determining the mechanism by which acupuncture exerts its beneficial effects. According to traditional Chinese medicine, concepts such as the meridian system, circulation of Qi, and other related theories remain difficult to define and characterize. Sato (1997) has shown somatovisceral reflexes evoked from somatic afferent stimulation may induce either excitatory or inhibitory changes in bladder function and sphincter activity. Bergström and associates (2000) hypothesized any one or a combination of (1) endorphinergic effects at the sacral spinal cord level or above, (2) inhibitory somatovisceral reflexes, and (3) increase in peripheral circulation as possible mechanisms of action. The true nature of how acupuncture exerts its beneficial effects remains incompletely understood.
Bergström and associates (2000) reported on the results of classic acupuncture performed twice weekly in a group of 15 elderly women with urgency or mixed incontinence. In this open, uncontrolled study, subjective assessments of improvement and objective measurements in the form of grams of leakage over 48 hours were encouraging, not only at the end of the study but also after 1 and 3 months. More recently, Emmons and Otto (2005) compared acupuncture versus placebo acupuncture in the treatment of women with overactive bladder and urinary urgency incontinence. The number of incontinent episodes decreased by 59% in the treatment group compared with 40% in the placebo group. The treatment group had a significant improvement in bladder capacity, urgency, frequency, and quality-of-life scores compared with the placebo group.
Many studies have been performed to determine the efficacy of acupuncture; however, the level of evidence continues to be limited secondary to difficulties in performing adequate placebo controlled, double-blind studies. In addition, acupuncture requires extensive training, is performed by few clinicians, and few teachers exist. Overall, the importance of acupuncture may not just be in learning or performing the procedure; it may be more important to learn the mechanisms by which it achieves these results, in order to develop easier and less invasive methods of achieving the same (or better) clinical results.
Bladder Distention Therapy
Initially described by Helmstein in 1972 for the treatment of patients with bladder carcinoma, this treatment modality has undergone several modifications. Bladder overdistention, most familiar for its use in the treatment of interstitial cystitis (see Chapter 12), has also been used for other diverse indications, including nocturnal enuresis, irritative symptoms following radiation or BCG, and for patients with overactive bladder.
No standard technique has been clearly defined for this procedure and, as such, several variations exist. Bladder distention may be performed under regional or general anesthesia. The bladder is then distended by either a balloon catheter or more commonly by saline infused cystoscopically or through a urethral catheter. The ideal pressure and duration needed to distend the bladder has not been well defined. Dunn and colleagues (1974) described prolonged bladder distention of several hours at a pressure equal to the patients’ systolic blood pressure. At this pressure, therapeutic benefit is thought to derive from ischemic changes induced in submucosal afferent nerve endings and stretch receptors (Dunn et al, 1977). In a primarily interstitial cystitis population, Hanno and Wein (1991) described distending the bladder to 80 cm H2O for several minutes with good results. Bladder rupture, historically as high as 5% to 10%, is the most significant complication after bladder distention therapy. Other possible complications include hematuria, back pain, and urinary retention.
Ramsden and colleagues (1976) reported good to excellent results in the treatment of patients with detrusor overactivity, while most others have reported inferior results with this treatment modality. In this same patient population, Jorgensen and coworkers (1985) reported a success rate of only 1 in 15 (6%) patients. In a retrospective review by Taub and Stein (1994), 8 of 22 (36%) of patients with frequency and urgency of various etiologies had subjective improvement, while no patient with detrusor overactivity improved. They also demonstrated that short duration distention therapy (15 to 30 minutes) was as efficacious as prolonged therapy (6 hours). Lloyd and colleagues (1992) reported that only 6 of 29 women with severe irritative symptoms, including 6 patients with interstitial cystitis and none with neurogenic detrusor overactivity, had a good response to treatment. In a study of 26 women with frequency and urgency, Liapis and associates (2001) found a statistically significant increase in bladder capacity after treatment; however, only 4 (15%) had any improvement in symptoms beyond 9 months. Although largely regarded as an ineffective procedure in patients with storage failure secondary to neurogenic detrusor overactivity, bladder overdistention may provide substantial, but usually temporary, improvement to some patients with non-neurogenic storage symptoms who have failed medical therapy.
Key Points: Storage Failure Due to Bladder Overactivity, Decreased Compliance, or Hypersensitivity
Increasing Outlet Resistance
See the Expert Consult website for a discussion of this topic, as well as Figures 75-2 and 75-3.![]()
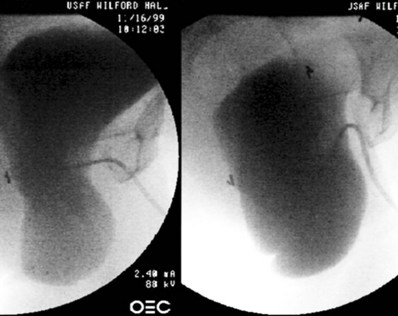
Figure 75–2 In this lateral fluoroscopic view, taken during urodynamics, the bladder is seen to prolapse significantly with Valsalva maneuver, with severe kinking of the urethra causing obstruction. When this prolapse is reduced during examination, the urethra is straightened, and occult incontinence may be elicited.
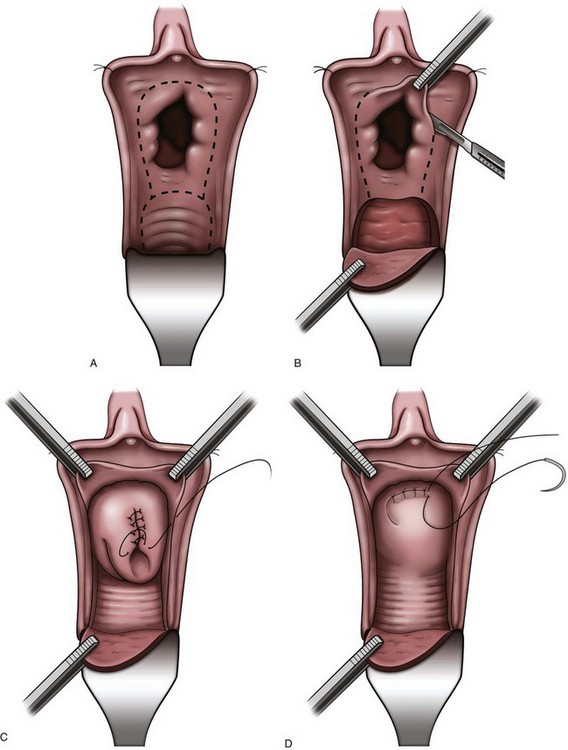
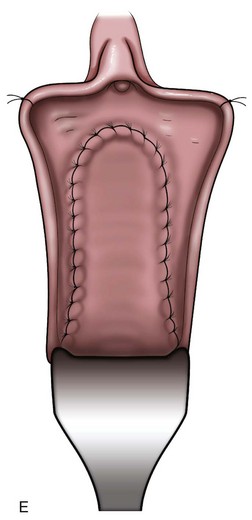
Figure 75–3 A, After injection of normal saline solution into the anterior vaginal wall, an incision is made around the urethral opening to create the vaginal wall flap. B, The urethral meatus is dissected free, and the vaginal wall flap is created. C, The bladder neck wall musculature is vertically closed in a running fashion after the mucosal layer has a watertight closure. D, The final muscular layer is closed in a horizontal fashion and rotated superiorly behind the pubis. E, The vaginal epithelium is advanced over the entire defect and closed with a running suture.
Key Points: Storage Failure Due to Sphincter Underactivity
Nonsurgical Compressive and Occlusive Devices
Urethral compression devices have been used to successfully control urinary incontinence in males for over 250 years, with little change in their construction during this time. Indeed, the Heister penile clamp was described in Institutiones Chirurgicale, a surgical textbook published in 1750. This device closely resembles the Cunningham clamp, a device commonly used today (Madjar et al, 2001). These devices are primarily used to treat patients with pure sphincteric incontinence, most commonly postprostatectomy incontinence (PPI), because normal bladder capacity and storage pressures are a relative requirement. Continence is achieved by mechanically compressing the urethra between two foam pads. The device is adjusted by slowly increasing the level of compression until continence is attained, because this allows the lowest pressure to be applied, keeping obstruction of blood flow to a minimum. Regardless, these devices should be unclamped regularly at 3- to 4-hour intervals, because prolonged or excessive compression can cause pressure-related injury to the penis. Additionally, these devices should not be worn during an erection or while sleeping. Pressure-related injuries may also be more prevalent in patients with impaired sensation or cognition; therefore penile clamp usage in this population should be considered a relative contraindication. Several other compression devices are available and are similar in both design and function to the Cunningham clamp. Moore and colleagues (2004) reported efficacy, comfort, and patient satisfaction with three penile compression devices: the Cunningham clamp, the C3, and the U-Tex. The study population included 12 men with postprostatectomy incontinence requiring continuous pad protection. Over 4 consecutive days, patients were randomly assigned to use either one of the three devices, or pads alone as a control group. Each group wore the device or pads continuously for 4 hours (maximum time recommended for continuous usage), and pad weights were obtained before and after the study period to quantify leakage. Additionally, patients were asked to complete a questionnaire at the conclusion of the study. Urine leakage was significantly reduced by all three devices evaluated; however, the Cunningham clamp was the most efficient of the three devices tested, reducing urine leakage by nearly 85%. The Cunningham clamp was also noted to reduce cavernosal blood flow significantly more than the other devices. Overall, patients rated the Cunningham clamp the most acceptable and preferable of the devices evaluated.
Although these devices manage sphincteric incontinence relatively well, they are rarely used today because they are inconvenient, and many minimally invasive options for male sphincteric incontinence now exist. These devices remain useful for patients who cannot undergo surgical therapy due to medical conditions, and for patients who have severe leakage during the early postprostatectomy period, while surgical treatment is contraindicated.
The renewed interest in conservative management of female sphincteric incontinence, and lower urinary tract symptoms associated with pelvic organ prolapse, has prompted the development and investigation of several nonsurgical devices. Unfortunately, due to the location and anatomy of the female urethra, no compressive or occluding device has been introduced that successfully treats sphincteric incontinence without causing significant side effects or patient discomfort. In addition, reliable external urinary collection devices do not exist for females, making the Foley catheter the most common method of treatment for intractable sphincteric incontinence. Therefore the role of these devices in the algorithm of conservative management of female sphincteric incontinence remains unclear. A more thorough description of these devices, along with the indications and efficacy, are discussed in Chapter 69.
Pelvic organ prolapse may be associated with sphincteric incontinence, overactive bladder symptoms, and/or urinary retention due to anatomic urethral obstruction. Vaginal support devices that support the anterior vaginal wall, thereby supporting the bladder neck and proximal urethra, should theoretically improve both stress incontinence due to urethral hypermobility and allow normal voiding by reducing urethral obstruction. Some devices may even improve stress incontinence due to intrinsic sphincter deficiency, if the urethra is sufficiently compressed. Intravaginal devices, such as tampons, contraceptive diaphragms, pessaries, and pessary-like devices specifically designed for bladder neck support have all been used with variable results. The issues that exist with these devices include the need for intermittent removal and cleaning, problems with device placement, and dislodgement due to the variable anatomy of the vagina, and patient discomfort.
Female urethral occlusive devices may be external or internal, depending on whether the device occludes the urethra or bladder neck from the outside or must be inserted into the urethra. Examples of these devices include urethral meatal occlusive devices and urethral inserts. These devices are most useful in patients with mild to moderate sphincteric incontinence without significant detrusor overactivity or decreased compliance. The characteristics of an ideal occlusive or supportive device include (1) efficacy, (2) comfort, (3) ease of application/insertion/removal, (4) no interference with voiding, (5) lack of tissue damage, (6) no increase of infection risk, (7) no compromise of subsequent therapy, (8) cosmetic acceptance, and (9) no interference with sexual activity. Unfortunately, no occlusive or supportive device currently fulfills all these requirements.
Sphincteric Injection Therapy
Injection therapy is commonly employed as a minimally invasive treatment option for male and female stress incontinence. Although it is theoretically a temporary treatment, typically lasting only 6 to 12 months, the ease of reinjection in a clinical setting renders it a potentially permanent solution in selected patients. Injectables treat stress incontinence by increasing the abdominal leak point pressure by several mechanisms, including increased mucosal sealing pressure and improvement in the mucosal seal mechanism (Appell, 2002). Females most likely to benefit from this procedure have intrinsic sphincter deficiency (ISD), a normally contractile bladder, and a well-supported urethra. This constellation of requirements is most commonly fulfilled by elderly females; however, several authors have demonstrated improvement in younger patients with stress urinary incontinence (SUI) due primarily to urethral hypermobility (Appell, 2009). The rarity of injectable use in this population really reflects the efficacy and widespread use of the newer minimally invasive slings, compared with the relative ineffectiveness of injectables.
The lack of standardization in defining success, largely attributable to the nonpermanent nature of this treatment, makes it difficult to accurately assess the true cure and improved rates after treatment. Nevertheless, the most extensively studied injectable, glutaraldehyde cross-linked (GAX) collagen, has consistently resulted in improvement and/or cure in 60% to 80% of selected female patients. Most patients will require two injections to achieve dryness and will require reinjection every 6 to 12 months to maintain continence. The injections can be performed indefinitely without any apparent side effects. If the patient fails injection therapy, it does not hinder other surgical procedures.
In males, postprostatectomy incontinence can also be managed with injection therapy. The best results are obtained with patients with mild incontinence (one to three pads per day), while patients who have undergone radiation, bladder neck incision, or cryotherapy have less favorable responses (Cespedes, 2000). Most patients require four to five injections, with reinjections every 6 to 12 months to maintain dryness (Cespedes, 2000). Westney and colleagues (2005) reported their results of collagen injection in 322 men with ISD after therapy for prostate carcinoma or benign prostatic hyperplasia. The authors reported that after a mean of 4.37 injections, and irrespective of therapy, overall improvement was approximately 50% with a mean duration of 6 months.
The ideal bulking agent should be durable, biocompatible, should not migrate, or cause significant inflammatory reaction at the injection site. Unfortunately, no current injectable has all of these qualities. In the future, advances in the field of tissue engineering, including autologous chondrocytes and cultured myoblasts, may offer new and exciting options in the treatment of stress urinary incontinence using injectables. For a more thorough discussion of the history, indications, techniques, and results of bulking procedures, including a review of the injectable agents currently available, refer to Chapter 77.
Surgical Compression/Closure of the Bladder Outlet
Vesicourethral Suspension and Prolapse Repair (Female)
Vesicourethral suspension procedures, such as the Burch and MMK (Marshall-Marchetti-Krantz) procedures, have been successfully used for many years to treat stress incontinence secondary to urethral hypermobility, most commonly defined as stress incontinence occurring with an abdominal leak point pressure (ALPP) greater than 100 cm H2O. More recently, with the increasing popularity of the minimally invasive mesh slings, their use has diminished substantially, and they are rarely used unless a simultaneous abdominal procedure, such as a hysterectomy or abdominal sacral colpopexy, is planned. In addition, the Burch colposuspension was recently shown to be inferior to the autologous pubovaginal sling in a randomized, controlled study (Albo et al, 2007). Although vaginal prolapse does not, per se, cause urinary continence, a deficiency of vaginal support is shared by both conditions. Therefore, urinary incontinence secondary to hypermobility would imply that vaginal prolapse, especially the anterior wall, likely coexists and should be repaired at the same time. Severe vaginal wall prolapse has also been associated with occult urinary incontinence, defined as stress incontinence that only occurs with reduction of the prolapse. Patients with severe vaginal prolapse, with or without complaints of incontinence, should be carefully assessed preoperatively by reduction of the prolapse during the stress incontinence evaluation (see Fig. 75–2). This condition and additional descriptions of these procedures are further discussed in Chapters 71 and 72.
Pubovaginal Slings and Tension-Free Tape Procedures
Autologous fascia pubovaginal slings, popularized by McGuire in the 1980s, were originally used for female patients with poor sphincteric function. Historically, female patients with stress incontinence have been divided into groups based on etiologies of either urethral hypermobility or intrinsic sphincter deficiency (ISD). It has become clearer over time that almost all patients leak due to a combination of hypermobility and intrinsic sphincter deficiency, with each component of incontinence on a continuum. Using this logic, a sling would be the ideal treatment because it successfully corrects both urethral hypermobility and ISD. More recently, pubovaginal slings have been supplanted by a wide variety of minimally invasive tension-free mesh sling procedures. These procedures, although less invasive, have never shown success rates superior to the 90% cure rate of the original pubovaginal slings, which are still considered by most to be the gold standard therapy. Autologous or cadaveric fascial slings are still commonly used in situations where the sling must be pulled more tightly, as with the obstructing slings sometimes used in patients with neurogenic incontinence. Theoretically, mesh slings are “tension- free” slings and therefore would be much more likely to erode into the urethra if pulled tightly. In cases where mesh is preferred, a “spiral” or “circumferential” sling, which wraps completely around the urethra, can be used; however, this requires a urethrolysis to gain access to the retropubic space and passage of the needles from the abdomen to the opposite side of the vagina to gain circumferential compression. This procedure and many others are more fully described in Chapter 73.
Artificial Urinary Sphincter and Male Perineal Sling
The introduction by Scott and coworkers (1974) of the artificial urinary sphincter (AUS) signified a major advancement in the treatment of sphincter-related incontinence. The AUS has been successfully used in the management of stress incontinence of various etiologies and can be implanted in adults and children of both sexes. Postprostatectomy incontinence remains the most common indication for AUS insertion, with female patients accounting for only 1% of the patients in whom the devices are implanted yearly, typically for neurogenic disease (Lee et al, 2009). Although the AUS is commonly used in patients with neurogenic incontinence, it is important to remember that because of the ability of the AUS to greatly increase outlet resistance, social continence may be gained at the expense of renal deterioration in patients with poor detrusor compliance. In general, storage pressures and bladder capacities should be normalized prior to placement of an AUS.
For treatment of PPI, the AUS has been quite successful and remains the gold standard therapy. Nevertheless, revisions may be necessary due to atrophy, infections, erosions, or mechanical problems. In addition, some patients are reluctant to have the AUS placed for a variety of reasons, including the desire to “void normally” without having to manipulate a device. More recently, the male sling has had a resurgence of popularity in the search for a minimally invasive, efficacious, “natural voiding” alternative to treat PPI. The male sling is a passively obstructive device that appears to function by increasing the urethral closure pressure and functional sphincteric length, thereby augmenting existing sphincteric function. This may be why it does not work as well in patients with severe sphincteric incontinence. Male slings are easily placed using a perineal approach and can be fixated using either bone anchors (into the inferior pubic ramus) or a transobturator approach. Although slings are more commonly used in patients with mild to moderate PPI, and the AUS with more severe PPI, high rates of continence and patient satisfaction have been reported using both the male sling and AUS in properly selected patients (Rajpurkar et al, 2005; Gallagher et al, 2007). A more thorough and in-depth discussion of the male sling and artificial urinary sphincter are covered in Chapter 79.
Closure of the Bladder Outlet
Complete closure of the bladder neck is rarely necessary, because a compressive bladder neck sling is more easily performed, is less morbid, and allows transurethral access if necessary. The main indication for bladder outlet closure is urethral destruction secondary to prolonged catheter drainage in neurogenic bladder patients. The clinical course is typically a progressive worsening of leakage around the catheter, requiring progressively larger-bore catheters and larger volumes of fluid in the balloon, ultimately resulting in a dilated, nonfunctional urethra and bladder neck. It is thought that a long-term Foley catheter and balloon can cause pressure necrosis and bladder neck erosion, especially in the setting of recurrent bladder spasms. In these patients, an obstructing sling or AUS is rarely feasible. For patients with some residual normal urethra, Chancellor and colleagues (1994a) reported on the use of a combined “tight” autologous pubovaginal sling and lower urinary tract reconstruction in 14 female patients with urethras destroyed by long-term Foley use. At 24 months, they reported excellent results with minimal incontinence or other complications. They concluded that at least 1 cm of normal urethra was required for proper functioning of the sling. For patients without residual normal urethra, management options include transvaginal, transabdominal, or combined closure of the bladder neck with simultaneous continent or incontinent urinary diversion, incontinent ileovesicostomy, or suprapubic catheter placement.
Feneley (1963) reported on 24 female patients with neurologic disease who underwent transvaginal bladder neck closure and suprapubic tube placement. Overall results were good; however, a vesicovaginal fistula developed in 4 patients, resulting in persistent incontinence. Zimmern and associates (1985) reported on 6 female patients using a transvaginal closure. At 21 months of follow-up, all patients were cured with no fistulas or incontinence reported. Levy and colleagues (1994) reported a 40% success rate using a transvaginal bladder neck closure. They subsequently modified the approach, using a combined transvaginal-transabdominal approach, and reported a 100% success rate at a mean 16-month follow-up for the subsequent 10 patients. Shpall and Ginsberg (2004) reported on 39 patients who underwent a combined transabdominal bladder neck closure and various continent and incontinent diversions. At a mean of 37 months, 6 patients (15%) developed fistulas; however, 4 patients were successfully repaired, for an overall 95% cure rate. Most recently, O’Connor and colleagues (2005) reported on 35 patients with a mean 79-month follow-up who underwent a transabdominal bladder neck closure for refractory incontinence. They were initially successful in 28 (83%) patients with an overall 94% cure rate after one revision.
As illustrated in this historical perspective, the risk of complications, specifically a vesicovaginal fistula, is relatively common and can be difficult to repair. It is important to remember that a bladder neck closure is much more difficult than a simple closure of the bladder wall. The bladder neck is usually hyperactive in patients with neurologic disease, and every voiding reflex includes active opening and closing of the bladder neck, which forcibly attempts to destroy the bladder neck closure. To reduce this risk, postoperative suppression of the voiding reflex using prolonged continuous catheter drainage (3 weeks) and liberal use of anticholinergics is imperative. In addition, to reduce the risk of fistula, the repair must be watertight from the beginning, and this requires a precise mucosal closure using a running suture and multiple additional layers of muscle to reinforce the strength of the repair (see Fig. 75–3). Lastly, a drain should be used to minimize fistula formation should suture line leakage occur. When performed correctly, a bladder neck closure with simultaneous lower urinary tract reconstruction remains an excellent treatment option in those patients with a destroyed bladder neck or urethra.
Bladder Outlet Reconstruction
Sphincteric incontinence with a fixed, nonvolitional, open bladder outlet may be congenital or acquired. In most cases, the sphincteric musculature is insufficient and denervated and therefore does not provide sufficient closure pressure to prevent incontinence. Techniques have been developed that concentrate on increasing urethral resistance by either tightening or lengthening the urethral segment, such as the Kropp and Pippi-Salle procedures (see Chapter 129) or by constructing a mechanism more similar to the native sphincter. In 1919, Hugh Hampton Young first described a technique to reconstruct the bladder outlet to improve sphincteric continence. Originally, the procedure included resecting a portion of bladder neck and reconstructing the bladder outlet with constricting sutures to effectively narrow its caliber. This technique was further modified by Dees (1949) and Leadbetter (1964). In theory, circularly arranged muscle fibers located in close proximity to the bladder neck are used to re-create a functional, tubularized neourethra that replicates the outlet resistance of a normal sphincteric outlet, but with variable volitional control. The Leadbetter modification involves proximal reimplantation of the ureters to allow more extensive tubularization of the trigone and, theoretically, improved outlet resistance. The construction of this neourethra may be formed from tissue of the anterior bladder neck as described by Tanagho (1981), or from the posterior surface of the bladder wall and trigone, according to any of the variations of the Young-Dees-Leadbetter technique. Leadbetter (1985) reported long-term success rates of 60% to 70% in 34 patients; however, the exact definition of success in this paper is unclear. Clinically, these procedures are most useful for patients with a good-capacity, acontractile bladder and who are mentally and physically able to perform CIC on a regular basis. In addition, care should be taken in the setting of poor bladder compliance or significant detrusor overactivity, because these patients commonly need bladder augmentation. For further discussion regarding the original technique described by Young, and its several variations, refer to Chapters 124 and 129.
Myoplasty for Functional Sphincter Reconstruction
The Holy Grail for sphincteric incontinence may well be the replacement of the dysfunctional sphincter with a new, functional native replacement. The first attempt at this type of sphincteric replacement was a skeletal muscle transposition reported by Deming (1926). He used unstimulated gracilis muscle wrapped around the urethra, which reportedly did improve sphincteric continence; however, the unstimulated skeletal muscle required the patient to voluntarily contract (adduct) the leg for prolonged periods to provide outlet resistance. Additional problems with the use of unstimulated graciloplasty include (1) poor sustainability of the contraction due to the fast twitch, non–fatigue-resistant fibers of the gracilis, (2) high passive resistance resulting in urethral obstruction, (3) postsurgical changes resulting in the loss of resting tension and reduced contractility, (4) the potential for fibrosis due to segmental vascularization (Stenzl, 1998). The next improvement in functional sphincteric replacement was the development of external stimulation of the gracilis using implanted electrodes (Janknegt et al, 1995). They developed an electrical stimulation program that was able to transform fatigable type 2 skeletal muscle fibers to the slow type I fibers that were able to sustain a long-lasting contraction. This early report described three patients who achieved “good results” and one patient who became dry at night. Two years later, Chancellor and colleagues (1997) reported their series using a gracilis urethral wrap in five men with neurogenic bladder dysfunction and severe sphincteric stress incontinence. The procedure was considered successful in four patients; however, three required CIC, and two of these patients had persistent stress incontinence.
The shortcomings of using the gracilis was noted by Stenzl (1998), who commented that the electrically stimulated gracilis muscle was an improvement over the unstimulated muscle; however, the bulky musculature and propensity for stricture formation caused an inherent passive obstruction. This shortcoming was scientifically overcome by Palacio and coworkers (1998) in an animal model using a free but innervated flap of well-vascularized proximal gracilis muscle in female dogs. The smaller size of the graft allowed it to be more easily transposed around the urethra while stimulation of the sphincter was carried out using the graft’s own motor innervation. Unfortunately, this apparently successful animal model has never been reported in a human clinical study.
The most recent twist in an attempt to improve the function of a neurologically damaged sphincter includes injecting allogenic muscle–derived progenitor cells (MDPC) directly into the sphincter (Cannon et al, 2003). The authors used a rat model in which the urethra was mechanically denervated and subsequently injected with MDPCs, which dramatically improved the fast-twitch muscle contraction amplitude from 8.8% to 87% (compared with normal amplitude). Although promising, this innovative and minimally invasive option for sphincteric incontinence has not yet been proven in follow-up human clinical studies.
Lower Urinary Tract Reconstruction, Diversion, and Bladder Substitution
![]() See the Expert Consult website for a discussion of the topic.
See the Expert Consult website for a discussion of the topic.
Key Points: Lower Urinary Tract Reconstruction, Diversion, and Bladder Substitution
After conservative measures have failed, patients with refractory storage problems may consider more invasive surgical options, such as bladder augmentation or urinary diversion. The common goal of these procedures is to maintain a low system or reservoir pressure, thereby preserving renal function, reducing infection risks, and improving continence. Although advances in experimental treatments, such as the artificial or tissue-engineered bladder, make these procedures potential future options. Currently, augmentation cystoplasty and diversions continue to play the most important role in lower urinary tract reconstruction.
Augmentation Cystoplasty
Bladder augmentation offers the best chance to construct a “normal” urinary system and, as such, should be considered before attempting urinary diversion. The ability to void without self- catheterization is not guaranteed, especially in men; therefore, this procedure should only be considered for patients who have the ability and motivation to perform CIC, because bladder rupture can be a life-threatening complication. CIC performed by a caretaker is rarely adequate and makes the patient dependent on a caretaker. If the urethra is not a reliable conduit—due to urethral stricture, or access to the native urethra is limited by spasticity, obesity, or contracture—a continent abdominal stoma or alternate procedure should be considered.
Originally, augmentation cystoplasty was used for the treatment of bladder fibrosis secondary to tuberculous cystitis (Smith et al, 1977). More recently, augmentation has been used for the treatment of other conditions associated with bladder fibrosis and limited bladder capacity, such as in patients with end-stage interstitial cystitis, neurogenic bladder or, less often, patients who have undergone pelvic radiation. Neurogenic bladder patients are a special group because they often have hydronephrosis, vesicoureteral reflux secondary to chronic elevated storage, and voiding pressures due to poor detrusor compliance and detrusor sphincter dyssynergia. Augmentation cystoplasty in this group can preserve renal function, whereas typically it serves to improve the quality of life in the other groups. Augmentation should also be considered for patients with incontinence secondary to intractable neurogenic detrusor overactivity (Linsenmeyer et al, 2006). Because no effective external collecting device exists for females, bladder augmentation has been used in female paraplegics, with encouraging results (Venn and Mundy, 1998), to treat incontinence due to poor compliance or neurogenic detrusor overactivity. Although augmentations are invasive, the value of these procedures cannot be discounted, because they are generally very effective, with significant improvement reported in up to 90% of patients with neurogenic lower urinary tract dysfunction or limited-capacity bladders due to other causes (Barrett, 1999).
Ureterocystoplasty and autoaugmentation are alternatives to conventional bladder augmentation. Autoaugmentation, as it was initially described by Cartwright and Snow (1989), involves excising the detrusor muscle over the bladder dome and leaving the urothelial mucosa intact. Since the original description in 1989, several modifications have been used. A review by Gurocak and associates (2007) summarizes these modifications and provides a good review of the ongoing research with the autoaugmentation procedure. The results after autoaugmentation are less favorable and of shorter duration than after conventional enterocystoplasty; however, some patients may benefit by avoiding the use of intestinal segments and from the lower morbidity associated with this procedure. In most cases, detrusor compliance can be reliably improved; however, the overall increase in bladder capacity is usually nominal. Patients most likely to benefit are those with poor compliance with a reasonable bladder capacity, usually over 200 mL. The specific issues regarding the type of procedure to be performed (enterocystoplasty, autoaugmentation, or ureterocystoplasty), the amount (if any) of bladder to be removed in different disease states, the effect of the loss of the bowel segment on the individual patient’s physiology, and the question of whether to perform a simultaneous procedure to increase the resistance of the bladder outlet is described more fully in Chapter 129.
Urinary Diversion
Supravesical diversion is a treatment modality common to disorders of both storage and emptying failure. Although it has been commonly used in the past for the treatment of neurogenic voiding dysfunction, it is now rarely indicated in any patient with voiding dysfunction alone. Indications may include (1) progressive, medically refractory hydronephrosis, commonly caused by obstruction at the ureterovesical junction from a thickened bladder or by vesicoureteral reflux; (2) recurrent episodes of urosepsis; and (3) intractable storage or emptying failure when CIC is impossible.
Both patient- and disease-related factors should be considered when deciding the type of urinary diversion to perform. The healthy, 60-year-old male with muscle-invasive bladder cancer clearly has more options for reconstruction than a bedridden spinal cord–injured patient. In addition, the decision to perform a continent or incontinent diversion is often fraught with physician bias and emotional preconception on the part of the patient. Continent reconstruction has been shown in some studies to result in a high level of patient satisfaction and improved quality of life in select patients (Zommick et al, 2003); however, the generalized notion that continent diversion is superior to any other form of urinary diversion has not been supported in the literature (Porter and Penson, 2005). Other potential complications include neobladder rupture and increased risk of upper tract deterioration secondary to high-pressure storage, with or without vesicoureteral reflux. In some cases, a continent orthotopic diversion may be considered; however, this procedure should rarely ever be performed in the neurologically impaired patient but is a reasonable option in patients who desire to avoid CIC, although normal voiding is not guaranteed.
The incontinent ileovesicostomy or “bladder chimney” is an excellent method for managing patients with neurogenic bladder who are unable to perform CIC independently (Mutchnik et al 1997; Leng et al 1999). This procedure avoids the complications of long-term indwelling catheters and maintains the native antireflux and urethral sphincteric mechanisms. In addition, this procedure may be reversed if necessary.
Overall, with all the possible choices for a diversion, an ileal conduit is still the most commonly performed. Most urologists are very familiar with this procedure and a conduit gives the patient the best chance of maintaining a system with low pressure while providing a reliable method of urine collection.
Perhaps one of the most controversial issues is what to do with the bladder that remains in situ. Pyocystis remains a potentially fatal condition, especially in the spinal cord–injured population. For in-depth discussions regarding patient selection, technique, postoperative care, and complications of urinary diversions refer to Chapters 85, 86, and 87.
The Artificial Bladder and Tissue Engineering
Prosthetic organs have been successful in many regions of the body, and it would seem to be a simple matter to replace a biologic reservoir such as urinary bladder with a mechanical storage/emptying device. Unfortunately, despite numerous attempts over the past 50 years, we are still far from achieving this goal. Desgrandchamps and Griffith (1999) described the overall goals to include providing adequate storage with complete volitional evacuation of urine while preserving renal function. The structure of the artificial bladder must be biocompatible and resistant to urinary encrustation and tolerant to bacterial infection. There have been numerous alloplastic materials (nonbiologicals) considered for such use, along with the consideration of various (mostly failed) design concepts for total artificial bladder replacement. The desirable qualities for future designs would include:
Presently, incorporation of intestinal segments in urologic reconstruction will continue to serve an important role, but the benefits obtained with their use must be weighed against the associated physiologic effects and complications that accompany those procedures. Further advances in the development of tissue-engineered bladders will likely make them a potential treatment option in the near future. As progress continues in the development of this new technology, it is fascinating to realize the implications it might have regarding our current methods of reconstruction. The processes involved in tissue engineering may include selective cell transplantation, expansion in culture, attachment to a support matrix, and reimplantation after expansion. These topics as well as other options in bladder substitution are discussed in Chapter 19.
Catheterization
Catheterization, in its many forms, is an effective method of bladder emptying, and is a useful adjunct when efforts to increase intravesical pressure and/or decrease outlet resistance have been unsuccessful. In addition, for those patients who have filling/storage failure caused by bladder overactivity and/or sphincteric incontinence, catheterization may also be used if the dysfunction can be converted solely or primarily to one of emptying by nonsurgical or surgical means (Wein and Barrett, 1988). The common goals, irrespective of the mode of catheterization, are to provide low-pressure storage, preserve continence, avoid renal deterioration, minimize complications, and maintain quality of life.
Indwelling urethral catheters are generally used for short-term bladder drainage, and careful use of a small-bore catheter for a short time is unlikely to adversely affect the ultimate outcome, especially if used in the initial bladder management in SCI (Lloyd et al, 1986). Long-term bladder drainage may be obtained by intermittent catheterization, or by an indwelling suprapubic or urethral catheter. Historically, the most appropriate form of bladder drainage in patients requiring prolonged bladder management has been debated. Most studies evaluating the different forms of long-term bladder management have been retrospective and in patients with SCI, because they represent the majority of patients requiring long-term catheterization. The main area of controversy concerns whether long-term indwelling catheterization in neurologically impaired patients is associated with an inferior outcome compared to clean intermittent catheterization, specifically regarding urinary tract complications or quality of life.
Although CIC is currently the preferred management for patients requiring prolonged bladder drainage, it is a recent innovation. Intermittent catheterization was first introduced as a sterile procedure in 1949 by Guttman and, at the time, challenged the beliefs of most urologists (Guttman, 1949; Guttman and Frankel, 1966). It was not until Lapides and colleagues (1972) introduced the concept of clean intermittent catheterization that widespread usage became more common. As the popularity of CIC grew, long-term indwelling catheterization was condemned, based on both infectious risks and a perceived increased risk of other urologic complications. Jacobs and Kaufman (1978) reported that there were more renal and other urologic complications with long-term (>10 years) catheterization use than with short-term use. Hackler (1982) also reported accelerated renal deterioration in patients with SCI managed with long-term suprapubic catheterization. McGuire and Savastano (1984) reported a poorer outcome in women with an indwelling urethral catheter than in those on CIC after 2 to 12 years. Of 13 in the catheter group, 54% had adverse changes on intravenous pyelography, as opposed to 0% in the CIC group. Other urologic complications were also more frequent and severe in the catheter group.
Conversely, more recent investigations have suggested the complications from chronic indwelling catheters may be lower than previously thought. Sekar and coworkers (1997) reported on the effect of different bladder management methods in 1114 patients with SCI using total and individual kidney-effective renal plasma flow as the primary outcome measure. Follow-up was relatively long with 51.3% followed 0 to 3 years, 40% at least 5 years, and 20% for at least 10 years. Unfortunately, many issues, such as unclear methods of urinary management at discharge, incomplete data on approximately 200 patients, and the fact that most men who were discharged using CIC later changed to condom catheter drainage, weaken the authors’ conclusions that there was very little change in renal function over time in patients using different bladder management methods. Dewire and associates (1992) reviewed the course of 32 quadriplegic patients managed with, and 25 without, an indwelling catheter. The groups were roughly comparable, and follow-up was for 10 years or longer. The incidences of upper and lower urinary tract complications and renal deterioration were not significantly different. Chao and coworkers (1993) did a similar review on 32 patients with SCI with an indwelling urethral (14 patients) or suprapubic catheter (18 patients) versus 41 patients without. Follow-up was 20 years or longer. Although the catheterized group had a higher prevalence of upper tract scarring and caliectasis, no significant differences were found in other indices of renal function or in the prevalence of other urologic complications. Jackson and DeVivo (1992) reported on the results of indwelling catheterization in 108 women (with SCI) followed for 2 to 5 years (56 women), 6 to 9 years (31 women), and 10 or more years (21 women) after injury. Compared with the male population, the majority being managed by condom drainage, there was no difference in upper or lower tract complications. MacDiarmid and colleagues (1995) reported on suprapubic catheterization in 44 patients with SCI, with follow-up ranging from 12 to 150 months (mean, 58 months). They reported that no patient had renal deterioration or vesicoureteral reflux and that the incidences of incontinence, infection, and calculi were acceptable. Eleven percent of the patients had leakage, 100% had bacteriuria, 41% developed bladder calculi, and 7% developed renal calculi. Thirty-six percent of patients developed episodes of catheter blockage, and gross hematuria resulting in hospitalization occurred in 5%.
Upper tract and infectious complications often occur regardless of whether intermittent or indwelling catheterization is used. Although bacteruria is common in all forms of bladder catheterization, symptomatic infection is not. The presence of asymptomatic bacteruria does not usually require treatment and should be distinguished from an invasive, symptomatic urinary tract infection (UTI). Treatment of asymptomatic bacteruria has not proven beneficial, and the use of continuous prophylactic antibiotics is rarely indicated (Gribble and Puterman, 1993). Nevertheless, infectious complications are the most common complications associated with prolonged catheterization, and procedures such as frequent catheter changes should be considered to reduce these complications (Weld and Dmochowski, 2000; Wyndaele, 2002). Much interest has recently been generated with catheter-associated infections in hospitalized patients. Under new rules by the Centers of Medicare and Medicaid Services, hospitals will not be compensated for catheter-associated UTIs, causing many hospitals to intensify their efforts in implementing preventive measures (Saint et al, 2009). For more information regarding bacteruria and catheter-associated UTI see Chapter 10.
More recent studies have shown the superiority of CIC over long-term indwelling catheter drainage. Weld and Dmochowski (2000) reported a retrospective review of 316 patients with SCI with a mean follow-up of 18.3 years. Bladder management methods included chronic urethral catheterization in 114 patients, CIC in 92, spontaneous voiding in 74, and suprapubic catheterization in 36. Complications were recorded in terms of infectious complications (epididymitis and pyelonephritis), renal and bladder calculi, urethral complications (stricture and periurethral abscess), and radiographic abnormalities (vesicoureteral reflux and abnormal urographic findings). Overall, there were 398 complications recorded, of which 236 developed in 61 patients (53.5%) on chronic urethral catheterization, 48 in 16 patients (44.4%) on suprapubic catheterization, 57 in 24 patients (32.4%) who voided spontaneously, and 57 in 25 patients (27.2%) on CIC. Separate bar graphs for each type of complication seem to confirm the overall superiority of CIC as the least problematic long-term form of bladder management (Fig. 75–4).
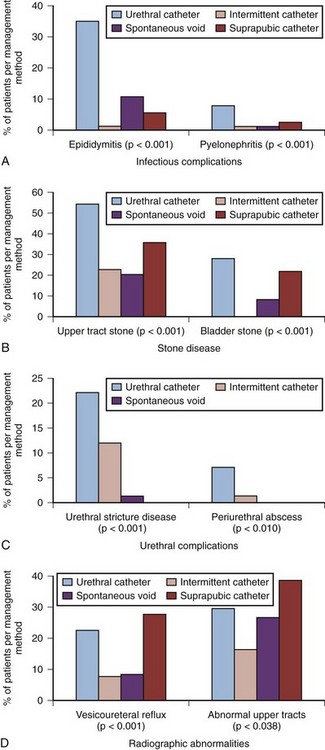
Figure 75–4 Complications related to specific bladder management methods: infectious (A); stone disease (B); urethral (C); radiographic abnormalities (D).
(Modified from Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol 2000;163:768–72.)
The exact etiology of upper tract deterioration in patients with long-term indwelling catheters is unclear, because the bladder should be well drained by a catheter; however, it is likely related to chronic “occult” or subclinical detrusor overactivity in the face of sphincteric dyssynergy providing a functional obstruction. Regardless of the etiology, it is clinically heralded by the development of poor detrusor compliance demonstrated on urodynamic studies. Weld and colleagues (2000) reported the effects of CIC versus indwelling urethral catheterization on bladder compliance in patients with SCI. Logistical regression analysis of compliance versus bladder management and interval because injury revealed that CIC and spontaneous voiding were associated more with normal compliance than indwelling urethral catheterization. Poor compliance was statistically associated with vesicoureteral reflux, radiographic upper tract abnormalities, clinical pyelonephritis, and upper tract calculi (Fig. 75–5). Jamil and associates (1999) reported on ambulatory urodynamics in 30 patients with SCI whose bladders were managed with an indwelling urethral catheter. They found that freely draining indwelling catheters did not guarantee consistently low intravesical pressure. Eleven of 30 patients demonstrated intermittent detrusor contractions causing intravesical pressure increases greater than 40 cm H2O for up to 4.5 minutes. These patients had used an indwelling catheter for a mean of 14.3 years (range, 4 to 36 years). Renal scarring was observed in 9 patients, and, of these, 6 were in the group with the abnormal bladder contractions, whereas only 5 of 21 patients with normal kidneys had such pressure rises. The clinical correlate emphasized by the authors was their belief that maintenance of a compliant bladder and suppression of high-pressure contractions in chronically catheterized patients may play a role in the prevention of renal deterioration. Kim (1997) demonstrated in a retrospective analysis that anticholinergic medications can reduce the incidence of hydronephrosis, improve bladder compliance, as well as decrease leak point pressures in patients with chronic catheters. The role of anticholinergics with various forms of prolonged bladder management and in the prevention of upper tract complications has not yet been clarified (Feifer, 2008).
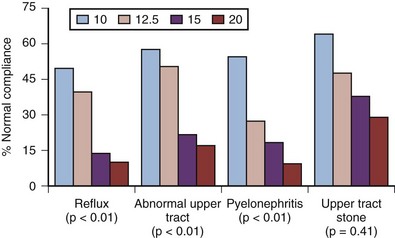
Figure 75–5 Incidence of patients with upper tract complications who had normal bladder compliance at various threshold values.
(From Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol 2000;163:1228–33.)
There is certainly some controversy about the classic teaching that long-term continuous bladder catheterization in patients with neurogenic bladder dysfunction should be avoided at all costs. There are clearly some situations in which such management is desirable and necessary. Most of the studies that purport to compare methods of management regarding lower and upper tract complications are often flawed and prevent total acceptance of their conclusions. In the absence of a prospective, randomized study or acceptable retrospective data, patient and family comfort, convenience, and quality of life must be strongly considered in the decision of which method of catheterization will be employed. Regardless of which method is used, periodic upper and lower tract evaluation using renal ultrasonography and cystoscopy should be considered in all patients who require prolonged bladder management. Lastly, the use of urodynamics to monitor the bladder in neurologically impaired individuals is controversial. At a minimum, we believe that urodynamics should be performed after the initial neurologic injury is stable and whenever any significant changes in continence or voiding function occur. The initial urodynamics is useful to direct early bladder management, while subsequent urodynamics evaluations are performed to determine if lower urinary tract changes, such as the development of dyssynergia, have occurred.
Clean Intermittent Catheterization (CIC)
CIC has proved to be the most effective and practical means of attaining a catheter-free state in the majority of patients with acute spinal cord lesions. CIC has revolutionized the treatment of difficult cases of neuromuscular dysfunction of the lower urinary tract by providing a safe and effective method that preserves the independence of the patient to empty the lower urinary tract in cases in which continence has been achieved pharmacologically or surgically, producing total or partial urinary retention. For example, without CIC, the successful outcomes achieved using augmentation cystoplasty or continent urinary diversion would have never been achieved. CIC is based on a theory proposed by Lapides that high intravesical pressure or bladder overdistention is primarily responsible for the development of UTI, not the bacteruria itself. Theoretically, reduced blood flow to the bladder can lead to increased host susceptibility to bacterial invasion and UTI. Bacteria introduced by CIC would be neutralized by the host, and relatively sterile urine would be maintained as long as bladder distention and high intraluminal pressures were avoided. The long-term efficacy and safety of such a program has been demonstrated by Lapides and others (Weld and Dmochowski, 2000).
Whereas indwelling catheter drainage requires little input from the patient, a cooperative, well-motivated patient or family is a requirement for CIC. The patient must have adequate hand control, or a family member must be willing to perform the catheterization. In addition, there must be adequate urethral exposure. Graham (1989) reported on the factors required to successfully develop a catheterization program for patients with functional limitations, which commonly exists in patients with neurogenic bladder dysfunction. It is advantageous to have a dedicated nurse who instructs the patients and families in the catheterization regimen; provides them with understandable written instructions to refresh their memory regarding technique, precautions, and danger signals; and provides continuing support for patients and families who call with questions or problems regarding their regimen. Many patients are initially reluctant to perform any procedure on their own genitalia. Patients need a thorough explanation of the advantages of CIC along with assurances that it is simple and that it will not tie them to their houses or to an absolute time schedule. Additionally, proper selection of equipment for the patient’s intelligence and financial level will increase patient acceptance of and compliance with a self-catheterization program. Patients who are reticent initially are continually amazed by the ease with which such a regimen is established. Successful CIC is intimately associated with patient compliance, and therefore patients should be monitored periodically to ensure catheterization is performed properly. Of note, CIC should be used cautiously in patients known to have autonomic dysreflexia.
Intermittent catheterization may be performed by clean, aseptic, or sterile techniques (Hudson and Murahata, 2005). Clean intermittent catheterization often includes reusing a catheter several times before disposal. It is washed, generally with soap and water, and allowed to air dry prior to storage. When reusing catheters, some have advocated boiling or microwaving for sterilization (Douglas et al, 1990). In April 2008, in an attempt to decrease the incidence of UTIs in patients performing intermittent catheterization, Medicare policy regarding intermittent catheterization changed. Any patient requiring intermittent catheterization may obtain up to 200 sterile catheters per month for one-time use. This change in policy has negated the need for reusing catheters in most patients, but it still may be necessary in patients without insurance coverage or other financial limitations. For adult patients, catheterization is typically performed at a minimum of every 4 to 6 hours to minimize bacterial dwell time. CIC may need to be more frequent if large volumes of fluid are ingested. In most cases, catheterizations should be timed to maintain bladder volumes below the normal 400- to 500-mL capacity to minimize bladder wall pressure. Even smaller volumes may be required if they have poor detrusor compliance. Catheter choice is variable, but a 12- to 16-Fr soft catheter may be used for males and short (6-inch “female”) 12- to 16-Fr catheters for females. Note that rigid catheters have the potential to injure the urethra in insensate males because they may not “make the bend” at the prostate, causing a false passage such that CIC may be quite difficult or impossible. Larger catheters may be required in patients with a prior bowel augmentation or those who require bladder irrigation. In men, hydrophilic coated catheters have been found to reduce the incidence of UTI and hematuria and have higher patient satisfaction rates than conventional plastic catheters (Vapnek et al, 2003; De Ridder et al, 2005). Patients with recurrent UTIs, despite the use of single-use sterile catheters, may obtain sterile catheter kits for sterile intermittent catheterization (IC). Anticholinergic medication should be considered when urine leakage occurs between catheterization intervals or if high storage pressures develop. Wyndaele (2002) reported that trauma from catheterization occurs frequently, but effects are usually not long standing, and urethral stricture and false passages are more common the longer that CIC is employed.
Continuous Catheterization
Long-term indwelling catheters should be considered when anatomical, functional, or familial limitations prohibit performance of intermittent catheterization. Continuous catheterization may also be indicated in patients with complications from persistent incontinence or autonomic dysreflexia, despite therapy or when a small bladder capacity prohibits effective CIC. Long-term catheterization may be accomplished by either a urethral or suprapubic catheter. The basics of urethral catheterization, including the technique of suprapubic tube (SPT) insertion are covered in Chapter 7.
In males, the benefits of an indwelling SPT over urethral catheterization include a lower incidence of epididymitis and urethral stricture disease (Weld and Dmochowski, 2000) and preserved sexual function (Rutkowski et al, 1995) (see Fig. 75–4). Overall, suprapubic catheters have been associated with high rates of patient satisfaction. Barnes and associates (1993) concluded that long-term suprapubic catheters were well tolerated by patients with neuropathic bladders. Based on the replies of 32 patients who expressed an opinion, 84% were satisfied; however, the follow-up was short (mean 23 months), and in 2 of 12 patients assessable at over 2 years, creatinine levels increased. Other problems occurred, including recurrent catheter blockage in 38%, recurrent symptomatic urinary infections in 23%, and displaced catheters requiring reinsertion in the operating room in 15%. Urethral leakage occurred in 8 of 14 females with a suprapubic catheter alone and in 6 of 16 males. Patients using a suprapubic catheter must be warned that urinary incontinence may commence or worsen in the setting of sphincteric incontinence or reflex detrusor overactivity, because active opening of the sphincteric unit will occur in the absence of dyssynergia. Sheriff and associates (1998) discussed the clinical outcome in a satisfaction survey of 185 patients with neuropathic bladder dysfunction treated with long-term suprapubic catheterization (follow-up, 3 to 68 months; mean, 24 months). The authors reported an 82% satisfaction rate; however, the main reason for this procedure was failed CIC from poor hand function. In addition, only 103 of the 185 patients filled out the satisfaction questionnaire, and only 8 patients had a suprapubic catheter for longer than 2 years. Complications in this group included 5 patients with a small-bowel injury during insertion, 2 developing significant hemorrhage, 2 requiring catheter repositioning, 1 requiring reinsertion because of dislodgement, 8 with persistent incontinence, and 18% with recurrent catheter blockage. Bacteriuria existed in 98% of the patients, but recurrent symptomatic infection occurred in only 4%. More recently, Ahluwalia and colleagues (2006) reported a satisfaction rating of 71% among 219 patients having indwelling suprapubic catheters for greater than 50 months.
A controversial issue, common to all long-term indwelling catheters, is the development of bladder cancer. The long-term risk of carcinoma in the SCI patient with a chronic catheter has been estimated to be 8% to 10% (Locke et al, 1985; Delnay et al, 1999). Kaufman and colleagues (1977) were among the first to recognize that patients with SCI and chronic indwelling catheters had an increased incidence of bladder cancer, particularly squamous cell carcinoma (SCC). Several others have reported similar findings (Chao et al, 1993; Stonehill et al, 1996). Since this report, the association between chronic indwelling catheterization and the development of bladder carcinoma has been debated. No associations with intermittent catheterization have been identified. Chronic inflammation is the most likely causative factor and may be caused by the indwelling catheter itself, bladder calculi, and/or recurrent infections. It is not clear whether earlier treatment of these conditions or better follow-up will lessen the risk for bladder carcinoma. A mechanism for the development of bladder carcinoma secondary to long-term inflammation was described by Wall and colleagues (2001). According to their report, inducible nitric oxide synthase expressed by inflammatory macrophages in areas of chronic inflammation may lead to the formation of potentially carcinogenic nitrosamines in the bladder. For more on the development of carcinoma and the associations with spinal cord injury and chronic catheterization, see Chapter 65.
When SCC is identified in SCI patients, it is often advanced and commonly fatal; however, routine screening cystoscopy for SCI patients at risk is controversial. Although some authors suggest that screening cystoscopy conveys a survival advantage by identifying bladder cancer at an earlier stage (Navon et al, 1997), others have found no advantage for screening (Yang and Clowers, 1999; Hamid et al, 2003). Cytology, although commonly obtained, is not useful for detecting squamous cell carcinoma (Bejany et al, 1987). Additionally, SCC may not always be cystoscopically detectable (Kaufman et al, 1977). For this reason, routine random biopsies have been advocated by some (Stonehill et al, 1996). Despite the lack of data in non-SCI patients, most urologists recommend all patients with chronic catheters undergo annual screening, generally starting after 8 years (Stonehill et al, 1996). Gross hematuria has been reported by several investigators as the most common presenting symptom for patients eventually diagnosed with bladder cancer (Hess et al, 2003). Despite the disparate opinions regarding screening, certainly all would agree that patients with new-onset gross hematuria should be evaluated with upper tract imaging, urinary cytology, cystoscopy, and perhaps random biopsies. Other patients to consider for close surveillance should include patients with known risk factors for the development of carcinoma, such as recurrent UTIs or recurrent bladder stones.
Key Points: Catheterization
Urinary Collection Devices and Continence Products
![]() See the Expert Consult website for a discussion of the topic.
See the Expert Consult website for a discussion of the topic.
Key Points: Urinary Collection Devices and Continence Products
For some patients, a collection device or pad may be their first consideration, because they may be unwilling or unable to pursue other modes of therapy. For others, it may be their last resort, having persistent urinary incontinence despite having undergone multiple anti-incontinence treatments. Whether these products are used temporarily, as is often the case in post-prostatectomy incontinence, or as a long-term solution in the cognitively impaired nursing home patient, the aim is the same: to minimize, control, and conceal urine leakage. It must be made clear to the patient that these devices are simply management strategies, not treatments, and, as such, are unlikely to yield uniformly satisfactory results.
External Collecting Devices
Despite the numerous patents for prospective female collection devices, no device has yet to perform effectively enough to distinguish itself as a reliable method of urine collection. Problems in application and failure of leak-proof collection have often limited the success and widespread use of these devices. As a result, females must often make due with pads, diapers, or other absorbent products. On the contrary, external collection devices for the male—the condom catheter, penile sheath or Texas catheter—are generally successful regarding urine collection. Unfortunately, they are also unacceptable to some patients because the device is visible (sometimes through their clothing) and due to odor issues secondary to leakage of often foul-smelling urine. Condom catheters are available in various sizes, and some include a measuring guide to assist in catheter selection. The inappropriate sizing of the condom catheter is often responsible for dislodgment and leakage of these catheters.
Condom catheters are generally perceived as more comfortable, less painful, and less restrictive on daily activities than indwelling catheters (Saint et al, 2006). Saint and colleagues (2006) reported the results of a prospective, randomized, controlled trial of 75 hospitalized men requiring a urinary collection device. In this study, condom catheters were associated with a lower incidence of complications, including bacteruria, symptomatic urinary tract infection, and death, than with use of indwelling catheters. It should be emphasized that despite the reduced incidence of these adverse outcomes, the use of external collection devices and absorbent products is associated with a higher risk for UTIs compared with cases where no appliances are used (Sturman et al, 1989). It is generally recommended that condom catheters be changed daily, because the risk for UTI is increased when catheters are changed less frequently (Waites et al, 1993; Zimakoff et al, 1996). The use of this device is not free of complications; it can cause allergic reactions, skin maceration, and/or penile edema. Furthermore, the penile skin and glans should be examined at every catheter change to ensure no skin breakdown or contact reactions have occurred (Newman, 1999). These devices have the potential to cause pressure necrosis of the penis and, when severe, may even damage the urethra (Golji, 1981). Pressure-related complications are more likely to occur in patients with impaired sensation, such as those with neurogenic lower urinary tract dysfunction (Golji, 1981). The authors suggest that many of these problems may be due to the hard ring on the condom catheter. Maintaining an external urinary collecting device can also be a major problem for patients with SCI. The inability to maintain a device during a vigorous voiding contraction, inadequate penile length (often due to obesity), or recurrent lacerations of the penile skin may dictate temporary use of an indwelling catheter. Van Arsdalen and coworkers (1981) described the use of a noninflatable penile prosthesis in this type of patient, because it increases the ease of applying and maintaining such a common catheter. This is also advantageous for those patients who are interested in a prosthesis for reasons of potency. Initial studies reported up to a 25% penile implant loss in these patients; however, more recent data have shown much lower rates of implant loss. Zermann and associates (2006) reported on the long-term follow-up of penile prosthetic surgery for neurologically impaired patients. Indications for implantation included erectile dysfunction, urinary management, or both. After a mean follow-up of 7.2 years, the overall infection rate was 5%, and the rate of explantation was 7.7%. This is not much higher than the explantation rate in non-neurologic patients. In addition, the rate of perforation was different depending on the model used, with the semirigid device having the highest occurrence (18.1%), the self-contained inflatable 2.4%, and 0% for the three-piece inflatable. The authors attribute these improvements to changes in antibiotic prophylaxis and the introduction of new softer implant materials. For similar problems resulting from a “receding phallus,” Binard and colleagues (1993) described a penoplasty procedure that basically lengthens the shortened penis. Early results are encouraging; however, no long-term results of this procedure have been reported.
Absorbent Products
Patients with incontinence are more likely to suffer from depression or anxiety (Shaw, 2001) and may adjust their lifestyle by limiting social activities for fear of odor, discovery, and embarrassment. Eventually, many patients will initially manage their urine leakage by wearing absorbent products. It is not known how many patients use this method to control incontinence, but consumers spend billions of dollars annually on these products (Getliffe et al, 2007; Erekson et al, 2008). Depending on the type of product used, the average patient may spend $40 to $150 a month, none of which is covered by Medicare, HMOs, or insurance companies (Newman, 1999). Consumers may be overwhelmed by the numerous brands and types of products available; options include pads, shields, drip collectors, guards, undergarments, briefs, diapers, or underpads. Further, products may be disposable or reusable. For the majority of patients, selection is often a matter of trial and error and is based on personal opinion, manufacturers’ claims, convenience of the caregiver, or product cost ( Baker and Norton, 1996; Fantl et al, 1996). Very few studies are available that provide evidence-based recommendations for any specific brand or type of product, and therefore choosing the most appropriate absorbent product is not a simple matter. The ideal substance for absorptive devices is one that is highly permeable and absorbent. Generally, a layer of hydrophobic material is immediately next to the patient’s skin. Through this layer, urine passes into an absorbent pad, which is, in turn, surrounded by a waterproof material to keep clothing dry. Ideally, the hydrophobic material next to the skin keeps the patient relatively dry and reduces chafing as much as possible. There is no standardization in the absorbent capacity or quality of materials used to construct these products (Newman et al, 2004). Also, several factors may influence a patient’s satisfaction, such as shape or contour of the pad, their capacity and speed of absorbency, amount of core fluff, fit, and comfort when dry or wet (Newman et al, 2004). Erekson and colleagues (2008) examined the difference in cost and performance of brand name and generic incontinence pads. The authors concluded that brand name products generally cost more, but often perform better than generic products. They also found that neither the cost nor the size of the pad significantly affects product performance. The authors acknowledge the limitations of the study, stating that pad performance was not always reliable and that it may be attributed to errors in measurement or product inconsistencies. To guide patient selection, Fader and associates (2007, 2008) compared the performance and cost-effectiveness of different designs of absorbent products. The authors concluded that although there are significant differences between designs of absorbent products, there is considerable individual variability in preferences. The authors also suggest that, depending on the degree of the patient’s incontinence, some designs may function better than others. Selection of the ideal absorbent product continues to be inadequately assessed and studied. Only recently have researchers started to investigate the factors that influence patients’ selections. In addition, results from any study must be interpreted in the context of the population that was evaluated, making widespread recommendations to the wide variety of patients with incontinence difficult. Indeed, as reported by Fader and associates (2008), there is no single-best design that is significantly better than all other designs and applicable to all users.
Often recognized as the authority in the management of the incontinent patient, urologists should acknowledge the basic nursing principles and skin care recommendations that may significantly contribute to patient care. Absorbent products should be changed frequently to help avoid buildup of odor and to limit the exposure of the skin to urine. Prolonged exposure of the skin to a wet environment may lead to supersaturation and disruption of the skin’s protective barriers, promoting skin maceration, dermatitis, and possibly infection. Incontinence-associated dermatitis (IAD) can be defined as inflammation of the surface of the skin with redness, edema, and, in some cases, bullae containing clear exudate (Gray et al, 2007). IAD predominately occurs in skin folds and may promote candidiasis or bacterial skin infections. Gray and associates (2007) published an excellent review of the epidemiology, pathophysiology, and management of IAD with recommendations for prevention and treatment of incontinence-associated dermatitis.
Further information regarding the types of absorbent products available for males and females, including a review of skin care management, external collection, and urethral compression devices can be found in the book by Newman and Wein (2009) and the 2008-to-2009 catalogue available from the National Association for Continence (2004) (http://www.nafc.org).
Methods to Improve Bladder Emptying
Low flow rates and elevated residual volumes are often signs of a bladder that fails to empty. Symptoms are less specific in identifying the underlying abnormality but may include incomplete emptying, straining to urinate, and decreased force of stream. Patients with emptying failure are prone to bladder stones, UTI, and urinary retention and, if not addressed, may lead to renal deterioration. Methods to improve bladder emptying consist of two main strategies: increasing intravesical pressure and decreasing outlet resistance. Unfortunately, management of poor detrusor contractility is limited and has not been overwhelmingly successful, obliging most patients to rely on chronic catheterization. Attempts to alter the contractility of the bladder through pharmacotherapy or electrical stimulation have also been described and are detailed in Chapters 68 and 70, respectively.
Conversely, methods of decreasing outlet resistance due to obstruction are more straightforward, more successful, and are thoroughly discussed in several chapters. Pharmacologic measures have been used to decrease outlet resistance both at sites of anatomic and sphincteric obstruction and are reviewed in Chapters 92 and 68. Chapter 36 outlines the management of urethral stricture disease; prostatic obstruction is discussed in Chapters 93 and 94. For patients with intractable emptying failure and inability to catheterize, urinary diversion may be considered (see earlier discussion in this chapter). In the following sections, we discuss further management options to improve bladder emptying. Some of these modalities are well known and established, while others are less familiar.
Increasing Intravesical Pressure or Facilitating Bladder Contractility
External Compression (Credé) and Valsalva Maneuver
The Credé maneuver (manual compression of the bladder) is most effective in patients with decreased bladder tone who can generate an intravesical pressure greater than 50 cm H2O and have decreased bladder outlet resistance (Wein and Barrett, 1988). The technique of voiding by the openhanded Credé method involves placement of the thumbs of each hand over the area of the anterior superior iliac spines and the digits over the suprapubic area, with slight overlap at the fingertips. The slightly overlapped digits are pressed into the lower abdomen and when they are located behind the symphysis, are pressed downward to compress the fundus of the bladder. For some patients, this maneuver can be accomplished more efficiently by using a closed fist of one hand or using a rolled-up towel. Straining as the Credé maneuver is applied is generally counterproductive because this increases intra-abdominal pressure and causes bulging of the abdominal wall, which then tends to lift the compressing hands off the fundus of the bladder. If the guarding reflex arc is intact, this may also produce a striated sphincter contraction. The Credé maneuver is much easier in a patient with a lax, lean abdominal wall than in one with a taut or obese one, and it is more readily performed in a child than an adult.
A similar increase in intravesical pressure may be achieved by abdominal straining (Valsalva maneuver). The proper technique involves sitting and letting the abdomen protrude forward on the thighs. During straining in this position, hugging of the knees and legs may be advantageous to prevent any bulging of the abdomen. To increase intravesical pressure in this manner requires voluntary control of the abdominal wall and diaphragmatic muscles.
“Voiding” by the Credé or Valsalva maneuver is generally discouraged, because it is nonphysiologic and is resisted by the same forces that normally resist stress incontinence. Reflex sphincteric opening of the bladder outlet does not occur with external compression maneuvers of any kind, and, in many cases, an increase in outlet resistance may occur reflexively. If adequate emptying does not occur in the properly selected patient, other types of therapy to decrease outlet resistance may be considered; however, these treatments may adversely affect urinary continence.
The best chance of success with this mode of therapy (some would say it should never be used) is in the patient with an areflexic bladder and some degree of outlet denervation. This is most commonly seen in the patient with an autonomous neurogenic bladder (Lapides classification), T11 through L2 spinal cord injury where the sympathetic nervous supply is damaged, or after outlet resistance is decreased surgically or by botulinum toxin injection. Most of these patients already have stress incontinence, and the Credé or Valsalva maneuver simply helps the patient overcome outlet resistance to empty the bladder at a convenient time. These techniques may also be useful to decompress a neobladder when the outlet resistance is low either by volitional sphincteric relaxation or when surgically induced.
Vesicoureteral reflux is a relative contraindication to external compression or the Valsalva maneuver, especially in patients capable of generating a high intravesical pressure. The most flagrant misuse of this form of management is in the patient with a neurogenic bladder and poor detrusor compliance, because the increased storage pressures can cause upper tract deterioration with minimal filling. External compression or Valsalva maneuvers will only further aggravate this already dangerous situation.
Even when the patient has a flaccid bladder and/or low detrusor leak point pressures, close follow-up and periodic evaluation are necessary to avoid upper tract deterioration. Chang and colleagues (2000) reported the long-term urologic complications and residual volumes of 74 SCI patients with flaccid bladders who routinely performed the Credé maneuver as their primary form of bladder management for more than 20 years. Residual urine volume was greater than 100 ml in 93% of patients, and 50% were greater than 300 ml. The authors also reported that 59.5% developed ureteral dilation, 35.1% developed hydronephrosis, and 16.2% suffered renal deterioration. Males were significantly more likely to develop these complications than females, probably due to increased outlet resistance. Other complications included recurrent UTIs, pyuria, urinary lithiasis, epididymo-orchitis, genital-rectal prolapse, and hemorrhoids (Chang et al, 2000; Wyndaele, 2008).
Promotion or Initiation of Reflex Contractions
In patients with SCI and an intact sacral spinal cord, voiding can be elicited by exploiting existing spinal reflexes. The sacral micturition reflex occurs when tension receptors within the bladder wall are stimulated by bladder filling and activate sensory afferent neurons. Motor efferents from the spinal cord respond by generating a reflexive bladder contraction and, when of adequate magnitude, will result in voiding. Bladder contractions obtained in this manner are often involuntary and sporadic. In some cases, patients with SCI may be able to trigger this reflex voluntarily by manual stimulation of areas within the sacral or lumbar dermatomes. According to the classic reference by Glahn (1974), the most effective method of initiating a reflex contraction is rhythmic suprapubic manual pressure (seven or eight pushes every 3 seconds). Quick repetitive stimulation in this manner is thought to produce a summation effect on the tension receptors in the bladder wall, resulting in activation of the bladder reflex arc. Ideally, the elicited contraction would be of sufficient magnitude and duration to empty the bladder. Other commonly used maneuvers to induce reflex detrusor contractions include pulling the skin or hair of the pubis, scrotum, or thigh; squeezing the clitoris; or by digital rectal stimulation (Wein and Barrett, 1988). Patients able to void in such a way are encouraged to find their own optimal trigger points and position for urination. If induced emptying can be carried out frequently enough to keep bladder volume and pressure below the threshold for activation of the involuntary micturition reflex, and below pressures that might cause upper tract deterioration, incontinence can be controlled, similar to timed voiding in normal individuals. Reflex voiding is dependent on the ability to stimulate detrusor contractions and may be most suitable for patients with SCI or conditions characterized by detrusor overactivity. To void reflexively, patients require manual dexterity and the ability to transfer to a commode, or at least the ability to use and maintain an external collecting device. For this reason, reflex voiding may not be suitable for many female patients. Surgical procedures to reduce outlet resistance should be considered if significant obstruction or sphincter dyssynergia are present. Complications with this voiding technique are most often related to the use of external collection devices or from the development of high-pressure storage resulting in upper tract deterioration. To ensure compliance, periodic surveillance should be performed with consideration of regular urodynamic evaluation.
Some clinicians believe that the micturition reflex can be “trained” by maintaining a copious fluid intake and periodically clamping and unclamping an indwelling catheter or by CIC at regular intervals. This cyclic pattern of filling and emptying may promote storage and emptying in a more physiologic manner, focuses attention on the urinary tract, and ensures an adequate fluid intake. It is true that balanced lower urinary tract function can be achieved using this program (Opitz, 1984; Menon and Tan, 1992), but whether this is a cause-and-effect relationship is unknown and difficult to prove.
One fascinating set of experiments that relates to the concept of establishing or promoting a reflex pathway for micturition is that reported by Xiao and de Groat (1999). They created a skin-to–central nervous system to bladder reflex pathway in cats by intradural microanastomosis of the left L7 ventral root to the S1 ventral root, leaving the L7 dorsal root intact to conduct cutaneous afferent signals. A detrusor contraction, without striated sphincter dyssynergia, could be initiated by scratching the skin or by percutaneous electrical stimulation in the L7 dermatome. The pathway was found to be mediated by cholinergic transmission at both ganglionic and peripheral levels. The importance of this experimental model is that somatic motor axons were able to innervate parasympathetic bladder ganglion cells and therefore transfer somatic reflex activity to the lower urinary tract. Xiao and colleagues (2003) reported on the first human clinical trial, in which 15 males with neurogenic overactivity and detrusor–external sphincter dyssynergia (DESD) secondary to complete suprasacral SCI underwent a ventral root microanastomosis between the L5 and the S2 and/or S3 ventral root. With a mean follow-up of 3 years, recovery in bladder storage and emptying function was reported in 10 (67%) patients. Average residual urine decreased from 332 cc to 31 cc with resolution of UTIs and overflow incontinence. Postoperative urodynamics demonstrated the resolution of detrusor overactivity and DESD to near-normal storage pressures, with synergic voiding and without DESD. Improvement in bowel function was also noted. Xiao and colleagues (2005) subsequently reported on 20 children with neurogenic bladder from spina bifida, among whom 17 of 20 (71.4%) demonstrated urodynamic improvements after surgery. Partial loss of L4 or L5 motor function, ranging from slight muscular weakness to visible foot drop, was reported in 5 of 20 patients. Xiao (2006) recently reviewed the initial human trials to date, and although encouraging results have been reported, experiences from other centers are needed to confirm these findings.
Reduction Cystoplasty
Myogenic decompensation of the bladder with excessive urinary capacity and chronic urinary retention may be idiopathic, of neurologic origin, the result of an infravesical obstruction, or exist as one of the components of the prune belly syndrome (Eagle-Barrett syndrome). The simplicity of partial cystectomy to reduce bladder capacity and, theoretically, restore contractility has often enticed surgeons to perform this procedure; however, if urodynamic evaluation after prolonged Foley catheter drainage shows no spontaneous detrusor contractions, this procedure is unlikely to be successful. If contractility is proven, surgical reduction is certainly an option in the appropriate patient. Some of the techniques that have been used in the past include fundus invagination (Stewart, 1966), transection and removal of the upper “free” part of the bladder (Klarskov et al, 1988), a detrusor wrap (Hanna, 1982), or detrusor duplication (Zoedler, 1964). Kinn (1985) reported on 10 patients with various etiologies and was successful in reducing the bladder capacity, reducing residual urine volume, and reducing frequency of urinary infection, but found no improvement of detrusor contractility after reduction cystoplasty. Similar results were obtained by Klarskov and colleagues (1988), who reported on 11 patients, with only 3 being subjectively cured, one proceeding to supravesical diversion, and 5 using CIC. Although patients with acontractile bladders generally do poorly, encouraging results have been attained in patients with hypocontractile detrusor function (Kinn, 1985; Klarskov et al, 1988). Kinn (1985) recommended that a radical incision of the bladder neck also be performed in men undergoing these procedures to reduce outlet resistance. Bukowski and Perlmutter (1994) reported on the long-term outcome of reduction cystoplasty in 11 boys with prune belly syndrome. They found that short-term reductions in bladder volume may have initially contributed to the decreased incidence of urinary infections, but bladder capacity and residual volumes tended to increase over time. Overall, reduction cystoplasty has not been definitively proven to yield long-term success in most patients, and its use remains debatable. When compared to properly performed CIC alone, reduction cystoplasty is unlikely to add much to the patient’s quality of life and should be used in only select patients.
Bladder Myoplasty
Patients with impaired or absent detrusor contractility have few dependable treatment options, except for continuous or clean intermittent catheterization. Although neurostimulator implantation has achieved modest success in patients with idiopathic detrusor hypotonicity, it has not shown significant benefit in neurogenic conditions where the bladder is unable to generate a contraction. To address this particularly difficult situation, several investigators have focused on attempting to restore bladder contractility through correction of the myogenic component of the disorder. Restoration of myogenic deficits by the transfer of an innervated, striated free muscle flap has been used successfully in reconstructive efforts involving other areas of the body, such as the heart (Blanc et al, 1993; Ninkovic et al, 2003). The augmentation of detrusor function with a skeletal muscle flap and cellular tissue engineering using injectable myoblasts represent the two current methodologies that have evolved.
In an animal feasibility study, Chancellor and associates (1994c) were among the first to report successful restoration of voluntary emptying of the bladder using a wrap of skeletal muscle. To augment the noncontractile detrusor, a segment of neurovascularly intact rectus muscle was wrapped around the bladder. Further experience was reported by Stenzl and colleagues (1998), when three patients managed with chronic catheterization for bladder acontractility were treated with a microneurovascular free transfer of autologous latissimus dorsi muscle. Short-term follow-up revealed peak flow rates of 18 to 26 mL/sec and residual urine volumes of 0 to 90 mL. In this procedure, the main neural and vascular supply to the latissimus dorsi was anastomosed to the lowermost motor branch of the intercostal nerve and to the inferior epigastric vessels supplying the rectus abdominis muscle. The transferred muscle was wrapped around the bladder with longitudinal tension and a slightly spiral configuration, ultimately covering about 75% of the mobilized bladder and leaving only the area of the trigone and the lateral pedicles uncovered. Patients were instructed to empty their bladders by actively contracting the lower abdominal musculature. Similarly, Stenzl and colleagues (2003) reported on 20 patients with bladder acontractility, with a mean follow-up of 44 months, and found that 14 of 20 (70%) were able to void spontaneously within 4 months, 4 more were able to void spontaneously after a bladder neck incision, while 2 had minimal improvement. Available urodynamic parameters revealed mean voided volumes of 447 mL (range, 250 to 728 mL), mean postvoid residual volumes of 38 mL (range, 0 to 100 mL), and mean maximum detrusor pressures of 72 cm H2O (range, 5 to 218 cm H2O). Recently, equally impressive results were reported in a multicenter trial of latissimus dorsi detrusor myoplasty by Gakis and associates (2009). In 24 patients with a mean follow-up of 46 months, 16 of 24 (67%) regained spontaneous micturition without the need for CIC, while 3 were able to reduce the frequency of CIC.
Both Von Heyden and colleagues (1998) and Van Savage and associates (2000) reported early studies with electrically stimulated detrusor myoplasty in dogs. Von Heyden and colleagues described anastomosing the thoracodorsal nerve to the obturator nerve and the vascular supply to the external iliac vessels. The graft was then stimulated by electrodes connected to the anastomosed neural supply and with direct muscle stimulation. Van Savage and associates described detrusor myoplasty with rectus muscle with the addition of stimulation being achieved with electrodes inserted into the muscle near the nerve entrance. Good results were reported initially with acute stimulation generating bladder pressures adequate for bladder emptying. Unfortunately, in a study of chronic contractile dysfunction, although detrusor compliance and flap viability were maintained, bladder emptying was not (Van Savage et al, 2002).
Yokoyama and coworkers (2000) reported on myoblast-mediated ex-vivo gene transfer to improve contractility. The authors demonstrated successful long-term survival of the injected myoblasts in the bladder, with preliminary histochemical evidence that these skeletal myoblasts can differentiate to smooth muscle. Further research in this area will be needed to determine whether tissue engineering using cellular myoplasty can improve impaired bladder contractility. For further discussion of the role of myoblast injection therapy for urologic disorders, including treatment of detrusor failure and stress urinary incontinence, see Chapter 19.
Key Points: Increasing Intravesical Pressure or Facilitating Detrusor Contractility
Decreasing Outlet Resistance at a Site of Anatomic Obstruction
Transurethral Resection or Incision of the Bladder Neck
The first transurethral bladder neck resection for neurogenic lower urinary tract dysfunction was performed by Emmett in 1937 (Wein and Barrett, 1988). Originally, the procedure had two main indications: a hypocontractile (or atonic bladder) and anatomic (or functional) obstruction at the level of the bladder neck and/or proximal urethra that prevented emptying despite abdominal straining or a sustained detrusor contraction. It was also used in areflexic bladder patients with sacral spinal cord lesions who could not adequately empty by straining or Credé maneuver; however, this simply created progressively severe stress incontinence. In these instances, other alternatives for improving bladder emptying should be sought first (Wein et al, 1976).
The most common modern indication for transurethral resection (TURBN) or incision (TUIBN) of the bladder neck is a urodynamic-confirmed anatomic or functional obstruction at the bladder neck or proximal urethra. Primary bladder neck obstruction (PBNO) is a videourodynamically diagnosed condition, characterized by high-pressure, low-flow voiding, with radiographic evidence of obstruction at the bladder neck, without striated sphincter contraction or distal obstruction (Padmanabhan and Nitti, 2007) (Fig. 75–6). Alternatively, the diagnosis can be made by a micturitional urethral profile (McGuire et al, 1984). The true prevalence and etiology of PBNO is unknown. The highest reported incidence is in younger males with severe lower urinary tract symptoms (LUTS) and ranges from 33% to 54% in this population (Huckabay and Nitti, 2005). Much lower incidences have been reported for females (4.6% to 8.7%). For more on bladder neck dysfunction refer to Chapter 65.
Figure 75–6 During urodynamics in a young male with severe lower urinary tract symptoms, a primary bladder neck obstruction was found. The bladder neck obstruction was accentuated by having the patient tighten his voluntary sphincter during voiding.
Currently, the preferred technique is incision of the bladder neck at the 5-o’clock and/or 7-o’clock positions. A single full-thickness incision is extended from 1 cm distal to each ureteral orifice and continued through the bladder neck and prostatic fossa to just proximal to the verumontanum. The incision is deepened until no ridge is visible at the bladder neck, and fat is visualized through the capsular fibers (Huckabay and Nitti, 2005). Other techniques include a limited resection of the dorsal tissue from the 3-o’clock to the 9-o’clock position and a resection limited to the posterior lip. In women, incisions may be performed at the same anatomic location as in men and extended from just inside the vesical neck through the proximal third of the urethra (Blaivas et al, 2004). Overly aggressive technique should be avoided in females, because of the risk of stress incontinence.
In this predominately young male population, one of the most significant concerns with bladder neck incision or resection is the development of retrograde ejaculation. A reduction in the incidence of retrograde ejaculation has been reported with the performance of a unilateral TUIBN (Kaplan et al, 1994; Kochakarn et al, 2003). Earlier studies reported the incidence of diminished ejaculate volume ranging from 10% (Turner-Warwick, 1984) to as high as 50% (Norlen and Blaivas, 1986). More recently, a 27% incidence was reported with bilateral incisions (Trockman et al, 1996), while antegrade ejaculation was preserved in all patients who underwent a unilateral incision (Kaplan et al, 1994).
Significant improvements in symptom relief and maximum flow rate have been reported with these techniques (Huckabay and Nitti, 2005). Norlen and Blaivis (1986) reported subjective improvement in 18 men with PBNO, with an increase in Qmax from 9.1 mL/sec to 26.1 mL/sec. Kaplan and colleagues (1994) performed a unilateral incision in 31 men, 30 of 31 had subjective improvement and a mean increase in Qmax from 9.2 to 15.6 mL/sec. Peng and Kuo (2005) reported their experience with TUIBN in 11 women with urodynamically confirmed bladder neck obstruction. After a mean follow-up of 10 months, significant urodynamic improvements were reported in Qmax (3.8 to 12.0 mL/sec), residual urine (355.5 to 84.5 mL), and voiding pressure (86.5 to 59.1 cm H2O). A similar study of 11 women with PBNO was reported by Goldman and Zimmern (2006). Ten had improvement or resolution of symptoms, and one developed mild SUI postoperatively.
YV-Plasty of the Bladder Neck
The objective of the YV-plasty is to reduce bladder outlet resistance by revision of the anterior bladder neck; however, this technique is now rarely performed. Bladder neck resection or incision performed endoscopically is now the preferred procedure and is capable of achieving the same objectives as the YV-plasty but with less morbidity. It would only seem appropriate to consider this method when a concurrent open procedure is already required for a concomitant urologic disorder. Significant incontinence may result following this procedure and, at times, was the intended consequence, especially when it was performed radically or in the setting of a refractory, poorly emptying bladder. Due to the infrequency and short-lived use of this procedure, the efficacy of YV-plasty and incidence of postoperative complications have not been reported.
Decreasing Outlet Resistance at the Level of the Striated Sphincter
Detrusor sphincter dyssynergia (DSD) is the most common cause of neurogenic sphincteric obstruction and is most often managed by intermittent catheterization. Avoidance of high intravesical pressure through appropriate bladder drainage protects the upper tracts, decreases provocation of autonomic dysreflexia, and lessens the risk for other complications such as UTI and stones. If improperly treated, complications from DSD will occur in greater than 50% of men (Chancellor and Rivas, 1995; Ku, 2006). For patients who are unwilling or unable to self-catheterize, the most effective treatment option is controversial. Historically, external sphincterotomy served as the primary surgical intervention for DSD, resulting in significant short-term improvement in bladder emptying in 70% to 90% of cases (Wein et al, 1976). Failure rates as high as 40% to 50%, however, have left the long-term efficacy of sphincterotomy in question (Santiago, 1993; Vapnek et al, 1994; Yang and Mayo, 1995). With favorable results being reported for less invasive alternatives such as laser sphincterotomy and urethral stenting, the role of traditional sphincterotomy continues to be debated. Sphincteric injection of botulinum toxin has also gained interest as an alternative to sphincterotomy. Other pharmacologic alternatives, including the use of Botox are discussed in Chapter 68.
Surgical Sphincterotomy
Therapeutic destruction of the external urethral sphincter was initially performed in 1936, but the first large clinical series was not reported until 1958 by Ross and colleagues (Wein and Barrett, 1988). Several techniques have been described; however, the 12-o’clock sphincterotomy, as originally proposed by Madersbacher and Scott (1975), remains the procedure of choice for a number of reasons. The anatomy of the striated sphincter is such that its main bulk is anteromedial. With the blood supply primarily lateral, a 12-o’clock incision is least likely to cause hemorrhage, with reported rates of 5% to 20%. The rate of postoperative erectile dysfunction with this incision is approximately 5% compared with a 5% to 30% incidence with a 3-o’clock and 9-o’clock technique. Significant urinary extravasation has also been reported. Sphincterotomy can be performed using a knife electrode, resection with a loop electrode, or laser ablation. The incision must extend from the level of the verumontanum at least to the bulbomembranous junction. Gradual deepening of the incision allows good visual control and minimizes the chance of significant hemorrhage and extravasation. An external collecting device is usually necessary postoperatively, precluding usage in females. Vapnek and associates (1994) reported that conversion to suprapubic bladder drainage was most commonly attributed to difficulty with the collection device. Continuous sphincteric incontinence or severe stress incontinence is uncommon, unless the urethra or bladder neck has been compromised by prior surgery or preexisting pathology. Noll and colleagues (1995) reported that laser sphincterotomy seems to have an advantage over conventional diathermy procedures, after finding that only 15.1% of laser-treated patients required resphincterotomy compared with 30% of those treated with a conventional procedure. Further advantages of laser sphincterotomy were reported by Perkash (1996) with the finding of less bleeding following procedures performed in this manner.
When early failure occurs, it is generally attributable to an inadequate surgical procedure (either not deep enough or not extensive enough), inadequate detrusor function, and bladder neck or prostatic obstruction. Late failure may occur because of fibrosis somewhere along the extent of the sphincterotomy, a change in detrusor function, the development of prostatic obstruction, or a change in neurologic status such that smooth sphincter dyssynergia develops (Lockhart and Pow-Sang, 1989). Yang and Mayo (1995) defined failure as (1) the presence of large postvoid residual urine volumes associated with urinary tract infections, (2) autonomic hyperreflexia symptomatology associated with bladder overdistention or high voiding pressures, and/or (3) progressive upper tract deterioration from persistent reflux or poor bladder emptying.
Failure rates following sphincterotomy have been reported to be as high as 40% to 50% in some series (Santiago, 1993; Vapnek et al, 1994; Yang and Mayo, 1995). In addition, the incidence of failure increases with time from the initial procedure (Vapnek et al, 1994; Yang and Mayo, 1995). In a more recently reported series, Pan and colleagues (2009) reported only a 32% primary success rate following external sphincterotomy, with eventual failure occurring in 57 of 84 (68%) patients at a mean of 42.7 months from the initial procedure. Of the 57 patients that failed, 30 underwent a repeat sphincterotomy with a mean response of 56 additional months. Kim and colleagues (1998) reported a significantly higher incidence of upper tract damage and persistent sphincter dyssynergia with detrusor leak point pressures of greater than 40 cm H2O. Juma and associates (1995) considered detrusor leak point pressure as the most reliable urodynamic parameter to predict the risk of upper tract complications after sphincterotomy. The authors reported a 30% incidence of significant upper tract complications (half of which developed more than 2 years after sphincterotomy), in their series of 63 patients with a mean follow-up of 11 years. Lower urinary tract complications (recurrent infection, calculi, urethral diverticula, stricture, bladder neck stenosis, and recurrent epididymitis) occurred in 48% of patients.
Urethral Stenting
The use of a urethral stent to bypass the striated sphincter was suggested by Shah and associates (1990). Chancellor and Rivas (1995) reported the initial multicenter North American data using the UroLume stent (American Medical Systems, Minnetonka, MN) in 153 patients at 15 centers. A significant decrease in detrusor leak pressure and residual urine volume was seen; however, 18% of patients required more than one procedure to adequately open the sphincter, hyperplasia within the lumen was seen in 42 patients (33.3%) at 3 months, and 10 devices were removed (7 for migration) and 7 were replaced. Bladder neck obstruction requiring treatment was seen in 13 patients (4 with α blockers, 7 with incision, 2 with CIC), and when removal was required (in 4) this was not a problem up to 12 months. Rivas and coworkers (1994) concluded that a stent prosthesis is as effective, easier, less morbid, and less expensive than a sphincterotomy.
Chancellor and colleagues (1999b) reported the long-term follow-up of the UroLume stent in for striated sphincter dyssynergia in a group of 160 men with SCI at 15 centers in North America. Mean voiding pressure decreased from 75.1 cm H2O to 37.4 cm H2O at year 1 and was maintained for up to 5 years. Residual urine volume also decreased and was maintained for 5 years; however, the residual urine volume was not less than 105 mL in any group, and mean cystometric bladder capacity did not change. Symptoms of autonomic hyperreflexia after stent placement were reduced in 53 of 89 patients at 1 year and 37 of 72 at 2 years. De novo detrusor overactivity developed in only 1 of 36 patients in the 1-year follow-up group and only 3 of 30 in the 2-year group. One obvious advantage using a sphincteric stent is that it is potentially reversible. Fifteen percent of patients (24 patients) required stent removal; another stent was implanted in 4 of these. Stent migration occurred in 12.4% of patients at 3 months after implantation and in 5% at 6 months, with correspondingly decreased percentages up to 4 and 5 years. Forty-seven patients (26.3%) were diagnosed with bladder neck obstruction after stent placement, and were managed by bladder neck incision in 20, α blockade in 10, CIC in 8, and watchful waiting in 9. The authors commented that in those patients who required stent removal, the procedure could be accomplished despite epithelialization but that a learning curve certainly exists before one becomes comfortable removing the stent.
Chancellor and colleagues reported a prospective, randomized, multicenter trial of sphincteric stent placement versus external sphincterotomy (Chancellor et al, 1999a). Fifty-seven men were randomized; the primary outcome indicator was maximum detrusor pressure. At 12 months, there was no significant difference; however, at 24 months, maximum detrusor pressure had decreased from 90.6 cm H2O to 41.6 cm H2O in the sphincterotomy group and from 101.9 cm H2O to 71.6 cm H2O in the stent group. Whether this is meaningful is uncertain. At 24 months, residual urine volume had decreased from 218 to 112 mL in the sphincterotomy group and from 164 to 132 mL in the stent group. Cystometric capacities did not appear to change up to 24 months. Bladder neck obstruction requiring treatment developed in 6 patients in each group. Six of the 31 stent patients required stent removal, all without difficulty.
The use of temporary urethral stents in 147 men with DSD was reported by Gamé and colleagues (2008). Patient satisfaction and treatment efficacy was assessed every 3 months postoperatively until a mean of 10 months after insertion. After the initial period of temporary stenting, patients who were subjectively satisfied with this treatment modality, who also had a significant decrease in urinary complications and in postvoid residual, went on to permanent urethral stenting, 70.7% in this case. Seven (4.7%) recovered enough neurologically to perform CIC upon removal of the temporary stent. The authors state that the use of temporary urethral stents allows identification of patients who are unlikely to benefit from permanent stent insertion.
The ideal patient and therapeutic role for urethral stenting remains controversial (Boone, 2009; Perkash, 2009). The reversibility of this procedure is attractive to some; however, stent removal, when necessary, may be difficult. Complications include obstruction by urothelial ingrowth, stent encrustation, stricture, stent migration, and UTI. Similar to sphincterotomy, a collection device is required with urethral stenting, precluding use in females.
An intraurethral sphincter prosthesis with a self-contained urinary pump has been developed for use in women with a hypocontractile or acontractile bladder (“inflow” intraurethral insert). Schurch and associates (1999) reported on the use of this device in 18 women with neurogenic voiding dysfunction and a hypocontractile bladder. Sixteen months after placement, only 6 of the women were still using the implant and were satisfied. In 10 patients, severe incontinence around the catheter or irritation necessitated removal. In 2 patients, the device was removed because the patients could not transfer to the toilet to empty their bladder. Fourteen of the devices had to be replaced early because of device dysfunction, and three external remote control units malfunctioned. At that time, the authors considered the device unsuitable for long-term use.
Madjar and colleagues (2000) reported on 92 women, having the device, who were followed for periods of longer than 1 year. Early removal of the device (<14 days) was required in 56.5% of patients, mostly because of local discomfort and urinary leakage. In an additional 20.6% of patients, the device was removed between 2 and 16 months after placement. Only 21 patients (22.8% of the original group) with the device still in place after more than 1 year (mean, 24.6 months) were being followed. In these patients, six device removals in 4 patients were required because of migration into the bladder, asymptomatic bacteriuria developed in 15 patients, and symptomatic urinary tract infections developed in 4 patients. The authors’ conclusion that further studies are necessary to compare this treatment with CIC and other modalities seems justified.
Pharmacologic Sphincterotomy
Botulinum toxin is a protein neurotoxin produced by the bacterium Clostridium botulinum. Urologic use of botulinum toxin for the treatment of detrusor striated sphincter dyssynergia was first reported by Dykstra and colleagues (Dykstra and Sidi, 1990; Dykstra et al, 1998). Further experiences followed, with several reports of botulinum toxin usage in diverse groups with voiding dysfunction (Schurch et al 1996; Gallien et al, 1998; Petit et al, 1998). Fowler and coworkers (1992) injected botulinum toxin into six women with difficult voiding/urinary retention secondary to what is now called the Fowler syndrome (manifested by abnormal myotonus-like electromyographic activity in the striated urethral sphincter). Kuo (2003) reported periurethral injections of botulinum A toxin in 103 patients with lower urinary tract dysfunction due to a number of different causes, including detrusor sphincter dyssynergia, dysfunctional voiding, nonrelaxing urethral sphincter, cauda equina lesion, peripheral neuropathy, and idiopathic detrusor underactivity. As the role of botulinum toxin in the treatment of sphincteric obstruction continues to evolve, its use remains uncommon and unclear. A more detailed discussion of the role of botulinum toxin in urologic practice is covered in Chapter 68.
Theoretically, any agent that promotes striated sphincter relaxation in a uroselective manner could be used to decrease outlet resistance and facilitate voiding dysfunction. Yoshiyama and associates (2000) reported improved voiding in rats with SCI by using intravenously administered α-bungarotoxin. This compound is a toxin, extracted from the venom of a Formosan snake, that selectively blocks nicotinic receptors without influencing transmission in autonomic ganglia. The nicotinic receptors in the striated sphincter have been shown to be a potential target for drug therapy for striated sphincter dyssynergia.
Urethral Overdilatation
Urethral overdilatation to 40 to 50 Fr in females can achieve the same objective as external sphincterotomy in males (see Wein and Barrett, 1988) but is rarely performed because of the lack of a suitable external collecting device. In young boys, a similar stretching of the posterior urethra can be performed using a perineal urethrostomy, obviating or postponing the need for a surgical sphincterotomy. Wang and associates (1989) reported the results of urethral dilatation, using sounds or balloon procedures, to 22 to 28 Fr in 11 myelodysplastic children with high intravesical pressures refractory to traditional forms of treatment. After dilatation, the intravesical pressures decreased, and upper tract function and bladder compliance improved. There was no discernible effect on continence. These observations demonstrated that urethral dilation can decrease outlet resistance and improve compliance without deterioration in urinary control beyond preoperative conditions.
Balloon dilatation of the external urethral sphincter was reported by Werbrouck and associates (1990). Using balloon dilatation to 90 Fr at 3 atm, Chancellor and colleagues (1994b) compared balloon dilatation (20 patients), sphincterotomy (15 patients), and stent placement (26 patients) in the treatment of striated sphincter dyssynergia. A significant decrease in detrusor leak point pressure and residual urine occurred in all three groups. Bladder capacity remained constant, renal function stabilized or improved, and autonomic hyperreflexia improved. Balloon dilatation and stent placement were associated with a significantly shorter surgery and hospitalization times and less blood loss. In the dilatation group, 3 patients developed recurrent obstruction (at 3, 8, and 12 months), 1 required transfusion, and 1 developed a 1-cm bulbar stricture. In the surgical sphincterotomy group, 2 patients required transfusion, 2 developed a stricture with obstruction, and 1 developed erectile dysfunction. In the stent group, 3 patients had migration requiring adjustment (1 had the stent replaced, 2 had a second overlapping stent), and 2 developed bladder neck obstruction requiring transurethral incision. McFarlane and colleagues (1997) reported on the use of balloon dilatation of the striated sphincter as an alternative to sphincterotomy in 14 patients followed for a mean of 55.5 months (8 to 68 months). Sphincter activity was initially abolished in all patients, and reflux, which was present in 1 patient, resolved. Long term, however, 62% failed within 1 year, and overall 85% failed. The authors concluded that this is an ineffective long-term treatment for striated sphincter dyssynergia in the majority of patients, thus recommending either surgical sphincterotomy or stenting as the surgical treatments of choice for this condition.
Pudendal Nerve Interruption
Relief of obstruction at the level of the striated sphincter can also be achieved by pudendal neurectomy, first described in 1899 by Rocket (see Wein and Barrett, 1988). This method is seldom used today due to potential undesirable effects, even after a unilateral nerve section. Bilateral nerve section results in an extremely high rate of impotence and may result in significant fecal and stress urinary incontinence. If this procedure is contemplated, the results of a block should precede the formal procedure, which should be performed only unilaterally. More recently, Changfeng and colleagues (2004) reported on the use of high-frequency electrical stimulation to provide a reversible pudendal nerve block in cats. The authors stated that the goal of this approach is to relax the external urethral sphincter only at the time of voiding, allowing normal function between voids to maintain continence. No human studies have been reported at this time.
Key Points: Decreasing Outlet Resistance
Summary
The possible etiologies of voiding and storage dysfunction are numerous and varied. Some conditions will require a complex treatment strategy including a combination of medications and diverse surgical procedures. It is important that we continue to advance our understanding of the complex pathophysiology of storage and voiding dysfunction to improve the quality of life of the patients under our care.
Lapides J, Diokno A, Silber S, et al. Clean intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458-465.
Madersbacher H. Denervation techniques. BJU Int. 2000;85:1-6.
Reynard JM, Vass J, Sullivan ME, et al. Sphincterotomy and the treatment of detrusor-sphincter dyssynergia: current status, future prospects. Spinal Cord. 2003;41:1-11.
Wein AJ, Barrett DM. Voiding function and dysfunction—a logical and practical approach. Chicago: Year Book Medical; 1988.
Wein AJ, Raezer DM, Benson GS. Management of neurogenic bladder dysfunction in the adult. Urology. 1976;8:432-438.
Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol. 2000;163:768-772.
Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol. 2000;163:1228-1233.
Ahluwalia RS, Johal N, Kouriefs C, et al. The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl. 2006;88:210-213.
Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. NEJM. 2007;356:2143-2155.
Appell RA. Injection therapy for urinary incontinence, 8th ed.. Walsh J, Retik AB, Vaughan EDJr, Wein AJ, editors. Campbell’s urology, vol 2. WB Saunders, Philadelphia, 2002, 1172-1186.
Appell RA. Bulking agents for female stress urinary incontinence. AUA Update Series. 2009;28:lesson 19.
Awad SA, Flood HD, Acker KL, et al. Selective sacral cryoneurolysis in the treatment of patients with detrusor instability/hyperreflexia and hypersensitive bladder. Neurourol Urodyn. 1987;6:307-315.
Baker J, Norton P. Evaluation of absorbent products for women with mild to moderate urinary incontinence. Appl Nurs Res. 1996;9:29-33.
Barnes DG, Shaw PJ, Timoney AG, et al. Management of the neuropathic bladder by suprapubic catheterization. Br J Urol. 1993;72:169-174.
Barrett D, et al. Surgery for the neuropathic patient (Committee 20). In: Abrams P, Khoury S, Wein A, editors. Incontinence. London: Health Publication Limited; 1999:813-816.
Bejany DE, Lockhart JL, Rhamy RK. Malignant vesical tumors following spinal cord injury. J Urol. 1987;138:1390.
Bergström K, Carlsson CPO, Lindholm C, et al. Improvement of urge cord mixed type incontinence after acupuncture treatment among elderly women—a pilot study. J Auton Nerv System. 2000;79:173-180.
Binard JE, Persky L, DeLeary G, et al. Penoplasty for receding phallus: a new surgical technique to prevent dislodging of external collecting device. J Am Paraplegia Soc. 1993;16:204-209.
Blackford HN, Murray K, Stephenson TP, et al. Results of transvesical infiltration of the pelvic plexuses with phenol in 116 patients. Br J Urol. 1984;56:647-650.
Blaivas JG, Flisser AJ, Tash JA. Treatment of primary bladder neck obstruction in women with transurethral resection of the bladder neck. J Urol. 2004;171:1172-1175.
Blanc P, Girard C, Vedrinne C, et al. Latissimus dorsi cardiomyoplasty. Perioperative management and postoperative evolution. Chest. 1993;103:214-220.
Boone TB. External urethral sphincter stent for dyssnergia. J Urol. 2009;181:1538-1539.
Brindley G. Control of the bladder and urethral sphincters by surgically implanted electrical stimulation. In: Chisolm GD, Fair WB, editors. Scientific foundations of urology. Chicago: Year Book Medical; 1990:336-339.
Brindley G. Electrical stimulation in vesicourethral dysfunction: general principles; practical devices. In: Mundy AR, Stephenson TP, Wein AJ, editors. Urodynamics: principles, practice, application. London: Churchill Livingstone; 1994:481-488.
Brindley G. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795-805.
Bukowski TP, Perlmutter AD. Reduction cystoplasty in the prune belly syndrome: a long-term follow up. J Urol. 1994;152:2113-2118.
Cameron-Strange A, Millard RJ. Management of refractory detrusor instability with transvesical phenol injection. Br J Urol. 1988;62:323-327.
Cannon TW, Lee JY, Somogyi G, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958-963.
Cartwright PC, Snow BW. Bladder autoaugmentation: early clinical experience. J Urol. 1989;142:505-508.
Cespedes RD. Collagen injection or artificial sphincter for postprostatectomy incontinence: collagen. Urology. 2000;55:5-7.
Cespedes RD, Cross CA, McGuire EJ. Modified Ingelman-Sundberg bladder denervation procedure for intractable urge incontinence. J Urol. 1996;156:1744-1747.
Chancellor MB, Bennett C, Simoneau AR, et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: prospective randomized multicenter trial. J Urol. 1999;161:1893-1898.
Chancellor MB, Erhard JM, Kilholma P, et al. Functional urethral closure with pubovaginal sling for destroyed female urethra after long-term catheterization. Urology. 1994;43:499-504.
Chancellor MB, Gajewski J, Ackman CFD, et al. Long-term follow-up of the North American Multicenter UroLume Trial for the treatment of external detrusor-sphincter dyssynergia. J Urol. 1999;161:1545-1550.
Chancellor MB, Hong RD, Rivas DA, et al. Gracilis urethromyoplasty—an autologous urinary sphincter for neurologically impaired patients with stress incontinence. Spinal Cord. 1997;35:546-548.
Chancellor MB, Rivas DA. Current management of detrusor-sphincter dyssynergia. In: McGuire E, editor. Advances in urology. St Louis: CV Mosby; 1995:291-324.
Chancellor MB, Rivas DA, Abdill CK, et al. Prospective comparison of external sphincter balloon dilatation and prosthesis placement with external sphincterotomy in spinal cord injured men. Arch Phys Med Rehabil. 1994;75:297-305.
Chancellor MB, Rivas DA, Acosta R, et al. Detrusor-myoplasty, innervated rectus muscle transposition study, and functional effect on the spinal cord injury rat model. Neurol Urodyn. 1994;13:547-557.
Chang SM, Hou CL, Dong DQ, Zhang H. Urologic status of 74 spinal cord injury patients from the 1976 Tangshan earthquake, and managed for over 20 years using the Credé maneuver. Spinal Cord. 2000;38:552-554.
Changfeng T, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172:2069-2072.
Chao R, Clowers D, Mayo ME. Fate of upper tracts in patients with indwelling catheter after spinal cord surgery. Urology. 1993;42:259-264.
Chapple CR, Hampson SJ, Turner-Warwick RT, et al. Subtrigonal phenol ingestion: how safe and effective is it? Br J Urol. 1991;68:483-485.
Crooks JE, Khan AB, Meddings RN. Bladder transection revisited. Br J Urol. 1995;75:590-591.
Dees J. Congenital epispadias with incontinence. J Urol. 1949;62:513.
Delnay K, Stonehill W, Goldman H, et al. Bladder histological changes associated with chronic indwelling urinary catheter. J Urol. 1999;161:1106-1109.
Deming CL. Transplantation of the gracilis muscle for incontinence of urine. JAMA. 1926;86:822-823.
De Ridder DJ, Everaert K, Fernández LG, et al. Intermittent catheterization with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomized parallel comparative trial. Eur Urol. 2005;48:991-995.
Desgrandchamps F, Griffith DP. The artificial bladder. Eur Urol. 1999;35:257-266.
Dewire DM, Owens RS, Anderson GA, et al. A comparison of the urological complications associated with long term management of quadriplegics with and without chronic indwelling urinary catheters. J Urol. 1992;147:1069-1073.
Douglas C, Burke B, Kessler OL, et al. Microwave: practical cost effective method for sterilizing urinary catheters in the home. Urology. 1990;35:219-224.
Dunn M, Ramsden PD, Roberts JB. Interstitial cystitis, treated by prolonged bladder distention. Br J Urol. 1977;49:641-645.
Dunn M, Smith JC, Ardran GM. Prolonged bladder distension as a treatment of urgency and urge incontinence of urine. Br J Urol. 1974;46:645-652.
Dykstra DD, Sidi AA. Treatment of detrusor-striated sphincter dyssynergia with botulinum A toxin. J Urol. 1990;138:1155-1160.
Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol. 1998;139:919-922.
Emmons SL, Otto L. Acupuncture for overactive bladder. Obstet Gynecol. 2005;106:138-143.
Erekson EA, Meyer SA, Melick C, McLennan MT. Incontinence pads: recommending the best product-based wetback performance and price. Int Urogynecol J. 2008;19:1411-1414.
Ewing R, Bultitude MI, Shuttleworth KE. Subtrigonal phenol injection therapy for incontinence in female patients with multiple sclerosis. Lancet. 1983;1:1304-1306.
Fader M, Clarke-O’Neill S, Green N, et al. Gender differences in performance of and preferences for absorbent products for men and women with moderate-heavy urinary incontinence: a randomized cross-over clinical trial. Neurourol Urodyn. 2007;26:635-636.
Fader M, Cottenden A, Getliffe K, et al. Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product designs. Health Technol Assess. 2008;12:iii-iv. ix–185
Fantl JA, Newman DK, Colling J, et al. Managing acute and chronic urinary incontinence. AHCPR urinary incontinence in adults guideline update panel. Am Fam Physician. 1996;54:1661-1672.
Feifer A, Corcos J. Contemporary role of suprapubic cystostomy in treatment of neuropathic bladder dysfunction in spinal cord injured patients. Neurourol Urodyn. 2008;27:475-479.
Feneley RCL. The management of female incontinence by suprapubic catheterization, with or without urethral closure. Br J Urol. 1963;55:203-207.
Fowler CJ, Betts C, Christmas T, et al. Botulinum toxin in the treatment of chronic urinary retention in women. Br J Urol. 1992;70:387-393.
Gakis G, van Koeveringe GA, Raina S, et al. Latissimus dorsi detrusor myoplasty in patients with acontractile bladder— long-term results of a multicenter trial. J Urol. 2009;181:339.
Gallagher B, Dwyer N, Gaynor-Krupnick D, et al. Objective and quality-of-life outcomes with bone-anchored male bulbourethral sling. Urology. 2007;69:1090-1094.
Gallien P, Robineau S, Verin M, et al. Treatment of detrusor sphincter dyssynergia by transperineal injection of botulinum toxin. Arch Phys Med Rehabil. 1998;79:715-717.
Gamé X, Chartier-Kastler E, Ayoub N, et al. Outcome after treatment of detrusor-sphincter dyssynergia by temporary stent. Spinal Cord. 2008;46:74-77.
Getliffe K, Fader M, Cottenden A, et al. Absorbent products for incontinence: ‘treatment effects’ and impact on quality of life. J Clin Nurs. 2007;16:1936-1945.
Gibbon N. Management of the bladder in acute and chronic disorders of the nervous system. Acta Neurol Scand. 1966;42(Suppl.):133.
Glahn BE. Neurogenic bladder in spinal cord injury: management of patients in Hornback, Denmark. Urol Clin North Am. 1974;1:163-176.
Goldman HB, Zimmern PE. The treatment of female bladder outlet obstruction. BJU Int. 2006;98:17-23.
Golji H. Complications of external condom drainage. Paraplegia. 1981;19:189-194.
Graham C. Making a catheterization program work in patients with functional limitations. Probl Urol. 1989;3:54-65.
Gray M, Bliss DZ, Doughty DB, et al. Incontinence-associated dermatitis. J Wound Ostomy Continence Nurs. 2007;34(1):45-54.
Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95:141-152.
Gurocak S, De Gier RP, Feitz W. Bladder augmentation without integration of intact bowel segments: critical review and future perspectives. J Urol. 2007;177:839-844.
Guttman L. Management of paralysis. Br Surg Pract. 1949;6:445.
Guttmann L, Frankel H. The value of intermittent catheterisation in the early management of traumatic paraplegia and tetraplegia. Paraplegia. 1966;4:63-83.
Hackler R. Long-term suprapubic cystotomy in spinal cord injury patients. Br J Urol. 1982;54:120-124.
Hamid R, Bycroft J, Arya M, Shah PJ. Screening cystoscopy and biopsy in patients with neuropathic bladder and chronic suprapubic indwelling catheters: is it valid? J Urol. 2003;170:425-427.
Hanna MK. New concept in bladder remodeling. Urology. 1982;19:6-11.
Hanno PM, Wein AJ. Conservative therapy of interstitial cystitis. Semin Urol. 1991;9:143-147.
Hasan ST, Robson WA, Ramsden PD, et al. Outcome of endoscopic bladder transection. Br J Urol. 75, 1995. 597-596
Heimburger RF, Freeman LW, Wilde NJ. Sacral nerve innervations of the human bladder. Int J Neurosurg. 1948;5:154.
Helmstein K. Treatment of bladder carcinoma by a hydrostatic pressure technique report on 43 cases. Br J Urol. 1972;44:434-450.
Hess MJ, Zhan EH, Foo DK, et al. Bladder cancer in patients with spinal cord injury. J Spinal Cord Med. 2003;26:322.
Huckabay C, Nitti VW. Diagnosis and treatment of primary bladder neck obstruction in men. Curr Urol Rep. 2005;6:271-275.
Hudson H, Murahata RI. The ‘no touch’ method of intermittent urinary catheter insertion: can it reduce the risk of bacteria entering the bladder? Spinal Cord. 2005;43:611-614.
Ingelman-Sunberg A. Partial denervation of the bladder: a new operation for the treatment of urge incontinence and similar conditions in women. Acta Obstet Gynecol Scand. 1959;38:487.
Jackson AB, DeVivo M. Urological long-term follow-up in women with spinal cord injuries. Arch Phys Med Rehabil. 1992;4:99-105.
Jacobs SC, Kaufman JM. Complications of permanent bladder catheter drainage in spinal cord injury patients. J Urol. 1978;119:740-746.
Jamil F, Williamson M, Ahmed YS, et al. Natural fill urodynamics in chronically catheterized patients with spinal cord injury. BJU Int. 1999;83:396-399.
Janknegt RA, Heesakkers JP, Weil EH, et al. Electrically stimulated gracilis sphincter (dynamic graciloplasty) for treatment of intrinsic sphincter deficiency: a pilot study on feasibility and side effects. J Urol. 1995;154:1830-1833.
Jorgensen L, Mortensen SO, Colstrup H, et al. Bladder distention in the management of detrusor instability. Eur Urol. 1985;8:107-111.
Juma S, Mostavi M, Joseph A. Sphincterotomy: long-term complications and warning signs. Neurourol Urodyn. 1995;14:33-41.
Kaplan SA, Te AE, Jacobs BZ. Urodynamic evidence of vesical neck obstruction in men with misdiagnosed chronic nonbacterial prostatitis and the therapeutic role of endoscopic incision of the bladder neck. J Urol. 1994;152:2063-2065.
Kaufman JM, Fam B, Jacobs SC, et al. Bladder cancer and squamous metaplasia in spinal cord injury patients. J Urol. 1977;118:967-972.
Kim YH, Bird ET, Priebe M, Boone TB. The role of oxybutynin in spinal cord injured patients with indwelling catheters. J Urol. 1997;158:2083-2086.
Kim YH, Kattan MW, Boone TB. Bladder leak point pressure: the measure for sphincterotomy success in the spinal cord injured patients with external detrusor sphincter dyssynergia. J Urol. 1998;159:493-497.
Kinn AC. The lazy bladder-appraisal of surgical reduction. Scand J Urol Nephrol. 1985;19:93.
Klarskov P, Holm-Bentzen M, Larsen S, et al. Partial cystectomy for the myogenous decompensated bladder with excessive residual urine. Scand J Urol Nephrol. 1988;22:251-256.
Kochakarn W, Lertsithichai P. Unilateral incision for primary bladder neck obstruction: symptom relief and fertility preservation. World J Urol. 2003;21:249-253.
Koldewijn E, Van Kerrebroeck PEV, Rosier PF, et al. Bladder compliance after posterior sacral root rhizotomies and anterior sacral root stimulation. J Urol. 1994;151:955-960.
Ku JH. The management of neurogenic bladder and quality of life in spinal cord injury. BJU Int. 2006;98:739-745.
Kuo HC. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J Urol. 2003;170:1908-1912.
Kutzenberger J, Domurath B, Sauerwein D. Spastic bladder and spinal cord injury: seventeen years of experience with sacral deafferentation and implantation of an anterior root stimulator. Artif Organs. 2005;29:239-241.
Lapides J, Diokno A, Silber S, et al. Clean intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458-465.
Leadbetter GWJr. Surgical reconstruction of total urinary incontinence. J Urol. 1964;91:261.
Leadbetter GWJr. Surgical reconstruction for complete urinary incontinence: A 10- to 22-year follow up. J Urol. 1985;133:205-212.
Lee R, Te A, Kaplan S, et al. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol. 2009;181:2622-2627.
Leng W, Faerber G, Del Terzo M, McGuire EJ. Long-term outcome of incontinent ileovesicostomy management of severe lower urinary tract dysfunction. J Urol. 1999;161:1803-1806.
Levy JB, Jacobs JA, Wein AJ. Combined abdominal and vaginal approach for bladder neck closure and permanent suprapubic tube: urinary diversion in the neurologically impaired woman. J Urol. 1994;152:2081-2084.
Liapis A, Bakas P, Creatsas G. The efficacy of bladder distention therapy in the treatment of frequency and urgency. Eur J Obstet Gynecol Reprod Biol. 2001;95:97-99.
Linsenmeyer TA. Management of long-term catheters in adults: II. Indwelling urethral and suprapubic catheters. AUA Update Series. 2006;25:lesson 38.
Lloyd LK, Kuhlemeier LV, Fine PR, et al. Initial bladder management in spinal cord injury: does it make a difference? J Urol. 1986;135:523-527.
Lloyd SN, Lloyd SM, Rogers K, et al. Is there still a place for prolonged bladder distention? Br J Urol. 1992;70:382-385.
Locke JR, Hall DE, Walzer Y. Incidence of squamous cell carcinoma in patients with long-term catheter drainage. J Urol. 1985;133:1034-1038.
Lockhart JL, Pow-Sang JM. Indications and problems with external urethral sphincterotomy. Probl Urol. 1989;3:44-53.
Lucas MG, Thomas DG. Endoscopic bladder transection for detrusor instability. Br J Urol. 1987;59:26-30.
MacDiarmid SA, Arnold EP, Palmer NB, Anthony A. Management of spinal cord injured patients by indwelling suprapubic catheterization. J Urol. 1995;154:492-494.
Madersbacher H. Denervation techniques. BJU Int. 2000;85:1-6.
Madersbacher H, Scott FB. Twelve o’clock sphincterotomy. Urol Int. 1975;30:75-81.
Madjar S, Halachmi S, Wald M, et al. Long-term follow-up of the in-flow intraurethral insert for the treatment of women with voiding dysfunction. Eur Urol. 2000;38:161-166.
Madjar S, Raz S, Gousse AE. Fixed and dynamic urethral compression for the treatment of post-prostatectomy urinary incontinence: is history repeating itself? J Urol. 2001;166:411-415.
McFarlane JP, Foley SJ, Shah PJR. Balloon dilatation of the external urethral sphincter in the treatment of detrusor-sphincter dyssynergia. Spinal Cord. 1997;35:96-98.
McGuire EJ, Savastano J. Urodynamic findings and clinical status following vesical denervation procedures for control of continence. J Urol. 1984;132:87-92.
McGuire EJ, Shi-Chun Z, Horwinski ER. Treatment for motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78-84.
Meirowsky AM, Scheibert CD, Hinchey TR. Studies on the reflex arc in paraplegia. Int J Neurosurg. 1950;7:33.
Menon EB, Tan ES. Bladder training in patients with spinal cord injury. Urology. 1992;40:425.
Misak SJ, Bunts RC, Ulmer JL, et al. Nerve interruption procedures in the urologic management of paraplegic patients. J Urol. 1962;88:392.
Moore KN, Schieman S, Ackerman T, et al. Assessing comfort, safety, and patient satisfaction with three commonly used penile compression devices. Urology. 2004;63:150-154.
Mulcahy JJ, Young AB. Percutaneous radiofrequency sacral rhizotomy in the treatment of the hyperreflexic bladder. J Urol. 1978;120:557-558.
Mundy AR. The surgical treatment of detrusor instability. Neurourol Urodyn. 1985;4:357-361.
Mutchnik SE, Hinson JL, Nickell KG, Boone TB. Ileovesicostomy as an alternative form of bladder management in tetraplegic patients. Urology. 1997;49:353-357.
National Association for Continence. National Association for Continence resource guide, 13th ed. Spartanburg (SC): National Association for Continence; 2004.
Navon JD, Soleman H, Khonsari F, et al. Screening cystoscopy and survival of spinal cord injured patients with squamous cell carcinoma of the bladder. J Urol. 1997;157:2109-2111.
Newman DK. The urinary incontinence sourcebook, 2nd ed. New York: McGraw-Hill; 1999.
Newman DK, Fader M, Bliss DZ. Managing incontinence using technology, devices, and products. Nurs Res. 2004;53:S42-S48.
Newman DK, Wein AJ. Managing and treating urinary incontinence, 2nd ed. Baltimore: Health Professions Press; 2009.
Ninkovic M, Stenzl A, Schwabegger A, et al. Free neurovascular transfer of latissimus dorsi muscle for the treatment of bladder acontractility: II. Clinical results. J Urol. 2003;169:1379-1383.
Noll F, Sauerwein D, Stöhrer M. Transurethral sphincterotomy in quadriplegic patients: long-term follow-up. Neurourol Urodyn. 1995;14:351-358.
Norlen LJ, Blaivas JG. Unsuspected proximal urethral obstruction in young and middle-aged men. J Urol. 1986;135:972-976.
O’Connor RC, Stapp EC, Donnellan SM, et al. Long-term results of suprapubic bladder neck closure for treatment of the devastated outlet. Urology. 2005;66:311-315.
Opitz JL. Treatment of voiding dysfunction in spinal cord injured patients: bladder retraining. In: Barrett DM, Wein AJ, editors. Controversies in neurourology. London: Churchill Livingstone; 1984:437-451.
Padmanabhan P, Nitti VW. Primary bladder neck obstruction in men, women, and children. Curr Urol Rep. 2007;8:379-384.
Palacio MM, Van Aalst VC, Prez Abadia GA, et al. Muscular urinary sphincter; electrically stimulated myoplasty for function and sphincter reconstruction. J Urol. 1998;160:1867-1871.
Pan D, Troy A, Rogerson J, et al. Long-term outcomes of external sphincterotomy in a spinal injured population. J Urol. 2009;181:705-709.
Parsons KF, Macher DG, Woolfender ICA, et al. Endoscopic bladder transection. Br J Urol. 1984;56:625-630.
Peng CH, Kuo HC. Transurethral incision of bladder neck in treatment of bladder neck obstruction in women. Urology. 2005;65:275-278.
Perkash I. Contact laser sphincterotomy: further experience and longer follow-up. Spinal Cord. 1996;34:227.
Perkash I. Transurethral sphincterotomy. J Urol. 2009;181:1539-1540.
Petit H, Wiart L, Gaujard E, et al. Botulinum A toxin treatment for detrusor sphincter dyssynergia in spinal cord disease. Spinal Cord. 1998;36:91-94.
Porter M, Penson D. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic and critical analysis of the literature. J Urol. 2005;173:1318-1322.
Rajpurkar A, Onur R, Singla A. Patient satisfaction and clinical efficacy of the new perineal bone-anchored male sling. Eur Urol. 2005;47:237-242.
Ramsden PD, Smith JC, Dunn M, et al. Distention therapy for the unstable bladder: later results including an assessment of repeat distention. Br J Urol. 1976;48:623-627.
Rivas DA, Chancellor MB, Bagley D. Prospective comparison of external sphincter prosthesis placement and external sphincterotomy in men with spinal cord injury. J Endourol. 1994;8:89-93.
Rockswold Gl, Bradley WE, Chou SN. Differential sacral rhizotomy in the treatment of neurogenic bladder dysfunction. J Neurosur. 1973;38:748-754.
Rutkowski SB, Middleton JW, Truman G, et al. The influence of bladder management on fertility in spinal cord injured males. Paraplegia. 1995;33:263-266.
Saint S, Kaufman SR, Rogers MA, et al. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54:1055-1061.
Saint S, Meddings JA, Calfee D, et al. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877-884.
Santiago JA. Sphincterotomy failure. J Am Paraplegia Soc. 1993;16:164-167.
Sato A. Neural mechanisms of autonomic responses elicited by somatic sensory stimulation. Neurosci Behav Physiol. 1997;27:610-621.
Sauerwein D. Die operative Behandlung der spastischen blasenlähmung bei, querschnittlähmung. Urologe. 1990;29:196-203.
Schumacher S, Scheepe JR, Bross S, et al. Sacral anterior root stimulation and cryotechnique: a new option for selective urethral sphincter block and reversible deafferentation in the future? Arch Physio Biochem. 1999;107:242-247.
Schurch B, Hauri D, Rodic B, et al. Botulinum-A toxin as treatment of detrusor sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol. 1996;155:1023-1029.
Schurch B, Knapp PA, Jeanmonod D, et al. Does sacral posterior rhizotomy suppress autonomic hyper-reflexia in patients with spinal cord injury? Br J Urol. 1998;81:73-82.
Schurch B, Suter S, Dubz M. Intraurethral sphincter prosthesis to treat hyporeflexic bladders in women: does it work? BJU Int. 1999;84:789-794.
Scott FB, Bradley W, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. 1974;112:75-80.
Seif C, Jünemann KP, Braun PM. Deafferentation of the urinary bladder and implantation of a sacral anterior root stimulator (SARS) for treatment of the neurogenic bladder in paraplegic patients. Biomed Tech. 2004;49:88-92.
Sekar P, Wallace DD, Waties KB, et al. Comparison of long term renal function after spinal cord injury using different management methods. Arch Phys Med Rehabil. 1997;78:992-997.
Shah PJR, Milroy EJ, Timoney AG, et al. Permanent external striated sphincter stents in spinal injured patients. Neurourol Urodyn. 1990;9:311-316.
Shaw C. A review of the psychosocial predictors of help-seeking behaviour and impact on quality of life in people with urinary incontinence. J Clin Nurs. 2001;10:15-24.
Sheriff MKM, Foley S, MacFarlane J. Long-term suprapubic catheterization: clinical outcome and satisfaction survey. Spinal Cord. 1998;36:171-176.
Shpall AI, Ginsberg DA. Bladder neck closure with lower urinary tract reconstruction: technique and long-term followup. J Urol. 2004;172:2296-2299.
Smith RB, van Cangh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
Stenzl A. Electrically stimulated myoplasty for functional sphincter reconstruction. J Urol. 1998;160:1615-1616.
Stenzl A, Ninkovic M, Kollé D, et al. Restoration of voluntary emptying of the bladder by transplantation of innervated free skeletal muscle. Lancet. 1998;351:1483-1485.
Stewart HH. The surgical treatment of severe chronic retention without large diverticula. Br J Urol. 1966;38:685.
Stonehill WH, Dmochowski RR, Patterson AL, et al. Risk factors for bladder tumors in spinal cord injury patients. J Urol. 1996;155:248-250.
Sturmann D, O’Grady S, D’mello V. Management of urinary incontinence: a comparative study in a continuing care facility. Infect Control Can. 4(4), 1989.
Tanagho EA. Bladder neck reconstruction for total urinary incontinence: ten years of experience. J Urol. 1981;125:321-326.
Tanagho EA, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331-1336.
Tanagho EA, Schmidt RA, Orvis BR. Neural stimulation for control of voiding disorders: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol. 1989;142:340-346.
Taub HC, Stein M. Bladder distention therapy for symptomatic relief. Urology. 1994;43:36-39.
Torrens M. The role of denervation in the treatment of detrusor instability. Neurourol Urodyn. 1985;4:353-358.
Trockman BA, Gerspach J, Dmochowski R, et al. Primary bladder neck obstruction: urodynamic findings and treatment results in 36 men. J Urol. 1996;156:1416-1420.
Turner-Warwick RT. Bladder outflow obstruction in the male. In: Mundy AR, Stephenson TP, Wein AJ, editors. Urodynamics: principles, practice and application. London: Churchill Livingstone; 1984:183-204.
Van Arsdalen KN, Klein FA, Hackler RH. Penile implants in spinal cord injury patients for maintaining external appliances. J Urol. 1981;126:1117-1123.
Van Savage JG, Perez-Abadia GP, Palanca LG, et al. Electrically stimulated detrusor myoplasty. J Urol. 2000;164:969-972.
Van Savage JG, Perez-Abadia G, Palanca LG, et al. Comparison of the experience with acute and chronic electrically stimulated detrusor myoplasty. Neuro Urodyn. 2002;21:516-521.
Vapnek JM, Couillard DR, Stone AR. Is sphincterotomy the best management of the spinal cord injured bladder? J Urol. 1994;151:961-967.
Vapnek JM, Maynard FM, Kim J. A prospective randomized trial of the lofric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization. J Urol. 2003;169:994-998.
Venn SN, Mundy AR. Long-term results of augmentation cystoplasty. Eur Urol. 1998;34(Suppl. 1):40-42.
von Heyden B, Anthon JP, Brock GB, et al. The latissimus dorsi bladder myoplasty to assist detrusor function. Urol Res. 1998;26:215-221.
Waites KB, Canupp KC, DeVivo MJ. Epidemiology and risk factors for urinary tract infection following spinal cord injury. Arch Phy Med Rehabil. 1993;74:691-695.
Wall BM, Dmochowski RR, Malecha M, et al. Inducible nitric oxide synthase in the bladder of spinal cord injured patients with a chronic indwelling urinary catheter. J Urol. 2001;165:1457-1461.
Wall LL, Stanton SL. Transvesical phenol injection of pelvic nerve plexuses in females with refractory urge incontinence. Br J Urol. 1989;63:645-650.
Wang SC, McGuire EJ, Bloom DA. Urethral dilation in the management of urological complications of myelodysplasia. J Urol. 1989;142:1054-1055.
Wein AJ, Barrett DM. Voiding function and dysfunction—a logical and practical approach. Chicago: Year Book Medical; 1988.
Wein AJ, Raezer DM, Benson GS. Management of neurogenic bladder dysfunction in the adult. Urology. 1976;8:432-438.
Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol. 2000;163:768-772.
Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol. 2000;163:1228-1233.
Werbrouck P, Baert L, Binard JE, et al. Balloon dilation of the external urethral sphincter: a clinical study. J Am Paraplegia Soc. 1990;13:13-19.
Westney OL, Bevan-Thomas R, Palmer JL, et al. Transurethral collagen injections for male intrinsic sphincter deficiency: the University of Texas-Houston experience. J Urol. 2005;174:994-997.
Westney OL, Lee JT, McGuire EJ, et al. Long-term results of Ingleman-Sundberg denervation procedure for urge incontinence refractory to medical therapy. J Urol. 2002;168:1044-1047.
Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord. 2002;40:536-541.
Wyndaele JJ. Conservative treatment of patients with neurogenic bladder. Eur Urol, Suppl. 7, 2008, 557-565.
Xiao C. Reinnervation for neurogenic bladder: historic review and introduction of a somatic-autonomic reflex pathway procedure for patients with spinal cord injury or spina bifida. Eur Urol. 2006;49:22-29.
Xiao C, Du M, Dai C, et al. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. J Urol. 2003;170:1237-1241.
Xiao C, Du M, Li B, et al. An artificial somatic-autonomic reflex pathway procedure for bladder control in children with spina bifida. J Urol. 2005;173:2112-2116.
Xiao CG, de Groat WC. “Skin-CNS-bladder” reflex pathway for micturition after spinal cord injury and its underlying mechanisms. J Urol. 1999;162:936-942.
Yang CC, Clowers DE. Screening cystoscopy in chronically catheterized spinal cord injury patients. Spinal Cord. 1999;37:204-207.
Yang CC, Mayo ME. External urethral sphincterotomy: long-term follow-up. Neurourol Urodyn. 1995;14:25-31.
Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress incontinence and bladder dysfunction. World J Urol. 2000;18:56-61.
Yoshiyama M, de Groat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956-960.
Zermann DH, Kutzenberge J, Sauerwein D, et al. Penile prosthetic surgery in neurologically impaired patients: long-term followup. J Urol. 2006;175(3 Pt. 1):1041-1044.
Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infections in Danish hospitals, nursing homes, and home care: a national prevalence study. Infect Control Hosp Epidemiol. 1996;17:215-221.
Zimmern PE, Hadley HR, Leach GE, Raz S. Transvaginal closure of the bladder neck and placement of a suprapubic catheter for destroyed urethra after long-term indwelling catheterization. J Urol. 1985;134:554-558.
Zoedler D. Zur operative behandlung der blasenatonie. Z Urol. 1964;19:743.
Zommick JN, Simoneau AR, Skinner DG, et al. Continent lower urinary tract reconstruction in the cervical spinal cord-injured population. J Urol. 2003;169:2184-2187.